Contents
DHEA-sulfate test
Dehydroepiandrosterone sulfate test or DHEAS blood test checks how much DHEA-sulfate (DHEAS) is in your blood. DHEA-sulfate (DHEAS) is a male sex hormone (androgen) that is found in both men and women. Dehydroepiandrosterone Sulfate (DHEAS) is a steroid precursor hormone (prohormone) made by your adrenal glands with weak androgenic effects that is converted into male sex hormones (androgens) and/or female sex hormones (estrogens) in peripheral tissues in your body to exert their effects 1, 2. The DHEA-sulfate test is generally done to check how well your adrenal glands are working and to look into conditions that might cause hormone production to be off.
Although DHEA-sulfate is the most abundant hormone in the body, its exact function is still not known. DHEA-sulfate (DHEAS) plays an important role in making the male sex hormone testosterone and androstenedione and the female sex hormone estrogen. DHEA-sulfate (DHEAS) is also responsible for the onset of sexual differentiation in males and females and the development of secondary male physical characteristics at puberty such as a deep voice and facial hair.
- In men, the DHEA-sulfate (DHEAS) male hormone effect may not be important if testosterone level is normal.
- In women, DHEA contributes to normal libido and sexual satisfaction.
- DHEA may also have effects on the immune system.
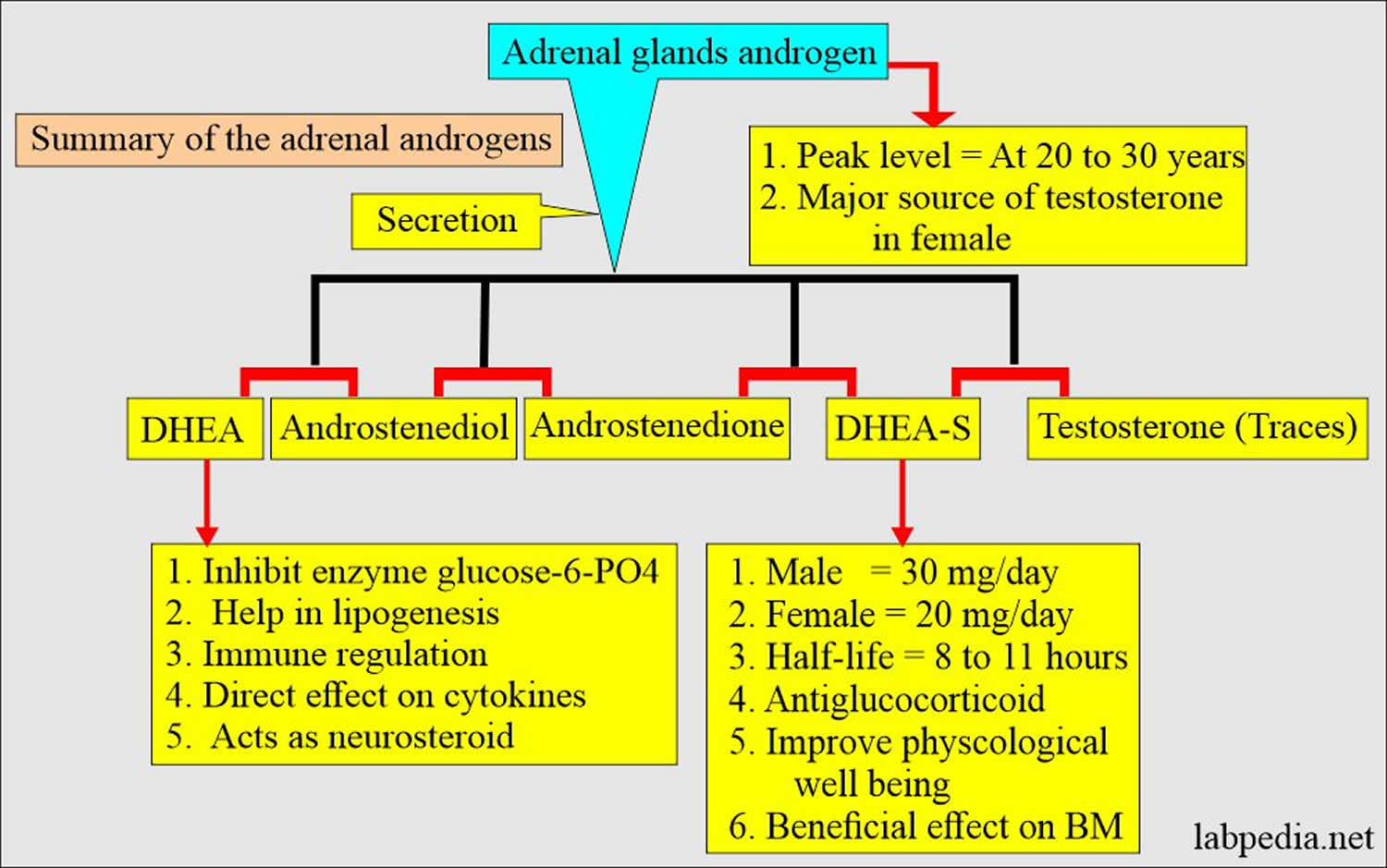
DHEA-sulfate (DHEAS) is mostly made in the adrenal glands, two small glands located above your kidneys (Figure 1). They help control heart rate, blood pressure, and other body functions. Smaller amounts of DHEAS are made in a man’s testicles and in a woman’s ovaries. If your DHEAS levels are not normal, it may mean there is a problem with your adrenal glands or sex organs (testicles or ovaries).
The DHEA-sulfate (DHEAS) test is used to determine whether or not your adrenal glands are functioning properly and to detect adrenal tumors or cancers 3. DHEAS blood test also helps determine the cause of masculine (male) physical characteristics in girls and women (virilization) or early puberty in boys (precocious puberty). DHEA-sulfate test can help figure out what’s causing your irregular periods, excessive hair growth (hirsutism), infertility, and a decreased sex drive (low libido) 4. DHEAS test is also used to find out if someone has a growth in their adrenal gland, congenital adrenal hyperplasia, diagnosis of hyperandrogenism (in conjunction with measurements of other sex steroids) or polycystic ovary syndrome (PCOS) or to assess delayed puberty or premature adrenarche. Babies may also need testing if they have genitals that are not clearly male or female in appearance (ambiguous genitalia). Boys may need this test if they have signs of early puberty (precocious puberty). People on long-term glucocorticoid medicine can also use the DHEAS test to check how well their adrenal glands are working or to rule out Cushing’s syndrome. It is an important test for people who think their male sex hormones (androgens) or female sex hormones (estrogens) might be out of order.
A DHEA-sulfate (DHEAS) test is most often used to 5:
- Find out if your adrenal glands are working right
- Diagnose adrenal glands tumors or cancers
- Diagnose disorders of the testicles or ovaries
- Find out the cause of early puberty in boys
- Find out the cause of excess body hair growth (hirsutism) and development of masculine features in women and girls (virilization)
A DHEAS test is often done along with other sex hormone tests. These include testosterone tests for men and estrogen tests for women.
The DHEAS test is ordered along with tests for testosterone and several other male hormones (androgens) to 3:
- Evaluate whether the adrenal glands are working properly
- Distinguish between DHEAS-secreting conditions caused by the adrenal glands and those that originate in the testicles — or rarely, in the ovaries (ovarian tumors)
- Help diagnose tumors in the outer layer (cortex) of the adrenal gland (adrenocortical tumors) and adrenal cancers
- Help diagnose congenital adrenal hyperplasia and enlargement of the adrenal glands (hyperplasia) in adults
In women, DHEAS levels are often measured, along with other hormones such as follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin, estrogen, and testosterone, to help diagnose polycystic ovary syndrome (PCOS) and rule out other causes of infertility, lack of menstrual period (amenorrhea), and excess hair on the face and body (hirsutism).
DHEAS levels may be ordered with other hormones to investigate and diagnose the cause of the development of masculine physical characteristics (virilization) in young girls and early (precocious) puberty in young boys.
An initial workup in adults might also include total testosterone and bioavailable testosterone. Depending on results, this may be supplemented with measurements of sex hormone-binding globulin (SHBG) and occasionally other androgenic steroids (eg, 17-hydroxyprogesterone).
