Contents
What is Addisonian crisis?
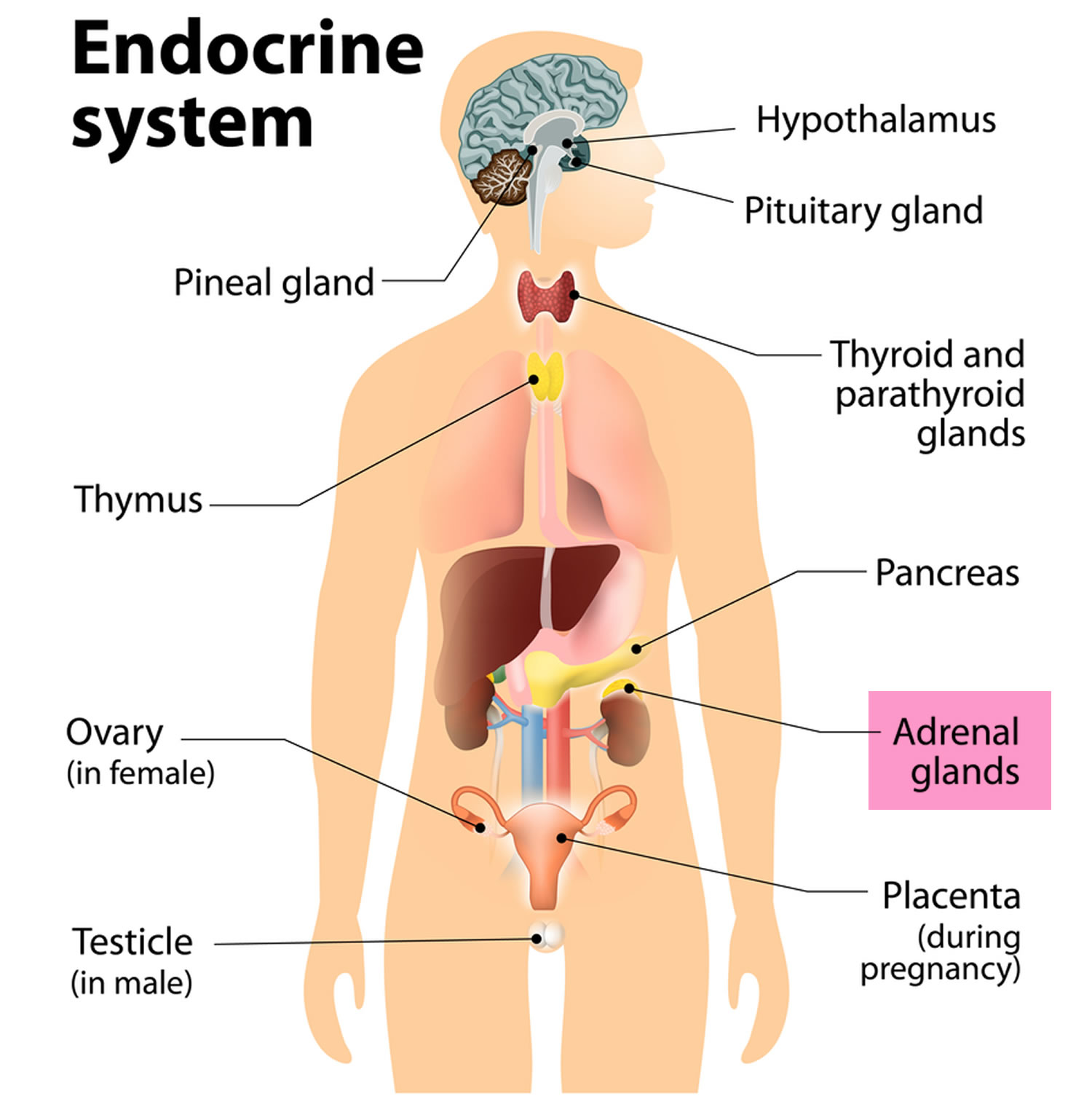
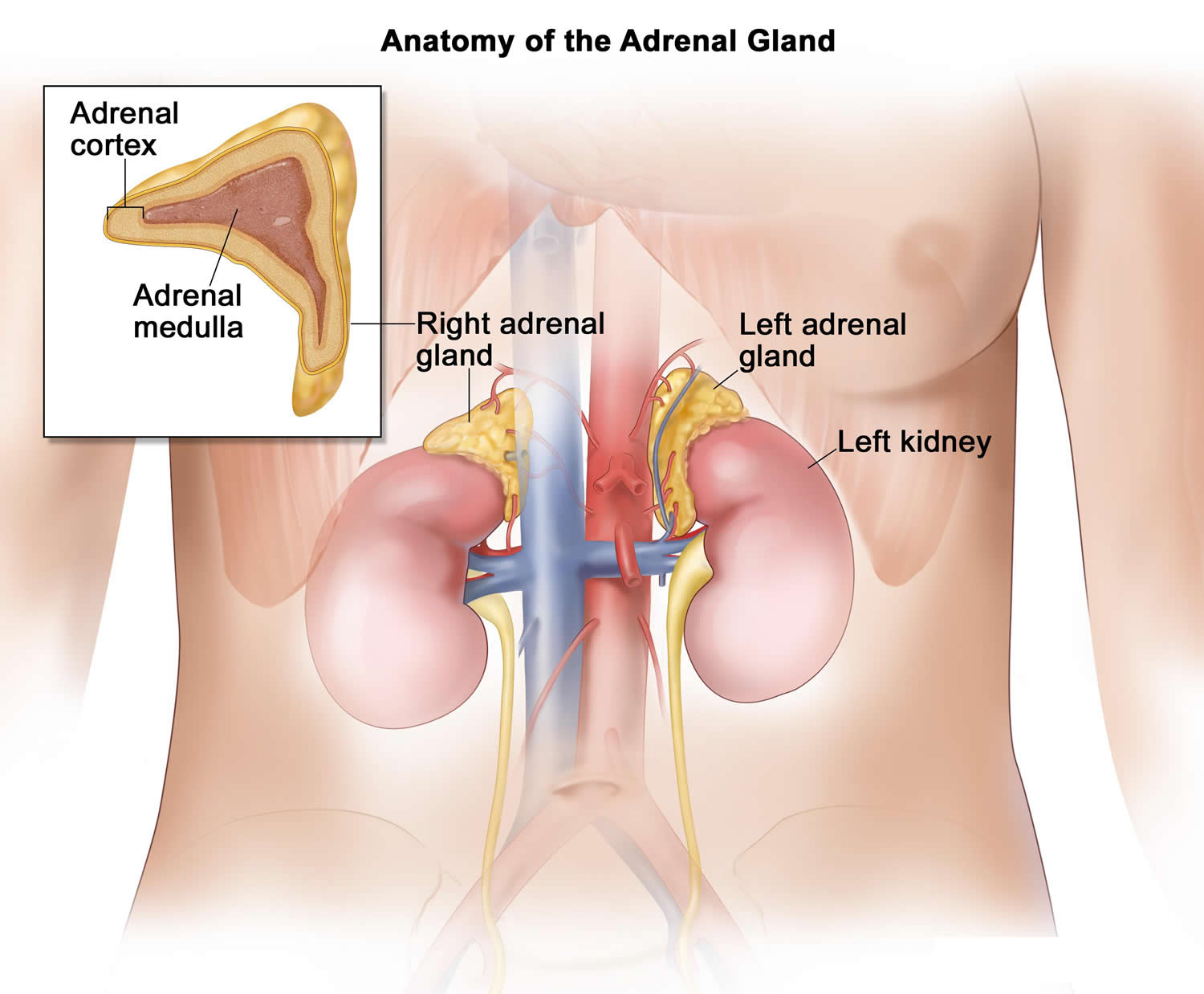
Addisonian crisis also called acute adrenal crisis, acute adrenal failure or acute adrenal insufficiency is a serious life-threatening condition that can cause death in people who lack the adrenal gland hormone cortisol 1, 2, 3. Cortisol belongs to a class of hormones called glucocorticoids. Glucocorticoids (cortisol and corticosterone) are secreted by the adrenal glands in larger amounts (10–15 mg cortisol/day) from the zona fasciculata in response to stimulation by adrenocorticotrophic hormone (ACTH) (Figure 3) 4, 5. Cortisol is a steroid hormone that is produced by your 2 adrenal glands, which sit on top of each of your kidneys (Figures 1 to 7). When you are stressed, increased cortisol is released into your bloodstream. Having the right cortisol balance is essential for your health, and producing too much or too little cortisol can cause health problems. Cortisol is also needed for the ‘fight or flight’ response, which is your healthy, natural response to perceived threats. The amount of cortisol produced is controlled by your body to ensure the balance is correct. Often, people are unaware that they lack cortisol hormone and therefore do not know about their risk of adrenal crisis.
Acute adrenal crisis can be the initial presentation of undiagnosed adrenal insufficiency, potentially occurring in up to 50% of patients who have already been diagnosed with this condition 6, 7, 8. In a previous study, approximately 10% of patients exhibited no identifiable cause for their adrenal crisis 9.
Adrenal crisis has a substantial death rate of 0.5 per 100 patient-years and remains a significant cause of death in individuals with adrenal insufficiency 10. Patients can experience rapid deterioration without timely treatment, potentially resulting in fatal outcomes either at home or shortly after hospital admission 11. An acute adrenal crisis signs and symptoms include vomiting, abdominal pain, and hypovolemic shock 12, 13. Due to its rare occurrence, adrenal crisis remains inadequately recognized, leading to substantial treatment delays and increased morbidity and mortality rates 14.
Adrenal crisis is defined by an acute deterioration in health status that is associated with the following conditions 15:
- Absolute hypotension (low blood pressure) with a systolic blood pressure <100 mm Hg.
- Relative hypotension (low blood pressure) with a systolic blood pressure ≥20 mm Hg lower than the patient’s usual baseline.
Typically, these features should improve and resolve within 1 to 2 hours after administering parenteral stress dose of hydrocortisone, marked by a significant reduction in hypotension within the first hour. Furthermore, there should be a gradual improvement in patients’ clinical symptoms over the subsequent 2 hours 15.
Administration of stress dose hydrocortisone (above physiological dose of glucocorticoid replacement therapy) and fluid resuscitation is the only definitive therapy for adrenal crisis 16,
Emergency management of adrenal crisis (Addisonian crisis) the first 24 hours 17, 18, 19, 20:
- For Adults, administer parenteral hydrocortisone 100mg stat (IM preferable) and repeat 6 hourly until the patient is hemodynamically stable and clinical improvement (alternative 200mg/24hrs by continuous IV infusion).
- Infants and children should receive an initial parenteral injection of 50mg hydrodortisone/m² (usually 25mg in infants and 50mg in children) followed by 50mg/24h in infants and 100mg/24h in children.
- Administer 1 liter IV 0.9% saline stat (adjust for infants and children), and continue saline resuscitation at an appropriate rate until hemodynamic stability and correction of any electrolyte disturbance and acute kidney injury
- Monitor urea and electrolytes at least 12 hourly during initial resuscitation and continue regular monitoring until any hyponatremia, hyperkalemia or renal impairment are corrected
- Identify and treat any precipitating cause for Addisonian crisis:
- vomiting/diarrheal illness
- infection
- heart attack (myocardial infarction)
Recent evidence indicates that continuous infusion is a superior delivery method for hydrocortisone in the management of adrenal crisis when compared to intermittent boluses 21. Continuous hydrocortisone infusion has shown better maintenance of cortisol levels within the therapeutic range.
Once there is clinical improvement, initiating a gradual tapering of steroids is advisable 22. This approach helps prevent abrupt discontinuation and enables a smoother transition to lower doses.
The necessity for mineralocorticoid replacement should be assessed individually and in consultation with an endocrinologist. If the glucocorticoid doses administered to patients exceed 50 mg, mineralocorticoid replacement is unnecessary 23.
