Contents
- What is a diaphragmatic hernia
- Diaphragmatic hernia types
- Diaphragmatic hernia vs Hiatal hernia
- Diaphragmatic hernia causes
- Diaphragmatic hernia symptoms
- Diaphragmatic hernia complications
- Diaphragmatic hernia diagnosis
- Congenital diaphragmatic hernia treatment
- Congenital diaphragmatic hernia prognosis
- Congenital diaphragmatic hernia life expectancy
What is a diaphragmatic hernia
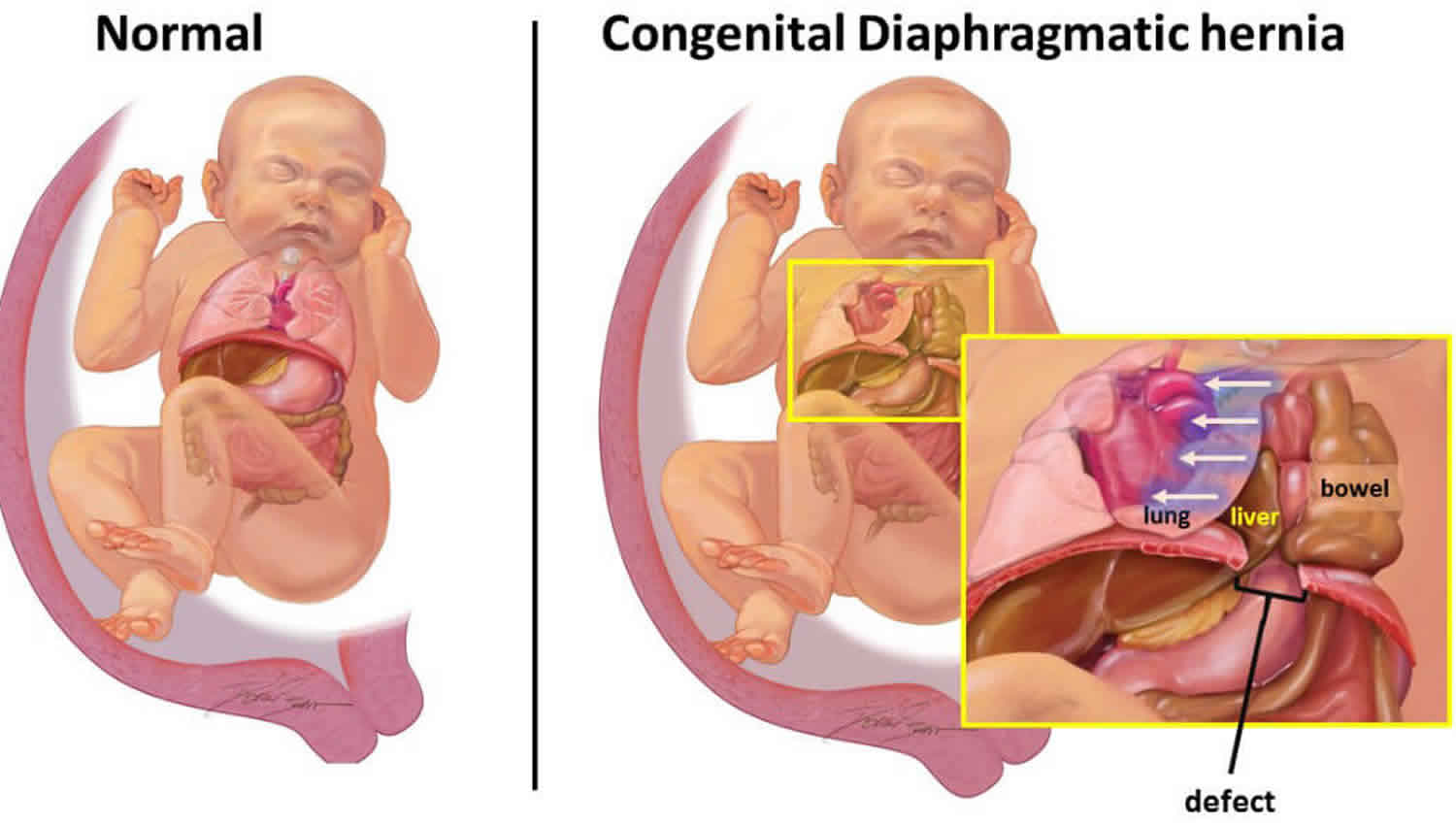
A diaphragmatic hernia is also called congenital diaphragmatic hernia, is a birth defect in which there is an abnormal opening in the diaphragm that allows part of the abdominal organs to migrate into the chest cavity 1. The diaphragm is the muscle between the chest and abdomen that helps you breathe. The opening allows part of the organs from the belly to move into the chest cavity near the lungs. Congenital diaphragmatic hernia affects approximately 1 in 2,500 newborns 2, although considerable variation has been reported with frequencies as low as one in 5000. The disparity in prevalence may be attributable to varying study methods such as different inclusion criteria and modes of ascertaining affected individuals.
