Contents
What is whey protein
Milk proteins have long been known for their nutritional and technological value. Proteins are important constituents of the human diet, since they comprise a principal source of nitrogen and essential amino acids. Milk proteins have high nutritional value compared to other proteins because of their relatively high content of essential amino acids and good digestibility 1. Cow’s milk contains about 3.5 g of protein per 100 mL, of which whey accounts for about 20% and casein 80% 2.
Whey proteins and caseins are the two main protein groups in milk 3. Caseins, representing about 80% of the protein content in cow’s milk, are isolated from milk by acid or by rennet precipitation. The acid or isoelectric, precipitation is performed at pH 4.6, where the caseins precipitate and the whey proteins remain soluble. Caseins are flexible and heat stable proteins.
Whey proteins comprise approximately 20% of the total milk proteins 3. Whey proteins (or cow’s milk serum proteins) are defined as proteins in milk that remain soluble after acid 4 or after rennet casein precipitation 5. Acid precipitation yields acid whey, while rennet casein precipitation yields sweet or rennet whey 6. Whey consists of a heterogeneous group of proteins, including beta-lactoglobulin (35%), alpha-lactalbumin (12%), proteose peptone (12%), immunoglobulins (8%), and bovine serum albumin (5%) 7, 8. When chymosin is used in the cheese-making process, glycomacropeptide – which is high in branched chain amino acids – accounts for about 12% of total protein in whey 9. Up to 1% of the total protein content of whey comprises “low abundance” proteins, including lactoferrin, and lactoperoxidase 8. All these proteins have been reported to have nutritional and/or physiological functions 10.
Whey proteins are considered globular proteins that are soluble over a broad pH range between pH 2 and 10 11.
From the nutritional point of view, milk whey proteins have been considered superior to casein in various aspects. They present amino acid profile superior to casein, being similar to human milk, is what recommends whey proteins for the formulation of humanized milk products for replacement of bovine milk in infant nutrition 12. Whey protein from cow’s milk is also a rich source of essential and branched chain amino acids 13. Some publications 14, 15 reported on important differential properties between caseins and the milk whey proteins. It was observed that the caseins undergo much lower digestion and absorption than the whey proteins.
Table 1. Whey proteins component and their biological activities
| Components of whey protein | Biological Function | Species | References |
|---|---|---|---|
| α-lactalbumin | Enhancement of antibody response to systematic antigen stimulation and used in manufacturing of infant food | Camel, bovine, and human | 16 |
| Lactoferrin | Antimicrobial activities against microorganisms, anticancer, anti- inflammatory | Camel, bovine, and human | 17 |
| β-lactoglobulin | Source of essential and branched chain amino acid, responsible for child allergy | Bovine, buffalo, caprine, and equine | 18 |
| Lysozymes | Antibacterial protein present in milk, tears, and saliva, and thus plays an important role in enhancing innate immunity | Camel and bovine | 19 |
| Immunoglobulin | Enhances immune functions | Camel, bovine, and human | 20 |
| Lactoperoxidase | Suppression of bacterial growth | Camel and bovine | 21 |
| Glycomacropeptide | Has an inhibitory effect on acid gastric secretion and modifies the concentration of blood which regulates digestive peptides | Camel and bovine | 22 |
The amino acid composition is the most important factor in defining food protein quality, followed by the digestibility of the protein and the bioavailability of its amino acids 3. Because of their amino acid composition the main cow’s milk proteins, caseins and whey proteins, can be regarded as a complete source of amino acids. Milk proteins are currently the main source of a range of biologically active peptides and the occurrence of their specific physiological properties which might have nutritional implication is another aspect of their nutritive value.
Table 2. Composition (%) of different whey protein forms
| Component | Whey Powder | Whey Concentrate | Whey Isolate |
|---|---|---|---|
| Protein | 11 – 14.5 | 25 – 89 | 90 |
| Lactose | 63 – 75 | 10 – 55 | 0.5 |
| Milk Fat | 1 – 1.5 | 2 – 10 | 0.5 |
- Whey Protein Powder
Whey protein powder has many applications throughout the food industry 24. As an additive it is seen in food products for beef, dairy, bakery, confectionery, and snack products. Whey powder itself has several different varieties including sweet whey, acid whey (seen in salad dressings), demineralized (seen primarily as a food additive including infant formulas), and reduced forms. The demineralized and reduced forms are used in products other than sports supplements.
- Whey Protein Concentrate
The processing of whey concentrate removes the water, lactose, ash, and some minerals 24. In addition, compared to whey isolates whey concentrate typically contains more biologically active components and proteins that make them a very attractive supplement for the athlete 24.
- Whey Protein Isolate (WPI)
Isolates are the purest protein source available 24. Whey protein isolates contain protein concentrations of 90% or higher. During the processing of whey protein isolate there is a significant removal of fat and lactose. As a result, individuals who are lactose-intolerant can often safely take these products 25. Although the concentration of protein in this form of whey protein is the highest, it often contain proteins that have become denatured due to the manufacturing process. The denaturation of proteins involves breaking down their structure and losing peptide bonds and reducing the effectiveness of the protein.
The nutritive value of whey protein concentrate and its hydrolysates was superior to that of sodium caseinate and its hydrolysates as indicated by some nutritional parameters such as the amino acid composition, chemical score, essential amino acid index and predicted biological value. However, the enzymic protein efficiency ratio was lower for the whey protein concentrate hydrolysates as compared to unhydrolyzed whey protein concentrate but sodium caseinate and its hydrolysates did not differ significantly. The nutritional qualities of whey protein concentrate, sodium caseinate and their hydrolysates were good and make them appropriate for food formulations or as nutritional supplements 3.
What are Amino Acids?
Amino acids are organic compounds that combine to form proteins 26. Amino acids and proteins are the building blocks of life that help maintain and repair muscles, organs, and other parts of the body.
Animal protein includes all of the building blocks that your body needs. Plant proteins need to be combined to get all of the building blocks that your body needs.
In the human body, certain amino acids can be converted to other amino acids, proteins, glucose, fatty acids or ketones. For example, in the human body, glucogenic amino acids can be converted to glucose in the process called gluconeogenesis; they include all amino acids except lysine and leucine 27, 28.
Ketogenic amino acids, are amino acids that can be converted into ketone bodies through ketogenesis. In humans, the ketogenic amino acids are leucine and lysine, while threonine, isoleucine, phenylalanine, tyrosine and tryptophan can be either ketogenic or glucogenic 29. Ketones can be used by the brain as a source of energy during fasting or in a low-carbohydrate diet.
When proteins are digested or broken down, amino acids are left.
The human body uses amino acids to make proteins to help the body:
- Break down food
- Grow
- Repair body tissue
- Perform many other body functions
- Amino acids can also be used as a source of energy by the body, like proteins, they can provide about 4 Calories per gram.
Other functions of amino acids:
- Chemical messengers (neurotransmitters) in the nervous system: aspartate, GABA, glutamate, glycine, serine
- Precursors of other neurotransmitters or amino acid-based hormones:
- Tyrosine is a precursor of dopamine, epinephrine, norepinephrine and thyroxine.
- Tryptophan is a precursor of melatonin and serotonin and nicotinic acid (vitamin B3).
- Histidine is a precursor of histamine.
- Glycine is a precursor of heme, a part of hemoglobin.
- Aspartate, glutamate and glycine are precursors of nucleic acids, which are parts of DNA.
Amino acids are classified into three groups:
- Essential amino acids.
- Nonessential amino acids.
- Conditional amino acids.
You do not need to eat essential and nonessential amino acids at every meal, but getting a balance of them over the whole day is important. A diet based on a single plant item will not be adequate, but we no longer worry about pairing proteins (such as beans with rice) at a single meal. Instead we look at the adequacy of the diet overall throughout the day.
Essential Amino Acids
The 9 amino acids are essential (vital), which means they are necessary for the human life and health but cannot be produced in your body so you need to get them from foods 30.
- Histidine (His)
- Isoleucine (Ile)
- Leucine (Leu)
- Lysine (Lys)
- Methionine (Met)
- Phenylalanine (Phe)
- Threonine (Thr)
- Tryptophan (Trp)
- Valine (Val).
Conditionally Essential Amino Acids
These amino acids can be synthesized in your body, but in certain circumstances, like young age, illness or hard exercise, you need to get them in additional amounts from foods to meet the body requirements for them. Ornithine is also considered conditionally essential amino acid, but it does not form proteins 26.
- Arginine (Arg)
- Cysteine (Cys)
- Glutamine (Gln)
- Glycine (Gly)
- Proline (Pro)
- Serine (Ser)
- Tyrosine (Tyr)
Nonessential Amino Acids
These amino acids can be synthesized in your body from other amino acids, glucose and fatty acids, so you do not need to get them from foods.
