Contents
- Sirolimus
- Sirolimus special precautions
- Sirolimus mechanism of action
- Sirolimus uses
- Sirolimus dosage
- Sirolimus side effects
- Sirolimus overdose
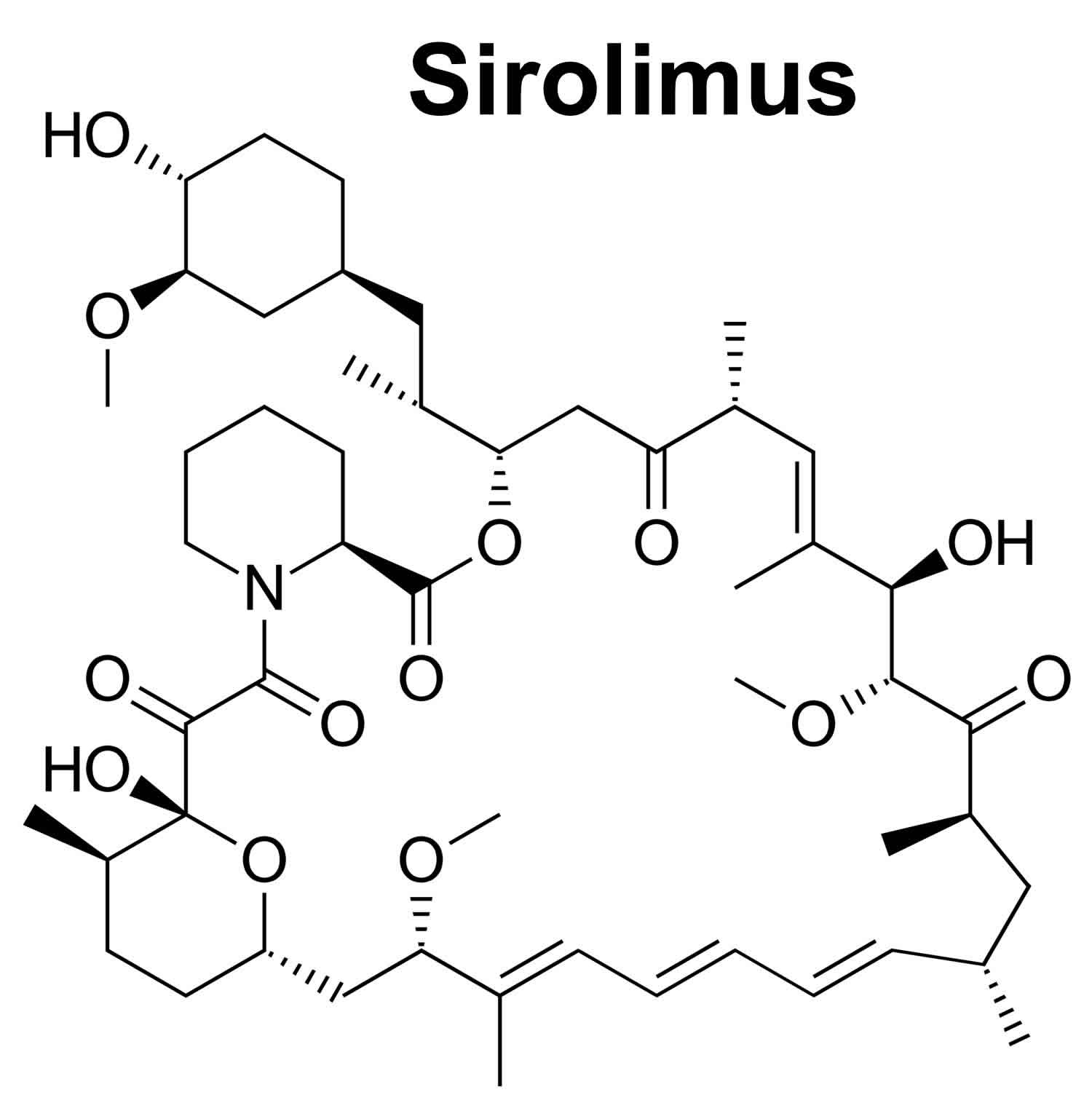
Sirolimus
Sirolimus also known as Rapamune or Rapamycin is an immunosupressant or a drug that weakens your body’s immune system in order to help keep it from “rejecting” a transplanted organ that is used along with other medications to prevent rejection (attack of a transplanted organ by the immune system of a person receiving the organ) in people who have received a kidney transplant 1. Sirolimus is also given without other medicines to treat a rare lung disorder called lymphangioleiomyomatosis or LAM. Lymphangioleiomyomatosis (LAM) is a condition that affects the lungs, the kidneys, and the lymphatic system 2. Lymphangioleiomyomatosis (LAM) is found almost exclusively in women. Lymphangioleiomyomatosis (LAM) often occurs as a feature of an inherited syndrome called tuberous sclerosis complex. When LAM occurs alone it is called isolated or sporadic LAM. Mutations in the TSC1 gene or more commonly the TSC2 gene, cause lymphangioleiomyomatosis (LAM). The TSC1 and TSC2 genes provide instructions for making the proteins hamartin and tuberin, respectively. Within cells, these two proteins, hamartin and tuberin, likely help regulate cell growth and size. The hamartin and tuberin proteins act as tumor suppressors, which normally prevent cells from growing and dividing too fast or in an uncontrolled way. Signs and symptoms of lymphangioleiomyomatosis (LAM) most often appear during a woman’s thirties. Affected women have an overgrowth of abnormal smooth muscle-like cells (LAM cells) in their lungs, resulting in the formation of lung cysts and the destruction of normal lung tissue. They may also have an accumulation of fluid in the cavity around the lungs (chylothorax). The lung abnormalities resulting from lymphangioleiomyomatosis (LAM) may cause difficulty breathing (dyspnea), chest pain, and coughing, which may bring up blood (hemoptysis). Many women with this disorder have recurrent episodes of collapsed lung (spontaneous pneumothorax). Women with lymphangioleiomyomatosis (LAM) may develop cysts in the lymphatic vessels of the chest and abdomen. These cysts are called lymphangioleiomyomas. Affected women may also develop tumors called angiomyolipomas made up of LAM cells, fat cells, and blood vessels. Angiomyolipomas usually develop in the kidneys. Internal bleeding is a common complication of angiomyolipomas. Sirolimus should be prescribed only by physicians with experience in immunosuppression and management of its complications.
