Contents
- Vernal keratoconjunctivitis
- Vernal conjunctivitis causes
- Vernal conjunctivitis types
- Vernal conjunctivitis signs and symptoms
- Vernal conjunctivitis complications
- Vernal conjunctivitis differential diagnosis
- Vernal conjunctivitis treatment
- Vernal conjunctivitis prognosis
Vernal keratoconjunctivitis
Vernal conjunctivitis also called vernal keratoconjunctivitis is a severe type of seasonal allergic conjunctivitis that results in small, raised lumps on the inside of the upper eyelid called Horner Tranta dots and a stringy (ropey), mucus discharge (Figures 3, 4 and 5) 1, 2, 3. Large bumps or papillae on the conjunctiva are a classic sign of vernal conjunctivitis. Histopathology shows eosinophils in the conjunctival secretions. Vernal conjunctivitis is frequently associated with allergic rhinitis, atopic dermatitis, or asthma. Vernal conjunctivitis may be associated with a single allergen but more usually with multiple sensitivities. Vernal conjunctivitis is often seen in areas of the world where the weather is hot and dry than in cold climates, most commonly in Asia, Central and West Africa and South America 4, 5, 6, 7, 8, 3. Vernal conjunctivitis is also seen commonly in the Middle East, Japan, India, the Mediterranean area, North America, and Australia 9, 10. At least one study showed prevalence ranges from 4.0 to 11.1% prevalence of vernal keratoconjunctivitis in African countries schoolchildren 6, 11. Vernal conjunctivitis is thought to be relatively unusual in North America and Western Europe 12, 9. One European study demonstrated the prevalence of vernal conjunctivitis was between 1.2 to 10.6 per 10,000 and 0.8/10,000 with corneal complications has been reported 7. The increased incidence in hot regions is speculated to be secondary to a higher level of pollution by pollens and various other allergens 3.
Vernal conjunctivitis is a T-helper-2 (Th2) lymphocyte-driven disease characterized by infiltration of the conjunctiva by a number of inflammatory cell types, including eosinophils, mast cells, and T lymphocytes 13, 14. This differs from atopic keratoconjunctivitis, which has been shown to involve both Th1 and Th2 inflammatory cascades 15. Increased levels of tumor necrosis factor (TNF) alpha, histamine, tryptase, IgE, and IgG antibodies are observed on pathologic examination of tears 16. It is believed that the exaggerated immunoglobulin E (IgE) response observed with vernal conjunctivitis in response to common allergens may be a secondary event 14. Mast cells and basophils cause the immediate reaction through the release of histamine and the recruitment of inflammatory cells lymphocytes and eosinophils 1. This results in the release of a number of pro-inflammatory cytokines, including but not limited to interleukin (IL)-4, IL-5, and IL-13, as well as other toxic cell mediators such as eosinophil cationic protein, eosinophil-derived neurotoxin/eosinophil protein X (EDN/EPX) that result in corneal damage 14, 17. Release of these factors mediates the remodeling, eye inflammation, and itch that are commonly associated with vernal conjunctivitis.
Diagnosis and treatment of vernal conjunctivitis is a challenge for many ophthalmologists, since no precise diagnostic criteria have been established, the pathogenesis of the disease is unclear, and anti-allergic treatments are often ineffective in patients with moderate or severe disease 1.
Vernal conjunctivitis usually affects both eyes and is severe, occurring seasonally and mainly in children and more common in young boys than girls, but this difference becomes smaller as age increases 18. Often, patients with vernal conjunctivitis also have asthma or eczema. The majority of vernal conjunctivitis occurs in patients between the ages of 5 to 25 years of age with an age of onset between 10 to 12 years old; however there are reports of patients as young as 5-months-old 10, 19. Symptoms typically occur throughout the year, but are worse in spring (vernal means springtime in Latin) and summer time. 23% of patients may have a perennial (all year round) form of them disease and many may have recurrences outside of the springtime 10, 18. Symptoms can be so bad that children need to be treated with topical (eye drops) or systemic (oral tablets) steroids in addition to allergy eye drops such as topical cromolyn or antihistamine preparations. However, vernal conjunctivitis is more difficult to treat than other types of allergic conjunctivitis, and may need special immune based medications such as cyclosporine drops to control the eye inflammation and prevent other eye problems. Sleeping in an air-conditioned room, ice packs and cold compresses can help with symptom relief. Furthermore, without adequate treatment, the seasonal vernal conjunctivitis can evolve into a chronic perennial vernal conjunctivitis after a mean of 3 years from disease onset 14.
Most vernal conjunctivitis patients eventually do grow out of vernal conjunctivitis usually lasts between five to ten years and usually resolves after puberty or adolescence and is rarely seen after the age of thirty. In contrast, children diagnosed with atopic keratoconjunctivitis will suffer from signs and symptoms throughout their life, and may develop more severe complications, as the atopic keratoconjunctivitis is progressive 20.
Figure 1. Human eye
Figure 2. Eye anatomy
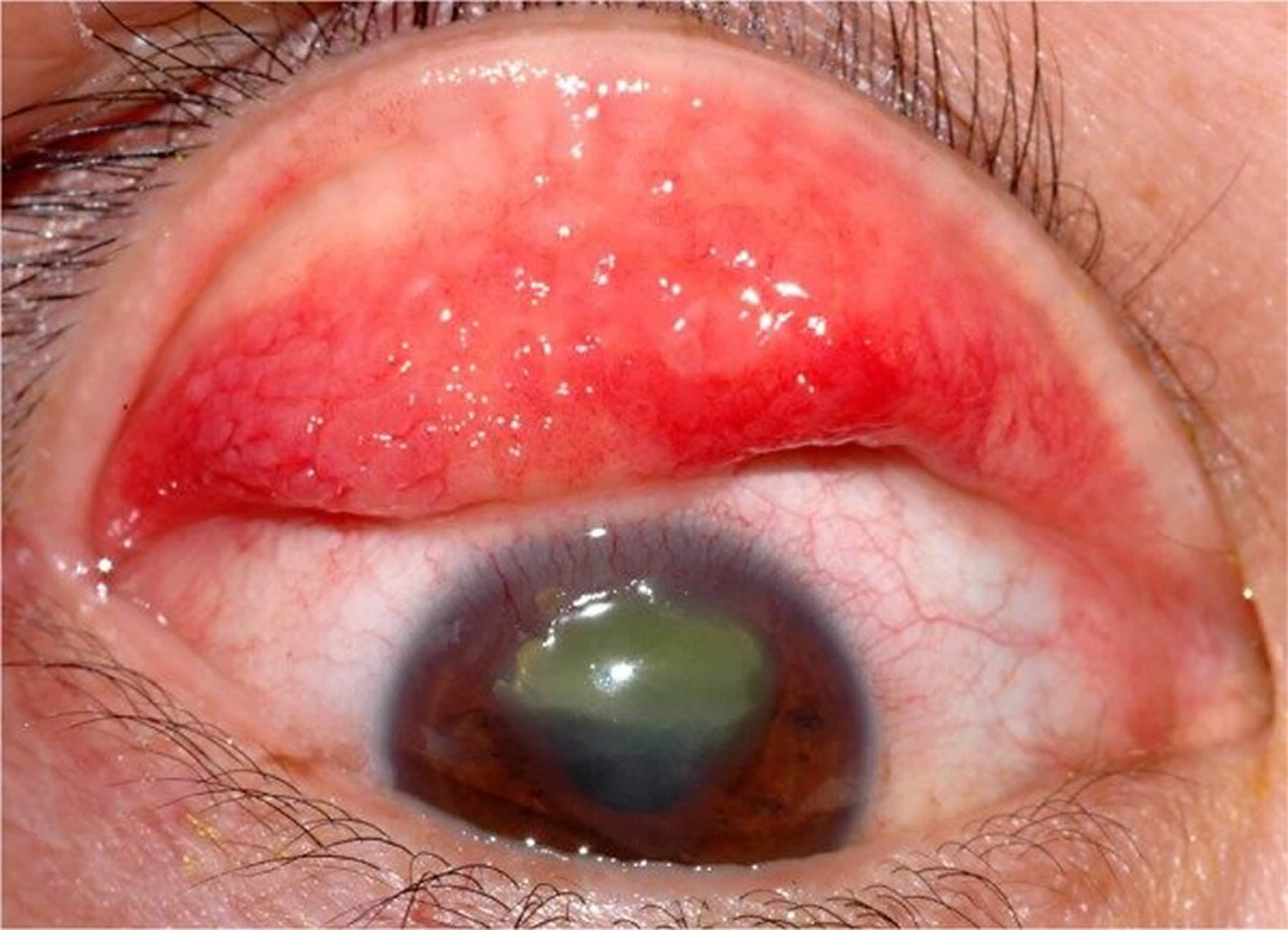
Figure 3. Vernal conjunctivitis
Figure 4. Vernal keratoconjunctivitis
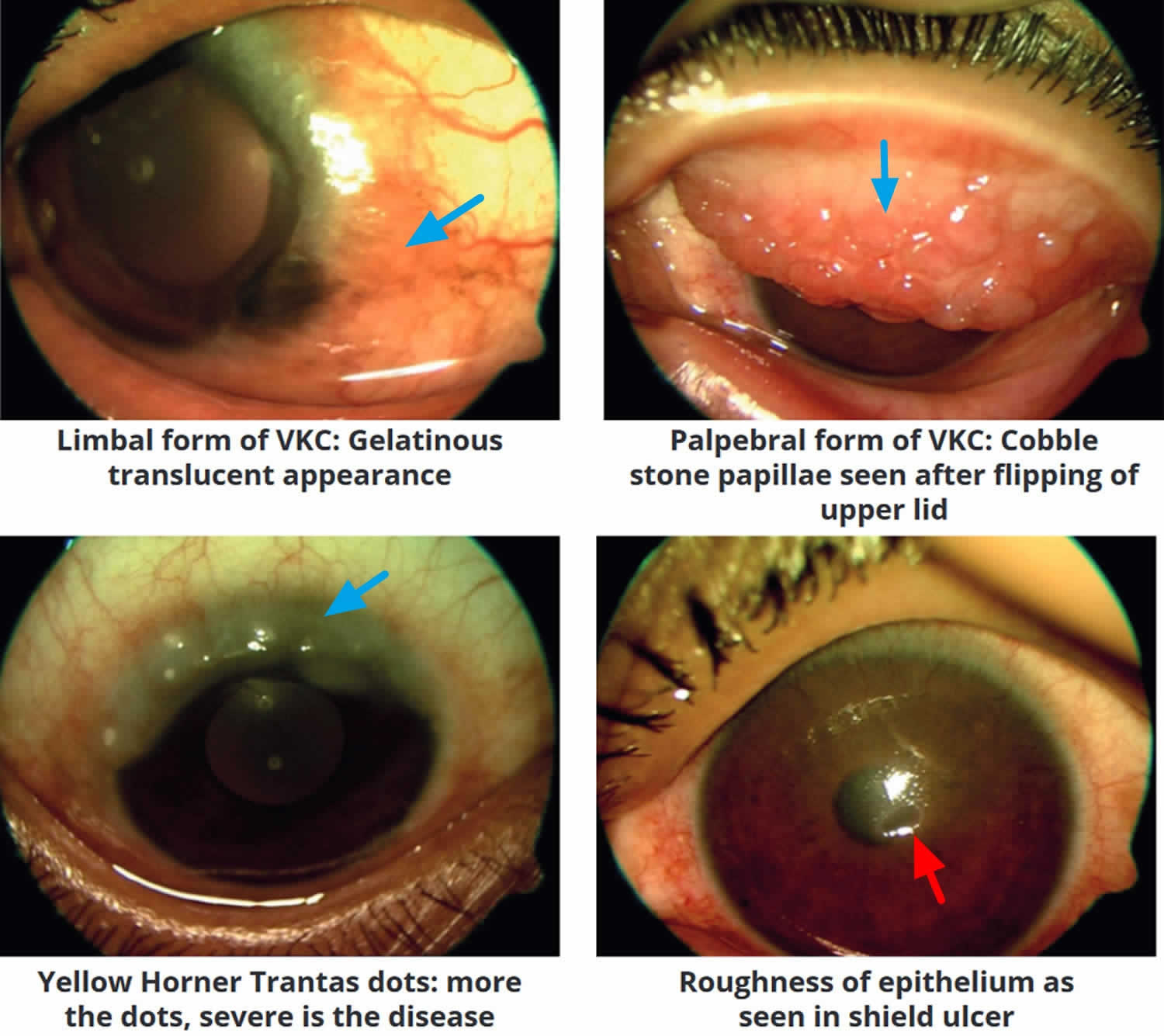
Footnote: Large cobblestone papillae. Upper tarsal giant papillae are typical of vernal conjunctivitis. These have characteristically flattened tops which sometimes demonstrate stain with fluorescein. Giant papillae can sometimes be seen near the limbus and, while relatively uncommon, symblepharon formation (an eye condition that causes the conjunctiva of the eye to stick to itself or the cornea) and conjunctival fibrosis (scar) can occur.
