Contents
Collapsing glomerulopathy
Collapsing glomerulopathy also known as collapsing variant of focal segmental glomerulosclerosis is a pattern of kidney injury from multiple causes that include HIV infection (HIV-associated nephropathy) and APOL1 gene mutations in African American patients 1, 2, 3, 4, 5, 6, 7, 8, 9, 10. Collapsing glomerulopathy is characterized by segmental or global collapse of the glomerular capillaries and hypertrophy and hyperplasia of podocytes migrating to the tuft to give the appearance of ‘pseudocrescents’ 11, 12, 5. The Columbia classification of 2004 classified collapsing glomerulopathy as a histological subtype of focal segmental glomerulosclerosis (FSGS) 13, 14.
The most common signs and symptoms of collapsing glomerulopathy is massive, severe proteinuria associated with pure nephrotic syndrome. Patients may present with high blood pressure, lipiduria, and hematuria on urinalysis 5. A significantly high number of patients present with renal failure on admission and rapidly progress to end-stage renal disease (ESRD) 15, 16, 17, 18, 19.
The normal kidney dimensions are usually preserved in collapsing glomerulopathy and presents as in other conditions, such as diabetic nephropathy, polycystic kidney disease, and amyloidosis. Ultrasonography may demonstrate normal or increased renal size and hyperechogenicity, which may be due to edema, fibrosis, and tubulointerstitial infiltrates resulting from the rapid progression to end-stage renal disease (ESRD) and absence of renal parenchymal contraction 16.
Collapsing glomerulopathy is the most common morphological pattern in HIV nephropathy 20. The increased incidence of HIV infection has been identified as a cause for the proportionate increase in the cases of collapsing glomerulopathy 16. Disease activity characterized by a high viral load and low CD4 lymphocyte count results in greater kidney damage 10; however, the virus has also been detected in the kidney tissue of patients with undetectable viral loads 21. Non-structural HIV proteins, such as viral protein R and negative factor, promote cell cycle dysregulation, which stimulates podocyte proliferation. In some studies, the prevalence of HIV infection in patients with collapsing glomerulopathy ranged from 30 to 55% 15, 22.
Other factors such as chronic inflammation are associated with the genetic predisposition for APOL1 and MHY9 mutations, which result in collapsing glomerulopathy 21.
Cytomegalovirus infection is associated with immunosuppression, but it can also occur in immunocompetent patients and progress to collapsing glomerulopathy, even in the acute phase of the disease. Specific treatment is associated with several benefits for prognosis in this population 23, 24, 25, 26.
Arboviruses were recently identified as an important causative factor for collapsing glomerulopathy, particularly in Brazil 27, 28. Eight of 13 collapsing glomerulopathy biopsy samples obtained from the first half of 2016 from a large Brazilian kidney biopsy center were positive for arbovirus, wherein six samples were positive for dengue, one for Zika, and one for a concomitant infection; only one case had APOL1 mutations. These findings suggested that direct viral action in tissues may be associated with other risk factors, such as G1 and G2 mutations 27, 28.
The association between SARS-CoV-2 (COVID-19) infection and collapsing glomerulopathy was initially demonstrated in a series of autopsies 29. A recent systematic review of 59 studies reporting COVID-19 related histopathological diagnoses from kidney biopsy identified collapsing glomerulopathy as the most common finding, followed by acute tubular injury and trombotic microangiopathy 30. Various mechanisms of acute kidney injury secondary to COVID-19 have been proposed—from direct intrarenal infection to dysregulation of the renin-angiotensin-aldosterone system, to altered hemodynamic control, coagulation and cytokine homeostasis 30. Although the association is multifactorial, it has been emphasized the influence of the hyperactive inflammatory process and participation of circulating interferons 30 and direct infection appears highly unlikely to play a significant pathogenic role.
APOL1 mutations have been detected in many patients with COVID-19, which suggested that the SARS-CoV-2 virus is a potential secondary trigger for glomerular damage 31. Studies have confirmed the strong association of collapsing glomerulopathy and non-collapsing podocytopathies with concurrent or recent COVID-19 in patients with APOL1 high-risk alleles 30, 31, 32.
Systemic lupus erythematosus (SLE) can trigger collapsing glomerulopathy as an extreme form of lupus podocytopathy in the absence of other lupus nephritis patterns 33. Collapsing glomerulopathy may also result from the association of SLE with other risk factors, such as black ethnicity and APOL1 mutations. collapsing glomerulopathy is occasionally present during the diagnosis of SLE, with low levels of therapeutic response 33.
Collapsing glomerulopathy can be drug-induced; bisphosphonates, especially pamidronate and zoledronic acid, inhibit the mevalonate synthesis pathways, which are essential for cell differentiation. This triggers podocyte proliferation and progression to collapsing glomerulopathy 34. Synthetic interferons (which are used to treat some infectious, autoimmune, and neoplastic diseases) can result in APOL1 overexpression in the glomerular epithelium, which triggers podocyte damage. This effect is evidenced by the presence of tubuloreticular inclusions on electron microscopy (48). Illicit drugs, such as cocaine and heroin, are also associated with collapsing glomerulopathy. The most probable mechanism involves ischemic glomerular damage from oxidative endothelial damage, accelerated atheromatosis, and direct vasoconstriction 35.
Acquired Hemophagocytic Syndrome results from a hyperactive immune system that develops secondary to infectious diseases or lymphatic hematological neoplasms. Excessive T lymphocyte and circulating cytokine activation can promote podocyte proliferation and progression to collapsing glomerulopathy 36. Monoclonal gammopathies can also encourage progression toward collapsing glomerulopathy. Histological analysis demonstrates diffuse Ig chain deposits, with glomerular collapse resulting from the deposition of extracellular elements instead of from podocyte proliferation 37. Once triggered, collapsing glomerulopathy progresses independently of the underlying disease. Further, while the underlying gammopathy may go into remission, collapsing glomerulopathy can continue to worsen until kidney dialysis becomes necessary 38.
Collapsing glomerulopathy is also associated with diseases characterized by microvascular damage, such as sickle cell anemia, intravascular hemolysis syndromes, drug reactions (calcineurin inhibitors), malignant arterial hypertension, and antiphospholipid syndrome. A total of 53 histological samples that demonstrated thrombotic microangiopathy were evaluated. These samples were acquired from 33 patients with focal segmental glomerulosclerosis, 19 of whom had collapsing glomerulopathy 39. Glomerular ischemia resulted in the loss of podocyte differentiation and reduced cell proliferation. The population in this study was predominantly of white ethnicity and was characterized by a lower incidence of nephrotic proteinuria.
Other conditions, such as hypertensive disorders of pregnancy, have also been implicated as a trigger for collapsing glomerulopathy. Hypertensive disorders of pregnancy are characterized by diffuse endothelial damage and possible glomerular ischemia. Kidney biopsy often shows association between collapsing glomerulopathy and thrombotic microangiopathy 32.
Collapsing glomerulopathy demonstrates no sex differences, but preferentially affects patients of African descent. Collapsing glomerulopathy also often affects young adult patients and has a varying predominance in the pediatric age group 40, 15, 16, 41, 17, 42.
Population-based studies in the United States, India, Pakistan, Macedonia, and Portugal proposed prevalence rates of collapsing glomerulopathy to 1.7, 0.75, 0.38, 1.7, and 0.29%, respectively 43, 40, 44, 45, 15. A study from Brazil, the São Paulo Registry of Glomerulopathies, indicates that 36% of focal segmental glomerulosclerosis cases comprise patients with collapsing glomerulopathy 46.
