Contents
- Drug induced liver injury
- Hy’s law
- Liver anatomy
- Types of Drug-Induced Liver Injury
- Drug induced liver injury causes
- Pathophysiology and mechanisms of drug-induced liver injury
- Drug induced liver injury signs and symptoms
- Drug induced liver injury complications
- Drug induced liver injury diagnosis
- Drug induced liver injury treatment
- Drug induced liver injury prognosis
Drug induced liver injury
Drug-induced liver injury or ‘DILI’, is a rare, unpredictable, and potentially life-threatening acute liver failure caused by adverse drug reaction 1, 2. Drug-induced liver injury (DILI) encompasses the unexpected harms that prescription and non-prescription drugs, herbal and dietary supplements can cause to the liver 3. Drug-induced liver injury (DILI) is a frequent cause of hepatitis (approximately 10% of all cases of acute hepatitis) 4 and hospitalization 5 and is implicated in 5%-10% of all patients hospitalized for jaundice 6, accounting for 95% of adverse drug reactions and 14.6% of drug fatalities in Denmark 7. In the United States, approximately 2000 cases of acute liver failure occur annually and drugs account for over 50% of them (39% are due to acetaminophen, 13% are idiosyncratic reactions due to other medications) 8.
Drug-induced liver injury (DILI), has been the major reason for denial of approval, withdrawal from the market, or “black box” warnings for many drugs and complementary and alternative medicines (CAMs), by the U.S. Food and Drug Administration (FDA) 9, 10, 11.
More than 1100 drugs, herbal remedies, natural products, vitamins, minerals, dietary supplements, and recreational and illicit compounds have been reported to cause DILI (albeit, some only occasionally) 12, 13, 14, 15. DILI prevalences are mostly less than 1 in 100,000 to 1 in 10,000 patients, but are occasionally more frequent 16. In a population-based study from a rural area in France, the crude global incidence of DILI was 13.9 cases/100,000 population—a rate 16-fold higher than reported to regulatory authorities 17. Four of 34 (11.8%) patients in that study were hospitalized, and two (5.9%) died 17. In a 1990s United Kingdom-based survey 5, DILI requiring specialist referral affected 2.4 cases/100,000 person-years (similar to the 1980s DILI incidence in Denmark) 7, of whom 36/128 (28.2%) were hospitalized, but only one required liver transplantation.
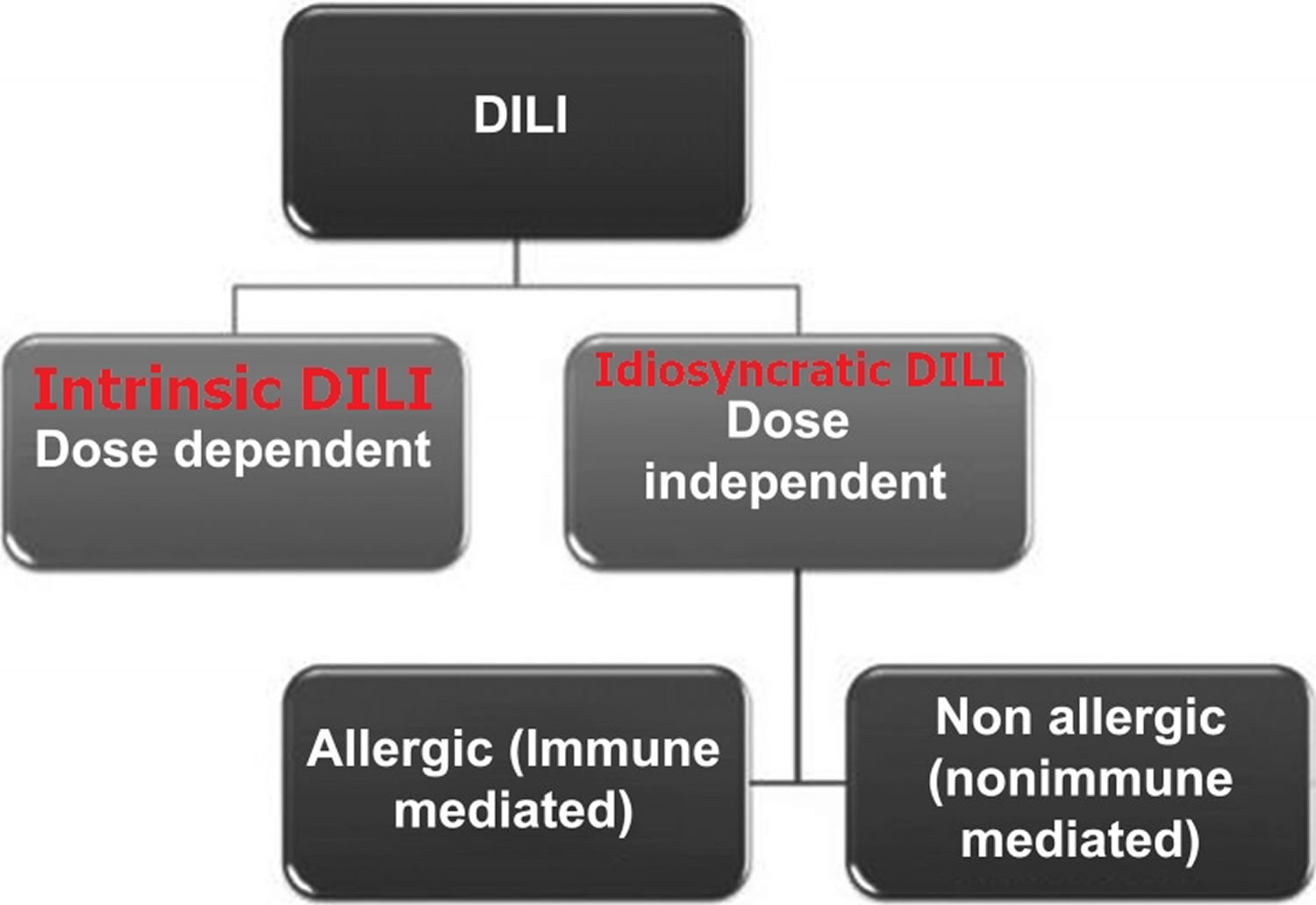
Drug‐induced liver injury (DILI) has been classically divided into two varieties based on the presumed mechanism of action of the chemical compound: intrinsic DILI and idiosyncratic DILI 18. The intrinsic DILI (or direct, predictable DILI) is dose‐related and occurs shortly after exposure (hours to days) in the majority of individuals exposed to the drug (which is toxic at a dose threshold level) 3. In contrast, the idiosyncratic DILI (unpredictable DILI) is multifactorial and unpredictable drug induced liver injury and does not correlate with the dose and usually occurs in less than 1 of every 1000 to 1 of every 100,000 exposed individuals 19, 20 and with a longer latency period (from a few days to several months) 21, 22. Idiosyncratic DILI is further subdivided into two sub-categories: allergic (immune-mediated) and non-allergic (nonimmune-mediated) as presented in Figure 1 21, 23, 24. If immune-mediated, the latency is shorter (1–6 weeks) compared with non-immune mediated reactions (1 month to 1 year) 25. However, exceptions do occur with immune reactions appearing after a very long latency with drugs such as nitrofurantoin 26 or even after drug cessation (eg sulfonamides, erythromycin and amoxicillin-clavulanate) 25. Immune-mediated idiosyncratic reactions can be characterized by presence of fever, rash, eosinophilia, autoantibodies (such as antinuclear and anti-smooth muscle antibodies) and Stevens-Johnson syndrome 27. Non-immune mediated reactions lack the aforementioned characteristics and importantly, as described, tend to have a long latency period (1 month to 1 year) 25, 28. The incidence of idiosyncratic DILI is higher in women (59%) when compared to men which may be due to hormonal interactions with immunomodulating drugs or differing pharmacokinetics 29.
Recently, a third type of DILI—indirect DILI—has been described as a liver injury caused by the mechanism of action of the drug (what it does; i.e., rituximab by depletion of B cells reactivates silent hepatitis B virus which is responsible for the damage), in contrast to its idiosyncratic properties (what it is; i.e., the unexpected hepatotoxic reaction of amoxicillin-clavulanate) 30.
Other forms of DILI include steatohepatitis (amiodarone, tamoxifen, methotrexate) 31, neoplasms (hepatic adenomas due to androgenic anabolic steroids) 32 and vascular (nodular regenerating hyperplasia due to azathioprine) 33.
Figure 1. Drug‐induced liver injury (DILI) types
[Source 12 ]Table 1. Classification of drug‐induced liver injury (DILI) based on liver enzyme derangement
| Phenotype | Case definition | Commonly implicated agents |
|---|---|---|
| Idiosyncratic | Hepatocellular: If ALT alone is elevated less than fivefold above ULN or R≥5. Cholestatic: ALP alone is elevated less than twofold above ULN or R≤2. Mixed: R>2 to<5 Chronic DILI: DILI with acute presentation, with evidence of persistent liver injury at >1 year after its onset | Antimicrobials, anticonvulsants, antiarrhythmic, androgens, oestrogens/progesterone, immunomodulatory and antineoplastic |
| Drug reaction with eosinophilia and systemic symptoms | Drug-related hypersensitivity with eosinophilia and systemic inflammation | Anticonvulsants, NRTIs |
| Drug-induced autoimmune hepatitis | Acute DILI with serological and/or histological features of AIH | NSAIDs, statins, minocycline and nitrofurantoin |
| Secondary sclerosing cholangitis | Presenting as acute DILI with histological/radiological features of sclerosing cholangiopathy | Inhalational anaesthetics, atorvastatin, 6-MP |
| Granulomatous hepatitis | Granulomas on histology with exposure to implicated agent(s) | Anticonvulsants, sulphonamides |
| Acute fatty liver | Acute development of microvesicular steatohepatitis | Reverse transcriptase inhibitors |
| Drug-associated fatty liver disease | Consistent with NAFLD and attributable exposure | Methotrexate, corticosteroids, 5-FU |
| Nodular regenerative hyperplasia | Diffuse nodularity organised around central hepatocytes | Antineoplastic/cytotoxic |
| Ductopaenia | Chronic cholestasis and ductular loss | Antimicrobials (β-lactams, tetracyclines and sulphonamides) |
| Liver tumors | Features of hepatocellular adenoma or carcinoma dependent of histological/imaging characteristics | Anabolic androgenic steroids and oral contraceptives |
Abbreviations: 5-FU = 5-fluorouracil; 6-MP = 6-mercaptopurine; AIH = autoimmune hepatitis; ALP = alkaline phosphatase; ALT = alanine transferase; DILI = drug-induced liver injury; NAFLD = non-alcoholic fatty liver disease; NRTI = nucleoside reverse transcriptase inhibitor; NSAID = non-steroidal antiinflammatory drug; ULN = upper limit of normal.