In situations where hydrocortisone is unavailable, alternative parenteral glucocorticoids can be considered 24, 15:
- Prednisolone: This can be a preferred alternative treatment, administered as an initial bolus of 25 mg and followed by 2 additional 25 mg doses within the first 24 hours. Subsequently, this regimen should be continued with a daily dose of 50 mg of prednisone.
- Methylprednisolone: This can be administered at a dosage of 40 mg every 24 hours.
- Dexamethasone: This is the least preferred alternative treatment, with a recommended dosage of 4 mg every 24 hours.
In patients with an infectious process as the precipitating event for adrenal crisis, prompt administration of appropriate antibiotics is necessary to address the underlying infection.
If patients do not adequately respond within the first 24 hours after glucocorticoid administration, an alternative diagnosis, such as sepsis or a cardiogenic event, or coexisting cause of hypotension should be considered 15.
An adrenal crisis can result in fatal outcomes, even with timely recognition and appropriate treatment 11. Besides the risk of death, adrenal crisis is associated with other potential complications. Electrolyte abnormalities, such as hyponatremia, hyperkalemia, and hypoglycemia, can lead to various complications, including seizures, arrhythmias, and coma 25. If left untreated, hypotension can lead to hypoperfusion, potentially resulting in multiple organ failure. Furthermore, the precipitating disease or event that triggered the adrenal crisis can introduce additional complications 1.
