Contents
- Streptococcus pneumoniae
- Streptococcus pneumoniae causes
- Risk factors for developing pneumococcal disease
- Streptococcus pneumoniae prevention
- Behavior modification and risk factors
- Pneumococcal vaccines
- Pneumococcal conjugate vaccines
- Pneumococcal 13-valent conjugate vaccine (Prevnar 13)
- Pneumococcal 15-valent conjugate vaccine (Vaxneuvance)
- Pneumococcal 20-valent conjugate vaccine (Prevnar20)
- Pneumococcal 21-valent conjugate vaccine (CAPVAXIVE)
- Pneumococcal polysaccharide vaccine (Pneumovax 23)
- Pneumococcal vaccine administration
- Side effects of pneumonia vaccine
- Pneumonia vaccine contraindications
- Which underlying medical conditions indicate that a child age 6 through 18 years should receive additional doses of pneumococcal vaccine beyond the routine schedule?
- What are the different serotypes of Streptococcus pneumoniae targeted by different pneumococcal vaccines?
- How effective are pneumococcal conjugate vaccines at preventing pneumococcal carriage or disease?
- How effective is pneumococcal polysaccharide vaccine at preventing pneumococcal carriage or disease?
- Streptococcus pneumoniae complications
- Streptococcus pneumoniae signs and symptoms
- Streptococcus pneumoniae diagnosis
- Streptococcus pneumoniae treatment
- Streptococcus pneumoniae prognosis
Streptococcus pneumoniae
Streptococcus pneumoniae also known as pneumococcus, is an encapsulated lancet-shaped diplococcus, Gram-positive, catalase-negative, alpha-hemolytic, facultative anaerobic bacteria pathogen of humans that colonizes the upper respiratory tract and causes life-threatening diseases such as pneumonia, bacteremia, and meningitis throughout the world 1, 2. There are 100 known serotypes of Streptococcus pneumoniae with most Streptococcus pneumoniae serotypes have been shown to cause serious disease, but only a few serotypes cause the majority of pneumococcal infections 3. Streptococcus pneumoniae is the most common cause of fatal community-acquired pneumonia in the elderly and is also one of the most common causes of middle ear infections (otitis media) and bacterial meningitis in children, as well as an important cause of sinusitis, bacteremia, septic arthritis, osteomyelitis, peritonitis, and endocarditis 4, 2. In the United States, prior to the widespread use of 7-valent pneumococcal conjugate vaccine (PCV7), the seven most common serotypes isolated from blood or cerebrospinal fluid (CSF) of children younger than age 5 years accounted for 80% of infections; these seven serotypes accounted for about 50% of isolates from older children and adults 3. The prominence of Streptococcus pneumoniae as a cause of disease is due to the combination of high carriage rates, its genetic adaptability and its ability to shift from a commensal to a pathogenic interaction with its host 5.
Streptococcus pneumoniae (pneumococci) commonly inhabit the upper respiratory tract (the nasopharynx) and are usually asymptomatic 6. Streptococcus pneumoniae bacteria may be isolated from the nasopharynx of 5% to 90% of healthy persons, depending on the population age, environment, and the presence of upper respiratory infections 7, 8, 9, 10:
- 5% to 10% of adults without children are carriers
- 20% to 60% of school-aged children may be carriers
- 50% to 60% of service personnel on military installations may be carriers
Risk factors associated with higher rates of Streptococcus pneumoniae carriage include race (particularly Australian Aboriginals and Native Americans) 11, 12, 13, infancy 14, 15, season with higher carriage during winter months 14 and crowded areas such as childcare centers with estimates suggesting 40–60% of children who attend childcare are colonized 16. Pneumococcal infections are more common during the winter and in early spring when respiratory diseases are more prevalent. Duration of colonization decreases with age and varies from 2 weeks to 4 months 17. The introduction of pneumococcal conjugate vaccines has reduced carriage rates for serotypes covered by the vaccine while non-vaccine serotypes have emerged to occupy this empty niche 18.
The duration of carriage varies and is generally longer in children than adults. In addition, researchers do not clearly understand the relationship of carriage to the development of natural immunity 7.
Streptococcus pneumoniae spreads between hosts through aerosol and potentially through the contamination of objects with mucosal secretions if the bacteria is living within a biofilm 19, 20. Streptococcus pneumoniae can cause a wide variety of clinical symptoms owing to its ability to cause disease by either direct extension from the nasopharynx into surrounding anatomic structures or vascular invasion with hematogenous spread. Features that should prompt the clinician to consider pneumococcal infection include the following 2:
- High-risk age groups (children younger than 5 years, particularly aged 2 years or younger; adults older than 55-65 years)
- Conditions that cause immune deficits (eg, HIV infection, malignancy, or diabetes mellitus)
- Conditions associated with decreased pulmonary clearance functions (eg, asthma, chronic bronchitis, or chronic obstructive pulmonary disease [COPD])
- Presentation from late fall to early spring
Conditions that may develop by direct extension of Streptococcus pneumoniae from the nasopharynx include the following 2:
- Conjunctivitis
- Otitis media
- Sinusitis
- Acute exacerbations of chronic bronchitis
- Pneumonia (which may be complicated by purulent pericarditis). In the era before antibiotics, Streptococcus pneumoniae was estimated to be the cause of 95% of all cases of pneumonia. Currently, however, Streptococcus pneumoniae accounts for up to 15% of pneumonia cases in the United States and 27% of cases worldwide today 21. Blood cultures are positive in only 20% to 25% of all pneumonia cases that are caused by Streptococcus pneumoniae making it a challenging diagnosis for the clinician 22.
Conditions that may result from vascular invasion and hematogenous spread of Streptococcus pneumoniae include the following 2:
- Meningitis
- Bacteremia (most common manifestation of invasive pneumococcal disease)
- Joint and bone infections (osteomyelitis and septic arthritis)
- Soft tissue infections (eg, myositis, periorbital cellulitis, abscess)
- Peritonitis
- Cardiac infections (eg, endocarditis)
Invasive pneumococcal disease occurs as a result of the spread of Streptococcus pneumoniae bacteria from the nasopharynx to other parts of the body including the lungs, blood, and brain. Infants, the elderly, and immunocompromised individuals are at an increased risk for developing invasive pneumococcal disease 23, 24. Colonization is a prerequisite for invasive pneumococcal disease and while the incidence of infection is relatively low, high rates of colonization result in extensive morbidity and mortality that is a global concern. Worldwide, it is estimated that Streptococcus pneumoniae is responsible for 15 cases of invasive pneumococcal disease per 100,000 persons per year 25, and over a million deaths annually. As of 2004, in the United States, it is estimated that the pneumococcus was responsible for greater than 1.5 million cases of otitis media and 800,000 cases of pneumonia 26. The World Health Organization (WHO) estimates that close to half a million children under the age of 5 years die annually as a result of Streptococcus pneumoniae infection 27. Pneumococcal bacteremia and meningitis are also responsible for significant mortality particularly in the elderly where rates may be as high as 60% and 80% respectively 28.
In 2017, the WHO included Streptococcus pneumoniae as one of 12 priority pathogens. The continued high burden of pneumococcal disease and rising rates of resistance to penicillin and other antibiotics have renewed interest in prevention. The widespread use of pneumococcal conjugate vaccines (PCVs) has reduced invasive disease of sero-types with the capsular polysaccharide (CPS) types that are included in the pneumococcal vaccine 29. The remarkable capacity of Streptococcus pneumoniae to remodel its genome through the uptake and incorporation of exogenous DNA ( natural competence) from other pneumococci or closely related oral streptococci has facilitated the spread of antibiotic resistance and evasion of vaccine-induced immunity 5.
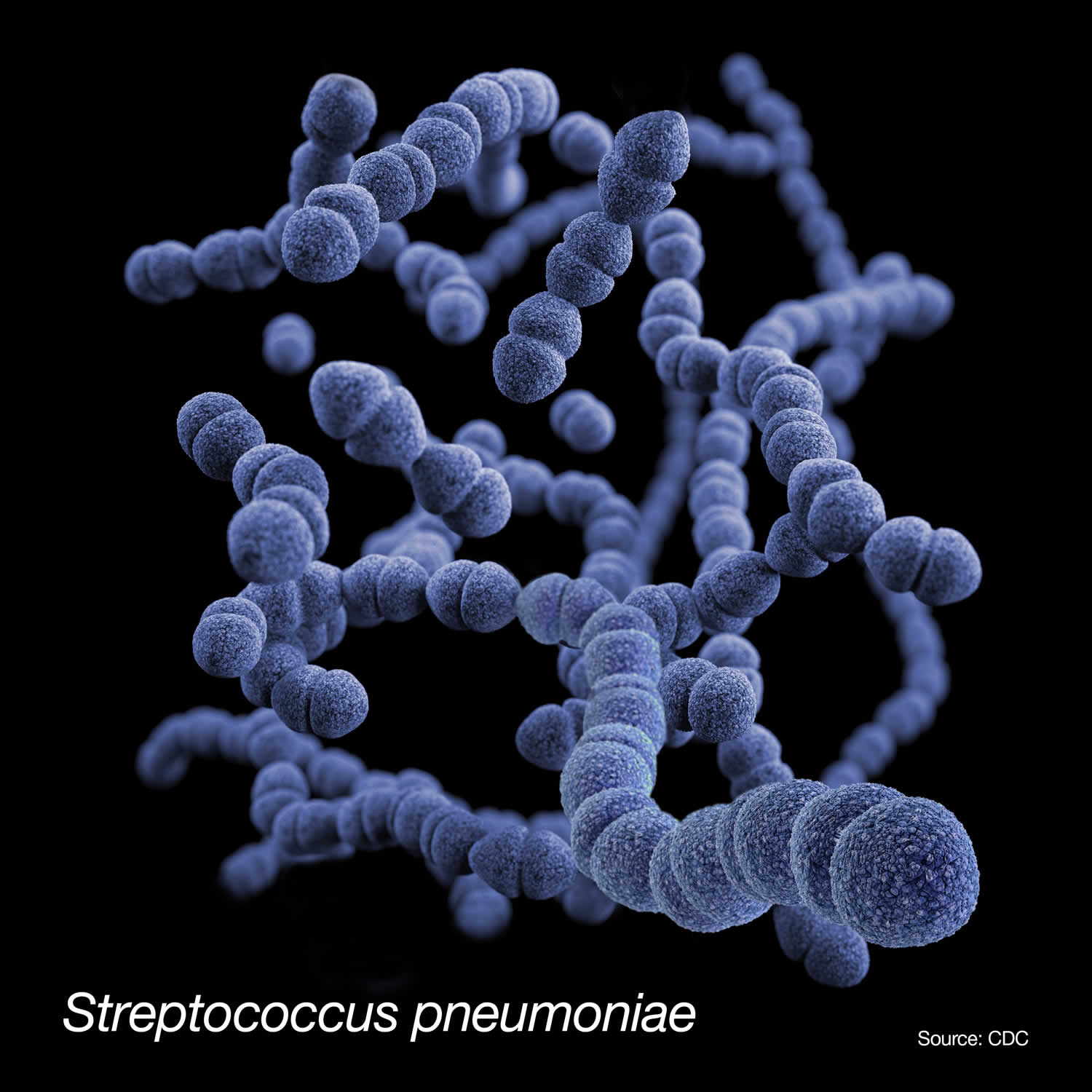
Figure 1. Streptococcus pneumoniae bacteria
Footnote: This is a medical illustration Streptococcus pneumoniae bacteria, presented in the Centers for Disease Control and Prevention (CDC) publication entitled, Antibiotic Resistance Threats in the United States, 2019.
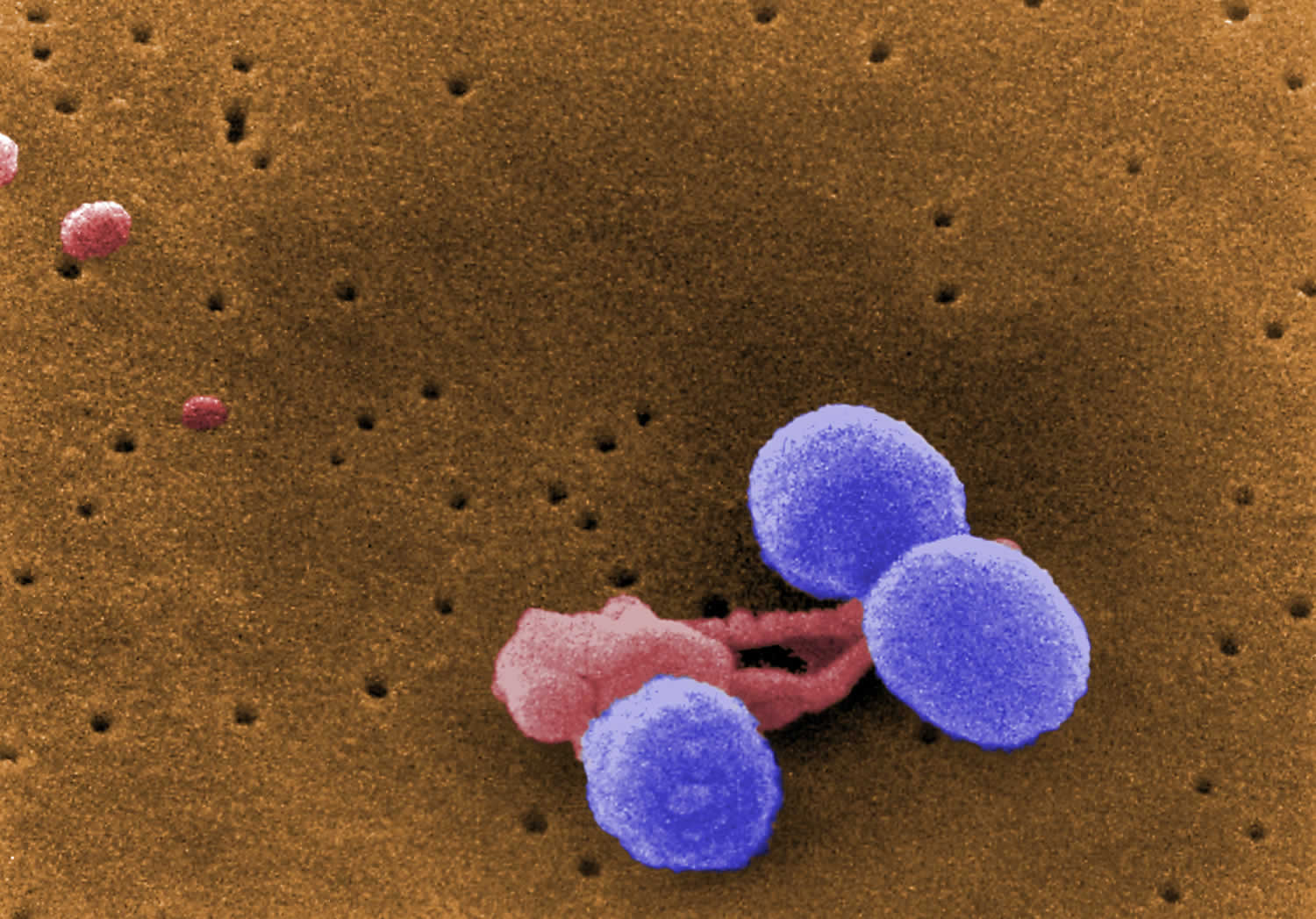
[Source 30 ]Figure 2. Streptococcus pneumoniae
Footnote: This digitally-colorized, scanning electron microscopic (SEM) image depicts what were three, round-shaped, Gram-positive, Streptococcus pneumoniae bacteria (lavender), as they were being attacked by an irregularly-shaped white blood cell (WBC) (pink). Note what appeared to be unidentified organisms in the upper left corner.
