Contents
What is caprylic acid
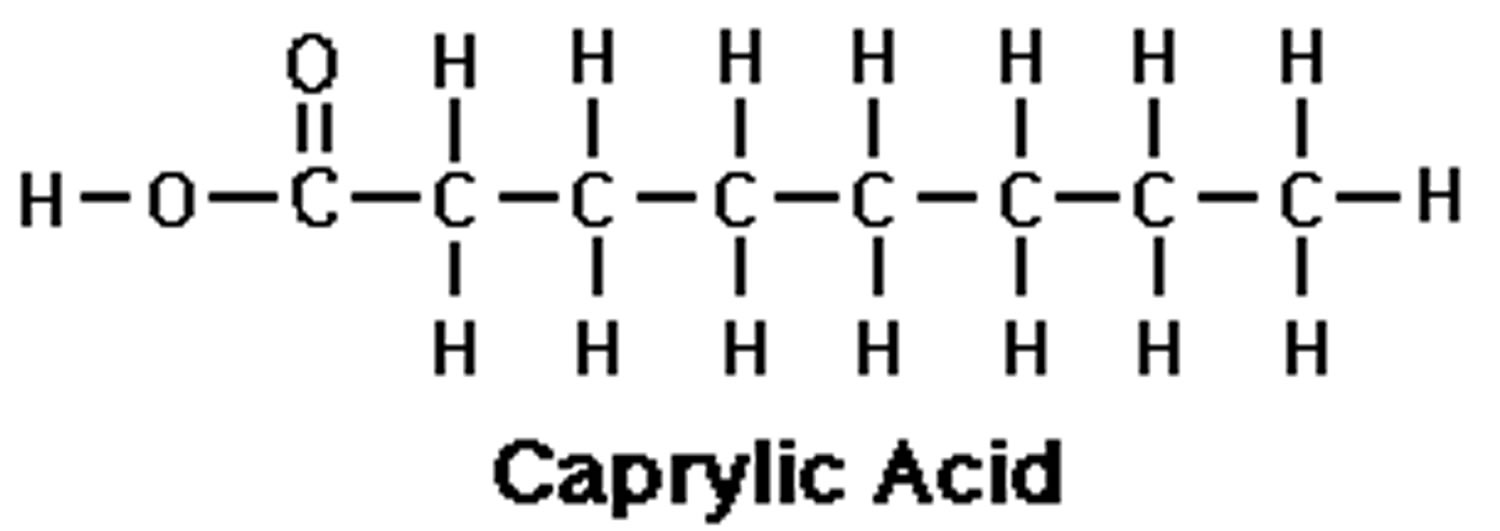
Caprylic acid (C8H16O2) is also referred to as octanoic acid or food additive E 570, which is the common name for the medium-chain saturated fatty acids (MCFAs) with eight-carbon backbone (C8:0). Caprylic acid belongs to a class of medium chain triglyceride (MCT) which also includes caproic acid (C6:0) and capric acid (C10:0). Caprylic acid is found naturally in breast milk, dairy products 1 and is a minor component of coconut oil and palm kernel oil 2. Caprylic acid is an oily liquid with a slightly unpleasant rancid taste that is minimally soluble in water. Caprylic acid is used commercially in the production of esters used in perfumery and also in the manufacture of dyes. Caprylic triglyceride is widely used in many skin products due to its rapid penetration ability 3. Caprylic triglyceride is metabolized into ketone bodies that can serve as an alternate energy substrate for neuronal metabolism 3. Caprylic acid is the main constituent of the medium-chain triglyceride diet advocated for seizure therapy 4 and it has been demonstrated to cross the blood-brain barrier 5, to exert antiepileptic effects 6 and to increase the effectiveness of the anticonvulsant drug, valproic acid 7, in mouse models of seizure. Although further research is needed to better understand the mechanism and magnitude of the clinical impact that caprylic acid has on mouse models of seizure.
According to Anneken et al. 8, the fatty acids – caprylic‐, capric‐, lauric‐, myristic‐, palmitic‐, stearic and oleic acid – are usually produced by hydrolysis of common animal and vegetable fats and oils and later fractionation of the individual fatty acids. Caprylic acid may be produced from coconut oil by saponification and fractional distillation of the coconut oil triglycerides 9.
Caprylic acid has been classified by the US Food and Drug Administration (FDA) as a direct food additive that is Generally Recognized as Safe (GRAS) when this naturally-occurring component of food is added as a flavoring agent or adjuvant to various foods. Dietary exposure is expected to occur from the FDA direct food additive uses as well as the Environmental Protection Agency (EPA) indirect food additive uses on dairy equipment, food processing equipment and utensils, and in eating establishments.
