Contents
Vitamin C deficiency
Vitamin C deficiency causes Scurvy a disease that occurs when you have a severe and prolonged lack of vitamin C (ascorbic acid) in your diet 1, 2, 3, 4, 5, 6, 7, 8. Scurvy develops in approximately four weeks in those who consume less than 10 mg/day of Vitamin C 9. Early features of the scurvy include general weakness, fatigue, lethargy, lassitude, irritability and aching limbs. If left untreated, more serious problems can develop such as muscle weakness, swollen and bleeding gums, loosening or loss of teeth, corkscrew hairs, petechial hemorrhaging, spontaneous bruises (ecchymoses), anemia, poor wound healing, hyperkeratosis, weakness, myalgia, arthralgia, and weight loss (there can also be a paradoxical weight increase due to swelling). Shortness of breath (dyspnea), generalized edema, severe jaundice, hemolysis, acute spontaneous bleeding, neuropathy, fever and convulsions can be observed as well. In children, bone growth is impaired with vitamin c deficiency and can be associated with bleeding into the periosteum and sub-periosteum 10. Scurvy is fatal if it is not treated and sudden death has occurred as a consequence of a cerebral/myocardial hemorrhage or pneumonia 11, 6.
Vitamin C deficiency or scurvy symptoms generally develop after at least 3 months of severe or total vitamin C deficiency. Scurvy can be cured with vitamin C supplements taken by mouth. Once recovery is complete, dietary modifications to ensure the “recommended daily intake” of vitamin C is reached will prevent relapse. Except in the case of severe dental disease, permanent damage from scurvy does not usually occur 12. Smokers have greater vitamin C requirements than non-smokers, which predisposes them to scurvy 13, 14. The recommended daily allowance (RDA) of vitamin C for men is 90 mg/day and women is 75 mg/day 15, 9. If you smoke, add 35 mg/day to the above values to calculate your total daily recommended amount 9. Daily requirements increase for patients who are pregnant (80 to 85 mg/day) and during lactation (115 to 120 mg/day), or patients who smoke, are on hemodialysis, or have trauma/infection 16, 9. Consuming five varied servings of fruits and vegetables a day can provide more than 200 mg of vitamin C 9.
Scurvy is relatively rare in the United States and Canada, however, scurvy continues to be a problem in malnourished populations around the world such as impoverished, underdeveloped third world countries. In developing countries such as northern India, the incidence of vitamin C deficiency can be as high as 73.9% due to limited access to Vitamin C-rich fruit and vegetables. People who get little or no vitamin C (below about 10 mg per day) for many weeks can get scurvy 17, 6, 18, 19. The prevalence of vitamin C deficiency in the United States is 7.1% 20. Studies estimate that among people with low incomes, there is a 40% prevalence of vitamin C deficiency 21. Populations at risk include low incomes, food insecurity, poor nutrition, alcohol use disorder (alcoholic patients), isolated elderly patients, gastrointestinal disorders and malabsorption syndromes, and eating disorders or people who voluntarily restrict their type of food intake 22. In addition to poor nutritional intake, alcoholic patients develop vitamin C deficiency secondary to increased excretion of vitamin C in the urine 23. In a retrospective chart review conducted at the Mayo Clinic in Rochester, NY, and Scottsdale, AZ, from 1976 to 2002, 10 of 12 scurvy cases were related to alcohol abuse, illicit drug use, and psychiatric disorders 4. Other risk factors include fad diets, routine diets, and severe allergies to food products 24. Anorexia nervosa, Crohn disease, celiac disease, and hemodialysis patients have also been documented as having increased susceptibility to ascorbic acid deficiency (as well as other common nutritional deficiencies).
In a small study of 9 healthy middle-aged men, alcohol consumption (0.58 g/kg) produced a 47% increase in urinary ascorbic acid excretion 4 hours after ingestion 23. A small comparative study of 13 healthy men pretreated with high doses of vitamin C (2 g/d for 2 weeks) before alcohol consumption (0.8 g/kg) demonstrated a significant increase in plasma alcohol clearance when compared with a non-pretreated group 25. The literature suggests a correlation between alcohol metabolism and ascorbic acid excretion; however, the mechanism remains unknown 26. These poorly understood interactions might leave individuals who abuse alcohol at higher risk of developing scurvy.