- Alanine (Ala)
- Asparagine (Asn)
- Aspartic acid (Asp)
- Glutamic acid (Glu)
- Selenocysteine (Sec)
The amino acid composition is the most important factor in defining food protein quality, followed by the digestibility of the protein and the bioavailability of its amino acids. Due to their amino acid composition, the major bovine milk proteins, which are caseins and whey proteins, are regarded as complete protein sources of essential amino acids 13. However, whey proteins are considered superior to other types of proteins such as casein due to their better digestibility, absorption and closer amino acid profile to human requirements 14, 31, 32. In addition to the study of their nutritive value, milk proteins are extensively being examined as the primary food sources for a variety of biologically-active peptides used in clinical applications such as hypertension, hypercholesterolemia, cancer, and inflammatory bowel disease 3. The major whey proteins are β-lactoglobulin (β-lb), α-lactalbumin (α-la), serum albumin, immunoglobulins, and glycomacropeptide (GMP), while minor proteins include lactoperoxidase, lactoferrin (Lf), β2-microglobulin, lysozyme, insulin-like growth factor, γ-globulins and several other small proteins [9]. A large number of human and animal feeding trials have indicated that whey proteins exert putative health benefits that include anti-inflammatory, immune-enhancement, antioxidative, and anabolic effects 33.
Table 2. Amino acid pattern (mg/g protein) of whey protein concentrate and its hydrolysates, human milk and the FAO/WHO/UNU reference standard
| Amino acid | Whey protein concentrate | Whey protein concentrate hydrolysates | Human milka | FAO/WHO/UNU 34 reference standardb | |||
| 5% DH | 10% DH | 15% DH | 20% DH | ||||
| Essential | |||||||
| Ile | 49.7 | 54.1 | 52.4 | 54.6 | 49.1 | 49 | 28 |
| Leu | 106.6 | 105.7 | 110.1 | 108.4 | 106.5 | 91 | 66 |
| Lys | 88.1 | 91.5 | 93.2 | 92.5 | 91.9 | 65 | 58 |
| Met+Cys | 79.7 | 40.7 | 43.5 | 41.1 | 42.2 | 37 | 25 |
| Phe+Tyr | 58.2 | 61.0 | 63.2 | 61.6 | 60.6 | 76 | 63 |
| Thr | 68.7 | 72.9 | 71.9 | 70.5 | 71.8 | 44 | 34 |
| Val | 18.4 | 53.5 | 51.7 | 54.6 | 47.8 | 52 | 35 |
| Trp | 17.3 | 21.4 | 17.9 | 19.7 | 17.1 | NAc | 11 |
| Non-essential | |||||||
| His | 7.8 | 17.8 | 18.4 | 17.4 | 16.7 | ||
| Ala | 55.5 | 54.1 | 54.8 | 54.0 | 54.7 | ||
| Arg | 27.1 | 29.0 | 29.0 | 29.4 | 28.2 | ||
| Asp | 91.8 | 91.1 | 93.0 | 90.4 | 93.4 | ||
| Glu | 158.4 | 172.5 | 170.8 | 167.6 | 169.4 | ||
| Gly | 53.2 | 19.1 | 19.6 | 19.6 | 19.9 | ||
| Pro | 66.6 | 60.5 | 55.1 | 64.5 | 74.8 | ||
| Ser | 53.0 | 55.1 | 55.3 | 54.0 | 55.6 | ||
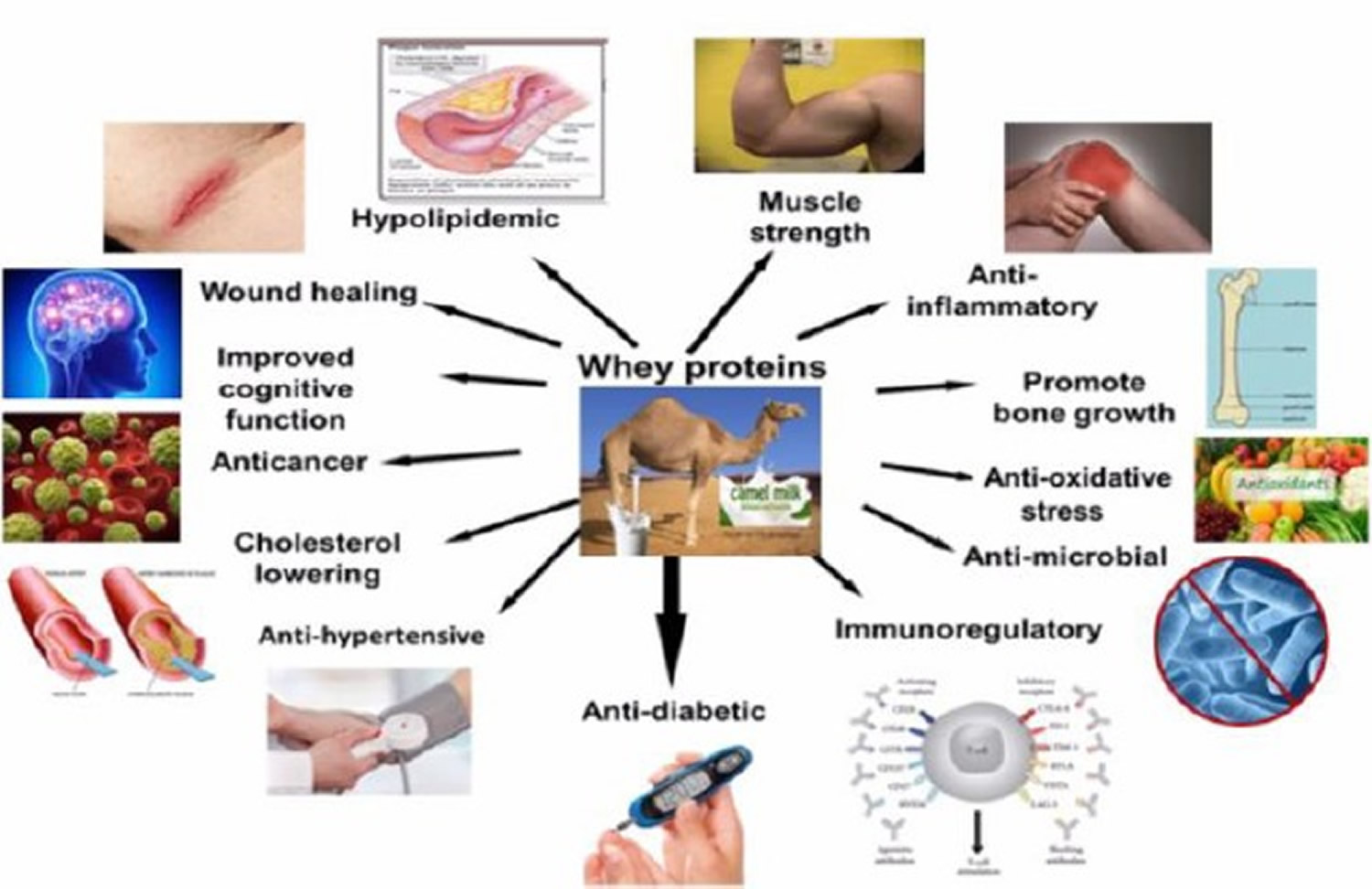
What does whey protein do?
Figure 1. Whey protein health benefits
Antioxidant and Anti-Inflammatory Effects of Whey Proteins
Several studies have demonstrated that whey protein intake can exert significant protective effects against oxidative stress imbalances associated with a variety of chronic and acute disease conditions including cancer, heart disease, diabetes, and inflammatory bowel disease 35, 36. Differences in essential amino acid composition between whole milk proteins with high biological value can greatly affect their biological and nutritional impact. For example, although both casein and whey are proteins with high biological value, several studies have indicated that the comparatively high concentration of cysteine in whey protein stimulates the synthesis of glutathione, an intracellular cysteine-containing peptide. This is not true for casein, which does not stimulate glutathione 37, 38. The main metabolic fates of cysteine are in synthesis of glutathione and specific acute phase proteins such as albumin and haptoglobin 39. Several studies have shown that sulphur amino acid requirements are increased in stress situations 40, 41 and that cysteine supplementation of the diet of septic rats had beneficial effects on recovery of protein status 41. Other authors suggested that these latter beneficial effects are associated with an increase in glutathione synthesis, since glutathione turnover may account for more than 50% of cysteine flux in healthy men 42. Another study has demonstrated that piglets with dextran sodium sulfate (DSS)-induced colitis supplemented with L-cysteine had markedly improved colon histology including lower inflammation, decreased crypt damage, and increased intestinal regeneration 43.
