Contents
What is azotemia
Azotemia is a serious medical condition characterized by abnormally high levels of toxic nitrogenous waste products normally excreted in your urine, build up in your blood; this is usually as a result of severe kidney disease or kidney failure. In azotemia there is an increase in your blood urea nitrogen (BUN) — nitrogen containing compounds (such as urea, creatinine, various body waste compounds, and other nitrogen-rich compounds) in the blood. Azotemia is largely related to insufficient or dysfunctional filtering of blood by the kidneys. Azotemia can lead to uremia and acute kidney injury (kidney failure) if not controlled. One major role of a healthy kidney is to get rid of the byproducts of nitrogen metabolism (from protein). Azotemia occurs when the kidneys are damaged and can no longer efficiently get rid of these metabolites. Blood urea nitrogen (BUN) and creatinine are just two easily measured markers of nitrogen accumulation. When their levels are increased one can be said to have renal dysfunction. Eight five to 90% of kidney function can be lost (if gradual) before one notices any obvious symptoms. For those who visit a doctor regularly and have blood drawn, kidney dysfunction can be detected by accident. For others, diagnosis comes when they develop symptoms at a late stage, and may require dialysis in the short term. The possible advantage of knowing early is applying the possible interventions that might slow progression and the ability to prepare psychologically (if that is possible).
Vigorous exercise can lead to a temporary increase in the blood urea nitrogen as a result of dehydration. Hydration during exercise should make this less of a possibility. Meat and protein in the diet does not increase protein in the urine (proteinuria).
The kidneys filter the blood. They also make urine to remove waste products. When the amount, or pressure, of blood flow through the kidney drops, filtering of the blood also drops. Or it may not occur at all. Waste products stay in the blood. Little or no urine is made, even though the kidney itself is working.
When nitrogen waste products, such as creatinine and urea, build up in the body, the condition is called azotemia. These waste products act as poisons when they build up. They damage tissues and reduce the ability of the organs to function.
Nitrogenous wastes
A waste is any substance that is useless to yout body or present in excess of your body’s needs. A metabolic waste, more specifically, is a waste substance produced by your body. Among the most toxic of your metabolic wastes are small nitrogen-containing compounds called nitrogenous wastes. Examples of nitrogenous wastes are ammonia, urea, creatinine and uric acid. About 50% of the nitrogenous waste is urea, a byproduct of protein catabolism. Proteins are hydrolyzed to amino acids, and then the —NH2 group is removed from each amino acid. The —NH2 forms ammonia, which is exceedingly toxic but which the liver quickly converts to urea, CO(NH2)2, a somewhat less toxic waste.
Other nitrogenous wastes in the urine include uric acid and creatinine, produced by the catabolism of nucleic acids and creatine phosphate, respectively. Although less toxic than ammonia and less abundant than urea, these too are potentially harmful.
The level of nitrogenous waste in the blood is typically expressed as blood urea nitrogen (BUN). The normal concentration of blood urea is 10 to 20 mg/dL. An elevated BUN (blood urea nitrogen) is called azotemia and may indicate renal insufficiency.
A diagnosis of renal failure is based on abnormalities in glomerular filtration rate (GFR) or creatinine clearance 1. Glomerular filtration rate (GFR) is the rate in milliliters per minutes at which substances in plasma are filtered through the glomerulus, in other words, the clearance of a substance from the blood. Glomerular filtration rate (GFR) is normally 105–125 mL/min. Glomerular filtration rate (GFR) must be precisely controlled. If it is too high, fluid flows through the renal tubules too rapidly for them to reabsorb the usual amount of water and solutes. Urine output rises and creates a threat of dehydration and electrolyte depletion. If glomerular filtration rate (GFR) is too low, fluid flows sluggishly through the tubules, they reabsorb wastes that should be eliminated in the urine, and azotemia may occur.
The only way to adjust glomerular filtration rate from moment to moment is to change glomerular blood pressure. This is achieved by three homeostatic mechanisms: renal autoregulation, sympathetic control, and hormonal control.
A GFR of less than 15 ml per minute is considered to be end-stage renal failure requiring renal replacement therapy, e.g., dialysis. The presence of a normal GFR does not exclude the presence of renal disease which may be evidenced by the presence of albuminuria/proteinuria or imaging.
Reference intervals for serum creatinine and urea are dependant on age and gender.
Presence of electrolytes in urine depends on the hydration status, duration of the collection of urine apart from pathological factors, and reference intervals are often wide and dependant on clinical context.
Creatinine
Serum creatinine is elevated when there is a significant reduction in the glomerular filtration rate (GFR) or when urine elimination is obstructed. About 50% of kidney function must be lost before a rise in serum creatinine can be detected. Thus serum creatinine is a late marker of acute kidney injury.
BUN
Serum urea/BUN is increased acute and chronic renal disease.
eGFR equations are used to determine the presence of renal disease, stage of chronic kidney disease and to monitor response to treatment.
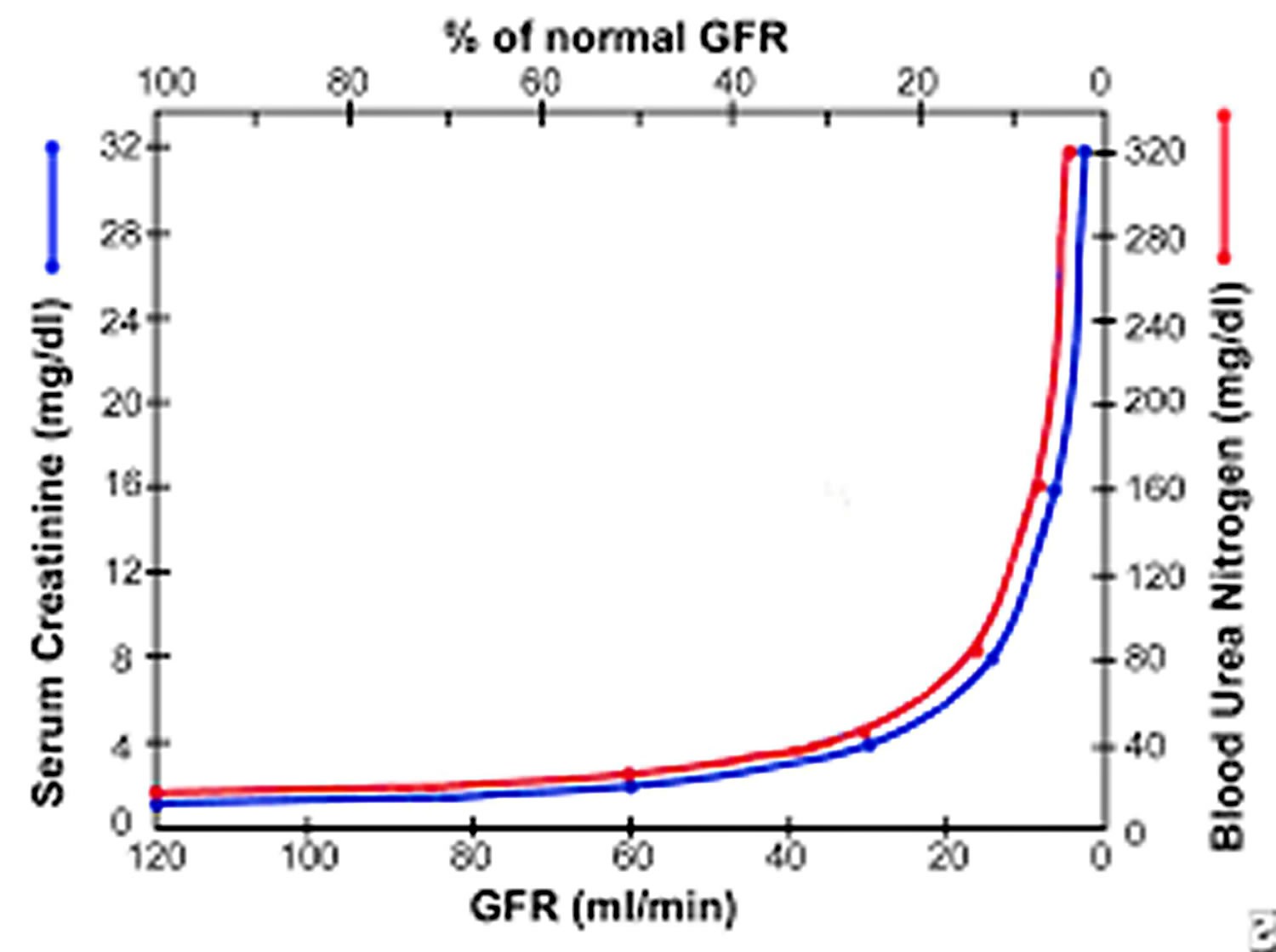
Figure 1. Glomerular filtration rate and blood urea nitrogen and creatinine
Footnote: Graph shows relation of glomerular filtration rate (GFR) to steady-state serum creatinine and blood urea nitrogen (BUN) levels. In early renal disease, substantial decline in GFR may lead to only slight elevation in serum creatinine. Elevation in serum creatinine is apparent only when GFR falls to about 70 mL/min.
Azotemia vs Uremia
Uremia, a clinical condition associated with worsening renal function, is characterized by fluid, electrolyte, and hormone imbalances in addition to metabolic abnormalities. The literal meaning of uremia is “urine in the blood,” and uremia develops most commonly in the setting of chronic and end-stage renal disease (ESRD), but may also occur as a result of acute kidney injury 2.
It is difficult to determine the exact prevalence of uremia in the United States because patients with end-stage renal disease (ESRD) typically begin dialysis before the development of uremic symptoms. Uremic symptoms typically arise once creatinine clearance is less than 10mL/min or 15mL/min in the case of diabetic patients 2.
Putative uremic toxins include parathyroid hormone, macroglobulin, advanced glycosylation end products, and beta2 microglobulin, though no specific uremic toxin has been identified as responsible for all clinical manifestations of uremia 3.
