Fibromyalgia syndrome
Fibromyalgia syndrome also called fibromyalgia, fibro or FMS, is a chronic musculoskeletal pain syndrome characterized widespread body pain, stiffness, and tenderness of the muscles, tendons, and joints 1. Fibromyalgia syndrome is also characterized by restless sleep, tiredness, fatigue, anxiety, depression, and disturbances in bowel functions. But all of these symptoms are common to many other conditions. People with fibromyalgia may be more sensitive to pain than people who don’t have it. This is called abnormal pain perception processing 2. People who have fibromyalgia often experience chronic pain (pain that lasts a long time – possibly your entire life). People with fibromyalgia also have “tender points” on their body. Tender points are specific places on the neck, shoulders, back, hips, arms, elbows and legs. These points hurt when pressure is put on them. And because fibromyalgia symptoms can occur alone or along with other conditions, it can take time to tease out which symptom is caused by what problem. To make things even more confusing, fibromyalgia symptoms can come and go over time. That’s why it can take a long time to go from fibromyalgia symptoms to a fibromyalgia diagnosis.
People with fibromyalgia may also have other symptoms or co-exists with other conditions, such as 3:
- Increased sensitivity to pain.
- Fatigue (extreme tiredness) or chronic fatigue syndrome
- Muscle stiffness
- Difficulty sleeping
- Trouble sleeping
- Morning stiffness
- Migraine and other types of headaches
- Painful menstrual periods
- Tingling or numbness in hands and feet
- Problems with mental processes known as “fibro-fog” – such as problems with memory, thinking and concentration
- Pain in the face or jaw, including disorders of the jaw know as temporomandibular joint syndrome (also known as TMJ disorders).
- Irritable bowel syndrome (IBS) – a digestive condition that causes abdominal pain, constipation and bloating
- Interstitial cystitis or painful bladder syndrome
- Anxiety
- Depression
- Postural tachycardia syndrome
There will be times when your fibromyalgia may “flare up” and your symptoms will be worse. Other times, you will feel much better. The good news is that your symptoms can be managed.
Fibromyalgia was first described in the 19th century. In the 1970s and 1980s, a cause of the disease involving the central nervous system was discovered 4. In 1950, Graham introduced the concept of “pain syndrome” in the absence of a specific organic disease 5. The term “fibromyalgia” was later coined by Smythe and Moldofsky following the identification of regions of extreme tenderness known as “pain points” 6. These points are defined as areas of hyperalgesia or allodynia when a pressure of about 4 kg causes pain 7. In 1990, the committee of the American College of Rheumatology drew up diagnostic criteria, which have only recently been modified 8, 9. According to the American College of Rheumatology, the diagnosis of fibromyalgia includes two variables: (1) bilateral pain above and below the waist, characterized by centralized pain, and (2) chronic generalized pain that lasts for at least three months, characterized by pain on palpation in at least 11 of 18 specific body sites (see Figure 1) 10.
No one knows what causes fibromyalgia. Anyone can get it, but fibromyalgia is most common in middle-aged women (7 times as many women as men). People with rheumatoid arthritis and other autoimmune diseases are particularly likely to develop fibromyalgia.
The prevalence of fibromyalgia syndrome in adults is about 2 to 3% in the USA and other countries 11. Fibromyalgia syndrome is higher among women (3.4%) than men (0.5%). It increases with age 12. The average age of diagnosis in adults is around 40-50 years, and 13-15 years for children and adolescents 13. Between the ages of 20 to 55 years, the cause of generalized, musculoskeletal pain in most women is fibromyalgia 14. The prevalence in adolescents has been found to be similar to those in adults in many studies. Amongst the patients referred to a tertiary care pain clinic, more than 40% met the criteria for fibromyalgia 15. The risk for fibromyalgia is higher if you have an existent rheumatic disease.
Fibromyalgia is more common in women than men because of the following 14:
- Higher levels of anxiety
- Use of maladaptive coping methods
- Altered behavior in response to pain
- Higher levels of depression
- Altered input to the central nervous system (CNS) and hormonal effects of the menstrual cycle.
Anyone can get fibromyalgia, though it occurs most often in women and often starts in middle age. If you have certain other diseases, you may be more likely to have fibromyalgia. These diseases include:
- Rheumatoid arthritis.
- Systemic lupus erythematosus (commonly called lupus or SLE).
- Ankylosing spondylitis (spinal arthritis).
If you have a family member with fibromyalgia, you may be more likely to get fibromyalgia.
The cause of fibromyalgia remains unknown, but recent advances and discoveries have helped to unravel some of the mysteries of this disease. Research highlights some of the biochemical, metabolic, and immunoregulatory abnormalities associated with fibromyalgia.
In fibromyalgia, there appears to a problem with the processing of pain in the brain. Patients often become hypersensitive to the perception of pain. The constant hypervigilance of pain is also associated with numerous psychological issues. Abnormalities noted in fibromyalgia include 14:
- Elevated levels of the excitatory neurotransmitters like glutamate and substance P
- Diminished levels of serotonin and norepinephrine in the descending anti-nociceptive pathways in the spinal cord
- Prolonged enhancement of pain sensations
- Dysregulation of dopamine
- Alteration in the activity of brain endogenous opioids.
Management of fibromyalgia syndrome at the present time is very difficult as it has multiple etiological factors and psychological predispositions; however, a patient centered approach is essential to handle this problem.
If you think you have fibromyalgia, visit your doctor. Treatment is available to ease some of its symptoms, although they’re unlikely to disappear completely.
It’s important to have a health care team that understands fibromyalgia and has experience treating fibromyalgia. Your team will probably include your family doctor, a rheumatologist, and a physical therapist. Other health care professionals may help you manage other symptoms, such as mood or sleep problems. However, the most important member of your health care team is you. The more active you are in your care, the better you will feel.
There is no cure for fibromyalgia, but fibromyalgia can be effectively treated and managed with a variety of medications and self-care strategies. It’s important for you to be responsible for your health. Getting enough sleep, exercising, stress-reduction and eating well may also help. No one treatment works for all symptoms, but trying a variety of treatment strategies can have a cumulative effect.
Exercise seems to be the most effective treatment, including yoga, tai chi, or other low-impact aerobic activity. Acupuncture, chiropractic, and massage may help ease symptoms. Psychotherapy may help patients manage stress and anxiety. A sleep specialist may help patients address sleep disorders.
Medications can help reduce the pain of fibromyalgia and improve sleep. Three drugs are FDA-approved for fibromyalgia: Duloxetine (Cymbalta) and Milnacipran (Savella) adjust brain chemicals to ease widespread pain and fatigue associated with fibromyalgia and Pregabalin (Lyrica), which blocks overactive nerve cells involved in pain. Pregabalin (Lyrica) was the first drug approved by the Food and Drug Administration to treat fibromyalgia.
- Pain relievers. Over-the-counter pain relievers such as acetaminophen (Tylenol, others), ibuprofen (Advil, Motrin IB, others) or naproxen sodium (Aleve, others) may be helpful. Opioid medications are not recommended, because they can lead to significant side effects and dependence and will worsen the pain over time.
- Older drugs, such as amitryptiline (Elavil), cyclobenzaprine (Flexeril) and other antidepressants may be used to help promote sleep. Opioids and sleep medicines like zolpidem (Ambien) are not recommended for use in treating fibromyalgia symptoms.
- Anti-seizure drugs. Medications designed to treat epilepsy are often useful in reducing certain types of pain. Gabapentin (Neurontin) is sometimes helpful in reducing fibromyalgia symptoms.
Self-care is critical in the management of fibromyalgia:
- Stress management. Develop a plan to avoid or limit overexertion and emotional stress. Allow yourself time each day to relax. That may mean learning how to say no without guilt. But try not to change your routine completely. People who quit work or drop all activity tend to do worse than do those who remain active. Try stress management techniques, such as deep-breathing exercises or meditation.
- Sleep hygiene. Because fatigue is one of the main components of fibromyalgia, getting good quality sleep is essential. In addition to allotting enough time for sleep, practice good sleep habits, such as going to bed and getting up at the same time each day and limiting daytime napping.
- Exercise regularly. At first, exercise may increase your pain. But doing it gradually and regularly often decreases symptoms. Appropriate exercises may include walking, swimming, biking and water aerobics. A physical therapist can help you develop a home exercise program. Stretching, good posture and relaxation exercises also are helpful.
- Pace yourself. Keep your activity on an even level. If you do too much on your good days, you may have more bad days. Moderation means not overdoing it on your good days, but likewise it means not self-limiting or doing too little on the days when symptoms flare.
- Maintain a healthy lifestyle. Eat healthy foods. Do not use tobacco products. Limit your caffeine intake. Do something that you find enjoyable and fulfilling every day.
Doctors usually treat fibromyalgia with a combination of treatments, which may include:
- Medications, including prescription drugs and over-the-counter pain relievers.
- Aerobic exercise and muscle strengthening exercise.
- Patient education classes, usually in primary care or community settings.
- Stress management techniques such as meditation, yoga, and massage.
- Good sleep habits to improve the quality of sleep.
- Cognitive behavioral therapy (CBT) to treat underlying depression. CBT is a type of talk therapy meant to change the way people act or think.
In addition to medical treatment, people can manage their fibromyalgia with the self-management strategies described below, which are proven to reduce pain and disability, so they can pursue the activities important to them.
Self-Management Resources:
- You can join a self-management education class, which helps people with arthritis or other conditions—including fibromyalgia—be more confident in how to control their symptoms, how to live well and understand how the condition affects their lives. You can find more info on Self-Management Resource Center here: https://www.selfmanagementresource.com/
- Chronic Disease Self-Management Program is an effective self-management education workshop for people with chronic health problems. The program specifically addresses arthritis, diabetes, lung and heart disease, but teaches skills useful for managing a variety of chronic diseases. This program was developed at Stanford University. Locate a Chronic Disease Self-Management Program in your area here: http://www.eblcprograms.org/evidence-based/map-of-programs/
However, there isn’t one treatment plan that works best for every person who has fibromyalgia. You’ll have to work with your care team to create a plan that’s right for you. After all, nobody knows more than you do about your feelings, your actions, and how your fibromyalgia symptoms affect you.
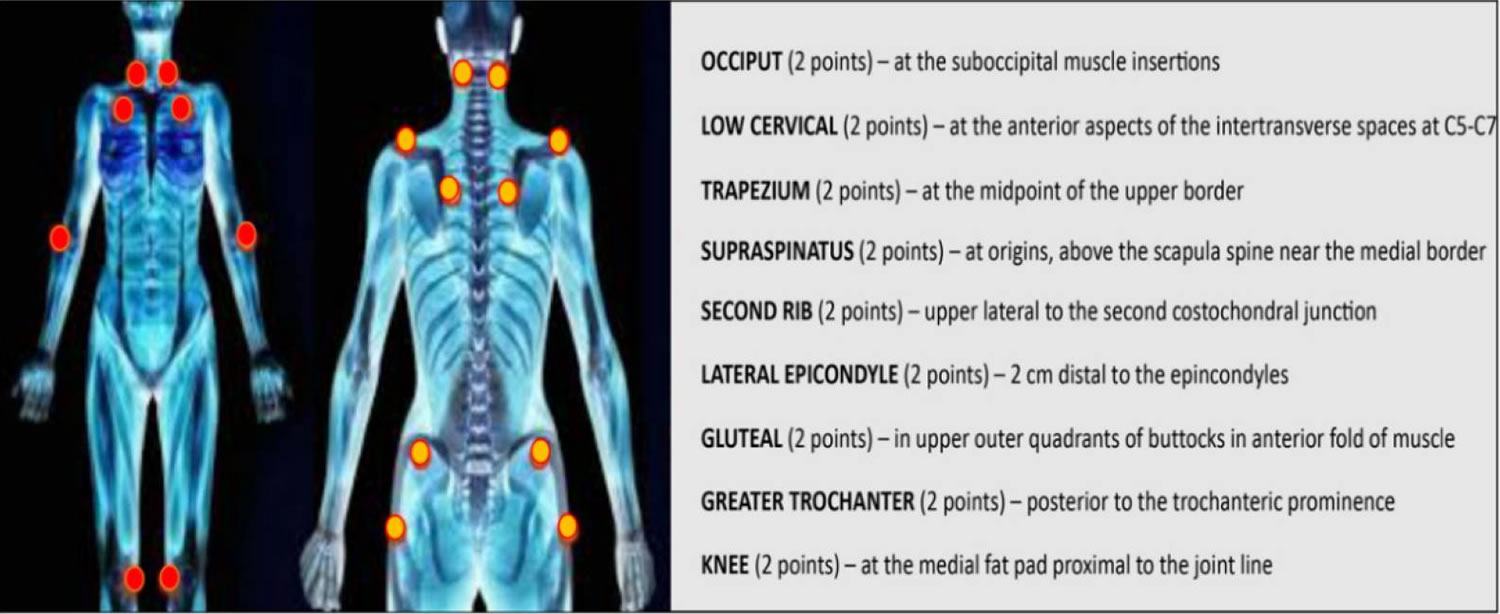
Figure 1. Fibromyalgia tender points
Footnote: The dots indicate the 18 tenderness points important for the diagnosis of fibromyalgia.
[Source 16 ]Is fibromyalgia real?
This is the top misconception where people think fibromyalgia isn’t a real medical problem or that it is “all in your head.” Despite there’s a lot that’s unknown about fibromyalgia, researchers have learned more about it in just the past few years.
In people who have fibromyalgia, the brain and spinal cord process pain signals differently. As a result, they react more strongly to touch and pressure, with a heightened sensitivity to pain. It is a real physiological and neurochemical problem.
The power of the mind is a real factor in pain perception. For example, studies have shown that anxiety that occurs in anticipation of pain is often more problematic than the pain experience itself. In that sense, the mind has a negative impact on symptoms. It takes lifestyle changes and small steps toward achieving wellness.
Why do I feel depressed?
Depression or anxiety may occur as a result of your constant pain and fatigue, or the frustration you feel with the condition. It is also possible that the same chemical imbalances in the brain that cause mood changes also contribute to fibromyalgia.
Does fibromyalgia cause permanent damage?
No. Although fibromyalgia causes symptoms that can be very painful and uncomfortable, your muscles and organs are not being damaged. Fibromyalgia is not life-threatening, but it is chronic (ongoing and lasting more than 3 months). Although there is no cure, there are many things you can do to feel better.
Is it hard to diagnose fibromyalgia?
Unfortunately, it can take years for some people who have fibromyalgia to get a correct diagnosis. This can happen for many reasons. The main symptoms of fibromyalgia are generalized muscle pain and fatigue. These are also common symptoms of many other health problems, such as chronic fatigue syndrome, hypothyroidism, and arthritis.
Currently, there is no laboratory test or X-ray that can diagnose fibromyalgia.
It may take some time for your doctor to understand all of your symptoms and rule out other health problems so he or she can make an accurate diagnosis. As part of this process, your family doctor may consult with a rheumatologist. This type of doctor specializes in pain in the joints and soft tissue.
Juvenile fibromyalgia syndrome
Juvenile fibromyalgia syndrome also called juvenile primary fibromyalgia syndrome, is a chronic condition characterized by symptoms of chronic diffuse musculoskeletal pain and multiple painful tender points on palpation 11. Juvenile fibromyalgia syndrome is often accompanied by fatigue, disorders of sleep, chronic headaches, irritable bowel syndrome, and subjective soft tissue swelling. The complexity of the presenting clinical picture in juvenile fibromyalgia syndrome has not been sufficiently defined in the literature 11. Similarities to adult fibromyalgia syndrome in juvenile fibromyalgia syndrome are often difficult to compare, because many of the symptoms are “medically unexplained” and often overlap frequently with other medical conditions. However, a valid diagnosis of juvenile fibromyalgia syndrome often decreases parents’ anxiety, reduces unnecessary further investigations, and provides a rational framework for a management plan. The diagnostic criteria proposed by Yunus and Masi in 1985 to define juvenile fibromyalgia syndrome were never validated or critically analyzed. In most cases, the clinical diagnosis is based on the history, the physical examination that demonstrates general tenderness (muscle, joints, tendons), the absence of other pathological conditions that could explain pain and fatigue, and the normal basic laboratory tests. Research and clinical observations defined that juvenile fibromyalgia syndrome may have a chronic course that impacts the functional status and the psychosocial development of children and adolescents.
The reported prevalence of juvenile fibromyalgia syndrome varies widely probably reflecting differences in ethnicity, socio-cultural background, psychological traits of the population and diverse methodologies that have been used in the published studies 17. The prevalence of juvenile fibromyalgia syndrome reported in the literature in different countries is summarized in Table 1. Juvenile fibromyalgia syndrome has a prevalence around 1-6%, more common in girls, and can be seen in children of all ages.
Table 1. Prevalence of juvenile fibromyalgia syndrome in children and adolescents: review of the literature
| References | Diagnostic criteria | Cohort variables | Country/prevalence |
|---|---|---|---|
| Buskila D, Press J, Gedalia A, Klein M, Neumann L, Boehm R, Sukenik S. Assessment of nonarticular tenderness and prevalence of fibromyalgia in children. J Rheumatol. 1993 Feb;20(2):368-70. | 1990 ACR criteria | 338 healthy school children, 179 boys and 159 girls, aged 9 to 15 yrs. | Israel Prevalence 6.2%. |
| Clark P, Burgos-Vargas R, Medina-Palma C, Lavielle P, Marina FF. Prevalence of fibromyalgia in children: a clinical study of Mexican children. J Rheumatol. 1998 Oct;25(10):2009-14. | 1990 ACR criteria. | 548 children, 264 boys and 284 girls, aged 9-15. | Mexico Prevalence 1.2%. |
| Mikkelsson M. One year outcome of preadolescents with fibromyalgia. J Rheumatol. 1999 Mar;26(3):674-82. | Structured pain questionnaire to assess the prevalence and persistence of self-reported musculo-skeletal pain symptoms and disability caused by pain. | 1626 third and fifth grade schoolchildren | Finland Prevalence 1.3% at baseline. |
| Weir PT, Harlan GA, Nkoy FL, Jones SS, Hegmann KT, Gren LH, Lyon JL. The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on International Classification of Diseases, 9th Revision codes. J Clin Rheumatol. 2006 Jun;12(3):124-8. doi: 10.1097/01.rhu.0000221817.46231.18 | ICD-9 criteria (*) | 2595 incident cases of adult and juvenile fibromyalgia syndrome | U.S.A The estimated prevalence per age group was: 0.5 to1% for 0-4 yrs; 1 to 1.4% for 5-9 yrs; 2 to 2.6% for 10-14 yrs; and 3.5 to 6.2% for 15-19 yrs. |
| Fuda A et al. Egypt Rheumatol Rehabil 2014; 41: 135-138 | A questionnaire was completed by students. A clinical diagnosis of fibromyalgia was established in only 25 cases. | 2000 students: 960 boys (48%) and 1040 girls (52%). Ages: 9-15 yrs, mean 11.9 yrs | Egypt prevalence 1.2%. |
Abbreviations: JFMS = juvenile fibromyalgia syndrome; FM = fibromyalgia; (*) International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes to identify fibromyalgia cases (ICD code 729.1).
[Source 11 ]Juvenile fibromyalgia syndrome in children and adolescents treatments
Goals of treatment should be pain relief, restoration of functioning, reduction of school absenteeism, dissolving social isolation, strengthening self-awareness, mobilizing domestic resources and the development of strategies for coping with pain. The inclusion of the family, the training of strategies in everyday life and the treatment of mental comorbidities are also important 18.
Evidence-based treatment guidelines in ibromyalgia include those developed by the American Pain Society (APS) in 2005 19 and the European League Against Rheumatism (EULAR) in 2008 20.
However, they were developed before FDA approved any medications for treating fibromyalgia and substantial heterogeneity exists between the recommendations. Furthermore, the studies evaluated during the development of these guidelines were not directly comparable as a result of variations in study design and short duration that limit their general applicability in clinical practice 21.
Little is known regarding treatment choices of youth diagnosed with juvenile fibromyalgia syndrome as they move into young adulthood. The management of juvenile fibromyalgia syndrome is centered on the issues of education, behavioral and cognitive change (cognitive-behavioral therapy [CBT]) with a strong emphasis on physical exercise), and a relatively minor role for pharmacological treatment with medications such as muscle relaxants, analgesics and tricyclic agents 22. Any patient being treated with a medication should be carefully evaluated for both efficacy as well as side effects, and medications should be discontinued unless there is evidence for definite benefit. More controlled studies are needed to investigate the effectiveness of these complementary methods to assist treatment providers in giving evidence-based treatment recommendations.
A recent Cochrane review has concluded that psychological treatments may improve pain control for children with a variety of pain conditions, including muscle pain, abdominal pain, headaches and fibromyalgia syndrome 23. Therefore, it appears that CBT should be offered as a preferred modality of non-pharmacological treatment for juvenile fibromyalgia syndrome.
If disease development is assumed to be linked to the family background, hospitalization aimed to temporarily isolate the patient from his or her home environment should be taken into consideration 24.
Prompt recognition of juvenile fibromyalgia syndrome may decrease problems for pediatric patients with chronic pain, while pediatric primary care providers’ lack of familiarity with juvenile fibromyalgia syndrome can cause a delay in diagnosis and management 25.
Fibromyalgia in children and adolescents prognosis
Initial studies indicated a positive long-term prognosis for juvenile fibromyalgia syndrome 26. By contrast, studies in subjects with juvenile fibromyalgia syndrome recruited from hospital settings have shown a chronic and fluctuating course, with symptoms persisting in ~70% of young people 27.
One controlled study published in 2010 of patients with juvenile fibromyalgia syndrome and matched healthy controls (mean age, 15 years) showed that about 50% of patients with juvenile fibromyalgia met the full American College of Rheumatology criteria for fibromyalgia at ~4 years follow-up (mean age, 19 years), and >70% had continuing symptoms of pain, fatigue or sleep difficulty 27.
Several authors report different prognoses between adults and youths with fibromyalgia 28. They suggested that the early detection of juvenile fibromyalgia syndrome is an indication of a better prognosis 29, with significant gains in quality of life 30, and functionality for individuals who receive adequate treatment, whereas those with widespread pain that are not treated adequately have a greater chance of developing fibromyalgia 31. On the other hand, in a large prospective longitudinal study of juvenile fibromyalgia syndrome patients, Kashikar-Zuck et al. 32 found that the majority of adolescent patients (~80%) with juvenile fibromyalgia syndrome seen in a pediatric specialty care setting continued to report persistent pain and other FM symptoms as they transitioned into young adulthood.
In conclusion, most of youth with juvenile fibromyalgia syndrome continue to experience symptoms into adulthood, which highlights the importance of early diagnosis and intervention. However, more research into the variability of outcomes within the juvenile primary fibromyalgia syndrome group, with closer examination of risk and protective factors associated with future outcomes is essential to designing focused interventions.