The timeline for the development of scurvy varies, depending on vitamin C body stores, but signs can appear within 1 month of little or no vitamin C intake (below 10 mg/day) 6. Scurvy symptoms are not seen unless the total vitamin C content in the body falls below 300–400 mg 27, 28, 29, 30, 31. Initial symptoms can include fatigue (probably the result of impaired carnitine biosynthesis), malaise, and inflammation of the gums 7. As vitamin C deficiency progresses, collagen synthesis becomes impaired and connective tissues become weakened, causing petechiae, ecchymoses, purpura, joint pain, poor wound healing, hyperkeratosis, and corkscrew hairs 32. Additional signs of scurvy include depression as well as swollen, bleeding gums and loosening or loss of teeth due to tissue and capillary fragility 6. Iron deficiency anemia can also occur due to increased bleeding and decreased nonheme iron absorption secondary to low vitamin C intake 7. In children, bone disease can be present 17.
The human body lacks the ability to synthesize and make vitamin C (ascorbic acid) and therefore depends on exogenous dietary sources to meet vitamin C needs. Your body’s pool of vitamin C can be depleted in 1-3 months. Ascorbic acid (vitamin C) is prone to oxidation in your body, and your body stores are affected by environmental and lifestyle factors (e.g., smoking), biological conditions (e.g., inflammation, iron excess), and pathologic conditions (e.g., malabsorption) that may alter its oxidation. Consumption of fruits and vegetables or diets fortified with vitamin C is essential to avoid ascorbic acid deficiency 33.
Scurvy develops 1 to 3 months after initiating a vitamin C-deficient diet 34. Individuals may complain of lethargy, fatigue, malaise, emotional lability, arthralgias (joint pain), weight loss, anorexia, and diarrhea. They also may experience easy bleeding, bruising, and poor wound healing.
Your body needs vitamin C to work properly. Without enough vitamin C in your body, you can start to feel very ill. The symptoms of scurvy include:
- feeling tired and weak
- aching legs and arms
- swollen and bleeding gums
- red or blue spots on the skin, usually on the shins
- bruising easily
- wounds taking a long time to heal
Sources of vitamin C
Vitamin C is found in many different fruits and vegetables, including:
- blackcurrants
- citrus fruits – oranges, limes and lemons
- berries
- kiwifruit
- tomatoes
- broccoli
- sprouts
- red, yellow and green capsicum
Cutting and heating foods changes vitamin C and makes it less effective. So it helps to eat fruits and vegetables raw, or lightly cooked, and don’t cut them too long before eating them.
You should be able to get all the vitamin C you need from your diet.
See a doctor if you’re at risk of scurvy and you:
- feel very tired and weak all the time
- feel irritable and sad all the time
- have severe joint or leg pain
- have swollen, bleeding gums – sometimes teeth can fall out
- develop red or blue spots on the skin, usually on your shins
- have skin that bruises easily
These might be symptoms of scurvy.
Scurvy is easily treated by adding some vitamin C to your diet, such as fresh fruit and vegetables.
Your doctor may also recommend taking vitamin C supplements until you feel better. The treatment for scurvy is vitamin C supplementation. Recommendations are that 1 to 2 grams of vitamin C be administered daily for the first 2 to 3 days followed by 500 mg per day for the next week 26. Afterward, a daily intake of 100 mg of vitamin C should be given for 1 to 3 months 26.
Your doctor might arrange a blood test to confirm you have scurvy if they’re not sure.
Most people treated for scurvy feel better within 48 hours and make a full recovery within 2 weeks.
Your doctor may refer you to a specialist for treatment, support or advice. This depends on what’s causing your scurvy.
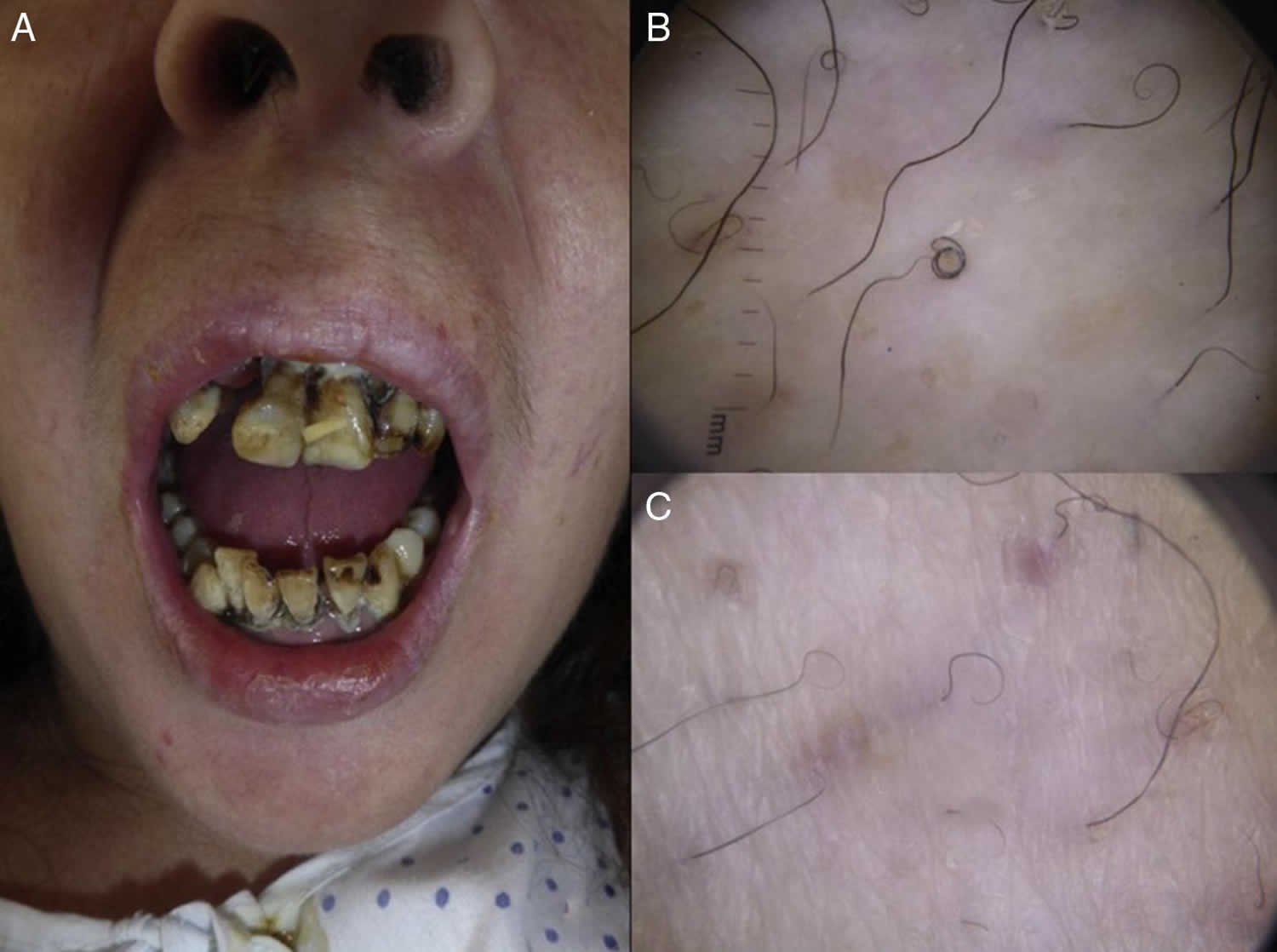
Figure 1. Corkscrew hair
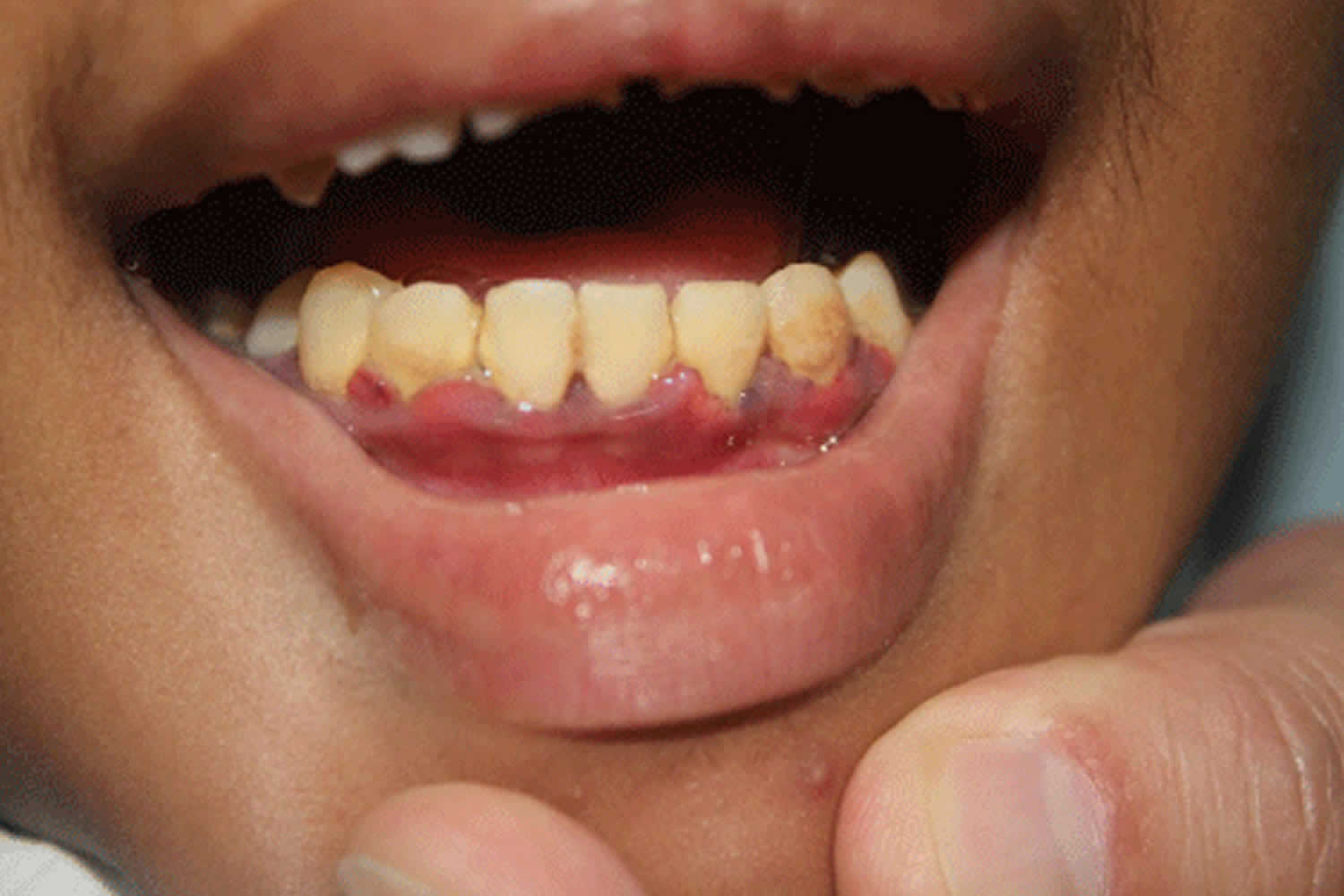
Figure 2. Inflamed marginal gingiva in scurvy
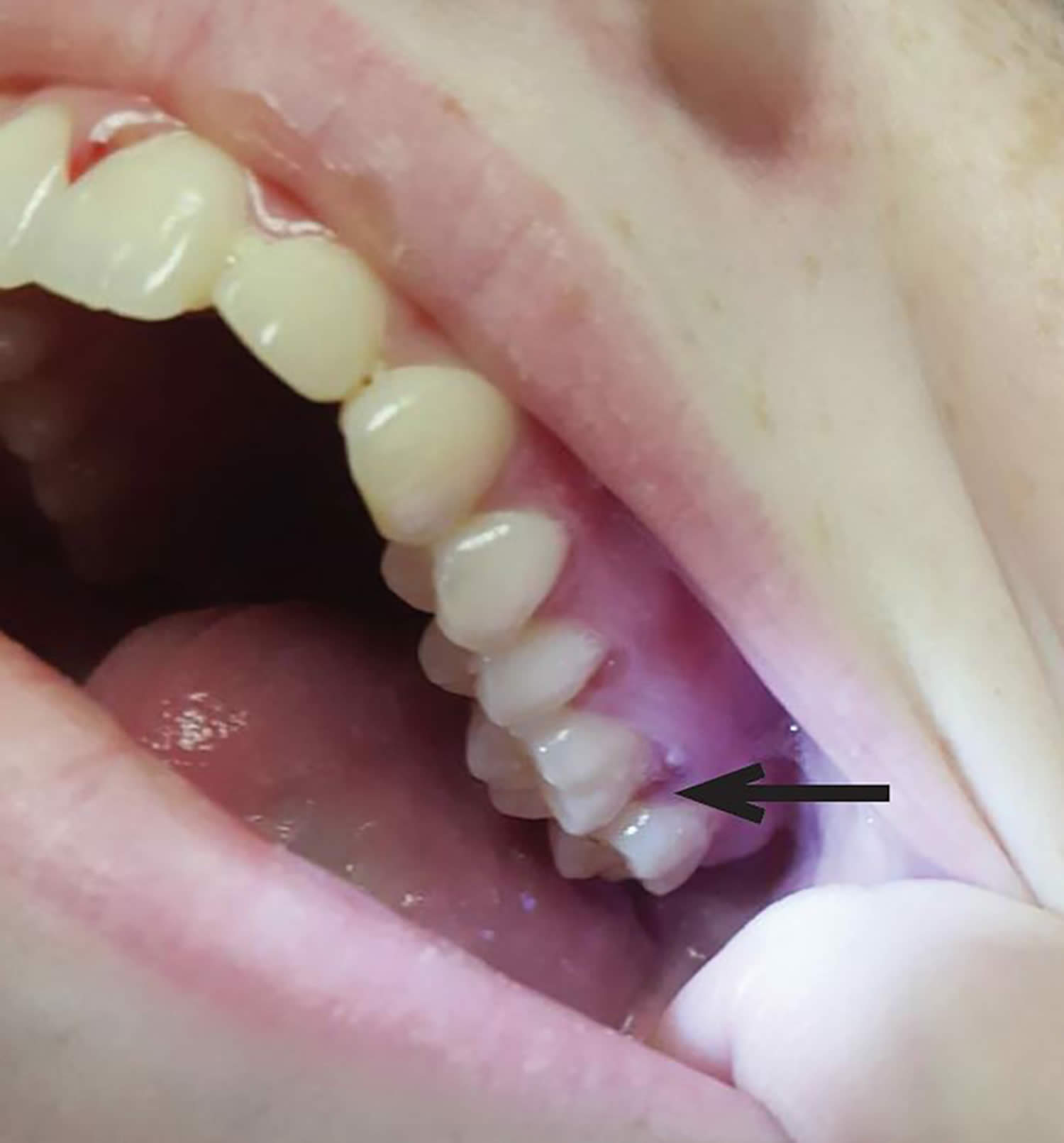
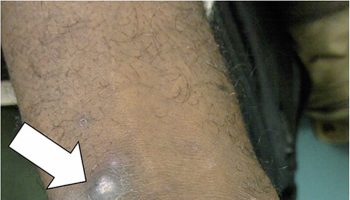
Figure 3. Gingival hyperplasia due to scurvy
Footnote: Gingival hyperplasia, most prominent in the patient’s left upper gum line (arrow), is a typical sign of scurvy.
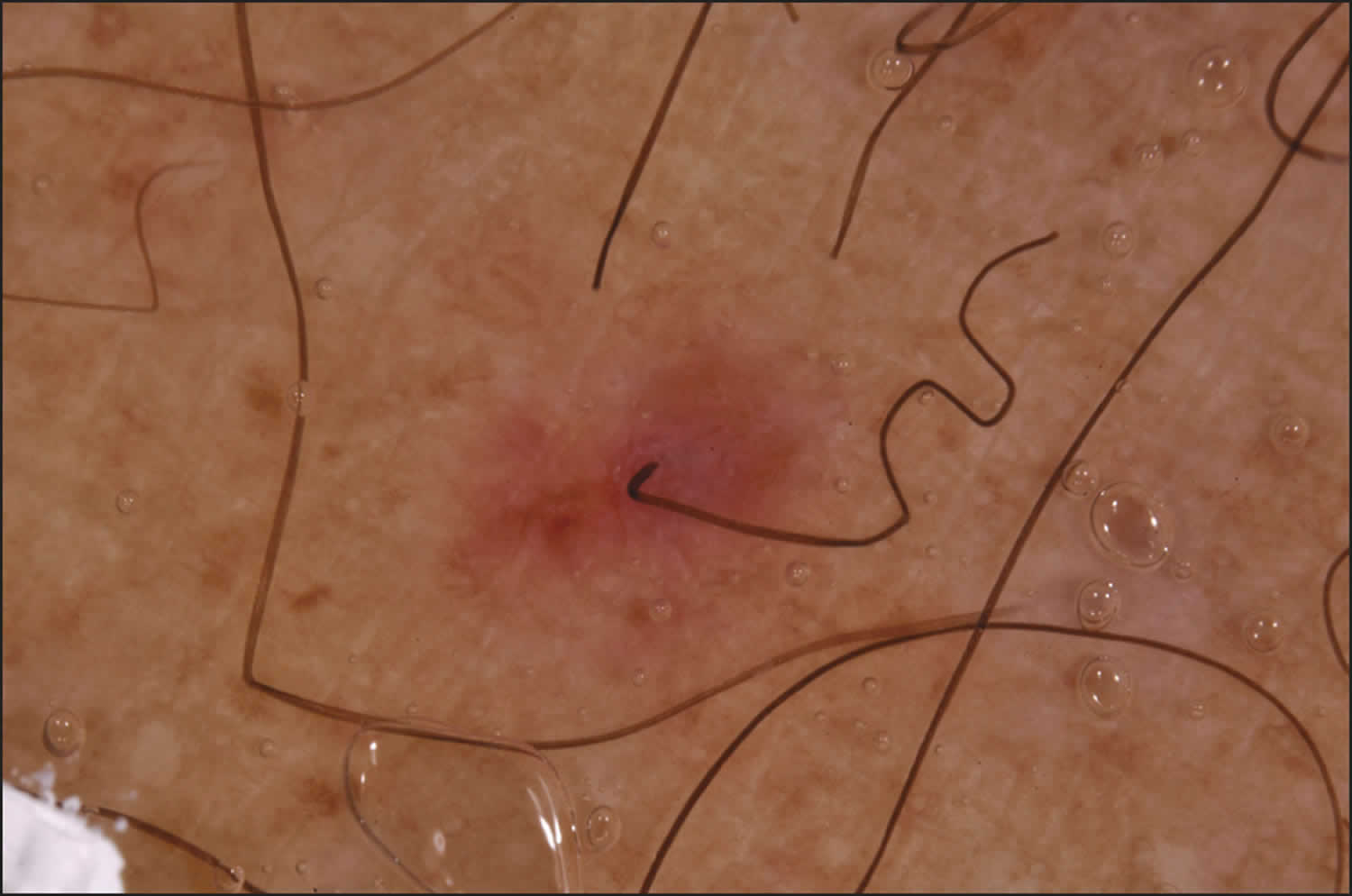
[Source 15 ]Figure 4. Perifollicular hemorrhagic papules
[Source 35 ]Figure 5. Hyperkeratotic hair follicles
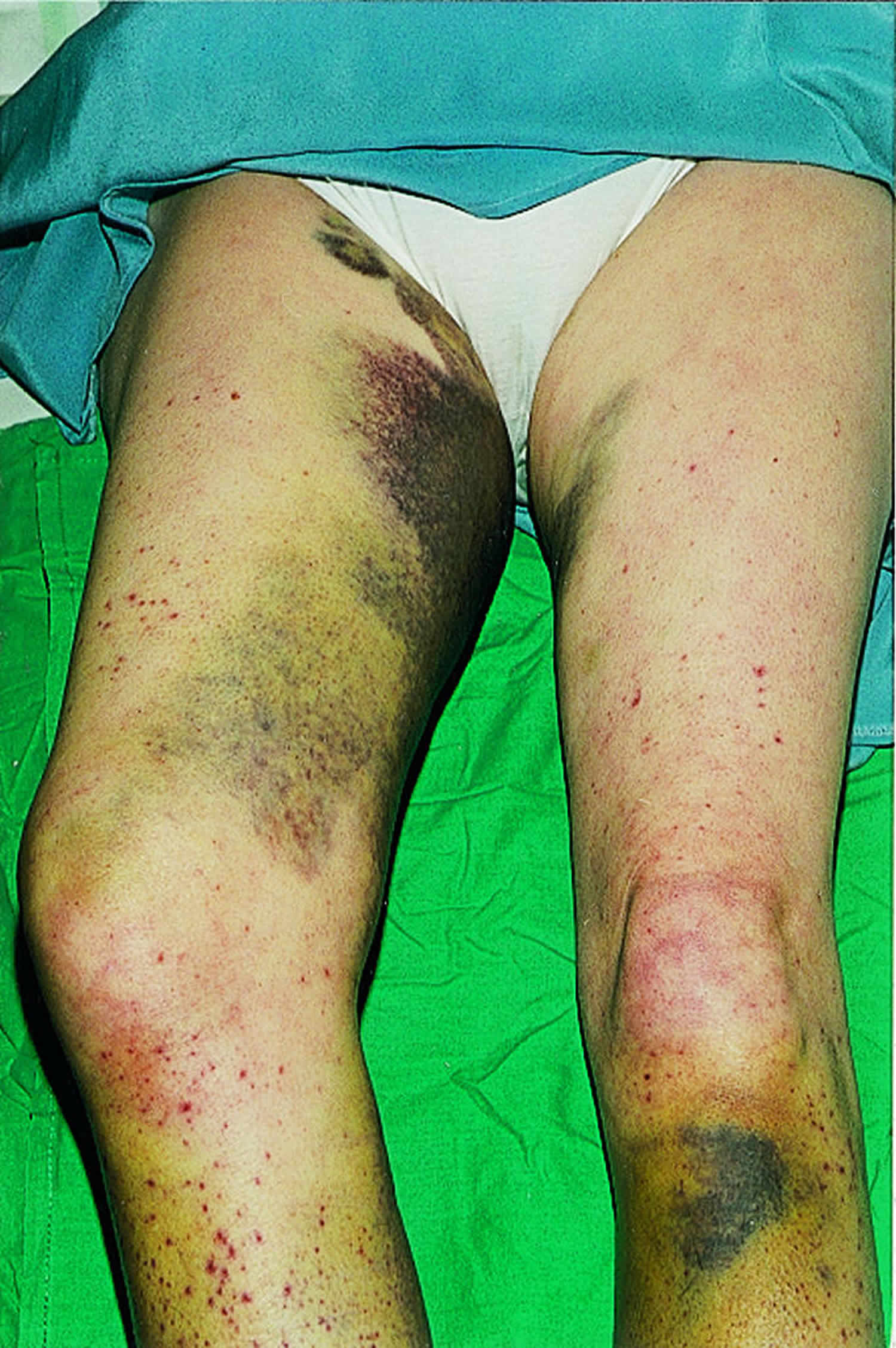
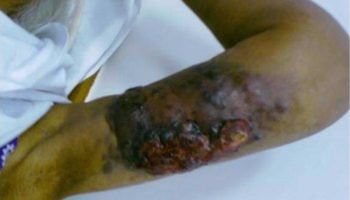
[Source 36 ]Figure 6. Extensive bruising
Footnote: A 59 year old woman presented to our department with a month’s history of extensive bruising of the legs and a more recent haemarthrosis of the right knee. Full blood count, blood film, and clotting screen were normal, but she had longstanding oesophageal reflux, a very restricted diet, and undetectable serum concentration of vitamin C. Her doctor diagnosed scurvy and treated her with vitamin C. Hemorrhagic symptoms settled within two weeks, as did her gastrointestinal symptoms. She now has a more normal diet.
