What is bilirubin
Bilirubin is an orange-yellow pigment, a waste product primarily produced by the normal breakdown of heme (ferroprotoporphyrin IX) 1, 2, 3, 4, 5. Approximately 85 percent of the heme is from the hemoglobin (Hb; an iron-containing protein in red blood cells that transports oxygen from the lungs to the body’s tissues) following the degradation of red blood cells (RBCs), while the remaining heme derives from the ineffective erythropoiesis and the breakdown of other hemoproteins such as cytochromes, myoglobin, and catalase 6. Bilirubin is ultimately processed by the liver to allow its elimination from the body.
There are 2 forms of bilirubin in the body: a toxic form called unconjugated bilirubin and a nontoxic form called conjugated bilirubin. Both forms can be measured or estimated by laboratory tests, and a total bilirubin result (a sum of these) may also be reported. Bilirubin is ultimately processed by the liver to allow its elimination from the body.
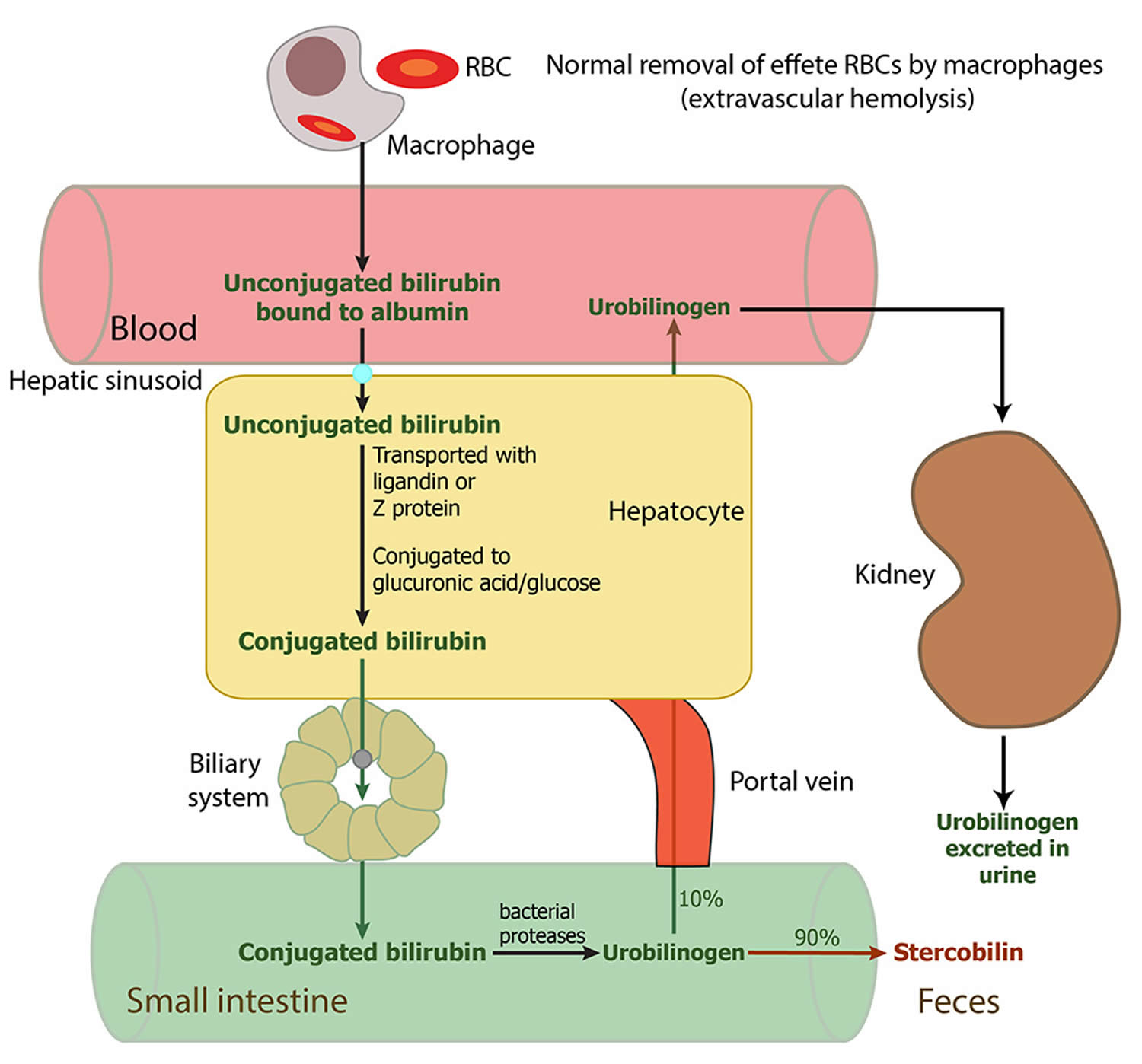
Red blood cells normally degrade after about 120 days in the blood circulation. As heme is released from hemoglobin, it is converted to bilirubin. The conversion of heme to bilirubin is a 2-step reaction, in the first step the microsomal heme oxygenase enzyme of the reticuloendothelial system (a network of phagocytic cells, including monocytes and macrophages, that is part of the immune system whose function is to engulf and destroy foreign particles, such as bacteria and toxins, and cellular debris, a process known as phagocytosis), converts heme to biliverdin, which in turn is reduced to unconjugated bilirubin (toxic form of bilirubin) by a second enzyme biliverdin reductase 7. The unconjugated bilirubin (toxic form of bilirubin) is lipophilic (dissolves readily in fats). Unconjugated bilirubin (toxic form of bilirubin) tightly bound to albumin is transported to the liver. The entry of unconjugated bilirubin into the liver has not been elucidated, and the best candidate appears to be a bilirubin transporter 2. In liver cells, unconjugated bilirubin dissociates from albumin and binds to proteins of the glutathione-S-transferases family that present it for conjugation and prevent it from effluxing from the liver 2. Next, unconjugated bilirubin gets conjugated (attached) with one or two molecules of glucuronic acid by the enzyme uridine diphospho-glucuronate glucuronosyltransferase (UGT1A1), which forms bilirubin monoglucuronide and bilirubin diglucuronide respectively 7, 8. Conjugation increases the solubility of bilirubin in plasma and thereby enhances its elimination from the body. The conjugated bilirubin (nontoxic bilirubin) is then pumped into bile via an energy-requiring process requiring the multidrug resistance-associated protein 2 (MRP2) 9. This process also reduces the ability of bilirubin to diffuse across the blood-brain barrier. Therefore, unconjugated hyperbilirubinemia can result from dysfunction of any of these conjugation steps. Conjugated bilirubin enters the bile and passes from the liver to the small intestines; there, it is further broken down by bacteria and eventually eliminated in the stool. Therefore, the breakdown products of bilirubin give stool its characteristic brown color. In newborns, inefficient conjugation of bilirubin leads to unconjugated hyperbilirubinemia (physiologic neonatal jaundice).
A small amount (approximately 250 to 350 milligrams) of bilirubin is produced daily in a normal, healthy adult. Most (85%) of bilirubin is derived from damaged or degraded red blood cells, with the remaining amount derived from the bone marrow or liver. Normally, small amounts of unconjugated bilirubin are released into the blood, but virtually no conjugated bilirubin is present.
Bilirubin concentrations tend to be slightly higher in males than females. African Americans routinely show lower bilirubin concentrations than non-African Americans. Strenuous exercise may increase bilirubin levels.
Drugs that can decrease total bilirubin include barbiturates, caffeine, penicillin, and high doses of salicylates. The drug atazanavir increases unconjugated (indirect) bilirubin.
If the bilirubin level increases in the blood, a person may appear jaundiced, with a yellowing of the skin and/or whites of the eyes. The pattern of bilirubin test results can give your doctor information regarding the condition that may be present. For example, unconjugated bilirubin may be increased when there is an unusual amount of red blood cell destruction (hemolysis) or when the liver is unable to process bilirubin (i.e., with liver diseases such as cirrhosis or inherited problems). Conversely, conjugated bilirubin can increase when the liver is able to process bilirubin but is not able to pass the conjugated bilirubin to the bile for removal; when this happens, the cause is often acute hepatitis or blockage of the bile ducts.
Increased total and unconjugated bilirubin levels are relatively common in newborns in the first few days after birth. This finding is called “physiologic jaundice of the newborn” and occurs because the newborn’s liver is not mature enough to process bilirubin yet. Usually, physiologic jaundice of the newborn resolves itself within a few days. However, in hemolytic disease of the newborn, red blood cells may be destroyed because of blood incompatibilities between the baby and the mother; in these cases, treatment may be required because high levels of unconjugated bilirubin can damage the newborn’s brain.
A rare (about 1 in 10,000 births) but life-threatening congenital condition called biliary atresia can cause increased total and conjugated bilirubin levels in newborns. This condition must be quickly detected and treated, usually with surgery, to prevent serious liver damage that may require liver transplantation within the first few years of life. Some children may require liver transplantation despite early surgical treatment.
Rare inherited disorders that cause abnormal bilirubin metabolism such as Rotor, Dubin-Johnson, and Crigler-Najjar syndromes, may also cause increased levels of bilirubin.
- Rotor syndrome is a rare, inherited genetic disorder characterized by elevated levels of bilirubin in the blood (hyperbilirubinemia). People with Rotor syndrome have a buildup of both unconjugated and conjugated bilirubin in their blood, but the majority is conjugated bilirubin 10, 11. Rotor syndrome is caused by SLCO1B1 and SLCO1B3 genes. Mutations in both SLCO1B1 and SLCO1B3 genes are required for Rotor syndrome to occur. The SLCO1B1 and SLCO1B3 genes provide instructions for making similar proteins, called organic anion transporting polypeptide 1B1 (OATP1B1) and organic anion transporting polypeptide 1B3 (OATP1B3), respectively. Both proteins are found in liver cells; they transport bilirubin and other compounds from the blood into the liver so that they can be cleared from the body. In the liver, bilirubin is dissolved in a digestive fluid called bile and then excreted from the body. In people with Rotor syndrome, jaundice is usually evident shortly after birth or in childhood and may come and go; yellowing of the whites of the eyes also called conjunctival icterus is often the only symptom. Rotor syndrome is a benign and harmless condition that typically does not require treatment and does not affect life expectancy. Jaundice may be noticeable shortly after birth or in childhood, but often it’s an incidental finding.
- Crigler–Najjar syndrome is a very rare inherited disorder in which bilirubin cannot be broken down. Mutations in the UGT1A1 gene cause Crigler-Najjar syndrome. The UGT1A1 gene provides instructions for making the bilirubin uridine diphosphate glucuronosyl transferase (bilirubin-UGT) enzyme, which is found primarily in liver cells and is necessary for the removal of bilirubin from the body. Mutations in the UGT1A1 gene that cause Crigler-Najjar syndrome result in reduced or absent function of the bilirubin-UGT enzyme. People with Crigler–Najjar Type 1 have no bilirubin uridine diphosphate glucuronosyl transferase (bilirubin-UGT) enzyme function, while people with Crigler–Najjar Type 2 have less than 20 percent of normal function. The loss of bilirubin-UGT function decreases glucuronidation of unconjugated bilirubin. Glucuronidation makes bilirubin dissolvable in water so that it can be removed from the body. This toxic unconjugated bilirubin then builds up in the body, causing unconjugated hyperbilirubinemia and jaundice. Crigler–Najjar Type 1 (CN1) is very severe, and affected individuals can die in childhood due to kernicterus, although with proper treatment, they may survive longer. Crigler–Najjar Type 2 (CN2) is less severe. People with Crigler–Najjar Type 2 are less likely to develop kernicterus, and most affected individuals survive into adulthood.
- Dubin-Johnson syndrome is a rare, inherited liver disorder characterized by an increase in conjugated bilirubin, leading to mild, chronic jaundice. Dubin-Johnson syndrome is caused by mutation in ABCC2 gene affecting the liver’s ability to excrete bilirubin, resulting in pigment deposits that make the liver appear black on medical imaging. The ABCC2 gene provides instructions for making a protein that transports certain substances out of cells so they can be released (excreted) from the body. For example, this protein transports bilirubin out of liver cells and into bile (a digestive fluid produced by the liver). Bilirubin is produced during the breakdown of old red blood cells and has an orange-yellow tint. As a result, bilirubin accumulates in the body, causing a condition called hyperbilirubinemia. The buildup of bilirubin in the body causes the yellowing of the skin and whites of the eyes in people with Dubin-Johnson syndrome. Dubin-Johnson syndrome is most often seen in Middle Eastern Jewish and Japanese people. In the Jewish population, about 60% of affected individuals also have an associated blood clotting abnormality, a prolonged prothrombin time (PT), caused by a decrease in factor VII (factor 7). In most affected people jaundice appears during adolescence or early adulthood. Infants with Dubin-Johnson syndrome typically also have enlarged livers (hepatomegaly) and a severely reduced ability to produce and release a digestive fluid called bile (cholestasis). As these children get older, their liver problems go away and they usually do not have any related health problems later in life. Jaundice is typically the only feature of Dubin-Johnson syndrome, but some people can experience weakness, mild abdominal pain, nausea, or vomiting. In most people with Dubin-Johnson syndrome, certain deposits build up in the liver but do not seem to impair liver function. The deposits make the liver appear black when viewed with medical imaging. Dubin-Johnson syndrome is benign, has a normal life expectancy, and usually requires no specific treatment, though precautions like avoiding birth control pills, alcohol, environmental factors that affect the liver, infection, pregnancy, fasting or dehydration and fatigue are recommended.
