Contents
- What is buprenorphine
- How does buprenorphine work?
- What is buprenorphine used for?
- Buprenorphine special precautions
- Buprenorphine administration
- Buprenorphine and Naloxone for Opioid Dependence
- Buprenorphine for pain
- Buprenorphine vs Methadone
- Buprenorphine dosage
- Adult dose for opiate dependence – maintenance
- Adult dose for pain
- Adult dose for chronic pain
- Adult dose for opiate dependence – induction
- Pediatric dose for pain
- Pediatric dose for opiate dependence – maintenance
- Pediatric dose for opiate dependence – induction
- Renal dose adjustments
- Liver dose adjustments
- Managing Missed Doses
- Buprenorphine side effects
What is buprenorphine
Buprenorphine is an orally available, semisynthetic opioid analgesic used in medication-assisted treatment to help people reduce or quit their use of heroin or other opiates, such as pain relievers like morphine. Buprenorphine is a semisynthetic opioid that is 25 to 50 times more potent than morphine and has been used as an analgesic as well as therapy of opioid addiction. Buprenorphine is a partial µ (mu)-opioid receptor agonist and a (kappa) κ-receptor antagonist accounting for its benefit for opioid deterrence. Buprenorphine competes with morphine and heroin for the µ (mu) receptor, but is only a partial agonist and has a “ceiling” effect. Buprenorphine and combination of buprenorphine/naloxone (Suboxone) was approved for treatment of opioid addiction in October 2002 by the U.S. Food and Drug Administration (FDA) and is a schedule 3 controlled substance, which means that it has some potential for moderate or low physical dependence or high psychological dependence 1. Current indications include treatment of moderate to severe pain (in low doses ~200 µg) and opiate addiction (in higher doses 2 to 16 mg daily).
Unlike methadone treatment, which must be performed in a highly structured clinic, buprenorphine is the first medication to treat opioid dependency that is permitted to be prescribed or dispensed in physician offices, significantly increasing treatment access. Under the Drug Addiction Treatment Act of 2016 2, qualified U.S. physicians can prescribe schedule III, IV, or V “narcotic” medications that are approved by the US Food and Drug Administration (FDA) for patients with opioid addiction. Physicians can offer buprenorphine for opioid dependency in various settings, including in an office, community hospital, health department, or correctional facility.
Buprenorphine treatment decreases withdrawal and craving. Patients who receive buprenorphine are less likely to overdose, die, use illicit opioids, spread hepatitis C or HIV and have fewer injection drug use complications and contacts with the criminal justice system 3.
Buprenorphine offers several benefits to those with opioid dependency and to others for whom treatment in a methadone clinic is not preferred or is less convenient. The FDA has approved the following buprenorphine products:
- Bunavail (buprenorphine and naloxone) buccal film
- Suboxone (buprenorphine and naloxone) film
- Zubsolv (buprenorphine and naloxone) sublingual tablets
- Buprenorphine-containing transmucosal products for opioid dependency
Buprenorphine is available as 2 and 8 mg tablets for sublingual administration under the brand name Subutex, and in 1 mL ampules of 0.3 mg/mL for intravenous (iv) or intramuscular (im) injection under the brand name Buprenex. For opioid addiction, the usually recommended dose is 12 to 16 mg in a single daily dose. Buprenorphine is also available in fixed combination with naloxone for sublingual administration generically and under the brand name Suboxone. Naloxone is not absorbed orally, but provides full opioid antagonism if the combination is administer intravenously, as might occur with intentional abuse. Finally, parenteral forms of buprenorphine are used for moderate to severe pain and administered IV (intravenous) or IM (intramuscular), the typical dose being 0.3 mg every 6 hours as needed.
Common side effects of buprenorphine include headache, dizziness, fatigue, sedation, dry mouth, urinary retention, diaphoresis and withdrawal symptoms.
Buprenorphine’s side effects are similar to those of opioids and can include:
- Nausea, vomiting, and constipation
- Muscle aches and cramps
- Cravings
- Inability to sleep
- Distress and irritability
- Fever
How does buprenorphine work?
Buprenorphine has unique pharmacological properties that help:
- Lower the potential for misuse
- Diminish the effects of physical dependency to opioids, such as withdrawal symptoms and cravings
- Increase safety in cases of overdose
Buprenorphine is an opioid partial agonist at the mu receptor (µ-opioid receptor), meaning that it only partially activates opiate receptors. This means that, like opioids, it produces effects such as euphoria or respiratory depression. With buprenorphine, however, these effects are weaker than those of full drugs such as heroin and methadone. Buprenorphine’s opioid effects increase with each dose until at moderate doses they level off, even with further dose increases. This “ceiling effect” lowers the risk of misuse, dependency, and side effects. Also, because of buprenorphine’s long-acting agent, many patients may not have to take it every day.
Buprenorphine is also a weak kappa receptor antagonist and delta receptor agonist. Buprenorphine is a potent analgesic that acts on the central nervous system (CNS). The partial agonism at the mu receptor is a unique quality to buprenorphine and the feature that gives it its many unique properties, specifically its analgesic effects plateau at higher doses, and then its effects become antagonistic. Buprenorphine exhibits ceiling effects on respiratory depression, which means that it is safer than methadone for agonist substitution treatment in addiction.
Buprenorphine has high-affinity binding to the mu-opioid receptors and slow-dissociation kinetics. In this way, it differs from other full-opioid agonists like morphine and fentanyl. This allows withdrawal symptoms to be milder and less uncomfortable for the patient.
When administered orally buprenorphine has poor bioavailability because of the first pass effect. The majority of the drug is broken down by the liver and intestine. Sublingual administration is the preferred route of administration. The absorption is fast, and this route also avoids the first pass effect. Once the tablet is placed under the tongue, it has a slow onset of action with the peak effect occurring at 3 to 4 hours after administration.
Once in the body, buprenorphine is broken down by the cytochrome CYP 34A enzymes to an active metabolite (norbuprenorphine) with weak intrinsic activity. The average half-life of buprenorphine is about 38 hours (25 to 70 hours) following sublingual administration. Strong inhibition of the 3A4 enzyme by drugs (such as ketoconazole or protease inhibitors) may cause increased levels of buprenorphine, while inducers of this enzyme (such as carbamazepine, topiramate, phenytoin, or barbiturates) may cause lower levels.
The majority of the drug and the metabolite are excreted in the feces, and the kidneys excrete less than 20%. Because of the slow onset of action and prolonged duration of action, the drug is used to treat opioid dependence. It may be prescribed on alternate days once the patient has stabilized on the daily dose.
What is buprenorphine half life?
Buprenorphine average half-life is about 38 hours (25 to 70 hours) following sublingual administration. Strong inhibition of the 3A4 enzyme by drugs (such as ketoconazole or protease inhibitors) may cause increased levels of buprenorphine, while inducers of this enzyme (such as carbamazepine, topiramate, phenytoin, or barbiturates) may cause lower levels.
The majority of the drug and the metabolite are excreted in the feces, and the kidneys excrete less than 20%. Because of the slow onset of action and prolonged duration of action, the drug is used to treat opioid dependence. It may be prescribed on alternate days once the patient has stabilized on the daily dose.
What is buprenorphine used for?
Buprenorphine and the combination of buprenorphine and naloxone are used to treat opioid dependence (addiction to opioid drugs, including heroin and narcotic painkillers). Buprenorphine is in a class of medications called opioid partial agonist-antagonists and naloxone is in a class of medications called opioid antagonists. Buprenorphine alone and the combination of buprenorphine and naloxone work to prevent withdrawal symptoms when someone stops taking opioid drugs by producing similar effects to these drugs.
Buprenorphine for opiod addiction indications:
- For managing opioid-dependent patients who have a contraindication to methadone
- No available methadone facilities or healthcare providers or there is a long wait list of more than 3 months to join a methadone clinic
- For opioid-dependent patients with intolerance to or have failed methadone treatment
- Other individuals who may benefit from buprenorphine are those with a short history of opioid dependence and/or have lower needs for opioid agonists
Buprenorphine treatment happens in three phases:
- The Induction Phase is the medically monitored startup of buprenorphine treatment performed in a qualified physician’s office or certified OTP using approved buprenorphine products. The medication is administered when a person with an opioid dependency has abstained from using opioids for 12 to 24 hours and is in the early stages of opioid withdrawal. It is important to note that buprenorphine can bring on acute withdrawal for patents who are not in the early stages of withdrawal and who have other opioids in their bloodstream.
- The Stabilization Phase begins after a patient has discontinued or greatly reduced their misuse of the problem drug, no longer has cravings, and experiences few, if any, side effects. The buprenorphine dose may need to be adjusted during this phase. Because of the long-acting agent of buprenorphine, once patients have been stabilized, they can sometimes switch to alternate-day dosing instead of dosing every day.
- The Maintenance Phase occurs when a patient is doing well on a steady dose of buprenorphine. The length of time of the maintenance phase is tailored to each patient and could be indefinite. Once an individual is stabilized, an alternative approach would be to go into a medically supervised withdrawal, which makes the transition from a physically dependent state smoother. People then can engage in further rehabilitation—with or without medication-assisted treatment—to prevent a possible relapse.
