Contents
- What is glyphosate
- Glyphosate mechanism of action
- Glyphosate uses
- Glyphosate health effects
- Is glyphosate safe?
- Table 1. Humans exposed to glyphosate Cancer Outcomes for Solid Tumors
- Table 2. Humans exposed to glyphosate Noncancer Outcomes
- Table 3. Toxicological profile of glyphosate metabolites found as residues in livestock and/or crops
- Animal Health Effects
- Glyphosate and cancer
- Glyphosate and death
- Glyphosate and respiratory effects
- Glyphosate and cardiovascular effects
- Glyphosate and gastrointestinal effects
- Glyphosate and hematological effects
- Glyphosate and liver effects
- Glyphosate and kidney effects
- Glyphosate and rheumatoid arthritis
- Glyphosate and skin effects
- Glyphosate and eye effects
- Glyphosate and endocrine effects
- Glyphosate and reproductive effects
- Glyphosate and neurological effects
- Glyphosate and developmental effects
What is glyphosate
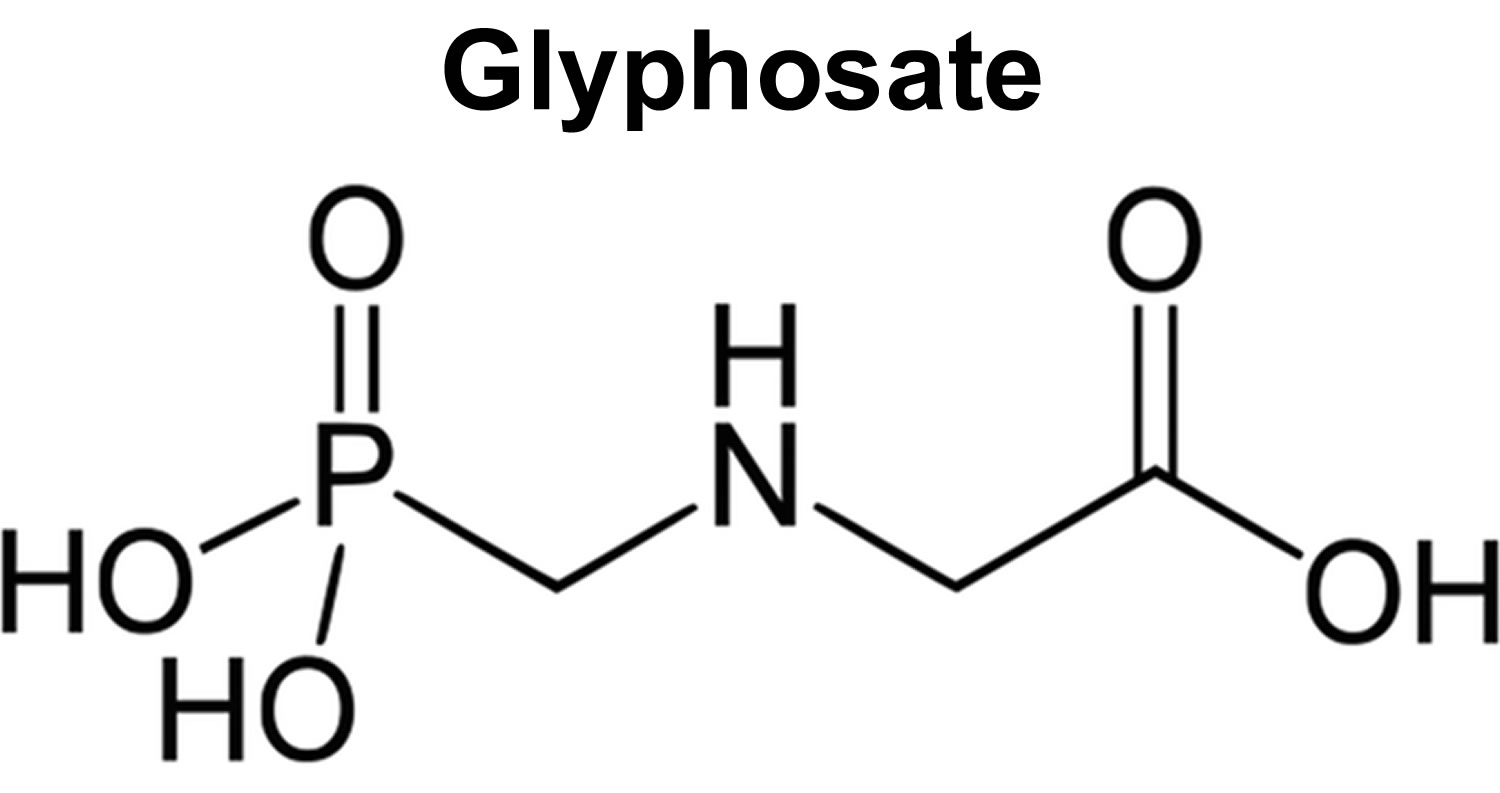
Glyphosate also known as N-phosphonomethyl glycine is a widely used non-selective herbicide that kills weeds, grasses and most plants by blocking a plant enzyme called 5-enolpyruvyl shikimate-3 phosphate (EPSP) synthase disturbing the shikimate pathway that’s essential for plant growth 1, 2, 3, 4, 5, 6. The 5-enolpyruvyl shikimate-3 phosphate (EPSP) synthase enzyme is not found in humans or animals 7. Glyphosate molecule was first synthesized by Henri Martin from Cilag, a small Swiss pharmaceutical company, but was only tested as a herbicide in 1970 by John E. Franz of Monsanto Co. 8. In 1974, after being patented for herbicide use, glyphosate (as an isopropylamine salt of glyphosate) reached the market as a non-selective herbicide 9 and has been used for total control of vegetation since it is a broad-spectrum, non-selective systemic herbicide 10.
Glyphosate comes in many forms, including an acid and several salts. These can be either solids or an amber-colored liquid. There are over 750 products containing glyphosate for sale in the United States. Glyphosate is often used in agriculture, forestry, lawn and garden care to control broad-leafed plants, weeds and grasses that compete with crops 11, 12. Glyphosate can be applied to plants by spraying using different spray equipment, depending on the situation. People can be exposed to glyphosate by getting it on their skin, in their eyes, or breathing it in while using it. People can also swallow it if they eat or smoke after applying it without washing their hands. In humans, glyphosate does not easily pass through the skin. In addition, certain trace amounts of glyphosate chemical residues may remain in or on some crops after they’re harvested. Glyphosate that is absorbed or ingested will pass through the body relatively quickly. The vast majority of glyphosate leaves the body in urine and feces without being changed into another chemical.
When glyphosate is applied to a plant it spreads to all parts of the plant. Glyphosate stops the plant’s cells from making some of the amino acids needed for plant growth. Humans and animals make these amino acids using a different process than plants.
Glyphosate has been detected in the air during spraying, in water, and in food. The general population is exposed primarily through residence near sprayed areas, home use, and diet, and the level that has been observed is generally low.
Once in soil, glyphosate may be adsorbed onto soil particles, degraded by microbes, or transferred to deeper soil horizons, migrating via soil pores or root canals. However, some agricultural practices, such as phosphorous amendment, may re-solubilize glyphosate in soils, making it available for leaching 13 and to the rhizosphere (the zone of soil surrounding a plant root where the biology and chemistry of the soil are influenced by the root) of non-target plants.
Recently, a number of studies have focused on the environmental presence of the major glyphosate metabolite aminomethylphosphonic acid (AMPA). Glyphosate and its metabolite, aminomethylphosphonic acid (AMPA), have been detected in water 14. The half-life of glyphosate in water can range from days to months, complicating efforts to fully understand its environmental persistence 15. Glyphosate is quickly degraded to aminomethylphosphonic acid (AMPA) in soils by microorganisms 8, 16. A similar mechanism of glyphosate degradation has been proposed in plants 17; therefore the co-occurrence of glyphosate and AMPA is expected in plant tissues due to glyphosate degradation and/or AMPA uptake from environmental matrices. Upon penetrating the plant tissues, glyphosate will reach active metabolic sites, such as root and shoot meristems, after being translocated through vascular tissues 18, following the same pathway as photoassimilates 19. Similarly, aminomethylphosphonic acid (AMPA) can also be translocated to diverse plant tissues 17. Therefore, plant organs such as nodules, root tips, and shoot apices, which show high rates of metabolism and growth, represent important sinks for glyphosate and aminomethylphosphonic acid (AMPA) 20, 21.
Figure 1. Glyphosate
[Source 22 ]What happens to glyphosate in the environment?
Glyphosate binds tightly to soil 11. It can persist in soil for up to 6 months depending on the climate and the type of soil it is in 11. Glyphosate is broken down by bacteria in the soil.
Glyphosate is not likely to get into groundwater because it binds tightly to soil. In one study, half the glyphosate in dead leaves broke down in 8 or 9 days. Another study found that some glyphosate was taken up by carrots and lettuce after the soil was treated with it.
Pure glyphosate is low in toxicity to fish and wildlife, but some products containing glyphosate may be toxic because of the other ingredients in them. Glyphosate may affect fish and wildlife indirectly because killing the plants alters the animals’ habitat.
Glyphosate mechanism of action
The herbicidal effects of glyphosate are due to the inhibition of the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), an enzyme from the shikimate pathway, which leads to prevention of the biosynthesis of the amino acids phenylalanine, tyrosine, and tryptophan 23. The shikimic acid pathway is necessary for plants and some microorganisms.
Glyphosate is usually applied to plants by spraying. Glyphosate is then absorbed through plant leaves. It is then carried by the sap stream into the plant roots, where it prevents them from absorbing nutrients from the soil – thereby killing the plant.
Glyphosate also has several secondary or indirect effects on plant physiology which may also explain its herbicidal effects 24. The toxicity of glyphosate could be related to its effects on other physiological processes such as mineral nutrition and photosynthesis, and to the plant’s hormone and oxidative status 24. The alteration of these cellular processes could be directly linked to the deleterious effects of glyphosate observed on plant growth and production. As a metal chelator, glyphosate could deprive plants from important nutrients which have important roles as enzymatic co-factors, biomolecular constituents, and anti-oxidative systems 24. Oxidative stress, more specifically lipid peroxidation induced by glyphosate, is known to severely damage the cell integrity which may lead to cell death 24. Moreover, increased reactive oxygen species (ROS) production can negatively interfere with photosynthetic processes, for example by decreasing the chlorophyll content, photochemical efficiency, and C metabolism, leading to reduction in plant growth 24.
Annual weeds, including grasses and most broad-leafed plants, are easily controlled using glyphosate. This is because they have soft tissue and when growing actively they quickly absorb
enough chemical to destroy the plant. However, weeds with bulbs and perennial weeds with woody stems are much harder to control. They will only die if sufficient glyphosate reaches each plant’s root system. In all cases, but particularly with bulbs and woody weeds, timing of the spray application is critical.
To get the best results from spraying with glyphosate make sure that 25:
- plants are actively growing
- there is plenty of fresh, green growth to absorb enough of the glyphosate spray mix to kill the plant – spray only when there is enough foliage to ensure adequate glyphosate intake
- leaves are:
- free of dust or dirt
- not covered with heavy dew or frost
- plants are not under stress due to:
- dry conditions
- waterlogging
- high temperatures (do not spray if the temperature is over 77 °F [25 °C])
- low temperatures (do not spray until the day temperature reaches 53.6 °F [12°C])
- water used for mixing is clean and free of dirt
- spray equipment has the right nozzle and operates at the right pressure to ensure good coverage
- no rain is expected for at least six hours.
Do not spray when there is a breeze that may cause spray droplets to drift onto other plants, including trees, or onto hard surface areas (paving, pathways and driveways) where it may be washed
into the gutters and from there into local drains, creeks and rivers.
If the breeze is strong enough to shake the foliage of trees or bushes, it is probably too windy to spray safely.
Allow enough time after spraying with glyphosate for the chemical to be absorbed and do its work.
- Do not pull, dig or mow weeds for a week after spraying.
- Do not respray because you fail to see an obvious effect within just a few days.
- With perennial weeds the visible effects of spraying (gradual wilting and yellowing) may not be evident for three weeks or more.
Glyphosate uses
Glyphosate is often used in agriculture, forestry, lawn and garden care to control broadleaf plants, weeds and grasses that compete with crops 11, 26, 27, 28. In agriculture, glyphosate is used in no-till cropping to kill weeds before planting the next crop. Glyphosate is also used to control invasive weeds to help with conservation of natural habitats. Glyphosate can be applied to plants by spraying using different spray equipment, depending on the situation.
Glyphosate products are supplied as concentrates for professional users and ready to use sprays for home garden users. Always put 80 per cent of the required water into your clean sprayer before adding glyphosate, then slowly add the other 20 per cent of the water. This avoids frothing of the spray mix.
According to data from the Pesticide Action Network (PAN) Pesticide Database, there are 102 products containing glyphosate (CASRN 1071-83-6) as the active ingredient, 94 of which have active registrations in the United States. There are 848 products containing glyphosate isopropylamine salt (CASRN 38641-94-0) as the active ingredient, of which 739 have active registrations in the United States 29, 30.
Dealing with Problem Weeds
The following guidelines are for particular problem weeds will help ensure that glyphosate works well for you without over using or respraying unnecessarily.
Soursobs (Bermuda buttercup or Oxalis corniculata)
For soursobs (Bermuda buttercup or Oxalis corniculata) the best results will be achieved by spraying glyphosate when the plants are about one third in flower. At this growth stage glyphosate will be effectively carried down to the bulbs of the plants.
Dealing with Perennial Plants
To kill perennial plants such as couch grass, glyphosate needs to be applied to fresh, actively growing green vegetation with sufficient leaf area to absorb enough glyphosate to kill the whole plant. Slashing or mowing and allowing ample regrowth (good lush green growth 10 to 15 cm long in the case of kikuyu or couch grass) before spraying can improve the chances of maximum control.
Glyphosate health effects
Ingesting glyphosate can cause mucous membrane irritation, abdominal pain, vomiting, diarrhea, and other effects. In fatal cases, it can cause hypovolemic shock, cardiac arrhythmias, metabolic acidosis, and pulmonary edema.
Is glyphosate safe?
Pure glyphosate is low in toxicity by the oral, dermal and inhalation exposure routes, but products usually contain other ingredients that help the glyphosate get into the plants 31. The other ingredients in the product can make glyphosate product more toxic. Products containing glyphosate may cause eye or skin irritation 32. People who breathed in spray mist from products containing glyphosate felt irritation in their nose and throat. Swallowing products with glyphosate can cause increased saliva, burns in the mouth and throat, nausea, vomiting, and diarrhea. Fatalities have been reported in cases of intentional ingestion. Pets may be at risk if they touch or eat plants that are still wet with spray from products containing glyphosate. Animals exposed to products with glyphosate may drool, vomit, have diarrhea, lose their appetite, ataxia, hunched posture, piloerection, convulsions, reduced activity, or seem sleepy. Clinical signs of glyphosate toxicity were observed in rats and mice following acute oral exposure to > 2,000 mg/kg body weight 33. Long-term feeding studies in animals were assessed by the U.S. Environmental Protection Agency (EPA) and other regulatory authorities. Based on these evaluations, they found there is no evidence glyphosate is toxic to the nervous or immune systems. They also found it is not a developmental or reproductive toxin 33.
in 2015, a committee of scientists working for the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) classified glyphosate as “probably carcinogenic to humans” (Group 2A) 34, 35. According to the International Agency for Research on Cancer (IARC) there was “limited evidence of carcinogenicity in humans for non-Hodgkin lymphoma” 34. The evidence in humans is from studies of exposures, mostly agricultural, in the USA, Canada, and Sweden published since 2001 36, 37, 38, 39. In addition, there is convincing evidence that glyphosate also can cause cancer in laboratory animals as well as strong evidence that exposure to glyphosate is genotoxic and can induce oxidative stress in experimental animals and in humans in vitro 34. “Limited evidence” means that a positive association has been observed between exposure to glyphosate and cancer but that other explanations for the observations called chance, bias, or confounding could not be ruled out. Group 2A category is also used when there is limited evidence of carcinogenicity in humans and strong data on how the agent causes cancer 34.
Glyphosate also caused DNA and chromosomal damage in human cells, although it gave negative results in tests using bacteria. One study in community residents reported increases in blood markers of chromosomal damage (micronuclei) after glyphosate formulations were sprayed nearby 34.
In 2005, an evaluation of glyphosate and cancer risk was conducted in the Agricultural Health Study 40. This evaluation considered glyphosate use reported at enrollment (1993–1997), and included 2088 cancers diagnosed between enrollment and 2001. No statistically significant associations were found for any cancer sites, including non-Hodgkin lymphoma, but there was an increased risk, though not statistically significant, of multiple myeloma in the highest exposure category based on a small number of cases 40.
In this large 2017 prospective cohort study 41, no association was apparent between glyphosate and any solid tumors or lymphoid malignancies overall, including non-Hodgkin lymphoma and its subtypes. There was some evidence of increased risk of acute myeloid leukemia (AML) among the highest glyphosate exposed group that requires confirmation 41.
Following the 2023 assessment by the European Food Safety Authority (EFSA), “based on the available evidence, glyphosate does not meet the criteria for endocrine disruption” 33.