[Source 34 ]Table 2. Drug‐induced liver injury (DILI) severity classifications
| Category | Severity | Description |
|---|---|---|
| US Drug-Induced Liver Injury Network 35 | ||
| 1 | Mild | Elevated ALT and/or ALP but total bilirubin <2.5 mg/dl and INR <1.5 |
| 2 | Moderate | Elevated ALT and/or ALP and total bilirubin ≥2.5 mg/dl or INR ≥1.5 |
| 3 | Moderate-severe | Elevated ALT, ALP, total bilirubin and/or INR and hospitalization or ongoing hospitalization prolonged due to DILI |
| 4 | Severe | Elevated ALT and/or ALP and total bilirubin ≥2.5 mg/dl and at least 1 of the following criteria: – Hepatic failure (INR >1.5, ascites or encephalopathy) – Other organ failure due to DILI |
| 5 | Fatal | Death or liver transplantation due to DILI |
| International DILI Expert Working Group 36 | ||
| 1 | Mild | ALT ≥5 or ALP ≥2 and total bilirubin <2 × upper limit of normal (ULN) |
| 2 | Moderate | ALT ≥5 or ALP ≥2 and total bilirubin ≥2 × upper limit of normal (ULN), or symptomatic hepatitis |
| 3 | Severe | ALT ≥5 or ALP ≥2 and total bilirubin ≥2 × upper limit of normal (ULN), or symptomatic hepatitis and 1 of the following criteria: – INR ≥1.5 – Ascites and/or encephalopathy, disease duration <26 weeks, and absence of underlying cirrhosis – Other organ failure due to DILI |
| 4 | Fatal/transplantation | Death or liver transplantation due to DILI |
Abbreviations: ALP = alkaline phosphatase; ALT = alanine aminotransferase; INR = international normalized ratio
[Source 37 ]Symptoms of drug induced liver injury (DILI) may vary, ranging from asymptomatic elevations of liver enzymes to severe hepatitis with jaundice, fever, nausea, vomiting, dark urine and itching and only a small fraction of patients develop chronic liver disease (defined as abnormal liver tests for longer than 6 months) 12. Drug-induced liver injury is responsible for 3 to 5% of hospital admissions for jaundice 38 and is the most frequent cause of acute liver failure in most Western countries, accounting for more than half of cases 39, 40. More than 90% of patients with jaundice recover after discontinuation of the drug, and about 10% of patients with severe DILI with jaundice will either die or need liver transplantation 41. Hy’s Law is based on clinical observations made by the late Hyman Zimmerman that DILI patients with hepatocellular type of liver injury and jaundice have a 10% to 50% higher risk of mortality or need for liver transplantation 42. Hy’s Law has since been defined more specifically as aspartate aminotransferase (AST) or alanine aminotransferase (ALT) > 3 x upper limit of normal (ULN) and total bilirubin > 2 x upper limit of normal (ULN) in the absence of alkaline phosphatase (ALP) elevations and alternative reasons for alanine aminotransferase (ALT) and total bilirubin elevations, and is endorsed by the American Food and Drug Administration as criteria for severe DILI 43.
In fact, up to 10% of patients with drug-induced jaundice fulfilling “Hy’s Law” criteria will go into acute liver failure 42. Some of these patients will require a liver transplantation or ultimately died, and others will develop chronic DILI (defined as abnormal liver tests for longer than 6 months). In addition, recently it has been observed that both the extent of drug metabolism and the levels of total bilirubin and alkaline phosphatase (ALP) at DILI onset are associated with the time course for DILI 44.
Biochemical signs of drug induced liver injury (DILI) mainly include increased levels of liver enzymes measured in blood, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and total bilirubin 3. Clinically, drug induced liver injury (DILI) is defined when one of the three following thresholds are met 36, 45:
- ALT (alanine aminotransferase) or AST (aspartate aminotransferase) elevation ≥5 times the upper limit of normal (ULN);
- ALP (alkaline phosphatase) ≥2 × upper limit of normal (ULN);
- The combination of ALT or AST ≥3 × upper limit of normal (ULN) with simultaneous elevation of total bilirubin exceeding 2 × upper limit of normal (ULN).
Depending on the extent of increase in those enzymes and the “R-value” of ALT/ALP or AST/ALP activity at drug induced liver injury (DILI) presentation, drug induced liver injury (DILI) is roughly classified as hepatocellular when ALT or AST ≥5 × upper limit of normal (ULN) alone or when R-value is ≥5, cholestatic when ALP ≥2 × upper limit of normal (ULN) alone or if R-value is ≤ 2, and mixed if the ratio is 2–5 36, 46. Age is a clear determinant of drug induced liver injury (DILI) phenotype, with older age more frequently showing the cholestatic phenotype across different drug induced liver injury (DILI) cohorts 47.
The “R-value” is very useful and beneficial for distinguishing acute liver damage 48. This value was formed by the Council for International Organizations of Medical Sciences (CIOMS) of the World Health Organization (WHO) for the differentiation of drug-induced types of acute liver injury 49. The R-value is found by proportioning the ratio of the ALT and ALP values, which are measured in the patient, to the upper limit of normal (ULN). If the R-value is ≥5, it can be said that hepatocellular damage occurs. If the R-value is ≤ 2, sepsis, choledocholithiasis and heart failure ocur, and if the R-value is between 2-5, it can be interpreted as mixed type damage 50, 51.
Early recognition of drug‐induced liver injury (DILI) is essential to minimizing injury 52. The first step is to discontinue the suspected drug. Specific therapy against acetaminophen-induced liver injury is limited to the use of N-acetyl-cysteine (NAC) in the early phases. In non-acetaminophen-induced acute liver failure, N-acetyl-cysteine (NAC) has been shown to be efficacious at improving overall survival, post-transplant survival, and survival without transplant while decreasing the overall length of hospital stays 53. The other specific therapy that is available is L-carnitine for valproic acid overdose 27.
No specific antidote is available for the vast majority of hepatotoxic agents. In general, corticosteroids have no definitive role in treatment. They may suppress the systemic features associated with hypersensitivity or allergic reactions. Corticosteroid therapy is usually used when the histological appearance of DILI resembles that of autoimmune hepatitis. For this reason, it has a limited role and usually does not change the course of recovery 54. Management of protracted drug-induced cholestasis is similar to that for primary biliary cirrhosis. Cholestyramine (a bile acid resin) or antihistamines may be used for alleviation of pruritus 55, 54. Ursodeoxycholic acid may be used.
Hospital admission is required for patients with signs or symptoms of DILI progression or acute liver failure 55. If acute liver failure is suspected, early liver transplant consideration is essential, because there is high mortality with acute liver failure 27. An important additional aspect of management is reporting cases of DILI to regulatory bodies to evaluate if the suspected drug needs to be withdrawn from the market 56.
Hy’s law
Hy’s law was developed from the clinical observations of Dr. Hyman Zimmerman is a sensitive and specific predictor of a drug’s potential to cause severe hepatotoxicity 57, 58, 59. Dr. Hyman Zimmerman noted that among patients with DILI, the presence of hepatocellular injury and jaundice conferred a 10% or higher mortality rate from liver failure or the need for liver transplantation 57, 58, 59. Dr. Robert Temple of the U.S. Food and Drug Administration (FDA) later specified formal biochemical criteria for Hy’s Law (alanine aminotransferase [ALT] or aspartate aminotransferase [AST] levels ≥3 times upper limit of normal [ULN] plus a total bilirubin ≥2 times ULN) 60, 58. Hy’s Law criteria at DILI diagnosis had high specificity and negative predictive value, but low sensitivity and positive predictive value, for incident acute liver failure 61.
Hy’s law consists of 3 components:
- A statistically significant higher incidence of 3-fold or greater elevations above upper limit of normal [ULN] of ALT or AST compared to (non-hepatotoxic) control or placebo
- Individuals showing ALT or AST >3 × upper limit of normal [ULN], combined with elevation of serum total bilirubin >2 × upper limit of normal [ULN], without initial findings of cholestasis, indicated by elevated ALP
- Absence of any alternative cause likely to explain the combination of increased ALT or AST and total bilirubin, such as viral hepatitis A, B, C, or E, pre-existing or acute liver disease, or another drug capable of causing the observed injury
Now used by the FDA, this ‘10% rule’ has been observed for many drugs and is used to predict the risk of hepatotoxicity of drugs 58. If more than one patient meets the criteria for Hy’s law in a clinical trial the implicated drug is unlikely to be marketed as this is likely to lead a hepatotoxicity problem post‐marketing 58. The validity of Hy’s law has been confirmed in several studies. Patients with hepatocellular type of jaundice were found to have the worst prognosis in two studies, with a fatality rate of 7‐13% 62, 41, whereas patients with cholestatic type were found to have highest fatality rate in the first report from the Drug Induced Liver Injury Network cohort of 14% 63, which was higher than in the Swedish and the Spanish DILI cohorts with fatality rate of approximately 5‐8% 62, 41. However, jaundice induced by different drugs can have different prognosis. For example, in one study of patients with jaundice due to idiosyncratic DILI the mortality rate varied from 40% (6 of 15) for halothane to 0% for erythromycin (0 of 32) 41. Recently, researchers from the Spanish hepatotoxicity network have tried to optimize the definition of Hy’s law and to develop a model for predicting acute liver failure in patients with DILI 42. These researchers were able to develop a prognostic algorithm that was found be more reliable than Hy’s law, in particular in predicting who will not develop acute liver failure 42.
Liver anatomy
Your liver is essential to your life. You cannot live without your liver. Your liver is the largest internal organ in your body. Your liver is about the size of a football and weighs about 3 to 3.5 pounds (1.36–1.59kg). Your liver lies under your right ribs just beneath your right lung. Your liver has two lobes (sections). Your liver is made up mainly of liver cells called hepatocytes. It also has other types of cells, including cells that line its blood vessels and cells that line small tubes in the liver called bile ducts. The bile ducts carry bile from the liver to the gallbladder or directly to the intestines.
Your liver has many important functions:
- It breaks down and stores many of the nutrients absorbed from the intestine that your body needs to function. Some nutrients must be changed (metabolized) in the liver before they can be used for energy or to build and repair body tissues.
- It makes most of the clotting factors that keep you from bleeding too much when you are cut or injured.
- It delivers bile into the intestines to help absorb nutrients (especially fats).
- It breaks down alcohol, drugs, and toxic wastes in the blood, which then pass from the body through urine and stool.
Figure 2. Liver anatomy
Types of Drug-Induced Liver Injury
Drug‐induced liver injury (DILI) has been classically divided into two varieties based on the presumed mechanism of action of the chemical compound: intrinsic DILI and idiosyncratic DILI 18. The intrinsic DILI (or direct, predictable DILI) is dose‐related and occurs shortly after exposure (hours to days) in the majority of individuals exposed to the drug (which is toxic at a dose threshold level) 3. In contrast, the idiosyncratic DILI (unpredictable DILI) is multifactorial and unpredictable drug induced liver injury and does not correlate with the dose and usually occurs in less than 1 of every 1000 to 1 of every 10,000 exposed individuals 19 and with a longer latency period (from a few days to several months) 21, 22. Idiosyncratic DILI is further subdivided into two sub-categories: allergic (immune-mediated) and non-allergic (nonimmune-mediated) as presented in Figure 1 21, 23, 24. If immune-mediated, the latency is shorter (1–6 weeks) compared with non-immune mediated reactions (1 month to 1 year) 25. However, exceptions do occur with immune reactions appearing after a very long latency with drugs such as nitrofurantoin 26 or even after drug cessation (eg sulfonamides, erythromycin and amoxicillin-clavulanate) 25. Immune-mediated idiosyncratic reactions can be characterized by presence of fever, rash, eosinophilia, autoantibodies (such as antinuclear and anti-smooth muscle antibodies) and Stevens-Johnson syndrome 27. Non-immune mediated reactions lack the aforementioned characteristics and importantly, as described, tend to have a long latency period (1 month to 1 year) 25, 28.