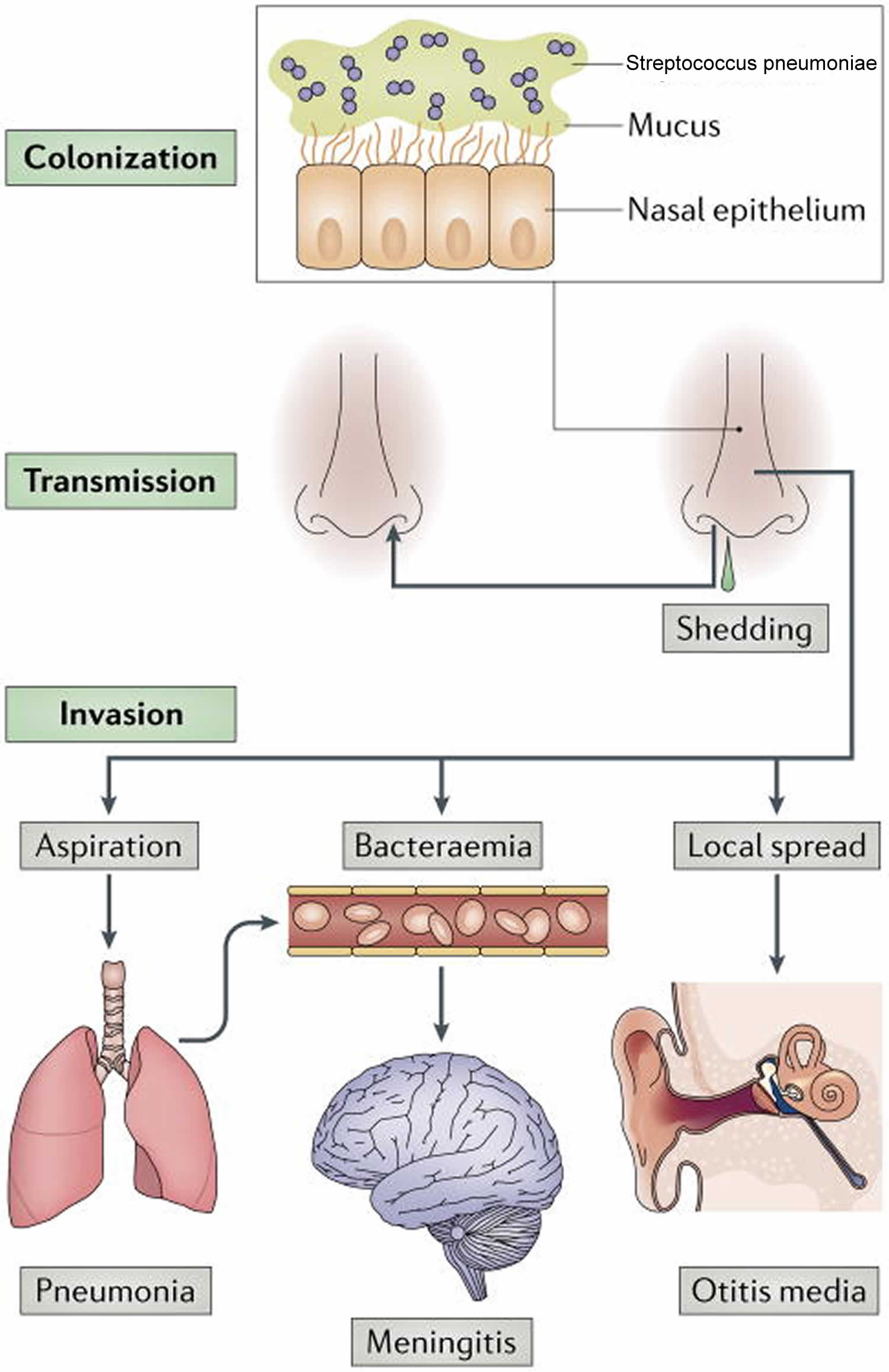
[Source 31 ]Figure 3. Streptococcus pneumoniae life cycle
Footnote: Streptococcus pneumoniae colonizes the mucosa of the upper respiratory tract (URT). This carriage is the prerequisite for both transmission to other individuals and invasive disease in the carrier. Carriers can shed Streptococcus pneumoniae in nasal secretions and thereby transmit the bacterium. Dissemination beyond its niche along the nasal epithelium, either by aspiration, bacteraemia or local spread, can lead to invasive diseases, such as otitis media, community-acquired pneumonia, sepsis and meningitis. As all of these diseases are ‘dead ends’ in the life cycle of the organism, the bacterial factors that cause invasive diseases must also be adaptive for colonization and/or transmission.
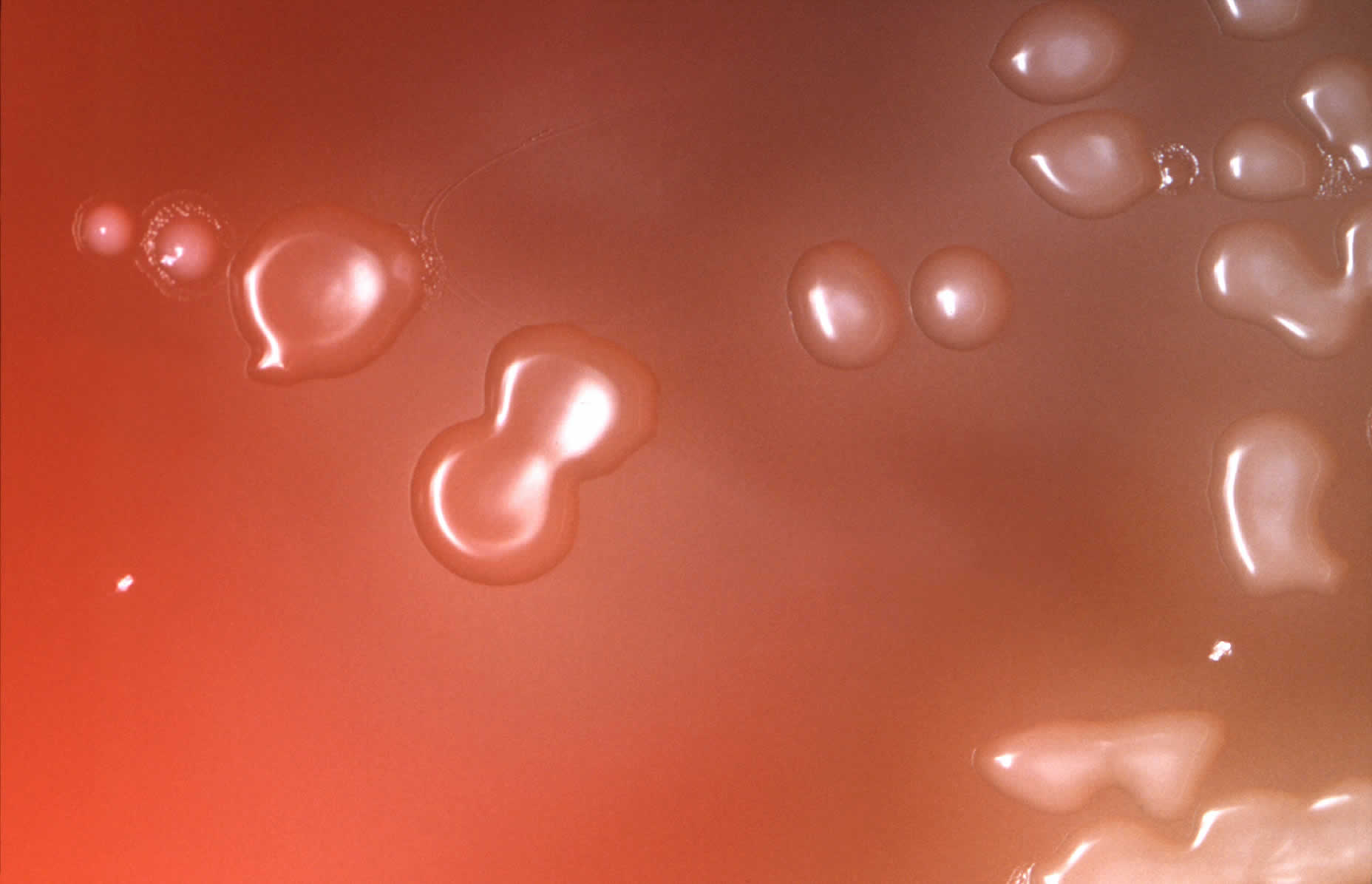
[Source 5 ]Figure 4. Streptococcus pneumoniae in blood agar
Footnote: Under an approximate 10X magnification, this image depicts the colonial characteristics displayed by Streptococcus pneumoniae bacterial colonies that were grown on primary isolation medium, consisting of trypticase-soy-agar, containing 5% sheep’s blood, as well as 5mg of gentamicin/ml. Note that the so called doughnut-shaped colonies are those of Streptococcus pneumoniae. Those that do not have depressed centers are not Streptococcus pneumoniae.
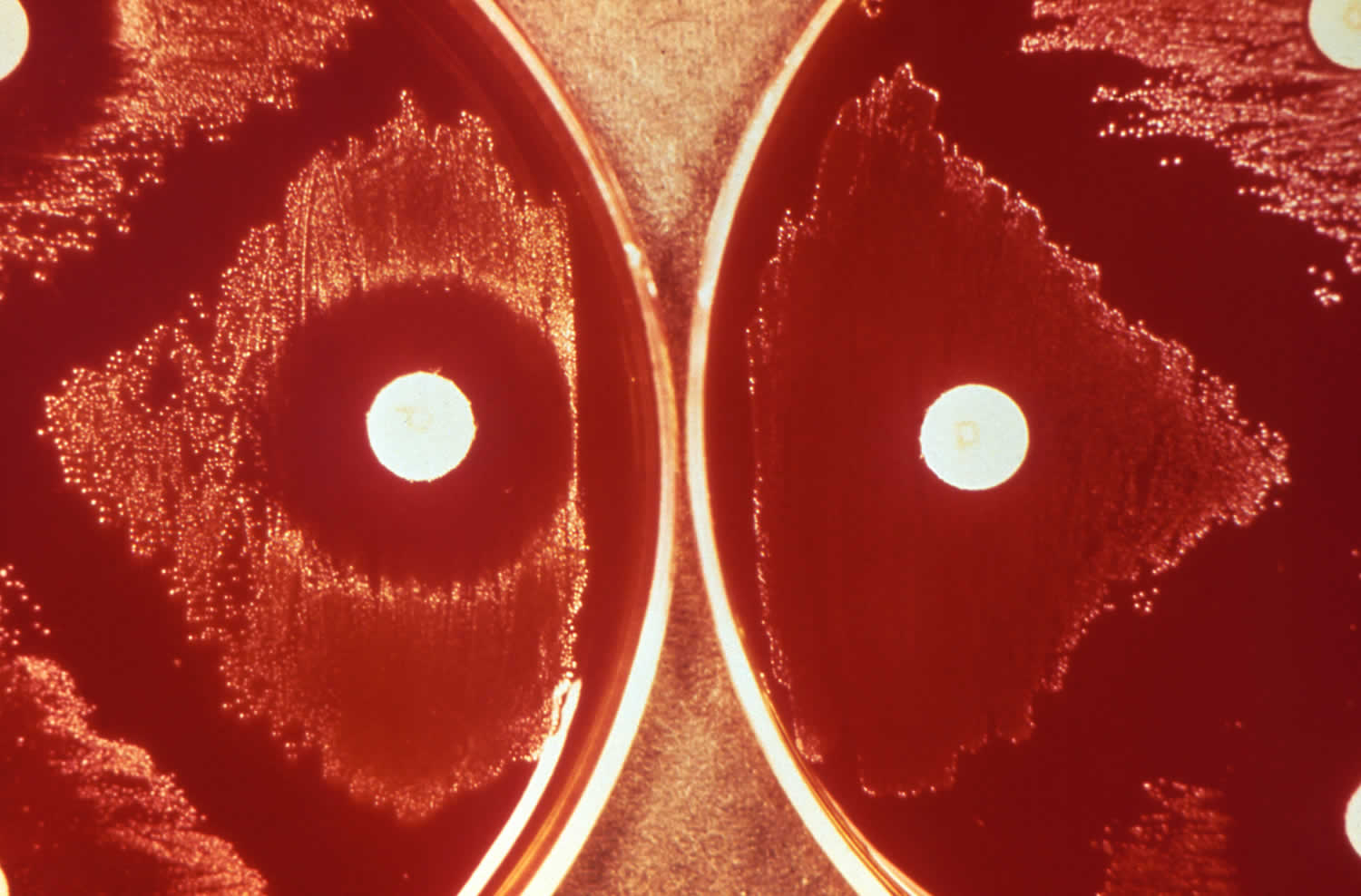
[Source 32 ]Figure 5. Streptococcus pneumoniae optochin test
Footnote: Depicted here, were the results of what is referred to as an optochin (ethylhydrocupreine) test, used to confirm the presence of Streptococcus pneumoniae bacteria, which are optochin-sensitive. In so doing, Streptococcus pneumoniae can then be differentiated from other alpha-hemolytic streptococcal organisms, such as Streptococcus viridans, which are optochin-resistant. The results, revealed that optochin-sensitive, S. pneumoniae, were present on the left, given the zone of inhibition around the optochin imbibed disc, while the optochin-resistant bacteria on the right grew uninhibitedly up to the disc’s edge.
[Source 33 ]Streptococcus pneumoniae causes
Streptococcus pneumoniae is the most common cause of fatal community-acquired pneumonia in the elderly and is also one of the most common causes of middle ear infections (otitis media) and bacterial meningitis in children, as well as an important cause of sinusitis, bacteremia, septic arthritis, osteomyelitis, peritonitis, and endocarditis 4, 2. In the United States, prior to the widespread use of 7-valent pneumococcal conjugate vaccine (PCV7), the seven most common serotypes isolated from blood or cerebrospinal fluid (CSF) of children younger than age 5 years accounted for 80% of infections; these seven serotypes accounted for about 50% of isolates from older children and adults 3. The prominence of Streptococcus pneumoniae as a cause of disease is due to the combination of high carriage rates, its genetic adaptability and its ability to shift from a commensal to a pathogenic interaction with its host 5. Scientists do not clearly understand the immunologic mechanism that allows pneumococcal disease to occur in a Streptococcus pneumoniae carrier.
Pneumococcal disease in Adults
- Pneumococcal pneumonia
- Most common clinical presentation
- Incubation period 1 to 3 days
- Symptoms: Fever, chills, pleuritic chest pain, cough, rusty sputum, dyspnea, tachypnea, hypoxia, tachycardia, malaise, weakness
- Pneumococcal bacteremia
- Can lead to arthritis, meningitis, and endocarditis
- 12% overall case fatality ratio
- Pneumococcal meningitis
- Symptoms: Headache, lethargy, vomiting, irritability, fever, nuchal rigidity, cranial nerve signs, seizures, coma
- 14% case fatality ratio
Pneumococcal disease in Children
- Pneumococcal pneumonia
- Accounts for 25% to 30% of invasive disease in children age 2 years or younger
- Pneumococcal bacteremia
- Accounts for 40% of invasive disease in children age 2 years or younger
- Pneumococcal meningitis
- S. pneumoniae leading cause of bacterial meningitis among children younger than age 5 years
- Streptococcus pneumoniae (pneumococci) are a common cause of acute otitis media and are detected in 24% to 31% of middle ear aspirates. By age 12 months, more than 60% of children have had at least one episode of acute otitis media. Middle ear infections are a leading reason for pediatric office visits in the United States, resulting in more than 10 million visits annually. Complications of pneumococcal otitis media may include mastoiditis and meningitis.
The main way people spread Streptococcus pneumoniae to others is through direct contact with respiratory droplets. Streptococcus pneumoniae bacteria often spread within households and in crowded conditions.
Transmission of Streptococcus pneumoniae occurs through 34:
- Direct person-to-person contact via respiratory droplets
- Autoinoculation in persons carrying the bacteria in their upper respiratory tract
The pneumococcal serotypes most often responsible for causing infection are those most frequently found in carriers. Although carriage does not necessarily lead to disease, it is an important precursor for pneumococcal disease.
The following factors influence the spread of the organism within a family or household:
- Crowding
- Season. Pneumococcal infections are more common during the winter and in early spring when respiratory diseases are more prevalent.
- Presence of upper respiratory infections or pneumococcal disease, such as pneumonia or otitis media.
The period of communicability for pneumococcal disease is unknown. Presumably, transmission can occur as long as the organism appears in respiratory secretions.
Pneumonia
Pneumococcal pneumonia is the most common clinical presentation of pneumococcal disease among adults.
Pneumococcal pneumonia U.S. statistics 35:
- Pneumococcal pneumonia causes an estimated 150,000 hospitalizations each year in the United States.
- Accounts for up to 30% of adult community-acquired pneumonia
- Sometimes (25–30%) occurs with bacteremia or meningitis
The incubation period of pneumococcal pneumonia is short, about 1 to 3 days. The symptoms of pneumonia can develop suddenly over 24 to 48 hours, or they may come on more slowly over several days.
Common symptoms of pneumonia include:
- a cough – which may be dry, or produce thick yellow, green, brown or blood-stained mucus (phlegm)
- difficulty breathing – your breathing may be rapid and shallow, and you may feel breathless, even when resting
- rapid heartbeat
- fever
- feeling generally unwell
- sweating and shivering
- loss of appetite
- chest pain – which gets worse when breathing or coughing
Less common symptoms include:
- coughing up blood (hemoptysis)
- headaches
- fatigue
- nausea or vomiting
- wheezing
- joint and muscle pain
- feeling confused and disorientated, particularly in elderly people
Streptococcus pneumoniae pneumonia can affect people of any age, but it’s more common and can be more serious in certain groups of people, such as the very young (younger than 2 years), the elderly (older than 65 years), those who smoke, abuse alcohol, have asthma or chronic obstructive pulmonary disease (COPD), or are asplenic (absence of a spleen) 21. People in these groups are more likely to need hospital treatment if they develop pneumonia. The case-fatality rate is 5–7% and may be much higher among older adults or people with underlying medical conditions. Complications of pneumococcal pneumonia include pleural effusion, empyema, pericarditis, and respiratory failure.
