Contents
What is leptospirosis
Leptospirosis is one of the most common zoonotic diseases (a disease that normally exists in animals but that can infect humans) worldwide. Leptospirosis is a bacterial disease that is caused by corkscrew-shaped bacteria belonging to the genus Leptospira that can infect humans and animals. Leptospirosis is an infection you can catch from animals through contact with infected animals, or soil or water that has been contaminated by the urine of infected animals. While leptospirosis occurs worldwide, it is more common in tropical or sub-tropical climates. In humans, leptospirosis can cause a wide range of symptoms, some of which may be mistaken for other diseases. Some infected persons, however, may have no symptoms at all.
Symptoms of leptospirosis include fever, headache, chills, muscle aches, vomiting/diarrhea, cough, conjunctivitis, jaundice, and sometimes a rash.
The incubation period is usually 5-14 days, with a range of 2-30 days. Most illnesses occur 5–14 days after exposure.
- Most infections are thought to be asymptomatic.
- Approximately 90% of clinical illnesses present as a nonspecific acute febrile illness, while approximately 10% progress to severe, potentially fatal illness with multi-organ dysfunction.
- Leptospirosis during pregnancy can cause fetal complications including fetal death or abortion.
Leptospirosis can be difficult to clinically distinguish from other causes of acute febrile illnesses or their severe complications; diagnosis may therefore be missed or delayed. A detailed history is important for identifying patients who have undertaken activities that place them at risk of exposure to infection. A travel history is also important for delineating possible diagnoses.
Leptospirosis is an occupational hazard for many people who work outdoors or with animals, such as:
- Farmers
- Mine workers
- Sewer workers
- Slaughterhouse workers
- Veterinarians and animal caretakers
- Fish workers
- Dairy farmers
- Military personnel
Leptospirosis has also been associated with swimming, wading, kayaking, and rafting in contaminated lakes and rivers. As such, it is a recreational hazard for campers or those who participate in outdoor sports. The risk is likely greater for those who participate in these activities in tropical or temperate climates.
In addition, incidence of Leptospirosis infection among urban children appears to be increasing.
- It is estimated that 100-150 Leptospirosis cases are identified annually in the United States. About 50% of cases occur in Puerto Rico.
- The largest recorded U.S. outbreak occurred in 1998, when 775 people were exposed to the disease. Of these, 110 became infected.
- Although incidence in the United States is relatively low, leptospirosis is considered to be the most widespread zoonotic disease in the world.
- It is estimated that more than 1 million leptospirosis cases occur worldwide annually, including almost 60,000 deaths 1.
- Outbreaks of leptospirosis tend to occur after heavy rainfall or flooding in endemic areas, especially areas with poor housing and sanitation conditions.
Without treatment, Leptospirosis can lead to kidney damage, meningitis (inflammation of the membrane around the brain and spinal cord), liver failure, respiratory distress, and even death.
However, leptospirosis clinical course is highly variable. Most cases involve flu-like symptoms (fever, chills, muscle aches, headaches). Other symptoms may include: conjunctivitis, vomiting, diarrhea, stomach pain, jaundice, cough, and rarely a skin rash. About 10 percent of people with leptospirosis develop severe disease, including kidney or liver failure, meningitis, difficulty breathing, bleeding, and meningitis. Case fatality rate is 5 to 15% in cases with severe clinical illness.
Key points
- For patients with risk factors, leptospirosis should be considered as a differential diagnosis of undifferentiated febrile illness and the complications discussed above.
- A high index of clinical suspicion is required to ensure early diagnosis and treatment, and to minimize the risk of complications.
- Patients involved in high-risk activities should be advised to seek medical care promptly if they develop a fever.
- Diagnosis is confirmed by serology or culture, but polymerase chain reaction (PCR) is valuable in the early phase of the infection.
- Severe cases and complications should be referred to a hospital for inpatient management.