Buprenorphine is also used for the management of severe pain in people who are expected to need pain medication around the clock for a long time and who cannot be treated with other medications.
Because of buprenorphine’s opioid effects, it can be misused, particularly by people who do not have an opioid dependency. Naloxone is added to buprenorphine to decrease the likelihood of diversion and misuse of the combination drug product. When these products are taken as sublingual tablets, buprenorphine’s opioid effects dominate and naloxone blocks opioid withdrawals. If the sublingual tablets are crushed and injected, however, the naloxone effect dominates and can bring on opioid withdrawals.
Buprenorphine special precautions
While you are using buprenorphine (Belbuca), you may be told to always have a rescue medication called naloxone available (e.g., home, office). Naloxone is used to reverse the life-threatening effects of an overdose. It works by blocking the effects of opiates to relieve dangerous symptoms caused by high levels of opiates in the blood. You will probably be unable to treat yourself if you experience an opiate overdose. You should make sure that your family members, caregivers, or the people who spend time with you know how to tell if you are experiencing an overdose, how to use naloxone, and what to do until emergency medical help arrives. Your doctor or pharmacist will show you and your family members how to use the medication. Ask your pharmacist for the instructions or visit the manufacturer’s website to get the instructions. If someone sees that you are experiencing symptoms of an overdose, he or she should give you your first dose of naloxone, call your local emergency services number immediately, and stay with you and watch you closely until emergency medical help arrives. Your symptoms may return within a few minutes after you receive naloxone. If your symptoms return, the person should give you another dose of naloxone. Additional doses may be given every 2 to 3 minutes, if symptoms return before medical help arrives.
Pregnant or Breastfeeding Women and Buprenorphine
Limited information exists on the use of buprenorphine in women who are pregnant and have an opioid dependency. But the few case reports available have not demonstrated any significant problems resulting from use of buprenorphine during pregnancy.
Pregnant Patients
It is well-known that in-utero exposure of infants to opioids can result in withdrawal symptoms after birth or what is referred to as the neonatal abstinence syndrome (NAS). Buprenorphine is classified by the FDA as Pregnancy Category C medications for use during pregnancy, which means that the risk of adverse effects on the fetus cannot be ruled out. Buprenorphine does cross the placenta, and use of opioids during pregnancy may result in neonatal withdrawals soon after birth. Symptoms of this may include irritability, apnea, increased tone, tremor, convulsions, or respiratory depression in the neonate. The onset of withdrawal in a neonate whose mother has taken buprenorphine during the pregnancy could be anywhere from the first day of life to the eighth day of life.
There is ample evidence indicating that methadone maintenance does improve maternal and newborn outcomes in pregnant opioid-dependent patients. Similarly, there is evidence suggesting that maintenance with buprenorphine may also improve fetal and maternal and outcomes and that the resultant NAS may be less intense than that observed after methadone. At present buprenorphine is listed as a category C drug in pregnancy; whereas methadone is listed as category B, in pregnant patients. Buprenorphine is classified as category C for use during pregnancy, which means that the risk of adverse effects on the fetus cannot be ruled out. Buprenorphine does cross the placenta, and use of opioids during pregnancy may result in neonatal withdrawals soon after birth. Symptoms of this may include irritability, apnea, increased tone, tremor, convulsions, or respiratory depression in the neonate. The onset of withdrawal in a neonate whose mother has taken buprenorphine during the pregnancy could be anywhere from the first day of life to the eighth day of life 4. Experts suggest that if methadone is not available and the patient needs treatment, then buprenorphine can be started, but the risks and benefit must be explained to the patient. Further, only buprenorphine can be administered during pregnancy and not the buprenorphine/naloxone combination.
Breastfeeding Women
It has been shown that buprenorphine does pass into breast milk, but because it has low bioavailability, it is not well established how much enters the systemic circulation in the breastfed infant. A few case reports indicate that the buprenorphine does not suppress neonatal abstinence syndrome (NAS) and that the syndrome doesn’t develop even after breastfeeding is discontinued. While the manufacturers of buprenorphine advise against the use of buprenorphine in breastfeeding women, the limited evidence to date reveals that buprenorphine appears to be safe and discontinuation may not be necessary.
Elderly
So far there is very little data on the use of buprenorphine in elderly patients. Because geriatric patients do have altered absorption, distribution and metabolism, one should exercise caution when prescribing buprenorphine to this population. Plus, the potential for drug interactions also exists.
HIV Patients
Common comorbidity in HIV patients is an opioid addiction. While highly active antiretroviral therapy (HAART) can prolong life and improve the quality of life, the opioid dependency still needs to be treated. In one study, buprenorphine-treated patients were found to be more compliant with HAART compared to untreated patients, but the drug does not change the effectiveness of HAART.
Since many HAART drugs also affect the liver microsomal enzymes, healthcare workers should closely monitor liver function and drug levels in patients who are prescribed buprenorphine at the same time. In some patients, the dose of buprenorphine may need to be altered.
Hepatitis
Both hepatitis B and C are common comorbid conditions in opioid-dependent patients. Since buprenorphine is broken down in the liver, these patients should have their liver function and drug levels closely monitored. Patients with hepatitis should be cautioned that intravenous use of buprenorphine has been linked to liver damage.
Patients with Pain
Even though buprenorphine is an opioid, it only has partial analgesic activity at the mu opioid receptor. The two reasons why buprenorphine is not used as an analgesic is it is only a partial agonist and has a ceiling effect, and it binds tightly to the mu receptors and will prevent the binding of full agonists at the mu receptor and prevent further analysis. Thus, in patients with pain who are being managed with buprenorphine, the options for analgesic include the use of non-steroidal-antiinflammatory drugs. If the patient is on a low dose of buprenorphine (2 to 8 mg), this can be increased to up to 24 mg every day. Other options include regional anesthesia, nerve blocks or use of anticonvulsants.
Before using buprenorphine:
- The only true contraindication to buprenorphine use is a hypersensitivity reaction to it. It should be used with caution in patients with respiratory depression, gastrointestinal obstruction.
- tell your doctor and pharmacist if you are allergic to buprenorphine, any other medications, or any of the ingredients in buprenorphine buccal films. Ask your pharmacist or check the Medication Guide for a list of the ingredients.
- tell your doctor and pharmacist what other prescription and nonprescription medications, vitamins, and nutritional supplements you are taking or plan to take. Be sure to mention the medications listed in the IMPORTANT WARNING section and any of the following: amiodarone (Cordarone, Nexterone, Pacerone); anticholinergics (atropine, belladonna, benztropine, dicyclomine, diphenhydramine, isopropamide, procyclidine, and scopolamine); butorphanol; carbamazepine (Carbatrol, Tegretol, Teril, others); clarithromycin (Biaxin, in Prevpac); cyclobenzaprine (Amrix); dextromethorphan (found in many cough medications; in Nuedexta); disopyramide (Norpace); diuretics (‘water pills’); dofetilide (Tikosyn); enzalutamide (Xtandi); human immunodeficiency virus (HIV) medications such as atazanavir (Reyataz, in Evotaz), delavirdine (Rescriptor), efavirenz (Sustiva, in Atripla), etravirine (Intelence), indinavir (Crixivan), nelfinavir (Viracept), nevirapine (Viramune), ritonavir (Norvir, in Kaletra), and saquinavir (Invirase); itraconazole (Onmel, Sporanox); ketoconazole (Nizoral); lithium (Lithobid); medications for migraine headaches such as almotriptan (Axert), eletriptan (Relpax), frovatriptan (Frova), naratriptan (Amerge), rizatriptan (Maxalt), sumatriptan (Imitrex, in Treximet), and zolmitriptan (Zomig); mirtazapine (Remeron); nalbuphine; nefazodone; pentazocine (Talwin); phenobarbital; phenytoin (Dilantin, Phenytek); pioglitazone (Actos); procainamide; quinidine (in Nuedexta); rifabutin (Mycobutin); rifampin (Rifadin, Rimactane); 5HT3 serotonin blockers such as alosetron (Lotronex), dolasetron (Anzemet), granisetron (Kytril), ondansetron (Zofran, Zuplenz), or palonosetron (Aloxi); selective serotonin-reuptake inhibitors such as citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac, Sarafem, in Symbyax), fluvoxamine (Luvox), paroxetine (Brisdelle, Prozac, Pexeva), and sertraline (Zoloft); serotonin and norepinephrine reuptake inhibitors such as duloxetine (Cymbalta), desvenlafaxine (Khedezla, Pristiq), milnacipran (Savella), and venlafaxine (Effexor); tramadol (Conzip, Ultram, in Ultracet); trazodone ; sotalol (Betapace, Sotylize, others); or tricyclic antidepressants (‘mood elevators’) such as amitriptyline, clomipramine (Anafranil), desipramine (Norpramin), doxepin (Silenor), imipramine (Tofranil), nortriptyline (Pamelor), protriptyline (Vivactil), and trimipramine (Surmontil). Also tell your doctor or pharmacist if you are taking or receiving the following monoamine oxidase (MAO) inhibitors or if you have stopped taking them within the past two weeks: isocarboxazid (Marplan), linezolid (Zyvox), methylene blue, phenelzine (Nardil), selegiline (Eldepryl, Emsam, Zelapar), or tranylcypromine (Parnate). Your doctor may need to change the doses of your medications or monitor you carefully for side effects. Many other medications may also interact with buprenorphine, so be sure to tell your doctor about all the medications you are taking, even those that do not appear on this list.
