What is hypovolemia
Hypovolemia is defined as a decrease in the blood volume resulting from loss of blood, plasma and/or plasma water, thereby causing a loss of intravascular content and resulting in a potential limitation of tissue perfusion 1. Hypovolemia is often seen in case of severe dehydration or blood loss owing to trauma or surgery. If left untreated, this ‘hypovolemic shock’ can result in hypoxic tissue damage, organ failure, and ultimately, death. Activation of sympathetic nervous system (homeostatic response) results in peripheral vasoconstriction and tachycardia thereby trying to preserve blood flow to vital organs and maintain blood pressure up to a certain degree of hypovolemia. Hence, in patients of trauma, only when the magnitude of blood loss approaches half the circulating volume or that occurs rapidly, there can be a relation between the cardiac output and blood pressure.
Reduction in circulating blood volume leads to lower venous return irrespective of its cause and, when hypovolemia is sufficiently severe, arterial hypotension 2. Compensatory systemic release of catecholamines promotes peripheral vasoconstriction, increased cardiac contractility and tachycardia. Systemic blood pressure may therefore remain stable in the face of continuing hypovolemia. Tachycardia promotes increased myocardial oxygen demand that, in conjunction with reduced tissue perfusion, may result in myocardial failure. Finally, anerobic metabolism occurring in response to reduced perfusion may produce acidosis and, together with myocardial dysfunction, contribute to multi-organ failure.
Up to 10% of the total blood volume can be lost without affecting either cardiac output or arterial pressure. Greater than 10% loss diminishes cardiac output due to decreased preload, and oxygen delivery to the tissues falls. Arterial pressure also declines with more than 20% loss of total blood volume.
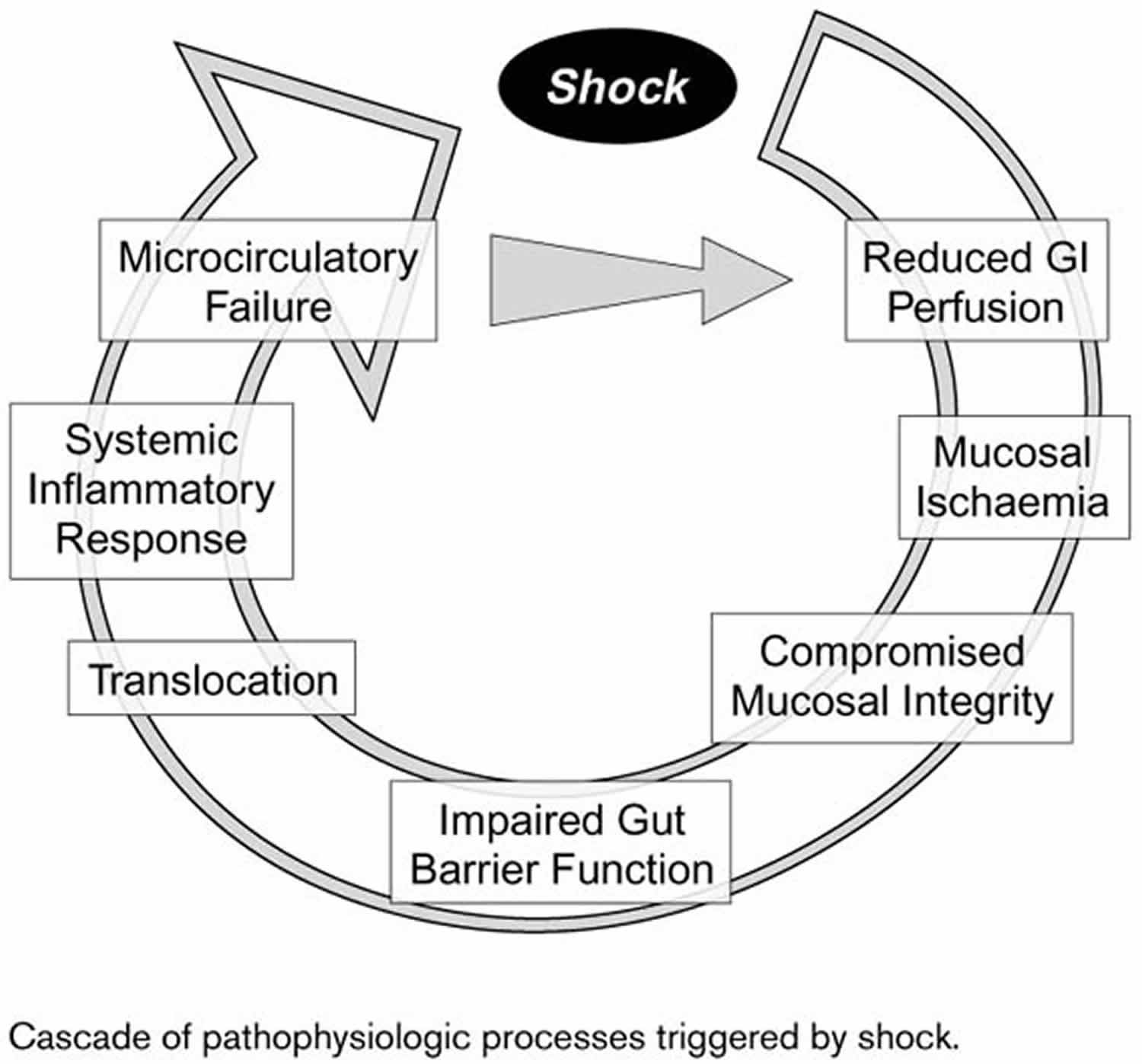
Hypovolemia and hypotension exert differential effects on organ function. In the splanchnic organs, α-adrenergic activity is relatively high 3, and the splanchnic region is highly vulnerable in patients with hypovolemic shock and hypotension. Ischemia develops with diminished gastrointestinal perfusion, especially in the mucosal layer of the gut (Figure 1). Mucosal integrity may be compromised. Impaired gut barrier function may allow translocation of bacteria and endotoxins, precipitating a systemic inflammatory response as a consequence of shock. In cases of severe hypovolemia, due for instance to trauma, these pathophysiologic processes may ultimately lead to the development of sepsis.