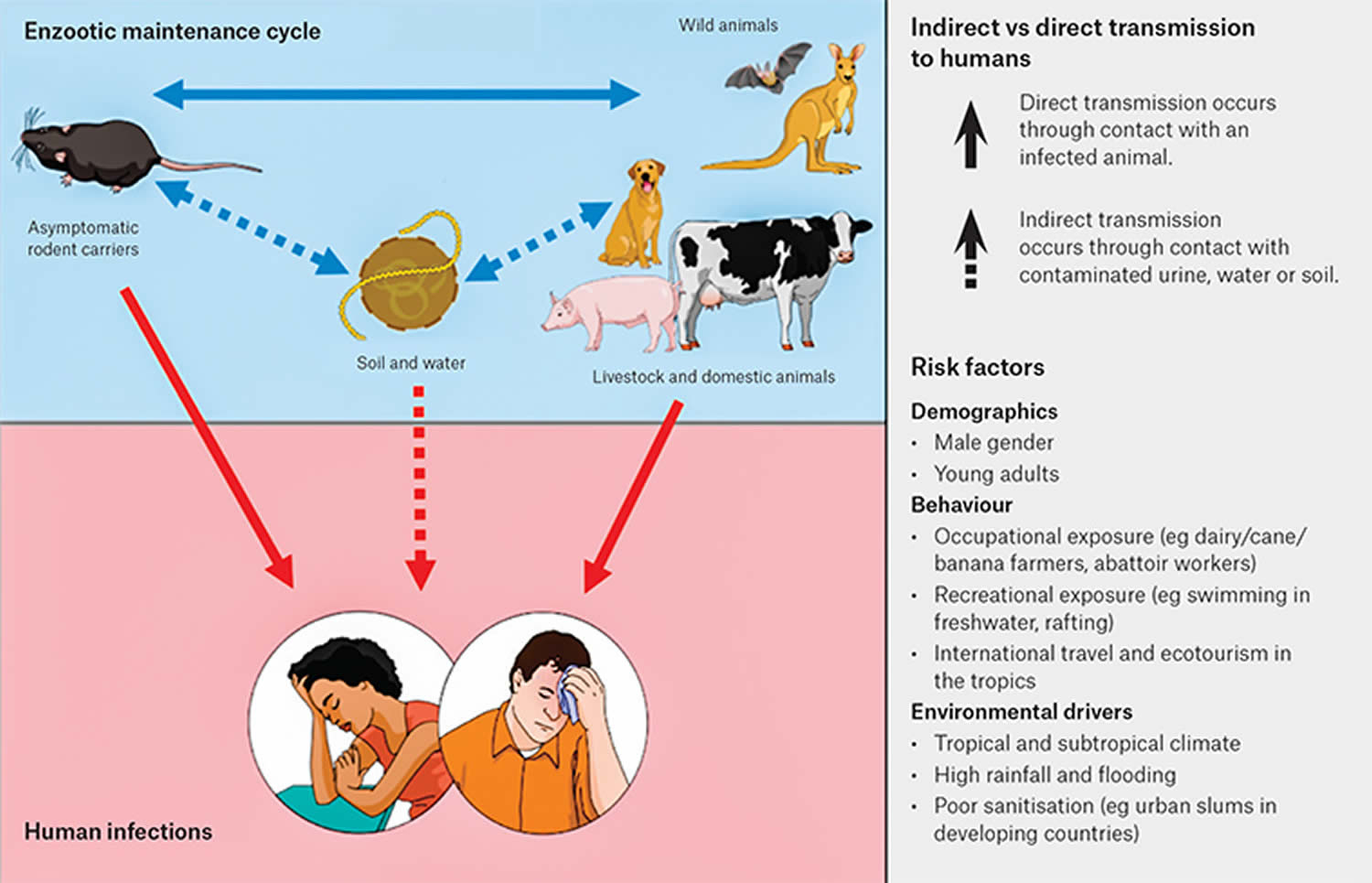
Leptospirosis transmission
Leptospirosis is spread through the urine of infected animals – most commonly rats, mice, cows, pigs and dogs, which can get into water or soil and can survive there for weeks to months. Many different kinds of wild and domestic animals carry the Leptospira bacterium.
These can include, but are not limited to:
- Cattle
- Pigs
- Horses
- Dogs
- Rodents
- Wild animals
- Raccoons
- Opossums
- Buffaloes
- Sheep
- Goats
When these animals are infected, they may have no symptoms of the disease.
Infected animals may continue to excrete the bacteria into the environment continuously or every once in a while for a few months up to several years.