The overall rate of confirmed Streptococcus pneumoniae infection in the United States is 5.16 to 6.11 cases/100,000 in adults with the rate for those older than 65 years being 36.4/100,000 and infants younger than 1 year being 34.2/100,000 36, 37. World Health Organization estimated that 1.6 million deaths in 2005 including 1 million children less than 5 years of age, occurred due to streptococcus pneumoniae. Streptococcus pneumoniae is a common co-infection in influenza patients and affects the morbidity and mortality in such patients.
Bacteremia
Bacteremia is the presence of viable bacteria in the circulating blood 38. Pneumococcal bacteremia can occur with or without pneumonia and lead to arthritis, meningitis, and endocarditis 3. The case fatality rate of pneumonia with bacteremia is around 10% to 20% but may be as high as 60% among patients who are older adults. More than 5,000 cases of pneumococcal bacteremia without pneumonia occur each year. The overall case fatality ratio for bacteremia is about 12%. Patients with asplenia who develop bacteremia may experience a fulminant clinical course.
Bacteremia without a known site of infection (occult bacteremia) is the most common invasive clinical presentation of pneumococcal infection among children 2 years old or younger 35. However, the overall incidence of Streptococcus pneumoniae bacteremia has been decreasing since the institution of routine pneumococcal immunization in infants 39. Bacteremia may present as fever greater than or equal to 102.2 °F (≥39 °C) in otherwise well-appearing children.
In adult patients, pneumococcal bacteremia is much more likely to be associated with another focus of infection, such as pneumonia or meningitis.
Clinical features of bacteremia with focus depend on the primary site of infection.
Complications, which develop in an estimated 10% of patients with occult bacteremia (bacteremia without a known site of infection), include meningitis, osteomyelitis, pneumonia, soft tissue and joint infections, and sepsis. Patients with higher white blood cell counts and fever, those who have not undergone prior antibiotic therapy, and children younger than 20 months are at a higher risk for persistent bacteremia or the development of focal infection 40.
Pneumococcal bacteremia without a known site of infection U.S. statistics:
- Causes an estimated 4,000 cases each year
- Accounts for up to 70% of invasive pneumococcal disease in children aged 2 years and younger
Pneumococcal bacteremic pneumonia U.S. statistics:
- Accounts for 12% to 16% of invasive pneumococcal disease in children aged 2 years.
- Pneumococcal meningitis and bacteremia killed approximately 3,250 people in the United States in 2019.
Meningitis
Meningitis is an inflammation or an infection of the membranes covering the brain and spinal cord called the meninges 41.
Pneumococcal meningitis U.S. statistics:
- Causes over 50% of all bacterial meningitis cases
- Is the leading cause of bacterial meningitis in children younger than 5 years old
- Causes an estimated 2,000 cases each year
The clinical symptoms, cerebrospinal fluid (CSF) profile, and neurologic complications are similar to other forms of purulent bacterial meningitis. Symptoms may include:
- A severe headache
- Sensitivity to light (photophobia)
- Lethargy
- Vomiting
- Irritability
- Sudden high fever
- Stiff neck
- Nausea or vomiting
- Upset stomach and diarrhea
- Fatigue
- Nuchal rigidity, cranial nerve signs, or seizures
- Coma
Systemic complications, such as septic shock, disseminated intravascular coagulation (DIC) or organ failure, can occur. The case-fatality rate of pneumococcal meningitis is about 8% among children and 22% among adults 42, 40.
Complications of pneumococcal meningitis such as intellectual and behavioral disabilities, seizures, hearing loss, and motor deficits, can happen in as many as 50% of pneumococcal meningitis survivors. In a study from Denmark 43, 240 patients who survived pneumococcal meningitis were examined using audiometry. More than half (54%) had a hearing deficit, with 39% of these not suspected of hearing loss at the time of hospital discharge. Of the 240 study participants, 14% demonstrated profound hearing loss—7% unilateral and 7% bilateral. Significant risk factors for hearing loss included advanced age, the presence of comorbidity, and higher severity of meningitis. Audiometry should be considered in all patients who survive pneumococcal meningitis.
Acute otitis media
Otitis media is an inflammation or infection of the middle ear. Acute otitis media (acute ear infection) occurs when there is bacterial or viral infection of the fluid of the middle ear, which causes production of fluid or pus. Streptococcus pneumoniae are a common cause of acute otitis media. Clinical manifestations may depend on the age of the patient. In young children, ear pain may be shown as irritability, change in sleeping or eating habits, or holding or tugging at the ear. Fever, ear drainage, and hearing loss may be present. Complications of pneumococcal otitis media may include mastoiditis and meningitis. Streptococcus pneumoniae infection is the most common cause of mastoiditis, a complication of otitis media that was more common in the pre-antibiotic era; this complication is now more commonly associated with untreated or improperly treated cases.
Several early studies demonstrated that otitis media due to Streptococcus pneumoniae is usually accompanied by fever and pain; the fever associated with pneumococcal otitis media tends to be higher than that caused by other common bacterial pathogens 40. Pneumococcal disease is less likely to resolve spontaneously.
Pneumococcal acute otitis media U.S. statistics:
- Causes up to 20% of all acute otitis media infections, which are the most frequent reasons for pediatric medical visits and pediatric antibiotic prescriptions
Sinusitis
Sinusitis is an inflammation of the mucous membrane (the lining of the sinuses) of one or more paranasal sinuses. The sinuses are small, air-filled cavities behind your cheekbones and forehead. The mucus produced by your sinuses usually drains into your nose through small channels. In sinusitis, these channels become blocked because the sinus linings are inflamed (swollen).
Sinusitis may be caused by a microbial infection (virus, bacterium, or fungus), allergic reactions, nasal polyps, or a severely deviated nasal septum.
Acute bacterial sinusitis is often triggered by obstruction of orifices by viral infection, pollutants, or allergens in the atmosphere, together with fluid accumulation in paranasal sinus cavities. Bacterial sinusitis tends to have longer duration of symptoms (e.g., ≥10 days), and more severe clinical manifestations (e.g., fever greater than or equal to 102.2 °F (≥39 °C), purulent discharge, pain ≥3 days) compared to viral sinusitis. Acute bacterial sinusitis is usually preceded by a viral upper respiratory infection that leads to mucosal edema, resulting in ostia obstruction. This is followed by the development of a purulent nasal discharge and cough. Halitosis and worsening cough at night due to postnasal drip are often noted.
Acute sinusitis manifestations may vary depending on the age of the patient and the developmental status of individual sinuses. In children younger than 5 years, infection is usually limited to the ethmoid and maxillary sinuses.
Sinusitis can rarely spread beyond the paranasal sinuses and nasal cavity into surrounding structures such as the central nervous system, orbit, or surrounding tissue.
Acute exacerbations of chronic bronchitis
Acute exacerbations of chronic bronchitis manifest as a change from baseline chronic symptoms. Symptoms include shortness of breath, increased production and/or purulence of sputum, increased sputum tenacity, and cough.
An estimated 80% of cases of acute exacerbations of chronic bronchitis are caused by infection, with about one half of those caused by aerobic bacteria, of which Streptococcus pneumoniae is the most commonly isolated organism.
Symptoms such as sore throat, rhinorrhea, nasal congestion, and dyspnea (shortness of breath) may indicate a viral cause 44.
Conjunctivitis
Conjunctivitis also called “pink eye”, is an inflammation or infection of the transparent membrane (conjunctiva) that lines your eyelid and covers the white part of your eyeball. When small blood vessels in the conjunctiva become inflamed, they’re more visible. This is what causes the whites of your eyes to appear reddish or pink. Conjunctivitis is commonly caused by a bacterial or viral infection, an allergic reaction, or — in babies — an incompletely opened tear duct.
Bacterial conjunctivitis is more likely to be bilateral and purulent than viral conjunctivitis.
Streptococcus pneumoniae is found in up to one third of patients with bacterial conjunctivitis; the rate of isolates that are not susceptible to penicillin is increasing.
Osteomyelitis and septic arthritis
Streptococcus pneumoniae infection is an uncommon cause of osteomyelitis (bone infection) and septic arthritis (joint infection), causing approximately 4% and 20% of cases in children, respectively.
- Septic arthritis: Pneumococcal septic arthritis usually manifests as painful, swollen, and hot joints. The ankles and knees are most commonly involved, and one or more joints may be affected. Blood or synovial cultures usually grow S pneumoniae. Up to half of patients with pneumococcal septic arthritis have concomitant osteomyelitis.
- Osteomyelitis: The femur and humerus are most often involved in cases of pneumococcal osteomyelitis in children; the vertebral bones are often involved in adult patients. Up to 20% of patients with pneumococcal osteomyelitis develop long-term sequelae, a figure similar to that of rates of osteomyelitis of other causes. One clinical study performed by the Pediatric Multicenter Pneumococcal Surveillance Study Group 45 showed that more than 40% of patients with joint and bone pneumococcal infections had associated bacteremia. Patients with joint prostheses or rheumatic fever are at increased risk for joint disease.
Soft tissue infections
Although uncommon, Streptococcus pneumoniae infection can be a cause of mild-to-serious soft tissue infections, including cellulitis, myositis, periorbital cellulitis, and abscess, particularly in some immunocompromised hosts (eg, those with SLE). Most patients have white blood cell counts greater than 15,000 cells/μL and elevated temperatures. Physical findings are related to the site of infection and usually include redness, warmth, and tenderness of the involved area. Movement may be limited by pain and/or swelling. The incidence of soft tissue infections is increased in persons with HIV infection or underlying connective tissue disease; however, most affected individuals are otherwise healthy and respond well to antibiotic therapy 40.
Peritonitis
Peritonitis is an inflammation (irritation) of the peritoneum. This is the thin tissue that lines the inner wall of the abdomen and covers most of the abdominal organs. Overall, primary peritonitis (peritonitis caused by the spread of organisms via blood or lymph to the peritoneal cavity) is rare, accounting for less than 20% of peritonitis cases.
Streptococcus pneumoniae is the most commonly isolated organism in patients with primary peritonitis. Primary peritonitis in children is usually associated with underlying conditions such as nephrotic syndrome or other immunocompromising diseases. In adults, primary peritonitis is usually associated with cirrhosis.
Females with severe pelvic inflammatory disease due to Streptococcus pneumoniae infection may develop peritonitis. In such cases, organisms may gain access to the peritoneum via the fallopian tubes from the female genital tract. This is the only invasive disease caused by Streptococcus pneumoniae infection that is more common in females. Other persons at risk for peritonitis include persons with gastrointestinal injury, ulcers, or malignancy.
Presenting symptoms of peritonitis include abdominal pain, anorexia, emesis, diarrhea, and fever. Children may present atypically with right lower quadrant abdominal pain that may be mistaken for appendicitis.
Cardiac infections
In the antibiotic era, pneumococcal cardiac infections are rare.
- Endocarditis: Involvement of native aortic and mitral valves are most common; infection can lead to valve destruction, heart failure, and embolization. Presenting signs and symptoms are typical of those seen in other causes of endocarditis and include fever, new or changing murmurs, muscle and/or joint pains, sweating, fatigue, anorexia, and skin findings. In alcoholics, may be part of the triad of endocarditis, pneumonia, and meningitis.
- Pericarditis: Prior to the widespread use of antibiotics, S pneumoniae infection was the most common cause of purulent pericarditis in children; now, infection in childhood is extremely rare, and nearly all cases of pneumococcal pericarditis occur in adults. Symptoms, signs, and examination findings may include chest and/or pleuritic pain; radiating pain to the neck, abdomen, shoulder, or back; orthopnea; dry cough; extremity swelling; anxiety; fatigue; fever; pericardial rub; and muffled heart sounds.
Risk factors for developing pneumococcal disease
Many health conditions and other factors can increase someone’s risk for pneumococcal disease, including severe infections.
Conditions and other factors that increase the risk for invasive pneumococcal disease include 46:
- Alcoholism
- Cerebrospinal fluid leak
- Chronic heart, lung, liver, or renal disease
- Cigarette smoking
- Cochlear implant
- Decreased immune function from disease or drugs
- Diabetes
- Functional or anatomic asplenia, including sickle cell disease
Conditions associated with decreased pulmonary clearance, such as asthma, chronic bronchitis, chronic obstructive pulmonary disease (COPD), viral infections, and active/passive cigarette smoke exposure, also predispose to Streptococcus pneumoniae infection.
Chronic lung conditions that increase someone’s risk include chronic obstructive pulmonary disease (COPD), emphysema, and asthma (in adults) or asthma treated with high-dose steroids (in children).
Persons with a cochlear implant appear to be at increased risk of pneumococcal meningitis.
Conditions that cause immune deficits, including HIV infection, malignancy, diabetes mellitus, functional or anatomic asplenia, humoral immunity defects, complement deficiencies, and neutrophil dysfunction, are associated with an increased risk of disease.
Children with HIV infection or functional or anatomic asplenia, particularly sickle cell disease, are at high risk for invasive pneumococcal disease. Some studies report rates more than 50 times higher than those among children of the same age without these conditions.
Experts do not know why, but children of certain racial and ethnic groups also have increased rates of disease:
- Alaska Native people
- African American people
- Certain American Indian people
Research shows that young children attending childcare are at increased risk for invasive pneumococcal disease and acute otitis media.
Children younger than 5 years, particularly aged 2 years or younger, are at an increased risk of pneumococcal disease 47. In addition, absence of breastfeeding, exposure to cigarette smoke, daycare attendance, and lack of immunization with the pneumococcal conjugate vaccine further increase the risk of disease. Adults older than 55-65 years are also at an increased risk of pneumococcal disease 47.
Children at risk for pneumococcal disease
Children at increased risk for pneumococcal disease include those younger than 2 years old and those with:
- Chronic heart, lung, or kidney disease
- Cerebrospinal fluid (CSF) leak (a health problem where fluid surrounding and protecting the brain and spinal cord leaks)
- Cochlear implant (a small electronic device that is surgically implanted to help people with severe hearing loss be able to hear)
- Diabetes
- HIV infection, cancer, solid organ transplant, or another condition or taking medicine that weakens the immune system
- Nephrotic syndrome (a kidney disorder)
- Sickle cell disease, a damaged spleen, or no spleen
Children with functional or anatomic asplenia, particularly those with sickle cell disease, and children with immunocompromising conditions are at very high risk for invasive disease, with rates in some studies more than 50 times higher than those among children of the same age without these conditions (i.e., incidence rates of 5,000 to 9,000 per 100,000 population). Other conditions that increase the risk of invasive pneumococcal disease in children include chronic heart disease, lung disease (including asthma if treated with high-dose oral corticosteroid therapy), liver disease, CSF leak, and having a cochlear implant. Rates are also increased among children of certain racial and ethnic groups, including Alaska Natives, African Americans, and certain American Indian groups (Navajo and White Mountain Apache). The reason for this increased risk by race and ethnicity is not known with certainty but has also been noted for invasive Haemophilus influenzae infection (also an encapsulated bacterium). Attendance at a childcare center has also been shown to increase the risk of invasive pneumococcal disease and acute otitis media 2- or 3-fold among children younger than age 5 years. Children with a cochlear implant are at increased risk for pneumococcal meningitis.
Adults at risk for pneumococcal disease
Adults 65 years or older are at increased risk for pneumococcal disease.
Adults of all ages are also at increased risk for pneumococcal disease if they have:
- Alcoholism
- Chronic heart, lung, kidney, or liver disease
- Cochlear implant
- CSF leak
- Diabetes
- HIV infection, cancer, solid organ transplant, or another condition or taking medicine that weakens the immune system
- Nephrotic syndrome
- Sickle cell disease, a damaged spleen, or no spleen
Adults with certain medical conditions are at highest risk for invasive pneumococcal disease. Adults who smoke cigarettes are also at increased risk for pneumococcal disease.
For adults age 18 through 64 years with hematologic cancer, the rate of invasive pneumococcal disease in 2013–2014 was 129 per 100,000 population. Other conditions that place adults at highest risk for invasive pneumococcal disease include other immunosuppressive conditions from disease or drugs, functional or anatomic asplenia, and renal disease. Other conditions that increase the risk of invasive pneumococcal disease in adults include chronic heart disease, chronic lung diseases (including chronic obstructive lung disease, emphysema, and asthma), liver disease, smoking cigarettes, alcoholism, CSF leak, and having a cochlear implant.
Streptococcus pneumoniae prevention
Behavior modification and risk factors
Cigarette smoking and passive cigarette smoke exposure have been linked to an increased risk for invasive pneumococcal disease in healthy adults; thus, smoking cessation should be encouraged. Optimal nutrition and living conditions may decrease the risk for pneumococcal disease. Breastfeeding of infants should also be encouraged, as the rates of invasive pneumococcal infection is lower in breastfed infants. Daycare attendance is associated with acquisition, carriage (of susceptible and drug-resistant strains), infection, and outbreaks of pneumococcal disease in proportion to the number of attendees.
