Contents
- What are bloodborne pathogens
- What to do if you are exposed?
- Bloodborne pathogens test
- HIV Tests for Screening and Diagnosis
- Hepatitis B tests for screening and diagnosis
- Hepatitis C tests for screening and diagnosis
- How soon after exposure to hepatitis C can HCV RNA be detected by PCR?
- Under what circumstances is a false-positive anti-HCV test result likely?
- Under what circumstances might a false-negative anti-HCV test result occur?
- Can a patient have a normal liver enzyme (e.g., ALT) level and still have chronic hepatitis C?
- How soon after exposure to hepatitis C can anti-HCV be detected?
- What is the risk for hepatitis C infection from a needlestick exposure to HCV-contaminated blood?
- Other than needlesticks, do other exposures, such as splashes to the eye, pose a risk to health care personnel for hepatitis C transmission?
- Should hepatitis C-infected health care personnel be restricted in their work?
- What is the recommended management of a health care worker with occupational exposure to hepatitis C?
- What is the treatment for acute hepatitis C?
- OSHA bloodborne pathogens standard
What are bloodborne pathogens
Bloodborne pathogens are infectious microorganisms in human blood that can cause disease in humans 1. These pathogens include, but are not limited to, hepatitis B (HBV), hepatitis C (HCV) and human immunodeficiency virus (HIV). Needlesticks and other sharps-related injuries may expose workers to bloodborne pathogens. Workers in many occupations, including first responders, housekeeping personnel in some industries, nurses and other healthcare personnel, all may be at risk for exposure to bloodborne pathogens.
The most common and dangerous pathogens spread through blood in the hospital are:
- Hepatitis B virus (HBV) and hepatitis C virus (HCV). These viruses cause infections and liver damage.
- HIV (human immunodeficiency virus). This virus causes HIV/AIDS.
You can be infected with human immunodeficiency virus (HIV), hepatitis B virus (HBV), or hepatitis C virus (HCV) if you are stuck with a needle or other sharp object that has touched the blood or bodily fluids of a person who has one of these infections.
These infections can also spread if infected blood or bloody bodily fluids touch mucous membranes or an open sore or cut. Mucous membranes are the moist parts of your body, such as in your eyes, nose, and mouth.
Exposures to blood and other body fluids occur across a wide variety of occupations. Health care workers, emergency response and public safety personnel, and other workers can be exposed to blood through needlestick and other sharps injuries, mucous membrane, and skin exposures.
HIV can also spread from one person to another through fluid in your joints or spinal fluid. And it can spread through semen, fluids in the vagina, breast milk, and amniotic fluid (the fluid that surrounds a baby in the womb).
What to do if you are exposed?
If you are stuck with a needle, get blood in your eye, or are exposed to any bloodborne pathogen:
- Wash the area. Use soap and water on your skin. If your eye is exposed, irrigate with clean water, saline, or a sterile irrigant.
- Tell your supervisor right away that you were exposed.
- Get medical help right away.
You may or may not need lab tests, a vaccine, or medicines.
HEPATITIS
- Symptoms of hepatitis B and hepatitis C may be mild, and not start until 2 weeks to 6 months after contact with the virus. Sometimes, there are no symptoms.
- Hepatitis B often gets better on its own and sometimes does not need to be treated. Some people develop a long-term infection that leads to liver damage.
- Most people who become infected with hepatitis C develop a long-term infection. After many years, they often have liver damage.
HIV
After someone is infected with HIV, the virus stays in the body. It slowly harms or destroys the immune system. Your body’s immune system fights disease and helps you heal. When it is weakened by HIV, you are more likely to get sick from other infections, including ones that would not normally make you sick.
Treatment can help people with all of these infections.
Hepatitis B can be prevented by a vaccine. There is no vaccine to prevent hepatitis C or HIV.
Bloodborne pathogens test
HIV Tests for Screening and Diagnosis
HIV tests are very accurate, but no test can detect the virus immediately after infection. How soon a test can detect infection depends upon different factors, including the type of test being used. There are three types of HIV diagnostic tests: nucleic acid tests (NAT), antigen/antibody tests, and antibody tests.
- Nucleic acid tests (NATs) look for the actual HIV virus in the blood. NAT test can give either a positive/negative result or an actual amount of virus present in the blood (known as a viral load test). This test is very expensive and not routinely used for screening individuals unless they recently had a high-risk exposure or a possible exposure with early symptoms of HIV infection.