Sirolimus comes as tablets of 0.5, 1 and 2 mg and a solution (liquid) of 1 mg/mL to be taken by mouth. Sirolimus is usually taken once a day, either always with food or always without food. To help you remember to take sirolimus, take it around the same time every day. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take sirolimus exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
Swallow the tablets whole; do not split, chew, or crush them.
The recommended initial dose of sirolimus is 1 mg orally once daily and the usual maintenance of dose in adults is 2 mg (~1 mg/m²) once daily to maintain a trough level ≤10 ng/mL (usually after the first week of initiating treatment) 3.
Your doctor will probably adjust your dose of sirolimus during your treatment, usually not more than once every 7 to 14 days.
Continue to take sirolimus even if you feel well. Do not stop taking sirolimus without talking to your doctor.
Sirolimus solution may develop a haze when refrigerated. If this happens, let the bottle stand at room temperature and gently shake it until the haze goes away. The haze does not mean that the medication is damaged or unsafe to use.
Sirolimus has less kidney damaging adverse effect (nephrotoxicity) than the calcineurin inhibitors. Common side effects of sirolimus include anxiety, weakness, depression, dizziness, headache, gastrointestinal upset (dyspepsia, nausea & diarrhea), oral ulcers, edema, bone marrow suppression and rash. Uncommon but potentially severe adverse events include hypercholesterolemia, kidney failure, severe and opportunistic infections, delayed wound healing, pneumonitis, embryo-fetal toxicity, infertility, lower-extremity swelling, allergic reactions (hives, rash, or peeling skin) and anemia 4. The adverse effects of sirolimus are reported to occur in the first few months of initiation of therapy, which are generally dose-related 5. Talk to your doctor about the possible risks of using sirolimus for your condition.
