Contents
What is BCAA
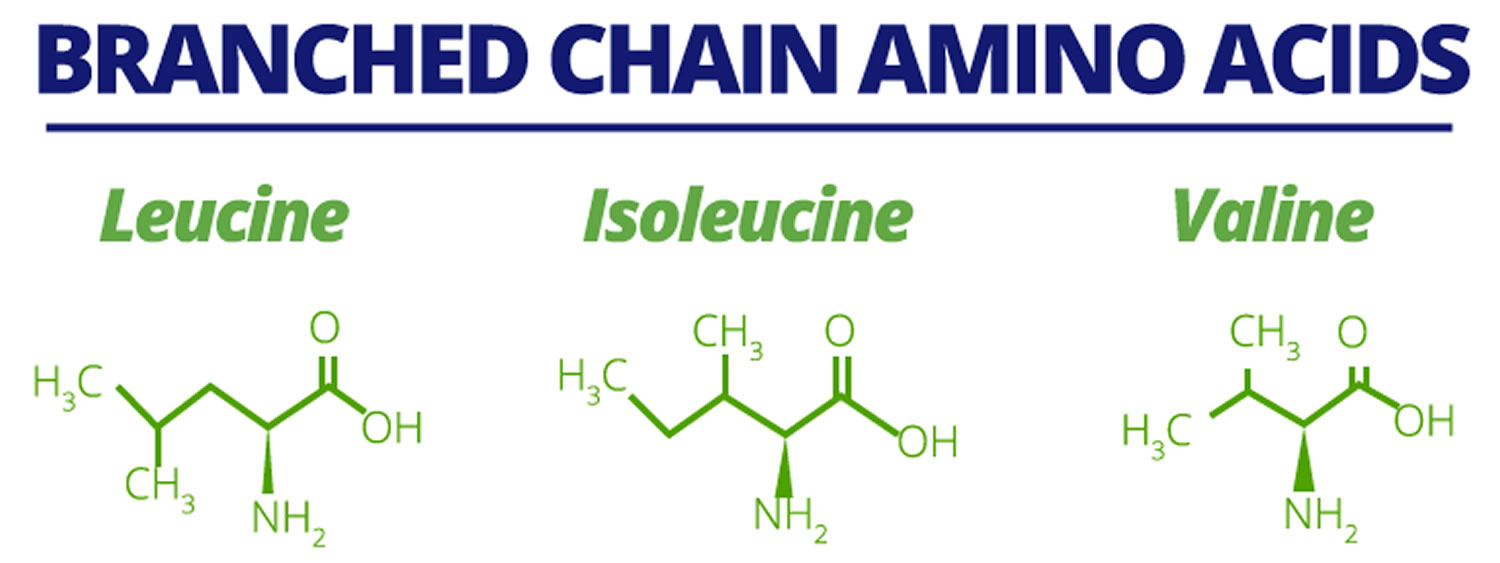
The essential amino acids (valine, leucine, and isoleucine) with aliphatic‐branched side chains (a branch) are known as branched‐chain amino acids (BCAAs). In addition to their role as key building blocks for peptide synthesis, BCAAs are also important sources for the biosynthesis of sterol, ketone bodies and glucose 1. BCAAs (branched‐chain amino acids) account for about 21% of the total body protein content and 35% of the dietary essential amino acids in muscle proteins 2. The free BCAA pool not bound to a peptide chain accounts for an extremely small proportion of the body’s total mass of BCAAs 3. Nevertheless, free BCAAs act as important nutrient signals and metabolic regulators. Skeletal muscle contains the largest amount of free BCAAs, corresponding to ~0.1 g/kg muscle 4.
The BCAAs (branched-chain amino acids) have been studied in a number of disorders, notably liver cirrhosis, renal failure, sepsis, trauma, burn injury, and cancer 5. BCAA supplementation has been thought to promote anabolic pathways and therefore mitigate cachexia, prevent or treat signs of hepatic encephalopathy, attenuate fatigue during exercise, promote wound healing, and stimulate insulin production. However, there is not consensus regarding their use as nutritional supplements 6.
Many studies have been carried out to understand whether sports performance can be enhanced by a BCAA supplementation. However, many of these researches have failed to confirm this hypothesis 7. In recent years investigators have changed their research target and focused on the effects of BCAA on the muscle protein matrix and the immune system. Data show that BCAA supplementation before and after exercise has beneficial effects for decreasing exercise-induced muscle damage and promoting muscle-protein synthesis 7. Muscle damage develops delayed onset muscle soreness: a syndrome that occurs 24-48 hours after intensive physical activity that can inhibit athletic performance. Other recent works indicate that BCAA supplementation recovers peripheral blood mononuclear cell proliferation in response to mitogens after a long distance intense exercise, as well as plasma glutamine concentration. The BCAA also modifies the pattern of exercise-related cytokine production, leading to a diversion of the lymphocyte immune response towards a Th1 type 7. According to these findings, it is possible to consider the BCAA as a useful supplement for muscle recovery and immune regulation for sports events 7.
In recent years, it has also become evident that the enzymes catalyzing the first step in BCAA degradation are overexpressed in many cancers 8. An accumulating body of evidence also demonstrates that BCAAs (branched‐chain amino acids) are essential nutrients for cancer growth and are used by tumors in various biosynthetic pathways and as a source of energy 9, 10. BCAAs can be used for protein synthesis or oxidized for energy purposes by tumors. BCAAs are essential amino acids and tumors must rely on dietary BCAA intake and their release from protein degradation 11. Several recent studies have found BCAA metabolism to be an important ‘module’ within cancer metabolism, but appear to drive cancer progression by diverse mechanisms 11. For example, transamination of BCAAs leads to formation of glutamate, which can be used for biosynthesis of other nonessential amino acids such as glutamine, or recycled to α-ketoglutarate via other aminotransferases 12. In non-small cell lung cancers, high glutamate and glutamine concentrations correlated with an increased expression of branched-chain amino acid transaminase 1 (BCAT1) and higher rates of BCAA uptake 13. Similarly, knockdown of branched-chain amino acid transaminase 1 (BCAT1) expression (or pharmacological BCAT1 inhibition) in glioblastoma cells reduced the formation of glutamate 14. However, not all cancers produced high levels of glutamate in response to overexpression of branched-chain amino acid transaminase 1 (BCAT1). In chronic myeloid leukemia blast crisis, high expression of branched-chain amino acid transaminase 1 (BCAT1) correlated with lower intracellular branched-chain α-keto acids and glutamate concentrations 15.
The past few years of in-depth research on BCAA metabolism in cancer has provided strong evidence for the essential role of BCAAs in tumor progression and has clearly established branched-chain amino acid transaminase 1 (BCAT1) as an important prognostic cancer marker. Moreover, BCAA supplementation and branched-chain amino acid transaminase 1 (BCAT1) status were tested in clinical trials for hepatocellular carcinoma and colorectal cancer 16. However, the recent research also revealed a complex addiction of cancer cells to BCAA metabolites, which appear dependent on both the tissue-of-origin and the cancer genetics. This heterogeneous reliance of cancer cells on BCAAs needs to be addressed with future studies so that therapeutic approaches aiming to target BCAA metabolism in cancer can be successfully developed 16.
Lastly, some epidemiological studies have correlated BCAA supplementation with a higher incidence of amyotrophic lateral sclerosis (ALS) among professional football players 17. Certain studies have shown that this effect could possibly be associated with BCAA‐induced hyperexcitability of the cortical motoneurons. In contrast, BCAA supplementation was given to ALS (amyotrophic lateral sclerosis) patients with the idea that BCAAs could activate the glutamate dehydrogenase enzyme, hence increase the catabolism of glutamate and reducing its harmful levels in the brain. A meta‐analysis concluded that BCAAs actually did not change the course of ALS 18. Although, to date, no scientific studies have clearly demonstrated that ALS (amyotrophic lateral sclerosis) is a direct consequence of BCAA supplementation, giving the popular usage of BCAAs among sportsman this risk should be taken into consideration. Pharmacovigilance studies assessing the risk of ALS (amyotrophic lateral sclerosis) incidence among cohorts of sportsman using BCAA supplementation are thus needed.
BCAA metabolism
You eat protein. BCAAs are readily absorbed and largely pass through the liver. Skeletal muscles represent the largest protein pool and reservoir of BCAAs in the body 19. Although the molecular basis and the regulation of BCAA uptake remain poorly studied, L‐type amino‐acid transporters and bidirectional transporters for L‐glutamine and L‐leucine essential amino acids in the enterocytes of proximal jejunum play a major role in the transport of BCAAs and activation of downstream signalling 20. BCAAs enter the blood circulation by absorption, largely escape the first‐pass hepatic metabolism and appear directly in the systemic circulation 21. About 95–99% of all circulating BCAAs are reabsorbed in the kidney nephrons, largely through the proximal convoluted tubules 20. Measurements of the arteriovenous exchanges have shown that muscles and the splanchnic bed extract over half and about one quarter of the circulating BCAAs, respectively, while the remainder is removed by the brain and other tissues 22. Both plasma and cerebrospinal BCAA levels are rapidly elevated after ingestion of a BCAA‐containing meal, and brain access to BCAAs is mediated by facilitative transport, which involves both saturable and unsaturable processes 23. Therefore, the BCAA increase rapidly in systemic circulation after protein intake and are readily available to extrahepatic tissues. This phenomenon gives a unique advantage to the BCAA-based nutritional formulas compared with others, especially those targeted on muscles and brain.
BCAA metabolism can be directed either to catabolic (i.e. oxidation) or anabolic (i.e. protein synthesis) fate by signals initiated by specific cellular energy/nutrient sensors. In the case of starvation, autonomic activation, glucagon, adrenaline, noradrenaline, cortisol and growth hormone increase intracellular BCAA uptake and favor BCAA oxidation 19. At the cellular level, the molecular events underlying these signals are mainly regulated by AMP activated kinase (AMPK), a master sensor of energy balance 24. In turn, high levels of amino acids are activated, through the Rag guanosine triphosphatases (GTPases), the mammalian/mechanistic target of rapamycin complex 1 (mTORC1) 24. This multiprotein complex acts at different levels, cooperates with other anabolic hormones [insulin and insulin‐like growth factor 1 (IGF1)] to increase the intracellular BCAA uptake, promotes protein synthesis, reduces protein degradation and increases cell growth 25. Intracellular BCAAs can synthesize new proteins, but can also be converted to glutamate, which detoxifies ammonia via glutamine synthesis in skeletal muscle 26. Importantly, in catabolic states, BCAAs are normally oxidized to generate ATP. Carbon originating from leucine enters the tricarboxylic acid (TCA) cycle as acetyl‐CoA for complete disposal as CO2, whereas isoleucine and valine mainly provide carbon for anaplerotic conversion of propionyl‐CoA to succinyl‐CoA 27.