- Antigen/antibody tests look for both HIV antibodies and antigens. Antigens are foreign substances that cause your immune system to activate. If you’re infected with HIV, an antigen called p24 is produced even before antibodies develop. Tests that detect both antigen and antibodies are recommended for testing done in labs and are now common in the United States. There is also a rapid antigen/antibody test available.
- Antibody tests detect the presence of antibodies, proteins that a person’s body makes against HIV, not HIV itself. Most rapid tests and home tests are antibody tests.
An initial HIV test usually will either be an antigen/antibody test or an antibody test. If the initial HIV test is a rapid test and it is positive, the individual will be sent to a health care provider to get follow-up testing. If the initial HIV test is a laboratory test and it is positive, the laboratory will usually conduct follow-up testing on the same blood sample as the initial test. Although HIV tests are generally very accurate, follow-up testing allows the health care provider to be sure the diagnosis is right.
How soon can clinicians rule out infection?
The Centers for Disease Control and Prevention (CDC) recently published research findings 2 that estimate the window period for 20 U.S. Food and Drug Administration (FDA)-approved HIV tests. The study showed that laboratory testing using antigen/antibody tests detects HIV infection sooner than other available tests that detect only antibodies. If a person gets a laboratory-based antigen/antibody test on blood plasma less than 45 days after a possible HIV exposure and the result is negative, follow-up testing can begin 45 days after the possible HIV exposure. For all other tests, CDC recommends testing again at least 90 days after exposure to be sure that a negative test result is accurate.
What is the window period?
The window period varies from person to person, and is also different depending upon the type of HIV test. Most HIV tests are antibody tests. It takes time for the body to produce enough antibodies for an HIV test to show that a person has HIV. The soonest an antibody test will detect infection is 3 weeks. Most, but not all people will develop detectable antibodies within 3 to 12 weeks of infection.
Most, but not all people will make enough antigens and antibodies for fourth generation or combination tests to accurately detect infection 13 to 42 days after infection.
Most, but not all people will have enough HIV in their blood for a NAT test to detect infection 7 to 28 days after infection. This is during the time when someone has acute HIV infection.
If you think you’ve recently been exposed to HIV during sex (e.g., if the condom breaks or comes off) or through sharing needles and works to prepare drugs (e.g., cotton, cookers, water), talk to your health care provider or an emergency room doctor about taking post-exposure prophylaxis (PEP) right away. Post-exposure prophylaxis (PEP) must begin as soon as possible, and always within 72 hours (3 days) of a recent possible exposure.
What is post-exposure prophylaxis (PEP)?
PEP (post-exposure prophylaxis) means taking antiretroviral medicines (ART) after being potentially exposed to HIV to prevent becoming infected.
Post-exposure prophylaxis (PEP) should be used only in emergency situations and must be started within 72 hours after a recent possible exposure to HIV. The sooner you start PEP, the better; every hour counts. If you think you’ve recently been exposed to HIV during sex or through sharing needles and works to prepare drugs or if you’ve been sexually assaulted, talk to your health care provider or an emergency room doctor about PEP right away.
Post-exposure prophylaxis (PEP) is not a substitute for regular use of other proven HIV prevention methods, such as pre-exposure prophylaxis (PrEP), which means taking HIV medicines daily to lower your chance of getting infected; using condoms the right way every time you have sex; and using only your own new, sterile needles and works every time you inject.
Post-exposure prophylaxis (PEP) is effective, but not 100%, so you should continue to use condoms with sex partners and safe injection practices while taking PEP. These strategies can protect you from being exposed to HIV again and reduce the chances of transmitting HIV to others if you do become infected while you’re on PEP.
All persons offered post-exposure prophylaxis (PEP) should be prescribed a 28-day course of a 3-drug antiretroviral regimen 3.
The preferred regimen for otherwise healthy adults and adolescents:
- tenofovir disoproxil fumarate (tenofovir DF or TDF) (300 mg) with emtricitabine (200 mg) once daily plus raltegravir (RAL) 400 mg twice daily or dolutegravir (DTG) 50 mg daily.
Alternative regimen for otherwise healthy adults and adolescents is:
- tenofovir disoproxil fumarate (300 mg) with emtricitabine (FTC) (200 mg) once daily plus darunavir (DRV) (800 mg) and ritonavira (RTV) (100 mg) once daily.
