Contents
The pineal gland
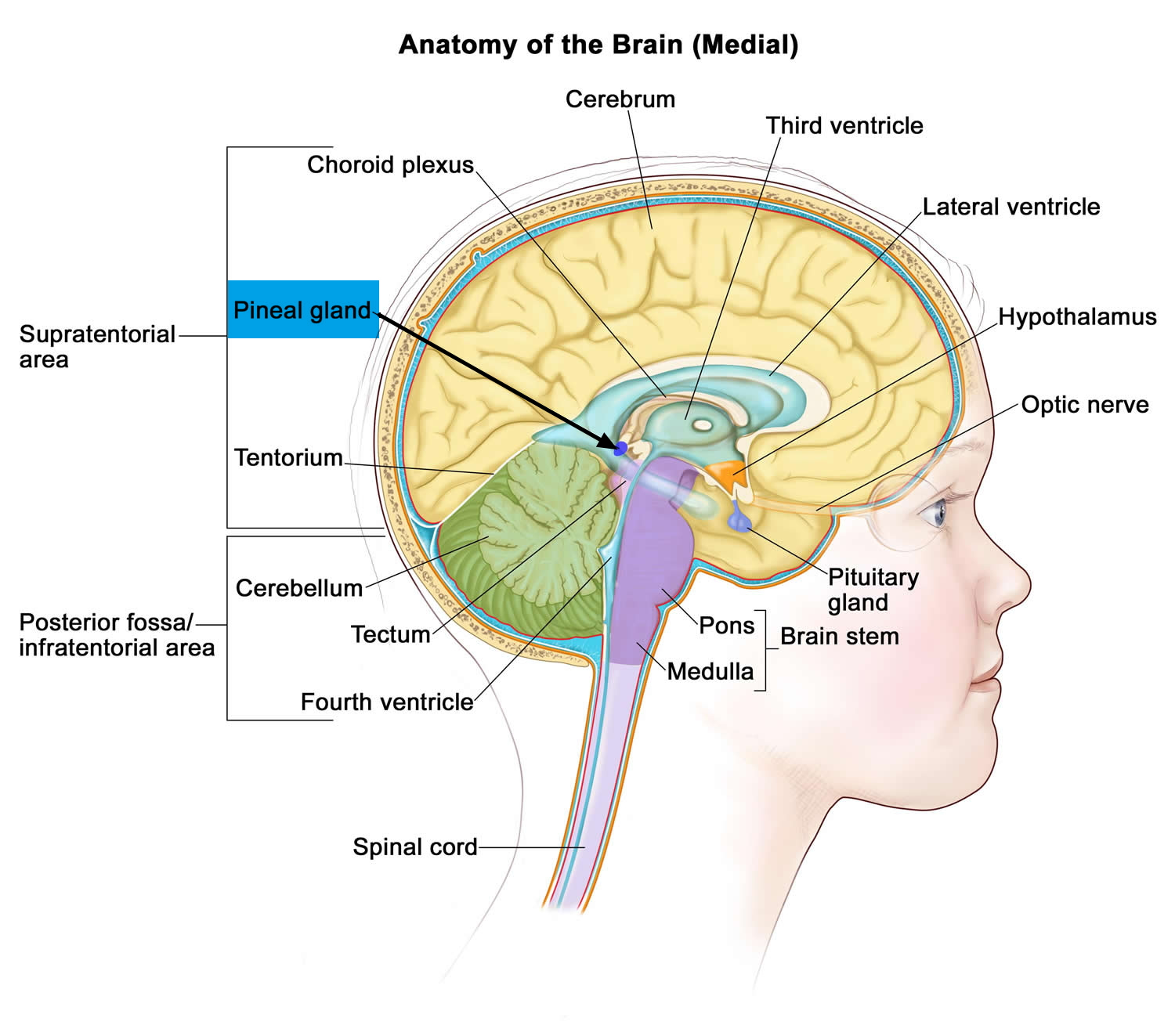
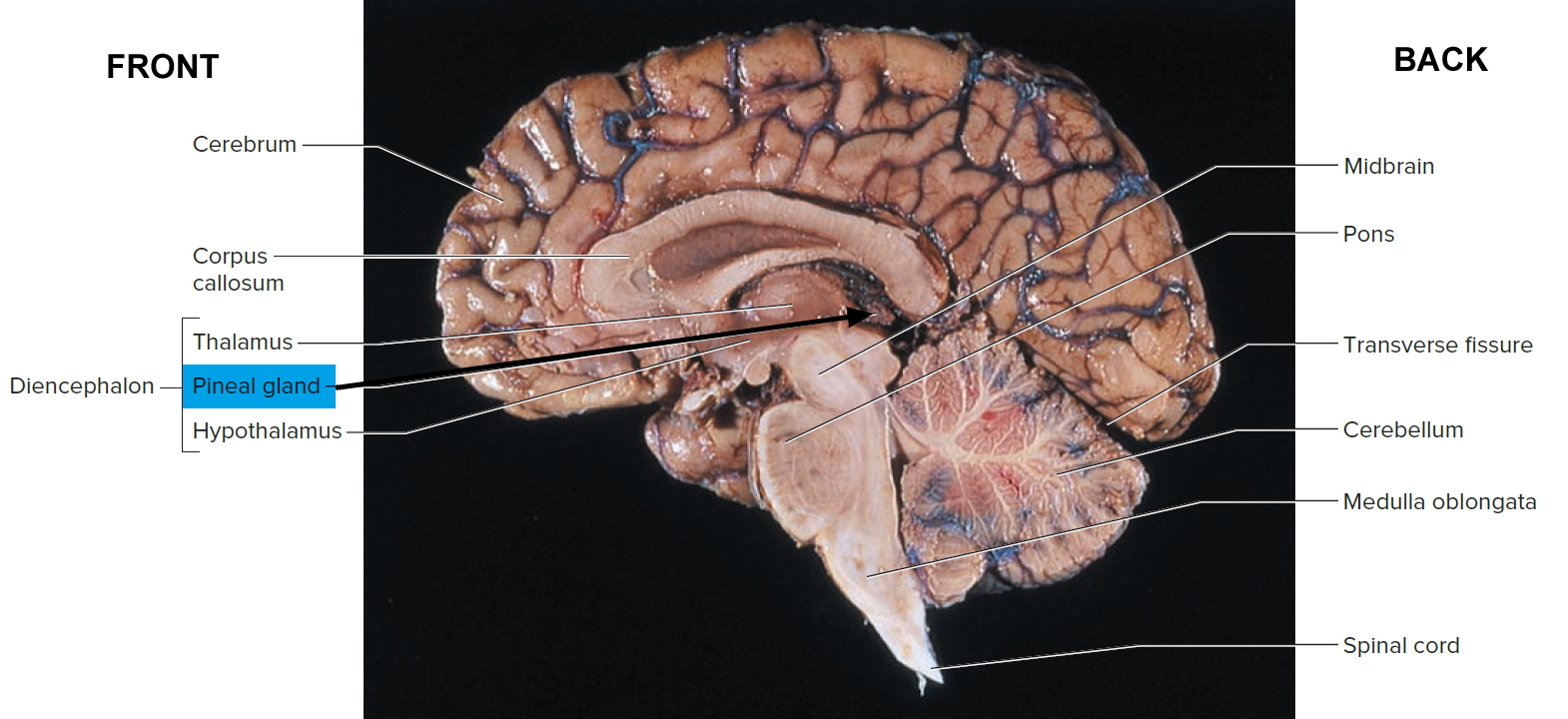
The pineal gland is a small pine cone like shape structure located deep between the cerebral hemispheres, where it is attached to the upper part of the thalamus near the roof of the third ventricle (see Figures 1 and 2). The normal pineal gland appears as a small reddish-brown structure and the normal size ranges between 10 and 14 mm 1). Two primary cell types make up the pineal gland. The pineocyte is the principal parenchyma cell and comprises 95 % of the pineal gland. The other 5 % are supporting cells referred to as astrocytes. Together, these two cell types are arranged in lobules, which are separated by a fibrovascular stroma. Calcifications commonly occur within the pineal gland and are often associated with increasing age 2).
To the present day, the functions of the pineal gland are not fully understood 3). Current knowledge indicates that by secretion of melatonin, the pineal gland plays an important role in the regulation of the sleep-wake cycle and of reproductive function (e.g. onset of puberty) 4), with melatonin also acting as a neuroprotector or antioxidant 5), 6).
The pineal gland translates the rhythmic cycles of night and day encoded by the retina into hormonal signals that are transmitted to the rest of the neuronal system in the form of serotonin and melatonin synthesis and release 7). The pineal gland secretes the hormone melatonin in response to changing light conditions outside the body (see Figure 3 below). Impulses originating in the retinas of the eyes are conducted along a complex pathway that eventually reaches the pineal gland. Melatonin secretion is suppressed during the day and increases in the dark of night.
Melatonin may help to regulate circadian rhythms. Circadian rhythms are patterns of repeated activity associated with the environmental cycles of day and night, including the sleep–wake cycle. The fact that melatonin secretion responds to day length may explain why traveling across several time zones produces the temporary insomnia of jet lag.
Previous studies have suggested a decline of melatonin secretion with age and an association between melatonin decrease and neurodegenerative diseases such as Alzheimer’s or Parkinson’s disease 8), 9), 10), 11). The amount of uncalcified pineal tissue was shown to predict total melatonin excretion with lack of melatonin being hypothesized to result from pineal gland calcification 12), 13). As a consequence, detection and measurement of pineal gland calcification might be of clinical interest by identifying patients with possible melatonin deficits and a risk for the development of neurodegenerative diseases 14), 15).