Fibromyalgia syndrome causes
The exact cause of fibromyalgia is unknown, but fibromyalgia is thought to be related to abnormal levels of certain chemicals in the brain and changes in the way the central nervous system (brain, spinal cord and nerves) processes pain messages carried around the body 33, 34, 35. Current evidence describes fibromyalgia pain as the result of a complex evaluative process of environmental and multisystem information 36, 37. For this reason, currently, pain is considered a personal somatic experience in response to a threat to bodily or existential integrity 38. Pain and sensory processing alterations in the central nervous system (brain and spinal cord) are present in fibromyalgia 35. Patients perceive noxious stimuli as being painful at lower levels of physical stimulation compared to healthy controls 39. With rapidly repetitive short noxious stimuli to fibromyalgia patients, they experience higher than normal increases in the perceived intensity of pain. There appears to be a deficiency in the endogenous analgesic systems in patients with fibromyalgia. There has been a demonstration of differences in activation of areas of the brain which are pain-sensitive areas by functional neuroimaging techniques 40.
There is no evidence of any single event cause of fibromyalgia syndrome; instead, it is triggered or aggravated by multiple physical and/or emotional stressors which include infections as well as emotional and physical trauma 40.
It’s also suggested that some people are more likely to develop fibromyalgia because of genes inherited from their parents, though there is no documentation of a definitive candidate gene 41.
There is most often some triggering factor that sets off fibromyalgia. It may be spine problems, arthritis, injury, or other type of physical stress. Emotional stress also may trigger fibromyalgia. The result is a change in the way your body “talks” with your spinal cord and brain. Levels of brain chemicals and proteins may change. More recently, fibromyalgia has been described as Central Pain Amplification disorder, meaning the volume of pain sensation in the brain is turned up too high.
In many cases, fibromyalgia appears to be triggered by a physically or emotionally stressful event, such as:
- an injury or infection
- giving birth
- having an operation
- the breakdown of a relationship
- the death of a loved one
- illness or other diseases
- post-traumatic stress disorder (PTSD)
- repetitive injuries
- obesity.
Here are some of the main factors thought to contribute to fibromyalgia:
Abnormal pain messages
One of the main theories is that people with fibromyalgia have developed changes in the way the central nervous system processes the pain messages carried around the body. This could be due to changes to chemicals in the nervous system.
The central nervous system (brain, spinal cord and nerves) transmits information all over your body through a network of specialized cells. Changes in the way this system works may explain why fibromyalgia results in constant feelings of, and extreme sensitivity to, pain.
Chemical imbalances
Research has found that people with fibromyalgia have abnormally low levels of the hormones serotonin, noradrenaline and dopamine in their brains.
Low levels of these hormones may be a key factor in the cause of fibromyalgia, as they’re important in regulating things such as:
- mood
- appetite
- sleep
- behavior
- your response to stressful situations
These hormones also play a role in processing pain messages sent by the nerves. Increasing the hormone levels with medication can disrupt these signals.
Some researchers have also suggested that changes in the levels of some other hormones, such as cortisol (which is released when the body is under stress), may contribute to fibromyalgia.
Sleep problems
It’s possible that disturbed sleep patterns may be a cause of fibromyalgia, rather than just a symptom.
Fibromyalgia can prevent you from sleeping deeply and cause fatigue (extreme tiredness). People with the condition who sleep badly can also have higher levels of pain, suggesting that these sleep problems contribute to the other symptoms of fibromyalgia.
Genetics
Research has suggested that genetics may play a small part in the development of fibromyalgia, with some people perhaps more likely than others to develop the condition because of their genes, although there is no evidence of a definitive gene has been found 41. Currently, about 100 genes that regulate pain are believed to be relevant to pain sensitivity or analgesia. The main genes are those encoding for voltage-dependent sodium channels, GABAergic pathway proteins, mu-opioid receptors, catechol-O-methyltransferase and GTP cyclohydrolase 1 42. Further studies are needed to understand the role of these genes in chronic pain conditions such as fibromyalgia.
Associated conditions
There are several other conditions often associated with fibromyalgia. Generally, these are rheumatic conditions (affecting the joints, muscles and bones), such as:
- Osteoarthritis – when damage to the joints causes pain and stiffness
- Lupus – when the immune system mistakenly attacks healthy cells and tissues in various parts of the body
- Rheumatoid arthritis – when the immune system mistakenly attacks healthy cells in the joints, causing pain and swelling
- Ankylosing spondylitis – pain and swelling in parts of the spine
- Temporomandibular disorder – a condition that can cause pain in the jaw, cheeks, ears and temples
Conditions such as these are usually tested for when diagnosing fibromyalgia.
Possible triggers
Fibromyalgia is often triggered by a stressful event, including physical stress or emotional (psychological) stress. Possible triggers for the condition include:
- an injury
- a viral infection
- giving birth
- having an operation
- the breakdown of a relationship
- being in an abusive relationship
- the death of a loved one
However, in some cases, fibromyalgia doesn’t develop after any obvious trigger.
Risk factors for fibromyalgia
Known risk factors for fibromyalgia include:
- Age. Fibromyalgia can affect people of all ages, including children. However, most people are diagnosed during middle age and you are more likely to have fibromyalgia as you get older.
- Lupus or Rheumatoid Arthritis. If you have lupus or rheumatoid arthritis (RA), you are more likely to develop fibromyalgia.
Some other factors have been weakly associated with onset of fibromyalgia, but more research is needed to see if they are real. These possible risk factors include:
- Sex. Women are seven times as likely to have fibromyalgia as men.
- Stressful or traumatic events, such as car accidents, post-traumatic stress disorder (PTSD).
- Repetitive injuries. Injury from repetitive stress on a joint, such as frequent knee bending.
- Illness (such as viral infections).
- Family history. You may be more likely to develop fibromyalgia if a parent or sibling also has fibromyalgia.
- Obesity.
Fibromyalgia syndrome pathophysiology
Fibromyalgia appears to be related to a pain-processing problem in your brain 43, 44, 16. In most cases, people with fibromyalgia become hypersensitive to the perception of pain 16. The constant hypervigilance to pain can also be associated with psychological problems 43.
Abnormalities noted in fibromyalgia include 43:
- Elevated levels of the excitatory neurotransmitters like glutamate and substance P
- Diminished levels of serotonin and norepinephrine (also known as noradrenaline) in the descending anti-nociceptive pathways in the spinal cord
- Prolonged enhancement of pain sensations
- Dysregulation of dopamine
- Alteration in the activity of brain endogenous opioids.
Fibromyalgia is more common in women than men because of the following 43:
- Higher levels of anxiety
- Use of maladaptive coping methods
- Altered behavior in response to pain
- Higher levels of depression
- Altered input to the central nervous system (brain and spinal cord)
- Hormonal effects of the menstrual cycle
The main alterations observed in fibromyalgia are dysfunctions in mono-aminergic neurotransmission, leading to elevated levels of excitatory neurotransmitters, such as glutamate and substance P, and decreased levels of serotonin and norepinephrine in the spinal cord at the level of descending anti-nociceptive pathways. Other anomalies observed are dopamine dysregulation and altered activity of endogenous cerebral opioids. Taken together, these phenomena seem to explain the central physiopathology of fibromyalgia 45.
Over the years, peripheral pain generators have also been recognized as a possible cause of fibromyalgia. In this case, patients manifest symptoms such as cognitive impairment (“fibro-fog”), chronic fatigue, sleep disturbances, intestinal irritability, interstitial cystitis and mood disorders 46, 47.
Peripheral abnormalities may contribute to increased nociceptive tonic supply in the spinal cord, which results in central sensitization. Other factors that appear to be involved in the pathophysiology of fibromyalgia are neuroendocrine factors, genetic predisposition, oxidative stress and environmental and psychosocial changes 48, 49.
Pain-processing problem in the brain
Fibromyalgia appears to be related to a pain-processing problem in your brain also known as central sensitization 43, 44, 16. In most cases, people with fibromyalgia become hypersensitive to the perception of pain 16. Central sensitization refers to a neuronal signal amplification mechanism within the central nervous system (brain and spinal cord) that leads to a greater perception of pain 50. For this reason, patients with fibromyalgia present an increase in the receptive field of pain, allodynia and hyperalgesia. Central sensitization is also implicated in persistent and chronic pain. Although central sensitization plays an important role in fibromyalgia, it is even more important to understand the initial cause, that is, the persistent nociceptive input associated with tissue damage, including peripheral sensitization 51. According to Vierck 51, if peripheral pain generators can be blocked, the symptoms of fibromyalgia should disappear or not even develop. Despite this, researchers focus more on central sensitization as the mechanism of pain sensitivity because there is less evidence to support the involvement of peripheral pain tissue abnormalities and nociceptive processes in fibromyalgia 52. Brosschot et al. 53 observed that fibromyalgia patients were selectively attentive to information regarding the body and the environment in relation to pain. In this regard, they introduced the term “cognitive-emotional sensitization” to explain how selective attention to certain body pain can increase the perception of that pain. Pain sensitivity is also linked to social groups. It has been suggested that the mechanism that underlies “interpersonal sensitization” could be linked to the shared neuronal representation of the experience of pain. In other words, a feed-forward effect occurs in which a family, in an attempt to reduce painful behaviors in one of its members, actually creates a state of anxiety in the person concerned by increasing the perception of pain 54, 55.
Patients with fibromyalgia present a lower pain threshold that generates a condition of diffuse hyperalgesia and/or allodynia. This indicates that there may be a problem with the amplification of pain or with sensory processing in the central nervous system (brain and spinal cord). These fibromyalgia phenomena have been confirmed in clinical studies that used functional neuroimaging or measured alterations in neurotransmitter levels that influence sensory transmission and pain 56, 57, 58. It was also observed that treatments with drugs aimed at increasing anti-nociceptive neurotransmitters in the central nervous system (brain and spinal cord) or at lowering the levels of pro-nociceptive excitatory neurotransmitters, such as glutamate, were able to improve these conditions in patients with fibromyalgia. Exercise has also proved useful for increasing anti-nociceptive neurotransmitters and reducing glutamate 59, 60. In contrast, patients with fibromyalgia do not respond to non-steroidal anti-inflammatory drug (NSAID) therapies aimed at resolving acute pain or pain induced by tissue damage or inflammation.
The chronic pain typical of fibromyalgia is due to alterations in central and peripheral sensitization. Over the years, researchers have searched for biomarkers that are capable of detecting these changes. In particular, they focused on factors capable of acting on the growth and survival of nerve cells, such as nerve growth factor (NGF). The nerve growth factor (NGF) is indeed involved in promoting the growth, proliferation and survival of sensory neurons that transmit pain, temperature and tactile sensations 61. Data obtained in earlier studies showed an increase in nerve growth factor (NGF) in the cerebrospinal fluid of fibromyalgia patients 62. However, these data disagree with those from a recent study in patients, in which plasma NGF levels were not found to differ between fibromyalgia and control subjects. In this regard, different statistical methods were used, which nonetheless led to the same conclusions 63. Further studies are therefore needed to understand the involvement of NGF in the pathophysiology of fibromyalgia.
Inflammation and Immunity
Increasing evidence indicates that neurogenic-derived inflammatory processes occurring in the peripheral tissues, spinal cord and brain are also responsible for the pathophysiology of fibromyalgia 64, 65, 66. The release of biologically active agents, such as chemokines and cytokines, leads to the activation of the innate and adaptive immune system. All of this translates into many of the peripheral clinical features reported by patients with fibromyalgia, such as swelling and dysesthesia, which can also affect central symptoms, including cognitive changes and fatigue. In addition, the physiological mechanisms related to stress and emotions are considered to be upstream drivers of neurogenic inflammation in fibromyalgia 67.
Studies conducted in patients have confirmed that inflammation is involved in fibromyalgia. Fibromyalgia patients have been shown to have enhanced circulating inflammatory cytokines and inflammatory cytokines released by circulating immune cells 66, 68.
Kadetoff et al. 69 described an increased concentration of IL-8 in the cerebrospinal fluid of fibromyalgia patients compared to healthy subjects. This finding could be due to the activation of glial cells, which play an important role in the central sensitization process, as they are activated in response to excitatory synaptic signals (glutamate) 70. Furthermore, since the synthesis of IL-8 is dependent on orthosympathetic activation, this could help explain the correlation between stress and fibromyalgia symptoms 71. In addition to this, some studies have shown an increase in serum concentrations of IL-6, IL-8, IL-1β and TNF-α in individuals with fibromyalgia, although no clear correlation with symptom severity has been identified, except, perhaps, for IL-6 66, 72, 73, 74. It appears that immune cells such as mast cells, monocytes and neutrophils, as mediators of inflammation processes, may also have a function in defining an inflammatory substrate of fibromyalgia 75. In animals, macrophages located in the muscle have been shown to contribute to the development of chronic widespread muscle pain. For example, the removal of macrophages at the acid injection site through a local injection of clodronate liposomes is capable of preventing the development of exercise-induced hyperalgesia 76. Another observation is that pro-inflammatory cytokines, such as interleukins (IL-1β, IL-6) and tumor necrosis factor (TNFα), can activate and sensitize nociceptors, induce pain in humans and trigger hyperalgesia in animals. Another potential source of these cytokines is adipose tissue; many studies suggest that diffuse or multifocal pain is more common in obese individuals 77 and obese animals show enhanced nociceptive responses 78, 79. Therefore, pro-inflammatory cytokines could play a role in the generation of chronic muscle pain, including fibromyalgia.
Smart et al. 80 described a subgroup of fibromyalgia patients characterized by ANA (anti-nuclear antibody) positivity, with the speckled pattern clearly predominating. The use of the Smart Index, which corrects the erythrocyte sedimentation rate value in relation to age, revealed that ANA-positive fibromyalgia patients had a more pronounced inflammatory response profile than the ANA-negative subgroup, suggesting that autoimmunity potentially contributes to sub-inflammatory fibromyalgia 80.
Genetic factors
Over the years, studies have shown the potential involvement of genetic factors in the onset of fibromyalgia 81, 82. Linkage studies have shown a correlation rate of 50% between genetic variants and the development of chronic pain 83. Currently, about 100 genes that regulate pain are believed to be relevant to pain sensitivity or analgesia. The main genes are those encoding for voltage-dependent sodium channels, GABAergic pathway proteins, mu-opioid receptors, catechol-O-methyltransferase and GTP cyclohydrolase 1 42. The small sample sizes did not allow the authors to confirm an association between single nucleotide polymorphisms and fibromyalgia susceptibility. However, a genome-wide linkage scan study found that first-degree relatives had an increased risk of developing fibromyalgia, reinforcing the genetic hypothesis. The serotonin transporter gene (SLC64A4) and the transient receptor 2 potential vanillic channel gene (TRPV2) are the major genes responsible for pain susceptibility in fibromyalgia 84. SLC64A4 is characterized by a single nucleotide polymorphism and is associated with chronic pain conditions (for example, mandibular joint disorder), as well as increased levels of depression and psychological disorders related to an alteration in serotonin reuptake 85. The TRPV2 gene is expressed in mechano- and thermo-responsive neurons in the dorsal root and trigeminal ganglia and appears to be responsible for reducing the pain threshold in fibromyalgia patients 86.
Other genetic polymorphisms that have been identified and associated with fibromyalgia susceptibility are in the serotonin transporter (5-HTT), catechol-O-methyltransferase (COMT) and serotonin 2A (5-HT2A) genes. However, subsequent meta-analyses could only confirm that the 102T/C polymorphism in the 5-HT2A receptor is connected with fibromyalgia 87. Therefore, further studies are needed to understand the role of these genes in chronic pain conditions such as fibromyalgia. A genome-wide association and copy number variant study in 952 fibromyalgia cases and 644 controls revealed the existence of two variables associated with fibromyalgia. One variable is the single nucleotide polymorphism rs11127292 in a gene similar to myelin transcription factor 1 (MYT1L), which is responsible for neuronal differentiation and involved in cognitive alterations. The second is an intron copy number variable in the neurexin 3 (NRXN3) gene, which normally acts as a receptor and cell adhesion molecule in the nervous system, and variations in this gene are involved in autism spectrum disorder 88.
Other researchers analyzed 350 other genes that are specifically involved in pain treatment. Among these is the TAAR1 gene, which mediates the availability of dopamine, whose reduction can increase the sensitivity to pain typical of fibromyalgia 89. Another widely studied gene is RGS4, which is expressed in the dorsal horn of the spinal cord, the locus coeruleus and the nuclei of the bed of the stria terminalis, and is responsible for modulating the descending inhibition of pain perception 90. One gene studied and related to pain disorders is CNR1, which encodes the cannabinoid receptor CB-1 91, 92. Another gene presumably involved in central sensitization is GRIA4, which mediates the rapid excitatory transmission of nociceptive signals in the central nervous system 93. Taken together, these studies have increased the current knowledge on fibromyalgia and support genes as a potential factor in the pathogenesis of this disease. However, as fibromyalgia remains a multifactorial disease, further studies are needed to examine haplotypes and combinations of different variants that could influence its development.
Endocrine factors
The role of stress in the worsening of fibromyalgia symptoms has been widely described from an epidemiological point of view through both self-reports and clinical questionnaires. On the basis of these data, the hypothalamic–pituitary–adrenal axis, central to the stress response, was examined. Despite the discrepancy between different studies on possible alterations in plasma cortisol levels in fibromyalgia patients, dysregulation of its circadian variation is frequently observed. In particular, flattening of the plasma cortisol concentration curve was observed during the day: this seems to manifest itself through a milder and more gradual descent compared to the morning peak of maximum concentration or through a lowering of the peak itself 94, 95, 96, 97, 98. In addition to this, decreased cortisol secretion has also been described in response to adrenocorticotropic hormone (ACTH) tests 99. The hypothalamic–pituitary–adrenal axis (HPA) comprises neurotransmitter and neuroendocrine response systems to stress and can be activated in fibromyalgia 100. This system may explain some of the symptoms seen in fibromyalgia.
A patient study looked at levels of corticotropin-releasing factor (CRF) in cerebrospinal fluid (CSF), heart rate variability (HRV) and pain symptoms (e.g., fatigue and depression) in subjects with fibromyalgia. The results obtained in this study showed that corticotropin-releasing factor (CRF) levels were associated with sensory and affective pain symptoms but not with symptoms of fatigue. Furthermore, an increase in heart rate variability (HRV) was associated with an increase in CRF and pain in patients with fibromyalgia. These results were subsequently adjusted for age, sex and depressive symptoms, and a correlation between CRF levels and sensory pain symptoms was confirmed. Another important finding was that women with fibromyalgia and self-reported histories of physical or sexual abuse did not have increased levels of CRF in their CSF. This indicates that there may be subgroups of fibromyalgia patients with different neurobiological characteristics. Therefore, further studies are needed to better understand the association between CRF and pain symptoms in fibromyalgia 101. In another study, the association between salivary cortisol levels and pain symptoms in patients with fibromyalgia was assessed at different times of day. The results obtained in this study revealed a strong relationship between salivary cortisol and pain symptoms only at the time of awakening and the 1 h that followed in women with fibromyalgia. Furthermore, no relationship was observed between the cortisol level and symptoms of fatigue or stress. These findings suggest that early-day pain symptoms are associated with changes in hypothalamic–pituitary–adrenal axis (HPA) function in women with fibromyalgia.
However, to date, the results regarding the involvement of the hypothalamic–pituitary–adrenal axis (HPA) in the pathophysiology of fibromyalgia have been conflicting, and new studies will be needed in the future to fully clarify this aspect 96. Furthermore, there are indications that total and free cortisol levels are dissociated in fibromyalgia patients. They have normal salivary and free plasma cortisol despite having reduced total cortisol levels. A possible explanation for this finding is a reduced concentration of glucocorticoid-binding globulin (CBG). Reduced levels of glucocorticoid-binding globulin have been reported in fibromyalgia patients compared to healthy patients. It is of particular interest that chronic social stress can lead to reduced levels of glucocorticoid-binding globulin, while IL-6 and IL-1β, which can also inhibit glucocorticoid-binding globulin production, may contribute further 102.
The possible pathogenetic role of the growth hormone (GH)/insulin-like growth factor 1 (IGF-1) axis was also investigated. Several studies have found that about one-third of individuals with fibromyalgia have lower IGF-1 levels than control groups 103. Serial measurements at 12 to 24 h also showed a reduction in growth hormone (GH) secretion in patients with fibromyalgia, particularly at night. Since GH secretion occurs mainly during phase 3 of sleep and 80% of patients have sleep disturbances, it remains to be clarified whether the nature of this alteration is primary or secondary 104. Given the higher prevalence of fibromyalgia in the female population, the role of estrogens in this pathology was investigated. However, the results of various studies suggest that this role is limited, and the only significant result is an increased serum concentration of the G protein-coupled estrogen receptor (GPER) in patients with fibromyalgia compared to healthy subjects 105. Although a strong correlation with the disease allows us to hypothesize the possible use of this receptor as a potential diagnostic biomarker, the exact mechanism by which it fits into the pathophysiological cascade remains unclear 106.
Psychopathological factors
Psychiatric comorbidities in fibromyalgia constitute a relevant aspect of the disease, and a close correlation between stress and fibromyalgia symptoms has been described several times. According to several studies, the prevalence of psychiatric comorbidities, such as anxiety disorders and depression, among patients with this pathology reaches 60% in certain subpopulations 107. The presence of depressive patterns has been shown to correlate with a worse prognosis: patients with comorbid symptoms of depression seem to report pain of greater severity and duration as well as a greater degree of hyperalgesia/allodynia than healthy controls. Furthermore, these psychiatric aspects seem to have a certain predictive value in relation to various somatic symptoms, including musculoskeletal pain and headaches 108. The impact of depression symptoms on pain processing is still unclear. A study in fibromyalgia patients attempted to evaluate this correlation by comparing the results of quantitative sensory tests and neuronal responses to pressure stimuli (assessed by functional magnetic resonance imaging (fMRI)) with the levels of symptoms of depression. The results showed that the symptom levels of depression were not associated with quantitative test results or with the extent of neuronal activation in brain areas, such as primary and secondary somatosensory cortices, that are associated with the sensory dimension of pain. However, symptoms of depression were observed to be associated with the extent of pain-evoked neuronal activation in the amygdala and contralateral anterior insula, which are brain areas associated with affective pain processing. Therefore, these findings suggest the existence of parallel, possibly independent, neuronal pain processing networks for sensory and affective pain elements 109.
The therapeutic aspect is an element that supports the pathogenetic overlap between depressive disorder and fibromyalgia. The effectiveness of treatment with antidepressant drugs (e.g., serotonin-norepinephrine reuptake inhibitors (SNRIs) and tricyclics) has in fact been described by numerous studies on fibromyalgia patients and constitutes one of the main therapeutic strategies in both fibromyalgia and other chronic pain conditions, such as chronic headache and irritable bowel (IBS), which are often symptoms of fibromyalgia 110, 111, 112. The effectiveness of serotonin-norepinephrine reuptake inhibitors (SNRIs) and other double-acting antidepressants, such as mirtazapine, suggests that neurotransmission dysfunction of both serotonin and norepinephrine exists in fibromyalgia 113. Similar to observations for the depressive pattern, stress also appears to be both a predictive and negative prognostic factor. It has been shown that stress can modulate pain sensitivity by inducing hyperalgesia or allodynia through alterations in the physiological circadian secretion of cholesterol, therefore indirectly inducing the release of pro-inflammatory cytokines and setting in motion the pathophysiological processes described above 114, 115, 116.