Next, it is essential to identify and treat the underlying cause for the adrenal crisis 26. Further thorough investigation should focus on identifying an underlying infection, since this is by far the most common precipitating factor for an adrenal crisis and a substantial cause of death 26. It is essential to take cultures adequately and start antibiotic treatment in case of a suspected bacterial infection.
Figure 1. Location of the adrenal glands on top of each kidneys
Figure 2. Adrenal gland anatomy
Figure 3. Adrenal gland hormones
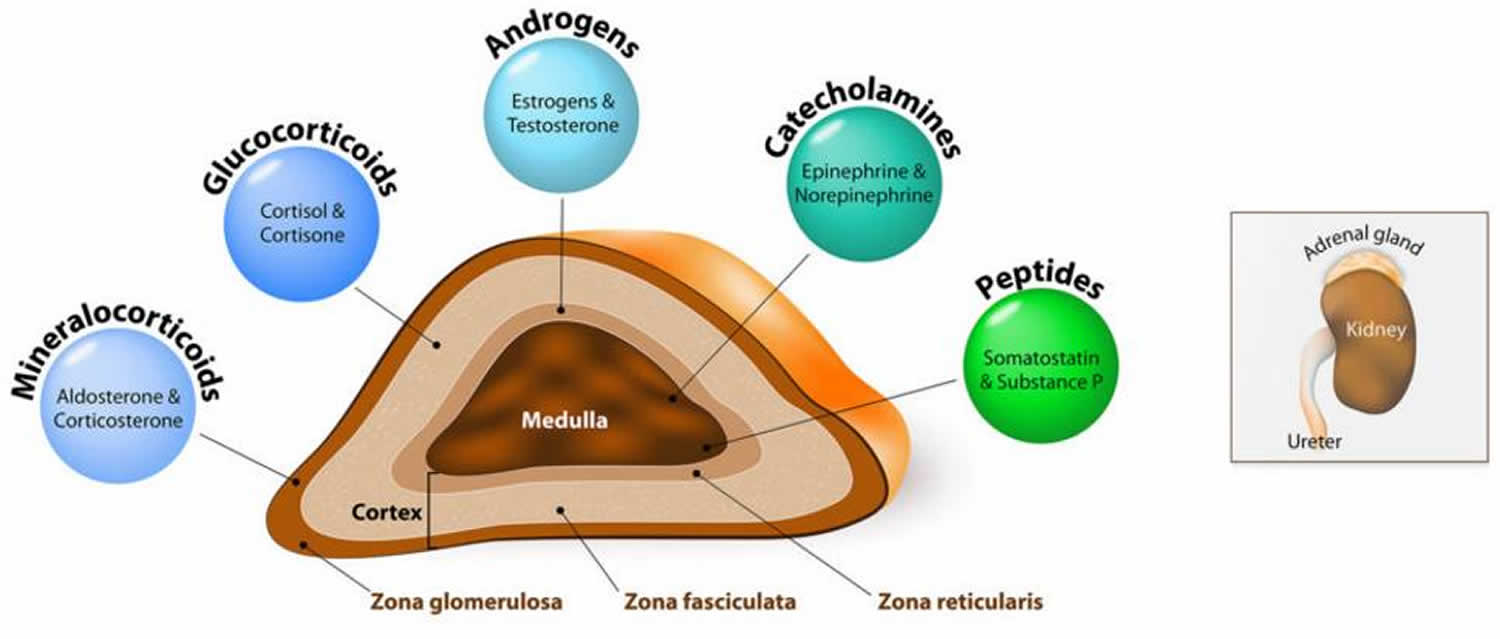
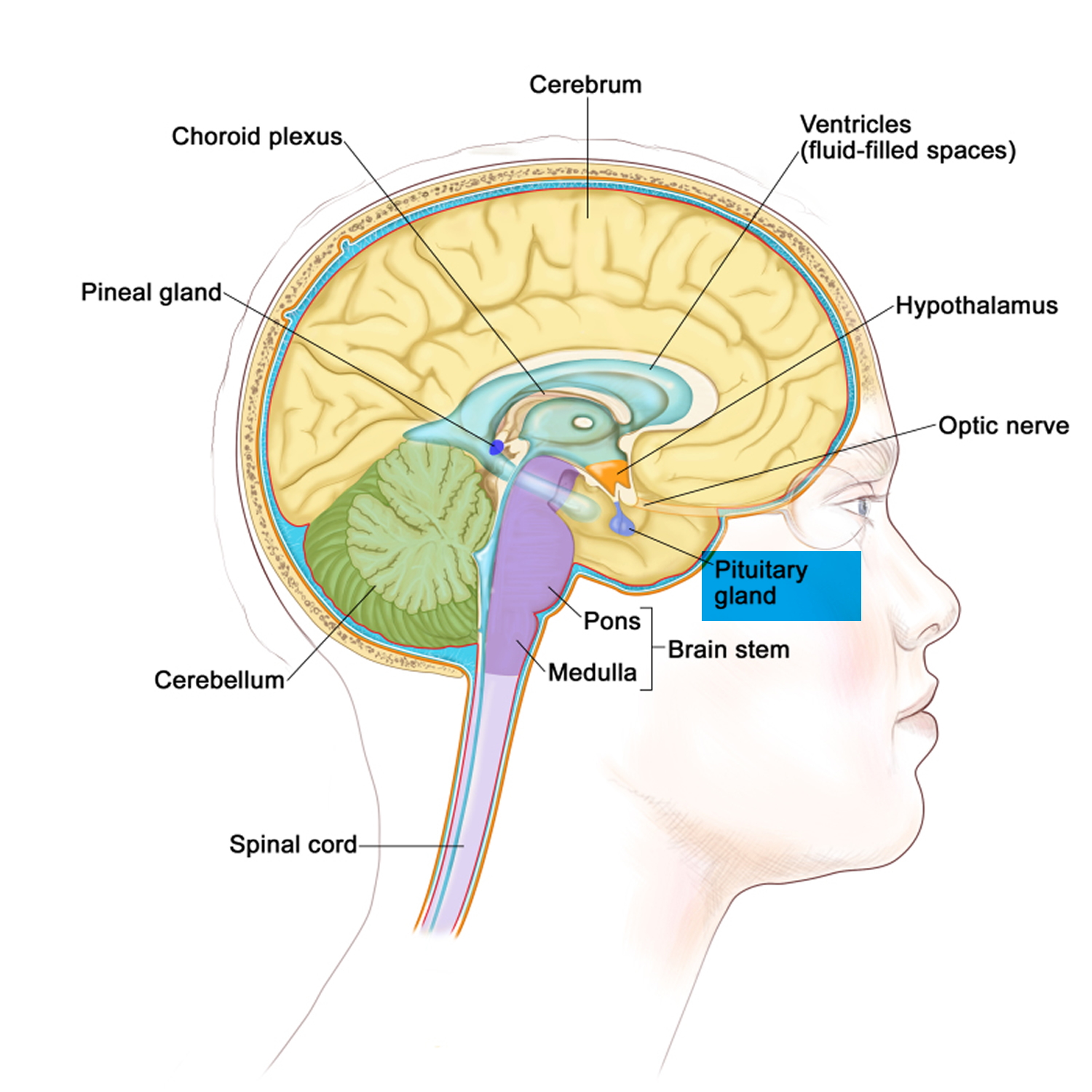
Figure 4. The pituitary gland location
Figure 5. Pituitary gland
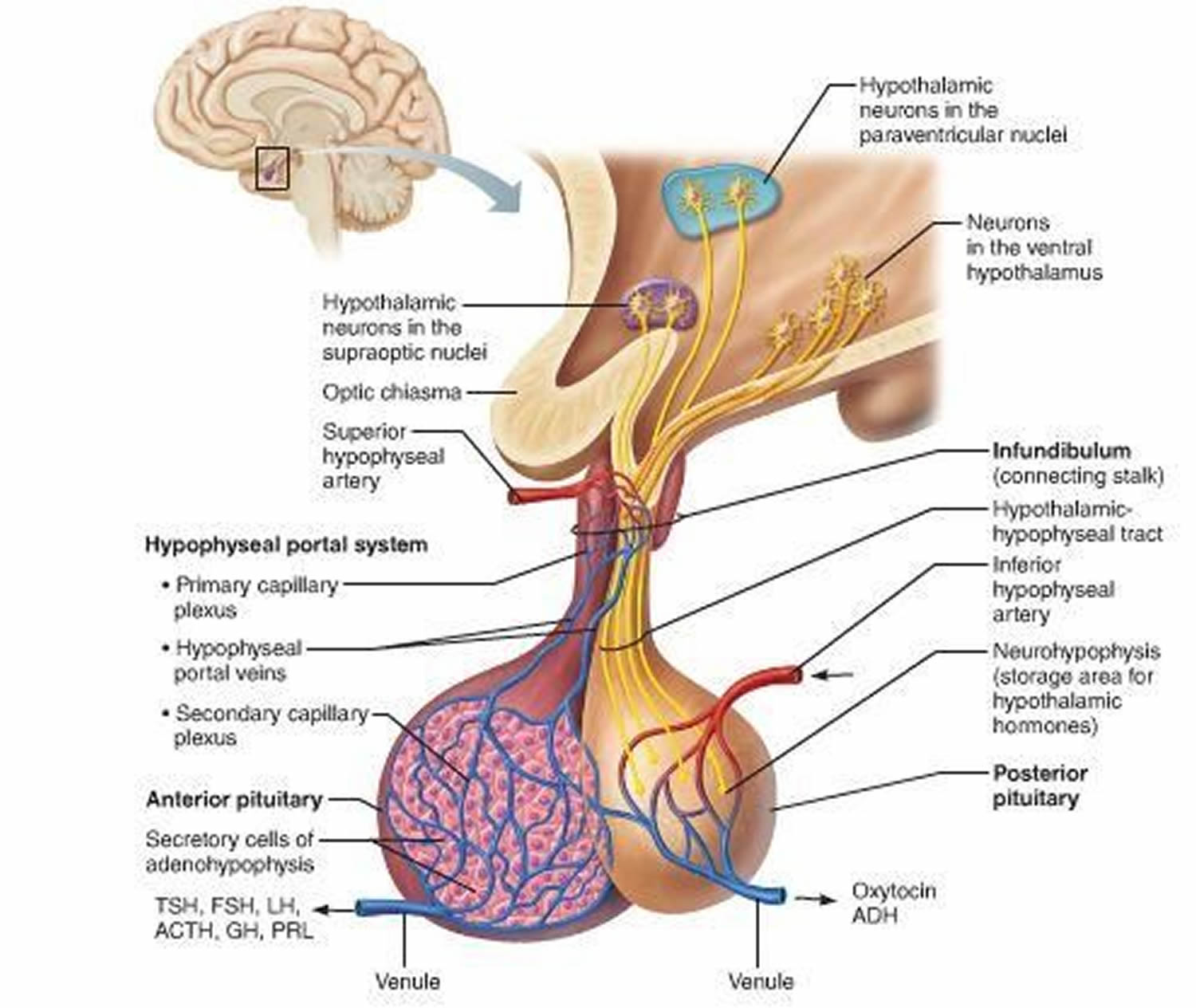
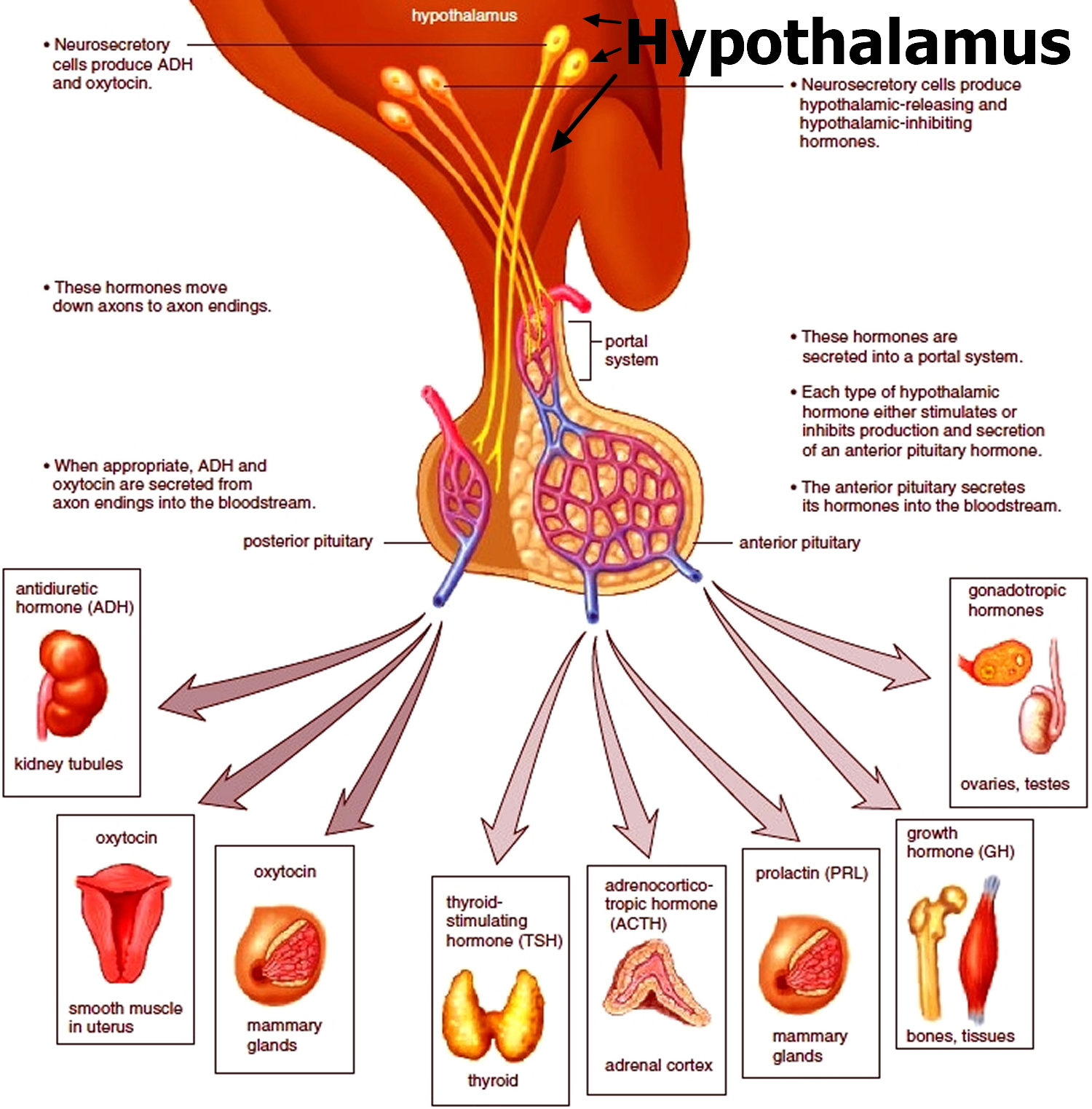
Figure 6. The hypothalamus and pituitary gland (anterior and posterior) endocrine pathways and target organs
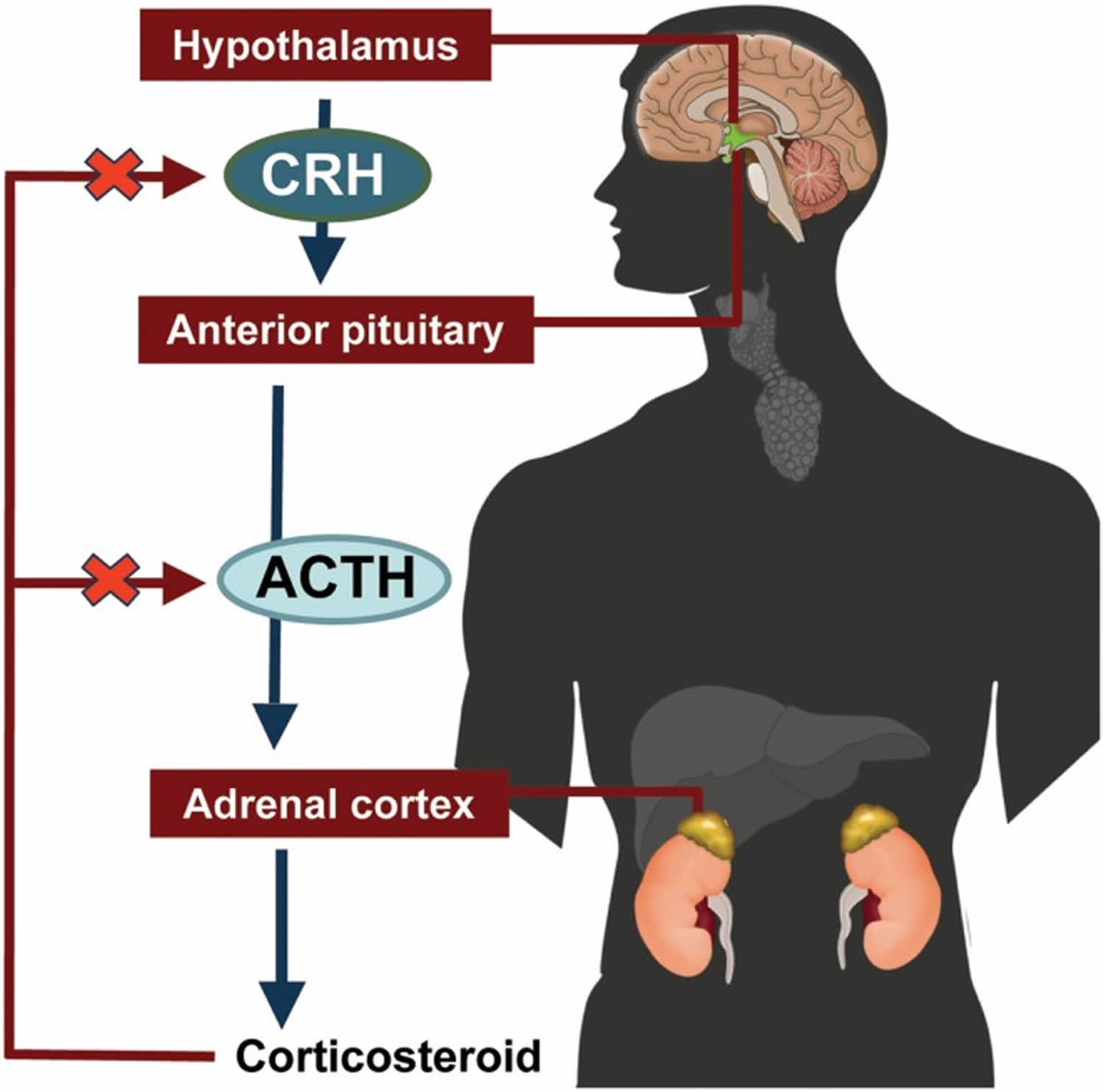
Figure 7. ACTH and Cortisol production and control by the hypothalamus (the Hypothalamic-Pituitary-Adrenal axis)
[Source 27 ]Figure 8. Negative feedback regulates cortisol secretion
See your doctor if you have common signs and symptoms of Addison’s disease, such as:
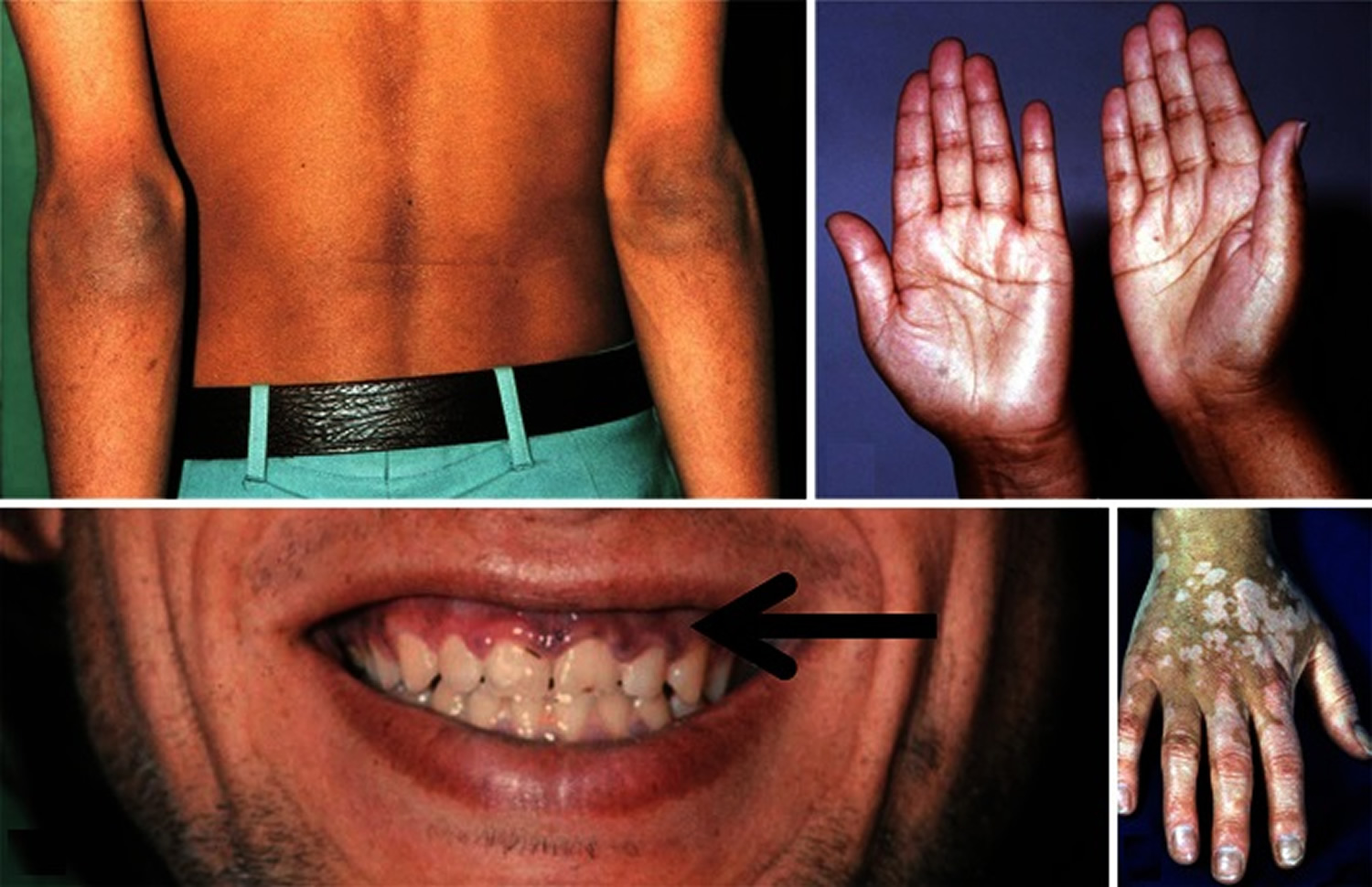
- Darkening areas of skin (hyperpigmentation)
- Severe fatigue
- Unintentional weight loss
- Gastrointestinal problems, such as nausea, vomiting and abdominal pain
- Lightheadedness or fainting
- Salt cravings
- Muscle or joint pains
Each year, typically 8% of people with Addison’s disease experience Addisonian crisis (adrenal crisis). This means they need extra steroid medication immediately, in the form of an emergency injection of intra-muscular hydrocortisone.
