Contents
- What is Pycnogenol
- Pycnogenol health benefits
- Table 1. Summary of randomized, double-blind placebo-controlled human clinical studies on Pycnogenol
- Anti-inflammatory activity
- Protection of articular cartilage
- Pycnogenol and ADHD
- Pycnogenol effects on human skin
- Pine bark extract and photoaged facial skin
- Modulation of skin pigmentation by oral ingestion of Pycnogenol
- Eye health
- Oral health
- Respiratory health and allergies
- Women’s health
- Sports endurance and performance
- Table 1. Summary of randomized, double-blind placebo-controlled human clinical studies on Pycnogenol
- Pycnogenol dosage
- Pycnogenol side effects
What is Pycnogenol
Pycnogenol is a patented, proprietary powder extract made exclusively from French maritime pine (Pinus pinaster Aiton) bark by Horphag Research (Geneva, Switzerland) 1, 2, 3. Pycnogenol is a standardized extract from the bark of the French maritime pine consists of a concentrate of polyphenols 4, 5. A pharmacokinetic study with volunteers ingesting Pycnogenol revealed that catechin, caffeic acid, ferulic acid, taxifolin and the metabolite M1 (δ-(3,4-dihydroxy-phenyl)-γ-valerolactone) were detectable in a nanomolar range in plasma 6. Moreover, about 65–75 % of the Pycnogenol extract are procyanidins that consist of catechin and epicatechin subunits of varying chain lengths 7, besides taxifolin, catechin, and phenol acids 8. The active ingredients in maritime pine can also be extracted from other sources, including peanut skin, grape seed, and witch hazel bark. Procyanidin is a powerful antioxidant also found in food such as grapes, berries, pomegranates, red wine and various nuts. Maritime pine bark extract, pycnogenol, is commonly used orally to treat and prevent diabetes, diabetes-related health issues, and problems of the heart and blood vessels among many other uses. Some people use skin creams that contain maritime pine bark extract as “anti-aging” products. Pycnogenol is also applied to the skin to treat foot ulcers in people with diabetes, hemorrhoids, and mouth ulcers caused by chemotherapy. There is limited scientific research to support most of these uses.
French maritime pine bark extract is a complex mixture of polyphenolic compounds 9. A catechin metabolite (M1) produced by human intestinal bacteria was found in the plasma samples. Subsequent investigations showed that M1 exerted various anti-inflammatory effects in vitro such as the inhibition of the activity of the matrix metalloproteinases MMP-1, −2 and −9, decrease of the release of MMP-9 from human monocytes or inhibition of the expression of the inducible NO synthase (iNOS) in RAW 264.7 macrophages 10. In other in vitro experiments using the whole extract a decrease of IL1B mRNA synthesis in RAW 264.7 cells was reported 11 as well as inhibitory effects on the expression of COX-2, IL-8 and iNOS in human chondrocytes and fibroblasts 12. However, since not all components of the French maritime pine extract are bioavailable and other bioactive molecules such as M1 are generated in vivo, it is not clear whether experiments using the whole extract would be indicative for cellular effects that actually occur in vivo.
Clinical studies indicate that pycnogenol is effective in the treatment of chronic venous insufficiency and retinal micro-hemorrhages 13. Pycnogenol protects against oxidative stress in several cell systems by doubling the intracellular synthesis of anti-oxidative enzymes and by acting as a potent scavenger of free radicals. Other anti-oxidant effects involve a role in the regeneration and protection of vitamin C and E. Anti-inflammatory activity has been demonstrated in vitro and in vivo in animals. Protection against UV-radiation-induced erythema was found in a clinical study following oral intake of pycnogenol 13. In asthma patients symptom scores and circulating leukotrienes are reduced and lung function is improved 13. Immunomodulation has been observed in both animal models as well as in patients with Lupus erythematosus. Pycnogenol antagonizes the vasoconstriction caused by epinephrine and norepinephrine by increasing the activity of endothelial nitric oxide synthase. Dilation of the small blood vessels has been observed in patients with cardiovascular disease, whereas in smokers, pycnogenol prevents smoking-induced platelet aggregation and reduces the concentration of thromboxane 13. The ability to inhibit angiotensin-converting enzyme is associated with a mild antihypertensive effect 13. Pycnogenol relieves premenstrual symptoms, including abdominal pain and this action may be associated with the spasmolytic action of some phenolic acids 13. An improvement in cognitive function has been observed in controlled animal experiments and these findings support anecdotal reports of improvement in ADHD patients taking pycnogenol supplements 13.
Chemical identification studies showed that pycnogenol is primarily composed of procyanidins and phenolic acids. Procyanidins are biopolymers of catechin and epicatechin subunits which are recognized as important constituents in human nutrition. Pycnogenol contains a wide variety of procyanidins that range from the monomeric catechin and taxifolin to oligomers with 7 or more flavonoid subunits 13. The phenolic acids are derivatives of benzoic and cinnamic acids. The ferulic acid and taxifolin components are rapidly absorbed and excreted as glucuronides or sulphates in men, whereas procyanidins are absorbed slowly and metabolized to valerolactones which are excreted as glucuronides. As all of these constituents of Pycnogenol and its metabolites exhibit anti-inflammatory actions, the progressing appearance of the diverse active substances provides a long-lasting pain relief, so that patients feel less pain, also during the night 14. Pycnogenol has low acute and chronic toxicity with mild unwanted effects occurring in a small percentage of patients following oral administration.
Pine bark extract vs Pycnogenol
There are many pine bark extracts on the market, from different pine tree species, from different countries and with different levels of efficacy for human health 15, 16, 17, 3. French maritime pine (Pinus pinaster Aiton) bark extract is a complex mixture of bioflavonoids, with oligometric proanthocyanidins as the major constituents. Oligometric proanthocyanidins are dimers or oligomers of catechin, epicatechin, and their gallic acid esters. The major oligometric proanthocyanidins in maritime pine bark are proanthocyanidin B1 (epicatechin-(4β→8)-catechin), catechin, and epicatechin 18.
Pycnogenol is a patented, proprietary powder extract made exclusively from French maritime pine (Pinus pinaster Aiton) bark by Horphag Research (Geneva, Switzerland) 1. The French maritime pine (Pinus pinaster Aiton) trees that are used for Pycnogenol grow in Les Landes de Gascogne, along the coast of southwest France 19. Pycnogenol is a standardized extract from the bark of the French maritime pine consists of a concentrate of polyphenols. A pharmacokinetic study with volunteers ingesting Pycnogenol revealed that catechin, caffeic acid, ferulic acid, taxifolin and the metabolite M1 (δ-(3,4-dihydroxy-phenyl)-γ-valerolactone) were detectable in a nanomolar range in their plasma 6. Moreover, about 65–75 % of the Pycnogenol extract are procyanidins that consist of catechin and epicatechin subunits of varying chain lengths 7, 20, as well as taxifolin, catechin, and phenolic acids 8. The active ingredients in maritime pine can also be extracted from other sources, including peanut skin, grape seed, and witch hazel bark. Procyanidin is a powerful antioxidant also found in food such as grapes, berries, pomegranates, red wine and various nuts.
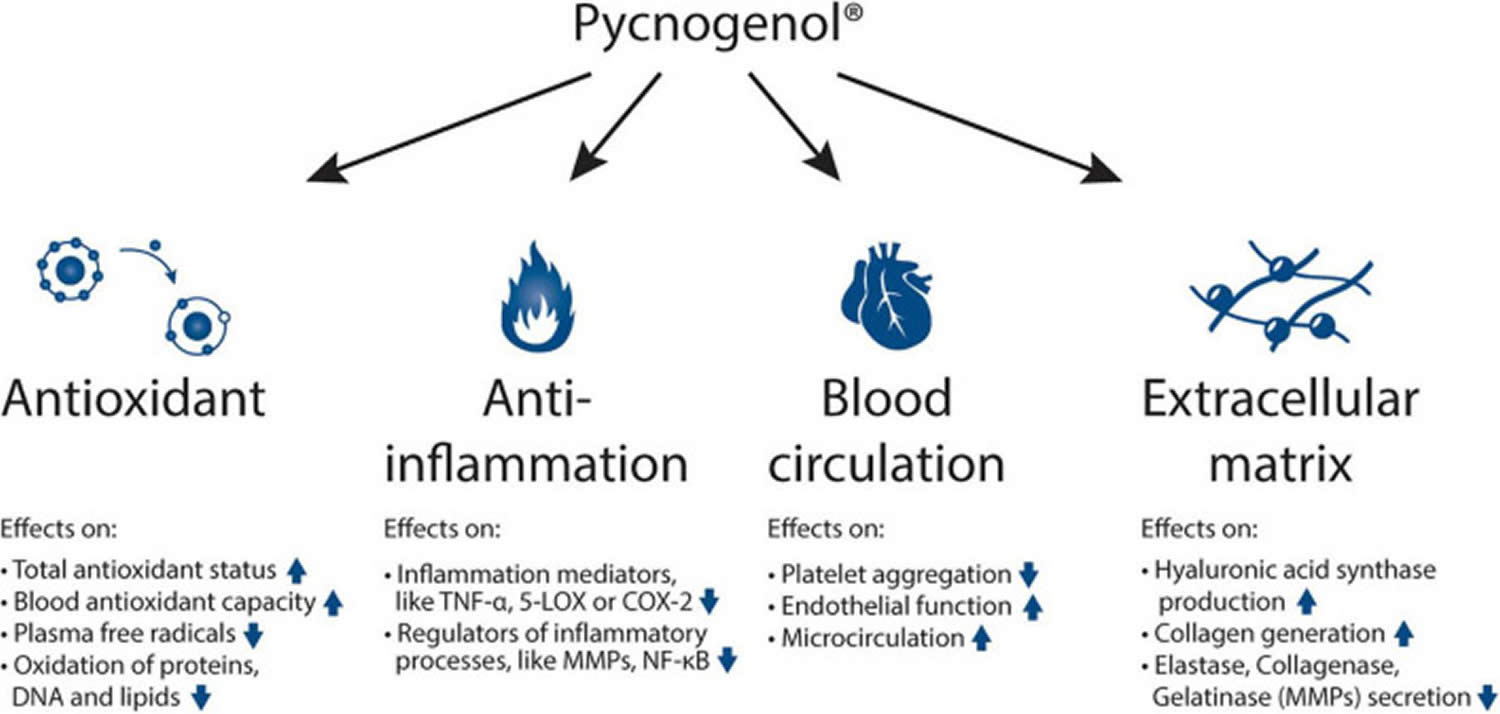
Figure 1. Pycnogenol mechanisms of action
Abbreviations: TNF-α = tumor necrosis factor alpha; 5-LOX = arachidonate 5-lipoxygenase; COX-2 = cyclooxygenase-2; MMP = matrix metallopeptidases; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells.
[Source 21 ]Pycnogenol health benefits
Clinical research on Pycnogenol started over 40 years ago and various health benefits of Pycnogenol have been observed and investigated 21. Due to Pycnogenol’s specific composition, unique specification and standardization processes, the research results obtained from studies with Pycnogenol cannot be extrapolated to other pine bark extracts 21. Many of the studies are conducted following a randomized, double-blind and placebo-controlled methodology to assess the efficacy of the extract in comparison to potential placebo effects.
Pycnogenol has been shown to have 4 main effects such as antioxidant effects, anti-inflammatory abilities, beneficial effects on blood circulation and reinforcing activities on the extracellular matrix (Figure 1) 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 effects on human skin: Clinical and molecular evidence. Skin Pharmacol Physiol. (2016) 29:13–7. 10.1159/000441039)). Mainly through these mechanisms 4, Pycnogenol supplementation has been shown in human clinical trials to beneficially affect cardiovascular health, chronic venous insufficiency, cognition, joint health, skin health, eye health, women’s health, respiratory health and allergies, oral health and sports performance 21.
Pycnogenol possibly effective for:
- Allergies: Some research shows that taking a standardized extract of maritime pine bark before allergy season begins reduces allergy symptoms in people with birch allergies.
- Asthma: Taking a standardized extract of maritime pine bark daily, along with asthma medications, seems to decrease asthma symptoms and the need for rescue inhalers in children and adults with asthma.
- Athletic performance: Young people (age 20-35 years) seem to be able to exercise on a treadmill for a longer time after taking a standardized extract of maritime pine bark daily for about a month. Also, athletes training for a physical fitness test or a triathalon seem to perform better in the tests and competitions when they take this extract daily for 8 weeks while training compared to only training.
- Circulation problems: Taking a standardized extract of maritime pine bark by mouth seems to reduce leg pain and heaviness, as well as fluid retention, in people with circulation problems. Some people also use horse chestnut seed extract to treat this condition, but using the extract alone appears to be more effective.
- Improving mental function: Research suggests that taking a standardized extract of maritime pine bark by mouth for 3 months improves mental function and memory in both young adults and the elderly.
- Disease of the retina in the eye: Taking a standardized extract of maritime pine bark by mouth for 2 months seems to slow or prevent further worsening of retinal disease caused by diabetes, atherosclerosis, or other diseases. It also seems to improve eyesight..
Insufficient evidence to rate effectiveness for:
- Attention deficit-hyperactivity disorder (ADHD). Taking a standardized extract of maritime pine bark by mouth does not seem to help ADHD symptoms in adults. However, taking it by mouth for one month appears to improve symptoms in children.
- Common cold. Taking a standardized extract of maritime pine bark by mouth twice daily starting at the beginning of a cold seems to reduce the number of days with a cold and the number of lost working days. It also seems to reduce the amount of over-the-counter cold products needed to manage symptoms.
- Clogged arteries (coronary artery disease). There is some evidence that taking a standardized extract of maritime pine bark three times daily for 4 weeks might help improve some complications associated with clogged arteries.
- Blood clots in deep veins (deep vein thrombosis, DVT). There is some evidence that taking a specific combination product containing maritime pine might help to prevent DVT during long-haul plane flights. The product combines a blend of standardized maritime pine bark extract plus nattokinase. Two capsules are taken 2 hours before the flight and then again 6 hours later. Also, taking the standardized maritime pine bark extract before a flight, 6 hours after the flight, and the following day appears to reduce the risk of blood clots forming in the veins during long flights. In addition, taking the extract for one year eems to reduce the risk of post-thrombotic syndrome. This condition can develop in people who already experienced a blood clot.
- Dental plaque. Early research suggests that chewing at least 6 pieces of gum with added extract from maritime pine bark for 14 days reduces bleeding and prevents increased plaque.