In a growing embryo, the diaphragm is completely formed by 10 weeks of gestation. However in cases of congenital diaphragmatic hernia, the process that leads to formation of the diaphragm is disrupted. Once there is a hole present in the diaphragm, abdominal contents can move into the chest. This is called herniation. Because fetal activity and breathing movements become more frequent and vigorous as a pregnancy continues, the amount of herniation can fluctuate or increase.
Sometimes congenital diaphragmatic hernia is caused by a problem with a baby’s chromosomes or by a genetic disorder. If this is the case, the baby may have additional medical problems or organ abnormalities. In other instances, congenital diaphragmatic hernia may occur without an identifiable genetic cause. This is called isolated congenital diaphragmatic hernia, and under these circumstances the primary concern is the degree of pulmonary hypoplasia caused by the defect. In order to determine if congenital diaphragmatic hernia is isolated and to provide the most correct information about the disease, genetic testing is required.
When the diaphragm develops with a hole in it, the abdominal organs can pass into the chest cavity. The lung tissue on the affected side is compressed, fails to grow normally, and is unable to expand after birth. As the child begins to breathe, cry, and swallow, air enters the intestines that are protruding into the chest. The increasing size of the intestines puts pressure on the other side of the chest, lung, and heart and can quickly cause a life-threatening situation.
Congenital diaphragmatic hernia most often involves only one side of the diaphragm. It is more common on the left side. Often, the lung tissue and blood vessels in the area do not develop normally either. It is not clear if the diaphragmatic hernia causes the underdeveloped lung tissue and blood vessels, or the other way around.
About 50%-60% of affected individuals have isolated congenital diaphragmatic hernia; the remainder have complex congenital diaphragmatic hernia – that is, congenital diaphragmatic hernia occurring with additional malformations or as part of a single-gene disorder or chromosome abnormality 1. Infants with congenital diaphragmatic hernia often present in the neonatal period with severe respiratory distress; pulmonary hypoplasia is common. Presenting symptoms after infancy can be acute onset of respiratory or gastrointestinal distress or abdominal pain from chronic intestinal obstruction or pleural effusion from entrapment of the bowel in the chest.
Diaphragmatic hernia types
Congenital diaphragmatic hernias are often classified by their position. A Bochdalek hernia is a defect in the side or back of the diaphragm. Between 80 and 90 percent of congenital diaphragmatic hernias are of this type. A Morgnani hernia is a defect involving the front part of the diaphragm. This type of congenital diaphragmatic hernia, which accounts for approximately 2 percent of cases, is less likely to cause severe symptoms at birth. Other types of congenital diaphragmatic hernia, such as those affecting the central region of the diaphragm, or those in which the diaphragm muscle is absent with only a thin membrane in its place, are rare.
All hernia types can present with a sac (e.g., membranous sheet of tissue) covering the herniated abdominal contents. There currently is no explanation for the development of a hernia sac; however, its presence is thought to portend a better prognosis. Because a thin and redundant membranous diaphragm resulting from an eventration defect may represent a “sac,” it is probable that eventration and “sac type” congenital diaphragmatic hernia diagnoses are often interchanged. For this reason, it is dangerous to assume that the prognosis is better when a sac is visualized.
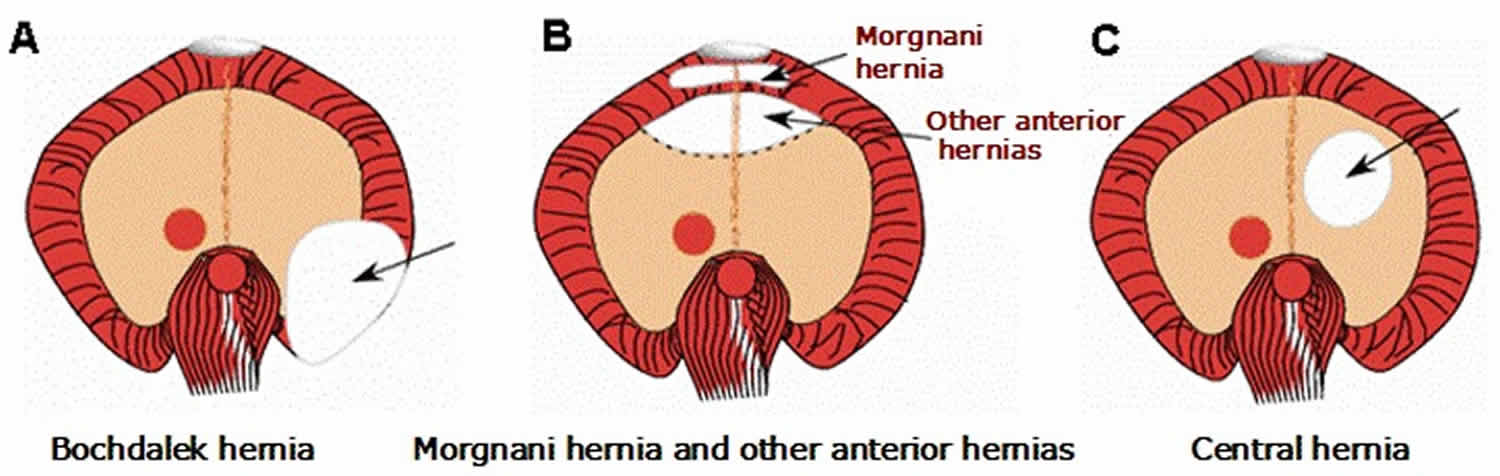
Figure 1. Diaphragmatic hernia types
Footnote: View of diaphragmatic defects from below.