Sirolimus may increase the risk that you will develop an infection or cancer, especially lymphoma (cancer of a part of the immune system) or skin cancer. To reduce your risk of skin cancer, plan to avoid unnecessary or prolonged exposure to sunlight and to wear protective clothing, sunglasses, and sunscreen during your treatment. If you experience any of the following symptoms, call your doctor immediately: fever, sore throat, chills, frequent or painful urination, or other signs of infection; new sores or changes on the skin; night sweats; swollen glands in the neck, armpits, or groin; unexplained weight loss; trouble breathing; chest pain; weakness or tiredness that does not go away; or pain, swelling, or fullness in the stomach.
Sirolimus may cause serious side effects or death in patients who have had liver or lung transplants. This medication should not be given to prevent rejection of liver or lung transplants.
Keep all appointments with your doctor and the laboratory. Your doctor will order certain tests to check your body’s response to sirolimus.
Talk to your doctor about the risks of taking sirolimus.
Sirolimus special precautions
Before taking sirolimus:
- tell your doctor and pharmacist if you are allergic to sirolimus, any other medications, or any of the ingredients in sirolimus tablets or solution. Ask your pharmacist for a list of the ingredients.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, and nutritional supplements you are taking. Be sure to mention any of the following: aminoglycoside antibiotics such as amikacin, gentamicin, kanamycin, neomycin (Neo-Fradin, Neo-Rx), streptomycin, and tobramycin (Tobi); amphotericin B (Abelcet, AmBisome, Amphocin, Fungizone); angiotensin-converting enzyme (ACE) inhibitors such as benazepril (Lotensin), captopril (Capoten), enalapril (Vasotec), fosinopril (Monopril), lisinopril (Prinivil, Zestril), moexipril (Univasc), perindopril (Aceon), quinapril (Accupril), ramipril (Altace), and trandolapril (Mavik); antifungals such as clotrimazole (Lotrimin), fluconazole (Diflucan), itraconazole (Sporanox), ketoconazole (Nizoral), and voriconazole (Vfend); bromocriptine (Cycloset, Parlodel); cimetidine (Tagamet); cisapride (Propulsid) (not available in the U.S.); clarithromycin (Biaxin); danazol (Danocrine); diltiazem (Cardizem, Dilacor, Tiazac); erythromycin (E.E.S., E-Mycin, Erythrocin); HIV protease inhibitors such as indinavir (Crixivan) and ritonavir (Norvir, in Kaletra); certain medications for cholesterol; medications for seizures such as carbamazepine (Tegretol), phenobarbital (Luminal), and phenytoin (Dilantin); metoclopramide (Reglan); nicardipine (Cardene); rifabutin (Mycobutin); rifampin (Rifadin, Rimactane); rifapentine (Priftin); telithromycin (Ketek); troleandomycin (TAO) (not available in the U.S.); and verapamil (Calan, Covera, Isoptin, Verelan). Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- if you are taking cyclosporine (Neoral) soft gelatin capsules or solution, take them 4 hours before sirolimus.
- tell your doctor what herbal products you are taking, especially St. John’s wort.
- tell your doctor if you have or have ever had high cholesterol or triglycerides or liver disease.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. You should use an effective method of birth control before starting to take sirolimus, while taking sirolimus, and for 12 weeks after stopping sirolimus. If you become pregnant while taking sirolimus, call your doctor.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking sirolimus.
- do not have any vaccinations without talking to your doctor.
You should not use sirolimus if you have ever had a lung transplant or liver transplant.
Sirolimus may cause your body to overproduce white blood cells. This can lead to cancer, severe brain infection causing disability or death, or a viral infection causing kidney transplant failure.
See your doctor right away if you have: fever, flu symptoms, burning when you urinate, a new skin lesion, any change in your mental state, decreased vision, weakness on one side of your body, problems with speech or walking, or pain around your transplant.
Sirolimus drug interactions
Tell your doctor about all your current medicines. Many drugs can interact with sirolimus, especially:
- bromocriptine (Cycloset, Parlodel)
- cyclosporine
- danazol
- St. John’s wort
- tacrolimus
- cholesterol-lowering medication
- an antibiotic or antifungal medicine
- antiviral medicine to treat HIV or hepatitis C
- heart or blood pressure medication
- medicine to reduce stomach acid or treat an ulcer
- seizure medicine.
