Contents
What is cryptococcosis
Cryptococcus is an invasive fungus that causes cryptococcosis an infection commonly associated with immunosuppressed individuals while being extremely rare in healthy individuals. There are more than 50 species of Cryptococcus; Cryptococcus neoformans and Cryptococcus gatti cause the majority of infections, and some consider them the only pathogens in humans 1. Infection with the fungus Cryptococcus (either Cryptococcus neoformans or Cryptococcus gatti) is called cryptococcosis. Cryptococcosis is a rare and fatal disease that preferentially infects immunosuppressed hosts 2. A previous study 3 reported that the rate of cryptococcal infection in immunosuppressed patients (5–10%, up to 30% in patients with AIDS) was higher than in immunocompetent patients (less than 5% in adults, and less than 1% in children). Cryptococcosis usually affects the lungs or the central nervous system (the brain and spinal cord), but it can also affect other parts of the body. Brain infections due to the fungus Cryptococcus are called cryptococcal meningitis. Cryptococcus fungus is widely prevalent in certain regions of the world. However, the most common forms of exposure include a history of exposure to soil contaminated with bird droppings.
Cryptococcus neoformans and Cryptococcus gattii are the fungi that cause cryptococcosis. Infection with Cryptococcus neoformans is seen worldwide. Infection with Cryptococcus gattii is mainly seen in the U.S. Pacific Northwest, British Columbia in Canada, Southeast Asia, and Australia. It is the more common fungus that causes the infection.
Both types of fungi are found in soil. If you breathe the fungus in, it infects your lungs. The infection may go away on its own, remain in the lungs only, or spread throughout the body (disseminate). Cryptococcosis is most often seen in people with a weak immune system, such as those who:
- Are infected with HIV/AIDS
- Take high doses of corticosteroid medicines
- Cancer
- Are on chemotherapy medicines for cancer
- Have Hodgkin disease
- Have had an organ transplant
Epidemiological data indicate that Cryptococcus neoformans occurs more frequently in immunocompromised individuals, particularly those with HIV/AIDS. By contrast, Cryptococcus gattii is more likely to cause disease in healthy people 4, as recently highlighted in the outbreaks in Canada (British Columbia) and the United States Pacific Northwest 5.
Cryptococcus gattii may affect people with normal immune system.
Cryptococcus neoformans is the most common life-threatening cause of fungal infection in people with HIV/AIDS. People can become infected with Cryptococcus neoformans after breathing in the microscopic fungus, although most people who are exposed to the fungus never get sick from it. Cryptococcus neoformans infections are rare in people who are otherwise healthy; most cases occur in people who have weakened immune systems, particularly those who have advanced HIV/AIDS.
Cryptococcus neoformans infections are not contagious. Humans and animals can get the infection after inhaling the microscopic fungus from the environment.
People between 20 to 40 years of age have this infection.
Cryptococcus species are fungal pathogens which are encapsulated yeasts morphologically. They are facultative intracellular organisms. Cryptococcal fungi exist in asexual forms (yeast) and sexual forms (telomorphs). Cryptococcal fungi produce mucoid colonies which are white in color when grown on a wide variety of agars 6. The two-predominant species of Cryptococcus are differentiated by growth features on canavanine-glycine-bromomethyl blue agar. Histological identification can be performed by methenamine silver stain for yeast forms, mucicarmine stain for yeast and capsule forms and Fontana-Masson stain for the melanin in the yeast.
Immune suppression is the major underlying mechanism that is involved in the causation of cryptococcal disease. Diseases like AIDS, diabetes, chronic liver disease, chronic renal disease, prolonged use of steroids and patients who undergo organ transplantation are commonly associated with the development of cryptococcal disease.
Globally approximately 1 million cases of cryptococcosis are reported each year resulting in 625,000 deaths approximately. In the United States incidence of cryptococcosis is estimated to be about 0.4-1.3 cases per 100,000 in general population and 2-7 cases per 100,000 in people affected with AIDS with a case fatality ratio of about 12%. The incidence of cryptococcal infections has declined drastically over the last two decades owing to advances in the anti-retroviral therapy. Cryptococcus neoformans is usually associated with infections in immunocompromised patients, while Cryptococcus gatti is associated with infections in immunocompetent patients.
Cryptococcosis transmission
Cryptococcus fungi are commonly found in soil contaminated by bird droppings and in decaying wood and in tree hollows 7, 8. The capsule of the fungus comprises of polysaccharides glucuronoxylomannan and glucuronoxylomannogalactan which are the major factors contributing to the virulence of pathogen. Infection usually occurs through inhalation of spores (basidiospores or desiccated yeast cells) from the environment. The initial infection is mostly asymptomatic and is contained in healthy individuals. Spread of the disease from initial site of infection occurs through blood dissemination in patients who are immune suppressed. Another mechanism through which the infection can develop is reactivation of the organism at the initial site of infection after several years when the patient becomes immunocompromised. Some research suggests that people may be exposed to Cryptococcus neoformans in the environment when they are children 9. Most people who breathe in Cryptococcus neoformans never get sick from it. However, in people who have weakened immune systems, Cryptococcus neoformans can stay hidden in the body and cause infection later when the immune system becomes too weak to fight it off 10.