- Diabetes. Early evidence suggests that taking a standardized extract of maritime pine bark daily for 3-12 weeks slightly decreases blood sugar in people with diabetes.
- Foot ulcers due to diabetes. Early research suggests that taking maritime pine bark extract by mouth and applying it to the skin helps heal foot ulcers related to diabetes.
- Circulation problems in diabetes. Early research shows that taking standardized maritime pine bark extract three times daily for 4 weeks improves circulation and symptoms in people with diabetes.
- Swelling (edema). Early research suggests that taking standardized extract of maritime pine bark before a flight, 6 hours after the flight, and once the next day reduces ankle swelling.
- Erectile dysfunction (ED). Early research suggests that standardized maritime pine bark extract, used alone or in combination with L-arginine, might improve sexual function in men with ED. It seems to take up to 3 months of treatment for significant improvement.
- Heart failure. Early research suggests that taking a specific combination product containing standardized maritime pine bark and coenzyme Q10 for 12 weeks improves some symptoms of heart failure.
- Hemorrhoids. Early research suggests that taking standardized extract of maritime pine bark by mouth, alone or in combination with a cream containing this same extract, improves quality of life and symptoms of hemorrhoids.
- High cholesterol. A standardized extract of maritime pine bark seems to lower “bad cholesterol” (low-density lipoprotein (LDL) cholesterol) in people with high cholesterol. However, the extract doesn’t seem to improve cholesterol levels in people with other conditions such as high blood pressure, type 2 diabetes, erectile dysfunction, and others.
- High blood pressure. One standardized extract of maritime pine bark (Pycnogenol, Horphag Research) seems to lower systolic blood pressure (the top number in a blood pressure reading) but does not significantly lower diastolic blood pressure (the bottom number). This extract might also help lower blood pressure in some patients already treated with the blood pressure-lowering drug ramipril. However, other maritime pine bark extract (Toyo-FVG, Toyo Bio-Pharma) does not appear to lower blood pressure in obese people with slightly high blood pressure.
- Leg cramps. There is some evidence that taking a standardized extract of maritime pine bark by mouth daily might decrease leg cramps.
- Menopausal symptoms. Early research shows that taking a standardized extract of maritime pine bark by mouth decreases menopausal symptoms, including tiredness, headache, depression and anxiety, and hot flashes.
- Metabolic syndrome. Early research suggests that taking a standardized extract of maritime pine bark by mouth three times dialy for 6 months lowers triglycerides, blood sugar levels, and blood pressure, and increases high-density lipoprotein (“good” or HDL) cholesterol in people with metabolic syndrome.
- Oral mucositis. Applying solution containing a standardized extract of maritime pine bark inside the mouth for one week seems to help heal mouth ulcers in children and adolescents undergoing chemotherapy treatment.
- Osteoarthritis. There is mixed evidence about the effectiveness of maritime pine for osteoarthritis. Taking a standardized extract of maritime pine bark by mouth might reduce overall symptoms, but it does not seem to reduce pain or improve the ability to perform daily tasks.
- Pain in late pregnancy. Early research suggests that taking a standardized extract of maritime pine by mouth daily during the last 3 months of pregnancy reduces lower back pain, hip joint pain, pelvic pain, and pain due to varicose veins or calf cramps.
- Pelvic pain in women. There is early evidence that taking a standardized extract of maritime pine bark by mouth might help reduce pelvic pain in women with endometriosis or severe menstrual cramps.
- Problems with sexual function. Early research suggests that taking a combination product containing a standardized extract of maritime pine bark, L-arginine, L-citrulline, and rose hip extract daily for 8 weeks can help improve sexual function in women.
- Improving symptoms of lupus (SLE). Early research suggests that taking a standardized extract of maritime pine bark by mouth reduces symptoms of SLE in some patients.
- Ringing in the ears (tinnitus). Early research suggests that taking a standardized extract of maritime pine bark by mouth reduces ringing in the ears.
- Stroke prevention.
- Muscle soreness.
- Other conditions.
More evidence is needed to rate pycnogenol for these uses.
Table 1. Summary of randomized, double-blind placebo-controlled human clinical studies on Pycnogenol
| References | Title | Study details | Main findings |
|---|---|---|---|
| Cardiovascular health and endothelial health | |||
| Trebaticky et al. 40 | Natural polyphenols improve erectile function and lipid profile in patients suffering from erectile dysfunction | 53 male subjects, 120 mg Pycnogenol® per day or placebo for 3 months | Total cholesterol and low density lipoprotein (LDL or bad cholesterol) levels were reduced in subjects taking Pycnogenol®. In diabetes type 2 patients, plasma glucose levels were decreased after Pycnogenol® intake. Erectile function was improved after Pycnogenol® supplementation. Placebo showed no significant effects. |
| Enseleit et al. 22 | Effects of Pycnogenol on endothelial function in patients with stable coronary artery disease: a double-blind, randomized, placebo-controlled, cross-over study | 23 subjects, 200 mg Pycnogenol® per day or placebo for 8 weeks | Flow-mediated dilation was significantly improved by 32% in the Pycnogenol® group compared to baseline and by 49% compared to placebo. Lipid peroxidation was decreased by 7% with Pycnogenol®. |
| Zibadi et al. 35 | Reduction of cardiovascular risk factors in subjects with type 2 diabetes by Pycnogenol supplementation | 48 subjects, 100 mg Pycnogenol® per day or placebo for 3 months | Serum endothelin 1-levels were lowered by 20% after Pycnogenol® intake compared to placebo. low density lipoprotein (LDL or bad cholesterol) cholesterol was reduced by 12% with Pycnogenol® (vs. +3% with placebo). Pycnogenol® was shown to lower glycated hemoglobin by 10% in the Pycnogenol® group, a significant effect compared to placebo. Fasting plasma glucose was lowered by 18.4% in type 2 diabetes subjects, taking Pycnogenol® compared to placebo-controlled subjects. |
| Nishioka et al. 34 | Pycnogenol®, French maritime pine bark extract, augments endothelium-dependent vasodilation in humans | 16 subjects, 180 mg Pycnogenol® per day or placebo for 2 weeks | Forearm blood flow in response to acetylcholine significantly increased by up to 41% after Pycnogenol® intake, while placebo had no effect. Forearm blood flow in response to an endothelium independent vasodilator was not influenced by Pycnogenol® intake showing the effect of Pycnogenol® is mediated by the endothelium. |
| Yang et al. 25 | A randomized, double-blind, placebo-controlled trial on the effect of Pycnogenol® on the climacteric syndrome in peri-menopausal women | 155 female subjects, 200 mg Pycnogenol® per day or placebo for 6 months | low density lipoprotein (LDL or bad cholesterol)-cholesterol was significantly lowered, and high density lipoprotein (HDL or good cholesterol)-cholesterol was significantly increased after Pycnogenol® intake compared to placebo. Systolic and diastolic blood pressure were significantly reduced after Pycnogenol® supplementation, compared to placebo. All climacteric symptoms improved with Pycnogenol® compared to placebo. |
| Liu et al. 33 | Pycnogenol®, French maritime pine bark extract, improves endothelial function of hypertensive patients | 58 subjects, 100 mg Pycnogenol® per day or placebo for 3 months | Endothelin 1-levels were significantly lowered by 16% after Pycnogenol® intake compared to placebo. 6-keto prostaglandin F1a-levels were increased after Pycnogenol®. 57% of the Pycnogenol® subjects and 13% of the placebo subjects could cut their individual anti-hypertensive drug medication by half. |
| Liu et al. 41 | Antidiabetic effect of Pycnogenol® French maritime pine bark extract in patients with diabetes type II | 77 subjects, 100 mg Pycnogenol® per day or placebo for 12 weeks | Plasma glucose levels of diabetes type 2 patients decreased significantly with Pycnogenol®, compared to placebo. Glycosylated hemoglobin and vasoconstrictive endothelin-1 in the blood were reduced and vaso-relaxant 6-keto prostaglandin f1 alpha was increased with Pycnogenol® but not in placebo. |
| uračková et al. 24 | Lipid metabolism and erectile function improvement by Pycnogenol®, extract from the bark of Pinus pinaster in patients suffering from erectile dysfunction-a pilot study | 21 male subjects, 120 mg Pycnogenol® per day or placebo for 3 months | Total and low density lipoprotein (LDL or bad cholesterol)-cholesterol were significantly reduced while high density lipoprotein (HDL or good cholesterol)-cholesterol was slightly increased after Pycnogenol® intake. |
| Hosseini et al. 42 | A randomized, double-blind, placebo-controlled, prospective, 16-week crossover study to determine the role of Pycnogenol in modifying blood pressure in mildly hypertensive patients. | 11 subjects, 200 mg Pycnogenol® per day or placebo for 8 weeks | Systolic blood pressure was significantly lowered after Pycnogenol® supplementation by 5% compared to placebo. In subjects with the highest systolic blood pressure, the reduction was greater (−11% compared to baseline). |
| Wang et al. 43 | The effect of Pycnogenol® on the microcirculation, platelet function and ischemic myocardium in patients with coronary artery diseases | 60 subjects, 150 mg Pycnogenol® per day or placebo for 4 weeks | The percentage of patients with improvement of the microcirculation at the fingertips was higher and platelet aggregation of the blood was reduced after Pycnogenol® intake compared to placebo. |
| Chronic venous insufficiency | |||
| Arcangeli 44 | Pycnogenol® in chronic venous insufficiency | 40 subjects, 300 mg Pycnogenol® per day or placebo for 2 months | Pycnogenol® supplementation reduced symptoms of chronic venous insufficiency. |
| Petrassi et al. 45 | Pycnogenol® in chronic venous insufficiency | 20 subjects, 300 mg Pycnogenol® per day or placebo for 2 months | Leg heaviness, swelling and evening edema were relieved in chronic venous insufficiency patients after Pycnogenol® intake, compared to placebo. Venous pressure was significantly decreased with Pycnogenol® not with placebo. |
| Cognitive function | |||
| Weyns et al. 46 | Clinical investigation of French maritime pine bark extract on attention-deficit hyperactivity disorder as compared to methylphenidate and placebo: Part 1: efficacy in a randomized trial | 88 children, 20 or 40 mg Pycnogenol® /day if < or ≥ 30 kg or 20 or 30 mg methylphenidate hydrochloride /day if < or ≥ 30 kg or placebo for 10 weeks | Hyperactivity and impulsivity were significantly improved with both Pycnogenol® (by 34%) and methylphenidate hydrochloride (by 36%) and deteriorated with placebo, according to teacher’s rating. Inattention (according to teachers) was improved with Pycnogenol® and significantly improved with methylphenidate hydrochloride. The rate of adverse events was statistically significant with methylphenidate hydrochloride (39%) and not with Pycnogenol® (8%) and placebo (9%). |
| Weyns et al. 47 | Clinical investigation of French maritime pine bark extract on attention-deficit hyperactivity disorder as compared to methylphenidate and placebo: Part 2: oxidative stress and immunological modulation | 88 children, 20 or 40 mg Pycnogenol® /day if < or ≥ 30 kg or 20 or 30 mg methylphenidate hydrochloride /day if < or ≥ 30 kg or placebo for 10 weeks | methylphenidate hydrochloride intake led to loss of appetite and a significant weight loss. After Pycnogenol® supplementation, children had physiologically appropriate weight gain. The orexigenic peptide, neuropeptide Y was significantly reduced after methylphenidate hydrochloride and insignificantly increased after Pycnogenol® intake. |
| Donovan et al. 48 | A placebo-controlled, pseudo-randomized, crossover trial of botanical agents for gulf war illness: curcumin (Curcuma longa), Boswellia (Boswellia serrata), and French maritime pine bark (Pinus pinaster) | 20 subjects, 400 mg Pycnogenol® per day or placebo for 4 weeks | The symptoms of gulf war illness after intake of Pycnogenol® were significantly reduced compared to placebo. |
| Ryan et al. 26 | An examination of the effects of the antioxidant Pycnogenol® on cognitive performance, serum lipid profile, endocrinological and oxidative stress biomarkers in an elderly population | 101 subjects, 150 mg Pycnogenol® per day or placebo for 3 months | Spatial working memory and numeric quality of working memory were significantly improved with Pycnogenol® compared with placebo. Lipid peroxidation products (plasma F2 isoprostane) were reduced after Pycnogenol® intake compared to placebo. |
| Dvorakova et al. 49 | Urinary catecholamines in children with attention deficit hyperactivity disorder (attention deficit and hyperactivity disorder (ADHD)): modulation by a polyphenolic extract from pine bark (Pycnogenol®) | 61 children, 1 mg Pycnogenol®/kg/day or placebo for 4 weeks | The levels of catecholamines (like adrenaline, noradrenaline and dopamine) in the urine were reduced after Pycnogenol® supplementation. |
| Chovanova et al. 27 | Effect of polyphenolic extract, Pycnogenol®, on the level of 8-oxoguanine in children suffering from attention deficit/hyperactivity disorder | 61 children, 1 mg Pycnogenol®/kg/day or placebo for 4 weeks | The increased levels of 8-oxoG, as a measure of oxidized DNA in attention deficit and hyperactivity disorder (ADHD) children were reduced after Pycnogenol® intake compared to baseline and placebo. |
| Dvorakova et al. 50 | The effect of polyphenolic extract from pine bark, Pycnogenol® on the level of glutathione in children suffering from attention deficit hyperactivity disorder (attention deficit and hyperactivity disorder (ADHD)) | 61 children, 1 mg Pycnogenol®/kg/day or placebo for 4 weeks | The levels of oxidized glutathione (GSSG) were significantly decreased by 22% after Pycnogenol® intake, while reduced glutathione (GSH) was significantly increased by 26.8%. Placebo had no significant effect on GSSH and GSH. |
| Trebaticka et al. 51 | Treatment of attention deficit and hyperactivity disorder (ADHD) with French maritime pine bark extract, Pycnogenol® | 61 children, 1 mg Pycnogenol®/kg/day or placebo for 4 weeks | As rated by parents and teachers, hyperactivity was reduced and attention was increased after Pycnogenol® intake compared to placebo. |
| Joint health | |||
| Belcaro et al. 52 | Treatment of osteoarthritis with Pycnogenol®. The SVOS (San Valentino Osteo-arthrosis Study). Evaluation of signs, symptoms, physical performance and vascular aspects | 156 subjects, 100 mg Pycnogenol® per day or placebo for 3 months | The joint discomfort scores were reduced after Pycnogenol® intake. Walking distance on a treadmill increased significantly more with Pycnogenol® supplementation compared to placebo. |