This list is not complete and many other drugs may affect sirolimus. This includes prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible drug interactions are listed here.
What should I avoid while taking sirolimus?
Avoid exposure to sunlight or tanning beds. Sirolimus may increase your risk of skin cancer. Wear protective clothing and use sunscreen (SPF 30 or higher) when you are outdoors.
Avoid being near people who are sick or have infections. Tell your doctor at once if you develop signs of infection.
Avoid getting sirolimus oral liquid on your skin. Wash the skin with soap and water if this happens. If sirolimus gets into your eyes, rinse them with plain water.
Grapefruit may interact with sirolimus and lead to unwanted side effects. Avoid the use of grapefruit and grapefruit juice while taking sirolimus.
Do not receive a “live” vaccine while using sirolimus. The vaccine may not work as well during this time, and may not fully protect you from disease. Live vaccines include measles, mumps, rubella (MMR), polio, rotavirus, typhoid, yellow fever, varicella (chickenpox), and zoster (shingles).
Sirolimus mechanism of action
Sirolimus is macrocyclic antibiotic with potent immunosuppressive activity that affects T cells and the cellular immune response 6. In cells, sirolimus binds to the immunophilin FK binding protein 12 (FKBP-12), which in turn blocks the activation of mammalian target of rapamycin (mTOR) that interrupts several signal transduction pathways for several cytokines and growth factors including interlukin-2 (IL2) thereby inhibiting downstream protein biosynthesis, cell proliferation, and angiogenesis 7, 8, 9, 6. Sirolimus does not block the process of T-cell activation through the T-cell receptor but rather inhibits T-cell proliferation by binding to the mammalian target of rapamycin (mTOR) 10. However, the mTOR inhibition also leads to inhibition of lymphocyte activation which results in immunosuppression and might therefore be associated with an increased susceptibility to infections 11. Sirolimus is used alone or in combination with calcineurin inhibitors and corticosteroids to prevent cellular rejection after kidney transplantation.
Sirolimus uses
Sirolimus (Rapamune or Rapamycin) was approved for use in the United States in 1999 and current US Food and Drug Administration (FDA) approvals are for prevention of organ rejection after kidney transplantation alone or in combination with calcineurin inhibitors or corticosteroids 12, 13, 14. Sirolimus is also FDA approved as therapy for lymphangioleiomyomatosis (LAM) 12, 13, 14. Sirolimus is an mTOR inhibitor that controls the abnormal proliferation and growth of smooth muscle cells in the lung tissue of patients with lymphangioleiomyomatosis (LAM). Sirolimus has shown in the MILES trial the ability to prevent the worsening of lung function (stabilization of FEV and improvement in FVC) and respiratory symptoms while improving the quality of life and reducing serum VEGF-D levels 15.
Sirolimus has not been FDA approved for use in liver or lung transplantation but is used off-label for prevention of rejection after other forms of organ transplantation after failure or intolerance to tacrolimus. Sirolimus also is used sometimes to treat psoriasis.
To use Sirolimus bottles of solution, follow these steps:
- Open the solution bottle. On first use, insert the plastic tube with stopper tightly into the bottle until it is even with the top of the bottle. Do not remove from the bottle once inserted.
- For each use, tightly insert one of the amber syringes, with the plunger fully pushed in, into the opening in the plastic tube.
- Draw up the amount of solution your doctor has prescribed by gently pulling out the plunger of the syringe until the bottom of the black line of the plunger is even with the correct mark on the syringe. Keep the bottle upright. If bubbles form in the syringe, empty the syringe into the bottle and repeat this step.
- Empty the syringe into a glass or plastic cup containing at least 2 ounces (60 milliliters [1/4 cup]) of water or orange juice. Do not use apple juice, grapefruit juice, or other liquids. Stir vigorously for 1 minute and drink immediately.
- Refill the cup with at least 4 ounces (120 milliliters [1/2 cup]) of water or orange juice. Stir vigorously and drink the rinse solution.
- Dispose of the used syringe.
If you need to carry a filled syringe with you, snap a cap onto the syringe and put the syringe in the carrying case. Use the medication in the syringe within 24 hours.