Furthermore, splanchnic organs may prove refractory to the effects of administered fluids. In a canine model of hemorrhagic shock, mean arterial blood pressure (MAP) was reduced to 40-45 mmHg for 30 min 4. Thereafter, autologous blood and saline were infused to restore mean arterial blood pressure (MAP) to preshock levels. Gastric intramucosal pH (pHi) declined sharply during shock. The intramucosal pH (pHi) initially rebounded only partially after resuscitation, and subsequently deteriorated progressively over the remainder of the study follow-up period. The mean arterial blood pressure (MAP) and intramucosal pH (pHi) data suggest persistent splanchnic hypoperfusion or reperfusion injury despite restoration of normovolemia by fluid administration.
Figure 1. Hypovolemic shock pathophysiology
[Source 5 ]Hypovolemia causes
Trauma
One frequent cause of hypovolemia is trauma. In Germany, for example, trauma is still the leading cause of death among persons aged under 45 years, accounting for a third of all deaths in this age range 5. Estimated blood loss of 100-800 ml has been reported in association with traumatic fracture of the humerus, 50-400 ml with that of the forearm, 500-5000 ml with that of the pelvis, 300-2000 ml with that of the femur and 100-1000 ml with traumatic fracture of the tibia 6. Significant hypovolemia requiring treatment can thus develop very early after trauma.
Dehydration
Another common source of hypovolemia is dehydration. Precipitating factors include: diarrhea; vomiting; ileus; gastrointestinal (GI) fistula; gastric tube; fever; hyperglycemia and azetonuria with diabetes mellitus; and renal dysfunction (polyuria, etc.). Lost fluid in this type of patient will be comprised primarily of plasma rather than whole blood as in the trauma patient.
Hypovolemia symptoms
When fluid loss is < 5% of extracellular fluid (ECF) (mild volume depletion), the only sign may be diminished skin turgor (best assessed at the upper torso). Skin turgor may be low in elderly patients regardless of volume status. Patients may complain of thirst. Dry mucous membranes do not always correlate with volume depletion, especially in the elderly and in mouth-breathers. Oliguria is typical.
When extracellular fluid (ECF) volume has diminished by 5 to 10% (moderate volume depletion), orthostatic tachycardia, hypotension, or both are usually, but not always, present. Also, orthostatic changes can occur in patients without ECF volume depletion, particularly patients deconditioned or bedridden. Skin turgor may decrease further.
When fluid loss exceeds 10% of extracellular fluid (ECF) volume (severe volume depletion), signs of hypovolemic shock (e.g., tachypnea, tachycardia, hypotension, confusion, poor capillary refill) can occur.