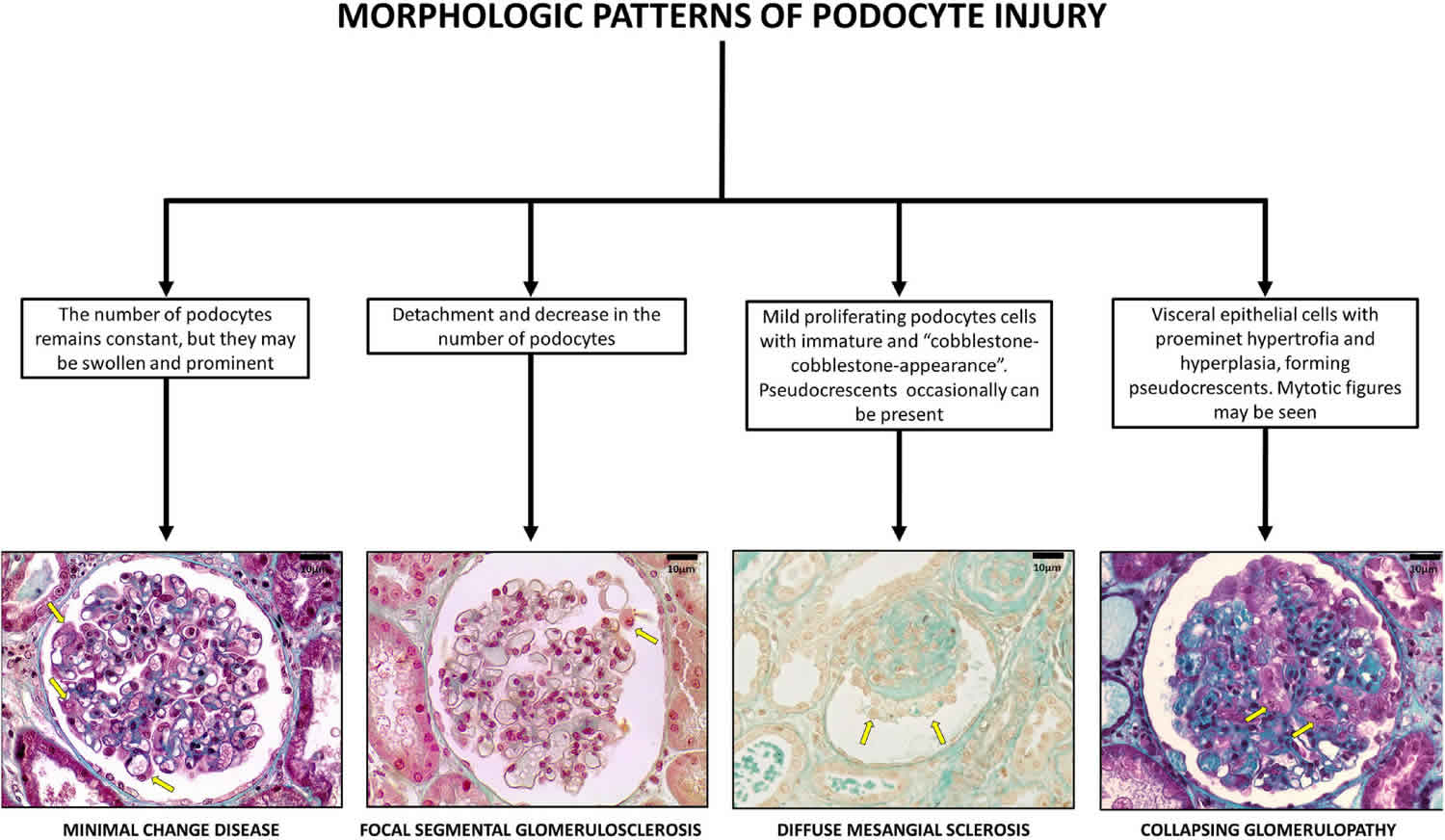
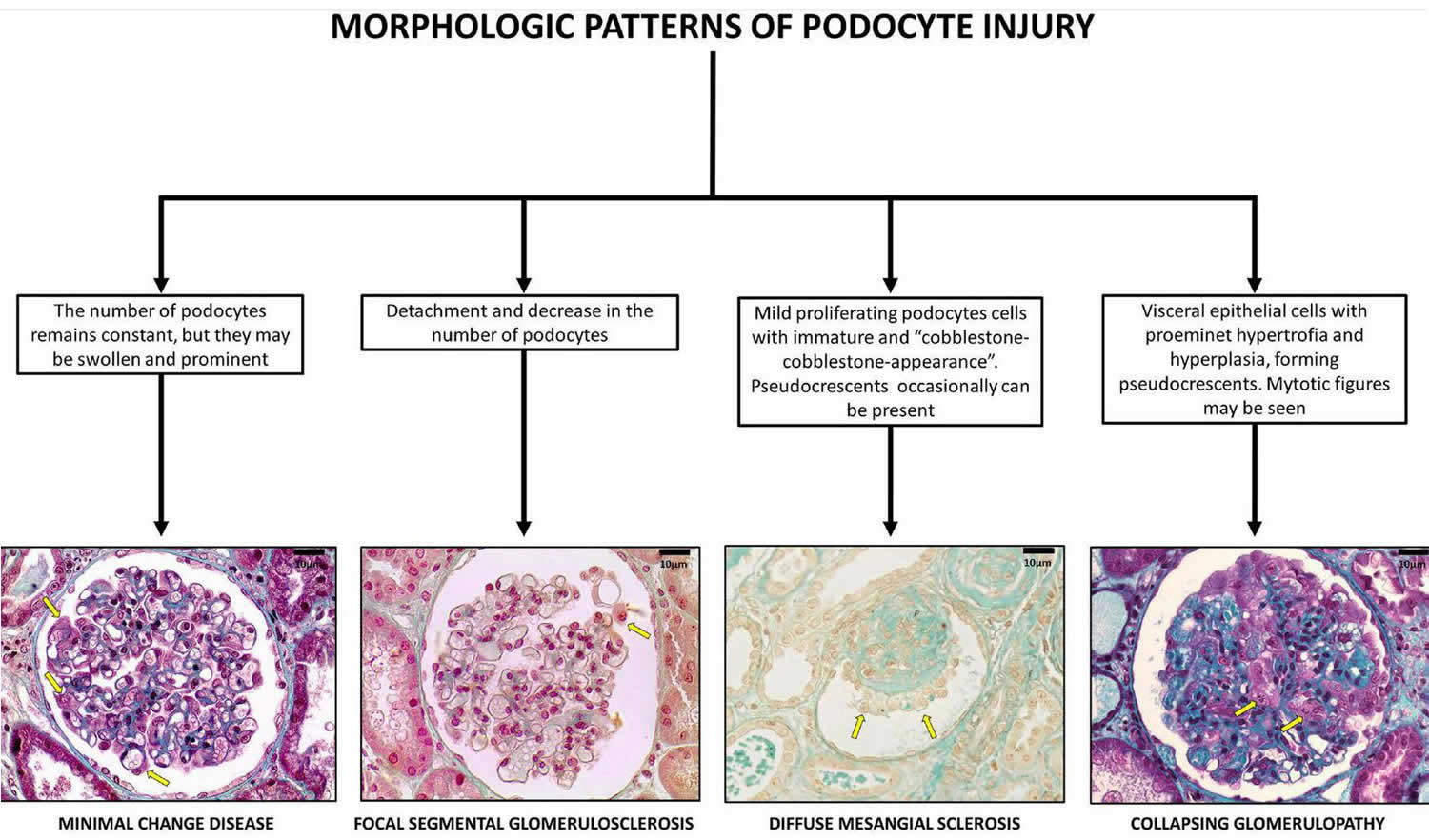
Figure 1. Collapsing glomerulopathy
Footnote: Glomerular pattern and podocytes morphological changes in human podocytopathies (bar = 10 μm). Podocytopathies are kidney diseases in which direct or indirect podocyte injury drives proteinuria or nephrotic syndrome 47.
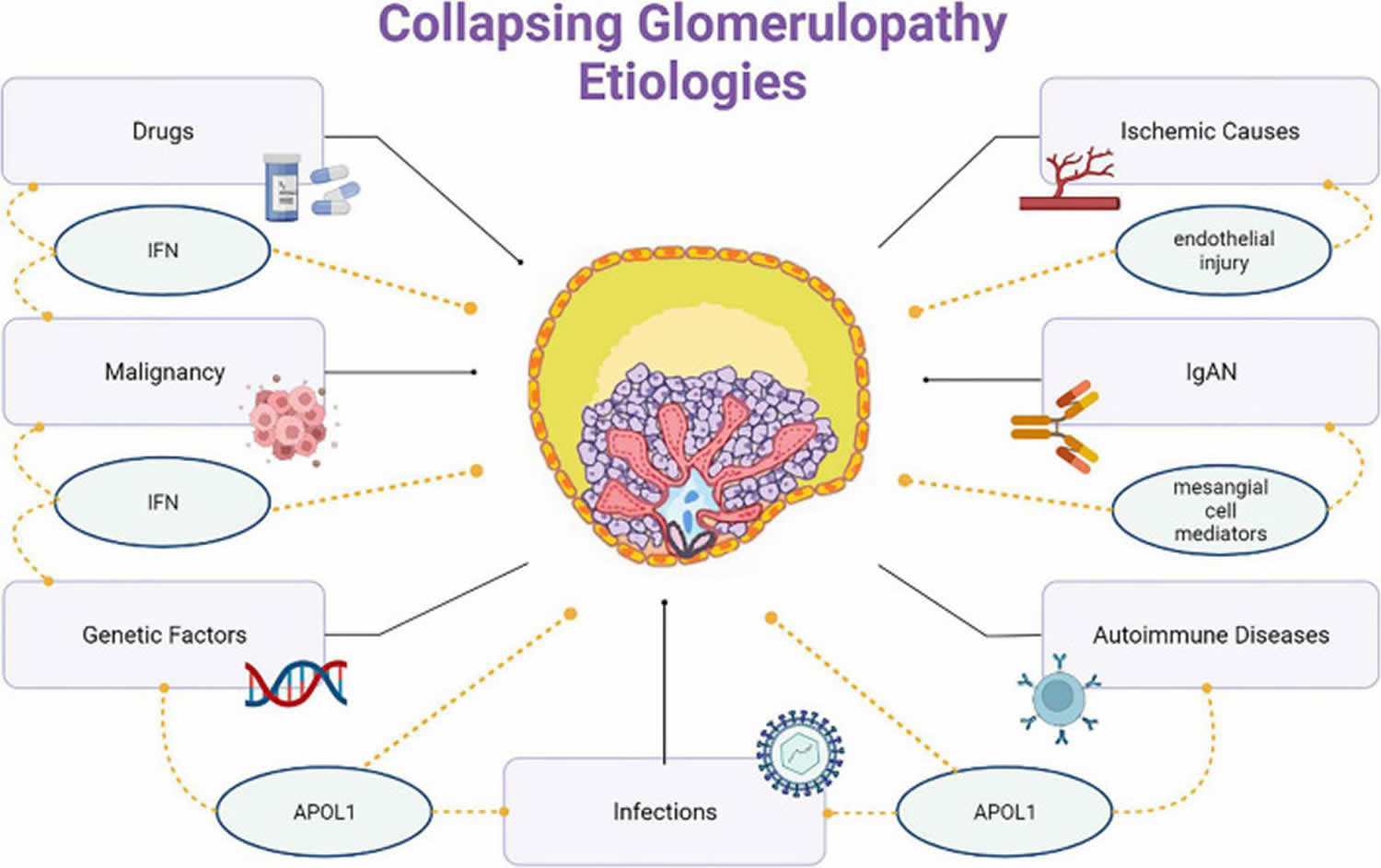
[Source 5 ]Figure 2. Collapsing glomerulopathy causes
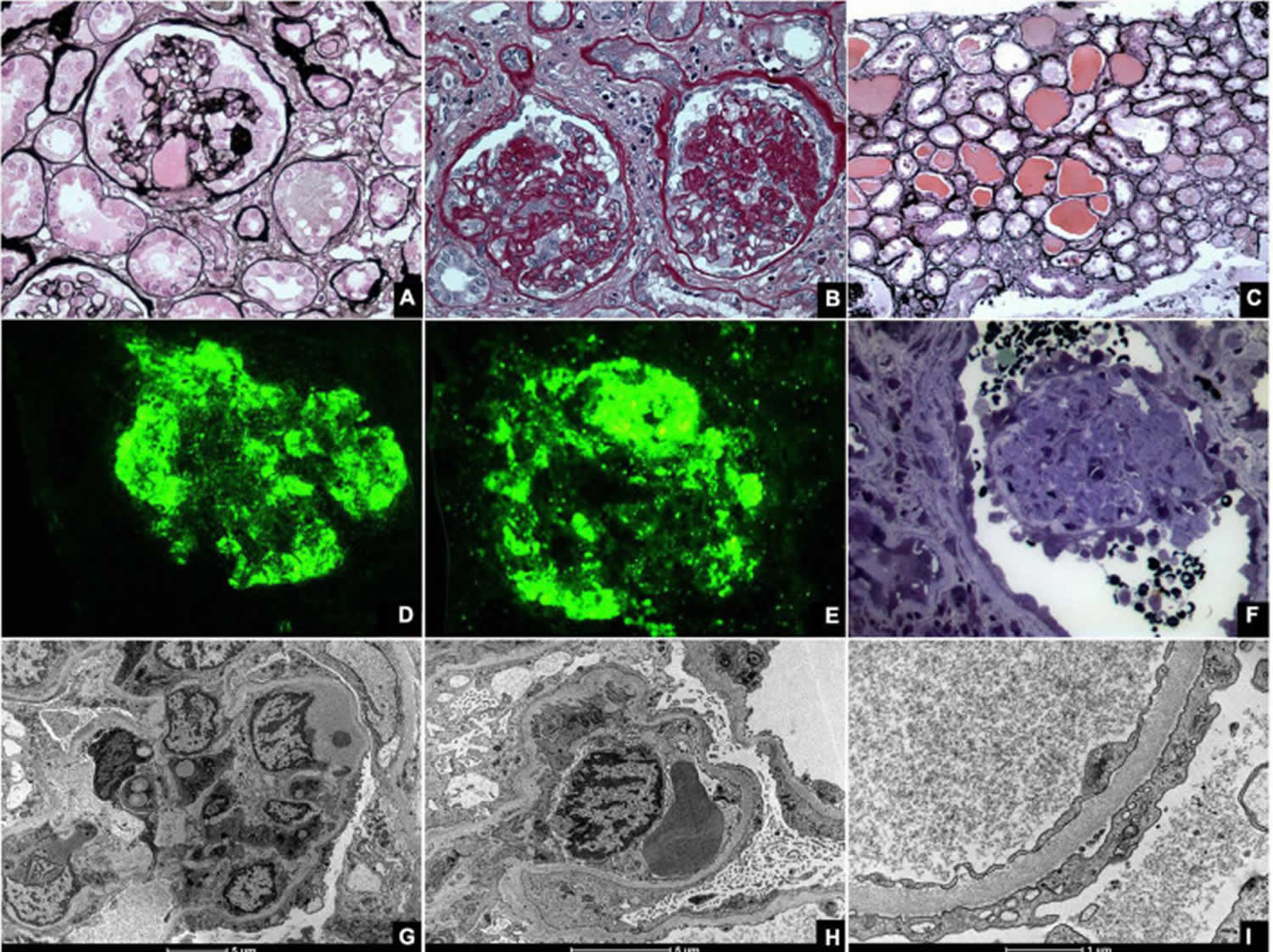
[Source 5 ]Figure 3. Collapsing glomerulopathy diagnosis
Footnotes: Kidney biopsies of collapsing glomerulopathy. (A,B) Periodic Acid Schiff (PAS) and Jones Methenamine Silver (JMS) (40×), respectively show intense podocyte hyperplasia and glomerular tuft collapse. (C) Jones Methenamine Silver (JMS) (20×) exhibits microcytic transformation of distal convoluted tubules with accumulations of hyaline material inside of those. (D,E) Fluorescence microscopy (40×) shows, respectively, IgM and C3 trapping in areas of collapse/sclerosis. (F) Semi-fine stained in Toluidine Blue (63×) with collapse of the entire glomerular tuft and hyperplasia of podocytes and dilated Bowman’s space. (G,H) Transmission electron microscopy contrasted with Osmium Tetroxide, Lead Citrate and Uranyl in block shows capillary loop collapse with hyalinosis in addition to diffuse fusion and flattening of the pedicels associated with microvillous transformation. (I) Electron microscopy tubes contrasted with osmium tetroxide, lead citrate, and uranyl in block with detail of disorganization of the cytoskeleton in the podocyte cytoplasm, with extensive effacement of the pedicels.