Sirolimus dosage
Follow all directions on your prescription label and read all medication guides or instruction sheets. Your doctor may occasionally change your dose. Use Sirolimus exactly as directed by your doctor. Sirolimus is usually taken once a day. If you also take cyclosporine, take it at least 4 hours before you take sirolimus. You may take sirolimus with or without food, but take it the same way every time.
Do not crush, chew, or break a sirolimus tablet. Tell your doctor if you have trouble swallowing the tablet whole.
Read and carefully follow any Instructions for Use provided with your medicine. Ask your doctor or pharmacist if you do not understand these instructions.
Sirolimus oral liquid must be mixed only with water or orange juice, no other juices or liquids. Measure the liquid carefully. Use the dosing syringe provided, or use a medicine dose-measuring device (not a kitchen spoon).
Sirolimus can increase your risk of infection by changing the way your immune system works. You will need frequent medical tests. Your dosing schedule may be delayed based on the results of these tests.
You should not stop using sirolimus without your doctor’s advice. Stopping suddenly could make your condition worse.
Store sirolimus tablets at room temperature, away from heat, moisture, and light.
Store sirolimus liquid in the refrigerator. Do not freeze. You may notice a slight haze to the liquid. This haze should disappear when the liquid reaches room temperature.
If you are using sirolimus oral liquid with a disposable syringe, you may store a loaded syringe in the carrying case provided. Keep the case at room temperature and use the medicine within 24 hours. Use a disposable syringe only once and then throw it away.
Adult dose for Organ Transplant
Use: As an immunosuppressive agent for the prevention of organ rejection in patients aged 13 years or older receiving kidney transplant.
For patients with low to moderate immunologic risk
Dosing by body weight
- Less than 40 kg:
- Loading dose: 3 mg/m² on day 1
- Maintenance dose: 1 mg/m² once daily
- Greater than or equal to 40 kg:
- Loading dose: 6 mg orally on day 1
- Maintenance dose: 2 mg orally once daily
For patients with high immunologic risk
Patients with high immunologic risk is defined as Black transplant recipients and/or repeat kidney transplant recipients who lost a previous allograft for immunologic reason and/or patients with high-panel reactive antibodies [PRA; peak PRA level greater than 80%]):
For patients receiving sirolimus with cyclosporine:
- Loading dose: Up to 15 mg on day one post-transplantation
- Maintenance dose: Beginning on day 2, an initial maintenance dose of 5 mg/day should be given. A trough level should be obtained between days 5 and 7, and the daily dose of sirolimus should be adjusted thereafter.
Antibody induction therapy may be used.
Comments:
- It is recommended that this sirolimus be used in a regimen with cyclosporine and corticosteroids.
- Sirolimus should be taken consistently with or without food.
- Once the sirolimus maintenance dose is adjusted, patients should continue on the new maintenance dose for at least 7 to 14 days before further dosage adjustment with concentration monitoring.
Maintenance therapy after withdrawal of cyclosporine
Cyclosporine withdrawal is not recommended in high-immunological risk patients. Following 2 to 4 months of combined therapy, withdrawal of cyclosporine may be considered in low-to-moderate risk patients. Cyclosporine should be discontinued over 4 to 8 weeks, and a necessary increase in the dosage of sirolimus (up to 4-fold) should be anticipated due to removal of metabolic inhibition by cyclosporine and to maintain adequate immunosuppressive effects. -Dose-adjusted trough target concentrations are typically 16 to 24 ng/mL for the first year post-transplant and 12 to 20 ng/mL thereafter (measured by chromatographic methodology).
Adult dose for Pulmonary Lymphangioleiomyomatosis (LAM)
Use: For the treatment of patients with lymphangioleiomyomatosis (LAM)
- Initial dose: 2 mg/day
- Sirolimus whole blood trough concentrations should be measured in 10 to 20 days, with dosage adjustment to maintain concentrations between 5 and 15 ng/mL.
Comment:
Sirolimus should be taken consistently with or without food.
Children dose for Organ Transplant
Use: As an immunosuppressive agent indicated for the prophylaxis of organ rejection in patients aged 13 years or older receiving renal transplant.