Figure 1. Adrenal gland androgens
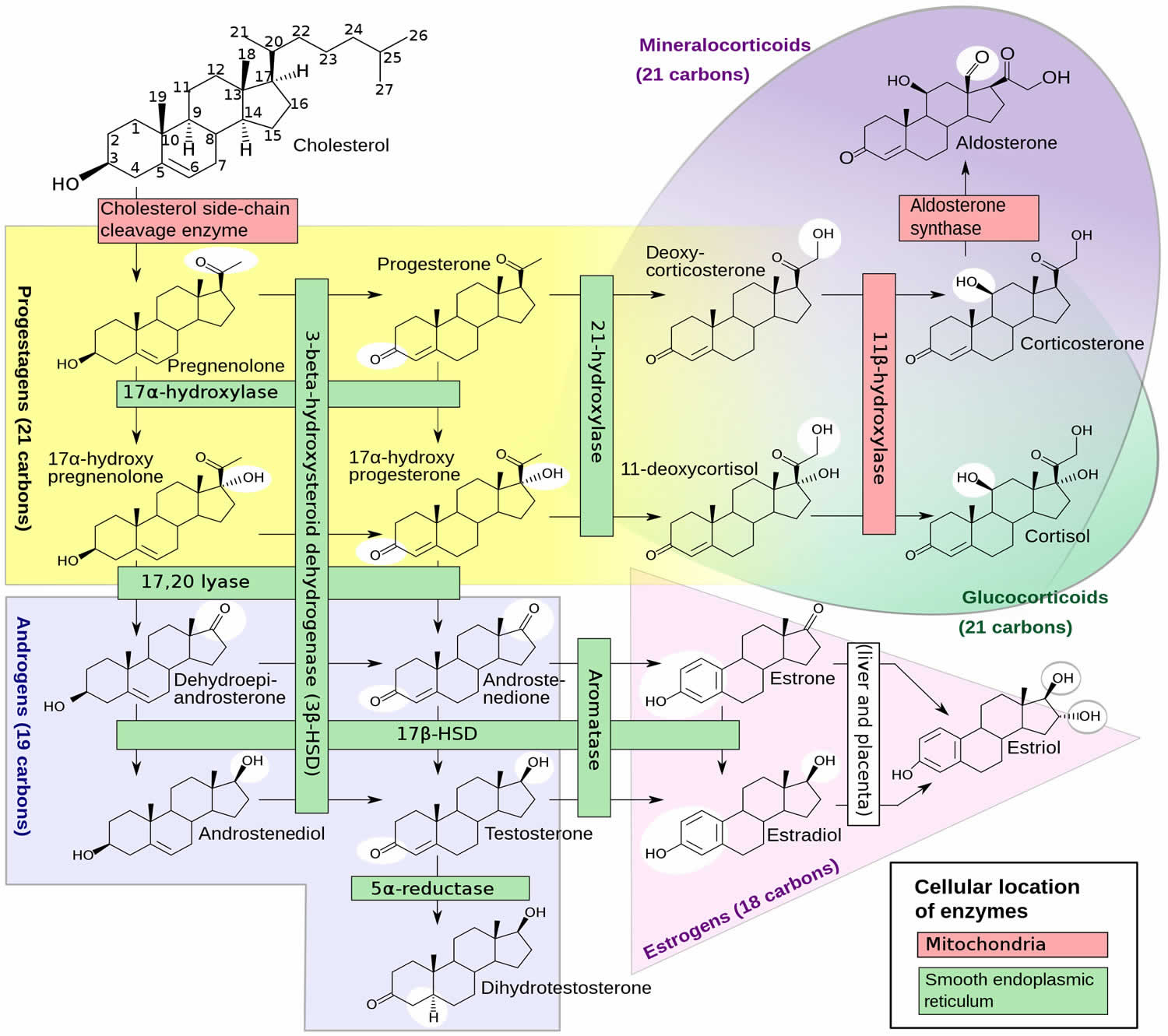
[Source 4 ]Figure 2. DHEA Biosynthesis
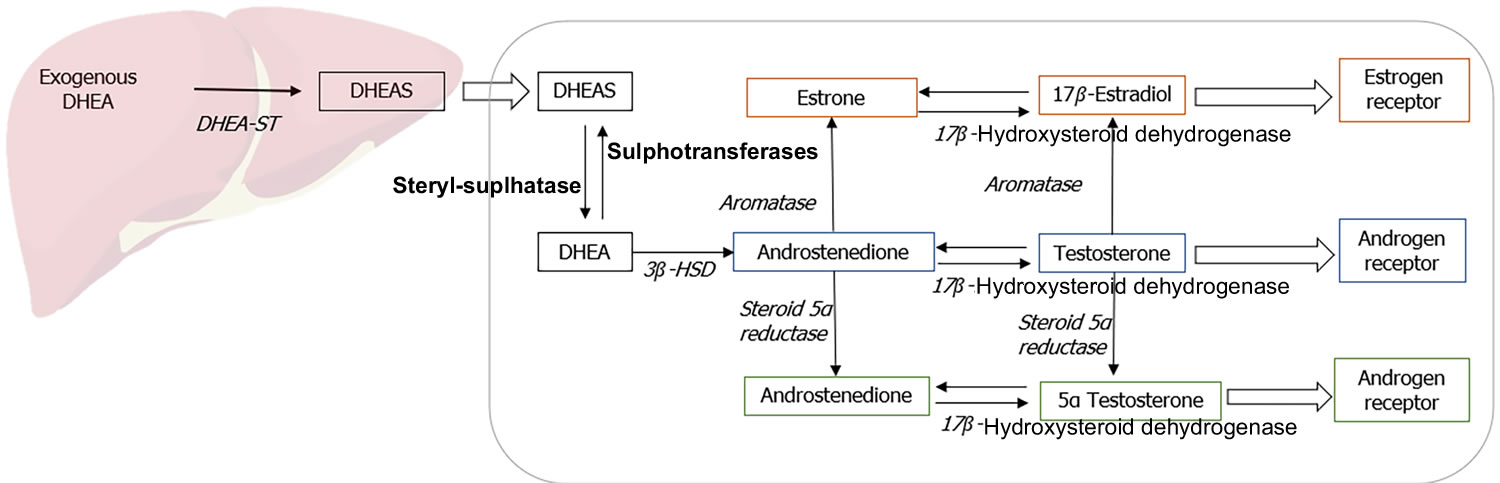
Figure 3. Metabolism of DHEA
Footnote: Metabolism of exogenously administered dehydroepiandrosterone (DHEA).
Abbreviations: DHEA = Dehydroepiandrosterone; DHEAS = Dehydroepiandrosterone sulfate.
[Spurce 6 ]Figure 4. Adrenal androgens summary
[Source 4 ]What is DHEA-sulfate?
DHEA also called Dehydroepiandrosterone and its metabolite Dehydroepiandrosterone Sulfate (DHEAS), are adrenal steroid precursor hormones (prohormones) with weak androgenic effects that is converted into male sex hormones (androgens) and/or female sex hormones (estrogens) in peripheral tissues in your body to exert their effects 1, 2. Both dehydroepiandrosterone (DHEA) and its metabolite dehydroepiandrosterone sulfate (DHEAS) hormones are albumin bound, but binding of DHEAS is much tighter. In gonads and several other tissues, most notably the skin, steroid sulfatases can convert DHEAS back to DHEA, which can then be metabolized to stronger androgens and to estrogens.
Dehydroepiandrosterone sulphate (DHEAS) has a long half-life of 8 to 11 hours and provides a stable pool of circulating DHEA 2. DHEA and DHEAS are involved in the development of male sexual characteristics at puberty. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) are made and secreted primarily by the zona reticularis of the adrenal glands (adrenal cortex) in response to adrenocorticotropic hormone (ACTH) 2. The ovaries also synthesize DHEA; however, the ovaries lack the enzyme DHEA-sulphotransferase so that dehydroepiandrosterone sulphate (DHEAS) is almost exclusively synthesized and secreted by the adrenal glands. Furthermore, dehydroepiandrosterone (DHEA) is claimed to be synthesized in the human brain as well 7. In fact, DHEA concentrations in the human brain have been shown to be higher than that in circulation, while DHEAS concentrations are lower 8, 9, which not only supports the theory of local synthesis, but also testifies to the importance of this hormone in the central nervous system (brain and spinal cord) 7. DHEA can also be made in a laboratory; this man-made synthetic form is known as prasterone 10. Although DHEA and DHEAS are secreted in greater quantities, androstenedione is qualitatively more important since it is more readily converted to testosterone in peripheral tissues 2. Androstenedione is also an androgen, one of several “male” sex hormones that are responsible for the onset of sexual differentiation in males and females and the development of secondary male physical characteristics such as a deep voice and facial hair. Though androstenedione is considered to be a “male” sex hormone, it is present in the blood of both men and women and is a precursor that can be converted by the body into more potent androgens, such as testosterone, or converted into the female hormone estrogen. Androstenedione is produced by the ovaries in women, the testicles in men, and by the adrenal glands in both. Roughly, the relative androgenic potency of DHEA, androstenedione, testosterone, and dihydrotestosterone (DHT) are 5:10:100:300, respectively 2. In the female, androstenedione from peripheral tissues and ovaries is converted into testosterone and estrogen. As adrenocorticotropic hormone (ACTH) is the main regulator of adrenal androgen production in adults, both DHEA and androstenedione exhibit circadian periodicity in concert with ACTH and cortisol and their plasma concentrations increase rapidly following ACTH administration; also, they are suppressed by glucocorticoid administration. Because of its slow metabolic clearance, DHEAS does not exhibit diurnal rhythm variation.