In 1999, the Joint FAO (Food and Agriculture Organization of the United Nations)/WHO (World Health Organization) Expert Committee on Food Additives (JECFA) reviewed caprylic acid, capric acid, lauric acid, myristic acid, palmitic acid and stearic acid as flavoring substances 10 and considered them as ‘no safety concern at current levels of intake when used as a flavoring agent’. The European Food Safety Authority Panel noted that the contribution of fatty acids (E 570) represented on average only 1% of the overall exposure to saturated fatty acids from all dietary sources (food additive and regular diet). In western countries, total dietary medium-chain saturated fatty acids (MCFAs) represent less than 2% of total dietary energy including 1 to 2% of caprylic acid in milk fat 11. The European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies proposed dietary reference values for fats was as follows: “intake of saturated fatty acid (caprylic‐, capric‐, lauric‐, myristic‐, palmitic‐ and stearic acid) as low as possible” within the context of a nutritionally adequate diet. Limiting the intake of saturated fatty acids should be considered when establishing nutrient goals and recommendations 12.
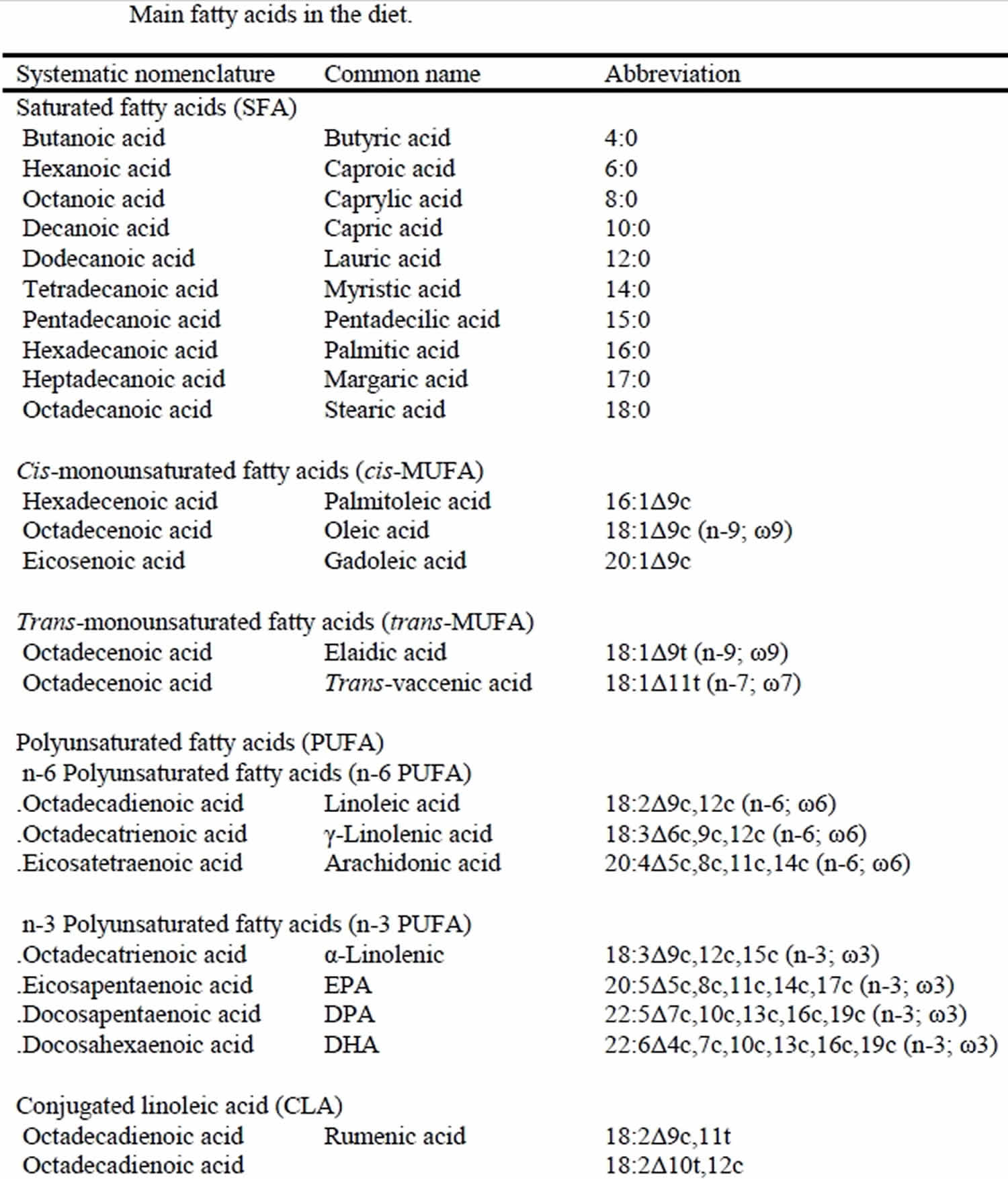
Figure 1. Main fatty acids in the diet
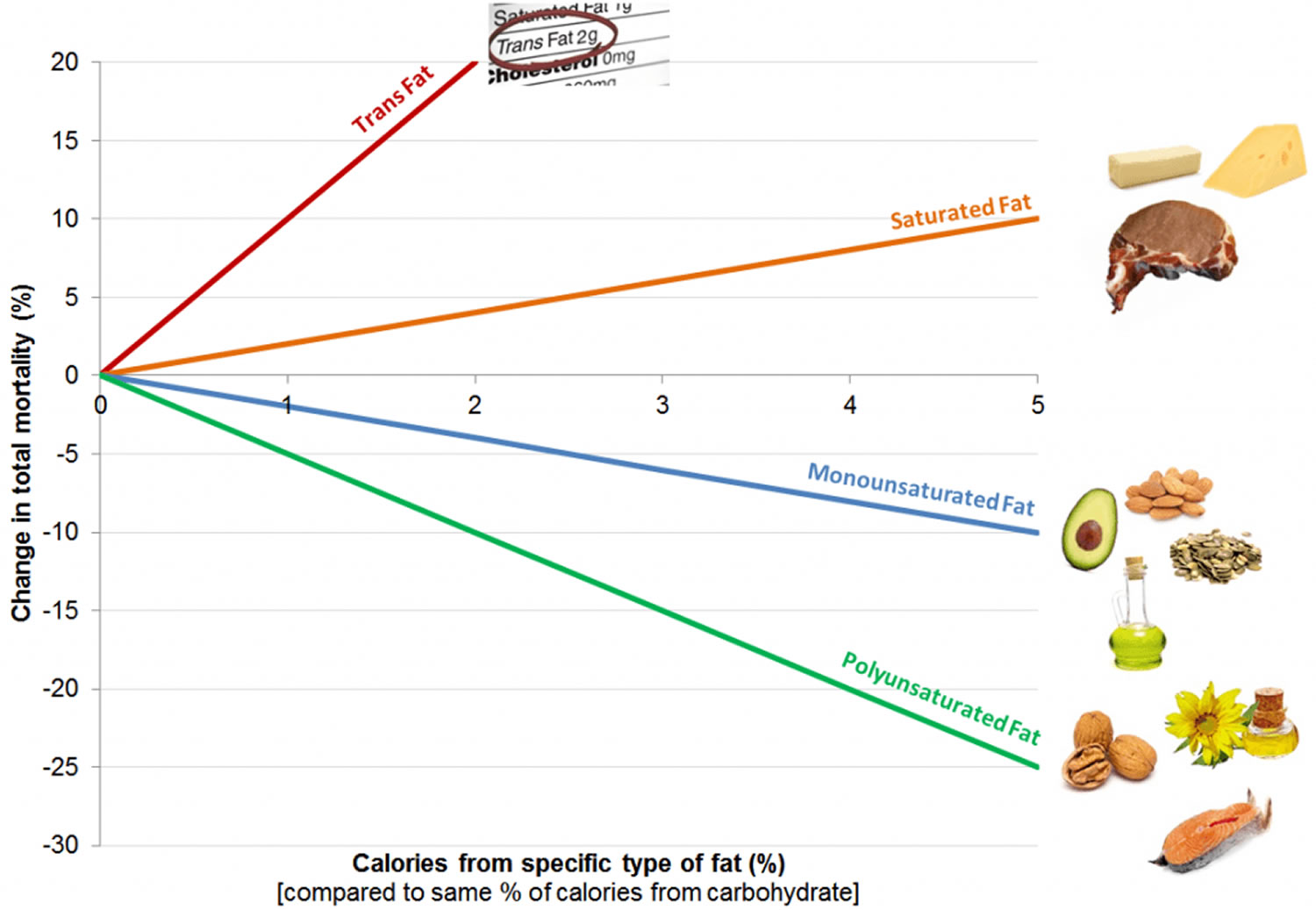
[Source 12]Figure 2. Dietary Fats and Mortality Rates
[Source 13]Caprylic acid benefits
In overweight humans, intakes of equal-caloric diets rich in medium-chain saturated fatty acids (MCFAs) were shown to decrease adiposity and increase energy expenditure compared to similar diets rich in long-chain saturated fatty acids (LCFAs ≥12 carbons) 14. Regarding other physiological parameters, epidemiological studies have also shown that intakes of short to medium-chain saturated fatty acids were not significantly associated with the risk of coronary heart disease (coronary artery disease) 15.
