Contents
What is dermatofibrosarcoma protuberans
Dermatofibrosarcoma protuberans is a rare type of cancer that causes a tumor in the deep layers of skin (the dermis). This condition is a type of soft tissue sarcoma (fibrohistiocytic neoplasm), which are cancers that affect skin, fat, muscle, and similar tissues. Dermatofibrosarcoma protuberans is estimated to occur in 1 in 100,000 to 1 in 1 million people per year. Dermatofibrosarcoma protuberans results from a new mutation that occurs in the body’s cells after conception and is found only in the tumor cells. This type of genetic change is called a somatic mutation and is generally not inherited.
Who is at risk of dermatofibrosarcoma protuberans?
- It usually presents in early or middle adult life between 20 and 59 years of age, but all ages can be affected. Although the tumor is rare in children, children get dermatofibrosarcoma protuberans. Sometimes, a child is born with dermatofibrosarcoma protuberans. In newborns, this skin cancer often looks like a birthmark.
- Most people are diagnosed when they are between 20 and 50 years of age.
- Males are affected slightly more frequently than females.
- Blacks were slightly more likely than whites to get dermatofibrosarcoma protuberans.
- Among whites, those living in Hawaii had the highest incidence of dermatofibrosarcoma protuberans.
- Cases of Dermatofibrosarcoma protuberans are increasing; this increase is greatest among whites.
- Dermatofibrosarcoma protuberans favors the trunk (40–60%), followed by the proximal extremities (20–30%) and the head and neck (10%–16%) 1).
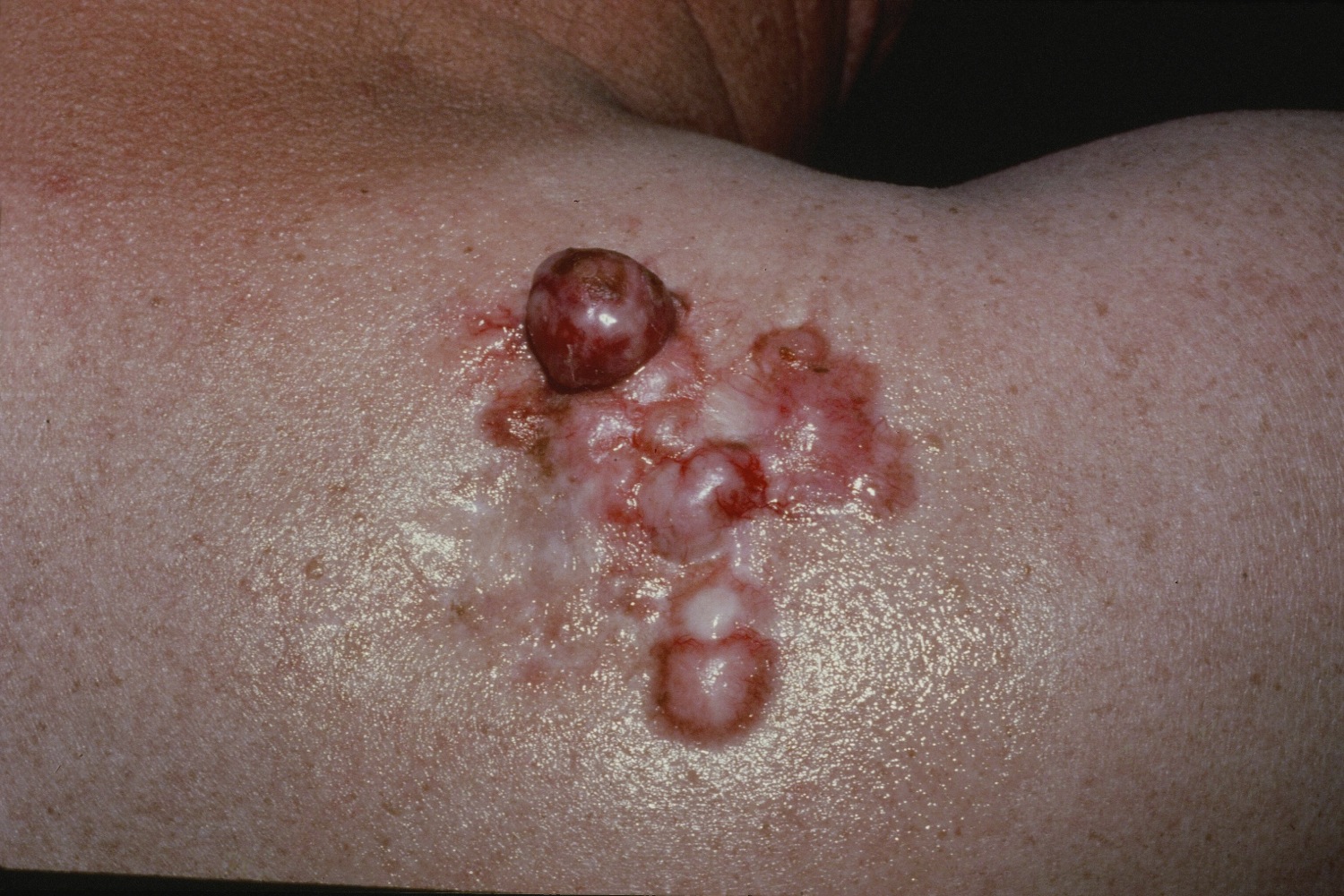
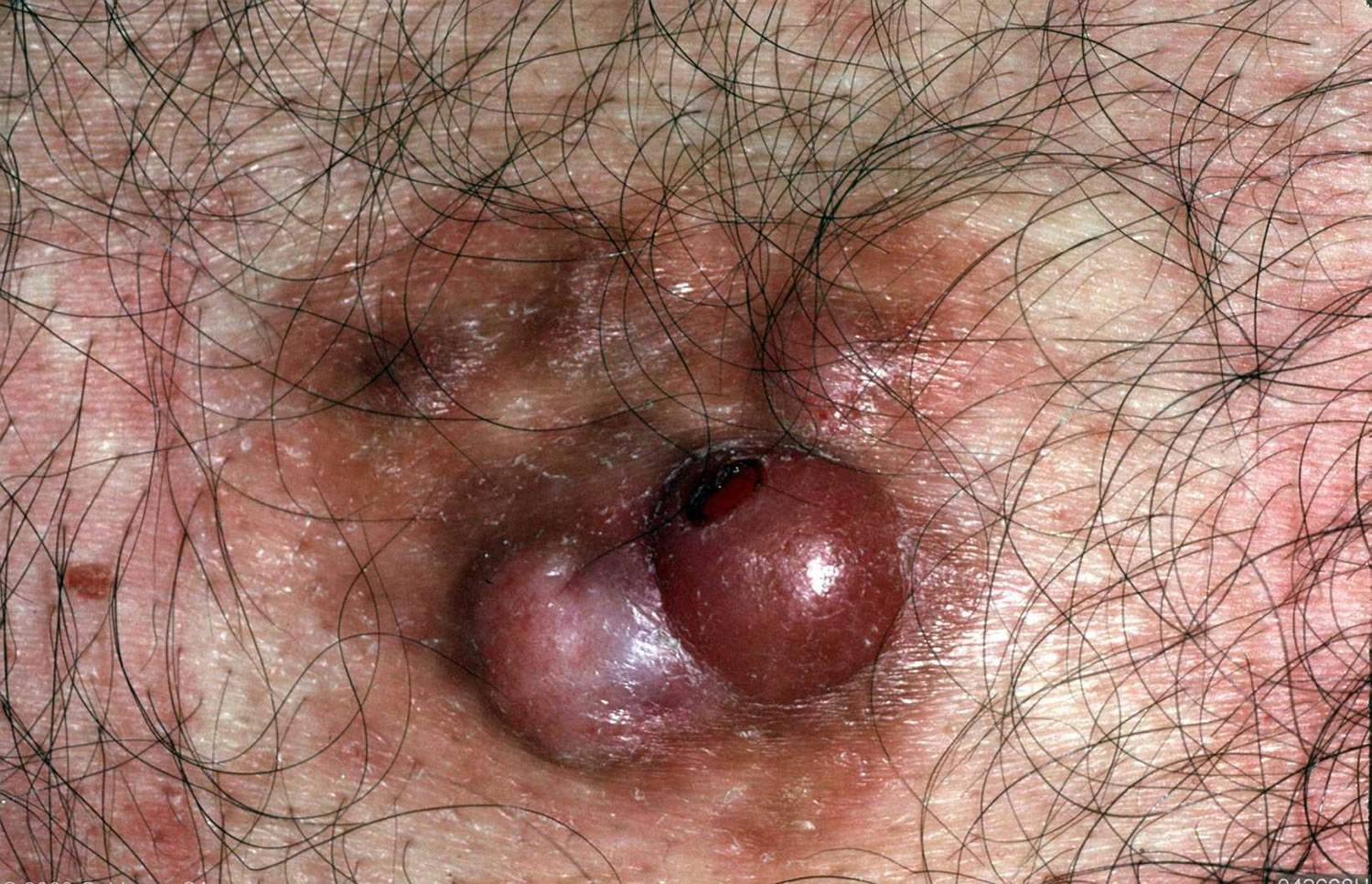
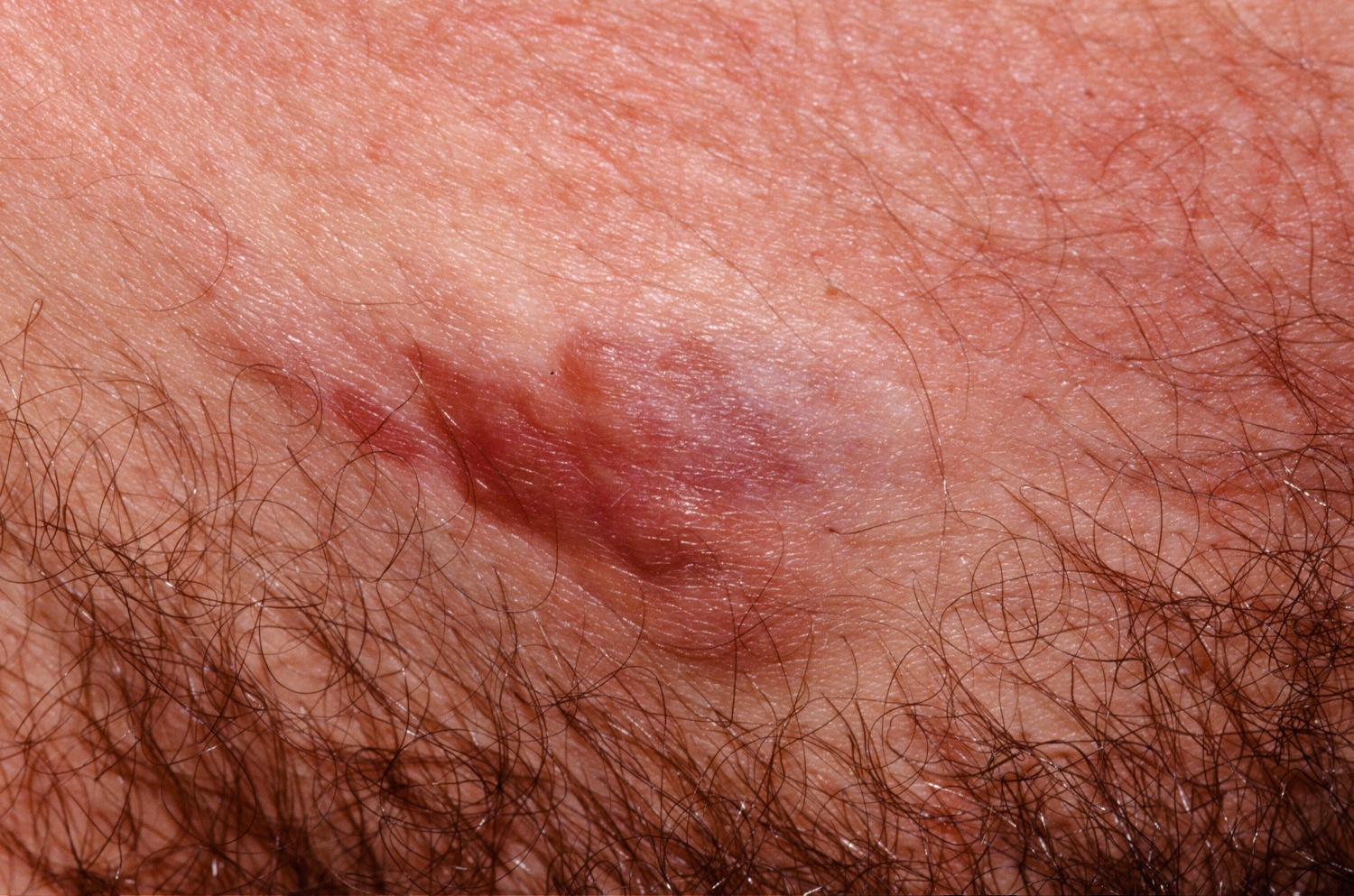
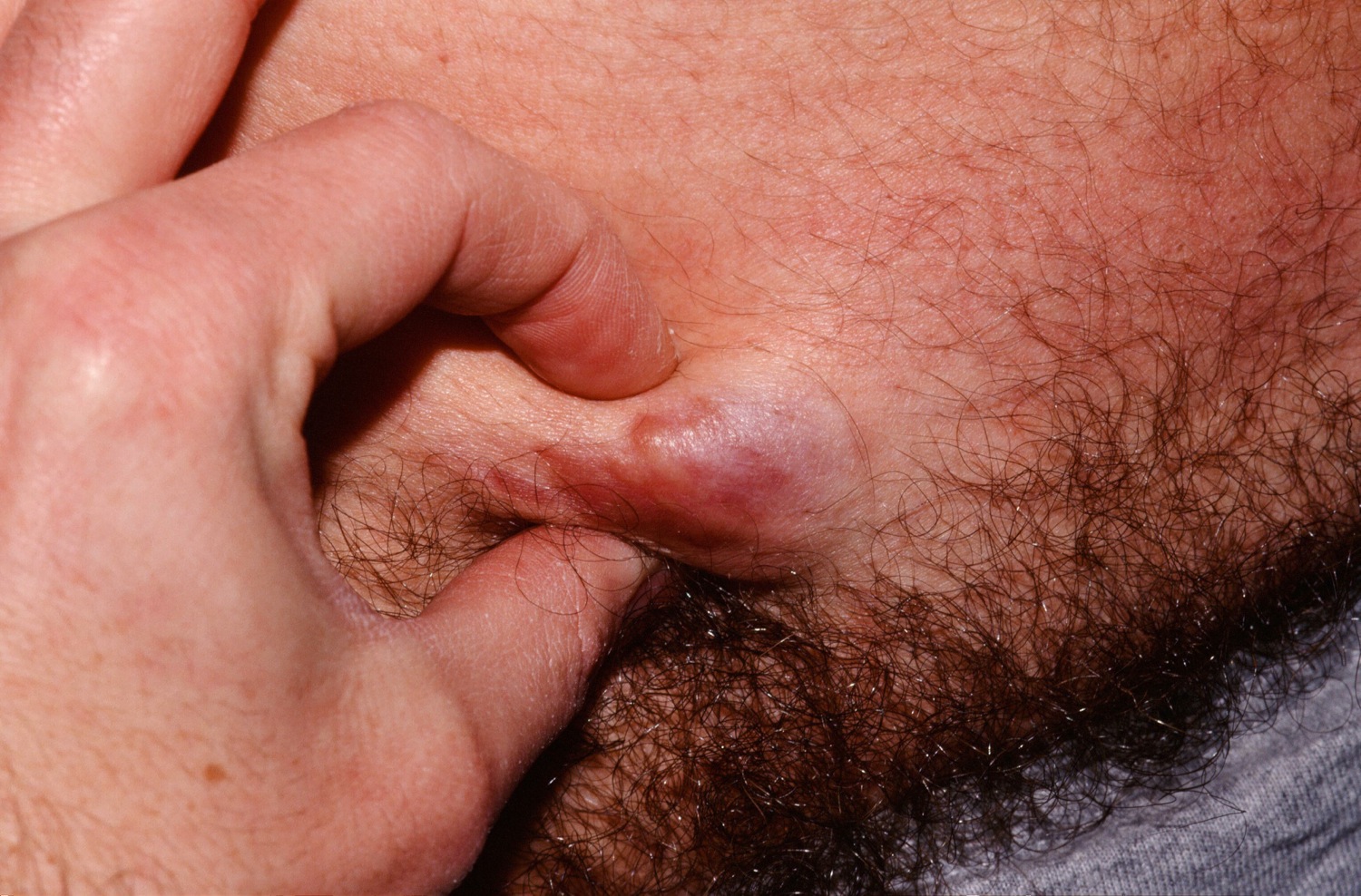
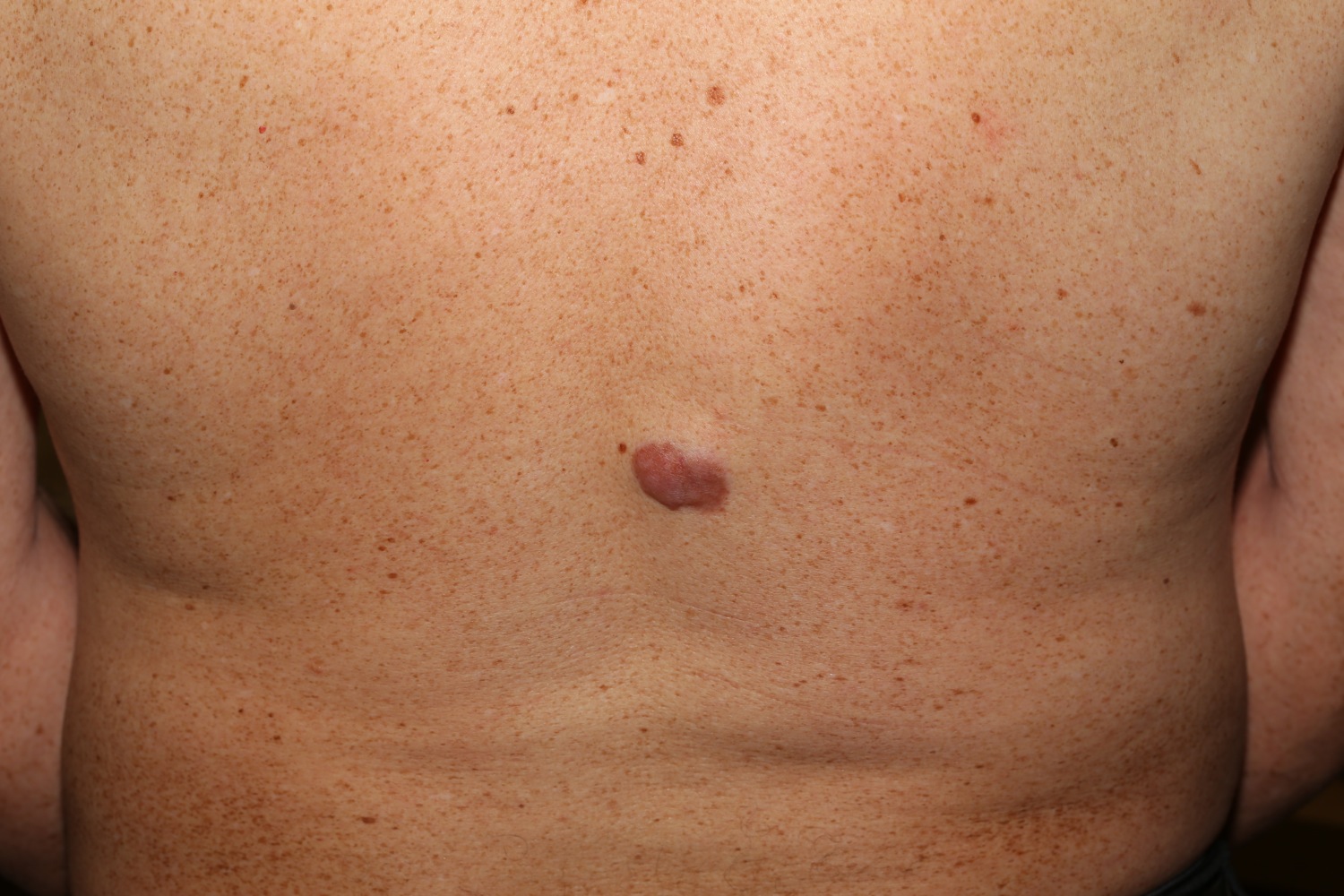
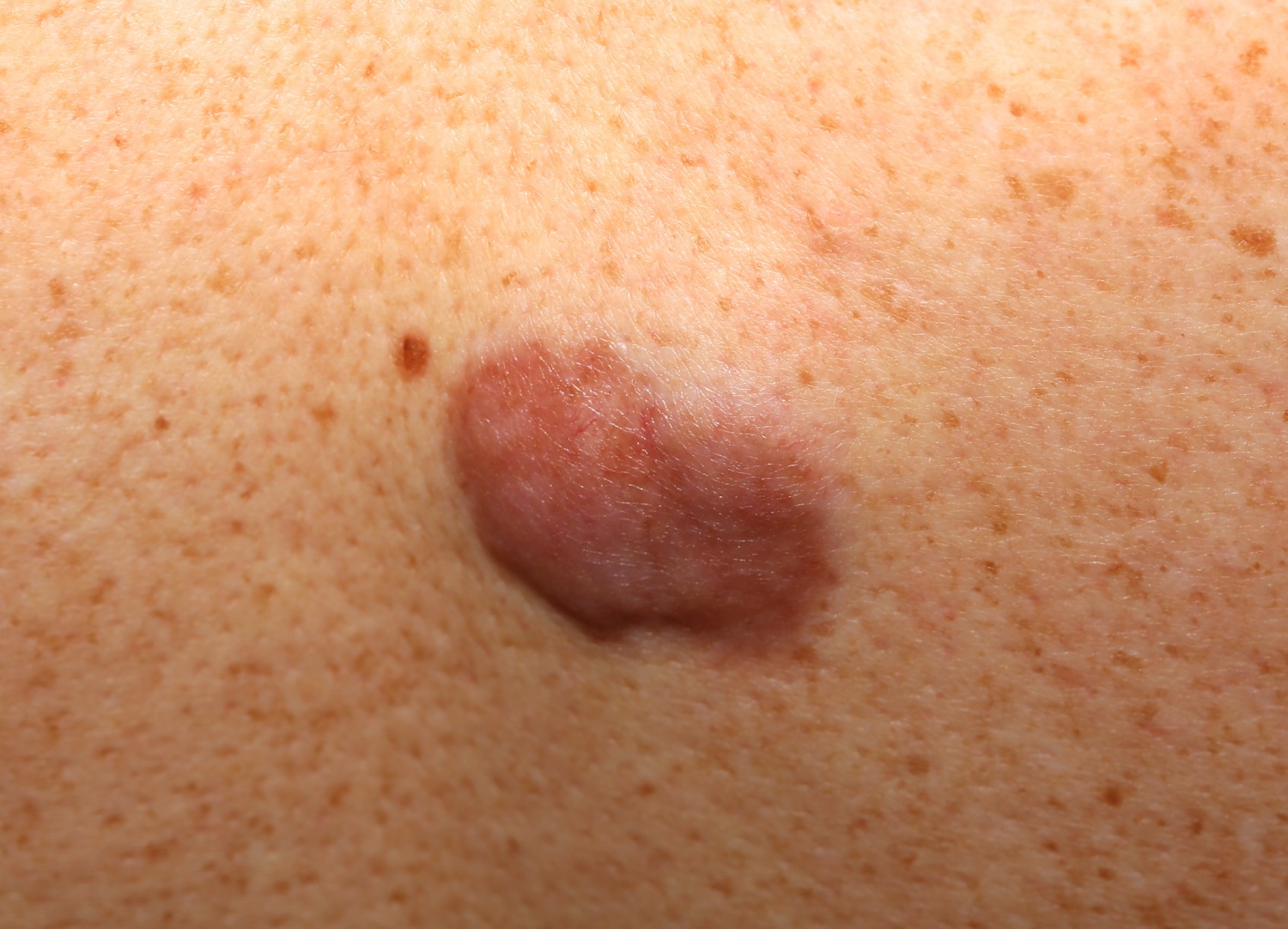