During pregnancy, DHEAS and its 16-hydroxylated metabolites are secreted by the fetal adrenal gland in large quantities. They serve as precursors for placental production of the dominant pregnancy-related estrogen, estriol. Within weeks after birth, DHEAS levels fall by 80% or more and remain low until the onset of adrenarche. The production of DHEA and DHEAS start increasing in boys and girls around the age of 6 to 8 years as a consequence of the maturation of the zona reticularis of the adrenal cortex and this increase in adrenal androgens is known as adrenarche and the concomitant clinical appearance of pubic hair is known as pubarche 11, 6. Adrenarche is a poorly understood phenomenon peculiar to higher primates, which is characterized by a gradual rise in adrenal androgen production. It precedes puberty but is not causally linked to it. Early adrenarche is not associated with early puberty or with any reduction in final height or overt androgenization and is generally regarded as a benign condition, not needing intervention. However, girls with early adrenarche may be at increased risk of polycystic ovarian syndrome (PCOS) as adults, and some boys may develop early penile enlargement.
Following adrenarche (increase in adrenal androgens production), DHEA and DHEAS levels rise steadily and peak in the second to third decade of life (serum DHEAS concentration can be as high as 10 μM in young adults), levels roughly comparable to that observed at birth 12, 13, 14, 15. Thereafter there is a progressive decline by around 2% to 5% each year with advancing age, such that levels decrease by 80% to 90% in the eighth to ninth decade of life 16. In a healthy aged 65- to 81-year-old population, plasma DHEAS concentration is 2.2 µM in women and 3.3 µM in men 17. However, the interindividual variability across adulthood is substantial, and the normal range of serum DHEA and DHEAS is therefore very wide at each decade of life 15. DHEAS levels in women in their mid 70s are about 77% lower than women in their third decade of life, with age alone explaining about 30% of the variation in DHEAS levels 15. Despite the overall decline in DHEAS with age, levels across the menopausal transition vary according to the transitional phase 18.
Between the early and late perimenopause, DHEAS appears to increase on average by 3.95% in most women and then decline to levels seen in the early perimenopause by the late postmenopause 19. Women who do not exhibit this rise in DHEAS across the menopause have lower levels as they enter menopause 18. Overall Chinese women tend to have higher DHEAS levels and African-American women have lower DHEAS levels than Caucasian women 18. The clinical significance of this DHEAS age-related drop is unknown. To compensate for the decrease in aged people, DHEA is available over the counter in the United States. However, clinical trials of DHEAS replacement in the elderly have not produced convincing benefits. On the other hand, in young and old patients with primary adrenal failure, the addition of DHEAS to corticosteroid replacement has been shown in some studies to improve mood, energy, and sex drive.
The potential clinical roles of dehydroepiandrosterone (DHEA) and its metabolite dehydroepiandrosterone sulfate (DHEAS) have been studied extensively, as previous epidemiologic and prospective studies found DHEA or DHEAS levels seem to go down as people get older and indicate an inverse relation between low circulating levels of dehydroepiandrosterone (DHEA) and its metabolite dehydroepiandrosterone sulphate (DHEAS) with a host of aging-associated pathologies such as sexual dysfunction, degenerative disorders and increased frailty, mood defects, poor sense of well-being and mortality from all causes in the elderly, attributing to DHEA or DHEAS anti-aging properties 20, 7, 21, 18, as well as higher risk of hospital admission 22, poor muscle strength 23 and mobility 22, 24, and higher prevalence of frailty 25, insulin resistance, obesity, cardiovascular disease 26 and mortality from cardiovascular disease 27. At the same time, a positive relation between higher levels of DHEAS and better health and well-being was documented 28. Furthermore, animal (primarily rats) studies have suggested many beneficial effects of DHEA treatment, including improved immune function and prevention of atherosclerosis, cancer, diabetes, and obesity. Therefore, the therapeutic role of DHEA replacement as an anti-aging factor for the prevention and/or treatment of the above conditions was studied; however, recent systematic reviews of the reports do not seem promising 29, 30, 31, 32, 33, 34, 35, 2.
- Thinning of vaginal tissue (vaginal atrophy). A prescription DHEA product is available for vaginal atrophy. Using vaginal inserts containing DHEA can reduce pain during sex by up to 15% after menopause.
- Aging. In theory, taking DHEA supplements to maintain DHEA levels could slow the aging process, possibly improving well-being, cognitive function and body composition. But so far research hasn’t proved this to be true. More studies are needed to better understand whether DHEA supplementation can counteract some of the effects of aging.
- Aging skin. Taking DHEA by mouth or applying it to the skin might improve skin appearance after menopause and in people over the age of 60 years. A small study suggested that taking DHEA supplements might improve skin hydration and firmness, and decrease aging spots in elderly adults.
- Depression. Changes in levels of DHEA have been linked to depression. Several preliminary studies show improvement in depression symptoms when taking DHEA as a dietary supplement, but more research is needed. Taking 30-500 mg of DHEA by mouth daily seems to improve symptoms of depression. Lower doses don’t seem to help.
- Inability to become pregnant within a year of trying to conceive (infertility). Taking DHEA by mouth before in-vitro fertilization (IVF) might improve the chances of pregnancy and having a baby. But it’s not clear if taking DHEA helps prevent miscarriage after IVF.
- Osteoporosis. Study findings on the effects of DHEA supplementation in the treatment of osteoporosis are mixed. More research is needed to determine whether taking DHEA supplements improves bone density in older adults with low DHEA.
DHEA levels seem to be lower in people with depression, Alzheimer’s disease and after menopause 36, 37, 38, 39, 40, 41, 42, 43, 9.
There is interest in using DHEA for a number of health purposes (e.g., for aging and aging related problems such as body shape, bone strength, muscle strength, physical performance or quality of life in people older than 60 or memory and thinking skills [cognitive function] in healthy older people, people with HIV, or in healthy young adults), but there isn’t enough reliable information to say whether it might be helpful. The authors of a Cochrane Systematic Review regarding the supplementation of DHEA in peri- and post-menopausal women, questioned the effectiveness of DHEA in women, and concluded that the overall quality of the studies analyzed was moderate to low and that the study outcomes were inconsistent and could not be pooled to obtain an overall effect due to the diversity of the measurement methods employed 44. Without exception, all recent reviews of the available data regarding DHEA replacement for the management of aging-related disorders do not support its use in clinical practice 29, 30, 31, 32, 33, 34, 35, 2.
There’s no scientific evidence to support taking DHEA to improve exercise or athletic performance. DHEA is banned by the National Collegiate Athletic Association (NCAA), the International Olympic Committee (IOC) and the World Anti-Doping Agency (WADA) 45, 46. Don’t confuse DHEA with 7-alpha-hydroxy-DHEA, 7-beta-hydroxy-DHEA, and 7-keto-DHEA. These are all different forms of DHEA but are not the same as DHEA.
DHEA supplementation has historically been used in the treatment of reproductive-related diseases, particularly in women, where it has been shown to alleviate menopause-related pathologies such as vaginal tissue thinning (vaginal atrophy) 47. DHEA supplements are also used for aging skin, depression, infertility, muscle strength, heart disease, erectile dysfunction (ED), and many other conditions, but there is no good scientific evidence to support many of these other uses 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59. Studies have shown quality control of DHEA supplement to often be low.
As an ingredient in dietary supplement products, DHEA is mostly marketed for bodybuilding as a “testosterone booster” (which it is not) or for nootropic (“cognitive enhancer”) actions. Research on the effects of DHEA on muscle strength and physical performance had mixed results, but most studies indicate DHEA supplementation has no effect on muscle strength in younger or older adults. DHEA might eventually prove to have benefits in treating people diagnosed with certain conditions, such as adrenal insufficiency and lupus. However, further studies are needed.
DHEA is considered as a hormone in Europe and available only by prescription, while in the United States DHEA is considered as a nutritional supplement and is sold over the counter without a prescription. This difference has no scientific foundation and is mostly a matter of declaration 2. Most DHEA supplements are made in laboratories from a substance called diosgenin, a plant sterol found in soy and wild yams. DHEA supplements were taken off the U.S. market in 1985 because of their unproven safety and effectiveness, but were reintroduced as a dietary supplement after the Dietary Supplement Health and Education Act was passed in 1994 2. At present, questionable over-the-counter DHEA preparations lacking pharmacokinetic and pharmacodynamic data are widely used in the United States 60, 61. There is no standard dosage of DHEA replacement; some studies have used between 25 and 200 milligrams a day, or sometimes even higher amounts. DHEA in current preparations has a long half-life 26, which allows a single intake a day. Target levels of DHEA are around the middle of normal range for healthy young subjects, measured in a blood sample 24 hour after the last intake 62.