Figure 1. Pineal gland
Note: The pineal gland is commonly located along the midline above the superior colliculi and inferior to the splenium of the corpus callosum. It is attached to the superior aspect of the posterior border of the third ventricle.
Figure 2. Pineal gland
Biological rhythms
Biological rhythms are changes that systematically recur in organisms. The period of any rhythm is the duration of one complete cycle. The frequency of a rhythm is the number of cycles per time unit.
Three common types of rhythms in humans are:
- Ultradian rhythm: Ultradian rhythms have periods shorter than 24 hours and include the cardiac cycle and the breathing cycle,
- Infradian rhythm: Periods of infradian rhythms, such as the female reproductive cycle, are longer than 24 hours,
- Circadian rhythm: Periods of circadian rhythms, such as the sleep–wake cycle, variation in body temperature, and changes in hormone secretion, are approximately 24 hours.
Both external (exogenous) and internal (endogenous) factors regulate human biological rhythms. Exogenous factors are environmental components, such as daily temperature changes and the light–dark cycle. Endogenous factors include “clock” genes. Many members of an extended family in Utah, for example, have “advanced sleep phase syndrome” due to a mutation in a gene called “period.” The effect is striking—they promptly fall asleep at 7:30 each night and awaken suddenly at 4:30 a.m 16).
The sleep–wake cycle is the most obvious circadian rhythm in humans. It is largely controlled by the pattern of daylight and night, but under laboratory conditions of constant light or dark, the human body eventually follows an approximately 25-hour cycle.
Using a backlit electronic device, such as a smartphone or tablet, can delay falling asleep long after the device is shut off. Experiments show that such light exposure decreases melatonin production by about 22 percent. Body temperature is mostly endogenously regulated, but light exposure and physical activity help
keep this rhythm on a 24- rather than 25-hour cycle. Body temperature is usually lowest between 4 and 6 a.m., and then increases and peaks between 5 and 11 p.m. It drops during the late evening hours and into the night.
Platelet cohesion, blood pressure, and pulse rate are typically highest 2 hours after awakening. This may explain why heart attacks and strokes are more likely to occur between 6 a.m. and noon than at other times. Plasma cortisol surges and peaks at about 6 a.m., and then gradually declines to its minimum level in late evening before increasing again in the early morning. Growth hormone secretion peaks during the night. Antidiuretic hormone secretion is greater at night, when it decreases urine formation.
Pineal gland function
Melatonin is the main hormone secreted by the pineal gland. Extrapineal sources of melatonin were reported in the retina, bone marrow cells, platelets, skin, lymphocytes, Harderian gland, cerebellum, and especially in the gastrointestinal tract of vertebrate species 17). Indeed, melatonin is present but can also be synthesized in the enterochromaffin cells; the release of gastrointestinal melatonin into the circulation seems to follow the periodicity of food intake, particularly tryptophan intake 18). It is noteworthy that the concentration of melatonin in the gastrointestinal tract surpasses blood levels by 10-100 times and there is at least 400 times more melatonin in the gastrointestinal tract than in the pineal gland 19). Melatonin in the gastrointestinal tract of newborn and infant mammals is of maternal origin given that melatonin penetrates easily the placenta and is after secreted into the mother’s milk 20). It has even been suggested that melatonin is involved in the production of mekonium 21). Melatonin in human breast milk follows a circadian rhythm in both preterm and term milk, with high levels during the night and undetectable levels during the day 22). No correlation was found between gestational age and concentration of melatonin. It is noteworthy that bottle milk composition does not contain melatonin in powder formula. Also, human colostrum, during the first 4 or 5 days after birth, contains immune – competent cells (colostral mononuclear cells) which are able to synthesize melatonin in an autocrine manner 23).
Melatonin is mainly synthesized by the pinealocytes from the amino acid tryptophan, which is hydroxylated (by the tryptophan-5-hydroxylase) in 5-hydroxytryptophan, then decarboxylated (by the 5-hydroxytryptophan decarboxylase) in serotonin (see Figure 3). Two enzymes, found mainly in the pineal gland, transform serotonin to melatonin 24): serotonin is first acetylated to form N-acetylserotonin by arylalkylamine-N-acetyltransferase (AA-NAT, also called “Timezyme”, is the rate-limiting enzyme for melatonin synthesis), and then N-acetylserotonin is methylated by acetylserotonin-O-methyltransferase (ASMT, also called hydroxyindole-O- methyltransferase or HIOMT) to form melatonin (Figure 2). Both AA-NAT and ASMT activities are controlled by noradrenergic and neuropeptidergic projections to the pineal gland 25). Norepinephrine, also called noradrenaline, activates adenylate cyclase which in turn promotes the melatonin biosynthesis enzymes, especially AA-NAT 26). Once synthesized, melatonin is quickly released into the systemic circulation to reach central and peripheral target tissues.
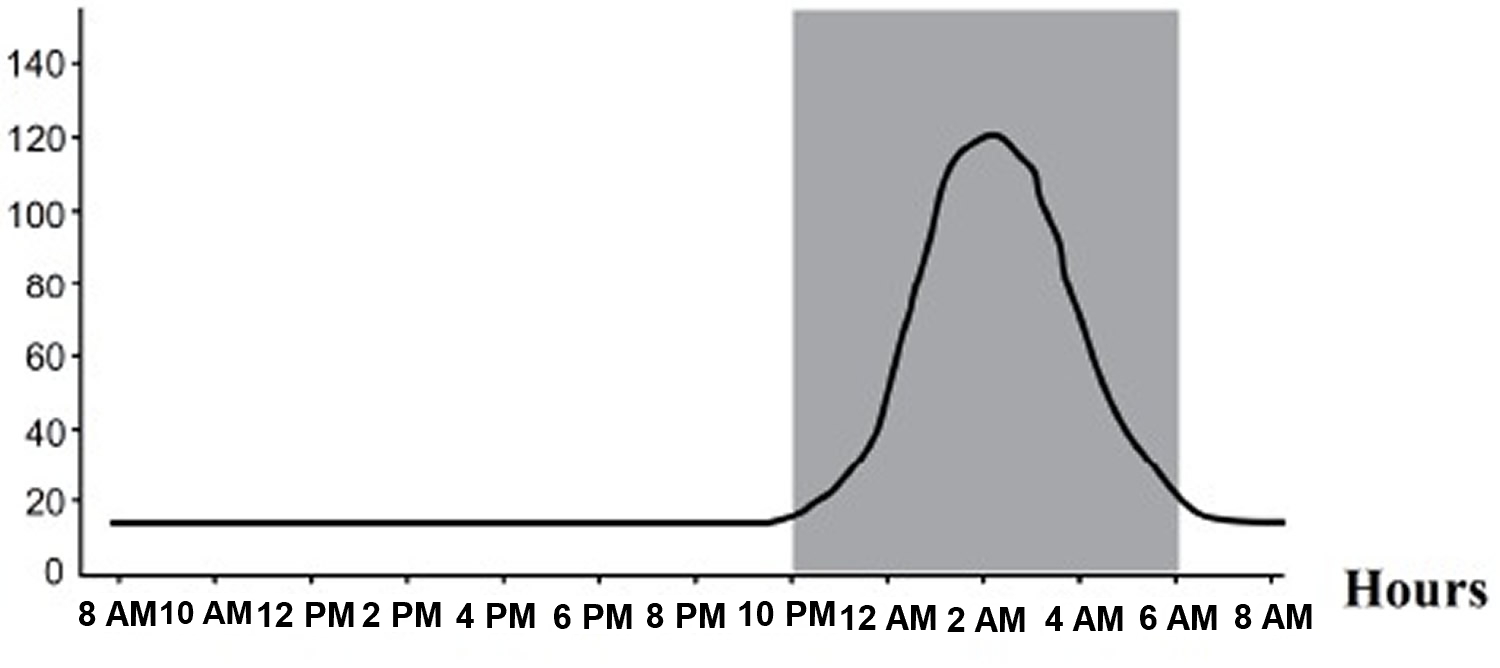
Melatonin synthesis and secretion is enhanced by darkness and inhibited by light (Figure 1) 27). Luminous information is transmitted from the retina to pineal gland through the suprachiasmatic nucleus (SCN) of the hypothalamus. In humans, its secretion starts soon after sundown, reaches a peak in the middle of the night (between 2 and 4 in the morning) and decreases gradually during the second half of the night 28). Nearly 80% of the melatonin is synthesized at night, with serum concentrations varying between 80 and 120 pg/ml. During daylight hours, serum concentrations are low (10-20 pg/ml) 29).
Serum concentrations of melatonin vary considerably with age, and infants secrete very low levels of melatonin before 3 months of age. Melatonin secretion increases and becomes circadian along with child development: Sadeh 30) reported an association between melatonin secretion and organization of sleep-wake rhythm from 6 months of age. However, more recent studies suggest that melatonin rhythm is set around 3 months of age in typical development, at the same time that infants begin to have more regular sleep–wake cycles associated with nighttime sleep lasting 6-8 h 31). In 3-years-old children, a stabilization of the sleep-wake rhythm is observed, which corresponds to a regular melatonin secretion rhythm [26]. Nocturnal concentration peaks are the highest between the 4th and 7th years of age 32) and then decline progressively 33).