Animal studies have shown that stress induction (e.g., swimming stress and cold stress) can produce muscle and skin hyperalgesia that lasts for weeks after the stressor 117, 118, 119. On the other hand, milder stressors (e.g., fatigue and acoustic stress), which do not produce hyperalgesia on their own, can cause an increase in and prolongation of the hyperalgesic response to a subthreshold or mild noxious stimulus 120, 121, 122. Other studies have shown that animals exposed to stressors also exhibit changes in the spinal cord. In particular, animals showed a greater expression of c-fos in response to formalin, as well as a reduction in the basal and induced release of the inhibitory neurotransmitter GABA; a reduction in mu-opioid agonist antinociception enhanced the basal and evoked the release of glutamate 123, 124, suggesting both increased central excitability and reduced central inhibition. In animals, stress-induced hyperalgesia was reduced by the spinal blockade of substance P, calcitonin gene-related peptide (CGRP), NMDA-glutamate receptors and neurokinin-1 receptors, all substances involved in the neurotransmission of pain 122. At the supraspinal level, cold stress-induced alterations were observed in the serotonergic system, with reductions in both serotonin (5-HT) and 5-hydroxy indoleacetic acid (5-HIAA) levels in the supraspinal regions 125. Therefore, stress and psychological factors are involved in the development and severity of fibromyalgia.
Poor Sleep
Sleep disorders are classically described within the symptomatic process of fibromyalgia. However, some recently reported data have generated the hypothesis that sleep disorders may be included among the causative factors of this pathology, rather than among its manifestations. Studies published in recent years have described a bidirectional correlation between sleep disturbances and widespread musculoskeletal pain, and it even seems that insomnia tends to precede the onset of pain and has predictive value regarding its onset and its persistence 126, 127. Studies carried out in healthy subjects also seem to show that total, partial and stage-specific sleep deprivation leads to hyperalgesia, an increased incidence of spontaneous pain and mood alterations, particularly anxiety and depression 128, 129. In a further study by Smith et al. 130, the authors hypothesized that the development or aggravation of somatic and psychiatric symptoms is secondary to sleep discontinuity rather than sleep deprivation. In addition to the number of awakenings, the cyclic alternating pattern (CAP) is a useful tool to analyze this discontinuity. It is represented by short cycles of periodic electroencephalographic activity of non-REM sleep, distinct from the background rhythm and with a periodicity of up to one minute 131. The cyclic alternating pattern (CAP) has been shown to be frequent in fibromyalgia patients and correlated with poor quality of sleep and with the severity of pain observed in these patients 132.
Findings in human studies carried out through the application of evoked potentials indicate that increased nociceptive sensitivity in response to sleep deprivation could derive from dysregulation of the descending pathways of pain control or from cognitive amplification of the central origin, thus excluding the mechanism of sensory amplification 133. Biochemical analyses suggest that an insufficient amount of sleep could also play a facilitating role in nociception through the elevation of the serum concentration of IL-6, thus entering the pathogenetic cascade with an inflammatory substrate 128. The structural analysis of sleep obtained by EEG studies provides additional support for the hypothesis that sleep alterations are among the causative factors of fibromyalgia. One of the first works on this aspect was a study by Moldofsky et al. 134, in which microstructural analysis identified the presence of a rhythm component typical of wakefulness within the non-REM sleep pattern, particularly during periods of slow delta rhythm (0.5 to 2 Hz, characteristic of deep sleep), among both fibromyalgia patients and healthy subjects deprived of the deeper stages of alpha sleep (8 to 13 Hz). Moreover, in healthy subjects, deprivation was accompanied by a set of musculoskeletal and psychological symptoms similar to those chronically reported by patients. In light of these data, a hypothesis was put forward that considered fibromyalgia, then called fibrositis, to be a “non-restorative sleep syndrome”, in which an arousal mechanism (presumably responsible for the alpha component) interferes with non-REM sleep and its restorative function, consequently generating mood alterations and characteristic somatic disturbances. More recent human studies have described the mechanisms underlying alpha-delta sleep (ADS, the intrusion of alpha rhythms in the deep phases of sleep), highlighting the role played by the thalamus, which, in turn, is modulated by GABAergic and cholinergic afferents 135, 136. It has been observed that the alpha-delta sleep (ADS, the intrusion of alpha rhythms in the deep phases of sleep) phenomenon manifests itself in three different patterns: phasic (contemporary with delta activity), tonic (continuous throughout NREM sleep) and low alpha activity. Among these, the phasic pattern appears to be the most common among fibromyalgia patients and is the one that correlates most strongly with symptoms such as insomnia and pain 137.
It should be noted, however, that alpha-delta sleep is not an exclusive feature of fibromyalgia: it is also seen in a number of chronic pain syndromes and in some healthy individuals. Unlike alpha activity, the functional anatomical substrate of sleep spindles has been extensively studied. These are trains of electroencephalographic waves with a frequency between 12 and 16 Hz, lasting between 0.5 and 1.5 s and recurring every 3 to 10 s, and characteristic of non-REM (NREM) sleep, particularly the N2 stage of sleep (intermediate sleep). Sleep spindles are generated by the rhythmic firing of thalamic relay neurons, and their role is central in the induction and maintenance of NREM sleep, as well as in the gating mechanism through which transmission and the consequent cortical response to both internal and external stimuli are attenuated during sleep (control of arousal status) 138, 139, 140, 141. The frequency of spindles during NREM sleep is modulated by a series of factors, including age (inverse proportionality) and a certain degree of interindividual variability, as well as various pathological conditions in the neuropsychiatric field, such as depression, anxiety and stress 142. A study by Landis et al. 143 described a reduction in the frequency and amplitude of sleep spindles in a population of women with fibromyalgia compared to a control group, proposing the hypothesis of a dysfunction of the thalamo-cortical circuits underlying this alteration.
In a rat study 144, a deep learning method, known as SpindleNet, was applied to characterize sleep spindle activity in animals with induced chronic pain. The results showed a correlation between a decrease in the frequency of spindles during NREM sleep and the level of chronic pain and allodynia, suggesting that this finding could be a biomarker of chronic pain as well as a target for neuromodulator therapy 145. It is therefore possible to hypothesize that a dysfunctional primitive thalamus causes an alteration in its spindle pacemaker activity and alpha activity, compromising the restorative function of sleep and consequently generating the somatic and psychological symptoms of fibromyalgia, similar to what was suspected by Moldofsky et al 134. In light of the important function of the thalamus in sensory transmission pathways, it can also be hypothesized that both sleep disturbances and hyperalgesia/allodynia are the direct result of thalamic alteration, representing independent manifestations of the same pathological process. The relationship between sleep disturbances and fibromyalgia has not yet been fully clarified, and new studies will be needed to better define the relationship between the two. At the moment, the main hypotheses converge in their proposal of a bidirectional correlation characterized by a positive feedback circuit.
Fibromyalgia syndrome symptoms
Fibromyalgia has many symptoms that tend to vary from person to person. The main symptom is widespread pain. The pain associated with fibromyalgia often is described as a constant dull ache that has lasted for at least three months. To be considered ‘widespread pain’, the pain must occur on both sides of your body and above and below your waist.
Symptoms of fibromyalgia can include the following:
- Increased sensitivity to pain.
- Fatigue (extreme tiredness) or chronic fatigue syndrome
- A deep ache or a burning pain that gets worse because of activity, stress, weather changes, or other factors.
- Muscle stiffness or spasms.
- Pain that moves around your body.
- Feelings of numbness or tingling in your hands, arms, or legs.
- Feeling very tired or fatigued (out of energy), even when you get enough sleep. People with fibromyalgia often awaken tired, even though they report sleeping for long periods of time. Sleep is often disrupted by pain, and many patients with fibromyalgia have other sleep disorders, such as restless legs syndrome and sleep apnea.
- Trouble sleeping.
There may be periods when your symptoms get better or worse, depending on factors such as:
- your stress levels
- changes in the weather
- how physically active you are
People who have fibromyalgia often also have one or more of the following:
- Anxiety.
- Depression.
- Irritable bowel syndrome (IBS).
- Restless legs syndrome.
- Increased sensitivity to odors, bright lights, loud noises, or medicines.
- Headaches, migraines, or jaw pain.
- Dry eyes or mouth.
- Dizziness and problems with balance.
- Problems with memory or concentration (sometimes called the “fibro fog”).
- For women, painful menstrual periods.
If you think you have fibromyalgia, visit your doctor. Treatment is available to ease some of the symptoms, although it’s unlikely they’ll ever disappear completely.
The main symptoms of fibromyalgia are outlined below.
The 1990 American College of Rheumatology fibromyalgia classification criteria included tenderness at least 11 of 18 defined tender points:
- Suboccipital muscle insertion bilaterally
- The anterior aspect of C5 to C7 intertransverse spaces bilaterally
- Mid-upper border of trapezius bilaterally
- Origin of supraspinatus muscle bilaterally
- Second costochondral junctions bilaterally
- 2cm distal to the lateral epicondyles bilaterally
- Upper outer quadrants of buttocks bilaterally
- Greater trochanteric prominence bilaterally
- Medial fat pad of the knees bilaterally
The pressure appropriate for detecting these tender points should be equal 4 kg/cm², enough to whiten the nail bed of the fingertip of the examiner.
However, given many limitations of the tender point examination, the 2010 American College of Rheumatology diagnostic criteria eliminated these findings. The new American College of Rheumatology criteria are mentioned below under diagnosis.
Mood disturbances, including depression, anxiety and heightened somatic concern, may often also occur. Approximately 25% of fibromyalgia patients have accompanying depression at the time of diagnosis, while 50-75% of patients have a lifetime history of depression. In addition, the lifetime prevalence of an anxiety disorder in fibromyalgia patients is approximately 60% 146. The levels of depression and anxiety in patients with fibromyalgia seem to be associated with the degree of cognitive impairment, as shown in a meta-analysis of 23 case-control studies 147.
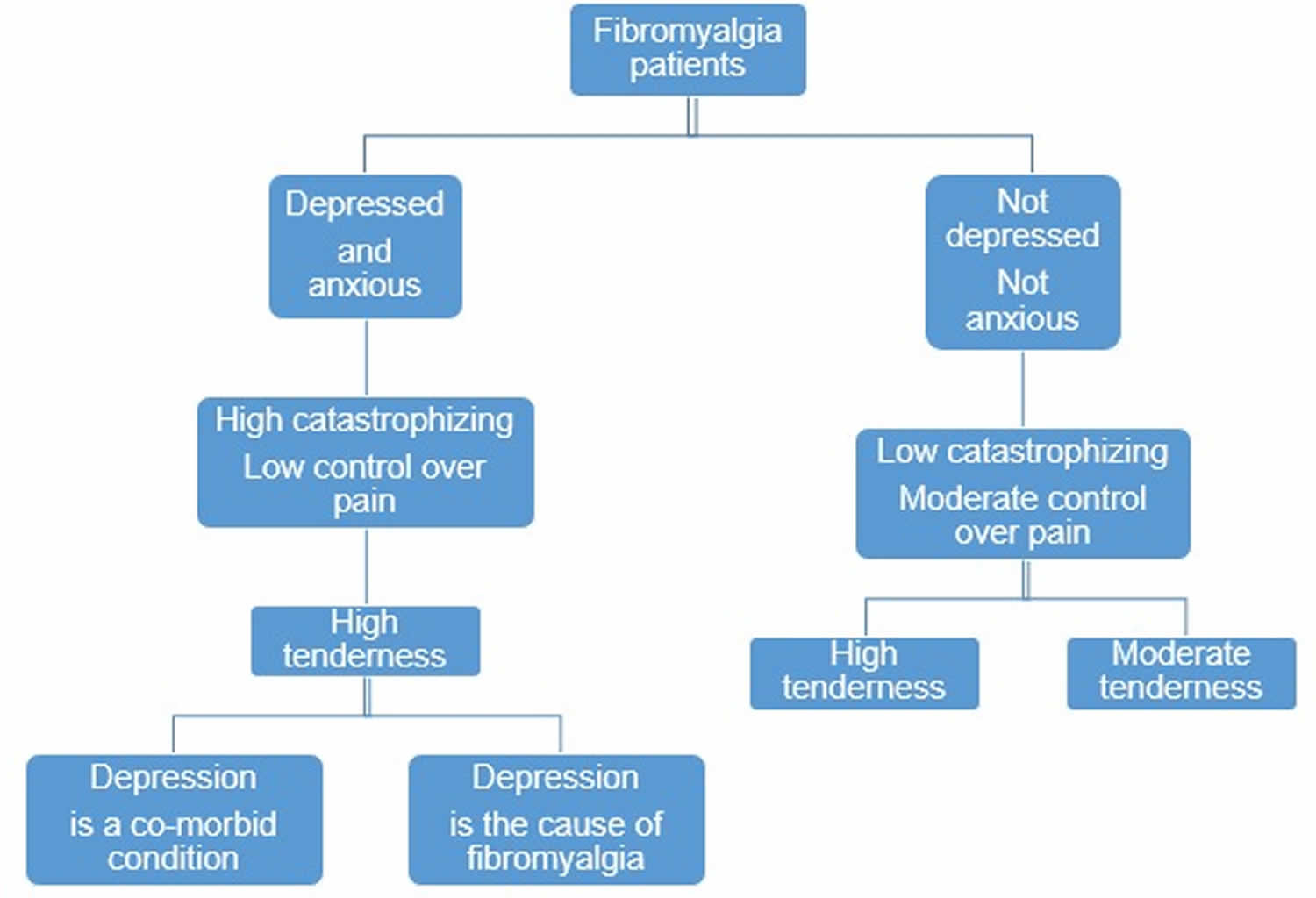
Based on the coexistence of depression and anxiety, fibromyalgia patients can be divided into 2 major groups. The first group comprises of patients without coexisting mood disorders, while the second of patients with concomitant depressive mood, often in combination with anxiety. According to the results of a study that intended to subgroup fibromyalgia patients based on: 1) mood status (evaluated by the Center for Epidemiologic Studies Depression Scale for depression and the State-Trait Personality Inventory for symptoms of trait-related anxiety), 2) cognition (by the catastrophizing and control of pain subscales of the Coping Strategies Questionnaire), and 3) hyperalgesia/tenderness (by dolorimetry and random pressure-pain applied at suprathreshold values), it was noted that fibromyalgia patients with depressive mood and anxiety are also ‘catastrophizing’. This term is used to indicate that such patients have a very negative, pessimistic view of what their pain is and what is causing, while they have no sense that they can control their pain. On the contrary fibromyalgia patients who are neither depressed nor anxious and therefore do not catastrophize, have a moderate sense that they can control their pain. These patients can be further divided into 2 subgroups based on the presence of hyperalgesia/tenderness as patients with high and lower levels of tenderness, (although they both fulfill the classification criteria for fibromyalgia) 148. In addition, it has been proposed that depressed fibromyalgia patients can also be divided into 2 subgroups, in the first one depression is a co-morbid condition, while in the second depression is the cause of fibromyalgia 149. All these fibromyalgia subgroups are illustrated in Figure 2.
Figure 2. Fibromyalgia patient subgroups
In another study fibromyalgia patients were classified as dysfunctional, inter-personally distressed or adaptive coppers, based on their responses to the Multidimensional Pain Inventory. The dysfunctional patients experienced more pain behaviors and overt expressions of pain, distress, and suffering, such as slowed movement, bracing, limping, and grimacing compared to the inter-personally distressed or the adaptive coppers 150. It is of interest though that up to 25% of patients correctly diagnosed with a systemic rheumatic disease (e.g. rheumatoid arthritis, systemic lupus erythematosus) will also fulfill the classification criteria for fibromyalgia 151. The most commonly encountered comorbid conditions in fibromyalgia patients are shown in Table 2.
Table 2. The most commonly encountered co-morbid conditions in fibromyalgia
| Sleep disorders | Non restorative sleep (alpha-delta sleep anomaly) Sleep apnea Restless leg syndrome Nocturnal myoclonus |
|---|---|
| Chronic fatigue syndrome (systemic exertion intolerance disease) | |
| Psychiatric disorders | Anxiety disorders Depression Obsessive compulsive disorder |
| Headache | Tension type headache Migraine |
| Irritable bowel syndrome | |
| Musculofascial pain syndrome | Temporomandibular joint syndrome and interstitial cystitis |
| Dysmenorrhea | |
| Premenstrual syndrome | |
| Non-cardiac chest pain | |
| Raynaud’s phenomenon | |
| Systemic autoimmune diseases | Rheumatoid arthritis Systematic lupus erythematosus Sjögren’s syndrome Ankylosing spondylitis and other seronegative spondyloarthritis Polymyalgia rheumatica |
Widespread pain
If you have fibromyalgia, one of the main symptoms is likely to be widespread pain. This may be felt throughout your body, but could be worse in particular areas, such as your back or neck. The pain is likely to be continuous, although it may be better or more severe at different times.
The pain could feel like:
- an ache
- a burning sensation
- a sharp, stabbing pain
Temporo-mandibular joint pain (TMJ pain)
This Temporo-mandibular Joint Dysfunction Syndrome, sometimes referred to as TMJD, causes tremendous face and head pain in one quarter of fibromyalgia patients. However, a 1997 report indicates that as many as 90% of fibromyalgia patients may have jaw and facial tenderness that could produce, at least intermittently, symptoms of temporo-mandibular joint dysfunction. Most of the problems associated with this condition are thought to be related to the muscles and ligaments surrounding the joint and not necessarily the joint itself.
Extreme sensitivity
Fibromyalgia can make you extremely sensitive to pain all over your body, and you may find that even the slightest touch is painful. If you hurt yourself – such as stubbing your toe – the pain may continue for much longer than it normally would.
You may hear the condition described in the following medical terms:
- Hyperalgesia – when you’re extremely sensitive to pain
- Allodynia – when you feel pain from something that shouldn’t be painful at all, such as a very light touch
You may also be sensitive to things such as smoke, certain foods and bright lights. Being exposed to something you’re sensitive to can cause your other fibromyalgia symptoms to flare up.
Multiple Chemical Sensitivity Syndrome
Sensitivities to odors, noise, bright lights, medications and various foods is common in roughly 50% of fibromyalgia patients.
Stiffness
Fibromyalgia can make you feel stiff. The stiffness may be most severe when you’ve been in the same position for a long period of time – for example, when you first wake up in the morning.
It can also cause your muscles to spasm, which is when they contract (squeeze) tightly and painfully.
Fatigue
Fibromyalgia can cause fatigue (extreme tiredness). This can range from a mild, tired feeling to the exhaustion often experienced during a flu-like illness.
Severe fatigue may come on suddenly and can drain you of all your energy. If this happens, you may feel too tired to do anything at all.
Poor sleep quality
Fibromyalgia can affect your sleep. You may often wake up tired, even when you’ve had plenty of sleep. This is because the condition can sometimes prevent you from sleeping deeply enough to refresh you properly.
You may hear this described as “non-restorative sleep”.
Cognitive problems (‘fibro-fog’)
Cognitive problems commonly referred to as “fibro fog” are issues related to mental processes (cognitive difficulties), such as thinking and learning. If you have fibromyalgia, you may have:
- trouble remembering and learning new things
- problems with attention (the ability to focus) and concentration on mental tasks
- slowed or confused speech
Headaches
If fibromyalgia has caused you to experience pain and stiffness in your neck and shoulders, you may also have frequent headaches.
These can vary from being mild headaches to severe migraines, and could also involve other symptoms, such as nausea (feeling sick).
Irritable bowel syndrome (IBS)
Some people with fibromyalgia also develop irritable bowel syndrome (IBS).
IBS is a common digestive condition that causes pain and bloating in your stomach. It can also lead to constipation or diarrhoea.
Other symptoms
Other symptoms that people with fibromyalgia sometimes experience include:
- dizziness and clumsiness
- feeling too hot or too cold – this is because you’re not able to regulate your body temperature properly
- restless legs syndrome (an overwhelming urge to move your legs)
- tingling, numbness, prickling or burning sensations in your hands and feet (pins and needles, also known as paresthesia)
- in women, unusually painful periods
- anxiety
- depression
Depression
In some cases, having the condition can lead to depression. This is because fibromyalgia can be difficult to deal with, and low levels of certain hormones associated with the condition can make you prone to developing depression.
Depression can cause many symptoms, including:
- constantly feeling low
- feeling hopeless and helpless
- losing interest in the things you usually enjoy
If you think you may be depressed, it’s important to get help from your doctor or your fibromyalgia healthcare professional, if you’ve been seeing one.
Fibromyalgia syndrome complications
Fibromyalgia can cause pain, disability, and lower quality of life. US adults with fibromyalgia may have complications such as:
- More hospitalizations. If you have fibromyalgia you are twice as likely to be hospitalized as someone without fibromyalgia.
- Lower quality of life. Women with fibromyalgia may experience a lower quality of life.
- Higher rates of major depression. Adults with fibromyalgia are more than 3 times more likely to have major depression than adults without fibromyalgia. Screening and treatment for depression is extremely important.
- Higher death rates from suicide and injuries. Death rates from suicide and injuries are higher among fibromyalgia patients, but overall mortality among adults with fibromyalgia is similar to the general population.
- Higher rates of other rheumatic conditions. Fibromyalgia often co-occurs with other types of arthritis such as osteoarthritis, rheumatoid arthritis, systemic lupus erythematosus, and ankylosing spondylitis.
Fibromyalgia syndrome diagnosis
If you think you have fibromyalgia, visit your doctor. Diagnosing fibromyalgia can be difficult, as there’s no specific test to diagnose the condition 153. And the symptoms of fibromyalgia, such as pain, sleep problems and fatigue, are common in many other conditions and can vary. It sometimes takes visits to several different health care providers to get a diagnosis.
During diagnosis, you’ll be asked about how your symptoms are affecting your daily life. If you have had any trouble sleeping or fatigue, tell your doctor how long you have had this problem. Your doctor may ask whether you have been feeling anxious or depressed since your symptoms began.
Your body will also be examined to check for visible signs of other conditions – for example, swollen joints may suggest arthritis, rather than fibromyalgia.
In the past, doctors would check 18 specific points on a person’s body to see how many of them were painful when pressed firmly (see Figure 1). Newer 2016 guidelines from the American College of Rheumatology don’t require a tender point exam. Instead, the main factor needed for a fibromyalgia diagnosis is widespread pain throughout your body for at least three months 154.