Previous studies have shown that the end products of DHEA supplementation depend on the patient’s gender, with a non-symmetrical transformation of DHEA favoring androgens in women and estrogens in men 29, 63, 64. The above refer to oral administration of DHEA supplements; percutaneous administration of DHEA seems to provoke similar increases in both estrogens and androgens in the two genders 65.
DHEA supplements are generally well tolerated in studies using oral or percutaneous administration, with daily doses ranging from 25 mg to 1,600 mg. In women DHEA when administered orally is mainly converted to androgen metabolites. As a result, some minimal androgenic adverse effects have been reported, including mild acne, seborrhea (a red, itchy rash and white scales skin), facial hair growth, and ankle swelling 26, 32, 66.
How does DHEA work?
The exact mechanism of action and clinical role of DHEA and DHEAS remain unclear 2, 6. As DHEA has minor steroidogenic activity, it acts predominantly by conversion to androgens and estrogens in peripheral target tissues (Figure 1 and 2). It also functions as a neurosteroid and acts via receptors for N methyl-D aspartate receptors (NMDA) and gamma amino butyric acid alpha (GABAα), peroxisome proliferator-activated receptor α (PPARα), or receptors for pregnane X, androstanol, and estrogen receptor β 67.
About 75%-90% of DHEA is produced by the zona reticularis of the adrenal gland while the rest is produced in the ovaries and the brain 6. DHEA sulfated form, dehydroepiandrosterone sulfate (DHEAS), is exclusively synthesized by the adrenals. DHEA has a shorter half-life and is secreted in a pulsatile manner, mirroring the circadian rhythm of corticotrophin (ACTH). In contrast, dehydroepiandrosterone sulfate (DHEAS) has a longer half-life and relatively more stable levels across the day, providing a continuous reservoir of DHEA.
The production of DHEA and DHEAS start increasing in boys and girls around the age of 6 to 8 years as a consequence of the maturation of the zona reticularis of the adrenal cortex and this increase in adrenal androgens is known as adrenarche and the concomitant clinical appearance of pubic hair is known as pubarche 11, 6. DHEA and DHEAS levels rise steadily and peak in the second to third decade of life 68, 14, 15. Serum DHEA concentration is around 20 nM in young adults and decreases with age to 7 nM 12. Thereafter there is a progressive decline by around 2%-5% each year with advancing age, such that levels decrease by 80%-90% in the eighth to ninth decade of life 16. However, the interindividual variability across adulthood is substantial, and the normal range of serum DHEA and DHEAS is therefore very wide at each decade of life 15. DHEAS levels in women in their mid 70s are about 77% lower than women in their third decade of life, with age alone explaining about 30% of the variation in DHEAS levels 15. Despite the overall decline in DHEAS with age, levels across the menopausal transition vary according to the transitional phase 18. Between the early and late perimenopause, DHEAS appears to increase on average by 3.95% in most women and then decline to levels seen in the early perimenopause by the late postmenopause 19. Women who do not exhibit this rise in DHEAS across the menopause have lower levels as they enter menopause 18. Overall Chinese women tend to have higher DHEAS levels and African-American women have lower DHEAS levels than Caucasian women 18.
In adult women, dehydroepiandrosterone sulfate (DHEAS) is the most abundant steroid hormone, with daily production rates being approximately 8 to 16 mg/day, which are almost exclusively adrenal glands 69. DHEAS is formed from DHEA in the highly specialized zona reticularis of the adrenals, which has high sulfuryl transferase activity. The hydrophilic DHEAS is the major circulating form of DHEA and is interconverted in various tissues with DHEA by DHEA sulfotransferases and hydroxysteroid sulfatases 69. In premenopausal women, the production rate of DHEA is approximately 6–8 mg/day. Approximately 50% of DHEA is secreted by the adrenal glands, with the ovaries producing approximately 1 to 2 mg of DHEA per day; the remaining DHEA production occurs in peripheral tissues 69. In contrast, the production of testosterone (T) in premenopausal women is approximately 0.2 to 0.25 mg/day, which is about 25–40 times less than that of DHEA and even much lower than that of DHEAS 70. The ovaries of some postmenopausal women continue to produce some testosterone (T), but not DHEA 70, 71. In the circulation, DHEAS and DHEA are found in low micromolar and low nanomolar concentrations, respectively. Rosenfeld et al. 72 reported a circulating half-life of 1 to 3 hours for DHEA and 10 to 20 hours for DHEAS. Sex hormone-binding globulin (SHBG) weakly binds DHEA but not DHEAS 73.
Regular exercise is known to increase DHEA production in the body 74, 75. Calorie restriction has also been shown to increase DHEA in rhesus monkeys 76. Some theorize that the increase in endogenous DHEA brought about by calorie restriction is partially responsible for the longer life expectancy known to be associated with calorie restriction 77.
Peripherally, DHEA appears to exert its primary effects through its estrogenic and androgenic metabolites because a unique DHEA receptor has not been characterized. However, DHEA has been shown to exhibit weak agonist effects on the estrogen receptors (ER) α and β and be a weak antagonist of the androgen receptor (AR) 78. There is evidence that DHEA is synthesized within the brain and may act locally as an excitatory neurosteroid by antagonizing the actions of the gamma-aminobutyric acid type A receptor (GABAα) and stimulating the N-methyl-d-aspartate receptor (NMDA), peroxisome proliferator-activated receptor α (PPARα), or receptors for pregnane X, androstanol, and estrogen receptor β 6, 79, 80. A putative plasma membrane-bound G coupled receptor activated by DHEA has been identified on bovine aortic endothelial cells 81, 82. Liu et al. 83 subsequently have shown that physiological concentrations of DHEA activate this plasma membrane receptor on vascular endothelial cells and stimulate endothelial proliferation and angiogenesis through extracellular signal-regulated kinase 1/2-mediated mechanisms.
DHEA and DHEAS can be converted to many different metabolites; Figure 2 depicts some of the more important ones. DHEA and DHEAS are metabolized in extragonadal target tissues such as the brain, bone, and body fat either by aromatization to estrone or by 5α-reduction to testosterone (T), with the testosterone (T) being converted to either estradiol or 5 alpha-dihydrotestosterone (DHT) in the same cells 84. The transformation of DHEA into active androgens and estrogens depends upon the level of expression of the various steroidogenic and metabolizing enzymes in each cell type. With the increase in aromatase gene expression in body fat with age 85, 86, older women potentially have greater capacity to synthesize estrone from DHEA and DHEAS. Furthermore, the ultimate effects of the complex metabolism of DHEA and DHEAS will involve the absolute levels of each metabolite, their receptor content within the target cell, the presence and levels of specific coactivator and corepressor proteins that modify the transcriptional response, and the up- or down-regulation of receptor levels by other hormones 19.
Why do I need a DHEA sulfate test?
DHEA-sulfate levels are not routinely measured. The DHEA-sulfate test, along with other hormone tests, is often done in women who show signs of having excess male hormones (or rarely, a deficiency in male hormones). Some signs of having excess male hormones in females are male body changes (virilization), a deeper voice, enlargement of the Adam’s apple, excess hair growth (hirsutism), loss of hair from the top of the head (male pattern baldness), oily skin, acne, irregular periods, no menstrual periods (amenorrhea), decreased breast size or problems becoming pregnant (infertility).
DHEA-sulfate test may also be done in women who are concerned about low libido or decreased sexual satisfaction who have pituitary or adrenal gland disorders.
DHEA-sulfate test is also done in young boys who are maturing too early (precocious puberty) who show signs of the development of a deeper voice, pubic hair, muscularity, and an enlarged penis well before the age of normal puberty (precocious puberty).
Babies may also need testing if they have genitals that are not clearly male or female in appearance (ambiguous external genitalia).
DHEA-sulfate test is also done in a young girl with ambiguous external genitalia.
Men may not have any symptoms of high levels of DHEA-sulfate, but through peripheral conversion of androgens to estrogens can occasionally experience mild estrogen excess.
