Contents
Paramyotonia congenita
Paramyotonia congenita also called PMC, Eulenburg disease, Von Eulenburg’s disease, paralysis periodica paramyotonica, paramyotonia congenita of von Eulenburg, is a form of periodic paralysis that results from a mutation in the sodium channel and produces muscle stiffness (myotonia) or rigidity and weakness in response to cold or exercise 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11. Myotonia is a disorder that resulted in the delayed relaxation of skeletal muscles after voluntary contraction 12. Beginning in infancy or early childhood, people with paramyotonia congenita experience bouts of sustained muscle stiffness (myotonia) that prevent muscles from relaxing normally. Paramyotonia congenita causes muscle stiffness that typically appears after exercise and can also be induced by muscle cooling. Exposure to cold initially causes muscle stiffness (myotonia) in these individuals, and prolonged cold exposure leads to temporary episodes of mild to severe muscle weakness that may last for several hours at a time. Some older people with paramyotonia congenita develop permanent muscle weakness that can be disabling. Paramyotonia congenita can make small everyday activities difficult, such as letting go of small objects (e.g. pens or door knobs).
The muscle stiffness (myotonia) mainly affects muscles in the eyelids, face, tongue, neck, arms, and hands, although it can also affect muscles used for breathing and muscles in the lower limbs 13. Unlike many other forms of myotonia, the muscle stiffness associated with paramyotonia congenita tends to worsen with repeated movements. Paramyotonia Congenita patients may complain of hand stiffness while shoveling snow or in the frozen food section of the supermarket 14. Parents will occasionally report that affected infants are unable to open their eyes after a crying spell, presumably due to the eyelids being “exercised” while crying 14.
Paramyotonia Congenita symptoms can begin during infancy, and are always apparent by the teenage years. Paramyotonia Congenita patients characteristically present in their childhood complaining of inability to open their eyes following rapid, forceful successive closures. Weakness and myotonia (muscle stiffness) last for minutes to hours. Even after the immediate rewarding of the muscles, cold-induced weakness usually persists for several hours. The disease is non-progressive, does not cause muscle wasting or hypertrophy.
Paramyotonia congenita is caused by mutations in the sodium channel gene SCN4A that codes for the alpha-subunit of the skeletal muscle sodium channels, i.e., voltage sensor domain 15. Paramyotonia congenita is inherited in an autosomal dominant pattern, which means one copy of the altered gene in each cell is sufficient to cause the disorder. In many cases, an affected person has one parent with the condition.
Paramyotonia congenita is a very rare disorder that affects males and females in equal numbers. It is estimated to affect fewer than 1 in 250,000 people 16, 11.
Paramyotonia Congenita (PMC) comes in two forms, one in which attacks are always associated with a rise in potassium (hyperkalemia) and a form called Paramyotonia von Eulenburg in which attacks can be associated with a fall in blood potassium levels or hypokalemia. Both result from mutations in the sodium channel. Both can accompany hyperkalemic periodic paralysis (HyperPP) or can occur alone.
When paramyotonia congenita is suspected, a test is administered to test the capacity of muscles to conduct electricity called electromyography (EMG). During the EMG (electromyography) test, the muscles are chilled and electrical signals are recorded before and after the muscle is cooled. The electromyography (EMG) taken during cooling of a muscle shows profuse myotonic discharges and reduced compound muscle action potential (CMAP) amplitudes. However, EMG cannot always diagnose paramyotonia congenita definitively, and further testing may be necessary.
Genetic testing on a blood sample will result in a definitive diagnosis by showing the presence of a characteristic mutation in the SCN4A gene.
The treatment of paramyotonia congenita is based on the individual’s symptoms. Paramyotonia congenita can be handled on a day-to-day basis and many patients can lead normal lives. Individuals must be cautious to sudden exposures to very cold weather, as well as avoiding sudden heavy physical activity.
Muscle stiffness could also be triggered or enhanced by potassium-rich foods. Patients will need to learn how to manage their potassium-intake. They should avoid potassium-rich foods, avoid skipping meals and take carbohydrate rich snacks in between meals.