In dermatofibrosarcoma protuberans, the tumor most often starts as a small, firm patch of skin, usually 1 to 5 centimeters in diameter, that is usually purplish, reddish, or flesh-colored. The cancer typically grows slowly and can become a raised nodule. Occasionally, the dermatofibrosarcoma protuberans cancer begins as a flat or depressed patch of skin (plaque). Tumors are most commonly found on the torso and can also be found on the arms, legs, head, or neck. Affected individuals usually first show signs of this condition in their thirties, but the age at which a tumor appears varies widely.
Dermatofibrosarcoma protuberans is a slow-growing infiltrative dermal tumor with little metastatic potential. However, it shows a relatively high recurrence rate after surgical resection, and distant metastasis is rarely reported 2).
There are several variants of dermatofibrosarcoma protuberans in which different cell types are involved in the tumor.
- Bednar tumors, often called pigmented dermatofibrosarcoma protuberans, contain dark-colored (pigmented) cells called melanin-containing dendritic cells.
- Myxoid dermatofibrosarcoma protuberans tumors contain an abnormal type of connective tissue known as myxoid stroma.
- Giant cell fibroblastoma, which is sometimes referred to as juvenile dermatofibrosarcoma protuberans because it typically affects children and adolescents, is characterized by giant cells in the tumor.
Rarely, the tumors involved in the different types of dermatofibrosarcoma protuberans can have regions that look similar to fibrosarcoma, a more aggressive type of soft tissue sarcoma. In these cases, the condition is called fibrosarcomatous dermatofibrosarcoma protuberans. Fibrosarcomatous dermatofibrosarcoma protuberans tumors are more likely to metastasize than tumors in the other types of dermatofibrosarcoma protuberans.
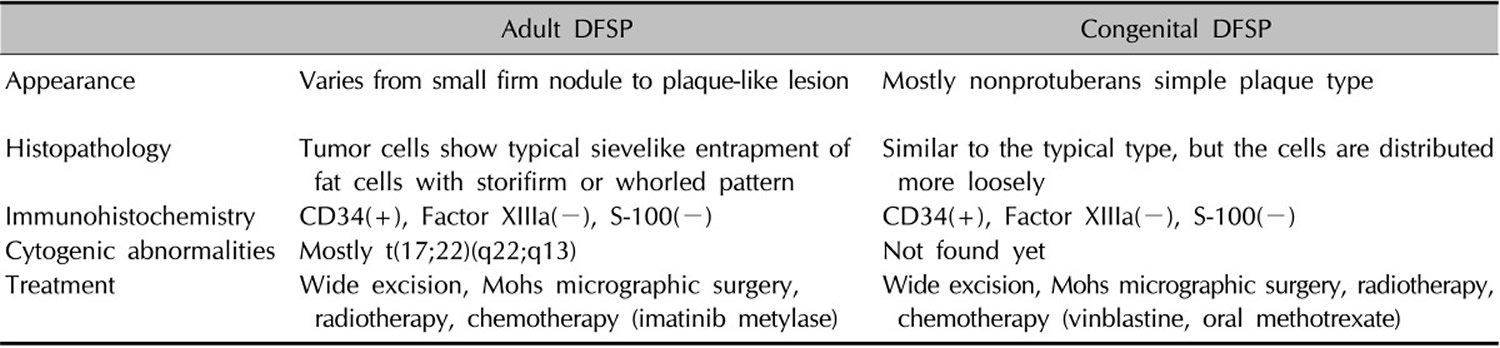
Table 1. The differences between adult dermatofibrosarcoma protuberans and congenital (present at birth) dermatofibrosarcoma protuberans through the literature review
Note: DFSP = ermatofibrosarcoma protuberans
[Source 3)]Other names for dermatofibrosarcoma protuberans:
- Darier-Ferrand tumor
- Darier-Hoffmann tumor
- dermatofibrosarcoma
- DFSP
Figure 1. Dermatofibrosarcoma protuberans
Figure 2. Dermatofibrosarcoma protuberans
Dermatofibrosarcoma protuberans causes
The cause is unknown, but an injury to the affected skin may be a predisposing factor. The injured skin may have a scar from a burn or surgery. Sometimes, dermatofibrosarcoma protuberans forms where a person received many radiation treatments or vaccines. More research is needed to know whether a skin injury plays a role in causing dermatofibrosarcoma protuberans.
Recent advances show tumor cells carry abnormal chromosomes within the tumor cells—t(17;22)(q22;q13)—resulting in the fusion gene COL1A1-PDGFB. This encodes a protein that causes the tumour to grow by autocrine overproduction of platelet derived growth factor (PDGF).
