Contents
What is alkalosis
Alkalosis is an abnormal condition in which a there is a shift in the acid-base balance of the body to have more base (alkali) than normal, often causing the pH of the blood and body tissues to rise above 7.45 (the healthy pH range 7.35-7.45, average 7.40). Alkalosis can be due to decreased acid or increased base (alkali). Alkalosis may have respiratory causes such as hyperventilation (respiratory alkalosis) and pneumonia or metabolic causes such as prolonged vomiting and severe dehydration (metabolic alkalosis). Decreased carbon dioxide (an acid) level or increased bicarbonate (a base) level makes the body too alkaline. There are different types of alkalosis. These are described below.
The kidneys and lungs maintain the proper balance (proper pH level) of chemicals called acids and bases in the body. People with healthy kidneys and lungs do not usually have serious alkalosis.
Respiratory alkalosis is caused by a low carbon dioxide level in the blood. This can be due to:
- Fever
- Being at a high altitude
- Lack of oxygen
- Liver disease
- Lung disease, which causes you to breathe faster (hyperventilate)
- Aspirin poisoning
Metabolic alkalosis is caused by too much bicarbonate in the blood. It can also occur due to certain kidney diseases.
- Hypochloremic alkalosis is caused by an extreme lack or loss of chloride, such as from prolonged vomiting.
- Hypokalemic alkalosis is caused by the kidneys’ response to an extreme lack or loss of potassium (hypokalemia). This can occur from taking certain water pills (diuretics) or from mineralocorticoid excess (e.g., hyperaldosteronism or Conn syndrome or renovascular hypertension).
Compensated alkalosis occurs when the body returns the acid-base balance to normal in cases of alkalosis, but bicarbonate [HCO3–] and carbon dioxide (CO2) levels remain abnormal.
Untreated alkalosis or alkalosis not treated properly, complications may include any of the following:
- Arrhythmias (heart beating too fast, too slow, or irregularly)
- Coma
- Electrolyte imbalance (such as low potassium level)
Acidosis vs alkalosis
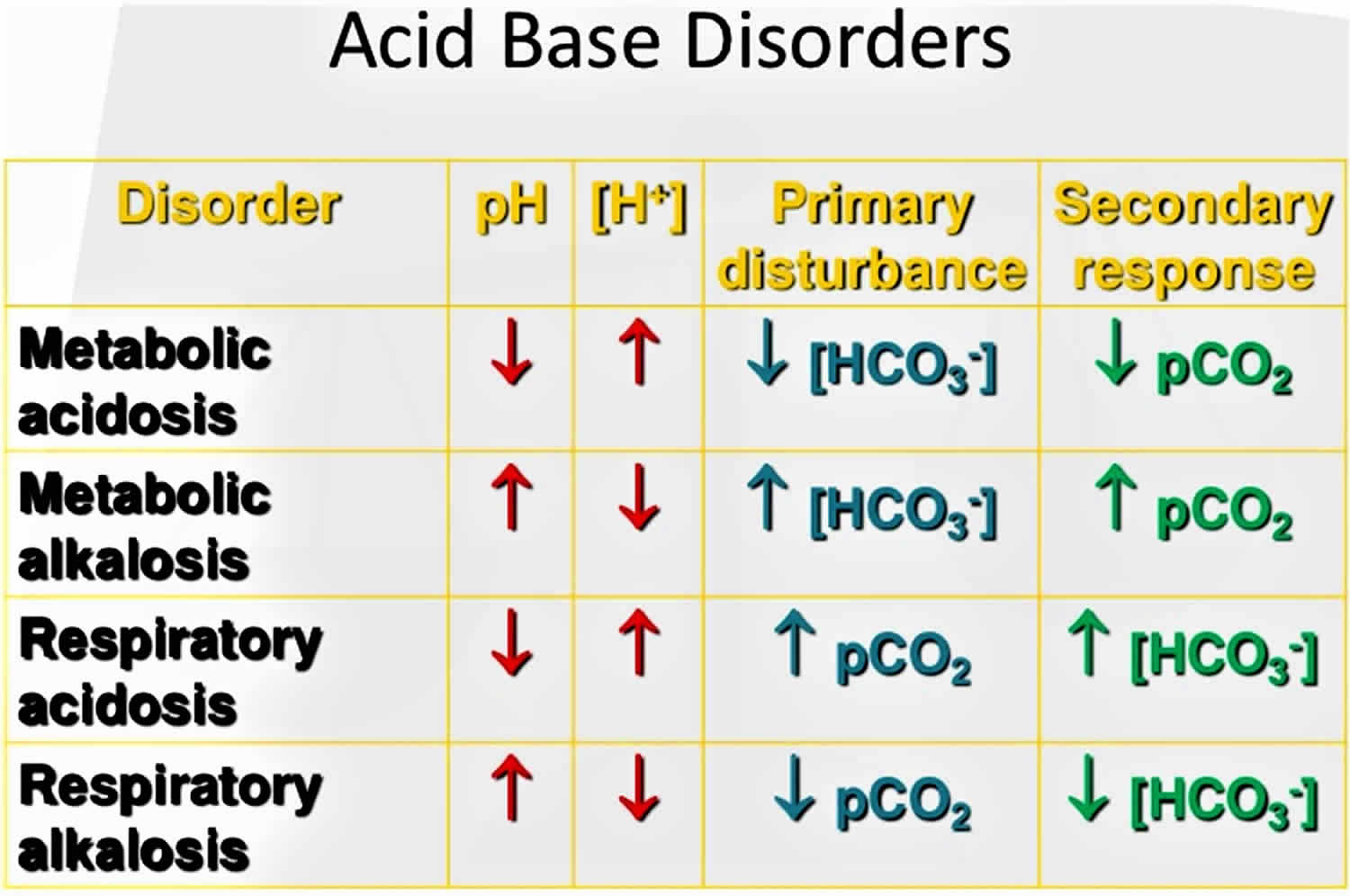
Normal blood pH must be maintained within a narrow range, typically 7.35-7.45 (average 7.40), to ensure the proper functioning of metabolic processes and the delivery of the right amount of oxygen to tissues. Acidosis refers to an excess of acid in the blood that causes the pH to fall below 7.35, and alkalosis refers to an excess of base in the blood that causes the pH to rise above 7.45. Many conditions and diseases can interfere with pH control in the body and cause a person’s blood pH to fall outside of healthy limits.
