Contents
What is taurine
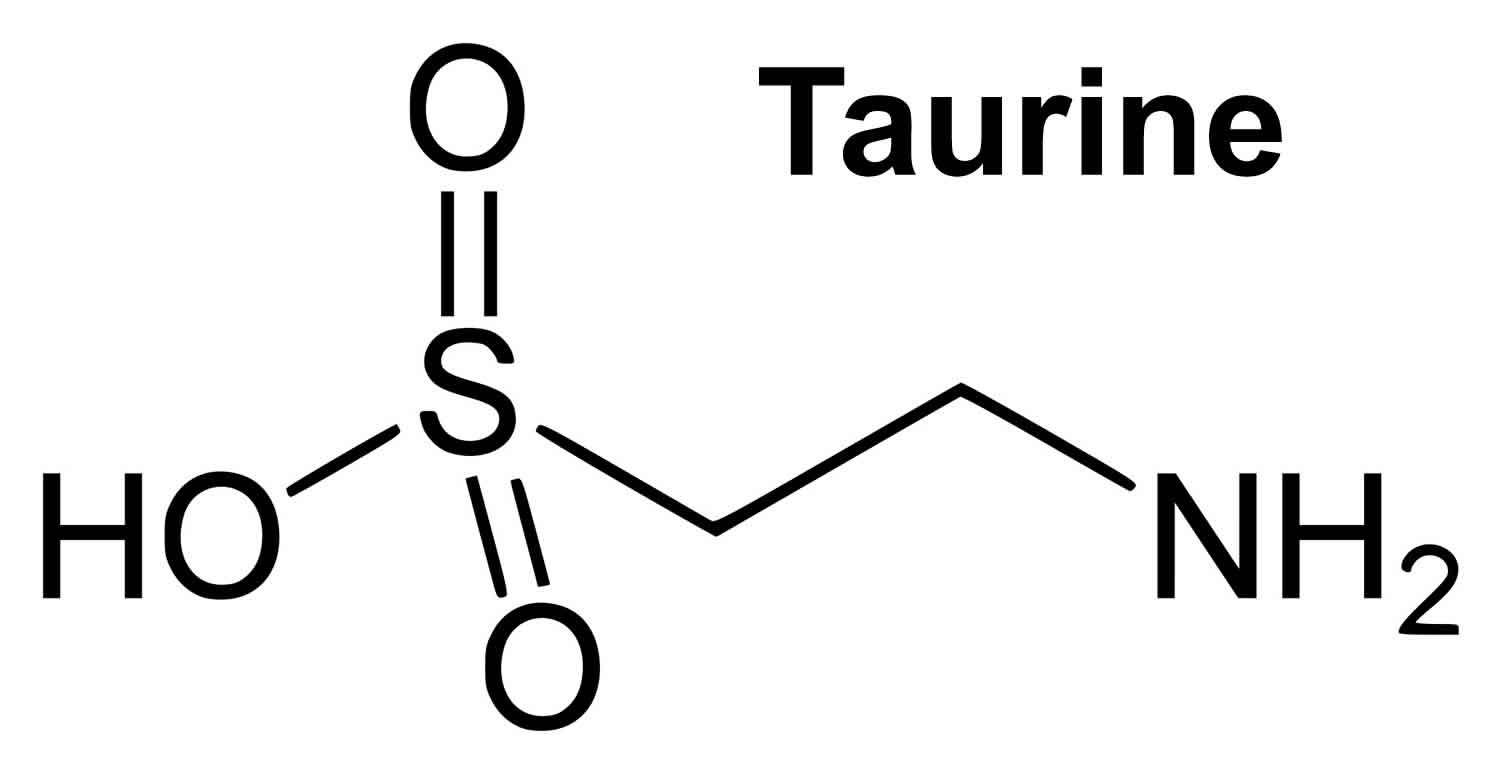
Taurine also called 2-aminoethanesulfonic acid or beta–amino acid, is found in very high concentration in most cells, with levels particularly high in excitable tissues 1. Taurine unlike other amino acid is not incorporated into into the structural building blocks of protein 2. Taurine is found naturally in animal and fish protein, dairy products and human milk, which are good sources of dietary taurine and it’s also available as a dietary supplement. Taurine is abundant in the brain, heart, breast, gallbladder and kidney and has important roles in health and disease in these organs. Taurine has many diverse biological functions serving as a neurotransmitter in the brain, a stabilizer of cell membranes and a facilitator in the transport of ions such as sodium, potassium, calcium and magnesium. While research is mixed, some studies suggest that taurine supplementation might improve athletic performance. And, in one study, people with congestive heart failure who took taurine supplements three times a day for two weeks showed improvement in their exercise capacity.
Adult human not only take up taurine from the diet but also synthesize it de novo from cysteine when vitamin B6 is present. Because human infants have low de novo taurine synthesis activity, dietary taurine is essential for normal human development 3. Deficiency of taurine occurs in premature infants and neonates fed formula milk, and in various disease states. Inborn errors of taurine metabolism called Perry syndrome, is an unusual neuropsychiatric disorder inherited in an autosomal dominant fashion through 3 generations of a family 4. Perry syndrome symptoms began late in the fifth decade in 6 affected persons and death occurred after 4 to 6 years. The earliest and most prominent symptom was mental depression not responsive to antidepressant drugs or electroconvulsive therapy. Sleep disturbances, exhaustion and marked weight loss were features. Parkinsonism developed later, and respiratory failure occurred terminally.
Functions and possible roles for taurine 5:
- Intestinal absorption of fat
- Osmoregulation
- Energy storage
- Pigmentation
- Reproduction
- Hypoglycemic agent
- Neurotransmitter and neuromodulator
- Antiepileptic agent
- Antiarrythmic agent and cardiac effects
- Calcium ion fluxes
- Protein phosphorylation
The discovery that taurine is an effective therapy against congestive heart failure led to the study of taurine as a therapeutic agent against other disease conditions 6. Today, taurine has been approved for the treatment of congestive heart failure in Japan and shows promise in the treatment of several other diseases 1. In addition, taurine is extremely effective in the treatment of the mitochondrial disease, mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes and offers a new approach for the treatment of metabolic diseases, such as diabetes, and inflammatory diseases, such as arthritis 1.
Is taurine bad for you?
Taurine is not genotoxic, teratogenic or carcinogenic 7. However, a tolerable upper intake level (UL) could not be derived from the available NOAELs (no observed adverse effects levels) by the European Scientific Committee on Food 8 or the European Food Safety Authority Panel on Food Additive and Nutrient Sources added to food 9 because the studies did not investigate all the relevant toxicological endpoints (e.g. some aspects of reproductive toxicity). Shao and Hathcock 10 performed observed safe level (OSL) risk assessments for taurine based on the available published human clinical trial data. Since the evidence for the absence of adverse effects is strong for taurine at supplemental intakes up to 3 g/day, this level was identified as the observed safe level (OSL) for normal healthy adults. Although much higher levels of taurine have been tested without adverse effects and may be safe, the data for intakes above these levels are not sufficient to draw any confident conclusions about long-term safety. It was noted by the European Food Safety Authority Panel on Food Additive and Nutrient Sources added to food 9 that the results of a large number of studies in adults, children and infants indicated that daily ingestion of taurine doses of up to 6 g/person per day for periods up to one year (including supplements) did not produce adverse health effects. On the basis of this information, an observed safe level (OSL) of 6 g/person per day (equal to 100 mg/kg body weight per day for a 60-kg person) can be identified. This value is taken as a reference for comparison with population exposure. The possibility of an increased susceptibility during pregnancy and/or nursing was not taken into consideration in the observed safe level. Exposure resulting from the consumption of foodstuffs and energy drinks together would amount to about one-third of the observed safe level (OSL) of 6 g/person per day.
Note: Observed safe level (OSL) is a model based on observations of exposed humans, in which the highest intake with convincing evidence of safety, even if there are no established adverse effects at any level‘ is taken without applying additional safety factors 11.
What does taurine do
Taurine is one of the most abundant amino acids in the brain, retina, muscle tissue, and organs throughout the body 12. Taurine serves a wide variety of cellular functions including bile salt synthesis and cholesterol metabolism, antioxidation, osmoregulation and modulation of neuronal excitability in the central nervous system from development to cytoprotection 13 and taurine deficiency is associated with cardiomyopathy, renal dysfunction, developmental abnormalities, and severe damage to retinal neurons 12. All ocular tissues contain taurine, and quantitative analysis of ocular tissue extracts of the rat eye revealed that taurine was the most abundant amino acid in the retina, vitreous, lens, cornea, iris, and ciliary body. In the retina, taurine is critical for photoreceptor development and acts as a cytoprotectant against stress-related neuronal damage and other pathological conditions 12. Despite its many functional properties, however, the cellular and biochemical mechanisms mediating the actions of taurine are not fully known.
Table 1. Mechanisms underlying cytoprotective actions of taurine to improve clinical and nutritional health of humans
| Cytoprotection | Functions of Taurine |
|---|---|
| Antioxidation | Anti-inflammation by neutralization of hypochlorous to produce taurine chloramine |
| Diminishes superoxide by conjugating with uridine of tRNALeu(UUR) in mitochondria | |
| Generates ATP by encoding mitochondrial ND6 protein. Prevents mitochondrial membrane permeability and apoptosis. | |
| Benefits mitochondrial disease, MELAS by providing substrate for taurine conjugation. | |
| Energy metabolism | Activates complex I and NADH sensitive enzymes by reducing NADH/NAD+ ratio during glycolysis. |
| Restores fatty acid oxidation by increasing PPARalpha levels. | |
| Conjugates bile acids to facilitate lipid absorption by intestines. | |
| Gene expression | Changes transcription profile of metabolism-related genes. |
| Modulates genes to induce longevity. | |
| Changes transcription factors. | |
| Modulates protein phosphorylation and cell signalling. | |
| ER stress | Attenuates ER stress by improving protein folding. |
| Ameliorates stroke brain injury by inhibiting ER stress. | |
| Protects neurons in stroke and Alzheimer’s disease. | |
| Neuromodulation | Protects CNS by agonizing GABAA, glycine and NMDA receptors. |
| Decreases seizures by binding with GABAA receptor. | |
| Protects against seizures by elevating glutamic acid decarboxylase. | |
| Quality control | Protects cardiomyocytes by activating ubiquitin-proteasome system and autophagy. |
| Attenuates toxin-mediated autophagy. | |
| Ca2+ homeostasis | Protects heart and brain during myocardial infarction and stroke by diminishing Ca2+ overload. |
| Taurine loss during ischemia-reperfusion protects heart by reducing hypoxia-induced Ca2+ overload. | |
| Taurine depletion leads to cardiomyopathy due to reduced activity of SR Ca2+ ATPase. | |
| Protects brain neurons during epilepsy by inducing Ca2+ binding proteins. | |
| Protects neurons against glutamate excitotoxicity by reducing glutamate-induced elevation of [Ca2+]i | |
| Osmoregulation | Serves as an organic osmolyte. |
Antioxidant activity
Recent studies have uncovered novel mechanisms responsible for taurine-mediated cytoprotection (Table 1). One of the primary mechanisms of taurine cytoprotection appears to involve its antioxidant activity, which is mediated by three distinct events. First, taurine is a proven anti-inflammatory agent that neutralizes the neutrophil oxidant, hypochlorous acid. The product of the reaction between taurine and hypochlorous acid, taurine chloramine, also interferes with the inflammatory process 14. Second, taurine diminishes the generation of superoxide by the mitochondria 15. In normal mitochondria, taurine forms a conjugate with a uridine residue of tRNALeu(UUR). Because the modified uridine residue is located in the Wobble position of the anticodon, the conjugation reaction is capable of enhancing the interaction of the AAU anticodon of tRNALeu(UUR) with the UUG codon of mitochondrial mRNAs. However, in certain mitochondrial diseases, the formation of the taurine conjugate is diminished, an effect that suppresses the expression of specific mitochondria encoded proteins, such as NADH-ubiquinone oxidoreductase chain 6 (ND6) 15. ND6 is a subunit of complex I that is required for maximal complex I activity and the proper assembly of the complex. Therefore, a reduction in ND6 biosynthesis decreases complex I activity, the utilization of NADH by the respiratory chain and mitochondrial ATP generation but it increases the generation of superoxide by the respiratory chain16. It is widely accepted that mitochondrial oxidative stress damages macromolecules within the mitochondria, but more importantly it is capable of triggering the mitochondrial permeability transition (permeabilization of the inner mitochondrial membrane) and mitochondria-dependent apoptosis 16. This sequence of events can be disrupted by taurine treatment. In the mitochondrial disease, mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS), the formation of the taurine conjugate is impaired. Taurine therapy provides a source of substrate for the taurine conjugation reaction, thereby restoring mitochondrial protein biosynthesis, improving mitochondrial function and reducing superoxide generation 17. In support of this theory, it has been shown that promoters of mitochondrial oxidative stress, including ozone, nitrogen dioxide, bleomycin, amiodarone, arsenic, iron, Adriamycin and catecholamines, to name a few, respond favorably to taurine therapy, 18. Third, reactive oxygen species (ROS) generated by the mitochondria can damage antioxidant enzymes that are capable of preventing oxidative stress. Because the activity of some of the antioxidant enzymes is sensitive to oxidative damage, taurine may limit oxidative stress by preventing damage to those sensitive enzymes.
Effect on energy metabolism
Taurine deficiency-mediated impairment of complex I activity also affects energy metabolism, largely through elevations in the NADH/NAD+ ratio, which regulate energy metabolism by feedback inhibiting key dehydrogenases. The citric acid cycle is very sensitive to increases in the NADH/NAD+ ratio, as three NADH sensitive enzymes (α-ketoglutarate dehydrogenase, isocitrate dehydrogenase and citrate synthase) are subject to inhibition by elevations in the NADH/NAD+ ratio. For example, oxidation of pyruvate by the taurine deficient heart falls, as elevations in the NADH/NAD+ ratio inhibits pyruvate dehydrogenase activity and causes a deficiency in pyruvate, arising from the massive conversion of pyruvate to lactate 19. Thus, despite stimulation of glycolysis, glucose oxidation is significantly reduced in the taurine deficient heart, dramatically decreasing the contribution of glucose metabolism toward overall ATP biosynthesis. The rate of taurine biosynthesis by the liver is low in humans, therefore, the major source of taurine in humans is the diet. For many years, diets rich in seafood were considered an excellent source of taurine, with meat containing some but less taurine than seafood. However, the recent introduction of taurine-containing supplements, such as Bacchus-D and Red Bull, provides an alternative source of taurine. The supplements have proven to be effective therapeutic agents, at least in the case of heart failure. According to Jeejeebhoy et al. 20, taurine is deficient in hearts of patients suffering from heart failure. Restoration of taurine levels in these patients through supplementation leads to improved contractile function. This study reinforces the view that taurine supplements are important therapeutic agents. However, the largest decline in energy metabolism occurs in fatty acid oxidation, which falls in part because of a decrease in citric acid cycle flux. Also suppressing fatty acid oxidation in taurine deficiency are the low levels of the transcription factor, PPARα 19. PPARα regulates several proteins and enzymes involved in fatty acid metabolism, with the most important being the long chain fatty acyl carnitine transporter complex 19. Another factor affecting lipid metabolism is taurine deficiency-mediated reductions in bile acid biosynthesis, as bile acids facilitate the absorption of lipids by the intestines (see section on atherosclerosis).
Regulation of gene expression
Park et al. 21 were the first investigators to recognize that taurine treatment triggers genetic changes. More recently, Ito et al. 22 have identified several taurine sensitive genes that contribute to a wide range of cellular functions (cell cycle progression, cell signaling, death and survival, amino acid metabolism, protein biosynthesis, protein folding and aging). Taurine-mediated changes in transcription factor content have also been reported 19. Although taurine is known to modulate protein phosphorylation and cell signaling 23, it remains to be determined if alterations in protein phosphorylation are involved in taurine-mediated genetic changes.