After intravenous or oral administration, melatonin is quickly metabolized, mainly in the liver and secondarily in the kidney. However, after intravenous administration, the hepatic bio-degradation is less important due to the absence of hepatic first pass. It undergoes hydroxylation to 6-hydroxymelatonin by the action of the cytochrome P450 enzyme CYP1A2, followed by conjugation with sulfuric acid (90%) or glucuronic acid (10%) and is excreted in the urine. About 5% of serum melatonin is excreted unmetabolized also in urine. The principal metabolite, the 6-sulfatoxy-melatonin, is inactive, and its urinary excretion reflects melatonin plasma concentrations 34). Plasma levels can be also measured directly or indirectly assessed through salivary measures. A reverse relation between bioavailability of melatonin and the 6-sulfatoxy-melatonin concentrations area under the curve has been shown, the low bioavailability being explained by an important hepatic first pass 35).
Figure 3. Melatonin plasma concentrations – Circadian profile (in grey is represented the period of darkness)
Figure 4. Melatonin chemical structure
Figure 5. Melatonin synthesis
Note: AA-NAT = arylalkylamine-N-acetyl-transferase; ASMT = acetylserotonin-O-methyltransferase
[Source 36)]Physiological effects of melatonin
Melatonin regulates circadian rhythms such as the sleep-wake rhythm, neuroendocrine rhythms or body temperature cycles through its action on melatonin receptors (MT-1 and MT-2) 37). Ingestion of melatonin induces fatigue, sleepiness and a diminution of sleep latency 38). Disturbed circadian rhythms are associated with sleep disorders and impaired health 39). For example, children with multiple developmental, neuro-psychiatric and health difficulties often show melatonin deficiency 40). When circadian rhythms are restored, behavior, mood, development, intellectual function, health, and even seizure control may improve 41). It should be noted that according to several studies, circadian rhythms are important for typical (normal) neurodevelopment and their absence suppresses neurogenesis in animal models 42).
Finally, melatonin may be involved in early fetal development, with direct effects on placenta, glial and neuronal development, and could play an ontogenic role in the establishment of diurnal rhythms and synchronization of the fetal biological clock 43). Iwasaki et al. 44) investigated the expression of the two enzymes involved in the conversion of serotonin to melatonin (AA-NAT and ASMT) (see Figure 3 above) and found that transcripts of these enzymes were present in the first-trimester human placenta. Moreover, they found also that melatonin significantly potentiated hCG (human chorionic gonadotropin) secretion at optimal concentrations on cultured human trophoblast cells. These results suggest that melatonin regulates in a paracrine/autocrine way human placental function with a potential role in human reproduction. Test tube studies have shown that neural stem/progenitor cells express melatonin MT1 receptors and melatonin induces glial cell-line derived neurotrophic factor (GDNF) expression in neural stem cells, suggesting an early role for melatonin in central nervous system development. Indeed, as indicated previously, melatonin of maternal origin crosses the placenta and can therefore influence fetal development. Studies in humans have repeatedly confirmed that the cycle of melatonin in maternal blood occurs also in fetal circulation 45). The maturation and synchronization of the fetal circadian system have not been thoroughly studied. However, studies in nonhuman primate fetus have shown that maternal melatonin stimulates growth of the primate fetal adrenal gland and entrains fetal circadian rhythms, including suprachiasmatic nucleus (SCN) rhythms 46). Furthermore, in mice, the suppression of melatonin rhythm by maternal exposure to constant light changes the rhythmic expression in fetal clock genes; these changes are reversed when melatonin is injected daily to the mother 47). These results document that the fetal clock is imprinted by melatonin, which under normal circumstances is of maternal origin. In addition, some studies in humans and nonhuman primates show 24h rhythms in fetal heart rate and respiratory movements during the latter half of pregnancy. Whether the circadian system of the human fetus, particularly in late pregnancy, is under the influence of maternal suprachiasmatic nucleus (SCN) remains to be better ascertained 48).
Besides the well-known effects of melatonin on the regulation of sleep-wake rhythms, melatonin is considered as an endogenous synchronizer and a chronobiotic molecule, i.e. a substance that reinforces oscillations or adjusts the timing of the central biological clock located in the suprachiasmatic nuclei of the hypothalamus to stabilize bodily rhythms 49). Furthermore, Pevet and Challet 50) view melatonin as both the master clock output and internal time-giver in the complex circadian clocks network: as a major hormonal output, melatonin distributes, through its daily rhythm of secretion, temporal cues to the numerous tissue targets with melatonin receptors, driving circadian rhythms in some tissue structures such as the adenohypophysis or synchronizing peripheral oscillators such as the fetal adrenal gland but also many other peripheral tissues (pancreas, liver, kidney, heart, lung, fat, gut, etc.). Circadian rhythms, and more precisely the circadian clocks network, allow temporal organization of biological functions in relation to periodic environmental changes and therefore reflect adaptation to the environment. Thus, the sleep–wake rhythm associated with biological circadian rhythms can be seen as an adaptation to the day–night cycle. Moreover, the synchronization by melatonin of peripheral oscillators reflects adaptation of the individual to his/her internal and external environment (for example, the synchronized effects of melatonin on cortisol and insulin secretion allow the individual to be fully awake at 8am and able to start the day by eating and getting some energy from food intake). Given the major synchronizing effects of melatonin on central and peripheral oscillators, measures of melatonin are considered the best peripheral indices of human circadian timing 51).
Futhermore, melatonin is involved in blood pressure and autonomic cardiovascular regulation, immune system regulation but also various physiological functions such as retinal functions, detoxification of free radicals and antioxidant actions through its action on melatonin MT3 receptors protecting the brain from oxidative stress 52). A through its action on MT3 receptors specific section is developed below on melatonin and brain protection. The antioxidant actions of melatonin protect also the gastrointestinal tract from ulcerations by reducing secretion of hydrochloric acid and the oxidative effects of bile acids on the intestinal epithelium, and by increasing duodenal mucosal secretion of bicarbonate through its action on MT2 receptors (this alkaline secretion is an important mechanism for duodenal protection against gastric acid); melatonin prevents also ulcerations of gastrointestinal tract by increasing microcirculation and fostering epithelial regeneration 53). Concerning the role of melatonin in immune regulation, melatonin has direct immuno-enhancement effects in animals and humans 54). Indeed, melatonin stimulates the production of cytokines and more specifically interleukins (IL-2, IL-6, IL-12) 55). In addition, melatonin enhances T helper immune responses 56). Furthermore, the melatonin antioxidant actions contribute to its immuno-enhancing effects 57) and have also an indirect effect by reducing nitric oxide formation which facilitates the decrease of the inflammatory response 58). As suggested by Esquifino et al. 59), melatonin might provide a time-related signal to the immune network.
In addition, effects of melatonin on body mass and bone mass regulation have been reported. Melatonin is known for its role in energy expenditure and body mass regulation in mammals by preventing the increase in body fat with age 60). These effects are mediated by MT2 receptors in adipose tissue 61). Moreover, melatonin increases bone mass by promoting osteoblast cell differentiation and bone formation 62). In humans, melatonin stimulates bone cell proliferation and Type I collagen synthesis in these cells, and inhibits bone resorption through down-regulation of the RANKL-mediated osteoclast formation and activation 63). Also, a deficit of melatonin has been found to be associated with animal scoliosis following pinealectomy and human idiopathic scoliosis 64).
Finally, melatonin has physiological effects on reproduction and sexual maturation in mammals through down-regulation of gonadotropin-releasing hormone (GnRH) gene expression in a cyclical pattern over a 24-hour period 65). The rhythmic release of GnRH controls luteinizing hormone (LH) and follicule-stimulating hormone (FSH) secretion. The daily profile of melatonin secretion conveys internal information used for both circadian and seasonal temporal organization 66). The melatonin rhythmic pattern entrains the reproductive rhythm via the influence of photoperiod on LH pulsatile secretion and therefore mediates the seasonal fluctuations of reproduction clearly observed in animals (seasonal breading as species-specific seasons for reproduction) and moderately observed in humans 67).
Calcified pineal gland
There is a wide range of lesions from different entities arises in the pineal region, which can be classified into tumors of germ cell origin, tumors of pineal cell origin and other tumors, and makes up for approximately 1 % of intracranial tumours in adults and 3–8 % in children 68), 69), 70), 71). Calcification of pineal region tumors is very common and tumor-specific patterns of calcification have been reported 72), 73). In germinomas, pineal calcifications tend to be engulfed by the tumor, whereas in pineoblastomas calcification is often not central, but “exploded” to the periphery 74). As a consequence, the assessment of calcification in the pineal region might also have a clinical benefit by narrowing differential diagnosis of pineal region tumors.
Pineal gland calcifications, also referred to as “brain sand”, involves the development of hydroxyapatite deposits and is very common with a reported prevalence of approximately 68–75% in adults 75), 76), 77). In all population groups, calcification of the pineal gland was found to increase with age 78), 79). Pineal gland calcification is frequently detected on computed tomography (CT) scans 80). As CT causes substantial radiation exposure, it would be of advantage to identify pineal gland calcification with MRI instead. Apart from the absence of ionizing radiation, MRI provides superior soft-tissue contrast and is the modality of choice to evaluate the pineal region, as it enables an accurate delineation of pineal tumors before surgery. However, in the case of calcifications of the pineal gland or tumor calcifications in the pineal region, conventional MRI sequences do not allow for a reliable identification and have a poor sensitivity, as calcifications appear hypointense on T1, T2 and T2*weighted sequences and consequently cannot be reliably differentiated from e.g. soft tissue artifacts or microbleeds 81).
