Contents
- What are lymphocytes
- B-lymphocytes
- T-lymphocytes
- Lymphocytes function
- Lymphocytes normal range
- Lymphocytes high
- Lymphocytes low
What are lymphocytes
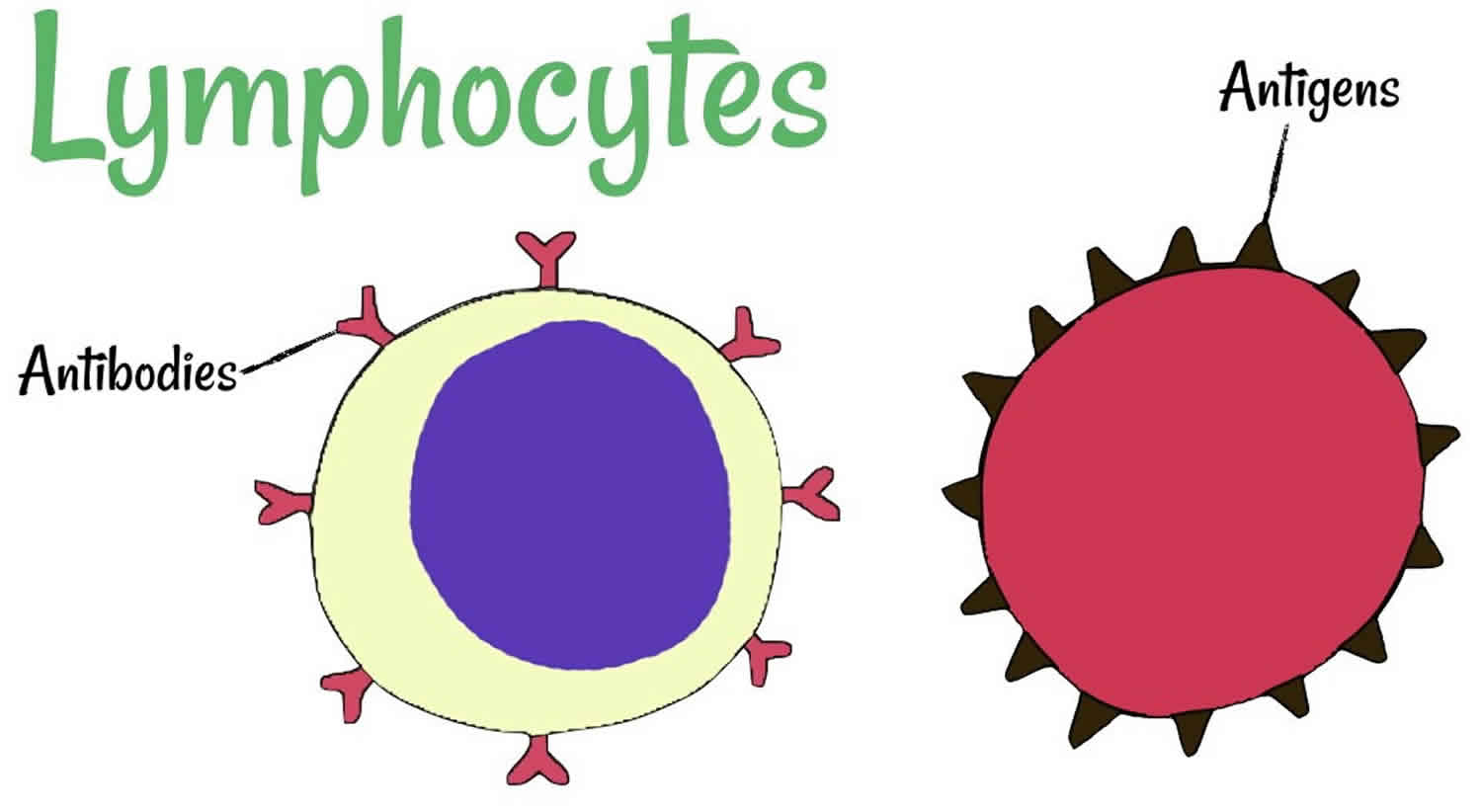
Lymphocyte is a type of white blood cell that is made in the bone marrow and is found in the blood and in lymph tissue. There are about 2 × 1012 lymphocytes in the human body, making the immune system comparable in cell mass to the liver or brain 1. The three types of lymphocytes are B lymphocytes, T lymphocytes, and natural killer cells. All of these cells help protect your body from infection. B lymphocytes (B cells) produce antibodies, and T lymphocytes (T cells) recognize foreign substances, process them for removal and help kill tumor cells and help control immune responses. About 20 to 40 percent of all white blood cells (leukocytes) are lymphocytes. A normal lymphocyte count for adults usually is between 1,000 and 4,800 lymphocytes per microliter of blood. For children, a normal lymphocyte count usually is between 3,000 and 9,500 lymphocytes per microliter of blood.
The T and B lymphocytes (T and B Cells) are involved in the acquired or antigen-specific immune response given that they are the only cells in the organism able to recognize and respond specifically to each antigenic epitope. The B lymphocytes (B cells) have the ability to transform into plasmocytes and are responsible for producing antibodies (immunoglobulins). Thus, humoral immunity depends on the B lymphocytes (B cells) while cell immunity depends on the T lymphocytes (T cells).
T lymphocytes and B lymphocytes derive their names from the organs in which they develop. T lymphocytes develop in the thymus, and B lymphocytes, in mammals, develop in the bone marrow in adults or the liver in fetuses. The greater part of lymphocyte development in mammals occurs in the specialized environments of the central lymphoid organs—the bone marrow (and the liver in the fetus) for B lymphocytes and the thymus for T lymphocytes. In the fetus and the juvenile, these tissues are the source of large numbers of new lymphocytes, which migrate to populate the peripheral lymphoid tissues. In mature individuals, development of new T lymphocytes in the thymus slows down and T-lymphocyte numbers are maintained through division of mature T lymphocytes outside of the central lymphoid organs. New B lymphocytes, on the other hand, are continually produced from the bone marrow, even in adults 2. As B lymphocytes differentiate from primitive stem cells, they proceed through stages that are marked by the sequential rearrangement of immunoglobulin gene segments to generate a diverse repertoire of antigen receptors. This developmental program also involves changes in the expression of other cellular proteins. Precursors of T lymphocytes migrate from the bone marrow and mature in the thymus. This process is similar to that for B lymphocytes, including the sequential rearrangement of antigen receptor gene segments. Developing T lymphocytes pass through a series of stages that can be distinguished by the differential expression of CD44 and CD25, the CD3:T-cell receptor complex (TCR) proteins, and the co-receptor proteins CD4 and CD8. The development of both T and B lymphocytes is guided by the environment, particularly by stromal cells that provide contact-dependent signals and growth factors for developing lymphocytes. In the case of T lymphocytes, development is compartmentalized, with different types of stromal cells in the thymic cortex and medulla. Most steps in T-lymphocyte differentiation occur in the cortex of the thymus. The thymic medulla contains mainly mature T lymphocytes. Lymphocyte development is accompanied by extensive cell death, reflecting intense selection and the elimination of those lymphocytes with inappropriate receptor specificities. B lymphocytes are produced throughout life, whereas T-lymphocyte production from the thymus slows down after puberty.
Despite their different origins, both T and B lymphocytes develop from the same pluripotent hemopoietic stem cells, which give rise to all of the blood cells, including red blood cells, white blood cells, and platelets 1. These stem cells are located primarily in hemopoietic tissues—mainly the liver in fetuses and the bone marrow in adults. T cells develop in the thymus from precursor cells that migrate there from the hemopoietic tissues via the blood. In most mammals, including humans and mice, B cells develop from stem cells in the hemopoietic tissues themselves. Because they are sites where lymphocytes develop from precursor cells, the thymus and hemopoietic tissues are referred to as central (primary) lymphoid organs 1.
