Contents
What is pneumonectomy
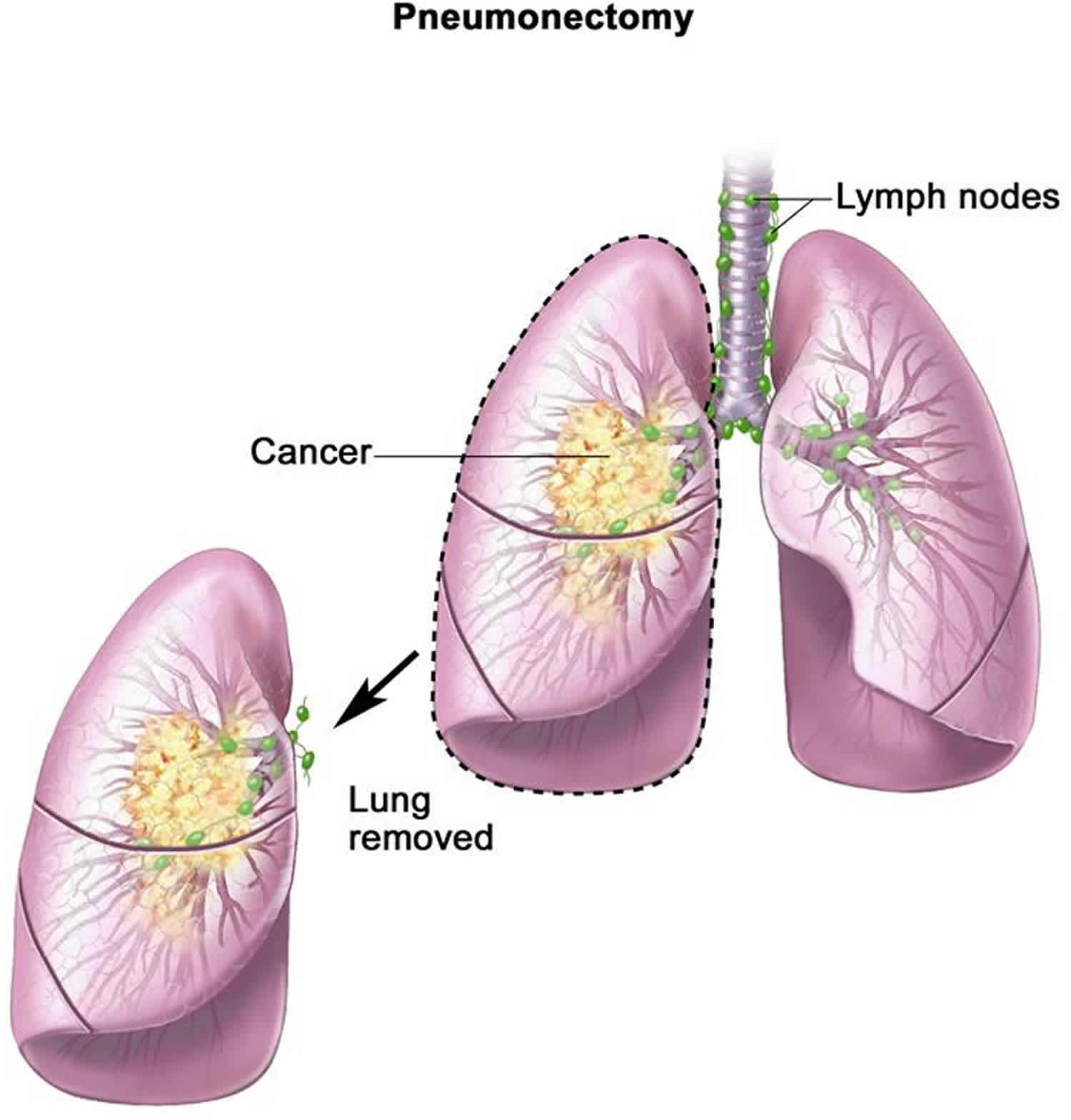
Pneumonectomy is a surgery to remove an entire lung. Pneumonectomy is most commonly performed for a primary lung cancer. The lung is removed in its entirety providing the patient has adequate pulmonary reserve from the opposite lung. Resection of part of the pericardium (intracardiac pneumonectomy) or chest wall may be required for tumor clearance.