Recent years have witnessed an increasing interest in studies regarding the impact of pineal gland calcifications on decreased secretory activity of the gland and on specific pathological entities such as Alzheimer’s disease 82). Mahlberg et al. 83) suggested, that pineal gland calcification was significantly higher in patients with Alzheimer’s disease compared to others types of cognitive impairment and that pineal gland calcifications might contribute to the pathogenesis of Alzheimer’s disease by reflecting a reduced level of crystallization inhibitors 84). With regard to the association between plasma melatonin and uncalcified solid pineal tissue, Liebrich et al. 85) reported that uncalcified pineal functional volume as derived from MRI was linked to the hormonal function of the pineal gland. On the other hand, there has also been a study suggesting that the levels of melatonin did not significantly differ by presence of cysts or calcification 86). Consequently, it is not yet fully resolved whether there is an association between the incidence of pineal gland calcifications and reduced melatonin excretion.
Pineal Gland Cyst
Pineal gland cysts are common. Pineal cysts are relatively common and may be found by chance in up to 10% of people who have a head CT scan or MRI 87). Most people with a pineal cyst do not have any signs or symptoms 88). Rarely, a pineal cyst may cause headaches, hydrocephalus, obstruction of the vein of Galen (a vein at the base of the brain), Parinaud syndrome (also known as dorsal midbrain syndrome, which leads to difficulty of upward vertical gaze, mydriasis, blepharospasm and impaired ocular convergence), or other symptoms 89), 90). The exact cause of pineal cysts is unknown. Treatment is usually only considered when a cyst causes symptoms. In most cases, no treatment is necessary for a pineal gland cyst 91). Treatment may involve open or stereotactic (a surgical technique for precisely directing the tip of a delicate instrument (e.g a needle) or beam of radiation in three planes using coordinates provided by medical imaging) removal of the cyst, stereotactic aspiration, and/or CSF diversion (a procedure used to drain fluid from the brain) 92).
A cyst is a sac that can form in any part of the body. Often cysts are filled with air, fluid or other material. Cysts that occur in the pineal gland almost never cause symptoms. So, it is unlikely that headaches are the result of a pineal gland cyst. In most cases, these cysts are discovered when a brain scan is done for an unrelated reason, such as a head trauma, migraine headaches or dizzy spells. Pineal gland cysts are most commonly found in women 20 to 30 years old, and are very rare before puberty or after menopause. This suggests hormones may play a role in causing the cysts.
Because they do not usually cause symptoms or lead to complications, the vast majority of pineal gland cysts do not require surgery or other treatment. Pineal cysts are best seen on brain magnetic resonance imaging (MRI). This type of brain imaging is typically reviewed by a specialist, such as a neuroradiologist, who is experienced in evaluating brain cysts and tumors. That physician can tell the difference between a simple pineal gland cyst and another condition that may require treatment, such as a pineal gland tumor.
In contrast to cysts, tumors are an abnormal mass of tissue. They can be either noncancerous or cancerous. If a pineal gland tumor is found, treatment depends on the specific type, size and location of the tumor, as well as the individual’s overall health and preferences. In many cases, surgery is often the first step in treating pineal gland tumors.
Pineoblastoma
Pineoblastoma is a rare, aggressive type of cancer that begins in the cells of the brain’s pineal gland 93). Your pineal gland, located in the center of your brain, produces a hormone (melatonin) that plays a role in your natural sleep-wake cycle.
Pineoblastoma can occur at any age, but it tends to occur most often in young children 94). Pineoblastoma may cause headaches, sleepiness and subtle changes in the way the eyes move 95).
Pineoblastoma can be very difficult to treat. It can spread within the brain and the fluid (cerebrospinal fluid) around the brain, but it rarely spreads beyond the central nervous system. Treatment usually involves surgery to remove as much of the cancer as possible. Additional treatments may also be recommended.
Diagnosis of pineoblastoma
Tests and procedures used to diagnose pineoblastoma include:
- Imaging tests. Imaging tests can help your doctor determine the location and size of your child’s brain tumor. Magnetic resonance imaging (MRI) is often used to diagnose brain tumors, and advanced techniques, such as perfusion MRI and magnetic resonance spectroscopy, may also be used.
Additional tests might include computerized tomography (CT) and positron emission tomography (PET).
- Removing a sample of tissue for testing (biopsy). A biopsy can be done with a needle before surgery or during surgery to remove the pineoblastoma. The sample of suspicious tissue is analyzed in a laboratory to determine the types of cells and their level of aggressiveness.
- Removing cerebrospinal fluid for testing (lumbar puncture). Also called a spinal tap, this procedure involves inserting a needle between two bones in the lower spine to draw out cerebrospinal fluid from around the spinal cord. The fluid is tested to look for tumor cells or other abnormalities. In certain situations, cerebrospinal fluid may instead be collected during a biopsy procedure to remove suspicious tissue from the brain.
Treatment for pineoblastoma
Pineoblastoma treatment options include:
- Surgery to relieve fluid buildup in the brain. A pineoblastoma may grow to block the flow of cerebrospinal fluid, which can cause a buildup of fluid that puts pressure on the brain (hydrocephalus). An operation to create a way for the fluid to flow out of the brain may be recommended. Sometimes this procedure can be combined with a biopsy or surgery to remove the tumor.
- Surgery to remove the pineoblastoma. The brain surgeon (neurosurgeon) will work to remove the pineoblastoma with the goal of removing as much of the tumor as possible. But it’s often impossible to remove the tumor entirely because pineoblastoma forms near critical structures deep within the brain. Most children with pineoblastoma receive additional treatments after surgery to target the remaining cells.
- Radiation therapy. Radiation therapy uses high-energy beams, such as X-rays or protons, to kill cancer cells. During radiation therapy, your child lies on a table while a machine moves around him or her, directing beams to the brain and spinal cord, with additional radiation to the tumor. Because there is a high risk the tumor cells can spread beyond the initial site to other areas of the central nervous system, radiation therapy directed to the entire brain and spinal cord is recommended for children older than 3.
- Chemotherapy. Chemotherapy uses drugs to kill cancer cells. Chemotherapy may be recommended after surgery or radiation therapy in children with pineoblastoma. In some cases, it’s used at the same time as radiation therapy. For larger tumors, chemotherapy may be used before surgery to shrink the tumor and make it easier to remove.
- Radiosurgery. Technically a type of radiation and not an operation, stereotactic radiosurgery focuses multiple beams of radiation on precise points to kill the tumor cells. Radiosurgery is sometimes used to treat pineoblastoma that recurs.
- Clinical trials. Clinical trials are studies of new treatments. These studies give you a chance to try the latest treatment options, but the risk of side effects may not be known. Ask your doctor whether your child might be eligible to participate in a clinical trial.
Melatonin supplement
Melatonin is a hormone secreted by the pineal gland in the brain. It helps regulate other hormones and maintains the body’s circadian rhythm. The circadian rhythm is an internal 24-hour “clock” that plays a critical role in when you fall asleep and when you wake up. When it is dark, your body produces more melatonin. When it is light, the production of melatonin drops. Being exposed to bright lights in the evening, or too little light during the day, can disrupt the body’s normal melatonin cycles. For example, jet lag, shift work, and poor vision can disrupt melatonin cycles.
Some scientific evidence supports use of melatonin to minimize the effects of jet lag, especially in people traveling eastward over 2 to 5 time zones 96), 97). However, in one well-designed study, melatonin supplements did not relieve symptoms of jet lag 98) and only a few small studies suggest that these supplements can relieve jet lag symptoms 99), 100), indicating that clinical trial results are inconsistent.
Standard dosage is not established and ranges from 0.5 to 5 mg orally taken 1 hour before usual bedtime on the day of travel and 2 to 4 nights after arrival. Evidence supporting use of melatonin as a sleep aid in adults and children with neuropsychiatric disorders (eg, pervasive developmental disorders) is less strong.
Melatonin also helps control the timing and release of female reproductive hormones 101). It helps determine when a woman starts to menstruate, the frequency and duration of menstrual cycles, and when a woman stops menstruating (menopause). Preliminary research suggests low levels of melatonin help identify women at risk of a pregnancy complication called pre-eclampsia 102).
Some researchers also believe that melatonin levels may be related to aging 103). For example, young children have the highest levels of nighttime melatonin. Researchers believe these levels drop as we age. Some people think lower levels of melatonin may explain why some older adults have sleep problems and tend to go to bed and wake up earlier than when they were younger. However, newer research calls this theory into question.
Melatonin has strong antioxidant effects. Preliminary evidence suggests that it may help strengthen the immune system 104).
Melatonin levels may also play a role in 105), 106):
- regulating the immune response or immune system function
- regulating development and aging
- temperature homeostasis
- the development of cardiovascular diseases, obesity, metabolic syndrome, and osteoporosis
- mood
However, it is not confirmed whether melatonin levels cause, or are a consequence of, specific conditions because they may strongly influence sleep 107). The most common use of melatonin as a supplement is to aid in sleep 108).