The anti-inflammatory effects of glycomacropeptide were investigated in a rat model of 2,4,6-trinitrobenzenesulfonic acid to induced colitis. Glycomacropeptide was administered orally to female Wistar rats 3 h post colitis induction. The results showed a decrease in body weight loss, anorexia, colonic damage, colonic alkaline phosphatase activity and IL-1β compared with control rats 44. Another study investigated the effect of whey protein in a rat model of DSS-induced colitis. Rats were fed diets containing casein (control), whey protein, or casein plus threonine and cysteine (positive control) for 14 day prior to DSS consumption for 7 day. The authors demonstrated that whey protein and positive control diets decreased colonic expression of IL-1β, calprotectin and iNOS, decreased diarrhea, and increased mucin secretion 45. The anti-oxidative and anti-inflammatory effects of Enprocal, a protein supplement containing a 41% content of whey protein concentrate (WPC), were investigated on gut cell proliferation. Caco-2 cells were treated with digested and undigested Enprocal. The results demonstrated a down regulation of TNF-α and IL-8 and upregulation of IL-2 and IL-10 secretion in Caco-2 cells fed digested Enprocal 46. These results demonstrated that digested whey protein products exert more potent bioactive properties than whole whey proteins, and promote a more physiological context in the cell culture system. Caco-2 intestinal epithelial cells have been used as an in vitro model of the gut epithelium 47. Caco-2 cell cultures have been used to model inflammatory bowel disease. Caco-2 cells exposed to inflammatory mediators such as IL-1β, TNF-α, IFN-γ and lipopolysaccharide (LPS) regulate gut maintenance and has a defence mechanism by controlling the permeability of intestinal epithelial and by acting as inflammatory mediators 48. The in vitro inflammatory response in the intestine is up-regulated by CXCL16 mRNA and protein expression, proposing an important role for this chemokine in the inflammation of the intestine 49.
β-lb is currently thought to be an important source of biologically active peptides that are inactive within the sequence of the precursor protein, but can be released by in vivo or in vitro enzymatic proteolysis. Once released and absorbed, these peptides may play important roles in human health promoting antihypertensive, antioxidant, and antimicrobial activities 50. The antioxidant activity of hydrolysates of β-lb was investigated following hydrolysis by different preparations of commercial proteases (pepsin, trypsin, chymotrypsin, thermolysin and corolase). The results demonstrated that a combination of pepsin, trypsin, and chymotrypsin was the most appropriate to produce β-lb hydrolysates having high oxygen radical scavenging activity, measured by oxygen radical absorbance capacity 51. The whey protein Lf binds to cationic metals such as Fe+2, Fe+3, Cu+2, Zn+2, Mn+2, and so could play a role in both stable iron delivery and scavenging of free iron and other minerals that would otherwise catalyze oxidative reactions 52. Moreover, studies carried out in mice have shown that administration of Lf can reduce gastritis and protect gut mucosal integrity during LPS-induced endotoxemia 53, 54. The above findings have been documented in a number of cell culture, animal and clinical trials and are summarized in Table 3.
Table 3. Antioxidant and anti-inflammatory effects of whey proteins and peptides
| Study Objective | Treatment | Overall Results | Reference |
|---|---|---|---|
| Supplementation with whey protein isolate (WPI) to augment intracellular glutathione (GSH) and enhance performance. | Adults received 20 g of WPI or casein for 3 months. | Enhanced lymphocyte GSH (35%); increased peak power and 30-s work capacity (13% ± 3.7%) in the WPI group. | 55 |
| Examine the importance of an adequate supply of cysteine and glycine to rats in a low and high protein diets. | Rats on a low protein diet (80 g/kg) supplemented with L-cysteine (4 g/kg) and glycine (5 g/kg), alone or in combination, or a high protein (200 g/kg) diet for 1 week before injection with tumor necrosis factor-alpha (TNF-α) or saline. | Increased liver weight, zinc and GSH concentrations in the high protein-fed rats; greater liver weight after TNF-α treatment in the rats supplemented with glycine and cysteine; enhanced ceruloplasmin, alpha-2-macroglobulin and alpha-1-acid glycoprotein in the TNF-α -treated rats than in saline controls in each dietary group. | 56 |
| Effect of glycomacropeptide (GMP) in a rat model of trinitrobenzene-sulfonic acid-induced colitis. | GMP orally administered to female Wistar rats 2 day prior or 3 h post colitis induction. | Dose-dependent decrease in body weight loss, anorexia, colonic damage, colonic alkaline phosphatase activity and interleukin-1 beta (IL-1β). | 44 |
| Effect of dietary cheese whey feeding in a rat model of dextran sodium sulfate (DSS)-induced colitis. | Male Wistar rats fed diets containing casein, cheese whey, or casein + threonine/cysteine for 14 day d prior to DSS consumption for 7 day. | Cheese whey and positive control diets decreased colonic expression of IL-1β, calprotectin and inducible nitric oxide synthase (iNOS); softened stools, decreased diarrhea, and increased mucin secretion. | 45 |
| Effect of supplementation of L-cysteine in piglets with DSS-induced colitis. | Piglets were fed 0.15 g/kg/day of L-cysteine or saline for 10 d. | Improved colon histology including lower inflammation, decreased crypt damage; increased intestinal regeneration in the piglets-fed L-cysteine. | 43 |
| Immunomodulatory, antioxidative and anti-inflammatory effects of Enprocal (41% whey protein concentrate (WPC) in gut cell proliferation. | Caco-2 cells treated with digested and undigested Enprocal. | Downregulation of TNF-α and IL-1β, upregulation of IL-2, IL-10 and interferon gamma (IFNγ) secretion; decreased adhesion of Jurkat E6-1 and Tamm-Horsfall Protein (THP)-1 cells to Caco-2 monolayer. | 46 |
| Effect of bovine and human lactoferrin (Lf) and lactoferricin B on a monocytic cell line. | Lipopolysaccharide (LPS)-stimulated THP-1 cells incubated with Lf or lactoferricin B. | Both Lf and lactoferricin B decreased IL-6 production. | 57 |
Effects of Whey Proteins on Strength and Lean Body Mass in Resistance-Trained Individuals
Even though the positive effects of whey protein-containing supplements for optimizing the anabolic responses and adaptations process in resistance-trained individuals have been supported by several investigations, their use continues to be controversial.
- This study compare muscle anabolic responses to a single bolus intake of whey or casein after performance of heavy resistance exercise 58. Young male individuals were randomly assigned to participate in two protein trials (n = 9) or one control trial (n = 8). Either whey, casein (0.3 g/kg lean body mass), or a noncaloric control drink was ingested immediately after exercise. The result was the immediate intake of whey and casein following heavy resistance exercise in young men results in similar muscle protein synthesis responses over the subsequent 6-hour recovery period. Myofibrillar protein synthesis was equally increased 1–6 h postexercise after whey and casein intake, both of which were higher compared with control. However, a trend toward a higher but temporally shorter muscle protein synthesis response with whey compared with a more moderate but prolonged muscle protein synthesis response with casein 58. These findings may have clinical relevance in situations where individuals face, e.g., restitution from rehabilitating resistance exercise, immobilization, or, in the case of an elderly individual, sarcopenia, in whom a protein-containing nutrient supplement might be needed. Milk, easily accessible and containing both whey and casein, could very likely be an optimal choice.
- This study 59 was designed to determine if the anabolic response of muscle to whey protein ingestion would be different depending on the timing of ingestion in relation to resistance exercise. The study examined the response of muscle protein balance to ingestion of whey proteins both before and following resistance exercise. Healthy volunteers were randomly assigned to one of two groups. A solution of whey proteins was consumed either immediately before exercise (n=8) or immediately following exercise (n=9). Each subject performed 10 sets of 8 repetitions of leg extension exercise. Phenylalanine concentrations were measured in femoral arteriovenous samples to determine balance across the leg. Arterial amino acid concentrations were elevated by ∼50%, and net amino acid balance switched from negative to positive following ingestion of proteins at either time. The results showed there was an anabolic response to ingestion of 20 g of whey proteins whether ingested before or 1 h following resistance exercise, and no differences were detected between time points. This result was in contrast to another study performed by the same researchers where they demonstrated that ingestion of essential amino acid plus carbohydrate before exercise resulted in a greater anabolic response than following exercise 60.
- Small dose (10 g) of whey protein with carbohydrate (21 g) in stimulating muscle protein synthesis following resistance exercise in trained young men 61.