| Cisar et al. 53 | Effect of pine bark extract (Pycnogenol®) on symptoms of knee osteoarthritis | 100 subjects, 150 mg Pycnogenol® per day or placebo for 3 months | Joint discomfort, stiffness and analgesics consumption were lowered, and physical function increased after Pycnogenol® intake compared to placebo. |
| Farid et al. 54 | Pycnogenol supplementation reduces pain and stiffness and improves physical function in adults with knee osteoarthritis | 37 subjects, 150 mg Pycnogenol® per day or placebo for 3 months | Reduced joint discomfort, stiffness and need for nonsteroidal anti-inflammatory drugs (NSAIDs) with Pycnogenol® supplementation compared to placebo. |
| Skin health | |||
| Cai et al. 55 | An oral French maritime pine bark extract improves hair density in menopausal women: a randomized, placebo-controlled, double-blind intervention study | 76 female subjects, 150 mg Pycnogenol® per day or placebo for 6 months | Hair density of menopausal women was significantly increased after Pycnogenol® supplementation compared to baseline. Scalp water loss was reduced with Pycnogenol® intake compared to placebo. Resting flux of the scalp skin was improved with Pycnogenol® compared to baseline. |
| Zhao et al. 56 | Oral Pycnogenol® intake benefits the skin in urban Chinese outdoor workers: a randomized, placebo-controlled, double-blind, and crossover intervention study | 78 subjects, 100 mg Pycnogenol® per day or placebo for 3 months | Skin water loss was decreased while skin elasticity and skin tone regularity increased after Pycnogenol® supplementation, compared to baseline and placebo. |
| Eye health | |||
| Steigerwalt et al. 57 | Pycnogenol® improves microcirculation, retinal edema, and visual acuity in early diabetic retinopathy | 46 subjects, 150 mg Pycnogenol® per day or placebo for 3 months | Retinal edema score and retinal thickness were reduced, and visual acuity was significantly improved after Pycnogenol® intake compared to baseline and placebo. |
| Spadea and Balestrazzi 58 | Treatment of vascular retinopathies with Pycnogenol® | 20 subjects, 150 mg Pycnogenol® per day or placebo for 2 months | Vascular permeability of the eyes and retinal vascularization decreased, and visual acuity increased after Pycnogenol® supplementation, compared to placebo. |
| Women’s health | |||
| Kohama and Negami 59 | Effect of low-dose French maritime pine bark extract on climacteric syndrome in 170 perimenopausal women | 170 female subjects, 60 mg Pycnogenol® per day or placebo for 3 months | Total menopause symptom score was significantly reduced with Pycnogenol® compared to placebo. Vasomotor symptoms, sleep problems and fatigue were improved with Pycnogenol®. Hormone levels were not changed after Pycnogenol® intake, compared to baseline or placebo. |
| Suzuki et al. 60 | French Maritime Pine Bark Extract significantly lowers requirement of analgesic medication in dysmenorrhea–a multi-center, randomized, double-blind, placebo-controlled study | 116 female subjects, 60 mg Pycnogenol® per day or placebo for 2 menstrual cycles | The need for analgesic medication and the number of days on which analgesics were required was significantly reduced with Pycnogenol® supplementation compared to placebo. |
| Yang et al. 25 | A randomized, double-blind, placebo-controlled trial on the effect of Pycnogenol® on the climacteric syndrome in peri-menopausal women | 155 female subjects, 200 mg Pycnogenol® per day or placebo for 6 months | Menopause symptoms according to the Women’s health questionnaire were improved after Pycnogenol® intake, significantly more than with placebo. The cholesterol profiles were significantly improved after Pycnogenol® intake compared to placebo. Systolic and diastolic blood pressure were significantly reduced after Pycnogenol® intake. |
| Respiratory health and allergies | |||
| Wilson et al. 61 | A randomized, double-blind, placebo-controlled exploratory study to evaluate the potential of Pycnogenol® for improving allergic rhinitis symptoms | 39 subjects, 100 mg Pycnogenol® per day or placebo for 5 to 8 weeks | Allergic rhinitis symptoms from pollen allergy, such as nasal and eye symptoms were reduced in subjects, taking Pycnogenol® at least 5 weeks before pollen season. The number of subjects, requiring rescue antihistamines was reduced with Pycnogenol®. |
| Lau et al. 62 | Pycnogenol® as an adjunct in the management of childhood asthma | 60 children, 2 mg Pycnogenol® per kg per day or placebo for 3 months | Asthma symptoms and forced expiration volume in 1 second (FEV1) improved after Pycnogenol® intake. Leukotriene levels were significantly reduced in the Pycnogenol® group. The need for albuterol rescue inhalers was reduced with Pycnogenol®. |
| Hosseini et al. 63 | Pycnogenol® in the management of asthma | 22 subjects, 2 mg Pycnogenol® per kg per day or placebo for 4 weeks | The forced expiration volume in 1 second (FEV1) was increased after Pycnogenol® intake, compared to baseline and placebo. Subjective asthma symptom rating and the level of plasma leukotrienes reduced after Pycnogenol® intake compared to placebo and baseline. |
| Oral health | |||
| Watanabe et al. 64 | Effects of French Pine Bark Extract Chewing Gum on Oral Malodor and Salivary Bacteria | 21 subjects, 30 mg Pycnogenol® per day in a gum or placebo gum for 4 weeks | The levels of volatile sulfur compounds in the mouth, the tongue-coating score and hydrogen sulfide-producing bacteria in saliva were reduced with a Pycnogenol® gum compared to a placebo gum. |
| Sports | |||
| Ackermann et al. 65 | The effect of an acute antioxidant supplementation compared with placebo on performance and hormonal response during a high-volume resistance training session | 15 subjects, 2 ml per kg body weight of a sports drink, containing 4.8 mg Pycnogenol® per 2 ml or placebo drink, single dose, 4 h before training | Muscle contractile performance and accumulated power output during lower limb hypertrophic resistance training was improved after Pycnogenol® supplementation compared to placebo. |
| Bentley et al. 66 | Acute antioxidant supplementation improves endurance performance in trained athletes | 9 subjects, 360 mg Pycnogenol® in a drink or placebo drink, single dose 4 h before training | Cycling time before exhaustion was increased by 80 s after Pycnogenol® drink consumption, compared to placebo subjects. |
| Mach et al. 67 | The effect of antioxidant supplementation on fatigue during exercise: potential role for nicotinamide adenine dinucleotide (NADH) | 13 subjects, 360 mg Pycnogenol® in a drink or placebo drink, single dose, 3 h before training | The physical work capacity until fatigue during cycling training was increased with Pycnogenol® compared to placebo and baseline. Serum nicotinamide adenine dinucleotide levels were increased significantly with Pycnogenol® compared to placebo. |
| Pavlovic 68 | Improved endurance by use of antioxidants | 24 subjects, 200 mg Pycnogenol® per day or placebo for 30 days | Performance time on a treadmill was increased with Pycnogenol® supplementation compared to placebo. |
Abbreviations: FMD = Flow-mediated dilation; LDL = low density lipoprotein; HDL = high density lipoprotein; CVI = chronic venous insufficiency; MPH = methylphenidate hydrochloride; NPY = neuropeptide Y; 8-oxo-G = 8-oxo-7,8-dihydroguanine; ADHD = attention deficit and hyperactivity disorder; NSAIDs = nonsteroidal anti-inflammatory drugs; FEV1 = forced expiration volume in 1 second; NAD+ = nicotinamide adenine dinucleotide.
[Source 21 ]Anti-inflammatory activity
The constituents of Pycnogenol act in concert, as all of its phenolic compounds are scavengers of free radicals and exhibit a range of anti-inflammatory actions, as documented for ferulic acid, caffeic acid, catechin, and taxifolin 69.
The metabolites, formed by ring fission of catechin units by microbiota, possess also remarkable anti-inflammatory activity. The procyanidin metabolite M1 showed in vitro a 100% higher activity than hydrocortisone 70. These initial in vitro effects could be proven in ex vivo experiments.
Plasma collected from volunteers subsequent to consumption of Pycnogenol, inhibited the activity of cyclooxygenases 1 and 2, as well as the activation of the inflammation “master switch” nuclear factor kappa B (NFκB) 70.
Human pharmacokinetic investigations indicated that the metabolite M1 is enriched in blood cells by active transport mechanisms, so that anti-inflammatory activity of blood is most probably 30 times higher than measured in plasma 71.
Furthermore, the active metabolite M1, ferulic acid, and caffeic acid are present in the synovial fluid, thus acting directly on the source of inflammation in case of synovitis 72.
Protection of articular cartilage
The progression of osteoarthritis is connected with a blockage of synthesis of proteoglycan components and type 2 collagen by inflammatory cytokines and tumor necrosis factor alpha 73. Furthermore, cartilage degrading proteolytic enzymes such as matrix metalloproteinases (MMPs) are liberated as MMP-1, MMP-2, and MMP-13 74. The metabolite M1 formed from the procyanidins of Pycnogenol inhibited the activity of MMP-1, MMP-2, and MMP-9 in vitro and blocked the release of MMP-9 from activated monocytes 70. The oral intake of Pycnogenol downregulated the gene expression of various cartilage degradation markers in the patients’ chondrocytes, the decrease of MMP3, MMP13 and the pro-inflammatory cytokine IL1B were statistically significant. Additionally, protein concentrations of ADAMTS-5 in serum were reduced significantly after three weeks intake of the pine bark extract 75. These chondroprotective effects were confirmed in ex vivo experiments. Plasma, taken from human volunteers following intake of Pycnogenol, inhibits the release of NFκB and MMP-9 from activated monocytes. The inhibition of the master switch of inflammation NFκB, acting together with inflammatory cytokines, reduces considerably the inflammatory process connected to osteoarthritis. Analysis of synovial fluid from osteoarthritis patients revealed the presence of ferulic acid, caffeic acid, taxifolin, catechin, and the metabolite M1 in serum, blood cells, and synovial fluid 72. The anti-inflammatory substances ferulic acid, caffeic acid, and the active metabolite M1 were enriched in the synovial fluid relative to serum. So, the chondroprotective and anti-inflammatory action take place locally in the synovia.
Pycnogenol acts like a sustained-release formulation by its combination of fast absorbed phenolic compounds and slowly metabolized procyanidins. Its constituent ferulic acid and the metabolite M1 are enriched in synovial fluid and contribute to local anti-inflammatory action.
Pycnogenol as an anti-inflammatory and chondroprotective add-on supplement provided long-lasting positive effects such as enhanced physical mobility and pain relief for patients with mild osteoarthritis. The use of NSAIDs could be significantly reduced, thus diminishing unwanted effects of NSAIDs. Studies involving more patients are needed to confirm the beneficial actions of Pycnogenol on an even broader basis.
The reduced anti-inflammatory activity in osteoarthritis with Pycnogenol is reflected in a decrease of C-reactive protein levels in osteoarthritis patients by 70%, with plasma-free radicals simultaneously scavenged by 30% 76.
The cooperation of the diverse anti-inflammatory effects of Pycnogenol in plasma and synovial fluid results in reduction of pain and increased mobility.
Three identically designed clinical trials investigating the role of Pycnogenol in osteoarthritis treatment have been published to date 77, 78, 79.
All studies were randomized, double blind, and placebo controlled. Middle-aged patients (48–54 years) suffering from mild osteoarthritis, stage I or II, verified by X-ray, were treated either with 3 × 50 mg Pycnogenol daily or placebo, added to existing therapy with NSAIDs. Success of the add-on supplementation was objectivated by the Western Ontario McMasters University (WOMAC) questionnaire for osteoarthritis during a period of 3 months. Results are summarized in Table 1. It has to be emphasized that patients were allowed to use their NSAIDs as concomitant medication when needed.
The first, small-scale study (n = 35) showed a clear reduction of scores for pain, functionality, and total WOMAC score, dependent on duration of treatment 78. After 3 months, significant differences to placebo were observed, symptoms were reduced by 43%, 35%, 52%, and 49% for pain, stiffness, physical function, and total WOMAC score, respectively.
In the second study, involving 100 patients, the pain score improved significantly with time compared to baseline 80. In this study, a considerable placebo effect was observed for pain, daily activity scores, and overall WOMAC scores, and although improvement relative to baseline was highly significant, the difference to placebo did not reach significance level in any case. Only scores for stiffness improved significantly both to baseline and versus placebo after 2 and 3 months, while placebo had no effect.
A total of 156 patients were included in the third study 81. Symptom scores dropped significantly: pain by 45%, stiffness by 47%, physical function by 43%, and overall WOMAC score by 44%. The decrease under placebo was not significant. A more detailed analysis of the WOMAC scores of the third study revealed a significant reduction of nocturnal pain and pain during troublesome stair climbing, particularly relevant for the quality of life of osteoarthritis patients 81.
Also, for joint stiffness during the day, only minor changes were observed with placebo, while Pycnogenol improved stiffness remarkably.
An example for an enhanced physical function refers to the onerous rising from sitting: with Pycnogenol the score dropped clearly from initial 3.1 to 0.8, with placebo the score remained nearly unchanged (3.0 to 3.1).
The improvement of osteoarthritis was impressively objectivated by performance of patients on a treadmill. Patients in the Pycnogenol group could increase their walking distance after 3 months from 68 to 198 m, whereas placebo expanded walking distance from 65 m just to 88 m 81.
These positive effects related to relief from daily pain, stiffness, and physical function had of course a great influence on the well-being of the patients in the Pycnogenol group. As the negative impact of osteoarthritis on daily activities subsided, the emotional status of patients shifted significantly from irritability, frustration, depression, and insomnia to better well-being 81. The sum of negative emotional scores dropped from 31.4 to 11.5, whereas placebo had a nonsignificant effect (28.4 to 24.1). Thus, quality of life was definitely improved in the Pycnogenol group.
Use of NSAIDs was significantly reduced in all three studies in the Pycnogenol group, in contrast to a slight increase of intake of NSAIDs in the placebo group. A more precise evaluation of NSAID use was performed in the third study 81.
Intake of Pycnogenol allowed patients to decrease intake of NSAID medication by 58% according to their diaries 81. Correspondingly, gastrointestinal complications decreased by 63% as well as days spent in hospital (60%). Values under placebo decreased by only 1% (reduction of use of NSAIDs) and 3% (reduction of hospital admissions and gastrointestinal problems).