What is hypovolemic shock
Hypovolemic shock results from loss of the intravascular circulating volume from fluid loss or blood loss 7. Hypovolemic shock may arise from bleeding due to trauma or atraumatic bleeding (such as an aortic aneurysm rupture or gastrointestinal bleed). Fluid losses from the gastrointestinal tract from excessive vomiting or diarrhea, malabsorption, or hormone imbalances, such as diabetes insipidus can result in excessive volume loss that may lead to shock if left untreated.
Patients with hypovolemic shock have severe hypovolemia with decreased peripheral perfusion. If left untreated, these patients can develop ischemic injury of vital organs, leading to multi-system organ failure. The first factor to be considered is whether the hypovolemic shock has resulted from hemorrhage or fluid losses, as this will dictate treatment. When cause (source) of hypovolemic shock has been determined, replacement of blood or fluid loss should be carried out as soon as possible to minimize tissue ischemia. Factors to consider when replacing fluid loss include the rate of fluid replacement and type of fluid to be used.
The annual incidence of shock of any etiology is 0.3 to 0.7 per 1000, with hemorrhagic shock being most common in the intensive care unit. Hypovolemic shock is the most common type of shock in children, most commonly due to diarrheal illness in the developing world.
The first changes in vital signs seen in hypovolemic shock include an increase in diastolic blood pressure with narrowed pulse pressure 8. As volume status continues to decrease, systolic blood pressure drops. As a result, oxygen delivery to vital organs is unable to meet oxygen demand. Cells switch from aerobic metabolism to anaerobic metabolism, resulting in lactic acidosis. As sympathetic drive increases, blood flow is diverted from other organs to preserve blood flow to the heart and brain. This propagates tissue ischemia and worsens lactic acidosis. If not corrected, there will be worsening hemodynamic compromise and, eventually, death.
The circulatory status of trauma patients is typically monitored in the prehospital phase of emergency by determinations of heart rate and systolic blood pressure. Central venous pressure devices or even a Swan-Ganz catheter may be inserted while in the intensive care unit 5.
The focus in the operating theater is frequently upon blood volume deficit, as estimated by blood absorbed on surgical sponges or suctioned during the procedure. Such estimates can unfortunately be inaccurate. Blood loss measured by red cell volume using nonradioactive marker sodium fluorescein was compared with that estimated intraoperatively in 30 patients undergoing gynecological operations 9. Estimated blood loss averaged nearly 300 ml less than measured blood loss. Blood loss in individual patients was underestimated by almost 1000 ml, and blood loss in other patients was overestimated. Thus, to the extent that fluid administration decisions are guided by estimated intraoperative blood loss, the volume infused may prove to be either insufficient or excessive.
Causes of hypovolemic shock
Hypovolemic shock occurs as a result of either blood loss or extracellular fluid loss 8. Hemorrhagic shock is hypovolemic shock from blood loss 8. Traumatic injury is by far the most common cause of hemorrhagic shock. Other causes of hemorrhagic shock include gastrointestinal (GI) bleed, bleed from an ectopic pregnancy, bleeding from surgical intervention, or vaginal bleeding. Elderly patients are more likely to experience hypovolemic shock due to fluid losses as they have a less physiologic reserve.
Common causes of hemorrhagic hypovolemic shock include:
- Gastrointestinal bleed (both upper and lower gastrointestinal bleed (e.g., variceal bleed, portal hypertensive gastropathy bleed, peptic ulcer, diverticulosis) trauma.
- Vascular etiologies (e.g., aortoenteric fistula, ruptured abdominal aortic aneurysm, tumor eroding into a major blood vessel).
- Spontaneous bleeding in the setting of anticoagulant use (in the setting of supratherapeutic INR from drug interactions).
Common causes of nonhemorrhagic hypovolemic shock include:
- Gastrointestinal losses – the setting of vomiting, diarrhea, NG suction or drains.
- Renal losses – medication induced diuresis, endocrine disorders such as hypoaldosteronism.
- Skin losses/insensible losses – burns, Steven Johnson syndrome, Toxic epidermal necrolysis, heat stroke, pyrexia.
- Third-space loss – in the setting of pancreatitis, cirrhosis, intestinal obstruction, trauma.
Gastrointestinal Losses
Gastrointestinal losses can occur via many different causes. The gastrointestinal tract usually secretes between 3 to 6 liters of fluid per day. However, most of this fluid is reabsorbed as only 100 to 200 mL are lost in the stool. Volume depletion occurs when the fluid ordinarily secreted by the gastrointestinal tract cannot be reabsorbed. This occurs when there is retractable vomiting, diarrhea, or external drainage via stoma or fistulas.