Pneumonectomy is most frequently performed for bronchogenic carcinoma involving the hilum. Occasionally pneumonectomy is performed for inflammatory lung disease, traumatic lung injury, congenital lung disease, and irreversible atelectatic conditions. Pneumonectomy is a major operation that results in changes in anatomy and cardiopulmonary physiology. Potentially serious and sometimes life-threatening postpneumonectomy pulmonary, cardiovascular, or other complications are relatively frequent.
Pneumonectomy plays an important role in the management of patients with early stage non-small cell lung cancer that is not amenable to less extensive surgical resection, with five-year survival rates up to 60% depending on patient age and stage of disease at the time of resection 1. These outcomes are significantly better than outcomes for matched non-surgical patients, with pneumonectomy offering 2- to 3-fold higher 5-year survival across age groups.
Right-sided pneumonectomy is associated with greater mortality compared with left-sided pneumonectomy (10% to 12% versus 1% to 3.5%). The indication for pneumonectomy may affect outcome; for example, pneumonectomy for lung cancer has a mortality of 3% to 4%, whereas that performed for benign disease may be as high as 26%. Emergent pneumonectomy in cases of trauma or massive hemoptysis is associated with mortality rates greater than 30%. Also, pneumonectomy performed by thoracic surgeons has a lower mortality than that performed by general surgeons. Associated lung disease, history of coronary artery disease, history of congestive heart failure, hypertension, atrial fibrillation, cerebrovascular accident, cigarette smoking, and a 10% or greater weight loss over the 6-month period before surgery all contribute to higher mortality.
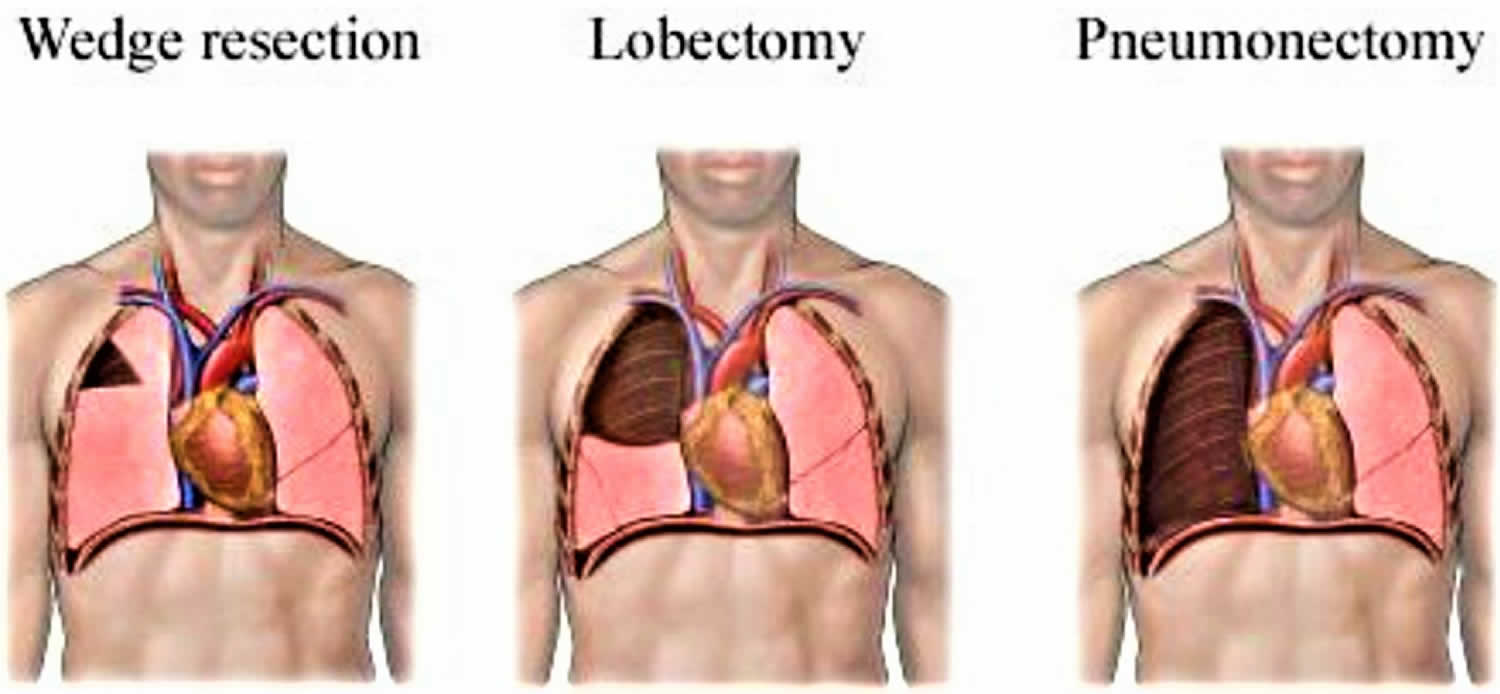
Types of lung resections