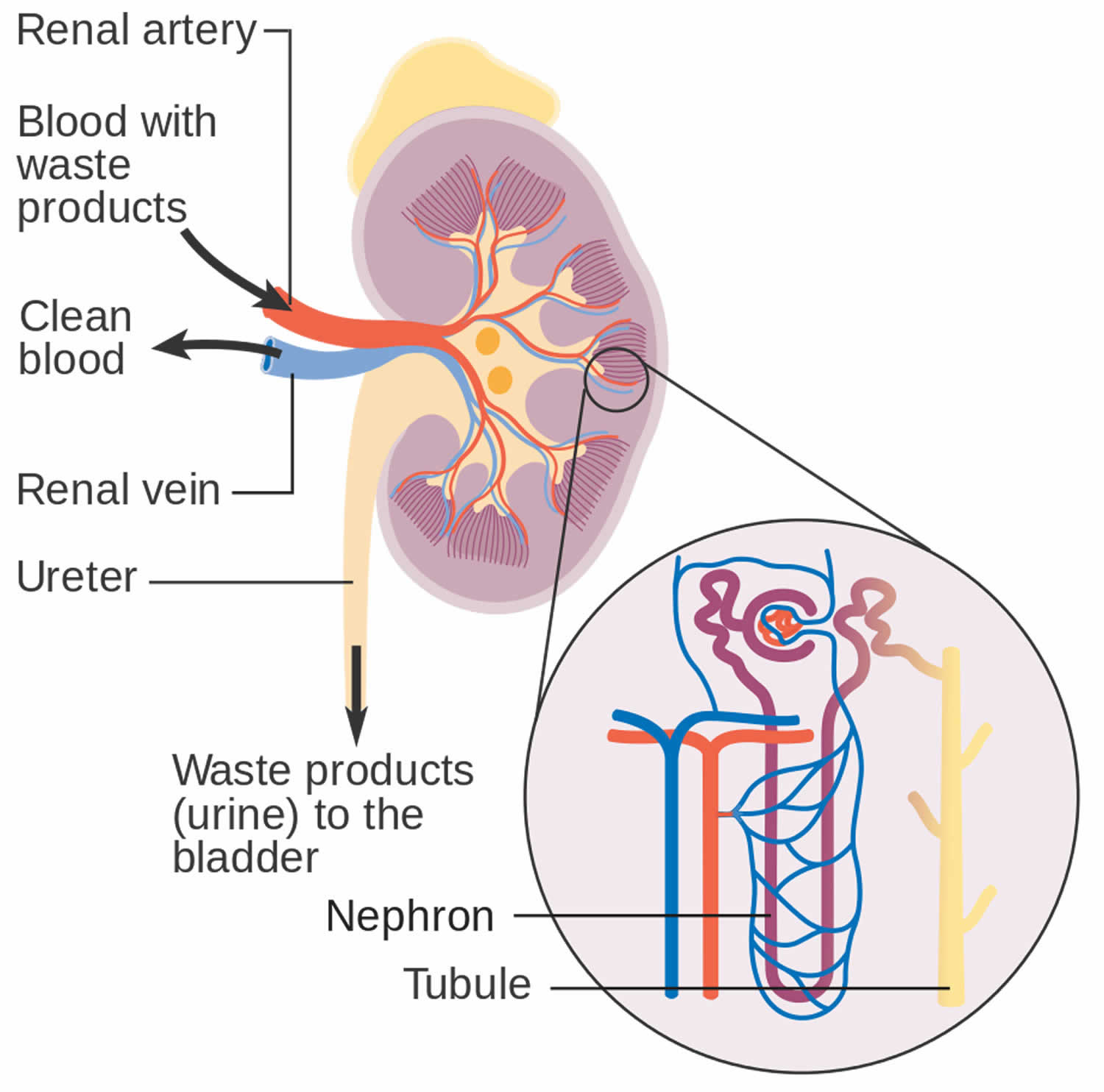
[Source 5 ]How your kidneys work
You have two kidneys, each about the size of an adult fist, located on either side of the spine just below the rib cage. Although they are small, your kidneys perform many complex and vital functions that keep the rest of the body in balance. Your kidneys remove waste and excess fluid from your blood through filtering units called nephrons. Each nephron contains a filter (glomerulus) that has a network of tiny blood vessels called capillaries. When blood flows into a glomerulus, tiny molecules — water, essential minerals and nutrients, and wastes — pass through the capillary walls. Large molecules, such as proteins and red blood cells, do not. The filtered solution then passes into another part of the nephron called the tubule. The water, nutrients and minerals your body needs are transferred back to the bloodstream. The excess water and waste become urine that flows to the bladder.
Kidney functions:
- Help remove waste and excess fluid
- Filter the blood, keeping some compounds while removing others
- Control the production of red blood cells
- Make vitamins that control growth
- Release hormones that help regulate blood pressure
- Help regulate blood pressure, red blood cells, and the amount of certain nutrients in the body, such as calcium and potassium.