Cryptococcus neoformans and Cryptococcus gatti both spread through inhalation and cause a similar spectrum of illness. Despite lung being the common site where the pathogen enters the body, meningoencephalitis is the most common clinical manifestation of cryptococcosis (cryptococcus infection). Clinical features of cryptococcal meningitis typically manifest with in a period of 1-2 weeks and include fever, malaise, headache, neck stiffness, photophobia, nausea, and vomiting. The disease may rarely progress to coma and death. Symptoms such as a cough, dyspnea, skin rash have been reported to occur rarely in the literature. Physical examination findings may sometimes reveal focal neurological deficits, and elevated diastolic pressure indicative of raised intracranial pressure.
Is cryptococcus infection contagious?
No. The cryptococcus infection can’t spread between people or between people and animals.
Who gets Cryptococcus neoformans infections?
Cryptococcus neoformans infections are rare among people who are otherwise healthy. Most cases of Cryptococcus neoformans infection occur in people who have weakened immune systems1–3, such as people who:
- Have advanced HIV/AIDS,
- Have had an organ transplant, or
- Are taking corticosteroids, medications to treat rheumatoid arthritis, or other medications that weaken the immune system.
How can I prevent a Cryptococcus neoformans infection?
It’s difficult to avoid breathing in Cryptococcus neoformans because it’s thought to be common in the environment. Most people who breathe in Cryptococcus neoformans never get sick from it. However, in people who have weakened immune systems, Cryptococcus neoformans can stay hidden in the body and cause infection later when the immune system becomes too weak to fight it off. This leaves a window of time when the silent infection can be detected and treated early, before symptoms develop
Cryptococcosis in humans
Pulmonary cryptococcosis
Pulmonary cryptococcosis is most commonly reported in immunocompromised patients, whereas immunocompetent hosts are rarely affected and may be asymptomatic 11. Pulmonary cryptococcosis is an important opportunistic fungal infection, with the majority of cases caused by Cryptococcus neoformans or Cryptococcus gattii infection 5. Pulmonary cryptococcosis may be the first manifestation of cryptococcosis since the respiratory tract is considered to be the entry site and is most frequently involved when cryptococcal infection develops 12. Depending on the host immune status, the infection may remain dormant in the lung or may undergo hematogenous spread to any organ system, including the central nervous system (CNS), bones and skin 13. However, pulmonary cryptococcosis does not present specific clinical manifestations and radiological findings. The clinical expression is variable and may present as coughing, expectoration, chest tightness, fever or asymptomatic. The most common radiological characteristics are known to be solitary or multiple pulmonary nodules, and segmental or lobar consolidation 14. For these reasons, pulmonary cryptococcosis is frequently misdiagnosed as other lung diseases, such as cancer and pneumonia. Thus, the main diagnostic methods include culture of clinical samples, detection of cryptococcal antigen in body fluids and/or histopathological examinations in tissue sections 15.
For treatment of pulmonary cryptococcosis, see the Infectious Diseases Society of America 2010 treatment guidelines below.
Cerebral cryptococcosis
Approximately 1 million cases of cryptococcal meningitis are reported annually 1. The incidence have markedly increased since 1950s because of corticosteroid use and improvement of survival in cancer patients. However, most of the cryptococcal reports come from the 1980s and are predominantly AIDS-related cases. Approximately 6% of patients with AIDS develop cryptococcal infections, and patients with AIDS-associated cryptococcosis account for 85% of all patients diagnosed with cryptococcosis 16.
The cryptococcus fungus is acquired by inhalation. After being deposited into the pulmonary alveoli, the yeast spores must survive the normal to high pH and the physiological concentrations of carbon dioxide before they are phagocytized by alveolar macrophages, a more acidic environment, and disseminated after a latent period of containment in the lung lymph nodes. The essential factor in the survival of Cryptococcus neoformans in this extracellular environment is glucosylceramide synthase 17.
The host response includes both cellular and humoral components. Possibly, the eradication of Cryptococci relies on natural killer cells and antibody-dependent cell-mediated killing 18.
While all organs can be involved, Cryptococcus have a strong affinity for the central nervous system. This neurotropism is linked to several cryptococcal-specific factors which facilitate the permeability of the blood-brain barrier; between them, metalloproteinases and ureases enzymes cause neuromodulation and mechanisms facilitate survival in the nutrient-deprived environment of the brain (autophagy and high-affinity sugar transporters) 19.
There can be little or no necrosis or organ damage until later disease. In patients with heavy infections, organ dysfunction may be accelerated, primarily due to tissue distortion caused by the growing fungal burden 20.
Cryptococcal meningitis usually presents as a subacute meningoencephalitis. The patient commonly presents with neurological symptoms such as a headache, altered mental status, and other signs and symptoms include lethargy along with fever, stiff neck (both associated with an aggressive inflammatory response), nausea and vomiting. Some patients who are HIV positive may have minimal or nonspecific symptoms at presentation.
The duration of symptoms from onset to a presentation is usually 1 to 2 weeks in HIV cases and 6 to 12 weeks in non-HIV cases.
Visual symptoms include diplopia and photophobia at the onset, and reduced acuity later in the disease (due to high cerebrospinal fluid (CSF) pressure or compression of the optic nerve and tracts). Other findings include hearing defects, ataxia, aphasia, seizures, and chorea.
Although Cryptococcus neoformans enters the body through the lungs, the central nervous system (CNS) is the main site of evident clinical infection 21.