For children with low to moderate immunologic risk
Greater than or equal to 13 years of age
Dosing by body weight:
- Less than 40 kg:
- Loading dose: 3 mg/m² on day 1
- Maintenance dose: 1 mg/m² once daily
- Greater than or equal to 40 kg:
- Loading dose: 6 mg orally on day 1
- Maintenance dose: 2 mg orally once daily
Sirolimus Dose Adjustments
Sirolimus dosages should be adjusted to maintain trough concentrations within the desired range based on risk and concomitant therapy. Maximum daily dose: 40 mg. The dosage should be adjusted at intervals of 7 to 14 days to account for the long half-life of sirolimus. In general, dose proportionality may be assumed. The new sirolimus dose equals current dose multiplied by (target concentration/current concentration).
If a large dose increase is required, consider loading dose calculated as:
- Loading dose equals (new maintenance dose minus current maintenance dose) multiplied by 3.
- Maximum dose in one day: 40 mg
If the required dose is greater than 40 mg (due to loading dose), then the dose should be divided over 2 days. Serum concentrations should not be used as the sole basis for dosage adjustment. Clinical signs/symptoms, tissue biopsy, and laboratory parameters should also be monitored.
Sirolimus narrow therapeutic index
- Sirolimus should be considered a narrow therapeutic index drug as small differences in dose or blood concentrations may lead to serious therapeutic failures or adverse drug reactions 16, 17.
- Recommendations:
- Generic substitution should be done cautiously, if at all, as current bioequivalence standards are generally insufficient for narrow therapeutic index drugs.
- Additional and/or more frequent monitoring should be done to ensure receipt of an effective dose while avoiding unnecessary toxicities.
The safety and efficacy of conversion from calcineurin inhibitors to sirolimus in maintenance renal transplant population has not been established.
Safety and efficacy have not been established in patients less than 13 years old, or in pediatric (less than 18 years) renal transplant patients considered at high-immunologic risk.
What should I do if I forget a dose?
Take the missed dose as soon as you remember it. However, if it is almost time for the next dose, skip the missed dose and continue your regular dosing schedule. Do not take a double dose to make up for a missed one.
Sirolimus side effects
Common side effects of sirolimus may include:
- fever, cold symptoms such as stuffy nose, sneezing, sore throat
- mouth sores
- nausea, stomach pain, diarrhea
- headache, muscle aches
- chest pain
- dizziness
- acne.
See your doctor if any of these symptoms are severe or do not go away.
Some side effects can be serious. The following symptoms are uncommon, but if you experience any of them or those listed in the IMPORTANT WARNING section, see your doctor immediately:
- unusual bleeding or bruising
- redness, oozing, or slow healing of a skin wound
- cough
- a new skin lesion, or a mole that has changed in size or color
- swollen, red, cracked, scaly skin
- hives
- rash
- itching
- sudden chest pain or discomfort, cough, feeling short of breath
- difficulty breathing or swallowing
- swelling of the face, throat, tongue, lips, eyes, hands, feet, ankles, or lower legs
- low red blood cells (anemia)–pale skin, unusual tiredness, feeling light-headed or short of breath, cold hands and feet
- hoarseness
- tenderness around the transplanted kidney
- signs of infection–fever, chills, painful mouth sores, skin sores, cold or flu symptoms, pain or burning when you urinate.
Sirolimus may cause a serious brain infection that can lead to disability or death. See your doctor right away if you have any change in your mental state, decreased vision, weakness on one side of your body, or problems with speech or walking. These symptoms may start gradually and get worse quickly.
Get emergency medical help if you have signs of an allergic reaction such as hives, rash, or peeling skin; wheezing, difficulty breathing, chest pain or tightness; feeling like you might pass out; swelling of your face, lips, tongue, or throat.
Sirolimus may cause other side effects. See your doctor if you have any unusual problems while taking sirolimus.