Symptomatic uremia tends to occur once creatinine clearance decreases below 10 mL/min unless kidney failure develops acutely, in which case, some patients may become symptomatic at higher clearance rates.
Patients presenting with uremia typically complain of nausea, vomiting, fatigue, anorexia, weight loss, muscle cramps, pruritus, or changes in mental status 2. The clinical presentation of uremia can be explained by the metabolic disturbances associated with the condition.
Fatigue as a result of anemia is considered one of the major components of the uremic syndrome.
Patients with a history of diabetes may report improved glycemic control but are at a greater risk of developing hypoglycemic episodes as kidney function worsens.
Hypertension, atherosclerosis, valvular stenosis and insufficiency, chronic heart failure, and angina may all develop as a result of a buildup of uremic toxins and metastatic calcification associated with uremia and end-stage renal disease (ESRD).
Occult gastrointestinal bleeding as a result of platelet abnormalities may present with nausea or vomiting. Uremic fetor, ammonia or urine-like odor of the breath, may also occur in uremic patients.
Uremia complications
- Hyperpigmented skin
- Severe itching
- Pericarditis plus effusion
- Pulmonary edema
- Valvular calcification
Uremic encephalopathy occurs in patients with acute or chronic renal failure, once the estimated GFR (eGFR) declines and stays below 15 mL/min. It is important to recognize the signs and symptoms early, as untreated uremic encephalopathy can progress to coma, while symptoms are easily reversible with dialysis. Early symptoms of uremic encephalopathy include nausea, anorexia, restlessness, drowsiness, and slowing of concentration and cognitive functions. As uremic encephalopathy progresses, patients typically become more disoriented, confused, and may exhibit bizarre behavior and emotional instability. Eventually, severe uremic encephalopathy will result in stupor and coma. Physical examination may reveal altered mental status, signs of cranial nerve involvement (e.g., nystagmus), or papilledema. Patients may additionally display hyperreflexia, clonus or asterixis, and eventually, coma.
A patient with uremic encephalopathy should improve clinically, following initiation of dialysis. However, electroencephalographic (EEG) findings such as slowing or loss of alpha frequency waves, disorganized signals, slow background activity with intermittent bursts of theta and delta waves may not improve instantly. Improvement may take several months, and one may not ever return completely back to normal. Treating uremic encephalopathy involves addressing many of the same parameters as are addressed when treating any patient with end-stage renal disease (ESRD) for example, correcting associated anemia, regulation of calcium or phosphate imbalances, monitoring the adequacy of dialysis.
Uremia diagnosis
It is important to determine whether a patient presenting with uremic symptoms is experiencing acute or chronic renal failure, as acute kidney injury is reversible. Laboratory studies to evaluate for abnormalities in hemoglobin, calcium, phosphate, parathyroid hormone, albumin, potassium, and bicarbonate in addition to urinalysis (with microscopic examination) will help point towards any potential abnormalities.
A 24-hour urine collection may provide insight to both GFR and creatinine clearance, though this method is both burdensome and often inaccurate. Alternatively, a nuclear medicine radioisotope (iothalamate) clearance assay may be used to measure GFR. However, this test is also time-consuming and expensive relative to the Cockcroft-Gault formula [creatinine clearance = Sex times 4 times (weight / 72)] or the Modification of Diet in Renal Disease formula [(GFR (mL/min/1.73 m) = 175 x (S) times (Age) times (0.742 if female) or times (1.212 if African American)] that are often used instead.
As per the National Kidney Foundation, patients presenting with chronic kidney disease are staged based on the estimated GFR (creatinine clearance) as calculated by the Modification of Diet in Renal Disease formula.
- Stage 1 – normal GFR (90 mL/min or greater)
- Stage 2 – mildly reduced GFR (60 mL/min to 90 mL/min)
- Stage 3 – moderately reduced GFR (30 mL/min to 59 mL/min)
- Stage 4 – severely reduced GFR (15 mL/min to -29 mL/min)
- Stage 5 – ESRD (GFR < 15 mL/min or patient is on dialysis)
A renal ultrasound may be useful to determine the size and shape of the kidneys, and to evaluate for hydronephrosis or ureteral and/or bladder obstruction. This may occur as a result of kidney stones, neurologic abnormalities, trauma, pregnancy, prostate enlargement, retroperitoneal fibrosis, abdominal tumors (secondary to cervical or prostate cancers) or additional structural abnormalities. Early diabetic nephropathy, multiple myeloma, polycystic kidney diseases, and glomerulonephritis associated with human immunodeficiency virus (HIV) are all associated with enlarged kidneys on ultrasound. Smaller kidneys are indicative of more chronic, irreversible changes as a result of long-standing kidney disease, ischemic nephropathy, or hypertensive nephrosclerosis.
If a patient presents with significant alterations in mental status, a brain computed tomography (CT) scan may be warranted. Uremic patients with a blood urea nitrogen (BUN) level greater than 150 mg/dL to 200 mg/dL are also at an increased risk of developing spontaneous subdural hematomas. Given the increased risk of bleeding and hemorrhage in uremia (especially in the setting of a fall or trauma), a CT scan of both the brain and abdomen may additionally be considered. An abdominal CT scan might help further elucidate the underlying cause of hydronephrosis if it was found on ultrasound without any obvious cause.
Finally, magnetic resonance imaging (MRI) may be considered to assess for renal artery stenosis or thrombosis, or aortic and renal artery dissection- all potentially reversible causes of renal failure.
A renal biopsy may be helpful in determining reversibility or treatability of the renal injury, and may ultimately be required to make an accurate diagnosis of acute kidney injury or chronic kidney disease. However, a biopsy should not be performed in the case of small kidneys because of the associated comorbidities and increased risk of bleeding.
Uremia treatment
Dialysis is indicated in a patient with symptomatic uremia (e.g., nausea, vomiting, hyperkalemia, metabolic acidosis) that is not treatable my medical means, and should be initiated as soon as possible, regardless of the patient’s GFR 5.
Patients presenting with a uremic emergency (e.g., hyperkalemia, acidosis, symptomatic pericardial effusion, or uremic encephalopathy) require emergent dialysis which should be initiated gently to avoid dialysis disequilibrium syndrome (neurologic symptoms secondary to cerebral edema occurring during or shortly after the initiation of dialysis).
Ultimately, the best renal replacement therapy is renal transplantation, although practitioners may also consider long-term hemodialysis and peritoneal dialysis. Renal transplantation is associated with improvements in both survival and quality of life, and should be considered early (before the need for dialysis) as the waiting list for transplantation is often longer than two to three years.
Iron replacement should be initiated in patients with anemia of chronic kidney disease and underlying iron deficiency (as long as serum ferritin is greater than 100 mcg/mL). This can be done with dialysis treatments, or as oral therapy, if dialysis has not yet been initiated. Erythropoietic stimulating agents, such as erythropoietin or darbepoetin, may additionally be used in low doses (due to the increased risk of cardiovascular mortality) once hemoglobin levels reach below 10 g/dL.
Hyperparathyroidism and associated or isolated hypocalcemia and hyperphosphatemia can be treated with oral calcium carbonate or calcium acetate, oral vitamin D therapy, and oral phosphate binders (e.g., calcium carbonate, calcium acetate, sevelamer or lanthanum carbonate).
A dietitian should be consulted if dietary alterations are being considered. Patients with chronic kidney disease should reduce potassium, phosphate and sodium intake to 2 g to 3 g, 2 g, and 2 g per day of each, respectively. Though there is some conflicting evidence regarding protein intake in patients with kidney failure, the current low-protein diet recommendations before initiation of dialysis are 0.8 g to 1 g of protein/kg of weight per day with an added gram of protein for each gram of protein lost in the urine in patients with nephrotic syndrome.
A low-protein diet is not recommended in patients with advanced uremia or malnutrition, as this type of diet can result in worsening of malnutrition and has been associated with increased risk of mortality with the initiation of dialysis.
Patients with a creatinine clearance of less than 20 mL/min should avoid excessive potassium intake and use certain medications with caution (e.g., potassium-sparing diuretics, angiotensin-converting enzymes (ACE) inhibitors, angiotensin-receptor blockers, beta blockers, NSAIDs).
Due to the buildup of uremic toxins and potentially increased risk of bleeding and hemorrhage, extra care needs to be taken when prescribing oral anticoagulants or antiplatelet medications to patients who have ESRD.
Finally, nephrotoxic medications (e.g., NSAIDs, aminoglycoside antibiotics) should be avoided in all patients with renal disease. To avoid nephrotoxicity, N-acetylcysteine may be administered before administration of intravenous contrast for radiologic imaging, although alternative modes of imaging like MRI should be considered in these patients, to avoid the risk of acute kidney injury altogether 6.
Uremia prognosis
In general, the prognosis for patients with uremia is poor unless they are treated with renal replacement therapy such as transplantation or dialysis. When the cause of uremia is a reversible cause, the prognosis is better than in patients with an irreversible cause. Uremic patients require frequent admission to the hospitals and have high morbidity and mortality without treatment. While dialysis has improved treatment, vascular access is still a major problem in the long run. In addition, there are not enough kidney donors. Patients with uremia are also at a high risk for adverse cardiac events and stroke compared to the general population.
Azotemia types
Azotemia has three classifications, depending on its causative origin:
- Prerenal azotemia,
- Renal azotemia, and
- Post renal azotemia.
The BUN:Creatinine ratio is a useful measure in determining the type of azotemia and will be discussed in each section below. A normal BUN:Creatinine is equal to 15.
What is prerenal azotemia
Prerenal azotemia refers to elevations in BUN and creatinine levels resulting from problems in the systemic circulation that decrease flow to the kidneys. In prerenal azotemia, decreased renal flow stimulates salt and water retention to restore volume and pressure. When volume or pressure is decreased, the baroreceptor reflexes located in the aortic arch and carotid sinuses are activated. This leads to sympathetic nerve activation, resulting in renal afferent arteriolar vasoconstriction and renin secretion through β1 receptors.
