Contents
What is HTP
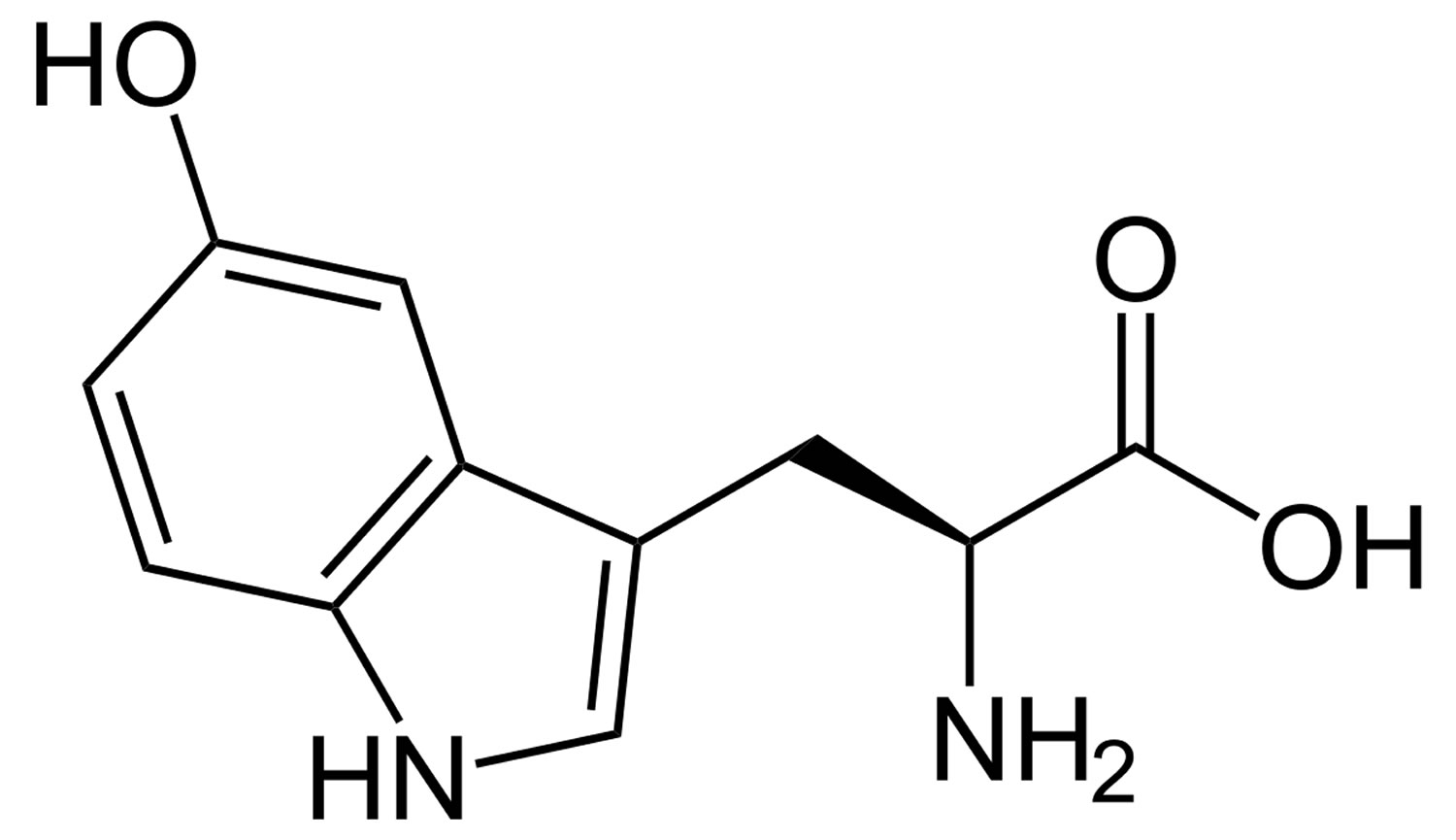
HTP is short for hydroxy-L-tryptophan and it is often called oxitriptan or 5-HTP (5-Hydroxy-L-tryptophan), which is the immediate precursor in the biosynthesis of serotonin (5-HT or 5-hydroxy-tryptamine) from the essential amino acid L-tryptophan 1. 5-HTP is often sold as an herbal supplement. There are no regulated manufacturing standards in place for many herbal compounds and some marketed supplements have been found to be contaminated with toxic metals or other drugs. Herbal/health supplements should be purchased from a reliable source to minimize the risk of contamination. HTP is used as an antiepileptic and antidepressant. Other uses not proven with clinical research have included insomnia, alcohol withdrawal, headaches, premenstrual syndrome, binge-eating related to obesity, attention deficit disorder, and muscle spasms in the mouth. 5-HTP may also be an appetite suppressant, but further clinical trials are needed. Medicinal use of HTP or 5-HTP has not been approved by the FDA. 5-HTP should not be used in place of medication prescribed for you by your doctor.
Endogenous HTP (oxitriptan) is produced from the essential amino acid L-tryptophan. The exogenous therapeutic form is isolated from the seeds of the African plant Griffonia simplicifolia. HTP is an aromatic amino acid with antidepressant activity. In your body, oxitriptan (or 5-HTP) is converted into serotonin (5-HT or 5-hydroxytryptamine) as well as other neurotransmitters. HTP may exert its antidepressant activity via conversion to serotonin or directly by binding to serotonin (5-HT) receptors within the central nervous system (CNS). Concurrent use of 5-HTP with a selective serotonin reuptake inhibitors (SSRI) such as citalopram, fluvoxamine maleate, fluoxetine, paroxetine, sertraline, venlafaxine may potentiate the antidepressant effect of the SSRI and may also increase the risk of adverse reactions 2. Theoretically, concurrent use of 5-HTP and Saint John’s wort may both potentiate the possible antidepressant activity of the herbal product and increase the risk of adverse reactions 2. Concurrent use of 5-HTP with an Monoamine Oxidase Inhibitor (MAOI type A) such as isocarboxazid, phenelzine sulfate, tranylcypromine may increase the risk of adverse effects 2. Carbidopa (amino acid decarboxylase inhibitor also known as DOPA decarboxylase) suppresses peripheral 5-HTP metabolism, allowing greater amounts of 5-HTP to reach the brain 2. Concurrent intake of 5-HTP and doses of vitamin B6 5 mg or greater, may enhance the peripheral decarboxylation of 5-HTP to serotonin. Avoid using 5-HTP with other herbal/health supplements that can cause drowsiness. This includes California poppy, catnip, chamomile, gotu kola, Jamaican dogwood, kava, melatonin, St. John’s wort, skullcap (or scullcap), valerian, yerba mansa, and others.