Here’s how kidneys perform their important work:
- Blood enters the kidneys through an artery from the heart
- Blood is cleaned by passing through millions of tiny blood filters
- Waste material passes through the ureter and is stored in the bladder as urine
- Newly cleaned blood returns to the bloodstream by way of veins
- Bladder becomes full and urine passes out of the body through the urethra.
The kidneys perform their life-sustaining job of filtering and returning to the bloodstream about 200 quarts of fluid every 24 hours. Approximately two quarts are eliminated from the body in the form of urine, while the remainder, about 198 quarts, is retained in the body. The urine we excrete has been stored in the bladder for approximately one to eight hours.
Figure 4. How kidneys work
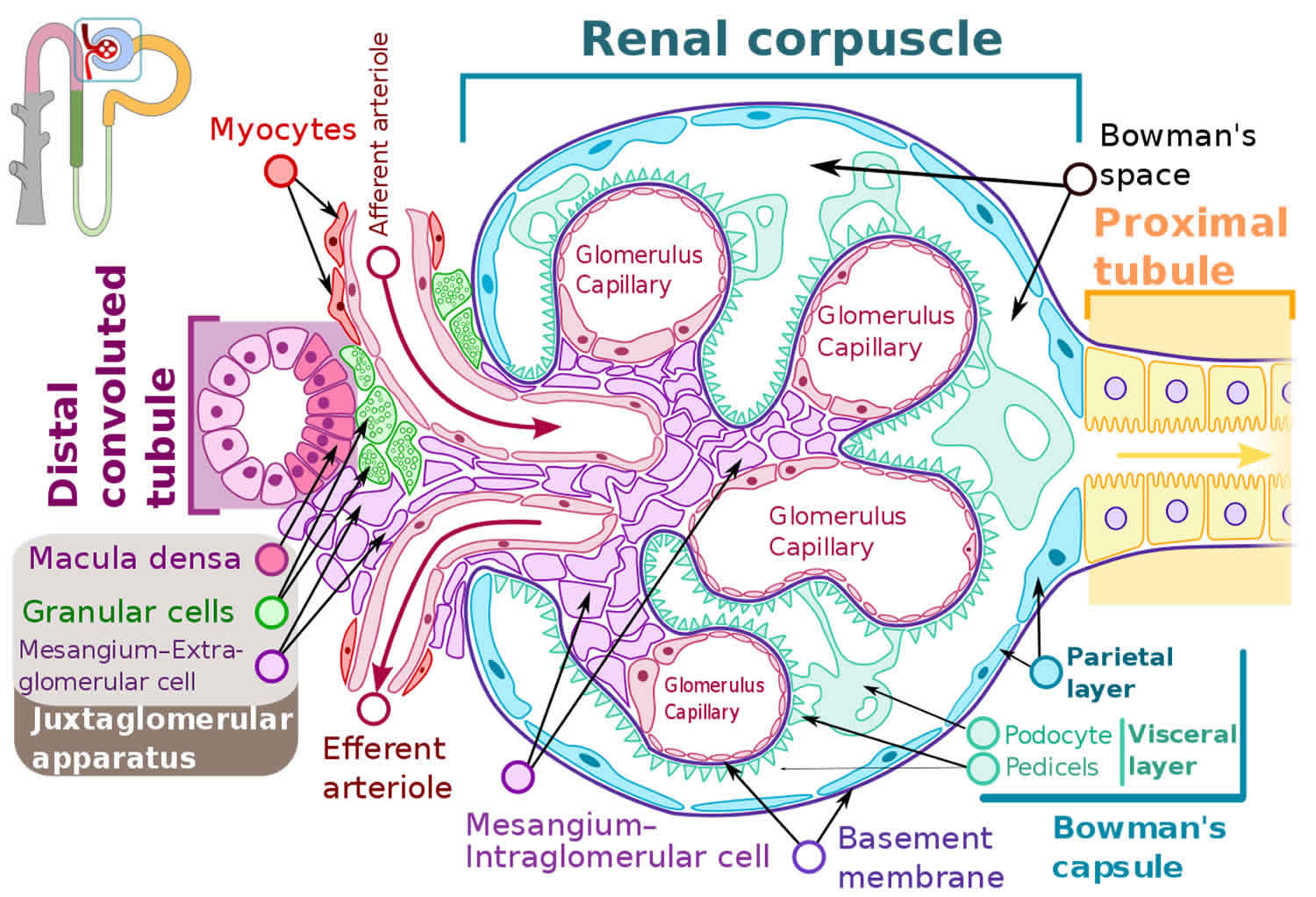
Figure 5. Glomerulus
How do glomerular diseases interfere with kidney function?
Glomerular diseases damage the glomeruli, letting protein and sometimes red blood cells leak into the urine. Sometimes a glomerular disease also interferes with the clearance of waste products by the kidney, so they begin to build up in the blood. Furthermore, loss of blood proteins like albumin in the urine can result in a fall in their level in the bloodstream. In normal blood, albumin acts like a sponge, drawing extra fluid from the body into the bloodstream, where it remains until the kidneys remove it. But when albumin leaks into the urine, the blood loses its capacity to absorb extra fluid from the body. Fluid can accumulate outside the circulatory system in the face, hands, feet, or ankles and cause swelling.
What are renal failure and end-stage renal disease?
Renal failure is any acute or chronic loss of kidney function and is the term used when some kidney function remains. Total kidney failure, sometimes called end-stage renal disease (ESRD), indicates permanent loss of kidney function. Depending on the form of glomerular disease, kidney function may be lost in a matter of days or weeks or may deteriorate slowly and gradually over
the course of decades.
Acute renal failure (acute kidney failure)
A few forms of glomerular disease cause very rapid deterioration of kidney function. For example, post-streptococcal glomerulonephritis (PSGN) can cause severe symptoms (hematuria, proteinuria, edema) within 2 to 3 weeks after a sore throat or skin infection develops. The patient may temporarily require dialysis to replace kidney function. This rapid loss of kidney function is called acute renal failure (acute kidney failure). Although acute renal failure (acute kidney failure) can be life-threatening while it lasts, kidney function usually returns after the cause of the kidney failure has been treated. In many patients, acute kidney failure is not associated with any permanent damage. However, some patients may recover from acute renal failure and subsequently develop chronic kidney disease (CKD).
Chronic kidney disease (CKD)
Most forms of glomerular disease develop gradually, often causing no symptoms for many years. Chronic kidney disease (CKD) is the slow, gradual loss of kidney function. Some forms of chronic kidney disease (CKD) can be controlled or slowed down. For example, diabetic nephropathy can be delayed by tightly controlling blood glucose levels and using angiotensin-converting enzyme inhibitors (ACE inhibitors) and angiotensin 2 receptor blockers (ARBs) to reduce proteinuria and control blood pressure. But chronic kidney disease (CKD) cannot be cured. Partial loss of kidney function means that some portion of the patient’s nephrons have been scarred, and scarred nephrons cannot be repaired. In many cases, CKD leads to total kidney failure.
Total kidney failure
To stay alive, a patient with total kidney failure must go on dialysis, either hemodialysis or peritoneal dialysis or receive a new kidney through kidney transplantation. Patients with chronic kidney disease (CKD) who are approaching total kidney failure should learn as much about their treatment options as possible so they can make an informed decision when the time comes. With the help of dialysis or kidney transplantation, many people continue to lead full, productive lives after reaching total kidney failure.
Collapsing glomerulopathy causes
Barisoni proposed a classification scheme for podocytopathies and characterized collapsing glomerulopathy as idiopathic/primary, genetic, and reactive/secondary 12. Podocytopathies are kidney diseases in which direct or indirect podocyte injury drives proteinuria or nephrotic syndrome 47. The primary conditions that cause collapsing glomerulopathy are highlighted here and classified according to the nature of injury:
- Infectious disease like HIV, T-cell lymphotropic virus, hepatitis B virus (HBV), hepatitis C virus (HCV), Epstein-Barr virus, cytomegalovirus (CMV), parvovirus B19, dengue, Zika virus, Chikungunya, tuberculosis, visceral leishmaniasis, filariasis, and SARS-COV-2 (COVID-19).
- Autoimmune diseases including systemic lupus erythematosus (SLE), adult Still’s disease, temporal arteritis, mixed connective tissue disease, and Behçet’s disease.
- Neoplastic causes, such as multiple myeloma, acute monoblastic leukemia, natural killer cell leukemia, and acquired hemophagocytic syndrome.
- Drug-related causes like bisphosphonates, interferons, anabolic steroids, heroin, valproic acid, and anthracyclines.
- Ischemic causes, that include thrombotic microangiopathy, atheroembolic disease, sickle cell anemia, and thromboembolism by hydrophilic polymers.
Collapsing glomerulopathy can also coexist with other nephropathies, such as immunoglobulin A (IgA) nephropathy, membranous glomerulopathy, and other histological types of focal segmental glomerulosclerosis and diffuse mesangial sclerosis.
Several case reports have linked collapsing glomerulopathy to infectious diseases. Chandra proposed the following criteria to assess the causal link between infection and collapsing glomerulopathy 23:
- Collapsing glomerulopathy demonstration in multiple cases of viral disease;
- Clear demonstration of collapsing glomerulopathy lesions, including glomerular tuft collapse and changed podocyte phenotype;
- Demonstration of viral proteins or nucleic acids in glomerular cells, especially in podocytes; and
- Experimental demonstration (in animal models) of some (or all) collapsing glomerulopathy findings.
