Contents
IgA nephropathy
IgA nephropathy also known as Berger’s disease, is a common kidney disease that occurs when an antibody called immunoglobulin A (IgA) builds up in your kidneys, causing local inflammation that damages kidney tissues, over time, can hamper your kidneys’ ability to filter waste from your blood 1, 2, 3. IgA nephropathy is one of the most common kidney diseases, other than those caused by diabetes or high blood pressure 4. About 1 in 10 kidney biopsies in the United States show IgA nephropathy 1. IgA nephropathy can occur at any age, although the first evidence of kidney disease most frequently appears when people are in their teens to late 30s 5. IgA nephropathy is rare in children younger than five years of age 6. The incidence of IgA nephropathy peaks in the second and third decades of life 7, 8. The incidence of IgA nephropathy varies substantially between ethnic or racial groups, being highest in East Asians and Caucasians where IgA nephropathy accounts for about 40% of all native-kidney biopsies in Japan, 25% in Europe, 12% in the United States, and less than 5% in central Africa 9, 10. IgA nephropathy in the United States and Europe is twice as likely to appear in men than in women but about 1:1 in East Asia 11, 12, 13, 14, 15, 16. A systematic study of biopsy-based literature from multiple countries reveals an overall IgA nephropathy incidence of over 2.5 per 100,000 17. In another study, IgA nephropathy is more common in Asian individuals (45 cases per million population/year in Japan) than in Whites (31 cases per million population/year in France) 18. Compelling data suggests a higher burden of IgA nephropathy in East and Pacific Asian countries 19.
IgA nephropathy usually progresses slowly over years, but the course of the disease varies from person to person. Some people leak blood in their urine (hematuria) without developing problems, some eventually achieve complete remission and others develop chronic kidney disease (CKD) and end-stage kidney failure 20. IgA nephropathy is a common cause of chronic kidney disease (CKD), particularly for patients with proteinuria persistently more than 1 g/day 21, 22, 23. In a study of 669 patients, the multivariable analysis revealed that people of Pacific Asian origin have a higher risk of end-stage renal disease (ESRD) 24.
In children and adolescents, painless visible blood in urine (hematuria), often at the same time with an infection of the upper respiratory or gastrointestinal tract, frequently heralds the onset of clinical IgA nephropathy disease 6. This sign and symptom of blood in urine (hematuria) may also accompany intense physical activity. Most patients with macroscopic (visible) hematuria have additional episodes over several years 25. Visible hematuria due to IgA nephropathy rarely begins after age 40 years 6. For patients in their 30s and 40s, microscopic hematuria, with or without, proteinuria may be discovered at the time of routine health screenings 6. The magnitude of proteinuria varies widely between patients, although proteinuria without microscopic hematuria is uncommon.
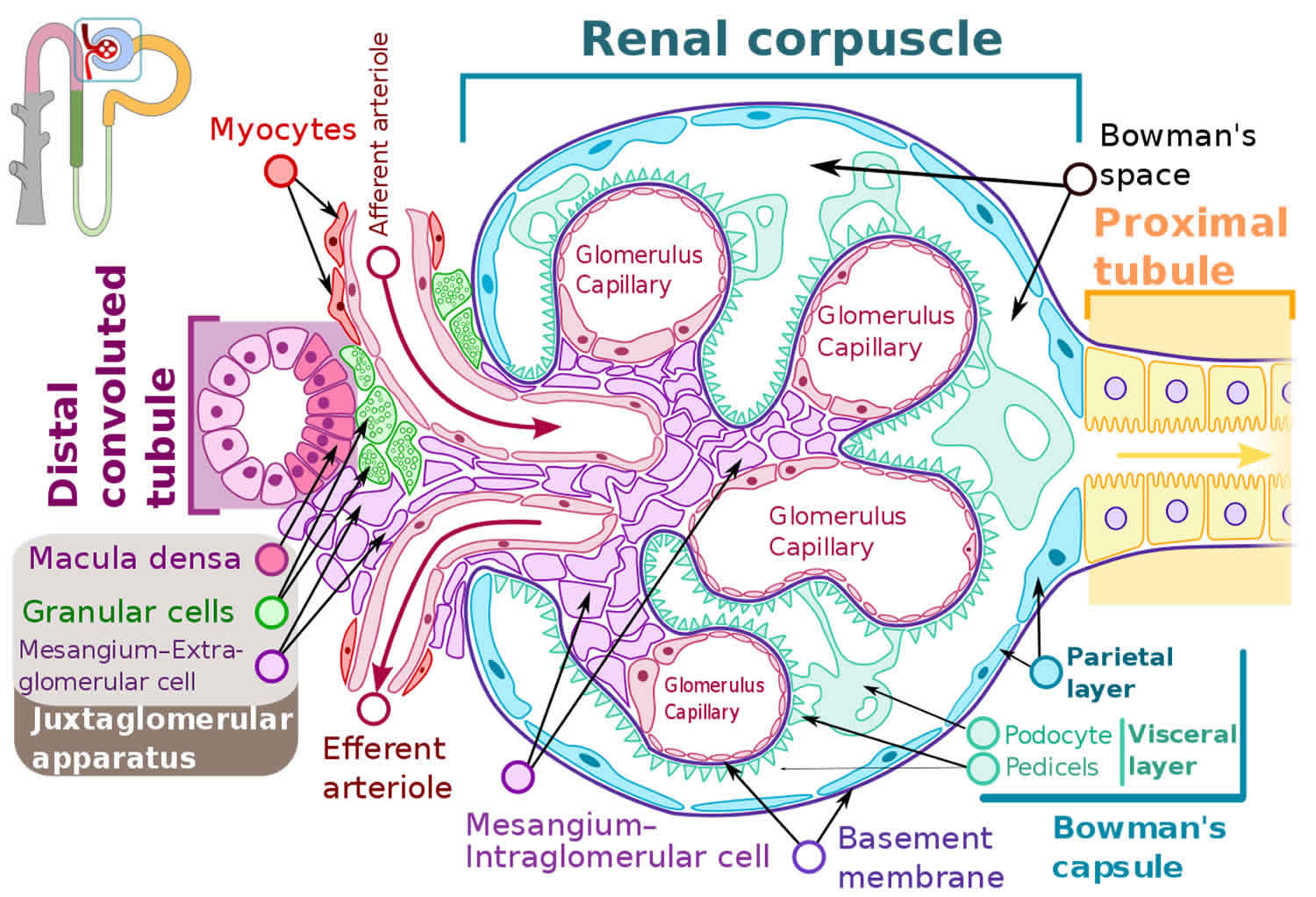
IgA (immunoglobulin A) is an antibody, a protein made by your immune system to protect your body from foreign substances such as bacteria or viruses. IgA nephropathy affects the kidneys by attacking the glomerulus (more than one glomerulus are called glomeruli) 1, 2. The glomeruli are sets of looping blood vessels in nephrons, the tiny working units of your kidneys that filter wastes and remove extra fluid from the blood. The buildup of IgA deposits inflames and damages the glomeruli, causing the kidneys to leak blood and protein into the urine. The damage may lead to scarring of the nephrons that progresses slowly over many years. Eventually, IgA nephropathy can lead to end-stage kidney disease, sometimes called ESRD, which means the kidneys no longer work well enough to keep a person healthy. When a person’s kidneys fail, he or she needs a kidney transplant or blood-filtering treatments called dialysis.
A person may be more likely to develop IgA nephropathy if 20, 1, 2:
- he or she has a family history of IgA nephropathy, IgA vasculitis or Henoch-Schönlein purpura—a disease that causes small blood vessels in the body to become inflamed and leak
- he is a male in his teens to late 30s
- he or she is Asian or white European ancestry
- he or she has certain health conditions, such as celiac disease, hepatitis, cirrhosis, and HIV infection
IgA nephropathy often becomes worse slowly over years. But the course of the disease varies from person to person. Some people leak blood into their urine (hematuria) without having other problems. Others might have complications such as losing kidney function and spilling protein into the urine. Still others develop kidney failure, which means the kidneys stop working well enough to filter the body’s waste on their own.
Sometimes, routine medical tests find signs of IgA nephropathy, such as protein and red blood cells in the urine that are seen under a microscope.
Most people with IgA nephropathy receive care from a nephrologist, a doctor who specializes in treating people with kidney disease.
No cure exists for IgA nephropathy and no sure way of knowing what course your disease will take, but certain medications can slow its course. Some people need treatment to lower inflammation, reduce the spilling of protein into the urine and prevent the kidneys from failing. Such treatments may help the disease become not active, a state called remission. Keeping your blood pressure under control and reducing your cholesterol levels also slow the disease.
Some people need only monitoring to determine whether the disease is getting worse. For others, a number of medications can slow disease progress and help manage symptoms.
Medications to treat IgA nephropathy include:
- High blood pressure medications. Taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) can lower your blood pressure and reduce protein loss.
- Omega-3 fatty acids. These fats, available in dietary fish oil supplements, might reduce inflammation in the glomeruli without harmful side effects. Talk to your doctor before you start supplements.
- Immunosuppressants. In some cases, corticosteroid medications, such as prednisone, and other potent drugs that suppress the immune response (immunosuppressants) might keep your immune system from attacking your glomeruli. These drugs can cause serious side effects, such as high blood pressure, high blood sugar and increased risk of infection.
- Statin therapy. If you have high cholesterol, cholesterol-lowering medications can help control it and slow the progression of kidney damage.
- Diuretics. These help remove extra fluid from your blood. Removing extra fluid can help improve blood pressure control.
The ultimate goal is to avoid the need for kidney dialysis or kidney transplantation. But in some cases, dialysis or transplantation is necessary.
IgA nephropathy key points
- Immunoglobulin A (IgA) nephropathy, also known as Berger’s disease, is a kidney disease that occurs when IgA deposits build up in the kidneys, causing inflammation that damages kidney tissues.
- Scientists think that IgA nephropathy is an autoimmune kidney disease, meaning that the disease is due to the body’s immune system attacking tissues in the kidney.
- IgA nephropathy is one of the most common kidney diseases, other than those caused by diabetes or high blood pressure.
- In its early stages, IgA nephropathy may have no symptoms; it can be silent for years or even decades.
- Once symptoms appear, the most common one is hematuria, or blood in the urine.
- Another symptom of IgA nephropathy is albuminuria—when a person’s urine contains an increased amount of albumin, a protein typically found in the blood, or large amounts of protein in the urine.