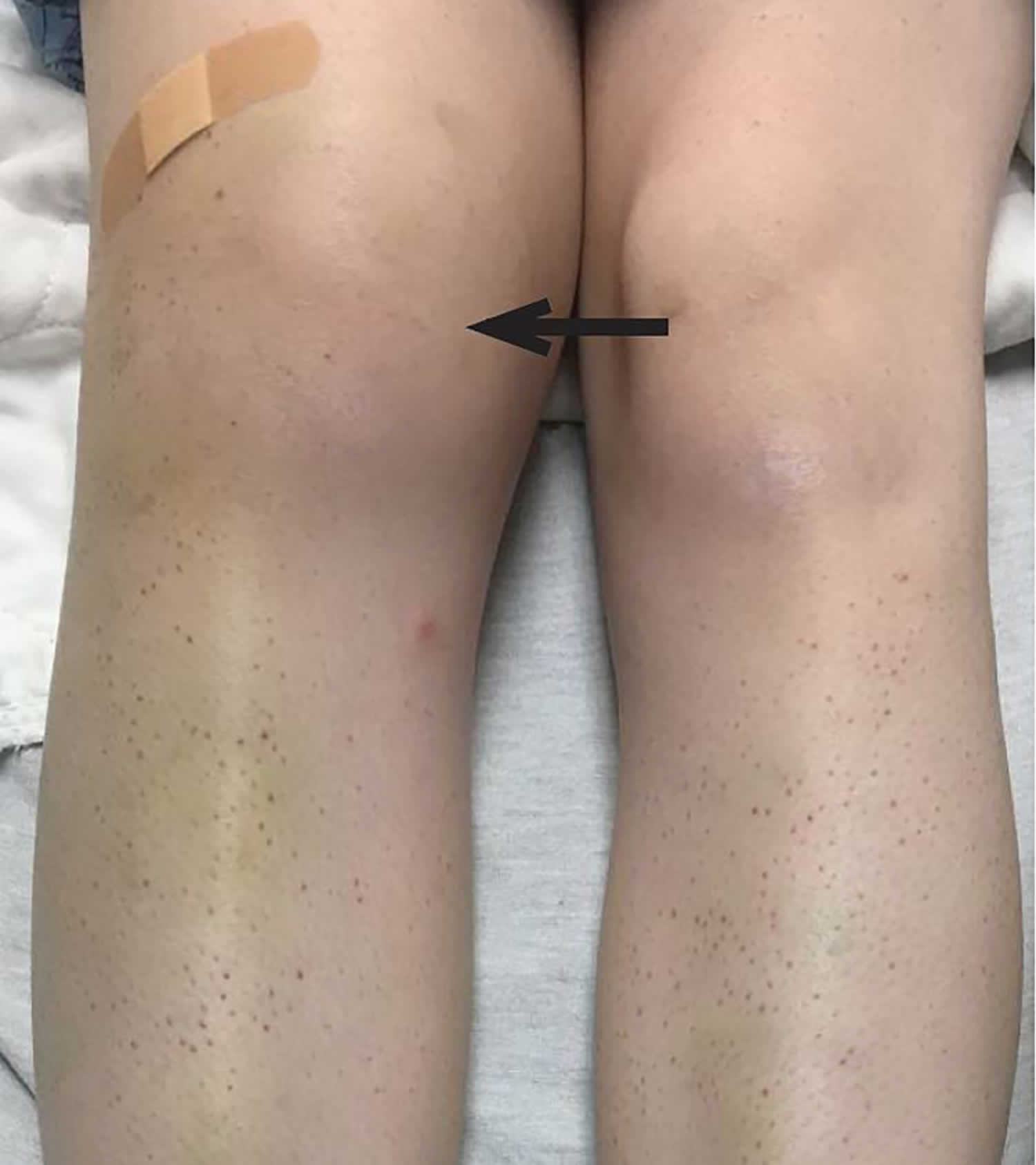
[Source 37 ]Figure 7. Hemarthrosis (bleeding into the joint cavity)
[Source 15 ]Is scurvy contagious?
No. Scurvy is a clinical syndrome that results from vitamin C deficiency.
Scurvy is caused by the deficiency of which vitamin?
Scurvy is a clinical syndrome that results from vitamin C deficiency.
Who is at risk of vitamin C deficiency?
Vitamin C deficiency is rare. Vitamin C deficiency usually arises in the setting of decreased intake or increased requirements or losses. Persons at risk for inadequate intake of vitamin include patients in the following groups:
- Find it difficult to maintain a healthy diet of fresh fruit and vegetables (e.g. elderly people, low-income households, people with an eating disorder)
- Those who smoke heavily or are dependent on alcohol or drugs
- Have a health condition that makes it difficult to digest food, such as celiac disease, ulcerative colitis or Crohn’s disease
- Those with alcoholism, anorexia, or cancer
- Eat very little food at all – possible reasons include treatments that make you feel very sick all the time (such as chemotherapy) or an eating disorder such as anorexia
- Practicing food fads – with very few or no sources of vitamin C
- Those with presumed food allergies
- Receiving unsupplemented parenteral nutrition
- Those on restricted diets secondary to inflammatory bowel disease, gastrointestinal reflux or Whipple disease
- Taking medications such as aspirin, indomethacin, oral contraceptives, tetracyclines, and corticosteroids.
- Those who have renal failure due to filtration of water-soluble vitamin C during dialysis
- Those with a complication of interleukin-2 treatment of metastatic renal cell carcinoma
- Receiving liver transplants
- Have a poor diet and are pregnant or breastfeeding – your body needs more vitamin C at these times
- Babies and young children who aren’t getting the recommended amount of vitamins
What is Vitamin C?
Vitamin C also known as ascorbic acid, L-ascorbic acid or ascorbate, is an essential, water-soluble vitamin that is naturally present in some foods, added to others and available as a dietary supplement. Vitamin C is synthesized from D-glucose or D-galactose by many plants and animals. However, humans lack the enzyme L-gulonolactone oxidase required for ascorbic acid synthesis and must obtain vitamin C through food or supplements 38, 39. Vitamin C is found in many fruits and vegetables, including citrus fruits, tomatoes, potatoes, red and green peppers, kiwifruit, broccoli, strawberries, brussels sprouts, and cantaloupe. In the body, vitamin C acts as an antioxidant, helping to protect cells from the damage caused by free radicals. Free radicals are compounds formed when our bodies convert the food you eat into energy. People are also exposed to free radicals in the environment from cigarette smoke, air pollution, and ultraviolet light from the sun.
The Recommended Dietary Allowance (RDA; average daily level of intake sufficient to meet the nutrient requirement of 97–98% healthy individuals) for vitamin C ranges from 15 to 115 mg for infants and children (depending on age) and from 75 to 120 mg for nonsmoking adults; people who smoke need 35 mg more per day 40. The intestinal absorption of vitamin C is regulated by at least one specific dose-dependent, active transporter 41. Cells accumulate vitamin C via a second specific transport protein. In vitro studies have found that oxidized vitamin C, or dehydroascorbic acid, enters cells via some facilitated glucose transporters and is then reduced internally to ascorbic acid. The physiologic importance of dehydroascorbic acid uptake and its contribution to overall vitamin C economy is unknown 9.
Fruits and vegetables are the best sources of vitamin C. You can get the recommended amounts of vitamin C by eating a variety of foods including the following:
- Citrus fruits (such as oranges and grapefruit) and their juices, as well as red and green pepper and kiwifruit, which have a lot of vitamin C.
- Other fruits and vegetables—such as broccoli, strawberries, cantaloupe, baked potatoes, and tomatoes—which also have vitamin C.
- Some foods and beverages that are fortified with vitamin C. To find out if vitamin C has been added to a food product, check the product labels.
The vitamin C content of food may be reduced by prolonged storage and by cooking because ascorbic acid is water soluble and is destroyed by heat 40, 17. Steaming or microwaving may lessen cooking losses. Fortunately, many of the best food sources of vitamin C, such as fruits and vegetables, are usually consumed raw. Consuming five varied servings of fruits and vegetables a day can provide more than 200 mg of vitamin C.
Vitamin C plays a role in collagen, carnitine, hormone, and amino acid formation. It is essential for wound healing and facilitates recovery from burns. Vitamin C is also an antioxidant, supports immune function, and facilitates the absorption of iron 42. Vitamin C also plays an important role in both innate and adaptive immunity, probably because of its antioxidant effects, antimicrobial and antiviral actions, and effects on immune system modulators 43. Vitamin C helps maintain epithelial integrity, enhance the differentiation and proliferation of B cells and T cells, enhance phagocytosis, normalize cytokine production, and decrease histamine levels 44. Vitamin C might also inhibit viral replication 45.
Vitamin C deficiency impairs immune function and increases susceptibility to infections 44. Some research suggests that supplemental vitamin C enhances immune function 46, but its effects might vary depending on an individual’s vitamin C status 47.
Vitamin C deficiency is uncommon in the United States, affecting only about 7% of individuals aged 6 years and older 48. People who smoke and those whose diets include a limited variety of foods (such as some older adults and people with alcohol or drug use disorders) are more likely than others to obtain insufficient amounts of vitamin C 46.
Vitamin C is required for the biosynthesis of collagen, L-carnitine, and certain neurotransmitters; vitamin C is also involved in protein metabolism 32. Collagen is an essential component of connective tissue, which plays a vital role in wound healing. Vitamin C is also an important physiological antioxidant 49 and has been shown to regenerate other antioxidants within the body, including vitamin E (alpha-tocopherol) 50. Ongoing research is examining whether vitamin C, by limiting the damaging effects of free radicals through its antioxidant activity, might help prevent or delay the development of certain cancers, cardiovascular disease, and other diseases in which oxidative stress plays a causal role. In addition to its biosynthetic and antioxidant functions, vitamin C plays an important role in immune function 50 and improves the absorption of nonheme iron 51, the form of iron present in plant-based foods. Insufficient vitamin C intake causes scurvy, which is characterized by fatigue or lassitude, widespread connective tissue weakness, and capillary fragility 17.
The intestinal absorption of vitamin C is regulated by at least one specific dose-dependent, active transporter 52. Cells accumulate vitamin C via a second specific transport protein. In vitro studies have found that oxidized vitamin C, or dehydroascorbic acid, enters cells via some facilitated glucose transporters and is then reduced internally to ascorbic acid. The physiologic importance of dehydroascorbic acid uptake and its contribution to overall vitamin C economy is unknown.
Oral vitamin C produces tissue and plasma concentrations that the body tightly controls. Approximately 70%–90% of vitamin C is absorbed at moderate intakes of 30–180 mg/day. However, at doses above 1 g/day, absorption falls to less than 50% and absorbed, unmetabolized ascorbic acid is excreted in the urine 50. Results from pharmacokinetic studies indicate that oral doses of 1.25 g/day ascorbic acid produce mean peak plasma vitamin C concentrations of 135 micromol/L, which are about two times higher than those produced by consuming 200–300 mg/day ascorbic acid from vitamin C-rich foods 53. Pharmacokinetic modeling predicts that even doses as high as 3 g ascorbic acid taken every 4 hours would produce peak plasma concentrations of only 220 micromol/L 53.
The total body content of vitamin C ranges from 300 mg (at near scurvy) to about 2 g 50. High levels of vitamin C (millimolar concentrations) are maintained in cells and tissues, and are highest in leukocytes (white blood cells), eyes, adrenal glands, pituitary gland, and brain. Relatively low levels of vitamin C (micromolar concentrations) are found in extracellular fluids, such as plasma, red blood cells, and saliva 50.
Even before the discovery of vitamin C in 1932, nutrition experts recognized that something in citrus fruits could prevent scurvy, a disease that killed as many as two million sailors between 1500 and 1800 54. Scurvy is a disease caused by a deficiency of vitamin C, characterized by swollen bleeding gums, malaise, lethargy, easy bruising, and spontaneous bleeding and the opening of previously healed wounds 55, which particularly affected poorly nourished sailors until the end of the 18th century 54. High-dose vitamin C has been studied as a treatment for patients with cancer since the 1970s. A Scottish surgeon named Ewan Cameron worked with Nobel Prize-winning chemist Linus Pauling to study the possible benefits of vitamin C therapy in clinical trials of cancer patients in the late 1970s and early 1980’s 56. In the 1970s, Linus Pauling promoted daily megadoses of vitamin C (the amount in 12 to 24 oranges) as a way to prevent colds and some chronic diseases 57. In the mid-20th century, a study hypothesized that cancer may be related to changes in connective tissue, which may be a consequence of vitamin C deficiency 58. A review of evidence published in 1974 suggested that high-dose ascorbic acid may increase host resistance and be a potential cancer therapy 59.
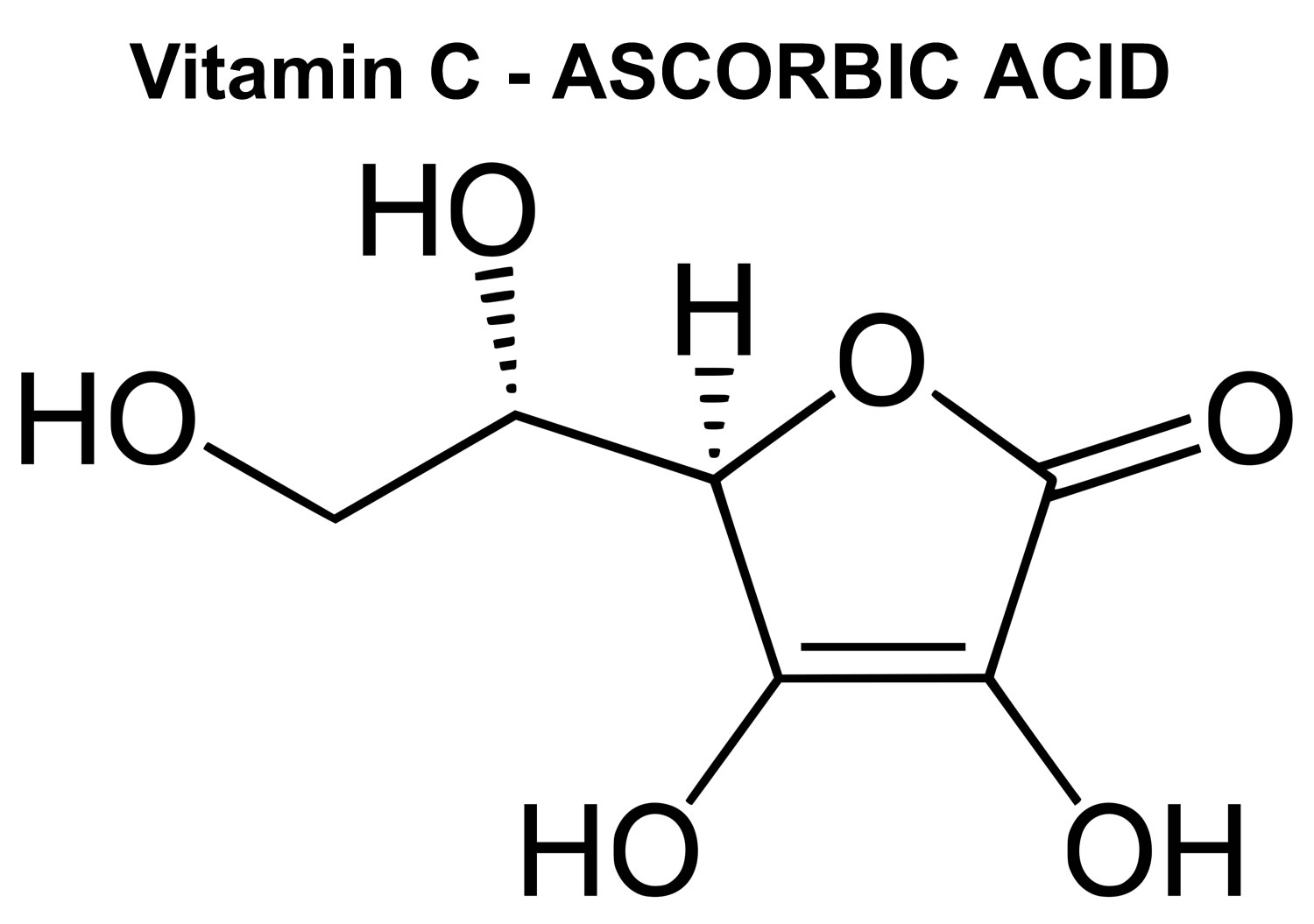
Figure 8. Vitamin C chemical structure
What does vitamin C do?