Symptoms of HIGH levels of DHEAS in women and girls may include:
- Excess body and facial hair growth (hirsutism)
- Deepening of voice
- Irregular periods or amenorrhea (absence of periods)
- Acne
- Increased muscularity
- Hair loss at the top of your head (male pattern baldness)
Symptoms of LOW levels of DHEAS may include the following signs of an adrenal gland disorder:
- Unexplained weight loss
- Nausea and vomiting
- Dizziness
- Dehydration
- Craving for salt
Other symptoms of LOW DHEAS are related to aging and may include:
- Decreased sex drive (low libido)
- Erectile dysfunction in men
- Thinning of vaginal tissues in women (vaginal atrophy)
How DHEA-sulfate test is performed
A blood sample is needed. A health care professional will take a blood sample from a vein in your arm, using a small needle. After the needle is inserted, a small amount of blood will be collected into a test tube or vial. You may feel a little sting when the needle goes in or out. This usually takes less than five minutes.
You don’t need any special preparations for a DHEA sulfate test.
Are there any risks to the DHEA sulfate?
There is little risk involved with having your blood taken. You may have slight pain or bruising at the spot where the needle was put in, but most symptoms go away quickly.
Other risks associated with having blood drawn are slight, but may include:
- Excessive bleeding
- Fainting or feeling lightheaded
- Multiple punctures to locate veins
- Hematoma (blood buildup under the skin)
- Infection (a slight risk any time the skin is broken)
What do the DHEA-sulfate test results mean?
A DHEA-sulfate (DHEAS) test is most often used to 5:
- Find out if your adrenal glands are working right
- Diagnose adrenal glands tumors or cancers
- Diagnose disorders of the testicles or ovaries
- Find out the cause of early puberty in boys
- Find out the cause of excess body hair growth (hirsutism) and development of masculine features in women and girls (virilization)
A DHEAS test is often done along with other sex hormone tests. These include testosterone tests for men and estrogen tests for women.
The DHEAS test is ordered along with tests for testosterone and several other male hormones (androgens) to 3:
- Evaluate whether the adrenal glands are working properly
- Distinguish between DHEAS-secreting conditions caused by the adrenal glands and those that originate in the testicles — or rarely, in the ovaries (ovarian tumors)
- Help diagnose tumors in the outer layer (cortex) of the adrenal gland (adrenocortical tumors) and adrenal cancers
- Help diagnose congenital adrenal hyperplasia and enlargement of the adrenal glands (hyperplasia) in adults
In women, DHEAS levels are often measured, along with other hormones such as follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin, estrogen, and testosterone, to help diagnose polycystic ovary syndrome (PCOS) and rule out other causes of infertility, lack of menstrual period (amenorrhea), and excess hair on the face and body (hirsutism).
DHEAS levels may be ordered with other hormones to investigate and diagnose the cause of the development of masculine physical characteristics (virilization) in young girls and early (precocious) puberty in young boys.
DHEAS normal range
Normal blood levels of DHEA-sulfate can differ by sex and age. Normal value ranges may vary slightly among different laboratories. Some labs use different measurements or test different specimens. Talk to your health care provider about the meaning of your specific test results.
Normal DHEA-sulfate levels in females
Typical normal DHEA-sulfate ranges for females are 87:
- Ages 18 to 19: 145 to 395 micrograms per deciliter (µg/dL) or 3.92 to 10.66 micromoles per liter (µmol/L)
- Ages 20 to 29: 65 to 380 µg/dL or 1.75 to 10.26 µmol/L
- Ages 30 to 39: 45 to 270 µg/dL or 1.22 to 7.29 µmol/L
- Ages 40 to 49: 32 to 240 µg/dL or 0.86 to 6.48 µmol/L
- Ages 50 to 59: 26 to 200 µg/dL or 0.70 to 5.40 µmol/L
- Ages 60 to 69: 13 to 130 µg/dL or 0.35 to 3.51 µmol/L
- Ages 69 and older: 17 to 90 µg/dL or 0.46 to 2.43 µmol/L
Normal DHEA-sulfate levels in males
Typical normal DHEA-sulfate ranges for males are 87:
- Ages 18 to 19: 108 to 441 µg/dL or 2.92 to 11.91 µmol/L
- Ages 20 to 29: 280 to 640 µg/dL or 7.56 to 17.28 µmol/L
- Ages 30 to 39: 120 to 520 µg/dL or 3.24 to 14.04 µmol/L
- Ages 40 to 49: 95 to 530 µg/dL or 2.56 to 14.31 µmol/L
- Ages 50 to 59: 70 to 310 µg/dL or 1.89 to 8.37 µmol/L
- Ages 60 to 69: 42 to 290 µg/dL or 1.13 to 7.83 µmol/L
- Ages 69 and older: 28 to 175 µg/dL or 0.76 to 4.72 µmol/L
High DHEA-sulfate levels
If your DHEAS blood test results show high levels of DHEA sulfate (DHEAS), it may mean you have one of the following conditions:
- Congenital adrenal hyperplasia (CAH), an inherited disorder of the adrenal glands
- A tumor of the adrenal gland. It may be benign (noncancerous) or cancerous.
- Polycystic ovary syndrome (PCOS). PCOS is a common hormone disorder affecting childbearing women. It is one of the leading causes of female infertility. About 25% to 50% of women with PCOS have elevated DHEAS.
- Body changes of a girl in puberty happening earlier than normal.
- Rarely, an ovarian tumor that produces DHEA-sulfate.
A high dehydroepiandrosterone sulfate (DHEAS) blood level may indicate that excess DHEA sulfate (DHEAS) production is causing or contributing to your symptoms. Elevated DHEA-sulfate levels can cause symptoms or signs of hyperandrogenism in women. However, an increased level of DHEAS is not diagnostic of a specific condition. Most mild to moderate elevations in DHEAS levels are idiopathic (unknown cause). It usually indicates the need for further testing to pinpoint the cause of the hormone imbalance. However, pronounced elevations of DHEA-sulfate with levels of 600 micrograms per deciliter (µg/dL) or more may be indicative of androgen-producing adrenal tumors. DHEA sulfate (DHEAS) levels are elevated in more than 90% of patients with adrenal tumors, usually well above 600 mcg/dL. This is particularly true for androgen-secreting adrenal carcinomas (adrenal gland cancer), as they have typically lost the ability to produce down-stream androgens, such as testosterone 88. By contrast, androgen-secreting adrenal adenomas (adrenal benign tumors) may also produce excess testosterone and secrete lesser amounts of DHEAS 88.
In small children, congenital adrenal hyperplasia (CAH) due to 3 beta-hydroxysteroid deficiency is associated with very high levels of DHEA sulfate (DHEAS) production, often 5- to 10-fold elevations. Lesser elevations may be observed in 21-hydroxylase deficiency (the most common form of congenital adrenal hyperplasia) and 11 beta-hydroxylase deficiency. By contrast, steroidogenic acute regulatory protein or 17 alpha-hydroxylase deficiencies are characterized by low DHEA-sulfate levels. Consequently, DHEAS testing should not be used as the primary
tool for congenital adrenal hyperplasia (CAH) diagnosis. Similarly, discovering a high DHEA sulfate (DHEAS) level in an infant or child with symptoms or signs of possible congenital adrenal hyperplasia (CAH) should prompt additional testing, as should the discovery of very high DHEAS levels in an adult. In the latter case, adrenal tumors need to be excluded and additional adrenal steroid profile testing may assist in diagnosing congenital adrenal hyperplasia (CAH). If you have questions about your results, talk to your doctor.
Girls below the age of 7 to 8 and boys before age 8 to 9, who present with early development of pubic hair, or, in boys, penile enlargement, may be suffering from either premature adrenarche or premature puberty or both 88. Measurement of DHEAS and androstenedione, alongside determination of sensitive estradiol,total testosterone and bioavailable testosterone or free testosterone, sex hormone-binding globulin (SHBG), luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels will allow correct diagnosis in most cases 88. In premature adrenarche, only the adrenal androgens, chiefly DHEA sulfate (DHEAS), will be above prepubertal levels, whereas early puberty will also show a fall in sex hormone-binding globulin (SHBG) levels and variable elevations of gonadotropins (luteinizing hormone [LH] and follicle-stimulating hormone [FSH]) and gonadal sex-steroids above the prepuberty reference range.
Many drugs and hormones can result in changes in DHEA sulfate (DHEAS) levels. Whether any of these secondary changes in DHEAS levels are of clinical significance and how they should be related to the established normal reference ranges is unknown 88. In most cases, the drug-induced changes are not large enough to cause diagnostic confusion, but when interpreting mild abnormalities in DHEAS levels, drug and hormone interactions should be taken into account. Examples of drugs that may increase DHEA sulfate (DHEAS) levels include metformin, troglitazone, prolactin, many neuroleptic drugs (by indirect implication)), danazol, calcium channel blockers (eg, diltiazem, amlodipine), and nicotine 88.
Low DHEA-sulfate levels
If your DHEAS blood test results show low levels of DHEA sulfate (DHEAS), it may mean you have one of the following conditions:
- Adrenal gland disorders that produce lower than normal amounts of adrenal hormones, including adrenal insufficiency and Addison disease
- Hypopituitarism, a condition in which the pituitary gland does not make enough pituitary hormones
- Taking glucocorticoid medicines (Cushing’s syndrome)
If you have questions about your results, talk to your doctor.