- Currently, health care providers do not use blood or urine tests as reliable ways to diagnose IgA nephropathy; therefore, the diagnosis of IgA nephropathy requires a kidney biopsy.
- Researchers have not yet found a specific cure for IgA nephropathy.
Who is more likely to have IgA nephropathy?
IgA nephropathy is more common in people who 20, 1, 2:
- have a family history of IgA nephropathy or of IgA vasculitis (Henoch-Schönlein purpura)
- have certain health conditions, such as celiac disease, hepatitis, cirrhosis, and HIV infection
- are ages 10 to 40
- are of East Asian or white European ancestry
- are male
Is IgA nephropathy genetic?
For some people, this condition runs in families. Researchers have discovered some genetic markers, meaning that a genetic mutation (change) may cause IgA nephropathy.
What’s the difference between IgA nephropathy and selective IgA deficiency?
Both conditions have to do with the protein IgA. People with selective IgA deficiency either don’t have enough IgA or have low levels of it.
What is the connection between IgA nephropathy and end-stage renal disease (ESRD)?
IgA nephropathy attacks the glomeruli. This type of glomerular disease occurs when IgA deposits build up and damage the glomeruli. The damage causes your kidneys to leak blood (hematuria) and protein (proteinuria) into your urine.
Eventually, the nephrons may scar, causing kidney disease. As the scarring progresses, you may develop end-stage kidney (renal) disease (ESRD) or total kidney failure. This process can happen quickly over the course of months or can take as long as 20 years after your initial diagnosis.
If you develop end-stage renal disease (ESRD), your kidneys can’t work well enough to keep you healthy. You may need:
- Dialysis, a machine that helps filter your blood.
- Kidney transplant, surgery to remove your damaged kidney and replace it with a healthy donor kidney.
How your kidneys work
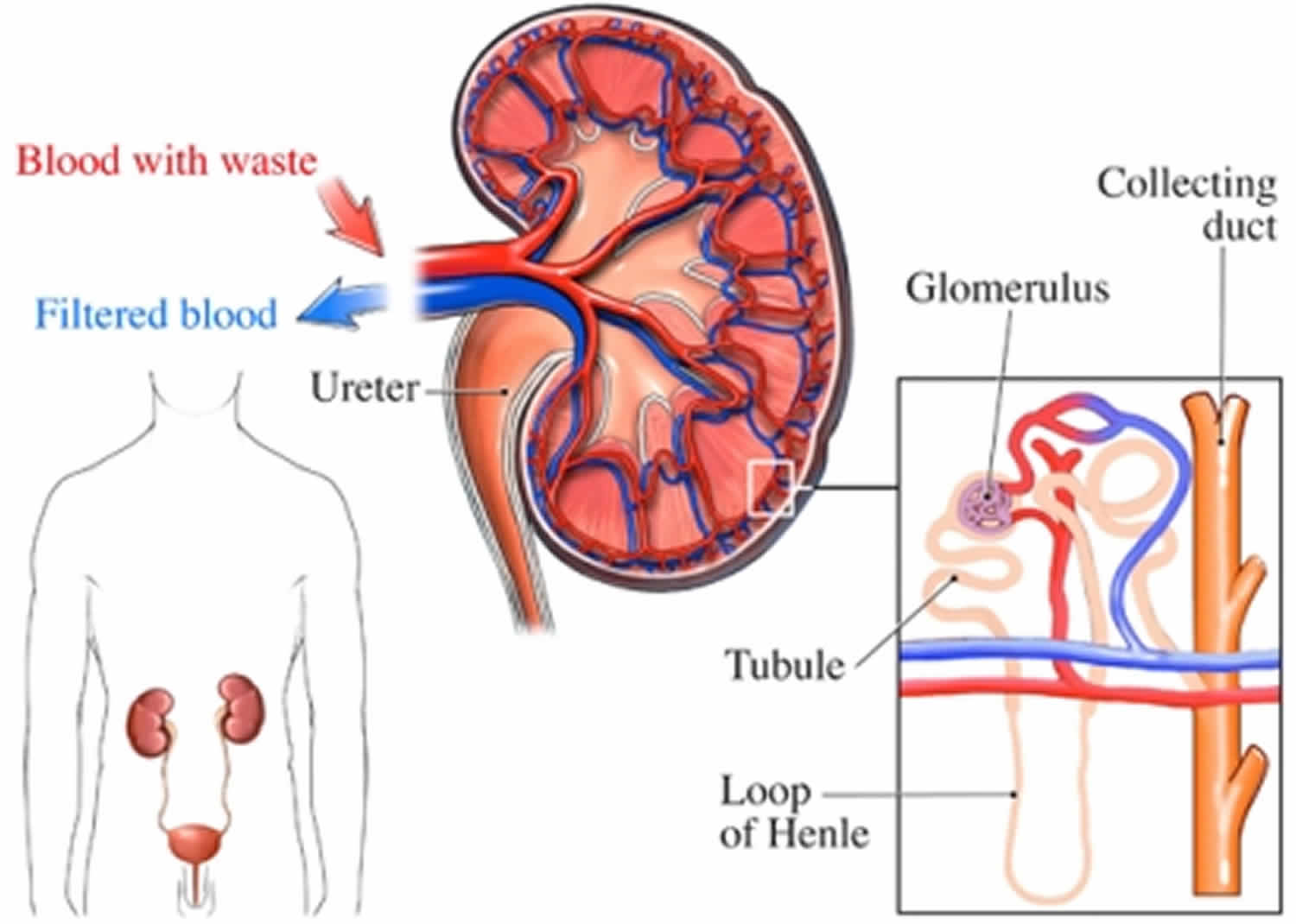
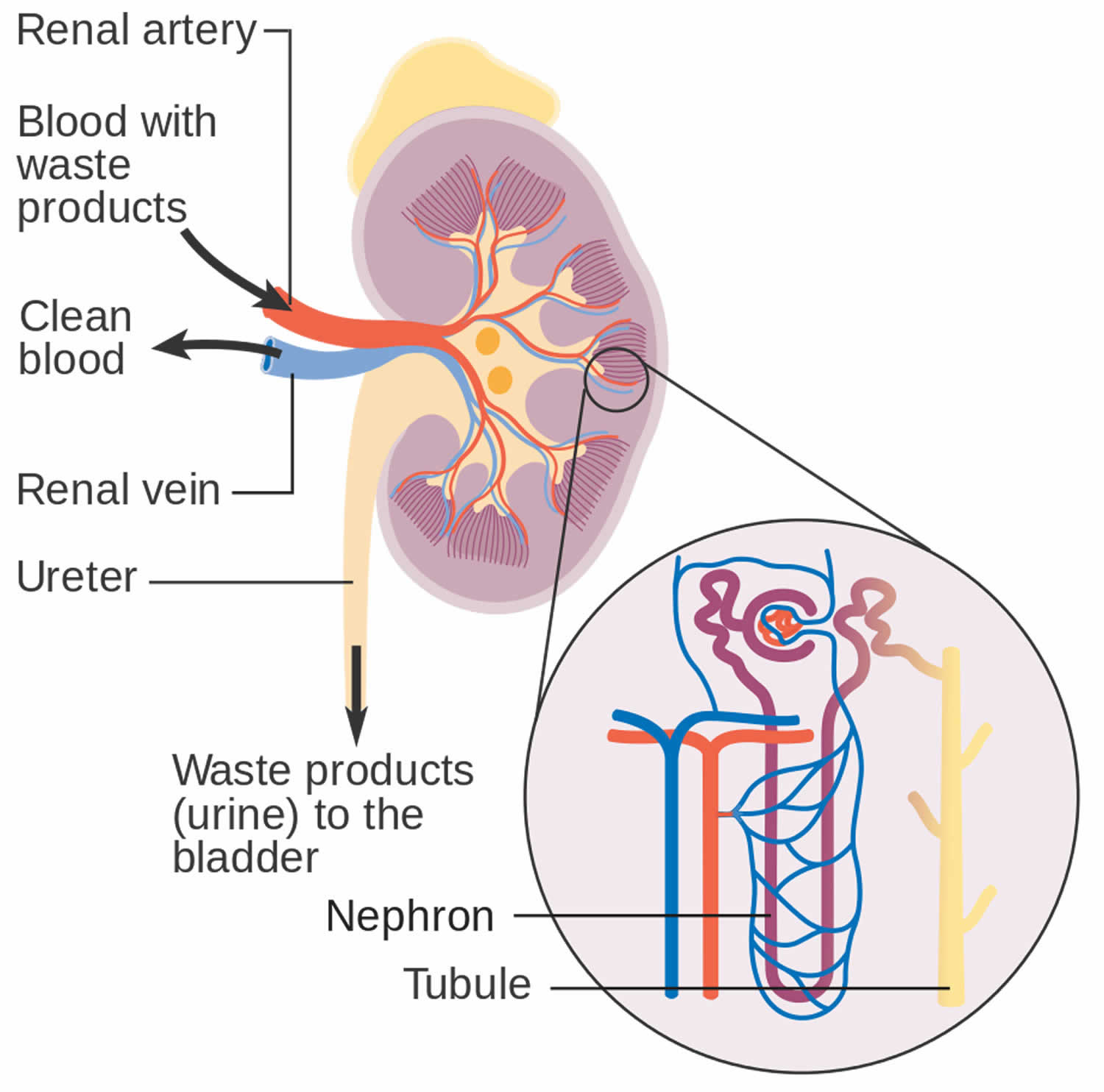
You have two kidneys, each about the size of an adult fist, located on either side of the spine just below the rib cage. Although they are small, your kidneys perform many complex and vital functions that keep the rest of the body in balance. Your kidneys remove waste and excess fluid from your blood through filtering units called nephrons. Each nephron contains a filter (glomerulus) that has a network of tiny blood vessels called capillaries. When blood flows into a glomerulus, tiny molecules — water, essential minerals and nutrients, and wastes — pass through the capillary walls. Large molecules, such as proteins and red blood cells, do not. The filtered solution then passes into another part of the nephron called the tubule. The water, nutrients and minerals your body needs are transferred back to the bloodstream. The excess water and waste become urine that flows to the bladder.
Kidney functions:
- Help remove waste and excess fluid
- Filter the blood, keeping some compounds while removing others
- Control the production of red blood cells
- Make vitamins that control growth
- Release hormones that help regulate blood pressure
- Help regulate blood pressure, red blood cells, and the amount of certain nutrients in the body, such as calcium and potassium.