Figure 1. Mechanisms of action of BCAAs (branched‐chain amino acids)
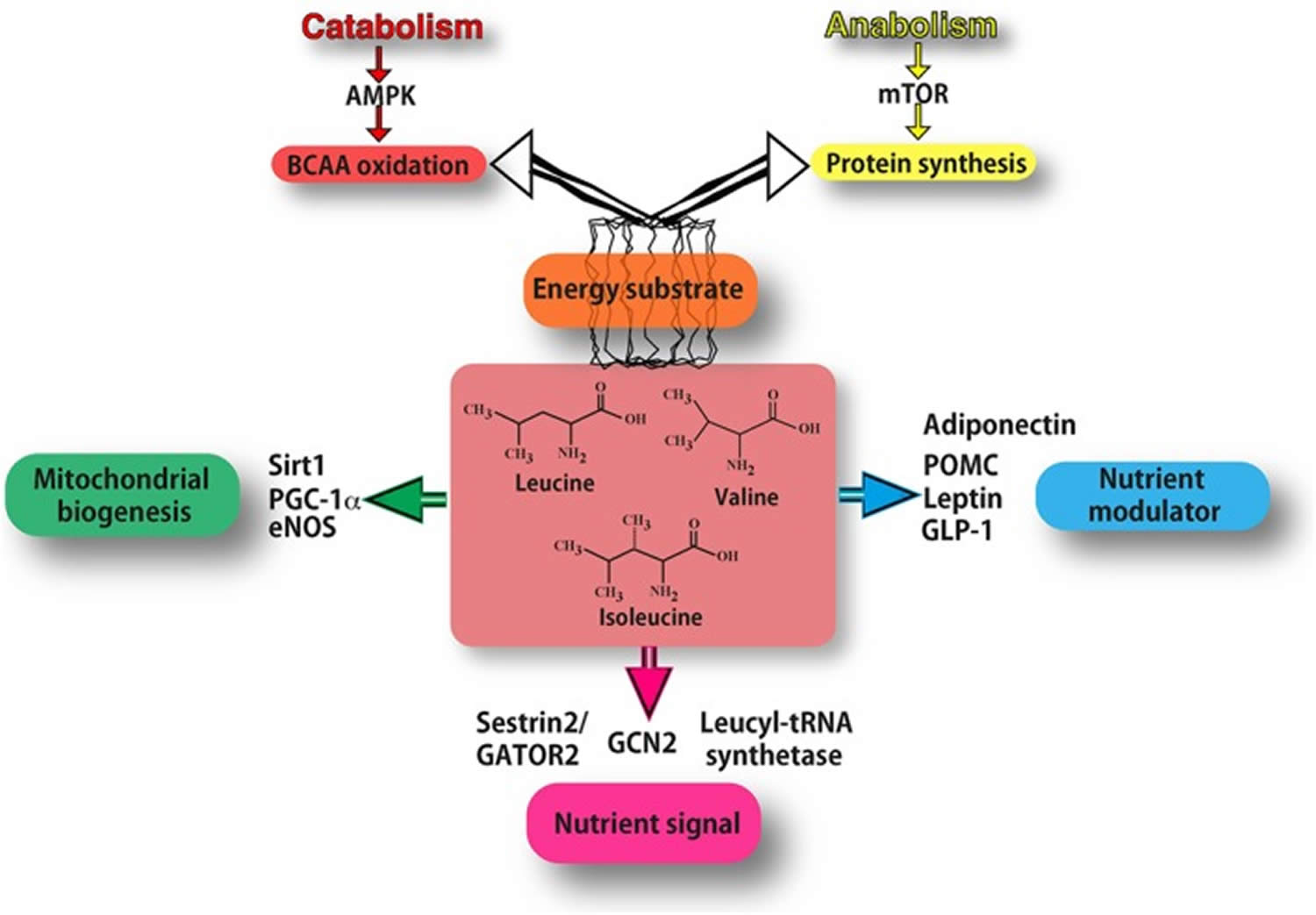 Footnotes: As energy substrate (orange box) BCAAs can be directed either to oxidation (through AMPK signalling in catabolic conditions) or to protein synthesis (through mTOR signalling in anabolic conditions). BCAAs act as nutrient signals to specific nutrient‐sensing systems (purple box). In particular, they may interact mostly with the general amino acid control non‐derepressible 2 (GCN2) pathway, specific leucyl‐tRNA synthetase and Sestrin2. Leucine inhibits the Sestrin2‐GATOR2 interaction and allows GATOR2 to activate mTOR signalling. BCAAs function also as nutrient modulators (blue box), by fine‐tuning the secretion of nutrient‐related hormones, such as leptin, adiponectin, GLP‐1 and pro‐opiomelanocortin (POMC). BCAAs increase the expression of PGC‐1α and sirtuin 1(Sirt1), thus promoting mitochondrial biogenesis (green box), partly through eNOS activity. [Source 19]
Footnotes: As energy substrate (orange box) BCAAs can be directed either to oxidation (through AMPK signalling in catabolic conditions) or to protein synthesis (through mTOR signalling in anabolic conditions). BCAAs act as nutrient signals to specific nutrient‐sensing systems (purple box). In particular, they may interact mostly with the general amino acid control non‐derepressible 2 (GCN2) pathway, specific leucyl‐tRNA synthetase and Sestrin2. Leucine inhibits the Sestrin2‐GATOR2 interaction and allows GATOR2 to activate mTOR signalling. BCAAs function also as nutrient modulators (blue box), by fine‐tuning the secretion of nutrient‐related hormones, such as leptin, adiponectin, GLP‐1 and pro‐opiomelanocortin (POMC). BCAAs increase the expression of PGC‐1α and sirtuin 1(Sirt1), thus promoting mitochondrial biogenesis (green box), partly through eNOS activity. [Source 19]Figure 2. Main pathways of BCAA catabolism
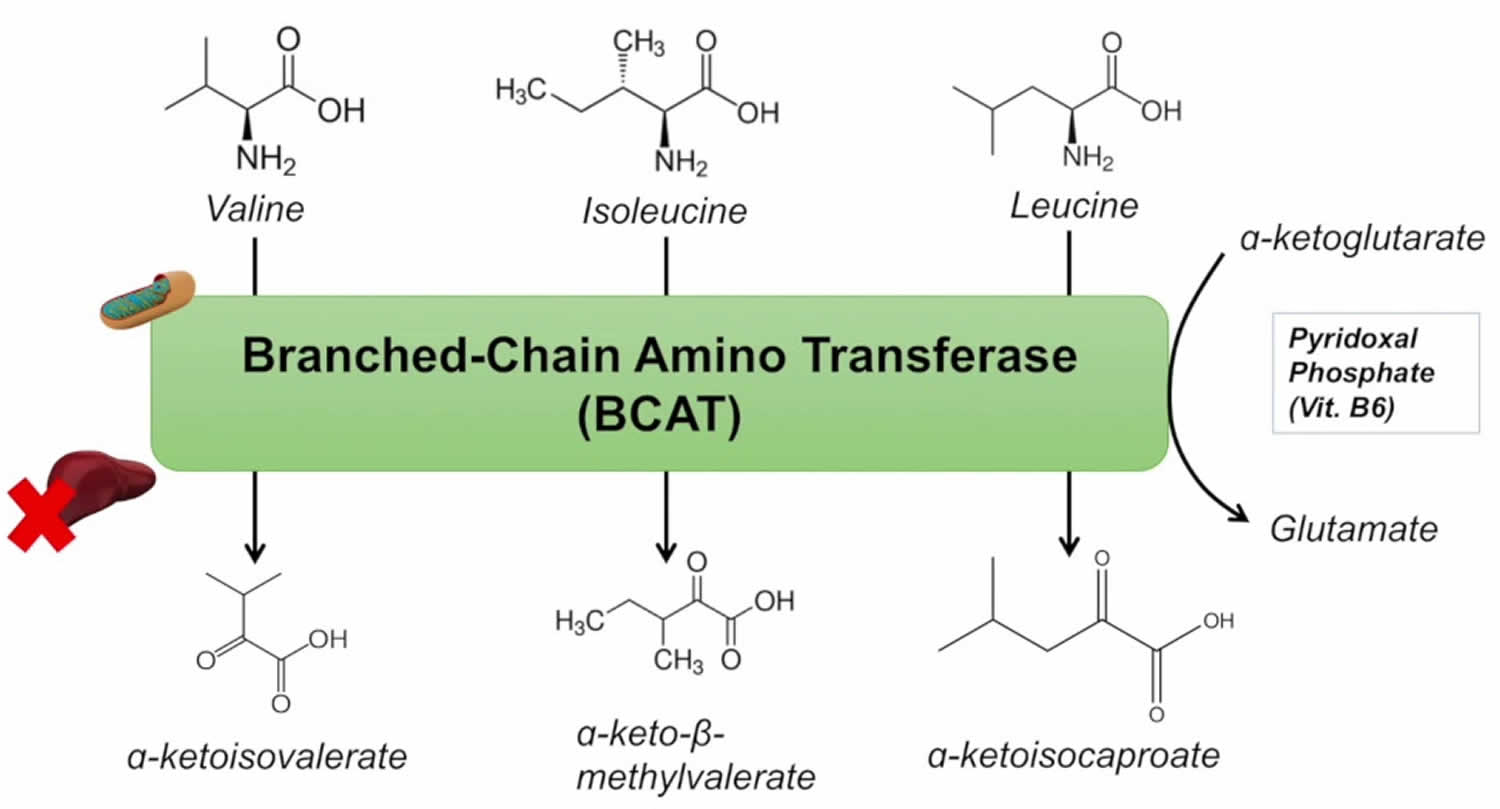
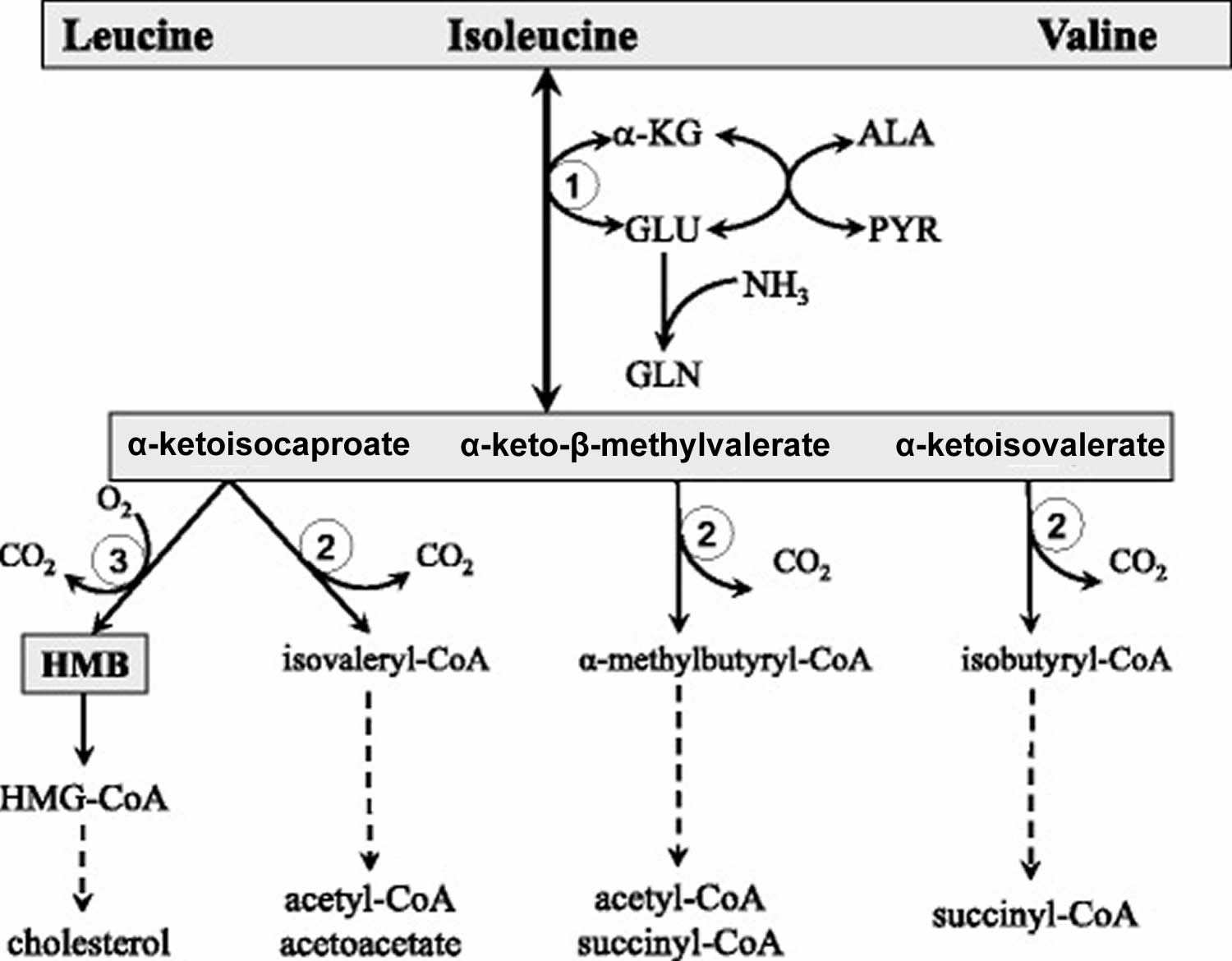
Abbreviations: ALA = alanine; GLU = glutamate; GLN = glutamine; HMB = β-hydroxy-β-methylbutyrate; HMG-CoA = 3-hydroxy-3-methyl-glutaryl-CoA; KIC = α-ketoisocaproate (ketoleucine); KIV = α-ketoisovalerate (ketovaline); KMV = α-keto-β-methylvalerate (ketoisoleucine); α-KG = α-ketoglutarate. (1) branched-chain-amino-acid aminotransferase (BCAT); (2) branched-chain α-keto acid dehydrogenase (BCKD); (3) α-ketoisocaproate dioxygenase
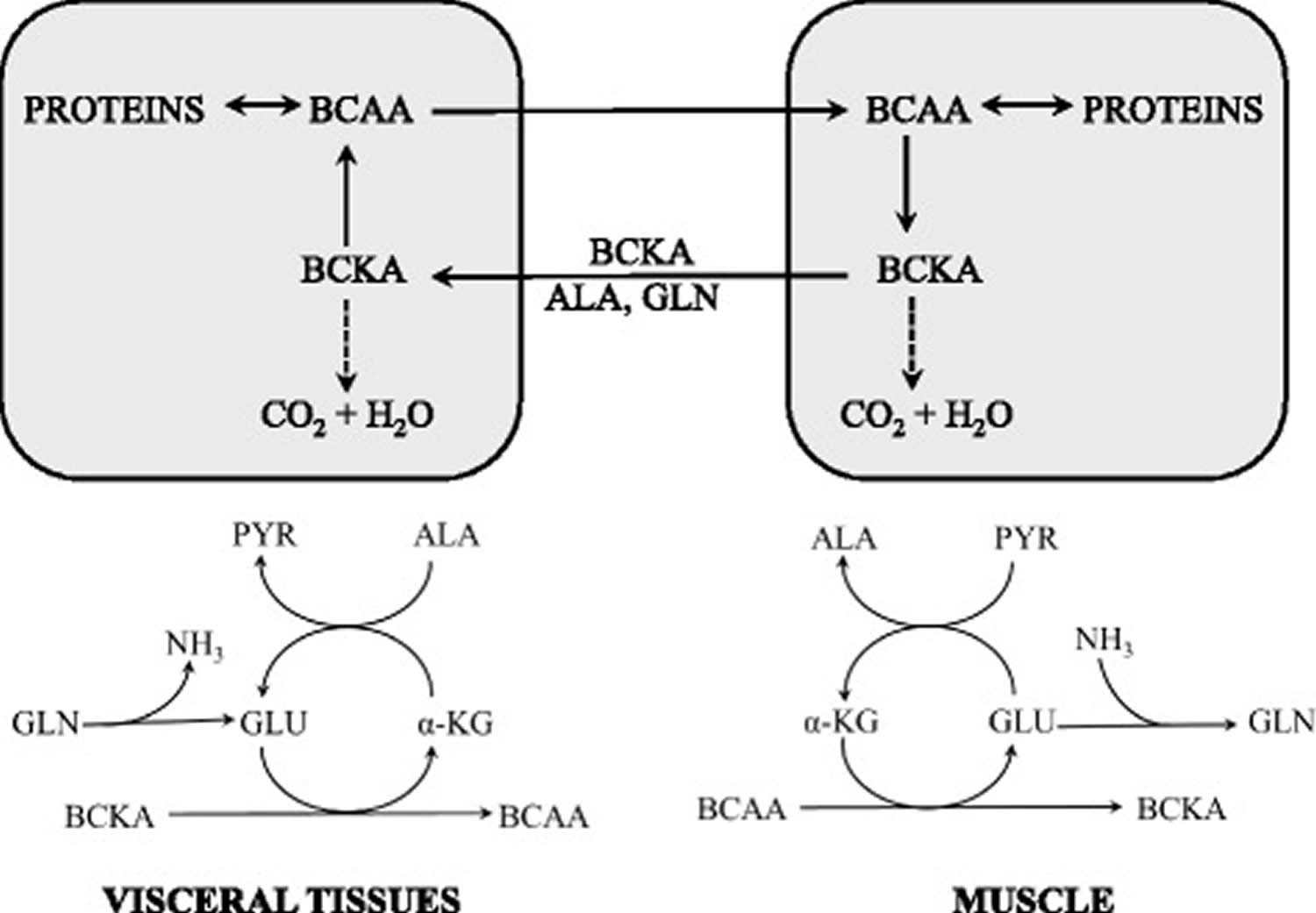
[Source 5]The initial site of most of the BCAA catabolism is skeletal muscle because of the branched-chain-amino-acid aminotransferase (BCAT) high activity. The branched-chain-amino-acid aminotransferase (BCAT) reaction involves the reversible transfer of the BCAA amino group to α-ketoglutarate (α-KG) to form glutamate and the corresponding branched-chain keto acids, α-ketoisocaproate (ketoleucine), α-keto-β-methylvalerate (ketoisoleucine), and α-ketoisovalerate (ketovaline). Glutamate then acts as an amino group source to form alanine (ALA) from pyruvate or as a substrate for ammonia detoxification to glutamine. Glutamine, alanine and a significant portion of the branched-chain keto acid are released from muscles to the blood.