Dermatofibrosarcoma protuberans is associated with a rearrangement (translocation) of genetic material between chromosomes 17 and 22. This translocation, written as t(17;22), fuses part of the COL1A1 gene from chromosome 17 with part of the PDGFB gene from chromosome 22. The translocation is found on one or more extra chromosomes that can be either the normal linear shape or circular. When circular, the extra chromosomes are known as supernumerary ring chromosomes. Ring chromosomes occur when a chromosome breaks in two places and the ends of the chromosome arms fuse together to form a circular structure. Other genes from chromosomes 17 and 22 can be found on the extra chromosomes, but the role these genes play in development of the condition is unclear. The translocation is acquired during a person’s lifetime and the chromosomes containing the translocation are present only in the tumor cells. This type of genetic change is called a somatic mutation.
In normal cells, the COL1A1 gene provides instructions for making part of a large molecule called type I collagen, which strengthens and supports many tissues in the body. The PDGFB gene provides instructions for making one version (isoform) of the platelet derived growth factor (PDGF) protein. By attaching to its receptor, the active PDGFB protein stimulates many cellular processes, including cell growth and division (proliferation) and maturation (differentiation).
The abnormally fused COL1A1-PDGFB gene provides instructions for making an abnormal combined (fusion) protein that researchers believe ultimately functions like the PDGFB protein. The gene fusion leads to the production of an excessive amount of protein that functions like the PDGFB protein. In excess, this fusion protein stimulates cells to proliferate and differentiate abnormally, leading to the tumor formation seen in dermatofibrosarcoma protuberans.
The COL1A1-PDGFB fusion gene is found in more than 90 percent of dermatofibrosarcoma protuberans cases. In the remaining cases, changes in other genes may be associated with this condition. These genes have not been identified.
Dermatofibrosarcoma protuberans signs and symptoms
Dermatofibrosarcoma protuberans usually presents as a painless thickened area of skin (plaque) and/or nodule that feels rubbery or firm to touch and is fixed to the underlying skin. It may be red-brown or skin colored. It usually grows very slowly over months to years.
Rarely it presents as a soft depressed area of skin making the diagnosis even more difficult. Dermatofibrosarcoma protuberans may range in size from 0.5 to 25 cm in diameter. Fifty to sixty percent of tumors arise on the trunk, often in the shoulder and chest area. The remaining 35% of tumors are found on the limbs and 10 to 15% on the head and neck region.
Dermatofibrosarcoma protuberans is often diagnosed when it enters a more rapid growth phase giving rise to larger lesions. Neglected tumors may reach large proportions.
Dermatofibrosarcoma protuberans diagnosis
The absence of symptoms often leads to a delay in diagnosis. Redness and pain only occur in 15% of cases. It is often mistaken for other skin conditions particularly in the early stages. Skin biopsy is needed to confirm the diagnosis.
It has a characteristic appearance under the microscope with densely arranged spindle shaped cells. It may be difficult to assess complete removal due to extensions widely in the skin and deeper structures. It is important to identify fibrosarcomatous Dermatofibrosarcoma protuberans, a more aggressive tumour, that requires more aggressive treatment.
Dermatofibrosarcoma protuberans’s chromosomal abnormalities can be detected using reverse transcription polymerase chain reaction (RT-PCR) or fluorescence in situ hybrydization (FISH).
In most cases no other investigations are necessary. However, if there is suspicion of metastasis or there is fibrosarcomatous transfomation, lymph node ultrasound, chest X-ray and pelvic ultrasound scan may be arranged.
Dermatofibrosarcoma protuberans staging
There is no dermatofibrosarcoma protuberans staging in the literature. So we have adopted the staging system for a soft tissue sarcoma. The following staging is from the American Cancer Council 4).
Soft Tissue Sarcoma Stages
The stages of soft tissue sarcomas range from stages I (1) through IV (4). As a rule, the lower the number, the less the cancer has spread. A higher number, such as stage IV, means cancer has spread more. And within a stage, an earlier letter means a lower stage. Although each person’s cancer experience is unique, cancers with similar stages tend to have a similar outlook and are often treated in much the same way.
How is the soft tissue sarcomas stage determined?
The staging system most often used for soft tissue sarcomas is the American Joint Committee on Cancer (AJCC) TNM system, which is based on 4 key pieces of information:
- The extent of the tumor (T): How large is the cancer?
- The spread to nearby lymph nodes (N): Has the cancer spread to nearby lymph nodes?
- The spread (metastasis) to distant sites (M): Has the cancer spread to distant organs such as the lungs?
- The grade (G) of the cancer: How much do the sarcoma cells look like normal cells?
The grade of a sarcoma is determined using a system known as the French or FNCLCC system, and is based on 3 factors:
- Differentiation: Cancer cells are given a score of 1 to 3, with 1 being assigned when they look similar to normal cells and 3 being used when the cancer cells look very abnormal. Certain types of sarcoma are given a higher score automatically.
- Mitotic count: How many cancer cells are seen dividing under the microscope; given a score from 1 to 3 (a lower score means fewer cells were seen dividing)
- Tumor necrosis: How much of the tumor is made up of dying tissue; given a score from 0 to 2 (a lower score means there was less dying tissue present).
The scores for each factor are added to determine the grade for the cancer. Higher-grade cancers tend to grow and spread faster than lower-grade cancers.
- GX: The grade cannot be assessed (because of incomplete information).
- Grade 1 (G1): Total score of 2 or 3
- Grade 2 (G2): Total score of 4 or 5
- Grade 3 (G3): Total score of 6, 7 or 8.
There are different staging systems for soft tissue sarcomas depending on where the cancer is in the body.
- Head and neck
- Trunk and extremities (arms and legs)
- Abdomen and thoracic (chest) visceral organs
- Retroperitoneum
Numbers or letters after T, N, and M provide more details about each of these factors. Higher numbers mean the cancer is more advanced. Once a person’s T, N, and M categories have been determined, this information is combined in a process called stage grouping to assign an overall stage. Of the 4 main locations, only 2 (Trunk and Extremities and Retroperitoneum) have stage groupings.