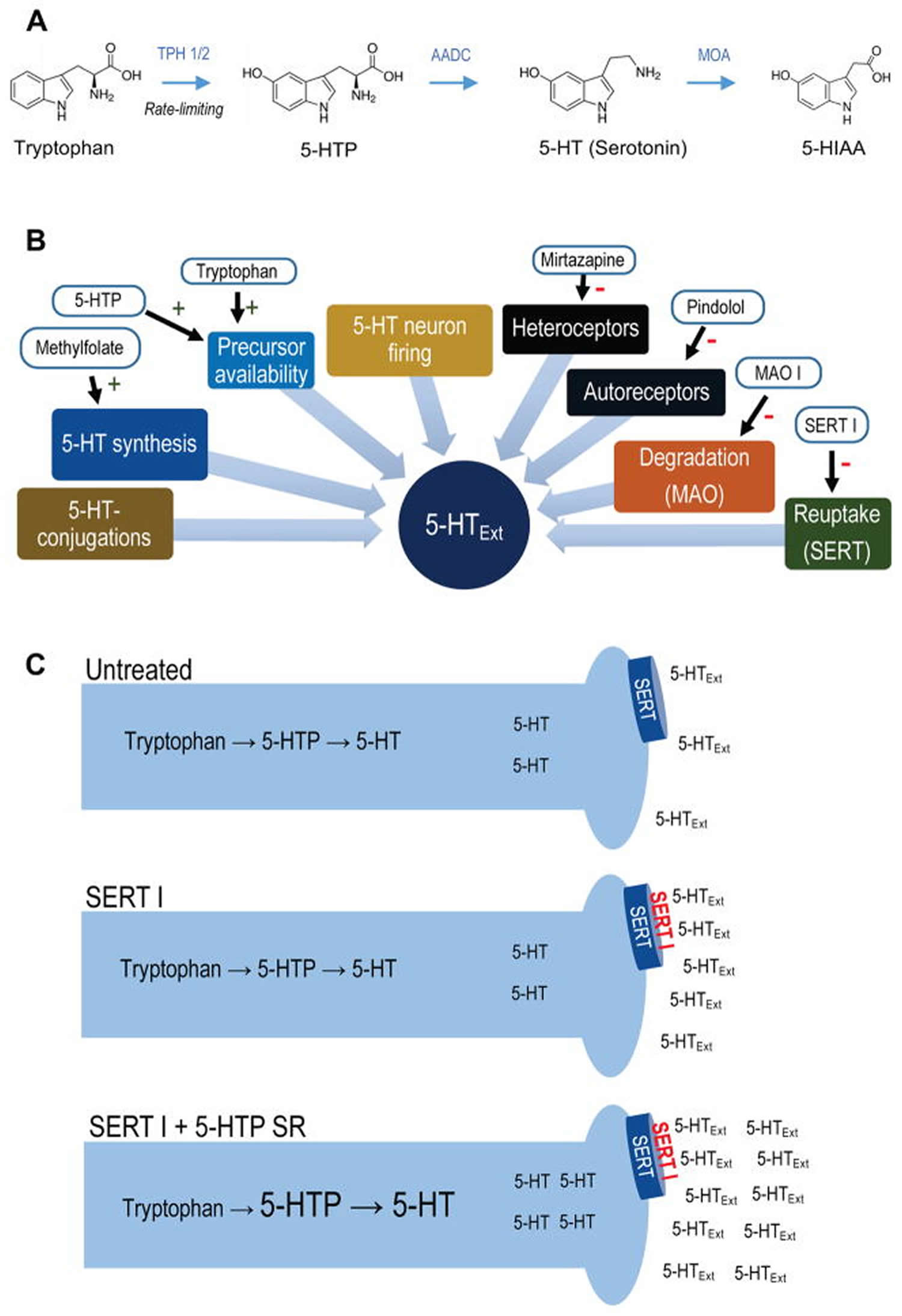
Figure 1. Synthesis of 5-HTP from tryptophan
Footnotes: (A) 5-HT metabolic pathway. Synthesis of 5-HTP from tryptophan via TPH 1 (periphery) or TPH 2 (CNS) is the rate-limiting step in 5-HT synthesis. 5-HTP is rapidly converted to 5-HT by the ubiquitous enzyme amino acid decarboxylase. 5-HT is metabolized to 5-HIAA, 5-HT’s main metabolite, by monoamine oxidase. (B) Simplified schematic of regulatory elements of CNS 5-HTExt. Drugs interacting with each element are indicated. (C) Schematic for adjunct 5-HTP SR mechanism-of-action. Adjunct exogenous 5-HTP increases endogenous 5-HT synthesis, increasing availability of 5-HT for net release by concomitant SERT inhibitor treatment.
Abbreviations: 5-HT = 5-hydroxytryptamine (serotonin); 5-HTExt = extracellular 5-HT; 5-HTP = 5-hydroxytryptopan; 5-HIAA = 5-hydroxyindoleacetic acid; AADC = aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase; MOA = monoamine oxidase; MOA I = monoamine oxidase inhibitor; SERT= serotonin transporter; SERT I = serotonin transporter inhibitor; SR = slow-release; TPH = tryptophan hydroxylase.
[Source 3]The use of the essential amino acid L-tryptophan as a dietary supplement was discontinued in 1989 due to an outbreak of eosinophilia-myalgia syndrome (EMS) that was traced to a contaminated synthetic L-tryptophan from a single manufacturer. 5-HTP has since become a popular dietary supplement in lieu of the removal of L-tryptophan from the market. Because of its chemical and biochemical relationship to L-tryptophan, 5-HTP has been under vigilance by consumers, industry, academia and government for its safety. However, no definitive cases of toxicity have emerged despite the worldwide usage of 5-HTP for last 20 years, with the possible exception of one unresolved case of a Canadian woman. Extensive analyses of several sources of 5-HTP have shown no toxic contaminants similar to those associated with L-tryptophan, nor the presence of any other significant impurities.
5-HTP has shown promising antidepressant effects, but poor pharmacokinetics limits the therapeutic potential 4. A slow-release delivery mode would be predicted to overcome the pharmacokinetic limitations of 5-HTP, substantially enhance the pharmacological action, and transform 5-HTP into a clinically viable drug.
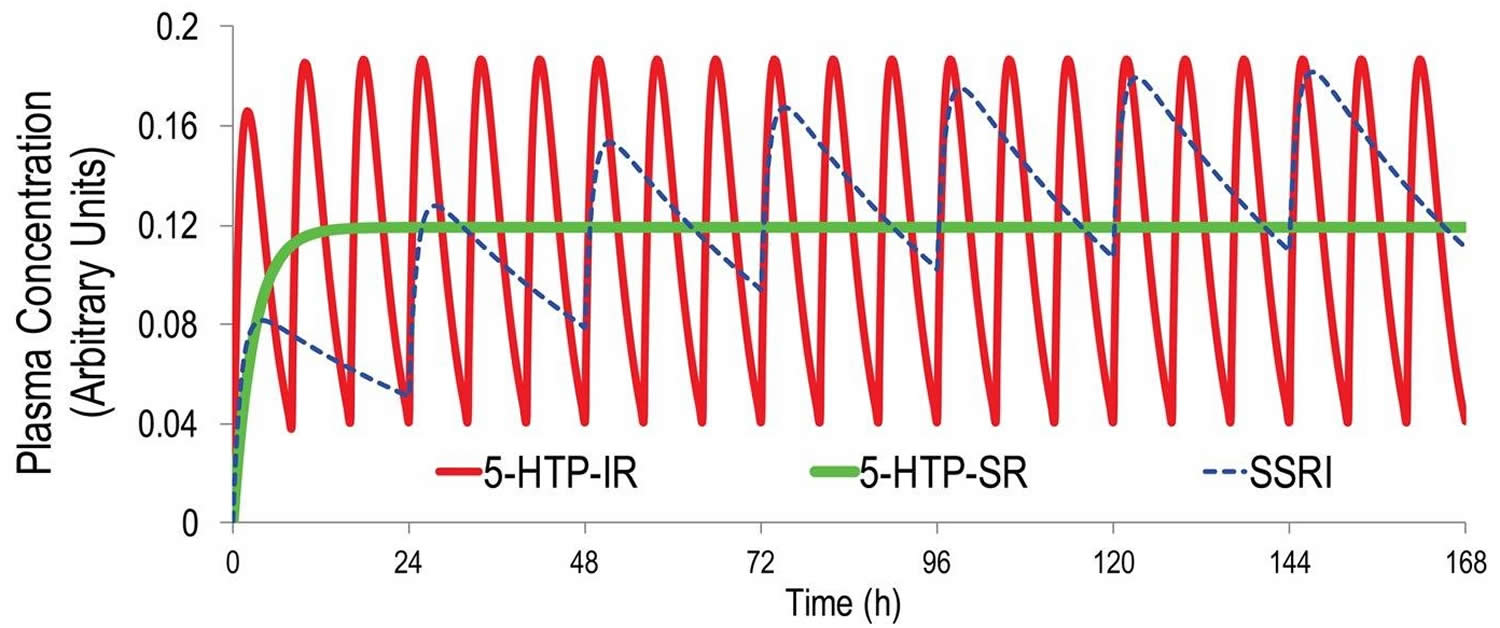
Native 5-HTP immediate release is a poor serotonergic antidepressant. As discussed below, effective antidepressant therapy requires sustained, minimally fluctuating serotonin (5-HT) elevation 5. A half life (T1/2) = 2 hours means that even at thrice-daily dosing 5-HTP plasma levels will fluctuate at least 5-fold at steady-state. This contrasts to the less than 0.3-fold steady-state plasma fluctuations of most SSRIs 6 (Figure 2). Further, 5-HTP’s fast-onset adverse events likely results from the rapid absorption and resultant 5-HT spikes upon administration. Co-administering a peripheral amino acid decarboxylase inhibitor (e.g. carbidopa) with 5-HTP will modestly extend the T1/2, several-fold enhance exposure, and not affect TMax 7. Including a DCI in a 5-HTP SR (slow release) drug could be beneficial, but could complicate formulation development, dosing, and safety.