Collapsing glomerulopathy pathogenesis
Regarding the origin of the hyperplastic podocytes in the collapsing glomerulopathy, one key study using immunofluorescent techniques indicates that cellular crescents in crescentic glomerulonephritis and hyperplastic podocytes in collapsing glomerulopathy all come from the same parietal epithelium of the glomeruli 48. The study also finds that there are 3 populations of hyperplastic podocytes in the collapsing glomerulopathy. Using confocal microscopy to evaluate several merged marker expressions, the study determined that there are approximately 60% immature podocytes (CD133+, CD24+, podocalyxin− [PDX, a podocyte marker] nestin− [another podocyte marker]), 30% mature podocytes (CD133−, CD24−, PDX+, nestin+), and 10% transitional podocytes (CD133+, CD24+, PDX+, nestin+) 48. The finding indicates that glomerular hyperplastic lesions are derived from the proliferation of renal progenitors at different stages of their differentiation toward mature podocytes 48.
Cell Behavior
From a histopathologic perspective, collapsing glomerulopathy falls within the spectrum of podocytopathies (see Figure 3). Podocytes are terminal cells that constitute an essential part of the glomerular structure. They consist of a cell body, primary, secondary, and tertiary podocyte processes, and interdigitations between these processes 49. The spaces between pedicels are filled with slit diaphragm proteins, which function as mechanical and electrical barriers for a plasma ultrafiltrate 50, 51, 52. The proteins that constitute the diaphragm are strongly anchored to the cytoskeleton of the podocytes 50 and comprise a strong functional part of the glomerular filtration barrier.
Barisoni 12 classified podocytopathies based on their morphological characteristics and pathogenesis. Minimal change disease presents with slit diaphragm involvement instead of podocyte damage, whereas FSGS presents with marked podocytopenia 18. In contrast, diffuse mesangial sclerosis and collapsing glomerulopathy are characterized by high proliferation rates 43. The proliferation rate is higher in collapsing glomerulopathy than in diffuse mesangial sclerosis 12.
Although podocytes are usually considered as terminal differentiation cells, they have a marked loss of differentiation and high mitotic rates in collapsing glomerulopathy. Shkeri et al 53 utilized the western blot technique to analyze the gene expressions of several glomerulopathies and demonstrated that collapsing glomerulopathy was associated with increased cell proliferation markers, such as T-telomerase, Ki-67 protein, and beta-cadherin 1; however, these findings were not observed in the other types of FSGS.
The human Wilms tumor 1 (WT1) gene is a tumor suppressor gene that spans ∼50 kb and consists of 10 exons. It encodes a protein that shares a high degree of structural homology with the early growth response family of transcription factors. Several lines of evidence suggest that Wilms tumor 1 (WT1) protein is important for normal podocyte function 54. Overexpression of the Wilms tumor 1 (WT1) protein results in an increased telomerase function, which promotes podocyte cell differentiation and renal histological organization. Inhibition of the WT1 protein is associated with terminal phenotype loss and podocyte hyperplasia 53. In collapsing glomerulopathy, WT1 inhibition results in podocytes that have similar gene expressions patterns with those of the glomerular parietal epithelium, which may represent a phenotypic return to the original cells that yielded the Bowman’s capsule epithelium 53. Wilms tumor 1 (WT1) protein has been demonstrated to activate transcription of the podocalyxin gene. The integral membrane protein podocalyxin connects to the cytoskeleton of the podocytes and is implicated in maintaining the complex three-dimensional shape of the cells 54. Gene inhibition of podocalyxin and other proteins that contribute to the structure of podocytes and slit diaphragms by determining protein binding and local electrostatic forces as synaptopodin, glomerular epithelial protein 1, common acute lymphoblastic leukemia antigen, and the C3b receptor has also been documented in collapsing glomerulopathy 51.
Other cells may also be associated with the pathophysiology of collapsing glomerulopathy. Under hypoxic conditions, glomerular endothelial cells have been reported to secrete paracrine factors that modify podocyte structure 39. HIV-affected T lymphocytes are also involved in cell proliferation 21.
Genetics
APOL1 gene, which is located on the long arm of chromosome 22 (22q12 region), contributes to the pathogenesis of collapsing glomerulopathy. APOL1 gene is responsible for forming high-density lipoproteins in different cell membranes and is associated with innate immunity. APOL1 gene confers resistance to Trypanosoma brucei rhodesiense, the etiologic agent for African sleeping sickness. APOL1 improves gene function and fights against infections through G1 (missense) and G2 (deletion of two amino acids) mutations. APOL1 is also associated with several cell damage mechanisms, such as mitochondrial damage, lysosomal degranulation, and cell pore formation 2. Lysosomal degranulation plays a particular role in destroying the cell membranes of foreign pathogens 55.
APOL1 gene mutations are correlated with the distribution of sleeping sickness. As such, these mutations are quite prevalent in sub-Saharan Africa, reaching over 40% in countries such as Ghana and Nigeria 2. In several countries including Brazil, APOL1 mutations are considered as risk factors and determinants of chronic kidney disease (CKD). A national case-control study demonstrated that these mutations are associated with an odds ratio of 10.95 for progression to chronic kidney disease compared to controls. APOL1 mutations have also been associated with an increased indication for hemodialysis within a mean period of 12 years or earlier 56.
Other genes have been implicated in the pathogenesis of collapsing glomerulopathy. The COQ2 gene (4q21.23) encodes the mitochondrial protein CoQ1, which plays a role in some neurologic, muscle, and renal syndromes. Mitochondrial gene mutations contribute to the proliferation of a poorly differentiated podocyte profile, which results in collapsing glomerulopathy 57, 58. In contrast, the MHY9 gene is responsible for synthesizing myosin microfilaments that maintain the podocyte structure and filtration barrier. Among patients with HIV, MHY9 gene mutations increase the risk of progression to collapsing glomerulopathy by 4–8-fold 59.
Collapsing glomerulopathy signs and symptoms
The most common signs and symptoms of collapsing glomerulopathy is massive, severe proteinuria associated with pure nephrotic syndrome. Nephrotic syndrome is a kidney disorder that causes your body to pass too much protein in your urine (proteinuria), low levels of a protein called albumin in your blood (hypoalbuminemia), swelling in parts of your body (edema) and high levels of cholesterol and other lipids (fats) in your blood (hyperlipidemia) 60. Patients may present with high blood pressure (hypertension), presence of lipids in the urine (lipiduria) and blood in urine (hematuria) on urinalysis 5. A significantly high number of patients present with kidney failure on admission and rapidly progresses to end-stage renal disease (ESRD) 15, 16, 17, 18, 19.
Signs and symptoms of nephrotic syndrome include:
- Severe swelling (edema), particularly around your eyes and in your ankles and feet
- Foamy urine, a result of excess protein in your urine
- Weight gain due to fluid retention
- Fatigue
- Loss of appetite
Collapsing glomerulopathy complications
Possible complications of nephrotic syndrome include:
- Blood clots. The inability of the glomeruli to filter blood properly can lead to loss of blood proteins that help prevent clotting. This increases your risk of developing a blood clot in your veins.
- High blood cholesterol and elevated blood triglycerides. When the level of the protein albumin in your blood falls, your liver makes more albumin. At the same time, your liver releases more cholesterol and triglycerides.
- Poor nutrition. Loss of too much blood protein can result in malnutrition. This can lead to weight loss, which can be masked by edema. You may also have too few red blood cells (anemia), low blood protein levels and low levels of vitamin D.
- High blood pressure. Damage to your glomeruli and the resulting buildup of excess body fluid can raise your blood pressure.
- Acute kidney injury. If your kidneys lose their ability to filter blood due to damage to the glomeruli, waste products can build up quickly in your blood. If this happens, you might need emergency dialysis — an artificial means of removing extra fluids and waste from your blood — typically with an artificial kidney machine (dialyzer).
- Chronic kidney disease. Nephrotic syndrome can cause your kidneys to lose their function over time. If kidney function falls low enough, you might need dialysis or a kidney transplant.
- Infections. People with nephrotic syndrome have an increased risk of infections.
Collapsing glomerulopathy diagnosis
Health care professionals diagnose glomerular disease by ordering tests, such as:
Blood tests:
- Blood tests can measure the levels of products in your blood, such as creatinine, urea nitrogen, and a protein called cystatin C, to find out how well your kidneys are working. Blood tests can also check for low levels of a protein in your blood, called albumin, which can happen when too much of that protein passes from your blood into your urine.
- Other blood test include:
- Antiglomerular basement membrane antibody test
- Antineutrophil cytoplasmic antibodies (ANCAs)
- Antinuclear antibodies
- Complement levels
A simple test of your urine can confirm if there is blood or protein in your urine.
Urinalysis, which examines a sample of your urine to find out if levels of protein and red blood cells are too high:
- Creatinine clearance
- Examination of the urine under a microscope
- Urine total protein
- Uric acid in the urine
- Urine concentration test
- Urine creatinine
- Urine protein
- Urine red blood cell
- Urine specific gravity
- Urine osmolality
Imaging tests that may be done include:
- Abdominal CT scan
- Kidney ultrasound
- Chest x-ray
- Intravenous pyelogram (IVP)
In some cases, a test called a kidney biopsy may be needed. In this test, a tiny piece of your kidney is removed with a special needle, and looked at under a microscope. A kidney biopsy can confirm you have glomerular disease and help find the cause in order to help your doctor plan the best treatment for you.
Treatment for glomerular disease varies by symptoms, causes, and how badly your kidneys are damaged. In some cases, glomerular disease may go away once its cause has been treated. In other cases, the disease may go away but later return. Less often, glomerular disease may not respond to treatment and lead to kidney failure over time.