For treatment of cerebral cryptococcosis, see the Infectious Diseases Society of America 2010 treatment guidelines below. The combination of amphotericin B and flucytosine has proved the most effective measure to clear the infection, and it showed a greater survival benefit over amphotericin alone. However, due to its cost, flucytosine is often unavailable in poor-resource settings where the disease burden is significant. Combinations of amphotericin B and fluconazole have been studied, and greater results have been found over amphotericin B alone 22.
Without treatment, the clinical course progresses to confusion, seizures, reduced level of consciousness, and coma.
Management of CNS cryptococcosis and in immune compromised hosts involves treatment targeting the fungal pathogen, reducing the intracranial pressure, and improvising the immune status of the patient. Antifungal therapy that targets Cryptococcus comprises of induction, consolidation, and maintenance phase. Amphotericin B and flucytosine, for a minimum duration of 2 weeks are recommended antifungal therapies that are used for induction phase and fluconazole for a duration of eight weeks is the recommended drug for consolidation phase of the therapy. The medication choices are usually similar in HIV positive patients, patients with organ transplantation and patients suffering from other conditions that cause immune suppression. After 2 weeks of induction therapy a repeat examination of cerebrospinal fluid is recommended and the duration of induction therapy can further be extended if the cultures remain positive. Maintenance therapy is recommended with fluconazole after eight weeks of induction and consolidation phases and the duration of maintenance phase is generally for a period of 1 year. Maintenance therapy can be discontinued if the patient achieves healthy immune status clinically correlated as CD4 cell count of more than 100 cells/microL.
Monitoring intracranial pressure and keeping it under check plays an important role in reducing the mortality associated with cryptococcal meningitis. Lumbar puncture is the recommended option for management of intracranial pressure and either a ventricular drain or ventriculo peritoneal shunt is used in patients who require frequent lumbar punctures. Use of medications such as acetazolamide, mannitol and dexamethasone which normally used in bacterial meningitis for lowering intracranial pressure is not recommended in cryptococcal meningitis. Another crucial aspect of the management of cryptococcosis includes improving the immune status of the patient. For HIV-positive patients, anti-retroviral therapy is recommended after a duration of 2-10 weeks after initiation of the anti-fungal therapy. Delayed initiation of the anti-retroviral therapy has been associated with better survival rates when compared to early initiation. For patients who have organ transplantations, reduction in the immunosuppressive therapy in a phased manner while maintaining the balance with risk of organ rejection would be a recommended approach to enhance the immune status.
Headache refractory to pain-killers can be treated with spinal decompression after an adequate neuroimaging evaluation with a CT scan or MRI. The safe maximum volume of CSF that can be drained at one lumbar puncture is unclear, but up to 30 mL are frequently removed with the pressure checked after each 10 mL removed 21.
Cerebral cryptococcosis prognosis
The initial prognosis depends on mortality predictors such as the following 23:
- CSF opening pressure of more than 25 cm of water
- Low CSF white cells count
- Sensory impairment
- Delayed diagnosis
- Elevated CSF antigen titers
- Rate of infection clearance
- CSF yeast count more than 10 mm^3 (a common practice in Brazil) 24
- Non-HIV-related patients and the prognostic factors in these patients, in addition to the already mentioned:
- Markers of a poor inflammatory response
- Absence of headache
- Underlying hematological malignancy
- Chronic renal or liver disease
Mortality varies from country to country depending on the resource settings. It remains high in the United States and France, with a 10-week mortality of 15% to 26%, and it is even higher in non-HIV patients because of the delayed diagnosis and dysfunctional immune responses. On the other hand, in poor-resource countries, mortality increase from 30% to 70% in 10 weeks because of the late presentation and lack of access to drugs, manometers, and optimal monitoring 25.
Cerebral cryptococcosis complications
Complications include the following 21:
- Persistent Infection – Persistently positive results of cultures of CSF after 4 weeks of proven antifungal therapy at an established effective dose is a reasonable starting point.
- Relapsed Infection (based upon two main characteristics)
- The recovery of viable Cryptococci from a previously checked sterile body site is essential.
- Recrudescence of signs and symptoms at the previous site of disease supports the presence of disease.
- Elevated CSF Pressure – Acute elevated symptomatic CSF pressure (sometimes due to initial antifungal therapy) should be managed rapidly by decompression via repeated lumbar taps, transitory lumbar drainage catheter, or ventriculostomy in some patients.
- Immune Reconstitution Inflammatory Syndrome:
- Unmasking Immune Reconstitution Inflammatory Syndrome , in which cryptococcal symptoms appear after the initiation of antiretroviral therapy.
- Paradoxical Immune Reconstitution Inflammatory Syndrome during the treatment of cryptococcosis and administration of antiretroviral therapy.
Cerebral cryptococcomas can cause significant short- and long-term neurological complications. They are hard to treat and normally require a long-term antifungal therapy.
Hydrocephalus is a later complication and can present as dementia or chronic headache.