More recently, caprylic acid was also shown to specifically acylate ghrelin 16, the only known peptide hormone with an orexigenic effect (appetite stimulant). Ghrelin is a 28 amino acid peptide expressed mainly in the different sections of the digestive tract and especially the stomach 17. Acylated ghrelin binds to the growth hormone secretagogue receptor 1a (GHSR-1a) located in the pituitary gland and hypothalamus 18 and regulates many relevant biological processes including the secretion of the growth hormone (GH), the stimulation of appetite and food intake, the modulation of gastric acid secretion and motility and the regulation of glucose homeostasis and adiposity 19. Ghrelin seems to have an essential function in blood glucose regulation in case of calorie restriction 20, even if the mechanisms mediating this effect remain poorly understood. During its maturation in the gastric mucosa and before secretion in the blood, part of the proghrelin is subjected to a unique modification consisting in the addition of an activated caprylic acyl-coA to the 3rd serine residue. The ghrelin O-acyltransferase (GOAT or MBOAT4), the gastric enzyme involved in ghrelin octanoylation, belongs to the family of membrane bound O-acyltransferases (MBOAT), a group of proteins involved in acetyltransferase and acyltransferase activity 21. Plasma ghrelin exists therefore in both unacylated and acylated forms, but only the latter can bind its growth hormone secretagogue receptor 1a (GHSR-1a) receptor. Because of the above-mentioned potential specific gastric absorption of medium-chain saturated fatty acids (MCFAs) following the consumption of medium-chain triglycerides (MCTs), dietary caprylic acid is now suspected to directly provide ghrelin O-acyltransferase (GOAT) enzyme with octanoyl-CoA co-substrate necessary for the acyl modification of ghrelin. Indeed, ingestion by mice of either medium-chain saturated fatty acids (MCFAs) or medium-chain triglycerides (MCTs) increased the stomach concentration of acylated ghrelin 22, without changing the total ghrelin amount. Using mice genetic models, Kirchner et al. 23 also suggested that GOAT was required to mediate the impact of dietary MCTs on body adiposity. Recent studies have suggested that ghrelin O-acyltransferase (GOAT) might be a therapeutic target against obesity and hyperphagia by inhibiting the ghrelin O-acyltransferase (GOAT) activity 24, in order to decrease the circulating level of acylated ghrelin (which may also be obtained by limiting the availability of its substrate caprylic acid) 24.
Focusing on the caprylic acid (C8:0), this study 11 aimed at investigating the discrepancy between the formerly described beneficial effects of dietary medium chain fatty acids on body weight loss and the caprylic acid (C8:0) newly reported effect on food intake via ghrelin octanoylation. During 6 weeks, Sprague-Dawley male rats were fed with three dietary caprylic acid (C8:0) levels (0, 8 and 21% of fatty acids) in three experimental conditions (moderate fat, caloric restriction and high fat) 11. A specific dose-response enrichment of the stomach tissue C8:0 was observed as a function of dietary caprylic acid (C8:0), supporting the hypothesis of an early preduodenal hydrolysis of medium chain triglycerides and a direct absorption at the gastric level. However, the octanoylated ghrelin concentration in the plasma was unchanged in spite of the increased caprylic acid (C8:0) availability. A reproducible decrease in the plasma concentration of unacylated ghrelin was observed, which was consistent with a decrease in the stomach preproghrelin mRNA and stomach ghrelin expression. The concomitant decrease of the plasma unacylated ghrelin and the stability of its acylated form resulted in a significant increase in the acylated/total ghrelin ratio which had no effect on body weight gain or total dietary consumption 11. Altogether, these results show that daily feeding with diets containing caprylic acid increased the caprylic acid level in the stomach more than all the other tissues, affecting the acylated/total ghrelin plasma ratio by decreasing the concentration of circulating unacylated ghrelin. Because adipose tissues are target organs for ghrelin 25 and are also influenced by consumption of fatty acids, the impact of the diets on fat pad masses and on histological parameters of subcutaneous adipose tissues was studied. Fat pad masses were measured for several adipose tissues (mesenteric, epididymal, subcutaneous, retroperitoneal and perirenal) in rats fed with the moderate amounts of fat (10% in mass, i.e. 21% of total energy), calorie restricted and high amounts of fat (25% in mass, i.e. 45% of total energy) diets and no significant differences were observed. In the subcutaneous adipose tissue of the moderate amounts of fat diet rats, shows that rats consuming both the moderate amounts of fat diet with 8% caprylic acid and moderate amounts of fat diet with 21% caprylic acid diets exhibited an increased frequency of larger adipocytes compared with the control group. As a consequence, the mean adipocyte size in the subcutaneous fat of rats fed with both moderate amounts of fat diet with 8% caprylic acid and moderate amounts of fat diet with 21% caprylic acid (respectively 63 ± 2 μm and 63 ± 2 μm) was significantly larger than in rats fed with the control diet (54 ± 1 μm). However, these modifications were not associated with increased body weight or food consumption 11.