Table 1. Humans exposed to glyphosate Cancer Outcomes for Solid Tumors
| Reference and study overview | Methods and outcomes | Results (with 95% confidence interval) | Study author conclusions and limitations |
|---|---|---|---|
| Andreotti et al 41 Prospective cohort study of 54,251 licensed pesticide applicators (97% white, 97% male) recruited between 1993 and 1997 in Iowa and North Carolina from the Agricultural Health Study to evaluate agricultural exposure to 50 pesticides (including glyphosate) and cancer incidence cases. 44,932 participants reported ever use of glyphosate, including 5,779 participants with incident cancer cases. | Exposure: Self-reported ever/never use of any glyphosate pesticides, lifetime days of glyphosate use (days per year × number of years), and intensity-weighted lifetime days (lifetime days × intensity score) at enrollment (1993–1997) or follow-up (1999–2005). Intensity-weighted lifetime days of glyphosate use was categorized into quartiles, tertiles, or the median, such that there were at least five exposed cases in each category. Outcome: Incident cancer diagnoses ascertained via linkage to cancer registries in Iowa (enrollment through 2013) and North Carolina (enrollment through 2012). Data analysis: Poisson regression Adjustments: Age, cigarette smoking status, alcohol drinks per month, family history of any cancer, state of recruitment, and the five pesticides (atrazine, alachlor, metolachlor, trifluralin, and 2,4- dichlorophenoxyacetic acid (2,4-D)). Confounders considered included body mass index (body mass index) and pack-years of cigarettes smoked. | Oral cavity: Q4: relative risk 0.84 (0.48–1.46) Colon: Q4: relative risk 1.01 (0.74–1.38) Rectum: Q4: relative risk 0.84 (0.52–1.34) Pancreas: Q4: relative risk 1.06 (0.57–1.97) Lung: Q4: relative risk 1.00 (0.76–1.33) Melanoma: Q4: relative risk 1.17 (0.78–1.74) Prostate: Q4: relative risk 0.99 (0.86–1.13) Testicular: T3: relative risk 0.57 (0.20–1.67) Bladder: Q4: relative risk 1.26 (0.87–1.82) Kidney: Q4: relative risk 1.03 (0.66–1.61) | Conclusions: The authors observed no associations between glyphosate use and overall cancer risk or risk of cancer of the oral cavity, colon, rectum, pancreas, lung, skin, prostate, testes, bladder or kidney. Risk estimates were similar in magnitude between the unlagged and lagged (5 or 20 years) exposure analyses for all sites evaluated. Limitations: Some misclassification of exposure undoubtedly occurred; because many cancer sites were evaluated, there is the possibility that results were observed by chance, and should be interpreted with caution. However, 37% of the participants did not respond to follow-up, which may have resulted in an underestimation of glyphosate exposure though imputation procedures were used in an attempt to mitigate this issue. |
| De Roos et al. 40 Prospective cohort study of 54,315 certified pesticide applicators (97% male, 97% Caucasian) in Iowa and North Carolina (Agricultural Health Study) to evaluate agricultural exposure to glyphosate and cancer incidence. Number cases (exposed percent) for different cancer sites:
| Exposure: Self-reported never/ever use of glyphosate. Cumulative exposure days (cumulative exposure days): 1–20 (reference), 21–56, and 57–2,678 days. Intensity weighted exposure days (intensity weighted exposure days) of 0.1–79.5 (reference), 79.6–337.1, and 337.2–18,241 units. Outcomes/endpoints: Cancer registry files in Iowa and North Carolina for case identification. Incident cases were identified from enrollment to 2001 (median follow-up time: 6.7 years). Data analysis: Poisson regression analyses for all cancers combined and 12 specific cancer sites (with at least 30 cases). Adjustments: Age at enrollment, education, pack-years of cigarette smoking, alcohol consumption, family history of cancer, state of residency, and co-exposure to 10 other pesticides (2,4- dichlorophenoxyacetic acid (2,4-D), alachlor, atrazine, metolachlor, trifluralin, benomyl, maneb, paraquat, carbaryl, and diazinon). | All cancers: Ever used: relative risk 1.0 (0.9–1.2) Lung: Ever used: relative risk 0.9 (0.6–1.3) Oral cavity: Ever used: relative risk 1.0 (0.5–1.8) Colon: Ever used: relative risk 1.4 (0.8–2.2) Rectum: Ever used: relative risk 1.3 (0.7–2.3) Pancreas: Ever used: relative risk 0.7 (0.3–2.0) Kidney: Ever used: relative risk 1.6 (0.7–3.8) Bladder: Ever used: relative risk 1.5 (0.7–3.2) Prostate: Ever used: relative risk 1.1 (0.9–1.3) Melanoma: Ever used: relative risk 1.6 (0.8–3.0) | Conclusions: No association between glyphosate exposure and all cancer incidence or most of the specific cancer subtypes, including non-Hodgkin’s lymphoma. A small number of cases suggested a positive association between multiple myeloma and glyphosate exposure. Limitations: Self-reported exposure information, few cases for many of the cancer subtypes, most applicators were male, there is no information on timing of pesticide use in relation to disease. |
| Engel et al. 42 Prospective cohort study of 30,454 wives (98% Caucasian) of private pesticide applicators (largely farmers) in Iowa and North Carolina (Agricultural Health Study) to evaluate breast cancer risk in relation to use of individual pesticides by the women themselves or by their husbands. Glyphosate analysis for wife’s pesticide use among all wives in the cohort included 82 exposed and 227 unexposed cases (n= 309) and 10,016 exposed and 20,129 (n= 30,145) unexposed controls. Further analysis of husband’s pesticide use among wives who reported never having used pesticides themselves included 109 “exposed” (husband used pesticide) and 43 “unexposed” cases and 9,304 “exposed” and 3,993 “unexposed” controls. | Exposure: Self-reported ever/never use of any glyphosate products at enrollment (1993–1997). Husband’s information was used as a measure of possible indirect pesticide exposure for their wives. Outcomes/endpoints: Breast cancer incident cases identified through state cancer registries from enrollment to 2000 (mean follow-up period: 4.8 years). Data analysis: Poisson regression Adjustments: Age, race, and state of residence. Confounders considered included body mass index, age at menarche, parity, age at first birth, menopausal status, age at menopause, family history of breast cancer, physical activity, smoking, alcohol consumption, fruit and vegetable consumption, and education. | Breast cancer: Wife’s pesticide use among all wives in cohort: relative risk 0.9 (0.7–1.1) Husband’s pesticide use among wives who never used pesticides: relative risk 1.3 (0.8–1.9) | Conclusions: No specific conclusion was given on glyphosate exposure and breast cancer. Limitations: Some associations may have occurred by chance, data on pesticide-specific exposure-response relations were only available for the husband, lack of information on how long each woman had been married to her current partner, limited power to assess associations for less commonly used pesticides, pesticide use was based on self-reporting. |
| Flower et al 43 Prospective and retrospective cohort study of 17,280 children (52% male, 96% Caucasian) of pesticide applicators in Iowa (Agricultural Health Study) to evaluate parental exposure to 50 pesticides (including glyphosate) and childhood cancer risk. Glyphosate analysis included 6,075 children (13 cases) with maternal use and 3,231 children (6 cases) with paternal use of glyphosate. | Exposure: Self-reported parental ever/never use of any glyphosate product by both applicators and spouses at enrollment (1993–1997). Outcomes/endpoints: Childhood cancer cases were both retrospectively and prospectively identified after parental enrollment through Iowa Cancer registries from 1975 to 1998. Data analysis: Multiple logistic regression. Adjustments: Child’s age at parent’s enrollment. Confounders considered included parental age at child’s birth, child’s sex, child’s birth weight, history of parental smoking, paternal history of cancer, and maternal history of miscarriage. | Childhood cancers: Maternal use (ever): odds ratio 0.61 (0.32–1.16) | Conclusions: No significant associations were observed between maternal (or paternal) pesticide including glyphosate application, including increased frequency of application, and risk of childhood cancer risk. Limitations: Small number of cases limits statistical power, maternal use is limited by lack of data on timing of exposure in relation to child’s birth, paternal prenatal use constitutes a broad window of exposure and not necessarily just prenatal. |
| Koutros et al. 44 Prospective cohort study of 54,412 certified pesticide applicators in Iowa and North Carolina (Agricultural Health Study) to evaluate agricultural exposure to 50 pesticides (including glyphosate) and prostate cancer risk. There were 1,962 incident prostate cancer cases, 919 of whom had aggressive prostate cancer. Glyphosate analysis included 1,464 exposed and 498 unexposed cases (n=1,962) and 42,420 exposed and 10,015 unexposed controls (n=52,435). | Exposure: Self-reported ever/never glyphosate use, lifetime days of glyphosate use (years of use × days/year used), intensity-weighted lifetime days of glyphosate use (lifetime days × exposure intensity) at enrollment (1993–1997). Exposure was categorized into non-exposed and quartiles exposure on the basis of the distribution of exposed cases. Outcomes/endpoints: Prostate cancer incidences determined through state cancer registries from enrollment to 2007. Data analysis: Poisson regression. Adjustments: Age at enrollment, race, state, family history of prostate cancer, smoking, fruit servings, and leisure-time physical activity in the winter. Separate glyphosate analyses were conducted by disease aggressiveness and family history of prostate cancer (yes, no). | Cumulative lifetime exposure based on intensity-weighted days: Total prostate cancer: Q4: relative risk 0.99 (0.86–1.15) Aggressive prostate cancer: Q4: relative risk 0.94 (0.75–1.18) Total prostate cancer, no family history: Q4: relative risk 1.02 (0.86–1.21) Total prostate cancer, with family history: Q4: relative risk 0.95 (0.64–1.40) | Conclusions: No significant association was found between any specific pesticide including glyphosate and risk of total prostate cancer. Limitations: Information on Gleason score of severity was missing for some and not standardized, which most likely led to an underestimation of advanced cases; use of take-home questionnaire could introduce selection bias and exposure misclassification; large number of pesticides investigated so cannot rule out the possibility that some findings may be due to chance. |
| Koutros et al 45 Prospective cohort study of 54,344 male pesticide applicators in Iowa and North Carolina (Agricultural Health Study) to evaluate agricultural exposure to 65 pesticides (including glyphosate) and bladder cancer risk (n=321 incident cases identified). Glyphosate analysis included 248 exposed and 73 unexposed cases (n=321) and 54,023 controls. | Exposure: Self-reported ever/never glyphosate use, lifetime days of glyphosate use (years of use × days/year used), intensity-weighted lifetime days of glyphosate use (lifetime days × exposure intensity) at enrollment (1993–1997). Outcomes/endpoints: Bladder cancer incidences determined through state-based cancer registries from enrollment through 2010 in North Carolina and 2011 in Iowa. Data analysis: Poisson regression. Adjustments: Age, race, state, cigarette smoking, and pipe smoking. | Bladder cancer: Ever use: relative risk 1.17 (0.78–1.77) Cumulative lifetime exposure based on intensity-weighted days: Overall Stratification by smoking status Never smoker: Former smoker: Current smoker: | Conclusions: No specific conclusion given on glyphosate exposure and bladder cancer. Never smokers who were heavy users of the glyphosate had increased risk of bladder cancer. Limitations: Potential for exposure misclassification, findings may be due to chance, due to small number of cases. |
| Lee et al 46 Prospective cohort study of 56,813 certified pesticide applicators (97% male, 97% Caucasian) in Iowa and North Carolina (Agricultural Health Study) to evaluate agricultural exposure to 50 pesticides (including glyphosate) and colorectal cancer risk. Glyphosate analysis included 225 exposed and 67 unexposed for colorectal cancer cases (n=305), 151 exposed and 49 unexposed for colon cancer cases (n=212), and 74 exposed and 18 unexposed for rectal cancers (n=93). | Exposure: Self-reported ever use of any glyphosate pesticides at enrollment (1993–1997). Outcomes/endpoints: Colorectal cancer incidences determined through cancer registries from enrollment to 2002 (mean follow-up period: 7.3 years). Data analysis: Unconditional multivariate logistic regressions. Adjustments: Age, state of residence, smoking history, total pesticide application days to any pesticide. Confounders considered included body mass index, race, license type, education level, aspirin intake, family history of colorectal cancer, physical activity, smoking, and intakes of meat, fruits, vegetables, and alcohol. | Colorectal cancer: odds ratio 1.2 (0.9–1.6) Colon cancer: odds ratio 1.0 (0.7–1.5) Rectal cancer: odds ratio 1.6 (0.9–2.9) | Conclusions: No specific conclusion was given on glyphosate exposure and colorectal cancers. Limitations: Since the study examined risks for 50 pesticides, it is possible that some significant findings might occur by chance alone due to the multiple comparisons. Potential recall bias and thus exposure misclassification associated with subjects recalling pesticide use from many years ago. |
| Andreotti et al 47 Nested case-control study of 93 cases of pancreatic cancer (64 applicators and 29 spouses) and 82,503 controls (52,721 applicators and 29,782 spouses) from the Agricultural Health Study, conducted in Iowa and North Carolina, to evaluate the association of pancreatic cancer and use of 24 pesticides (including glyphosate). Glyphosate analysis included 55 exposed and 35 unexposed cases (n= 90) and 48,461 exposed and 31,282 unexposed controls (n= 79,743). | Exposure: Self-reported ever/never use of any glyphosate product for applicators and spouses and intensity-weighted lifetime exposure days for applicators at enrollment (1993–1997). Outcomes/endpoints: Pancreatic cancer incidences identified through state cancer registries from enrollment to 2004 (over 9 years of follow-up time). Data analysis: Unconditional logistic regression. Adjustments: Age, cigarette smoking, diabetes, and subject type for ever/never pesticide exposure (applicator versus spouse). | Pancreatic cancer: Ever/never among applicators and spouses: odds ratio 1.1 (0.6–1.7) Intensity weighted pesticide exposure among applicators: Never: 1.0 (reference) | Conclusions: No specific conclusion given on glyphosate exposure and pancreatic cancer. Limitations: There was a limited number of exposed cases and limited in generalizability due to predominantly white male study population. |
| Band et al 48 Case-control study on male cancer patients (96.8% Caucasian) in British Columbia, Canada, to evaluate exposure to 139 specific active compounds in pesticides (including glyphosate) and prostate cancer risk. Glyphosate analysis included 25 exposed and 1,128 unexposed cases (n=1,153) and 60 exposed and 3,939 age-matched internal controls (patients with cancer of other primary site) controls (n=3,999). | Exposure: Self-reported ever/never use of glyphosate pesticides from questionnaire. Agricultural job exposure matrix (job exposure matrix) was developed for farm workers in British Columbia for the period of 1950–1998. Outcomes/endpoints: Prostate cancer cases identified through British Columbia Cancer Registry for 1983–1990 and histologically confirmed. Data analysis: Conditional logistic regression on age-matched sets of cases and controls. Adjustments: Alcohol consumption, cigarette years, education level, p-years, and respondent. Confounders considered included marital status, smoking (age started smoking, average number of cigarettes, pipe or cigars smoked per day, total years smoked), and ethnicity. | Prostate cancer: odds ratio 1.36 (0.83–2.25) | Conclusions: No specific conclusion given on glyphosate exposure and prostate cancer. job exposure matrix likely to result in non-differential misclassification and may underestimate the true association; thus, negative findings should be regarded as inconclusive. Limitations: Lack of information on familial history, potential for misclassification of exposure due to use of job exposure matrix, use of cancer controls may result in selection bias, statistically significant associations could have occurred by chance as a result of multiple comparisons since 142 active chemicals were examined. |
| Lee et al 49 Case control study of white men and women (ages ≥21 years) diagnosed with stomach adenocarcinoma (n=170) or esophagus adenocarcinoma (n=137) and 502 controls in eastern Nebraska to evaluate the risk of the stomach and esophageal adenocarcinomas associated with farming and agricultural use of 16 insecticides and 14 herbicides (including glyphosate). Glyphosate analysis included 12 cases of stomach cancer and 12 cases of esophageal cancer among farmers, and 46 controls compared to non-farmers (59 stomach cancer, 62 esophageal cancer cases and 184 controls). Controls were randomly selected from a group of controls interviewed in 1986–1987 for a previous population-based case-control study. Controls were frequency-matched by sex and age to the combined distribution of the stomach and esophagus cases. | Exposure: Self- or proxy-reported ever use of glyphosate pesticide at enrollment (1992–1994). Outcomes: Stomach and esophageal cancer cases were identified from the Nebraska Cancer Registry (1988–1990) or by review of discharge diagnosis and pathology records at 14 hospitals (1991–1993). Data analysis: Unconditional logistic regression. Adjustments: Age, sex. Confounders considered included body mass index, smoking, alcohol consumption, educational level, family history of stomach or esophageal cancer, respondent type, dietary intake of vitamin A and C, b-cryptoxanthin, riboflavin, folate, zinc, dietary fiber, protein, and carbohydrate. | Stomach cancer: odds ratio 0.8 (0.4–1.5) Esophageal cancer: odds ratio 0.7 (0.3–1.4) | Conclusions: “No significant associations were found between specific agricultural pesticide exposures (including glyphosate) and the risk of stomach or esophageal adenocarcinomas among Nebraska farmers.” Limitations: Possible misclassification of pesticide exposure and generally small number of farmers exposed to some of the individual pesticides. |
| Lee et al 50 Case control study of 251 white men and women (ages ≥21 years) diagnosed with gliomas and 498 controls in eastern Nebraska (Nebraska Health Study II) to evaluate adult glioma associated with farming and agricultural use of 20 insecticides and 17 herbicides (including glyphosate). Glyphosate analysis (only conducted among male farmers) included 17 cases and 32 controls among farmers compared to non-farmers (49 cases and 112 controls). Among these, self-reported respondents included 4 cases/17 controls for glyphosate users and 20 cases/40 controls for reference non-farmers; proxy-reported respondents included 13 cases/15 controls for glyphosate users and 29 cases/72 controls for reference non-farmers. Controls were randomly selected from a group of controls interviewed in 1986–1987 for a previous population-based case-control study. Controls were frequency-matched by sex, age, and vital status to the combined distribution of the cases. | Exposure: Self- or proxy-reported ever use of glyphosate pesticide at enrollment (1992–1994). Outcomes: Incident primary adult glioma cases diagnosed between 1988 and 1993 were identified from the Nebraska Cancer Registry or from 11 hospitals. Data analysis: Unconditional logistic regression. Separate analyses by sex and respondent type (self- versus proxy-reported) were also conducted. Adjustments: Age, sex, and respondent type. Confounders considered included history of head injury, marital status, education level, alcohol consumption, medical history of diabetes mellitus, dietary intake of a- and b-carotene, and dietary fiber. | Glioma among male farmers: odds ratio 1.5 (0.7–3.1), all reported glyphosate use odds ratio 0.4 (0.1–1.6), self-reported glyphosate use odds ratio 3.1 (1.2–8.2), proxy-reported glyphosate use | Conclusions: “Glioma risk was also significantly increased among men who used specific pesticides (including glyphosate) and pesticide chemical classes; however, the positive results were mostly limited to proxy respondents.” Limitations: The major limitation was the large proportion of proxy respondents. Most of the associations observed were limited to proxy respondents. |
| Pahwa et al 51 Case control study of 357 soft tissue sarcoma cases and 1,506 controls in Canada (all males, ≥19 years of age) to investigate the putative associations of pesticides (including glyphosate) with soft-tissue sarcoma (soft tissue sarcoma). Glyphosate analysis included 36 exposed and 321 unexposed cases and 147 exposed and 1,359 unexposed controls. | Exposure: Self-reported ever use of glyphosate herbicides collected through self-administered postal questionnaire and telephone interviews. Outcomes: soft tissue sarcoma cases (first diagnosed in 1991–1994) ascertained from provincial cancer registries, except in Quebec, where hospital ascertainment was used. Data analysis: Conditional logistic regression. Adjustments: Age, province of residence, medical history. | Soft tissue sarcoma: odds ratio 0.93 (0.60–1.42), stratified by age group and province of residence odds ratio 0.90 (0.58–1.40), adjusted for medical history and with strata for age group and province of residence | Conclusions: “No association between herbicides (individual compound or major chemical class) (including glyphosate) and soft tissue sarcoma.” Limitations: Limitations common to epidemiological case-control studies. |
| Yiin et al 52 Case control study of 798 cases of glioma and 1,175 controls (98% white, aged 18–80 years) in Iowa, Michigan, Minnesota, and Wisconsin (Upper Midwest Health Study) to investigate association between exposure to pesticides (including glyphosate) and risk of glioma in male and female participants. House and garden pesticide use: Glyphosate analysis included 51 exposed and 747 unexposed cases and 76 exposed and 1,099 unexposed controls. Analysis included 28 exposed and 410 unexposed cases and 75 exposed and 1,066 unexposed controls excluding proxy respondents. Randomly-selected, population-based controls were frequency-matched within a state. | Exposure: Self- or proxy-reported ever/never use of glyphosate pesticide through 1992. Outcomes: Cases with a histologically confirmed primary intracranial glioma were identified through medical facilities, oncologists, neurosurgeons, and cancer registries (1995–1997). Data analysis: Unconditional logistic regression. Analyses were separately conducted with or without proxy respondents. Adjustments: Age, sex, education. | Glioma Non-farm job use: odds ratio 0.83 (0.39–1.73) including proxy respondents; odds ratio 0.79 (0.33–1.86) excluding proxy respondents. House and garden use: odds ratio 0.98 (0.67–1.43) including proxy respondents; odds ratio 0.84 (0.52–1.33) excluding proxy respondents | Conclusions: “No individual pesticides (including glyphosate) or broader category of pesticides, with or without proxy respondent, was associated with a statistically significant decrease or elevation in glioma risk.” Limitations: A limitation of this study is the high proportion (45%) of proxy interviews for case participants compared to 2.9% control interviews that were with proxies. The accuracy and completeness of information given by proxy respondents varies by many factors. Another concern is the validity and reliability of the pesticide exposure assessment. |
Footnotes: Relative risk (RR) is a ratio of the probability of an event occurring in the exposed group versus the probability of the event occurring in the non-exposed group. A relative risk (RR) of 1 implies there is no difference of the event if the exposure has or has not occurred. If the relative risk (RR) > 1, then the event is more likely to occur if there was exposure. If the relative risk (RR) < 1, then the event is less likely to occur if there was exposure. For example, when the relative risk (RR) is 2 the chance of a bad outcome is twice as likely to occur with the treatment as without it; whereas an relative risk (RR) of 0.5 means that the chance of a bad outcome is twice as likely to occur without the intervention. When the relative risk (RR) is exactly 1, the risk is unchanged.
