Contents
What is rhodiola rosea
Rhodiola rosea is also known as golden root, Arctic root or rose root, is a succulent herbaceous, perennial flowering plant in the family Crassulaceae 1. Rhodiola rosea grows naturally in the cooler regions of the world—the Arctic, Scandinavia, North Russia, in the mountains of Asia (the Himalayas, the Altai, and the Ural mountains) and Europe (the Alps, the Pyrenees, the Carpathian Mountains), and in other higher mountainous regions (980 to 2000 m altitude) like those in Bulgaria (the mountains of Sredna Gora, Rila, Pirin, and the Balkan) 2. Rhodiola rosea plant prefers the scree, grassy, or rocky slopes, from mountain to subalpine zone of heights up to 2280 meters altitude 3. Rhodiola rosea is a dioecious plant (having separate male or female plants) with a thick quite branched scaly rhizome (rootstock) with average weight of 70–400 g, but reaching 3.5 kg, too. The Rhodiola rosea root and rhizomes have rose scent. Several shoots grow from the same thick root. The stem is straight, 10–30 cm in height 4. The leaves are oblong, elliptic-oblanceolate, or obovate, entire. Inflorescences are corymbiform or capitates, the flowers unisexual, flowers are set from April to August. Propagation is vegetative or by seeds 5.
Figure 1. Rhodiola rosea
Historically, people in northern regions have used Rhodiola rosea for anxiety, fatigue, anemia, impotence, infections, headache, and depression related to stress. Traditional folk medicine used Rhodiola rosea to promote work endurance, increase longevity, and to promote resistance to high altitude sickness, fatigue, depression and other health conditions 6. Rhodiola rosea may enhance mood and affect via its complex effect on central biogenic amines and β-endorphins. For example, Rhodiola rosea appears to stimulate noradrenalin, serotonin, dopamine, and acetylcholine receptors in brain regions involved in mood and affect 7. In in-vitro bioassay studies, Rhodiola rosea has also been shown to inhibit monoamine oxidase A and B enzymes 8. Further, studies suggest that Rhodiola rosea may have antidepressant activity via its ability to increase endogenous β-endorphin levels while preventing stress-induced elevation of β-endorphin 9 and via its action in prolonging the ‘forced swim test’ in rats 10. Today, people use Rhodiola rosea as a dietary supplement to increase energy, stamina, and strength, to improve attention and memory, and to enhance the ability to cope with stress. Rhodiola rosea root extracts are also available in capsule or tablet form.
The chemical composition of the extracts from the Rhodiola rosea root and rhizomes was studied by East-European research groups mainly 11. A decade of investigation 12 revealed evidences about the presence of different biological active substances in the rhizome of golden root unlike some other Rhodiola species.
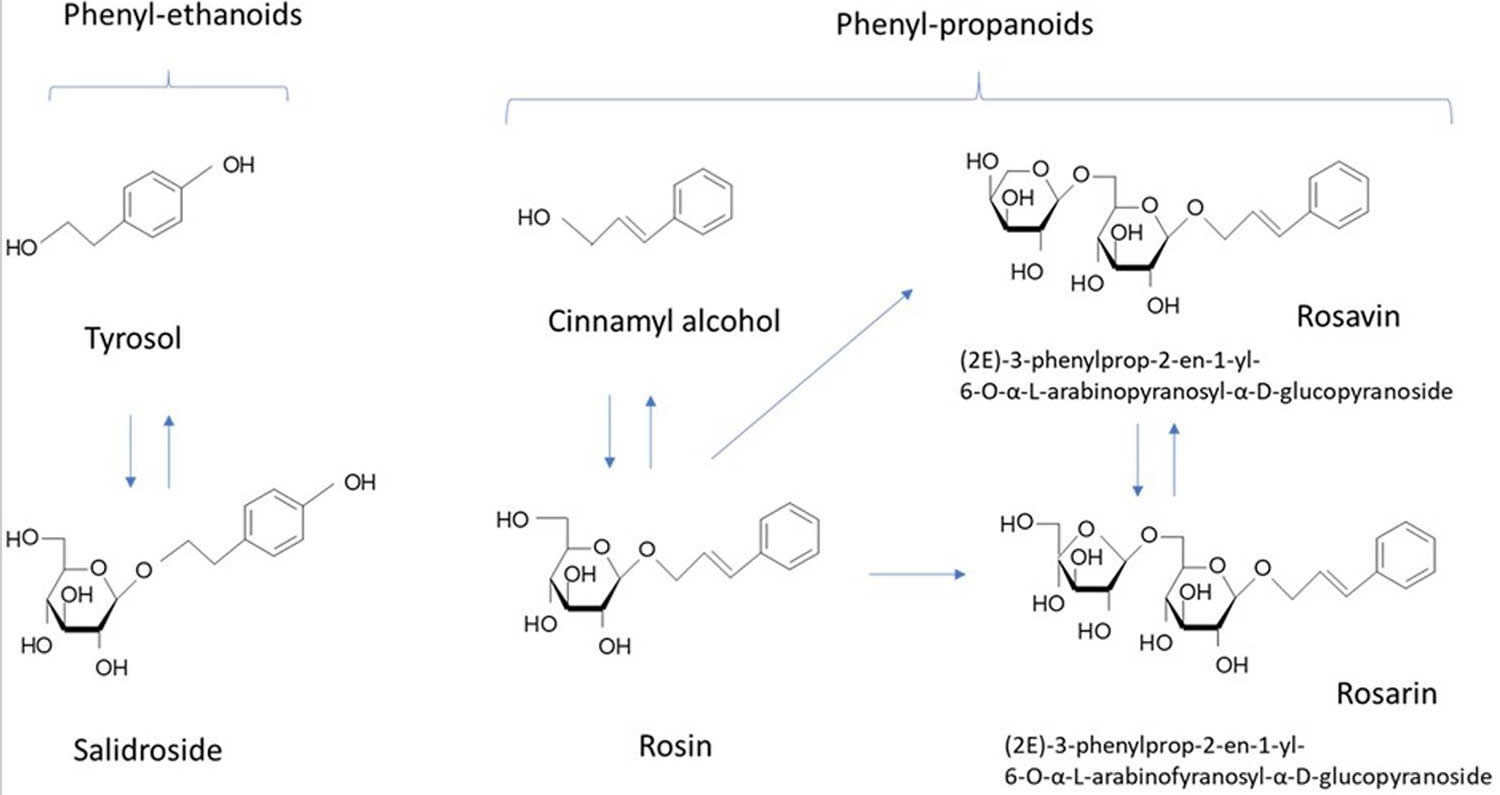
Six different groups of chemical components with pharmaceutical interest could be found in the Rhodiola rosea roots and rhizomes: (1) phenylpropanoids—alcohol derivatives of the cinnamon acid and glycosides like rosavin (2.1%) 13, rosin and rosarin (which are classified under the general name of “rosavins”) 14; (2) phenylethanoids—salidroside (rhodioloside) (0.8%), p-tyrosol15; (3) flavonoids—including rodiolin, rodionin, rodiosin, acetylrodalgin, tricin 16, and tannins 16–18% 17; (4) monoterpenes, including rosiridol and rosidarin; (5) triterpenes, such as daucosterol and betasitosterol; (6) phenolic acids such as chlorogenic, hydroxycinnamic, gallic acids 18 and essential oils 19. All these substances determine the specificity of the Rhodiola extracts. However, the roots have many other substances like phenolic antioxidant, including proanthocyanidins, quercetin, gallic acid, and chlorogenic acid 20.