Figure 2. 5-HTP pharmacokinetics simulation
Footnotes: Pharmacokinetics simulation using one-compartment modeling and published human pharmacokinetic parameters for 5-HTP immediate release (IR) 7 and the canonical SSRI escitalopram 8. Even at thrice-daily dosing at 8 hour intervals, an unrealistic level of adherence in outpatients, 5-HTP plasma levels will fluctuate 5-fold between doses. In contrast, during steady-state once-daily dosing of escitalopram, plasma escitalopram levels will fluctuate only about 0.3-fold. Also shown are 5-HTP plasma levels obtained during steady-state dosing with an ideal 5-HTP slow release (SR) dosage form producing zero-order, constant, 5-HTP delivery.
[Source 3]What is Serotonin (5-Hydroxytryptamine)?
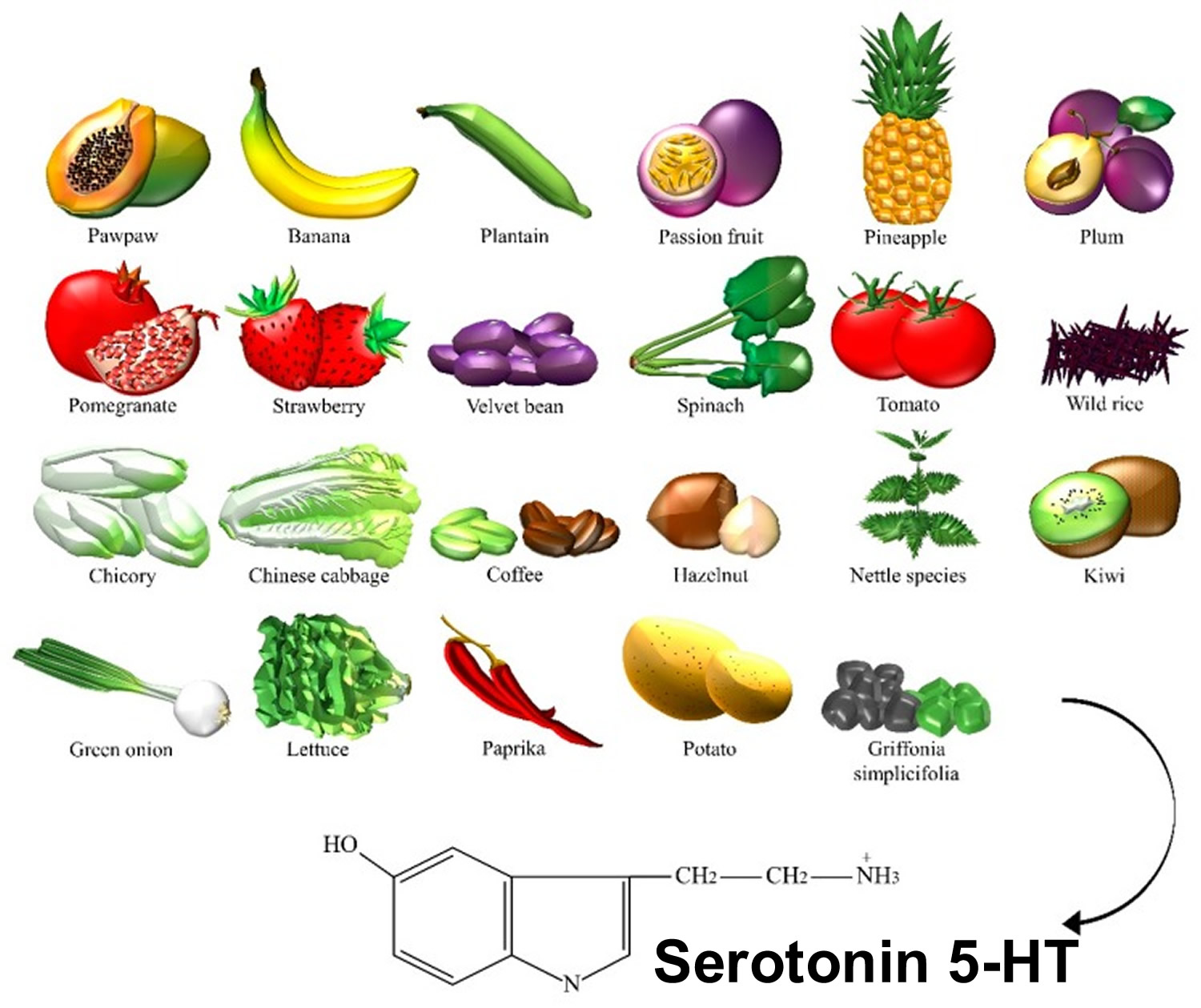
Serotonin or 5-HT (5-Hydroxytryptamine) is a neurotransmitter (a messenger chemical that carries signals between nerve cells in the brain). Serotonin (5-HT) is thought to have a good influence on mood, emotion and sleep. In the central nervous system, serotonin (5-HT) pathways modulate behaviors, eating, and sleep, whereas, in the gut, they are involved in the regulation of gastrointestinal motility 9. Serotonin is a chemical derived from the amino acid tryptophan. It is produced as needed by the nervous system, mainly the brain, but also by special cells in the bronchial tubes (lungs) and gastrointestinal tract. More than 90% of serotonin in the blood is found in the platelets. Serotonin helps transmit nerve impulses and constrict blood vessels, is a participant in the wake-sleep cycle, and affects mood. Serotonin is metabolized by the liver and its metabolites, primarily 5-HIAA (5-hydroxyindoleacetic acid), are eliminated in the urine. Fruits, vegetables, and seeds are major sources of 5-HT (serotonin) (Figure 3). In recent years, the number of studies on the content of serotonin (5-HT) in plants has increased, greatly encouraged by the discovery of melatonin, which stimulates the late vegetative growth of different tissue sections 10. Serotonin (5-HT) appeared to be prevalent in the green fruit of the Musa genus (that is, prata banana, and other species), containing about 7100–21,000 ng/g of fresh weight, followed by a significant decrease during ripening 11. Higher concentrations were found in banana peels compared to the pulp 12. The accumulation of 5-HT was also detected in Capsicum annuum L. (that is, pepper) 13, and paprika 14. 5-HT was identified in Corylus avellana L. (that is, hazelnut) 15, fruits of tomato and cherry tomato 14, Ananas comosus L. (that is, pineapple) 16, Prunus domestica L. (that is, plum) 12, Passiflora edulis S. (that is, passion fruit), Carica papaya L. (that is, pawpaw) 17, and in fruits of the Actinidia genus (that is, kiwi) 18. Similar to dopamine, 5-HT was found in the Mucuna pruriens or velvet bean 19. Ly et al. detected about 34,400 ng/g of dry weight in spinach 14. Brassica rapa L. (that is, Chinese cabbage) 14, potato leaves 20, rice plant, and seeds of Oryza sativa L. (that is, wild rice) 21, were also considered sources of 5-HT. This neurotransmitter was found in green coffee beans and, because of its high resistance to roasting, even in coffee powders 22. Traces were found in Punica granatum L. (that is, pomegranate), fruits of the Fragaria genus (that is, strawberry) 23, Cichorium intybus L. (that is, chicory), Allium ascalonicum L. (that is, green onion), and Lactuca sativa L. (that is, lettuce) 14. Some plants, such as nettle 24 and Griffonia simplicifolia DC were found to contain 5-HT. Griffonia was marketed for its presumptive anxiolytic effects that were later associated with the content of 5-hydroxy-l-tryptophan, a direct precursor in the synthesis of serotonin 25.