Abbreviations: BMI = body mass index; CED = cumulative exposure day; CI = confidence interval; IWED = intensity weighted exposure day; JEM = job exposure matrix; NHL = non-Hodgkin’s lymphoma; OR = odds ratio; RR = relative risk; Q = quartile; STS = soft tissue sarcoma; T = tertile
[Source 53 ]Table 2. Humans exposed to glyphosate Noncancer Outcomes
| Reference and study population | Exposure | Outcomes |
|---|---|---|
| Death | ||
| Cho et al 54 Retrospective cohort study of 150 adults who presented with glyphosate poisoning at a Korean hospital between January 2006 and April 2017. | Exposure: ingestion of glyphosate-based herbicides Multivariate logistic regression: age, amount of glyphosate-based herbicide ingested, elapsed time since ingestion, qSOFA score | 14/150 patients died (9.3% mortality), and age was determined to be a statistically significant factor (p<0.001). Higher qSOFA scores were associated with greater odds of death (odds ratio (OR):2.73, 95% confidence interval: 1.41–5.76) and life threatening complications (odds ratio (OR): 17.19, 95% confidence interval: 6.25–72.65). |
| Respiratory | ||
| Camacho and Mejia 55 Cross-sectional study examining individual health records from the general public over a five-year period (2003 to 2007) merged with data of aerial spraying events. The study examined the data under several specifications:
| Exposure: aerial spraying of glyphosate on coca crops and the general population living in the spray areas within study period 2003 to 2007 Regression Adjustments: age, age square, health regime, municipal tax income, population, area in square km, rurality index, average monthly rainfall, municipal spending on education and health, subsidized regime coverage, year and month dummy | Increased number of respiratory illnesses consistent across all specifications analyzed (only statistically significant p values were presented). |
| Hoppin et al 56 Cohort study of 20,468 participants in the Agricultural Health Study in Iowa and North Carolina | Exposure: glyphosate ever use and application frequency categories Logistic regression adjustments: age, state, smoking history, asthma-atopy status | Wheeze, self-reported odds ratio (OR) 1.05 (0.95–1.17), p=0.04 for trend of increasing exposure days |
| Hoppin et al 57 Prospective cohort study of 20,175 participants in the Agricultural Health Study in Iowa and North Carolina (17,920 farmers and 2,255 commercial pesticide applicators) | Exposure: glyphosate ever use in the year prior to enrollment Logistic regression adjustments: age, state, smoking history, body mass index (BMI) | Wheeze, self-reported
|
| Hoppin et al 58 Cohort study of 2,255 commercial pesticide applicators participating in the Agricultural Health Study in Iowa and North Carolina | Exposure: glyphosate ever use in the year prior to enrollment Logistic regression adjustments: age, smoking status, asthma and atopy history, body mass index (BMI) | Wheeze, self-reported odds ratio (OR) 1.38 (1.03–1.86) odds ratio (OR) 1.14 (0.83–1.57), with adjustment for use of chlorimuron-ethyl pesticide |
| Hoppin et al 59 Prospective cohort study of 20,908 participants in the Agricultural Health Study in Iowa and North Carolina | Exposure: glyphosate ever use Logistic regression adjustments: age, state, sex, smoking (pack-years) | Chronic bronchitis odds ratio (OR) 0.99 (0.82–1.19) |
| Hoppin et al 60 Prospective cohort study of 25,814 farm women participating in the Agricultural Health Study in Iowa and North Carolina | Exposure: glyphosate ever use Logistic regression adjustments: age, state, smoking status, “grew up on farm” | Atopic asthma odds ratio (OR) 1.31 (1.02–1.67) Nonatopic asthma odds ratio (OR) 1.13 (0.92–1.39) |
| Hoppin et al 61 Prospective cohort study of 19,704 male farmers participating in the Agricultural Health Study in Iowa and North Carolina | Exposure: glyphosate ever use Logistic regression adjustments: age, state, smoking status, body mass index (BMI) | Allergic asthma odds ratio (OR) 1.37 (0.86–2.17) Nonallergic asthma odds ratio (OR) 1.15 (0.87–1.51) |
| Hoppin et al 62 Prospective cohort study of 22,134 male farmers participating in the Agricultural Health Study in Iowa and North Carolina | Exposure: glyphosate ever use Logistic regression adjustments: age, body mass index (BMI), state, smoking status, current asthma, days applying pesticides, days driving diesel tractors | Allergic wheeze odds ratio (OR) 1.56 (1.19–2.03), higher prevalence with increasing use days per year Nonallergic wheeze odds ratio (OR) 1.24 (1.07–1.44) |
| Slager et al 63 Prospective cohort study of 2,245 commercial applicators participating in the Agricultural Health Study in Iowa | Exposure: any glyphosate use and application frequency categories during the past year Logistic regression adjustments: age, education, “growing up on farm” | Current rhinitis odds ratio (OR) 1.32 (1.08–1.61), p=0.735 for trend for increasing use days per year |
| Slager et al 64 Prospective cohort study of 19,565 farmers participating in the Agricultural Health Study in Iowa and North Carolina | Exposure: any glyphosate use and application frequency categories during the past year Logistic regression adjustments: age; race; education; state; body mass index (BMI); currently working on farm; years mixing pesticides, repairing engines or pesticide equipment, welding, painting, handling stored grain or hay, working in swine areas, working with hogs or other farm animals, butchering animals, and growing cabbage, Christmas trees, field corn, sweet corn, and hay | Current rhinitis odds ratio (OR) 1.09 (1.05–1.13) |
| Cardiovascular Effects | ||
| Dayton et al 65 Case control study of 168 cases of nonfatal myocardial infarction and 22,257 controls in women in Iowa and North Carolina participating in the Agricultural Health Study | Exposure: glyphosate ever use Logistic regression adjustments: age, body mass index (BMI), smoking, state | Nonfatal heart attack odds ratio (OR) 0.8 (0.6–1.2) |
| Mills et al 66 Prospective study of male participants in the Agricultural Health Study in Iowa and North Carolina (n=54,069 for fatal myocardial infarction and 32,024 for nonfatal incidence) | Exposure: glyphosate ever use Cox proportional regression adjustments: age, state, smoking, body mass index (BMI) (nonfatal analysis only) | Fatal heart attack hazard ratio (HR) 0.99 (0.80–1.23) Nonfatal heart attack hazard ratio (HR) 1.10 (0.93–1.31) |
| Musculoskeletal Effects | ||
| De Roos et al 67 Nested case control study of 135 cases of physician-confirmed rheumatoid arthritis and 675 controls participating in the Agricultural Health Study in Iowa and North Carolina (female participants only) | Exposure: glyphosate ever use Logistic regression adjustments: birth date, state | Rheumatoid arthritis odds ratio (OR) 1.2 (0.8–1.8) |
| Parks et al 68 Nested case-control study of cases of physician-confirmed rheumatoid arthritis or self-reported use of disease modifying antirheumatic drugs and noncases participating in the Agricultural Health Study in Iowa and North Carolina (female spouses of licensed pesticide applicators only); enrolled between 1993 and 1997 and followed through 2010 | Exposure: glyphosate ever use Logistic regression adjustments: age, state, pack-years smoking | Rheumatoid arthritis odds ratio (OR) 1.2 (0.95–1.6); based on 100 prevalent cases odds ratio (OR) 1.4 (1.0–2.0); based on 54 incident cases |
| Liver Effects | ||
| Mills et al 69 Case-control study of 97 participants, 63 with nonalcoholic steatohepatitis (NASH) and 34 without NASH | Exposure: glyphosate and glyphosate residue measured from fasting urine; glyphosate residue (calculated) which estimates dietary intake and exposure to residues Multivariable linear adjustments: age, sex, body mass index | Glyphosate residue significantly higher in patients with nonalcoholic steatohepatitis (NASH) compared with patents without nonalcoholic steatohepatitis (NASH) (p=0.008) Significant dose-dependent increase of glyphosate exposure with increase in fibrosis stage when comparing patients without advanced fibrosis to those with Stage 2,3 or 4 fibrosis (glyphosate: 0.230 mg/L, SD: 0.19 vs. 0.351 mg/L, SD: 0.45; glyphosate residue: 0.525 mg/L, SD 0.38 vs. 0.938 mg/L, SD: 0.372,p <0.001). Nonalcoholic steatohepatitis (NASH) cases confirmed through liver biopsy. |
| Skin Effects | ||
| Camacho and Mejia 70 Cross-sectional study examining individual health records from the general public over a five-year period (2003 to 2007) merged with data of aerial spraying events. The study examined the data under several specifications:
| Exposure: aerial spraying of glyphosate on coca crops and the general population living in the spray areas within study period 2003 to 2007 Regression Adjustments: age, age square, health regime, municipal tax income, population, area in square km, rurality index, average monthly rainfall, municipal spending on education and health, subsidized regime coverage, year and month dummy | Increased number of skin conditions consistent across all specifications analyzed (only statistically significant p values were presented). |
| Maibach 71 Experimental study of 24 males and females | Exposure: 0.1 mL applied to intact and Draize-type abraded skin; patch removed after 24 hours | No skin irritation 24 or 48 hours after application to intact skin Irritancy scores 24 hours after application to abraded skin were negative in 10 subjects, equivocal in 4 subjects and erythema was noted in 10 subjects; at 48 hours, the scores were negative in 10 subjects, equivocal in 6 subjects, and erythema was noted in 8 subjects |
| Maibach 71 Experimental study of 23 males and females | Exposure: 0.1 mL applied 5 days/week for 21 days | The average score was 1.4 where a score of 1 indicates redness and 2 indicates redness and swelling; none of the subjects reported burning, stinging, or itching from the test compound |
| Maibach 71 Experimental study of 204 males and females | Exposure: 0.2 mL applied 3 days/week for 3 weeks with patches remaining in place for 48–72 hours; a challenge patch was applied after a 2-week rest period | No skin irritation was observed |
| Maibach 71 Experimental study of 15 males and females | Exposure: Full-strength glyphosate was applied to skin stripped of the stratum corneum; the test site received irradiation with ultraviolet A and ultraviolet B light | No positive results for photoirritation or photosensitization were found |
| Eye Effects | ||
| Kirrane et al 72 Prospective study of 31,173 female spouses of commercial pesticide applicators participating in the Agricultural Health Study in Iowa and North Carolina | Exposure: glyphosate ever use Hierarchical regression adjustments: age, state | Retinal degeneration odds ratio (OR) 1.1 (0.8–1.5) |
| Endocrine Effects | ||
| Goldner et al 73 Prospective study of 16,529 participants (female spouses only) in the Agricultural Health Study in Iowa and North Carolina | Exposure: glyphosate ever use Polytomous logistic regression adjustments: age, education, smoking status, hormone replacement therapy, body mass index (BMI) | Hyperthyroid disease (overactive thyroid) odds ratio (OR) 0.98 (0.78–1.2) Hypothyroid disease (underactive thyroid) odds ratio (OR) 1.0 (0.91–1.2) Other thyroid disease odds ratio (OR) 0.97 (0.81–1.2) |
| Kongtip et al 74 Cross sectional study of 195 conventional farmers and 222 organic farmers in Thailand. Sera were collected and analyzed for the following thyroid levels: TSH, T3, T4, free T3 and free T4 | Exposure: glyphosate use, amount recorded in a diary Generalized linear regression adjustments: sex, current smoking, current alcohol use, insecticide use at home in the past year, triglyceride levels, and any stress symptoms in the past 2–4 weeks | Log(e) estimates of thyroid hormone levels predicted by models of glyphosate sprayed in past year Expβ (95%confidence interval):
Increased use of glyphosate sprayed (measured in moles) was found to increase T4 levels. |
| Shrestha et al 75 Prospective study of 35,150 male and female pesticide applicators in the Agricultural Health Study in Iowa and North Carolina followed over 20 years | Exposure: glyphosate ever use Logistic regression adjustments: sex, education, state of residence, smoking | Hypothyroid disease (underactive thyroid disease)
|
| Neurological Effects | ||
| Kamel et al 76 Case control study of cases of self-reported Parkinson’s disease (n=83 prevalent cases and 78 incident cases) and controls (n=79,557 prevalent controls and 55,931 incident controls) participating in the Agricultural Health Study in Iowa and North Carolina | Exposure: glyphosate ever use Logistic regression adjustments: age, state, type of participant | Parkinson’s disease
Prevalent disease defined as reporting Parkinson’s disease at enrollment and incident disease defined as Parkinson’s disease reported at the study follow-up |
| Zhang et al 77 A cross-sectional study of 218 farmers in China. Abnormalities of peripheral nerve conduction including nerve conduction velocity, distal motor latency, and amplitude were measured with a conventional nerve conduction assessment. | Exposure: glyphosate agriculture use Logistic regression adjustments: age, gender, smoking habit, alcohol consumption, whether adopting personal protective equipment, and whether suffering from diabetes mellitus | Abnormalities in nerve conduction velocity
Abnormalities in nerve conduction distal motor latency odds ratio (OR) 1.05 (0.81-1.37) Abnormalities in nerve conduction amplitude odds ratio (OR) 1.21 (0.75-1.97) |
| Reproductive Effects | ||
| Camacho and Mejia 70 Cross-sectional study examining individual health records from the general public over a five-year period (2003 to 2007) merged with data of aerial spraying events. The study examined the data under several specifications:
| Exposure: aerial spraying of glyphosate on coca crops and the general population living in the spray areas within study period 2003 to 2007 Regression Adjustments: age, age square, health regime, municipal tax income, population, area in square km, rurality index, average monthly rainfall, municipal spending on education and health, subsidized regime coverage, year and month dummy | Increased number of miscarriages consistent across all specifications analyzed (only statistically significant p values were presented). |
| Curtis et al 78 Retrospective cohort study of 2,012 planned pregnancies among participants in the Canadian Ontario Farm Family Health Study | Exposure: glyphosate use on the farm Cox proportional hazard adjustments: age when beginning to try to conceive, recent oral contraceptive use, men’s and women’s smoking, and use of other pesticides | Fecundability (the likelihood of becoming pregnant during a menstrual cycle or within a month)
|
| Developmental Effects | ||
| Arbuckle et al 79 Retrospective cohort study of 2,110 female participants in the Canadian Ontario Farm Family Health Study | Exposure: glyphosate use during gestation Logistic regression adjustments: none | Spontaneous abortion, preconception exposure
Spontaneous abortion, postconception exposure
|
| Garcia et al 80 Case control study of 261 cases of congenital malformations and 261 matched controls in Spain | Exposure: paternal glyphosate use Conditional logistic regression adjustments: paternal age and paternal job and maternal history of spontaneous abortion, twins, drug consumption, heavy smoking, education, occupation | Congenital malformations odds ratio (OR) 0.94 (0.37–2.34) for the acute risk period (during 3 months preceding conception or during the first trimester of pregnancy or both for the father and during 1 month preceding conception or during the first trimester of pregnancy or both for the mother) |
| Garry et al 81 Cross sectional study of 695 families and 1,532 children in Minnesota | Exposure: glyphosate ever use Regression adjustments: maternal age, smoking status, alcohol use, season of conception | Attention deficit disorder/attention deficit hyperactivity disorder (ADD/ADHD), parent reported odds ratio (OR) 3.6 (1.35–9.65) |
| Parvez et al 82 Birth-cohort study of 71 women with singleton pregnancies in Central Indiana | Exposure: maternal glyphosate levels in urine specimens Nonparametric Spearman correlation adjustments: maternal age, pre-pregnancy body mass index (BMI), tobacco use, alcohol use, trimester of pregnancy | Shortened gestational length (r = −0.30, p = 0.01) Reduced birth weight (r = −0.14, p = 0.27) Head circumference (r = −0.06, p = 0.64) |
| Rull et al 83 Case control study of 731 cases of neural tube defects and 940 controls in California | Exposure: maternal residential proximity to glyphosate application (within 1,000 m) Logistic regression adjustments: maternal ethnicity, education, periconceptional smoking, vitamin use | Neural tube defects
|
| Sathyanarayana et al 84 Prospective study of 2,246 women whose most recent singleton birth occurred within 5 years of enrollment in the Agricultural Health Study in Iowa and North Carolina | Exposure: maternal glyphosate ever use (n=700) Linear regression adjustments: maternal body mass index (BMI) and height, parity, preterm status, state, maternal smoking during pregnancy | Multiple regression estimates of change in birth weight (g) in relation to maternal self-reported glyphosate use (coefficient = 4 g; 95% confidence interval −40 to +48 g) indicate no significant association between birth weight and maternal use of glyphosate |
| Savitz et al 85 Retrospective cohort study of 1,898 couples participating in the Canadian Ontario Farm Family Health Study | Exposure: any paternal glyphosate use from 3 months prior to conception through the month of conception Logistic regression adjustments: maternal age, parity, maternal and paternal education, income, maternal and paternal off farm job, maternal smoking and alcohol use during pregnancy, conception to interview interval | Miscarriage odds ratio (OR) 1.5 (0.8–2.7) Preterm delivery odds ratio (OR) 2.4 (0.8–7.9) Small for gestational age odds ratio (OR) 0.8 (0.2–2.3) |
| Other Noncancer Effects | ||
| Montgomery et al 86 Prospective study of 33,457 participants (white males only) in the Agricultural Health Study in Iowa and North Carolina | Exposure: glyphosate ever use Logistic regression adjustments: age, state, body mass index (BMI) | Diabetes incidence odds ratio (OR) 0.85 (0.74–0.98) |
| Saldana et al 87 Prospective study of 11,273 participants in the Agricultural Health Study in Iowa and North Carolina | Exposure: any agricultural glyphosate exposure during the first trimester Logistic regression adjustments: body mass index (BMI) at enrollment, mother’s age at pregnancy, parity, race, state, commonly used pesticides by women | Gestational diabetes mellitus odds ratio (OR) 0.7 (0.2–1.75) |
Footnotes:
- Odds ratio (OR) is a measure of association between an exposure and an outcome. Odds ratio (OR) represents the odds that an outcome will occur given a particular exposure, compared to the odds of the outcome occurring in the absence of that exposure. Odds ratio (OR) > 1 means the event is more likely to occur with exposure; while an Odds ratio (OR) < 1 means the event is less likely to occur with exposure.
- Hazard ratio (HR) is a statistical measure that compares the rate of an event occurring in one group to the rate of the same event occurring in another group over time. Hazard ratios (HRs) are often used interchangeably with relative risk (RR) ratios, but there are important differences between the two. Relative risks (RR) are cumulative over an entire study, while hazard ratios (HRs) define instantaneous risk over a study period. Hazard ratio (HR) of 1 means there’s no difference in survival between the groups, while a hazard ratio (HR) greater than or less than 1 means one group had better survival.
Abbreviations: ADD/ADHD = attention deficit disorder/attention deficit hyperactivity disorder; BMI = body mass index; CFR = conditional fecundability ratio; CI = confidence interval; HR = hazard ratio; OR = odds ratio
[Source 53 ]Table 3. Toxicological profile of glyphosate metabolites found as residues in livestock and/or crops
| Metabolite | Genotoxicity | General toxicity Toxicological reference values (TRVs) | Additional source of human exposure (e.g. groundwater) |
|---|---|---|---|
| AMPA | Unlikely to be genotoxic | TRVs of glyphosate apply | No |
| N-acetyl AMPA | Unlikely to be genotoxic | TRVs of glyphosate apply | No |
| N-acetyl glyphosate | Negative for both mutagenicity and clastogenicity; aneugenicity not sufficiently investigated (data gap) | TRVs of glyphosate apply | No |
| N-methyl AMPA | Unlikely to be genotoxic | No data, not needed for consumer risk assessment | No |
| N-glyceryl AMPA | Negative for mutagenicity. Clastogenicity and aneugenicity not sufficiently investigated(data gap) | No data, not needed for consumer risk assessment | No |
| N-malonyl AMPA | Negative for mutagenicity. Clastogenicity and aneugenicity not sufficiently investigated(data gap) | No data, not needed for consumer risk assessment | No |
Abbreviation: AMPA = aminomethylphosphonic acid; TRVs = toxicological reference values
[Source 33 ]Animal Health Effects
Results from available animal studies identify the following glyphosate toxicity 53:
- Developmental effects: Histopathologic testicular lesions, decreased sperm production, and increased incidence of fetal skeletal malformations were reported in response to oral dosing of rat weanlings or pregnant rats with selected glyphosate formulations in the range of 5–500 mg/kg/day.
- Endocrine effects: Decreased serum testosterone was noted in male rat weanlings administered a glyphosate formulation orally at 5 mg/kg/day.
- Body weight effects: Seriously depressed body weight gain was observed in mice administered a glyphosate formulation orally at 50 mg/kg/day.
