Contents
What is pulmonary vein
The pulmonary veins drain oxygenated blood from the lungs to the left atrium. A small amount of blood is also drained from the lungs by the bronchial veins
There are typically 4 pulmonary veins, two draining each lung:
- Right superior pulmonary vein: drains the right upper and middle lobes
- Right inferior pulmonary vein: drains the right lower lobe
- Left superior pulmonary vein: drains the left upper lobe
- Left inferior pulmonary vein: drains the left lower lobe
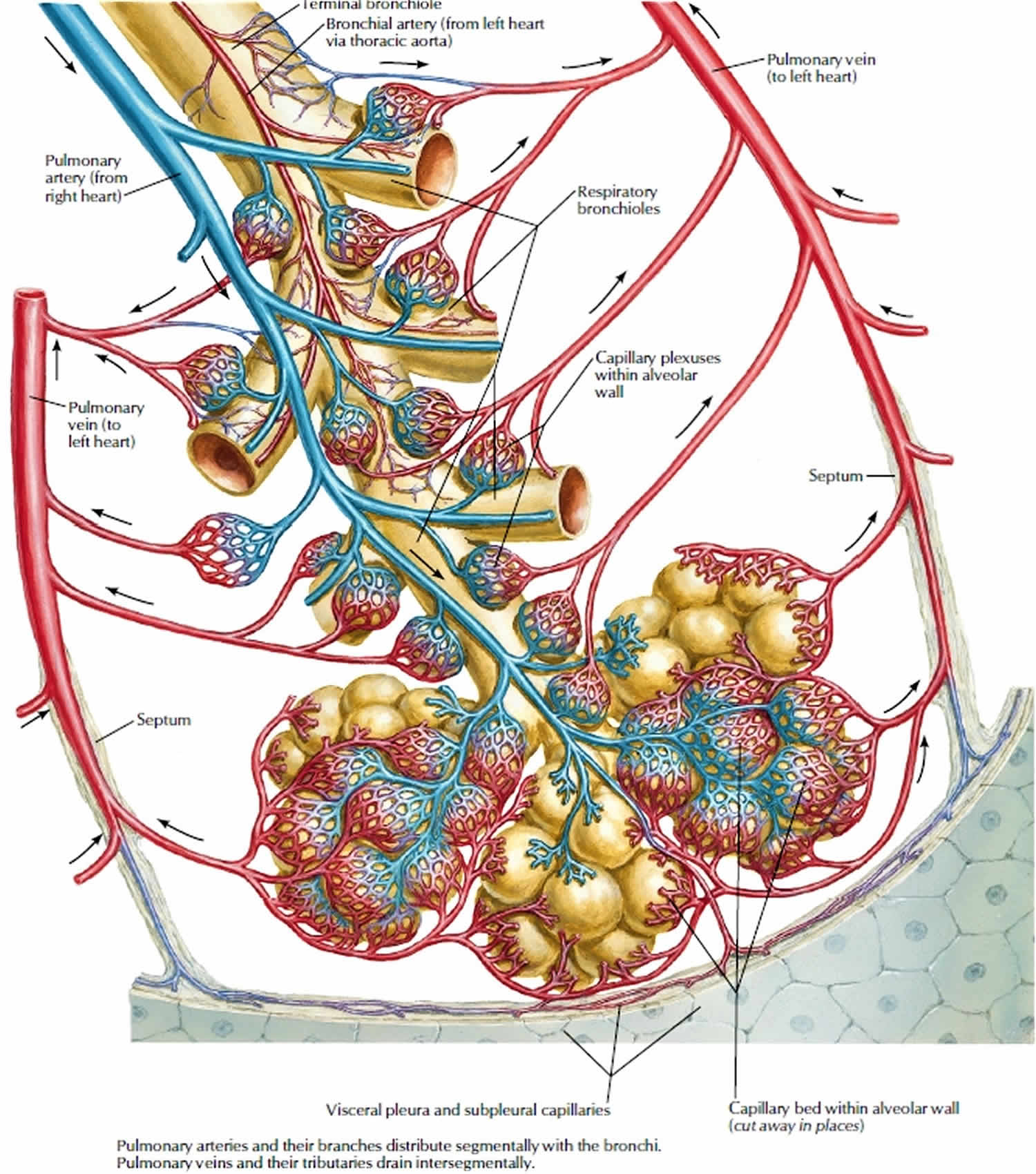
The pulmonary veins course in the intersegmental septa and as such do not run with the bronchi like the pulmonary arteries do.
The superior pulmonary veins take an oblique inferomedial course whereas the inferior pulmonary veins run horizontally peripherally before taking a more vertical course. They pass through the lung hilum, antero-inferiorly to the pulmonary arteries, forming a short intrapericardial segment, to drain into the left atrium. The ostia of the inferior pulmonary veins are more posteromedial and the left pulmonary veins being more superior.
There is extensive communication with deep bronchial veins within the lung and with the superficial bronchial veins at the hilum.
The pulmonary veins are covered by a short (~9 mm) myocardial layer, which is often the electrical focus of atrial fibrillation with the left superior pulmonary vein being the foci for almost half of cases. These abnormal foci can be treated with radiofrequency ablation.
Typical anatomy described above is found in ~70% of patients 1. Variant configurations are more common on the right and include:
- Common trunks
- common draining trunk of left superior and inferior pulmonary veins
- Accessory (additional pulmonary veins)
- single accessory right middle pulmonary vein (~10%)
- two accessory right middle pulmonary veins