The aim of treatment is to reduce the intensity of acute symptoms and to prevent, as far as possible, further attacks. Some attacks are so mild that treatment is not necessary. However, in other instances drug therapy is required.
Treatment with medications that block the sodium channels such as mexiletine and lamotrigine may help reduce the stiffness related to myotonia. Some patients with paramyotonia congenita may benefit from acetazolamide or thiazide diuretic drugs to reduce the number of paralytic attacks.
Genetic counseling is recommended for patients and their families.
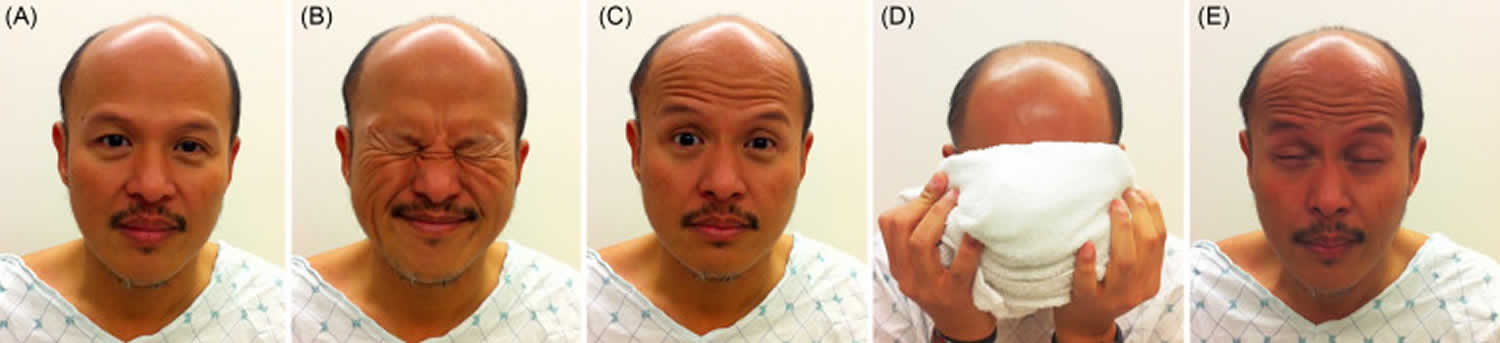
Figure 1. Paramyotonia congenita
Footnotes: A man with paramyotonia congenita at rest (A) is told to forcefully close his eyes (B). Mild myotonia of the eyelid muscles follows (C). Frontalis muscle is activated to help with eye opening. He then applies a cold cloth to the face for 30 seconds (D). This produces severe myotonia of the eyelid (E), much worse than with just forceful closure. He has difficulty opening his eyes for 30 seconds, even with the help of the frontalis muscle.
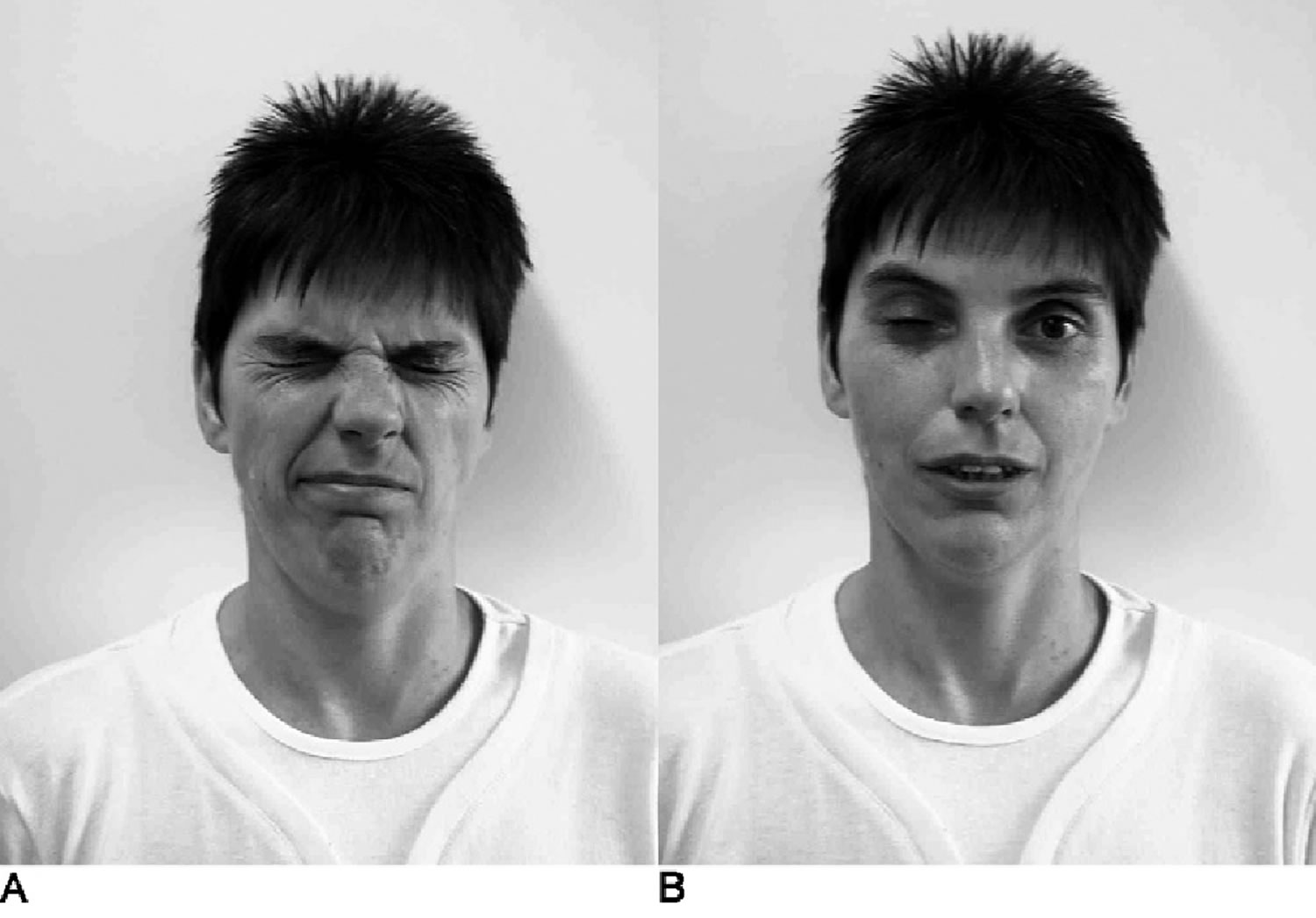
[Source 17 ]Figure 2. Paramyotonia congenita
Footnotes: Effects of local cooling on a paramyotonia congenita patient. After the patient’s right eye was cooled for 10 minutes, she was asked to close her eyes forcefully (A) and the open them fast (B). The cooled right eyelid remained involuntarily closed for almost a minute.
[Source 18 ]Paramyotonia congenita cause
Mutations in the SCN4A gene on chromosome 17 cause paramyotonia congenita 11, 19, 20, 21. The SCN4A gene provides instructions for making sodium channels, that are abundant in muscles used for movement (skeletal muscles), which transport positively charged sodium atoms (sodium ions) into cells, play a key role in a cell’s ability to generate and transmit electrical signals that is critical for the normal function of skeletal muscle cells. For your body to move normally, skeletal muscles must tense (contract) and relax in a coordinated way. Muscle contractions are triggered by the flow of positively charged atoms (ions), including sodium, into skeletal muscle cells. The SCN4A protein forms channels that control the flow of sodium ions into these cells.
Mutations in the SCN4A gene alter the usual structure and function of sodium channels. The altered channels cannot effectively regulate the flow of sodium ions into skeletal muscle cells. The resulting increase in ion flow interferes with normal muscle contraction and relaxation, leading to episodes of muscle stiffness and weakness.