Alkalosis symptoms
Symptoms of alkalosis can include any of the following:
- Confusion (can progress to stupor or coma)
- Hand tremor
- Lightheadedness
- Muscle twitching
- Nausea, vomiting
- Numbness or tingling in the face, hands, or feet
- Prolonged muscle spasms (tetany)
Alkalosis diagnosis
Your health care provider will perform a physical exam and ask about your symptoms.
Laboratory tests that may be ordered include:
- Arterial blood gas analysis.
- Electrolytes test, such as basic metabolic panel to confirm alkalosis and show whether it is respiratory or metabolic alkalosis.
Other tests may be needed to determine the cause of the alkalosis. These may include:
- Urinalysis
- Urine pH
Arterial blood gas (ABG) sampling, is a test often performed in an inpatient setting to assess the acid-base status of a patient. A needle is used to draw blood from an artery, often the radial, and the blood is analyzed to determine parameters such as the pH, the arterial partial pressure of oxygen (PaO2), the arterial partial pressure of carbon dioxide (PaCO2), bicarbonate [HCO3–], oxygen (O2) saturation, total CO2, and base excess. This allows the physician to understand the status of the patient better. Arterial blood gases are especially important in the critically ill. Urine chloride is a direct measurement of chloride being excreted into urine. Arterial blood gas (ABG) test is useful to help determine the cause of metabolic alkalosis. They are the main tool utilized in adjusting to the needs of a patient on a ventilator. The following are the most important normal values on an arterial blood gas:
- pH = 7.35 to 7.45 (normal range, average 7.40)
- PaCO2 = 35 to 45 mmHg
- PaO2 = 75 to 100 mmHg
- HCO3- = 22 to 26 mEq/L
- O2 (oxygen) saturation = greater than 95%
The ability to quickly and efficiently read an arterial blood gas, especially in reference to inpatient medicine, is paramount to quality patient care.
- Look at the pH
- Decide whether it is acidotic, alkalotic, or within the physiological range
- The arterial partial pressure of oxygen (PaO2) level determines respiratory contribution; a high level means the respiratory system is lowering the pH and vice versa.
- Bicarbonate (HCO3-) level denotes metabolic/kidney effect. An elevated HCO3- is raising the pH and vice versa.
- If the pH is acidotic, look for the number that corresponds with a lower pH. If it is a respiratory acidosis, the CO2 should be high. If the patient is compensating metabolically, the bicarbonate (HCO3-) should be high as well. A metabolic acidosis will be depicted with an bicarbonate (HCO3-) that is low.
- If the pH is alkalotic, again, determine which value is causing this. A respiratory alkalosis will mean the CO2 is low; a metabolic alkalosis should lend an HCO3- that is high. Compensation with either system will be reflected oppositely; for a respiratory alkalosis the metabolic response should be a low HCO3- and for metabolic alkalosis, the respiratory response should be a high CO2.
- If the pH level is in the physiological range but the PaCO2 and/or bicarb are not within normal limits, there is likely a mixed disorder. Also, compensation does not always occur; this is when clinical information becomes paramount.
- Sometimes it is difficult to ascertain whether a patient has a mixed disorder.
Other tests that are important to perform when analyzing the acid-base status of a patient include those that measure electrolyte levels and renal function. This helps the clinician gather information that can be used to determine the exact mechanism of the acid-base imbalance as well as the factors contributing to the disorders.
Alkalosis treatment
To treat alkalosis, your doctor needs to first find the underlying cause.