- Kidney effects: Histopathologic kidney lesions were noted in male rats gavaged once with a glyphosate formulation at 250 mg/kg.
- Liver effects: Increased serum liver enzyme activity and histopathologic liver lesions were reported in male rats repeatedly gavaged with a glyphosate formulation at 487 mg/kg/day.
- Hematological effects: Decreases in red blood cells, hematocrit, and hemoglobin, and increases in mean corpuscular volume and neutrophils were reported in mice administered a glyphosate formulation orally at 500 mg/kg/day.
- Reproductive effects: Increased percentage of morphologically abnormal sperm was reported among rats receiving a glyphosate formulation from the drinking water for 8 days at 640 mg/kg/day.
Results from the oral animal studies identify glyphosate toxicity at relatively high dose levels 53:
- Gastrointestinal effects: Clinical signs and/or pathological evidence of glyphosate-induced irritation were observed in several animal studies; the lowest dose level resulting in gastrointestinal effects was 175 mg/kg/day for diarrhea and few feces in pregnant rabbits administered glyphosate acid by gavage. Gastrointestinal disturbances are signs and/or symptoms following ingestion of large amounts of glyphosate-containing products.
- Developmental effects: Glyphosate treatment-related developmental effects were noted in a few studies at dose levels (≥1,234 mg/kg/day) resulting in maternal toxicity as well.
- Body weight effects: Depressed body weight and/or depressed body weight gain resulted from repeated dosing of glyphosate technical at dose levels ≥1,183 mg/kg/day.
- Liver effects: Increases in liver weight and serum ALT activity were observed in one repeated-dose study at a dose level of 1,678 mg/kg/day.
- Eye effects: Lens abnormalities were observed in one repeated-dose study at a dose level of 940 mg/kg/day.
- Kidney effects: Indicators of renal toxicity were noted in rats and mice administered glyphosate technical in the diet for 2 years at high doses (940 and 6,069 mg/kg/day, respectively).
- Other effects: Neurological, hematological, immunological, and reproductive endpoints have been evaluated, but do not appear to be particular targets of glyphosate toxicity.
- Cancer: Upon evaluation of available carcinogenicity studies in laboratory rodents, multiple agencies or organizations have concluded that glyphosate technical does not appear to be an animal carcinogen. In contrast, International Agency for Research on Cancer (IARC) considered the animal data to provide “sufficient evidence” of glyphosate carcinogenicity.
Body weight
Evidence of treatment-related effects on body weight among laboratory animals receiving lower oral doses of glyphosate or glyphosate-based herbicides varied by study. Studies using doses of glyphosate at ≤1,000 mg/kg/day during acute-, intermediate-, or chronic-duration exposure found no effects on body weight 88, 89, 90, 91, 92, 93. In contrast, body weight changes were sometimes observed in lower dose oral studies using glyphosate formulations 94, 95, 96, 97, 98, 99.
Several studies evaluated effects of oral exposure to glyphosate formulations on body weight. Limited results indicate that mice may be more sensitive than rats to body weight effects from repeated oral exposure to glyphosate formulations. In intermediate studies on male mice, significantly reduced body weight gain (>70%) occurred at 250 mg/kg/day after 6-12 week exposure 94 and in offspring exposed in utero, during lactation and then orally from post-natal day 21 to 60 (14% less than controls) 96. However, in another intermediate oral study, pregnant C57B1/6 mice showed a 17% decrease in body weight and an approximate 25% decrease in body weight gain, while their male offspring exposed in utero and during lactation did not have significantly different body weight or body weight gain when compared with controls 99. Seriously-depressed mean body weight gain (60–66% less than controls) was reported for albino Swiss mice gavaged with Roundup Original® at 50 mg/kg/day for 15 days and approximately 10% body weight loss for mice dosed at 500 mg/kg/day 100. When compared to controls, male rats orally exposed to a range of concentrations of Roundup® for two weeks showed an estimated 37% decrease in body weight when exposed to 100 mg/kg/day and an estimated 33% decrease in body weight when exposed to 250 mg/kg/day. Pregnant rats fed 500 mg/kg/day Roundup via gavage for 7 days had 10% less body weight gain compared to controls, when exposed simultaneously with paraquat 95. In rats, 8-10% less body weight gain was seen after exposure to 126 mg/kg/day in feed for 60 days 97.
Oral exposure of rats to glyphosate at relatively high doses resulted in significant effects on body weight and/or body weight gain. Pregnant rats gavaged at 3,500 mg/kg/day during gestational days 6–19 exhibited as much as 28.5% lower mean body weight gain than controls 101. Body weight gain was 12–18% less than that of controls in two generations of parental male and female rats exposed via the diet for 14–19 weeks at 2,219 or 3,134 mg/kg/day, respectively 102. No treatment-related effects on body weight were seen among young female mice treated for 28 days at estimated doses up to 1,447.5 mg/kg/day 91. In 13-week oral studies, body weight and/or body weight gain among rats and mice at oral doses in the range of 2,273–11,977 mg/kg/day were 10–18% less than controls 103. In a 2-year study, female rats dosed at 1,183 mg/kg/day exhibited 13% lower mean body weight than controls at treatment week 81 104.
However, other studies found no effects of oral glyphosate exposure on body weight. No significant effects on body weight were observed among Wistar rats gavaged with Roundup at 56 or 560 mg/kg/day for up to 13 weeks 105, male mice orally exposed to Roundup for 35 days 106. Pregnant Wistar rats gavaged with Roundup at 1,000 mg/kg/day during gestational days 6–15 107, or maternal Wistar rats gavaged with Roundup at 50–450 mg/kg/day during gestation and lactation 108. No effects on body weight were observed among male Wistar rats gavaged with Roundup Transorb® at 250 mg/kg/day during postnatal days 23–53 109. After exposure to 1.75 mg/kg/day of glyphosate or Roundup Bioflow® during pregnancy and lactation, no weight changes were observed in Sprague-Dawley F0 females or their male offspring 93. At higher exposure levels to glyphosate formulations (3.69 mg/kg/day and 352.2 mg/kg/day) during pregnancy, F0 and F1 female Wistar rats showed no signs of glyphosate-associated weight changes 110.
Non-oral exposure to glyphosate and glyphosate formulations was not associated with changes in body weight 53. No significant body weight effects occurred in a 4-week inhalation study of rats intermittently exposed to Roundup at exposure levels as high as 360 mg/m3 (approximately 36 mg Roundup/m³) 111. A study on male albino rats intraperitoneally exposed to 269.9 mg/kg/day of Roundup for up to two weeks also found no changes in body weight 89. No significant treatment-related effects on body weight were observed among rabbits administered repeated skin applications of glyphosate at doses in the range of 100–5,000 mg/kg/application for 21 days 112. When mice were acutely exposed subcutaneously to 2 mg/kg body weight, no effects on body weight were seen 113, 114, 115. No effects on body weight were seen in male mice after intranasal exposure to 50 mg/kg/day for 3 times a week for 4 weeks 88.
Respiratory effects
Available data regarding respiratory effects in laboratory animals exposed to glyphosate are limited. In mice, a 50% decrease in relative lung weight was observed following exposure to 250 mg/kg/day for 12 weeks 94. No other observations were made in the lungs. Kumar et al. 116 reported an inflammatory respiratory response (evidenced by increased eosinophil and neutrophil counts, mast cell degranulation, and production of IL-33, TSLP, IL-13, and IL-5) in anesthetized mice exposed intranasally to glyphosate. Adam et al. 117 designed a study to evaluate the effects of glyphosate (200 mg/kg), glyphosate + polyethoxylated tallow amine (POEA) 200 and 100 mg/kg, respectively. Polyethoxylated tallow amine (POEA) alone (100 mg/kg), and Roundup in rats evaluated for 24 hours following intratracheal instillation 117. Control rats received normal saline. Obvious clinical signs of adverse lung effects and mortalities occurred in each group except the saline controls. The study authors stated that the pulmonary effects were more severe and lasted longer in rats treated with polyethoxylated tallow amine (POEA) alone or in combination with glyphosate compared to responses in glyphosate only-treated rats. These results suggest polyethoxylated tallow amine (POEA) was more acutely toxic than glyphosate to the lungs. No respiratory effects occurred in a 120-day study where rats were exposed to 250 mg/kg/day 118. No respiratory effects occurred in a 4-week study of rats intermittently exposed to Roundup at exposure levels as high as 360 mg/m3 (approximately 36 mg Roundup/m³) 111.
Gastrointestinal effects
Limited information was located regarding gastrointestinal effects in laboratory animals following oral exposure to glyphosate 53. Rats exposed daily for 6-12 weeks to glyphosate 250 mg/kg/day exhibited a decreased in total bacterial count in the gut 119. Lozano et al. 120 reported significant differences in microbiome genomic diversity, characterized by an increase in the Bacteroidetes family S24-7 and a decrease in Lactobacillaceae, between treated female rats exposed to 5,000 ppm of Roundup for 673 days when compared to control males, control females, and treated males. The study found that Roundup had a direct selective bactericidal action on isolated gastrointestinal strains 120. In a study designed to evaluate the effects of glyphosate technical (2,000 mg/kg), rats were administered glyphosate + polyethoxylated tallow amine (POEA) 2,000 and 1,000 mg/kg, respectively, polyethoxylated tallow amine (POEA) alone (1,000 mg/kg), or Roundup by gavage, followed by 24 hours of posttreatment observation 117. Control rats received normal saline. Two rats in the polyethoxylated tallow amine (POEA)-only treatment group died. Diarrhea was noted in all groups except the control group. The study authors stated that the groups given polyethoxylated tallow amine (POEA) or mixtures that included polyethoxylated tallow amine (POEA) experienced more rapid and severe diarrhea than those given glyphosate alone. These results suggest that polyethoxylated tallow amine (POEA) was more acutely toxic than glyphosate to the gastrointestinal system. Mao et al. 121 reported that Roundup added to the drinking water of rat dams from gestational day 6 through lactation and to F1 offspring up to PND 125 at a concentration designed to deliver a daily dose of 1.75 mg glyphosate/kg/day (the U.S. glyphosate ADI) resulted in modifications to the gut microbiota in early development, particularly among prepubertal rats.
Glyphosate oral exposure in laboratory animals found the most common effect was clinical signs of gastrointestinal disturbances. Such clinical signs are commonly observed in studies of laboratory animals receiving bolus gavage doses of test substances, in which cases the clinical signs may be at least partially the result of the method of gavage dosing. Diarrhea was observed among rats gavaged once with glyphosate technical at 2,000 mg/kg 122. Gastrointestinal disturbances (e.g., soft stool, diarrhea, few feces) were reported among pregnant rats gavaged at 3,500 mg/kg/day during gestational days 6–19 101 and pregnant rabbits gavaged at 350 mg/kg/day during gestational days 6–27 123 or 175 mg/kg/day during gestational days 8–20 92. A slight increase in observations of soft stool and/or diarrhea was noted in the rabbits dosed at 175 mg/kg/day 123. Soft stools were observed in rats exposed via the diet for 2 generations at concentrations resulting in estimated doses in the range of 2,219–2,633 and 3,035–3,134 mg/kg/day for parental males and females, respectively 102. Mao et al. 121 reported that glyphosate added to the drinking water of rat dams from gestational day 6 through lactation and to F1 offspring up to PND 125 at a concentration resulting in a daily dose of 1.75 mg/kg/day (the U.S. acceptable daily intake [ADI]) resulted in modifications to the gut microbiota in early development, particularly among prepubertal rats.
In a 2-year study of rats exposed via the diet 104, 124, inflammation of gastric squamous mucosa was observed in females at an estimated dose level of 457 mg/kg/day; there were no signs of gastrointestinal effects in males at estimated doses as high as 940 mg/kg/day. In another chronic-duration oral rat study 125, there were no signs of treatment-related gastrointestinal effects at the highest estimated dose level (31.45–34.02 mg/kg/day). No clinical signs or histopathological evidence of treatment-related gastrointestinal effects were seen among male or female mice exposed via the diet for 24 months at estimated doses as high as 4,945 and 6,069 mg/kg/day, respectively 126, 127. Increased incidence of exocrine hyperplasia in the pancreas was reported for male rats receiving glyphosate technical from the diet for up to 2 years at an estimated dose of 1,214 mg/kg/day 128. Increased severity of cytoplasmic changes in salivary gland cells (basophilia and hypertrophy of acinar cells in parotid and submandibular salivary glands) was reported for male and female rats receiving glyphosate from the diet for 13 weeks at 410 and 421 mg/kg/day, respectively 103 and other rats similarly treated at 300 mg/kg/day for up to 2 years 128. Similar effects on salivary glands were observed in male and female mice treated for 13 weeks at much higher doses (1,065 and 2,707 mg/kg/day, respectively; not observed at 507 and 753 mg/kg/day, respectively) 103. Although salivary gland cytoplasmic changes were noted in rats at doses <300 mg/kg/day as well, the changes were reported to be only of minimal or mild severity; therefore, they are not considered adverse effects. The toxicological significance of the glyphosate treatment-related effects on salivary glands is uncertain.
Liver effects
The potential for glyphosate to cause liver toxicity was evaluated in studies of rats and mice 53. In a 7-day study of pregnant rats, the liver had a 19-23% increase in thiobarbituric acid reactive substances (TBARS) levels in liver tissue compared to controls, following exposure to 500 mg/kg/day by oral gavage 95. In rats orally administered glyphosate for 28-days up to 10 mg/kg body weight per day, no treatment related findings were reported after gross necropsy. Furthermore, no significant differences in liver weights were reported between glyphosate treated groups and the control 129. However, reactive oxygen species (ROS) levels in the liver were significantly increased at 10 mg/kg body weight per day, while thiobarbituric acid reactive substances (TBARS) concentrations decreased at 0.5, 1.75 and 10 mg/kg body weight per day compared to controls. GHS levels in liver decreased by 22.7% and 27% at 1.75 and 10 mg/kg body weight per day concentrations, respectively 129. Similarly, GHS peroxidase (GSH-Px) activity in the liver was noticeably higher among rats exposed to 0.5, 1.75 and 10 mg/kg body weight per day glyphosate compared to controls. Elevated levels of GSH-Px is reflective of glyphosate inducing the antioxidant defense system in the liver 129.
In a 13-week rat dietary study of glyphosate, increases in liver weight and serum alanine aminotransferase (ALT) were observed in males at 1,678 mg/kg/day; increased liver weight and increased serum alkaline phosphatase (ALP), alanine aminotransferase (ALT) and bile acids were noted in females at 3,393 mg/kg/day 90. There were no indications of treatment-related liver effects among male and female rats treated via the diet for 2 generations at estimated doses as high as 1,234–1,273 mg/kg/day 90 or other rats treated for 2 years to doses as high as 940–1,183 mg/kg/day 104, 124. Male mice exposed via the diet for 13 weeks at doses ≥2,273 mg/kg/day exhibited increased mean relative liver weight (4–9% greater than controls) in the absence of histopathologic liver lesions; there were no effects on liver weight in similarly-treated female mice at doses up to and including 11,977 mg/kg/day 103. Male mice exposed via the diet for 2 years at an estimated dose of 4,945 mg/kg/day exhibited increased incidence of histopathologic central lobular hepatocyte necrosis; there was no evidence of treatment-related liver effects in similarly-treated female mice at an estimated dose of 6,069 mg/kg/day 127.
Following oral exposure to glyphosate (1.0 g/L in drinking water) for 72 weeks, wild type and multiple myeloma model mice (Vk*MYC strain) showed liver fibrosis and blood abnormalities including decreases in hemoglobin levels (i.e. anemia); lower levels of platelet counts and hematocrit were observed in model multiple myeloma mice 130. This study was part of a larger effort to understand the effect of glyphosate on multiple myeloma development 130.
Available information regarding liver endpoints in animals exposed to glyphosate formulations is limited. No liver effects occurred in a 4-week study of rats intermittently exposed to Roundup at exposure levels as high as 360 mg/m³ (approximately 36 mg Roundup/m³) 111. Increased serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity and histopathologic liver lesions (increased Kupffer cells in hepatic sinusoids and deposition of reticulin fibers) were seen in male rats treated with Glyphosate-Biocarb® by gavage for 75 days (one dose every 2 days) at 487 mg/kg/dosing 131. In an acute oral exposure study, a significant increase in liver index, liver weight, and cytokine expression (IL-1β and TNF-α) was observed in male rats exposed to 100 or 250 mg/kg/day Roundup. Lower doses of Roundup, including exposure to 25 or 50 mg/kg/day, resulted in increased liver weight, higher numbers of liver macrophages, and changes in glycogen storage. However, these results were less consistent and did not adhere to a dose-response relationship 98. Following a 120-day exposure to 500 mg/kg/day Roundup, rats exhibited increased mean liver malondialdehyde levels, decreased superoxide dismutase concentration, catalase activity, increased alanine aminotransferase, aspartate amino transferase, lactate dehydrogenase and enzymatic activity of GSH-Px, moderate hepatocytic degeneration and necrosis 118. Following 6-12 weeks of daily exposure to ≥250 mg/kg/day of Roundup ®, mice showed a 44% decrease in relative liver weight, no other liver observations were made 94.
Rabbits administered repeated skin applications of glyphosate at doses in the range of 100–5,000 mg/kg/application for 21 days exhibited no evidence of treatment-related liver effects 112. However, male albino rats intraperitoneally exposed to 134.95 mg/kg/day glyphosate for up to two weeks showed significantly increased serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP), decreased serum GSH, increased hepatic nitric oxide, and increased hepatic lipid peroxidation, which the author attributed to impairment of liver enzymes and hepatic metabolism 89.
Two-week intraperitoneal exposure of albino rats to 269.9 mg/kg/day Roundup® demonstrated the same liver effects as intraperitoneal exposure to 134.95 mg/kg/day glyphosate technical, including increased serum AST, ALT, and ALP, decreased serum GSH, increased hepatic nitric oxide, and increased hepatic lipid peroxidation. Most of these observed effects were similar in both Roundup®-exposed and glyphosate-exposed rats. However, compared to controls, rats exposed to glyphosate technical showed a larger increase in hepatic nitric oxide than rats exposed to Roundup® (72% increase and 21% increase respectively). Conversely, the increase in hepatic lipid peroxidation compared to controls was much more pronounced in Roundup ®-exposed rats than in glyphosate-exposed rats (630% increase and 432% increase respectively) 89.
In vivo metabolome and proteome profiling of liver obtained from rats chronically exposed to long-term exposure at low levels of Roundup (4 ng/kg bw/day) for two years indicate effects to the liver including metabolite alterations associated with non-alcoholic fatty liver disease and steatohepatosis 132. Metabolome profiling, or the analysis of metabolites characterizing the range of chemical processes, analogous to chemical fingerprinting, revealed a lipotoxic condition, oxidative stress, and markers of hepatotoxicity in the liver 132. Results from the proteome analysis, which characterizes the expression of protein products and their interaction, reported rats exposed to Roundup ® had alterations reflective of peroxisomal proliferation, steatosis, and necrosis 132.
Kidney effects
Several studies evaluated possible kidney toxicity in laboratory animals treated with glyphosate 53. In a 2-generation reproductive toxicity study 90, slightly increased absolute and relative kidney weights (7–11% greater than controls) were reported among female rats dosed at 1,273 mg/kg/day; there was no evidence of histopathologic kidney lesions 53. Therefore, the slightly increased kidney weight was not considered to represent an adverse effect 53. During 2 years of glyphosate dietary treatment of rats, urinalysis revealed increased specific gravity of urine and decreased urinary pH among males treated at an estimated dose of 940 mg/kg/day (no-observed-adverse-effect level (NOAEL) =362 mg/kg/day); there were no signs of treatment-related renal effects in urinalysis results from females treated at an estimated dose as high as 1,183 mg/kg/day 104, 124. Papillary necrosis (males and females) and decreased pH of urine (males only) were observed in a study of rats administered glyphosate in the diet for up to 2 years at estimated doses of 1,214 mg/kg/day (males) and 1,498 mg/kg/day (females); respective no-observed-adverse-effect levels (NOAELs) were 361 and 437 mg/kg/day 128. Another 2-year rat study reported decreased pH of urine among males treated at 1,000 mg/kg/day (NOAEL=300 mg/kg/day); no kidney effects were observed in females at doses as high as 1,000 mg/kg/day 128. Female mice treated for 2 years at an estimated dose of 6,069 mg/kg/day exhibited significantly increased incidence of renal proximal tubule epithelial basophilia and hypertrophy (NOAEL=968 mg/kg/day); there was no evidence of renal effects in similarly-treated male mice at doses as high as 4,945 mg/kg/day 127.
