Contents
Talaromycosis
Talaromycosis previously known as penicilliosis penicilliosis, is a fungus infection caused by the thermally dimorphic fungus called Talaromyces marneffei (previously called Penicillium marneffei), that are endemic to South and Southeast Asia 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12. The fungus Talaromyces marneffei was described by Professor Gabriel Segretain in 1959 in Vietnam from the hepatic lesions of a bamboo rat (Rhizomys sinensis), originally as a member of the genus Penicillium marneffei, which is an important fungal pathogen endemic in Southeast Asia especially to HIV-positive patients 13, 14. In highly endemic regions in northern Thailand, Vietnam, Philippines, Taiwan, India and southern China (Guangdong, Guangxi and Hong Kong), the prevalence of talaromycosis as an human immunodeficiency virus (HIV)-associated opportunistic infection is only lower than that of tuberculosis and cryptococcosis 15, 16, 17, 18, 19. Talaromycosis affects people who live in or visit Southeast Asia, southern China, or northeastern India. Talaromycosis is increasingly being diagnosed in immunocompromised individuals who are returning travelers or immigrants from the endemic regions, and it has been reported in many non-endemic countries including Japan, Australia, Belgium, France, Germany, the Netherlands, Sweden, Switzerland, the United Kingdom, Oman (in the Middle East), and the United States 20, 21. Talaromycosis is increasingly recognized in individuals who have a primary immunodeficiency condition (e.g., idiopathic CD4 lymphopenia; anti-interferon-gamma autoantibody-associated immunodeficiency; conditions due to mutations in CYBB or CD40L; or gain-of-function mutation in STAT1/STAT3 pathways) or secondary immunodeficiency conditions (e.g., autoimmune diseases in people on corticosteroids and/or other immunosuppressive therapy; solid and hematological malignancies; solid organ transplantation; hematopoietic stem cell transplantation; and therapy with novel target therapies, such as monoclonal antibodies against CD20 and kinase inhibitors).
Most people who get talaromycosis have a weakened immune system 22, 23, 24, 25, 26, 27, 28, 23, 29, 26. Talarmycosis typically affects people with HIV differently than other people who get infected. Talaromycosis is increasingly diagnosed among patients who are not infected with HIV but who have other immunodeficiency conditions 11 and is reported to be the second most common cause of all bloodstream infections in southern Vietnam 30. People living with HIV are particularly at risk. Talaromycosis is seen almost exclusively in children and adults with weakened immune system. Infection was initially identified in HIV/AIDS patients in tropical areas of Southeast Asia in the late 1980s and 1990s. Talaromycosis was classified as an AIDS-defining infection and one of the HIV clinical stage 4 conditions. Talaromycosis has also been reported to develop during the immune-reconstitution syndrome.
The severity and clinical signs and symptoms of talaromycosis depend on the degree of host immunosuppression 5, 31, 32, 11. Talaromyces marneffei mostly causes mild and localized infections in patients with normal immunity, but it can cause severe disseminated infections with generalized lymph node enlargement (lymphadenopathy), respiratory symptoms, skin lesions, digestive symptoms, enlarged liver and spleen (hepatosplenomegaly) and persistent fever in AIDS patients 33, 11, 24. However, many previous studies have reported that varying clinical signs and symptoms of talaromycosis, including fever, lymphadenopathy, hepatosplenomegaly, malaise, weight loss, skin and soft tissue lesions, cough and shortness of breath (dyspnea), commonly occurred among these non-HIV patients 22, 11. Because of nonspecific clinical signs and symptoms, talaromycosis can be easily misdiagnosed as tuberculosis, histoplasmosis, cryptococcosis and lymphoma in patients with fever and generalized lymphadenopathy 11, 34.
Tuberculosis coinfection is common (occurring in up to 22% of patients in highly endemic regions) and complicates disease management because of itraconazole and rifampin drug interactions 35.
Talaromycosis can be diagnosed by using a sample from the body part that is affected. For example:
- Bone marrow
- Blood
- Fluid in and around the lungs
- Lymph node
- Skin
Microscopy, histology, and culture are the current gold standard diagnostic methods. The sample is sent to a laboratory for a fungal culture or to be examined under the microscope. The sample can also be tested for the presence of a protein or DNA of the Talaromyces marneffei fungus.
Talaromycosis must be treated with prescription antifungal medicine. The most common treatments are amphotericin B and itraconazole. Amphotericin B is given through a vein for two weeks, followed by itraconazole, given by mouth for 10 weeks. Other antifungal medicines that can be used include voriconazole or posaconazole.
Talaromycosis-related mortality, despite antifungal therapy in people both with and without HIV, is up to 30% (children 55%, HIV-negative adults 30%, and HIV-positive adults 20%) 25, 35, 8, 11, 36, 37.
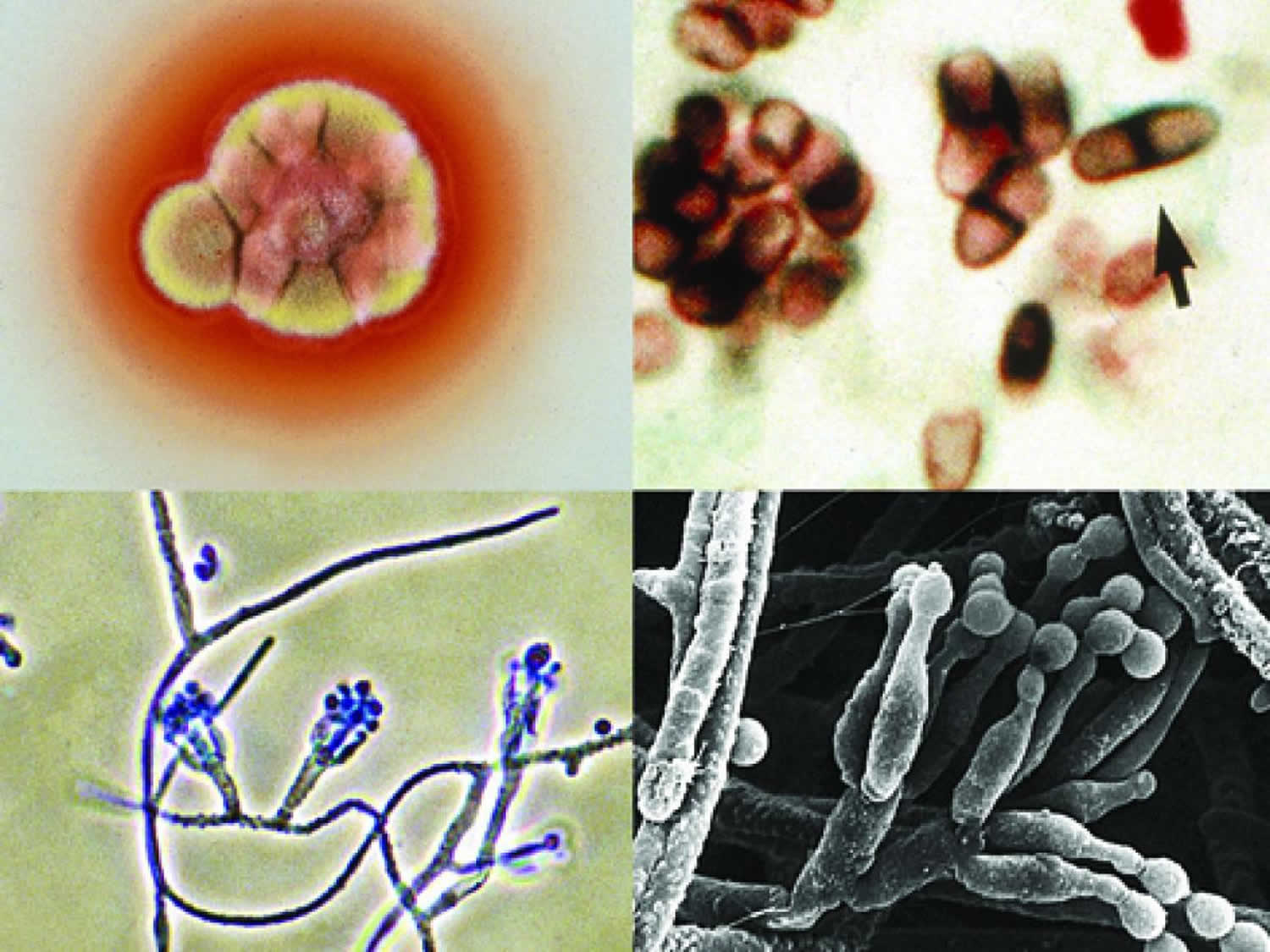
Figure 1. Talaromyces marneffei fungus
Footnotes: Talaromyces marneffei culture showing red soluble pigment; a Giemsa stained touch smear showing typical septate yeast-like cells (arrow), phialides and conidia. The conidiophores are usually biverticillate. Oval, smooth-walled conidia are produced in chains.