Recently, a third type of DILI—indirect DILI—has been described as a liver injury caused by the mechanism of action of the drug (what it does; i.e., rituximab by depletion of B cells reactivates silent hepatitis B virus which is responsible for the damage), in contrast to its idiosyncratic properties (what it is; i.e., the unexpected hepatotoxic reaction of amoxicillin-clavulanate) 30.
Other forms of DILI include steatohepatitis (amiodarone, tamoxifen, methotrexate) 31, neoplasms (hepatic adenomas due to androgenic anabolic steroids) 32 and vascular (nodular regenerating hyperplasia due to azathioprine) 33.
Idiosyncratic drug induced liver injury
Idiosyncratic DILI (unpredictable DILI) is multifactorial and unpredictable drug induced liver injury and does not correlate with the dose and usually occurs in less than 1 of every 1000 to 1 of every 100,000 exposed individuals 19, 20 and with a longer latency period (from a few days to several months) 21, 22. Idiosyncratic DILI is further subdivided into two sub-categories: allergic (immune-mediated) and non-allergic (nonimmune-mediated) as presented in Figure 1 21, 23, 24. If immune-mediated, the latency is shorter (1–6 weeks) compared with non-immune mediated reactions (1 month to 1 year) 25. However, exceptions do occur with immune reactions appearing after a very long latency with drugs such as nitrofurantoin 26 or even after drug cessation (eg sulfonamides, erythromycin and amoxicillin-clavulanate) 25. Immune-mediated idiosyncratic reactions can be characterized by presence of fever, rash, eosinophilia, autoantibodies (such as antinuclear and anti-smooth muscle antibodies) and Stevens-Johnson syndrome 27. Non-immune mediated reactions lack the aforementioned characteristics and importantly, as described, tend to have a long latency period (1 month to 1 year) 25, 28.
Idiosyncratic DILI cases are caused by 20, 56:
- Antibiotics (45.4%): amoxicillin-clavulanate (most common), sulfamethoxazole-trimethoprim, ciprofloxacin, isoniazid (INH)
- Nonsteroidal anti-inflammatory drugs (NSAIDs)
- Herbal and dietary supplements (16.1%): green tea extract, anabolic steroids, multi-ingredient nutritional supplements
- Cardiovascular drugs (10%): statins, amiodarone
- Central nervous system (CNS) agents: valproate, phenytoin
- Antineoplastic drugs: tyrosine kinase inhibitors, tumor necrosis factor inhibitors, alpha inhibitors, methotrexate
Idiosyncratic liver injury is categorized as hepatocellular, cholestatic, or both (mixed) on the basis of the “R-value” of ALT/ALP or AST/ALP, calculated by dividing the alanine aminotransferase level by the alkaline phosphatase level from the time of initial presentation, with both values expressed as multiples of the upper limit of the normal range 64. Hepatocellular injury is defined as an R value of ALT/ALP or AST/ALP more than 5, cholestatic injury as a value of less than 2, and mixed injury as a value of 2 to 5.
Acute hepatocellular hepatitis is the most common manifestation of idiosyncratic liver injury 20. The latency period generally ranges from 5 to 90 days. The symptoms and course resemble those of acute viral hepatitis, with prominent alanine aminotransferase elevations (increased by a factor of 5 to 50), whereas alkaline phosphatase levels are only modestly increased. Liver histologic studies show changes suggestive of acute viral hepatitis, the major disorder in the differential diagnosis, but eosinophils may be prominent. The rate of death from icteric hepatocellular injury due to medications is high, usually 10% or higher, a feature first stressed by the late Hyman J. Zimmerman, for which reason it is called Hy’s law 65. A key feature of Hy’s law is jaundice with hepatocellular rather than cholestatic injury. Drug-induced idiosyncratic acute liver injury is an important cause of acute liver failure, accounting for 11 to 15% of cases in series from the United States and Europe 40. Common causes of drug-induced idiosyncratic acute hepatocellular injury are isoniazid, nitrofurantoin, and diclofenac 20, 66, 67.
Chronic hepatitis is an uncommon form of drug-induced liver injury; the chronicity occurs if the agent is continued and typically resolves slowly once the agent has been stopped. Many agents that cause acute hepatocellular injury can also cause a chronic hepatocellular pattern 67. The injury arises after months or years of exposure. Autoantibodies are frequently present, and the differential diagnosis often focuses on ruling out spontaneous autoimmune hepatitis. Common causes of drug-induced, autoimmune-like chronic liver injury are nitrofurantoin, minocycline, hydralazine, methyldopa, statins, and fenofibrate 66, 67, 68. Glucocorticoids, which are frequently used to manage chronic hepatitis (starting dose, 20 to 60 mg of prednisone or its equivalent daily), may alleviate symptoms and speed recovery, but the injury will often resolve without intervention. If prednisone is used, the dose and duration should be kept to a minimum. Monitoring for evidence of relapse should be performed for at least 6 months after the withdrawal of glucocorticoids. Ultimately, spontaneous autoimmune hepatitis is best ruled out by evidence of resolution of the liver injury after withdrawal of the medication and, if glucocorticoids are used, by the absence of relapse when they are discontinued 69.
Cholestatic hepatitis is characterized by prominent symptoms of pruritus and jaundice accompanied by moderate-to-marked elevations in alkaline phosphatase levels. Drug-induced cholestatic liver injury is usually self-limited, and although often protracted, it ultimately resolves 70. Liver histologic studies show bile duct injury and cholestasis in small bile canaliculi 71. Exceptions to the usual benign course occur when there is bile duct loss, which is associated with delayed resolution of jaundice and elevated enzyme levels 72. Some cases evolve into vanishing bile duct syndrome, with prolonged jaundice, liver failure, need for liver transplantation, or death. Common causes of drug-induced cholestatic hepatitis are amoxicillin–clavulanate, cephalosporins, terbinafine, azathioprine, and temozolomide 73, 68.
Drug-induced mixed hepatitis is caused by many agents, some of which also cause hepatocellular or cholestatic hepatitis 20. The mixed forms of drug-induced liver injury tend to have the most benign outcomes, rarely leading to liver failure. Common causes of drug-induced mixed hepatitis include the fluoroquinolone and macrolide antibiotics, phenytoin, and sulfonamides 74.
All forms of idiosyncratic drug-induced hepatitis can be accompanied by immunoallergic features, such as rash, fever, and eosinophilia — signs of drug hypersensitivity 20. More extreme examples include drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, toxic epidermal necrolysis, and the Stevens–Johnson syndrome 75. Prominent causes of idiosyncratic drug-induced hepatitis with immunoallergic features include allopurinol, carbamazepine, phenytoin, sulfonamides, and macrolide antibiotics 75. Immunoallergic hepatitis is more common among black Americans than among non-Hispanic white Americans 76.
Bland cholestasis represents a distinctive phenotype of drug-induced liver injury, characterized by marked and prolonged jaundice with pruritus. In women, bland cholestasis is typically caused by estrogens or oral contraceptives 77 and in men it is typically caused by anabolic steroids, usually obtained illicitly for bodybuilding or improving athletic performance 78. Jaundice and itchy skin arise within 30 to 90 days, and elevations in enzyme levels are minimal or modest, despite marked and prolonged jaundice 79. Liver biopsy shows bland cholestasis with scant inflammation and hepatocellular necrosis. The cholestasis can be prolonged, but the injury is almost always self-limited and deaths are rare. The pathogenesis remains unclear.
Drug induced liver injury causes
More than 1100 drugs, herbal compounds, natural products, vitamins, minerals, dietary supplements, and recreational and illicit compounds have been reported to cause DILI (albeit, some only occasionally) 12, 13, 14, 15. In addition to specific drug-related properties, there are important host predisposing factors including advancing age, sex, alcohol intake and underlying liver disease. Female sex appears to confer risk for drug‐induced liver injury (DILI) and have a higher risk of progression to acute liver failure 45.
Acetaminophen (paracetamol) is one of the most studied drugs that cause intrinsic DILI (predictable DILI), while it is less often seen in aspirin, tetracycline, and vitamin A 27. Acetaminophen (paracetamol) hepatotoxicity is dose-dependent and the hepatic injury occurs due to the activity of the toxic N-acetyl-p-benzoquinone Imine (NAPQI) metabolite, produced by the CYP2E1 pathway of acetaminophen metabolism 80. Exposure of Reactive Oxygen Species (ROS) to organelles can lead to apoptosis and necrosis of hepatocytes 80. Interestingly, a significant proportion of acetaminophen hepatotoxicity cases occurs unintentionally at doses slightly above the maximum recommended daily dose of 4 g, or even with repeated doses below this safety threshold 81. Supposedly, a number of factors including fasting, alcoholism, concomitant use of other drugs and coexisting diseases, can decrease the toxic acetaminophen threshold dose by activating the generation of reactive drug metabolites via CYP2E1 and/or by depleting the hepatic glutathione concentration, which is the main detoxification pathway for acetaminophen toxic intermediates.
Bromfenac, cyclophosphamide and methotrexate are also examples of drugs known for induction of dose-related hepatotoxicity (intrinsic DILI) 80.
Data from prospective DILI registries suggest that antibiotics remain the most common cause of idiosyncratic DILI (unpredictable DILI) 27. The American DILI Network reported antibiotics to be implicated in 45.4% of cases 20. Other common drug classes reported by the American Drug Induced Liver Injury Network were herbal and dietary supplements (16.1% – a significant increase over the last 10 years), cardiovascular agents (9.8%), central nervous system agents (9.1%), anti-neoplastic agents (5.5%) and analgesics (3.7%) 20. Amoxicillin-clavulanate antibiotic is the most common individual drug implicated in DILI as confirmed by both European and American studies (Table 4) 27. In some Asian countries, however, herbal and dietary supplements are implicated in more than 70% of all DILI 82.
The drugs most commonly implicated in acute liver failure include anti-tuberculosis therapies, herbal and dietary supplements, sulpha-containing drugs, nitrofurantoin, phenytoin, sodium valproate, flutamide and amoxicillin-clavulanate 42, 40.
Novel causative potentiator of DILI is those related to the classes of novel immunomodulatory therapeutic classes 83, 84. These molecules are being increasingly used to treat cancer through restitution of strong humoral, antitumor immune response, thereby improving patient survival. The reduction in tumor tolerance induced by immune checkpoint inhibitors can lead to inflammatory side effects, and an increase in immune related adverse events, including hepatotoxicity. Risk factors include the type of check point inhibitor, higher dose, autoimmune predisposition, pre-existing liver disease and the use of combination agents 83, 84. Hepatotoxicity is heterogeneous ranging from mild transaminase derangement to pronounced acute hepatitis and fulminant liver failure.