A. Bochdalek hernia
B. Morgnani hernia and other anterior hernias
C. Central hernia
[Source 1 ]Posterolateral (Bochdalek) hernia
This posterolateral defect in the diaphragm, commonly referred to as a Bochdalek hernia, is often accompanied by herniation of the stomach, intestines, liver, and/or spleen into the chest cavity. An extremely large defect, or apparent absence of the hemidiaphragm, is called agenesis of the diaphragm; this defect probably represents the severe end of the Bochdalek hernia spectrum rather than a distinct entity.
Posterolateral hernias comprise approximately 80%-90% of all congenital diaphragmatic hernia and appear to fall into two types:
- A diaphragmatic defect accompanied by absent or extremely deficient rim of posterior and lateral musculature (see Figure 1A and 1B)
- A diaphragmatic defect with an intact rim of posterior and lateral musculature
About 85% of Bochdalek hernias occur on the left side, about 10% on the right, and approximately 5% are bilateral.
Non-posterolateral (non-Bochdalek) hernia
Anterior defects of the diaphragm can occur in the midline, on the left side, or the right side. Several distinct “subtypes” are described, but considerable overlap in the anatomic location among these defects exists. Furthermore, it is not clear whether these “subtypes” share a common embryologic mechanism.
- Morgagni (Morgagni-Larrey) hernia. Morgagni hernia is an anterior retrosternal or parasternal hernia that can result in the herniation of liver or intestines into the chest cavity (see Figure 1B). Morgagni hernias comprise approximately 2% of all congenital diaphragmatic hernia, are generally accompanied by a hernia sac, and often do not cause symptoms in the newborn period.
- Other anterior hernias associated with Pentalogy of Cantrell. These rare and severe types of hernias, possibly derived from the septum transversum, are found in individuals with Pentalogy of Cantrell (which also includes defects in the supraumbilical midline abdominal wall, lower sternum, diaphragmatic pericardium, and heart).
- Central hernia. This hernia is a rare diaphragm defect involving the central tendinous (e.g., amuscular) portion of the diaphragm. The entire rim of diaphragmatic musculature is present (see Figure 1C). Whether abnormal septum transversum development results in central as well as more anteriorly located hernias, and also whether central hernias can be distinguished from posterolateral hernias with a complete rim of musculature, is still a matter of debate.
Diaphragmatic eventration
Diaphragmatic eventration is incomplete muscularization of the diaphragm resulting in a thin membranous sheet of tissue. It is difficult to estimate the frequency of eventration because it may coexist with and/or be misdiagnosed as a Bochdalek hernia. Severe diaphragmatic eventration is associated with pulmonary hypoplasia and respiratory distress during infancy. Milder degrees of diaphragmatic eventration can present later in life with respiratory symptoms such as cough and pneumonias, or without symptoms so that the diagnosis is made incidentally on chest x-ray. Increasingly, it is observed that eventration of the diaphragm and “true” congenital diaphragmatic hernia can occur in the same individual, suggesting that in some instances they share a common cause.
Diaphragmatic hernia vs Hiatal hernia
A hiatal hernia is a condition in which the upper part of your stomach bulges through an opening in your diaphragm. Your diaphragm is the thin muscle that separates your chest from your abdomen. Your diaphragm helps keep acid from coming up into your esophagus. When you have a hiatal hernia, it’s easier for the acid to come up. This leaking of acid from your stomach into your esophagus is called GERD (gastroesophageal reflux disease). GERD (gastroesophageal reflux disease) may cause symptoms such as
- Heartburn
- Problems swallowing
- A dry cough
- Bad breath
- Nausea and/or vomiting
- Breathing problems
- The wearing away of your teeth
Often, the cause of a hiatal hernia is unknown. It may have to do with weakness in the surrounding muscles. Sometimes the cause is an injury or a birth defect. Your risk of getting a hiatal hernia goes up as you age; they are common in people over age 50. You are also at higher risk if you have obesity or smoke.