Here’s how kidneys perform their important work:
- Blood enters the kidneys through an artery from the heart
- Blood is cleaned by passing through millions of tiny blood filters
- Waste material passes through the ureter and is stored in the bladder as urine
- Newly cleaned blood returns to the bloodstream by way of veins
- Bladder becomes full and urine passes out of the body through the urethra.
The kidneys perform their life-sustaining job of filtering and returning to the bloodstream about 200 quarts of fluid every 24 hours. Approximately two quarts are eliminated from the body in the form of urine, while the remainder, about 198 quarts, is retained in the body. The urine we excrete has been stored in the bladder for approximately one to eight hours.
Figure 1. How kidneys work
Figure 2. Glomerulus
How do glomerular diseases interfere with kidney function?
Glomerular diseases damage the glomeruli, letting protein and sometimes red blood cells leak into the urine. Sometimes a glomerular disease also interferes with the clearance of waste products by the kidney, so they begin to build up in the blood. Furthermore, loss of blood proteins like albumin in the urine can result in a fall in their level in the bloodstream. In normal blood, albumin acts like a sponge, drawing extra fluid from the body into the bloodstream, where it remains until the kidneys remove it. But when albumin leaks into the urine, the blood loses its capacity to absorb extra fluid from the body. Fluid can accumulate outside the circulatory system in the face, hands, feet, or ankles and cause swelling.
What are renal failure and end-stage renal disease?
Renal failure is any acute or chronic loss of kidney function and is the term used when some kidney function remains. Total kidney failure, sometimes called end-stage renal disease (ESRD), indicates permanent loss of kidney function. Depending on the form of glomerular disease, kidney function may be lost in a matter of days or weeks or may deteriorate slowly and gradually over
the course of decades.
Acute renal failure (acute kidney failure)
A few forms of glomerular disease cause very rapid deterioration of kidney function. For example, post-streptococcal glomerulonephritis (PSGN) can cause severe symptoms (hematuria, proteinuria, edema) within 2 to 3 weeks after a sore throat or skin infection develops. The patient may temporarily require dialysis to replace kidney function. This rapid loss of kidney function is called acute renal failure (acute kidney failure). Although acute renal failure (acute kidney failure) can be life-threatening while it lasts, kidney function usually returns after the cause of the kidney failure has been treated. In many patients, acute kidney failure is not associated with any permanent damage. However, some patients may recover from acute renal failure and subsequently develop chronic kidney disease (CKD).
Chronic kidney disease (CKD)
Most forms of glomerular disease develop gradually, often causing no symptoms for many years. Chronic kidney disease (CKD) is the slow, gradual loss of kidney function. Some forms of chronic kidney disease (CKD) can be controlled or slowed down. For example, diabetic nephropathy can be delayed by tightly controlling blood glucose levels and using angiotensin-converting enzyme inhibitors (ACE inhibitors) and angiotensin 2 receptor blockers (ARBs) to reduce proteinuria and control blood pressure. But chronic kidney disease (CKD) cannot be cured. Partial loss of kidney function means that some portion of the patient’s nephrons have been scarred, and scarred nephrons cannot be repaired. In many cases, CKD leads to total kidney failure.
Total kidney failure
To stay alive, a patient with total kidney failure must go on dialysis, either hemodialysis or peritoneal dialysis or receive a new kidney through kidney transplantation. Patients with chronic kidney disease (CKD) who are approaching total kidney failure should learn as much about their treatment options as possible so they can make an informed decision when the time comes. With the help of dialysis or kidney transplantation, many people continue to lead full, productive lives after reaching total kidney failure.
IgA nephropathy causes
The exact cause of IgA nephropathy is unknown. Scientists think that IgA nephropathy is an autoimmune kidney disease causing antibody-mediated destruction of the glomerular basement membrane, meaning that the disease is due to the body’s immune system harming the kidneys 26. Usually, there is an infectious disease preceding IgA nephropathy, which leads to the dysregulated immune response, but IgA nephropathy per se is not of an infectious origin. There has been no evidence to suggest that IgA nephropathy is secondary to any specific infectious agents despite the association between macroscopic hematuria and mucosal inflammation 1.
More than 90% of IgA nephropathy cases are sporadic, and the underlying cause is unknown 27.
People with IgA nephropathy have an increased blood level of IgA that contains less of a special sugar, galactose, than normal circulating IgA1 in healthy persons 27, 28, 29, 30. This galactose-deficient IgA (Gd-IgA1) is considered “foreign” by other antibodies circulating in the blood. As a result, these other antibodies attach to the galactose-deficient IgA and form a clump 27, 30. This clump is also called an immune complex. Some of the clumps become stuck in the glomerulus of the nephron and cause inflammation and damage 27. The immune proteins in the glomeruli of patients with IgA nephropathy generally include complement C3; IgG, IgM, or both, are often present 31, 7. Light microscopy typically shows glomerular injury as mesangial hypercellularity and increased mesangial matrix 32.
Less than 10% of IgA nephropathy cases are due to familial IgA nephropathy where IgA nephropathy runs in families. Scientists have recently found several genetic markers that may play a role in the development of the disease 33. IgA nephropathy may also be related to respiratory or intestinal infections and the immune system’s response to these infections. In some people, the first signs or symptoms of IgA nephropathy may become noticeable after a cold, sore throat, or other respiratory infection.
The following things might be linked with IgA nephropathy 34:
- Genes. IgA nephropathy is more common in some families and in certain ethnic groups, such as people of Asian and European descent.
- Genome-wide linkage analysis revealed an association of IgA nephropathy to 6q22-23 and gene locus IGAN1 33
- There are no obvious genes within the linked interval
- It is unclear that genetic findings in these families will have a direct bearing on typical sporadic cases of IgA nephropathy
- Liver diseases. These include scarring of the liver called cirrhosis and chronic hepatitis B and C infections.
- Celiac disease. Eating gluten, a protein found in most grains, triggers this digestive condition.
- Infections. These include hepatitis, cirrhosis, human immunodeficiency virus (HIV) infection and some bacterial infections 26, 35
Risk factors for IgA nephropathy
Although the exact cause of IgA nephropathy is unknown, these factors might increase your risk of developing IgA nephropathy 34:
- Sex. In North America and Western Europe, IgA nephropathy affects at least twice as many men as it does women.
- Ethnicity. IgA nephropathy is more common in whites and Asians than it is in blacks.
- Age. IgA nephropathy most often develops between the late teens and late 30s.
- Family history. In some cases, IgA nephropathy appears to run in families, indicating that genetic factors contribute to the disease.
IgA nephropathy pathophysiology
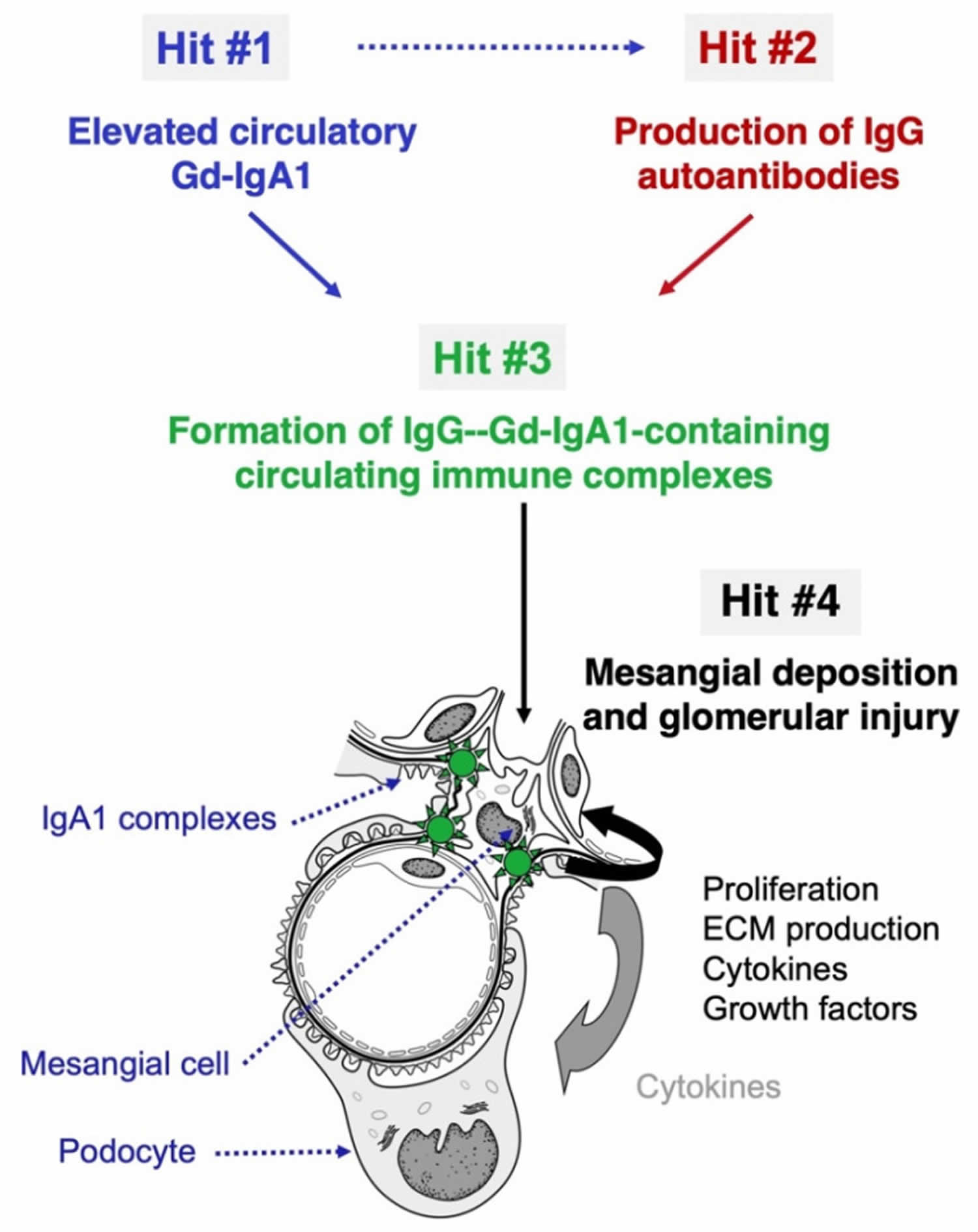
The current understanding is that IgA nephropathy occurs due to a multi-hit mechanism (Figure 3) 36, 6, 37. The first ‘hit’ is a genetically susceptible host predisposed to developing a dysregulated immune response. The next ‘hit’ is a precipitating factor producing the immunological attack. Infections are potential precipitants of IgA nephropathy 26. Trivial mucosal infections, chronic exposure to pathogens, and abnormal handling of commensals in the gut have all been hypothesized to trigger the abnormal immune response in IgA nephropathy 26. The damage to the basement membrane results in the ultrafiltration of larger molecules and produces hematuria. The pathophysiology of how some develop asymptomatic hematuria while some develop rapidly progressive glomerulonephritis, culminating in kidney failure, is poorly understood 38.