Modulation of ER stress
Another important mechanism of taurine cytoprotection is attenuation of endoplasmic reticular (ER) stress. ER stress is an important regulatory mechanism designed to restore ER function and re-establish a balance between protein degradation and protein biosynthesis/folding. When a cell experiences excessive ER stress, pathways are stimulated that can kill the cell. A common initiator of ER stress is the accumulation of defective proteins, whose levels increase as a result of improper protein folding, inadequate protein degradation or ER dysfunction. To restore ER function and the balance between protein degradation and protein biosynthesis/folding, unfolded or misfolded proteins activate three stress sensors (PERK, ATF6 and IRE1) that initiate distinct pathways known as the unfolded protein response (UPR) pathways. Together, the UPR pathways are capable of suppressing protein biosynthesis, enhancing protein degradation, generating chaperones to improve protein folding and initiating either autophagy or apoptosis. During a stroke, taurine decreases glutamate toxicity, thereby reducing both oxidative stress and calcium overload. However, taurine also suppresses two of the three UPR pathways. Although the mechanisms underlying the actions of taurine against ER stress and the UPR pathways remain to be determined, it is relevant that taurine deficiency is associated with ER stress 24. It has been proposed that taurine might alter protein folding, either by reducing oxidative stress or providing a better osmotic environment for protein folding 24. The initial studies describing the effect of taurine on ER stress used cellular and animal models of stroke 25. According to the authors of those studies, ER stress, along with oxidative stress and mitochondrial dysfunction, are characteristic features of stroke and neurodegenerative diseases, including Alzheimer’s, Huntington’s and Parkinson’s diseases 26. During stroke, massive amounts of the neurotransmitter, glutamate, are released which overstimulates postsynaptic neurons leading to a neuroexcitotoxic response, characterized by oxidative stress, calcium overload, ER stress and in some cases cell death 26.
Taurine as an inhibitory neuromodulator
Although ER stress assumes an important role in the cytoprotective actions of taurine in the central nervous system (CNS), another important mechanism affecting the CNS is the neuromodulatory activity of taurine. Toxicity in the CNS commonly occurs when an imbalance develops between excitatory and inhibitory neurotransmitters. GABA is one of the dominant inhibitory neurotransmitters, therefore, reductions in either the CNS levels of GABA or the activity of the GABA receptors can favor neuronal hyperexcitability. Taurine serves as a weak agonist of the GABAA, glycine and NMDA receptors 27. Therefore, taurine can partially substitute for GABA by causing inhibition of neuronal excitability. However, the regulation of the GABAA receptor by taurine is complex. While acute taurine administration activates the GABAA receptor, chronic taurine feeding promotes the downregulation of the GABAA receptor 28 and the upregulation of glutamate decarboxylase, the rate-limiting step in GABA biosynthesis 29. Therefore, complex interactions within the GABAeric system, as well as in the glycine and NMDA receptors, largely define the actions of taurine in the CNS.
Regulation of quality control processes
Taurine also regulates quality control processes, such as the ubiquitin-proteasome system and autophagy. These processes either rejuvenate damaged cells and subcellular organelles or eliminate them through degradation or cell death. In taurine deficient cells, a reduction in the activity of the proteasome leads to an accumulation of ubiquitinated proteins, an effect abolished by the mitochondrial specific antioxidant, mitoTEMPO 30. Taurine deficiency is also associated with diminished autophagy, a condition that allows damaged cells and organelles to accumulate 30. Inactivation of these quality control processes is extremely damaging to cells and tissue. However, excessive autophagy is also damaging because it can elevate cell death. Although there have only been a few studies examining the effect of taurine treatment on autophagy, the actions of taurine are compatible with its cytoprotective activity, as taurine attenuates toxin-mediated autophagy 31.
Modulation of Ca2+ homeostasis
Excessive accumulation of Ca2+ by the heart and brain during a myocardial infarction or stroke, is also cytotoxic. Not only does high [Ca2+]i activate proteases and lipases, but it also initiates the mitochondrial permeability transition, an event that permeabilizes the inner mitochondrial membrane and provokes the release of pro-apoptotic factors from the mitochondria that kill the cell 16. Taurine protects the cell by diminishing Ca2+ overload through three mechanisms 26. First, the loss of taurine from cells during an ischemia-reperfusion insult appears to be mediated by the taurine transporter, as taurine loss is also accompanied by the loss of Na+ from the cell. Consequently, upon taurine release, less Na+ is available for Ca2+ entry via the Na+/Ca2+ exchanger, which minimizes the degree of Ca2+ overload 32. Second, taurine indirectly regulates the activity of the sarcoplasmic reticular Ca2+ ATPase, which is responsible for maintaining cytosolic Ca2+ homeostasis through the removal of Ca2+ from the cytosol 23. This action of taurine involves alterations of protein phosphorylation, however, the mechanism by which taurine modulates protein phosphorylation has not been ascertained and warrants further study. Third, taurine treatment is associated with changes in the presence of calbindin D28k, calretinin and parvalbumin 33. Fourth, taurine inhibits glutamateinduced Ca2+ influx through the L-, P/Q- and N-type voltage gated Ca2+ channels, as well as the NMDA receptor channel 34.
Osmoregulation
The concentration of taurine within most cells is quite high. In response to elevations in an osmotic load, intracellular taurine levels increase while they decrease in response to hypo-osmotic stress. These are important mechanisms to protect the cell from excessive stretching in response to osmotic imbalances. Because taurine serves as an organic osmolyte, it also modulates the levels of other osmolytes, such as Na+, which not only carries a charge (unlike taurine which is a neutral zwitterion) but is also involved in many important cellular functions, such as transport and membrane potential 32. In the kidney, taurine serves as a weak diuretic and natriuretic agent, important properties for normal renal function.
Taurine benefits
In some species, such as the cat and fox, taurine is an essential nutrient 35. Not only does taurine deficiency cause pathology in those animals but it also shortens their lifespan 22. In contrast, taurine is classified as a conditionally essential nutrient or a functional nutrient in man 36. Although humans are incapable of synthesizing large amounts of taurine, the retention of taurine by human tissues is greater than that of cats or fox. Thus, unlike cats, humans do not readily develop overt signs of taurine deficiency although parenteral feeding can be associated with taurine deficiency 37. Nonetheless, human studies have revealed the nutritional value of taurine. Particularly noteworthy is a World Health Association (WHO) study involving middle aged subjects in 50 population groups in 25 different countries throughout the world, which reports that elevated dietary taurine consumption is associated with decreased risk of hypertension and hypercholesterolemia 38. Taurine supplementation is also linked to diminished body mass index 39 and reduced levels of inflammation markers in obese women 40. Thus, the cytoprotective actions of taurine contribute to an improvement in the clinical and nutritional health of humans.
Effect of taurine on the central nervous system
Stroke
Stroke, whose incidence is on the rise, is presently a leading cause of death and disability. Under pathological conditions, such as ischemic stroke and hypo-osmotic stress, taurine is released from various cells in the central nervous system (CNS) and function as a neuroprotective agent 41). For many brain areas, concentrations of taurine are below 1 mM, which are adequate to activate glycine receptors but not most GABA receptors; the GABA receptors of the ventrobasal thalamus are an exception, as they exhibit especially high affinity for taurine. Taurine plays an important role in neuronal development in the cerebral cortex 42. Although concentrations of taurine below 1 mM do not activate the GABA receptor in many regions of the CNS, taurine deficiency leads to impaired GABAergic inhibition in the striatum, a condition associated with the development of a disease mimicking hepatic encephalopathy 43.
A major cause of cellular damage and death during stroke is the release of large amounts of glutamate, which overstimulate glutamate receptors, resulting in hyper-excitability 26. Among the damaging events associated with glutamate toxicity are calcium overload, oxidative stress, ATP depletion and mitochondrial dysfunction 26. The combination of calcium excess and oxidative stress is capable of triggering the mitochondrial permeability transition and the release of pro-apoptotic factors that initiate mitochondrial apoptosis. Apoptosis can also be initiated by caspase 12, CHOP (C/EBP homologous protein) and JNK, which are end products of unfolded protein response (UPR) pathways that arise from ER stress. Interestingly, taurine is also released from brain slices in response to glutamate receptor stimulation 44. The release of taurine helps counteract the adverse effects of glutamate toxicity by reducing [Ca2+]i, increasing the Bcl-2/Bad ratio and suppressing ER (endoplasmic reticulum) stress 45. Moreover, taurine reduces cell swelling in rat brain cortical slices subjected to a hypoxic-reoxygenation insult 46. However, it remains to be determined whether taurine-mediated increases in ATP generation and suppression of mitochondrial ROS (reactive oxygen species) generation might also contribute to the reductions in the severity of stroke. There is reason to believe that the mitochondrial actions of taurine should reverse stroke-mediated suppression of complex I activity. Taurine might also attenuate another source of ROS in stroke, namely those from NADPH oxidase 47, as taurine treatment is known to reduce the levels of the substrate, NADPH 19 and mediate the downregulation of Nox2/Nox4 48. Using an animal model of focal cerebral ischemia, Gharibani et al. 25 found that the combination of taurine and a N-methyl-D-aspartate (NMDA) partial antagonist (5-methyl-N,N-diethylthiolcarbamate-sulfoxide) reduced apoptosis and ER stress while the partial antagonist alone exhibited no neuroprotection.
While there is abundant evidence that taurine is effective in treating stroke in animals 49, only a few studies have addressed its effect on the risk of stroke in humans. In a prospective-case study based on the New York University Women’s Health Study, which examined 14,274 women, no association was observed between serum taurine levels and stroke risk 50. However, among non-smokers there may be a link that deserves further consideration, particularly in light of evidence that the incidence of stroke was reduced 90% in a genetic model of stroke (stroke-prone spontaneously hypertensive rats) fed a diet rich in taurine 51. Clearly, clinical studies examining the effectiveness of taurine against stroke in humans is warranted (Figure 1).
Figure 1. Taurine health benefits
Footnote: High concentrations of taurine in most cells regulate physiological function of excitable tissues and mitochondria. Taurine protects CNS (central nervous system = brain + spinal cord) by decreasing ER (endoplasmic reticulum) stress and antagonizing neurotransmitter receptors of GABAA, glycine and N-methyl-D-aspartate (NMDA). Protection of the cardiovascular system by taurine occurs through regulation of cell signaling, such as Ca2+ transport, ROS (reactive oxygen species) generation and protein phosphorylation. Supplementation of taurine ameliorates symptoms of MELAS (mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes) and diabetes mellitus. The anti-inflammatory activity of taurine involves either the formation of taurochloramine in neutrophils or the attenuation of nitric oxide and prostaglandin E2 in inflammatory diseases, such as rheumatoid arthritis and osteoarthritis. Taurine depletion or taurine transporter KO leads to cardiac and skeletal muscle dysfunction. Taurine prevents sarcopenia in aged person by minimizing gradual muscle loss.
Abbreviations: CNS: central nervous system; FXS = fragile X syndrome; SSDD = succinic semialdehyde dehydrogenase deficiency; MELAS = mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes.
[Source 1]Neurodegenerative diseases, such as Alzheimer’s, Huntington’s and Parkinson’s disease
The neurodegenerative diseases share many of the pathological features of stroke caused by glutamate-mediated activation of N-methyl-D-aspartate (NMDA) receptors, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) inotropic glutamate receptors and metabotropic glutamate receptors 52. A major cause of cell death from glutamate-mediated hyperexcitability in neurodegenerative diseases appears to involve the collapse of the mitochondrial membrane potential 53. Activation of glutamate receptors leads to calcium overload and an increase in ROS production. Because mitochondrial function is adversely affected by neurodegeneration, one would expect that mitochondrial ROS generation should be a major cause of cellular damage. Indeed, respiratory chain defects have been detected in degenerative diseases, although the site of the defect is disease specific, with Parkinson’s disease affecting complex I, Huntington’s disease affecting complex II and Alzheimer’s disease affecting complex IV 54. Interestingly, one of the animal models of Parkinson’s disease is generated by treating rodents with the complex I inhibitor, rotenone 55. Taurine deficiency and rotenone actions are similar, as both lead to reductions in complex I activity, inhibition of NADH dehydrogenase activity, reductions in respiratory activity and elevations in NADH. Because a primary physiological function of taurine is the maintenance of complex I activity, there is reason to believe that taurine therapy should reduce the severity of Parkinson’s disease 56. Indeed, it has recently been reported that reduced plasma taurine content is associated with motor severity in Parkinson’s disease 57. Moreover, a single blind, randomized, controlled study of 47 patients with Parkinson’s disease revealed effectiveness in reducing excessive sleepiness upon treatment with the taurine analog, homotaurine 58.
Fragile X Syndrome and succinic semialdehyde dehydrogenase deficiency
Fragile X syndrome is a genetic disease characterized by behavioral disorders and moderate to severe intellectual disabilities. The learning deficit (memory retention) of the fragile X mouse responds favorably to chronic oral administration of taurine (0.05% w/v) for 4 weeks, an effect seemingly linked to taurine’s GABAergic activity 59.
Succinic semialdehyde dehydrogenase (SSADH) deficiency is a rare autosomal genetic disease involving a key enzyme in GABA catabolism. Patients with SSADH deficiency showed symptoms such as ataxia, hypotonia, language deficits, and intellectual disability. Nearly half of the SSADH patients suffer from seizures. Because the disease is associated with disruption of GABA homeostasis, the effect of taurine therapy on symptoms of the disease before and after treatment have been examined. In a single case study of a 2-year old boy, taurine therapy (200 mg/kg/day) for 12 months improved social behavior, coordination and activity 60. However, in a subsequent open-label study of 18 SSADH deficient subjects administered taurine (50-200 mg/kg/day) for a period up to one year there was no major improvement in adaptive behavior with taurine 61. Clearly, a controlled, randomized double-blind study of a larger number of patients receiving either placebo or taurine therapy is warranted.
Epilepsy
Imbalances between excitatory and inhibitory neurotransmitters underlie the mechanism of seizures. Taurine is an abundant amino acid in the brain, where it serves as an inhibitory neuromodulator 62. Although taurine levels can suppress specific types of seizures, taurine deficiency is not required for initiation of seizures. In animal studies, taurine administration has been found to abolish seizures evoked by a wide range of stimulants, including [D-Ala,Met ]-enkphalinamide, opioids, kainite, isoniazid, picrotoxin, penicillin and hypoxia. Nonetheless, clinical trials examining the effect of taurine treatment on human epilepsy have been mixed, with only about 1/3 of the patients responding favorably to taurine therapy 63.
The dominant inhibitory neurotransmitter in the brain is GABA, therefore, regulation of neuroexcitability by GABA plays a prominent role in preventing neuronal hyperexcitability and seizures 28. Taurine serves as an agonist of the GABAA receptor, an action that enhances chloride influx into postsynaptic neurons, which causes hyperpolarization that inhibits hyperexcitability. Suppression of kainic acid, isoniazid and picrotoxin-mediated seizures has been attributed to the actions of taurine on the GABAeric system 28. Like GABA, glycine is a major inhibitory neurotransmitter that activates chloride conductance and hyperpolarizes neurons. The inhibitory neuromodulator activity of taurine also extends to the glycine receptor, as the binding of taurine to the glycine receptor evokes chloride current and suppresses neuronal firing 64.
Retinal degeneration
The landmark studies showing that taurine is an essential nutrient for cats focused on the link between taurine deficiency and the development of photoreceptor loss and retinal degeneration 65. More recently, Hadj-Said et al. 66 found that taurine deficiency also causes nuclear ganglion cell degeneration and loss. Moreover, the anti-epileptic agent, vigabatrin, can provoke retinal degeneration, including both photoreceptor and ganglion cell loss, accompanied by taurine deficiency 67. In two patients with succinic semialdehyde dehydrogenase deficiency exhibiting abnormal ERGs at baseline and after 6 months of vigabatrin treatment, taurine therapy partially prevents retinal damage triggered by vigabatrin treatment 68. Because taurine is required for normal retinal ganglion cell survival 69, it has been proposed that taurine therapy may serve an important role in the prevention of retinal degeneration 67.
Effect of taurine on the cardiovascular system
Congestive heart failure
Taurine has been approved for the treatment of congestive heart failure in Japan 70. Like other heart failure medication, taurine not only diminishes the common symptoms of congestive heart failure (breathlessness on exertion and edema) but also eliminates or decreases the need for administering other heart failure medication, such as digoxin 70. Although taurine exerts a mild positive inotropic effect on the hypodynamic heart and promotes natriuresis and diuresis, the major therapeutic effect of chronic taurine administration appears to involve a reduction in the actions of norepinephrine and angiotensin II, which are known to decrease myocardial performance through elevations in afterload pressure, ventricular remodeling and fluid remodeling 71. Taurine is effective in reducing the adverse actions of norepinephrine through its ability to both decrease catecholamine overflow (through alterations in Ca2+ transport) and diminish cell signaling (through changes in Ca2+ transport, ROS content and protein phosphorylation). Although recent studies have shown that taurine therapy improves exercise capacity of patients with heart failure 72, it remains to be determined whether taurine supplementation also reduces the risk of developing overt heart failure in the general population. Moreover, the possibility that taurine supplementation might lower the mortality rate of heart failure patients has not been examined. There is reason to believe that taurine might prolong lifespan of heart failure patients because it elevates high energy phosphate content of the heart, which is an important determinant of mortality among patients suffering from congestive heart failure 19.