Medium-chain triglyceride ketogenic diet as treatment for refractory epilepsy
High-fat, low-carbohydrate diets, known as ketogenic diets, have been used as a non-pharmacological treatment for refractory epilepsy. A key mechanism of this treatment is thought to be the generation of ketones, which provide brain cells (neurons and astrocytes) with an energy source that is more efficient than glucose, resulting in beneficial downstream metabolic changes, such as increasing adenosine levels, which might have effects on seizure control.
The ketogenic diet is a high-fat, low-carbohydrate diet that was developed as a treatment for epilepsy 26. The diet aims to mimic the metabolic profile of fasting by reducing blood glucose concentration and increasing blood ketone concentration because starvation has long been reported to reduce the frequency of seizures. Under normal dietary conditions, the brain uses glucose as an energy source; by contrast, during fasting conditions, ketones are used as the main energy source. Hence, starvation (associated with seizure control) induces ketone generation, which might be the therapeutic mechanism of action. Despite the common use of the ketogenic diet to treat epilepsy, the mechanisms underlying its efficacy have remained unclear. However, advances in our understanding of the mechanisms of action of medium-chain fatty acids have resulted in a paradigm shift in the hypothesis behind the mechanisms of the diet, away from ketones as a therapeutic mechanism and focusing on fatty acids instead, paving the way for novel dietary and drug therapies for epilepsy and other disorders.
There are two forms of the ketogenic diet. The so-called classic ketogenic diet provides 60–80% of dietary energy through long-chain fats, which have 16–20 carbon atoms 27. This diet is particularly stringent, with very low carbohydrate content, and consequently, it is difficult to maintain. As such, an alternative medium-chain triglyceride (MCT) ketogenic diet was developed 27, in which fats are provided though triglycerides comprising about 60% caprylic acid (octanoic acid, an eight-carbon fatty acid) and about 40% decanoic acid (a ten-carbon fatty acid). By contrast with the classic ketogenic diet, only about 45% of dietary energy is provided by these medium-chain fats (allowing a larger carbohydrate component) 27 and the more rapid metabolism of the shorter fatty acids results in more efficient generation of ketones.
The MCT ketogenic diet is used worldwide to treat drug-resistant epilepsy, mainly in children 26, but also in adults 28. Both the classic and medium-chain triglyceride (MCT) ketogenic diets have garnered increased interest as potential treatments for other diet-sensitive disorders, including Alzheimer’s disease 29, cancer 30 and diabetes 31. As with epilepsy, the main therapeutic mechanism was assumed to occur through replacing carbohydrates with ketones as an energy source 32. However, despite the efficacy of the ketogenic diet in controlling seizures in patients with epilepsy, several studies have shown a poor correlation between blood plasma ketone concentrations and seizure control 33 and ketones do not acutely block seizure activity in an animal model 34. An additional study 35 has shown seizure control in the absence of ketosis. These observations challenge the view that ketones alone have a role in seizure control and raise the question of the roles of other components of the diet, particularly fats that are provided at high levels in the diet. Additionally, several studies 36 have indicated that medium-chain fatty acids provided in the MCT ketogenic diet can have a direct action on seizure activity and mitochondrial function.