Fibromyalgia may now be diagnosed in adults when all of the following criteria are met 154:
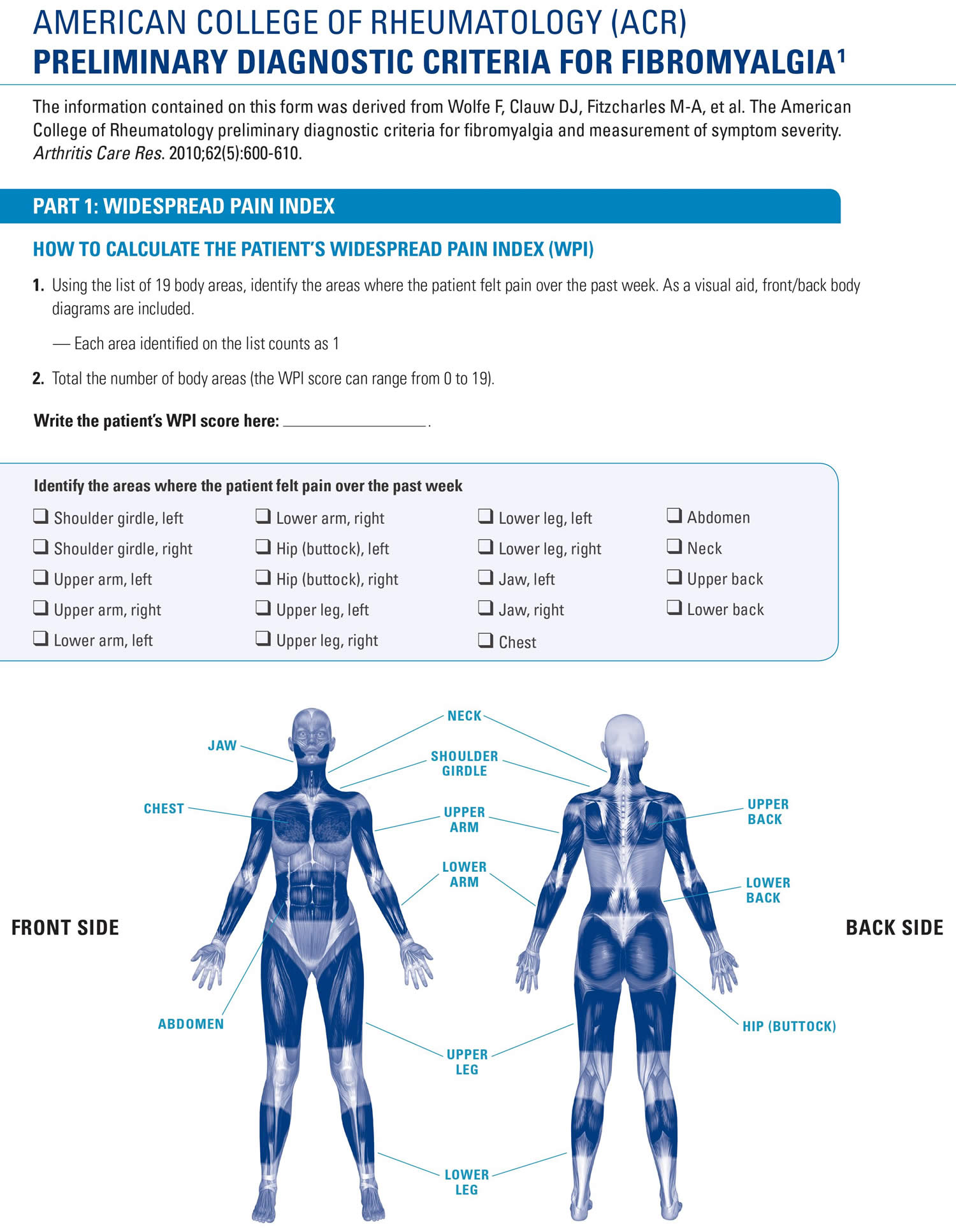
- Generalized pain, defined as pain in at least 4 of 5 regions, is present.
- Symptoms have been present at a similar level for at least 3 months.
- Widespread pain index (WPI) ≥ 7 and symptom severity scale (SSS) score ≥ 5 OR WPI of 4–6 and SSS score ≥ 9.
- A diagnosis of fibromyalgia is valid irrespective of other diagnoses. A diagnosis of fibromyalgia does not exclude the presence of other clinically important illnesses.
To meet the American College of Rheumatology 2016 criteria, you must have pain in at least four of these five areas:
- Left upper region, including shoulder, arm or jaw
- Right upper region, including shoulder, arm or jaw
- Left lower region, including hip, buttock or leg
- Right lower region, including hip, buttock or leg
- Axial region, which includes neck, back, chest or abdomen.
Old guidelines required tender points
Fibromyalgia is also often characterized by additional pain when firm pressure is applied to specific areas of your body, called tender points. In the past, at least 11 of these 18 spots had to test positive for tenderness to diagnose fibromyalgia.
But fibromyalgia symptoms can come and go, so a person might have 11 tender spots one day but only eight tender spots on another day. And many family doctors were uncertain about how much pressure to apply during a tender point exam. While specialists or researchers may still use tender points, an alternative set of guidelines has been developed for doctors to use in general practice.
These newer diagnostic criteria include:
- Widespread pain lasting at least three months
- Presence of other symptoms such as fatigue, waking up tired and trouble thinking
- No other underlying condition that might be causing the symptoms.
Criteria for diagnosing fibromyalgia
1990 American College of Rheumatology classification criteria was used in many clinical and therapeutic trials but has not been useful in diagnosing fibromyalgia in clinical practice.
The 1990 American College of Rheumatology fibromyalgia classification criteria included:
- Symptoms of widespread pain, present on both sides of the body and both above and below the waist
- Physical findings of at minimum 11 of 18 defined tender points:
- Suboccipital muscle insertion bilaterally
- Anterior aspect of C5 to C7 intertransverse spaces bilaterally
- Mid upper border of trapezius bilaterally
- Origin of supraspinatus muscle bilaterally
- Second costochondral junctions bilaterally
- 2cm distal to the lateral epicondyles bilaterally
- Upper outer quadrants of buttocks bilaterally
- Greater trochanteric prominence bilaterally
- Medial fat pad of the knees bilaterally
The pressure appropriate for detecting these tender points should be equal to 4 kg/cm², enough to whiten the nail bed of the fingertip of the examiner.
For the purposes of classification, the patient is said to have fibromyalgia if both criteria are met.
There were a number of limitations of the 1990 1990 American College of Rheumatology diagnostic criteria, which include the following:
- Physicians do not know how to examine tender points, perform the exam incorrectly, or refuse to do so.
- A number of symptoms that were previously not considered were increasingly appreciated as key symptoms of fibromyalgia.
- The criteria set the bar so high that it left little room for variation among fibromyalgia patients. Also, the patient whose symptoms improved failed to satisfy the 1990 criteria.
Criteria Needed for a Fibromyalgia Diagnosis (American College of Rheumatology Fibromyalgia Diagnostic Criteria 2010) 155
- Pain and symptoms over the past week, based on the total of number of painful areas out of 19 parts of the body plus level of severity of these symptoms:
- a. Fatigue
- b. Waking unrefreshed
- c. Cognitive (memory or thought) problems
- Plus number of other general physical symptoms
- Symptoms lasting at least three months at a similar level
- No other health problem that would explain the pain and other symptoms
Revised 2016 American College of Rheumatology guidelines don’t require a tender point exam. Instead, the main factor needed for a fibromyalgia diagnosis is widespread pain throughout your body for at least three months 154.
Fibromyalgia may now be diagnosed in adults when all of the following criteria are met 154:
- Generalized pain, defined as pain in at least 4 of 5 regions, is present.
- Symptoms have been present at a similar level for at least 3 months.
- Widespread pain index (WPI) ≥ 7 and symptom severity scale (SSS) score ≥ 5 OR WPI of 4–6 and SSS score ≥ 9.
- A diagnosis of fibromyalgia is valid irrespective of other diagnoses. A diagnosis of fibromyalgia does not exclude the presence of other clinically important illnesses.
Note:
- Widespread pain index (WPI): note the number of areas where and how many areas the patient has had pain in the prior week. The score will be between 0 and 19. Shoulder girdle, left hip (buttock, trochanter), left jaw, left upper back shoulder girdle, right hip (buttock, trochanter), right jaw, right lower back upper arm, left upper leg, left chest neck upper arm, right upper leg, right abdomen lower arm, left lower leg, left lower arm, right lower leg, right
- Symptom severity scale (SSS): Fatigue, waking unrefreshed, and cognitive symptoms. For each of the three symptoms above, indicate the severity level over the past week utilizing the following scale: 0 no problem; 1 slight or mild problems, generally mild or intermittent; 2 moderate, considerable problems, often present and/or at a moderate level; 3 severe: pervasive, continuous, life-disturbing problems. Considering somatic symptoms in general, indicate whether the patient has: 0 for no symptoms, 1 for a few symptoms, 2 for a moderate number of symptoms, and 3 for many symptoms. The symptom severity scale (SSS) sums the severity of the 3 symptoms (fatigue, waking unrefreshed, cognitive symptoms) plus the severity) of general somatic symptoms. The final score is between 0 and 12.
To meet the American College of Rheumatology 2016 criteria, you must have pain in at least four of these five areas:
- Left upper region, including shoulder, arm or jaw
- Right upper region, including shoulder, arm or jaw
- Left lower region, including hip, buttock or leg
- Right lower region, including hip, buttock or leg
- Axial region, which includes neck, back, chest or abdomen
Figure 3. Fibromyalgia diagnostic criteria
Ruling out other conditions
If your doctor thinks you may have fibromyalgia, they’ll first have to rule out all other conditions that could be causing your symptoms. These conditions may include:
- Chronic fatigue syndrome (also known as myalgic encephalomyelitis) – a condition that causes long-term tiredness
- Rheumatic diseases. Certain conditions — such as rheumatoid arthritis, Sjogren’s syndrome and lupus — can begin with generalized aches and pain.
- Mental health problems. Disorders such as depression and anxiety often feature generalized aches and pain.
- Neurological disorders. In some people, fibromyalgia causes numbness and tingling, symptoms that mimic those of disorders such as multiple sclerosis and myasthenia gravis.
Tests to check for some of these conditions include urine and blood tests, although you may also have X-rays and other scans.
Blood tests may include:
- Complete blood count
- Erythrocyte sedimentation rate
- Cyclic citrullinated peptide test
- Rheumatoid factor
- Thyroid function tests
- Anti-nuclear antibody
- Celiac serology
- Vitamin D
If there’s a chance that you may be suffering from sleep apnea, your doctor may recommend an overnight sleep study.
If you’re found to have another condition, you could still have fibromyalgia as well.
Fibromyalgia test
Fibromyalgia is usually diagnosed by documenting the patient’s medical history, ruling out disorders and diseases that may be mimicking or exacerbating fibromyalgia, and by utilizing the criteria last updated by the American College of Rheumatology in 2016 8, 9, 154, 156.
Revised American College of Rheumatology 2016 guidelines don’t require a tender point exam. Instead, the main factor needed for a fibromyalgia diagnosis is widespread pain throughout your body for at least three months 154.
Fibromyalgia may now be diagnosed in adults when all of the following criteria are met 154:
- Generalized pain, defined as pain in at least 4 of 5 regions, is present.
- Symptoms have been present at a similar level for at least 3 months.
- Widespread pain index (WPI) ≥ 7 and symptom severity scale (SSS) score ≥ 5 OR WPI of 4–6 and SSS score ≥ 9.
- A diagnosis of fibromyalgia is valid irrespective of other diagnoses. A diagnosis of fibromyalgia does not exclude the presence of other clinically important illnesses.
To meet the American College of Rheumatology 2016 criteria, you must have pain in at least four of these five areas 154:
- Left upper region, including shoulder, arm or jaw
- Right upper region, including shoulder, arm or jaw
- Left lower region, including hip, buttock or leg
- Right lower region, including hip, buttock or leg
- Axial region, which includes neck, back, chest or abdomen.
Laboratory Tests
Laboratory tests can be useful to help diagnose conditions with symptoms similar to fibromyalgia, such as rheumatoid arthritis, Sjögren syndrome, thyroid disease, and lupus. It is not usually cost effective or necessary to do extensive screening. General tests that may be ordered include:
- Comprehensive metabolic panel – to examine electrolytes, proteins, liver and kidney function, calcium, and glucose
- CBC (complete blood count) – to look for anemia, a possible cause of weakness and fatigue
- TSH (thyroid stimulating hormone) and/or other thyroid testing since hypothyroidism can cause symptoms similar to fibromyalgia
- ANA (anti-nuclear antibody) – to rule out autoimmune disorders, such as lupus or Sjogren syndrome
- CK (creatine kinase) – to rule out other conditions that can cause muscle weakness or pain
- Erythrocyte sedimentation rate (ESR)
- Cyclic citrullinated peptide test
- Rheumatoid factor
- Celiac serology
- Vitamin D
A healthcare practitioner will typically consider the following in developing a diagnosis: results of the general tests, the patient’s history (including family history and risk factors for certain diseases), and results of the physical examination. Based on these findings, some additional tests could be done.
Meanwhile, researchers continue to look for new testing protocols that may be more specific for fibromyalgia.
Non-Laboratory Tests
Electromyography (EMG) may be performed to assess the health of muscles and the nerves that control them. Occasionally, an imaging scan such as an MRI (magnetic resonance imaging) may be ordered to help rule out the possibility of multiple sclerosis or other diseases that may cause symptoms similar to fibromyalgia.
More clues for fibromyalgia diagnosis
People who have fibromyalgia also often wake up tired, even after they’ve slept continuously for more than eight hours. Brief periods of physical or mental exertion may leave them exhausted. They may also have problems with short-term memory and the ability to concentrate. If you have these problems, your doctor may ask you to rank how severely they affect your day-to-day activities.
Fibromyalgia often coexists with other health problems, so your doctor may also ask if you experience:
- Irritable bowel syndrome (IBS)
- Headaches
- Jaw pain
- Anxiety or depression
- Frequent or painful urination
Possible fibromyalgia triggers
In some cases, fibromyalgia symptoms begin shortly after a person has experienced a mentally or physically traumatic event, such as a car wreck. People who have post-traumatic stress disorder appear to be more likely to develop fibromyalgia, so your doctor may ask if you’ve experienced any traumatic events recently.
Because a genetic factor appears to be involved in fibromyalgia, your doctor may also want to know if any other members of your immediate family have experienced similar symptoms.
All this information taken together will give your doctor a much better idea of what may be causing your symptoms. And that determination is crucial to developing an effective treatment plan.
2010 American College of Rheumatology Preliminary Diagnostic Criteria
To address the aforementioned issues the American College of Rheumatology in 2010 proposed the preliminary diagnostic criteria for fibromyalgia (Table 3), that were not meant to replace the 1990 American College of Rheumatology classification criteria, but to represent an alternative simple and easy method of diagnosis in clinical practice (40). These diagnostic criteria do not require a tender point count. Instead they rely only on symptoms for the diagnosis of fibromyalgia. They introduced the widespread pain index (WPI), which counts the areas that the patient feels pain during one week preceding the examination, and the symptom severity (SS) scale, which describes the severity of fatigue, unrefreshing sleep, cognitive problems, and a number of associated somatic fibromyalgia symptoms. These symptoms need to be assessed and rated by a physician. The 2010 American College of Rheumatology preliminary diagnostic criteria are in-adequate for patient self-diagnosis.
Two more conditions need to be fulfilled. The symptoms need to be present at a similar level for at least 3 months while alternate disorders that would otherwise explain the pain need to be excluded (Table 3). The authors of the 2010 American College of Rheumatology preliminary diagnostic criteria have clarified that the latter condition does not mean that fibromyalgia is an exclusion diagnosis according to these criteria. The diagnosis of fibromyalgia should not be made only when there is not another disease that could explain the pain that would otherwise be attributed to fibromyalgia. It should be noted that rheumatic diseases usually do not cause pain that can be confused with fibromyalgia 157.
There is evidence that there is good agreement between the 1990 American College of Rheumatology classification criteria and the 2010 American College of Rheumatology preliminary diagnostic criteria 158. However, these criteria are expected not to agree completely, as the former are focused on the presence of tender points while the latter on the presence of symptoms. The 1990 criteria can diagnose fibromyalgia in patients who do not have sufficiently high symptom score according to the 2010 criteria, while the 2010 criteria can diagnose fibromyalgia in patients who do not have sufficient tender points according to the 1990 criteria.
The introduction of the 2010 American College of Rheumatology preliminary diagnostic criteria was surrounded by controversy too. In particular, they have been criticized for being completely symptom focused, ill-defined, and lucking some mechanistic features of fibromyalgia, such as hyperalgesia, central sensitization and dysfunctional pain modulation 159. Additionally, these diagnostic criteria are based on the subjective assessment of the patient’s somatic symptoms by the physician, adding ambiguity and influencing repeatability among different physicians 160. A self-reported version of the 2010 American College of Rheumatology preliminary diagnostic criteria was developed, so as to be used in survey research, and not in clinical practice 161. These criteria are known as the modified 2010 American College of Rheumatology preliminary diagnostic criteria or the research criteria. They introduced the fibromyalgia severity (FS) score (originally called fibromyalgianess scale) which is the sum of the self-reported widespread pain index (WPI) and symptom severity (SS) score. This score can be used as an approximate measure of the severity of fibromyalgia. The fibromyalgia severity (FS) score has also been called polysymptomatic distress (PSD) scale. It has been proposed that the markers of physical and psychological distress have a continuous distribution in the general population with fibromyalgia patients being at the extreme end of this distribution 162. The polysymptomatic distress (PSD) scale could be useful to define the position of each individual in this continuum, without having to differentiate between patients with fibromyalgia and those without, as this distinction can sometimes be unclear if not arbitrary 163.
Table 3. 2010 American College of Rheumatology Preliminary Diagnostic Criteria
| Criteria: A patient satisfies diagnostic criteria for fibromyalgia if the following 3 conditions are met: Widespread pain index (WPI) ≥7 and symptom severity (SS) scale score ≥5 or Widespread pain index (WPI) 3-6 and symptom severity (SS) scale score ≥9. Symptoms have been present at a similar level for at least 3 months. The patient does not have a disorder that would otherwise explain the pain. Ascertainment: WPI Note the number of areas in which the patient has had pain over the last week. In how many areas has the patient had pain? (Score will be between 0 and 19) | |||||
| -Neck | -Upper arm, left | -Abdomen | -Upper leg, left | ||
| -Jaw, left | -Upper arm, right | -Upper back | -Upper leg, right | ||
| -Jaw, right | -Lower arm, left | -Lower back | -Lower leg, left | ||
| -Shoulder girdle, left | -Lower arm, right | -Hip (buttock, trochanter), left | -Lower leg, right | ||
| -Shoulder girdle, right | -Chest | -Hip (buttock, trochanter), right | |||
| SS scale score The SS scale score is the sum of the severity of the 3 symptoms (fatigue, waking unrefreshed, cognitive symptoms) plus the extent (severity) of somatic symptoms in general. (The final score is between 0 and 12) -For the each of the 3 symptoms below, indicate the level of severity over the past week using the following scale: 0 = no problem 1 = slight or mild problems, generally mild or intermittent 2 = moderate, considerable problems, often present and/or at a moderate level 3 = severe: pervasive, continuous, life-disturbing problems Fatigue (0-3) Waking unrefreshed (0-3) Cognitive symptoms (0-3) -Considering somatic symptoms in general, indicate whether the patient has: muscle pain, irritable bowel syndrome, fatigue/tiredness, thinking or remembering problem, muscle weakness, headache, pain/cramps in the abdomen, numbness/tingling, dizziness, insomnia, depression, constipation, pain in the upper abdomen, nausea, nervousness, chest pain, blurred vision, fever, diarrhea, dry mouth, itching, wheezing, Raynaud’s phenomenon, hives/welts, ringing in ears, vomiting, heartburn, oral ulcers, loss of/change in taste, seizures, dry eyes, shortness of breath, loss of appetite, rash, sun sensitivity, hearing difficulties, easy bruising, hair loss, frequent urination, painful urination, and bladder spasms 0 = no symptoms 1 = few symptoms 2 = a moderate number of symptoms 3 = a great deal of symptoms | |||||
2016 Revisions to the 2010/2011 Fibromyalgia Diagnostic Criteria
A limitation of the widespread pain index (WPI) is the fact that it counts the number of painful areas without considering their distribution in the body. Patients with regional pain disorders can fulfill the 2010 American College of Rheumatology preliminary diagnostic criteria since pain can be located in 3 or more areas in the same region 164. To overcome this issue the 2016 revision of the diagnostic criteria require the pain to be generalized (multisite pain). The areas widespread pain index (WPI) assesses are divided in 5 regions (Table 4) and the diagnosis of fibromyalgia requires the distribution of pain in 4 out of 5 regions 165. The jaw, the chest and the abdomen area are problematic when they are used to define a region. In this way they are excluded from the definition of generalized pain 166. Since pain needs to be located in at least 4 areas according to the 2016 revision, the previous criterion for diagnosis, widespread pain index (WPI) of 3-6 and symptom severity (SS) scale score ≥9 was changed to widespread pain index (WPI) of 4-6 and symptom severity (SS) scale score ≥9.
The 2010 and 2011 criteria are extremely similar. Their difference is that the 2010 criteria are physician-based and can be used in clinical practice for the diagnosis of fibromyalgia, while the 2011 criteria are self-reported and can be used only in survey research. According to the 2010 criteria the symptom severity (SS) scale assesses a wide range of somatic symptoms, which makes them impractical for use in questionnaires. With the 2016 revision the assessment of somatic symptoms that is included in the symptom severity (SS) scale is limited to headaches, pain and cramps in the lower abdomen and depression. In this way, there is no longer need for different criteria for clinical practice and for survey research. The same criteria can be used in both settings having 2 different methods of administration.
One prerequisite for diagnosis of fibromyalgia according to the 2010 American College of Rheumatology preliminary diagnostic criteria is the patient not to have a condition that would otherwise explain the pain. The authors of these criteria clarified that this does not mean that the diagnosis of fibromyalgia is an exclusion diagnosis. However, this phrasing was not considered clear enough and caused significant misunderstanding. In this way this criterion was removed in the 2016 revision. The diagnosis of fibromyalgia can be valid even if there is another condition that can cause the pain that is attributed to fibromyalgia. According to this definition fibromyalgia can coexist with other clinically significant conditions that can cause pain.
