Contents
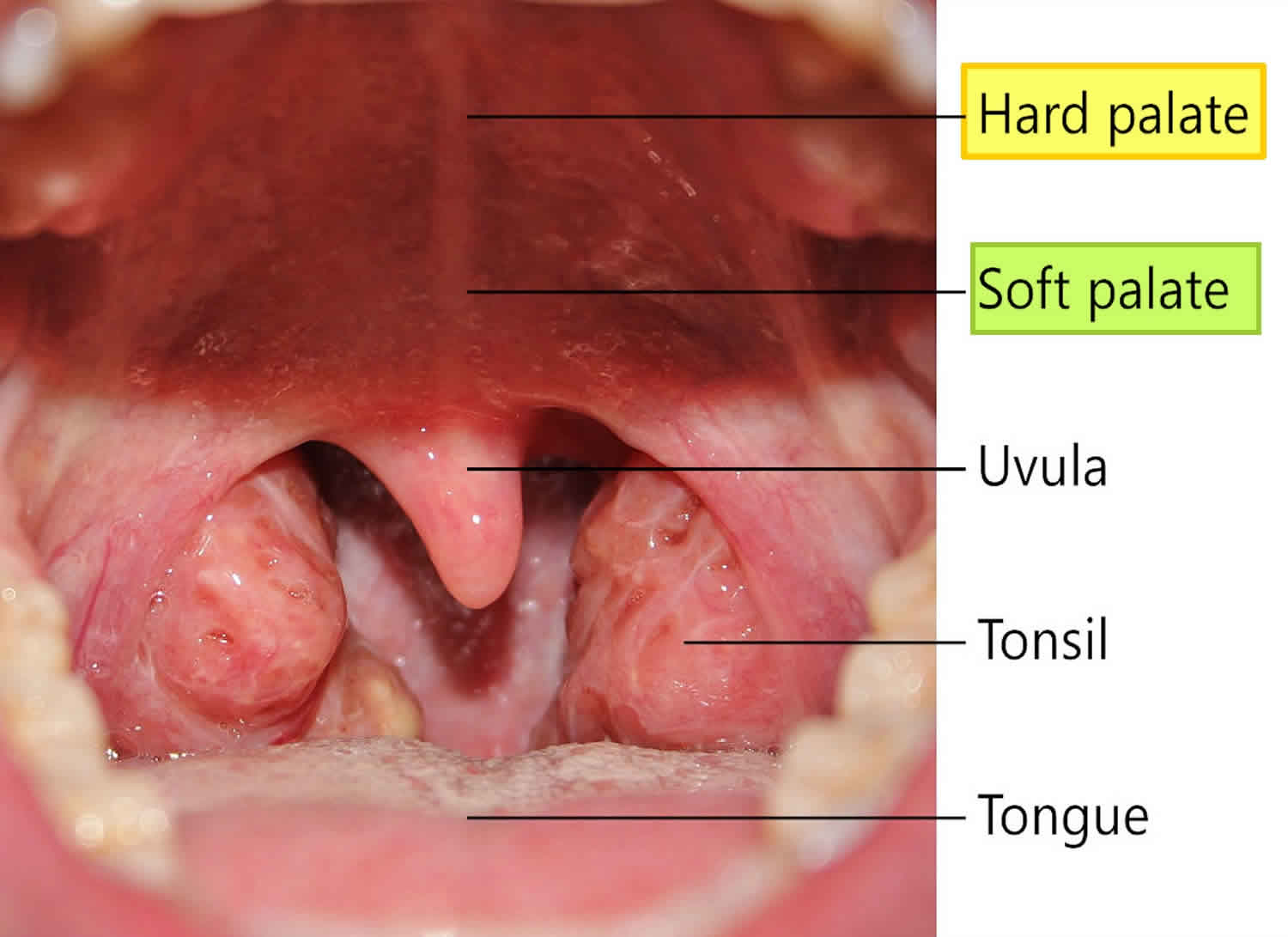
What is hard palate
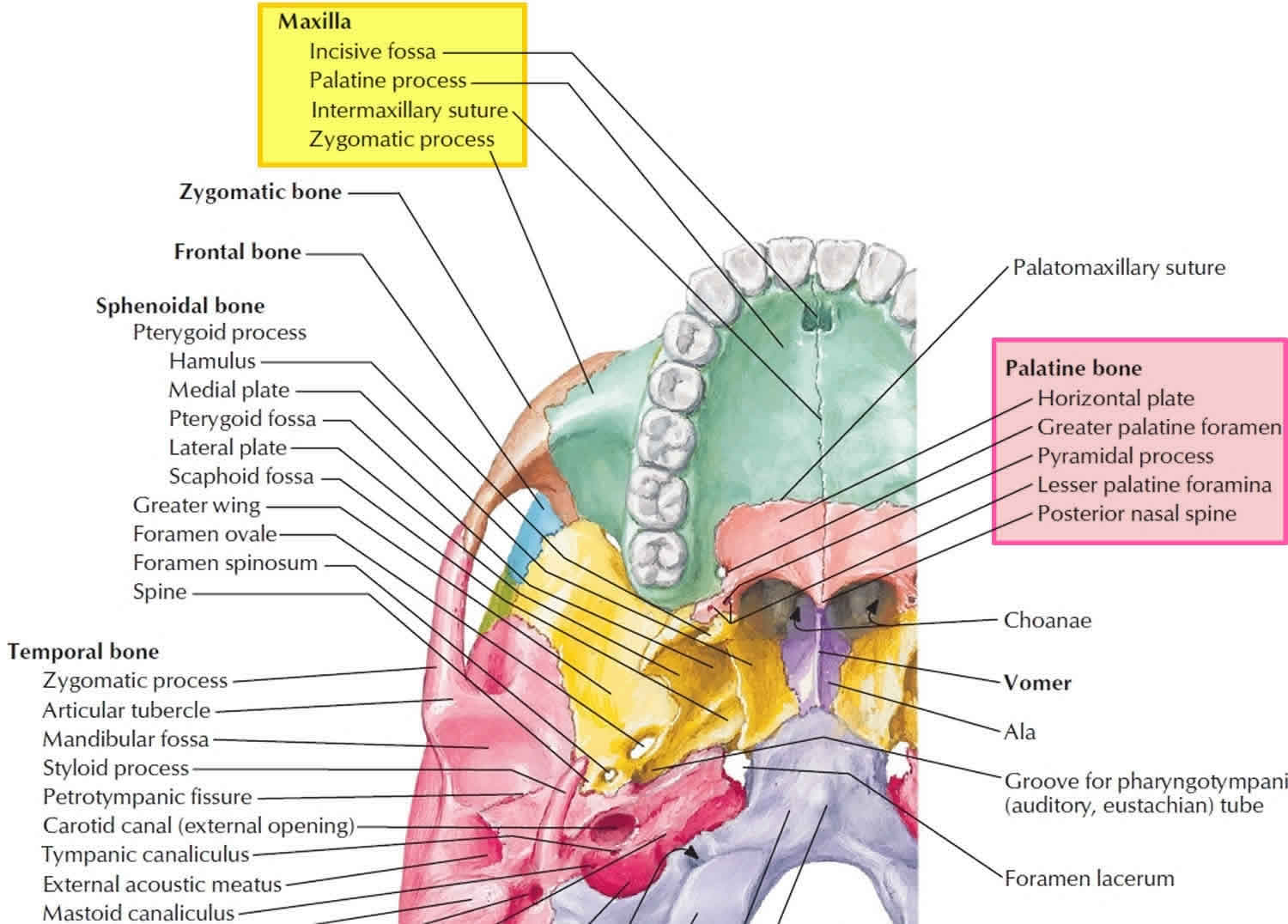
The palate forms the roof of the mouth and floor of the nasal cavity. The palate is divided anatomically into the bony hard palate anteriorly (part of the oral cavity) and the fleshy soft palate posteriorly (part of the oropharynx). Most of the hard palate is formed by horizontal extensions of the maxilla called palatine processes. The L-shaped palatine bones are located behind the maxillae (see Figure 1). The horizontal portions form the posterior section of the hard palate and the floor of the nasal cavity. The palate’s function is to separate the nasal cavity from the oral cavity, enabling you to continue breathing while chewing. The high metabolic rate of humans requires rapid digestion of food, which in turn is aided by prolonged and thorough mastication into small, easily digested particles. This would be difficult if such prolonged mastication required an interruption of airflow.
Just behind the incisors (front teeth) is a median pit, the incisive fossa, which is a passage for an artery to the palate and a nerve to the lower part of the nasal septum and the six front teeth of the maxilla. One or two pairs of incisive foramina open into this fossa (Figure 1) but are difficult to see. The palatine processes normally meet at the intermaxillary suture at about 12 weeks of gestation. Failure to join results in a cleft palate, often accompanied by a cleft lip lateral to the midline. A cleft palate and lip can be surgically corrected with good cosmetic results, but a cleft palate makes it difficult for an infant to generate the suction needed for nursing.
