Contents
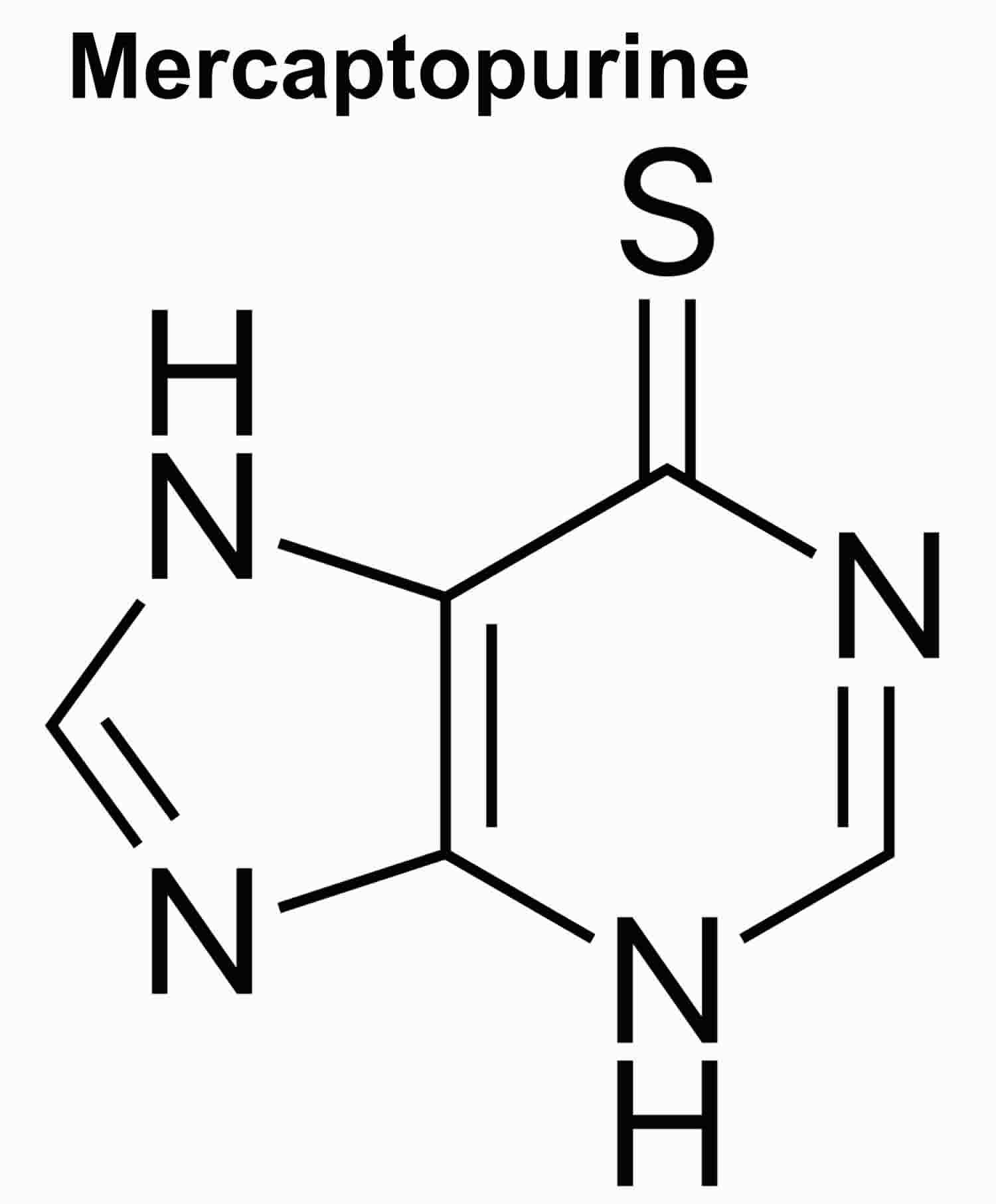
Mercaptopurine
Mercaptopurine also referred to as 6-mercaptopurine or 6-MP, is a purine analogue that is effective both as an anticancer and an immunosuppressive agent, and is used to treat leukemia (acute lymphocytic leukemia or acute lymphoblastic leukemia) and autoimmune diseases as a corticosteroid-sparing agent. Mercaptopurine is a purine analogue that acts as an antimetabolite by antagonism of purine metabolism which results in a general inhibition of DNA, RNA and subsequent protein synthesis. Mercaptopurine works by stopping the growth of cancer cells. Mercaptopurine also has antiinflammatory and immunosuppressive activity, inhibiting the maturation of T cells and blocking delayed hypersensitivity reactions.
