Contents
- What is astaxanthin
- Astaxanthin food sources
- Natural astaxanthin vs synthetic astaxanthin
- Astaxanthin Metabolism: Absorption and Tissue Distribution
- What is astaxanthin used for?
- Astaxanthin health benefits
- Table 4. Human clinical study for astaxanthin
- Astaxanthin a Unique Cell Membrane Antioxidant
- Table 5. Health benefits of astaxanthin in human subjects
- Antioxidant Effects
- Anti-inflammatory Benefits
- Skin health
- Metabolic Syndrome
- Anti-Diabetic Activity
- Cardiovascular health
- Anticancer Activity
- Memory and other Higher Brain Functions
- Effect on Vision and Eye Fatigue
- Muscle Performance and Endurance
- Male Fertility and Reproduction
- Immuno-Modulation
- Astaxanthin dosage
- Astaxanthin side effects
What is astaxanthin
Astaxanthin (3,3′-dihydroxy-β, β′-carotene-4,4′-dione) is a lipid-soluble red-orange pigment belonging to the xanthophyll carotenoid family created by micro-algae (Haematococcus pluvialis, Chlorella zofingiensis, Chlorococcum, and Phaffia rhodozyma) in times of stress 1, 2, 3, 4, 5, 6. Haematococcus pluvialis is a green microalga, which accumulates high astaxanthin content under stress conditions such as high salinity, nitrogen deficiency, high temperature and light 7. When the Haematococcus pluvialis algae are exposed to change in their environment such as direct sunlight, starved of food or being eaten, it produces astaxanthin to protect itself. As a potent antioxidant, astaxanthin protects the algae from free radicals and oxidative stress, keeping it alive and well when stressed. Astaxanthin accumulates up to 3.8% on the dry weight basis in Haematococcus pluvialis. Astaxanthin produced from haematococcus pluvialis is a main source for human consumption 8. Astaxanthin carotenoid is what gives many sea creatures such as salmon, trout, krill, crayfish, shrimp and crab their red color 9, 10, 11. Carotenoids contribute to the yellow, orange, and red colors of the skin, shell, or exoskeleton of aquatic animals. Indeed, carotenoids are the most widespread pigments found in nature, as they occur in bacteria, yeasts, mold, all green plants, and many animals, and therefore various functions have been attributed to them. From anthropocentric consideration, the most significant aspect of carotenoids is the color they impart to our food and environment.
Astaxanthin is closely related to beta-carotene, lutein, and zeaxanthin, sharing with them many of the general metabolic and physiological functions attributed to carotenoids. Mammals, including humans, lack the ability to synthesize astaxanthin or to convert dietary astaxanthin into vitamin A.
Astaxanthin is a carotenoid widely used in salmon, trout, red seabream, shrimp, lobster, fish eggs and crustacean aquaculture to provide the pink color characteristic of that species 9, 10, 12, 13. Astaxanthin is also found in some birds, such as flamingoes, quails, and other species. Astaxanthin, similar to other carotenoids, cannot be synthesized by animals and must be provided in the diet. Salmon farming increased substantially in the 1980s, which created a large market for astaxanthin, the principal pigment of salmon. Since animals lack the ability to synthesize carotenoids, the pigments must be supplemented to feeds, usually at considerable expense to the farmer (10 to 15% of total feed costs). This application has been well documented for over two decades and is currently the major market driver for the astaxanthin pigment. Currently, chemically synthesized astaxanthin and canthaxanthin (β,β-carotene4,4′-dione) are added to salmon feeds as pigmenters, but there is considerable interest within the aquaculture industry in using natural sources of astaxanthin. Additionally, astaxanthin also plays a key role in animals as an intermediary in reproductive processes, the carotenoids are associated with reproductive organs and hence the hatching success and survival of alevins (newly spawned salmons or trouts still carrying the yolk).
Synthetic astaxanthin dominates the world market but recent interest in natural sources of the pigment has increased substantially. Common sources of natural astaxanthin are the green algae Haematococcus pluvialis, the red yeast Phaffia rhodozyma, as well as crustacean byproducts. Each natural pigment source has its limitations and they currently cannot compete economically with the synthetic additive 14. The algae Haematococcus pluvialis has a high concentration of astaxanthin (0.2 to 2%), but industrial application is limited by the lengthy autotrophic cultivation in open freshwater ponds and the requirement for disruption of the cell wall to liberate the carotenoid 14. The red yeast Phaffia rhodozyma has desirable properties as a biological source of pigment, including rapid heterotrophic metabolism and production of high cell densities in fermentors, but its content of astaxanthin in wild strains is only 200 to 300 μg/g yeast (0.02 to 0.03%) 14. Mutants have been isolated that produce >3000 μg total carotenoid per gram of yeast (>0.30%) in shake flasks after 5 d growth, and measurement of carotenoid fluorescence in individual cells indicates that levels of 10,000 to 15,000 μg/g can be obtained 14. High producers, however, are often unstable and further strain development is required.
Astaxanthin possesses an unusual antioxidant activity which has caused a surge in the nutraceutical market for the encapsulated product. Also, health benefits such as cardiovascular disease prevention, immune system boosting, bioactivity against Helycobacter pylori, and cataract prevention, have been associated with astaxanthin consumption 15. Research on the health benefits of astaxanthin is very recent and has mostly been performed in test tube study or at the pre-clinical level with humans.
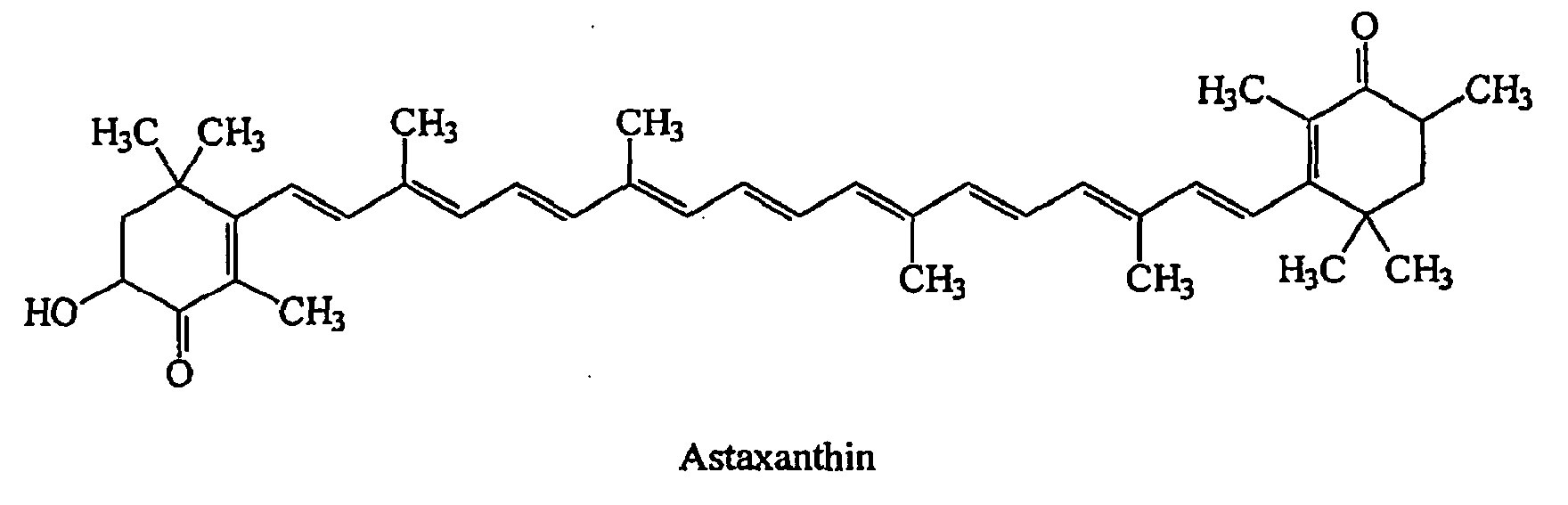
Figure 1. Astaxanthin chemical structure
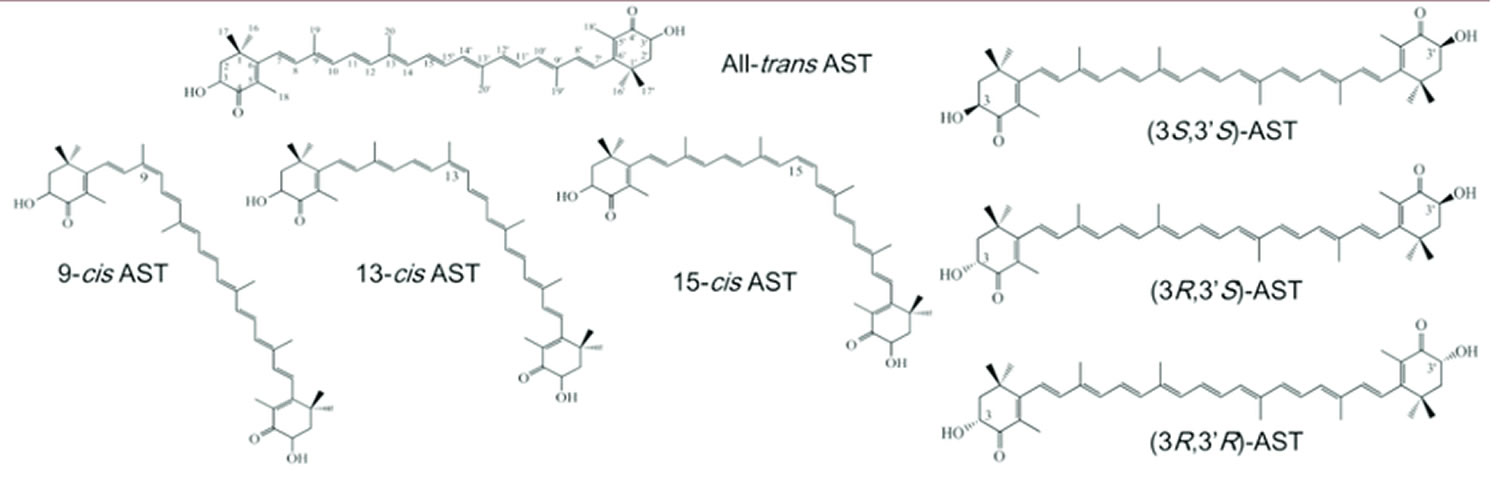
Figure 2. Astaxanthin isomers
Footnotes: Astaxanthin optical isomers and major geometric isomers. Most natural astaxanthin exists as a mixture of geometrical isomers, including small amounts of 9-cis, 13-cis, and 15-cis forms, along with the all-trans form.
Abbreviation: AST = astaxanthin
[Source 6 ]Figure 3. Astaxanthin used in supplement
Figure 4. Natural sources of astaxanthin production
[Source 16 ]Astaxanthin food sources
Astaxanthin was first discovered in European lobster (Astacus gammarus) by the Nobel laureate Richard Kuhn 17. Since this pioneer work, astaxanthin has been found to be naturally synthesized by land plants (Adonis annua [pheasant’s-eye] and Adonis aestivalis [summer pheasant’s-eye]), microalgae (Haematococcus lacustris, Chromochloris zofingiensis, Chlorococcum species, Bracteacoccus aggregatus, Coelastrella rubescens, Chlamydomonas nivalis), yeasts (Phaffia rhodozyma) and some bacteria eg. Paracoccus carotinifaciens, a motile aerobic Gram-negative bacterium 18, 19, 20, 21, 22, 23, 24, 25, 26.
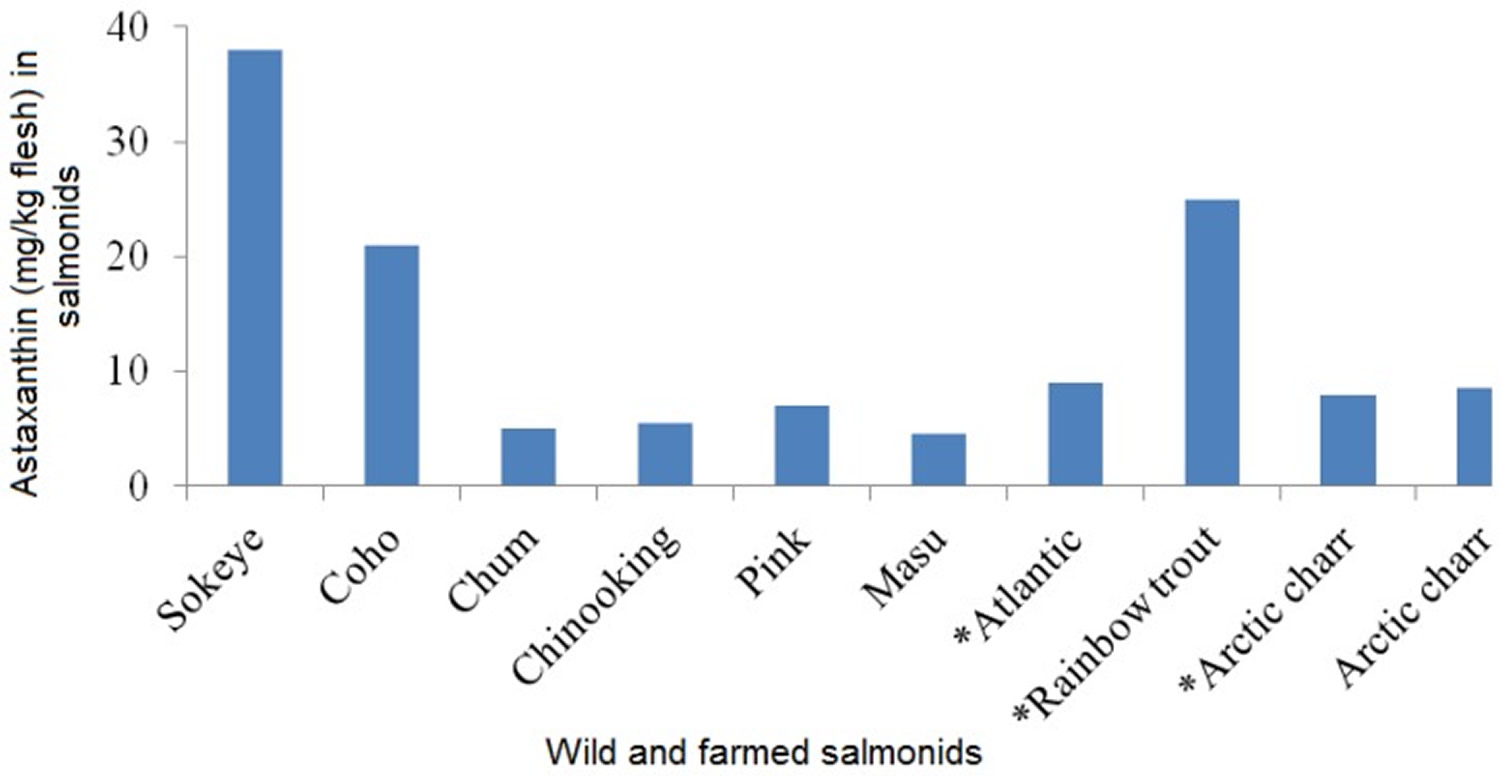
Certain, plants, algae, fungi, yeast and bacteria constituted the primary natural sources of astaxanthin (Table 1 and 2) 27, 28. Besides, the astaxanthin-containing organisms that are consumed by aquatic animals for the acquisition of glamorous coloration store astaxanthin in their tissues. For example, aquatic zooplanktons fed on marine algae (rich in beta-carotene, fucoxanthin, and diatoxanthin) are in turn converting beta-carotene, which accumulated from marine algae to astaxanthin in their body; consequently, they are ingested by nautical fish (e.g., salmonids and trouts) and crustacean organisms (e.g., shrimp, crabs, crayfish, lobsters, and krill) at higher nutritive levels 29, 30. Man-made (synthetic) astaxanthin is commercially obtained by either chemical composition or natural microbial resources, such as red yeast Xanthophyllomyces dendrorhous (Phaffia rhodozyma) and green microalga Haematococcus pluvialis (H. pluvialis) 31, 32, 33, 34, 35, 36, 37. The leading role is undoubtedly achieved by Haematococcus pluvialis (H. pluvialis) which is one of the most promising sources of natural astaxanthin, and so many studies have investigated the best conditions to synthesize and extract astaxanthin from Haematococcus pluvialis (H. pluvialis) 38, 39, 40. Furthermore, other significant sources of astaxanthin include the flesh of wild and farmed marine fish, such as salmonids and trout. However, considerable variations were assayed in the muscle astaxanthin content among species. For instance, astaxanthin concentrations in wild Oncorhynchus species varied from 3 mg/kg flesh in chum salmon Oncorhynchus keta up to 38 mg/kg flesh in sockeye salmon Oncorhynchus nerka 41. Astaxanthin contents in wild and farmed Atlantic salmon Salmo salar were reported as 3–10 mg/kg flesh and 1–9 mg/kg flesh, respectively 41. Besides, astaxanthin concentrations were significantly higher in fresh salmon compared to pouch packaged and canned products 42. Therefore, salmonid fillets can be served as a good dietary source of natural astaxanthin 1. In general, the primary natural sources of astaxanthin in high concentrations are displayed in Figure 5 43.
Among crustaceans, shrimps have been widely studied due to their capacity in producing astaxanthin and so they are important dietary sources of astaxanthin 44, 45, 46. The astaxanthin contents of 1.41 mg/100 g and 1.69 mg/100 g were found in the muscles of wild Penaeus semisulcatus and Metapenaues monoceros shrimps, respectively 47. Cultured Whiteleg shrimp (Litopenaeus vannamei) fed a basal diet, which contained 2.24 mg of astaxanthin per 100 g 45. Also, dried Indian prawn (Penaeus indicus) had 6.16 mg of astaxanthin per 100 g 44. The Antarctic krill Euphausia superba (E. superba) is an excellent source of astaxanthin diester (55–64%), astaxanthin monoester (25–35%), and astaxanthin (7–8%), especially in the carapace, flesh, and eyes of the Antarctic krill. The amounts of astaxanthin in Antarctic krill carapace 1.13 mg/100 g, flesh 1.06 mg/100 g, and eyes 90.82 mg/100 g 48, 49. As well, Pacific krill (Euphausia pacifica) possesses a higher concentration of astaxanthin 50. Additionally, astaxanthin concentrations are mostly detected in the processed wastes of the cephalothorax, abdominal epidermal layer, and abdominal exoskeleton (shells), particularly those ranging from 4.79 mg/100 g in Indian prawn (Penaeus indicus) to 9.17 mg/100 g in Atlantic seabob shrimp (Xiphopenaeus kroyeri) 51, 52, 53. On the other hand, common seabream (Pagrus pagrus) skin contains higher astaxanthin levels in fish fed Haematococcus pluvialis (freshwater microalgae) (4.89 mg/100 g) than in the fish’s skin fed synthetic astaxanthin (2.91 mg/100 g). Based on these data, Haematococcus pluvialis (freshwater microalgae) provide adequate concentrations of esterified astaxanthin to imbue the skin of red porgy (common seabream) more efficiently which may be indicated by the higher intestinal solubility and easier incorporation of astaxanthin esters into mixed micelles when compared with synthetic, unesterified astaxanthin 54.