Pneumococcal vaccines
Pneumococcal vaccines are the best way to protect against serious pneumococcal infections 48. Pneumococcal vaccines help protect against some of the more than 100 serotypes of pneumococcal bacteria 49. The Centers for Disease Control and Prevention (CDC) recommends pneumococcal vaccination for children younger than 5 years and adults 65 years or older 50.
There are 2 types of pneumococcal vaccines used in the United States 51, 52:
- Pneumococcal conjugate vaccines (PCVs). A conjugate vaccine is a type of vaccine that joins a protein to an antigen (in the case of pneumococcal vaccines, the protein is connected to unique polysaccharides [long chains of sugar molecules] from the surface of each of the pneumococcal serotypes). The conjugate vaccines have the polysaccharides for different serotypes attached (or conjugated) to a carrier protein. The protein helps improve the quality of the immune system response to the vaccine compared to the response to an unconjugated polysaccharide (PPV). Immune response to pneumococcal conjugate vaccine (PCV) is a T-cell dependent response that produces memory B-cells and reduces carriage of the bacteria in the respiratory track. Pneumococcal conjugate vaccines (PCVs) are given to children younger than 5 years old and to older children who need it. Vaccine providers also give pneumococcal conjugate vaccines (PCVs) to adults 65 years or older and other adults who need it.
- Pneumococcal polysaccharide vaccine (PPV). A polysaccharide vaccine is a type of vaccine that is composed of long chains of sugar molecules called polysaccharides, that resemble the surface of certain serotypes of pneumococcal bacteria in order to help the immune system mount a response. The immune response to the Pneumovax23 (PPSV23) vaccine is a T-cell independent immune response but does not reduce bacterial carriage. Vaccine providers may give Pneumovax23 (PPSV23) to children 2 through 18 years old with certain medical conditions. Vaccine providers give it to adults who receive PCV15 (Vaxneuvance). They also may give it to adults who have received an earlier vaccine called PCV13 (Prevnar 13). PCV13 (Prevnar 13) is FDA-licensed and may still be available in some clinics. PCV13 (Prevnar 13) is no longer routinely recommended; however, CDC guidance allows for its use as previously recommended in situations where PCV15, PCV20, or PCV21 is indicated but unavailable and the alternative is that the patient would not be vaccinated 56.
- PPSV23 (Pneumovax23) helps protect against 23 types of Streptococcus pneumoniae bacteria 57. Following the 2022 changes to the pneumococcal vaccination schedule for adults, PPSV23 (Pneumovax23) is no longer recommended alone, however PPSV23 (Pneumovax23) is recommended for adults following PCV13 (Prevnar 13) or PCV15 (Vaxneuvance) vaccination. PPSV23 (Pneumovax23) is not recommended for people who have previously received a PCV20 (Prevnar 20) or PCV21 (Capvaxive) vaccination.
Each of these vaccines helps protect against specific serotypes, or strains of Streptococcus pneumoniae bacteria. The number at the end of the vaccine name tells how many serotypes the pneumococcal vaccine includes.
The Centers for Disease Control and Prevention (CDC) recommends pneumococcal vaccination for 58:
- Children
- All children younger than 5 years old
- Children 5 through 18 years old with certain risk conditions
- Adults
- All adults 65 years or older
- 19 through 64 years old with certain risk conditions
Pneumococcal conjugate vaccines
Pneumococcal conjugate vaccine is a type of vaccine that joins (conjugates) a protein to an antigen and in the case of pneumococcal vaccines, the protein is connected to unique polysaccharides (long chains of sugar molecules) from the surface of each of the pneumococcal serotypes. The conjugate vaccines have the polysaccharides for different serotypes attached (conjugated) to a carrier protein. The protein helps improve the quality of the immune system response to the vaccine compared to the response to an unconjugated polysaccharide (PPV). Immune response to pneumococcal conjugate vaccine (PCV) is a T-cell dependent response that produces memory B-cells and reduces carriage of the bacteria in the respiratory track. Pneumococcal conjugate vaccines (PCVs) are given to children younger than 5 years old and to older children who need it. Vaccine providers also give pneumococcal conjugate vaccines (PCVs) to adults 65 years or older and other adults who need it.
In United States there are 3 types of pneumococcal conjugate vaccines (PCVs) that are differentiated by the number of serotypes they provide protection against — PCV15 (Vaxneuvance), PCV20 (Prevnar 20) and PCV21 (Capvaxive) 51, 52. PCV13 (Prevnar 13) is no longer routinely recommended; however, CDC guidance allows for its use as previously recommended in situations where PCV15, PCV20, or PCV21 is indicated but unavailable and the alternative is that the patient would not be vaccinated 56.
The latest Pneumococcal Vaccine Recommendation by the Centers for Disease Control and Prevention (CDC) is to use PCV15 (Vaxneuvance) or PCV20 (Prevnar 20) for routine pneumococcal vaccination for all children younger than 5 years of age 50, 59. And for all adults 65 years or older who have never received any pneumococcal conjugate vaccine or whose previous vaccination history is unknown administer PCV15 (Vaxneuvance), PCV20 (Prevnar 20) or PCV21 (Capvaxive) 50, 59.
Pneumococcal vaccine schedule for children
Administer a 4-dose pneumococcal conjugate vaccine series (PCV15 [Vaxneuvance] or PCV20 [Prevnar 20]), 1 dose at each of the following ages 50:
- 2 months
- 4 months
- 6 months
- with a booster at age 12 through 15 months
Catch-up guidance
Vaccinate children younger than 5 years of age who miss their shots or start the pneumococcal conjugate vaccine series later than recommended. The number of doses recommended and the intervals between doses will depend on the child’s age when vaccination begins. Otherwise healthy children who fall behind should be given catch-up vaccination through age 59 months; if they have certain underlying medical conditions they should be given catch-up vaccination through age 71 months.
Children may be at increased risk for febrile seizures if a pneumococcal conjugate vaccine is administered with inactivated influenza vaccine. However, clinicians may give either pneumococcal conjugate vaccine at the same time as an influenza vaccine.
Currently, no data from clinical trials are available for co-administration of Pneumovax23 (PPSV23) with other childhood vaccines during the same visit.
Pneumococcal vaccine schedule for adults 65 years or older
The CDC recommends PCV15 (Vaxneuvance), PCV20 (Prevnar 20) or PCV21 (Capvaxive) for all adults 65 years or older 50:
- Who have never received any pneumococcal conjugate vaccine (PCV)
- Whose previous vaccination history is unknown
If PCV15 (Vaxneuvance) is used, administer a dose of Pneumovax23 (PPSV23) one year later, if needed. Only one dose of Pneumovax23 (PPSV23) is indicated. If previously administered, another dose isn’t needed. If Pneumovax23 (PPSV23) is not available, one dose of PCV20 (Prevnar 20) or PCV21 (Capvaxive) may be given. Their pneumococcal vaccinations are complete 50.
The minimum interval is 8 weeks and can be considered in adults with 50:
- An immunocompromising condition
- A cochlear implant
- A cerebrospinal fluid leak
If PCV20 (Prevnar 20) or PCV21 (Capvaxive) is used, a dose of Pneumovax23 (PPSV23) isn’t indicated. Regardless of which vaccine is used (PCV20 or PCV21), their pneumococcal vaccinations are complete 50.
Recommendation for shared clinical decision-making
Based on shared clinical decision-making, adults 65 years or older have the option to get no additional pneumococcal vaccines, PCV20 (Prevnar 20) or PCV21 (Capvaxive). They can get PCV20 (Prevnar 20) or PCV21 (Capvaxive) if they have received both 50:
- PCV13 (Prevnar 13) (but not PCV15, PCV20, or PCV21) at any age and
- Pneumovax23 (PPSV23) at or after the age of 65 years old
Pneumococcal 13-valent conjugate vaccine (Prevnar 13)
Pneumococcal 13-valent conjugate vaccine (Prevnar 13) includes purified capsular polysaccharide of 13 serotypes of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 19A, 19F, 18C, and 23F) conjugated to a nontoxic variant of diphtheria toxin known as CRM197. A 0.5 milliliter (mL) pneumococcal 13-valent conjugate vaccine (Prevnar 13) dose contains approximately 2.2 micrograms (mcg) of polysaccharide from each of 12 serotypes and approximately 4.4 mcg of polysaccharide from serotype 6B; the total concentration of CRM197 is approximately 34 mcg. The vaccine contains 0.02% polysorbate 80, 0.125 milligrams of aluminum as aluminum phosphate adjuvant, and 5 mL of succinate buffer. Pneumococcal 13-valent conjugate vaccine (Prevnar 13) is administered by intramuscular injection.
Researchers conducted a randomized placebo-controlled trial (CAPiTA trial) in the Netherlands among approximately 85,000 adults 65 years or older from 2008 through 2013 60. This trial evaluated the clinical benefit of pneumococcal 13-valent conjugate vaccine (Prevnar 13) in the prevention of pneumococcal pneumonia. The results of the CAPiTA trial demonstrated:
- 46% efficacy against vaccine-type pneumococcal pneumonia
- 45% efficacy against vaccine-type non-bacteremic pneumococcal pneumonia
- 75% efficacy against vaccine-type invasive pneumococcal disease
Studies show that getting at least 1 shot of pneumococcal 13-valent conjugate vaccine (Prevnar 13) protects 61, 62, 60:
- At least 8 in 10 babies from serious infections called invasive pneumococcal disease
- 3 in 4 adults 65 years or older against invasive pneumococcal disease
- 9 in 20 adults 65 years or older against pneumococcal pneumonia
Substantial evidence demonstrates routine infant pneumococcal 7-valent conjugate vaccine (PCV7) and pneumococcal 13-valent conjugate vaccine (Prevnar 13) vaccination reduced carriage and transmission of Streptococcus pneumoniae vaccine serotypes. This resulted in lower invasive pneumococcal disease incidence among unvaccinated persons of all ages, including infants too young to receive the vaccine 51.
The Centers for Disease Control and Prevention (CDC) recommends routine administration of pneumococcal 13-valent conjugate vaccine (Prevnar 13) for all children younger than 2 years of age 63:
- Give pneumococcal 13-valent conjugate vaccine (Prevnar 13) to infants as a series of 4 doses, one dose at each of these ages: 2 months, 4 months, 6 months, and 12 through 15 months.
- Children who miss their shots or start the series later should still get their pneumococcal 13-valent conjugate vaccine (Prevnar 13). The number of doses recommended and the intervals between doses will depend on the child’s age when vaccination begins.
- 1 dose for healthy children age 24–59 months with any incomplete* pneumococcal 13-valent conjugate vaccine (Prevnar 13) series (* Not having received all doses in either the recommended series or an age-appropriate catch-up series in the pneumococcal vaccine recommendations)
The CDC also recommends pneumococcal vaccination for children 2 through 5 years old who have certain medical conditions.
- For a child with any of these conditions 64:
- Cerebrospinal fluid leak
- Chronic heart disease, particularly cyanotic congenital heart disease and cardiac failure
- Chronic lung disease, including asthma if treated with prolonged high-dose oral corticosteroid therapy
- Cochlear implant
- Diabetes mellitus
- The CDC recommends you:
- Give 2 doses of pneumococcal 13-valent conjugate vaccine (Prevnar 13) if they are unvaccinated or received an incomplete pneumococcal 13-valent conjugate vaccine (Prevnar 13) series with <3 doses. Give the second dose at least 8 weeks after the first.
- Give 1 dose of pneumococcal 13-valent conjugate vaccine (Prevnar 13) if they received 3 doses of pneumococcal 13-valent conjugate vaccine (Prevnar 13) but none were given after 12 months of age.
- Give 1 dose of pneumococcal polysaccharide vaccine (Pneumovax23 or PPSV23) at least 8 weeks after the pneumococcal 13-valent conjugate vaccine (Prevnar 13) series is complete.
- For a child with any of these conditions 64:
- Chronic renal failure or nephrotic syndrome
- Congenital immunodeficiency
- B- (humoral) or T-lymphocyte deficiency
- Complement deficiency, particularly C1, C2, C3, or C4 deficiency
- Phagocytic disorder, excluding chronic granulomatous disease
- Congenital or acquired asplenia, or splenic dysfunction
- Diseases associated with treatment of immunosuppressive drugs or radiation therapy
- Hodgkin disease
- Leukemia
- Lymphoma
- Malignant neoplasm
- Solid organ transplant
- HIV infection
- Sickle cell disease or other hemoglobinopathies
- The CDC recommends you:
- Give 2 doses of pneumococcal 13-valent conjugate vaccine (Prevnar 13) if they are unvaccinated or received an incomplete pneumococcal 13-valent conjugate vaccine (Prevnar 13) series with <3 doses. Give the second dose at least 8 weeks after the first.
- Give 1 dose of pneumococcal 13-valent conjugate vaccine (Prevnar 13) if they received 3 doses of pneumococcal 13-valent conjugate vaccine (Prevnar 13) but none were given after 12 months of age.
- Give 2 doses of pneumococcal polysaccharide vaccine (Pneumovax23 or PPSV23) after the pneumococcal 13-valent conjugate vaccine (Prevnar 13) series is complete. Give the first dose at least 8 weeks after any prior pneumococcal 13-valent conjugate vaccine (Prevnar 13) dose, then give the second dose of PPSV23 at least 5 years after the first PPSV23 dose.
The CDC recommends pneumococcal vaccination for children 6 through 18 years old who have certain medical conditions.
- For a child with any of these conditions 64:
- Cerebrospinal fluid leak
- Cochlear implant
- CDC recommends you:
- Give 1 dose of pneumococcal 13-valent conjugate vaccine (Prevnar 13) if they have not received any doses of pneumococcal 13-valent conjugate vaccine (Prevnar 13). Administer pneumococcal 13-valent conjugate vaccine (Prevnar 13) before giving any recommended doses of pneumococcal polysaccharide vaccine (Pneumovax23 or PPSV23).
- Give 1 dose of pneumococcal polysaccharide vaccine (Pneumovax23 or PPSV23) (if not already given earlier in childhood) at least 8 weeks after pneumococcal 13-valent conjugate vaccine (Prevnar 13).
- For a child with any of these conditions 64:
- Chronic renal failure or nephrotic syndrome
- Congenital immunodeficiency
- B- (humoral) or T-lymphocyte deficiency
- Complement deficiency, particularly C1, C2, C3, or C4 deficiency
- Phagocytic disorder, excluding chronic granulomatous disease
- Congenital or acquired asplenia
- Diseases associated with treatment of immunosuppressive drugs or radiation therapy
- Hodgkin disease
- Leukemia
- Lymphoma
- Malignant neoplasm
- Solid organ transplant
- HIV infection
- Sickle cell disease or other hemoglobinopathies
- CDC recommends you:
- Give 1 dose of pneumococcal 13-valent conjugate vaccine (Prevnar 13) if they have not received any doses of pneumococcal 13-valent conjugate vaccine (Prevnar 13). Administer pneumococcal 13-valent conjugate vaccine (Prevnar 13) before giving any recommended doses of pneumococcal polysaccharide vaccine (Pneumovax23 or PPSV23).
- Ensure the child receives 2 doses of pneumococcal polysaccharide vaccine (Pneumovax23 or PPSV23). The first dose of PPSV23 should be given at least 8 weeks after any prior pneumococcal 13-valent conjugate vaccine (Prevnar 13) dose, then the second dose of PPSV23 should be given at least 5 years after the first dose of PPSV23.
- For a child with any of these conditions 64:
- Chronic heart disease, particularly cyanotic congenital heart disease and cardiac failure
- Chronic lung disease, including asthma if treated with prolonged high-dose oral corticosteroid therapy
- Diabetes mellitus
- CDC recommends you:
- Give 1 dose of pneumococcal polysaccharide vaccine (Pneumovax23 or PPSV23) (if not already given earlier in childhood). * One dose of pneumococcal 20-valent conjugate vaccine (Prevnar 20) may be used if pneumococcal polysaccharide vaccine (Pneumovax23 or PPSV23) is not available
Pneumococcal 15-valent conjugate vaccine (Vaxneuvance)
Pneumococcal 15-valent conjugate vaccine (Vaxneuvance) is a sterile suspension of purified capsular polysaccharides from 15 serotypes of S. pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F) individually conjugated to a nontoxic variant of diphtheria toxin known as CRM197. A 0.5 mL PCV15 dose contains 2.0 mcg of polysaccharide from each of 14 serotypes and 4.0 µg of polysaccharide from serotype 6B, 30 mcg of CRM197 carrier protein, 1.55 mg L-histidine, 1 mg of polysorbate 20, 4.50 mg sodium chloride, and 125 mcg of aluminum as aluminum phosphate adjuvant. The vaccine does not contain any preservatives.
Pneumococcal 20-valent conjugate vaccine (Prevnar20)
Pneumococcal 20-valent conjugate vaccine (Prevnar20) is a sterile suspension of saccharides from 20 serotypes of S. pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, and 33F) individually linked to a nontoxic variant of diphtheria toxin known as CRM197. A 0.5 mL dose contains approximately 2.2 mcg of saccharides from each of 19 serotypes, approximately 4.4 mcg of saccharides from serotype 6B, 51 μg CRM197 carrier protein, 100 mcg polysorbate 80, 295 mcg succinate buffer, 4.4 mg sodium chloride, and 125 mcg aluminum as aluminum phosphate adjuvant.