In conclusion, the underlying mechanisms of the ketogenic diet are still largely unknown. Understanding the role of AMPA receptors, PPARγ (peroxisome proliferator-activated receptor γ), and mitochondrial biosynthesis in relation to MCT ketogenic diet-responsive disorders might provide new therapeutic targets and facilitate the development of new pharmacological and dietary treatments (eg, different fatty acid content in MCT diets) or chemical modification of fats to reduce metabolism clearance. The AMPA receptor (AMPA-R) is a subtype of the ionotropic glutamate receptor coupled to ion channels that modulate cell excitability by gating the flow of calcium and sodium ions into the cell 37. The proposed mechanism of AMPA-receptor inhibition, PPARγ activation, and mitochondrial biosynthesis provides a rationale for efficacy in other conditions, and several clinical studies are currently validating the use of the MCT ketogenic diet in the treatment of other disorders. Additionally, further clinical studies are needed to either decrease or mitigate potential adverse effects of ketogenic diets, such as the low-grade acidosis resulting from elevation in β-hydroxybutyric and acetoacetic acids 38. Furthermore, whether other ketogenic diets, such as the classic diet, are also associated with elevated concentrations of medium-chain fatty acids remains to be elucidated, and monitoring of these components in clinical studies will help to investigate these mechanisms. Validation of these fats provided in the diet as therapeutic targets might both improve and widen the use of the diet as a treatment for epilepsy, Alzheimer’s disease, cancer, diabetes, and other disorders.
Caprylic acid toxicity study
Acute oral toxicity LD50 (lethal dose 50 where 50 percent of test subjects die) values for caprylic acid range from 1283 mg/kg to 10,080 mg/kg of body weight in rats, and a dermal LD50 value greater than 5000 mg/kg was reported in rabbits 39. No acute inhalation data are available for caprylic acid; however, studies have been conducted on heptanoic acid (98.5%) and nonanoic acid (97%). The LC50 (the lethal concentration required to kill 50% of the population) values were greater than 4.6 mg/l for heptanoic acid and 0.46 mg/l – 3.8 mg/l for nonanoic acid. The test substance caused a moderate dermal reaction when 0.5 ml was applied to the skin 39.
No data on caprylic acid are available for subchronic and chronic toxicity. However, there are subchronic and chronic toxicity data for heptanoic acid and nonanoic acid (pelargonic acid). In a 14-day rat oral toxicity study, no systemic toxicity was observed in either sex dosed with pelargonic acid (nonanoic acid) as high as 20,000 ppm (1,834 mg/kg/day), the highest dose tested 39. In addition, no adverse effects were caused on survival, clinical signs, body weight gain, food consumption, hematology, clinical chemistry or gross pathology. For each dose, three animals per sex were tested; however, the study did not report organ weights and histopathology. This was considered a deficiency in this study. Nevertheless, the Environmental Protection Agency (EPA) determined that, because no systemic toxic effects were observed at a very high dose level approaching 2,000 mg/kg/day, a 90-day oral study was not necessary 39.
Groups (10/sex/group) of rats (Sprague-Dawley) 45 days of age were given heptanoic acid by gavage in corn oil (10 ml/kg at doses of 0, 875, 1750, and 3500 mg/kg body weight/day) daily for 27 days. Clinical signs included languid behavior, dyspnea, polypnea, tremors, wheezing, ataxia and excess salivation. Significant decreases in body weight and food consumption (male only) were observed compared to those of the control group. Hyperkeratosis of the non-glandular stomach was reported in high-dose males and females at necropsy. No significant findings were noted in low- and mid-dose groups that could be related to administration of the test material. Clinical chemistry and hematological examinations revealed no significant changes compared to those for the control group. A NOAEL (No-Observed-Adverse-Effect Level) of 1750 mg/kg/day and a LOAEL (Lowest-Observed-Adverse-Effect Level) of 3500 mg/kg/day were determined based on decreased body weights and food consumption and gross lesions of the stomach 39.
A supplemental study on chronic toxicity/carcinogenicity in mice was conducted for 80 weeks. A dose of 50 mg of pelargonic (nonanoic acid) acid was dermally applied to each mouse twice/day for 80 weeks. Histopathology showed no non-neoplastic or neoplastic lesions on skins and internal organs of mice. The Environmental Protection Agency (EPA) concluded that although this study was not conducted according to the guideline specifications, it adequately assesses the chronic toxicity and the carcinogenic potential of pelargonic (nonanoic acid) acid via the dermal route 39.
The Environmental Protection Agency (EPA) does not have any information with respect to potential endocrine effects of caprylic acid in mammalian systems. There is no information from the available scientific literature to suggest that this fatty acid would have endocrine effects. The Environmental Protection Agency (EPA) has no knowledge of caprylic acid being an endocrine disruptor. When the appropriate screening and/or testing protocols being considered under the Agency’s Endocrine Disruptor Screening Program have been developed and vetted, caprylic acid may be subjected to additional screening and/or testing to better characterize effects related to endocrine disruption 39.