Figure 1. Bilirubin metabolism
Unconjugated bilirubin
Though unconjugated bilirubin may be toxic to brain development in newborns (up to 2-4 weeks of age), it does not pose the same threat to older children and adults. In older children and adults, the “blood-brain barrier” is more developed and prevents bilirubin from gaining access to brain cells. Nevertheless, elevated bilirubin strongly suggests that a medical condition is present that must be evaluated and treated.
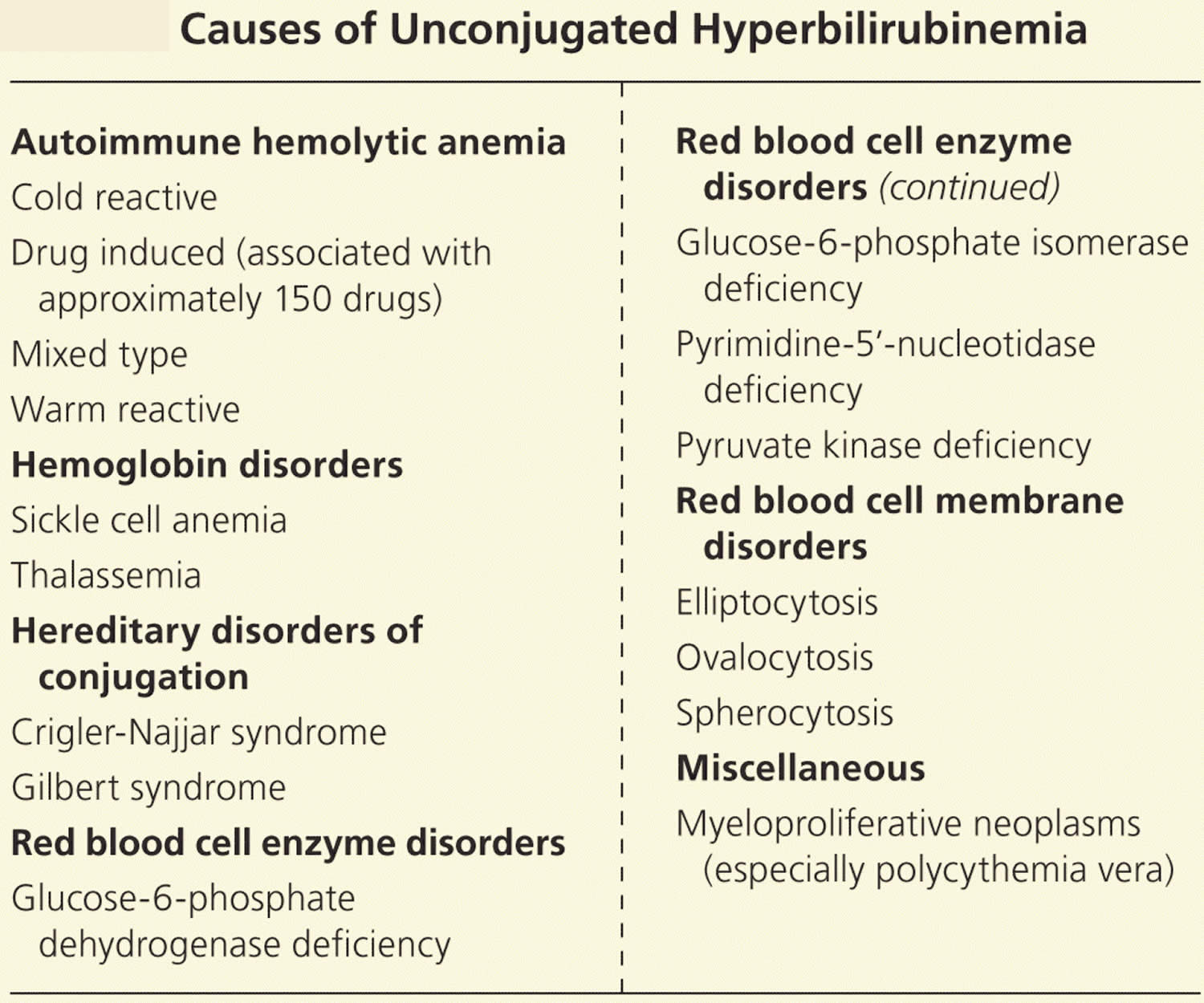
Table 1. Causes of Unconjugated Hyperbilirubinemia
Unconjugated hyperbilirubinemia
Increased bilirubin production
Unconjugated hyperbilirubinemia is usually a result of too much bilirubin presented to the conjugating machinery (from increased red blood cell destruction). Increased red blood cell breakdown may be caused by red blood cell membrane disorders, red blood cell enzyme disorders, hemoglobin disorders (Thalassemias), autoimmune red blood cell destruction (autoimmune hemolytic anemia), or some cancers 13, 14, 15, 16. The excess turnover of red blood cells results in increased heme metabolism, producing large amounts of bilirubin that overwhelm the conjugating machinery, leading to decreased excretion and clinical jaundice.
Impaired bilirubin conjugation
Deficiencies in the same conjugating machinery may also lead to jaundice in individuals with normal red blood cell turnover. Gilbert syndrome involves a deficiency in uridine diphosphate-glucuronosyltransferase, and it affects 10% of the white population 17. This is a benign condition that may be exacerbated by physical or emotional stress such as illness, strenuous exercise, or fasting. Crigler-Najjar syndrome is a more severe variant of the same uridine diphosphate-glucuronosyltransferase enzyme deficiency 17. Patients with impaired conjugation due to low levels of the bilirubin-UGT enzyme are particularly susceptible to jaundice from medications that inhibit this enzyme, such as protease inhibitors 18. Table 1 lists the causes of unconjugated hyperbilirubinemia.
Conjugated bilirubin
Conjugated bilirubin is a water-soluble form of bilirubin formed in the liver by the chemical addition of sugar molecules to unconjugated bilirubin; when present in the blood, conjugated bilirubin can become chemically bound to albumin, forming delta-bilirubin also known as biliprotein.
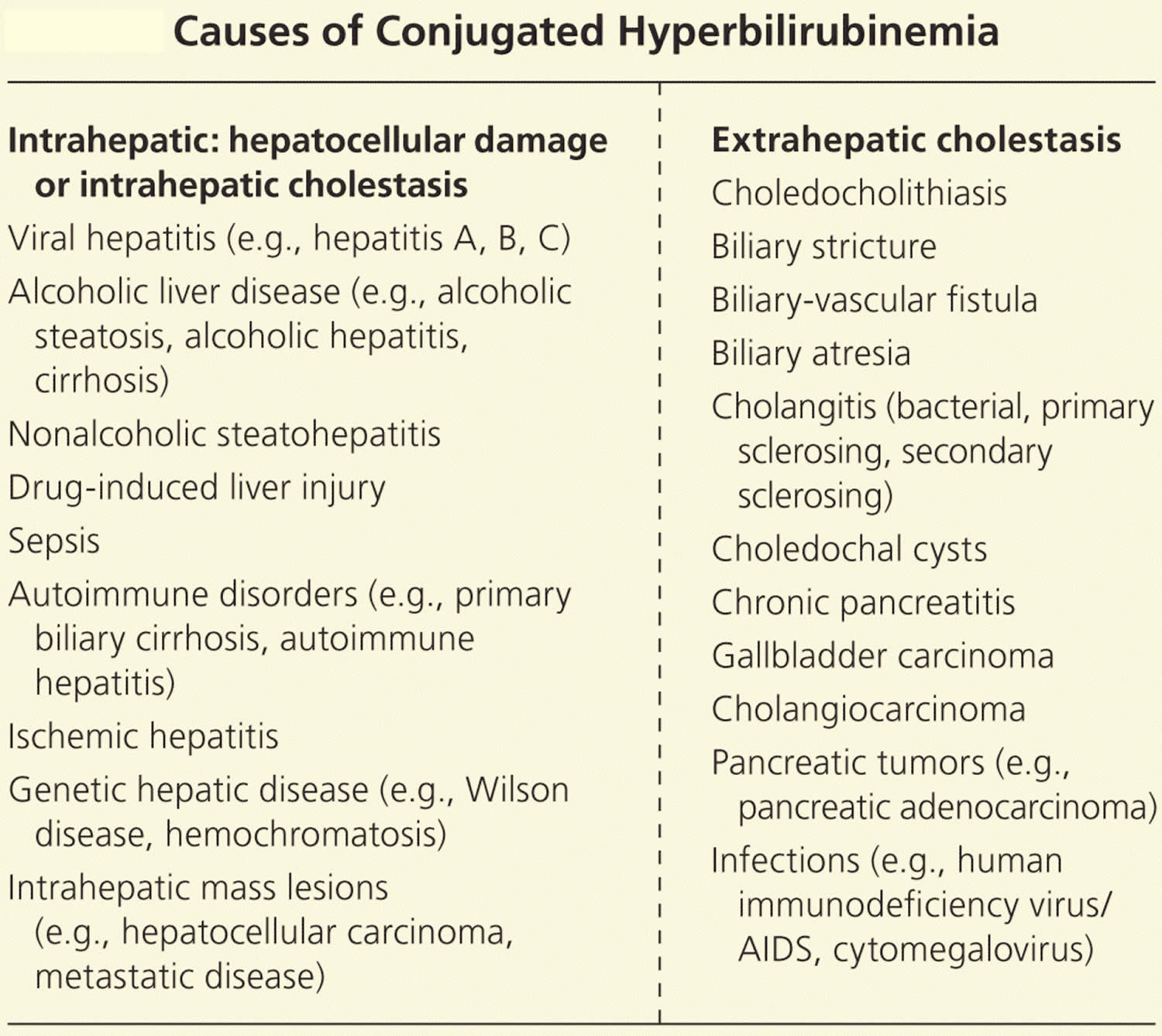
Table 2. Causes of Conjugated Hyperbilirubinemia
[Source 12 ]Conjugated hyperbilirubinemia
Intrahepatic disorders and intrahepatic cholestasis
The largest worldwide contributor to liver disease is viral hepatitis, mostly from hepatitis C 19. Viral hepatitis causes increased oxidative stress within liver cells (hepatocytes), leading to cell death, scarring, and diminished liver mass available for normal function 20, 21. Chronic alcohol consumption can cause various hepatic disorders, including fatty liver disease (steatosis) with minimal symptoms and often no jaundice; alcoholic hepatitis with acute onset jaundice and more severe symptoms; and cirrhosis, which is often associated with decompensation and liver failure in the setting of jaundice 22. Jaundice in persons with alcoholic liver disease can occur via multiple mechanisms, such as direct hepatocellular damage caused by ethanol metabolites or from alcohol’s effect on bile acid uptake and secretion contributing to cholestasis (reduced or stopped bile flow) 22, 23.
Approximately 30% to 40% of patients with nonalcoholic fatty liver disease (NAFLD) progress to nonalcoholic steatohepatitis (NASH), and approximately 40% to 50% of these patients develop fibrosis or cirrhosis that may lead to hyperbilirubinemia 24. Although the exact mechanism is poorly understood, liver lipid deposition may trigger inflammation and fibrosis, particularly when coupled with type 2 diabetes 24. Sepsis may also induce hyperbilirubinemia as circulating acute phase reactants and bacterial endotoxins disrupt bilirubin transport, leading to cholestasis and elevated bile salt levels 25, 26.
Extrahepatic disorders
Conjugated hyperbilirubinemia may also arise from extrahepatic obstruction. Patients with biliary obstruction may present with multiple signs and symptoms, including fever, itch (pruritus), abdominal pain, weight loss, muscle wasting, dark urine, and pale stools. Choledocholithiasis or the presence of gallstones within the common bile duct, is the most common non-neoplastic cause of biliary obstruction, accounting for 14% of all new cases of jaundice 29. An estimated 20 million Americans have gallstones, and risk factors for gallstones within the common bile duct (choledocholithiasis) include female sex, older age, increasing body mass index, and rapid weight loss 30.
Gallstones may cause jaundice by obstructing the biliary tree (typically the common bile duct) or by inducing a biliary stricture 31. Less commonly, stones in the gallbladder or cystic duct may mechanically compress the common hepatic duct causing jaundice, and, rarely, stones may cause the formation of a biliary-vascular fistula with accompanying jaundice 32. Biliary stricture causing postoperative jaundice is a rare complication of cholecystectomy (0.6% of cases) 31, 33.
Jaundice may be caused by surgeries such as liver transplantation and the Whipple and Billroth procedures, which both involve the creation of a choledochojejunostomy. Chronic pancreatitis may cause biliary strictures and jaundice, as may different forms of cholangitis 33, 34. In children, biliary atresia and choledochal cysts are the main causes of extrahepatic biliary obstruction 35.