Rosavins (rosavin, rosarin, and rosin) and the salidroside or rhodioloside, as well as rodiolin, rodonizid, and roziridine are the most important, and are mainly used as active substances for production of medical preparations. Tyrosol is also a crucial active ingredient; though to a less extent than the other two standards. The rosavins complex is specific for Rhodiola rosea unlike salidroside which presents in other Rhodiola species and in some plants from other genera 21.
One of the major results of the research is the detection of differences in the content of biologically active substances in the roots of Rhodiola rosea depending on their habitats 22. Investigation of Rhodiola rosea root from different Bulgarian mountains areas indicated the highest amount of salidroside of 1.55% in Rila mountain sites, and, respectively, the lowest of 0.72% in Pirin Mountain sites 23. The stem and the leaves contain less salidroside, while there is no substantial difference in the levels of polyphenols accumulation in the epigeous parts of the plant.
Rhodiola species contain a range of antioxidant compounds, including p-tyrosol, organic acids (gallic acid, caffeic acid, and chlorogenic acid), and flavonoids (catechins and proanthocyanidins) 24. The stimulating and adaptogenic properties of Rhodiola rosea are attributed to p-tyrosol, salidroside (synonym: rhodioloside and rhodosin), rhodioniside, rhodiolin, rosin, rosavin, rosarin, and rosiridin 25. Rosavin is the constituent currently selected for standardization of extracts 26. p-Tyrosol has been shown to be readily and dose-dependently absorbed after an oral dose 27; however, pharmacokinetic data on the other adaptogenic compounds found in Rhodiola rosea is unavailable.
Figure 2. Rhodiola rosea extracts bioactive compounds
[Source 28]
Rhodiola rosea benefits
Rhodiola rosea roots and rhizome extracts are active ingredients in adaptogenic herbal medicinal products and dietary supplements for temporary relief of symptoms of stress, such as fatigue and weakness 29.
There have been some studies of Rhodiola rosea in people; however, the quality of research is limited so firm conclusions about its effectiveness can’t be made.
- Two review articles—published in 2011 30 and 2012 31 — looked at 15 studies that tested Rhodiola rosea on physical and mental performance in 575 people. Both reviews found evidence that Rhodiola rosea may enhance physical performance and ease mental fatigue, but emphasized that the limited quantity, methodological flaws and quality of available evidence did not allow firm conclusions to be made.
- A small, National Center for Complementary and Integrative Health-supported study 32 tested Rhodiola rosea against the drug sertraline and a placebo in people with mild-to-moderate major depressive disorder. The patients were given either pharmaceutical grade Rhodiola rosea powdered extract 340 mg (standardized to a content of rosavin 3.07% and rhodioloside 1.95%) or sertraline 50 mg HCl. The 2015 study results showed Rhodiola rosea produced less antidepressant effect versus sertraline in reducing depressive symptoms, but people who took Rhodiola rosea had fewer side effects than those who took sertraline 32. These findings suggest that Rhodiola rosea, although less effective than sertraline, may possess a more favorable risk to benefit ratio for individuals with mild to moderate depression. However rhodiola’s effectiveness and safety for depression need testing in larger, more powerful studies.
A growing body of evidence has indicated the Rhodiola rosea extract’s potential use in the prevention and treatment of stress and age-related impairments of cognitive functions and mental disorders 33. The stimulating effects of Rhodiola rosea on the CNS (central nervous system) were demonstrated long ago and suggested there were potential benefits on cognitive functions, memory, learning, and attention 34. An active compound, named rhodioloside was isolated and identified as salidroside 35. A pilot study of rhodioloside (syn. salidroside) in 46 healthy human volunteers showed that 2.5 mg salidroside increased attention in cognitive tests 1 hour after a single dose was administered in 83% of subjects, compared with 54% of volunteers who were administered placebo 35. Further studies provided evidence that R. rosea and salidroside exhibit neuroprotective activity 36, suggesting they may be effective in treating neurodegenerative disorders, such as Alzheimer’s disease 37.