Pneumococcal 21-valent conjugate vaccine (CAPVAXIVE)
Pneumococcal 21-valent conjugate vaccine (CAPVAXIVE) was approved by the FDA on June 17, 2024 65, 55. Pneumococcal 21-valent conjugate vaccine (CAPVAXIVE) is indicated for the prevention of invasive pneumococcal disease caused by Streptococcus pneumoniae serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A,15B, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F, and 35B inindividuals 18 years of age and older 55.
Active ingredient(s): Bacterial sugars from 21 types of pneumococcus; each linked to a protein (CRM197). The sugars from these bacteria and the protein are not alive and do not cause disease 65.
Inactive ingredient(s): L-histidine, polysorbate 20, sodium chloride, water 65.
CAPVAXIVE does not have any preservatives 65. The tip cap and plunger stopper of the prefilled syringe are not made with natural rubber latex 65.
The most common side effects of CAPVAXIVE are 55:
- Pain, redness, or swelling where you got the injection
- Feeling tired
- Headache
- Muscle aches
- Fever
These side effects usually last less than 3 days. Tell your vaccine provider about these side effects or any unusual symptoms that develop after you get this vaccine. Get medical care right away if you have symptoms of an allergic reaction, which may include:
- Wheezing or trouble breathing
- Swelling of the face, lips, or tongue
- Hives
- Rash
There may be side effects not listed here. Ask your vaccine provider for more information.
Pneumococcal polysaccharide vaccine (Pneumovax 23)
Pneumococcal polysaccharide vaccine (Pneumovax23 or PPSV23) includes purified preparations of pneumococcal capsular polysaccharide. PPSV23 contains polysaccharide antigen from 23 types of pneumococcal bacteria (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F) 57. Pneumococcal polysaccharide vaccine (Pneumovax23) contains 25 mcg of each antigen per dose and contains 0.25% phenol as a preservative. Pneumococcal polysaccharide vaccine (Pneumovax 23) is administered by either intramuscular or subcutaneous injection.
More than 80% of healthy adults who receive the pneumococcal polysaccharide vaccine (Pneumovax23) develop antibodies against the serotypes contained in the vaccine 51. This immune response usually occurs within 2 to 3 weeks after vaccination. Older adults and persons with some chronic illnesses or immunodeficiency may not respond as well. Elevated antibody levels persist for at least 5 years in healthy adults but decline more quickly in persons with certain underlying illnesses. Children younger than 2 years of age generally have a poor antibody response to pneumococcal polysaccharide vaccine (Pneumovax23).
Pneumococcal polysaccharide vaccine (Pneumovax23) vaccine efficacy studies have resulted in various estimates of clinical effectiveness 66, 67, 68, 69. Overall, the vaccine is 60% to 70% effective in preventing invasive disease caused by serotypes in the vaccine 51. Pneumococcal polysaccharide vaccine (Pneumovax23) shows reduced effectiveness among immunocompromised persons; however, because of their increased risk of invasive pneumococcal disease, the CDC recommends pneumococcal polysaccharide vaccine (Pneumovax23) for people in these groups who receive pneumococcal 15-valent conjugate vaccine (Vaxneuvance). There is no consensus regarding the ability of pneumococcal polysaccharide vaccine (Pneumovax23) to prevent non-bacteremic pneumococcal pneumonia.
Studies comparing patterns of asymptomatic pneumococcal carriage before and after pneumococcal polysaccharide vaccine (Pneumovax23) vaccination have not shown decreases in carrier rates among those vaccinated 51.
Pneumococcal polysaccharide vaccine (Pneumovax 23) side effects
Problems following pneumococcal polysaccharide vaccine (Pneumovax23) can include:
- Reactions where the shot was given
- Redness
- Pain
- Feeling tired
- Fever
- Muscle aches
If these problems occur, they usually go away within about 2 days.
Problems that could happen after getting any injected vaccine
- People sometimes faint after medical procedures, including vaccination. Sitting or lying down for about 15 minutes can help prevent fainting and injuries caused by a fall. Tell your doctor if you or your child:
- Feel dizzy
- Have vision changes
- Have ringing in the ears
- As with any medicine, there is a very remote chance of a vaccine causing a severe allergic reaction, other serious injury, or death.
Pneumococcal vaccine administration
Use a needle length appropriate for the age and size (22–25 Gauge) of the person receiving the pneumococcal vaccine. NEVER administer a pneumococcal conjugate vaccine (PCV15, PCV20, or PCV21) and Pneumovax23 (PPSV23) during the same visit 70. If someone is indicated to receive PCV15 (Vaxneuvance) and Pneumovax23 (PPSV23), administer PCV15 (Vaxneuvance) first followed by Pneumovax23 (PPSV23).
- Administer pneumococcal polysaccharide vaccine Pneumovax23 (PPSV23) intramuscularly or subcutaneously 70.
- Administer pneumococcal conjugate vaccines (PCV15 [Vaxneuvance] or PCV20 [Prevnar 20]) intramuscularly 70.
- For infants and young children, use the vastus lateralis muscle in the anterolateral thigh.
- For older children and adults, use the deltoid muscle.
- Administer pneumococcal conjugate vaccine (PCV21 [Capvaxive]) to adults intramuscularly using the deltoid muscle.
The primary series of PCV15 (Vaxneuvance) or PCV20 (Prevnar 20) consists of 3 doses routinely given at 2, 4, and 6 months of age. You can administer the first dose as early as 6 weeks of age. CDC recommends a fourth (booster) dose at 12 through 15 months of age. For children vaccinated when they are younger than 12 months of age, the minimum interval between doses is 4 weeks. Separate doses given at 12 months of age and older by at least 8 weeks.
The number and timing of doses for older children and adults depends on the medical indication, prior pneumococcal vaccination, and age.
For children (2 through 18 years old), the interval between PCV15 (Vaxneuvance) and Pneumovax23 (PPSV23) should be at least 8 weeks. If Pneumovax23 (PPSV23) is inadvertently administered first, wait at least 8 weeks to administer PCV15 (Vaxneuvance).
For adults, the recommended interval is at least 1 year. An 8-week minimum interval can be considered for adults with an immunocompromising condition, cochlear implant, or cerebrospinal fluid leak. If Pneumovax23 (PPSV23) is inadvertently administered first, wait at least 1 year to administer PCV15 (Vaxneuvance).
In adults, you can administer a pneumococcal vaccine (PCV15, PCV20, PCV21, or PPSV23) during the same visit with influenza vaccination or other recommended vaccines. Administer each vaccine with a separate syringe and, if feasible, at a different injection site. Annual influenza vaccination is important to help prevent the flu. Additionally, since having the flu increases the risk of getting pneumococcal disease, receiving a flu vaccine is important for preventing pneumococcal disease.
Side effects of pneumonia vaccine
Redness, swelling, pain, or tenderness where the pneumonia vaccine is given, and fever, loss of appetite, fussiness (irritability), feeling tired, headache, muscle aches, joint pain, and chills can happen after pneumococcal conjugate vaccination (PCV) 71.
PCV15 (Vaxneuvance), PCV20 (Prevnar 20) or PCV21 (Capvaxive) side effects:
- Redness, swelling, pain, or tenderness where the vaccine provider gave the shot
- Fever or chills
- Loss of appetite
- Fussiness (irritability) in young children
- Feeling tired
- Headache
- Muscle aches or joint pain
Pneumovax23 (PPSV23) side effects:
- Redness or pain where the vaccine provider gave the shot
- Feeling tired
- Fever
- Muscle aches
Young children may be at increased risk for seizures caused by fever after a pneumococcal conjugate vaccine (PCV) if it is administered at the same time as inactivated influenza vaccine. Ask your doctor for more information.
People sometimes faint after medical procedures, including vaccination. Tell your doctor if you feel dizzy or have vision changes or ringing in the ears. As with any medicine, there is a very remote chance of a vaccine causing a severe allergic reaction, other serious injury, or death 71.
An allergic reaction could occur after the vaccinated person leaves the clinic. If you see signs of a severe allergic reaction (hives, swelling of the face and throat, difficulty breathing, a fast heartbeat,
dizziness, or weakness), call your local emergency services number and get the person to the nearest hospital. For other signs that concern you, see your doctor.
Pneumonia vaccine contraindications
You shouldn’t get PCV15 (Vaxneuvance), PCV20 (Prevnar 20) or PCV21 (Capvaxive) if you’ve:
- Had a life-threatening allergic reaction after any type of pneumococcal conjugate vaccination (PCV)
- Had a life-threatening allergic reaction to any vaccine containing diphtheria toxoid (DTaP)
- Have a severe allergy to any part of these vaccines
You shouldn’t get Pneumovax23 (PPSV23) if you:
- Are younger than 2 years old
- Had a life-threatening allergic reaction after getting Pneumovax23 (PPSV23)
- Have a severe allergy to any part of Pneumovax23 (PPSV23)
Which underlying medical conditions indicate that a child age 6 through 18 years should receive additional doses of pneumococcal vaccine beyond the routine schedule?
Medical conditions that increase the risk of pneumococcal disease and are indications for additional pneumococcal vaccine doses beyond the routine schedule are broken down into two categories: non-immunocompromising (non-IC) and immunocompromising (IC) 72. Recommendations differ slightly under certain circumstances by non-immunocompromising (non-IC) or immunocompromising (IC) category.
Non-immunocompromising (non-IC) conditions include 72:
- Cerebrospinal fluid (CSF) leak
- Chronic heart disease (especially cyanotic congenital heart disease and heart failure)
- Chronic kidney disease (except as specified in the immunocompromising (IC) list below)
- Chronic liver disease
- Chronic lung disease (including moderate persistent or severe persistent asthma)
- Diabetes mellitus
- Cochlear implant
Immunocompromising (IC) conditions include 72:
- Kidney disease and on maintenance dialysis
- Kidney disease with nephrotic syndrome
- Asplenia or splenic dysfunction
- Congenital or acquired immunodeficiency, including B-(humoral) or T-lymphocyte deficiency; complement deficiencies, particularly C1, C2, C3, and C4 deficiency; and phagocytic disorders (excluding chronic granulomatous disease)
- Treatment with immunosuppressive drugs or radiation therapy (including treatment for Hodgkin disease, leukemias, lymphomas, malignant neoplasm, and solid organ transplant)
- HIV infection
- Sickle cell disease or other hemoglobinopathies
An older child through age 18 years with any high-risk condition who completed a pneumococcal conjugate vaccine (PCV) series before age 6 years that included any dose of PCV20 (Prevnar 20), is not recommended to receive any additional pneumococcal conjugate vaccine (PCV) doses.
Children with non-immunocompromising (non-IC) or immunocompromising (IC) conditions who completed a pneumococcal conjugate vaccine (PCV) series before age 6 years with PCV13 (Prevnar 13) or PCV15 (Vaxneuvance) but who have not received PCV20 (Prevnar 20) or pneumococcal polysaccharide vaccine Pneumovax23 (PPSV23) should receive additional pneumococcal vaccination with a single dose of PCV20 at least 8 weeks after the most recent pneumococcal conjugate vaccine (PCV) dose. If PCV20 (Prevnar 20) is not available, a non-IC or IC child in this circumstance may, alternatively, receive a single dose of Pneumovax23 (PPSV23) at least 8 weeks after the most recent pneumococcal conjugate vaccine (PCV) dose. An immunocompromised (IC) child given Pneumovax23 (PPSV23) in this circumstance would also be due for a dose of either PCV20 (Prevnar 20) or a second dose of Pneumovax23 (PPSV23) at least 5 years after the first Pneumovax23 (PPSV23).
Doses of the PCV7 (Prevnar 7) do not count toward pneumococcal conjugate vaccine (PCV) vaccination when determining the current pneumococcal vaccination needs of a child or teen with a qualifying non-IC or IC condition.
When feasible, administer any needed pneumococcal vaccination at least two weeks before initiating planned interventions that place a child at high risk (such as a cochlear implant or spleen removal).
What are the different serotypes of Streptococcus pneumoniae targeted by different pneumococcal vaccines?
Streptococcus pneumoniae bacteria are serotyped based on the polysaccharides in the outer capsule of the bacteria. Serotypes vary in how common they are and in what percentage of pneumococcal disease they cause.
Among the pneumococcal conjugate vaccines (PCVs), PCV13 (Prevnar 13) includes serotypes: 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F. PCV15 (Vaxneuvance) includes all PCV13 serotypes plus 22F and 33F. PCV20 (Prevnar 20) includes all PCV15 serotypes plus 8, 10A, 11A, 12F, and 15B.
Pneumovax23 (PPSV23) vaccine does not contain serotype 6A, but contains 19 other serotypes present in PCV20, plus serotypes 2, 9N, 17F, and 20.
PCV21 (Capvaxive) is designed to target additional Streptococcus pneumoniae serotypes causing a significant proportion of disease in adults that are not prevented by the vaccines approved for children. PCV21 (Capvaxive)does not contain 10 serotypes found in other pneumococcal vaccines approved for children (1, 4, 5, 6B, 9V, 14, 18C, 19F, 23F, 15B, or 2). Instead, it contains an additional 11 serotypes not found in PCV20 (Prevnar 20): 9N, 17F, 20, 15A, 15C, 16F, 23A, 23B, 24F, 31, and 35B. Because of these differences, CDC estimates that PCV20 (Prevnar 20) targets serotypes that cause between 54% and 65% of invasive pneumococcal disease in adults, and PCV21 (Capvaxive) targets serotypes that cause between 77% and 85% of invasive pneumococcal disease in adults 72.
How effective are pneumococcal conjugate vaccines at preventing pneumococcal carriage or disease?
The United States Food and Drug Administration (FDA) licensed the first pneumococcal conjugate vaccine (PCV) against seven pneumococcus bacteria serotypes (PCV7, Prevnar 7) in 2000. A large clinical trial showed PCV7 (Prevnar7) reduced invasive disease caused by vaccine serotypes by 97%. Compared to unvaccinated children, children who received PCV7 (Prevnar 7) 72:
- Had 20% fewer episodes of chest X-ray confirmed pneumonia
- Had 7% fewer episodes of acute otitis media
- Underwent 20% fewer tympanostomy tube placements
FDA licensed PCV13 (Prevnar 13) based on studies comparing the serologic response of children who received PCV13 (Prevnar 13) to those who received PCV7 (Prevnar 7). Substantial evidence demonstrates that routine infant PCV7 and PCV13 vaccination reduces the carriage and transmission of vaccine serotypes.
Researchers conducted a randomized placebo-controlled trial (CAPiTA trial) in the Netherlands among approximately 85,000 adults 65 years or older from 2008 through 2013 73. The Community-Acquired Pneumonia Immunization Trial in Adults (CAPiTA trial) evaluated the clinical benefit of PCV13 (Prevnar 13) in the prevention of pneumococcal pneumonia. The results of the CAPiTA trial demonstrated 73:
- 46% efficacy against vaccine-type pneumococcal pneumonia
- 45% efficacy against vaccine-type non-bacteremic pneumococcal pneumonia
- 75% efficacy against vaccine-type invasive pneumococcal disease (IPD, i.e., bacteremia or meningitis)
FDA licensed PCV15 (Vaxneuvance) and PCV20 (Prevnar 20) in 2021 based on studies comparing the serologic response of adults who received either PCV15 or PCV20 to those who received PCV13 (Prevnar 13). These studies showed PCV15 (Vaxneuvance) and PCV20 (Prevnar 20) induced antibody levels comparable to those induced by PCV13 (Prevnar 13) and shown to be protective against invasive disease. FDA subsequently expanded the indication for use of PCV15 and PCV20 to include children starting at age 6 weeks in 2022 and 2023, respectively, based on serologic studies. PCV21 (Capvaxive) was licensed in 2024 based on a similar evaluation of serologic response to vaccination.
How effective is pneumococcal polysaccharide vaccine at preventing pneumococcal carriage or disease?
According to CDC, more than 80% of healthy adults who receive Pneumovax23 (PPSV23) develop antibodies against the serotypes contained in the vaccine that persist for at least 5 years 72. Older adults and people with some chronic illnesses or immunodeficiency may not respond as well and their antibody levels may decline more quickly.
Overall, the Pneumovax23 (PPSV23) vaccine is 60% to 70% effective in preventing invasive pneumococcal disease caused by serotypes in the vaccine 72. Pneumovax23 (PPSV23) shows less effectiveness among immunocompromised people; however, because of their increased risk of invasive pneumococcal disease, CDC recommends Pneumovax23 (PPSV23) for people in these groups who receive PCV15 (Vaxneuvance). There has not been consensus regarding the ability of Pneumovax23 (PPSV23) to prevent non-bacteremic pneumococcal pneumonia; however, recent observational studies reported 21%–46% effectiveness against Pneumovax23 (PPSV23)-type pneumococcal pneumonia when PPSV23 was given less than 5 years before illness onset.
