Contents
- Hyperkalemic periodic paralysis
- Why do I get weak during hyperkalemic periodic paralysis episodes?
- My potassium level is always normal yet the doctor says I have hyperkalemic periodic paralysis. Why?
- No one in my family has hyperkalemic periodic paralysis. How did I get it?
- Can I have a DNA test for hyperkalemic periodic paralysis?
- Will I pass hyperkalemic periodic paralysis on to my children?
- What is Potassium?
- Hyperkalemic periodic paralysis cause
- Hyperkalemic periodic paralysis pathophysiology
- Hyperkalemic periodic paralysis prevention
- Hyperkalemic periodic paralysis symptoms
- Hyperkalemic periodic paralysis complications
- Hyperkalemic periodic paralysis differential diagnosis
- Hyperkalemic periodic paralysis diagnosis
- Hyperkalemic periodic paralysis treatment
- Hyperkalemic periodic paralysis life expectancy
Hyperkalemic periodic paralysis
Hyperkalemic periodic paralysis also known as Familial hyperkalemic periodic paralysis, Primary hyperkalemic periodic paralysis, HyperPP or HyperKPP, is a rare, autosomal dominant condition that causes episodes of extreme muscle weakness (adynamia) or paralysis, usually beginning in infancy or early childhood (onset before age 20 years) caused by a mutation in the SCN4A gene that codes for voltage-gated sodium channel causing potassium to shift into the extracellular space due to impaired sodium channel function in skeletal muscle 1, 2, 3, 4, 5. However, in 30 to 40 percent of cases, the cause of hyperkalemic periodic paralysis is unknown 6. Changes in other genes, which have not been identified, likely cause hyperkalemic periodic paralysis in these cases 7. Hyperkalemic periodic paralysis (hyperPP) is characterized by attacks of generalized or focal flaccid muscle weakness, which may also include weakness of the muscles of the eyes, throat, breathing muscles, and trunk. In addition, most people with hyperkalemic periodic paralysis have increased levels of potassium in their blood (hyperkalemia) during attacks with serum potassium concentration greater than 5 mmol/L or an increase of serum potassium concentration of at least 1.5 mmol/L during an attack of weakness 8, 2. Hyperkalemia results when the weak or paralyzed muscles release potassium ions into the bloodstream. In other cases, attacks are associated with normal blood potassium levels (normokalemia). Ingesting potassium can trigger attacks in affected individuals, even if blood potassium levels do not go up. Most often, these episodes involve a temporary inability to move muscles in the arms and legs. Episodes tend to increase in frequency until mid-adulthood, after which they occur less frequently in many people with hyperkalemic periodic paralysis 7. Factors that can trigger attacks include rest after exercise, potassium-rich foods such as bananas and potatoes, stress, fatigue, alcohol, pregnancy, exposure to hot or cold temperatures, certain medications, and periods without food (fasting) 7. Muscle strength usually returns to normal between attacks, although many affected people continue to experience mild stiffness (myotonia), particularly in muscles of the face and hands 7.
Hyperkalemic periodic paralysis affects an estimated 1 in 200,000 to and 1 in 500 000 people 9, 10, 7. In approximately half of affected individuals, attacks of flaccid muscle weakness begin in the first decade of life, with 25% reporting their first attack at age 10 years or older 2. Initially infrequent, the attacks then increase in frequency and severity over time until approximately age 50 years, after which the frequency of attacks declines considerably 2. The major attack trigger is eating potassium-rich foods; other triggers include: cold environment; rest after exercise, stress, or fatigue; alcohol; hunger; and changes in activity level. A spontaneous attack commonly starts in the morning before breakfast, lasts for 15 minutes to one hour, and then passes. Individuals with hyperPP frequently have myotonia (muscle stiffness), especially around the time of an episode of weakness. Paramyotonia (muscle stiffness aggravated by cold and exercise) is present in about 45% of affected individuals. Although most affected individuals have normal muscle strength between attacks, some develop fixed or chronic progressive weakness, independently of the presence of episodic attacks 4, 11, 12. More than 80% of individuals with hyperkalemic periodic paralysis (hyperPP) older than age 40 years with a long disease duration report permanent muscle weakness and about one third develop a chronic progressive myopathy 2, 4, 11. It has been proposed that sodium (Na+) overload in muscle fibers can lead to muscle degeneration that increases with age 13. However, little is known about the development of permanent muscle weakness in hyperKPP, including the pattern of muscle involvement 14.
Hyperkalemic periodic paralysis (hyperPP) is caused by a mutation in the SCN4A gene that codes for voltage-gated sodium channel Na1.4 1. Diagnosis is based on clinical symptoms including the increase of blood potassium level during an episode, but normal levels of blood potassium level in between episodes. Genetic testing can confirm the diagnosis, although they are not always definitive. In case of diagnostic uncertainty, a provocative test such as the potassium challenge test can be employed, although the availability of genetic testing and electrophysiologic studies largely obviates the need for such dangerous tests 2, 15, 16.
Treatment for hyperkalemic periodic paralysis (hyperPP) is focused on avoiding triggers, decreasing the severity of an episode, preventing further attacks and relieving symptoms. Attacks are seldom severe enough to require emergency treatment. At the first sign of muscle weakness, attacks may be prevented or aborted with mild exercise and/or eating carbohydrates (sugars), intravenously injected glucocorticoids, inhalation of salbutamol, or intravenous calcium gluconate 2. Hyperkalemic attacks of weakness can be prevented by frequent meals rich in carbohydrates; continuous use of a thiazide diuretic or a carbonic anhydrase inhibitor; and avoidance of potassium-rich medications and foods, fasting, strenuous work, and exposure to cold 2. But irregular heartbeats (heart arrhythmias) may also occur during attacks, for which emergency treatment is needed. Muscle weakness can become worse with repeated attacks, so treatment to prevent the attacks should occur as soon as possible.
Why do I get weak during hyperkalemic periodic paralysis episodes?
During attacks of muscle weakness, potassium moves from the muscle cells into the blood, causing an imbalance in the ratio of potassium inside and outside muscle cells. This makes the cells unable to contract and/or relax properly.
My potassium level is always normal yet the doctor says I have hyperkalemic periodic paralysis. Why?
The term ‘hyperkalemic’ is somewhat confusing, because in most patients the level of potassium in the blood does not rise above normal during attacks. ‘Hyperkalemic’ refers to the fact that attacks may be triggered by eating potassium-rich foods or by giving the patient potassium. Patients whose potassium does not rise during attacks of weakness or paralysis are sometimes referred to as having Normokalemic Periodic Paralysis (normoPP). Normokalemic Periodic Paralysis (normoPP) is caused by a mutation in the sodium channel and is managed and treated in the same way as hyperkalemic periodic paralysis (hyperPP).
No one in my family has hyperkalemic periodic paralysis. How did I get it?
In a majority of cases hyperkalemic periodic paralysis (hyperPP) is inherited, but a person may carry, and pass on the gene mutation without ever experiencing any symptoms at all. But some cases the mutation happens at conception, like any other gene mutation, for reasons which are unclear.
Can I have a DNA test for hyperkalemic periodic paralysis?
Genetic testing is available, but it is not yet reliable enough to diagnose all hyperkalemic periodic paralysis (hyperKPP) patients. While a genetic test may be able to say you have hyperkalemic periodic paralysis (hyperKPP), if the results of the test is negative that does NOT mean you do not have the disorder, as not all mutations have been identified yet. If you plan to be tested it is wise to talk about this issue with your physician and genetics professional ahead of time, just in case your test comes back negative (as many are still doing at this point).
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Will I pass hyperkalemic periodic paralysis on to my children?
The chances that a child of an affected person will inherit the gene mutation is ‘statistically’ 50%, but not all who inherit the mutation will have symptoms. The degree to which children are affected may vary from one child to the next. One child may be mildly affected, the next seriously affected and the next unaffected. Even identical twins may be affected to different degrees.
What is Potassium?
Potassium (K+) is a mineral that is vital to cell metabolism. Potassium is a major intracellular cation (positively charged ion) and a type of electrolyte that plays a significant role in the regulation of fluid volume and maintenance of the water-electrolyte balance 17, 18, 19. Potassium is present in all body tissues and is required for normal cell function because of its role in maintaining intracellular fluid volume and transmembrane electrochemical gradients 20, 21. Potassium helps transport nutrients into cells and removes waste products out of cells. Potassium is essential for the proper functioning of the heart, kidneys, muscles, nerves, and digestive system. Potassium is also important in muscle function, helping to transmit messages between nerves and muscles 20.
Potassium (K+) is a positively charged ion (cation), which is present throughout your body in both intracellular and extracellular fluids. The majority of body potassium, > 90%, are intracellular. It moves freely from intracellular fluid (ICF) to extracellular fluid (ECF) and vice versa when adenosine triphosphate (ATP) increases the permeability of the cell membrane. Potassium (K+) is mainly replaced inside or outside the cells by another cation, sodium (Na+). The movement of potassium into or out of the cells is linked to certain body hormones and also to certain physiological states. Standard laboratory tests measure extracellular fluid (ECF) potassium. Potassium enters the body rapidly during food ingestion. Insulin is produced when a meal is eaten; this causes the temporary movement of potassium from extracellular fluid (ECF) to intracellular fluid (ICF). Over the ensuing hours, the kidneys excrete the ingested potassium and homeostasis is returned. In the critically ill patient, suffering from high potassium level or hyperkalemia, this mechanism can be manipulated beneficially by administering high concentration (50%) intravenous glucose. Insulin can be added to the glucose, but glucose alone will stimulate insulin production and cause movement of potassium from extracellular fluid (ECF) to intracellular fluid (ICF). The stimulation of alpha receptors causes increased movement of potassium from intracellular fluid (ICF) to extracellular fluid (ECF). A noradrenaline infusion can elevate serum potassium levels. An adrenaline infusion, or elevated adrenaline levels, can lower serum potassium levels. Metabolic acidosis causes a rise in extracellular potassium levels (hyperkalemia). In this situation, excess of hydrogen ions (H+) are exchanged for intracellular potassium ions, probably as a result of the cellular response to a falling blood pH. Metabolic alkalosis causes the opposite effect, with potassium moving into the cells 22.
Potassium (K+), along with other electrolytes such as sodium (Na+), chloride (Cl–), and bicarbonate (HCO3–), helps regulate the amount of fluid in your body and maintains a stable acid-base balance. Potassium is present in all body fluids, but most potassium is found within the cells (intracellularly). Only a small amount is present in fluids outside the cells and in the liquid part of the blood (called serum or plasma).
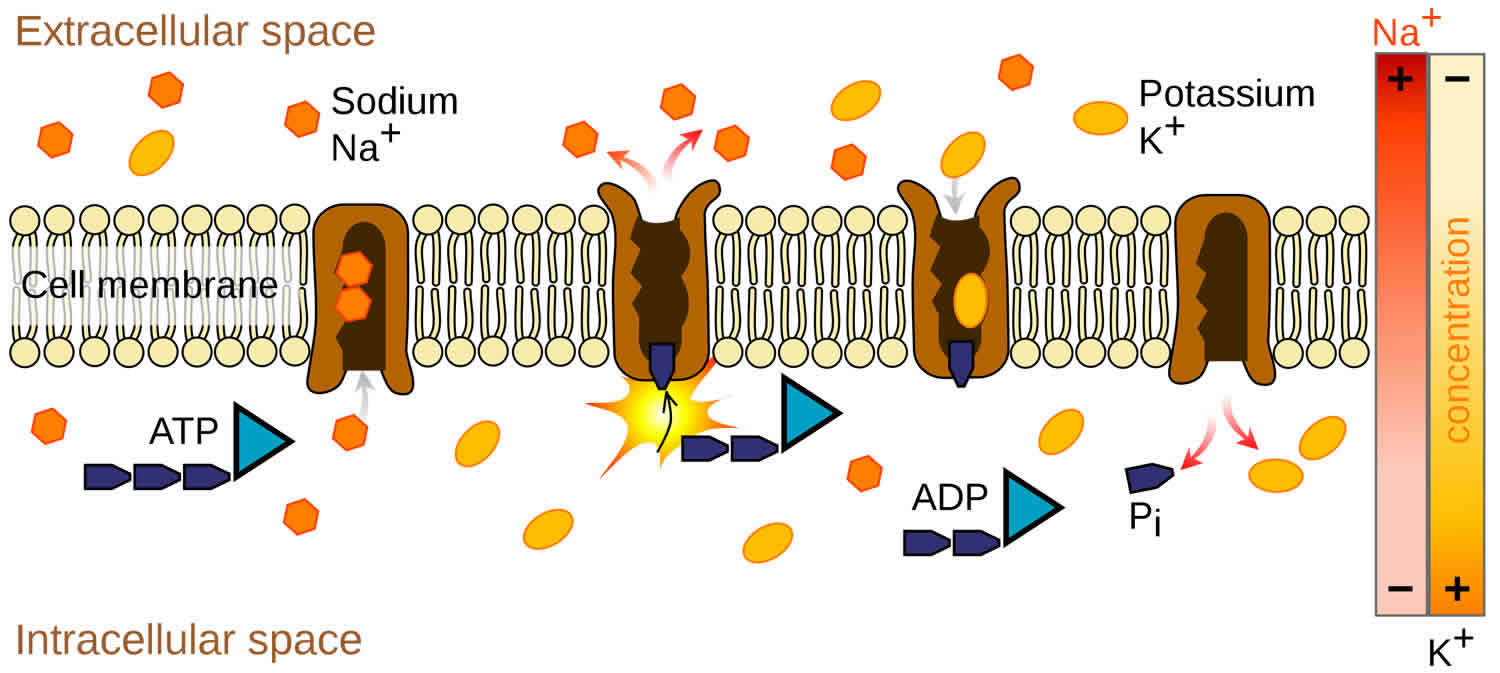
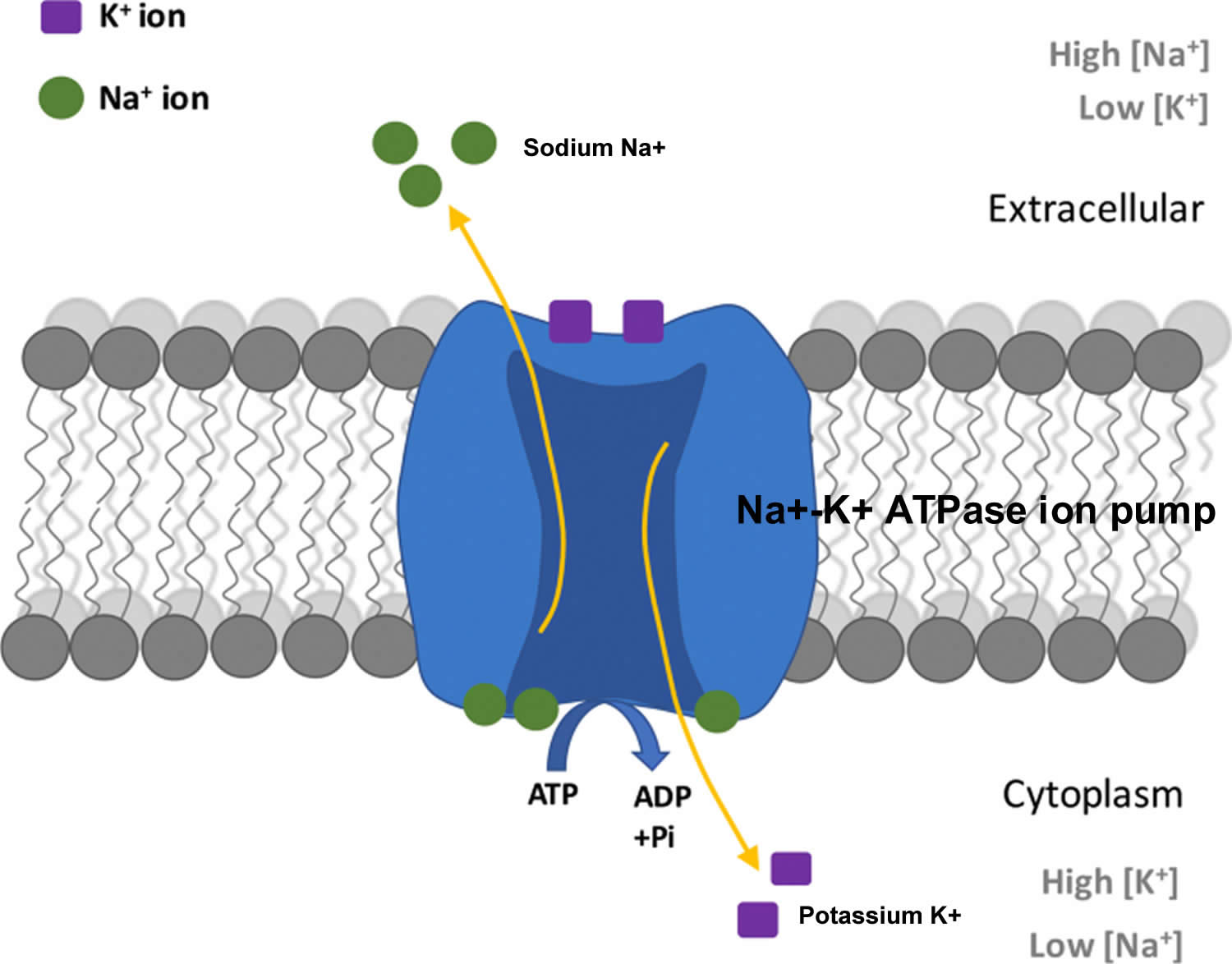
The total amount of potassium (K+) in the adult body is about 45 millimole (mmol)/kg body weight (about 140 g for a 175 pound adult; 1 millimole [mmol] = 1 milliequivalent [mEq] = 39.1 mg of potassium) 23. Most potassium are found within the cells (intracellularly) and a small amount is in extracellular fluid. The intracellular concentration of potassium is about 30 times higher than the extracellular concentration, and this difference forms a transmembrane electrochemical gradient that is maintained via the Sodium-Potassium ATPase pumps (Na+-K+ ATPase ion pumps) 24. When activated, the sodium-potassium ATPase pump (Na+-K+ ATPase ion pumps) exchanges 2 extracellular potassium (K+) ions for 3 intracellular sodium (Na+) ions, influencing membrane potential based on physiological excitation or inhibition. These sodium-potassium ATPase pumps (Na+-K+ ATPase ion pumps) are partially responsible, along with the sodium-potassium-chloride (Na+-K+-2Cl) co-transporter and sodium-calcium (Ca) exchanger, for maintaining the potential difference across the resting cell membrane as well. In addition to maintaining cellular tonicity, this gradient is required for proper nerve transmission, muscle contraction, and kidney function 20, 25, 26.