There is very limited information in the literature about the influence of a pineal cyst on melatonin secretion. A recent study concluded that their patients with a pineal cyst retained a pattern of melatonin secretion comparable to those without a pineal cyst. However, this study had several limitations, including a small sample size of 4 patients. It should be noted that those who have a pineal cyst removed will no longer produce melatonin. Possible consequences of melatonin deficiency other than expected sleep disturbances are difficult to identify 109).
If you are considering using melatonin supplements, talk to your doctor first.
How much melatonin should I take?
The typical adult dose ranges from 0.3 mg to 5 mg at bedtime. Lower doses often work as well as higher doses. Intake of an usual dose (i.e., 1 to 5 mg), allows within the hour after ingestion, melatonin concentrations 10 to 100 times higher than the physiological nocturnal peak to be obtained, with a return to basal concentrations in 4 to 8 hours 110). A bioavailability study in four male healthy volunteers 111) showed a plasma melatonin peak varying between 2 and 395 nmol/L and an elimination half-life of 47± 3 min (mean ± SD) after oral administration of a 0.5 mg dose. Bioavailability varied from 10 to 56% (mean 33%).
Read the directions on the label of the pill bottle. These will tell you how much melatonin to take and how often to take it. If you have questions about how to take melatonin, call your doctor or pharmacist. Do not take more than the recommended amount. Taking more melatonin does not make it work quicker or better. Overdosing on any medicine can be dangerous.
Keep a record of all medicines and supplements you take and when you take them. Take this list with you when you go to the doctor. Ask your doctor if it’s okay to take melatonin if:
- You take other prescription or over the counter (OTC) medicines.
- You have ongoing health problems.
- You are pregnant or nursing (it is unclear what effect melatonin can have on an unborn baby or nursing infant).
Melatonin for sleep
How to Take Melatonin for Sleep (Insomnia):
Dosage: Take melatonin 0.1 mg to 0.5 mg thirty minutes before bedtime. Studies suggest melatonin for sleep may be effective in promoting but not maintaining sleep (early morning awakening).
How to Take Melatonin for Shift-Work Sleep Disorders
Dosage: Take melatonin 1.8 mg to 3 mg thirty minutes prior to the desired onset of daytime sleep; melatonin may NOT lead to improved alertness during the nighttime work shift and may only improve daytime sleep time by about 30 minutes.
How to Take Melatonin for Delayed Sleep Phase Disorder
Delayed sleep phase disorder most often occurs in adolescents, possibly due to reduced melatonin production and melatonin deficiency at this age. Sleep onset is delayed by 3 to 6 hours compared with conventional bedtimes (10 to 11 pm). Delayed sleep phase disorder can negatively affect school performance, daily activities, and lead to morning drowsiness which can be dangerous for teen drivers. Any sleep disorder in an adolescent should be evaluated by a physician.
Dosage: Take melatonin 1 mg four to six hours before set bedtime. Once a set bedtime is achieved, use maintenance doses of 0.5 mg melatonin 2 hours before expected sleep onset. Bright light therapy and behavioral management may enhance results. Be aware drowsiness may occur after melatonin dose, so avoid hazardous activities such as driving.
How to Take Melatonin for Non-24-Hour Sleep Wake Disorder (Non-24)
More than 70% of people who are totally blind have Non-24, a circadian rhythm disorder. For people who are totally blind, there are no light cues to help reset the biological clock. The sleep time and wake up time of people who have Non-24-Hour Sleep Wake Disorder shifts a little later every day. Sleep times go in and out of alignment compared to a normal sleep-wake phase. Extra minutes add up each day by day and disrupt the normal wake-sleep pattern.
Use of melatonin in Non-24 is to aid in stimulation to reset the biological clock with one long sleep time at night and one long awake time during the day. However, no large-scale clinical trials of melatonin therapy for Non-24 have been conducted to date.
Dosage: Studies on the blind suggest that 0.5 mg/day melatonin is an effective dose.
Hetlioz, a prescription-only melatonin agonist is also approved for use in Non-24-Hour Sleep Wake Disorder in blind individuals.
Hetlioz (tasimelteon)
Fast-dissolving Melatonin
Some melatonin tablets are available in fast-dissolving formulations in the U.S. To take the orally disintegrating tablet:
- Use dry hands to remove the tablet and place it in your mouth.
- Do not swallow the tablet whole. Allow it to dissolve in your mouth without chewing. If desired, you may drink liquid to help swallow the dissolved tablet.
Call your doctor if the condition you are treating with melatonin does not improve, or if it gets worse while using this product.
Store at room temperature away from moisture and heat.
Melatonin for children
Parents may consider using melatonin to help their child who has a trouble falling asleep. Only use melatonin for your child under the care of a pediatrician or other medical sleep specialist. Insomnia or other sleeping disorders in children should always be evaluated by a medical professional. Children 6 months to 14 years of age with sleep disorders : Melatonin 2 to 5 mg has been used.
Melatonin should not be used as a substitute for good sleep hygiene and consistent bedtime routines in children.
Products containing lower-dose melatonin for kids do exist on the U.S. market. However, long-term use of melatonin has not been studied in children and possible side effects with prolonged use are not known. Use for children with autism spectrum disorder or attention-deficit hyperactivity disorder should involve behavioral interventions and should be directed by a physician.
Delayed sleep phase disorder often occurs in teenagers and young adults, possibly due to alterations in endogenous melatonin production. Sleep onset is delayed by 3 to 6 hours compared with normal bedtime hours of 10 to 11 PM. Maintaining a consistent bedtime free of electronics for at least one hour prior to bedtime is especially important for children and adolescents.
Melatonin Side Effects in Children
The most common melatonin side effect in children is morning drowsiness. Other common side effects in children include:
- Bedwetting
- Headache
- Dizziness
- Nausea
- Diarrhea
- Possible increased risk for seizures in children with severe neurological disorders.
Dietary melatonin supplements can still have drug interactions or health risks if you have certain medical conditions, upcoming surgery, or other health concerns.
Melatonin benefits
Melatonin supplements may help some people with certain sleep disorders, including jet lag, sleep problems related to shift work, and delayed sleep phase disorder (one in which people go to bed but can’t fall asleep until hours later), and insomnia. Unlike many other sleep medications, with melatonin you are unlikely to become dependent, have a diminished response after repeated use (habituation), or experience a hangover effect.
If melatonin for sleep isn’t helping after a week or two, stop using it. And if your sleep problems continue, talk with your health care provider.
If melatonin does seem to help, it’s safe for most people to take nightly for one to two months. After that, stop and see how your sleep is. Be sure you’re also relaxing before bed, keeping the lights low and sleeping in a cool, dark, comfortable bedroom for optimal results.
Sleep Disorders
Studies suggest that melatonin may help with certain sleep disorders, such as jet lag, delayed sleep phase disorder (a disruption of the body’s biological clock in which a person’s sleep-wake timing cycle is delayed by 3 to 6 hours), sleep problems related to shift work, and some sleep disorders in children. It’s also been shown to be helpful for a circadian rhythm sleep disorders in the blind that causes changes in blind peoples’ sleep and wake times. Melatonin can help improve these disorders in adults and children.
However, study results are mixed on whether melatonin is effective for insomnia in adults, but some studies suggest it may slightly reduce the time it takes to fall asleep.
Jet lag
Jet lag is caused by rapid travel across several time zones; its symptoms include disturbed sleep, daytime fatigue, indigestion, and a general feeling of discomfort. To ease jet lag, try taking melatonin two hours before your bedtime at your destination, starting a few days before your trip.
- In a 2009 research review, results from six small studies and two large studies suggested that melatonin may ease jet lag symptoms, such as alertness.
- In a 2007 clinical practice guideline, the American Academy of Sleep Medicine supported using melatonin to reduce jet lag symptoms and improve sleep after traveling across more than one time zone.
You can also adjust your sleep-wake schedule to be in sync with your new time zone by simply staying awake when you reach your destination—delaying sleep until your usual bedtime in the new time zone. Also, get outside for natural light exposure.
Melatonin for Jet Lag:
- Eastbound: If you are traveling east, say from the US to Europe, take melatonin after dark, 30 minutes before bedtime in the new time zone or if you are on the plane. Then take it for the next 4 nights in the new time zone, after dark, 30 minutes before bedtime. If drowsy the day after melatonin use, try a lower dose.
- Westbound: If you are heading west, for example, from the US to Australia, a dose is not needed for your first travel night, but you then may take it for the next 4 nights in the new time zone, after dark, 30 minutes before bedtime. Melatonin may not always be needed for westbound travel.
Given enough time (usually 3 to 5 days), jet lag will usually resolve on its own, but this is not always optimal when traveling.
Delayed Sleep Phase Disorder
In this disorder your sleep pattern is delayed two hours or more from a conventional sleep pattern, causing you to go to sleep later and wake up later. Adults and teens with delayed sleep-wake phase sleep disorder have trouble falling asleep before 2 a.m. and have trouble waking up in the morning. Research shows that melatonin reduces the length of time needed to fall asleep and advances the start of sleep in young adults and children with this condition. Talk to your child’s doctor before giving melatonin to a child.
- In a 2007 review of the literature, researchers suggested that a combination of melatonin supplements, a behavioral approach to delay sleep and wake times until the desired sleep time is achieved, and reduced evening light may even out sleep cycles in people with this sleep disorder.
- In a 2007 clinical practice guideline, the American Academy of Sleep Medicine recommended timed melatonin supplementation for this sleep disorder.