Unlike pneumococcal conjugate vaccines (PCVs), Pneumovax23 (PPSV23) vaccination has not been shown to decrease nasal carriage of pneumococcal bacteria among those vaccinated 72.
Streptococcus pneumoniae complications
Although exact rates are difficult to determine, the World Health Organization (WHO) estimates that, worldwide, 1.6 million deaths were caused by pneumococcal disease in 2005, with 700,000 to 1 million of these occurring in children younger than 5 years 74. Even in patients in developed countries, invasive pneumococcal disease carries a high mortality rate—an average of 10-20% in adults with pneumococcal pneumonia, with much higher rates in those with risk factors for disease 75.
Worldwide, the most common cause of death due to pneumococcal disease is pneumonia. In adults admitted to the hospital in the United States for pneumonia treatment, Streptococcus pneumoniae remains the most common organism isolated. Until 2000, 100,000-135,000 patients were hospitalized for pneumonia proven to be caused by Streptococcus pneumoniae infection in the United States annually. These numbers are likely a gross underestimate, as a definite cause is not determined in most cases of pneumonia treated each year. In addition, the actual rates are also likely decreasing owing to implementation of pneumococcal conjugate vaccination. Pneumococcal pneumonia kills about 1 in 20 who get it.
Complications of pneumococcal pneumonia include:
- Empyema (infection around the lungs and in the chest cavity)
- Pericarditis (inflammation of the outer lining of the heart)
- Endobronchial obstruction (blockage of the airway that allows air into the lungs), with atelectasis (collapse within the lungs) and abscess (collection of pus) in the lungs.
Sinus infections (sinusitis)
Sinus infections (sinusitis) complications are rare, but include infection of the tissue surrounding the eyes, bone infection, and a painful abscess (collection of pus).
Pneumococcal meningitis
About 1 in 12 children and 1 in 6 older adults who get pneumococcal meningitis dies of the infection. Those who survive may have long-term problems, such as hearing loss or developmental delay.
Pneumococcal bacteremia
About 1 in 30 children with pneumococcal bacteremia die of it. Pneumococcal bacteremia kills about 1 in 8 adults who get it. For those who survive, pneumococcal bacteremia can lead to loss of limb(s).
Sepsis
Sepsis is a potentially life-threatening condition that occurs when the body’s response to an infection damages its own tissues. When the infection-fighting processes turn on the body, they cause organs to function poorly and abnormally. Complications of sepsis include kidney failure and damage to the brain, lungs, or heart.
Sepsis may progress to septic shock. This is a dramatic drop in blood pressure that can lead to severe organ problems and death.
Early treatment with antibiotics and intravenous fluids improves chances for survival.
Streptococcus pneumoniae signs and symptoms
Pneumococcal disease can include many different types of infections. Symptoms depend on the part of the body that is infected. Most pneumococcal infections are mild. However, some can be deadly or result in long-term problems.
After successful colonization, Streptococcus pneumoniae can cause a wide variety of clinical symptoms.
- By direct extension from the nasopharynx, Streptococcus pneumoniae infection can spread and then manifest as otitis media, sinusitis, tracheobronchitis, bronchitis, and pneumonia.
- By invasion and hematogenous spread, Streptococcus pneumoniae infection can cause primary bacteremia, meningitis, osteomyelitis, pericarditis, endocarditis, myositis, septic arthritis, and peritonitis.
Streptococcus pneumoniae (pneumococcus) bacteria can cause infections in many parts of the body, including:
- Lungs (pneumonia)
- Ears (otitis)
- Sinuses (sinusitis)
- Brain and spinal cord tissue (meningitis)
- Blood (bacteremia)
Symptoms of pneumococcal infection depend on the part of the body affected. Symptoms can include fever, cough, shortness of breath, chest pain, stiff neck, confusion, increased sensitivity to light, joint pain, chills, ear pain, sleeplessness, and irritability. In severe cases, pneumococcal disease can cause hearing loss, brain damage, and death.
Symptoms of pneumococcal pneumonia, a lung infection, include:
- Fever and chills
- Cough
- Rapid breathing or difficulty breathing
- Chest pain
Older adults with pneumococcal pneumonia may experience confusion or low alertness, rather than the more common symptoms listed above.
Symptoms of pneumococcal meningitis, an infection of the lining of the brain and spinal cord, include:
- Stiff neck
- Fever
- Headache
- Photophobia (eyes being more sensitive to light)
- Confusion
In babies, meningitis may cause poor eating and drinking, low alertness, and vomiting.
Symptoms of pneumococcal bacteremia, a blood infection, include:
- Fever
- Chills
- Low alertness
Symptoms of sepsis, the body’s extreme response to an infection, include:
- Confusion or disorientation
- Shortness of breath
- High heart rate
- Fever, shivering, or feeling very cold
- Extreme pain or discomfort
- Clammy or sweaty skin
Symptoms of otitis media (middle ear infection), which pneumococcal bacteria commonly cause, include:
- Ear pain
- A red, swollen ear drum
- Fever
- Sleepiness
Ear infections are usually mild and are more common than the more severe forms of pneumococcal disease. However, some children develop repeated ear infections and may need ear tubes.
Symptoms of sinus infections (sinusitis) include:
- Headache
- Stuffy or runny nose
- Loss of the sense of smell
- Facial pain or pressure
- Postnasal drip (mucus building up in the back of the throat or nose)
Streptococcus pneumoniae diagnosis
If Streptococcus pneumoniae infection is suspected or considered, Gram stain and culture of appropriate specimens should be obtained, when possible. Potential specimens may include 1 or more of the following 2:
- Blood
- Cerebrospinal fluid (CSF)
- Sputum
- Pleural fluid or lung aspirate
- Joint fluid
- Bone
- Other abscess or tissue specimens
Specimens should be obtained prior to the initiation of antibiotic therapy and inoculated directly into blood-culture bottles, when possible.
Antibiotic susceptibilities should be obtained routinely on all cultures with growth of Streptococcus pneumoniae. Note that minimum inhibitory concentration (MIC) breakpoints are different depending on the specimen type.
Other laboratory values that may be helpful in diagnosis and treatment include a complete blood cell (CBC) count and differential, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP).
In children who do not produce sputum and in adults with a nonproductive cough, the diagnosis may be made based on urine antigen testing for Streptococcus pneumoniae. As with urinary antigen testing for Legionella, antigenuria may not be present in early infection or in patients without bacteremia, but, if present, may persist after clinical resolution of infection. Evaluation of sputum via a combination of culture, Gram stain, and pneumococcal antigen was found to be the most useful way of achieving an etiologic diagnosis of community acquired pneumonia. Pneumococcal antigen detection in sputum has been shown to have high sensitivity (90%), even compared with urinary antigen detection essays, in early and mild community acquired pneumonia in pretreatment patients whose sputum specimens can be obtained 76. The pneumococcal urinary antigen assay may augment the standard diagnostic methods of blood culture and sputum culture, as it provides rapid results 77.
A commercially available urinary antigen test can detect the C-polysaccharide antigen of Streptococcus pneumoniae as a cause of community-acquired pneumonia. The urinary antigen test:
- Is rapid and simple to use
- Has a reasonable specificity in adults
- Can detect pneumococcal pneumonia after initiation of antibiotic therapy
In the diagnosis of Streptococcus pneumoniae community acquired pneumonia, the urinary antigen test has a sensitivity of 77%-88% and a specificity of 67%-100% 78. However, the clinical usefulness of this pneumococcal urinary antigen test is not well defined. The urinary antigen test is unable to provide antimicrobial susceptibility data, so it does not supplant traditional culture methods. The 2019 Infectious Diseases Society of America community acquired pneumonia guidelines do not recommend routine urinary testing for pneumococcal antigen in adults with community acquired pneumonia, except in adults with severe pneumonia 79.
The role of fiberoptic bronchoscopy is best done in the absence of adequate sputum for culture or when the patient is not responding to current therapy 80.
Culture and sensitivity
Antimicrobial susceptibility testing should be performed on all isolates of Streptococcus pneumoniae, regardless of the isolation site, because of the increasing prevalence of antibiotic resistant isolates. All isolates should be tested for susceptibility to penicillin and either cefotaxime or ceftriaxone. In addition, cerebrospinal fluid (CSF) isolates should be tested for susceptibility to vancomycin and meropenem. Cerebrospinal fluid (CSF) isolates that are found to be nonsusceptible to penicillin should also be tested for susceptibility to rifampin.
Microbiology laboratories should follow established guidelines regarding inoculum size and media (Mueller-Hinton agar with sheep, horse, or lysed horse red blood cells). Isolates from patients with invasive disease should undergo testing with quantitative minimal inhibitory concentration (MIC) techniques (eg, broth microdilution, antibiotic gradient strips).
The Clinical and Laboratory Institute (CLSI) (2010) has defined Streptococcus pneumoniae susceptibility as follows 81, 82:
- Pneumonia: For penicillin-sensitive Streptococcus pneumoniae (MIC < 2 μg/mL), penicillin G or amoxicillin is considered first-line therapy. For penicillin-resistant Streptococcus pneumoniae (MIC ≥2 μg/mL), the choice of antimicrobial agent should be directed by susceptibility testing.
- Cefotaxime or ceftriaxone considerations are as follows:
- Susceptible (non-CNS/CNS): MIC is ≤1/0.5 µg/mL, respectively.
- Intermediate (non-CNS/CNS): MIC is 2/1 µg/mL, respectively.
- Resistant (non-CNS/CNS): MIC is ≥4/2 µg/mL, respectively.
Strains with intermediate or resistant susceptibility patterns should be considered nonsusceptible and alternate therapy used.
Imaging studies
- Chest radiography: Chest radiography should be performed in most patients with evidence of invasive pneumococcal infection and in those with pneumococcal pneumonia. The typical chest radiography finding in adolescents and adults with pneumococcal pneumonia is lobar consolidation. Infants and young children with pneumococcal pneumonia more often have a pattern of scattered parenchymal consolidation and bronchopneumonia. Other chest radiography findings may include air bronchograms, pleural effusions/empyema, pneumatoceles, and, rarely, abscesses. Cavitation is not a feature of Streptococcus pneumoniae pneumonia and, if present, should prompt investigation for other pathogens.
- Ultrasonography/CT scanning:
- Chest ultrasonography or chest CT scanning may be obtained to provide information on the presence and/or extent of pleural effusion/empyema and parenchymal disease. Studies investigating the diagnostic utility of lung ultrasonography to diagnose pneumonia have also been promising 83.
- Echocardiography should be performed in patients in whom endocarditis is suspected.
- Sinus CT scanning may provide information about the presence and extent of sinus disease. Positive findings include opacification or air-fluid levels.
- Facial CT scanning should be obtained in patients with periorbital or orbital cellulitis to look for evidence of soft tissue swelling, bony involvement, cranial nerve impingement, or proptosis.
- MRI/CT scanning:
- MRI or CT scanning of affected bones or joints should be obtained to evaluate for evidence of joint destruction, periosteal elevation, or a mass.
- An MRI of the brain may be obtained in patients with meningitis to determine the location and extent of involvement but is not required by Infectious Disease Society of America (IDSA) guidelines.
Procedures
- Middle ear fluid aspiration
- Pleural fluid aspiration
- Chest tube thoracostomy or catheter placement
- Video-assisted thoracoscopy (VATS) or pleural decortication
- Lumbar puncture
- Joint fluid aspiration and/or wash-out of joint space
- Bone biopsy
- Soft tissue/muscle biopsy
Streptococcus pneumoniae treatment
Antibiotics are the mainstay of treatment in Streptococcus pneumoniae infections. Antibiotic treatment for serious pneumococcal infections typically includes ‘broad-spectrum’ antibiotics until results of antibiotic sensitivity testing are available. Antibiotic sensitivity testing shows which antibiotics will be most successful at treating a bacterial infection. Broad-spectrum antibiotics work against a wide range of bacteria. Once the sensitivity of the bacteria is known, doctors may choose a more targeted (or ‘narrow-spectrum’) antibiotic.
Streptococcus pneumoniae bacteria are resistant to one or more antibiotics in more than 30% of cases 84. The CDC, as well as many state health departments, maintain a population-based surveillance system (the Active Bacterial Core surveillance system) that investigates the epidemiology and susceptibility patterns of invasive pneumococcal infections in the United States. In 2019, Active Bacterial Core surveillance estimated there were about 30,300 cases of invasive pneumococcal disease 85. 3.8% and 2.4% of the isolates obtained showed intermediate or resistant susceptibility patterns to penicillin and cefotaxime, respectively 85. One hundred percent of the isolated were susceptible to vancomycin 85. The prevalence of resistance varies greatly among countries, states, counties, and within populations in particular cities and may be as high as 30%-40% in some locations 86. Resistance rates are generally higher in most European countries, as well as in Hong Kong and Thailand 87.
Penicillin-resistant pneumococci are often resistant to multiple additional classes of antibiotics, including other penicillin derivatives, cephalosporins, sulfonamides, trimethoprim-sulfamethoxazole (through amino acid changes), macrolides (through methylation or via an efflux pump), quinolones (through decreased permeability, efflux pumps, and alteration of enzymes), and chloramphenicol (through inactivating enzymes).
Resistance rates of pneumococcal isolates in the United States to trimethoprim-sulfamethoxazole, tetracycline, and the macrolides are relatively high. Some isolates (< 10% in the United States) that are resistant to macrolides are also resistant to clindamycin.
State and local health departments have reported outbreaks of drug-resistant Streptococcus pneumoniae in 88:
- Long-term care settings
- Institutions for people living with HIV
- Childcare centers
Streptococcus pneumoniae antibiotics
Penicillin and its derivatives are inexpensive effective antibiotics for treating pneumococcal infections when they are used against susceptible isolates. Penicillins can be administered orally or parenterally and work by inhibiting cell wall synthesis. Penicillin G is the parenteral drug of choice for susceptible Streptococcus pneumoniae infections, and other parenteral beta-lactams do not provide additional or improved coverage (nor do beta-lactamase inhibitor combinations).
Cephalosporins’ mechanism of action and modes of resistance are the same as for all other beta-lactams. First-generation cephalosporins provide similar coverage in the treatment of penicillin-susceptible strains, although many of them have higher MICs. Penicillin- and cefotaxime-susceptible strains of Streptococcus pneumoniae were estimated at 96.2% and 97.6%, respectively 85.
In most cases, macrolides have activity against penicillin-susceptible strains of Streptococcus pneumoniae. However, between 1998 and 2011, resistance rates have increased to an estimated 25%-45% in the United States 89. In 2019, the CDC’s active bacterial core surveillance report found the erythromycin resistance rate to be 29.3% 85.
Macrolides have poor CSF penetration and should not be used to treatment meningitis 90.
Most pneumococcal isolates in the United States remain susceptible to respiratory fluoroquinolones. In the United States, less than 1% of Streptococcus pneumoniae isolates are resistant to levofloxacin, moxifloxacin, or gemifloxacin 91. Ciprofloxacin and ofloxacin have limited activity against pneumococcal infections. Fluoroquinolones achieve excellent serum drug levels and tissue penetration. Specific populations in whom the use of fluoroquinolones is traditionally increased (eg, nursing home residents) have shown increased rates of pneumococcal resistance to fluoroquinolones, serving as a reminder that consideration of their empiric use in uncomplicated respiratory infections should be tempered by concern for the promotion of further antimicrobial resistance.
Vancomycin, dalbavancin, and telavancin are glycopeptide antibiotics that have demonstrated efficacy against pneumococcal infections 92. To date, no clinical or in vitro evidence of pneumococcal resistance to vancomycin has been reported in the United States, and it is the drug of choice (with a third-generation cephalosporin) in the treatment of penicillin-resistant pneumococcal meningitis.
The increasing number of pneumococcal isolates resistant to trimethoprim-sulfamethoxazole precludes its use unless susceptibilities are known and beta-lactam use is contraindicated.
Clindamycin may also be used to treat nonmeningeal Streptococcus pneumoniae infections. Approximately 5%-10% of Streptococcus pneumoniae strains in the United States are resistant to clindamycin 93. As such, clindamycin should be used only after susceptibility testing has confirmed activity on clinical isolates. Penicillin or macrolide resistance may also be associated with clindamycin resistance in individual isolates.
Carbapenems are also effective against Streptococcus pneumoniae but should be reserved for specific cases given their broad coverage and the potential for development of resistance by multiple organisms.