Tumors are associated with 6.2% of new-onset cases of jaundice 29. Cholangiocarcinoma may affect the proximal or distal portions of the biliary tree by causing biliary strictures. Five-year survival for persons who have resection is 20% to 40%; survival in unresectable disease is less than one year 36, 37. Primary sclerosing cholangitis confers a 1,500-fold increased risk of cholangiocarcinoma, but more than 80% of cases have no risk factors for cholangiocarcinoma 36.
Gallbladder cancer, although rare, is the most common biliary tract malignancy; risk factors include gallstones, infection (Salmonella typhi), and female sex. Median survival is six to 12 months, depending on the stage at diagnosis 38. Ampullary cancers and bile duct compression from lymphadenopathy, or external tumors such as pancreatic cancer, may also cause bile duct obstruction.
Normal bilirubin levels
Direct (conjugated) Bilirubin
- > or =12 months: 0.0-0.3 mg/dL (less than 5.1 µmol/L)
Reference values have not been established for patients who are <12 months of age.
Total Bilirubin
- 0-6 days: Refer to www.bilitool.org for information on age-specific (postnatal hour of life) serum bilirubin values.
- 7-14 days: <15.0 mg/dL
- 15 days to 17 years: < or =1.0 mg/dL
- > or =18 years: 0.1 to 1.2 mg/dL (1.71 to 20.5 µmol/L)
Normal value ranges may vary slightly among different laboratories. Some labs use different measurements or may test different samples. Talk to your provider about the meaning of your specific test results.
Bilirubin in urine
Bilirubin is not normally present in the urine. However, conjugated bilirubin is water-soluble and may be eliminated from the body through the urine if it cannot pass into the bile. Measurable bilirubin in the urine usually indicates blockage of liver or bile ducts, hepatitis, or some other form of liver damage and may be detectable early in disease; for this reason, bilirubin testing is integrated into common dipstick testing used for routine urinalysis.
Are some people more at genetic risk of abnormal bilirubin levels?
Several inherited chronic conditions increase bilirubin levels in the blood and include Gilbert syndrome, Dubin-Johnson syndrome, Rotor syndrome, and Crigler-Najjar syndrome. The first three are usually mild, chronic conditions that can be aggravated under certain conditions but in general cause no significant health problems. For example, Gilbert syndrome is very common; about 1 in every 6 people has this genetic abnormality, but usually people with Gilbert syndrome do not have elevated bilirubin. Crigler-Najjar syndrome is the most serious inherited condition listed; this disorder is relatively rare, and some people with it may die.
How do you treat abnormal bilirubin levels and/or jaundice?
Treatment depends on the cause of the jaundice. In newborns, phototherapy (special light therapy), blood exchange transfusion, and/or certain drugs may be used to reduce the bilirubin level. In Gilbert, Rotor, and Dubin-Johnson syndromes, no treatment is usually necessary. Crigler-Najjar syndrome may respond to certain enzyme drug therapy or may require a liver transplant. Jaundice caused by an obstruction is often resolved by surgery. Jaundice due to cirrhosis is a result of long-term liver damage and does not respond well to any type of therapy other than liver transplantation.
Bilirubin test
Bilirubin test measures the amount of bilirubin in your blood to evaluate your liver function or to help diagnose anemias caused by red blood cell destruction (hemolytic anemia).
A bilirubin test is used to detect an elevated bilirubin level in the blood. Bilirubin test may be used to help determine the cause of jaundice and/or help diagnose conditions such as liver disease, hemolytic anemia, and blockage of the bile ducts.
Bilirubin is an orange-yellow pigment, a waste product primarily produced by the normal breakdown of heme. Heme is a component of hemoglobin, which is found in red blood cells. Bilirubin is ultimately processed by the liver to allow its elimination from the body. Any condition that accelerates the breakdown of red blood cells or affects the processing and elimination of bilirubin may cause an elevated blood level.
Two forms of bilirubin can be measured or estimated by laboratory tests:
- Unconjugated bilirubin—when heme is released from hemoglobin, it is converted to unconjugated bilirubin. It is carried by proteins to the liver. Small amounts may be present in the blood.
- Conjugated bilirubin—formed in the liver when sugars are attached (conjugated) to bilirubin. It enters the bile and passes from the liver to the small intestines and is eventually eliminated in the stool. Normally, no conjugated bilirubin is present in the blood.
Usually, a chemical test is used to first measure the total bilirubin level (unconjugated plus conjugated bilirubin). If the total bilirubin level is increased, the laboratory can use a second chemical test to detect water-soluble forms of bilirubin, called “direct” bilirubin. The direct bilirubin test provides an estimate of the amount of conjugated bilirubin present. Subtracting direct bilirubin level from the total bilirubin level helps estimate the “indirect” level of unconjugated bilirubin. The pattern of bilirubin test results can give your healthcare provider information regarding the condition that may be present.
In adults and older children, bilirubin is measured to:
- Diagnose and/or monitor diseases of the liver and bile duct (e.g., cirrhosis, hepatitis, or gallstones)
- Evaluate people with sickle cell disease or other causes of hemolytic anemia; these people may have episodes called crises when excessive red blood cell destruction increases bilirubin levels.
In newborns with jaundice, bilirubin is used to distinguish the causes of jaundice.
- In both physiologic jaundice of the newborn and hemolytic disease of the newborn, only unconjugated (indirect) bilirubin is increased.
- In much less common cases, damage to the newborn’s liver from neonatal hepatitis and biliary atresia will increase conjugated (direct) bilirubin concentrations as well, often providing the first evidence that one of these less common conditions is present.
It is important that an elevated level of bilirubin in a newborn be identified and quickly treated because excessive unconjugated bilirubin damages developing brain cells. The consequences of this damage include mental retardation, learning and developmental disabilities, hearing loss, eye movement problems, and death.
When is bilirubin test ordered?
A healthcare practitioner usually orders a bilirubin test in conjunction with other liver function tests (alkaline phosphatase [ALP], aspartate aminotransferase [AST], alanine aminotransferase [ALT]) when someone shows signs of abnormal liver function.
A bilirubin level may be ordered when a person:
- Shows evidence of jaundice
- Has a history of drinking excessive amounts of alcohol
- Has suspected drug toxicity
- Has been exposed to hepatitis-causing viruses
Other symptoms that may be present include:
- Dark, amber-colored urine
- Nausea/vomiting
- Abdominal pain and/or swelling
- Fatigue and general malaise that often accompany chronic liver disease
Measuring and monitoring bilirubin in newborns with jaundice is considered standard medical care.
Tests for bilirubin may also be ordered when someone is suspected of having (or known to have) hemolytic anemia as a cause of anemia. In this case, it is often ordered along with other tests used to evaluate hemolysis, such as complete blood count, reticulocyte count, haptoglobin, and lactate dehydrogenase (LDH).
What does high bilirubin levels mean?
Unconjugated hyperbilirubinemia arises in one of the 3 major pathophysiologic conditions or a combination of them 39, 12, 40 41:
- Increased bilirubin production. Increased bilirubin production and consequential unconjugated hyperbilirubinemia can result from increased catabolic degradation of hemoglobin and other heme proteins, typically due to accelerated hemolysis, a large hematoma, dyserythropoiesis (e.g., megaloblastic and sideroblastic anemias), or sometimes due to destruction of transfused erythrocytes. In these conditions, patients with normal liver function efficiently conjugate and excrete the excess bilirubin. As a result, the serum levels of unconjugated bilirubin remain modest (1 to 4 mg/dL) and rarely exceed 4 mg/dL. Prolonged hemolysis can lead to severe unconjugated hyperbilirubinemia in patients with concurrent hepatic dysfunction.
- Impaired bilirubin uptake. The impaired hepatic uptake of bilirubin can be the result of decreased bilirubin delivery to the liver and inefficient uptake of bilirubin by hepatocytes, usually resulting from reduced hepatic blood flow (congestive heart failure and portosystemic shunts) and drugs/contrast administration. The unconjugated hyperbilirubinemia induced by several drugs (rifampin, flavaspidic acid, novobiocin, and various cholecystographic contrast agents), generally resolves within 48 hours of drug discontinuation 42.
- Impaired bilirubin conjugation. Impaired bilirubin conjugation can result from hereditary defects, including Gilbert syndrome and the Crigler-Najjar syndrome type 1 and 2, that cause a decrease or loss of UDP-glucuronosyltransferase (UGT1A1) activity, an enzyme responsible for conjugation of bilirubin with glucuronic acid 43. Lucy-Driscoll syndrome, also known as maternal serum jaundice, a form of transient familial neonatal unconjugated hyperbilirubinemia, is a rare metabolic disorder caused by a UGT1A1 inhibitor usually present in the maternal serum 44. Most newborns develop unconjugated hyperbilirubinemia (neonatal jaundice) because of hepatic immaturity and low activity of UGT1A1 during days 2 to 5. Breast milk feeding increases bilirubin levels in infants which results in maternal milk jaundice 45, 46. Lactation failure also results in hyperbilirubinemia due to insufficient caloric intake, which results in decreased bilirubin clearance, and increased enterohepatic circulation 47. Drugs such as novobiocin, pregnanediol, chloramphenicol, gentamycin, and several HIV protease inhibitors can induce hyperbilirubinemia by inhibiting the UGT1A1 enzyme 48. In newborns, ABO incompatibility or Rh incompatibility may lead to hyperbilirubinemia and consequently, neonatal jaundice 41.
Adults and children
Increased total bilirubin that is mainly unconjugated (indirect) bilirubin may be a result of:
- Hemolytic anemia or pernicious anemia
- Megaloblastic anemia
- A blood disorder called erythroblastosis fetalis
- Transfusion reaction in which red blood cells that were given in a transfusion are destroyed by the person’s immune system
- Cirrhosis (scarring of the liver)
- A relatively common inherited condition called Gilbert syndrome, due to low levels of the enzyme that produces conjugated bilirubin
If conjugated (direct) bilirubin is elevated more than unconjugated (indirect) bilirubin, there typically is a problem associated with decreased elimination of bilirubin by the liver cells. Some conditions that may cause this include:
- Viral hepatitis
- Drug reactions
- Alcoholic liver disease
Conjugated (direct) bilirubin is also elevated more than unconjugated (indirect) bilirubin when there is blockage of the bile ducts. This may occur, for example, with:
- Gallstones present in the bile ducts
- Tumors
- Scarring of the bile ducts or abnormal narrowing of the common bile duct (biliary stricture)
- Cancer of the pancreas or gallbladder cancer
In hepatobiliary diseases of various causes, bilirubin uptake, storage, and excretion are impaired to varying degrees. Thus, both conjugated and unconjugated bilirubin are retained and a wide range of abnormal serum concentrations of each form of bilirubin may be observed. Both conjugated and unconjugated bilirubins are increased in hepatitis and space-occupying lesions of the liver; and obstructive lesions such as carcinoma of the head of the pancreas, common bile duct, or ampulla of Vater.
Newborns
An elevated bilirubin level in a newborn may be temporary and resolve itself within a few days to two weeks. However, if the bilirubin level is above a critical threshold or increases rapidly, an investigation of the cause is needed so appropriate treatment can be initiated. Increased bilirubin concentrations may result from the accelerated breakdown of red blood cells due to:
- Blood type incompatibility between the mother and her newborn
- Certain congenital infections
- Lack of oxygen (hypoxia)
- Diseases that can affect the liver
In most of these conditions, only unconjugated (indirect) bilirubin is increased. An elevated conjugated (direct) bilirubin is seen in the rare conditions of biliary atresia and neonatal hepatitis. Biliary atresia requires surgical intervention to prevent liver damage.
Physiologic jaundice should resolve in 5 to 10 days in full-term infants and by 14 days in preterm infants.
Neonatal jaundice
Neonatal jaundice or infant jaundice is the yellow coloring of a newborn baby’s skin and eyes. Neonatal jaundice is caused by a buildup of pigment called bilirubin in the baby’s blood. Infant jaundice is a common condition, especially in babies born before 37 weeks’ gestation (preterm babies) and some breastfed babies. It usually happens because a baby’s liver isn’t mature enough to get rid of bilirubin in the bloodstream. In some babies, an underlying disease may cause infant jaundice. Most infants born between 35 weeks’ gestation and full term need no treatment for jaundice. Rarely, an unusually high blood level of bilirubin can place a newborn at risk of brain damage, particularly in the presence of certain risk factors for serious jaundice.