- 13 male, recreational bodybuilders supplemented their normal diet with either hydrolyzed whey isolate or casein (1.5 g/kg body wt per day) 62. Strength was assessed by 1-RM (1-repetition maximum) in three exercises (barbell bench press, squat, and cable pull-down). Body composition was assessed by dual energy X-ray absorptiometry. All assessments occurred in the week before and the week following 10 week of training. The hydrolyzed whey isolate group achieved a significantly greater gain in lean mass than the casein group and a significant change in fat mass compared to the casein group. The hydrolyzed whey isolate group also achieved significantly greater improvements in strength compared to the casein group in each assessment of strength. When the strength changes were expressed relative to body weight, the hydrolyzed whey isolate group still achieved significantly greater improvements in strength compared to the casein group 62.
- 6 weeks of resistance training in 36 males randomly assigned to supplementation with whey protein (1.2 g/kg body wt per day), whey protein and creatine monohydrate (0.1 g/kg body wt per day), or placebo (1.2 g/kg body wt per day maltodextrin) 63. Measures included lean tissue mass by dual energy x-ray absorptiometry, bench press and squat strength 1-RM (1-repetition maximum), and knee extension/flexion peak torque. Lean tissue mass increased to a greater extent with training in creatine monohydrate compared to the other groups, and in the whey protein compared to the placebo group. Bench press strength increased to a greater extent for creatine monohydrate compared to whey protein and placebo. Knee extension peak torque increased with training for creatine monohydrate and whey protein, but not for placebo. All other measures increased to a similar extent across groups. Continued training without supplementation for an additional 6 weeks resulted in maintenance of strength and lean tissue mass in all groups. Males that supplemented with whey protein while resistance training demonstrated greater improvement in knee extension peak torque and lean tissue mass than males engaged in training alone 63. Males that supplemented with a combination of whey protein and creatine had greater increases in lean tissue mass and bench press than those who supplemented with only whey protein or placebo 63. However, not all strength measures were improved with supplementation, since subjects who supplemented with creatine and/or whey protein had similar increases in squat strength and knee flexion peak torque compared to subjects who received placebo 63.
- Meta-analysis to examine the effect of whey protein, with or without resistance exercise, on body weight and body composition in randomized controlled trials conducted in generally healthy adult study populations 64. Based on the meta-analysis of the current body of literature supports the use of whey protein, either as a supplement combined with resistance exercise or as part of a weight loss or weight maintenance diet, to improve body composition parameters 64.
- Meta-analysis to examine the effects of whey protein alone or as part of a multi-ingredient formulation on strength, fat-free mass, or lean body mass in resistance-trained individuals 65. The literature showed whey protein alone or as a part of a multi-ingredient appears to maximize lean body mass or fat-free mass gain, as well as upper and lower body strength improvement with respect to the ingestion of an iso-energetic equivalent carbohydrate or non-whey protein supplement in resistance-training individuals. This enhancement effect seems to be more evident when whey proteins are consumed within a multi-ingredient containing creatine 65..
- This study compared the post-exercise consumption of rice protein isolate and whey protein isolate in terms of recovery and changes in body composition following exercise 66. 24 college-aged, resistance trained males were recruited for this study. Subjects were randomly and equally divided into two groups, either consuming 48 g of rice or whey protein isolate (isocaloric and isonitrogenous) on training days. Subjects trained 3 days per week for 8 weeks as a part of a daily undulating periodized resistance-training program. The rice and whey protein supplements were consumed immediately following exercise. Ratings of perceived recovery, soreness, and readiness to train were recorded prior to and following the first training session. Ultrasonography determined muscle thickness, dual emission x-ray absorptiometry determined body composition, and bench press and leg press for upper and lower body strength were recorded during weeks 0, 4, and 8. The result of this study was no detectable differences were present in perceived recovery, soreness, or readiness to train. In other words, rice protein isolate supports changes in strength and body composition similarly to whey protein isolate. Rice protein isolate consumption post resistance exercise decreases fat-mass and increases lean body mass, skeletal muscle hypertrophy, power and strength comparable to whey protein isolate 66.
- Tang et al. 67 studied the acute response of muscle protein synthesis to rapidly (i.e., whey hydrolysate and soy) and slowly (i.e., micellar casein) digested proteins both at rest and after resistance exercise. Three groups of healthy young men (n = 6 per group) performed a bout of unilateral leg resistance exercise followed by the consumption of a drink containing an equivalent content of essential amino acids (10 g) as either whey hydrolysate, micellar casein, or soy protein isolate. Mixed muscle protein synthesis was determined by a primed constant infusion of l-[ring-13C6]phenylalanine. Ingestion of whey protein resulted in a larger increase in blood essential amino acid, branched-chain amino acid, and leucine concentrations than either casein or soy 67. Mixed muscle protein synthesis at rest (determined in the nonexercised leg) was higher with ingestion of faster proteins (whey = 0.091%/hour, soy = 0.078%/hour, casein = 0.047%/h); muscle protein synthesis after consumption of whey was ∼93% greater than casein and ∼18% greater than soy. A similar result was observed after exercise (whey > soy > casein); muscle protein synthesis following whey consumption was ∼122% greater than casein and 31% greater than soy. Muscle protein synthesis was also greater with soy consumption at rest (64%) and following resistance exercise (69%) compared with casein. They concluded that the feeding-induced simulation of muscle protein synthesis in young men is greater after whey hydrolysate or soy protein consumption than casein both at rest and after resistance exercise; moreover, despite both being fast proteins, whey hydrolysate stimulated muscle protein synthesis to a greater degree than soy after resistance exercise. These differences may be related to how quickly the proteins are digested (i.e., fast vs. slow) or possibly to small differences in leucine content of each protein 67.
- This study investigated the effects of soy and whey protein supplementation on sex hormones following an acute bout of heavy resistance exercise in resistance trained men 68. Ten resistance-trained men (age 21.7 years; height 175.0; weight 84.2) volunteered to participate in an investigation. All subjects completed 3 experimental treatment conditions supplementing with whey protein isolate, soy protein isolate, and maltodextrin placebo control for 14 days with participants ingesting 20 g of their assigned supplement each morning at approximately the same time each day. Following supplementation, subjects performed an acute heavy resistance exercise test consisting of 6 sets of 10 repetitions in the squat exercise at 80% of the subject’s 1-RM (one repetition maximum). This investigators observed lower testosterone responses following supplementation with soy protein in addition to a positive blunted cortisol response with the use of whey protein at some recovery time points. Although sex hormone binding globulin was proposed as a possible mechanism for understanding changes in androgen content, sex hormone binding globulin did not differ between experimental treatments. Importantly, there were no significant differences between groups in changes in estradiol concentrations. The conclusion was that 14 days of supplementation with soy protein does appear to partially blunt serum testosterone 68. In addition, whey influences the response of cortisol following an acute bout of resistance exercise by blunting its increase during recovery 68. Protein supplementation alters the physiological responses to a commonly used exercise modality with some differences due to the type of protein utilized. Many male exercisers avoid soy protein because there is a perception that it is inferior to proteins like whey for supporting lean boss mass gain 69 . This perception persists even though there are no studies comparing whey and soy for effects on lean body mass gain.
- Another study 69 comparing soy versus whey protein bars effects on on lean body mass and antioxidant status. Male subjects, aged 19–25, were recruited from the Sport, Fitness and Health Program courses at The Ohio State University to participate in the present 9-week study. All subjects were considered experienced weightlifters with at least 1 year or more experience in strength training. Subjects were reported to be non-smokers, non-vegetarians, not currently taking supplements of any kind, and having no major health problems (i.e., diabetes, cardiovascular disease, etc.). All subjects had a body mass index (BMI) of less than 30 kg/m2. The strength training protocol was 3 sets of 4–6 repetitions for 14 exercises so that strength was the variable being maximized. The following exercises were performed to work all major muscle groups: 1) chest press; 2) chest fly; 3) incline press; 4) lat pull-down; 5) seated row; 6) military press; 7) lateral raise; 8) preacher curl; 9) bicep curl; 10) supine tricep extension; 11) seated tricep extension; 12) leg press; 13) calf raise; and 14) abdominal crunches 69. Subjects were randomly assigned in a double-blind manner to either a soy, whey, or control group. The controls did the exercise program but did not consume a protein product (n = 9/each group). The soy protein bar contained 11 grams of protein and an assortment of micronutrients. The whey bars were made using the same recipe as the soy bars except that whey protein was substituted for soy protein. Each subject was instructed to consume 3 bars per day (total protein intake 33 g per day) for the 9-week training period. This was in addition to the subjects’ self-selected diet. The results were exercise training plus soy or whey treatments each produced a statistically significant increase in lean body mass, but the training alone did not 69. A comparison of the change in lean body mass for the soy group versus the change in the whey group did not show a significant difference, but the soy had the added benefit of preserving two aspects of antioxidant function. It should however be noted that the current study diet intervention used bars which included added micronutrients. Thus, this study did not determine if the effects of the soy or whey protein required co-administration of micronutrients. Another unresolved issue is whether the effects on lean body mass seen here for the two proteins were due to increased total protein intake or other factors e.g. the participants eating habits.