People usually find out that they have a hiatal hernia when they are getting tests for GERD (gastroesophageal reflux disease), heartburn, chest pain, or abdominal pain. The tests may be a chest x-ray, an x-ray with a barium swallow, or an upper endoscopy.
You don’t need treatment if your hiatal hernia does not cause any symptoms or problems. If you do have symptoms, some lifestyle changes may help. They include eating small meals, avoiding certain foods, not smoking or drinking alcohol, and losing weight. Your health care provider may recommend antacids or other medicines. If these don’t help, you may need surgery.
Diaphragmatic hernia causes
Congenital diaphragmatic hernia has many different causes. In 10 to 15 percent of affected individuals, the condition appears as a feature of a disorder that affects many body systems, called a syndrome. Donnai-Barrow syndrome, Fryns syndrome, and Pallister-Killian mosaic syndrome are among several syndromes in which congenital diaphragmatic hernia may occur. Some of these syndromes are caused by changes in single genes, and others are caused by chromosomal abnormalities that affect several genes.
About 25 percent of individuals with congenital diaphragmatic hernia that is not associated with a known syndrome also have abnormalities of one or more major body systems. Affected body systems can include the heart, brain, skeleton, intestines, genitals, kidneys, or eyes. In these individuals, the multiple abnormalities likely result from a common underlying disruption in development that affects more than one area of the body, but the specific mechanism responsible for this disruption is not clear.
Approximately 50 to 60 percent of congenital diaphragmatic hernia cases are isolated, which means that affected individuals have no other major malformations.
More than 80 percent of individuals with congenital diaphragmatic hernia have no known genetic syndrome or chromosomal abnormality. In these cases, the cause of the condition is unknown. Researchers are studying changes in several genes involved in the development of the diaphragm as possible causes of congenital diaphragmatic hernia. Some of these genes are transcription factors, which provide instructions for making proteins that help control the activity of particular genes (gene expression). Others provide instructions for making proteins involved in cell structure or the movement (migration) of cells in the embryo. Environmental factors that influence development before birth may also increase the risk of congenital diaphragmatic hernia, but these environmental factors have not been identified.
Isolated congenital diaphragmatic hernia is rarely inherited. In almost all cases, there is only one affected individual in a family.
When congenital diaphragmatic hernia occurs as a feature of a genetic syndrome or chromosomal abnormality, it may cluster in families according to the inheritance pattern for that condition.
Diaphragmatic hernia symptoms
Severe breathing problems almost always develop shortly after the baby is born. This is due in part to poor movement of the diaphragm muscle and crowding of the lung tissue. Problems with breathing and oxygen levels are often due to underdeveloped lung tissue and blood vessels as well.
Other symptoms include:
- Bluish colored skin due to lack of oxygen
- Rapid breathing (tachypnea)
- Fast heart rate (tachycardia)
Congenital diaphragmatic hernia and pulmonary hypoplasia
A baby with congenital diaphragmatic hernia may suffer from a form of underdeveloped lungs known as pulmonary hypoplasia.
When pulmonary hypoplasia occurs, there are abnormalities that impact:
- The number of air sacs (alveoli) available for air entry into the lungs
- The distance that oxygen has to travel to reach the blood vessels in the lungs
- The amount of blood that can be carried in the blood vessels in the lungs (pulmonary hypertension)
Before birth, the placenta takes over all functions of the lungs so a fetus can grow in the womb without suffering low oxygen levels (hypoxemia). However, after birth, the baby depends on the function of the lungs, and if their underdevelopment is severe, artificial ventilation techniques will be necessary. congenital diaphragmatic hernia can appear on the left side, right side or rarely on both sides of the chest. congenital diaphragmatic hernia occurs in about 1 in 2500 live births.
In 5 to 10 percent of affected individuals, signs and symptoms of congenital diaphragmatic hernia appear later in life and may include breathing problems or abdominal pain from protrusion of the intestine into the chest cavity 2. In about 1 percent of cases, congenital diaphragmatic hernia has no symptoms; it may be detected incidentally when medical imaging is done for other reasons 2.
Diaphragmatic hernia complications
Diaphragmatic hernia complications may include:
- Lung infections
- Other congenital problems
Associated anomalies are present in 10-50% of patients with congenital diaphragmatic hernia; patients with these anomalies have a twofold relative risk of mortality when compared with patients with isolated congenital diaphragmatic hernias 3. Frequently associated anomalies include cardiac defects, chromosomal anomalies (i.e., trisomies 21, 18, and 13), renal anomalies, genital anomalies, and neural tube defects.