Hyperbilirubinemia in newborn also called neonatal hyperbilirubinemia, is a medical condition in which there is a build up of bilirubin in the blood, defined as a total serum bilirubin level above 5 mg per dL (86 μmol per L), causing yellow discoloration of the eyes and skin, called jaundice. Jaundice typically results from the deposition of unconjugated bilirubin pigment in the skin and mucus membranes. Depending on the underlying cause, this condition may present throughout the neonatal period. Approximately 60% of term babies and 80% of preterm newborns develop jaundice in the first week of life, and about 10% of breastfed babies are still jaundiced at 1 month of age 49, 50, 51, 52, 53. In most babies with jaundice there is no underlying disease, and this early jaundice termed ‘physiological jaundice’ is generally harmless and does not cause any trouble and will resolve on its own in the first week of life 54. However, there are pathological causes of hyperbilirubinemia in the newborn period, which, although rare, need to be detected. Such pathological jaundice may co-exist with physiological jaundice. Hyperbilirubinemia in the newborn period can be associated with severe illnesses such as premature infants (babies born before 37 weeks’ gestation), certain blood disorders, metabolic and endocrine disorders, anatomic abnormalities of the liver, and infections 55. Although low levels of bilirubin are not usually a concern, large amounts of bilirubin can circulate to tissues in the brain and may cause seizures and brain damage. This condition is called kernicterus or bilirubin encephalopathy, which refers to the ‘yellow staining of the basal nuclei of the brain’ caused by bilirubin 56. This is seen in parts of the brain on autopsy. Poor-quality studies have shown a link between kernicterus (acute and chronic brain effects of severe hyperbilirubinemia) and both high serum bilirubin levels and free bilirubin levels in all babies.
Physiological jaundice is mild, unconjugated bilirubinemia, and affects nearly all newborns. Physiological jaundice levels typically peak at 5 to 6 mg/dL (86 to 103 micromol/L) at 72 to 96 hours of age, and do not exceed 17 to 18 mg/dL (291 to 308 micromol/L) 57. The level of bilirubinemia that results in kernicterus in a given infant is unknown. The level of bilirubinemia that causes kernicterus varies by infant, but risk increases significantly when unconjugated bilirubin levels exceed 25 to 30 mg/dL (428 to 513 micromol/L) in term or near-term infants 58, 59, 56, 60. Factors like prematurity, hypoxia, acidosis, and certain infections can lower this threshold, making some infants more vulnerable at lower bilirubin levels
Depending on the cause of the hyperbilirubinemia, jaundice may appear at birth or at any time afterward. Factors significantly associated with hyperbilirubinemia are gestational age < 38 weeks, visible jaundice within 24 hours of birth, mother’s intention to breastfeed exclusively and family history of neonatal jaundice requiring treatment with phototherapy.
Bilirubin is a natural byproduct produced when red blood cells breakdown. The adult liver converts unconjugated bilirubin into a conjugated form, that be excreted. During pregnancy, the placenta excretes bilirubin but when the baby is born, the baby’s immature liver must assume that role. There are several causes of hyperbilirubinemia and jaundice in newborn, including the following:
- Physiologic jaundice. Physiologic jaundice occurs as a “normal” response to the baby’s limited ability to excrete bilirubin in the first days of life due to the immaturity of the liver. This will usually resolve by the first week of life.
- Breastfeeding failure jaundice. During the first few days of breastfeeding when the maternal breast milk supply is low and the baby is having trouble latching and feeding, the baby may become dehydrated. Since bilirubin is eliminated in the urine and stool, decreased urination and infrequent stools result in a buildup of bilirubin. While common in full term infants, premature infants and late preterm infants are more susceptible to breastfeeding failure jaundice because they may have uncoordinated suck as well as easy fatigability. Once effective breastfeeding is established, this problem will resolve.
- Breast milk jaundice. About 2 percent of breastfed babies develop jaundice after the first week. It peaks about two weeks of age and can persist up to three to twelve weeks. Breast milk jaundice is thought to be caused by glucuronidase enzyme in the breast milk that deconjugates bilirubin into unconjugated bilirubin 61. Breastfeeding can usually continue or only be interrupted briefly.
- Jaundice from breakdown of red blood cells (hemolysis). Jaundice may occur if there is an increase of red blood cell breakdown (hemolysis) such as that seen when there is a mismatch of maternal and fetal blood type, resulting in ABO incompatibility (a condition where a mother’s antibodies attack a baby’s red blood cells due to a difference in blood type, with most common type when a mother is blood type O and her baby is blood type A or B), hemolytic disease of the newborn (Rh disease) or abnormalities of the red cell itself such as hereditary spherocytosis, elliptocytosis, glucose-6-phosphate dehydrogenase deficiency (G6PD deficiency), pyruvate kinase deficiency; and hemoglobinopathies, such as alpha and beta thalassemias 62. Increased hemolysis (red blood cell breakdown) can also occur if the baby is bruised or develops a hematoma during delivery.
- Birth Trauma such as cephalohematoma (a collection of blood under the scalp that forms on a newborn’s skull, usually caused by ruptured blood vessels during childbirth), subgaleal hemorrhage (a serious, potentially life-threatening condition in newborns caused by bleeding into the space between the skull’s periosteum and the galea aponeurosis [the scalp’s tough, fibrous tissue layer]), or peripheral bruises from birth trauma 63.
- Jaundice related to inadequate liver function. Jaundice may be related to prolonged liver dysfunction due to infection and other factors.
- Disruption or Obstruction in the Biliary System.
- Hereditary Bilirubin Conjugation Defects – Crigler-Najjar syndrome and Gilbert syndrome are due to the deficiency of the enzyme uridine 5′-diphospho-glucuronosyltransferase (UDGPT), which conjugates the bilirubin in the liver cells 64.
- Rotor syndrome is a rare, inherited genetic disorder characterized by elevated levels of bilirubin in the blood (hyperbilirubinemia). People with Rotor syndrome have a buildup of both unconjugated and conjugated bilirubin in their blood, but the majority is conjugated bilirubin 10, 11. Rotor syndrome is caused by SLCO1B1 and SLCO1B3 genes. Mutations in both SLCO1B1 and SLCO1B3 genes are required for Rotor syndrome to occur. The SLCO1B1 and SLCO1B3 genes provide instructions for making similar proteins, called organic anion transporting polypeptide 1B1 (OATP1B1) and organic anion transporting polypeptide 1B3 (OATP1B3), respectively. Both proteins are found in liver cells; they transport bilirubin and other compounds from the blood into the liver so that they can be cleared from the body. In the liver, bilirubin is dissolved in a digestive fluid called bile and then excreted from the body. In people with Rotor syndrome, jaundice is usually evident shortly after birth or in childhood and may come and go; yellowing of the whites of the eyes also called conjunctival icterus is often the only symptom. Rotor syndrome is a benign and harmless condition that typically does not require treatment and does not affect life expectancy. Jaundice may be noticeable shortly after birth or in childhood, but often it’s an incidental finding.
- Crigler–Najjar syndrome is a very rare inherited disorder in which bilirubin cannot be broken down. Mutations in the UGT1A1 gene cause Crigler-Najjar syndrome. The UGT1A1 gene provides instructions for making the bilirubin uridine diphosphate glucuronosyl transferase (bilirubin-UGT) enzyme, which is found primarily in liver cells and is necessary for the removal of bilirubin from the body. Mutations in the UGT1A1 gene that cause Crigler-Najjar syndrome result in reduced or absent function of the bilirubin-UGT enzyme. People with Crigler–Najjar Type 1 have no bilirubin uridine diphosphate glucuronosyl transferase (bilirubin-UGT) enzyme function, while people with Crigler–Najjar Type 2 have less than 20 percent of normal function. The loss of bilirubin-UGT function decreases glucuronidation of unconjugated bilirubin. Glucuronidation makes bilirubin dissolvable in water so that it can be removed from the body. This toxic unconjugated bilirubin then builds up in the body, causing unconjugated hyperbilirubinemia and jaundice. Crigler–Najjar Type 1 (CN1) is very severe, and affected individuals can die in childhood due to kernicterus, although with proper treatment, they may survive longer. Crigler–Najjar Type 2 (CN2) is less severe. People with Crigler–Najjar Type 2 are less likely to develop kernicterus, and most affected individuals survive into adulthood.
- Dubin-Johnson syndrome is a rare, inherited liver disorder characterized by an increase in conjugated bilirubin, leading to mild, chronic jaundice. Dubin-Johnson syndrome is caused by mutation in ABCC2 gene affecting the liver’s ability to excrete bilirubin, resulting in pigment deposits that make the liver appear black on medical imaging. The ABCC2 gene provides instructions for making a protein that transports certain substances out of cells so they can be released (excreted) from the body. For example, this protein transports bilirubin out of liver cells and into bile (a digestive fluid produced by the liver). Bilirubin is produced during the breakdown of old red blood cells and has an orange-yellow tint. As a result, bilirubin accumulates in the body, causing a condition called hyperbilirubinemia. The buildup of bilirubin in the body causes the yellowing of the skin and whites of the eyes in people with Dubin-Johnson syndrome. Dubin-Johnson syndrome is most often seen in Middle Eastern Jewish and Japanese people. In the Jewish population, about 60% of affected individuals also have an associated blood clotting abnormality, a prolonged prothrombin time (PT), caused by a decrease in factor VII (factor 7). In most affected people jaundice appears during adolescence or early adulthood. Infants with Dubin-Johnson syndrome typically also have enlarged livers (hepatomegaly) and a severely reduced ability to produce and release a digestive fluid called bile (cholestasis). As these children get older, their liver problems go away and they usually do not have any related health problems later in life. Jaundice is typically the only feature of Dubin-Johnson syndrome, but some people can experience weakness, mild abdominal pain, nausea, or vomiting. In most people with Dubin-Johnson syndrome, certain deposits build up in the liver but do not seem to impair liver function. The deposits make the liver appear black when viewed with medical imaging. Dubin-Johnson syndrome is benign, has a normal life expectancy, and usually requires no specific treatment, though precautions like avoiding birth control pills, alcohol, environmental factors that affect the liver, infection, pregnancy, fasting or dehydration and fatigue are recommended.
- Neonatal polycythemia also called polycythemia in newborns is a condition where there are too many red blood cells, defined by a venous hematocrit greater than 65% 65, 66, 67. Polycythemia means there are too many red blood cells (RBCs) in an infant’s blood. Polycythemia in newborn can lead to increased blood viscosity and decreased blood flow, causing symptoms like lethargy, poor feeding, and respiratory distress. Causes of polycythemia in newborn include delayed cord clamping, twin-to-twin transfusion, and intrauterine hypoxia. Treatment may involve fluid administration for mild cases or a partial exchange transfusion for more severe cases.
- Hypoalbuminemia is a condition where the level of albumin, a protein in the blood, is abnormally low. Unconjugated bilirubin is lipid-soluble and is normally bound to albumin; a decrease in the albumin level leads to an increase in free unconjugated bilirubin level 68
Prematurity, hemolytic disease of the newborn and glucose-6-phosphate dehydrogenase (G6PD) deficiency are the most common risk factors 60, 69. Newborns who develop neurological complications of hyperbilirubinemia usually have at least two neurotoxicity risk factors 70. There is no clear correlation between bilirubin level alone and the risk of developing brain toxicity 71, 62.
The timing of when your child’s jaundice first starts matters. It may help their your baby’s doctor make a diagnosis.
- First 24 hours. This type of jaundice is often serious. Your child will likely need treatment right away.
- Second or third day. This is often physiologic jaundice. Sometimes it can be a more serious type of jaundice. It’s important to be sure the baby is getting enough milk at this point.
- Toward the end of the first week. This type of jaundice may be from breastmilk jaundice but may be due to an infection or other rare, serious problems.
- In the second week. This is often caused by breastmilk jaundice but may be caused by rare liver problems.
In the United States Kernicterus Registry, 56% had abnormalities known to increase the bilirubin concentration in the blood. Glucose-6-phosphate dehydrogenase deficiency (G6PD deficiency) was diagnosed in 21.3%, severe hemolytic processes were recognized in 20.5%, birth trauma was identified in 15%, and other causes such as galactosemia, Crigler-Najjar syndrome, and sepsis were diagnosed in 7% 56. However, no cause was identified in 43.4% of the infants 72.
Severe jaundice requiring exchange transfusion (bilirubin > 340 micromol/liter) and early onset of jaundice (within 24 hours) are statistically significant risk factors for hearing loss 49, 73. Hemolytic disorders such as G6PD deficiency and ABO incompatibility (ABO incompatible blood) may cause a rapid increase in bilirubin level, and these disorders have been over-represented in international kernicterus registries and population studies of significant hyperbilirubinemia. A study of low-birthweight babies found a weak association between high serum bilirubin levels (> 340 micromol/L) and neurodevelopmental impairment, hearing impairment and psychomotor impairment.
Rarely, bilirubin crosses the blood-brain barrier (BBB) and can cause serious consequences 74. One study performed in Canada found that acute bilirubin encephalopathy occurs in approximately 1 out of 10,000 infants 75. Symptoms include lethargy, hypotonia or hypertonia, back and neck arching, irritability, and high-pitched crying 75, 76. Acute bilirubin encephalopathy fully resolves in most cases but can progress to kernicterus 62, 77.
Kernicterus describes the irreversible, chronic effects of bilirubin toxicity 54. Kernicterus occurs in approximately 1 out of 100,000 infants in high-income countries and presents as cerebral palsy, hearing loss, gaze paralysis, dental dysplasia, and developmental disability 78, 60, 69.
Complications of kernicterus include 79, 80, 81:
- Hearing loss – most common abnormality
- Extrapyramidal symptoms like athetosis and chorea
- Visual abnormalities including gaze palsies
- Abnormalities in dentition
Because of the serious and irreversible complications of kernicterus, it is important to be aware of risk factors for neurotoxicity and routinely examine newborns for jaundice.