- This small study 70 involving the elderly combined regular resistance muscle training and whey protein and vitamin D intervention to prevent or reverse this biological deterioration in elderly people. The effects of the regular resistance muscle training (n = 17) alone and the combined exercise + special nutrition therapy containing whey protein and vitamin D (n = 17) were monitored for 3 months in 34 elderly patients (12 men and 22 women; mean age: 66.47 years) randomly distributed into two groups at a long-term care facility. Physical exercise alone did not result in significant improvement in skeletal muscle mass or strength, whereas combined intervention significantly increased the muscle strength 70. When therapeutic responses to the intervention were compared, a significant advantage of combined exercise + special nutrition therapy containing whey protein and vitamin D over physical exercise was found. In conclusion, combined intervention (physical exercise + whey protein and vitamin D) is necessary for the efficient protection of the musculature in the high-risk elderly patients 70.
Benefits of whey protein
This study evaluated the effects of a whey protein supplementation on intrahepatocellular lipids (fatty liver), and fasting plasma triglycerides in obese non diabetic women 71. Eleven obese women received a 60 g/day whey protein supplement for 4-weeks, while otherwise nourished on their normal diet. Fatty liver (intrahepatocellular lipids) concentrations, visceral body fat, total liver volume, fasting total-triglyceride and cholesterol concentrations, glucose tolerance (standard 75 g OGTT), insulin sensitivity, creatinine clearance, blood pressure and body composition (bio-impedance analysis) were assessed before and after 4-week 60 g/day whey protein supplement. The results showed 60 g/day whey protein supplement decreased significantly fatty liver by 20.8%, fasting total triglyceride by 15 ± 6.9%, and total cholesterol by 7.3 ± 2.7%. Whey protein supplement slightly increased fat free mass from 54.8 ± 2.2 kg to 56.7 ± 2.5 kg. Visceral fat, total liver volume, glucose tolerance, creatinine clearance and insulin sensitivity were not changed. In conclusions 60 g/day whey protein supplement improves hepatic steatosis and plasma lipid profiles in obese non diabetic patients, without adverse effects on glucose tolerance or creatinine clearance 71. On a study on mice with nonalcoholic fatty liver disease (NAFLD) showed that mice given whey proteins was accompanied by an improvement in fatty infiltration in the liver cells and a reduction of oxidative stress parameters 72.
In a study by Pal and colleagues 73 evaluating the effects of whey protein supplementation on body composition, lipids, insulin and glucose in comparison to casein and glucose (control) supplementation in overweight/obese individuals for 12 weeks. The subjects were randomised to whey protein, casein or glucose supplementation for 12 weeks. Fasting blood samples and dual-energy X-ray absorptiometry measurements were taken. Seventy men and women with a mean age of 48.4 years and a mean BMI of 31.3 kg/m2 completed the study. Subjects supplemented with whey protein had no significant change in body composition or serum glucose at 12 weeks compared with the control or casein group. Fasting triglyceride levels were significantly lowered in the whey group compared with the control group at 6 weeks and 12 weeks. There was a significant decrease in total cholesterol and LDL cholesterol at week 12 in the whey group compared with the casein and control groups. Fasting insulin levels and homeostasis model assessment of insulin resistance scores were also significantly decreased in the whey group compared with the control group. The present study demonstrated that supplementation with whey proteins improves fasting lipids and insulin levels in overweight and obese individuals 73.
This study 74 tested the hypothesis that nutritional supplementation with whey protein (22 g), essential amino acids (10.9 g, including 4 g leucine), and vitamin D [2.5 μg (100 IU)] concurrent with regular, controlled physical activity would increase fat-free mass, strength, physical function, and quality of life, and reduce the risk of malnutrition in sarcopenic elderly persons. A total of 130 sarcopenic elderly people (53 men and 77 women; mean age: 80.3 y) participated in a 12-wk randomized, double-blind, placebo-controlled supplementation trial. All participants concurrently took part in a controlled physical activity program. We examined body composition with dual-energy X-ray absorptiometry, muscle strength with a handgrip dynamometer, and blood biochemical indexes of nutritional and health status, and evaluated global nutritional status, physical function, and quality of life before and after the 12 wk of intervention. The results were compared with physical activity and placebo, supplementation plus physical activity increased fat-free mass (1.7-kg gain), relative skeletal muscle mass, android distribution of fat, handgrip strength, activities of daily living and insulin-like growth factor I and lowered C-reactive protein. In conclusion, supplementation with whey protein, essential amino acids, and vitamin D, in conjunction with age-appropriate exercise, not only boosts fat-free mass and strength but also enhances other aspects that contribute to well-being in sarcopenic elderly 74.
Another study 75 on the elderly to determine if the effects of whey protein ingestion on muscle protein accrual in elderly are due solely to its constituent essential and nonessential amino acid content. Fifteen elderly humans were randomly assigned to ingest a bolus of either 15 g of whey protein, 6.72 g of essential amino acids or 7.57 g of non-essential amino acids. In conclusion, 15 gram whey protein ingestion improves skeletal muscle protein accrual through mechanisms that are beyond those attributed to its essential amino acid content. This finding may have practical implications for the formulation of nutritional supplements to enhance muscle anabolism in older individuals 75.
Best whey protein
Determining the quality of a protein is determined by assessing its essential amino acid composition, digestibility and bioavailability of amino acids 76. There are several measurement scales and techniques that are used to evaluate the quality of protein.
Protein Rating Scales
Numerous methods exist to determine protein quality. These methods have been identified as protein efficiency ratio, biological value, net protein utilization, and protein digestibility corrected amino acid score.
- Protein Efficiency Ratio
The protein efficiency ratio (PER) determines the effectiveness of a protein through the measurement of animal growth. This technique requires feeding rats a test protein and then measuring the weight gain in grams per gram of protein consumed. The computed value is then compared to a standard value of 2.7, which is the standard value of casein protein. Any value that exceeds 2.7 is considered to be an excellent protein source. However, this calculation provides a measure of growth in rats and does not provide a strong correlation to the growth needs of humans 24.
- Biological Value
Biological value measures protein quality by calculating the nitrogen used for tissue formation divided by the nitrogen absorbed from food. This product is multiplied by 100 and expressed as a percentage of nitrogen utilized. The biological value provides a measurement of how efficient the body utilizes protein consumed in the diet. A food with a high value correlates to a high supply of the essential amino acids. Animal sources typically possess a higher biological value than vegetable sources due to the vegetable source’s lack of one or more of the essential amino acids. There are, however, some inherent problems with this rating system. The biological value does not take into consideration several key factors that influence the digestion of protein and interaction with other foods before absorption 24. The biological value also measures a protein’s maximal potential quality and not its estimate at requirement levels 24.
- Net Protein Utilization
Net protein utilization is similar to the biological value except that it involves a direct measure of retention of absorbed nitrogen 24. Net protein utilization and biological value both measure the same parameter of nitrogen retention, however, the difference lies in that the biological value is calculated from nitrogen absorbed whereas net protein utilization is from nitrogen ingested 24.
- Protein Digestibility Corrected Amino Acid Score (PDCAAS)
In 1989, the Food & Agriculture Organization and World Health Organization (FAO/WHO) in a joint position stand stated that protein quality could be determined by expressing the content of the first limiting essential amino acid of the test protein as a percentage of the content of the same amino acid content in a reference pattern of essential amino acids 76. The reference values used were based upon the essential amino acids requirements of preschool-age children. The recommendation of the joint FAO/WHO statement was to take this reference value and correct it for true fecal digestibility of the test protein. The value obtained was referred to as the protein digestibility corrected amino acid score (PDCAAS). This method has been adopted as the preferred method for measurement of the protein value in human nutrition 77. Table 3 provides a measure of the quantity of various proteins using these protein rating scales.
Although the protein digestibility corrected amino acid score (PDCAAS) is currently the most accepted and widely used method, limitations still exist relating to overestimation in the elderly (likely related to references values based on young individuals), influence of ileal digestibility, and antinutritional factors 78.
Amino acids that move past the terminal ileum may be an important route for bacterial consumption of amino acids, and any amino acids that reach the colon would not likely be utilized for protein synthesis, even though they do not appear in the feces 77. Thus, to get truly valid measure of fecal digestibility the location at which protein synthesis is determined is important in making a more accurate determination. Thus, ileal digestibility would provide a more accurate measure of digestibility. PDCAAS, however, does not factor ileal digestibility into its equation. This is considered to be one of the shortcomings of the PDCAAS 77.