Three main types of surgery are used in metastatic lung cancer treatment. The choice depends on the size and location of the tumor, the extent of the cancer, and the general health of the patient. The surgeon will remove only the diseased portion of the lung. All types of lung operations require a thoracotomy which is an incision (cut) into the chest wall.
- Centrally located lung cancer tumor (bronchogenic carcinoma involving the hilum): Pneumonectomy
- Tumor that involves bronchus: “Sleeve lobectomy”
- Benign tumor, metastases, cancer in those who would not tolerate full lobectomy: Limited wedge resection
For tumors involving a bronchus, a “sleeve lobectomy” may be performed; in this surgery a segment of bronchus along with a lobe are excised. The remaining lobes are then reattached to the residual bronchus. Sleeve lobectomy is performed as an alternative to pneumonectomy with the aim of preserving lung function. Bronchotracheal resection with bronchial reattachment is required in cases in which tumors involve the carina. Limited wedge or segmental resection is appropriate for benign tumors, for metastases, and for primary lung cancer in a patient who would not tolerate full lobectomy. However, in patients with primary lung cancer, sublobar resection is associated with increased local recurrence rates compared to lobectomy. Concurrent with pulmonary resection, mediastinal lymph node sampling is usually performed for the purpose of staging the tumor.
Figure 1. Surgical procedure for lung cancer
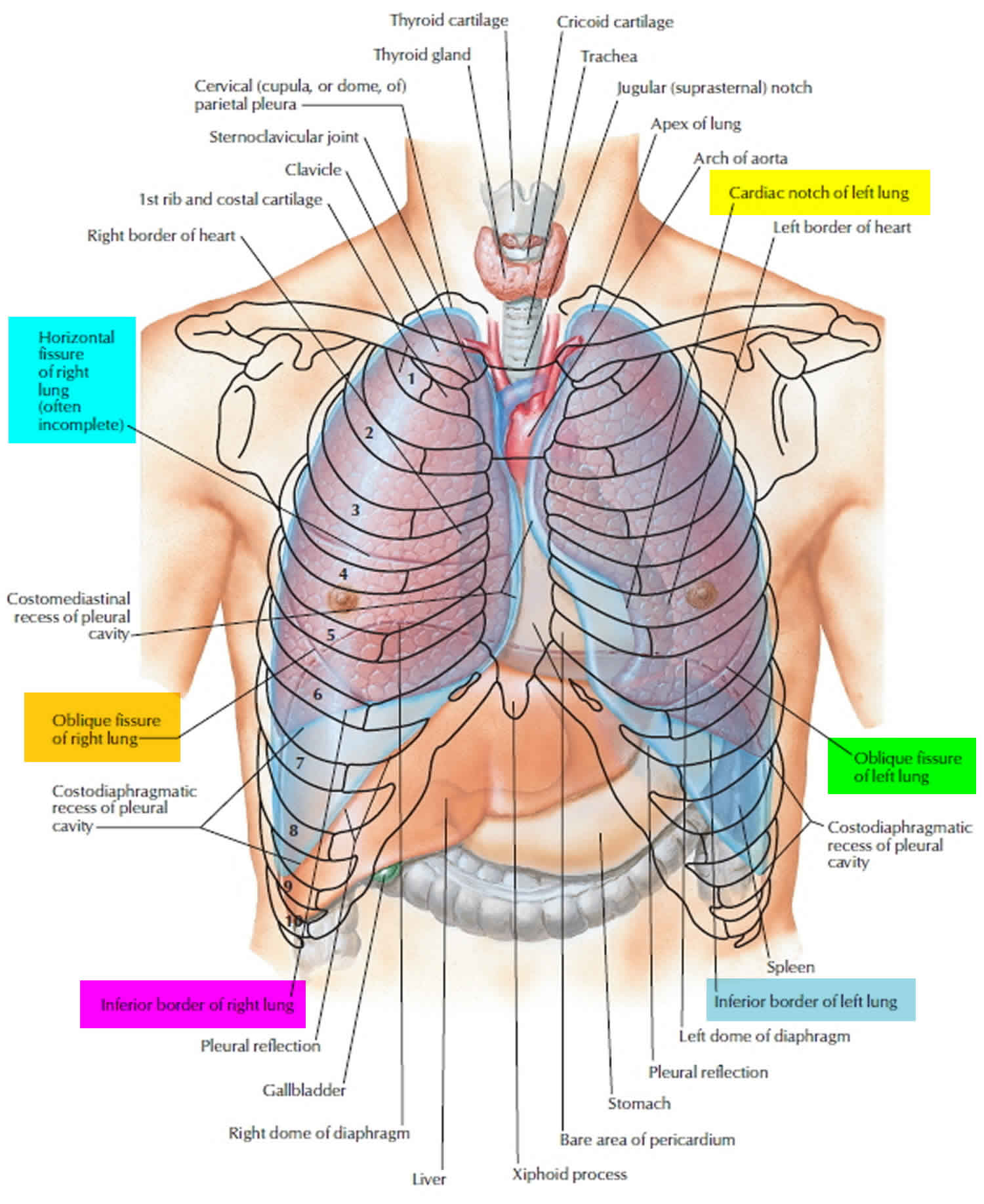
Lungs anatomy
Several deep fissures divide the two lungs into different patterns of lobes.
- The left lung is divided into two lobes, the superior lobe and the inferior lobe, by the oblique fissure. The left lung is somewhat smaller than the right and has a cardiac notch, a deviation in its anterior border that accommodates the heart.