Table 4. 2016 Fibromyalgia Diagnostic Criteria
| Criteria: A patient satisfies diagnostic criteria for fibromyalgia if the following 3 conditions are met: Widespread pain index (WPI) ≥7 and symptom severity (SS) scale score ≥5 or Widespread pain index (WPI) 4-6 and symptom severity (SS) scale score ≥9. Generalized pain: Pain must be present in at least 4 of 5 regions. Jaw, chest, and abdominal pain are not included in generalized pain definition. Symptoms have been generally for at least 3 months. A diagnosis of fibromyalgia is valid irrespective of other diagnoses. A diagnosis of fibromyalgia does not exclude the presence of other clinically important illnesses. Ascertainment: WPI Note the number of areas in which the patient has had pain over the last week. In how many areas has the patient had pain? (Score will be between 0 and 19) | |||
| Region 1: Left Upper Region -Jaw, left * -Shoulder girdle, left -Upper arm, left -Lower arm, left | Region 2: Right Upper Region -Jaw, right * -Shoulder girdle, right -Upper arm, right -Lower arm, right | Region 5: Axial Region -Neck -Upper back -Lower back -Chest * -Abdomen * | |
| Region 3: Left Lower Region -Hip (buttock, trochanter), left -Upper leg, left -Lower leg, left | Region 4: Right Lower Region -Hip (buttock, trochanter), right -Upper leg, right -Lower leg, right | ||
| |||
| SS scale score The SS scale score is the sum of the severity of the 3 symptoms (fatigue, waking unrefreshed, cognitive symptoms) plus the sum of the number of 3 symptoms (headaches, pain or cramps in lower abdomen, depression) (The final score is between 0 and 12)
0 = no problem
The fibromyalgia severity (FS) scale is the sum of the WPI and the SS scale | |||
AAPT Diagnostic Criteria
In an attempt to improve the recognition of fibromyalgia in clinical practice, the ACTTION-APS Pain Taxonomy (AAPT) fibromyalgia working group proposed new diagnostic criteria in 2018 167. These criteria are similar to the American College of Rheumatology criteria as they require the pain to be generalized (multisite), require the presence of non-pain symptoms and require the symptoms to be present for at least 3 months. These diagnostic criteria are more simple than the American College of Rheumatology criteria and they can be easily implemented in primary clinical practice, but some of their aspects have been criticized 168. According to the AAPT criteria the head, the abdomen and the chest are included in the areas that are assessed for the presence of generalized musculoskeletal pain. However, these regions are problematic since pain originating from the teeth, the heart and the bowel can be referred to these areas. Additionally, the AAPT criteria do not have the ability to quantify the severity of fibromyalgia as, apart from the generalized pain, they only assess the presence or absence of the 2 most common non-pain symptoms of fibromyalgia, abolishing all other somatic symptoms.
ACTTION-APS Pain Taxonomy (AAPT) Diagnostic Criteria 169
- Multisite pain defined as 6 or more pain sites from a total of 9 possible sites:
- Head
- Left arm
- Right arm
- Chest
- Abdomen
- Upper back and spine
- Lower back and spine, including buttocks
- Left leg
- Right leg
- Moderate to severe sleep problems or fatigue
- Multisite pain plus fatigue or sleep problems must have been present for at least 3 months
NOTE. The presence of another pain disorder or related symptoms does not rule out a diagnosis of fibromyalgia. However, a clinical assessment is recommended to evaluate for any condition that could fully account for the patient’s symptoms or contribute to the severity of the symptoms.
Fibromyalgia syndrome treatment
Not all health care providers are familiar with fibromyalgia and its treatment. You should see a doctor who specializes in the treatment of fibromyalgia. In some cases, fibromyalgia may require a healthcare team that may include your primary care physician, a rheumatologist, a physical therapist and a mental health professional. The treatment should be individualized based on the symptoms, the comorbidities and the preferences of the patient, who should be encouraged to participate in the decision-making process of selecting the optimal therapies 170.
The treatment of fibromyalgia is challenging because of current limited understanding of its pathogenesis and the poor response of patients to conventional pain treatments. The goal of treatment is to tackle different symptoms (pain, fatigue, sleep and emotional problems) at the same time. Treatment options for fibromyalgia help to reduce pain, stress and fatigue, treat depression, improve sleep and help people understand what triggers symptoms and how to manage them.
Your doctor may recommend treating your symptoms with acetaminophen (paracetamol) first. He or she may also recommend an anti-depressant, such as duloxetine or milnacipran. Anti-seizure medicines, such as preglabin, may also be effective in managing your pain. Nonsteroidal anti-inflammatory medicines (which include ibuprofen, aspirin, and naproxen) are not usually effective in treating fibromyalgia when taken alone.
No one treatment works for all symptoms, but trying a variety of treatment strategies can have a cumulative effect.
Making changes in your lifestyle and daily habits can help you feel better. Remember, your treatment won’t be as effective if you don’t take an active role in your health care. The following are some ways you can take an active role in managing your fibromyalgia symptoms.
Patient education
The first step should be the education of the patient. The patients with fibromyalgia need to understand their illness before any treatment modality is used 171. Providing a diagnosis, “labeling” the patient with fibromyalgia, may have beneficial effects. It has been shown that fewer symptoms and an improvement in health status is noted after the patients are informed of their diagnosis 172. The physician should clarify that fibromyalgia is a real illness and the symptoms the patient experiences are not imaginary. The role of neurotransmitters and neuromodulators in pain perception, fatigue, abnormal sleep and mood disturbances should be discussed, so as the patient to understand the rationale of the pharmacologic therapy, especially when antidepressant drugs are used. The patient also needs to acknowledge that fibromyalgia is a chronic relapsing condition without though being life-threatening nor deforming.
Self-help
If you have fibromyalgia, there are several ways to change your lifestyle to help relieve your symptoms and make your condition easier to live with.
Your doctor, or another healthcare professional treating you, can offer advice and support about making these changes part of your everyday life.
There are organizations to support people with fibromyalgia that may also be able to offer advice.
- Self-Management Resource Center: https://www.selfmanagementresource.com/
- Chronic Disease Self-Management Program in your area here: http://www.eblcprograms.org/evidence-based/map-of-programs/
- UK Fibromyalgia’s support group: http://ukfibromyalgia.com/index.php
- Fibromyalgia Action UK: https://healthunlocked.com/fibromyalgia-action-uk
Exercise
One of the best things you can do if you have fibromyalgia is engage in moderate exercise on a regular basis. Exercise can reduce your pain, give you more energy, reduce stress, and help you sleep better. If you’re not used to exercising, be sure to talk to your doctor before you start. If you have a physical therapist on your health care team, he or she can help you develop an exercise routine that’s right for you. It’s usually best to start with low-impact aerobic exercise (for example, walking or water aerobics) for a short period of time a few days a week. As your pain decreases and your energy increases, you can gradually increase the intensity and frequency of your exercise.
It has been reported that an exercise program incorporating aerobic, strengthening and flexibility elements can lead to greater benefits than a relaxation program. Exercise in fibromyalgia patients should have two major components: strengthening to increase soft-tissue length and joint mobility, and aerobic conditioning to increase fitness and function, reduce fibromyalgia symptoms and improve quality of life 173. Exercise should be of low impact and of sufficient intensity so as to be able to change aerobic capacity 174. Successful interventions include fast walking, biking, swimming, water aerobics, tai chi and yoga. Land and aquatic training appears to be equally beneficial 175. It has been suggested that in the presence of exercise-induced pain, the intensity and duration of exercise should be reduced, while its frequency should be maintained, so as to avoid any further decrease in exercise tolerance 176. The type and intensity of the exercise program should be individualized and should be based upon patient preference and the presence of any other cardiovascular, pulmonary, or musculoskeletal comorbidities.
Aerobic exercise
Aerobic activities are any kind of rhythmic, moderate-intensity exercises that increase your heart rate and make you breathe harder. Examples include:
- walking
- cycling
- swimming
Research suggests that aerobic fitness exercises should be included in your personalised exercise plan, even if you can’t complete these at a high level of intensity. For example, if you find jogging too difficult, you could try brisk walking instead.
A review of a number of studies found that aerobic exercises may improve quality of life and relieve pain. As aerobic exercises increase your endurance (how long you can keep going), these may also help you function better on a day-to-day basis.
Resistance and strengthening exercises
Resistance and strengthening exercises are those that focus on strength training, such as lifting weights. These exercises need to be planned as part of a personalised exercise programme; if they aren’t, muscle stiffness and soreness could be made worse.
A review of a number of studies concluded that strengthening exercises may improve:
- muscle strength
- physical disability
- depression
- quality of life
People with fibromyalgia who completed the strengthening exercises in these studies said they felt less tired, could function better and experienced a boost in mood.
Improving the strength of your major muscle groups can make it easier to do aerobic exercises.
Pacing yourself
If you have fibromyalgia, it’s important to pace yourself. This means balancing periods of activity with periods of rest, and not overdoing it or pushing yourself beyond your limits.
If you don’t pace yourself, it could slow down your progress in the long term. Over time, you can gradually increase your periods of activity, while making sure they’re balanced with periods of rest.
If you have fibromyalgia, you will probably have some days when your symptoms are better than others. Try to maintain a steady level of activity without overdoing it, but listen to your body and rest whenever you need to.
Avoid any exercise or activity that pushes you too hard, because this can make your symptoms worse. If you pace your activities at a level that’s right for you, rather than trying to do as much as possible in a short space of time, you should make steady progress.
For example, it may help to start with gentler forms of exercise – such as tai chi, yoga and pilates – before attempting more strenuous aerobic or strengthening exercises.
Cognitive-behavioral therapy
Cognitive-behavioral therapy (CBT) has been proven effective for managing fibromyalgia symptoms. One of the goals of the fibromyalgia treatment should be to help patients understand the effect of thoughts, beliefs and expectations on their symptoms. With the help of a trained mental health counselor, a person learns how to change negative thought patterns and behaviors to relieve pain, promote better sleep and improve functioning. This can help them to abolish the perception of helplessness and the catastrophizing thoughts that can adversely influence their condition. Patients with greater self-efficacy are more likely to have a good response to treatment programs and experience better outcomes. The beneficial effect of cognitive-behavioral therapies in fibromyalgia patients with anxiety and depression disorders is limited to a reduction of negative mood, while the rest of the patients also demonstrate a reduction of pain and fatigue. It is worth mentioning that psychologically based interventions, have been proven to be useful when they are compared to no treatment or treatment other than aerobic exercise 177. Preliminary data from functional MRI studies suggest that cognitive-behavioral therapies have the ability to restore the alterations in the functional connectivity of brain areas responsible for pain processing observed in fibromyalgia patients 178.
Acupuncture
Acupuncture is the insertion of needles in the human body. There are different styles of acupuncture depending on the location and the depth the needles are inserted. The inserted needles can be stimulated by heat, electrical current (electro-acupuncture), mechanical pressure (acupressure), or laser (laser acupuncture). The most common type of acupuncture involves skin penetration without stimulation (manual acupuncture). Sham or fake acupuncture is a research tool to control the effects of real acupuncture. It can involve skin contact with the needles without actual penetration or needle insertion in areas other than the ones usually targeted.
In a high-quality meta-analysis, it was demonstrated that the effects of manual acupuncture on pain, sleep quality and global well-being did not differ significantly from the effects of sham acupuncture. On the contrary electro-acupuncture significantly reduced pain, fatigue and stiffness, while it improved sleep quality and global well-being when compared to sham acupuncture. Additionally, electro-acupuncture significantly improved pain, stiffness and global well-being when compared to non-acupuncture. The beneficial effects of acupuncture could be observed at 1 month after treatment, but they were not maintained at 6-7 months 179.
Alternative therapies
The effectiveness of meditative movement therapies (qigong, yoga, tai chi) on sleep and fatigue improvement and of hydrotherapy on pain reduction has been supported by some studies 180. A number of other modalities has also been utilized for the treatment of fibromyalgia including biofeedback, chiropractic therapy, massage therapy, hypnotherapy, guided imagery, electrothermal therapy, phototherapeutic therapy, music therapy, journaling / storytelling, static magnet therapy, transcutaneous electrical nerve stimulation and transcranial direct current stimulation. However there are no well-designed studies to advocate their general use 170.
Massage therapy
In massage therapy, a massage therapist rubs and kneads the soft tissues of your body. The soft tissues include muscle, connective tissue, tendons, ligaments and skin. The massage therapist varies the amount of pressure and movement. Massage can reduce your heart rate, relax your muscles, improve range of motion in your joints and increase production of your body’s natural painkillers. It often helps relieve stress and anxiety.
Yoga and tai chi
Yoga and tai chi combine meditation, slow movements, deep breathing and relaxation. Both have been found to be helpful in controlling fibromyalgia symptoms.
Chiropractic adjustment
Chiropractic adjustment is a procedure in which trained specialists (chiropractors) use their hands or a small instrument to apply a controlled, sudden force to a spinal joint. The goal of this procedure, also known as spinal manipulation, is to improve spinal motion and improve your body’s physical function.
Aromatherapy
Aromatherapy or the therapeutic use of essential oils extracted from plants. Aromatherapy is thought to work by stimulating smell receptors in your nose, which then send messages through your nervous system to the limbic system — the part of your brain that controls emotions. Aromatherapy might have health benefits, including:
- Relief from anxiety and depression.
- Improved quality of life, particularly for people with chronic health conditions.
Physical therapy
A physical therapist can teach you exercises that will improve your strength, flexibility and stamina. Water-based exercises might be particularly helpful.
Occupational therapy
An occupational therapist can help you make adjustments to your work area or the way you perform certain tasks that will cause less stress on your body.
Counseling
Talking with a counselor can help strengthen your belief in your abilities and teach you strategies for dealing with stressful situations.
Recognize stress and take steps to reduce it
Because stress makes the symptoms of fibromyalgia worse, it’s important to recognize when you’re feeling stressed. Signs of stress may include a feeling of tension in your shoulders or neck, an upset stomach, or a headache. Unfortunately, there isn’t a way to completely get rid of stress in your life. However, you can focus on changing the way you react to stress. For example, you might set aside time each day to practice deep-breathing techniques or meditation.
Relaxation
If you have fibromyalgia, it’s important to regularly take time to relax or practice relaxation techniques. Stress can make your symptoms worse or cause them to flare up more often. It could also increase your chances of developing depression.
There are many relaxation aids available, including books, tapes and courses, although deep-breathing techniques or meditation may be just as effective. Try to find time each day to do something that relaxes you. Taking time to relax before bed may also help you sleep better at night.
Talking therapies, such as counseling, can also be helpful in combating stress and learning to deal with it effectively. Your doctor may recommend you try this as part of your treatment.
Establish healthy sleep habits
Lack of sleep can make your fibromyalgia symptoms worse. And increased pain makes it hard to get restful sleep. To avoid getting caught in this cycle, try to have healthy sleeping habits. Avoid caffeine and alcohol before bedtime, go to bed and wake up at the same time each day (including weekends), and limit naps during the day.
Fibromyalgia can make it difficult to fall asleep or stay asleep (known as insomnia). If you have problems sleeping, it may help to:
- get up at the same time every morning
- try to relax at least 1 hour before going to bed
- try to create a bedtime routine, such as taking a bath and drinking a warm, milky drink every night
- avoid caffeine, nicotine and alcohol before going to bed
- avoid eating a heavy meal late at night
- make sure your bedroom is a comfortable temperature, and is quiet and dark – use thick curtains, blinds, an eye mask or ear plugs
- exercise regularly during the day
make sure your mattress, pillows and covers are comfortable - avoid checking the time throughout the night
Everyone needs different amounts of sleep. On average we need:
- adults – 7 to 9 hours
- children – 9 to 13 hours
- toddlers and babies – 12 to 17 hours
You probably don’t get enough sleep if you’re constantly tired during the day.
Get into a routine
Many people who have fibromyalgia do better when their schedule follows a routine pattern. This usually means that each day they have meals at the same times, go to bed and get up at the same times, and exercise at the same time. Try to keep your weekend and holiday schedules as similar to your weekday schedule as possible.
Make healthy lifestyle choices
By making healthy choices, you’ll have more energy, you’ll feel better, and you’ll lower your risk for other health problems. Eat a healthy, balanced diet. Limit the amount of alcohol you drink. If you use tobacco products, stop. Lose weight if you are overweight.
Medications
A wide range of drugs has been used in the treatment of fibromyalgia including antidepressants, sedatives, muscle relaxants and antiepileptic drugs. The choice of medication is influenced by patient preference; prominence of particular symptoms, including fatigue, insomnia, and depression; potential adverse effects; patient tolerance of individual medications; cost and regulatory limitations on prescription choice 181. Nonsteroidal anti-inflammatory drugs and opioids, although often prescribed for fibromyalgia, are not an effective form of treatment 182.
Three medications are specifically approved to treat fibromyalgia. Duloxetine (Cymbalta) and milnacipran(Savella) work by changing the levels of certain chemicals in the brain that help control pain. Pregabalin (Lyrica) targets brain chemicals that affect how much pain you experience. Duloxetine (Cymbalta) and milnacipran (Savella) may help ease the pain and fatigue associated with fibromyalgia. Your doctor may prescribe amitriptyline or the muscle relaxant cyclobenzaprine to help promote sleep.
Other medications can be used to treat pain, sleep and mood. These include anti-inflammatories, antidepressants and sleep medicines.
Anti-seizure medications designed to treat epilepsy are often useful in reducing certain types of pain. Gabapentin (Neurontin) is sometimes helpful in reducing fibromyalgia symptoms, while pregabalin (Lyrica) was the first drug approved by the Food and Drug Administration to treat fibromyalgia.
Patients should be informed that for most pharmacologic therapies several weeks may be needed until they experience a benefit. Initially a single drug should be administered. However, in the case of non-responsiveness combination therapy should be considered. Since therapeutic responses are rarely durable, physicians should not be surprised when the initial efficacy of a medication is abolished. Successful treatment of fibromyalgia may require regular reassessment and possible rotation of medications 183. The doses of the most commonly used medications with strong and moderate evidence of effectiveness are shown in Table 5. Adequate dose prescription and patient adherence are significant for the effectiveness and tolerability of pharmacologic treatment 184.
Table 5. The doses of the most commonly used medications with strong and moderate evidence of effectiveness in fibromyalgia
| Drugs | Doses |
|---|---|
| Tricyclic antidepressants | |
| Amitriptyline | Start 10 mg at bedtime, increase up to 25-50 mg |
| Cyclobenzaprine | Start 10 mg at bedtime, increase up to 30-40mg, decrease to 5mg if 10mg too sedating |
| Serotonin-norepinephrine reuptake inhibitors | |
| Duloxetine | Start 10-15mg twice daily, gradually increased to 30 mg twice daily |
| Milnacipran | Start 12.5mg in the morning, gradually increase to 50mg twice daily |
| Venlafaxine | 167 mg per day |
| Anticonvulsants | |
| Gabapentin | Start 100mg at bedtime, increase to 1200-2400 mg per day |
| Pregabalin | Start 25-50mg at bedtime, increase to 300-450 mg/day |
| Other | |
| Tramadol | 37.5 mg four times daily |
The U.S. Food and Drug Administration has approved three drugs for the treatment of fibromyalgia. In June 2007, Lyrica (pregabalin) became the first FDA-approved drug for specifically treating fibromyalgia; a year later, in June 2008, Cymbalta (duloxetine hydrochloride) became the second; and in January 2009, Savella (milnacipran HCI) became the third 185. Older drugs that affect these same brain chemicals also may be used to treat fibromyalgia. These include amitriptyline (Elavil) and cyclobenzaprine (Flexeril). Other antidepressant drugs can be helpful in some patients. Side effects vary by the drug. Ask your doctor about the risks and benefits of your medicine.
Lyrica, Cymbalta and Savella reduce pain and improve function in some people with fibromyalgia. While those with fibromyalgia have been shown to experience pain differently from other people, the mechanism by which these drugs produce their effects is unknown. There is data suggesting that these drugs affect the release of neurotransmitters in the brain. Neurotransmitters are chemicals that transmit signals from one neuron to another. Treatment with Lyrica, Cymbalta, and Savella may reduce the level of pain experienced by some people with fibromyalgia.
Lyrica, marketed by Pfizer Inc., was previously approved to treat seizures, as well as pain from damaged nerves that can happen in people with diabetes (diabetic peripheral neuropathy) and in those who develop pain following the rash of shingles. Side effects of Lyrica including sleepiness, dizziness, blurry vision, weight gain, trouble concentrating, swelling of the hands and feet, and dry mouth. Allergic reactions, although rare, can occur.
Cymbalta, marketed by Eli Lilly and Co., was previously approved to treat depression, anxiety, and diabetic peripheral neuropathy. Cymbalta’s side effects include nausea, dry mouth, sleepiness, constipation, decreased appetite, and increased sweating. Like some other antidepressants, Cymbalta may increase the risk of suicidal thinking and behavior in people who take the drug for depression. Some people with fibromyalgia also experience depression.
Savella, marketed by Forest Pharmaceuticals, Inc., is the first drug introduced primarily for treating fibromyalgia. Savella is not used to treat depression in the United States, but acts like medicines that are used to treat depression (antidepressants) and other mental disorders. Antidepressants may increase suicidal thoughts or actions in some people. Side effects include nausea, constipation, dizziness, insomnia, excessive sweating, vomiting, palpitations or increased heart rate, dry mouth and high blood pressure.
Studies of both drugs showed that a substantial number of people with fibromyalgia received good pain relief, but there were others who didn’t benefit.
Lyrica and Cymbalta are approved for use in adults 18 years and older 185. The drug manufacturers have agreed to study their drugs in children with fibromyalgia and in breastfeeding women.
Tricyclic antidepressants
Tricyclic antidepressants (TCAs) are often used as initial treatment for fibromyalgia. Their analgesic effect is independent of their antidepressant action and is thought to be mediated by inhibition of norepinephrine (rather than serotonin) reuptake at spinal dorsal horn synapses, with secondary activity at the sodium channels. The most widely studied drugs of this group are amitriptyline and cyclobenzaprine. They should be administered at lower doses than those required for the treatment of depression, a few hours before bedtime, and their dose should be escalated very slowly. A clinically important improvement is observed in 25-45% of patients treated with TCAs compared to 20% in those taking placebo 186. However their use is limited by the fact that they are ineffective or intolerable in 60-70% of patients 176, while their efficacy may decrease over time 187.
In a systematic review and meta-analysis, amitriptyline was shown to be more efficient compared to the serotonin-norepinephrine reuptake inhibitors duloxetine and milnacipran in reducing pain, sleep disturbance, and fatigue, without differences in acceptability 188. The combination of 20 mg of fluoxetine in the morning with 25 mg of amitriptyline at bedtime has been shown to be more effective than either medication alone 189. Side effects of amitriptyline include dry mouth, constipation, fluid retention, weight gain, grogginess, difficulty in concentrating and possibly cardiotoxicity.
Cyclobenzaprine has a similar tricyclic structure and presumed mode of action with amitriptyline in fibromyalgia, but is thought to have minimal antidepressant effect 181. A meta-analysis of five placebo-controlled trials has revealed improvement of the global functioning, with a similar effect size as this reported for amitriptyline. The group that received cyclobenzaprine had a significant decrease in pain for 4 weeks, compared to those treated with placebo, but the decrease in pain was not significantly different after 8 and 12 weeks. Sleep was improved at all time points in both cyclobenzaprine and placebo groups, while no effect was noted on fatigue 186. It has been demonstrated that the use of very low-dose cyclobenzaprine (1 to 4 mg at bedtime) can improve the symptoms of fibromyalgia, including pain, fatigue, and depression, compared to symptoms at baseline and to placebo. Significantly more patients who received the very low-dose of cyclobenzaprine experienced improved restorative sleep, based upon analysis of cyclic alternating pattern of sleep by electroencephalography. The increase in nights with improved sleep by this measure correlated with improvements in fatigue and depression 190.