Vitamin C plays a role in collagen, carnitine, hormone, and amino acid formation 11. Vitamin C is essential for wound healing and facilitates recovery from burns. Vitamin C is also an antioxidant [substance that prevents or reduces damage caused by reactive oxygen species (ROS) or reactive nitrogen species (RNS) by donating electron], supports immune function, and facilitates the absorption of iron 29, 42. Vitamin C is a potent antioxidant (reducing agent), meaning that it readily donates electrons to recipient molecules, which can reduce, and thereby neutralize, reactive oxygen species (ROS) (see Figure 9). Vitamin C also plays an important role in both innate and adaptive immunity, probably because of its antioxidant effects, antimicrobial and antiviral actions, and effects on immune system modulators 43. Vitamin C helps maintain epithelial integrity, enhance the differentiation and proliferation of B cells and T cells, enhance phagocytosis, normalize cytokine production, and decrease histamine levels 44. Vitamin C might also inhibit viral replication 45.
Vitamin C deficiency impairs immune function and increases susceptibility to infections 44. Some research suggests that supplemental vitamin C enhances immune function 46, but its effects might vary depending on an individual’s vitamin C status 47.
High-Dose vitamin C, when taken by intravenous (IV) infusion, vitamin C can reach much higher levels in the blood than when it is taken by mouth. Studies suggest that these higher levels of vitamin C may cause the death of cancer cells in the laboratory. Surveys of healthcare practitioners at United States complementary and alternative medicine conferences in recent years have shown that high-dose IV vitamin C is frequently given to patients as a treatment for infections, fatigue, and cancers, including breast cancer 60.
Vitamin C is required for the biosynthesis of collagen, L-carnitine, and certain neurotransmitters; vitamin C is also involved in protein metabolism 61, 62. Collagen is an essential component of connective tissue, which plays a vital role in wound healing. Vitamin C is also an important physiological antioxidant 63 and has been shown to regenerate other antioxidants within the body, including alpha-tocopherol (vitamin E) 52. Ongoing research is examining whether vitamin C, by limiting the damaging effects of free radicals through its antioxidant activity, might help prevent or delay the development of certain cancers, cardiovascular disease, and other diseases in which oxidative stress plays a causal role. In addition to its biosynthetic and antioxidant functions, vitamin C plays an important role in immune function 52 and improves the absorption of nonheme iron 64, the form of iron present in plant-based foods. Insufficient vitamin C intake causes scurvy, which is characterized by fatigue or lassitude, widespread connective tissue weakness, and capillary fragility 61, 62, 52, 65, 66, 40, 67.
Vitamin C is the primary water-soluble, non-enzymatic antioxidant in plasma and tissues. Even in small amounts, vitamin C can protect indispensable molecules in the body, such as proteins, lipids (fats), carbohydrates, and nucleic acids (DNA and RNA), from damage by free radicals and reactive oxygen species (ROS) that are generated during normal metabolism, by active immune cells, and through exposure to toxins and pollutants (e.g., certain chemotherapy drugs and cigarette smoke). Vitamin C also participates in redox recycling of other important antioxidants; for example, vitamin C is known to regenerate vitamin E from its oxidized form.
The role of vitamin C as a cofactor is also related to its redox potential (another term for an oxidation-reduction reaction). A redox reaction is any reaction in which electrons are removed from one molecule or atom and transferred to another molecule or atom. In such a reaction one substance is oxidized (loses electrons) while the other is reduced (gains electrons). By maintaining enzyme-bound metals in their reduced forms, vitamin C assists mixed-function oxidases in the synthesis of several critical biomolecules 68. These enzymes are either monooxygenases or dioxygenases. Symptoms of vitamin C deficiency, such as poor wound healing and lethargy, likely result from the impairment of these vitamin C-dependent enzymatic reactions leading to the insufficient synthesis of collagen, carnitine, and catecholamines. Moreover, several dioxygenases involved in the regulation of gene expression and the maintenance of genome integrity require vitamin C as a cofactor. Indeed, research has recently uncovered the crucial role played by enzymes, such as the Ten-eleven translocation (TET) dioxygenases and Jumonji domain-containing histone demethylases, in the fate of cells and tissues. These enzymes contribute to the epigenetic regulation of gene expression by catalyzing reactions involved in the demethylation of DNA and histones. The capacity of vitamin C to influence the methylation status of DNA and histones in mammalian cells supports a role for the vitamin in health and disease beyond what was previously understood, in particular by safeguarding genome integrity 69.
Numerous in vitro studies (test tube studies) demonstrate vitamin C’s ability to prevent oxidative stress in human cell lines, a process which has also been shown to occur in the human body. Cooke and colleagues 70 measured urinary and serum levels of 8-oxo-2′-deoxyguanosine (8-oxodG) to evaluate oxidative stress. They measured serum and urinary 8-oxodG after the supplementation of 500 mg of vitamin C in both experimental and control subjects over the course of 25 weeks. Vitamin C supplementation began 3 weeks after a baseline of 8-oxodG was established. After the vitamin C washout period, where no vitamin C was supplemented, there was a significant increase in the levels of 8-oxodG in DNA, enforcing its antioxidant effects Cooke M.S., Evans M.D., Podmore I.D., Herbert K.E., Mistry N., Mistry P., Hickenbotham P.T., Hussieni A., Griffiths H.R., Lunec J. Novel repair action of vitamin C upon in vivo oxidative DNA damage. FEBS Lett. 1998;439:363–367. doi: 10.1016/S0014-5793(98)01403-3. These results were negatively correlated, but the authors did not report the experimental or control 8-oxodG levels in DNA Cooke M.S., Evans M.D., Podmore I.D., Herbert K.E., Mistry N., Mistry P., Hickenbotham P.T., Hussieni A., Griffiths H.R., Lunec J. Novel repair action of vitamin C upon in vivo oxidative DNA damage. FEBS Lett. 1998;439:363–367. doi: 10.1016/S0014-5793(98)01403-3. This study was performed with only 30 healthy volunteers, making it difficult to generalize; however, other studies have shown similar results. Fraga et al. 71 illustrated that with a decrease in the intake of vitamin C, there were elevated levels of 8-oxo-dG in human sperm. In another study, 14 healthy human volunteers who had taken vitamin C had a decrease in H2O2 damage in isolated white blood cells 72. However, there was no change in endogenous DNA damage. Brennan et al. 72 had their participants take 1000 mg vitamin C daily for 42 days or 800 mg vitamin E for 42 days. Peripheral blood was taken and treated with 200 micromolar H2O2, 10 micromolar H2O2, or used as a control. They analyzed DNA damage using ELISA after a 3-week and 6-week wash out period. Cells that were treated with 200 micromolar H2O2 showed a significantly decreased DNA oxidative damage when supplementing with vitamin C 72. For vitamin C, the DNA decreased from roughly 78% to 45%. The control did not have hydrogen peroxide added nor did it have vitamins added. The DNA damage was consistent between 10 and 20% 72. Another study examined lung cancer prevention, demonstrating that smokers who supplemented their diet with vitamin C had less oxidative DNA damage than prior to supplementation 73. The researchers obtained results comparing 500 mg slow-release and plain release tablets of vitamin C paired with an average dose of vitamin E (91 mg), and assessed how this protocol changed the levels of endonuclease 3 and formamidopyrimidine DNA glycosylase enzymes, which mediate DNA repair after oxidative damage. The result was that the slow-release tablet prolonged the protective effect of oxidative DNA damage after a 4-week trial 74. Bo and colleagues 75 performed a meta-analysis of the existing literature to assess the impact of dietary vitamin C on esophageal cancer risk. Their meta-analysis included 15 studies, encompassing 7063 controls and 3955 cancer cases. Their results demonstrate that higher dietary vitamin C intake is inversely associated with esophageal cancer risk 75. Similar results were shown with bladder cancer 76, breast cancer 76 and prostate cancer 77. However, a number of meta-analyses demonstrate non-significant results. One meta-analysis of 47 studies found no association between dietary vitamin C intake and colorectal cancer risk 78. These results support the notion that vitamin C may have site-specific effects, inhibiting certain cancers with no impact on others. Generalization of the results of these studies may be difficult due to the number of confounders that limit each study.
Additionally, many studies have evaluated the impact of supplemental vitamin C and cancer prevention. The Iowa Women’s Health Study published by Kushi et al. 79, followed 34,387 eligible women ages 55–69 through questionnaires for four years. They assessed the antioxidant vitamins A, C, and E. Women that consumed more than 10,000 IU/day of vitamin A demonstrated a slight decrease in age-adjusted risk of breast cancer 79. Those who took vitamin C supplements between 500 and 1000 mg/day had a relative risk of 0.79, but those who took over 1000 mg had a relative risk of 0.77 which showed insignificant differences between the two. After following the women who supplemented vitamin C, there was no significant decrease in risk of developing breast cancer and no significant protective factors against breast cancer 79. In a case control study with 261 women with cervical cancer and 498 controls, diet was assessed and analyzed to see if there was change in cancer after the addition of different supplements 80. No correlation was found between vitamin C and cervical cancer risk 80. One case control study assessed vitamin supplementation and risk of oral or pharyngeal cancer risk 81. After controlling for other risk factors such as smoking and alcohol, there was a significant decrease in risk when supplementing vitamin C 81. However, when adjusting for other use of supplements, the only vitamin that was still associated with a decreased risk was vitamin E 81. Additionally, the PROTEUS study 82, which was a case-control study including 1916 patients with prostate cancer matched with 1915 controls, failed to demonstrate any relationship between dietary or supplemental vitamin C and cancer prevention. In summary, the current body of evidence surrounding the supplementation of vitamin C for cancer prevention fails to demonstrate any definite conclusions. Furthermore, if there is a benefit to supplemental vitamin C for cancer prevention, the potential mechanism may occur through a broad variety of pathways, which may or may not include its antioxidant properties. Future case-control or prospective cohort studies should be designed and control for the impact of multiple vitamin supplements in carcinogenic risk.
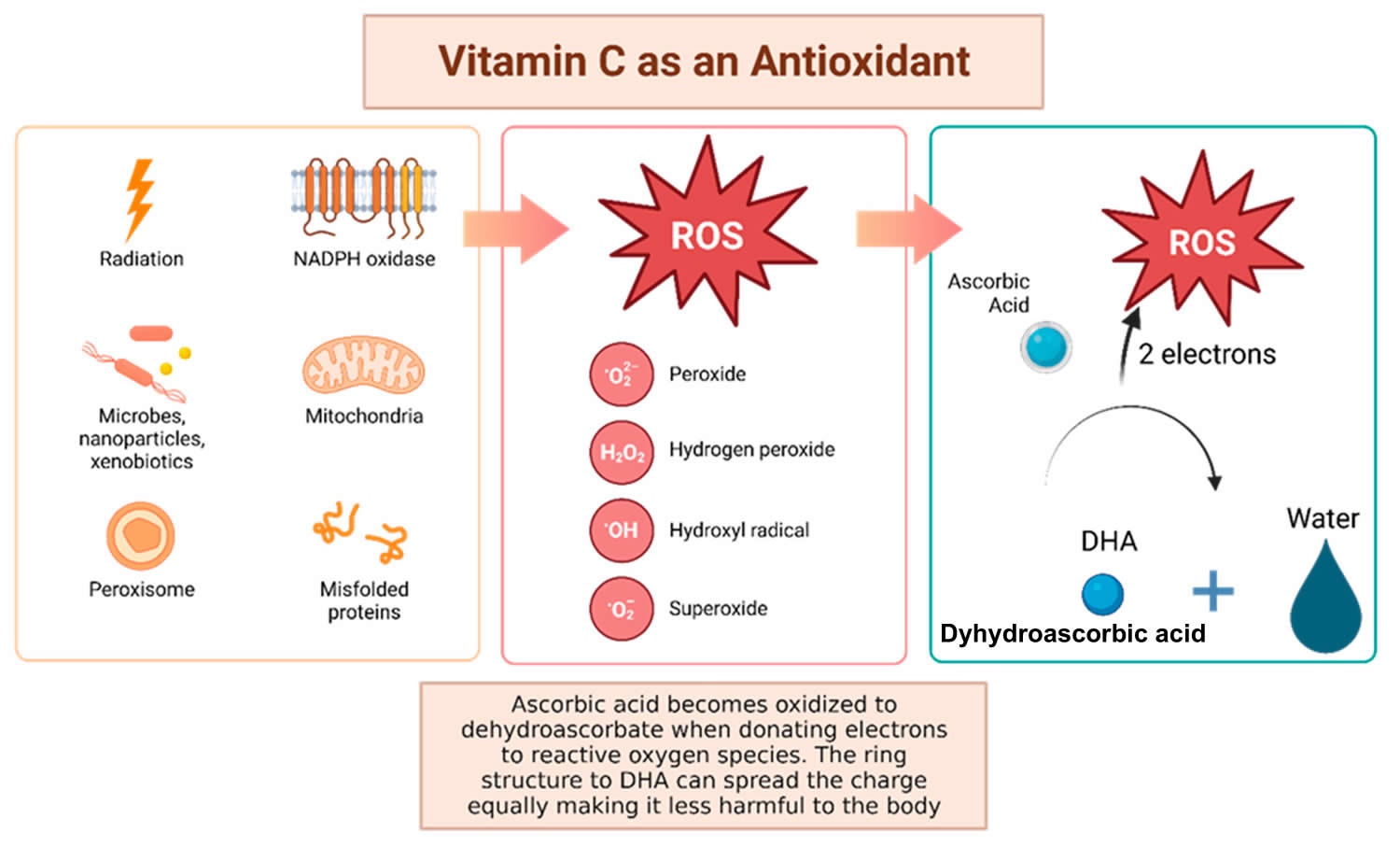
Figure 9. Vitamin C antioxidant properties
Footnotes: Vitamin C has been shown to have antioxidant properties, allowing it to reduce free radicals that may cause harmful damage to DNA. Reactive oxygen species (ROS) may be made by peroxisomes, radiation, the mitochondria, and more biological processes which result in ROS. Vitamin C (ascorbic acid), when ingested, contains electrons that it can give to reactive oxygen species (ROS). These will be reduced to water, and therefore will not be harmful to the body 83. The oxidized version of vitamin C, or dehydroascorbate (DHA), has the ability to even out the positive charge with its ring structure ensuring that it, itself, is not going to damage cells 84.