Hard palate anatomy
The bones that comprise the hard palate are the palatine processes of the maxillae anteriorly and the horizontal plates of the palatine bones posteriorly. The hard palate is bounded in front and laterally by the superior alveolar ridge. The soft palate is attached to the free posterior border of the hard palate. Sensory fibers reach the palate in branches of the maxillary (V2) division of the trigeminal nerve. The nasopalatine nerve emerges from the incisive foramen and supplies the anterior part of the hard palate. The greater palatine nerve gains the hard palate via the greater palatine foramen and innervates its posterior portion.
Figure 1. Hard palate anatomy
Hard palate function
The hard and soft palates separate the oral cavity from the nasal cavity. The combination of the two palates also helps humans breathe and chew simultaneously. That’s because the passage remains open when a person is not swallowing food, thus allowing him to breathe through the mouth and nose. However, the soft palate moves upward and blocks off the entrance to the back nasal passage when a person is swallowing food.
Hard palate cancer
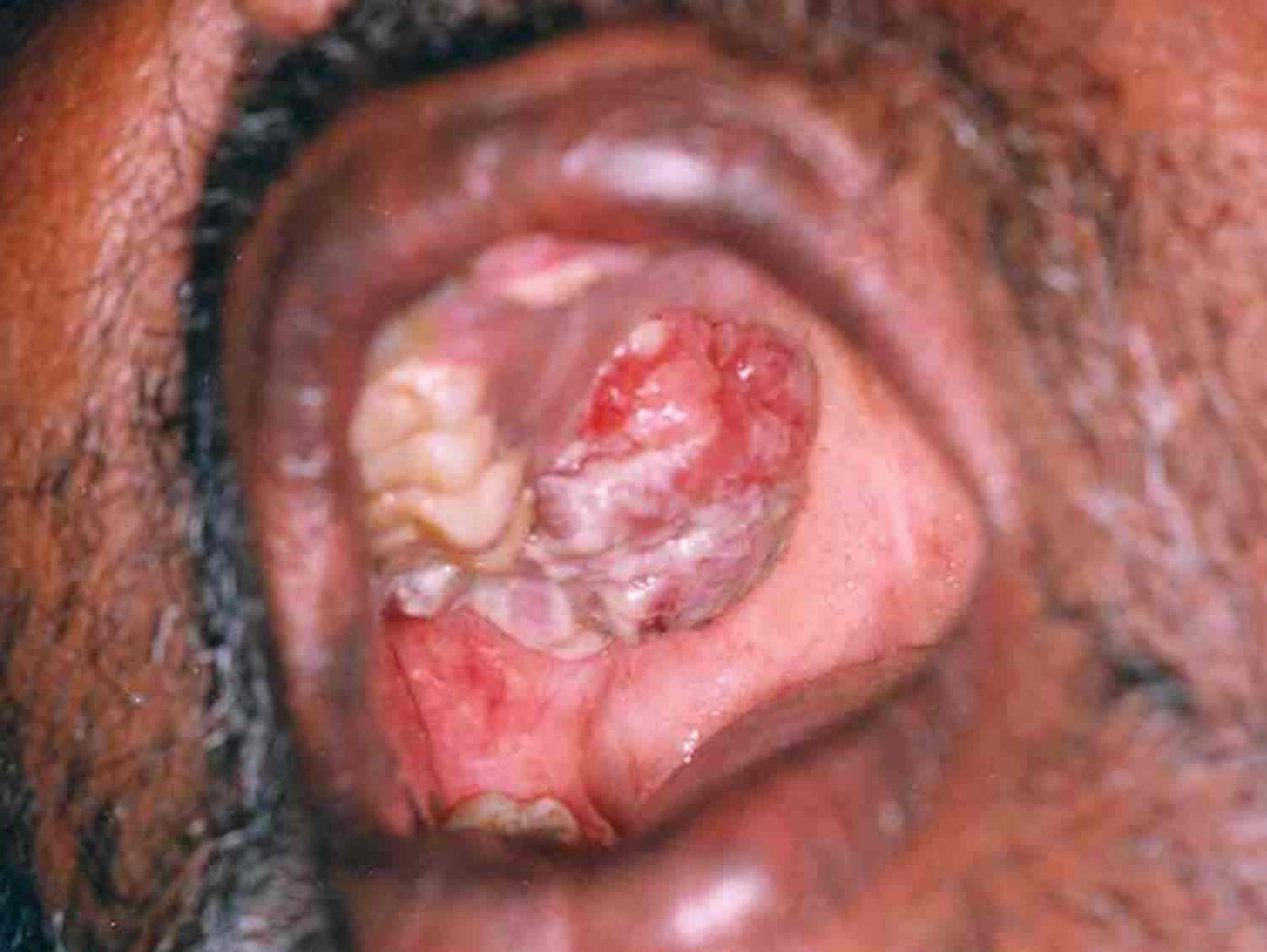
Half of all hard palate cancers are squamous cell carcinomas (SCCs) as seen in the image below. Nonsquamous cell cancers, including minor salivary gland cancers, sarcomas, and melanomas, account for the other half (see the histologic distribution of hard palate malignant neoplasms and the histologic types and frequencies of minor salivary gland neoplasms of the hard palate below).