Collapsing glomerulopathy histology
Although initially treated as a single entity, focal segmental glomerulosclerosis (FSGS) encompasses several distinct histological patterns. FSGS is heterogenous in terms of etiology, histological characteristics, clinical presentation, treatment response, and prognosis. For this reason, a classification of FSGS histological subtypes was proposed in 2004 61. The diagnosis of collapsing glomerulopathy requires the presence of at least one glomerulus with segmental or global collapse and podocyte hypertrophy and hyperplasia (Figure 8). Collapsing glomerulopathy can occur with other morphological subtypes, and a single characteristic lesion is adequate for a diagnosis of collapsing glomerulopathy.
Light microscope shoes partial or total obliteration of the lumen of the glomerular capillaries secondary to podocyte hypertrophy and hyperplasia 62, 63. Podocyte crowns may contain intracytoplasmic deposits, which denote protein reabsorption. Pseudocrescents, which resemble glomerular crescents, may temporally precede glomerular sclerosis 18. Other findings, such as glomerulomegaly, hyalinosis, hypercellularity, and adhesions, are unusual but more common in the final stages of the disease 64.
As a rule, tubulointerstitial damage in collapsing glomerulopathy is more intense than in other FSGS subtypes 16. Important specific findings include tubular dilatation with molding and the formation of tubular microcysts 16, 40. Tissue and lymphatic macrophages, specifically CD4 and CD8, can also be found in interstitial infiltrates 40.
Immunofluorescence (IF) findings are non-specific and, in many cases, negative. If present, they usually comprise granular or mesangial IgM and C3 deposits. IF may suggest associated conditions such as Berger’s disease, which is characterized by mesangial IgA deposits 63, or SLE, which is associated with several antibody deposits.
Electron microscopy (EM) is mostly unnecessary; however, it can demonstrate collapsed and ruptured capillary membranes and swollen, hypertrophic, and/or hyperplastic podocytes 63. Electron microscopy may also identify intracellular inclusions that are often associated with HIV or SLE. In these conditions, the podocyte processes are greatly compromised with loss of glomerular filtration barrier integrity.
Collapsing glomerulopathy treatment
The treatment of collapsing glomerulopathy consists of the following:
- Targeted therapy for disorders resulting from nephrotic syndrome, such as dyslipidemia, hypertension, and edema;
- Treatment of the underlying disease when collapsing glomerulopathy is associated with other conditions; and
- Immunosuppressive therapy.
Symptoms of nephrotic syndrome are most often treated with these medicines 65, 66:
- Blood pressure medications. Drugs called angiotensin-converting enzyme (ACE) inhibitors reduce blood pressure and the amount of protein released in urine. Medications in this category include lisinopril (Prinivil, Qbrelis, Zestril), benazepril (Lotensin), captopril and enalapril (Vasotec). Another group of drugs that works similarly is called angiotensin 2 receptor blockers (ARBs) and includes losartan (Cozaar) and valsartan (Diovan). Other medications, such as renin inhibitors, also might be used, though angiotensin-converting enzyme (ACE) inhibitors and angiotensin 2 receptor blockers (ARBs) are generally used first.
- Water pills (diuretics). These help control swelling by increasing your kidneys’ fluid output. Diuretic medications typically include furosemide (Lasix). Others include spironolactone (Aldactone, Carospir) and thiazides, such as hydrochlorothiazide or metolazone (Zaroxolyn).
- Cholesterol-reducing medications. Statins can help lower cholesterol levels. However, it’s not clear whether cholesterol-lowering medications can improve the outcomes for people with nephrotic syndrome, such as avoiding heart attacks or decreasing the risk of early death. Statins include atorvastatin (Lipitor), fluvastatin (Lescol XL), lovastatin (Altoprev), pravastatin (Pravachol), rosuvastatin (Crestor, Ezallor) and simvastatin (Zocor).
- Blood thinners (anticoagulants). These might be prescribed to decrease your blood’s ability to clot, especially if you’ve had a blood clot. Anticoagulants include heparin, warfarin (Coumadin, Jantoven), dabigatran (Pradaxa), apixaban (Eliquis) and rivaroxaban (Xarelto).
- Immune system-suppressing medications. Medications to control the immune system, such as corticosteroids, can decrease the inflammation that accompanies some of the conditions that can cause nephrotic syndrome. Medications include rituximab (Rituxan), cyclosporine and cyclophosphamide.
According to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines on the management of glomerulopathies 67, 68, the measures to control complications associated with nephrotic syndrome consist of edema control through a low-sodium diet (<2 g/day), fluid restriction, diuretic therapy (water pill), and, if necessary, hemodialysis or ultrafiltration. Systolic blood pressure should be maintained at <120 mmHg with angiotensin-converting enzyme inhibitors or angiotensin 2 receptor blockers, unless renal function worsens on these medications 65.
The severity of dyslipidemia in patients with nephrotic syndrome is proportional to the degree of proteinuria. Once the proteinuria in patients with collapsing glomerulopathy is very high, the serum levels of cholesterol and its fractions as well as lipoproteins can reach high elevated values. The potential contributors to dyslipidemia in patients with nephrotic syndrome are the patient’s diet, use of drugs (such as corticosteroids, calcineurin inhibitors, and mTOR inhibitors), in addition to genetic predisposition 69, 70. According to the new KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases 65 in patients with nephrotic syndrome the dyslipidemia should be controlled, especially in those ones with other cardiovascular risk factors (e.g., hypertension and diabetes) and/or diseases that respond poorly to stabilized therapy.
As non-pharmacological measures, lifestyle changes (diet, smoking cessation, weight loss, and physical activity) must be encouraged in all patients. The tratment with statins as a first-line treatment should be indicated in patients at increased risk of atherosclerotic cardiovascular disease, such as those with GFR <60 ml/min/1.73m² and/or albuminuria >30 mg/g, in addition to treating infectious/inflammatory diseases that contribute to increased cardiovascular risk. For patients who do not tolerate the use of statins or cannot achieve the recommended lipid levels targets, other drugs as bile acid chelators, fibrates, nicotinic acid, ezetimibe, PCSK9 inhibitors and lipid apheresis can be used.
Special attention should also be given to the risk of thrombotic events; prophylactic anticoagulation should be considered for susceptible populations. Immunocompromised patients should be given full immunizations; tested for HIV, hepatisis B (HBV), hepatisis C (HCV), tuberculosis (TB), and syphilis; and initiated on strongyloidiasis and pneumocystis prophylaxis.
When an underlying cause is identified, such as drug-related, infectious, genetic, autoimmune, and other causes, the patient should undergo specific therapy for the underlying cause, when available. In drug-induced collapsing glomerulopathy, the offending drug must be discontinued whenever possible 71. In cases associated with infections, specific antimicrobial treatments can reduce collapsing glomerulopathy progression or even promote remission. Some HIV-associated cases demonstrate 38% delay in the progression to ESRD following the initiation of antiretroviral therapy (ART) 72; however, other studies proposed that up to 50% of patients progress toward the initiation of kidney dialysis despite adequate treatment 73. Nevertheless, HIV-associated nephropathy should always be treated with ART because it is very effective at controlling the disease 74.
Intravenous immunoglobulin (IVIg) and cidofovir can be used to treat parvovirus B19 infection, which is particularly important from the perspective of renal transplantation 75. CMV infections should be treated with ganciclovir, which also promotes collapsing glomerulopathy remission and renal function improvement 24.
Immunosuppressant therapy was administered to most patients. While there are no specific recommendations for collapsing glomerulopathy, the immunotherapy guidelines for collapsing glomerulopathy are extrapolated from the FSGS protocol 17. Several types of immunosuppressants have been proposed 76, 16, 22, 17, but the initial therapeutic regimen consists of high-dose of oral corticosteroids for 4–16 weeks or until remission is achieved. Calcineurin inhibitors or cyclophosphamide are considered second-line medications for patients that exhibit corticosteroid resistance, corticosteroid dependence, or frequent relapses; they should be given for at least 12 weeks. Other immunosuppressants (such as rituximab and mycophenolate mofetil) are indicated for patients who do not respond to other immunosuppressive regimens. Patients with extensive kidney damage may choose to forego treatment for collapsing glomerulopathy because of the lack of benefits and risks associated with immunosuppression therapy.
Collapsing glomerulopathy self care
If you have developed nephrotic syndrome, your health care professional may recommend that you change your diet. Your doctor might refer you to a dietitian, who might recommend that you do the following:
- Choose lean sources of protein. Plant-based protein is helpful in kidney disease.
- Reduce the amount of fat and cholesterol in your diet to help control your blood cholesterol levels.
- Eat a low-salt diet to help control swelling.
- Reduce the amount of liquid in your diet.
Collapsing glomerulopathy prognosis
Collapsing glomerulopathy has a poor prognosis 5. Most collapsing glomerulopathy cases present with refractory proteinuria, severe loss of renal function, and progression to permanent kidney dialysis 77, 62, 63. While other FSGS subtypes have a kidney survival time of 62.5 months, collapsing glomerulopathy has a kidney survival time of 13 months 16.