Figure 5. Astaxanthin concentrations from natural sources
Table 1. Microorganism sources of astaxanthin
| Microorganism sources | Astaxanthin (%) on the Dry Weight Basis |
|---|---|
| Chlorophyceae | |
| Haematococcus pluvialis | 3.8 |
| Haematococcus pluvialis (K-0084) | 3.8 |
| Haematococcus pluvialis (Local isolation) | 3.6 |
| Haematococcus pluvialis (AQSE002) | 3.4 |
| Haematococcus pluvialis (K-0084) | 2.7 |
| Chlorococcum | 0.2 |
| Chlorella zofingiensis | 0.001 |
| Neochloris wimmeri | 0.6 |
| Ulvophyceae | |
| Enteromorpha intestinalis | 0.02 |
| Ulva lactuca | 0.01 |
| Florideophyceae | |
| Catenella repens | 0.02 |
| Alphaproteobacteria | |
| Agrobacterium aurantiacum | 0.01 |
| Paracoccus carotinifaciens (NITE SD 00017) | 2.2 |
| Tremellomycetes | |
| Xanthophyllomyces dendrorhous (JH) | 0.5 |
| Xanthophyllomyces dendrorhous (VKPM Y2476) | 0.5 |
| Labyrinthulomycetes | |
| Thraustochytrium sp. CHN-3 (FERM P-18556) | 0.2 |
| Malacostraca | |
| Pandalus borealis | 0.12 |
| Pandalus clarkia | 0.015 |
Table 2. Astaxanthin microorganism sources
| Taxon | Scientific Name | Common Name | Astaxanthin | |||
|---|---|---|---|---|---|---|
| Form † | Stereoisomer (3R,3′R, meso, 3S,3′S) | Content (mg/100 g) | Origin | |||
| Bacteria, Prtoteobacteria, Alphaproteobacteria | ||||||
| Paracoccus carotinifaciens (w/. mutation) | PanaFerd-AX | Free form | 3S,3′S | 2180 (50.2% of total Car) | De novo synthesis | |
| Paracoccus sp. strain N81106 (NBRC 101723) (Agrobacterium auranticum) (w/. mutation) | N/A | Free form and glycoside | 3S,3′S | ~800 (63.2% of total Car) | De novo synthesis | |
| Brevundimonas sp. M7 (w/. mutation) | N/A | Free form ** | 3S,3′S ** | 130 | De novo Synthesis ** | |
| Sphingomonas astaxanthinifaciens TDMA-17 | N/A | Free form | 3S,3′S ** | 96.0 (34.3% of total Car) | De novo Synthesis ** | |
| Paracoccus haeundaensis KCCM 10460 (Co-culture w/. Lactic Acid Bacteria) | N/A | Free form | 3S,3′S ** | 82.1 | De novo synthesis | |
| Paracoccus bogoriensis BOG6T (DSM16578, LMG2279) | N/A | Free form | 3S,3′S | 40 (10.8% of total Car) | De novo synthesis | |
| Brevundimonas spp. | N/A | Free form ** | 3S,3′S ** | 2.8~36.5 | De novo Synthesis ** | |
| Sphingomicrobium astaxanthinifaciens CC-AMO-30B | N/A | Free form | 3S,3′S ** | 4 | De novo Synthesis ** | |
| Brevundimonas sp. strain SD212 (NBRC 101024) | N/A | Free form | 3S,3′S | N/A (9.9% of total Car) | De novo synthesis | |
| Archaea | ||||||
| Halobacterium salinarium NRC-1 | N/A | Free form ‡ | N/D | 26.5 (c.a.73% of total Car) | De novo Synthesis * | |
| Haloarcula hispanica ATCC 33960 | N/A | Free form ‡ | N/D | 1.7 (c.a.1.3% of total Car) | De novo Synthesis* | |
| Eukaryota, Fungi | ||||||
| Xanthophyllomyces dendrorhous (ATCC SD 5340) | Phaffia Yeast | Free form | 3R,3′R | 723.5~1247.8 (c.a. 73% of total Car) | De novo synthesis | |
| Eukaryota, Plantae | ||||||
| Adonis amurensis (Reddish flower varieties) | Amur adonis, pheasant’s eye | Fatty acid esters | 3S,3′S ** | ~3310 (in upper red part of petal) (c.a. 70% of total Car) | De novo Synthesis ** | |
| Adonis annua | Autumn pheasant’s eye, blooddrops | Fatty acid esters | 3S,3′S | 120~1000 (in dry petal) (c.a. 75% of total Car) | De novo Synthesis ** | |
| Adonis aestivalis | Summer pheasant’s eye | Fatty acid esters | 3S,3′S | 166 (in wet petal) (87.4% of total Car) | De novo synthesis | |
| Eukaryota, Plantae, Chlorophyta | ||||||
| Haematococcus lacustris | Haematococcus pluvilialis, Haematococcus algae | Fatty acid esters | 3S,3′S | ~9800 (in red cyst) (>90% of total Car) | De novo synthesis | |
| Neochloris wimmeri CCAP-213/4 | N/A | Fatty acid esters | 3S,3′S ** | ~1920 (c.a 85% of total Car) | De novo Synthesis ** | |
| Asterarcys quadricellulare PUMCC 5.1.1 | N/A | N/A | 3S,3′S** | ~1550 (c.a 13% of total Car) | De novo Synthesis ** | |
| Protosiphon botryoides SAG-731/1a | N/A | Fatty acid esters | 3S,3′S** | ~1430 (c.a 80% of total Car) | De novo Synthesis ** | |
| Scotiellopsis oocystiformis SAG-277/1 | N/A | Fatty acid esters | 3S,3′S ** | ~1090 (c.a 70% of total Car) | De novo Synthesis ** | |
| Chlorococcum sp. | N/A | Fatty acid esters | 3S,3′S ** | ~c.a. 700 (c.a. 32% of total Car) | De novo Synthesis ** | |
| Chlorella zofingiensis SAG-211/14 | Chlorella | Fatty acid esters | 3S,3′S ** | ~680 (c.a. 75% of total Car) | De novo Synthesis ** | |
| Scenedesmus vacuolatus SAG-211/15 | N/A | Fatty acid esters | 3S,3′S ** | ~270 (40–50% of total Car) | De novo Synthesis ** | |
| Chlamydocapsa spp. Strain 101-99/R2 | N/A | N/A | 3S,3′S ** | ~44.4 (20.3% of total Car) | De novo Synthesis ** | |
| Neochloris oleoabundans UTEX#1185 | N/A | Fatty acid esters | 3S,3′S ** | N/A | De novo Synthesis ** | |
| Dysmorphococcus globosus-HI | N/A | Free form/ Fatty acid esters | 3S,3′S ** | ~517,090?? | De novo Synthesis ** | |
| Eukaryota, Chromista, Bigyra, Labyrinthulomycetes | ||||||
| Aurantiochytrium sp. RH-7A-7 (w/. mutation) | Labyrinthulomycetes | N/A | 3S,3′S ** | -470 (c.a. 85% of total Car) | De novo Synthesis * | |
| Thraustochytrium sp. CHN-3 (FERM P-18556) | Labyrinthulomycetes | Free form ** | 3S,3′S ** | ~280 (~60% of total Car) | De novo Synthesis * | |
| Aurantiochytrium sp. KH-10 | Labyrinthulomycetes | Fatty acid esters/ Free form | 3S,3′S ** | ~81 (28% of total Car) | De novo Synthesis * | |
| Thraustochytrium sp. CHN-1 | Labyrinthulomycetes | Free form | 3S,3′S | 50 (c.a. 50% of total Car) | De novo Synthesis * | |
| Eukaryota, Chromista, Gyrista , Eustigmatales | ||||||
| Nannochloropsis gaditana strain S4 (w/. mutation) | Nannochloropsis | Free form | 3S,3′S? | ~219 (14.4% of total Car) | De novo Synthesis | |
| Nannochloropsis oculata | Nannochloropsis | Free form | 3S,3′S? | 3.4 ng/106 cells | De novo Synthesis | |
| Nannochloropsis salina | Nannochloropsis | Free form | 3S,3′S? | 9.6 ng/106 cells | De novo Synthesis | |
| Eukaryota, Excavata, Euglenozoa | ||||||
| Euglena sanguinea | Euglena | Fatty acid esters/free | 3S,3′S | ~1.9 (80% of total Car) | De novo Synthesis | |
| Trachelomonas volvocina | Euglena | Fatty acid esters/ Free form | 3S,3′S * | N/A | De novo Synthesis | |
| Animals (Invertebrate), Coelenterata | ||||||
| Velella velella | By-the-wind sailor (Jerry fish) | Free form | Mixtures of stereoisomers | N/A | Accumulated from dietary Crustaceans | |
| Aurelia aurita | (Jerry fish) | Fee form/ Fatty acid esters (minor) | N/A | 12.2 (c.a.67% of total Car) | N/A | |
| Metridium senile var. fimbriatum | Frilled anemone (Sea anemone) | Fatty acid esters (in ovary) | Mixtures of stereoisomers | N/A | Oxidative metabolite of β-carotene | |
| Corynactis californica | Strawberry anemone (Sea anemone) | Fatty acid esters | N/A | N/A | Oxidative metabolite of β-carotene | |
| Animals (Invertebrate), Mollusca , Gastropoda | ||||||
| Clione limacina | Sea angel | Free form | 3S,3′S | 0.051 (1.1% of total Car) | Oxidaive metabolite of zeaxanthin | |
| Paedoclione doliiformis | Sea angel | Free form | 3S,3′S | 0.8 (5.5% of total Car) | Oxidaive metabolite of zeaxanthin | |
| Semisulcospira libertina | Terestorial Snail (Kawanina in Japanese) | Free form | 3S,3′S | 0.2 (6.5% of total Car) | Oxidaive metabolite of zeaxanthin | |
| Fushinus perplexu | Spindle shell | Free form | 3S,3′S | 0.2 (4.0% of total Car) | Oxidative metabolite of β-carotene | |
| Pomacea canaliculata | Apple snail | Free form | 3S,3′S | 5.0 in gonad, 2.31 in egg (~75% of total Car) | Oxidative metabolite of β-carotene | |
| Animals (Invertebrate), Mollusca , Cephalopoda | ||||||
| Octopus vulgaris | Common octopus | Fatty acid esters/ Free form | Mixtures of stereoisomers (46:22:32) | 3.2 in liver (c.a.80% of total Car) | Accumulated from dietary crustaceans | |
| Watasenia scintillans | Firefly squid | Fatty acid esters/ Free form | Mixtures of stereoisomers (40:6:54) | 5.0 in liver (>90% of total Car) | Accumulated from dietary crustaceans | |
| Animals (Invertebrate), Mollusca, Polyplacophora | ||||||
| Placiphorella japoonica | Chiton | Free form | Mixtures of stereoisomers (5:3:2) | 1.25 (~34% of total Car) | Oxidative metabolite of β-carotene | |
| Acanthochitona defilippii | Chiton | Free form | 3S,3′S | 1.55 in gonad (~4.0% of total Car) | Oxidative metabolite of β-carotene | |
| Liolophura japonica | Chiton | Free form | 3S,3′S | 0.8 in viscera (~10% of total Car) | Oxidative metabolite of β-carotene | |
| Animals (Invertebrate), Echinodermata | ||||||
| Peronella japonica | Sea urchin | Free form | Mixtures of stereoisomers (3:7:90) | ~3.0 in gonad (c.a.43% of total Car) | Oxidative metabolite of β-carotene | |
| Asteria pectinifera | Starfish | Free form | Mixtures of stereoisomers (50:25:25) | ~1.35 (% of total Car) | Oxidative metabolite of β-carotene | |
| Asterias amurensis | Starfish | Free form | Mixtures of stereoisomers (48:25:27) | ~4.64 (% of total Car) | Oxidative metabolite of β-carotene | |
| Animals (Invertebrate), Arthropoda, Crustacea, Decapoda (Lobsters, rock lobsters and crawfishes) | ||||||
| Procambarus clarkii | Louisiana crawfish | Fatty acid ester/ Free form | Mixtures of stereoisomers | 7.9–19.8 in carapace | Oxidative metabolite of β-carotene | |
| Pontastacus leptodactylus (Astacus leptodactylus) | Turkish crayfish | Fatty acid esters /Free form | Mixtures of stereoisomers ** | 5.0 in carapace 0.13 in muscle 0.98 in intestine (82.5% of total Car) | Oxidative metabolite of β-carotene ** | |
| Panulirus japonicus | Japanese Spiny Lobster (Ise-ebi) | Free form/ Fatty acid esters | Mixtures of stereoisomers (20:20:56) | 3.3 in carapace (65% of total Car) | Oxidative metabolite of β-carotene | |
| Animals (Invertebrate), Arthropoda, Crustacea, Copepoda | ||||||
| Tigriopus californicus | Red marine copepod | Free form (major) | 3S,3′S (major) | ~423 | Oxidative metabolite of β-carotene | |
| Animals (Invertebrate), Arthropoda, Crustacea, Eucarida | ||||||
| Euphausia superba | Antarctic krill | Fatty acid esters/ Free form | 3R,3′R (Major, ~70%) | ~566 in eye | Oxidative metabolite of β-carotene ? | |
| Euphausia pacifica | Pacific krill (Isada) | Fatty acid esters/ Free form | 3R,3′R (Major) | ~ 252 in eye | Oxidative metabolite of β-carotene ? | |
| Animals (Invertebrate), Arthropoda, Crustacea, Decapoda (Prawns and shrimps) *** | ||||||
| Pandalus borealis | Atlantic shrimp (Northern prawn) | Fatty acid esters/ Free form | Mixtures of stereoisomers (25:52:23) | ~28.48 in carapace | Oxidative metabolite of β-carotene | |
| Penaeus japonicus | Japanese tiger prawn (Kuruma-ebi) | Fatty acid esters/ Free form | Mixtures of stereoisomers (12:40:48) | ~13 in carapace | Oxidative metabolite of β-carotene | |
| Penaeus semisulcatus | Green tiger prawn | Fatty acid esters/ Free form | Mixtures of stereoisomers (19:44:57) | ~15.6 in carapace | Oxidative metabolite of β-carotene | |
| Penaeus monodon | Black tiger prawn | Fatty acid esters/ Free form | Mixtures of stereoisomers (16:43:41) | ~7.3 in carapace | Oxidative metabolite of β-carotene | |
| Litopenaeus vannamei | Whiteleg shrimp | Fatty acid esters/ Free form | Mixtures of stereoisomers (23:44:32) | ~5.8 in carapace | Oxidative metabolite of β-carotene | |
| Metapenaeus joyneri | Shiba shrimp | Fatty acid esters/ Free form | Mixtures of stereoisomers (14:46:40) | ~3.3 in carapace | Oxidative metabolite of β-carotene | |
| Animals (Invertebrate), Arthropoda, Crustacea, Decapoda , Brachyura (Crabs) *** | ||||||
| Chionoecetes japonicus | Red snow crab (Beni-zuwai crab) | Fatty acid esters/ Free form ** | Mixtures of stereoisomers ** | ~23 in carapace (with demineralization treatment) | Oxidative metabolite of β-carotene? | |
| Chionoecetes opilio | Snow crab (Zuwai crab) | Fatty acid esters/ Free form | Mixtures of stereoisomers? | ~11.9 in carapace (~91.7% of total Car) | Oxidative metabolite of β-carotene? | |
| Callinectes sapidus | Blue crab | Fatty acid esters/ Free form | Mixtures of stereoisomers? | ~9.8 (with demineralization treatment) | Oxidative metabolite of β-carotene? | |
| Cancer pagurus | Brown crab | Fatty acid esters/ Free form | Mixtures of stereoisomers (56:24:20) | 0.37 in carapace | Oxidative metabolite of β-carotene? | |
| Animals (Invertebrate), Arthropoda, Crustacea, Decapoda (Others) *** | ||||||
| Paralithodes brevipes | Hanasaki crab | Fatty acid esters/ Free form | Mixtures of stereoisomers (26:9:6) | ~2.4 in carapace (~39.9% of total Car) | Oxidative metabolite of β-carotene | |
| Paralithodes camtschaticus | Red king crab | Fatty acid esters/ Free form | Mixtures of stereoisomers (45–55:7–19:27–48) | ~0.35 in carapace (~97% of total Car) | Oxidative metabolite of β-carotene | |
| Cervimunida princeps | Squat lobster | Fatty acid esters/ Free form | Mixtures of stereoisomers (26:9:65) | ~ 0.45 in carapace (~100% of total Car) | Oxidative metabolite of β-carotene | |
| Upogebia major | Japanese mud shrimp | Fatty acid esters/ Free form | Mixtures of stereoisomers (72:21:7) | ~ 0.25 in carapace (~100% of total Car) | Oxidative metabolite of β-carotene | |
| Birgus latro | Coconut crab | Fatty acid esters/ Free form | Mixtures of stereoisomers (9:41:50) | ~ 0.3 in carapace (~96% of total Car) | Oxidative metabolite of β-carotene | |
| Asellus aquaticus | Isopoda | Free form/ Fatty acid esters? | N/A | ~0.52 (~37.5% of total Car) | Oxidative metabolite of β-carotene? | |
| Pleuroncodes planipes | Red crab langostilla | Fatty acid esters/ Free form | Mixtures of stereoisomers (3–4:1:3–4) | N/A | Oxidative metabolite of β-carotene? | |
| Animals (Invertebrate), Arthropoda, Arachnida, Acari | ||||||
| Balaustium murorum | Red velvet mite | Free form/ Fatty acid esters | 3S,3′S ** | ~61,530 mg/ 100 g protein (60% of total Car) | Oxidative metabolite of zeaxanthin (De novo Synthesis **) | |
| Panonychus citri | Citrus red mite | Fatty acid esters | 3S,3′S ** | ~263 mg/ 100 g protein (42.5% of total Car) | De novo Synthesis ** | |
| Tetranychus kanzawai | Kanzawa spider mite | Fatty acid esters | 3S,3′S ** | Undefined | De novo Synthesis | |
| Tetranychus urticae | Two-spotted spider mite | Fatty acid esters | 3S,3′S ** | Undefined | De novo Synthesis | |
| Eylais hamata | Hydracarina | Free form/ Fatty acid esters (minor) | N/A | 12.2 (c.a.67% of total Car) | N/A | |
| Eylais extendens | Hydracarina | N/A | N/A | Undefined (c.a.70% of total Car) | N/A | |
| Schizonobia sycophanta | Parasite mite | Fatty acid ester | 3S,3′S | Undefined (30% of total Car) | De novo Synthesis ** | |
| Animals (Invertebrate), Arthropoda, Arachnida, Araneae | ||||||
| Trichonephila clavata | Arachnida spider | ?? | Mixtures of stereoisomers (2:1:1) | 0.02 (1.9% of total Car) | Oxidative metabolite of β-carotene | |
| Animals (Invertebrate), Arthropoda, Insecta | ||||||
| Locusta migratoria | Migratory locust | Free form | Mixtures of stereoisomers (2:1:1) | 0.25 in brown form (12.5% of total Car) | Oxidative metabolite of β-carotene | |
| Aiolopus thalassinus tamulus | Grasshopper | Free form | Mixtures of stereoisomers (2:1:1) | 0.09 in brown form (3.0% of total Car) | Oxidative metabolite of β-carotene | |
| Schistocerca gregaria | Desert locust | Free form | Mixtures of stereoisomers * | N/A | Oxidative metabolite of β-carotene | |
| Animals (Vertebrate), Fish (Salmonidae) | ||||||
| Oncorhynchus nerka (Wild, anadromous form) | Sockeye salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers (depending on AX source) | 2.6–3.8 in muscle in egg (% of total Car) | Accumulated from dietary crustaceans | |
| Oncorhynchus nerka (Wild, non-anadromous form) | Kokanee salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers (depending on AX source) | −0.8 in muscle 0.4–2.8 in skin −1.1 in golnad (−94% of total Car) | Accumulated from dietary crustaceans | |
| Oncorhynchus kisutch | Coho salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers (depending on AX source) | 1.0–2.1 in muscle in egg (% of total Car) | Accumulated from dietary crustaceans | |
| Salvelinus alpinus (Wild) | Arctic char | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers (depending on AX source) | 0.86 in muscle in egg (−30% of total Car) | Accumulated from dietary crustaceans | |
| Salmo salar (Wild) | Atlantic salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers (depending on AX source) | 0.6–0.8 in muscle in egg (% of total Car) | Accumulated from dietary crustaceans | |
| Oncorhynchus keta | Chum salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mainly 3S,3′S (in ovary) | 0.1–0.5 in muscle −0.7 in egg 0.1 in skin (male) (4.8–90% of total Car) | Accumulated from dietary crustaceans | |
| Oncorhynchus gorbuscha | Pink salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers | 0.4–0.7 in muscle in egg (% of total Car) | Accumulated from dietary crustaceans | |
| Oncorhynchus tshawytscha | Chinook salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers | 0.54 in muscle in egg (% of total Car) | Accumulated from dietary crustaceans | |
| Oncorhynchus masou (Wild, anadromous form) | Masu salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers | 0.3–0.8 in muscle 0.03–0.8 in skin 0.7–1.7 in egg (1.9–80% of total Car) | Accumulated from dietary crustaceans | |
| Oncorhynchus masou ishikawae (Wild, anadromous form) | Red-spotted masu salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers | 0.2 in muscle trace in skin N/D in egg (1.9–68.5% of total Car) | Accumulated from dietary crustaceans | |
| Oncorhynchus masou rhodurus | Biwa trout | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers | 0.2 in muscle −0.1 in skin (3.2–58.3% of total Car) | Accumulated from dietary crustaceans | |
| Oncorhynchus mykiss (Wild, pigmented phenotype) | Rainbow trout | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers | trace in muscle 0.8 in skin trace in egg (1.9–42.3% of total Car) | Accumulated from dietary crustaceans | |
| Animals (Vertebrate), Fish (Non-Salmonidae ) | ||||||
| Sebastolobus macrochir | Broadbanded thornyhead (Kichiji rockfish) | Fatty acid esters (skin) | N/A | 26 in skin (>90% of total Car) | Accumulated from dietary crustaceans or Oxidative metabolite of β-carotene/ zeaxanthin? | |
| Plectropomus leopardus | Coral trout (Suziara) | Fatty acid esters/ Free form (skin) | Mixtures of stereoisomers (13:7:80) | 19.5 in skin (84.8% of total Car) | Accumulated from dietary crustaceans or Oxidative metabolite of β-carotene/ zeaxanthin? | |
| Epinephelus fasciatus | Blacktip grouper (Akahata) | Fatty acid esters (skin) | N/A | 2.27 in skin (74% of total Car) | Accumulated from dietary crustaceans or Oxidative metabolite of β-carotene/ zeaxanthin? | |
| Beryx splendens | Splendid alfonsino (Kinmedai) | Fatty acid esters (skin) | Mixtures of stereoisomers | 0.9 in skin (c.a. 100% of total Car) | Accumulated from dietary crustaceans or Oxidative metabolite of β-carotene/ zeaxanthin? | |
| Pagrus malor | Red sea bream (Madai) | Fatty acid esters (skin) | Mixtures of stereoisomers (38:0:62) | ~2 in skin (wild) 0.25 in skin (firmed w/o. AX) / 0.98 (firmed with. AX) (~c.a 45% of total Car) | Accumulated from dietary crustaceans and supplementary pigment | |
| Carassius auratus | Goldfish (Kingyo/Hibuna) | Free/ Fatty acid esters (skin) | 3S,3′S | 0.58 (whole body) (~47% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin | |
| Branchiostegus japonicus | Red tilefish (Red amadai) | Fatty acid esters (skin) | Mixtures of stereoisomers (24:24:52) | 0.39 in skin (35.8% of total Car) | Accumulated from dietary crustaceans | |
| Animals (Vertebrate), Amphibian | ||||||
| Cynops pyrrhogaster | Japanese newt | Free form/ Fatty acid esters | N/A | 4.55 in skin (c.a.21% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin | |
| Salamandra salamandra | Fire salamander | Free form/ Fatty acid esters | N/A | 0.23 (37.5% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin | |
| Lissotriton vulgaris (Triturus vulgaris) | Smooth newt/common newt | Free form | N/A | 0.1 (−23.5% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin | |
| Ranitomeya sirensis | Sira poison frog | Free form/ Fatty acid esters ? | N/A | N/A (-c.a. 40% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin | |
| Bufo bufo | Common toad | Free form/ Fatty acid esters | N/A | N/D in muscle and liver 0.02 in skin 0.35 in intestine (25.8–57.4% of total Car) 0.23 in gonads (95.8% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin | |
| Bufotes viridis (Bufo viridis) | European green toad | Free form/ Fatty acid esters | N/A | N/D in muscle and liver 0.02 in skin 0.35 in intestine (25.8–57.4% of total Car) 0.23 in gonads (95.8% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin | |
| Pelobates fuscus | European common spadefoot toad | Free form | N/A | 1.1 in liver (19.6% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin ** | |
| Melanophryniscus rubriventris | (Aposematic poison toad) | N/A (Free form/ ester form?) | N/A | Undefined | Oxidative metabolite of β-carotene ** | |
| Animals (Invertebrate), Reptile | ||||||
| Chlamydosaurus kingii. (the western red-frilled form) | Frillneck lizard | N/A (Free form?) | N/A | Undefined | Oxidative metabolite of β-carotene | |
| Chrysemys picta | Painted turtle | N/A | N/A | c.a 0.11 in leg skin | Oxidative metabolite of β-carotene | |
| Trachemys scripta | Red-eared slider | N/A | N/A | c.a 0.06 in skin around eye spot | Oxidative metabolite of β-carotene | |
| Animals (Invertebrate), Aves *** | ||||||
| Lagopus lagopus scoticus | Red grouse | Free form/ Fatty acid esters | N/A | 317.8 in combs N/D in plasma (-c.a.81.6% of total Car) | Oxidative metabolite of β-carotene | |
| Pygoscelis papua | Gentoo penguins | Free form | N/A | 2.19 in blood, breeding adults and chicks | Accumulated from dietary crustaceans, fishes | |
Footnotes: The respective number was quoted from the reference(s), and it may vary depending on the collection location and season.†, the presence of binding forms to carotenoproteins would not be mentioned in this table; ‡, the identification method of the compounds remains uncertain; *, the biosynthetic pathways have not been fully characterized; **, based on the information on close species/genus;*** Since astaxanthin is diversely found in the skin, feathers, and retinas of birds, only the characteristic reports are described. ?; based on the information on close taxa.