For alkalosis caused by hyperventilation, breathing into a paper bag allows you to keep more carbon dioxide in your body, which improves the alkalosis. If your oxygen level is low, you may receive oxygen.
Medicines may be needed to correct chemical loss (such as chloride and potassium). Your provider will monitor your vital signs (temperature, pulse, rate of breathing, and blood pressure).
Metabolic alkalosis
Metabolic alkalosis is defined as a disease state where the body’s pH is elevated to greater than 7.45 secondary to some metabolic process. Metabolic alkalosis results from increased bicarbonate [HCO3–] concentration from renal or gastrointestinal hydrogen ion loss, or from an increased intake of bicarbonate [HCO3–] ions for example with administration of bicarbonate containing compounds (e.g., calcium bicarbonate supplement). The kidney can rapidly compensate for an increased bicarbonate load and so for a metabolic alkalosis to be maintained there is likely to be impairment of this compensatory mechanism, resulting in inappropriately high bicarbonate re-absorption. This can result from hypovolaemia which stimulates renal sodium re-absorption. To maintain electrochemical neutrality, either chloride or bicarbonate ions also have to be re-absorbed. Therefore in a state of chloride deficiency, bicarbonate re-absorption occurs. Sodium can also be re-absorbed in exchange for hydrogen and potassium ions, and hence hypovolaemia can cause a hypokalaemic metabolic alkalosis. Hypokalaemia alone can also lead to the maintenance of a metabolic alkalosis, because hydrogen ions are excreted by the kidney in exchange for potassium ions. Mineralocorticoid excess has similar consequences as aldosterone leads to re-absorption of sodium via the epithelial sodium channel in the collecting duct and hydrogen and potassium ions are then secreted into the lumen.
People with a metabolic alkalosis usually have low chloride (Cl-) and potassium (K+) values, which again provides clues as to the cause of the acid-base disturbance.
Metabolic alkalosis also can be divided into two main categories that help ascertain the cause: chloride responsive vs. non-chloride responsive. In non-chloride-responsive metabolic alkalosis, the urine chloride is < 20 mEq/L. Some causes include vomiting, hypovolemia, and diuretic use.
Bartter and Gitelman syndromes are autosomal recessive conditions which cause hypokalemia, metabolic alkalosis, hyperaldosteronism and in some patients, hypomagnaesemia, as a result of impaired sodium chloride re-absorption in the kidney in the loop of Henle and distal tubule, respectively. Bartter syndrome tends to present in childhood, whereas Gitelman syndrome may present later, including during pregnancy 1. If a patient has these features, in the absence of another cause such as vomiting, the diagnosis can be supported by measuring the urinary calcium (this is normal or high in Bartter syndrome and below normal in Gitelman syndrome).
Diuretic abuse can also cause hypokalemia and metabolic alkalosis, and is important to exclude in patients who present with these clinical features.
Metabolic alkalosis causes
There is a multitude of disease states that can cause metabolic alkalosis.
Table 1. Causes of metabolic alkalosis
| Exogenous administration | |
| Alkali administration (e.g. milk alkali syndrome) | |
| Intravenous penicillin | |
| Current use of diuretics | |
| Low urinary chloride (<20 mEq/L) | |
| Loss of gastric secretions, e.g. vomiting, villous adenoma | |
| Congenital chloridorrhea | |
| Following an episode of hypercapnea | |
| Previous diuretic treatment | |
| Increased urinary chloride without hypertension | |
| Hypokalemia | |
| Hypomagnesemia | |
| Bartter’s syndrome | |
| Gitelman’s syndrome | |
| Increased urinary chloride with hypertension | |
| Cushing syndrome (or exogenous steroid use) | |
| Congenital adrenal hyperplasia | Low-plasma renin activity |
| 11 β hydroxysteroid dehydrogenase deficiency | Low-aldosterone level |
| Liquorice consumption | |
| Congenital adrenal hyperplasia | |
| Liddle’s syndrome | |
| Renal artery stenosis | High plasma renin activity |
| Diuretic use | High aldosterone level |
| Renin-secreting tumours | |
| Primary hyperaldosteronism (adrenal adenoma, bilateral adrenal hyperplasia or rarely adrenal carcinoma) | Low plasma renin activity High aldosterone level |
Causes of metabolic alkalosis can include:
- Loss of acid from extracellular space
- A. Loss of acid from gastric juice: vomiting; gastric suction or fistula
- B. Loss of acid into urine: increased distal Na delivery in presence of hyperaldosteronism.