From the morphological point of view, nonactivated T and B lymphocytes are indistinguishable (even in an electron microscope) since they are both small cells (8–10 microns in diameter) and each possesses a large nucleus with dense hetero-chromatin and a cytoplasmic border that contains few mitochondria, ribosomes, and lyzosomes 3. Both T and B lymphocytes are small, only marginally bigger than red blood cells, and contain little cytoplasm. T and B lymphocytes become morphologically distinguishable from each other only after they have been activated by antigen to proliferate and mature into effector cells 1. Effector B lymphocytes secrete antibodies. In their most mature form, called plasma cells, they are filled with an extensive rough endoplasmic reticulum. In contrast, effector T lymphocytes contain very little endoplasmic reticulum and do not secrete antibodies. When lymphocytes are activated by the antigenic stimulus, they may enlarge, thus increasing their cytoplasm and organelle number. Lymphocytes present receptors for antigen (Ag) recognition (T Cell receptor (TCR) and B Cell receptor (BCR) respectively) with different specificities on their surfaces. The genes that encode for these structures undergo a series of DNA recombinations, which provides them with immense phenotypic diversity.
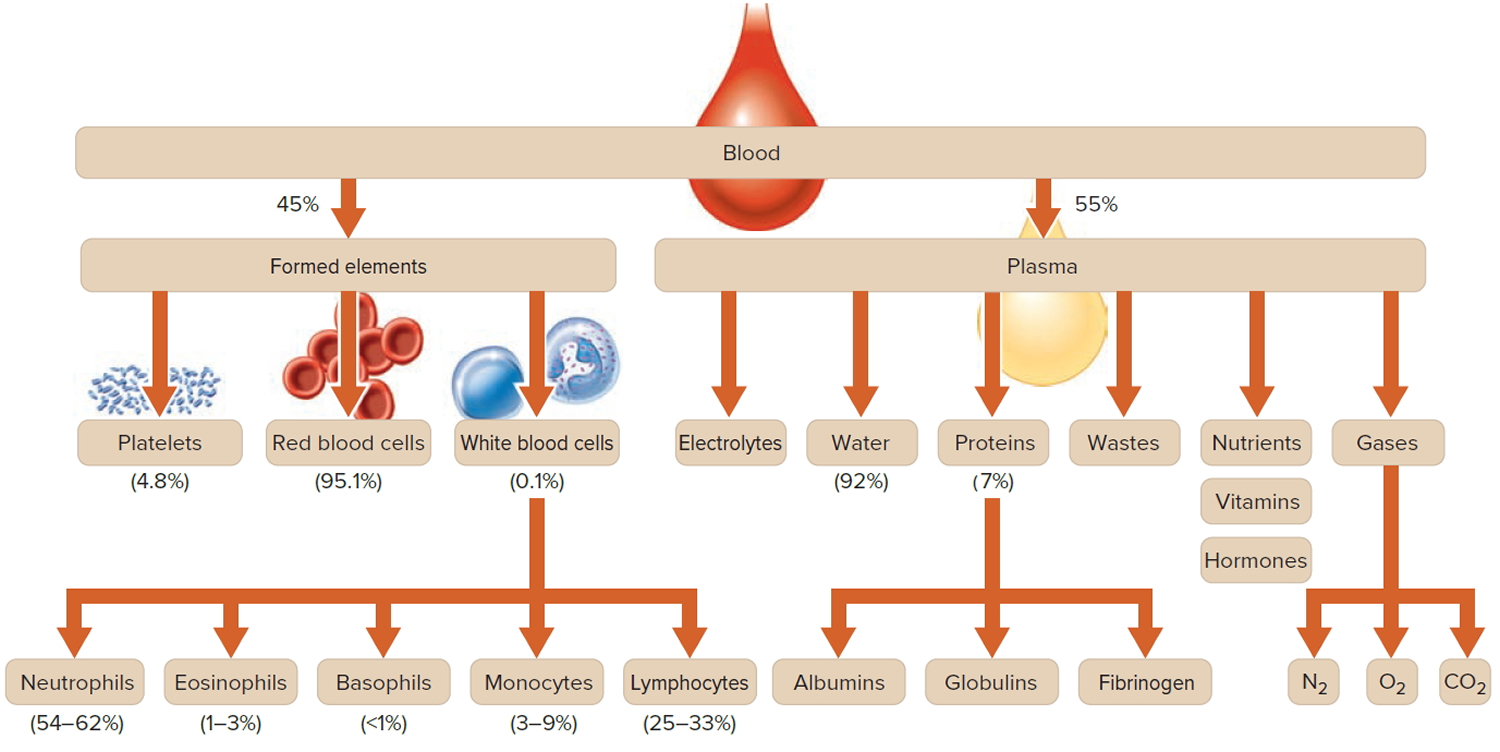
Figure 1. Blood composition
Note: Blood is a complex mixture of formed elements in a liquid extracellular matrix, called blood plasma. Note that water and proteins account for 99% of the blood plasma.
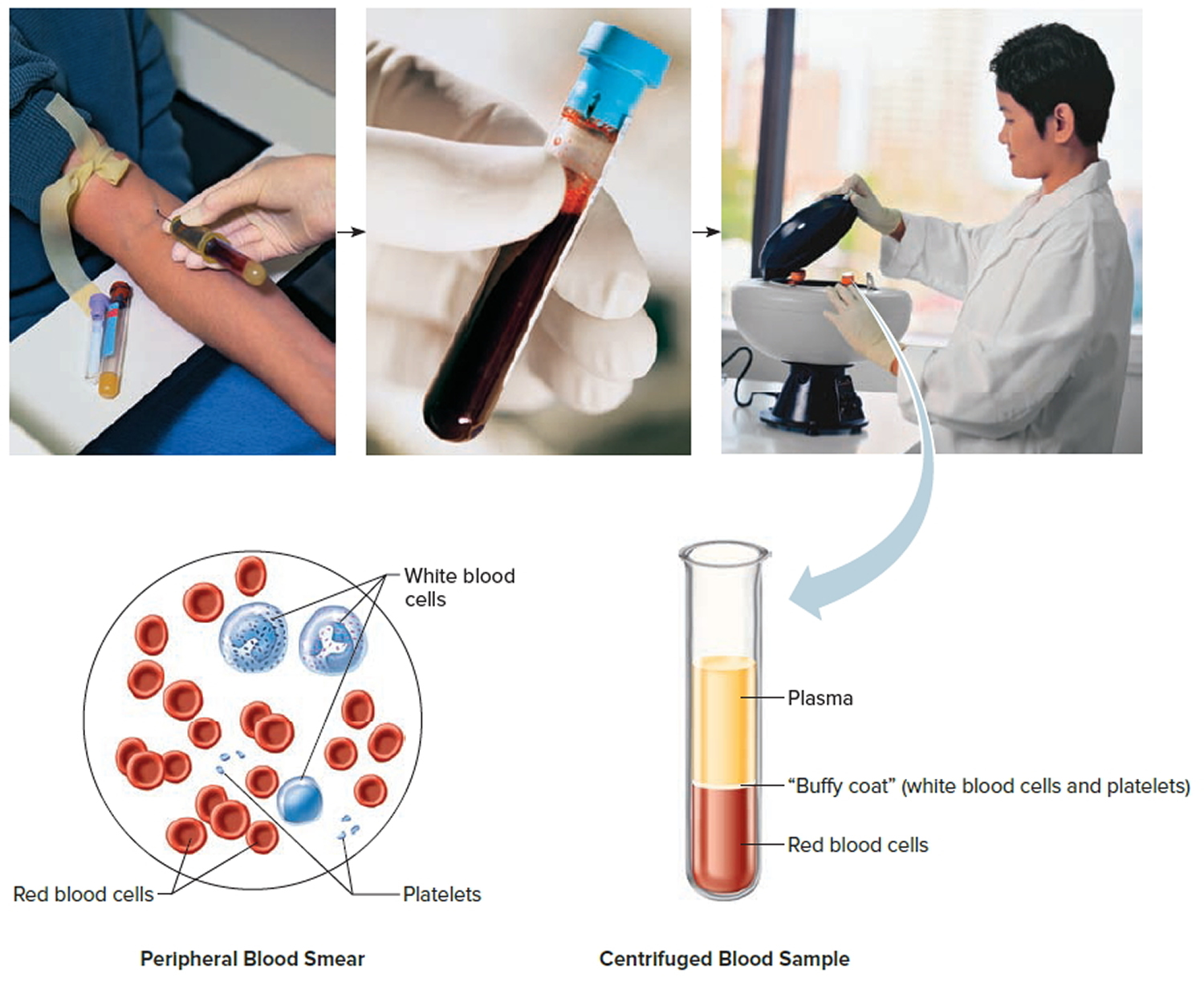
Footnote: Blood consists of a liquid portion called plasma and a solid portion (the formed elements) that includes red blood cells, white blood cells, and platelets. When blood components are separated by centrifugation, the white blood cells and platelets form a thin layer, called the “buffy coat,” between the plasma and the red blood cells, which accounts for about 1% of the total blood volume. Blood cells and platelets can be seen under a light microscope when a blood sample is smeared onto a glass slide.