Skeletal muscles move the body; muscle contractions pull on tendons, which are attached to the bones and causes the body to move. Muscle contractions are triggered by the flow of positively charged atoms (e.g. potassium and sodium) through channels into the skeletal muscles. These atoms carry electrical impulses necessary for normal function of the muscle cells. However, a mutation of the SCN4A gene alters the structure of the sodium channels. The sodium channels fail to regulate the flow of atoms into the muscles cells, and the ratio of sodium and potassium becomes unbalanced. The abnormal ratio interferes with normal muscle contraction and relaxation, causing bouts of muscle weakness and stiffness.
Paramyotonia congenita inheritance
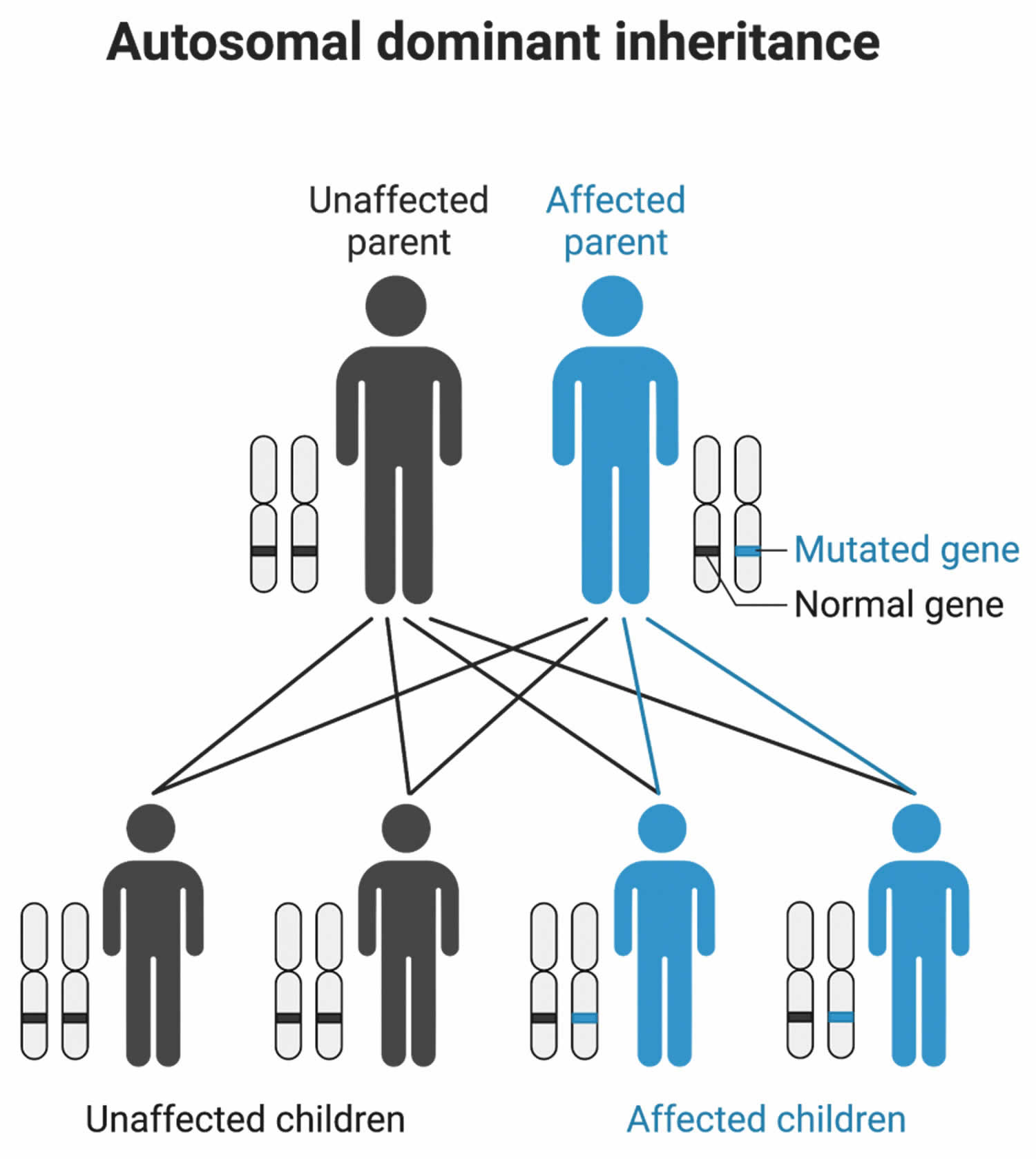
Paramyotonia congenita is inherited in an autosomal dominant pattern, which means one copy of the altered SCN4A gene in each cell is sufficient to cause the disorder (Figure 3). The SCN4A gene can be inherited from either parent or can be the result of a mutated gene in the affected individual. The risk of passing the non-working gene from an affected parent to an offspring is 50% for each pregnancy. The risk is the same for males and females. Most individuals with a SCN4A gene mutation have symptoms; however, there are a few who remain unaffected and are known as “carriers”.