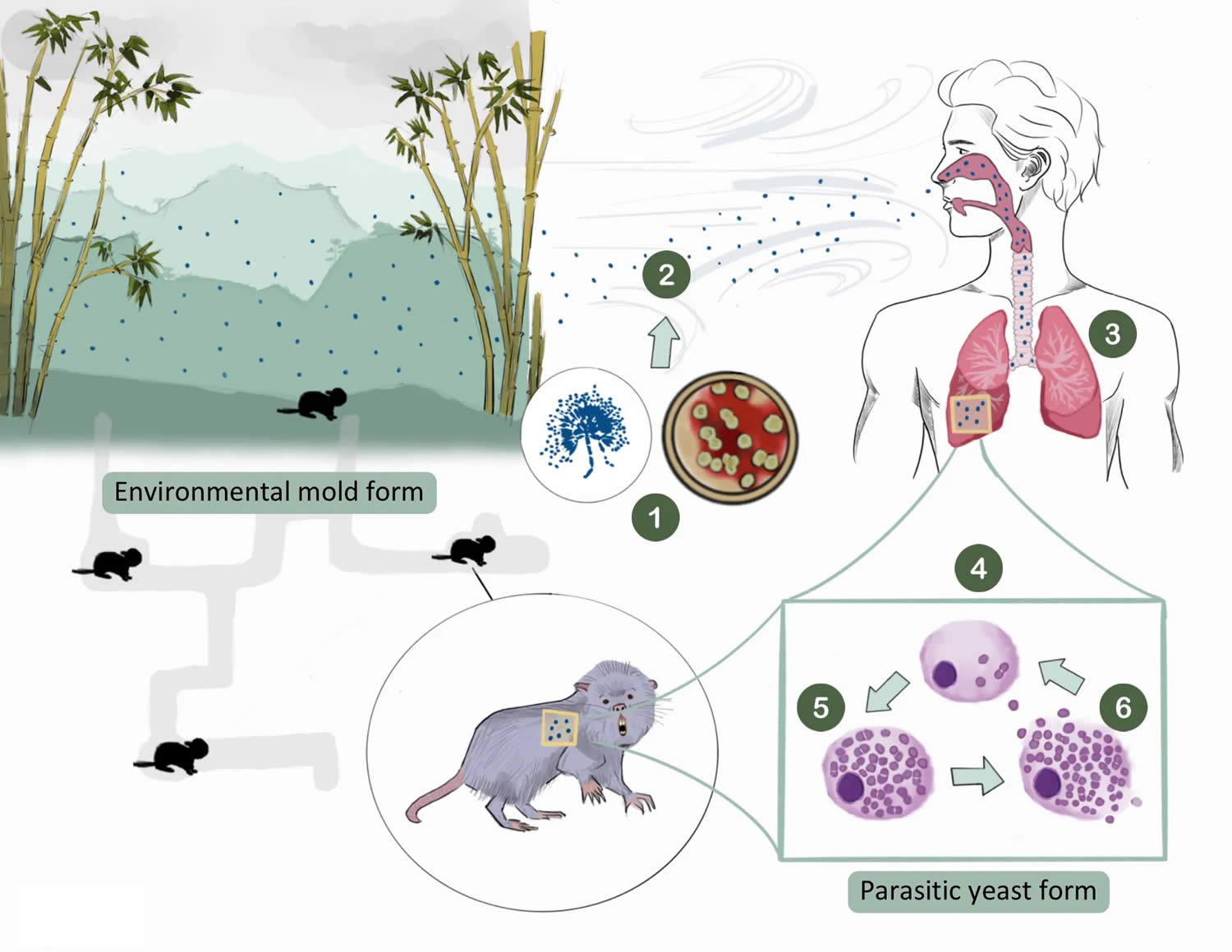
[Source 38 ]Figure 2. Talaromycosis lifecycle
Footnotes: (1) The Talaromyces marneffei fungus produces spores that are easily aerosolized (2). The spores are inhaled by the host and are settled into the lungs (3), where they are phagocytosed by the host’s alveolar macrophages (4). The higher body temperature (at 37°C) triggers a morphologic change from the mold to a yeast form, which uniquely divides by binary fission forming a characteristic midline septum (5). The yeasts multiply until they cause rupture of the macrophage (6), releasing yeasts that can spread within the lungs and can disseminate to other organs. At the demise of the hosts, the yeast returns to the mold, in abundance, and the cycle repeats.
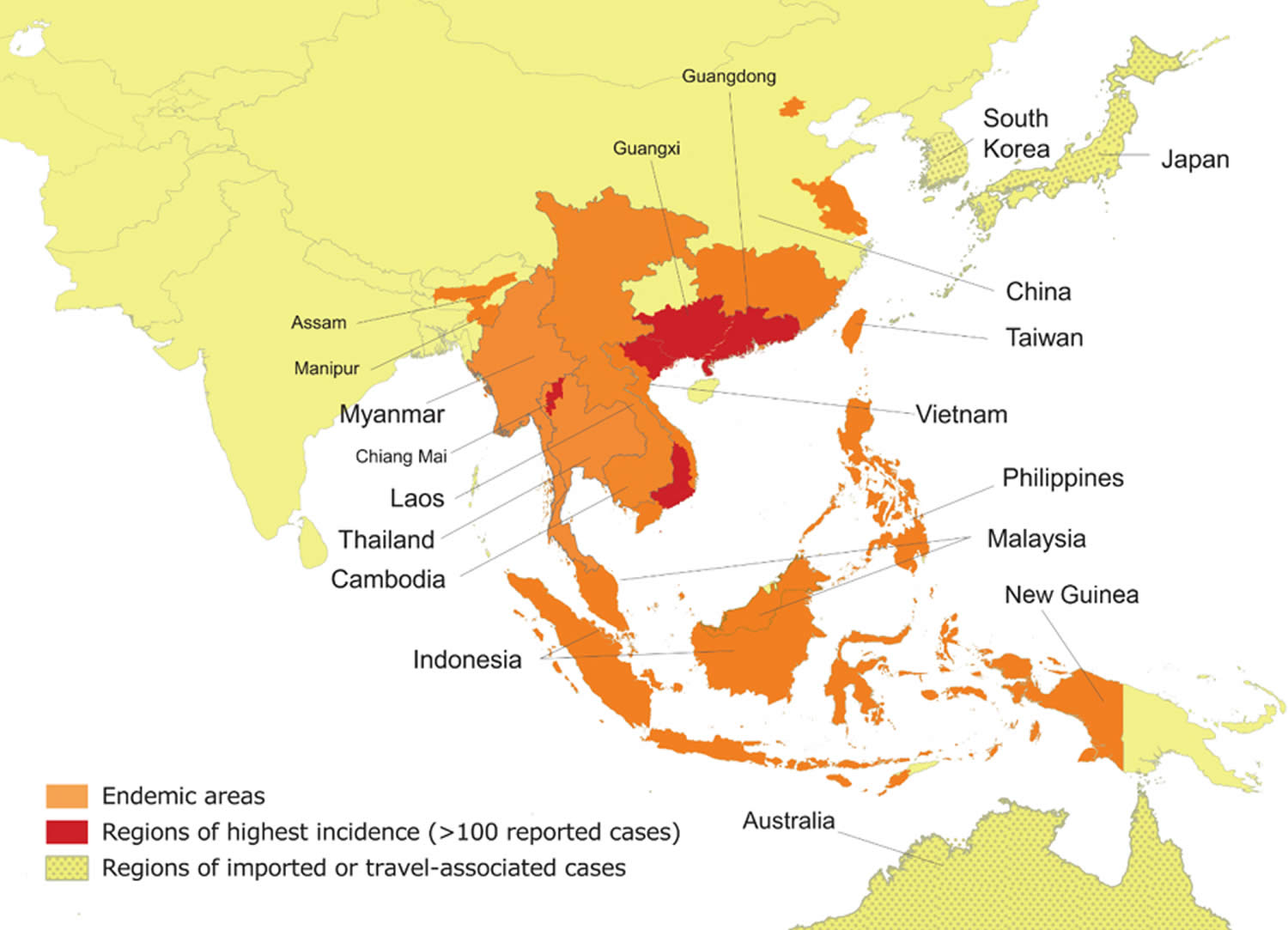
[Source 39 ]Figure 3. Talaromycosis endemic region
Footnotes: Talaromycosis is endemic in Southeast Asia (northern Thailand, Vietnam, and Myanmar), East Asia (southern China, Hong Kong, and Taiwan), and South Asia (northeastern India).