Figure 3. Serotonin dietary sources
[Source 9]What is depression?
Depression is characterized by persistent depressed mood and/or anhedonia in conjunction with other mood and physical symptoms 26. According to statistics from the USA National Institute of Mental Health, 6.6 % of the population will suffer from depression each year. There are numerous causal hypotheses for major depressive disorder, and they are not necessarily exclusive. The serotonin (5-HT or 5-hydroxytryptamine) hypothesis for depression has been intensely investigated since the 1967 seminal paper of Alec Coppen, laying down the foundation for such a hypothesis 27. These two types of issues need not, however, be linked. One possibility is that there may not be any anomaly in the serotonin (5-HT) system in major depressive disorder, while a therapeutic approach may still work by enhancing serotonin (5-HT) transmission above normal. Another possibility is that a deficient serotonin (5-HT) system may indeed contribute to the manifestation of major depressive disorder, and an enhancement of serotonin (5-HT) transmission by antidepressant strategies may restore normal, tranquil mental state or mood. An example for this therapeutic principle is the use of dopamine (DA) precursors or agonists in the presence of dopamine (DA) neuronal cell loss in Parkinson’s disease. The latter hypothesis for a causal role of serotonin (5-HT) in major depressive disorder has often been dismissed over the years, because of a lack of consistency of observations on a decrease of serotonin (5-HT) concentrations in post-mortem brain samples, and the fact that acute tryptophan depletion does not produce depression in healthy controls, tryptophan being the essential amino acid precursor of 5-HT 28. Nevertheless, in unaffected individuals with a family history of depression, mild dysphoric symptoms can be triggered by this acute serotonin (5-HT)-lowering challenge 29. Moreover, the mainstay of antidepressant therapy remains the serotonin transporter inhibitors, predominantly selective serotonin reuptake-inhibitors (SSRIs) and dual serotonin and norepinephrine reuptake inhibitors (SNRIs). Serotonin transporter inhibitors block reuptake of serotonin (5-HT) from the extracellular space. This causes sustained elevation of brain extracellular serotonin (5-HT), which over time leads to an antidepressant response 30. Unfortunately, serotonin transporter inhibitors achieve remission in only a third of patients 31. As such, an estimated 2 % of the population suffers from treatment-resistant depression 32. Current treatments for treatment-resistant depression are of limited benefit 33, and new treatments are needed.
Over the years, further electrical and pharmacological treatments with antidepressant properties have been studied and all shown to enhance overall serotonin (5-HT) transmission in the rat hippocampus 34. A critical observation supporting the necessity of sustained elevation of serotonin (5-HT) is that acutely lowering brain serotonin (5-HT) by eliminating dietary tryptophan, a precursor of serotonin (5-HT), precipitates a return of depression symptoms in 50 % of patients otherwise remitted on a serotonin transporter inhibitor. The relapse occurs within hours 35. In rat models of tryptophan depletion – where the procedure lowers plasma tryptophan as in humans (by 80 %) – brain serotonin (5-HT) rapidly drops by 50 %, from the initial, serotonin transporter inhibitor-elevated level 36. Thus, it appears an acute 50 % drop in brain serotonin (5-HT) will trigger acute relapse in 50 % of depression patients otherwise treated to remission with a serotonin transporter inhibitor.
An additional consideration is that lapse of sustained elevation in 5-HT (serotonin) can precipitate specific adverse events. Specifically, missing even a single dose of a serotonin transporter inhibitor can occasionally precipitate discontinuation syndrome 37, characterized by dizziness, nausea, lethargy and headache. In animals, SSRI (selective serotonin reuptake inhibitor)-induced 5-HT (serotonin) elevation rapidly reverts to baseline upon SSRI-withdrawal 38. For the short-acting serotonin and norepinephrine reuptake inhibitor (SNRI) venlafaxine (T1/2 = 8h [average for parent compound and active metabolite]), the discontinuation syndrome is more frequent, and can occur within hours 39. Because of the short T1/2, venlafaxine is used predominantly in its SR version. In a head-to-head antidepressant trial, venlafaxine SR was superior to venlafaxine IR 40. All marketed SSRIs have T1/2 > 20h. This leads to < 0.3 fold steady-state drug level fluctuations, hence minimal fluctuations in serotonin transporter occupancy, and hence essentially stable 5-HT (serotonin), so that discontinuation do not occur with once-daily dosing 6.
Nevertheless, other neuronal systems can also play a role in the antidepressant response. For instance, tricyclic antidepressants (TCAs) also sensitize postsynaptic α1- and/or α2-adrenoceptors in regions such as the thalamus, the amygdala and the facial motor nucleus, and most tricyclic antidepressants (TCAs) block norepinephrine reuptake 41. The clinical parallel to these basic observations is that catecholamine depletion, but not serotonin (5-HT) depletion, worsens depressive symptoms in patients who had responded to an norepinephrine reuptake inhibitor 42. Similarly, tryptophan depletion does not cause a re-emergence of depressive symptoms in patients who responded to electroconvulsive shock 43. In contrast, patients who responded to mirtazapine were observed to have a recurrence of their depressive symptoms with both tryptophan depletion and catecholamine depletion 44.
A novel antidepressant strategy producing strikingly rapid therapeutic benefits in major depressive disorder has provided credence to the theory that neuronal hyperactivity in limbic structures may account in large part for the pathology of major depressive disorder. The injection of a sub-anaesthetic dose of the glutamate N-methyl-d-aspartate (NMDA) antagonist ketamine can produce, within hours, a robust antidepressant response, presumably in part by blocking excitatory NMDA receptors 45. In other words, within hours ketamine may produce a dampening of the excessive neuronal activity in limbic structures that antidepressants take weeks to build up by enhancing inhibitory monoaminergic transmission. It is also noteworthy that even before the first controlled observations of the antidepressant action of ketamine infusion, it had been reported that a variety of classical antidepressants induce a decreased function of NMDA receptors, probably through their monoaminergic properties 46.