Normal Leukocyte Life Cycle and Responses
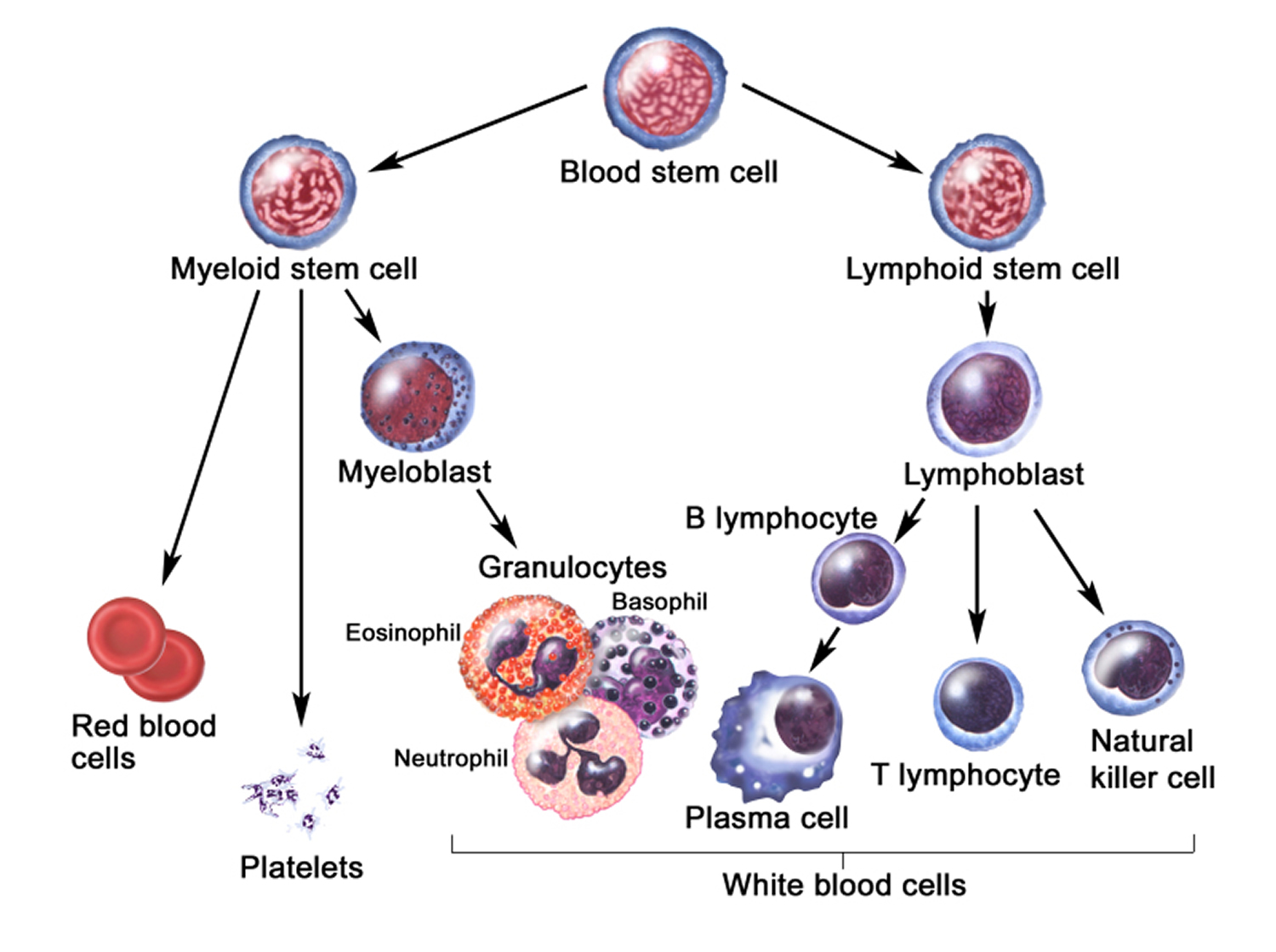
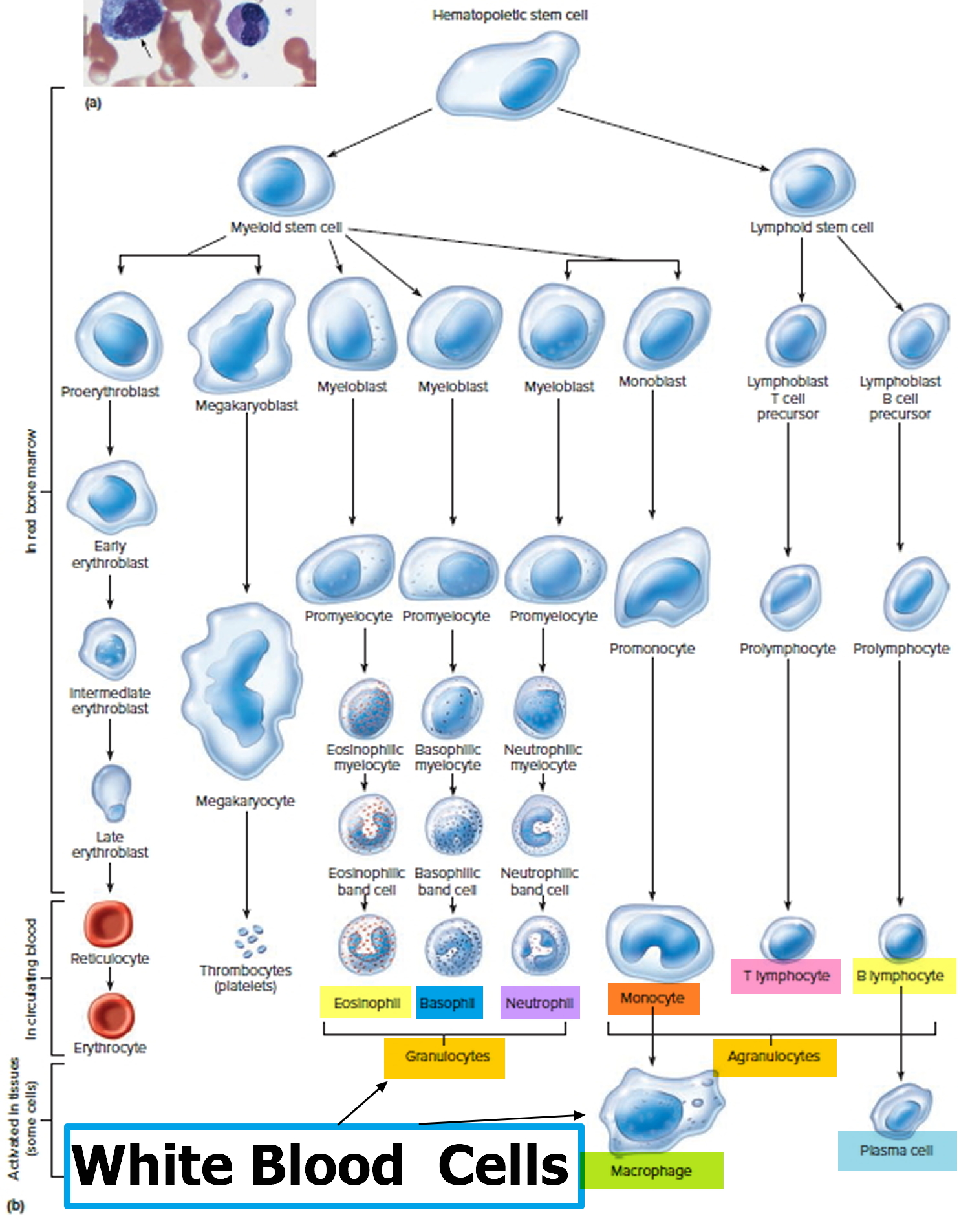
The life cycle of leukocytes includes development and differentiation, storage in the bone marrow, margination within the vascular spaces, and migration to tissues. Stem cells in the bone marrow produce cell lines of erythroblasts, which become red blood cells; megakaryoblasts, which become platelets; lymphoblasts; and myeloblasts. Lymphoblasts develop into various types of T and B cell lymphocytes. Myeloblasts further differentiate into monocytes and granulocytes, a designation that includes neutrophils, basophils, and eosinophils (Figure 2 and 3). Once white blood cells have matured within the bone marrow, 80% to 90% remain in storage in the bone marrow. This large reserve allows for a rapid increase in the circulating white blood cell count within hours. A relatively small pool (2% to 3%) of leukocytes circulate freely in the peripheral blood 4; the rest stay deposited along the margins of blood vessel walls or in the spleen. Leukocytes spend most of their life span in storage. Once a leukocyte is released into circulation and peripheral tissues, its life span ranges from two to 16 days, depending on the type of cell.
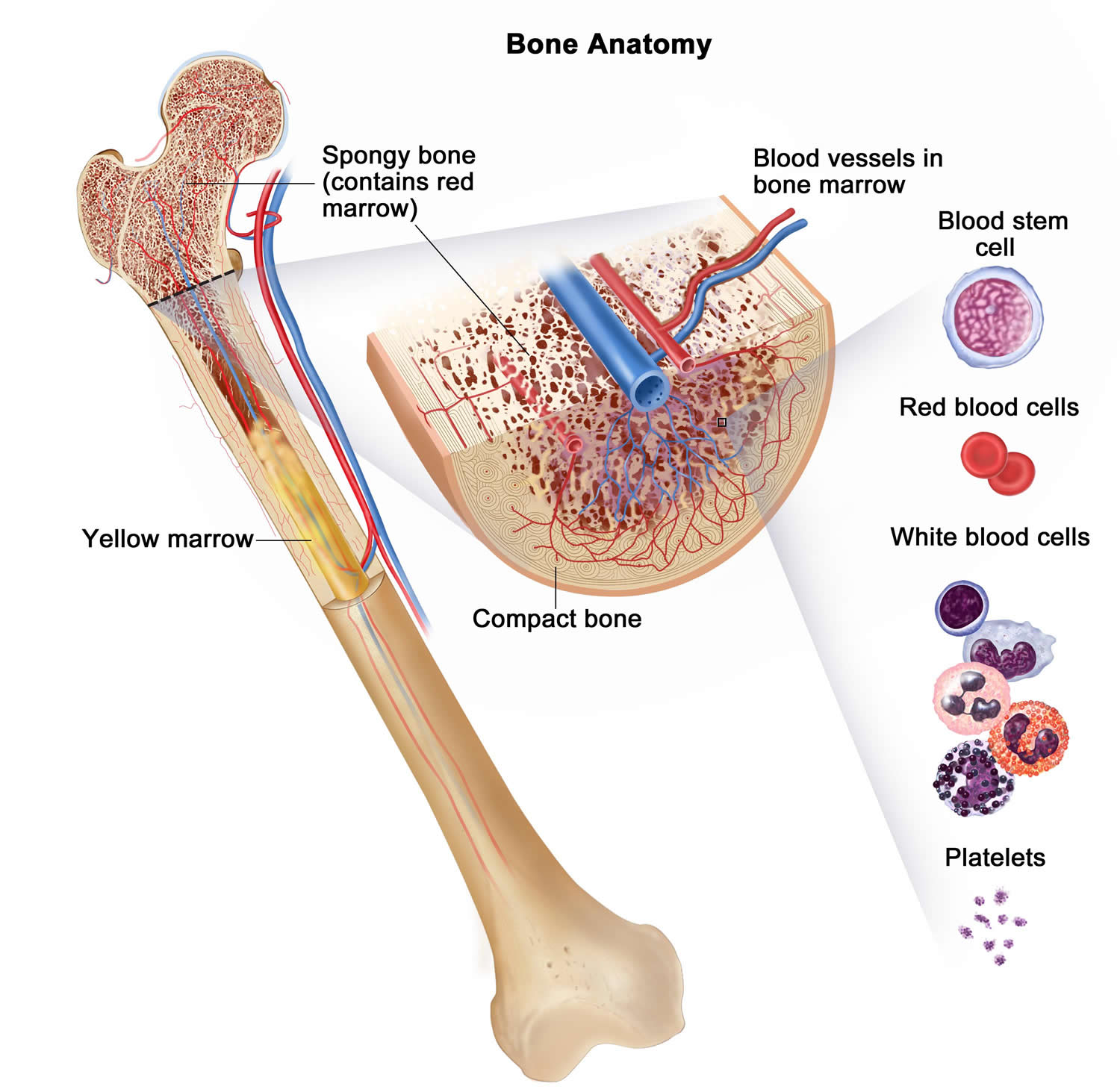
Figure 2. Bone marrow anatomy
Footnote: Anatomy of the bone. The bone is made up of compact bone, spongy bone, and bone marrow. Compact bone makes up the outer layer of the bone. Spongy bone is found mostly at the ends of bones and contains red marrow. Bone marrow is found in the center of most bones and has many blood vessels. There are two types of bone marrow: red and yellow. Red marrow contains blood stem cells that can become red blood cells, white blood cells, or platelets. Yellow marrow is made mostly of fat.
Figure 3. White blood cells development. A blood stem cell goes through several steps to become a red blood cell, platelet, or white blood cell
Figure 4. White blood cells development
B-lymphocytes
B lymphocytes are the effectors of humoral immunity, providing defense from pathogens through different functions including antibody production. B lymphocytes (B cells) constitute approximately 15% of peripheral blood leukocytes and arise from hemopoietic stem cells in the bone marrow 5. It is here that their antigen receptors (surface immunoglobulin) are assembled. In the context of autoimmune diseases defined by B and/or T lymphocyte auto-reactive that upon activation lead to chronic tissue inflammation and often irreversible structural and functional damage; B lymphocytes play an essential role by not only producing auto-antibodies but also functioning as Antigen-Presenting Cells (APC) and as a source of cytokines.