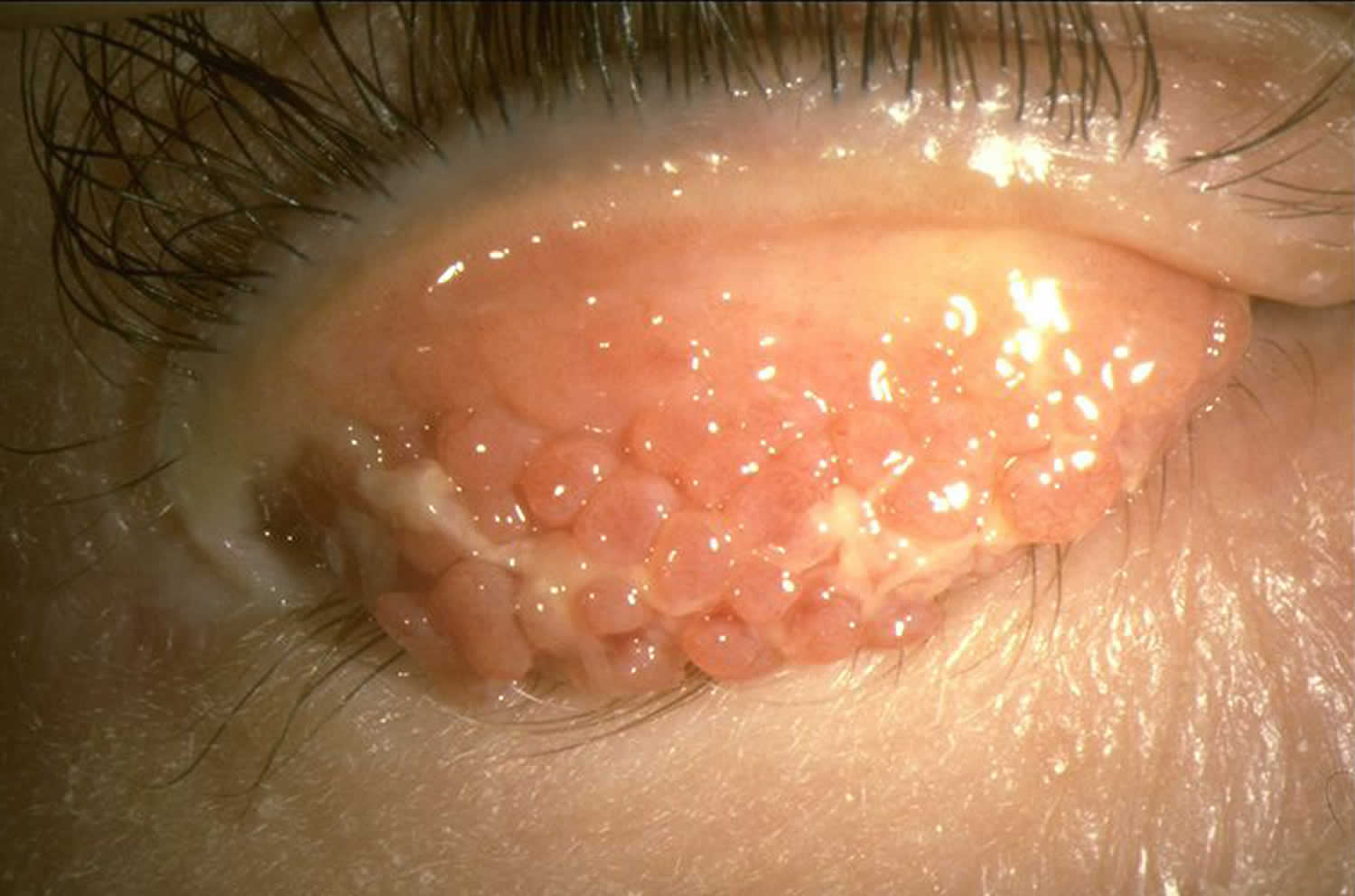
[Source 3 ]Figure 5. Horner–Trantas dots
Footnote: Peri-limbal Horner–Trantas dots are focal white dots consisting of degenerated epithelial cells and eosinophils and are indicative of vernal keratoconjunctivitis.
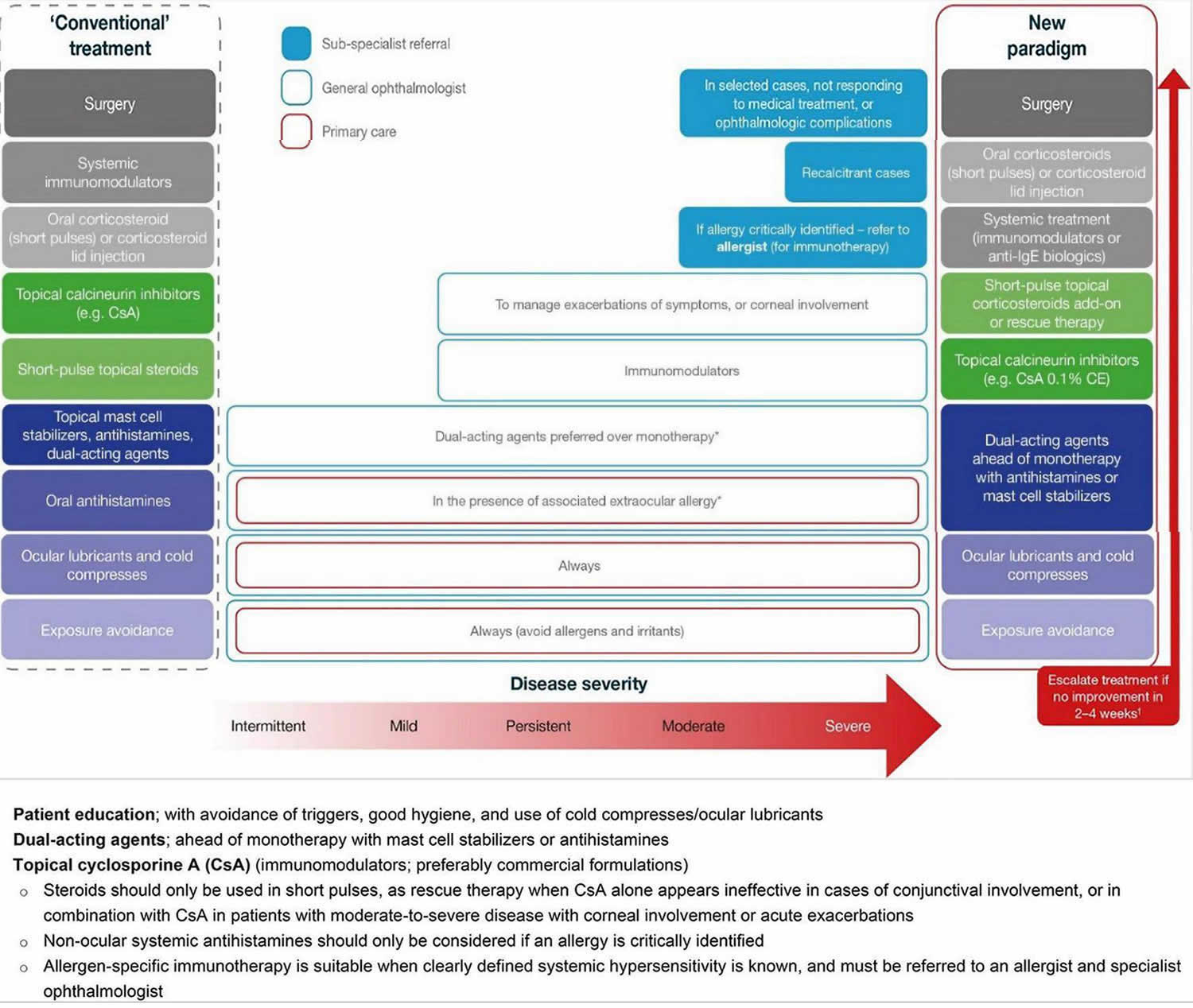
[Source 1 ]Figure 6. Vernal conjunctivitis treatment options
Footnotes: Treating vernal keratoconjunctivitis should entail a stepwise approach, identifying triggers, educating patients/caregivers on good ocular health, and addressing symptoms. In a new paradigm of management, the use of immunomodulators (e.g., topical cyclosporine A) should be considered early to tackle the inflammatory and chronic nature of vernal keratoconjunctivitis, with topical corticosteroids reserved as an add-on, short-pulse therapy for persistent disease, during acute exacerbations, or in patients with corneal involvement. Any use of corticosteroids requires tapering once symptoms have been controlled to avoid adverse events. For patients with an identified allergy, referral to an allergist is recommended for additional systemic treatment. In the rare patients who do not respond to medical treatment, surgery may be required.
*In the case of associated rhinitis, consider treatment according to Allergic Rhinitis and its Impact on Asthma (ARIA) protocol;
†No improvement is defined as no improvement in symptoms or changes in conjunctival, papillary or ocular surface clinical signs.
Abbreviations: CE = cationic emulsion; CsA = cyclosporine A; IgE = immunoglobulin E.
[Source 1. Adapted from Leonardi et al. 21]Vernal conjunctivitis causes
Vernal conjunctivitis is a severe type of seasonal allergic conjunctivitis that results in small, raised lumps on the inside of the upper eyelid called Horner Tranta dots and a stringy, mucus discharge 22, 23. A personal or family history of atopy is seen in a large proportion of vernal conjunctivitis patients 24. Atopy is a genetic tendency to develop allergic diseases, such as asthma, allergic rhinitis, and atopic dermatitis (eczema). Vernal conjunctivitis was originally thought to be due to a solely immunoglobulin E (IgE) mediate reaction via mast cell release and cell-mediated immune mechanisms 25. It has now been shown that IgE is not enough to cause the varied inflammatory response that is seen with vernal conjunctivitis 12. Activated eosinophils have also been implicated to play a significant role in the pathophysiology of vernal conjunctivitis and these can be shown consistently in conjunctival scrapings of vernal conjunctivitis patients; however mononuclear cells and neutrophils are also seen 25, 12. Additionally, the role of type 4 hypersensitivity mediated by CD4 T-helper-2 (Th2) lymphocytes with immunomodulators such as IL-4, IL-5, and bFGF has also been highlighted through a few studies 13, 14, 10, 12. This differs from atopic keratoconjunctivitis, which has been shown to involve both Th1 and Th2 inflammatory cascades 15. Immunomodulators like interleukins 4 (IL-4), IL-5, IL-13, and fibroblast growth factors have also been implicated. Increased levels of tumor necrosis factor (TNF) alpha, histamine, tryptase, IgE, and IgG antibodies are observed on pathologic examination of tears 16. There is also reported over-expression of cytokines and chemokines in the conjunctiva of these patients 26. Thought has been given to a possible endocrine predisposing factor as well as there is a decrease in symptoms and prevalence after puberty 25, 10.