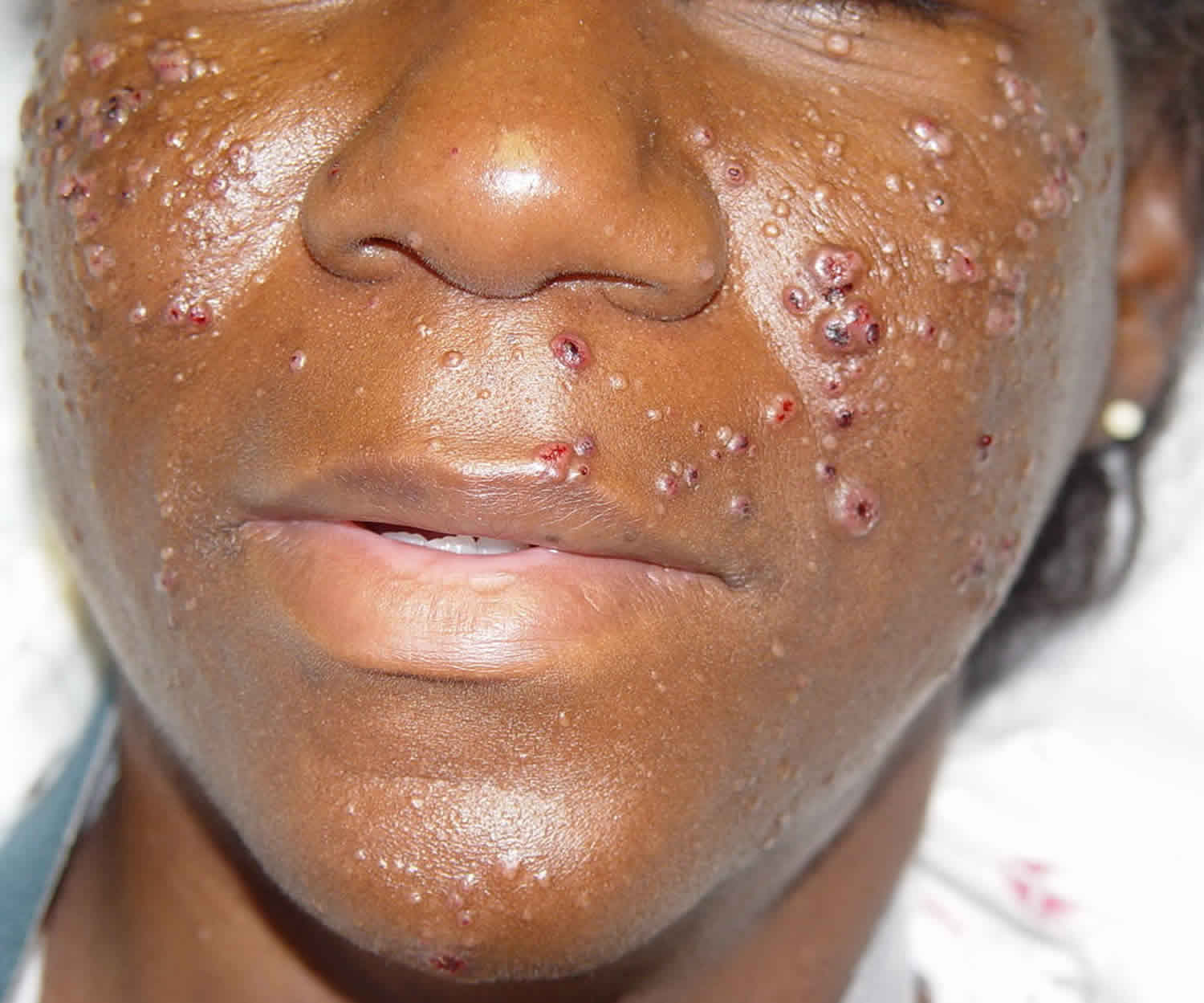
Cutaneous cryptococcosis
Skin is the third most common organ system to be involved after lungs and the central nervous system. Skin involvement is rare, but it is nearly all the time a sign of disseminated cryptococcosis; it may precede the systemic symptoms even eight months before 26. Cryptococcus infections present with a wide variety of skin lesions. Skin lesions are frequently a sentinel for disseminated disease; however, primary cutaneous lesions do occur in immunocompetent persons 27. The primary cutaneous lesion may be a papule, maculopapular lesion with an ulcerated center or a violaceous nodular lesion. Often there is a discharging sinus that connects to the underlying deep abscess or the underlying bone. Some strains of C. neoformans show dermatropism. However, primary cutaneous cryptococcosis is a diagnosis of exclusion 27.
Skin lesions in HIV patients can be identical to a bacterial abscess in appearance and clinical presentation. Cutaneous cryptococcosis may present as a cold abscess, without fever and signs of inflammation. Therefore, it is important to send cultures from any abscess drained from an immunocompromised patient. Cutaneous cryptococcal lesions in an AIDS patient may present as crusty, ulcerative lesions that develop slowly. Sometimes the skin lesions of Cryptococcus are umbilicated and can be mistaken for Molluscum contagiosum. Frequently there is simultaneous pulmonary and central nervous system (CNS) involvement.
Cryptococcosis is the third most common invasive fungal infection in organ transplant recipients, after candidiasis and aspergillosis. It is often a late disease occurring at three to ten years post-transplant. Solid organ transplant patients have a greater propensity for Cryptococcus skin infections when compared to hematopoietic stem cell transplant patients, for unclear reasons. Cutaneous cryptococcosis can mimic acute bacterial cellulitis in solid organ transplant patients. Cutaneous manifestations can be protean, may mimic an abscess, present as cellulitis that later ulcerates, blisters and shows necrosis, or mimic panniculitis. Primary cutaneous lesions do occur in solid organ transplant patients, but a disseminated disease is more common. In a study of 146 patients with cutaneous cryptococcosis, the following lesions were present in one-third of the patients: nodular mass, papule, ulcer/abscess, and cellulitis. Two-thirds of the skin lesions occurred in the lower extremities, and in 70% of cases, the patients had disseminated disease. The CNS was involved in 90% of cases with disseminated disease.