Together, the three clinical studies demonstrate an improvement of symptoms of mild osteoarthritis under Pycnogenol, despite the reduced intake of NSAIDs. No unwanted effects of Pycnogenol were reported in the three studies. This is in line with the safety profile of Pycnogenol. Unwanted effects such as headache, dizziness, nausea, sleepiness, skin irritation, and gastric troubles were mild with a rate of 1.9% in clinical trials involving 7000 patients 82.
Table 2. Overview of 3 clinical trials demonstrating efficacy of pycnogenol for arthritis
| Belcaro et al.81 | Cisar et al.80 | Farid et al.78 | |
|---|---|---|---|
| N = 156 | N = 100 | N = 35 | |
| % improvement | 100 mg/day | 150 mg/day | 150 mg/day |
| Pain | −45% | −40% | −43% |
| Stiffness | −53% | −40% | −35% |
| Physical performance | +56% | +22% | +52% |
| Global score | +50% | +49% |
Pycnogenol and ADHD
Attention-deficit and hyperactivity disorder (ADHD) is a complex and multifactorial disorder, influenced by both genetics and the environment. Its exact pathophysiology remains, however, unclear. Dopaminergic dysfunction is involved, but also associations with immune and oxidant-antioxidant imbalances exist 84. Various studies demonstrated, for example, increased levels of plasma malondialdehyde (MDA) and exhalant ethane (oxidative stress markers) and decreased activity of antioxidant enzymes such as glutathione peroxidase (GPX) and catalase (CAT) 85. ADHD has also been hypothesized to be a hypersensitivity disorder, with a disrupted immune regulation contributing to its cause 86; i.e. ADHD has comorbidity with both Th1- and Th2-mediated disorders and several related genes have immune functions 86. Ceylan et al. 84 observed increased levels of adenosine deaminase, a marker of cellular immunity, and of the oxidative enzymes xanthine oxidase and nitric oxide synthase, and decreased levels of the antioxidant enzymes glutathione-S-transferase and paraoxonase-1. These results indicate the involvement of oxidative changes and cellular immunity in ADHD 84.
Methylphenidate, the first-choice medication for ADHD, is a central nervous system stimulant. It increases attentiveness and reduces hyperactivity and impulsivity by inhibition of dopamine reuptake in the striatum, without triggering its release. methylphenidate is prescribed for chronic use to a large proportion of ADHD patients, but is linked to possible publication bias in reported efficacy 87. In addition, parents are often disinclined to use methylphenidate due to its negative publicity and its frequent side effects, including serious side effects like arrhythmia, and, subsequently, nonadherence to therapy is high 87. A recent review reports adverse effects, like insomnia and decreased appetite, in about 25% of patients using methylphenidate 88. Other therapeutic options are therefore warranted, at least for a subgroup of patients 87.
In one previous trial on twenty-four adults (24 to 53 years old) with attention-deficit/hyperactivity disorder (ADHD), the effect of Pycnogenol was compared to methylphenidate and placebo. However, neither methylphenidate nor Pycnogenol outperformed placebo, possibly due to the short treatment period of 3 weeks 89. Further research is needed to investigate its efficacy, mechanism of action and value, especially compared to methylphenidate treatment. For example, dietary polyphenols and their metabolites exert prebiotic-like effects, stimulating the growth of intestinal microbiota, which play a fundamental role in immunity 90. Also the Pycnogenol dosage is based upon this previous clinical trial, using 1 mg/kg body weight 91.
Pycnogenol effects on human skin
A number of studies provide compelling evidence that oral supplementation with Pycnogenol protects human skin against UV radiation 56, 55. Accordingly, in a study on 21 fair-skinned volunteers, Saliou et al. 92 demonstrated that oral ingestion of 1.10 mg or 1.66 mg/kg body weight/day Pycnogenol is effective in reducing UV-induced erythema. In this study, the UV protective effect of Pycnogenol was found to be dose dependent, to develop after 4-8 weeks of oral intake and to almost double the individual minimal erythema dose which was determined prior to Pycnogenol intake. The strength of this study is the intraindividual comparison of minimal erythema doses before, during and after Pycnogenol intake as well as the observed dose dependency of minimal erythema dose increases. Weaknesses of the study include the lack of a placebo treatment, e.g. in a crossover design or a comparator group. Although the study has been conducted during winter/spring time, the study has not been controlled for the seasonal increase in exogenous antioxidants in the regular diet which is often observed during summer and autumn 93. Also, it should be noted that solar radiation-induced erythema responses mainly result from the formation of DNA photoproducts such as cyclobutane pyrimidine dimers in human skin, which can be reduced by antioxidants only to some extent. In other words, photoprotection by Pycnogenol might be even greater than observed here, if other biological end points, which more strongly depend on UV radiation-induced oxidative damage, would have been studied. Accordingly, oral ingestion of the carotenoid lycopene was previously shown to only moderately reduce solar UV radiation-induced erythema by 37% 94, whereas long-wave UVA radiation-induced gene transcription, which strictly depends on the generation of reactive oxygen species in human skin, was almost completely inhibited 95. It should also be noted that in vivo animal studies show that oral ingestion by mice significantly reduces the number and growth rate of skin tumors which were induced either by chronic UVB irradiation or by a combination of UVB radiation with topical treatment of skin with the polyaromatic hydrocarbon 7,12-dimethylbenzanthracene 96. As UVB- as well as polyaromatic hydrocarbon-mediated skin carcinogeneses both critically involve activation of the aryl hydrocarbon receptor 97 and since flavonoids such as catechin and epicatechin, which are a main constituent of Pycnogenol, have been shown to inhibit aryl hydrocarbon receptor activation 98, it is tempting to speculate that oral ingestion of Pycnogenol may help to suppress environmentally induced aryl hydrocarbon receptor activation in skin cells.
A recently published randomized double-blind, placebo-controlled study identified Pycnogenol as a natural, safe and effective supplement for women who face hair thinning 55. 76 healthy menopausal women between 45 and 60 years were randomly assigned to either take 150 mg Pycnogenol per day or placebo for six months 55. Oral intake of Pycnogenol led to a significant increase of hair density of 30%, compared to baseline and of 15% compared to placebo after two months. The effects of Pycnogenol stayed on a highly improved level after six months of supplementation 55. In addition, the study showed that Pycnogenol significantly reduced water loss from the skin of subjects’ scalp, compared to the placebo group. This leads to a better regulated scalp skin moisture balance for healthier hair and scalp 55. The study also confirmed that Pycnogenol intake positively affects microcirculation in the skin. Using photoplethysmography, Pycnogenol was found to decrease resting flux in the scalp by 21.7% after two, and by 43.5% after six months. In the placebo subjects, the observed decrease was only 5.1 and 20.5%, respectively 55. This effect of Pycnogenol leads to a better supply of nutrients and oxygen to hair follicles. In several previously published studies, Pycnogenol was shown to improve circulation in small blood vessels in the body, like the very fine micro vessels in the fingertips or in the retinal capillaries of the eye 43, 57. In addition to an improvement of microcirculation, two other mechanisms of action explain Pycnogenol’s efficacy for hair health and beauty. Pycnogenol’s anti-inflammatory and antioxidant activities contribute to protecting hair follicles by capturing free radicals, generated either by stress, sun rays, pollution, or inflammation 22, 23, 24, 25, 26, 27, 28, 29, 30, 31.
Pine bark extract and photoaged facial skin
In a randomized double-blind, placebo-controlled study, Pycnogenol’s effects on the skin of 78 urban outdoor workers was investigated 56. Water loss of the skin during the hot summer season was significantly reduced by 14% with Pycnogenol supplementation for three months and only by 5% with placebo 56. Accordingly, skin elasticity was shown to be improved by 13% after Pycnogenol supplementation, compared to an increase of 1% in the placebo group.
During the dry autumn season, Pycnogenol helped achieve a more even skin tone by 7.2% after six weeks and by 13.8% after twelve weeks of Pycnogenol supplementation. These effects were statistically significant versus the placebo control. In the placebo group, subjects experienced a decrease in skin tone regularity because of pollution and sun radiation during the dry season. Skin tone regularity was assessed on the cheeks of the participants by the individual typology angle, which is an objective classification of the skin tone in dermatology and cosmetology.
Several mechanisms behind Pycnogenol’s effects on skin have been researched 38, 39 effects on human skin: Clinical and molecular evidence. Skin Pharmacol Physiol. (2016) 29:13–7. 10.1159/000441039)), 99. Clinical investigations of Pycnogenol supplementation for twelve weeks in women aged 55 to 68 years found increased hyaluronic acid synthase mRNA levels by 44% and collagen type 1 mRNA levels by 40% in skin biopsies 38. These mechanisms explain the effects of Pycnogenol on skin hydration and elasticity.
Another study investigated the depigmenting action of Pycnogenol and found a significant reduction of the tyrosinase activity by 66.5%. Tyrosinase is an enzyme that activates the production of melanin, responsible for melasma (a common skin problem caused by brown to gray-brown patches on the face). In addition, Pycnogenol downregulated other pigmentation-related mediators in UV-light treated human melanocytes 99. Pycnogenol’s ability to counteract skin hyperpigmentation was clinically validated in another study 39 effects on human skin: Clinical and molecular evidence. Skin Pharmacol Physiol. (2016) 29:13–7. 10.1159/000441039)). In this clinical trial with 20 women, oral supplementation with Pycnogenol was shown to significantly lower UV-induced expression of the pigment synthesizing enzymes TRP1 by 75%, tyrosinase by 51%, MITF by 67% and MART-1 by 67% 39 effects on human skin: Clinical and molecular evidence. Skin Pharmacol Physiol. (2016) 29:13–7. 10.1159/000441039)). These markers are associated with long-lasting pigmentation. From these results, it was concluded that Pycnogenol “contributes to the inhibition of pathways associated with skin hyperpigmentation” 39 effects on human skin: Clinical and molecular evidence. Skin Pharmacol Physiol. (2016) 29:13–7. 10.1159/000441039)), 99.
Furumura et al. 100 enrolled 112 healthy women younger than 60 years with age spots, mostly diagnosed as solar lentigines, and multiple symptoms of photodamaged skin, including mottled pigmentation, roughness (including dry flaky skin), wrinkles, and swelling. All women enrolled in this study had mild to moderate facial photodamage graded on the Glogau scale between II and III and Fitzpatrick skin phototypes III to IV. After approval by the institutional ethics committee of Fukuoka University, which adheres to the principles of the Declaration of Helsinki, informed consent was obtained from all participants in the study. None of the subjects took topical/systemic retinoids, health food supplements, oral medications such as hormone replacement therapy, or topical medications, or were pregnant 4 weeks prior to enrolling in this study.
Twenty-four women were enrolled in an open-label, high-dose pine bark extract (Flavangenol®) trial and were treated with 100 mg/day pine bark extract (Flavangenol®) for 12 weeks, while a further 88 women were enrolled in part 1 of a separate low-dose trial and treated with pine bark extract (Flavangenol®) 40 mg/day for a total of 24 weeks in an open-label, randomized, parallel-group comparative fashion. Group 1 participants were asked to take pine bark extract (Flavangenol®) 40 mg/day once daily, and to use a cleanser and sunscreen for 24 weeks throughout part 1 of the study. Group 2 participants were merely placed under observation without taking pine bark extract (Flavangenol®) for the first 12 weeks before starting oral treatment with pine bark extract (Flavangenol®) 40 mg/day once daily for the next 12 weeks, and were instructed to use a cleanser and sunscreen for 24 weeks throughout part 1 of the study.
Furumura et al. 100 examined the efficacy of pine bark extract in the treatment of photodamaged facial skin, and significant improvement was suggested from multiple dermatological score assessments during this study.
A subject questionnaire concerning subjective facial symptoms demonstrated that a relatively large number of subjects felt that the roughness of their facial skin had improved in the high-dose trial. Although improvement in age spots was only recognized by a relatively small number of subjects in the high-dose trial [100 mg/day pine bark extract (Flavangenol®)], detailed evaluation of digital images revealed that 71% of participants had improvement of their age spots, albeit to a varying extent. In part 1 of the low-dose trial [40 mg/day pine bark extract (Flavangenol®)], there was significant improvement in scores for solar lentigines, mottled pigmentation, skin roughness, and swelling only when subjects were on treatment with pine bark extract. Further, subjects treated with pine bark extract had significantly lower scores for solar lentigines and skin roughness when compared with the untreated patients. Therefore, we consider that both the high-dose and low-dose arms in this study demonstrate a similar trend of improvement in symptoms of photodamaged facial skin. Further significant improvements were seen during the long-term 18-month study (part 2 of the low-dose trial) in almost every photoaging score. This improvement was maintained and enhanced by continuous administration of pine bark extract over a long period. Finally, 72% of the subjects receiving pine bark extract for 12 weeks (group 2) showed improvement versus 87% of those receiving pine bark extract for 24 weeks (group 1). All subjects who completed treatment with pine bark extract for 15–18 months showed improvement in symptoms. In line with the score assessment results, objective biophysical measurements demonstrated a significant gradual decrease in average melanin index during treatment with pine bark extract in both trials.
In an earlier study of treatment of photoaged skin with oral polyphenols 101, a popular polyphenol-rich green tea extract containing (−)-epigallocatechin gallate (EGCG) was used. Although facial photoaging scores improved on treatment with the green tea extract for the first 12 months, there was no significant antiphotoaging effect after 24 months of treatment. In contrast, gradual improvement of photoaging scores even at 18 months was confirmed in their pine bark extract trial. Demographic diversity in subject age and race might account for the different results seen in these two studies. The mean age of the subjects in the previous study was around 12 years older than in Furumura et al. 100 study, so a less favorable outcome would be expected because of the exponential decline of intrinsic antioxidative potency in the elderly. Furumura et al. 100 findings in Japanese women might be positively biased by a racial difference, ie, age spots in East Asians often appear as early as in the 20 s and 30 s, while age spots in Caucasians tend to become apparent between the ages of 50 and 60 years 102.
In the skin, pine bark extract has been found to protect capillary walls 103 and to inhibit matrix metalloproteinases 104. Direct assessment of the antioxidant effects of pine bark extract by electron spin resonance spectroscopy showed that pine bark extract had significant antioxidant effects on the facial skin of ultraviolet B-irradiated hairless mice in vivo 105. Oligometric proanthocyanidins have also been reported to be effective inhibitors of tyrosinase in skin-derived melanocytes and in the hyperpigmented skin of ultraviolet-irradiated mice and guinea pigs 106. Oral oligometric proanthocyanidin supplements are expected to have desirable effects on photoaging because they promote tissue elasticity, help heal microinjuries, reduce bruising and swelling by strengthening blood vessels, prevent postinflammatory skin pigmentation, restore dermal collagen, and improve the peripheral circulation 107. In fact, oligometric proanthocyanidins from grape seeds have previously been reported to improve melasma to a significant extent 108.