Apart from personal allergy history, other predisposing factors include male gender, close contact with animals, and increased exposure to dust and sunlight 27.
A hereditary association or genetic link has been suggested, but no direct genetic associations have been made. Vernal conjunctivitis is seen more often in patients who have family history of atopy and might be positive in close to 49% of these cases. But no clear correlation with specific genetic loci has been identified 10.
Vernal conjunctivitis types
Vernal conjunctivitis or vernal keratoconjunctivitis is a subtype of allergic conjunctivitis 22. The episodes are often periodic and have seasonal recurrences. Seasonal exacerbations characterize vernal keratoconjunctivitis in the initial stages with a peak incidence during spring and summer. Over time, vernal conjunctivitis tends to become perennial (all year round).
Additional types of vernal conjunctivitis include perennial and seasonal rhinoconjunctivitis, atopic keratoconjunctivitis, and giant papillary conjunctivitis 2.
Vernal conjunctivitis is classified into three clinical subtypes based on the location of the papillae into palpebral (tarsal), limbal (bulbar), and mixed forms 13, 28, 1:
- Palpebral vernal conjunctivitis (tarsal vernal conjunctivitis). Palpebral vernal conjunctivitis is characterized by large, cobblestone-like papillae on the upper tarsal conjunctiva. These can differ in shape and size, but are usually defined as >1.0 mm in diameter 29, 13. There is a close association between the inflamed conjunctiva and the corneal epithelium, often leading to significant corneal disease.
- Limbal vernal conjunctivitis (bulbar vernal conjunctivitis). Limbal vernal conjunctivitis typically involves Horner–Trantas dots indicating lymphocytic and eosinophilic infiltration of the limbal conjunctiva 29, 13. Limbal vernal conjunctivitis typically affects the Black and Asian populations.
- Mixed vernal conjunctivitis. Mixed vernal conjunctivitis has features of both the palpebral (tarsal) and limbal (bulbar) subtypes in only one eye (as signs are often heterogeneous between eyes) 29.
According to the Management of Vernal Keratoconjunctivitis in Asia (MOVIA) Expert Working Group, the most common form of vernal conjunctivitis seen in clinics across Asia is the tarsal form; however, up to one-third of patients are assumed to have the mixed form 1. The limbal form of vernal conjunctivitis is considered less common in Asia (based on clinical experience) 1.
Figure 7. Vernal keratoconjunctivitis types
Footnote: Clinical forms of vernal keratoconjunctivitis: Limbal or bulbar, palpebral and mixed vernal keratoconjunctivitis.
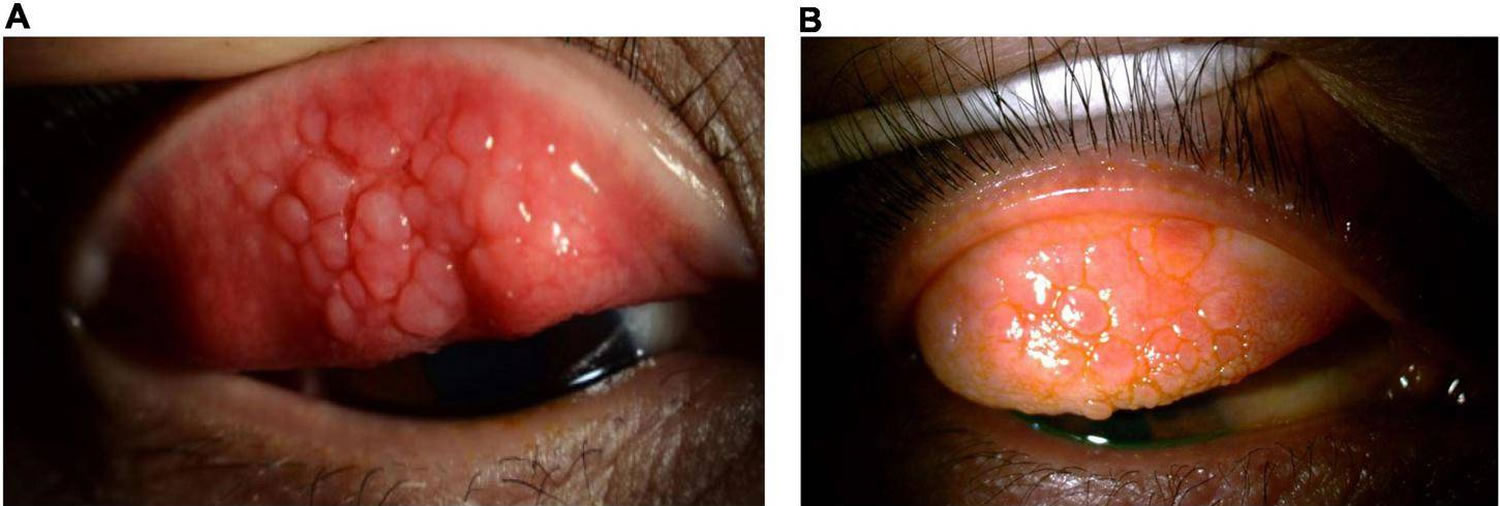
[Source 30 ]Figure 8. Palpebral vernal conjunctivitis
Footnote: Palpebral vernal conjunctivitis also called tarsal vernal conjunctivitis is characterized by large, cobblestone-like papillae on the upper tarsal conjunctiva.
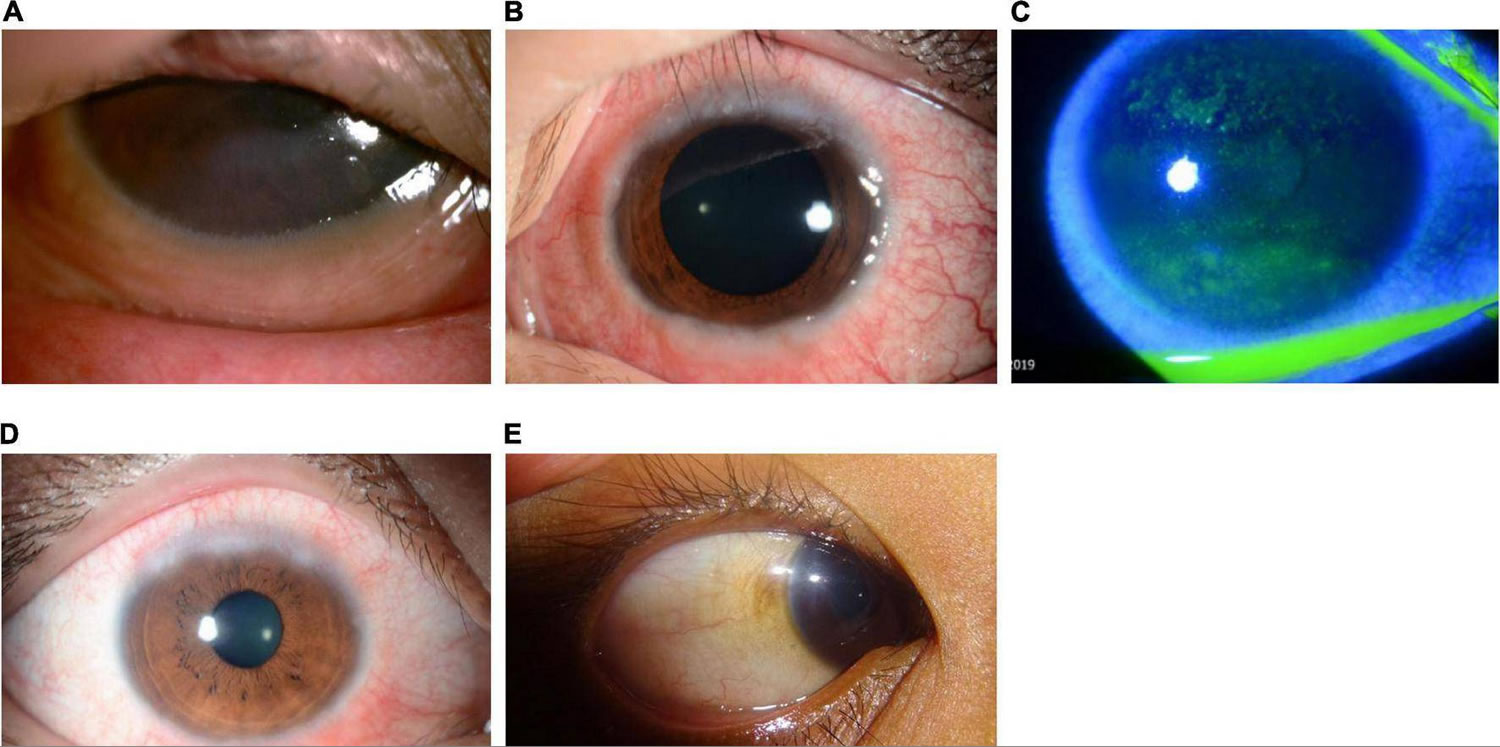
[Source 1 ]Figure 9. Limbal vernal conjunctivitis
Footnote: Limbal vernal conjunctivitis typically involves Horner–Trantas dots, indicating lymphocytic and eosinophilic infiltration of the limbal (bulbar) conjunctiva. The mixed form is characterized by the presence of both tarsal and limbal subtypes in only one eye.
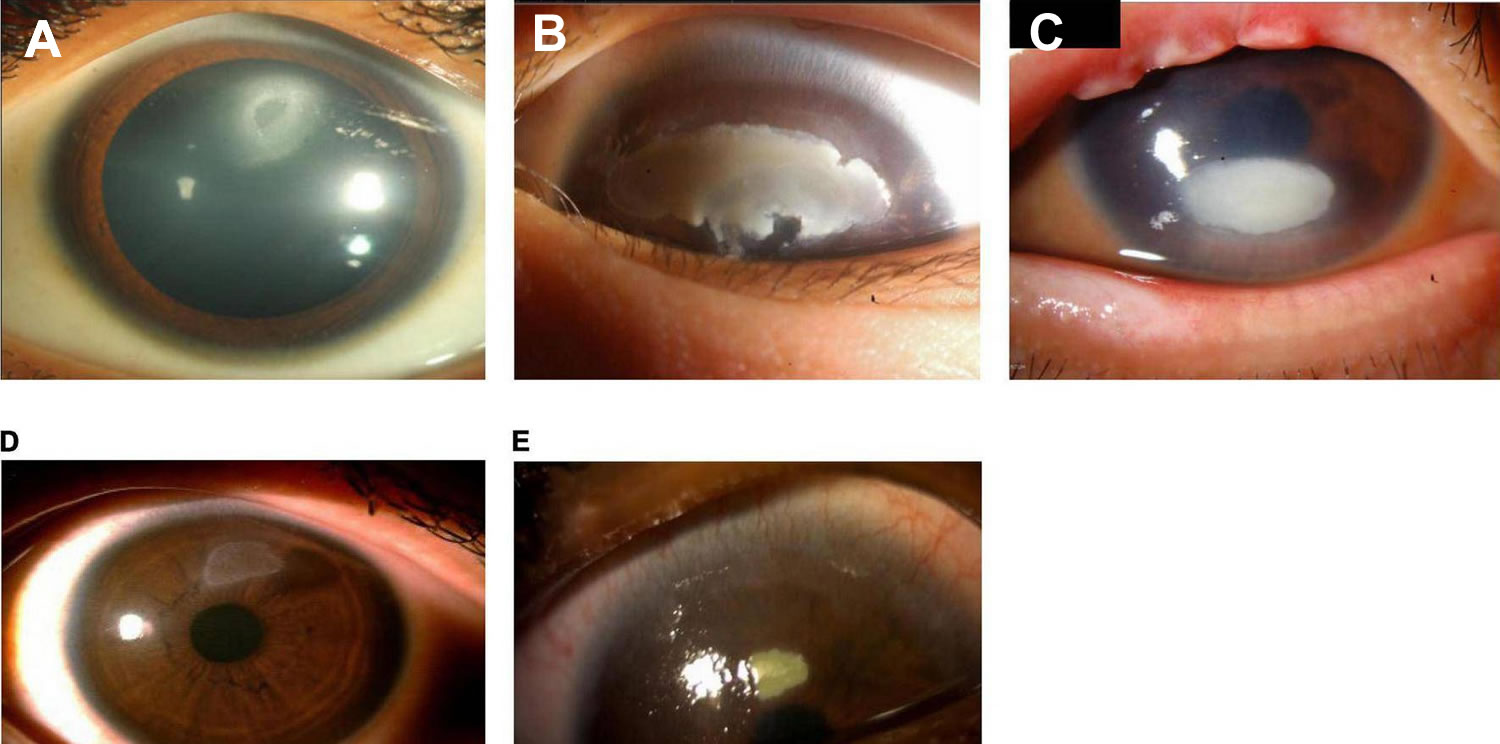
[Source 1 ]Figure 10. Shield ulcer
Footnote: Shield ulcer formation. Shield ulcers usually form on the upper third of the cornea. Plaques can also form when inflammatory debris accumulates at the base of a shield ulcer.