Constriction of the afferent arterioles causes a decrease in intraglomerular pressure, which reduces the GFR proportionally. Reduction in renal blood flow results in the generation of renin, which converts angiotensinogen to angiotensin I. Angiotensin-converting enzyme then converts angiotensin I to angiotensin II, which, in turn, stimulates aldosterone release. The increase in aldosterone levels results in salt and water absorption in the distal collecting tubule.
A decrease in volume or pressure is a nonosmotic stimulus for hypothalamic production of antidiuretic hormone, which exerts its effect in the medullary collecting duct for water reabsorption. Through unknown mechanisms, activation of the sympathetic nervous system leads to enhanced proximal tubular reabsorption of salt and water, as well as BUN, creatinine, calcium, uric acid, and bicarbonate. The net result of these 4 mechanisms of salt and water retention is decreased output and decreased urinary excretion of sodium (< 20 mEq/L).
Prerenal azotemia causes
Prerenal azotemia is common, especially in people who are in the hospital. Prerenal azotemia occurs as a consequence of impaired renal blood flow or decreased perfusion resulting from decreased blood volume, decreased cardiac output (congestive heart failure), decreased systemic vascular resistance, decreased effective arterial volume from sepsis or hepatorenal syndrome 7 or renal artery abnormalities. Prerenal azotemia may be superimposed on a background of chronic renal failure. Iatrogenic factors, such as excessive diuresis and treatment with ACE inhibitors, should be ruled out.
Some common causes of prerenal azotemia include hypovolemic states such as diarrhea, vomiting, bleeding, dehydration, burns, renal losses via diuretics or osmotic diuresis, and third fluid sequestration 8. Edematous states such as heart failure and cirrhosis cause reduced kidney perfusion. Sepsis or anaphylaxis leads to systemic vasodilation. Coagulopathy, such as disseminated intravascular coagulation, can also cause acute tubular necrosis.
Prerenal azotemia is the most common form of kidney failure in hospitalized people. Any condition that reduces blood flow to the kidney may cause it, including:
- Burns
- Conditions that allow fluid to escape from the bloodstream
- Long-term vomiting, diarrhea, or bleeding
- Heat exposure
- Decreased fluid intake (dehydration)
- Loss of blood volume
Conditions in which the heart cannot pump enough blood or pumps blood at a low volume also increase the risk for prerenal azotemia. These conditions include:
- Heart failure
- Shock (septic shock)
Prerenal azotemia also can be caused by conditions that interrupt blood flow to the kidney, such as:
- Certain types of surgery
- Injury to the kidney
- Blockage of the artery that supplies blood to the kidney (renal artery occlusion)
Prerenal azotemia prevention
Quickly treating any condition that reduces the volume or force of blood flow through the kidneys may help prevent prerenal azotemia.
Prerenal azotemia symptoms
Prerenal azotemia may have no symptoms. Or, symptoms of the causes of prerenal azotemia may be present.
Symptoms of dehydration may be present and include any of the following:
- Confusion
- Decreased or no urine production
- Dry mouth due to thirst
- Fast pulse
- Fatigue
- Pale skin color
- Swelling
Prerenal azotemia possible complications
Prerenal azotemia complications may include:
- Acute kidney failure
- Acute tubular necrosis (tissue death)
Prerenal azotemia diagnosis
An examination may show:
- Collapsed neck veins
- Dry mucus membranes
- Little or no urine in the bladder
- Low blood pressure
- Low heart function or hypovolemia
- Poor skin turgor
- Rapid heart rate
- Reduced pulse pressure
- Signs of acute kidney failure
The following tests may be done:
- Blood creatinine
- Blood urea nitrogen (BUN)
- Urine osmolality and specific gravity
- Urine tests to check sodium and creatinine levels and to monitor kidney function
In prerenal azotemia, hemoconcentration results in elevation of the hematocrit and total protein/albumin, calcium, bicarbonate, and uric acid levels from baseline values.
Oliguria (urine volume < 500 mL/day) or anuria (< 100 mL/day), high urine specific gravity (>1.015), normal urinary sediment, and low urinary sodium (< 20 mEq/L; fractional excretion of sodium [FENa] < 1%) are seen.
When volume depletion is predominant, exaggerated proximal tubular reabsorption results in azotemia, hypernatremia, and elevated levels of calcium, uric acid, and bicarbonate, whereas hemoconcentration results in elevation of total protein, albumin, and hematocrit levels from baselines. When hypoperfusion due to decreased cardiac output or effective arterial volume is present, patients exhibit edema, hyponatremia, and hypoalbuminemia. Hematocrit and calcium, uric acid, and bicarbonate levels vary widely. These patients often are critically ill.
The fractional excretion of sodium (FENa) has traditionally been used to differentiate prerenal azotemia from acute tubular necrosis. An fractional excretion of sodium (FENa) below 1% suggests a prerenal cause (eg, volume depletion), whereas an fractional excretion of sodium (FENa) above 2% suggests acute tubular necrosis (ATN). Because the fractional excretion of sodium (FENa) is based on the fact that sodium reabsorption is enhanced in the setting of volume depletion, active use of diuretics may elevate the FENa even when volume depletion is present, yielding misleading values.
Alternatives to the FENa in this setting include the fractional excretion of urea or urea nitrogen (FEUrea) and the fractional excretion of uric acid (FEUA); excretion of urea and uric acid excretion is not influenced by diuretics. An fractional excretion of urea or urea nitrogen (FEUrea) below 35% or an fractional excretion of urea or urea nitrogen (FEUrea) below 9-10 % suggests a prerenal etiology of acute renal failure, whereas an FEUrea above 50% or an fractional excretion of uric acid (FEUA) above 10-12 % suggests ATN 9
Prerenal azotemia treatment
The main goal of treatment is to quickly correct the cause before the kidney becomes damaged. People often need to stay in the hospital.
Intravenous (IV) fluids, including blood or blood products, may be used to increase blood volume. After blood volume has been restored, medicines may be used to:
- Increase blood pressure
- Improve the pumping of the heart
If the person has symptoms of acute kidney failure, treatment will likely include:
- Dialysis
- Diet changes
- Medicines
Prerenal azotemia prognosis
Prerenal azotemia can be reversed if the cause can be found and corrected within 24 hours. If the cause is not fixed quickly, damage may occur to the kidney (acute tubular necrosis).
Intrarenal azotemia
Intrarenal azotemia, also known as acute renal failure, renal-renal azotemia, and acute kidney injury, refers to elevations in BUN and creatinine resulting from problems in the kidney itself. There are several definitions, including a rise in serum creatinine levels of about 30% from baseline or a sudden decline in output below 500 mL/day. If output is preserved, acute kidney injury is nonoliguric; if output falls below 500 mL/day, acute renal failure is oliguric. Any form of acute kidney injury may be so severe that it virtually stops formation; this condition is called anuria (< 100 mL/day) 10.
The most common causes of nonoliguric acute kidney injury are acute tubular necrosis (ATN), aminoglycoside nephrotoxicity, lithium toxicity, and cisplatin nephrotoxicity. Tubular damage is less severe than it is in oliguric acute kidney injury. Normal output in nonoliguric acute kidney injury does not reflect a normal GFR. Patients may still make 1440 mL/day of urine even when the GFR falls to about 1 mL/min because of decreased tubular reabsorption.
Some studies indicate that nonoliguric forms of acute kidney injury are associated with less morbidity and mortality than is oliguric acute kidney injury. Uncontrolled studies also suggest that volume expansion, potent diuretic agents, and renal vasodilators can convert oliguric acute kidney injury to nonoliguric acute kidney injury if administered early.
The pathophysiology of acute oliguric or nonoliguric acute kidney injury depends on the anatomic location of the injury. In acute tubular necrosis, epithelial damage leads to functional decline in the ability of the tubules to reabsorb salt, water, and other electrolytes. Excretion of acid and potassium is also impaired. In more severe acute tubular necrosis, the tubular lumen is filled with epithelial casts, causing intraluminal obstruction and resulting in a declining GFR.
Acute interstitial nephritis is characterized by inflammation and edema, which result in azotemia, hematuria, sterile pyuria, white blood cell (WBC) casts with variable eosinophiluria, proteinuria, and hyaline casts. The net effect is a loss of urinary concentrating ability, with low osmolality (< 500 mOsm/L), low specific gravity (< 1.015), high urinary sodium (>40 mEq/L), and, occasionally, hyperkalemia and renal tubular acidosis. However, if there is superimposed prerenal azotemia, the specific gravity, osmolality, and sodium may be misleading.
Glomerulonephritis or vasculitis is suggested by the presence of hematuria, red blood cells (RBCs), white blood cells, granular and cellular casts, and a variable degree of proteinuria. Nephrotic syndrome usually is not associated with active inflammation and often presents as proteinuria greater than 3.5 g/24 hours.
Glomerular diseases may reduce GFR by changing basement membrane permeability and stimulating the renin-aldosterone axis. Such diseases are often manifested as nephrotic or nephric syndrome. In nephrotic syndrome, the urinary sediment is inactive, and there is gross proteinuria (>3.5 g/day), hypoalbuminemia, hyperlipidemia, and edema. Azotemia and hypertension are uncommon initially, but their presence may indicate advanced disease.
Some patients with nephrotic syndrome may present with acute renal failure. Impairment of capillary circulation in the kidney due to edema (nephrosarca) and tubular obstruction from protein casts, as well as decreased effective circulating volume, have been proposed as potential mechanisms for the development of ARF in patients with nephrotic syndrome.
In nephritic syndrome, the urinary sediment is active with white blood cell or red blood cell casts, granular casts, and azotemia. Proteinuria is less obvious, but increased salt and water retention in glomerulonephritis can lead to hypertension, edema formation, decreased output, low urinary excretion of sodium, and increased specific gravity.
Acute vascular diseases include vasculitis syndromes, malignant hypertension, scleroderma renal crisis, and thromboembolic disease, all of which cause renal hypoperfusion and ischemia leading to azotemia. Chronic vascular diseases are due to hypertensive benign nephrosclerosis, which has not been conclusively associated with end-stage renal disease (ESRD) and ischemic renal disease from bilateral renal artery stenosis 11.