- tell your doctor what herbal products you are taking, especially St. John’s wort and tryptophan.
- tell your doctor if you have any of the conditions listed in the IMPORTANT WARNING section or paralytic ileus (condition in which food does not move through the intestines) or a blockage in the stomach or intestines. Your doctor may tell you not to use buprenorphine (Belbuca).
- tell your doctor if you or an immediate family member have or have ever had prolonged QT syndrome (condition that increases the risk of developing an irregular heartbeat that may cause loss of consciousness or sudden death); if you have low levels of potassium or magnesium in the blood; and if you have or have ever had a slow or irregular heartbeat; heart failure; low blood pressure; any condition that causes difficulty urinating; seizures; mouth sores; or gallbladder, pancreas, kidney, thyroid, or liver disease.
- tell your doctor if you are breastfeeding.
- you should know that this medication may decrease fertility in men and women. Talk to your doctor about the risks of using buprenorphine.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are using buprenorphine (Belbuca).
- you should know that buprenorphine (Belbuca) may make you drowsy. Do not drive a car or operate machinery until you know how this medication affects you.
- you should know that buprenorphine (Belbuca) may cause dizziness, lightheadedness, and fainting when you get up too quickly from a lying position. To avoid this problem, get out of bed slowly, resting your feet on the floor for a few minutes before standing up.
- you should know that buprenorphine (Belbuca) may cause constipation. Talk to your doctor about changing your diet or using other medications to prevent or treat constipation while you are using buprenorphine (Belbuca).
Follow up
As with patients treated with methadone, patients prescribed buprenorphine also need close monitoring from a multidisciplinary group of healthcare professionals as part of a comprehensive opioid dependence treatment protocol. In some parts of the country, pharmacists have also taken an active role in the supervision and monitoring of patients treated with buprenorphine. The pharmacist further communicates with the healthcare providers and plays an active role in dispensing take-home doses.
Monitoring
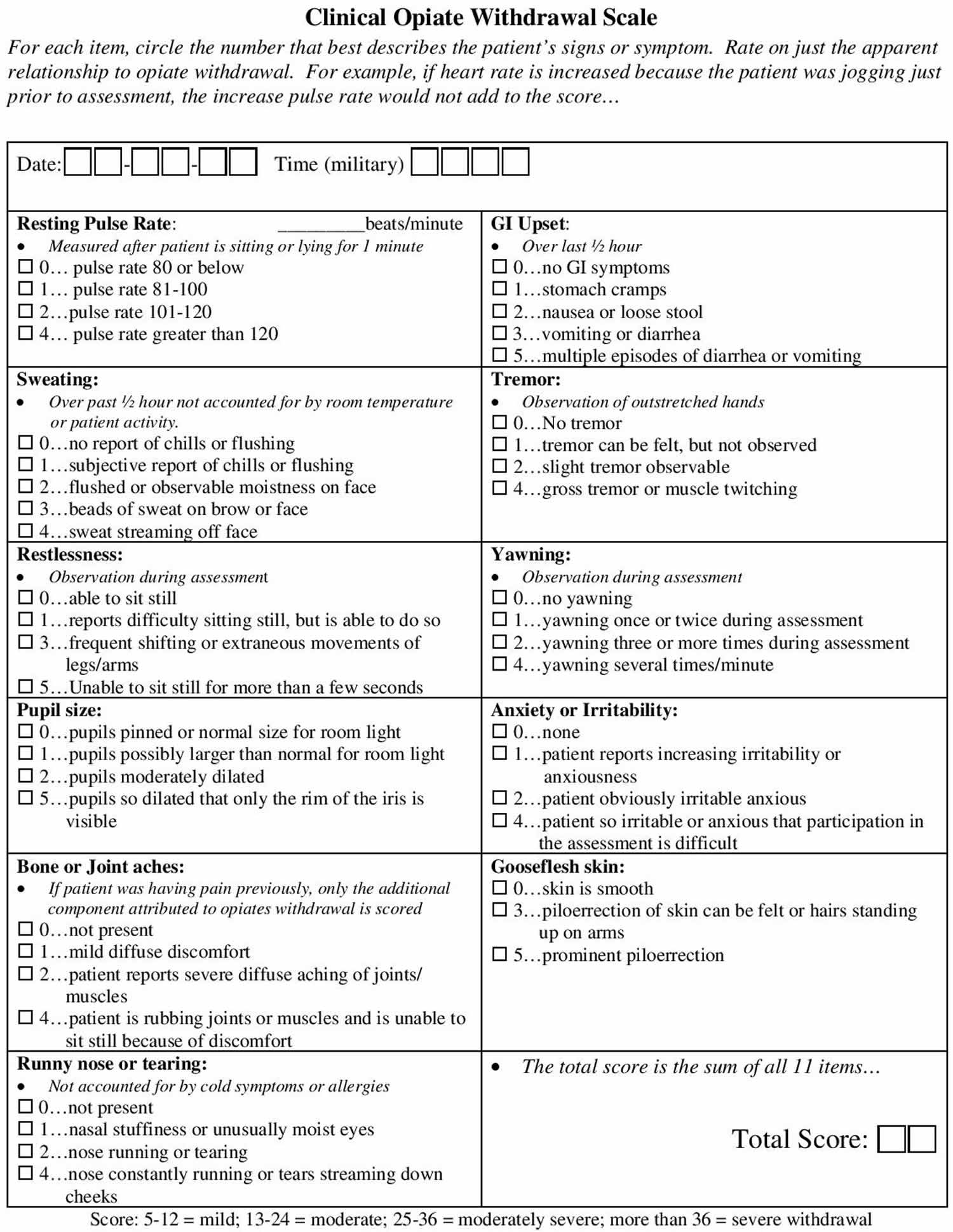
It is important to keep in mind how buprenorphine, a partial agonist, behaves when administered with other opioid receptor agonists. When in the presence of a full agonist, buprenorphine use results in a blockade effect and doesn’t allow the high of the full opioid agonist to occur. If taken too soon after a full agonist, the patient may enter into withdrawals. This is why it is important to perform a simple assessment such as the Clinical Opiate Withdrawal Scale, or COWS (see Figure 1), before giving buprenorphine. It is suggested that the patient is in at least mild to moderate withdrawal, which translates of a score of at least 5 to 24 on the Clinical Opiate Withdrawal Scale. This ensures that a patient who is intoxicated with opioids will not receive a partial agonist that may push them into withdrawal 5.
Before buprenorphine is prescribed one should closely look at all the medications, the patient is taking because serious drug interactions can occur. When buprenorphine is combined with CNS depressants like the benzodiazepines, alcohol, certain antidepressants, antihistamines, hypnotics or sedatives, it can lead to life-threatening respiratory depression, coma, and even death. The patient should be warned not to combine buprenorphine with other opioids or alcohol.
Buprenorphine is broken down in the liver by the CYP3A4 microsomal enzymes. Hence if the patient is on medications that induce these enzymes (e.g., carbamazepine, phenytoin or rifampin), therapeutic levels of buprenorphine may not be reached. On the other hand, if the patient is on inhibitors of CYP3A4 (e.g., fluvoxamine, ketoconazole, indinavir, erythromycin, saquinavir), levels of buprenorphine will remain elevated, and there is potential for toxicity.
At each clinic visit, the patient’s drug list has to be checked to make sure that no new drug has been added.
Figure 1. Clinical Opiate Withdrawal Scale
Buprenorphine administration
Buprenorphine can be administered in many ways. For chronic pain relief, a transdermal patch can be used. Oral forms include a buccal film and sublingual tablets. Parenteral routes include a subdermal or subcutaneous implant, and intravenous (IV) or intramuscular (IM) injections.
Buprenorphine is also available combined with naloxone in a sublingual tablet called Suboxone. Naloxone is not absorbed orally, so when Suboxone is taken, the effect is predominantly of buprenorphine. Naloxone is added to buprenorphine in order to reduce its abuse potential when injected. When Suboxone is taken in an IV form, the naloxone is absorbed as well and works to prevent the high of buprenorphine and may even precipitate a withdrawal. This is why buprenorphine alone has a higher potential for abuse than Suboxone does 6.
Federal Regulations for Prescribing Buprenorphine
Sublingual buprenorphine preparations are often used in the management of opioid-dependence (such as heroin, oxycodone, hydrocodone, morphine). The use of buprenorphine replacement therapy in the management of opioid dependence is regulated and highly monitored. In the United States, a special federal waiver is required to prescribe buprenorphine on an outpatient basis. Each federally approved physician is allowed to manage only 30 patients on buprenorphine for opioid addiction as outpatients.