Many drugs and hormones can result in changes in DHEA sulfate (DHEAS) levels. Whether any of these secondary changes in DHEAS levels are of clinical significance and how they should be related to the established normal reference ranges is unknown 88. In most cases, the drug-induced changes are not large enough to cause diagnostic confusion, but when interpreting mild abnormalities in DHEAS levels, drug and hormone interactions should be taken into account. Examples of drugs and hormones that can reduce DHEA sulfate (DHEAS) levels include: insulin, oral contraceptive drugs,
corticosteroids, central nervous system agents that induce hepatic enzymes (eg, carbamazepine, clomipramine, imipramine, phenytoin), many antilipemic drugs (eg, statins, cholestyramine), dopaminergic drugs (eg, levodopa/dopamine, bromocriptine), fish oil, and vitamin E 88.
DHEA sulfate levels normally decline with age in both men and women. The production of DHEA and DHEAS start increasing in boys and girls around the age of 6 to 8 years as a consequence of the maturation of the zona reticularis of the adrenal cortex and this increase in adrenal androgens is known as adrenarche and the concomitant clinical appearance of pubic hair is known as pubarche 11, 6. DHEA and DHEAS levels rise steadily and peak in the second to third decade of life (serum DHEAS concentration can be as high as 10 μM in young adults) 12, 13, 14, 15. Thereafter there is a progressive decline by around 2%-5% each year with advancing age, such that levels decrease by 80%-90% in the eighth to ninth decade of life 16. In a healthy aged 65- to 81-yr-old population, plasma DHEAS concentration is 2.2 µM in women and 3.3 µM in men 17. However, the interindividual variability across adulthood is substantial, and the normal range of serum DHEA and DHEAS is therefore very wide at each decade of life 15. DHEAS levels in women in their mid 70s are about 77% lower than women in their third decade of life, with age alone explaining about 30% of the variation in DHEAS levels 15. Despite the overall decline in DHEAS with age, levels across the menopausal transition vary according to the transitional phase 18. Between the early and late perimenopause, DHEAS appears to increase on average by 3.95% in most women and then decline to levels seen in the early perimenopause by the late postmenopause 19. Women who do not exhibit this rise in DHEAS across the menopause have lower levels as they enter menopause 18. Overall Chinese women tend to have higher DHEAS levels and African-American women have lower DHEAS levels than Caucasian women 18. To compensate for the decrease in aged people, over-the-counter DHEA sulfate supplements are available and are sometimes promoted as an anti-aging therapy. But there is no reliable evidence to support these anti-aging claims of DHEA supplements. In fact, DHEA supplements may cause serious side effects. If you have questions about DHEA supplements, talk to your health care provider.
How much does the DHEA-sulfate test cost?
The cost of a DHEA-sulfate test will vary depending on factors such as where the test is done and whether you have health insurance. When ordered by a doctor, insurance typically covers the test, although you may have to pay a copay or deductible. Your doctor’s office, lab, and health plan can provide information about any out-of-pocket costs that may be your responsibility.
- Labrie F, Luu-The V, Bélanger A, Lin SX, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005 Nov;187(2):169-96. https://joe.bioscientifica.com/view/journals/joe/187/2/1870169.xml[↩][↩]
- Papadopoulou-Marketou N, Kassi E, Chrousos GP. Adrenal Androgens and Aging. [Updated 2023 Jan 18]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279006[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- DHEA-S Test. https://www.testing.com/tests/dheas[↩][↩][↩]
- Androgens: Androstenedione (AD), DHEA- S (Dehydroepiandrosterone sulphate), DHEA (dehydroepiandrosterone). https://labpedia.net/androgens-androstenedione-ad-dhea-s-dehydroepiandrosterone-sulphate-dhea-dehydroepiandrosterone[↩][↩][↩]
- DHEA Sulfate Test. https://medlineplus.gov/lab-tests/dhea-sulfate-test[↩][↩]
- Jethwani P, Rastogi A, Shukla R. Dehydroepiandrosterone sulfate supplementation in health and diseases. World J Meta-Anal 2023; 11(4): 102-111 https://www.wjgnet.com/2308-3840/full/v11/i4/102.htm[↩][↩][↩][↩][↩][↩][↩]
- Powrie YSL, Smith C. Central intracrine DHEA synthesis in ageing-related neuroinflammation and neurodegeneration: therapeutic potential? J Neuroinflammation. 2018 Oct 16;15(1):289. doi: 10.1186/s12974-018-1324-0[↩][↩][↩]
- Stárka L, Dušková M, Hill M. Dehydroepiandrosterone: a neuroactive steroid. J Steroid Biochem Mol Biol. 2015;145:254–260. doi: 10.1016/j.jsbmb.2014.03.008[↩]
- Arbo BD, Bennetti F, Ribeiro MF. Astrocytes as a target for neuroprotection: modulation by progesterone and dehydroepiandrosterone. Prog Neurobiol. 2016;144:27–47. doi: 10.1016/j.pneurobio.2016.03.010[↩][↩]
- DHEA: CAN I USE IT? https://www.opss.org/article/dhea-can-i-use-it[↩]
- Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004 Nov;22(4):337-47. doi: 10.1055/s-2004-861550[↩][↩][↩]
- Labrie F, Luu-The V, Bélanger A, Lin S-X, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol 187: 169–196, 2005. doi: 10.1677/joe.1.06264[↩][↩][↩]
- Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab 59: 551–555, 1984. doi: 10.1210/jcem-59-3-551[↩][↩]
- Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997 Aug;82(8):2396-402. doi: 10.1210/jcem.82.8.4160[↩][↩][↩]
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005 Jul;90(7):3847-53. doi: 10.1210/jc.2005-0212[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Mazat L, Lafont S, Berr C, Debuire B, Tessier JF, Dartigues JF, Baulieu EE. Prospective measurements of dehydroepiandrosterone sulfate in a cohort of elderly subjects: relationship to gender, subjective health, smoking habits, and 10-year mortality. Proc Natl Acad Sci U S A. 2001 Jul 3;98(14):8145-50. doi: 10.1073/pnas.121177998[↩][↩][↩]
- Geisler C, Schweitzer L, Müller MJ. Functional correlates of detailed body composition in healthy elderly subjects. J Appl Physiol 124: 182–189, 2018. doi: 10.1152/japplphysiol.00162.2017[↩][↩]
- Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009 Aug;94(8):2945-51. doi: 10.1210/jc.2009-0386[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Susan R. Davis and others, DHEA Replacement for Postmenopausal Women, The Journal of Clinical Endocrinology & Metabolism, Volume 96, Issue 6, 1 June 2011, Pages 1642–1653, https://doi.org/10.1210/jc.2010-2888[↩][↩][↩][↩]
- Burns BR. Hormone Therapy: Aging-Related Hormone Replacement Therapy and Supplementation. FP Essent. 2023 Aug;531:22-26.[↩]
- Lasley BL, Crawford SL, Laughlin GA, Santoro N, McConnell DS, Crandall C, Greendale GA, Polotsky AJ, Vuga M. Circulating dehydroepiandrosterone sulfate levels in women who underwent bilateral salpingo-oophorectomy during the menopausal transition. Menopause. 2011 May;18(5):494-8. doi: 10.1097/gme.0b013e3181fb53fc[↩]
- Forti P, Maltoni B, Olivelli V, Pirazzoli GL, Ravaglia G, Zoli M. Serum dehydroepiandrosterone sulfate and adverse health outcomes in older men and women. Rejuvenation Res. 2012 Aug;15(4):349-58. doi: 10.1089/rej.2011.1248[↩][↩]
- Valenti G, Denti L, Maggio M, Ceda G, Volpato S, Bandinelli S, Ceresini G, Cappola A, Guralnik JM, Ferrucci L. Effect of DHEAS on skeletal muscle over the life span: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004 May;59(5):466-72. doi: 10.1093/gerona/59.5.m466[↩]