The current antimicrobial indoor uses of caprylic acid are not expected to result in residues in drinking water supplied by residential wells or municipal sources. In addition all use sites are indoors except for the registered ornamental use which includes the option to apply their product on ornamental plants raised outdoors. As a result, dietary exposure via drinking water may occur but is likely to be very low. Based on the low toxicity, knowledge that caprylic acid is naturally-occurring, is a component of the human diet, and is recognized by the FDA as a GRAS chemical, a dietary and drinking water risk assessment is not needed 39.
Exposure to caprylic acid could result from food, drinking water, and postapplication/bystander sources; all of these could contribute to aggregate risk. As caprylic acid induces no adverse systemic effects via any route of exposure, an aggregate risk assessment is not needed 39.
Those handling the undiluted antimicrobial product directly, i.e., during pouring and mixing the end-use product in/with water prior to application, would be most at risk. Current labels bear the following precautionary statements: “Causes irreversible eye damage and skin burns. May be fatal if inhaled or absorbed through the skin. Harmful if swallowed. Do not get in eyes, on skin, or on clothing. Do not breathe vapor or spray mist. Wear protective eyewear (goggles, face shield, or safety glasses), protective clothing, and rubber gloves” and “When spraying or fogging, wear a mask or pesticide respirator jointly approved by Mine Safety and Health Administration and the National Institute for Occupational Safety and Health” 39.
What is caprylic acid used for?
Caprylic acid is used commercially in the production of esters used in perfumery and also in the manufacture of dyes.
Caprylic acid is also used as an antimicrobial pesticide that is used as a food contact surface sanitizer in commercial food handling establishments 39. Caprylic acid is also used as a disinfectant in health care facilities and as an algaecide in greenhouses and interiorscapes on ornamentals. In addition, caprylic acid is characterized by low toxicity, is biodegradable, and is found extensively in nature.
- Lipids of bovine and human milks: a comparison. Jensen RG, Ferris AM, Lammi-Keefe CJ, Henderson RA. J Dairy Sci. 1990 Feb; 73(2):223-40.[↩]
- The application of medium-chain fatty acids: edible oil with a suppressing effect on body fat accumulation. Takeuchi H, Sekine S, Kojima K, Aoyama T. Asia Pac J Clin Nutr. 2008; 17 Suppl 1():320-3.[↩]
- Zhao W, Varghese M, Vempati P, et al. Caprylic Triglyceride as a Novel Therapeutic Approach to Effectively Improve the Performance and Attenuate the Symptoms Due to the Motor Neuron Loss in ALS Disease. Pandey U, ed. PLoS ONE. 2012;7(11):e49191. doi:10.1371/journal.pone.0049191. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3492315/[↩][↩]
- Sills MA, Forsythe WI, Haidukewych D, MacDonald A, Robinson M (1986) The medium chain triglyceride diet and intractable epilepsy. Arch Dis Child 61: 1168–1172. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1778211/pdf/archdisch00703-0026.pdf[↩]
- Spector R (1988) Fatty acid transport through the blood-brain barrier. J Neurochem 50: 639–643.[↩]
- Perlman BJ, Goldstein DB (1984) Membrane-disordering potency and anticonvulsant action of valproic acid and other short-chain fatty acids. Mol Pharmacol 26: 83–89.[↩]
- Wlaz P, Socala K, Nieoczym D, Luszczki JJ, Zarnowska I, et al. (2012) Anticonvulsant profile of caprylic acid, a main constituent of the medium-chain triglyceride (MCT) ketogenic diet, in mice. Neuropharmacology 62: 1882–1889.[↩]
- Anneken DJ, Both S, Christoph R, Fieg G, Steinberner U and Westfechtel A, 2012. Fatty acids. Ullmann’s Encyclopedia of Industrial Chemistry, 14, 73–116.[↩]
- Lewis RJ, 2007b. Octanoic acid. In: Lewis RJ (ed.). Hawley’s Condensed Chemical Dictionary. 15th Edition. John Wiley & Sons, Inc., New York. 919 pp.[↩]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1999. Safety evaluation of certain food additives. WHO Food Additives Series 42. Prepared by the Fifty‐first meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Linear and branched‐chain aliphatic, unsaturated, unconjugated alcohols, aldehydes, acids, and related esters. Geneva, Switzerland.[↩]