Along with salidroside and its aglycone tyrosol (Figure 2), cinnamyl alcohol, glycosides, and rosavins (collective name of rosavin, rosarin, and rosin) also exhibited stress-protective 38, stimulating, and neurotropic activities in rodents; reduced sleep induced by barbital, hexanal, and chloral hydrate in mice 39; increased locomotor activity in mice 39; and induced anti-depressant-like effects in animal models of depression 40. Salidroside is common for all species of Rhodiola, while phenylpropanoids, rosavin, rosarin, and rosin are specific only for Rhodiola rosea and Rhodiola sachalinensis 41. Many publications have reported on the neuroprotective and neurotropic activity of salidroside 36; while, there is limited evidence supporting the importance of rosavin, the major active marker 42. Rosavin was inactive is rats during a behavioral test of binge eating; while, salidroside dose-dependently reduced or abolished binge eating for the period in which it was elicited 43. In another study, salidroside was more effective than rosarin and rosin in inhibiting the expression of IL-1β, and IL-6 in microglial cells, while rosavin was not tested 36. Rosavin inhibited the expression of the TNF-related apoptosis-inducing ligand in concanavalin A activated Jurkat T cells, while salidroside was inactive and rosarin had an opposite effect 42.
It is unclear which analytical markers are important for assessing the quality and efficacy of Rhodiola rosea herbal preparations intended for treating aging-induced mild cognitive disorders, such as attenuated memory, attention, and learning ability 28. Rhodiola rosea preparations are usually standardized for salidroside (1%) and rosavin (3%). The content of active ingredients in herbal preparations depends on many factors, such as the geographic and climate zone it was grown in, which season and under what conditions it was harvested, and how it was dried, extracted, and prepared to give the final dosage form. For example, a high degree of inter-clonal variation was found for all tested constituents (salidroside, tyrosol, rosavin, rosarin, rosin, and cinnamyl alcohol) in six samples of Rhodiola rosea roots collected in various regions of Norway. The highest variation was found for salidroside and tyrosol, showing inter-clonal variations of 92.8 and 87.8%, respectively 44. Therefore, the preparations obtained by various producers can have quite different active dose levels. Furthermore, the contribution of these active markers to the overall activity of the total extracts was not systematically assessed. It was suggested that these phenolic compounds (rosavin, rosarin, rosin, salidroside/rhodioloside, and tyrosol) have no impact on activity of CYP450 enzymes and do not inhibit CYP3A4, CYP2D6, or CYP1A2 45. The presence of minor amounts of herbacetin rhamnosides (rhodiosin and rhodionin) may presumably induce inhibition of CYP2D6 46 in some commercial preparations of Rhodiola rosea 47.
It is a challenge to obtain reproducible efficacy and quality of herbal medicinal products, particularly for preparations of the underground parts of Rhodiola rosea 48. There may be unpredictable, complex interactions between the active constituents of the Rhodiola rosea extracts that affect the regulation of molecular networks playing an important role in cellular and physiological functions of human organisms 49. The pharmacological activity of Rhodiola rosea crude extract is related to many compounds, such as salidroside, tyrosol, rosavin, and other phenolic compounds 42. The batch to batch reproducible content of key active markers and the UPLC fingerprint are not a guaranty of reproducible efficacy and safety. Additional bioassays are required to assure reproducible pharmacological activity of herbal medicinal products. These bioassays may serve as validation tools for the quality assurance of complex herbal medicinal products where the total extract contains active pharmaceutical ingredients. In this context, assessment of the correlation between the content of active markers and pharmacological activity of herbal medicinal products is important. Although the dose-response relationship of salidroside and rosavin and dietary supplements was studied 42, the correlation between the content and biological activity of various commercial Rhodiola rosea extracts has not been investigated. The standardized content of active markers is necessary for the quality control of herbal preparations containing Rhodiola extracts.
The efficacy of Rhodiola rosea enthanol extract containing 3.1% rosavin, 2.1% salidroside (rhodioloside) and 4.8% rosavins was demonstrated previously on healthy subjects 50; subjects experiencing stress and fatigue 1 and patients with chronic fatigue 51 and major depressive disorder 52. In a double blind, placebo-controlled study on 20 healthy subjects, Dimpfel 50 demonstrated that a single dose administration of two capsules containing 200 mg Rhodiola rosea enthanol extract containing 3.1% rosavin, 2.1% salidroside (rhodioloside) and 4.8% rosavins changed the spectral signature of electric brain activity in a stimulating way compared with placebo. The effect was regarded as a safe booster of mental activity during cognitive and emotional challenges. Rhodiola rosea enthanol extract containing 3.7% rosavin, 3.1% salidroside (rhodioloside) and 5.1% rosavins was also studied earlier in isolated skeletal muscle cells 53, animals 54, and healthy human subjects 55. Oral administration of 100 mg/kg of Rhodiola rosea root extract led to significant attenuation of α1, α2, β1, β2, δ, and θ waves of the electropharmacograms, which are associated with the activation of dopamine, serotonin, glutamate, GABA, acetylcholine, and norepinephrine-mediated signaling pathways 54. The most affected were α2 (dopaminergic transmission – CNS stimulating effect) and β1 (glutaminergic transmission – CNS stimulating effect) in the frontal cortex. The next strongest changes were seen in the striatum, and the weakest changes in the reticular formation. Spectral changes lasted up to 4 hours after administration. A concentration of Rhodiola rosea extract 5 mg/L induced a slight increase in the amplitude of the population spike and an increase in long-term potentiation. Further increases were observed by increasing the concentration up to 30 mg/L. During theta burst stimulation, amplitudes of more than 4 mV were measured, indicating their effect on long-term potentiation.
A meta-analysis on the putative antidepressant action of Rhodiola extract revealed it was effective on major depressive disorder (146 subjects) and stress-induced mild depression (714 individuals) 56. Rosavin was not included in that study. In mice, the treatment for 5 days (12 and 24 mg/kg) with salidroside (rhodioloside) or fluoxetine prevented the development of the depression-like behavior and of the downregulation of brain-derived neurotrophic factor (BDNF) protein levels in the hippocampus induced by a single injection of lipopolysaccharide 57.
Rhodiola rosea for physical fatigue
Rhodiola rosea as single ingredient versus placebo
A three arm double blind randomized controlled trial compared the effect of Rhodiola rosea (as a single ingredient) to placebo, or nothing 58. The study examined muscle recovery in 30 adults by measuring C-reactive protein (CRP) and creatinine kinase (CK) levels in blood. Subjects underwent an exhausting physical exercise test on day 30 which consisted of cycling at 20 W on a bicycle ergometer with power increased by 10 W/min until volitional exhaustion (i.e. subject could no longer pedal at 60 rpm). Findings indicate that Rhodiola rosea significantly lowered CRP levels at 5 hours and 5 days after the test but that creatinine kinase (CK) levels were not significantly different between groups. Adverse events were not reported.