- The right lung is partitioned into three lobes, the superior, middle, and inferior lobes, by the oblique and horizontal fissures.
Right lung
The right lung has three lobes and two fissures. Normally, the lobes are freely movable against each other because they are separated, almost to the hilum, by invaginations of visceral pleura. These invaginations form the fissures:
- The oblique fissure separates the inferior lobe (lower lobe) from the superior lobe and the middle lobe of the right lung.
- The horizontal fissure separates the superior lobe (upper lobe) from the middle lobe.
The approximate position of the oblique fissure on a patient, in quiet respiration, can be marked by a curved line on the thoracic wall that begins roughly at the spinous process of the vertebra T4 level of the spine, crosses the fifth interspace laterally, and then follows the contour of rib 6 anteriorly.
The horizontal fissure follows the fourth intercostal space from the sternum until it meets the oblique fissure as it crosses rib 5.
The orientations of the oblique and horizontal fissures determine where clinicians should listen for lung sounds from each lobe. The largest surface of the superior lobe is in contact with the upper part of the anterolateral wall and the apex of this lobe proj ects into the root of the neck. The surface of the middle lobe lies mainly adjacent to the lower anterior and lateral wall. The costal surface of the inferior lobe is in contact with the posterior and inferior walls.
The medial surface of the right lung lies adjacent to a number of important structures in the mediastinum and the root of the neck. These include the:
- heart,
- inferior vena cava,
- superior vena cava,
- azygos vein, and
- esophagus.
The right subclavian artery and vein arch over and are related to the superior lobe of the right lung as they pass over the dome of the cervical pleura and into the axilla.