[Source 1 ]Vernal conjunctivitis signs and symptoms
The most common eye symptoms of vernal conjunctivitis are initially severe itching, redness, and tearing. Other common symptoms include blurred vision, photophobia, foreign body sensation, burning, blinking or eye twitching (blepharospasm) and a characteristic ropey, stringy mucus, and/or serous discharge 29, 31, 25, 10. Other typical signs and symptoms include moderate-to-intense conjunctival hyperemia, mild-to-moderate chemosis, foreign-body sensation, and pain, all of which can be very intense upon awakening, causing what is called “the morning misery” 29. Vernal conjunctivitis typically affect both eyes but may also affect one eye only.
Clinical signs of vernal conjunctivitis include a papillary reaction of the upper tarsal conjunctiva and throughout the limbus. The signs of vernal conjunctivitis can be divided into conjunctival, limbal and corneal signs 3. Moreover, the clinical examination findings also vary dependent on the geographical location 32.
- Conjunctival signs include diffuse conjunctival injection and upper tarsal giant papillae. These are discrete >1mm in diameter that characteristically have flattened tops which sometimes demonstrate stain with fluorescein 25, 33. Additionally, these giant papillae can sometimes be seen near the limbus and, while relatively uncommon, symblepharon formation (an eye condition that causes the conjunctiva of the eye to stick to itself or the cornea) and conjunctival fibrosis can occur 34.
- Palpebral disease: In the early stages, there is conjunctival hyperemia and velvety papillary hypertrophy of the superior tarsal plate. In the intense disease, flat polygonal macropapillae, which are <1mm in size, are seen along with the whitish inflammatory disease. With further disease progression, these can form giant papillae that are>1mm in size and result from rupture of the dividing septa. Mucoid deposits can occur between giant papillae. The milder form of the pathology is characterized by minimal conjunctival congestion and decreased mucus production 35.
- Bulbar disease: The bulbar disease is also called limbal disease and is particularly more common and severe in tropical regions. This is characterized by congestion of the bulbar conjunctiva in the interpalpebral area. Gelatinous thickened papillae can form around the limbus and are associated with apically located whitish cellular collections known as Horner Tranta Spots 36.
- Limbal signs include thickening and opacification of the limbal conjunctiva as well as gelatinous appearing and sometime confluent limbal papillae. Peri-limbal Horner-Trantas dots are focal white limbal dots consisting of degenerated epithelial cells and eosinophils 25. Limbal disease can result in a limbal stem cell deficiency which can lead to pannus formation with corneal neovascularization 34.
- Corneal signs vary according to the severity of the disease process 25. Punctate epithelial erosions or keratitis can coalesce into macro-erosions of the epithelium 12.
- Plaques containing fibrin and mucous can accumulate into macro-erosions forming Shield ulcers (see Figure 10). Corneal neovascularization can ensue and resolution can leave a characteristic ring-like scar 33, 9.
- A waxing and waning gray-white lipid depositing in the peripheral, superficial stroma can occur and is known as pseudogerontoxon 25. Pseudogerontoxon is characterized by a paralimbal band of superficial scarring adjacent to the inflamed limbus resulting from recurrent limbal disease. It usually mimics arcus senalis.
- Vernal keratoconjunctivitis is also associated with corneal ectasias, particularly keratoconus, which results from persistent eye rubbing and occasionally superficial vascularization. Keratoconus has been shown to be more prominent in vernal conjunctivitis patients as well; possibly due to increased eye rubbing of chronically irritated patients 37.
- Associated bilateral herpes simplex keratitis has also been reported 38
- Lid signs. Blepharitis is often associated with patients with vernal keratoconjunctivitis. Some cases might be seen with eczema or maceration of the lid. Subconjunctival fibrosis and symblepharon can rarely develop due to the inflammatory complications 39.
Severe vernal conjunctivitis can result in sight-threatening complications 1. Eye surface damage as a result of repetitive eyelid trauma and vernal conjunctivitis-associated inflammatory activity can lead to corneal complications such as superficial punctate keratopathy, shield ulcers, corneal scarring, keratoconus, dry eyes, limbal stem-cell deficiency, and secondary infections 29, 4, 40, 41. Shield ulcers, which can be self-limiting or associated with bacterial keratitis, usually form on the upper third of the cornea, and can lead to loss of vision (see Figure 10) 13. Plaques can also form when inflammatory debris accumulates at the base of a shield ulcer 40 and can be particularly resistant to topical therapy or require surgical intervention 13. Limbal stem-cell deficiency can occur with longstanding inflammation 13. Keratoconus and irregular astigmatism can result from frequent eye rubbing in the pediatric population 42, 43, 44. In these patients, there is a fine balance between the benefits of corticosteroids and the risk for vision loss as a result of overtreatment. Patients with severe vernal conjunctivitis may also develop lid complications and acquire ptosis often with atopic dermatitis 1. The variable severity of these complications in each patient presents a challenge to ophthalmologists to not only manage acute episodes, but also to prevent reappearances.
Vernal conjunctivitis complications
The ocular complications reported among vernal keratoconjunctivitis patients include steroid-induced cataract, steroid-induced glaucoma, irregular astigmatism, keratoconus, acute hydrops, shield ulcer, central corneal scars, and limbal tissue hyperplasia 2.
Vernal conjunctivitis differential diagnosis
The close differentials of vernal keratoconjunctivitis include all types of allergic conjunctivitis, including seasonal allergic conjunctivitis, perennial allergic conjunctivitis, atopic keratoconjunctivitis (AKC) and giant papillary conjunctivitis 2. Most of these are IgE mediated responses except giant papillary conjunctivitis. None have gender predilection, except atopic keratoconjunctivitis (AKC) and vernal keratoconjunctivitis, which are more prevalent among males. History and physical examination often help in distinguishing between these clinical entities.
Atopic keratoconjunctivitis (AKC) typically has an older age of onset in 20-50 years of age, as opposed to onset prior to age 10 years with vernal conjunctivitis 2. Conjunctival involvement is classically on the upper tarsus in vernal conjunctivitis and on the lower tarsus in atopic keratoconjunctivitis. Additionally, atopic keratoconjunctivitis is typically more chronic in nature and more commonly results in scarring of the cornea and conjunctival cicatrization, whereas vernal conjunctivitis is typically more self-limiting and do not result in severe vision-threatening complications 45, 33. Atopic keratoconjunctivitis (AKC) is more commonly associated with asthma, rhinitis, dermatitis. Vernal keratoconjunctivitis is associated with increased goblet cells compared to decrease goblet cells in atopic keratoconjunctivitis.
Vernal conjunctivitis treatment
Management should follow a stepwise approach with active patient education 1:
- Address triggers and aggravating factors (e.g., environment and allergens).
- Maintain eye health, including frequent hand, face, and hair washing.
- Use of eye lubricants and cold compresses should always be considered.
- For the use of any eye drops on the eye surface, it is recommended to use preservative-free compounds, where possible, to minimize eye surface toxicity.
- Conventional topical anti-allergic drugs
- Dual-acting agents, antihistamines, and mast cell stabilizers are all effective for reducing signs and symptoms of mild or moderate vernal conjunctivitis.
- Dual-acting agents should be considered ahead of monotherapy with antihistamines or mast cell stabilizers.
- Cyclosporine A 0.1% cationic emulsion
- Topical cyclosporine A 0.1% cationic emulsion should be considered for patients with in moderate-to-severe or persistent vernal conjunctivitis.
- Patients should be instructed on how to apply cyclosporine A eye drops to minimize stinging or burning on instillation, such as using artificial tears prior to instillation.
- Topical corticosteroid eye drops
- In patients with only conjunctival involvement, topical corticosteroids should be reserved for use after loss of control or persistence of symptoms with immunomodulators such as cyclosporine A.
- Topical corticosteroids are effective for the management of acute exacerbations, or when the cornea is involved, and preferably only introduced in patients with more severe disease. In these individuals, corticosteroids should be used in combination with cyclosporine A to account for the fact that cyclosporine A may require ≥1 week to act.
- Because of an increased risk of adverse events/or vision loss with chronic use, topical corticosteroid eye drops should be used in short pulses alone or in combination with cyclosporine A under the supervision of an ophthalmologist and tapered rapidly.
- Tacrolimus
- In regions where available, tacrolimus should be reserved for patients with severe vernal conjunctivitis that is refractory to cyclosporine A.
- Tacrolimus can be considered as a treatment for moderate-to-severe vernal conjunctivitis in patients with allergy of the eyelid, but note this may be off-label.
- Vasoconstrictors
- Vasoconstrictors are not recommended for the treatment of vernal conjunctivitis. Topical vasoconstrictors can be effective at alleviating hyperemia but offer little to no relief from itchiness and have a short duration of action. If used to address hyperemia, vasoconstrictors should be used with caution and only for a short period (no longer than 5–7 days) due to adverse events and tachyphylaxis 46.
- Vasoconstrictors may cause several side effects including rebound redness, chronic follicular conjunctivitis, and tachyphylaxis. Vasoconstrictors are rarely used in the pediatric population. It is recommended that these agents should be avoided.
- Topical decongestants do not reduce the allergic response because they do not antagonize any of the mediators of allergic inflammation. Burning or stinging on instillation is a common adverse event, and prolonged use and/or discontinuation following longer-term use can lead to rebound hyperemia and conjunctivitis medicamentosa 47. These events are usually associated with topical combinations of vasoconstrictors and first-generation antihistamines, such as pheniramine and antazoline, which are available as over-the-counter products.
- Non-steroidal anti-inflammatory drugs (NSAIDs)
- Non-steroidal anti-inflammatory drugs (NSAIDs) are not recommended as they do not target the specific inflammatory mechanism associated with vernal conjunctivitis.
- Non-steroidal anti-inflammatory drugs (NSAIDs) used in the treatment of eye allergy typically inhibit cyclooxygenase (COX)-1 and COX-2 enzymes 29. Some have demonstrated a slight effect in the treatment of ocular allergy, by targeting itching, intercellular adhesion molecule-1 expression, and tear tryptase levels 29, 46. However, use of NSAIDs is not encouraged in vernal keratoconjunctivitis because of their local side effects, such as burning or stinging after application, increased risk of inducing keratitis on the ocular surface, and because they do not target the specific inflammatory mechanism associated with vernal keratoconjunctivitis.