Morbidity
Multi-organ morbidity is considerable among survivors, even those with seemingly isolated congenital diaphragmatic hernia. For many survivors, congenital diaphragmatic hernia is truly a chronic disease, though reports of essentially normal or near-normal long-term outcomes are increasing. It can be difficult to determine whether certain abnormalities are intrinsic to the condition or secondary to treatment. The most vulnerable organ systems include the following:
- Pulmonary. Almost all individuals with congenital diaphragmatic hernia have some degree of pulmonary hypoplasia. Many infants require oxygen supplementation and diuretics following surgical correction of congenital diaphragmatic hernia. Given the remarkable growth and recuperative capacity of the lung, these treatments can usually be discontinued within the first two years of life. By early childhood, few children have respiratory symptoms at rest; however, formal testing even in older children shows small airway obstruction and diminished blood flow on ventilation-perfusion (V-Q) scan, especially to the lung ipsilateral to the hernia. Limited exercise tolerance can be a lifelong problem. Intermittent wheezing requiring bronchodilator use is common in persons with congenital diaphragmatic hernia, and they are at risk for respiratory decompensation with intercurrent illness. It is not clear whether the severity of long term pulmonary morbidity can be predicted based on the severity of the perinatal respiratory disease. The long-term clinical significance of abnormal pulmonary function testing in these children will be better defined as a greater number of severely affected children survive 4.
- Gastrointestinal. “Failure to thrive” with growth parameters less than the third centile of normal is common among infants with more significant pulmonary hypoplasia and/or a more prolonged hospitalization following surgical repair of congenital diaphragmatic hernia. Growth failure is caused, in large part, by oral aversion and feeding difficulties (often requiring gastrostomy tube insertion for the first few years of life) and gastroesophageal reflux (frequently requiring pharmacotherapy and/or surgical fundoplication). Some infants and children require long-term high-calorie nutritional supplements.
- Neurologic/developmental. In early reports of long-term survivors, neurologic abnormalities and mild-to-moderate developmental delay were common, especially among those receiving ECMO (extra-corporeal membrane oxygenation) treatment. Information about developmental outcomes using more current practice standards is limited by lack of prospective studies testing with standard developmental assessment tools. However, emerging data indicate that most children who are not diagnosed with a chromosome abnormality or syndrome have full-scale IQ scores in the low-average or average range, with virtually none having intellectual disability (i.e., IQ score <70) 5. However, these children with normal IQs remain vulnerable to learning disabilities, attention problems, and behavior problems 6. Non-focal neurologic abnormalities such as hypotonia are common, as are motor problems, especially in ECMO survivors 7. Nonspecific findings such as cortical atrophy, ventriculomegaly, and intracranial hemorrhage can be seen on neuroimaging studies 8.
- Musculoskeletal. Chest asymmetry is found in as many as half of individuals with congenital diaphragmatic hernia. Pectus, most often of the excavatum type, and scoliosis (≥10° Cobb’s angle) are found in approximately 25% of individuals. These musculoskeletal abnormalities occur more often following repair of large diaphragmatic defects, possibly as a result of the extra tension exerted on the chest wall during surgical repair.
- Hearing loss. Sensorineural hearing loss has been found in 25% of individuals with congenital diaphragmatic hernia and as many as 100% of individuals treated with ECMO in some series 9. One study of ECMO survivors showed that sensorineural hearing loss, often of delayed onset, was 2.5 times more common among those requiring ECMO for congenital diaphragmatic hernia than for other indications. Prolonged treatment with aminoglycosides and ECMO increased the risk for sensorineural hearing loss, independent of the indication for the use of ECMO. Among this cohort, 60% of the congenital diaphragmatic hernia survivors had sensorineural hearing loss that was often severe. Other factors such as use of nitric oxide, prolonged or high-frequency mechanical ventilation, and/or metabolic alkalosis could also contribute to the development of sensorineural hearing loss 10.
- Reherniation. At least 10% of individuals reherniate; the risk is considerably greater among those whose hernia repair required a prosthetic patch.
Diaphragmatic hernia diagnosis
More than 50% of cases with congenital diaphragmatic hernia are detected prenatally by ultrasound examination. Detection of congenital diaphragmatic hernia may come during a routine ultrasound, which may reveal excess amniotic fluid and/or abdominal contents in the fetal chest cavity. To confirm a prenatal diagnosis of congenital diaphragmatic hernia, doctors may perform a very detailed ultrasound, conduct testing of the fetus’s chromosomes and take measurements of its lung size. During the ultrasound examination, doctors focus on specific findings that may point to the presence of a syndrome. The genetic testing is performed by amniocentesis.
The lung size is then measured and compared to the expected size at this stage of a pregnancy. This can be done by measuring the lung area to head circumference ratio (LHR) or comparing the observed/expected LHR (o/e LHR). It is also important to determine whether the liver has also moved into the chest. Based on these measurements, specialists can grade the severity of congenital diaphragmatic hernia as mild, moderate or severe. Specialized imaging techniques including magnetic resonance imaging (MRI) are used to help achieve the most accurate assessment.
MRI is especially useful for the prenatal diagnosis of thoracic lesions that are atypical or complicated by multiple abnormalities and for assessing lung volumes 11.