The second enzyme of BCAA catabolism, branched-chain α-keto acid dehydrogenase, is a multienzyme complex located on the inner surface of the inner mitochondrial membrane, which catalyzes irreversible decarboxylation of the branched-chain keto acid to the corresponding branched-chain acyl-CoA esters. The branched-chain α-keto acid dehydrogenase is regulated by the phosphorylation-dephosphorylation mechanism. Phosphorylation mediated by a specific kinase results in inactivation, while dephosphorylation by a specific phosphatase activates the enzyme. Changes in kinase activity may play a main role.
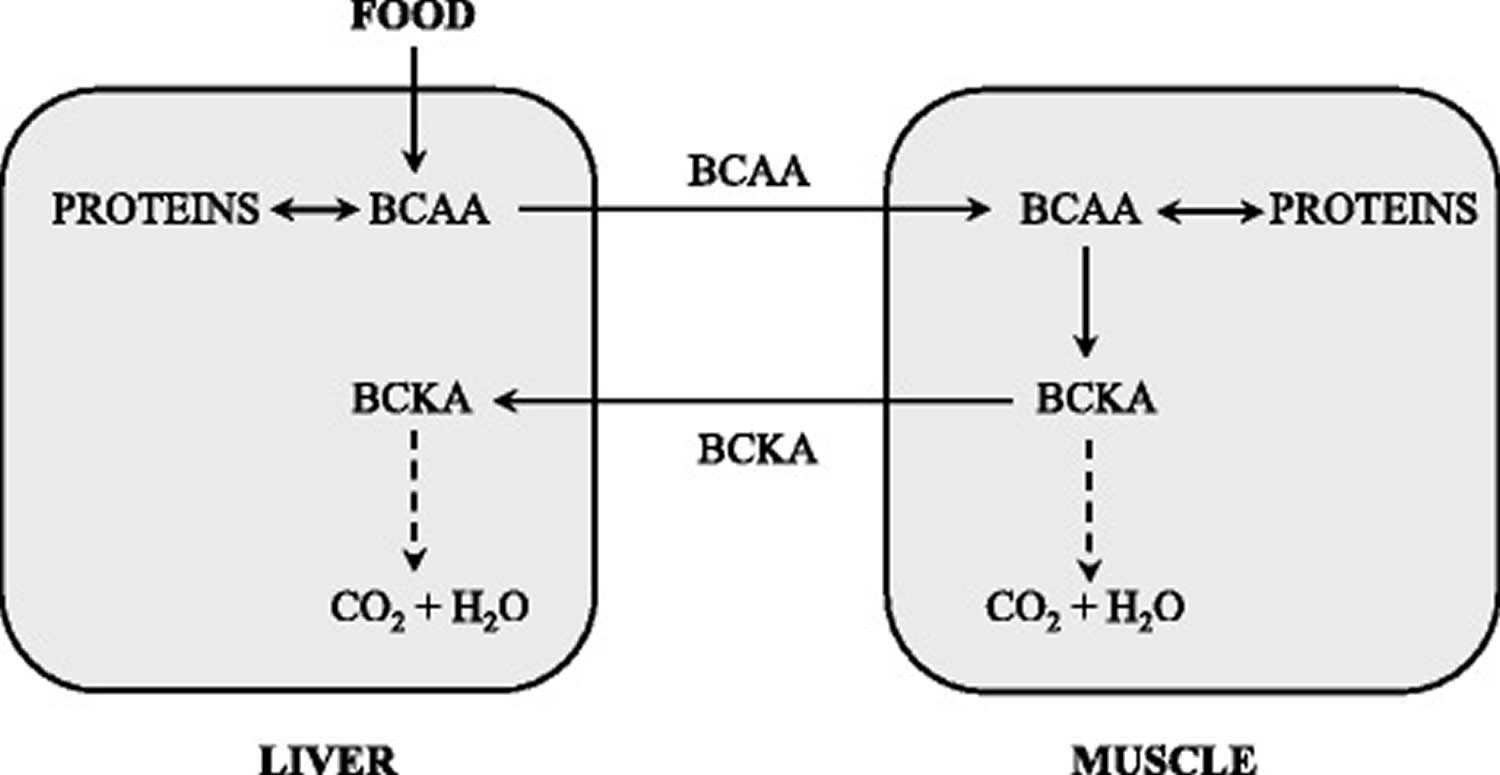
The branched-chain α-keto acid dehydrogenase activity is highest in the liver, intermediate in kidneys and heart, and low in muscles, adipose tissue, and brain 28. When the weights of individual tissues are taken into consideration, muscles, which make up 35 to 40% of total body weight, should contribute substantially to total body BCAA utilization. Thus, BCAA degradation is under joint control of a number of tissues, among which the muscle and liver play a dominant role (Figure 3). Many influences including cytokines, hormones, nutrients, and various metabolites affect the activity state of the enzyme 28. The remarkable rise in branched-chain α-keto acid dehydrogenase activity in muscles induces endotoxin or tumor necrosis factor alpha (TNF-α) administration 29.
Beyond the branched-chain α-keto acid dehydrogenase reaction, the metabolism of the BCAA diverges into separate pathways. Catabolism of α-ketoisocaproate leads to acetyl-CoA and acetoacetate (α-ketoisocaproate (ketoleucine) is ketogenic), α-ketoisovalerate (ketovaline) is catabolized to succinyl-CoA (α-ketoisovalerate (ketovaline) is glucogenic), and α-keto-β-methylvalerate to acetyl-CoA and succinyl-CoA (α-keto-β-methylvalerate (ketoisoleucine) is both glycogenic and ketogenic). A special product of α-ketoisocaproate catabolism is β-hydroxy-β-methylbutyrate (HMB) synthesized in the reaction catalyzed by α-ketoisocaproate dioxygenase.
Figure 3. BCAA catabolism in muscles and liver
Abbreviation: BCKA = branched-chain keto acid
[Source 5]Figure 4. Supposed cycling of the BCAA and branched-chain keto acid among organs
Abbreviation: ALA = alanine; BCAA = branched-chain amino acids; BCKA = branched-chain keto acid; GLU = glutamate; GLN = glutamine; PYR = pyruvate; α-KG = α-ketoglutarate
[Source 5]What does BCAA do
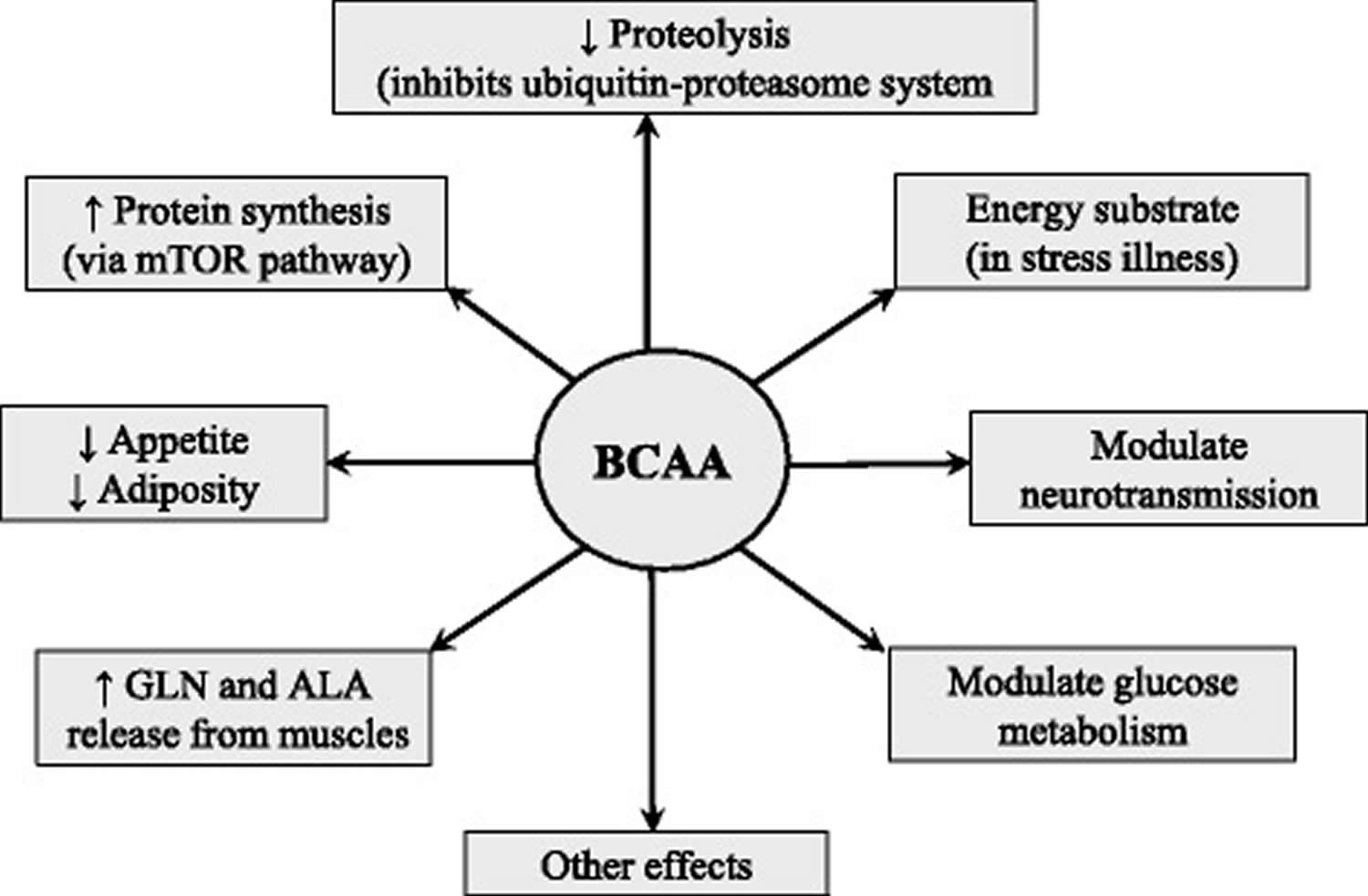
The BCAAs serve as substrates for protein synthesis or energy production and perform several metabolic and signaling functions, particularly via activation of the mammalian target of rapamycin (mTOR) signaling pathway. The following roles of the BCAA should be considered as crucial for their use as nutritional supplements (Figure 5).