- Ravaglia G, Forti P, Maioli F, Boschi F, Cicognani A, Bernardi M, Pratelli L, Pizzoferrato A, Porcu S, Gasbarrini G. Determinants of functional status in healthy Italian nonagenarians and centenarians: a comprehensive functional assessment by the instruments of geriatric practice. J Am Geriatr Soc. 1997 Oct;45(10):1196-202. doi: 10.1111/j.1532-5415.1997.tb03769.x[↩]
- Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, Walston JD. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004 Apr;16(2):153-7. doi: 10.1007/BF03324545[↩]
- Legrain S, Massien C, Lahlou N, Roger M, Debuire B, Diquet B, Chatellier G, Azizi M, Faucounau V, Porchet H, Forette F, Baulieu EE. Dehydroepiandrosterone replacement administration: pharmacokinetic and pharmacodynamic studies in healthy elderly subjects. J Clin Endocrinol Metab. 2000 Sep;85(9):3208-17. doi: 10.1210/jcem.85.9.6805[↩][↩][↩]
- Ohlsson C, Labrie F, Barrett-Connor E, Karlsson MK, Ljunggren O, Vandenput L, Mellström D, Tivesten A. Low serum levels of dehydroepiandrosterone sulfate predict all-cause and cardiovascular mortality in elderly Swedish men. J Clin Endocrinol Metab. 2010 Sep;95(9):4406-14. doi: 10.1210/jc.2010-0760[↩]
- Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J Clin Pharmacol. 1999 Apr;39(4):327-48. doi: 10.1177/00912709922007903[↩]
- Nair KS, Rizza RA, O’Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ 3rd, Smith GE, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006 Oct 19;355(16):1647-59. doi: 10.1056/NEJMoa054629[↩][↩][↩]
- Baker WL, Karan S, Kenny AM. Effect of dehydroepiandrosterone on muscle strength and physical function in older adults: a systematic review. J Am Geriatr Soc. 2011 Jun;59(6):997-1002. doi: 10.1111/j.1532-5415.2011.03410.x[↩][↩]
- Kritz-Silverstein D, von Mühlen D, Laughlin GA, Bettencourt R. Effects of dehydroepiandrosterone supplementation on cognitive function and quality of life: the DHEA and Well-Ness (DAWN) Trial. J Am Geriatr Soc. 2008 Jul;56(7):1292-8. doi: 10.1111/j.1532-5415.2008.01768.x[↩][↩]
- Panjari M, Bell RJ, Jane F, Wolfe R, Adams J, Morrow C, Davis SR. A randomized trial of oral DHEA treatment for sexual function, well-being, and menopausal symptoms in postmenopausal women with low libido. J Sex Med. 2009 Sep;6(9):2579-90. doi: 10.1111/j.1743-6109.2009.01381.x[↩][↩][↩]
- Alkatib AA, Cosma M, Elamin MB, Erickson D, Swiglo BA, Erwin PJ, Montori VM. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. J Clin Endocrinol Metab. 2009 Oct;94(10):3676-81. doi: 10.1210/jc.2009-0672[↩][↩]
- Labrie F, Archer D, Bouchard C, Fortier M, Cusan L, Gomez JL, Girard G, Baron M, Ayotte N, Moreau M, Dubé R, Côté I, Labrie C, Lavoie L, Berger L, Gilbert L, Martel C, Balser J. Effect of intravaginal dehydroepiandrosterone (Prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause. 2009 Sep-Oct;16(5):923-31. doi: 10.1097/gme.0b013e31819e85c6[↩][↩]
- van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, Marshall JR, Miller AB, Rohan T, Smith-Warner SA, Speizer FE, Willett WC, Wolk A, Hunter DJ. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000 Sep 15;152(6):514-27. doi: 10.1093/aje/152.6.514[↩][↩]
- Maninger Nicole, Wolkowitz Owen M., Reus Victor I., Epel Elissa S., Mellon Synthia H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Frontiers in Neuroendocrinology. 2009;30(1):65–91. doi: 10.1016/j.yfrne.2008.11.002[↩]
- Sunderland Trey, Merril CarlR., Harrington MichaelG., Lawlor BrianA., Molchan SusanE., Martinez Rick, Murphy DennisL. REDUCED PLASMA DEHYDROEPIANDROSTERONE CONCENTRATIONS IN ALZHEIMER’S DISEASE. The Lancet. 1989;334(8662):570. doi: 10.1016/S0140-6736(89)90700-9[↩]
- Yanase T, Fukahori M, Taniguchi S, Nishi Y, Sakai Y, Takayanagi R, et al. Serum dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEA-S) in Alzheimer’s disease and in cerebrovascular dementia. Endocr J. 1996;43:119–123. doi: 10.1507/endocrj.43.119[↩]
- Bernardi F, Lanzone A, Cento RM, Spada RS, Pezzani I, Genazzani AD, et al. Allopregnanolone and dehydroepiandrosterone response to corticotropin-releasing factor in patients suffering from Alzheimer’s disease and vascular dementia. Eur J Endocrinol. 2000;142:466–471. doi: 10.1530/eje.0.1420466[↩]
- Hillen T, Lun A, Reischies FM, Borchelt M, Steinhagen-Thiessen E, Schaub RT. DHEA-S plasma levels and incidence of Alzheimer’s disease. Biol Psychiatry. 2000;47:161–163. doi: 10.1016/S0006-3223(99)00217-6[↩]
- Murialdo G, Barreca A, Nobili F, Rollero A, Timossi G, Gianelli MV, et al. Relationships between cortisol, dehydroepiandrosterone sulphate and insulin-like growth factor-I system in dementia. J Endocrinol Invest. 2001;24:139–146. doi: 10.1007/BF03343833[↩]
- Genedani S, Rasio G, Cortelli P, Antonelli F, Guidolin D, Galantucci M, et al. Studies on homocysteine and dehydroepiandrosterone sulphate plasma levels in Alzheimer’s disease patients and in Parkinson’s disease patients. Neurotox Res. 2004;6:327–332. doi: 10.1007/BF03033443[↩]
- Aldred S, Mecocci P. Decreased dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) concentrations in plasma of Alzheimer’s disease (AD) patients. Arch Gerontol Geriatr. 2010;51:e16–e18. doi: 10.1016/j.archger.2009.07.001[↩]
- Scheffers CS, Armstrong S, Cantineau AEP, Farquhar C, Jordan V. Dehydroepiandrosterone for women in the peri‐ or postmenopausal phase. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No.: CD011066. DOI: 10.1002/14651858.CD011066.pub[↩]
- NCAA Banned Substances. https://www.ncaa.org/sports/2015/6/10/ncaa-banned-substances.aspx[↩]
- The Prohibited List. https://www.wada-ama.org/en/prohibited-list?item-id=5027[↩]
- Labrie Fernand, Archer David, Bouchard Céline, Fortier Michel, Cusan Leonello, Gomez José-Luis, Girard Ginette, Baron Mira, Ayotte Normand, Moreau Michèle, Dubé Robert, Côté Isabelle, Labrie Claude, Lavoie Lyne, Berger Louise, Gilbert Lucy, Martel Céline, Balser John. Intravaginal dehydroepiandrosterone (Prasterone), the physiological and a highly efficient treatment of vaginal atrophy. Menopause. 2009;16(5):907–922. doi: 10.1097/gme.0b013e31819e8e2d[↩]
- Chen H, Jin Z, Sun C, Santos HO, Kord Varkaneh H. Effects of dehydroepiandrosterone (DHEA) supplementation on cortisol, leptin, adiponectin, and liver enzyme levels: A systematic review and meta-analysis of randomised clinical trials. Int J Clin Pract. 2021 Nov;75(11):e14698. doi: 10.1111/ijcp.14698[↩]
- Zhu Y, Qiu L, Jiang F, Găman MA, Abudoraehem OS, Okunade KS, Zhang M. The effect of dehydroepiandrosterone (DHEA) supplementation on estradiol levels in women: A dose-response and meta-analysis of randomized clinical trials. Steroids. 2021 Sep;173:108889. doi: 10.1016/j.steroids.2021.108889[↩]
- Krysiak R, Szkróbka W, Okopień B. Impact of dehydroepiandrosterone on thyroid autoimmunity and function in men with autoimmune hypothyroidism. Int J Clin Pharm. 2021 Aug;43(4):998-1005. doi: 10.1007/s11096-020-01207-w[↩]
- Wang X, Feng H, Fan D, Zou G, Han Y, Liu L. The influence of dehydroepiandrosterone (DHEA) on fasting plasma glucose, insulin levels and insulin resistance (HOMA-IR) index: A systematic review and dose response meta-analysis of randomized controlled trials. Complement Ther Med. 2020 Dec;55:102583. doi: 10.1016/j.ctim.2020.102583[↩]