Hypertension
Supplementation with taurine prevents the development of hypertension in several animal models 73. In those models, taurine-mediated reductions in blood pressure appear to be mediated by a combination of diminished [Ca2+]i, oxidative stress, sympathetic activity and inflammatory activity, as well as an improvement in renal function 74.
Two recent clinical studies 74, 75 support the view that taurine therapy reduces blood pressure in hypertensive subjects. Katakawa attributed the beneficial effects of taurine therapy against hypertension in humans to improved endothelial function secondary to a decline in oxidative stress. By comparison, Sun et al. 75 focused on the vasodilatory effects of pre-hypertensive patients receiving taurine therapy. The most important feature of the study reported by Sun et al. 75 was its size and design, as it is a single-center, double-blind, randomized, placebo-controlled trial of 120 pre-hypertensive subjects aged 18–75 with systolic pressure varying from 120–139 mm Hg and diastolic pressure from 80–89 mm Hg. After administration of taurine (1.6 g/day) for 12 weeks, systolic pressure of the pre-hypertensive subjects was reduced 7.2 mm Hg and diastolic pressure fell 4.7 mm Hg while pre-hypertensive subjects treated with placebo exhibited no significant reduction in blood pressure over the same treatment period 75. The taurine effect was greater in pre-hypertensive subjects with higher blood pressure than with the subjects with lower blood pressure at the time of initial taurine administration. The administration of taurine resulted in a 1.5-fold increase in plasma taurine concentration, an effect correlated with the improvement in blood pressure. This observation was consistent with an earlier epidemiological study by Yamori et al. 76, who found that individuals with elevated amounts of taurine intake exhibit lower blood pressure values than individuals consuming less taurine. Moreover, Ogawa et al. 77 had previously observed that plasma taurine content is reduced in essential hypertension. The human studies are consistent with animal studies showing that taurine deficiency accelerates the onset of hypertension in rats lacking a kidney and maintained on a high salt diet 78. Moreover, a negative correlation has been detected between plasma taurine content and blood pressure in spontaneously hypertensive rats 79. In their clinical study, Sun et al. 75 attributed the taurine-mediated reduction in blood pressure to improved flow- and nitroglycerin-mediated dilation, a change not observed in the placebo treated group. In addition to elevating plasma taurine content, the taurine treated group exhibited elevated H2S content, the latter that promotes hypotension by inhibiting transient receptor potential channel 3 (TRPC3)-induced signaling in the vasculature. Further studies are warranted to compare the importance of H2S content on blood pressure control relative to the other common regulators of vascular function, such as Ca2+, neurohumoral factors and nitric oxide.
Atherosclerosis
Atherosclerosis is the primary cause of pathology in stroke, myocardial infarction and peripheral artery disease. The process of atherosclerosis is complex, involving multiple factors and steps 80. One of the key steps in the initiation of atherosclerosis is the uptake of the cholesterol-enriched lipoprotein, LDL, by the intima of the arterial wall. Also recruited in the arterial wall are monocytes, which normally exhibit little adhesion to endothelial cells and are not readily accumulated by smooth muscle. However, upon exposure to inflammatory factors and chemoattractants (chemokines), monocytes begin to adhere to endothelial cells, where they are taken up by the intima of the arterial wall. Upon exposure to factors, such as macrophage-colony factor, most of the monocytes in the early atheromata are differentiated into macrophages. Within the intima, LDL can undergo oxidation and glycation, which individually enhance the uptake of the lipoprotein by macrophages. While the uptake and handling of LDL by macrophages is considered an early event in the formation of foam cells, it is now apparent that there are multiple pathways involved in foam cell formation and atherosclerosis 80.
Taurine treatment diminishes atherogenesis through several possible mechanisms. First, in most, but not all studies, taurine supplementation accelerates the regression in serum cholesterol levels in atherogenic animals 81. During the regression period, hepatic cholesterol levels fall more rapidly in taurine treated animals, largely because of an increase in 7α-hydroxylase activity, which accelerates the degradation of cholesterol. Because there is a correlation between lower serum cholesterol and the dose of taurine used during treatment, the upregulation of the CYP7A1 gene in the liver is thought to regulate serum cholesterol levels 81. At the same time, taurine treatment is associated with diminished activity of 3-hydroxy-3-methylglutaryl CoA reductase, the rate-limiting step of cholesterol biosynthesis 82. Second, exposure of hepatic cells for 24 hrs with medium containing taurine leads to a decline in the biosynthesis of cholesterol esters and triglycerides. Because the hepatic content of triglycerides and cholesterol esters is a determinant of lipoprotein assembly in the endoplasmic reticulum of the liver, taurine specifically decreases the assembly and secretion of lipoproteins containing the structural protein, apolipoprotein B100 81. Apolipoprotein B100 is the primary structural protein of both LDL and its precursor, VLDL. Third, taurine protects endothelial cells of vascular tissue from glucose-induced and oxidized LDL-induced toxicity, which is an early step in the development of atherosclerosis 83. It has also been suggested that taurine protects endothelial cells from homocysteine-induced ER stress and apoptosis by reducing hyperhomocysteinemia 84. While oxidative stress is a primary adverse response to hyperglycemia and homocysteinemia, oxidized LDL-induced toxicity has been attributed to the accumulation of asymmetric dimethylarginine, an inhibitor of nitric oxide synthase 85. Fourth, taurine suppresses platelet-derived growth factor-BB (PDGF-BB) induced vascular smooth muscle cell proliferation, which plays an important role in atherosclerosis 86. Taurine appears to alter the activity of a phosphatase that dephosphorylates the PDGF-β receptor, which is a potent chemoattractant and proliferative factor for vascular smooth muscle cells. However, taurine does not suppress phorbol ester-induced activation of the Raf/MEK/ERK pathway of vascular smooth muscle proliferation, leading to the suggestion that taurine’s actions are specific for the PDGF-β pathway. Nonetheless, the field remains controversial. According to Terashima et al. 87, taurine suppresses mesenchymal cell proliferation by inhibiting ERK activity and immediate early gene expression. Clearly, studies clarifying the effect of taurine on smooth muscle cell proliferation are warranted. Fifth, taurine diminishes the expression of LOX-1, which mediates the uptake of oxidized LDL by endothelial cells and decreases the rate of stenosis in oxidatively stressed rabbits subjected to balloon injury of the iliac artery 88. The authors also recognized the important role of taurine-mediated attenuation of oxidative stress in their findings. Finally, the possibility that taurine-mediated inhibition of atherosclerosis may involve its anti-inflammatory activity deserves consideration.
The epidemiological WHO-CARDIAC study showed that dietary taurine intake is correlated with reduced mortality of ischemic heart disease patients 89. In support of that study, Elvevoll et al. 90 found that taurine enhances the beneficial effects of n-3 fatty acid supplementation on total cholesterol, LDL cholesterol and triglycerides. Recently, Katakawa et al. 74 attributed the diminished risk of atherogenesis in humans maintained on a diet supplemented with taurine and magnesium to reductions in oxidative stress and improvement in endothelial function. These studies have revealed the importance of taurine supplementation in human health.
Ischemia-reperfusion injury
Several actions of taurine (antioxidant, modulation of [Ca2+]i. osmoregulation, protein phosphorylation and high energy phosphate regulation) alter the outcome of an ischemia-reperfusion insult. A discussion of the differing effects of these factors in various models of ischemia-reperfusion injury and of the importance of taurine treatment and loss during an ischemia-reperfusion insult, appeared in a recent review 17. Because of variable effects, it is unlikely that taurine would be adopted as an acute cardioprotective agent to diminish infarct size and minimize injury during a myocardial infarction. Rather, taurine’s use may be restricted to cardiac transplantation and bypass surgery. Several investigators have reported a benefit of taurine as a component of cardioplegic solutions 91 or of loading hearts with taurine prior to their use as donor hearts 92. Besides reducing oxidative stress and swelling, taurine loss during an ischemia-reperfusion insult leads to a decrease in [Na+]i, which not only diminishes osmotic stress but also Ca2+ overload 93. In addition, rapid intravenous infusion of taurine before bypass surgery protects against oxidative stress and cellular necrosis 94.
Myocardial arrhythmias
One of the earliest reported cardiovascular actions of taurine is its antiarrhythmic actions against a broad range of pro-arrhythmic agents (digoxin or related cardiac glycoside, epinephrine, ouabain, CsCl, hypokalemia) 95. This effect is likely related to the modulation of [K+]i, [Na+]i and [Ca2+]i. According to a clinical report, oral administration of taurine and L-arginine dramatically reduced cardiac arrhythmias of three subjects. Thus, under appropriate conditions, taurine is a very effective antiarrhythmic agent 96. Nonetheless, taurine is presently not used in the treatment of cardiac arrhythmias.
Role of taurine in metabolic diseases
Mitochondrial disease – MELAS
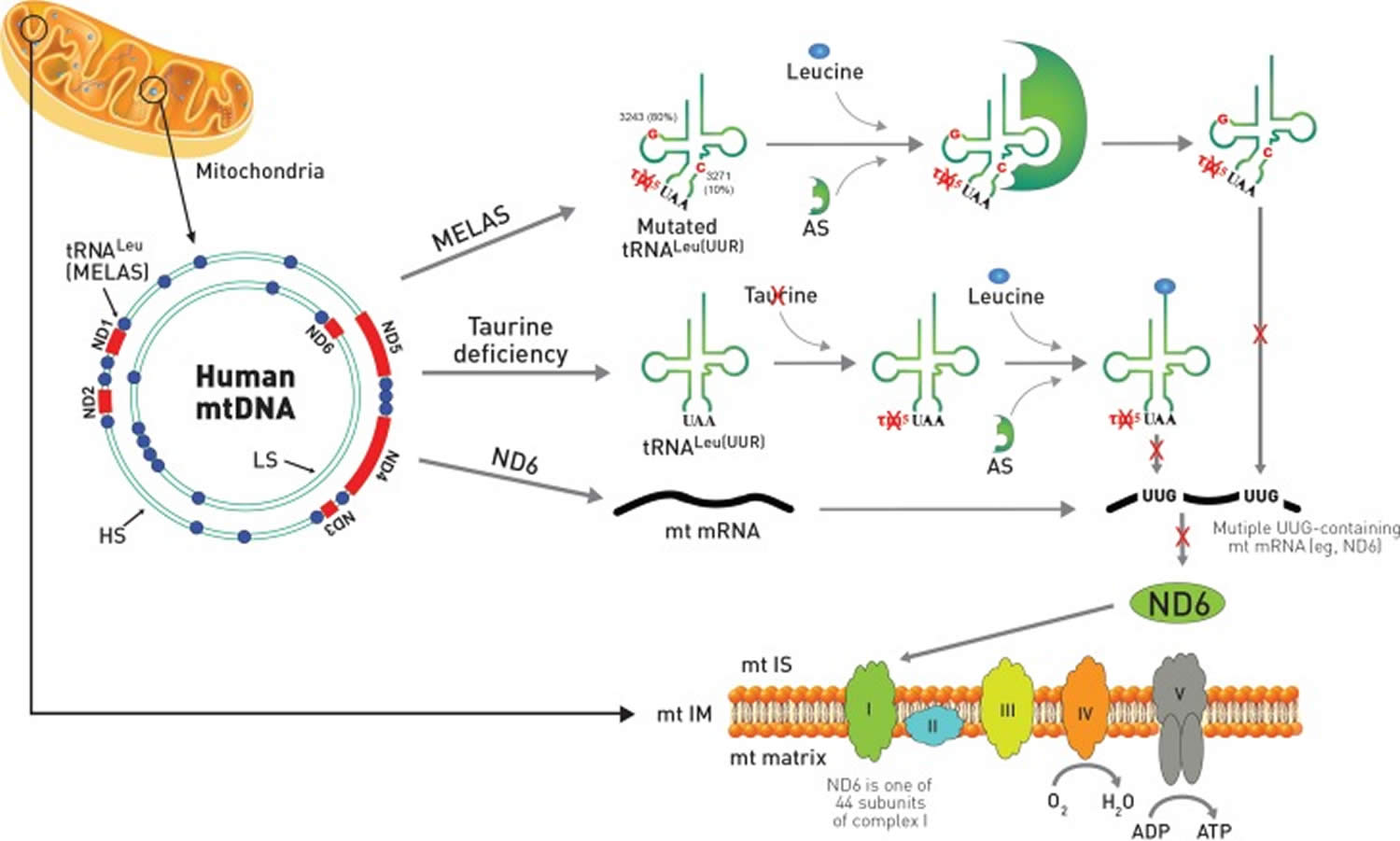
A remarkable similarity exists between the symptoms of taurine deficiency and that of the mitochondrial disease, MELAS 97. Indeed, the characteristic symptoms of MELAS (myopathy, encephalopathy, lactic acidosis and stroke-like episodes) are also present in taurine deficiency. This is not surprising, as the pathophysiology of the two conditions is similar. MELAS is caused by specific point mutations in the region of DNA that codes for tRNALeu(UUR) 15. The mutations appear to alter the structure of the tRNA, preventing the conjugation of taurine with the uridine base of tRNALeu(UUR) 98. Modification of the uridine base alters the interaction of the UUG codon with the AAU anticodon of tRNALeu(UUR), thereby altering UUG decoding 99. Taurine deficiency also appears to reduce the formation of the taurine conjugate, 5-taurinomethyluridine-tRNALeu(UUR), but the effect is related to a reduction in mitochondrial taurine content 15. Taurine deficiency reduces the expression of UUG-dependent proteins, with one of the most UUG-dependent mitochondria encoded protein being ND6, a subunit of complex I. Because ND6 plays a prominent role in the assembly of complex I, taurine-mediated reductions in ND6 levels lead to several features seen in MELAS, including lactic acidosis, reduced complex I activity and diminished oxygen consumption. Impaired respiratory chain function causes elevations in superoxide generation and reduced ATP generation, effects that likely play a central role in the development of the myopathy and encephalopathy of MELAS (Figure 2). Seizures, which are another symptom of MELAS, could depend on the inhibitory neuromodulatory activity of taurine although the mitochondrial activity of taurine might also be a determinant of seizures.
Figure 2. Mitochondrial disease and taurine deficiency
Footnote: Comparison of MELAS and taurine deficiency in mitochondria. The mitochondrial disease, MELAS, is caused by specific point mutations in mitochondrial DNA (mtDNA) that codes for tRNALeu(UUR). Most of the point mutations of MELAS with 80% frequency occur at A3243G while mutations at T3271C exist with 10% frequency. In mtDNA, ND genes are shown in red color and tRNA genes are depicted as blue circles. The gene of tRNALeu(UUR) responsible for MELAS is located adjacent to ND1. The mutation in MELAS alters the structure of the tRNALeu(UUR) preventing the conjugation of taurine with the uridine base of the UAA anti-codon from forming 5-taurinomethyluridine (τm5U). MELAS patients also show reduced aminoacylation of taurine deficient tRNALeu(UUR) by leucine catalyzed by aminoacyl-tRNA synthetase (AS). Both reduced aminoacylation of tRNALeu(UUR) by leucine and formation of the taurine conjugate of τm5UAA-tRNALeu(UUR) prevent decoding of mitochondrial UUG-dependent proteins, including ND6, which is one of 44 protein subunits of complex I of the electron transport chain located in the mitochondria inner membrane. On the other hand, taurine deficiency has normal aminoacylation of tRNALeu(UUR) by leucine, but exhibits reduced formation of the taurine conjugate of τm5UAA-tRNALeu(UUR), which also prevents decoding of mitochondrial ND6 mRNA, resulting in increased superoxide generation and reduced ATP generation.
Abbreviations: MELAS = mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes; ND6 = NADH-ubiquinone oxidoreductase chain 6; mt IS = motochodrial intermembrane space; mtIM = mitochondrial inner membrane; LS = light strand; HS = heavy strand.