If you’re a health care worker and think you’ve had a possible HIV exposure at work, see the following question.
Post-exposure prophylaxis (PEP) should be considered if you’ve had a recent possible exposure to HIV at work. Report your exposure to your supervisor, and seek medical attention immediately.
Occupational transmission of HIV to health care workers is extremely rare, and the proper use of safety devices and barriers can help minimize the risk of exposure while caring for patients with HIV.
A health care worker who has a possible exposure should see a doctor or visit an emergency room immediately. PEP must be started within 72 hours after a recent possible exposure to HIV. The sooner, the better; every hour counts.
CDC issued updated guidelines in 2013 (https://www.cambridge.org/core/journals/infection-control-and-hospital-epidemiology/article/updated-us-public-health-service-guidelines-for-the-management-of-occupational-exposures-to-human-immunodeficiency-virus-and-recommendations-for-postexposure-prophylaxis/FACD234C788E0D541E37BC3DEFB4C248) for the management of health care worker exposures to HIV and recommendations for PEP.
The following is a summary of recommendations:
- PEP is recommended when occupational exposures to HIV occur;
- The HIV status of the exposure source patient should be determined, if possible, to guide need for HIV PEP;
- PEP medication regimens should be started as soon as possible after occupational exposure to HIV, and they should be continued for a 4-week duration;
- New recommendation—PEP medication regimens should contain 3 (or more) antiretroviral drugs (see above) for all occupational exposures to HIV;
- Expert consultation is recommended for any occupational exposures to HIV;
- Close follow-up for exposed personnel should be provided that includes counseling, baseline and follow-up HIV testing, and monitoring for drug toxicity; follow-up appointments should begin within 72 hours of an HIV exposure; and
- New recommendation—if a newer fourth-generation combination HIV p24 antigen-HIV antibody test is utilized for follow-up HIV testing of exposed healthcare worker, HIV testing may be concluded 4 months after exposure; if a newer testing platform is not available, follow-up HIV testing is typically concluded 6 months after an HIV exposure.
Clinicians caring for health care workers who’ve had a possible exposure can call the PEPline (1-888-448-4911), which offers around-the-clock advice on managing occupational exposures to HIV, as well as hepatitis B and C. Exposed health care workers may also call the PEPline, but they should seek local medical attention first.
When should post-exposure prophylaxis (PEP) be taken?
Post-exposure prophylaxis (PEP) must be started within 72 hours after a possible exposure. The sooner you start PEP, the better; every hour counts.
Starting PEP as soon as possible after a potential HIV exposure is important. Research has shown that PEP has little or no effect in preventing HIV infection if it is started later than 72 hours after HIV exposure.
If you’re prescribed PEP, you’ll need to take it once or twice daily for 28 days.
Where can I get PEP?
Your health care provider or an emergency room doctor can prescribe PEP. Talk to them right away if you think you’ve recently been exposed to HIV.
When should clinicians start treatment for HIV?
Data from a National Institutes of Health sponsored trial, the Strategic Timing of AntiRetroviral Treatment (START) 4 indicates there is a clear personal advantage to diagnosis soon after HIV infection and starting combination antiretroviral treatment (ART) early in the course of infection. The study further highlights the importance of routine HIV testing and the potential impact of early treatment on better health outcomes.
Hepatitis B tests for screening and diagnosis
Screening should include testing to three HBV screening seromarkers (HBsAg, antibody to HBsAg [anti-HBs], and antibody to hepatitis B core antigen [anti-HBc]) so that persons can be classified into the appropriate hepatitis B category and properly recommended to receive vaccination, counseling, and linkage to care and treatment.
What do the different hepatitis B serologic markers mean?
Hepatitis B surface antigen (HBsAg): The presence of HBsAg, a protein on the surface of HBV, indicates that the person is infectious. It can be detected in high levels in serum during acute or chronic HBV infection. The presence of HBsAg indicates that the person is infectious. The body normally produces antibodies to HBsAg as part of the normal immune response to infection. HBsAg is the antigen used to make hepatitis B vaccine.
Hepatitis B surface antibody (anti-HBs): The presence of anti-HBs is generally interpreted as indicating recovery and immunity from HBV infection. Anti-HBs also develops in a person who has been successfully vaccinated against hepatitis B.
Total hepatitis B core antibody (anti-HBc): Appears at the onset of symptoms in acute hepatitis B and persists for life. The presence of anti-HBc indicates previous or ongoing infection with HBV in an undefined time frame.