The danger signs of Addisonian crisis include:
- Extreme weakness, feeling terrible, vomiting, headache
- Light-headedness or dizziness on sitting up or standing up
- Feeling very cold, uncontrollable shaking; back, limb or abdominal pain
- Confusion, drowsiness, loss of consciousness
If this happens to yourself or someone you are caring for, do not delay:
- inject yourself (or the person you are caring for) with your hydrocortisone ampoule (100mg)
- seek immediate medical attention – call your local emergency services number, stating “Addisonian crisis”
- Other key phrases to use are: Steroid-dependent, risk of adrenal crisis, adrenal insufficiency crisis, Addison’s or Addisonian emergency
- AND describe symptoms (vomiting, diarrhea, dehydration, injury/shock).
What do adrenal hormones do?
Adrenal hormones, such as cortisol and aldosterone, play key roles in the functioning of the human body, such as regulating blood pressure; metabolism, the way the body uses digested food for energy; and the body’s response to stress. In addition, the body uses the adrenal hormone dehydroepiandrosterone (DHEA) to make androgens and estrogens, the male and female sex hormones.
Cortisol
Cortisol belongs to the class of hormones called glucocorticoids, which affect almost every organ and tissue in the body. Cortisol’s most important job is to help the body respond to stress. Among its many tasks, cortisol helps:
- maintain blood pressure and heart and blood vessel function
- slow the immune system’s inflammatory response—how the body recognizes and defends itself against bacteria, viruses, and substances that appear foreign and harmful
- regulate metabolism
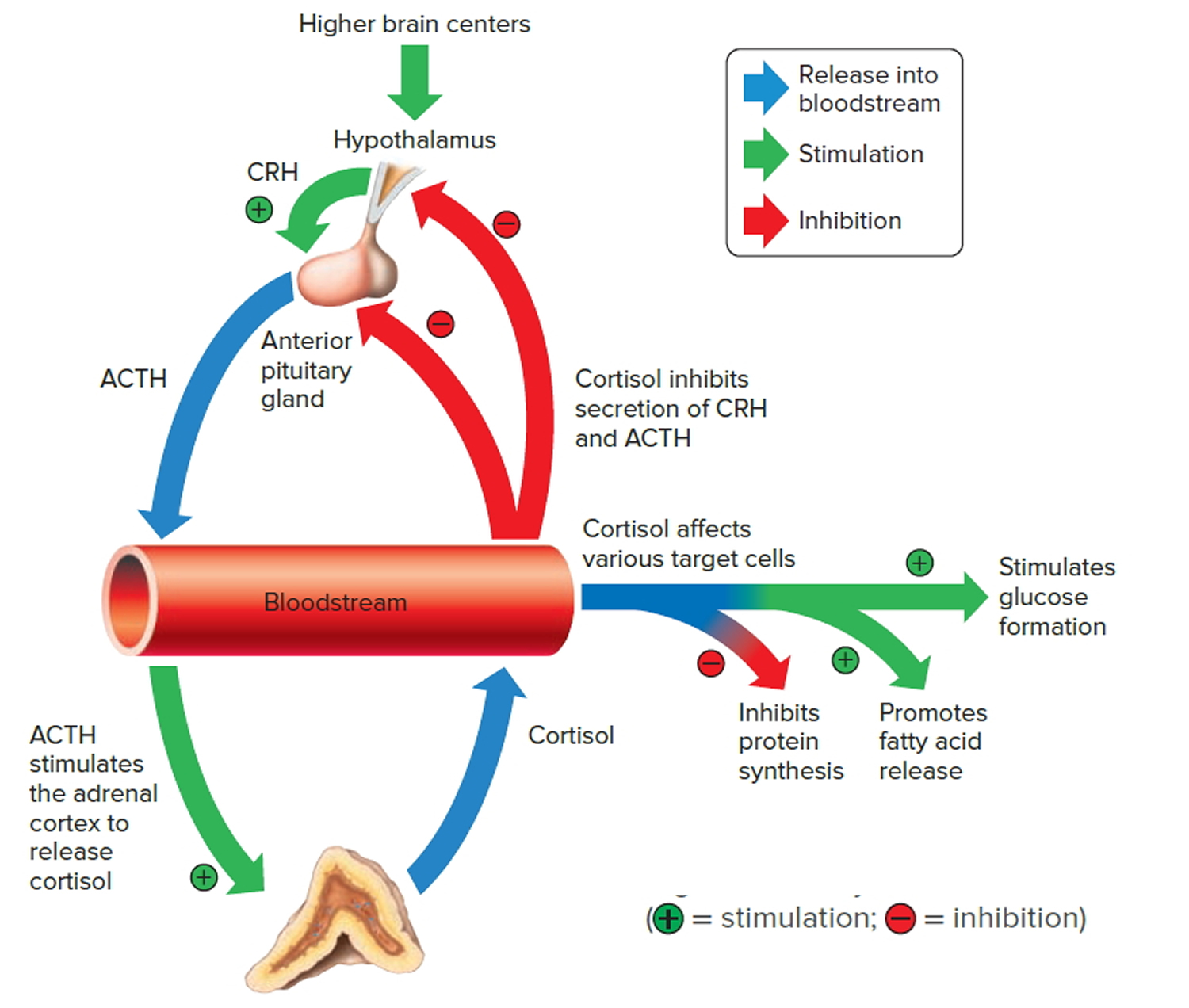
The amount of cortisol produced by the adrenal glands is precisely balanced. Like many other hormones, cortisol is regulated by the hypothalamus, which is a part of the brain, and the pituitary gland. First, the hypothalamus releases a “trigger” hormone called corticotropin-releasing hormone (CRH), which signals the pituitary gland to send out adrenocorticotropic hormone (ACTH). ACTH stimulates the adrenal glands to produce cortisol. Cortisol then signals back to both the pituitary gland and hypothalamus to decrease these trigger hormones.
Figure 9. Cortisol
What happens when you produce too much or little cortisol?
Your body usually produces the right amount of cortisol. In a condition such as Cushing’s syndrome, it produces too much. In a condition such as Addison’s disease, it produces too little.
Symptoms of too much cortisol include:
- weight gain, particularly around the abdomen and face
- thin and fragile skin that is slow to heal
- acne
- for women, facial hair and irregular menstrual periods.
Symptoms of not enough cortisol include:
- continual tiredness
- nausea and vomiting
- weight loss
- muscle weakness
- pain in the abdomen.
If you experience any of these symptoms, your doctor may suggest you have a blood test to measure your cortisol levels.
If your body does not produce enough cortisol, your doctor may prescribe corticosteroids for you. Corticosteroids are synthetic versions of cortisol that can be used to treat a variety of conditions including:
- inflammatory conditions (such as asthma)
- Addison’s disease
- skin conditions (such as psoriasis).
Some people take anabolic steroids to build muscles, without a doctor’s prescription. This is risky. Anabolic steroids are different to corticosteroids.
Because corticosteroids are powerful medications, side effects are quite common. These may include:
- thinning skin
- osteoporosis
- weight gain, especially around the face, and increased appetite
- high blood sugar or diabetes
- rapid mood changes, feeling irritable and anxious
- an increased chance of infections like chickenpox or measles
- Cushing’s syndrome
- eye conditions, such as glaucoma and cataracts
- depression or suicidal thoughts
- high blood pressure.
If you experience these side effects, it is important to talk to your doctor before stopping your medication.
Aldosterone
Aldosterone belongs to the class of hormones called mineralocorticoids, also produced by the adrenal glands. Aldosterone helps maintain blood pressure and the balance of sodium and potassium in the blood. When aldosterone production falls too low, the body loses too much sodium and retains too much potassium.
The decrease of sodium in the blood can lead to a drop in both blood volume—the amount of fluid in the blood—and blood pressure. Too little sodium in the body also can cause a condition called hyponatremia. Symptoms of hyponatremia include feeling confused and fatigued and having muscle twitches and seizures.
Too much potassium in the body can lead to a condition called hyperkalemia. Hyperkalemia may have no symptoms; however, it can cause irregular heartbeat, nausea, and a slow, weak, or an irregular pulse.
Dehydroepiandrosterone (DHEA)
Dehydroepiandrosterone (DHEA) is another hormone produced by your adrenal glands. Dehydroepiandrosterone (DHEA) is a precursor hormone, which means it has little biological effect on its own, but has powerful effects when converted into sex hormones such as testosterone and estradiol. The body uses dehydroepiandrosterone (DHEA) to make the sex hormones, androgen and estrogen. Dehydroepiandrosterone (DHEA) is produced from cholesterol mainly by the outer layer of the adrenal glands (the adrenal cortex), although it is also made by the testes and ovaries in small amounts. With adrenal insufficiency, the adrenal glands may not make enough dehydroepiandrosterone (DHEA). Healthy men derive most androgens from the testes. Healthy women and adolescent girls get most of their estrogens from the ovaries. In women, dehydroepiandrosterone (DHEA) is an important source of estrogens in the body – it provides about 75% of estrogens before the menopause, and 100% of estrogens in the body after menopause. However, women and adolescent girls may have various symptoms from DHEA insufficiency, such as loss of pubic hair, dry skin, a reduced interest in sex, and depression.
What causes Addisonian crisis?
Adrenal crisis can occur from any of the following:
- The adrenal gland is damaged due to, for example, Addison’s disease or other adrenal gland disease, and surgery
- The pituitary is injured and cannot release adrenocorticotropic hormone (ACTH)
- Adrenal insufficiency is not properly treated
- You’ve been taking glucocorticoid medicines for a long time, and suddenly stop
- You’ve become very dehydrated
- Infection or other physical stress
Adrenal crisis results from an acute lack of glucocorticoids (cortisol and corticosterone) or mineralocorticoids (aldosterone & corticosterone). Adrenal crisis most often occurs in a patient with known adrenal insufficiency, either primary or secondary.
- Primary Adrenal Insufficiency (Addison’s disease) is caused by impairment of function of the adrenal gland itself, most commonly caused by autoimmune-mediated adrenalitis, infection or due to inborn disruption of adrenal cortisol production in congenital adrenal hyperplasia resulting in decreased cortisol as well as aldosterone 28. Some causes of primary adrenal insufficiency (Addison’s disease) include autoimmune causes, adrenal hemorrhage, medications, infection and congenital adrenal hyperplasia. Conventional treatment of adrenal insufficiency involves lifelong glucocorticoid replacement therapy.
- Secondary adrenal insufficiency is caused by disruption of the hypothalamic-pituitary-adrenal (HPA) axis with insufficient stimulation of the adrenal gland due to inadequate secretion or synthesis of adrenocorticotropic hormone (ACTH) production by the pituitary gland. Causes of secondary adrenal insufficiency include cessation of steroid therapy, pituitary disease or pituitary tumors or their subsequent treatment including surgery and radiotherapy, head trauma, postpartum pituitary necrosis, pituitary apoplexy and infiltrative disorders of the pituitary gland or hypothalamus 29.