- Oral antihistamines
- Second-generation systemic non-sedative antihistamines are preferred over older first-generation antihistamines.
- Allergen-specific immunotherapy
- Allergen-specific immunotherapy is only recommended when clearly defined systemic hypersensitivity to an identified allergen exists.
- Patients requiring allergen-specific immunotherapy should be referred to an allergist or specialist ophthalmologist.
- In selected patients with ocular complications or persisting symptoms following prior treatments
- Surgery, oral corticosteroids (short pulses) or corticosteroid lid injection, or systemic treatment with immunomodulators or biologics may be appropriate options for use by corneal specialists.
In patients with a history of vernal conjunctivitis, drug treatment should be planned early and started at the beginning of spring or continued all year, depending on allergen exposure and duration of symptoms 29.
Table 1. Overview of medications currently available for the management of vernal conjunctivitis
| Class | Drug | Standard dosing | Indication | Considerations |
|---|---|---|---|---|
| Topical antihistamines (second generation) | Antazoline Emedastine Levocabastine | QID | Relief of itching Relief of signs and symptoms | • Short duration of action • Frequently not enough alone to treat the entire disease |
| Mast cell stabilizers | DSCG Lodoxamide NAAGA Pemirolast potassium | BID to QID | Relief of signs and symptoms | • Long-term use • Slow onset of action • Prophylactic dosing • Frequently not enough alone to treat the entire disease |
| Dual-acting agents (antihistamine + mast cell stabilizers) | Alcaftadine Azelastine Bepotastine Epinastine Ketotifen Olopatadine | QD QD to BID BID BID BID to QID BID | Relief of itching Relief of signs and symptoms | • Side effects • Bitter taste (azelastine) • Frequently not enough alone to treat the entire disease |
| Immunomodulators (calcineurin inhibitors) | Cyclosporine A Tacrolimus | QD to QID QD | Treatment of severe vernal keratoconjunctivitis and AKC not responding to anti-allergic drugs | • Pharmacy-compounded preparations vary from center to center • Quality control and availability of pharmacy-compounded preparations are poor • Tacrolimus is largely used off-label in ocular allergy (tacrolimus is approved for vernal keratoconjunctivitis only in Japan) |
| Corticosteroids | Betamethasone Desonide Dexamethasone Fluorometholone Hydrocortisone Loteprednol Prednisolone Rimexolone | As required (up to 7 days) | Treatment of allergic inflammation Use in moderate-to-severe forms | • Risk for long-term adverse events • No mast cell stabilization • Potential for inappropriate patient use • Requires close monitoring |
| Vasoconstrictors (vasoconstrictor–antihistamine combinations) | Naphazoline/ pheniramine | BID to QID | Episodic itching and redness | • Rapid onset but short duration of action • Only addresses hyperemia • Associated with a range of side effects • Potential for inappropriate patient use |
Footnotes: * Availability and access to treatments may vary across clinics, hospitals, regions, and countries. Each treatment option should be considered in accordance with the level of evidence available at the time.
Abbreviations: AKC = atopic keratoconjunctivitis; BID = twice daily; DSCG = disodium cromoglycate; NAAGA = N-acetyl-aspartyl glutamic acid; QD = once daily; QID = four times daily; VKC = vernal keratoconjunctivitis.
[Source 1 ]Vernal conjunctivitis treatment options
Removal of any and all possible allergens as well as conservative management such as cool compresses and lid scrubs make up the first line of therapy 48. A topical antihistamine only may work in mild cases 48. Topical mast cell stabilizers (cromolyn sodium, nedocromil sodium, and lodoxamide) are typically used with topical antihistamines and have been shown to be effective in moderate presentations of vernal conjunctivitis 48. Mast-cell stabilizers have a loading period to reach their full therapeutic effect 12. Overall, topical antihistamines (instilled four times daily) appear to be safe and well tolerated in patients with vernal conjunctivitis 1. Alongside mast cell stabilizers and dual-acting agents, they are often the first-choice treatment and are effective in reducing the signs and symptoms of vernal conjunctivitis 46. Antihistamine drugs with eye activity are a good therapeutic option for patients with allergic conjunctivitis, including vernal conjunctivitis, since they can inhibit proinflammatory cytokine secretion from conjunctival epithelial cells 49.
Antihistamines
When you have an allergic reaction your body releases histamine from mast cells, which leads to hay fever. Antihistamines block this reaction. Antihistamines act via histamine receptor antagonism to block the inflammatory effects of histamine and relieve any associated signs and symptoms. Most antihistamines used in the treatment of eye allergy are histamine-1 (H1) receptor antagonists, although some agents have affinity for other subtypes. Histamine-2 (H2) antagonists have been shown to modulate both cell growth and migration. Animal model studies have shown that antihistamines may even reduce infiltration of eosinophils and thus reduce the clinical aspects of the late-phase reaction 29.
First-generation antihistamines are well tolerated and associated with a favorable long-term safety record, but are associated with instillation pain, short duration of action, and limited potency 50. First-generation antihistamines remain available in over-the-counter products, particularly in combination with vasoconstrictors (substances that cause the walls of blood vessels to narrow, or constrict). While newer antihistamines are also H1 antagonists, they have a longer duration of action (4–6 hours) and are better tolerated than their predecessors 29.
Topical antihistamines are widely available without a prescription. Antihistamines competitively block histamine receptors (e.g., H1 or H4) on nerve endings and blood vessels of the mucosal surface, thereby reducing itchiness and conjunctival redness 51. First-generation antihistamines were associated with a range of systemic side effects (e.g., sedation, dizziness, cognitive impairment, blurred vision) caused by anticholinergic actions and nonspecific binding to histamine H2 receptors in addition to drying of the ocular surface. Newer oral, intranasal, and topical ocular antihistamines demonstrate improved H1 receptor selectivity, with fewer adverse effects; however, eye side effects, such as burning and dryness, remain a concern. Topical antihistamines (e.g., levocabastine, emedastine difumarate) are useful for providing rapid relief of allergic conjunctivitis symptoms, but their duration of action is limited; most topical antihistamines require dosing four times.
Mast cell stabilizers
Alongside antihistamines and dual-acting agents, mast cell stabilizers (instilled two to four times daily) form the first line of treatment and are effective in reducing the signs and symptoms of vernal keratoconjunctivitis. Mast cell stabilizers have been shown to act on multiple cells involved in allergic inflammation, including eosinophils, neutrophils, macrophages, mast cells, and monocytes. For example, lodoxamide is known to have an effect on eosinophil activation (shown in studies by measuring tear eosinophil cationic protein before and after therapy), while N-acetyl-aspartyl glutamic acid (NAAGA) is known to inhibit leukotriene synthesis and release of histamine by mast cells 29. Overall, mast cell stabilizers are generally well tolerated especially preservative-free options; however, there are tolerability concerns with some agents, including burning and stinging sensations upon instillation, blurred vision, and an undesirable aftertaste. Data on long-term efficacy and safety of mast cell stabilizersare lacking 46.
Dual-Acting Antihistamine–Mast Cell Stabilizing Agents
Dual-acting agents are the combination of mast cell stabilizing properties and histamine receptor antagonism (instilled twice daily) has shown clear benefits in treating forms of eye allergy 1. This dual action rapidly alleviates multiple signs and symptoms of allergic conjunctivitis in the short term and blocks the feed-forward cycle of persistent inflammation caused by continuous mast cell activation in the long term to promote regression of allergic conjunctivitis. Antihistamine–mast cell stabilizing agents (e.g., olopatadine, alcaftadine, epinastine, bepotastine besilate) are currently considered first-line therapeutics for allergic conjunctivitis because they offer acute symptomatic relief and control inflammation, and can be used chronically without long-term safety concerns. Most dual-acting agents require twice-daily dosing. Olopatadine 0.2% and alcaftadine are indicated for once-daily dosing and maintain effectiveness through 16 hours after administration in conjunctival allergen challenge studies 52, 53, 54.
Dual-acting agents are well tolerated and are not associated with significant ocular drying effects 29, and should be preferred over monotherapy with antihistamines or mast cell stabilizers alone. A key benefit of dual-acting agents is the provision of rapid symptomatic relief by alleviating itching and redness 29. Olopatadine and ketotifen, for example, have shown to be effective in relieving itching, tearing, conjunctival hyperemia, mucus discharge, and photophobia 55.
If seasonal recurrence is known, it is suggested that mast-cell stabilization therapy be initiated prior to the season in which symptoms are encountered and continued throughout the season 12. Dual-action agents with both histamine-1 (H1) blocking mechanism and mast-cell stabilization have the benefits of working immediately and having long-term disease modifying effects. Topical corticosteroids are typically the most effective. High pulse dose with quick tapering and use of low-absorptions corticosteroids (fluoromethelone, loteprednol, remexolone, etc.) is preferred when using topical corticosteroid therapy 48. Oral corticosteroids can be considered in sight threatening conditions 25, 9. Supratarsal injection of local corticosteroid (triamcinolone or dexamethasone) into the upper tarsal papillae can sometimes offer short term relief as well 56.
Cyclosporine A
Long term immunomodulation (a process in which the immune system is modified or modulated in a beneficial way) with steroid sparing agents such as cyclosporine and tacrolimus is often needed. Topical calcineurin inhibitors (topical immunomodulators) are frequently prescribed for patients with vernal conjunctivitis 46. Topical calcineurin inhibitors are recommended in patients with acute-phase or persistent, moderate or severe vernal conjunctivitis that is not responding to anti-allergic drugs 46. Topical Cyclosporine A is effective in controlling ocular surface inflammation in vernal conjunctivitis and is thought to work by inhibiting Th2 proliferation and IL-2 production, and by reducing levels of immune cells and mediators acting on the ocular surface and conjunctiva 57. Different concentrations of cyclosporine A, ranging from 0.05 to 2.0%, are currently available in different countries with different clinical indications 58, 59. The 0.1% cationic emulsion (CE) formulation increases the bioavailability of cyclosporine A in the tear film compared with available oil-based (often used when cyclosporine A is pharmacy-compounded) or anionic emulsion cyclosporine A formulations 60. Clinical studies on the efficacy of topical cyclosporine A 0.1% cationic emulsion (CE) for treating vernal conjunctivitis have consistently shown a beneficial effect of cyclosporine A, with safety and efficacy demonstrated for up to 7 years and comparable with those seen in patients with dry eye disease 57, 61, 62, 63.
There is no consensus on the minimum effective concentration of cyclosporine A to treat vernal conjunctivitis, as this can depend on the emulsion vehicle 1. Topical cyclosporine A in concentrations of 0.05% to 2% has been shown to decrease inflammatory cytokines and the signs and symptoms of treated vernal conjunctivitis patients 64, 65. Currently, cyclosporine A 0.1% cationic emulsion (CE) is approved based on significant improvements in signs, symptoms, and quality of life in patients with severe vernal conjunctivitis, and is commercially available across Asia. It is recommended that cyclosporine A 0.1% CE should be initiated twice daily for patients with mild-to-moderate disease and four times daily for those with moderate-to-severe disease for up to 6 months. Other commercially available cyclosporine A formulations exist but are not approved for use in vernal conjunctivitis (as of December 2020) 1. In addition, cyclosporine A may be compounded in hospital-based pharmacies to provide variable concentrations and formulations; however, its quality may be compromised, and availability is limited to specialist centers 1.