- one accessory right middle pulmonary vein and one accessory right upper pulmonary vein
- superior segment right lower lobe vein
- basilar segment right lower lobe vein
- right top pulmonary vein (drains right superior basal segment)
There may also be partial anomalous pulmonary venous return where the pulmonary veins drain into a structure besides the left atrium and rarely total anomalous pulmonary venous return occurs where there is no drainage of pulmonary veins into the left atrium.
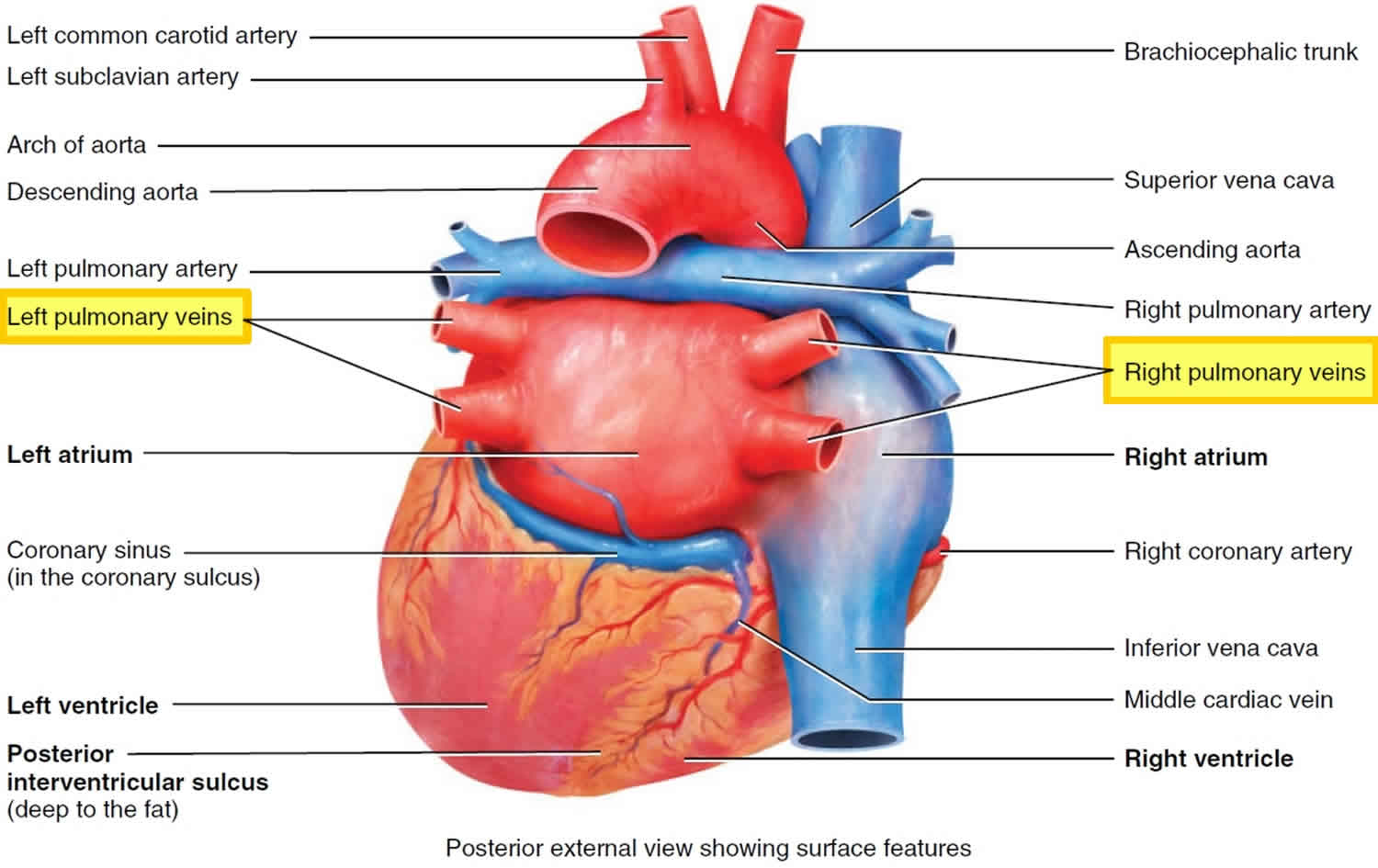
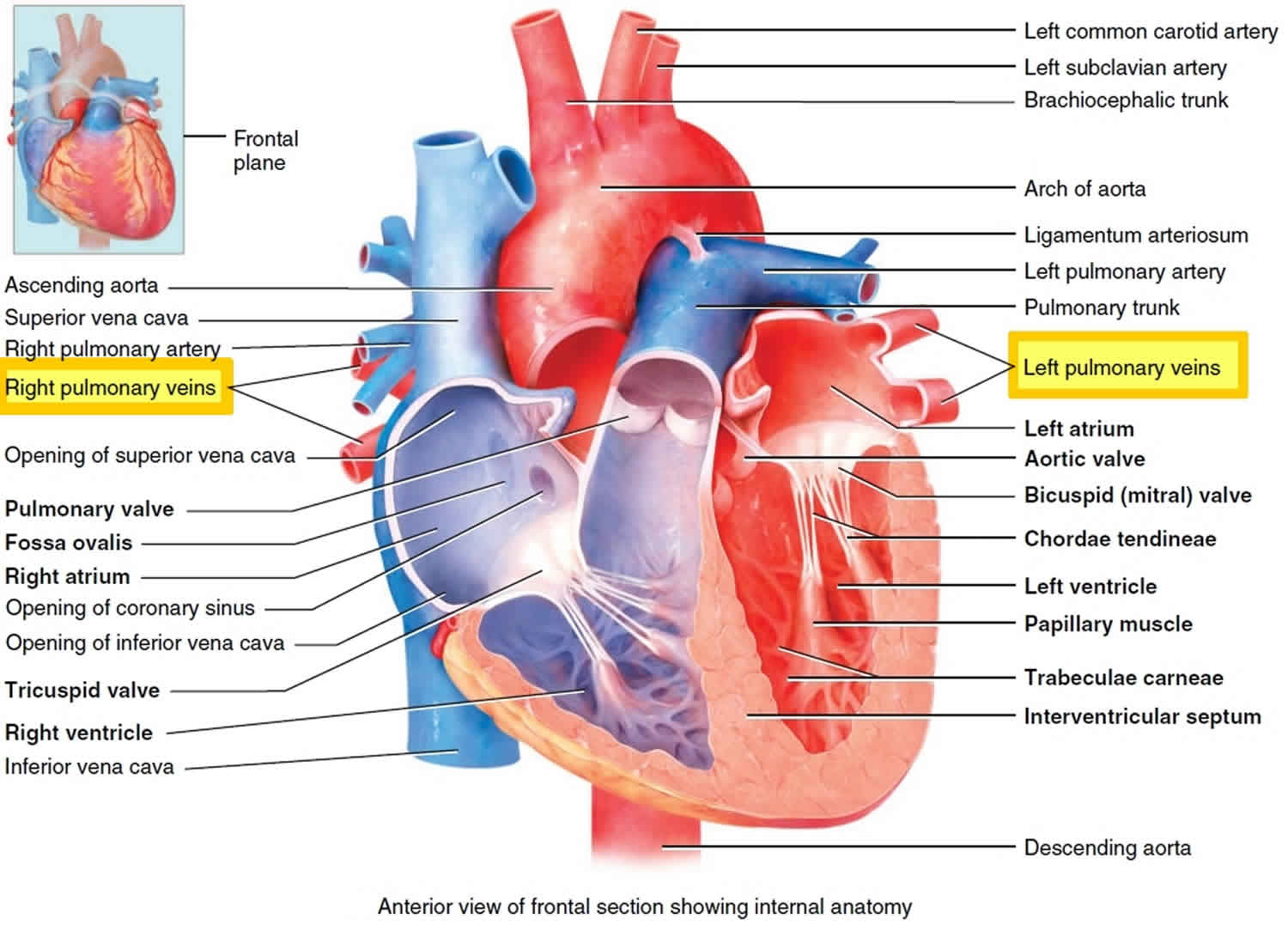
Pulmonary vein anatomy
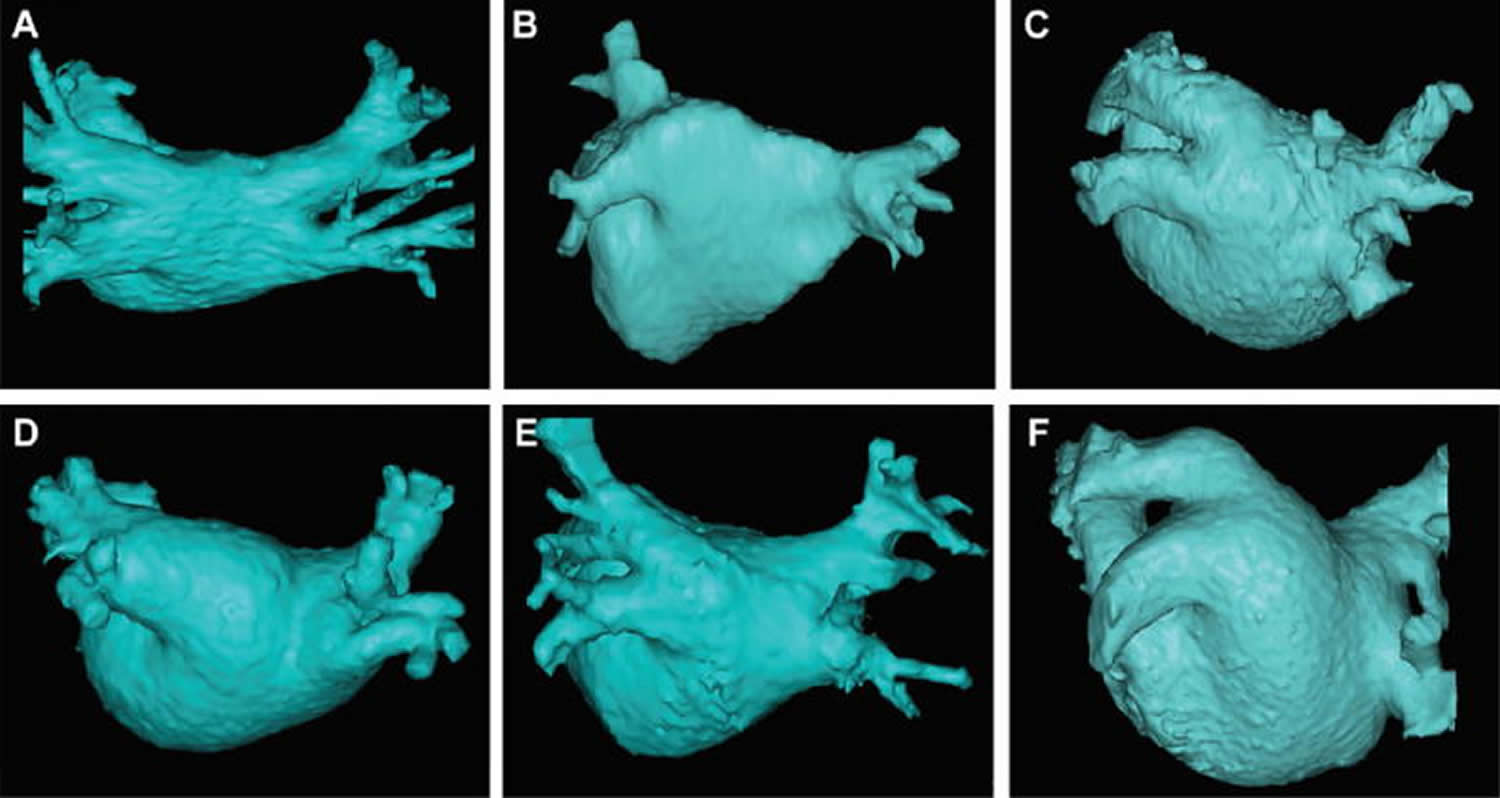
Pulmonary vein anatomy is highly variable between patients (Figure 3). Four distinct pulmonary vein openings (ostia) are present in approximately 60% of patients, whereas variant anatomy is observed in 40% of patients undergoing pulmonary vein ablation 2. In approximately 80% of cases, the anterior part of the ostium of the left pulmonary veins is common, separated from the appendage by a ridge 3. The most frequent type of variant anatomy is a left common pulmonary vein, and the second most frequent variant anatomy is a right middle pulmonary vein. Anomalous pulmonary veins can also be observed arising from the roof of the atrium. The orifices of the left pulmonary veins are located more superior than those of the right pulmonary veins. The right superior (RS) pulmonary vein and the left superior (LS) pulmonary vein project forward and upward, whereas the right inferior (RI) pulmonary vein and the left inferior (LI) pulmonary vein project backward and downward. The right superiorpulmonary vein lies just behind the superior vena cava (SVC) or right atrium, and the left pulmonary veins are positioned between the left atrial appendage (LAA) and the descending aorta.