In some individuals, paramyotonia congenita is due to a spontaneous (de novo) genetic mutation that occurs in the egg or sperm cell. In such situations, Paramyotonia congenita is not inherited from the parents.
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Figure 3. Paramyotonia congenita autosomal dominant inheritance pattern
Paramyotonia congenita symptoms
Muscle stiffness in paramyotonia congenita is the inability for the muscles to relax in a timely manner after contracting (myotonia). The muscles most commonly affected are located in your eyelids, face, tongue, neck, arms, and hands, although it can affect the muscles used for breathing and swallowing, as well as muscles in the lower legs 20, 22, 14. Paramyotonia congenita is usually apparent during infancy and always presents by teenage years 19, 23, 22. Symptoms do not progress with age. Individuals with paramyotonia congenita do not have wasting of muscles (atrophy) but often have an increase of muscle bulk (hypertrophy) 22.
The severity of the muscle stiffness depends on the individual; some patients experience painful myotonia, while others experience painless myotonia. Paramyotonia congenita becomes worse with exposure to cold and alleviated by warm temperatures 24, 23. In addition, it can become more severe with exercise. Sudden overexertion can trigger muscle stiffness and overall weakness that can take several days to completely resolve. Paramyotonia congenita can make small everyday activities difficult, such as letting go of small objects (e.g. pens or door knobs).
Some episodes of muscle stiffness can coincide with pregnancy, menstruation, potassium intake and anesthetics 25, 22; affected individuals are instructed to avoid certain food products rich in potassium.
Some patients with more severe paramyotonia congenita can experience shortness of breath or tightness in their chest muscles.
Paramyotonia congenita diagnosis
When paramyotonia congenita is suspected, a test is administered to test the capacity of muscles to conduct electricity called electromyography (EMG). During the EMG (electromyography) test, the muscles are chilled and electrical signals are recorded before and after the muscle is cooled. The electromyography (EMG) taken during cooling of a muscle shows profuse myotonic discharges and reduced compound muscle action potential (CMAP) amplitudes 26. However, EMG cannot always diagnose paramyotonia congenita definitively, and further testing may be necessary.
Genetic testing on a blood sample will result in a definitive diagnosis by showing the presence of a characteristic mutation in the SCN4A gene 13, 27.
Muscle biopsy demonstrates non-specific myopathic changes with occasional vacuoles 13, 27.
Paramyotonia congenita treatment
The treatment of paramyotonia congenita is based on the individual’s symptoms. Paramyotonia congenita can be handled on a day-to-day basis and many patients can lead normal lives. Individuals must be cautious to sudden exposures to very cold weather, as well as avoiding sudden heavy physical activity.
Muscle stiffness could also be triggered or enhanced by potassium-rich foods. Patients will need to learn how to manage their potassium-intake. They should avoid potassium-rich foods, avoid skipping meals and take carbohydrate rich snacks in between meals.
The aim of treatment is to reduce the intensity of acute symptoms and to prevent, as far as possible, further attacks. Some attacks are so mild that treatment is not necessary. However, in other instances drug therapy is required.
Treatment with medications that block the sodium channels such as mexiletine and lamotrigine may help reduce the stiffness related to myotonia. Some patients with paramyotonia congenita may benefit from acetazolamide or thiazide diuretic drugs to reduce the number of paralytic attacks.