Susceptibility to IgA nephropathy is dependent on many genetic and environmental factors. The pathogenesis of this disease is a multi-“hit” process (see Figure 3) 36, 6, 37. These “hits” are understood from the IgA moieties found in biopsies and the circulation of patients with IgA nephropathy. A central finding in patients with IgA nephropathy is the presence of immune complexes in circulation and glomeruli comprised of galactose-deficient IgA1, which is an IgG autoantibody against C3 and the hinge region O-glycans. The presence of abnormally glycosylated IgA1 is a heritable trait 39. In a quarter of blood relatives of IgA nephropathy patients, galactose-deficient IgA1 levels are elevated, and segregation analysis reveals a major dominant gene with a polygenic background 40. A recent study outlines the cellular mechanisms causing IgA glycosylation 41.
The levels of galactose-deficient IgA1 could also be influenced by environmental factors. For instance, these antibodies are prone to bacteria-derived proteases 42. Recent studies suggest that anti-glycan autoantibodies could target the IgA VH gene segment due to somatic hypermutation and not sequences found in the host germline 43. Glomerular inflammation and mesangial proliferation are thought to occur because these immune complexes are nephritogenic. Activation of the renin-angiotensin and complement systems also leads to glomerulosclerosis and tubulointerstitial fibrosis, causing deranged renal function. Other risk factors, such as smoking and hypertension, contribute to disease progression through microvascular injury 44. Glmoerulomegaly and maladaptive hyperfiltration injury attributed to obesity may also be implicated in the nonimmunologic progression of the disease 45.
Experimental studies in mice suggest that exposure to bacteria is needed for excess IgA production, which is enabled by the mediators of B cell differentiation and proliferation. Although the application of this theory to IgA nephropathy in humans must be made cautiously, this idea is further aided by genome-wide association studies and studies of disease progression 46.
It is hypothesized that cytokine APRIL (a proliferation-inducing ligand) contributes to IgA nephropathy by propagating B cell class switch to IgA-producing plasma cells through actions on the TACI receptor. APRIL gene polymorphism confers IgA nephropathy susceptibility, and various risk alleles linked to IgA nephropathy are also associated with several diseases of mucosal immunity 47.
Activation of complement is described as a significant pathogenic contributor to IgA nephropathy, particularly the lectin pathway. Polymeric IgA1 can activate this pathway, and the mannose-binding lectin pathway is detected in glomerular deposits 48, 49. Immune complexes contain C3, as seen in the immunofluorescence study of kidney biopsies 50. Complement factor H (CFH) and properdin are also observed in immune deposits 51. Genome-wide association studies report an allele localized to the CFH gene conferring protection against the development of IgA nephropathy. Further analysis indicates that the deletion of complement factor H–related (CFHR) genes is in linkage disequilibrium with the observed risk allele. The CFHR1 and CFHR3 titrate CFH activity, and their absence leads to altered CFH levels and increased CFH activity 52.
Figure 3. IgA nephropathy pathogenesis
Footnotes: The “four hit” hypothesis of IgA nephropathy. IgA nephropathy (IgAN) is an autoimmune disease with a genetically and environmentally co-determined multi-hit process 36. (Hit #1) Appearance in the circulation of increased levels poorly O-galactosylated IgA1 (galactose-deficient IgA1; Gd-IgA1). (Hit #2) Generation of IgG and IgA autoantibodies directed against Gd-IgA1. (Hit #3) Formation of anti-Gd-IgA1-Gd-IgA1 immune complexes with other serum proteins being added (e.g., complement). Blood levels of the autoantigen (Gd-IgA1) and the corresponding IgG autoantibodies correlate in IgA nephropathy patients, suggesting that elevated circulating levels of Gd-IgA1 are associated with the production of IgG autoantibodies specific for Gd-IgA1 (dashed arrow). Some of the immune complexes formed in the circulation deposit in the kidneys, activate mesangial cells, and induce glomerular injury (Hit #4).
[Source 53 ]IgA nephropathy prevention
Researchers have not found a way to prevent IgA nephropathy. People with a family history of IgA nephropathy should talk with their health care provider to find out what steps they can take to keep their kidneys healthy, such as controlling their blood pressure and keeping their blood cholesterol at healthy levels.
You may reduce your risk of kidney disease by taking care of your kidneys:
- Pay attention to labels when taking over-the-counter (OTC) pain medications. Follow the instructions for OTC pain medications, such as aspirin, acetaminophen (Tylenol, others), ibuprofen (Advil, Motrin IB, others) and naproxen sodium (Aleve, others). Taking too much of these medications may increase your risk of kidney injury. This is especially true if you have pre-existing kidney disease, diabetes or high blood pressure.
- Work with your doctor to manage kidney and other chronic conditions. If you have kidney disease or another condition that increases your risk of acute kidney failure, such as diabetes or high blood pressure, stay on track with treatment goals and follow your doctor’s recommendations to manage your condition.
- Make a healthy lifestyle a priority. Be active; eat a sensible, balanced diet; and drink alcohol only in moderation — if at all.
Eating, diet, and nutrition have not been shown to play a role in causing or preventing glomerular disease. But if you have glomerular disease, your health care professional may recommend you
- Limit salt (sodium) intake
- Reduce calories if your doctor advises you to lose excess weight
- Limit saturated fats if your cholesterol is high
- Make a healthy lifestyle a priority. Be active; eat a sensible, balanced diet; and drink alcohol only in moderation — if at all.
IgA nephropathy symptoms
In its early stages, IgA nephropathy may have no symptoms; it can be silent for years or even decades. Once symptoms appear, the most common one is hematuria, or blood in the urine. Hematuria can be a sign of damaged glomeruli. Blood in the urine may appear during or soon after a cold, sore throat, or other respiratory infection. The amount of blood may be:
- visible with the naked eye. The urine may turn pink or the color of tea or cola. Sometimes a person may have dark or bloody urine.
- so small that it can only be detected using special medical tests.
Another symptom of IgA nephropathy is albuminuria (proteinuria), where a person’s urine contains an increased amount of albumin, a protein typically found in the blood, or large amounts of protein in the urine. Albumin is the main protein in the blood. Healthy kidneys keep most proteins in the blood from leaking into the urine. However, when the glomeruli are damaged, large amounts of protein leak out of the blood into the urine.
When albumin leaks into the urine, the blood loses its capacity to absorb extra fluid from the body. Too much fluid in the body may cause edema, or swelling, usually in the legs, feet, or ankles and less often in the hands or face. Foamy urine is another sign of albuminuria. Some people with IgA nephropathy have both hematuria and albuminuria (proteinuria).
When IgA nephropathy causes symptoms, they might include:
- Cola- or tea-colored urine caused by blood. You might notice these color changes after a cold, sore throat or respiratory infection.
- Blood that can be seen in the urine.
- Foamy urine from protein leaking into the urine. This is called proteinuria.
- Pain on one or both sides of the back below the ribs.
- Swelling in the hands and feet called edema.
- High blood pressure.
- Weakness and tiredness.
After 10 to 20 years with IgA nephropathy, about 20 to 40 percent of adults develop end-stage kidney disease 54. Signs and symptoms of end-stage kidney disease may include:
- high blood pressure
- little or no urination
- edema
- feeling tired
- drowsiness
- confusion
- generalized itching or numbness
- dry skin
- headaches
- weight loss
- appetite loss
- metallic taste in the mouth
- nausea
- upset stomach and vomiting
- sleep problems
- trouble concentrating
- darkened skin
- rashes and itchy skin
- muscle cramps.
Kidney failure is life-threatening without treatment. But dialysis or a kidney transplant can help people live for many more years.
IgA nephropathy complications
The course of IgA nephropathy varies from person to person. Some people have IgA nephropathy for years with few or no problems. Many don’t get diagnosed. Complications of IgA nephropathy include 1, 2:
- High blood pressure (hypertension). Damage to your kidneys from IgA deposits can raise your blood pressure, and high blood pressure can cause further damage to your kidneys.
- Acute kidney failure—sudden and temporary loss of kidney function. If your kidneys lose their filtering ability due to IgA deposits, waste products build up quickly in your blood. And if kidney function gets worse very quickly, health care professionals may use the term rapidly progressive glomerulonephritis.
- Chronic kidney failure (CKD)—reduced kidney function over a period of time. IgA nephropathy can cause your kidneys to gradually stop functioning. Then permanent dialysis or a kidney transplant is needed to live.
- Nephrotic syndrome—a collection of symptoms that indicate kidney damage; symptoms include albuminuria (high urine protein levels or proteinuria), low blood protein levels, and high blood cholesterol or lipids levels (hypercholesterolemia or hyperlipidemia) and fluid can accumulate outside your circulatory system, leading to swelling in your face, hands, feet, or ankles and cause swelling also called edema. About 1 in 5 people with IgA nephropathy develop kidney failure within 10 years of diagnosis 1.
- Heart or cardiovascular problems —the slow loss of kidney function over many years—which can lead to heart disease or stroke.
- Henoch-Schönlein purpura or IgA vasculitis
- High cholesterol. High levels of cholesterol can increase your risk of a heart attack.
Side effects and complications of steroid and steroid-sparing therapy are common 1. Increased risk of infections, hypertension, fluid retention, weight gain, diabetes mellitus, osteoporosis, and iatrogenic Cushing syndrome is the most frequent side effects of steroid therapy 55. Immunosuppression, anaphylaxis, renal, and hepatotoxicity are complications of steroid-sparing agents.