- Tertiary adrenal insufficiency caused by chronic exogenous glucocorticoid treatment which can also impair pituitary regulation of cortisol production 30, 31. This subgroup is, especially in the untreated phase, at specific risk of adrenal crisis during stressful situations, despite a residual endogenous cortisol production in many patients with the ability to (partially) recover within a variable period after cessation of steroids. However, literature on the incidence of adrenal crisis in tertiary adrenal insufficiency is scarce. Available studies are very heterogenous with respect to cause of tertiary adrenal insufficiency (i.e. steroids for rheumatologic diseases, high-dose steroids lymphoblastic leukemia or inhalation steroids for asthma) 32, 33 and criteria for adrenal insufficiency and adrenal crisis, and report inconsistent results 34, 35. A study by Smans et al. 34 reports a remarkable high incidence of adrenal crisis among patients with glucocorticoid-induced adrenal insufficiency, namely 15.1 per 100 patient-years, although the number of patients with tertiary adrenal insufficiency was very small in this study (only 28 patients). In the study of Li et al. 35 the risk of adrenal crisis in tertiary adrenal insufficiency was comparable with the risk observed in secondary adrenal insufficiency patients. Further studies are needed to investigate the exact risk of adrenal crisis in the different subgroups of patients with glucocorticoid-induced adrenal insufficiency. The focus should be on identification of individual patients with long-term exogenous steroid exposure that are most at risk for adrenal crisis in order to give adequate individualized stress instructions 31.
Primary Adrenal Insufficiency (Addison’s disease) 36, 1:
- Autoimmune adrenalitis
- Medication-induced
- Infiltrative diseases
- Sarcoidosis
- Amyloidosis
- Hemochromatosis
- Adrenal metastasis (from lung, breast, kidney) rare
- Infections
- Adrenal hemorrhage
- Waterhouse-Friderichsen syndrome
- Other:
- Trauma
- Pregnancy
- Sepsis
- Myocardial infarction
- Strenuous physical activity and dehydration 42
- Significant emotional distress 9
- Gastrointestinal and flu-like illnesses 15, 43
- Surgery
- Congenital adrenal hyperplasia
- Adrenomyeloneuropathy or adrenoleukodystrophy
- Bilateral adrenalectomy
- Other endocrinopathies (diabetic ketoacidosis [DKA], thyrotoxicosis, myxedema coma). Thyrotoxicosis accelerates cortisol metabolism
- Levothyroxine therapy initiation in a previously untreated case of adrenal insufficiency
- Nonadherence to glucocorticoid replacement therapy 44
Secondary Adrenal Insufficiency 36, 1:
- Iatrogenic after abrupt cessation of chronic glucocorticoid therapy or rapid withdrawal of corticosteroids (oral and inhaled steroids) without tapering (exogenous steroids e.g., glucocorticoid therapy, megestrol acetate, medroxyprogesterone) 44
- Antiadrenal medications, including mitotane, metyrapone, ketoconazole
- Pituitary causes
- Pituitary adenoma
- Metastatic tumor to the pituitary
- Other cranial tumors (craniopharyngioma, meningioma, germinoma)
- Central nervous system infection
- Pituitary surgery or radiation
- Primary and Secondary hypophysitis
- Pituitary infiltration (sarcoidosis, histiocytosis)
- Lymphocytic hypophysitis
- Sheehan’s syndrome
- Pituitary apoplexy
- Trauma
- Head trauma
- Empty-sella syndrome
An adrenal crisis may develop when adrenal insufficiency goes untreated or the patient experiences stress such as from infection, surgery, trauma, myocardial infarction, dehydration, exposure to cold, burns, or overexertion 45. An adrenal crisis can also occur in patients with previously normal adrenal functions in situations that result in adrenal or pituitary injury or when suddenly stopping long-term steroid treatment without tapering. One of the more common presentations of the Addisonian crisis is a patient who is on chronic steroid therapy, who abruptly stop their usual doses of corticosteroid due to the long-term suppression of the hypothalamic-pituitary axis 46.
The most common precipitating cause of Addisonian crisis is acute infection, such as from a gastrointestinal upset 43. Uncommonly, a patient will have no prior history or family history of autoimmune disease or adrenal insufficiency but will have acquired adrenal insufficiency due to chronic infection, such as from mycobacterium tuberculosis leading to chronic infectious adrenalitis and fibrosis, or have acute Meningococcal meningitis causing Waterhouse-Friderichsen Syndrome and massive bilateral adrenal hemorrhage.
The differentiation between Primary Adrenal Insufficiency (Addison’s disease) and Secondary Adrenal Insufficiency is crucial as it allows clinicians to target therapy to associated deficiencies 1. In Primary Adrenal Insufficiency (Addison’s disease), all steroid hormones synthesized by the adrenal gland are deficient (aldosterone, cortisol and sex steroids) whereas in Secondary Adrenal Insufficiency only hormones that are predominantly controlled by adrenocorticotrophic hormone (ACTH) (cortisol and sex steroids) are deficient, however, aldosterone replacement is not required as it is controlled by the renin–angiotensin–aldosterone system 1.
Adrenal crisis risk factors
The risk factors associated with adrenal crisis include the following:
- A known history of adrenal insufficiency or previous adrenal crisis
- Primary adrenal insufficiency diagnosis, which carries a higher risk than secondary adrenal insufficiency 6, 47
- Ongoing glucocorticoid therapy therapy, including topical and inhalation forms, which poses a risk for an adrenal crisis due to the potential suppression of the hypothalamic-pituitary-adrenal (HPA) axis, if abruptly discontinued
- Medications, including levothyroxine, phenytoin, phenobarbital, rifampin, carbamazepine, St John’s wort, ketoconazole, etomidate, and fluconazole, which affect cortisol metabolism or reduce its production 48, 49, 24, 50
- Anticoagulation agents, which increase the risk of adrenal hemorrhage
- Additional medications, including megestrol acetate and medroxyprogesterone 51, 52, 53
- Pregnancy, particularly during the third trimester 54
- Advanced age 15
- The presence of comorbidities 6, 47
- Patients with type 1 diabetes 15
- Adrenal metastasis or adrenal hemorrhage 55, 56
- Polyglandular autoimmune syndromes 1 and 2 57, 58
Adrenal crisis pathophysiology
The pathophysiology of adrenal crisis is not fully understood; however, examining the functions of glucocorticoids can provide insight into clinical manifestations 2. Adrenal crisis refers to an acute status of severe cortisol and aldosterone deficiency. Adrenal corticosteroids aid the body’s ability to retain sodium, excrete potassium and handle stress 59. Aldosterone deficiency is usually seen in more severe cases as the primary cause of electrolyte arrangements in cases of more severe adrenal gland dysfunction 59. Deficient levels of aldosterone cause increased renal sodium loss and potassium reabsorption, resulting in decreased intravascular volume, vascular tone, cardiac output, and renal perfusion. This, in turn, lowers arterial blood pressure, which may lead to postural hypotension, compensatory tachycardia, and eventual vascular collapse. Reduced renal perfusion causes water retention, which dilutes the extracellular fluid and causes the cells to leak potassium, leading to hyperkalemia and metabolic acidosis. Circulatory collapse impairs urinary excretion of waste products, causing elevated levels of blood urea nitrogen and creatinine.
As a response to an environmental stressor, the hypothalamus releases corticotropin-releasing hormone (CRH). CRH stimulates the anterior pituitary to release adrenocorticotropin hormone (ACTH), which travels through the bloodstream to the adrenal cortex and upregulates cortisol production. Cortisol is a glucocorticoid hormone synthesized from cholesterol by enzymes of the cytochrome P450 family in the zona fasciculate, the middle area of the adrenal cortex 59. Cortisol is the primary hormone involved in the human stress response and has multiple effects throughout the body. It helps regulate the supply of glucose in the body by triggering gluconeogenesis or stimulating glycogen synthesis in the liver. It affects pH by regulating sodium and potassium levels. It weakens the immune response by preventing T-cell proliferation and preventing T-cells from recognizing interleukin signals 60. Additionally, excess cortisol may cause atrophy of the hippocampus, leading to memory loss.
Cardiovascular system
Glucocorticoids have a permissive effect on the functioning of adrenergic receptors in the heart and vasculature 2. Without glucocorticoids, catecholamines cannot exert their full impact on these receptors 61. Consequently, during an adrenal crisis, patients often experience hypotension, and in severe cases, they may develop profound shock that remains unresponsive to fluid resuscitation and vasopressor therapy.
Immune system
Infectious or noninfectious stressors can trigger the activation of the immune system, leading to an elevated release of cytokines. Interleukin (IL)-1, IL-2, IL-6, tumor necrosis factor (TNF)-α, and TNF-γ have pivotal roles in this immune response 2. This immune activation subsequently leads to the activation of the hypothalamic-pituitary-adrenal (HPA) axis, resulting in elevated glucocorticoid levels 62, 63.
Glucocorticoids reduce immune response by inhibiting cytokine production, release, and effects, thereby playing a crucial role in immune regulation 64, 65. During an adrenal crisis, any significant stressor can trigger an uncontrolled cytokine response, such as inflammation, resulting in fever, widespread vasodilation, and heightened capillary permeability 2. This can result in hypovolemia and shock as a consequence of fluid shifting from capillaries into tissues, thereby contributing to the development of hypovolemic shock 2.
In summary, during an adrenal crisis, the dysregulation of the immune response, marked by an excessive cytokine release, can result in systemic inflammation, fever, vasodilation, capillary leakage, hypovolemia, and shock 2.
Intravascular volume
Glucocorticoids can potentially suppress the expression and secretion of antidiuretic hormone (ADH) in the hypothalamic neurons 66, 67. During an adrenal crisis, there is an upsurge in the activity of ADH (antidiuretic hormone), leading to increased diuresis and volume depletion 2.
Glucose homeostasis
Glucocorticoids increase glucose levels in response to stress through various mechanisms, including promoting glycogenolysis and stimulating gluconeogenesis 68. In addition, glucocorticoids also induce insulin resistance, which reduces glucose uptake by the peripheral cells 68. During an adrenal crisis, the deficiency of glucocorticoids hinders these normal stress responses. As a result of the glucocorticoid-deficient state, hypoglycemia can occur due to insufficient glucose production and improved peripheral utilization 2.
Appetite regulation
Corticotropin-releasing hormone (CRH) is a potent appetite suppressant in response to stress 69. Glucocorticoids are potent inhibitors of corticotropin-releasing hormone (CRH) release that can lead to increased appetite 66. During an adrenal crisis, the release of CRH (corticotropin-releasing hormone) remains uninhibited in the glucocorticoid-deficient state and leads to anorexia 2.
Electrolyte disturbances
Primary adrenal insufficiency (Addison’s disease) causes mineralocorticoid deficiency due to direct destruction of the adrenal cortex 2. However, the cortex remains intact in secondary and tertiary adrenal insufficiency. The renin-angiotensin-aldosterone (RAS) system regulates aldosterone production and secretion 70. An isolated deficiency of adrenocorticotropic hormone (ACTH) secretion observed in secondary or tertiary adrenal insufficiency does not significantly affect aldosterone levels 70, 71. In primary adrenal insufficiency (Addison’s disease), aldosterone deficiency leads to volume loss, hyponatremia, and hyperkalemia 8.
Adrenal crisis prevention
Severe cortisol deficiency is the main driver of acute adrenal insufficiency. Cortisol is usually released during times of stress to assist with homeostasis, vasoconstriction, and immune function. Aldosterone deficiency is also present, contributing to electrolyte imbalances such as hyperkalemia, hyponatremia, and hypercalcemia. If you have Addison disease, learn to recognize the signs of potential stress that may cause an acute adrenal crisis. The most common triggering event is gastrointestinal infection. Any other condition that can cause stress on the body can lead to an acute crisis. If you have been instructed by your doctor, be prepared to give yourself an emergency shot of glucocorticoid or to increase your dosage of oral glucocorticoid medicine in times of stress. Parents should learn to do this for their children who have adrenal insufficiency.
Always carry medical ID (card, bracelet, or necklace) that says you have adrenal insufficiency. The ID should also say the type of medicine and dosage you need in case of an emergency.
Never miss taking your medicines.
Prevention strategies to avoid Addisonian crisis include:
- Close routine follow-up with the physician managing their adrenal insufficiency
- Individualized care plans
- Educating the patient in the event of acute illness; for example, to increase their usual steroid dose by 2 to 3 times.
- Those who plan for elective surgeries should be aware that they may need post-operative stress-dose steroids and notify the operating surgeon or anesthesiologist.