You can catch leptospirosis if:
- soil or freshwater (such as from a river, canal or lake) containing infected urine gets in your mouth, eyes or a cut – usually during activities like kayaking, outdoor swimming or fishing
- you touch an infected animal’s blood or flesh – usually from working with animals or animal parts
- you have direct contact with the urine or reproductive fluids from infected animals
- you come in contact with urine-contaminated water (floodwater, rivers, streams, sewage) and wet soil
Human-to-human transmission is very rare but has been documented through sexual intercourse and breastfeeding. Transmission has also rarely occurred through animal bites.
The bacteria can enter the body through skin or mucous membranes (eyes, nose, or mouth), especially if the skin is broken from a cut or scratch. Drinking contaminated water can also cause infection. Outbreaks of leptospirosis are usually caused by exposure to contaminated water, such as floodwaters. Person to person transmission is rare.
It’s very rare to get leptospirosis from pets, other people or bites.
Figure 1. Leptospirosis transmission pathways and risk factors
Who is at risk of leptospirosis?
Leptospirosis occurs worldwide, but is most common in temperate or tropical climates.
There is always a risk of infection for people who have contact with infected animals or soil/water where the bacteria are present.
People who work outdoors or with animals may be at increased risk for infection.
You are at risk of leptospirosis if you:
- are a farmer, especially of sugar cane or bananas
- dairy farmers
- work in an abattoir or slaughterhouse workers
- are a veterinarian/animal caretaker
- are exposed to water, mud or soil contaminated with animal urine
- come into close contact with animals.
- mine workers
- sewer workers
- fishermen and people who work with fish
- military personnel
Those involved in outdoor freshwater activities may also face an increased risk.
Activities may include:
- Swimming
- Rafting
- Kayaking
Leptospirosis symptoms
Symptoms of leptospirosis can develop anywhere from 2 days to 4 weeks after being exposed to the bacteria and becoming sick.
After an incubation period of two to 30 days (usually five to 14 days), leptospirosis manifests as a biphasic illness with an acute/bacteremic phase (seven to 10 days from symptom onset), characterized by sudden onset of fever, muscle ache and headache 2. Calf tenderness and conjunctival suffusion are characteristic but not always present. Other non-specific symptoms include anorexia, nausea, vomiting, abdominal pain, dizziness, lethargy, arthralgia, eye pain, photophobia and rashes. This is followed by a late/immune phase (>7 days from symptom onset), when immunologically mediated organ damage occurs, and severe complications include acute renal failure, pulmonary haemorrhage, myocarditis, arrhythmias, shock, liver failure, coagulopathy and neurological complications 2.
Leptospirosis may occur in two phases:
- After the first phase (with fever, chills, headache, muscle aches, vomiting, or diarrhea) the patient may recover for a time but become ill again.
- If a second phase occurs, it is more severe; the person may have kidney or liver failure or meningitis.
The illness lasts from a few days to 3 weeks or longer. Without treatment, recovery may take several months.
Common symptoms of leptospirosis in humans include:
- Fever
- Chills
- Headache
- Muscle Aches
- Vomiting
- Diarrhea
- Abdominal Pain
- Jaundice (yellowing of the skin and eyes)
- Skin Rash
- Red Eyes
Many of these symptoms can be mistaken for other diseases. In addition, some infected persons may have no symptoms at all.
Severe symptoms can include jaundice, renal failure, hemorrhage (especially pulmonary), aseptic meningitis, cardiac arrhythmias, pulmonary insufficiency, and hemodynamic collapse.
Weil’s disease is the classic triad of jaundice, renal failure and hemorrhage, but these manifestations do not always occur together. Aseptic meningitis, encephalitis, convulsions, Guillain–Barré syndrome, transverse myelitis and other neurological syndromes have also been reported 2. Leptospirosis can also present with acute or chronic optic manifestations, including subconjunctival and retinal haemorrhages, optic neuritis and chronic uveitis.
The case fatality rate for leptospirosis is approximately 5%–15% among patients with severe illness. Among patients with severe pulmonary hemorrhagic syndrome, the case fatality rate can exceed 50%.