In bilateral renal artery stenosis, maintenance of adequate intraglomerular pressure for filtration greatly depends on efferent arteriolar vasoconstriction. Azotemia sets in when angiotensin-converting enzyme (ACE) inhibitors or angiotensin 2 receptor blockers (ARBs) cause efferent arteriolar dilatation, thereby decreasing intraglomerular pressure and filtration. Therefore, ACE inhibitors and angiotensin 2 receptor blockers (ARBs) are contraindicated in bilateral renal artery stenosis.
In addition to accumulation of urea creatinine and other waste products, a substantial reduction in GFR in chronic kidney disease results in the following:
- Decreased production of erythropoietin (causing anemia) and vitamin D-3 (causing hypocalcemia, secondary hyperparathyroidism, hyperphosphatemia, and renal osteodystrophy)
- Reduction in acid, potassium, salt, and water excretion (causing acidosis, hyperkalemia, hypertension, and edema)
- Platelet dysfunction (leading to increased bleeding tendencies)
The syndrome associated with the signs and symptoms of accumulation of toxic waste products (uremic toxins) is termed uremia and often occurs at a GFR of about 10 mL/min. Some of the uremic toxins (eg, urea, creatinine, phenols, and guanidines) have been identified, but none have been found responsible for all the manifestations of uremia.
Intrarenal azotemia causes
Intrarenal azotemia occurs as a result of injury to the glomeruli, tubules, interstitium, or small vessels. It may be acute oliguric, acute nonoliguric, or chronic. Systemic disease, nocturia, proteinuria, loss of urinary concentrating ability (low urine specific gravity), anemia, and hypocalcemia are suggestive of chronic intrarenal azotemia.
Intrarenal azotemia diagnosis
Anemia, thrombocytopenia, hypocalcemia, and high–anion gap metabolic acidosis may suggest intrarenal azotemia. Low urine specific gravity (< 1.015), active urinary sediment (see Pathophysiology), high urinary sodium (>40 mEq/L; fractional excretion of sodium (FENa) >5%), a plasma BUN–creatinine ratio of less than 20, and low urine osmolality may also suggest intrarenal azotemia.
In patients with long-standing chronic kidney disease, renal ultrasonography usually shows small, contracted kidneys. Some causes of chronic kidney disease can be associated with normal-sized or large kidneys, such as HIV nephropathy, diabetes, and renal amyloidosis. The renal sonogram usually is diagnostic for patients with polycystic kidney disease. In patients with active urinary sediment, progressive azotemia, proteinuria, or normal-sized kidneys on ultrasonography, a renal biopsy should be considered. Consultation with a nephrologist is imperative in all such patients.
Post renal azotemia
Postrenal azotemia refers to elevations in BUN and creatinine levels resulting from obstruction in the collecting system. Obstruction to flow leads to reversal of the Starling forces responsible for glomerular filtration. Progressive bilateral obstruction causes hydronephrosis with an increase in the Bowman capsular hydrostatic pressure and tubular blockage that leads to progressive decline in and ultimate cessation of glomerular filtration, azotemia, acidosis, fluid overload, and hyperkalemia.
Unilateral obstruction rarely causes azotemia. There is evidence that if complete ureteral obstruction is relieved within 48 hours of onset, relatively complete recovery of GFR can be achieved within a week; little or no further recovery occurs after 12 weeks. Complete or prolonged partial obstruction can lead to tubular atrophy and irreversible renal fibrosis. Hydronephrosis may be absent if obstruction is mild or acute or if the collecting system is encased by retroperitoneal tumor or fibrosis.
Postrenal azotemia causes
Postrenal azotemia occurs when an obstruction to urine flow is present. It is observed in bilateral ureteral obstruction from tumors or stones, retroperitoneal fibrosis, neurogenic bladder, and bladder neck obstruction from prostatic hypertrophy or carcinoma and posterior urethral valves. It may be superimposed on a background of chronic renal failure.
Postrenal azotemia diagnosis
Urinary indices in postrenal azotemia due to complete bilateral obstruction are usually nondiagnostic. The prima facie finding here is anuria, occasionally accompanied by hypertension. Urine output still may be present if overflow (in bladder outlet obstruction) or partial ureteral obstruction is present.
A Foley catheter should be inserted as part of the initial evaluation to rule out obstruction below the bladder outlet. Unilateral ureteral obstruction rarely leads to azotemia; it occurs acutely (as a result of obstruction from calculi, papillary necrosis, or hematoma), producing renal colic, or may be chronic and asymptomatic, producing hydronephrosis.
Bilateral partial obstruction may be associated with azotemia in the presence of normal urine output. When patients are subjected to maneuvers that increase urinary flow (eg, diuretic renography or perfusion pressure flow studies), they may exhibit an increase in size or pressure of the collecting system or experience pain.
In addition to azotemia, polyuria due to loss of concentrating ability and type 1 renal tubular acidosis, with hyperkalemia, hypercalcemia from a metastatic pelvic tumor, and elevated prostate-specific antigen (PSA) levels, may be clues to postrenal azotemia. Hydronephrosis in the absence of hydroureter may be seen in early (< 3 days) obstruction, retroperitoneal process, or partial obstruction.
Renal ultrasonography (see below) is the test of choice for ruling out obstructive uropathy. If the renal sonogram is equivocal, a furosemide (Lasix) washout scan (see Radionuclide Studies) should be performed.
Azotemia causes
Prerenal azotemia occurs as a consequence of impaired renal blood flow or decreased perfusion resulting from decreased blood volume, decreased cardiac output (congestive heart failure), decreased systemic vascular resistance, decreased effective arterial volume from sepsis or hepatorenal syndrome 7 or renal artery abnormalities. It may be superimposed on a background of chronic renal failure. Iatrogenic factors, such as excessive diuresis and treatment with ACE inhibitors, should be ruled out.
Intrarenal azotemia occurs as a result of injury to the glomeruli, tubules, interstitium, or small vessels. It may be acute oliguric, acute nonoliguric, or chronic. Systemic disease, nocturia, proteinuria, loss of urinary concentrating ability (low urine specific gravity), anemia, and hypocalcemia are suggestive of chronic intrarenal azotemia.
Postrenal azotemia occurs when an obstruction to urine flow is present. It is observed in bilateral ureteral obstruction from tumors or stones, retroperitoneal fibrosis, neurogenic bladder, and bladder neck obstruction from prostatic hypertrophy or carcinoma and posterior urethral valves. It may be superimposed on a background of chronic renal failure.
Azotemia symptoms
- Oliguria or anuria (decreased or absent urine output)
- Fatigue
- Asterixis (flapping tremor)
- Decreased alertness
- Confusion
- Pale skin
- Tachycardia (rapid pulse)
- Xerostomia (dry mouth)
- Thirst
- Edema, anasarca (swelling)
- Orthostatic blood pressure (fluctuates depending on body position)
- Uremic frost, a condition that occurs when urea and urea derivatives are secreted through the skin in sweat, which evaporates away to leave solid uric compounds, resembling a frost.
Azotemia diagnosis
It is necessary to quickly establish if azotemia is acute or chronic and whether it is due to prerenal, intrarenal, or postrenal causes. This is vital in initiating treatment and in preventing progression. Clinical evaluation requires a thorough history, physical examination, and specific laboratory tests (including serologies, urinalysis, and, if indicated, radiologic studies and kidney biopsy; see Workup).
Patients with prerenal azotemia commonly have a history of diarrhea, vomiting, profound heat exhaustion, excessive sweat loss, concurrent illness that impairs their ability to eat and drink adequately, hemorrhage, liver disease, congestive heart failure, and polyuria (eg, caused by lithium intoxication, diuretics, diabetes, or diabetes insipidus).
Patients with intrarenal azotemia may have a history of nocturia, polyuria, proteinuria, shock, and edema. There may be a personal or family history of congenital or systemic diseases, especially diabetes, hypertension, systemic lupus erythematosus (SLE), other collagen vascular diseases, hepatitis B (HBV), hepatitis C (HCV), syphilis, multiple myeloma, and AIDS.
Obtain a detailed medication history, looking for nephrotoxic medications (especially antibiotics, nonsteroidal anti-inflammatory drugs [NSAIDs], angiotensin-converting enzyme [ACE] inhibitors, diuretics, and herbal remedies), chemical exposure, and intravenous (IV) drug abuse (associated with exposure to HIV, HBV, and HCV infections).
Patients with postrenal azotemia frequently have a history of renal colic, dysuria, frequency, hesitancy, urgency incontinence, pelvic malignancy or irradiation, or benign prostatic hypertrophy.
Assessment of Renal Function
There are a number of clinical laboratory tests that are useful in investigating and evaluating kidney function. Clinically, the most practical tests to assess renal function is to get an estimate of the glomerular filtration rate (GFR) and to check for proteinuria (albuminuria).
Glomerular Filtration Rate
The best overall indicator of the glomerular function is the glomerular filtration rate (GFR). The normal GFR for an adult male is 105 to 125 mL per minute. GFR is the rate in milliliters per minutes at which substances in plasma are filtered through the glomerulus, in other words, the clearance of a substance from the blood.
Creatinine
The most commonly used endogenous marker for assessment of glomerular function is creatinine. The calculated clearance of creatinine is used to provide an indicator of GFR. This involves the collection of urine over a 24-hour period or preferably over an accurately timed period of 5 to 8 hours since 24-hour collections are notoriously unreliable. Creatinine clearance is then calculated using the equation:
C = (U x V) / P
C = clearance, U = urinary concentration, V = urinary flow rate (volume/time ie ml/min), and P = plasma concentration
Creatinine clearance should be corrected for body surface area. Improper or incomplete urine collection is one of the major issues affecting the accuracy of this test, hence timed collection is advantageous. Furthermore, due to tubular secretion, creatinine overestimates GFR by around 10% to 20%.