Streptococcus pneumoniae pneumonia
Streptococcus pneumoniae pneumonia treatment is antibiotic therapy and supportive care including mechanical ventilation if necessary. Treatment of community-acquired pneumonia varies based on the area of practice and severity of the disease. In 2019, the Infectious Disease Society of America (IDSA) guidelines for diagnosis and treatment of community-acquired pneumonia were published 79. In healthy outpatient adults without comorbidities (eg, chronic heart, lung, liver or renal disease; diabetes mellitus, alcoholism, malignancy and asplenia), monotherapy with amoxicillin, doxycycline, or a macrolide (clarithromycin or azithromycin) is recommended; however, the use of macrolides should be limited to areas with less than 25% pneumococcal resistance to macrolides. Outpatients with the comorbidities listed above should receive either (1) combination therapy with amoxicillin/clavulanate or a cephalosporin (cefpodoxime or cefuroxime) plus a macrolide or doxycycline or (2) monotherapy with a respiratory fluoroquinolone (moxifloxacin, levofloxacin, or gemifloxacin). Regarding the treatment of hospitalized patients with CAP, the recommended regimens consist of (1) combination therapy with a beta-lactam and a macrolide or (2) monotherapy with a respiratory fluoroquinolone. There was not enough evidence to support beta-lactam monotherapy over fluoroquinolone therapy or combination beta-lactam/macrolide in hospitalized patients.
A recent Cochrane review shows the non-superiority of any outpatient antibiotic regimen for community-acquired pneumonia concerning the other drug classes 94, 95.
The first dose of antibiotics should be given as quickly as possible after the definitive diagnosis. The American College of Emergency Physicians policy statement gives a level B recommendation that there is not enough evidence to establish a benefit in mortality or morbidity from starting the antibiotics in less than 4, 6, or 8 hours 96. The American College of Emergency Physicians clinical policy also notes that there is not enough evidence to determine if there is a benefit in morbidity or mortality from antibiotics being administered within any specific time course but recommends to begin antibiotics as soon as the diagnosis is made.
Patients with pneumococcal pneumonia who do not respond or respond slower than usual to initial treatment should undergo follow-up chest radiography. Worsening disease and/or the presence of a pleural effusion may indicate the need for consultation with a pulmonologist, an infectious disease specialist, and/or a surgeon for further intervention. Oral therapy can be initiated when patients have clinically improved and become afebrile. Repeat chest radiography should be performed 4-8 weeks after therapy is completed to ensure resolution of disease. Chest radiography findings may remain abnormal for weeks to months, particularly following severe disease or complicated pneumonias.
Patients with complicated pneumonia having parapneumonic effusion may need a tube thoracostomy and if it further progresses to empyema and is resistant to drainage by tube thoracostomy then decortication is required with video-assisted thoracoscopic surgery (VATS).
Streptococcus pneumoniae meningitis
Patients with Streptococcus pneumoniae meningitis should be admitted to the hospital and treated with parenteral antibiotics. Per Infectious Disease Society of America guidelines, the initial treatment for pneumonococcal meningitis should be vancomycin and a third-generation cephalosporin 97.
For the treatment of pneumococcal meningitis in children who are allergic to beta-lactams, a combination of vancomycin and rifampin should be considered. Monotherapy with vancomycin should not be attempted, as it is difficult to achieve sustained adequate bactericidal concentrations of vancomycin in the CSF. Monotherapy with rifampin should also not be attempted owing to the high potential for rapid development of resistance in this setting.
In patients infected with rifampin-sensitive pneumococcal isolates, the addition of rifampin should be considered after 48 hours if (1) the clinical condition has worsened despite treatment with vancomycin and cefotaxime/ceftriaxone, (2) repeat lumbar puncture repeatedly yields positive culture results, and/or (3) the isolate displays an minimum inhibitory concentration (MIC) to cefotaxime/ceftriaxone of ≥4 µg/mL.
The recommendations for treatment of bacterial meningitis in adults are similar to those in children.
The use of systemic steroids within 15 minutes of initiating infusion of antibiotics in adult patients with bacterial meningitis is usually recommended with caution, as they may decrease cerebrospinal fluid (CSF) antibiotic concentration; patients with meningitis treated with steroids should be monitored closely 98.
Steroids can be considered prior to antibiotic therapy in children aged 6 weeks and older with possible pneumococcal meningitis. If used, they should be given before or at the time of the first dose of antibiotics.
A repeat lumbar puncture should be considered after 48 hours of therapy in the following circumstances:
- Patients whose isolates are not susceptible to penicillin based on oxacillin disc diffusion testing or minimum inhibitory concentration (MIC) testing without pending results of cefotaxime and/or ceftriaxone susceptibilities
- Patients whose condition has worsened or has not improved
- Patients who received steroid therapy (which could alter the ability to observe clinical improvement/worsening)
Intravenous fluids, parenteral/enteral nutrition, and other medications should be used as indicated in appropriate clinical instances.
Streptococcus pneumoniae bacteremia and sepsis
Patients with pneumococcal bacteremia should be treated with appropriate antibiotics and supportive care.
Repeat blood cultures should always be obtained in patients with Streptococcus pneumoniae bacteremia until culture results are negative.
Patients with signs or symptoms of sepsis should be admitted to the hospital and treated aggressively with antibiotics and other medical therapies, as indicated.
Streptococcus pneumoniae conjunctivitis, otitis media, sinusitis, bronchitis, and tracheobronchitis
Most patients with conjunctivitis, otitis media, sinusitis, bronchitis, and tracheobronchitis due to Streptococcus pneumoniae infection can be treated on an outpatient basis with appropriate antibiotics. Compliance and follow-up should be ensured.
Infants and elderly patients, as well as those with immunodeficiencies, underlying disease, or signs of severe disease, should be treated more aggressively and hospitalized when indicated.
Otitis media
The guideline produced by the American Academies of Pediatrics and Family Practitioners for the treatment of pneumococcal otitis media recommends first-line treatment of most patients with amoxicillin 80-90 mg/kg/day or amoxicillin-clavulanate (amoxicillin 90 mg/kg/day with clavulanate 6.4 mg/kg/day). Alternatives include cefdinir, cefuroxime, cefpodoxime, or ceftriaxone. These alternative antibiotics vary in their efficacy against the different pathogens known to cause otitis media. US data on in vitro susceptibility of Streptococcus pneumoniae to cefdinir and cefuroxime are 70% to 80%, respectively, compared with 84% to 92% to amoxicillin 99.
Patients who do not improve within 48-72 hours should be re-evaluated and their antibiotics switched to amoxicillin-clavulanate or a second- or third-generation oral cephalosporin, although highly resistant pneumococci may require treatment with parenteral ceftriaxone in order to achieve adequate serum levels of antibiotics.
Sinusitis
The typical pathogens that cause sinusitis mimic those of otitis media; therefore, initial therapeutic recommendations are similar. In adult allergic patients and in adults who do not respond to initial therapy, fluoroquinolones provide appropriate coverage. In this clinical situation, this class of antibiotics is not approved for children.
Other invasive infections
Purulent pneumococcal pericarditis and endocarditis are serious diseases and should be treated aggressively with appropriate courses of parenteral antibiotics.
Blood cultures should be obtained until multiple negative sets are documented. Repeat chest radiography, echocardiography, and other imaging tests may be repeated as recommended to monitor disease resolution.
Patients with osteomyelitis and joint infections caused by S pneumoniae infection should be monitored closely for a decrease in pain and inflammatory markers and improved use of the affected limb or joint. Failure to improve should prompt re-evaluation of the area via aspiration, washout, biopsy, or repeat imaging.
Surgical care
Patients with complicated pneumonia may require a chest tube for drainage of pleural fluid; video-assisted thoracoscopic surgery (VATS) or decortication may be required in more severe cases or in those with empyema.
In patients with suspected septic arthritis or osteomyelitis, synovial fluid or bone tissue should be obtained for Gram stain, cell count, histology, and culture.
Patients with recurrent or chronic otitis media, periorbital or orbital cellulitis, or facial cellulitis may require surgical intervention.
Streptococcus pneumoniae prognosis
Pneumococcal conjunctivitis, otitis media, and sinusitis in developed countries where appropriate antibiotics are available usually carry an excellent prognosis 100.
The prognosis of pneumococcal pneumonia depends largely on underlying factors, including age, immunosuppression, availability of antibiotics, and extent of lung involvement. It appears that most adults (mean age, 64.6 years) who survive invasive pneumococcal pneumonia lose a mean 9.9 years of longevity 101.
The prognosis of pneumococcal meningitis is also related in part to host factors. Most studies have shown that morbidity rates in otherwise healthy US children with meningitis are usually less than 10%; however, neurological complications are common 100.
- Mufson M A. Streptococcus pneumoniae. In: Mandell G L, Douglas R G Jr, Bennett J E, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone, Inc.; 1990. pp. 1539–1550.[↩]
- Pneumococcal Infections (Streptococcus pneumoniae). https://emedicine.medscape.com/article/225811-overview[↩][↩][↩][↩][↩][↩][↩]
- Pneumococcal Disease. https://www.cdc.gov/vaccines/pubs/pinkbook/pneumo.html[↩][↩][↩][↩]
- Jedrzejas M. J. (2001). Pneumococcal virulence factors: structure and function. Microbiology and molecular biology reviews : MMBR, 65(2), 187–207. https://doi.org/10.1128/MMBR.65.2.187-207.2001[↩][↩]
- Weiser, J. N., Ferreira, D. M., & Paton, J. C. (2018). Streptococcus pneumoniae: transmission, colonization and invasion. Nature reviews. Microbiology, 16(6), 355–367. https://doi.org/10.1038/s41579-018-0001-8[↩][↩][↩][↩]
- Nunes, S., Sá-Leão, R., Carriço, J., Alves, C. R., Mato, R., Avô, A. B., Saldanha, J., Almeida, J. S., Sanches, I. S., & de Lencastre, H. (2005). Trends in drug resistance, serotypes, and molecular types of Streptococcus pneumoniae colonizing preschool-age children attending day care centers in Lisbon, Portugal: a summary of 4 years of annual surveillance. Journal of clinical microbiology, 43(3), 1285–1293. https://doi.org/10.1128/JCM.43.3.1285-1293.2005[↩]
- Streptococcus pneumoniae. https://www.cdc.gov/pneumococcal/clinicians/streptococcus-pneumoniae.html[↩][↩]
- Melegaro, A., Gay, N. J., & Medley, G. F. (2004). Estimating the transmission parameters of pneumococcal carriage in households. Epidemiology and infection, 132(3), 433–441. https://doi.org/10.1017/s0950268804001980[↩]
- Wyllie, A. L., Rümke, L. W., Arp, K., Bosch, A., Bruin, J. P., Rots, N. Y., Wijmenga-Monsuur, A. J., Sanders, E., & Trzciński, K. (2016). Molecular surveillance on Streptococcus pneumoniae carriage in non-elderly adults; little evidence for pneumococcal circulation independent from the reservoir in children. Scientific reports, 6, 34888. https://doi.org/10.1038/srep34888[↩]
- Wyllie, A. L., Wijmenga-Monsuur, A. J., van Houten, M. A., Bosch, A., Groot, J. A., van Engelsdorp Gastelaars, J., Bruin, J. P., Bogaert, D., Rots, N. Y., Sanders, E., & Trzciński, K. (2016). Molecular surveillance of nasopharyngeal carriage of Streptococcus pneumoniae in children vaccinated with conjugated polysaccharide pneumococcal vaccines. Scientific reports, 6, 23809. https://doi.org/10.1038/srep23809[↩]
- Mackenzie, G. A., Leach, A. J., Carapetis, J. R., Fisher, J., & Morris, P. S. (2010). Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC infectious diseases, 10, 304. https://doi.org/10.1186/1471-2334-10-304[↩]
- Morris, P. S., Leach, A. J., Silberberg, P., Mellon, G., Wilson, C., Hamilton, E., & Beissbarth, J. (2005). Otitis media in young Aboriginal children from remote communities in Northern and Central Australia: a cross-sectional survey. BMC pediatrics, 5, 27. https://doi.org/10.1186/1471-2431-5-27[↩]
- Smith-Vaughan, H., Marsh, R., Mackenzie, G., Fisher, J., Morris, P. S., Hare, K., McCallum, G., Binks, M., Murphy, D., Lum, G., Cook, H., Krause, V., Jacups, S., & Leach, A. J. (2009). Age-specific cluster of cases of serotype 1 Streptococcus pneumoniae carriage in remote indigenous communities in Australia. Clinical and vaccine immunology : CVI, 16(2), 218–221. https://doi.org/10.1128/CVI.00283-08[↩]
- Gray BM, Turner ME, Dillon HC Jr. Epidemiologic studies of Streptococcus pneumoniae in infants. The effects of season and age on pneumococcal acquisition and carriage in the first 24 months of life. Am J Epidemiol. 1982 Oct;116(4):692-703. doi: 10.1093/oxfordjournals.aje.a113452[↩][↩]
- Gray BM, Converse GM 3rd, Dillon HC Jr. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980 Dec;142(6):923-33. doi: 10.1093/infdis/142.6.923[↩]
- Dunais B, Pradier C, Carsenti H, Sabah M, Mancini G, Fontas E, Dellamonica P. Influence of child care on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae. Pediatr Infect Dis J. 2003 Jul;22(7):589-92. doi: 10.1097/01.inf.0000073203.88387.eb[↩]
- Högberg, L., Geli, P., Ringberg, H., Melander, E., Lipsitch, M., & Ekdahl, K. (2007). Age- and serogroup-related differences in observed durations of nasopharyngeal carriage of penicillin-resistant pneumococci. Journal of clinical microbiology, 45(3), 948–952. https://doi.org/10.1128/JCM.01913-06[↩]
- Davis SM, Deloria-Knoll M, Kassa HT, O’Brien KL. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine. 2013 Dec 17;32(1):133-45. doi: 10.1016/j.vaccine.2013.05.005[↩]
- Marks, L. R., Reddinger, R. M., & Hakansson, A. P. (2014). Biofilm formation enhances fomite survival of Streptococcus pneumoniae and Streptococcus pyogenes. Infection and immunity, 82(3), 1141–1146. https://doi.org/10.1128/IAI.01310-13[↩]
- Austrian R. Some aspects of the pneumococcal carrier state. J Antimicrob Chemother. 1986 Jul;18 Suppl A:35-45. doi: 10.1093/jac/18.supplement_a.35[↩]
- Dion CF, Ashurst JV. Streptococcus Pneumoniae. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470537[↩][↩]
- Luna, C. M., Pulido, L., Niederman, M. S., Casey, A., Burgos, D., Leiva Agüero, S. D., Grosso, A., Membriani, E., Entrocassi, A. C., Rodríquez Fermepin, M., Vay, C. A., Garcia, S., & Famiglietti, A. (2018). Decreased relative risk of pneumococcal pneumonia during the last decade, a nested case-control study. Pneumonia (Nathan Qld.), 10, 9. https://doi.org/10.1186/s41479-018-0053-6[↩]
- Grau, I., Ardanuy, C., Calatayud, L., Rolo, D., Domenech, A., Liñares, J., & Pallares, R. (2012). Invasive pneumococcal disease in healthy adults: increase of empyema associated with the clonal-type Sweden(1)-ST306. PloS one, 7(8), e42595. https://doi.org/10.1371/journal.pone.0042595[↩]
- Lehmann D, Willis J, Moore HC, Giele C, Murphy D, Keil AD, Harrison C, Bayley K, Watson M, Richmond P. The changing epidemiology of invasive pneumococcal disease in aboriginal and non-aboriginal western Australians from 1997 through 2007 and emergence of nonvaccine serotypes. Clin Infect Dis. 2010 Jun 1;50(11):1477-86. doi: 10.1086/652440[↩]