For treatment of cutaneous cryptococcosis, see the Infectious Diseases Society of America 2010 treatment guidelines below.
Cutaneous cryptococcosis diagnosis
No skin lesion is characteristic of cutaneous cryptococcosis; therefore, a biopsy is important 27. A good epidemiological history is important. Patients must be evaluated for an underlying immunosuppressed state. Patients with cutaneous cryptococcosis should be evaluated for the involvement of other organ systems such as the lungs and the CNS.
The polysaccharide capsule antigen is a large molecule, and assays have been developed to detect the antigen in serum and other body fluids. If cryptococcal polysaccharide antigen is present in cerebrospinal fluid (CSF), then there is a good chance that the yeast is present as well. The cryptococcal polysaccharide antigen assay is nearly 100% sensitive and 96% to 99.5% specific in serum and 96% to 100% sensitive and 93.5% to 99.8% specific in CSF.
Other microbiological tests to identify Cryptococcus are India-ink preparations and Grocott’s methenamine silver stain. Cryptococcemia can be easily detected by automated systems and seldom results in shock or signs and symptoms of sepsis.
Disseminated cryptococcosis
Disseminated cryptococcosis is a well‐recognized condition among patients infected with HIV, but it can also occur in non‐HIV patients 28. When cryptococcus is inhaled into the respiratory tract, it might localize in immunocompetent hosts 2. Once individuals show immune defects, cryptococcus could disseminate hematogenously to any part of the body, including the central nervous system (CNS), lymph nodes, liver, spleen, kidney, and skin, especially in patients with profound cellular immune deficiency 29. If 2 or more are invaded, patients would be diagnosed with disseminated cryptococcosis 30. Joshi et al. 31 reported that cryptococcosis usually occurs in immunocompromised subjects, accounting for nearly 80% of patients with cryptococcal infection, especially in those with human immunodeficiency virus (HIV) infection. This is followed by primary immunodeficiencies, diabetes, and requiring glucocorticosteroid therapy or organ transplantation. On the contrary, studies 32 in China demonstrated that 50–77% of patients with cryptococcal meningitis exhibited normal immune function. However, disseminated cryptococcosis is rare in immunocompetent children, and is mostly localized in the brain or lungs 33. It is usually misdiagnosed as tuberculosis or other diseases.
Cryptococcal infection commonly presents as meningitis. However, infection of the skin and other organs may also be involved in disseminated cryptococcosis. Cutaneous infections are reported in 10–20% of patients 34. Primary cutaneous infections are rare, and cutaneous manifestations are often signs of disseminated disease. Dermatological manifestations of cryptococcal infections include ulcers, abscess, granulomas, and pustules. Necrotizing fasciitis is rare 35.
For treatment of disseminated cryptococcosis, see the Infectious Diseases Society of America 2010 treatment guidelines below.
Cryptococcosis prevention
Prophylactic treatment for cryptococcal infection is recommended in HIV-positive patients with a CD4 count of fewer than 100 cells/microL. Fluconazole and Itraconazole are the recommended drugs for prophylaxis against cryptococcosis 7.
Detecting silent cryptococcal infections in people who have HIV/AIDS
One approach to prevent cryptococcal meningitis is called “targeted screening.” Research suggests that Cryptococcus neoformans is able to live in the body undetected, especially when a person’s immune system is weaker than normal. In a targeted screening program, a simple blood test is used to detect cryptococcal antigen (an indicator of cryptococcal infection) in HIV-infected patients before they begin taking antiretroviral treatment (ART). A patient who tests positive for cryptococcal antigen can take fluconazole, an antifungal medication, to fight off the silent fungal infection and prevent it from developing into life-threatening meningitis.
Current research
Efficacy of sertraline an antidepressant as an adjunct therapy with fluconazole is being currently studied in preventing cryptococcal infections in asymptomatic patients who test positive for cryptococcal antigen 7. A long-term prospective study is being performed to have a better understanding of the immunogenetic defects that might play a role in the pathogenesis of cryptococcal infections.
Cryptococcosis symptoms
Cryptococcosis (cryptococcus infection) may spread to the brain in people who have a weakened immune system. Neurological (brain) symptoms start slowly. Most people have swelling and irritation of the brain and spinal cord when they are diagnosed.
Symptoms of cerebral cryptococcosis (brain infection) may include:
- Fever and headache
- Neck stiffness or neck pain
- Nausea and vomiting
- Blurred vision or double vision
- Confusion or changes in behavior
- Sensitivity to light
The infection can also affect the lungs and other organs.
Pulmonary cryptococcosis (lung infection) symptoms may include:
- Difficulty in breathing or shortness of breath
- Cough
- Chest pain
- Fever
Other symptoms may include:
- Bone pain or tenderness of the breastbone
- Fatigue
- Skin rash, including pinpoint red spots (petechiae), ulcers, or other skin lesions
- Sweating — unusual, excessive at night
- Swollen glands
- Unintentional weight loss
People with a healthy immune system may have no symptoms at all.