Unlike methadone, buprenorphine/naloxone does not necessitate special prescribing exemptions, and thus the prescription can be written by any healthcare worker who is licensed to prescribe narcotics. However, most states highly recommend that health care workers who wish to prescribe buprenorphine to treat their opioid dependent patients undertake some training or extra-education to know more about this agent, before offering the drug to patients. Further, most insurers also recommend that health care workers who prescribe this drug must have completed an approved course of buprenorphine treatment for opioid dependence 7.
Just like the prescription of other narcotics like morphine, the healthcare worker must maintain good medical records when prescribing buprenorphine. Each time the drug is prescribed the medical notes should contain the following:
- Reason for prescribing
- Start and end date
- Which pharmacy will dispense the drug?
- Who will supervise the administration?
- Will the drug be taken at home or at the pharmacy?
- What type of follow up and who will be in charge of follow up
- How will compliance be monitored?
Further, the prescriber must comply with all the DEA requirements and actively monitor the patient.
DEA Rules
All healthcare workers who prescribe must have an active DEA registration number and a waiver to prescribe buprenorphine. The parenteral formula, Buprenex, is not FDA-approved for the management of opioid dependence, and hence Intravenous use is not permitted, except under extraordinary circumstances and with permission; otherwise, such use can be illegal, and the prescriber can lose his or her DEA number and ability to write any future prescriptions for controlled substances.
Buprenorphine and Naloxone for Opioid Dependence
Buprenorphine and naloxone combinations are used to treat opioid dependence (addiction to opioid drugs, including heroin and narcotic painkillers). Buprenorphine is in a class of medications called opioid partial agonist-antagonists and naloxone is in a class of medications called opioid antagonists. Buprenorphine alone and the combination of buprenorphine and naloxone work to prevent withdrawal symptoms when someone stops taking opioid drugs by producing similar effects to these drugs.
The combination of buprenorphine and naloxone comes as a sublingual tablet (Zubsolv) and as a sublingual film (Suboxone) to take under the tongue and as a buccal film (Bunavail) to apply between the gum and cheek. After your doctor determines an appropriate dose, these products are usually taken once a day. To help you remember to take or apply buprenorphine or buprenorphine and naloxone, take or apply it around the same time every day. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take or apply buprenorphine or buprenorphine and naloxone exactly as directed. Do not take or apply more or less of it or take or apply it more often than prescribed by your doctor.
Your doctor may decide to start your treatment with buprenorphine, which you will take in the doctor’s office. You will start on a low dose of buprenorphine and your doctor will increase your dose for 1 or 2 days before switching you to buprenorphine and naloxone. Depending on the type of opioid that you were taking, a different option that your doctor may choose is to start you on treatment with buprenorphine and naloxone right away. Your doctor may increase or decrease your buprenorphine and naloxone dose depending on your response.
If you are taking the sublingual tablets, place the tablets under your tongue until they completely melt. If you are taking more than two tablets, either place them all under your tongue at the same time or place them under your tongue up to two at a time. Do not chew the tablets or swallow them whole. Do not eat, drink, or talk until the tablet dissolves completely.
If you are using the buccal film, use your tongue to wet the inside of your cheek or rinse your mouth with water before you apply the film. Apply the film with a dry finger against the inside of the cheek. Then remove your finger and the film will stick to the inside of your cheek. If you are to use two films, place another film on the inside of your other cheek at the same time. Do not apply films on top of each other and do not apply more than two films to the inside of the mouth at one time. Leave the film(s) in the mouth until they dissolve. Do not cut, tear, chew, swallow, touch or move the film while it dissolves. Do not eat or drink anything until the film dissolves completely.
If you are using the sublingual film, rinse your mouth with water before you place the film. Place the film with a dry finger under your tongue to the right or left of the center and hold the film in place for 5 seconds. If you are using two films, place the other one on the opposite side under the tongue. Do not put the films on top of or near each other. Do not use more than two films at one time. Do not cut, tear, chew, swallow, touch or move the film while it dissolves. Do not eat or drink anything until the film dissolves completely.
If you need to switch from one buprenorphine or buprenorphine and naloxone product to another, your doctor may need to adjust your dose. Each time you receive your medication, check to be sure that you have received the buprenorphine product that was prescribed for you. Ask your pharmacist if you have are not sure that you received the right medication.
Do not stop taking or using buprenorphine or buprenorphine and naloxone without talking to your doctor. Stopping buprenorphine or buprenorphine and naloxone too quickly can cause withdrawal symptoms. Your doctor will tell you when and how to stop taking or using buprenorphine or buprenorphine and naloxone. If you suddenly stop taking or using buprenorphine or buprenorphine and naloxone, you may experience withdrawal symptoms such as hot or cold flushes, restlessness, teary eyes, runny nose, sweating, chills, muscle pain, vomiting, or diarrhea.
Buprenorphine and naloxone special precautions
Before taking buprenorphine and naloxone:
- tell your doctor and pharmacist if you are allergic to buprenorphine, naloxone, any other medications, or any of the other ingredients in buprenorphine or buprenorphine and naloxone sublingual tablets or film. Ask your pharmacist or check the Medication Guide for a list of the ingredients.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking. Be sure to mention any of the following: antihistamines (found in cold and allergy medications); antipsychotics such as aripiprazole (Abilify), asenapine (Saphris), cariprazine (Vraylar), chlorpromazine, clozapine (Versacloz), fluphenazine, haloperidol (Haldol), iloperidone (Fanapt), loxapine, lurasidone (Latuda), molindone, olanzapine (Zyprexa), paliperidone (Invega), perphenazine, pimavanserin (Nuplazid), quetiapine (Seroquel), risperidone (Risperdal), thioridazine, thiothixene, trifluoperazine, and ziprasidone (Geodon); benzodiazepines such as alprazolam (Xanax), chlordiazepoxide (Librium), clobazam (Onfi), clonazepam (Klonopin), clorazepate (Gen-Xene, Tranxene), diazepam (Diastat, Valium), estazolam, flurazepam, lorazepam (Ativan), oxazepam, quazepam (Doral), temazepam (Restoril), and triazolam (Halcion); diuretics (‘water pills’); erythromycin (E.E.S., Eryc, Erythrocin, others); certain HIV medications such as atazanavir (Reyataz, in Evotaz), delavirdine (Rescriptor), efavirenz (Sustiva, in Atripla), etravirine (Intelence), indinavir (Crixivan), nelfinavir (Viracept), nevirapine (Viramune), and ritonavir (Norvir, in Kaletra, in Technivie); ipratropium (Atrovent); medications for irritable bowel disease, motion sickness, Parkinson’s disease, ulcers, or urinary problems; ketoconazole; medications for migraine headaches such as almotriptan (Axert), eletriptan (Relpax), frovatriptan (Frova), naratriptan (Amerge), rizatriptan (Maxalt), sumatriptan (Alsuma, Imitrex, in Treximet), and zolmitriptan (Zomig); mirtazapine (Remeron); muscle relaxants; opiate (narcotic) medications for pain control and cough; rifampin (Rifadin, Rimactane, in Rifater, in Rifamate); medications for seizures such as carbamazepine (Epitol, Tegretol, Teril, others), phenobarbital, and phenytoin (Dilantin, Phenytek); 5HT3 serotonin blockers such as alosetron (Lotronex), granisetron (Sancuso, Sustol), ondansetron (Zofran, Zuplenz), or palonosetron (Aloxi); selective serotonin-reuptake inhibitors such as citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac, Sarafem, in Symbyax), fluvoxamine (Luvox), paroxetine (Brisdelle, Prozac, Pexeva), and sertraline (Zoloft); serotonin and norepinephrine reuptake inhibitors such as duloxetine (Cymbalta), desvenlafaxine (Khedezla, Pristiq), milnacipran (Savella), and venlafaxine (Effexor); sleeping pills; tramadol (Conzip); trazodone; or tricyclic antidepressants (‘mood elevators’) such as amitriptyline, clomipramine (Anafranil), desipramine (Norpramin), doxepin (Silenor), imipramine (Tofranil), nortriptyline (Pamelor), protriptyline (Vivactil), and trimipramine (Surmontil). Also tell your doctor or pharmacist if you are taking or receiving the following monoamine oxidase (MAO) inhibitors or if you have stopped taking them within the past two weeks: isocarboxazid (Marplan), linezolid (Zyvox), methylene blue, phenelzine (Nardil), selegiline (Eldepryl, Emsam, Zelapar), or tranylcypromine (Parnate). Many other medications may also interact with buprenorphine or buprenorphine and naloxone, so be sure to tell your doctor about all the medications you are taking, even those that do not appear on this list. Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- tell your doctor what herbal products you are taking, especially St. John’s wort and tryptophan.
- tell your doctor if you drink or have ever drunk large amounts of alcohol and if you have or have ever had adrenal problems such as Addison’s disease (condition in which the adrenal gland produces less hormone than normal); benign prostatic hypertrophy (BPH, enlargement of the prostate gland); difficulty urinating; a head injury; hallucinations (seeing things or hearing voices that do not exist); a curve in the spine that makes it hard to breathe; gallbladder disease; chronic obstructive pulmonary disease (COPD; a group of diseases that affect the lungs and airways); or thyroid, kidney, liver, or lung disease.