Deep brain stimulation is another novel, yet still experimental, approach to treat refractory major depressive disorder. Thus far, stimulations at high frequencies of the subgenual anterior cingulate cortex have been the most studied 47. Consistent with the attenuated hyperactivity that decreases with effective antidepressant treatments, deep brain stimulation also produces the same phenomenon. Similar stimulations applied to the equivalent region in the rat brain leads to an enhancement of the firing rate of serotonin (5-HT) neurons in the dorsal raphe nucleus and an increase in the extracellular levels of serotonin (5-HT) in the hippocampus 48. Furthermore, the antidepressant-like action of deep brain stimulation is abolished in rats in which serotonin (5-HT) neurons have been lesioned 49. This is yet another example of the important connectivity between various brain structures involved in mood disorders.
5-HTP as an antidepressant
5-HTP has never been formally developed as a drug and optimized dosage forms and dosing regimens are unavailable. Furthermore, all previous 5-HTP trials were small, including at most a few dozen subjects. In contrast, to ensure reasonable statistical power, a typical antidepressant proof-of-concept Phase II trial includes 50–100 subjects per arm 50. Most trials used 5-HTP monotherapy; but 5-HTP may be more relevant as an adjunctive, augmentation therapy. For these reasons in aggregate, previous trials may inherently have underestimated the antidepressant potential of 5-HTP. Nevertheless, most 5-HTP antidepressant reports are positive 51. Scholarly reviews conclude 5-HTP has shown promise as an antidepressant, and that more and better trials are warranted 52. Consistent with its pharmacology, 5-HTP antidepressant effect appears to be more consistent when adjunctive to another 5-HT (serotonin) elevating antidepressant 52. In table 1 are 5-HTP trials published in English.
In a double-blind trial in depressed inpatients, Alino et al. 53 found that nialamide (MAO inhibitor) + 5-HTP (200 mg/day) was superior to nialamide alone. The worst reported adverse events were diarrhea. In an open-label case-series of 99 chronic treatment-resistant depression patients, most already on serotonin transporter inhibitor therapy, van Hiele 54 found that 5-HTP (average dose 200 mg/day) + peripheral amino acid decarboxylase inhibitor treatment induced a “remarkable recovery” in ~50 % of patients. Antidepressant responses tended to be all-or-none. Few adverse events were reported, mostly nausea. Hypomania occurred in 15 patients, which reversed upon lowering the 5-HTP dose. In a four-arm double-blind placebo-controlled trial in depressed inpatients, van Praag et al. 55 compared placebo with clomipramine (a SERT inhibitor), 5-HTP (200 mg/day) + peripheral amino acid decarboxylase inhibitor, and clomipramine + 5-HTP + peripheral amino acid decarboxylase inhibitor. Clomipramine + 5-HTP + peripheral amino acid decarboxylase inhibitor was superior to all other arms. Nausea was the most common adverse events. In a double-blind trial in depressed inpatients, Nardini et al. 56 found that clomipramine + 5-HTP (300 mg/day) was superior to clomipramine alone. Adverse events were reported to be few.
Experience from >100 published clinical trials and widespread nutraceutical use suggests that oral 5-HTP – in high milligram to low gram doses, alone or as an adjunct to other serotonergic drugs, with or without a peripheral amino acid decarboxylase inhibitor – has a low propensity to cause severe adverse events in humans 52.
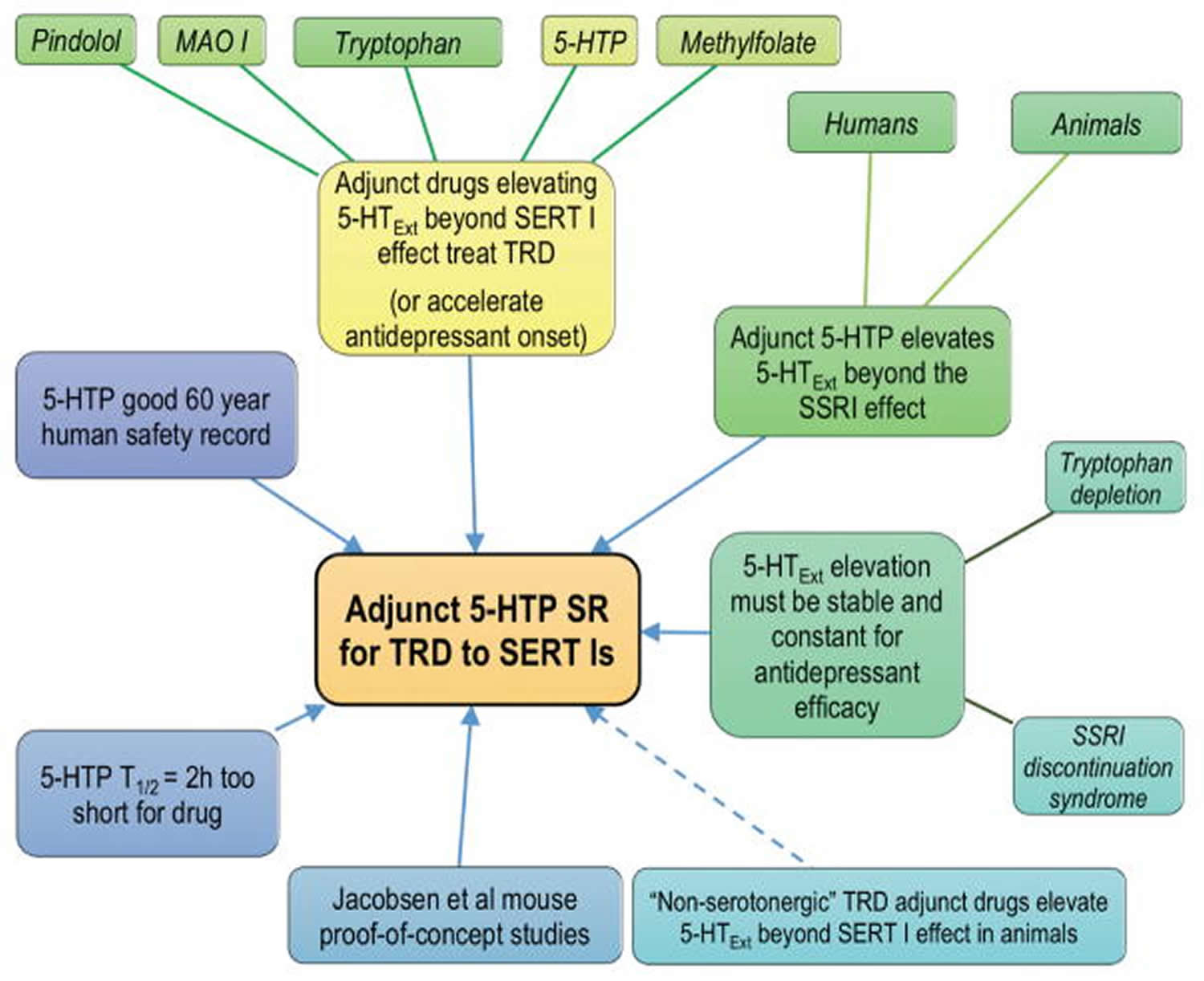
These pilot data on efficacy and safety of adjunct (add-on) 5-HTP in treatment-resistant depression are encouraging. Similar pilot trials provided initial evidence of antidepressant efficacy of tricyclic antidepressants and ketamine 57. The data add to the larger rationale supporting adjunctive 5-HTP SR (slow release) as a novel therapy for patients who respond inadequately to serotonin transporter inhibitors (Figure 4).