Renal Losses
Renal losses of salt and fluid can lead to hypovolemic shock. The kidneys usually excrete sodium and water in a manner that matches intake. Diuretic therapy and osmotic diuresis from hyperglycemia can lead to excessive renal sodium and volume loss. In addition, there are several tubular and interstitial diseases beyond the scope of this article that cause severe salt-wasting nephropathy.
Skin Losses
Fluid loss also can occur from the skin. In a hot and dry climate, skin fluid losses can be as high as 1 to 2 liters/hour. Patients with a skin barrier interrupted by burns or other skin lesions also can experience large fluid losses that lead to hypovolemic shock.
Third-Space Sequestration
Sequestration of fluid into a third-space also can lead to volume loss and hypovolemic shock. Third-spacing of fluid can occur in intestinal obstruction, pancreatitis, obstruction of a major venous system, or any other pathological condition that results in a massive inflammatory response.
Hypovolemic shock pathophysiology
Hypovolemic shock results from depletion of intravascular volume, whether by extracellular fluid loss or blood loss 8. The body compensates with increased sympathetic tone resulting in increased heart rate, increased cardiac contractility, and peripheral vasoconstriction. The first changes in vital signs seen in hypovolemic shock include an increase in diastolic blood pressure with narrowed pulse pressure 8. As volume status continues to decrease, systolic blood pressure drops. As a result, oxygen delivery to vital organs is unable to meet oxygen demand. Cells switch from aerobic metabolism to anaerobic metabolism, resulting in lactic acidosis. As sympathetic drive increases, blood flow is diverted from other organs to preserve blood flow to the heart and brain. This propagates tissue ischemia and worsens lactic acidosis. If not corrected, there will be worsening hemodynamic compromise and, eventually, death.
Hypovolemic shock stages
There are four stages of hypovolemic shock based on how much blood volume has been lost. All stages require early treatment, but it is helpful to recognize the stage of hypovolemia a person is in, so they receive appropriate treatment quickly.
Stage 1 shock (<500-750 mL)
Small volumes of fluid loss are well tolerated due to the compensatory mechanisms of the body. The venous system increases resistance to increase circulating blood volume and decrease venous capacitance. This results in the first vital sign change – a narrowed pulse pressure due to an increase of the diastolic blood pressure relative to the systolic blood pressure. Heart rate, blood pressure, and urine output are maintained.
Stage 2 shock (750-1500mL)
As the body detects lower circulatory volumes, the heart rate increases to augment cardiac output. The body attempts to compensate for the lack of blood volume by diverting blood flow away from the extremities and intestinal circulation in favor of the heart and brain. Blood pressure and urine output are maintained. Patients may experience mild anxiety, sweating and restless.
Stage 3 shock (1500-2000 mL)
Ongoing volume loss greater than 1500-2000 ml overcomes the ability of the heart to maintain blood pressure, given that this equates to a 30-40% change in circulating volume, blood pressure decreases and urine output drops to preserve remaining circulatory volume. The mental status may also decline with stage 3 shock. Patient is nearing irreversible shock and immediate, aggressive intervention with volume and blood replacement is necessary. Physical exam findings show peripheral vasoconstriction and cold, clammy extremities, dry mucous membranes, and pallor associated with extreme anemia.
Stage 4 shock (> 2000mL blood or >40% of the circulating volume is loss)
Stage 4 shock is reached with > 2000ml of blood or >40% of the circulating volume is loss. Patients are lethargic, with extreme tachycardia, profound hypotension, and oliguria. The patient may be moribund once stage 4 shock commences.
Certain patient populations may manifest hypovolemic shock differently than the aforementioned pattern. Children and pregnant patients will often guard their physiology until the point of collapse. Tachycardia will be prominent feature of severe shock before hypotension manifests in late stage 3 to 4 hemorrhage, just before circulatory collapse. On the other extreme of age, the elderly may not be able to mount a tachycardic response to hemorrhage because of beta blockade, medications, and pacer dependence. Additionally, the elderly typically have baseline hypertension, thus, they may not manifest the traditional level of hypotension systolic BP <100 mm Hg or mean arterial blood pressure (MAP) <65 mmHg despite profound volume loss.
Hypovolemic shock vital signs
Vital signs are important indicators of the patient’s physiologic status.