Bilirubin encephalopathy
Bilirubin encephalopathy is a rare neurological condition that occurs in some newborns with severe jaundice. Bilirubin encephalopathy is a serious condition. Many infants with late-stage nervous system complications die.
Bilirubin encephalopathy most often develops in the first week of life, but may be seen up until the third week. Some newborns with Rh hemolytic disease are at high risk for severe jaundice that can lead to this condition. Rarely, bilirubin encephalopathy can develop in seemingly healthy babies.
Acute bilirubin encephalopathy
Bilirubin is toxic to cells of the brain. If a baby has severe jaundice, there’s a risk of bilirubin passing into the brain, a condition called acute bilirubin encephalopathy 82. Prompt treatment may prevent significant lasting damage.
Bilirubin encephalopathy is a rare neurological condition that occurs in some newborns with severe jaundice due to severe unconjugated hyperbilirubinemia 82. Bilirubin encephalopathy is a serious condition. Many infants with late-stage nervous system complications die.
Bilirubin encephalopathy most often develops in the first week of life, but may be seen up until the third week. Some newborns with Rh hemolytic disease are at high risk for severe jaundice that can lead to this condition. Rarely, bilirubin encephalopathy can develop in seemingly healthy babies.
Signs of acute bilirubin encephalopathy in a baby with jaundice include:
- Listlessness.
- Difficulty waking.
- High-pitched crying.
- Poor sucking or feeding.
- Backward arching of the neck and body.
- Fever.
The signs and symptoms of bilirubin encephalopathy in neonates with severe unconjugated hyperbilirubinemia depend on when the symptoms appear. The level at which unconjugated bilirubin becomes neurotoxic is unclear, and kernicterus has been reported in infants without markedly elevated bilirubin levels on autopsy. There are 3 phases of acute bilirubin encephalopathy, including:
Phase 1: The symptoms of phase 1 appear during the first 1 to 2 days of illness and are notable for poor feeding, lethargy, hypotonia, irritability, or frank seizures.
Early stage symptoms:
- Extreme jaundice
- Absent startle reflex
- Poor feeding or sucking
- Extreme sleepiness (lethargy) and low muscle tone (hypotonia)
Phase 2: If infants continue to deteriorate, they progress to phase 2, characterized by increased extensor muscle tone, exhibiting opisthotonus and retrocollis. This typically occurs during the middle of the first week of illness.
Middle stage:
- High-pitched cry
- Irritability
- May have arched back with neck hyperextended backwards, high muscle tone (hypertonia)
- Poor feeding
Phase 3: After the first week, muscle rigidity, stupor or coma, apnea, or seizures may occur.
Late stage:
- Stupor or coma
- No feeding
- Shrill cry
- Muscle rigidity, markedly arched back with neck hyperextended backwards
- Seizures.
Kernicterus
Kernicterus is the syndrome that happens if acute bilirubin encephalopathy causes permanent damage to the brain 83. Extreme hyperbilirubinemia (total bilirubin of 25 to 30 mg/dL) can cause kernicterus. Kernicterus is usually characterized by the deposition of unconjugated bilirubin (yellow stain) in brain cells. Neuronal necrosis or damage occurs in the basal ganglia, hippocampus, hypothalamic nuclei, diencephalon, midbrain responsible for neurohumoral and electrolyte control, brainstem nuclei accounting for oculomotor and auditory function, and in the cerebellum depositing as a bilirubin-phosphatidylcholine precipitate. Vision, hearing, speech, cognition, gait, and language are typically affected 84, 85, 86, 87. Kernicterus clinical features include cerebral palsy, deafness, seizures, abnormalities of the gaze, and hypoplasia of the dental enamel 83.
The symptoms depend on the stage of bilirubin encephalopathy. Not all babies with kernicterus on autopsy have had definite symptoms.
Kernicterus usually present in 2 forms, depending on the timing of symptoms 41:
- In the first year: These patients present with hypotonia, exaggerated deep tendon reflexes, obligatory tonic neck reflexes, and delayed motor milestones.
- Beyond the first year: Patients exhibit movement disorders, most commonly choreo-athetoid cerebral palsy, dental enamel hypoplasia, upward gaze abnormality, and sensorineural hearing loss.
Kernicterus may result in:
- Permanent brain damage
- Movements that are not controlled or intended, known as athetoid cerebral palsy.
- Permanent upward gaze.
- Hearing loss.
- Improper development of tooth enamel.
- Death.
Bilirubin encephalopathy Prevention
Pregnant women should be screened with Rhesus factor (Rh factor) and treated appropriately. Rhesus factor (Rh factor) is a type of protein on the outside or surface of your red blood cells. You inherit the Rh protein, which means you get your Rh factor from your biological parents. If you have the Rh protein, you’re Rh-positive (Rh+). If you don’t have the Rh protein, you’re Rh-negative. The majority of people, about 85%, are Rh-positive (Rh+). During pregnancy, complications may occur if a mother is Rh-negative and the fetus is Rh-positive (Rh+). This is called Rh factor incompatibility. With Rh incompatibility, a mother who is Rh-negative reacts to this difference known as incompatibility and creates antibodies. These antibodies drive an immune system attack against the fetus’s red blood cells that are Rh-positive (Rh+), which a mother who is Rh-negative thinks are foreign objects. This is called Rh sensitization. Obstetricians (physicians who specialize in delivering babies) can prevent this from happening by giving you a shot (injection) of immune globulin called Rh immune globulin (RhIg or RhoGAM®). Rh immune globulin (RhIg or RhoGAM®) is a medication that stops a mother who is Rh-negative from making Rh antibodies. It’s only helpful if your body hasn’t already made Rh antibodies. You receive it as a shot (injection). Rh immunoglobulin shots are usually very successful in treating Rh-incompatibility during pregnancy. Detecting Rh incompatibility early in pregnancy is the best way to prevent serious complications.
If a mother who is Rh-negative already has Rh antibodies, the fetus is at risk for Rh disease. Since Rh immune globulin won’t be helpful, the best treatment is close monitoring for the remainder of your pregnancy. There’s a small chance your obstetrician will want to deliver early, but this depends on how severe the fetus’s Rh disease is.
During pregnancy, a mother don’t share blood with the fetus she’s carrying. However, a small amount of blood from the fetus can mix with a mother’s blood during labor and delivery (either vaginal or cesarean). It can also happen during:
- Tests like amniocentesis and chorionic villus sampling (CVS).
- Any type of vaginal bleeding during pregnancy.
- Injury or trauma to your abdomen.
- Early pregnancy complications like miscarriage or ectopic pregnancy.
- After external cephalic version, a maneuver to turn a breech baby.
Rh incompatibility doesn’t affect the pregnant woman. In a fetus, Rh disease can cause hemolytic anemia. Hemolytic anemia destroys the fetus’s red blood cells faster than it can replace them.
The effects of Rh incompatibility can range from mild to severe. These effects may also include:
- Jaundice.
- Liver failure.
- Heart failure.
- Stillbirth.
For mild Rh disease, the fetus may not need any treatment. Most fetuses recover fully if they have a mild case of Rh disease.
For severe Rh disease cases, the fetus may receive a blood transfusion. This procedure helps replace its red blood cells. Doctors can use special lights to help reduce bilirubin levels in fetuses that have jaundice. You may need to give birth early to avoid serious complications of hemolytic anemia.
Since the development of Rh immune globulin injections, Rh disease occurs infrequently.
Because suboptimal feeding plays a role in developing hyperbilirubinemia, all breastfeeding mothers should receive support to promote adequate feeding. Breastfeeding within the first hour of life is recommended, followed by on-demand nursing at least 8 times daily. Furthermore, staff should visually assess all hospitalized neonates for jaundice at least every 12 hours. The AAP guidelines recommend universal bilirubin screening before discharge with total serum bilirubin 88.
For all babies, diagnosing jaundice early and getting treatment right away are key. This can stop your baby’s bilirubin levels from rising to dangerous levels.
Treating jaundice or conditions that may lead to it can help prevent neonatal hyperbilirubinemia. Infants with the first signs of jaundice have bilirubin level measured within 24 hours. If the level is high, the infant should be screened for diseases that involve the destruction of red blood cells (hemolysis).
The best preventive of infant jaundice is adequate feeding. Feedings should start within the first hour of life and continue at least every 2 or 3 hours, or sooner if the baby shows signs of wanting to eat. The more premature the baby, the more likely they are to need supplements of expressed milk or formula at first. Make sure you feed your baby early and often. Breastfed infants should have 8 to 12 feedings a day for the first several days of life.
All newborns have a follow-up appointment within 2 to 3 days after leaving the hospital. This is very important for late preterm or early term babies (born more than 2 to 3 weeks before their due date).
Bilirubin encephalopathy treatment
Treatment depends on how old the baby is (in hours) and whether the baby has any risk factors (such as prematurity). It may include:
- Light therapy (phototherapy)
- Exchange transfusions (removing the child’s blood and replacing it with fresh donor blood or plasma)
In preterm infants, the risk of a handicap increases by 30% for each 2.9 mg/dL increase of maximal total bilirubin concentration. While central nervous system damage is rare when total serum bilirubin is less than 20 mg/dL, premature infants may be affected at lower levels. The decision to institute therapy is based on a number of factors including total serum bilirubin, age, clinical history, physical examination, and coexisting conditions. Phototherapy typically is discontinued when total serum bilirubin level reaches 14 to 15 mg/dL.