Congenital diaphragmatic hernia may also be diagnosed after birth — often if a newborn is having trouble breathing.
In the newborn, the abdomen is scaphoid; chest x-ray confirms the diagnosis of congenital diaphragmatic hernia when bowel gas visible above the diaphragm is accompanied by a mediastinal shift.
An exam of the infant shows:
- Irregular chest movements
- Lack of breath sounds on side with the hernia
- Bowel sounds that are heard in the chest
- Abdomen that looks less protuberant than a normal newborn’s and feels less full when touched
A chest x-ray may show abdominal organs in the chest cavity.
Congenital diaphragmatic hernia treatment
A diaphragmatic hernia is an emergency that requires surgery. Surgery is done to place the abdominal organs into the proper position and repair the opening in the diaphragm. However, surgery after delivery does not address the lung damage that has already occurred. For this reason, fetal therapeutic procedures are recommended in some pregnancies. These procedures may help decrease the amount of lung damage that can occur during the pregnancy. The goal of fetal treatment is to reverse some of the lung damage that results from compression of the lungs.
The infant will need breathing support during the recovery period. Some infants are placed on a heart/lung bypass machine to help deliver enough oxygen to the body.
Newborns with congenital diaphragmatic hernia are intubated immediately to avoid bag-mask ventilation and inflation of the bowel that has herniated into the chest; care is taken to minimize barotraumas induced by positive pressure ventilation. Correction of hypercapnea and pre-ductal hypoxemia are focused on assuring adequate end-organ perfusion. Infants with congenital diaphragmatic hernia are treated with minimal sedation and pressure support modes of ventilation; some centers use high-frequency oscillatory ventilation. Extra-corporeal membrane oxygenation (ECMO) is used in some centers for neonates with critical cardiopulmonary deterioration. The ex-utero intrapartum treatment (EXIT) procedure transitions a newborn directly onto cardiopulmonary bypass when oxygenation and ventilation by intubation and mechanical ventilation are either not expected to be possible, or are likely to exacerbate pulmonary barotrauma. Other therapies that have been introduced in the acute neonatal treatment phase for congenital diaphragmatic hernia include nitric oxide (NO), delay of surgical repair, and use of surfactant and perflubron. Fetal surgery has been attempted for severely affected infants but is controversial. Tracheal occlusion is being offered to high-risk fetuses in Europe and in few specialized centers in the United States. Long-term follow-up for infants with congenital diaphragmatic hernia is best provided at specialized centers.
If a diaphragmatic hernia is diagnosed early during pregnancy (before 24 to 28 weeks), fetal surgery may be an option in some situations.
The indications for a diaphragmatic hernia repair include:
- chest X-rays showing diaphragmatic hernia
- severe breathing difficulty (respiratory distress) shortly after birth
- prenatal ultrasound often identifies a diaphragmatic hernia
Complications observed in the early postoperative period include recurrent pulmonary hypertension and deterioration in respiratory mechanics and gaseous exchange. Less commonly observed complications include recurrence of the congenital diaphragmatic hernia, which is more common with patch repair 12; leakage of peritoneal fluid and blood into the thorax; and development of an ipsilateral hydrothorax. Small-bowel obstruction may occur secondary to adhesions or volvulus.
Fetal Treatment for Congenital Diaphragmatic Hernia
Fetoscopic tracheal occlusion (FETO)
The fetal lungs produce fluid that leaves the body through the baby’s mouth. If this outflow of fluid is blocked, it has nowhere to go and swells up in the affected lung. When this occurs over a period of four to five weeks, the lung expands and its function appears to improve. This type of blockage can be achieved by temporarily blocking the fetal windpipe (trachea) with a balloon for a period of time. This is done by performing operative fetoscopy, known as fetoscopic tracheal occlusion. It is believed that fetoscopic tracheal occlusion works by increasing the lung maturation and reversing some of the damaging effects of congenital diaphragmatic hernia on lung function.
Fetal surveillance and delivery planning
There is a high possibility that a baby with congenital diaphragmatic hernia will get worse before the anticipated due date. Part of a comprehensive treatment plan will involve close fetal and maternal monitoring to avoid severe fetal deterioration and to determine the circumstances and timing for optimal delivery.
Congenital Diaphragmatic Hernia Delivery
Extracorporeal membrane oxygenation (ECMO) is an advanced form of respiratory support where a machine takes over all the functions of the lungs. Some newborns with congenital diaphragmatic hernia will require neonatal ECMO to help them breathe.
Ex-utero intrapartum treatment (EXIT) is a specialized delivery method where the baby is placed on breathing or circulation support before the umbilical cord is cut. This allows the mother to provide oxygen to the baby through the placenta until the machine is ready to take over. EXIT or ECMO may be recommended for some babies with congenital diaphragmatic hernia.