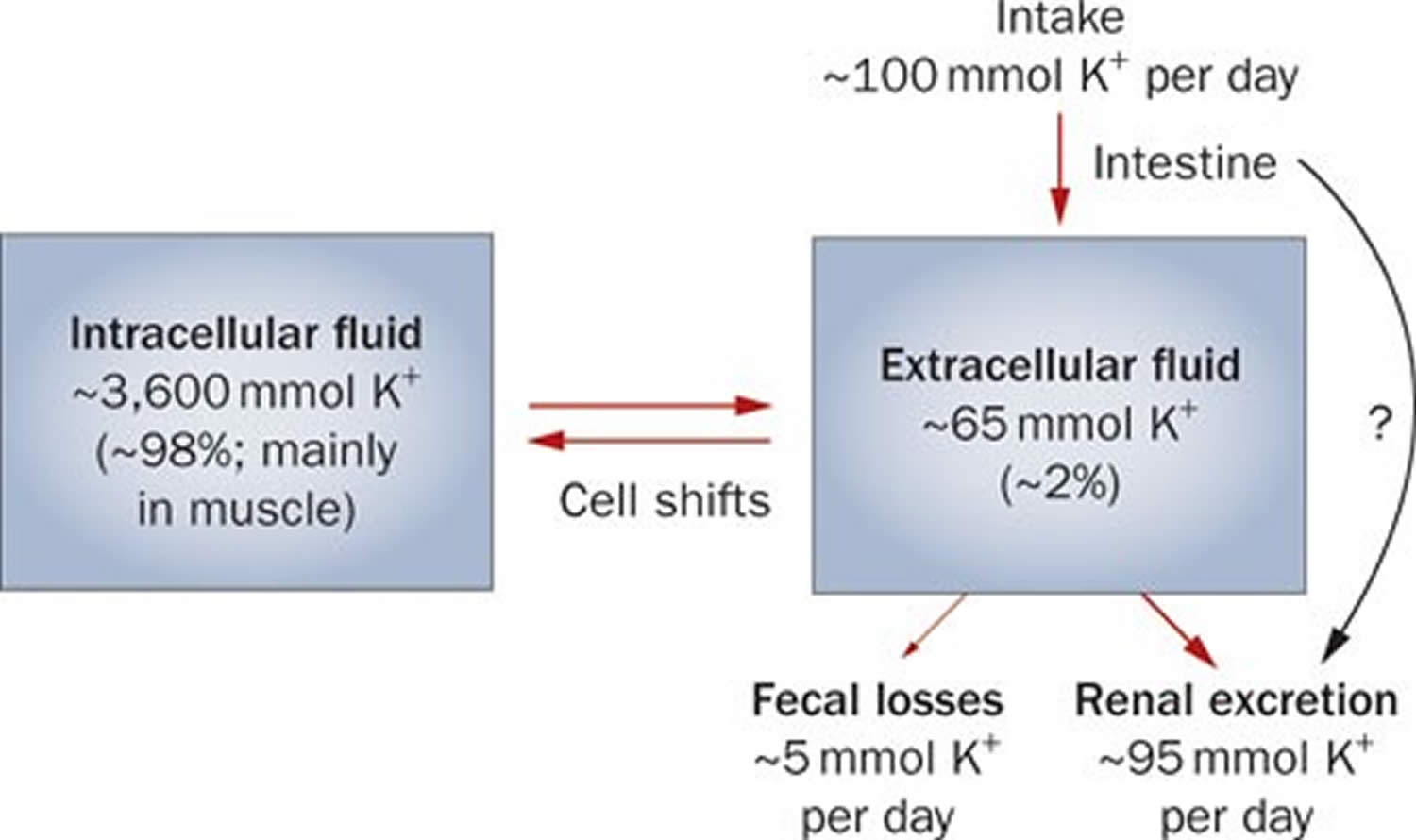
Potassium (K+) homeostasis depends on external balance (dietary intake [typically 100 mmol per day] versus excretion [95% via the kidney; 5% via the colon]) and internal balance (the distribution of potassium (K+) between intracellular and extracellular fluid compartments). The uneven distribution of potassium (K+) across cell membranes means that a mere 1% shift in its distribution can cause a 50% change in plasma potassium (K+) concentration 19. Hormonal mechanisms involving insulin, beta-adrenergic agonists and aldosterone modulate potassium (K+) distribution by promoting rapid transfer of potassium (K+) across the plasma membrane 27. Your body uses what potassium (K+) it requires and your kidneys eliminate the rest in the urine. Your body tries to keep the blood potassium level within a very narrow range. Levels are mainly controlled by aldosterone, a hormone produced by the adrenal glands in the kidneys. Extrarenal potassium (K+) losses from the body are usually small, but can be marked in individuals with chronic diarrhea, severe burns or prolonged sweating 19, 27. Under normal circumstances, the kidney’s distal nephron secretes potassium (K+) and determines final urinary excretion. In patients with low potassium levels or hypokalemia (plasma K+ concentration <3.5 mmol/l), after the exclusion of extrarenal causes, alterations in sodium ion (Na+) delivery to the distal nephron, aldosterone status, or a specific inherited or acquired defect in distal nephron function (each of which affects distal nephron K+ secretion), should be considered 19.
Figure 1. Potassium physiology
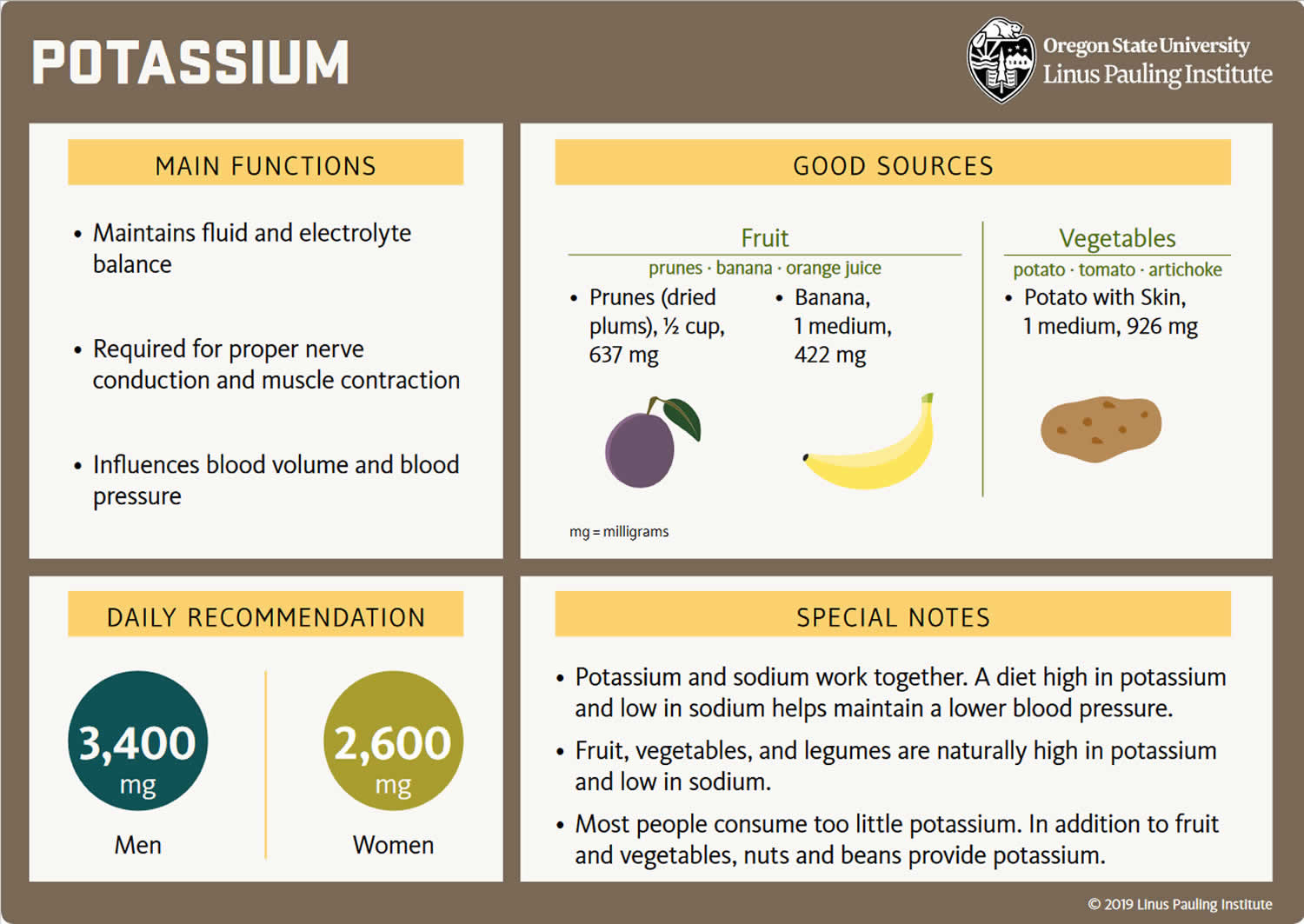
Because most potassium (K+) ions are found within the cells (a major intracellular cation), it is widely distributed in foods once derived from living tissues. Potassium concentration is higher in fruits and vegetables than in cereals and meat. You get most of the potassium you need from the foods that you eat and most people have an adequate intake of potassium. Recommended adequate intakes for potassium were set by the Food and Nutrition Board of the Institute of Medicine at 4700 mg/day 21. However it should be noted that the Food and Nutrition Board of the Institute of Medicine Recommended adequate intakes (AIs) for potassium at 4700 mg/day for adults is substantially higher than the World Health Organization’s (WHO) guidelines, which recommend 3150 mg/day for adults 28. The National Health and Nutrition Examination Survey (NHANES) data indicates that 99.2% of potassium in the US diet is naturally occurring, with the remaining 0.8% coming from fortified foods 29. These naturally occurring potassium sources include milk and other non-alcoholic beverages, as well as potatoes and fruit, which rank highest as sources of potassium intake among American adults 30. In addition, Western dietary practices with higher consumption of cereal, low nutrient density processed foods and lower consumption of fruits and vegetables has led to a diet lower in potassium and higher in sodium in recent decades 21. Salting foods and discarding the liquid induces sodium (Na+) for potassium (K+) exchange and reduces the potassium content of foods. Few Americans meet the recommended intakes; the average intake is 2591 ± 9 mg/day 29. This large gap between potassium intakes and recommended intakes led to potassium being called a shortfall nutrient in the Dietary Guidelines for Americans 31.
Actual potassium requirements would vary with an individual’s genetics, blood pressure (BP) status, and sodium intake 20. Blood pressure is currently the primary criterion for determining potassium requirements, with African Americans being more vulnerable to high blood pressure (hypertension) and more responsive to potassium supplementation than whites; individuals with high blood pressure (hypertension) are more responsive to increasing potassium intakes than individuals with normal blood pressure, and potassium having a greater benefit for those consuming a high salt diet 32. Other benefits of increasing potassium consumption may include improved blood sugar (glucose) control, glucose intolerance and insulin resistance becoming a concern for individuals with high blood pressure (hypertension) prescribed potassium wasting diuretics (water pills) 33. These differences support personalized nutrition approaches. Understanding movement of potassium within the body may help to improve these health outcomes.
Potassium is absorbed via passive diffusion, primarily in the small intestine 24. About 90% of ingested potassium is absorbed and used to maintain its normal intracellular and extracellular concentrations 34. There is around 50 mEq/kg of potassium (K+) in the body such that total body potassium (K+) in a 70-kg person is 3,500 mEq. Around 98% of potassium (K+) is found mainly within cells, and about 2% of the bodies’ potassium (K+) is in the extracellular fluid. The normal concentration of potassium (K+) in the extracellular fluid is 3.5–5.3 mEq/L. Large deviations from these values are not compatible with life.
Approximately 90% of the daily potassium (K+) intake is excreted in the urine, whereas a smaller percentage (10%) is excreted by the gastrointestinal tract in the stool and a very small amount is lost in sweat 35, 27, 36. Therefore, within the body, the kidney is the major organ responsible for potassium (K+) homeostasis. The kidneys control potassium excretion in response to changes in dietary intakes, and potassium excretion increases rapidly in healthy people after potassium consumption, unless body stores are depleted 20. The kidney facilitates potassium (K+) homeostasis by adjusting renal potassium (K+) excretion over several hours in response to a potassium load. Initial changes in extracellular potassium (K+) concentration are buffered by movement of potassium (K+) into or out of skeletal muscle cells. Internal potassium (K+) balance is a term used to refer to regulation of potassium (K+) distribution between the intracellular and extracellular space. Insulin, catecholamines, and, to a lesser extent, aldosterone are critical factors responsible for maintaining the normal internal distribution of potassium (K+) 27, 36.