Antinutritional factors such as trypsin inhibitors, lectins, and tannins present in certain protein sources such as soybean meal, peas and fava beans have been reported to increase losses of endogenous proteins at the terminal ileum 79. These antinutritional factors may cause reduced protein hydrolysis and amino acid absorption. This may also be more effected by age, as the ability of the gut to adapt to dietary nutritional insults may be reduced as part of the aging process 78.
Table 3. Protein quality rankings
| Protein Type | Protein Efficiency Ratio | Biological Value | Net Protein Utilization | Protein Digestibility Corrected Amino Acid Score |
|---|---|---|---|---|
| Beef | 2.9 | 80 | 73 | 0.92 |
| Black Beans | 0 | 0 | 0.75 | |
| Casein | 2.5 | 77 | 76 | 1.00 |
| Egg | 3.9 | 100 | 94 | 1.00 |
| Milk | 2.5 | 91 | 82 | 1.00 |
| Peanuts | 1.8 | 0.52 | ||
| Soy protein | 2.2 | 74 | 61 | 1.00 |
| Wheat gluten | 0.8 | 64 | 67 | 0.25 |
| Whey protein | 3.2 | 104 | 92 | 1.00 |
Adapted from: U.S Dairy Export Council, Reference Manual for U.S. Whey Products 2nd Edition, 1999 and Sarwar, 1997 78.
Whey protein and weight loss
In a randomized, double-blind, 12-week study evaluating a specialized whey fraction for use as a dietary supplement to enhance weight loss together with reduced calorie intake of 500 calories per day 80. One-hundred and fifty-eight subjects were recruited for this study through local advertising. Subjects were 25–50 years old with a body mass index (BMI) of 30–42 kg/m2. Subjects were assigned a diet plan with a certain number of servings for various food groups similar to the standard paradigm set by the American Heart Association 81. The composition of the planned diet was approximately 55% of calories as carbohydrate, 15% as protein, and 30% as fat. These percentages were distributed into 3 meals and 2 snacks per day. Each subject was instructed to consume one whey protein + minerals supplement 20 minutes before breakfast and one whey protein + minerals supplement 20 minutes before dinner. The whey protein + minerals supplement contained 10 grams of protein per serving as a combination of intact whey protein and peptides. It also contained minerals that were purified from milk. The control group received an iso-caloric beverage containing maltodextrin.
The composition of the whey protein + minerals supplement were:
| Component | Daily Intake |
| Protein (g) | 20 |
| Leucine (g) | 2.24 |
| Isoleucine (g) | 1.44 |
| Valine (g) | 1.26 |
| Total BCAA | 4.94 |
| Lysine | 1.91 |
| Cysteine | 0.50 |
| Methionine | 0.41 |
| Tryptophan | 0.44 |
| Phenylalanine | 0.64 |
| Histidine | 0.37 |
| Threonine | 1.59 |
| Tyrosine | 0.62 |
| Lipid (g) | 0.12 |
| Carbohydrate (g) | 0.83 |
| Lactose (g) | 0.27 |
| Minerals | |
| Calcium (mg) | 482 |
| Phosphorus (mg) | 253 |
| Sodium (mg) | 213 |
| Potassium (mg) | 100 |
| Magnesium (mg) | 50 |
| Zinc (ug) | 60 |
The results from this study was the particpants in both the control (iso-caloric beverage containing maltodextrin) and treatment group (20 gram whey protein + minerals supplement) lost a significant amount of weight with a 500 calorie reduced diet. In other words, there is no difference in weight loss between the control and subjects taking 20 gram whey protein + minerals. However, subjects taking 20 gram whey protein + minerals supplement lost significantly more body fat and showed a greater preservation of lean muscle compared to subjects consuming the control beverage 80. Because subjects taking 20 gram whey protein + minerals supplement lost 6.1% of their body fat mass and because a 5% reduction of body fat mass has been shown to reduce the risk of obesity related disease 82, 83, the results have practical significance.
Whey protein side effects
Too much protein can make the kidneys work harder, so people with chronic kidney disease may need to eat less protein 84.
- Hambraeus L. Nutritional Aspects of Milk Proteins. In: Fox PF, editor. Advanced Dairy Chemistry–1: Proteins. London: Elsevier Applied Science; 1992. pp. 457–490.[↩]
- Philanto A. Whey proteins and peptides: Emerging properties to promote health. NUTRA Foods. 2011;10:29–42.[↩]
- Sindayikengera S, Xia W. Nutritional evaluation of caseins and whey proteins and their hydrolysates from Protamex . Journal of Zhejiang University Science B. 2006;7(2):90-98. doi:10.1631/jzus.2006.B0090. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1363751/[↩][↩][↩][↩][↩][↩]
- Walstra P, Jenness R. Dairy Chemistry and Physics. New York: John Willey & Sons Inc; 1984. pp. 114–122.[↩]
- [Nutritional physiology of whey and whey components]. Barth CA, Behnke U. Nahrung. 1997 Feb; 41(1):2-12. https://www.ncbi.nlm.nih.gov/pubmed/9157293/[↩]
- Fuente M.A., Hemar Y., Tamehana M., Munro P.A., Singh H. Process-induced changes in whey proteins during the manufacture of whey protein concentrates. Int. Dairy J. 2002;12:361–369. doi: 10.1016/S0958-6946(02)00031-6.[↩]
- Marschall Rhône-Poulenc Award Lecture. Nutritional and functional characteristics of whey proteins in food products. de Wit JN. J Dairy Sci. 1998 Mar; 81(3):597-608. https://www.ncbi.nlm.nih.gov/pubmed/9565865/[↩]
- Emerging health properties of whey proteins and their clinical implications. Krissansen GW. J Am Coll Nutr. 2007 Dec; 26(6):713S-23S. https://www.ncbi.nlm.nih.gov/pubmed/18187438/[↩][↩]
- Therapeutic applications of whey protein. Marshall K. Altern Med Rev. 2004 Jun; 9(2):136-56. https://www.ncbi.nlm.nih.gov/pubmed/15253675/[↩]
- Smithers GW. Whey and whey proteins – from ‘gutter to gold’ Int Dairy J. 2008;18:695–704.[↩]
- Mulvihill DM. Production, Functional Properties and Utilization of Milk Protein Products. In: Fox PF, editor. Advanced Dairy Chemistry–1: Proteins. London: Elsevier Science Publishers; 1992. pp. 369–404.[↩]
- Hambraeus L. Nutritional Aspects of Milk Proteins. In: Fox PF, editor. Developments in Dairy Chemistry–1: Proteins. London: Applied Science Publishers; 1982. pp. 289–313.[↩][↩]
- Piccolomini AF, Kubow S, Lands LC. Clinical Potential of Hyperbaric Pressure-Treated Whey Protein. Samman S, Darnton-Hill I, eds. Healthcare. 2015;3(2):452-465. doi:10.3390/healthcare3020452. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4939533/[↩][↩][↩]
- Boirie Y, Dangin M, Gachon P, Vasson M-P, Maubois J-L, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14930-14935. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC25140/[↩][↩]
- Protein metabolism. Slow and fast dietary proteins. Frühbeck G. Nature. 1998 Feb 26; 391(6670):843, 845. https://www.ncbi.nlm.nih.gov/pubmed/9495333/[↩]
- Bounous G, Batist G, Gold P. Immunoenhancing property of dietary whey protein in mice: role of glutathione. Clin Invest Med. 1989 Jun;12(3):154-61.[↩]
- Legrand D, Pierce A, Elass E, Carpentier M, Mariller C, Mazurier J. Lactoferrin structure and functions. In Bioactive components of milk. Springer; 2008. pp. 163–194.[↩]
- Guimont C, Marchall E, Girardet JM, Linden G. Biologically active factors in bovine milk and dairy byproducts: influence on cell culture. Crit Rev Food Sci Nutr. 1997 Jun;37(4):393-410. doi: 10.1080/10408399709527780[↩]
- El-Agamy EI, Nawar M, Shamsia SM, Awad S, Haenlein GF. Are camel milk proteins convenient to the nutrition of cow milk allergic children?Small Rumin Res. 2009;82:1–6.[↩]
- Laleye LC, Jobe B, Wasesa AA. Comparative study on heat stability and functionality of camel and bovine milk whey proteins. J Dairy Sci. 2008 Dec;91(12):4527-34. doi: 10.3168/jds.2008-1446[↩]