[Source 1]Because of the direct link between taurine content and the development of MELAS, it is not surprising that patients with MELAS respond favorably to taurine therapy 100. In one such case study, laboratory tests revealed that a 29-year-old woman presented at the hospital with stroke-like episodes had a MELAS-like A3243G point mutation in a left biceps brachii muscle biopsy. Anticonvulsants, both phenytoin and valproate, were administered over the next 7 months but failed to halt the epileptic and stroke-like episodes. Shortly after beginning oral taurine supplementation, which increased blood taurine content about 5-fold, the epileptic and stroke-like episodes completely ceased. Also corrected by taurine therapy was lactic acidosis. In a related case study, a 21-year-old male was admitted to a hospital with right homonymous hemianopsia. He was diagnosed with A3243G-linked MELAS and lactic acidosis. Anticonvulsion therapy was used over the next several years, but the patient still experienced several stroke-like episodes, including sensory aphasia and visual impairment. After taurine supplementation, which elevated blood levels approximately 10-fold, the stroke-like episodes ceased. The same group examined the effect of taurine treatment on cytoplasmic hybrids (cybrids) harboring a 3243G-MELAS mutation; incubation of the cybrids for 4 days with medium containing 40 mM taurine partially normalized oxygen consumption and mitochondrial membrane potential, which were suppressed in the A3243G containing cybrids. Taurine treatment also attenuated the degree of oxidative stress in cybrids harboring the 3243 mutant. Thus, taurine therapy not only abolishes stroke-like episodes in MELAS patients but also restores normal mitochondrial respiratory function. These data reveal promise for the use of taurine as a therapeutic agent against MELAS.
Diabetes mellitus
Diabetes mellitus is a disease characterized by elevated blood glucose and either a decrease in plasma insulin (type 1 diabetes) or resistance to the actions of insulin (type 2 diabetes). Type 1 diabetes is an autoimmune disease that is caused by T-cell-mediated destruction of pancreatic β-cells that dramatically reduces insulin biosynthesis. Thus, the disease is characterized by hypoinsulinemia and hyperglycemia. By contrast, type 2 diabetes is a progressive disorder characterized initially by insulin resistance, in which the actions of insulin at target organs are reduced, although in later stages of the disease insulin secretion is also impaired. Diabetes is associated with multiple complications, including cardiovascular disease, neuropathy, nephropathy, retinopathy, foot ulcers, skin lesions and hearing impairment. Although restrictions in the degree of hyperglycemia are central to the control of these complications, several downstream factors can contribute to the severity of the complications. One of the most important of these factors is ROS. According to seminal studies by Brownlee 101, it was proposed that glucose oxidation, which involves pyruvate metabolism by the mitochondria, produces ROS, which in turn enhances flux through several damaging pathways (advanced glycosylation end product generation, protein kinase C activation, polyol formation and hexosamine pathway stimulation) involved in the development of diabetic complications.
Two important concepts arose from the work of Brownlee 101. First, his unifying hypothesis provided a logical link between glucose-mediated oxidative damage and several pathways implicated in diabetic complications. Moreover, it introduced the insightful idea that mitochondria-derived ROS play an important role in the development of certain diabetic complications. However, it did not take into consideration the effect of diabetes-mediated oxidative stress on mitochondrial function itself 102.
The major source of ROS in most diabetic tissues is complexes I and III of the respiratory chain. Interestingly, ROS generated by complex I remain within the matrix of the mitochondria while complex III-derived ROS are distributed between the matrix and extra-mitochondrial locations. In the matrix, ROS provokes damage, as evidenced by decreased activity of the oxidant-sensitive enzyme, aconitase, increased mitochondrial DNA oxidation and if severe enough, death via apoptosis 103. Although complex III-mediated ROS generation can affect targets outside of the mitochondria, the intra-mitochondrial effects appear most important in the development of diabetic complications. In leptin deficient, obese, type 2 diabetic db/db mice, elevations in mitochondrial ROS production of the heart lead to reduced ATP production, as respiratory function and energy metabolism are impaired, which in turn diminishes myocardial performance 104. Respiration and ATP generation are also defective in diabetic renal mesangial and tubular cells. Because ATP is required for reabsorption in the proximal tubules while energy deficiency leads to renal failure, hyperglycemia-mediated mitochondrial damage and ATP deficiency appear to play a prominent roles in the development of the diabetic nephropathy 105.
Mitochondrial DNA damage, which has been detected in humans suffering from diabetes melllitus 106, invariably alters the expression of mitochondria encoded proteins, leading to diminished respiratory chain activity. As a result, more ROS is produced, which triggers further mitochondrial DNA damage, ultimately leading to a vicious cycle of damage. Evidence of severe mitochondrial structural changes and dysfunction (morphology, biogenesis, respiratory chain function, fatty acid and citric acid cycle metabolism, oxidative stress, apoptosis and uncoupling activity) support a role of mitochondrial damage in the development of diabetic complications 105.
Plasma and platelet taurine levels are reduced in subjects with type 1 diabetes 107. Based on 711 overweight, diabetic subjects, plasma taurine levels are related to the decline in insulin sensitivity 108. In fact, most of the major targets of diabetes (kidney, retina and neurons) undergo hyperglycemic-mediated reductions in taurine content 18. Nonetheless, at an early age, the metabolic pattern of subjects with diabetes is very different from that of the taurine deficient mouse 19. The initial defect in type 1 diabetes is insulinopenia, which reduces glucose metabolism and elevates fatty acid metabolism, while taurine deficient mice exhibit impaired respiratory chain function, increased glycolysis and decreased glucose and fatty acid oxidation 19. Nonetheless, taurine is required for normal β-cell viability, therefore, the number of pancreatic β-cells is reduced in 12-month-old TauTKO mice 109. Moreover, the properties of the diabetic animal are altered with age, as mitochondrial oxidative injury leads to impaired respiratory chain function in the late phases of the disease. Also affecting mitochondrial function and biogenesis is diet and physical activity 110. Therefore, upon aging and dietary modification, the diabetic and taurine phenotypes adopt similar features. In this regard it is interesting that Han et al. 111 claim that taurine deficiency is a necessary requirement for the development of an appropriate model of diabetic nephropathy.
There is overwhelming evidence that taurine therapy reduces pathology associated with diabetes, obesity and the metabolic syndrome 112. In many animal studies, particularly of type II diabetes, taurine treatment diminishes the degree of hypoglycemia, an effect that in turn attenuates diabetic complications 113. Several mechanisms may contribute to the regulation of hyperglycemia in diabetic animals treated with taurine. First, taurine improves respiratory function and increases ATP production, effects that should improve pancreatic β-cell function and insulin secretion 19. Second, hyperglycemia and lipidemia are associated with elevations in mitochondrial ROS generation. In pancreatic β-cells, fatty acid-mediated ROS generation appears to decrease insulin secretion, an effect attenuated by taurine treatment 114. Third, mitochondrial dysfunction can provoke insulin resistance 115. Haber et al. 116 found that taurine treatment prevents hyperglycemia-induced insulin resistance and oxidative stress. Together, these findings indicate that taurine protects against type 2 diabetes-mediated complications, but the mechanism by which taurine diminishes the development of the complications of type 2 diabetes remain unclear, largely because it is virtually impossible to separate the mitochondrial actions of taurine from its effects on insulin secretion and action.
On the other hand, in the streptozotocin-induced model of type 1 diabetes, plasma glucose levels remain unaltered by taurine treatment while the severity of the diabetic complications are diminished. Because diabetic status in the streptozotocin model of type 1 diabetes is unaffected by taurine, one can readily establish the mechanism underlying taurine’s effectiveness against the development of diabetic complications. According to several investigators, taurine-mediated reductions in the severity of type 2 diabetic complications are more closely linked to improvements in cellular stresses (ER, oxidative and inflammatory) and mitochondrial dysfunction 117. Trachtman et al. 118 were the first group to recognize the benefit of taurine treatment against the development of diabetic complications. They reported that male rats administered streptozotocin developed a diabetic nephropathy characterized by elevated glomerular filtration rate, glomerular hypertrophy and proteinuria and albuminuria. Administration of taurine (1% in the drinking water) reduced proteinuria by 50% and dramatically suppress glomerular hypertrophy and tubointerstitial fibrosis without affecting blood glucose. Because the amino acid also abolished the elevation in renal cortical malondialdehyde, a marker of oxidative stress, and of advanced glycoloxidation products, a marker of advanced glycosylation end products, the protective effects of taurine were attributed to suppression of oxidative stress and advanced glycosylation. Recently, the effectiveness of taurine therapy against the development of diabetic nephropathy has been confirmed 113. In a related study, Ikubo et al. 119 found that streptozotocin-treated diabetic rats developed vascular defects that were associated with oxidative stress without a change in blood glucose. Interestingly, taurine also protects against apoptosis in cellular models of glucose toxicity 83.
Obesity is a disorder characterized by insulin resistance, hyperlipidemia, hyperglycemia and inflammatory responses related to enlarged adipocytes. Taurine has been effective in decreasing body weight of obese animals and in suppressing inflammatory responses. These effects and their mechanism are not reviewed here but are covered in a complete review by Murakami 120.
Role of taurine in inflammatory diseases
Arthritis is a term used to describe over 100 diseases, the most common being rheumatoid arthritis and osteoarthritis. The major symptoms of arthritis are joint stiffness and pain with inflammation of the joints, the tissues surrounding the joint and connective tissue. Rheumatoid arthritis is characterized by synovial inflammation and proliferation, bone erosions and thinning of articular cartilage.
The distinctive features of the acute inflammatory phase, which can last up to several days, are vascular dilation, microvascular leakage and leukocyte recruitment, the latter mediated by adhesion factors that facilitate the interaction of leukocytes with activated endothelium. Acute inflammation, which is triggered by damaged or diseased tissue, as well as irritants and pathogens, is an early phase in the repair of damage and the removal of microorganisms and harmful agents by the innate immune system. During acute inflammation, leukocytes adhere to the endothelium prior to transmigrating across the endothelium into the interstitium. Chemotactic factors and cytokines recruit the neutrophils to the site of inflammation. Activation of the leukocytes, in particular neutrophils and mononuclear phagocytes, leads to the secretion of a host of proinflammatory mediators, including microbicidal peptides, cationic microbial proteins, lytic enzymes, ROS and lysosomal granule constituents. When these proinflammatory mediators are released into phagolysosomes, they destroy engulfed microbes and other pathogens. However, when they are released into the extracellular millieu, they can cause tissue damage. In rheumatoid arthritis, activation of the inflammatory process is part of the autoimmune disorder contributing to joint deformation, erosion of bone and disruption of the cartilagebone interface.
The content of taurine in the neutrophil is high, representing about 50% of the total free amino acid pool. The two primary functions of taurine in the neutrophil are anti-inflammatory and antioxidant actions. ROS are produced by the neutrophil as a weapon to kill pathogens, with one of those ROS being hypochlorous acid (HOCl). Myeloperoxidase-catalyzes the formation of taurine chloramine (TauCl) from taurine and HOCl. Because TauCl is a less potent oxidant than HOCl, the neutralization of HOCl represents one of the important antioxidant mechanisms of taurine. The myelperoxidase-catalyzed reaction is also responsible for the anti-inflammatory activity of taurine, as TauCl inhibits the production of proinflammatory cytokines 121, attenuates elevations in nitric oxide and prostaglandin E2 122, decreases the activity of matrix metalloproteinases and initiates leukocyte apoptosis to terminate acute inflammation 123. For a detailed discussion of the anti-inflammatory and anti-arthritic actions of taurine please refer to the extensive review by Marcinkiewicz and Kontny 14.
Effect of taurine on muscle
Modulation of muscle contraction
Taurine deficiency leads to impaired contractile function of both cardiac and skeletal muscle 124. Hamilton et al. 125 found that exposure to the taurine transport inhibitor, guanidinoethanesulfonate, led to a 60% reduction in extensor digitorum longus taurine content, which was associated with a decrease in peak force contraction. More severe muscular dysfunction is seen in taurine transporter knockout mice, whose muscle taurine content is reduced by over 90%, which leads to a decline in muscle mass and muscular dysfunction 126. Also associated with severe taurine deficiency are histological changes, including disruption of the myofibrils.
There are abundant reports that taurine administration enhances exercise performance of both humans 127 and animals 128. Besides improving contractile function of rodents, taurine administration was found to increase the time until exhaustion, reduce exercise-induced fatigue and diminish damage from intense exercise. When taurine is administered prior to heavy exercise, the levels of pro-inflammatory factors are reduced and exercising muscle is protected 129. Also noteworthy is a trial of 36 male subjects that were administered either a branched-chain amino acid supplement, a taurine supplement, placebo or the combination of taurine and branched-chain amino acid supplements 130. The least muscle damage after eccentric exercise was observed in the combination group, as muscle soreness was diminished two days after exercise and there was less lactate dehydrogenase and aldolase released into the blood. Interestingly, serum levels of 8-hydroxydeoxyguanosine, a measure of DNA oxidative damage, were also reduced in the combination group, implicating the antioxidant activity of taurine in the beneficial effects of the combination supplement. The authors suggested that taurine potentiates the beneficial effect of the branched-chain amino acid supplement. The likely candidates for the protective activity of taurine are suppression of oxidative stress and inflammation. In another study by the same laboratory, the end point of the study was arterial stiffness after eccentric exercise 127. Serum malondialdehyde remained elevated for 4 days after exercise in the control placebo group while oxidative stress was decreased in the taurine treated group. There was a parallel between the increase in malondialdehyde and arterial stiffness, which led the authors to suggest that taurine-mediated reductions in oxidative stress helped attenuate the degree of arterial stiffness. For a more complete review of the role of taurine in muscle function and disorders, please refer to DeLuca et al. 131.
Sarcopenia
Sarcopenia, which is the gradual loss of skeletal muscle tissue related to an imbalance between protein biosynthesis and degradation. Because sarcopenia occurs with aging and leads to physical impairment among the elderly it is a serious health problem. Interestingly, taurine deficiency is associated with a reduction in cell size 132. In a recent review, Scicchitano and Sica 133 raised the possibility that taurine might counteract the adverse effects of sarcopenia. This is an interesting condition that could extend the utilization of taurine to another application.
Duchenne muscular dystrophy
Encouraging results have been reported using taurine therapy for the treatment of mdx mice, a model for Duchenne muscular dystrophy, which is a fatal muscle wasting disease characterized by oxidative stress and inflammation 134. In humans suffering from the disease, there are no symptoms at birth, however, symptoms in the form of waddling gait and difficulty climbing steps, begin to appear at a young age. The primary cause of the disease in humans are mutations in dystrophin, a cytoskeletal protein that connects the cytoskeleton and the extracellular matrix, while in the mdx mouse model of Duchenne muscular dystrophy the mutations are replaced by inadequate expression of dystrophin.
In the disease, structural changes within the sarcolemma increase the permeability of the cell membrane and facilitate the accumulation of Ca2+, contributing to the development of Ca2+ overload in which the rate of [Ca2+]i rise after depolarization is enhanced, peak [Ca2+]i is increased and the relaxation phase is prolonged 135. Because the protease inhibitor, leupeptin, abolishes the exaggerated increase in [Ca2+]i, it has been proposed that proteases contribute to the severity of the disorder. Another factor that contributes to the development of the disorder is a lack of nNOS, which normally produces nitric oxide to ensure adequate vasodilation. Because nitric oxide levels fall in an oxidatively stressed environment, it has been proposed that oxidative stress and acute inflammation may also contribute to pathology of the disease.
Taurine has a potential to disrupt several of the steps in the development of Duchenne’s muscular dystrophy, in part by restoring taurine levels that decline in the muscle disorder 134. ln fact, addition of 4% taurine to mouse chow of mdx mice after 14 days of age increases muscle taurine content, an effect correlated with reductions in inflammation (neutrophil infiltration) and mitigation of severe bouts of myocyte necrosis after exercise 136. According to DeLuca et al. 137 taurine treatment also significantly enhances forelimb strength of exercising dmx mice. This effect of taurine is largely attributed to an improvement in Ca2+ homeostasis, although taurine supplementation has no effect on the expression of key E-C coupling proteins 138. Creatine treatment is nearly as effective as taurine in enhancing muscle strength, indicating that delivery of more energy to the muscle prevents the decline in muscle function. In this regard, the effect of taurine on improvement in high-energy phosphate metabolism and respiratory chain function may also contribute to its beneficial actions 19.