5-HTP has not been reported to cause serotonin syndrome in humans
Serotonin syndrome is a toxic state caused by excessive 5-HT (serotonin). Severe serotonin syndrome is rare, and almost exclusively caused by Serotonin transporter inhibitor + MAO inhibitor co-treatment 58. 5-HTP has never been associated with serotonin syndrome in humans. In published reports, > 250 humans have been dosed with 5-HTP + a Serotonin transporter inhibitor, with no serious adverse events 54. A Monoamine Oxidase Inhibitor (MAO inhibitor) blocks the metabolic flow through the 5-HT pathway, at the point of degradation, which might lead to extreme build-up of serotonin. In contrast, 5-HTP increases 5-HT (serotonin) synthesis and the dynamic flow through the 5-HT (serotonin) pathway, which might not easily lead to 5-HT (serotonin) build-up.
In rodents, high parenteral acute bolus doses of 5-HTP, e.g. 100 mg/kg, in combination with an SSRI can cause transient serotonin syndrome [71]. However, this is an artefact of preclinical pharmacology methodology, i.e. extreme doses and non-oral routes of administration. Similar high parenteral doses of fluoxetine, methylphenidate, and caffeine often kill rodents [72], whereas in humans these compounds are extremely safe, in their appropriate oral doses and dosage forms.
Table 1. Clinical trials with adjunct 5-HTP immediate release in treatment-resistant depression
| Reference | Design | Arms & total daily dose | Dosing | DCI | Duration | Population | Finding | Safety | Comment |
|---|---|---|---|---|---|---|---|---|---|
| Alino et al 1976 53 | Double-blind; HAMD | Nialamide (MAO I) 200 mg (N=15) vs. Nialamide + 5-HTP 200 mg (N=15) | BID, at breakfast and lunch | None noted | 15 days | Inpatients | Nialamide + 5-HTP superior (p<0.05) to Nialamide at day 15 | 2 patients in Nialamide + 5-HTP arm reported diarrhea | Doses of both drug titrated up over 5 days. |
| Hiele 1980 54 | Open-label | Tricyclics (mostly) + 5-HTP (~200 mg/day) (N=99) | TID | Carbidopa, 150 mg/day | Variable | Outpatients; treatment-resistant on average for 18 months | 50% of patients full recovery | Transient hypomania in 1/3; “no significant side-effects” | Patients resistant to multiple drug treatments; dichotomous all-or-none response to adjunct 5-HTP |
| van Praag et al 1982 55 | Double-blind; HAMD | Placebo vs. clomipramine 225 mg (tricyclic) vs. HTP 200 mg vs. clomipramine + 5-HTP (N=10, all groups) | TID | Carbidopa, 150 mg/day, 5-HTP groups only | 21 days | Inpatients | Both clomipramine and 5-HTP superior to placebo; clomipramine + 5-HTP superior to 3 other groups | Nausea | 5-HTP doses titrated up |
| Nardini et al 1983 56 | Double-blind; HAMD | Clomipramine 50 mg (tricyclic) (N=13) vs. clomipramine + 5-HTP (300 mg/day) (N=13) | ? | None noted | 28 days | Inpatients | Clomipramine + 5-HTP superior (p<0.05) to Clomipramine at day 28 | Few adverse effects | No details on dosing regimen |
Figure 4. 5-HTP SR (slow release) as add on new therapy for treatment-resistant depression
 [Source 3]
[Source 3]
HTP side effects
In humans, acute and long term treatment with 5-HTP, even at high doses, has minimal effects on cardiovascular, hepatic, renal, hematological, or urinalysis parameters 59. Similar, in rats, a 1-year toxicology study found no effects of oral high-dose 5-HTP (875 mg/kg/day, via the drinking water) on cardiovascular, hepatic, renal, hematological, body weight gain, organ histology, and organ weight parameters 60. In humans, common adverse events seen with oral 5-HTP are mild to moderate, and gastrointestinal, e.g. nausea or stomach cramps, and less frequently diarrhea and vomiting 61. Occasional adverse events include hypomania, headaches, lightheadedness, and palpitations. Often onset is rapid, which is likely due to rapid conversion of 5-HTP to 5-HT (serotonin) upon dosing with standard 5-HTP IR 62. Interestingly, two studies report that using enteric coated 5-HTP capsules, which delays 5-HTP delivery until the intestine, substantially reduces gastrointestinal adverse events 63. This suggests a direct irritating effect of 5-HTP on the stomach. Most studies do not specify if they administered 5-HTP with enteric coating. On the other hand, vomiting and nausea could be centrally, rather than peripherally, mediated. Evidence for this is that upon acute bolus 5-HTP administration, peripheral amino acid decarboxylase inhibitor co-treatment (for example carbidopa, which reduces peripheral and increases central 5-HTP conversion to 5-HT) can induce nausea and vomiting at 5-HTP doses (100–200 mg) otherwise devoid of adverse events 64. Further, acute co-treatment of 5-HTP immediate release 200 mg + SSRI causes vomiting and nausea 65, but acute 5-HTP immediate release 200 mg causes no adverse events when added after 4 weeks prior SSRI treatment 66. In any case, 5-HTP gastrointestinal adverse events lessen or disappear over time 67, as occurs with SSRIs 68. Overall, the evidence suggests that gastrointestinal adverse events after adjunctive 5-HTP can be greatly reduced if (i) the 5-HTP Cmax in plasma is minimized, (ii) appropriate 5-HTP formulations are used, and (iii) SSRI treatment has lasted several weeks prior to the start of 5-HTP dosing.
Although not all side effects are known, 5-hydroxytryptophan is thought to be possibly safe when taken for a short period of time.
Stop using 5-hydroxytryptophan and call your doctor at once if you have:
- severe tingling or numbness;
- skin rash, bruising, fever; or
- muscle pain or weakness.
Common side effects may include:
- drowsiness;
- nausea, vomiting, stomach pain, heartburn;
- diarrhea; or
- loss of interest in sex.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects.
Get emergency medical help if you have any of these signs of an allergic reaction while taking 5-hydroxytryptophan: hives; difficult breathing; swelling of your face, lips, tongue, or throat.
What other drugs will affect 5-HTP?
Taking HTP with any medicine that makes you sleepy can worsen this effect. Ask your doctor before taking 5-HTP with a sleeping pill, narcotic pain medicine, muscle relaxer, or medicine for anxiety, depression, or seizures.
Do NOT take 5-HTP without medical advice if you are using any of the following medications:
- an antidepressant;
- carbidopa;
- narcotic medicine; or
- cough medicine that contains dextromethorphan.
This list is not complete. Other drugs may interact with 5-HTP, including prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible interactions are listed here.