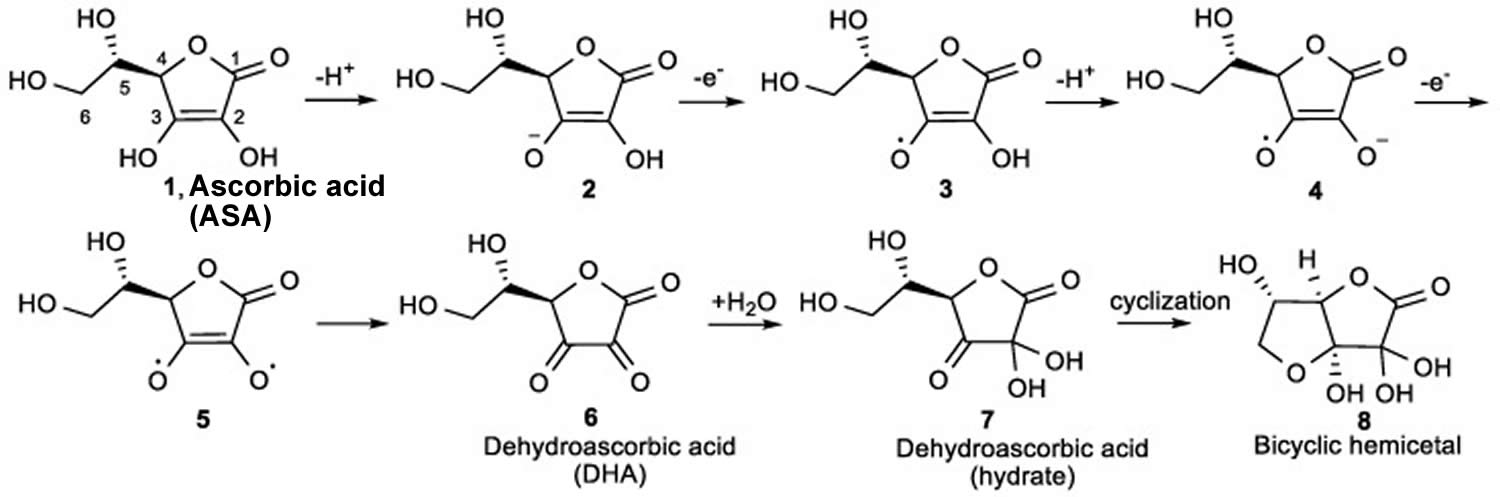
[Source 85 ]Figure 10. Vitamin C Antioxidant Effects
Footnote: The mechanism for the ionization and oxidation of ascorbic acid (ASA) to the inactive dehydroascorbic acid (DHA) and bicyclic hemiacetal.
[Source 86 ]How much vitamin C do I need?
The amount of vitamin C you need each day depends on your age. Average daily recommended amounts for different ages are listed below in milligrams (mg).
You should be able to get all the vitamin C you need from your daily diet.
Vitamin C can’t be stored in the body, so you need it in your diet every day.
Table 1. Vitamin C requirement by age group
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months* | 40 mg |
| Infants 7–12 months* | 50 mg |
| Children 1–3 years | 15 mg |
| Children 4–8 years | 25 mg |
| Children 9–13 years | 45 mg |
| Teens 14–18 years (boys) | 75 mg |
| Teens 14–18 years (girls) | 65 mg |
| Adults (men) | 90 mg |
| Adults (women) | 75 mg |
| Pregnant teens | 80 mg |
| Pregnant women | 85 mg |
| Breastfeeding teens | 115 mg |
| Breastfeeding women | 120 mg |
Footnote: If you smoke, add 35 mg to the above values to calculate your total daily recommended amount.
* Adequate Intake (intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an recommended dietary allowance (RDA) – the RDA is the average daily dietary intake level of a nutrient sufficient to meet the requirements of nearly all healthy individuals in a specific life stage and gender group)
[Source 87 ]What foods provide vitamin C?
Fruits and vegetables are the best sources of vitamin C (see Table 2) 9. You can get the recommended amounts of vitamin C by eating a variety of foods including the following:
- Citrus fruits (such as oranges and grapefruit) and their juices, as well as red and green pepper and kiwifruit, which have a lot of vitamin C.
- Other fruits and vegetables—such as broccoli, strawberries, cantaloupe, baked potatoes, and tomatoes—which also have vitamin C.
- Some foods and beverages that are fortified with vitamin C. To find out if vitamin C has been added to a food product, check the product labels.
Citrus fruits, tomatoes and tomato juice, and potatoes are major contributors of vitamin C to the American diet 40. Other good food sources include red and green peppers, kiwifruit, broccoli, strawberries, Brussels sprouts, and cantaloupe (see Table 2) 40. Although vitamin C is not naturally present in grains, it is added to some fortified breakfast cereals.
The vitamin C content of food may be reduced by prolonged storage and by cooking because ascorbic acid is water soluble and is destroyed by heat 40, 17. Steaming or microwaving may lessen cooking losses. Fortunately, many of the best food sources of vitamin C, such as fruits and vegetables, are usually consumed raw. Consuming five varied servings of fruits and vegetables a day can provide more than 200 mg of vitamin C.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central website (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing vitamin C arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitaminC-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitaminC-Food.pdf).
Table 2. Vitamin C content of selected foods
| Food | Milligrams (mg) per serving | Percent (%) DV* |
|---|---|---|
| Red pepper, sweet, raw, ½ cup | 95 | 158 |
| Orange juice, ¾ cup | 93 | 155 |
| Orange, 1 medium | 70 | 117 |
| Grapefruit juice, ¾ cup | 70 | 117 |
| Kiwifruit, 1 medium | 64 | 107 |
| Green pepper, sweet, raw, ½ cup | 60 | 100 |
| Broccoli, cooked, ½ cup | 51 | 85 |
| Strawberries, fresh, sliced, ½ cup | 49 | 82 |
| Brussels sprouts, cooked, ½ cup | 48 | 80 |
| Grapefruit, ½ medium | 39 | 65 |
| Broccoli, raw, ½ cup | 39 | 65 |
| Tomato juice, ¾ cup | 33 | 55 |
| Cantaloupe, ½ cup | 29 | 48 |
| Cabbage, cooked, ½ cup | 28 | 47 |
| Cauliflower, raw, ½ cup | 26 | 43 |
| Potato, baked, 1 medium | 17 | 28 |
| Tomato, raw, 1 medium | 17 | 28 |
| Spinach, cooked, ½ cup | 9 | 15 |
| Green peas, frozen, cooked, ½ cup | 8 | 13 |
Footnote: *DV = Daily Value. The DV (Daily Value) for vitamin C is 90 mg for adults and children age 4 years and older. Foods providing 20% or more of the DV (Daily Value) are considered to be high sources of a nutrient, but foods providing lower percentages of the DV (Daily Value) also contribute to a healthful diet.
[Source 9 ]Vitamin C deficiency causes
You can develop scurvy if you don’t have enough vitamin C in your diet for at least 3 months, which reduces total body vitamin C stores from 1500mg to less than 300mg and the plasma vitamin C level is less than 0.2 mg/dL (less than 11 umol/L) 88. You can’t store vitamin C in your body for long, so you need to take it in regularly. Vitamin C, which is also known as ascorbic acid, is found mainly in fruit and vegetables. Unlike nearly all other animals, humans are unable to synthesize Vitamin C which must be obtained from the diet.
Good sources of vitamin C include oranges, lemons, limes, guavas and kiwi fruit. Vegetables like broccoli, cauliflower, cabbage, Asian greens and tomatoes also have a lot of vitamin C. You can find vitamin C in many other fruits and vegetables and in fresh fruit and vegetable juices. The richest source is the bush food known as the Kakadu plum, salty plum or gubinge.
You are more at risk of getting scurvy if you:
- eat a diet without many fruit and vegetables
- cook your vegetables for long periods, since this destroys vitamin C
- have a health condition that makes it difficult to digest food, like Crohn’s disease or ulcerative colitis
- are on a very restrictive diet
- have an eating disorder like anorexia nervosa
- smoke, drink a lot of alcohol, or take drugs
You can also develop scurvy if you have a bad diet and you are pregnant or breastfeeding. Young children can get scurvy too.
You can even develop scurvy if you are overweight. You might consume a lot of calories, but if you don’t eat enough fresh fruit and vegetables you still might not be getting enough vitamin C.
Signs and symptoms of vitamin C deficiency or scurvy usually develop after 1 to 3 months of insufficient vitamin C 89. Vitamin C plays an integral role in several biochemical pathways, such as collagen biosynthesis and iron absorption 26. Mature collagen is composed of three polypeptide molecules that form a triple helix. Vitamin C is needed as a cofactor in the hydroxylation of lysine and proline residues on the polypeptides to allow for the formation of the triple helix structure 90. If this reaction does not occur, the polypeptides are unstable and unable to form rigid, triple helices. This collagen abnormality leads to blood vessel fragility and poor wound healing 26. Defective collagen synthesis and poor iron absorption are responsible for scurvy’s many clinical signs and symptoms, such as easy bruising, petechiae, bleeding gums, myalgia, anemia, hemarthrosis, perifollicular hemorrhages, and corkscrew hairs 91, 92. Extracutaneous hemorrhage may also occur in muscles, bones, eyes, heart, and the nervous system 93, 94, 95, 96, resulting in hematomas, subperiosteal bleeding, fractures due to osteopenia, loosening and subsequent loss of teeth, conjunctival varicosities, retrobulbar hemorrhages, hemopericardium, cardiac tamponade, and neuropathy due to hemorrhage into nerve sheaths 94, 16, 97, 10.
Groups at Risk of Vitamin C Deficiency
Acute vitamin C deficiency leads to scurvy 6. The timeline for the development of scurvy varies, depending on vitamin C body stores, but signs can appear within 1 month of little or no vitamin C intake (below 10 mg/day) 98. Initial symptoms can include fatigue (probably the result of impaired carnitine biosynthesis), malaise, and inflammation of the gums 7. As vitamin C deficiency progresses, collagen synthesis becomes impaired and connective tissues become weakened, causing petechiae, ecchymoses, purpura, joint pain, poor wound healing, hyperkeratosis, and corkscrew hairs 32. Additional signs of scurvy include depression as well as swollen, bleeding gums and loosening or loss of teeth due to tissue and capillary fragility 99. Iron deficiency anemia can also occur due to increased bleeding and decreased nonheme iron absorption secondary to low vitamin C intake 17. In children, bone disease can be present 17. Left untreated, scurvy is fatal 17.
Today, vitamin C deficiency and scurvy are rare in developed countries 100. Overt deficiency symptoms occur only if vitamin C intake falls below approximately 10 mg/day for many weeks 6. Vitamin C deficiency is uncommon in developed countries but can still occur in people with limited food variety.
In developed countries vitamin C deficiency affects up to 10% of women and 14% of men. Groups affected include:
- Children with very restricted diets such as may be seen with autism spectrum disorder, eating disorders, or food-fads; sickle cell disease or thalassaemia requiring blood transfusions resulting in iron overload; haemodialysis; bowel disease such as coeliac disease or Crohn disease
- Adults associated with:
- Pregnancy (due to an increased requirement)
- Psychiatric and behavioral disorders including depression, anorexia nervosa, alcoholism
- Malabsorption due to bowel disease, iron overload, or alcohol excess
- Cancer patients due to anorexia related to the malignancy, treatment, or depression.
- Elderly and others living alone, on a low income, homeless, or with poor dentition who are not eating a well-balanced diet
Smokers and passive smokers
Studies consistently show that smokers have lower plasma and leukocyte vitamin C levels than nonsmokers, due in part to increased oxidative stress 100. For this reason, the Institute of Medicine (IOM) of the National Academies concluded that smokers need 35 mg more vitamin C per day than nonsmokers 100. Exposure to secondhand smoke also decreases vitamin C levels. Although the Institute of Medicine (IOM) of the National Academies was unable to establish a specific vitamin C requirement for nonsmokers who are regularly exposed to secondhand smoke, these individuals should ensure that they meet the RDA for vitamin C 52, 100.
Infants fed evaporated or boiled milk
Most infants in developed countries are fed breastmilk and/or infant formula, both of which supply adequate amounts of vitamin C 100, 101. For many reasons, feeding infants evaporated or boiled cow’s milk is not recommended. This practice can cause vitamin C deficiency because cow’s milk naturally has very little vitamin C and heat can destroy vitamin C 65, 102.
Individuals with limited food variety
Although fruits and vegetables are the best sources of vitamin C, many other foods have small amounts of this nutrient 100. Thus, through a varied diet, most people should be able to meet the vitamin C RDA or at least obtain enough to prevent scurvy. People who have limited food variety—including some elderly, indigent individuals who prepare their own food; people who abuse alcohol or drugs; food faddists; people with mental illness; and, occasionally, children—might not obtain sufficient vitamin C 100.
People with malabsorption and certain chronic diseases
Some medical conditions can reduce the absorption of vitamin C and/or increase the amount needed by the body. People with severe intestinal malabsorption or cachexia and some cancer patients might be at increased risk of vitamin C inadequacy 103. Low vitamin C concentrations can also occur in patients with end-stage renal disease on chronic hemodialysis 104.
Vitamin C deficiency prevention
Eating a healthy, balanced diet, with plenty of fruit and vegetables, is the best way to prevent scurvy.
It’s best to steam vegetables rather than boil them. Or, you could eat vegetables raw or in a soup or stew. If you prefer to boil them, do it lightly.
Your doctor might refer you to a nutritionist, or another specialist, to help you improve your diet.
The amount of vitamin C you need each day depends on your age. Average daily recommended amounts for different ages are listed below in milligrams (mg). Intake recommendations for vitamin C and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by the Institute of Medicine 100. DRI is the general term for a set of reference values used for planning and assessing nutrient intakes of healthy people. These values, which vary by age and gender, include:
- Recommended Dietary Allowance (RDA): average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals.
- Adequate Intake (AI): established when evidence is insufficient to develop an RDA and is set at a level assumed to ensure nutritional adequacy.
- Estimated Average Requirement (EAR): Average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals.
- Tolerable Upper Intake Level (UL): maximum daily intake unlikely to cause adverse health effects.
Table 1 lists the current RDAs for vitamin C 100. For infants from birth to 12 months, the Institute of Medicine Food and Nutritional Board established an AI for vitamin C that is equivalent to the mean intake of vitamin C in healthy, breastfed infants.
The amount of vitamin C you need each day depends on your age. Average daily recommended amounts for different ages are listed below in milligrams (mg).
If you smoke, add 35 mg to the above values to calculate your total daily recommended amount.
Vitamin C status is typically assessed by measuring plasma vitamin C levels. Other measures, such as leukocyte vitamin C concentration, could be more accurate indicators of tissue vitamin C levels, but they are more difficult to assess and the results are not always reliable.