Myotonic dystrophy
Myotonia is a condition characterized by delayed relaxation following skeletal muscle contraction. Conte-Camerino et al. 139 found that acute administration of taurine was effective in attenuating myotonic discharges by 20,25 diazacholesterol-treated rats but not by rats treated with anthracene-9-carboxylic acid. The positive effect of taurine against the 20,25 diazacholesterol model of myotonia was attributed to an improvement in flux through voltage-gated chloride channels of extensor digitorum longus myofibers. It has been suggested that the actions of anthracene-9-carboxylate preclude an effect of taurine. Taurine requires functional chloride channels, but anthracene-9-carboxylate inhibits those channels.
Several clinical trials examining the effect of chronic taurine therapy on the severity of myotonia have been evaluated. In a double-blind, single crossover study of 9 patients with dystrophia myotonica, chronic taurine therapy diminished the severity of myotonia while decreasing potassium-induced hyperexcitability 140. The possibility that taurine might also be effective against sodium channel myotonia and paramyotonia congenita has also been proposed, as taurine modulates sodium transients of the Nav1.4 channel 131.
Taurine also increases the sensitivity of myofibrils to Ca2+ 141, an effect also seen in the TauTKO heart, where it has been attributed to enhanced phosphorylation of troponin 23. It is also possible that taurine affects muscular activity of the sarcoplasmic reticular Ca2+ pump, an effect in the heart caused by alterations in the phosphorylation state of the regulator protein, phospholamban 23.
Where does taurine come from?
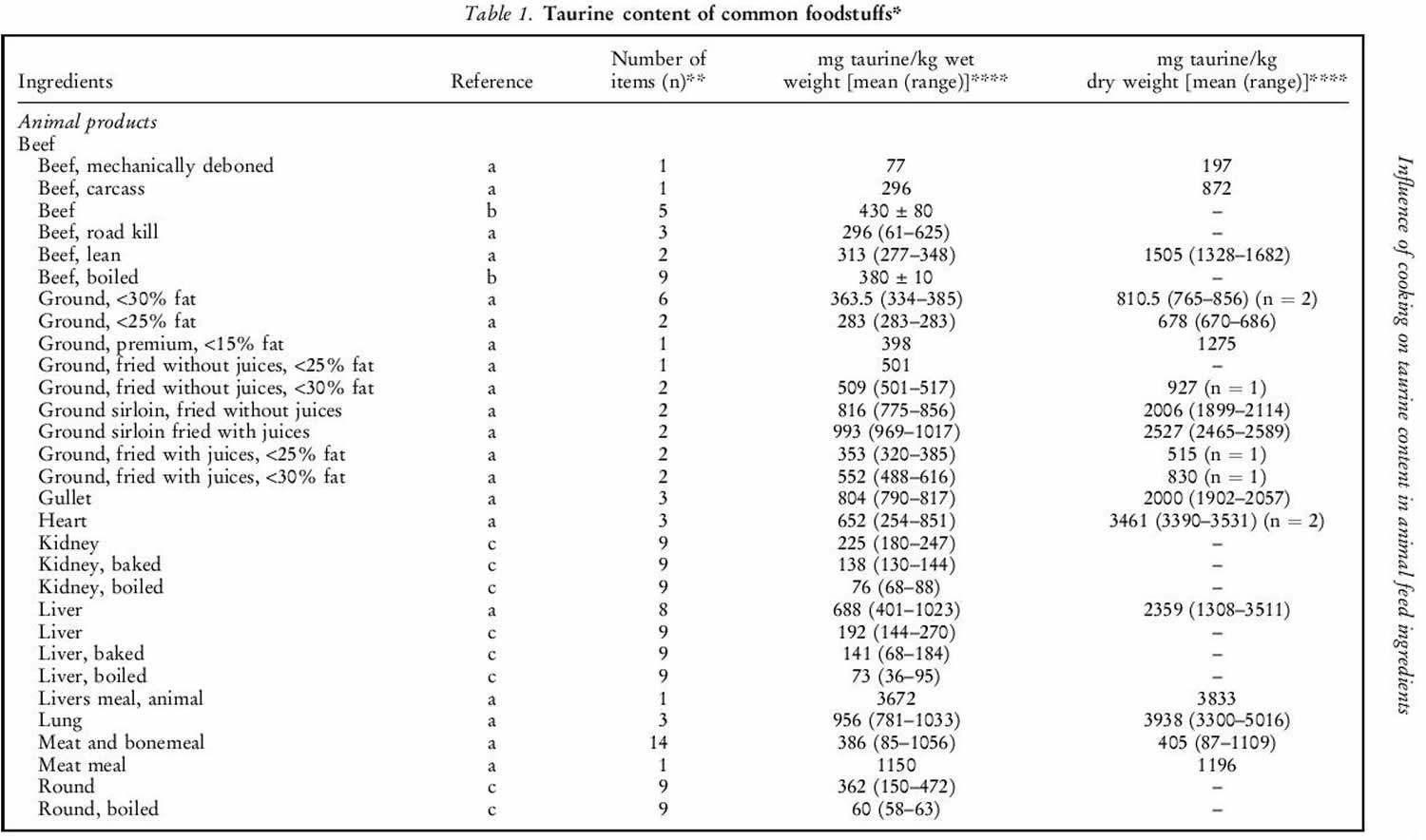
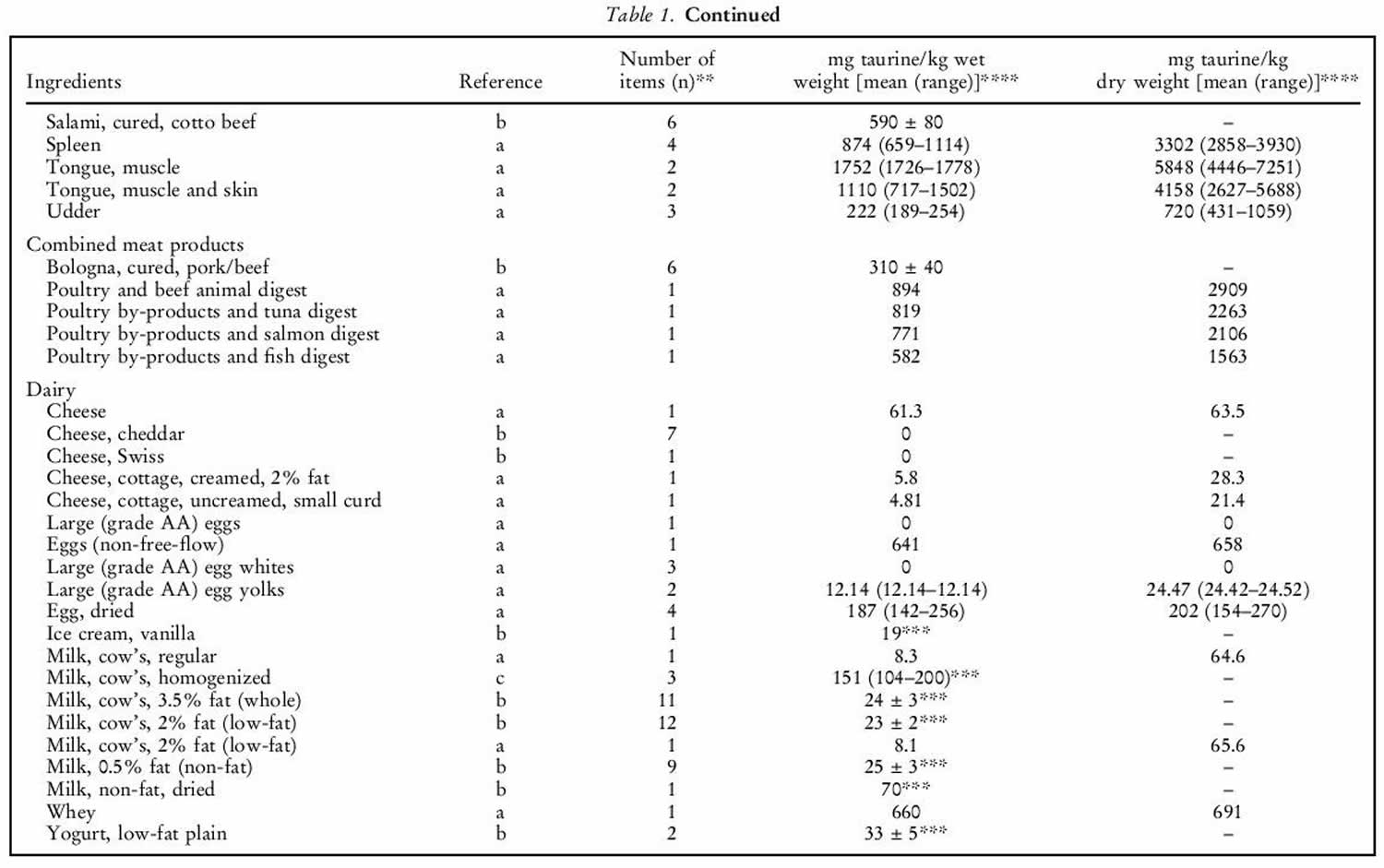
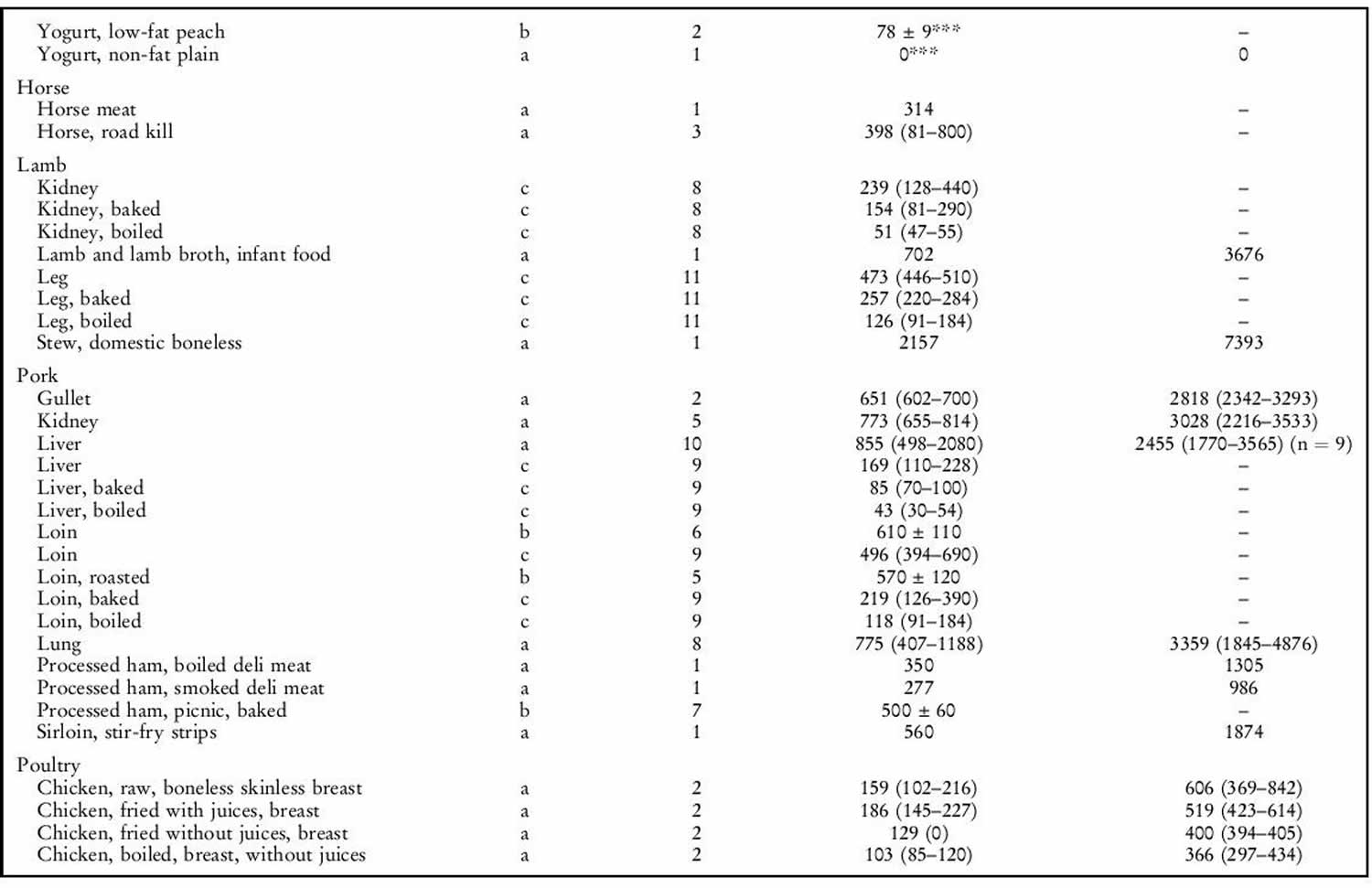
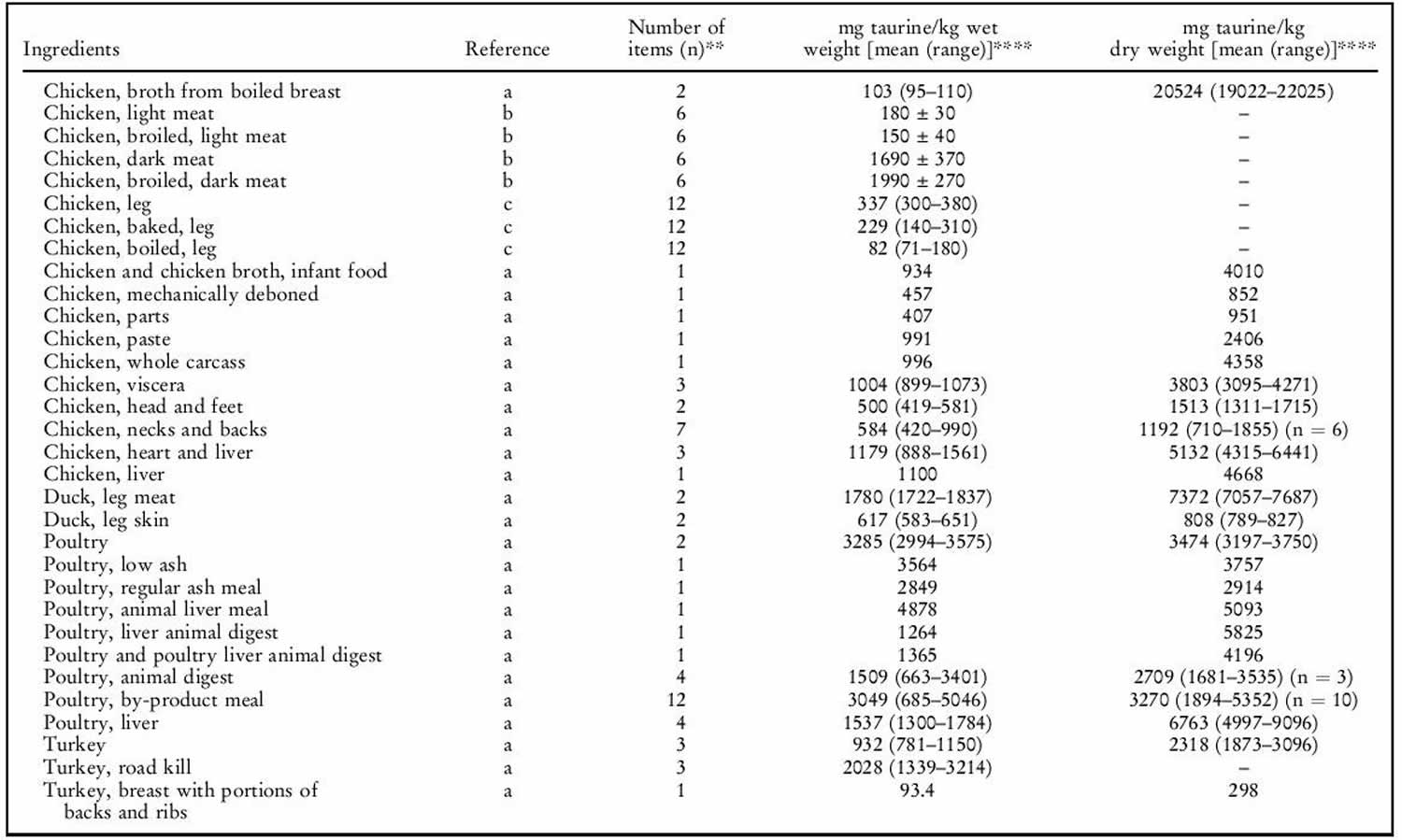
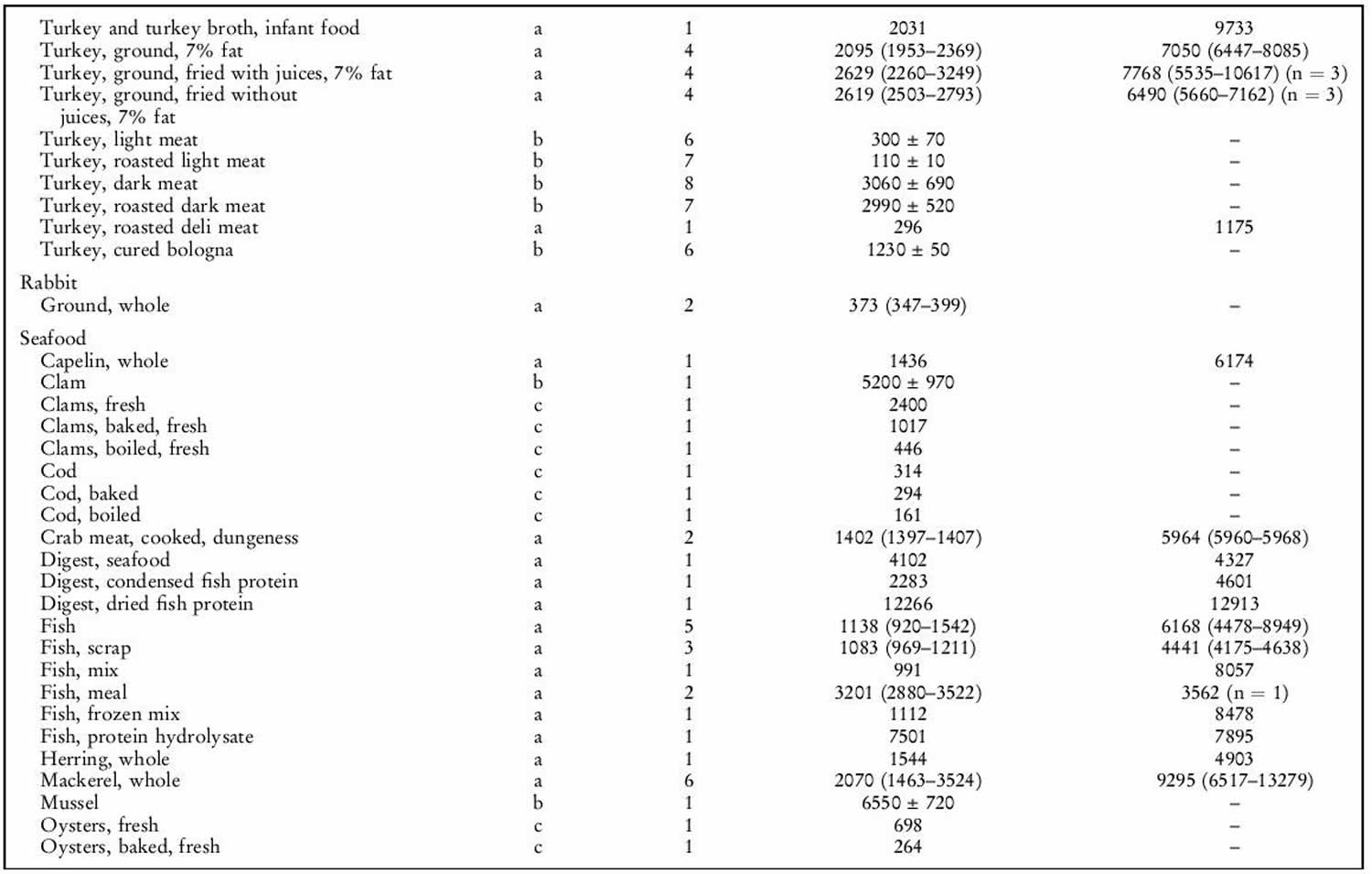
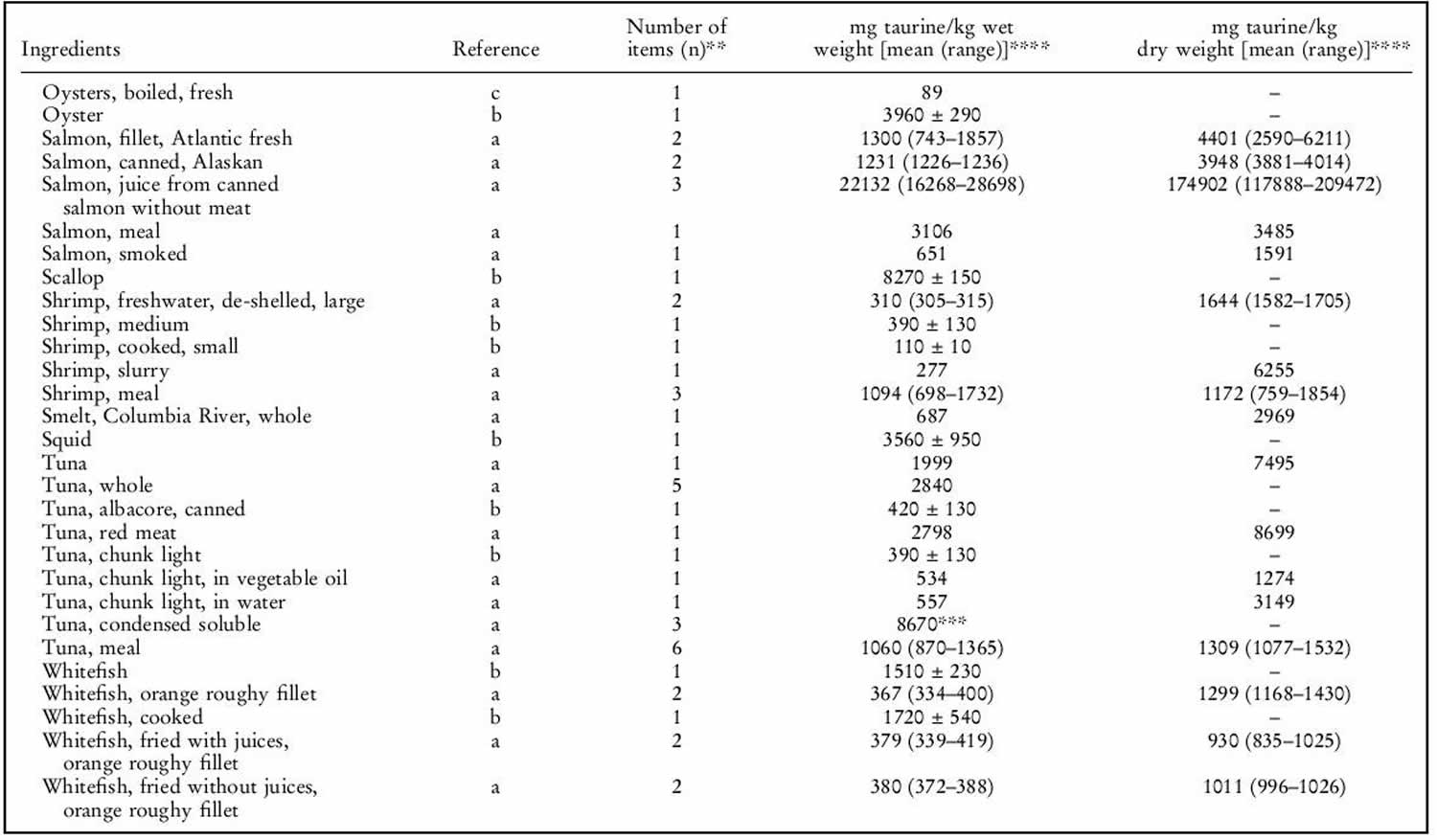
Human exposure to taurine is unavoidable as it is a natural constituent of the body and is present in foods of animal origin. Spitze et al. 142 determined the taurine content of a variety of foodstuffs. Animal muscle tissue, particularly fish flesh, contained high concentrations of taurine. Plant products contained either low or undetectable amounts of taurine. An important property of taurine is its high water solubility. Most of the taurine contained in tissues will be dissolved into water if exposed 142. Therefore, how a diet is
prepared will affect the taurine that is retained in the food ingredient for consumption by the animal. As shown in table 1 below, there was a trend of increasing taurine loss by method of preparation in the order of raw (no preparation at all), frying with juices retained, frying without juices retained, baking or boiling. Cooking in water, such as in boiling or basting, resulted in the greatest taurine loss because the food was surrounded by water, thereby leeching the taurine from the product. Unless the water is included in the meal, you’re consuming less taurine than predicted. Whereas food preparation methods that minimized water loss, such as baking or frying, resulted in higher rates of taurine retention.
The mean daily exposure to taurine from omnivore diets has been estimated to range from 9–40 (lowest range values) to up to 200–400 mg/person per day (top range values) 143. Taurine intake was negligibly low in subjects following a strict vegetarian diet 144. The highest, as well as the most recent, of the top range estimates is 400 mg/person per day 143, equal to 6.7 mg/kg body weight per day for a 60-kg person. This value is 150 times lower than the NOAEL (no observed adverse effects level) of 1 000 mg/kg body weight per day in laboratory animals and 15 times lower than the human observed safe level (100 mg/kg body weight per day) 9. Consumers of energy drinks can ingest quite high levels of taurine (for example, the 50th and 95th percentiles are 8.3 and 23.3 mg/kg body weight, respectively 9. Thus, regular consumption of energy drinks would lead to a taurine intake (mean 500 mg/person per day, 95th percentile 1 400 mg/person per day) higher than the upper range of daily intake from omnivore diets (eats both animals and plants) 9. The results of study carried out in laboratory rodents 145 suggest that dietary taurine does not affect taurine levels in tissues except liver.
Taurine sources
Generally, seafood products were found to contain the highest concentrations of taurine. High concentrations of taurine in seafood have also been previously reported 146. Poultry products, especially turkey, also contained high concentrations of taurine. All plant products tested in our laboratory contained undetectable levels of taurine. This is in agreement with the results of Laidlaw et al. 146. It was also not unexpected that all fruits, grains, legumes, nuts, seeds and vegetables tested contained no detectable taurine. The only exception was the high-protein infant cereal. Although only considered a conditionally essential amino acid in pre-mature newborns, taurine is now added to many human infant and toddler formulas as a measure of prudence to provide improved nourishment.
Although taurine is most commonly concentrated in animal muscle tissue, small amounts were detected in certain dairy products such as milk, yogurt, cheese, ice cream and eggs 146. As the nutrients in milk and eggs are provided primarily for the well-being of the infant or embryo, the presence of taurine was not unexpected.
It was interesting to note that taurine was found in certain plants. Seaweeds contained higher amounts of taurine than land plants, which had undetectable levels. Plants within the same evolutionary division had much greater differences in taurine content than expected. The taurine content of yeast, a fungus, differed greatly compared with other fungus samples analysed. The physiological significance of taurine in these particular plants is unknown, as it does not appear to play the same role in animal tissues. Likewise, it is not known why taurine occurs in lower plant divisions while it is absent in upper plant divisions 147.
Table 1. Taurine food sources
[Source 148]- Schaffer S, Kim HW. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomolecules & Therapeutics. 2018;26(3):225-241. doi:10.4062/biomolther.2017.251. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5933890/[↩][↩][↩][↩][↩][↩]
- http://www.hmdb.ca/metabolites/HMDB0000251[↩]
- Taurine in development. Sturman JA. Physiol Rev. 1993 Jan; 73(1):119-47. https://www.physiology.org/doi/pdf/10.1152/physrev.1993.73.1.119[↩]
- Perry syndrome. http://omim.org/entry/168605?search=168605&highlight=168605[↩]
- https://ods.od.nih.gov/pubs/conferences/taurine_supplementation.pdf[↩]
- Schaffer SW, Ju Jong C, KC R, Azuma J. Physiological roles of taurine in heart and muscle. Journal of Biomedical Science. 2010;17(Suppl 1):S2. doi:10.1186/1423-0127-17-S1-S2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2994395[↩]
- EC (European Commission), 1999, online Opinion of the Scientific Committee on Food on caffeine, taurine and D-glucurono-γ-lactone as constituents of the so-called ―energy‖ drinks. Expressed on 21 January 1999. https://ec.europa.eu/info/departments/health-and-food-safety_en[↩]
- EC (European Commission), 2003, online. Opinion of the Scientific Committee on Food on additional information on ―energy‖ drinks. Expressed on 5 March 2003. https://ec.europa.eu/info/departments/health-and-food-safety_en[↩]
- EFSA Panel on Food Additive and Nutrient Sources added to food (ANS), 2009a. Scientific opinion on the use of taurine and D-glucurono-γ-lactone as constituents of the so-called ―energy‖ drinks. The EFSA Journal, 935, 1–31.[↩][↩][↩][↩][↩]
- Shao A and Hathcock JN, 2008. Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Regulatory Toxicology and Pharmacology, 50, 367–399.[↩]
- Hathcock JN and Shao A, 2008. Expanded approach to tolerable upper intake guidelines for nutrients and bioactive substances. Journal of Nutrition, 138, 1992S–1995S.[↩]
- Ripps H, Shen W. Review: Taurine: A “very essential” amino acid. Molecular Vision. 2012;18:2673-2686. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3501277/[↩][↩][↩]
- Is taurine a functional nutrient? Bouckenooghe T, Remacle C, Reusens B. Curr Opin Clin Nutr Metab Care. 2006 Nov; 9(6):728-33. https://www.ncbi.nlm.nih.gov/pubmed/17053427/[↩]
- Taurine and inflammatory diseases. Marcinkiewicz J, Kontny E. Amino Acids. 2014 Jan; 46(1):7-20.[↩][↩]
- Role of taurine in the pathologies of MELAS and MERRF. Schaffer SW, Jong CJ, Ito T, Azuma J. Amino Acids. 2014 Jan; 46(1):47-56.[↩][↩][↩][↩]
- Mitochondrial defects associated with β-alanine toxicity: relevance to hyper-beta-alaninemia. Shetewy A, Shimada-Takaura K, Warner D, Jong CJ, Mehdi AB, Alexeyev M, Takahashi K, Schaffer SW. Mol Cell Biochem. 2016 May; 416(1-2):11-22.[↩][↩][↩]
- Effect of taurine on ischemia-reperfusion injury. Schaffer SW, Jong CJ, Ito T, Azuma J. Amino Acids. 2014 Jan; 46(1):21-30.[↩][↩]