Desipramine has fewer anticholinergic and sedative effects than other tricyclic antidepressants, which can make it a possible alternative, although its efficacy is not well studied in fibromyalgia.
Serotonin-norepinephrine reuptake inhibitors
Serotonin-norepinephrine reuptake inhibitors (SNRIs) are similar to tricyclic antidepressants in their ability to inhibit the reuptake of both serotonin and norepinephrine, but they differ from tricyclic antidepressants in being devoid of significant activity at other receptor systems, resulting in diminished side effects and increased tolerance. Venlafaxine, duloxetine and milnacipran have been shown to be effective in diminishing fibromyalgia symptoms 191. These drugs can be used in fibromyalgia patients who do not respond to a trial of low-dose tricyclic antidepressants or who have intolerable side effects. They can also be administered as an alternative to amitriptyline for initial therapy. Of these medications’ duloxetine and milnacipran are better studied and they are preferred to be administered to patients with fibromyalgia. There are more limited data regarding the efficacy of venlafaxine for fibromyalgia, while withdrawal symptoms if a dose is missed occur more often, because of the short half-life of this medication 192. A meta-analysis has shown that fibromyalgia patients treated with duloxetine at 60mg daily are more likely to have more than 50% reduction in pain, compared to patients taking placebo 193. However, duloxetine at 30mg daily does not significantly reduce pain 194. The efficacy of duloxetine can be maintained at 3 and 6 months of treatment 195. In a 2018 systematic review and meta-analysis it was shown that duloxetine and milnacipran were not superior to placebo in the frequency of pain relief of at least 50%, but there was a benefit in reducing the pain at least by 30% and in the patient’s global impression to be much or very much improved. Additionally, there was not a significant difference in the reduction of fatigue, in the reduction of sleep problems, nor in the improvement of health-related quality of life 196. Another meta-analysis has shown that duloxetine, pregabalin and milnacipran were superior to placebo for pain relief, while duloxetine and pregabalin were superior to milnacipran. These drugs also differed in their effects on sleep disturbances, depression and fatigue. Headaches, nausea and diarrhea were more common with duloxetine and milnacipran treatment, while cognitive defects and weight gain were more common with pregabalin 197.
Monoamine oxidase inhibitors
Monoamine oxidase inhibitors block the catabolism of serotonin, increasing its levels in the brain. It has been indicated that pirlindole and moclobemide have a significant beneficial effect on pain, without a significant effect on sleep nor fatigue 198.
Anticonvulsants
Pregabalin has been reported to be efficient against pain, sleep disturbances and fatigue in fibromyalgia. In a meta-analysis a reduction of pain of at least 50% was found in 22% of patients treated with pregabalin, compared to 14% of those taking placebo. There was a small benefit in sleep disturbances, while no improvement could be found on fatigue 199. Side effects such as somnolence and dizziness have been reported 182. It is preferred in patients with more severe problems with sleep, and it is administered at bedtime.
Gabapentin has been shown to be efficient in treating fibromyalgia associated pain, while it was well tolerated 200. Side effects include dizziness, sedation, lightheadedness, and weight gain. It can be considered as an acceptable alternative in case pregabalin cannot be administered due to its cost or due to regulatory limitations.
Muscle relaxants
Carisoprodol in combination with acetaminophen and caffeine has been shown to improve pain, sleep quality and the overall feeling of well-being in fibromyalgia patients 201.
Sedative hypnotic agents
Zopiclone and zolpidem have been used in fibromyalgia. It has been suggested that they can improve the sleep and perhaps fatigue, without any significant effects on pain 176.
Sodium oxibate, a precursor of GABA with powerful sedative properties has been shown to improve pain, fatigue and sleep architecture in fibromyalgia 202. However, in view of safety concerns the European Medicines Agency and the US Food and Drug Administration have not approved it for use in fibromyalgia patients.
Tramadol
Tramadol has multiple analgesic effects, since it inhibits norepinephrine and serotonin reuptake, and its major metabolite binds weakly to opioid μ receptors 176. The use of tramadol (with or without acetaminophen) is both effective and well tolerated for the management of pain in fibromyalgia 203. There are some concerns regarding the long-term potential of abuse of tramadol, although the risk is less than that of more potent narcotic analgesics that have also been used in fibromyalgia.
Other treatment options
As well as medication, there are other treatment options that can be used to help cope with the pain of fibromyalgia, such as:
- swimming, sitting or exercising in a heated pool or warm water (known as hydrotherapy or balneotherapy)
- an individually tailored exercise programme
- cognitive behavioral therapy (CBT) – a talking therapy that aims to change the way you think about things, so you can tackle problems more positively
- psychotherapy – a talking therapy that helps you understand and deal with your thoughts and feelings
- relaxation techniques
- psychological support – any kind of counseling or support group that helps you deal with issues caused by fibromyalgia
See self-help for fibromyalgia for more information about exercise and relaxation techniques.
Treating other conditions
If you’ve been diagnosed with fibromyalgia and another condition, such as depression or irritable bowel syndrome (IBS), you may need to have separate treatment for these. For example, additional counseling or medication may be recommended.
Fibromyalgia diet
Some people say their fibromyalgia symptoms are worsened by certain foods or food additives — such as refined flour, dairy products, sugar, sugar substitutes or MSG — but there’s no clear research-based evidence to support this. Fibromyalgia patients produce higher levels of harmful free radicals than healthy people and have a decreased antioxidant ability, contributing to oxidative stress 204.
Some studies show a benefit in avoiding certain foods or additives, while other studies don’t show such a correlation. Scientists are investigating possible connections between the consumption of gluten and fibromyalgia symptoms, but more research is needed.
People who have fibromyalgia are also more likely to be overweight or obese, and both problems impact quality of life. For some people, losing weight can help reduce fibromyalgia symptoms.
The progression of fibromyalgia may be dependent on increased reactive oxygen species (ROS). Reactive oxygen species (ROS) are highly reactive chemicals, containing oxygen, that react easily with other molecules, resulting in potentially damaging modifications. Treatment with antioxidants and vitamins, in addition to antidepressants and structural analogs of gamma-aminobutyric acid (GABA), was able to change the symptoms of fibromyalgia patients 205. Certain groups of bioactive compounds derived from medicinal plants have also demonstrated analgesic activity and antioxidant properties with respect to fibromyalgia: these include essential oils 206, extracts 207, monoterpenes 208, sesquiterpenes 209 and alkaloids 210.
Table 6. Compounds with antioxidant and analgesic properties for fibromyalgia management
| Compound | Effects | References |
|---|---|---|
| Melatonin | In an animal study, melatonin was able to improve behavioral defects, oxidative and nitrosative stress, mast cell infiltration and activation of microglia in a reserpine-induced fibromyalgia model. | 211 |
| In a clinical trial, the exogenous administration of 10 mg of melatonin once every 24 h increased endogenous pain inhibition, assessed on a numerical scale (0–10). The combination of amitriptyline and melatonin provided better results than amitriptyline alone, as calculated by the visual analog pain scale, in subjects with fibromyalgia. | 212 | |
| A randomized trial found that melatonin alone or in combination with fluoxetine was beneficial for the treatment of fibromyalgia. Using melatonin (3 or 5 mg/day) in combination with 20 mg/day fluoxetine caused a significant reduction in both total and individual components of the Fibromyalgia Impact Questionnaire score compared to the pretreatment values. | 213 | |
| Coenzyme Q10 | Coenzyme Q10 treatment showed effects on clinical symptoms, blood mononuclear cells and markers of mitochondrial and oxidative stress in women with fibromyalgia. | 214 |
| The results of this clinical study suggest that Coenzyme Q10 supplementation plays a role in the modulation of mitochondrial dysfunction and oxidative stress that induce headaches in individuals with fibromyalgia. | 215 | |
| In a clinical study, Coenzyme Q10 supplementation was shown to provide additional benefits for relieving pain sensation in fibromyalgia patients treated with pregabalin, possibly by improving mitochondrial function, reducing inflammation and decreasing brain activity. | 216 | |
| Vitamins D and E | A clinical study found that women with fibromyalgia had a lower qualitative and quantitative intake than control subjects. In particular, an association has been found between vitamin D deficiency and fibromyalgia. However, its role in fibromyalgia pathophysiology and the clinical relevance of its identification and treatment requires further clarification. Only vitamin E appears to be related to quality of life and pain sensation. | 81, 217, 218 |
| Palmitoylethanolamide (PEA) | Palmitoylethanolamide (PEA) is a major anti-inflammatory, analgesic and neuroprotective mediator in central and peripheral organs and systems and acts on several molecular targets. | 219, 220 |
| Palmitoylethanolamide (PEA) is emerging as a candidate biomarker due to its anti-inflammatory and anti-hyperalgesic effects via the downregulation of mast cell activation. Preclinical and clinical studies support the idea that Palmitoylethanolamide (PEA) merits further study as a therapeutic approach for controlling inflammatory responses, pain, related peripheral neuropathic pain and symptoms of fibromyalgia. | 221, 222, 223, 224, 225 |
Living with fibromyalgia syndrome
There are many things you can do to while living with fibromyalgia, including:
- Getting enough sleep.
- Exercising.
- Adjusting your work demands.
- Eating well.
Getting enough sleep
Getting enough sleep and the right kind of sleep can help ease the pain and fatigue of fibromyalgia. You may have problems such as pain, restless legs syndrome, or brainwave changes that interfere with restful sleep. It is important to discuss any sleep problems with your doctor, who can prescribe or recommend treatment.
Tips for good sleep
- Keep regular sleep habits. Try to get to bed and wake up at the same time every day.
- Avoid caffeine and alcohol in the late afternoon and evening. Even though alcohol can make you feel sleepy, drinking any close to bedtime can disturb your sleep.
- Time your exercise. Regular daytime exercise can help improve your nighttime sleep. However, exercise within 3 hours of bedtime can keep you awake.
- Avoid daytime naps. Sleeping in the afternoon can interfere with nighttime sleep. If you feel like you cannot get by without a nap, set an alarm for 1 hour. When it goes off, get up and start moving.
- Reserve your bed for sleeping. Watching TV, reading, or using a laptop or phone in bed can keep you awake.
- Keep your bedroom comfortable. Try to keep your bedroom dark, quiet, and cool.
- Avoid drinking liquids and eating spicy meals before bed. Heartburn and late-night trips to the bathroom can interfere with your sleep.
- Wind down before bed. Avoid working right up to bedtime. Try some relaxing activities that get you ready for sleep, such as listening to soft music or taking a warm bath.
Exercising
Although pain and fatigue may make exercise and daily activities difficult, it is important for you to be as physically active as possible. Research shows that regular exercise is one of the most useful treatments for fibromyalgia. If you have too much pain or fatigue to do exercise, you should begin with walking or other gentle exercise. Over time you can build your strength.
Adjusting your work life
You can continue to work when you have fibromyalgia, but may have to make some changes to do so. For example, you may need to cut down the number of hours they work, switch to a less demanding job, or adapt your current job. An occupational therapist can help you make changes at work. For example, they can help design a more comfortable workstation or find more efficient and less painful ways to lift.
Eating well
Although some people with fibromyalgia report feeling better when they eat or avoid certain foods, no specific diet has been proven to influence fibromyalgia. Of course, it is important to have a healthy, balanced diet. Not only will proper nutrition give you more energy and make you generally feel better, it will also help you avoid other health problems.
Fibromyalgia diet
Some people say their fibromyalgia symptoms are worsened by certain foods or food additives — such as refined flour, dairy products, sugar, sugar substitutes or MSG — but there’s no clear research-based evidence to support this.
Some studies show a benefit in avoiding certain foods or additives, while other studies don’t show such a correlation. Scientists are investigating possible connections between the consumption of gluten and fibromyalgia symptoms, but more research is needed.
People who have fibromyalgia are also more likely to be overweight or obese, and both problems impact quality of life. For some people, losing weight can help reduce fibromyalgia symptoms.
Fibromyalgia syndrome prognosis
Most longitudinal long-term studies have shown that most fibromyalgia patients continue to have chronic pain and fatigue, but the majority of these studies have been from tertiary referral centers 43. In contrast, patients treated by primary care physicians in the community have a much better prognosis 43. Many demographic and psychosocial factors significantly impact the prognosis and outcome in patients with fibromyalgia. Female gender, low socioeconomic status, unemployment, obesity, depression, and history of abuse had negative effects on the outcome 43.
Overall fibromyalgia prognosis is poor for many patients. Factors associated with poor prognosis include:
- A long duration of disease
- High-stress levels
- Presence of depression or anxiety that has not been adequately treated
- Long-standing avoidance of work
- Alcohol or drug dependence
- Moderate to severe functional impairment
Patients with fibromyalgia are more likely to be hospitalized for any reason compared to the general population.
- Jahan F, Nanji K, Qidwai W, Qasim R. Fibromyalgia syndrome: an overview of pathophysiology, diagnosis and management. Oman Med J. 2012;27(3):192–195. doi:10.5001/omj.2012.44 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3394355[↩]
- Fibromyalgia. https://medlineplus.gov/fibromyalgia.html[↩]
- Bellato E., Marini E., Castoldi F., Barbasetti N., Mattei L., Bonasia D.E., Blonna D. Fibromyalgia syndrome: Etiology, pathogenesis, diagnosis, and treatment. Pain Res. Treat. 2012;2012:426130. doi: 10.1155/2012/426130[↩]
- Gowers WR. A Lecture on Lumbago: Its Lessons and Analogues: Delivered at the National Hospital for the Paralysed and Epileptic. Br Med J. 1904 Jan 16;1(2246):117-21. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2352601/pdf/brmedj08152-0001.pdf[↩]
- GRAHAM W. The fibrosits syndrome. Bull Rheum Dis. 1953 Apr;3(8):33-4.[↩]
- Smythe HA, Moldofsky H. Two contributions to understanding of the “fibrositis” syndrome. Bull Rheum Dis. 1977-1978;28(1):928-31.[↩]
- Staud R., Vierck C.J., Cannon R.L., Mauderli A.P., Price D.D. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/S0304-3959(00)00432-2[↩]
- Wolfe F. New American College of Rheumatology criteria for fibromyalgia: A twenty-year journey. Arthritis Care Res. 2010;62:583–584. doi: 10.1002/acr.20156[↩][↩]
- Wolfe F., Clauw D.J., Fitzcharles M.A., Goldenberg D.L., Katz R.S., Mease P., Russell A.S., Russell I.J., Winfield J.B., Yunus M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600–610. doi: 10.1002/acr.20140[↩][↩]
- Wolfe F., Clauw D.J., Fitzcharles M.A., Goldenberg D.L., Hauser W., Katz R.L., Mease P.J., Russell A.S., Russell I.J., Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016;46:319–329. doi: 10.1016/j.semarthrit.2016.08.012[↩]
- De Sanctis V, Abbasciano V, Soliman AT, et al. The juvenile fibromyalgia syndrome (JFMS): a poorly defined disorder. Acta Biomed. 2019;90(1):134–148. Published 2019 Jan 23. doi:10.23750/abm.v90i1.8141 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6502146[↩][↩][↩][↩]
- Jones GT, Atzeni F, Beasley M, Flüß E, Sarzi-Puttini P, Macfarlane GJ. The prevalence of fibromyalgia in the general population: a comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis & rheumatology (Hoboken, N.J.). 2015 Feb;67(2):568-75.[↩]
- Häuser W, Fitzcharles MA. Facts and myths pertaining to fibromyalgia. Dialogues Clin Neurosci. 2018;20:53–62.[↩]
- Bhargava J, Hurley JA. Fibromyalgia. [Updated 2019 Nov 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK540974[↩][↩][↩]
- Brill S, Ablin JN, Goor-Aryeh I, Hyat K, Slefer A, Buskila D., Tel Aviv-Sourasky Medical Center. Prevalence of fibromyalgia syndrome in patients referred to a tertiary pain clinic. J. Investig. Med. 2012 Apr;60(4):685-8.[↩]
- Siracusa R, Paola RD, Cuzzocrea S, Impellizzeri D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int J Mol Sci. 2021 Apr 9;22(8):3891. doi: 10.3390/ijms22083891[↩][↩][↩][↩][↩][↩]
- Eraso RM, Bradford NJ, Fontenot CN, Espinoza LR, Gedalia A. Fibromyalgia syndrome in young children: onset at age 10 years and younger. Exp Rheumatol. 2007;25:639–644.[↩]
- Zernikow B, Gerhold K, Bürk G, Häuser W, Hinze CH, Hospach T, Illhardt A, Mönkemöller K, Richter M, Schnöbel-Müller E, Häfner R. Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften. Definition, diagnosis and therapy of chronic widespread pain and so-called fibromyalgia syndrome in children and adolescents. Systematic literature review and guideline. Schmerz. 2012;26:318–330.[↩]
- American Pain Society. Glenview: American Pain Society; 2005. Guideline for the management of fibromyalgia syndrome pain in adults and children.[↩]
- Carville SF, Arendt-Nielsen L, Bliddal H, Blotman F, Branco JC, Buskila D, Da Silva JA, Danneskiold-Samsøe B, Dincer F, Henriksson C, Henriksson KG, Kosek E, Longley K, McCarthy GM, Perrot S, Puszczewicz M, Sarzi-Puttini P, Silman A, Späth M, Choy EH. EULAR. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67:536–541.[↩]
- Navarro RP. Contemporary management strategies for fibromyalgia. Am J Manag Care. 2009;15(7 suppl):S197–218.[↩]
- Tran ST, Guite JW, Pantaleao A, Pfeiffer M, Myer GD, Sil S, Thomas SM, Ting TV, Williams SE, Edelheit B, Ounpuu S, Rodriguez-MacClintic J, Zemel L, Zempsky W, Kashikar-Zuck S. Preliminary Outcomes of a Cross-Site Cognitive-Behavioral and Neuromuscular Integrative Training Intervention for Juvenile Fibromyalgia. Arthritis Care Res (Hoboken) 2017;69:413–420.[↩]
- Eccleston C, Palermo TM, Williams AC, Lewandowski A, Morley S. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2009 Apr 15;(2):CD003968. doi: 10.1002/14651858[↩]
- Yokota S, Kikuchi M, Miyamae T. Juvenile fibromyalgia: Guidance for management. Pediatr Int. 2013;55:403–409.[↩]
- McLeod JD. Juvenile fibromyalgia syndrome and improved recognition by pediatric primary care providers. J Pediatr Health Care. 2014;28:e9–18.[↩]
- Mikkelsson M. One year outcome of preadolescents with fibromyalgia. J Rheumatol. 1999;26:674–682.[↩]
- Kashikar-Zuck S, Parkins IS, Ting TV, Verkamp E, Lynch-Jordan A, Passo M, Graham TB. Controlled follow-up study of physical and psychosocial functioning of adolescents with juvenile primary fibromyalgia syndrome. Rheumatology (Oxford) 2010;49:2204–2209.[↩][↩]