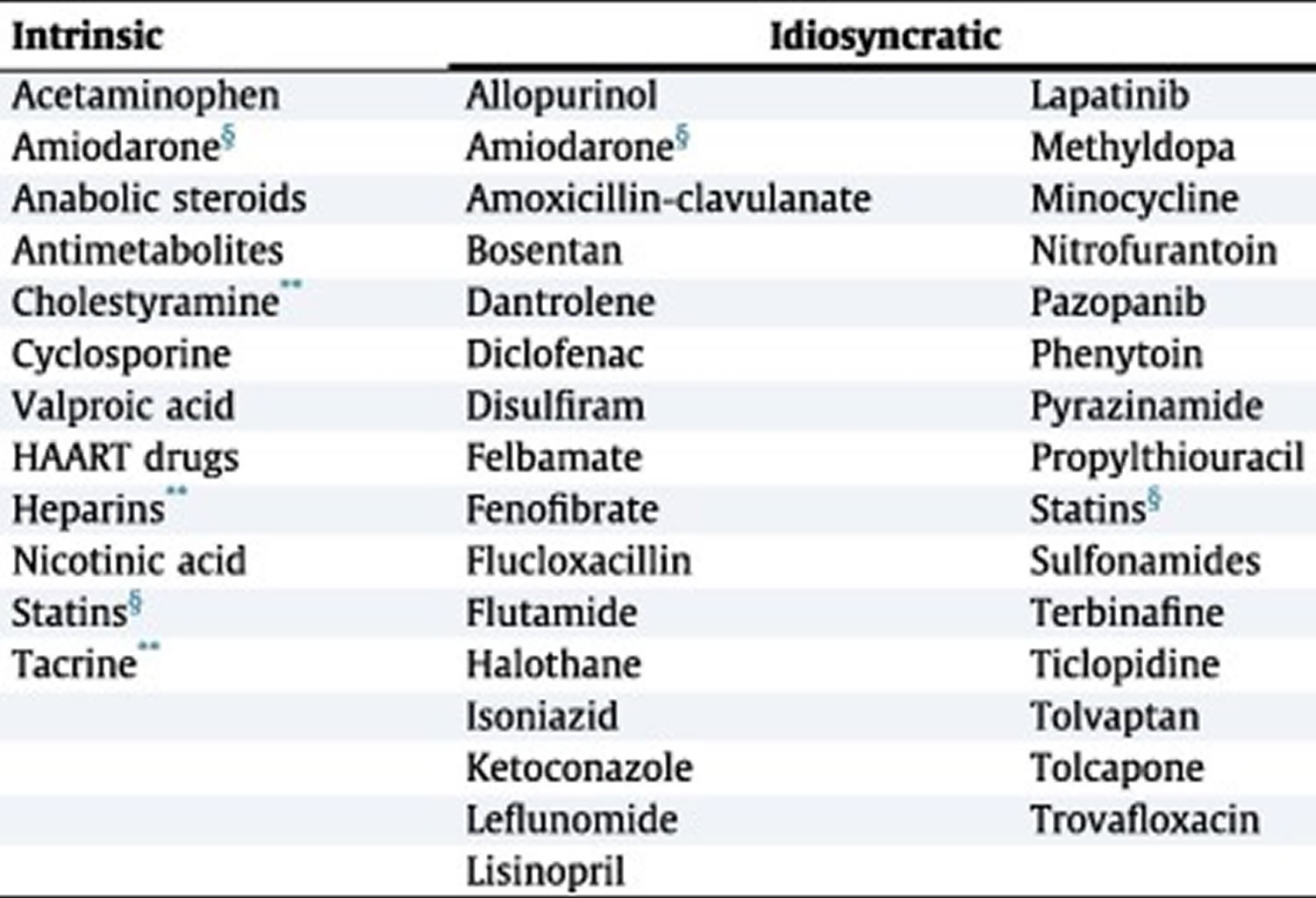
Table 3. Drugs associated with intrinsic DILI and idiosyncratic DILI
Footnotes: HAART = highly active antiretroviral therapy; *Known examples; withdrawn or unapproved drugs not listed; **Mild ALT elevations without jaundice; §Both intrinsic and idiosyncratic.
[Source 45 ]Table 4. Common drugs implicated in idiosyncratic drug-induced liver injury according to studies from different countries
| Iceland 19 | American drug-induced liver injury network 20 | Spanish registry 42 | UK 5 |
|---|---|---|---|
| Amoxicillin-clavulanate | Amoxicillin-clavulanate | Amoxicillin-clavulanate | Amoxicillin-clavulanate |
| Diclofenac | Isoniazid | Isoniazid | Diclofenac |
| Azathioprine | Nitrofurantoin | Combined anti-tuberculous therapy | Tricyclic antidepressants |
| Infliximab | Trimethoprim-sulfamethoxazole | Flutamide | Macrolides |
| Nitrofurantoin | Minocycline | Ibuprofen | Chlorpromazine |
Risk factors for drug-induced liver injury
- Race. Some drugs appear to have different toxicities based on race. For example, blacks and Hispanics may be more susceptible to isoniazid (INH) toxicity. The rate of metabolism is under the control of P-450 enzymes and can vary from individual to individual 85.
- Age. Apart from accidental exposure, hepatic drug reactions are rare in children. Older patients have an almost threefold increase in the incidence of DILI as shown in a study from Iceland 19. This was attributed to the increased prescription rate of drugs in older patients 19. Elderly persons are at increased risk of hepatic injury because of decreased clearance, drug-to-drug interactions, reduced hepatic blood flow, variation in drug binding, and lower hepatic volume. In addition, poor diet, infections, and multiple hospitalizations are important reasons for drug-induced hepatotoxicity 86. In general, higher drug dose and longer duration of therapy have also been implicated in poorer outcomes 87. In the Spanish Registry, 77% of patients with idiosyncratic DILI received medications with daily doses of ≥50 mg 87. Ninety percent of patients with serious DILI needing a liver transplant and reported to the Swedish Adverse Drug Reactions Advisory Committee were consuming ≥50 mg/day of the drug 88. A longer duration of drug therapy (135±31 days versus 53±3 days) has been associated with a higher liver-related morbidity and mortality 89.
- Female Sex. Although the reasons are unknown, hepatic drug reactions are more common in females 86. The American drug-induced liver injury network found a higher prevalence of DILI in women (59% female versus 41% male) 20, although this was not corroborated by the Spanish DILI registry (49% female versus 51% male) 42. Potential reasons for increased female susceptibility to DILI include differences in various aspects of drug pharmacokinetics or pharmacodynamics; hormonal effects or interactions with immunomodulating agents or signaling molecules; and differences in the adverse response of the immune system to some drugs, reactive drug metabolites or drug-protein adducts 90. It is consistently observed, however, that women tend to be younger and develop hepatocellular DILI whereas men tend to be older and develop cholestatic DILI 87, 20.
- Alcohol ingestion. Alcoholic persons are susceptible to drug toxicity because alcohol induces liver injury and cirrhotic changes that alter drug metabolism. Alcohol causes depletion of glutathione (hepatoprotective) stores that make the person more susceptible to toxicity by drugs.
- Liver disease. Preexisting liver disease has not been thought to make patients more susceptible to drug-induced liver injury, but it may be that a diminished liver reserve or the ability to recover could make the consequences of injury worse 91. Although the total cytochrome P-450 is reduced in chronic liver disease, some may be affected more than others. The modification of doses in persons with liver disease should be based on the knowledge of the specific enzyme involved in the metabolism. Patients with HIV infection who are co-infected with hepatitis B or C virus are at increased risk for hepatotoxic effects when treated with antiretroviral therapy. Similarly, patients with cirrhosis are at increased risk of decompensation by toxic drugs. In the American drug-induced liver injury network, 10% of patients with DILI had pre-existing liver disease, mostly due to hepatitis C and non-alcoholic fatty liver disease (NAFLD) 20. This group was characterized by more severe liver injury and higher overall mortality (16% verus 5.2%) although they had similar liver-related mortality (57% versus 46%) 20. The higher overall mortality in those with pre-existing liver disease may have been due to significantly higher prevalence of comorbidities, specifically diabetes mellitus (38% versus 23%) 20. Additionally, alcohol excess and/or risk factors for NAFLD (non-alcoholic fatty liver disease) increase susceptibility to methotrexate toxicity by three- to fourfold 92. Furthermore, some studies have shown that patients with chronic hepatitis B or C could have a threefold increased risk of abnormal liver tests after receiving anti-tuberculosis treatment 93. Nonetheless, the interpretation of liver injury in patients with chronic hepatitis B and C receiving anti-tuberculosis treatment remains complex. It could be due to anti-tuberculosis treatment-induced DILI, viral reactivation and/or immune reconstitution 94.
- Genetic factors. Genetic factors also increase susceptibility to DILI. A unique gene encodes each P-450 protein. Genetic differences in the P-450 enzymes can result in abnormal reactions to drugs, including idiosyncratic reactions 95. Debrisoquine is an antiarrhythmic drug that undergoes poor metabolism because of abnormal expression of P-450-II-D6. This can be identified by polymerase chain reaction amplification of mutant genes. This has led to the possibility of future detection of persons who can have abnormal reactions to a drug 96. Another example is human leukocyte antigen (HLA) B*5701 that confers an eightyfold increased risk to flucloxacillin induced DILI, whereas the absence of this genotype has an 88% negative predictive value for flucloxacillin-related DILI 97.
- Drug formulation. Long-acting drugs may cause more injury than shorter-acting drugs.
- Other comorbidities. Persons with AIDS, persons who are malnourished, and persons who are fasting may be susceptible to drug reactions because of low glutathione stores.
- Host factors. Factors that may enhance susceptibility to drugs, possibly inducing liver disease, are as follows:
- Female – Halothane, nitrofurantoin, sulindac
- Male – Amoxicillin-clavulanic acid (Augmentin)
- Old age – Acetaminophen, halothane, isoniazid (INH), amoxicillin-clavulanic acid
- Young age – Salicylates, valproic acid
- Fasting or malnutrition – Acetaminophen
- Large body mass index/obesity – Halothane
- Diabetes mellitus – Methotrexate, niacin
- Renal failure – Tetracycline, allopurinol
- AIDS – Dapsone, trimethoprim-sulfamethoxazole
- Hepatitis C – Ibuprofen, ritonavir, flutamide
- Preexisting liver disease – Niacin, tetracycline, methotrexate
Pathophysiology and mechanisms of drug-induced liver injury
The pathogenesis of idiosyncratic DILI remains unknown but is most likely due to a complex interplay between drug (eg dose, duration of therapy, hepatic metabolism, lipophilicity) and host factors (eg age, gender, genetic polymorphisms) 27. The drug may form a reactive metabolite that triggers DILI or may form covalent adducts with tissue proteins, eliciting an immune response and subsequent DILI 98. The following are some of the mechanisms that have been described 99:
- Disruption of the hepatocyte: Covalent binding of the drug to intracellular proteins can cause a decrease in ATP levels, leading to actin disruption. Disassembly of actin fibrils at the surface of the hepatocyte causes blebs and rupture of the membrane.
- Disruption of the transport proteins: Drugs that affect transport proteins at the canalicular membrane can interrupt bile flow. Loss of villous processes and interruption of transport pumps such as multidrug resistance–associated protein 3 prevent the excretion of bilirubin, causing cholestasis.
- Cytolytic T-cell activation: Covalent binding of a drug to the P-450 enzyme acts as an immunogen, activating T cells and cytokines and stimulating a multifaceted immune response.
- Apoptosis of hepatocytes: Activation of the apoptotic pathways by the tumor necrosis factor-alpha receptor of Fas may trigger the cascade of intercellular caspases, which results in programmed cell death.