Cryptococcosis diagnosis
Your health care provider will perform a physical exam and ask about symptoms and travel history.
The physical exam may reveal:
- Abnormal breath sounds
- Fast heart rate
- Fever
- Mental status changes
- Stiff neck
Patients presenting with symptoms of central nervous system are evaluated with radiographic imaging of the brain to rule out the presence of elevated cerebrospinal fluid pressure. Cerebrospinal fluid analysis, culture, staining and immunodiagnostic tests of cerebrospinal fluid are the primary diagnostic tests that are performed to diagnose meningitis caused by Cryptococcus. Analysis of the fluid usually shows low white blood cell count, low glucose, and elevated protein but could also be normal in approximately 25-30% of the cases. The culture of the infected fluid reveals cream colored colonies in about 3-7 days while staining with Indian ink helps in identifying the organism rapidly. Detection of cryptococcal antigen through immunodiagnostic tests of the serum and the cerebrospinal fluid provide a definitive diagnosis of the infection. Different techniques such as latex agglutination, enzyme-linked immunosorbent assay (ELISA) and lateral flow assay can be used to confirm the diagnosis of cryptococcal antigen.
Disseminated Cryptococcus infection is defined by a positive blood culture or a positive culture from at least two different sites. Disseminated infection is commonly associated with HIV infection or several other immunocompromised states.
Tests that may be done include:
- Culture: The gold standard for diagnosing cryptococcal infection; culture is traditionally identify Cryptococcus from human body samples.
- Blood culture to differentiate between the two fungi
- Antigen detection: Cryptococcal antigen test (looks for a certain molecule that is shed from the cell wall of the Cryptococcus neoformans fungus into the bloodstream). Can be used on CSF or serum for detection of early, asymptomatic cryptococcal infection in HIV-infected patients; higher sensitivity than microscopy or culture.
- Latex agglutination (LA)
- Enzyme immunoassay (EIA)
- Lateral flow assay (LFA)
- Microscopy: India Ink can be performed on CSF to quickly visualize Cryptococcus cells under a microscope; however, it can have limited sensitivity. Histopathology for detection of narrow-based budding yeasts in tissue can also be used.
- CT scan of the head
- Sputum culture and stain
- Lung biopsy
- Bronchoscopy and bronchoalveolar lavage
- Spinal tap to obtain a sample of cerebrospinal fluid (CSF)
- Cerebrospinal fluid (CSF) culture and other tests to check for signs of infection
- Chest x-ray
Cryptococcosis treatment
Some infections require no treatment. Even so, there should be regular checkups for a full year to make sure the infection has not spread. If there are lung lesions or the disease spreads, your provider will prescribe you antifungal medicines. These medicines may need to be taken for a long time.
Medicines include:
- Amphotericin B (can have severe side effects)
- Flucytosine
- Fluconazole
Fluconazole is the recommended treatment for asymptomatic or mild-to-moderate pulmonary infections. For severe pulmonary or central nervous system infections, amphotericin B in combination with flucytosine is the preferred initial treatment; after that, patients usually need to take fluconazole for an extended time to clear the infection.
There has been a steep decline in the mortality rates associated with cryptococcal infections because of advancements in the anti-retroviral therapy. The prognostic predictors of the disease associated with poor outcomes include altered mental status, cerebrospinal fluid antigen titer greater than 1:1024 and cerebrospinal fluid white blood cell count less than 20/microL.
Pharmacological treatment for cryptococcal infections is dependent upon the site of infection and the severity of symptoms. Per the 2010 Infectious Diseases Society of America Guidelines 36, non-immunosuppressed patients suspected of having a pulmonary cryptococcal infection with mild-to-moderate symptoms can be treated with fluconazole at a dose of 400mg daily for 6-12 months. This is also the treatment recommendation for non-meningeal, non-pulmonary cryptococcosis in patients where CNS disease was ruled out. In non-immunocompromised patients, a lumbar puncture should be considered to rule out asymptomatic CNS involvement, but the guidelines state that an lumbar puncture can be avoided in asymptomatic, immunocompetent patients with no CNS symptoms.