IgA nephropathy diagnosis
IgA nephropathy is often detected after you notice blood in your urine or when a routine test shows that you have protein or blood in your urine. Currently, doctors do not use blood or urine tests as reliable ways to diagnose IgA nephropathy; therefore, the diagnosis of IgA nephropathy requires a kidney biopsy. A kidney biopsy is a procedure that involves taking a small piece of kidney tissue for examination with a microscope. A doctor performs a kidney biopsy in a hospital or an outpatient center with light sedation and a local anesthetic. The health care provider uses imaging techniques such as ultrasound or a computerized tomography scan to guide the biopsy needle into the kidney. A pathologist—a doctor who specializes in examining tissues to diagnose diseases—examines the kidney tissue with a microscope. Only a biopsy can show the IgA deposits in the glomeruli. The biopsy can also show how much kidney damage has already occurred. The biopsy results can help the health care provider determine the best course of treatment.
These tests can help identify which kidney disease you have:
- Urine tests. Blood or protein in the urine, a possible first sign of IgA nephropathy, might be discovered during a routine checkup. If your doctor suspects that you have problems with your kidneys, you might be asked to collect your urine for 24 hours for additional kidney function tests.
- Dipstick test for albumin and blood. A dipstick test performed on a urine sample can detect the presence of albumin and blood. The patient provides a urine sample in a special container in a health care provider’s office or a commercial facility. A nurse or technician can test the sample in the same location, or he or she can send it to a lab for analysis. The test involves placing a strip of chemically treated paper, called a dipstick, into the patient’s urine sample. Patches on the dipstick change color when albumin or blood is present in urine.
- Urine albumin-to-creatinine ratio (UACR). A health care provider uses this measurement, which compares the amount of albumin with the amount of creatinine in a urine sample, to estimate 24-hour albumin excretion. A patient may have chronic kidney disease if the urine albumin-to-creatinine ratio is greater than 30 milligrams (mg) of albumin for each gram (g) of creatinine (30 mg/g).
- Blood tests. If you have kidney disease, a blood test might show increased blood levels of the waste product creatinine or the protein cystatin C. A blood test involves having blood drawn at a health care provider’s office or a commercial facility and sending the sample to a lab for analysis. A health care provider may order a blood test to estimate how much blood a patient’s kidneys filter each minute—a measurement called the estimated glomerular filtration rate (eGFR). Depending on the results, the test can indicate the following:
- eGFR of 60 or above is in the normal range
- eGFR below 60 may indicate kidney disease
- eGFR of 15 or below may indicate kidney failure
- Iothalamate clearance test. Your doctor may also recommend this test, which uses a special contrast agent to track how well your kidneys are filtering wastes.
Histologically, IgA nephropathy is characterized by the following 1:
- The diffuse proliferation of mesangial cells and matrix
- Hypercellular or normal glomeruli with diffuse necrotizing crescentic glomerulonephritis
- Mesangial involvement resembling focal and segmental glomerulosclerosis
- Immunofluorescence will reveal a diffuse granular pattern of IgA deposits in the mesangium
Light Microscopy
The most commonly seen light microscopy findings are focal or diffuse mesangial proliferation and expansion of the extracellular matrix 56. Morphology shows intracapillary and extracapillary proliferative lesions. Occasionally, focal glomerular sclerosis is seen as indistinguishable from focal segmental glomerulosclerosis. In advanced diseases, interstitial fibrosis, vascular sclerosis, and tubular atrophy can be seen. A few patients show segmental necrotizing areas with crescent formation because of extensive disruption of the capillaries.
Electron Microscopy
Electron microscopy reveals mesangial hypercellularity and excess mesangial matrix. An important finding is the presence of mesangial electron-dense deposits of IgA; however, subepithelial and subendothelial deposits of the glomerular capillary wall are seen in a minority of patients, particularly those with the more severe form of the disease 57.
Immunofluorescence
Immunofluorescence reveals mesangial IgA deposits in a diffuse granular pattern. These deposits are majorly polymeric IgA of the IgA1 subclass. Additionally, IgG is found in 43% of patients and IgM in 54% 37. C3 is also often present. The presence of C4d imparts a worse prognosis.
IgA nephropathy treatment
Researchers have not yet found a specific cure for IgA nephropathy. The amount of proteinuria, eGFR, blood pressure, and histological appearance is important in formulating the management plan. Once the kidneys are scarred, they cannot be repaired. Therefore, the ultimate goal of IgA nephropathy treatment is to prevent or delay end-stage kidney disease 58. Your kidney specialist may prescribe medications to:
- Control your blood pressure and slow the progression of kidney disease. Angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin 2 receptor blockers (ARBs) are used to manage proteinuria and lower blood pressure. Salt intake is restricted to control blood pressure. A sodium-glucose cotransporter 2 (SGLT2) inhibitor may be added for persistent proteinuria despite ACE inhibitors or ARBs 59. In a pre-specified subgroup analysis of patients with IgA nephropathy, sodium-glucose cotransporter 2 (SGLT2) inhibitors (which improve outcomes in patients with proteinuria due to diabetic kidney disease) reduced the risk of chronic kidney disease progression 60.
- Remove extra fluid from your blood
- Control your immune system. Immunosuppression with corticosteroids or steroid-sparing agents is used to reduce the rate of progression, including increasing proteinuria, especially into the nephrotic range, and increasing serum creatinine level 61. Steroids have the most benefit if there is heavy proteinuria. Various regimens of oral prednisolone and methylprednisolone are available 55. If there are contraindications for steroids or if the risks of therapy outweigh the anticipated benefits of steroid therapy, steroid-sparing agents may be an option. Cyclophosphamide, azathioprine, and cyclosporine are potential steroid-sparing agents 62, 63. Corticosteroids and cyclophosphamide for proliferative injury or rapidly progressive glomerulonephritis 59
- Lower your blood cholesterol levels
- Weight reduction may reduce proteinuria in IgA nephropathy 64.
- Given the association between smoking and IgA nephropathy progression, smoking cessation should also be advised 44.
Normotensive patients with intact renal function (serum creatinine < 1.2 mg/dL [106.08 micromol/L]) and only mild proteinuria (< 0.5 g/day) usually are not treated beyond angiotensin inhibition (with an ACE inhibitor or ARB) and an SGLT2 inhibitor 59. Patients with renal insufficiency or more severe proteinuria and hematuria are usually offered corticosteroids, which ideally should be started before significant renal insufficiency develops 59.
For the few who progress to develop end-stage renal disease (ESRD), renal transplantation is an option. There is still the risk of IgA nephropathy in the transplanted kidney. Treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker may delay the progression of recurrent disease in allografts 65.
High blood pressure drugs
People with IgA nephropathy that has high blood pressure may need to take high blood pressure medications to lower the blood pressure which can also significantly slow the progression of kidney disease 66. Two types of blood pressure-lowering medications, angiotensin-converting enzyme inhibitors (ACE inhibitors) and angiotensin 2 receptor blockers (ARBs)—have proven effective in slowing the progression of kidney disease 66. Many people require two or more medications to control their blood pressure. A person may also need beta-blockers, calcium channel blockers, sodium-glucose cotransporter 2 inhibitors and other blood pressure medications. The blood pressure target is 130/80 mmHg 67.
Diuretics (water pills)
Your doctor may prescribe a diuretic or water pill, a medication that helps the kidneys remove extra fluid from your blood. Removing the extra fluid can control swelling in your hands and feet called edema and also improve the control of your blood pressure. Taking a diuretic along with an ACE inhibitor or an angiotensin receptor blocker often increases the effectiveness of these medications.
Corticosteroids and immunosuppressants
Doctors sometimes use medications called immunosuppressants to control a person’s immune system. Since inflammation is the immune system’s normal response, controlling the immune system can decrease inflammation. Health care providers may prescribe the following medications:
- corticosteroids, such as prednisone
- cyclophosphamide
There are many studies supporting that corticosteroids reduce proteinuria in IgA nephropathy. In an old study, patients received corticosteroid therapy or supportive therapy alone. The use of renin-angiotensin system (RAS) blockade was random, and the run-in period of optimizing conservative treatment was not carried out. In this trial, 5-year renal survival was greater in the corticosteroid group 68. This finding suggested that corticosteroids for a period of 6 months have a “legacy effect” with a sustained risk reduction in progressive renal dysfunction. In another similar study of patients with biopsy-proven IgA nephropathy, corticosteroids were reported to have reduced proteinuria 69.
Corticosteroids, in combination with an additional agent, are generally reserved for progressive disease. A single-center, randomized, prospective trial with “high-risk” IgA nephropathy demonstrated better renal survival in patients receiving prednisone in combination with cyclophosphamide/azathioprine as opposed to no immunotherapy 70. In contrast, combination therapy does not always provide an advantage. A multicenter trial of 207 patients with IgA nephropathy demonstrated no difference between patients receiving corticosteroids alone and those taking combination therapy 71.
There is no consensus among experts regarding the optimal corticosteroid regimen. One protocol uses methylprednisolone 1 g IV once a day for 3 days at the beginning of months 1, 3, and 5 plus prednisone 0.5 mg/kg orally every other day for 6 months. Another regimen uses prednisone beginning 1 mg/kg orally once a day with dose gradually tapered over 6 months.
Because of the risk of adverse effects, corticosteroids should probably be reserved for patients with any of the following 59:
- Worsening or persistent proteinuria (> 1 g/day), especially if in the nephrotic range despite maximal ACE inhibitor or ARB therapy
- Increasing serum creatinine level
Combinations of IV corticosteroids and cyclophosphamide plus oral prednisone are used for severe disease, such as proliferative or crescentic (rapidly progressive) nephropathy. Evidence for mycophenolate mofetil is conflicting; it should not be used as first-line treatment. None of these medications, however, prevents recurrence in transplant patients. Immunosuppressive therapy should also be avoided in patients with advanced fibrotic kidney disease, which is not reversible.
A novel potential therapy for IgA nephropathy is a formulation of oral budesonide. This agent can potentially act locally at the lymphoid tissue of the mucosa in the distal ileum and proximal large intestine to modulate IgA production. Higher first-pass metabolism theoretically minimizes systemic effects.
Although evidence for rituximab efficacy in other glomerular diseases is significant, early results in IgA nephropathy are not encouraging. A pilot study assessed the efficacy of rituximab versus conservative management in patients with proteinuria. No favorable effects on proteinuria or renal function were observed 72.
Data around the efficacy of mycophenolate are indefinite, such that current guidelines recommend against using mycophenolate in IgA nephropathy 73. The majority of studies are limited by small sample size. There is also possible race-specific variation in response to mycophenolate, as in lupus nephritis 74. However, systematic reviews and meta-analyses of the randomized trials of mycophenolate and systematic reviews suggest mixed results 75, 76.