The histologic distribution of hard palate malignant neoplasms is as follows 1:
- Squamous cell carcinoma – 53%
- Adenoid cystic carcinoma – 15%
- Mucoepidermoid carcinoma – 10%
- Adenocarcinoma – 4%
- Anaplastic carcinoma – 4%
- Other – 14%
Although a strong correlation is established between tobacco and alcohol consumption and squamous cell carcinoma of the oral cavity and soft palate, the relationship to hard palate cancer is not as clear. Reverse smoking is a specific risk factor for squamous cell carcinoma of the hard palate. In reverse smoking, the lit end of the cigarette is placed in the mouth so that an intense heat is generated during smoking. Other factors, including ill-fitting dentures, poor oral hygiene, mechanical irritation, and mouthwash, are implicated in oral cavity squamous cell carcinoma; however, the evidence is less convincing 1.
Hard palate cancer signs and symptoms
Squamous cell carcinomas of the hard palate manifest as hard palate sore or ulcers. Often, patients are asymptomatic in the early stages, but they may experience pain in advanced stages. A palate mass, bleeding, a foul odor, ill-fitting dentures in edentulous patients, or loose teeth may be the presenting symptoms for patients with hard palate cancer. Because the area is easily visualized, tumors are often found at early stages incidentally by the patient or the physician.
On the other hand, minor salivary gland tumors manifest as submucosal lesions, with a smooth, normal mucosal covering. Melanomas are smooth, black lesions but may be brown or brownish gray. Kaposi sarcomas are bluish lesions that are commonly seen in patients with HIV infection.
Pseudoepitheliomatous hyperplasia and necrotizing sialometaplasia are benign self-limited lesions that can mimic squamous cell carcinoma and need to be distinguished histologically. Torus palatinus (i.e, bony hyperplasia of the palate) are hard midline masses that produce no symptoms and should not be confused with tumors.
Figure 2. Hard palate cancer
Footnote: Carcinoma on the right side of the hard palate. An exophytic growth of 5×4 cm extending from the right maxillary alveolus to the adjacent hard palate
[Source 2 ]Biopsy of an ulcerative lesion may be easily obtained in the office transorally using biopsy forceps with the patient under local anesthesia. Alternatively, fine-needle aspiration cytology studies may be performed if an experienced cytopathologist is available.
- For ulcerative lesions, obtaining a biopsy specimen from closer to the edge of the tumor is important to avoid the necrotic central component.
- In large, nonulcerated palatal lumps, an incision through the intact mucosa may be required prior to biopsy. Place the biopsy incision in a manner that allows for subsequent removal of the biopsy scar in continuity with the tumor.
- Smaller submucosal lesions may be managed with excisional biopsy. If the pathology results indicate malignancy, further treatment is initiated.
Preoperative examination
Approximately 10-15% of patients with head and neck squamous cell carcinoma (squamous cell carcinoma) have a synchronous second primary cancer in the upper aerodigestive tract, lung, or esophagus. Hence panendoscopy, including esophagoscopy, bronchoscopy, and laryngopharyngoscopy, are performed on these patients. Alternatively, a complete flexible nasopharyngolaryngoscopy, chest radiography, and barium esophagography may suffice for synchronous tumor assessment.
The results of one study found that the use of panendoscopy may help identify synchronous second primary tumors in patients with a history of tobacco use but not in nonsmoking patients 3.
Hard palate cancer treatment
Specific treatment of palate cancer depends on the location of the tumor (hard vs soft palate), stage of the tumor and pathologic type of the cancer. For this reason, management of squamous cell carcinoma and carcinomas of minor salivary gland origin are discussed separately.
Treatment of T1-T2 squamous cell carcinoma of the hard palate
Surgery is the preferred treatment for squamous cell carcinoma of the hard palate. However, megavoltage radiation has also been used with some success as a viable alternative in treating patients with these tumors.
- T1 – Tumor 2 cm or smaller in greatest dimension
- T2 – Tumor larger than 2 cm but not larger than 4 cm in greatest dimension
Small T1 and T2 lesions can be managed with either surgery or radiation therapy. Radiation therapy is given to a total dose of 60-70 Gy. The proximity of the tumor to the bone and potential complications of osteoradionecrosis make radiation therapy less desirable for managing these lesions. On the other hand, surgery for these lesions is simple, with low morbidity and no loss of function.