T-lymphocytes
T lymphocytes are a diverse and important group of lymphocytes that mature and undergo a positive and negative selection processes in the thymus, hence, the name T cells. T lymphocytes play a vital role in both components of active immunity (cell-mediated and to some extent humoral immunity). T lymphocytes function by recognizing short peptides of antigens presented on the surface of “antigen presenting cells.” T lymphocytes can only recognize protein based, receptor-bound antigens. This is achieved by use of the major histocompatibility complex (MHC) also known as human leukocyte antigen (HLA) 1 and 2 receptors, which along with the TCRs (T-cell receptors) bind the antigen in question and form a complex that allows the T cell to recognize the antigen. T cells cannot recognize soluble, free antigens 6. T cells are identified via flow cytometry for their cluster of differentiation (CD) markers.
There are two major T lymphocyte subsets: CD8+ T Cells (cytotoxic T cells, or killer T cells), which typically recognize major histocompatibility complex (MHC) class 1 molecules and express the CD8 coreceptor exclusively; and CD4+ T cells (helper T cells), which recognize MHC class 2 molecules and express the CD4 coreceptor exclusively 6. Maintaining the proper number and proportion of CD4 and CD8 T cells in the periphery is critical for an optimal immune response and is enforced in the first instance by stringent and complex T cell development programs in the thymus. Elucidating how this strict correlation between MHC specificity and lineage choice is orchestrated has been a major research area for over three decades. However, the molecular basis of differentiation of immature thymocyte precursors to alternate CD4 or CD8 lineage cells remains poorly understood, but appears to be controlled by differences in T lymphocyte receptors (TCR) signaling 7.
T lymphocytes play a dual role in autoimmunity, on one hand, T lymphocytes (T cells) play a crucial role in the maintenance of tolerance to self, while on the other, autoreactive T lymphocytes (T cells) play an important effector role in the cell and tissue damage that encompasses autoimmune disease 8.
T lymphocytes development
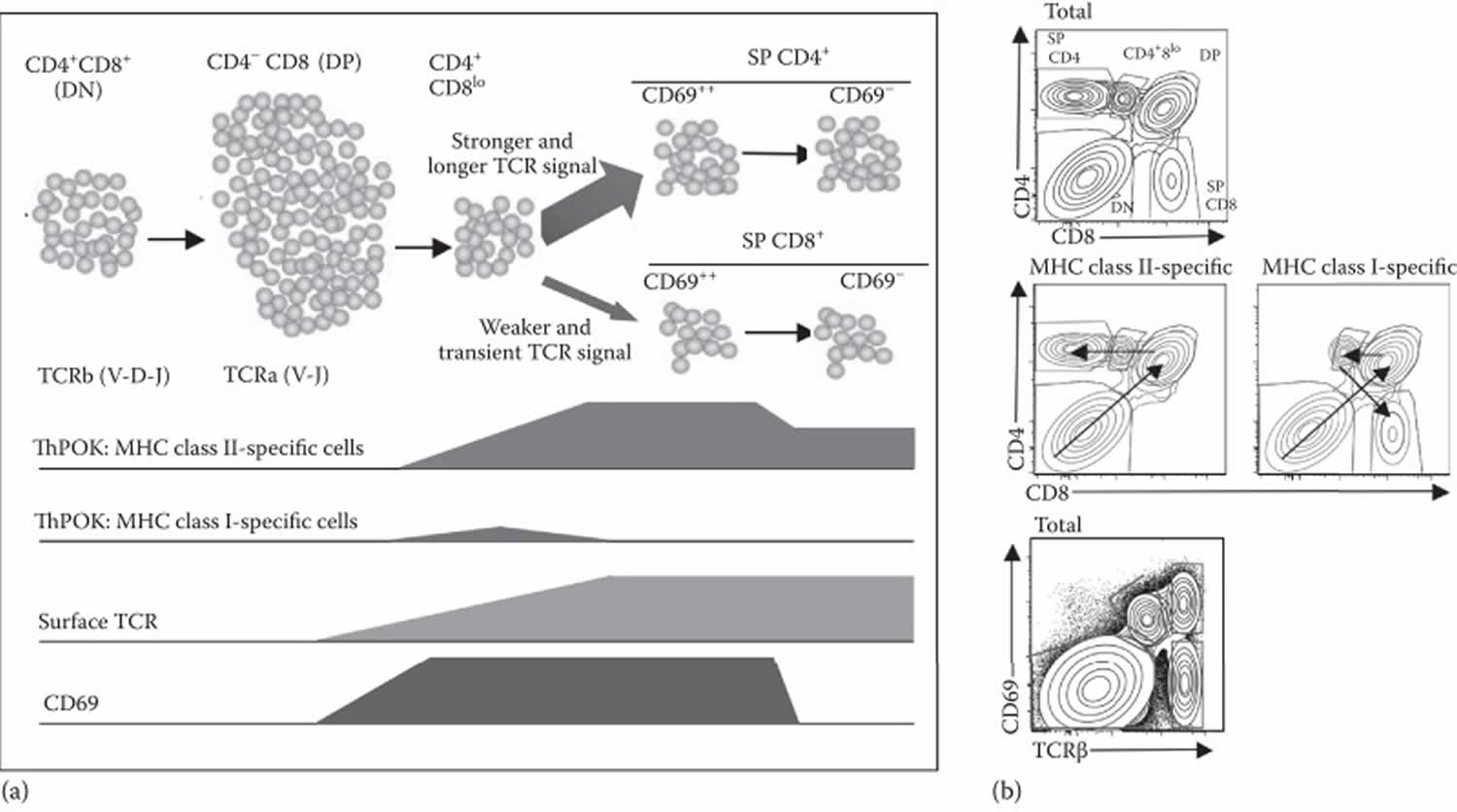
T-lymphocyte development is more complicated than B-lymphocyte development as it has to accommodate the production of two distinct lineages of T lymphocytes with different types of T-lymphocyte receptor—α:β and γ:δ TCR 2. The α:β and γ:δ T lymphocytes diverge early in T-cell development. There is a further division of the α:β T cells into CD4 and CD8 T cells that occurs in immature T cells after the T-cell receptor genes have been assembled and expressed. Thymic development of T lymphocytes is a highly complex and closely controlled program, beginning with early progenitor cells from the bone marrow, which express neither CD4 nor CD8 surface markers, known as double negative (DN) thymocytes, and ends with mature single positive (SP) CD4 and CD8 thymocytes. Within the double negative (DN) compartment, four sequential stages are marked by differential expression of the CD44 and CD25 markers: DN1 (CD25–CD44hi), DN2 (CD25+CD44+), DN3 (CD25+CD44–), and DN4 (CD25–CD44–). Cells belonging to the major αβ T cell lineage develop further to the double positive CD4+CD8+ (DP) stage, where the αβ T cell receptor (TCR) complex is first expressed at the cell surface, allowing engagement by intrathymic peptide/MHC ligands (Figure 5a). At this stage, cells with high self-affinity undergo death by apoptosis (negative selection), whereas cells with lower self-affinity undergo activation and further differentiation into single positive T cells (positive selection). These processes are extremely stringent leading to the programmed cell death of >90% of DP thymocytes. Defects in these selection processes can result in survival of strongly self-reactive T cells and consequent autoimmune disease. The few thymocytes that are selected for survival undergo alternate commitment to either SP CD4 or CD8 lineages 9. Regardless of the final lineage commitment, DP cells initially downregulate the CD8 coreceptor to give rise to intermediate CD4+8lo cells (Figure 5b) 10. From this common CD4+8lo stage, cells can mature directly into SP CD4 cells or into SP CD8 cells, via additional intermediate stages (CD4loCD8lo and CD4loCD8+) 11. There is an almost perfect correlation between commitment to the CD8 or CD4 lineages, and specificity of a developing thymocyte to MHC class I or II, respectively. The correct match between lineage choice and MHC class specificity is highly important for proper T cell function. How this near perfect correlation between MHC specificity and functional lineage choice is achieved is still not fully understood.