A white complexion is a highly desirable symbol of beauty among Asian women, who believe that it is powerful enough to hide a number of faults. Pine bark extract did not modify facultative skin color in Furumura et al. 100 trial, suggesting that the skin lightening elicited by pine bark extract is confined to solar lentigines that appear with chronic inflammation, and can persist long after exposure to ultraviolet light. Recent profiling of solar lentigines with cDNA microarrays and immunohistochemical assays revealed a number of upregulated genes for the enzymes that synthesize arachidonic acid, as well as melanogenic and inflammatory genes in those lesions 109.
Modulation of skin pigmentation by oral ingestion of Pycnogenol
There is now also more and more evidence that Pycnogenol may affect pigmentation of human skin. In 2002, Ni et al. 110 were first to provide evidence that Pycnogenol intake may reduce hyperpigmentation in women with melasma. In this study, a total of 30 Chinese female patients with melasma orally ingested 75 mg Pycnogenol/day for a total of 1 month. The impact of Pycnogenol intake on preexisting melasma was assessed by means of a clinical score, i.e. the melasma area index, which was based on assessing the diameter of the lesional skin area by means of a ruler. In addition, the pigmentary intensity index was determined by means of a color chart. It was found that after the 30-day treatment period both parameters were significantly reduced. Pycnogenol was well tolerated in all patients, and standard blood and urine parameters did not change. The authors concluded that Pycnogenol is therapeutically effective and safe in patients with melasma, and they attributed the observed beneficial effects to the well-known antioxidative properties of Pycnogenol 110. It has to be noted that the design of this study was open, and efficacy parameters were based on subjective assessments. Nevertheless, this study was first to indicate that Pycnogenol intake might be effective to downregulate skin hyperpigmentation. In line with this assumption are in vitro experiments which showed that treatment of cells from the human melanoma cell line B16 with Pycnogenol reduced tyrosinase activity and melanin synthesis in this tumor cell line 111. Even more important are results from a recent human in vivo study which provide molecular evidence that the oral intake of Pycnogenol downregulates the expression of genes in human skin which are critically involved in melanin synthesis. The design and part of the results of this clinical trial have previously been published in this journal 112. This study is unique because it is the only one to provide in vivo molecular evidence that Pycnogenol uptake is beneficial for human skin. In this clinical trial, a total of 20 healthy postmenopausal Caucasian women were supplemented with 3 × 25 mg Pycnogenol daily for a total of 12 weeks. It was found that this intervention significantly improved skin elasticity and skin hydration, and that this improvement of skin physiological parameters was associated with a significant upregulation of mRNA steady-state levels for hyaluronic acid synthase-1, an enzyme which is important for hyaluronic acid synthesis in skin, as well as genes involved in collagen de novo synthesis. Further RT-PCR analysis of RNA purified from biopsies obtained in this study additionally revealed a significant effect of intake of Pycnogenol on the transcriptional expression of genes which are critically involved in skin pigmentation 113. Accordingly, these yet unpublished data, demonstrate that oral Pycnogenol intake was able to significantly inhibit UV radiation-induced upregulation of microphthalmia-associated transcription factor, tyrosinase-related protein 1 and melanoma antigen recognized by T cells, and mRNA expression of tyrosinase was inhibited by trend. These changes provide a molecular basis to explain the previous notion that Pycnogenol intake benefits patients with melasma. The exact mechanism through which Pycnogenol may inhibit the expression of genes involved in skin hyperpigmentation is currently not known. We previously discussed the possibility that Pycnogenol may at least in part function by antagonizing aryl hydrocarbon receptor activation. In this regard it should be noted that aryl hydrocarbon receptor activation in human as well as murine melanocytes has recently been reported to be critically involved in UV radiation-induced skin pigmentation 114. Specifically, aryl hydrocarbon receptor activation was shown to cause upregulation of tyrosinase-related proteins 1 and 2 as well as tyrosinase in primary human melanocytes. In aggregate, these studies indicate that the oral intake of Pycnogenol may be used to reduce skin pigmentation in humans in general and hyperpigmentation caused by melasma in particular. Given the central role of microphthalmia-associated transcription factor in the pathogenesis of skin hyperpigmentation and pigmented skin lesions, this should prompt further controlled clinical trials to assess the effect of oral Pycnogenol intake on pigment spot formation in chronically UV-exposed skin areas.
As discussed above, additional studies being done which take into account very recent evidence that skin damage in general and skin hyperpigmentation/skin aging in particular can also be caused by other environmental factors such as nonionizing radiation in the visible as well as infrared range 115 and ambient air pollution including traffic-related particulate matter 116.
Eye health
Owing to Pycnogenol’s effects on microcirculation, inflammation and oxidation, Pycnogenol was found to have positive effects on eye health 58, 57. As the retina is the tissue with the highest metabolic rate in the body, it is particularly susceptible to oxidative stress. The eye tissue is particularly exposed to UV light which generates reactive oxygen species in cells. Furthermore, metabolic conditions like diabetes involve a pathological oxidative stress, which can lead to diabetic retinopathy 117.
Pycnogenol showed effects to stop further progression of retinopathy of diabetics by stabilizing and sealing leaky capillaries of the retina, stopping further outflow of blood 58, 57.
In an randomized double-blind, placebo-controlled study, Pycnogenol was shown to reduce capillary leaking in the eyes 58. Using fluorescein angiography as a measurement tool, vascular permeability was found to have decreased by 16 and 23% for left and right eye, respectively, compared to baseline. Retinal vascularization and the presence of macular edema was assessed by ophthalmoscopy, determining the severity of retinal damage. After Pycnogenol supplementation for two months, the ophthalmoscopy score significantly improved by 9% in the left eyes and by 17% in the right eyes compared to baseline, whereas there was no change observed in the placebo group. Furthermore, Pycnogenol® supplementation led to increased visual acuity by 5.7% (left eye) and 7% (right eye). The improvement was statistically significant compared to placebo. Whereas, with placebo treatment, retinopathy continued to progress as the visual acuity was reduced by 3.2% (left) and 7.5% (right), assessed by the Snellen Chart.
Another study showed that Pycnogenol reduced retinal edema and improved visual acuity in early diabetic retinopathy 57. The score assessing retinal edema was significantly reduced by 30% in mild edema cases and by 35% in moderate edema cases compared to placebo patients after three months of Pycnogenol supplementation. The retinal thickness, assessed by high resolution ultrasound, significantly decreased by 11% in mild cases and by 25% in moderate cases in Pycnogenol subjects 57. The most significant outcome observed in this study was the improvement of the visual acuity by 21% in Pycnogenol patients compared to baseline and placebo patients, which was assessed with the Snellen chart. Furthermore, the retinal blood flow was improved by around 30% after Pycnogenol supplementation.
Pycnogenol’s effects on endothelial function 22 might explain the observed effects on perfusion of the retinal tissue and the resulting restoration of vision loss in diabetic retinopathy patients.
Oral health
A study on oral health, specifically on persistent bad breath (halitosis) was conducted with Pycnogenol 64. The 21 healthy participants used either two placebo gums or two gums with 2.5 mg Pycnogenol each, six times daily for 15 min each. After two weeks of Pycnogenol gum usage, the levels of volatile sulfur compounds (hydrogen sulfide, methyl mercaptan and dimethyl sulfide) were significantly reduced compared to baseline and after four weeks, these levels were reduced significantly compared to placebo 64. In addition, the Pycnogenol group had a lowered tongue-coating score and significantly reduced hydrogen sulfide-producing bacteria in saliva after four weeks. The mechanism behind these effects has been suggested to be the previously observed bacteriostatic properties of Pycnogenol 118.
Respiratory health and allergies
Chronic respiratory diseases, such as chronic obstructive pulmonary disease (COPD), asthma and pulmonary hypertension are among the five disease areas with the highest global mortality and morbidity rates 119. With almost 300 million people affected, asthma is one of the most common respiratory diseases worldwide 120. Asthma is a chronic inflammatory disease that can be triggered by allergies, by medication such as aspirin or it can arise within the body through yet unclear molecular mechanisms 121. Recurrent episodes of coughing, shortness of breath, wheezing and chest tightness are typical symptoms for asthma 122.
Supplementation with Pycnogenol has been shown to have several beneficial effects on asthma and allergic rhinitis symptoms 63, 62, 61. Especially Pycnogenol’s anti-inflammatory effects are suggested to be responsible for its anti-allergic and anti-asthma efficacy.
In an randomized double-blind, placebo-controlled cross-over study, the effects of Pycnogenol supplementation for four weeks on 22 chronic asthma patients were investigated 63. The patients’ lung function was assessed by analysis of the “forced expiration volume in one second” (FEV1), representing the percentage of lung volume exhaled in a second. In asthmatics, the FEV1 is generally reduced as their breathing is restricted. After Pycnogenol supplementation, the all-adult patients could exhale 70% of their lung volume as compared to 59% at trial start and 63% in response to placebo 63. Additionally, the study participants rated their asthma symptoms regarding severity. Four weeks of Pycnogenol intake led to a reduction of symptom severity by 20%, whereas it was reduced by 4.5% in placebo subjects 63. Furthermore, Pycnogenol® supplementation significantly reduced pro-inflammatory mediators (leukotrienes) in the blood of patients, as compared to both baseline values and placebo.
Most asthmatics develop the disease already during childhood, often before the age of five years. An randomized double-blind, placebo-controlled study investigated the effects of Pycnogenol on 60 children aged six to eighteen years with mild to moderate asthma 62. The study showed that the breathing capacity improved significantly already after one month of Pycnogenol supplementation, as assessed by measuring FEV1 62. The severity of asthma symptoms as well as leukotriene levels in the urine decreased drastically after one month of Pycnogenol intake and further decreased significantly throughout the trial period 62. The treatment with placebo had no significant effect on leukotriene levels or asthma symptoms. Another interesting outcome of the study is the reduced necessity of using albuterol rescue inhalers as severe asthma attacks appeared less frequently. After one month, eight out of thirty children taking Pycnogenol didn’t require rescue inhalers anymore and eighteen children were completely off the inhaler after the three-month supplementation with Pycnogenol.
In an randomized double-blind, placebo-controlled trial, researchers found that Pycnogenol supplementation improved allergic rhinitis symptoms from birch and other pollen allergy when the supplementation started at least five weeks before the pollen season 61. Eye symptoms decreased by 35% and nasal symptoms by 20.5% compared to placebo 61. The number of patients requiring rescue antihistamines was 26% lower in the Pycnogenol group than in the placebo group 61.
Women’s health
Investigating the efficacy of Pycnogenol on the health of women, two randomized double-blind, placebo-controlled studies on menopausal symptoms 25, 59 and one on menstrual discomfort 60 have been performed.
An randomized double-blind, placebo-controlled study with 155 peri-menopausal women found the menopause symptoms assessed by the Women’s Health Questionnaire (WHQ) to be significantly improved after six month of Pycnogenol supplementation, as compared to placebo controls 25. The symptoms on the WHQ include somatic (tiredness, headache) and vasomotor problems (hot flashes, sweating), depressed mood, memory and concentration issues, attractiveness, anxiety, sexual behavior (including vaginal dryness), sleep, and menstrual problems. After one month, all symptoms of the Pycnogenol supplemented subjects were significantly improved compared to enrollment and most items significantly compared to placebo 25. In the placebo group, several symptoms also improved significantly compared to baseline after one month; however, after six months, only memory/concentration remained significantly increased compared to baseline. Interestingly, systolic and diastolic blood pressure, as well as LDL-cholesterol levels were found to be significantly reduced after 6 months of Pycnogenol supplementation, compared to placebo 25. The increase of total antioxidant status, measured as Trolox equivalent antioxidant capacity, after Pycnogenol intake was highly significant compared to baseline and placebo. From these results, the authors attributed a positive, protective role to Pycnogenol on the vascular health of peri-menopausal women, which also “clearly reduced the frequency as well as the severity of climacteric symptoms” 25.
Significant efficacy of a low dosage of Pycnogenol (60 mg daily) on menopause symptoms could be shown in an randomized double-blind, placebo-controlled investigation with 170 women 59. In this study, the total menopause symptom score of the women was reduced by 17% compared to placebo control after three months 59. Vasomotor symptoms (hot flashes), sleep problems and fatigue were significantly improved in the Pycnogenol group compared to placebo 59. After 3 months, no significant effects on blood pressure or cholesterol levels were detected but positive trends were observed. In this study, blood plasma levels of different sexual hormones were investigated as well. Interestingly, after Pycnogenol intake, none of the hormone levels showed significant changes compared to baseline or placebo, suggesting non-hormonal mechanisms of Pycnogenol on menopause symptoms 59.
The results from these studies demonstrated interesting efficacy of Pycnogenol supplementation in addressing various menopause symptoms. The underlying mechanism has not been investigated fully yet, however, Pycnogenol’s validated effects on blood circulation and endothelial health are good explanations for its beneficial effects on symptoms like hot flashes and sexual behavior 21.
An randomized double-blind, placebo-controlled multicenter study from 2008 showed that Pycnogenol supplementation significantly reduced abdominal discomfort in women with menstrual cramps (dysmenorrhea) 60. The women took Pycnogenol for two menstrual cycles, during which they needed significantly less analgesic medication than in the registration period of two months before supplementation (−46%) and less than placebo controls (−28%) 60. Interestingly, after discontinuing Pycnogenol and placebo intake in the fifth cycle of the study, subjects of the Pycnogenol group reported a continuation of the trend, as their need for analgesics was even more reduced (−50% compared to baseline), while women of the placebo group experienced a rapid return to analgesics 60. The number of days on which analgesics were required was significantly reduced with Pycnogenol from 2.1 to 1.3 days and changed less in the placebo group, from 1.9 to 1.7 days.
Inflammatory processes were found to be a key mechanism in menstrual cramps (dysmenorrhea) 123. During menstruation, the tissue lining of the uterine cavity is replaced, leading to wound healing and inflammation. Pycnogenol was shown to have potent anti-inflammatory activities in several studies 28, 29, 30, 31.