- C. Loss of acid into cells: potassium (K) deficiency
- D. Loss of acid into stool: congenital alkalosis with diarrhea
- Excessive bicarbonate [HCO3–] loads
- A. Absolute
- 1. Oral or parenteral loads of NaHCO3 or alkalinizing Na salts
- 2. Metabolic conversion of endogenous acid anions (e.g. ketones, lactate) to bicarbonate [HCO3–]
- B. Relative
- 1. Alkaline loads in renal failure
- A. Absolute
- Contraction of extracellular space
- A. Diuretic loss of NaCI without commensurate loss of NaHCO3
- Post-hypercapneic state
In general, the cause of metabolic alkalosis can be narrowed down to an intracellular shift of hydrogen ions (H+) (e.g., hypokalemia or low blood potassium), gastrointestinal (GI) loss of hydrogen ions (e.g., excessive vomiting, excessive intake of calcium carbonate supplement), excessive renal hydrogen ion loss, retention or addition of bicarbonate ions, or volume contraction around a constant amount of extracellular bicarbonate known as contraction alkalosis. All of which leads to the net result of increased levels of bicarbonate in the blood. As long as renal function is maintained, excess bicarbonate is excreted in the urine fairly rapidly.
As a result, metabolic alkalosis will persevere if the ability to eliminate bicarbonate is impaired due to one of the following causes:
- hypovolemia,
- reduced effective arterial blood volume,
- chloride depletion,
- hypokalemia,
- reduced glomerular filtration rate (GFR), and/or hyperaldosteronism.
Intracellular Shift of Hydrogen
Anytime that hydrogen ions (H+) are shifted intracellularly, this imbalance in the acid-base buffer system has a relative increase in bicarbonate. Processes that drive hydrogen intracellularly include hypokalemia (low blood potassium).
Gastrointestinal Loss of Hydrogen
Stomach fluids are highly acidic at a pH of approximately 1.5 to 3.5. Hydrogen secretion is accomplished via parietal cells in the gastric mucosa. Therefore, the large volume loss of gastric secretions will correlate as a loss of hydrogen chloride (HCl), an acidic substance, leading to a relative increase in bicarbonate in the blood, thus driving alkalosis. Losses can occur pathologically via vomiting or nasogastric suctioning.
Renal Loss of Hydrogen
Hydrogen is used within the kidneys are an antiporter energy gradient to retain a multitude of other elements. Of interest here, sodium is reabsorbed through an exchange for hydrogen in the renal collecting ducts under the influence of aldosterone. Therefore, pathologies that increase the levels of mineralocorticoids or increase the effect of aldosterone, such as Conn syndrome will lead to hypernatremia, hypokalemia, and hydrogen loss in the urine. In a similar vein of thought, loop and thiazide diuretics are capable of inducing secondary hyperaldosteronism by increasing sodium and fluid load to the distal nephron, which encourages the renin-angiotensin-aldosterone system. Genetic defects that lead to decreased expression of ion transporters in the Loop of Henle are possible but less common. These syndromes are known as Bartter and Gitelman disease. The net effect of these genetic defects is akin to the action of loop diuretics.
Retention/Addition of Bicarbonate
Several etiologies lead to increases in bicarbonate within the blood. The simplest of which is an overdose of exogenous sodium bicarbonate in a medical setting. Milk-alkali syndrome is a pathology where the patient consumes excessive quantities of oral calcium antacids, which leads to hypercalcemia and varying degrees of renal failure. Additionally, since antacids are neutralizing agents, they add alkaline substances to the body while reducing acid levels thus increasing pH. A pathology that is in line with normal physiology is the body’s natural compensation mechanism for hypercarbia. When a patient hypoventilates, CO2 retention occurs in the lungs and subsequently reduces pH. Over time, the renal system compensates by retaining bicarbonate to balance pH. This is a slower process. Once the hypoventilation is corrected, such as with a ventilator-assisted respiratory failure patient CO2 levels will quickly decrease, but bicarbonate levels will lag in reducing. This causes post-hypercapnia metabolic alkalosis, which is self-correcting. It is possible to calculate the expected pCO2 in the setting of metabolic alkalosis to determine if it is a compensatory increase in bicarbonate, or if there is an underlying pathology driving alkalosis using the following equation:
Expected pCO2 = 0.7 [HCO3–] + 20 mmHg +/- 5
If the expected pCO2 does not match the measured value, an underlying metabolic alkalosis is a likely present.
Contraction Alkalosis
This phenomenon occurs when a large volume of sodium-rich, bicarbonate low fluid is lost from the body. This occurs with diuretic use, cystic fibrosis, congenital chloride diarrhea, among others. The net concentration of bicarbonate increases as a result. This pathology is easily offset by the release of hydrogen from intracellular space to balance the pH in most incidences.
The exact cause, if unknown or not obvious, can be elucidated in part by evaluation of urinary chloride. Metabolic alkalosis is split into 2 main categories: Chloride responsive with urine chloride less than 10 mEq/L and chloride resistant with urine chloride greater than 20 mEq/L. Chloride responsive etiologies include loss of hydrogen via the gastrointestinal tract, congenital chloride diarrhea syndrome, contraction alkalosis, diuretic therapy, post-hypercapnia syndrome, cystic fibrosis, and exogenous alkalotic agent use. Chloride-resistant causes include retention of bicarbonate, the shift of hydrogen into intracellular spaces, hyperaldosteronism, Bartter syndrome, and Gitelman syndrome.