Mercaptopurine was introduced into use in the 1950s for the therapy of leukemia and lymphoma and was formally approved for use in the United States in 1953. It is still used in therapy of acute lymphocytic leukemia (ALL) and off-label for autoimmune conditions such as Crohn’s disease and ulcerative colitis. Mercaptopurine is available generically and under the brand name of Purinethol as tablets of 50 mg. The usual dose is 1 to 3 mg per kilogram or 50 to 150 mg daily, and it is typically given long term. Common side effects include nausea, abdominal upset, rash, aphthous ulcers and dose related bone marrow suppression.
Mercaptopurine comes as a tablet and a suspension (liquid) to take by mouth. It is usually taken once a day. Take mercaptopurine at around the same time every day. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take mercaptopurine exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
If you are taking the suspension, shake the bottle very well for 30 seconds before each use to mix the medication evenly. It is important to use an oral syringe (measuring device) to accurately measure and take your dose of mercaptopurine. If you do not find an oral syringe with your medication, ask your pharmacist to give you one. After you use the oral syringe to take your medication, remove the plunger from the rest of the measuring device, wash both parts with warm soapy water, and rinse under running tap water. Allow the parts to air dry before putting back together for the next use.
Continue to take mercaptopurine even if you feel well. Do not stop taking mercaptopurine without talking to your doctor.
Mercaptopurine special precautions
Before taking mercaptopurine:
- tell your doctor and pharmacist if you are allergic to mercaptopurine, any other medications, or any of the ingredients in mercaptopurine tablets or suspension. Ask your pharmacist for a list of the ingredients.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Be sure to mention any of the following: allopurinol (Lopurin, Zyloprim); aminosalicylates such as mesalamine (Apriso, Asacol, Canasa, Lialda, Delzicol, Pentasa, others), olsalazine (Dipentum), and sulfasalazine (Azulfidine); anticoagulants (‘blood thinners’) such as warfarin (Coumadin, Jantoven); doxorubicin (Doxil); and trimethoprim and sulfamethoxazole (Bactrim, Septra). Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- tell your doctor if you have already taken mercaptopurine or thioguanine to treat your cancer. Your doctor may tell you not to take mercaptopurine if either of these medications did not work well against your cancer in the past.
- tell your doctor if you have any type of infection and if you have or have ever had liver or kidney disease.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. You should use birth control to avoid pregnancy during your treatment with mercaptopurine. If you become pregnant while taking mercaptopurine, call your doctor immediately. Mercaptopurine may harm the fetus.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking mercaptopurine.
- do not have any vaccinations without talking to your doctor.
- you should know that the risk that you will develop serious side effects of mercaptopurine may be higher if you have a genetic (inherited) risk factor. Your doctor may order tests before or during your treatment to see if you have this risk factor.
It is very important that your doctor check your progress at regular visits to make sure that mercaptopurine is working properly. Blood tests may be needed to check for unwanted effects. Genetic testing may also be performed to check your levels of thiopurine S-methyltransferase (an enzyme needed to metabolize mercaptopurine).
Using mercaptopurine while you are pregnant can harm your unborn baby. Use an effective form of birth control to keep from getting pregnant during therapy. If you think you have become pregnant while using mercaptopurine, tell your doctor right away.
Mercaptopurine can temporarily lower the number of white blood cells in your blood, increasing the chance of getting an infection. It can also lower the number of platelets, which are necessary for proper blood clotting. If this occurs, there are certain precautions you can take, especially when your blood count is low, to reduce the risk of infection or bleeding:
- If you can, avoid people with infections. Check with your doctor immediately if you think you are getting an infection or if you get a fever or chills, cough or hoarseness, lower back or side pain, or painful or difficult urination.
- Check with your doctor immediately if you notice any unusual bleeding or bruising, black, tarry stools, blood in the urine or stools, or pinpoint red spots on your skin.
- Be careful when using a regular toothbrush, dental floss, or toothpick. Your medical doctor, dentist, or nurse may recommend other ways to clean your teeth and gums. Check with your medical doctor before having any dental work done.