The kidneys can adapt to variable potassium intakes in healthy individuals even in the setting of high dietary intake, but a minimum of 5 mmol (about 195 mg) potassium is excreted daily in urine 23. To demonstrate this, studies have shown potassium (K+) levels are kept within the normal range even when there are increases to ~15 g daily of dietary potassium (K+) intake sustained for 20 days 37, 38. Recent findings have identified the presence of an enteric potassium (K+) sensing mechanism that initiates the renal secretory process upon K+ entry into the gastrointestinal tract 36. The distal convoluted tubule has been identified as a site critical for potassium (K+) homeostasis, where it acts as a potassium (K+) sensor capable of initiating potassium (K+) excretion independent of mineralocorticoid activity 36. Combined with other obligatory losses, potassium balance cannot be achieved with intakes less than about 400–800 mg/day 27, 36.
Assessing potassium status is not routinely done in clinical practice, and it is difficult to do because most potassium in the body is inside cells 39. Although blood potassium levels can provide some indication of potassium status, they often correlate poorly with tissue potassium stores 23, 40, 41. Other methods to measure potassium status include collecting balance data (measuring net potassium retention and loss); measuring the total amount of potassium or the total amount of exchangeable potassium in the body; and conducting tissue analyses (e.g., muscle biopsies), but all have limitations 40.
Normal serum concentrations of potassium range from about 3.6 to 5.0 mmol/L and are regulated by a variety of mechanisms 23, 42. Diarrhea, vomiting, kidney disease, use of certain medications, and other conditions that alter potassium excretion or cause transcellular potassium shifts can cause low potassium level also called hypokalemia (serum potassium levels below 3.6 mmol/L) or high potassium level also called hyperkalemia (serum potassium levels above 5.0 mmol/L) 42. Otherwise, in healthy individuals with normal kidney function, abnormally low or high blood levels of potassium are rare.
Because the blood concentration of potassium is so small, minor changes can have significant consequences. If potassium levels are too low (serum potassium levels below 3.6 mmol/L) or too high (serum potassium levels above 5.0 mmol/L), there can be serious health consequences; a person may be at risk for developing shock, respiratory failure, or heart rhythm disturbances. An abnormal potassium level can alter the function of the nerves and muscles; for example, the heart muscle may lose its ability to contract.
Your body needs potassium to:
- Build proteins
- Break down and use carbohydrates
- Build muscle
- Maintain normal body growth
- Control the electrical activity of the heart
- Control the acid-base balance
Reduced potassium consumption has been associated with hypertension and cardiovascular diseases, and appropriate consumption levels could be protective against these conditions 43. A recent meta-analysis including 11 cohort studies reported an inverse association between potassium intake and risk of stroke 44. Additionally, two meta-analyses of trials comparing increased potassium to lower potassium intake found that increased potassium intake lowers blood pressure 45, 46. These results were further supported by a systematic review without a meta-analysis, which concluded that increased potassium intake results in decreased blood pressure in adults 47. Thus, a public health intervention aimed at increasing potassium intake from food could be a cost-effective strategy to reduce the burden of cardiovascular morbidity and mortality. Moreover, increasing potassium consumption from food in the population is safe; in individuals without renal impairment caused by medical conditions or drug therapy, the body is able to efficiently adapt and excrete excess potassium via the urine when consumption 48.
The American Heart Association recommended potassium intake for an average adult is 4,700 milligrams (mg) per day. Most of us aren’t getting nearly that much. On average, adult males eat almost 3,200 mg/day, and adult females eat about 2,400 mg/day 49. Remember that potassium is only part of an overall heart-healthy eating pattern. Other dietary factors that may affect blood pressure include amount and type of dietary fat; cholesterol; protein, sugar and fiber; calcium and magnesium, and of course, sodium.
For example, the DASH (Dietary Approaches to Stop Hypertension) diet study found that a diet rich in fruits, vegetables, fat-free or low-fat (1 percent) milk and milk products, whole-grain foods, fish, poultry, beans, seeds and unsalted nuts reduced blood pressure compared to a typical American diet. The DASH eating plan also had less sodium; sweets, added sugars and sugar-containing beverages; saturated and trans fats; and red meats than the typical American diet.
People with kidney problems, especially those on dialysis, should not eat too many potassium-rich foods. Your health care provider will recommend a special diet.
What does potassium do?
Potassium (K+) is the principal positively charged ion (cation) in the fluid inside of cells, while sodium (Na+) is the principal cation in the extracellular fluid. Potassium (K+) concentrations are about 30 times higher inside than outside cells, while sodium (Na+) concentrations are more than 10 times lower inside than outside cells 50. The concentration differences of these charged particles causes a difference in electric potential between the inside and outside of cells, known as the membrane potential. A cell’s membrane potential is maintained by ion pumps in the cell membrane, especially the Sodium-Potassium ATPase pumps (Na+-K+ ATPase ion pumps). These sodium-potassium ATPase pumps (Na+-K+ ATPase ion pumps) use ATP (energy) to pump sodium (Na+) of the cell and potassium (K+) into the cell, leading to a potassium (K+) gradient across the cell membrane [potassium (K+) in > potassium (K+) out], which is partially responsible for maintaining the cell membrane potential (Figure 2). The sodium-potassium ATPase pumps (Na+-K+ ATPase ion pumps) activity has been estimated to account for 20%-40% of the resting energy consumption in a typical adult 50. The large proportion of energy dedicated to maintaining sodium/potassium concentration gradients emphasizes the importance of this function in sustaining life 50. The cell membrane potential created by potassium and sodium ions allows the cell generate an action potential–a “spike” of electrical discharge. The ability of cells to produce electrical discharge is critical for body functions such as nerve impulse transmission, muscle contraction, and heart function 51, 52, 53.
Potassium is also an essential mineral needed to regulate water balance, blood pressure and levels of acidity 54. The more potassium you eat, the more sodium you pass out of the body through urine. Increased potassium intake has no adverse effect on blood lipid concentration, catecholamine concentrations, or renal function in apparently healthy adults without impaired renal handling of potassium 49. The largest benefit was detected when sodium intake was more than 4 g/day, which is the intake of most populations globally 55, so increased potassium intake should benefit most people in most countries. However, the authors also found a statistically significant decrease in blood pressure with increased potassium when sodium intake was 2-4 g/day. Therefore, increased potassium can continue to be beneficial in terms of blood pressure even as individuals and populations decrease their sodium intake. Studies examining both nutrients simultaneously support this concept, showing an increased benefit with simultaneous reduction in sodium and increase in potassium compared with changes in one nutrient individually 56, 57.
Potassium also helps relax blood vessel walls, which helps lower blood pressure 49.
World Health Organization recommends an increase in potassium intake from food to reduce blood pressure and risk of cardiovascular disease, stroke and coronary heart disease in adults. World Health Organization suggests a potassium intake of at least 90 mmol/day (3510 mg/day) for adults (conditional recommendation) 48.
Potassium also acts as a cofactor for some enzymes activity. For example, the activation of Na+/K+-ATPase requires the presence of sodium and potassium. The presence of potassium is also required for the activity of pyruvate kinase, an important enzyme in carbohydrate metabolism 58.
Figure 2. Sodium-Potassium ATPase pump
What are normal potassium levels?
Normal serum potassium values are between 3.5 to 5.0 millimoles/L (mmol/L) or 3.5 to 5.0 milliequivalent/L (mEq/L) 17, 59, 60, 61. However, there can be slight variation between laboratories and for this reason, it is important to look for the specific reference interval listed on your test report. Potassium levels outside this range, 3.5 to 5.0 millimoles/L (mmol/L) or 3.5 to 5.0 milliequivalent/L (mEq/L), are not compatible with life with increased rates of death from several causes 62, 63.
Your health care provider may order a potassium blood test as part of your regular checkup or to monitor an existing condition, such as diabetes, kidney disease, or adrenal gland disorders. You may also need this test if you take medicines that could affect your potassium levels or if you have symptoms of having too much or too little potassium.
Interpretation of a potassium test requires carefully considering the result, the laboratory reference range, and your health situation. Because potassium is frequently measured with other electrolytes, levels may be evaluated together. For a blood test, the report should list the amount of potassium measured in either milliequivalents per liter (mEq/L) or millimoles per liter (mmol/L). The test report will also show a reference range, which the laboratory considers an expected range for potassium levels.
What are high potassium levels?
High potassium levels also known as hyperkalemia is defined as serum potassium level greater than 5 mEq/L or greater than 5 mmol/L (Kim MJ, Valerio C, Knobloch GK. Potassium Disorders: Hypokalemia and Hyperkalemia. Am Fam Physician. 2023 Jan;107(1):59-70.https://www.aafp.org/pubs/afp/issues/2023/0100/potassium-disorders-hypokalemia-hyperkalemia.html)).
If your potassium levels are too high or hyperkalemia, your symptoms may include 64:
- Arrhythmia (a problem with the rate or rhythm of your heartbeat)
- Fatigue
- Muscle weakness
- Nausea
- Numbness or tingling
Too much potassium in the blood or hyperkalemia is often the result of two or more causes. High potassium levels may be a sign of 64:
- Kidney disease. Your kidneys remove extra potassium from your body. Too much potassium may mean your kidneys aren’t working well.
- Addison disease also called adrenal insufficiency, is a disorder in which your immune system mistakenly attacks your adrenal glands, damaging the adrenal cortex. Other causes include infections and cancer. The damage causes the adrenal glands cortex not to make enough of the hormone cortisol and sometimes the hormone aldosterone.
- Injuries, burns, or surgery that can cause your cells to release extra potassium into your blood
- Type 1 diabetes that is not well controlled
- The side effects of certain medicines, such as diuretics (water pills) or antibiotics
- A diet too high in potassium (not common). Bananas, apricots, green leafy vegetables, avocados and many other foods are good sources of potassium that are part of a healthy diet. But eating very large amounts of potassium-rich foods or taking potassium supplements can lead to health problems.
Hyperkalemic periodic paralysis cause
Hyperkalemic periodic paralysis is caused by mutations in the sodium voltage-gated channel alpha subunit 4 gene or SCN4A gene on chromosome 17q23-25 (long arm of chromosome 17) 65, 66, 67, 7. The SCN4A gene belongs to a family of genes that provide instructions for making a critical part (the alpha subunit) sodium channels (Nav1.4) that are abundant in muscles that plays an essential role in muscles used for movement (skeletal muscles) 68, 68. These voltage-gated sodium channel type 4 alpha, which transport positively charged sodium atoms (sodium ions Na+) into cells, play a key role in a cell’s ability to generate and transmit electrical signals. For your body to move normally, your skeletal muscles must tense (contract) and relax in a coordinated way. One of the changes that helps trigger muscle contractions is the flow of positively charged atoms (cations), including sodium ions (Na+), into muscle cells. The SCN4A protein forms channels that control the flow of sodium ions (Na+) into these cells. Mutations in the SCN4A gene alter the usual structure and function of sodium channels. The altered sodium channels stay open too long or do not stay closed long enough, allowing more sodium ions to flow into muscle cells 7. This increase in sodium ions triggers the release of potassium from muscle cells, which causes more sodium channels to open and stimulates the flow of even more sodium ions into these cells. These changes in ion transport reduce the ability of skeletal muscles to contract, leading to episodes of muscle weakness or paralysis.
In 30 to 40 percent of cases, the cause of hyperkalemic periodic paralysis is unknown. Changes in other genes, which have not been identified, likely cause hyperkalemic periodic paralysis in these cases.
Hyperkalemic periodic paralysis triggers
The major attack trigger is eating potassium-rich foods such as bananas and potatoes in affected individuals, even if blood potassium levels do not go up (see Table 1). Fruits high in potassium include cantaloupes, apricots (fresh and dried) dried figs, kiwi fruit, peaches, raisins, banana and prunes. Fruit juices are high in potassium, especially orange and pineapple juice and apricot and peach nectars. High potassium vegetables include artichoke, parsnip, potato, pumpkin, spinach, broccoli, Brussel sprouts, cauliflower, tomato juice, tomato paste and V-8 juice. Lentils and beans are high in potassium. Other foods that are high in potassium include nuts, peanut butter and chocolate.
The other major attack triggers include: exposure to hot or cold temperatures; changes in humidity; potassium supplements; rest after exercise; stress, or fatigue; alcohol; hunger or periods without food (fasting); specific foods or beverages; extra sleep; pregnancy; certain medications; illness of any type; menstruation; and changes in activity level 7, 69, 11. A spontaneous attack commonly starts in the morning before breakfast, lasts for 15 minutes to one hour, and then passes. Individuals with hyperPP frequently have myotonia (muscle stiffness), especially around the time of an episode of weakness. Paramyotonia (muscle stiffness aggravated by cold and exercise) is present in about 45% of affected individuals. Muscle strength usually returns to normal between attacks, although many affected people continue to experience mild stiffness (myotonia), particularly in muscles of the face and hands 7. More than 80% of individuals with hyperkalemic periodic paralysis (hyperPP) older than age 40 years report permanent muscle weakness and about one third develop a chronic progressive myopathy 2.
Of note, attacks occur more frequently on holidays and weekends when people rest in bed longer than usual 2.