Creatinine is the by-product of creatine phosphate in muscle, and it is produced at a constant rate by the body. For the most part, creatinine is cleared from the blood entirely by the kidney. Decreased clearance by kidney results in an increased blood creatinine. The amount of creatinine produced per day depends on muscle bulk, and thus, there is a difference in creatinine ranges between males and females with lower creatinine values in children and those with decreased muscle bulk. Diet also influences creatinine values. Creatinine can change as much as 30% after ingestion of red meat. As GFR increases in pregnancy lower creatinine values are found in pregnancy. Additionally, serum creatinine is a later indicator of renal impairment-renal function is decreased by 50% before a rise in serum creatinine is observed.
Serum creatinine is also utilized in GFR estimating equations such as the Modified Diet in Renal Disease (MDRD) and the CKD-EPI equation. These eGFR equations are superior to serum creatinine alone since they include race, age, and gender variables. GFR is classified into the following stages based on the kidney disease.
Improving Global Outcomes (KDIGO) stages of chronic kidney disease (CKD):
- Stage 1 GFR greater than 90 ml/min/1.73 m
- Stage 2 GFR-between 60 to 89 ml/min/1.73 m
- Stage 3a GFR 45 to 59 ml/min/1.73 m
- Stage 3b GFR 30 to 44 ml/min/1.73 m
- Stage 4 GFR of 15 to 29 ml/min/1.73 m
- Stage 5-GFR less than 15 ml/min/1.73 m (end-stage renal disease)
These provide an easier estimation of GFR without collection of urine or use of exogenous materials. However, as they utilize serum creatinine, they are also affected by the issues around serum creatinine measurement, hence the correction for race, gender, and age.
Blood Urea Nitrogen (BUN)
Urea or BUN is a nitrogen-containing compound formed in the liver as the end product or protein metabolism and urea cycle. About 85% of urea is eliminated via kidneys; the rest is excreted via the gastrointestinal (GI) tract. Serum urea is increased in conditions where renal clearance decreased (in acute and chronic renal failure/impairment). Urea may also increase in other conditions not related to renal diseases such as upper GI bleeding, dehydration, catabolic states, and high protein diets. Urea may be decreased in starvation, low-protein diet, and severe liver disease. Serum creatinine is a more accurate assessment of renal function than urea; however, urea is increased earlier in renal disease.
The ratio of BUN:
Creatinine can be useful to differentiate prerenal from renal causes when the BUN is increased. In pre-renal disease the ratio is close to 20:1, while in intrinsic renal disease it is closer to 10:1.
Cystatin C
Cystatin C is a low-molecular-weight protein which functions as a protease inhibitor produced by all nucleated cells in the body. It is formed at a constant rate and freely filtered by the kidneys. Serum levels of cystatin C are inversely correlated with the glomerular filtration rate (GFR). In other words, high values indicate low GFRs, while lower values indicate higher GFRs, similar to creatinine. The renal handling of cystatin C differs from creatinine. While both are freely filtered by glomeruli, once cystatin C is filtered, it is reabsorbed and metabolized by proximal renal tubules, unlike creatinine. Thus, under normal conditions, cystatin C does not enter the final excreted urine to any significant degree. Cystatin C is measured in serum and urine. The advantages of cystatin C over creatinine are that it is not affected by age, muscle bulk, or diet, and various reports have indicated that it is a more reliable marker of GFR than creatinine particularly in early renal impairment. Cystatin C has also be incorporated into eGFR equations such as the combined creatinine-cystatin KDIGO CKD-EPI equation.
Cystatin C concentration may be affected by the presence of cancer, thyroid disease, and smoking.
Albuminuria and Proteinuria
Albuminuria refers to the presence of urine albumin 30 to 300 mg per day. Microalbumin, considered an obsolete term as there is no such biochemical molecule, is now referred to simply as urine albumin. Albuminuria is used as a marker for detection of incipient nephropathy in diabetics; it is an independent marker for the cardiovascular disease since it connoted increased endothelial permeability and is also a marker of chronic renal impairment. Urine albumin may be measured in 24-hour urine collections or early morning/random specimens as an albumin/creatinine ratio. Presence of albuminuria on two occasions with the exclusion of a urinary infection indicates glomerular dysfunction. The presence of albuminuria for 3 or more months is indicative of chronic kidney disease. Frank proteinuria is defined as greater than 300 mg per day of protein. Normal urine protein up to 150 mg per day (30% albumin; 30% globulins; 40% Tamm Horsfall protein). Increased amounts of protein in urine may be due to:
- Glomerular proteinuria: Caused by defects in perm selectivity of the glomerular filtration barrier to plasma proteins (for example, glomerulonephritis or nephrotic syndrome)
- Tubular proteinuria: Caused by incomplete tubular reabsorption of proteins (for example, interstitial nephritis)
- Overflow proteinuria: Caused by increased plasma concentration of proteins (for example, multiple myeloma-Bence Jones protein, myoglobinuria)
- Urinary tract inflammation or tumor
Urine protein may be measured using either a 24-hour urine collection or random urine protein: creatinine ratio (early morning sample preferred and more representative of the 24-hour sample).
The KDIGO classification defines 3 stages of albuminuria:
- A1: Less than 30 mg/g creatinine
- A2: 30 to 300 mg/g creatinine
- A3: Greater than 300 mg/g creatinine
In nephrotic syndrome, urine protein excretion exceeds 3.5 g per day and is associated with edema, hypoalbuminemia, and hypercholesterolemia.
Tests of Tubular Function
The renal tubules play an important role in reabsorption of electrolytes, water, and maintaining acid-base balance. Electrolytes, sodium, potassium, chloride, magnesium, phosphate can be measured in urine as well as glucose. Measurement of urine osmolality allows for assessment of concentrating ability of urine tubules. A urinary osmolality greater than 750 mOsmol/Kg H2O implies a normal concentrating ability of tubules. A water deprivation test can be used to exclude nephrogenic diabetes insipidus. Also in distal renal tubular acidosis (dRTA), an ammonium chloride test can be used to confirm the diagnosis of distal RTA with failure to acidify the urine to a pH less than 5.3. In Fanconi’s syndrome, there is aminoaciduria, glycosuria, and phosphaturia and bicarbonate wasting (proximal RTA).
Urine Analysis
Urine analysis involves assessment of urine characteristics to aid in disease diagnosis and consists of physical observation, chemical, and microscopic analysis. Physical observation involves assessing color and clarity. The normal color of urine is straw colored in the presence of dehydration urine is a darker color. Red urine may indicate hematuria or porphyria or represent the dietary intake of food like beets. Cloudy urine may be seen in the presence of pyuria due to urinary tract infection. Specific gravity is an indicator of renal concentrating ability may be measured using refractometry or chemically by use of urine dipstick. The physiologic range for specific gravity is 1.003 to 1.030 and is increased with concentrated urine and decreased with dilute urine.
Urine dipstick provides qualitative analysis of different analytes in urine using chemical analysis.
Dipstick uses dry chemistry methods to detect for the presence of protein, glucose, blood, ketones, bilirubin, urobilinogen, nitrite, and leukocyte esterase. These may be performed as a point-of-care test near a patient. The color changes following interaction of the urine with the chemical reagents impregnated on the paper of the dipstick are compared to the color chart guide to interpret the results.
Analytes tested on urine dipstick-protein should not be detectable in normal urine specimens. Bilirubin is not detected in normal urine. Glucose is not detected in healthy patients but may be seen in diabetes mellitus, pregnancy, and renal glycosuria when the renal threshold of 180 mg/dl is decreased. The presence of ascorbic acid (vitamin C) and some antibiotics may affect results. Blood may be present after renal tract injury or infection, with ascorbic acid causing a falsely negative result. Urine dipstick detects the globin portion of hemoglobin, and thus cannot detect the difference between the presence of myoglobin or hemoglobin in urine. Additionally, both intact red blood cells (RBC) and hemoglobinuria are detected. In normal urine RBC per high-power field is between 0 to 3 and white blood cells (WBC) between 0 to 5. Ketones are present in fasting, severe vomiting, or diabetic ketoacidosis. Urine dipstick only detects acetoacetate and acetone, not the ketone beta-hydroxybutyrate. Bilirubin is detected in the presence of conjugated hyperbilirubinemia, urobilinogen may normally be present but is absent in conjugated hyperbilirubinemia and increased in the presence of prehepatic jaundice and hemolysis. Nitrite and leucocyte esterase are indicators of urinary tract infection. Some bacteria, for example, Enterobacteriaceae, convert nitrates to nitrites.
The microscopic analysis involves wet-prep analysis of urine to assess in the presence of cells, casts, and crystals as well as micro-organisms. Red blood casts usually denote glomerulonephritis while white blood cell casts are consistent with pyelonephritis. Presence of white blood cells and WBC casts indicates infection; red blood cells indicate renal injury; RBC casts indicate tubular damage or glomerulonephritis. Hyaline casts consist of protein and may occur in glomerular disease. Crystals may also be identified in urine and are indicative of the following conditions:
- Triple phosphate crystals are rectangular are seen in pseudogout.
- Uric acid crystals are needle-shaped and are associated with gout.
- Oxalate crystals are envelope shaped and are present in ethylene glycol poisoning or primary and secondary hyperoxaluria.
- Cystine crystals are hexagonal and observed in cystinuria.
The best specimen for urine analysis is a freshly voided midstream urine. Midstream urine is utilized as it is less likely to be contaminated by commensal bacteria and epithelial cells.