The staging system in the table below uses the pathologic stage (also called the surgical stage). It is determined by examining tissue removed during an operation. Sometimes, if surgery is not possible right away or at all, the cancer will be given a clinical stage instead. This is based on the results of a physical exam, biopsy, and imaging tests. The clinical stage will be used to help plan treatment. Sometimes, though, the cancer has spread further than the clinical stage estimates, and may not predict the patient’s outlook as accurately as a pathologic stage.
The system described below is the most recent AJCC system, effective January 2018. Cancer staging can be complex, so ask your doctor to explain it to you in a way you understand.
Table 1. Trunk and Extremities Sarcoma Stages 5)
| AJCC stage | Stage grouping | Trunk and Extremities Sarcoma Stage description* |
| IA | T1 N0 M0 G1 or GX | The cancer is 5 cm (2 inches) or smaller (T1). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). The cancer is grade 1 (G1) or the grade cannot be assessed (GX). |
| IB | T2, T3, T4 N0 M0 G1 or GX | The cancer is:
It has not spread to nearby lymph nodes (N0) or to distant sites (M0). The cancer is grade 1 (G1) or the grade cannot be assessed (GX). |
| II | T1 N0 M0 G2 or G3 | The cancer is 5 cm (2 inches) or smaller (T1). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). The cancer is grade 2 (G2) or grade 3 (G3). |
| IIIA
| T2 N0 M0 G2 or G3 | The cancer is larger than 5 cm (2 inches) but not more than 10 cm (T2). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). The cancer is grade 2 (G2) or grade 3 (G3). |
| IIIB | T3 or T4 N0 M0 G2 or G3 | The cancer is:
It has not spread to nearby lymph nodes (N0) or to distant sites (M0). The cancer is grade 2 (G2) or grade 3 (G3). |
| IV | Any T N1 M0 Any G | The cancer is any size (Any T) AND it has spread to nearby lymph nodes (N1). It has not spread to distant sites (M0). It can be any grade. |
| OR | ||
| Any T Any N M1 Any G | The cancer is any size (Any T) AND it has spread to nearby lymph nodes (N1). It has spread to distant sites such as the lungs (M1). It can be any grade. | |
*The following additional categories are not listed in the table above:
- TX: Main tumor cannot be assessed due to lack of information.
- T0: No evidence of a primary tumor.
- NX: Regional lymph nodes cannot be assessed due to lack of information.
Survival by Stage of Soft Tissue Sarcoma
Survival rates are often used by doctors as a standard way of discussing a person’s prognosis (outlook).
The 5-year survival rate (or observed survival rate) refers to the percentage of patients who live at least 5 years after their cancer is diagnosed. Of course, many people live much longer than 5 years (and many are cured).
Five-year relative survival rates assume that some people will die of other causes and compare the observed survival with that expected for people without the cancer. This is a better way to see the effect of the cancer on survival.
To get 5-year survival rates, doctors have to look at people who were treated at least 5 years ago. If treatment has improved since then, people now being diagnosed with soft tissue sarcoma may have a more favorable outlook.
Survival rates are often based on previous outcomes of large numbers of people who had the disease, but they cannot predict what will happen in any individual’s case. Many other factors might affect a person’s outlook, like the type of sarcoma, the location of the tumor, the treatment received, and the age of the patient. For example, sarcomas of the arms or legs have a better outcome than those found in other places. Also, older patients tend to have worse outcomes than younger people. Your doctor can tell you how the numbers below may apply to you, as he or she is familiar with your particular situation.
The rates below are based on the stage of the cancer at the time of diagnosis. When looking at survival rates, it’s important to understand that the stage of a cancer does not change over time, even if the cancer progresses. A cancer that comes back or spreads is still referred to by the stage it was given when it was first found and diagnosed, but more information is added to explain the current extent of the cancer. (And the treatment plan is adjusted based on the change in cancer status.)
The overall relative 5-year survival rate of people with soft tissue sarcomas is around 50% according to statistics from the National Cancer Institute (NCI). These statistics include people with Kaposi sarcoma, which has a poorer outlook than many sarcomas. The National Cancer Institute doesn’t use the AJCC staging system. Instead, they group sarcomas only by whether they are still confined to the primary site (called localized) have spread to nearby lymph nodes or tissues (called regional); or have spread (metastasized) to sites away from the main tumor (called distant). The 5-year survival rates for soft tissue sarcomas have not changed much for many years.
The corresponding 5-year relative survival rates were:
- 83% for localized sarcomas (56% of soft tissue sarcomas were localized when they were diagnosed)
- 54% for regional stage sarcomas; (19% were in this stage)
- 16% for sarcomas with distant spread (16% were in this stage)
The 10-year relative survival rate is only slightly worse for these stages, meaning that most people who survive 5 years are probably cured.
For sarcomas of the arms and legs, Memorial Sloan-Kettering Cancer Center has survival rates broken down by AJCC stage (these are for observed, not relative survival):
| Stage | 5-year observed survival rate |
| I | 90% |
| II | 81% |
| III | 56% |
| IV | Not available |
Survival is worse when the sarcoma has developed somewhere other than the arms or legs. For example, the 5-year survival for retroperitoneal sarcomas is around 40% to 60%.
Dermatofibrosarcoma protuberans survival rate
Dermatofibrosarcoma protuberans skin cancer rarely spreads to other parts of the body, so people often live for many years after treatment.
Lifelong follow-up with your doctors is essential though. dermatofibrosarcoma protuberans can return after treatment. It is very important to keep all follow-up appointments.
Follow-up with clinical examination of the site of the dermatofibrosarcoma protuberans is recommended every 6 months for 5 years, and then annually.