The most commonly associated side effects with cyclosporine A is a stinging or burning sensation on instillation, and no new safety issues were identified in long-term follow-ups 1. Instillation pain can be minimized by following a standard technique for eye drop instillation, which includes using artificial tears prior to instillation (avoiding any potential washout effect of the subsequently administered cyclosporine A, by waiting at least 10 minutes between administrations), followed by one drop into the lower conjunctival sac of each eye in the morning, at noon, in the afternoon, and in the evening, approximately 4 hours apart 63. Patients should also be counseled to expect stinging and burning when the drops are applied, but that the sensation should taper off with regular use as prescribed.
After long cycles of treatment, cyclosporine A can allow control of symptoms without corticosteroids 1. There is a trend in the Asia-Pacific region, in using cyclosporine A as a first-line treatment for patients who present with moderate-to-severe or persistent vernal conjunctivitis 1. In patients presenting with conjunctival involvement only, cyclosporine A alone is recommended for treatment, while corticosteroids should only be prescribed (as short pulses) if there is an inadequate response to cyclosporine A 1. Otherwise, cyclosporine A can be used in combination with corticosteroids in patients with corneal complications (or limbitis) or acute exacerbations, in the understanding that cyclosporine A may take over a week to act due to its immunosuppressive mechanism of action 1.
Tacrolimus
Tacrolimus is a strong, non-steroidal immunosuppressant that binds to FK506-binding proteins in T lymphocytes and inhibits calcineurin activity 66. Tacrolimus has been investigated in a small randomized controlled trial in vernal keratoconjunctivitis, where it demonstrated improvements in symptoms of itching, foreign-body sensation, and photophobia, as well as in signs of limbal inflammatory activity and keratitis, with maintenance of disease control 67, 68. Tacrolimus may be administered as eye drops or an ointment. The use of tacrolimus (0.03%) ointment in ophthalmic and dermatologic form is currently off-label in Asia for patients with vernal keratoconjunctivitis. However, there are findings in published studies of small population sizes demonstrating effectiveness and tolerability 67, 68, 69, 70, 71, 72.
Tacrolimus 0.1% topically has also shown to improved signs and symptoms of vernal conjunctivitis, with one study showing improvement even in patients unresponsive to 0.1% topical cyclosporine A 73, 74. A study comparing the use of 0.1% tacrolimus ophthalmic ointment dosed twice a day versus 2% cyclosporine A eye drops four times a day for the treatment of vernal conjunctivitis, did not find a statistically significant difference in signs or symptoms or side effects between the two groups 75. Additionally, adult patients with vernal conjunctivitis may respond more favorably to topical cyclosporine A therapy 76. Oral anti-histamines are sometimes used, but there is no real evidence in their support 3.
Similar to cyclosporine A, tacrolimus may be associated with instillation pain 66. As a strong immunosuppressant, topical tacrolimus may be associated with an increased risk for corneal infections with prolonged use, and close monitoring of patients on long-term therapy is necessary 72. Topical tacrolimus (0.02–0.1%) should be reserved for patients with severe vernal keratoconjunctivitis involving allergy of the eyelid or whose disease is refractory to cyclosporine A 46.
Topical corticosteroid
Topical corticosteroid eye drops should be used as short, pulsed therapy to provide symptomatic relief in patients with more persistent vernal keratoconjunctivitis or acute exacerbations, or when the cornea is involved (e.g., patients with shield ulcers or hyperplasia in limbal vernal keratoconjunctivitis), and under an ophthalmologist’s supervision 46. Topical corticosteroids can be administered at a low or high dose, depending on the severity of vernal keratoconjunctivitis, and can be given up to four times daily with or without cyclosporine A. Corticosteroids act by suppressing the late-phase reaction in both experimental and clinical settings. Corticosteroids, in part, limit the inflammatory cascade by inhibiting phospholipase A2, and consequently act to prevent migration of leukocytes, release of hydrolytic enzymes, growth of fibroblasts, and lead to changes in vascular permeability 77.
Corticosteroids with low intraocular absorption (“soft steroids”), such as hydrocortisone, fluorometholone and loteprednol, may be preferred 29. Dosages are chosen based on the inflammatory state of the eye, with therapy prescribed in pulses of 3–5 days (1). Loteprednol is usually indicated for 7–8 days in the treatment of the acute phase 29. Prednisolone, dexamethasone, and betamethasone are sometimes considered only as second-line options, or as first-line treatment in more severe cases, due to their potential effect on intraocular pressure 29. Steroid–antibiotic combination eye drops are not recommended, since vernal keratoconjunctivitis is an allergic inflammation and not an infection 29.
Moderate-to-severe vernal keratoconjunctivitis may require repeated topical corticosteroid treatment to downregulate conjunctival inflammation 29. Persistent and severe symptoms, thick mucus discharge with moderate-to-severe corneal involvement, numerous and inflamed limbal infiltrates, and/or giant papillae may indicate a need for corticosteroids in addition to cyclosporine A use 29. However, use of corticosteroids as first-line therapy is not recommended in those with only conjunctival involvement 29.
Corticosteroids are not recommended for long-term use because of the increased risk for ocular adverse events, including increased intraocular pressure (IOP), glaucoma, cataracts, and susceptibility to infection 29. These adverse events depend, in part, on the structure of the steroid, the dose, and duration of treatment 78. Treatment with corticosteroids requires careful monitoring for the development of intraocular pressure (IOP), which should be promptly brought under control to prevent deterioration or loss of vision 79.
Supratarsal corticosteroid injections have demonstrated some value in treating adults with vernal keratoconjunctivitis but may not be an appropriate approach for children or for application by general ophthalmologists 79, 80. For more specialist ophthalmologists, this may be an option in children with refractory, severe, or challenging vernal keratoconjunctivitis.
Oral antihistamines or antileukotrienes
Systemic treatment with oral antihistamines or antileukotrienes can reduce the severity of flare-ups and generalized hyperreactivity 29. Oral antihistamines are good for treating hay fever symptoms, especially if you have a lot of different symptoms. You can also take oral antihistamines in advance if you know you are going to be exposed to allergens or triggers. Newer second-generation antihistamines are preferred over older first-generation antihistamines, in order to avoid the sedative and anticholinergic effects that are associated with first-generation agents 81.
Newer, less-sedating antihistamines
Newer antihistamines may rarely cause drowsiness; do not drive or operate machinery if you are affected e.g. cetirizine (Zilarex, Zyrtec), desloratadine (Aerius), fexofenadine (Fexotabs, Telfast), loratadine (Claratyne, Lorano).
Cetirizine and loratadine are available as syrups for children; check correct doses for different age groups. Cetirizine is more likely to cause drowsiness than other less sedating antihistamines.
Older, sedating antihistamines
Older sedating antihistamines can cause drowsiness, sometimes the next day; it is important you do not drive or operate machinery e.g. chlorpheniramine + pseudoephedrine (Demazin 6 Hour Relief Tablets), dexchlorpheniramine (Polaramine), loratadine + pseudoephedrine (Claratyne-D with Decongestant Repetabs), promethazine (Phenergan, Sandoz Fenezal)
Older antihistamines are not available without a prescription for children under 2 years old. Do not drink alcohol with antihistamines that make you drowsy.
If you have other medical conditions, such as glaucoma, epilepsy or prostate problems, or you take antidepressants, check with your doctor before taking these medicines.
Systemic immunotherapy
Systemic immunotherapy is also an option, especially for those patients with underlying systemic atopic disease such as atopic dermatitis and asthma. There are case reports of the successful use of omalizumab, an anti-IgE monoclonal antibody, in patients with vernal conjunctivitis recalcitrant to other treatment modalities, but this remains an off-label use 82, 83. Dupilumab is a human monoclonal antibody against interleukin-4 receptor alpha used in the treatment of atopic dermatitis. There is currently a multi-center, double-masked, randomized, and placebo-controlled, parallel-group study that is evaluating the safety and efficacy of dupilumab in the treatment of signs and symptoms of individuals with atopic keratoconjunctivitis (NCT04296864), and may also play a role in treating vernal disease in those patients older than 12 years 84. However, dupilumab has been found to itself induce atopic keratoconjunctivitis compared to placebo in treated patients, so its role as a primary treatment is debatable 3.
Allergen-specific immunotherapy
Allergen-specific immunotherapy is indicated only when a clearly defined systemic hypersensitivity to an identified allergen exists 46 and should be managed by an allergist or specialist ophthalmologist. The combination of clinical history with the results of a skin-prick test and specific serum IgE should be taken into consideration when the choice of immunotherapy is made 46. There are currently no robust studies of allergen-specific immunotherapy in vernal keratoconjunctivitis 46. A meta-analysis of several double-blind, randomized, placebo-controlled trials investigating sublingual immunotherapy for allergic conjunctivitis, reported a significant reduction in total ocular symptom scores and ocular signs (redness, itchiness, and tearing) vs. placebo in pollen-induced allergic conjunctivitis, but not in allergic conjunctivitis associated with house dust mites, in the pediatric population 85.
Surgical management
Case reports and ongoing clinical practice have indicated that, in rare instances, specialist surgical management may be beneficial in patients with vernal keratoconjunctivitis 1. Surgical approaches that focus on conjunctival cobblestone papillae, shield ulcers, corneal plaques, limbal insufficiency, and other ocular surface presentations that do not respond to medical treatment can be beneficial 86, 87, 88.
Surgical approaches can include excision of giant papillae, debridement of the corneal plaque to remove cytotoxic cells, and amniotic membrane transplantation 89. Surgical treatment, especially corneal plaque removal, may be appropriate for patients with ulcers of moderate-to-severe severity, because short-term re-epithelialization rates are higher and the number of complications is lower than that associated with medical therapy 89. Other reports suggest surgical treatment, such as giant papillae excision, may be appropriate in cases of corneal involvement and in the presence of coarse giant tarsal papillae resulting in ptosis (or mechanical pseudoptosis) 29, 90. Amniotic membrane transplantation following keratectomy has been described as a successful treatment for deep ulcers, in severe allergic patients with slight stromal thinning 91.
Patients with vernal keratoconjunctivitis who may benefit from surgical intervention should be referred to a corneal specialist first. Leonardi et al. 29 have suggested that cryotherapy and/or giant papillae excision papillae should otherwise be avoided because they only treat the complications of vernal keratoconjunctivitis and not the underlying disease, and may induce unnecessary scarring.
Vernal conjunctivitis prognosis
Generally vernal conjunctivitis is a rather benign and self-limiting disease that may resolve with age or spontaneously at puberty 25, 92, 93. Sometimes the debilitating nature of vernal conjunctivitis when it is active necessitates therapy to control symptoms. Complications typically arise from occasional corneal scarring and the unsupervised used of topical corticosteroids can result in cataracts, or glaucoma can lead to permanent visual impairment 25, 92. Rarely, complications in the form of keratoconus, shield ulcers, corneal scarring, and vascularisation may significantly affect visual acuity. Corneal ulcers are reported in close to 9.7% of patients 94, 95. In some patients (as many as 12% of cases) symptoms may persist beyond childhood, which in some cases may represent a conversion to an adult form of atopic keratoconjunctivitis 25. This persistence into adulthood has been shown to be as high as 12% 96.