- Hassett AL, Hilliard PE, Goesling J, Clauw DJ, Harte SE, Brummett CM. Reports of chronic pain in childhood and adolescence among patients at a tertiary care pain clinic. J Pain. 2013;14:1390–7.27.[↩]
- Gedalia A, García CO, Molina JF, Bradford NJ, Espinoza LR. Fibromyalgia syndrome in pediatric patients. Clin Exp Rheumatol. 2000;18:415–419.[↩]
- Alfvén G. Recurrent pain, stress, tender points and fibromyalgia in childhood: an exploratory descriptive clinical study. Acta Paediatr. 2012;101:283–291.[↩]
- Black WR, Kashikar-Zuck S. Exercise interventions for juvenile fibromyalgia: current state and recent advancements. Pain Manag. 2017;7:143–148.[↩]
- Kashikar-Zuck S, Cunningham N, Sil S, Bromberg MH, Lynch-Jordan AM, Strotman D, Peugh J, Noll J, Ting TV, Powers SW, Lovell DJ, Arnold LM. Long-term outcomes of adolescents with juvenile-onset fibromyalgia in early adulthood. Pediatrics. 2014;133:e592–600.[↩]
- Sarzi-Puttini P, Atzeni F, Mease PJ. Chronic widespread pain: from peripheral to central evolution. Best Pract Res Clin Rheumatol. 2011 Apr;25(2):133-9. doi: 10.1016/j.berh.2011.04.001[↩]
- Schmidt-Wilcke T, Clauw DJ. Fibromyalgia: from pathophysiology to therapy. Nat Rev Rheumatol. 2011 Jul 19;7(9):518-27. doi: 10.1038/nrrheum.2011.98[↩]
- Dadabhoy D, Crofford LJ, Spaeth M, Russell IJ, Clauw DJ. Biology and therapy of fibromyalgia. Evidence-based biomarkers for fibromyalgia syndrome. Arthritis Res Ther. 2008;10(4):211. doi: 10.1186/ar2443[↩][↩]
- Apkarian A.V. Definitions of nociception, pain, and chronic pain with implications regarding science and society. Neurosci. Lett. 2019;702:1–2. doi: 10.1016/j.neulet.2018.11.039[↩]
- Baliki M.N., Apkarian A.V. Nociception, Pain, Negative Moods, and Behavior Selection. Neuron. 2015;87:474–491. doi: 10.1016/j.neuron.2015.06.005[↩]
- Cohen M., Quintner J., Van Rysewyk S. Reconsidering the International Association for the study of pain definition of pain. Pain Rep. 2018;3:e634. doi: 10.1097/PR9.0000000000000634[↩]
- Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003 May;48(5):1420-9. https://onlinelibrary.wiley.com/doi/epdf/10.1002/art.10893[↩]
- Bhargava J, Hurley JA. Fibromyalgia. [Updated 2022 Oct 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK540974[↩][↩]
- Buskila D, Sarzi-Puttini P. Biology and therapy of fibromyalgia. Genetic aspects of fibromyalgia syndrome. Arthritis Res Ther. 2006;8(5):218. doi: 10.1186/ar2005[↩][↩]
- Oertel B., Lotsch J. Genetic mutations that prevent pain: Implications for future pain medication. Pharmacogenomics. 2008;9:179–194. doi: 10.2217/14622416.9.2.179[↩][↩]
- Bhargava J, Hurley JA. Fibromyalgia. [Updated 2023 Jun 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK540974[↩][↩][↩][↩][↩][↩][↩][↩]
- Staud R. Abnormal pain modulation in patients with spatially distributed chronic pain: fibromyalgia. Rheum Dis Clin North Am. 2009 May;35(2):263-74. doi: 10.1016/j.rdc.2009.05.006[↩][↩]
- Meyer H.P. Myofascial pain syndrome and its suggested role in the pathogenesis and treatment of fibromyalgia syndrome. Curr. Pain Headache Rep. 2002;6:274–283. doi: 10.1007/s11916-002-0048-z[↩]
- Clauw D.J. Fibromyalgia and related conditions. Mayo Clin. Proc. 2015;90:680–692. doi: 10.1016/j.mayocp.2015.03.014[↩]
- Malatji B.G., Mason S., Mienie L.J., Wevers R.A., Meyer H., van Reenen M., Reinecke C.J. The GC-MS metabolomics signature in patients with fibromyalgia syndrome directs to dysbiosis as an aspect contributing factor of FMS pathophysiology. Metabolomics. 2019;15:54. doi: 10.1007/s11306-019-1513-6[↩]
- Bradley L.A. Pathophysiology of fibromyalgia. Am. J. Med. 2009;122(Suppl. S12):S22–S30. doi: 10.1016/j.amjmed.2009.09.008[↩]
- Yunus M.B., Khan M.A., Rawlings K.K., Green J.R., Olson J.M., Shah S. Genetic linkage analysis of multicase families with fibromyalgia syndrome. J. Rheumatol. 1999;26:408–412.[↩]
- Muir W.W., 3rd, Woolf C.J. Mechanisms of pain and their therapeutic implications. J. Am. Vet. Med. Assoc. 2001;219:1346–1356. doi: 10.2460/javma.2001.219.1346[↩]
- Vierck C.J., Jr. Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia) Pain. 2006;124:242–263. doi: 10.1016/j.pain.2006.06.001[↩][↩]
- Staud R., Nagel S., Robinson M.E., Price D.D. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: A randomized, double-blind, placebo-controlled study. Pain. 2009;145:96–104. doi: 10.1016/j.pain.2009.05.020[↩]
- Brosschot J.F. Cognitive-emotional sensitization and somatic health complaints. Scand. J. Psychol. 2002;43:113–121. doi: 10.1111/1467-9450.00276[↩]
- Jackson P.L., Meltzoff A.N., Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006[↩]
- Collado A., Gomez E., Coscolla R., Sunyol R., Sole E., Rivera J., Altarriba E., Carbonell J., Castells X. Work, family and social environment in patients with Fibromyalgia in Spain: An epidemiological study: EPIFFAC study. BMC Health Serv. Res. 2014;14:513. doi: 10.1186/s12913-014-0513-5[↩]
- O’Brien A.T., Deitos A., Trinanes Pego Y., Fregni F., Carrillo-de-la-Pena M.T. Defective Endogenous Pain Modulation in Fibromyalgia: A Meta-Analysis of Temporal Summation and Conditioned Pain Modulation Paradigms. J. Pain. 2018;19:819–836. doi: 10.1016/j.jpain.2018.01.010[↩]
- Harris R.E., Clauw D.J. Imaging central neurochemical alterations in chronic pain with proton magnetic resonance spectroscopy. Neurosci. Lett. 2012;520:192–196. doi: 10.1016/j.neulet.2012.03.042[↩]
- Clauw DJ. Fibromyalgia: A Clinical Review. JAMA. 2014;311(15):1547–1555. doi:10.1001/jama.2014.3266[↩]
- Sluka K.A., O’Donnell J.M., Danielson J., Rasmussen L.A. Regular physical activity prevents development of chronic pain and activation of central neurons. J. Appl. Physiol. 2013;114:725–733. doi: 10.1152/japplphysiol.01317.2012[↩]
- Bobinski F., Ferreira T.A.A., Cordova M.M., Dombrowski P.A., da Cunha C., Santo C., Poli A., Pires R.G.W., Martins-Silva C., Sluka K.A., et al. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain. 2015;156:2595–2606. doi: 10.1097/j.pain.0000000000000372[↩]
- Nugraha B., Karst M., Engeli S., Gutenbrunner C. Brain-derived neurotrophic factor and exercise in fibromyalgia syndrome patients: A mini review. Rheumatol. Int. 2012;32:2593–2599. doi: 10.1007/s00296-011-2348-2[↩]
- Giovengo SL, Russell IJ, Larson AA. Increased concentrations of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia. J Rheumatol. 1999 Jul;26(7):1564-9.[↩]
- Baumeister D., Eich W., Saft S., Geisel O., Hellweg R., Finn A., Svensson C.I., Tesarz J. No evidence for altered plasma NGF and BDNF levels in fibromyalgia patients. Sci. Rep. 2019;9:13667. doi: 10.1038/s41598-019-49403-7[↩]
- Slade G.D., Conrad M.S., Diatchenko L., Rashid N.U., Zhong S., Smith S., Rhodes J., Medvedev A., Makarov S., Maixner W., et al. Cytokine biomarkers and chronic pain: Association of genes, transcription, and circulating proteins with temporomandibular disorders and widespread palpation tenderness. Pain. 2011;152:2802–2812. doi: 10.1016/j.pain.2011.09.005[↩]
- Sturgill J., McGee E., Menzies V. Unique cytokine signature in the plasma of patients with fibromyalgia. J. Immunol. Res. 2014;2014:938576. doi: 10.1155/2014/938576[↩]
- Mendieta D., De la Cruz-Aguilera D.L., Barrera-Villalpando M.I., Becerril-Villanueva E., Arreola R., Hernandez-Ferreira E., Perez-Tapia S.M., Perez-Sanchez G., Garces-Alvarez M.E., Aguirre-Cruz L., et al. IL-8 and IL-6 primarily mediate the inflammatory response in fibromyalgia patients. J. Neuroimmunol. 2016;290:22–25. doi: 10.1016/j.jneuroim.2015.11.011[↩][↩][↩]
- Littlejohn G., Guymer E. Neurogenic inflammation in fibromyalgia. Semin. Immunopathol. 2018;40:291–300. doi: 10.1007/s00281-018-0672-2[↩]
- Bote M.E., Garcia J.J., Hinchado M.D., Ortega E. An exploratory study of the effect of regular aquatic exercise on the function of neutrophils from women with fibromyalgia: Role of IL-8 and noradrenaline. Brain Behav. Immunol. 2014;39:107–112. doi: 10.1016/j.bbi.2013.11.009[↩]
- Kadetoff D., Lampa J., Westman M., Andersson M., Kosek E. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J. Neuroimmunol. 2012;242:33–38. doi: 10.1016/j.jneuroim.2011.10.013[↩]
- Coskun Benlidayi I. Role of inflammation in the pathogenesis and treatment of fibromyalgia. Rheumatol. Int. 2019;39:781–791. doi: 10.1007/s00296-019-04251-6[↩]
- Kosek E., Altawil R., Kadetoff D., Finn A., Westman M., Le Maitre E., Andersson M., Jensen-Urstad M., Lampa J. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain–interleukin-8 in fibromyalgia and interleukin-1 beta in rheumatoid arthritis. J. Neuroimmunol. 2015;280:49–55. doi: 10.1016/j.jneuroim.2015.02.002[↩]
- Bote M.E., Garcia J.J., Hinchado M.D., Ortega E. Inflammatory/stress feedback dysregulation in women with fibromyalgia. Neuroimmunomodulation. 2012;19:343–351. doi: 10.1159/000341664[↩]
- Ranzolin A., Duarte A.L., Bredemeier M., da Costa Neto C.A., Ascoli B.M., Wollenhaupt-Aguiar B., Kapczinski F., Xavier R.M. Evaluation of cytokines, oxidative stress markers and brain-derived neurotrophic factor in patients with fibromyalgia–A controlled cross-sectional study. Cytokine. 2016;84:25–28. doi: 10.1016/j.cyto.2016.05.011[↩]
- Imamura M, Targino RA, Hsing WT, Imamura S, Azevedo RS, Boas LS, Tozetto-Mendoza TR, Alfieri FM, Filippo TR, Battistella LR. Concentration of cytokines in patients with osteoarthritis of the knee and fibromyalgia. Clin Interv Aging. 2014 Jun 12;9:939-44. doi: 10.2147/CIA.S60330[↩]
- Mastrangelo F., Frydas I., Ronconi G., Kritas S.K., Tettamanti L., Caraffa A.C., DOvidio C., Younes A., Gallenga C.E., Conti P. Low-grade chronic inflammation mediated by mast cells in fibromyalgia: Role of IL-37. J. Biol. Regul. Homeost. Agents. 2018;32:195–198.[↩]
- Gregory N.S., Brito R.G., Fusaro M., Sluka K.A. ASIC3 Is Required for Development of Fatigue-Induced Hyperalgesia. Mol. Neurobiol. 2016;53:1020–1030. doi: 10.1007/s12035-014-9055-4[↩]
- Cicuttini F.M., Wluka A.E. Not just loading and age: The dynamics of osteoarthritis, obesity and inflammation. Med. J. Aust. 2016;204:47. doi: 10.5694/mja15.01069[↩]
- Rossi H.L., Luu A.K., DeVilbiss J.L., Recober A. Obesity increases nociceptive activation of the trigeminal system. Eur. J. Pain. 2013;17:649–653. doi: 10.1002/j.1532-2149.2012.00230.x[↩]
- Rossi H.L., Luu A.K., Kothari S.D., Kuburas A., Neubert J.K., Caudle R.M., Recober A. Effects of diet-induced obesity on motivation and pain behavior in an operant assay. Neuroscience. 2013;235:87–95. doi: 10.1016/j.neuroscience.2013.01.019[↩]
- Smart P.A., Waylonis G.W., Hackshaw K.V. Immunologic profile of patients with fibromyalgia. Am. J. Phys. Med. Rehabil. 1997;76:231–234. doi: 10.1097/00002060-199705000-00014[↩][↩]
- Batista E.D., Andretta A., de Miranda R.C., Nehring J., Dos Santos Paiva E., Schieferdecker M.E. Food intake assessment and quality of life in women with fibromyalgia. Rev. Bras. Reumatol. Engl. Ed. 2016;56:105–110. doi: 10.1016/j.rbr.2015.03.012[↩][↩]
- Rokyta R., Holecek V., Pekarkova I., Krejcova J., Racek J., Trefil L., Yamamotova A. Free radicals after painful stimulation are influenced by antioxidants and analgesics. Neuro Endocrinol. Lett. 2003;24:304–309.[↩]
- Mogil J.S. Pain genetics: Past, present and future. Trends Genet. 2012;28:258–266. doi: 10.1016/j.tig.2012.02.004[↩]
- Arnold L.M., Fan J., Russell I.J., Yunus M.B., Khan M.A., Kushner I., Olson J.M., Iyengar S.K. The fibromyalgia family study: A genome-wide linkage scan study. Arthritis Rheum. 2013;65:1122–1128. doi: 10.1002/art.37842[↩]
- Mergener M., Becker R.M., dos Santos A.F., dos Santos G.A., de Andrade F.M. Influence of the interaction between environmental quality and T102C SNP in the HTR2A gene on fibromyalgia susceptibility. Rev. Bras. Reum. 2011;51:594–602.[↩]
- Mickle AD, Shepherd AJ, Mohapatra DP. Sensory TRP channels: the key transducers of nociception and pain. Prog Mol Biol Transl Sci. 2015;131:73-118. doi: 10.1016/bs.pmbts.2015.01.002[↩]
- Lee Y.H., Choi S.J., Ji J.D., Song G.G. Candidate gene studies of fibromyalgia: A systematic review and meta-analysis. Rheumatol. Int. 2012;32:417–426. doi: 10.1007/s00296-010-1678-9[↩]
- Docampo E., Escaramis G., Gratacos M., Villatoro S., Puig A., Kogevinas M., Collado A., Carbonell J., Rivera J., Vidal J., et al. Genome-wide analysis of single nucleotide polymorphisms and copy number variants in fibromyalgia suggest a role for the central nervous system. Pain. 2014;155:1102–1109. doi: 10.1016/j.pain.2014.02.016[↩]
- Wood P.B., Schweinhardt P., Jaeger E., Dagher A., Hakyemez H., Rabiner E.A., Bushnell M.C., Chizh B.A. Fibromyalgia patients show an abnormal dopamine response to pain. Eur. J. Neurosci. 2007;25:3576–3582. doi: 10.1111/j.1460-9568.2007.05623.x[↩]
- Gold S.J., Ni Y.G., Dohlman H.G., Nestler E.J. Regulators of G-protein signaling (RGS) proteins: Region-specific expression of nine subtypes in rat brain. J. Neurosci. 1997;17:8024–8037. doi: 10.1523/JNEUROSCI.17-20-08024.1997[↩]
- Lu A.T., Ogdie M.N., Jarvelin M.R., Moilanen I.K., Loo S.K., McCracken J.T., McGough J.J., Yang M.H., Peltonen L., Nelson S.F., et al. Association of the cannabinoid receptor gene (CNR1) with ADHD and post-traumatic stress disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147B:1488–1494. doi: 10.1002/ajmg.b.30693[↩]
- Park J.M., Choi M.G., Cho Y.K., Lee I.S., Kim S.W., Choi K.Y., Chung I.S. Cannabinoid receptor 1 gene polymorphism and irritable bowel syndrome in the Korean population: A hypothesis-generating study. J. Clin. Gastroenterol. 2011;45:45–49. doi: 10.1097/MCG.0b013e3181dd1573[↩]
- Bleakman D., Alt A., Nisenbaum E.S. Glutamate receptors and pain. Semin. Cell Dev. Biol. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008[↩]
- Crofford L.J., Pillemer S.R., Kalogeras K.T., Cash J.M., Michelson D., Kling M.A., Sternberg E.M., Gold P.W., Chrousos G.P., Wilder R.L. Hypothalamic-pituitary-adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheum. 1994;37:1583–1592. doi: 10.1002/art.1780371105[↩]
- McCain G.A., Tilbe K.S. Diurnal hormone variation in fibromyalgia syndrome: A comparison with rheumatoid arthritis. J. Rheumatol. Suppl. 1989;19:154–157.[↩]
- McLean S.A., Williams D.A., Harris R.E., Kop W.J., Groner K.H., Ambrose K., Lyden A.K., Gracely R.H., Crofford L.J., Geisser M.E., et al. Momentary relationship between cortisol secretion and symptoms in patients with fibromyalgia. Arthritis Rheum. 2005;52:3660–3669. doi: 10.1002/art.21372[↩][↩]
- Weissbecker I., Floyd A., Dedert E., Salmon P., Sephton S. Childhood trauma and diurnal cortisol disruption in fibromyalgia syndrome. Psychoneuroendocrinology. 2006;31:312–324. doi: 10.1016/j.psyneuen.2005.08.009[↩]
- Crofford L.J., Young E.A., Engleberg N.C., Korszun A., Brucksch C.B., McClure L.A., Brown M.B., Demitrack M.A. Basal circadian and pulsatile ACTH and cortisol secretion in patients with fibromyalgia and/or chronic fatigue syndrome. Brain Behav. Immun. 2004;18:314–325. doi: 10.1016/j.bbi.2003.12.011[↩]
- Abeles A.M., Pillinger M.H., Solitar B.M., Abeles M. Narrative review: The pathophysiology of fibromyalgia. Ann. Intern. Med. 2007;146:726–734. doi: 10.7326/0003-4819-146-10-200705150-00006[↩]
- Rea K., Dinan T.G., Cryan J.F. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress. 2016;4:23–33. doi: 10.1016/j.ynstr.2016.03.001[↩]
- McLean S.A., Williams D.A., Stein P.K., Harris R.E., Lyden A.K., Whalen G., Park K.M., Liberzon I., Sen A., Gracely R.H., et al. Cerebrospinal fluid corticotropin-releasing factor concentration is associated with pain but not fatigue symptoms in patients with fibromyalgia. Neuropsychopharmacology. 2006;31:2776–2782. doi: 10.1038/sj.npp.1301200[↩]
- Wingenfeld K., Heim C., Schmidt I., Wagner D., Meinlschmidt G., Hellhammer D.H. HPA axis reactivity and lymphocyte glucocorticoid sensitivity in fibromyalgia syndrome and chronic pelvic pain. Psychosom. Med. 2008;70:65–72. doi: 10.1097/PSY.0b013e31815ff3ce[↩]
- Bennett R.M., Cook D.M., Clark S.R., Burckhardt C.S., Campbell S.M. Hypothalamic-pituitary-insulin-like growth factor-I axis dysfunction in patients with fibromyalgia. J. Rheumatol. 1997;24:1384–1389.[↩]
- Paul-Savoie E., Marchand S., Morin M., Bourgault P., Brissette N., Rattanavong V., Cloutier C., Bissonnette A., Potvin S. Is the deficit in pain inhibition in fibromyalgia influenced by sleep impairments? Open Rheumatol. J. 2012;6:296–302. doi: 10.2174/1874312901206010296[↩]
- Okifuji A., Turk D.C. Sex hormones and pain in regularly menstruating women with fibromyalgia syndrome. J. Pain. 2006;7:851–859. doi: 10.1016/j.jpain.2006.04.005[↩]
- Koca T., Kocyigit B., Seyithanoglu M., Berk E. The Importance of G-protein Coupled Estrogen Receptor in Patients with Fibromyalgia. Arch. Rheumatol. 2019;34:419–425. doi: 10.5606/ArchRheumatol.2019.7236[↩]
- Epstein S.A., Kay G., Clauw D., Heaton R., Klein D., Krupp L., Kuck J., Leslie V., Masur D., Wagner M., et al. Psychiatric disorders in patients with fibromyalgia. A multicenter investigation. Psychosomatics. 1999;40:57–63. doi: 10.1016/S0033-3182(99)71272-7[↩]
- Leino P., Magni G. Depressive and distress symptoms as predictors of low back pain, neck-shoulder pain, and other musculoskeletal morbidity: A 10-year follow-up of metal industry employees. Pain. 1993;53:89–94. doi: 10.1016/0304-3959(93)90060-3[↩]
- Giesecke T., Gracely R.H., Williams D.A., Geisser M.E., Petzke F.W., Clauw D.J. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577–1584. doi: 10.1002/art.21008[↩]
- O’Malley P.G., Balden E., Tomkins G., Santoro J., Kroenke K., Jackson J.L. Treatment of fibromyalgia with antidepressants: A meta-analysis. J. Gen. Intern. Med. 2000;15:659–666. doi: 10.1046/j.1525-1497.2000.06279.x[↩]
- Jackson J.L., O’Malley P.G., Tomkins G., Balden E., Santoro J., Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: A meta-analysis. Am. J. Med. 2000;108:65–72. doi: 10.1016/S0002-9343(99)00299-5[↩]
- Tomkins G.E., Jackson J.L., O’Malley P.G., Balden E., Santoro J.E. Treatment of chronic headache with antidepressants: A meta-analysis. Am. J. Med. 2001;111:54–63. doi: 10.1016/S0002-9343(01)00762-8[↩]
- Moret C., Briley M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr. Dis. Treat. 2006;2:537–548. doi: 10.2147/nedt.2006.2.4.537[↩]
- Jennings E.M., Okine B.N., Roche M., Finn D.P. Stress-induced hyperalgesia. Prog. Neurobiol. 2014;121:1–18. doi: 10.1016/j.pneurobio.2014.06.003[↩]
- Michaux G.P.N., Magerl W., Anton F., Treede R.D. Experimental characterization of the effects of acute stresslike doses of hydrocortisone in human neurogenic hyperalgesia models. Pain. 2012;153:420–428. doi: 10.1016/j.pain.2011.10.043[↩]
- Kuehl L.K., Michaux G.P., Richter S., Schachinger H., Anton F. Increased basal mechanical pain sensitivity but decreased perceptual wind-up in a human model of relative hypocortisolism. Pain. 2010;149:539–546. doi: 10.1016/j.pain.2010.03.026[↩]
- Suarez-Roca H., Silva J.A., Arcaya J.L., Quintero L., Maixner W., Pinerua-Shuhaibar L. Role of mu-opioid and NMDA receptors in the development and maintenance of repeated swim stress-induced thermal hyperalgesia. Behav. Brain Res. 2006;167:205–211. doi: 10.1016/j.bbr.2005.09.006[↩]
- Nasu T., Taguchi T., Mizumura K. Persistent deep mechanical hyperalgesia induced by repeated cold stress in rats. Eur. J. Pain. 2010;14:236–244. doi: 10.1016/j.ejpain.2009.05.009[↩]
- Nishiyori M., Uchida H., Nagai J., Araki K., Mukae T., Kishioka S., Ueda H. Permanent relief from intermittent cold stress-induced fibromyalgia-like abnormal pain by repeated intrathecal administration of antidepressants. Mol. Pain. 2011;7:69. doi: 10.1186/1744-8069-7-69[↩]
- Gregory N.S., Gibson-Corley K., Frey-Law L., Sluka K.A. Fatigue-enhanced hyperalgesia in response to muscle insult: Induction and development occur in a sex-dependent manner. Pain. 2013;154:2668–2676. doi: 10.1016/j.pain.2013.07.047[↩]
- Khasar S.G., Dina O.A., Green P.G., Levine J.D. Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J. Pain. 2009;10:1073–1077. doi: 10.1016/j.jpain.2009.04.005[↩]
- Sluka K.A., Danielson J., Rasmussen L., DaSilva L.F. Exercise-induced pain requires NMDA receptor activation in the medullary raphe nuclei. Med. Sci. Sports Exerc. 2012;44:420–427. doi: 10.1249/MSS.0b013e31822f490e[↩][↩]