- Kaufman L, Yang G, Hayashi K, Ashby JR, Huang L, Ross MJ, et al.. The homophilic adhesion molecule sidekick-1 contributes to augmented podocyte aggregation in HIV-associated nephropathy. FASEB J. (2007) 21:1367–75. 10.1096/fj.06-7191com[↩]
- Freedman BI, Limou S, Ma L, Kopp JB. APOL1-associated nephropathy: a key contributor to racial disparities in CKD. Am J Kidney Dis. (2018) 72(5 Suppl. 1):S8–16. 10.1053/j.ajkd.2018.06.020[↩][↩][↩]
- Laurinavicius A, Hurwitz S, Rennke HG. Collapsing glomerulopathy in HIV and non-HIV patients: a clinicopathological and follow-up study. Kidney Int. 1999 Dec;56(6):2203-13. https://doi.org/10.1046/j.1523-1755.1999.00769.x[↩]
- Smith KD, Prince DK, Henriksen KJ, Nicosia RF, Alpers CE, Akilesh S. Digital spatial profiling of collapsing glomerulopathy. Kidney Int. 2022 May;101(5):1017-1026. doi: 10.1016/j.kint.2022.01.033[↩]
- Cutrim ÉMM, Neves PDMM, Campos MAG, Wanderley DC, Teixeira-Júnior AAL, Muniz MPR, Ladchumananandasivam FR, Gomes OV, Vasco RFV, Brito DJA, Lages JS, Salgado-Filho N, Guedes FL, de Almeida JB, Magalhães M, Araújo SA, Silva GEB. Collapsing Glomerulopathy: A Review by the Collapsing Brazilian Consortium. Front Med (Lausanne). 2022 Mar 3;9:846173. doi: 10.3389/fmed.2022.846173[↩][↩][↩][↩][↩][↩][↩][↩]
- Amoura A, Moktefi A, Halfon M, Karras A, Rafat C, Gibier JB, Gleeson PJ, Servais A, Argy N, Maillé P, Belenfant X, Gueutin V, Delpierre A, Tricot L, El Karoui K, Jourde-Chiche N, Houze S, Sahali D, Audard V. Malaria, Collapsing Glomerulopathy, and Focal and Segmental Glomerulosclerosis. Clin J Am Soc Nephrol. 2020 Jul 1;15(7):964-972. doi: 10.2215/CJN.00590120[↩]
- Wu H, Larsen CP, Hernandez-Arroyo CF, Mohamed MMB, Caza T, Sharshir M, Chughtai A, Xie L, Gimenez JM, Sandow TA, Lusco MA, Yang H, Acheampong E, Rosales IA, Colvin RB, Fogo AB, Velez JCQ. AKI and Collapsing Glomerulopathy Associated with COVID-19 and APOL1 High-Risk Genotype. J Am Soc Nephrol. 2020 Aug;31(8):1688-1695. doi: 10.1681/ASN.2020050558[↩]
- Weiss MA, Daquioag E, Margolin EG, Pollak VE. Nephrotic syndrome, progressive irreversible renal failure, and glomerular “collapse”: a new clinicopathologic entity? Am J Kidney Dis. (1986) 7:20–8. 10.1016/s0272-6386(86)80052-x[↩]
- Cohen AH, Nast CC. HIV-associated nephropathy. A unique combined glomerular, tubular, and interstitial lesion. Mod Pathol. 1988 Mar;1(2):87-97.[↩]
- Wyatt CM, Klotman PE, D’Agati VD. HIV-associated nephropathy: clinical presentation, pathology, and epidemiology in the era of antiretroviral therapy. Semin Nephrol. (2008) 28:513–22. 10.1016/j.semnephrol.2008.08.005[↩][↩]
- Rosenberg AZ, Kopp JB. Focal Segmental Glomerulosclerosis. Clin J Am Soc Nephrol. 2017 Mar 7;12(3):502-517. doi: 10.2215/CJN.05960616. Epub 2017 Feb 27. Erratum in: Clin J Am Soc Nephrol. 2018 Dec 7;13(12):1889.[↩]
- Barisoni L, Schnaper HW, Kopp JB. A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol. 2007 May;2(3):529-42. doi: 10.2215/CJN.04121206[↩][↩][↩][↩]
- D’Agati V. D., Fogo A. B., Bruijn J. A., Jennette J. C. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. American Journal of Kidney Diseases. 2004;43(2):368–382. doi: 10.1053/j.ajkd.2003.10.024[↩]
- Stokes M. B., D’Agati V. D. Morphologic variants of focal segmental glomerulosclerosis and their significance. Advances in Chronic Kidney Disease. 2014;21(5):400–407. doi: 10.1053/j.ackd.2014.02.010[↩]
- Ferreira AC, Carvalho D, Carvalho F, Galvo MJ, Nolasco F. Collapsing glomerulopathy in Portugal: a review of the histological and clinical findings in HIV and non-HIV patients. Nephrol Dial Transplant. (2011) 26:2209–15. 10.1093/ndt/gfq686[↩][↩][↩][↩][↩]
- Valeri A, Barisoni L, Appel GB, Seigle R, D’Agati V. Idiopathic collapsing focal segmental glomeruloscierosis: a clinicopathologic study. Kidney Int. (1996) 50:1734–46. 10.1038/ki.1996.493[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Laurin LP, Gasim AM, Derebail VK, McGregor JG, Kidd JM, Hogan SL, et al. Renal survival in patients with collapsing compared with not otherwise specified FSGS. Clin J Am Soc Nephrol. (2016) 11:1752–9. 10.2215/CJN.13091215[↩][↩][↩][↩][↩]
- Albaqumi M, Soos TJ, Barisoni L, Nelson PJ. Collapsing glomerulopathy. J Am Soc Nephrol. (2006) 17:2854–63. 10.1681/ASN.2006030225[↩][↩][↩][↩]
- Albaqumi M, Barisoni L. Current views on collapsing glomerulopathy. J Am Soc Nephrol. (2008) 19:1276–81. 10.1681/ASN.2007080926[↩][↩]
- Husain NE, Ahmed MH, Almobarak AO, Noor SK, Elmadhoun WM, Awadalla H, et al. HIV-associated nephropathy in Africa: pathology, clinical presentation and strategy for prevention. J Clin Med Res. (2018) 10:1–8. 10.14740/jocmr3235w[↩]
- Ross MJ. Advances in the pathogenesis of HIV-associated kidney diseases. Kidney Int. (2014) 86:266–74. 10.1038/ki.2014.167[↩][↩][↩]
- Laurinavicius A, Hurwitz S, Rennke HG. Collapsing glomerulopathy in HIV and non-HIV patients: a clinicopathological and follow-up study. Kidney Int. (1999) 56:2203–13. 10.1046/j.1523-1755.1999.00769[↩][↩]
- Chandra P, Kopp JB. Viruses and collapsing glomerulopathy: a brief critical review. Clin Kidney J. (2013) 6:1–5. 10.1093/ckj/sft002[↩][↩]
- Grover V, Gaiki MR, DeVita MV, Schwimmer JA. Cytomegalovirus-induced collapsing focal segmental glomerulosclerosis. Clin Kidney J. (2013) 6:71–3. 10.1093/ckj/sfs097[↩][↩]
- Freitas GRR, Praxedes MRG, Malheiros D, Testagrossa L, Dias CB, Woronik V. Collapsing variant of focal segmental glomerulosclerosis by parvovirus B19: case report. J Bras Nefrol. (2015) 37:121–6. 10.5935/0101-2800.20150017[↩]
- Besse W, Mansour S, Jatwani K, Nast CC, Brewster UC. Collapsing glomerulopathy in a young woman with APOL1 risk alleles following acute parvovirus B19 infection: a case report investigation. BMC Nephrol. (2016) 17:125. 10.1186/s12882-016-0330-7[↩]
- Araújo SDA, Cordeiro TM, Belisário AR, Araújo RFA, Marinho PES, Kroon EG, et al. First report of collapsing variant of focal segmental glomerulosclerosis triggered by arbovirus: dengue and zika virus infection. Clin Kidney J. (2019) 12:355–61. 10.1093/ckj/sfy104[↩][↩]
- Queiroz PC, Jorge AES, Mourão PHV, Penido MGMG. Collapsing focal segmental glomerulosclerosis probably triggered by dengue virus infection – two case reports. J Bras Nefrol. (2020) 42:489–93. 10.1590/2175-8239-JBN-2019-0237[↩][↩]
- Nasr SH, Kopp JB. COVID-19–associated collapsing glomerulopathy: an emerging entity. Kidney Int Rep. (2020) 5:759–61. 10.1016/j.ekir.2020.04.030[↩]
- Oliveira P, Cunha K, Neves P, Muniz M, Gatto G, Salgado-Filho N, et al. Renal morphology in coronavirus disease: a literature review. Medicina (Kaunas). (2021) 57:258. 10.3390/medicina57030258[↩][↩][↩][↩]
- Teixeira Júnior AAL, Neves PDMM, Lages JS, Cunha KA, Muniz MRP, Brito DJA, et al. Brazilian consortium for the study on renal diseases associated with COVID-19: a multicentric effort to understand SARS-CoV-2-related nephropathy. Front Med. (2020) 7:584235. 10.3389/fmed.2020.584235[↩][↩]
- Gopalakrishnan N, Dhanapriya J, Padmakumar C, Dineshkumar T, Kurien AA, Sakthirajan R, et al. Collapsing glomerulopathy and thrombotic microangiopathy in postpartum period: two case reports. Indian J Nephrol. (2018) 28:157–9. 10.4103/ijn.IJN_242_16[↩][↩]
- Salvatore SP, Barisoni LMC, Herzenberg AM, Chander PN, Nickeleit V, Seshan SV. Collapsing glomerulopathy in 19 patients with systemic lupus erythematosus or lupus-like disease. Clin J Am Soc Nephrol. (2012) 7:914–25. 10.2215/CJN.11751111[↩][↩]
- Markowitz GS, Bomback AS, Perazella MA. Drug-induced glomerular disease: direct cellular injury. Clin J Am Soc Nephrol. (2015) 10:1291–9. 10.2215/CJN.00860115[↩]
- Jaffe JA, Kimmel PL. Chronic nephropathies of cocaine and heroin abuse: a critical review. Clin J Am Soc Nephrol. (2006) 1:655–67. 10.2215/CJN.00300106[↩]