A double-blind cross-over randomized controlled trial examined the effect of Rhodiola rosea on exercise performance in twelve male subjects 59. Subjects received Rhodiola rosea or identical placebo for 3 days before outcomes were measured by an exercise test and another dose on the day of the test. A wash-out period of 7 to 14 days separated cross-over to the opposite treatment. The primary outcome was muscle recovery measured by ATP levels and secondary outcomes were time to exhaustion and perceived exertion; all outcomes were measured at baseline, during the exercise test and during recovery. There was no significant difference between groups in Pi, phosphocreatine and ATP levels, time to exhaustion and perceived exhaustion. Adverse events and drop-outs were not mentioned.
Rhodiola rosea plus starch versus starch alone
One cross-over randomized controlled trial and one controlled clinical trial described in a single report examined the acute and long-term effects, respectively, of Rhodiola rosea on exercise performance 60. In both studies, endurance capacity was the primary outcome and muscle strength, speed of limb movement, reaction time and sustained attention were secondary outcomes.
In the first study on acute effects, Rhodiola rosea combined with starch or placebo was taken on each of 2 days 60. One hour after ingestion on each day, outcomes were measured while subjects underwent a physical functioning test. After a five day washout period, subjects switched to the alternate treatment and performed the same tests. Baseline measurements were not taken. Three out of six parameters of endurance capacity (time to exhaustion, O2 uptake and CO2 output) significantly improved in the Rhodiola rosea group. There was no difference between groups in any secondary outcomes. After five days, authors stated that 12 subjects were reassigned to intervention and control groups for the long-term evaluation study. The long term study evaluated subjects receiving same intervention and control as in the acute study twice per day over a four week period 60. The same outcomes as in the acute study were measured. Long term supplementation produced no significant difference in any outcomes between treatment groups; one participant on Rhodiola rosea dropped out during long term supplementation for medical reasons unrelated to the study protocol (reason not stated). One subject with strong headaches during acute supplementation and one with minor headaches during long term supplementation were both on placebo. One subject experienced a minor headache and another had insomnia during long term supplementation of Rhodiola rosea. It is unclear why the long-term study was not randomized.
Rhodiola rosea plus cordyceps versus placebo
Two double blind randomized controlled trials conducted evaluate the effect of Rhodiola rosea combined with other herbs on exercise performance 61. Both studies were conducted by the same group of authors using slightly different protocols and populations. In both studies, intervention capsules were described as every 3 capsules containing 300 mg of Rhodiola rosea (standardized to 3.0% rosavins and 2.5% salidrosidesminimum), 1000 mg of Cordyceps sinensis, a Chinese herb reported to improve circulation 62, and 800 mg of the manufacturers ‘proprietary blend’ of substances (undisclosed).
In one of the randomized controlled trials, 17 male were randomly assigned to either the Rhodiola rosea-containing formulation or placebo for 15 days 61. Subjects took six capsules per day for 4 days (loading dose) then three capsules per day 11 days (maintenance dose). Endurance capacity was measured by multiple parameters including peak CO2 output, power output, time to exhaustion and peak heart rate, which were measured at the beginning and end of the study period. The authors found that the herbal formulation did not have any significant effect on exercise endurance or capacity. Adverse events were not reported.
The second study involving eight male cyclists randomized to either Rhodiola rosea-containing formulation (33.0 ± 12.6) years) or placebo (23.8 ± 2.9 years), followed the same protocol as above, however the study period was only 13 days – 6 days of the loading dose and 7 days of the maintenance dose 63. Respiratory parameters were measured in the participants. This study also found no significant difference in outcomes between groups. There were no drop outs; adverse events were not mentioned.
Rhodiola rosea for mental fatigue
A double blinded randomized controlled trial assessed the efficacy of a Rhodiola rosea extract, 3.1% rosavin, 2.1% salidroside and 4.8% rosavins, for stress related fatigue 64. Sixty subjects were randomized to receive 576 mg of Rhodiola rosea preparation or placebo per day for 28 days. Mental fatigue, measured by the Pines burnout scale, was the primary outcome. Other outcomes evaluated were depression (Montgomery-Asberg Depression Rating Scale, MADRS), quality of life (Medical Outcomes Study Short form 36-item questionnaire, SF-36), attention (Conners’ Computerized Continuous Performance Test II, CCCPT II) and “anti-fatigue” effect (saliva cortisol response after awakening). All outcomes were measured before and after the treatment period. The Pines burnout scale scores and two out of five indices of CCCPT II improved in favour or Rhodiola rosea. While investigators conclude that the treatment appears to have beneficial effect, they report excluding follow-up data for at least 5 participants due to physical loss of data and protocol deviations. Per-protocol analyses (i.e. analysis of only participants who followed the protocol for the entirety of the study) may overestimate treatment effect if the reasons for incomplete data are related to the treatment effect 65 – in this case, it is not explicitly stated what “protocol deviations” occurred. No adverse effects occurred during the study period.
Rhodiola rosea for non-specific fatigue was evaluated in a double-blind crossover randomized controlled trial in 56 Armenian physicians 66. Participants were randomized to either 170 mg Rhodiola rosea (standardized to 2.6% salidroside) or placebo. The study period lasted for two weeks followed by a two-week wash-out period, after which participants were crossed over for two weeks. The primary outcome was fatigue, measured using a fatigue index developed for use in this study; the tool does not appear to be validated. Measurements were carried out before and after the treatment period. Authors 66 state that they found a significant improvement in the fatigue index after two weeks of Rhodiola rosea supplementation, but only present data for the 5 individual test scores. Since we are unable to replicate and confirm their analysis, findings of this study must be interpreted as inconclusive. Authors indicate that no adverse events occurred; whether or not anyone dropped out of the study was not reported.