How to manage hyperbilirubinemia in newborn babies
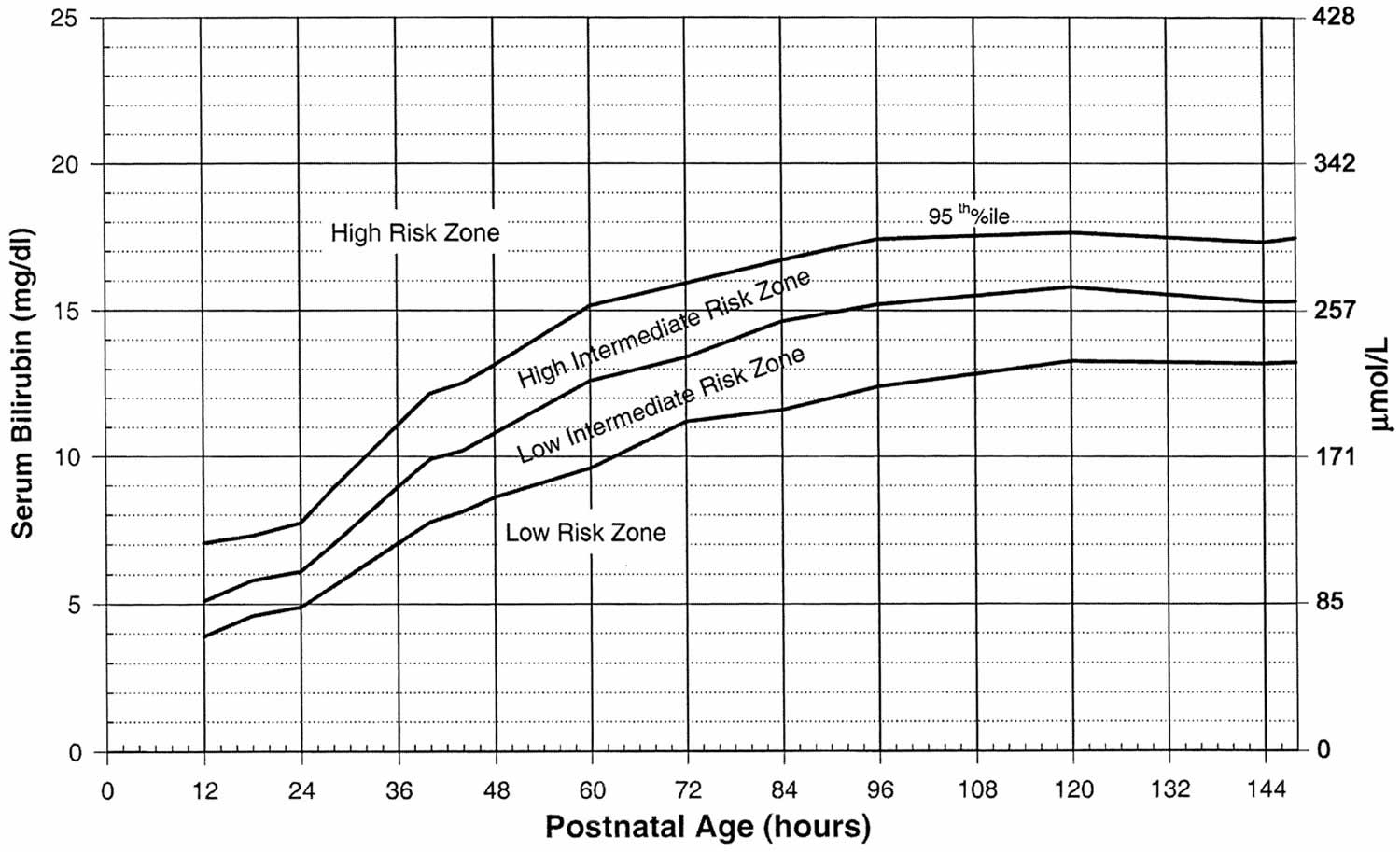
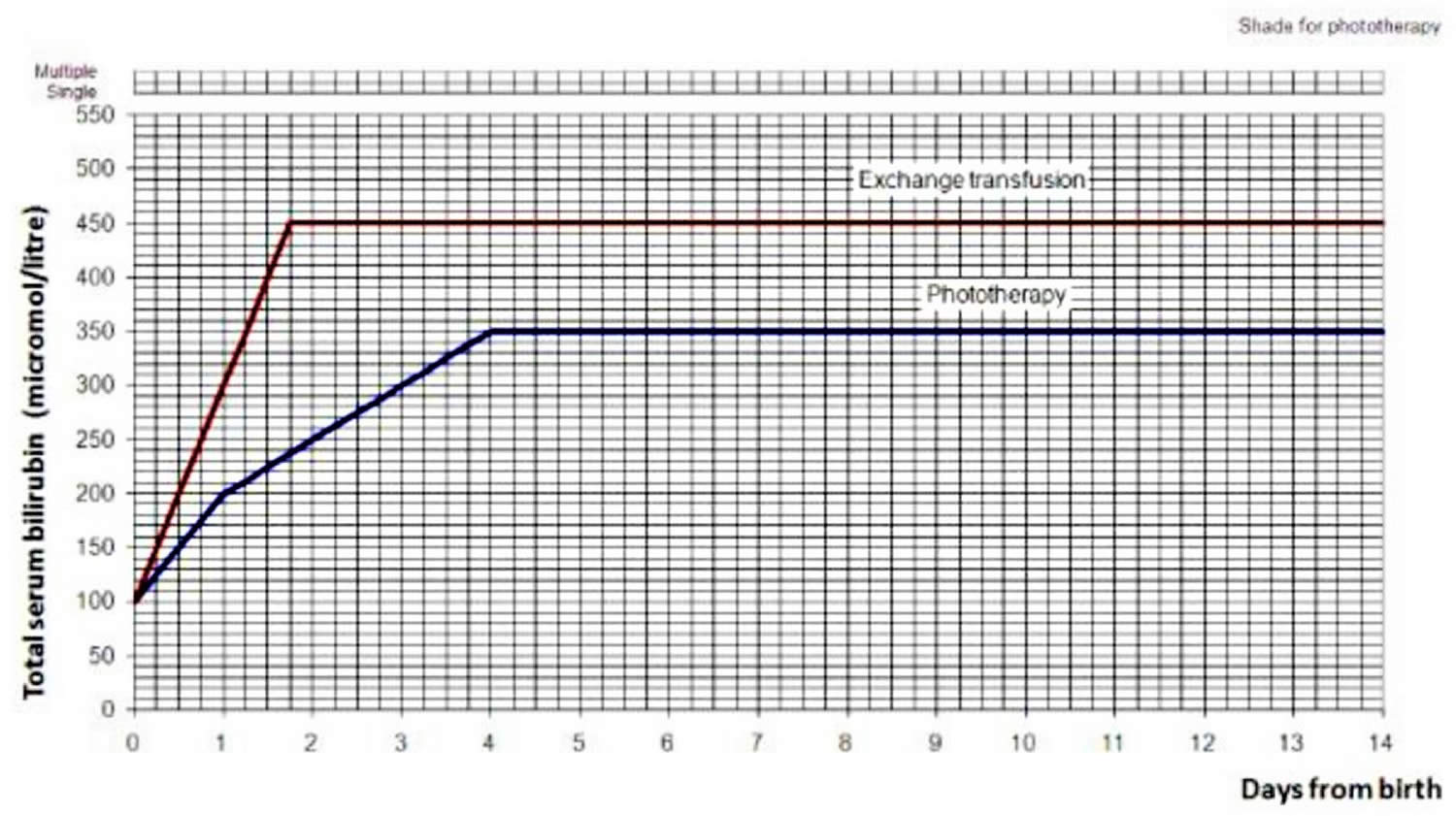
Treatment will depend on your child’s symptoms, age, and general health. It will also depend on how bad the condition is. Mild infant jaundice often disappears on its own within two or three weeks. For moderate or severe jaundice, a baby may need to stay longer in the newborn nursery or be readmitted to the hospital. Use the bilirubin level to determine the management of hyperbilirubinemia in all babies, see threshold table (see Table 1) and treatment threshold graphs (Figures 4 and 5).
Treatment depends on how old the baby is (in hours) and whether the baby has any risk factors (such as prematurity). It may include:
- Light therapy also called phototherapy. Your baby may be placed under a special lamp that emits light in the blue-green spectrum. The light changes the shape and structure of bilirubin molecules in such a way that they can be excreted in both the urine and stool. During treatment, your baby will wear only a diaper and protective eye patches. Light therapy may be supplemented with the use of a light-emitting pad or mattress.
- Fiber optic blanket is another form of phototherapy. The blanket is usually put under your baby. It may be used alone or with regular phototherapy.
- Exchange transfusions (removing the child’s blood and replacing it with fresh donor blood or plasma). Rarely, when severe jaundice doesn’t respond to other treatments, a baby may need an exchange transfusion of blood. This involves repeatedly withdrawing small amounts of blood and replacing it with donor blood. The procedure dilutes the bilirubin and maternal antibodies and is done in a newborn intensive care unit.
- Enhanced nutrition. To prevent weight loss, your baby’s doctor may recommend more-frequent feeding or supplementation to ensure that your baby receives enough nutrition.
- Intravenous immunoglobulin (IVIg). Jaundice may be related to blood type differences between mother and baby. This condition results in the baby carrying antibodies from the mother that contribute to the rapid breakdown of the baby’s red blood cells. Intravenous transfusion of an immunoglobulin (IVIg), a blood protein that can reduce levels of antibodies, may decrease jaundice and lessen the need for an exchange transfusion, although results are not conclusive.
- Treating any underlying cause of the hyperbilirubinemia.
Use the bilirubin level to determine the management of hyperbilirubinemia in all babies see threshold table (see Table 3) and treatment threshold graphs (Figures 2 and 3).
Table 3. Bilirubin threshold table for the management of babies of 38 weeks or more gestational age with hyperbilirubinemia
| Age (hours) | Bilirubin measurement (micromol/liter) | |
|---|---|---|
| 0 | >100 | >100 |
| 6 | >125 | >150 |
| 12 | >150 | >200 |
| 18 | >175 | >250 |
| 24 | >200 | >300 |
| 30 | >212 | >350 |
| 36 | >225 | >400 |
| 42 | >237 | >450 |
| 48 | >250 | >450 |
| 54 | >262 | >450 |
| 60 | >275 | >450 |
| 66 | >287 | >450 |
| 72 | >300 | >450 |
| 78 | >312 | >450 |
| 84 | >325 | >450 |
| 90 | >337 | >450 |
| 96 | >350 | >450 |
| Action | Start phototherapy | Perform an exchange transfusion unless the bilirubin level falls below threshold while the treatment is being prepared |
Footnotes: Note that there is variability between assays from different manufacturers in reported bilirubin measurement. Healthcare professionals should consult their local pathology laboratory when interpreting threshold tables.
[Source 89 ]Figure 2. Hyperbilirubinemia threshold graph for babies
Figure 3. Hyperbilirubinemia treatment threshold graph for babies with neonatal jaundice: >=38 weeks gestation
[Source 49 ]Phototherapy
Bilirubin absorbs light. High bilirubin levels often decrease when a baby is put under special blue spectrum lights. This is called phototherapy 91, 82, 92. Your child may get this treatment in the day and night. It may take several hours for it to start working. During light treatment, your baby’s eyes will be protected. Your baby’s doctor will check your baby’s temperature. They will also test your baby’s bilirubin levels. This will tell if phototherapy is working.
Bilirubin optimally absorbs light in the blue-green range (ie, 460-490 nm). The underlying mechanism of phototherapy involves inducing photoisomerization and converting bilirubin into lumirubin, which is readily excreted into bile and urine 93. During phototherapy, the neonate’s maximum body surface area should be exposed to the light source while keeping the eyes covered to avoid retinal injury, and interruptions should be minimized. The maintenance of hydration is necessary to ensure adequate urine output, as most bilirubin is excreted in the urine as lumirubin, a structural isomer of bilirubin formed during phototherapy. Therefore, breastfeeding support should be offered to all nursing mothers as early initiation of breastfeeding and frequent, on-demand feeding decreases the likelihood of dehydration. Although supplemental oral water and dextrose water are not recommended, supplemental pumped breastmilk or infant formula can be considered for feeding issues, including infants with ineffective sucking or latching or inadequate maternal milk production 88.
After phototherapy is discontinued, there may be an increase in the total serum bilirubin level, known as the rebound bilirubin. This level is usually lower than the pretreatment level and rarely requires reinitiation of phototherapy 94. Phototherapy is considered safe, but recent evidence suggests a possible association with long-term complications, including a small risk of childhood seizures. However, no studies have proven causation 95. A few studies also have reported a possible association between solid organ tumors and nonlymphocytic leukemias and children treated with phototherapy 96, 97. Adverse effects of phototherapy include rashes, dehydration, hypocalcemia, retinal damage, hemolysis due to oxidative damage, delay in patent ductus arteriosis closure in preterm infants, and allergic reactions 98. Bronze baby syndrome is a self-limited condition associated with elevated levels of conjugated bilirubin that rarely occurs with phototherapy, resulting in irregular, bronze-gray pigmentation of the skin, mucous membranes, and urine. The Bronze baby syndrome’s mechanism is unclear but appears to be related to the accumulation of bilirubin and biliverdin photoisomers. Bronze baby syndrome usually resolves within a few days of discontinuing phototherapy; however, the prognosis depends upon the underlying cause of the conjugated hyperbilirubinemia 99, 100.
Fiber optic blanket
A fiber optic blanket is another form of phototherapy. The blanket is usually put under your baby. It may be used alone or with regular phototherapy.
Exchange transfusion
Exchange transfusion is the treatment that involves removing your baby’s blood that has a high bilirubin level and replacing it with fresh donor blood or plasma 101. This raises your baby’s red blood cell count. It also lowers their bilirubin level. During the procedure, your baby will switch between giving and getting small amounts of blood. This will be done through a vein or artery in the baby’s umbilical cord. It is only done in an intensive care nursery when bilirubin levels are extremely high. Your baby may need to have this procedure again if their bilirubin levels stay high.
Exchange transfusion is now the second-line treatment for severe unconjugated hyperbilirubinemia since phototherapy was developed in the 1950s 102, 103. Indications for exchange transfusion include neonatal failure to respond to phototherapy or a total serum bilirubin level at the exchange transfusion threshold. The threshold to initiate exchange transfusion is calculated based on several factors, including the total serum bilirubin level and rate of rise, neonatal age (ie, hours or days since birth), and risk factors for neurologic complications 88. Exchange transfusion rapidly removes bilirubin and hemolysis-causing antibodies from the infant’s circulation. A double-volume exchange blood transfusion (160-180 ml/kg) is performed, replacing aliquots of the neonate’s blood with crossed-matched donor blood. Since most of the total body bilirubin is extravascular, the total serum bilirubin level immediately following exchange transfusion is approximately 60% of the pre-exchange level, but that later increases to 70% to 80% of the pretreatment level as a result of equilibrium. During exchange transfusion, the neonate’s vital signs should be monitored closely. Following the procedure, total serum bilirubin, complete blood count, serum calcium, glucose, and electrolytes should be rechecked due to potential complications, including electrolyte abnormalities (eg, hypocalcemia and hyperkalemia), cardiac arrhythmias, thrombocytopenia, blood-borne infections, portal vein thrombosis, graft versus host disease, and necrotizing enterocolitis (NEC) 104, 105. Phototherapy should resume after exchange transfusion until the bilirubin reaches a level where phototherapy can be safely discontinued.
Intravenous immunoglobulin (IVIg)
Jaundice may be related to blood type differences between mother and baby. This condition results in the baby carrying antibodies from the mother that contribute to the rapid breakdown of the baby’s red blood cells. Intravenous transfusion of an immunoglobulin (IVIg), a blood protein that can reduce levels of antibodies, may decrease jaundice and lessen the need for an exchange transfusion, although results are not conclusive. The American Academy of Pediatrics recommends intravenous immunoglobulin (IVIg) infusion in immune-mediated hemolysis if total serum bilirubin remains within 2 to 3 mg/dL of the exchange threshold despite intensive phototherapy 106, 107. However, the evidence that intravenous immunoglobulin (IVIg) reduces the need for exchange transfusion is unclear. Nonetheless, intravenous immunoglobulin (IVIg) is often used in clinical practice to manage severe unconjugated hyperbilirubinemia 82.
Feeding with breastmilk
The American Academy of Pediatrics says that you should keep breastfeeding a baby with jaundice. If your baby has not been getting enough milk at the breast, you may need to supplement with pumped breastmilk or formula.