T lymphocyte receptors (TCR) signaling is believed to represent the major driving force underlying alternate commitment to the CD4 and CD8 lineages 7. Accumulating genetic and biochemical evidence suggests that the strength and duration of the TCR-MHC interaction during thymic T cell development are critical for determining alternate lineage choice, such that relatively long and strong TCR signals give rise to SP CD4 cells, whereas weak and transient signals give rise to CD8 T cells. Two alternative models have been proposed, based on differential TCR signal strength (signal strength model) or duration (kinetic model).
Figure 5. T lymphocyte development
Footnotes: T lymphocyte development. (a) Simplified diagram of stages in thymic development, including, in sequential order, double negative (DN), double positive (DP), intermediate CD4+8lo, and finally single-positive (SP) CD4 and CD8. Thymic development is also marked by changes in expression of TCR and CD69 surface markers. TCR and CD69 surface markers are both upmodulated as thymocytes mature from the DP to the CD4+8lo stage and even further during subsequent transition to the SP CD4 and CD8 stages. Finally, just before exiting the thymus, SP thymocytes lose CD69 expression but still maintain high TCR expression. At the CD4+8lo stage, MHC class II- and class I-specific cells express high or low levels of ThPOK, respectively, and diverge into alternate CD4 and CD8 lineages. (b) Schematics of the surface expression pattern of CD4, CD8, CD69, and T cell receptors (TCR) by total thymocytes, or by MHC class I- or II-specific thymocytes, as indicated.
[Source 7 ]Lymphocytes function
Lymphocytes play important roles in cellular and humoral immunity. Lymphocytes usually respond to foreign antigens only if the innate immune system is first activated. The innate immune responses to an infection are rapid. They depend on pattern recognition receptors that recognize patterns of pathogen-associated molecules (immunostimulants) that are not present in the host organism, including microbial DNA, lipids, and polysaccharides, and proteins that form bacterial flagella. Some of these receptors are present on the surface of professional phagocytic cells such as macrophages and neutrophils, where they mediate the uptake of pathogens, which are then delivered to lysosomes for destruction. Others are secreted and bind to the surface of pathogens, marking them for destruction by either phagocytes or the complement system. Still others are present on the surface of various types of host cells and activate intracellular signaling pathways in response to the binding of pathogen-associated immunostimulants; this leads to the production of extracellular signal molecules that promote inflammation and help activate adaptive immune responses.
Some cells of the innate immune system directly present microbial antigens to T lymphocytes to initiate an adaptive immune response. The cells that do this most efficiently are called dendritic cells, which are present in most vertebrate tissues. They recognize and phagocytose invading microbes or their products at a site of infection and then migrate with their prey to a nearby peripheral lymphoid organ. There they act as antigen-presenting cells, which directly activate T lymphocytes to respond to the microbial antigens. Once activated, some of the T lymphocytes then migrate to the site of infection, where they help other phagocytic cells, mainly macrophages, destroy the microbes. Other activated T lymphocytes remain in the lymphoid organ and help B lymphocytes respond to the microbial antigens. The activated B lymphocytes secrete antibodies that circulate in the body and coat the microbes, targeting them for efficient phagocytosis.
Thus, innate immune responses are activated mainly at sites of infection, whereas adaptive immune responses are activated in peripheral lymphoid organs. The two types of responses work together to eliminate invading pathogens.
B lymphocytes are the effectors of humoral immunity, providing defense from pathogens through different functions including antibody production 5. In the context of autoimmune diseases defined by B and/or T lymphocyte auto-reactive that upon activation lead to chronic tissue inflammation and often irreversible structural and functional damage; B lymphocytes play an essential role by not only producing auto-antibodies but also functioning as Antigen-Presenting Cells (APC) and as a source of cytokines.
Cytotoxic T cells (CD8+ T Cells or killer T cells) kill infected cells, whereas helper T cells (CD4+ T cells) help activate macrophages, B lymphocytes, and cytotoxic T cells. Effector helper T cells secrete a variety of signal proteins called cytokines, which act as local mediators. They also display a variety of costimulatory proteins on their surface. By means of these cytokines and membrane-bound costimulatory proteins, they can influence the behavior of the various cell types they help. Effector cytotoxic T cells kill infected target cells also by means of proteins that they either secrete or display on their surface. Thus, whereas B lymphocytes can act over long distances by secreting antibodies that are distributed by the bloodstream, T lymphocytes can migrate to distant sites, but there they act only locally on neighboring cells.
T lymphocytes play a crucial role in the development of autoimmune diseases, for they are the main responsible of self-tolerance maintenance but, additionally, they actively participate in the mechanisms of cell and tissue damage in autoimmune mediated diseases 12.
Innate immune system
Innate immune responses are triggered at sites of infection by microbe-specific molecules associated with invading pathogens. In addition to fighting infection directly, these responses help activate adaptive immune responses in peripheral lymphoid organs. Unlike innate immune responses, adaptive responses provide specific and long-lasting protection against the particular pathogen that induced them.
Adaptive immune system
The adaptive immune system is composed of millions of lymphocyte clones, with the cells in each clone sharing a unique cell-surface receptor that enables them to bind a particular antigen. The binding of antigen to these receptors, however, is usually not sufficient to stimulate a lymphocyte to proliferate and differentiate into an effector cell that can help eliminate the pathogen. Costimulatory signals provided by another specialized cell in a peripheral lymphoid organ are also required. Helper T cells provide such signals for B cells, while antigen-presenting dendritic cells usually provide them for T cells. Effector B cells secrete antibodies, which can act over long distances to help eliminate extracellular pathogens and their toxins. Effector T cells, by contrast, act locally at sites of infection to either kill infected host cells or help other cells to eliminate pathogens. As part of the adaptive immune response, some lymphocytes proliferate and differentiate into memory cells, which are able to respond faster and more efficiently the next time the same pathogen invades. Lymphocytes that would react against self molecules are either induced to alter their receptors, induced to kill themselves, inactivated, or suppressed, so that the adaptive immune system normally reacts only against foreign antigens. Both B and T cells circulate continuously between the blood and lymph. Only if they encounter their specific foreign antigen in a peripheral lymphoid organ do they stop migrating, proliferate, and differentiate into effector cells or memory cells.
Lymphocytes normal range
The lymphocyte reference ranges absolute lymphocytes counts [Abs, cells/μl] unadjusted for gender differences for 13:
Adolescents
- CD3: 939-2959 cells/μl
- CD4: 467-1563 cells/μl
- CD8: 259-1262 cells/μl
- CD19+ B cells: 169-1297 cells/μl
- CD16+CD56+ NK cells: 59-1178 cells/μl
Adults
- CD3: 983-3572 cells/μl
- CD4: 491-2000 cells/μl
- CD8: 314-2,087 cells/μl
- CD19+ B cells: 64-800 cells/μl
- CD16+CD56+ NK cells: 27-693 cells/μl
Note: The reference values of peripheral blood lymphocyte subsets were analyzed in healthy adolescent and adult population of South Florida 13.
The ranges for CD4:CD8 ratio for adolescents and adults are 0.7-2.6 and 0.6-4.4, respectively 13. Gender based analysis of relative percentages of lymphocyte subsets showed no significant differences between adult and adolescent males and females. The mean CD4:CD8 ratio was significantly higher in adult females than males and in adolescents this difference was not significant between genders. The mean CD3 and CD4 T cell percentages were higher and CD19 cell percentages were lower in adults compared to adolescents. Absolute lymphocyte counts showed a positive correlation with the absolute counts of CD3+, CD4+, CD8+, CD19+, CD16+CD56+, CD45RO+ and CD45RA+ cells 13.