Abbreviations: N/A = not available; N/D = not detected.
[Source 2 ]Natural astaxanthin vs synthetic astaxanthin
Many differences are documented between synthetic (man-made) astaxanthin and natural astaxanthin 1. Firstly, synthetic astaxanthin is cheaper than natural microalgal astaxanthin since microalgal cultivation and harvesting are cost-consuming 1. Secondly, synthetic astaxanthin is mostly unesterified while microalgal astaxanthin is esterified 38, 56. Thirdly, synthetic astaxanthin and microalgal astaxanthin contain different geometrical and optical isomers 56. Previous research assayed that microalgal astaxanthin could be better than synthetic astaxanthin in astaxanthin accumulation, safety, and potential nutritive quality of Chinese mitten crab (Eriocheir sinensis) 57, 56. Besides, synthetic astaxanthin is markedly inferior to algal natural astaxanthin as an antioxidant 58. Therefore, natural astaxanthin from algae and aquatic animals has shown better benefits than synthetic astaxanthin 1.
Synthetic astaxanthin
Astaxanthin production has relied mostly on most microalgal strains, such as Chlorella zofingiensis, Haematococcus pluvialis, and Scenedesmus obliquus. Generally, no obvious morphological changes are observed during the cultivation of green-colored microalgae 59. Under adverse conditions, the microalgae cells are transformed into resting cysts, in which microalgae growth is prohibited but the survival efficiency of algal cells is intensified 60. In the resting stage, the blood-red color of microalgal cells and astaxanthin content originated in a harsh environment 59. Therefore, astaxanthin synthesis in microalgal strains can be regarded as a self-protection mechanism, which enhances the survival of algal cells at the expense of microalgal biomass accumulation. Beta-carotene forms a general precursor for astaxanthin from microalgal cells. The precursor is catalyzed by the enzymatic activity of beta-carotene ketolase and hydroxylase, resulting in metabolic intermediates canthaxanthin and zeaxanthin, respectively 61. Therefore, the synthesis process of astaxanthin is performed through different pathways according to microalgal species and enzymatic activities of beta-carotene 62, 63. Besides, the natural contents of three geometric isomers (all-trans, 9-cis, and 13-cis-astaxanthin) of astaxanthin differ in microalgae. For example, the content of all-trans astaxanthin is higher than that of cis astaxanthin in Haematococcus pluvialis and Chlorella zofingiensis; however, cis-astaxanthin has much higher antioxidative properties 64. Under the natural environment, the astaxanthin synthesis in microalgae is too low to neet the market demand. Therefore, many studies reported production technologies to promote astaxanthin productivity and alleviate the conflict between produced microalgal biomass and astaxanthin synthesis. In recent years, astaxanthin productivity can be improved by genetic modification technology 65.
Astaxanthin Metabolism: Absorption and Tissue Distribution
In pharmacokinetic studies, after ingestion of esterified natural astaxanthin, only unesterified astaxanthin appears in the blood 66. This is most likely due to breaking the ester bonds by digestive enzymes via their hydrolytic activity. Absorption into the intestinal lining cells (enterocytes) is thought to occur by passive diffusion and is facilitated in the presence of fat or other lipids 67. The enterocytes then incorporate the unesterified astaxanthin into chylomicrons, which transport it to the liver 68. The liver does not convert this molecule to vitamin A or otherwise biochemically transform it 69. Instead unesterified astaxanthin becomes incorporated into low-density lipoprotein (LDL) and high-density lipoprotein (HDL), which then distribute it to the tissues via the circulation 66.
When astaxanthin is fed to human subjects, detailed pharmacokinetic data are difficult to obtain for single doses of less than 10 mg, due to limitations of assay precision 66. However, there is good data to indicate a single 10-mg dose can persist in the blood for 24 hours and a 100-mg dose for 76 hours 66. Doses as low as 1 mg can significantly increase blood levels when taken once daily for four weeks 70.
Astaxanthin’s bioavailability is substantially affected by meal timing and by smoking. In a 2009 study, a single 48-mg dose was much better absorbed when taken just after a meal than on an empty stomach, and was about 40-percent less bioavailable in subjects who smoked 67.
What is astaxanthin used for?
Astaxanthin is a red fat-soluble red-orange pigment which does not have pro-Vitamin A activity in the human body, although some of the studies reported that astaxanthin has more potent biological activity than other carotenoids 55. Unlike many other antioxidant supplements, astaxanthin does not develop into a pro-oxidant once exhausted,unlike beta-carotene and lycopene 71. Therefore, astaxanthin is often described as a “pure antioxidant”. In fact, it has been demonstrated that astaxanthin, in contrast to beta-carotene and lycopene, exhibited significant antioxidant activity and reduced lipid peroxidation in a liposomal model membrane 72. When applied to biological membranes, astaxanthin may allow Haematococcus cyst cells to resist oxidative stress resulting from adverse environmental conditions 73, 74. Astaxanthin may also exert a protective role in muscle cell membranes during the extreme physical exertion experienced by salmon, during migration from the sea to their spawning ground. Based on this scenario in salmon, astaxanthin has also been investigated as an intervention for oxidative muscle damage during and after endurance exercise 75. Although it is still unclear whether the observed effects of astaxanthin are a result of its direct and/or indirect antioxidant activity, several clinical studies have shown that astaxanthin reduced oxidative stress markers in humans (Table 4).
Astaxanthin is currently only used as a food ingredient 76, 16. The United States Food and Drug Administration (FDA) has approved the use of astaxanthin as food colorant in animal and fish feed 77. The European Commission considers natural astaxanthin as a food dye 78. Astaxanthin is used as a source of pigment in the feed for salmon, trout and shrimp 79. For dietary supplement in humans and animals, astaxanthin is obtained from seafood or extracted from Haematococcus pluvialis 80. The consumption of astaxanthin can prevent or reduce risk of various disorders in humans and animals 8.
Table 3. Astaxanthin added as an antioxidant in different food products
| Food Products | Addition Form | Concentration | Source | Results |
|---|---|---|---|---|
| Wholemeal cookie | Astaxanthin powder | 5%, 10% and 15% | Haematococcus pluvialis | Antioxidant properties (DPPH radical scavenging and oxygen radical absorbance capacity value) of the cookies increased significantly with increasing astaxanthin content. |
| Formulated cookies | Astaxanthin powder | 10%, 15% and 20% | Hematococcus pluvialis | Astaxanthin could stabilize lipid oxidation in cookies. |
| Rainbow trout | Adonis aestivalis extract | 50, 100 and 200 mg/kg | Adonis aestivalis | Dietary Adonis aestivalis extract improved the flesh colour and the antioxidative status of rainbow trout, and the supplemental level was suggested to be 3.4 g/kg with astaxanthin inclusion of 100 mg/kg diet. |
| Minced Tilapia | Green solvents containing astaxanthin | 1.00% | Shrimp wastes | Adding astaxanthin to surimi samples can prolong its shelf life. |
| Tilapia fillets | Added in vegetable oil | 3% of astaxanthin doses of 100 and 200 mg/kg of feed | Haematococcus pluvialis | Tilapia supplemented with astaxanthin can reduce lipid oxidation index. |
| Coral trout (Plectropomus leopardus) | Haematococcus pluvialis powder | 0, 0.5, 1.0 and 2.0 g/kg, | Haematococcus pluvialis | Adding 1.0 g/kg astaxanthin-rich Haematococcus pluvialis powder (the content of astaxanthin is 0.091 g/kg) can improve the activity of antioxidant enzymes as well as the total antioxidant capacity of coral trout, and significantly reduced malondialdehyde content. |
| Ready-to-cook shrimp surimi products | Astaxanthin Extract | 30 g/kg | Shrimp by-product powder | Astaxanthin extract from shrimp by-products had positive effects on the antioxidant activity and color difference of ready-to-cook shrimp surimi products. |
| Black Tiger Prawn | Astaxanthin powder | 0.02%, 0.04%, 0.08% and 0.16% | Chemical synthesis | Dietary synthetic astaxanthin is a suitable feed additive to improve growth, body color and antioxidant capacity of black tiger prawn. |
| Meatballs | Astaxanthin powder | 0.5% and 1% w/w | Haematococcus pluvialis | Astaxanthin use in meatball production can enhance lipid oxidative stability and colour characteristics. |
| Lamb meat | Astaxanthin-commercial powder | 25 mg of pure astaxanthin/kg milk-replacer powder | AstaReal®EL25, Nacka, Sweden, containing 2.5% natural astaxanthin | Astaxanthin improved lipid oxidative stability in lamb meat frozen for 3 months and it can reduce butylated hydroxytoluene levels in suckling lamb meat. |
| Male broilers | Astaxanthin powder | 0, 20, 40 and 80 ppm | Haematococcus pluvialis | Astaxanthin improved meat quality and antioxidant status of male broilers exposed to heat stress. |
| Diets of laying hens | 10% oil extract of astaxanthin | 10, 20 and 30 mg/kg feed | Haematococcus pluvialis | Astaxanthin supplements in the diets had a greater enriching effect on carotenoids in egg yolks. |
| Egg | Carotenoid-rich dried cell powders were added to hens’ diet | 8 mg/kg diet | Paracoccus carotinifaciens | Feeding hens with dried Paracoccus carotinifaciens cell powders increased the concentrations of valuable carotenoids (astaxanthin, adonirubin, and adonixanthin) in their egg yolk and enhanced the egg yolk pigmentation. |
| Staple crop maize | Astaxanthin biosynthesis | 46.76–73.65 mg/kg dry weight | Haematococcus pluvialis | Astaxanthin-rich maize directly applied to chicken feed and laying hens successfully accumulated astaxanthin in the egg yolk. Astaxanthin rich corn retained most of the astaxanthin when stored at 4 °C for 7 months compared to traditional algae powder. |
| Chia oil | Added in chia oil | 400 µg/g oil | Haematococcus pluvialis | Blends of chia oil and astaxanthin stored at 25 °C showed good stability and the content of α-linolenic acid in chia oil did not change significantly. |
| Turkish delight | Astaxanthin pigment extract | 3.75, 7.50, 11.25 and 15 mg | Clementine peels | Adding astaxanthin pigment with high essential oil content in Turkish delight can improve its antioxidant activity. |
| Yogurt | Astaxanthin oleoresin | 0.055 ± 0.001 g in 750 g yogurt | Haematococcus pluvialis | The results shows that it is possible to use oleoresin of astaxanthin complex to simulate the apricot color and is well-packed in the lipid–protein matrix of the final yogurt products. |
Astaxanthin health benefits
The use of astaxanthin (3,3’-dihydroxy-beta,beta-carotene-4,4’-dione) as a nutritional supplement has been rapidly growing in foods, feeds, nutraceuticals and pharmaceuticals. Astaxanthin, a xanthophyll carotenoid, has excellent antioxidant properties and is considered to play an important role in the regulation of oxidative stress, inflammation, cell life cycle management and lipid metabolism in the body, thereby positively affecting skin health, visual function, cardiovascular function, nervous system function, physical performance and immune system efficacy. In addition, based on data from previous preclinical studies, astaxanthin has shown promising potential for regulating gut microbial ecological balance and anti-diabetic activity.