Shorter-term studies on rodents exposed to glyphosate found signs of potential kidney damage. Myeloma model mice orally exposed to glyphosate (1.0 g/L in drinking water) for 72 weeks showed kidney damage, including tubular obstruction by casts 130. This study was part of a larger effort to understand the effect of glyphosate on myeloma development 130. In a two-week study albino rats exposed intraperitoneally to 134.95 mg/kg/day glyphosate showed serum changes indicative of potential kidney effects 89. Compared to control rats, glyphosate-exposed rats had 33.8% decreased serum creatinine, 110% increased serum urea, and 146% increased uric acid. Similar results were seen in the male rats exposed to 269.9 mg/kg/day Roundup 89.
A multigenerational study on reproductive effects measured dam kidney weight and found no difference between controls and dams exposed to 420 mg/kg/day of Roundup 99. In mice, decreased relative kidney weight (50% less than controls) was reported after daily exposure to ≥250 mg/kg/day for 12 weeks 94. No other kidney observations were made. No kidney effects occurred in a 4-week study of rats intermittently exposed to Roundup at exposure levels as high as 360 mg/m³ (approximately 36 mg Roundup/m³) 111. Histopathologic kidney lesions (necrotic and apoptotic cells, localized primarily in tubular epithelium of the proximal straight tubule and thick ascending limb of the loop of Henle) were reported in male rats gavaged once with Concentrate Roundup Weedkiller at dose levels ranging from 250 to 2,500 mg/kg 133. There is some uncertainty regarding the role of glyphosate in the reported effects.
Hematological effects
Available information regarding hematological effects related to glyphosate formulations is limited. No hematological effects occurred in a 4-week study of rats intermittently exposed to Roundup at exposure levels as high as 360 mg/m3 (approximately 36 mg Roundup/m³) 111. Decreases in red blood cell count, hematocrit, and hemoglobin, and increases in corpuscular volume and neutrophil count were reported in mice gavaged with Roundup Original for 15 days at 500 mg/kg/day 100.
There were no apparent treatment-related hematological effects in chronic-duration oral studies of rats, mice, or dogs administered glyphosate at oral doses as high as 940–1,183 mg/kg/day for rats 104, 124, 125, 4,945–6,069 mg/kg/day for mice 127 and 500 mg/kg/day for dogs 134, 135. Rabbits administered repeated dermal applications of glyphosate technical at doses in the range of 100–5,000 mg/kg/application for 21 days exhibited no evidence of treatment-related hematological effects 112. Small changes in hematological parameters were seen in both male and female rats following dietary exposure to glyphosate technical in the 13-week National Toxicology Program study 103. These were considered to be unremarkable and most likely due to mild dehydration 53.
Skin effects
Minor skin irritation was reported in response to topically-applied glyphosate 53. At the application site, very slight redness and swelling were observed in rabbits during 21 days of repeated dermal application of glyphosate at 5,000 mg/kg/application; no skin effects were seen at doses ≤1,000 mg/kg/application 112.
Eye effects
Two chronic-duration oral studies included ophthalmoscopic examinations of laboratory animals exposed to glyphosate. The Environmental Protection Agency 104, 124 reported significantly increased incidence of lens abnormalities in male rats treated via the diet for 2 years at an estimated dose of 940 mg/kg/day; there were no indications of a treatment-related ocular effect in female rats at the highest estimated dose level (1,183 mg/kg/day). No signs of treatment-related ocular effects were seen among dogs treated via capsule for 1 year at estimated doses as high as 500 mg/kg/day 134.
According to Food and Agriculture Organization of the United Nations and World Health Organization 136, glyphosate was moderate to severely irritating to the rabbit eye. There were no signs of exposure-related effects in ophthalmologic examinations of rats intermittently exposed to Roundup for 4 weeks at exposure levels as high as 360 mg/m³ (approximately 36 mg Roundup/m³) 111.
Endocrine effects
Limited information was located regarding the potential for glyphosate to affect the endocrine system in animal studies. While one study reported degeneration of pancreatic acinar cells and islets of Langerhans after male Wistar rats were exposed to 14.4, 375, or 750 mg/kg/day of the herbicide Bushfire via drinking water 137, most of the observed endocrine effects in glyphosate animal studies involved changes in hormone levels. Romano et al. 109 reported dose-related 30–50% decreased serum testosterone in young male rats gavaged with Roundup Transorb® at 5–250 mg/kg/day during postpartum days 23–53. Romano et al. 138 implicated disruption of gonadotropin expression as a mechanism of action for glyphosate-induced effects on male rat sexual development. Pregnant C57B1/6 mice exposed to 420 mg/kg/day Roundup in their drinking water showed no difference in pancreas weight compared to controls, but their male F1 offspring had 195% higher intratesticular testosterone, an estimated 60% increase in plasma LH, and a 11% increase in β-LH pituitary protein compared with male F1 controls 99.
A multi-generational study of F0 Sprague-Dawley rats exposed to either 1.75 mg/kg/day glyphosate or Roundup Bioflow® found no evidence of endocrine organ lesions in F1 offspring for either exposure 93. However, exposure to Roundup Bioflow® was associated with changes in hormone ratios in F1 offspring when compared to controls. Specifically, after 13 weeks of exposure to Roundup Bioflow® in addition to exposure during gestation and lactation, female F1 rats had increased serum total testosterone (TT), decreased 5α-dihydrotestosterone (DHT)/TT ratios, and increased 17β-estradiol (E2)/TT ratios, while male F1 rats had decreased serum DHT and increased plasma thyroid stimulating hormone (TSH) 93. A 12-week study on male albino rats orally exposed to Roundup® also found changes in hormone levels (13% decrease in testosterone, 17% decrease in follicle stimulating hormone, approximately 14% decrease in luteinizing hormone, and approximately 31% increase in prolactin) at doses as low as 3.6 mg/kg/day 139.
However, in an exposure study that exposed male Sprague-Dawley rats to either glyphosate (2.5 mg/kg/day or 25 mg/kg/day) or the glyphosate formulation Glyfonova (25 mg/kg/day) via their drinking water, no changes in intratesticular testosterone levels were observed 140. While the rats exposed to Glyfonova showed a statistically significant upregulation in P450 encoding genes in the testes, the authors concluded this was not indicative of endocrine effects 140. Nevertheless, other studies reported changes in gene and protein expression attributed to glyphosate exposures 138, 99, 141.
Immunological effects
There was no evidence of treatment-related effects on spleen or thymus of mice administered glyphosate in their diet for 28 days at estimated doses as high as 1,447.5 mg/kg/day and no evidence of treatment-related effects on splenic anti-sheep red blood cell (anti-SRBC) anti-body forming cell (AFC) responses to sheep red blood cell (SRBC) 91. The Environmental Protection Agency 125 reported significantly increased incidences of lymphocytic hyperplasia in the thymus from female rats administered glyphosate technical in the diet for up to 26 months at doses of 3.37, 11.22, and 34.02 mg/kg/day (13/32, 18/37, and 17/34, respectively, versus 5/25 controls). However, the Environmental Protection Agency 125 did not consider the lesion to be compound-related because the lesion occurs spontaneously in older rats and is quite variable in the thymus, there was no apparent effect on lymphocytes in the spleen (a much less variable indicator for lymphocytic hyperplasia), and the severity of the lesion was similar among controls and glyphosate-treated groups. Kumar et al. 116 reported an inflammatory respiratory response (evidenced by increased eosinophil and neutrophil counts, mast cell degranulation, and production of interleukin-33, thymic stromal lymphopoietin, interleukin-13, and interleukin-5) in anesthetized mice exposed intranasally to glyphosate.
Neurological effects
Several animal studies in rats and mice have evaluated the neurological effects of glyphosate. Mice exposed once to 250 mg/kg/day Roundup via gavage showed a decrease in aversive memory performance 142. However, no neurological effects were seen in mice given ≤500 mg/kg/day once by oral gavage 94, 119. At 6-12 weeks of daily exposure, mice showed behavioral changes in locomotor activity, increase of anxiety and depression-like behavior levels, decreased memory performance and decreased grooming time with 250 mg/kg/day RoundUp exposure 94, 119, 142. The observed neurobehavioral changes were attributed to neurodevelopmental impairment as evidenced by a reduction in serotonin (5-HT) immunoreactivity following 6 or 12 weeks of oral exposure to 250 or 500 mg/kg/day RoundUp and a reduction in tyrosine hydroxylase (TH) immunoreactivity following 12 weeks of exposure to 250 or 500 mg/kg/day RoundUp 94.
Ait Bali et al. 142, measured acetylcholinesterase activity in rat whole brain, prefrontal cortex and hippocampus, which was inhibited at ≥250 mg/kg/day by >25%, >35%, >25%, respectively. Inhibition in all measured parts was dose-dependent142. In pregnant rats, glutathione was decreased by 16-26% following exposure to 500 mg/kg/day RoundUp on days 1 to 7 of pregnancy 95. In male offspring exposed in utero, during lactation then from post-natal day 21 to 60 to 70 mg/kg/day RoundUp, there was a 23% inhibition of hippocampus cholinesterase after post-natal day 60 96. Oxidative damage, astrocyte dysfunction, glutamate excitotoxicity, and misregulation of cholinergic transmission were also seen 143.
In rats treated via the diet for 13 weeks at doses as high as 1,547–1,631 mg/kg/day and in rats administered glyphosate technical once by gavage at up to 2,000 mg/kg, there was no evidence of treatment-related neurotoxicity seen by functional observational battery, motor activity testing, and gross and histopathologic examination of brain and peripheral nervous tissue 122. However, clinical signs included decreased activity, subdued behavior, and hunched posture.
Glyphosatewas observed to affect monoaminergic neurotransmitters in the central nervous system. In rats administered glyphosate orally up to 800 mg/kg bw/day for 6 days, serotonin neurotransmitter levels were significantly decreased in a dose dependent manner at 75, 150 and 800 mg/kg body weight in various regions of the brain including the striatum, hippocampus, prefrontal cortex, hypothalamus and midbrain region 144. Similarly, dopamine and norepinephrine levels were reported to decrease with increasing concentrations of glyphosate starting at 75 mg/kg body weight per day. Turnover rates of the neurotransmitters were measured, and their metabolites were altered; there was a significant increase in turnover between serotonin and dopamine, and a decrease in turnover with norepinephrine 144.
Rats orally exposed to 5 mg/kg/day of glyphosate or glyphosate-based formulation perinatally from day 9 gestation to day 22 post-natal were found to have increased expression of synaptophysin a marker of synaptic terminals in the hippocampus of both groups 145. Maternal behavior was also observed; at day 1 post-natal, dams were observed to spend less time licking and grooming offspring whereas between day 2 and 6 post-natal, more time was spent with offspring.
Test tube studies have also examined glyphosate and glyphosate-based herbicides for neurotoxicity. Glyphosate and an herbicide containing glyphosate isopropylamine as its active ingredient were tested in vitro at concentrations of 0.005% to 0.0005%. Although no effect was observed for pure glyphosate, glyphosate-based herbicides were reported to interfere with myelination and also as a demyelinating agent in a dose-dependent manner 146. However, after testing for demyelination using glyphosate and isopropylamine additively (rather than as formulated), the authors note that this effect may be due to undisclosed additives 146. Neither glyphosate (pure) nor glyphosate-based herbicide were found to impair neurite development 146.
Glyphosate and cancer
Animal and human studies were evaluated by regulatory agencies in the USA, Canada, Japan, Australia, and the European Union, as well as the Joint Meeting on Pesticide Residues of the United Nations and World Health Organization (WHO). These agencies looked at cancer rates in humans and studies where laboratory animals were fed high doses of glyphosate. Based on these studies, they determined that glyphosate is not likely to be carcinogenic 147, 148, 149, 150, 136, 151, 152, 153, 154. However, in 2015, a committee of scientists working for the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) classified glyphosate as “probably carcinogenic to humans” (Group 2A) 34, 35. According to the International Agency for Research on Cancer (IARC) there was “limited evidence of carcinogenicity in humans for non-Hodgkin lymphoma” 34. The evidence in humans is from studies of exposures, mostly agricultural, in the USA, Canada, and Sweden published since 2001 36, 37, 38, 39. In addition, there is convincing evidence that glyphosate also can cause cancer in laboratory animals as well as strong evidence that exposure to glyphosate is genotoxic and can induce oxidative stress in experimental animals and in humans in vitro 34. “Limited evidence” means that a positive association has been observed between exposure to glyphosate and cancer but that other explanations for the observations called chance, bias, or confounding could not be ruled out. Group 2A category is also used when there is limited evidence of carcinogenicity in humans and strong data on how the agent causes cancer 34.
Glyphosate also caused DNA and chromosomal damage in human cells, although it gave negative results in tests using bacteria. One study in community residents reported increases in blood markers of chromosomal damage (micronuclei) after glyphosate formulations were sprayed nearby 34. The International Agency for Research on Cancer (IARC) also concluded that there is strong evidence for glyphosate-induced oxidative stress based on results from studies of animal models in vivo and human cells in vitro 155.
In 2005, an evaluation of glyphosate and cancer risk was conducted in the Agricultural Health Study 40. This evaluation considered glyphosate use reported at enrollment (1993–1997), and included 2088 cancers diagnosed between enrollment and 2001. No statistically significant associations were found for any cancer sites, including non-Hodgkin lymphoma, but there was an increased risk, though not statistically significant, of multiple myeloma in the highest exposure category based on a small number of cases 40.
In this large 2017 prospective cohort study 41, no association was apparent between glyphosate and any solid tumors or lymphoid malignancies overall, including non-Hodgkin lymphoma and its subtypes. There was some evidence of increased risk of acute myeloid leukemia (AML) among the highest glyphosate exposed group that requires confirmation 41.
Glyphosate and death
Several case report have reported deaths in individuals intentionally ingesting glyphosate products 156, 157, 158, 159, 160, 161, 162. The main cause of death from glyphosate ingestion was shock (hypovolemic or cardiogenic), low blood pressure (hypotension) and respiratory failure often due to aspiration 156, 157, 158, 161. Among 107 people who ingested glyphosate isopropylamine salt, eleven fatalities were reported (10.3% rate) while none were observed in the glyphosate ammonium salt group, for which there were 40 patients 158. A retrospective cohort study reported that 14 of 150 patients with glyphosate surfactant herbicide poisoning died and that non-surviving patients were statistically older compared to surviving patients 163.
LD50 also called “median lethal dose” and refers to the amount of a substance that is lethal to half of a group of test animals. LD50 is a way to measure how toxic a material is in the short term, or its acute toxicity. An acute oral glyphosate LD50 value of 4,320 mg/kg/day was reported following single oral dosing of rats with glyphosate 164. In a developmental toxicity study, 6/25 pregnant rats died during oral dosing of glyphosate at 3,500 mg/kg/day; there were no deaths during treatment at 1,000 mg/kg/day 101. No adequate sources were located regarding death in laboratory animals exposed to glyphosate by inhalation or dermal routes 53.
In a study of female Wistar rats exposed to 126 or 315 mg/kg/day of a glyphosate for 60 days, 6/12 rats died 97. In a study that employed oral dosing of pregnant rats with Roundup, 8/15 dams died during the first 8 days of treatment at 1,000 mg/kg/day glyphosate 107. No deaths occurred in a 4-week study of rats intermittently exposed to Roundup at exposure levels as high as 360 mg/m3 (approximately 36 mg Roundup/m³) 111. No adequate sources were located regarding death in laboratory animals exposed to glyphosate formulations by the skin route.
Glyphosate and respiratory effects
The Agricultural Health Study participants have examined the possible associations between use of glyphosate-containing products and increased risk of rhinitis, wheezing, atopic asthma, allergic asthma, or chronic bronchitis 56, 58, 57, 165, 60, 61, 63, 64. No associations were found for diagnosed chronic bronchitis 165 or for wheezing after adjusting for confounding exposure to other pesticides 56, 58, 57. Current rhinitis was associated with glyphosate use among commercial applicators 63 and farmers 64, but no relationship between risk and the number of days of use per year was found among the commercial applicators 63. The relationship seen in commercial applicators was limited to applicators that also reported using 2,4- dichlorophenoxyacetic acid (2,4-D) herbicide. Applicators using 2,4- dichlorophenoxyacetic acid (2,4-D) herbicide or glyphosate alone did not show an increased risk of rhinitis 63. An association between glyphosate use and the risk of atopic asthma was found among farm women, but there was no association with nonatopic asthma 60. No associations were found between glyphosate use by male farmers and risk of allergic or nonallergic asthma 61. An association between glyphosate use and the risk of both allergic and nonallergic wheeze was found among male farmers, with an increase in risk for allergic wheeze with increasing days of use per year 62. It is noted that many of these studies did not account for use of other pesticides 53.
A study by Camacho and Mejia 70 investigated the association between aerial applications of glyphosate in Colombia and health effects of individuals living in the sprayed areas. The association was assessed using individual medical record data and information about Colombia’s aerial spraying program. Several health outcomes including respiratory illnesses was examined and a positive association was observed. For each additional square kilometer increase in area sprayed with glyphosate there was an increase in the proportion of respiratory diagnoses 7 to 15 days following exposure 70.
Respiratory failure or distress was reported in about 10–25% of the cases of intentional ingestion of glyphosate products 166, 167, 168, 162. Cho et al. 163 found that the most common complication occurring in patients who had ingested glyphosate surfactant herbicide was acute respiratory distress syndrome (ARDS).
Glyphosate and cardiovascular effects
Two studies of Agricultural Health Study participants did not find associations between the use of glyphosate-containing products and the risk of myocardial infarctions (Dayton et al. 2010; Mills et al. 2009); see Table 2-5 for details. An association was found between using a glyphosate-based herbicide and vasculitic neuropathy in a 70 year old man who sprayed approximately 2,000 mL of the herbicide for several hours without using protective gear 4 months before presenting with symptoms (Kawagashira et al. 2017). In case series reports, abnormal electrocardiogram (EKG) readings have been found in patients ingesting large doses of glyphosate-containing products (Kim et al. 2014; Lee et al. 2000, 2008; Moon and Chun 2010; Moon et al. 2018; Talbot et al. 1991). The most commonly reported alterations included prolonged QTc interval and sinus tachycardia. In the most severe poisoning cases, hypotension and shock have been reported (Picetti et al. 2018; Roberts et al. 2010; Sawada et al. 1988; Tominack et al. 1991). Additionally, adverse cardiovascular events (myocardial injury, shock, ventricular dysrhythmia, or cardiac arrest) have been reported among patients who ingested glyphosate (Moon et al. 2018).
No data were available regarding evaluation of cardiovascular endpoints in laboratory animals exposed to glyphosate technical or glyphosate formulations by any exposure route.
Glyphosate and gastrointestinal effects
Gastrointestinal symptoms are commonly reported in case series reports of patients who ingested glyphosate products 53. In numerous reports, over 40% of the patients reported nausea/vomiting 169, 170, 167, 159, 171. Other gastrointestinal effects reported included abdominal pain 166, 159, 160, 168, 161, sore throat 168, 162, poor appetite 170 and damage to mucosal tissue in the mouth and esophagus 167, 160, 172, 161, 162. One case study reported gastric ulcer and a large pyloric antrum ulcer 170. In a woman who accidentally ingested glyphosate-surfactant herbicide, a CT scan showed aspiration pneumonitis and ileus of the intestine 173.
Glyphosate and hematological effects
After the accidental ingestion of 100 mL of glyphosate-surfactant herbicide a woman exhibited hypoxemia, hyperkalemia, hypotension, and hemoconcentration 173.