- Konuspayeva G, Faye B, Loiseau G, Levieux D. Lactoferrin and immunoglobulin contents in camel’s milk (Camelus bactrianus, Camelus dromedarius, and Hybrids) from Kazakhstan. J Dairy Sci. 2007 Jan;90(1):38-46. doi: 10.3168/jds.S0022-0302(07)72606-1[↩]
- El-Hatmi H, Girardet JM, Gaillard JL, Yahyaoui MH, Attia H. Characterisation of whey proteins of camel (camelus dromedarius) milk and colostrum. SmallRumin Res. 2007;70:267–271.[↩]
- Badr G, Ramadan NK, Sayed LH, Badr BM, Omar HM, Selamoglu Z. Why whey? Camel whey protein as a new dietary approach to the management of free radicals and for the treatment of different health disorders. Iranian Journal of Basic Medical Sciences. 2017;20(4):338-349. doi:10.22038/IJBMS.2017.8573. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5425915/[↩]
- Hoffman JR, Falvo MJ. Protein – Which is Best? Journal of Sports Science & Medicine. 2004;3(3):118-130. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3905294/[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Geiser M. (2003) The wonders of whey protein. NSCA’s Performance Training Journal 2, 13-15[↩]
- U.S. National Library of Medicine. Medline Plus. Amino acids. https://medlineplus.gov/ency/article/002222.htm[↩][↩]
- Brosnan J (2003). “Interorgan amino acid transport and its regulation”. J Nutr 133 (6 Suppl 1): 2068S-2072S.[↩]
- Young V, Ajami A (2001). “Glutamine: the emperor or his clothes?”. J Nutr 131 (9 Suppl): 2449S-59S; discussion 2486S-7S.[↩]
- Wikipedia. Ketogenic amino acid. https://en.wikipedia.org/wiki/Ketogenic_amino_acid[↩]
- Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) ( 2005 ) /10 Protein and Amino Acids. https://www.nap.edu/read/10490/chapter/12#593[↩]
- Fruhbeck G. Protein metabolism—Slow and fast dietary proteins. Nature. 1998;391:843–845. doi: 10.1038/35993. https://www.ncbi.nlm.nih.gov/pubmed/9495333[↩]
- Steijns J., Schaafsma G. Nutritional quality and health aspects of milk proteins. Agro Food Ind. HiTech. 2009;20:29–31.[↩]
- Huppertz T., Kelly A.L., Fox P.F. Effects of high pressure on constituents and properties of milk. Int. Dairy J. 2002;12:561–572. doi: 10.1016/S0958-6946(02)00045-6.[↩]
- FAO/WHO/UNU. Geneva: WHO; 1985. Energy and Protein Requirements. Report of a Joint FAO/WHO/UNU Expert Consultation, WHO Technical Report, No. 724.[↩]
- Nicodemo A., Ismail A.A., Lands L., Kubow S. The effects of whey proteins on lipoprotein metabolism and oxidative stress: A review of human and animal studies. Recent Res. Dev. Lipids. 2000;4:245–302.[↩]
- Smithers G.W. Whey and whey proteins—From “gutter-to-gold” Intern. Dairy J. 2008;18:695–704. doi: 10.1016/j.idairyj.2008.03.008.[↩]
- Grey V., Mohammed S.R., Smountas A.A., Bahlool R., Lands L.C. Improved glutathione status in young adult patients with cystic fibrosis supplemented with whey protein. J. Cyst. Fibros. 2003;2:195–198. doi: 10.1016/S1569-1993(03)00097-3. https://www.ncbi.nlm.nih.gov/pubmed/15463873[↩]
- Tseng Y.M., Lin S.K., Hsiao J.K., Chen I.J., Lee J.H., Wu S.H., Tsai L.Y. Whey protein concentrate promotes the production of glutathione (GSH) by GSH reductase in the PC12 cell line after acute ethanol exposure. Food Chem. Toxic. 2006;44:574–578. doi: 10.1016/j.fct.2005.09.003. https://www.ncbi.nlm.nih.gov/pubmed/16360258[↩]
- Malmezat T., Breuille D., Pouyet C., Mirand P.P., Obled C. Metabolism of cysteine is modified during the acute phase of sepsis in rats. J. Nutr. 1998;128:97–105. http://jn.nutrition.org/content/128/1/97.long[↩]
- Voisin L, Breuillé D, Combaret L, et al. Muscle wasting in a rat model of long-lasting sepsis results from the activation of lysosomal, Ca2+ -activated, and ubiquitin-proteasome proteolytic pathways. Journal of Clinical Investigation. 1996;97(7):1610-1617. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC507224/[↩]
- Vary T.C., Dardevet D., Obled C., Pouyet C., Breuille D., Grizard J. Modulation of skeletal muscle lactate metabolism following bacteremia by insulin or insulin-like growth factor-I: Effects of pentoxifylline. Shock. 1997;7:432–438. doi: 10.1097/00024382-199706000-00008. https://www.ncbi.nlm.nih.gov/pubmed/9185244[↩][↩]
- Fukagawa N.K., Ajami A.M., Young V.R. Plasma methionine and cysteine kinetics in response to an intravenous glutathione infusion in adult humans. Am. J. Phys. Endocr. Metab. 1996;33:E209–E214. https://www.ncbi.nlm.nih.gov/pubmed/8779940[↩]
- Kim C.J., Kovacs-Nolan J., Yang C., Archbold T., Fan M.Z., Mine Y. L-Cysteine supplementation attenuates local inflammation and restores gut homeostasis in a porcine model of colitis. Biochim. Biophys. Acta. 2009;1790:1161–1169. doi: 10.1016/j.bbagen.2009.05.018. https://www.ncbi.nlm.nih.gov/pubmed/19520150[↩][↩]
- Daddaoua A., Puerta V., Zarzuelo A., Suarez M.D., de Medina F.S., Martinez-Augustin O. Bovine glycomacropeptide is anti-inflammatory in rats with hapten-induced colitis. J. Nutr. 2005;135:1164–1170. http://jn.nutrition.org/content/135/5/1164.long[↩][↩]
- Sprong R.C., Schonewille A.J., van der Meer R. Dietary cheese whey protein protects rats against mild dextran sulfate sodium-induced colitis: Role of mucin and microbiota. J. Dairy Sci. 2010;93:1364–1371. doi: 10.3168/jds.2009-2397. https://www.ncbi.nlm.nih.gov/pubmed/20338413[↩][↩]
- Kanwar JR, Kanwar RK. Gut health immunomodulatory and anti-inflammatory functions of gut enzyme digested high protein micro-nutrient dietary supplement-Enprocal. BMC Immunology. 2009;10:7. doi:10.1186/1471-2172-10-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2667481/[↩][↩]
- Araki Y., Katoh T., Ogawa A., Bamba S., Andoh A., Koyama S., Fujiyama Y., Bamba T. Bile acid modulates transepithelial permeability via the generation of reactive oxygen species in the caco-2 cell line. Free Radic. Biol. Med. 2005;39:769–780. doi: 10.1016/j.freeradbiomed.2005.04.026. https://www.ncbi.nlm.nih.gov/pubmed/16109307[↩]
- Van de Walle J., Hendrickx A., Romier B., Larondelle Y., Schneider Y.J. Inflammatory parameters in Caco-2 cells: Effect of stimuli nature, concentration, combination and cell differentiation. Toxicol. Vitro. 2010;24:1441–1449. doi: 10.1016/j.tiv.2010.04.002. https://www.ncbi.nlm.nih.gov/pubmed/20406675[↩]
- Diegelmann J, Seiderer J, Niess J-H, et al. Expression and regulation of the chemokine CXCL16 in Crohn’s disease and models of intestinal inflammation. Inflammatory bowel diseases. 2010;16(11):1871-1881. doi:10.1002/ibd.21306. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2981128/[↩]
- Hernandez-Ledesma B., Recio I., Amigo L. Beta-lactoglobulin as source of bioactive peptides. Amino Acids. 2008;35:257–265. doi: 10.1007/s00726-007-0585-1. https://www.ncbi.nlm.nih.gov/pubmed/17726638[↩]
- Hernandez-Ledesma B., Davalos A., Bartolome B., Amigo L. Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and beta-lactoglobulin. Identification of active peptides by HPLC-MS/MS. J. Agric. Food Chem. 2005;53:588–593. doi: 10.1021/jf048626m. https://www.ncbi.nlm.nih.gov/pubmed/15686406[↩]
- Conneely O.M. Antiinflammatory activities of lactoferrin. J. Am. Coll. Nutr. 2001;20:389s–395s. doi: 10.1080/07315724.2001.10719173. https://www.ncbi.nlm.nih.gov/pubmed/11603648[↩]
- Kruzel M.L., Harari Y., Chen C.Y., Castro G.A. The gut—A key metabolic organ protected by lactoferrin during experimental systemic inflammation in mice. Adv. Lactoferrin Res. 1998;443:167–173. https://www.ncbi.nlm.nih.gov/pubmed/9781356[↩]