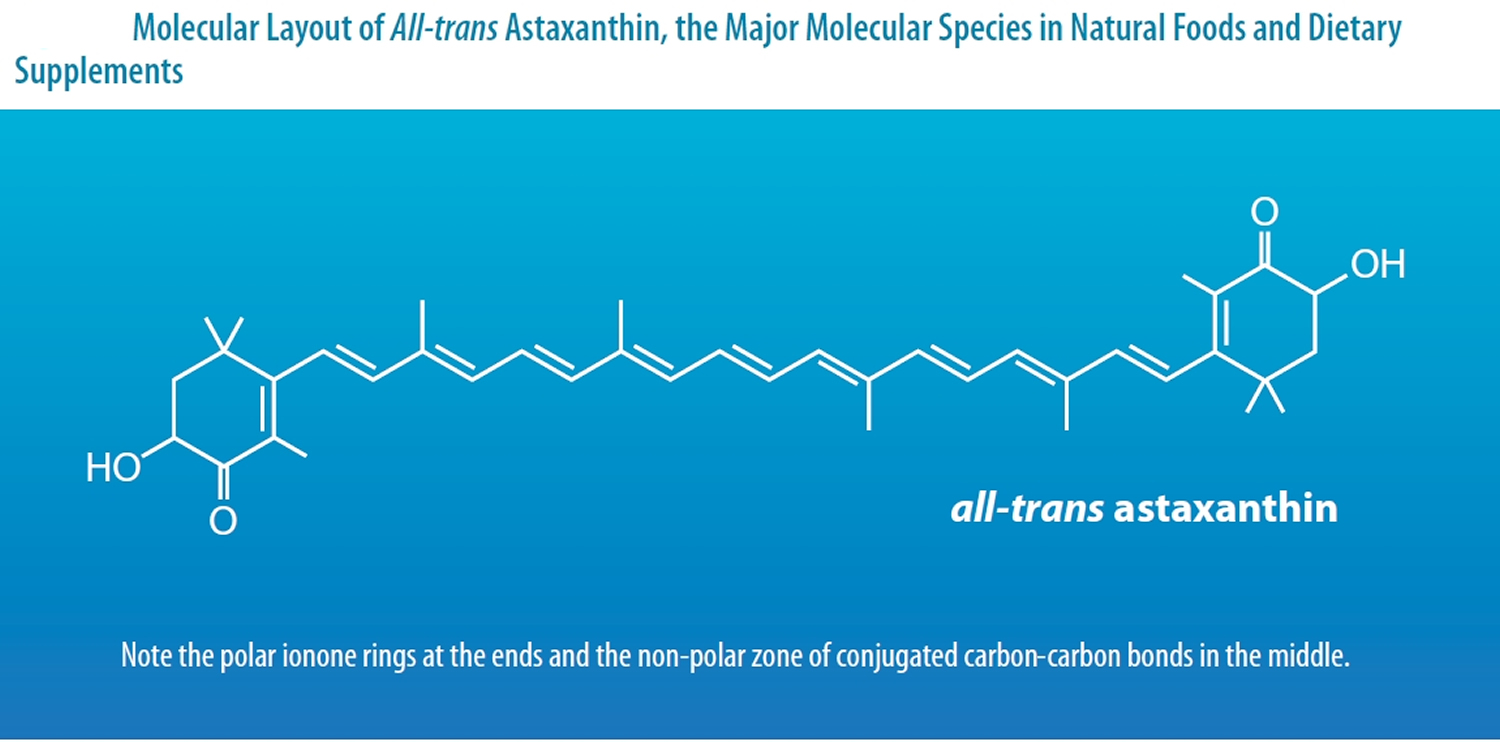
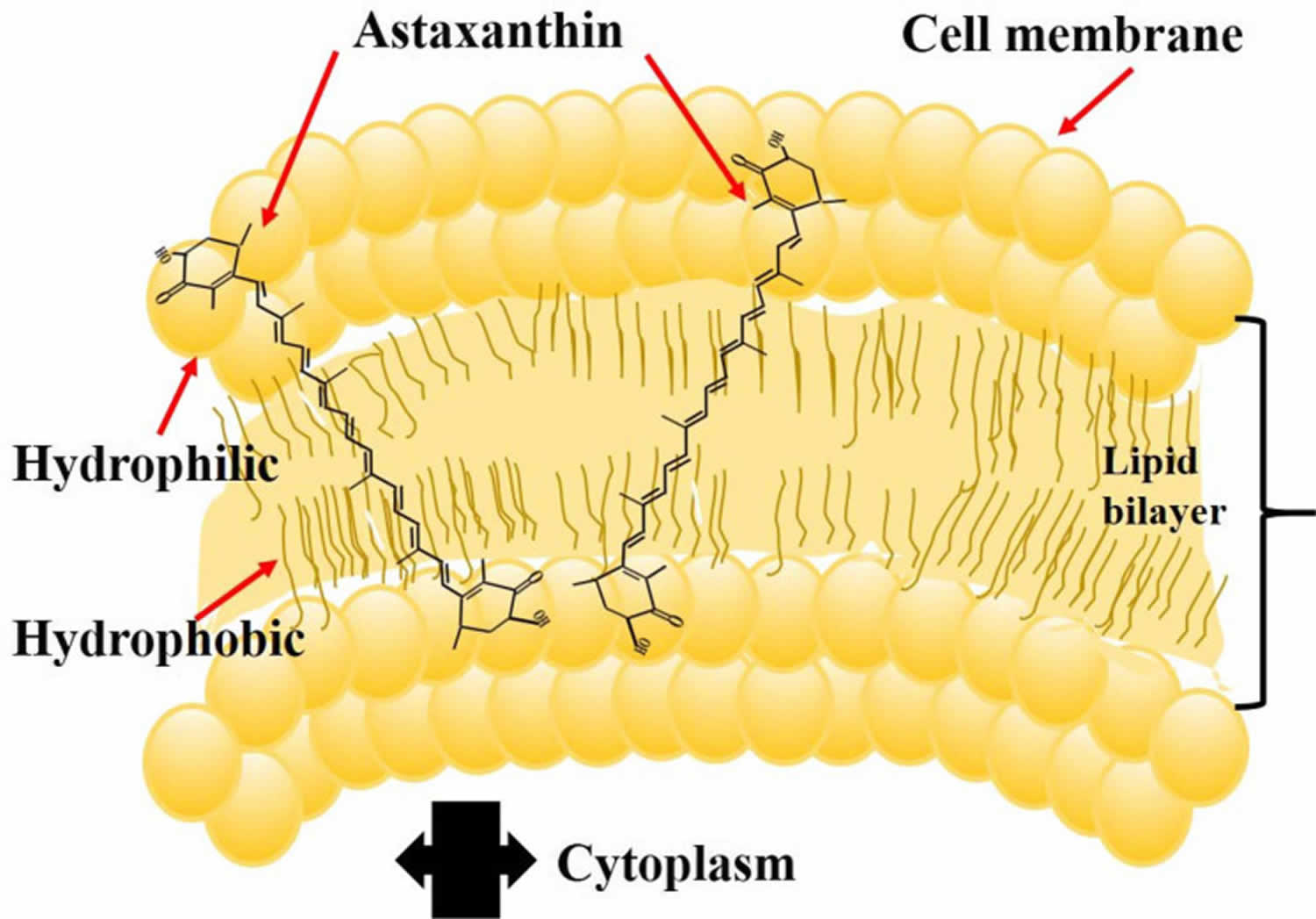
Astaxanthin molecule neutralizes free radicals or other oxidants by either accepting or donating electrons, and without being destroyed or becoming a pro-oxidant in the process. The astaxanthin molecule has an extended shape, with a polar structure at either end of the molecule and a nonpolar zone in the middle (Figure 1). The polar structures are ionone rings that have potent capacity for quenching free radicals or other oxidants, primarily in an aqueous environment, but possibly also in the absence of water 81. Astaxanthin’s linear, polar-nonpolar-polar molecular layout equips it to precisely insert into the membrane and span its entire width. In this position, astaxanthin can intercept reactive molecular species within the membrane’s hydrophobic interior and along its hydrophilic boundaries (see Figure 6).
The red color of Astaxanthin is due to the conjugated double bonds at the center of the compound 82, 38. This type of conjugated double bond acts as a strong antioxidant by donating the electrons and reacting with free radicals to convert them to be more stable product and terminate free radical chain reaction in a wide variety of living organisms 83, 53, 84, 85. Astaxanthin showed better biological activity than other antioxidants such as beta-carotene, lycopene, and vitamin C, because it could link with cell membrane from inside to outside and can be incorporated into liposome phospholipid bilayers (Figure 6) 86, 87, 88, 89, 90.
Astaxanthin has unique chemical properties based on its molecular structure. The presence of the hydroxyl (OH) and keto (CdO) moieties on each ionone ring explains some of its unique features, namely, the ability to be esterified and a higher antioxidant activity and a more polar nature than other carotenoids 82, 38. In its free form, astaxanthin is considerably unstable and particularly susceptible to oxidation. Hence it is found in nature either conjugated with proteins (e.g., salmon muscle or lobster exoskeleton) or esterified with one or two fatty acids (monoester and diester forms), which stabilize the molecule. Various astaxanthin isomers have been characterized on the basis of the configuration of the two hydroxyl groups on the molecule. the geometrical and optical isomers of astaxanthin are distributed selectively in different tissues and that levels of free astaxanthin in the liver are greater than the corresponding concentration in the plasma, suggesting concentrative uptake by the liver. Astaxanthin, similar to other carotenoids, is a very lipophilic compound and has a low oral bioavailability.
Another complication in the chemistry of natural astaxanthin is that the molecule in its free form is relatively uncommon within the various organisms that produce it. Instead, most of this astaxanthin is either conjugated with proteins or esterified with one or two fatty acids (as astaxanthin acyl monoesters or diesters) 91. Acyl esters make up more than 99 percent of the astaxanthin from H. pluvialis and approximately 80 percent of astaxanthin in krill 91. Thus, acyl monoester and diester forms make up virtually all the astaxanthin currently available in dietary supplements.
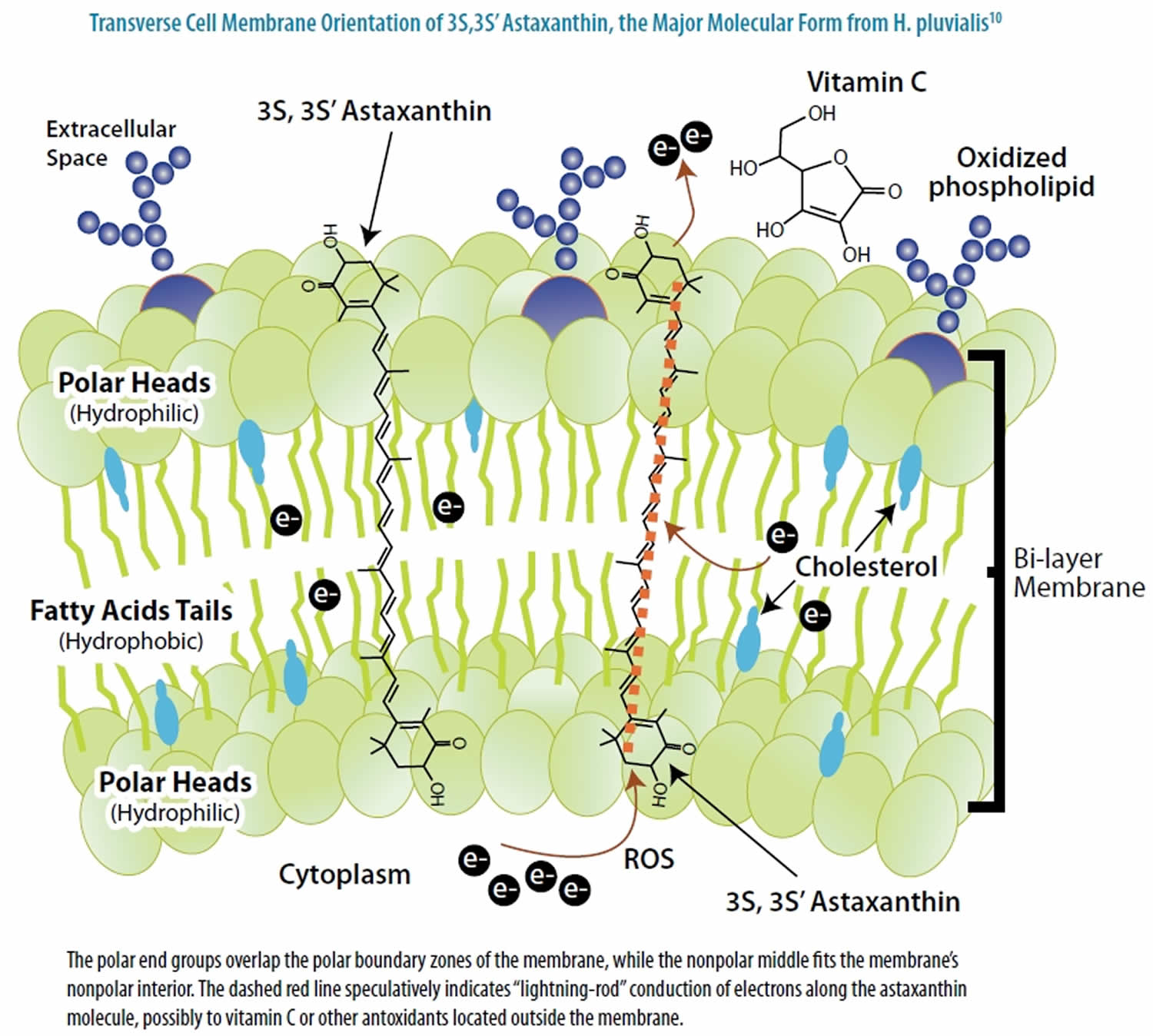
The bonding patterns of natural astaxanthin generate many different molecular forms (isomers), each with its unique three-dimensional shape. Pertaining to its use as a dietary supplement, virtually all commercially available natural astaxanthin is predominantly in the all-trans geometric form 3S,3S’ Astaxanthin, as occurs in Haematococcus pluvialis and as illustrated in Figure 3. This is the predominant natural astaxanthin used in all clinical trials to date. Natural astaxanthin has been extensively studied for its various health benefits. It is known to be a powerful antioxidant and anti-inflammatory phytonutrient that contributes to healthy aging in several critical areas, including improved mobility, locomotive activities, enhanced vision, mental fitness, cognitive functions and skin health.
Figure 6. Astaxanthin spanning across a cell membrane
In its position spanning the membrane, astaxanthin provides versatile antioxidant actions, including 92:
- donating electrons to unpaired electrons to neutralize free radicals;
- pulling away (“abstracting”) an unpaired electron, which also can neutralize a radical;
- bonding with the radical to form an unreactive “adduct”;
- conducting electrons or electronic energy out of the membrane (Figure 6);
- neutralizing radical species of nitrogen, sulfur, or carbon, in addition to oxygen; and
- carrying very low net molecular energy, therefore providing resistance to transformation into a pro-oxidant molecule.
Table 4. Human clinical study for astaxanthin
| Study Design | Subjects | Dose | Duration | Outcome | Author/Year/Reference |
|---|---|---|---|---|---|
| Randomized, double-blind, placebo-controlled, crossover study | 14 healthy subjects | 0, 6 mg/day | 4 weeks | Glutathione was ∼7% higher following AX compared with placebo (p < 0.05). No effect on plasma hydrogen peroxide or malondialdehyde (MDA; p > 0.05). Advanced oxidation protein products (AOPP) reduced by ∼28% (N.S.; p = 0.45). | McAllister M.J. et al., 2021 93 |
| Randomized, blinded, four-arm, prospective study | 32 subjects with oxidative stress, 8 subjects taking AX only | 0, 7 mg/day * | 4 weeks | Reduced serum oxidized LDL by 55.4% after 4 weeks (p < 0.05). Reduced MDA by 52.7% after 4 weeks (p < 0.05). | Petyaev I.M., et al., 2018 94 |
| Open-label, prospective study | 31 subjects; 18 obese, 8 overweight, 5 healthy weight | 4 mg/day | 92 days | Plasma MDA decreased with AX by 11.2% on day 15 and by 21.7% on day 29 (N.S.) | Chalyk, N. et al., 2017 95 |
| Open-label, prospective study | 35 subjects during cataract surgery | 6 mg/day | 2 weeks | Superoxide anion scavenging activity (U/mL) 18.2 ± 4.1 at 0 weeks reduced to 19.9 ± 3.6 after 2 weeks of supplementation compared with baseline, p < 0.05. Total hydroperoxides (U CARR) from 1.16 ± 0.18 at 0 weeks reduced to 1.04 ± 0.31 after 2 weeks of supplementation compared with baseline, p < 0.05 | Hashimoto H. et al., 2016 96 |
| Randomized, double-blind, placebo-controlled, prospective study | 40 healthy subjects (soccer players) | 0, 4 mg/day | 90 days | Improved prooxidant-antioxidant balance (PAB; p < 0.05) | Baralic, I. et al., 2015 97 |
| Randomized, double-blind, prospective study | 40 healthy subjects (soccer players) | 0, 4 mg/day | 90 days | Protected thiol groups against oxidative modification (increase in -SH groups, p < 0.05; improved PON1 activity towards paraoxon and diazoxon, p < 0.05 and p < 0.01, respectively) | Baralic I. et al., 2013 98 |
| Open-label, prospective study | 35 cataract patients | 6 mg/day | 2 weeks | Reduced total hydroperoxides (hydrogen peroxides, lipid peroxides, and peroxides of protein in aqueous humor; p < 0.05), increased superoxide scavenging activity (p< 0.05) | Hashimoto, H. et al., 2013 99 |
| Randomized, two-arm, prospective study | 23 obese and overweight subjects | 5 and 20 mg/day | 3 weeks | 5 mg/day: MDA decreased by 34.6%, isoprostane (ISP) decreased by 64.9%, superoxide dismutase (SOD) increased by 193%, and total antioxidant capacity (TAC) increased by 121% after 3 weeks compared with baseline (p < 0.01). 20 mg/day: MDA decreased by 35.2%, ISP decreased by 64.7%, SOD increased by 194%, and TAC increased by 125% after 3 weeks compared with baseline (p < 0.01). | Choi H.D. et al., 2011 100 |
| Randomized, double-blind, placebo-controlled, prospective study | 27 overweight subjects | 0, 20 mg/day | 12 weeks | MDA reduced by 17.3% and 29% after 8 and 12 weeks compared with placebo (p < 0.01), isoprostane (ISP) reduced by 40.2% and 52.9% after 8 and 12 weeks compared with placebo (p < 0.01), superoxide dismutase (SOD) increased by 124.8% after 12 weeks compared with placebo (p < 0.01), and total antioxidant capacity (TAC) increased by 130.1% after 12 weeks compared with placebo (p < 0.05) | Choi, H.D. et al., 2011 101 |
| Open-label, prospective study | 35 cataract patients | 6 mg/day | 2 weeks | Reduced total hydroperoxides (hydrogen peroxides, lipid peroxides, and peroxides of protein in aqueous humor; p < 0.05) | Hashimoto H. et al., 2011 102 |
| Randomized, Repeated, measured, prospective study | 39 heavy smokers, 39 non-smokers | 0, 5, 20, or 40 mg/day | 3 weeks | 5 mg/day: MDA and ISP significantly lower after 2 and 3 weeks compared with baseline in smokers (p < 0.05). SOD and TAC significant increase after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05) 20 mg/day: MDA and ISP significantly lower after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05). SOD and TAC significant increase after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05). 40 mg/day: MDA and ISP significantly lower after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05). SOD and TAC significant increase after 2 and 3 weeks compared with baseline in smokers (p < 0.05) | Kim, J.H. et al., 2011 103 |
| Randomized, double-blind, placebo-controlled, prospective study | 30 healthy subjects | 0, 6, 12 mg/day | 12 weeks | 6 mg/day: reduction in total phospholipid hydroperoxides (PLOOH) after 12 weeks compared with baseline (p < 0.01) and compared with placebo (p < 0.05). Reduced phosphatidyl-ethanolamine hydroperoxide (PEOOH) after 12 weeks compared with baseline (p < 0.05) and compared with placebo (p < 0.05). 12 mg/day: 48% reduction in total PLOOH after 12 weeks compared with baseline (p < 0.01) and 35% less total PLOOH at 12 weeks compared with the control group (p < 0.05). The 12 mg/day group had 46% less phosphatidylcholine hydroperoxide (PCOOH) at 12 weeks compared with baseline (p < 0.01). | Nakagawa K. et al., 2011 104 |
| Randomized, placebo-controlled study | 115 healthy subjects | 0, 40 mg/day | 90 days | Comparing with the control group, MDA contents in the test group decreased significantly (p < 0.01), and SOD and GSH-Px activities increased significantly (p < 0.01). | Peng L. et al., 2011 105 |
| Randomized, double-blind, placebo-controlled, prospective study | 42 healthy subjects | 2 or 8 mg/day | 8 weeks | 2 mg/day: Concentrations of plasma 8-hydroxy-2′-deoxyguanosine reduced after 4 weeks and 8 weeks compared with placebo (p < 0.05). 8 mg/day: Concentrations of plasma 8-hydroxy-2′-deoxyguanosine reduced after 4 weeks and 8 weeks compared with placebo (p < 0.05) | Park J.S. et al., 2010 106 |
| Open-label, prospective study | 35 healthy subjects (with high oxidative stress) | 12 mg/day | 8 weeks | Increased blood biological antioxidant potential (BAP; +4.6%, p < 0.05) | Iwabayashi M. et al., 2009 107 |
| Open-label,prospective study | 6 healthy subjects and 6 Sjoegren’s syndrome subjects | 12 mg/day | 2 weeks | Reduced protein oxidation (−10%, p < 0.05) | Yamada T. et al., 2010 108 |
| Randomized, double-blind, placebo-controlled, prospective study | 58 renal transplant recipients | 0, 12 mg/day | 12 months | Total plasma F2-isoprostanes reduced by 23.0% in placebo and 29.7% in AX groups (N.S.) | Fassett, R.G. et al., 2008 109 |
| Randomized, double-blind, placebo-controlled, prospective study | 39 healthy subjects | 0, 8 mg/day | 3 months | Decreased oxidation of fatty acids in healthy men (p < 0.05) | Karppi, J. et al., 2007 110 |
| Open-label, prospective study | 15 healthy postmenopausal women | 0, 2, 8 mg/day | 8 weeks | Decreased plasma TBARS levels: 2 mg group from 1.42 ± 0.18 to 1.13 ± 0.18 nM/mg (p < 0.05). 8 mg AX group from 1.62 ± 0.14 nM/mg to 1.13 ± 0.12 nM/mg after 8 weeks (p < 0.05). Increased TAS from 0.85 ± 0.42 mM/L to 1.90 ± 0.58 mM/L in the 8 mg group. Urinary 8-isoprostanes excretion did not decrease significantly. | Kim Y.K. et al., 2004 111 |
Astaxanthin a Unique Cell Membrane Antioxidant
Astaxanthin is believed to exert its antioxidant activity through both direct quenching and scavenging of reactive chemical species, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS). Additionally, it employs indirect mechanisms by inducing a group of antioxidant enzymes in biological systems. Astaxanthin provides cell membranes with potent protection against free radical or other oxidative attack. Experimental studies confirm that this nutrient has a large capacity to neutralize free radical or other oxidant activity in the nonpolar (“hydrophobic”) zones of phospholipid aggregates, as well as along their polar (hydrophilic) boundary zones 112. A particularly elegant experimental study by McNulty et al conclusively established astaxanthin’s membrane protection capacity 92.