Long-Term Monitoring
Continued care is provided for survivors of congenital diaphragmatic hernia by a multidisciplinary team consisting of a social worker, a nutritionist, a physiotherapist, a pediatrician/neonatologist, a neurologist, and a pediatric surgeon.
The following screening tests could be performed before discharge:
- Chest radiography
- Arterial blood gas evaluation
- Brainstem auditory evoked potentials
- Computed tomography (CT) or ultrasonography of the head
- Developmental evaluation
In the outpatient clinic, chest radiography, pulmonary function tests, nutritional and developmental assessments, and repeated auditory, ophthalmology, and neurology evaluations are performed.
Congenital diaphragmatic hernia prognosis
The prognosis of surgery depends on how well the baby’s lungs have developed. It also depends on whether there are any other congenital problems. Most often the outlook is good for infants who have a sufficient amount of working lung tissue and have no other problems.
Medical advances have made it possible for over half of infants with this condition to survive. The babies survived will often have ongoing challenges with breathing, feeding, and growth.
Long-term pulmonary disease depends on the degree of pulmonary hypoplasia, barotrauma, and volutrauma sustained in the neonatal period. Bronchopulmonary dysplasia and restrictive and/or obstructive lung disease may be observed 13.
Failure to thrive is often observed in the presence of optimal feeding regimes.
Functional and anatomic esophageal abnormalities are associated with significant gastroesophageal reflux (GER) in 40% of survivors 14. Prophylactic fundoplication at the time of primary repair for infants requiring a patch repair is advocated by some team as a means of preventing growth disorders or failure to thrive in this subset of patients. Other patients who may go on to require fundoplication include the neurologically impaired and those with chronic lung disease. Most other infants outgrow gastroesophageal reflux 15.
The use of extracorporeal membrane oxygenation (ECMO), hyperventilation treatment, and ototoxic medication places this population at a higher risk for sensorineural hearing loss, as well as for neurodevelopmental abnormalities (ie, cognitive and developmental delay, cerebral palsy, seizure disorders, impaired vision).
Altered musculoskeletal development results in thoracic scoliosis, pectus deformities, and a decreased thoracic cavity on the affected side.
In late childhood and adolescence, learning disability, developmental disability, and attention deficit hyperactivity disorder are noted in approximately 50% of patients. In addition to cognitive and attention deficits, behavioral problems are seen. Only a third of these children require placement in a special educational class 16.
Congenital diaphragmatic hernia life expectancy
Mortality from congenital diaphragmatic hernia continues to be high, ranging from 20% to 60% 1. Data from neonatal or referral centers operating on relatively selected cases, primarily those with isolated left-sided Bochdalek hernia, report 80%-90% survival 17. However, population-based studies of outcome for all prenatally diagnosed congenital diaphragmatic hernia cases report mortality of at least 50%, if pregnancy terminations are included 18. In a meta-analysis, Stege et al 19 observed that approximately one quarter of all prenatally diagnosed cases were electively terminated, 3% spontaneously miscarried, and 3% were stillborn; 31% of the liveborns died, the majority within the first 24 hours of life.
The key determinants of mortality are:
- Whether the congenital diaphragmatic hernia is isolated or complex. Higher mortality occurs with complex congenital diaphragmatic hernia associated with a chromosome abnormality, a single-gene disorder, and/or the coexistence of major malformations. The presence of a cardiovascular malformation also indicates a worse prognosis 20.
- The size of the diaphragm defect 21
- The degree of pulmonary hypoplasia
- Whether the liver is up in the chest or remains down below the diaphragm 22. Individuals with a large amount of “liver up” congenital diaphragmatic hernia have higher mortality than those whose liver remains down below the diaphragm.
- The severity of pulmonary hypertension in the perinatal period. Pulmonary hypertension, which may progress to a late or chronic phase, is often not responsive to medical therapy 23.
- Whether the hernia is right-sided, left-sided, or bilateral. Some, but not all, studies show that a right-sided hernia is associated with greater mortality than a left-sided hernia 24. Bilateral congenital diaphragmatic hernia always confers a high mortality.