Glyphosate and liver effects
Human studies evaluating the relationship between glyphosate and liver endpoints is limited 53. One retrospective cohort study reported acute liver failure as a complication associated with organ injury 163. In a case-control study evaluating the association between glyphosate and patients with nonalcoholic steatohepatitis (NASH), Mills et al. 69 reported significantly higher levels of glyphosate residue in nonalcoholic steatohepatitis (NASH) patients compared to non-NASH patients. Furthermore, a dose-dependent increase of glyphosate exposure was observed with advanced stages of fibrosis (stage 2, 3 or 4). No other information was located regarding liver effects in humans exposed to glyphosate-containing products 53.
Glyphosate and kidney effects
Available human data regarding the possible association between exposure to glyphosate and risk of kidney effects is limited to a few case-control studies, two case reports and one retrospective cohort study 53. One case-control study of patients with chronic kidney disease (CKD) found an increased risk of chronic kidney disease among glyphosate applicators 174. However, uncertainty regarding an association between exposure to glyphosate and risk of chronic kidney disease includes the finding that the glyphosate applicators were also exposed to high levels of calcium, magnesium, barium, strontium, iron, titanium, and vanadium by drinking water from abandoned wells 53. In the case of a 55 year old man who ingested 200 mL of a glyphosate mixture, acute renal failure occurred 167. Acute kidney injury and metabolic acidosis occurred in a woman who accidentally ingested glyphosate-surfactant herbicide 173. Acute kidney injury associated with glyphosate-based herbicide ingestion was also reported in a retrospective cohort study as a complication associated with organ injury 163.
Glyphosate and rheumatoid arthritis
De Roos et al. 67 did not find an association between glyphosate use and the risk of rheumatoid arthritis among participants of the Agricultural Health Study. In a subsequent study of female spouses of licensed pesticide applicators, Parks et al. 68 reported a weakly positive association between spousal use of glyphosate and risk of rheumatoid arthritis (based on incident cases).
Glyphosate and skin effects
One woman developed skin lesions after accidental dermal exposure to glyphosate in an herbicide and was diagnosed with toxic hand dermatitis 175. After treatment did not fully resolve the lesions, she was diagnosed with koebnerization of psoriasis caused by acute irritant contact dermatitis. According to the Environmental Protection Agency 176, glyphosate is considered a slight skin irritant following acute skin application.
In another study, Camacho and Mejia 70 evaluated the association between aerial applications of glyphosate in Colombia and health effects of individuals living in the sprayed areas. The association was assessed using individual medical record data and information about Colombia’s aerial spraying program. Several health outcomes including dermatological effects. Their results observed that for each additional square kilometer increase in area sprayed with glyphosate there was an increase in the proportion of skin disorders 7 to 15 days following exposure 70.
One study evaluated the potential skin toxicity of glyphosate in humans. In an experimental study 71, a single application of Roundup to intact skin for 24 hours did not result in irritation. When applied to abraded skin, redness was noted in 42% of the subjects after 24 hours. Mild skin irritation was observed in a repeated glyphosate exposure test study 71. No skin irritation was observed in a Draize skin sensitization test or in a photosensitivity/photoirritation test 71.
Glyphosate and eye effects
In a study of wives of commercial pesticide applicators, no association was found between glyphosate use among the wives and retinal degeneration 72. In a case series report of 1,513 eye exposures to glyphosate, minor symptoms (primarily transient irritation) were observed in 70% of the cases; most (99%) complained of eye pain 177. Moderate effects, such as persistent irritation or low-grade corneal burns or abrasions, were observed in about 2% of the cases 177. Among the cases with moderate effects, 93% reported eye pain, 20% reported lacrimation, and 27% reported blurred vision 177. According to the Environmental Protection Agency 176, glyphosate is considered mildly irritating to the eye following ocular instillation. The European Food Safety Authority 149 stated that glyphosate acid was a severe eye irritant, but that salts of glyphosate do not require classification as eye irritants.
Glyphosate and endocrine effects
Available human information regarding possible associations between exposure to glyphosate or glyphosate-containing products and risk of endocrinological effects is limited to 3 studies with conflicting results 53. Goldner et al. 73 reported no associations between any glyphosate exposure and the risks of thyroid diseases. Shrestha et al. 75 found a significant association between ever-use of glyphosate and hypothyroidism (underactive thyroid) (hazard ratio = 1.28). Kongtip et al. 74 reported a positive association between increasing use of glyphosate and increased T4 thyroid hormone levels.
To evaluate the potential for glyphosate to affect the endocrine system, the Environmental Protection Agency 178 subjected glyphosate to the Endocrine Disruptor Screening Program Tier 1 (a battery of in vitro assays designed assist in evaluation of the potential for a substance to interact with estrogen, androgen, or thyroid signaling pathways). Environmental Protection Agency evaluated results from the battery of in vitro assays and relevant laboratory mammalian and wildlife studies. Using this approach, EPA determined that there is no convincing evidence of potential interaction between glyphosate and estrogen, androgen, or thyroid pathways in mammals or wildlife 178. Included in the evaluation of the estrogen pathway were estrogen receptor (ER) binding assays, an ER transactivation assay, aromatase and steroidogenesis assays, a fish short-term reproduction assay, and mammalian and wildlife studies that assessed female reproductive parameters. Included in the evaluation of the androgen pathway were androgen receptor (AR) binding and steroidogenesis assays, a fish short-term reproduction assay, Hershberger and male pubertal assays, an androgen receptor (AR) transactivation assay, and mammalian and wildlife studies that assessed male reproductive parameters. Included in the evaluation of the thyroid pathway were male and female pubertal assays, an amphibian metamorphosis assay, and mammalian and wildlife studies that assessed thyroid parameters 178, 179.
Glyphosate and reproductive effects
No association between glyphosate use and fecundability (the likelihood of becoming pregnant during a menstrual cycle or within a month) was found among women living at farms in which pesticides were used and were involved in pesticide activities 180. This study also reported an association with improved fecundability when the women were not involved in pesticide activities 180. Sanin et al. 181 examined the fertility of women living in regions of Columbia with varying agricultural usage of glyphosate. Although time to pregnancy varied widely by region, no significant associations were found with level of glyphosate usage.
In another study, Camacho and Mejia 70 evaluated the association between aerial applications of glyphosate in Colombia and miscarriages by women living in the sprayed areas. Their results observed a positive association. For each additional square kilometer increase in area sprayed with glyphosate there was an increase in the number of miscarriage diagnoses. However, the authors note the way miscarriage was defined in the study may overestimate the number of actual miscarriages 70. In the study, miscarriage was defined when a mother was observed to have attended a prenatal care visit, but a corresponding birth registry was not located after 10 months 70.
Glyphosate and neurological effects
Available information regarding possible associations between exposure to glyphosate-containing products and risk of neurological effects in humans is limited. One case-control study did not find an association between glyphosate exposure and Parkinson’s disease 76. In a cross-sectional study conducted on farmers in China, Zhang et al. 77 reported glyphosate use was not associated with increased odds or incidence rates of abnormalities of peripheral nerve conduction.
One case study found that a man who ingested 200 ml of glyphosate developed Parkinson’s symptoms 4 years after exposure 169. A spatial analysis of exposure to pesticides in Washington State found an association between glyphosate exposure and increased odds of premature mortality attributable to Parkinson’s disease (odds ratio = 1.33) 182.
Test tube studies have also examined glyphosate and glyphosate-based herbicides for neurotoxicity. Glyphosate and an herbicide containing glyphosate isopropylamine as its active ingredient were tested in test tube at concentrations of 0.005% to 0.0005%. Although no effect was observed for pure glyphosate, glyphosate-based herbicides were reported to interfere with myelination and also as a demyelinating agent in a dose-dependent manner 146. However, after testing for demyelination using glyphosate and isopropylamine additively (rather than as formulated), the authors note that this effect may be due to undisclosed additives 146. Neither glyphosate (pure) nor glyphosate-based herbicide were found to impair neurite development 146.
Glyphosate and developmental effects
Several epidemiology studies have examined possible associations between glyphosate use and developmental toxicity. Given that only one study examined each endpoint and the lack of quantification of glyphosate exposure across studies, these results were not considered sufficient for drawing conclusions on the risk of developmental toxicity associated with glyphosate exposure in humans 53. Arbuckle et al. 79 reported a positive association between maternal preconception exposure to glyphosate and increased risk of spontaneous abortion (miscarriage). Garry et al. 81 reported a positive association between phosphonamino- herbicides (glyphosate, Roundup) exposure and parent-reported attention deficit disorder/attention deficit hyperactivity disorder (ADD/ADHD). No associations were found between paternal exposure and risk of miscarriages, preterm delivery, small for gestational age risk 85, or congenital malformations 80. Similarly, no associations were found between maternal glyphosate exposure and birth weight 84, 82, neural tube defects 83 or head circumference 82.
- What is glyphosate? https://www.apvma.gov.au/resources/chemicals-news/glyphosate/glyphosate-australia[↩]
- Richmond, M. E. Glyphosate: A review of its global use, environmental impact, and potential health effects on humans and other species. J. Environ. Stud. Sci.8, 416–434 (2018).[↩]
- Clapp, J. Explaining growing glyphosate use: The political economy of herbicide-dependent agriculture. Glob. Environ. Change67, 102239 (2021).[↩]
- Araujo, L. G., Zordan, D. F., Celzard, A. & Fierro, V. Glyphosate uses, adverse effects and alternatives: focus on the current scenario in Brazil. Environ. Geochem. Health45, 9559–9582 (2023).[↩]
- Tauhata, S. B. F. et al. The glyphosate controversy: An update. Arq. Inst. Biol. (Sao. Paulo)87, 1–8 (2020).[↩]
- Kraus EC, Stout MJ. Direct and Indirect Effects of Herbicides on Insect Herbivores in Rice, Oryza sativa. Sci Rep. 2019 May 6;9(1):6998. doi: 10.1038/s41598-019-43361-w[↩]
- Gomes MP, Smedbol E, Chalifour A, Hénault-Ethier L, Labrecque M, Lepage L, Lucotte M, Juneau P. Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: an overview. J Exp Bot. 2014 Sep;65(17):4691-703. doi: 10.1093/jxb/eru269.[↩]
- Franz J Mao M Sikorski J . 1997. Glyphosate: a unique and global herbicide. American Chemical Society Monograph 189: Washington DC.[↩][↩]
- Duke SO, Powles SB. Glyphosate: a once-in-a-century herbicide. Pest Manag Sci. 2008 Apr;64(4):319-25. doi: 10.1002/ps.1518[↩]
- Cobb A . 1992. Herbicides and plant physiology. London: Chapman and Hall.[↩]
- Glyphosate. http://npic.orst.edu/factsheets/glyphogen.html[↩][↩][↩][↩]
- Xu J, et al. Glyphosate contamination in grains and foods: an overview. Food Control; 2019. p. 106710.[↩]
- Borggaard OK Gimsing AL . 2008. Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Management Science456, 441–456.[↩]
- Pompermaier A, Alves C, Chagas FB, Tamagno WA, Bridi C, Ferreira GF, Hartmann PA, Hartmann M. Effects of glyphosate based herbicide exposure in early developmental stages of Physalaemus gracilis. Sci Rep. 2024 Oct 27;14(1):25652. doi: 10.1038/s41598-024-76338-5[↩]
- Grunewald, K., Schmidt, W., Unger, C. & Hanschmann, G. Behavior of glyphosate and aminomethylphosphonic acid (AMPA) in soils and water of reservoir Radeburg II catchment (Saxony/Germany). J. plant Nutr. soil Sci.164, 65–70 (2001).[↩]
- Van Eerd LL Hoagland RE Zablotowicz RM Hall JC . 2003. Pesticide metabolism in plants and microorganisms. Weed Science51, 472–495.[↩]
- Reddy KN Rimando AM Duke SO . 2004. Aminomethylphosphonic acid, a metabolite of glyphosate, causes injury in glyphosate-treated, glyphosate-resistant soybean. Journal of Agricultural and Food Chemistry52, 5139–5143.[↩][↩]
- Satchivi NM Wax LM Stoller EW Briskin DP . 2000. Absorption and translocation of glyphosate isopropylamine and trimethylsulfonium salts in Abutilon theophrasti and Setaria faberi. Weed Science48, 675–679.[↩]
- Monquero PA Christoffoleti PJ Osuna MD De Prado RA . 2004. Absorção, translocação e metabolismo do glyphosate por plantas tolerantes e suscetíveis a este herbicida. Planta Daninha22, 445–451.[↩]
- Hetherington PR Reynolds TL Marshall G Kirkwood RC . 1999. The absorption, translocation and distribution of the herbicide glyphosate in maize expressing the CP-4 transgene. Journal of Experimental Botany50, 1567–1576.[↩]
- Feng PCC Chiu T Douglas Sammons R . 2003. Glyphosate efficacy is contributed by its tissue concentration and sensitivity in velvetleaf (Abutilon theophrasti). Pesticide Biochemistry and Physiology77, 83–91.[↩]
- Viirlaid E, Ilisson M, Kopanchuk S, Mäeorg U, Rinken A, Rinken T. Immunoassay for rapid on-site detection of glyphosate herbicide. Environ Monit Assess. 2019 Jul 24;191(8):507. doi: 10.1007/s10661-019-7657-z[↩]
- Siehl D . 1997. Inhibitors of EPSPS synthase, glutamine synthetase and histidine synthesis. In: Roe R Burton J Kuhr R, eds. Herbicide activity: toxicology, biochemistry and molecular biology. Amsterdam: IOS Press, 37–67.[↩]
- Marcelo P. Gomes, Elise Smedbol, Annie Chalifour, Louise Hénault-Ethier, Michel Labrecque, Laurent Lepage, Marc Lucotte, Philippe Juneau, Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: an overview, Journal of Experimental Botany, Volume 65, Issue 17, September 2014, Pages 4691–4703, https://doi.org/10.1093/jxb/eru269[↩][↩][↩][↩][↩]
- Using Glyphosate. https://cdn.environment.sa.gov.au/landscape/docs/hf/responsible-chemical-use-using-glyphosate-fact.pdf[↩]
- Muller F, Applebyke AP. 2010. Weed control, 2. Individual herbicides. In: Ullmann’s encyclopedia of industrial chemistry. Wiley VCH, Verlag GmbH & Co. KGaA. 10.1002/14356007.o28_o01[↩]
- Plimmer JR, Bradow JM, Dionigi CP, et al. 2004. Herbicides. In: Kirk-Othmer encyclopedia of chemical technology. John Wiley & Sons, Inc. 10.1002/0471238961.0805180202180104.a01.pub2[↩]
- Dow. 2017. Rodeo herbicide. Specimen label revised 01-05-17. Dow Chemical Company, Dow Agrosciences.[↩]
- PAN. 2016a. PAN pesticides database-California pesticide use. Oakland, CA: Pesticide Action Network, North America. http://www.pesticideinfo.org/Detail_ChemUse.jsp?Rec_Id=PC33138[↩]
- PAN. 2016b. PAN pesticides database-Pesticide products. Oakland, CA: Pesticide Action Network, North America. http://www.pesticideinfo.org/List_Products.jsp?Rec_Id=PC33138&Chem_Name=Glyphosate&PC_Code=417300,%20471300[↩]
- Kováčik J, Novotný V, Bujdoš M, Dresler S, Hladký J, Babula P. Glyphosate does not show higher phytotoxicity than cadmium: Cross talk and metabolic changes in common herb. J Hazard Mater. 2020 Feb 5;383:121250. doi: 10.1016/j.jhazmat.2019.121250[↩]
- ECHA (European Chemicals Agency), 2022. Committee for Risk Assessment (RAC) Opinion proposing harmonised classification and labelling at EU level of glyphosate (ISO); N-(phosphonomethyl)glycine. CLH-O-0000007122-85-01/F[↩]
- Álvarez, F., Arena, M., Auteri, D., Binaglia, M., Castoldi, A. F., Chiusolo, A., Crivellente, F., Egsmose, M., Fait, G., Ferilli, F., Gouliarmou, V., Nogareda, L. H., Ippolito, A., Istace, F., Jarrah, S., Kardassi, D., Kienzler, A., Lanzoni, A., … Villamar-Bouza, L. (2023). Peer review of the pesticide risk assessment of the active substance glyphosate. EFSA Journal, 21(7), 1–52. http://doi.org.10.2903/j.efsa.2023.8164[↩][↩][↩][↩]
- IARC Monographs Volume 112: evaluation of five organophosphate insecticides and herbicides. https://www.iarc.who.int/wp-content/uploads/2018/07/MonographVolume112-1.pdf[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- IARC Monograph on Glyphosate. https://www.iarc.who.int/featured-news/media-centre-iarc-news-glyphosate/[↩][↩]
- De Roos AJ, Zahm SH, Cantor KP, Weisenburger DD, Holmes FF, Burmeister LF, Blair A. Integrative assessment of multiple pesticides as risk factors for non-Hodgkin’s lymphoma among men. Occup Environ Med. 2003 Sep;60(9):E11. https://pmc.ncbi.nlm.nih.gov/articles/instance/1740618/pdf/v060p00e11.pdf[↩][↩]
- Hardell L, Eriksson M. A case-control study of non-Hodgkin lymphoma and exposure to pesticides. Cancer. 1999 Mar 15;85(6):1353-60. doi: 10.1002/(sici)1097-0142(19990315)85:6<1353::aid-cncr19>3.0.co;2-1[↩][↩]
- Hardell L, Eriksson M, Nordstrom M. Exposure to pesticides as risk factor for non-Hodgkin’s lymphoma and hairy cell leukemia: pooled analysis of two Swedish case-control studies. Leuk Lymphoma. 2002 May;43(5):1043-9. doi: 10.1080/10428190290021560[↩][↩]