For tumors that do not involve the periosteum or bone, through-and-through excision of the palate, opening the sinonasal fossa, is not necessary. For these lesions, a simple transoral excision into and including the periosteum is sufficient. A 1-cm margin is taken with the tumor. The periosteum serves as the superior margin. The periosteum may be spared only in very superficial tumors that are not close to the periosteum. This is an intraoperative decision. With surgical management, the 5-year survival rates are 75% for stage I and 50% for stage II tumors.
In most cases, the defect from such lesions may be left open to heal by secondary intention and granulation. Skin grafting is discouraged. Consider placing a palatal acrylic prosthesis (healing plate), which can be fabricated by a dentist or prosthodontist prior to resection. This helps protect the palate wound during the healing process. In some cases, palatal and/or buccal mucosal flaps are necessary to restore tissue deficiency, especially when dealing with patients’ postradiation therapy and/or those with larger soft palate defects 4.
- Clinical and radiological N0 (no regional lymph node metastasis) necks in these patients do not require elective treatment. When occult neck metastasis is suggested, staging functional neck dissection (including levels 1, 2, and 3) is performed. Recently, a 27% rate of occult cervical metastasis was reported in a series of 26 patients with maxillary alveolar ridge and hard palate squamous cell carcinoma. The authors thus suggest an elective neck dissection for such cancers with clinically N0 (no regional lymph node metastasis) neck 5. Other authors have suggested the use of sentinel lymph node biopsy in such situations 6.
- Treatment of an N1 (metastasis in a single ipsilateral lymph node, 3 cm or smaller in greatest dimension) neck is controversial. A pathological N1 (metastasis in a single ipsilateral lymph node, 3 cm or smaller in greatest dimension) node is considered adequately treated with neck dissection alone when no extracapsular extension is present. However, in many centers, any pathological N1 node is treated with postoperative radiotherapy; this is recommended. Definitely initiate postoperative radiation therapy for patients with extracapsular extension.
- If the pathological stage of the neck is N2 or higher, initiate postoperative radiotherapy.
Treatment of T3-T4 squamous cell carcinoma of the hard palate
T3 and T4 lesions frequently require combined oncologic treatment, including surgery and radiation therapy to both the primary site and the neck. N1 (metastasis in a single ipsilateral lymph node, 3 cm or smaller in greatest dimension) necks may be treated with radiotherapy or neck dissection. Necks that are N2 or higher are treated with planned combined surgery and radiotherapy. Larger palatal cancers have a poor prognosis and require multimodality oncologic therapy. Radiation is given using high-voltage equipment to a total of 60-70 Gy.
- T3 – Tumor larger than 4 cm in greatest dimension
- T4 – Tumor invades adjacent structures (e.g, through cortical bone, soft tissues of neck, deep [extrinsic] muscle of tongue)
A study by Givi et al 7 indicated that in patients with squamous cell carcinoma of the maxillary alveolus/hard palate who are clinically node negative (cN0), elective lymph neck dissection is linked to lower recurrence rates and improved survival. The neck recurrence rate was 6% in those study patients who underwent dissection and 21% in those who did not, with occult nodal metastases found in 29% of necks in the dissection group. (T3 and T4 tumors were more prevalent in the dissection group than in the nondissection patients [62% vs 34%, respectively].) Five-year recurrence-free survival was 68% in patients who underwent dissection versus 45% in the nondissection group, while overall survival was 86% vs 62%, respectively.
Importantly, when planning surgery for lesions that extend beyond the hard palate, determine the deficit that will result from resection. Resection of the soft palate can cause significant velopharyngeal insufficiency. Because the soft palate is a dynamic structure, it is difficult to reconstruct. Lesions that invade the palatine bone require partial palatectomy, with resulting oroantral and oronasal fistula. Invasion into the nasal cavity or the maxillary sinus requires inferior maxillectomy, partial maxillectomy, or total maxillectomy, depending on the extent of the lesion. Prosthetic rehabilitation is highly effective in these patients. However, use of vascularized free flaps, such as the scapular osteocutaneous flap or free fibula osteocutaneous flap, are highly effective in functional as well as aesthetic reconstruction and restoration of maxillary buttresses 8.
Extension into the pterygopalatine and infratemporal fossa requires skull-base approaches to effectively extirpate the tumor.