Lower blood cholesterol levels
People with IgA nephropathy may develop high blood cholesterol levels. Cholesterol is a type of fat found in the body’s cells, in blood, and in many foods. People who take medications for high blood cholesterol levels can lower their blood cholesterol levels. A health care provider may prescribe one of several cholesterol-lowering medications called statins.
IgA nephropathy diet
Researchers have not found that eating, diet, and nutrition play a role in causing or preventing IgA nephropathy. Health care providers may recommend that people with kidney disease, such as IgA nephropathy, make dietary changes such as:
- limiting dietary sodium, often from salt, to help reduce edema and lower blood pressure
- decreasing liquid intake to help reduce edema and lower blood pressure
- eating a diet low in saturated fat and cholesterol to help control high levels of lipids, or fats, in the blood
Health care providers may also recommend that people with kidney disease eat moderate or reduced amounts of protein, although the benefit of reducing protein in a person’s diet is still being researched. Proteins break down into waste products the kidneys must filter from the blood. Eating more protein than the body needs may burden the kidneys and cause kidney function to decline faster. However, protein intake that is too low may lead to malnutrition, a condition that occurs when the body does not get enough nutrients. People with kidney disease on a restricted protein diet should receive blood tests that can show nutrient levels.
Some researchers have shown that fish oil supplements containing omega-3 fatty acids may slow kidney damage in some people with kidney disease by lowering blood pressure. Omega-3 fatty acids may help reduce inflammation and slow kidney damage due to IgA nephropathy. To help ensure coordinated and safe care, people should discuss their use of complementary and alternative medical practices, including their use of dietary supplements and probiotics, with their health care provider. Ask your doctor if prescription fish oil supplements might help you.
People with IgA nephropathy should talk with a health care provider about dietary changes to best manage their individual needs.
Other treatments
Although other interventions have been tried to lower IgA overproduction and to inhibit mesangial proliferation, data supporting any of these are limited or absent, and none can be recommended for routine treatment. These interventions include elimination of gluten, dairy products, eggs, and meat from the diet; tonsillectomy; and immune globulin 1 g/kg IV 2 days a month for 3 months followed by immune globulin 0.35 mL/kg of 16.5% solution IM every 2 weeks for 6 months all theoretically reduce IgA production. Heparin, dipyridamole, and statins are just a few examples of in vitro mesangial cell inhibitors.
For patients who progress to end-stage renal disease (ESRD), kidney transplantation is preferred over dialysis because of improved long-term disease-free survival. The condition recurs in approximately 30% of graft recipients 77.
IgA nephropathy prognosis
Frequently IgA nephropathy patients have a benign course 78. Infrequently, IgA nephropathy patients may gradually progress to end-stage renal disease (ESRD), with the frequency of ESRD increasing with age 79, 80. In general, about 1 in 4 adults with IgA nephropathy eventually get end-stage renal disease (ESRD). About 1 in every 10 to 20 children with IgA nephropathy develop end-stage renal disease (ESRD). About 20% of patients will progress to end-stage renal disease (ESRD) within ten years.
Efforts have been made to determine clinical and histological features associated with progression to end-stage renal disease 81. IgA nephropathy prognosis is predictable to some extent, based on the Oxford classification called the MEST-C score 82. Additionally, nephrotic range proteinuria, hypertension, high serum creatinine level, and widespread intestinal fibrosis of the kidneys on presentation indicate a poor prognosis 83.
Currently, the MEST-C score published in 2009, comprises four histological features is often used to predict IgA nephropathy prognosis 82. The IgA Nephropathy Classification Working Group added crescents to the Oxford classification, to form the MEST-C score 84.
The Oxford classification of IgA nephropathy or MEST score includes the following features 82, 84, 1:
- M = Mesangial cellularity, defined as greater than 4 mesangial cells in any mesangial segment of the glomerulus. M0 is mesangial cellularity in <50% of glomeruli; M1 ≥50%
- E = Endocapillary proliferation is the degree of hypercellularity due to an increased number of cells within glomerular capillary lumina: E0 is absence of hypercellularity; E1 is hypercellularity in any glomeruli
- S = Segmental glomerulosclerosis is defined as sclerosis or adhesions in the glomerular (obliteration of capillary lumina by matrix) in part of but not the whole glomerular tuft: S0 is absence of segmental glomerulosclerosis, S1 is presence of segmental glomerulosclerosis in any glomerulus
- T = Tubular atrophy or interstitial fibrosis, defined as the estimated percentage of cortical area showing tubular atrophy or interstitial fibrosis, whichever is greater: T0 is 0-25%; T1 is 25-50%; T2 is >50%
- C = Presence or absence of crescents. C0 (no crescents), C1 (crescents in less than one-fourth of glomeruli), and C2 (crescents in over one-fourth of glomeruli).
The clinical significance of the individual MEST-C features is as follows:
- M1 – Worse outcomes than M0
- E1 – Worse renal survival in patients not on immunosuppression and improved renal survival with immunosuppression
- S1 – Predictive of worse outcomes
- T – Strongest predictor of worse outcomes
- C1 – Predictive of worse outcomes if no immunosuppression is given, but not if immunosuppression is used; C2 is predictive of worse outcomes regardless of Immunosuppression
Other predictors of poor renal outcomes include the following:
- High serum creatinine level (>120 mmol/L) at presentation
- Hypertension (diastolic >95 mm hg or need for antihypertensive treatment)
- Proteinuria: Urinary protein excretion 3.5 g/24 hr with 7% renal survival 85
- Extensive interstitial fibrosis and tubular atrophy on renal biopsy
- C4d staining on biopsy
A calculator for estimating the risk of progression to end-stage renal disease (ESRD) in patients with IgA nephropathy has been developed by Xie et al 86, based on a cohort of 619 Chinese patients. It has yet to be validated in other ethnic groups. The calculator uses four variables: glomerular filtration rate, hemoglobin level, serum albumin level, and systolic blood pressure.
In general, if any of the above features are seen (i.e., GFR of less than 60 mL/minute, proteinuria greater than 0.5 g/day, hypertension greater than 140/90 mm Hg, more than 50% glomeruli affected by mesangial hypercellularity), then the prognosis is poor. Other factors that determine outcomes include elevated creatinine, hypertension, need for antihypertensive treatment, proteinuria, decreased eGFR at diagnosis, significant interstitial fibrosis, and CD4 staining 87, 88. Isolated microscopic hematuria with mild proteinuria is considered favorable, particularly in Whites. Race may also be a significant determinant of outcome 24.
Will I need a kidney transplant?
Everyone’s IgA nephropathy disease progression is different. Some people respond well to treatment and can live with IgA nephropathy for a long time.
If IgA nephropathy progresses to kidney failure, you may need to consider a kidney transplant. Your care team will discuss dialysis and kidney transplant with you.
In patients with kidney failure due to IgA nephropathy, IgA deposits can recur in a subsequent kidney transplant. If only patients who had undergone a post-transplant kidney biopsy are analyzed, the recurrence rate was 42% after 10 years 89. Cumulative risk of IgA nephropathy recurrence increased after transplant and was associated with a 3.7-fold greater risk of graft loss 89.
If you have kidney disease such as IgA nephropathy, it’s important to:
- Limit sodium (salt) in your foods to help lower blood pressure and reduce swelling.
- Decrease how much liquid you drink, another way to lower blood pressure and reduce swelling.
- Eat foods low in saturated fat and cholesterol to reduce levels of fat in your blood.
- Lifestyle modification such as quitting smoking, diet and exercise, if needed, can also be beneficial.
- Some healthcare providers recommend fish oil supplements that contain omega-3 fatty acids. Research suggests this approach may lower blood pressure and slow the progress of the disease. Speak to your healthcare provider before you start taking any supplements.
IgA nephropathy life expectancy
Although IgA nephropathy usually follows a benign course, end-stage renal disease (ESRD) develops in 15-20% of patients within 10 years of onset and in about 25-30% of patients by 20 years 16. 30–40% of IgA nephropathy patients progress to kidney failure that reduces life expectancy by about 10 years 90. Cumulative incidence of recurrent IgA nephropathy was 19% at 10 years and 23% at 15 years after kidney transplantation 89.