Table 1. Potassium rich foods
| Food | Milligrams (mg) per serving | Percent DV* |
|---|---|---|
| Apricots, dried, ½ cup | 755 | 16 |
| Lentils, cooked, 1 cup | 731 | 16 |
| Squash, acorn, mashed, 1 cup | 644 | 14 |
| Prunes, dried, ½ cup | 635 | 14 |
| Raisins, ½ cup | 618 | 13 |
| Potato, baked, flesh only, 1 medium | 610 | 13 |
| Kidney beans, canned, 1 cup | 607 | 13 |
| Orange juice, 1 cup | 496 | 11 |
| Soybeans, mature seeds, boiled, ½ cup | 443 | 9 |
| Banana, 1 medium | 422 | 9 |
| Milk, 1%, 1 cup | 366 | 8 |
| Spinach, raw, 2 cups | 334 | 7 |
| Chicken breast, boneless, grilled, 3 ounces | 332 | 7 |
| Yogurt, fruit variety, nonfat, 6 ounces | 330 | 7 |
| Salmon, Atlantic, farmed, cooked, 3 ounces | 326 | 7 |
| Beef, top sirloin, grilled, 3 ounces | 315 | 7 |
| Molasses, 1 tablespoon | 308 | 7 |
| Tomato, raw, 1 medium | 292 | 6 |
| Soymilk, 1 cup | 287 | 6 |
| Yogurt, Greek, plain, nonfat, 6 ounces | 240 | 5 |
| Broccoli, cooked, chopped, ½ cup | 229 | 5 |
| Cantaloupe, cubed, ½ cup | 214 | 5 |
| Turkey breast, roasted, 3 ounces | 212 | 5 |
| Asparagus, cooked, ½ cup | 202 | 4 |
| Apple, with skin, 1 medium | 195 | 4 |
| Cashew nuts, 1 ounce | 187 | 4 |
| Rice, brown, medium-grain, cooked, 1 cup | 154 | 3 |
| Tuna, light, canned in water, drained, 3 ounces | 153 | 3 |
| Coffee, brewed, 1 cup | 116 | 2 |
| Lettuce, iceberg, shredded, 1 cup | 102 | 2 |
| Peanut butter, 1 tablespoon | 90 | 2 |
| Tea, black, brewed, 1 cup | 88 | 2 |
| Flaxseed, whole, 1 tablespoon | 84 | 2 |
| Bread, whole-wheat, 1 slice | 81 | 2 |
| Egg, 1 large | 69 | 1 |
| Rice, white, medium-grain, cooked, 1 cup | 54 | 1 |
| Bread, white, 1 slice | 37 | 1 |
| Cheese, mozzarella, part skim, 1½ ounces | 36 | 1 |
| Oil (olive, corn, canola, or soybean), 1 tablespoon | 0 | 0 |
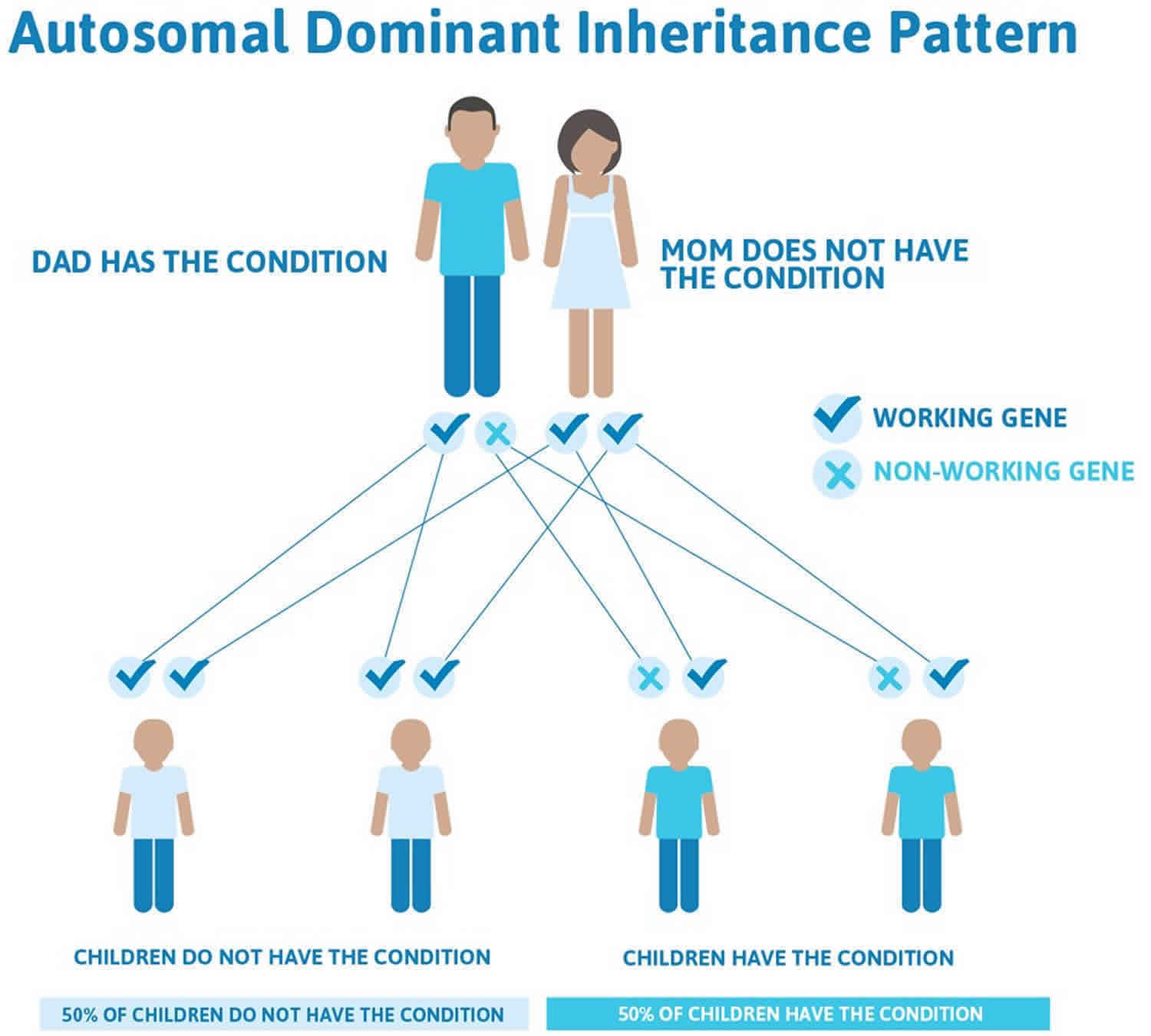
Hyperkalemic periodic paralysis inheritance pattern
Hyperkalemic periodic paralysis is inherited in an autosomal dominant pattern, which means one copy of the altered gene in each cell is sufficient to cause the disorder. In most cases, an affected person inherits the SCN4A gene mutation from one affected parent.
In 30 to 40 percent of cases, the cause of hyperkalemic periodic paralysis is unknown. Changes in other genes, which have not been identified, likely cause hyperkalemic periodic paralysis in these cases.
Figure 3. Hyperkalemic periodic paralysis autosomal dominant inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Hyperkalemic periodic paralysis pathophysiology
Hyperkalemic periodic paralysis (HyperPP) is an autosomal dominantly inherited disease characterized by episodic paralytic attacks with hyperkalemia, and is caused by mutations of the SCN4A gene encoding the skeletal muscle type voltage-gated sodium channel Nav1.4, which is composed of four repeated domains (Domain I–IV) consisting of six transmembrane segments (S1–S6), with S1–S4 forming the voltage-sensing domain (VSD) and S5 and S6 forming the pore domain (PD) 71. The skeletal muscle type voltage-gated sodium channel Nav1.4 is co-expressed with voltage-gated sodium channel beta subunit 1 encoded by the SCN1B gene 72, and a recent study revealed the structure of the Nav1.4 complex by cryo-electron microscopy 73.
Nav1.4 has three major functional states; the closed state, the open state, and the inactivated state 71. The ion conductive pore of Nav1.4 remains closed at the resting potentials of the myofibers (the closed state). Depolarization of the membrane potential induces the conformational change of the Nav1.4 proteins mainly in the voltage-sensing domain (VSD), resulting in the pore gate opening which allows the passage of sodium ions (Na+) (the open state). Prolonged depolarizing stimulus induces another conformational state, the inactivated state, which prevents the passage of sodium ions (Na+) through the pore by additional structural rearrangement. The inactivated state is divided into two types according to the time course; fast inactivation induced by sustained depolarization for hundreds of milliseconds and slow inactivation induced by sustained depolarization for seconds to minutes. Fast inactivation has been shown to be related to the structural rearrangement in voltage-sensing domain (VSD) of Domain IV followed by that in the cytosolic linker between Domain III and Domain IV 74, 75. Although the structure–function relationship in slow inactivation has not been fully elucidated, previous reports indicated that the structural rearrangement in pore domain (PD) would be associated with it 76.
The pathological mechanism of hyperkalemic periodic paralysis was suggested to be associated with gain-of-function changes for Nav1.4 gating, some of which are defects of slow inactivation. Excitable cells at the motor endplate have a resting membrane potential of −70 mV and a threshold potential of −55 mV. These gross voltages are predominantly due to the resting potentials of potassium (Ek −75 mV) and sodium (Ena +55 mV). HyperKPP pathophysiology is triggered by a slight increase in extracellular potassium (K+), most commonly resulting from the ingestion of potassium-rich food, rest after a heavy workout, periods of fasting, emotional stress, pregnancy, exposure to cold, surgery and anaesthesia 11. This slight potassium (K+) increase may still be within normal laboratory values but causes a minor membrane depolarisation. If an impulse is generated, an unknown percentage of mutated NaV 1.4 channels may fail to inactivate, leading to a prolonged increase in intracellular sodium ion (Na+) and persistent cell depolarisation 77. If intervention occurs at this point, often paralysis can be avoided, and weakness will be transient. If this continues, the cycle undergoes a pathologic feedback loop with worsening membrane excitability at baseline from (1) the higher intracellular Na+ concentration driving K+ extracellularly and (2) continuation of the initial hyperkalemic trigger event. The net result of this cycle is the subsequent loss of electrical excitability and thus paralysis 78.
The sodium channel has an alpha subunit and a beta subunit. The alpha subunit of the sodium channel is a 260-kd glycoprotein comprising about 1800-2000 amino acids. This channel is highly conserved evolutionarily from Drosophila to human. It has 4 homologous domains (I-IV) that fold to form a central pore, each with 225-325 amino acids. Each domain consists of 6 hydrophobic segments (S1-S6) traversing the cell membrane. The main functions of the sodium channel include voltage-sensitive gating, inactivation, and ion selectivity. The extracellular loop between S5 and S6 dips into the plasma membrane and participates in the formation of the pore. The S4 segment contains positively charged amino acids at every third position and functions as a voltage sensor. Conformation changes may occur during depolarization, resulting in activation and inactivation of the channel. The cellular loop between domain III-S6 and domain IV-S1 acts as an inactivating gate.
Many SCN4A mutations associated with diseases have been found in functional studies using cultured cell lines and Xenopus oocytes expressing mutant Nav1.4 channels 71, and most of them showed gain-of-function mutations in Nav1.4. For paramyotonia congenita or sodium channel myotonia, the mutant channels represent defective fast inactivation and/or enhancement of activation, leading to pathological myotonia 71. For HyperPP, two representative variants have been reported so far, T704M and M1592V, and they are located in the transmembrane segments of the PD and represent defective slow inactivation 79, 80.
Two populations of channels exist, mutant and wild-type; the impaired fast-inactivation results in prolonged depolarization of the mutant muscle fiber membranes and can explain the 2 cardinal symptoms of these disorders, myotonia and weakness. In hyperkalemic periodic paralysis, a gain of function occurs in mutant channel gating, resulting in an increased sodium current excessively depolarizing the affected muscle. Mild depolarization (5-10 mV) of the myofiber membrane, which may be caused by increased extracellular potassium concentrations, results in the mutant channels being maintained in the noninactivated mode. The persistent inward sodium current causes repetitive firing of the wild-type sodium channels, which is perceived as stiffness (ie, myotonia).
If a more severe depolarization (20-30 mV) is present, both normal and abnormal channels are fixed in a state of inactivation, causing weakness or paralysis. Thus, subtle differences in severity of membrane depolarization may make the difference between myotonia and paralysis. Temperature sensitivity is a hallmark of paramyotonia congenita. Cold exacerbates myotonia and induces weakness. A number of mutations are associated with this condition, 3 of them at the same site in the S4 segment. These mutations replace arginine with other amino acids and neutralize this highly conserved S4 positive charge. Mutations of these residues are the most common cause of paramyotonia congenita. Some of the possible mechanisms responsible for temperature sensitivity include the following:
- Temperature may differentially affect the conformational change in the mutant channel.
- Lower temperatures may stabilize the mutant channels in an abnormal state.
- Mutations may alter the sensitivity of the channel to other cellular processes, such as phosphorylation or second messengers.
Most cases of hyperkalemic periodic paralysis are due to 2 mutations in SCN4A, T704M, and M1592V. Mutations in the sodium channel, especially at residues 1448 and 1313, are responsible for paramyotonia congenita.
Hyperkalemic periodic paralysis prevention
Preventive measures for individuals with hyperkalemic periodic paralysis (hyperPP) consist of frequent meals rich in carbohydrates and avoidance of the following 2:
- Potassium-rich medications and foods (e.g., fruits, fruit juices)
- Fasting
- Strenuous work
- Exposure to cold
- Opioids or depolarizing agents such as potassium, anticholinesterases, and succinylcholine as part of general anesthesia. These can aggravate a myotonic reaction and induce masseter spasms and stiffness of respiratory muscles, which may impair intubation; mechanical ventilation may also be impaired.
- Drugs known as ACE-inhibitors for the treatment of arterial hypertension. These may lead to hyperkalemia as a side effect, especially if they are combined with potassium-sparing diuretics (e.g., spironolactone) and/or renal function is impaired.
- Alterations of serum osmolarity, pH, and hypothermia-induced muscle shivering and mechanical stimuli during general anesthesia 81. These can exacerbate the myotonic reaction in individuals with hyperkalemic periodic paralysis.
A low potassium, high carbohydrate diet, and light exercise may help prevent attacks. Avoiding fasting, strenuous activity, or cold temperatures also may help.
Early start to the day
As attacks occur more frequently on holidays and weekends when people rest in bed longer than usual, individuals are advised to rise early and have a full breakfast.
Lifestyle
Individuals should prioritize avoidance or minimization of triggers whenever possible by keeping stress levels low and avoiding exercise that is overly intense (as rest after such exercise is a trigger).
Diuretics (water pills)
It is often advisable to prevent hyperkalemic attacks of weakness by the continuous use of a thiazide diuretic or a carbonic anhydrase inhibitor, such as acetazolamide or dichlorphenamide.
Note: In a trial of dichlorphenamide, the median attack rate was lower in participants with hyperkalemic periodic paralysis on dichlorphenamide than in participants with hyperkalemic periodic paralysis on placebo (0.9 vs 4.8), but the difference in median attack rate was not significant 82. Diuretics are used in modest dosages at intervals from twice daily to twice weekly.
- Thiazide diuretics are preferable because they have fewer side effects than either acetazolamide or dichlorphenamide therapy.
- The dosage should be kept as low as possible (e.g., 25 mg hydrochlorothiazide daily or every other day). In severe cases, 50 mg or 75 mg of hydrochlorothiazide should be taken daily very early in the morning.
- Individuals should be monitored so that the serum potassium concentration does not fall below 3.3 mmol/L or the serum sodium concentration below 135 mmol/L 83. Thiazides may be helpful even if the serum potassium concentration is in the normal range 84.
- Four weeks after start of diuretic treatment, effects should be evaluated by muscle strength measurement and MRI of proximal leg muscles.
Evaluation of relatives
It is appropriate to evaluate apparently asymptomatic older and younger at-risk relatives of an individual with hyperkalemic periodic paralysis in order to identify as early as possible those who would benefit from initiation of preventive measures, particularly those that would decrease the risk of unexpected acute paralysis or anesthetic events. Evaluations include:
- Molecular genetic testing if the pathogenic variant in the family is known;
- Full neurologic examination to rule out muscular weakness and electromyogram (EMG) to rule out myotonia if the pathogenic variant in the family is not known.
At-risk relatives who have not undergone molecular genetic testing or clinical evaluation (i.e., neurologic examination and electromyogram (EMG)) must be considered at risk for hyperkalemic periodic paralysis-related complications and precautions are indicated – particularly during anesthesia.
Hyperkalemic periodic paralysis symptoms
Hyperkalemic periodic paralysis symptoms include attacks of muscle weakness or loss of muscle movement (paralysis) that come and go. There is normal muscle strength between attacks. Attacks usually begin in childhood. How often the attacks occur varies. Some people have several attacks a day. They are usually not severe enough to need therapy. Some people have associated myotonia, in which they cannot immediately relax their muscles after use.
The muscle weakness or paralysis:
- Most commonly occurs at the shoulders, back, and hips
- May also involve the arms and legs, but does not affect muscles of the eyes and muscles that help with breathing and swallowing
- Most commonly occurs while resting after activity or exercise
- May occur on awakening
- Occurs on and off
- Usually lasts 15 minutes to 1 hour, but may last up to an entire day
Triggers may include:
- Eating potassium-rich foods or taking medicines that contain potassium
- Rest after exercise
- Exposure to cold
- Skipping meals
- Stress
Hyperkalemic periodic paralysis (hyperPP) has three clinically distinct signs and symptoms:
- Without myotonia (muscle stiffness),
- With clinical or electromyographic (EMG) myotonia, or
- With paramyotonia congenita (muscle stiffness aggravated by cold and exercise beginning in infancy or early childhood).