IgM antibody to hepatitis B core antigen (IgM anti-HBc): Positivity indicates recent infection with hepatitis B virus (≤6 months). Its presence indicates acute infection.
Hepatitis B e antigen (HBeAg): The presence indicates that the virus is replicating and the infected person has high levels of HBV. HBeAg is a secreted product of the nucleocapsid gene of HBV that is found in serum during acute and chronic hepatitis B.
Hepatitis B e antibody (HBeAb or anti-HBe): Spontaneous conversion from e antigen to e antibody (a change known as seroconversion) is a predictor of long-term clearance of HBV in patients undergoing antiviral therapy and indicates lower levels of HBV. HBeAb is produced by the immune system temporarily during acute HBV infection or consistently during or after a burst in viral replication.
HBV DNA: HBV DNA concentration correlates with levels of HBV virus particles. HBV DNA is measured as IU/mL or copies/ml by the polymerase chain reaction assay. HBV viral DNA can be detected and quantified in serum. There are several commercial assays that can detect and quantify HBV DNA, some to limits as low as 10 IU/ml.
How long does it take for blood to test HBsAg-positive after exposure to hepatitis B?
HBsAg will be detected in an infected person’s blood an average of 4 weeks (range: 1–9 weeks) after exposure to the virus. About 1 of 2 patients will no longer be infectious by 7 weeks after onset of symptoms, and all patients who do not remain chronically infected will be HBsAg-negative by 15 weeks after onset of symptoms 5.
How do I interpret hepatitis B serologic test results?
The following table provides interpretations for different combinations and results of hepatitis B serologic markers.
Table 1. Antibody and Antigen Biomarkers for Hepatitis B Infection
| Clinical state | HBsAg | Total Anti-HBs | Total anti-HBc | Action |
|---|---|---|---|---|
| Chronic infection | Positive | Negative | Positive | Link to hepatitis B-directed care |
| Acute | Positive | Negative | Positive (IgM anti-HBc) | Link to hepatitis B-directed care |
| Resolved infection | Negative | Positive | Positive | Counseling, reassurance |
| Immune (immunization) | Negative | Positive | Negative | Reassurance |
| Susceptible (never infected and no evidence of immunization) | Negative | Negative | Negative | Vaccinate |
| *Isolated core antibody | Negative | Negative | Positive | Depends on situation |
Footnote: *can be a result of:
- 1 False positive: Repeat testing required
- 2 Past infection: No action needed
- 3 Occult HBV infection: Needs to be known if patient ever becomes immunosuppressed or given chemotherapy or treated with antiviral therapy for hepatitis C virus infection. Consider monitoring HBV DNA.
- 4 Passive transfer to infant born to HBsAg-positive mother No specific action needed.
Hepatitis C tests for screening and diagnosis
Several blood tests are performed to test for hepatitis C infection, including:
- Screening tests for antibody to HCV (anti-HCV)
- enzyme immunoassay (EIA)
- enhanced chemiluminescence immunoassay (CIA)
- Qualitative tests to detect presence or absence of virus (HCV RNA polymerase chain reaction [PCR])
- Quantitative tests to detect amount (titer) of virus (HCV RNA PCR)
Table 2. Interpretation of Blood Tests for Diagnosis of Acute Hepatitis C Infection
| TEST | INTERPRETATION FOR DIAGNOSIS OF ACUTE Hepatitis C Infection |
|---|---|
| HCV Antibody |
|
| HCV RNA |
|
| ALT |
|
How soon after exposure to hepatitis C can HCV RNA be detected by PCR?
HCV RNA appears in blood and can be detected as early as 2–3 weeks after infection 7.
Under what circumstances is a false-positive anti-HCV test result likely?
False-positive anti-HCV tests appear more often when people at low risk for HCV infection (e.g., blood donors) are tested. Therefore, it is important to follow-up all positive anti-HCV tests with an RNA test to establish current infection.
Under what circumstances might a false-negative anti-HCV test result occur?
People with early HCV infection might not yet have developed antibody levels high enough that the test can measure. In addition, some people might lack the (immune) response necessary for the test to work well. In these people, further testing such as PCR for HCV RNA may be considered.
Can a patient have a normal liver enzyme (e.g., ALT) level and still have chronic hepatitis C?