- Pober BR, Russell MK, Ackerman KG. Congenital Diaphragmatic Hernia Overview. 2006 Feb 1 [Updated 2010 Mar 16]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2019. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1359[↩][↩][↩][↩]
- Congenital diaphragmatic hernia. https://ghr.nlm.nih.gov/condition/congenital-diaphragmatic-hernia[↩][↩][↩]
- Tonks A, Wyldes M, Somerset DA, Dent K, Abhyankar A, Bagchi I, et al. Congenital malformations of the diaphragm: findings of the West Midlands Congenital Anomaly Register 1995 to 2000. Prenat Diagn. 2004 Aug. 24 (8):596-604.[↩]
- Basek P, Bajrami S, Straub D, Moeller A, Baenziger O, Wildhaber J, Bernet V. The pulmonary outcome of long-term survivors after congenital diaphragmatic hernia repair. Swiss Med Wkly. 2008;138:173–9[↩]
- Bouman NH, Koot HM, Tibboel D, Hazebroek FW. Children with congenital diaphragmatic hernia are at risk for lower levels of cognitive functioning and increased emotional and behavioral problems. Eur J Pediatr Surg. 2000;10:3–7[↩]
- Peetsold MG, Huisman J, Hofman VE, Heij HA, Raat H, Gemke RJ. Psychological outcome and quality of life in children born with congenital diaphragmatic hernia. Arch Dis Child. 2009;94:834–40[↩]
- Nijhuis-van der Sanden MW, van der Cammen-van Zijp MH, Janssen AJ, Reuser JJ, Mazer P, van Heijst AF, Gischler SJ, Tibboel D, Kollée LA. Motor performance in five-year-old extracorporeal membrane oxygenation survivors: a population-based study. Crit Care. 2009;13:R47[↩]
- Rasheed A, Tindall S, Cueny DL, Klein MD, Delaney-Black V. Neurodevelopmental outcome after congenital diaphragmatic hernia: Extracorporeal membrane oxygenation before and after surgery. J Pediatr Surg. 2001;36:539–44.[↩]
- Robertson CM, Tyebkhan JM, Hagler ME, Cheung PY, Peliowski A, Etches PC. Late-onset, progressive sensorineural hearing loss after severe neonatal respiratory failure. Otol Neurotol. 2002;23:353–6.[↩]
- Fligor BJ, Neault MW, Mullen CH, Feldman HA, Jones DT. Factors associated with sensorineural hearing loss among survivors of extracorporeal membrane oxygenation therapy. Pediatrics. 2005;115:1519–28[↩]
- Matsuoka S, Takeuchi K, Yamanaka Y, Kaji Y, Sugimura K, Maruo T. Comparison of magnetic resonance imaging and ultrasonography in the prenatal diagnosis of congenital thoracic abnormalities. Fetal Diagn Ther. 2003;18:447–53[↩]
- St Peter SD, Valusek PA, Tsao K, Holcomb GW 3rd, Ostlie DJ, Snyder CL. Abdominal complications related to type of repair for congenital diaphragmatic hernia. J Surg Res. 2007 Jun 15. 140 (2):234-6.[↩]
- Diaphragmatic Hernias. https://emedicine.medscape.com/article/934824-overview[↩]
- Koivusalo AI, Pakarinen MP, Lindahl HG, Rintala RJ. The cumulative incidence of significant gastroesophageal reflux in patients with congenital diaphragmatic hernia-a systematic clinical, pH-metric, and endoscopic follow-up study. J Pediatr Surg. 2008 Feb. 43(2):279-82.[↩]
- Dariel A, Rozé JC, Piloquet H, Podevin G, French CDH Study Group. Impact of prophylactic fundoplication on survival without growth disorder in left congenital diaphragmatic hernia requiring a patch repair. J Pediatr. 2010 Oct. 157 (4):688-90, 690.e1.[↩]
- Frisk V, Jakobson LS, Unger S, Trachsel D, O’Brien K. Long-term neurodevelopmental outcomes of congenital diaphragmatic hernia survivors not treated with extracorporeal membrane oxygenation. J Pediatr Surg. 2011 Jul. 46 (7):1309-18.[↩]
- Downard CD, Jaksic T, Garza JJ, Dzakovic A, Nemes L, Jennings RW, Wilson JM. Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg. 2003;38:729–32[↩]
- Colvin J, Bower C, Dickinson JE, Sokol J. Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics. 2005;116:e356–63.[↩]
- Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics. 2003;112:532–5.[↩]
- Cohen MS, Rychik J, Bush DM, Tian ZY, Howell LJ, Adzick NS, Flake AW, Johnson MP, Spray TL, Crombleholme TM. Influence of congenital heart disease on survival in children with congenital diaphragmatic hernia. J Pediatr. 2002;141:25–30.[↩]
- Congenital Diaphragmatic Hernia Study Group. Defect size determines survival in infants with congenital diaphragmatic hernia. Pediatrics. 2007;120:e651–7[↩]
- Albanese CT, Lopoo J, Goldstein RB, Filly RA, Feldstein VA, Calen PW, Jennings RW, Farrell JA, Harrison MR. Fetal liver position and perinatal outcome for congenital diaphragmatic hernia. Prenat Diagn. 1998;18:1138–42[↩]
- Kinsella JP, Ivy DD, Abman SH. Pulmonary vasodilator therapy in congenital diaphragmatic hernia: acute, late, and chronic pulmonary hypertension. Semin Perinatol. 2005;29:123–8.[↩]
- Skari H, Bjornland K, Haugen G, Egeland T, Emblem R. Congenital diaphragmatic hernia: a meta-analysis of mortality factors. J Pediatr Surg. 2000;35:1187–97[↩]