- Mitochondrial disruption: Certain drugs inhibit mitochondrial function by a dual effect on both beta-oxidation energy production by inhibiting the synthesis of nicotinamide adenine dinucleotide and flavin adenine dinucleotide, resulting in decreased ATP production.
- Bile duct injury: Toxic metabolites excreted in bile may cause injury to the bile duct epithelium.
Drug toxicity mechanisms
The classic division of drug reactions is into at least two major groups, (1) drugs that directly affect the liver and (2) drugs that mediate an immune response, as follows 99:
- Intrinsic or predictable drug reactions: Drugs that fall into this category cause reproducible injuries in animals, and the injury is dose related. The injury can be due to the drug itself or to a metabolite. Acetaminophen is a classic example of a known intrinsic or predictable hepatotoxin at supertherapeutic doses. Another classic example is carbon tetrachloride.
- Idiosyncratic drug reactions: Idiosyncratic drug reactions can be subdivided into those that are classified as hypersensitivity or immunoallergic and those that are metabolic-idiosyncratic. Regarding hypersensitivity reactions, phenytoin is a classic, if not common, cause of hypersensitivity reactions. The response is characterized by fever, rash, and eosinophilia and is an immune-related response with a typical short latency period of 1-4 weeks. A metabolic-idiosyncratic reaction occurs through an indirect metabolite of the offending drug. Unlike intrinsic hepatotoxins, the response rate is variable and can occur within a week or up to one year later. It occurs in a minority of patients taking the drug, and no clinical manifestations of hypersensitivity are noted. Isoniazid (INH) toxicity is considered to fall into this class. Not all drugs fall neatly into one of these categories, and overlapping mechanisms may occur with some drugs (eg, halothane).
Drug induced liver injury signs and symptoms
Symptoms of drug induced liver injury (DILI) may vary, ranging from asymptomatic elevations of liver enzymes to severe hepatitis with jaundice, fever, nausea, vomiting, dark urine and itching and only a small fraction of patients develop chronic liver disease 12. More than 90% of patients with jaundice recover after discontinuation of the drug, and about 10% of patients with severe DILI with jaundice will either die or need liver transplantation 41. Hy’s Law states that hepatocellular drug-induced liver injury (DILI) with jaundice indicates a serious reaction, is used widely to determine risk for acute liver failure 42. In fact, up to 10% of patients with drug-induced jaundice fulfilling “Hy’s Law” criteria will go into acute liver failure 42. Some of these patients will require a liver transplantation or ultimately died, and others will develop chronic DILI. In addition, recently it has been observed that both the extent of drug metabolism and the levels of total bilirubin and alkaline phosphatase (ALP) at DILI onset are associated with the time course for DILI 44.
Drug induced liver injury complications
There is a 17% risk of drug induced liver injury (DILI) progresing to chronic liver disease, mostly seen in patients with prolonged cholestatic injury such as vanishing duct syndrome 20, 27. Acute liver failure results more often from hepatocellular injury than cholestatic 100.
Drug induced liver injury diagnosis
The diagnosis of drug-induced liver injury is particularly challenging, because the presentation is similar to many hepatobiliary disorders and is based largely on exclusion of other causes. Drug-induced liver injury (DILI) is complex, with no unifying criteria and a relatively high index of suspicion is necessary.
The timing of the onset of injury after the implicated agent has been started (latency), resolution after the agent is stopped (“dechallenge”), recurrence on re-exposure (rechallenge), knowledge of the agent’s potential for hepatotoxicity (likelihood), and clinical features (phenotype) are the major diagnostic elements 101. With few exceptions, there are no specific diagnostic markers for drug-induced liver injury, and special tests (liver biopsy, imaging, and testing for serologic markers) are helpful mostly in ruling out other causes of liver injury. The large number of agents that can cause liver injury highlights these challenges. LiverTox website on hepatotoxicity (https://www.ncbi.nlm.nih.gov/books/NBK547852), has descriptions of more than 1200 agents (prescription and over-the-counter medications, herbal products, nutritional supplements, metals, and toxins), along with their potential to cause liver injury. Among the 971 prescription drugs described, 447 (46%) have been implicated in causing liver injury in at least one published case report 101.
Investigations should be appropriate to the severity of the condition in a particular patient. They include:
Blood tests:
- coagulation studies, glucose, and potassium as soon as possible
- full blood count, group and save, bilirubin, albumin, AST, amylase
- hepatitis serology, paracetamol levels, serum copper and caeruloplasmin, plus 24 hour copper where appropriate
Radiology:
- Chest radiograph
- Ultrasound scan of liver and pancreas. Ultrasonography is effective to evaluate the gall bladder, bile ducts, and hepatic tumors.
- CT scanning can help detect focal hepatic lesions 1 cm or larger and some diffuse conditions. It can also be used to visualize adjacent structures in the abdomen.
MRI provides excellent contrast resolution. It can be used to detect cysts, hemangiomas, and primary and secondary tumors. The portal vein, hepatic veins, and biliary tract can be visualized without contrast injections.
Others:
- blood cultures, even if the patient is afebrile
- arterial blood gases – a CNS induced respiratory alkalosis or metabolic acidosis may occur
- ECG
- A liver biopsy is not essential in every case, but a morphologic pattern consistent with the expected pattern provides supportive evidence and could exclude other causes of liver disease.
Patients with DILI should undergo testing for hepatotropic viruses including hepatitis A to E, particularly in those with acute hepatocellular injury 34. For completeness an autoantibody screen (antinuclear antibody [ANA], anti-smooth muscle antibody [ASMA], M2-anti-mitochondrial antibody [AMA], liver microsomal antibody, immunoglobulins) should be undertaken. DILI assessment should also include coagulation profiles, as elevated prothrombin time ratio values may suggest impending acute liver failure and prompt referral to a liver transplant unit should be considered.
Abdominal ultrasound should be undertaken in all patients suspected of DILI to exclude any biliary, parenchymal or vasculopathy, additional imaging is dependent on the clinical context 34.
Liver biopsy may be reasonable to consider in DILI, as histology may provide information pertaining to severity of liver injury and provide mechanistic insights by identifying specific patterns of injury. Liver biopsy is also warranted in those patients suspected of DILI when serology raises the possibility of autoimmune hepatitis 45. Liver biopsy may also be considered in patients whereby suspected DILI progresses, or fails to resolve on withdrawal of the causal agent, since histology may provide prognostic information assisting clinical decision particularly regarding immunosuppression 102. There are characteristic histological patterns associated with individual check-point inhibitors including presence of ring granulomas and endotheliitis which may aid decision making around immunosuppression 45. In select cases of DILI, human leucocyte antigen genotyping can be used, whereby genetic determination may aid diagnosis and management, particularly those with features compatible with autoimmune hepatitis.
DILI should be classified according to the dominant pattern of liver enzyme derangement; hepatocellular, cholestatic and mixed injury.
Biochemical signs of drug induced liver injury (DILI) mainly include increased levels of liver enzymes measured in blood, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and total bilirubin 3. Clinically, drug induced liver injury (DILI) is defined when one of the three following thresholds are met 36, 45:
- ALT (alanine aminotransferase) or AST (aspartate aminotransferase) elevation ≥5 times the upper limit of normal (ULN);
- ALP (alkaline phosphatase) ≥2 × upper limit of normal (ULN);
- The combination of ALT or AST ≥3 × upper limit of normal (ULN) with simultaneous elevation of total bilirubin exceeding 2 × upper limit of normal (ULN).
Depending on the extent of increase in those enzymes and the “R-value” of ALT/ALP or AST/ALP activity at drug induced liver injury (DILI) presentation, drug induced liver injury (DILI) is roughly classified as hepatocellular when ALT or AST ≥5 × upper limit of normal (ULN) alone or when R-value is ≥5, cholestatic when ALP ≥2 × upper limit of normal (ULN) alone or if R-value is ≤ 2, and mixed if the ratio is 2–5 36, 46. Age is a clear determinant of drug induced liver injury (DILI) phenotype, with older age more frequently showing the cholestatic phenotype across different drug induced liver injury (DILI) cohorts 47.
Given the non-specific nature of traditionally employed liver enzyme measurements there is increasing interest in determining novel serum biomarkers. These markers include glutamate dehydrogenase, keratin 18, glutathione S-transferase, sorbitol dehydrogenase, bile acids, cytochrome P450 and osteopontin 34. These markers may help to improve the specificity of DILI diagnosis, and aid prognostication. Presently, however, none are routinely employed, but represent exciting future avenues of research.
Rechallenging patients who had initial drug-related liver injury can lead to rapid, progressive liver insult often worse than previous with fulminant hepatic failure. A positive rechallenge is defined as an ALT>3 ULN and is the strongest proof of drug causality 103. Some essential, irreplaceable medications may be used to rechallenge patients including; antituberculous and chemotherapy agents, however, this should only be considered with meticulous monitoring arrangements under specialist supervision.
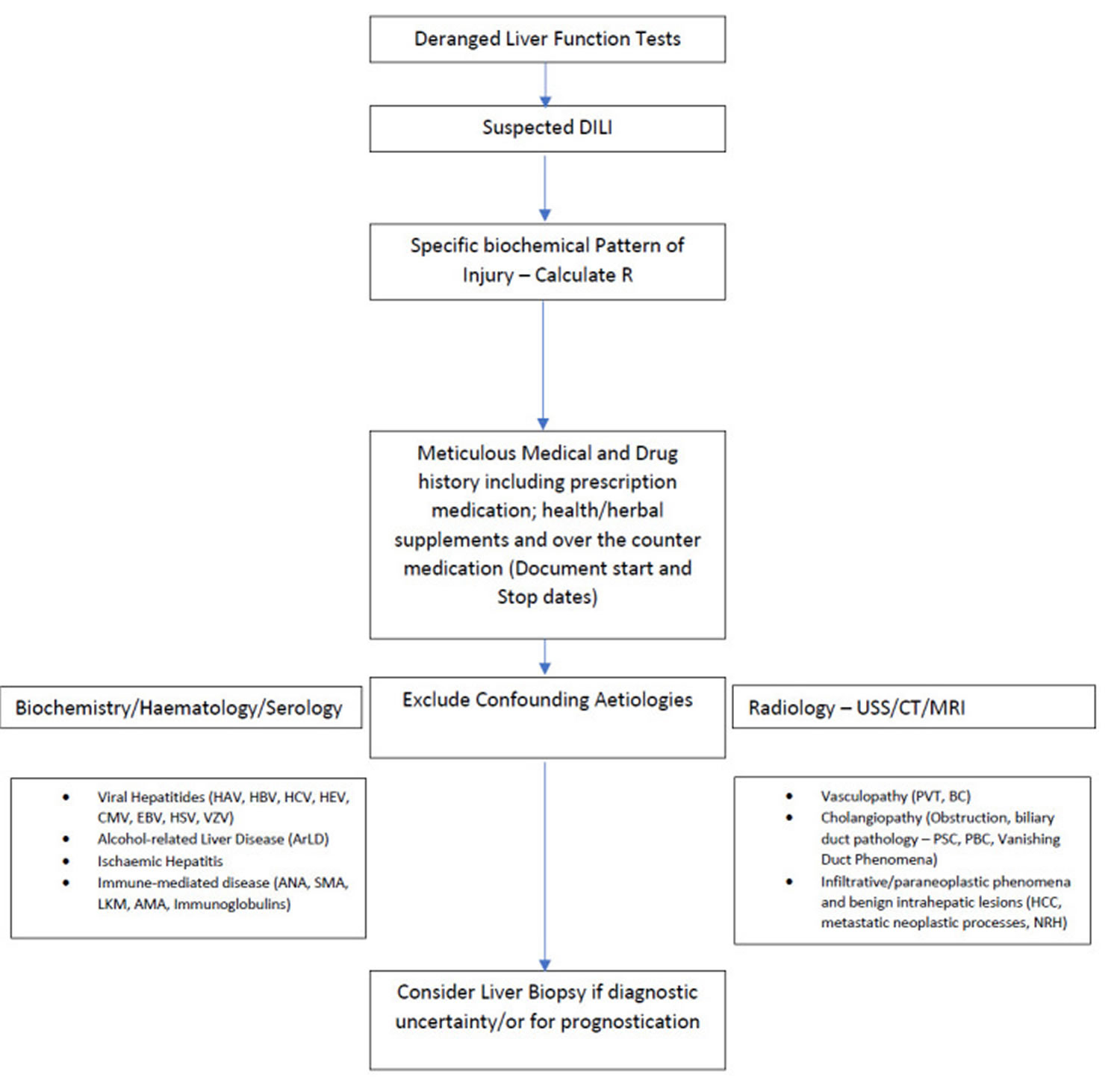
Figure 3. Drug-induced liver injury (DILI) diagnostic algorithm
Footnote: Suggested approach to presentation of drug-induced liver injury (DILI).