Shift Work Disorder
Shift work refers to job-related duties conducted outside of morning to evening working hours. About 2 million Americans who work afternoon to nighttime or nighttime to early morning hours are affected by shift work disorder.
- A 2007 clinical practice guideline and 2010 review of the evidence concluded that melatonin may improve daytime sleep quality and duration, but not nighttime alertness, in people whose jobs require them to work outside the traditional morning to evening schedule.
- The American Academy of Sleep Medicine recommended taking melatonin prior to daytime sleep for night shift workers with shift work disorder to enhance daytime sleep.
Insomnia
Insomnia is a general term for a group of problems characterized by an inability to fall asleep and stay asleep. Research suggests that melatonin might provide relief from the inability to fall asleep and stay asleep (insomnia) by slightly improving your total sleep time, sleep quality and how long it takes you to fall asleep.
- In adults. A 2013 analysis of 19 studies of people with primary sleep disorders found that melatonin slightly improved time to fall asleep, total sleep time, and overall sleep quality. In a 2007 study of people with insomnia, aged 55 years or older, researchers found that prolonged-release melatonin significantly improved quality of sleep and morning alertness.
- In children. There’s limited evidence from rigorous studies of melatonin for sleep disorders among young people. A 2011 literature review suggested a benefit with minimal side effects in healthy children as well as youth with attention-deficit hyperactivity disorder, autism, and several other populations. There’s insufficient information to make conclusions about the safety and effectiveness of long-term melatonin use.
Sleep-wake cycle disturbances in children
Sleep problems are one of the most common problems parents encounter with their children. There are some simple steps parents can take to improve their children’s sleep, such as having a set bedtime and bedtime routine, avoiding foods or drinks with caffeine, and limiting the amount of screen time. Melatonin can help treat these sleep-wake cycle disturbances in children with a number of disabilities. For example, children with multiple developmental, neuro-psychiatric and health difficulties often show melatonin deficiency 112). When circadian rhythms are restored, behavior, mood, development, intellectual function, health, and even seizure control may improve 113).
Other therapeutic effects of melatonin
Therapeutic effects of melatonin have been reported in several disorders such as certain tumors, cardiovascular diseases or psychiatric disorders. The part concerning melatonin and psychiatric disorders is in particular developed given our past and current work on this topic.
Indeed, oncostatic effects of melatonin have been reported in several tumors (breast cancer, ovarian and endometrial carcinoma, human uveal melanoma, prostate cancer, hepatomas, and intestinal tumors) 114). These oncostatic effects have been attributed to the anti-oxidative role of melatonin given that oxidative stress is involved in the initiation, promotion and progression of carcinogenesis 115). Also, decreased melatonin levels (measures of blood melatonin or urinary excretion of 6-SM) were reported in patients with cardiovascular diseases 116). Inversely, melatonin treatment reduces blood pressure in patients with hypertension 117).
Concerning psychiatric disorders, secretion disturbances of the pineal gland have been described in child and adult psychiatry, with notably in most studies a decreased nocturnal melatonin secretion observed in major depressive disorder, bipolar disorder, schizophrenia or autism spectrum disorder 118).
Also, a phase-shift of melatonin has been reported in major depressive disorder and bipolar disorder, including in particular a delayed melatonin peak secretion 119). It is noteworthy that increased melatonin levels (measures of blood melatonin and urinary excretion of 6-SM) were found when clinical therapeutic benefits were observed following the use of antidepressants 120). Furthermore, significant improvement of major depressive disorder and anxiety was described following administration of 25mg per day of agomelatine, a MT1/MT2 melatonin agonist and selective antagonist of 5-HT2C receptors 121).
Autism spectrum disorder
Concerning autism spectrum disorder (ASD), abnormalities in the serotoninergic system and sleep-wake rhythm disturbances observed in children with autism spectrum disorder suggest altered melatonin secretion in autism 122). Sleep disorders (mostly increased sleep latency, reduced total sleep and nocturnal awakenings with insomnia) are observed in 50-80% of individuals with autism 123). It is noteworthy that sleep problems are not specific of autism and are also observed in children with intellectual disability associated or not with autism 124). However, melatonin measures in children with intellectual disability not associated with autism, such as some children with Down syndrome and Fragile X syndrome, showed respectively normal melatonin production despite delayed nocturnal melatonin peak secretion and increased levels of melatonin 125), whereas decreased nocturnal melatonin secretion was mostly observed in children with autism 126). Scientists reported in two different large samples of children with autism significant relationships between decreased nocturnal urinary excretion and severity of autistic impairments in social communication 127), 128). These results suggest that abnormalities in melatonin physiology might contribute not only to sleep problems in autism, but also to biological and psychopathological mechanisms involved in the development of autism spectrum disorder (for example, certain immunological abnormalities found in autism, such as a decrease number of T lymphocytes, might be explained by the hypo-functioning of the melatonin system).
Schizophrenia
Concerning schizophrenia, as suggested by Morera-Fumero and Abreu-Gonzalez 129), a possible explanation for the “low melatonin syndrome” present in some individuals with schizophrenia may stem from the study of the melatonin-synthesizing enzymes, the AA-NAT and ASMT. Furthermore, according to some authors, MT3 might be involved in the melatonin disturbances observed in schiozophrenia 130). Finally, melatonin secretion was also studied in obsessive compulsive disorder but no abnormalities in melatonin levels were reported.
Brain Protection
Neurological and neuropsychological disabilities caused by brain injuries are a major public health concern. Thus, reducing deficits after a stroke is a major issue. In this line, a number of recent studies have reported the important role of melatonin in neuroprotection in animal models of stroke 131). Indeed, melatonin administration after an experimental stroke in animals reduces infarction volume 132). Such a protective effect can be seen in both gray and white matter 133) and melatonin reduces also inflammatory response 134), cerebral oedema formation 135), and blood-brain barrier permeability 136). In addition, Kilic et al. 137) investigated how sub-acute delivery of melatonin, starting at 24 hours after stroke onset, and continuing for 29 days can influences neuronal survival, endogenous neurogenesis, motor recovery and locomotor activity in mice submitted to an occlusion of the middle cerebral artery during 30 minutes. Furthermore, melatonin improved neuronal survival and enhanced neurogenesis, even when applied one day after stroke. In addition, the authors showed both motor as well as behavioral improvements after melatonin administration. Indeed, the results indicate that cell survival was associated with a long-lasting improvement of motor and coordination deficits as well as with attenuation of hyperactivity and anxiety of the animals as revealed in open field tests. Its neuroprotective activity in animal models of ischemic stroke, as well as its lack of serious toxicity suggests that melatonin could be used for human stroke treatment in the future.
In addition to its protective effect after stroke, experimental data obtained in various independent animal models of brain lesions in neonates support the notion of a neuroprotective effect of melatonin in preterm neonates {see for a review, Biran et al., 138)]. In infants, a major source of brain injury is preterm birth, often associated to long-term neurological, cognitive, educational, and social problems. Neurodevelopmental disorders are not only seen in extremely preterm birth 139) but also in late prematurity 140). A large number of infants who survive very preterm birth develop cerebral palsy 141) with a high occurrence of associated motor, perceptual and cognitive deficiencies in childhood 142). Nowadays, the most common brain damage observed in preterm children is diffuse white matter damage as well as reduced neural connectivity 143) in the context of infection, inflammation, and hypoxia-ischemia 144). Although a number of treatments have been tested in preclinical animal models of perinatal brain injury, none of them had been proved to be efficient as a neuroprotector nor translated in clinical practice. Among the molecules proposed, melatonin is a very good candidate, given its effect on brain development, neuroprotection as well as regarding its absence of adverse effects 145). As discussed by Biran et al., 146), in addition to its good safety profile, melatonin easily crosses the placenta as the blood-brain barriers and blocks oxidative, excitotoxic and inflammatory pathways, all involved in the pathogenesis of perinatal brain damage caused by preterm birth. However, only a few studies have looked at the synthesis of melatonin in preterm and term neonates. These studies point to a reduced urinary concentration of melatonin during the first 3 months after birth in preterm infants 147). As these authors discussed, compared with term neonates, preterm neonates show a delayed secretion of melatonin which persists after correction for gestational age up to 8 to 9 months of age. In the absence of maternal melatonin, the appearance of circadian rhythms depends principally on neurological maturation, and very little on the environment 148).
Since, melatonin easily crosses the blood–brain and placental barriers, it can be administered antenatally in order to reduce or prevent the impact of
brain lesions in preterm neonates. Currently, two therapeutic trials testing the neuroprotective properties of melatonin administration in the immediate prepartum period in very preterm infants are under way in France and in the United Kingdom149). The French trial aims to determine the dose of melatonin to be administered in prepartum by parenteral route to mothers at risk of preterm delivery, to decrease the extent of white matter damage detected by diffusion tensor imaging in infants born preterm. The objective of the English trial is to prove that melatonin is capable of reducing brain injury and white matter disease as defined by magnetic resonance imaging at term. These trials will probably lead to a clinical use of melatonin before preterm birth (in case of at risk mother) of just after birth in preterm neonates in order to prevent neurodevelopmental deficits in these children.
Interestingly, from a functional point of view, abnormalities in melatonin physiology associated with sleep disorders, and in particular sleep deprivation, are seen to endanger cerebral and more specifically hippocampal integrity, leading to cognitive dysfunction and contributing to the development of mood disorders 150). The involvement of melatonin in the development of mood disorders was discussed in the previous section.