Left lung
The left Iung is smaller than the right lung and has two lobes separated by an oblique fissure. The oblique fissure of the left lung is slightly more oblique than the corresponding fissure of the right lung. During quiet respiration, the approximate position of the left oblique fissure can be marked by a curved line on the thoracic wall that begins between the spinous processes of vertebrae T 3 and T 4, crosses the fifth interspace laterally, and follows the contour of rib 6 anteriorly.
As with the right lung, the orientation of the oblique fissure determines where to listen for lung sounds from each lobe. The largest surface of the superior lobe is in contact with the upper part of the anterolateral wall, and the apex of this lobe projects into the root of the neck. The costal surface of the inferior lobe is in contact with the posterior and inferior walls.
The inferior portion of the medial surface of the left lung, unlike the right lung, is notched because of the heart’s projection into the left pleural cavity from the middle mediastinum. From the anterior border of the lower part of the superior lobe a tongue-like extension (the lingula of the left lung) projects over the heart bulge.
The medial surface of the left lung lies adjacent to a number of important structures in the mediastinum and root of the neck. These include the:
- heart,
- aortic arch,
- thoracic aorta, and
- esophagus.
The left subclavian artery and vein arch over and are related to the superior lobe of the left lung as they pass over the dome of the cervical pleura and into the axilla.
Figure 2. Lungs anatomy
Pneumonectomy vs Lobectomy
Lobectomy is a surgery to remove the entire lobe of one lung. Pneumonectomy results in a greater loss of functioning lung tissue compared with a lobectomy.
Extrapleural pneumonectomy
Extrapleural pneumonectomy is a surgery to remove the entire afflicted lung together with the parietal pleura, pericardium and diaphragm.
Extrapleural pneumonectomy is usually performed for pleural malignancies such as diffuse malignant pleural mesothelioma, locally advanced lung cancer and thymoma. Diffuse malignant pleural mesothelioma is a rare aggressive cancer associated with asbestos exposure 2. Approximately 2000 to 3000 cases occur annually in the United States 3. The natural history is defined by a median survival of 4 to 12 months with no treatment.
Before the pneumonectomy procedure
You will have several visits with your health care provider and undergo medical tests before your surgery. Your provider will:
- Do a complete physical exam
- Make sure other medical conditions you may have, such as diabetes, high blood pressure, or heart or lung problems are under control
- Perform tests to make sure that you will be able to tolerate the removal of your lung tissue, if necessary
If you are a smoker, you should stop smoking several weeks before your surgery. Ask your provider for help.
Always tell your doctor:
- Which drugs, vitamins, herbs, and other supplements you are taking, even ones you bought without a prescription
- If you have been drinking a lot of alcohol, more than 1 or 2 drinks a day
During the week before your surgery:
- You may be asked to stop taking drugs that make it hard for your blood to clot. Some of these are aspirin, ibuprofen (Advil, Motrin), vitamin E, warfarin (Coumadin), clopidogrel (Plavix), or ticlopidine (Ticlid).
- Ask your doctor which drugs you should still take on the day of your surgery.
- Prepare your home for your return from the hospital.
On the day of your surgery:
- Do not eat or drink anything after midnight the night before your surgery.
- Take the medicines your doctor prescribed with small sips of water.
- Your doctor will tell you when to arrive at the hospital.
Pneumonectomy procedure
You will have general anesthesia before surgery. You will be asleep and unable to feel pain.
During the pneumonectomy procedure your chest wall is opened, ribs are spread and your lung is entered to remove the diseased portion, thereby causing the lung to collapse.
An incision (cut) will usually extend from just below your underarm to around the back. The incision is closed with dissolvable sutures (thread).
After lung surgery, air and fluid tend to collect in the chest. The air and fluid are drained out through a tube (chest tube) which is connected to a drainage system.
Lung surgery using a thoracotomy is called open surgery. In this surgery:
- You will lie on your side on an operating table. Your arm will be placed above your head.
- Your surgeon will make a surgical cut between two ribs. The cut will go from the front of your chest wall to your back, passing just underneath the armpit.
- These ribs will be separated or a rib may be removed.
- Your lung on this side will be deflated so that air will not move in and out of it during surgery. This makes it easier for the surgeon to operate on the lung.