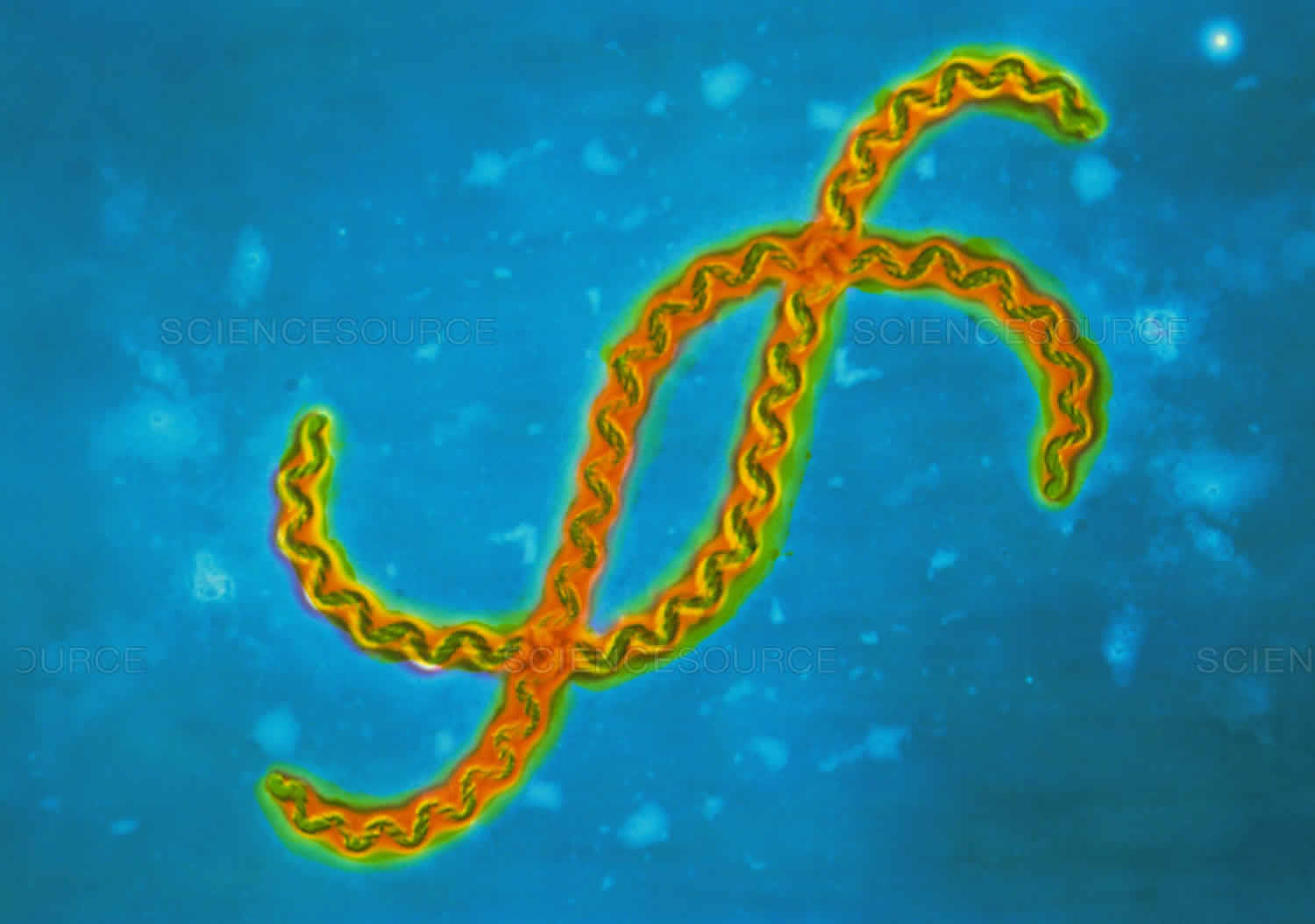
Leptospirosis causes
Leptospires are long, thin, motile spirochetes. They may be free-living or associated with animal hosts and survive well in fresh water, soil, and mud in tropical areas. Organisms are antigenically complex, with over 250 known pathogenic serologic variants. Although certain geographic regions contain specific leptospiral serovars and species, the serologic characterization of an isolate is not an absolute predictor of its species designation.