Finally, based on the brain protective role of melatonin against oxidative stress previously described in this article, there is also increased experimental evidence showing the therapeutic potential of melatonin in neurodegenerative conditions such as Alzheimer disease, Parkinson disease, Huntington’s disease and amyotrophic lateral sclerosis 151). Additional studies and clinical trials are now required both in preterm neonates and aging adults to test the clinical efficacy of melatonin supplementation in such disorders, and to identify the specific therapeutic concentrations needed regarding the subject’s age, disease and brain lesion as well as the short and long-term effects of melatonin both on physiological, functional and cognitive outcomes.
Uses of melatonin supplement
- Insomnia
Studies suggest that melatonin supplements may help people with disrupted circadian rhythms (such as people with jet lag or those who work the night shift), and those with low melatonin levels (such as some seniors and people with schizophrenia) to sleep better 152). A review of the scientific literature suggests that melatonin supplements may help prevent jet lag, particularly in people who cross 5 or more time zones.
A few clinical studies suggest that, when taken for short periods of time (days to weeks), melatonin is more effective than a placebo in reducing the time it takes to fall asleep, increasing the number of sleeping hours, and boosting daytime alertness. It is not clear how well melatonin works, however. Some studies suggest that it only reduces the amount of time to fall asleep by a few minutes.
Several human studies have measured the effects of melatonin supplements on sleep in healthy people. A wide range of doses has been used, often taken by mouth 30 to 60 minutes prior to sleep time. Results have been mixed. Some evidence suggests that melatonin may work best for people over 55 who have insomnia 153). One study of 334 people aged 55 and older found that sustained-release melatonin seemed to help people with primary insomnia fall asleep faster, sleep better, be more alert in the morning, and improve quality of life in people with primary insomnia 154).
- Heart Disease
Several studies show melatonin has cardioprotective properties, including antioxidant and anti-inflammatory effects 155), 156). Research also suggests that melatonin may help lower blood pressure levels and improve cholesterol profiles 157). More research is needed.
- Menopause
Melatonin supplements may improve sleep problems associated with menopause. Other studies suggest it may help restore quality of life 158) and prevent bone loss among perimenopausal women 159). However, it does not appear to relieve other symptoms of menopause, such as hot flashes. Peri- or postmenopausal women who use melatonin supplements should do so only for a short period of time since long-term effects are not known.
- Benzodiazepine Withdrawal
Some research suggests that melatonin may help elderly people with insomnia who are tapering off or stopping benzodiazepines such as diazepam (Valium), alprazolam (Xanax), or lorazepam (Ativan). Taking controlled-release melatonin improved sleep quality in those stopping benzodiazepine use. More research is needed. You should never combine melatonin with sedative medications unless you are under the strict supervision of a health care provider.
- Breast Cancer
Several studies suggest that low melatonin levels may be associated with breast cancer risk 160), 161). For example, women with breast cancer tend to have lower levels of melatonin than those without the disease 162). Laboratory experiments have found that low levels of melatonin stimulate the growth of certain types of breast cancer cells, while adding melatonin to these cells slows their growth 163). Preliminary evidence also suggests that melatonin may strengthen the effects of some chemotherapy drugs used to treat breast cancer 164). In a study that included a small number of women with breast cancer, melatonin (given 7 days before beginning chemotherapy) prevented the lowering of platelets in the blood 165). This is a common complication of chemotherapy that can lead to bleeding.
In another small study of women who were taking tamoxifen for breast cancer but seeing no improvement, adding melatonin caused tumors to modestly shrink in more than 28% of the women 166). Women with breast cancer should ask their doctors before taking melatonin.
- Prostate Cancer
Studies show that men with prostate cancer have lower melatonin levels than men without the disease 167), 168). In test tube studies, melatonin blocks the growth of prostate cancer cells. In one small-scale study, melatonin, combined with conventional medical treatment, improved survival rates in 9 out of 14 men with metastatic prostate cancer. Interestingly, since meditation may cause melatonin levels to rise it appears to be a valuable addition to the treatment of prostate cancer. More research is needed before doctors can make recommendations in this area. Men with prostate cancer should talk to their doctor before taking medication.
- Attention Deficit Hyperactivity Disorder (ADHD) and Autism
Some evidence suggests that melatonin may help promote sleep in children with ADHD 169) or autism, although it does not seem to improve the behavioral symptoms of ADHD or autism 170), 171).
- Fibromyalgia and Chronic Pain
A randomized, placebo-controlled study found that people with fibromyalgia experienced a significant reduction in their symptoms when they took a melatonin supplement either alone or in conjunction with fluoxetine (Prozac) 172). Other studies suggest that melatonin may play a role in other painful conditions, such as migraines 173). People with chronic pain should speak to their physicians before using melatonin as it can interact with some medications.
Other Uses
- Sunburn. Preliminary studies suggest that gels, lotions, or ointments containing melatonin may protect against sunburn and other skin damage. Studies examined using melatonin alone or combined with topical vitamin E prior to UV light exposure from the sun.
- Irritable Bowel Syndrome (IBS). Preliminary research suggests that people with IBS who take melatonin reduce some symptoms, such as abdominal pain 174). Results are mixed as to whether melatonin may help improve other symptoms, such as bloating and frequency of bowel movements.
- Epilepsy. Some studies suggest melatonin may reduce the frequency and duration of seizures in children with epilepsy, but other studies suggest melatonin may increase the frequency of seizures 175), 176). DO NOT take melatonin for epilepsy, or give it to a child, without talking to your doctor first.
- Sarcoidosis. Some researchers suggest that melatonin may be effective in the treatment of pulmonary sarcoidosis 177). Talk to your doctor.
- Assisted Reproduction. Interestingly, preliminary studies suggest melatonin supplementation in the eggs of women with polycystic ovarian syndrome could improve egg maturation and pregnancy rates 178).
- Other Uses. Preliminary evidence suggests that melatonin may play a role in pain modulation and digestive function. More research is needed.
Available Forms
Melatonin is available as tablets, capsules, cream, and lozenges that dissolve under the tongue.
How to Take It
There is currently no recommended dose for melatonin supplements. Different people will have different responses to its effects. Lower doses appear to work better in people who are especially sensitive. Higher doses may cause anxiety and irritability.
The best approach for any condition is to begin with very low doses of melatonin. Keep the dose close to the amount that our bodies normally produce (< 0.3 mg per day). You should only use the lowest amount possible to achieve the desired effect. Your doctor can help you determine the most appropriate dose for your situation, including how to increase the amount, if needed.
Effective starting doses for jet lag range from 0.3 to 0.5 mg. One milligram tablets can be cut in half to achieve a 0.5 mg dose if smaller doses are not available for purchase. Lower doses may work for some people, while others may need a higher dose, up to 3 to 5 mg. However, higher doses may be associated with more side effects such as headache, next day grogginess, or vivid dreams.
Always start with the lowest melatonin dose. According to a Cochrane review 179), doses over 5 mg appear to be no more effective than lower doses. It is important to note that much higher doses are available for sale in the U.S., but these doses may result in excessively high levels of physiologic melatonin.
Pediatric
Always ask your child’s doctor before giving melatonin to a child. In fact, doses between 1 to 5 mg may cause seizures in this age group.
Adult
You should work with your doctor to find the safest and most effective dose for you. The right dose for you should produce restful sleep with no daytime irritability or fatigue.
Jet lag: 0.5 to 5 mg of melatonin 1 hour prior to bedtime at final destination has been used in several studies. Another approach that has been used is 1 to 5 mg 1 hour before bedtime for 2 days prior to departure and for 2 to 3 days upon arrival at final destination.
Precautions
Because of the potential for side effects and interactions with medications, people should take dietary supplements only under the supervision of a knowledgeable health care provider.
Some people may have vivid dreams or nightmares when they take melatonin. Taking too much melatonin may disrupt circadian rhythms (your “body clock”).
Melatonin can cause drowsiness if taken during the day. If you are drowsy the morning after taking melatonin, try taking a lower dose.
Additional side effects include stomach cramps, dizziness, headache, irritability, decreased libido, breast enlargement in men (called gynecomastia), and reduced sperm count.
Pregnant or nursing women should not take melatonin because it could interfere with their fertility, or their pregnancy.
Melatonin is a hormone so patients with a history of hormonal-related issues should only use melatonin under the supervision of their physicians.
Some studies show that melatonin supplements worsened symptoms of depression. For this reason, people with depression should consult their doctor before using melatonin supplements.
Although many researchers believe that melatonin levels go down with age, newer evidence has brought this theory into question. People older than 65 should ask their doctor before taking melatonin supplements, so blood levels of this hormone can be monitored.
Possible Interactions
If you are taking prescription medications, you should not use melatonin without first discussing it with your health care provider. Below is a partial list of medications that may interact with melatonin.
- Antidepressant medications. In an animal study, melatonin supplements reduced the antidepressant effects of desipramine and fluoxetine (Prozac). More research is needed to know if the same thing would happen in people. In addition, fluoxetine (a member of a class of drugs called selective serotonin reuptake inhibitors, or SSRIs) can cause low levels of melatonin in people.
- Antipsychotic medications. A common side effect of antipsychotic medications used to treat schizophrenia is a condition called tardive dyskinesia, which causes involuntary movements. In a study of 22 people with schizophrenia and tardive dyskinesia caused by antipsychotic medications, those who took melatonin supplements had fewer symptoms compared to those who did not take the supplements.