Table 1. Vitamin C requirement by age group
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months* | 40 mg |
| Infants 7–12 months* | 50 mg |
| Children 1–3 years | 15 mg |
| Children 4–8 years | 25 mg |
| Children 9–13 years | 45 mg |
| Teens 14–18 years (boys) | 75 mg |
| Teens 14–18 years (girls) | 65 mg |
| Adults (men) | 90 mg |
| Adults (women) | 75 mg |
| Pregnant teens | 80 mg |
| Pregnant women | 85 mg |
| Breastfeeding teens | 115 mg |
| Breastfeeding women | 120 mg |
Footnote: If you smoke, add 35 mg to the above values to calculate your total daily recommended amount.
* Adequate Intake (intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an RDA)
[Source 87 ]Vitamin C deficiency signs and symptoms
Other nutritional deficiencies are commonly associated with vitamin C deficiency or scurvy and clinical features may therefore be mixed and therefore confusing. Given that vitamin C and folate are often found in the same foods and that vitamin C also promotes iron absorption, patients who are deficient in vitamin C are often iron and folate-deficient 105.
Vitamin C deficiency manifests symptomatically after 8 to 12 weeks of inadequate intake and patients initially complain of weakness, fatigue, listlessness and aching limbs, especially in the legs. If left untreated, scurvy can progress to the following more severe problems. After these initial symptoms, dermatologic findings include poor wound healing, gingival swelling with loss of teeth, mucocutaneous petechiae, ecchymosis, and hyperkeratosis. Because of the disruption of disulfide bond formation both corkscrew and swan-neck hairs occur. Perifollicular hemorrhages often are localized to the lower extremities, as capillary fragility is unable to withstand the gravity-dependent hydrostatic pressure. This can result in “woody edema.” Nail findings include koilonychia and splinter hemorrhages. Beyond mucocutaneous manifestations, multiple other organ systems also are involved. Rheumatologic problems occur, including painful hemarthrosis and subperiosteal hemorrhage. This bleeding results from vascular fragility from impaired collagen formation. Osseous pathology also occurs and presents with fractures in brittle bones from disrupted endochondral bone formation. A “scorbutic rosary” at the costochondral junction and sternal depression may occur. Ocular manifestations of hemorrhage include flame hemorrhages, cotton-wool spots, and retrobulbar bleeding into optic nerves, resulting in atrophy and papilledema. The late disease may be life-threatening with anasarca, hemolysis, jaundice, and convulsions.
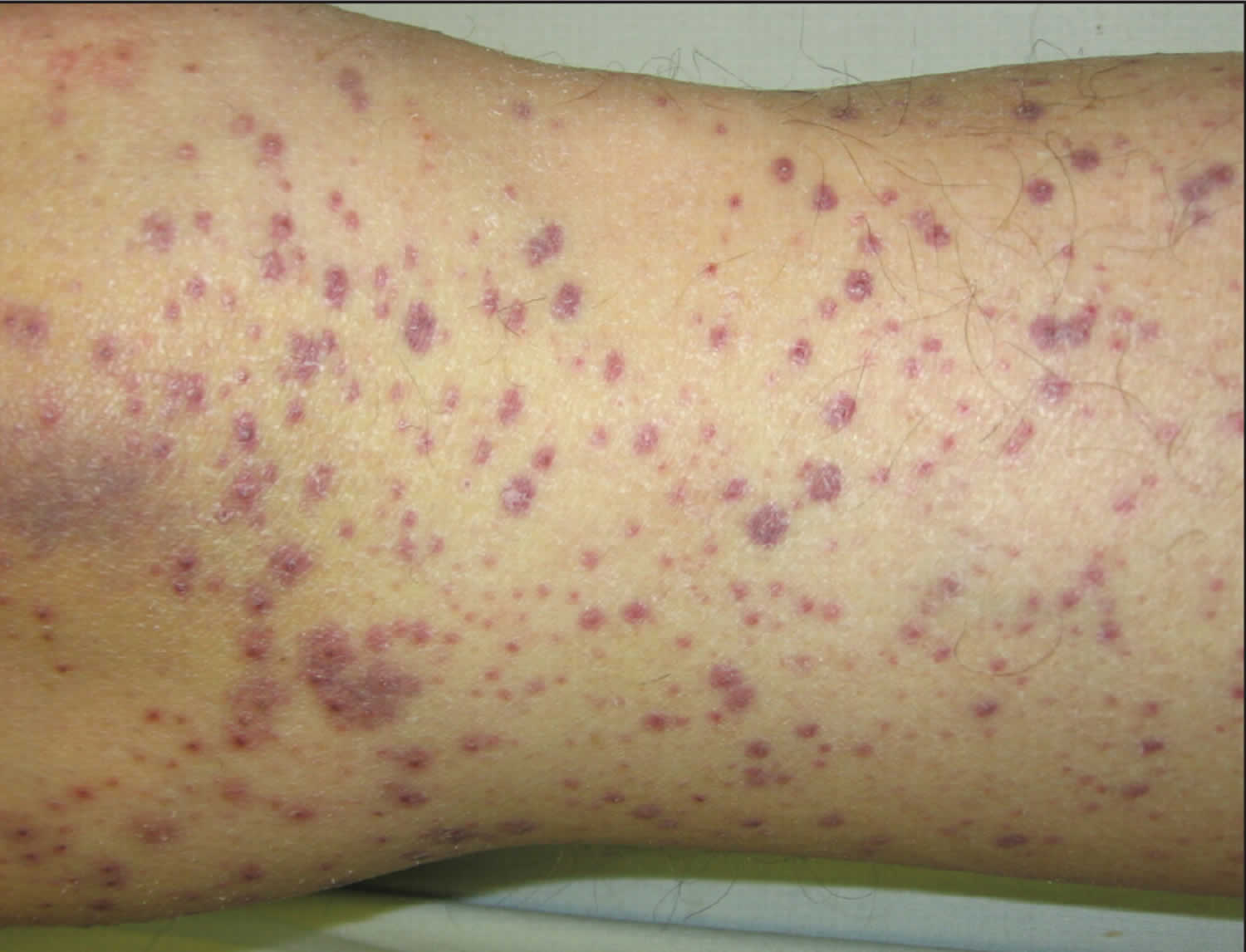
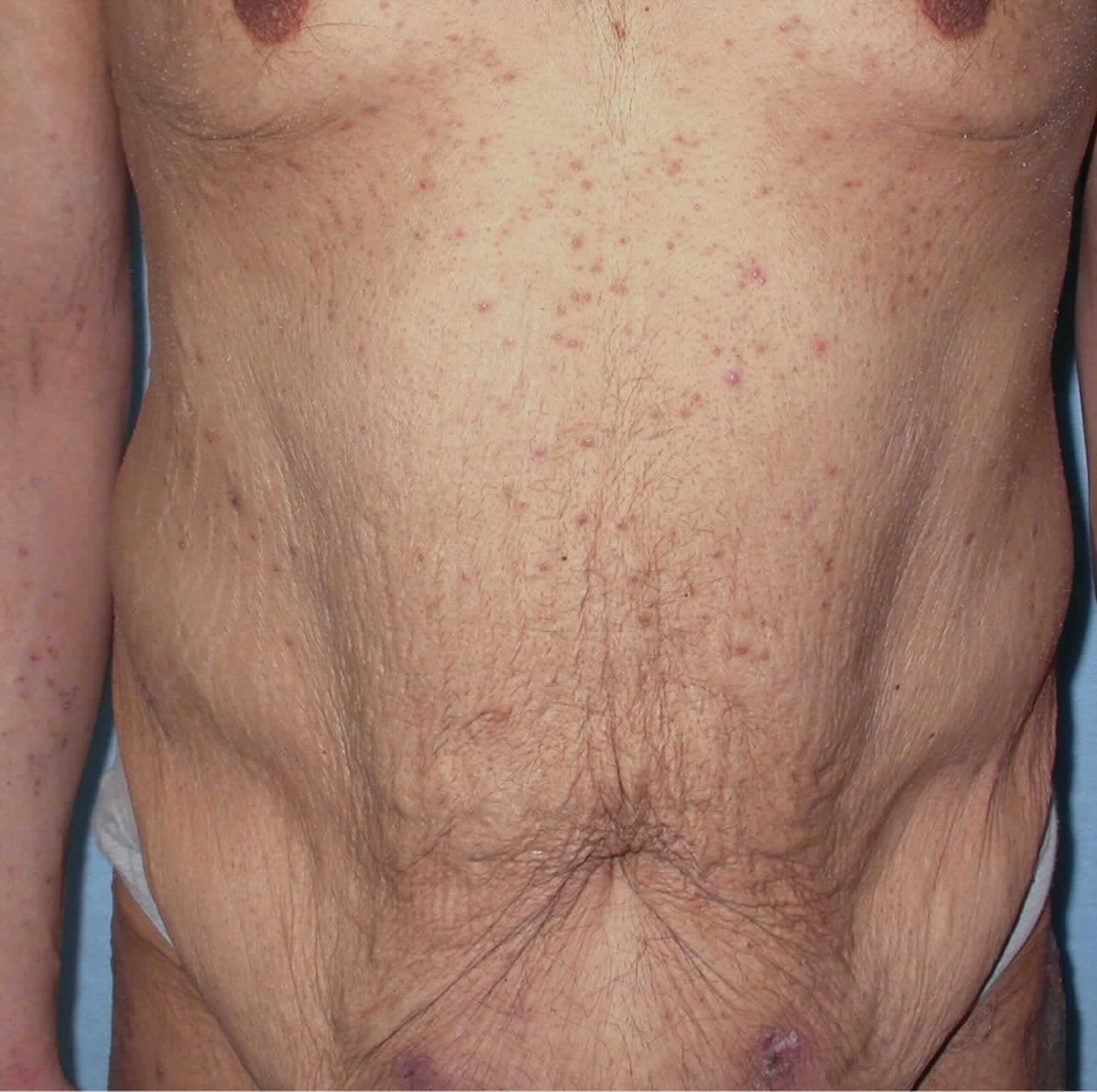
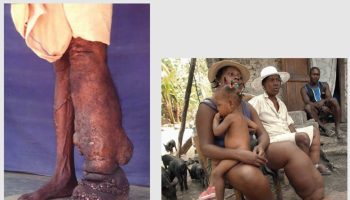
- Skin problems – one of the first signs of scurvy is the development of perifollicular hyperkeratotic papules (phrynoderma), often on the shins. These appear as reddish/bluish bruise-like spots surrounding hair follicles. The central hairs are twisted like corkscrews that may break easily. The papules may join together to form large areas of palpable purpura or ecchymoses (bruises).
- Oral problems – gums may swell and become red, soft and spongy. Any slight friction may cause the gums to bleed. Often this results in poor oral hygiene and dental diseases.
- Musculoskeletal problems – bleeding in the joints causes extreme discomfort and pain. Joints may be swollen and tender and the pain can be so severe that patients cannot walk.
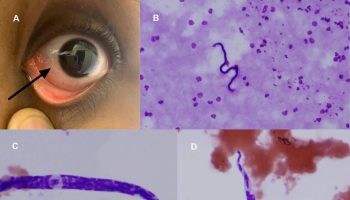
- Eye problems – patients may complain of dryness, irritation, light intolerance, transient visual blurring and stickiness. Haemorrhaging (bleeding) beneath the conjunctiva and within the optic nerve sheath may also occur.
- Anemia – this develops in 75% of patients as a result of blood loss into tissue, altered absorptions and metabolism of iron and folate, gastrointestinal bleeding and intravascular hemolysis 106, 10.
- Heart and lung problems – shortness of breath, low blood pressure, and chest pain leading to shock and death.
The cutaneous manifestations of scurvy include phrynoderma, corkscrew hairs, perifollicular hemorrhage and purpura, edema of the lower extremities, and splinter hemorrhages. Phrynoderma, or enlarged hyperkeratotic hair follicles, initially present on the posterolateral arms. This subsequently generalizes to involve the buttocks, posterior thighs, calves, shins, and back. Corkscrew hairs represent fractured and coiled hairs due to impaired keratin cross-links by disulfide bonds. With time, significant vascular congestion occurs, particularly in the lower extremities, leading to perifollicular hemorrhage and edema. This purpura is occasionally palpable, mimicking a cutaneous vasculitis. Blood vessel wall fragility also results in splinter hemorrhages of the nail bed. Oral disease is prominent among those with pre-existing poor dentition. Individuals may develop a hemorrhagic gingivitis, where the gingiva is initially red, swollen, and shiny and later becomes purple, necrotic, and prone to bleeding. Additionally, poorly formed soft teeth are prone to infection. Musculoskeletal disease is frequently seen in children. Hemorrhage can be intramuscular, intra-articular, or subperiosteal, leading to pain and pseudoparalysis. Bowing of the long bones, depression of the sternum, and swelling of the costochondral junctions are noted on physical examination. Radiographic findings include a transverse metaphyseal radiolucent band (scurvy line or Trummerfeld zone), widening at the zone of calcification (white line of Frankel), a ring of increased density around the epiphysis (Wimberger ring) and metaphyseal spurs with marginal fractures (Pelkan spurs). Conjunctival, intraocular, intracerebral, and gastrointestinal bleeding have been reported.
Mucocutaneous features of scurvy
- Follicular hyperkeratotic papules appear first on the upper arms, often spreading to the legs and buttocks
- Perifollicular haemorrhages, purpura (which can be palpable), and ecchymoses on the legs
- Hairs are often twisted and fragile (corkscrew hairs, swan-neck hairs)
- Poor wound healing and re-opening of old healed scars
- Splinter hemorrhages in the nails
- Red, swollen gums in patients with teeth (particularly around the upper incisors) which may later become purple or black
- Bleeding from the gums
- Loosening and loss of teeth
Ocular effects of scurvy
- Eye dryness and irritation
- Subconjunctival, periorbital, or orbital hemorrhage
Musculoskeletal effects of scurvy
- Painful hemarthosis (bleeding into the joint cavity)
- Subperiosteal haemorrhage, particularly femur and proximal tibia
- Costochondral junction beading – ‘scorbutic rosary’
- Intramuscular bleeding leading to woody oedema
- Severe pain of arms and legs (the major feature of scurvy in infants) and reluctance to walk.
Vitamin C deficiency complications
The predominant morbidity associated with vitamin C deficiency or scurvy is a result of bleeding into various tissues and depends on the site of involvement 107. Subperiosteal hemorrhages cause pain and tenderness, resulting in pseudoparalysis. Loss of function at the site of the hemorrhage and anemia are typical sequelae of the hemorrhages observed in scurvy. Subperiosteal hemorrhage in the tibia and femur causes excruciating pain.
Laboratory data suggest that the neonatal brain is particularly susceptible to vitamin C deficiency and that this condition may adversely affect early brain development 108.