Figure 2. Leptospira bacteria (under scanning electron microscope)
Leptospirosis prevention
There are several steps you can take to help prevent getting leptospirosis. These include:
- See a veterinarian to get vaccines for your pets that can protect against this disease.
- Avoid contact with animal urine or body fluids, especially if there are any cuts or abrasion of the skin
- Do not swim in, walk in, swallow or submersing head in potentially contaminated freshwater (rivers, streams) especially after periods of heavy
rainfall or flooding that may contain animal urine - Wear protective clothing or footwear near soil or water that may be contaminated with animal urine. Appropriate personal protective equipment
includes (rubber boots, waterproof coveralls/clothing, gloves). Cover open wounds with waterproof dressings. - Avoid contact with floodwater, and do not eat food contaminated with floodwater.
- Treat unsafe or potentially contaminated drinking water by boiling or chemically treating.
- Keep rodent populations (rats and mice) or other animal pests under control. Do not eat food that may have been exposed to rodents and possibly contaminated with their urine.
- As a general rule, always wash your hands after handling your pet or anything that might have your pet’s excrement on it.
- If you are cleaning surfaces that may be contaminated or have urine from an infected pet on them, use an antibacterial cleaning solution or a solution of 1 part household bleach in 10 parts water.
- Some studies have shown that chemoprophylaxis with doxycycline might be effective in preventing clinical disease and could be considered for people at high risk and with short-term exposures. But a systematic review concluded that regular use at 200 mg weekly was associated with nausea and vomiting, and there was no clear benefit for reducing the risk of leptospirosis 3. Routine use is therefore not recommended, but might be considered if short-term intense exposures are anticipated (e.g., military personnel, outbreak response personnel, recreational exposure) or after high-risk exposures (e.g., floodwaters). Doxycycline can also provide protection against other infectious disease (e.g., malaria, rickettsia). For travelers to malaria-endemic areas who may be at risk of leptospirosis, using doxycycline for malaria prophylaxis (in preference to other antimalarial medications) could be considered.
The risk of acquiring leptospirosis can be greatly reduced by not swimming or wading in water that might be contaminated with animal urine, or eliminating contact with potentially infected animals.
Protective clothing or footwear should be worn by those exposed to contaminated water or soil because of their job or recreational activities.
Leptospirosis vaccine
Leptospirosis vaccine does not provide 100% protection. This is because there are many strains (types) of leptospires (the bacteria that causes Leptospirosis), and the vaccine does not provide immunity against all strains. It is important to get your pet vaccinated again even if it gets leptospirosis because it can still get infected with a different strain of leptospires.
Human leptospirosis vaccines are commercially available only in few countries like France, Cuba and Japan 4. Human vaccines are rarely used because of limited effectiveness, adverse reactions and short duration of protection 5.
Available literature have indicated that current human leptospirosis vaccines contain whole-cell of inactivated leptospires 6. The monovalent vaccine from France contains an inactive strain of Leptospira icterohaemorrhagiae, and initially given in 3 shots followed by biannual revaccination 7. The trivalent vaccine from Cuba contains serovars of Canicola, Copenhageni and Mozdok 8. Vaccination is given in 2 shots at 6-week intervals. The polyvalent vaccine from Japan contains serovars of Autralis, Autumnalis, Hebdomadis, and Copenhageni 9. It is given in 2 shots followed by a booster injection after 5 years.
The current leptospirosis vaccines are still facing major issues related to short-term and serovar-specific protection, including concerns on efficacy and safety with the use of inactivated whole-cell preparation 10. Their duration of immunity varied from less than 2 years to a maximum of 7 years after vaccination, while their efficacies range from 60% to 100% 11. The side-effects have been reported to include: mild local reactions such as skin redness, hard lump, swelling, local pain and itching; systemic reactions such as fever, headache, fainting, nausea and vomiting; and serious autoimmune disease like inflation of vascular structure of the eyes 12. The use of recombinant, lipopolysaccharide and DNA-based vaccines may offer solutions to some of the limitations of current leptospirosis vaccine 10. However, their applications are still in exploratory and may take years until new leptospirosis vaccines will be commercially available.