Figure 1. Cryptococcosis treatment – strength of recommendation and quality of evidence
[Source 36 ]The first-line antifungal treatment, according to recommendations from the 2010 IDSA (Infectious Diseases Society of America) update 36, is based on the induction, consolidation, and maintenance of the following three types of patients:
HIV-Related Disease
Induction therapy
- Amphotericin B deoxycholate (0.7 to 1.0 mg/kg/day) + flucytosine (100 mg/kg/day orally in 4 divided doses; IV formulations may be used in severe cases and in those without oral intake where the preparation is available) for 2 weeks (A1 Evidence)
- Liposomal amphotericin B (3 to 4 mg/kg/day) or amphotericin B lipid complex (5 mg/kg/day; kidney function surveillance) + flucytosine (100 mg/kg/day) for 2 weeks (B2 Evidence)
- Amphotericin B deoxycholate (0.7 to 1.0 mg/kg/day) or liposomal amphotericin B (3 to 4 mg/kg/day) or amphotericin B lipid complex (5 mg/kg/day, for patients who do not tolerate flucytosine) for 4 to 6 weeks (B2 Evidence)
Induction therapy alternatives
- Amphotericin B deoxycholate + fluconazole (B1 Evidence)
- Fluconazole + flucytosine (B2 Evidence)
- Fluconazole (B2 Evidence)
- Itraconazole (C2 Evidence)
Consolidation therapy
- Fluconazole (400 mg/day) for 8 weeks (A1 Evidence)
Maintenance therapy
- Fluconazole (200 mg/day) for 1 or more years (A1 Evidence)
Maintenance therapy alternatives
- Itraconazole (200 mg twice per day orally; drug-level monitoring strongly advised) for 1 or more years (C1 Evidence)
- Amphotericin B deoxycholate (1 mg/kg/week) for 1 or more years (C1 Evidence)
Transplant Related Disease
Induction therapy
- Liposomal amphotericin B (3 to 4 mg/kg/day IV) or amphotericin B lipid complex (5 mg/kg/day) + flucytosine (100 mg/kg/day) for 2 weeks (B3 Evidence)
Induction therapy alternatives
- Liposomal amphotericin B (6 mg/kg/day) or amphotericin B lipid complex (5 mg/kg/day) for 4 to 6 weeks (B3 Evidence)
- Amphotericin B deoxycholate (0.7 mg/kg/day) for 4 to 6 weeks (B3 Evidence)
Consolidation therapy
- Fluconazole (400 to 800 mg/day) for 8 weeks (B3 Evidence)
Maintenance therapy
- Fluconazole (200 to 400 mg/day) for 6 months to 1 year (B3 Evidence)
Not HIV/Transplant Related Disease
Induction therapy
- Amphotericin B deoxycholate (0.7 to 1.0 mg/kg/day) + flucytosine (100 mg/kg/day) for 4 or more weeks (B2 Evidence)
- Amphotericin B deoxycholate (0.7 to 1.0 mg/kg/day) for 6 weeks (B2 Evidence)
- Liposomal amphotericin B (3 to 4 mg/kg/day) or amphotericin B lipid complex (5 mg/kg/day) combined with flucytosine, 4 weeks (B3 Evidence)
- Amphotericin B deoxycholate (0.7 mg/kg/day) + flucytosine (100 mg/kg/day) for 2 weeks (B2 Evidence)
Consolidation therapy
- Fluconazole (400 to 800 mg/day) for 8 weeks (B3 Evidence)
Maintenance therapy
- Fluconazole (200 mg/day) for 6 to 12 months (B3 Evidence)
Cryptococcosis prognosis
Central nervous system involvement often causes death or leads to permanent damage.
- Pescador Ruschel MA, Thapa B. Meningitis, Cryptococcal. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK525986[↩][↩]
- Gao LW, Jiao AX, Wu XR, et al. Clinical characteristics of disseminated cryptococcosis in previously healthy children in China. BMC Infect Dis. 2017;17(1):359. Published 2017 May 22. doi:10.1186/s12879-017-2450-5 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5440943/[↩][↩]
- Lizarazo J, Escandón P, Agudelo CI, Castañeda E. Cryptococcosis in Colombian children and literature review. Mem Inst Oswaldo Cruz. 2014;109(6):797–804. doi: 10.1590/0074-0276130537[↩]
- Environmental isolation, biochemical identification, and antifungal drug susceptibility of Cryptococcus species. Teodoro VL, Gullo FP, Sardi Jde C, Torres EM, Fusco-Almeida AM, Mendes-Giannini MJ. Rev Soc Bras Med Trop. 2013 Nov-Dec; 46(6):759-64.[↩]
- Cryptococcosis due to Cryptococcus gattii in Germany from 2004-2013. Smith IM, Stephan C, Hogardt M, Klawe C, Tintelnot K, Rickerts V. Int J Med Microbiol. 2015 Oct; 305(7):719-23.[↩][↩]
- Mada PK, Alam MU. Cryptococcus (Cryptococcosis) [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431060[↩]
- Mada PK, Alam MU. Cryptococcus (Cryptococcosis) [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431060/[↩][↩][↩]
- Lazera MS, Salmito Cavalcanti MA, Londero AT, Trilles L, Nishikawa MM, Wanke B. Possible primary ecological niche of Cryptococcus neoformans. Med Mycol. 2000 Oct;38(5):379-83.[↩]
- Goldman DL, Khine H, Abadi J, Lindenberg DJ, Pirofski L, Niang R, et al. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics. 2001 May;107(5):E66.[↩]
- Saha DC, Goldman DL, Shao X, Casadevall A, Husain S, Limaye AP, et al. Serologic evidence for reactivation of cryptococcosis in solid-organ transplant recipients. Clin Vaccine Immunol. 2007 Dec;14(12):1550-4.[↩]
- Yang R, Yan Y, Wang Y, Liu X, Su X. Plain and contrast-enhanced chest computed tomography scan findings of pulmonary cryptococcosis in immunocompetent patients. Exp Ther Med. 2017;14(5):4417-4424. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5658728/[↩]