Until minimal daily requirements of vitamin C were supplied, scurvy plagued prolonged naval voyages and military campaigns as personnel succumbed to its devastating effects. Lethargy, fatigue, and hemorrhagic manifestations of impaired collagen synthesis affecting oral, ophthalmic, musculoskeletal, cardiac, and gastrointestinal structures and functions incapacitated or killed more people than enemy action in many cases.
Vitamin C deficiency diagnosis
The diagnosis of vitamin C deficiency or scurvy is primarily a clinical one, based on a dietary history of inadequate vitamin C intake and the signs and symptoms described above. To diagnose scurvy, your doctor will examine you and ask questions about your diet. They might also arrange a blood test to test for vitamin C. Adults vitamin C concentrations in serum should be 4-15 mg/L 109. Measurement of serum level of ascorbic acid before and after treatment, although seldom done, can confirm the diagnosis when symptoms improve or resolve within weeks 94. However, serum vitamin C measurements may not correlate well with vitamin C levels in tissue 110. Furthermore, the diagnosis of scurvy is frequently delayed or overlooked because of its rarity and can lead to unnecessary exhaustive workups 111. The differential diagnosis can be broad, encompassing other causes of hemorrhage, purpura, and joint effusion. This includes coagulation disorders, vasculitis, idiopathic thrombocytopenic purpura, rheumatoid arthritis, and septic arthritis 106.
Vitamin C deficiency or scurvy diagnosis begins with the evaluation of risk factors and a physical examination. Dermoscopy can be used to aid in diagnosis, confirming follicular purpura and corkscrew hairs with a 4 mm punch biopsy of affected areas showing similar findings by histopathology. Biopsy specimens of skin lesions often demonstrate follicular hyperkeratosis, perifollicular hemorrhage, a proliferation of blood vessels, and coiled hair follicles 94. Serum testing for low plasma vitamin C (less than 0.2 mg/dL or less than 10 μmol/L) is usually consistent with scurvy 112; however, as stated above, recent intake or supplementation may elevate plasma levels and not be reflective of a prior prolonged deficit. The level of vitamin C in leukocytes is more accurate when assessing the sparse vitamin C stores as they are less affected by acute dietary changes. A leukocyte vitamin C level of 0 mg/dL is indicative of latent scurvy. Zero to 7 mg/dL is consistent with vitamin C deficiency, and greater than 15 mg/dl is adequate.
In addition to assessing vitamin C levels, screening for concomitant other vitamin deficiencies should be undertaken. As deficiency is primarily related to poor intake, those affected also may have poor intake of other essential vitamins and minerals. Vitamin B12, folate, calcium, zinc, and iron have been notably low in this patient population. Additionally, vitamin C’s role in iron absorption cause those with scurvy to be more prone to bleeding and iron deficiency, in particular, should be assessed.
Vitamin C deficiency treatment
Direct replacement of vitamin C is standard, with up to 300 mg daily for children and 500 mg to 1000 mg daily for adults. Vitamin C may be given by intravenous (IV) infusion or taken by mouth. When given by IV infusion, vitamin C can reach much higher levels in the blood than when it is taken by mouth. The endpoint of replacement is one month or upon resolution of clinical signs and symptoms. Alternative treatment regimens for adults include 1 g to 2 g for up to 3 days followed by 500 mg daily for a week followed by 100 mg daily for up to 3 months. In addition to immediate supplementation, educate the patient on lifestyle modifications to ensure adequate intake, and recommend cessation of alcohol, and tobacco use.
In children, The American Academy of Paediatrics recommends children receive 100 mg 3 times daily for at least 1 week, followed by 100 mg daily until symptoms have resolved.
Transfusion is sometimes required for severe anemia, especially if acute related to hemorrhage.
Children with bone disease may require surgery if symptoms do not resolve with vitamin C supplements.
Identifying and treating comorbid nutritional deficiencies (eg, iron deficiency anemia, folate deficiency, other vitamin deficiencies) are integral parts of management.
As patients with scurvy are often deficient in other nutrients, close attention is needed to prevent the development of refeeding syndrome, which is a result of profound hypophosphatemia and is common in patients after prolonged starvation 113. Refeeding syndrome can produce rhabdomyolysis, hypotension, arrhythmias, seizures, and may result in multiorgan failure and death in 0.43% to 34% of these patients if untreated 114, 115. Therefore electrolytes, especially serum phosphate levels, need to be monitored at least three times a week during hospital treatment 116.
Vitamin C deficiency prognosis
Improvement of symptoms of fatigue, lethargy, pain, anorexia, and confusion usually within 24 hours of vitamin C supplementation 26, 117. Bruising, perifollicular hemorrhages, gingival bleeding, and weakness usually improve within 1 to 2 weeks 26, 117. Corkscrew hairs take up to 4 weeks to resolve, and complete resolution is usually seen after approximately 3 months of regular vitamin C supplementation 118. Bone abnormalities may require surgical intervention 117.
- Magiorkinis E, Beloukas A, Diamantis A: Scurvy: past, present and future. Eur J Intern Med. 2011, 22:147-52. https://doi.org/10.1016/j.ejim.2010.10.006[↩]
- Kluesner NH, Miller DG. Scurvy: malnourishment in the land of plenty. J Emerg Med. 2014 Apr;46(4):530-2. doi: 10.1016/j.jemermed.2013.09.027[↩]
- Al-Dabagh A, Milliron BJ, Strowd L, Feldman SR. A disease of the present: scurvy in “well-nourished” patients. J Am Acad Dermatol. 2013 Nov;69(5):e246-e247. doi: 10.1016/j.jaad.2013.04.051[↩]
- Olmedo JM, Yiannias JA, Windgassen EB, Gornet MK. Scurvy: a disease almost forgotten. Int J Dermatol. 2006 Aug;45(8):909-13. doi: 10.1111/j.1365-4632.2006.02844.x[↩][↩]
- Popovich D, McAlhany A, Adewumi AO, Barnes MM. Scurvy: forgotten but definitely not gone. J Pediatr Health Care. 2009 Nov-Dec;23(6):405-15. doi: 10.1016/j.pedhc.2008.10.008[↩]
- Wang AH, Still C. Old world meets modern: a case report of scurvy. Nutr Clin Pract. 2007 Aug;22(4):445-8. doi: 10.1177/0115426507022004445[↩][↩][↩][↩][↩][↩][↩]
- Francescone MA, Levitt J. Scurvy masquerading as leukocytoclastic vasculitis: a case report and review of the literature. Cutis. 2005 Oct;76(4):261-6.[↩][↩][↩][↩]
- Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academies of Sciences, Engineering, and Medicine. 2000. Washington, DC: The National Academies Press. https://nap.nationalacademies.org/read/9810/chapter/1[↩]
- Vitamin C. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional[↩][↩][↩][↩][↩][↩][↩][↩]
- Hafez D, Saint S, Griauzde J, Mody R, Meddings J. CLINICAL PROBLEM-SOLVING. A Deficient Diagnosis. N Engl J Med. 2016 Apr 7;374(14):1369-74. doi: 10.1056/NEJMcps1407520[↩][↩][↩]
- Doseděl M, Jirkovský E, Macáková K, Krčmová LK, Javorská L, Pourová J, Mercolini L, Remião F, Nováková L, Mladěnka P, On Behalf Of The Oemonom. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients. 2021 Feb 13;13(2):615. doi: 10.3390/nu13020615[↩][↩]
- Scurvy. https://emedicine.medscape.com/article/125350-overview[↩]
- Schectman G. Estimating ascorbic acid requirements for cigarette smokers. Ann N Y Acad Sci. 1993 May 28;686:335-45; discussion 345-6. doi: 10.1111/j.1749-6632.1993.tb39197.x[↩]
- Weber P, Bendich A, Schalch W. Vitamin C and human health—a review of recent data relevant to human requirements. Int J Vitam Nutr Res 1996;66:19-30.[↩]
- Perry ME, Page N, Manthey DE, Zavitz JM. Scurvy: Dietary Discretion in a Developed Country. Clin Pract Cases Emerg Med. 2018 Mar 14;2(2):147-150. doi: 10.5811/cpcem.2018.1.36860[↩][↩][↩]
- Pimentel L. Scurvy: historical review and current diagnostic approach. Am J Emerg Med. 2003 Jul;21(4):328-32. doi: 10.1016/s0735-6757(03)00083-4[↩][↩]
- Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics. 2001 Sep;108(3):E55. doi: 10.1542/peds.108.3.e55[↩][↩][↩][↩][↩][↩][↩][↩]
- Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3704-9. doi: 10.1073/pnas.93.8.3704[↩]
- Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999 Apr 21;281(15):1415-23. doi: 10.1001/jama.281.15.141[↩]
- Schleicher RL, Carroll MD, Ford ES, Lacher DA: Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009, 90:1252-63. 10.3945/ajcn.2008.27016[↩]
- Mosdøl A, Erens B, Brunner EJ: Estimated prevalence and predictors of vitamin C deficiency within UK’s low-income population. J Public Health (Oxf). 2008, 30:456-60. 10.1093/pubmed/fdn076[↩]
- Khalife R, Grieco A, Khamisa K, Tinmouh A, McCudden C, Saidenberg E: Scurvy, an old story in a new time: the hematologist’s experience. Blood Cells Mol Dis. 2019, 76:40-4. 10.1016/j.bcmd.2019.01.004[↩]
- Faizallah R, Morris AI, Krasner N, Walker RJ. Alcohol enhances vitamin C excretion in the urine. Alcohol Alcohol. 1986;21(1):81-4.[↩][↩]
- Des Roches A, Paradis L, Paradis J, Singer S. Food allergy as a new risk factor for scurvy. Allergy. 2006 Dec;61(12):1487-8. doi: 10.1111/j.1398-9995.2006.01200.x[↩]
- Chen MF, Boyce HW Jr, Hsu JM. Effect of ascorbic acid on plasma alcohol clearance. J Am Coll Nutr. 1990 Jun;9(3):185-9. doi: 10.1080/07315724.1990.10720368[↩]
- Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician. 2008 Oct;54(10):1403-6.[↩][↩][↩][↩][↩][↩][↩]
- Granger M., Eck P. Dietary vitamin C in human health. Adv. Food Nutr. Res. 2018;83:281–310. doi: 10.1016/bs.afnr.2017.11.006[↩]
- World Health Organization . Scurvy and its Prevention and Control in Major Emergencies/Prepared by Zita Weise Prinzo. World Health Organization; Geneva, Switzerland: 1999.[↩]
- Padayatty S.J., Levine M. Vitamin C: The known and the unknown and Goldilocks. Oral. Dis. 2016;22:463–493. doi: 10.1111/odi.12446[↩][↩]
- Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9:1211. doi: 10.3390/nu9111211[↩]
- Hodges R.E., Hood J., Canham J.E., Sauberlich H.E., Baker E.M. Clinical manifestations of ascorbic acid deficiency in man. Am. J. Clin. Nutr. 1971;24:432–443. doi: 10.1093/ajcn/24.4.432[↩]
- Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007 Oct;137(10):2171-84. doi: 10.1093/jn/137.10.2171[↩][↩][↩]
- Valerio E, Meneghel A, Masiero S, Zangardi T, Zanconato S. Scurvy: just think about it. J Pediatr. 2013 Dec. 163 (6):1786-7.[↩]
- Abdullah M, Attia FN. Vitamin C (Ascorbic Acid) [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499877[↩]
- Scurvy in a man with schizophrenia. Mark Dubé. CMAJ Aug 2011, 183 (11) E760; DOI: 10.1503/cmaj.080505 http://www.cmaj.ca/content/183/11/E760[↩]
- Phrynoderma: a cutaneous sign of an inadequate diet. Alessandro Di Stefani, Augusto Orlandi, Sergio Chimenti, Luca Bianchi. CMAJ Oct 2007, 177 (8) 855-856; DOI: 10.1503/cmaj.070086 http://www.cmaj.ca/content/177/8/855[↩]
- BMJ. 2001;322(7280):246. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1119499/[↩]
- Naidu KA: Vitamin C in human health and disease is still a mystery? An overview. Nutr J 2: 7, 2003. https://www.ncbi.nlm.nih.gov/pubmed/14498993[↩]
- Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr 2007;137:2171-84. https://www.ncbi.nlm.nih.gov/pubmed/17884994[↩]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington (DC): National Academies Press (US); 2000. 5, Vitamin C. Available from: https://www.ncbi.nlm.nih.gov/books/NBK225480[↩][↩][↩][↩][↩][↩]
- Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care 2002;5:66-74. https://www.ncbi.nlm.nih.gov/pubmed/12134712[↩]
- Merck Sharp & Dohme Corp., Merck Manual. Vitamin C (Ascorbic Acid). https://www.merckmanuals.com/professional/nutritional-disorders/vitamin-deficiency,-dependency,-and-toxicity/vitamin-c[↩][↩]
- Holford P, Carr AC, Jovic TH, Ali SR, Whitaker IS, Marik PE, Smith AD. Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients. 2020 Dec 7;12(12):3760. doi: 10.3390/nu12123760[↩][↩]
- Carr AC, Maggini S. Vitamin C and Immune Function. Nutrients. 2017 Nov 3;9(11):1211. doi: 10.3390/nu9111211[↩][↩][↩][↩]
- Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013 Jan 31;2013(1):CD000980. doi: 10.1002/14651858.CD000980.pub4[↩][↩]
- Johnston CS. Vitamin C. In: Marriott BP, Birt DF, Stallings VA, Yates AA, eds. Present Knowledge in Nutrition 11th ed. Cambridge, MA: Elsevier; 2020:155-69.[↩][↩][↩]
- Hemilä H. Vitamin C and Infections. Nutrients. 2017 Mar 29;9(4):339. doi: 10.3390/nu9040339[↩][↩]
- Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009 Nov;90(5):1252-63. doi: 10.3945/ajcn.2008.27016[↩]