Temperature
Fever may point the examiner to search for signs of infection. Hypothermia may accompany poor perfusion but may also be a paradoxical manifestation of infection or a symptom of endocrine dysfunction.
Heart rate
Due to compensatory mechanisms, heart rate is typically elevated in hypotension. In distributive and hypovolemic shock, the heart rate will be elevated to compensate for the low stroke volume while maintaining cardiac output per the equation:
- Cardiac Output = Heart Rate x Stroke Volume
Bradycardic or normal heart rates may be observed with neurogenic and cardiogenic shock. Inappropriately low heart rates may also occur when the patient is unable to augment heart rate due to medications (beta blockers), or the presence of a pacemaker that may be set to a low heart rate.
Blood pressure
Hypotension defined as mean arterial pressure (MAP) <65 mm Hg is often a prominent feature of shock. However, patients may present with hypertension or swings of hyper and hypotension as their physiology decompensates. Alternatively, patients who ‘live” at higher blood pressure ranges may present with a “normal” systolic and mean arterial pressures reading but be hypotensive compared to their baseline. Thus, as with all numeric vital sign values, the interpretation of trends and understanding the patient’s physiology is essential to interpreting the clinical findings.
Respiratory rate
Tachypnea is commonly observed in patients with shock. An elevated respiratory rate helps alleviate systemic acidosis by neutralizing excess hydrogen ion by tipping the buffer system to make CO2 per the Henderson Hasselbalch equation:
- CO2+H20 ←—> HCO3 – +H+
This process increases ventilatory drive leading to increased respiration rate.
Oxygen saturation
Oxygen saturation is typically preserved by increasing oxygen extraction when delivery to tissue is diminished. Saturations fall only at very late stages of hypoperfusion, or when ventilation perfusion mismatch occurs, as in pulmonary emboli or pneumothorax.
Signs and symptoms of hypovolemic shock
For patients with hemorrhagic shock, a history of trauma or recent surgery is present. For hypovolemic shock due to fluid losses, history and physical should attempt to identify possible gastrointestinal, renal, skin, or third-spacing as a cause of extracellular fluid loss. Symptoms of hypovolemic shock can be related to volume depletion, electrolyte imbalances, or acid-base disorders that accompany hypovolemic shock.
Patients with volume depletion may complain of thirst, muscle cramps, and/or orthostatic hypotension. Severe hypovolemic shock can result in mesenteric and coronary ischemia that can cause abdominal or chest pain. Agitation, lethargy, or confusion may result from brain malperfusion.
Hypovolemic shock diagnosis
History and Physical
History and physical can often make the diagnosis of hypovolemic shock. A history of trauma, recent surgery, or evidence of bleeding may help diagnose acute blood loss. Alternatively, vomiting, and diarrhea or gastrointestinal illness will point to fluid loss as the etiology of volume loss. On exam, the patient initially appears to have a cold shock picture, and pallor may be evident in the setting of bleeding.
Physical findings suggestive of volume depletion include dry mucous membranes, decreased skin turgor, and low jugular venous distention. Tachycardia and hypotension can be seen along with decreased urinary output. Patients in shock can appear cold, clammy, and cyanotic.
Lab tests
Various laboratory values can be abnormal in hypovolemic shock. Patients can have increased BUN (blood urea nitrogen) and serum creatinine as a result of prerenal kidney failure. Hypernatremia or hyponatremia can result, as can hyperkalemia or hypokalemia. Lactic acidosis can result from increased anaerobic metabolism. However, the effect of acid-base balance can be variable as patients with large gastrointestinal losses can become alkalotic. In cases of hemorrhagic shock, hematocrit and hemoglobin can be severely decreased. However, with a reduction in plasma volume, hematocrit and hemoglobin can be increased due to hemoconcentration.
Complete blood count (CBC): Hemoglobin and hematocrit (Hct) are lab values that measure the iron-oxygen containing protein in blood and the volume of red blood cells compared to volume of blood, respectively. They are each a measure of concentration. Hemoglobin and hematocrit may be decreased in acute blood loss; however, the lab values are not a reliable indicator of the amount of blood loss in early exsanguinating hemorrhage. As patient bleeds, the concentration of remaining blood remains stable until compensatory mechanisms of the body and fluid resuscitation dilute the relative concentration of the protein or red cells. Thus, in early blood loss, the hemoglobin may remain preserved while the circulating blood volume may be significantly reduced. Trends in hemoglobin, hematocrit are a better assessment of blood loss than a single value. Conversely with severe fluid loss, hemoconcentration may occur elevating the concentration of the hemoglobin and hematocrit relative to circulating plasma volume.