McNulty’s group 92 assembled model membranes from phosphatidylcholine and a small amount of cholesterol, at ratios similar to natural cell membranes. They then introduced varying amounts of astaxanthin, zeaxanthin, lutein, beta-carotene, and lycopene to these model membranes and monitored the packing of the phospholipids by X-ray diffraction. They also induced peroxidative processes by gently increasing the temperature of the system. With the exception of astaxanthin, the carotenoids all disrupted the phospholipid packing and exacerbated peroxidative breakdown. The greater the membrane disruption by a carotenoid, the greater was its peroxidative effect. Only astaxanthin reduced peroxidation (by 41 percent) and preserved the membrane structure. The researchers gave particular credit to astaxanthin’s unique ionone polar groups.
Astaxanthin has also protected human LDL against oxidative attack. In a Japanese study 113, astaxanthin purified from krill was provided to healthy volunteers (average age 28 years; n=24) at 0 mg/day, 1.8 mg/day, 3.6 mg/day, 14.4 mg/day, or 21.6 mg/day, for 14 days. After supplementation, venous blood was drawn and the LDL analyzed for susceptibility to oxidation (lag time after a chemical peroxidative trigger) for each subject compared to baseline. Astaxanthin significantly increased lag time, which indicated a protective effect, at the dose levels of 3.6 mg/day and higher.
Supplementation with astaxanthin may lower lipid peroxidation in vivo 114. A double-blind, randomized controlled trial investigated astaxanthin (8 mg/day) versus a placebo for three months in Finnish men ages 19-33. Plasma 12- and 15-hydroxy fatty acids, both markers of lipid peroxidation, were statistically significantly reduced in the astaxanthin group, but not in the placebo group. The reduction in 15-hydroxy fatty acid in the astaxanthin group fell just short of significance when compared to the placebo group.
Table 5. Health benefits of astaxanthin in human subjects
| Duration of Experiment | Subjects in Humans | Dosage (mg/day) | Benefits of Astaxanthin | References |
|---|---|---|---|---|
| 2 weeks | Volunteers | 1.8, 3.6, 14.4 and 21.6 | Reduction of LDL oxidation | 115 |
| Single dose | Middle aged male volunteers | 100 | Astaxanthin take up by VLDL chylomicrons | 116 |
| 8 weeks | Healthy females | 0.2 and 8 | Decreased plasma 8-hydoxy-2′-deoxyguanosine and lowered in CRP levels | 117 |
| 8 weeks | Healthy adults | 6 | Assessed by blood pressure | 118 |
| 10 days | Healthy males | 6 | Improved blood rheology | 119 |
| 12 weeks | Healthy non-smoking finnish males | 8 | Decreased oxidation of fatty acids | 120 |
| 12 months | Age related macular degeneration | 4 | Improved central retinal dysfunction in age related macular degeneration | 121 |
| 12 weeks | Middle aged/elderly | 12 | Improved Cog health battery scores | 122 |
| 12 weeks | Middle aged/elderly | 6 | Improved groton maze learning test scores | 122 |
| 8 or 6 weeks | Healthy female or male | 6 | Improved skin winkle, corneocyte layer, epidermis and dermis | 123 |
| 2 weeks | Disease (bilateral cataract) | 6 | Improved superoxide scavenging activity and lowered hydroperoxides in the human aqueous humor | 124 |
Abbreviations: LDL, Low-density lipoproteins, VLDL, Very low-density lipoprotein, CRP, C-reactive protein.
Antioxidant Effects
Significant antioxidant powers have been ascribed to astaxanthin, based primarily on experimental findings. The real breakthrough with this nutrient, however, is that it produces clinically significant antioxidant benefits in human subjects, including groups especially vulnerable to oxidative stress, such as smokers, the obese, and the overweight.
Oxidative stress can be defined as a relative excess of free radical activity over antioxidant capacity, which in human subjects can be determined using blood samples 125. Alternately, oxidative breakdown products can be measured in the blood 126. People who are overweight or obese tend to manifest greater “oxidative stress” when compared to individuals within the normal weight range 127. In a Korean double-blind randomized controlled trial 126, astaxanthin “normalized” oxidative stress in individuals with weight challenges. In this three-week study, overweight and obese individuals (body mass index [BMI] >25.0 kg/m²; n=23) were randomized to receive astaxanthin at 5 mg/day or 20 mg/day and compared to a control group (n=10) with normal body weight (BMI <25.0 kg/m²) who received no intervention 126. At baseline, the plasma levels in overweight and obese individuals were significantly higher than normal weight individuals on two oxidative biomarkers – malondialdehyde (MDA) and isoprostanes (ISP), while plasma levels in overweight and obese individuals were significantly lower on two antioxidant measures – superoxide dismutase (SOD) and total antioxidant capacity. At the three-week mark, when compared against baseline, both astaxanthin groups showed significant lowering of oxidative markers malondialdehyde and isoprostanes (p<0.01 for 5 mg/day; p<0.001 for 20 mg/day). The astaxanthin groups also had significant increases in superoxide dismutase (SOD) and total antioxidant capacity. Marked improvements on all four measures caused the overweight and obese subjects to become statistically indistinguishable from the control group, suggesting that supplementation lowered oxidative stress and improved aspects of the antioxidant defense system. The improvements were not significantly better for the 20 mg/day group than the 5 mg/day group.
In another randomized controlled trial conducted by the same group 128, heavy smokers (n=39) were randomly allocated to receive astaxanthin at 5-, 20-, or 40 mg/day for three weeks. Compared with baseline, the plasma malondialdehyde and isoprostane levels decreased, whereas superoxide dismutase (SOD) and total antioxidant capacity increased in all intervention groups over the three-week period. In particular, isoprostane levels showed a significant dose-dependent decrease after astaxanthin intake.
Astaxanthin also can protect against oxidative DNA damage. In a 2010 double-blind randomized controlled trial conducted by Park et al, 129 healthy women (ages 20-23; n=42) received either a placebo or astaxanthin at doses of 2 mg/day or 8 mg/day for eight weeks. Both astaxanthin dosages significantly lowered plasma 8-hydroxy-2’-deoxyguanosine (8-OHdG), an indicator of oxidative DNA breakdown. Plasma 8-isoprostane, a marker of lipid peroxidation, was not significantly lowered. The authors attributed this finding to a lack of sensitivity and accuracy in their assay method.
Anti-inflammatory Benefits
In the Park double-blind randomized controlled trial 129, astaxanthin also significantly lowered C-reactive protein (CRP), a biomarker of systemic inflammation 130. Although the 2-mg/day dose had a significant CRP-lowering effect, the 8-mg/day dose fell short of statistical significance. Compared with the lower dose, the 8-mg/day dose significantly increased the cytokine interferon-gamma, which may indicate an anti-inflammatory effect, but also significantly increased interleukin-6 (IL-6), which can have a pro-inflammatory effect. The clinical significance of these findings is unclear, particularly since none of these, except CRP, is a generally accepted inflammatory marker.
Astaxanthin’s effect on CRP was also investigated in a small double-blind trial that was published without peer review 131. Subjects (ages 40-60 years; n=19), with no diagnosis of cardiovasculardisease, kidney disease, diabetes, or cancer, received three softgel capsules daily supplying either astaxanthin at 12 mg/day (with 120 mcg of lutein, 195 IU of vitamin A activity [in the form of beta-carotene), and 150 IU of vitamin E) or a placebo (safflower oil) for eight weeks. The astaxanthin combination lowered CRP levels by about 20 percent (from 1.35 mg/dL to 1.07 mg/dL), which was significantly better than placebo.
An eight-week, double-blind randomized controlled trial conducted on rheumatoid arthritis subjects was published in abstract form 132. One group (n=14) received the same combination as in the previously described study – 12 mg/day astaxanthin plus 120 mcg of lutein, 195 IU vitamin A activity (from beta-carotene), and 150 IU of vitamin E, while the other group (n=7) received a placebo. The improvement in self-reported scores of pain and satisfaction for the astaxanthin group was significantly better than for the placebo group, which suggests a possible anti-inflammatory effect.
Astaxanthin has been reported to benefit other inflammatory conditions such as canker sores, carpal tunnel syndrome, and “tennis elbow,” but the evidence currently available to support these claims is insufficient.
Skin health
Since the very early days of human use of astaxanthin, it has been expected to contribute to “skin health” due to its strong reactive oxygen species (ROS) quenching activity and lipid peroxidation inhibitory effect 2. In the very early days of human skin application studies, Yamashita 133 evaluated the effects of krill-derived astaxanthin diester on sunburn-induced redness and pigmentation and found it potentially beneficial. Subsequently, the evidence for the prevention of inflammation and pigmentation against UV-induced skin redness from various different research groups has been accumulating 2. Most of these actions could be expected to maintain barrier function in the epidermis through topical application or oral administration of astaxanthin 2. In parallel, several research groups have reported that astaxanthin may contribute to the maintenance of skin wrinkles and elasticity since its action in the dermis, where the impact of singlet oxygen seems to be strongest, is expected to protect the matrix that maintains the elasticity of the epidermis, such as collagen and elastin, from reactive oxygen species (ROS) 2. Recently, Honda et al 134 found that astaxanthin, especially its cis-isomers, directly inhibits elastase, suggesting that there may be a pathway that is not mediated by ROS. However, little is known about the dynamics of astaxanthin in the skin, and future studies are needed.
Metabolic Syndrome
Astaxanthin improved certain blood lipids in subjects with moderately elevated serum triglycerides (TGs). Healthy non-obese subjects (BMI <28 kg/m²), ages 20-65 years (n=61) with fasting triglycerides in the range 120-200 mg/dL, were recruited into a double-blind randomized controlled trial 135. They were randomly allocated to receive astaxanthin at 6 mg/day, 12 mg/day, or 18 mg/day, or a placebo for 12 weeks. Astaxanthin, as compared to placebo, significantly elevated HDL-cholesterol at the doses of 6 mg/day and 12 mg/day. It also significantly lowered triglycerides at doses of 12 mg/day and 18 mg/day compared to placebo. There was no effect on LDL-cholesterol at any dose. Astaxanthin also significantly increased blood adiponectin levels.
Adiponectin is a hormone produced by adipose tissue, cardiac and skeletal muscle, and vessel endothelia. Serum levels of adiponectin tend to be reduced in obese and/or diabetic subjects, smokers, patients with coronary heart disease, and individuals with metabolic syndrome 136. Although the results of this study suggest a normalization of adiponectin levels, 12 weeks of supplementation had no effect on BMI.
In a small, open-label trial (16 subjects), astaxanthin did not produce clinically significant benefits on any of the criteria for metabolic syndrome 137. Further investigation is required under better controlled conditions in order to clarify astaxanthin’s utility for this condition.
Anti-Diabetic Activity
Generally, oxidative stress levels are very high in diabetes mellitus patients. It is induced by hyperglycemia, due to the dysfunction of pancreatic β-cells and tissue damage in patients. Astaxanthin could reduce the oxidative stress caused by hyperglycemia in pancreatic β-cells and also improve glucose and serum insulin levels 138. Astaxanthin can protect pancreatic β-cells against glucose toxicity. It was also shown to be a good immunological agent in the recovery of lymphocyte dysfunctions associated with diabetic rats 139. In another study, ameliorate oxidative stress in streptozotocin-diabetes rats were inhibited by the combination of astaxanthin with α-tocopherol 140. It is also inhibited glycation and glycated protein induced cytotoxicity in human umbilical vein endothelial cells by preventing lipid/protein oxidation 141. Improved insulin sensitivity in both spontaneously hypertensive corpulent rats and mice on high fat plus high fructose diets was observed after feeding with astaxanthin 142. The urinary albumin level in astaxanthin treated diabetic mice was significantly lower than the control group 138. Some of the studies demonstrated that astaxanthin prevents diabetic nephropathy by reduction of the oxidative stress and renal cell damage 143.
Cardiovascular health
As people age, their red blood cells (RBCs) can be more susceptible to oxidative attack, resulting in peroxidative damage to the red blood cell membrane phospholipids 144, impairing its oxygen-carrying capacity. In a 2011 double-blind randomized controlled trial 145, healthy subjects, ages 50-69 years (n=30), were randomly allocated to receive astaxanthin at 6 mg/day or 12 mg/day or a placebo for 12 weeks. Both astaxanthin intakes significantly lowered red blood cell hydroperoxide levels; the 12 mg/day dose did not work significantly better than the 6 mg/day dose.
Astaxanthin also improved an experimental measure of “rheology” (blood flow capacity) in healthy men 146. Venous blood was drawn with heparin to protect against coagulation, then forced using mild pressure through tiny “microchannels,” each just seven millionths of a meter wide, approximating the diameter of an red blood cell and the width of a capillary. The time required to traverse these capillary-type tubes under a set pressure was termed the transit time. A total of 20 men were selected whose blood demonstrated transit times in the range of 45-70 seconds per 100 microliters. They were then randomly allocated to receive either astaxanthin (6 mg/day) or a placebo for 10 days. Upon retest, the astaxanthin group had significantly faster transit time compared to placebo. This finding suggests astaxanthin could potentially improve microcirculation.
Anticancer Activity
The specific antioxidant dose may be helpful for the early detection of various degenerative disorders. Reactive oxygen species such as superoxide, hydrogen peroxide and hydroxyl radical are generated in normal aerobic metabolism. Singlet oxygen is generated by photochemical events whereas peroxyl radicals are produced by lipid peroxidation. These oxidants contribute to aging and degenerative diseases such as cancer and atherosclerosis through oxidation of DNA, proteins and lipids 147. Antioxidant compounds decrease mutagenesis and carcinogenesis by inhibiting oxidative damage to cells. Cell–cell communication through gap junctions is lacking in human tumors and its restoration tends to decrease tumor cell proliferation. Gap junctional communication occurs due to an increase in the connexin-43 protein via upregulation of the connexin-43 gene. Gap junctional communication was improved in between the cells by natural carotenoids and retinoids 148. Canthaxanthin and astaxanthin derivatives enhanced gap junctional communication between mouse embryo fibroblasts 149. Increased connexin-43 expression in murine fibroblast cells by β-carotene was reported 150. Astaxanthin showed significant antitumor activity when compared to other carotenoids like canthaxanthin and β-carotene 151. It also inhibited the growth of fibrosarcoma, breast, and prostate cancer cells and embryonic fibroblasts 152. Increased gap junctional intercellular communication in primary human skin fibroblasts cells were observed when treated with astaxanthin 153. Astaxanthin inhibited cell death, cell proliferation and mammary tumors in chemically induced male/female rats and mice 154. H. pluvialis extract inhibited the growth of human colon cancer cells by arresting cell cycle progression and promoting apoptosis reported by Palozza et al. 152. Nitroastaxanthin and 15-nitroastaxanthin are the products of astaxanthin with peroxynitrite, 15-nitroastaxanthin anticancer properties were evaluated in a mouse model. Epstein-Barr virus and carcinogenesis in mouse skin papillomas were significantly inhibited by astaxanthin treatment 154.
Memory and other Higher Brain Functions
Astaxanthin might improve cognitive functions. Astaxanthin has shown partially beneficial effects when cognitive function was evaluated as a brain function in the elderly. In particular, it is thought to be very beneficial for improving cognitive function in subjects with moderate cognitive impairment (MCI). In addition to the antioxidant effect in the brain, this effect is thought to be related to the improvement of blood flow and mitochondrial function. It is expected to be very useful for improving muscle function in the elderly as well as preventing so-called frailty. Concurrently, astaxanthin has also been reported to have beneficial effects on fatigue during brain overload. Similarly, a subjective but stress-reducing effect has also been reported. Although there are still many scientifically unclear aspects of fatigue, it is expected that as the scientific analysis of fatigue progresses, it will become clearer what kind of effects astaxanthin has on what kind of fatigue.
In a small, open-label trial, 10 healthy men ages 50-69, who had been complaining of forgetfulness, received astaxanthin (12 mg/day) for 12 weeks 155. On a computerized test designed to accurately detect early cognitive deterioration (“CogHealth” from CogState Ltd, Melbourne, Australia), they showed improvement on measures of reaction time, attention, and working memory.
Although this trial clearly was only preliminary, astaxanthin has shown a variety of brain benefits under experimental conditions. Unlike much of the experimental research conducted with this supplement, the following studies employed levels readily achieved by oral intake in humans.
Astaxanthin:
- significantly improved the memory performance of mice in the Morris water maze 156;
- effectively protected cultured nerve cells against hydrogen peroxide toxicity 157 and down-regulated genes linked to cell death and up-regulated genes linked to cell survival 158;
- specifically protected the mitochondria of cultured nerve cells against toxic attack 157,
- 158; and
- stimulated the proliferation of cultured nerve stem cells 159.
Effect on Vision and Eye Fatigue
Unlike lutein and zeaxanthin, astaxanthin has not been reported to be detected in the macula under normal dietary conditions. However, it has been reported that astaxanthin migrates into the retina in studies using monkeys (Macaca fascicularis) treated with astaxanthin and its precursor, adonixanthin 160.
Astaxanthin has been extensively researched for its benefits for vision, especially in Japan. It has been reported that astaxanthin prevents choroidal neovascularization and protects photoreceptor cells and retinal ganglion cells from hypoxia and light stress 161, 162, 163, 164, 165. Therefore, its efficacy in retinal degeneration, including age-related macular degeneration (AMD) and glaucoma involving neuronal cell death, is expected. Unfortunately, in human clinical trials, astaxanthin has been evaluated in combination with lutein and zeaxanthin in age-related macular degeneration (AMD) and its efficacy remains unclear. However, preclinical studies are actively being conducted in these fields, and further research is expected in the future. In contrast, many clinical trials have been conducted on asthenopia (eyestrain), and all have shown significant benefits. Since eyestrain can also lead to stiff shoulders and other symptoms, astaxanthin has been shown to be useful for these expanded conditions. The main mechanism for this is thought to be the improvement of blood flow in microvasculature 166, 167, 168, including the uveal tissue such as the ciliary body and the iris, which regulate the focus of the eye. Moreover, in the same group, astaxanthin has been reported to be beneficial against oxidative damage and inflammation during cataract surgery. The authors believe that astaxanthin may have some protective effects, just as various xanthophylls have ocular benefits in other organisms 166, 167, 168.
Yuan 91 and Kajita 169 discussed double-blind and other controlled trials that were published in Japanese with English abstracts. They concluded that astaxanthin taken at 6 mg/day consistently improved visual sharpness, even in healthy subjects.
Astaxanthin also might relieve eye fatigue in persons using computer monitors. Extended work at computer monitors is linked to eyes strain and to blurred vision, often accompanied by tensing of the muscles of the shoulder and low back 170. One double-blind randomized controlled trial used instrumentation to measure eye muscle endurance, the usual basis for eye strain 171. Japanese visual display terminal workers, ages 38-53 (n=26), received astaxanthin (5 mg/day) or a placebo for four weeks. A non-visual display terminal control group received neither astaxanthin nor placebo. After supplementation, eye strain was significantly more improved in the astaxanthin group than in the placebo group.
Many individuals, as they age, suffer decline in the eye’s ability to focus on near objects (presbyopia). In an open-label Japanese trial 169, papillary constriction capacity was determined using instrumentation in presbyopic middle-aged and older subjects (ages 46-65 years; n=22). They then received astaxanthin (6 mg/day) for four weeks. Astaxanthin significantly improved pupillary constriction, and more than 60 percent of the subjects indicated on a questionnaire that they also experienced improvement in the categories of “difficulty to see near objects,” “eye strain,” “blurred vision,” and “shoulder and low-back stiffness”.