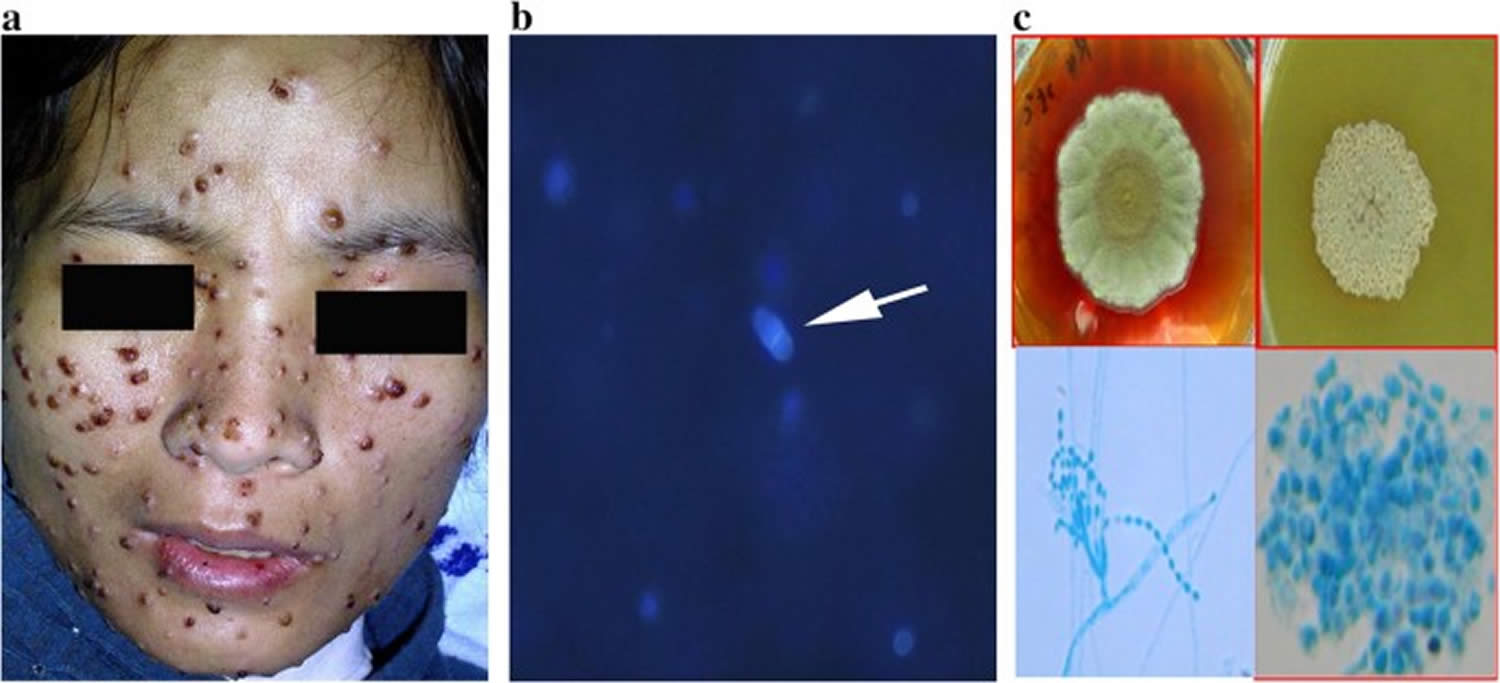
[Source 39 ]Figure 4. Talaromycosis
Footnotes: Multiple umbilicated papules on the face seen in a patient with talaromycosis (Penicilliosis). Skin lesions in talaromycosis have typical central-necrotic appearance and can be a diagnostic sign. However, skin lesions are a late manifestation of talaromycosis and are absent in up to 60% of patients 22, 35, 34.
[Source 40 ]Talaromycosis cause
Talaromycosis is caused by Talaromyces marneffei, a fungus found in wild bamboo rats, their feces, and the soil around their burrows. Scientitsts believe that people get talaromycosis after breathing in Talaromyces marneffei fungus from the environment 41, 42. Bamboo rats can also get talaromycosis but there is no evidence that Talaromyces marneffei spreads from rats to people. Talaromycosis does not spread from person to person. In Thailand, infections are more common during rainy seasons so rain might promote the growth of the fungus. Acute infection has an incubation period of 1–3 weeks, but reactivation of latent infection may present years later.
The wild bamboo rat in highland areas in the endemic regions is the known animal reservoir of Talaromyces marneffei fungus 43, 44; however, case-control studies suggest that human infection results from inhalation of fungal spores released from a soil-related environmental reservoir (plants and farmed animals) rather than from direct bamboo rat-to-human transmission 41, 42. Talaromycosis incidence increased 30% to 50% during the rainy months in southern Vietnam and northern Thailand 35, 45 and was associated with increased humidity and not precipitation 46, 47, which suggests that humidity facilitates an expansion of the environmental reservoir, resulting in increased exposure to the Talaromyces marneffei fungus.
Reactivation of latent infections has been demonstrated in non-autochthonous cases with a history of remote travel to the endemic countries and can occur many years after exposure 32, 20, 21. One case of presumed laboratory-acquired talaromycosis was reported in an African man with HIV who was at the Pasteur Institute in Paris 48; however, laboratory-acquired infection has never been reported from the endemic regions. Donor-acquired transmission has been reported in a lung-transplant recipient from Belgium 49.
Risk factors for talaromycosis
Talaromycosis affects people who live in or visit Southeast Asia, southern China, or eastern India. Healthy people rarely get talaromycosis.
Most people who get talaromycosis have a medical condition that weakens their immune system, such as:
- Adult-onset immunodeficiency syndrome
- Cancer
- HIV/AIDS
- Organ transplant recipients
- Other autoimmune diseases
Agricultural workers and farmers, particularly those working during the rainy season, appear to be more likely to get talaromycosis.
HIV or AIDS is a major risk factor for talaromycosis in highly endemic regions, accounting for approximately 88% of the disease 25. Talaromyces marneffei fungus is also a major cause of HIV-associated opportunistic infections in these regions, making up to 16% of hospital admissions due to AIDS and is a leading cause of HIV-associated bloodstream infections and deaths in Vietnam and southern China 25, 35, 18, 50, 51, 52, 30, 53. Talaromycosis occurs predominantly in individuals who have very advanced HIV disease with a CD4 T lymphocyte (CD4) cell count of <100 cells/mm³ 25, 35, 8. Talaromycosis affects people who live in or visit Southeast Asia, southern China, or northeastern India. Talaromycosis is increasingly being diagnosed in immunocompromised individuals who are returning travelers or immigrants from the endemic regions, and it has been reported in many non-endemic countries including Japan, Australia, Belgium, France, Germany, the Netherlands, Sweden, Switzerland, the United Kingdom, Oman (in the Middle East), and the United States 20, 21. Talaromycosis is increasingly recognized in individuals who have a primary immunodeficiency condition (e.g., idiopathic CD4 lymphopenia; anti-interferon-gamma autoantibody-associated immunodeficiency; conditions due to mutations in CYBB or CD40L; or gain-of-function mutation in STAT1/STAT3 pathways) or secondary immunodeficiency conditions (e.g., autoimmune diseases in people on corticosteroids and/or other immunosuppressive therapy; solid and hematological malignancies; solid organ transplantation; hematopoietic stem cell transplantation; and therapy with novel target therapies, such as monoclonal antibodies against CD20 and kinase inhibitors).
With highly active antiretroviral therapy (HAART), talaromycosis is now increasingly recognized in HIV-negative patients. Infected adults may have known associated conditions including:
- Autoimmune conditions, such as systemic lupus erythematosus (SLE) and mixed connective tissue disease, are associated with impaired cell-mediated immunity
- Autoimmune conditions treated with immunosuppressants include primary biliary cirrhosis and autoimmune hemolytic anaemia
- Hematological conditions (e.g, non-Hodgkin lymphoma, Waldenström macroglobulinaemia) and following hematological stem cell transplant
- Solid organ transplant, particularly renal transplants
- Adult-onset immunodeficiency syndrome due to neutralizing anti-interferon gamma autoantibodies
- Treatment with biological agents, specifically kinase inhibitors (e.g, ruxolitinib, sorafenib) and anti-CD30 monoclonal antibodies (eg, rituximab, obinutuzumab).
HIV-negative children with talaromycosis should be suspected to have an underlying immunodeficiency syndrome if secondary immunosuppression has been excluded. Talaromycosis has been reported in children with:
- Hyper-IgM syndrome
- Hyper-IgE syndrome (Job syndrome)
- Severe combined immunodeficiency disease (SCID)
- Congenital neutropenia
- Common variable immunodeficiency disease (CVID).