- Role of antioxidant activity of taurine in diabetes. Schaffer SW, Azuma J, Mozaffari M. Can J Physiol Pharmacol. 2009 Feb; 87(2):91-9.[↩][↩]
- Impaired energy metabolism of the taurine‑deficient heart. Schaffer SW, Shimada-Takaura K, Jong CJ, Ito T, Takahashi K. Amino Acids. 2016 Feb; 48(2):549-58.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Nutritional supplementation with MyoVive repletes essential cardiac myocyte nutrients and reduces left ventricular size in patients with left ventricular dysfunction. Jeejeebhoy F, Keith M, Freeman M, Barr A, McCall M, Kurian R, Mazer D, Errett L. Am Heart J. 2002 Jun; 143(6):1092-100.[↩]
- Park SH, Lee H, Park KK, Kim HW, Lee DH, Park T. Taurine-induced changes in transcription profiling of metabolism-related genes in human hepatoma cells HepG2. Adv Exp Med Biol. 2006;583:119–128. doi: 10.1007/978-0-387-33504-9_12[↩]
- Tissue depletion of taurine accelerates skeletal muscle senescence and leads to early death in mice. Ito T, Yoshikawa N, Inui T, Miyazaki N, Schaffer SW, Azuma J. PLoS One. 2014; 9(9):e107409.[↩][↩]
- Ramila KC, Jong CJ, Pastukh V, Ito T, Azuma J, Schaffer SW. Role of protein phosphorylation in excitation-contraction coupling in taurine deficient hearts. Am J Physiol. 2015;308:H232–H239[↩][↩][↩][↩]
- Ito T, Miyazaki N, Schaffer S, Azuma J. Potential antiaging role of taurine via proper protein folding: a study from taurine transporter knockout mouse. Adv Exp Med Biol. 2015a;803:481–487. doi: 10.1007/978-3-319-15126-7_38[↩][↩]
- Gharibani P, Modi J, Menzie J, Alexandrescu A, Ma Z, Tao R, Prentice H, Wu JY. Comparison between single and combined post-treatment with S-Methyl-N, N-diethylthiolcarbamate sulfoxide and taurine following transient focal cerebral ischemia in rat brain. Neuroscience. 2015;300:460–473. doi: 10.1016/j.neuroscience.2015.05.042[↩][↩]
- Prentice H, Modi JP, Wu JY. Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxid Med Cell Longev. 2015;2015:964518. doi: 10.1155/2015/964518[↩][↩][↩][↩][↩]
- Chan CY, Sun HS, Shah SM, Agovic MS, Ho I, Friedman E, Banerjee SP. Direct interaction of taurine with the NMDA glutamate receptor subtype via multiple mechanisms. Adv Exp Med Biol. 2013;775:45–52. doi: 10.1007/978-1-4614-6130-2_4[↩]
- L’Amoreaux WJ, Marsillo A, El Idrissi A. Pharmacological characterization of GABAA receptors in taurine-fed mice. J Biomed Sci. 2010;17:S14. doi: 10.1186/1423-0127-17-S1-S14[↩][↩][↩]
- El Idrissi A, L’Amoreaux WJ. Selective resistance of taurine-fed mice to isoniazide-potentiated seizures: in vivo functional test for the activity of glutamic acid decarboxylase. Neuroscience. 2008;156:693–699. doi: 10.1016/j.neuroscience.2008.07.055[↩]
- Jong CJ, Ito T, Schaffer SW. The ubiquitin-proteasome system and autophagy are defective in the taurine-deficient heart. Amino Acids. 2015;47:2609–2622. doi: 10.1007/s00726-015-2053-7[↩][↩]
- Bai J, Yao X, Jiang L, Zhang Q, Guan H, Liu S, Wu W, Qiu T, Gao N, Yang L, Yang G, Sun X. Taurine protects against As2O3-induced autophagy in livers of rat offsprings through PPARY pathway. Sci Rep. 2016;6:27733. doi: 10.1038/srep27733[↩]
- Schaffer SW, Solodushko V, Kakhniashvili D. Beneficial effect of taurine depletion on osmotic sodium and calcium loading during chemical hypoxia. Am J Physiol. 2002;282:C1113–C1120. doi: 10.1152/ajpcell.00485.2001[↩][↩]
- Junyent F, Romero R, De Lemos L, Ultrera J, Camins A, Pallas M, Auladell C. Taurine treatment inhibits CaMKII activity and modulates the presence of calbindin D28k, calretinin and parvalbumin in the brain. J Neurosci Res. 2010;88:136–142. doi: 10.1002/jnr.22192[↩]
- Wu H, Jin Y, Wei J, Jin H, Sha D, Wu JY. Mode of action of taurine as a neuroprotector. Brain Res. 2005;1038:123–131. doi: 10.1016/j.brainres.2005.01.058[↩]
- Review: taurine: a “very essential” amino acid. Ripps H, Shen W. Mol Vis. 2012; 18():2673-86.[↩]
- Is taurine a functional nutrient? Bouckenooghe T, Remacle C, Reusens B. Curr Opin Clin Nutr Metab Care. 2006 Nov; 9(6):728-33.[↩]
- Phase IV prospective clinical study to evaluate the effect of taurine on liver function in postsurgical adult patients requiring parenteral nutrition. Arrieta F, Balsa JA, de la Puerta C, Botella JI, Zamarrón I, Elías E, Del Río JI, Alonso P, Candela A, Blanco-Colio LM, Egido J, Navarro P, Vázquez C. Nutr Clin Pract. 2014 Oct; 29(5):672-80.[↩]
- Taurine in 24-h Urine Samples Is Inversely Related to Cardiovascular Risks of Middle Aged Subjects in 50 Populations of the World. Sagara M, Murakami S, Mizushima S, Liu L, Mori M, Ikeda K, Nara Y, Yamori Y. Adv Exp Med Biol. 2015; 803():623-36. https://www.ncbi.nlm.nih.gov/pubmed/25833532/[↩]
- Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. Yamori Y, Taguchi T, Hamada A, Kunimasa K, Mori H, Mori M. J Biomed Sci. 2010 Aug 24; 17 Suppl 1():S6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2994368/[↩]
- Oxidative stress and inflammation in obesity after taurine supplementation: a double-blind, placebo-controlled study. Rosa FT, Freitas EC, Deminice R, Jordão AA, Marchini JS. Eur J Nutr. 2014 Apr; 53(3):823-30.[↩]
- Taurine interaction with neurotransmitter receptors in the CNS: an update. Albrecht J, Schousboe A. Neurochem Res. 2005 Dec; 30(12):1615-21.[↩]
- Roles of taurine-mediated tonic GABAA receptor activation in the radial migration of neurons in the fetal mouse cerebral cortex. Furukawa T, Yamada J, Akita T, Matsushima Y, Yanagawa Y, Fukuda A. Front Cell Neurosci. 2014; 8():88.[↩]
- GABAA-receptor modification in taurine transporter knockout mice causes striatal disinhibition. Sergeeva OA, Fleischer W, Chepkova AN, Warskulat U, Häussinger D, Siebler M, Haas HL. J Physiol. 2007 Dec 1; 585(Pt 2):539-48.[↩]
- Modulation of taurine release by glutamate receptors and nitric oxide. Oja SS, Saransaari P. Prog Neurobiol. 2000 Nov; 62(4):407-25.[↩]
- Role of taurine in the central nervous system. Wu JY, Prentice H. J Biomed Sci. 2010 Aug 24; 17 Suppl 1():S1.[↩]
- Protection by taurine of rat brain cortical slices against oxygen glucose deprivation- and reoxygenation-induced damage. Ricci L, Valoti M, Sgaragli G, Frosini M. Eur J Pharmacol. 2009 Oct 25; 621(1-3):26-32.[↩]
- Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. Abramov AY, Scorziello A, Duchen MR. J Neurosci. 2007 Jan 31; 27(5):1129-38.[↩]
- Neuroprotection of taurine against reactive oxygen species is associated with inhibiting NADPH oxidases. Han Z, Gao LY, Lin YH, Chang L, Wu HY, Luo CX, Zhu DY. Eur J Pharmacol. 2016 Apr 15; 777():129-35.[↩]
- Neuroprotective Mechanisms of Taurine against Ischemic Stroke. Menzie J, Prentice H, Wu JY. Brain Sci. 2013 Jun 3; 3(2):877-907.[↩]
- Serum Taurine and Stroke Risk in Women: A Prospective, Nested Case-Control Study. Wu F, Koenig KL, Zeleniuch-Jacquotte A, Jonas S, Afanasyeva Y, Wójcik OP, Costa M, Chen Y. PLoS One. 2016; 11(2):e0149348.[↩]
- Taurine as the nutritional factor for the longevity of the Japanese revealed by a world-wide epidemiological survey. Yamori Y, Liu L, Mori M, Sagara M, Murakami S, Nara Y, Mizushima S. Adv Exp Med Biol. 2009; 643():13-25.[↩]
- Cell signaling and neuronal death. Hara MR, Snyder SH. Annu Rev Pharmacol Toxicol. 2007; 47():117-41.[↩]
- Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Abramov AY, Duchen MR. Biochim Biophys Acta. 2008 Jul-Aug; 1777(7-8):953-64.[↩]
- Unraveling the role of metal ions and low catalytic activity of cytochrome C oxidase in Alzheimer’s disease. Alleyne T, Mohan N, Joseph J, Adogwa A. J Mol Neurosci. 2011 Mar; 43(3):284-9.[↩]
- A highly reproducible rotenone model of Parkinson’s disease. Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. Neurobiol Dis. 2009 May; 34(2):279-90.[↩]
- Attenuation of rotenone toxicity in SY5Y cells by taurine and N-acetyl cysteine alone or in combination. Alkholifi FK, Albers DS. Brain Res. 2015 Oct 5; 1622():409-13.[↩]
- Reduced plasma taurine level in Parkinson’s disease: association with motor severity and levodopa treatment. Zhang L, Yuan Y, Tong Q, Jiang S, Xu Q, Ding J, Zhang L, Zhang R, Zhang K. Int J Neurosci. 2016; 126(7):630-6.[↩]
- Homotaurine in Parkinson’s disease. Ricciardi L, De Nigris F, Specchia A, Fasano A. Neurol Sci. 2015 Sep; 36(9):1581-7.[↩]
- Taurine recovers mice emotional learning and memory disruptions associated with fragile x syndrome in context fear and auditory cued-conditioning. Neuwirth LS, Volpe NP, Ng S, Marsillo A, Corwin C, Madan N, Ferraro AM, El Idrissi A. Adv Exp Med Biol. 2015; 803():425-38.[↩]
- Saronwala A, Tournay A, Garguss JJ. Taurine treatment of succinate semialdehyde dehydrogenase (SSADH) deficiency reverses MRI-documented globus lesions and clinical syndrome.. Proc. Am. Coll. Med. Genet., 15th Ann. Clinical Genet Meeting; March 12–16 2008; Phoenix, AZ, USA. 2008[↩]
- Taurine trial in succinic semialdehyde dehydrogenase deficiency and elevated CNS GABA. Pearl PL, Schreiber J, Theodore WH, McCarter R, Barrios ES, Yu J, Wiggs E, He J, Gibson KM. Neurology. 2014 Mar 18; 82(11):940-4.[↩]
- Taurine and epilepsy. Oja SS, Saransaari P. Epilepsy Res. 2013 May; 104(3):187-94.[↩]