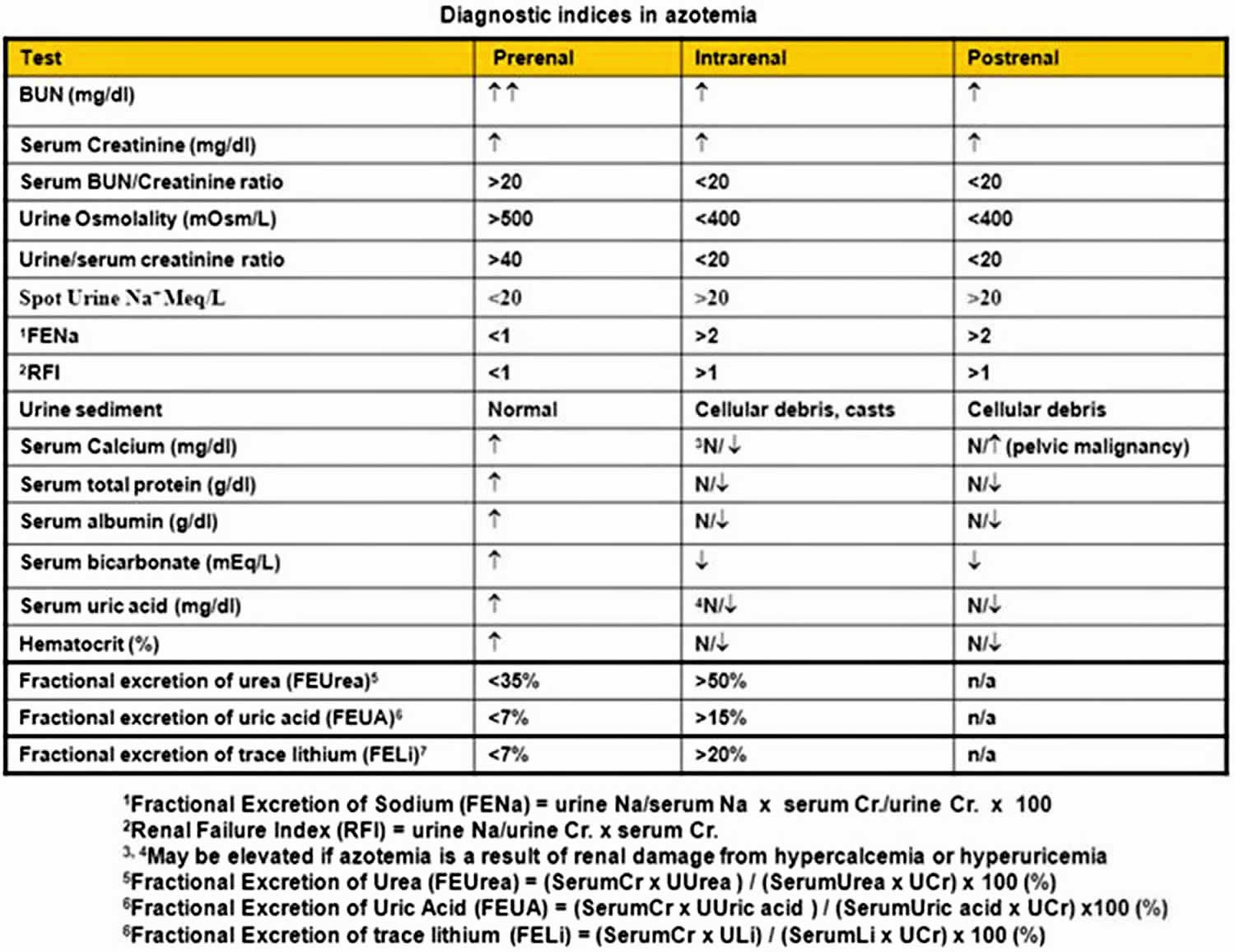
Table 1. Diagnostic indices in azotemia
Footnote: Diagnostic indices in azotemia. Although such indices are helpful, it is not necessary to perform all these tests on every patient. Comparison should always be made with patients’ baseline values to identify trends consistent with increase or decrease in effective circulating volume. Use of some of these indices may be limited in certain clinical conditions, such as anemia (hematocrit), hypocalcemia (serum calcium), decreased muscle mass (serum creatinine), liver disease (blood urea nitrogen [BUN], total protein, and albumin), poor nutritional state (BUN, total protein, and albumin), and use of diuretics (urine sodium). Fractional excretion of urea and fractional excretion of trace lithium appear to be superior for assessing prerenal status in patients on diuretics.
Ultrasonography
Renal ultrasonography is the most commonly used renal imaging study because of its ease of use and broad applicability for the following purposes 12:
- Determination of renal size and echogenicity, which is important when considering renal biopsy; small echogenic kidneys (< 9 cm) may suggest scarring from advanced renal disease, whereas normal or large kidneys with smooth contours may indicate a potentially reversible process
- Differentiation of cystic lesions from solid lesions
- Diagnosis of urinary tract obstruction (for which it is the test of choice)
- Detection of kidney stones
Doppler renal ultrasonography can be used to evaluate renal vascular flow (eg, for identification of renal vein thrombosis, renal infarction, or renal artery stenosis).
Computed Tomography and Magnetic Resonance Imaging
Computed tomography (CT) 13 is complementary to ultrasonography, especially when the diagnosis is uncertain. Contrast nephrotoxicity should be weighed against the benefits. CT is used for the following purposes:
- Differentiation of neoplastic lesions from simple cysts (in most cases)
- Radiologic diagnosis of renal stone disease, including radiolucent stones
- Evaluation and staging of renal cell carcinoma
- Diagnosis of renal vein thrombosis
- Diagnosis of polycystic kidney disease; it is more sensitive than ultrasonography for this task, particularly in younger patients
Knipp et al 14 describe successful use of a technique for computed tomographic angiography (CTA) of the abdomen and pelvis in azotemic patients that uses a reduced iodinated contrast volume and low kilovolt (peak) [80-kV(p)] with iterative reconstruction. Their retrospective study in 103 patients with end-stage renal disease found that this technique allows for satisfactory abdominal/pelvic CTA with a 50% reduction in contrast volume and a 43% mean radiation dose reduction, , compared with a standard 120-kV(p) CTA protocol.
Magnetic resonance imaging (MRI) or magnetic resonance angiography (MRA) is used only when CT and ultrasonography are nondiagnostic. These modalities are standard for diagnosis of renal vein thrombosis and are also used in the evaluation of renal cell carcinoma and renal artery stenosis or vasculitis.
Abdominal Radiography, Pyelography, and Angiography
If symptoms suggest nephrolithiasis, a plain film of the abdomen is performed to screen for presence of a radiopaque stone. Calcium-containing, struvite, and cystine stones can be identified, but radiolucent ones, such as uric acid stones, will be missed.
Intravenous pyelography (IVP) can provide detailed information concerning calyceal anatomy and the size and shape of the kidney. It is extremely useful for detecting renal stones. IVP is the preferred technique for evaluation and diagnosis of certain structural disorders (eg, chronic pyelonephritis, medullary sponge kidney, and papillary necrosis). It can provide data on the degree of obstruction. The risk of contrast nephrotoxicity should be weighed against the benefits of making a diagnosis that will not change management.
Retrograde or anterograde pyelography is of limited usefulness now that renal ultrasonography is more widely available. It may be used in patients with a high index of suspicion for hydronephrosis in whom sonograms appear normal, such as those with retroperitoneal fibrosis.
Renal arteriography is used in polyarteritis nodosa and renal artery stenosis to demonstrate multiple aneurysms or stenoses. Because of the availability of procedures that do not require contrast material (eg, ultrasonography, MRI, and MRA) do not carry a risk of contrast nephrotoxicity, this test is less commonly used than it once was.
Renal venography is the standard for diagnosis of renal vein thrombosis. However, there is a risk of contrast nephrotoxicity.
Radionuclide Studies
Technetium-99m dimercaptosuccinic acid (99m Tc DMSA) is heavily distributed within the renal parenchyma at first pass and so is best for detecting renal parenchymal scarring.
Technetium diethylenetriamine pentaacetic acid (99m Tc DTPA) is heavily filtered at first pass and therefore is best for qualitative assessment of renal function (filtration and excretion). Because it is heavily filtered, it is most sensitive in detecting urine leaks after renal transplant. For the same reason, it is also used concomitantly with a furosemide washout scan (see below) for assessing functional obstruction of the collecting system.
Mercaptoacetyltriglycine (MAG3) is evenly distributed at first pass in the kidney and so is best for qualitative assessment of perfusion, filtration, and excretion. It is the preferred test for assessing the 3 aspects of function after renal transplantation. It can be used to detect urine leaks or functional obstruction with furosemide, though99m Tc DTPA scanning remains the test of choice for these conditions. Voiding cystourethrography can be performed with a radionuclide study to detect vesicoureteral reflux.
In a furosemide washout scan, the renal scan usually is performed first. Then, if needed, the furosemide washout is done after the radionuclide has accumulated in the collecting system. Furosemide is used as a part of the renogram to separate nonobstructive hydronephrosis from obstructive hydronephrosis. If there is no obstruction, furosemide-induced flow containing little or no radionuclide will fill the collecting system, washing out radionuclide-containing urine. If obstruction is present, the radionuclide is not washed out as quickly.
The half-life or clearance of the radioisotope is plotted on a curve. A half-life shorter than 10 minutes is considered normal, one longer than 20 minutes is considered obstruction, and one of between 10-20 minutes is subject to further interpretation.
Conditions that can make it difficult to interpret the furosemide washout curve include a megaureter or pelvis that accepts a large bolus of urine and poor renal function. In patients with a megaureter, it can be difficult to determine when the renal pelvis is full, and in patients with renal disease, the onset of furosemide action may be delayed. To overcome the problem of poor renal function or relative hypovolemia if a patient has been fasting, the patient should be well hydrated with intravenous (IV) fluids before the study.
The test also is operator dependent, in that the furosemide should be administered at a time when the renal pelvis is believed to be full. A full bladder also delays washout of isotope. Therefore, the patient’s bladder must be catheterized before the study can be performed.
Renal Biopsy
When glomerulonephritis, vasculitis, and (occasionally) interstitial nephritis are suspected, renal biopsy is indicated to establish the correct diagnosis and guide therapy. The following are common indications for renal biopsy:
- Isolated glomerular proteinuria or hematuria
- Nephrotic syndrome
- Acute nephritic syndrome
- Unexplained acute or subacute renal failure
Percutaneous renal biopsy is associated with potential complications. Severe bleeding causing hypotension occurs in 1-2% of patients. Bleeding necessitating transfusion occurs in about 0.1-0.3% of patients. Bleeding complications can be minimized by using data obtained from tests for bleeding time, prothrombin time (PT), partial thromboplastin time (PTT), and platelet count.