- An emergency dose of parenteral hydrocortisone at home when the patient cannot tolerate oral steroids but still needs the cortisol replacement. The family should be taught how to give an emergency steroid injection via IV administration.
- Having some identification detailing the patient having adrenal insufficiency or a history of Addisonian crisis, such as a bracelet or ID card
Essential measures in preventing adrenal crises include educating patients and their families about sick day rules and ensuring the availability of intramuscular hydrocortisone at home 72.
Addisonian crisis signs and symptoms
Adrenal crisis signs and symptoms can include any of the following:
- Abdominal pain or flank pain
- Confusion, loss of consciousness, or coma
- Dehydration
- Dizziness or lightheadedness
- Fatigue, severe weakness
- Headache
- High fever
- Loss of appetite
- Low blood pressure
- Nausea, vomiting
- Rapid heart rate
- Rapid respiratory rate
- Slow, sluggish movement
- Unusual and excessive sweating on face or palms
The most common signs and symptoms of adrenal crisis are weakness, severe fatigue, unintentional weight loss, nausea, vomiting, abdominal pain, reduced appetite, back or limb pain, dizziness, somnolence, confusion, and loss of consciousness 15, 26
In children, adrenal crisis can manifest as weight loss with failure to thrive and are accompanied by other features, such as hypoglycemic crises, which may result in seizures 2. Less frequently observed presentations in individuals of all age groups include clinical scenarios resembling surgical emergencies, such as an acute abdomen, and symptoms, such as salt cravings, amenorrhea, loss of libido, and depression 2.
Addisonian crisis complications
If left untreated, an adrenal crisis can lead to complications such as:
- Cardiac arrhythmias from multiple electrolyte abnormalities
- Cardiac arrest
- Hypotension can lead to orthostatic hypotension and syncope, with progression to a shock state with hypoperfusion to the organs causing sequelae such as elevated transaminitis (shock liver), bradycardia, and myocardial infarction, respiratory failure, ileus, hypoxic brain injury.
- Hypoglycemia and hypoglycemic coma
Addisonian crisis diagnosis
Tests that may be ordered to help diagnose acute adrenal crisis include:
- ACTH (cosyntropin) stimulation test
- Cortisol level
- Blood sugar
- Potassium level
- Sodium level
- pH level
When evaluating a patient suspected of adrenal crisis, it is crucial to conduct a comprehensive review of the patient’s medical history and past surgical history. In addition, the healthcare practitioner should perform a comprehensive review of the patient’s list of home medications and supplements 73. It is important to determine if the patient has a history of adrenal insufficiency. If the patient is on chronic steroids, it is important to know whether the patient has been compliant or not, because abrupt cessation or acute illness in these patients can precipitate adrenal crisis 74. Identifying any precipitating factors that may trigger an adrenal crisis in individuals is essential. Useful elements of the history include any recent illnesses, surgeries, traumatic injuries or other stressors.
Evaluating other autoimmune diseases in patients while obtaining their history is crucial, as individuals with autoimmune polyglandular endocrinopathy can manifest multiple autoimmune disorders. Furthermore, recurrent hypoglycemia in patients with type 1 diabetes on insulin can indicate adrenal insufficiency. Patients with adrenal crisis usually present with an unexplained shock resistant to standard fluid resuscitation and vasopressor therapy 75.
Assessing a patient’s vital signs when evaluating for an adrenal crisis is essential. The salient feature of patients in acute adrenal crisis is circulatory collapse 72, 26. They might have symptoms like nausea, vomiting, fever, tachycardia, and lower chest or abdominal pain with orthostatic hypotension (low blood pressure that happens when standing after sitting or lying down) 72. During physical examination, patients may appear visibly unwell. They may appear cyanotic due to shock. Sometimes the patients are confused, delirious, or lethargic. Abdominal symptoms may take on features of an acute abdomen 76. Patients may have hyperpyrexia, with temperatures reaching 105 °F (40.6 °C) or higher, and may be comatose. In acute adrenal hemorrhage, the patient can deteriorate with sudden collapse, abdominal or flank pain, and nausea with or without hyperpyrexia 77. Individuals with primary adrenal insufficiency might display signs such as skin and buccal mucosa hyperpigmentation and scarring 1.
Initial laboratory testing should include serum chemistry (particularly sodium, potassium, glucose, and calcium), blood count, cortisol level, adrenocorticotropic hormone (ACTH), aldosterone, renin, and thyroid function 76. The serum chemistry is used to guide initial therapy. Patients often have hypoglycemia, hyponatremia, hyperkalemia, and hypercalcemia. Although a reliable diagnosis of underlying adrenal insufficiency is not possible during the adrenal crisis, measurement of blood ACTH and cortisol during the crisis (before treatment with corticosteroids is given) is often enough to make a preliminary diagnosis. Healthcare providers should not delay treatment pending these results. Primary adrenal insufficiency (Addison’s disease) is characterized by low cortisol and high ACTH. Secondary adrenal insufficiency is characterized by low cortisol and low to normal ACTH. Once the crisis is controlled, an ACTH stimulation test can be performed to help make a specific diagnosis 76. More complex lab tests are sometimes used if the diagnosis remains unclear.
An EKG may show peaked T waves from hyperkalemia or short QT interval from hypercalcemia 78. A chest radiograph, urine studies and blood cultures should be ordered to evaluate any infection. A CT scan of the abdomen may show hemorrhage in the adrenals, calcification of the adrenals (seen with tuberculosis) or metastasis. In cases of secondary adrenal insufficiency, a head CT scan may show the destruction of the pituitary (i.e., empty sella syndrome) or a pituitary mass lesion.
Blood tests
Treatment of adrenal crisis should never be delayed to obtain blood work for etiological assessment. Prompt administration of hydrocortisone is of utmost importance in managing adrenal crisis. However, if there is no anticipated delay in treatment, blood work can be done quickly before initiating hydrocortisone administration.
Classic laboratory features of adrenal crisis that may be revealed include:
- Hyponatremia, resulting from mineralocorticoid deficiency
- Hyperkalemia, resulting from mineralocorticoid deficiency
- Hypoglycemia, stemming from decreased gluconeogenesis and glycogenolysis
- Low or low normal ACTH levels, as observed in secondary adrenal insufficiency
- High or high normal ACTH levels, as observed in primary adrenal insufficiency
- Hypercalcemia, resulting from hypovolemia
- Elevated creatinine levels, attributed to prerenal failure
- Low aldosterone levels, due to mineralocorticoid deficiency in primary adrenal insufficiency
- High renin levels, as typically seen in primary adrenal insufficiency due to increased urinary sodium loss and reduced blood volume
- Normocytic normochromic anemia, lymphocytosis, and eosinophilia, resulting from glucocorticoid deficiency
- Increased thyroid-stimulating hormone (TSH) levels, owing to coexisting hypothyroidism in autoimmune polyglandular endocrinopathy or the absence of cortisol’s inhibitory effect on TSH production.
Cortisol Levels
Experts suggest the below-mentioned blood tests for the treatment of adrenal crisis 2:
- ACTH: High ACTH levels with low cortisol and aldosterone indicate primary adrenal insufficiency, whereas low ACTH levels with low cortisol suggest secondary or tertiary adrenal insufficiency.
- Basic metabolic panel: A basic metabolic profile blood test, including glucose, should also be included in the recommended blood work.
- Other blood tests: Additional blood work includes determining cortisol, aldosterone, and renin levels.
In situations with uncertainty regarding the diagnosis of adrenal crisis and borderline cortisol levels, performing an ACTH stimulation test in the acute setting is not recommended until the patient’s condition has stabilized 79.
Addisonian crisis treatment
The diagnosis and management of adrenal crisis (Addisonian crisis) requires a multidisciplinary team for quick identification and treatment; any delay in diagnosis can lead to a very high mortality. In cases of adrenal crisis, involving an endocrinologist as soon as possible is crucial to ensure appropriate management of the condition and guidance in patient care. Fluids and glucocorticoid replacement are the mainstays of adrenal crisis emergency therapy. Two to three liters of normal saline or 5% dextrose in normal saline should be infused in the first 12 to 24 hours 24. The dextrose-containing solution should be used in the setting of hypoglycemia. Volume status and urine output should be used to guide the resuscitation. Dexamethasone (4 mg IV bolus) or hydrocortisone (100 mg IV bolus) can be used in patients with known adrenal insufficiency presenting in adrenal crisis 80, 81. In patients without known adrenal insufficiency, dexamethasone is preferred because it does not interfere with the diagnostic testing, unlike hydrocortisone. Maintenance steroid replacement is required – dexamethasone 4 mg IV every 12 hours or hydrocortisone 50 mg IV every 6 hours until vital signs have stabilized and the patient can take medication orally 80. If the patient does not have known adrenal insufficiency, an ACTH stimulation test should be performed. It can then be followed by testing to determine the cause of the adrenal insufficiency. After initial stabilization of the patient using the above measures, the underlying cause of the crisis should be identified and treated 76.
Emergency management of adrenal crisis (Addisonian crisis) the first 24 hours 17:
- For Adults, administer parenteral hydrocortisone 100mg stat (IM preferable) and repeat 6 hourly until the patient is hemodynamically stable and clinical improvement (alternative 200mg/24hrs by continuous IV infusion).
- Infants and children should receive an initial parenteral injection of 50mg hydrodortisone/m² (usually 25mg in infants and 50mg in children) followed by 50mg/24h in infants and 100mg/24h in children.
- Administer 1 liter IV 0.9% saline stat (adjust for infants and children), and continue saline resuscitation at an appropriate rate until hemodynamic stability and correction of any electrolyte disturbance and acute kidney injury
- Monitor urea and electrolytes at least 12 hourly during initial resuscitation and continue regular monitoring until any hyponatremia, hyperkalemia or renal impairment are corrected
- Identify and treat any precipitating cause for Addisonian crisis:
- vomiting/diarrheal illness
- infection
- heart attack (myocardial infarction)
Recent evidence indicates that continuous infusion is a superior delivery method for hydrocortisone in the management of adrenal crisis when compared to intermittent boluses 21. Continuous hydrocortisone infusion has shown better maintenance of cortisol levels within the therapeutic range.
Once there is clinical improvement, initiating a gradual tapering of steroids is advisable 22. This approach helps prevent abrupt discontinuation and enables a smoother transition to lower doses.
The necessity for mineralocorticoid replacement should be assessed individually and in consultation with an endocrinologist. If the glucocorticoid doses administered to patients exceed 50 mg, mineralocorticoid replacement is unnecessary 23.
In situations where hydrocortisone is unavailable, alternative parenteral glucocorticoids can be considered 24, 15:
- Prednisolone: This can be a preferred alternative treatment, administered as an initial bolus of 25 mg and followed by 2 additional 25 mg doses within the first 24 hours. Subsequently, this regimen should be continued with a daily dose of 50 mg of prednisone.
- Methylprednisolone: This can be administered at a dosage of 40 mg every 24 hours.
- Dexamethasone: This is the least preferred alternative treatment, with a recommended dosage of 4 mg every 24 hours.
In patients with an infectious process as the precipitating event for adrenal crisis, prompt administration of appropriate antibiotics is necessary to address the underlying infection.
If patients do not adequately respond within the first 24 hours after glucocorticoid administration, an alternative diagnosis, such as sepsis or a cardiogenic event, or coexisting cause of hypotension should be considered 15.
Once the Addisonian crisis (adrenal crisis) is controlled, an ACTH stimulation test can be performed to diagnose the cause of the adrenal insufficiency in those patients with no prior history of adrenal disorders 82. After the initial stabilization of the patient using the above measures, the underlying cause of the crisis should be identified and treated 82. This assessment may encompass the evaluation for conditions such as sepsis, septic shock, circulatory shock, myxedema coma, infection, trauma, physical or emotional stress, myocardial infarction, and other potential triggers. In patients without a known adrenal pathology and exhibiting hypotension resistant to fluid administration and vasopressor support, the diagnosis of adrenal crisis should be strongly considered 72.