In all three forms, the course of the paralytic attacks is the same 85, 15. Electrical myotonia (muscle stiffness) can be demonstrated on EMG in 50 to 75% of patients with hyperPP, while less than 20% manifest clinically 86. In those with muscle stiffness (myotonia), i.e., a tonic spasm of muscle 87, the myotonia is often mild and can be provoked with percussion or activity in the face, tongue, forearms, and thenar eminence 11. The myotonia eases with repetitive activity 15. From birth onwards, those with paramyotonia congenita (muscle stiffness aggravated by cold and exercise beginning in infancy or early childhood) experience muscle stiffness that increases with continued activity (paramyotonia) and is cold-induced 9. Paralytic attacks can occur at any time, though often occur spontaneously in the morning prior to breakfast, last up to an hour, and unpredictably subside 15, 2. Attacks may be provoked or worsened by anesthesia 16, rest after exercise, potassium loading, cold environments, hunger, emotional stress, glucocorticoids, or pregnancy. During attacks, individuals may be hyperkalemic (high potassium levels) or normal serum potassium levels (normokalemic) 7. The concomitant rise in serum potassium levels can range from upper normal values to those in the cardiotoxic spectrum 6. After an attack, serum potassium levels may be transiently low due to the elimination of potassium from the kidneys and the reuptake of potassium by muscle 85. Usually, individuals do not experience cardiac arrhythmias or respiratory insufficiency during attacks 2. After an attack, individuals may feel pain for up to several days in the involved muscle groups 15. Between attacks, affected individuals have normal serum potassium levels (normokalemia), normal sensation and muscle stretch reflexes, and normal muscle strength, although they may experience minor myotonia that does not hinder voluntary movement 11. “Lid lag” secondary to eyelid myotonia may be the only clinical sign present between attacks 15, 2.
The attacks of flaccid muscle weakness associated with hyperkalemic periodic paralysis (hyperPP) usually begin in the first decade of life and increase in frequency and severity over time, with 25% experiencing their sentinel attack in the second decade of life. Initially infrequent, the attacks increase in frequency and severity over time until approximately age 50 years, after which the frequency declines considerably.
Pattern of attacks
A spontaneous attack commonly starts in the morning before breakfast, lasts for 15 minutes to an hour, and then passes and may not happen often, though some patients have several episodes per day 2. In about 20% of affected individuals the attacks last considerably longer, from more than two days to more than a week 2.
In some individuals, a burning or prickling sensation or “pins-and-needles” sensation that is usually felt in the hands, arms, legs, or feet (paresthesias) probably induced by the hyperkalemia herald the weakness. During an attack of weakness, the muscle stretch reflexes are abnormally diminished or absent. Difficulty swallowing (dysphagia) during an attack of weakness has also been described 88. The strength of the attacks is not always consistent; sometimes the patient only feels fatigued, but can still move around slowly. Other times the patients are completely paralyzed. Sometimes attacks may come very suddenly.
Individuals most commonly describe their attacks as stiffness followed by weakness, although many have described their attacks as some other permutation of weakness and/or stiffness. The arms and hands are just as frequently affected as the thighs and calves 11.
Frequency of attacks can vary greatly among individuals. Some have attacks every day, others several times a month; others have them every few months or less often.
Usually, cardiac arrhythmia or respiratory insufficiency does not occur during the attacks. When present, respiratory insufficiency manifests as shortness of breath. In a study by Charles et al 11, 26% of subjects reported that their breathing musculature was affected and 62% reported that their face was affected during attacks. The mouse model has demonstrated a resistance to weakness triggered by hyperkalemia in diaphragmatic muscle as compared to skeletal muscle 89.
Individuals learn that they have to stay warm, avoid unaccustomed heavy exercise, and not sit still too long at a time. Many find they can abort a developing episode by drinking a sweet beverage or eating some hard candy at the first sign of an attack. Unfortunately this can lead to weight gain.
Between paralytic attacks findings
After an attack, affected individuals report clumsiness, weakness, and irritability, and in 62% muscle pain secondary to the attack 2. One observational study identified fibromyalgia in half of the individuals surveyed who had hyperPP 90. Between attacks, the majority report no or mild symptoms. However, 12% report severe symptoms between attacks that impair activities of daily living 90.
Muscle issues
Individuals with hyperPP frequently (i.e., >50% of the time) have myotonia (muscle stiffness), especially around the time of an episode of weakness. Mild myotonia (muscle stiffness) that does not impede voluntary movements is often present between attacks 2. Myotonia is most readily observed in the facial, lingual, thenar, and finger extensor muscles; eyelid myotonia (lid lag myotonia) has been rarely reported. Paramyotonia (muscle stiffness aggravated by cold and exercise) is present in about 45% of affected individuals 2. Of individuals with myotonia, 37% have experienced progressive myopathy, while of those reporting absence of myotonia, 33% have experienced progressive myopathy 11.
Bradley et al 4 reported more than 80% of the affected individuals older than 40 years to have permanent muscle weakness and approximately one third of older affected individuals developed a chronic progressive myopathy. The myopathy mainly affects the pelvic girdle and proximal and distal lower-limb muscles. A more recent study using MRI reveals an even earlier onset of progressive myopathy: progressive myopathy was observed even in individuals at the second and third decades of life with myopathic findings prominent in the gastrocnemius muscle. Muscle atrophy, edematous change, and fatty change were prominent in the superficial posterior compartment of the lower leg 91.
Thyroid dysfunction
As shown by an observational study, individuals with hyperPP appear to be at higher risk for thyroid dysfunction than those in the general population 11.
Hyperkalemic periodic paralysis complications
Health problems that may be due to hyperkalemic periodic paralysis include:
- Kidney stones (a side effect of medicine used to treat the condition)
- Irregular heart beat
- Muscle weakness that slowly continues to get worse
Hyperkalemic periodic paralysis differential diagnosis
In addition to the pathogenic variants in SCN4A gene, other conditions with periodic paralysis or with hyperkalemia to consider when making the diagnosis of hyperkalemic periodic paralysis (hyperPP) are discussed below.
Several types of myotonia and periodic paralyses are caused by pathogenic variants in SCN4A gene. All of the phenotypes occur in association with a heterozygous pathogenic variant in SCN4A except for congenital myasthenic syndrome, which is associated with biallelic pathogenic variants (autosomal recessive inheritance) (see Table 2). Some SCN4A pathogenic variants may be associated with more than one phenotype. For example, the clinical overlap of paramyotonia congenita and hyperkalemic periodic paralysis (hyperPP) is extensive, and family members with the same pathogenic variant may have a syndrome typical of paramyotonia congenita or hyperPP 92.
Adult onset periodic paralysis points to other diagnoses such as the Andersen-Tawil syndrome or secondary acquired forms of hyperkalemic periodic paralysis.
The following signs and symptoms suggest a diagnosis other than hyperkalemic periodic paralysis (hyperPP):
- Associated sensory symptoms, including pain or tenderness
- Sensory loss could suggest polyneuropathy such as Guillain-Barré syndrome.
- Pain could suggest myositis; however, some individuals with hyperkalemic periodic paralysis (hyperPP) report paralytic episodes as painful and show symptoms of fibromyalgia.
- Urinary retention or constipation, which may be observed in other causes of acute or subacute paralysis, but can occur rarely in hyperkalemic periodic paralysis (hyperPP). Bowel incontinence and bladder incontinence during attacks are reported in hyperkalemic periodic paralysis (hyperPP).
- Associated symptoms that suggest myasthenia or involvement of the neuromuscular junction, including:
- Ptosis (Lid lag myotonia, which may mimic ptosis, may rarely be reported in hyperPP; see Clinical Description.)
- Diplopia
- Dysphagia (may rarely be reported in hyperkalemic periodic paralysis)
- Dysarthria
- Alteration or loss of consciousness
- Abnormal movement
- History of fever days before an attack, which could suggest poliomyelitis or other virus-caused paralysis
- History of back pain days before an attack, which could suggest acute transverse myelitis or Guillain-Barré syndrome
- History of tick bite, which could suggest tick paralysis
The four major differential diagnoses of hyperkalemic periodic paralysis (hyperPP) are (see Table 3):
- Hypokalemic periodic paralysis (hypoPP). HypoPP is the most common cause of periodic paralysis.
- Normokalemic potassium-sensitive periodic paralysis (normoPP),
- Thyrotoxic periodic paralysis (TPP). Thyrotoxic periodic paralysis (TPP) typically mimics hypokalemic periodic paralysis (hypoPP). Individuals with paralytic attacks associated with hypokalemia and hyperthyroidism should be evaluated for thyrotoxic periodic paralysis (TPP).
- Andersen-Tawil syndrome (ATS). Andersen-Tawil syndrome is characterized by a triad of episodic flaccid muscle weakness (i.e., periodic paralysis), ventricular arrhythmias and prolonged QT interval, and anomalies including low-set ears, widely spaced eyes, small mandible, fifth-digit clinodactyly, syndactyly, short stature, and scoliosis. The periodic paralysis may be accompanied by hypokalemia, normokalemia, or hyperkalemia. Affected individuals present in the first or second decade with either cardiac symptoms (palpitations and/or syncope) or weakness that occurs spontaneously following prolonged rest or following rest after exertion. Long-lasting interictal weakness is common. Mild learning difficulties and a distinct neurocognitive phenotype (i.e., deficits in executive function and abstract reasoning) have been described. Incomplete clinical presentations are possible. An electrocardiogram or a Holter-EKG recording between attacks of weakness is necessary to evaluate for the possibility of ATS. EKG should be performed in an interictal period in order to evaluate for a U wave, which is observed in Andersen-Tawil syndrome. Pathogenic variants in KCNJ2 gene are causative 93. Andersen-Tawil syndrome inheritance is autosomal dominant with reduced penetrance and variable expressivity.
Normokalemic periodic paralysis (normoPP) and hyperkalemic periodic paralysis (hyperPP) differ in several ways from hypokalemic periodic paralysis (hypoPP):
- Serum concentration of potassium during the paralytic attacks is normal or elevated.
- Some triggering factors for hypokalemic periodic paralysis (hypoPP) attacks (e.g., carbohydrate-rich meals) are not found.
- Age of onset of paralytic attacks is lower.
- Duration of attacks is assumed to be shorter. However, this is questionable, according to surveys of affected individuals.
- Electromyography (EMG) shows myotonic discharges in most individuals between attacks; however, the response patterns for short exercise test (SET) and long exercise test (LET) may be indiscernible; i.e., pattern IV or V defined by Fournier et al 94 may be caused by both hypokalemic and normo/hyperkalemic periodic paralysis.
- In normokalemic periodic paralysis (normoPP), the reaction to oral potassium administration may be different from that in hypokalemic periodic paralysis (hypoPP) – anything from amelioration to worsening of the weakness 95.
- Usually, the distinction between hypokalemic periodic paralysis (hypoPP) and normokalemic periodic paralysis (normoPP) or hyperkalemic periodic paralysis (hyperPP) can be made on the basis of clinical, laboratory (i.e., kalemia during an attack), and EMG findings, and confirmed by molecular genetic testing 12, 96, 97.
Table 2. Selected SCN4A Allelic Disorders
| Phenotype | Main Findings |
|---|---|
| Hypokalemic periodic paralysis (hypoPP) | See Table 3 |
| Normokalemic periodic paralysis (normoPP) | See Table 3 |
| Paramyotonia congenita 98 | Cold-induced muscle stiffness that increase with continued activity; inability to reopen the eyes after several forceful closures in rapid succession; usually not induced or aggravated by potassium. Often stiffness gives way to flaccid weakness or even paralysis on intensive exercise & cooling. |
| Sodium channel myotonias known as potassium-aggravated myotonia (SCM/PAM) | Development of severe stiffness following vigorous exercise or oral ingestion of potassium. Spectrum ranges from mild (myotonia fluctuans in which affected persons either are not aware of muscle stiffness or may experience stiffness that tends to fluctuate from day to day) to very severe (myotonia permanens in which continuous myotonic activity is noticeable on EMG & generalized muscle hypertrophy including face muscles). |
| Congenital myasthenic syndrome (CMS) | Fatigable generalized muscle weakness & recurrent attacks of respiratory & bulbar paralysis from birth |
| Alternating hemiplegia of childhood 99 | Alternating hemiplegia of childhood is a neurological condition characterized by recurrent episodes of temporary paralysis, often affecting one side of the body (hemiplegia). During some episodes, the paralysis alternates from one side of the body to the other or affects both sides at the same time. These episodes begin in infancy or early childhood, usually before 18 months of age, and the paralysis lasts from minutes to days. In addition to paralysis, affected individuals can have sudden attacks of uncontrollable muscle activity; these can cause involuntary limb movements (choreoathetosis), muscle tensing (dystonia), movement of the eyes (nystagmus), or shortness of breath (dyspnea). People with alternating hemiplegia of childhood may also experience sudden redness and warmth (flushing) or unusual paleness (pallor) of the skin. These attacks can occur during or separately from episodes of hemiplegia. The episodes of hemiplegia or uncontrolled movements can be triggered by certain factors, such as stress, extreme tiredness, cold temperatures, or bathing, although the trigger is not always known. A characteristic feature of alternating hemiplegia of childhood is that all symptoms disappear while the affected person is sleeping but can reappear shortly after awakening. The number and length of the episodes initially worsen throughout childhood but then begin to decrease over time. The uncontrollable muscle movements may disappear entirely, but the episodes of hemiplegia occur throughout life. Alternating hemiplegia of childhood also causes mild to severe cognitive problems. Almost all affected individuals have some level of developmental delay and intellectual disability. Their cognitive functioning typically declines over time. |
| Essential tremor 100 | Essential tremor is a movement disorder that causes involuntary, rhythmic shaking (tremor), especially in the hands. It is distinguished from tremor that results from other disorders or known causes, such as Parkinson’s disease or head trauma. Essential tremor usually occurs alone, without other neurological signs or symptoms. However, some experts think that essential tremor can include additional features, such as mild balance problems. Essential tremor usually occurs with movements and can occur during many different types of activities, such as eating, drinking, or writing. Essential tremor can also occur when the muscles are opposing gravity, such as when the hands are extended. It is usually not evident at rest. In addition to the hands and arms, muscles of the trunk, face, head, and neck may also exhibit tremor in this disorder; the legs and feet are less often involved. Head tremor may appear as a “yes-yes” or “no-no” movement while the affected individual is seated or standing. In some people with essential tremor, the tremor may affect the voice (vocal tremor). Essential tremor does not shorten the lifespan. However, it may interfere with fine motor skills such as using eating utensils, writing, shaving, or applying makeup, and in some cases these and other activities of daily living can be greatly impaired. Symptoms of essential tremor may be aggravated by emotional stress, anxiety, fatigue, hunger, caffeine, cigarette smoking, or temperature extremes. Essential tremor may appear at any age but is most common in older adults. Some studies have suggested that people with essential tremor have a higher than average risk of developing neurological conditions including Parkinson’s disease or sensory problems such as hearing loss, especially in individuals whose tremor appears after age 65. |
Footnote: 1. Duan et al 99; 2. Bergareche et al 100
Table 3. Different Categories of Periodic Paralyses and Associated Findings
| Hypokalemic periodic paralysis (HypoPP) | Normokalemic periodic paralysis (NormoPP) | Hyperkalemic periodic paralysis (HyperPP) | Thyrotoxic periodic paralysis (TPP) | Andersen-Tawil syndrome | |
|---|---|---|---|---|---|
| Main clinical features | Weakness episodes lasting hrs to days w/concomitant hypokalemia | Weakness episodes lasting hrs to days w/concomitant normokalemia | Weakness episodes lasting mins to hrs w/concomitant normo- or hyperkalemia | Identical to that of the paralytic episodes of hypoPP | Episodic PP, ventricular arrhythmias, prolonged QT interval, characteristic anomalies 2 |
| Age at first attacks | Late in 1st decade or in 2nd decade | Late in 1st decade or in 2nd decade | 1st years of life | Variable, dependent on onset of thyrotoxicosis | Late in 1st decade or in 2nd decade (usually after cardiac events) |
| Main triggers | Rest after exercise, carbohydrate-rich meal, salt intake, stress, cold | Rest after exercise, carbohydrate-rich meal, salt intake, stress, cold | Cold; rest after exercise, stress, & fatigue; alcohol; hunger; changes in activity level; potassium in food; specific foods | Thyrotoxicosis | Prolonged rest, rest after exertion |
| EMG: myotonic discharges | No | Some | Some | No | No |
| EMG tests | Late decrement w/LET (pattern IV, V) | Late decrement w/LET (pattern IV, V) | Pattern IV, V | Initial CMAP ↑ + ↓ | Variable (CMAP ↑ + ↓, normal CMAP + ↓, etc) |
| Extramuscular expression | None | None | None | Possible manifestations of thyrotoxicosis | Cardiac arrhythmia, dysmorphy |
| Prevention of paralysis attacks | ACZ, DCP | ACZ, DCP | ACZ, DCP | Normal thyroid function | ACZ, DCP |
| Curative treatment | None | None | None | Treatment of thyroid disorder | None |
| Known causative or susceptibility gene(s) 3 | CACNA1S; SCN4A | SCN4A | SCN4A | KCNJ18 | KCNJ2 |
| Defective ion channel(s) | Cav 1.1; Nav 1.4; Kir 6.2 | Nav 1.4 | Nav 1.4 | Kir 6.2 | Kir 2.1 |
Footnote:
- Thyrotoxic periodic paralysis (TPP) 101, 102, 103, 104, 105
- Andersen-Tawil syndrome anomalies include low-set ears, widely spaced eyes, small mandible, fifth-digit clinodactyly, syndactyly, short stature, and scoliosis.