- Your surgeon may not know how much of your lung needs to be removed until your chest is open and the lung can be seen.
- Your surgeon may also remove lymph nodes in this area.
- After surgery, one or more drainage tubes will be placed into your chest area to drain out fluids that build up. These tubes are called chest tubes.
- After the surgery on your lung, your surgeon will close the ribs, muscles, and skin with sutures.
- Open lung surgery may take from 2 to 6 hours.
Video-assisted thoracoscopic surgery (minimally invasive technique)
- Your surgeon will make several small surgical cuts over your chest wall. A videoscope (a tube with a tiny camera on the end) and other small tools will be passed through these cuts.
- Then, your surgeon may remove part or all of your lung, drain fluid or blood that has built up, or do other procedures.
- One or more tubes will be placed into your chest to drain fluids that build up.
- This procedure leads to much less pain and a faster recovery than open lung surgery.
Video-assisted thoracoscopic surgery (VATS):
VATS lobectomy – Lobectomy (removal of a large section of the lung) can be performed using a minimally invasive approach. During video-assisted lobectomy, three 2.5cam incisions and one 7.5cm to 10cm incision are made to provide access to the chest cavity without spreading of the ribs. The patient experiences a more rapid recovery with less pain and a shorter hospital stay (usually 3 days) than traditional thoracotomy surgery. The surgical outcomes of video-assisted lobectomy are comparable to traditional lobectomy outcomes. Although minimally invasive approaches are considered for every patient, in some cases, patients who have a large or more central tumor may not be candidates for video-assisted lobectomy.
VATS wedge resection – as previously stated, a wedge resection is the surgical removal of a wedge-shaped portion of tissue from one, or both, lungs which can also be accomplished using this minimally invasive technique. A wedge resection is typically performed for the diagnosis or treatment of small lung nodules.
Pneumonectomy recovery
Most people stay in the hospital for 5 to 7 days after open thoracotomy. Hospital stay for a video-assisted thoracoscopic surgery is most often shorter. You may spend time in the intensive care unit (ICU) after either surgery.
During your hospital stay, you will:
- Be asked to sit on the side of the bed and walk as soon as possible after surgery.
- Have tube(s) coming out of the side of your chest to drain fluids and air.
- Wear special stockings on your feet and legs to prevent blood clots.
- Receive shots to prevent blood clots.
- Receive pain medicine through an IV (a tube that goes into your veins) or by mouth with pills. You may receive your pain medicine through a special machine that gives you a dose of pain medicine when you push a button. This allows you to control how much pain medicine you get. You may also have an epidural placed. This is a catheter in the back that delivers pain medicine to numb the nerves to the surgical area.
- Be asked to do a lot of deep breathing to help prevent pneumonia and infection. Deep breathing exercises also help inflate the lung that was operated on.
- Your chest tube(s) will remain in place until your lung has fully inflated.
Pneumonectomy complications
Pneumonectomy complications may include:
Pulmonary
- Hypoxemia
- Postoperative respiratory failure
- Chronic pulmonary debility or deficiency
- Postpneumonectomy pulmonary edema
- Postpneumonectomy syndrome
- Bronchopleural fistula
- Pulmonary embolism
- Empyema
- Esophagopleural fistula
- Hemothorax
- Chylothorax
- Contralateral pneumothorax
- Pneumomediastinum
- Mediastinal infection (mediastinitis)
- Vocal cord paralysis
Cardiovascular
- Arrhythmias
- Myocardial infarction
- Intracardiac shunt
- Cardiac tamponade or herniation
- Pneumopericardium
Miscellaneous
- Postpneumonectomy paralysis
- Postpneumonectomy scoliosis
- Difficulty interpreting pulmonary artery catheter data
Risks of pneumonectomy surgery include:
- Failure of the lung to expand
- Injury to the lungs or blood vessels
- Need for a chest tube after surgery
- Pain
- Prolonged air leak
- Repeated fluid buildup in the chest cavity
- Bleeding
- Infection
- Heart rhythm disturbances
- Damage to the diaphragm, esophagus, or trachea
- Death
Recognized post-pneumonectomy complications include:
- Post pneumonectomy syndrome
- Bronchopleural fistula formation
- Cardiac herniation
- Recurrent pneumothorax
Post-pneumonectomy syndrome
Post-pneumonectomy syndrome is a rare delayed complication of pneumonectomy characterized by respiratory compromise caused by severe mediastinal shift and counterclockwise rotation of the heart and great vessels.