- Pulmonary manifestations of cryptococcosis in patients with AIDS: CT features. Sider L, Westcott MA. J Thorac Imaging. 1994 Spring; 9(2):78-84. https://www.ncbi.nlm.nih.gov/pubmed/8207784/[↩]
- Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. Brizendine KD, Baddley JW, Pappas PG. PLoS One. 2013; 8(3):e60431.[↩]
- Clinical features and high-resolution CT findings of pulmonary cryptococcosis in non-AIDS patients. Kishi K, Homma S, Kurosaki A, Kohno T, Motoi N, Yoshimura K. Respir Med. 2006 May; 100(5):807-12.[↩]
- Current trends in the prevalence of Cryptococcus gattii in the United States and Canada. Espinel-Ingroff A, Kidd SE. Infect Drug Resist. 2015; 8():89-97.[↩]
- May RC, Stone NR, Wiesner DL, Bicanic T, Nielsen K. Cryptococcus: from environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016 Feb;14(2):106-17.[↩]
- Rittershaus PC, Kechichian TB, Allegood JC, Merrill AH, Hennig M, Luberto C, Del Poeta M. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J. Clin. Invest. 2006 Jun;116(6):1651-9.[↩]
- Schmidt S, Zimmermann SY, Tramsen L, Koehl U, Lehrnbecher T. Natural killer cells and antifungal host response. Clin. Vaccine Immunol. 2013 Apr;20(4):452-8[↩]
- Vu K, Tham R, Uhrig JP, Thompson GR, Na Pombejra S, Jamklang M, Bautos JM, Gelli A. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. MBio. 2014 Jun 03;5(3):e01101-14[↩]
- Perfect JR, Bicanic T. Cryptococcosis diagnosis and treatment: What do we know now. Fungal Genet. Biol. 2015 May;78:49-54.[↩]
- Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A, Harrison TS. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol. 2017 Jan;13(1):13-24.[↩][↩][↩]
- Campitelli M, Zeineddine N, Samaha G, Maslak S. Combination Antifungal Therapy: A Review of Current Data. J Clin Med Res. 2017 Jun;9(6):451-456.[↩]
- Jitmuang A, Panackal AA, Williamson PR, Bennett JE, Dekker JP, Zelazny AM. Performance of the Cryptococcal Antigen Lateral Flow Assay in Non-HIV-Related Cryptococcosis. J. Clin. Microbiol. 2016 Feb;54(2):460-3[↩]
- Vidal JE, Gerhardt J, Peixoto de Miranda EJ, Dauar RF, Oliveira Filho GS, Penalva de Oliveira AC, Boulware DR. Role of quantitative CSF microscopy to predict culture status and outcome in HIV-associated cryptococcal meningitis in a Brazilian cohort. Diagn. Microbiol. Infect. Dis. 2012 May;73(1):68-73[↩]
- Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 2010 Feb 01;50(3):291-322[↩]
- Ni W, Huang Q, Cui J. Disseminated cryptococcosis initially presenting as cellulitis in a patient suffering from nephrotic syndrome. BMC Nephrol. 2013;14:20. Published 2013 Jan 22. doi:10.1186/1471-2369-14-20 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3556306/[↩]
- Akram SM, Koirala J. Cryptococcus (Cryptococcosis), Cutaneous. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448148[↩][↩][↩]
- Hoshino T, Omura K, Kimura S, Takahashi H, Kamei K, Ohkusu M. A case of disseminated cryptococcosis with necrotizing fasciitis in a non-HIV patient. Acute Med Surg. 2017;4(4):454-457. Published 2017 Jul 13. doi:10.1002/ams2.298 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5649293/[↩]
- Tabone MD, Leverger G, Landman J, Aznar C, Boccon-Gibod L, Lasfargues G. Disseminated lymphonodular cryptococcosis in a child with X-linked hyper-IgM immunodeficiency. Pediatr Infect Dis J. 1994;13:77–79. doi: 10.1097/00006454-199401000-00020[↩]
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of cancer/invasive fungal infections cooperative group and the National Institute of Allergy and Infectious Diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660[↩]
- Joshi NS, Fisher BT, Prasad PA, Zaoutis TE. Epidemiology of cryptococcal infection in hospitalized children. Pediatr Infect Dis J. 2010;29:e91–e95. doi: 10.1097/INF.0b013e3181fbc83d[↩]
- Chen YY, Lai CH. Nationwide population-based epidemiologic study of cryptococcal meningitis in Taiwan. Neuroepidemiology. 2011;36:79–84. doi: 10.1159/000323390[↩]
- Huang KY, Huang YC, Hung IJ, Lin TY. Cryptococcosis in nonhuman immunodeficiency virus-infected children. Pediatr Neurol. 2010;42:267–270. doi: 10.1016/j.pediatrneurol.2009.10.015[↩]
- Chaya R, Padmanabhan S, Anandaswamy V, Moin A. Disseminated Cryptococcosis presenting as cellultis in a renal transplant recipient. J. Infect. Dev. Ctries. 2013; 7: 060–3.[↩]
- Capoor MR, Khanna G, Malhotra R et al Disseminated cryptococcosis with necrotizing fasciitis in an apparently immunocompetent host: a case report. Med. Mycol. 2008; 46: 269–73.[↩]
- John R. Perfect, William E. Dismukes, Francoise Dromer, David L. Goldman, John R. Graybill, Richard J. Hamill, Thomas S. Harrison, Robert A. Larsen, Olivier Lortholary, Minh-Hong Nguyen, Peter G. Pappas, William G. Powderly, Nina Singh, Jack D. Sobel, Tania C. Sorrell; Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America, Clinical Infectious Diseases, Volume 50, Issue 3, 1 February 2010, Pages 291–322, https://doi.org/10.1086/649858[↩][↩][↩]