- In a cohort of 60 Chinese individuals with primary periodic paralysis, 92.5% of those with a genetic diagnosis had pathogenic variants in CACNA1S, KCNJ2, or SCN4A 106.
Abbreviations: ACZ = acetazolamide; CMAP = compound muscle action potential; DCP = dichlorphenamide; LET = long exercise test
Hereditary disorders characterized by hyperkalemia
- Adrenal insufficiency is characterized by hyperkalemia, hyponatremia, and hypoglycemia. Adrenal insufficiency in infancy may be caused by congenital adrenal hyperplasia (most commonly caused by 21-hydroxylase deficiency, associated with biallelic pathogenic variants in CYP21A2) and congenital adrenal hypoplasia including X-linked adrenal hypoplasia congenita, associated with pathogenic variants in NR0B1.
- Adrenal cortical hypofunction (Addison disease) can be an autoimmune disorder with familial aggregation or combined with other endocrinopathies, particularly hypoparathyroidism. Addison disease also occurs in X-linked adrenoleukodystrophy.
- Recessive infantile hypoaldosteronism (corticosterone methyloxidase type 2 deficiency), another hyperkalemic disorder, leads to a rare form of salt wasting that may be life threatening during the first years of life 107. Recurrent dehydration and severe failure to thrive, associated with mild hyponatremia and hyperkalemia, are typical features. Laboratory tests reveal elevated plasma renin-to-serum aldosterone ratios and serum 18-hydroxycorticosterone to aldosterone ratios.
- Pseudohypoaldosteronism type 1 is characterized by neonatal salt-wasting resistant to mineralocorticoids. The autosomal recessive form with symptoms persisting into adulthood is caused by pathogenic loss-of-function variants in SCNN1A, SCNN1B, and SCNN1G 108. The autosomal dominant form associated with pathogenic variants in NR3C2, shows milder symptoms that remit with age 109.
- Pseudohypoaldosteronism type 2 (PHAII), also known as Gordon’s syndrome or familial hyperkalemia and hypertension, is characterized by hypertension, increased renal salt reabsorption, and impaired potassium and hydrogen excretion resulting in hyperkalemia that may be improved by thiazide diuretics. PHAII is caused by pathogenic variants in CUL3, KLHL3, WNK1, or WNK4. PHAII is frequently inherited in an autosomal dominant manner; PHAIID (caused by pathogenic variants in KLHL3) may also be inherited in an autosomal recessive manner.
Periodic paralysis secondary to acquired sustained hyperkalemia
Periodic paralysis secondary to acquired sustained hyperkalemia can occur in any individual when the serum potassium concentration exceeds 7 mmol/L. Weakness can be accompanied by glove-and-stocking paresthesias. Hyperkalemia can cause cardiac arrhythmia, usually tachycardia, and typical EKG abnormalities (i.e., T-wave elevation, disappearance of P waves). Rest after exercise provokes weakness as in hyperkalemic periodic paralysis (hyperPP). The diagnosis is suggested by very high serum potassium concentration during the attack, persistent hyperkalemia between attacks, and the underlying disorder. Serum potassium concentrations are far higher than those in hyperkalemic periodic paralysis (hyperPP). The usual cause is chronic use of medications such as spironolactone, ACE inhibitors, trimethoprim, nonsteroidal anti-inflammatory drugs, heparin, and nonselective beta blockers. Myopathies associated with paroxysmal myoglobinuria (e.g., glycogen storage disease type 5 [McArdle disease], carnitine palmitoyltransferase 2 deficiency) can damage the kidneys and thus also lead to potassium retention. Therapy of acquired sustained hyperkalemia involves restricting dietary potassium intake and treating the underlying cause of the hyperkalemia.
Hyperkalemic periodic paralysis diagnosis
The diagnosis of hyperkalemic periodic paralysis is established based on the clinical presentation of muscle weakness or paralysis, hyperkalemia and a positive family history and sometimes with the use of molecular genetic testing in cases of diagnostic uncertainty 2, 11. The diagnosis is suggested by a history of attacks of weakness or paralysis, a positive family history, and the presence of myotonia (muscle stiffness) or paramyotonia (muscle stiffness aggravated by cold and exercise). Serum creatine kinase (CK) values may be elevated, and some individuals exhibit calf hypertrophy 11. The muscles are typically well-developed 6, 15; however, a large proportion of individuals with hyperkalemic periodic paralysis develop a chronic progressive proximal myopathy as they age 110, 86. Individuals without muscle stiffness (myotonia) between attacks are much more susceptible to developing this progressive myopathy than are individuals with muscle stiffness (myotonia) 9, 2.
Muscle biopsy is non-specific, though will frequently reveal muscle fiber atrophy with vacuoles 15, 16. Genetic testing is positive in approximately 60% of individuals who meet clinical diagnostic criteria. An electromyogram (EMG) may show myotonic signs, which strongly support the diagnosis, although approximately half of those with the most common mutation show no such signs 2. Provocative tests, such as the potassium challenge test, pose obvious risks to the patient but may be done to support the diagnosis 11. The availability of genetic testing and electrophysiologic studies largely obviates the need for such dangerous tests 2, 15, 16.
Clinical findings
- History of at least two attacks of flaccid limb weakness (which may also include weakness of the muscles of the eyes, throat, breathing muscles, and trunk)
- Onset or worsening of an attack as a result of oral potassium intake
- Disease manifestations before age 20 years
- Absence of cardiac arrhythmia between attacks
- Normal psychomotor development
Family history
- Typically, at least one affected first-degree relative
- Note: Absence of a family history suggestive of hyperkalemic periodic paralysis does not preclude the diagnosis.
Electromyogram (EMG)
- During the attack, EMG demonstrates a reduced number of motor units or may be silent (no insertional or voluntary activity).
- In the intervals between attacks, EMG may reveal myotonic activity (bursts of muscle fiber action potentials with amplitude and frequency modulation, firing rate generally between 20 and 150 Hz), even though myotonic stiffness may not be clinically present.
- In some individuals, especially in those with permanent weakness, a myopathic pattern may be visible.
- Note: Approximately 50% of affected individuals have no detectable electric myotonia.
Suggestive laboratory findings during attacks
- Hyperkalemia (serum potassium concentration >5 mmol/L) or an increase of serum potassium concentration of at least 1.5 mmol/L.
- Note: Serum potassium concentration seldom reaches cardiotoxic levels, but changes in the EKG (increased amplitude of T waves) may occur.
- Elevated serum creatine kinase (CK) concentration (sometimes 5-10x the normal range)
Suggestive laboratory findings between attacks
- Normal serum potassium concentration and muscle strength between attacks
- Note: At the end of an attack of weakness, elimination of potassium via the kidney and reuptake of potassium by the muscle can cause transient hypokalemia that may lead to the misdiagnosis of hypokalemic periodic paralysis.
- Elevated serum creatine kinase (CK) concentration with normal serum sodium concentration
Genetic testing
The diagnosis of hyperkalemic periodic paralysis (hyperPP) is established in affected individual with suggestive clinical and lab findings and a heterozygous pathogenic variant in SCN4A ionic channel gene identified by molecular genetic testing, but up to 20% of the cases would be tested negative for the gene mutation 2. In cases of diagnostic uncertainty, provocative tests such as a potassium challenge test can be employed but simultaneously carrying risks of triggering a severe attack 2. Muscle biopsy is generally not advisable as the findings are non-specific 2.
Provocative testing
In case of diagnostic uncertainty (i.e., in the absence of a measurement of during an attack serum potassium concentration and normal molecular genetic studies), a provocative test may be employed to ensure the diagnosis 2. The availability of genetic testing and electrophysiologic studies largely obviates the need for such dangerous tests 2. Systemic provocative testing carries the risk of inducing a severe attack; therefore, such testing must be performed by an experienced physician and a stand-by anesthetist, with close monitoring of the EKG and serum concentration of potassium 2.
The classic provocative test consists of the administration of 2-10 g potassium under clinical surveillance with serum potassium concentration and strength measured at 20-minute intervals 2. Usually, an attack is induced within an hour and lasts approximately 30 to 60 minutes, accompanied by an increase in serum potassium concentration, similar to spontaneously occurring attacks of weakness.
Note: This test is contraindicated in individuals who already have hyperkalemia and in those individuals who do not have adequate renal or adrenal function.
Hyperkalemic periodic paralysis treatment
Treatment for hyperkalemic periodic paralysis is symptomatic, as there is no curative treatment for hyperPP. Hyperkalemic periodic paralysis treatment is focused on correcting the levels of potassium in your blood and preventing episodes with lifestyle changes.
A tablespoonful of calcium gluconate syrup stirred into a glass of sweet beverage may stop a mild episode in the early stages. Calcium gluconate syrup is a mineral supplement available off the shelf and is found in most pharmacies. Mild exercise with intake of carbohydrates may be helpful to relieve symptoms. Some patients find that drinking 1/4 cup of tonic water daily (available at the grocer’s) helps ease symptoms. Albuterol, administered by nebulizer or inhaler (puffer), is an effective treatment for some patients. For those who have frequent episodes more aggressive treatment is advisable, especially since some patients with hyperkalemic periodic paralysis may develop permanent muscle weakness after years of episodes.
Preventive measures such as avoidance of potassium-rich foods and medications, fasting, strenuous work and exposure to cold are equally important 11, 2.
The carbonic anhydrase inhibitors ‘Diamox’ (acetazolomide) and ‘Keveyis’ (dichlorphenamide) are often prescribed for hyperkalemic periodic paralysis patients. About 25% of patients do not respond to carbonic anhydrase inhibitors and must take other medication. Other medications used to treat hyperkalemic periodic paralysis include the potassium-wasting diuretics (thiazide diuretics) – Lasix (furosemide), Hydrodiuril (hydrochlorothiazide), etc. For those who have significant myotonia, the anti-arrhythmic drug Mexitil (mexiletine) is used in low doses; 50-150 mg daily is reported to be adequate by patients. Very low doses of Paxil (paroxetine) (10-20 mg daily) have also been reported to help relieve myotonia.
The individuals’ serum electrolytes should be monitored, and their serum potassium and sodium levels should not fall below 3.3 mmol/L and 135 mmol/L, respectively 111.
Individuals with hyperPP are at risk of complications from surgery and anaesthetics 11. Non-anaesthetic surgical complications were reported in 22.8% of cases, including temporary complete paralysis postoperatively, respiratory difficulties and hyperthermia. Up to 29.9% of patients experienced attacks during general anaesthesia. During anaesthesia, non-depolarizing muscle relaxants are preferred 11, 2. The use of depolarizing muscle relaxants such as suxamethonium as well as anticholinesterase types of reversal agents should be avoided as there will be a risk of masseter spasm and respiratory muscle stiffness rendering endotracheal intubation and mechanical ventilation difficult 112. Perioperatively, maintaining normoglycemia (normal blood sugar level) and normal body temperature are crucial to prevent attacks. Postoperatively, the affected patient needs to be closely monitored for signs of respiratory distress attributable to the disease itself, anesthetic drugs that depress respiration and hypothermia 2. There is currently no agreed recommendation for the choice of regional or general anaesthesia for patients with hyperPP.
Table 4. Treatment of Hyperkalemic Periodic Paralysis (hyperPP)
| Signs and symptoms | Treatment | Considerations/Other |
|---|---|---|
| Attacks of flaccid muscle weakness | Continuing mild exercise &/or oral ingestion of carbohydrates (2 g glucose per kg body weight) at onset of weakness | May prevent or abort attacks |
| Intravenous (IV) glucocorticoids or inhalation of 2 puffs of 0.1 mg salbutamol | May abort or attenuate attacks | |
| Calcium gluconate (0.5-2 g taken intravenously) 1 | May terminate attacks in some persons | |
| Myotonia associated with general anesthesia 2 | An induction sequence incorporating inhalation of oxygen, cricoid pressure, thiopental, & 2x the ED95 dose of an intermediate or short-action non-depolarizing muscle relaxant, followed by intubation, is a reasonable approach to securing the airway in persons with hyperPP. | Avoid opioids or depolarizing agents incl potassium, anticholinesterases, & succinylcholine, which can aggravate a myotonic reaction & induce masseter spasms & stiffness of respiratory muscles. Alterations of serum osmolarity, pH, & hypothermia-induced muscle shivering & mechanical stimuli can exacerbate myotonic reaction. |
| Inhalational induction may also be possible for hyperPP & is well tolerated in those undergoing elective surgery. | ||
| Post anesthesia general & respiratory muscle weakness | To prevent this complication the following are recommended: 3 Glucose infusion Maintain normal body temperature. Maintain serum potassium at low level. | |
| Myotonia | Mexiletine has been used to treat myotonia in this disorder 113. |
Footnotes:
- One case report suggested that intravenous magnesium is beneficial as well 114.
- Because the generalized muscle spasms associated with such attacks may lead to an increase in body temperature, individuals with hyperkalemic periodic paralysis have been considered to be susceptible to malignant hyperthermia. Most likely, anesthesia-related complications suggestive of a malignant hyperthermia crisis result from severe myotonic reactions 83, 115.