Figure 2. Pulmonary vein anatomy
Figure 3. Pulmonary vein anatomy
Footnote: This figure includes six CT or MR images of the left atrium and pulmonary veins viewed from the posterior perspective. Common and uncommon variations in pulmonary vein anatomy are shown. (A) Standard pulmonary vein anatomy with 4 distinct pulmonary vein ostia. (B) Variant pulmonary vein anatomy with a right common and a left common pulmonary vein. (C) Variant pulmonary vein anatomy with a left common pulmonary vein with a short trunk and an anomolous pulmonary vein arising from the right posterior left atrial wall. (D) and (E) Variant pulmonary vein anatomy with a common left pulmonary vein with a long trunk. (F) Variant pulmonary vein anatomy with a massive left common pulmonary vein.
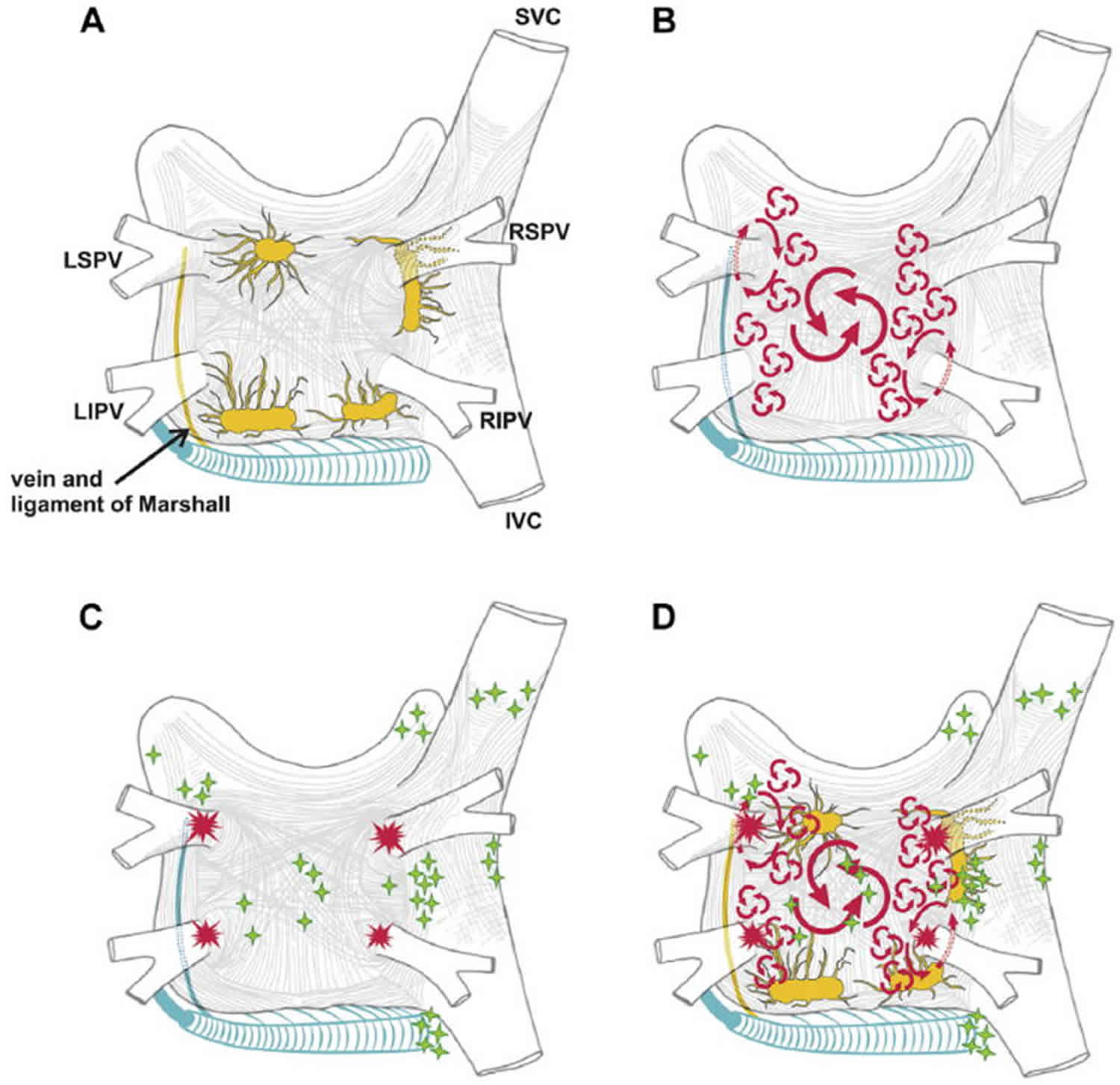
[Source 4 ]Nathan and Eliakim first drew attention to the presence of sleeves of cardiac tissue that extend onto the pulmonary veins (Figure 1) 5. Myocardial muscle fibers extend from the left atrium into all the pulmonary veins for 1–3 cm; the thickness of the muscular sleeve is highest at the proximal ends (1–1.5 mm), and then gradually decreases distally 6. The orientation of the major atrial muscular bundles (e.g., Bachmann’s bundle or Crista terminalis) has been recognized from anatomical dissections, with mostly circular bundles around the ostia of the pulmonary veins, atrioventricular valves, and left atrial appendage 7. Studies have described how premature firing from the pulmonary veins can initiate atrial fibrillation (AF) by interacting with tissue mechanisms, using diffusion tensor imaging (at present, in vitro) 8. These findings have been reproduced by cardiac magnetic resonance imaging (MRI), highlighting the very variable individual pattern of fiber orientation 9. Future in vivo implementation (in addition to identification of fibrosis), combined with simultaneous mapping techniques, could allow individual tailoring of interruption of potential reentrant “pathways” 10.
The greater coronary venous system drains approximately 85% of the venous flow into the right atrium, with the most proximal part being called the coronary sinus. The great cardiac vein ascends into the left atrioventricular groove, where it passes close to the circumflex artery and under the cover of the left atrial appendage. The juncture between the great cardiac vein and the coronary sinus is marked by the entrance of the vein of Marshall (which is typically obliterated in adults and is referred to as the ligament of Marshall), which descends along the epicardium between the left atrial appendage and the left superior pulmonary vein and can contain sympathetic nerves and ganglia 11. Especially around the coronary sinus itself, muscular bundles are present that interconnect to the left atrium, thereby serving as additional interatrial electrical “conductors” 12.