- Fedson DS, Musher DM, Eskola J. 1998. Pneumococcal vaccine In Plotkin SA, Ordenstein WA (ed), Vaccines, 3rd ed. WB Saunders, Philadelphia.[↩]
- Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, Finkelstein JA. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011 Apr 18;29(18):3398-412. doi: 10.1016/j.vaccine.2011.02.088[↩]
- Loughran, A. J., Orihuela, C. J., & Tuomanen, E. I. (2019). Streptococcus pneumoniae: Invasion and Inflammation. Microbiology spectrum, 7(2), 10.1128/microbiolspec.GPP3-0004-2018. https://doi.org/10.1128/microbiolspec.GPP3-0004-2018[↩]
- Kramer MR, Rudensky B, Hadas-Halperin I, Isacsohn M, Melzer E. Pneumococcal bacteremia–no change in mortality in 30 years: analysis of 104 cases and review of the literature. Isr J Med Sci. 1987 Mar;23(3):174-80.[↩]
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, Facklam RR, Jorgensen JH, Schuchat A; Active Bacterial Core Surveillance of the Emerging Infections Program Network. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003 May 1;348(18):1737-46. doi: 10.1056/NEJMoa022823[↩]
- https://phil.cdc.gov/Details.aspx?pid=23253[↩]
- https://phil.cdc.gov/Details.aspx?pid=9996[↩]
- https://phil.cdc.gov/Details.aspx?pid=10864[↩]
- https://phil.cdc.gov/Details.aspx?pid=19332[↩]
- Pneumococcal Disease Transmission. https://www.cdc.gov/pneumococcal/clinicians/transmission.html[↩]
- Pneumococcal Disease Clinical Features. https://www.cdc.gov/pneumococcal/clinicians/clinical-features.html[↩][↩]
- Alqahtani AS, Tashani M, Ridda I, Gamil A, Booy R, Rashid H. Burden of clinical infections due to S. pneumoniae during Hajj: A systematic review. Vaccine. 2018 Jul 16;36(30):4440-4446. doi: 10.1016/j.vaccine.2018.04.031[↩]
- Boeddha, N. P., Schlapbach, L. J., Driessen, G. J., Herberg, J. A., Rivero-Calle, I., Cebey-López, M., Klobassa, D. S., Philipsen, R., de Groot, R., Inwald, D. P., Nadel, S., Paulus, S., Pinnock, E., Secka, F., Anderson, S. T., Agbeko, R. S., Berger, C., Fink, C. G., Carrol, E. D., Zenz, W., … EUCLIDS consortium (2018). Mortality and morbidity in community-acquired sepsis in European pediatric intensive care units: a prospective cohort study from the European Childhood Life-threatening Infectious Disease Study (EUCLIDS). Critical care (London, England), 22(1), 143. https://doi.org/10.1186/s13054-018-2052-7[↩]
- Bacteremia. https://emedicine.medscape.com/article/961169-overview[↩]
- Waddle E, Jhaveri R. Outcomes of febrile children without localising signs after pneumococcal conjugate vaccine. Arch Dis Child. 2009 Feb;94(2):144-7. doi: 10.1136/adc.2007.130583[↩]
- Dagan R, Greenberg D, Jacobs MR. Pneumococcal Infections. Feigin RD, Cherry JD, Demmler GJ, Kaplan SL. Textbook of Pediatric Infectious Diseases. 5th. Philadelphia, Pennsylvania: Saunders (Elsevier Science); 2004. 1: 1204-1258/90.[↩][↩][↩][↩]
- Meningitis. https://medlineplus.gov/meningitis.html[↩]
- Musher DM. Streptococcus pneumoniae. Mandell GL, Bennett JE, Dolin R. Principles and Practice of Infectious Diseases. 6th. Philadelphia, Pennsylvania: Elsevier, Churchill Livingstone; 2005. 2: 197.[↩]
- Worsøe L, Cayé-Thomasen P, Brandt CT, Thomsen J, Østergaard C. Factors associated with the occurrence of hearing loss after pneumococcal meningitis. Clin Infect Dis. 2010 Oct 15;51(8):917-24. doi: 10.1086/656409[↩]
- Brunton S, Carmichael BP, Colgan R, Feeney AS, Fendrick AM, Quintiliani R, Scott G. Acute exacerbation of chronic bronchitis: a primary care consensus guideline. Am J Manag Care. 2004 Oct;10(10):689-96.[↩]
- Bradley JS, Kaplan SL, Tan TQ, Barson WJ, Arditi M, Schutze GE, Wald ER, Givner LB, Mason EO. Pediatric pneumococcal bone and joint infections. The Pediatric Multicenter Pneumococcal Surveillance Study Group (PMPSSG). Pediatrics. 1998 Dec;102(6):1376-82. doi: 10.1542/peds.102.6.1376[↩]
- Pneumococcal Disease Risk Factors. https://www.cdc.gov/pneumococcal/clinicians/risk-factors.html[↩]
- Pneumococcal Infections (Streptococcus pneumoniae) Clinical Presentation. https://emedicine.medscape.com/article/225811-clinical[↩][↩]
- Pilishvili T., Bennett N.M. Pneumococcal disease prevention among adults: Strategies for the use of pneumococcal vaccines. Vaccine. 2015;33:D60–D65. doi: 10.1016/j.vaccine.2015.05.102[↩]
- Pneumococcal Vaccination: Information for Healthcare Professionals. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/index.html[↩]
- Pneumococcal Vaccine Recommendations. https://www.cdc.gov/pneumococcal/hcp/vaccine-recommendations/index.html[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- About Pneumococcal Vaccines. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html[↩][↩][↩][↩][↩][↩]
- Types of Pneumococcal Vaccines. https://www.cdc.gov/pneumococcal/vaccines/types.html[↩][↩]
- VAXNEUVANCE. https://www.fda.gov/vaccines-blood-biologics/vaccines/vaxneuvance[↩]
- PREVNAR 20. https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar-20[↩]
- CAPVAXIVE. https://www.fda.gov/vaccines-blood-biologics/capvaxive[↩][↩][↩][↩]
- Centers for Disease Control and Prevention (CDC). Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older. MMWR Morb Mortal Wkly Rep. 2012 Jun 1;61(21):394-5. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6121a3.htm[↩][↩]
- PNEUMOVAX 23 – Pneumococcal Vaccine, Polyvalent. https://www.fda.gov/vaccines-blood-biologics/vaccines/pneumovax-23-pneumococcal-vaccine-polyvalent[↩][↩]
- Pneumococcal Vaccination. https://www.cdc.gov/pneumococcal/vaccines/index.html[↩]
- ACIP Recommendations: Pneumococcal Vaccine. https://www.cdc.gov/acip-recs/hcp/vaccine-specific/pneumococcal.html[↩][↩]
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, Patton M, McDonough A, Moradoghli-Haftvani A, Smith H, Mellelieu T, Pride MW, Crowther G, Schmoele-Thoma B, Scott DA, Jansen KU, Lobatto R, Oosterman B, Visser N, Caspers E, Smorenburg A, Emini EA, Gruber WC, Grobbee DE. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015 Mar 19;372(12):1114-25. doi: 10.1056/NEJMoa1408544[↩][↩]
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Holtzman C, Harrison LH, Zansky SM, Rosen JB, Reingold A, Scherzinger K, Thomas A, Guevara RE, Motala T, Eason J, Barnes M, Petit S, Farley MM, McGee L, Jorgensen JH, Whitney CG. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med. 2016 May;4(5):399-406. doi: 10.1016/S2213-2600(16)00052-7[↩]
- Pilishvili T, Bennett NM. Pneumococcal disease prevention among adults: Strategies for the use of pneumococcal vaccines. Vaccine. 2015 Nov 27;33 Suppl 4:D60-5. doi: 10.1016/j.vaccine.2015.05.102[↩]
- Pneumococcal Vaccine Recommendations. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/recommendations.html[↩]
- Pneumococcal Vaccination: Summary of Who and When to Vaccinate. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html[↩][↩][↩][↩][↩]
- https://www.fda.gov/media/179428/download[↩][↩][↩][↩][↩]
- Gutierrez Rodriguez MA, Ordobas Gavin MA, Garcia-Comas L, Sanz Moreno JC, Cordoba Deorador E, Lasheras Carbajo MD, Taveira Jimenez JA, Martin Martinez F, Iniesta Fornies D, Arce Arnaez A. Effectiveness of 23-valent pneumococcal polysaccharide vaccine in adults aged 60 years and over in the Region of Madrid, Spain, 2008-2011. Euro Surveill. 2014 Oct 9;19(40):20922. doi: 10.2807/1560-7917.es2014.19.40.20922[↩]
- Kim JH, Chun BC, Song JY, Kim HY, Bae IG, Kim DM, Choi YH, Jun YH, Choi WS, Kang SH, Kwon HH, Jeong HW, Kee SY, Hur J, Chung JW, Yoon YK, Sohn JW, Yang KS, Kim MJ. Direct effectiveness of pneumococcal polysaccharide vaccine against invasive pneumococcal disease and non-bacteremic pneumococcal pneumonia in elderly population in the era of pneumococcal conjugate vaccine: A case-control study. Vaccine. 2019 May 9;37(21):2797-2804. doi: 10.1016/j.vaccine.2019.04.017[↩]
- Andrews NJ, Waight PA, George RC, Slack MP, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012 Nov 6;30(48):6802-8. doi: 10.1016/j.vaccine.2012.09.019[↩]
- Shimbashi, R., Suzuki, M., Chang, B., Watanabe, H., Tanabe, Y., Kuronuma, K., Oshima, K., Maruyama, T., Takeda, H., Kasahara, K., Fujita, J., Nishi, J., Kubota, T., Tanaka-Taya, K., Matsui, T., Sunagawa, T., Oishi, K., & Adult IPD Study Group (2020). Effectiveness of 23-Valent Pneumococcal Polysaccharide Vaccine against Invasive Pneumococcal Disease in Adults, Japan, 2013-2017. Emerging infectious diseases, 26(10), 2378–2386. https://doi.org/10.3201/eid2610.191531[↩]
- Administering Pneumococcal Vaccines. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/administering-vaccine.html[↩][↩][↩]
- Pneumococcal Conjugate Vaccine: What You Need to Know. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/pcv.pdf[↩][↩]
- Ask the Experts: Pneumococcal. https://www.immunize.org/ask-experts/topic/pneumococcal[↩][↩][↩][↩][↩][↩][↩][↩]
- Theilacker C, Fletcher MA, Jodar L, Gessner BD. PCV13 Vaccination of Adults against Pneumococcal Disease: What We Have Learned from the Community-Acquired Pneumonia Immunization Trial in Adults (CAPiTA). Microorganisms. 2022 Jan 8;10(1):127. doi: 10.3390/microorganisms10010127. Erratum in: Microorganisms. 2022 May 12;10(5):1018. doi: 10.3390/microorganisms10051018[↩][↩]
- Weekly Epidemiological Record. https://www.who.int/publications/journals/weekly-epidemiological-record[↩]
- Rudan I, Campbell H. The deadly toll of S pneumoniae and H influenzae type b. Lancet. 2009 Sep 12;374(9693):854-6. doi: 10.1016/S0140-6736(09)61608-1[↩]
- Fukushima K, Nakamura S, Inoue Y, Higashiyama Y, Ohmichi M, Ishida T, Yoshimura K, Sawai T, Takayanagi N, Nakahama C, Kakugawa T, Izumikawa K, Aoki N, Nishioka Y, Kosaka O, Kohno S. Utility of a Sputum Antigen Detection Test in Pneumococcal Pneumonia and Lower Respiratory Infectious Disease in Adults. Intern Med. 2015;54(22):2843-50. doi: 10.2169/internalmedicine.54.4082[↩]
- Mandell, L. A., Wunderink, R. G., Anzueto, A., Bartlett, J. G., Campbell, G. D., Dean, N. C., Dowell, S. F., File, T. M., Jr, Musher, D. M., Niederman, M. S., Torres, A., Whitney, C. G., Infectious Diseases Society of America, & American Thoracic Society (2007). Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 44 Suppl 2(Suppl 2), S27–S72. https://doi.org/10.1086/511159[↩]
- Blaschke A. J. (2011). Interpreting assays for the detection of Streptococcus pneumoniae. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 52 Suppl 4(Suppl 4), S331–S337. https://doi.org/10.1093/cid/cir048[↩]
- Metlay, J. P., Waterer, G. W., Long, A. C., Anzueto, A., Brozek, J., Crothers, K., Cooley, L. A., Dean, N. C., Fine, M. J., Flanders, S. A., Griffin, M. R., Metersky, M. L., Musher, D. M., Restrepo, M. I., & Whitney, C. G. (2019). Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. American journal of respiratory and critical care medicine, 200(7), e45–e67. https://doi.org/10.1164/rccm.201908-1581ST[↩][↩]
- van der Eerden MM, Vlaspolder F, de Graaff CS, Groot T, Jansen HM, Boersma WG. Value of intensive diagnostic microbiological investigation in low- and high-risk patients with community-acquired pneumonia. Eur J Clin Microbiol Infect Dis. 2005 Apr;24(4):241-9. doi: 10.1007/s10096-005-1316-8[↩]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 18th Informational Supplement. 2008.[↩]
- Effects of New Penicillin Susceptibility Breakpoints for Streptococcus pneumoniae — United States, 2006–2007. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5750a2.htm[↩]
- Chavez, M. A., Shams, N., Ellington, L. E., Naithani, N., Gilman, R. H., Steinhoff, M. C., Santosham, M., Black, R. E., Price, C., Gross, M., & Checkley, W. (2014). Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respiratory research, 15(1), 50. https://doi.org/10.1186/1465-9921-15-50[↩]
- ANTIBIOTIC RESISTANCE THREATS IN THE UNITED STATES 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf[↩]
- Active Bacterial Core Surveillance (ABCs) Report Emerging Infections Program Network. Streptococcus pneumoniae, 2019. https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2019.pdf[↩][↩][↩][↩][↩]
- Karlowsky JA, Thornsberry C, Jones ME, Evangelista AT, Critchley IA, Sahm DF; TRUST Surveillance Program. Factors associated with relative rates of antimicrobial resistance among Streptococcus pneumoniae in the United States: results from the TRUST Surveillance Program (1998-2002). Clin Infect Dis. 2003 Apr 15;36(8):963-70. doi: 10.1086/374052[↩]
- Song, J. H., Jung, S. I., Ko, K. S., Kim, N. Y., Son, J. S., Chang, H. H., Ki, H. K., Oh, W. S., Suh, J. Y., Peck, K. R., Lee, N. Y., Yang, Y., Lu, Q., Chongthaleong, A., Chiu, C. H., Lalitha, M. K., Perera, J., Yee, T. T., Kumarasinghe, G., Jamal, F., … Shibl, A. (2004). High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrobial agents and chemotherapy, 48(6), 2101–2107. https://doi.org/10.1128/AAC.48.6.2101-2107.2004[↩]
- Pneumococcal Disease Drug Resistance. https://www.cdc.gov/pneumococcal/clinicians/drug-resistance.html[↩]
- Jones RN, Sader HS, Mendes RE, Flamm RK. Update on antimicrobial susceptibility trends among Streptococcus pneumoniae in the United States: report of ceftaroline activity from the SENTRY Antimicrobial Surveillance Program (1998-2011). Diagn Microbiol Infect Dis. 2013 Jan;75(1):107-9. doi: 10.1016/j.diagmicrobio.2012.08.024[↩]
- Hawser SP. Activity of tigecycline against Streptococcus pneumoniae, an important causative pathogen of community-acquired pneumonia (CAP). J Infect. 2010 Apr;60(4):306-8. doi: 10.1016/j.jinf.2010.02.003[↩]
- Jones RN, Jacobs MR, Sader HS. Evolving trends in Streptococcus pneumoniae resistance: implications for therapy of community-acquired bacterial pneumonia. Int J Antimicrob Agents. 2010 Sep;36(3):197-204. doi: 10.1016/j.ijantimicag.2010.04.013[↩]
- Jones, R. N., Schuchert, J. E., & Mendes, R. E. (2016). Dalbavancin Activity When Tested against Streptococcus pneumoniae Isolated in Medical Centers on Six Continents (2011 to 2014). Antimicrobial agents and chemotherapy, 60(6), 3419–3425. https://doi.org/10.1128/AAC.00116-16[↩]
- Henriques-Normark, B., & Tuomanen, E. I. (2013). The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harbor perspectives in medicine, 3(7), a010215. https://doi.org/10.1101/cshperspect.a010215[↩]
- Osowicki J, Steer AC. International survey of paediatric infectious diseases consultants on the management of community-acquired pneumonia complicated by pleural empyema. J Paediatr Child Health. 2019 Jan;55(1):66-73. doi: 10.1111/jpc.14128[↩]
- Jakhar SK, Pandey M, Shah D, Ramachandran VG, Saha R, Gupta N, Gupta P. Etiology and Risk Factors Determining Poor Outcome of Severe Pneumonia in Under-Five Children. Indian J Pediatr. 2018 Jan;85(1):20-24. doi: 10.1007/s12098-017-2514-y[↩]
- American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Community-Acquired Pneumonia, Smith MD, Fee C, Mace SE, Maughan B, Perkins JC Jr, Kaji A, Wolf SJ. Clinical Policy: Critical Issues in the Management of Adult Patients Presenting to the Emergency Department With Community-Acquired Pneumonia. Ann Emerg Med. 2021 Jan;77(1):e1-e57. https://www.acep.org/globalassets/new-pdfs/clinical-policies/cp-pneumonia.pdf[↩]
- Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004 Nov 1;39(9):1267-84. doi: 10.1086/425368[↩]
- van de Beek D, de Gans J, McIntyre P, Prasad K. Steroids in adults with acute bacterial meningitis: a systematic review. Lancet Infect Dis. 2004 Mar;4(3):139-43. doi: 10.1016/S1473-3099(04)00937-5[↩]
- Lieberthal AS, Carroll AE, Chonmaitree T, Ganiats TG, Hoberman A, Jackson MA, Joffe MD, Miller DT, Rosenfeld RM, Sevilla XD, Schwartz RH, Thomas PA, Tunkel DE. The diagnosis and management of acute otitis media. Pediatrics. 2013 Mar;131(3):e964-99. doi: 10.1542/peds.2012-3488. Epub 2013 Feb 25. Erratum in: Pediatrics. 2014 Feb;133(2):346. Dosage error in article text.[↩]
- Pneumococcal Infections (Streptococcus pneumoniae). https://emedicine.medscape.com/article/225811-overview#a3[↩][↩]
- Ajayi OO, Norton NB, Gress TW, Stanek RJ, Mufson MA. Three Decades of Follow-up of Adults After Recovery From Invasive Pneumococcal Pneumonia. Am J Med Sci. 2017 May;353(5):445-451. doi: 10.1016/j.amjms.2017.03.002[↩]