- Mehta JS, Chen WL, Cheng ACK, Cung LX, Dualan IJ, Kekunnaya R, Khaliddin N, Kim TI, Lam DK, Leo SW, Manurung F, Tesavibul N, Bremond-Gignac D. Diagnosis, Management, and Treatment of Vernal Keratoconjunctivitis in Asia: Recommendations From the Management of Vernal Keratoconjunctivitis in Asia Expert Working Group. Front Med (Lausanne). 2022 Aug 1;9:882240. doi: 10.3389/fmed.2022.882240[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Kaur K, Gurnani B. Vernal Keratoconjunctivitis. [Updated 2023 Jun 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK576433[↩][↩][↩][↩][↩]
- Vernal Keratoconjunctivitis. https://eyewiki.org/Vernal_Keratoconjunctivitis[↩][↩][↩][↩][↩][↩][↩]
- Katelaris CH. Ocular allergy in the Asia Pacific region. Asia Pac Allergy. (2011) 1:108–14. 10.5415/apallergy.2011.1.3.108[↩][↩]
- Zicari AM, Capata G, Nebbioso M, De Castro G, Midulla F, Leonardi L, et al. Vernal keratoconjunctivitis: an update focused on clinical grading system. Ital J Pediatr. (2019) 45:64. 10.1186/s13052-019-0656-4[↩]
- Miyazaki D, Fukagawa K, Okamoto S, Fukushima A, Uchio E, Ebihara N, et al. Epidemiological aspects of allergic conjunctivitis. Allergol Int. (2020) 69:487–95. 10.1016/j.alit.2020.06.004[↩][↩]
- Bremond-Gignac D, Donadieu J, Leonardi A, Pouliquen P, Doan S, Chiambarretta F, et al. Prevalence of vernal keratoconjunctivitis: a rare disease? Br J Ophthalmol. (2008) 92:1097–102. 10.1136/bjo.2007.117812[↩][↩]
- Leonardi A. Management of vernal keratoconjunctivitis. Ophthalmol Ther. 2013 Dec;2(2):73-88. doi: 10.1007/s40123-013-0019-y[↩]
- De Smedt, S., G. Wildner, and P. Kestelyn, Vernal keratoconjunctivitis: an update. Br J Ophthalmol, 2013. 97(1): p. 9-14.[↩][↩][↩][↩]
- Kumar, S., Vernal keratoconjunctivitis: a major review. Acta Ophthalmologica, 2009. 87(2): p. 133-147.[↩][↩][↩][↩][↩][↩][↩]
- Smedt, S.D., et al., Vernal keratoconjunctivitis in school children in Rwanda and its association with socio-economic status: a population-based survey. Am J Trop Med Hyg, 2011. 85(4): p. 711-7.[↩]
- La Rosa, M., et al., Allergic conjunctivitis: a comprehensive review of the literature. Ital J Pediatr, 2013. 39: p. 18.[↩][↩][↩][↩][↩][↩][↩]
- Addis H, Jeng BH. Vernal keratoconjunctivitis. Clin Ophthalmol. (2018) 12:119–23. 10.2147/OPTH.S129552[↩][↩][↩][↩][↩][↩][↩][↩]
- Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye. (2004) 18:345–51. 10.1038/sj.eye.6700675[↩][↩][↩][↩][↩]
- Baudouin C, Liang H, Bremond-Gignac D, Hamard P, Hreiche R, Creuzot-Garcher C, et al. CCR4 and CCR5 expression in conjunctival specimens as differential markers of T(H)1/T(H)2 in ocular surface disorders. J Allergy Clin Immunol. (2005) 116:614–9. 10.1016/j.jaci.2005.05.033[↩][↩]
- Mishra GP, Tamboli V, Jwala J, Mitra AK. Recent patents and emerging therapeutics in the treatment of allergic conjunctivitis. Recent Pat Inflamm Allergy Drug Discov. (2011) 5:26–36. 10.2174/187221311794474883[↩][↩]
- Leonardi A. Vernal keratoconjunctivitis: pathogenesis and treatment. Prog Retin Eye Res. (2002) 21:319–39. 10.1016/S1350-9462(02)00006-X[↩]
- Bonini, S., et al., Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followup. Ophthalmology, 2000. 107(6): p. 1157-63.[↩][↩]
- Ukponmwan, C.U., Vernal keratoconjunctivitis in Nigerians: 109 consecutive cases. Trop Doct, 2003. 33(4): p. 242-5.[↩]
- Bremond-Gignac D. Atopic and vernal keratoconjunctivitis: differences and similarities. Acta Ophthalmologica. (2016) 94:S256. 10.1111/j.1755-3768.2016.0005[↩]
- Leonardi A, Silva D, Perez Formigo D, Bozkurt B, Sharma V, Allegri P, Rondon C, Calder V, Ryan D, Kowalski ML, Delgado L, Doan S, Fauquert JL. Management of ocular allergy. Allergy. 2019 Sep;74(9):1611-1630. doi: 10.1111/all.13786[↩]
- Vichyanond P, Kosrirukvongs P. Use of cyclosporine A and tacrolimus in treatment of vernal keratoconjunctivitis. Curr Allergy Asthma Rep. 2013 Jun;13(3):308-14. doi: 10.1007/s11882-013-0345-0[↩][↩]
- Oray M, Toker E. Tear cytokine levels in vernal keratoconjunctivitis: the effect of topical 0.05% cyclosporine a therapy. Cornea. 2013 Aug;32(8):1149-54. doi: 10.1097/ICO.0b013e31828ffdf8[↩]
- Zicari, A.M., et al., Vernal keratoconjunctivitis: atopy and autoimmunity. Eur Rev Med Pharmacol Sci, 2013. 17(10): p. 1419-23.[↩]
- Buckley, R.J., Vernal keratoconjunctivitis. Int Ophthalmol Clin, 1988. 28(4): p. 303-8.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Leonardi A. Vernal keratoconjunctivitis: pathogenesis and treatment. Prog Retin Eye Res. 2002 May;21(3):319-39. doi: 10.1016/s1350-9462(02)00006-x[↩]
- Alemayehu AM, Yibekal BT, Fekadu SA. Prevalence of vernal keratoconjunctivitis and its associated factors among children in Gambella town, southwest Ethiopia, June 2018. PLoS One. 2019 Apr 18;14(4):e0215528. doi: 10.1371/journal.pone.0215528[↩]
- Barot RK, Shitole SC, Bhagat N, Patil D, Sawant P, Patil K. Therapeutic effect of 0.1% Tacrolimus Eye Ointment in Allergic Ocular Diseases. J Clin Diagn Res. 2016 Jun;10(6):NC05-9. doi: 10.7860/JCDR/2016/17847.7978[↩]
- Leonardi A. Management of vernal keratoconjunctivitis. Ophthalmol Ther. (2013) 2:73–88. 10.1007/s40123-013-0019-y[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Rathi, V.M., & Murthy, S.I. (2017). Allergic conjunctivitis. Community Eye Health, 30, S7 – S10. https://pdfs.semanticscholar.org/d820/987003b832dc90f868f531c9616a4444ac4c.pdf[↩]
- Sacchetti M, Baiardini I, Lambiase A, Aronni S, Fassio O, Gramiccioni C, et al. Development and testing of the quality of life in children with vernal keratoconjunctivitis questionnaire. Am J Ophthalmol. (2007) 144:557–63. 10.1016/j.ajo.2007.06.028[↩]
- Brindisi G, Cinicola B, Anania C, De Castro G, Nebbioso M, Miraglia Del Giudice M, Licari A, Caffarelli C, De Filippo M, Cardinale F, Duse M, Zicari AM. Vernal keratoconjunctivitis: state of art and update on treatment. Acta Biomed. 2021 Nov 29;92(S7):e2021517. doi: 10.23750/abm.v92iS7.12419[↩]
- Krachmer, Jay H., Mark J. Mannis, and Edward J. Holland. Cornea. 2nd ed. Philadelphia: Elsevier/Mosby, 2005. Print. p667-674.[↩][↩][↩]
- Bonini, S., et al., Vernal keratoconjunctivitis. Eye (Lond), 2004. 18(4): p. 345-51.[↩][↩]
- Lai B, Phan K, Lewis N, Shumack S. A rare case of vernal keratoconjunctivitis in a patient with atopic dermatitis treated with tralokinumab. J Eur Acad Dermatol Venereol. 2022 May;36(5):e343-e345. doi: 10.1111/jdv.17824[↩]
- Zengarini C, Roda M, Schiavi C, Bruni F, Bardazzi F, Bellusci C, Raone B. Successful treatment of severe recalcitrant vernal keratoconjunctivitis and atopic dermatitis associated with elevated IgE levels with omalizumab. Clin Exp Dermatol. 2022 Mar;47(3):604-606. doi: 10.1111/ced.15000[↩]
- Cameron, J.A., A.A. Al-Rajhi, and I.A. Badr, Corneal ectasia in vernal keratoconjunctivitis. Ophthalmology, 1989. 96(11): p. 1615-23.[↩]
- Jeng BH, Whitcher JP, Margolis TP. Pseudogerontoxon. Clin Exp Ophthalmol. 2004 Aug;32(4):433-4. doi: 10.1111/j.1442-9071.2004.00849.x[↩]
- Turgut B, Kurt J, Catak O, Demir T. Phthriasis palpebrarum mimicking lid eczema and blepharitis. J Ophthalmol. 2009;2009:803951. doi: 10.1155/2009/803951[↩]
- Cameron JA. Shield ulcers and plaques of the cornea in vernal keratoconjunctivitis. Ophthalmology. (1995) 102:985–93. 10.1016/S0161-6420(95)30925-6[↩][↩]
- Reddy JC, Basu S, Saboo U, Murthy SI, Vaddavalli PK, Sangwan VS. Management, clinical outcomes, and complications of shield ulcers in vernal keratoconjunctivitis. Am J Ophthalmol. (2013) 155:550–9. 10.1016/j.ajo.2012.09.014[↩]
- Bonini S, Bonini S, Lambiase A, Marchi S, Pasqualetti P, Zuccaro O, et al. Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followup. Ophthalmology. (2000) 107:1157–63. 10.1016/s0161-6420(00)00092-0[↩]
- Tabbara KF. Ocular complications of vernal keratoconjunctivitis. Can J Ophthalmol. (1999) 34:88–92.[↩]
- Sen P, Jain S, Mohan A, Shah C, Sen A, Jain E. Pattern of steroid misuse in vernal keratoconjunctivitis resulting in steroid induced glaucoma and visual disability in Indian rural population: an important public health problem in pediatric age group. Indian J Ophthalmol. (2019) 67:1650–5. 10.4103/ijo.IJO_2143_18[↩]
- La Rosa M, Lionetti E, Reibaldi M, Russo A, Longo A, Leonardi S, Tomarchio S, Avitabile T, Reibaldi A. Allergic conjunctivitis: a comprehensive review of the literature. Ital J Pediatr. 2013 Mar 14;39:18. doi: 10.1186/1824-7288-39-18[↩]
- Leonardi A, Silva D, Perez Formigo D, Bozkurt B, Sharma V, Allegri P, et al. Management of ocular allergy. Allergy. (2019) 74:1611–30. 10.1111/all.13786[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Spector SL, Raizman MB. Conjunctivitis medicamentosa. J Allergy Clin Immunol. 1994 Jul;94(1):134-6. doi: 10.1016/0091-6749(94)90081-7[↩]
- Leonardi, A., Management of vernal keratoconjunctivitis. Ophthalmol Ther, 2013. 2(2): p. 73-88.[↩][↩][↩][↩]
- Yanni JM, Weimer LK, Sharif NA, Xu SX, Gamache DA, Spellman JM. Inhibition of histamine-induced human conjunctival epithelial cell responses by ocular allergy drugs. Arch Ophthalmol. (1999) 117:643–7. 10.1001/archopht.117.5.643[↩]
- Yanni JM, Sharif NA, Gamache DA, Miller ST, Weimer LK, Spellman JM. A current appreciation of sites for pharmacological intervention in allergic conjunctivitis: effects of new topical ocular drugs. Acta Ophthalmol Scand Suppl. (1999) 228:33–7. 10.1111/j.1600-0420.1999.tb01171.x[↩]