Basic metabolic panel (BMP):
- Electrolyte assessment:
- Gastrointestinal losses may lead to hypokalemia. Massive hemorrhage with transfusion of banked blood may lead to low ionized calcium levels that need aggressive repletion for hemostasis.
- Acid/ Base:
- Large volume loss leads to poor oxygen delivery to the tissue and a transition to anaerobic metabolism in the tissue bed. Lactate is produced and lactic acid level elevate leading to a metabolic anion gap acidosis
- Resuscitation with sodium chloride solutions may contribute to a hyperchloremic acidosis. Chloride rises more than sodium and results in increased production of HCL to maintain electroneutrality, hence metabolic acidosis results.
- Loss of hydrochloric acid with excessive vomiting leads to a hypochloremic-hypokalemic metabolic alkalosis that is treated with potassium repletion and normal saline.
- Gastrointestinal losses from diarrhea, ileostomy output or high output pancreatic fistula may result in the loss of bicarbonate and contribute to metabolic acidosis.
- Renal function: Blood Urea Nitrogen (BUN), Creatinine
- In severe hypovolemic shock the blood urea nitrogen (BUN): creatinine ratio is often > 20. As shock progresses, renal failure may ensue from acute tubular necrosis and cause further elevation of these parameters.
Coagulation studies (PT/ INR; PTT, fibrinogen, fibrin related markers)
- In severe hemorrhagic shock coagulopathy, secondary to an overactivation of clot breakdown (termed fibrinolysis) may occur.
Mixed venous oxygen saturation (SvO2):
- Mixed venous oxygen saturation due to poor delivery of O2 to the periphery, the body will maximally extract oxygen leading to a decreased SvO2.
Imaging:
- Chest X-Ray (CXR) and Pelvic X-Ray (PXR) are mainstays of the trauma workup. Identification of hemothorax, or unstable pelvic fractures can be immediately addressed with chest tube placement or the application of a pelvic binder, respectively.
- Focused Assessment with Sonography for Trauma (FAST exam), a bedside four view ultrasound that evaluates the hepatorenal and splenorenal recesses, the pelvis, and the pericardial free fluid may help triage the abdomen for intraperitoneal bleeding.
- CT Scan: In the hemodynamically stable patients CT may help identify injury and source of blood loss.
- Angiography may localize sources of bleeding and be a therapeutic measure to stop ongoing blood loss.
- Direct peritoneal aspiration/lavage (DPA/ DPL): Largely replaced by increased utilization of the non-invasive FAST exam, direct peritoneal aspiration and lavage rapidly diagnose intraperitoneal bleeding at the bedside in blunt and penetrating trauma. A catheter is placed in the umbilical or supraumbilical position and the abdominal cavity is aspirated and then lavaged with 1 liter of normal saline. Aspiration of free blood or a red blood cell count >100,000/mm³, white blood cell count >500/mm³, elevated fluid amylase, or the presence of succus is deemed an indication for emergent laparotomy.
Low urinary sodium is commonly found in hypovolemic patients as the kidneys attempt to conserve sodium and water to expand the extracellular volume. However, sodium urine can be low in a euvolemic patient with heart failure, cirrhosis, or nephrotic syndrome. A fractional excretion of sodium under 1% is also suggestive of volume depletion. Elevated urine osmolality can also suggest hypovolemia. However, this number also can be elevated in the setting of impaired concentrating ability by the kidneys.
Central venous pressure (CVP) is often used to assess volume status. However, its usefulness in determining volume responsiveness has recently come into question. Ventilator settings, chest wall compliance, and right-sided heart failure can compromise central venous pressures accuracy as a measure of volume status. Measurements of pulse pressure variation via various commercial devices has also been postulated as a measure of volume responsiveness. However, pulse pressure variation as a measure of fluid responsiveness is only valid in patients without spontaneous breaths or arrhythmias. The accuracy of pulse pressure variation also can be compromised in right heart failure, decreased lung or chest wall compliance, and high respiratory rates.
Similar to examining pulse pressure variation, measuring respiratory variation in inferior vena cava diameter as a measure of volume responsiveness has only been validated in patients without spontaneous breaths or arrhythmias. Measuring the effect of passive leg raises on cardiac contractility by echo appears to be the most accurate measurement of volume responsiveness, although it is also subject to limitations.