Metabolic alkalosis diagnosis
A history of severe vomiting, previous gastrointestinal procedures, or other features such as hypertension, all help narrow the differential diagnosis. Examination is important because the volume status of the patient also helps identify the cause of the metabolic derangement and can guide treatment.
Urinary chloride concentration is a useful diagnostic tool as a low result reflects appropriate chloride handling in the kidney in response to low plasma chloride. Appropriate reduction in renal chloride excretion is seen in volume deplete states and makes other causes such as mineralocorticoid excess less likely.
A raised urinary chloride is not specific for one diagnosis, but an elevation is seen in disorders that are not related to volume depletion, such as mineralocorticoid excess. Measurement of plasma renin activity and aldosterone concentration therefore aid the diagnosis and alongside the presence or absence of hypertension, may distinguish between the less common causes (see Table 1).
Metabolic alkalosis symptoms
Symptoms of alkalosis are often due to associated potassium (K+) loss and may include irritability, weakness, and muscle cramping.
Symptoms of alkalosis can include any of the following:
- Confusion (can progress to stupor or coma)
- Hand tremor
- Lightheadedness
- Muscle twitching
- Nausea, vomiting
- Numbness or tingling in the face, hands, or feet
- Prolonged muscle spasms (tetany)
Severe alkalosis is associated with significant morbidity and mortality, particularly in critically ill patients 3. An increase in pH results in a shift of the oxygen dissociation curve to the left, which represents increased oxygen affinity to hemoglobin and therefore reduced oxygen delivery to the tissues, which is exacerbated by the hypoxia that may result from compensatory physiological respiratory depression. Arrhythmias, confusion and impaired myocardial contractility can result from reduced oxygen delivery. Cerebral blood flow may be impaired but can be partially balanced by increased partial pressure of the carbon dioxide that is present.
Metabolic alkalosis compensation
The lungs and kidneys are the major organs involved in regulating blood pH. And to compensate for the metabolic alkalosis, you slowed your breathing (hypoventilation) to decrease CO2 elimination.
- The lungs flush acid out of the body by exhaling CO2. Raising and lowering the respiratory rate alters the amount of CO2 that is breathed out, and this can affect blood pH within minutes.
- The kidneys excrete acids in the urine, and they regulate the concentration of bicarbonate (HCO3–, a base) in blood. Acid-base changes due to increases or decreases in bicarbonate [HCO3–] concentration occur more slowly than changes in CO2, taking hours or days.
Both of these processes are always at work, and they keep the blood pH in healthy people tightly controlled.
Buffering systems that resist changes in pH also contribute to the regulation of acid and base concentrations. The main buffers in blood are hemoglobin (in red blood cells), plasma proteins, CO2, bicarbonate, and phosphates.
The absolute quantities of acids or bases are less important than the balance between the two and its effect on blood pH.
Carbon dioxide (CO2) plays a remarkable role in the human body mainly through pH regulation of the blood. The pH is the primary stimulus to initiate ventilation. In its normal state, the body maintains CO2 in a well-controlled range from 38 to 42 mm Hg by balancing its production and elimination. In a state of hypoventilation, the body produces more CO2 than it can eliminate, causing a net retention of CO2. The increased CO2 is what leads to an increase in hydrogen ions and a slight increase in bicarbonate, as seen by a right shift in the following equilibrium reaction of carbon dioxide:
CO2 + H2O -> H2CO3 (carbonic acid) -> HCO3- + H+
The buffer system created by carbon dioxide consists of the following three molecules in equilibrium: CO2, H2CO3-, and HCO3-. When H+ is high, bicarbonate [HCO3–] buffers the low pH. When OH- is high, H2CO3 (carbonic acid) buffers the high pH. Bicarbonate [HCO3–] functions as an alkalotic substance. CO2 functions as an acidic substance. Therefore, increases in bicarbonate [HCO3–] or decreases in CO2 will make blood more alkalotic. The opposite is also true where decreases in bicarbonate [HCO3–] or an increase in CO2 will make blood more acidic. CO2 levels are physiologically regulated by the pulmonary system through respiration, whereas the bicarbonate [HCO3–] levels are regulated through the renal system with reabsorption rates. Therefore, metabolic alkalosis is an increase in serum bicarbonate [HCO3–].
Metabolic alkalosis treatment
Treatment of metabolic alkalosis depends on the patient’s clinical condition and the potential cause of the alkalosis.
Metabolic alkalosis associated with hypochloremia and hypovolaemia requires the administration of sodium chloride containing fluid. Histamine receptor antagonists or proton pump inhibitors reduce the volume and acidity of secretions in patients with large volume gastric fluid loss and therefore may aid resolution of the metabolic abnormalities 4.
In extreme cases hemodialysis has been used, initially with an acid dialysate 5, but more recently case reports have described successful resolution with normal bicarbonate dialysate 6.
Acetazolamide can also be used to correct serum pH in alkalotic patients. In one study, a single dose was administered to 15 consecutive patients on an intensive care unit, which caused an increased ratio for renal excretion of sodium to chloride, resulting in an increase in serum chloride and a resolution of the alkalosis 7.