Feeding more frequently will provide your baby with more milk and cause more bowel movements, increasing the amount of bilirubin eliminated in your baby’s stool. Infants who are breastfed should have 8 to 12 feedings a day for the first several days of life. Formula-fed infants usually should have 1 to 2 ounces (about 30 to 60 milliliters) of formula every 2 to 3 hours for the first week.
Supplemental feedings
If your baby is having trouble breastfeeding, is losing weight or is dehydrated, your baby’s doctor may suggest giving your baby formula or expressed milk to supplement breastfeeding. Depending on the situation, your baby’s doctor may recommend using formula alone for a couple of days and then resuming breastfeeding. Ask your baby’s doctor what feeding options are right for your baby.
Treating any underlying cause of the hyperbilirubinemia
This may include treating an infection.
What does low bilirubin levels mean?
Low levels of bilirubin are generally not of any concern and are not monitored.
- Kalakonda A, Jenkins BA, John S. Physiology, Bilirubin. [Updated 2022 Sep 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470290[↩]
- Singh A, Koritala T, Jialal I. Unconjugated Hyperbilirubinemia. [Updated 2023 Feb 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549796[↩][↩][↩]
- Kosmachevskaya OV, Topunov AF. Alternate and Additional Functions of Erythrocyte Hemoglobin. Biochemistry (Mosc). 2018 Dec;83(12):1575-1593. doi: 10.1134/S0006297918120155[↩]
- Dosch AR, Imagawa DK, Jutric Z. Bile Metabolism and Lithogenesis: An Update. Surg Clin North Am. 2019 Apr;99(2):215-229. doi: 10.1016/j.suc.2018.12.003[↩]
- Shen H, Zeng C, Wu X, Liu S, Chen X. Prognostic value of total bilirubin in patients with acute myocardial infarction: A meta-analysis. Medicine (Baltimore). 2019 Jan;98(3):e13920. doi: 10.1097/MD.0000000000013920[↩]
- Franchini M, Targher G, Lippi G. Serum bilirubin levels and cardiovascular disease risk: a Janus Bifrons? Adv Clin Chem. 2010;50:47-63. doi: 10.1016/s0065-2423(10)50003-9[↩]
- Vítek L, Schwertner HA. The heme catabolic pathway and its protective effects on oxidative stress-mediated diseases. Adv Clin Chem. 2007;43:1-57. doi: 10.1016/s0065-2423(06)43001-8[↩][↩]
- Vítek L, Ostrow JD. Bilirubin chemistry and metabolism; harmful and protective aspects. Curr Pharm Des. 2009;15(25):2869-83. doi: 10.2174/138161209789058237[↩]
- Jedlitschky G, Hoffmann U, Kroemer HK. Structure and function of the MRP2 (ABCC2) protein and its role in drug disposition. Expert Opin Drug Metab Toxicol. 2006 Jun;2(3):351-66. doi: 10.1517/17425255.2.3.351[↩]
- Hashmi MF, Mehta D. Rotor Syndrome. [Updated 2023 Feb 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532306[↩][↩]
- Rotor syndrome. https://medlineplus.gov/genetics/condition/rotor-syndrome[↩][↩]
- Fargo MV, Grogan SP, Saguil A. Evaluation of Jaundice in Adults. Am Fam Physician. 2017 Feb 1;95(3):164-168. https://www.aafp.org/pubs/afp/issues/2017/0201/p164.html[↩][↩][↩]
- Gallagher PG. Abnormalities of the erythrocyte membrane. Pediatr Clin North Am. 2013 Dec;60(6):1349-62. doi: 10.1016/j.pcl.2013.09.001[↩]
- Koralkova, P., van Solinge, W.W. and van Wijk, R. (2014), Rare hereditary red blood cell enzymopathies associated with hemolytic anemia – pathophysiology, clinical aspects, and laboratory diagnosis. Int. Jnl. Lab. Hem., 36: 388-397. https://doi.org/10.1111/ijlh.12223[↩]
- Martin A, Thompson AA. Thalassemias. Pediatr Clin North Am. 2013 Dec;60(6):1383-91. doi: 10.1016/j.pcl.2013.08.008[↩]
- Bass GF, Tuscano ET, Tuscano JM. Diagnosis and classification of autoimmune hemolytic anemia. Autoimmun Rev. 2014 Apr-May;13(4-5):560-4. doi: 10.1016/j.autrev.2013.11.010[↩]
- Strassburg CP. Hyperbilirubinemia syndromes (Gilbert-Meulengracht, Crigler-Najjar, Dubin-Johnson, and Rotor syndrome). Best Pract Res Clin Gastroenterol. 2010 Oct;24(5):555-71. doi: 10.1016/j.bpg.2010.07.007[↩][↩]
- Erlinger S, Arias IM, Dhumeaux D. Inherited disorders of bilirubin transport and conjugation: new insights into molecular mechanisms and consequences. Gastroenterology. 2014 Jun;146(7):1625-38. doi: 10.1053/j.gastro.2014.03.047[↩]
- Sun S, Song Z, Cotler SJ, Cho M. Biomechanics and functionality of hepatocytes in liver cirrhosis. J Biomech. 2014 Jun 27;47(9):2205-10. doi: 10.1016/j.jbiomech.2013.10.050[↩]
- Suhail M, Abdel-Hafiz H, Ali A, Fatima K, Damanhouri GA, Azhar E, Chaudhary AG, Qadri I. Potential mechanisms of hepatitis B virus induced liver injury. World J Gastroenterol. 2014 Sep 21;20(35):12462-72. doi: 10.3748/wjg.v20.i35.12462[↩]
- Levitt DG, Levitt MD. Quantitative assessment of the multiple processes responsible for bilirubin homeostasis in health and disease. Clin Exp Gastroenterol. 2014 Sep 2;7:307-28. doi: 10.2147/CEG.S64283[↩]
- Roche SP, Kobos R. Jaundice in the adult patient. Am Fam Physician. 2004;69(2):299-304. https://www.aafp.org/pubs/afp/issues/2004/0115/p299.html[↩][↩][↩]
- Rocco A, Compare D, Angrisani D, Sanduzzi Zamparelli M, Nardone G. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014 Oct 28;20(40):14652-9. doi: 10.3748/wjg.v20.i40.14652[↩]
- Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015 Apr;62(1 Suppl):S47-64. doi: 10.1016/j.jhep.2014.12.012[↩][↩]
- Bauer M, Press AT, Trauner M. The liver in sepsis: patterns of response and injury. Curr Opin Crit Care. 2013 Apr;19(2):123-7. doi: 10.1097/MCC.0b013e32835eba6d[↩]
- Kosters A, Karpen SJ. The role of inflammation in cholestasis: clinical and basic aspects. Semin Liver Dis. 2010 May;30(2):186-94. doi: 10.1055/s-0030-1253227[↩]
- Chen M, Suzuki A, Borlak J, Andrade RJ, Lucena MI. Drug-induced liver injury: Interactions between drug properties and host factors. J Hepatol. 2015 Aug;63(2):503-14. doi: 10.1016/j.jhep.2015.04.016[↩]
- Wooton-Kee CR, Jain AK, Wagner M, Grusak MA, Finegold MJ, Lutsenko S, Moore DD. Elevated copper impairs hepatic nuclear receptor function in Wilson’s disease. J Clin Invest. 2015 Sep;125(9):3449-60. doi: 10.1172/JCI78991[↩]
- Vuppalanchi R, Liangpunsakul S, Chalasani N. Etiology of new-onset jaundice: how often is it caused by idiosyncratic drug-induced liver injury in the United States? Am J Gastroenterol. 2007 Mar;102(3):558-62; quiz 693. doi: 10.1111/j.1572-0241.2006.01019.x[↩][↩]
- Cafasso DE, Smith RR. Symptomatic cholelithiasis and functional disorders of the biliary tract. Surg Clin North Am. 2014 Apr;94(2):233-56. doi: 10.1016/j.suc.2013.12.001[↩]
- Fang Y, Gurusamy KS, Wang Q, Davidson BR, Lin H, Xie X, Wang C. Pre-operative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev. 2012 Sep 12;9(9):CD005444. doi: 10.1002/14651858.CD005444.pub3[↩][↩]
- Luu MB, Deziel DJ. Unusual complications of gallstones. Surg Clin North Am. 2014 Apr;94(2):377-94. doi: 10.1016/j.suc.2014.01.002[↩]
- Winger J, Michelfelder A. Diagnostic approach to the patient with jaundice. Prim Care. 2011 Sep;38(3):469-82; viii. doi: 10.1016/j.pop.2011.05.004[↩][↩]
- Mortelé KJ, Wiesner W, Cantisani V, Silverman SG, Ros PR. Usual and unusual causes of extrahepatic cholestasis: assessment with magnetic resonance cholangiography and fast MRI. Abdom Imaging. 2004 Jan-Feb;29(1):87-99. doi: 10.1007/s00261-003-0062-6[↩]
- Krishna RP, Lal R, Sikora SS, Yachha SK, Pal L. Unusual causes of extrahepatic biliary obstruction in children: a case series with review of literature. Pediatr Surg Int. 2008 Feb;24(2):183-90. doi: 10.1007/s00383-007-2087-3[↩]
- Dickson PV, Behrman SW. Distal cholangiocarcinoma. Surg Clin North Am. 2014 Apr;94(2):325-42. doi: 10.1016/j.suc.2013.12.004[↩][↩]
- Brown KM, Geller DA. Proximal biliary tumors. Surg Clin North Am. 2014 Apr;94(2):311-23. doi: 10.1016/j.suc.2013.12.003[↩]
- Wernberg JA, Lucarelli DD. Gallbladder cancer. Surg Clin North Am. 2014 Apr;94(2):343-60. doi: 10.1016/j.suc.2014.01.009[↩]
- Roche SP, Kobos R. Jaundice in the adult patient. Am Fam Physician. 2004 Jan 15;69(2):299-304. https://www.aafp.org/pubs/afp/issues/2004/0115/p299.html[↩]
- Nelson M, Mulani SR, Saguil A. Evaluation of Jaundice in Adults. Am Fam Physician. 2025 Jan;111(1):25-30. https://www.aafp.org/pubs/afp/issues/2025/0100/jaundice.html[↩]
- Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. N Engl J Med. 2001 Feb 22;344(8):581-90. doi: 10.1056/NEJM200102223440807[↩][↩][↩]
- Kenwright S, Levi AJ. Sites of competition in the selective hepatic uptake of rifamycin-SV, flavaspidic acid, bilirubin, and bromsulphthalein. Gut. 1974 Mar;15(3):220-6. https://pmc.ncbi.nlm.nih.gov/articles/instance/1412884/pdf/gut00616-0068.pdf[↩]
- Radlović N. Hereditary hyperbilirubinemias. Srp Arh Celok Lek. 2014 Mar-Apr;142(3-4):257-60. doi: 10.2298/sarh1404257r[↩]
- ARIAS IM, WOLFSON S, LUCEY JF, MCKAY RJ Jr. TRANSIENT FAMILIAL NEONATAL HYPERBILIRUBINEMIA. J Clin Invest. 1965 Sep;44(9):1442-50. https://pmc.ncbi.nlm.nih.gov/articles/instance/292625/pdf/jcinvest00257-0028.pdf[↩]
- Grunebaum E, Amir J, Merlob P, Mimouni M, Varsano I. Breast mild jaundice: natural history, familial incidence and late neurodevelopmental outcome of the infant. Eur J Pediatr. 1991 Feb;150(4):267-70. doi: 10.1007/BF01955528[↩]
- Maisels MJ, Clune S, Coleman K, Gendelman B, Kendall A, McManus S, Smyth M. The natural history of jaundice in predominantly breastfed infants. Pediatrics. 2014 Aug;134(2):e340-5. doi: 10.1542/peds.2013-4299[↩]
- Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998 Jun;101(6):995-8. doi: 10.1542/peds.101.6.995[↩]
- Zhang D, Chando TJ, Everett DW, Patten CJ, Dehal SS, Humphreys WG. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos. 2005 Nov;33(11):1729-39. doi: 10.1124/dmd.105.005447[↩]
- National Collaborating Centre for Women’s and Children’s Health (UK). Neonatal Jaundice. London: RCOG Press; 2010 May. (NICE Clinical Guidelines, No. 98.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK65119[↩][↩][↩]
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004 Jul;114(1):297-316. doi: 10.1542/peds.114.1.297. Erratum in: Pediatrics. 2004 Oct;114(4):1138.[↩]
- Maisels MJ. Neonatal jaundice. Pediatr Rev. 2006 Dec;27(12):443-54. doi: 10.1542/pir.27-12-443[↩]
- Practice parameter: management of hyperbilirubinemia in the healthy term newborn. American Academy of Pediatrics. Provisional Committee for Quality Improvement and Subcommittee on Hyperbilirubinemia. Pediatrics. 1994 Oct;94(4 Pt 1):558-65. Erratum in: Pediatrics 1995 Mar;95(3):458-61.[↩]