- Kruzel M.L., Harari Y., Chen C.Y., Castro G.A. Lactoferrin protects gut mucosal integrity during endotoxemia induced by lipopolysaccharide in mice. Inflammation. 2000;24:33–44. doi: 10.1023/A:1006935908960. https://www.ncbi.nlm.nih.gov/pubmed/10704062[↩]
- Lands L.C., Grey V.L., Smountas A.A. Effect of supplementation with a cysteine donor on muscular performance. J. Appl. Phys. 1999;87:1381–1385. http://jap.physiology.org/content/87/4/1381.long[↩]
- Grimble R.F., Jackson A.A., Persaud C., Wride M.J., Delers F., Engler R. Cysteine and glycine supplementation modulate the metabolic response to tumor-necrosis-factor-alpha in rats fed a low protein-diet. J. Nutr. 1992;122:2066–2073. https://www.ncbi.nlm.nih.gov/pubmed/1279141[↩]
- Mattsby-Baltzer I., Roseanu A., Moras C., Elverfors J., Engberg I., Hanson L.A. Lactoferrin or a fragment thereof inhibits the endotoxin-induced interleukin-6 response in human monocytic cells. Ped. Res. 1996;40:257–262. doi: 10.1203/00006450-199608000-00011. https://www.ncbi.nlm.nih.gov/pubmed/8827774[↩]
- Whey and casein labeled with l-[1-13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. American Journal of Physiology – Endocrinology and Metabolism Published 28 December 2010 Vol. 300 no. 1, E231-E242 DOI: 10.1152/ajpendo.00513.2010. http://ajpendo.physiology.org/content/300/1/E231[↩][↩]
- Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. American Journal of Physiology – Endocrinology and Metabolism Published 3 January 2007 Vol. 292 no. 1, E71-E76 DOI: 10.1152/ajpendo.00166.2006. http://ajpendo.physiology.org/content/292/1/E71.long[↩]
- Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. American Journal of Physiology – Endocrinology and Metabolism Published 1 August 2001 Vol. 281 no. 2, E197-E206. http://ajpendo.physiology.org/content/281/2/E197[↩]
- Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Appl Physiol Nutr Metab. 2007 Dec;32(6):1132-8. https://www.ncbi.nlm.nih.gov/pubmed/18059587[↩]
- The effect of whey isolate and resistance training on strength, body composition, and plasma glutamine. Int J Sport Nutr Exerc Metab. 2006 Oct;16(5):494-509. https://www.ncbi.nlm.nih.gov/pubmed/17240782[↩][↩]
- The effect of whey protein supplementation with and without creatine monohydrate combined with resistance training on lean tissue mass and muscle strength. Int J Sport Nutr Exerc Metab. 2001 Sep;11(3):349-64. https://www.ncbi.nlm.nih.gov/pubmed/11591884/[↩][↩][↩][↩]
- Effects of whey protein and resistance exercise on body composition: a meta-analysis of randomized controlled trials. J Am Coll Nutr. 2014;33(2):163-75. doi: 10.1080/07315724.2013.875365. https://www.ncbi.nlm.nih.gov/pubmed/24724774[↩][↩]
- Effects of Whey Protein Alone or as Part of a Multi-ingredient Formulation on Strength, Fat-Free Mass, or Lean Body Mass in Resistance-Trained Individuals: A Meta-analysis. Sports Med. 2016 Jan;46(1):125-37. doi: 10.1007/s40279-015-0403-y. https://www.ncbi.nlm.nih.gov/pubmed/26403469[↩][↩]
- Joy JM, Lowery RP, Wilson JM, et al. The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutrition Journal. 2013;12:86. doi:10.1186/1475-2891-12-86. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3698202/[↩][↩]
- Jason E. Tang, Daniel R. Moore, Gregory W. Kujbida, Mark A. Tarnopolsky, Stuart M. Phillips. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. Journal of Applied Physiology Published 1 September 2009 Vol. 107 no. 3, 987-992 DOI: 10.1152/japplphysiol.00076.2009. http://jap.physiology.org/content/107/3/987.long[↩][↩][↩]
- The effects of soy and whey protein supplementation on acute hormonal reponses to resistance exercise in men. J Am Coll Nutr. 2013;32(1):66-74. doi: 10.1080/07315724.2013.770648. https://www.ncbi.nlm.nih.gov/pubmed/24015701[↩][↩][↩]
- Brown EC, DiSilvestro RA, Babaknia A, Devor ST. Soy versus whey protein bars: Effects on exercise training impact on lean body mass and antioxidant status. Nutrition Journal. 2004;3:22. doi:10.1186/1475-2891-3-22. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC539287/[↩][↩][↩][↩]
- Special nutrition intervention is required for muscle protective efficacy of physical exercise in elderly people at highest risk of sarcopenia. Physiol Int. 2016 Sep;103(3):368-376. doi: 10.1556/2060.103.2016.3.12. https://www.ncbi.nlm.nih.gov/pubmed/28229646[↩][↩][↩]
- Effects of a whey protein supplementation on intrahepatocellular lipids in obese female patients. Clin Nutr. 2011 Aug;30(4):494-8. doi: 10.1016/j.clnu.2011.01.006. Epub 2011 Feb 1. https://www.ncbi.nlm.nih.gov/pubmed/21288612[↩][↩]
- Hamad EM, Taha SH, Abou Dawood A-GI, Sitohy MZ, Abdel-Hamid M. Protective effect of whey proteins against nonalcoholic fatty liver in rats. Lipids in Health and Disease. 2011;10:57. doi:10.1186/1476-511X-10-57. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3096574/[↩]
- Pal S, Ellis V, Dhaliwal S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br J Nutr. 2010 Sep;104(5):716-23. doi: 10.1017/S0007114510000991. Epub 2010 Apr 9. https://www.ncbi.nlm.nih.gov/pubmed/20377924[↩][↩]
- Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr. 2016 Mar;103(3):830-40. doi: 10.3945/ajcn.115.113357. Epub 2016 Feb 10. http://ajcn.nutrition.org/content/103/3/830.long[↩][↩]
- Katsanos CS, Chinkes DL, Paddon-Jones D, Zhang X, Aarsland A, Wolfe RR. Whey protein ingestion in elderly results in greater muscle protein accrual than ingestion of its constituent essential amino acid content. Nutrition research (New York, NY). 2008;28(10):651-658. doi:10.1016/j.nutres.2008.06.007. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2612691/[↩][↩]
- Food and Agriculture Organization/World Health Organization (1990) Protein quality evaluation; report of the joint FAO/WHO expert consultation. FAO Food and Nutrition Paper 52, Rome, Italy[↩][↩]
- The protein digestibility-corrected amino acid score. Schaafsma G. J Nutr. 2000 Jul; 130(7):1865S-7S. http://jn.nutrition.org/content/130/7/1865S.long[↩][↩][↩]
- Sarwar G. (1997) The protein digestibility-corrected amino acid score method overestimates quality of proteins containing antinutritional factors and of poorly digestible proteins supplemented with limiting amino acids in rats. Journal of Nutrition 127, 758-764. http://jn.nutrition.org/content/127/5/758.long[↩][↩][↩]
- Legume grains enhance ileal losses of specific endogenous serine-protease proteins in weaned pigs. Salgado P, Montagne L, Freire JP, Ferreira RB, Teixeira A, Bento O, Abreu MC, Toullec R, Lallès JP. J Nutr. 2002 Jul; 132(7):1913-20. http://jn.nutrition.org/content/132/7/1913.long[↩]
- Frestedt JL, Zenk JL, Kuskowski MA, Ward LS, Bastian ED. A whey-protein supplement increases fat loss and spares lean muscle in obese subjects: a randomized human clinical study. Nutrition & Metabolism. 2008;5:8. doi:10.1186/1743-7075-5-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2289832/[↩][↩]
- AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL. Circulation. 2000 Oct 31; 102(18):2284-99. http://circ.ahajournals.org/content/102/18/2284.long[↩]
- Freedman MR, King J, Kennedy E. Popular diets: a scientific review. Obes Res. 2001;9:1S–40S. https://www.ncbi.nlm.nih.gov/pubmed/11374180[↩]
- Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. https://www.ncbi.nlm.nih.gov/pubmed/15181027[↩]
- The National Institute of Diabetes and Digestive and Kidney Diseases, Health Information Center. Protein: Tips for People with Chronic Kidney Disease. https://www.niddk.nih.gov/health-information/health-communication-programs/nkdep/a-z/nutrition-protein/Pages/nutrition-protein.aspx[↩]