Respiratory alkalosis
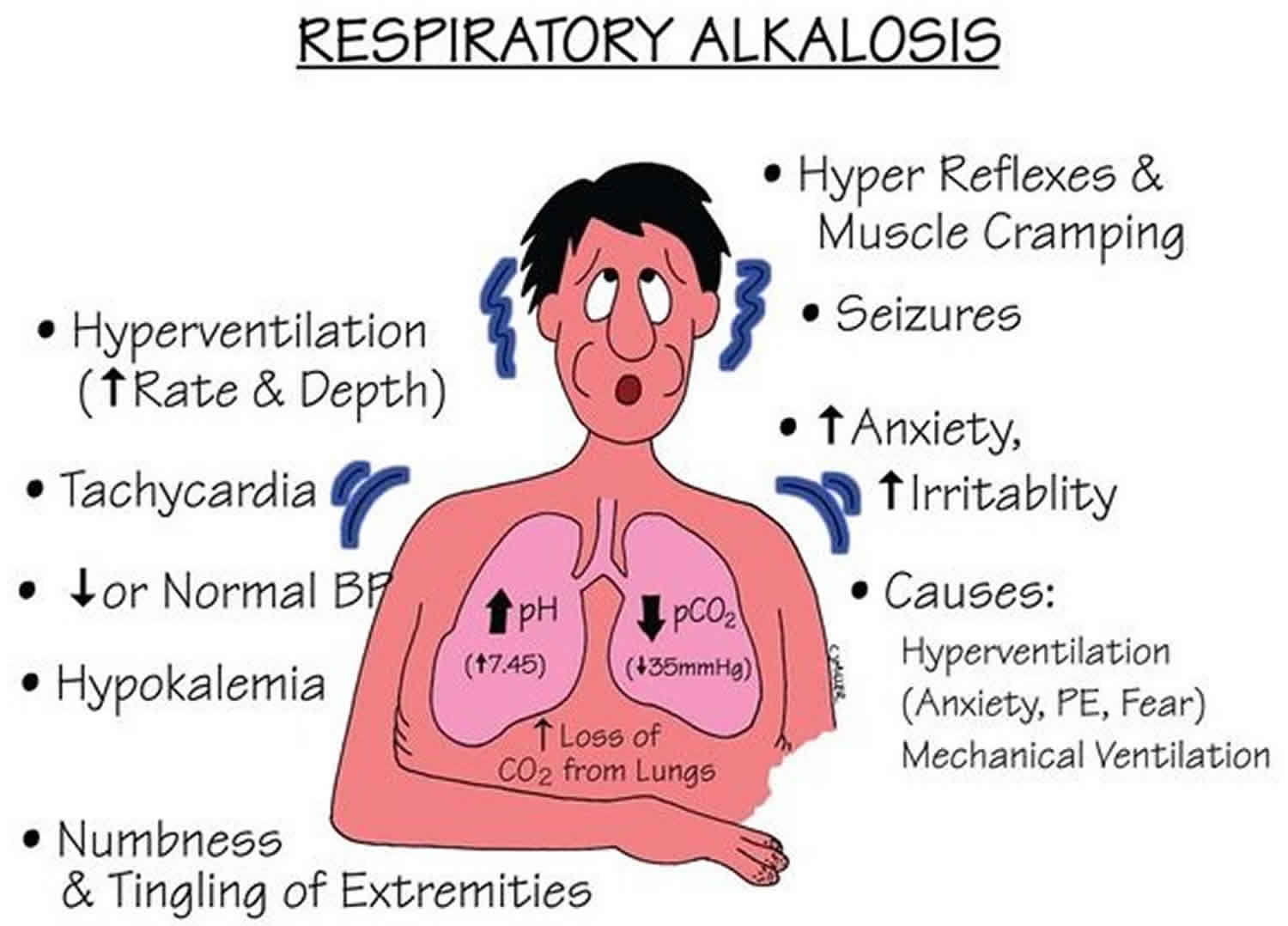
Respiratory alkalosis is a pathology that is secondary to hyperventilation. Hyperventilation typically occurs in response to an insult such as hypoxia, metabolic acidosis, pain, anxiety, or increased metabolic demand.
Respiratory alkalosis is a condition marked by a low level of carbon dioxide (CO2) in the blood due to breathing excessively. Any condition that leads to the increased expiration of carbon dioxide (CO2) can result in respiratory alkalosis. When excess carbon dioxide (CO2) is expired, the pH of the human body is increased due to less carbonic acid being created. Physiologically, the appropriate compensation is a decreased amount of bicarbonate being created by the renal system. Some causes of respiratory alkalosis include panic attacks with hyperventilation, pulmonary embolism, pneumonia, and salicylate intoxication.
Respiratory alkalosis in itself is not life-threatening; however, the underlying cause may be. Always look for and treat the source of the illness. Interventions to reduce pH directly are typically not necessary as there is no mortality benefit to this therapy.