Muscle Performance and Endurance
Early preclinical studies from Aoi et al 172 suggested that astaxanthin could efficiently prevent oxidative damage to skeletal muscle during strenuous exercise and may accelerate recovery. Many studies have been conducted with athletes, and results vary considerably, with some studies finding benefit and others finding no benefit in ameliorating post-exercise injuries. The reasons for this are speculative but may depend on the protocol of administration, the characteristics of the subject’s skeletal muscles, and other physical functions. Skeletal muscles with sufficient endurance for exercise may not be as effective as expected. Future studies are needed to determine which subjects should be treated with astaxanthin and how it should be administered. Similarly, the same thing can be observed with regard to energy metabolism in athletes’ physical activity functions. However, in subjects with relatively low exercise habits and in the elderly, astaxanthin has provided beneficial effects, such as improvement of muscle function, including muscle strength, enhanced endurance, and changes in body composition. Therefore, the effects of astaxanthin may have beneficial effects on muscle weakness, including sarcopenia, in the elderly and obesity.
In a double-blind randomized controlled trial 173, young healthy male students (ages 17-19 years; n=40) were subjected to fitness, strength, and endurance testing, then randomized to receive astaxanthin (4 mg/day) or a placebo for six months. Astaxanthin significantly improved performance in the assessment designed to measure strength/endurance (maximum number of knee bends [“squats”] performed while carrying a 42.5 kg barbell) over the placebo group. The maximum number of knee bends increased by more than 50 percent in the astaxanthin group versus 19 percent in the placebo group. No benefit was detected for the assessments of strength/explosivity or overall fitness. This finding (and probably others asserted from unpublished trials) may have motivated the double-blind trials currently in progress with astaxanthin for protection against oxidative stress 174 and for improving speed and endurance in soccer players 175.
Male Fertility and Reproduction
Astaxanthin has been reported to have beneficial effects on polycystic ovary syndrome (PCOS), endometriosis, and heavy menstrual bleeding in females. In males, astaxanthin has been reported to improve sperm dysplasia and activity. Additionally, as in the case of livestock animals, there is a beneficial effect on sperm cryopreservation, and it is expected that beneficial effects on assisted reproductive technology (ART) will be reported in the future.
In a double-blind randomized controlled trial, astaxanthin was evaluated for protecting sperm function and fertility 176. Thirty men were recruited, all from infertile couples in which the female partner showed no demonstrable cause of infertility. They were randomized to receive either astaxanthin (16 mg/day) or a placebo for three months. During that period they were allowed to provide semen for intrauterine insemination, and pregnancy occurrence was recorded.
By the end of three months, sperm linear velocity was significantly increased in the astaxanthin group but not in the placebo group 176. Semen oxygen radical generation (upon stimulation by the oxidant phorbol ester) was markedly decreased in the astaxanthin group. However, the most telling outcome of this trial was the pregnancy rate, which was 54.5 percent for the astaxanthin group compared to 10.5 percent for the placebo group.
Immuno-Modulation
Immune system cells are very sensitive to free radical damage. The cell membrane contains poly unsaturated fatty acids (PUFA). Antioxidants in particular astaxanthin offer protection against free radical damage to preserve immune-system defenses and in boosting the immune response 177. There are reports on astaxanthin and its effect on immunity in animals under laboratory conditions however clinical research is lacking in humans. Astaxanthin showed higher immuno-modulating effects in mouse model when compared to beta-carotene 178. Enhanced antibody production and decreased humoral immune response in older animals after dietary supplementation of astaxanthin was reported 178. Astaxanthin produced immunoglobulins in human cells in a laboratory study 179. Eight week-supplementation of astaxanthin in humans 180 resulted in increased blood levels of astaxanthin and improved activity of natural killer cells which targeted and destroyed cells infected with viruses. In this study, T and B cells were increased, DNA damage was low, and C-reactive protein (CRP) was significantly lower in the astaxanthin supplemented group. Astaxanthin also helps in T helpers 1 cytokines production, such as IL-2 and Mouse interferon Y (IFN-Y) 181. It also reduces nuclear factor-κB (NF-κB) and other down-stream mediators such as IL-1β, interleukin (IL)-6, matrix metalloproteinase (MMP-9). In addition, it modulates phosphoinositide 3-kinases (PI3K)/Akt, ERK/MAPK, and the up-stream macrophage migration inhibitory factor (MIF) 182.
Astaxanthin is also reported to have anti-inflammatory effects in several conditions such as neurodegenerative disorders, gastrointestinal disease, diabetes, and renal inflammation, which makes it a potential anti-inflammatory agent 183.
Astaxanthin dosage
The doses of astaxanthin used in clinical trials have ranged from 1 mg/day to 40 mg/day (with the majority in the 6-12 mg range); single-dose pharmacokinetic studies used up to 100 mg per dose. Recommended dose of astaxanthin is 2–4 mg/day 15. As a dietary supplement, astaxanthin should be taken along with fats, with or immediately prior to meals, to ensure its optimal absorption 184. It is recommended to administer astaxanthin with omega-3 rich seed oils such as chia, flaxseed, fish, nutella, walnuts and almonds.
Astaxanthin content in wild and farmed salmons are shown in Figure 7. Among the wild salmonids, the maximum astaxanthin content in wild Oncorhynchus species was reported in the range of 26–38 mg/kg flesh in sockeye salmon whereas low astaxanthin content was reported in chum 185. Astaxanthin content in farmed Atlantic salmon was reported as 6–8 mg/kg flesh. Astaxanthin is available in the European (6 mg/kg flesh) and Japanese market (25 mg/kg flesh) from large trout. Shrimp, crab and salmon can serve as dietary sources of astaxanthin 185. Wild caught salmon is a good source of astaxanthin. In order to get 3.6 mg of astaxanthin you can eat 165 grams of salmon per day. Astaxanthin supplement at 3.6 mg per day can be beneficial to health as reported by Iwamoto et al. 186.
The European Food Safety Authority recommends that the acceptable daily intake (ADI) for synthetic astaxanthin in food is 2.0 mg/day for a 60-kg adult, and the estimated acceptable daily intake (ADI) for nature astaxanthin is also about 2.0 mg/day for a 60-kg adult. Moreover, Brendler and Williamson 187 reviewed 87 human studies involving over 2000 participants and found that natural astaxanthin can be consumed at short-term intake up to 100 mg/day, and the long-term average intake is 8 and 12 mg/day with excellent clinical safety.
Figure 7. Astaxanthin levels (mg/kg flesh) of wild and farmed (*) salmons
Astaxanthin side effects
Astaxanthin is safe, with no side effects when it is consumed with food. It is lipid soluble, accumulates in animal tissues after feeding of astaxanthin to rats and no toxic effects were found 188. Short-term overdose administration studies involving daily supplementation of approximately 45 mg astaxanthin for up to 4 weeks have also reported no adverse safety effects 189, 190, 191, 192, 193. A comprehensive list of human clinical studies investigating the effects of astaxanthin includes over a hundred studies found no clinical safety concerns in short-term studies using doses up to 24 mg or astaxanthin per day for 12 weeks and/or in long-term studies of more than 6 months with 12 mg astaxanthin per day in healthy subjects 2. Excessive astaxanthin consumption leads to yellow to reddish pigmentation of the skin in animals.
Astaxanthin has demonstrated safety in numerous human clinical trials. In one open-label clinical study on subjects with metabolic syndrome (n=17) 137, astaxanthin (16 mg/day, for three months) significantly raised blood bilirubin, potassium and creatine kinase, although all three values remained within normal range. Also, astaxanthin significantly lowered the liver enzyme gamma-glutamyl transpeptidase (GGTP). Since the researchers noted this enzyme was abnormally elevated in 11 of the 17 subjects at baseline, this astaxanthin effect may have been beneficial.
Animal experiments have investigated astaxanthin at levels well over 120 mg/day in human equivalents 194, without causing apparent harm.
Following a request from the European Commission, the European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies (NDA) was asked to deliver a scientific opinion on the safety of astaxanthin‐rich ingredients AstaREAL A1010 and AstaREAL L10 as novel food ingredients in the context of Regulation (EC) No 258/97 195. The novel food ingredients are produced from astaxanthin‐rich alga Haematococcus pluvialis. Astaxanthin content is 5.0‐5.6 % in AstaREAL A1010 powder, 10.0‐12.0 % in AstaREAL L10 oil and 2.5‐2.7 % in AstaREAL L10 encapsulated oil. Sufficient information was provided regarding the composition, specification, manufacture and stability of the novel food ingredients. The novel food ingredients are intended to be used in fermented liquid dairy products, non‐fermented liquid dairy products, fermented soya products and fruit drinks for healthy adults at a maximum incorporation level of 1.6 mg astaxanthin per 100 g or 100 mL 195.
The European Food Safety Authority Panel considers that the composition of the ingredients and results from available studies do not indicate that the consumption of the novel food ingredients is nutritionally disadvantageous at the proposed daily intake 195.
Astaxanthin is absorbed in the human gastro-intestinal tract. Its bioavailability and distribution seem to depend on a variety of factors, including its form, its mode of consumption and the smoking habits of the consumer. Astaxanthin is also absorbed in rodents. The metabolic fate of astaxanthin involves the cleavage of the polyene chain at the C9, C9 positions and stepwise reduction in both rats and humans. On the basis of similarities in absorption and metabolic fate, the European Food Safety Authority Panel considers that rats are an acceptable species for toxicity testing of astaxanthin 195.
Based on the results of in vitro and in vivo genotoxicity studies on the biomass of Haematococcus pluvialis and other astaxanthin products, the European Food Safety Authority Panel concludes that it has no safety concerns regarding genotoxicity of the novel food ingredients 195.
In the human studies provided, which addressed safety endpoints, no clinically relevant changes or adverse effects were observed after consumption of the novel food ingredients or other astaxanthin-rich ingredients from Haematococcus pluvialis at doses ranging from 2 to 40 mg/day astaxanthin for 10 days to 3 months 195. However, as these studies were of only short duration, the European Food Safety Authority Panel considers that no conclusion can be drawn as regards long-term effects 195.
Supplemental intakes of β-carotene have been shown to increase the risk of lung cancer in smokers, and concerns were expressed that the novel food ingredients may induce similar effects. There are neither human nor animal studies that have investigated astaxanthin and smoking with regard to the risk of lung cancer. There are differences in structure, metabolism and function between astaxanthin and β-carotene. In contrast to β-carotene, astaxanthin is more polar, is not a precursor of vitamin A and is considered as an antioxidant with no indication of pro-oxidative properties. The European Food Safety Authority Panel concludes that the available data do not indicate that the novel food ingredients at the proposed level of use would increase the risk of lung cancer in smokers 195.
The Panel considers that the likelihood of adverse allergic reactions to the novel food ingredients is low.
Biomass of Haematococcus pluvialis (algal meal containing 3 % astaxanthin) and astaxanthin-rich oil obtained by solvent extraction from Haematococcus pluvialis biomass (containing ca. 5 % astaxanthin) were tested for subchronic toxicity in rats. There is no indication from these studies that the novel food ingredients would be more toxic than astaxanthin. Therefore, the Panel bases the evaluation of the novel food ingredients on astaxanthin and considers the acceptable daily intake (ADI) of 0.034 mg/kg body weight for astaxanthin derived by the European Food Safety Authority Panel on Additives and Products or Substances used in Animal Feed.
The European Food Safety Authority Panel notes that the maximum intake of 4 mg astaxanthin per day (0.06 mg/kg body weight per day for a 70 kg person) from the novel food ingredients as proposed by the applicant and the estimated mean intake based on the use levels in the proposed food categories (0.106 mg/kg body weight per day) exceed the acceptable daily intake (ADI) for astaxanthin of 0.034 mg/kg body weight per day by approximately two- and three-fold, respectively. The European Food Safety Authority Panel therefore concludes that the safety of the novel food ingredients AstaREAL A1010 and AstaREAL L10 at the proposed use and use levels has not been established.
- Elbahnaswy S, Elshopakey GE. Recent progress in practical applications of a potential carotenoid astaxanthin in aquaculture industry: a review. Fish Physiol Biochem. 2024 Feb;50(1):97-126. doi: 10.1007/s10695-022-01167-0[↩][↩][↩][↩][↩]
- Nishida Y, Berg PC, Shakersain B, Hecht K, Takikawa A, Tao R, Kakuta Y, Uragami C, Hashimoto H, Misawa N, Maoka T. Astaxanthin: Past, Present, and Future. Mar Drugs. 2023 Sep 28;21(10):514. doi: 10.3390/md21100514[↩][↩][↩][↩][↩][↩][↩]
- Wang S, Qi X. The Putative Role of Astaxanthin in Neuroinflammation Modulation: Mechanisms and Therapeutic Potential. Front Pharmacol. 2022 Jun 24;13:916653. doi: 10.3389/fphar.2022.916653[↩]
- Nair A, Ahirwar A, Singh S, Lodhi R, Lodhi A, Rai A, Jadhav DA, Harish, Varjani S, Singh G, Marchand J, Schoefs B, Vinayak V. Astaxanthin as a King of Ketocarotenoids: Structure, Synthesis, Accumulation, Bioavailability and Antioxidant Properties. Mar Drugs. 2023 Mar 13;21(3):176. doi: 10.3390/md21030176[↩]
- Astaxanthin: a review of its chemistry and applications. Higuera-Ciapara I, Félix-Valenzuela L, Goycoolea FM. Crit Rev Food Sci Nutr. 2006; 46(2):185-96. https://www.tandfonline.com/doi/abs/10.1080/10408690590957188[↩]
- Li Y, Gong F, Guo S, Yu W, Liu J. Adonis amurensis Is a Promising Alternative to Haematococcus as a Resource for Natural Esterified (3S,3’S)-Astaxanthin Production. Plants (Basel). 2021 May 25;10(6):1059. doi: 10.3390/plants10061059[↩][↩]
- Sarada R., Ranga Rao A., Sandesh B.K., Dayananda C., Anila N., Chauhan V.S., Ravishankar G.A. Influence of different culture conditions on yield of biomass and value added products in microalgae. Dyn. Biochem. Proc. Biotechnol. Mol. Biol. 2012;6:77–85.[↩]
- Kidd P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011;16:355–364. http://archive.foundationalmedicinereview.com/publications/16/4/355.pdf[↩][↩][↩]
- Nishida Y. Astaxanthin: Commercial production and its potential health-promoting effects. Oleoscience. 2012;12:525–531. doi: 10.5650/oleoscience.12.525[↩][↩]
- Ambati R.R., Phang S.-M., Ravi S., Aswathanarayana R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs. 2014;12:128–152. doi: 10.3390/md12010128[↩][↩]
- Stachowiak B., Szulc P. Astaxanthin for the Food Industry. Molecules. 2021;26:2666. doi: 10.3390/molecules26092666[↩]
- Maoka T. Carotenoids in Marine Animals. Mar. Drugs. 2011;9:278–293. doi: 10.3390/md9020278[↩]
- Matsuno T. Aquatic animal carotenoids. Fish. Sci. 2001;67:771–783. doi: 10.1046/j.1444-2906.2001.00323.x[↩]
- Eric A. Johnson & Gil-Hwan An (1991) Astaxanthin from Microbial Sources, Critical Reviews in Biotechnology, 11:4, 297-326, DOI: 10.3109/07388559109040622 https://www.tandfonline.com/doi/abs/10.3109/07388559109040622[↩][↩][↩][↩]
- Ambati RR, Siew Moi P, Ravi S, Aswathanarayana RG. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Marine Drugs. 2014;12(1):128-152. doi:10.3390/md12010128. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3917265/[↩][↩]
- Patil AD, Kasabe PJ, Dandge PB. Pharmaceutical and nutraceutical potential of natural bioactive pigment: astaxanthin. Nat Prod Bioprospect. 2022 Jul 7;12(1):25. doi: 10.1007/s13659-022-00347-y[↩][↩]
- Kuhn R., Sörensen N.A. Über astaxanthin und ovoverdin. Ber. Der Dtsch. Chem. Ges. A B Ser. 1938;71:1879–1888. doi: 10.1002/cber.19380710918[↩]
- Seybold A., Goodwin T. Occurrence of astaxanthin in the flower petals of Adonis annua L. Nature. 1959;184:1714–1715. doi: 10.1038/1841714a0[↩]
- Egger K., Kleinig H. Die ketocarotinoide in Adonis annua L.—II.: Zur struktur der ester. Phytochemistry. 1967;6:437–440. doi: 10.1016/S0031-9422(00)86302-5[↩]
- Fucikova K., Lewis L.A. Intersection of Chlorella, Muriella and Bracteacoccus: Resurrecting the genus Chromochloris kol et chodat (Chlorophyceae, Chlorophyta) Fottea. 2012;12:83–93. doi: 10.5507/fot.2012.007[↩]
- Kopecky J., Schoefs B., Loest K., Stys D., Pulz O. Microalgae as a source for secondary carotenoid production: A screening study. Algol. Stud. 2000;98:153–168. doi: 10.1127/algol_stud/98/2000/153[↩]
- Chekanov K., Litvinov D., Fedorenko T., Chivkunova O., Lobakova E. Combined production of astaxanthin and β-carotene in a new strain of the microalga Bracteacoccus aggregatus BM5/15 (IPPAS C-2045) cultivated in photobioreactor. Biology. 2021;10:643. doi: 10.3390/biology10070643[↩]