- Klingler et al 115, Mackenzie et al 116, Jurkat-Rott & Lehmann-Horn 117, Barker 118
Table 5. Recommended monitoring of individuals with Hyperkalemic Periodic Paralysis (hyperPP)
| System/Concern | Evaluation | Frequency |
|---|---|---|
| Diuretic-induced hypokalemia 1 | Serum potassium concentration (target: between 3.0 & 3.5 mmol/L) | Every 6 mos |
| Muscle weakness | Neurologic exam w/focus on muscle strength in the legs in order to detect permanent weakness | Annually |
| MRI of the leg muscles 2 | Every 1-3 yrs | |
| Thyroid dysfunction | Thyroid function testing | Annually |
Footnote:
- If on continuous prophylactic diuretic treatment
- To judge how much normal muscle tissue is preserved and whether edema is present. MRI is ideal, but only investigational at this time.
Pregnancy management
More than 90% of affected women report an increase in attack frequency during pregnancy. While approximately 80% reported improved muscle weakness during attacks, 75% also reported worse muscle stiffness during attacks 11, 112. In contrast, however, are case reports describing improvement during pregnancy [Finsterer et al 2017, Huang et al 2019].
Women who are chronically treated with a diuretic may continue treatment in pregnancy. Human data on prenatal exposure to acetazolamide have not demonstrated an increased risk of fetal malformations. Human data on the use of oral dichlorphenamide therapy during pregnancy – and whether it leads to an increased risk of malformations in exposed fetuses – are limited.
Hyperkalemic periodic paralysis life expectancy
Most people with hyperkalemic periodic paralysis lead reasonably normal lives. Many cases of hyperKPP are mild, and even those who are severely affected can have their symptoms eased through medication and attention to diet and lifestyle issues. There is no cure, but most people manage to lead well-rounded and fulfilling lives. Several people with hyperkalemic periodic paralysis have lived well into their 80’s, in complete control of their mental faculties and capable of independent life. However, some people with hyperKPP have physically-compromised lives, especially patients with frequent and severe attacks which may lead to permanent muscle weakness. But many are quite active. Furthermore, permanent muscle weakness is a fact of life for many older patients, and activities like climbing stairs and walking long distances may become very fatiguing. Some old people with hyperkalemic periodic paralysis use a wheelchair or ‘scooter’ outside their home, but are mobile and on their feet inside the house. Very few rely on a wheelchair full time unless their condition is complicated by other problems.
- Sekhon DS, Vaqar S, Gupta V. Hyperkalemic Periodic Paralysis. [Updated 2023 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK564319[↩][↩]
- Weber F. Hyperkalemic Periodic Paralysis. 2003 Jul 18 [Updated 2021 Jul 1]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1496[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Gamstorp, I. (1963), ADYNAMIA EPISODICA HEREDITARIA AND MYOTONIA. Acta Neurologica Scandinavica, 39: 41-58. https://doi.org/10.1111/j.1600-0404.1963.tb05386.x[↩]
- Bradley WG, Taylor R, Rice DR, Hausmanowa-Petruzewicz I, Adelman LS, Jenkison M, Jedrzejowska H, Drac H, Pendlebury WW. Progressive myopathy in hyperkalemic periodic paralysis. Arch Neurol. 1990 Sep;47(9):1013-7. doi: 10.1001/archneur.1990.00530090091018[↩][↩][↩][↩]
- CARL M. PEARSON, THE PERIODIC PARALYSES: DIFFERENTIAL FEATURES AND PATHOLOGICAL OBSERVATIONS IN PERMANENT MYOPATHIC WEAKNESS, Brain, Volume 87, Issue 2, June 1964, Pages 341–354, https://doi.org/10.1093/brain/87.2.341[↩]
- Schapira A, Griggs R. Muscle disease: blue books of practical neurology. New York: Elsevier; 1991.[↩][↩][↩]
- Hyperkalemic periodic paralysis. https://medlineplus.gov/genetics/condition/hyperkalemic-periodic-paralysis[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Statland JM, Fontaine B, Hanna MG, Johnson NE, Kissel JT, Sansone VA, Shieh PB, Tawil RN, Trivedi J, Cannon SC, Griggs RC. Review of the Diagnosis and Treatment of Periodic Paralysis. Muscle Nerve. 2018 Apr;57(4):522-530. doi: 10.1002/mus.26009[↩]
- Lehmann-Horn F, Rüdel R, Jurkat-Rott K. Nondystrophic myotonias and periodic paralyses. In: Engel A, Franzini-Armstrong C, editors. Myology. 3. New York: McGraw-Hill Professional; 2004. pp. 1257–1300.[↩][↩][↩]
- Jurkat-Rott K, Lehmann-Horn F. Genotype-Phenotype correlation and therapeutic rationale in hyperkalemic periodic paralysis. Neurotherapeutics 2007;4:216–24. 10.1016/j.nurt.2007.02.001[↩]
- Charles G, Zheng C, Lehmann-Horn F, Jurkat-Rott K, Levitt J. Characterization of hyperkalemic periodic paralysis: a survey of genetically diagnosed individuals. J Neurol. 2013 Oct;260(10):2606-13. doi: 10.1007/s00415-013-7025-9[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Miller TM, Dias da Silva MR, Miller HA, Kwiecinski H, Mendell JR, Tawil R, McManis P, Griggs RC, Angelini C, Servidei S, Petajan J, Dalakas MC, Ranum LP, Fu YH, Ptácek LJ. Correlating phenotype and genotype in the periodic paralyses. Neurology. 2004 Nov 9;63(9):1647-55. doi: 10.1212/01.wnl.0000143383.91137.00[↩][↩]
- Amarteifio E, Nagel AM, Weber MA, Jurkat-Rott K, Lehmann-Horn F. Hyperkalemic periodic paralysis and permanent weakness: 3-T MR imaging depicts intracellular 23Na overload–initial results. Radiology. 2012 Jul;264(1):154-63. doi: 10.1148/radiol.12110980[↩]
- Lee YH, Lee HS, Lee HE, Hahn S, Nam TS, Shin HY, Choi YC, Kim SM. Whole-Body Muscle MRI in Patients with Hyperkalemic Periodic Paralysis Carrying the SCN4A Mutation T704M: Evidence for Chronic Progressive Myopathy with Selective Muscle Involvement. J Clin Neurol. 2015 Oct;11(4):331-8. doi: 10.3988/jcn.2015.11.4.331[↩]
- Amato A, Russell J. Neuromuscular disorders. New York: McGraw Hill Professional; 2008.[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Hyperkalemic periodic paralysis. https://www.orpha.net/consor/cgi-bin/Disease_Search.php?lng=EN&data_id=212[↩][↩][↩][↩]
- Sur M, Mohiuddin SS. Potassium. [Updated 2022 Dec 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539791[↩][↩]
- Potassium. https://pubchem.ncbi.nlm.nih.gov/compound/potassium[↩]
- Unwin, R., Luft, F. & Shirley, D. Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol 7, 75–84 (2011). https://doi.org/10.1038/nrneph.2010.175[↩][↩][↩][↩]
- Stone MS, Martyn L, Weaver CM. Potassium Intake, Bioavailability, Hypertension, and Glucose Control. Nutrients. 2016 Jul 22;8(7):444. doi: 10.3390/nu8070444[↩][↩][↩][↩][↩]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium; Oria M, Harrison M, Stallings VA, editors. Dietary Reference Intakes for Sodium and Potassium. Washington (DC): National Academies Press (US); 2019 Mar 5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538102[↩][↩][↩]
- Brooks G. Potassium additive algorithm for use in continuous renal replacement therapy. Nurs Crit Care. 2006 Nov-Dec;11(6):273-80. doi: 10.1111/j.1478-5153.2006.00185.x[↩]
- Preuss HG, Clouatre DL. Sodium, chloride, and potassium. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:475-92.[↩][↩][↩][↩]
- Hinderling PH. The Pharmacokinetics of Potassium in Humans Is Unusual. J Clin Pharmacol. 2016 Oct;56(10):1212-20. doi: 10.1002/jcph.713[↩][↩]
- Palmer B.F. Regulation of potassium homeostasis. Clin. J. Am. Soc. Nephrol. 2015;10:1050–1060. doi: 10.2215/CJN.08580813[↩]
- Unwin R.J., Luft F.C., Shirley D.G. Pathophysiology and management of hypokalemia: A clinical perspective. Nat. Rev. Nephrol. 2011;7:75–84. doi: 10.1038/nrneph.2010.175[↩]
- Palmer BF, Clegg DJ. Physiology and Pathophysiology of Potassium Homeostasis: Core Curriculum 2019. Am J Kidney Dis. 2019 Nov;74(5):682-695. doi: 10.1053/j.ajkd.2019.03.427. Erratum in: Am J Kidney Dis. 2022 Nov;80(5):690.[↩][↩][↩][↩][↩]
- World Health Organisation (WHO) Guideline: Potassium Intake for Adults and Children. WHO; Geneva, Switzerland: 2012.[↩]
- Fulgoni VL 3rd, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J Nutr. 2011 Oct;141(10):1847-54. doi: 10.3945/jn.111.142257[↩][↩]
- O’Neil C.E., Keast D.R., Fulgoni V.L., Nicklas T.A. Food sources of energy and nutrients among adults in the us: Nhanes 2003–2006. Nutrients. 2012;4:2097–2120. doi: 10.3390/nu4122097[↩]
- DeSalvo K.B., Olson R., Casavale K.O. Dietary guidelines for americans. JAMA. 2016;315:457–458. doi: 10.1001/jama.2015.18396[↩]
- Weaver C.M. Potassium and health. Adv. Nutr. (Bethesda Md.) 2013;4:S368–S377. doi: 10.3945/an.112.003533[↩]
- He F.J., MacGregor G.A. Beneficial effects of potassium on human health. Physiol. Plant. 2008;133:725–735. doi: 10.1111/j.1399-3054.2007.01033.x[↩]
- Bailey JL, Sands JM, Franch HA. Water, electrolytes, and acid-based metabolism. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:102-32.[↩]
- Shils M.E., Shike M. Modern Nutrition in Health and Disease. Lippincott Williams & Wilkins; Baltimore, MD, USA: 2006.[↩]
- Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. 2016 Dec;40(4):480-490. doi: 10.1152/advan.00121.2016[↩][↩][↩][↩][↩]
- Hené RJ, Koomans HA, Boer P, Dorhout Mees EJ. Adaptation to chronic potassium loading in normal man. Miner Electrolyte Metab. 1986;12(3):165-72.[↩]
- Rabelink TJ, Koomans HA, Hené RJ, Dorhout Mees EJ. Early and late adjustment to potassium loading in humans. Kidney Int. 1990 Nov;38(5):942-7. doi: 10.1038/ki.1990.295[↩]
- Potassium. https://ods.od.nih.gov/factsheets/Potassium-HealthProfessional[↩]
- Patrick J. Assessment of body potassium stores. Kidney Int 1977;11:476-90. https://www.kidney-international.org/article/S0085-2538(15)31757-9/pdf[↩][↩]
- Chatterjee R, Yeh HC, Edelman D, Brancati F. Potassium and risk of Type 2 diabetes. Expert Rev Endocrinol Metab. 2011 Sep;6(5):665-672. doi: 10.1586/eem.11.60[↩]
- Viera AJ, Wouk N. Potassium Disorders: Hypokalemia and Hyperkalemia. Am Fam Physician. 2015 Sep 15;92(6):487-95. https://www.aafp.org/pubs/afp/issues/2015/0915/p487.html[↩][↩]
- WHO. Diet, nutrition and the prevention of chronic disease. Report of a Joint WHO/FAO Expert Consultation. Geneva, World Health Organization (WHO), 2003. http://whqlibdoc.who.int/trs/WHO_TRS_916.pdf [↩]
- D’Elia L, Barba G, Cappuccio FP et al. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. Journal of the American College of Cardiology, 2011, 57(10):1210–1219. http://www.ncbi.nlm.nih.gov/pubmed/21371638[↩]
- Whelton PK, He J, Cutler JA et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. Journal of the American Medical Association, 1997, 277(20):1624–1632. http://www.ncbi.nlm.nih.gov/pubmed/9168293[↩]
- Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. Journal of Human Hypertension, 2003, 17(7):471–480. http://www.ncbi.nlm.nih.gov/pubmed/12821954[↩]
- Dietary Guidelines Advisory Committee. The report of the Dietary Guidelines Advisory Committee on Dietary Guidelines for Americans. Washington, D.C., Department of Health and Human Services and Department of Agriculture, 2005. http://www.health.gov/dietaryguidelines/dga2005/report/default.htm[↩]
- World Health Organization. Guideline: Potassium intake for adults and children. http://apps.who.int/iris/bitstream/10665/77986/1/9789241504829_eng.pdf[↩][↩]
- American Heart Association. A Primer on Potassium. https://sodiumbreakup.heart.org/a_primer_on_potassium?utm_source=SRI&utm_medium=HeartOrg&utm_term=Website&utm_content=SodiumAndSalt&utm_campaign=SodiumBreakup[↩][↩][↩]
- Potassium. https://lpi.oregonstate.edu/mic/minerals/potassium[↩][↩][↩]
- Clausen T. Quantification of Na+,K+ pumps and their transport rate in skeletal muscle: functional significance. J Gen Physiol. 2013 Oct;142(4):327-45. doi: 10.1085/jgp.201310980[↩]
- Larsen BR, Stoica A, MacAulay N. Managing Brain Extracellular K(+) during Neuronal Activity: The Physiological Role of the Na(+)/K(+)-ATPase Subunit Isoforms. Front Physiol. 2016 Apr 22;7:141. doi: 10.3389/fphys.2016.00141[↩]
- Shattock MJ, Ottolia M, Bers DM, Blaustein MP, Boguslavskyi A, Bossuyt J, Bridge JH, Chen-Izu Y, Clancy CE, Edwards A, Goldhaber J, Kaplan J, Lingrel JB, Pavlovic D, Philipson K, Sipido KR, Xie ZJ. Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J Physiol. 2015 Mar 15;593(6):1361-82. doi: 10.1113/jphysiol.2014.282319[↩]
- DrugBank. Potassium. http://www.drugbank.ca/drugs/DB01345[↩]
- Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol2009;38:791-813.[↩]
- Kawasaki T, Itoh K, Kawasaki M. Reduction in blood pressure with a sodium-reduced, potassium- and magnesium-enriched mineral salt in subjects with mild essential hypertension. Hypertens Res1998;21:235-43. https://www.ncbi.nlm.nih.gov/pubmed/9877516?access_num=9877516&link_type=MED&dopt=Abstract[↩]