- McDuffie HH, Pahwa P, McLaughlin JR, Spinelli JJ, Fincham S, Dosman JA, Robson D, Skinnider LF, Choi NW. Non-Hodgkin’s lymphoma and specific pesticide exposures in men: cross-Canada study of pesticides and health. Cancer Epidemiol Biomarkers Prev. 2001 Nov;10(11):1155-63.[↩][↩]
- De Roos AJ, Blair A, Rusiecki JA, Hoppin JA, Svec M, Dosemeci M, Sandler DP, Alavanja MC. Cancer incidence among glyphosate-exposed pesticide applicators in the Agricultural Health Study. Environ Health Perspect. 2005 Jan;113(1):49-54. doi: 10.1289/ehp.7340[↩][↩][↩][↩][↩]
- Andreotti G, Koutros S, Hofmann JN, Sandler DP, Lubin JH, Lynch CF, Lerro CC, De Roos AJ, Parks CG, Alavanja MC, Silverman DT, Beane Freeman LE. Glyphosate Use and Cancer Incidence in the Agricultural Health Study. J Natl Cancer Inst. 2018 May 1;110(5):509-516. doi: 10.1093/jnci/djx233[↩][↩][↩][↩][↩]
- Engel LS, Hill DA, Hoppin JA, Lubin JH, Lynch CF, Pierce J, Samanic C, Sandler DP, Blair A, Alavanja MC. Pesticide use and breast cancer risk among farmers’ wives in the agricultural health study. Am J Epidemiol. 2005 Jan 15;161(2):121-35. doi: 10.1093/aje/kwi022[↩]
- Flower KB, Hoppin JA, Lynch CF, Blair A, Knott C, Shore DL, Sandler DP. Cancer risk and parental pesticide application in children of Agricultural Health Study participants. Environ Health Perspect. 2004 Apr;112(5):631-5. doi: 10.1289/ehp.6586[↩]
- Koutros S, Beane Freeman LE, Lubin JH, Heltshe SL, Andreotti G, Barry KH, DellaValle CT, Hoppin JA, Sandler DP, Lynch CF, Blair A, Alavanja MC. Risk of total and aggressive prostate cancer and pesticide use in the Agricultural Health Study. Am J Epidemiol. 2013 Jan 1;177(1):59-74. doi: 10.1093/aje/kws225[↩]
- Koutros S, Silverman DT, Alavanja MC, Andreotti G, Lerro CC, Heltshe S, Lynch CF, Sandler DP, Blair A, Beane Freeman LE. Occupational exposure to pesticides and bladder cancer risk. Int J Epidemiol. 2016 Jun;45(3):792-805. doi: 10.1093/ije/dyv195[↩]
- Lee WJ, Sandler DP, Blair A, Samanic C, Cross AJ, Alavanja MC. Pesticide use and colorectal cancer risk in the Agricultural Health Study. Int J Cancer. 2007 Jul 15;121(2):339-46. doi: 10.1002/ijc.22635[↩]
- Andreotti G, Freeman LE, Hou L, Coble J, Rusiecki J, Hoppin JA, Silverman DT, Alavanja MC. Agricultural pesticide use and pancreatic cancer risk in the Agricultural Health Study Cohort. Int J Cancer. 2009 May 15;124(10):2495-500. doi: 10.1002/ijc.24185[↩]
- Band PR, Abanto Z, Bert J, Lang B, Fang R, Gallagher RP, Le ND. Prostate cancer risk and exposure to pesticides in British Columbia farmers. Prostate. 2011 Feb 1;71(2):168-83. doi: 10.1002/pros.21232[↩]
- Lee WJ, Lijinsky W, Heineman EF, Markin RS, Weisenburger DD, Ward MH. Agricultural pesticide use and adenocarcinomas of the stomach and oesophagus. Occup Environ Med. 2004 Sep;61(9):743-9. doi: 10.1136/oem.2003.011858[↩]
- Lee WJ, Colt JS, Heineman EF, McComb R, Weisenburger DD, Lijinsky W, Ward MH. Agricultural pesticide use and risk of glioma in Nebraska, United States. Occup Environ Med. 2005 Nov;62(11):786-92. doi: 10.1136/oem.2005.020230[↩]
- Pahwa P, Karunanayake CP, Dosman JA, Spinelli JJ, McLaughlin JR; Cross-Canada Group. Soft-tissue sarcoma and pesticides exposure in men: results of a Canadian case-control study. J Occup Environ Med. 2011 Nov;53(11):1279-86. doi: 10.1097/JOM.0b013e3182307845[↩]
- Yiin JH, Ruder AM, Stewart PA, Waters MA, Carreón T, Butler MA, Calvert GM, Davis-King KE, Schulte PA, Mandel JS, Morton RF, Reding DJ, Rosenman KD; Brain Cancer Collaborative Study Group. The Upper Midwest Health Study: a case-control study of pesticide applicators and risk of glioma. Environ Health. 2012 Jun 12;11:39. doi: 10.1186/1476-069X-11-39[↩]
- Toxicological Profile for Glyphosate. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2020 Aug. CHAPTER 2, HEALTH EFFECTS. Available from: https://www.ncbi.nlm.nih.gov/books/NBK591692[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Cho YS, Moon JM, Chun BJ, Lee BK. Use of qSOFA Score in Predicting the Outcomes of Patients With Glyphosate Surfactant Herbicide Poisoning Immediately Upon Arrival at the Emergency Department. Shock. 2019 Apr;51(4):447-452. doi: 10.1097/SHK.0000000000001201[↩]
- Camacho A, Mejía D. The health consequences of aerial spraying illicit crops: The case of Colombia. J Health Econ. 2017 Jul;54:147-160. doi: 10.1016/j.jhealeco.2017.04.005[↩]
- Hoppin JA, Umbach DM, London SJ, et al. 2002. Chemical predictors of wheeze among farmer pesticide applicators in the Agricultural Health Study. Am J Respir Crit Care Med 165(5):683–689. 10.1164/ajrccm.165.5.2106074[↩][↩][↩]
- Hoppin JA, Umbach DM, London SJ, Lynch CF, Alavanja MC, Sandler DP. Pesticides and adult respiratory outcomes in the agricultural health study. Ann N Y Acad Sci. 2006 Sep;1076:343-54. doi: 10.1196/annals.1371.044[↩][↩][↩]
- Hoppin JA, Umbach DM, London SJ, et al. 2006b. Pesticides associated with wheeze among commercial pesticide applicators in the Agricultural Health Study. Am J Epidemiol 163(12):1129–1137. 10.1093/aje/kwj138[↩][↩][↩]
- Hoppin JA, Valcin M, Henneberger PK, Kullman GJ, Umbach DM, London SJ, Alavanja MC, Sandler DP. Pesticide use and chronic bronchitis among farmers in the Agricultural Health Study. Am J Ind Med. 2007 Dec;50(12):969-79. doi: 10.1002/ajim.20523[↩]
- Hoppin JA, Umbach DM, London SJ, et al. 2008. Pesticides and atopic and nonatopic asthma among farm women in the Agricultural Health Study. Am J Respir Crit Care Med 177(1):11–18. 10.1164/rccm.200706-821OC[↩][↩][↩]
- Hoppin JA, Umbach DM, London SJ, et al. 2009. Pesticide use and adult-onset asthma among male farmers in the Agricultural Health Study. Eur Respir J 34(6):1296–1303. 10.1183/09031936.00005509[↩][↩][↩]
- Hoppin JA, Umbach DM, Long S, et al. 2017. Pesticides are associated with allergic and non-allergic wheeze among male farmers. Environ Health Perspect 125(4):535–543. 10.1289/EHP315[↩][↩]
- Slager RE, Poole JA, LeVan TD, et al. 2009. Rhinitis associated with pesticide exposure among commercial pesticide applicators in the Agricultural Health Study. Occup Environ Med 66(11):718–724. 10.1136/oem.2008.041798[↩][↩][↩][↩][↩]
- Slager RE, Simpson SL, Levan TD, et al. 2010. Rhinitis associated with pesticide use among private pesticide applicators in the Agricultural Health Study. J Toxicol Environ Health A 73(20):1382–1393. 10.1080/15287394.2010.497443[↩][↩][↩]
- Dayton SB, Sandler DP, Blair A, et al. 2010. Pesticide use and myocardial infarction incidence among farm women in the Agricultural Health Study. J Occup Environ Med 52(7):693–697. 10.1097/JOM.0b013e3181e66d25[↩]
- Mills KT, Blair A, Freeman LE, et al. 2009. Pesticides and myocardial infarction incidence and mortality among male pesticide applicators in the Agricultural Health Study. Am J Epidemiol 170(7):892–900. 10.1093/aje/kwp214[↩]
- De Roos AJ, Cooper GS, Alavanja MC, et al. 2005b. Rheumatoid arthritis among women in the Agricultural Health Study: Risk associated with farming activities and exposures. Ann Epidemiol 15(10):762–770. 10.1016/j.annepidem.2005.08.001[↩][↩]
- Parks CG, Hoppin JA, De Roos AJ, Costenbader KH, Alavanja MC, Sandler DP. Rheumatoid Arthritis in Agricultural Health Study Spouses: Associations with Pesticides and Other Farm Exposures. Environ Health Perspect. 2016 Nov;124(11):1728-1734. doi: 10.1289/EHP129[↩][↩]
- Mills PJ, Caussy C, Loomba R. 2019. Glyphosate excretion is associated with steatohepatitis and advanced liver fibrosis in patients with fatty liver disease. Clin Gastroenterol Hepatol. 10.1016/j.cgh.2019.03.045[↩][↩]
- Camacho A, Mejia D. 2017. The health consequences of aerial spraying illicit crops: The case of Colombia. J Health Econ 54147–160. 10.1016/j.jhealeco.2017.04.005[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Maibach HI. Irritation, sensitization, photoirritation and photosensitization assays with a glyphosate herbicide. Contact Dermatitis. 1986 Sep;15(3):152-6. doi: 10.1111/j.1600-0536.1986.tb01316.x[↩][↩][↩][↩][↩][↩][↩]
- Kirrane EF, Hoppin JA, Kamel F, et al. 2005. Retinal degeneration and other eye disorders in wives of farmer pesticide applicators enrolled in the Agricultural Health Study. Am J Epidemiol 161(11):1020–1029. 10.1093/aje/kwi140[↩][↩]
- Goldner WS, Sandler DP, Yu F, et al. 2010. Pesticide use and thyroid disease among women in the Agricultural Health Study. Am J Epidemiol 171(4):455–464. 10.1093/aje/kwp404[↩][↩]
- Kongtip P, Nankongnab N, Kallayanatham N, et al. 2019. Thyroid hormones in conventional and organic farmers in Thailand. Int J Environ Res Public Health 16(15). 10.3390/ijerph16152704[↩][↩]
- Shrestha S, Parks CG, Goldner WS, et al. 2018. Pesticide use and incident hypothyroidism in pesticide applicators in the agricultural health study. Environ Health Perspect 126(9):097008. 10.1289/EHP3194[↩][↩]
- Kamel F, Tanner C, Umbach D, et al. 2007. Pesticide exposure and self-reported Parkinson’s disease in the Agricultural Health Study. Am J Epidemiol 165(4):364–374. 10.1093/aje/kwk024[↩][↩]
- Zhang C, Sun Y, Hu R, et al. 2018. A comparison of the effects of agricultural pesticide uses on peripheral nerve conduction in China. Sci Rep 8(1):9621. 10.1038/s41598-018-27713-6[↩][↩]
- Curtis KM, Savitz DA, Weinberg CR, Arbuckle TE. The effect of pesticide exposure on time to pregnancy. Epidemiology. 1999 Mar;10(2):112-7. Erratum in: Epidemiology 1999 Jul;10(4):470[↩]
- Arbuckle TE, Lin Z, Mery LS. An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environ Health Perspect. 2001 Aug;109(8):851-7. doi: 10.1289/ehp.01109851[↩][↩]
- García AM, Benavides FG, Fletcher T, Orts E. Paternal exposure to pesticides and congenital malformations. Scand J Work Environ Health. 1998 Dec;24(6):473-80. doi: 10.5271/sjweh.371[↩][↩]
- Garry VF, Harkins ME, Erickson LL, Long-Simpson LK, Holland SE, Burroughs BL. Birth defects, season of conception, and sex of children born to pesticide applicators living in the Red River Valley of Minnesota, USA. Environ Health Perspect. 2002 Jun;110 Suppl 3(Suppl 3):441-9. doi: 10.1289/ehp.02110s3441[↩][↩]
- Parvez S, Gerona RR, Proctor C, et al. 2018. Glyphosate exposure in pregnancy and shortened gestational length: a prospective Indiana birth cohort study. Environ Health 17(1):23. 10.1186/s12940-018-0367-0[↩][↩][↩]
- Rull RP, Ritz B, Shaw GM. 2006. Neural tube defects and maternal residential proximity to agricultural pesticide applications. Am J Epidemiol 163(8): 743–753. 10.1093/aje/kwj101[↩][↩]
- Sathyanarayana S, Basso O, Karr CJ, et al. 2010. Maternal pesticide use and birth weight in the Agricultural Health Study. J Agromedicine 15(2):127–136. 10.1080/10599241003622699[↩][↩]
- Savitz DA, Arbuckle T, Kaczor D, Curtis KM. Male pesticide exposure and pregnancy outcome. Am J Epidemiol. 1997 Dec 15;146(12):1025-36. doi: 10.1093/oxfordjournals.aje.a009231[↩][↩]
- Montgomery MP, Kamel F, Saldana TM, et al. 2008. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study, 1993-2003. Am J Epidemiol 167(10):1235–1246. 10.1093/aje/kwn028[↩]
- Saldana TM, Basso O, Hoppin JA, et al. 2007. Pesticide exposure and self-reported gestational diabetes mellitus in the Agricultural Health Study. Diabetes Care 30(3):529–534. 10.2337/dc06-1832[↩]
- Baier CJ, Gallegos CE, Raisman-Vozari R, et al. 2017. Behavioral impairments following repeated intranasal glyphosate-based herbicide administration in mice. Neurotoxicol Teratol 64:63–72. 10.1016/j.ntt.2017.10.004[↩][↩]
- El-Shenawy NS. 2009. Oxidative stress responses of rats exposed to Roundup and its active ingredient glyphosate. Environ Toxicol Pharmacol 28(3):379–385. 10.1016/j.etap.2009.06.001[↩][↩][↩][↩][↩][↩]
- EPA. 2013a. Memorandum. 14-Mar-2013. Memorandum: Glyphosate. Review and generation of data evaluation records. Reproduction and fertility effects study-rat OCSPP870.3800; OECD 416. MRID numbers 48865101, 48865102, 48865103, 48865104, 48865105 [Received via FOIA request]. U.S. Environmental Protection Agency.[↩][↩][↩][↩]
- EPA. 2013b. Memorandum. February 27, 2013. Memorandum: Glyphosate. Immunotoxicity study in mice. MRID 48934207 [Received via FOIA request]. U.S. Environmental Protection Agency.[↩][↩][↩]
- EPA. 2017b. Memorandum. December 13, 2017. Glyphosate: Preparation of data evaluation records for developmental rat and rabbit toxicity studies. MRID numbers 43320615, 43320616. Washington, DC: U.S. Environmental Protection Agency, Office of Chemical Safety and Pollution Prevention.[↩][↩]
- Manservisi F, Lesseur C, Panzacchi S, et al. 2019. The Ramazzini Institute 13-week pilot study glyphosate-based herbicides administered at human-equivalent dose to Sprague Dawley rats: effects on development and endocrine system. Environ Health 18(1):15. 10.1186/s12940-019-0453-y[↩][↩][↩][↩]
- Ait Bali Y, Ba-Mhamed S, Bennis M. 2017. Behavioral and immunohistochemical study of the effects of subchronic and chronic exposure to glyphosate in mice. Front Behav Neurosci 11:146. 10.3389/fnbeh.2017.00146[↩][↩][↩][↩][↩][↩][↩][↩]
- Almeida LL, Teixeira AAC, Soares AF, et al. 2017. Effects of melatonin in rats in the initial third stage of pregnancy exposed to sub-lethal doses of herbicides. Acta Histochem 119(3):220–227. 10.1016/j.acthis.2017.01.003[↩][↩][↩][↩]
- Cattani D, Cesconetto PA, Tavares MK, et al. 2017. Developmental exposure to glyphosate-based herbicide and depressive-like behavior in adult offspring: Implication of glutamate excitotoxicity and oxidative stress. Toxicology 38767–80. 10.1016/j.tox.2017.06.001[↩][↩][↩]
- Hamdaoui LN, M.; Rahmouni, F.; Harrabi, B.; Ayadi, F.; Sahnoun, Z.; Rebai, T. 2018. Subchronic exposure to kalach 360 SL-induced endocrine disruption and ovary damage in female rats. Arch Physiol Biochem 124(1):27–34. 10.1080/13813455.2017.1352606[↩][↩][↩]
- Pandey A, Dhabade P, Kumarasamy A. 2019. Inflammatory effects of subacute exposure of roundup in rat liver and adipose tissue. Dose Response 17(2):1559325819843380. 10.1177/1559325819843380[↩][↩]
- Teleken JL, Gomes ECZ, Marmentini C, et al. 2019. Glyphosate-based herbicide exposure during pregnancy and lactation malprograms the male reproductive morphofunction in F1 offspring. J Dev Orig Health Dis: 1–8. 10.1017/S2040174419000382[↩][↩][↩][↩][↩]
- Jasper R, Locatelli GO, Pilati C, et al. 2012. Evaluation of biochemical, hematological and oxidative parameters in mice exposed to the herbicide glyphosate-Roundup. Interdiscip Toxicol 5(3):133–140. I 10.2478/v10102-012-0022-5[↩][↩]
- EPA. 1992e. Data evaluation report. Test material: Glyphosate, technical; 98.7% purity; lot XHJ-64; white powder. Teratology study in rats. MRID 00098460. In: July 22, 1992. Memorandum. Glyphosate-List A chemical for reregistration-rereview of toxicology studies for acceptability. The attached 7-22-92 memorandum contains the Agency reviews for the following glyphosate studies: MRID numbers 0046362, 00046363, 00046364, 00067039, 00078619, 00078620, 00093879, 00098460, 00105995, 00132681, 00132683, 00132685 and 00132686 [Received via FOIA request]. HED pages 41–49. U.S. Environmental Protection Agency.[↩][↩][↩]
- EPA. 1992a. July 29, 1992. Memorandum. 48 Page(s). William Dykstra. Toxicology Branch I. Glyphosate (Roundup); review of 2-generation rat reproduction study; PP #0F03865, 2H05635 – Glyphosate in/on wheat. MRID 416215-01. Pages 9-10, 21-48 removed, registrant data. Tox review 009634. U.S. Environmental Protection Agency. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/103601/103601-273.pdf[↩][↩]
- NTP. 1992. NTP technical report on the toxicity studies of glyphosate (CAS No. 1071-83-6) administered in dosed feed To F344/N rats and B6C3F1 mice. Toxicity Report Series, No. 16. National Toxicology Program, U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health.[↩][↩][↩][↩][↩]
- EPA. 1991a. June 03, 1991. Memorandum. 40 Page(s). William Dykstra. Toxicology Branch. Glyphosate; 2-Year combined chronic toxicity/carcinogenicity study in Sprague-Dawley rats – List A Pesticide for Reregistration Pages 29-40 removed-registrant data. MRID 416438-01. Tox review 008390. U.S. Environmental Protection Agency. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/103601/103601-263.pdf[↩][↩][↩][↩][↩][↩]
- Caglar S, Kolankaya D. 2008. The effect of sub-acute and sub-chronic exposure of rats to the glyphosate-based herbicide Roundup. Environ Toxicol Pharmacol 25(1):57–62. 10.1016/j.etap.2007.08.011[↩]
- Jiang X, Zhang N, Yin L, et al. 2018. A commercial Roundup(R) formulation induced male germ cell apoptosis by promoting the expression of XAF1 in adult mice. Toxicol Lett 296:163–172. 10.1016/j.toxlet.2018.06.1067[↩]
- Dallegrave E, Mantese FD, Coelho RS, Pereira JD, Dalsenter PR, Langeloh A. The teratogenic potential of the herbicide glyphosate-Roundup in Wistar rats. Toxicol Lett. 2003 Apr 30;142(1-2):45-52. doi: 10.1016/s0378-4274(02)00483-6[↩][↩]
- Dallegrave E, Mantese FD, Oliveira RT, et al. 2007. Pre- and postnatal toxicity of the commercial glyphosate formulation in Wistar rats. Arch Toxicol 81(9):665–673. 10.1007/s00204-006-0170-5[↩]
- Romano RM, Romano MA, Bernardi MM, et al. 2010. Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Arch Toxicol 84(4):309–317. 10.1007/s00204-009-0494-z[↩][↩]
- Milesi MM, Lorenz V, Pacini G, et al. 2018. Perinatal exposure to a glyphosate-based herbicide impairs female reproductive outcomes and induces second-generation adverse effects in Wistar rats. Arch Toxicol 92(8):2629–2643. 10.1007/s00204-018-2236-6[↩]
- EPA. 1985c. March 27, 1985. Memorandum. 5 Page(s). Stephen Saunders. Toxicology Branch. 4-week subchronic inhalation in rats with Roundup 33 1/3% use-dilution. EPA Reg. No. 524-308; Accession No. 252621. U.S. Environmental Protection Agency. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/103601/103601-178.pdf[↩][↩][↩][↩][↩][↩][↩]