- Ali H.E.A., Vorisek F., Dowd S.E., Kesner S., Song Y., Qian D., Crocker M. Formation of Lutein, β-Carotene and Astaxanthin in a Coelastrella sp. Isolate. Molecules. 2022;27:6950. doi: 10.3390/molecules27206950[↩]
- Viala G. Recherches sur le Chlamydomonas nivalis Wille dans les Pyrénées. Bull. De La Société Bot. De Fr. 1967;114:75–79. doi: 10.1080/00378941.1967.10838330[↩]
- Yuan J.P., Peng J., Yin K., Wang J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011;55:150–165. doi: 10.1002/mnfr.201000414[↩]
- Tsubokura A., Yoneda H., Mizuta H. Paracoccus carotinifaciens sp. nov., a new aerobic gram-negative astaxanthin-producing bacterium. Int. J. Syst. Evol. Microbiol. 1999;49:277–282. doi: 10.1099/00207713-49-1-277[↩]
- Brotosudarmo THP, Limantara L, Setiyono E, Heriyanto. Structures of Astaxanthin and Their Consequences for Therapeutic Application. Int J Food Sci. 2020 Jul 20;2020:2156582. doi: 10.1155/2020/2156582[↩]
- Oslan SNH, Shoparwe NF, Yusoff AH, Rahim AA, Chang CS, Tan JS, Oslan SN, Arumugam K, Ariff AB, Sulaiman AZ, Mohamed MS. A Review on Haematococcus pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin. Biomolecules. 2021 Feb 10;11(2):256. doi: 10.3390/biom11020256[↩]
- Johnson EA, An G-H. Astaxanthin from microbial sources. Crit Rev Biotechnol. 1991;11:297–326. doi: 10.3109/07388559109040622[↩]
- Lim KC, Yusoff FM, Shariff M, Kamarudin MS. Astaxanthin as feed supplement in aquatic animals. Rev Aquac. 2018;10:738–773. doi: 10.1111/raq.12200[↩]
- Li J, Zhu D, Niu J, Shen S, Wang G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol Adv. 2011;29:568–574. doi: 10.1016/j.biotechadv.2011.04.001[↩]
- Cheng C-H, Guo Z-X, Ye C-X, Wang A-L. Effect of dietary astaxanthin on the growth performance, non-specific immunity, and antioxidant capacity of pufferfish (Takifugu obscurus) under high temperature stress. Fish Physiol Biochem. 2018;44:209–218. doi: 10.1007/s10695-017-0425-5[↩]
- Rodríguez-Sáiz M, de la Fuente JL, Barredo JL. Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Appl Microbiol Biotechnol. 2010;88:645–658. doi: 10.1007/s00253-010-2814-x[↩]
- Hara KY, Morita T, Mochizuki M, Yamamoto K, Ogino C, Araki M, Kondo A. Development of a multi-gene expression system in Xanthophyllomyces dendrorhous. Microb Cell Fact. 2014;13:1–8. doi: 10.1186/s12934-014-0175-3[↩]
- Dursun D, Dalgıç AC. Optimization of astaxanthin pigment bioprocessing by four different yeast species using wheat wastes. Biocatal Agric Biotechnol. 2016;7:1–6. doi: 10.1016/j.bcab.2016.04.006[↩]
- Wang N, Guan B, Kong Q, Sun H, Geng Z, Duan L. Enhancement of astaxanthin production from Haematococcus pluvialis mutants by three-stage mutagenesis breeding. J Biotechnol. 2016;236:71–77. doi: 10.1016/j.jbiotec.2016.08.009[↩]
- Li Y, Huang J, Sandmann G, Chen F. Glucose sensing and the mitochondrial alternative pathway are involved in the regulation of astaxanthin biosynthesis in the dark-grown Chlorella zofingiensis (Chlorophyceae) Planta. 2008;228:735–743. doi: 10.1007/s00425-008-0775-4[↩]
- Ambati RR, Phang S-M, Ravi S, Aswathanarayana RG. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Mar Drugs. 2014;12:128–152. doi: 10.3390/md12010128[↩][↩][↩][↩]
- Shah M, Mahfuzur R, Liang Y, Cheng JJ, Daroch M. Astaxanthin-producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Front Plant Sci. 2016;7:531. doi: 10.3389/fpls.2016.00531[↩]
- Zhao Y, Yue C, Geng S, Ning D, Ma T, Yu X. Role of media composition in biomass and astaxanthin production of Haematococcus pluvialis under two-stage cultivation. Bioprocess Biosyst Eng. 2019;42:593–602. doi: 10.1007/s00449-018-02064-8[↩]
- EFSA Panel on Additives and Products or Substances used in Animal Feed, 2005. Opinion of the Scientific Panel on additives and products or substances used in animal feed (FEEDAP) on the safety of use of colouring agents in animal nutrition – PART I. General Principles and Astaxanthin, EFSA Journal 2005; 3(12):291, 40 pp. doi:10.2903/j.efsa.2005.291[↩][↩]
- Sutliff A, O’Connor L, Hendrick A, Tang M, Quinn K, Doenges K, Westcott J, Borengasser S, Reisdorph R, Frank D. Astaxanthin levels are higher in fresh salmon compared to canned and pouch varieties. Curr Dev Nutr. 2020;4:128–128. doi: 10.1093/cdn/nzaa041_032[↩]
- Ekpe L, Inaku K, Ekpe V. Antioxidant effects of astaxanthin in various diseases—a review. J Mol Pathophysiol. 2018;7:1–6. doi: 10.5455/jmp.20180627120817[↩]
- Niamnuy C, Devahastin S, Soponronnarit S, Raghavan GV. Kinetics of astaxanthin degradation and color changes of dried shrimp during storage. J Food Eng. 2008;87:591–600. doi: 10.1016/j.jfoodeng.2008.01.013[↩][↩]
- Ju Z, Forster I, Dominy W. Effects of supplementing two species of marine algae or their fractions to a formulated diet on growth, survival and composition of shrimp (Litopenaeus vannamei) Aquaculture. 2009;292:237–243. doi: 10.1016/j.aquaculture.2009.04.040[↩][↩]
- Tume R, Sikes A, Tabrett S, Smith D. Effect of background colour on the distribution of astaxanthin in black tiger prawn (Penaeus monodon): effective method for improvement of cooked colour. Aquaculture. 2009;296:129–135. doi: 10.1016/j.aquaculture.2009.08.006[↩]
- Yanar Y, Çelik M, Yanar M. Seasonal changes in total carotenoid contents of wild marine shrimps (Penaeus semisulcatus and Metapenaeus monoceros) inhabiting the eastern Mediterranean. Food Chem. 2004;88:267–269. doi: 10.1016/j.foodchem.2004.01.037[↩]
- Yamaguchi K, Miki W, Toriu N, Kondo Y, Murakami M. S, KONOSU, M, SATAKE, and T. FuJITA: Bull. Japan Soc Sci Fish. 1983;49:1411–1415. doi: 10.2331/suisan.49.1411[↩]
- Maoka T, Katsuyama M, Kaneko N, Matsuno T. Stereochemical investigation of carotenoids in the Antarctic krill Euphausia superba. Nippon Suisan Gakkaishi. 1985;51:1671–1673. doi: 10.2331/suisan.51.1671[↩]
- Koomyart I, Nagamizu H, Khuwijitjaru P, Kobayashi T, Shiga H, Yoshii H, Adachi S. Astaxanthin stability and color change of krill during subcritical water treatment. J Food Sci Technol. 2017;54:3065–3072. doi: 10.1007/s13197-017-2742-1[↩]
- De Holanda HD, Netto FM. Recovery of components from shrimp (Xiphopenaeus kroyeri) processing waste by enzymatic hydrolysis. J Food Sci. 2006;71:C298–C303. doi: 10.1111/j.1750-3841.2006.00040.x[↩]
- Sachindra N, Bhaskar N, Siddegowda G, Sathisha A, Suresh P. Recovery of carotenoids from ensilaged shrimp waste. Biores Technol. 2007;98:1642–1646. doi: 10.1016/j.biortech.2006.05.041[↩]
- Seabra LMAJ, Pedrosa LFC. Astaxanthin: structural and functional aspects. Rev Nutr. 2010;23:1041–1050. doi: 10.1590/S1415-52732010000600010[↩][↩]
- Tejera N, Cejas JR, Rodríguez C, Bjerkeng B, Jerez S, Bolaños A, Lorenzo A. Pigmentation, carotenoids, lipid peroxides and lipid composition of skin of red porgy (Pagrus pagrus) fed diets supplemented with different astaxanthin sources. Aquaculture. 2007;270:218–230. doi: 10.1016/j.aquaculture.2007.01.019[↩]
- Ambati RR, Siew Moi P, Ravi S, Aswathanarayana RG. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Marine Drugs. 2014;12(1):128-152. doi:10.3390/md12010128. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3917265[↩][↩]
- Su F, Yu W, Liu J. Comparison of effect of dietary supplementation with Haematococcus pluvialis powder and synthetic astaxanthin on carotenoid composition, concentration, esterification degree and astaxanthin isomers in ovaries, hepatopancreas, carapace, epithelium of adult female Chinese mitten crab (Eriocheir sinensis) Aquaculture. 2020;523:735146. doi: 10.1016/j.aquaculture.2020.735146[↩][↩][↩]
- Yang C, Zhang L, Zhang H, Sun Q, Liu R, Li J, Wu L, Tsao R. Rapid and efficient conversion of all-E-astaxanthin to 9Z- and 13Z-isomers and assessment of their stability and antioxidant activities. J Agric Food Chem. 2017;65:818–826. doi: 10.1021/acs.jafc.6b04962[↩]
- Capelli B, Bagchi D, Cysewski GR. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods. 2013;12:145–152. doi: 10.1007/s13749-013-0051-5[↩]
- Ranjbar R, Inoue R, Shiraishi H, Katsuda T, Katoh S. High efficiency production of astaxanthin by autotrophic cultivation of Haematococcus pluvialis in a bubble column photobioreactor. Biochem Eng J. 2008;39:575–580. doi: 10.1016/j.bej.2007.11.010[↩][↩]
- Kobayashi M. Astaxanthin biosynthesis enhanced by reactive oxygen species in the green alga Haematococcus pluvialis. Biotechnol Bioprocess Eng. 2003;8:322–330. doi: 10.1007/BF02949275[↩]
- Rajesh K, Rohit M, Mohan SV (2017) Microalgae-based carotenoids production, Algal green chemistry. Elsevier, 139–147.[↩]
- Li M-Y, Guo W-Q, Guo G-L, Zhu X-M, Niu X-T, Shan X-F, Tian J-X, Wang G-Q, Zhang D-M. Effects of dietary astaxanthin on lipopolysaccharide-induced oxidative stress, immune responses and glucocorticoid receptor (GR)-related gene expression in Channa argus. Aquaculture. 2020;517:734816. doi: 10.1016/j.aquaculture.2019.734816[↩]
- Qin S, Liu G-X, Hu Z-Y. The accumulation and metabolism of astaxanthin in Scenedesmus obliquus (Chlorophyceae) Process Biochem. 2008;43:795–802. doi: 10.1016/j.procbio.2008.03.010[↩]
- Liu F, Shi H-Z, Guo Q-S, Yu Y-B, Wang A-M, Lv F, Shen W-B. Effects of astaxanthin and emodin on the growth, stress resistance and disease resistance of yellow catfish (Pelteobagrus fulvidraco) Fish Shellfish Immunol. 2016;51:125–135. doi: 10.1016/j.fsi.2016.02.020[↩]
- Lu Q, Yang L, Deng X. Critical thoughts on the application of microalgae in aquaculture industry. Aquaculture. 2020;528:735538. doi: 10.1016/j.aquaculture.2020.735538[↩]
- Coral-Hinostroza GN, Ytrestoyl T, Ruyter B, Bjerkeng B. Plasma appearance of unesterified astaxantin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3’R/S isomers of astaxanthin fatty acyl diesters. Comp Biochem Physiol C Toxicol Pharmacol 2004;139:99-110[↩][↩][↩][↩]
- Okada Y, Ishikura M, Maoka T. Bioavailability of astaxanthin in Haematococcus algal extract: the effects of timing of diet and smoking habits. Biosci Biotechnol Biochem 2009;73:1928-1932.[↩][↩]
- Coral-Hinostroza GN, Ytrestoyl T, Ruyter B, Bjerkeng B. Plasma appearance of unesterified astaxantin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3’R/S isomers of astaxanthin fatty acyl diesters. Comp Biochem Physiol C Toxicol Pharmacol 2004;139:99-110.[↩]
- Kistler A, Liechti H, Pichard L, et al. Metabolism and CYP-inducer properties of astaxanthin in man and primary human hepatocytes. Arch Toxicol 2002;75:665-675.[↩]
- Miyazawa T, Nakagawa K, Kimura F, et al. Plasma carotenoid concentrations before and after supplementation with astaxanthin in middle-aged and senior subjects. Biosci Biotechnol Biochem 2011;75:1856-1858.[↩]
- Martin H.D., Ruck C., Schmidt M., Sell S., Beutner S., Mayer B., Walsh R. Chemistry of carotenoid oxidation and free radical reactions. Pure Appl. Chem. 1999;71:2253–2262. doi: 10.1351/pac199971122253[↩]
- McNulty H.P., Byun J., Lockwood S.F., Jacob R.F., Mason R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta Biomembr. 2007;1768:167–174. doi: 10.1016/j.bbamem.2006.09.010[↩]
- Focsan A.L., Polyakov N.E., Kispert L.D. Photo Protection of Haematococcus pluvialis Algae by Astaxanthin: Unique Properties of Astaxanthin Deduced by EPR, Optical and Electrochemical Studies. Antioxidants. 2017;6:80. doi: 10.3390/antiox6040080[↩]
- Kobayashi M., Katsuragi T., Tani Y. Enlarged and Astaxanthin-Accumulating Cyst Cells of the Green Alga Haematococcus pluvialis. J. Biosci. Bioeng. 2001;92:565–568. doi: 10.1016/S1389-1723(01)80317-0[↩]
- Aoi W., Naito Y., Sakuma K., Kuchide M., Tokuda H., Maoka T., Toyokuni S., Oka S., Yasuhara M., Yoshikawa T. Astaxanthin Limits Exercise-Induced Skeletal and Cardiac Muscle Damage in Mice. Antioxid. Redox Signal. 2003;5:139–144. doi: 10.1089/152308603321223630[↩]
- Dang Y, Li Z, Yu F. Recent Advances in Astaxanthin as an Antioxidant in Food Applications. Antioxidants (Basel). 2024 Jul 22;13(7):879. doi: 10.3390/antiox13070879[↩][↩]
- U.S. Food and Drug Administration. GRAS Notices. https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices[↩]
- Roche F. Astaxanthin As a Pigmenter in Salmon Feed, Color Additive Petition 7C02 1 1, United States Food and Drug Administration. Hoffman-La Roche Ltd.; Basel, Switzerland: 1987. Astaxanthin: Human food safety summary; p. 43.[↩]
- Higuera-Ciapara I., Felix-Valenzuela L., Goycoolea F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188 https://www.ncbi.nlm.nih.gov/pubmed/16431409[↩]
- Guerin M., Huntley M.E., Olaizola M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003;21:210–216. doi: 10.1016/S0167-7799(03)00078-7. https://www.ncbi.nlm.nih.gov/pubmed/12727382[↩]
- Goto S, Kogure K, Abe K, et al. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim Biophys Acta 2001;1512:251-258.[↩]
- Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea F. Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188[↩][↩]
- Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 2003;21:210–216. doi: 10.1016/S0167-7799(03)00078-7[↩]
- Donoso A, González-Durán J, Muñoz AA, González PA, Agurto-Muñoz C. “Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials”. Pharmacol Res. 2021 Apr;166:105479. doi: 10.1016/j.phrs.2021.105479[↩]
- Haematococcus astaxanthin: applications for human health and nutrition. Guerin M, Huntley ME, Olaizola M. Trends Biotechnol. 2003 May; 21(5):210-6. https://www.ncbi.nlm.nih.gov/pubmed/12727382/[↩]
- Miki W. Biological functions and activities of animal carotenoids. Pure Appl Chem. 1991;63:141–146. doi: 10.1351/pac199163010141[↩]
- Britton G (2008) Functions of intact carotenoids. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids. Carotenoids, vol 4. Birkhäuser Basel. 10.1007/978-3-7643-7499-0_10[↩]
- McNulty HP, Byun J, Lockwood SF, Jacob RF, Mason RP. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochimica et biophysica acta (BBA)-biomembranes. 2007;1768:167–174. doi: 10.1016/j.bbamem.2006.09.010[↩]
- Yamashita E. Let Astaxanthin Be Thy Medicine. Pharmanutrition. 2015;3:115–122. doi: 10.1016/j.phanu.2015.09.001[↩]
- Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Yuan JP, Peng J, Yin K, Wang JH. Mol Nutr Food Res. 2011 Jan; 55(1):150-65. https://www.ncbi.nlm.nih.gov/pubmed/21207519/[↩]
- Yuan JP, Peng J, Yin K, Wang JH. Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol Nutr Food Res 2011;55:150-165.[↩][↩][↩]
- McNulty HP, Byun J, Lockwood SF, et al. Differential effects of carotenoids on lipid peroxidation due to membrane interactions. X-ray diffraction analysis. Biochim Biophys Acta 2007;1768:167-174.[↩][↩][↩]
- McAllister M.J., Mettler J.A., Patek K., Butawan M., Bloomer R.J. Astaxanthin supplementation increases glutathione concentrations but does not impact fat oxidation during exercise in active young men. Int. J. Sport Nutr. Exerc. Metab. 2021;1:1–8. doi: 10.1123/ijsnem.2021-0138[↩]
- Petyaev I.M., Klochkov V.A., Chalyk N.E., Pristensky D.V., Chernyshova M.P., Kyle N.H., Bashmakov Y.K. Markers of Hypoxia and Oxidative Stress in Aging Volunteers Ingesting Lycosomal Formulation of Dark Chocolate Containing Astaxanthin. J. Nutr. Health Aging. 2018;22:1092–1098. doi: 10.1007/s12603-018-1063-z[↩]
- Chalyk N.E., Klochkov V., Bandaletova T.Y., Kyle N.H., Petyaev I.M. Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017;48:40–48. doi: 10.1016/j.nutres.2017.10.006[↩]
- Hashimoto H., Arai K., Hayashi S., Okamoto H., Takahashi J., Chikuda M. The effect of astaxanthin on vascular endothelial growth factor (VEGF) levels and peroxidation reactions in the aqueous humor. J. Clin. Biochem. Nutr. 2016;59:10–15. doi: 10.3164/jcbn.15-137[↩]
- Baralic I., Andjelkovic M., Djordjevic B., Dikic N., Radivojevic N., Suzin-Zivkovic V., Radojevic-Skodric S., Pejic S. Effect of Astaxanthin Supplementation on Salivary IgA, Oxidative Stress, and Inflammation in Young Soccer Players. Evid.-Based Complement. Altern. Med. 2015;2015:1–9. doi: 10.1155/2015/783761[↩]
- Baralic I., Djordjevic B., Dikic N., Kotur-Stevuljevic J., Spasic S., Jelic-Ivanovic Z., Radivojevic N., Andjelkovic M., Pejic S. Effect of Astaxanthin Supplementation on Paraoxonase 1 Activities and Oxidative Stress Status in Young Soccer Players. Phytother. Res. 2012;27:1536–1542. doi: 10.1002/ptr.4898[↩]
- Hashimoto H., Arai K., Hayashi S., Okamoto H., Takahashi J., Chikuda M., Obara Y. Effects of astaxanthin on antioxidation in human aqueous humor. J. Clin. Biochem. Nutr. 2013;53:1–7. doi: 10.3164/jcbn.13-6[↩]
- Choi H.D., Kim J.H., Chang M.J., Kyu-Youn Y., Shin W.G. Effects of Astaxanthin on Oxidative Stress in Overweight and Obese Adults. Phytother. Res. 2011;25:1813–1818. doi: 10.1002/ptr.3494[↩]
- Choi H.D., Youn Y.K., Shin W.G. Positive Effects of Astaxanthin on Lipid Profiles and Oxidative Stress in Overweight Subjects. Plant Foods Hum. Nutr. 2011;66:363–369. doi: 10.1007/s11130-011-0258-9[↩]
- Hashimoto H.A.K., Okamoto Y., Takahashi J., Chikuda M., Obara Y. Effect of astaxanthin consumption on hydroper-oxides in the aqueous. Jpn. J. Clin. Ophthalmol. 2011;65:465–470. doi: 10.11477/mf.1410103607[↩]
- Kim J.H., Chang M.J., Choi H.D., Youn Y.-K., Kim J.T., Oh J., Shin W.G. Protective Effects of Haematococcus Astaxanthin on Oxidative Stress in Healthy Smokers. J. Med. Food. 2011;14:1469–1475. doi: 10.1089/jmf.2011.1626[↩]
- Nakagawa K., Kiko T., Miyazawa T., Burdeos G.C., Kimura F., Satoh A., Miyazawa T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011;105:1563–1571. doi: 10.1017/S0007114510005398[↩]
- Peng L., Zhao P., Li B., Zhang J., Huang C. Antioxidant effects and impact on human health of astaxanthin. Chin. J. Food Hyg. 2011;23:313–316.[↩]
- Park J.S., Chyun J.H., Kim Y.K., Line L.L., Chew B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010;7:18. doi: 10.1186/1743-7075-7-18[↩]
- Iwabayashi M., Fujioka N., Nomoto K., Miyazaki R., Takahashi H., Hibino S., Takahashi Y., Nishikawa K., Nishida M., Yonei Y. Efficacy and safety of eight-week treatment with astaxanthin in individuals screened for increased oxidative stress burden. Anti-Aging Med. 2009;6:15–21. doi: 10.3793/jaam.6.15[↩]