Treatment of minor salivary gland cancers of the palate
Seventy-four percent of minor salivary gland tumors are malignant. The palate is the most common site for minor salivary gland carcinomas. Most of these occur in the hard palate. Minor salivary gland malignancies are divided into high- and low-grade tumors. High-grade tumors include adenoid cystic carcinoma, high-grade mucoepidermoid carcinoma, high-grade adenocarcinoma, malignant mixed tumor, and carcinoma expleomorphic adenoma. Low-grade malignancies include low-grade mucoepidermoid carcinoma, polymorphous low-grade adenocarcinoma (with its propensity to occur in the hard palate), acinic cell carcinoma, and other rare tumors.
The most important poor prognostic factors for malignant minor salivary gland tumors of the palate are grade 3 histology, tumor size larger than 3 cm, and positive margins.
Surgery is the mainstay of treatment for minor salivary gland tumors of the palate. For minor salivary gland tumors of the palate in which perineural invasion is suggested, identify and evaluate the greater palatine nerve by frozen section. If the nerve is involved, follow it with proximal resection until negative margins are attained. If negative margins cannot be attained at the foramen rotundum, postoperative radiation therapy must include the trigeminal ganglion.
Postoperative radiotherapy with or without chemotherapy is indicated for high-grade tumors, large T3 or T4 lesions, positive margins, tumors showing perineural invasion, and cervical lymph node metastasis.
- T3 – Tumor larger than 4 cm in greatest dimension
- T4 – Tumor invades adjacent structures (e.g, through cortical bone, soft tissues of neck, deep [extrinsic] muscle of tongue)
Radiotherapy, possibly combined with chemotherapy, is used as the primary treatment if the patient refuses surgery or is not a candidate for surgery because of extensive unresectable disease.
Cervical node metastasis is a rare event for salivary gland tumors of the palate, occurring in approximately 3% of cases. Therefore, elective neck dissection is not indicated in these tumors in the absence of clinical or radiological signs of nodal metastasis.
For adenoid cystic carcinoma, surgery followed by radiation therapy is the treatment of choice. Wide surgical margins are taken because this tumor is known for microscopic extension beyond the gross tumor margins. The propensity for perineural extension requires resection along the greater palatine nerves with frozen section control to achieve negative margins. Postoperative radiation is preferred because preoperative radiation therapy increases surgical complications.
Bump on hard palate
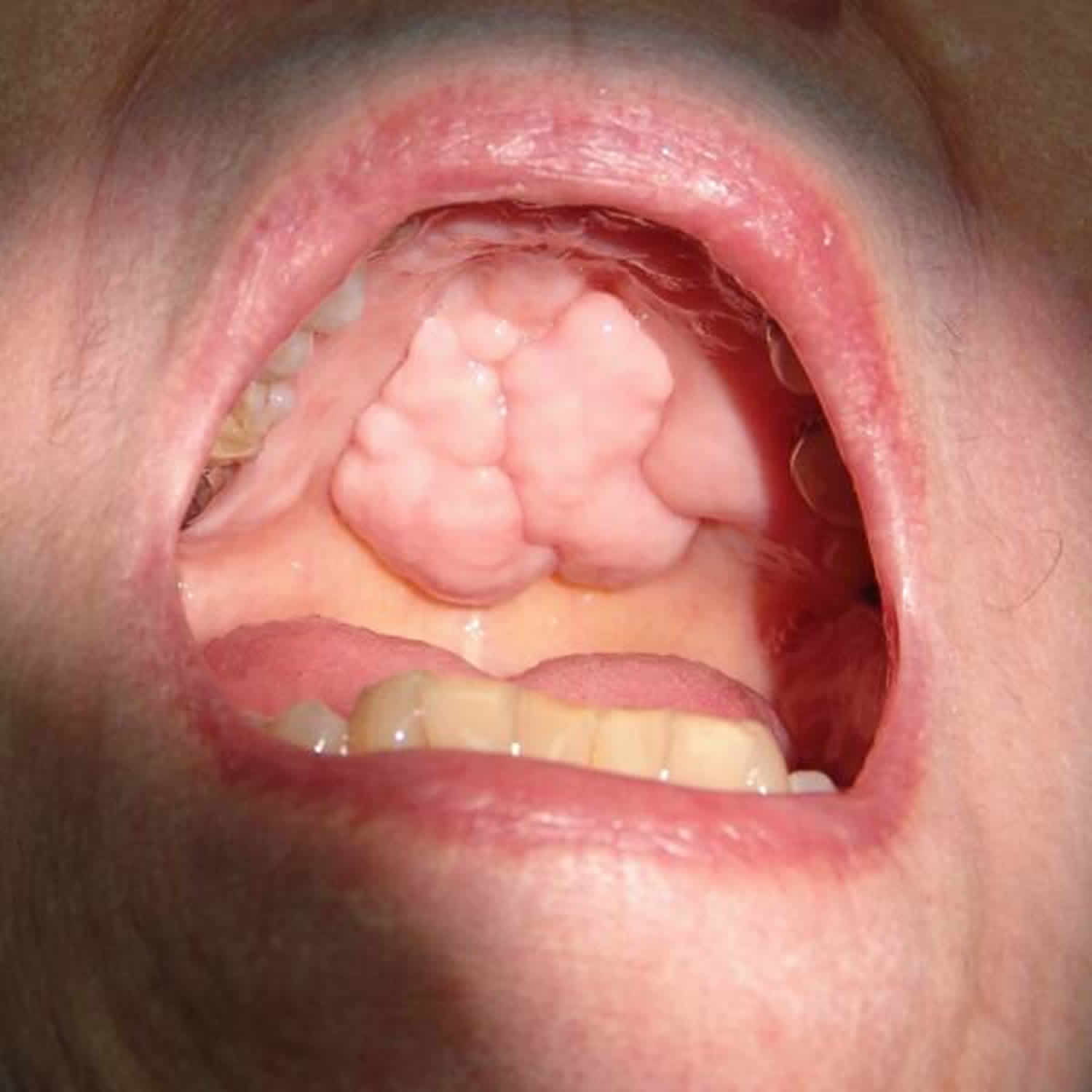
Torus palatinus is a common developmental exostosis or outgrowth of the bone of the hard palate that manifests itself in adults during the second or third decade of life 9. It is more common in women and is always located in the midline. Torus palatinus is an extension of the bone of the hard palate and and not a true neoplasm. Torus palatinus are benign anatomical bony protuberances 10 and rarely needs treatment. Occasionally, it is removed because it interferes with the fitting of dentures. In rare instances, multiple tori grow on the hard palate.
Figure 3. Torus Palatinus