- Do not touch your eyes or the inside of your nose unless you have just washed your hands and have not touched anything else in the meantime.
- Be careful not to cut yourself when you are using sharp objects such as a safety razor or fingernail or toenail cutters.
- Avoid contact sports or other situations where bruising or injury could occur.
Check with your doctor right away if you have pain or tenderness in the upper stomach, pale stools, dark urine, loss of appetite, nausea, vomiting, or yellow eyes or skin. These could be symptoms of a serious liver problem.
While you are being treated with mercaptopurine, and after you stop treatment with it, do not have any immunizations (vaccines) without your doctor’s approval. Mercaptopurine may lower your body’s resistance and the vaccine may not work as well or you might get the infection the vaccine is meant to prevent. In addition, you should not be around other persons living in your household who receive live virus vaccines because there is a chance they could pass the virus on to you. Some examples of live vaccines include measles, mumps, influenza (nasal flu vaccine), poliovirus (oral form), rotavirus, and rubella. Do not get close to them and do not stay in the same room with them for very long. If you have questions about this, talk to your doctor.
Mercaptopurine may increase your risk of getting certain types of cancer, including skin cancer and cervical cancer. Some teenagers and young adults with Crohn’s disease or ulcerative colitis developed a rare type of cancer called hepatosplenic T-cell lymphoma (HSTCL). Check with your doctor right away if you have unusual bleeding, bruising, or weakness, swollen lymph nodes in the neck, underarms, or groin, or unexplained weight loss.
Mercaptopurine may cause a life-threatening condition called macrophage activation syndrome (MAS). This usually occurs in patients with an autoimmune disease (e.g., inflammatory bowel disease) or virus infection (e.g., Epstein-Barr, cytomegalovirus), and must be treated immediately. Tell your doctor right away if you have a fever, cough that does not go away, redness in one part of your body, or warm feeling or swelling of your skin.
Mercaptopurine may make your skin more sensitive to sunlight. Wear sunscreen. Do not use sunlamps or tanning beds.
Do not take other medicines unless they have been discussed with your doctor. This includes prescription or nonprescription (over-the-counter [OTC]) medicines and herbal or vitamin supplements.
Mercaptopurine uses
Mercaptopurine is used to treat acute lymphoblastic leukemia (ALL). Mercaptopurine is sometimes given with other cancer medications. Mercaptopurine is also used off-label for autoimmune conditions such as Crohn’s disease and ulcerative colitis. Crohn’s disease (a condition in which the body attacks the lining of the digestive tract causing pain, diarrhea, weight loss, and fever), and ulcerative colitis (condition in which sores develop in the intestines causing pain and diarrhea). Talk to your doctor about the possible risks of using mercaptopurine for your condition.
Mercaptopurine dosage
The dose of mercaptopurine will be different for different patients. Follow your doctor’s orders or the directions on the label. The following information includes only the average doses of mercaptopurine. If your dose is different, do not change it unless your doctor tells you to do so.
The amount of mercaptopurine that you take depends on the strength of the medicine. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take the medicine depend on the medical problem for which you are using the medicine.
- For oral dosage forms (suspension or tablets):
- For maintenance treatment of acute lymphatic leukemia:
- Adults—Dose is based on body weight and must be determined by your doctor. At first, 1.5 to 2.5 milligrams (mg) per kilogram (kg) of body weight per day, taken as a single dose. Your doctor may adjust your dose as needed.
- Children—Use and dose must be determined by your doctor.
- For maintenance treatment of acute lymphatic leukemia:
Adult dose for acute lymphoblastic leukemia
Use:
- Tablets: For maintenance therapy of acute lymphatic (lymphocytic, lymphoblastic) leukemia as part of a combination regimen; response to this agent depends upon the subclassification of acute lymphatic leukemia and the age of the patient (pediatric or adult)
- Suspension: For acute lymphoblastic leukemia as part of a combination regimen
Tablets:
- MAINTENANCE THERAPY: When a complete hematologic remission is obtained, maintenance therapy is considered essential. Maintenance doses vary. The usual daily maintenance dose is 1.5 to 2.5 mg/kg/day orally as a single dose. This drug should rarely be relied upon as a single agent for the maintenance of remissions induced in acute leukemia.