Genetic counseling is recommended for patients and their families.
Paramyotonia congenita prognosis
There is no cure to paramyotonia congenita. Paramyotonia congenita patients usually live a normal life and the condition does not affect their lifespan 28. With the proper management of diet that avoid potassium-rich foods, avoidance of cold exposure and physical overactivity and medication, patients can lead normal lives 15.
- Lee MJ, Lin PC, Lin MH, Chiou HC, Wang K, Huang CW. Kinetic Alterations in Resurgent Sodium Currents of Mutant Nav1.4 Channel in Two Patients Affected by Paramyotonia Congenita. Biology (Basel). 2022 Apr 18;11(4):613. doi: 10.3390/biology11040613[↩]
- Featherstone DE, Fujimoto E, Ruben PC. A defect in skeletal muscle sodium channel deactivation exacerbates hyperexcitability in human paramyotonia congenita. J Physiol. 1998 Feb 1;506 ( Pt 3)(Pt 3):627-38. doi: 10.1111/j.1469-7793.1998.627bv.x[↩]
- Huang CW, Lai HJ, Lin PC, Lee MJ. Changes in Resurgent Sodium Current Contribute to the Hyperexcitability of Muscles in Patients with Paramyotonia Congenita. Biomedicines. 2021 Jan 8;9(1):51. doi: 10.3390/biomedicines9010051[↩]
- Siow WS, Chiew WA. Anaesthesia challenges of a parturient with paramyotonia congenita and terminal filum lipoma presenting for labour and caesarean section under epidural anaesthesia – a case report. BMC Anesthesiol. 2021 Feb 18;21(1):57. doi: 10.1186/s12871-021-01262-4[↩]
- Wang Q, Zhao Z, Shen H, Bing Q, Li N, Hu J. The Clinical, Myopathological, and Genetic Analysis of 20 Patients With Non-dystrophic Myotonia. Front Neurol. 2022 Mar 8;13:830707. doi: 10.3389/fneur.2022.830707[↩]
- Brooks EK, Schweitzer D, Robinson HL. A case of paramyotonia congenita in pregnancy. Obstet Med. 2020 Dec;13(4):192-194. doi: 10.1177/1753495X18816171[↩]
- Huang S, Zhang W, Chang X, Guo J. Overlap of periodic paralysis and paramyotonia congenita caused by SCN4A gene mutations two family reports and literature review. Channels (Austin). 2019 Dec;13(1):110-119. doi: 10.1080/19336950.2019.1600967[↩]
- Najid NM, Razak TA, Günaydın DB. Analgesia and Anaesthesia Management of Labour and Caesarean Delivery for a Parturient with Paramyotonia Congenita. Turk J Anaesthesiol Reanim. 2019 Aug;47(4):345-347. doi: 10.5152/TJAR.2019.69094[↩]
- Trivedi JR, Bundy B, Statland J, Salajegheh M, Rayan DR, Venance SL, et al. Non-dystrophic myotonia: prospective study of objective and patient reported outcomes. Brain. 2013;136(Pt 7):2189–200. doi: 10.1093/brain/awt133[↩]
- Matthews E, Fialho D, Tan SV, Venance SL, Cannon SC, Sternberg D, Fontaine B, Amato AA, Barohn RJ, Griggs RC, Hanna MG; CINCH Investigators. The non-dystrophic myotonias: molecular pathogenesis, diagnosis and treatment. Brain. 2010 Jan;133(Pt 1):9-22. doi: 10.1093/brain/awp294[↩]
- Ptacek LJ, Trimmer JS, Agnew WS, Roberts JW, Petajan JH, Leppert M. Paramyotonia congenita and hyperkalemic periodic paralysis map to the same sodium-channel gene locus. Am J Hum Genet. 1991 Oct;49(4):851-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1683172/pdf/ajhg00081-0159.pdf[↩][↩][↩]
- Roberts K, Kentris M. Myotonia. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559272[↩]