Figure 5. Assumed effects of BCAA supplementation
Abbreviation: ALA = alanine; BCAA = branched-chain amino acids; GLN = glutamine; ↑ = increase; ↓ = decrease
[Source 5]BCAA benefits
BCAA effects on protein metabolism
BCAAs not only serve as substrates for protein synthesis, but also exert stimulatory effect on protein synthesis and an inhibitory effect on proteolysis. The effects are realized by the BCAAs themselves, especially by leucine, and their metabolites. Leucine stimulates protein synthesis through the mTOR signaling pathway and phosphorylation of translation initiation factors and ribosomal proteins 30. A role in protein anabolic effect of leucine plays also its stimulatory effect on insulin secretion 31. The inhibitory effect of the BCAA on proteolysis is mediated mainly by branched-chain keto acids and β-hydroxy-β-methylbutyrate (HMB). Branched-chain keto acids have been shown to prevent proteolysis in muscles under in vitro conditions 32. Infusions of α-ketoisocaproate (ketoleucine) were more effective than leucine in maintaining nitrogen balance in fasted subjects and in patients undergoing major abdominal surgery 33. HMB (β-hydroxy-β-methylbutyrate) decreases the activity of the ubiquitin-proteasome proteolytic pathway and exerts beneficial effects on muscle in various conditions of health and disease 34.
BCAA effects on the immune system
The link between metabolism and immunity has been clearly established from an evolutionary point of view. In fact, the functions of adipose tissue and bone marrow, specialized organs that regulate lipid stores and immune cell production in mammals, are sustained by a single organ in Drosophila, the fat body 35. The activation of immune responses is a metabolically demanding process. It has been estimated that sepsis can increase the human metabolic rate by 30–60% and nitrogen excretion by two to threefold 36. Catabolic states such as starvation and malnutrition can actually impair the functions of the immune system, but, on the other hand, most infections suppress the host’s appetite, possibly by inducing the synthesis of leptin 37. Two crucial cell types of the immune and metabolic systems, macrophages and adipocytes, share similar functions and properties as far as they both secrete cytokines and can be activated by lipopolysaccharide. Nutrients may directly induce inflammation through activation of toll‐like receptor signalling by free fatty acids 38. Among the better characterized intracellular points of crosstalk between nutrient sensing multiprotein complexes (i.e. AMPK, mTOR), anabolic hormones, inflammatory pathways, IκB kinase‐α and JNK play important roles 39. The anti‐inflammatory cytokine IL‐22 is able to regulate oxidative stress pathways and increase mouse and human insulin secretion from beta cells of the pancreas 40.
Protein malnutrition is one of the major causes of decreased immune function in the elderly 41. Although glutamine has been considered the most important amino acid for immune function 42, human immune cells express BCK dehydrogenase complex and decarboxylase activities 43 and, thus, oxidize BCAAs. The BCAA uptake rate in lymphocytes varies according to the cell cycle phase, most likely reflecting the timing of protein synthetic activity 44. Moreover, dietary restriction of BCAAs impairs different aspects of the immune function, including the activity of cytotoxic T lymphocytes, natural killer cells and lymphocyte proliferation. The BCAA supplementation partially restored the immunosuppression occurring after intense long‐duration exercise 45.
BCAA supplementation reduces by 30% the incidence of infections acquired in geriatric long‐term rehabilitation centres 46. Moreover, BCAAs can reduce the risk of bacterial and viral infection in patients with decompensated cirrhosis by restoring the impaired innate immune responses of these patients 47. Furthermore, the BCAA supplementation in haemodialysis patients on low‐protein diet has been found to be associated with a reduction in inflammatory markers and correction of nephropathy‐linked anaemia 48.
BCAA effects on brain
BCAAs compete for large, neutral amino‐acid transport at the blood–brain barrier and can influence brain neurotransmitter synthesis 22. Ingestion of BCAAs causes a rapid elevation of their plasma concentrations, increases their uptake into the brain and decreases the brain uptake and level of the aromatic amino acids tryptophan, phenylalanine and tyrosine. This interaction may interfere with the synthesis of the amine neurotransmitters serotonin, and the catecholamines dopamine and noradrenaline. Experimental studies show that BCAAs have favourable effects on cognitive functions. BCAA supplementation has been reported to improve cognitive performance in active dogs, with greater benefit to senior dogs 49. BCAA transamination plays an essential role in the synthesis of glutamate and subsequently of GABA. Mice subjected to traumatic brain injury had a significant reduction in BCAA concentration and neurotransmitter changes in the hippocampus 50. Dietary delivery of BCAAs to brain‐injured mice restored hippocampal BCAA levels, synaptic glutamate and GABA pools, and net synaptic efficacy, and eradicated injury‐induced cognitive impairments 50. Parenteral supplementation of BCAAs was shown to enhance the cognitive recovery of patients with traumatic brain injury 51, even when in a vegetative or minimally conscious state 52. BCAAs were administered to bipolar subjects during periods of mania for 7 days and produced a significant reduction in manic symptoms, possibly reducing brain tyrosine and phenylalanine uptake and, thus, catecholamine synthesis 22.
BCAA effects on neurotransmission
BCAAs are transported into the brain via the same carrier that transports aromatic amino acids (phenylalanine, tyrosine, tryptophan), and competition between BCAAs and aromatic amino acids may influence synthesis of some neurotransmitters, notably dopamine, norepinephrine, and 5-hydroxytryptamine (serotonin). Therefore, elevation of the BCAA in blood plasma is able to influence neurotransmitter levels in the brain with effects on behavior and brain function. This phenomenon is the rationale for use of the BCAAs in patients with liver cirrhosis, in which a decreased ratio of BCAAs to aromatic amino acids plays a role in pathogenesis of hepatic encephalopathy 53. It is believed that BCAA supplementation attenuates production of serotonin, which is responsible for fatigue during exercise. Furthermore, BCAA transamination in the brain plays a role in the synthesis of glutamate and gamma-aminobutyric acid, and in ammonia detoxification to glutamine in astrocytes. The studies have shown that leucine decreases appetite and may decrease body adiposity 54.
BCAA effects on glucose metabolism
There are close associations between BCAAs and plasma glucose levels. The fact that BCAAs upregulate glucose transporters and activate insulin secretion has been widely demonstrated 55. However, several researchers have suggested that excessive intake of amino acids could lead to inhibition of insulin signaling 56. Recent studies have suggested differential effects of each BCAA on glucose utilization and that BCAAs may induce insulin resistance through mTOR activation 57. Further investigation is needed to understand variable reports ranging from improving glucose utilization to inducing insulin resistance.
Effects mediated by alanine and glutamine
The rate of BCAA degradation in skeletal muscle is highly responsive to their availability 58. The consequences of this phenomenon are that the primary effects of the consumption of a BCAA-enriched diet are activated catabolism of the BCAAs and enhanced levels of the branched-chain keto acids, alanine and glutamine in peripheral circulation 59. Therefore, a number of effects of BCAA supplementation are mediated by alanine and glutamine. Alanine is the main gluconeogenic amino acid, and glutamine availability is essential for immune system, glutathione production, maintenance of acid-base balance by the kidneys, and expression of heat shock proteins.
BCAA supplementation in burn, trauma, and sepsis
Rationales for the use of BCAA supplements in conditions with systemic inflammatory response syndrome are their enhanced oxidation, which may limit their availability in tissues and their protein anabolic properties. Benefits of BCAAs may also be related to their role as a precursor of glutamine, which is a key factor in maintaining immune functions and gut integrity, and has a favorable influence on protein balance.
Various solutions containing different amounts and proportions of individual BCAA have been used to examine their effects in trauma, burn, or sepsis. A number of investigators have reported that BCAA ameliorate negative nitrogen balance 60. However, the results of other investigators have not been impressive, and there is no scientific consensus regarding the effect BCAA-enriched formulas on protein balance, length of hospital stay, and mortality 61. A serious shortcoming of most of the studies is the lack of information regarding BCAA concentrations in blood and tissues, which may be suggested as a possible criterion of eligibility of the indication.
The low effectiveness of the BCAA in disorders with the presence of systemic inflammatory response syndrome may be related to insulin resistance and metabolic alteration associated with inflammation. Studies have shown that inflammatory response blunts the anabolic response to BCAA administration. Lang and Frost 62 demonstrated that leucine induced activation of eukaryotic initiation factor eIF4E is abrogated in endotoxin-treated rats and that endotoxin treatment antagonized the leucine-induced phosphorylation of ribosomal protein S6 and mTOR.
In recent years articles have emerged suggesting positive effects of BCAA in traumatic brain injury. In rodents, BCAAs have demonstrated to ameliorate injury-induced cognitive impairment 63, and clinical studies have demonstrated that BCAAs enhance the cognitive recovery in patients with severe traumatic brain injury 64.
Other BCAA effects
During recent years, a number of novel functions of BCAAs, including benefits for mammary health and milk quality, intestinal development, immune response, mitochondrial biogenesis and oxidative stress have been reported 55.
BCAA dosage
The recommended dietary allowance (RDA) of BCAAs is 19 mg/kg body weight per day of isoleucine, 42 mg/kg body weight per day of leucine and 24 mg/kg body weight per day of valine 19. In humans, the daily BCAA requirement is estimated to be in a range between 10.3 and 22% required for the maintenance protein 65. Extremely elevated blood concentrations of BCAAs (leucine greater than 1000 μmol/L), as in the maple syrup urine disease, causes, within a few hours, a severe clinical condition that includes anorexia, vomiting, dehydration, lethargy, hypotonia, seizures, hypoglycaemia, ketoacidosis, pancreatitis, coma and cerebral oedema 66.
BCAAs or leucine alone have been administered to humans in a variety of studies. The amounts of the BCAAs administered were typically double to triple the normal turnover of the BCAAs 67. The administration periods ranged from hours to months. None of these studies reported any untoward effects of BCAA administration 65. However, both BCAA and/or leucine administration significantly reduced the plasma concentration of several essential amino acids 4. This effect has been identified predominantly in muscle tissue 4. Both infusion of the BCAAs at three times the basal flux and dietary intake at six times the normal flux did not have any adverse effects. There are no reports of side effects associated with normal diets containing BCAAs nor with healthy subjects receiving single, infused supplemental BCAA doses as high as 9.75 g 65.
The tolerable upper intake level of leucine, in both healthy young (20–35 years) and elderly (72.2 ± 3.5 years) subjects, has been shown to be similar at a dose of 500 mg kg−1 day−1 or ~35 g day−1 for an individual weighing 70 kg 68. In a dose‐ranging study in normal volunteers, ingestion of 60 g BCAAs was well‐ tolerated and markedly raised the BCAA plasma concentration 300 min after administration (leucine went up to about 2000 nmol /L). The same BCAA dose was administered to bipolar subjects during periods of mania for 7 days and ameliorated their symptoms, without any side effects 22.
BCAAs in pregnancy
The Recommended Dietary Allowance of proteins in pregnancy increases by 24%. Dietary supplementation with an individual BCAA (2 g/kg body weight per day) in pregnant rats fed a low‐protein diet (6% casein) reduces fetal body weight and relative brain weights 69. When BCAA supplementation (2250 mg kg body weight per day) was given 2 weeks before mating and continued through three generations, pup brain weights were reduced starting from the second generation 70. Moreover, concentrations of aspartate decreased in the brain of these animals, although the dose–response effect was not considered.
Effects of starvation and diets with a different protein content on BCAA
BCAA metabolism is very sensitive to changes in the amount and composition of the food, which may occur in both healthy and disease states.
Starvation
Brief starvation uniquely increases BCAA concentrations in plasma. In humans, the increase is evident within a day, and reaches maximum by the second or third day 71.
Both increased proteolysis and reduced protein synthesis in muscles have been reported during brief starvation and may explain the enhanced availability of BCAAs for muscles 72. In this condition, BCAAs in muscles act as a source of nitrogen for synthesis of alanine and glutamine, which are released into the blood and used in visceral tissues, especially as gluconeogenic substrates. Increased BCAT activity in muscles during starvation has been reported by several laboratories 73.
Together with alanine and glutamine, the branched-chain keto acids generated in branched-chain-amino-acid aminotransferase (BCAT) reaction are released into circulation and their concentration in the blood increases 59. It may be supposed that a portion of nitrogen released during catabolism of alanine and glutamine in visceral tissues escapes utilization in the urea cycle and is used for amination of branched-chain keto acids. Higher rates of BCAA synthesis from the branched-chain keto acids were observed by the liver perfused with glutamine-containing medium than that perfused with glutamine-deficient medium 74. A role in the increase of BCAAs may have also their decreased uptake from the blood due to the decreased levels of insulin. An unresolved possibility is the activated breakdown of proteins in the liver, which may, due to low activity of hepatic branched-chain-amino-acid aminotransferase (BCAT), result in the release of the BCAA into the blood.
Prolonged starvation lowers the BCAA concentration to basal levels and gradually increases the activity of the branched-chain α-keto acid dehydrogenase complex. Marked increase in branched-chain α-keto acid dehydrogenase activity in muscles and heart occurs in the terminal phase of starvation, when amino acids replace fatty acids and ketone bodies as the predominant energy substrate 75.
Effects of a low-protein diet
Feeding healthy human volunteers or animals a diet devoid of protein, but adequate in caloric content, lowered the plasma BCAA concentrations below basal levels 76. The amino acid pattern of children with severe kwashiorkor shows severe decrease of BCAAs 77.