Hypovolemic shock treatment
Aggressive replacement of volume while attending to the underlying etiology is the mainstay of treatment of hypovolemic shock. In traumatic bleeding, patients should be triaged per the ABCDE’s of Advanced Trauma Life Support (ATLS).
A. Airway
The airway should be assessed for patency. Tracheal deviation may be a sign of tension physiology from obstructive mechanisms of shock such as a tension pneumothorax or hemothorax. Mental status changes that often accompany severe forms of shock may impede the ability of the patient to protect their airway. Care must be taken to secure an endotracheal tube in patient with inability to protect the airway or a Glasgow Coma Scale of <8.
B. Breathing
The breath sounds should be equal on both sides of the chest on auscultation. Decreased breath sounds may alert the clinician to blood in the chest, pneumothorax, or tension physiology. If clinically warranted, placement of a chest tube may be required to address such findings.
C. Circulation
Circulation is assessed with an evaluation of the peripheral pulses. IV access should be secured with large bore (14 or 16 gauge IV access or resuscitative lines). Crystalloid infusion of at least 30cc/ kg should be administered to support the intravascular volume while assessment of acute blood loss is determined. Active bleeding should be promptly tended to with hemostatic measures such as pressure, tourniquet, or procedural/ operative intervention. Attention should be paid to distension of the jugular veins, as this can signal obstructive physiology in the chest with tension or tamponade physiology. Finally, the perfusion of the distal extremities is a physical exam finding to help differentiate the types of shock. Acral cyanosis of the extremities with a cold, clammy feel is consistent with obstructive, hypovolemic, or cardiogenic shock. A warm dilated shock may be seen with distributive shock due to vasodilation of the peripheral vessels.
D. Disability
A full neurologic exam is also important to evaluate. In trauma, disability is routinely assessed using the Glasgow Coma Scale (GCS), but an assessment of neurologic status is necessary in all forms of shock. With poor perfusion, the patient’s mental status will deteriorate, risking airway compromise due to loss of the usual reflexes that allow secretion management and protect from aspiration. Low GCS (<8) is an indication for intubation, and a low threshold for intubation is required for any patient who is not protecting the airway. Motor and sensation deficits, paresthesia, priapism, and decreased rectal tone suggest injury to the spinal cord and the possibility of neurogenic shock.
E. Exposure and secondary evaluation
An exam of the patient’s entire body is important in the critically ill patient. Evaluation of sources of infection, signs of bleeding, extremity perfusion and capillary refill, and volume status are essential to determining the etiology of hypoperfusion. Attention to body temperature is also important to help best maintain normothermia.
Temporary measures to control bleeding such as pressure, tourniquets, and pelvic binders may be applied to help with immediate hemorrhage control until definitive management can stop the bleeding.
For patients in hemorrhagic shock, early use of blood products over crystalloid resuscitation results in better outcomes. Balanced transfusion using 1:1:1 or 1:1:2 of plasma to platelets to packed red blood cells results in better hemostasis 8. Anti-fibrinolytic administration to patients with severe bleed within 3 hours of traumatic injury appears to decrease death from major bleed as shown in the CRASH-2 trial 10. Research on oxygen-carrying substitutes as an alternative to packed red blood cells is ongoing, although no blood substitutes have been approved for use in the United States.
In atraumatic bleeding, the identification of the source must be expeditiously sought. All forms of hemorrhage necessitate large bore IV access (14 or 16 gauge IV or short, large diameter resuscitation lines). Crystalloid resuscitation should be limited to 1-2 liters of IV fluid, with a transition to early blood and plasma resuscitation in the exsanguinating patient.
This recent paradigm shift in management has been guided by recent evidence suggesting that a 1:2 resuscitation (1 unit of plasma for every 2 units of packed blood cells) leads to less overall product transfused, improved coagulopathy, and a mortality benefit in trauma and non-trauma patients 11. With massive transfusion, the citrate in stored blood will lead to low free plasma calcium; thus repletion of calcium, an essential element for coagulation, vasoconstriction, and cardiac function, is critical. Avoidance of hypothermia and correction of acidosis are also key factors that improve the patient’s physiology and homeostatic response to therapy.