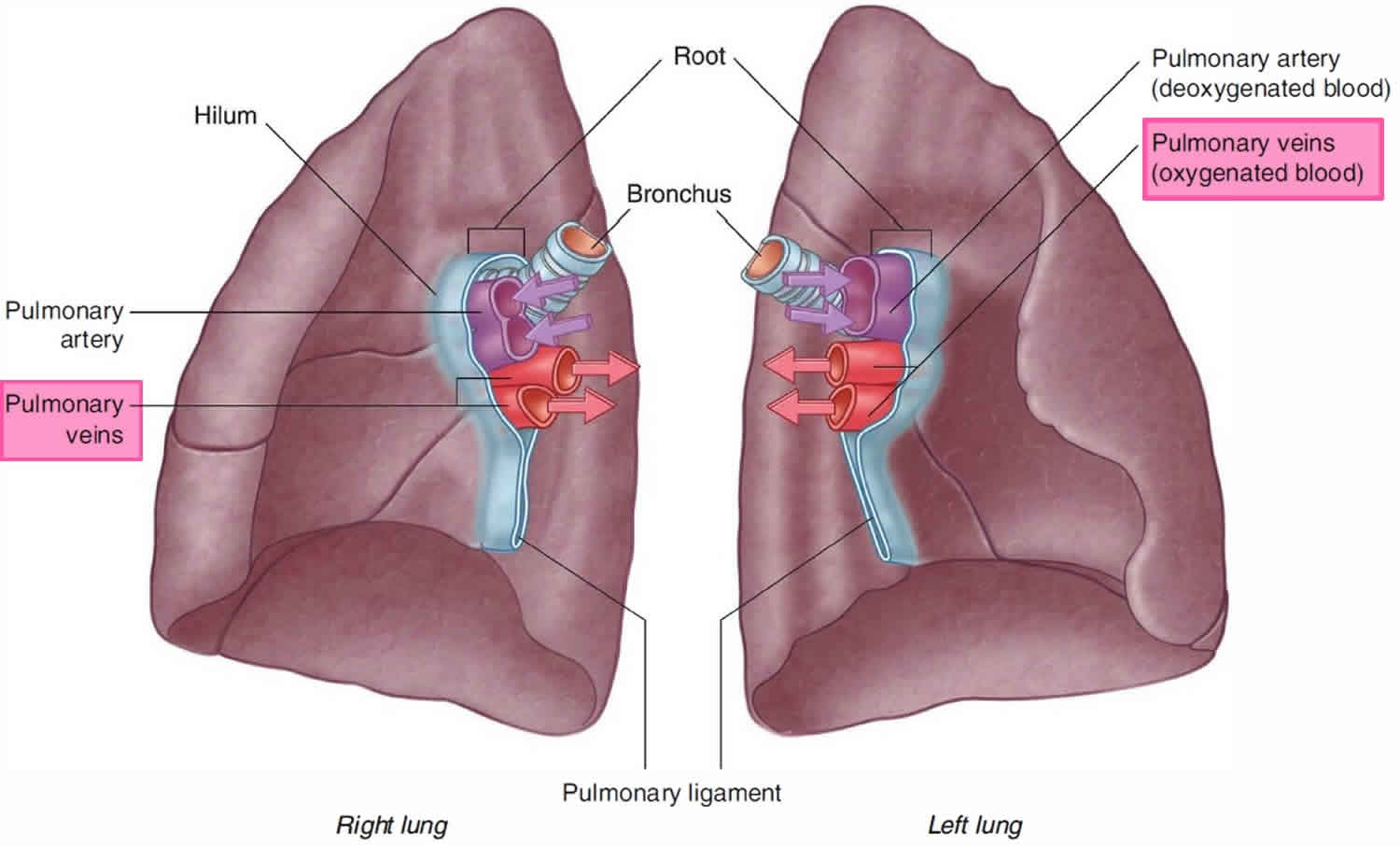
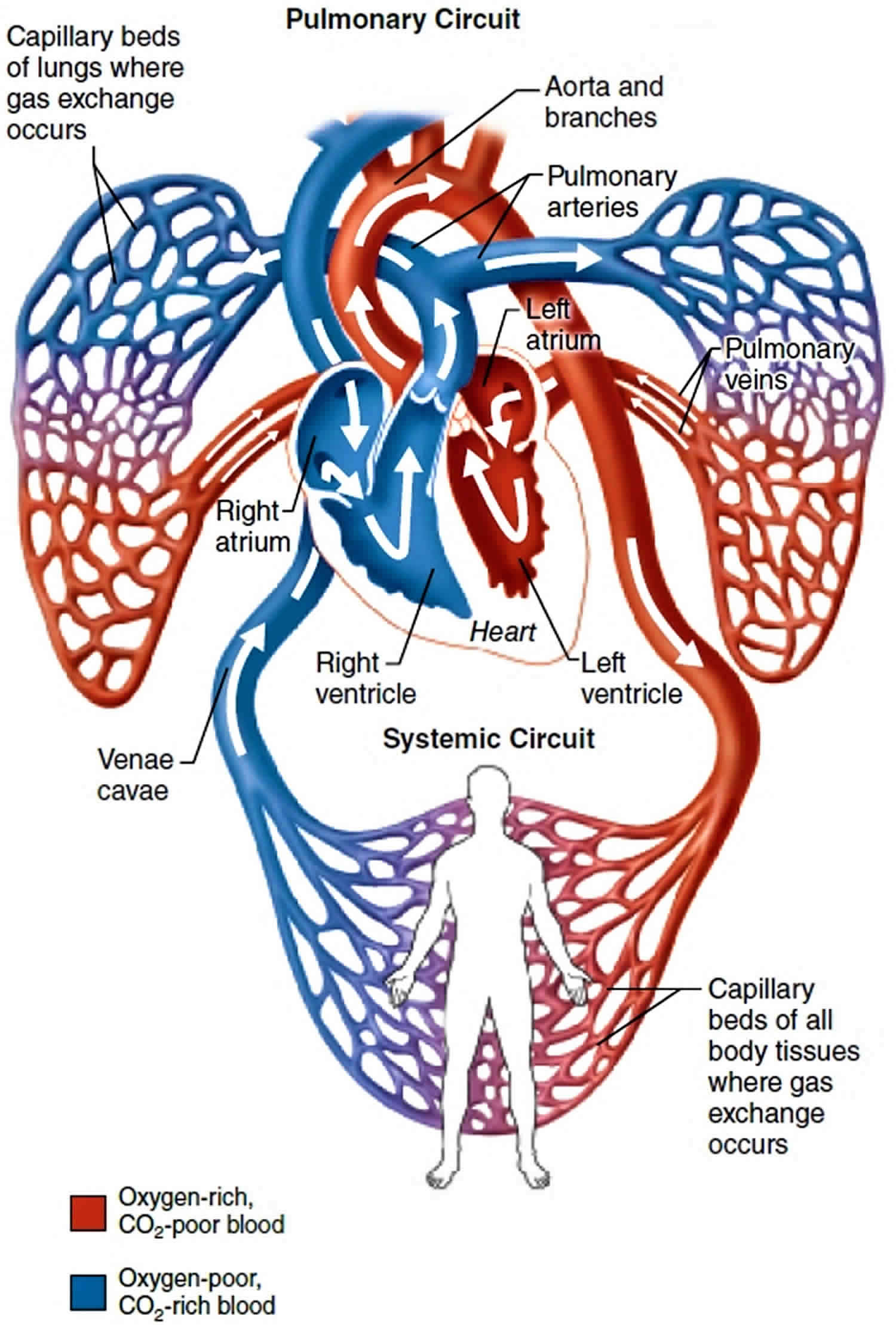
Pulmonary vein function
Superior pulmonary vein and an inferior pulmonary vein carry oxygenated blood from the lungs back to the heart. The right pulmonary vein and left pulmonary vein begin at the hilum of the lung, pass through the root of the lung, and immediately drain into the left atrium.
Figure 4. Pulmonary vein function
Pulmonary artery vs Pulmonary vein
The pulmonary arteries deliver deoxygenated blood to the lungs from the right ventricle of the heart. Oxygenated blood returns to the left atrium via the left and right superior and inferior pulmonary veins.
Pulmonary vein thrombosis
Pulmonary vein thrombosis is a rare but potentially serious condition with a number of underlying possible causes.
Pulmonary vein thrombosis causes
Pulmonary vein thrombosis causes include:
- intrapulmonary neoplasm: considered most frequent cause 13
- as a complication of lung transplantation or lobectomy 14
- as a complication of radiofrequency ablation
- fibrosing mediastinitis
- mitral stenosis with a left atrial clot
- hilar torsion 15
- hypercoagulable state
- idiopathic
Pulmonary vein thrombosis signs and symptoms
Pulmonary vein thrombosis signs and symptoms are non-specific and can range from acute (pulmonary infarction) to more insidious (progressive or recurrent pulmonary edema). Patients may have dyspnea, hemoptysis, chest pain, fever, and/or hypoxemia.
Pulmonary vein thrombosis treatment
Anticoagulation use is thought to be doubtful in terms of clinical outcome but may avoid clot development in the left atrium and possibly promote recanalisation of the affected pulmonary vein 15.
Pulmonary vein stenosis
Pulmonary vein stenosis refers to a spectrum of conditions characterized by narrowing of the pulmonary veins. Pulmonary vein stenosis can be congenital (present at birth) or acquired.
- Primary pulmonary vein stenosis – occurs in children
- Secondary pulmonary vein stenosis – occurs in adults and usually associated with some identifiable underlying causative process
- Involvement of the pulmonary veins by an extrinsic process:
- neoplasm growth
- sarcoidosis
- fibrosing mediastinitis
- Iatrogenic procedures – radiofrequency ablation for atrial fibrillation (1-3% of cases 3).
- Post surgical e.g. post-repair of anomalous pulmonary venous return
- Involvement of the pulmonary veins by an extrinsic process:
Pulmonary vein stenosis complications
Recognized complications of pulmonary vein stenosis include:
- pulmonary hypertension – pulmonary hypertensive crisis
- recurrent intercurrent pulmonary infection
- hemoptysis
Pulmonary vein atresia
Pulmonary vein atresia represents to a spectrum of disorders where the pulmonary veins fail to form to varying degrees.
Pulmonary vein atresia can be broadly divided into:
- Unilateral pulmonary vein atresia
- Bilateral pulmonary vein atresia or common pulmonary vein atresia
Unilateral pulmonary vein atresia
The condition usually present in infancy or childhood with recurrent episodes of pneumonia and/or hemoptysis. Presentation in adulthood does occur but is uncommon.
Unilateral pulmonary vein atresia results from failure of incorporation of the common pulmonary vein into the left atrium. There is no recognized right or left predilection.