- EPA. 1992c. Data evaluation report. Test material: Glyphosate technical, white powder. 21-Day dermal toxicity study in rats, MRID 00098460. In: July 22, 1992. Memorandum. Glyphosate-List A chemical for reregistration-rereview of toxicology studies for acceptability. The attached 7-22-92 memorandum contains the Agency reviews for the following glyphosate studies: MRID numbers 0046362, 00046363, 00046364, 00067039, 00078619, 00078620, 00093879, 00098460, 00105995, 00132681, 00132683, 00132685 and 00132686 [Received via FOIA request]. HED pages 9–16. U.S. Environmental Protection Agency.[↩][↩][↩][↩]
- Altamirano GA, Delconte MB, Gomez AL, et al. 2018. Postnatal exposure to a glyphosate-based herbicide modifies mammary gland growth and development in Wistar male rats. Food Chem Toxicol 118:111–118. 10.1016/j.fct.2018.05.011[↩]
- Guerrero Schimpf M, Milesi MM, Ingaramo PI, et al. 2017. Neonatal exposure to a glyphosate based herbicide alters the development of the rat uterus. Toxicology 376:2–14. 10.1016/j.tox.2016.06.004[↩]
- Guerrero Schimpf M, Milesi MM, Luque EH, et al. 2018. Glyphosate-based herbicide enhances the uterine sensitivity to estradiol in rats. J Endocrinol. 10.1530/JOE-18-0207[↩]
- Kumar S, Khodoun M, Kettleson EM, et al. 2014. Glyphosate-rich air samples induce (L-33, TSLP and generate IL-13 dependent airway inflammation. Toxicology 325:42–51. 10.1016/j.tox.2014.08.008[↩][↩]
- Adam A, Marzuki A, Abdul Rahman H, Abdul Aziz M. The oral and intratracheal toxicities of ROUNDUP and its components to rats. Vet Hum Toxicol. 1997 Jun;39(3):147-51.[↩][↩][↩]
- Dar MA, Sultana M, Mir AH, et al. 2019. Effect of Repeated Oral Administration of Roundup and Ammonium Nitrate on Liver of Wistar Rats. Proc Natl Acad Sci India Sect B 89(2):505–510. 10.1007/s40011-017-0961-x[↩][↩]
- Aitbali Y, Ba-M’hamed S, Elhidar N, et al. 2018. Glyphosate based-herbicide exposure affects gut microbiota, anxiety and depression-like behaviors in mice. Neurotoxicol Teratol 67:44–49. 10.1016/j.ntt.2018.04.002[↩][↩][↩]
- Lozano VL, Defarge N, Rocque L-M, et al. 2018. Sex-dependent impact of Roundup on the rat gut microbiome. Toxicol Rep 5:96–107. 10.1016/j.toxrep.2017.12.005[↩][↩]
- Mao Q, Manservisi F, Panzacchi S, et al. 2018. The Ramazzini Institute 13-week pilot study of glyphosate and Roundup administered at human-equivalent dose to Sprague Dawley rats: Effects on the microbiome. Environ Health 17(1):50. 10.1186/s12940-018-0394-x[↩][↩]
- EPA. 2013c. Memorandum. 04-Jun-2013. Memorandum: Glyphosate. Review and generation of data evaluation records. MRID numbers 44320610, 44320612. [Received via FOIA request]. U.S. Environmental Protection Agency.[↩][↩]
- EPA. 1992f. Data evaluation report. Test material: Glyphosate, technical; 98.7% purity; lot XHJ-64; white powder. Teratology study in rabbits. MRID 00046363. In: July 22, 1992. Memorandum. Glyphosate-List A chemical for reregistration-rereview of toxicology studies for acceptability. The attached 7-22-92 memorandum contains the Agency reviews for the following glyphosate studies: MRID numbers 0046362, 00046363, 00046364, 00067039, 00078619, 00078620, 00093879, 00098460, 00105995, 00132681, 00132683, 00132685 and 00132686 [Received via FOIA request]. HED pages 50–58. U.S. Environmental Protection Agency.[↩][↩]
- EPA. 1991b. December 13, 1991. Memorandum. 38 Page(s). William Dykstra. Toxicology Branch I. Glyphosate – EPA Registration No. 524-308 – 2-Year chronic feeding/oncogenicity study in rats with technical glyphosate. MRID 416438-01. Tox review 008897. U.S. Environmental Protection Agency. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/103601/103601-268.pdf[↩][↩][↩][↩][↩]
- EPA. 1992d. Data evaluation report. Test material: Glyphosate, technical; 98.7% purity; lot XHJ-64; white powder. A lifetime feeding study of glyphosate in rats. MRID 00098460. In: July 22, 1992. Memorandum. Glyphosate-List A chemical for reregistration-rereview of toxicology studies for acceptability. The attached 7-22-92 memorandum contains the agency reviews for the following glyphosate studies: MRID numbers 0046362, 00046363, 00046364, 00067039, 00078619, 00078620, 00093879, 00098460, 00105995, 00132681, 00132683, 00132685 and 00132686 [Received via FOIA request]. HED pages 17–40. U.S. Environmental Protection Agency.[↩][↩][↩][↩]
- EPA. 1985a. April 03, 1985. Memorandum. 4 Page(s). William Dykstra. Toxicology Branch. Glyphosate; EPA Reg. # 524-308; mouse oncogenicity study Accession No. 251007-014. Tox review 004370. U.S. Environmental Protection Agency. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/103601/103601-183.pdf[↩]
- EPA. 2015a. Memorandum. November 17, 2015. Glyphosate, updated data evaluation record for a mouse carcinogenicity study. MRID 00130406 [Received via FOIA request]. U.S. Environmental Protection Agency.[↩][↩][↩][↩]
- EPA. 2015c. Memorandum. November 17, 2015. Glyphosate. Review and generation of data evaluation records for three rodent carcinogenicity studies. MRID numbers 49707601, 49631701, 49631702. U.S. Environmental Protection Agency.[↩][↩][↩][↩]
- Milic M, Zunec S, Micek V, et al. 2018. Oxidative stress, cholinesterase activity, and DNA damage in the liver, whole blood, and plasma of Wistar rats following a 28-day exposure to glyphosate. Arh Hig Rada Toksikol 69(2):154–168. 10.2478/aiht-2018-69-3114[↩][↩][↩]
- Wang L, Deng Q, Hu H, et al. 2019. Glyphosate induces benign monoclonal gammopathy and promotes multiple myeloma progression in mice. J Hematol Oncol 12(1):70. 10.1186/s13045-019-0767-9[↩][↩][↩][↩]
- Benedetti AL, de Lourdes C, Trentin AG, et al. 2004. The effects of sub-chronic exposure of Wistar rats to the herbicide Glyphosate-Biocarb. Toxicol Lett 153(2):227–232. 10.1016/j.toxlet.2004.04.008[↩]
- Mesnage R, Antoniou MN, Renney G, et al. 2017. Multiomics reveal non-alcoholic fatty liver disease in rats following chronic exposure to an ultra-low dose of Roundup herbicide. Sci Rep 7:39328. 10.1038/srep39328[↩][↩][↩]
- Wunnapuk K, Gobe G, Endre Z, et al. 2014. Use of a glyphosate-based herbicide-induced nephrotoxicity model to investigate a panel of kidney injury biomarkers. Toxicol Lett 225(1):192–200. 10.1016/j.toxlet.2013.12.009[↩]
- EPA. 1986a. March 12, 1986. Memorandum. 4 Page(s). William Dykstra. Toxicology Branch. EPA Reg. No. 524-308; Glyphosate; miscellaneous data; one-year dog study. Accession No. 260021 Tox review 004975. U.S. Environmental Protection Agency. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/103601/103601-212.pdf[↩][↩]
- EPA. 1987. January 12, 1987. Memorandum. 5 Page(s). William Dykstra. Toxicology Branch. Glyphosate; Roundup; EPA Reg. No. 524-308; Addendum to one year dog study with glyphosate; PP# 6F3380/6H5502; Glyphosate in/on soybeans; revised Section F; and amended label text. Acc 264334. Tox review 005651 (excerpt). U.S. Environmental Protection Agency. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/103601/103601-229.pdf[↩]
- FAO and WHO. 2016. Pesticides residues in food 2016. Special session of the Joint FAO/WHO meeting on pesticide residues. FAO plant production and protection paper. Food and Agriculture Organization of the United Nations, World Health Organization. http://www.fao.org/3/a-i5693e.pdf[↩][↩]
- Tizhe E, Ibrahim N, Fatihu M, et al. 2018. Pancreatic function and histoarchitecture in Wistar rats following chronic exposure to Bushfire(R): the mitigating role of zinc. J Int Med Res 46(8):3296–3305. 10.1177/0300060518778640[↩]
- Romano MA, Romano RM, Santos LD, et al. 2012. Glyphosate impairs male offspring reproductive development by disrupting gonadotropin expression. Arch Toxicol 86(4):663–673. 10.1007/s00204-011-0788-9[↩][↩]
- Owagboriaye FO, Dedeke GA, Ademolu KO, et al. 2017. Reproductive toxicity of Roundup herbicide exposure in male albino rat. Exp Toxicol Pathol 69(7):461–468. 10.1016/j.etp.2017.04.007[↩]
- Johansson HKL, Schwartz CL, Nielsen LN, et al. 2018. Exposure to a glyphosate-based herbicide formulation, but not glyphosate alone, has only minor effects on adult rat testis. Reprod Toxicol 82:25–31. 10.1016/j.reprotox.2018.09.008[↩][↩]
- Varayoud J, Durando M, Ramos JG, et al. 2017. Effects of a glyphosate-based herbicide on the uterus of adult ovariectomized rats. Environ Toxicol 32(4):1191–1201. 10.1002/tox.22316[↩]
- Ait Bali Y, Kaikai N, Ba-M’hamed S, et al. 2019. Learning and memory impairments associated to acetylcholinesterase inhibition and oxidative stress following glyphosate based-herbicide exposure in mice. Toxicology 415:18–525. 10.1016/j.tox.2019.01.010[↩][↩][↩][↩]
- (Cattani D, Cesconetto PA, Tavares MK, et al. 2017. Developmental exposure to glyphosate-based herbicide and depressive-like behavior in adult offspring: Implication of glutamate excitotoxicity and oxidative stress. Toxicology 38767–80. 10.1016/j.tox.2017.06.001[↩]
- Martinez MA, Ares I, Rodriguez JL, et al. 2018. Neurotransmitter changes in rat brain regions following glyphosate exposure. Environ Res 161:212–219. 10.1016/j.envres.2017.10.051[↩][↩]
- Dechartres J, Pawluski JL, Gueguen MM, Jablaoui A, Maguin E, Rhimi M, Charlier TD. Glyphosate and glyphosate-based herbicide exposure during the peripartum period affects maternal brain plasticity, maternal behaviour and microbiome. J Neuroendocrinol. 2019 Sep;31(9):e12731. doi: 10.1111/jne.12731[↩]
- Szepanowski F, Szepanowski LP, Mausberg AK, et al. 2018. Differential impact of pure glyphosate and glyphosate-based herbicide in a model of peripheral nervous system myelination. Acta Neuropathol 136(6):979–982. 10.1007/s00401-018-1938-4[↩][↩][↩][↩][↩][↩]
- APVMA. 2017. Final regulatory position: Consideration of the evidence for a formal reconsideration of glyphosate. Australian Pesticides and Veterinary Medicines Authority. http://apvma.gov.au/sites/default/files/publication/26561-glyphosate-final-regulatory-position-report-final_0.pdf[↩]
- Brusick D, Aardema M, Kier L, et al. 2016. Genotoxicity Expert Panel review: Weight of evidence evaluation of the genotoxicity of glyphosate, glyphosate-based formulations, and aminomethylphosphonic acid. Crit Rev Toxicol 46(Supp1):56–74. 10.1080/10408444.2016.1214680[↩]
- EFSA. 2015. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA (Journal European Food Safety Authority) 13(11):4302. http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2015.4302/epdf[↩][↩]
- EPA. 2017c. Revised glyphosate issue paper. Evaluation of carcinogenic potential. December 12, 2017. U.S. Environmental Protection Agency, Office of Pesticide Programs.[↩]
- Health Canada. 2017. Re-evaluation decision. Glyphosate. Ottawa, Ontario: Pest Management Regulatory Agency, Health Canada. http://publications.gc.ca/collections/collection_2017/sc-hc/H113-28/H113-28-2017-1-eng.pdf[↩]
- Kier LD, Kirkland DJ. 2013. Review of genotoxicity studies of glyphosate and glyphosate-based formulations. Crit Rev Toxicol 43(4):283–315. 10.3109/10408444.2013.770820[↩]
- NZ EPA. 2016. Review of the evidence relating to glyphosate and carcinogenicity. New Zealand Environmental Protection Agency. http://www.epa.govt.nz/Publications/EPA_glyphosate_review.pdf[↩]
- Williams GM, Aardema M, Acquavella J, Berry SC, Brusick D, Burns MM, de Camargo JLV, Garabrant D, Greim HA, Kier LD, Kirkland DJ, Marsh G, Solomon KR, Sorahan T, Roberts A, Weed DL. A review of the carcinogenic potential of glyphosate by four independent expert panels and comparison to the IARC assessment. Crit Rev Toxicol. 2016 Sep;46(sup1):3-20. doi: 10.1080/10408444.2016.1214677. Erratum in: Crit Rev Toxicol. 2018 Nov;48(10):907-908. doi: 10.1080/10408444.2018.1522175[↩]
- IARC. 2017. Glyphosate. Some organophosphate insecticides and herbicides. In: IARC monographs on the evaluation of carcinogenic risks to humans. Volume 112. International Agency for Research on Cancer. http://monographs.iarc.fr/ENG/Monographs/vol112/mono112.pdf[↩]
- Chen YJ, Wu ML, Deng JF, et al. 2009. The epidemiology of glyphosate-surfactant herbicide poisoning in Taiwan, 1986-2007: A poison center study. Clin Toxicol (Phila) 47(7):670–677. 10.1080/15563650903140399[↩][↩]
- Kim YH, Lee JH, Hong CK, et al. 2014. Heart rate-corrected QT interval predicts mortality in glyphosate-surfactant herbicide-poisoned patients. Am J Emerg Med 32(3):203–207. 10.1016/j.ajem.2013.09.025[↩][↩]
- Moon JM, Chun BJ, Cho YS, et al. 2018. Cardiovascular effects and fatality may differ according to the formulation of glyphosate salt herbicide. Cardiovasc Toxicol 18(1):99–107. 10.1007/s12012-017-9418-y[↩][↩][↩]
- Roberts DM, Buckley NA, Mohamed F, et al. 2010. A prospective observational study of the clinical toxicology of glyphosate-containing herbicides in adults with acute self-poisoning. Clin Toxicol (Phila) 48(2):129–136. 10.3109/15563650903476491[↩][↩][↩]
- Sawada Y, Nagai Y, Ueyama M, Yamamoto I. Probable toxicity of surface-active agent in commercial herbicide containing glyphosate. Lancet. 1988 Feb 6;1(8580):299. doi: 10.1016/s0140-6736(88)90379-0[↩][↩][↩]
- Talbot AR, Shiaw MH, Huang JS, Yang SF, Goo TS, Wang SH, Chen CL, Sanford TR. Acute poisoning with a glyphosate-surfactant herbicide (‘Roundup’): a review of 93 cases. Hum Exp Toxicol. 1991 Jan;10(1):1-8. doi: 10.1177/096032719101000101[↩][↩][↩][↩]
- Tominack RL, Yang GY, Tsai WJ, Chung HM, Deng JF. Taiwan National Poison Center survey of glyphosate–surfactant herbicide ingestions. J Toxicol Clin Toxicol. 1991;29(1):91-109. doi: 10.3109/15563659109038601[↩][↩][↩][↩]
- Cho YS, Moon JM, Chun BJ, et al. 2019. Use of qSOFA score in predicting the outcomes of patients with glyphosate surfactant herbicide poisoning immediately upon arrival at the emergency department. Shock 51(4):447–452. 10.1097/SHK.0000000000001201[↩][↩][↩][↩]
- EPA. 1992b. Data evaluation report. Test material: Glyphosate, technical; sample No. 96. Toxicological investigation of: CP67573-3, MRID 00067039. In: July 22, 1992. Memorandum. Glyphosate-List A chemical for reregistration-rereview of toxicology studies for acceptability. The attached 7-22-92 memorandum contains the agency reviews for the following glyphosate studies: MRID numbers 0046362, 00046363, 00046364, 00067039, 00078619, 00078620, 00093879, 00098460, 00105995, 00132681, 00132683, 00132685 and 00132686 [Received via FOIA request]. HED pages 3–5. U.S. Environmental Protection Agency.[↩]
- Hoppin JA, Valcin M, Henneberger PK, et al. 2007. Pesticide use and chronic bronchitis among farmers in the Agricultural Health Study. Am J Ind Med 50(12):969–979. 10.1002/ajim.20523[↩][↩]
- Moon JM, Chun BJ. 2010. Predicting acute complicated glyphosate intoxication in the emergency department. Clin Toxicol (Phila) 48(7):718–724. 10.3109/15563650.2010.488640[↩][↩]
- Picetti E, Generali M, Mensi F, et al. 2018. Glyphosate ingestion causing multiple organ failure: A near-fatal case report. Acta Biomed 88(4):533–537. 10.23750/abm.v88i4.6322[↩][↩][↩][↩]
- Lee HL, Chen KW, Chi CH, Huang JJ, Tsai LM. Clinical presentations and prognostic factors of a glyphosate-surfactant herbicide intoxication: a review of 131 cases. Acad Emerg Med. 2000 Aug;7(8):906-10. doi: 10.1111/j.1553-2712.2000.tb02069.x[↩][↩][↩]
- Eriguchi M, Iida K, Ikeda S, et al. 2019. Parkinsonism relating to intoxication with glyphosate. Intern Med 58(13):1935–1938. 10.2169/internalmedicine.2028-18[↩][↩]
- Luo W, Lu T, Li F, et al. 2019. Surgical treatment of pyloric stenosis caused by glyphosate poisoning: A case report. Medicine (Baltimore) 98(30):e16590. 10.1097/MD.0000000000016590[↩][↩][↩]
- Lee CH, Shih CP, Hsu KH, et al. 2008. The early prognostic factors of glyphosate-surfactant intoxication. Am J Emerg Med 26(3):275–281. 10.1016/j.ajem.2007.05.011[↩]
- Chang CY, Peng YC, Hung DZ, Hu WH, Yang DY, Lin TJ. Clinical impact of upper gastrointestinal tract injuries in glyphosate-surfactant oral intoxication. Hum Exp Toxicol. 1999 Aug;18(8):475-8. doi: 10.1191/096032799678847078[↩]
- Ozaki T, Sofue T, Kuroda Y. 2017. Severe glyphosate-surfactant intoxication successfully treated with continuous hemodiafiltration and direct hemoperfusion: Case report. Ther Apher Dial 21(3):296–297. 10.1111/1744-9987.12565[↩][↩][↩]
- Jayasumana C, Paranagama P, Agampodi S, et al. 2015. Drinking well water and occupational exposure to herbicides is associated with chronic kidney disease in Padavi-Sripura, Sri Lanka. Environ Health 14(1):6. 10.1186/1476-069x-14-6[↩]
- Elsner P, Darr-Foit S, Schliemann S. 2018. Occupational koebnerization of psoriasis caused by glyphosate. J Dtsch Dermatol Ges 16(1):70–71. 10.1111/ddg.13393[↩]
- EPA. 1993. Reregistration Eligibility Decision (RED): Glyphosate. U.S. Environmental Protection Agency. EPA738R93014. https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-417300_1-Sep-93.pdf[↩][↩]
- Acquavella JF, Weber JA, Cullen MR, Cruz OA, Martens MA, Holden LR, Riordan S, Thompson M, Farmer D. Human ocular effects from self-reported exposures to Roundup herbicides. Hum Exp Toxicol. 1999 Aug;18(8):479-86. doi: 10.1191/096032799678847087[↩][↩][↩]
- EPA. 2015b. Memorandum. June 29, 2015. EDSP weight of evidence conclusions on the tier 1 screening assays for the list 1 chemicals. EDSP: Weight of evidence analysis of potential Minteraction with the estrogen, androgen or thyroid pathways. U.S. Environmental Protection Agency. https://www.regulations.gov/contentStreamer?documentId=EPA-HQ-OPP-2009-0361-0047&disposition=attachment&contentType=pdf[↩][↩][↩]
- EPA. 2015d. Memorandum. June 29, 2015. Glyphosate. Data Evaluation Records (DERs) for EDSP Tier 1 Assays. U.S. Environmental Protection Agency. https://www.regulations.gov/contentStreamer?documentId=EPA-HQ-OPP-2009-0361-0047&disposition=attachment&contentType=pdf[↩]
- Curtis KM, Savitz DA, Weinberg CR, Arbuckle TE. The effect of pesticide exposure on time to pregnancy. Epidemiology. 1999 Mar;10(2):112-7. Erratum in: Epidemiology 1999 Jul;10(4):470.[↩][↩]
- Sanin LH, Carrasquilla G, Solomon KR, et al. 2009. Regional differences in time to pregnancy among fertile women from five Colombian regions with different use of glyphosate. J Toxicol Environ Health A 72(15–16):949–960. 10.1080/15287390902929691[↩]
- Caballero M, Amiri S, Denney JT, et al. 2018. Estimated residential exposure to agricultural chemicals and premature mortality by Parkinson’s disease in Washington State. Int J Environ Res Public Health 15(12). 10.3390/ijerph15122885[↩]