- Effects of taurine treatment on epileptic patients. Airaksinen EM, Oja SS, Marnela KM, Leino E, Pääkkönen L. Prog Clin Biol Res. 1980; 39():157-66.[↩]
- Taurine activates glycine and gamma-aminobutyric acid A receptors in rat substantia gelatinosa neurons. Wu J, Kohno T, Georgiev SK, Ikoma M, Ishii H, Petrenko AB, Baba H. Neuroreport. 2008 Feb 12; 19(3):333-7.[↩]
- Retinal degeneration associated with taurine deficiency in the cat. Hayes KC, Carey RE, Schmidt SY. Science. 1975 May 30; 188(4191):949-51.[↩]
- Quantitative and Topographical Analysis of the Losses of Cone Photoreceptors and Retinal Ganglion Cells Under Taurine Depletion. Hadj-Saïd W, Froger N, Ivkovic I, Jiménez-López M, Dubus É, Dégardin-Chicaud J, Simonutti M, Quénol C, Neveux N, Villegas-Pérez MP, Agudo-Barriuso M, Vidal-Sanz M, Sahel JA, Picaud S, García-Ayuso D. Invest Ophthalmol Vis Sci. 2016 Sep 1; 57(11):4692-703.[↩]
- Taurine: the comeback of a neutraceutical in the prevention of retinal degenerations. Froger N, Moutsimilli L, Cadetti L, Jammoul F, Wang QP, Fan Y, Gaucher D, Rosolen SG, Neveux N, Cynober L, Sahel JA, Picaud S. Prog Retin Eye Res. 2014 Jul; 41():44-63.[↩][↩]
- The effect of taurine and β-alanine supplementation on taurine transporter protein and fatigue resistance in skeletal muscle from mdx mice. Horvath DM, Murphy RM, Mollica JP, Hayes A, Goodman CA. Amino Acids. 2016 Nov; 48(11):2635-2645.[↩]
- Taurine provides neuroprotection against retinal ganglion cell degeneration. Froger N, Cadetti L, Lorach H, Martins J, Bemelmans AP, Dubus E, Degardin J, Pain D, Forster V, Chicaud L, Ivkovic I, Simonutti M, Fouquet S, Jammoul F, Léveillard T, Benosman R, Sahel JA, Picaud S. PLoS One. 2012; 7(10):e42017.[↩]
- Usefulness of taurine in chronic congestive heart failure and its prospective application. Azuma J, Sawamura A, Awata N. Jpn Circ J. 1992 Jan; 56(1):95-9.[↩][↩]
- Tissue taurine depletion alters metabolic response to exercise and reduces running capacity in mice. Ito T, Yoshikawa N, Schaffer SW, Azuma J. J Amino Acids. 2014; 2014():964680.[↩]
- Taurine Supplementation Improves Functional Capacity, Myocardial Oxygen Consumption, and Electrical Activity in Heart Failure. Ahmadian M, Dabidi Roshan V, Ashourpore E. J Diet Suppl. 2017 Jul 4; 14(4):422-432.[↩]
- Antihypertensive effect of taurine in rat. Hu J, Xu X, Yang J, Wu G, Sun C, Lv Q. Adv Exp Med Biol. 2009; 643():75-84.[↩]
- Taurine and magnesium supplementation enhances the function of endothelial progenitor cells through antioxidation in healthy men and spontaneously hypertensive rats. Katakawa M, Fukuda N, Tsunemi A, Mori M, Maruyama T, Matsumoto T, Abe M, Yamori Y. Hypertens Res. 2016 Dec; 39(12):848-856.[↩][↩][↩]
- Taurine Supplementation Lowers Blood Pressure and Improves Vascular Function in Prehypertension: Randomized, Double-Blind, Placebo-Controlled Study. Sun Q, Wang B, Li Y, Sun F, Li P, Xia W, Zhou X, Li Q, Wang X, Chen J, Zeng X, Zhao Z, He H, Liu D, Zhu Z. Hypertension. 2016 Mar; 67(3):541-9.[↩][↩][↩][↩][↩]
- Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. Yamori Y, Taguchi T, Hamada A, Kunimasa K, Mori H, Mori M. J Biomed Sci. 2010 Aug 24; 17 Suppl 1():S6[↩]
- Decrease of plasma sulfur amino acids in essential hypertension. Ogawa M, Takahara A, Ishijima M, Tazaki S. Jpn Circ J. 1985 Dec; 49(12):1217-24[↩]
- Accelerated NaCl-induced hypertension in taurine-deficient rat: role of renal function. Mozaffari MS, Patel C, Abdelsayed R, Schaffer SW. Kidney Int. 2006 Jul; 70(2):329-37.[↩]
- Effect of dietary taurine on blood pressure in spontaneously hypertensive rats. Nara Y, Yamori Y, Lovenberg W. Biochem Pharmacol. 1978; 27(23):2689-92.[↩]
- Macrophages in the pathogenesis of atherosclerosis. Moore KJ, Tabas I. Cell. 2011 Apr 29; 145(3):341-55.[↩][↩]
- Prevention of hypercholesterolemia and atherosclerosis in the hyperlipidemia- and atherosclerosis-prone Japanese (LAP) quail by taurine supplementation. Murakami S, Sakurai T, Tomoike H, Sakono M, Nasu T, Fukuda N. Amino Acids. 2010 Jan; 38(1):271-8.[↩][↩][↩]
- Taurine increases bile acid pool size and reduces bile saturation index in the hamster. Bellentani S, Pecorari M, Cordoma P, Marchegiano P, Manenti F, Bosisio E, De Fabiani E, Galli G. J Lipid Res. 1987 Sep; 28(9):1021-7.[↩]
- Protective effects of taurine on endothelial cells impaired by high glucose and oxidized low density lipoproteins. Ulrich-Merzenich G, Zeitler H, Vetter H, Bhonde RR. Eur J Nutr. 2007 Dec; 46(8):431-8.[↩][↩]
- High dietary taurine reduces apoptosis and atherosclerosis in the left main coronary artery: association with reduced CCAAT/enhancer binding protein homologous protein and total plasma homocysteine but not lipidemia. Zulli A, Lau E, Wijaya BP, Jin X, Sutarga K, Schwartz GD, Learmont J, Wookey PJ, Zinellu A, Carru C, Hare DL. Hypertension. 2009 Jun; 53(6):1017-22.[↩]
- Taurine protects against low-density lipoprotein-induced endothelial dysfunction by the DDAH/ADMA pathway. Tan B, Jiang DJ, Huang H, Jia SJ, Jiang JL, Hu CP, Li YJ. Vascul Pharmacol. 2007 May; 46(5):338-45.[↩]
- Taurine suppresses platelet-derived growth factor (PDGF) BB-induced PDGF-beta receptor phosphorylation by protein tyrosine phosphatase-mediated dephosphorylation in vascular smooth muscle cells. Yoshimura H, Nariai Y, Terashima M, Mitani T, Tanigawa Y. Biochim Biophys Acta. 2005 Sep 30; 1745(3):350-60.[↩]
- Suppressive effect of taurine on platelet-derived growth factor (PDGF) BB-induced c-fos and c-jun mRNA expressions through extracellular signal-regulated kinase (ERK) in mesenchymal cell lines. Terashima M, Mitani T, Hosokawa Y, Nariai Y, Imada K, Kageyama E, Tanigawa Y. J Nutr Sci Vitaminol (Tokyo). 2003 Jun; 49(3):187-94[↩]
- Taurine suppresses oxidative stress-potentiated expression of lectin-like oxidized low-density lipoprotein receptor and restenosis in balloon-injured rabbit iliac artery. Gokce G, Ozsarlak-Sozer G, Oran I, Oktay G, Ozkal S, Kerry Z. Clin Exp Pharmacol Physiol. 2011 Dec; 38(12):811-8.[↩]
- Distribution of twenty-four hour urinary taurine excretion and association with ischemic heart disease mortality in 24 populations of 16 countries: results from the WHO-CARDIAC study. Yamori Y, Liu L, Ikeda K, Miura A, Mizushima S, Miki T, Nara Y, WHO-Cardiovascular Disease and Alimentary Comprarison (CARDIAC) Study Group. Hypertens Res. 2001 Jul; 24(4):453-7.[↩]
- Seafood diets: hypolipidemic and antiatherogenic effects of taurine and n-3 fatty acids. Elvevoll EO, Eilertsen KE, Brox J, Dragnes BT, Falkenberg P, Olsen JO, Kirkhus B, Lamglait A, Østerud B. Atherosclerosis. 2008 Oct; 200(2):396-402.[↩]
- Taurine prevents myocardial ischemia/reperfusion-induced oxidative stress and apoptosis in prolonged hypothermic rat heart preservation. Oriyanhan W, Yamazaki K, Miwa S, Takaba K, Ikeda T, Komeda M. Heart Vessels. 2005 Nov; 20(6):278-85.[↩]
- Is there any cardioprotective role of Taurine during cold ischemic period following global myocardial ischemia? Sahin MA, Yucel O, Guler A, Doganci S, Jahollari A, Cingoz F, Arslan S, Gamsizkan M, Yaman H, Demirkilic U. J Cardiothorac Surg. 2011 Mar 18; 6():31.[↩]
- Myocardial taurine, development and vulnerability to ischemia. Modi P, Suleiman MS. Amino Acids. 2004 Feb; 26(1):65-70.[↩]
- Reduction of reperfusion injury with preoperative rapid intravenous infusion of taurine during myocardial revascularization. Milei J, Ferreira R, Llesuy S, Forcada P, Covarrubias J, Boveris A. Am Heart J. 1992 Feb; 123(2):339-45.[↩]
- Taurine and electrical activity of the heart. Chazov EI, Malchikova LS, Lipina NV, Asafov GB, Smirnov VN. Circ Res. 1974 Sep; 35 Suppl 3():11-21.[↩]
- Elimination of cardiac arrhythmias using oral taurine with l-arginine with case histories: Hypothesis for nitric oxide stabilization of the sinus node. Eby G, Halcomb WW. Med Hypotheses. 2006; 67(5):1200-4.[↩]
- Taurine deficiency and MELAS are closely related syndromes. Schaffer SW, Jong CJ, Warner D, Ito T, Azuma J. Adv Exp Med Biol. 2013; 776():153-65.[↩]
- Specific correlation between the wobble modification deficiency in mutant tRNAs and the clinical features of a human mitochondrial disease. Kirino Y, Goto Y, Campos Y, Arenas J, Suzuki T. Proc Natl Acad Sci U S A. 2005 May 17; 102(20):7127-32.[↩]
- Decoding property of C5 uridine modification at the wobble position of tRNA anticodon. Kurata S, Ohtsuki T, Wada T, Kirino Y, Takai K, Saigo K, Watanabe K, Suzuki T. Nucleic Acids Res Suppl. 2003; (3):245-6.[↩]
- Taurine ameliorates impaired the mitochondrial function and prevents stroke-like episodes in patients with MELAS. Rikimaru M, Ohsawa Y, Wolf AM, Nishimaki K, Ichimiya H, Kamimura N, Nishimatsu S, Ohta S, Sunada Y. Intern Med. 2012; 51(24):3351-7.[↩]
- The pathobiology of diabetic complications: a unifying mechanism. Brownlee M. Diabetes. 2005 Jun; 54(6):1615-25.[↩][↩]
- Hyperglycemia induced damage to mitochondrial respiration in renal mesangial and tubular cells: Implications for diabetic nephropathy. Czajka A, Malik AN. Redox Biol. 2016 Dec; 10():100-107.[↩]
- Targeting Mitochondria and Reactive Oxygen Species-Driven Pathogenesis in Diabetic Nephropathy. Lindblom R, Higgins G, Coughlan M, de Haan JB. Rev Diabet Stud. 2015 Spring-Summer; 12(1-2):134-56.[↩]
- Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Diabetes. 2007 Oct; 56(10):2457-66.[↩]
- The Role of Mitochondria in Diabetic Kidney Disease. Hallan S, Sharma K. Curr Diab Rep. 2016 Jul; 16(7):61.[↩][↩]
- Altered circulating mitochondrial DNA and increased inflammation in patients with diabetic retinopathy. Malik AN, Parsade CK, Ajaz S, Crosby-Nwaobi R, Gnudi L, Czajka A, Sivaprasad S. Diabetes Res Clin Pract. 2015 Dec; 110(3):257-65.[↩]
- Plasma and platelet taurine are reduced in subjects with insulin-dependent diabetes mellitus: effects of taurine supplementation. Franconi F, Bennardini F, Mattana A, Miceli M, Ciuti M, Mian M, Gironi A, Anichini R, Seghieri G. Am J Clin Nutr. 1995 May; 61(5):1115-9.[↩]
- Plasma Taurine, Diabetes Genetic Predisposition, and Changes of Insulin Sensitivity in Response to Weight-Loss Diets. Zheng Y, Ceglarek U, Huang T, Wang T, Heianza Y, Ma W, Bray GA, Thiery J, Sacks FM, Qi L. J Clin Endocrinol Metab. 2016 Oct; 101(10):3820-3826.[↩]
- Impact of taurine depletion on glucose control and insulin secretion in mice. Ito T, Yoshikawa N, Ito H, Schaffer SW. J Pharmacol Sci. 2015 Sep; 129(1):59-64.[↩]
- Posttranslational modification of mitochondrial transcription factor A in impaired mitochondria biogenesis: implications in diabetic retinopathy and metabolic memory phenomenon. Santos JM, Mishra M, Kowluru RA. Exp Eye Res. 2014 Apr; 121():168-77.[↩]
- Knockout of the TauT gene predisposes C57BL/6 mice to streptozotocin-induced diabetic nephropathy. Han X, Patters AB, Ito T, Azuma J, Schaffer SW, Chesney RW. PLoS One. 2015; 10(1):e0117718.[↩]
- The beneficial effects of taurine in preventing metabolic syndrome. Chen W, Guo J, Zhang Y, Zhang J. Food Funct. 2016 Apr; 7(4):1849-63.[↩]
- Taurine alleviates the progression of diabetic nephropathy in type 2 diabetic rat model. Koh JH, Lee ES, Hyun M, Kim HM, Choi YJ, Lee EY, Yadav D, Chung CH. Int J Endocrinol. 2014; 2014():397307.[↩][↩]
- Free fatty acid-induced reduction in glucose-stimulated insulin secretion: evidence for a role of oxidative stress in vitro and in vivo. Oprescu AI, Bikopoulos G, Naassan A, Allister EM, Tang C, Park E, Uchino H, Lewis GF, Fantus IG, Rozakis-Adcock M, Wheeler MB, Giacca A. Diabetes. 2007 Dec; 56(12):2927-37.[↩]
- Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Sivitz WI, Yorek MA. Antioxid Redox Signal. 2010 Apr; 12(4):537-77.[↩]
- N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: possible role of oxidative stress. Haber CA, Lam TK, Yu Z, Gupta N, Goh T, Bogdanovic E, Giacca A, Fantus IG. Am J Physiol Endocrinol Metab. 2003 Oct; 285(4):E744-53.[↩]
- Potential role of taurine in the prevention of diabetes and metabolic syndrome. Imae M, Asano T, Murakami S. Amino Acids. 2014 Jan; 46(1):81-8.[↩]
- Taurine ameliorates chronic streptozocin-induced diabetic nephropathy in rats. Trachtman H, Futterweit S, Maesaka J, Ma C, Valderrama E, Fuchs A, Tarectecan AA, Rao PS, Sturman JA, Boles TH. Am J Physiol. 1995 Sep; 269(3 Pt 2):F429-38.[↩]
- Protective effect of taurine on diabetic rat endothelial dysfunction. Ikubo N, Saito M, Tsounapi P, Dimitriadis F, Ohmasa F, Inoue S, Shimizu S, Kinoshita Y, Satoh K. Biomed Res. 2011 Jun; 32(3):187-93.[↩]
- Role of taurine in the pathogenesis of obesity. Murakami S. Mol Nutr Food Res. 2015 Jul; 59(7):1353-63.[↩]
- Taurine chloramine inhibits inducible nitric oxide synthase and TNF-alpha gene expression in activated alveolar macrophages: decreased NF-kappaB activation and IkappaB kinase activity. Barua M, Liu Y, Quinn MR. J Immunol. 2001 Aug 15; 167(4):2275-81.[↩]
- Taurine chloramine differentially inhibits matrix metalloproteinase 1 and 13 synthesis in interleukin-1beta stimulated fibroblast-like synoviocytes. Kim KS, Park EK, Ju SM, Jung HS, Bang JS, Kim C, Lee YA, Hong SJ, Lee SH, Yang HI, Yoo MC. Arthritis Res Ther. 2007; 9(4):R80.[↩]
- Taurine chloramine, an oxidant derived from neutrophils, induces apoptosis in human B lymphoma cells through mitochondrial damage. Klamt F, Shacter E. J Biol Chem. 2005 Jun 3; 280(22):21346-52.[↩]
- Effects of guandinoethane sulfonate on contraction of skeletal muscle. Cuisinier C, Gailly P, Francaux M, Lebacq J. Adv Exp Med Biol. 2000; 483():403-9.[↩]
- The effect of taurine depletion on the contractile properties and fatigue in fast-twitch skeletal muscle of the mouse. Hamilton EJ, Berg HM, Easton CJ, Bakker AJ. Amino Acids. 2006 Oct; 31(3):273-8.[↩]
- Tissue taurine depletion alters metabolic response to exercise and reduces running capacity in mice. Ito T, Yoshikawa N, Schaffer SW, Azuma J. J Amino Acids. 2014; 2014():964680[↩]
- Taurine supplementation attenuates delayed increase in exercise-induced arterial stiffness. Ra SG, Choi Y, Akazawa N, Ohmori H, Maeda S. Appl Physiol Nutr Metab. 2016 Jun; 41(6):618-23.[↩][↩]
- Effects of taurine supplementation following eccentric exercise in young adults. da Silva LA, Tromm CB, Bom KF, Mariano I, Pozzi B, da Rosa GL, Tuon T, da Luz G, Vuolo F, Petronilho F, Cassiano W, De Souza CT, Pinho RA. Appl Physiol Nutr Metab. 2014 Jan; 39(1):101-4.[↩]
- The effects of taurine administration against inflammation in heavily exercised skeletal muscle of rats. Kato T, Okita S, Wang S, Tsunekawa M, Ma N. Adv Exp Med Biol. 2015; 803():773-84.[↩]
- Combined effect of branched-chain amino acids and taurine supplementation on delayed onset muscle soreness and muscle damage in high-intensity eccentric exercise. Ra SG, Miyazaki T, Ishikura K, Nagayama H, Komine S, Nakata Y, Maeda S, Matsuzaki Y, Ohmori H. J Int Soc Sports Nutr. 2013 Nov 6; 10(1):51.[↩]
- Taurine: the appeal of a safe amino acid for skeletal muscle disorders. De Luca A, Pierno S, Camerino DC. J Transl Med. 2015 Jul 25; 13():243.[↩][↩]
- Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. Ito T, Kimura Y, Uozumi Y, Takai M, Muraoka S, Matsuda T, Ueki K, Yoshiyama M, Ikawa M, Okabe M, Schaffer SW, Fujio Y, Azuma J. J Mol Cell Cardiol. 2008 May; 44(5):927-37.[↩]
- The Beneficial Effects of Taurine to Counteract Sarcopenia. Scicchitano BM, Sica G. Curr Protein Pept Sci. 2018; 19(7):673-680.[↩]
- Increasing taurine intake and taurine synthesis improves skeletal muscle function in the mdx mouse model for Duchenne muscular dystrophy. Terrill JR, Pinniger GJ, Graves JA, Grounds MD, Arthur PG. J Physiol. 2016 Jun 1; 594(11):3095-110.[↩][↩]
- Function and genetics of dystrophin and dystrophin-related proteins in muscle. Blake DJ, Weir A, Newey SE, Davies KE. Physiol Rev. 2002 Apr; 82(2):291-329.[↩]
- Increased taurine in pre-weaned juvenile mdx mice greatly reduces the acute onset of myofibre necrosis and dystropathology and prevents inflammation. Terrill JR PhD, Grounds MD, Arthur PG. PLoS Curr. 2016 Apr 29; 8[↩]
- Enhanced dystrophic progression in mdx mice by exercise and beneficial effects of taurine and insulin-like growth factor-1. De Luca A, Pierno S, Liantonio A, Cetrone M, Camerino C, Fraysse B, Mirabella M, Servidei S, Rüegg UT, Conte Camerino D. J Pharmacol Exp Ther. 2003 Jan; 304(1):453-63.[↩]
- The effect of taurine and β-alanine supplementation on taurine transporter protein and fatigue resistance in skeletal muscle from mdx mice. Horvath DM, Murphy RM, Mollica JP, Hayes A, Goodman CA. Amino Acids. 2016 Nov; 48(11):2635-2645[↩]
- The effects of taurine on pharmacologically induced myotonia. Conte Camerino D, De Luca A, Mambrini M, Ferrannini E, Franconi F, Giotti A, Bryant SH. Muscle Nerve. 1989 Nov; 12(11):898-904.[↩]
- The treatment of myotonia: evaluation of chronic oral taurine therapy. Durelli L, Mutani R, Fassio F. Neurology. 1983 May; 33(5):599-603.[↩]
- Acute effects of taurine on sarcoplasmic reticulum Ca2+ accumulation and contractility in human type I and type II skeletal muscle fibers. Dutka TL, Lamboley CR, Murphy RM, Lamb GD. J Appl Physiol (1985). 2014 Oct 1; 117(7):797-805.[↩]
- Spitze AR, Wong DL, Rogers QR and Fascetti AJ, 2003. Taurine concentrations in animal feed ingredients; cooking influences taurine content. Journal of Animal Physiology and Animal Nutrition, 87, 251–262.[↩][↩]
- Hayes KC and Trautwein EA, 1994. Modern nutrition in health and disease. In: Taurine. Lea & Febiger, Philadelphia, 477–485.[↩][↩]
- Rana SK and Sanders TAB, 1986. Taurine concentrations in the diet, plasma and breast milk of vegans compared with omnivores. British Journal of Nutrition, 56, 17–27.[↩]
- Sved DW, Godsey JL, Ledyard SL, Mahoney AP, Stetson PL, Ho S, Myers NR, Resnis P and Renwick AG, 2007. Absorption, tissue distribution, metabolism and elimination of taurine given orally to rats. Amino Acids, 32, 459–466.[↩]
- Laidlaw, S. A.; Grosvenor, M.; Kopple, J. D., 1990: J. Parent. Ent. Nutr. 14, 183.[↩][↩][↩]
- Kataoka, H.; Ohnishi, N., 1986: Agric. Biol. Chem. 50, 1887.[↩]
- Spitze AR, Wong DL, Rogers QR and Fascetti AJ, 2003. Taurine concentrations in animal feed ingredients; cooking influences taurine content. Journal of Animal Physiology and Animal Nutrition, 87, 251–262. https://www.vetmed.ucdavis.edu/sites/g/files/dgvnsk491/files/aal/pdfs/spitze.pdf[↩]