Unilateral pulmonary vein atresia complications include the development of pulmonary hypertension 16
Unilateral pulmonary vein atresia treatment
Pneumonectomy is sometimes indicated once symptoms or complications are present.
Pulmonary vein ablation
In 1998, Haïssaguerre et al. 17 showed that pulmonary vein ectopy can trigger atrial fibrillation (AF) and electrical isolation of these pulmonary vein triggers can suppress atrial fibrillation (AF). They found that in 29 patients there was a single point of origin for the atrial fibrillation, whereas two points were identified in nine patients, and 3-4 points in another seven patients. Of these ectopic sites, 94% originated in the pulmonary veins. The study by Haïssaguerre and colleagues heralded the current era of non-pharmacologic treatment approaches to atrial fibrillation management, which are founded on pulmonary vein isolation.
Atrial fibrillation is a common supraventricular arrhythmia that is characterized by rapid and irregular activation in the atria without discrete P waves on the surface electrocardiogram (ECG). Atrial fibrillation can be diagnosed with a surface ECG, an intracardiac atrial electrogram, or both. An arrhythmia that has the ECG characteristics of atrial fibrillation and lasts sufficiently long for a 12-lead ECG to be recorded, or is otherwise documented to last for at least 30 seconds, should be considered to be an atrial fibrillation episode 4. The 30-second duration was selected based on previous published consensus statements and is used as the minimal duration to define recurrence of atrial fibrillation after catheter ablation 18. This duration of atrial fibrillation has not been linked to a specific outcome of atrial fibrillation. In addition to the duration requirements listed above, the diagnosis of atrial fibrillation requires an ECG or rhythm strip demonstrating: (1) “absolutely” irregular R-R intervals (in the absence of complete atrioventricular [AV] block); (2) no distinct P waves on the surface ECG; and (3) an atrial cycle length (when visible) that is usually less than 200 ms 19.
Initial isolation approaches focused on identifying specific pulmonary vein triggers utilizing induction strategies. These pulmonary vein triggers were then targeted with segmental isolation 20. Unfortunately an induction strategy as a stepwise approach to pulmonary vein isolation was challenging due to difficulty in initiating the arrhythmogenic foci during the electrophysiology study. In addition, during subsequent studies in patients with atrial fibrillation recurrence, different ectopic foci from the same or different pulmonary veins were often found to be active contributors to the arrhythmia. As such, empiric isolation of all pulmonary veins became the preferred strategy 21. However, it is important to recognize that a close examination of long-term outcomes does not demonstrate that empiric isolation of all pulmonary veins is superior to treating only those veins that are arrhythmogenic 22.
Based upon the initial work by Haïssaguerre et al. 17 long-term success rates with pulmonary vein isolation alone should be much higher. For example, in the initial studies not all identified triggers were from the pulmonary veins, but over 90% were. However, success rates after catheter ablation were significantly less than 90% at 1 year. Furthermore, in studies with follow-up to 3-5 years success rates are even lower at 30-50% 23. In non-paroxysmal atrial fibrillation, recurrence rates are also very high with recurrence rates of 70% at 3 years despite aggressive ablation approaches 24. These long-term success rates should prompt a careful inquiry into why catheter ablation for atrial fibrillation is fraught with such high recurrence rates. Failure may stem from inadequate initial therapy or it may reflect an inadequate understanding of atrial fibrillation and how to utilize nonpharmaceutic therapy.
Electrophysiological basis of atrial fibrillation ablation
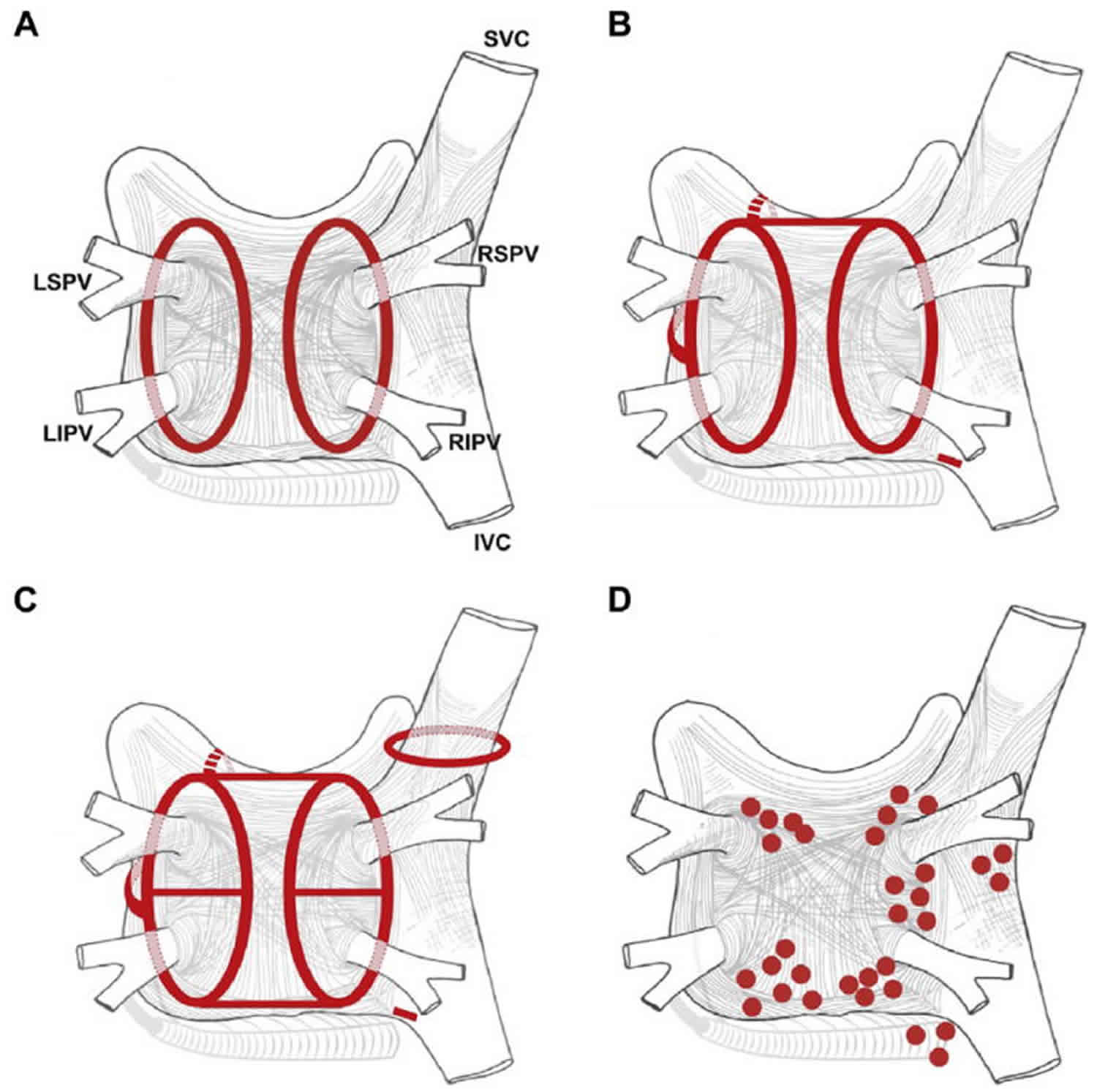
It is generally accepted that development of atrial fibrillation requires both a trigger and a susceptible substrate. Figures 5 and and 6 summarize the many mechanisms of atrial fibrillation. Over time, atrial fibrillation progresses from a trigger-driven to a more substrate-mediated arrhythmia due to structural remodeling of the atria 25. Ablative therapy is therefore aimed at either eliminating the trigger initiating atrial fibrillation or modifying the arrhythmogenic substrate. The most commonly employed ablation strategy consists of electrical isolation of the pulmonary veins by creation of circumferential lesions around the right and the left pulmonary vein 26. A schematic overview of common lesion sets created during an atrial fibrillation ablation procedure is shown in Figure 7. The effects of these lesions have been attributed to isolation of atrial fibrillation triggering pulmonary vein foci, elimination of non-pulmonary vein triggering foci, and/or as the result of modification of the arrhythmogenic substrate 27. The latter might include interruption of crucial pathways of conduction between pulmonary-atrial junctions, which play a role in sustenance of atrial fibrillation, reduction of the amount of mass available for the number of simultaneously circulating wavelets (atrial debulking), or partial vagal denervation by interruption of vagal stimulation from the autonomic ganglia 28. Adjuvant substrate modification is targeted at patient-specific atrial fibrillation sources in the right atrium and left atrium 29. Atrial fibrillation recurrences after an initially successful atrial fibrillation ablation procedure are typically associated with pulmonary vein isolation reconnection. A more recent strategy for atrial fibrillation ablation involves mapping and ablation of rotational activity 30.