- Safety of 5-hydroxy-L-tryptophan. Toxicol Lett. 2004 Apr 15;150(1):111-22. https://www.sciencedirect.com/science/article/pii/S0378427404000438[↩]
- Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.5, 2001.[↩][↩][↩][↩]
- Jacobsen JPR, Krystal AD, Krishnan KRR, Caron MG. Adjunctive 5-hydroxytryptophan slow-release for treatment-resistant depression: Clinical and pre-clinical rationale. Trends in pharmacological sciences. 2016;37(11):933-944. doi:10.1016/j.tips.2016.09.001 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5728156/[↩][↩][↩][↩]
- Jacobsen JPR, Krystal AD, Krishnan KRR, Caron MG. Adjunctive 5-hydroxytryptophan slow-release for treatment-resistant depression: Clinical and pre-clinical rationale. Trends in pharmacological sciences. 2016;37(11):933-944. doi:10.1016/j.tips.2016.09.001 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5728156[↩]
- Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24:183–197.[↩]
- van Harten J. Clinical pharmacokinetics of selective serotonin reuptake inhibitors. Clin Pharmacokinet. 1993;24:203–220.[↩][↩]
- Gijsman HJ, et al. Placebo-controlled comparison of three dose-regimens of 5-hydroxytryptophan challenge test in healthy volunteers. J Clin Psychopharmacol. 2002;22:183–189.[↩][↩]
- Nilausen DO, et al. The perception and pharmacokinetics of a 20-mg dose of escitalopram orodispersible tablets in a relative bioavailability study in healthy men. Clin Ther. 2011;33:1492–1502.[↩]
- Briguglio M, Dell’Osso B, Panzica G, et al. Dietary Neurotransmitters: A Narrative Review on Current Knowledge. Nutrients. 2018;10(5):591. doi:10.3390/nu10050591 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5986471/[↩][↩]
- Huang X., Mazza G. Application of LC and LC-MS to the analysis of melatonin and serotonin in edible plants. Crit. Rev. Food Sci. Nutr. 2011;51:269–284. doi: 10.1080/10408398.2010.529193[↩]
- Adão R.C., Glória M.B.A. Bioactive amines and carbohydrate changes during ripening of ‘Prata’ banana (Musa acuminata × M. balbisiana) Food Chem. 2005;90:705–711. doi: 10.1016/j.foodchem.2004.05.020[↩]
- Udenfriend S., Lovenberg W., Sjoerdsma A. Physiologically active amines in common fruits and vegetables. Arch. Biochem. Biophys. 1959;85:487–490. doi: 10.1016/0003-9861(59)90516-8[↩][↩]
- Kang S., Back K. Enriched production of N-hydroxycinnamic acid amides and biogenic amines in pepper (Capsicum annuum) flowers. Sci. Hortic. 2006;108:337–341. doi: 10.1016/j.scienta.2006.01.037[↩]
- Ly D., Kang K., Choi J.Y., Ishihara A., Back K., Lee S.G. HPLC analysis of serotonin, tryptamine, tyramine, and the hydroxycinnamic acid amides of serotonin and tyramine in food vegetables. J. Med. Food. 2008;11:385–389. doi: 10.1089/jmf.2007.514[↩][↩][↩][↩][↩]
- Lavizzari T., Teresa Veciana-Nogues M., Bover-Cid S., Marine-Font A., Carmen Vidal-Carou M. Improved method for the determination of biogenic amines and polyamines in vegetable products by ion-pair high-performance liquid chromatography. J. Chromatogr. A. 2006;1129:67–72. doi: 10.1016/j.chroma.2006.06.090[↩]
- Foy J.M., Parratt J.R. 5-Hydroxytryptamine in pineapples. J. Pharm. Pharmacol. 1961;13:382–383. doi: 10.1111/j.2042-7158.1961.tb11840.x[↩]
- Council N.R. Toxicants Occurring Naturally in Foods. National Academy of Sciences; Washington, DC, USA: 1973.[↩]
- Feldman J.M., Lee E.M. Serotonin content of foods: Effect on urinary excretion of 5-hydroxyindoleacetic acid. Am. J. Clin. Nutr. 1985;42:639–643. doi: 10.1093/ajcn/42.4.639[↩]
- Bowden K., Brown B.G., Batty J.E. 5-Hydroxytryptamine: Its occurrence in cowhage. Nature. 1954;174:925–926. doi: 10.1038/174925a0[↩]
- Engstrom K., Lundgren L., Samuelsson G. Bioassay-guided isolation of serotonin from fruits of Solanum tuberosum L. Acta Pharm. Nord. 1992;4:91–92[↩]
- Kang S., Kang K., Lee K., Back K. Characterization of tryptamine 5-hydroxylase and serotonin synthesis in rice plants. Plant Cell Rep. 2007;26:2009–2015. doi: 10.1007/s00299-007-0405-9[↩]
- Ramakrishna A., Giridhar P., Sankar K.U., Ravishankar G.A. Melatonin and serotonin profiles in beans of Coffea species. J. Pineal Res. 2012;52:470–476. doi: 10.1111/j.1600-079X.2011.00964.x[↩]
- Badria F. Melatonin, serotonin, and tryptamine in some egyptian food and medicinal plants. J. Med. Food. 2002;5:153–157. doi: 10.1089/10966200260398189[↩]
- Collier H.O., Chesher G.B. Identification of 5-hydroxytryptamine in the sting of the nettle (urtica dioica) Br. J. Pharmacol. Chemother. 1956;11:186–189. doi: 10.1111/j.1476-5381.1956.tb01051.x[↩]
- Carnevale G., Di Viesti V., Zavatti M., Zanoli P. Anxiolytic-like effect of Griffonia simplicifolia Baill. seed extract in rats. Phytomedicine. 2011;18:848–851. doi: 10.1016/j.phymed.2011.01.016[↩]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association; 2013.[↩]
- Coppen A. 1967. The biochemistry of affective disorders. Br. J. Pharmacol. 113, 1237–126410.1192/bjp.113.504.1237 https://www.cambridge.org/core/journals/the-british-journal-of-psychiatry[↩]
- Delgado P, Charney DS, Price LH, Landis H, Heninger GS. 1989. Neuroendocrine and behavioral effects of dietary tryptophan restriction in healthy subjects. Life Sci. 45, 2323–233210.1016/0024-3205(89)90114-8 https://www.sciencedirect.com/science/article/pii/0024320589901148[↩]
- Benkelfat C, Ellenbogen MA, Dean P, Palmour R, Young S. 1994. Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch. Gen. Psychiat. 51, 687–69710.1001/archpsyc.1994.03950090019003 https://jamanetwork.com/journals/jamapsychiatry/article-abstract/496756[↩]
- Serotonin and beyond: therapeutics for major depression. Blier P, El Mansari M. Philos Trans R Soc Lond B Biol Sci. 2013; 368(1615):20120536. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3638389/[↩]
- Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, STAR*D Study Team. Am J Psychiatry. 2006 Jan; 163(1):28-40. https://www.ncbi.nlm.nih.gov/pubmed/16390886/[↩]
- Nemeroff CB. Prevalence and management of treatment-resistant depression. The Journal of clinical psychiatry. 2007;68(Suppl 8):17–25.[↩]
- Thase ME. Using adjunctive treatments when first-line antidepressants fail. J Clin Psychiatry. 2012;73:e01[↩]
- Blier P, El Mansari M. Serotonin and beyond: therapeutics for major depression. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368(1615):20120536. doi:10.1098/rstb.2012.0536 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3638389/[↩]
- Delgado PL. Monoamine depletion studies: implications for antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(Suppl 4):22–26[↩]
- Bel N, Artigas F. Reduction of serotonergic function in rat brain by tryptophan depletion: effects in control and fluvoxamine-treated rats. Journal of neurochemistry. 1996;67:669–676[↩]
- Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24:183–197[↩]