The tumor only metastasises (spread beyond the skin) in 5% of cases. It spreads in 1% via lymphatic vessels to the regional lymph glands and in 4% via the blood stream, most commonly to the lung followed by the brain, bone and heart.
Local recurrences arise in 11–20% of cases, usually within 3 years of initial surgery, so follow-up is important.
If dermatofibrosarcoma protuberans returns, it is often treated with one of the surgeries described below. Some patients receive radiation treatments after surgery. Taking the drug imatinib mesylate may be an option for some patients.
Dermatofibrosarcoma protuberans treatment
Your dermatologist may create the treatment plan. Sometimes, doctors from different medical specialties team up to create the treatment plan. The doctors may include a dermatologist, surgical oncologist (cancer specialist), and plastic surgeon.
Most treatment plans include surgery to remove the cancer. Because dermatofibrosarcoma protuberans can grow deep, other treatment may be necessary. A treatment plan for dermatofibrosarcoma protuberans usually includes one of more of the following treatments:
Chemotherapy is ineffective.
Excision (surgery)
Treatment of dermatofibrosarcoma protuberans and dermatofibrosarcoma protuberans with fibrosarcomatous transformation consists of wide excision of the lesion including deep fascia, with 1–3 cm margin of normal skin. This may take more than one surgical procedure to ensure complete removal of the tumor.
Mohs surgery
Mohs micrographic surgery, which is a special surgical technique to control tumor margins, is sometimes used to check that all the abnormal cells have been excised.
During Mohs surgery, the Mohs surgeon cuts out the tumor plus a very small amount of healthy-looking tissue surrounding the tumor. While the patient waits, the Mohs surgeon uses a microscope to look at what was removed. The surgeon is looking for cancer cells.
If the Mohs surgeon finds cancer cells at the edge of the removed tissue, the surgeon will remove another small amount of tissue and look at it under the microscope. This process continues until the surgeon no longer sees cancer cells along the edge of the removed tissue. Your Mohs surgeon may refer to this edge as the “margin.” When the Mohs surgeon no longer sees cancer cells along the edges, the surgeon may tell you that the “margins look clear.”
Recurrence after Mohs surgery is reported to be around 1%.
Reducing the risk of dermatofibrosarcoma protuberans returning
When dermatofibrosarcoma protuberans grows into the lower layer of skin, the cancer often spreads out like the roots of a plant. This root-like growth can make it difficult to remove all of the cancer.
To reduce the risk of dermatofibrosarcoma protuberans returning after surgery, your dermatologist may include a second treatment. The second treatment helps to kill cancer cells.
Dermatologists also are studying new treatment options. One way to reduce dermatofibrosarcoma protuberans from returning may be to treat patients with both excision and Mohs surgery. In one small study, the cancer did not return when patients received both excision and Mohs.
More research is needed to find out whether this can reduce the risk of dermatofibrosarcoma protuberans returning.
Radiation treatments
Radiotherapy is sometimes used in addition to surgery if the tumor cannot be completely removed by surgery 6). The results from a small study show that radiation may reduce the risk of dermatofibrosarcoma protuberans from returning. Researchers followed 14 patients who received radiation treatments, mostly after surgery. Most patients, 86%, remained cancer-free. The average follow-up period exceeded 10 years.
When surgery is not possible
Some patients cannot undergo surgery. When this happens, other treatment options are used. Some patients have radiation treatments. Other options include: imatinib mesylate.
On October 19, 2006, the US Food and Drug Administration granted approval for imatinib mesylate (Gleevec) as a single agent for the treatment of Dermatofibrosarcoma protuberans. The tyrosine kinase inhibitor imatinib mesylate is indicated for the treatment of adult patients with unresectable, recurrent, and/or metastatic dermatofibroscarcoma characterized by COL1A1-PDGFB fusion gene, with 50% response rates. For this medicine to work, patients must have certain DNA. Testing is required to find out whether a patient has that COL1A1-PDGFB fusion gene.
Imatinib mesylate is designed to target specific cancer-causing molecules. This gives it the power to kill cancer cells while preventing serious damage to non-cancerous cells.
If your doctor prescribes imatinib mesylate, you will need to be carefully supervised.
After taking imatinib mesylate, some patients are able to have surgery because the drug shrinks the dermatofibrosarcoma protuberans enough so that the cancer can be surgically removed.
Some people have used it preMohs to shrink the tumor however this runs the risk of causing skip areas that may decrease the success of surgery.
Tips for managing dermatofibrosarcoma protuberans
Findings from research studies suggest that patients treated for dermatofibrosarcoma protuberans should:
- Keep all follow-up appointments with your doctors. This skin cancer can return after treatment. If dermatofibrosarcoma protuberans returns, it usually returns within 3 years of treatment. dermatofibrosarcoma protuberans can return later, too. It has appeared 10 years or more after treatment. Some patients develop new dermatofibrosarcoma protuberanss or other skin cancers. For this reason, dermatologists recommend lifelong follow-up exams. These follow-up exams help find skin cancer in the earliest stage. The sooner skin cancer is found and treated, the more likely the cancer can be treated successfully.
- Perform skin self-exams as often as your dermatologist recommends. If you have received treatment for dermatofibrosarcoma protuberans, it is essential that you learn how to perform a skin self-exam. Your dermatologist or someone in your dermatologist’s office can teach you exactly what you need to know.
- When examining your skin, you should immediately contact your dermatologist if you:
- Find any new growth or rough patch on your skin
- Feel a change in your lymph nodes
- Think about joining a support group. Many people live long lives after receiving treatment for dermatofibrosarcoma protuberans. Knowing that dermatofibrosarcoma protuberans can return may be stressful. Some people who have had dermatofibrosarcoma protuberans say a support group helps them feel better emotionally. You may be able to find a local support group by asking your doctor or contacting a local hospital.
- Ask your doctors what else you can do to improve your outcome. Asking questions can help you feel more in control. Your doctors may have insight that can help you feel better or reduce your risk for getting another cancer.
References [ + ]