Abbreviations: ANA, antinuclear antibody; BC, Budd-Chiari syndrome; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HEV, hepatitis E virus; HSV, hepes simplex virus; HCC, hepatocellular carcinoma; LKM, liver microsomal antibody; NRH, nodular regenerative hyperplasia; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; PVT, portal vein thrombosis; SMA, smooth muscle antibody; USS, ultransound scan; VZV, varicella zoster virus.
[Source 45 ]Drug induced liver injury treatment
The principal treatment for drug-induced hepatotoxicity is the discontinuation of the likely causative agent 29. In the majority of DILI, spontaneous recovery occurs, without any need for treatment or specific supportive measures. This aspect of spontaneous recovery following discontinuation of an offending substance forms an important criterion in the causality assessment of DILI.
N-acetyl-cysteine (NAC) is the treatment for intrinsic DILI (direct, predictable DILI) secondary to acetaminophen toxicity, as this promotes the regeneration of glutathione leading detoxification of the toxic metabolite 104. In non-acetaminophen-induced acute liver failure, N-acetyl-cysteine (NAC) has been shown to be efficacious at improving overall survival, post-transplant survival, and survival without transplant while decreasing the overall length of hospital stays 53.
The other specific therapy that is available is L-carnitine for valproic acid overdose 27. Corticosteroid therapy is usually used when the histological appearance of DILI resembles that of autoimmune hepatitis. For this reason, it has a limited role and usually does not change the course of recovery 54. Symptomatic therapies such as cholestyramine (a bile acid resin) for cholestatic DILI or antihistamines for pruritis can be used with some efficacy 55, 54. Hospital admission is required for patients with signs or symptoms of DILI progression or acute liver failure 55. If acute liver failure is suspected, early liver transplant consideration is essential, because there is high mortality with acute liver failure 27. An important additional aspect of management is reporting cases of DILI to regulatory bodies to evaluate if the suspected drug needs to be withdrawn from the market 56.
Emergency liver transplantation has utility in the setting of drug-induced fulminant hepatic injury. The Model for End-Stage Liver Disease score can be used to evaluate short-term survival in an adult with end-stage liver disease. This can help stratify candidates for liver transplantation. The parameters used are serum creatinine, total bilirubin, international normalized ratio, and the cause of the cirrhosis. Another criterion commonly used for liver transplantation is the Kings College criteria.
Kings College criteria for liver transplantation in cases of acetaminophen toxicity are as follows:
- pH less than 7.3 (irrespective of grade of encephalopathy)
- Prothrombin time (PT) greater than 100 seconds or international normalized ratio greater than 7.7
- Serum creatinine level greater than 3.4 mg/dL in patients with grade III or IV encephalopathy
- Measurement of lactate levels at 4 and 12 hours also helps in early identification of patients who require liver transplantation.
Kings College criteria for liver transplantation in other cases of drug-induced liver failure (DILI) are as follows:
- Prothrombin time (PT) greater than 100 seconds (irrespective of grade of encephalopathy) or
- Any three of the following criteria:
- Age younger than 10 years or older than 40 years;
- Etiology of non-A/non-B hepatitis, halothane hepatitis, or idiosyncratic drug reactions;
- Duration of jaundice of more than 7 days before onset of encephalopathy;
- Prothrombin time (PT) greater than 50 seconds; (5) serum bilirubin level greater than 17 mg/dL
Drug induced liver injury prognosis
The prognosis of drug‐induced liver injury (DILI) is highly variable depending on the patient’s presentation, several host factors, stage of liver damage and the drug type 18. Some patients develop acute liver failure and may require liver transplantation, whereas others can develop chronic DILI. In general, most patients (90% of cases) make a full recovery after discontinuation of the offending drug 29. Mortality risk can be predicted through “Hy’s law,” which include the following criteria that are associated with a poorer prognosis: ALT/AST more than 3 times upper limit of normal (ULN), total bilirubin up to 2 times upper limit of normal (ULN) in the absence of obstruction, and no other explanation of the mentioned laboratory values 56. Individuals more than 65 years old had a higher incidence of Hy’s law; however, mortality does not differ 20. Hepatocellular injury is more likely than cholestatic injury to have a 10% to 50% higher risk of mortality or to require a liver transplant 27, 56. In general, about 10% of patients progress to requiring liver transplantation. Following liver transplantation, survival is 66% 27, 12. In a prospective study conducted in the United States from 1998-2001, the overall survival rate of patients (including those who received a liver transplant) was 72%. The outcome of acute liver failure is determined by cause, the degree of hepatic encephalopathy present upon admission, and complications such as infections.
- Suh JI. Drug-induced liver injury. Yeungnam Univ J Med. 2020 Jan;37(1):2-12. doi: 10.12701/yujm.2019.00297[↩]
- Goldberg DS, Forde KA, Carbonari DM, Lewis JD, Leidl KB, Reddy KR, Haynes K, Roy J, Sha D, Marks AR, Schneider JL, Strom BL, Corley DA, Lo Re V 3rd. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology. 2015 Jun;148(7):1353-61.e3. doi: 10.1053/j.gastro.2015.02.050[↩]
- Segovia-Zafra A, Di Zeo-Sánchez DE, López-Gómez C, Pérez-Valdés Z, García-Fuentes E, Andrade RJ, Lucena MI, Villanueva-Paz M. Preclinical models of idiosyncratic drug-induced liver injury (iDILI): Moving towards prediction. Acta Pharm Sin B. 2021 Dec;11(12):3685-3726. doi: 10.1016/j.apsb.2021.11.013[↩][↩][↩][↩][↩]
- Galan MV, Potts JA, Silverman AL, Gordon SC. The burden of acute nonfulminant drug-induced hepatitis in a United States tertiary referral center [corrected]. J Clin Gastroenterol. 2005 Jan;39(1):64-7. Erratum in: J Clin Gastroenterol. 2005 Feb;39(2):176.[↩]
- de Abajo FJ, Montero D, Madurga M, García Rodríguez LA. Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br J Clin Pharmacol. 2004 Jul;58(1):71-80. doi: 10.1111/j.1365-2125.2004.02133.x[↩][↩][↩]
- Björnsson E, Ismael S, Nejdet S, Kilander A. Severe jaundice in Sweden in the new millennium: causes, investigations, treatment and prognosis. Scand J Gastroenterol. 2003 Jan;38(1):86-94. doi: 10.1080/00365520310000492[↩]
- Friis H, Andreasen PB. Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med. 1992 Aug;232(2):133-8. doi: 10.1111/j.1365-2796.1992.tb00562.x[↩][↩]
- Vega M, Verma M, Beswick D, Bey S, Hossack J, Merriman N, Shah A, Navarro V; Drug Induced Liver Injury Network (DILIN). The Incidence of Drug- and Herbal and Dietary Supplement-Induced Liver Injury: Preliminary Findings from Gastroenterologist-Based Surveillance in the Population of the State of Delaware. Drug Saf. 2017 Sep;40(9):783-787. doi: 10.1007/s40264-017-0547-9[↩]
- Wysowski DK, Swartz L. Adverse Drug Event Surveillance and Drug Withdrawals in the United States, 1969-2002: The Importance of Reporting Suspected Reactions. Arch Intern Med. 2005;165(12):1363–1369. doi:10.1001/archinte.165.12.1363[↩]
- Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of New Black Box Warnings and Withdrawals for Prescription Medications. JAMA. 2002;287(17):2215–2220. doi:10.1001/jama.287.17.2215[↩]
- Bakke, O.M., Manocchia, M., de Abajo, F., Kaitin, K.I. and Lasagna, L. (1995), Drug safety discontinuations in the United Kingdom, the United States, and Spain from 1974 through 1993: A regulatory perspective. Clinical Pharmacology & Therapeutics, 58: 108-117. https://doi.org/10.1016/0009-9236(95)90078-0[↩]
- Kuna L, Bozic I, Kizivat T, Bojanic K, Mrso M, Kralj E, Smolic R, Wu GY, Smolic M. Models of Drug Induced Liver Injury (DILI) – Current Issues and Future Perspectives. Curr Drug Metab. 2018;19(10):830-838. doi: 10.2174/1389200219666180523095355[↩][↩][↩][↩][↩][↩]
- Zimmerman H. Drug Hepatotoxicity. 2nd ed. Lippincott; Philadelphia: 1999.[↩][↩]
- Larrey D. Hepatotoxicity of herbal remedies. J Hepatol. 1997;26 Suppl 1:47-51. doi: 10.1016/s0168-8278(97)82333-1[↩][↩]
- Aithal GP, Rawlins MD, Day CP. Accuracy of hepatic adverse drug reaction reporting in one English health region. BMJ. 1999 Dec 11;319(7224):1541. doi: 10.1136/bmj.319.7224.1541[↩][↩]
- Larrey D. Epidemiology and individual susceptibility to adverse drug reactions affecting the liver. Semin Liver Dis. 2002;22(2):145-55. doi: 10.1055/s-2002-30105[↩]
- Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, Lenoir C, Lemoine A, Hillon P. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002 Aug;36(2):451-5. doi: 10.1053/jhep.2002.34857[↩][↩]
- Andrade RJ, Chalasani N, Björnsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, Devarbhavi H, Merz M, Lucena MI, Kaplowitz N, Aithal GP. Drug-induced liver injury. Nat Rev Dis Primers. 2019 Aug 22;5(1):58. https://www.zora.uzh.ch/id/eprint/180564/1/NRDP-18-063_Drug-induced_liver_injury_ZORA.pdf[↩][↩][↩]