- Ceglia I, et al. Effects of chronic treatment with escitalopram or citalopram on extracellular 5-HT in the prefrontal cortex of rats: role of 5-HT1A receptors. British journal of pharmacology. 2004;142:469–478.[↩]
- Dallal A, Chouinard G. Withdrawal and rebound symptoms associated with abrupt discontinuation of venlafaxine. J Clin Psychopharmacol. 1998;18:343–344.[↩]
- Cunningham LA. Once-daily venlafaxine extended release (XR) and venlafaxine immediate release (IR) in outpatients with major depression. Venlafaxine XR 208 Study Group. Annals of clinical psychiatry: official journal of the American Academy of Clinical Psychiatrists. 1997;9:157–164.[↩]
- Tatsumi M, Groshan K, Blakely R, Richelson E. 1999. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol. 340, 249–25810.1016/S0014-2999(97)01393-9[↩]
- Delgado PL, Miller HL, Salomon RM, Licinio J, Krystal JH, Moreno FA, Heninger GR, Charney DS. 1999. Tryptophan-depletion challenge in depressed patients treated with desipramine or fluoxetine: implications for the role of serotonin in the mechanism of antidepressant action. Biol. Psychiat. 46, 212–22010.1016/S0006-3223(99)00014-1[↩]
- Cassidy F, Weiner RD, Cooper TB, Carroll BJ. 2010. Combined catecholamine and indolamine depletion following response to ECT. Br. J. Psychiat. 196, 493–49410.1192/bjp.bp.109.070573 https://www.cambridge.org/core/journals/the-british-journal-of-psychiatry/article/combined-catecholamine-and-indoleamine-depletion-following-response-to-ect/C3B96CFFB3798A02AA8C01A592E703B8/core-reader[↩]
- Delgado PL, Moreno FA, Onate L, Gelenberg AJ. 2002. Sequential catecholamine and serotonin depletion in mirtazapine-treated depressed patients. Int. J. Neuropsychopharmacol. 5, 63–6610.1017/S1461145702002778 https://watermark.silverchair.com/5-1-63.pdf?token=AQECAHi208BE49Ooan9kkhW_Ercy7Dm3ZL_9Cf3qfKAc485ysgAAAaEwggGdBgkqhkiG9w0BBwagggGOMIIBigIBADCCAYMGCSqGSIb3DQEHATAeBglghkgBZQMEAS4wEQQM8pkew712qRFoEd51AgEQgIIBVFLke46mlLmt8wJvCehZAz_4Ewhhm4a5zo9WLZt2WWh5Tb6Vsk3F6gniyTRN8S1Rw6b4IenGcxzXRi60hEtybdJ18clMuodFBtepq6E0pu95A1__J2m-W3MFepGOgfzmtHGxTsuTKYOkLSRciO3xDby6ObLBbtSQ1a1iTCjdKTi_lEwUMzek1mcR6T5TOKyza7SFpLotkmKKqeIb9bDcTImZT-0DNVIh-4tywGOdWHFS7h1a3KCwUnT7BNa575dEEC8b4B3C4sCA4U46G4YiosqtslSngd4VLXbBuVKt3pHUdTXh_OK_FkZ6pTfJLe8vj_rZRfREwgAHmXStF_npGVYL4aSmUH4rJB4-deMPx8nT7sxy_6BzmWSB9pzPS3Up-HCP_6snPfY5XHK-J66jODDULdtCpeyKrConfQevCUQo8XeA949V1e_Uqm3nDwd-OnzWgWY[↩]
- Diazgranados N, et al. 2010. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiat. 67, 793–80210.1001/archgenpsychiatry.2010.90 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3000408[↩]
- Paul IA, Nowak G, Layer RT, Popik P, Skolnick P. 1993. Adaptation of the N-methyl-D-aspartate receptor complex following chronic antidepressant treatments. J. Pharmacol. Exp. Ther. 269, 95–102 https://www.ncbi.nlm.nih.gov/pubmed/8169857[↩]
- Holtzheimer PE, Mayberg HS. 2011. Deep brain stimulation for psychiatric disorders. Ann. Rev. Neurosci. 34, 289–30710.1146/annurevneuro-061010-113638 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4413475/[↩]
- Etiévant A, Oosterhof CA, Bétry C, Abrial E, Lambas-Senas L, Scarna H, Lucas G, Haddjeri N. 2011. Antidepressant-like action of medial prefrontal cortex deep brain stimulation is modulated by glial system. Eur. Neuropsychopharmacol. 21(Suppl. 2), 405–40610.1016/S0924-977X(11)70654-X[↩]
- Hamani C, Diwan M, Macedo CE, Brandao MI, Shumake J, Gonzalez-Lima F, Raymond R, Lozano AM, Fletcher PJ. 2010. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol. Psychiat. 67, 117–12410.1016/j.biopsych.2009.08.025[↩]
- Post A, et al. A Selective Nociceptin Receptor Antagonist to Treat Depression: Evidence from Preclinical and Clinical Studies. Neuropsychopharmacology. 2016;41:1803–1812 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4869049/[↩]
- Turner EH, et al. Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol Ther. 2006;109:325–338. https://www.ncbi.nlm.nih.gov/pubmed/16023217[↩]
- Turner EH, et al. Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol Ther. 2006;109:325–338. https://escholarship.org/uc/item/58h866d5[↩][↩][↩]
- Alino JJ, et al. 5-Hydroxytryptophan (5-HTP) and a MAOI (nialamide) in the treatment of depressions. A double-blind controlled study. Int Pharmacopsychiatry. 1976;11:8–15.[↩][↩]
- van Hiele LJ. l-5-Hydroxytryptophan in depression: the first substitution therapy in psychiatry? The treatment of 99 out-patients with ‘therapy-resistant’ depressions. Neuropsychobiology. 1980;6:230–240.[↩][↩][↩]
- van Praag HM. Serotonin precursors in the treatment of depression. Advances in biochemical psychopharmacology. 1982;34:259–286.[↩][↩]
- Nardini M, et al. Treatment of depression with L-5-hydroxytryptophan combined with chlorimipramine, a double-blind study. Int J Clin Pharmacol Res. 1983;3:239–250.[↩][↩]
- Lopez-Munoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15:1563–1586[↩]
- Buckley NA, et al. Serotonin syndrome. British Medical Journal. 2014;348:g1626[↩]
- Das YT, et al. Safety of 5-hydroxy-L-tryptophan. Toxicol Lett. 2004;150:111–122. https://www.ncbi.nlm.nih.gov/pubmed/15068828[↩]
- Preuss HG, et al. Does 5-hydroxytryptophan cause acute and chronic toxic perturbations in rats? Toxicol Mech Methods. 2006;16:281–286[↩]
- Byerley WF, et al. 5-Hydroxytryptophan: a review of its antidepressant efficacy and adverse effects. J Clin Psychopharmacol. 1987;7:127–137. https://www.ncbi.nlm.nih.gov/pubmed/3298325[↩]
- van Hiele LJ. l-5-Hydroxytryptophan in depression: the first substitution therapy in psychiatry? The treatment of 99 out-patients with ‘therapy-resistant’ depressions. Neuropsychobiology. 1980;6:230–240. https://www.karger.com/Article/Abstract/117757[↩]
- van Praag HM. Serotonin precursors in the treatment of depression. Advances in biochemical psychopharmacology. 1982;34:259–286. https://www.ncbi.nlm.nih.gov/pubmed/6753514[↩]
- Gijsman HJ, et al. Placebo-controlled comparison of three dose-regimens of 5-hydroxytryptophan challenge test in healthy volunteers. J Clin Psychopharmacol. 2002;22:183–189 https://www.ncbi.nlm.nih.gov/pubmed/11910264[↩]
- Lowe SL, et al. L-5-Hydroxytryptophan augments the neuroendocrine response to a SSRI. Psychoneuroendocrinology. 2006;31:473–484.[↩]
- Meltzer H, et al. Fluoxetine, but not tricyclic antidepressants, potentiates the 5-hydroxytryptophan-mediated increase in plasma cortisol and prolactin secretion in subjects with major depression or with obsessive compulsive disorder. Neuropsychopharmacology. 1997;17:1–11.[↩]
- Byerley WF, et al. 5-Hydroxytryptophan: a review of its antidepressant efficacy and adverse effects. J Clin Psychopharmacol. 1987;7:127–137.[↩]
- Peretti S, et al. Safety and tolerability considerations: tricyclic antidepressants vs. selective serotonin reuptake inhibitors. Acta psychiatrica Scandinavica Supplementum. 2000;403:17–25.[↩]