It has been suggested there may be a genetic susceptibility to infection with HLA-associations reported particularly in people of Southeast Asian descent.
Talaromycosis prevention
Your doctor might prescribe medicine to prevent talaromycosis in high-risk individuals. This includes people with weakened immune systems and who live in places where the fungus is present. Itraconazole is the most commonly used anti-fungal medicine to prevent talaromycosis. However, your doctor also can prescribe other antifungal medicines.
Exposure prevention
Two case-controls studies in Thailand and Vietnam demonstrated that people with World Health Organization Stage 4 HIV disease or a CD4 count <100 cells/mm³ who had an occupational exposure to plants and farmed animals were at increased risk for infection 41, 42. The risk was higher in the rainy and humid months 45, 35.
Residency or a history of traveling to the highland regions (as short as 3 days) was a risk factor for talaromycosis in people with advanced HIV disease in southern Vietnam 41. These data suggest that people with advanced HIV should avoid visiting the areas where talaromycosis is highly endemic, particularly highland regions during the rainy and humid months.
Primary prophylaxis
Primary prophylaxis is only recommended for people with HIV with CD4 counts <100 cells/mm³ who reside in the highly endemic regions in northern Thailand, southern China, and northern and southern Vietnam who are unable to have antiretroviral therapy (ART) for whatever reasons or have treatment failure without access to effective antiretroviral options. The drug choices for prophylaxis are oral itraconazole 200 mg once daily or oral fluconazole 400 mg once weekly.
Primary prophylaxis is not recommended in people with HIV who are on or about to start effective antiretroviral therapy (ART) and is not recommended in geographic areas outside of the mentioned highly endemic regions.
For people with HIV who are from the United States and from countries outside of the endemic region who are not on effective antiretroviral therapy (ART), have a CD4 count <100 cells/mm³, and must travel to the highly-endemic areas mentioned, primary prophylaxis with either itraconazole or fluconazole should begin 3 days prior to travel to allow serum drug level to reach steady state and may continue for 1 week after travel.
For individuals residing in endemic areas
- Preferred therapy: Itraconazole 200 mg orally once daily.
- Alternative therapy: Fluconazole 400 mg orally once weekly.
For individuals traveling to endemic areas
- Preferred therapy: Begin itraconazole 200 mg orally once daily 3 days before travel and continue for 1 week after leaving the endemic area.
- Alternative therapy: Begin fluconazole 400 mg 3 days before travel, then continue 400 mg once weekly while in the area and take final dose after leaving the endemic area.
Indication for Discontinuing Primary Prophylaxis for People Who Reside in Endemic Areas
- CD4 count >100 cells/mm³ for ≥6 months in response to antiretroviral therapy (ART).
- Viral load suppression for ≥6 months on antiretroviral therapy (ART).
Indication for Restarting Primary Prophylaxis
CD4 count decreases to <100 cells/mm³ and the person still resides in or travels to high-risk areas. Primary prophylaxis for travelers may begin 3 days prior to travel to allow serum drug level to reach steady state and may continue for 1 week after travel.
Primary prophylaxis has been shown to reduce the incidence of talaromycosis and other invasive fungal infections. A double-blind, placebo-controlled trial in Chiang Mai, Thailand, demonstrated that oral itraconazole 200 mg daily for primary prophylaxis significantly reduced the occurrence of invasive fungal infections (predominantly cryptococcosis and talaromycosis) in people with HIV with a CD4 count <200 cells/mm³ 54.
In a retrospective study also in Chiang Mai, fluconazole (400 mg weekly) was shown to be as effective as itraconazole (200 mg daily) for primary prophylaxis 55. However, these studies were conducted prior to the widespread use of ART, and had small sample sizes, and a mortality benefit was not observed.
Therefore, primary prophylaxis has not been widely adopted given concerns about long-term toxicity, drug–drug interactions, and costs.
Talaromycosis symptoms
The severity and clinical signs and symptoms of talaromycosis depend on the degree of host immunosuppression 5, 31, 32, 11. Talaromyces marneffei mostly causes mild and localized infections in patients with normal immunity, but it can cause severe disseminated infections with generalized lymph node enlargement (lymphadenopathy), respiratory symptoms, skin lesions, digestive symptoms, enlarged liver and spleen (hepatosplenomegaly) and persistent fever in AIDS patients 33, 11, 24. However, many previous studies have reported that varying clinical signs and symptoms of talaromycosis, including fever, lymphadenopathy, hepatosplenomegaly, malaise, weight loss, skin and soft tissue lesions, cough and shortness of breath (dyspnea), commonly occurred among these non-HIV patients 22, 11. Because of nonspecific clinical signs and symptoms, talaromycosis can be easily misdiagnosed as tuberculosis, histoplasmosis, cryptococcosis and lymphoma in patients with fever and generalized lymphadenopathy 11, 34.
Common symptoms of talaromycosis
Bumps on the skin are a common symptom of talaromycosis. They are usually small and painless. The bumps usually appear on the face and neck but can also appear in other places on the body.
Other symptoms of talaromycosis include:
- Fever
- General discomfort
- Weight loss
- Cough or shortness of breath (dyspnea)
- Swollen lymph nodes (lymphadenopathy), liver, or spleen (hepatosplenomegaly)
- Diarrhea
- Abdominal pain
Talaromyces marneffei fungus can make people sick weeks to years after they come in contact with it.
Talaromycosis in people who have HIV
In people with advanced HIV disease, talaromycosis is more likely to spread to the bloodstream and other parts of the body (disseminated infection). Skin bumps, fever and swelling of the spleen are also more common symptoms in people with HIV. Skin bumps have a slightly different appearance and have a dent in the middle.
The infection frequently begins as a subacute illness characterized by fever, weight loss, hepatosplenomegaly, lymphadenopathy, and respiratory and gastrointestinal abnormalities 35, 56. These clinical features are nonspecific and are indistinguishable from those of disseminated tuberculosis, other systemic mycoses, or infections due to intracellular pathogens such as Salmonella species.
Skin lesions are the most specific but late manifestations of talaromycosis, with central-necrotic papules on the face, trunk, and extremities occurring in 40% to 70% of patients 22, 35, 34. Lung involvement manifested as cough or shortness of breath occurs in 40% of patients. Gastrointestinal involvement presenting as diarrhea or abdominal pain occurs in 30% of patients. Significant hepatosplenomegaly is present in 70% of patients and together with intra-abdominal lymphadenopathy cause abdominal distention and pain 35, 50. Meningoencephalitis is a rare manifestation that occurs in <1% of patients and has a rapid disease course with a mortality of 80% 57. Concurrent infections with other opportunistic pathogens occur in up to 60% of patients, with oropharyngeal candidiasis being the most common 25.
Talaromycosis in people who do not have HIV
Talaromycosis more commonly affects the mouth, throat, lungs, liver, and bone in people who do not have HIV. However it is still possible for infections to spread into the blood and other parts of hte body. Skin bumps are smooth without indentation in people with talaromycosis who do not have HIV.
Talaromycosis complications
Diagnosis of talaromycosis is often delayed, particularly in HIV-negative patients from non-endemic countries. Talaromycosis is rapidly progressive once the infection has disseminated.
Disseminated talaromycosis can involve the bone marrow, causing anaemia and thrombocytopenia, and the bones and joints, resulting in osteolytic lesions. In HIV-positive patients, talaromycosis is often associated with other opportunistic infections, such as tuberculous and non-tuberculous mycobacteria, which may obscure and delay diagnosis.
Talaromycosis diagnosis
Talaromycosis can be diagnosed by using a sample from the body part that is affected. For example:
- Bone marrow
- Blood
- Fluid in and around the lungs
- Lymph node
- Skin
The sample is sent to a laboratory for a fungal culture or to be examined under the microscope. The sample can also be tested for the presence of a protein or DNA of the Talaromyces marneffei fungus.
The current diagnostic methods for talaromycosis are still based on conventional microscopy, histology, and culture. Culture results usually return within 4 to 5 days but can take up to 28 days. Diagnostic delay, particularly in patients presenting without fever or skin lesions, is associated with increased mortality 11, 24, 25, 35. Antigen detection and polymerase chain reaction (PCR)–based methods are promising rapid diagnostics currently being evaluated.
A presumptive diagnosis of talaromycosis can be made based on the microscopic examination of Giemsa-, Wright-, or Gomori Methenamine Silver (GMS)–stained samples of skin lesion scrapings, lymph node aspirate, bone marrow aspirate, or tissue sections showing round-to-oval extracellular and intramacrophage yeast-like organisms measuring 3 to 6 µm in diameter. Identification of a clear midline septum in a dividing yeast cell is what distinguishes Talaromyces marneffei fungus from Histoplasma or Candida species 22. In some patients, Talaromyces marneffei fungus can be identified by microscopic examination of a Wright’s-stained peripheral blood smear 58.