A double-blinded randomized controlled trial conducted in Russia evaluated the effect of two different single doses of Rhodiola rosea on mental fatigue 67. Subjects were randomized to take Rhodiola rosea or placebo. A non-treatment group was also included, however subjects were not randomized into this group and comparisons against this group will not be considered in this review. The intervention was taken at 4:00 am while participants were on an overnight shift. Capacity for mental work, measured using a fatigue index of unknown origins and pulse pressure and rate were evaluated before night duty and one hour after taking the study medication. A self-report questionnaire evaluating general well-being was completed after taking the study medication. The fatigue index was comprised of three parameters: visual perception, short-term memory and perception of order. Improvements in favour of both doses of Rhodiola rosea were apparent in the fatigue index; no significant differences between groups occurred for other outcomes. The method of randomization was unclear. One subject in the placebo group experienced hypersalivation; whether or drop-outs occurred was not reported.
A double-blinded randomized controlled trial pilot study examined the effect of a repeated low dose of Rhodiola rosea on foreign students’ mental and physical well-being during their examination period 68. Subjects were randomized into 2 groups to receive either 100 mg Rhodiola rosea once per day or identical placebo for 20 days. Hand-eye coordination (maze test), motoric speed (tapping test), mental work capacity (correction of text test), fatigue and well-being (self-evaluation questionnaire), heart rate and physical work capacity (bicycle ergometer test) were assessed. Significant improvements were observed in hand-eye coordination, mental fatigue and general-well being in favour of Rhodiola rosea. Students on placebo had a significantly higher heart rate. Drop-outs and adverse events were not reported by authors.
Another randomized controlled trial conducted by the same group examined 60 male students in their first year of study at a Russian high school 69. Students were randomized into 3 groups to receive either of Rhodaxon (Rhodiola rosea extract with no ethyl alcohol per day; proportions of active constituents not given), placebo or nothing for 20 days. Participants underwent the same tests for mental and physical capacity as above as well as a psychophysiological test 70 to determine level of anxiety, psychological fatigue and need to rest. A comparative analysis between groups was not conducted leaving the effect of Rhodiola rosea indeterminable. Adverse events and drop-outs were not reported.
Rhodiola rosea on endurance exercise performance
The results of this study 71 show that acute ingestion of Rhodiola rosea by recreationally fit women can lower the perception of effort during high-intensity endurance exercise and improve performance when the goal is to complete a given distance as quickly as possible. The study authors observed a tendency for the subjects to be able to sustain a higher workload at a heart rate of 170 beats per minute after 20 days of supplementation of 100 mg/day of Rhodiola rosea. Spasov et al. 72 also found that heart rate recovered significantly faster after exercise in the Rhodiola rosea group. It is unclear from the present data what caused the reduction in heart rate during the warm-up after Rhodiola rosea treatment. This has obvious implications for recreational athletes, or “weekend warriors,” who may choose to compete in endurance events. However, caution should be used when extrapolating these results to other populations. For example, it is unclear whether similar results would be seen in highly trained endurance athletes who consumed Rhodiola rosea before races. It is also unclear whether the effects seen from an acute dose would be seen with chronic dosing. Because a previous study has shown a loss of effect with chronic treatment 73, athletes might want to use Rhodiola rosea sparingly in an attempt to maintain the ergogenic effects. That clinical trial 71 also shows that acute ingestion of Rhodiola rosea can lower the heart rate response to submaximal exercise. Although this effect was very pronounced from a statistical standpoint, the real-world benefits of this to athletes who consume Rhodiola rosea before competition or training is currently unknown. It is a common popular belief that Rhodiola rosea can decrease the body’s response to stress, and many athletes consume Rhodiola in the hopes that it can help them deal with a heavy training schedule and the stress of modern life. Although it cannot be ruled out that chronic supplementation may be beneficial in this respect, this study found no evidence that Rhodiola rosea can reduce the activation of the hypothalamic-pituitary-adrenal system’s after intense exercise 71.
Rhodiola rosea dosage
Dosage varies depending upon standardization. For chronic administration, a daily dose of 360-600 mg Rhodiola extract standardized for 1% rosavin, 180-300 mg of an extract standardized for 2% rosavin, or 100-170 mg of an extract standardized for 3.6% rosavin is suggested. Clinical studies reporting a positive effect of Rhodiola rosea on physical performance reported doses of 100 mg/day 71 and 680 mg/day and those reporting a positive effect on mental fatigue reported doses between 100–576 mg/day 74. For depressive disorder, pharmaceutical grade Rhodiola rosea powdered extract 340 mg/day (standardized to a content of rosavin 3.07% and rhodioloside 1.95%) for 12 weeks 75.
Administration is normally begun several weeks prior to a period of expected increased physiological, chemical, or biological stress, and continued throughout the duration of the challenging event or activity.
When using Rhodiola rosea as a single dose for acute purposes (e.g., for an exam or athletic competition), the suggested dose is three times the dose used for chronic supplementation.
Rhodiola rosea has been administered for periods ranging from as little as one day (acute administration) up to four months. Until more specific information is available, a dosing regimen following the established patterns used with other plant adaptogens – with periodic intervals of abstinence – seems warranted when Rhodiola rosea is being used chronically.
Rhodiola rosea side effects
When taken orally (by mouth), Rhodiola rosea may cause dizziness and dry mouth. Clinical feedback indicates, at doses of 1.5-2.0 grams and above, Rhodiola rosea extract standardized for 2% rosavin might cause some individuals to experience an increase in irritability and insomnia within several days.
Out of 446 subjects examined in the 11 included clinical studies 74, five adverse events were mentioned in three studies. Two subjects on 200 mg of Rhodiola rosea over a 4-week period each experienced a minor and serious headache 68; there appear to be few side effects associated with Rhodiola rosea supplementation; those identified are of a mild nature 74.
Evidence on the safety and appropriateness of Rhodiola rosea supplementation during pregnancy and lactation is currently unavailable.