Nonsteroidal anti-inflammatory drugs (NSAIDs) should be stopped at least 1 week before a scheduled elective biopsy. Patients on warfarin should be started on heparin at least 3 days before renal biopsy. Patients who are taking heparin for other reasons should stop the drug for at least 1 day.
Contraindications for percutaneous renal biopsy include the following:
- Uncorrectable bleeding diathesis
- Small kidneys
- Severe hypertension
- Multiple bilateral cysts or renal tumor
- Hydronephrosis
- Active renal or perirenal infection
- Uncooperative patient
Percutaneous biopsy may be performed in selected patients with a solitary kidney because of the generally low risk of bleeding. Open renal biopsy may be performed if a percutaneous attempt is either unsuccessful or contraindicated and if the benefits of diagnosis outweigh the risks. When percutaneous biopsy is contraindicated but a diagnosis is necessary, a transvenous transjugular renal core biopsy can be performed 15. With this approach, bleeding occurs intravascularly, thereby reducing the risk of hematoma.
Acute versus Chronic Renal Impairment
Acute renal impairment or acute kidney injury refers to the sudden onset of kidney injury within a period of a few hours or days. Chronic kidney disease (CKD) is caused by long-term diseases such as hypertension and diabetes. Causes of acute kidney injury can be divided into The following:
- Causes that result in decreased blood flow to the kidneys (pre-renal causes), for example, hypotensive and cardiogenic shock, dehydration, and blood loss from major trauma
- Causes that result in direct damage to the kidneys (renal /intrinsic causes) such as damage to kidneys by nephrotoxic medication and other toxins, sepsis, cancers such as myeloma, autoimmune diseases or conditions that cause inflammation, or damage to the kidney tubules
- Causes that result in blockage of the urinary tract such as bladder, prostate, or cervical cancer, large kidney stones, and blood clots in the urinary tract
It is important to note that pre-renal kidney injury may progress to acute tubular necrosis and cause intrinsic renal injury.
Urine output is a good tool for evaluating kidney function and is used in guidelines to define (acute kidney injury). Patients with acute kidney injury present with oliguria (less than 400 ml per day). The RIFLE classification of (risk, injury, failure, loss of kidney function, and end-stage kidney disease) is based on serum creatinine, GFR changes, and urine output determinants. The Acute Kidney Injury Network classification criteria for acute kidney injury also uses serum creatinine changes and urine output; however, it does not rely on GFR changes and does not require a baseline serum creatinine.
Other laboratory investigations apart from serum creatinine play a key role in the diagnosis of acute kidney injury and assist in differentiating between different types of acute kidney injury. This is important, as it will determine the appropriate patient management, with patients that have pre-renal causes being treated with fluid replacement, while those with renal and post-renal causes would be given fluids more conservatively.
Investigations that assist in determining if the renal injury is pre-renal, renal, or post-renal include the measurement of urine specific gravity increased (greater than 1.020) in dehydration and pre-renal causes. The presence of white and red blood cells, tubular epithelial cells, casts, or crystals in the urinary sediment under light microscopy can assist in the differential diagnosis.
Fractional excretion of sodium (FeNa) is useful in distinguishing acute tubular necrosis from pre-renal uremia. It requires the measurement of serum creatinine and sodium and measurement of creatinine and sodium in spot urine specimens. Fractional excretion is calculated using the following formula: FeNa = 100 x ( urinary sodium x serum creatinine) / (serum sodium x urinary creatinine). A value less than 1% indicates a pre-renal cause and values greater than 2% indicate intrinsic causes. However, in patients receiving diuretic therapy, the FeNa is not reliable. Spot urine sodium concentrations of less than 20 mmol/l are an indicator of pre-renal acute kidney injury. Fractional excretion of urea calculated similarly to FeNa using serum urea and urine urea instead of sodium can also be used to determine the presence of prerenal versus intrinsic acute kidney injury, with values less than 35% suggesting pre-renal injury. A urine osmolality of greater than 500 mOsm/Kg is associated with pre-renal causes, while an osmolality similar to serum (approximately 300 mOsm/kg) reflects an intrinsic cause.
Novel Biomarkers
Several new biomarkers have been reported to be useful for the determination of acute kidney injury and have utility in differentiation between acute kidney injury and stable chronic kidney disease and pre-renal and intrinsic acute kidney injury. These include low-molecular-weight proteins, which are present in the systemic circulation and undergo glomerular filtration (for example, cystatin C, beta2-microglobulin, and retinol binding protein) and proteins that are produced in response to cellular/tissue injury (NGAL , Kidney injury molecule 1 (KIM-1), L-type fatty acid-binding protein (L-FABP), FGF23, and beta-trace protein). Their optimum clinical utility will be realized with ongoing studies.
Azotemia treatment
The main goal of treatment is to quickly correct the cause before the kidney becomes damaged. People often need to stay in the hospital. Prompt treatment of some causes of azotemia can result in restoration of kidney function; delayed treatment may result in permanent loss of renal function.
Intravenous (IV) fluids, including blood or blood products, may be used to increase blood volume. After blood volume has been restored, medicines may be used to:
- Increase blood pressure
- Improve the pumping of the heart
- Treat the condition that caused the azotemia
If the person has symptoms of acute kidney failure, treatment will likely include:
- Hemodialysis
- Diet changes
- Medicines to increase renal perfusion and to maintain urine output. Drugs used in the management of patients with azotemia include diuretics, adrenergic agents, plasma volume expanders, and corticosteroids.
Prerenal azotemia
If volume depletion is due to free water loss, the serum sodium is often elevated by 10 mEq/L from baseline. The amount of fluid replacement in liters—that is, the free water deficit—can be estimated from serum sodium (mg/dL) and patient weight (kg) as follows:
[(Na/140) – 1] × 0.5 × weightThe volume of fluid to be administered is equal to the sum of the free-water deficit and daily maintenance fluids. Fifty percent of this total volume should be administered in the first 24 hours, and a new calculation should be performed at 24 hours based on new laboratory results.
Maintenance fluid can be roughly estimated at 1.5-2 L/day; however, it can also be estimated from caloric intake since 1 kCal requires 1 mL of water in the metabolic process. Normal caloric intake is about 30 Kcal/kg (low catabolic state requires < 30 kCal/kg and high catabolic state requires >40 kCal/kg). A 70-Kg person at normal caloric intake requires 2100 Kcal/day or 2.1 L of fluid intake. This volume should be added to the free-water deficit and administered as noted above.
Alternatively, the total free water deficit is usually quite close to the sum of 50% free-water deficit and daily maintenance fluids. Therefore, for all practical purposes, the total free-water deficit can be administered intravenously in 24 hours.
The fluid to be administered should consist of hypotonic solutions such as 0.5% saline or 5% dextrose in water. Alert patients should be encouraged to drink as much free water as they can tolerate; otherwise, free water can be administered via a nasogastric tube.
Serum sodium should be measured every 6-8 hours, and fluid replacement should be adjusted to avoid a precipitous decline in the serum sodium. To prevent brain edema, the rate of decrease in serum sodium should be no more than 0.7 mEq/h (17 mEq/24 h). Volume depletion due to blood loss requires IV saline and transfusion to maintain pressure (as well as interventions to halt further loss).
Diarrhea often causes isotonic volume loss that necessitates replacement with normal saline. Normal–anion gap metabolic acidosis occurring with diarrhea warrants infusion of bicarbonate in 0.5% normal saline.
Diuretic-induced volume depletion, especially in the elderly, manifests as dehydration, hyponatremia 16 and, occasionally, hypokalemia. The treatment of choice consists of normal saline infusion and correction of hypokalemia.
Decreased cardiac output requires optimization of cardiac performance through careful use of diuretics, an angiotensin-converting enzyme (ACE) inhibitor, beta blockers, nitrates, positive inotropic agents (including dobutamine), and, when indicated, specific therapy for the cause of impaired cardiac function.
When ACE inhibitors are contraindicated because of hyperkalemia, the combination of nitrates and hydralazine offers an alternative. Because these patients tend to have risk factors for macrovascular disease, the diagnosis of ischemic nephropathy or atheroembolic disease should be entertained when renal function continues to worsen despite optimization of cardiac function.
Reduced effective arterial volume due to systemic shunting can result from sepsis or liver failure. Severe edema, hyponatremia, and hypoalbuminemia often pose management problems. Decreased oncotic pressure, increased vascular permeability, and exaggerated salt and water retention shift the Starling forces toward formation of interstitial fluid. Effective treatment of sepsis with antibiotics and of hypotension with dopamine and norepinephrine is mandated. Crystalloid replacement can be tried, but it often leads to more edema.
In severely hypoalbuminemic patients, salt-poor albumin infusion may be undertaken, but there is no conclusive evidence of benefit.
Adequate nutrition and effective treatment of sepsis may improve oncotic pressure and normalize vascular permeability, thereby decreasing the systemic shunting. The net result is improved renal perfusion, decreased salt and water retention, improved output, and edema. In hepatorenal syndrome, the average survival is 1-2 weeks; however, there is evidence that the kidneys will recover with early liver transplantation. Occasionally, renal function is advanced, necessitating replacement therapy.
Intrarenal azotemia
Acute kidney injury (acute renal failure)
For ischemic or nephrotoxic acute tubular necrosis (ATN) due to shock (hypovolemic, cardiogenic, septic), the initial approach is to restore volume and pressure (with fluid replacement and vasopressors, respectively) and to withdraw any nephrotoxic drugs 17 . If the patient becomes oliguric or anuric from shock, volume in the form of crystalloids should be aggressively administered as boluses (eg, 300 mL every 2 hours, rather than 150 mL every hour). Bolus infusion leads to acute intravascular volume expansion, release of atrial natriuretic peptide from the heart, increased renal blood flow, and natriuresis, all of which are favorable in recovery from acute tubular necrosis compared with slow intravenous hydration.