- tell your doctor if you are pregnant or plan to become pregnant. If you become pregnant while taking or using buprenorphine or buprenorphine and naloxone, call your doctor. If you take or use buprenorphine or buprenorphine and naloxone tablets or film regularly during your pregnancy, your baby may experience life-threatening withdrawal symptoms after birth. Tell your baby’s doctor right away if your baby experiences any of the following symptoms: irritability, seizures, uncontrollable shaking of a part of the body, vomiting, diarrhea, or failure to gain weight.
- tell your doctor if you are breastfeeding. Tell your baby’s doctor right away if your baby is sleepier than usual or has trouble breathing while you are taking this medication.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking or using buprenorphine or buprenorphine and naloxone.
- you should know that this medication may decrease fertility in men and women. Talk to your doctor about the risks of using buprenorphine or buprenorphine and naloxone.
- you should know that buprenorphine or buprenorphine and naloxone may make you drowsy. Do not drive a car or operate machinery until you know how this medication affects you.
- you should not drink alcohol or take prescription or nonprescription medications that contain alcohol while taking or using this medication.
- you should know that buprenorphine or buprenorphine and naloxone may cause dizziness, lightheadedness, and fainting when you get up too quickly from a lying position. This is more common when you first start taking or using buprenorphine or buprenorphine and naloxone. To avoid this problem, get out of bed slowly, resting your feet on the floor for a few minutes before standing up.
Buprenorphine for pain
Buprenorphine (Belbuca) is used to relieve severe pain in people who are expected to need pain medication around the clock for a long time and who cannot be treated with other medications. Buprenorphine (Belbuca) should not be used to treat pain that can be controlled by medication that is taken as needed. Buprenorphine (Belbuca) in a class of medications called opiate partial agonists. It works by changing the way the brain and nervous system respond to pain.
Buprenorphine (Belbuca) comes as a buccal film to apply inside the cheek. It is usually applied twice a day. Apply buprenorphine (Belbuca) at around the same times every day. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Use buprenorphine (Belbuca) exactly as directed.
Your doctor will probably start you on a low dose of buprenorphine (Belbuca), either once daily or every 12 hours, and gradually increase your dose, not more than once every 4 days. Your doctor may decrease your dose if you experience side effects. Tell your doctor if you feel that your pain is not controlled or if you experience side effects during your treatment with buprenorphine (Belbuca). Do not change the dose of your medication without talking to your doctor.
Do not stop using buprenorphine (Belbuca) without talking to your doctor. Your doctor will probably decrease your dose gradually. If you suddenly stop using buprenorphine (Belbuca), you may have symptoms of withdrawal. Call your doctor if you experience any of these symptoms of withdrawal: restlessness, teary eyes, runny nose, yawning, sweating, chills, muscle and back aches, large pupils (black circles in the center of the eyes), irritability, anxiety, difficulty falling asleep or staying asleep, diarrhea, nausea, vomiting, decreased appetite, stomach cramps, pain in the joints, weakness, fast heartbeat, or rapid breathing.
Buprenorphine (Belbuca) is sealed in a foil package. Do not open the package until ready to use. Do not apply buprenorphine (Belbuca) if the package seal is broken or the buccal film is cut, damaged, or changed in any way.
To apply the buccal film, follow these steps:
- Fold along the dotted line at the top of the foil package. Keep folded and tear down or cut with scissors at the notch in the direction of the scissors on the dotted line. Tear all the way to the bottom. Be careful to avoid cutting and damaging the buccal film when using scissors.
- Use your tongue to wet the inside of your cheek or rinse your mouth with water to moisten the area in your mouth where you will apply the buccal film.
- Avoid placing the buccal film in areas with open sores.
- Remove the buccal film from the package and hold it with clean, dry fingers with the yellow side facing up.
- Immediately place the yellow side of the buccal film against the inside of your moistened cheek. Press and hold the buccal film in place for 5 seconds and then take your finger away.
- The buccal film should stick against your cheek. Leave the buccal film in place until it has completely dissolved, usually within 30 minutes after you apply it.
- Avoid touching or moving the buccal film with your tongue or fingers after you apply it. Do not eat or drink anything until the buccal film has dissolved completely. Do not chew or swallow the buccal film.
Buprenorphine (Belbuca) may be habit forming, especially with prolonged use. Apply buprenorphine exactly as directed. Do not apply more buprenorphine buccal films, use the buccal films more often, or use the buccal films in a different way than prescribed by your doctor. While using buprenorphine, discuss with your health care provider your pain treatment goals, length of treatment, and other ways to manage your pain. Tell your doctor if you or anyone in your family drinks or has ever drunk large amounts of alcohol, uses or has ever used street drugs, or has overused prescription medications, or if you have or have ever had depression or another mental illness. There is a greater risk that you will overuse buprenorphine if you have or have ever had any of these conditions. Talk to your health care provider immediately and ask for guidance if you think that you have an opioid addiction or call the U.S. Substance Abuse and Mental Health Services Administration (SAMHSA) National Helpline at 1-800-662-HELP (4357).
Buprenorphine (Belbuca) may cause serious or life-threatening breathing problems, especially during the first 24 to 72 hours and any time that your dose is increased. Your doctor will monitor you carefully during your treatment. Your doctor will adjust your dose carefully to control your pain and decrease the risk that you will experience serious breathing problems. Tell your doctor if you have breathing difficulties and if you have or have ever had asthma. Your doctor may tell you not to use buprenorphine (Belbuca.) Also tell your doctor if you have or have ever had chronic obstructive pulmonary disease (COPD; a group of diseases that affect the lungs and airways), other lung diseases, a head injury, a brain tumor, or any condition that increases the amount of pressure in your brain. The risk that you will develop breathing problems may also be higher if you are an older adult or are weakened or malnourished due to disease. If you have any of the following symptoms, call your doctor immediately: difficulty breathing, shortness of breath, extreme drowsiness, fainting, or loss of consciousness.
Taking certain medications with buprenorphine (Belbuca) may increase the risk of serious or life-threatening breathing problems, sedation, or coma. Tell your doctor and pharmacist if you are taking or plan to take any of the following medications: benzodiazepines such as such as alprazolam (Xanax), chlordiazepoxide (Librium), clonazepam (Klonopin), diazepam (Diastat, Valium), estazolam, flurazepam, lorazepam (Ativan), oxazepam, temazepam (Restoril), and triazolam (Halcion); medications for mental illness and nausea; other medications for pain; muscle relaxants; sedatives; sleeping pills; or tranquilizers. Your doctor may need to change the dosages of your medications and will monitor you carefully. If you use buprenorphine with any of these medications and develop any of the following symptoms, call your doctor immediately or seek emergency medical care: unusual dizziness, lightheadedness, extreme sleepiness, slowed or difficult breathing, or unresponsiveness. Be sure that your caregiver or family members know which symptoms may be serious so they can call the doctor or emergency medical care if you are unable to seek treatment on your own.
Drinking alcohol, taking prescription or nonprescription medications that contain alcohol, or using street drugs during your treatment with buprenorphine increases the risk that you will experience these serious, life-threatening side effects. Do not drink alcohol or use street drugs during your treatment.
Buprenorphine (Belbuca) may cause serious harm or death if used accidentally by a child or by an adult who has not been prescribed the medication. Do not allow anyone else to use your medication. Store buprenorphine (Belbuca) in a safe place so that no one else can use it accidentally or on purpose. Keep track of how many buccal films are left so you will know if any are missing.
Tell your doctor if you are pregnant or plan to become pregnant. If you use buprenorphine regularly during your pregnancy, your baby may experience life-threatening withdrawal symptoms after birth. Tell your baby’s doctor right away if your baby experiences any of the following symptoms: irritability, hyperactivity, abnormal sleep, high-pitched cry, uncontrollable shaking of a part of the body, vomiting, diarrhea, or failure to gain weight.
Your doctor or pharmacist will give you the manufacturer’s patient information sheet (Medication Guide) when you begin treatment with buprenorphine (Belbuca) and each time you refill your prescription. Read the information carefully and ask your doctor or pharmacist if you have any questions. You can also visit the Food and Drug Administration (FDA) website (https://www.fda.gov/Drugs/DrugSafety/ucm085729.htm) or the manufacturer’s website to obtain the Medication Guide.
Talk to your doctor about the risks of taking buprenorphine (Belbuca).
Buprenorphine vs Methadone
Methadone is a fully synthetic long acting opioid that has similar, but not fully equal potency to morphine, which is used widely as an analgesic as well as maintenance therapy for persons with opioid dependency. Methadone has been used for decades to treat people who are addicted to heroin and narcotic pain medicines. When taken as prescribed, it is safe and effective. Methadone allows people to recover from their addiction and to reclaim active and meaningful lives. For optimal results, patients should also participate in a comprehensive medication-assisted treatment program that includes counseling and social support.