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013 Jun;144(7):1419-25, 1425.e1-3; quiz e19-20. doi: 10.1053/j.gastro.2013.02.006[↩][↩][↩][↩][↩][↩]
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, Watkins PB, Navarro V, Barnhart H, Gu J, Serrano J; United States Drug Induced Liver Injury Network. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015 Jun;148(7):1340-52.e7. doi: 10.1053/j.gastro.2015.03.006[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005 Jun;4(6):489-99. doi: 10.1038/nrd1750[↩][↩][↩][↩][↩][↩]
- Chen M, Suzuki A, Borlak J, Andrade RJ, Lucena MI. Drug-induced liver injury: Interactions between drug properties and host factors. J Hepatol. 2015 Aug;63(2):503-14. doi: 10.1016/j.jhep.2015.04.016[↩][↩][↩]
- Han D, Shinohara M, Ybanez MD, Saberi B, Kaplowitz N. Signal transduction pathways involved in drug-induced liver injury. Handb Exp Pharmacol. 2010;(196):267-310. doi: 10.1007/978-3-642-00663-0_10[↩][↩][↩]
- Holt M, Ju C. Drug-induced liver injury. Handb Exp Pharmacol. 2010;(196):3-27. doi: 10.1007/978-3-642-00663-0_1[↩][↩][↩]
- Abboud G, Kaplowitz N. Drug-induced liver injury. Drug Saf. 2007;30(4):277-94. doi: 10.2165/00002018-200730040-00001[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication. South Med J. 2014 Feb;107(2):107-13. doi: 10.1097/SMJ.0000000000000059[↩][↩][↩]
- Katarey D, Verma S. Drug-induced liver injury. Clin Med (Lond). 2016 Dec;16(Suppl 6):s104-s109. doi: 10.7861/clinmedicine.16-6-s104[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Verma S, Kaplowitz N. Diagnosis, management and prevention of drug-induced liver injury. Gut. 2009 Nov;58(11):1555-64. doi: 10.1136/gut.2008.163675[↩][↩][↩]
- Francis P, Navarro VJ. Drug Induced Hepatotoxicity. [Updated 2022 Mar 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557535[↩][↩][↩]
- Hoofnagle JH, Björnsson ES. Drug-Induced Liver Injury – Types and Phenotypes. N Engl J Med. 2019 Jul 18;381(3):264-273. doi: 10.1056/NEJMra1816149[↩][↩]
- Amacher DE, Chalasani N. Drug-induced hepatic steatosis. Semin Liver Dis. 2014 May;34(2):205-14. doi: 10.1055/s-0034-1375960[↩][↩]
- Gorayski P, Thompson CH, Subhash HS, Thomas AC. Hepatocellular carcinoma associated with recreational anabolic steroid use. Br J Sports Med. 2008 Jan;42(1):74-5; discussion 75. doi: 10.1136/bjsm.2007.03932. Erratum in: Br J Sports Med. 2009 Oct 1;43(10):764. Erratum in: Br J Sports Med. 2010 Oct;44(13):e5.[↩][↩]
- Musumba CO. Review article: the association between nodular regenerative hyperplasia, inflammatory bowel disease and thiopurine therapy. Aliment Pharmacol Ther. 2013 Nov;38(9):1025-37. doi: 10.1111/apt.12490[↩][↩]
- Brennan PN, Cartlidge P, Manship T, Dillon JF. Guideline review: EASL clinical practice guidelines: drug-induced liver injury (DILI). Frontline Gastroenterol. 2021 Jul 29;13(4):332-336. doi: 10.1136/flgastro-2021-101886[↩][↩][↩][↩]
- Fontana RJ, Seeff LB, Andrade RJ, Björnsson E, Day CP, Serrano J, Hoofnagle JH. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010 Aug;52(2):730-42. doi: 10.1002/hep.23696[↩]
- Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, Hunt CM, Wilke RA, Avigan M, Kaplowitz N, Bjornsson E, Daly AK. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011 Jun;89(6):806-15. doi: 10.1038/clpt.2011.58[↩][↩][↩][↩][↩]
- EASL Clinical Practice Guidelines: Drug-induced liver injury. Clinical Practice Guidelines. Volume 70, ISSUE 6, P1222-1261, June 01, 2019. https://doi.org/10.1016/j.jhep.2019.02.014[↩]
- Vuppalanchi R, Liangpunsakul S, Chalasani N. Etiology of new-onset jaundice: how often is it caused by idiosyncratic drug-induced liver injury in the United States? Am J Gastroenterol. 2007 Mar;102(3):558-62; quiz 693. doi: 10.1111/j.1572-0241.2006.01019.x[↩]
- Wei G, Bergquist A, Broomé U, Lindgren S, Wallerstedt S, Almer S, Sangfelt P, Danielsson A, Sandberg-Gertzén H, Lööf L, Prytz H, Björnsson E. Acute liver failure in Sweden: etiology and outcome. J Intern Med. 2007 Sep;262(3):393-401. doi: 10.1111/j.1365-2796.2007.01818.x[↩]
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010 Dec;52(6):2065-76. doi: 10.1002/hep.23937[↩][↩][↩]
- Björnsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005 Aug;42(2):481-9. doi: 10.1002/hep.20800[↩][↩][↩][↩][↩]
- Robles-Diaz M, Lucena MI, Kaplowitz N, Stephens C, Medina-Cáliz I, González-Jimenez A, Ulzurrun E, Gonzalez AF, Fernandez MC, Romero-Gómez M, Jimenez-Perez M, Bruguera M, Prieto M, Bessone F, Hernandez N, Arrese M, Andrade RJ; Spanish DILI Registry; SLatinDILI Network; Safer and Faster Evidence-based Translation Consortium. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology. 2014 Jul;147(1):109-118.e5. doi: 10.1053/j.gastro.2014.03.050[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Aithal GP. Pharmacogenetic testing in idiosyncratic drug-induced liver injury: current role in clinical practice. Liver Int. 2015 Jul;35(7):1801-8. doi: 10.1111/liv.12836[↩]
- Ashby K, Zhuang W, González-Jimenez A, Alvarez-Alvarez I, Lucena MI, Andrade RJ, Aithal GP, Suzuki A, Chen M. Elevated bilirubin, alkaline phosphatase at onset, and drug metabolism are associated with prolonged recovery from DILI. J Hepatol. 2021 Aug;75(2):333-341. doi: 10.1016/j.jhep.2021.03.021[↩][↩]
- European Association for the Study of the Liver. Electronic address: [email protected]; Clinical Practice Guideline Panel: Chair:; Panel members; EASL Governing Board representative:. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol. 2019 Jun;70(6):1222-1261. doi: 10.1016/j.jhep.2019.02.014[↩][↩][↩][↩][↩][↩][↩]
- Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990 Sep;11(2):272-6. doi: 10.1016/0168-8278(90)90124-a[↩][↩]
- Lucena MI, Sanabria J, García-Cortes M, Stephens C, Andrade RJ. Drug-induced liver injury in older people. Lancet Gastroenterol Hepatol. 2020 Sep;5(9):862-874. doi: 10.1016/S2468-1253(20)30006-6[↩][↩]
- Kayacan, Y. , Yazar, H. & Özdin, M. (2021). COULD THE R-RATIO BE AN INDICATOR OF ACUTE LIVER INJURY FOR THE TREATMENT OF COVID-19? . Journal of Istanbul Faculty of Medicine , 84 (3) , 311-317 . DOI: 10.26650/IUITFD.2021.824074[↩]
- Zimmerman HJ, Ishak KG. General aspects of drug-induced liver disease. Gastroenterol Clin North Am. 1995 Dec;24(4):739-57.[↩]
- Norris W, Paredes AH, Lewis JH. Drug-induced liver injury in 2007. Curr Opin Gastroenterol. 2008 May;24(3):287-97. doi: 10.1097/MOG.0b013e3282f9764b[↩]
- Andrade RJ, Lucena MI, Kaplowitz N, García-Muņoz B, Borraz Y, Pachkoria K, García-Cortés M, Fernández MC, Pelaez G, Rodrigo L, Durán JA, Costa J, Planas R, Barriocanal A, Guarner C, Romero-Gomez M, Muņoz-Yagüe T, Salmerón J, Hidalgo R. Outcome of acute idiosyncratic drug-induced liver injury: Long-term follow-up in a hepatotoxicity registry. Hepatology. 2006 Dec;44(6):1581-8. doi: 10.1002/hep.21424[↩]
- Gonzalez HC, Jafri SM, Gordon SC. Management of Acute Hepatotoxicity Including Medical Agents and Liver Support Systems. Clin Liver Dis. 2017 Feb;21(1):163-180. doi: 10.1016/j.cld.2016.08.012[↩]
- Walayat S, Shoaib H, Asghar M, Kim M, Dhillon S. Role of N-acetylcysteine in non-acetaminophen-related acute liver failure: an updated meta-analysis and systematic review. Ann Gastroenterol. 2021;34(2):235-240. doi: 10.20524/aog.2021.0571[↩][↩]
- Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology. 2017 Jan;65(1):363-373. doi: 10.1002/hep.28813[↩][↩][↩][↩]
- Tujios SR, Lee WM. Acute liver failure induced by idiosyncratic reaction to drugs: Challenges in diagnosis and therapy. Liver Int. 2018 Jan;38(1):6-14. doi: 10.1111/liv.13535[↩][↩][↩][↩]
- Real M, Barnhill MS, Higley C, Rosenberg J, Lewis JH. Drug-Induced Liver Injury: Highlights of the Recent Literature. Drug Saf. 2019 Mar;42(3):365-387. doi: 10.1007/s40264-018-0743-2[↩][↩][↩][↩][↩]
- Zimmerman HJ. Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. Philadelphia: Lippincott; 1999.[↩][↩]
- Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006 Apr;15(4):241-3. doi: 10.1002/pds.1211[↩][↩][↩][↩][↩]
- Senior JR. Alanine aminotransferase: a clinical and regulatory tool for detecting liver injury-past, present, and future. Clin Pharmacol Ther. 2012 Sep;92(3):332-9. doi: 10.1038/clpt.2012.108[↩][↩]
- Reuben A. Hy’s law. Hepatology. 2004 Feb;39(2):574-8. doi: 10.1002/hep.20081[↩]
- Lo Re V 3rd, Haynes K, Forde KA, Goldberg DS, Lewis JD, Carbonari DM, Leidl KB, Reddy KR, Nezamzadeh MS, Roy J, Sha D, Marks AR, De Boer J, Schneider JL, Strom BL, Corley DA. Risk of Acute Liver Failure in Patients With Drug-Induced Liver Injury: Evaluation of Hy’s Law and a New Prognostic Model. Clin Gastroenterol Hepatol. 2015 Dec;13(13):2360-8. doi: 10.1016/j.cgh.2015.06.020[↩]
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, González-Grande R, Pizarro A, Durán JA, Jiménez M, Rodrigo L, Romero-Gomez M, Navarro JM, Planas R, Costa J, Borras A, Soler A, Salmerón J, Martin-Vivaldi R; Spanish Group for the Study of Drug-Induced Liver Disease. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005 Aug;129(2):512-21. doi: 10.1016/j.gastro.2005.05.006. Erratum in: Gastroenterology. 2005 Nov;129(5):1808.[↩][↩]