- Chang HY, Hu YW, Yue CS, Wen YW, Yeh WT, Hsu LS, et al. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr2006;83:1289-96. http://ajcn.nutrition.org/content/83/6/1289?ijkey=52dc92efe22c9a7ff1bd4e7366b4fcecb7f45e7e&keytype2=tf_ipsecsha[↩]
- Sheng H-W. Sodium, chloride and potassium. In: Stipanuk M, ed. Biochemical and Physiological Aspects of Human Nutrition. Philadelphia: W.B. Saunders Company; 2000:686-710.[↩]
- Weiss JN, Qu Z, Shivkumar K. Electrophysiology of Hypokalemia and Hyperkalemia. Circ Arrhythm Electrophysiol. 2017 Mar;10(3):e004667. doi: 10.1161/CIRCEP.116.004667[↩]
- Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 8: advanced challenges in resuscitation: section 1: life-threatening electrolyte abnormalities. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000 Aug 22;102(8 Suppl):I217-22.[↩]
- Rosano GMC, Tamargo J, Kjeldsen KP, Lainscak M, Agewall S, Anker SD, Ceconi C, Coats AJS, Drexel H, Filippatos G, Kaski JC, Lund L, Niessner A, Ponikowski P, Savarese G, Schmidt TA, Seferovic P, Wassmann S, Walther T, Lewis BS. Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the Working Group on Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J Cardiovasc Pharmacother. 2018 Jul 1;4(3):180-188. doi: 10.1093/ehjcvp/pvy015[↩]
- Goyal A, Spertus JA, Gosch K, Venkitachalam L, Jones PG, Van den Berghe G, Kosiborod M. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012 Jan 11;307(2):157-64. doi: 10.1001/jama.2011.1967[↩]
- Smyth A, Dunkler D, Gao P, Teo KK, Yusuf S, O’Donnell MJ, Mann JF, Clase CM; ONTARGET and TRANSCEND investigators. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int. 2014 Dec;86(6):1205-12. doi: 10.1038/ki.2014.214[↩]
- Potassium Blood Test. https://medlineplus.gov/lab-tests/potassium-blood-test[↩][↩]
- George AL Jr, Ledbetter DH, Kallen RG, Barchi RL. Assignment of a human skeletal muscle sodium channel alpha-subunit gene (SCN4A) to 17q23.1-25.3. Genomics. 1991 Mar;9(3):555-6. https://doi.org/10.1016/0888-7543(91)90425-E[↩]
- Han JY, Kim JB. Familial hyperkalemic periodic paralysis caused by a de novo mutation in the sodium channel gene SCN4A. Korean J Pediatr. 2011 Nov;54(11):470-2. doi: 10.3345/kjp.2011.54.11.470[↩]
- SCN4A gene. https://medlineplus.gov/genetics/gene/scn4a[↩]
- Lee SC, Kim HS, Park YE, Choi YC, Park KH, Kim DS. Clinical Diversity of SCN4A-Mutation-Associated Skeletal Muscle Sodium Channelopathy. J Clin Neurol. 2009 Dec;5(4):186-91. doi: 10.3988/jcn.2009.5.4.186[↩][↩]
- Hyperkalemic periodic paralysis. https://rarediseases.info.nih.gov/diseases/195/hyperkalemic-periodic-paralysis[↩]
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 28. https://ndb.nal.usda.gov/ndb/[↩]
- Segawa K, Nishiyama M, Mori I, Kubota T, Takahashi MP. Hyperkalemic periodic paralysis associated with a novel missense variant located in the inner pore of Nav1.4. Brain Dev. 2023 Apr;45(4):205-211. doi: 10.1016/j.braindev.2022.12.003[↩][↩][↩][↩]
- Kokunai Y, Goto K, Kubota T, Fukuoka T, Sakoda S, Ibi T, Doyu M, Mochizuki H, Sahashi K, Takahashi MP. A sodium channel myotonia due to a novel SCN4A mutation accompanied by acquired autoimmune myasthenia gravis. Neurosci Lett. 2012 Jun 21;519(1):67-72. doi: 10.1016/j.neulet.2012.05.023[↩]
- Cummins TR, Sigworth FJ. Impaired slow inactivation in mutant sodium channels. Biophys J. 1996 Jul;71(1):227-36. doi: 10.1016/S0006-3495(96)79219-6[↩]
- Takahashi MP, Cannon SC. Enhanced slow inactivation by V445M: a sodium channel mutation associated with myotonia. Biophys J. 1999 Feb;76(2):861-8. doi: 10.1016/S0006-3495(99)77249-8[↩]
- von Stein RT, Soderlund DM. Compound-specific effects of mutations at Val787 in DII-S6 of Nav 1.4 sodium channels on the action of sodium channel inhibitor insecticides. Neurotoxicology. 2012 Oct;33(5):1381-9. doi: 10.1016/j.neuro.2012.09.003[↩]
- Cannon SC. Channelopathies of skeletal muscle excitability. Compr Physiol. 2015 Apr;5(2):761-90. doi: 10.1002/cphy.c140062[↩]
- Lehmann-Horn F, Jurkat-Rott K. Voltage-Gated ion channels and hereditary disease. Physiol Rev 1999;79:1317–72. 10.1152/physrev.1999.79.4.1317[↩]
- Spier SJ. Hyperkalemic periodic paralysis: 14 years later. AAEP Proceedings 2006;52:347–50.[↩]
- Lehmann-Horn F, Küther G, Ricker K, Grafe P, Ballanyi K, Rüdel R. Adynamia episodica hereditaria with myotonia: a non-inactivating sodium current and the effect of extracellular pH. Muscle Nerve. 1987 May;10(4):363-74. doi: 10.1002/mus.880100414[↩]
- Lehmann-Horn F, Iaizzo PA, Hatt H, Franke C. Altered gating and conductance of Na+ channels in hyperkalemic periodic paralysis. Pflugers Arch. 1991 Apr;418(3):297-9. doi: 10.1007/BF00370530[↩]
- Sender D, Doyal A. Hyperkalemic periodic paralysis with paramyotonia and the anaesthetic implications. BMJ Case Rep. 2023 Jan 3;16(1):e251699. doi: 10.1136/bcr-2022-251699[↩]
- Sansone VA, Burge J, McDermott MP, Smith PC, Herr B, Tawil R, Pandya S, Kissel J, Ciafaloni E, Shieh P, Ralph JW, Amato A, Cannon SC, Trivedi J, Barohn R, Crum B, Mitsumoto H, Pestronk A, Meola G, Conwit R, Hanna MG, Griggs RC; Muscle Study Group. Randomized, placebo-controlled trials of dichlorphenamide in periodic paralysis. Neurology. 2016 Apr 12;86(15):1408-1416. doi: 10.1212/WNL.0000000000002416[↩]
- Lehmann-Horn F, Rudel R, Jurkat-Rott K. Non-dystrophic myotonias and periodic paralyses. In: Engel AG, Franzini-Armstrong C, eds. Myology: Basic and Clinical. 3 ed. New York, NY: McGraw-Hill. 2004:1257-300.[↩][↩]
- Akaba Y, Takahashi S, Sasaki Y, Kajino H. Successful treatment of normokalemic periodic paralysis with hydrochlorothiazide. Brain Dev. 2018 Oct;40(9):833-836. doi: 10.1016/j.braindev.2018.05.011[↩]
- Lehmann-Horn F, Jurkat-Rott K. Genotype-phenotype correlation and therapeutic rationale in hyperkalemic periodic paralysis. Neurotherapeutics. 2007;4:216–224. doi: 10.1016/j.nurt.2007.02.001[↩][↩]
- Venance SL, Cannon SC, Fialho D, Fontaine B, Hanna MG, Ptacek LJ, Tristani-Firouzi M, Tawil R, Griggs RC; CINCH investigators. The primary periodic paralyses: diagnosis, pathogenesis and treatment. Brain. 2006 Jan;129(Pt 1):8-17. doi: 10.1093/brain/awh639[↩][↩]
- Dorland’s Illustrated Medical Dictionary. W.B. Saunders Company, Philadelphia 1974[↩]
- Benhammou JN, Phan J, Lee H, Ghassemi K, Parsons W, Grody WW, Pisegna JR. A Sodium Channel Myotonia Presenting with Intermittent Dysphagia as a Manifestation of a Rare SCN4A Variant. J Mol Neurosci. 2017 Mar;61(3):312-314. doi: 10.1007/s12031-016-0878-5[↩]
- Ammar T, Lin W, Higgins A, Hayward LJ, Renaud JM. Understanding the physiology of the asymptomatic diaphragm of the M1592V hyperkalemic periodic paralysis mouse. J Gen Physiol. 2015 Dec;146(6):509-25. doi: 10.1085/jgp.201511476[↩]
- Giacobbe A, Subramony S, Chuquilin M. Pain as a significant symptom in patients with periodic paralysis-A cross-sectional survey. Muscle Nerve. 2021 Jun;63(6):897-901. doi: 10.1002/mus.27241[↩][↩]
- Jeong HN, Yi JS, Lee YH, Lee JH, Shin HY, Choi YC, Kim SM. Lower-extremity magnetic resonance imaging in patients with hyperkalemic periodic paralysis carrying the SCN4A mutation T704M: 30-month follow-up of seven patients. Neuromuscul Disord. 2018 Oct;28(10):837-845. doi: 10.1016/j.nmd.2018.06.008[↩]
- Cannon SC. Sodium Channelopathies of Skeletal Muscle. Handb Exp Pharmacol. 2018;246:309-330. doi: 10.1007/164_2017_52[↩]
- Plaster NM, Tawil R, Tristani-Firouzi M, Canún S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptácek LJ. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell. 2001 May 18;105(4):511-9. doi: 10.1016/s0092-8674(01)00342-7[↩]
- Fournier E, Arzel M, Sternberg D, Vicart S, Laforet P, Eymard B, Willer JC, Tabti N, Fontaine B. Electromyography guides toward subgroups of mutations in muscle channelopathies. Ann Neurol. 2004 Nov;56(5):650-61. doi: 10.1002/ana.20241[↩]
- Jurkat-Rott K, Groome J, Lehmann-Horn F. Pathophysiological role of omega pore current in channelopathies. Front Pharmacol. 2012 Jun 11;3:112. doi: 10.3389/fphar.2012.00112[↩]
- Vicart S, Sternberg D, Fournier E, Ochsner F, Laforet P, Kuntzer T, Eymard B, Hainque B, Fontaine B. New mutations of SCN4A cause a potassium-sensitive normokalemic periodic paralysis. Neurology. 2004 Dec 14;63(11):2120-7. doi: 10.1212/01.wnl.0000145768.09934.ec[↩]
- Fan C, Lehmann-Horn F, Weber MA, Bednarz M, Groome JR, Jonsson MK, Jurkat-Rott K. Transient compartment-like syndrome and normokalaemic periodic paralysis due to a Ca(v)1.1 mutation. Brain. 2013 Dec;136(Pt 12):3775-86. doi: 10.1093/brain/awt300[↩]
- PARAMYOTONIA CONGENITA OF VON EULENBURG; PMC. https://www.omim.org/entry/168300[↩]
- Duan BC, Wong LC, Lee WT. Alternating hemiplegia and paroxysmal torticollis caused by SCN4A mutation: A new phenotype? Neurology. 2019 Oct 8;93(15):673-674. doi: 10.1212/WNL.0000000000008212[↩][↩]
- Bergareche A, Bednarz M, Sánchez E, Krebs CE, Ruiz-Martinez J, De La Riva P, Makarov V, Gorostidi A, Jurkat-Rott K, Marti-Masso JF, Paisán-Ruiz C. SCN4A pore mutation pathogenetically contributes to autosomal dominant essential tremor and may increase susceptibility to epilepsy. Hum Mol Genet. 2015 Dec 15;24(24):7111-20. doi: 10.1093/hmg/ddv410[↩][↩]
- Siddamreddy S, Dandu VH. Thyrotoxic Periodic Paralysis. [Updated 2022 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560670[↩]
- Lin C, Lin CS, Lee DJ, Lee CC, Chen SJ, Tsai SH, Kuo FC, Chau T, Lin SH. Artificial Intelligence-Assisted Electrocardiography for Early Diagnosis of Thyrotoxic Periodic Paralysis. J Endocr Soc. 2021 Jun 29;5(9):bvab120. doi: 10.1210/jendso/bvab120[↩]
- Verma V, Kumar Y, Kotwal N, Upreti V, Hari Kumar KVS, Singh Y, Menon AS. Thyrotoxic periodic paralysis: A retrospective, observational study from India. Indian J Med Res. 2020 Jan;151(1):42-46. doi: 10.4103/ijmr.IJMR_335_18[↩]
- Zhao SX, Liu W, Liang J, Gao GQ, et al. China Consortium for the Genetics of Autoimmune Thyroid Disease. Assessment of Molecular Subtypes in Thyrotoxic Periodic Paralysis and Graves Disease Among Chinese Han Adults: A Population-Based Genome-Wide Association Study. JAMA Netw Open. 2019 May 3;2(5):e193348. doi: 10.1001/jamanetworkopen.2019.3348[↩]
- Heydorn A, Bertelsen B, Nolsöe RLM, Eiken P, Kristensen PL. Thyrotoxic periodic paralysis in a Caucasian man without identifiable genetic predisposition: a case report. Thyroid Res. 2023 May 1;16(1):10. doi: 10.1186/s13044-023-00152-w[↩]
- Luo S, Xu M, Sun J, Qiao K, Song J, Cai S, Zhu W, Zhou L, Xi J, Lu J, Ni X, Dou T, Zhao C. Identification of gene mutations in patients with primary periodic paralysis using targeted next-generation sequencing. BMC Neurol. 2019 May 8;19(1):92. doi: 10.1186/s12883-019-1322-6[↩]
- CORTICOSTERONE METHYLOXIDASE TYPE II DEFICIENCY. https://www.omim.org/entry/610600[↩]
- PSEUDOHYPOALDOSTERONISM, TYPE IB1, AUTOSOMAL RECESSIVE; PHA1B1. https://omim.org/entry/264350[↩]
- PSEUDOHYPOALDOSTERONISM, TYPE I, AUTOSOMAL DOMINANT; PHA1A. https://omim.org/entry/177735[↩]
- Amarteifio E, Nagel AM, Weber MA, Jurkat-Rott K, Lehmann-Horn F. Hyperkalemic periodic paralysis and permanent weakness: 3-T MR imaging depicts intracellular 23Na overload—initial results. Radiology. 2012;264:154–163. doi: 10.1148/radiol.12110980[↩]
- Lehmann-Horn F, Rudel R, Jurkat-Rott K. Non-dystrophic myotonias and periodic paralyses : Engel AG, Franzini-Armstrong C, Myology: Basic and Clinical. 3 ed New York, NY: McGraw-Hill, 2004:1257–300.[↩]
- Yong SL, Sin TH, Tang EB, Chai MC. Hyperkalaemic periodic paralysis in pregnancy. BMJ Case Rep. 2018 Jun 4;2018:bcr2017223588. doi: 10.1136/bcr-2017-223588[↩][↩]
- Modoni A, D’Amico A, Primiano G, Capozzoli F, Desaphy JF, Lo Monaco M. Long-Term Safety and Usefulness of Mexiletine in a Large Cohort of Patients Affected by Non-dystrophic Myotonias. Front Neurol. 2020 May 20;11:300. doi: 10.3389/fneur.2020.00300[↩]
- Mankodi A, Grunseich C, Skov M, Cook L, Aue G, Purev E, Bakar D, Lehky T, Jurkat-Rott K, Pedersen TH, Childs RW. Divalent cation-responsive myotonia and muscle paralysis in skeletal muscle sodium channelopathy. Neuromuscul Disord. 2015 Nov;25(11):908-12. doi: 10.1016/j.nmd.2015.08.007[↩]
- Klingler W, Lehmann-Horn F, Jurkat-Rott K. Complications of anaesthesia in neuromuscular disorders. Neuromuscul Disord. 2005 Mar;15(3):195-206. doi: 10.1016/j.nmd.2004.10.017[↩][↩]
- Mackenzie MJ, Pickering E, Yentis SM. Anaesthetic management of labour and caesarean delivery of a patient with hyperkalaemic periodic paralysis. Int J Obstet Anesth. 2006 Oct;15(4):329-31. doi: 10.1016/j.ijoa.2006.01.003[↩]
- Jurkat-Rott K, Lehmann-Horn F. Genotype-phenotype correlation and therapeutic rationale in hyperkalemic periodic paralysis. Neurotherapeutics. 2007 Apr;4(2):216-24. doi: 10.1016/j.nurt.2007.02.001[↩]
- Barker MC. Combined spinal/general anesthesia with postoperative femoral nerve block for total knee replacement in a patient with familial hyperkalemic periodic paralysis: a case report. AANA J. 2010 Jun;78(3):191-4.[↩]