Torus palatinus are usually considered an anatomical variation rather than a pathological entity and do not normally require treatment 10. Although torus palatinus are not considered to be pathologically significant, surgical removal is indicated in cases where they may cause chronic trauma or interfere with the function of oral cavity, or when there is a need for the replacement of a denture base or its framework 11. The size of the torus palatinus can change throughout life, and may range from a few millimeters to centimeters. An increase in size may be observed in the early adulthood, but it may show a decrease in size in the older age-group due to bone resorption. Reichart et al 12 have classified torus palatinus, based on their size, as small (less than 3 mm), medium (3–6 mm), and large (more than 6 mm) tori.
Torus palatinus are composed of dense cortical bony structure and minimal amount of bone marrow, and are covered with a fragile and limited vascularized mucosa. Tori usually become apparent Tori may develop at the midline of palate (torus palatinus) or the lingual aspect of the mandible (torus mandibularis). Jaw exostosis at any other location has no precise designation and may be seen as a bony protrusion on the buccal aspect of the maxilla and mandible or palatal aspect of the maxilla 13.
Torus palatinus may be defined as an exostosis of the hard palate located along the median palatine suture, involving both the processi palatini and the os palatinum 11. Torus mandibularis is observed as a bony protuberance on the lingual surface of the mandible, which is situated mostly in the canine and the premolar region, above the mylohyoid ridge 14. According to the shape, torus palatinus can be classified as flat, spindle-shaped, nodular, and lobular, whereas torus mandibularis can be classified as unilateral and bilateral solitary, unilateral and bilateral multiple, and bilateral combined 15. The size of torus palatinus is highly variable, varying from that of a small pea to an enormous enlargement that may cover the entire palate to the extent of occlusal plane.
Till date, no exact cause has been identified for the presence of tori. The most widely accepted theory today is that that this condition has a multifactorial cause, which includes mainly genetics and environmental factors 15. Many predisposing factors such as trauma, drugs, discontinued growth, infection, masticatory stress, and environmental and nutritional factors have been identified by various researchers 16. Correlation between torus palatinus and torus mandibularis and chronic phenytoin administration was reported by Sasaki et al. 17. Tori were also known to be associated with other dental anomalies such as exostosis, unerupted and impacted tooth, mandibular canines, and sclerosteosis 18. The prevalence and degree of expression of tori might be related to the masticatory demand that increases with increasing age, and possibly, subsides after the fifth decade of life due to reduction in the number of teeth 19.
According to a study by Eggen et al. 20, who investigated the influence of nutrients in the cause of tori, dietary components also seem to play a role in the prevalence of tori. It was suggested that consumption of saltwater fish in Norway is possibly responsible for supplying increased amounts of polyunsaturated fatty acids and vitamin D, which is associated with growth of the bone, and this may escalate the prevalence of tori 20. Significant racial differences have also been observed with Mongoloids having a higher prevalence rate than Caucasians, and Caucasians having a higher prevalence rate than blacks 21.