- Miller TM, Dias da Silva MR, Miller HA, Kwiecinski H, Mendell JR, Tawil R, McManis P, Griggs RC, Angelini C, Servidei S, Petajan J, Dalakas MC, Ranum LP, Fu YH, Ptácek LJ. Correlating phenotype and genotype in the periodic paralyses. Neurology. 2004 Nov 9;63(9):1647-55. doi: 10.1212/01.wnl.0000143383.91137.00[↩][↩][↩]
- Hahn C, Salajegheh MK. Myotonic disorders: A review article. Iran J Neurol. 2016 Jan 5;15(1):46-53. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4852070[↩][↩][↩]
- Finsterer J. Primary periodic paralyses. Acta Neurol Scand. 2008 Mar;117(3):145-58. doi: 10.1111/j.1600-0404.2007.00963.x[↩][↩]
- Phillips L, Trivedi JR. Skeletal Muscle Channelopathies. Neurotherapeutics. 2018 Oct;15(4):954-965. doi: 10.1007/s13311-018-00678-0[↩]
- Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease. Volume 2, Sixth Edition, 2020. https://www.sciencedirect.com/book/9780128138663/rosenbergs-molecular-and-genetic-basis-of-neurological-and-psychiatric-disease[↩]
- https://periodicparalysis.org/wp-content/uploads/2018/09/Lehmann-Horn-2007-Chap.3-Myotonic-disorders-Handbook-of-Clincal-Neurology.pdf[↩]
- Simkin D, Bendahhou S. Skeletal muscle na channel disorders. Front Pharmacol. 2011 Oct 14;2:63. doi: 10.3389/fphar.2011.00063[↩][↩]
- Palma C, Prior C, Gómez-González C, Rodríguez-Antolin C, Martínez-Montero P, Pérez de Ayala L, Pascual SI, Molano Mateos J. A SCN4A mutation causing paramyotonia congenita. Neuromuscul Disord. 2017 Dec;27(12):1123-1125. doi: 10.1016/j.nmd.2017.09.008[↩][↩]
- Paramyotonia congenita. https://medlineplus.gov/genetics/condition/paramyotonia-congenita[↩]
- Matthews E, Tan SV, Fialho D, Sweeney MG, Sud R, Haworth A, Stanley E, Cea G, Davis MB, Hanna MG. What causes paramyotonia in the United Kingdom? Common and new SCN4A mutations revealed. Neurology. 2008 Jan 1;70(1):50-3. doi: 10.1212/01.wnl.0000287069.21162.94[↩][↩][↩][↩]
- Grace RF, Roach VJ. Caesarean section in a patient with paramyotonia congenita. Anaesth Intensive Care. 1999 Oct;27(5):534-7.[↩][↩]
- Kaneda T, Iwahashi M, Suzuki T. Anesthetic management for subtotal gastrectomy in a patient with paramyotonia congenita. J Anesth. 2007;21:500–3. doi: 10.1007/s00540-007-0553-7[↩]
- Trivedi JR, Bundy B, Statland J, Salajegheh M, Rayan DR, Venance SL, Wang Y, Fialho D, Matthews E, Cleland J, Gorham N, Herbelin L, Cannon S, Amato A, Griggs RC, Hanna MG, Barohn RJ; CINCH Consortium. Non-dystrophic myotonia: prospective study of objective and patient reported outcomes. Brain. 2013 Jul;136(Pt 7):2189-200. doi: 10.1093/brain/awt133[↩]
- Nielsen VK, Friis ML, Johnsen T. Electromyographic distinction between paramyotonia congenita and myotonia congenita: effect of cold. Neurology. 1982 Aug;32(8):827-32. doi: 10.1212/wnl.32.8.827[↩]
- Thrush DC, Morris CJ, Salmon MV. Paramyotonia congenita: a clinical, histochemical and pathological study. Brain. 1972;95(3):537-52. doi: 10.1093/brain/95.3.537[↩][↩]
- Péréon Y, Lande G, Demolombe S, Nguyen The Tich S, Sternberg D, Le Marec H, David A. Paramyotonia congenita with an SCN4A mutation affecting cardiac repolarization. Neurology. 2003 Jan 28;60(2):340-2. doi: 10.1212/01.wnl.0000042093.96309.5a[↩]