- DOSAGE WITH CONCOMITANT ALLOPURINOL: When this drug is given concomitantly with allopurinol, the dose of this drug should be reduced to one-third to one-quarter of the usual dose to avoid severe toxicity.
Suspension:
- MAINTENANCE THERAPY: The recommended starting dose of this drug in multi-agent combination chemotherapy maintenance regimens is 1.5 to 2.5 mg/kg (50 to 75 mg/m2) orally as a single daily dose.
- HOMOZYGOUS DEFICIENCY IN EITHER TPMT OR NUDT15: Patients with homozygous deficiency of either enzyme typically require 10% or less of the standard oral suspension dose. Reduce initial dose in patients who are known to have homozygous TPMT or NUDT15 deficiency.
- HETEROZYGOUS DEFICIENCY IN TPMT AND/OR NUDT15: Reduce the oral suspension dose based on tolerability. Most patients with heterozygous TPMT or NUDT15 deficiency tolerate recommended doses, but some require dose reduction based on toxicities. Patients who are heterozygous for both TPMT and NUDT15 may require more substantial dosage reductions.
Comments:
- The dosage recommendations presented here are manufacturer suggested; consult local institutional guidelines for alternate dosing options.
- After initiating therapy, monitor complete blood counts (CBCs), transaminases, and bilirubin.
- Maintain ANC at a desirable level by reducing the dose in patients with excessive hematological toxicity.
- Evaluate the bone marrow in patients with prolonged or repeated marrow suppression to assess leukemia status and marrow cellularity.
- Evaluate thiopurine S-methyltransferase (TPMT) and nucleotide diphosphatase (NUDT15) status in patients with clinical or laboratory evidence of severe bone marrow toxicity, or repeated episodes of myelosuppression.
- There is no general agreement that the procedures recommended in the guidelines are necessary or appropriate.
Pediatric dose for intestinal arterial insufficiency
Use:
- Tablets: For maintenance therapy of acute lymphatic (lymphocytic, lymphoblastic) leukemia as part of a combination regimen; response to this agent depends upon the subclassification of acute lymphatic leukemia and the age of the patient (pediatric or adult)
- Suspension: For acute lymphoblastic leukemia as part of a combination regimen
Tablets:
- MAINTENANCE THERAPY: When a complete hematologic remission is obtained, maintenance therapy is considered essential. Maintenance doses vary. The usual daily maintenance dose is 1.5 to 2.5 mg/kg/day orally as a single dose. This drug should rarely be relied upon as a single agent for the maintenance of remissions induced in acute leukemia.
- DOSAGE WITH CONCOMITANT ALLOPURINOL: When this drug is given concomitantly with allopurinol, the dose of this drug should be reduced to one-third to one-quarter of the usual dose to avoid severe toxicity.
Suspension:
- MAINTENANCE THERAPY: The recommended starting dose of this drug in multi-agent combination chemotherapy maintenance regimens is 1.5 to 2.5 mg/kg (50 to 75 mg/m2) orally as a single daily dose.
- HOMOZYGOUS DEFICIENCY IN EITHER TPMT OR NUDT15: Patients with homozygous deficiency of either enzyme typically require 10% or less of the standard oral suspension dose. Reduce initial dose in patients who are known to have homozygous TPMT or NUDT15 deficiency.
- HETEROZYGOUS DEFICIENCY IN TPMT AND/OR NUDT15: Reduce the oral suspension dose based on tolerability. Most patients with heterozygous TPMT or NUDT15 deficiency tolerate recommended doses, but some require dose reduction based on toxicities. Patients who are heterozygous for both TPMT and NUDT15 may require more substantial dosage reductions.
Comments:
- The dosage recommendations presented here are manufacturer suggested; consult local institutional guidelines for alternate dosing options.
- After initiating therapy, monitor complete blood counts (CBCs), transaminases, and bilirubin.
- Maintain ANC at a desirable level by reducing the dose in patients with excessive hematological toxicity.
- Evaluate the bone marrow in patients with prolonged or repeated marrow suppression to assess leukemia status and marrow cellularity.
- Evaluate thiopurine S-methyltransferase (TPMT) and nucleotide diphosphatase (NUDT15) status in patients with clinical or laboratory evidence of severe bone marrow toxicity, or repeated episodes of myelosuppression.
- There is no general agreement that the procedures recommended in the guidelines are necessary or appropriate.