- Malignant Tumors of the Palate. https://emedicine.medscape.com/article/847807-overview[↩][↩]
- http://screening.iarc.fr/atlasoral_detail.php?flag=1&lang=1&Id=B2b00024&cat=B2b[↩]
- Rodriguez-Bruno K, Ali MJ, Wang SJ. Role of panendoscopy to identify synchronous second primary malignancies in patients with oral cavity and oropharyngeal squamous cell carcinoma. Head Neck. 2011 Jul. 33(7):949-53.[↩]
- Stassen L, Khosa AD, Israr M. The value of the ‘buccal pad of fat’ in the reconstruction of oral defects following removal of intraoral tumours–a clinical assessment. Ir Med J. 2013 Jan. 106(1):13-5[↩]
- Simental AA Jr, Johnson JT, Myers EN. Cervical metastasis from squamous cell carcinoma of the maxillary alveolus and hard palate. Laryngoscope. 2006 Sep. 116(9):1682-4.[↩]
- Sanchez-Fernandez JM, Santaolalla-Montoya F, Sanchez-del Rey A, et al. In reference to: “Cervical metastasis from squamous cell carcinoma of the maxillary alveolus and hard palate.”. Laryngoscope. 2007 Mar. 117(3):565-6; author reply 566.[↩]
- Givi B, Eskander A, Awad MI, et al. Impact of elective neck dissection on the outcome of oral squamous cell carcinomas arising in the maxillary alveolus and hard palate. Head Neck. 2016 Apr. 38 Suppl 1:E1688-94[↩]
- Germain MA, Hartl DM, Marandas P, et al. Free flap reconstruction in the treatment of tumors involving the hard palate. Eur J Surg Oncol. 2006 Apr. 32(3):335-9.[↩]
- Al-Sebaie D, Alwrikat M. Prevalence of torus palatinus and torus mandibularis in Jordanian population. Pak Oral Dent J. 2011;31:214–216.[↩]
- Prevalence and pattern of torus palatinus and torus mandibularis among edentulous patients of Saudi Arabia. Clin Interv Aging. 2016;11:209-13. Published 2016 Feb 24. doi:10.2147/CIA.S100282 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4771409/[↩][↩]
- Sisman Y, Ertas ET, Gokce C, Akgunlu F. Prevalence of torus palatinus in cappadocia region population of Turkey. Eur J Dent. 2008;2:269–275.[↩][↩]
- Reichart PA, Neuhaus F, Sookasem M. Prevalence of torus palatinus and torus mandibularis in Germans and Thais. Commun Dent Oral Epidemiol. 1988;16:61–64.[↩]
- Sawair FA, Shayyab MH, Al-Rababah MA, Saku T. Prevalence and clinical characteristics of tori and jaw exostoses in a teaching hospital in Jordan. Saudi Med J. 2009;30:1557–1562.[↩]
- Axelsson G, Hedegård B. Torus mandibularis among Icelanders. Am J Phys Anthropol. 1981;54:383–389.[↩]
- Simunković SK, Bozić M, Alajbeg IZ, Dulcić N, Boras VV. Prevalence of torus palatinus and torus mandibularis in the Split-Dalmatian County, Croatia. Coll Antropol. 2011;35:637–641.[↩][↩]
- Patil S, Maheshwari S, Khandelwal SK. Prevalence of torus palatinus and torus mandibularis in an Indian population. Saudi J Oral Sci. 2014;1:94–97.[↩]
- Pronounced palatal and mandibular tori observed in a patient with chronic phenytoin therapy: a case report. Sasaki H, Ikedo D, Kataoka M, Kido J, Kitamura S, Nagata T. J Periodontol. 1999 Apr; 70(4):445-8.[↩]
- Epidemiological aspects of oral tori in a Ghanaian community. Bruce I, Ndanu TA, Addo ME. Int Dent J. 2004 Apr; 54(2):78-82.[↩]
- Relationship between oral tori and temporomandibular disorders. Sirirungrojying S, Kerdpon D. Int Dent J. 1999 Apr; 49(2):101-4.[↩]
- Eggen S, Natvig B, Gåsemyr J. Variation in torus palatinus prevalence in Norway. Scand J Dent Res. 1994;102:54–59.[↩][↩]
- Agbaje JO, Arowojolu MO, Kolude N, Lawoyin JO. Torus palatinus and torus mandibularis in a Nigerian population. Afr J Oral Health. 2005;2:30–36.[↩]