Lymphocytes high
High lymphocyte count is also called lymphocytosis, is an increase in white blood cells called lymphocytes. Lymphocytes are an important part of the immune system. They help fight off diseases, so it’s normal to see a temporary rise in the number of lymphocytes after an infection.
A count significantly higher than 3,000 lymphocytes in a microliter of blood is generally considered to be lymphocytosis in adults. In children, the threshold for lymphocytosis varies with age. It may be as high as 9,000 lymphocytes per microliter. The exact thresholds for lymphocytosis may vary slightly from one lab to another.
A high lymphocyte count is usually found when your doctor has ordered tests to help diagnose a condition you’re already experiencing. It’s rarely an unexpected finding or simply discovered by chance. Talk with your doctor about what your test results mean. A high lymphocyte count and results from other tests may indicate the cause of your illness. Or your doctor may suggest other tests to further check your condition.
High lymphocyte count causes
You may have a lymphocyte count that is higher than would normally be expected but have few, if any, symptoms. It’s usually a harmless, temporary situation, as can occur after an illness. But it may represent something more serious, such as a blood cancer or a chronic infection. Your doctor may need to perform other tests to determine if your lymphocyte count is a cause for concern.
If your doctor determines that your lymphocyte count is high, the test result may be evidence of one of the following conditions:
- Infection (bacterial, viral, other)
- Cancer of the blood or lymphatic system
- An autoimmune disorder causing ongoing (chronic) inflammation
Specific causes of lymphocytosis include:
- Acute lymphocytic leukemia
- Chronic lymphocytic leukemia
- Cytomegalovirus (CMV) infection
- HIV/AIDS
- Mononucleosis
- Other viral infections
- Tuberculosis
- Vasculitis (blood vessel inflammation)
- Whooping cough
Causes shown here are commonly associated with this symptom. Work with your doctor or other health care professional for an accurate diagnosis.
Lymphocytes low
Low lymphocyte count is also called lymphopenia or lymphocytopenia, is a disorder in which your blood doesn’t have enough white blood cells called lymphocytes. Lymphocytes help protect your body from infection. Low numbers of lymphocytes can raise your risk of infection.
The term “lymphocytopenia” refers to a count of less than 1,000 lymphocytes per microliter of blood in adults, or less than 3,000 lymphocytes per microliter of blood in children.
Most people who have low lymphocyte count (lymphocytopenia) have low numbers of T lymphocytes. Sometimes they also have low numbers of the other types of lymphocytes.
Certain factors can cause a low lymphocyte count, such as:
- The body doesn’t make enough lymphocytes.
- The body makes enough lymphocytes, but they’re destroyed.
- The lymphocytes get trapped in the spleen or lymph nodes. Lymphocytes normally pass through these organs into the blood.
- A combination of the above factors.
Many diseases, conditions, and factors can cause the above problems that lead to low lymphocyte count (lymphocytopenia). These causes can be acquired or inherited.
“Acquired” means you aren’t born with the condition, but you develop it. One of the most common acquired causes of lymphocytopenia is AIDS.
“Inherited” means your parents passed the gene for the condition on to you. Inherited causes include DiGeorge anomaly, Wiskott-Aldrich syndrome, severe combined immunodeficiency syndrome, and ataxia-telangiectasia. These inherited conditions are rare.
Low lymphocyte count (lymphocytopenia) can range from mild to severe. Low lymphocyte count (lymphocytopenia) alone may not cause any signs, symptoms, or serious problems.
How long lymphocytopenia lasts depends on its cause. The treatment for this condition depends on its cause and severity. Mild low lymphocyte count (lymphocytopenia) may not require treatment. If an underlying condition is successfully treated, lymphocytopenia will likely improve.
If lymphocytopenia causes serious infections, you may need medicines or other treatments.
Low lymphocytes causes
In general, low lymphocyte count (lymphocytopenia) occurs because:
- The body doesn’t make enough lymphocytes.
- The body makes enough lymphocytes, but they’re destroyed.
- The lymphocytes get stuck in the spleen or lymph nodes.
A combination of these factors also may cause a low lymphocyte count.
Many diseases, conditions, and factors can lead to a low lymphocyte count. These conditions can be acquired or inherited. “Acquired” means you aren’t born with the condition, but you develop it. “Inherited” means your parents passed the gene for the condition on to you.
Exactly how each disease, condition, or factor affects your lymphocyte count isn’t known. Some people have low lymphocyte counts with no underlying cause.
Acquired Causes
Many acquired diseases, conditions, and factors can cause lymphocytopenia. Examples include:
- Infectious diseases, such as AIDS, viral hepatitis, tuberculosis, and typhoid fever.
- Autoimmune disorders, such as lupus. (Autoimmune disorders occur if the body’s immune system mistakenly attacks the body’s cells and tissues.)
- Steroid therapy.
- Blood cancer and other blood diseases, such as Hodgkin’s disease and aplastic anemia.
- Radiation and chemotherapy (treatments for cancer).
Inherited Causes
Certain inherited diseases and conditions can lead to lymphocytopenia. Examples include DiGeorge anomaly, Wiskott-Aldrich syndrome, severe combined immunodeficiency syndrome, and ataxia-telangiectasia. These inherited conditions are rare.
Risk factors for developing low lymphocytes
People at highest risk for low lymphocyte count (lymphocytopenia) have one of the diseases, conditions, or factors that can cause a low lymphocyte count. This includes people who have:
- AIDS or other infectious diseases
- Autoimmune disorders
- Blood cancers or other blood diseases
- Certain inherited diseases or conditions
People who have had steroid therapy or radiation or chemotherapy (treatments for cancer) also are at increased risk.
Low lymphocytes prevention
You can’t prevent low lymphocyte count (lymphocytopenia) that’s caused by an inherited condition. However, you can take steps to control lymphocytopenia. Follow your treatment plan and take all medicines as your doctor advises.
Early diagnosis also can help control lymphocytopenia. In the United States, newborns are routinely screened for an immune condition that can lead to lymphocytopenia. This allows doctors to diagnose the disorder before serious problems develop.
You may be able to lower your risk for acquired conditions that cause low lymphocyte count (lymphocytopenia).
Low lymphocytes signs and symptoms
A low lymphocyte count alone may not cause any signs or symptoms. Low lymphocyte count (lymphocytopenia) usually is found when a person is tested for other diseases or conditions, such as AIDS.
If you have unusual infections, repeat infections, and/or infections that won’t go away, your doctor may suspect that you have lymphocytopenia. Fever is the most common symptom of infection.
Low lymphocytes diagnosis
Your doctor will diagnose low lymphocyte count (lymphocytopenia) based on your medical history, a physical exam, and test results.
A low lymphocyte count alone may not cause any signs or symptoms. Thus, the condition often is diagnosed during testing for other diseases or conditions.
Specialists involved
Your primary care doctor may notice that you have unusual infections, repeat infections, and/or infections that won’t go away. These infections may be signs of low lymphocyte count (lymphocytopenia). Your primary care doctor may refer you to an infectious disease specialist to find out what’s causing the infections.
You also may see a hematologist (blood disease specialist) or an immunologist (immune disorders specialist). Blood diseases and immune disorders can cause low lymphocyte count (lymphocytopenia).
Medical history
To assess your risk for a low lymphocyte count, your doctor may ask:
- About your risk for AIDS, including questions about blood transfusions, sexual partners, intravenous (IV) drug use, and exposure to infectious blood or bodily fluids at work
- Whether you’ve ever had radiation or chemotherapy (treatments for cancer)
- Whether you’ve ever been diagnosed with a blood disease or immune disorder, or whether you have a family history of such illnesses
Physical exam
Your doctor will do a physical exam to look for signs of infection, such as fever. He or she may check your abdomen for signs of an enlarged spleen and your neck for signs of enlarged lymph nodes.