- Li Y, Ren J, Li N, Liu J, Tan SC, Low TY, Ma Z. A dose-response and meta-analysis of dehydroepiandrosterone (DHEA) supplementation on testosterone levels: perinatal prediction of randomized clinical trials. Exp Gerontol. 2020 Nov;141:111110. doi: 10.1016/j.exger.2020.111110[↩]
- Peixoto C, José Grande A, Gomes Carrilho C, Nardi AE, Cardoso A, Barciela Veras A. Dehydroepiandrosterone for depressive symptoms: A systematic review and meta-analysis of randomized controlled trials. J Neurosci Res. 2020 Dec;98(12):2510-2528. doi: 10.1002/jnr.24721[↩]
- Qin Y, O Santos H, Khani V, Tan SC, Zhi Y. Effects of dehydroepiandrosterone (DHEA) supplementation on the lipid profile: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2020 Aug 28;30(9):1465-1475. doi: 10.1016/j.numecd.2020.05.015[↩]
- Sandoughi M, Kaykhaei MA, Langarizadeh E, Dashipour A. Effects of dehydroepiandrosterone on quality of life in premenopausal women with rheumatoid arthritis: A preliminary randomized clinical trial. Int J Rheum Dis. 2020 Dec;23(12):1692-1697. doi: 10.1111/1756-185X.13975[↩]
- Lin H, Li L, Wang Q, Wang Y, Wang J, Long X. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA supplementation of bone mineral density in healthy adults. Gynecol Endocrinol. 2019 Nov;35(11):924-931. doi: 10.1080/09513590.2019.1616175[↩]
- Chen SN, Tsui KH, Wang PH, Chern CU, Wen ZH, Lin LT. Dehydroepiandrosterone Supplementation Improves the Outcomes of in vitro Fertilization Cycles in Older Patients With Diminished Ovarian Reserve. Front Endocrinol (Lausanne). 2019 Nov 15;10:800. doi: 10.3389/fendo.2019.00800[↩]
- Gravisse N, Vibarel-Rebot N, Labsy Z, Do MC, Gagey O, Dubourg C, Audran M, Collomp K. Short-term Dehydroepiandrosterone Intake and Supramaximal Exercise in Young Recreationally-trained Women. Int J Sports Med. 2018 Sep;39(9):712-719. doi: 10.1055/a-0631-3008[↩]
- Chern CU, Tsui KH, Vitale SG, Chen SN, Wang PH, Cianci A, Tsai HW, Wen ZH, Lin LT. Dehydroepiandrosterone (DHEA) supplementation improves in vitro fertilization outcomes of poor ovarian responders, especially in women with low serum concentration of DHEA-S: a retrospective cohort study. Reprod Biol Endocrinol. 2018 Sep 17;16(1):90. doi: 10.1186/s12958-018-0409-z[↩]
- HEALTH PRODUCTS FOR SENIORS. “Anti-Aging” Products Pose Potential for Physical and Economic Harm. September 2001. https://ods.od.nih.gov/pubs/gao-01-1129.pdf[↩]
- Dietary Supplements for Exercise and Athletic Performance. https://ods.od.nih.gov/factsheets/ExerciseAndAthleticPerformance-HealthProfessional[↩]
- Arlt W. The approach to the adult with newly diagnosed adrenal insufficiency. J Clin Endocrinol Metab. 2009 Apr;94(4):1059-67. doi: 10.1210/jc.2009-0032[↩]
- Panjari M, Davis SR. DHEA for postmenopausal women: a review of the evidence. Maturitas. 2010 Jun;66(2):172-9. doi: 10.1016/j.maturitas.2009.12.017[↩]
- Labrie F, Bélanger A, Bélanger P, Bérubé R, Martel C, Cusan L, Gomez J, Candas B, Chaussade V, Castiel I, Deloche C, Leclaire J. Metabolism of DHEA in postmenopausal women following percutaneous administration. J Steroid Biochem Mol Biol. 2007 Feb;103(2):178-88. doi: 10.1016/j.jsbmb.2006.09.034[↩]
- Labrie F, Cusan L, Gomez JL, Martel C, Bérubé R, Bélanger P, Chaussade V, Deloche C, Leclaire J. Changes in serum DHEA and eleven of its metabolites during 12-month percutaneous administration of DHEA. J Steroid Biochem Mol Biol. 2008 May;110(1-2):1-9. doi: 10.1016/j.jsbmb.2008.02.003[↩]
- Marwah A, Marwah P, Lardy H. Ergosteroids. VI. Metabolism of dehydroepiandrosterone by rat liver in vitro: a liquid chromatographic-mass spectrometric study. J Chromatogr B Analyt Technol Biomed Life Sci. 2002 Feb 15;767(2):285-99. doi: 10.1016/s1570-0232(01)00570-0[↩]
- Prough RA, Clark BJ, Klinge CM. Novel mechanisms for DHEA action. J Mol Endocrinol. 2016 Apr;56(3):R139-55. doi: 10.1530/JME-16-0013[↩]
- Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984 Sep;59(3):551-5. doi: 10.1210/jcem-59-3-551[↩]
- Kalimi M, Regelson M 1990 The biological role of dehydroepiandrosterone (DHEA). New York: Walter de Gryter[↩][↩][↩]
- Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab. 1986 May;15(2):213-28. doi: 10.1016/s0300-595x(86)80021-4[↩][↩]
- Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007 Aug;92(8):3040-3. doi: 10.1210/jc.2007-0581[↩]
- Rosenfeld RS, Rosenberg BJ, Hellman L. Direct analysis of dehydroisoandrosterone in plasma. Steroids. 1975 Jun;25(6):799-805. doi: 10.1016/0039-128x(75)90044-6[↩]
- Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab. 1981 Jul;53(1):58-68. doi: 10.1210/jcem-53-1-58[↩]
- Filaire E, Duché P, Lac G. Effects of amount of training on the saliva concentrations of cortisol, dehydroepiandrosterone and on the dehydroepiandrosterone: cortisol concentration ratio in women over 16 weeks of training. Eur J Appl Physiol Occup Physiol. 1998 Oct;78(5):466-71. doi: 10.1007/s004210050447[↩]
- Copeland JL, Consitt LA, Tremblay MS. Hormonal responses to endurance and resistance exercise in females aged 19-69 years. J Gerontol A Biol Sci Med Sci. 2002 Apr;57(4):B158-65. doi: 10.1093/gerona/57.4.b158[↩]
- Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003 Jan-Feb;38(1-2):35-46. doi: 10.1016/s0531-5565(02)00146-8[↩]
- Roberts E. The importance of being dehydroepiandrosterone sulfate (in the blood of primates): a longer and healthier life? Biochem Pharmacol. 1999 Feb 15;57(4):329-46. doi: 10.1016/s0006-2952(98)00246-9[↩]
- Chen F, Knecht K, Birzin E, Fisher J, Wilkinson H, Mojena M, Moreno CT, Schmidt A, Harada S, Freedman LP, Reszka AA. Direct agonist/antagonist functions of dehydroepiandrosterone. Endocrinology. 2005 Nov;146(11):4568-76. doi: 10.1210/en.2005-0368[↩]
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998 Nov;23(8):963-87. doi: 10.1016/s0306-4530(98)00071-7[↩]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol. 2009 Jan;30(1):65-91. doi: 10.1016/j.yfrne.2008.11.002[↩]
- Liu D, Dillon JS. Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Galpha(i2,3). J Biol Chem. 2002 Jun 14;277(24):21379-88. doi: 10.1074/jbc.M200491200[↩]
- Liu D, Dillon JS. Dehydroepiandrosterone stimulates nitric oxide release in vascular endothelial cells: evidence for a cell surface receptor. Steroids. 2004 Apr;69(4):279-89. doi: 10.1016/j.steroids.2004.02.004[↩]
- Liu D, Iruthayanathan M, Homan LL, Wang Y, Yang L, Wang Y, Dillon JS. Dehydroepiandrosterone stimulates endothelial proliferation and angiogenesis through extracellular signal-regulated kinase 1/2-mediated mechanisms. Endocrinology. 2008 Mar;149(3):889-98. doi: 10.1210/en.2007-1125[↩]
- Labrie F, Luu-The V, Labrie C, Bélanger A, Simard J, Lin SX, Pelletier G. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003 Apr;24(2):152-82. doi: 10.1210/er.2001-0031[↩]
- Bulun SE, Simpson ER. Competitive reverse transcription-polymerase chain reaction analysis indicates that levels of aromatase cytochrome P450 transcripts in adipose tissue of buttocks, thighs, and abdomen of women increase with advancing age. J Clin Endocrinol Metab. 1994 Feb;78(2):428-32. doi: 10.1210/jcem.78.2.8106632[↩]
- Misso ML, Jang C, Adams J, Tran J, Murata Y, Bell R, Boon WC, Simpson ER, Davis SR. Adipose aromatase gene expression is greater in older women and is unaffected by postmenopausal estrogen therapy. Menopause. 2005 Mar;12(2):210-5. doi: 10.1097/00042192-200512020-00016[↩]
- DHEA-sulfate test. https://medlineplus.gov/ency/article/003717.htm[↩][↩]
- Dehydroepiandrosterone Sulfate. https://www.mayocliniclabs.com/api/sitecore/TestCatalog/DownloadTestCatalog?testId=113595[↩][↩][↩][↩][↩][↩][↩][↩]