- Gane J, Buckley R. Leukotriene receptor antagonists in allergic eye disease: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2013 Jan;1(1):65-74. doi: 10.1016/j.jaip.2012.07.001[↩]
- Abelson M.B., and Gomes P.J. Olopatadine 0.2% ophthalmic solution: The first ophthalmic antiallergy agent with once-daily dosing. Expert Opin Drug Metab Toxicol 4: 453–461, 2008.[↩]
- Greiner JV, Edwards-Swanson K, Ingerman A. Evaluation of alcaftadine 0.25% ophthalmic solution in acute allergic conjunctivitis at 15 minutes and 16 hours after instillation versus placebo and olopatadine 0.1%. Clin Ophthalmol. 2011 Jan 13;5:87-93. doi: 10.2147/OPTH.S15379[↩]
- Ackerman S, D’Ambrosio F Jr, Greiner JV, Villanueva L, Ciolino JB, Hollander DA. A multicenter evaluation of the efficacy and duration of action of alcaftadine 0.25% and olopatadine 0.2% in the conjunctival allergen challenge model. J Asthma Allergy. 2013 Apr 8;6:43-52. doi: 10.2147/JAA.S38671[↩]
- Hida WT, Nogueira DC, Schaefer A, Dantas PEC, Dantas MCN. Comparação entre o uso tópico do fumarato de cetotifeno 0,025% e do cloridrato de olopatadina 0,1% no tratamento da ceratoconjuntivite primaveril [comparative study between 0.025% ketotifen fumarate and 0.1% olopatadine hydrochloride in the treatment of vernal keratoconjunctivitis]. Arq Bras Oftalmol. (2006) 69:851–6. 10.1590/S0004-27492006000600013[↩]
- Holsclaw, D.S.; Whitcher, J.P.; Wong, I.G.; Margolis, T.P. Supratarsal injection of corticosteroid in the treatment of refractory vernal keratoconjunctivitis. Am. J. Ophthalmol. 1996, 121, 243–249.[↩]
- Gokhale NS. Systematic approach to managing vernal keratoconjunctivitis in clinical practice: severity grading system and a treatment algorithm. Indian J Ophthalmol. (2016) 64:145–8. 10.4103/0301-4738.179727[↩][↩]
- Lallemand F, Schmitt M, Bourges JL, Gurny R, Benita S, Garrigue JS. Cyclosporine A delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm. (2017) 117:14–28. 10.1016/j.ejpb.2017.03.006[↩]
- Nebbioso M, Alisi L, Giovannetti F, Armentano M, Lambiase A. Eye drop emulsion containing 0.1% cyclosporin (1 mg/mL) for the treatment of severe vernal keratoconjunctivitis: an evidence-based review and place in therapy. Clin Ophthalmol. (2019) 13:1147–55. 10.2147/OPTH.S181811[↩]
- Lallemand F, Daull P, Benita S, Buggage R, Garrigue JS. Successfully improving ocular drug delivery using the cationic nanoemulsion, novasorb. J Drug Dev. (2012) 2012:6044204. 10.1155/2012/604204[↩]
- Wan KH, Chen LJ, Rong SS, Pang CP, Young AL. Topical cyclosporine in the treatment of allergic conjunctivitis: a meta-analysis. Ophthalmology. (2013) 120:2197–203. 10.1016/j.ophtha.2013.03.044[↩]
- Bremond-Gignac D, Doan S, Amrane M, Ismail D, Montero J, Németh K, et al. Twelve-month results of cyclosporine a cationic emulsion in a randomized study in patients with pediatric vernal keratoconjunctivitis. Am J Ophthalmol. (2020) 212:116–26. 10.1016/j.ajo.2019.11.020[↩]
- Leonardi A, Doan S, Amrane M, Ismail D, Montero J, Németh J, et al. A randomized, controlled trial of cyclosporine a cationic emulsion in pediatric vernal keratoconjunctivitis: the VEKTIS study. Ophthalmology. (2019) 126:671–81. 10.1016/j.ophtha.2018.12.027[↩][↩]
- Oray, M. and E. Toker, Tear cytokine levels in vernal keratoconjunctivitis: the effect of topical 0.05% cyclosporine a therapy. Cornea, 2013. 32(8): p. 1149-54.[↩]
- Vichyanond, P. and P. Kosrirukvongs, Use of cyclosporine A and tacrolimus in treatment of vernal keratoconjunctivitis. Curr Allergy Asthma Rep, 2013. 13(3): p. 308-14.[↩]
- Al-Amri AM, Mirza AG, Al-Hakami AM. Tacrolimus ointment for treatment of vernal keratoconjunctivitis. Middle East Afr J Ophthalmol. (2016) 23:135–8. 10.4103/0974-9233.164616[↩][↩]
- Müller EG, Santos MSD, Freitas D, Gomes JAP, Belfort R., Jr Tacrolimus eye drops as monotherapy for vernal keratoconjunctivitis: a randomized controlled trial. Arq Bras Oftalmol. (2017) 80:154–8. 10.5935/0004-2749.20170038[↩][↩]
- Müller GG, José NK, de Castro RS, de Holanda EC. Long-term use of topical tacrolimus ointment: a safe and effective option for the treatment of vernal keratoconjunctivitis. Arq Bras Oftalmol. (2019) 82:119–23. 10.5935/0004-2749.20190026[↩][↩]
- Ohashi Y, Ebihara N, Fujishima H, Fukushima A, Kumagai N, Nakagawa Y, et al. A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J Ocul Pharmacol Ther. (2010) 26:165–74. 10.1089/jop.2009.0087[↩]
- Chatterjee S, Agrawal D. Tacrolimus in corticosteroid-refractory vernal keratoconjunctivitis. Cornea. (2016) 35:1444–8. 10.1097/ICO.0000000000000918[↩]
- Liu FY, Liu HY, Chu HS, Chen WL, Hu FR, Wang IJ. Dermatologic tacrolimus ointment on the eyelids for steroid-refractory vernal keratoconjunctivitis. Graefes Arch Clin Exp Ophthalmol. (2019) 257:967–74. 10.1007/s00417-019-04287-1[↩]
- Fukushima A, Ohashi Y, Ebihara N, Uchio E, Okamoto S, Kumagai N, et al. Therapeutic effects of 0.1% tacrolimus eye drops for refractory allergic ocular diseases with proliferative lesion or corneal involvement. Br J Ophthalmol. (2014) 98:1023–7.[↩][↩]
- Barot, RK et al., Therapeutic effect of 0.1% Tacrolimus Eye Ointment in Allergic Ocular Diseases. J Clin Diagn Res. 2016 Jun;10(6):NC05-9.[↩]
- Wan Q et al., Therapeutic effect of 0.1% Tacrolimus Eye Drops in the Tarsal Form of Vernal Keratoconjunctivitis. Ophthalmic Res. 2018;59(3):126-134.[↩]
- Labcharoenwongs P, Jirapongsananuruk O, Visitsunthorn N, Kosrirukvongs P, Saengin P, Vichyanond P. A double-masked comparison of 0.1% tacrolimus ointment and 2% cyclosporine eye drops in the treatment of vernal keratoconjunctivitis in children. Asian Pac J Allergy Immunol. 2012 Sep;30(3):177-84. https://apjai-journal.org/wp-content/uploads/2017/12/2AdoublemaskedcomparisonVol30No3September2012P177.pdf[↩]
- Leonardi, A., et al., Vernal keratoconjunctivitis-like disease in adults. Am J Ophthalmol, 2013. 155(5): p. 796-803.[↩]
- Niederkorn JY, Dana MR. Immune System and the Eye Ocular Therapeutics. Amsterdam: Elsevier; (2008). p. 199–237. 10.1016/b978-012370585-3.50012-1[↩]
- McGhee CNJ, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. (2002) 25:33–55.[↩]
- Holsclaw DS, Whitcher JP, Wong IG, Margolis TP. Supratarsal injection of corticosteroid in the treatment of refractory vernal keratoconjunctivitis. Am J Ophthalmol. (1996) 121:243–9.[↩][↩]
- Zaouali S, Kahloun R, Attia S, Jelliti B, Trigui M, Yahia SB, et al. Supratarsal injection of triamcinolone acetonide and childhood allergic keratoconjunctivitis. Int Ophthalmol. (2012) 32:99–106. 10.1007/s10792-011-9421-4[↩]
- Bielory L, Lien KW, Bigelsen S. Efficacy and tolerability of newer antihistamines in the treatment of allergic conjunctivitis. Drugs. (2005) 65:215–28.[↩]
- de Klerk, T.A., et al., Severe vernal keratoconjunctivitis successfully treated with subcutaneous omalizumab. J aapos, 2013. 17(3): p. 305-6.[↩]
- Doan, S., Amat, F., Gabison, E. et al. Omalizumab in Severe Refractory Vernal Keratoconjunctivitis in Children: Case Series and Review of the Literature. Ophthalmol Ther 6, 2017, 195–206.[↩]
- Evaluation of Dupilumab in Patients With Atopic Keratoconjunctivitis (AKC). https://clinicaltrials.gov/study/NCT04296864[↩]
- Calderon MA, Penagos M, Sheikh A, Canonica GW, Durham SR. Sublingual immunotherapy for allergic conjunctivitis: cochrane systematic review and meta-analysis. Clin Exp Allergy. (2011) 41:1263–72.[↩]
- Lin HY, Yeh PT, Shiao CS, Hu FR. Surgical management and immunohistochemical study of corneal plaques in vernal keratoconjunctivitis. J Formos Med Assoc. (2013) 112:569–73. 10.1016/j.jfma.2012.07.017[↩]
- Pelegrin L, Gris O, Adán A, Plazas A. Superficial keratectomy and amniotic membrane patch in the treatment of corneal plaque of vernal keratoconjunctivitis. Eur J Ophthalmol. (2008) 18:131–3. 10.1177/112067210801800123[↩]
- Solomon A, Zamir E, Levartovsky S, Frucht-Pery J. Surgical management of corneal plaques in vernal keratoconjunctivitis: a clinicopathologic study. Cornea. (2004) 23:608–12. 10.1097/01.ico.0000121710.58571.c4[↩]
- Stock RA, Lazzari SL, Martins IP, Bonamingo EL. Surgical debridement of corneal shield ulcers in pediatric patients: two case reports and a review of the literature. J Med Case Rep. (2020) 14:70. 10.1186/s13256-020-02407-8[↩][↩]
- AlHaran DH. Management of vernal keratoconjunctivitis in children in Saudi Arabia. Oman J Ophthalmol. (2020) 13:3–12.[↩]
- Tanaka M, Dogru M, Takano Y, Miyake-Kashima M, Asano-Kato N, Fukagawa K, et al. Quantitative evaluation of the early changes in ocular surface inflammation following MMC-aided papillary resection in severe allergic patients with corneal complications. Cornea. (2006) 25:281–5. 10.1097/01.ico.0000183533.14899.8d[↩]
- Kumar S. Vernal keratoconjunctivitis: a major review. Acta Ophthalmol. 2009 Mar;87(2):133-47. doi: 10.1111/j.1755-3768.2008.01347.x[↩][↩]
- De Smedt S, Wildner G, Kestelyn P. Vernal keratoconjunctivitis: an update. Br J Ophthalmol. 2013 Jan;97(1):9-14. doi: 10.1136/bjophthalmol-2011-301376[↩]
- NEUMANN E, GUTMANN MJ, BLUMENKRANTZ N, MICHAELSON IC. A review of four hundred cases of vernal conjunctivitis. Am J Ophthalmol. 1959 Feb;47(2):166-72. doi: 10.1016/s0002-9394(14)76417-7[↩]
- Cameron JA. Shield ulcers and plaques of the cornea in vernal keratoconjunctivitis. Ophthalmology. 1995 Jun;102(6):985-93. doi: 10.1016/s0161-6420(95)30925-6[↩]
- Saboo, U.S., et al., Demographic and clinical profile of vernal keratoconjunctivitis at a tertiary eye care center in India. Indian J Ophthalmol, 2013. 61(9): p. 486-9.[↩]