If at least 2 L of fluids has been administered in a relatively short period (approximately 12 hours) with no improvement in urine output, a trial of high-dose intravenous furosemide at 100-160 mg can be tried, prior to preparation for renal replacement. This approach, called “tank and blast” in shock, is clinically useful but almost no evidence supports it. In one small study, hemodynamic and renal support with a continuous infusion of noradrenaline (0.06-0.12 microg/kg/min) and furosemide (10-30 mg/hr) induced polyuria and reversed acute tubular necrosis to nonoliguric acute renal failure in 11 of 14 cancer patients who had severe sepsis and multiorgan dysfunction syndrome 18. If the patient does not respond within 6 hours of this approach, dialysis or continuous renal replacement therapy should be considered as soon as possible.
If the patient responds by restoration of urine output to greater than 30 mL/h, continue on the appropriate amounts of intravenous fluids, vasopressors, and as-needed diuretics to keep the patient at the desired fluid balance (negative, positive, or match intake to output).
This approach is not indicated in nonshock patients with acute kidney injury. Nonshock patients with acute kidney injury require maintenance fluids, if needed, and avoidance of nephrotoxicity.
In both scenarios, early initiation of renal replacement therapy if azotemia sets in has a better prognosis than late initiation.
Albumin can be administered in combination with high-dose furosemide to enhance the diuretic effect of furosemide. The use of albumin in this context is not for volume expansion; rather, it allows more furosemide to be bound to albumin for delivery to the organic anion transporter in the kidney, thereby enabling more furosemide to enter the tubule than would otherwise do so.
Although this approach is widely used, research on the combination of albumin and a loop diuretic has principally studied its use for improving diuretic-resistant edema in patients with nephrotic syndrome 19. Other therapies that have not been conclusively shown to be beneficial are renal-dose dopamine and synthetic atrial natriuretic peptide.
The renal failure phase usually lasts 7-21 days if the primary insult can be corrected. Postischemic polyuria can be seen in the recovery phase and represents an attempt to excrete excess water and solute. Saline may be replaced (75% of output) as a maintenance fluid, owing to salt wasting during this phase, and to allow the patient to lose excess water retained while the patient was oliguric. Hypokalemia may result from the saline diuresis, and potassium should be replaced. Recovery is marked by the return of blood urea nitrogen (BUN) and creatinine levels to near-baseline values.
Acute interstitial nephritis is managed by withdrawing the offending nephrotoxin, avoiding further nephrotoxic exposure, and dehydration. The creatinine level begins to improve within 3-5 days. Renal biopsy may be indicated if renal failure is severe or azotemia is not improving.
Once the diagnosis is confirmed, a trial of oral prednisone (starting at 1 mg/kg/day and tapering over 6 weeks) or IV pulse methylprednisolone (1 g for 3 days) in severe cases may be considered. If the patient is a poor candidate for biopsy but the diagnosis is strongly suspected, therapy should be started.
Contrast-induced azotemia, which typically becomes evident 3-5 days after exposure, is best prevented by adequate hydration with half-normal saline at 1 mL/kg/h 12 hours before contrast administration and the use of smaller amounts of contrast. Clearly explain the risks of such procedures to the patient.
The benefits of N-acetylcysteine and sodium bicarbonate for prevention of contrast-induced azotemia are still being debated 20. A systematic review and meta-analysis 21 of prevention strategies found that the greatest clinically and statistically significant reduction in contrast-induced nephropathy occurred with N-acetylcysteine in patients receiving low-osmolar contrast media (compared with IV saline) and with statins plus N-acetylcysteine (compared with N-acetlycysteine alone).
The Prevention of Serious Adverse Events Following Angiography (PRESERVE) trial 22, which included 5177 patients at high risk for renal complications who were undergoing angiography, found no benefit of IV sodium bicarbonate over IV saline or of oral acetylcysteine over placebo. Outcomes measured included the prevention of death, need for dialysis, or persistent decline in kidney function at 90 days, as well as the prevention of contrast-associated acute kidney injury.
Chronic kidney disease
It is important that patients with chronic kidney disease (CKD) be referred early to a nephrologist for the management of complications and for the transition to renal replacement therapy (ie, hemodialysis, peritoneal dialysis, and renal transplantation). There is some evidence that early referral of patients with CKD improves short-term outcome.
Disease progression can be slowed by means of various maneuvers, such as aggressive control of diabetes, hypertension, and proteinuria; dietary protein and phosphate restriction; and specific therapies for some of the glomerular diseases, such as lupus. Anemia, hyperphosphatemia, acidosis, and hypocalcemia should be aggressively managed before renal replacement therapy.
Postrenal azotemia
Relief of the obstruction is the mainstay of therapy for postrenal azotemia. In anuria, bladder catheterization is mandatory to rule out bladder neck obstruction, whereas in progressive azotemia, catheterization should be done after the patient has voided to determine the postvoid residual volume. A postvoid residual volume of 100 mL or more suggests obstructive uropathy, and the cause should be further investigated.
Surgical Relief of Obstruction
If hydronephrosis is due to ureteral obstruction, unilateral or bilateral stenting or percutaneous nephrostomy is performed. Recovery of renal function takes 7-10 days, but renal function may be severely impaired, necessitating dialysis until such time as partial recovery is adequate for withdrawal of dialysis.
Up to 500-1000 mL/min of postobstructive polyuria can be seen with relief of obstruction. This is an appropriate response and represents an attempt to excrete the excess fluid accumulated during the period of obstruction.
Because of salt wasting during this phase, dehydration and hypokalemia are likely. Thus, two thirds of the urine output should be replaced with half-normal saline and potassium chloride if the patient is hypokalemic. Close monitoring is indicated to prevent hypotension and prerenal azotemia.
Matching the hourly urine output with IV replacement fluid is not recommended, because the excess water retained during the period of obstruction cannot be offloaded if hourly urine output is matched.
- Massy ZA, Liabeuf S. Middle-Molecule Uremic Toxins and Outcomes in Chronic Kidney Disease. Contrib Nephrol. 2017;191:8-17.[↩]
- Foris LA, Bashir K. Uremia. [Updated 2018 Nov 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441859[↩][↩][↩]
- Tsai HM. Atypical Hemolytic Uremic Syndrome: Beyond Hemolysis and Uremia. Am. J. Med. 2018 Aug 24[↩]
- 140 – Age) / (serum creatinine[↩]
- Leong SC, Sao JN, Taussig A, Plummer NS, Meyer TW, Sirich TL. Residual Function Effectively Controls Plasma Concentrations of Secreted Solutes in Patients on Twice Weekly Hemodialysis. J. Am. Soc. Nephrol. 2018 Jul;29(7):1992-1999.[↩]
- Leurs P, Machowska A, Lindholm B. Timing of dialysis initiation: when to start? Which treatment? J Ren Nutr. 2015 Mar;25(2):238-41.[↩]
- Mindikoglu AL, Weir MR. Current concepts in the diagnosis and classification of renal dysfunction in cirrhosis. Am J Nephrol. 2013. 38(4):345-54.[↩][↩]
- Hanif MO, Ramphul K. Renal Tubular Necrosis, Acute. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507815[↩]
- Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002 Dec. 62(6):2223-9.[↩]
- Azotemia. https://emedicine.medscape.com/article/238545-overview[↩]
- Eklof H, Bergqvist D, Hagg A, et al. Outcome after endovascular revascularization of atherosclerotic renal artery stenosis. Acta Radiol. 2009 Apr. 50(3):256-64.[↩]
- Faubel S, Patel NU, Lockhart ME, Cadnapaphornchai MA. Renal relevant radiology: use of ultrasonography in patients with AKI. Clin J Am Soc Nephrol. 2014 Feb. 9(2):382-94.[↩]
- Holmquist F, Hansson K, Pasquariello F, et al. Minimizing contrast medium doses to diagnose pulmonary embolism with 80-kVp multidetector computed tomography in azotemic patients. Acta Radiol. 2009 Mar. 50(2):181-93.[↩]
- Knipp D, Lane BF, Mitchell JW, Daly BD. Computed Tomographic Angiography of the Abdomen and Pelvis in Azotemic Patients Utilizing 80-kV(p) Technique and Reduced Dose Iodinated Contrast: Comparison With Routine 120-kV(p) Technique. J Comput Assist Tomogr. 2017 Jan. 41 (1):141-147.[↩]
- Sofocleous CT, Bahramipour P, Mele C, et al. Transvenous transjugular renal core biopsy with a redesigned biopsy set including a blunt-tipped needle. Cardiovasc Intervent Radiol. 2002 Mar-Apr. 25(2):155-7.[↩]
- Fenske W, Stork S, Koschker AC, et al. Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab. 2008 Aug. 93(8):2991-7.[↩]
- Liu KD, Matthay MA, Chertow GM. Evolving practices in critical care and potential implications for management of acute kidney injury. Clin J Am Soc Nephrol. 2006 Jul. 1(4):869-73.[↩]
- Zahorec R, Setvak D, Cintula D, Belovicova C, Blaskova A. Renal rescue therapy in early stage of severe sepsis: a case study approach. Bratisl Lek Listy. 2004. 105 (10-11):345-52.[↩]
- Duffy M, Jain S, Harrell N, Kothari N, Reddi AS. Albumin and Furosemide Combination for Management of Edema in Nephrotic Syndrome: A Review of Clinical Studies. Cells. 2015 Oct 7. 4 (4):622-30.[↩]
- Recio-Mayoral A, Chaparro M, Prado B, et al. The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the RENO Study. J Am Coll Cardiol. 2007 Mar 27. 49(12):1283-8.[↩]
- Subramaniam RM, Suarez-Cuervo C, Wilson RF, Turban S, Zhang A, Sherrod C, et al. Effectiveness of Prevention Strategies for Contrast-Induced Nephropathy: A Systematic Review and Meta-analysis. Ann Intern Med. 2016 Feb 2[↩]
- Weisbord SD, et al; PRESERVE Trial Group. Outcomes after Angiography with Sodium Bicarbonate and Acetylcysteine. N Engl J Med. 2018 Feb 15. 378 (7):603-614.[↩]