A definitive diagnosis of talaromycosis can be made by the histopathologic demonstration of the organism in biopsy specimens. There are three histopathological forms. The granulomatous reaction is formed by histiocytes, lymphocytes, and plasma; epithelioid and giant cells and can be seen in reticuloendothelial organs in patients who are HIV-negative or immunocompetent. The suppurative reaction develops with the joining of multiple abscesses seen in the lung and subcutaneous tissues of immunocompetent patients. The anergic and necrotizing reaction is characterized by focal necrosis surrounded by distended histiocytes containing proliferating fungi seen in the lung, liver, and spleen of immunocompromised patients 7.
Most frequently, a definitive diagnosis of talaromycosis is based on isolation of the organism from cultures of clinical specimens.
Compared to other endemic dimorphic fungi, Talaromyces marneffei fungus grows more readily in standard BACTEC blood culture media and Sabouraud dextrose agar but takes 5 to 14 days to grow and to demonstrate temperature dimorphism. At 25 ºC to 30 ºC, the fungus grows as a mold, producing yellow-green colonies with sulcate folds and a red diffusible pigment in the media. Microscopically, filamentous hyphae with characteristic spore-bearing structures called conidiophores and conidia can be seen. At 32 ºC to 37 ºC, the fungus makes the morphological transition from a mold to a yeast, producing tan-colored colonies without a red diffusible pigment. In laboratory media, only the transitional sausage-shaped cells can be seen microscopically. The round-to-oval yeast cells are only seen in natural tissue 22.
Culture yield is the highest from bone marrow (100%), followed by skin lesions (90%) and blood (70%) 35, 59. Less commonly, talaromycosis has been diagnosed from sputum, pleural fluid, peritoneal fluid, cerebrospinal fluid, pericardium fluid, stool, and urine.
Common laboratory findings associated with talaromycosis include anemia and thrombocytopenia due to bone marrow infiltration. Anemia can be profound and may require multiple red cell transfusions. Elevation of aminotransferase is common, with a serum aspartate aminotransferase (AST) over alanine aminotransferase (ALT) ratio of approximately 2 35.
The median CD4 count in multiple cohorts is <50 cells/mm³ 25, 35.
The chest radiographical findings are broad, ranging from diffuse interstitial disease to reticulonodular infiltrates to alveola infiltrates causing respiratory failure 60.
Talaromycosis differential diagnosis
The differential diagnosis of the skin lesions of talaromycosis depends on the lesion morphology.
- Umbilicated papules are seen in molluscum contagiosum and other fungal infections, such as histoplasmosis and cryptococcosis.
- A subcutaneous nodule may be an epidermoid cyst, lipoma, mycetoma, or other deep atypical infection.
- Verrucous lesions may be viral warts, cutaneous tuberculosis, sporotrichosis, or chromoblastomycosis.
Intracellular fungi on histology may be confused with histoplasmosis.
Talaromycosis treatment
Talaromycosis must be treated with prescription antifungal medicine. The most common treatments are amphotericin B and itraconazole. Amphotericin B is given through a vein for two weeks, followed by itraconazole, given by mouth for 10 weeks. Other antifungal medicines that can be used include voriconazole or posaconazole.
Antifungal therapy for talaromycosis is divided into induction, consolidation, and maintenance phases. The treatment recommendations are based on several observational studies in Thailand and China and the recent Itraconazole versus Amphotericin B for talaromycosis randomized, controlled trial in Vietnam 15, 61, 62, 63, 64.
Preferred therapy
- Induction therapy
- Liposomal amphotericin B 3–5 mg/kg/day IV for 2 weeks, followed by
- Consolidation therapy
- Itraconazole 200 mg oral twice daily for 10 weeks, followed by
- Maintenance therapy or secondary prophylaxis
- Itraconazole 200 mg oral daily
Alternative Therapy (If Liposomal Amphotericin B Is Not Available)
- Induction therapy
- Deoxycholate amphotericin B 0.7 mg/kg/day IV for 2 weeks, followed by
- Consolidation therapy
- Itraconazole 200 mg oral twice daily for 10 weeks, followed by
- Maintenance therapy or secondary prophylaxis
- Itraconazole 200 mg oral daily
Alternative Therapy (If Amphotericin B Is Not Available)
- Induction therapy
- Voriconazole 6 mg/kg IV every 12 hours for 1 day (loading dose) and then voriconazole 4 mg/kg IV every 12 hours for 2 weeks, or
- Oral voriconazole 600 mg every 12 hours on day 1 (loading dose) and then voriconazole 400 mg oral every 12 hours for 2 weeks; followed by
- Consolidation therapy
- Voriconazole 200 mg oral twice daily, or
- Itraconazole 200 mg oral twice daily for a maximum of 10 weeks; followed by
- Maintenance therapy or secondary prophylaxis
- Itraconazole 200 mg oral daily
Note: Itraconazole is not recommended as induction therapy for talaromycosis.
Criteria for Discontinuing Chronic Maintenance Therapy
- CD4 count >100 cells/mm³ for ≥6 months in response to antiretroviral (ART)
- Virologic suppression for ≥6 months on antiretroviral (ART)
Criteria for Restarting Chronic Maintenance Therapy
- CD4 count decreases to <100 cells/mm³
In an earlier noncomparative prospective study of 74 patients in Thailand, induction therapy with deoxycholate amphotericin B for 2 weeks followed by consolidation therapy with itraconazole for 10 weeks was shown to be highly effective. Treatment success rate (defined by negative blood culture and resolution of fever and skin lesions at the end of a 12-week treatment course) was 97% 61.
Voriconazole has been used for induction therapy in patients who could not tolerate amphotericin B and was shown to have favorable clinical and microbiological outcomes in 8 of 9 patients in Thailand 63 and 10 of 14 patients in China 62.
The Itraconazole versus Amphotericin B for talaromycosis randomized trial of 440 patients across 5 hospitals in Vietnam and demonstrated that induction therapy with amphotericin B was superior to itraconazole with respect to 6-month mortality (absolute risk of death was 11% and 21%, respectively; hazard ratio of death in the itraconazole arm was 1.88 15. Patients in the amphotericin B arm had significantly lower rates of disease complications, including disease relapse and immune reconstitution inflammatory syndrome (IRIS), and had a fourfold faster rate of blood fungal clearance. The difference in mortality between the arms was not dependent on disease severity (based on positive blood culture, blood fungal count, or requirement for oxygen support at presentation) or by a participant’s immune status (CD4 count <50 cells/mm³ or ≥50 cells/mm³), ART status, or intravenous (IV) drug use 15.
The recommended induction therapy for all patients, regardless of disease severity, is amphotericin B, preferably liposomal amphotericin B 3 to 5 mg/kg/day where available, or deoxycholate amphotericin B 0.7 mg/kg body weight/day, IV for 2 weeks.
Induction therapy should be followed by consolidation therapy with oral itraconazole, 200 mg every 12 hours for a subsequent duration of 10 weeks 15. After this period, maintenance therapy (or secondary prophylaxis) with oral itraconazole 200 mg/day is recommended to prevent recurrence until the CD4 count rises above 100 cells/mm3 for ≥6 months 63.
For patients who are unable to tolerate any form of amphotericin, induction therapy with IV voriconazole 6 mg/kg every 12 hours on Day 1 (loading dose), then 4 mg/kg every 12 hours or with oral voriconazole 600 mg every 12 hours on Day 1 (loading dose), then 400 mg every 12 hours for 2 weeks is recommended 62, 63.
Thereafter, either oral voriconazole or oral itraconazole 200 mg every 12 hours can be used for consolidation therapy for 10 weeks, followed by itraconazole 200 mg/day for secondary prophylaxis. The optimal dose of voriconazole for secondary prophylaxis beyond 12 weeks has not been studied.
Itraconazole is not recommended as an induction therapy for talaromycosis, regardless of disease severity 15.
Monitoring of response to therapy and side effects
Patients treated with amphotericin B should be monitored for infusion-related adverse reactions (fever, rigors, nausea, vomiting), electrolyte disturbances (particularly hypokalemia and hypomagnesemia), nephrotoxicity (rise in creatinine), and anemia. Hydration with 500 mL to 1,000 mL of normal saline and potassium supplementation before each amphotericin B infusion reduces the risk of nephrotoxicity during treatment. Infusion-related adverse reactions can be ameliorated by pre-treatment with acetaminophen and diphenhydramine.
Drug-Drug Interactions and Therapeutic Drug Monitoring
Itraconazole and voriconazole and antiretroviral (ART) drugs—such as protease inhibitors (PIs), some integrase strand transfer inhibitors, and non-nucleoside reverse transcriptase inhibitors can have bidirectional interactions with each other, leading to increased or decreased drug concentrations. Close monitoring is recommended when using these drugs together.