It is believed that the principal factors in the decrease of BCAAs during protein deprivation are the absence of exogenous amino acids as well as curtailed muscle protein breakdown. Lowered branched-chain α-keto acid dehydrogenase activities in muscles and liver of protein-depleted rats indicate the effort of the body to conserve BCAAs 78.
BCAA or branched-chain keto acid supplementation should be recommended when a low-protein diet is prescribed to patients with chronic renal failure or urea cycle disorders.
Effects of a high-protein diet
Increased intake of protein may increase protein synthesis, decrease protein breakdown, reduce fat accumulation, and increase fat-free mass. Therefore protein supplementation or a high-protein diet is recommended to build the muscles in athletes, to prevent muscle wasting in severe illness, and to lose fat in the treatment of obesity.
High concentrations of BCAAs and urea are found in the postprandial state in the peripheral blood and muscles after intake of a protein meal and in subjects consuming a high-protein diet. In contrast to increased BCAA levels, the increments in arterial concentrations of most remaining amino acids of the ingested protein are small or insignificant 79.
The main cause of the specific BCAA increase is the unique distribution of the enzymes, which control BCAA catabolism. While complete oxidation of most individual amino acids occurs in the liver, the initial site of BCAA catabolism is skeletal muscle. Therefore, a significant portion of ingested BCAA escapes hepatic uptake and appears in peripheral circulation. The effects of protein ingestion on BCAA levels are not observed in a postabsorptive state 80.
Disorders with decreased BCAA levels
The studies have shown that BCAA deficiency impairs mRNA translation and dietary inadequacies of BCAA result in impaired growth and protein wasting 81. In addition, studies in human subjects have shown that decreased BCAA level may influence synthesis of neurotransmitters and adversely affect brain function 82. Therefore, BCAA supplementation seems rational in disorders with decreased BCAA levels, which occur in liver cirrhosis, urea cycle disorders, and chronic renal insufficiency.
Muscle sarcopenia
Dietary BCAAem supplementation was found to preserve muscle fibre size, improve physical endurance and motor coordination in middle‐aged mice 83. Accordingly, BCAAs have been shown to improve sarcopenia, that is the age‐associated loss of muscle mass and function 84, an effect possibly due to the recovery of the altered Akt/mTOR signalling in skeletal muscles of aged rats 85. BCAAem‐mediated improvement of muscle functional capacity was further enhanced by exercise training 83. Similarly, other groups have reported that BCAAs decrease protein breakdown and protect against dexamethasone‐induced soleus muscle atrophy in rats 86. BCAAem has recently been reported to protect mice from rosuvastatin‐induced myopathy without impairing the ability of this drug to lower plasma cholesterol levels. These positive effects seem to be due to multiple mechanisms, including rescue of de‐novo protein synthesis and reduction of protein breakdown, improvement of mitochondrial dysfunction and strengthening of the anti‐ROS defence mechanisms of statin, with an effective prevention of oxidative stress in muscle 87. These findings may be important considering the high number of statin prescriptions over the world, also because they suggest that the modulation of different molecular and cellular processes, at the same time, can successfully affect degenerative myopathies.
Liver cirrhosis
The decrease in BCAAs and an increase in aromatic amino acids are characteristic alterations in the blood of subjects with liver cirrhosis, which play a role in pathogenesis of hepatic encephalopathy and muscle wasting 88. Several studies have shown an inverse relationship between plasma ammonia and BCAA concentrations in patients with cirrhosis and that ammonia infusion decreases BCAA levels 89. BCAAs decrease because they are rapidly consumed to form glutamate from α-ketoglutarate as a pivotal step in ammonia detoxification to glutamine in muscles and in the brain 90. Accelerated consumption of α-ketoglutarate (cataplerosis) may disturb the function of the tricarboxylic acid (TCA) cycle. The aromatic amino acid increase is due to the decreased ability of the diseased liver to metabolize these amino acids. The BCAA levels do not decrease in acute liver injury due the leaking of amino acids from dying hepatocytes into the circulatory system 91.
BCAA supplement
The pathogenesis of hepatic encephalopathy is not completely understood. Ammonia plays a central role 92. Most interventions for hepatic encephalopathy are directed against a reduction of ammonia 93. Hyperammonaemia is one of the main causes of decreased levels of BCAA in liver cirrhosis 94. In cirrhosis, BCAA are consumed in skeletal muscle 95. Biochemically, BCAA supply muscle tissue with carbon skeletons for replenishment of α‐ketoglutarate, which may be depleted during hyperammonaemia through enhanced amination to glutamate and subsequently, amidation of glutamate to glutamine. BCAA and glutamate concentrations in plasma and muscle tissue are reduced in people with cirrhosis and hyperammonaemia, and the removal of ammonia in muscle is proportional to the removal of BCAA in people with cirrhosis 96. Skeletal muscle is believed to play a key role in ammonia detoxification in people with cirrhosis and several studies have indicated that BCAA enhance this detoxification 95. However, the effects of BCAA supplements are complex. BCAA supplementation may reduce malnutrition and revert the loss of muscle cell mass that is common in severe liver disease and the breakdown of protein that occurs in people with hepatic encephalopathy 97. The increased muscle mass may increase extrahepatic ammonia detoxification 98. Glutamine synthetase activity is high in muscle tissue, which promotes detoxification of ammonia to glutamine. BCAA supplementation also increases plasma levels of BCAA and reduce the ratio between aromatic amino acids and BCAA 99. BCAA may further enhance detoxification of ammonia in skeletal muscle by the amidation process for glutamine synthesis 100. The addition of BCAA reduces cerebral efflux of aromatic amino acids across the blood‐brain barrier and the imbalance of the synthesis of dopamine, noradrenaline, and serotonin 101.
BCAAs are recommended to ameliorate cachexia and the decreased ratio of BCAAs to aromatic amino acids, which plays a role in the pathogenesis of hepatic encephalopathy. Potential benefits also include positive effects of the BCAA on ammonia detoxification to glutamine in muscles, liver regeneration, albumin synthesis, immune and hepatic function, glucose metabolism, and physical and mental fatigue 102.
Unfortunately, the results from clinical trials do not provide strong evidence of their beneficial effects 103 and adverse effects of BCAA supplementation, which may compete with their benefits, have also been suggested 104. The positive effects of BCAAs in subjects with liver cirrhosis may be blunted by enhanced catabolism of glutamine produced in muscles to ammonia in visceral tissues, especially in the gut and kidneys. The draining of α-KG from tricarboxylic acid (TCA) cycle may also be detrimental. Therefore, therapeutic strategies are needed to avoid potential adverse effects of BCAAs on ammonia production and cataplerosis. Options include substitution of α-ketoglutarate, glutamate related substrates (e.g. L-ornithine-L-aspartate), glutamine elimination from the body by phenylbutyrate, replacement of BCAAs by branched-chain keto acids, and optimizing dose, proportions, and timing of BCAA supplementation 104.
Urea cycle disorders (UCD) and phenylbutyrate
Urea cycle disorders (UCD) result from inherited enzymatic defects in the ammonia detoxification pathway in the liver, leading to low levels of urea and high levels of ammonia in the blood. The disorders are characterized by seizures, lethargy, coma, and death in the neonatal period or severe long-term neurological impairment.
In addition to altered levels of ammonia and urea, common finding in patients with urea cycle disorders (UCD) is an increase in glutamine and a decline in BCAA levels, notably during acute metabolic decompensation 105. These alterations support the theory that BCAAs play a unique role in ammonia detoxification to GLN and that hyperammonemia is the cause of decreased BCAA levels in subject with liver cirrhosis 106.
At present, the management of urea cycle disorders (UCD) is achieved by dietary protein restriction and the use of compounds that remove nitrogen, notably benzoate and phenylbutyrate. Benzoate conjugates glycine to promote the synthesis of hippuric acid that is eliminated in urine and thus attenuates catabolism of glycine to ammonia. Phenylbutyrate is converted by β-oxidation into phenylacetate that is conjugated with glutamine to form phenacetylglutamine, which is excreted in the urine. Unfortunately, it has been shown that phenylbutyrate activates the branched-chain α-keto acid dehydrogenase, resulting in decreases in BCAA and branched-chain keto acid levels in blood plasma 107. Marked decrease of BCAAs in urea cycle disorders (UCD) after phenylbutyrate treatment has been reported by Scaglia et al. 108.
Effects of BCAA supplementation
Low BCAA levels in subjects with urea cycle disorders (UCD), especially those treated by phenylbutyrate, indicate the rationale to use BCAAs as a therapeutic agent. Unfortunately, the reports of attempts to use BCAAs in urea cycle disorders (UCD) are unique. Cross-sectional data from 41 European Inherited Metabolic Disorder centers reported that only 16 (3%) patients (from 8 centers in 5 countries) received BCAA supplements. The two most common conditions were ornithine transcarbamylase deficiency and citrullinaemia 109.
Chronic renal failure
Most studies of amino acid patterns in chronic renal failure reported decreased BCAA and branched-chain keto acid levels in the blood plasma 110 and reduced concentrations of valine in muscles 110. The derangements are caused by the action of multiple factors, notably acidosis and glucocorticoids. Decreased intake of proteins and hemodialysis, resulting in low concentrations of most essential and nonessential amino acids, is also a factor. In contrast to chronic renal failure, inconsistent alterations have been reported in acute renal failure.
Several articles have suggested that metabolic acidosis is responsible for accelerated proteolysis and enhanced activity of the branched-chain α-keto acid dehydrogenase in muscles and liver 111. More significant increases in proteolysis and leucine oxidation were reported in rats with chronic uremia and acidosis when compared with uremic rats without acidosis. A significant decrease in valine concentration in the gastrocnemius muscle was found only in rats with acidosis 110.
Effects of BCAA supplementation
BCAAs and branched-chain keto acids are supplied to patients with chronic renal failure together with other essential amino acids and their ketoanalogues to decrease protein intake as much as possible to maintain protein balance and avoid its deleterious effects on urea levels 112.
Disorders with elevated BCAA levels
Increased BCAA concentrations are found in various insulin-deficient and -resistant states, especially diabetes and obesity. Very high BCAA and branched-chain keto acid concentrations are found in maple syrup urine disease (MSUD).
Type 1 diabetes
High BCAA levels in subjects with defective insulin secretion were first described in dogs with experimental diabetes 113. Further studies have shown that in addition to the increase of BCAAs, there is a decrease in levels of gluconeogenic amino acids, especially alanine 114. Most data on pathogenesis of high levels of the BCAA in diabetes type 1 originate from studies using animals with diabetes induced by streptozotocin or alloxan.
There are some similarities in the pathogenesis of the increased BCAAs in diabetes and short-term starvation, which is also an insulin deficient state. As in starvation, a role play activated amination of the branched-chain keto acids in the liver and impaired uptake of the BCAA by muscles. The branched-chain keto acid levels increase in blood plasma and muscles of rats with chemically-induced diabetes, but decline in the liver 115. The role of the liver as a source of BCAAs is supported by observations of reduced activity of hepatic branched-chain α-keto acid dehydrogenase in rats with severe ketotic diabetes 116.
However, unlike brief starvation, the changes in diabetes are associated with marked increase in proteolysis and branched-chain α-keto acid dehydrogenase activity in muscles, resulting in severe cachexia 117. While muscle nitrogen repletion occurs and BCAA levels are normalized after feeding of previously starving subjects, the BCAAs accumulate and diminished nitrogen repletion remains after feeding in subjects with type 1 diabetes Sherwin R, Palaiologos G. Amino acid and protein metabolism in diabetes mellitus. Arch Intern Med. 1977;137:507–513.)).
Obesity and type 2 diabetes
Plasma concentrations of BCAAs are frequently elevated in obesity and type 2 diabetes ((Felig P, Wahren J, 118. The mechanism responsible for the increased BCAAs in these insulin-resistant states is not completely clear. A major cause might be reduced activity of the branched-chain keto acid dehydrogenase, which was reported in the liver and adipose tissue in genetically obese ob/ob mice, Zucker rats and spontaneous type 2 diabetes Otsuka Long-Evans Tokushima Fatty rats ((Felig P, Wahren J, 118.
The studies have shown that the BCAA levels in obesity correlate with insulin resistance and are a sensitive predictor of diabetes in the future 119. Recent studies have suggested that high levels of the BCAA interfere with oxidation of fatty acids in muscles, leading to accumulation of various acylcarnitines and insulin resistance 120.
BCAA supplementation
Conflicting results have been reported concerning the effects of BCAA supplementation in subjects with insulin resistance. Leucine improved glucose tolerance, decreased hepatic steatosis, and decreased inflammation in adipose tissue in mice fad a high-fat diet 121 and rescued insulin signaling in adipose tissue obtained from insulin resistant db/db mice 122. Arakawa et al. 123 reported that BCAAs reduced hepatic and triglyceride concentrations in mice fed a high-fat diet. On the other hand, Newgard et al. 124 showed that administration of a mixture of BCAA to rats on a high-fat diet increased insulin resistance. White et al. 120 demonstrated that the BCAA-restricted diet improved muscle insulin sensitivity in Zucker-fatty rats.