Figure 5. Atrial fibrillation mechanisms (various hypotheses and proposals concerning the mechanisms of atrial fibrillation)
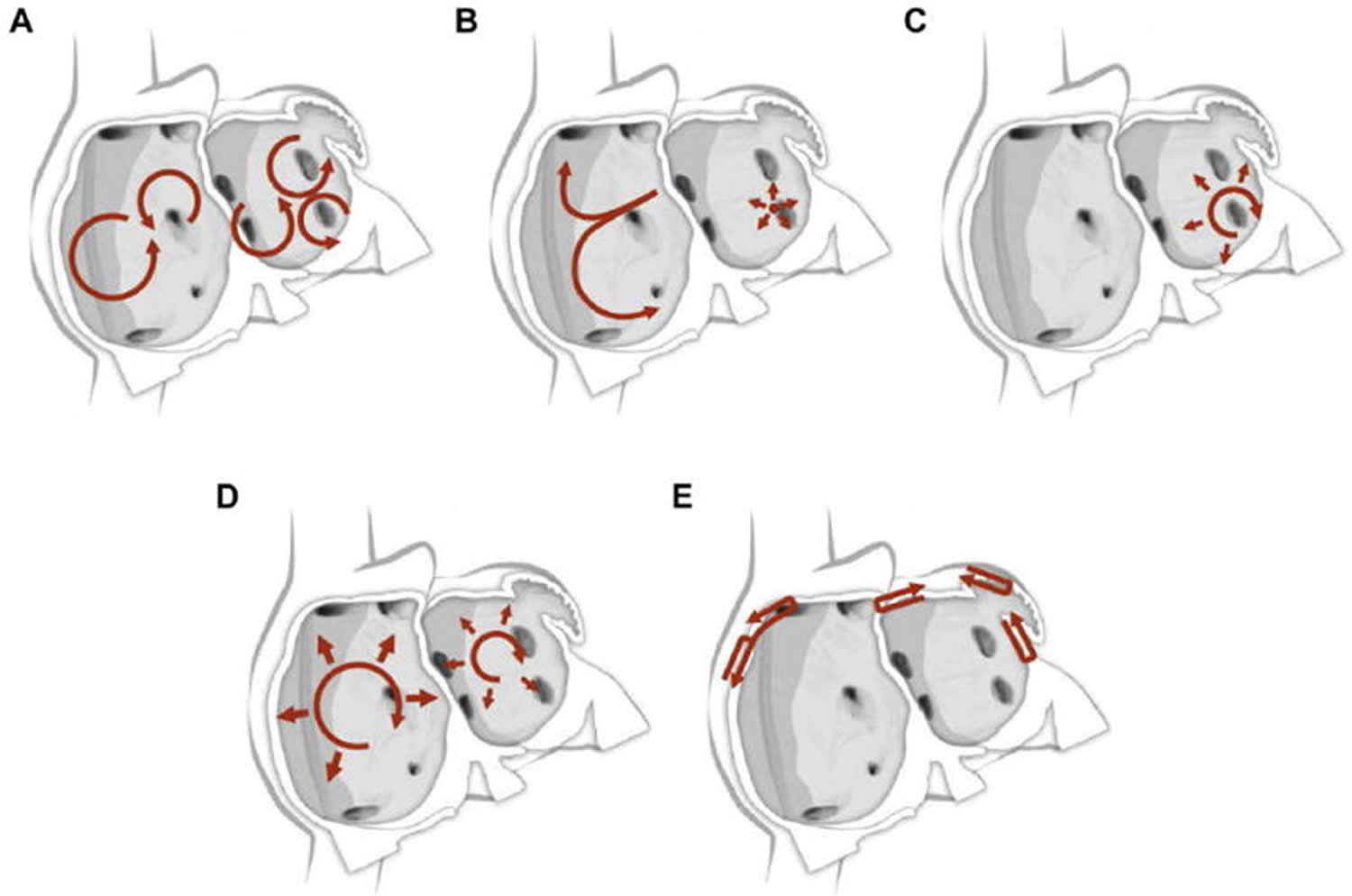
Footnote: Schematic drawing showing various hypotheses and proposals concerning the mechanisms of atrial fibrillation. (A) Multiple wavelets hypothesis. (B) Rapidly discharging automatic foci. (C) Single reentrant circuit with fibrillatory conduction. (D) Functional reentry resulting from rotors or spiral waves. (E) AF maintenance resulting from dissociation between epicardial and endocardial layers, with mutual interaction producing multiplying activity that maintains the arrhythmia.
Figure 6. Structure and mechanisms of atrial fibrillation
Footnote: (A) Schematic drawing of the left and right atria as viewed from the posterior perspective. The extension of muscular fibers onto the pulmonary veins can be appreciated. Shown in yellow are the five major left atrial autonomic ganglionic plexi (GP) and axons (superior left ganglionic plexus, inferior left ganglionic plexus, anterior right ganglionic plexus, inferior right ganglionic plexus, and ligament of Marshall). Shown in blue is the coronary sinus, which is enveloped by muscular fibers that have connections to the atria. Also shown in blue is the vein and ligament of Marshall, which travels from the coronary sinus to the region between the left superior pulmonary vein and the left atrial appendage. (B) The large and small reentrant wavelets that play a role in initiating and sustaining atrial fibrillation. (C) The common locations of pulmonary vein (red) and also the common sites of origin of non-pulmonary vein triggers (shown in green). (D) Composite of the anatomic and arrhythmic mechanisms of atrial fibrillation.
Figure 7. Structure and mechanisms of atrial fibrillation
Footnote: Schematic of common lesion sets employed in atrial fibrillation ablation. (A) The circumferential ablation lesions that are created in a circumferential fashion around the right and the left pulmonary veins. The primary endpoint of this ablation strategy is the electrical isolation of the pulmonary vein musculature. (B) Some of the most common sites of linear ablation lesions. These include a “roof line” connecting the lesions encircling the left and/or right pulmonary veins, a “mitral isthmus” line connecting the mitral valve and the lesion encircling the left pulmonary veins at the end of the left inferior pulmonary vein, and an anterior linear lesion connecting either the “roof line” or the left or right circumferential lesion to the mitral annulus anteriorly. A linear lesion created at the cavotricuspid isthmus is also shown. This lesion is generally placed in patients who have experienced cavotricuspid isthmus-dependent atrial flutter clinically or have it induced during electrophysiology (EP) testing. (C) Similar to 7B, but also shows additional linear ablation lesions between the superior and inferior pulmonary veins resulting in a figure of eight lesion sets as well as a posterior inferior line allowing for electrical isolation of the posterior left atrial wall. An encircling lesion of the superior vena cava (SVC) directed at electrical isolation of the superior vena cava is also shown. Superior vena cava isolation is performed if focal firing from the superior vena cava (SVC) can be demonstrated. A subset of operators empirically isolates the superior vena cava. (D) Representative sites for ablation when targeting rotational activity or complex fractionated atrial electrograms are targeted.
[Source 4 ]Catheter and surgical ablation of atrial fibrillation are highly complex procedures, and a careful assessment of the benefit and risk must be considered for each patient. Second, these indications stratify patients based only on the type of atrial fibrillation and whether the procedure is being performed prior to or following a trial of one or more Class I or III antiarrhythmic medications. There are many other additional clinical and imaging-based variables that can be used to further define the efficacy and risk of ablation in a given patient. Some of the variables that can be used to define patients in whom a lower success rate or a higher complication rate can be expected include the presence of concomitant heart disease, obesity, sleep apnea, LA size, patient age and frailty, as well as the duration of time the patient has been in continuous atrial fibrillation. Each of these variables needs to be considered when discussing the risks and benefits of atrial fibrillation ablation with a particular patient. Third, it is important to consider patient preference and values. Some patients are reluctant to consider a major procedure or surgery and have a strong preference for a pharmacological approach. In these patients, trials of antiarrhythmic agents including amiodarone might be preferred to catheter ablation. On the other hand, some patients prefer a nonpharmacological approach. Fourth, it is important to recognize that some patients early in the course of their atrial fibrillation journey might have only infrequent episodes for many years and/or could have atrial fibrillation that is responsive to well-tolerated antiarrhythmic drug therapy. And finally, it is important to bear in mind that a decision to perform catheter or surgical atrial fibrillation ablation should only be made after a patient carefully considers the risks, benefits, and alternatives to the procedure.