Patients taking methadone to treat opioid addiction must receive the medication under the supervision of a physician. After a period of stability (based on progress and proven, consistent compliance with the medication dosage), patients may be allowed to take methadone at home between program visits. By law, methadone can only be dispensed through an opioid treatment program certified by the U.S. Substance Abuse and Mental Health Services Administration (SAMHSA).
Methadone, like other opioids, acts by engagement in cell surface opiate receptors (predominant µ type receptors) that are found in the central nervous system, but also heart, lung, vascular and intestinal cells. Methadone may also act as an antagonist of the N-methyl-D-aspartate (NMDA) receptor accounting for some of its different effects. Methadone is a full opiate agonist and is well absorbed orally and has a longer half-life than morphine, features that can be used to help stabilize patients with opiate dependency by preventing withdrawal symptoms. In addition, the withdrawal syndrome from methadone is slower in onset, more prolonged and less severe than that with morphine.
Methadone was first approved for use as an opioid analgesic in the United States in 1947. Since then, its indications have expanded and now include management of acute or chronic moderate-to-severe pain not responsive to nonnarcotic analgesics, detoxification of opioid dependency and maintenance treatment of opioid addiction.
Methadone is available generically and under the brand names Dolophine and Methadose among others, in tablets of 5, 10 and 40 mg, oral solutions of 5 and 10 mg/5 mL (and concentrate of 10 mg/mL), and as a solution for injection in concentrations of 10 mg/mL. Typical oral doses vary by indication and clinical response. For opiate abstinence syndrome the dose is highly individualized, but maintenance doses are generally in the range of 20 to 120 mg daily.
Methadone side effects include sedation, respiratory depression, confusion, euphoria, agitation, itching, sweating, abdominal bloating, nausea, vomiting and constipation, adverse effects which are typical of the opioids. Methadone is a controlled substance and classified as a Schedule II drug, indicating that it has medical usefulness, but also a high potential for physical and psychological dependency and abuse. Use of methadone as a part of an opiate abstinence program requires special certification.
Benefits of Buprenorphine compared to Methadone
Use of buprenorphine has been shown to be effective than detoxification in improving outcomes in patients with opioid dependence. When compared to methadone, buprenorphine has the following advantages:
- Buprenorphine is safer even at high doses
- Optional therapeutic doses can be achieved relatively quickly
- There is less risk of abuse and diversion
- Buprenorphine is easier to taper
- There is less stigma associated with buprenorphine than methadone.
- Patients can get the medication from any healthcare provider and does not have to go to special methadone clinics
Buprenorphine, because of its partial opioid receptor agonist activity is said to cause less euphoria compared to full agonists like methadone or morphine, and thus is less likely to be abused or diverted.
The buprenorphine treatment typically lasts 3-6 months (or sometimes 1 to 2 years); on the other hand, methadone treatment is often lifelong.
Switch from Methadone to Buprenorphine
Patients can possibly switch from methadone to buprenorphine treatment, but because the two medications are so different, patients may not always be satisfied with the results. Studies indicate that buprenorphine is equally as effective as moderate doses of methadone. However, because buprenorphine is unlikely to be as effective as more optimal-dose methadone, it may not be the treatment of choice for patients with high levels of physical dependency.
A number of factors affect whether buprenorphine is a good choice for someone who is currently receiving methadone. Patients receiving buprenorphine can possibly be switched to methadone. Patients interested in learning more about switching their treatment should discuss this with their doctor.
Methadone safety
Methadone can be addictive, so it must be used exactly as prescribed. This is particularly important for patients who are allowed to take methadone at home and aren’t required to take medication under supervision at an opioid treatment program certified by the U.S. Substance Abuse and Mental Health Services Administration (SAMHSA).. Methadone medication is specifically tailored for the individual patient (as doses are often adjusted and readjusted) and is never to be shared with or given to others. Patients should share their complete health history with health providers to ensure the safe use of the medication.
Other medications may interact with methadone and cause heart conditions. Even after the effects of methadone wear off, the medication’s active ingredients remain in the body for much longer. Taking more methadone can cause unintentional overdose.
The following tips can help achieve the best treatment results:
- Never use more than the amount prescribed, and always take at the times prescribed. If a dose is missed, or if it feels like it’s not working, do not take an extra dose of methadone.
- Do not consume alcohol while taking methadone.
- Be careful driving or operating machinery on methadone.
- Call your local emergency services number if too much methadone is taken or if an overdose is suspected.
- Take steps to prevent children from accidentally taking methadone.
- Store methadone at room temperature and away from light.
- Dispose of unused methadone by flushing it down the toilet.
Pregnant or Breastfeeding Women and Methadone
Women who are pregnant or breastfeeding can safely take methadone. When withdrawal from an abused drug happens to a pregnant woman, it causes the uterus to contract and may bring on miscarriage or premature birth. Methadone’s ability to prevent withdrawal symptoms helps pregnant women better manage their addiction while avoiding health risks to both mother and baby.
Undergoing methadone maintenance treatment while pregnant will not cause birth defects, but some babies may go through withdrawal after birth. This does not mean that the baby is addicted. Infant withdrawal usually begins a few days after birth but may begin two to four weeks after birth.
Mothers taking methadone can still breastfeed. Research has shown that the benefits of breastfeeding outweigh the effect of the small amount of methadone that enters the breast milk. A woman who is thinking of stopping methadone treatment due to breastfeeding or pregnancy concerns should speak with her doctor first.
Methadone side effects
Side effects should be taken seriously, as some of them may indicate an emergency. Patients should stop taking methadone and contact a doctor or emergency services right away if they:
- Experience difficulty breathing or shallow breathing
- Feel lightheaded or faint
- Experience hives or a rash; swelling of the face, lips, tongue, or throat
- Feel chest pain
- Experience a fast or pounding heartbeat
- Experience hallucinations or confusion
Buprenorphine dosage
Buprenorphine can be administered in many different ways. For chronic pain relief, a transdermal patch can be used (this formula is only available in Europe). Oral forms include a buccal film and sublingual tablets. Parenteral routes include a subdermal or subcutaneous implant, and IV or IM injections, but are not used routinely. The sublingual formula is widely used to treat opioid addiction. It contains buprenorphine and naloxone in 4:1 ratio. Buprenorphine is available in 2-mg and 8-mg sublingual formula combined with naloxone 0.5 mg and 2 mg, respectively to deter drug abuse by injection. Once placed underneath the tongue, the drug formula dissolves in 2 to 10 minutes.
Naloxone is an opioid antagonist, and its use in the formula is to prevent injection of the liquid obtained by dissolving the pills. This may help decrease abuse of buprenorphine and also limit diversion. Because naloxone is poorly absorbed sublingually, its systemic effects when buprenorphine is taken properly are minimal. However, if the tablet is dissolved and injected, the naloxone blocks mu receptors and prevents receptor activation or precipitates withdrawal in opioid-dependent patients.
Initial Dosing
The initial treatment dose of buprenorphine/naloxone should be at the lowest dose and gradually titrated on a weekly basis until a response is seen. The minimum duration of treatment is 8 weeks. In the majority of cases, the drug is administered under supervision by a pharmacist, except when the pharmacy is not open on the weekends, then the patient can receive a take-home dose. Take home doses are only suitable for patients who are compliant and are clinically motivated to treat their opioid-dependence. However, before take-home dosing is agreed upon, the patient must be educated on the consequences of the following:
- Potential for overdose
- Unintended dosing by others
- Diversion
- Consequences of careless storage
If take-home dosing is agreed upon, initially it should be limited to weekend doses only and then gradually increased as the patient shows more reliability. At all times the patient must be monitored for compliance 8.
Induction Therapy
Induction with buprenorphine is initiated when the patient is experiencing mild to moderate symptoms of opioid withdrawal. The treatment is usually started 6 to 12 hours after use of short-acting opioids (e.g., heroin, oxycodone) or at least 24 hours or longer after the use of a long-acting opioid (e.g., morphine or oxycodone controlled-release formulations. For those methadone maintenance patients who prefer to be switched to buprenorphine, it is recommended that one wait at least 72 hours or more after the dose of methadone before initiating treatment. The dose of methadone should be tapered down to less than 30 mg before buprenorphine treatment to decrease the risk of precipitating intense withdrawal symptoms.
For patients on the fentanyl patch, at least 48 to 72 hours is required after discontinuation before starting treatment.
In most patients with opioid dependence, the initial dose is 2 to 4 mg. For those who are on high doses of opioids or potent agents like oxycodone, an additional dose of 2 to 4 mg may be required on the same day. After the supervised dosing, the patient is monitored by the healthcare provider in the clinic.
During this time the patient is monitored for withdrawal symptoms. Tools like the Clinical Opiate Withdrawal Scale is used to determine the presence and intensity of the withdrawal symptoms. Additional doses of buprenorphine may be required for symptomatic management of the withdrawal symptoms. Once the symptoms have subsided the patient is discharged, and the induction is rescheduled the following day. The patient should be encouraged to abstain from opioids while at home.