BCAA supplementation during workout
BCAAs (branched-chain amino acids) are essential amino acids that participate in skeletal muscle protein synthesis and can be oxidized in skeletal muscle and can be used as energy substrates during physical exercise 125. Circulating BCAAs, in particular leucine, have been shown to act as potent nutrient signals in muscle where they induce protein synthesis 4. A dietary supplementation of amino‐acid mixtures enriched in BCAAs preserves muscle fiber size and improves physical endurance and motor coordination in middle‐aged mice 83. Moreover, amino‐acid mixtures enriched in BCAAs increases the expression of PPARγ coactivator‐1α (PGC‐1α) and sirtuin 1(SIRT1) and promotes mitochondrial biogenesis and function in cardiac and skeletal muscles through an mTORC1‐dependent effect 83. BCAAem‐activated mTOR signalling can enhance mitochondrial biogenesis partially through increasing of the NO generating system. Endothelial NOS (eNOS) gene silencing decreased the activation of mTOR by amino‐acid mixtures enriched in BCAAs in vitro and in vivo 83. Thus, a positive feedback mechanism between eNOS and mTOR pathways could promote the effects of BCAAs. Finally, exercise training further enhanced the amino‐acid mixtures enriched in BCAAs‐mediated improvement in muscle functional capacity.
It is known that physical exercise is associated with enhanced BCAA oxidation and glutamine release from muscles 126. Therefore, since the 1980s, there has been high interest in BCAA by sports nutrition scientists 127. And now BCAA has been commonly used by Chinese athletes as nutritional supplements 127.
What benefits can you get from the BCAA supplementation?
Studies have shown that BCAA supplementation before and after exercise has beneficial effects for decreasing exercise-induced muscle damage and promoting muscle-protein synthesis 128. However, many researchers have not been able to confirm that BCAA supplementation can enhance sports performance 129. BCAAs act as donors of nitrogen and carbon skeleton for the synthesis of other amino acids, e.g., glutamine that are important in supporting immune cell function 130. Evidence suggests that branched-chain α-keto acid dehydrogenase, which catalyzes the second-step reaction of the BCAA catabolic pathway and is the rate-limiting enzyme in the pathway, is activated by dephosphorylation mediated by falling ATP levels within the muscles during exercise. Training appears to increase mRNA expression of this enzyme 131. However, results are conflicting, the plasma BCAA levels during or after exercise have been reported to be unchanged 132, to decrease 133, or to increase 134. The cause of inconsistent response can be explained by different work load and duration of exercise.
Effects of BCAA supplementation
BCAAs are recognized as supplements for athletes with a number of benefits, notably on muscle protein synthesis, fatigue recovery, and exercise-induced muscle damage 135. BCAA supplementation before and after exercise has beneficial effects for decreasing exercise-induced muscle damage and promoting muscle-protein synthesis; this suggests that BCAAs may be a useful supplement in relation to exercise and sports. Although in many human exercise studies, a dose of >5 g of BCAA was used as a supplement, the minimum dose to produce the beneficial effects of BCAA supplementation remains to be established. Furthermore, the most effective ratio of the three BCAAs (leucine:isoleucine:valine ratio) is unclear 136. In addition to the positive reports, there are a number of reports showing no benefits of BCAA supplementation 137. Of special interest should be findings of enhanced blood ammonia levels after BCAA administration during exercise suggesting that exogenous BCAA may exert negative effects on muscle performance via ammonia 138. A number of research groups examined whether BCAA supplementation might have a beneficial effect on endurance performance 139, but the results are inconsistent. In conclusion, additional studies are needed to assess the true efficacy of BCAA supplementation on muscle performance and fatigue.
BCAA dosage
The results obtained in this preliminary study 128 indicate that the ingestion of 5 g of BCAAs before exercise can reduce delayed-onset muscle soreness (DOMS) and muscle fatigue for several days after exercise. The mechanisms that underlie these BCAA effects have not yet been examined. However, one possibility is that BCAA may attenuate exercise-induced protein breakdown, while leucine may stimulate muscle protein synthesis. If the finding is substantiated, the results could support the usefulness of BCAA in muscle recovery from exercise. Further studies are required to elucidate the mechanisms responsible for the effects of BCAA supplementation.
Summary
The BCAAs (branched-chain amino acids), valine, leucine, and isoleucine are essential amino acids, which have been studied in a number of disorders, notably liver cirrhosis, renal failure, sepsis, trauma, burn injury, and cancer 5. BCAA supplementation has been thought to promote anabolic pathways and therefore mitigate cachexia, prevent or treat signs of hepatic encephalopathy, attenuate fatigue during exercise, promote wound healing, and stimulate insulin production. However, there is not consensus regarding their use as nutritional supplements 6.
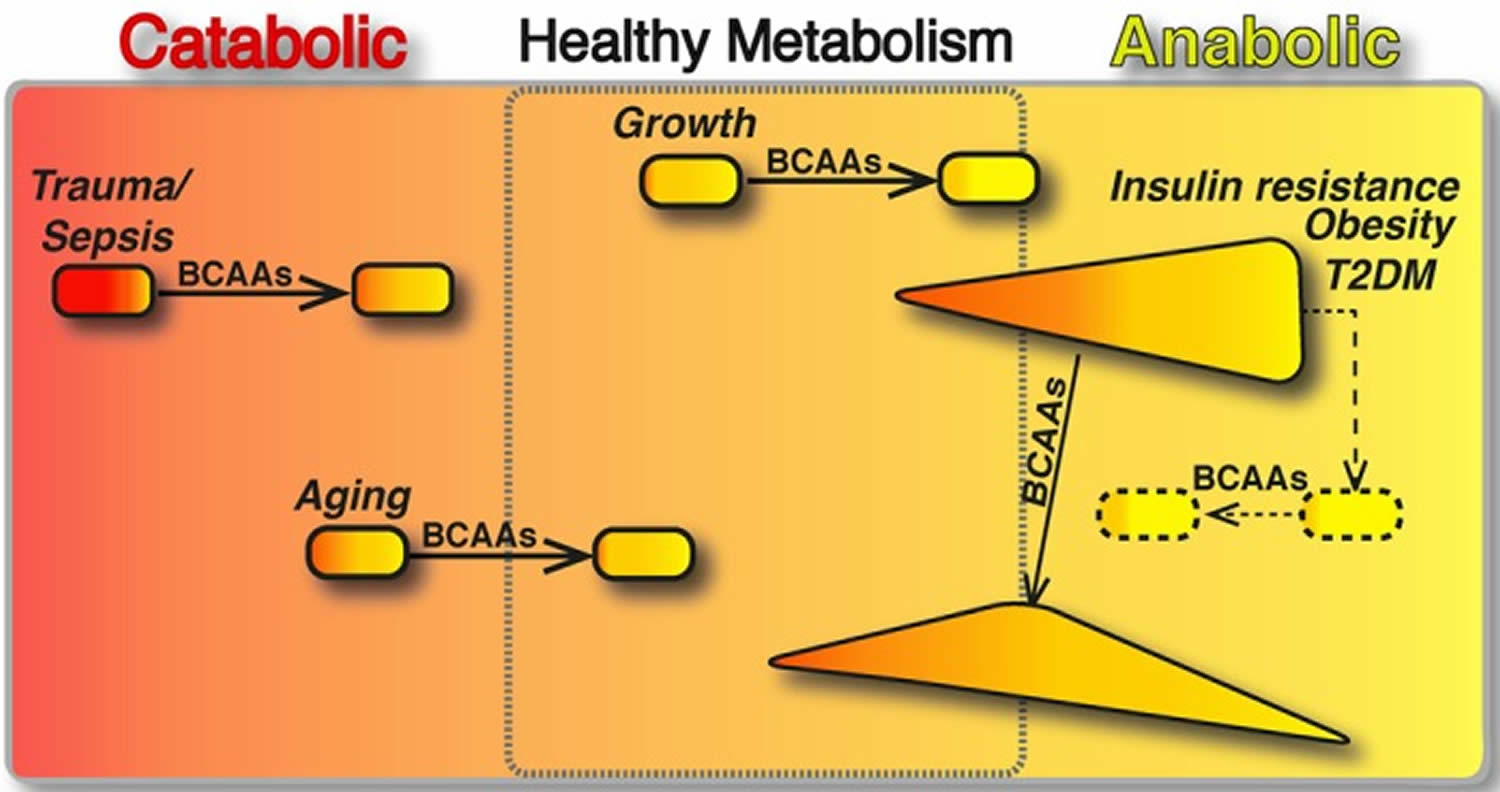
BCAAs have been shown to play an important role in the regulation of metabolism and energy balance by directly affecting peripheral tissues, such as white adipose tissue, liver and muscle 19. The effect of BCAAs drastically changes when they act in catabolic or anabolic conditions. In catabolic states, BCAAs can behave as energy substrate, which can be directly oxidized in the muscle or converted to gluconegenic‐chetogenic substrates. In contrast, in anabolic conditions, BCAAs stimulate protein synthesis and cell growth (Figure 6). In exclusively catabolic disorders, such as muscle sarcopenia, burn and trauma, BCAA supplementation improves muscle function and clinical outcomes (Figure 6). However, in metabolic disorders, in which different amounts of anabolic and catabolic signals coexist, the effects of BCAA supplementation are difficult to predict. This might be the case in obesity and insulin resistance, where BCAA supplementation seems to exert opposing effects depending on the prevalence of the catabolic or anabolic signals (Figure 6). Thus, a future challenge in this field will be to approach systemically the complex network of molecules and metabolites, beyond the environmental signals (i.e. foods, nutrient composition, calorie restriction, exercise, gut microbiome, etc.), that regulates BCAA metabolism and is regulated by BCAAs themselves.
Figure 6. BCAAs differently modulate catabolic and anabolic states
Footnotes: The boxed area represents the catabolic (red, left) or anabolic (yellow, right) states of the organisms. In catabolic (sepsis, trauma and ageing) and anabolic (growth) conditions, BCAAs have been shown to restore the energy balance and to improve the clinical outcomes (arrows). In contrast, in metabolic disorders, different amounts of anabolic and catabolic signals coexist. Here, we present insulin resistance, obesity and T2DM (type 2 diabetes mellitus), which are primarily anabolic conditions, in which several catabolic signals are co‐expressed with anabolic ones (e.g. exaggerated sympathetic nervous system activity, inadequate suppression of counter‐regulatory hormones, such as glucagon, cortisol and growth hormone). In these pathological conditions, the effects of BCAA supplementation seem (arrow) to exert opposing effects depending on the prevalence of the catabolic or anabolic signals. Multiple interventions capable of balancing the aberrant metabolic signals (dashed line) may be required to potentiate the healthy effects of BCAAs (dashed arrow).
BCAAs and cancer
Leucine and isoleucine have been shown to promote bladder neoplasms originated by the oncogenic agent N‐butyl‐N‐(4‐hydroxybutyl) nitrosamine in rats at dietary levels of 2% and above (Nishio et al., 1986). However, there is no evidence that either of these amino acids could possibly be carcinogenic in the absence of an initiating agent, and no dose–response studies have actually identified any effective BCAA carcinogenic concentration. Positron emission tomography with 11C–leucine points to the high avidity of amino acid uptake of some tumours (Smith et al., 2005). On the other hand, BCAA supplementation improved the metabolic parameters, morbidity and quality of life in patients with hepatocellular carcinoma (Kawaguchi et al., 2011). The few data available on BCAAs in the tumour‐bearing state are not conclusive, and further work is needed to clarify the effects of BCAAs on cancer (Baracos and Mackenzie, 2006).
BCAAs and amyotrophic lateral sclerosis
Some epidemiological studies have correlated BCAA supplementation with a higher incidence of amyotrophic lateral sclerosis (ALS) among professional football players 17. Certain studies have shown that this effect could possibly be associated with BCAA‐induced hyperexcitability of the cortical motoneurons. In contrast, BCAA supplementation was given to ALS (amyotrophic lateral sclerosis) patients with the idea that BCAAs could activate the glutamate dehydrogenase enzyme, hence increase the catabolism of glutamate and reducing its harmful levels in the brain. A meta‐analysis concluded that BCAAs actually did not change the course of ALS 18. Although, to date, no scientific studies have clearly demonstrated that ALS (amyotrophic lateral sclerosis) is a direct consequence of BCAA supplementation, giving the popular usage of BCAAs among sportsman this risk should be taken into consideration. Pharmacovigilance studies assessing the risk of ALS (amyotrophic lateral sclerosis) incidence among cohorts of sportsman using BCAA supplementation are thus needed.
In conclusion, alterations in BCAA metabolism are common in a number of disease states and the BCAA have therapeutic potential due to their proven protein anabolic effects. However, many controversies about the use of BCAAs in clinical practice still exist, and careful studies are needed to elucidate the effectiveness of BCAAs in most indications given BCAAs may be associated with increased risk of cancer and the possibility of developing amyotrophic lateral sclerosis (ALS).