Table 1. Indications for catheter (A and B) and surgical (C, D, and E) ablation of atrial fibrillation
| Recommendation | |
| Indications for catheter ablation of atrial fibrillation | |
| A. Indications for catheter ablation of atrial fibrillation | |
| Symptomatic AF refractory or intolerant to at least one Class I or III antiarrhythmic medication | Paroxysmal: Catheter ablation is recommended. |
| Persistent: Catheter ablation is reasonable. | |
| Long-standing persistent: Catheter ablation may be considered. | |
| Symptomatic AF prior to initiation of antiarrhythmic therapy with a Class I or III antiarrhythmic medication | Paroxysmal: Catheter ablation is reasonable. |
| Persistent: Catheter ablation is reasonable. | |
| Long-standing persistent: Catheter ablation may be considered. | |
| B. Indications for catheter atrial fibrillation ablation in populations of patients not well represented in clinical trials | |
| Congestive heart failure | It is reasonable to use similar indications for AF ablation in selected patients with heart failure as in patients without heart failure. |
| Older patients (>75 years of age) | It is reasonable to use similar indications for AF ablation in selected older patients with AF as in younger patients. |
| Hypertrophic cardiomyopathy | It is reasonable to use similar indications for AF ablation in selected patients with HCM as in patients without HCM. |
| Young patients (<45 years of age) | It is reasonable to use similar indications for AF ablation in young patients with AF (<45 years of age) as in older patients. |
| Tachy-brady syndrome | It is reasonable to offer AF ablation as an alternative to pacemaker implantation in patients with tachy-brady syndrome. |
| Athletes with AF | It is reasonable to offer high-level athletes AF as first-line therapy due to the negative effects of medications on athletic performance. |
| Asymptomatic AF** | Paroxysmal: Catheter ablation may be considered in select patients.** |
| Persistent: Catheter ablation may be considered in select patients. | |
| Indications for surgical ablation of atrial fibrillation | |
| C. Indications for concomitant open (such as mitral valve) surgical ablation of atrial fibrillation | |
| Symptomatic AF refractory or intolerant to at least one Class I or III antiarrhythmic medication | Paroxysmal: Surgical ablation is recommended. |
| Persistent: Surgical ablation is recommended. | |
| Long-standing persistent: Surgical ablation is recommended. | |
| Symptomatic AF prior to initiation of antiarrhythmic therapy with a Class I or III antiarrhythmic medication | Paroxysmal: Surgical ablation is recommended. |
| Persistent: Surgical ablation is recommended. | |
| Long-standing persistent: Surgical ablation is recommended. | |
| D. Indications for concomitant closed (such as CABG and AVR) surgical ablation of atrial fibrillation | |
| Symptomatic AF refractory or intolerant to at least one Class I or III antiarrhythmic medication | Paroxysmal: Surgical ablation is recommended. |
| Persistent: Surgical ablation is recommended. | |
| Long-standing persistent: Surgical ablation is recommended. | |
| Symptomatic AF prior to initiation of antiarrhythmic therapy with a Class I or III antiarrhythmic medication | Paroxysmal: Surgical ablation is reasonable. |
| Persistent: Surgical ablation is reasonable. | |
| Long-standing persistent: Surgical ablation is reasonable. | |
| E. Indications for stand-alone and hybrid surgical ablation of atrial fibrillation | |
| Symptomatic AF refractory or intolerant to at least one Class I or III antiarrhythmic medication | Paroxysmal: Stand-alone surgical ablation can be considered for patients who have failed one or more attempts at catheter ablation and also for those who are intolerant or refractory to antiarrhythmic drug therapy and prefer a surgical approach, after review of the relative safety and efficacy of catheter ablation versus a stand-alone surgical approach. |
| Persistent: Stand-alone surgical ablation is reasonable for patients who have failed one or more attempts at catheter ablation and also for those patients who prefer a surgical approach after review of the relative safety and efficacy of catheter ablation versus a stand-alone surgical approach. | |
| Long-standing persistent: Stand-alone surgical ablation is reasonable for patients who have failed one or more attempts at catheter ablation and also for those patients who prefer a surgical approach after review of the relative safety and efficacy of catheter ablation versus a sstand-alone surgical approach. | |
| It might be reasonable to apply the indications for stand-alone surgical ablation described above to patients being considered for hybrid surgical AF ablation. | |
Footnote: **A decision to perform AF ablation in an asymptomatic patient requires additional discussion with the patient because the potential benefits of the procedure for the patient without symptoms are uncertain.
Abbreviations: AF = atrial fibrillation; LOE = Level of Evidence; HCM = hypertrophic cardiomyopathy.
[Source 4 ]Catheter ablation of atrial fibrillation as first-line therapy
The role of catheter ablation as first-line therapy, prior to a trial of a Class I or III antiarrhythmic agent, is an appropriate indication for catheter ablation of atrial fibrillation in patients with symptomatic paroxysmal or persistent atrial fibrillation. These recommendations are consistent with the indications for atrial fibrillation ablation recommended in the recently published 2016 ESC guidelines for the management of atrial fibrillation as well as in the 2014 ACC/AHA/HRS Guidelines for atrial fibrillation management 4. For patients with paroxysmal symptomatic atrial fibrillation who have not failed drug therapy, catheter ablation has a Class IIa, LOE B-R indication; for patients with persistent symptomatic atrial fibrillation who have not failed drug therapy, catheter ablation has a Class IIa, LOE C-EO indication; and for patients with longstanding persistent atrial fibrillation who have not failed drug therapy, catheter ablation has a Class IIb, LOE C-EO indication.
Class and Level of Evidence (LOE). A Class I recommendation means that the benefits of the atrial fibrillation ablation procedure markedly exceed the risks, and that atrial fibrillation ablation should be performed; a Class IIa recommendation means that the benefits of an atrial fibrillation ablation procedure exceed the risks, and that it is reasonable to perform atrial fibrillation ablation; a Class IIb recommendation means that the benefit of atrial fibrillation ablation is greater or equal to the risks, and that atrial fibrillation ablation may be considered; and a Class III recommendation means that atrial fibrillation ablation is of no proven benefit and is not recommended.
Level A if the data were derived from high-quality evidence from more than one randomized clinical trial, meta-analyses of high-quality randomized clinical trials, or one or more randomized clinical trials corroborated by high-quality registry studies. The writing group ranked available evidence as Level B-R when there was moderate-quality evidence from one or more randomized clinical trials, or meta-analyses of moderate-quality randomized clinical trials. Level B-NR was used to denote moderate-quality evidence from one or more well-designed, well-executed nonrandomized studies, observational studies, or registry studies. This designation was also used to denote moderate-quality evidence from meta-analyses of such studies. Evidence was ranked as Level C-LD when the primary source of the recommendation was randomized or nonrandomized observational or registry studies with limitations of design or execution, meta-analyses of such studies, or physiological or mechanistic studies of human subjects. Level C-EO was defined as expert opinion based on the clinical experience of the writing group.
There have been three prospective randomized clinical trials that have examined the relative efficacy and safety of first-line atrial fibrillation ablation vs pharmacological therapy 31, 32, 33. The outcomes of these three trials have recently been summarized in a meta-analysis 34. A total of 491 young, generally healthy patients with predominantly paroxysmal atrial fibrillation were randomized to atrial fibrillation ablation vs pharmacological therapy. Catheter ablation of atrial fibrillation was associated with a significantly higher freedom from atrial fibrillation recurrence, as compared with drug therapy. The difference in the rate of symptomatic atrial fibrillation recurrences was not significant. There was one procedure-related death due to a stroke in the ablation arm. The main major complication was cardiac tamponade which occurred in 1.7% of patients in the ablation arm. Taken as a whole, these findings provide evidence to support the role of atrial fibrillation ablation as first-line therapy.
It is important to recognize that there are certain situations in which first-line atrial fibrillation ablation is a preferred treatment option. For example, first-line atrial fibrillation ablation is often recommended in patients with Patrial fibrillation who have symptomatic pauses (tachybrady syndrome). For these patients, initiation of pharmacological therapy would be inappropriate in the absence of a permanent pacemaker. Over the past 15 years, a body of literature has been published demonstrating that catheter ablation is effective, without concomitant need of a permanent pacemaker in the great majority of patients with tachy-brady syndrome 35. Based on this body of literature and the cumulative experience of the writing group, we believe that it is appropriate to consider atrial fibrillation ablation as first-line therapy in this clinical situation. Another specific population of patients in whom first-line atrial fibrillation ablation is often recommended as an initial approach are high-level competitive athletes with paroxysmal or persistent atrial fibrillation. These individuals often are strongly against taking a medication, which could potentially reduce their peak heart rate and/or impair cardiac function; they often have a marked resting bradycardia. Several studies have reported favorable outcomes of atrial fibrillation ablation in this subgroup of patients 36.
Pulmonary vein isolation
Pulmonary vein isolation is a procedure used to stop abnormal electrical signals in your heart that cause heart rhythm problems. The goal of pulmonary vein isolation is to cause scar tissue near where the pulmonary veins connect to your heart. This limits or stops the chaotic electrical signals from reaching the upper chambers of your heart.
Pulmonary vein isolation is a type of cardiac ablation. Cardiac ablation works by scarring or destroying tissue in your heart that triggers an abnormal heart rhythm. In some cases, cardiac ablation prevents abnormal electrical signals from traveling through your heart and, thus, stops the heart rhythm problem.
In pulmonary vein isolation, the procedure creates scar tissue in the part of the left upper chamber of your heart where each of your four pulmonary veins connects. Your pulmonary veins bring oxygen-rich blood from your lungs to your heart. Pulmonary vein isolation can reduce the signs and symptoms of atrial fibrillation, which affects the upper chambers of the heart.