Hypovolemic shock from fluid loss must also aggressively repleted with like fluid. The source of volume loss must also be addressed. Oral rehydration may be attempted in early forms of hypovolemia. Solutions with sucrose, and minerals, such as potassium and magnesium, are required for severe dehydration with volume of oral rehydration of 70-100 ml/kg over 12 hours to restore hydration. In patients with significant shock or those unable to take or absorb PO intake, isotonic fluid can be administered IV with Normal Saline (NS) or Lactated Ringers (LR). Administered as a bolus of 20-30 ml/kg, and repeated every 5-10 minutes, may quickly restore circulating volume. Care must be taken to avoid fluid overload in certain patient populations. Patients with heart failure, severe malnutrition, diabetic ketoacidosis (DKA), and Syndrome of Inappropriate Antidiuretic Hormone (SIADH), and extremes of age must be judiciously rehydrated with care not to overcorrect their volume status. Colloid resuscitation, while safe in most patient populations, has failed to show benefit over crystalloid except in patients with liver disease, in whom it may be indicated 12
For patients in hypovolemic shock due to fluid losses, the exact fluid deficit cannot be determined. Therefore, it is prudent to start with 2 liters of isotonic crystalloid solution infused rapidly as an attempt to quickly restore tissue perfusion. Fluid repletion can be monitored by measuring blood pressure, urine output, mental status, and peripheral edema. Multiple modalities exist for measuring fluid responsiveness such as ultrasound, central venous pressure monitoring, and pulse pressure fluctuation as described above. In general, for hypovolemic shock, vasopressors should not be used because they can worsen tissue perfusion.
Crystalloid fluid resuscitation is preferred over colloid solutions for severe volume depletion not due to bleeding. The type of crystalloid used to resuscitate the patient can be individualized based on the patients’ chemistries, estimated volume of resuscitation, acid/base status, and physician or institutional preferences. Isotonic saline is hyperchloremic relative to blood plasma, and resuscitation with large amounts can lead to a hyperchloremic metabolic acidosis. Several other isotonic fluids with lower chloride concentrations exist, such as lactated Ringer’s solution or PlasmaLyte. These solutions are often referred to as buffered or balanced crystalloids. Some evidence suggests that patients who need large volume resuscitation may have a less renal injury with restrictive chloride strategies and use of balanced crystalloids. Crystalloid solutions are equally as effective and much less expensive than colloid. Commonly used colloid solutions include those containing albumin or hyperoncotic starch. Studies examining albumin solutions for resuscitation have not shown improved outcomes, while other studies have shown resuscitation with hyperoncotic starch leads to increased mortality and renal failure.
- Mandal M. Ideal resuscitation fluid in hypovolemia: The quest is on and miles to go!. Int J Crit Illn Inj Sci. 2016;6(2):54-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4901826/[↩]
- Baskett PJ. ABC of major trauma. Management of hypovolaemic shock. BMJ. 1990;300:1453–1457. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1663124/pdf/bmj00181-0053.pdf[↩]
- Meβmer K. Intestinal factors in shock: intestinal circulation. Langenbecks Arch Chir. 1967;319:890–909.[↩]
- Oud L, Kruse JA. Progressive gastric intramucosal acidosis follows resuscitation from hemorrhagic shock. Shock. 1996;6:61–65.[↩]
- Kreimeier U. Pathophysiology of fluid imbalance. Crit Care. 2000;4 Suppl 2(Suppl 2):S3-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3226173/[↩][↩][↩]
- Burri C, Beck H, Ecke H, Unfallchirurgie, edn 3. Berlin: Springer Verlag, 1982.[↩]
- Shock. https://www.facs.org/~/media/files/education/core%20curriculum/shock.ashx[↩]
- Taghavi S, Askari R. Shock, Hypovolemic. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513297[↩][↩][↩][↩][↩][↩]
- Orth VH, Rehm M, Thiel M. et al. First clinical implications of perioperative red cell volume measurement with a nonradioactive marker (sodium fluorescein). Anesth Analg. 1998;87:1234–1238. doi: 10.1097/00000539-199812000-00003[↩]
- Gayet-Ageron A, Prieto-Merino D, Ker K, et al. Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: a meta-analysis of individual patient-level data from 40 138 bleeding patients. Lancet. 2018;391(10116):125-132. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5773762/[↩]
- Holcomb JB, Tilley BC, Baraniuk S, et al. PROPPR Study Group Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471.[↩]
- Bernardi M. Maggioli C, Zaccherini G. Human albumin in the management of complications of liver cirrhosis. Critical Care 2012;16:211.[↩]