Yes. It is common for patients with chronic hepatitis C to have liver enzyme levels that go up and down, with periodic returns to normal or near normal levels. Liver enzyme levels can remain normal for over a year despite chronic liver disease 8.
How soon after exposure to hepatitis C can anti-HCV be detected?
HCV infection can be detected by anti-HCV screening tests (enzyme immunoassay) 4–10 weeks after infection. Anti-HCV can be detected in >97% of people by 6 months after exposure 7.
What is the risk for hepatitis C infection from a needlestick exposure to HCV-contaminated blood?
After a needlestick or sharps exposure to HCV-positive blood, the risk of HCV infection is 0.1% 9.
Other than needlesticks, do other exposures, such as splashes to the eye, pose a risk to health care personnel for hepatitis C transmission?
Although a few cases of hepatitis C transmission via blood splash to the eye have been reported, the risk for such transmission is expected to be very low. Avoiding occupational exposure to blood is the primary way to prevent transmission of bloodborne illnesses among health care personnel. All health care personnel should adhere to Standard Precautions. Depending on the medical procedure involved, Standard Precautions may include the appropriate use of personal protective equipment (e.g., gloves, masks, and protective eyewear).
Should hepatitis C-infected health care personnel be restricted in their work?
There are no CDC recommendations to restrict a health care worker who is infected with hepatitis C. The risk of transmission from an infected health care worker to a patient appears to be very low. All health care personnel, including those who are hepatitis C positive, should follow a strict aseptic technique and Standard Precautions, including appropriate hand hygiene, use of protective barriers, and safe injection practices.
What is the recommended management of a health care worker with occupational exposure to hepatitis C?
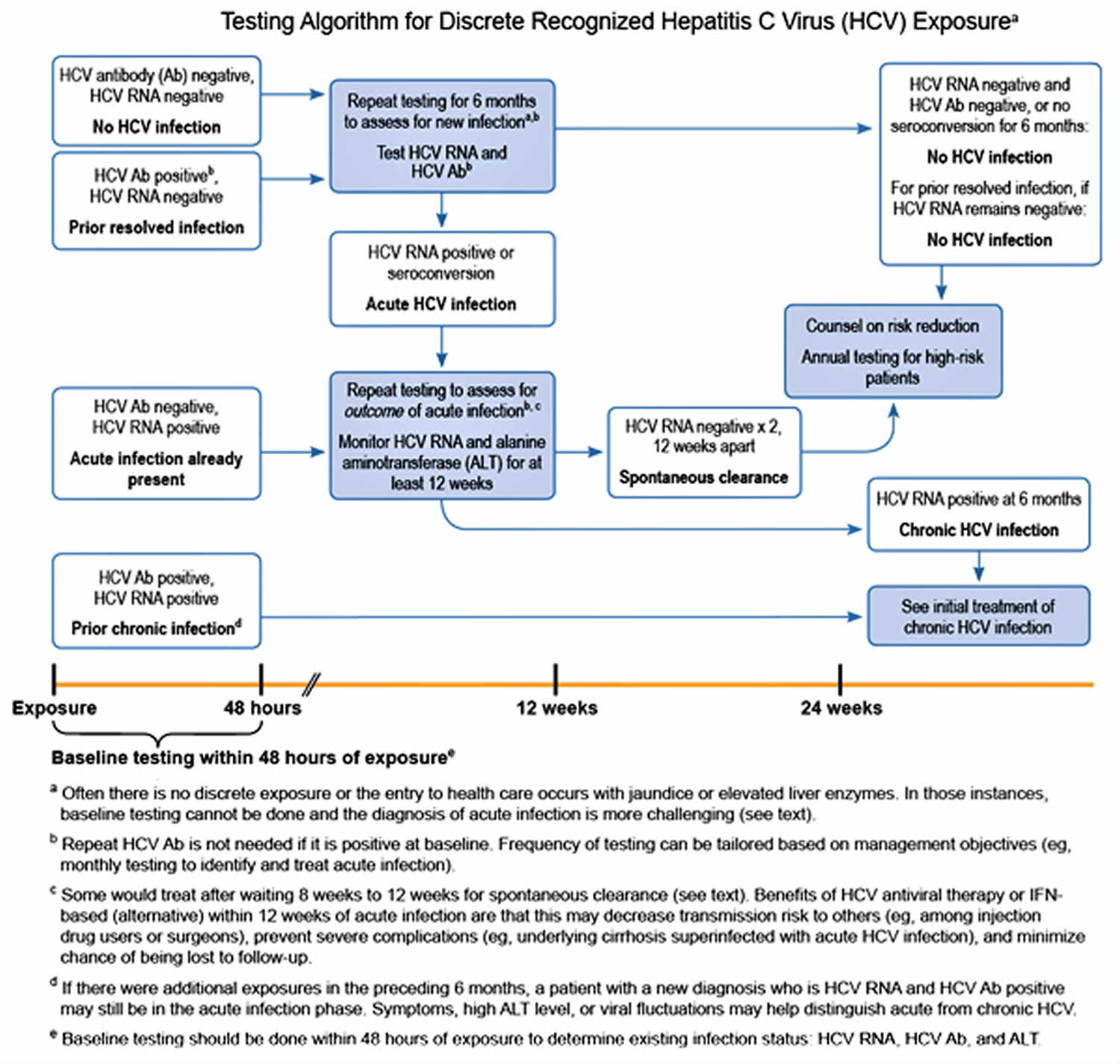
Postexposure prophylaxis (PEP) for hepatitis C is not recommended, as outlined in the 2001 MMWR on management of health-care personnel 10 who have occupational exposure to blood and other body fluids. Test the source for hepatitis C RNA. If the source is hepatitis C RNA positive, or if hepatitis C infection status is unknown, follow this testing algorithm.
After a needlestick or sharps exposure to hepatitis C-positive blood, the risk of HCV infection is 0.1% 9. If the health care worker does become infected, follow American Association for the Study of Liver Diseases or Infectious Diseases Society of America (IDSA) guidelines for management and treatment of hepatitis C.
Figure 1. Hepatitis C testing algorithm
What is the treatment for acute hepatitis C?
New treatment guidelines recommend no treatment of acute hepatitis C. Patients with acute HCV infection should be followed and only considered for treatment if hepatitis C RNA persists after 6 months. For more information see (https://www.hcvguidelines.org/).
OSHA bloodborne pathogens standard