Leptospirosis diagnosis
Multiple diagnostic tests are available, and it is important to order the appropriate test(s) and understand their interpretation for each phase of the illness. Tests that detect the presence of bacteria (e.g., polymerase chain reaction [PCR], culture) will only yield positive results in the acute/bacteraemic phase. Tests that detect antibodies will produce positive results later, from days six to eight from illness onset for immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA), and from day 10 to 12 from illness onset for microscopic agglutination test (MAT). A confirmed case is based on culture or microscopic agglutination test (MAT), the gold standard tests in the acute/bacteremic and late/immune phases respectively 13. Positive polymerase chain reaction (PCR) alone or immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA) alone are considered probable rather than confirmatory, but are nevertheless valuable for guiding clinical diagnosis in patients who present with suspected leptospirosis.
A ‘confirmed case’ requires definitive laboratory evidence of leptospirosis infection by one of the following:
- Isolation of pathogenic Leptospira species
- A fourfold or greater rise in Leptospira microscopic agglutination test (MAT) titer between acute-and convalescent-phase sera obtained at least two weeks apart, and preferably conducted at the same laboratory
- A single Leptospira microscopic agglutination test (MAT) titer ≥400, supported by a positive ELISA IgM result.
In the acute/bacteraemic phase, blood should be collected for PCR (in a serum-separating tube) and IgM ELISA before commencing antibiotics. During the early acute/bacteraemic phase, IgM ELISA has lower sensitivity, compared with PCR,18 but the results are helpful in determining the phase of illness. Blood cultures should be taken if the specific culture medium is available (Ellinghausen–McCullough–Johnson–Harris medium).19 However, it is important to check the availability of the culture medium with the local pathology laboratory, and whether they are able to conduct cultures. Cultures are examined for six weeks by dark-field microscopy, so this method is generally not useful for informing immediate clinical management.
During the late/immune phase, when antibodies are present, both IgM ELISA and MAT tests should be requested. Reactive IgM ELISAs are sent to reference laboratories for confirmation by MAT. A convalescent sample should be collected 14 days later to confirm rising MAT titers, particularly if the initial IgM ELISA was reactive but MAT was found to be non-reactive. MAT combines diluted serum with a panel of serovars from different serogroups 14. The World Health Organization’s (WHO’s) Leptospirosis Reference Laboratory, uses a routine panel of 22 serovars, which includes a representative from each major serogroup. Any history of overseas travel by the patient should be communicated to the laboratory so that appropriate serovars are included in the panel.
Depending on the clinical presentation, other relevant investigations should include full blood count, biochemistry, arterial blood gases, electrocardiography (ECG), chest X-ray and lumbar puncture. The most common laboratory findings in a patient with leptospirosis are neutrophilia and mildly abnormal liver function tests. Other possible investigation findings, depending on severity and complications, are summarized in Table 1.
Table 1. Possible investigation findings in leptospirosis, depending on severity and complications
| Investigations | Findings |
| Full blood count | Leucocytosis, neutrophilia with left shift, lymphopenia, normochromic anaemia, thrombocytopenia |
| Urea, electrolytes, creatinine | Raised urea and creatinine if renal impairment. Potassium is usually normal or low, high potassium is associated with poor outcomes (an indicator of impaired renal function, and might lead to arrhythmias). Low sodium |
| Liver function tests | Raised bilirubin (mainly direct), may take time to resolve. Normal or raised liver enzymes. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are typically three to five times above normal, but could be much higher in cases with fulminant hepatic failure |
| Urinalysis | Proteinuria, microscopic haematuria, pyuria, granular casts |
| Creatine phosphokinase | Raised in patients with myalgia |
| Coagulation | Prothrombin time, partial thromboplastin time and international normalized ratio may be raised because of impaired liver function |
| Arterial blood gases | Low partial pressure of O2 (PaO2), arterial O2 saturation (SaO2), ratio of partial pressure arterial oxygen and fraction of inspired oxygen (PaO2/FiO2) ratio, metabolic acidosis (ie low pH, low HCO3) |
| Electrocardiograph | Atrial fibrillation, supraventricular or ventricular extrasystoles, atrioventricular block, other arrhythmias |
| Chest X-ray | Variable findings, including alveolar infiltrates, nodular densities and consolidation. Changes could be diffuse or lobar, unilateral or bilateral. Findings could represent a range of pathology, including alveolar haemorrhage, acute respiratory distress syndrome, pulmonary oedema |
| Lumbar puncture | Neutrophilic or lymphocytic pleocytosis, mild elevations in protein, normal glucose |
Leptospirosis test
Antibodies for leptospirosis develop between 3-10 days after symptom onset, thus any serologic test must be interpreted accordingly – negative serologic test results from samples collected in the first week of illness do not rule out disease, and serologic testing should be repeated on a convalescent sample collected 7-14 days after the first.