- Quintero J.E., Dooley D.J., Pomerleau F., Huettl P., Gerhardt G.A. Amperometric measurement of glutamate release modulation by gabapentin and pregabalin in rat neocortical slices: Role of voltage-sensitive Ca2+ alpha2delta-1 subunit. J. Pharm. Exp. 2011;338:240–245. doi: 10.1124/jpet.110.178384[↩]
- Suarez-Roca H., Leal L., Silva J.A., Pinerua-Shuhaibar L., Quintero L. Reduced GABA neurotransmission underlies hyperalgesia induced by repeated forced swimming stress. Behav. Brain Res. 2008;189:159–169. doi: 10.1016/j.bbr.2007.12.022[↩]
- Hata T., Itoh E., Kawabata A. Changes in CNS levels of serotonin and its metabolite in SART-stressed (repeatedly cold-stressed) rats. Jpn. J. Pharm. 1991;56:101–104. doi: 10.1016/S0021-5198(19)39903-2[↩]
- Finan P.H., Goodin B.R., Smith M.T. The association of sleep and pain: An update and a path forward. J. Pain. 2013;14:1539–1552. doi: 10.1016/j.jpain.2013.08.007[↩]
- Mundal I., Grawe R.W., Bjorngaard J.H., Linaker O.M., Fors E.A. Prevalence and long-term predictors of persistent chronic widespread pain in the general population in an 11-year prospective study: The HUNT study. BMC Musculoskelet. Disord. 2014;15:213. doi: 10.1186/1471-2474-15-213[↩]
- Haack M., Sanchez E., Mullington J.M. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–1152. doi: 10.1093/sleep/30.9.1145[↩][↩]
- Irwin M.R., Olmstead R., Carrillo C., Sadeghi N., Fitzgerald J.D., Ranganath V.K., Nicassio P.M. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. 2012;35:537–543. doi: 10.5665/sleep.1742[↩]
- Smith M.T., Edwards R.R., McCann U.D., Haythornthwaite J.A. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494[↩]
- Parrino L., Grassi A., Milioli G. Cyclic alternating pattern in polysomnography: What is it and what does it mean? Curr. Opin. Pulm. Med. 2014;20:533–541. doi: 10.1097/MCP.0000000000000100[↩]
- Rizzi M., Sarzi-Puttini P., Atzeni F., Capsoni F., Andreoli A., Pecis M., Colombo S., Carrabba M., Sergi M. Cyclic alternating pattern: A new marker of sleep alteration in patients with fibromyalgia? J. Rheumatol. 2004;31:1193–1199.[↩]
- Tiede W., Magerl W., Baumgartner U., Durrer B., Ehlert U., Treede R.D. Sleep restriction attenuates amplitudes and attentional modulation of pain-related evoked potentials, but augments pain ratings in healthy volunteers. Pain. 2010;148:36–42. doi: 10.1016/j.pain.2009.08.029[↩]
- Moldofsky H., Scarisbrick P., England R., Smythe H. Musculosketal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom. Med. 1975;37:341–351. doi: 10.1097/00006842-197507000-00008[↩][↩]
- Gottshall J.L., Adams Z.M., Forgacs P.B., Schiff N.D. Daytime Central Thalamic Deep Brain Stimulation Modulates Sleep Dynamics in the Severely Injured Brain: Mechanistic Insights and a Novel Framework for Alpha-Delta Sleep Generation. Front. Neurol. 2019;10:20. doi: 10.3389/fneur.2019.00020[↩]
- Vijayan S., Klerman E.B., Adler G.K., Kopell N.J. Thalamic mechanisms underlying alpha-delta sleep with implications for fibromyalgia. J. Neurophysiol. 2015;114:1923–1930. doi: 10.1152/jn.00280.2015[↩]
- Roizenblatt S., Moldofsky H., Benedito-Silva A.A., Tufik S. Alpha sleep characteristics in fibromyalgia. Arthritis Rheum. 2001;44:222–230. doi: 10.1002/1529-0131(200101)44:1<222::AID-ANR29>3.0.CO;2-K[↩]
- Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101:243–276. doi: 10.1016/S0306-4522(00)00353-5[↩]
- Steriade M., Deschenes M. The thalamus as a neuronal oscillator. Brain Res. 1984;320:1–63. doi: 10.1016/0165-0173(84)90017-1[↩]
- Steriade M., McCormick D.A., Sejnowski T.J. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588[↩]
- Steriade M., Amzica F. Coalescence of sleep rhythms and their chronology in corticothalamic networks. Sleep Res. Online. 1998;1:1–10.[↩]
- Principe J.C., Smith J.R. Sleep spindle characteristics as a function of age. Sleep. 1982;5:73–84.[↩]
- Landis C.A., Lentz M.J., Rothermel J., Buchwald D., Shaver J.L. Decreased sleep spindles and spindle activity in midlife women with fibromyalgia and pain. Sleep. 2004;27:741–750. doi: 10.1093/sleep/27.4.741[↩]
- Kulkarni P.M., Xiao Z., Robinson E.J., Jami A.S., Zhang J., Zhou H., Henin S.E., Liu A.A., Osorio R.S., Wang J., et al. A deep learning approach for real-time detection of sleep spindles. J. Neural Eng. 2019;16:036004. doi: 10.1088/1741-2552/ab0933[↩]
- Caravan B., Hu L., Veyg D., Kulkarni P., Zhang Q., Chen Z.S., Wang J. Sleep spindles as a diagnostic and therapeutic target for chronic pain. Mol. Pain. 2020;16:1744806920902350. doi: 10.1177/1744806920902350[↩]
- Løge-Hagen JS, Sæle A, Juhl C, Bech P, Stenager E, Mellentin AI. Prevalence of depressive disorder among patients with fibromyalgia: Systematic review and meta-analysis. J Affect Disord. 2019;245:1098–1105.[↩]
- Wu Y-L, Huang C-J, Fang S-C, Ko L-H, Tsai P-S. Cognitive Impairment in Fibromyalgia: A Meta-Analysis of Case-Control Studies. Psychosom Med. 2018;80(5):432–438.[↩]
- Giesecke T, Williams DA, Harris RE, et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis & Rheumatism. 2003;48(10):2916–2922.[↩]
- Müller W, Schneider EM, Stratz T. The classification of fibromyalgia syndrome. Rheumatol Int. 2007;27(11):1005–1010.[↩]
- Thieme K, Spies C, Sinha P, Turk DC, Flor H. Predictors of pain behaviors in fibromyalgia syndrome. Arthritis Rheum. 2005;53(3):343–350.[↩]
- Goldenberg DL. Fibromyalgia syndrome a decade later: What have we learned? Arch Intern Med. 1999;159(8):777–785.[↩]
- Kaltsas G, Tsiveriotis K. Fibromyalgia. [Updated 2020 Jan 14]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279092[↩]
- Wolfe F., Clauw D.J., Fitzcharles M.-A., Goldenberg D.L., Häuser W., Katz R.L., Mease P.J., Russell A.S., Russell I.J., Walitt B. 2016 Revisions to the 2010/2011 Fibromyalgia Diagnostic Criteria. Semin. Arthritis Rheum. 2016;46:319–329. doi: 10.1016/j.semarthrit.2016.08.012[↩]
- Wolfe F., Clauw D.J., Fitzcharles M.-A., Goldenberg D.L., Häuser W., Katz R.L., Mease P.J., Russell A.S., Russell I.J., Walitt B. 2016 Revisions to the 2010/2011 Fibromyalgia Diagnostic Criteria. Semin. Arthritis Rheum. 2016;46:319–329. https://doi.org/10.1016/j.semarthrit.2016.08.012[↩][↩][↩][↩][↩][↩][↩][↩]
- Wang S.-M., Han C., Lee S.-J., Patkar A.A., Masand P.S., Pae C.-U. Fibromyalgia Diagnosis: A Review of the Past, Present and Future. Expert Rev. Neurother. 2015;15:667–679. doi: 10.1586/14737175.2015.1046841[↩]
- Galvez-Sánchez CM, Reyes Del Paso GA. Diagnostic Criteria for Fibromyalgia: Critical Review and Future Perspectives. J Clin Med. 2020 Apr 23;9(4):1219. doi: 10.3390/jcm9041219[↩]
- Wolfe F. Editorial: the status of fibromyalgia criteria. Arthritis & Rheumatology (Hoboken, NJ). 2015;67(2):330–333.[↩]
- Jones GT, Atzeni F, Beasley M, Flüß E, Sarzi-Puttini P, Macfarlane GJ. The prevalence of fibromyalgia in the general population: a comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis & Rheumatology (Hoboken, NJ). 2015;67(2):568–575.[↩]
- Staud R, Price DD, Robinson ME. The provisional diagnostic criteria for fibromyalgia: One step forward, two steps back: Comment on the article by Wolfe et al. Arthritis Care & Research. 2010;62(11):1675–1676.[↩]
- Toda K. Preliminary diagnostic criteria for fibromyalgia should be partially revised: Comment on the article by Wolfe et al. Arthritis Care & Research. 2011;63(2):308–309.[↩]
- Wolfe F, Clauw DJ, Fitzcharles M-A, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38(6):1113–1122.[↩]
- Häuser W, Schmutzer G, Brähler E, Glaesmer H. A cluster within the continuum of biopsychosocial distress can be labeled “fibromyalgia syndrome”–evidence from a representative German population survey. J Rheumatol. 2009;36(12):2806–2812.[↩]
- Wolfe F, Brähler E, Hinz A, Häuser W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis Care Res (Hoboken). 2013;65(5):777–785.[↩]
- Egloff N, von Känel R, Müller V, et al. Implications of proposed fibromyalgia criteria across other functional pain syndromes. Scand J Rheumatol. 2015;44(5):416–424.[↩]
- Wolfe F, Egloff N, Häuser W. Widespread Pain and Low Widespread Pain Index Scores among Fibromyalgia-positive Cases Assessed with the 2010/2011 Fibromyalgia Criteria. J Rheumatol. 2016;43(9):1743–1748.[↩]
- Wolfe F, Clauw DJ, Fitzcharles M-A, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–329.[↩]
- Arnold LM, Bennett RM, Crofford LJ, et al. AAPT Diagnostic Criteria for Fibromyalgia. J Pain. 2019;20(6):611–628[↩]
- Wolfe F. Letter to the editor, “Fibromyalgia Criteria. J Pain. 2019;20(6):739–740.[↩]
- Arnold LM, Bennett RM, Crofford LJ, Dean LE, Clauw DJ, Goldenberg DL, Fitzcharles MA, Paiva ES, Staud R, Sarzi-Puttini P, Buskila D, Macfarlane GJ. AAPT Diagnostic Criteria for Fibromyalgia. J Pain. 2019 Jun;20(6):611-628. doi: 10.1016/j.jpain.2018.10.008[↩]
- Macfarlane GJ, Kronisch C, Dean LE, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76(2):318–328.[↩][↩]
- Burckhardt CS, Bjelle A. Education programmes for fibromyalgia patients: description and evaluation. Baillieres Clin Rheumatol. 1994;8(4):935–955.[↩]
- Ehrlich GE. Pain is real; fibromyalgia isn’t. J Rheumatol. 2003;30(8):1666–1667.[↩]
- McDowell CP, Cook DB, Herring MP. The Effects of Exercise Training on Anxiety in Fibromyalgia Patients: A Meta-analysis. Med Sci Sports Exerc. 2017;49(9):1868–1876.[↩]
- Chakrabarty S, Zoorob R. Fibromyalgia. Am Fam Physician. 2007;76(2):247–254.[↩]
- Bidonde J, Busch AJ, Webber SC, et al. Aquatic exercise training for fibromyalgia. Cochrane Database Syst Rev. 2014;(10):CD011336[↩]
- Sarzi-Puttini P, Buskila D, Carrabba M, Doria A, Atzeni F. Treatment strategy in fibromyalgia syndrome: where are we now? Semin Arthritis Rheum. 2008;37(6):353–365.[↩][↩][↩][↩]
- Bernardy K, Klose P, Busch AJ, Choy EHS, Häuser W. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev. 2013;(9):CD009796[↩]
- Lazaridou A, Kim J, Cahalan CM, et al. Effects of Cognitive-Behavioral Therapy (CBT) on Brain Connectivity Supporting Catastrophizing in Fibromyalgia. Clin J Pain. 2017;33(3):215–221.[↩]
- Deare JC, Zheng Z, Xue CCL, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070[↩]
- Langhorst J, Klose P, Dobos GJ, Bernardy K, Häuser W. Efficacy and safety of meditative movement therapies in fibromyalgia syndrome: a systematic review and meta-analysis of randomized controlled trials. Rheumatol Int. 2013;33(1):193–207.[↩]
- Schmidt-Wilcke T, Clauw DJ. Fibromyalgia: from pathophysiology to therapy. Nat Rev Rheumatol. 2011;7(9):518–527.[↩][↩]
- Clauw DJ. Fibromyalgia: update on mechanisms and management. J Clin Rheumatol. 2007;13(2):102-109. doi:10.1097/01. rhu.0b013e318053d9bc[↩][↩]
- Abeles M, Solitar BM, Pillinger MH, Abeles AM. Update on fibromyalgia therapy. Am J Med. 2008;121(7):555–561.[↩]
- Liu Y, Qian C, Yang M. Treatment Patterns Associated with ACR-Recommended Medications in the Management of Fibromyalgia in the United States. J Manag Care Spec Pharm. 2016;22(3):263–271.[↩]
- Living with Fibromyalgia, Drugs Approved to Manage Pain. https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm107802.htm[↩][↩]
- Tofferi JK, Jackson JL, O’Malley PG. Treatment of fibromyalgia with cyclobenzaprine: A meta-analysis. Arthritis Rheum. 2004;51(1):9–13.[↩][↩]
- Uçeyler N, Häuser W, Sommer C. A systematic review on the effectiveness of treatment with antidepressants in fibromyalgia syndrome. Arthritis Rheum. 2008;59(9):1279–1298.[↩]
- Häuser W, Petzke F, Üçeyler N, Sommer C. Comparative efficacy and acceptability of amitriptyline, duloxetine and milnacipran in fibromyalgia syndrome: a systematic review with meta-analysis. Rheumatology (Oxford). 2011;50(3):532–543.[↩]
- Goldenberg D, Mayskiy M, Mossey C, Ruthazer R, Schmid C. A randomized, double-blind crossover trial of fluoxetine and amitriptyline in the treatment of fibromyalgia. Arthritis Rheum. 1996;39(11):1852–1859.[↩]
- Moldofsky H, Harris HW, Archambault WT, Kwong T, Lederman S. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol. 2011;38(12):2653–2663.[↩]
- Branco JC, Zachrisson O, Perrot S, Mainguy Y. A European multicenter randomized double-blind placebo-controlled monotherapy clinical trial of milnacipran in treatment of fibromyalgia. J Rheumatol. 2010;37(4):851–859.[↩]
- Sayar K, Aksu G, Ak I, Tosun M. Venlafaxine treatment of fibromyalgia. Ann Pharmacother. 2003;37(11):1561–1565.[↩]
- Lunn MPT, Hughes RAC, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115[↩]
- Arnold LM, Zhang S, Pangallo BA. Efficacy and safety of duloxetine 30 mg/d in patients with fibromyalgia: a randomized, double-blind, placebo-controlled study. Clin J Pain. 2012;28(9):775–781.[↩]
- Russell IJ, Mease PJ, Smith TR, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: Results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain. 2008;136(3):432–444.[↩]
- Welsch P, Üçeyler N, Klose P, Walitt B, Häuser W. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst Rev. 2018;2:CD010292[↩]
- Häuser W, Petzke F, Sommer C. Comparative efficacy and harms of duloxetine, milnacipran, and pregabalin in fibromyalgia syndrome. J Pain. 2010;11(6):505–521.[↩]
- Häuser W, Bernardy K, Uçeyler N, Sommer C. Treatment of fibromyalgia syndrome with antidepressants: a meta-analysis. JAMA. 2009;301(2):198–209.[↩]
- Üçeyler N, Sommer C, Walitt B, Häuser W. Anticonvulsants for fibromyalgia. Cochrane Database Syst Rev. 2013;10:CD010782[↩]
- Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336–1344.[↩]
- Vaerøy H, Abrahamsen A, Førre O, Kåss E. Treatment of fibromyalgia (fibrositis syndrome): a parallel double blind trial with carisoprodol, paracetamol and caffeine (Somadril comp) versus placebo. Clin Rheumatol. 1989;8(2):245–250.[↩]
- Perrot S, Russell IJ. More ubiquitous effects from non-pharmacologic than from pharmacologic treatments for fibromyalgia syndrome: a meta-analysis examining six core symptoms. Eur J Pain. 2014;18(8):1067–1080.[↩]
- MacLean AJB, Schwartz TL. Tramadol for the treatment of fibromyalgia. Expert Rev Neurother. 2015;15(5):469–475.[↩]
- Bagis S., Tamer L., Sahin G., Bilgin R., Guler H., Ercan B., Erdogan C. Free radicals and antioxidants in primary fibromyalgia: An oxidative stress disorder? Rheumatol. Int. 2005;25:188–190. doi: 10.1007/s00296-003-0427-8[↩]
- Miranda-Díaz A.G., Rodríguez-Lara S.Q. The Role of Oxidants/Antioxidants, Mitochondrial. Dysfunction, and Autophagy in Fibromyalgia. In: Wilke W.S., editor. Discussions of Unusual Topics in Fibromyalgia. Inthech; London, UK: 2018.[↩]
- Quintans-Junior L.J., Brito R.G., Quintans J.S.S., Santos P.L., Camargo Z.T., Barreto P.A., Arrigoni-Blank M.F., Lucca-Junior W., Scotti L., Scotti M.T., et al. Nanoemulsion Thermoreversible Pluronic F127-Based Hydrogel Containing Hyptis pectinata (Lamiaceae) Leaf Essential Oil Produced a Lasting Anti-hyperalgesic Effect in Chronic Noninflammatory Widespread Pain in Mice. Mol. Neurobiol. 2018;55:1665–1675. doi: 10.1007/s12035-017-0438-1[↩]
- Lister R.E. An open, pilot study to evaluate the potential benefits of coenzyme Q10 combined with Ginkgo biloba extract in fibromyalgia syndrome. J. Int. Med. Res. 2002;30:195–199. doi: 10.1177/147323000203000213[↩]
- Oliveira M.G., Brito R.G., Santos P.L., Araujo-Filho H.G., Quintans J.S., Menezes P.P., Serafini M.R., Carvalho Y.M., Silva J.C., Almeida J.R., et al. Alpha-Terpineol, a monoterpene alcohol, complexed with beta-cyclodextrin exerts antihyperalgesic effect in animal model for fibromyalgia aided with docking study. Chem. Biol. Interact. 2016;254:54–62. doi: 10.1016/j.cbi.2016.05.029[↩]
- Quintans-Junior L.J., Araujo A.A., Brito R.G., Santos P.L., Quintans J.S., Menezes P.P., Serafini M.R., Silva G.F., Carvalho F.M., Brogden N.K., et al. Beta-caryophyllene, a dietary cannabinoid, complexed with beta-cyclodextrin produced anti-hyperalgesic effect involving the inhibition of Fos expression in superficial dorsal horn. Life Sci. 2016;149:34–41. doi: 10.1016/j.lfs.2016.02.049[↩]
- Casanueva B., Rodero B., Quintial C., Llorca J., Gonzalez-Gay M.A. Short-term efficacy of topical capsaicin therapy in severely affected fibromyalgia patients. Rheumatol. Int. 2013;33:2665–2670. doi: 10.1007/s00296-012-2490-5[↩]
- Fusco R., Siracusa R., D’Amico R., Peritore A.F., Cordaro M., Gugliandolo E., Crupi R., Impellizzeri D., Cuzzocrea S., Di Paola R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants. 2019;8:628. doi: 10.3390/antiox8120628[↩]
- de Zanette S.A., Vercelino R., Laste G., Rozisky J.R., Schwertner A., Machado C.B., Xavier F., de Souza I.C., Deitos A., Torres I.L., et al. Melatonin analgesia is associated with improvement of the descending endogenous pain-modulating system in fibromyalgia: A phase II, randomized, double-dummy, controlled trial. BMC Pharm. Toxicol. 2014;15:40. doi: 10.1186/2050-6511-15-40[↩]
- Hussain S.A., Al K., II, Jasim N.A., Gorial F.I. Adjuvant use of melatonin for treatment of fibromyalgia. J. Pineal Res. 2011;50:267–271. doi: 10.1111/j.1600-079X.2010.00836.x[↩]
- Cordero M.D., Cotan D., del-Pozo-Martin Y., Carrion A.M., de Miguel M., Bullon P., Sanchez-Alcazar J.A. Oral coenzyme Q10 supplementation improves clinical symptoms and recovers pathologic alterations in blood mononuclear cells in a fibromyalgia patient. Nutrition. 2012;28:1200–1203. doi: 10.1016/j.nut.2012.03.018[↩]
- Cordero M.D., Cano-Garcia F.J., Alcocer-Gomez E., De Miguel M., Sanchez-Alcazar J.A. Oxidative stress correlates with headache symptoms in fibromyalgia: Coenzyme Q(1)(0) effect on clinical improvement. PLoS ONE. 2012;7:e35677. doi: 10.1371/journal.pone.0035677[↩]
- Sawaddiruk P., Apaijai N., Paiboonworachat S., Kaewchur T., Kasitanon N., Jaiwongkam T., Kerdphoo S., Chattipakorn N., Chattipakorn S.C. Coenzyme Q10 supplementation alleviates pain in pregabalin-treated fibromyalgia patients via reducing brain activity and mitochondrial dysfunction. Free Radic. Res. 2019;53:901–909. doi: 10.1080/10715762.2019.1645955[↩]
- Joustra M.L., Minovic I., Janssens K.A.M., Bakker S.J.L., Rosmalen J.G.M. Vitamin and mineral status in chronic fatigue syndrome and fibromyalgia syndrome: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0176631. doi: 10.1371/journal.pone.017663[↩]
- Ellis S.D., Kelly S.T., Shurlock J.H., Hepburn A.L.N. The role of vitamin D testing and replacement in fibromyalgia: A systematic literature review. BMC Rheumatol. 2018;2:28. doi: 10.1186/s41927-018-0035-6[↩]
- Gugliandolo E., Peritore A.F., Piras C., Cuzzocrea S., Crupi R. Palmitoylethanolamide and Related ALIAmides: Prohomeostatic Lipid Compounds for Animal Health and Wellbeing. Vet. Sci. 2020;7:78. doi: 10.3390/vetsci7020078[↩]
- Peritore A.F., Siracusa R., Crupi R., Cuzzocrea S. Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets. Nutrients. 2019;11:2175. doi: 10.3390/nu11092175[↩]
- D’Amico R., Impellizzeri D., Cuzzocrea S., Di Paola R. ALIAmides Update: Palmitoylethanolamide and Its Formulations on Management of Peripheral Neuropathic Pain. Int. J. Mol. Sci. 2020;21:5330. doi: 10.3390/ijms21155330[↩]
- Impellizzeri D., Bruschetta G., Cordaro M., Crupi R., Siracusa R., Esposito E., Cuzzocrea S. Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. J. Neuroinflam. 2014;11:136. doi: 10.1186/s12974-014-0136-0[↩]
- Schweiger V., Martini A., Bellamoli P., Donadello K., Schievano C., Balzo G.D., Sarzi-Puttini P., Parolini M., Polati E. Ultramicronized Palmitoylethanolamide (um-PEA) as Add-on Treatment in Fibromyalgia Syndrome (FMS): Retrospective Observational Study on 407 Patients. CNS Neurol. Disord. Drug Targets. 2019;18:326–333. doi: 10.2174/1871527318666190227205359[↩]
- Passavanti M.B., Fiore M., Sansone P., Aurilio C., Pota V., Barbarisi M., Fierro D., Pace M.C. The beneficial use of ultramicronized palmitoylethanolamide as add-on therapy to Tapentadol in the treatment of low back pain: A pilot study comparing prospective and retrospective observational arms. BMC Anesth. 2017;17:171. doi: 10.1186/s12871-017-0461-9[↩]
- Siracusa R., Fusco R., Cordaro M., Peritore A.F., D’Amico R., Gugliandolo E., Crupi R., Genovese T., Evangelista M., Di Paola R., et al. The Protective Effects of Pre- and Post-Administration of Micronized Palmitoylethanolamide Formulation on Postoperative Pain in Rats. Int. J. Mol. Sci. 2020;21:7700. doi: 10.3390/ijms21207700[↩]