Table 1. Guidelines for Managing Adrenal Crisis During Emergency
| Age Groups | Stress Dosing | Fluid Resuscitation |
|---|---|---|
| Adults | The recommended treatment regimen includes administering an initial dose of 100 mg of hydrocortisone through intravenous or intramuscular (IV/IM) bolus injection. This is followed by administering an additional 200 mg of hydrocortisone as IM or IV over the next 24 hours, with a dosage of 50 mg every 6 hours or as a continuous infusion 15 | The suggested treatment approach for hypoglycemia is administering either 1 L of normal saline or 5% dextrose in 1 L of normal saline, followed by maintenance fluids 15 |
| Children | Hydrocortisone dosage is initially calculated as 50 to 100 mg/m² of body surface area. This is followed by an additional 50 to 100 mg/m² over the next 24 hours, administered as IM/IV, divided into equal doses every 6 hours, or as a continuous infusion 24, 83 | The suggested treatment regimen is administering a normal saline bolus at 20 mL/kg of body weight, with repeated doses up to 60 mL/kg within the first hour. If hypoglycemia is present, additional dextrose should be considered at a dose of 0.5 to 1 g/kg 24 |
Addisonian crisis prognosis
Shock may occur if treatment is not provided early, and adrenal crisis can be life threatening. Each year roughly 8% of those with known adrenal insufficiency have an Addisonian crisis (adrenal crisis) and the rate of death is around 6% 36. A retrospective study in the United Kingdom revealed that adrenal crisis contributed to 10% of the deaths in patients with primary and secondary adrenal insufficiency 84. If the Addisonian crisis is quickly identified and given prompt treatment with IV fluid and steroids, patients have a good prognosis and recovery. Those who present critically ill with significantly altered mental status or had advanced endocrinopathies (severe diabetes, uncontrolled thyroid disease) or multiple comorbidities had increased mortality and residual disability. The patient may need physical or occupational therapy and rehabilitation to regain independent function.
- Dineen R, Thompson CJ, Sherlock M. Adrenal crisis: prevention and management in adult patients. Ther Adv Endocrinol Metab. 2019 Jun 13;10:2042018819848218. doi: 10.1177/2042018819848218[↩][↩][↩][↩][↩][↩][↩]
- Elshimy G, Chippa V, Kaur J, et al. Adrenal Crisis. [Updated 2023 Sep 13]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499968[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Alexandraki KI, Sanpawithayakul K, Grossman A. Adrenal Insufficiency. [Updated 2022 Nov 7]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279083[↩]
- Kerrigan JR, Veldhuis JD, Leyo SA, Iranmanesh A, Rogol AD. Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab. 1993 Jun;76(6):1505-10. doi: 10.1210/jcem.76.6.8501158[↩]
- Esteban NV, Loughlin T, Yergey AL, Zawadzki JK, Booth JD, Winterer JC, Loriaux DL. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab. 1991 Jan;72(1):39-45. doi: 10.1210/jcem-72-1-39[↩]
- Smans LC, Van der Valk ES, Hermus AR, Zelissen PM. Incidence of adrenal crisis in patients with adrenal insufficiency. Clin Endocrinol (Oxf). 2016 Jan;84(1):17-22. doi: 10.1111/cen.12865[↩][↩][↩]
- Bleicken B, Hahner S, Ventz M, Quinkler M. Delayed diagnosis of adrenal insufficiency is common: a cross-sectional study in 216 patients. Am J Med Sci. 2010 Jun;339(6):525-31. doi: 10.1097/MAJ.0b013e3181db6b7a[↩]
- Erichsen MM, Løvås K, Skinningsrud B, Wolff AB, Undlien DE, Svartberg J, Fougner KJ, Berg TJ, Bollerslev J, Mella B, Carlson JA, Erlich H, Husebye ES. Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: observations from a Norwegian registry. J Clin Endocrinol Metab. 2009 Dec;94(12):4882-90. doi: 10.1210/jc.2009-1368[↩][↩]
- Hahner S, Spinnler C, Fassnacht M, Burger-Stritt S, Lang K, Milovanovic D, Beuschlein F, Willenberg HS, Quinkler M, Allolio B. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J Clin Endocrinol Metab. 2015 Feb;100(2):407-16. doi: 10.1210/jc.2014-3191[↩][↩][↩]
- Rushworth RL, Torpy DJ, Stratakis CA, Falhammar H. Adrenal Crises in Children: Perspectives and Research Directions. Horm Res Paediatr. 2018;89(5):341-351. doi: 10.1159/000481660[↩]
- Hahner S. Acute adrenal crisis and mortality in adrenal insufficiency: Still a concern in 2018! Ann Endocrinol (Paris). 2018 Jun;79(3):164-166. doi: 10.1016/j.ando.2018.04.015[↩][↩]
- Loriaux DL, Fleseriu M. Relative adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2009 Oct;16(5):392-400. doi: 10.1097/MED.0b013e3283307d53[↩]
- Katherine White, Wiebke Arlt, Adrenal crisis in treated Addison’s disease: a predictable but under-managed event, European Journal of Endocrinology, Volume 162, Issue 1, Jan 2010, Pages 115–120, https://doi.org/10.1530/EJE-09-0559[↩]
- Higham CE, Olsson-Brown A, Carroll P, Cooksley T, Larkin J, Lorigan P, Morganstein D, Trainer PJ; Society for Endocrinology Clinical Committee. SOCIETY FOR ENDOCRINOLOGY ENDOCRINE EMERGENCY GUIDANCE: Acute management of the endocrine complications of checkpoint inhibitor therapy. Endocr Connect. 2018 Jul;7(7):G1-G7. doi: 10.1530/EC-18-0068[↩]
- Rushworth RL, Torpy DJ, Falhammar H. Adrenal Crisis. N Engl J Med. 2019 Aug 29;381(9):852-861. doi: 10.1056/NEJMra1807486[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Arlt W. The approach to the adult with newly diagnosed adrenal insufficiency. J Clin Endocrinol Metab. 2009 Apr;94(4):1059-67. doi: 10.1210/jc.2009-0032[↩]
- A&E management of adrenal crisis: the first 24 hours. Addison’s Clinical Advisory Panel. https://www.addisonsdisease.org.uk/emergency[↩][↩]
- Allolio B. Extensive Expertise in Endocrinology. Adrenal Crisis Eur J Endocrinol (2015) 172(3):R115–24. 10.1530/EJE-14-0824[↩]
- Husebye ES, Allolio B, Arlt W, Badenhoop K, Bensing S, Betterle C, et al.. Consensus Statement on the Diagnosis, Treatment and Follow-Up of Patients With Primary Adrenal Insufficiency. J Intern Med (2014) 275(2):104–15. 10.1111/joim.12162[↩]
- Nowotny H, Ahmed SF, Bensing S, Beun JG, Brösamle M, Chifu I, et al.. Therapy Options for Adrenal Insufficiency and Recommendations for the Management of Adrenal Crisis. Endocrine (2021) 71(3):1–9. 10.1007/s12020-021-02649-6[↩]
- Prete A, Taylor AE, Bancos I, Smith DJ, Foster MA, Kohler S, Fazal-Sanderson V, Komninos J, O’Neil DM, Vassiliadi DA, Mowatt CJ, Mihai R, Fallowfield JL, Annane D, Lord JM, Keevil BG, Wass JAH, Karavitaki N, Arlt W. Prevention of Adrenal Crisis: Cortisol Responses to Major Stress Compared to Stress Dose Hydrocortisone Delivery. J Clin Endocrinol Metab. 2020 Jul 1;105(7):2262–74. doi: 10.1210/clinem/dgaa133[↩][↩]
- Guignat L. Therapeutic patient education in adrenal insufficiency. Ann Endocrinol (Paris). 2018 Jun;79(3):167-173. doi: 10.1016/j.ando.2018.03.002[↩][↩]
- Shenker Y, Skatrud JB. Adrenal insufficiency in critically ill patients. Am J Respir Crit Care Med. 2001 Jun;163(7):1520-3. doi: 10.1164/ajrccm.163.7.2012022[↩][↩]
- Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89. doi: 10.1210/jc.2015-1710[↩][↩][↩][↩][↩][↩]
- Betterle C, Presotto F, Furmaniak J. Epidemiology, pathogenesis, and diagnosis of Addison’s disease in adults. J Endocrinol Invest. 2019 Dec;42(12):1407-1433. doi: 10.1007/s40618-019-01079-6[↩]
- Claessen KMJA, Andela CD, Biermasz NR, Pereira AM. Clinical Unmet Needs in the Treatment of Adrenal Crisis: Importance of the Patient’s Perspective. Front Endocrinol (Lausanne). 2021 Jul 20;12:701365. doi: 10.3389/fendo.2021.701365[↩][↩][↩][↩]
- Ross AP, Ben-Zacharia A, Harris C, Smrtka J. Multiple Sclerosis, Relapses, and the Mechanism of Action of Adrenocorticotropic Hormone. Frontiers in Neurology. 2013;4:21. doi:10.3389/fneur.2013.00021. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3591751/[↩]
- Bancos I, Hahner S, Tomlinson J, Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015 Mar;3(3):216-26. doi: 10.1016/S2213-8587(14)70142-1[↩]
- Grossman AB. Clinical Review#: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982[↩]
- Mebrahtu TF, Morgan AW, Keeley A, Baxter PD, Stewart PM, Pujades-Rodriguez M. Dose Dependency of Iatrogenic Glucocorticoid Excess and Adrenal Insufficiency and Mortality: A Cohort Study in England. J Clin Endocrinol Metab (2019) 104(9):3757–67. 10.1210/jc.2019-00153[↩]
- Erskine D, Simpson H. Exogenous Steroids, Adrenal Insufficiency and Adrenal Crisis-Who Is at Risk and How Should They Be Managed Safely. Clin Med (2020) 20(371). 10.7861/clinmed.2019-0324[↩][↩]
- Lapi F, Kezouh A, Suissa S, Ernst P. The Use of Inhaled Corticosteroids and the Risk of Adrenal Insufficiency. Eur Respir J (2013) 42(1):79–86. 10.1183/09031936.00080912[↩]
- Rensen N, Gemke RJ, van Dalen EC, Rotteveel J, Kaspers GJ. Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression After Treatment With Glucocorticoid Therapy for Childhood Acute Lymphoblastic Leukaemia. Cochrane Database Syst Rev (2017) 11(11):Cd008727. 10.1002/14651858.CD008727.pub4[↩]
- Smans LC, van der Valk ES, Hermus AR, Zelissen PM. Incidence of Adrenal Crisis in Patients With Adrenal Insufficiency. Clin Endocrinol (Oxf) (2016) 84(1):17–22. 10.1111/cen.12865[↩][↩]
- Li D, Genere N, Behnken E, Xhikola M, Abbondanza T, Vaidya A, et al.. Determinants of Self-Reported Health Outcomes in Adrenal Insufficiency: A Multi-Site Survey Study. J Clin Endocrinol Metab (2021) 106(3):e1408–19. 10.1210/clinem/dgaa668[↩][↩]
- Rathbun KM, Nguyen M, Singhal M. Addisonian Crisis. [Updated 2021 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441933[↩][↩][↩]