OSHA’s Bloodborne Pathogens Standard requirements state what employers must do to protect workers who are occupationally exposed to blood or other potentially infectious materials, as defined in the standard. All of the requirements of OSHA’s Bloodborne Pathogens Standard can be found in Title 29 of the Code of Federal Regulations at (https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1030). In order to reduce or eliminate the hazards of occupational exposure to bloodborne pathogens, an employer must implement an exposure control plan for the worksite with details on employee protection measures. The plan must also describe how an employer will use engineering and work practice controls, personal protective clothing and equipment, employee training, medical surveillance, hepatitis B vaccinations, and other provisions as required by OSHA’s Bloodborne Pathogens Standard (https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1030). Engineering controls are the primary means of eliminating or minimizing employee exposure and include the use of safer medical devices, such as needleless devices, shielded needle devices, and plastic capillary tubes.
In general, the OSHA’s Bloodborne Pathogens Standard requires employers to:
- Establish an exposure control plan. This is a written plan to eliminate or minimize occupational exposures. The employer must prepare an exposure determination that contains a list of job classifications in which all workers have occupational exposure and a list of job classifications in which some workers have occupational exposure, along with a list of the tasks and procedures performed by those workers that result in their exposure.
- Employers must update the plan annually to reflect changes in tasks, procedures, and positions that affect occupational exposure, and also technological changes that eliminate or reduce occupational exposure. In addition, employers must annually document in the plan that they have considered and begun using appropriate, commercially-available effective safer medical devices designed to eliminate or minimize occupational exposure. Employers must also document that they have solicited input from frontline workers in identifying, evaluating, and selecting effective engineering and work practice controls.
- Implement the use of universal precautions (treating all human blood and other potentially infectious materials as if known to be infectious for bloodborne pathogens).
- Identify and use engineering controls. These are devices that isolate or remove the bloodborne pathogens hazard from the workplace. They include sharps disposal containers, selfsheathing needles, and safer medical devices, such as sharps with engineered sharps-injury protection and needleless systems.
- Identify and ensure the use of work practice controls. These are practices that reduce the possibility of exposure by changing the way a task is performed, such as appropriate practices for handling and disposing of contaminated sharps, handling specimens, handling laundry, and cleaning contaminated surfaces and items.
- Provide personal protective equipment, such as gloves, gowns, eye protection, and masks. Employers must clean, repair, and replace this equipment as needed. Provision, maintenance, repair and replacement are at no cost to the worker.