- Gebregeorgis W, Patel I, Thakur M, Bhutani D, Woldie I. Collapsing glomerulopathy associated with hemophagocytic syndrome in a patient with NK/T cell lymphoma. Clin Nephrol Case Stud. (2016) 4:11. 10.5414/CNCS108586[↩]
- Korbet SM, Schwartz MM. Multiple myeloma. J Am Soc Nephrol. (2006) 17:2533–45. 10.1681/ASN.2006020139[↩]
- Bhowmik D, Dinda AK, Gupta S, Agarwal SK, Tiwari SC, Dash SC. Multiple myeloma presenting as collapsing glomerulopathy. Indian J Pathol Microbiol. 2003 Apr;46(2):233-4.[↩]
- Buob D, Decambron M, Gnemmi V, Frimat M, Hoffmann M, Azar R, et al. Collapsing glomerulopathy is common in the setting of thrombotic microangiopathy of the native kidney. Kidney Int. (2016) 90:1321–31. 10.1016/j.kint.2016.07.021[↩][↩]
- Kanodia KV, Vanikar AV, Patel RD, Shutar KS, Nigan LK, Patel HV, et al. Collapsing glomerulopathy: a single centre clinicopathologic study of seven years. J Clin Diagn Res. (2016) 10:EC15–7. 10.7860/JCDR/2016/17297.7646[↩][↩][↩][↩]
- Ahuja A, Gupta R, Sharma A, Bagga A, Bohwmik DN, Agarwal SK, et al. Idiopathic collapsing glomerulopathy: a clinicopathologic analysis of 30 cases. Indian J Nephrol. (2014) 24:239–42. 10.4103/0971-4065.133009[↩]
- Watanabe A, Guaragna MS, Belangero VMS, Casimiro FMS, Pesquero JB, Feltran LS, et al. APOL1 in an ethnically diverse pediatric population with nephrotic syndrome: implications in focal segmental glomerulosclerosis and other diagnoses. Pediatr Nephrol. (2021) 36:1–10. 10.1007/s00467-021-04960-w[↩]
- Cossey NL, Larsen CP, Liapis H. Collapsing glomerulopathy: a 30-year perspective and single, large center experience. Clin Kidney J. (2017) 10:443–9. 10.1093/ckj/sfx029[↩][↩]
- Mubarak M, Kazi JI. Collapsing FSGS: a clinicopathologic study of 10 cases from Pakistan. Clin Exp Nephrol. (2010) 14:222–7. 10.1007/s10157-010-0275-2[↩]
- Grcevska L, Polenakovik M. Collapsing glomerulopathy: clinical characteristics and follow-up. Am J Kidney Dis. (1999) 33:652–7. 10.1016/s0272-6386(99)70215-5[↩]
- De Abreu Testagrossa L, Malheiros DMAC. Estudo brasileiro das variantes morfológicas da glomerulosclerose segmentar e focal. J Bras Patol Med Lab. (2012) 48:211–5. 10.1590/S1676-24442012000300009[↩]
- Kopp JB, Anders HJ, Susztak K, Podestà MA, Remuzzi G, Hildebrandt F, Romagnani P. Podocytopathies. Nat Rev Dis Primers. 2020 Aug 13;6(1):68. doi: 10.1038/s41572-020-0196-7[↩][↩]
- Smeets B, Angelotti ML, Rizzo P, et al. Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J Am Soc Nephrol. 2009;20(12):2593–2603. doi: 10.1681/ASN.2009020132[↩][↩][↩]
- Kopp JB, Anders HJ, Susztak K, Posteda MA, Remuzzi G, Friedhelm H, et al. Podocytopathies. Nat Rev Dis Primers. (2020) 6:1–24. 10.1038/s41572-020-0196-7[↩]
- Cheng H, Harris RC. The glomerulus – a view from the outside – the podocyte. Int J Biochem Cell Biol. (2010) 42:1380–7. 10.1016/j.biocel.2010.05.014[↩][↩]
- Barisoni L, Kriz W, Mundel P, D’agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. (1999) 10:51–61. 10.1681/ASN.V10151[↩][↩]
- Chugh SS, Clement LC. Telomerase at the center of collapsing glomerulopathy. Nat Med. (2012) 18:26–7. 10.1038/nm.2602[↩]
- Shkreli M, Sarin KY, Pech MF, Papeta N, Chang W, Brockmann SA, et al. Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat Med. (2012) 18:111–9. 10.1038/nm.2550[↩][↩][↩]
- Scholz H, Kirschner KMA. Role for the Wilms’ tumor protein WT1 in organ development. Physiology. (2005) 20:54–9. 10.1152/physiol.00048.2004[↩][↩]
- Siemens TA, Riella MC, Moraes TP, Riella CV. APOL1 risk variants and kidney disease: what we know so far. J Braz Nephrol. (2018) 40:388–402. 10.1590/2175-8239-JBN-2017-0033[↩]
- Riella C, Siemens TA, Wang M, Campos RP, Moraes TP, Riella LV, et al. APOL1-associated kidney disease in Brazil. Kidney Int Rep. (2019) 4:923–9. 10.1016/j.ekir.2019.03.006[↩]
- Barisoni L, Diomedi-camassei F, Santorelli FM, Caridi G, Thomas DB, Emma F, et al. Collapsing glomerulopathy associated with inherited mitochondrial injury. Kidney Int. (2008) 74:237–43. 10.1038/sj.ki.5002767[↩]
- Starr MC, Chang IJ, Finn LS, Sun A, Larson AA, Goebel J, et al. COQ2 nephropathy: a treatable cause of nephrotic syndrome in children. Pediatr Nephrol. (2018) 33:1257–61. 10.1007/s00467-018-3937-z[↩]
- Winkler CA, Nelson G, Oleksyk TK, Nava MB, Kopp JB. Genetics of focal segmental glomerulosclerosis and human immunodeficiency virus-associated collapsing glomerulopathy: the role of MYH9 genetic variation. Semin Nephrol. (2010) 30:111–25. 10.1016/j.semnephrol.2010.01.003[↩]
- Nephrotic Syndrome in Adults. https://www.niddk.nih.gov/health-information/kidney-disease/nephrotic-syndrome-adults[↩]
- D’Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004 Feb;43(2):368-82. doi: 10.1053/j.ajkd.2003.10.024[↩]
- D’Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. (2004) 43:368–82. 10.1053/j.ajkd.2003.10.024[↩][↩]
- Fogo AB, Lusco MA, Najafian B, Alpers CE. AJKD Atlas of renal pathology: collapsing glomerulopathy. Am J Kidney Dis. (2015) 66:e3–4. 10.1053/j.ajkd.2015.04.009[↩][↩][↩][↩]
- Nieto Ríos JF, Milena Brand S, Serna Higuita LM, Fernando Arias L. The collapsing variant of focal segmental glomerulosclerosis in children. Iatreia. (2013) 26:481–6.[↩]
- Cheung AK, Chang TI, Cushman WC, Furth SL, Hou FF, Ix JH, et al. Executive summary of the KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. (2021) 99:559–69. 10.1016/j.kint.2020.10.026[↩][↩][↩]
- Kodner C. Diagnosis and Management of Nephrotic Syndrome in Adults. Am Fam Physician. 2016 Mar 15;93(6):479-85. https://www.aafp.org/pubs/afp/issues/2016/0315/p479.html[↩]
- Floege J, Barbour SJ, Cattran DC, Hogan JJ, Nachman PH, Tang SCW, et al. Management and treatment of glomerular diseases (part 1): conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. (2019) 95:268–80. 10.1016/j.kint.2018.10.018[↩]
- Rovin BH, Caster DJ, Cattran DC, Gibson KL, Hogan JJ, Moeller MJ, et al. Management and treatment of glomerular diseases (part 2): conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. (2019) 95:268–80. 10.1016/j.kint.2018.11.008[↩]
- Vaziri ND. Disorders of lipid metabolism in nephrotic syndrome: mechanisms and consequences. Kidney Int. (2016) 90:41–52. 10.1016/j.kint.2016.02.026[↩]
- Agrawal S, Zaritsky JJ, Fornoni A, Smoyer WE. Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat Rev Nephrol. (2018) 14:57–70. 10.1038/nrneph.2017.155[↩]
- Morales E, Alonso M, Gutiérrez E. Collapsing glomerulopathy: update. Med Clin (Barc). (2019) 152:361–7. 10.1016/j.medcli.2018.10.021[↩]
- Schwartz EJ, Szczech LA, Ross MJ, Klotman ME, Winston JA, Klotman PE. Highly active antiretroviral therapy and the epidemic of HIV end-stage renal disease. J Am Soc Nephrol. (2005) 16:2412–20. 10.1681/ASN.2005040340[↩]
- Szczech LA, Gupta SK, Habash R, Guasch A, Kalayjian R, Appel A, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. (2004) 66:1145–52. 10.1111/j.1523-1755.2004.00865.x[↩]
- Atta MG, Gallant JE, Rahman MH, Nagajothi N, Racusen LC, Scheel PL, et al. Antiretroviral therapy in the treatment of HIV-associated nephropathy. Nephrol Dial Transplant. (2006) 21:2809–13. 10.1093/ndt/gfl337[↩]
- Nair V, Jandovitz N, Jhaveri KD, Hirschwerk D, Grodstein L, Bijol V, et al. Treatment of parvovirus B19 viremia to facilitate kidney transplantation in a patient with collapsing glomerulopathy. Clin Nephrol Case Stud. (2020) 8:41–5. 10.5414/CNCS110113[↩]
- Detwiler RK, Falk RJ, Hogan SL, Jennette JC. Collapsing glomerulopathy: a clinically and pathologically distinct variant of focal segmental glomeruloscierosis. Kidney Int. (1994) 45:1416–24. 10.1038/ki.1994.185[↩]
- Haas M, Spargo BH, Coventry S. Increasing incidence of focal-segmental glomerulosclerosis among adult nephropathies: a 20-year renal biopsy study. Am J Kidney Dis. (1995) 26:740–50. 10.1016/0272-6386(95)90437-9[↩]