- Bifari F, Nisoli E. Branched‐chain amino acids differently modulate catabolic and anabolic states in mammals: a pharmacological point of view. British Journal of Pharmacology. 2017;174(11):1366-1377. doi:10.1111/bph.13624. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5429325/[↩]
- Harper AE, Miller RH, Block KP (1984). Branched‐chain amino acid metabolism. Annu Rev Nutr 4: 409–454.[↩]
- Waterlow JC, Golden MH, Garlick PJ (1978). Protein turnover in man measured with 15 N: comparison of end products and dose regimes. Am J Physiol 235: E165–E174.[↩]
- Shimomura Y, Yamamoto Y, Bajotto G, Sato J, Murakami T, Shimomura N et al. (2006). Nutraceutical effects of branched‐chain amino acids on skeletal muscle. J Nutr 136: 529S–532S.[↩][↩][↩][↩]
- Holeček M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutrition & Metabolism. 2018;15:33. doi:10.1186/s12986-018-0271-1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5934885/[↩][↩][↩][↩][↩][↩]
- Bifari F, Nisoli E. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: a pharmacological point of view. Br J Pharmacol. 2017;174:1366–1377 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5429325/[↩][↩]
- Branched-chain amino acid supplementation does not enhance athletic performance but affects muscle recovery and the immune system. J Sports Med Phys Fitness. 2008 Sep;48(3):347-51. https://www.ncbi.nlm.nih.gov/pubmed/18974721/[↩][↩][↩][↩]
- Genomic deletion of malic enzyme 2 confers collateral lethality in pancreatic cancer. Dey P, Baddour J, Muller F, Wu CC, Wang H, Liao WT, Lan Z, Chen A, Gutschner T, Kang Y, Fleming J, Satani N, Zhao D, Achreja A, Yang L, Lee J, Chang E, Genovese G, Viale A, Ying H, Draetta G, Maitra A, Wang YA, Nagrath D, DePinho RA. Nature. 2017 Feb 2; 542(7639):119-123.[↩]
- Ananieva EA, Wilkinson AC. Branched-chain amino acid metabolism in cancer. Current Opinion in Clinical Nutrition and Metabolic Care. 2018;21(1):64-70. doi:10.1097/MCO.0000000000000430. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5732628/[↩]
- Targeting amino acid metabolism in cancer growth and anti-tumor immune response. Ananieva E. World J Biol Chem. 2015 Nov 26; 6(4):281-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4657121/[↩]
- Nature and Nurture: What Determines Tumor Metabolic Phenotypes? Mayers JR, Vander Heiden MG. Cancer Res. 2017 Jun 15; 77(12):3131-3134. http://cancerres.aacrjournals.org/content/77/12/3131.long[↩][↩]
- Cluntun AA, Lukey MJ, Cerione RA, et al. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer 2017; 3:169–180.[↩]
- Mayers JR, Torrence ME, Danai LV, et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 2016; 353:1161–1165.[↩]
- Tonjes M, Barbus S, Park YJ, et al. BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat Med 2013; 19:901–908. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4916649/[↩]
- Hattori A, Tsunoda M, Konuma T, et al. Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature 2017; 545:500–504.[↩]
- Ananieva EA, Wilkinson AC. Branched-chain amino acid metabolism in cancer. Current Opinion in Clinical Nutrition and Metabolic Care. 2018;21(1):64-70. doi:10.1097/MCO.0000000000000430 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5732628/[↩][↩]
- Chio A, Benzi G, Dossena M, Mutani R, Mora G (2005). Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain 128: 472–476[↩][↩]
- Parton M, Mitsumoto H, Leigh PN (2003). Amino acids for amyotrophic lateral sclerosis / motor neuron disease. Cochrane Database Syst Rev 4: CD003457.[↩][↩]
- Bifari F, Nisoli E. Branched‐chain amino acids differently modulate catabolic and anabolic states in mammals: a pharmacological point of view. British Journal of Pharmacology. 2017;174(11):1366-1377. doi:10.1111/bph.13624. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5429325[↩][↩][↩][↩][↩]
- Broer S (2008). Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88: 249–286.[↩][↩]
- Brosnan JT, Brosnan ME (2006). Branched‐chain amino acids: enzyme and substrate regulation. J Nutr 136: 207S–211S.[↩]
- Fernstrom JD (2005). Branched‐chain amino acids and brain function. J Nutr 135: 1539S–1546S.[↩][↩][↩][↩]
- Smith QR, Momma S, Aoyagi M, Rapoport SI (1987). Kinetics of neutral amino acid transport across the blood–brain barrier. J Neurochem 49: 1651–1658.[↩]
- Yuan HX, Xiong Y, Guan KL (2013). Nutrient sensing, metabolism, and cell growth control. Mol Cell 49: 379–387.[↩][↩]
- Rennie MJ, Bohe J, Smith K, Wackerhage H, Greenhaff P (2006). Branched‐chain amino acids as fuels and anabolic signals in human muscle. J Nutr 136: 264S–268S.[↩]
- Kawaguchi T, Izumi N, Charlton MR, Sata M (2011). Branched‐chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology 54: 1063–1070.[↩]
- Harris RA, Joshi M, Jeoung NH, Obayashi M (2005). Overview of the molecular and biochemical basis of branched‐chain amino acid catabolism. J Nutr 135: 1527S–1530S.[↩]
- Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–454.[↩][↩]
- Holecek M, Sprongl L, Skopec F, Andrýs C, Pecka M. Leucine metabolism in TNF-α- and endotoxin-treated rats: contribution of hepatic tissue Am J Phys 1997;273: E1052–E1058.[↩]
- Nair KS, Short KR. Hormonal and signaling role of branched-chain amino acids. J Nutr. 2005;135:1547S–1552S.[↩]
- Floyd JC, Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966;45:1487–1502.[↩]
- Tischler ME, Desautels M, Goldberg AL. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem. 1982;257:1613–1621.[↩]
- Sapir DG, Stewart PM, Walser M, Moreadith C, Moyer ED, Imbembo AL, et al. Effects of alpha-ketoisocaproate and of leucine on nitrogen metabolism in postoperative patients. Lancet. 1983;1(8332):1010–1014.[↩]
- Holeček M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J Cachexia Sarcopenia Muscle. 2017;8:529–541[↩]
- Hotamisligil GS, Erbay E (2008). Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 8: 923–934.[↩]
- Romanyukha AA, Rudnev SG, Sidorov IA (2006). Energy cost of infection burden: an approach to understanding the dynamics of host‐pathogen interactions. J Theor Biol 241: 1–13[↩]
- Demas GE, Drazen DL, Nelson RJ (2003). Reductions in total body fat decrease humoral immunity. Proc Biol Sci 270: 905–911.[↩]
- Konner AC, Bruning JC (2011). Toll‐like receptors: linking inflammation to metabolism. Trends Endocrinol Metab 22: 16–23.[↩]
- Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y et al. (2007). IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell 130: 440–455.[↩]
- Hasnain SZ, Borg DJ, Harcourt BE, Tong H, Sheng YH, Ng CP et al. (2014). Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med 20: 1417–1426.[↩]
- Lesourd B (1995). Protein undernutrition as the major cause of decreased immune function in the elderly: clinical and functional implications. Nutr Rev 53: S86–91; discussion S92–84[↩]
- Roth E (2008). Nonnutritive effects of glutamine. J Nutr 138: 2025S–2031S.[↩]
- Calder PC (2006). Branched‐chain amino acids and immunity. J Nutr 136: 288S–293S. https://www.ncbi.nlm.nih.gov/pubmed/16365100[↩]
- Glassy MC, Furlong CE (1981). Neutral amino acid transport during the cell cycle of cultured human lymphocytes. J Cell Physiol 107: 69–74.[↩]
- Bassit RA, Sawada LA, Bacurau RF, Navarro F, Martins E Jr, Santos RV et al. (2002). Branched‐chain amino acid supplementation and the immune response of long‐distance athletes. Nutrition 18: 376–379.[↩]
- Aquilani R, Zuccarelli GC, Dioguardi FS, Baiardi P, Frustaglia A, Rutili C et al. (2011). Effects of oral amino acid supplementation on long‐term‐care‐acquired infections in elderly patients. Arch Gerontol Geriatr 52: e123–e128.[↩]
- Nakamura I, Ochiai K, Imai Y, Moriyasu F, Imawari M (2007). Restoration of innate host defense responses by oral supplementation of branched‐chain amino acids in decompensated cirrhotic patients. Hepatol Res 37: 1062–1067.[↩]
- Bolasco P, Caria S, Cupisti A, Secci R, Saverio Dioguardi F (2011). A novel amino acids oral supplementation in hemodialysis patients: a pilot study. Ren Fail 33: 1–5.[↩]
- Fretwell LK, McCune S, Fone JV, Yates DJ (2006). The effect of supplementation with branched‐chain amino acids on cognitive function in active dogs. J Nutr 136: 2069S–2071S.[↩]
- Cole JT, Mitala CM, Kundu S, Verma A, Elkind JA, Nissim I et al. (2010). Dietary branched chain amino acids ameliorate injury‐induced cognitive impairment. Proc Natl Acad Sci U S A 107: 366–371.[↩][↩]
- Aquilani R, Iadarola P, Contardi A, Boselli M, Verri M, Pastoris O et al. (2005). Branched‐chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil 86: 1729–1735.[↩]
- Aquilani R, Boselli M, Boschi F, Viglio S, Iadarola P, Dossena M et al. (2008). Branched‐chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil 89: 1642–1647.[↩]
- Fischer JE, Funovics JM, Aguirre A, James JH, Keane JM, Wesdorp RI, et al. The role of plasma amino acids in hepatic encephalopathy. Surgery. 1975;78:276–290.[↩]
- Pedroso JA, Zampieri TT, Donato J. Reviewing the effects of L-leucine supplementation in the regulation of food intake, energy balance, and glucose homeostasis. Nutrients. 2015;7:3914–3937.[↩]
- Zhang S, Zeng X, Ren M, Mao X, Qiao S. Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol. 2017;8:10.[↩][↩]
- Tremblay F, Lavigne C, Jacques H, Marette A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr. 2007;27:293–310.[↩]
- White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol Metab. 2016;5:538–551.[↩]
- Manchester KL. Oxidation of amino acids by isolated rat diaphragm and the influence of insulin. Biochim Biophys Acta. 1965;100:295–298.[↩]
- Holecek M, Siman P, Vodenicarovova M, Kandar R. Alterations in protein and amino acid metabolism in rats fed a branched-chain amino acid- or leucine-enriched diet during postprandial and postabsorptive states. Nutr Metab (Lond) 2016;13:12.[↩][↩]
- Jiménez Jiménez FJ, Ortiz Leyba C, Morales Ménedez S, Barros Pérez M, Muñoz GJ. Prospective study on the efficacy of branched-chain amino acids in septic patients. J Parenter Enter Nutr. 1991;15:252–261.[↩]
- Mattick JSA, Kamisoglu K, Ierapetritou MG, Androulakis IP, Berthiaume F. Branched-chain amino acid supplementation: impact on signaling and relevance to critical illness. Wiley Interdiscip Rev Syst Biol Med. 2013;5:449–460.[↩]
- Lang CH, Frost RA. Endotoxin disrupts the leucine-signaling pathway involving phosphorylation of mTOR, 4E-BP1, and S6K1 in skeletal muscle. J Cell Physiol. 2005;203:144–155.[↩]
- Cole JT, Mitala CM, Kundu S, Verma A, Elkind JA, Nissim I, Cohen AS. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107:366–371.[↩]
- Jeter CB, Hergenroeder GW, Ward NH, Moore AN, Dash PK. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30:671–679.[↩]
- Kamin H (1985). Status of the 10th edition of the Recommended Dietary Allowances‐‐prospects for the future. Am J Clin Nutr 41: 165–170.[↩][↩][↩]
- Strauss KA, Puffenberger EG, Morton DH (1993). Maple Syrup Urine Disease In: Pagon RA, editor; , Adam MP, editor; , Ardinger HH, editor; , Wallace SE, editor; , Amemiya A, editor; , Bean LJH, editor. et al. (eds). GeneReviews(R): Seattle (WA) Bookshelf ID: NBK1319[↩]
- Matthews DE (2005). Observations of branched‐chain amino acid administration in humans. J Nutr 135: 1580S–1584S[↩]
- Rasmussen B, Gilbert E, Turki A, Madden K, Elango R (2016). Determination of the safety of leucine supplementation in healthy elderly men. Amino Acids 48: 1707–1716.[↩]
- Matsueda S, Niiyama Y (1982). The effects of excess amino acids on maintenance of pregnancy and fetal growth in rats. J Nutr Sci Vitaminol (Tokyo) 28: 557–573.[↩]
- Thoemke F, Huether G (1984). Breeding rats on amino acid imbalanced diets for three consecutive generations affects the concentrations of putative amino acid transmitters in the developing brain. Int J Dev Neurosci 2: 567–574.[↩]
- Schauder P, Herbertz L, Langenbeck U. Serum branched chain amino and keto acid response to fasting in humans. Metabolism. 1985;34:58–61.[↩]
- Holecek M, Sprongl L, Tilser I. Metabolism of branched-chain amino acids in starved rats: the role of hepatic tissue. Physiol Res. 2001;50:25–33.[↩]
- Adibi SA, Peterson JA, Krzysik BA. Modulation of leucine transaminase activity by dietary means. Am J Phys. 1975;228:432–435.[↩]