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6377-81. doi: 10.1073/pnas.86.16.6377[↩]
- Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002 Mar-Apr;5(2):66-74. doi: 10.1046/j.1523-5408.2002.00005.x[↩][↩][↩][↩][↩]
- Gershoff SN. Vitamin C (ascorbic acid): new roles, new requirements? Nutr Rev. 1993 Nov;51(11):313-26. doi: 10.1111/j.1753-4887.1993.tb03757.x[↩]
- Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care 2002;5:66-74. https://www.ncbi.nlm.nih.gov/pubmed/12134712?dopt=Abstract[↩][↩][↩][↩][↩]
- Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 2004;140:533-7. https://www.ncbi.nlm.nih.gov/pubmed/15068981[↩][↩]
- Carpenter KJ. The history of scurvy and vitamin C. Cambridge: Cambridge University Press, 1986.[↩][↩]
- Padayatty S, Espey MG, Levine M: Vitamin C. In: Coates PM, Betz JM, Blackman MR, et al., eds.: Encyclopedia of Dietary Supplements. 2nd ed. New York, NY: Informa Healthcare, 2010, pp 821-31.[↩]
- National Cancer Institute. High-Dose Vitamin C. https://www.cancer.gov/about-cancer/treatment/cam/patient/vitamin-c-pdq[↩]
- Pauling L. The significance of the evidence about ascorbic acid and the common cold. Proc Natl Acad Sci U S A 1971;68:2678-81. https://www.ncbi.nlm.nih.gov/pubmed/4941984?dopt=Abstract[↩]
- McCormick WJ: Cancer: a collagen disease, secondary to a nutritional deficiency. Arch Pediatr 76 (4): 166-71, 1959. https://www.ncbi.nlm.nih.gov/pubmed/13638066?dopt=Abstract[↩]
- Cameron E, Pauling L: The orthomolecular treatment of cancer. I. The role of ascorbic acid in host resistance. Chem Biol Interact 9 (4): 273-83, 1974. https://www.ncbi.nlm.nih.gov/pubmed/4609626?dopt=Abstract[↩]
- National Cancer Institute. High-Dose Vitamin C–Patient Version. https://www.cancer.gov/about-cancer/treatment/cam/patient/vitamin-c-pdq#link/_5[↩]
- Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr 2007;137:2171-84. https://www.ncbi.nlm.nih.gov/pubmed/17884994?dopt=Abstract[↩][↩]
- Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr 1999;69:1086-107. https://www.ncbi.nlm.nih.gov/pubmed/10357726?dopt=Abstract[↩][↩]
- Proc Natl Acad Sci U S A. 1989 Aug;86(16):6377-81. Ascorbate is an outstanding antioxidant in human blood plasma. https://www.ncbi.nlm.nih.gov/pubmed/2762330%20?dopt=Abstract[↩]
- Gershoff SN. Vitamin C (ascorbic acid): new roles, new requirements? Nutr Rev 1993;51:313-26. https://www.ncbi.nlm.nih.gov/pubmed/8108031?dopt=Abstract[↩]
- Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics 2001;108:E55. https://www.ncbi.nlm.nih.gov/pubmed/11533373?dopt=Abstract[↩][↩]
- Wang AH, Still C. Old world meets modern: a case report of scurvy. Nutr Clin Pract 2007;22:445-8. https://www.ncbi.nlm.nih.gov/pubmed/17644699?dopt=Abstract[↩]
- Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med 2001;21:235-7. https://www.ncbi.nlm.nih.gov/pubmed/11604276?dopt=Abstract[↩]
- Levine M, Padayatty SJ. Vitamin C. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease, 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:416-426.[↩]
- Camarena V, Wang G. The epigenetic role of vitamin C in health and disease. Cell Mol Life Sci. 2016 Apr;73(8):1645-58. doi: 10.1007/s00018-016-2145-x[↩]
- Cooke M.S., Evans M.D., Podmore I.D., Herbert K.E., Mistry N., Mistry P., Hickenbotham P.T., Hussieni A., Griffiths H.R., Lunec J. Novel repair action of vitamin C upon in vivo oxidative DNA damage. FEBS Lett. 1998;439:363–367. doi: 10.1016/S0014-5793(98)01403-3[↩]
- Fraga C.G., Motchnik P.A., Shigenaga M.K., Helbock H.J., Jacob R.A., Ames B.N. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc. Natl. Acad. Sci. USA. 1991;88:11003–11006. doi: 10.1073/pnas.88.24.11003[↩]
- Brennan L.A., Morris G.M., Wasson G.R., Hannigan B.M., Barnett Y.A. The effect of vitamin C or vitamin E supplementation on basal and H2O2-induced DNA damage in human lymphocytes. Br. J. Nutr. 2000;84:195–202. doi: 10.1017/S0007114500001422[↩][↩][↩][↩]
- Moller P., Viscovich M., Lykkesfeldt J., Loft S., Jensen A., Poulsen H.E. Vitamin C supplementation decreases oxidative DNA damage in mononuclear blood cells of smokers. Eur. J. Nutr. 2004;43:267–274. doi: 10.1007/s00394-004-0470-6[↩]
- Moller P., Viscovich M., Lykkesfeldt J., Loft S., Jensen A., Poulsen H.E. Vitamin C supplementation decreases oxidative DNA damage in mononuclear blood cells of smokers. Eur. J. Nutr. 2004;43:267–274. doi: 10.1007/s00394-004-0470-6). In summary, vitamin C may be a promising supplement for individuals who have a predisposition for DNA damage. These studies demonstrate that the antioxidant role of vitamin C is not limited to in vitro (test tube studies) contexts, but occurs within the human body as well, providing some rationale for an anti-cancer effect. However, the evidence of this anti-cancer effect is unclear.
A number of observational studies have assessed dietary vitamin C intake and cancer risk, with mixed results ((Chen Z., Huang Y., Cao D., Qiu S., Chen B., Li J., Bao Y., Wei Q., Han P., Liu L. Vitamin C Intake and Cancers: An Umbrella Review. Front. Nutr. 2021;8:812394. doi: 10.3389/fnut.2021.812394[↩]
- Bo Y., Lu Y., Zhao Y., Zhao E., Yuan L., Lu W., Cui L., Lu Q. Association between dietary vitamin C intake and risk of esophageal cancer: A dose-response meta-analysis. Int. J. Cancer. 2016;138:1843–1850. doi: 10.1002/ijc.29838[↩][↩]
- Zhang D., Xu P., Li Y., Wei B., Yang S., Zheng Y., Lyu L., Deng Y., Zhai Z., Li N., et al. Association of vitamin C intake with breast cancer risk and mortality: A meta-analysis of observational studies. Aging. 2020;12:18415–18435. doi: 10.18632/aging.103769[↩][↩]
- Bai X.Y., Qu X., Jiang X., Xu Z., Yang Y., Su Q., Wang M., Wu H. Association between Dietary Vitamin C Intake and Risk of Prostate Cancer: A Meta-analysis Involving 103,658 Subjects. J. Cancer. 2015;6:913–921. doi: 10.7150/jca.12162[↩]
- Liu Y., Yu Q., Zhu Z., Zhang J., Chen M., Tang P., Li K. Vitamin and multiple-vitamin supplement intake and incidence of colorectal cancer: A meta-analysis of cohort studies. Med. Oncol. 2015;32:434. doi: 10.1007/s12032-014-0434-5[↩]
- Kushi L.H., Fee R.M., Sellers T.A., Zheng W., Folsom A.R. Intake of vitamins A, C, and E and postmenopausal breast cancer. The Iowa Women’s Health Study. Am. J. Epidemiol. 1996;144:165–174. doi: 10.1093/oxfordjournals.aje.a008904[↩][↩][↩]
- Ziegler R.G., Brinton L.A., Hamman R.F., Lehman H.F., Levine R.S., Mallin K., Norman S.A., Rosenthal J.F., Trumble A.C., Hoover R.N. Diet and the risk of invasive cervical cancer among white women in the United States. Am. J. Epidemiol. 1990;132:432–445. doi: 10.1093/oxfordjournals.aje.a115678[↩][↩]
- Gridley G., McLaughlin J.K., Block G., Blot W.J., Gluch M., Fraumeni J.F., Jr. Vitamin supplement use and reduced risk of oral and pharyngeal cancer. Am. J. Epidemiol. 1992;135:1083–1092. doi: 10.1093/oxfordjournals.aje.a116208[↩][↩][↩]
- Parent M.E., Richard H., Rousseau M.C., Trudeau K. Vitamin C Intake and Risk of Prostate Cancer: The Montreal PROtEuS Study. Front. Physiol. 2018;9:1218. doi: 10.3389/fphys.2018.01218[↩]
- Vissers M.C.M., Das A.B. Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. Front. Physiol. 2018;9:809. doi: 10.3389/fphys.2018.00809[↩]
- Kazmierczak-Baranska J., Boguszewska K., Adamus-Grabicka A., Karwowski B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients. 2020;12:1501. doi: 10.3390/nu12051501[↩]
- Didier AJ, Stiene J, Fang L, Watkins D, Dworkin LD, Creeden JF. Antioxidant and Anti-Tumor Effects of Dietary Vitamins A, C, and E. Antioxidants (Basel). 2023 Mar 3;12(3):632. doi: 10.3390/antiox12030632[↩]
- Meščić Macan A, Gazivoda Kraljević T, Raić-Malić S. Therapeutic Perspective of Vitamin C and Its Derivatives. Antioxidants (Basel). 2019 Jul 26;8(8):247. doi: 10.3390/antiox8080247[↩]
- Vitamin C. https://ods.od.nih.gov/factsheets/VitaminC-Consumer/[↩][↩]
- Moser MA, Chun OK. Vitamin C and Heart Health: A Review Based on Findings from Epidemiologic Studies. Int J Mol Sci. 2016 Aug 12;17(8):1328. doi: 10.3390/ijms17081328[↩]
- Tembunde Y, Ge S, Turney K, Driscoll M. Scurvy: A Diagnosis Not to Be Missed. Cureus. 2022 Dec 28;14(12):e33050. doi: 10.7759/cureus.33050[↩]
- Hall SL, Greendale GA. The relation of dietary vitamin C intake to bone mineral density: results from the PEPI study. Calcif Tissue Int. 1998 Sep;63(3):183-9. doi: 10.1007/s002239900512[↩]
- Smith A, Di Primio G, Humphrey-Murto S. Scurvy in the developed world. CMAJ. 2011 Aug 9;183(11):E752-5. doi: 10.1503/cmaj.091938[↩]
- Velandia B, Centor RM, McConnell V, Shah M. Scurvy is still present in developed countries. J Gen Intern Med. 2008 Aug;23(8):1281-4. doi: 10.1007/s11606-008-0577-1[↩]
- Pangan AL, Robinson D. Hemarthrosis as initial presentation of scurvy. J Rheumatol. 2001 Aug;28(8):1923-5.[↩]
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999 Dec;41(6):895-906; quiz 907-10. doi: 10.1016/s0190-9622(99)70244-6[↩][↩][↩][↩]
- Leggett J, Convery R. Images in clinical medicine. Scurvy. N Engl J Med. 2001 Dec 20;345(25):1818. doi: 10.1056/NEJMicm010202[↩]
- Blanchard MS, Romero JM, Hoang MP. Case records of the Massachusetts General Hospital. Case 1-2014. A 32-year-old man with loss of vision and a rash. N Engl J Med. 2014 Jan 9;370(2):159-66. doi: 10.1056/NEJMcpc1214217[↩]
- Chang CY, Rosenthal DI, Mitchell DM, Handa A, Kattapuram SV, Huang AJ. Imaging Findings of Metabolic Bone Disease. Radiographics. 2016 Oct;36(6):1871-1887. doi: 10.1148/rg.2016160004[↩]
- Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999 Apr 21;281(15):1415-23. doi: 10.1001/jama.281.15.1415[↩]
- Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001 Oct;21(3):235-7. doi: 10.1016/s0736-4679(01)00377-8[↩]
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press, 2000. https://www.nap.edu/catalog/9810/dietary-reference-intakes-for-vitamin-c-vitamin-e-selenium-and-carotenoids[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Bates CJ. Bioavailability of vitamin C. Eur J Clin Nutr 1997;51 (Suppl 1):S28-33. https://www.ncbi.nlm.nih.gov/pubmed/9023477?dopt=Abstract[↩]
- U.S. Department of Agriculture, Agricultural Research Service. 2011. USDA National Nutrient Database for Standard Reference, Release 24. Nutrient Data Laboratory Home Page, https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/[↩]
- Hoffman FA. Micronutrient requirements of cancer patients. Cancer. 1985;55 (1 Suppl):295-300. https://www.ncbi.nlm.nih.gov/pubmed/3917362?dopt=Abstract[↩]
- Deicher R, Hörl WH. Vitamin C in chronic kidney disease and hemodialysis patients. Kidney Blood Press Res 2003;26:100-6. https://www.ncbi.nlm.nih.gov/pubmed/12771534%20?dopt=Abstract[↩]
- Reuler JB, Broudy VC, Cooney TG. Adult scurvy. JAMA. 1985 Feb 8;253(6):805-7.[↩]
- Montalto M, Porceddu E, Pero E, Lupascu A, Gallo A, De Simone C, Nucera E, Aruanno A, Giarretta I, Pola R, Landolfi R. Scurvy: A Disease not to be Forgotten. Nutr Clin Pract. 2021 Oct;36(5):1063-1067. doi: 10.1002/ncp.10616[↩][↩]
- Scurvy. https://emedicine.medscape.com/article/125350-overview#a7[↩]
- Tveden-Nyborg P, Lykkesfeldt J. Does vitamin C deficiency result in impaired brain development in infants? Redox Rep. 2009;14(1):2-6. doi: 10.1179/135100009X392412[↩]
- An HIV-Infected Man with Odynophagia and Rash, Clinical Infectious Diseases, Volume 41, Issue 5, 1 September 2005, Pages 686–688, https://doi.org/10.1086/432575[↩]
- Emadi-Konjin P, Verjee Z, Levin AV, Adeli K. Measurement of intracellular vitamin C levels in human lymphocytes by reverse phase high performance liquid chromatography (HPLC). Clin Biochem. 2005 May;38(5):450-6. doi: 10.1016/j.clinbiochem.2005.01.018[↩]
- Khalife R, Grieco A, Khamisa K, Tinmouh A, McCudden C, Saidenberg E. Scurvy, an old story in a new time: The hematologist’s experience. Blood Cells Mol Dis. 2019 May;76:40-44. doi: 10.1016/j.bcmd.2019.01.004[↩]
- Levavasseur M, Becquart C, Pape E, Pigeyre M, Rousseaux J, Staumont-Sallé D, Delaporte E. Severe scurvy: an underestimated disease. Eur J Clin Nutr. 2015 Sep;69(9):1076-7. doi: 10.1038/ejcn.2015.99[↩]
- Choh CT, Rai S, Abdelhamid M, Lester W, Vohra RK. Unrecognised scurvy. BMJ. 2009 Sep 17;339:b3580. doi: 10.1136/bmj.b3580[↩]
- Hearing SD. Refeeding syndrome. BMJ. 2004 Apr 17;328(7445):908-9. doi: 10.1136/bmj.328.7445.908[↩]
- Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008 Jun 28;336(7659):1495-8. doi: 10.1136/bmj.a301[↩]
- Nutrition support in adults. Quality standard [QS24]. https://www.nice.org.uk/guidance/qs24[↩]
- Maxfield L, Crane JS. Vitamin C Deficiency. [Updated 2022 Oct 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493187[↩][↩][↩]
- De Luna RH, Colley BJ 3rd, Smith K, Divers SG, Rinehart J, Marques MB. Scurvy: an often forgotten cause of bleeding. Am J Hematol. 2003 Sep;74(1):85-7. doi: 10.1002/ajh.10354[↩]