Sports endurance and performance
In four randomized double-blind, placebo-controlled investigations, Pycnogenol supplementation was shown to enhance sport endurance and performance 68, 66, 65, 67.
An randomized double-blind, placebo-controlled crossover study found that the endurance time of a group of recreational athletes was significantly increased after Pycnogenol supplementation 68. For the measurements, the subjects performed on a treadmill with individual setting adjusted to 85% of a person’s maximal oxygen consumption. The 20 to 35 years old participants received 200 mg Pycnogenol per day or placebo for 30 days, then switched to the other group for another 30 days 68. Endurance was evaluated by measuring the running time in seconds on the treadmill after 30 and after 60 days. The composite performance time of the Pycnogenol groups increased significantly by 23.2% compared to baseline and by 7.1% compared to placebo. The time of placebo subjects was increased by 15% compared to baseline 68.
A small randomized double-blind, placebo-controlled study with nine trained 25 to 45 year old cyclists examined the effects of a single dose of a fitness drink with Pycnogenol as the only active ingredient on performance 66. The placebo drink consisted of the same ingredients (pineapple pulp, molasses, sodium chloride, flavor, steviol glycosides, sodium benzoate, potassium sorbate), except Pycnogenol. The study protocol included two separate sets of training of five minute cycling at 50% peak power output, eight minute at 70% peak power output and cycling until fatigue at 95% peak power output. The Pycnogenol or placebo drink was consumed four hours before the training. On average, the Pycnogenol supplemented cyclists rode 80 seconds longer until they reached exhaustion compared to subjects, taking a placebo drink 66. In addition, considerable evidence was given for positive effects of Pycnogenol intake on performance related variables like the rating of perceived exertion, heart rate and blood lactate concentration at 70% peak power output 66.
In a similar randomized double-blind, placebo-controlled study, 15 resistance trained subjects in their twenties took a single dose of the same fitness drink, containing Pycnogenol as the active ingredient 65. The participants consumed the placebo or Pycnogenol drink four hours prior to a lower limb hypertrophic resistance training, consisting of six sets of 70% of the individual’s repetition maximum of back squats 65. This exercise was repeated at a second visit. The researchers found improved muscle contractile performance after the Pycnogenol drink supplementation during resistance training compared to a placebo drink. Accumulated power output after Pycnogenol intake was significantly higher by 4% compared to placebo controls. Over the course of the six sets, power and velocity during the resistance training sessions was stable after Pycnogenol intake but was reduced significantly with each set in the placebo group 65.
Another randomized double-blind, placebo-controlled study with six trained and seven untrained subjects investigated NAD+/NADH levels after continuous progressive exercise and after having taken the fitness drink with Pycnogenol or placebo 67. The training setup was comparable to the cycling training in the study of Bentley et al. 66. The physical work capacity until fatigue was increased by 17% in the Pycnogenol® group compared to placebo. In addition, serum NAD+ levels were increased significantly with the Pycnogenol drink compared to placebo drink in trained and untrained subjects 67.
These results confirm the antioxidant properties of Pycnogenol and its protection against the high post-exercise oxidative stress 21.
There are several underlying mechanisms of Pycnogenol to explain these effects. During exercise, heart rate, blood flow and ventilation rapidly accelerate, and to do so, the cardio-pulmonary system adjusts to allow more nutrients, energy and oxygen to muscle tissues and avoid anaerobic build-up of lactic acid 21. Sufficient muscle oxygenation is facilitated by relaxed blood vessels and improved blood flow, which warrants aerobic energy generation. This increase in oxygen supply translates into acute oxidative stress due to higher production of free radicals in blood and muscles 124. Pycnogenol was shown to have potent antioxidant efficacy and protects lipids, DNA and proteins from oxidation within the body in addition to its ability to increase blood antioxidant capacity 21.
Another property of Pycnogenol explaining its effect of increased performance is the improved tissue perfusion by enhancing microcirculation 43 and its effects on endothelial function 37, 36, 35, 34, 33, 32, 22 contributing to improved blood flow by relaxing blood vessels.
Pycnogenol dosage
Typically, preparations of Pycnogenol are orally ingested in form of capsules or tablets. Pycnogenol can also be added to drinking solutions 125. Furthermore, a skin application has been explored 126. Daily Pycnogenol oral doses of 30–360 mg have been investigated in clinical studies 127.
The following doses have been studied in scientific research:
Adults
By mouth:
- Allergies: 50 mg of a standardized maritime pine bark extract has been used twice daily starting 5 weeks before allergy season.
- Asthma: 1 mg of a standardized maritime pine bark extract per pound of body weight, up to a maximum of 200 mg/day, has been given in two divided doses for one month. Also, 50 mg of the same extract has been used twice daily for 6 months.
- Athletic performance: 100-200 mg a standardized maritime pine bark extract has been used daily for 1-2 months.
- Circulation problems: 45-360 mg of a standardized maritime pine bark extract has been taken daily in up to three divided doses for 3-12 weeks.
- Improving mental function: 150 mg of a standardized maritime pine bark extract has been used daily for 3 months.
- Diseases of the retina in the eye: 50 mg of a standardized maritime pine bark extract has been used three times daily for 2 months.
- Photoaged skin: 40 to 100 mg/day pine bark extract (Flavangenol®) for 12 up to 24 weeks
Children
By mouth:
- Asthma: 1 mg of a standardized maritime pine bark extract per pound of body weight has been taken in two divided doses for 3 months by children and adolescents aged 6-18 years.
Pycnogenol side effects
Pycnogenol is possibly safe when taken by mouth in doses of 50 mg to 450 mg daily for up to one year, and when applied to the skin as a cream for up to 7 days or as a powder for up to 6 weeks. Pycnogenol may can cause dizziness, stomach problems, headache, mouth sores, and bad breath.
Based on data from 70 human clinical studies on 5723 healthy subjects and patients, the overall frequency of adverse side effects due to Pycnogenol is very low (1.8%) and unrelated to dose or duration of use 1. The majority of adverse effects observed are mild. Gastrointestinal discomfort, the most frequently occurring adverse effect, may be avoided by taking Pycnogenol with or after meals. In children with ADHD, 2 of 41 Pycnogenol-supplemented participants experienced side effects (rise of slowness and moderate gastric discomfort). Pycnogenol did not cause any significant changes in blood pressure or heart rate in four clinical studies (total n = 185). There have been no reports of serious adverse effects since its introduction into the European market around 1970 128. Safety trials have demonstrated the absence of mutagenic and teratogenic effects, no perinatal toxicity and no negative effects on fertility 129. Therefore, the use of Pycnogenol in children is considered to be safe 1.
Special precautions and warnings
Pregnancy and breast-feeding: Early research suggests that a standardized extract of maritime pine bark (Pycnogenol, Horphag Research) is possibly safe when used in late pregnancy. However, until more is known, it should be used cautiously or avoided by women who are pregnant.
There is not enough reliable information about the safety of taking maritime pine products if you are breast-feeding. Stay on the safe side and avoid use.
Children: A standardized extract of maritime pine bark (Pycnogenol, Horphag Research) is possibly safe when taken by mouth, short-term.
“Auto-immune diseases” such as multiple sclerosis (MS), lupus (systemic lupus erythematosus, SLE), rheumatoid arthritis (RA), or other conditions: Maritime pine might cause the immune system to become more active, and this could increase the symptoms of auto-immune diseases. If you have one of these conditions, it’s best to avoid using maritime pine..
Bleeding conditions: In theory, high doses of maritime pine might increase the risk of bleeding in people with bleeding conditions.
Diabetes: In theory, high doses of maritime pine might decrease blood sugar too much in people with diabetes.
Hepatitis: In theory, taking maritime pine might worsen liver function in people with hepatitis.
Surgery: Maritime pine might slow blood clotting and reduce blood sugar. There is some concern that it might cause blood sugar to go too low and increase the chance of bleeding during and after surgery. Stop using maritime pine at least 2 weeks before a scheduled surgery.
Interactions with medications
Moderate
Be cautious with this combination.
Medications for diabetes (Antidiabetes drugs)
Maritime pine might decrease blood sugar levels. Diabetes medications are also used to lower blood sugar. Taking maritime pine along with diabetes medications might cause your blood sugar to be too low. Monitor your blood sugar closely. The dose of your diabetes medication might need to be changed.
Some medications used for diabetes include glimepiride (Amaryl), glyburide (DiaBeta, Glynase PresTab, Micronase), insulin, pioglitazone (Actos), rosiglitazone (Avandia), and others. The mechanism of action is unclear.
Medications that decrease the immune system (Immunosuppressants)
Maritime pine seems to increase the immune system. By increasing the immune system, maritime pine might decrease the effectiveness of medications that decrease the immune system.
Some medications that decrease the immune system include azathioprine (Imuran), basiliximab (Simulect), cyclosporine (Neoral, Sandimmune), daclizumab (Zenapax), muromonab-CD3 (OKT3, Orthoclone OKT3), mycophenolate (CellCept), tacrolimus (FK506, Prograf), sirolimus (Rapamune), prednisone (Deltasone, Orasone), corticosteroids (glucocorticoids), and others.
Medications that slow blood clotting (Anticoagulant / Antiplatelet drugs)
Maritime pine might slow blood clotting. Taking maritime pine along with medications that also slow clotting might increase the chances of bruising and bleeding.
Some medications that slow blood clotting include aspirin, clopidogrel (Plavix), dalteparin (Fragmin), enoxaparin (Lovenox), heparin, ticlopidine (Ticlid), warfarin (Coumadin), and others.
Interactions with herbs and supplements
Herbs and supplements that might lower blood sugar
Maritime pine might lower blood glucose levels. Using it with other herbs or supplements that have the same effect might cause blood sugar levels to drop too low. Some herbs and supplements that can lower blood sugar include alpha-lipoic acid, chromium, devil’s claw, fenugreek, garlic, guar gum, horse chestnut, Panax ginseng, psyllium, Siberian ginseng, and others.
Herbs and supplements that might slow blood clotting
Using maritime pine along with herbs that can slow blood clotting could increase the risk of bleeding in some people. These herbs include angelica, clove, danshen, garlic, ginger, ginkgo, Panax ginseng, and others.
- Verlaet AAJ, Ceulemans B, Verhelst H, et al. Effect of Pycnogenol® on attention-deficit hyperactivity disorder (ADHD): study protocol for a randomised controlled trial. Trials. 2017;18:145. doi:10.1186/s13063-017-1879-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5370458/[↩][↩][↩][↩]
- Oliff H. Scientific and clinical monograph for Pycnogenol. Austin, TX: American Botanical Council; (2019). p. 1–46.[↩]
- Lee EL, Barnes J. Pine bark. J Prim Health Care. 2023 Jun;15(2):192-194. doi: 10.1071/HC23064[↩][↩]
- Nattagh-Eshtivani E, Gheflati A, Barghchi H, Rahbarinejad P, Hachem K, Shalaby MN, et al. The role of Pycnogenol in the control of inflammation and oxidative stress in chronic diseases: Molecular aspects. Phytother Res. (2022) 36:2352–74. 10.1002/ptr.7454[↩][↩]
- D’Andrea G. Pycnogenol: A blend of procyanidins with multifaceted therapeutic applications? Fitoterapia. (2010) 81:724–36. 10.1016/j.fitote.2010.06.011[↩]
- Grimm T, Skrabala R, Chovanova Z, Muchova J, Sumegova K, Liptakova A, et al. Single and multiple dose pharmacokinetics of maritime pine bark extract (pycnogenol) after oral administration to healthy volunteers. BMC Clin Pharmacol. 2006;6:4. doi: 10.1186/1472-6904-6-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1559639/[↩][↩]
- Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002;40:158–168.[↩][↩]
- A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Rohdewald P. Int J Clin Pharmacol Ther. 2002 Apr; 40(4):158-68.[↩][↩]