- Jaundice and hyperbilirubinemia in the newborn. In: Behrman RE, Kliegman RM, Jenson HB, eds. Nelson Textbook of pediatrics. 16th ed. Philadelphia: Saunders, 2000:511–28.[↩]
- Moerschel SK, Cianciaruso LB, Tracy LR. A practical approach to neonatal jaundice. Am Fam Physician. 2008 May 1;77(9):1255-62. https://www.aafp.org/pubs/afp/issues/2008/0501/p1255.html[↩][↩]
- Porter ML, Dennis BL. Hyperbilirubinemia in the term newborn. Am Fam Physician. 2002 Feb 15;65(4):599-606. https://www.aafp.org/pubs/afp/issues/2002/0215/p599.html[↩]
- Reddy DK, Pandey S. Kernicterus. [Updated 2023 Jun 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559120[↩][↩][↩]
- Ali R, Ahmed S, Qadir M, Ahmad K. Icterus Neonatorum in Near-Term and Term Infants: An overview. Sultan Qaboos Univ Med J. 2012 May;12(2):153-60. doi: 10.12816/0003107[↩]
- Ding Y, Wang S, Guo R, Zhang A, Zhu Y. High levels of unbound bilirubin are associated with acute bilirubin encephalopathy in post-exchange transfusion neonates. Ital J Pediatr. 2021 Sep 15;47(1):187. doi: 10.1186/s13052-021-01143-z. Erratum in: Ital J Pediatr. 2023 Feb 15;49(1):22. doi: 10.1186/s13052-023-01432-9[↩]
- Kernicterus. https://emedicine.medscape.com/article/975276-overview[↩]
- Alkén J, Håkansson S, Ekéus C, Gustafson P, Norman M. Rates of Extreme Neonatal Hyperbilirubinemia and Kernicterus in Children and Adherence to National Guidelines for Screening, Diagnosis, and Treatment in Sweden. JAMA Netw Open. 2019 Mar 1;2(3):e190858. doi: 10.1001/jamanetworkopen.2019.0858[↩][↩][↩]
- Leung AK, Sauve RS. Breastfeeding and breast milk jaundice. J R Soc Health. 1989 Dec;109(6):213-7. doi: 10.1177/146642408910900615[↩]
- Gamaleldin R, Iskander I, Seoud I, Aboraya H, Aravkin A, Sampson PD, Wennberg RP. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011 Oct;128(4):e925-31. doi: 10.1542/peds.2011-0206[↩][↩][↩]
- Raines DA, Krawiec C, Weisbrod LJ, et al. Cephalohematoma. [Updated 2024 Jun 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470192[↩]
- Cichoz-Lach H, Celiński K, Słomka M. Congenital nonhemolytic hyperbilirubinemias. Ann Univ Mariae Curie Sklodowska Med. 2004;59(1):449-52.[↩]
- Bashir BA, Othman SA. Neonatal polycythaemia. Sudan J Paediatr. 2019;19(2):81-83. doi: 10.24911/SJP.106-1566075225[↩]
- Polycythemia – newborn. https://www.mountsinai.org/health-library/diseases-conditions/polycythemia-newborn[↩]
- Wiswell TE, Cornish JD, Northam RS. Neonatal polycythemia: frequency of clinical manifestations and other associated findings. Pediatrics. 1986 Jul;78(1):26-30.[↩]
- Watchko JF, Spitzer AR, Clark RH. Prevalence of Hypoalbuminemia and Elevated Bilirubin/Albumin Ratios in a Large Cohort of Infants in the Neonatal Intensive Care Unit. J Pediatr. 2017 Sep;188:280-286.e4. doi: 10.1016/j.jpeds.2017.06.004[↩]
- Donneborg ML, Hansen BM, Vandborg PK, Rodrigo-Domingo M, Ebbesen F. Extreme neonatal hyperbilirubinemia and kernicterus spectrum disorder in Denmark during the years 2000-2015. J Perinatol. 2020 Feb;40(2):194-202. doi: 10.1038/s41372-019-0566-8[↩][↩]
- Wu YW, Kuzniewicz MW, Wickremasinghe AC, Walsh EM, Wi S, McCulloch CE, Newman TB. Risk for cerebral palsy in infants with total serum bilirubin levels at or above the exchange transfusion threshold: a population-based study. JAMA Pediatr. 2015 Mar;169(3):239-46. doi: 10.1001/jamapediatrics.2014.3036[↩]
- US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Services Task Force recommendation statement. Pediatrics. 2009 Oct;124(4):1172-7. doi: 10.1542/peds.2009-0128[↩]
- Johnson L, Bhutani VK, Karp K, Sivieri EM, Shapiro SM. Clinical report from the pilot USA Kernicterus Registry (1992 to 2004). J Perinatol. 2009 Feb;29 Suppl 1:S25-45. doi: 10.1038/jp.2008.211[↩]
- Bhutani VK, Johnson LH, Keren R. Treating acute bilirubin encephalopathy—before it’s too late. Contemp Pediatr. 2005;22(5):57-74.[↩]
- Par EJ, Hughes CA, DeRico P. Neonatal Hyperbilirubinemia: Evaluation and Treatment. Am Fam Physician. 2023 May;107(5):525-534. https://www.aafp.org/pubs/afp/issues/2023/0500/neonatal-hyperbilirubinemia.html[↩]
- Sgro M, Campbell D, Shah V. Incidence and causes of severe neonatal hyperbilirubinemia in Canada. CMAJ. 2006 Sep 12;175(6):587-90. doi: 10.1503/cmaj.060328[↩][↩]
- Usman F, Diala UM, Shapiro SM, et al. Acute bilirubin encephalopathy and its progression to kernicterus: current perspectives. Res Rep Neonatol. 2018;8:33-44. https://www.researchgate.net/publication/323611016_Acute_bilirubin_encephalopathy_and_its_progression_to_kernicterus_current_perspectives[↩]
- Kemper AR, Newman TB, Slaughter JL, et al. Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics. 2022 Sep 1;150(3):e2022058859. doi: 10.1542/peds.2022-058859[↩]
- Sgro M, Campbell DM, Kandasamy S, Shah V. Incidence of chronic bilirubin encephalopathy in Canada, 2007-2008. Pediatrics. 2012 Oct;130(4):e886-90. doi: 10.1542/peds.2012-0253[↩]
- Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010 Jun;15(3):157-63. doi: 10.1016/j.siny.2009.12.004[↩]
- Le Pichon JB, Riordan SM, Watchko J, Shapiro SM. The Neurological Sequelae of Neonatal Hyperbilirubinemia: Definitions, Diagnosis and Treatment of the Kernicterus Spectrum Disorders (KSDs). Curr Pediatr Rev. 2017;13(3):199-209. doi: 10.2174/1573396313666170815100214[↩]
- Amin SB, Karp JM, Benzley LP. Unconjugated hyperbilirubinemia and early childhood caries in a diverse group of neonates. Am J Perinatol. 2010 May;27(5):393-7. doi: 10.1055/s-0029-1243314[↩]
- Ansong-Assoku B, Adnan M, Daley SF, et al. Neonatal Jaundice. [Updated 2024 Feb 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532930[↩][↩][↩][↩]
- Amin SB, Saluja S, Saili A, Orlando M, Wang H, Laroia N, Agarwal A. Chronic Auditory Toxicity in Late Preterm and Term Infants With Significant Hyperbilirubinemia. Pediatrics. 2017 Oct;140(4):e20164009. doi: 10.1542/peds.2016-4009[↩][↩]
- Good WV, Hou C. Visuocortical bilirubin-induced neurological dysfunction. Semin Fetal Neonatal Med. 2015 Feb;20(1):37-41. doi: 10.1016/j.siny.2014.12.007[↩]
- Olds C, Oghalai JS. Audiologic impairment associated with bilirubin-induced neurologic damage. Semin Fetal Neonatal Med. 2015 Feb;20(1):42-46. doi: 10.1016/j.siny.2014.12.006[↩]
- Rose J, Vassar R. Movement disorders due to bilirubin toxicity. Semin Fetal Neonatal Med. 2015 Feb;20(1):20-25. doi: 10.1016/j.siny.2014.11.002[↩]
- Wusthoff CJ, Loe IM. Impact of bilirubin-induced neurologic dysfunction on neurodevelopmental outcomes. Semin Fetal Neonatal Med. 2015 Feb;20(1):52-57. doi: 10.1016/j.siny.2014.12.003[↩]
- Beal JA. American Academy of Pediatrics’ Updated Clinical Guidelines for Managing Neonatal Hyperbilirubinemia. MCN Am J Matern Child Nurs. 2023 Jan-Feb 01;48(1):49. doi: 10.1097/NMC.0000000000000874[↩][↩][↩]
- Jaundice in newborn babies under 28 days. London: National Institute for Health and Care Excellence (NICE); 2023 Oct 31. (NICE Guideline, No. 98.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK553311[↩]
- https://bilitool.org/[↩]
- Gottimukkala SB, Lobo L, Gautham KS, Bolisetty S, Fiander M, Schindler T. Intermittent phototherapy versus continuous phototherapy for neonatal jaundice. Cochrane Database Syst Rev. 2023 Mar 2;3(3):CD008168. doi: 10.1002/14651858.CD008168.pub2[↩]
- Horn D, Ehret D, Gautham KS, Soll R. Sunlight for the prevention and treatment of hyperbilirubinemia in term and late preterm neonates. Cochrane Database Syst Rev. 2021 Jul 6;7(7):CD013277. doi: 10.1002/14651858.CD013277.pub2[↩]
- Bhutani VK; Committee on Fetus and Newborn; American Academy of Pediatrics. Phototherapy to prevent severe neonatal hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2011 Oct;128(4):e1046-52. doi: 10.1542/peds.2011-1494[↩]
- Yetman RJ, Parks DK, Huseby V, Mistry K, Garcia J. Rebound bilirubin levels in infants receiving phototherapy. J Pediatr. 1998 Nov;133(5):705-7. doi: 10.1016/s0022-3476(98)70117-9[↩]
- Newman TB, Wu YW, Kuzniewicz MW, Grimes BA, McCulloch CE. Childhood Seizures After Phototherapy. Pediatrics. 2018 Oct;142(4):e20180648. doi: 10.1542/peds.2018-0648[↩]
- Newman TB, Wickremasinghe AC, Walsh EM, Grimes BA, McCulloch CE, Kuzniewicz MW. Retrospective Cohort Study of Phototherapy and Childhood Cancer in Northern California. Pediatrics. 2016 Jun;137(6):e20151354. doi: 10.1542/peds.2015-1354[↩]
- Auger N, Laverdière C, Ayoub A, Lo E, Luu TM. Neonatal phototherapy and future risk of childhood cancer. Int J Cancer. 2019 Oct 15;145(8):2061-2069. doi: 10.1002/ijc.32158[↩]
- Wang J, Guo G, Li A, Cai WQ, Wang X. Challenges of phototherapy for neonatal hyperbilirubinemia (Review). Exp Ther Med. 2021 Mar;21(3):231. doi: 10.3892/etm.2021.9662[↩]
- Itoh S, Okada H, Kuboi T, Kusaka T. Phototherapy for neonatal hyperbilirubinemia. Pediatr Int. 2017 Sep;59(9):959-966. doi: 10.1111/ped.13332[↩]
- Kar S, Mohankar A, Krishnan A. Bronze baby syndrome. Indian Pediatr. 2013 Jun 8;50(6):624. doi: 10.1007/s13312-013-0161-6[↩]
- Pearson CH. Replacement transfusion as a treatment of erythroblastosis fetalis, by Louis K. Diamond, MD, Pediatrics, 1948;2:520-524. Pediatrics. 1998 Jul;102(1 Pt 2):203-5.[↩]
- Hansen TWR, Maisels MJ, Ebbesen F, Vreman HJ, Stevenson DK, Wong RJ, Bhutani VK. Sixty years of phototherapy for neonatal jaundice – from serendipitous observation to standardized treatment and rescue for millions. J Perinatol. 2020 Feb;40(2):180-193. doi: 10.1038/s41372-019-0439-1[↩]
- DIAMOND LK, ALLEN FH Jr, THOMAS WO Jr. Erythroblastosis fetalis. VII. Treatment with exchange transfusion. N Engl J Med. 1951 Jan 11;244(2):39-49. doi: 10.1056/NEJM195101112440201[↩]
- Jackson JC. Adverse events associated with exchange transfusion in healthy and ill newborns. Pediatrics. 1997 May;99(5):E7. doi: 10.1542/peds.99.5.e7[↩]
- Patra K, Storfer-Isser A, Siner B, Moore J, Hack M. Adverse events associated with neonatal exchange transfusion in the 1990s. J Pediatr. 2004 May;144(5):626-31. doi: 10.1016/j.jpeds.2004.01.054[↩]
- Alpay F, Sarici SU, Okutan V, Erdem G, Ozcan O, Gökçay E. High-dose intravenous immunoglobulin therapy in neonatal immune haemolytic jaundice. Acta Paediatr. 1999 Feb;88(2):216-9. doi: 10.1080/08035259950170420[↩]
- Gottstein R, Cooke RW. Systematic review of intravenous immunoglobulin in haemolytic disease of the newborn. Arch Dis Child Fetal Neonatal Ed. 2003 Jan;88(1):F6-10. doi: 10.1136/fn.88.1.f6[↩]