Pulmonary vein isolation is used to reduce signs and symptoms and improve quality of life for people living with atrial fibrillation.
Pulmonary vein isolation usually isn’t your fist treatment option. Your doctor may recommend that you try to control your atrial fibrillation with other treatments first.
Pulmonary vein isolation risks
Pulmonary vein isolation carries a risk of complications, including:
- Bleeding or infection at the site where your catheter was inserted
- Damage to your blood vessels where the catheter may have scraped as it traveled to your heart
- Puncture of your heart
- Damage to your heart valves
- Damage to your heart’s electrical system, which could worsen your arrhythmia and require a pacemaker to correct
- Blood clots in your legs or lungs (venous thromboembolism)
- Stroke or heart attack
- Narrowing of the veins that carry blood between your lungs and heart (pulmonary vein stenosis)
- Death in rare cases
Discuss the risks and benefits of cardiac ablation with your doctor to understand if this procedure is right for you.
How you prepare for a pulmonary vein isolation
Your doctor will evaluate you and may order several tests to evaluate your atrial fibrillation. Your doctor will discuss with you the risks and benefits of pulmonary vein isolation.
You’ll need to stop eating and drinking the night before your procedure. If you take any medications, ask your doctor if you should continue taking them before your procedure.
Your doctor will let you know if you need to follow any other special instructions before or after your procedure. In some cases, you’ll be instructed to stop taking medications to treat a heart arrhythmia several days before your procedure.
If you have an implanted heart device, such as a pacemaker or implantable cardioverter-defibrillator, talk to your doctor to see if you need to take any special precautions.
During pulmonary vein isolation
Pulmonary vein isolation is performed in the hospital. Before your procedure begins, a specialist will insert an intravenous line into your forearm or hand, and you’ll be given a sedative to help you relax. In some situations, general anesthesia may be used instead to place you in a sleep-like state.
After your sedative takes effect, your doctor or another specialist will numb a small area near a vein in your groin, neck or shoulder. Your doctor will insert a needle into the vein and place a tube (sheath) through the needle.
Your doctor will thread catheters through the sheath and guide them to several places within your heart. Your doctor may inject dye into the catheter, which helps your care team see your blood vessels and heart using X-ray imaging. The catheters have electrodes at the tips that can be used to send electrical impulses to your heart and record your heart’s electrical activity.
This process of using imaging and other tests to determine what’s causing your arrhythmia is called an electrophysiology (EP) study.
Catheters are moved from the upper right chamber of your heart to the upper left chamber of your heart where your pulmonary veins connect. Heat (radiofrequency ablation) or cold (cryoablation) energy will travel through the catheter tip to the target area and create a scar or destroy the tissue. In most cases, each of the four pulmonary veins is treated during pulmonary vein isolation.
In some cases, ablation blocks the electrical signals traveling through your heart to stop the atrial fibrillation and allow signals to travel over a normal pathway instead.
Pulmonary vein isolation usually takes three to six hours to complete, but complicated procedures may take longer.
During the procedure, it’s possible you’ll feel some minor discomfort when the dye is injected in your catheter or when energy is run through the catheter tips. If you experience any type of severe pain or shortness of breath, let your doctor know.
After pulmonary vein isolation
Following your procedure, you’ll be moved to a recovery area to rest quietly for four to six hours to prevent bleeding at your catheter site. Your heartbeat and blood pressure will be monitored continuously to check for complications of the procedure.
Depending on your condition, you may be able to go home the same day as your procedure, or you may need to stay in the hospital. If you go home the same day, plan to have someone else drive you home after your procedure.
You may feel a little sore after your procedure, but the soreness shouldn’t last more than a week. You’ll usually be able to return to your normal activities within a few days after having pulmonary vein isolation.
Pulmonary vein isolation results
Although pulmonary vein isolation can be successful, some people need repeat procedures.
Pulmonary vein isolation may reduce the signs and symptoms of atrial fibrillation and improve your quality of life. However, it’s not been shown to reduce your risk of a stroke, so your doctor may recommend that you continue blood-thinning medications.
- Porres DV, Morenza OP, Pallisa E et-al. Learning from the pulmonary veins. Radiographics. 2013;33 (4): 999-1022. https://pubs.rsna.org/doi/pdf/10.1148/rg.334125043[↩]
- Kato R, et al. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: lessons learned by use of magnetic resonance imaging. Circulation. 2003;107(15):2004–2010[↩]
- Jongbloed MR, et al. Noninvasive visualization of the cardiac venous system using multislice computed tomography. J Am Coll Cardiol. 2005;45(5):749–753.[↩]
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6019327[↩][↩][↩][↩][↩]
- Nathan H, Eliakim M. The junction between the left atrium and the pulmonary veins. An anatomic study of human hearts. Circulation. 1966;34(3):412–422.[↩]
- Weiss C, et al. Impact of the distribution and structure of myocardium in the pulmonary veins for radiofrequency ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2002;25(9):1352–1356.[↩]
- Wang K, et al. Architecture of atrial musculature in humans. Br Heart J. 1995;73(6):559–565.[↩]
- Anne W, et al. Matrix metalloproteinases and atrial remodeling in patients with mitral valve disease and atrial fibrillation. Cardiovasc Res. 2005;67(4):655–666.[↩]
- Pashakhanloo F, et al. Myofiber Architecture of the Human Atria as Revealed by Submillimeter Diffusion Tensor Imaging. Circ Arrhythm Electrophysiol. 2016;9(4):e004133.[↩]
- Hansen BJ, et al. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J. 2015;36(35):2390–2401.[↩]
- Kim DT, et al. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol. 2000;36(4):1324–1327[↩]
- Han S, et al. Electrophysiological characteristics of the Marshall bundle in humans. Heart Rhythm. 2010;7(6):786–793[↩]
- Porres DV, Morenza OP, Pallisa E et-al. Learning from the pulmonary veins. Radiographics. 2013;33 (4): 999-1022. doi:10.1148/rg.334125043[↩]
- Burri E, Duwe J, Kull C et-al. Pulmonary vein thrombosis after lower lobectomy of the left lung. J Cardiovasc Surg (Torino). 2006;47 (5): 609-12.[↩]
- Genta PR, Ho N, Beyruti R, Takagaki TY, Terra-Filho M. Pulmonary vein thrombosis after bilobectomy and development of collateral circulation. Thorax. 2003;58(6):550-1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1746717/pdf/v058p00550.pdf[↩][↩]
- Zhang M, Wu QC, Zhang C et-al. Isolated unilateral pulmonary vein atresia in adult patients: a case report and literature review. Heart Surg Forum. 2010;13 (6): E370-2. doi:10.1532/HSF98.20101078[↩]
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66.[↩][↩]
- Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12(10):1360–1420.[↩]
- Calkins H, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm. 2012;9(4):632–696.e21[↩]
- Chen SA, Hsieh MH, Tai CT, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999;100:1879-86[↩]
- Oral H, Scharf C, Chugh A, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation 2003;108:2355-60.[↩]
- Zhang B, Zhen Y, Tao A, et al. Efficacy of selective arrhythmogenic pulmonary veins isolation versus empirical all pulmonary veins isolation for atrial fibrillation: a meta-analysis of randomized and observational studies. J Interv Card Electrophysiol 2014;39:233-40[↩]
- Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation 2010;122:2368-77.[↩]
- Chao TF, Tsao HM, Lin YJ, et al. Clinical outcome of catheter ablation in patients with nonparoxysmal atrial fibrillation: results of 3-year follow-up. Circ Arrhythm Electrophysiol 2012;5:514-20.[↩]
- Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54(2):230–246.[↩]
- Haissaguerre M, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–666[↩]
- Kottkamp H, et al. Time courses and quantitative analysis of atrial fibrillation episode number and duration after circular plus linear left atrial lesions: trigger elimination or substrate modification: early or delayed cure? J Am Coll Cardiol. 2004;44(4):869–877[↩]
- Pappone C, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109(3):327–334.[↩]
- Narayan SM, et al. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: on-treatment analysis of the CONFIRM trial (Conventional ablation for AF with or without focal impulse and rotor modulation) J Am Coll Cardiol. 2013;62(2):138–147[↩]
- Haissaguerre M, et al. Driver domains in persistent atrial fibrillation. Circulation. 2014;130(7):530–538.[↩]
- Wazni OM, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293(21):2634–2640.[↩]
- Cosedis Nielsen J, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367(17):1587–1595.[↩]
- Morillo CA, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311(7):692–700[↩]
- Hakalahti A, et al. Radiofrequency ablation vs antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace. 2015;17(3):370–378.[↩]
- Chen YW, et al. Pacing or ablation: which is better for paroxysmal atrial fibrillation-related tachycardia-bradycardia syndrome? Pacing Clin Electrophysiol. 2014;37(4):403–411[↩]
- Pathak RK, et al. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation: The CARDIO-FIT Study. J Am Coll Cardiol. 2015;66(9):985–996.[↩]