- Yamada T., Ryo K., Tai Y., Tamaki Y., Inoue H., Mishima K., Tsubota K., Saito I. Evaluation of Therapeutic Effects of Astaxanthin on Impairments in Salivary Secretion. J. Clin. Biochem. Nutr. 2010;47:130–137. doi: 10.3164/jcbn.10-31[↩]
- Fassett R.G., Healy H., Driver R., Robertson I.K., Geraghty D.P., Sharman J.E., Coombes J.S. Astaxanthin vs placebo on arterial stiffness, oxidative stress and inflammation in renal transplant patients (Xanthin): A randomised controlled trial. BMC Nephrol. 2008;9:17. doi: 10.1186/1471-2369-9-17[↩]
- Karppi J., Rissanen T.H., Nyyssönen K., Kaikkonen J., Olsson A.G., Voutilainen S., Salonen J.T. Effects of Astaxanthin Supplementation on Lipid Peroxidation. Int. J. Vitam. Nutr. Res. 2007;77:3–11. doi: 10.1024/0300-9831.77.1.3[↩]
- Kim Y.K., Chyun J.H. The Effects of Astaxanthin Supplements on Lipid Peroxidation and Antioxidant Status in Postmeno-pausal Women. Nutr. Sci. 2004;7:41–46.[↩]
- Fassett RG, Coombes JS. Astaxanthin: a potential therapeutic agent in cardiovascular disease. Mar Drugs 2011;9:447-465.[↩]
- Iwamoto T, Hosoda K, Hirano R, et al. Inhibition of low-density lipoprotein oxidation by astaxanthin. J Atheroscler Throm 2000;7:216-222.[↩]
- Karppi J, Rissanen TH, Nyyssonen K, et al. Effects of astaxanthin supplementation on lipid peroxidation. Int J Vitam Nutr Res 2007;77:3-11.[↩]
- Iwamoto T., Hosoda K., Hirano R., Kurata H., Matsumoto A., Miki W., Kamiyama M., Itakura H., Yamamoto S., Kondo K. Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheroscler. Thromb. 2000;7:216–222. https://www.ncbi.nlm.nih.gov/pubmed/11521685[↩]
- Osterlie M., Bjerkeng B., Liaaen-Jensen S. Plasma appearance and distribution of astaxanthin E/Z isomers in plasma lipoproteins of after single dose administration of astaxanthin. J. Nutr. Biochem. 2000;11:482–492. doi: 10.1016/S0955-2863(00)00104-2 https://www.ncbi.nlm.nih.gov/pubmed/11120445[↩]
- Park J.S., Chyun J.H., Kim Y.K., Line L.L., Chew B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010;7:1–10 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2845588/[↩]
- Spiller G.A., Dewell A. Safety of an astaxanthin rich Haemaotoccu pluvialis algal extract: A randomized clinical trial. J. Med. Food. 2003;6:51–56. doi: 10.1089/109662003765184741 https://www.ncbi.nlm.nih.gov/pubmed/12804020[↩]
- Miyawaki H., Takahashi J., Tsukahara H., Takehara I. Effects of astaxanthin on human blood rheology. J. Clin. Biochem. Nutr. 2008;43:69–74. doi: 10.3164/jcbn.2008048 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2533721/[↩]
- Karppi J., Rissanen T.H., Nyyssonen K., Kaikkonen J., Olsson A.G., Voutilainen S., Salonen J.T. Effects of astaxanthin supplementation on lipid peroxidation. Int. J. Vitam. Nutr. Res. 2007;77:3–11. doi: 10.1024/0300-9831.77.1.3[↩]
- Parisi V., Tedeschi M., Gallinaro G., Varano M., Saviano S., Piermarocchi S. Carotenoids and antioxidants in age-related maculopathy italian study: multifocal electroretinogram modifications after one year. Ophthalmology. 2008;115:324–333. doi: 10.1016/j.ophtha.2007.05.029[↩]
- Katagiri M., Satoh A., Tsuji S., Shirasawa T. Effects of astaxanthin rich Haematococcus pluvialis extact on cognitive function: Arandomised double blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2012;51:102–107. doi: 10.3164/jcbn.D-11-00017[↩][↩]
- Tominaga K., Hongo N., Karato M., Yamashita E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012;59:43–47[↩]
- Hashimoto H., Arai K., Hayashi S., Okamoto H., Takahashi J., Chikuda M., Obara Y. Effects of astaxanthin on antioxidation in human aqueous humor. J. Clin. Biochem. Nutr. 2013;53:1–7. doi: 10.3164/jcbn.13-6 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3705160/[↩]
- Iwabayashi M, Fujioka N, Nomoto K, et al. Efficacy and safety of eight-week treatment with astaxanthin in individuals screened for increased oxidative stress burden. Anti Aging Med 2009;6:15-21.[↩]
- Choi HD, Kim JH, Chang MJ, et al. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother Res 2011 Apr 8. doi: 10.1002/ptr.3494[↩][↩][↩]
- Grattagliano I, Palmieri VO, Portincasa P, et al. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem 2008;19:491-504.[↩]
- Kim JH, Chang MJ, Choi HD, et al. Protective effects of Haematococcus astaxanthin on oxidative stress in healthy smokers. J Med Food 2011;14:1469-1475.[↩]
- Park JS, Chyun JH, Kim YK, et al. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (Lond) 2010;7:18. doi:10.1186/1743-7075-7-18[↩][↩]
- Genest J. C-reactive protein: risk factor, biomarker and/or therapeutic target? Can J Cardiol 2010;26:41A-44A.[↩]
- Spiller GA. Effect of daily use natural astaxanthin on C-reactive protein. http://www.cyanotech.com/pdfs/bioastin/batl43.pdf[↩]
- Nir Y, Spiller G, Multz C. Effect of an astaxanthin containing product on rheumatoid arthritis. J Am Coll Nutr 2002;21:490[↩]
- Yamashita E. The Effects of a Dietary Supplement Containing. Astaxanthin on Skin Condition. Carotenoid Sci. 2006;10:91–95.[↩]
- Honda M., Nishida Y. In Vitro Evaluation of Skin-Related Physicochemical Properties and Biological Activities of Astaxanthin Isomers. ACS Omega. 2023;8:19311–19319. doi: 10.1021/acsomega.2c08173[↩]
- Yoshida H, Yanai H, Ito K, et al. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010;209:520-523.[↩]
- Kajikawa Y, Ikeda M, Takemoto S, et al. Association of circulating levels of leptin and adiponectin with metabolic syndrome and coronary heart disease in patients with various coronary risk factors. Int Heart J 2011;52:17-22.[↩]
- Uchiyama A, Okada Y. Clinical efficacy of astaxanthin-containing Haematococcus pluvialis extract for the volunteers at risk of metabolic syndrome. J Clin Biochem Nutr 2008;43:390-393[↩][↩]
- Uchiyama K., Naito Y., Hasegawa G., Nakamura N., Takahashi J., Yoshikawa T. Astaxanthin protects β-cells against glucose toxicity in diabetic db/db mice. Redox Rep. 2002;7:290–293. doi: 10.1179/135100002125000811[↩][↩]
- Otton R., Marin D.P., Bolin A.P., Santos R.C., Polotow T.G., Sampaio S.C., De Barros M.P. Astaxanthin ameliorates the redox imbalance in lymphocytes of experimental diabetic rats. Chem. Biol. Interact. 2010;186:306–315. doi: 10.1016/j.cbi.2010.05.011[↩]
- Nakano M., Onodera A., Saito E., Tanabe M., Yajima K., Takahashi J., Nguyen V.C. Effect of astaxanthin in combination with α-tocopherol or ascorbic acid against oxidative damage in diabetic ODS rats. J. Nutr. Sci. Vitaminol. 2008;54:329–334. doi: 10.3177/jnsv.54.329[↩]
- Nishigaki I., Rajendran P., Venugopal R., Ekambaram G., Sakthisekaran D., Nishigaki Y. Cytoprotective role of astaxanthin against glycated protein/iron chelate-induced toxicity in human umbilical vein endothelial cells. Phytother. Res. 2010;24:54–59. doi: 10.1002/ptr.2867[↩]
- Bhuvaneswari S., Yogalakshmi B., Sreeja S., Anuradha C.V. Astaxanthin reduces hepatic endoplasmic reticulum stress and nuclear factor-κB-mediated inflammation in high fructose and high fat diet-fed mice. Cell Stress Chaperones. 2013 in press[↩]
- Kim Y.J., Kim Y.A., Yokozawa T. Protection against oxidative stress, inflammation, and apoptosis of high glucose- exposed proximal tubular epithelial cells by astaxanthin. J. Agric. Food Chem. 2009;57:8793–8797. doi: 10.1021/jf9019745[↩]
- Marotta F, Pavasuthipaisit K, Yoshida C, et al. Relationship between aging and susceptibility of erythrocytes to oxidative damage: in view of nutraceutical interventions. Rejuvenation Res 2006;9:227-230.[↩]
- Nakagawa K, Kiko T, Miyazawa T, et al. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br J Nutr 2011;105:1563-1571.[↩]
- Miyawaki H, Takahashi J, Tsukahara H, Takehara I. Effects of astaxanthin on human blood rheology. J Clin Biochem Nutr 2008;43:69-74.[↩]
- Ryu S.K., King T.J., Fujioka K., Pattison J., Pashkow F.J., Tsimikas S. Effect of an oral astaxanthin prodrug (CDX-085) on lipoprotein levels and progression of atherosclerosis in LDLR and ApoE mice. Atherosclerosis. 2012;222:99–105. doi: 10.1016/j.atherosclerosis.2012.02.002[↩]
- Wolf G. Retinoids and carotenoids as inhibitors of carcinogenesis and inducers of cell-cell communication. Nutr. Rev. 1992;50:270–274. doi: 10.1111/j.1753-4887.1992.tb01345.x[↩]
- Hix L.M., Lockwood S.F., Bertram J.S. Upregulation of connexin 43 protein expression and increased gap junctional communication by water soluble disodium disuccinate astaxanthin derivatives. Cancer Lett. 2006;211:25–37[↩]
- Zhang L.X., Cooney R.V., Bertram J.S. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/2 cells: relationship to their cancer chemopreventive action. Carcinogenesis. 1991;12:2109–2114. doi: 10.1093/carcin/12.11.2109[↩]
- Chew B.P., Park J.S. Carotenoid action on the immune response. J. Nutr. 2004;134:257S–261S.[↩]
- Palozza P., Torelli C., Boninsegna A., Simone R., Catalano A., Mele M.C., Picci N. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 2009;283:108–117. doi: 10.1016/j.canlet.2009.03.031[↩][↩]
- Daubrawa F., Sies H., Stahl W. Astaxanthin diminishes gap junctional intercellular communication in primary human fibroblasts. J. Nutr. 2005;135:2507–2511[↩]
- Maoka T., Tokuda H., Suzuki N., Kato H., Etoh H. Anti-oxidative, anti-tumor-promoting, and anti-carcinogenesis activities of nitroastaxanthin and nitrolutein, the reaction products of astaxanthin and lutein with peroxynitrite. Mar. Drugs. 2012;10:1391–1399. doi: 10.3390/md10061391[↩][↩]
- Satoh A, Tsuji S, Okada Y, et al. Preliminary clinical evaluation of toxicity and efficacy of a new astaxanthin-rich Haematococcus pluvialis extract. J Clin Biochem Nutr 2009;44:280-284.[↩]
- Zhang X, Pan L, Wei X, et al. Impact of astaxanthin-enriched algal powder of Haematococcus pluvialis on memory improvement in BALB/c mice. Environ Geochem Health 2007;29:483-489.[↩]
- Lu YP, Liu SY, Sun H, et al. Neuroprotective effect of astaxanthin on H(2)O(2)-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res 2010;1360:40-48.[↩][↩]
- Kim JH, Choi W, Lee JH, et al. Astaxanthin inhibits H2O2-mediated apoptotic cell death in mouse neural progenitor cells via modulation of P38 and MEK signaling pathways. J Microbiol Biotechnol 2009;19:1355-1363.[↩][↩]
- Kim JH, Nam SW, Kim BW, et al. Astaxanthin improves the proliferative capacity as well as the osteogenic and adipogenic differentiation potential in neural stem cells. Food Chem Toxicol 2010;48:1741-1745.[↩]
- Nakamura S., Maoka T., Tsuji S., Hayashi M., Shimazawa M., Hara H. Central Nervous System Migration of Astaxanthin and Adonixanthin Following Their Oral Administration in Cynomolgus Monkeys. J. Nutr. Sci. Vitaminol. 2020;66:488–494. doi: 10.3177/jnsv.66.488[↩]
- Izumi-Nagai K., Nagai N., Ohgami K., Satofuka S., Ozawa Y., Tsubota K., Ohno S., Oike Y., Ishida S. Inhibition of Choroidal Neovascularization with an Anti-Inflammatory Carotenoid Astaxanthin. Investig. Opthalmology Vis. Sci. 2008;49:1679–1685. doi: 10.1167/iovs.07-1426[↩]
- Inoue Y., Shimazawa M., Nagano R., Kuse Y., Takahashi K., Tsuruma K., Hayashi M., Ishibashi T., Maoka T., Hara H. Astaxanthin analogs, adonixanthin and lycopene, activate Nrf2 to prevent light-induced photoreceptor degeneration. J. Pharmacol. Sci. 2017;134:147–157. doi: 10.1016/j.jphs.2017.05.011[↩]
- Nakajima Y., Inokuchi Y., Shimazawa M., Otsubo K., Ishibashi T., Hara H. Astaxanthin, a dietary carotenoid, protects retinal cells against oxidative stress in-vitro and in mice in-vivo. J. Pharm. Pharmacol. 2008;60:1365–1374. doi: 10.1211/jpp.60.10.0013[↩]
- Otsuka T., Shimazawa M., Inoue Y., Nakano Y., Ojino K., Izawa H., Tsuruma K., Ishibashi T., Hara H. Astaxanthin Protects Against Retinal Damage: Evidence from In Vivo and In Vitro Retinal Ischemia and Reperfusion Models. Curr. Eye Res. 2016;41:1465–1472. doi: 10.3109/02713683.2015.1127392[↩]
- Yamagishi R, Aihara M. Neuroprotective effect of astaxanthin against rat retinal ganglion cell death under various stresses that induce apoptosis and necrosis. Mol Vis. 2014 Dec 31;20:1796-805. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4287717[↩]
- Nagaki Y., Mihara M., Takahashi J., Kitamura A., Horita Y., Sugiura Y., Tsukahara H. The effect of astaxanthin on retinal capillary blood flow in normal volunteers. J. Clin. Ther. Med. 2005;21:5.[↩][↩]
- Saito M., Yoshida K., Saito W., Fujiya A., Ohgami K., Kitaichi N., Tsukahara H., Ishida S., Ohno S. Astaxanthin increases choroidal blood flow velocity. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012;250:239–245. doi: 10.1007/s00417-011-1843-1[↩][↩]
- Miyawaki H., Takahashi J., Tsukahara H., Takehara I. Effects of Astaxanthin on Human Blood Rheology. J. Clin. Biochem. Nutr. 2008;43:69–74. doi: 10.3164/jcbn.2008048[↩][↩]
- Kajita M, Tsukahara H, Kato M. The effects of a dietary supplement containing astaxanthin on the accommodation function of the eye in middle-aged and older people. Med Consult New Remedies 2009;46:89-93[↩][↩]
- Thomson WD. Eye problems and visual display terminals – the facts and the fallacies. Ophthalmic Physiol Opt 1998;18:111-119.[↩]
- Nagaki Y, Hayasaka S, Yamada T, et al. Effects of astaxanthin on accommodation, critical flicker fusion, and pattern visual evoked potential in visual display terminal workers. J Tradit Med 2002;19:170-173.[↩]
- Aoi W., Naito Y., Sakuma K., Kuchide M., Tokuda H., Maoka T., Toyokuni S., Oka S., Yasuhara M., Yoshikawa T. Astaxanthin limits exercise-induced skeletal and cardiac muscle damage in mice. Antioxid. Redox Signal. 2003;5:139–144. doi: 10.1089/152308603321223630[↩]
- Malmsten CL, Lignell A. Dietary supplementation with astaxanthin-rich algal meal improves strength endurance – a double blind placebo controlled study on male students. Carotenoid Sci 2008;13:20-22.[↩]
- Baralic I, Djordjevic B, Kotur-Stevuljevic J, et al. Astaxanthin supplementation prevents muscle and oxidative damage induced by training in elite young soccer players. European Database of Sports Science (EDSS). 16th Annual ECSS-Congress, Liverpool 2011.[↩]
- Radivojevic N, Dikic N, Baralic I, et al. Effects of astaxanthin supplementation on sports performance in young elite soccer players. European Database of Sports Science (EDSS). 16th Annual ECSS-Congress, Liverpool 2011[↩]
- Comhaire FH, El Garem Y, Mahmoud A, et al. Combined conventional/antioxidant “Astaxanthin” treatment for male infertility: a double blind, randomized trial. Asian J Androl 2005;7:257-262.[↩][↩]
- Park J.S., Chyun J.H., Kim Y.K., Line L.L., Chew B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010;7:1–10. doi: 10.1186/1743-7075-7-18[↩]
- Jyonouchi H., Hill R., Tomita Y., Good R. Studies of immunomodulating actions of carotenoids. I. Effects of β-carotene and astaxanthin on murine lymphocyte functions and cell surface marker expression in in vitro culture system. Nutr. Cancer. 1991;16:93–105. doi: 10.1080/01635589109514148[↩][↩]
- Jyonouchi H., Sun S., Gross M. Effect of carotenoids on in vitro immunoglobulin production by human peripheral blood mononuclear cells: astaxanthin, a carotenoid without vitamin A activity, enhances in vitro immunoglobulin production in response to a T-dependent stimulant and antigen. Nutr. Cancer. 1995;23:171–183. doi: 10.1080/01635589509514373[↩]
- Park J.S., Chyun J.H., Kim Y.K., Line L.L., Chew B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010;7:1–10[↩]
- Fakhri S., Yosifova Aneva I., Farzaei M.H., Sobarzo-Sánchez E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules. 2019;24:2640. doi: 10.3390/molecules24142640[↩]
- Fakhri S., Nouri Z., Moradi S.Z., Farzaei M.H. Astaxanthin, COVID-19 and immune response: Focus on oxidative stress, apoptosis and autophagy. Phytother. Res. 2020;34:2790. doi: 10.1002/ptr.6797[↩]
- Chang M.X., Xiong F. Astaxanthin and its effects in inflammatory responses and inflammation-associated diseases: Recent advances and future directions. Molecules. 2020;25:5342. doi: 10.3390/molecules25225342[↩]
- Osterlie M, Bjerkeng B, Liaaen-Jensen S. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin. J Nutr Biochem 2000;11:482-490.[↩]
- EFSA (European Food Safety Authority) Opinion of the scientific panel on additives and products or substances used in animal feed on the request from the European commission on the safety of use of colouring agents in animal human nutrition. EFSA J. 2005;291:1–40.[↩][↩]
- Iwamoto T., Hosoda K., Hirano R., Kurata H., Matsumoto A., Miki W., Kamiyama M., Itakura H., Yamamoto S., Kondo K. Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheroscler. Thromb. 2000;7:216–222. https://www.ncbi.nlm.nih.gov/pubmed/11521685[↩]
- Brendler T., Williamson E.M. Astaxanthin: How much is too much? A safety review. Phytother. Res. 2019;33:3090–3111. doi: 10.1002/ptr.6514[↩]
- Ranga Rao A., Baskaran V., Sarada R., Ravishankar G.A. In vivo bioavailability and antioxidant activity of carotenoids from micro algal biomass—A repeated dose study. Food Res. Int. 2013;54:711–717. doi: 10.1016/j.foodres.2013.07.067.[↩]
- Ohgami K., Shiratori K., Nitta T., Shinmei Y., Chin S., Yoshida K., Tsukahara H., Ohno S. Study on the Safety of High Dose Administration of Astaxanthin. J. Clin. Ther. Med. 2005;21:651–659. (In Japanese).[↩]
- Kajita M., Tsukahara H., Kato M., Yoshimoto T. Safety of excessive intake of astaxanthin. Journal of Clinical Therapeutics & Medicines [in Japanese] 2009;25:691–698.[↩]
- Kajita M., Kato T., Yoshimoto T., Masuda K. Study on the safety of high dose administration of astaxanthin. Folia Jpn. De Ophthalmol. Clin. 2010;4:365–370. (In Japanese).[↩]
- Tsukahara H., Fukuhara I., Takehara I. Evaluation of long-term safty of natural astaxanthin in healthy volunteers. Journal of Nutr. Food. 2005;8:1–11. (In Japanese).[↩]
- Matsuyama A., Takahashi J., Itakura H. A Safety Study on the Long-Term Consumption of Astaxanthin in Healthy Human Volunteers. Jpn. J. Complement. Altern. Med. 2010;7:43–50. doi: 10.1625/jcam.7.43[↩]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008;22:659-661[↩]
- Scientific Opinion on the safety of astaxanthin‐rich ingredients (AstaREAL A1010 and AstaREAL L10) as novel food ingredients. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2014.3757[↩][↩][↩][↩][↩][↩][↩][↩]