- Rhodiola rosea in stress induced fatigue–a double blind cross-over study of a standardized extract SHR-5 with a repeated low-dose regimen on the mental performance of healthy physicians during night duty. Darbinyan V, Kteyan A, Panossian A, Gabrielian E, Wikman G, Wagner H. Phytomedicine. 2000 Oct; 7(5):365-71.[↩][↩]
- Flora of Republic of Bulgaria. Vol. 4. Sofia, Bulgaria: Bulgarian Academy of Sciences; 1970.[↩]
- Igosheva NI, Shurova EA. Spread of some wild grown medicinal and strawberry species in Tyumen. Plant Resources. 2003;2:57–62. (Rus).[↩]
- Komarova V. Flora USSR. 1961;9[↩]
- Flora of Canadian Artic Archipelago. ver. 29, 2003[↩]
- Mao JJ, Xie SX, Zee J, et al. Rhodiola rosea versus sertraline for major depressive disorder: A randomized placebo-controlled trial. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2015;22(3):394-399. doi:10.1016/j.phymed.2015.01.010 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4385215/[↩]
- Brown R, G P, Ramazanov Z. Rhodiola rosea: a phytomedicinal overview. HerbalGram. 2002:40–52.[↩]
- Monoamine oxidase inhibition by Rhodiola rosea L. roots. van Diermen D, Marston A, Bravo J, Reist M, Carrupt PA, Hostettmann K. J Ethnopharmacol. 2009 Mar 18; 122(2):397-401.[↩]
- [Plasma beta-endorphin and stress hormones in stress and adaptation]. Lishmanov IuB, Trifonova ZhV, Tsibin AN, Maslova LV, Dement’eva LA. Biull Eksp Biol Med. 1987 Apr; 103(4):422-4.[↩]
- The adaptogens rhodiola and schizandra modify the response to immobilization stress in rabbits by suppressing the increase of phosphorylated stress-activated protein kinase, nitric oxide and cortisol. Panossian A, Hambardzumyan M, Hovhanissyan A, Wikman G. Drug Target Insights. 2007; 2():39-54.[↩]
- Panossian A, Wikman G, Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010;17(7):481–493[↩]
- Kurkin VA, Zapesochnaya GG. Chemical composition and pharmacological properties of Rhodiola rosea . Chemical and Pharmaceutical Journal (Moscow) 1986;20(10):1231–1244.[↩]
- Patov SA, Punegov VV, Kuchin AV. Synthesis of the Rhodiola rosea glycoside rosavin. Chemistry of Natural Compounds. 2006;42(4):397–399.[↩]
- Kurkin VA, Zapesochnaya GG, Shchavlinskii AN, Nukhimovskii EL, Vandyshev VV. Method for determination of Rhodiola rosea rhizomes authenticity and quality. Khimiko-Farmatsevticheskii Zhurnal. 1985;19:185–190.[↩]
- Linh PT, Kim YH, Hong SP, Jian JJ, Kang JS. Quantitative determination of salidroside and tyrosol from the underground part of Rhodiola rosea by high perfomance liquid chromatography. Eksperimental’naia i Klinicheskaia Farmakologiia. 2002;65(6):57–59. (Rus).[↩]
- Zapesochnaya GG, Kurkin VA. Glycosides from alcohol extract from rhizomes of Rhodiola rosea . Chemical Natural Products. 1982;6:723–727. (Rus).[↩]
- Nekratova NA, Krasnov EA, Nekratov NF, Mikhailova SI. Changes of quantitative contents of salidroside and tannins in underground organs of Rhodiola rosea L. in its natural habitats in Altai. Plant Resources. 1992;28:40–48. (Rus).[↩]
- Dubichev AG, Kurkin VA, Zapesochnaya GG, Vorontsov ED. Chemical composition of the rhizomes of the Rhodiola rosea by the HPLC method. Chemistry of Natural Compounds. 1991;27(2):161–164.[↩]
- Belov VN, Lavrova TV, Vashkevich NG, Mikhailov AY. Extraction of essential oils from plant raw material by steam distillation. Russian Journal of Applied Chemistry. 1994;67:154–156.[↩]
- Yousef GG, Grace MH, Cheng DM, Belolipov IV, Raskin I, Lila MA. Comparative phytochemical characterization of three Rhodiola species. Phytochemistry. 2006;67(21):2380–2391[↩]
- Ganzera M, Yaylaq Y, Khan IA. Analysis of the marker copounds of Rhodiola rosea L. (golden root) by reversed phase high performance liquid chromatography. Archives of Pharmacal Research. 2000;23(4):349–352[↩]
- Rohloff J. Volatiles from rhizomes of Rhodiola rosea L. Phytochemistry. 2002;59(6):655–661.[↩]
- Evstatieva LN, Revina TA. Investigation of Polyphenols in Rhodiola rosea Groupe polyphenols. Journees Internationales d’Etudes. 1984;12:127–128.[↩]
- Lee MW, Lee YA, Park HM, et al. Antioxidative phenolic compounds from the roots of Rhodiola sachalinensis A. Bor. Arch Pharm Res 2000;23:455-458.[↩]
- Linh PT, Kim YH, Hong SP, et al. Quantitative determination of salidroside and tyrosol from the underground part of Rhodiola rosea by high performance liquid chromatography. Arch Pharm Res 2000;23:349-352.[↩]
- Boon-Niermeijer EK, van den Berg A, Wikman G, Wiegant FA. Phyto-adaptogens protect against environmental stress-induced death of embryos from the freshwater snail Lymnaea stagnalis. Phytomedicine 2000;7:389-399.[↩]
- Visioli F, Galli C, Bornet F, et al. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett 2000;468:159-160.[↩]
- Dimpfel W, Schombert L, Panossian AG. Assessing the Quality and Potential Efficacy of Commercial Extracts of Rhodiola rosea L. by Analyzing the Salidroside and Rosavin Content and the Electrophysiological Activity in Hippocampal Long-Term Potentiation, a Synaptic Model of Memory. Frontiers in Pharmacology. 2018;9:425. doi:10.3389/fphar.2018.00425. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5976749/[↩][↩]