- Benzodiazepines. The combination of melatonin and triazolam (Halcion) improved sleep quality in one study. In addition, a few reports have suggested that melatonin supplements may help people stop using long-term benzodiazepine therapy. (Benzodiazepines are habit forming.)
- Birth control pills. Birth control pills may increase the amount of melatonin your body makes. Taking additional melatonin could increase your levels of melatonin above the healthy range.
- Blood pressure medications. Melatonin may make blood pressure medications like methoxamine (Vasoxyl) and clonidine (Catopres) less effective. In addition, medications in a class called calcium channel blockers may lower melatonin levels. Calcium channel blockers include:
- Nifedipine (Procardia)
- Amlodipine (Norvasc)
- Verapamil (Calan, Isoptin)
- Diltiazem (Cardizem)
- Felodipine (Plendil)
- Nisoldipine (Sular)
- Bepridil (Vascor)
Beta-blockers. Use of beta-blockers may lower melatonin levels in the body. Beta-blockers include:
- Acebutolol (Sectral)
- Atenolol (Tenormin)
- Bisoprolol (Zebeta)
- Carteolol (Cartrol)
- Metoprolol (Lopressor, Toprol XL)
- Nadolol (Corgard)
- Propranolol (Inderal)
Blood-thinning medications (anticoagulants). Melatonin may increase the risk of bleeding from anticoagulant medications such as warfarin (Coumadin).
Interleukin-2. In one study of 80 cancer patients, use of melatonin along with interleukin-2 led to more tumor regression and better survival rates than treatment with interleukin-2 alone.
Nonsteroidal anti-inflammatory drugs (NSAIDs). NSAIDs such as ibuprofen (Advil, Motrin) may lower levels of melatonin in the blood.
Steroids and immunosuppressant medications. Melatonin may cause these medication to lose their effectiveness. DO NOT take melatonin with corticosteroids or other medications used to suppress the immune system.
Tamoxifen. Preliminary research suggests that the combination of tamoxifen (a chemotherapy drug) and melatonin may benefit some people with breast and other cancers. More research is needed to confirm these results.
Other. Caffeine, tobacco, and alcohol can all lower levels of melatonin in the body.
Melatonin side effects
Melatonin taken orally in appropriate amounts is generally safe. Side effects of melatonin are uncommon but can include:
- drowsiness,
- a “heavy-head” feeling,
- headache,
- dizziness,
- feeling hungover,
- nausea.
There have been no reports of significant side effects of melatonin in children.
Other, less common melatonin side effects might include short-lasting feelings of depression, mild tremor, mild anxiety, abdominal cramps, irritability, reduced alertness, confusion or disorientation, and abnormally low blood pressure (hypotension). Because melatonin can cause daytime drowsiness, don’t drive or use machinery within five hours of taking the supplement.
- Do not use melatonin if you are pregnant or breastfeeding or have an autoimmune disorder, a seizure disorder or depression. Talk to your health care provider if you have diabetes or high blood pressure. Melatonin supplements may also raise blood-sugar levels and increase blood pressure levels in people taking some hypertension medications.
In addition, melatonin supplements can interact with various medications, including:
- Anticoagulants and anti-platelet drugs. These types of drugs, herbs and supplements reduce blood clotting. Combining use of melatonin with them might increase the risk of bleeding.
- Anticonvulsants. Melatonin might inhibit the effects of anticonvulsants in neurologically disabled children.
- Blood pressure drugs. Melatonin might worsen blood pressure in people taking blood pressure medications.
- CNS depressants. Melatonin use with use of these medications might cause an additive sedative effect.
- Diabetes medications. Melatonin might affect sugar levels. If you use diabetes medications, use melatonin cautiously.
- Contraceptive drugs. Use of contraceptive drugs with melatonin might increase the effects and possible side effects of melatonin.
- Cytochrome P450 1A2 (CYP1A2) and cytochrome P450 2C19 (CPY2C19) substrates. Use melatonin cautiously if you take drugs such as diazepam (Valium) and others that are affected by these enzymes.
- Fluvoxamine (Luvox). This selective serotonin reuptake inhibitor can increase melatonin levels, causing unwanted excessive drowsiness.
- Medications that suppress the immune system (immunosuppressants). Melatonin can stimulate immune function and interfere with immunosuppressive therapy.
- Seizure threshold lowering drugs. Taking melatonin with these drugs might increase the risk of seizures.
If you’re considering taking melatonin supplements, check with your doctor first — especially if you have any health conditions. He or she can help you determine if melatonin is right for you.
Is melatonin safe
Melatonin supplements appear to be safe when used short-term; less is known about its long-term safety. Further study is needed to find out more about melatonin’s side effects, especially the delayed or long-term effects. It is unknown if melatonin causes problems when taken with other medicines. It also is unknown if melatonin affects people who have certain diseases and conditions.
- In one study, researchers noted that melatonin supplements may worsen mood in people with dementia.
- The U.S. Food and Drug Administration (FDA) regulates dietary supplements such as melatonin, but the regulations for dietary supplements are different and less strict than those for prescription or over-the-counter drugs. In 2011, the U.S. Food and Drug Administration (FDA) issued a warning to a company that makes and sells “relaxation brownies,” stating that the melatonin in them hasn’t been deemed a safe food additive.
- Most dietary supplements haven’t been tested in pregnant women, nursing mothers, or children. If you’re pregnant or nursing a child, it’s especially important to see your health care provider before taking any medication or supplement, including melatonin.
Melatonin overdose
Melatonin is thought to be very safe in the short-term with a low risk for overdose. Melatonin is not known to be a potential cause of death, but you need to be aware that it could lead to certain complications if you overdose. Taking too much melatonin can disrupt your circadian rhythms (sleep-wake cycle). It may also cause other unwanted side effects. So, yes, you can technically overdose on melatonin. However, a melatonin overdose can be hard to define since there isn’t an official standard safe dose for everyone.
The acute toxicity of melatonin as seen in both animal and human studies is extremely low. Melatonin may cause minor adverse effects, such as headache, insomnia, rash, upset stomach, and nightmares 180). In animals, an LD50 (the lethal dose which suggests that this is an amount of which at least 50% of the experimental animals (rat or mouse) would die of exposure) could not be established 181). Even 800 mg/kg bodyweight (high dose) was not lethal 182). Studies of human subjects given varying doses of melatonin (1–6.6 g/day) for 30–45 days, and followed with an elaborate battery of biochemical tests to detect potential toxicity, have concluded that, aside from drowsiness, all findings were normal at the end of the test period 183).
Melatonin is widely available as an over-the-counter supplement marketed by different companies. These supplements may not be similar in dosage and/or composition, and some of them may contain additional vitamins. Moreover, melatonin may interact with other over-the-counter drugs, although such interactions have not been systematically evaluated and, therefore, remain unreported. Some people are more sensitive than others to the effects of melatonin. A dose that might trigger side effects in one person may have little effect on someone else.
Young children should avoid melatonin unless otherwise directed by a doctor. Doses between 1 and 5 milligrams (mg) may cause seizures or other complications for young children. In adults, doses in the 30-mg range may be harmful. In general, it’s better to start low and move up slowly and carefully if you see encouraging results.
Some people can have side effects from melatonin that may include:
- daytime drowsiness, dizziness, weakness, or confusion
- vivid dreams, nightmares
- feeling depressed, anxious, irritable
- headache
- loss of appetite, diarrhea, nausea, stomach pain
- blood pressure changes
- joint or back pain
- elevated risk for seizures
Animal studies suggest that melatonin can downregulate the pituitary/gonadal axis resulting in hypogonadism and/or delayed puberty. However chronic administration of low-dose melatonin in men did not alter blood levels of testosterone or luteinizing hormone 184). One case of extremely high melatonin levels associated with delayed puberty and hypogonadism has been reported 185). Pubertal development and resolution of the hypogonadism occurred spontaneously as melatonin levels declined over several years. Recent experimental evidence demonstrates that melatonin reduces sperm motility 186) and that long-term administration inhibits testicular aromatase levels 187).
Melatonin has also been suggested for use as a contraceptive for women 188), which might raise the question of whether melatonin damages the female reproductive system. Notably, no side effects were reported in a report of a phase 2 clinical trial in which 1400 women were treated with 75 mg of melatonin nightly for 4 years 189).
Preliminary animal studies suggest that melatonin may accelerate the development of autoimmune conditions 190). Melatonin transiently exacerbated neurologic symptoms in 1 patient with multiple sclerosis 191).
Although melatonin is a potential adjunctive agent in the treatment of cancer and immune deficiency, poorly timed administration can produce opposite effects. Melatonin injections given in the morning stimulate tumor growth 192), whereas the same doses in mid-afternoon have no effect but in the evening have a retarding effect. And although some people with depression may suffer from a “low melatonin syndrome” 193), melatonin administration that unduly prolongs the nocturnal melatonin rise, or that is given throughout the day, may exacerbate seasonal affective disorder 194) and bipolar and classic depression 195). Finally, animal studies have shown that moderately large doses of melatonin (equivalent in one study to about 30 mg in adult humans) increased light-induced damage to retinal photoreceptors 196).
There is also some concern regarding increased atherosclerosis in the aorta in hypercholesterolemic rats caused by melatonin 197). Moreover, in these animals LDL “bad” cholesterol were less well recognized by LDL-receptor metabolic pathways when melatonin was administered.
References [ + ]