Maintenance Phase
During this phase, the dose of buprenorphine is gradually increased according to the patient’s physical and psychological needs but should not exceed a maximum of 24 mg in one day. Most patients respond to doses between 8 to 12 mg per day. In most patients, the maintenance dose can be reached within 2 to 4 days. Once stabilized, the dosing frequency may be reduced, especially in reliable patients or those who need to travel. Some patients may benefit from alternate dosing by doubling the dose at each visit.
Adult dose for opiate dependence – maintenance
MAINTENANCE: Buprenorphine is prescribed as part of a comprehensive treatment plan that includes counseling and participation in social support programs.
SUBLINGUAL Tablets:
- Following 2-day induction: Adjust dose in 2 to 4 mg increments/decrements to a level that holds patient in treatment and suppresses opioid withdrawal signs and symptoms
- Target dose: 16 mg sublingually once a day; range 4 to 24 mg/day
- Maximum dose: 24 mg/day; higher doses have not shown a clinical advantage
Comment:
- Buprenorphine with naloxone is the preferred drug for maintenance treatment; unsupervised maintenance treatment with buprenorphine should be limited to those who cannot tolerate buprenorphine-naloxone.
EXTENDED-RELEASE SUBCUTANEOUS Injection (Sublocade[R]):
Following a minimum of 7-days of treatment with a transmucosal product delivering the equivalent of 8 to 24 mg buprenorphine per day:
- Initial dose: 300 mg subcutaneously once a month for 2 months
- Maintenance dose: 100 mg subcutaneously once a month
- Maintenance dose may be increased to 300 mg monthly for those tolerating lower dose and demonstrating a less than satisfactory clinical response, e.g. self-reported illicit opioid use or positive urine drug screens
Comments:
- Initiating therapy with subcutaneous injections has not been studied; subcutaneous injections should only be initiated following induction and dose-adjustment with a transmucosal buprenorphine-containing product.
- Monthly doses should allow for a minimum of 26 days between doses; occasional delays in dosing of up to 2 weeks are not expected to have a clinically significant impact on treatment effect.
SUBDERMAL IMPLANT (Probuphine[R]):
For use in opioid-tolerant patients who meet ALL of the following criteria:
- Achieved and sustained prolonged clinical stability on transmucosal buprenorphine as evidenced by a stable dose for 3 months or longer without any need for supplemental dosing or dose adjustments.
- Currently receiving buprenorphine maintenance (with or without naloxone) at doses of 8 mg/day or less, or equivalent transmucosal product (e.g. Bunavail[R] buprenorphine 4.2 mg/naloxone 0.7 mg per day or less; or Zubsolv[R] buprenorphine 5.7 mg/naloxone 1.4 mg per day or less)
-Patients should not be tapered to a lower dose for the sole purpose of transitioning to the implant.
Insert 1 dose subdermally in the inner side of the upper arm
Remove at the end of the sixth month
Comments:
- Implant insertions and removals should be performed by certified healthcare providers.
- Each dose consists of 4 implants; each implant contains 74.2 mg of buprenorphine
- After 1 insertion in each arm, most patients should be transitioned back to transmucosal products for continued treatment as there is no experience with inserting additional implants into other sites in the arm or into a previously-used site.
There is no maximum recommended duration for maintenance therapy as indefinite treatment may be required; when the decision is made to discontinue, doses should be tapered.
Adult dose for pain
Use: For the management of pain severe enough to require an opioid analgesic and for which alternate treatments are inadequate.
- Initial dose: 0.3 mg deep IM or slow IV (over at least 2 minutes); may repeat this dose once after 30 to 60 minutes if needed; then, 0.3 mg IV/IM every 6 hours as needed
- A single 0.6 mg IM dose may be given to patients who are not in a high risk category (see Warnings)
- Maximum single dose: 0.3 mg (IV) or 0.6 mg (IM)
Comments:
- Use extra caution with IV administration, especially the first dose.
- Monitor closely for respiratory depression, especially within the first 24 to 72 hours.
- Use the lowest effective dose for the shortest duration consistent with the individual patient’s treatment goals.
Adult dose for chronic pain
Use: For the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment for which alternative treatment options are inadequate.
BUCCAL Film:
Opioid-Naive and Opioid Non-Tolerant:
- As Initial Opioid Analgesic: 75 mcg buccally once a day, or if tolerated every 12 hours for at least 4 days; then increase to 150 mcg every 12 hours
- Titrate individually in increments of up to 150 mcg every 12 hours no more frequently than every 4 days to a dose that provides adequate analgesia and minimizes adverse reactions
- Maximum dose: 900 mcg every 12 hours due to potential for QTc interval prolongation
CONVERSION from Other Opioids to BUCCAL Film:
To reduce the risk of opioid withdrawal, taper patients to no more than 30 mg/day oral morphine mg equivalents (MME) before beginning therapy
Discontinue all around-the-clock opioid drugs when initiating buccal film
For opioid dose of less than 30 morphine mg equivalents (MME)/day prior to taper:
- Initial dose: 75 mcg buccally once a day, or every 12 hours
For opioid dose between 30 and 89 morphine mg equivalents (MME)/day prior to taper:
- Initial dose: 150 mcg buccally every 12 hours
For opioid dose between 90 and 160 morphine mg equivalents (MME)/day prior to taper:
- Initial dose: 300 mcg buccally every 12 hours
- Following initiation, titrate as above
Buccal Film NOTES:
- For opioid doses greater than 160 morphine mg equivalents (MME)/day: Consider alternate analgesics as buccal film may not provide adequate analgesia.
- Film strengths of 600 mcg, 750 mcg, and 900 mcg are only for use following titration from lower doses.
TRANSDERMAL System:
Opioid-Naive and Opioid Non-Tolerant:
- As Initial Opioid Analgesic: Apply 5 mcg/hr patch transdermally; change every 7 days
- Individually titrate to a dose that provides adequate analgesia and minimizes adverse reactions; minimal titration interval is 72 hours
- Maximum dose: 20 mcg/hr
CONVERSION from Other Opioids to TRANSDERMAL System:
Discontinue all other around-the-clock opioid drugs when initiating therapy
- For prior opioid dose of less than 30 oral morphine mg equivalents (MME) per day: Initiate with 5 mcg/hr patch applied transdermally at next dosing interval
- For prior opioid dose between 30 and 80 morphine mg equivalents (MME)/day: Taper around-the-clock opioids for up to 7 days to no more than 30 morphine mg equivalents (MME)/day; then, initiate with 10 mcg/hr patch applied transdermally at next dosing interval; may use short-acting analgesics as needed until analgesic efficacy is attained
- Following initiation, titrate as above
Transdermal NOTES:
- For prior opioid dose greater than 80 morphine mg equivalents (MME)/day: Consider an alternate analgesic as transdermal therapy may not provide adequate analgesia
- Transdermal patches of 7.5, 10, 15, and 20 mcg/hour are only for use in patients who are opioid-tolerant
Comments:
- Conversion from Methadone: Close monitoring will be of particular importance as methadone has a long half-life and accumulates in the plasma; no specific conversion is provided with an understanding that there is wide interpatient variability.
- The buccal film and transdermal system should be prescribed by healthcare providers knowledgeable in the use of potent opioids for chronic pain; these products are not for use as as-needed analgesics.
- Monitor patients closely for respiratory depression, especially within 24 to 72 hours after initiating therapy and with dose increases.
- If pain level increases after dose stabilization, attempt to identify source of increased pain before increasing dose; rescue medication with an immediate-release analgesic may be needed.
- Because of the risks of opioid addiction, abuse, and misuse, this drug should be reserved for patients for whom alternative treatment options have not or are not expected to be tolerated; or have not or are not expected to provide adequate analgesia.
- Upon discontinuation, use a gradual downward titration; for patients on transdermal system, may need to switch to an immediate-release opioid.
Adult dose for opiate dependence – induction
Treatment should be initiated when objective and clear signs of moderate opioid withdrawal appear, and
- at least 4 hours have elapsed since last use of heroin or other short-acting opioids
- at least 24 hours have elapsed since last use of methadone or other long-acting opioids
INDUCTION:
- Day 1: 8 mg sublingually once a day (may give in 2 to 4 mg increments, if preferred)
- Day 2: 16 mg sublingually once a day
Comments:
- Buprenorphine should be used as part of a complete treatment plan to include counseling and psychosocial support.
- Buprenorphine (without naloxone) is the preferred drug for induction.
- Adequate treatment doses should be given as soon as possible as gradual induction over several days has led to higher dropout rates.
Pediatric dose for pain
Use: For the management of pain severe enough to require an opioid analgesic and for which alternate treatments are inadequate.
2 to 12 years:
- Initial dose: 2 to 6 mcg/kg IM or slow IV every 4 to 6 hours
- Some patients may not need to be remedicated for 6 to 8 hours; fixed interval or round the clock dosing should not be used until the proper inter-dose interval has been established
Over 12 years:
- Initial dose: 0.3 mg deep IM or slow IV (over at least 2 minutes); may repeat this dose once after 30 to 60 minutes if needed; then, 0.3 mg IV/IM every 6 hours as needed
- Maximum single dose: 0.3 mg
Comments:
- Use extra caution with IV administration, especially the first dose.