Respiratory alkalosis causes
Common respiratory alkalosis causes include:
- Anxiety or panic
- Fever
- Overbreathing (hyperventilation)
- Pregnancy (this is normal)
- Being at a high altitude
- Lack of oxygen
- Pain
- Drugs, such as early in a overdose of aspirin (salicylates)
- Pneumonia, pulmonary (lung) congestion (due to heart failure), or embolism
- Exercise
- Central nervous system tumor, trauma, infection (meningitis, encephalitis)
- Liver failure
In almost every scenario, respiratory alkalosis is induced by a process involving hyperventilation. These include central causes, hypoxemic causes, pulmonary causes, and iatrogenic causes. Central sources are a head injury, stroke, hyperthyroidism, anxiety-hyperventilation, pain, fear, stress, drugs, medications such as salicylates, and various toxins. Hypoxic stimulation leads to hyperventilation in an attempt to correct hypoxia at the expense of a CO2 loss. Pulmonary causes include pulmonary embolisms, pneumothorax, pneumonia, and acute asthma or chronic obstructive pulmonary disease (COPD) exacerbations. Iatrogenic causes are primarily due to hyperventilation in intubated patients on mechanical ventilation.
Respiratory alkalosis may be an acute process or a chronic process. These are determined based on the level of metabolic compensation for the respiratory disease. Excess bicarbonate [HCO3–] levels are buffered to reduce levels and maintain a physiological pH through the renal decrease of H+ secretion and increasing bicarbonate [HCO3–] secretion; however, this metabolic process occurs over the course of days whereas respiratory disease can adjust CO2 levels in minutes to hours. Therefore, acute respiratory alkalosis is associated with high bicarbonate [HCO3–] levels since there has not been sufficient time to lower the bicarbonate [HCO3–] levels and chronic respiratory alkalosis is associated with low to normal bicarbonate [HCO3–] levels.
Respiratory alkalosis symptoms
The symptoms of respiratory alkalosis may include:
- Dizziness
- Lightheadedness
- Numbness of the hands and feet
- Seizures may occur if the alkalosis is extremely severe. This is very rare.
Respiratory alkalosis compensation
The lungs and kidneys are the major organs involved in regulating blood pH. And to compensate for the respiratory alkalosis, your kidney decreases retention of bicarbonate [HCO3–] and excretion of acid.
- The lungs flush acid out of the body by exhaling CO2. Raising and lowering the respiratory rate alters the amount of CO2 that is breathed out, and this can affect blood pH within minutes.
- The kidneys excrete acids in the urine, and they regulate the concentration of bicarbonate (HCO3–, a base) in blood. Acid-base changes due to increases or decreases in bicarbonate [HCO3–] concentration occur more slowly than changes in CO2, taking hours or days.
Both of these processes are always at work, and they keep the blood pH in healthy people tightly controlled.
Buffering systems that resist changes in pH also contribute to the regulation of acid and base concentrations. The main buffers in blood are hemoglobin (in red blood cells), plasma proteins, CO2, bicarbonate, and phosphates.
The absolute quantities of acids or bases are less important than the balance between the two and its effect on blood pH.
Carbon dioxide (CO2) plays a remarkable role in the human body mainly through pH regulation of the blood. The pH is the primary stimulus to initiate ventilation. In its normal state, the body maintains CO2 in a well-controlled range from 38 to 42 mm Hg by balancing its production and elimination. In a state of hypoventilation, the body produces more CO2 than it can eliminate, causing a net retention of CO2. The increased CO2 is what leads to an increase in hydrogen ions and a slight increase in bicarbonate, as seen by a right shift in the following equilibrium reaction of carbon dioxide:
CO2 + H2O -> H2CO3 (carbonic acid) -> HCO3- + H+
The buffer system created by carbon dioxide consists of the following three molecules in equilibrium: CO2, H2CO3-, and HCO3-. When H+ is high, bicarbonate [HCO3–] buffers the low pH. When OH- is high, H2CO3 (carbonic acid) buffers the high pH. Bicarbonate [HCO3–] functions as an alkalotic substance. CO2 functions as an acidic substance. Therefore, increases in bicarbonate [HCO3–] or decreases in CO2 will make blood more alkalotic. The opposite is also true where decreases in bicarbonate [HCO3–] or an increase in CO2 will make blood more acidic. CO2 levels are physiologically regulated by the pulmonary system through respiration, whereas the bicarbonate [HCO3–] levels are regulated through the renal system with reabsorption rates. Therefore, respiratory alkalosis is a decrease in serum CO2. While it is theoretically possible to have decreased CO2 production, in every scenario this illness is a result of hyperventilation where CO2 is breathed away.