Pediatric dose for acute lymphoblastic leukemia
Use:
- Tablets: For maintenance therapy of acute lymphatic (lymphocytic, lymphoblastic) leukemia as part of a combination regimen; response to this agent depends upon the subclassification of acute lymphatic leukemia and the age of the patient (pediatric or adult)
- Suspension: For acute lymphoblastic leukemia as part of a combination regimen
Tablets:
- MAINTENANCE THERAPY: When a complete hematologic remission is obtained, maintenance therapy is considered essential. Maintenance doses vary. The usual daily maintenance dose is 1.5 to 2.5 mg/kg/day orally as a single dose. This drug should rarely be relied upon as a single agent for the maintenance of remissions induced in acute leukemia.
- DOSAGE WITH CONCOMITANT ALLOPURINOL: When this drug is given concomitantly with allopurinol, the dose of this drug should be reduced to one-third to one-quarter of the usual dose to avoid severe toxicity.
Suspension:
- MAINTENANCE THERAPY: The recommended starting dose of this drug in multi-agent combination chemotherapy maintenance regimens is 1.5 to 2.5 mg/kg (50 to 75 mg/m2) orally as a single daily dose.
- HOMOZYGOUS DEFICIENCY IN EITHER TPMT OR NUDT15: Patients with homozygous deficiency of either enzyme typically require 10% or less of the standard oral suspension dose. Reduce initial dose in patients who are known to have homozygous TPMT or NUDT15 deficiency.
- HETEROZYGOUS DEFICIENCY IN TPMT AND/OR NUDT15: Reduce the oral suspension dose based on tolerability. Most patients with heterozygous TPMT or NUDT15 deficiency tolerate recommended doses, but some require dose reduction based on toxicities. Patients who are heterozygous for both TPMT and NUDT15 may require more substantial dosage reductions.
Comments:
- The dosage recommendations presented here are manufacturer suggested; consult local institutional guidelines for alternate dosing options.
- After initiating therapy, monitor complete blood counts (CBCs), transaminases, and bilirubin.
- Maintain ANC at a desirable level by reducing the dose in patients with excessive hematological toxicity.
- Evaluate the bone marrow in patients with prolonged or repeated marrow suppression to assess leukemia status and marrow cellularity.
- Evaluate thiopurine S-methyltransferase (TPMT) and nucleotide diphosphatase (NUDT15) status in patients with clinical or laboratory evidence of severe bone marrow toxicity, or repeated episodes of myelosuppression.
- There is no general agreement that the procedures recommended in the guidelines are necessary or appropriate.
Renal dose adjustments
- Starting at the low end of the dosing range, or increasing the dosing interval to 36 to 48 hours should be considered in patients with baseline renal impairment.
Dialysis
- Data not available
Liver dose adjustments
- Consideration should be given to reducing the dosage in patients with impaired hepatic function.
What should I do if I forget a dose?
Take the missed dose as soon as you remember it. However, if it is almost time for the next dose, skip the missed dose and continue your regular dosing schedule. Do not take a double dose to make up for a missed one.
Mercaptopurine side effects
Mercaptopurine may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- nausea
- vomiting
- darkening of the skin
- hair loss
- rash
Some side effects can be serious. If you experience any of these symptoms, call your doctor immediately:
- pale skin
- weakness
- shortness of breath
- sore throat, fever, chills, or other signs of infection
- swelling in the legs, ankles, or feet
- unusual bruising or bleeding
- yellowing of the skin or eyes
- loss of appetite
- diarrhea
- swelling of the stomach area
- pain in the upper right part of the stomach
Taking mercaptopurine may increase the risk that you will develop a new cancer. Some people who took mercaptopurine to treat Crohn’s disease or ulcerative colitis developed hepatosplenic T cell lymphoma (HSTCL), a very serious form of cancer that often causes death within a short time. Tell your doctor if you experience any of the following symptoms: stomach pain; fever; unexplained weight loss; night sweats or easy bruising or bleeding. Talk to your doctor about the risks of taking this medication.
Mercaptopurine may cause other side effects. Call your doctor if you have any unusual problems while you are taking mercaptopurine.
Symptoms of mercaptopurine overdose may include:
- loss of appetite
- nausea
- vomiting
- diarrhea
- pale skin
- weakness
- shortness of breath
- sore throat, fever, chills, and other signs of infection
- unusual bruising or bleeding.