- Panossian A., Wikman G. (2014). “Evidence based efficacy and effectiveness of Rhodiola SHR-5 extract in treating stress- and age-associated disorders,” in , eds Cuerrier C., Ampong-Nyarko K., editors. (Boca Raton, FL: CRC Press; ), 203–221.[↩]
- Hung SK, Perry R, Ernst E. The effectiveness and efficacy of Rhodiola rosea L.: a systematic review of randomized clinical trials. Phytomedicine. 2011;18(4):235-244. https://www.ncbi.nlm.nih.gov/pubmed/21036578[↩]
- Ishaque S, Shamseer L, Bukutu C, et al. Rhodiola rosea for physical and mental fatigue: a systematic review. BMC Complementary and Alternative Medicine. 2012;12:70 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3541197/[↩]
- Mao JJ, Xie SX, Zee J, et al. Rhodia rosea versus sertraline for major depressive disorder: a randomized placebo-controlled trial. Phytomedicine. 2015;22(3):394-399. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4385215/[↩][↩]
- Rhodiola rosea L. as a putative botanical antidepressant. Amsterdam JD, Panossian AG. Phytomedicine. 2016 Jun 15; 23(7):770-83. https://www.ncbi.nlm.nih.gov/pubmed/27013349/[↩]
- Saratikov A. S., Krasnov E. A. (2004). Tomsk: Tomsk University Press.[↩]
- Aksenova R. A., Zotova M. I., Nekhoda M. F., Cherdintsev S. G. (1968). “Comparative characteristics of the stimulating and adaptogenic effects of Rhodiola rosea preparations,” in Vol. 2 ed. Saratikov A. S., editor. (Tomsk: Tomsk University Press; ), 3–12.[↩][↩]
- Anti-Inflammatory and Neuroprotective Effects of Constituents Isolated from Rhodiola rosea. Lee Y, Jung JC, Jang S, Kim J, Ali Z, Khan IA, Oh S. Evid Based Complement Alternat Med. 2013; 2013():514049.[↩][↩][↩]
- Rhodiola rosea L. and Alzheimer’s Disease: From Farm to Pharmacy. Nabavi SF, Braidy N, Orhan IE, Badiee A, Daglia M, Nabavi SM. Phytother Res. 2016 Apr; 30(4):532-9. https://onlinelibrary.wiley.com/doi/full/10.1002/ptr.5569[↩]
- Barnaulov O. D., Limarenko A. Y., Kurkin V. A., Zapesochnaya G. G., Shchavlinskij A. N. (1986). A comparative evaluation of the biological activity of compounds isolated from species of Rhodiola. 23 1107–1112.[↩]
- Sokolov S. Y., Boyko V. P., Kurkin V. A., Zapesochnaya G. G., Rvantsova N. V., Grinenko H. A. (1990). A comparative study of the stimulant property of certain phenylpropanoids. 24 66–68.[↩][↩]
- Comparative study of Rhodiola preparations on behavioral despair of rats. Panossian A, Nikoyan N, Ohanyan N, Hovhannisyan A, Abrahamyan H, Gabrielyan E, Wikman G. Phytomedicine. 2008 Jan; 15(1-2):84-91.[↩]
- From Traditional Resource to Global Commodities:-A Comparison of Rhodiola Species Using NMR Spectroscopy-Metabolomics and HPTLC. Booker A, Zhai L, Gkouva C, Li S, Heinrich M. Front Pharmacol. 2016; 7():254.[↩]
- Altered expression of TRAIL on mouse T cells via ERK phosphorylation by Rhodiola rosea L. and its marker compounds. Marchev AS, Dimitrova P, Koycheva IK, Georgiev MI. Food Chem Toxicol. 2017 Oct; 108(Pt B):419-428.[↩][↩][↩][↩]
- Effect of salidroside, active principle of Rhodiola rosea extract, on binge eating. Cifani C, Micioni Di B MV, Vitale G, Ruggieri V, Ciccocioppo R, Massi M. Physiol Behav. 2010 Dec 2; 101(5):555-62.[↩]
- Potent in vitro inhibition of CYP3A4 and P-glycoprotein by Rhodiola rosea. Hellum BH, Tosse A, Hoybakk K, Thomsen M, Rohloff J, Georg Nilsen O. Planta Med. 2010 Mar; 76(4):331-8.[↩]
- In vitro inhibition of cytochrome P-450 activities and quantification of constituents in a selection of commercial Rhodiola rosea products. Thu OK, Nilsen OG, Hellum B. Pharm Biol. 2016 Dec; 54(12):3249-3256.[↩]
- Two potent cytochrome P450 2D6 inhibitors found in Rhodiola rosea. Xu W, Zhang T, Wang Z, Liu T, Liu Y, Cao Z, Sui Z. Pharmazie. 2013 Dec; 68(12):974-6.[↩]
- Noncompetitive inhibition of human CYP2C9 in vitro by a commercial Rhodiola rosea product. Thu OKF, Spigset O, Hellum B. Pharmacol Res Perspect. 2017 Aug; 5;4[↩]
- The authenticity and quality of Rhodiola rosea products. Booker A, Jalil B, Frommenwiler D, Reich E, Zhai L, Kulic Z, Heinrich M. Phytomedicine. 2016 Jun 15; 23(7):754-62.[↩]
- Mechanism of action of Rhodiola, salidroside, tyrosol and triandrin in isolated neuroglial cells: an interactive pathway analysis of the downstream effects using RNA microarray data. Panossian A, Hamm R, Wikman G, Efferth T. Phytomedicine. 2014 Sep 25; 21(11):1325-48.[↩]
- Dimpfel W. (2014). Neurophysiological effects of Rhodiola rosea extract containing capsules (A double-blind, randomized, placebo-controlled study). 3 157–165.[↩][↩]
- A randomised, double-blind, placebo-controlled, parallel-group study of the standardised extract shr-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Olsson EM, von Schéele B, Panossian AG. Planta Med. 2009 Feb; 75(2):105-12.[↩]
- Rhodiola rosea versus sertraline for major depressive disorder: A randomized placebo-controlled trial. Mao JJ, Xie SX, Zee J, Soeller I, Li QS, Rockwell K, Amsterdam JD. Phytomedicine. 2015 Mar 15; 22(3):394-9.[↩]