In settings where therapeutic drug monitoring is available, serum itraconazole and voriconazole levels should be obtained in all patients to ensure adequate drug exposure. This is because itraconazole and voriconazole can interact with some antiretroviral (ART) drugs and absorption of itraconazole can be erratic, and because of the extensive interindividual variability and nonlinear pharmacokinetics of voriconazole. The target serum trough concentration should be >0.5 μg/mL for itraconazole and >1 μg/mL for voriconazole. Because it is more bioavailable, itraconazole solution is preferred over the capsule formulation.
Prevention and Management of Immune Reconstitution Inflammatory Syndrome (IRIS)
Both unmasking and paradoxical Immune Reconstitution Inflammatory Syndrome (IRIS) have been described in patients with talaromycosis when ART is initiated 65, 66, 67. In the Itraconazole versus Amphotericin B for talaromycosis randomized, controlled trial in Vietnam, 188 of 432 (44%) patients had started ART a median of 3 to 4 months before developing talaromycosis, indicating the role of ART in the unmasking of subclinical infection in a significant proportion of patients 15. This finding highlights the need for a sensitive assay to screen for subclinical infection and the importance of pre-emptive antifungal therapy to prevent disease and unmasking Immune Reconstitution Inflammatory Syndrome (IRIS). In patients starting ART after a diagnosis of talaromycosis, paradoxical IRIS events only occurred in patients treated with itraconazole induction therapy, demonstrating the role of effective induction therapy with amphotericin B in the prevention of paradoxical IRIS 15. ART should not be withheld because of concerns for possible development of Immune Reconstitution Inflammatory Syndrome (IRIS).
Patients with paradoxical IRIS typically present with inflammatory manifestations that include erythematous or immunological skin lesions such as erythema nodosum, as well as large and painful peripheral lymph nodes and synovitis of small joints. Most symptoms can be managed by judicious use of nonsteroid anti-inflammatory medicine. Corticosteroids are reserved for synovitis that interferes with daily function 67. Although the IRIS events in the Itraconazole versus Amphotericin B for talaromycosis randomized, controlled trial in Vietnam were not associated with increased mortality and were managed effectively with continuation of ART and antifungal therapy, they were associated with higher morbidity, including lower quality of life and increased diagnostic testing, duration of hospitalization, and cost 15.
Treatment Failure and Relapse
Talaromycosis treatment failure and disease relapse were associated with ineffective induction therapy with itraconazole, highlighting the importance of amphotericin B induction therapy 15. On the basis of case series that included very few patients and on clinical experiences, voriconazole is an alternative therapy for patients who are unable to tolerate amphotericin B treatment.
Disease relapse is associated with higher mortality and occurs mainly in patients who are not adherent to ART or have virologic failure, as well as in those who are not adherent to itraconazole consolidation or maintenance therapy 15. Therapy adherence counseling and therapeutic drug monitoring for itraconazole and voriconazole, if available, are recommended.
Special Considerations During Pregnancy
The diagnosis and treatment of talaromycosis during pregnancy is similar to that in nonpregnant adults, with the following considerations regarding antifungal use in pregnancy. Amphotericin B has not been shown to be teratogenic in animals, and no increase in fetal anomalies has been seen with its use in humans. Neonates born to people on chronic amphotericin B at delivery should be evaluated for renal dysfunction and hypokalemia.
Itraconazole at high doses has been shown to be teratogenic in animals, but because humans lack the metabolic mechanism accounting for these defects, the animal teratogenicity data are not applicable to humans. Case series in humans do not suggest an increased risk of birth defects with itraconazole, but experience is very limited 68.
Voriconazole is Food and Drug Administration Category D because of teratogenicity (cleft palate and renal defects) seen in rats and embryotoxicity in rabbits. No human data on use of voriconazole are available, so use in the first trimester is not recommended.
Substitution of amphotericin B for high-dose azoles in the first trimester is recommended. People on secondary prophylaxis with itraconazole or other azoles should postpone pregnancy until their CD4 counts have been restored with ART, such that prophylaxis can be discontinued. If a person becomes pregnant while receiving itraconazole prophylaxis, the decision as to whether to continue should be individualized based on current CD4 count and viral suppression and patient preference.
Talaromycosis prognosis
Disseminated talaromycosis is fatal if untreated 69. Talaromycosis-related mortality, despite antifungal therapy in people both with and without HIV, is up to 30% (children 55%, HIV-negative adults 30%, and HIV-positive adults 20%) 25, 35, 8, 11, 36, 37.
- Ying, R., Le, T., Cai, W., Li, Y., Luo, C., Cao, Y., Wen, C., Wang, S., Ou, X., Chen, W., Chen, S., Guo, P., Chen, M., Guo, Y., Tang, X. and Li, L. (2020), Clinical epidemiology and outcome of HIV-associated talaromycosis in Guangdong, China, during 2011–2017. HIV Med., 21: 729-738. https://doi.org/10.1111/hiv.13024[↩]
- Wang M, Zhang Z, Yan J, Shi J, Liu S, Wan H. The Presence of Secondary Evans Syndrome in AIDS Patients with Talaromyces marneffei Infection. Infect Drug Resist. 2021 Mar 29;14:1265-1271. doi: 10.2147/IDR.S300082[↩]
- Pan M, Qiu Y, Zeng W, Tang S, Feng X, Deng J, Wei X, He Z, Zhang J. Talaromycosis-Associated Secondary Hemophagocytic Lymphohistiocytosis in Nine Human Immunodeficiency Virus-Negative Patients: A Multicenter Retrospective Study. Infect Drug Resist. 2019 Dec 4;12:3807-3816. doi: 10.2147/IDR.S232713[↩]
- Vergidis P, Rao A, Moore CB, et al. Talaromycosis in a renal transplant recipient returning from South China. Transpl Infect Dis. 2021; 23:e13447. https://doi.org/10.1111/tid.13447[↩]
- Cao C, Xi L, Chaturvedi V. Talaromycosis (Penicilliosis) Due to Talaromyces (Penicillium) marneffei: Insights into the Clinical Trends of a Major Fungal Disease 60 Years After the Discovery of the Pathogen. Mycopathologia. 2019 Dec;184(6):709-720. doi: 10.1007/s11046-019-00410-2[↩][↩][↩]
- Chen ZM, Li ZT, Li SQ, Guan WJ, Qiu Y, Lei ZY, Zhan YQ, Zhou H, Lin S, Wang X, Li Z, Yang F, Zeng W, Lin Y, Liu J, Zhang JQ, Ye F. Clinical findings of Talaromyces marneffei infection among patients with anti-interferon-γ immunodeficiency: a prospective cohort study. BMC Infect Dis. 2021 Jun 19;21(1):587. doi: 10.1186/s12879-021-06255-9[↩]
- Deng Z, Ribas JL, Gibson DW, Connor DH. Infections caused by Penicillium marneffei in China and Southeast Asia: review of eighteen published cases and report of four more Chinese cases. Rev Infect Dis. 1988 May-Jun;10(3):640-52. doi: 10.1093/clinids/10.3.640[↩][↩]
- Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated Penicillium marneffei infection in southeast Asia. Lancet. 1994 Jul 9;344(8915):110-3. doi: 10.1016/s0140-6736(94)91287-4[↩][↩][↩][↩]