- Holecek M, Rysava R, Safranek R, Kadlcikova J, Sprongl L. Acute effects of decreased glutamine supply on protein and amino acid metabolism in hepatic tissue: a study using isolated perfused rat liver. Metabolism. 2003;52:1062–1067.[↩]
- Holecek M. Effect of starvation on branched-chain alpha-keto acid dehydrogenase activity in rat heart and skeletal muscle. Physiol Res. 2001;50:19–24.[↩]
- Grimble RF, Whitehead RG. Changes in the concentration of specific amino acids in the serum of experimentally malnourished pigs. Br J Nutr. 1970;24:557–564.[↩]
- Holt LE, Snyderman SE, Norton PM, Roitman E, Finch J. The plasma aminogram in kwashiorkor. Lancet. 1963;2(7322):1342–1348.[↩]
- Reeds PJ. The catabolism of valine in the malnourished rat. Studies in vivo and in vitro with different labelled forms of valine. Br J Nutr. 1974;31:259–270.[↩]
- Wahren J, Felig P, Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976;57:987–999.[↩]
- Holecek M, Kovarik M. Alterations in protein metabolism and amino acid concentrations in rats fed by a high-protein (casein-enriched) diet – effect of starvation. Food Chem Toxicol. 2011;49:3336–3342.[↩]
- Watford M. Lowered concentrations of branched-chain amino acids result in impaired growth and neurological problems: insights from a branched-chain alpha-keto acid dehydrogenase complex kinase-deficient mouse model. Nutr Rev. 2007;65:167–172.[↩]
- Blomstrand E. Amino acids and central fatigue. Amino Acids. 2001;20:25–34.[↩]
- D’Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F et al. (2010). Branched‐chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle‐aged mice. Cell Metab 12: 362–372.[↩][↩][↩][↩][↩]
- Pansarasa O, Flati V, Corsetti G, Brocca L, Pasini E, D’Antona G (2008). Oral amino acid supplementation counteracts age‐induced sarcopenia in elderly rats. Am J Cardiol 101: 35E–41E.[↩]
- Flati V, Caliaro F, Speca S, Corsetti G, Cardile A, Nisoli E et al. (2010). Essential amino acids improve insulin activation of AKT/MTOR signaling in soleus muscle of aged rats. Int J Immunopathol Pharmacol 23: 81–89.[↩]
- Yamamoto D, Maki T, Herningtyas EH, Ikeshita N, Shibahara H et al. (2010). Branched‐chain amino acids protect against dexamethasone‐induced soleus muscle atrophy in rats. Muscle Nerve 41: 819–827.[↩]
- D’Antona G, Tedesco L, Ruocco C, Corsetti G, Ragni M, Fossati A et al. (2016). A peculiar formula of essential amino acids prevents rosuvastatin myopathy in mice. Antioxid Redox Signal 25: 595–608.[↩]
- Dasarathy S, Hatzoglou M. Hyperammonemia and proteostasis in cirrhosis. Curr Opin Clin Nutr Metab Care. 2018;21:30–36.[↩]
- Holeček M, Šprongl L, Tichý M. Effect of hyperammonemia on leucine and protein metabolism in rats. Metabolism. 2000;49:1330–1334[↩]
- Holecek M, Kandar R, Sispera L, Kovarik M. Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Amino Acids. 2011;40:575–584.[↩]
- Holeček M, Mráz J, Tilšer I. Plasma amino acids in four models of experimental liver injury in rats. Amino Acids. 1996;10:229–241.[↩]
- Holecek M. Evidence of a vicious cycle in glutamine synthesis and breakdown in pathogenesis of hepatic encephalopathy‐therapeutic perspectives. Metabolic Brain Disease 2014;29(1):9‐17.[↩]
- García‐Martínez R, Córdoba J. Acute‐on‐chronic liver failure: the brain. Current Opinion in Critical Care 2011;17(2):177‐83.[↩]
- Holecek M, Kandar R, Sispera L, Kovarik M. Acute hyperammonemia activates branched‐chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Amino Acids 2011;40(2):575‐84.[↩]
- Holecek M. Branched‐chain amino acids and ammonia metabolism in liver disease: therapeutic implications. Nutrition (Burbank, Los Angeles County, Calif.) 2013;29(10):1186‐91.[↩][↩]
- Gluud LL, Dam G, Les I, Córdoba J, Marchesini G, Borre M, Aagaard NK, Vilstrup H. Branched‐chain amino acids for people with hepatic encephalopathy. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No.: CD001939. DOI: 10.1002/14651858.CD001939.pub3[↩]
- Kachaamy T, Bajaj JS. Diet and cognition in chronic liver disease. Current Opinion Gastroenterology 2011;27:174‐9.[↩]
- Olde Damink SW, Jalan R, Redhead DN, Hayes PC, Deutz NE, Soeters PB. Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology 2002;36(5):1163‐71.[↩]
- Holecek M. Three targets of branched‐chain amino acid supplementation in the treatment of liver disease. Nutrition (Burbank, Los Angeles County, Calif.) 2010;26(5):482‐90.[↩]
- Kawaguchi T, Taniguchi E, Sata M. Effects of oral branched‐chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutrition in Clinical Practice 2013;28(5):580‐8.[↩]
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA‐glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. Journal of Neurochemistry 2006;98:641‐53.[↩]
- Holecek M. Three targets of branched-chain amino acid supplementation in the treatment of liver disease. Nutrition. 2010;26:482–490.[↩]
- Gluud LL, Dam G, Les I, Córdoba J, Marchesini G, Borre M, et al. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2015;9:CD001939.[↩]
- Holeček M. Branched-chain amino acid supplementation in treatment of liver cirrhosis: updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrition. 2017;41:80–85.[↩][↩]
- Rodney S, Boneh A. Amino acid profiles in patients with urea cycle disorders at admission to hospital due to metabolic decompensation. JIMD Rep. 2013;9:97–104.[↩]
- Holecek M. Evidence of a vicious cycle in glutamine synthesis and breakdown in pathogenesis of hepatic encephalopathy-therapeutic perspectives. Metab Brain Dis. 2014;29:9–17.[↩]
- Holecek M, Vodenicarovova M, Siman P. Acute effects of phenylbutyrate on glutamine, branched-chain amino acid and protein metabolism in skeletal muscles of rats. Int J Exp Pathol. 2017;98:127–133.[↩]
- Scaglia F, Carter S, O’Brien WE, Lee B. Effect of alternative pathway therapy on branched chain amino acid metabolism in urea cycle disorder patients. Mol Genet Metab. 2004;81:S79–S85.[↩]
- Adam S, Almeida MF, Assoun M, Baruteau J, Bernabei SM, Bigot S, et al. Dietary management of urea cycle disorders: European practice. Mol Genet Metab. 2013;110:439–445.[↩]
- Holecek M, Sprongl L, Tilser I, Tichý M. Leucine and protein metabolism in rats with chronic renal insufficiency. Exp Toxicol Pathol. 2001;53:71–76.[↩][↩][↩]
- May RC, Masud T, Logue B, Bailey J, England BK. Metabolic acidosis accelerates whole body protein degradation and leucine oxidation by a glucocorticoid-dependent mechanism. Miner Electrolyte Metab. 1992;18:245–249.[↩]
- Kovesdy CP, Kopple JD, Kalantar-Zadeh K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: reconciling low protein intake with nutritional therapy. Am J Clin Nutr. 2013;97:1163–1177.[↩]
- Ivy JH, Svec M, Freeman S. Free plasma levels and urinary excretion of eighteen amino acids in normal and diabetic dogs. Am J Phys. 1951;167:182–192.[↩]
- Jensen-Waern M, Andersson M, Kruse R, Nilsson B, Larsson R, Korsgren O, Essén-Gustavsson B. Effects of streptozotocin-induced diabetes in domestic pigs with focus on the amino acid metabolism. Lab Anim. 2009;43:249–254.[↩]
- Hutson SM, Harper AE. Blood and tissue branched-chain amino and alpha-keto acid concentrations: effect of diet, starvation, and disease. Am J Clin Nutr. 1981;34:173–183.[↩]
- Gibson R, Zhao Y, Jaskiewicz J, Fineberg SE, Harris RA. Effects of diabetes on the activity and content of the branched-chain alpha-ketoacid dehydrogenase complex in liver. Arch Biochem Biophys. 1993;306:22–28.[↩]
- Aftring RP, Miller WJ, Buse MG. Effects of diabetes and starvation on skeletal muscle branched-chain alpha-keto acid dehydrogenase activity. Am J Phys. 1988;254:E292–E300.[↩]
- Kuzuya T, Katano Y, Nakano I, Hirooka Y, Itoh A, Ishigami M, et al. Regulation of branched-chain amino acid catabolism in rat models for spontaneous type 2 diabetes mellitus. Biochem Biophys Res Commun. 2008;373:94–98.[↩][↩]
- Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453.[↩]
- White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol Metab. 2016;5:538–551[↩][↩]
- Macotela Y, Emanuelli B, Bång AM, Espinoza DO, Boucher J, Beebe K, et al. Dietary leucine – an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6:e21187.[↩]
- Hinault C, Mothe-Satney I, Gautier N, Lawrence JC, Jr, Van Obberghen E. Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/db mice. FASEB J. 2004;18:1894–1896.[↩]
- Arakawa M, Masaki T, Nishimura J, Seike M, Yoshimatsu H. The effects of branched-chain amino acid granules on the accumulation of tissue triglycerides and uncoupling proteins in diet-induced obese mice. Endocr J. 2011;58:161–170.[↩]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326[↩]
- Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Holecek M, Kandar R, Sispera L, Kovarik M. Amino Acids. 2011 Feb; 40(2):575-84.[↩]
- dos Santos RV, Caperuto EC, de Mello MT, Batista ML, Jr, Rosa LF. Effect of exercise on glutamine synthesis and transport in skeletal muscle from rats. Clin Exp Pharmacol Physiol. 2009;36:770–775.[↩]
- Xiao W, Chen P, Liu X, Zhao L. The Impaired Function of Macrophages Induced by Strenuous Exercise Could Not Be Ameliorated by BCAA Supplementation. Nutrients. 2015;7(10):8645-8656. doi:10.3390/nu7105425. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4632445/[↩][↩]
- Yoshiharu Shimomura, Yuko Yamamoto, Gustavo Bajotto, Juichi Sato, Taro Murakami, Noriko Shimomura, Hisamine Kobayashi, Kazunori Mawatari; Nutraceutical Effects of Branched-Chain Amino Acids on Skeletal Muscle, The Journal of Nutrition, Volume 136, Issue 2, 1 February 2006, Pages 529S–532S, https://doi.org/10.1093/jn/136.2.529S[↩][↩]
- Negro M., Giardina S., Marzani B., Marzatico F. Branched-chain amino acid supplementation does not enhance athletic performance but affects muscle recovery and the immune system. J. Sports Med. Phys. Fit. 2008;48:347–351. https://www.ncbi.nlm.nih.gov/pubmed/18974721[↩]
- Newsholme P. Why is l-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 2001;131:2515S–2522S. https://www.ncbi.nlm.nih.gov/pubmed/11533304[↩]
- Shimomura Y, Fujii H, Suzuki M, Murakami T, Fujitsuka N, Nakai N. Branched-chain alpha-keto acid dehydrogenase complex in rat skeletal muscle: regulation of the activity and gene expression by nutrition and physical exercise. J Nutr. 1995;125:1762S–1765S.[↩]
- Poortmans JR, Siest G, Galteau MM, Houot O. Distribution of plasma amino acids in humans during submaximal prolonged exercise. Eur J Appl Physiol Occup Physiol. 1974;32:143–147.[↩]
- Refsum HE, Gjessing LR, Strømme SB. Changes in plasma amino acid distribution and urine amino acids excretion during prolonged heavy exercise. Scand J Clin Lab Invest. 1979;39:407–413.[↩]
- Ahlborg G, Felig P, Hagenfeldt L, Hendler R, Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest. 1974;53:1080–1090.[↩]
- Shimomura Y, Murakami T, Nakai N, Nagasaki M, Harris RA. Exercise promotes BCAA catabolism: effects of BCAA supplementation on skeletal muscle during exercise. J Nutr. 2004;134:1583S–1587S. https://www.ncbi.nlm.nih.gov/pubmed/15173434[↩]
- Yoshiharu Shimomura, Taro Murakami, Naoya Nakai, Masaru Nagasaki, Robert A. Harris; Exercise Promotes BCAA Catabolism: Effects of BCAA Supplementation on Skeletal Muscle during Exercise, The Journal of Nutrition, Volume 134, Issue 6, 1 June 2004, Pages 1583S–1587S, https://doi.org/10.1093/jn/134.6.1583S[↩]
- Spillane M, Emerson C, Willoughby DS. The effects of 8 weeks of heavy resistance training and branched-chain amino acid supplementation on body composition and muscle performance. Nutr Health. 2012;21:263–273.[↩]
- Falavigna G, de Araújo AJ, Rogero MM, Pires IS, Pedrosa RG, Martins E, et al. Effects of diets supplemented with branched-chain amino acids on the performance and fatigue mechanisms of rats submitted to prolonged physical exercise. Nutrients. 2012;4:1767–1780.[↩]
- Smriga, M., Kameishi, M., Tanaka, T., Kondoh, T. & Torii, K. (2002) Preference for a solution of branched-chain amino acids plus glutamine and arginine correlates with free running activity in rats: involvement of serotonergic-dependent processes of lateral hypothalamus. Nutr. Neurosci. 5: 189–199.[↩]