- Hernández-Santana A., Pérez-López V., Zubeldia J. M., Jiménez-del-Rio M. (2014). A Rhodiola rosea root extract protects skeletal muscle cells against chemically induced oxidative stress by modulating heat shock protein 70 (HSP70) expression. 28 623–628. 10.1002/ptr.5046[↩]
- Dimpfel W., Schombert L., Vega-Morales T., Wiebe J. (2016b). Neuropharmacological characterization of extracts from Rhodiola rosea, Oenothera paradoxa and Paullinia cupana in comparison to caffeine. 7 290–303. 10.4236/pp.2016.77036[↩][↩]
- Ahmed M., Henson D. A., Sanderson M. C., Nieman D. C., Zubeldia J. M., Shanely R. A. (2015). Rhodiola rosea exerts antiviral activity in athletes following a competitive marathon race. 2:24. 10.3389/fnut.2015.00024[↩]
- Rhodiola rosea L. as a putative botanical antidepressant. Amsterdam JD, Panossian AG. Phytomedicine. 2016 Jun 15; 23(7):770-83.[↩]
- Zhu L. P., Wei T. T., Gao J., et al. Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neuroscience Letters. 2015;606:1–6. doi: 10.1016/j.neulet.2015.08.025[↩]
- Abidov M, Grachev S, Seifulla RD, Ziegenfuss TN. Extract of Rhodiola rosea radix reduces the level of C-reactive protein and creatinine kinase in the blood. BE Biol Med. 2004;138(1):63–64.[↩]
- Walker TB, Altobelli SA, Caprihan A, Robergs RA. Failure of Rhodiola rosea to alter skeletal muscle phosphate kinetics in trained men. Metab Clin Exp. 2007;56(8):1111–1117. doi: 10.1016/j.metabol.2007.04.004[↩]
- De Bock K, Eijnde BO, Ramaekers M, Hespel P. Acute Rhodiola rosea intake can improve endurance exercise performance. Int J Sport Nutr Exerc Metab. 2004;14(3):298–307.[↩][↩][↩]
- Earnest CP, Morss GM, Wyatt F, Jordan AN, Colson S, Church TS. et al. Effects of a commercial herbal-based formula on exercise performance in cyclists. Med Sci Sports Exerc. 2004;36(3):504–509. doi: 10.1249/01.MSS.0000125157.49280.AF[↩][↩]
- Mattioli L, Perfumi M. Rhodiola rosea L. extract reduces stress- and CRF-induced anorexia in rats. J Psychopharmacol. 2007;21(7):742–750.[↩]
- Colson SN, Wyatt FB, Johnston DL, Autrey LD, FitzGerald YL, Earnest CP. Cordyceps sinensis- and Rhodiola rosea-based supplementation in male cyclists and its effect on muscle tissue oxygen saturation. J Strength Cond Res. 2005;19(2):358–363[↩]
- Olsson EM, von Scheele B, Panossian AG. A randomised, double-blind, placebo-controlled, parallel-group study of the standardised extract shr-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Planta Med. 2009;75(2):105–112. doi: 10.1055/s-0028-1088346 https://www.ncbi.nlm.nih.gov/pubmed/19016404[↩]
- Gluud LL. Bias in clinical intervention research. Am J Epidemiol. 2006;163(6):493. doi: 10.1093/aje/kwj069[↩]
- Darbinyan V, Kteyan A, Panossian A, Gabrielian E, Wikman G, Wagner H. Rhodiola rosea in stress induced fatigue–a double blind cross-over study of a standardized extract SHR-5 with a repeated low-dose regimen on the mental performance of healthy physicians during night duty. Phytomedicine. 2000;7(5):365–371. doi: 10.1016/S0944-7113(00)80055-0[↩][↩]
- Shevtsov VA, Zholus BI, Shervarly VI, Vol’skij VB, Korovin YP, Khristich MP. et al. A randomized trial of two different doses of a SHR-5 Rhodiola rosea extract versus placebo and control of capacity for mental work phytomedicine. Phytomedicine. 2003;10(2–3):95–105[↩]
- Spasov AA, Wikman GK, Mandrikov VB, Mironova IA, Neumoin VV. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine. 2000;7(2):85–89. doi: 10.1016/S0944-7113(00)80078-1[↩][↩]
- Spasov AA, Mandrikov VB, Mironova IA. The effect of the preparation rodakson on the psychophysiological and physical adaptation of students to an academic load. Eksperimentalnaia i Klinicheskaia Farmakologiia. 2000;63(1):76–78[↩]
- Williamson EM. Interactions between herbal and conventional medicines. Expert Opin Drug Saf. 2005;4(2):355–378. doi: 10.1517/14740338.4.2.355[↩]
- The Effects of an Acute Dose of Rhodiola rosea on Endurance Exercise Performance. Journal of Strength and Conditioning Research: March 2013 – Volume 27 – Issue 3 – p 839–847. https://journals.lww.com/nsca-jscr/fulltext/2013/03000/The_Effects_of_an_Acute_Dose_of_Rhodiola_rosea_on.37.aspx[↩][↩][↩][↩]
- Spasov AA, Wikman GK, Mandrikov VB, Mironova IA, Neumoin VV. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine 7: 85–89, 2000.[↩]
- De Bock K, Eijnde BO, Ramaekers M, Hespel P. Acute Rhodiola rosea intake can improve endurance exercise performance. Int J Sport Nutr Exerc Metab 14: 298–307, 2004.[↩]
- Ishaque S, Shamseer L, Bukutu C, Vohra S. Rhodiola rosea for physical and mental fatigue: a systematic review. BMC Complementary and Alternative Medicine. 2012;12:70. doi:10.1186/1472-6882-12-70. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3541197/[↩][↩][↩]
- Mao JJ, Xie SX, Zee J, et al. Rhodiola rosea versus sertraline for major depressive disorder: A randomized placebo-controlled trial. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2015;22(3):394-399. doi:10.1016/j.phymed.2015.01.010. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4385215/[↩]