- Manaka K, Sato J, Takeuchi M, Watanabe K, Kage H, Kawai T, Sato Y, Miyagawa T, Yamada D, Kume H, Sato S, Nagase T, Iiri T, Nangaku M, Makita N. Immune checkpoint inhibitor combination therapies very frequently induce secondary adrenal insufficiency. Sci Rep. 2021 Jun 2;11(1):11617. doi: 10.1038/s41598-021-91032-6[↩]
- Joshi MN, Whitelaw BC, Palomar MT, Wu Y, Carroll PV. Immune checkpoint inhibitor-related hypophysitis and endocrine dysfunction: clinical review. Clin Endocrinol (Oxf). 2016 Sep;85(3):331-9. doi: 10.1111/cen.13063[↩]
- Elshimy G, Gandhi A, Guo R, Correa R. Tyrosine Kinase Inhibitors’ Newly Reported Endocrine Side Effect: Pazopanib-Induced Primary Adrenal Insufficiency in a Patient With Metastatic Renal Cell Cancer. J Investig Med High Impact Case Rep. 2020 Jan-Dec;8:2324709620936808. doi: 10.1177/2324709620936808[↩]
- Lodish MB. Clinical review: kinase inhibitors: adverse effects related to the endocrine system. J Clin Endocrinol Metab. 2013 Apr;98(4):1333-42. doi: 10.1210/jc.2012-4085[↩]
- Heidarpour M, Vakhshoori M, Abbasi S, Shafie D, Rezaei N. Adrenal insufficiency in coronavirus disease 2019: a case report. J Med Case Rep. 2020 Aug 24;14(1):134. doi: 10.1186/s13256-020-02461-2[↩]
- Lousada LM, Mendonca BB, Bachega TASS. Adrenal crisis and mortality rate in adrenal insufficiency and congenital adrenal hyperplasia. Arch Endocrinol Metab. 2021 Nov 3;65(4):488-494. doi: 10.20945/2359-3997000000392[↩]
- Hahner S, Loeffler M, Bleicken B, Drechsler C, Milovanovic D, Fassnacht M, Ventz M, Quinkler M, Allolio B. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur J Endocrinol. 2010 Mar;162(3):597-602. doi: 10.1530/EJE-09-0884[↩][↩]
- Allolio B. Extensive expertise in endocrinology. Adrenal crisis. Eur J Endocrinol. 2015 Mar;172(3):R115-24. doi: 10.1530/EJE-14-0824[↩][↩]
- Koo DJ, Jackman D, Chaudry IH, Wang P. Adrenal insufficiency during the late stage of polymicrobial sepsis. Crit. Care Med. 2001 Mar;29(3):618-22.[↩]
- Santos, A. R., Bello, C. T., Sousa, A., Duarte, J. S., & Campos, L. (2019). Pituitary Apoplexy Following Systemic Anticoagulation. European journal of case reports in internal medicine, 6(12), 001254. https://doi.org/10.12890/2019_001254[↩]
- Iwasaku M, Shinzawa M, Tanaka S, Kimachi K, Kawakami K. Clinical characteristics of adrenal crisis in adult population with and without predisposing chronic adrenal insufficiency: a retrospective cohort study. BMC Endocr Disord. 2017 Sep 11;17(1):58. doi: 10.1186/s12902-017-0208-0[↩][↩]
- Arlt W, Allolio B. Adrenal insufficiency. Lancet. 2003 May 31;361(9372):1881-93. doi: 10.1016/S0140-6736(03)13492-7[↩]
- Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med. 2009 May 28;360(22):2328-39. doi: 10.1056/NEJMra0804635[↩]
- Shibata S, Kami M, Kanda Y, Machida U, Iwata H, Kishi Y, Takeshita A, Miyakoshi S, Ueyama J, Morinaga S, Mutou Y. Acute adrenal failure associated with fluconazole after administration of high-dose cyclophosphamide. Am J Hematol. 2001 Apr;66(4):303-5. doi: 10.1002/ajh.1063[↩]
- Orme LM, Bond JD, Humphrey MS, Zacharin MR, Downie PA, Jamsen KM, Mitchell SL, Robinson JM, Grapsas NA, Ashley DM. Megestrol acetate in pediatric oncology patients may lead to severe, symptomatic adrenal suppression. Cancer. 2003 Jul 15;98(2):397-405. doi: 10.1002/cncr.11502[↩]
- Naing KK, Dewar JA, Leese GP. Megestrol acetate therapy and secondary adrenal suppression. Cancer. 1999 Sep 15;86(6):1044-9.[↩]
- Hellman L, Yoshida K, Zumoff B, Levin J, Kream J, Fukushima DK. The effect of medroxyprogesterone acetate on the pituitary-adrenal axis. J Clin Endocrinol Metab. 1976 May;42(5):912-7. doi: 10.1210/jcem-42-5-912[↩]
- Yuen KC, Chong LE, Koch CA. Adrenal insufficiency in pregnancy: challenging issues in diagnosis and management. Endocrine. 2013 Oct;44(2):283-92. doi: 10.1007/s12020-013-9893-2[↩]
- Tallis PH, Rushworth RL, Torpy DJ, Falhammar H. Adrenal insufficiency due to bilateral adrenal metastases – A systematic review and meta-analysis. Heliyon. 2019 May 29;5(5):e01783. doi: 10.1016/j.heliyon.2019.e01783[↩]
- Ramon I, Mathian A, Bachelot A, Hervier B, Haroche J, Boutin-Le Thi Huong D, Costedoat-Chalumeau N, Wechsler B, Karmali R, Velkeniers B, Touraine P, Coussieu C, Bennani A, Renard-Penna R, Grenier PA, Wahl D, Piette JC, Amoura Z. Primary adrenal insufficiency due to bilateral adrenal hemorrhage-adrenal infarction in the antiphospholipid syndrome: long-term outcome of 16 patients. J Clin Endocrinol Metab. 2013 Aug;98(8):3179-89. doi: 10.1210/jc.2012-4300[↩]
- Bain A, Stewart M, Mwamure P, Nirmalaraj K. Addison’s disease in a patient with hypothyroidism: autoimmune polyglandular syndrome type 2. BMJ Case Rep. 2015 Aug 3;2015:bcr2015210506. doi: 10.1136/bcr-2015-210506[↩]
- Puttanna A, Cunningham AR, Dainty P. Addison’s disease and its associations. BMJ Case Rep. 2013 Jul 26;2013:bcr2013010473. doi: 10.1136/bcr-2013-010473[↩]
- Gupta P, Bhatia V. Corticosteroid physiology and principles of therapy. Indian J Pediatr. 2008 Oct;75(10):1039-44.[↩][↩][↩]
- Barber AE, Coyle SM, Marano MA, Fischer E, Calvano SE, Fong Y, Moldawer LL, Lowry SF. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J. Immunol. 1993 Mar 01;150(5):1999-2006.[↩]
- Malbon CC, Rapiejko PJ, Watkins DC. Permissive hormone regulation of hormone-sensitive effector systems. Trends Pharmacol Sci. 1988 Jan;9(1):33-6. doi: 10.1016/0165-6147(88)90240-4[↩]
- Turnbull AV, Rivier C. Regulation of the HPA axis by cytokines. Brain Behav Immun. 1995 Dec;9(4):253-75. doi: 10.1006/brbi.1995.1026[↩]
- Bellavance MA, Rivest S. The HPA – Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front Immunol. 2014 Mar 31;5:136. doi: 10.3389/fimmu.2014.00136[↩]
- Pariante CM, Pearce BD, Pisell TL, Sanchez CI, Po C, Su C, Miller AH. The proinflammatory cytokine, interleukin-1alpha, reduces glucocorticoid receptor translocation and function. Endocrinology. 1999 Sep;140(9):4359-66. doi: 10.1210/endo.140.9.6986[↩]
- Quatrini L, Ugolini S. New insights into the cell- and tissue-specificity of glucocorticoid actions. Cell Mol Immunol. 2021 Feb;18(2):269-278. doi: 10.1038/s41423-020-00526-2[↩]
- Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab. 1998 Jun;83(6):2066-73. doi: 10.1210/jcem.83.6.4881[↩][↩]
- Kim JK, Summer SN, Wood WM, Schrier RW. Role of glucocorticoid hormones in arginine vasopressin gene regulation. Biochem Biophys Res Commun. 2001 Dec 21;289(5):1252-6. doi: 10.1006/bbrc.2001.6114[↩]
- Kuo T, McQueen A, Chen TC, Wang JC. Regulation of Glucose Homeostasis by Glucocorticoids. Adv Exp Med Biol. 2015;872:99-126. doi: 10.1007/978-1-4939-2895-8_5[↩][↩]
- Sominsky L, Spencer SJ. Eating behavior and stress: a pathway to obesity. Front Psychol. 2014 May 13;5:434. doi: 10.3389/fpsyg.2014.00434[↩]
- Bollag WB. Regulation of aldosterone synthesis and secretion. Compr Physiol. 2014 Jul;4(3):1017-55. doi: 10.1002/cphy.c130037[↩][↩]
- Esposito D, Pasquali D, Johannsson G. Primary Adrenal Insufficiency: Managing Mineralocorticoid Replacement Therapy. J Clin Endocrinol Metab. 2018 Feb 1;103(2):376-387. doi: 10.1210/jc.2017-01928[↩]
- Puar TH, Stikkelbroeck NM, Smans LC, Zelissen PM, Hermus AR. Adrenal Crisis: Still a Deadly Event in the 21st Century. Am J Med. 2016 Mar;129(3):339.e1-9. doi: 10.1016/j.amjmed.2015.08.021[↩][↩][↩][↩]
- Robati S, Shahid MK, Vella A, Rang S. Importance of a thorough drug history in presurgical patients. BMJ Case Rep. 2014 Mar 14;2014:bcr2013202667. doi: 10.1136/bcr-2013-202667. Erratum in: BMJ Case Rep. 2014;2014. doi: 10.1136/bcr-2013-202667corr1[↩]
- Dinsen S, Baslund B, Klose M, Rasmussen AK, Friis-Hansen L, Hilsted L, Feldt-Rasmussen U. Why glucocorticoid withdrawal may sometimes be as dangerous as the treatment itself. Eur J Intern Med. 2013 Dec;24(8):714-20. doi: 10.1016/j.ejim.2013.05.014. Epub 2013 Jun 25. Erratum in: Eur J Intern Med. 2014 Oct;25(8):781-3.[↩]
- Hopkins RL, Leinung MC. Exogenous Cushing’s syndrome and glucocorticoid withdrawal. Endocrinol Metab Clin North Am. 2005 Jun;34(2):371-84, ix. doi: 10.1016/j.ecl.2005.01.013[↩]
- Bouillon R. Acute adrenal insufficiency. Endocrinol. Metab. Clin. North Am. 2006 Dec;35(4):767-75, ix[↩][↩][↩][↩]
- Vella A, Nippoldt TB, Morris JC 3rd. Adrenal hemorrhage: a 25-year experience at the Mayo Clinic. Mayo Clin Proc. 2001 Feb;76(2):161-8. doi: 10.1016/S0025-6196(11)63123-6[↩]
- Sherlock M, Gittoes NJ, Arlt W. Adrenal crisis causing critical illness related reversible myocardial dysfunction. Clin. Endocrinol. (Oxf). 2008 Apr;68(4):667-9[↩]
- Kazlauskaite R, Evans AT, Villabona CV, Abdu TA, Ambrosi B, Atkinson AB, Choi CH, Clayton RN, Courtney CH, Gonc EN, Maghnie M, Rose SR, Soule SG, Tordjman K; Consortium for Evaluation of Corticotropin Test in Hypothalamic-Pituitary Adrenal Insufficiency. Corticotropin tests for hypothalamic-pituitary- adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008 Nov;93(11):4245-53. doi: 10.1210/jc.2008-0710[↩]
- Gupta P, Bhatia V. Corticosteroid physiology and principles of therapy. Indian J Pediatr. 2008 Oct;75(10):1039-44[↩][↩]
- Oelkers W. Adrenal insufficiency. N Engl J Med. 1996 Oct 17;335(16):1206-12. doi: 10.1056/NEJM199610173351607[↩]
- Bouillon R. Acute adrenal insufficiency. Endocrinol Metab Clin North Am. 2006 Dec;35(4):767-75, ix. doi: 10.1016/j.ecl.2006.09.004[↩][↩]
- Malikova J, Flück CE. Novel insight into etiology, diagnosis and management of primary adrenal insufficiency. Horm Res Paediatr. 2014;82(3):145-57. doi: 10.1159/000363107[↩]
- Ngaosuwan K, Johnston DG, Godsland IF, Cox J, Majeed A, Quint JK, Oliver N, Robinson S. Increased Mortality Risk in Patients With Primary and Secondary Adrenal Insufficiency. J Clin Endocrinol Metab. 2021 Jun 16;106(7):e2759-e2768. doi: 10.1210/clinem/dgab096[↩]