Your doctor also will look for signs and symptoms of diseases and conditions that can affect your lymphocyte count, such as AIDS and blood cancers.
Diagnostic tests
Your doctor may recommend one or more of the following tests to help diagnose a low lymphocyte count.
Complete Blood Count With Differential
A complete blood count (CBC) measures many parts of your blood. The test checks the number of red blood cells, white blood cells, and platelets in your blood. The complete blood count (CBC) will show whether you have a low number of white blood cells.
Lymphocytes account for 20 to 40 percent of all white blood cells. Although a complete blood count will show an overall low white blood cell count, it won’t show whether the number of lymphocytes is low.
You may need a more detailed test, called a complete blood count with differential, to find out whether you have a low lymphocyte count. This test shows whether you have low levels of certain types of white blood cells, such as lymphocytes. The test results can help your doctor diagnose lymphocytopenia.
Flow cytometry
Flow cytometry looks at many types of blood cells. It’s even more detailed than a complete blood count with differential. Flow cytometry can measure the levels of the different types of lymphocytes—T lymphocytes, B lymphocytes, and natural killer cells.
The test can help diagnose the underlying cause of lymphocytopenia. Some underlying conditions cause low levels of T lymphocytes. Others may cause low levels of B lymphocytes or natural killer cells.
Tests for underlying conditions
Many diseases and conditions can cause lymphocytopenia. Your doctor will want to find the cause of the disorder. You may be tested for HIV/AIDS, tuberculosis, blood diseases, and immune disorders.
Tests for these underlying conditions might include blood tests, bone marrow tests, and lymph node tests.
Lymph nodes are part of the immune system. They’re found in many places in your body. During a physical exam, your doctor may find that certain lymph nodes are swollen. In lymphocytopenia, the lymph nodes may hold on to too many lymphocytes instead of releasing them into the bloodstream.
To test a lymph node, you may need to have it removed. Removing a lymph node involves minor surgery.
Low lymphocytes treatment
If you have mild low lymphocyte count (lymphocytopenia) with no underlying cause, you may not need treatment. Mild low lymphocyte count (lymphocytopenia) may improve on its own.
If you have unusual infections, repeat infections, and/or infections that won’t go away due to low lymphocyte count (lymphocytopenia), you’ll need treatment for the infections.
If you have a disease or condition that’s causing lymphocytopenia, your doctor will prescribe treatment for that illness. Treating the underlying problem will help treat the lymphocytopenia.
Treatment for infections
A low lymphocyte count makes it hard for your body to fight infections. You may get infections caused by viruses, fungi, parasites, or bacteria.
Treatment for an infection will depend on its cause. You also may need treatment after an infection is gone to help prevent repeat infections.
Children who have serious, ongoing bacterial infections may get a medicine called immune globulin. This medicine helps boost the immune system and fight infections.
Treatment for underlying diseases or conditions
Many diseases and conditions can cause lymphocytopenia. Examples include infectious diseases, such as AIDS; blood diseases, such as aplastic anemia; and inherited diseases, such as Wiskott-Aldrich syndrome.
Your treatment will depend on your underlying disease or condition.
Emerging treatments
Researchers are looking at ways to increase lymphocyte production in people who have lymphocytopenia with serious underlying conditions.
For example, some studies are looking into blood and marrow stem cell transplants. This procedure may help treat or cure some of the conditions that can cause a low lymphocyte count.
Other studies are looking at medicines and other substances that can help the body make more lymphocytes.
Living with low lymphocytes
If you have mild low lymphocytes count with no underlying cause, you may not need treatment. The disorder may improve on its own.
If an underlying condition is causing your lymphocytopenia, you’ll need treatment for that condition. You’ll also need treatment for infections if your body is unable to fight them because of lymphocytopenia.
Treating and preventing Infections
The main risk of lymphocytopenia is getting unusual infections, repeat infections, and/or infections that won’t go away. If you have the disorder, you may get treatments to prevent infections or to treat infections you already have.
You also can take other steps to prevent infections. For example:
- Stay away from people who are sick, and avoid large crowds of people.
- Avoid foods that can expose you to bacteria, such as uncooked foods.
- Wash your hands often.
- Brush and floss your teeth and get regular dental care to reduce the risk of infection in your mouth and throat.
- Ask your doctor whether you should get a yearly flu shot and the pneumonia vaccine.
Know the signs of an infection, such as a fever. Call your doctor right away if you think you have an infection.
Treating an underlying disease or condition
If you have a disease or condition that’s causing lymphocytopenia, you’ll need treatment for that condition.
You’ll likely have regular tests to show how the treatment is working. For example, you may have blood tests to check the number of lymphocytes in your blood.
If the treatments for the underlying condition are working, the number of lymphocytes in your blood may go up.
Physical activity
Talk with your doctor about what types and amounts of physical activity are safe for you. You may want to avoid activities that could result in injuries or increase your risk of infections.
- Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Lymphocytes and the Cellular Basis of Adaptive Immunity. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26921[↩][↩][↩][↩]
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. Generation of lymphocytes in bone marrow and thymus. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27123[↩][↩]
- Cano RLE, Lopera HDE. Introduction to T and B lymphocytes. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, et al., editors. Autoimmunity: From Bench to Bedside [Internet]. Bogota (Colombia): El Rosario University Press; 2013 Jul 18. Chapter 5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459471[↩]
- Cerny J, Rosmarin AG. Why does my patient have leukocytosis? Hematol Oncol Clin North Am. 2012;26(2):303–319, viii.[↩]
- Izquierdo JH, Cañas CA, Tobón GJ. B lymphocytes in autoimmunity. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, et al., editors. Autoimmunity: From Bench to Bedside [Internet]. Bogota (Colombia): El Rosario University Press; 2013 Jul 18. Chapter 6. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459431[↩][↩]
- Sauls RS, Taylor BN. Histology, T-Cell Lymphocyte. [Updated 2018 Dec 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535433[↩][↩]
- Mookerjee-Basu J, Chemmannur SV, Qin L, et al. ThPOK, a Key Regulator of T Cell Development and Function. In: Soboloff J, Kappes DJ, editors. Signaling Mechanisms Regulating T Cell Diversity and Function. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532329[↩][↩][↩]
- Janeway C, Travers P, Walport MJ, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. 5th ed. Garland Pub; New York: 2001.[↩]
- Rothenberg, E. V., and Taghon, T. (2005). Molecular genetics of T cell development. Annual Review of Immunology, 23, 601–649.[↩]
- Kydd, R., Lundberg, K., Vremec, D., Harris, A. W., and Shortman, K. (1995). Intermediate steps in thymic positive selection. Generation of CD4-8+ T cells in culture from CD4+ 8+, CD4int8+, and CD4+ 8int thymocytes with up-regulated levels of TCR-CD3. The Journal of Immunology, 155(8), 3806–3814.[↩]
- Brugnera, E., Bhandoola, A., Cibotti, R., Yu, Q., Guinter, T. I., Yamashita, Y., Singer, A. (2000). Coreceptor reversal in the thymus: Signaled CD4+ 8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity, 13(1), 59–71.[↩]
- Ruiz-Argüelles A. T lymphocytes in autoimmunity. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, et al., editors. Autoimmunity: From Bench to Bedside [Internet]. Bogota (Colombia): El Rosario University Press; 2013 Jul 18. Chapter 7. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459477[↩]
- Reference ranges of lymphocyte subsets in healthy adults and adolescents with special mention of T cell maturation subsets in adults of South Florida. Immunobiology Volume 219, Issue 7, July 2014, Pages 487-496. https://doi.org/10.1016/j.imbio.2014.02.010[↩][↩][↩][↩]