In the acute phase of illness, leptospires are present in the blood (septicemia) for approximately the first 4–6 days of illness.
Leptospires may be shed intermittently in the urine after approximately the first week of illness onset. Due to the transience of leptospires in body fluids, a negative polymerase chain reaction (PCR) test does not rule out leptospisosis.
It is best to submit as many specimen types as possible. Recommended specimens based on collection timing:
- Acute illness (first week): whole blood and serum
- Convalescent illness (after first week): serum +/- urine
Supportive diagnostic tests
IgM-based commercial assays, such as
- ELISA IgM
- ImmunoDOT
- Lateral flow tests
IgM assays are screening tests and results should be confirmed using one of the confirmatory methods below.
Confirmatory diagnostic tests
1) Microscopic agglutination test (MAT) — confirmatory serologic testing, available at Centers for Disease Control and Prevention (CDC)
- Acute and convalescent serum samples collected 7–14 days apart is ideal.
- If only one serum sample can be sent for testing, a sample collected after the first 7–10 days of illness is preferred.
2) Polymerase chain reaction (PCR) – available at Centers for Disease Control and Prevention (CDC) and some commercial labs
Recommended samples:
- Whole blood collected in the first week of illness (in the first 4 days is ideal)
- Urine (collected at least 1 week after symptom onset is ideal)
- Cerebrospinal fluid from a patient with signs of meningitis
- Fresh frozen kidney and/or liver (if available from deceased patients) — kidney preferred
3) Pathology (immunohistochemistry) — available at CDC
- Formalin-fixed tissues: from the kidney (preferred), liver, lung, heart, or spleen
Leptospirosis treatment
Early treatment may decrease the severity and duration of disease. In patients with a high clinical suspicion of leptospirosis, initiating antibiotic treatment as soon as possible without waiting for laboratory results is recommended.
Leptospirosis is treated with antibiotics, such as doxycycline or penicillin, which should be given early in the course of the disease.
For patients with mild symptoms, doxycycline is the drug of choice (100 mg orally, twice daily), if not contraindicated, because it is also effective against rickettsial infections, which can have a similar presentation 15. Other options include azithromycin (500 mg orally, once daily), ampicillin (500-750 mg orally, every 6 hours), amoxicillin (500 mg orally, every 6 hours) 16.
Intravenous antibiotics may be required for persons with more severe symptoms.
For patients with severe disease, IV penicillin is the drug of choice (1.5 MU IV, every 6 hours), and ceftriaxone (1 g IV, every 24 hours) can be equally effective 16.
Jarish–Herxheimer reactions can occur one to 48 hours after starting antibiotics 17. Common features include sudden onset of shivers or rigors, fever and hypotension. Jarish–Herxheimer is caused by an acute inflammatory response mediated by the release of large amounts of cytokines in response to the sudden and rapid death of Leptospira.
Patients with suspected leptospirosis who present with life-threatening complications and/or evidence of end-organ failure (e.g., massive pulmonary hemorrhage, acute respiratory distress syndrome, renal failure, shock) should be referred to a hospital because of the risk of further deterioration.
Depending on the complications, management might include intravenous fluids and inotropes, ventilation, hemodialysis, and treatment of arrhythmias and coagulopathies. Currently, corticosteroids are not recommended because there is conflicting evidence about their effectiveness, and their use has been associated with increased risk of nosocomial infections in patients with severe leptospirosis 18.
- Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: A systematic review. PLoS Negl Trop Dis 2015;9(9):e0003898[↩]
- Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect Dis 2003;3(12):757–71[↩][↩][↩]
- Brett-Major DM, Lipnick RJ. Antibiotic prophylaxis for leptospirosis. Cochrane Database Syst Rev 2009;(3):CD007342[↩]
- Laurichesse H, Gourdon F, Smits HL, Abdoe TH, Estavoyer JM, Rebika H, Pouliquen P, Catalina P, Dubray C, Beytout J. Safety and immunogenicity of subcutaneous or intramuscular administration of a monovalent inactivated vaccine against Leptospira interrogans serogroup Icterohaemorrhagiae in healthy volunteers. Clin Microbiol Infect 2007; 13:395-403; PMID:17359323; http://dx.doi.org/10.1111/j.1469-0691.2007.01662.x [↩]
- Adler B. Vaccines against leptospirosis. In: Adler B ed. Leptospira and leptospirosis. Current topics in microbiology and immunology, vol 387. Berlin, Heidelberg: Springer, 2015.[↩]
- Verma R, Khanna P, Chawla S. Whole-cell inactivated Leptospirosis vaccine: future prospects. Hu Vaccin Immunother 2013; 9:763; PMID:23295984; http://dx.doi.org/10.4161/hv.23059[↩]
- Laurichesse H, Gourdon F, Smits HL, Abdoe TH, Estavoyer JM, Rebika H, Pouliquen P, Catalina P, Dubray C, Beytout J. Safety and immunogenicity of subcutaneous or intramuscular administration of a monovalent inactivated vaccine against Leptospira interrogans serogroup Icterohaemorrhagiae in healthy volunteers. Clin Microbiol Infect 2007; 13:395-403; PMID:17359323; http://dx.doi.org/10.1111/j.1469-0691.2007.01662.x[↩]
- González M, Martínez R, Paz RCdl, Bourzac JFI, Novo IG, Suárez MB, Sierra AP, González GS, Rodríguez OF, Gutiérrez RB, et al. vax-Spiral®. Trivalent antileptospirosis vaccine for human use: research, development and impact on the disease in Cuba. MEDICC Rev 2004; 2:33.[↩]
- Watanabe H, Koizumi N. Leptospirosis vaccines: past, present, and future. J Postgrad Med 2005; 51:210-4[↩]
- Dellagostin OA, Grassmann AA, Hartwig DD, Félix SR, da Silva ÉF, McBride AJ. Recombinant vaccines against leptospirosis. Hum Vaccin 2011; 7:1215-24; PMID:22048111; http://dx.doi.org/10.4161/hv.7.11.17944[↩][↩]
- Fernández LAR, Santiesteban NB, Arencibia DF, Arrebola BYVA, Toruño WJ, Duttman C. Efficacy of Leptospiral vaccine (vax-SPIRAL) against challenge with strains isolated from leptospirosis epidemic in Nicaragua using the hamster as biomodel. Veter World 2012; 5:5-12; http://dx.doi.org/10.5455/vetworld.2012.5-12[↩]
- Rathinam S. Ocular leptospirosis. Curr Opin Ophthalmol 2002; 13:381-6[↩]
- Department of Health. Australian national notifiable diseases case definitions – Leptospirosis. Canberra: DoH, 2004.[↩]
- Tulsiani SM, Lau CL, Graham GC, et al. Emerging tropical diseases in Australia. Part 1. Leptospirosis. Ann Trop Med Parasitol 2010;104(7):543–56.[↩]
- Therapeutic Guidelines Limited. Leptospirosis [revised 2014 Nov]. eTG Complete. Melbourne: Therapeutic Guidelines Limited, 2017.[↩]
- LEPTOSPIROSIS Fact Sheet for Clinicians. https://www.cdc.gov/leptospirosis/pdf/fs-leptospirosis-clinicians-eng-508.pdf[↩][↩]
- Guerrier G, D’Ortenzio E. The Jarisch–Herxheimer reaction in leptospirosis: A systematic review. PLoS One 2013;8(3):e59266[↩]
- Rodrigo C, Lakshitha de Silva N, Goonaratne R, et al. High dose corticosteroids in severe leptospirosis: A systematic review. Trans R Soc Trop Med Hyg 2014;108(12):743–50.[↩]