- Make available hepatitis B vaccinations to all workers with occupational exposure. This vaccination must be offered after the worker has received the required bloodborne pathogens training and within 10 days of initial assignment to a job with occupational exposure.
- Make available post-exposure evaluation and follow-up to any occupationally exposed worker who experiences an exposure incident. An exposure incident is a specific eye, mouth, other mucous membrane, non-intact skin, or parenteral contact with blood or other potentially infectious materials. This evaluation and follow-up must be at no cost to the worker and includes documenting the route(s) of exposure and the circumstances under which the exposure incident occurred; identifying and testing the source individual for HBV and HIV infectivity, if the source individual consents or the law does not require consent; collecting and testing the exposed worker’s blood, if the worker consents; offering postexposure prophylaxis; offering counseling; and evaluating reported illnesses. The healthcare professional will provide a limited written opinion to the employer and all diagnoses must remain confidential.
- Use labels and signs to communicate hazards. Warning labels must be affixed to containers of regulated waste; containers of contaminated reusable sharps; refrigerators and freezers containing blood or other potentially infectious materials; other containers used to store, transport, or ship blood or other potentially infectious materials; contaminated equipment that is being shipped or serviced; and bags or containers of contaminated laundry, except as provided in the standard. Facilities may use red bags or red containers instead of labels. In HIV and HBV research laboratories and production facilities, signs must be posted at all access doors when other potentially infectious materials or infected animals are present in the work area or containment module.
- Provide information and training to workers. Employers must ensure that their workers receive regular training that covers all elements of the standard including, but not limited to: information on bloodborne pathogens and diseases, methods used to control occupational exposure, hepatitis B vaccine, and medical evaluation and post-exposure follow-up procedures. Employers must offer this training on initial assignment, at least annually thereafter, and when new or modified tasks or procedures affect a worker’s occupational exposure. Also, HIV and HBV laboratory and production facility workers must receive specialized initial training, in addition to the training provided to all workers with occupational exposure. Workers must have the opportunity to ask the trainer questions. Also, training must be presented at an educational level and in a language that workers understand.
- Maintain worker medical and training records. The employer also must maintain a sharps injury log, unless it is exempt under Part 1904 –Recording and Reporting Occupational Injuries and Illnesses, in Title 29 of the Code of Federal Regulations.
- Bloodborne Pathogens and Needlestick Prevention. https://www.osha.gov/SLTC/bloodbornepathogens/index.html[↩]
- Kevin P. Delaney, Debra L. Hanson, Silvina Masciotra, Steven F. Ethridge, Laura Wesolowski, Sherry Michele Owen; Time Until Emergence of HIV Test Reactivity Following Infection With HIV-1: Implications for Interpreting Test Results and Retesting After Exposure, Clinical Infectious Diseases, Volume 64, Issue 1, 1 January 2017, Pages 53–59, https://doi.org/10.1093/cid/ciw666[↩]
- https://www.cdc.gov/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf[↩]
- Babiker AG, Emery S, Fätkenheuer G, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials. 2012;10(1 Suppl):S5-S36. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3664112/[↩]
- Krugman S, Overby LR, Mushahwar IK, Ling CM, Frosner GG, Deinhardt F. Viral hepatitis, type B. Studies on natural history and prevention re-examined. N Engl J Med. 1979;300(3):101-6.[↩]
- Abara WE, Qaseem A, Schillie S, McMahon BJ, Harris AM, High Value Care Task Force of the American College of P, et al. Hepatitis B Vaccination, Screening, and Linkage to Care: Best Practice Advice From the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2017;167(11):794-804.[↩]
- Hoofnagle JH. Hepatitis C: the clinical spectrum of disease. Hepatology. 1997;26(3 Suppl 1):15S-20S.[↩][↩]
- Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, Alexander WJ, Hu PY, Miller JK, Gerber MA, Sampliner RE, et al. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med. 1992;327(27):1899-1905.[↩]
- Egro FM, Nwaiwu CA, Smith S, Harper JD, Spiess AM. Seroconversion rates among health care workers exposed to hepatitis C virus-contaminated body fluids: the University of Pittsburgh 13-year experience. Am J Infect Control. 2017;45(9):1001-5.[↩][↩]
- Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV and Recommendations for Postexposure Prophylaxis. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5011a1.htm[↩]