- Rohdewald PA. Review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002;40:158–168. doi: 10.5414/CPP40158.[↩]
- Uhlenhut K, Högger P. Facilitated cellular uptake and suppression of inducible nitric oxide synthase by a metabolite of maritime pine bark extract (Pycnogenol) Free Radic Biol Med. 2012;53:305–313. doi: 10.1016/j.freeradbiomed.2012.04.013[↩]
- Cho KJ, Yun CH, Yoon DY, Cho YS, Rimbach G, Packer L, et al. Effect of bioflavonoids extracted from the bark of Pinus Maritima on proinflammatory cytokine interleukin-1 production in lipopolysaccharide-stimulated RAW 264.7. Toxicol Appl Pharmacol. 2000;168:64–71. doi: 10.1006/taap.2000.9001[↩]
- Peng YJ, Lee CH, Wang CC, Salter DM, Lee HS. Pycnogenol attenuates the inflammatory and nitrosative stress on joint inflammation induced by urate crystals. Free Radic Biol Med. 2012;52:765–774. doi: 10.1016/j.freeradbiomed.2011.12.003[↩]
- A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002 Apr;40(4):158-68. https://www.ncbi.nlm.nih.gov/pubmed/11996210/[↩][↩][↩][↩][↩][↩][↩][↩]
- Treatment of osteoarthritis with Pycnogenol. The SVOS (San Valentino Osteo-arthrosis Study). Evaluation of signs, symptoms, physical performance and vascular aspects. Belcaro G, Cesarone MR, Errichi S, Zulli C, Errichi BM, Vinciguerra G, Ledda A, Di Renzo A, Stuard S, Dugall M, Pellegrini L, Errichi S, Gizzi G, Ippolito E, Ricci A, Cacchio M, Cipollone G, Ruffini I, Fano F, Hosoi M, Rohdewald P. Phytother Res. 2008 Apr; 22(4):518-23.[↩]
- Karaçelik AA, Şeker ME, Karaköse M. Determination of antioxidant activity of different extracts from bark of Pinus spp. grown in Giresun (Turkey) province – phenolic analysis by RP-HPLC-DAD. KSU J Agric Nat. (2022) 25:10–8. 10.18016/ksutarimdoga.vi.875313[↩]
- Dridi W, Bordenave N. Pine bark phenolic extracts, current uses, and potential food applications: A review. Curr Pharm Des. (2020) 26:1866–79. 10.2174/1381612826666200212113903[↩]
- Li YY, Feng J, Zhang XL, Cui YY. Pine bark extracts: Nutraceutical, pharmacological, and toxicological evaluation. J Pharmacol Exp Ther. (2015) 353:9–16. 10.1124/jpet.114.220277[↩]
- Direct assessment by electron spin resonance spectroscopy of the antioxidant effects of French maritime pine bark extract in the maxillofacial region of hairless mice. Yoshida A, Yoshino F, Tsubata M, Ikeguchi M, Nakamura T, Lee MC. J Clin Biochem Nutr. 2011 Sep; 49(2):79-86. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3171680/[↩]
- Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. (2002) 40:158–68. 10.5414/CPP40158[↩]
- The United States Pharmacopeia. USP monograph. Maritime pine extract. (Vol. 39). North Bethesda, MD: The United States Pharmacopeia; (2016). p. 6748–9.[↩]
- Weichmann F, Rohdewald P. Pycnogenol® French maritime pine bark extract in randomized, double-blind, placebo-controlled human clinical studies. Front Nutr. 2024 May 2;11:1389374. doi: 10.3389/fnut.2024.1389374[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Enseleit F, Sudano I, Periat D, Winnik S, Wolfrum M, Flammer AJ, et al. Effects of Pycnogenol on endothelial function in patients with stable coronary artery disease: A double-blind, randomized, placebo-controlled, cross-over study. Eur Heart J. (2012) 33:1589–97. 10.1093/eurheartj/ehr482[↩][↩][↩][↩][↩]
- Devaraj S, Vega-Lopez S, Kaul N, Schonlau F, Rohdewald P, Jialal I. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters plasma lipoprotein profile. Lipids. (2002) 37:931–4. 10.1007/s11745-006-0982-3[↩][↩]
- D̆uračková Z, Trebatický B, Novotný V, Žitňanová I, Breza J. Lipid metabolism and erectile function improvement by Pycnogenol®, extract from the bark of Pinus pinaster in patients suffering from erectile dysfunction-a pilot study. Nutr Res. (2003) 23:1189–98. 10.1016/S0271-5317(03)00126-X[↩][↩][↩]
- Yang HM, Liao MF, Zhu SY, Liao MN, Rohdewald P. A randomised, double-blind, placebo-controlled trial on the effect of Pycnogenol on the climacteric syndrome in peri-menopausal women. Acta Obstet Gynecol Scand. (2007) 86:978–85. 10.1080/00016340701446108[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Ryan J, Croft K, Mori T, Wesnes K, Spong J, Downey L, et al. An examination of the effects of the antioxidant Pycnogenol® on cognitive performance, serum lipid profile, endocrinological and oxidative stress biomarkers in an elderly population. J Psychopharmacol. (2008) 22:553–62. 10.1177/0269881108091584[↩][↩][↩]
- Chovanova Z, Muchova J, Sivonova M, Dvorakova M, Zitnanova I, Waczulikova I, et al. Effect of polyphenolic extract, Pycnogenol, on the level of 8-oxoguanine in children suffering from attention deficit/hyperactivity disorder. Free Radic Res. (2006) 40:1003–10. 10.1080/10715760600824902[↩][↩][↩]
- Canali R, Comitato R, Schonlau F, Virgili F. The anti-inflammatory pharmacology of Pycnogenol in humans involves COX-2 and 5-LOX mRNA expression in leukocytes. Int Immunopharmacol. (2009) 9:1145–9. 10.1016/j.intimp.2009.06.001[↩][↩][↩]
- Grimm T, Chovanova Z, Muchova J, Sumegova K, Liptakova A, Durackova Z, et al. Inhibition of NF-kappaB activation and MMP-9 secretion by plasma of human volunteers after ingestion of maritime pine bark extract (Pycnogenol). J Inflamm. (2006) 3:1. 10.1186/1476-9255-3-1[↩][↩][↩]
- Grimm T, Schäfer A, Högger P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (Pycnogenol). Free Radic Biol Med. (2004) 36:811–22. 10.1016/j.freeradbiomed.2003.12.017[↩][↩][↩]
- Schäfer A, Chovanova Z, Muchova J, Sumegova K, Liptakova A, Durackova Z, et al. Inhibition of COX-1 and COX-2 activity by plasma of human volunteers after ingestion of French maritime pine bark extract (Pycnogenol). Biomed Pharmacother. (2005) 60:5–9. 10.1016/j.biopha.2005.08.006[↩][↩][↩]
- Fitzpatrick DF, Bing B, Rohdewald P. Endothelium-dependent vascular effects of Pycnogenol. J Cardiovasc Pharmacol. (1998) 32:509–15. 10.1097/00005344-199810000-00001[↩][↩]
- Liu X, Wei J, Tan F, Zhou S, Wurthwein G, Rohdewald P. Pycnogenol, French maritime pine bark extract, improves endothelial function of hypertensive patients. Life Sci. (2004) 74:855–62. 10.1016/j.lfs.2003.07.037[↩][↩][↩]
- Nishioka K, Hidaka T, Nakamura S, Umemura T, Jitsuiki D, Soga J, et al. Pycnogenol, French maritime pine bark extract, augments endothelium-dependent vasodilation in humans. Hypertens Res. (2007) 30:775–80. 10.1291/hypres.30.775[↩][↩][↩]
- Zibadi S, Rohdewald PJ, Park D, Watson RR. Reduction of cardiovascular risk factors in subjects with type 2 diabetes by Pycnogenol supplementation. Nutr Res. (2008) 28:315–20. 10.1016/j.nutres.2008.03.003[↩][↩][↩]
- Uhlenhut K, Högger P. Facilitated cellular uptake and suppression of inducible nitric oxide synthase by a metabolite of maritime pine bark extract (Pycnogenol). Free Radic Biol Med. (2012) 53:305–13. 10.1016/j.freeradbiomed.2012.04.013[↩][↩]
- Kurlbaum M, Mülek M, Högger P. Facilitated uptake of a bioactive metabolite of maritime pine bark extract (Pycnogenol) into human erythrocytes. PLoS One. (2013) 8:e63197. 10.1371/journal.pone.0063197[↩][↩]
- Marini A, Grether-Beck S, Jaenicke T, Weber M, Burki C, Formann P, et al. Pycnogenol(R) effects on skin elasticity and hydration coincide with increased gene expressions of collagen type I and hyaluronic acid synthase in women. Skin Pharmacol Physiol. (2012) 25:86–92. 10.1159/000335261[↩][↩][↩]
- Grether-Beck S, Marini A, Jaenicke T, Krutmann J. French maritime pine bark extract (Pycnogenol(R[↩][↩][↩][↩][↩]
- Trebaticky B, Muchova J, Ziaran S, Bujdak P, Breza J, Durackova Z. Natural polyphenols improve erectile function and lipid profile in patients suffering from erectile dysfunction. Bratisl Med J. (2019) 120:941–4. 10.4149/BLL_2019_158[↩]
- Liu X, Wei J, Tan F, Zhou S, Wurthwein G, Rohdewald P. Antidiabetic effect of Pycnogenol® French maritime pine bark extract in patients with diabetes type II. Life Sci. (2004) 75:2505–13. 10.1016/j.lfs.2003.10.043[↩]
- Hosseini S, Lee J, Sepulveda RT, Rohdewald P, Watson RR. A randomized, double-blind, placebo-controlled, prospective, 16 week crossover study to determine the role of Pycnogenol in modifying blood pressure in mildly hypertensive patients. Nutr Res. (2001) 21:1251–60. 10.1016/S0271-5317(01)00342-6[↩]
- Wang S, Tan D, Zhao Y, Gao G, Gao X, Hu L. The effect of Pycnogenol® on the microcirculation, platelet function and ischaemic myocardium in patients with coronary artery diseases. Eur Bull Drug Res. (1999) 7:19–25.[↩][↩][↩]
- Arcangeli P. Pycnogenol in chronic venous insufficiency. Fitoterapia. (2000) 71:236–44. 10.1016/S0367-326X(99)00164-1[↩]
- Petrassi C, Mastromarino A, Spartera C. Pycnogenol in chronic venous insufficiency. Pyctomedicine. (2000) 7:383–8. 10.1016/S0944-7113(00)80059-8[↩]
- Weyns A-S, Verlaet AAJ, Breynaert A, Naessens T, Fransen E, Verhelst H, et al. Clinical investigation of French maritime pine bark extract on attention-deficit hyperactivity disorder as compared to methylphenidate and placebo: Part 1: Efficacy in a randomised trial. J Funct Foods. (2022) 97:105246. 10.1016/j.jff.2022.105246[↩]
- Weyns A-S, Verlaet AAJ, Van Herreweghe M, Breynaert A, Fransen E, De Meester I, et al. Clinical investigation of French maritime pine bark extract on attention-deficit hyperactivity disorder as compared to methylphenidate and placebo: Part 2: Oxidative stress and immunological modulation. J Funct Foods. (2022) 97:105247. 10.1016/j.jff.2022.105247[↩]
- Donovan EK, Kekes-Szabo S, Lin JC, Massey RL, Cobb JD, Hodgin KS, et al. A placebo-controlled, pseudo-randomized, crossover trial of botanical agents for gulf war illness: Curcumin (Curcuma longa), Boswellia (Boswellia serrata), and French maritime pine bark (Pinus pinaster). Int J Environ Res Public Health. (2021) 18:2468. 10.3390/ijerph18052468[↩]
- Dvorakova M, Jezova D, Blazicek P, Trebaticka J, Skodacek I, Suba J, et al. Urinary catecholamines in children with attention deficit hyperactivity disorder (ADHD): Modulation by a polyphenolic extract from pine bark (Pycnogenol). Nutr Neurosci. (2007) 10:151–7. 10.1080/09513590701565443[↩]
- Dvorakova M, Sivonova M, Trebaticka J, Skodacek I, Waczulikova I, Muchova J, et al. The effect of polyphenolic extract from pine bark, Pycnogenol on the level of glutathione in children suffering from attention deficit hyperactivity disorder (ADHD). Redox Rep. (2006) 11:163–72. 10.1179/135100006X116664[↩]
- Trebaticka J, Kopasova S, Hradecna Z, Cinovsky K, Skodacek I, Suba J, et al. Treatment of ADHD with French maritime pine bark extract, Pycnogenol. Eur Child Adolesc Psychiatry. (2006) 15:329–35. 10.1007/s00787-006-0538-3[↩]
- Belcaro G, Cesarone MR, Errichi S, Zulli C, Errichi BM, Vinciguerra G, et al. Treatment of osteoarthritis with Pycnogenol. The SVOS (San Valentino Osteo-arthrosis Study). Evaluation of signs, symptoms, physical performance and vascular aspects. Phytother Res. (2008) 22:518–23. 10.1002/ptr.2376[↩]
- Cisar P, Jany R, Waczulikova I, Sumegova K, Muchova J, Vojtassak J, et al. Effect of pine bark extract (Pycnogenol) on symptoms of knee osteoarthritis. Phytother Res. (2008) 22:1087–92. 10.1002/ptr.2461[↩]
- Farid R, Mirfeizi Z, Mirheidari M, Rezaieyazdi Z, Mansouri H, Esmaelli H, et al. Pycnogenol supplementation reduces pain and stiffness and improves physical function in adults with knee osteoarthritis. Nutr Res. (2007) 27:692–7. 10.1016/j.nutres.2007.09.007[↩]
- Cai C, Zeng B, Lin L, Zheng M, Burki C, Grether-Beck S, et al. An oral French maritime pine bark extract improves hair density in menopausal women: A randomized, placebo-controlled, double blind intervention study. Health Sci Rep. (2023) 6:e1045. 10.1002/hsr2.1045[↩][↩][↩][↩][↩][↩][↩]
- Zhao H, Wu J, Wang N, Grether-Beck S, Krutmann J, Wei L. Oral Pycnogenol(R) intake benefits the skin in urban Chinese outdoor workers: A randomized, placebo-controlled, double-blind, and crossover Intervention study. Skin Pharmacol Physiol. (2021) 56:1–11. 10.1159/000514323[↩][↩][↩][↩]
- Steigerwalt R, Belcaro G, Cesarone MR, Di Renzo A, Grossi MG, Ricci A, et al. Pycnogenol improves microcirculation, retinal edema, and visual acuity in early diabetic retinopathy. J Ocul Pharmacol Ther. (2009) 25:537–40. 10.1089/jop.2009.0023[↩][↩][↩][↩][↩][↩]
- Spadea L, Balestrazzi E. Treatment of vascular retinopathies with Pycnogenol. Phytother Res. (2001) 15:219–23. 10.1002/ptr.853[↩][↩][↩][↩]
- Kohama T, Negami M. Effect of low-dose French maritime pine bark extract on climacteric syndrome in 170 perimenopausal women: a randomized, double-blind, placebo-controlled trial. J Reprod Med. 2013 Jan-Feb;58(1-2):39-46.[↩][↩][↩][↩][↩][↩]
- Suzuki N, Uebaba K, Kohama T, Moniwa N, Kanayama N, Koike K. French maritime pine bark extract significantly lowers the requirement for analgesic medication in dysmenorrhea: a multicenter, randomized, double-blind, placebo-controlled study. J Reprod Med. 2008 May;53(5):338-46.[↩][↩][↩][↩][↩]
- Wilson D, Evans M, Guthrie N, Sharma P, Baisley J, Schonlau F, et al. A randomized, double-blind, placebo-controlled exploratory study to evaluate the potential of Pycnogenol for improving allergic rhinitis symptoms. Phytother Res. (2010) 24:1115–9. 10.1002/ptr.3232[↩][↩][↩][↩][↩]