- Rawla P, Limaiem F, Hashmi MF. IgA Nephropathy. [Updated 2023 Jul 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538214[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Tashakkorinia N, Muco E, Tudor ME. Berger Disease. [Updated 2023 Feb 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499998[↩][↩][↩][↩][↩]
- IgA Nephropathy. https://www.niddk.nih.gov/health-information/kidney-disease/iga-nephropathy[↩]
- Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013 Jun 20;368(25):2402-14. doi: 10.1056/NEJMra1206793[↩]
- IgA nephropathy. https://medlineplus.gov/ency/article/000466.htm[↩]
- Knoppova B, Reily C, King RG, Julian BA, Novak J, Green TJ. Pathogenesis of IgA Nephropathy: Current Understanding and Implications for Development of Disease-Specific Treatment. J Clin Med. 2021 Sep 29;10(19):4501. doi: 10.3390/jcm10194501[↩][↩][↩][↩][↩][↩]
- Wyatt R.J., Julian B.A. IgA nephropathy. N. Engl. J. Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793[↩][↩]
- D’Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 1987 Sep;64(245):709-27.[↩]
- Woo KT, Lau YK, Chan CM, Wong KS. Angiotensin-converting enzyme inhibitor versus angiotensin 2 receptor antagonist therapy and the influence of angiotensin-converting enzyme gene polymorphism in IgA nephritis. Ann Acad Med Singap. 2008 May;37(5):372-6. https://annals.edu.sg/pdf/37VolNo5May2008/V37N5p372.pdf[↩]
- Satpathy HK. IgA nephropathy. In: Ferri FF, ed. Ferri’s Clinical Advisor 2013. 1st ed. St. Louis: Mosby; 2012: 570–571.[↩]
- Geddes C.C., Rauta V., Gronhagen-Riska C., Bartosik L.P., Jardine A.G., Ibels L.S., Pei Y., Cattran D.C. A tricontinental view of IgA nephropathy. Nephrol. Dial. Transplant. 2003;18:1541–1548. doi: 10.1093/ndt/gfg207[↩]
- Shen A.Y., Brar S.S., Khan S.S., Kujubu D.A. Association of race, heart failure and chronic kidney disease. Future Cardiol. 2006;2:441–454. doi: 10.2217/14796678.2.4.441[↩]
- Wyatt R.J., Julian B.A., Baehler R.W., Stafford C.C., McMorrow R.G., Ferguson T., Jackson E., Woodford S.Y., Miller P.M., Kritchevsky S. Epidemiology of IgA nephropathy in central and eastern Kentucky for the period 1975 through 1994. Central Kentucky Region of the Southeastern United States IgA Nephropathy DATABANK Project. J. Am. Soc. Nephrol. 1998;9:853–858. doi: 10.1681/ASN.V95853[↩]
- Schena F.P. A retrospective analysis of the natural history of primary IgA nephropathy worldwide. Am. J. Med. 1990;89:209–215. doi: 10.1016/0002-9343(90)90300-3[↩]
- Wyatt R.J., Kritchevsky S.B., Woodford S.Y., Miller P.M., Roy S., Holland N.H., Jackson E., Bishof N.A. IgA nephropathy: Long-term prognosis for pediatric patients. J. Pediatr. 1995;127:913–919. doi: 10.1016/S0022-3476(95)70027-7[↩]
- IgA Nephropathy. https://emedicine.medscape.com/article/239927-overview[↩][↩]
- McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011 Feb;26(2):414-30. doi: 10.1093/ndt/gfq665[↩]
- Schena FP, Nistor I. Epidemiology of IgA Nephropathy: A Global Perspective. Semin Nephrol. 2018 Sep;38(5):435-442. doi: 10.1016/j.semnephrol.2018.05.013[↩]
- Kiryluk K, Li Y, Sanna-Cherchi S, Rohanizadegan M, et al. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 2012;8(6):e1002765. doi: 10.1371/journal.pgen.1002765[↩]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney International. 2021;100(4S):S1–S276. doi: 10.1016/j.kint.2021.05.021[↩][↩][↩]
- D’Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin. Nephrol. 2004;24:179–196. doi: 10.1016/j.semnephrol.2004.01.001[↩]
- Barratt J., Feehally J. IgA nephropathy. J. Am. Soc. Nephrol. 2005;16:2088–2097. doi: 10.1681/ASN.2005020134[↩]
- Reich H.N., Troyanov S., Scholey J.W., Cattran D.C., Registry T.G. Remission of proteinuria improves prognosis in IgA nephropathy. J. Am. Soc. Nephrol. 2007;18:3177–3183. doi: 10.1681/ASN.2007050526[↩]
- Barbour SJ, Cattran DC, Kim SJ, Levin A, Wald R, Hladunewich MA, Reich HN. Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int. 2013 Nov;84(5):1017-24. doi: 10.1038/ki.2013.210. Epub 2013 Jun 5. Erratum in: Kidney Int. 2015 Jan;87(1):242.[↩][↩]
- D’Amico G, Colasanti G, Barbiano di Belgioioso G, Fellin G, Ragni A, Egidi F, Radaelli L, Fogazzi G, Ponticelli C, Minetti L. Long-term follow-up of IgA mesangial nephropathy: clinico-histological study in 374 patients. Semin Nephrol. 1987 Dec;7(4):355-8.[↩]
- Rollino C, Vischini G, Coppo R. IgA nephropathy and infections. J Nephrol. 2016 Aug;29(4):463-8. doi: 10.1007/s40620-016-0265-x[↩][↩][↩][↩]
- Lai KN, Tang SC, Schena FP, Novak J, Tomino Y, Fogo AB, Glassock RJ. IgA nephropathy. Nat Rev Dis Primers. 2016 Feb 11;2:16001. doi: 10.1038/nrdp.2016.1[↩][↩][↩][↩]
- Allen A.C., Bailey E.M., Brenchley P.E., Buck K.S., Barratt J., Feehally J. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: Observations in three patients. Kidney Int. 2001;60:969–973. doi: 10.1046/j.1523-1755.2001.060003969.x[↩]
- Hiki Y., Odani H., Takahashi M., Yasuda Y., Nishimoto A., Iwase H., Shinzato T., Kobayashi Y., Maeda K. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59:1077–1085. doi: 10.1046/j.1523-1755.2001.0590031077.x[↩]
- Conley M.E., Cooper M.D., Michael A.F. Selective deposition of immunoglobulin A1 in immunoglobulin A nephropathy, anaphylactoid purpura nephritis, and systemic lupus erythematosus. J. Clin. Investig. 1980;66:1432–1436. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC371631/pdf/jcinvest00696-0242.pdf[↩][↩]
- Jennette J.C. The immunohistology of IgA nephropathy. Am. J. Kidney Dis. 1988;12:348–352. doi: 10.1016/S0272-6386(88)80022-2[↩]
- Roberts I.S. Pathology of IgA nephropathy. Nat. Rev. Nephrol. 2014;10:445–454. doi: 10.1038/nrneph.2014.92[↩]
- Woo KT, Lau YK, Choong HL, Tan HK, Foo MW, Lee EJ, Anantharaman V, Lee GS, Yap HK, Yi Z, Fook-Chong S, Wong KS, Chan CM. Genomics and disease progression in IgA nephritis. Ann Acad Med Singap. 2013 Dec;42(12):674-80. https://annals.edu.sg/pdf/42VolNo12Dec2013/V42N12p674.pdf[↩][↩]
- IgA nephropathy (Berger disease). https://www.mayoclinic.org/diseases-conditions/iga-nephropathy/symptoms-causes/syc-20352268[↩][↩]
- Han SH, Kang EW, Kie JH, Yoo TH, Choi KH, Han DS, Kang SW. Spontaneous remission of IgA nephropathy associated with resolution of hepatitis A. Am J Kidney Dis. 2010 Dec;56(6):1163-7. doi: 10.1053/j.ajkd.2010.08.018[↩]
- Suzuki H., Kiryluk K., Novak J., Moldoveanu Z., Herr A.B., Renfrow M.B., Wyatt R.J., Scolari F., Mestecky J., Gharavi A.G., et al. The pathophysiology of IgA nephropathy. J. Am. Soc. Nephrol. 2011;22:1795–1803. doi: 10.1681/ASN.2011050464[↩][↩][↩]
- Magistroni R, D’Agati VD, Appel GB, Kiryluk K. New developments in the genetics, pathogenesis, and therapy of IgA nephropathy. Kidney Int. 2015 Nov;88(5):974-89. doi: 10.1038/ki.2015.252[↩][↩][↩]
- Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011 Oct;22(10):1795-803. doi: 10.1681/ASN.2011050464[↩]
- Kiryluk K, Moldoveanu Z, Sanders JT, Eison TM, Suzuki H, Julian BA, Novak J, Gharavi AG, Wyatt RJ. Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schönlein purpura nephritis. Kidney Int. 2011 Jul;80(1):79-87. doi: 10.1038/ki.2011.16[↩]
- Gharavi AG, Moldoveanu Z, Wyatt RJ, Barker CV, Woodford SY, Lifton RP, Mestecky J, Novak J, Julian BA. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol. 2008 May;19(5):1008-14. doi: 10.1681/ASN.2007091052[↩]
- Knoppova B, Reily C, Maillard N, Rizk DV, Moldoveanu Z, Mestecky J, Raska M, Renfrow MB, Julian BA, Novak J. The Origin and Activities of IgA1-Containing Immune Complexes in IgA Nephropathy. Front Immunol. 2016 Apr 12;7:117. doi: 10.3389/fimmu.2016.00117[↩]
- Lamm ME, Emancipator SN, Robinson JK, Yamashita M, Fujioka H, Qiu J, Plaut AG. Microbial IgA protease removes IgA immune complexes from mouse glomeruli in vivo: potential therapy for IgA nephropathy. Am J Pathol. 2008 Jan;172(1):31-6. doi: 10.2353/ajpath.2008.070131[↩]
- Huang ZQ, Raska M, Stewart TJ, Reily C, King RG, Crossman DK, Crowley MR, Hargett A, Zhang Z, Suzuki H, Hall S, Wyatt RJ, Julian BA, Renfrow MB, Gharavi AG, Novak J. Somatic Mutations Modulate Autoantibodies against Galactose-Deficient IgA1 in IgA Nephropathy. J Am Soc Nephrol. 2016 Nov;27(11):3278-3284. doi: 10.1681/ASN.2014101044[↩]
- Yamamoto R, Nagasawa Y, Shoji T, Iwatani H, Hamano T, Kawada N, Inoue K, Uehata T, Kaneko T, Okada N, Moriyama T, Horio M, Yamauchi A, Tsubakihara Y, Imai E, Rakugi H, Isaka Y. Cigarette smoking and progression of IgA nephropathy. Am J Kidney Dis. 2010 Aug;56(2):313-24. doi: 10.1053/j.ajkd.2010.02.351[↩][↩]
- Kataoka H, Ohara M, Honda K, Mochizuki T, Nitta K. Maximal glomerular diameter as a 10-year prognostic indicator for IgA nephropathy. Nephrol Dial Transplant. 2011 Dec;26(12):3937-43. doi: 10.1093/ndt/gfr139[↩]
- Zhai YL, Zhu L, Shi SF, Liu LJ, Lv JC, Zhang H. Increased APRIL Expression Induces IgA1 Aberrant Glycosylation in IgA Nephropathy. Medicine (Baltimore). 2016 Mar;95(11):e3099. doi: 10.1097/MD.0000000000003099[↩]
- Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014 Nov;46(11):1187-96. doi: 10.1038/ng.3118[↩]