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008 Dec;135(6):1924-34, 1934.e1-4. doi: 10.1053/j.gastro.2008.09.011[↩]
- Danan G, Benichou C. Causality assessment of adverse reactions to drugs–I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993 Nov;46(11):1323-30. doi: 10.1016/0895-4356(93)90101-6[↩]
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 1999.[↩]
- Schmeltzer PA, Kosinski AS, Kleiner DE, Hoofnagle JH, Stolz A, Fontana RJ, Russo MW; Drug-Induced Liver Injury Network (DILIN). Liver injury from nonsteroidal anti-inflammatory drugs in the United States. Liver Int. 2016 Apr;36(4):603-9. doi: 10.1111/liv.13032[↩][↩]
- de Boer YS, Kosinski AS, Urban TJ, Zhao Z, Long N, Chalasani N, Kleiner DE, Hoofnagle JH; Drug-Induced Liver Injury Network. Features of Autoimmune Hepatitis in Patients With Drug-induced Liver Injury. Clin Gastroenterol Hepatol. 2017 Jan;15(1):103-112.e2. doi: 10.1016/j.cgh.2016.05.043[↩][↩][↩]
- Ahmad J, Odin JA, Hayashi PH, Chalasani N, Fontana RJ, Barnhart H, Cirulli ET, Kleiner DE, Hoofnagle JH. Identification and Characterization of Fenofibrate-Induced Liver Injury. Dig Dis Sci. 2017 Dec;62(12):3596-3604. doi: 10.1007/s10620-017-4812-7[↩][↩]
- Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, Neuhauser M, Lindor K. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010 Jun;51(6):2040-8. doi: 10.1002/hep.23588[↩]
- deLemos AS, Ghabril M, Rockey DC, Gu J, Barnhart HX, Fontana RJ, Kleiner DE, Bonkovsky HL; Drug-Induced Liver Injury Network (DILIN). Amoxicillin-Clavulanate-Induced Liver Injury. Dig Dis Sci. 2016 Aug;61(8):2406-2416. doi: 10.1007/s10620-016-4121-6[↩]
- Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, Hayashi PH, Davern TJ, Navarro V, Reddy R, Talwalkar JA, Stolz A, Gu J, Barnhart H, Hoofnagle JH; Drug-Induced Liver Injury Network (DILIN). Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology. 2014 Feb;59(2):661-70. doi: 10.1002/hep.26709[↩]
- Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, Fontana RJ, Ghabril MS, Barnhart H, Hoofnagle JH; U.S. Drug Induced Liver Injury Network Investigators. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017 Apr;65(4):1267-1277. doi: 10.1002/hep.28967[↩]
- Fontana RJ, Cirulli ET, Gu J, Kleiner D, Ostrov D, Phillips E, Schutte R, Barnhart H, Chalasani N, Watkins PB, Hoofnagle JH. The role of HLA-A*33:01 in patients with cholestatic hepatitis attributed to terbinafine. J Hepatol. 2018 Dec;69(6):1317-1325. doi: 10.1016/j.jhep.2018.08.004[↩]
- Martinez MA, Vuppalanchi R, Fontana RJ, Stolz A, Kleiner DE, Hayashi PH, Gu J, Hoofnagle JH, Chalasani N. Clinical and histologic features of azithromycin-induced liver injury. Clin Gastroenterol Hepatol. 2015 Feb;13(2):369-376.e3. doi: 10.1016/j.cgh.2014.07.054[↩]
- Devarbhavi H, Raj S, Aradya VH, Rangegowda VT, Veeranna GP, Singh R, Reddy V, Patil M. Drug-induced liver injury associated with Stevens-Johnson syndrome/toxic epidermal necrolysis: Patient characteristics, causes, and outcome in 36 cases. Hepatology. 2016 Mar;63(3):993-9. doi: 10.1002/hep.28270[↩][↩]
- Chalasani N, Reddy KRK, Fontana RJ, Barnhart H, Gu J, Hayashi PH, Ahmad J, Stolz A, Navarro V, Hoofnagle JH. Idiosyncratic Drug Induced Liver Injury in African-Americans Is Associated With Greater Morbidity and Mortality Compared to Caucasians. Am J Gastroenterol. 2017 Sep;112(9):1382-1388. doi: 10.1038/ajg.2017.215[↩]
- Pauli-Magnus C, Meier PJ, Stieger B. Genetic determinants of drug-induced cholestasis and intrahepatic cholestasis of pregnancy. Semin Liver Dis. 2010 May;30(2):147-59. doi: 10.1055/s-0030-1253224[↩]
- Robles-Diaz M, Gonzalez-Jimenez A, Medina-Caliz I, Stephens C, García-Cortes M, García-Muñoz B, Ortega-Alonso A, Blanco-Reina E, Gonzalez-Grande R, Jimenez-Perez M, Rendón P, Navarro JM, Gines P, Prieto M, Garcia-Eliz M, Bessone F, Brahm JR, Paraná R, Lucena MI, Andrade RJ; Spanish DILI Registry; SLatinDILI Network. Distinct phenotype of hepatotoxicity associated with illicit use of anabolic androgenic steroids. Aliment Pharmacol Ther. 2015 Jan;41(1):116-25. doi: 10.1111/apt.13023[↩]
- Stolz A, Navarro V, Hayashi PH, Fontana RJ, Barnhart HX, Gu J, Chalasani NP, Vega MM, Bonkovsky HL, Seeff LB, Serrano J, Avula B, Khan IA, Cirulli ET, Kleiner DE, Hoofnagle JH; DILIN Investigators. Severe and protracted cholestasis in 44 young men taking bodybuilding supplements: assessment of genetic, clinical and chemical risk factors. Aliment Pharmacol Ther. 2019 May;49(9):1195-1204. doi: 10.1111/apt.15211[↩]
- Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003 Jul 31;349(5):474-85. doi: 10.1056/NEJMra021844[↩][↩][↩]
- Donnelly MC, Davidson JS, Martin K, Baird A, Hayes PC, Simpson KJ. Acute liver failure in Scotland: changes in aetiology and outcomes over time (the Scottish Look-Back Study). Aliment Pharmacol Ther. 2017 Mar;45(6):833-843. doi: 10.1111/apt.13943[↩]
- Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, Kim YS, Baik GH, Kim JB, Kweon YO, Kim BI, Kim SH, Kim IH, Kim JH, Nam SW, Paik YH, Suh JI, Sohn JH, Ahn BM, Um SH, Lee HJ, Cho M, Jang MK, Choi SK, Hwang SG, Sung HT, Choi JY, Han KH. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012 Sep;107(9):1380-7. doi: 10.1038/ajg.2012.138[↩]
- Wang W, Lie P, Guo M, He J. Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis of published data. Int J Cancer. 2017 Sep 1;141(5):1018-1028. doi: 10.1002/ijc.30678[↩][↩]
- Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, Lanoy E, Texier M, Libenciuc C, Eggermont AM, Soria JC, Mateus C, Robert C. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016 Aug;13(8):473-86. doi: 10.1038/nrclinonc.2016.58[↩][↩]
- The Effect of Cytochrome P450 Metabolism on Drug Response, Interactions, and Adverse Effects. Am Fam Physician. 2007;76(3):391-396. https://www.aafp.org/pubs/afp/issues/2007/0801/p391.html[↩]
- Hunt CM, Westerkam WR, Stave GM. Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol. 1992 Jul 22;44(2):275-83. doi: 10.1016/0006-2952(92)90010-g[↩][↩]
- Lucena MI, Andrade RJ, Kaplowitz N, García-Cortes M, Fernández MC, Romero-Gomez M, Bruguera M, Hallal H, Robles-Diaz M, Rodriguez-González JF, Navarro JM, Salmeron J, Martinez-Odriozola P, Pérez-Alvarez R, Borraz Y, Hidalgo R; Spanish Group for the Study of Drug-Induced Liver Disease. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology. 2009 Jun;49(6):2001-9. doi: 10.1002/hep.22895[↩][↩][↩]
- Lammert C, Einarsson S, Saha C, Niklasson A, Bjornsson E, Chalasani N. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008 Jun;47(6):2003-9. doi: 10.1002/hep.22272[↩]
- Björnsson E, Davidsdottir L. The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepatol. 2009 Mar;50(3):511-7. doi: 10.1016/j.jhep.2008.10.021[↩]
- Amacher DE. Female gender as a susceptibility factor for drug-induced liver injury. Hum Exp Toxicol. 2014 Sep;33(9):928-39. doi: 10.1177/0960327113512860[↩]
- Mayoral W, Lewis JH, Zimmerman H. Drug-induced liver disease. Curr Opin Gastroenterol. 1999 May;15(3):208-16. doi: 10.1097/00001574-199905000-00005[↩]
- Rosenberg P, Urwitz H, Johannesson A, Ros AM, Lindholm J, Kinnman N, Hultcrantz R. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007 Jun;46(6):1111-8. doi: 10.1016/j.jhep.2007.01.024[↩]
- Chien JY, Huang RM, Wang JY, Ruan SY, Chien YJ, Yu CJ, Yang PC. Hepatitis C virus infection increases hepatitis risk during anti-tuberculosis treatment. Int J Tuberc Lung Dis. 2010 May;14(5):616-21.[↩]
- Verma S. Kaplowitz N. Hepatotoxicity of antituberculosis drugs. In: Kaplowitz N, editor; DeLeve LD, editor. Drug-induced liver disease. 3rd. London: Academic Press; 2013. pp. 483–504.[↩]
- Larrey D, Pageaux GP. Genetic predisposition to drug-induced hepatotoxicity. J Hepatol. 1997;26 Suppl 2:12-21. doi: 10.1016/s0168-8278(97)80492-8[↩]
- Ahmad J, Odin JA. Epidemiology and Genetic Risk Factors of Drug Hepatotoxicity. Clin Liver Dis. 2017 Feb;21(1):55-72. doi: 10.1016/j.cld.2016.08.004[↩]
- Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe’er I, Floratos A, Daly MJ, Goldstein DB, John S, Nelson MR, Graham J, Park BK, Dillon JF, Bernal W, Cordell HJ, Pirmohamed M, Aithal GP, Day CP; DILIGEN Study; International SAE Consortium. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009 Jul;41(7):816-9. doi: 10.1038/ng.379[↩]
- Fontana RJ. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology. 2014 Apr;146(4):914-28. doi: 10.1053/j.gastro.2013.12.032[↩]
- Drug-Induced Hepatotoxicity. https://emedicine.medscape.com/article/169814-overview[↩][↩]
- Robles-Díaz M, Medina-Caliz I, Stephens C, Andrade RJ, Lucena MI. Biomarkers in DILI: One More Step Forward. Front Pharmacol. 2016 Aug 22;7:267. doi: 10.3389/fphar.2016.00267[↩]
- Björnsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: Critical assessment based on published case reports. Hepatology. 2016 Feb;63(2):590-603. doi: 10.1002/hep.28323[↩][↩]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005 Jun;41(6):1313-21. doi: 10.1002/hep.20701[↩]
- Andrade RJ, Robles M, Lucena MI. Rechallenge in drug-induced liver injury: the attractive hazard. Expert Opin Drug Saf. 2009 Nov;8(6):709-14. doi: 10.1517/14740330903397378[↩]
- Fisher K, Vuppalanchi R, Saxena R. Drug-Induced Liver Injury. Arch Pathol Lab Med. 2015 Jul;139(7):876-87. doi: 10.5858/arpa.2014-0214-RA[↩]