- Monitor closely for respiratory depression, especially within the first 24 to 72 hours.
- Use the lowest effective dose for the shortest duration consistent with the individual patient’s treatment goals.
Pediatric dose for opiate dependence – maintenance
16 years or older:
MAINTENANCE: Buprenorphine should be used as part of a complete treatment plan to include counseling and psychosocial support.
SUBLINGUAL Tablets:
- Following 2-day induction: Adjust dose in 2 to 4 mg increments/decrements to a level that holds patient in treatment and suppresses opioid withdrawal signs and symptoms
- Target dose: 16 mg sublingually once a day; range 4 to 24 mg/day
- Maximum dose: 24 mg/day; higher doses have not shown a clinical advantage
Comment:
- Buprenorphine with naloxone is the preferred drug for maintenance treatment; unsupervised maintenance treatment with buprenorphine should be limited to those who cannot tolerate buprenorphine-naloxone.
SUBDERMAL IMPLANT (Probuphine[R]):
- For use in opioid-tolerant patients who meet ALL of the following criteria:
- Achieved and sustained prolonged clinical stability on transmucosal buprenorphine as evidenced by a stable dose for 3 months or longer without any need for supplemental dosing or dose adjustments.
- Currently receiving buprenorphine maintenance (with or without naloxone) at doses of 8 mg/day or less, or equivalent transmucosal product (e.g. Bunavail[R] buprenorphine 4.2 mg/naloxone 0.7 mg per day or less; or Zubsolv[R] buprenorphine 5.7 mg/naloxone 1.4 mg per day or less)
- Patients should not be tapered to a lower dose for the sole purpose of transitioning to the implant.
Insert 1 dose subdermally in the inner side of the upper arm
Remove at the end of the sixth month
Comments:
- Implant insertions and removals should be performed by certified healthcare providers.
- Each dose consists of 4 implants; each implant contains 74.2 mg of buprenorphine
- After 1 insertion in each arm, most patients should be transitioned back to transmucosal products for continued treatment as there is no experience with inserting additional implants into other sites in the arm or into a previously-used site.
There is no maximum recommended duration for maintenance therapy as indefinite treatment may be required; when the decision is made to discontinue, doses should be tapered.
Pediatric dose for opiate dependence – induction
16 years or older:
- Treatment should be initiated when objective and clear signs of moderate opioid withdrawal appear, and
- at least 4 hours have elapsed since last use of heroin or other short-acting opioids
- at least 24 hours have elapsed since last use of methadone or other long-acting opioids
INDUCTION:
- Day 1: 8 mg sublingually once a day (may give in 2 to 4 mg increments, if preferred)
- Day 2: 16 mg sublingually once a day
Comments:
- Buprenorphine should be used as part of a complete treatment plan to include counseling and psychosocial support.
- Buprenorphine (without naloxone) is the preferred drug for induction.
- Adequate treatment doses should be given as soon as possible as gradual induction over several days has led to higher dropout rates.
Renal dose adjustments
- No adjustment recommended
Liver dose adjustments
Transdermal:
- Severe hepatic impairment: Consider analgesics that permit more flexibility in dosing
Buccal Film:
- Severe hepatic impairment: Reduce starting dose and titration dose by one-half (i.e. from 150 mcg to 75 mcg)
Sublingual Tablets:
- Mild hepatic impairment: No adjustment recommended
- Moderate hepatic impairment: Use with caution and close monitoring
- Severe hepatic impairment: Consider reducing the starting and titration dose by one-half; monitor closely for signs and symptoms of toxicity
Extended-release subcutaneous injection:
- Moderate to severe hepatic impairment: Not recommended
- For patients developing moderate to severe hepatic impairment while on treatment, monitor for signs and symptoms of toxicity or overdose for several months.
Implant:
- Moderate to severe hepatic impairment: Not recommended
- For patients developing moderate to severe hepatic impairment while on treatment, monitor for signs and symptoms of toxicity or overdose, if signs and symptoms of toxicity or overdose are observed, the implant should be removed.
Managing Missed Doses
When dealing with opioid-dependent patients and their treatment, one must be prepared to deal with missed doses. Today most pharmacists who dispense buprenorphine keep track of the drug, the dose, time and day. This information is vital as it helps with compliance anytime the individual misses a dose of buprenorphine, the healthcare provider must be notified since ti maybe the first sign of instability in the patient. To prevent loss of compliance a new treatment plan must be developed.
Buprenorphine side effects
Buprenorphine may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- diarrhea
- dry mouth
- sleepiness
- headache
Some side effects can be serious. If you experience any of these symptoms or those listed in the IMPORTANT WARNING section, call your doctor immediately or get emergency medical treatment:
- changes in heartbeat
- agitation, hallucinations (seeing things or hearing voices that do not exist), fever, sweating, confusion, fast heartbeat, shivering, severe muscle stiffness or twitching, loss of coordination, nausea, vomiting, or diarrhea
- nausea, vomiting, loss of appetite, weakness, or dizziness
- inability to get or keep an erection
- irregular menstruation
- decreased sexual desire
- chest pain
- swelling of your face, tongue or throat
- rash
- hives
Buprenorphine may cause other side effects. Call your doctor if you have any unusual problems while using buprenorphine.
Symptoms of buprenorphine overdose may include the following:
- slowed or difficulty breathing
- extreme sleepiness or drowsiness
- coma (loss of consciousness for a period of time)
- slow heartbeat
- cold, clammy skin
- muscle weakness
- narrowing or widening of the pupils (black circles in the center of the eye)
- unusual snoring
Buprenorphine and naloxone side effects
Buprenorphine and naloxone may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- headache
- stomach pain
- constipation
- difficulty falling asleep or staying asleep
- mouth numbness or redness
- tongue pain
- blurred vision
- back pain
Some side effects can be serious. If you experience any of these symptoms or those listed in the IMPORTANT WARNINGS or SPECIAL PRECAUTIONS sections, call your doctor immediately:
- hives
- rash
- itching
- difficulty breathing or swallowing
- swelling of the face, throat, tongue, lips, eyes, hands, feet, ankles, or lower legs
- agitation, hallucinations (seeing things or hearing voices that do not exist), fever, sweating, confusion, fast heartbeat, shivering, severe muscle stiffness or
- twitching, loss of coordination, nausea, vomiting, or diarrhea
- nausea, vomiting, loss of appetite, weakness, or dizziness
- inability to get or keep an erection
- irregular menstruation
- decreased sexual desire
- slowed breathing
- upset stomach
- extreme tiredness
- confusion
- blurred vision
- slurred speech
- unusual bleeding or bruising
- lack of energy
- pain in the upper right part of the stomach
- yellowing of the skin or eyes
- dark-colored urine
- light-colored stools
Buprenorphine and naloxone may cause other side effects. Call your doctor if you have any unusual problems while taking or using buprenorphine and naloxone.
Symptoms of buprenorphine and naloxone overdose may include the following:
- pinpoint pupils
- extreme drowsiness
- dizziness
- blurred vision
- slowed breathing
- Noble F, Marie N. Management of Opioid Addiction With Opioid Substitution Treatments: Beyond Methadone and Buprenorphine. Front Psychiatry. 2018;9:742[↩]
- Implementation of the Provision of the Comprehensive Addiction and Recovery Act of 2016 Relating to the Dispensing of Narcotic Drugs for Opioid Use Disorder. Final rule. Fed Regist. 2018 Jan 23;83(15):3071-5. https://www.govinfo.gov/content/pkg/FR-2018-01-23/pdf/2018-01173.pdf[↩]
- Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No.: CD002207. DOI: 10.1002/14651858.CD002207.pub4 [↩]
- Kumar R, Saadabadi A. Buprenorphine. [Updated 2019 Feb 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459126[↩]
- Wen H, Hockenberry JM, Pollack HA. Association of Buprenorphine-Waivered Physician Supply With Buprenorphine Treatment Use and Prescription Opioid Use in Medicaid Enrollees. JAMA Netw Open. 2018 Sep 07;1(5):e182943[↩]
- Shi JM, Henry SP, Dwy SL, Orazietti SA, Carroll KM. Randomized pilot trial of Web-based cognitive-behavioral therapy adapted for use in office-based buprenorphine maintenance. Subst Abus. 2019 Feb 04;:1-4.[↩]
- Latif ZE, Solli KK, Opheim A, Kunoe N, Benth JŠ, Krajci P, Sharma-Haase K, Tanum L. No increased pain among opioid-dependent individuals treated with extended-release naltrexone or buprenorphine-naloxone: A 3-month randomized study and 9-month open-treatment follow-up study. Am J Addict. 2019 Feb;28(2):77-85.[↩]
- Buprenorphine/Naloxone Versus Methadone for the Treatment of Opioid Dependence: A Review of Comparative Clinical Effectiveness, Cost-Effectiveness and Guidelines [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2016 Sep 2. Available from: https://www.ncbi.nlm.nih.gov/books/NBK385163[↩]