- Duong TA. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis. 1996 Jul;23(1):125-30. doi: 10.1093/clinids/23.1.125[↩]
- Hien TV, Loc PP, Hoa NT, Duong NM, Quang VM, McNeil MM, Dung NT, Ashford DA. First cases of disseminated penicilliosis marneffei infection among patients with acquired immunodeficiency syndrome in Vietnam. Clin Infect Dis. 2001 Feb 15;32(4):e78-80. doi: 10.1086/318703[↩]
- Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. 2016 Mar 9;5(3):e19. doi: 10.1038/emi.2016.18[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Ranjana KH, Priyokumar K, Singh TJ, Gupta ChC, Sharmila L, Singh PN, Chakrabarti A. Disseminated Penicillium marneffei infection among HIV-infected patients in Manipur state, India. J Infect. 2002 Nov;45(4):268-71. doi: 10.1053/jinf.2002.1062[↩]
- Segretain G. Description d’une nouvelle espèce de Penicillium: Penicillium marneffei n.sp. Bull Soc Mycol Fr. 1959;75:412–6.[↩]
- Tsang, CC., Lau, S.K.P. & Woo, P.C.Y. Sixty Years from Segretain’s Description: What Have We Learned and Should Learn About the Basic Mycology of Talaromyces marneffei?. Mycopathologia 184, 721–729 (2019). https://doi.org/10.1007/s11046-019-00395-y[↩]
- Le T, Kinh NV, Cuc NTK, Tung NLN, Lam NT, Thuy PTT, Cuong DD, Phuc PTH, Vinh VH, Hanh DTH, Tam VV, Thanh NT, Thuy TP, Hang NT, Long HB, Nhan HT, Wertheim HFL, Merson L, Shikuma C, Day JN, Chau NVV, Farrar J, Thwaites G, Wolbers M; IVAP Investigators. A Trial of Itraconazole or Amphotericin B for HIV-Associated Talaromycosis. N Engl J Med. 2017 Jun 15;376(24):2329-2340. doi: 10.1056/NEJMoa1613306[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Pruksaphon K, Intaramat A, Ratanabanangkoon K, Nosanchuk JD, Vanittanakom N, Youngchim S. Development and characterization of an immunochromatographic test for the rapid diagnosis of Talaromyces (Penicillium) marneffei. PLoS One. 2018 Apr 11;13(4):e0195596. doi: 10.1371/journal.pone.0195596[↩]
- Lei HL, Li LH, Chen WS, Song WN, He Y, Hu FY, Chen XJ, Cai WP, Tang XP. Susceptibility profile of echinocandins, azoles and amphotericin B against yeast phase of Talaromyces marneffei isolated from HIV-infected patients in Guangdong, China. Eur J Clin Microbiol Infect Dis. 2018 Jun;37(6):1099-1102. doi: 10.1007/s10096-018-3222-x[↩]
- Jiang J, Meng S, Huang S, Ruan Y, Lu X, Li JZ, Wu N, Huang J, Xie Z, Liang B, Deng J, Zhou B, Chen X, Ning C, Liao Y, Wei W, Lai J, Ye L, Wu F, Liang H. Effects of Talaromyces marneffei infection on mortality of HIV/AIDS patients in southern China: a retrospective cohort study. Clin Microbiol Infect. 2019 Feb;25(2):233-241. doi: 10.1016/j.cmi.2018.04.018[↩][↩]
- Wu TC, Chan JW, Ng CK, Tsang DN, Lee MP, Li PC. Clinical presentations and outcomes of Penicillium marneffei infections: a series from 1994 to 2004. Hong Kong Med J. 2008 Apr;14(2):103-9.[↩]
- Antinori S, Gianelli E, Bonaccorso C, Ridolfo AL, Croce F, Sollima S, Parravicini C. Disseminated Penicillium marneffei infection in an HIV-positive Italian patient and a review of cases reported outside endemic regions. J Travel Med. 2006 May-Jun;13(3):181-8. doi: 10.1111/j.1708-8305.2006.00039.x[↩][↩][↩]
- Cristofaro P, Mileno MD. Penicillium marneffei infection in HIV-infected travelers. AIDS Alert. 2006 Dec;21(12):140-2.[↩][↩][↩]
- Vanittanakom N, Cooper CR Jr, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006 Jan;19(1):95-110. doi: 10.1128/CMR.19.1.95-110.2006[↩][↩][↩][↩][↩][↩][↩]
- Wong SSY, Siau H, Yuen KY. Penicilliosis marneffei–West meets East. J Med Microbiol. 1999 Nov;48(11):973-975. doi: 10.1099/00222615-48-11-973[↩][↩]
- Zheng J, Gui X, Cao Q, Yang R, Yan Y, Deng L, Lio J. A Clinical Study of Acquired Immunodeficiency Syndrome Associated Penicillium Marneffei Infection from a Non-Endemic Area in China. PLoS One. 2015 Jun 17;10(6):e0130376. doi: 10.1371/journal.pone.0130376[↩][↩][↩][↩]
- Hu Y, Zhang J, Li X, Yang Y, Zhang Y, Ma J, Xi L. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia. 2013 Feb;175(1-2):57-67. doi: 10.1007/s11046-012-9577-0[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Ching-López R, Rodríguez Pavón S. Talaromycosis in a Lung Cancer Patient: A Rare Case. Cureus. 2020 Sep 23;12(9):e10615. doi: 10.7759/cureus.10615[↩][↩]
- Wang YG, Cheng JM, Ding HB, Lin X, Chen GH, Zhou M, Ye SN. Study on the Clinical Features and Prognosis of Penicilliosis marneffei Without Human Immunodeficiency Virus Infection. Mycopathologia. 2018 Jun;183(3):551-558. doi: 10.1007/s11046-017-0236-3[↩]
- Chen D, Chang C, Chen M, Zhang Y, Zhao X, Zhang T, Wang Z, Yan J, Zhu H, Zheng L, Zhao K. Unusual disseminated Talaromyces marneffei infection mimicking lymphoma in a non-immunosuppressed patient in East China: a case report and review of the literature. BMC Infect Dis. 2020 Oct 28;20(1):800. doi: 10.1186/s12879-020-05526-1[↩]
- Peng J, Chen Z, Cai R, Huang X, Lin L, Liang W, Xiong Z, Chen J, Chen H, Yang Y, Liu S, Jiang Q. Recovery from Talaromyces marneffei involving the kidney in a renal transplant recipient: A case report and literature review. Transpl Infect Dis. 2017 Aug;19(4). doi: 10.1111/tid.12710[↩]
- Nga TV, Parry CM, Le T, Lan NP, Diep TS, Campbell JI, Hoang NV, Dung le T, Wain J, Dolecek C, Farrar JJ, Chau NV, Hien TT, Day JN, Baker S. The decline of typhoid and the rise of non-typhoid salmonellae and fungal infections in a changing HIV landscape: bloodstream infection trends over 15 years in southern Vietnam. Trans R Soc Trop Med Hyg. 2012 Jan;106(1):26-34. doi: 10.1016/j.trstmh.2011.10.004[↩][↩]
- Vanittanakom N, Cooper CR, Jr, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95–110. doi: 10.1128/CMR.19.1.95-110.2006[↩][↩]
- Castro-Lainez MT, Sierra-Hoffman M, LLompart-Zeno J, Adams R, Howell A, Hoffman-Roberts H, Fader R, Arroliga AC, Jinadatha C. Talaromyces marneffei infection in a non-HIV non-endemic population. IDCases. 2018 Mar 3;12:21-24. doi: 10.1016/j.idcr.2018.02.013[↩][↩][↩]
- Yap FB, Thevarajah S, Asmah J. Penicillium marneffei infection in an African man. Dermatol Online J. 2010 Jul 15;16(7):2. https://escholarship.org/uc/item/6j96f72f[↩][↩]
- Chen J, Zhang R, Shen Y, Liu L, Qi T, Wang Z, Song W, Tang Y, Lu H. Clinical Characteristics and Prognosis of Penicilliosis Among Human Immunodeficiency Virus-Infected Patients in Eastern China. Am J Trop Med Hyg. 2017 Jun;96(6):1350-1354. doi: 10.4269/ajtmh.16-0521[↩][↩][↩][↩]
- Le T, Wolbers M, Chi NH, Quang VM, Chinh NT, Lan NP, Lam PS, Kozal MJ, Shikuma CM, Day JN, Farrar J. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin Infect Dis. 2011 Apr 1;52(7):945-52. doi: 10.1093/cid/cir028[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Son VT, Khue PM, Strobel M. Penicilliosis and AIDS in Haiphong, Vietnam: evolution and predictive factors of death. Med Mal Infect. 2014 Dec;44(11-12):495-501. doi: 10.1016/j.medmal.2014.09.008[↩][↩]
- Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis. 2013 Oct 5;13:464. doi: 10.1186/1471-2334-13-464[↩][↩]
- Talaromyces marneffei. https://www.adelaide.edu.au/mycology/fungal-descriptions-and-antifungal-susceptibility/hyphomycetes-conidial-moulds/talaromyces[↩]
- Talaromycosis (Penicilliosis) Basics. https://www.cdc.gov/talaromycosis/about/[↩][↩]