- Lau BH, Riesen SK, Truong KP, Lau EW, Rohdewald P, Barreta RA. Pycnogenol as an adjunct in the management of childhood asthma. J Asthma. (2004) 41:825–32. 10.1081/JAS-200038433[↩][↩][↩][↩][↩]
- Hosseini SPS, Sadrzadeh SMH, Farid F, Farid R, Watson RR. Pycnogenol in the management of asthma. J Med Food. (2001) 4:201–9. 10.1089/10966200152744472[↩][↩][↩][↩][↩]
- Watanabe K, Hiramine H, Hamada N. Effects of French pine bark extract chewing gum on oral Malodor and salivary bacteria. J Nutr Sci Vitaminol. (2018) 64:185–91. 10.3177/jnsv.64.185[↩][↩][↩]
- Ackerman J, Clifford T, McNaughton LR, Bentley DJ. The effect of an acute antioxidant supplementation compared with placebo on performance and hormonal response during a high volume resistance training session. J Int Soc Sports Nutr. (2014) 11:10. 10.1186/1550-2783-11-10[↩][↩][↩][↩][↩]
- Bentley DJ, Coupland R, Midgley A, Spence I. Acute antioxidant supplementation improves endurance performance in trained athletes. Res Sports Med. (2012) 20:1–12. 10.1080/15438627.2011.608050[↩][↩][↩][↩][↩][↩]
- Mach JMA, Dank S, Grant RS, Bentley DJ. The effect of antioxidant supplementation on fatigue during exercise: Potential role for NAD+(H). Nutrients. (2010) 2:319–29. 10.3390/nu2030319[↩][↩][↩][↩]
- Pavlovic P. Improved Endurance By Use Of Antioxidants. Eur Bull Drug Res. (1999) 7:26–9.[↩][↩][↩][↩][↩]
- Lapi F, Azoulay L, Suissa S: Author’s reply to Moore. BMJ 2013;346:f1016. https://www.bmj.com/content/346/bmj.f1016.long[↩]
- Grimm T, Chovanova Z, Muchova J, et al. : Inhibition of NF-κB activation and MMP-9 secretion by plasma of human volunteers after ingestion of maritime pine bark extract (Pycnogenol). J Inflamm 2006;3:1[↩][↩][↩]
- Kurlbaum M, Mülek M, Högger P: Facilitated uptake of a bioactive metabolite of maritime pine bark extract (Pycnogenol) into human erythrocytes. PLoS One 2013;8:1–10[↩]
- Mülek M, Seefried L, Genest F, et al. : Distribution of constituents and metabolites of maritime pine bark extract (Pycnogenol) into serum, blood cells, and synovial fluid of patients with severe osteoarthritis: A randomized controlled trial. Nutrients 2017;9:443[↩][↩]
- Wojdasiewicz P, Poniatowski LA, Szukiewicz D: The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm 2014;2014:561459[↩]
- Daheshia M, Yao JQ: The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol 2008;35:2306–2312[↩]
- Jessberger S, Högger P, Genest F, Salter DM, Seefried L. Cellular pharmacodynamic effects of Pycnogenol® in patients with severe osteoarthritis: a randomized controlled pilot study. BMC Complementary and Alternative Medicine. 2017;17:537. doi:10.1186/s12906-017-2044-1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5732384/[↩]
- Belcaro G, Cesarone MR, Errichi S, Zulli C, Errichi BM, Vinciguerra G, et al. : Variations in C-reactive protein, plasma free radicals and fibrinogen values in patients with osteoarthritis treated with Pycnogenol®. Redox Rep 2008;13:271–276[↩]
- Belcaro G, Cesarone MR, Errichi S, et al. : Treatment of osteoarthritis with Pycnogenol®. The SVOS (San Valentino Osteoarthritis Study). Evaluation of signs, symptoms, physical performance and vascular aspects. Phytother Res 2008;22:518–523[↩]
- Farid R, Mirfeizi Z, Mirheidari M, et al. : Pycnogenol supplementation reduces pain and stiffness and improves physical function in adults with knee arthritis. Nutr Res 2007;27:692–697[↩][↩][↩]
- Cisar P, Jany R, Waczulikova I, et al. : Effect of pine bark extract (Pycnogenol®) on symptoms of knee osteoarthritis. Phytother Res 2008;22:1087–1092[↩]
- Cisar P, Jany R, Waczulikova I, et al. : Effect of pine bark extract (Pycnogenol®) on symptoms of knee osteoarthritis. Phytother Res 2008;22:1087–1092 https://www.ncbi.nlm.nih.gov/pubmed/18570266[↩][↩]
- Belcaro G, Cesarone MR, Errichi S, et al. : Treatment of osteoarthritis with Pycnogenol®. The SVOS (San Valentino Osteoarthritis Study). Evaluation of signs, symptoms, physical performance and vascular aspects. Phytother Res 2008;22:518–523 https://www.ncbi.nlm.nih.gov/pubmed/18386255[↩][↩][↩][↩][↩][↩][↩]
- Rohdewald P: Update on the clinical pharmacology of Pycnogenol®. Med Res Arch 2015;3:1–11[↩]
- Rohdewald PJ. Review on Sustained Relief of Osteoarthritis Symptoms with a Proprietary Extract from Pine Bark, Pycnogenol. Journal of Medicinal Food. 2018;21(1):1-4. doi:10.1089/jmf.2017.0015. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5775113/[↩]
- Ceylan M, et al. Changes in oxidative stress and cellular immunity serum markers in attention-deficit/hyperactivity disorder. Psychiatry Clin Neurosci. 2012;66:220–226. doi: 10.1111/j.1440-1819.2012.02330.x[↩][↩][↩]
- Kawatani M, Tsukahara H, Mayumi M. Evaluation of oxidative stress status in children with pervasive developmental disorder and attention deficit hyperactivity disorder using urinary-specific biomarkers. Redox Rep. 2011;16(1):45–46. doi: 10.1179/174329211X12968219310873[↩]
- Pelsser LMJ, Buitelaar JK, Savelkoul HFJ. ADHD as a (non) allergic hypersensitivity disorder: a hypothesis. Pediatr Allergy Immunol. 2009;20(2):107–112. doi: 10.1111/j.1399-3038.2008.00749.x[↩][↩]
- Antshel KM, et al. Advances in understanding and treating ADHD. BMC Med. 2011;9:72. doi: 10.1186/1741-7015-9-72.[↩][↩][↩]
- Storebø OJ, et al. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ. 2015;351:h5203. doi: 10.1136/bmj.h5203[↩]
- Tenenbaum S, et al. An experimental comparison of Pycnogenol and methylphenidate in adults with attention-deficit/hyperactivity disorder (ADHD) J Atten Disord. 2002;6:49–60. doi: 10.1177/108705470200600201 https://www.ncbi.nlm.nih.gov/pubmed/12142861[↩]
- Booijink CC, et al. High temporal and inter-individual variation detected in the human ileal microbiota. Environ Microbiol. 2010;12(12):3213–3227. doi: 10.1111/j.1462-2920.2010.02294.x[↩]
- Treatment of ADHD with French maritime pine bark extract, Pycnogenol. Trebatická J, Kopasová S, Hradecná Z, Cinovský K, Skodácek I, Suba J, Muchová J, Zitnanová I, Waczulíková I, Rohdewald P, Duracková Z. Eur Child Adolesc Psychiatry. 2006 Sep; 15(6):329-35.[↩]
- Saliou C, Rimbach G, Moini H, McLaughlin L, Hosseini S, Lee J, Watson RR, Packer L: Solar ultraviolet-induced erythema in human skin and nuclear factor-kappa-B-dependent gene expression in keratinocytes are modulated by a French maritime pine bark extract. Free Radic Biol Med 2001;30:154-160.[↩]
- Darvin M, Sterry W, Lademann J, Vergou T: The role of carotenoids in human skin. Molecules 2011;16:10491-10506.[↩]
- Heinrich U, Gartner C, Wiebusch M, Eichler O, Sies H, Tronnier H, Stahl W: Supplementation with beta-carotene or a similar amount of mixed carotenoids protects humans from UV-induced erythema. J Nutr 2003;133:98-101.[↩]
- Grether-Beck S, Marini A, Jaenicke T, Krutmann J: Effect of nutritional lycopene on UV induced gene expression in human skin in vivo. Unpublished study.[↩]
- Kyriazi M, Yova D, Rallis M, Lima A: Cancer chemopreventive effects of Pinus maritima bark extract on ultraviolet radiation and ultraviolet radiation-7,12-dimethylbenz(a)anthracene induced skin carcinogenesis of hairless mice. Cancer Lett 2006;237:234-241.[↩]
- Haarmann-Stemmann T, Esser C, Krutmann J: The Janus faced role of aryl hydrocarbon receptor (AHR) signaling in skin: consequences for prevention and treatment of skin disorders. J Invest Dermatol 2015, Epub ahead of print.[↩]
- Palermo CM, Hernando JI, Dertinger SD, Kende AS, Gasiewicz TA: Identification of potential aryl hydrocarbon receptor antagonists in green tea. Chem Res Toxicol 2003;16:865-872.[↩]
- Ayres EL, Silva JDS, Eberlin S, Facchini G, Vasconcellos C, Costa A. In-vitro effect of pine bark extract on melanin synthesis, tyrosinase activity, production of endothelin-1 and PPAR in cultured melanocytes exposed to ultraviolet, infrared, and visible light radiation. J Cosmet Dermatol. (2021) 21:1234–42. 10.1111/jocd.14202[↩][↩][↩]
- Furumura M, Sato N, Kusaba N, Takagaki K, Nakayama J. Oral administration of French maritime pine bark extract (Flavangenol®) improves clinical symptoms in photoaged facial skin. Clinical Interventions in Aging. 2012;7:275-286. doi:10.2147/CIA.S33165. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3426262/[↩][↩][↩][↩][↩]
- Chiu AE, Chan JL, Kern DG, Kohler S, Rehmus WE, Kimball AB. Double-blinded, placebo-controlled trial of green tea extracts in the clinical and histologic appearance of photoaging skin. Dermatol Surg. 2005;31(7 Pt 2):855–860.[↩]
- Goh SH. The treatment of visible signs of senescence: the Asian experience. Br J Dermatol. 1990;122(Suppl 35):105–109.[↩]
- Belcaro G, Cesarone MR, Ricci A, et al. Control of edema in hypertensive subjects treated with calcium antagonist (nifedipine) or angiotensin-converting enzyme inhibitors with Pycnogenol. Clin Appl Thromb Hemost. 2006;12(4):440–444.[↩]
- Grimm T, Schafer A, Hogger P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (pycnogenol) Free Radic Biol Med. 2004;36(6):811–822.[↩]
- Yoshida A, Yoshino F, Tsubata M, Ikeguchi M, Nakamura T, Lee MC. Direct assessment by electron spin resonance spectroscopy of the antioxidant effects of French maritime pine bark extract in the maxillofacial region of hairless mice. J Clin Biochem Nutr. 2011;49(2):79–86.[↩]
- Shoji T, Masumoto S, Moriichi N, et al. Procyanidin trimers to pentamers fractionated from apple inhibit melanogenesis in B16 mouse melanoma cells. J Agric Food Chem. 2005;53(15):6105–6111.[↩]
- Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(Suppl 1):230S–242S[↩]
- Yamakoshi J, Sano A, Tokutake S, et al. Oral intake of proanthocyanidin-rich extract from grape seeds improves chloasma. Phytother Res. 2004;18(11):895–899[↩]
- Aoki H, Moro O, Tagami H, Kishimoto J. Gene expression profiling analysis of solar lentigo in relation to immunohistochemical characteristics. Br J Dermatol. 2007;156(6):1214–1223[↩]
- Ni Z, Mu Y, Gulati O: Treatment of melasma with Pycnogenol. Phytother Res 2002;16:567-571.[↩][↩]
- Kim YJ, Kang KS, Yokozawa T: The anti-melanogenic effect of pycnogenol by its anti-oxidative actions. Food Chem Toxicol 2008;46:2466-2471.[↩]
- Marini A, Grether-Beck S, Jaenicke T, Weber M, Burki C, Formann P, Brenden H, Schonlau F, Krutmann J: Pycnogenol® effects on skin elasticity and hydration coincide with increased gene expressions of collagen type I and hyaluronic acid synthase in women. Skin Pharmacol Physiol 2012;25:86-92.[↩]
- Grether-Beck S, Marini A, Jaenicke T, Krutmann J: An intervention study to evaluate the in vivo cosmetic effects of a dietary supplement (Pycnogenol) in postmenopausal healthy women: additional gene expression studies. 2013.[↩]
- Jux B, Kadow S, Luecke S, Rannug A, Krutmann J, Esser C: The aryl hydrocarbon receptor mediates UVB radiation-induced skin tanning. J Invest Dermatol 2011;131:203-210.[↩]
- Grether-Beck S, Marini A, Jaenicke T, Krutmann J: Photoprotection of human skin beyond ultraviolet radiation. Photodermatol Photoimmunol Photomed 2014;30:167-174.[↩]
- Krutmann J, Liu W, Li L, Pan X, Crawford M, Sore G, Seite S: Pollution and skin: from epidemiological and mechanistic studies to clinical implications. J Dermatol Sci 2014;76:163-168.[↩]
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. (2010) 376:124–36. 10.1016/S0140-6736(09)62124-3[↩]
- Torras MA, Faura CA, Schonlau F, Rohdewald P. Antimicrobial activity of Pycnogenol. Phytother Res. (2005) 19:647–8. 10.1002/ptr.1662[↩]
- Gould GS, Hurst JR, Trofor A, Alison JA, Fox G, Kulkarni MM, et al. Recognising the importance of chronic lung disease: A consensus statement from the global alliance for chronic diseases (lung diseases group). Respir Res. (2023) 24:15. 10.1186/s12931-022-02297-y[↩]
- Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. (2019) 7:246. 10.3389/fped.2019.00246[↩]
- Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. (2019) 56:219–33. 10.1007/s12016-018-8712-1[↩]
- Schoettler N, Strek ME. Recent advances in severe asthma: From phenotypes to personalized medicine. Chest. (2020) 157:516–28. 10.1016/j.chest.2019.10.009[↩]
- Barcikowska Z, Rajkowska-Labon E, Grzybowska ME, Hansdorfer-Korzon R, Zorena K. Inflammatory Markers in Dysmenorrhea and Therapeutic Options. Int J Environ Res Public Health. (2020) 17:1191. 10.3390/ijerph17041191[↩]
- Kawamura T, Muraoka I. Exercise-induced oxidative stress and the effects of antioxidant intake from a physiological viewpoint. Antioxidants (Basel). (2018) 7:119. 10.3390/antiox7090119[↩]
- Frontela C, Ros G, Martínez C, Sánchez-Siles LM, Canali R, Virgili F. Stability of Pycnogenol® as an ingredient in fruit juices subjected to in vitro gastrointestinal digestion. J Sci Food Agric. (2011) 91:286–92. 10.1002/jsfa.4183[↩]
- Sarikaki V, Rallis M, Tanojo H, Panteri I, Dotsikas Y, Loukas YL, et al. In vitro percutaneous absorption of pine bark extract (Pycnogenol) in human skin. J Toxicol. (2005) 23:149–58.[↩]
- American Botanical Council. Pycnogenol® proprietary botanical ingredient monograph. Austin, TX: American Botanical Council; (2019).[↩]
- González TJB, et al. Study of the aminoglycoside subsistence phenotype of bacteria residing in the gut of humans and zoo animals. Front Microbiol. 2015;6:1–7.[↩]
- Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002;40(4):158–168. doi: 10.5414/CPP40158[↩]