- Lemarié F, Beauchamp E, Dayot S, Duby C, Legrand P, Rioux V. Dietary Caprylic Acid (C8:0) Does Not Increase Plasma Acylated Ghrelin but Decreases Plasma Unacylated Ghrelin in the Rat. Blachier F, ed. PLoS ONE. 2015;10(7):e0133600. doi:10.1371/journal.pone.0133600. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4509905/[↩][↩][↩][↩][↩]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2010. Scientific Opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA Journal 2010;8(3):1461, 107 pp. https://doi.org/10.2903/j.efsa.2010.1461[↩][↩]
- Harvard University, Harvard School of Public Health. Different Dietary Fat, Different Risk of Mortality. https://www.hsph.harvard.edu/nutritionsource/2016/07/05/different-dietary-fat-different-risk-of-mortality/[↩]
- St-Onge MP, Bosarge A, Goree LL, Darnell B. Medium chain triglyceride oil consumption as part of a weight loss diet does not lead to an adverse metabolic profile when compared to olive oil. J Am Coll Nutr. 2008;27: 547–52. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2874191/[↩]
- Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, et al. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr. 1999;70: 1001–8[↩]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402: 656–60. https://www.ncbi.nlm.nih.gov/pubmed/10604470[↩]
- Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279: 909–913. doi: 10.1006/bbrc.2000.4039[↩]
- Wang Q, Liu C, Uchida A, Chuang J-C, Walker A, Liu T, et al. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab. 2014;3: 64–72. doi: 10.1016/j.molmet.2013.10.001[↩]
- Delporte C. Structure and physiological actions of ghrelin. Scientifica. 2013;2013: 518909 doi: 10.1155/2013/518909[↩]
- Zhao T-J, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci. 2010;107: 7467–7472. doi: 10.1073/pnas.1002271107[↩]
- Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132: 387–96. doi: 10.1016/j.cell.2008.01.017[↩]
- Nishi Y, Hiejima H, Hosoda H, Kaiya H, Mori K, Fukue Y, et al. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology. 2005;146: 2255–64[↩]
- Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15: 741–5. doi: 10.1038/nm.1997[↩]
- Barnett BP, Hwang Y, Taylor MS, Kirchner H, Pfluger PT, Bernard V, et al. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science. 2010;330: 1689–92. doi: 10.1126/science.1196154[↩][↩]
- Ghrelin in the regulation of body weight and metabolism. Castañeda TR, Tong J, Datta R, Culler M, Tschöp MH. Front Neuroendocrinol. 2010 Jan; 31(1):44-60.[↩]
- Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev, 2 (2016). CD001903.[↩][↩]
- “Alternative” ketogenic diets” SA Masino (Ed.), Ketogenic diet and metabolic therapies, Oxford University Press, New York (2017), pp. 5-15[↩][↩][↩]
- Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: a meta-analysis J Clin Neurol, 11 (2015), pp. 26-31[↩]
- A cross-sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer’s disease Exp Gerontol (2017) published online July 12. DOI:10.1016/j.exger.2017.07.004[↩]
- Anti-tumour effects of ketogenic diets in mice: a meta-analysis PLoS One, 11 (2016), p. e0155050[↩]
- Medium-chain triglyceride ameliorates insulin resistance and inflammation in high fat diet-induced obese mice. Eur J Nutr, 55 (2016), pp. 931-940[↩]
- Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab, 25 (2017), pp. 262-284[↩]
- Dietary fat, ketosis, and seizure resistance in rats on the ketogenic diet. Epilepsia, 41 (2000), pp. 1400-1410[↩]
- P Chang, K Augustin, K Boddum, et al. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain, 139 (2016), pp. 431-443[↩]
- G Dallerac, J Moulard, JF Benoist, et al. Non-ketogenic combination of nutritional strategies provides robust protection against seizures. Sci Rep, 7 (2017), p. 5496[↩]
- P Chang, N Terbach, N Plant, PE Chen, MC Walker, RS Williams. Seizure control by ketogenic diet-associated medium chain fatty acids. Neuropharmacology, 69 (2013), pp. 105-114[↩]
- Chapter 5 – Physicochemical Properties for Potential Alzheimer’s Disease Drugs. Drug Discovery Approaches for the Treatment of Neurodegenerative Disorders Alzheimer’s Disease 2017, Pages 59-82. https://doi.org/10.1016/B978-0-12-802810-0.00005-2[↩]
- AWC Yuen, IA Walcutt, JW Sander. An acidosis-sparing ketogenic (ASK) diet to improve efficacy and reduce adverse effects in the treatment of refractory epilepsy. Epilepsy Behav, 74 (2017), pp. 15-21[↩]
- Caprylic (Octanoic) Acid Final Registration Review Decision Registration Review Case 5028. Docket Number: EPA-HQ-OPP-2008-0477. https://www.regulations.gov/document?D=EPA-HQ-OPP-2008-0477-0009[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]