Respiratory alkalosis diagnosis
Your health care provider will perform a physical exam. Tests that may be done include:
- Arterial blood gas, which measures oxygen and carbon dioxide levels in the blood
- Basic metabolic panel
- Chest x-ray
- Pulmonary function tests to measure breathing and how well the lungs are functioning
Arterial blood gas (ABG) sampling, is a test often performed in an inpatient setting to assess the acid-base status of a patient. A needle is used to draw blood from an artery, often the radial, and the blood is analyzed to determine parameters such as the pH, the arterial partial pressure of oxygen (PaO2), the arterial partial pressure of carbon dioxide (PaCO2), bicarbonate [HCO3–], oxygen (O2) saturation, total CO2, and base excess. This allows the physician to understand the status of the patient better. Arterial blood gases are especially important in the critically ill. Urine chloride is a direct measurement of chloride being excreted into urine. Arterial blood gas (ABG) test is useful to help determine the cause of metabolic alkalosis. They are the main tool utilized in adjusting to the needs of a patient on a ventilator. The following are the most important normal values on an arterial blood gas:
- pH = 7.35 to 7.45 (normal range, average 7.40)
- PaCO2 = 35 to 45 mmHg
- PaO2 = 75 to 100 mmHg
- HCO3- = 22 to 26 mEq/L
- O2 (oxygen) saturation = greater than 95%
The ability to quickly and efficiently read an arterial blood gas, especially in reference to inpatient medicine, is paramount to quality patient care.
- Look at the pH
- Decide whether it is acidotic, alkalotic, or within the physiological range
- The arterial partial pressure of oxygen (PaO2) level determines respiratory contribution; a high level means the respiratory system is lowering the pH and vice versa.
- Bicarbonate (HCO3-) level denotes metabolic/kidney effect. An elevated HCO3- is raising the pH and vice versa.
- If the pH is acidotic, look for the number that corresponds with a lower pH. If it is a respiratory acidosis, the CO2 should be high. If the patient is compensating metabolically, the bicarbonate (HCO3-) should be high as well. A metabolic acidosis will be depicted with an bicarbonate (HCO3-) that is low.
- If the pH is alkalotic, again, determine which value is causing this. A respiratory alkalosis will mean the CO2 is low; a metabolic alkalosis should lend an HCO3- that is high. Compensation with either system will be reflected oppositely; for a respiratory alkalosis the metabolic response should be a low HCO3- and for metabolic alkalosis, the respiratory response should be a high CO2.
- If the pH level is in the physiological range but the PaCO2 and/or bicarb are not within normal limits, there is likely a mixed disorder. Also, compensation does not always occur; this is when clinical information becomes paramount.
- Sometimes it is difficult to ascertain whether a patient has a mixed disorder.
Other tests that are important to perform when analyzing the acid-base status of a patient include those that measure electrolyte levels and renal function. This helps the clinician gather information that can be used to determine the exact mechanism of the acid-base imbalance as well as the factors contributing to the disorders.
Respiratory alkalosis treatment
Treatment is aimed at the condition that causes respiratory alkalosis. Breathing into a paper bag — or using a mask that causes you to re-breathe carbon dioxide — sometimes helps reduce symptoms when anxiety is the main cause of the condition. In anxious patients, anxiolytics may be necessary.
In infectious disease, antibiotics targeting sputum or blood cultures are appropriate. In embolic disease, anticoagulation is necessary. Ventilator support may be necessary for patients with acute respiratory failure, acute asthma, or acute, chronic obstructive pulmonary disease (COPD) exacerbation if they show signs of respiratory fatigue. In ventilator controlled patients, it may be necessary to reevaluate their ventilator settings to reduce respiratory rate. If hyperventilation is intentional, monitor the arterial or venous blood gas values closely. In severe cases, pH may be directly reduced using acidic agents. However, this is not routinely done.
- de Bustros A, Aleppo G, Zikos D. Hypokalemia in pregnancy: clue to Gitelman syndrome. Endocrinologist 2001; 11: 447–450.[↩]
- Frise C, Noori M, Williamson C. Severe metabolic alkalosis in pregnancy. Obstet Med. 2013;6(3):138-140. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5032930/[↩]
- Alkalemia-associated morbidity and mortality in medical and surgical patients. Anderson LE, Henrich WL. South Med J. 1987 Jun; 80(6):729-33.[↩]
- Hixson R, Christmas D. Use of omeprazole in life-threatening metabolic alkalosis. Intensive Care Med 1999; 25: 1201–1201.[↩]
- Ponce P, Santana A, Vinhas J. Treatment of severe metabolic alkalosis by ‘acid dialysis’. Crit Care Med 1991; 19: 583–585.[↩]
- Hsu S-C, Wang M-C, Hsin-Liang L, Tsai M-C, Huang J-J. Extreme metabolic alkalosis treated with normal bicarbonate hemodialysis. Am J Kidney Dis 2001; 37: 1–4.[↩]
- Moviat M, Pickkers P, van der Voort PHJ, van der Hoeven JG. Acetazolamide-mediated decrease in strong ion difference accounts for the correction of metabolic alkalosis in critically ill patients. Crit Care 2006; 10: R14–R14.[↩]