- Cao, C., Xi, L. & Chaturvedi, V. Talaromycosis (Penicilliosis) Due to Talaromyces (Penicillium) marneffei: Insights into the Clinical Trends of a Major Fungal Disease 60 Years After the Discovery of the Pathogen. Mycopathologia 184, 709–720 (2019). https://doi.org/10.1007/s11046-019-00410-2[↩]
- Le T, Jonat B, Kim Cuc N, al E. The exposure and geospatial risk factors for AIDS-associated penicilliosis in Vietnam. Presented at: Conference on Retroviruses and Opportunistic Infections; 2015; Seattle, WA.[↩][↩][↩][↩]
- Chariyalertsak S, Sirisanthana T, Supparatpinyo K, Praparattanapan J, Nelson KE. Case-control study of risk factors for Penicillium marneffei infection in human immunodeficiency virus-infected patients in northern Thailand. Clin Infect Dis. 1997 Jun;24(6):1080-6. doi: 10.1086/513649[↩][↩][↩]
- Cao C, Liang L, Wang W, Luo H, Huang S, Liu D, Xu J, Henk DA, Fisher MC. Common reservoirs for Penicillium marneffei infection in humans and rodents, China. Emerg Infect Dis. 2011 Feb;17(2):209-14. doi: 10.3201/eid1702.100718[↩]
- Huang X, He G, Lu S, Liang Y, Xi L. Role of Rhizomys pruinosus as a natural animal host of Penicillium marneffei in Guangdong, China. Microb Biotechnol. 2015 Jul;8(4):659-64. doi: 10.1111/1751-7915.12275[↩]
- Chariyalertsak S, Sirisanthana T, Supparatpinyo K, Nelson KE. Seasonal variation of disseminated Penicillium marneffei infections in northern Thailand: a clue to the reservoir? J Infect Dis. 1996 Jun;173(6):1490-3. doi: 10.1093/infdis/173.6.1490[↩][↩]
- Bulterys PL, Le T, Quang VM, Nelson KE, Lloyd-Smith JO. Environmental predictors and incubation period of AIDS-associated penicillium marneffei infection in Ho Chi Minh City, Vietnam. Clin Infect Dis. 2013 May;56(9):1273-9. doi: 10.1093/cid/cit058[↩]
- Wang YF, Xu HF, Han ZG, Zeng L, Liang CY, Chen XJ, Chen YJ, Cai JP, Hao W, Chan JF, Wang M, Fu N, Che XY. Serological surveillance for Penicillium marneffei infection in HIV-infected patients during 2004-2011 in Guangzhou, China. Clin Microbiol Infect. 2015 May;21(5):484-9. doi: 10.1016/j.cmi.2014.12.014[↩]
- Hilmarsdottir I, Coutellier A, Elbaz J, Klein JM, Datry A, Guého E, Herson S. A French case of laboratory-acquired disseminated Penicillium marneffei infection in a patient with AIDS. Clin Infect Dis. 1994 Aug;19(2):357-8. doi: 10.1093/clinids/19.2.357[↩]
- Hermans F, Ombelet S, Degezelle K, Testelmans D, Van Raemdonck DE, Verleden GM, Verbeken EK, Van Bleyenbergh P, Lagrou K, Vos R. First-in-man observation of Talaromyces marneffei-transmission by organ transplantation. Mycoses. 2017 Mar;60(3):213-217. doi: 10.1111/myc.12574[↩]
- Larsson M, Nguyen LH, Wertheim HF, Dao TT, Taylor W, Horby P, Nguyen TV, Nguyen MH, Le T, Nguyen KV. Clinical characteristics and outcome of Penicillium marneffei infection among HIV-infected patients in northern Vietnam. AIDS Res Ther. 2012 Aug 16;9(1):24. doi: 10.1186/1742-6405-9-24[↩][↩]
- Wu TC, Chan JW, Ng CK, Tsang DN, Lee MP, Li PC. Clinical presentations and outcomes of Penicillium marneffei infections: a series from 1994 to 2004. Hong Kong Med J. 2008 Apr;14(2):103-9. https://www.hkmj.org/system/files/hkm0804p103.pdf[↩]
- Feng RF, Ma Y, Liu ZF, Zhang FJ, Yang Y, Huang SB, He HL, Lu J, Lei SY, Zhao HX, Dai LL, He Y. [Specific causes of death among 381 AIDS patients who died in hospitals]. Zhonghua Liu Xing Bing Xue Za Zhi. 2013 Dec;34(12):1237-41. Chinese.[↩]
- Qi T, Zhang R, Shen Y, Liu L, Lowrie D, Song W, Chen J, Wang Z, Shen J, Cai R, Guan L, Luo B, Tang Y, Lu H. Etiology and clinical features of 229 cases of bloodstream infection among Chinese HIV/AIDS patients: a retrospective cross-sectional study. Eur J Clin Microbiol Infect Dis. 2016 Nov;35(11):1767-1770. doi: 10.1007/s10096-016-2724-7[↩]
- Chariyalertsak S, Supparatpinyo K, Sirisanthana T, Nelson KE. A controlled trial of itraconazole as primary prophylaxis for systemic fungal infections in patients with advanced human immunodeficiency virus infection in Thailand. Clin Infect Dis. 2002 Jan 15;34(2):277-84. doi: 10.1086/338154[↩]
- Chaiwarith R, Fakthongyoo A, Praparattanapan J, Boonmee D, Sirisanthana T, Supparatpinyo K. Itraconazole vs fluconazole as a primary prophylaxis for fungal infections in HIV-infected patients in Thailand. Curr HIV Res. 2011 Jul;9(5):334-8. doi: 10.2174/157016211797635991[↩]
- Sirisanthana T. Penicillium marneffei infection in patients with AIDS. Emerg Infect Dis. 2001;7(3 Suppl):561. doi: 10.3201/eid0707.017734[↩]
- Le T, Huu Chi N, Kim Cuc NT, Manh Sieu TP, Shikuma CM, Farrar J, Day JN. AIDS-associated Penicillium marneffei infection of the central nervous system. Clin Infect Dis. 2010 Dec 15;51(12):1458-62. doi: 10.1086/657400[↩]
- Supparatpinyo K, Sirisanthana T. Disseminated Penicillium marneffei infection diagnosed on examination of a peripheral blood smear of a patient with human immunodeficiency virus infection. Clin Infect Dis. 1994 Feb;18(2):246-7. doi: 10.1093/clinids/18.2.246[↩]
- Supparatpinyo K, Chiewchanvit S, Hirunsri P, Uthammachai C, Nelson KE, Sirisanthana T. Penicillium marneffei infection in patients infected with human immunodeficiency virus. Clin Infect Dis. 1992 Apr;14(4):871-4. doi: 10.1093/clinids/14.4.871[↩]
- Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis. 2017 Nov;17(11):e334-e343. doi: 10.1016/S1473-3099(17)30303-1[↩]
- Sirisanthana T, Supparatpinyo K, Perriens J, Nelson KE. Amphotericin B and itraconazole for treatment of disseminated Penicillium marneffei infection in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998 May;26(5):1107-10. doi: 10.1086/520280[↩][↩]
- Ouyang Y, Cai S, Liang H, Cao C. Administration of Voriconazole in Disseminated Talaromyces (Penicillium) Marneffei Infection: A Retrospective Study. Mycopathologia. 2017 Jun;182(5-6):569-575. doi: 10.1007/s11046-016-0107-3[↩][↩][↩]
- Supparatpinyo K, Schlamm HT. Voriconazole as therapy for systemic Penicillium marneffei infections in AIDS patients. Am J Trop Med Hyg. 2007 Aug;77(2):350-3.[↩][↩][↩][↩]
- Supparatpinyo K, Chiewchanvit S, Hirunsri P, Baosoung V, Uthammachai C, Chaimongkol B, Sirisanthana T. An efficacy study of itraconazole in the treatment of Penicillium marneffei infection. J Med Assoc Thai. 1992 Dec;75(12):688-91.[↩]
- Hall C, Hajjawi R, Barlow G, Thaker H, Adams K, Moss P. Penicillium marneffei presenting as an immune reconstitution inflammatory syndrome (IRIS) in a patient with advanced HIV. BMJ Case Rep. 2013 Jan 28;2013:bcr2012007555. doi: 10.1136/bcr-2012-007555[↩]
- Liu X, Wu H, Huang X. Disseminated Penicillium marneffei infection with IRIS. IDCases. 2015 Aug 31;2(4):92-3. doi: 10.1016/j.idcr.2015.08.001[↩]
- Thanh NT, Vinh LD, Liem NT, Shikuma C, Day JN, Thwaites G, Le T. Clinical features of three patients with paradoxical immune reconstitution inflammatory syndrome associated with Talaromyces marneffei infection. Med Mycol Case Rep. 2016 Dec 9;19:33-37. doi: 10.1016/j.mmcr.2016.12.005[↩][↩]
- Pilmis B, Jullien V, Sobel J, Lecuit M, Lortholary O, Charlier C. Antifungal drugs during pregnancy: an updated review. J Antimicrob Chemother. 2015 Jan;70(1):14-22. doi: 10.1093/jac/dku355[↩]
- Supparatpinyo K, Nelson KE, Merz WG, Breslin BJ, Cooper CR Jr, Kamwan C, Sirisanthana T. Response to antifungal therapy by human immunodeficiency virus-infected patients with disseminated Penicillium marneffei infections and in vitro susceptibilities of isolates from clinical specimens. Antimicrob Agents Chemother. 1993 Nov;37(11):2407-11. doi: 10.1128/AAC.37.11.2407[↩]