- Roos A, Rastaldi MP, Calvaresi N, Oortwijn BD, Schlagwein N, van Gijlswijk-Janssen DJ, Stahl GL, Matsushita M, Fujita T, van Kooten C, Daha MR. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006 Jun;17(6):1724-34. doi: 10.1681/ASN.2005090923[↩]
- Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR. Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol. 2001 Sep 1;167(5):2861-8. doi: 10.4049/jimmunol.167.5.2861[↩]
- Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, Novak J. Current Understanding of the Role of Complement in IgA Nephropathy. J Am Soc Nephrol. 2015 Jul;26(7):1503-12. doi: 10.1681/ASN.2014101000[↩]
- Gharavi AG, Kiryluk K, Choi M, Li Y, et al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011 Mar 13;43(4):321-7. doi: 10.1038/ng.787[↩]
- Zhu L, Zhai YL, Wang FM, Hou P, Lv JC, Xu DM, Shi SF, Liu LJ, Yu F, Zhao MH, Novak J, Gharavi AG, Zhang H. Variants in Complement Factor H and Complement Factor H-Related Protein Genes, CFHR3 and CFHR1, Affect Complement Activation in IgA Nephropathy. J Am Soc Nephrol. 2015 May;26(5):1195-204. doi: 10.1681/ASN.2014010096[↩]
- Novak J., Julian B.A., Mestecky J., Renfrow M.B. Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin. Immunopathol. 2012;34:365–382. doi: 10.1007/s00281-012-0306-z[↩]
- IgA Nephropathy. https://www.kidney.org/atoz/content/iganeph[↩]
- Lv J, Xu D, Perkovic V, Ma X, Johnson DW, Woodward M, Levin A, Zhang H, Wang H; TESTING Study Group. Corticosteroid therapy in IgA nephropathy. J Am Soc Nephrol. 2012 Jun;23(6):1108-16. doi: 10.1681/ASN.2011111112[↩][↩]
- Hassler JR. IgA nephropathy: A brief review. Semin Diagn Pathol. 2020 May;37(3):143-147. doi: 10.1053/j.semdp.2020.03.001[↩]
- Kusaba G, Ohsawa I, Ishii M, Inoshita H, Takagi M, Tanifuji C, Takahashi K, Nakamoto J, Yoshida M, Ohi H, Horikoshi S, Kurihara H, Tomino Y. Significance of broad distribution of electron-dense deposits in patients with IgA nephropathy. Med Mol Morphol. 2012 Dec;45(1):29-34. doi: 10.1007/s00795-011-0538-3[↩]
- Pozzi C. Treatment of IgA nephropathy. J Nephrol. 2016 Feb;29(1):21-5. doi: 10.1007/s40620-015-0248-3[↩]
- Immunoglobulin A Nephropathy. https://www.msdmanuals.com/professional/genitourinary-disorders/glomerular-disorders/immunoglobulin-a-nephropathy[↩][↩][↩][↩][↩]
- Wheeler DC, Toto RD, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, McMurray JJV, Pecoits-Filho R, Correa-Rotter R, Rossing P, Sjöström CD, Umanath K, Langkilde AM, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021 Jul;100(1):215-224. doi: 10.1016/j.kint.2021.03.033[↩]
- Gharavi AG, Yan Y, Scolari F, Schena FP, Frasca GM, Ghiggeri GM, Cooper K, Amoroso A, Viola BF, Battini G, Caridi G, Canova C, Farhi A, Subramanian V, Nelson-Williams C, Woodford S, Julian BA, Wyatt RJ, Lifton RP. IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22-23. Nat Genet. 2000 Nov;26(3):354-7. doi: 10.1038/81677[↩]
- Coppo R. Treatment of IgA nephropathy: Recent advances and prospects. Nephrol Ther. 2018 Apr;14 Suppl 1:S13-S21. doi: 10.1016/j.nephro.2018.02.010[↩]
- Lai KN, Leung JC, Tang SC. The Treatment of IgA Nephropathy. Kidney Dis (Basel). 2015 May;1(1):19-26. doi: 10.1159/000381508[↩]
- Kittiskulnam P, Kanjanabuch T, Tangmanjitjaroen K, Chancharoenthana W, Praditpornsilpa K, Eiam-Ong S. The beneficial effects of weight reduction in overweight patients with chronic proteinuric immunoglobulin a nephropathy: a randomized controlled trial. J Ren Nutr. 2014 May;24(3):200-7. doi: 10.1053/j.jrn.2014.01.016[↩]
- Courtney AE, McNamee PT, Nelson WE, Maxwell AP. Does angiotensin blockade influence graft outcome in renal transplant recipients with IgA nephropathy? Nephrol Dial Transplant. 2006 Dec;21(12):3550-4. doi: 10.1093/ndt/gfl506[↩]
- Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR, Kuhlmann U, Witzke O, Gross O, Vielhauer V, Mann JF, Hilgers RD, Floege J; STOP-IgAN Investigators. Intensive Supportive Care plus Immunosuppression in IgA Nephropathy. N Engl J Med. 2015 Dec 3;373(23):2225-36. doi: 10.1056/NEJMoa1415463[↩][↩]
- Appel GB, Waldman M. The IgA nephropathy treatment dilemma. Kidney Int. 2006 Jun;69(11):1939-44. doi: 10.1038/sj.ki.5000434[↩]
- Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, Locatelli F. Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet. 1999 Mar 13;353(9156):883-7. doi: 10.1016/s0140-6736(98)03563-6[↩]
- Manno C, Torres DD, Rossini M, Pesce F, Schena FP. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant. 2009 Dec;24(12):3694-701. doi: 10.1093/ndt/gfp356. Erratum in: Nephrol Dial Transplant. 2010 Apr;25(4):1363-4.[↩]
- Ballardie FW, Roberts ISD. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol. 2002 Jan;13(1):142-148. doi: 10.1681/ASN.V131142[↩]
- Pozzi C, Andrulli S, Pani A, Scaini P, Roccatello D, Fogazzi G, Pecchini P, Rustichelli R, Finocchiaro P, Del Vecchio L, Locatelli F. IgA nephropathy with severe chronic renal failure: a randomized controlled trial of corticosteroids and azathioprine. J Nephrol. 2013 Jan-Feb;26(1):86-93. doi: 10.5301/jn.5000110[↩]
- Lafayette RA, Canetta PA, Rovin BH, Appel GB, Novak J, Nath KA, Sethi S, Tumlin JA, Mehta K, Hogan M, Erickson S, Julian BA, Leung N, Enders FT, Brown R, Knoppova B, Hall S, Fervenza FC. A Randomized, Controlled Trial of Rituximab in IgA Nephropathy with Proteinuria and Renal Dysfunction. J Am Soc Nephrol. 2017 Apr;28(4):1306-1313. doi: 10.1681/ASN.2016060640[↩]
- Beck L, Bomback AS, Choi MJ, Holzman LB, Langford C, Mariani LH, Somers MJ, Trachtman H, Waldman M. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am J Kidney Dis. 2013 Sep;62(3):403-41. doi: 10.1053/j.ajkd.2013.06.002. Erratum in: Am J Kidney Dis. 2017 Mar;69(3):485.[↩]
- Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, Li LS, Mysler E, Sánchez-Guerrero J, Solomons N, Wofsy D; Aspreva Lupus Management Study Group. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009 May;20(5):1103-12. doi: 10.1681/ASN.2008101028[↩]
- Chen Y, Li Y, Yang S, Li Y, Liang M. Efficacy and safety of mycophenolate mofetil treatment in IgA nephropathy: a systematic review. BMC Nephrol. 2014 Dec 5;15:193. doi: 10.1186/1471-2369-15-193[↩]
- Tian L, Shao X, Xie Y, Wang L, Wang Q, Che X, Ni Z, Mou S. The long-term efficacy and safety of immunosuppressive therapy on the progression of IgA nephropathy: a meta-analysis of controlled clinical trials with more than 5-year follow-up. Expert Opin Pharmacother. 2015 Jun;16(8):1137-47. doi: 10.1517/14656566.2015.1038238[↩]
- Jäger C, Stampf S, Molyneux K, Barratt J, Golshayan D, Hadaya K, Huynh-Do U, Binet FI, Mueller TF, Koller M, Kim MJ. Recurrence of IgA nephropathy after kidney transplantation: experience from the Swiss transplant cohort study. BMC Nephrol. 2022 May 10;23(1):178. doi: 10.1186/s12882-022-02802-x[↩]
- Walsh M, Sar A, Lee D, Yilmaz S, Benediktsson H, Manns B, Hemmelgarn B. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010 Mar;5(3):425-30. doi: 10.2215/CJN.06530909[↩]
- Shen PC, He LQ, Tang Y, Wang Q, Wang W, Li J. Clinicopathological characteristics and prognostic factors of asymptomatic IgA nephropathy. J Investig Med. 2010 Mar;58(3):560-5. doi: 10.231/JIM.0b013e3181d20aa1[↩]
- Herlitz LC, Bomback AS, Stokes MB, Radhakrishnan J, D’Agati VD, Markowitz GS. IgA nephropathy with minimal change disease. Clin J Am Soc Nephrol. 2014 Jun 6;9(6):1033-9. doi: 10.2215/CJN.11951113[↩]
- Walsh M, Sar A, Lee D, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010 Mar. 5(3):425-30.[↩]
- Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009 Sep;76(5):534-45. doi: 10.1038/ki.2009.243[↩][↩][↩]
- Soares MF, Roberts IS. IgA nephropathy: an update. Curr Opin Nephrol Hypertens. 2017 May;26(3):165-171. doi: 10.1097/MNH.0000000000000312[↩]
- Haas M, Verhave JC, Liu ZH, Alpers CE, Barratt J, Becker JU, Cattran D, Cook HT, Coppo R, Feehally J, Pani A, Perkowska-Ptasinska A, Roberts IS, Soares MF, Trimarchi H, Wang S, Yuzawa Y, Zhang H, Troyanov S, Katafuchi R. A Multicenter Study of the Predictive Value of Crescents in IgA Nephropathy. J Am Soc Nephrol. 2017 Feb;28(2):691-701. doi: 10.1681/ASN.2016040433. Epub 2016 Sep 9. Erratum in: J Am Soc Nephrol. 2017 May;28(5):1665.[↩][↩]
- Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012 Apr. 27 (4):1479-85.[↩]
- Xie J, Kiryluk K, Wang W, Wang Z, Guo S, Shen P, Ren H, Pan X, Chen X, Zhang W, Li X, Shi H, Li Y, Gharavi AG, Chen N. Predicting progression of IgA nephropathy: new clinical progression risk score. PLoS One. 2012;7(6):e38904. doi: 10.1371/journal.pone.0038904[↩]
- Barbour SJ, Reich HN. Risk stratification of patients with IgA nephropathy. Am J Kidney Dis. 2012 Jun;59(6):865-73. doi: 10.1053/j.ajkd.2012.02.326[↩]
- D’Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis. 2000 Aug;36(2):227-37. doi: 10.1053/ajkd.2000.8966[↩]
- Uffing A, Pérez-Saéz MJ, Jouve T, Bugnazet M, et al. Recurrence of IgA Nephropathy after Kidney Transplantation in Adults. Clin J Am Soc Nephrol. 2021 Aug;16(8):1247-1255. doi: 10.2215/CJN.00910121[↩][↩][↩]
- Hastings M.C., Bursac Z., Julian B.A., Villa Baca E., Featherston J., Woodford S.Y., Bailey L., Wyatt R.J. Life expectancy for patients from the southeastern United States with IgA nephropathy. Kidney Int. Rep. 2018;3:99–104. doi: 10.1016/j.ekir.2017.08.008[↩]