Post-pneumonectomy syndrome more commonly involves the left main stem bronchus after a right pneumonectomy. Very rarely it involves the right main stem bronchus post left pneumonectomy in the presence of a right aortic arch.
Post-pneumonectomy syndrome primarily involves children and young adults and women within a year after pneumonectomy surgery, probably related to increased elasticity and compliance of their lungs and mediastinum in these groups, compared with older patients and men.
Post-pneumonectomy syndrome symptoms include exertional dyspnea, inspiratory stridor, and recurrent cases of pneumonia.
Post-pneumonectomy syndrome is best diagnosed with a chest CT scan. CT scan better demonstrates the plain chest X-ray findings and may additionally show abnormal stretching and narrowing of the distal part of the trachea and the left main bronchus that is entrapped between the pulmonary artery anteriorly and the aorta and spine posteriorly. May also show the heart descending in the involved hemithorax and rotated counterclockwise along its main axis.
Post-pneumonectomy syndrome treatment and prognosis
Several surgical options described; the main aim is to reposition the mediastinal structures to the midline. One of the options is Silicone breast implants have been used in the involved pleural space to prevent ipsilateral rotational shifting of mediastinum.
Broncho-pleural fistula
Broncho-pleural fistulas are communications between the bronchial tree and the pleural space.
Broncho-pleural fistulas are usually divided as:
- central: when the fistula involves the trachea or a lobar bronchus
- peripheral: when a distal airway, either segmental bronchi or the lung parenchyma, communicates to the pleural space
CT scan is considered the imaging technique of choice for visualizing and characterizing bronchopleural fistulae 4. CT may show:
- pneumothorax
- hydropneumothorax
- pneumomediastinum
- underlying lung pathology
- demonstration of an actual fistulous communication
Cardiac herniation
Cardiac herniation is refers to herniation of heart outside its expected position. It can be intrathoracic or extrathoracic.
CT has shown pericardial tears before cardiac herniation and herniation itself.
Signs of a tear include focal pericardial discontinuity; pneumopericardium; and interposition of lung between the aorta and pulmonary artery, heart and diaphragm, or right atrium and right ventricular outflow tract; signs similar to congenital absence of the pericardium.
The primary sign of cardiac herniation is cardiac displacement when no large pleural effusion, atelectasis, or tension pneumothorax accounts for this displacement.
Pneumopericardium is non-specific, but a large volume of unilateral gas within the pericardium is more diagnostic of cardiac herniation, and has been termed the empty pericardial sac.
Pneumothorax
Pneumothorax refers to the presence of gas (air) in the pleural space. When this collection of gas is constantly enlarging with resulting compression of mediastinal structures, it can be life-threatening and is known as a tension pneumothorax (if no tension is present it is a simple pneumothorax).
Presentation is variable and may range from no symptoms to severe dyspnea with tachycardia and hypotension. In patients who have a tension pneumothorax, presentation may be with distended neck veins and tracheal deviation, cardiac arrest and in the most severe cases, death.
- Speicher PJ, Ganapathi AM, Englum BR, Onaitis MW, D’Amico TA, Berry MF. Survival in the elderly after pneumonectomy for early-stage non-small cell lung cancer: a comparison with nonoperative management. J Am Coll Surg. 2013;218(3):439-49. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3934839[↩]
- Selikoff, IJ, Hammond, EC, and Seidman, H. Latency of asbestos disease among insulation workers in the United States and Canada. Cancer. 1980; 46: 2736–2740[↩]
- Price, B and Ware, A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol. 2009; 39: 576–588[↩]
- Stern EJ, Sun H, Haramati LB. Peripheral bronchopleural fistulas: CT imaging features. AJR Am J Roentgenol. 1996;167 (1): 117-20.[↩]