Sirolimus overdose
- Ferrer IR, Araki K, Ford ML. Paradoxical aspects of rapamycin immunobiology in transplantation. Am J Transplant. (2011) 11:654–9. 10.1111/j.1600-6143.2011.03473.x[↩]
- Lymphangioleiomyomatosis. https://medlineplus.gov/genetics/condition/lymphangioleiomyomatosis[↩]
- Khaddour K, Sankari A, Shayuk M. Lymphangioleiomyomatosis. [Updated 2023 Jun 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534231[↩]
- Gupta N, Lee HS, Young LR, Strange C, Moss J, Singer LG, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, Brown KK, Lynch JP 3rd, Goldberg HJ, Downey GP, Taveira-DaSilva AM, Krischer JP, Setchell K, Trapnell BC, Inoue Y, McCormack FX; NIH Rare Lung Disease Consortium. Analysis of the MILES cohort reveals determinants of disease progression and treatment response in lymphangioleiomyomatosis. Eur Respir J. 2019 Apr 4;53(4):1802066. doi: 10.1183/13993003.02066-2018[↩]
- Neurohr C, Hoffmann AL, Huppmann P, Herrera VA, Ihle F, Leuschner S, von Wulffen W, Meis T, Baezner C, Leuchte H, Baumgartner R, Zimmermann G, Behr J. Is sirolimus a therapeutic option for patients with progressive pulmonary lymphangioleiomyomatosis? Respir Res. 2011 May 21;12(1):66. doi: 10.1186/1465-9921-12-66[↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Sirolimus. [Updated 2020 Feb 17]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548028[↩][↩]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12(1):9–22. doi: 10.1016/j.ccr.2007.05.008[↩]
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589–3594. doi: 10.1242/jcs.051011[↩]
- Wang J, Tran J, Wang H, Guo C, Harro D, Campbell AD, Eitzman DT. mTOR Inhibition improves anaemia and reduces organ damage in a murine model of sickle cell disease. Br J Haematol. 2016 Aug;174(3):461-9. doi: 10.1111/bjh.14057[↩]
- Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999 Mar 1;162(5):2775-84.[↩]
- Nguyen LS, Vautier M, Allenbach Y, Zahr N, Benveniste O, Funck-Brentano C, Salem JE. Sirolimus and mTOR Inhibitors: A Review of Side Effects and Specific Management in Solid Organ Transplantation. Drug Saf. 2019 Jul;42(7):813-825. doi: 10.1007/s40264-019-00810-9[↩]
- Sirolimus (marketed as Rapamune) Information. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/sirolimus-marketed-rapamune-information[↩][↩]
- Zhao DQ, Li SW, Sun QQ. Sirolimus-Based Immunosuppressive Regimens in Renal Transplantation: A Systemic Review. Transplant Proc. 2016 Jan-Feb;48(1):3-9. doi: 10.1016/j.transproceed.2016.01.002[↩][↩]
- Rossano JW, Jefferies JL, Pahl E, Naftel DC, Pruitt E, Lupton K, Dreyer WJ, Chinnock R, Boyle G, Mahle WT; Pediatric Heart Transplant Study Investigators. Use of sirolimus in pediatric heart transplant patients: A multi-institutional study from the Pediatric Heart Transplant Study Group. J Heart Lung Transplant. 2017 Apr;36(4):427-433. doi: 10.1016/j.healun.2016.09.009[↩][↩]
- McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, Brown KK, Lynch JP 3rd, Goldberg HJ, Young LR, Kinder BW, Downey GP, Sullivan EJ, Colby TV, McKay RT, Cohen MM, Korbee L, Taveira-DaSilva AM, Lee HS, Krischer JP, Trapnell BC; National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011 Apr 28;364(17):1595-606. doi: 10.1056/NEJMoa1100391[↩]
- Wang Z, Yao W, Sun H, Dong K, Ma Y, Chen L, Zheng S, Li K. Sirolimus therapy for kaposiform hemangioendothelioma with long-term follow-up. J Dermatol. 2019 Nov;46(11):956-961. doi: 10.1111/1346-8138.15076[↩]
- Ying H, Qiao C, Yang X, Lin X. A Case Report of 2 Sirolimus-Related Deaths Among Infants With Kaposiform Hemangioendotheliomas. Pediatrics. 2018 Apr;141(Suppl 5):S425-S429. doi: 10.1542/peds.2016-2919[↩]