Contents
Membranous glomerulopathy
Membranous glomerulopathy also known as membranous nephropathy or membranous glomerulonephritis, is one of the many glomerular diseases causing nephrotic syndrome 1. Nephrotic syndrome includes significant amounts of protein in the urine (at least 3.5 grams per day), low blood protein (albumin) levels, and swelling (edema). Membranous glomerulopathy or membranous glomerulonephritis is the second most common glomerular disease in adults after focal segmental glomerulosclerosis (FSGS) 2. Membranous glomerulopathy or membranous nephropathy is characterized histomorphologically by the presence of immune deposits in the subepithelial space of the glomerular filtration barrier 3. Membranous glomerulopathy or membranous nephropathy is the most common cause of primary nephrotic syndrome in White adults 4. Membranous glomerulopathy most commonly occurs above 40 years of age, with the peak incidence between 50 to 60 years in the USA 5. It is more common in males than females and tends to show a better outcome in females. The incidence is 8 to 10 cases per 1 million population worldwide, and 12 per 1 million population per year in the USA. Membranous nephropathy is less common in children and mostly due to secondary causes 6. Membranous glomerulopathy can occur by itself (primary) or due to another disease or underlying cause (secondary). Membranous glomerulopathy can also present in conjunction with other types of glomerulonephritis like IgA nephropathy, FSGS, and lupus nephritis 1.
Membranous glomerulopathy or membranous nephropathy is considered an autoimmune disease, which means that it caused by your body’s immune system turning against you and harming your body when it should be protecting you 7, 8. Membranous glomerulopathy is caused by the build-up of immune complexes within the filters (glomeruli) of the kidney itself. Figure 1 below is a diagram of how the immune complexes deposit in the kidney. The immune system normally creates antibodies to recognize and attach to something called an antigen. When an antibody attaches to an antigen, this is called an immune complex. Antigens are normally foreign to the body, like a virus or bacteria. However, sometimes, the body can make antibodies that recognize and attach to something in the body itself (not foreign) – these types of antibodies are called autoantibodies. Immune complexes are normally cleared from the blood before causing any problems, but under certain conditions they can accumulate in different parts of the body. In membranous nephropathy these immune complexes (antibodies made by the immune system attached to antigens) get caught in the kidney filters (glomeruli). In most cases of membranous nephropathy, antibodies are made to an antigen that is part of the kidney filter (glomerulus) itself. When your immune system attacks the glomeruli in membranous nephropathy, it causes changes to the filters that lead you to lose large amount of protein into the urine. Together these antibodies and antigens create immune complexes that get stuck in the kidney filter (glomerulus) and it can eventually lead to kidney failure.
Recently the antibody that causes most cases of membranous glomerulopathy was discovered and identified 9. In about 70-80% of patients with primary membranous glomerulopathy (meaning their membranous nephropathy is not associated with or due to other diseases or causes), an antibody called anti‐phospholipase‐A2‐receptor‐antibodies (anti‐PLA2R) is found in the kidney and/or bloodstream 10, 11. The anti-PLA2R antibody (short for anti-phospholipase A2 receptor antibody) attaches to the phospholipase A2 receptor (the antigen). The phospholipase A2 receptor is a protein found in the kidney filter (glomerulus), specifically within a cell called the podocyte which makes up part of this filter (see below). Another antibody called anti‐thrombospondin type‐1 domain‐containing protein 7A‐antibodies (anti‐THSD7A) was also discovered but is found in a much smaller number of patients with primary membranous nephropathy, only about 2-3% 12. This is an antibody to a different antigen, THSD7A, that is also found in the kidney filter (another protein in the podocyte) 11. Identifying presence or absence of PLA2R antibody and the subclass of immunoglobulin G (IgG) deposits may help to differentiate idiopathic from secondary membranous nephropathy. For example, the deposits in idiopathic membranous nephropathy are PLA2R antibody positive and predominantly IgG 4, whereas PLA2R antibody is typically negative and IgG 1 and 2 predominate in cancer-associated membranous nephropathy 10.
Patients with membranous nephropathy typically present with edema and nephrotic-range proteinuria (greater than 3.5 g/day) and occasionally with microscopic hematuria and hypertension. Symptoms and signs of a disorder causing membranous nephropathy (eg, a cancer) may be present initially.
Membranous nephropathy is characterized by proteinuria (greater than 3.5 g/day) found on routine urinalysis performed as part of a physical examination, or may present with peripheral edema and frothy urine 1. Other features of the nephrotic syndrome are often present, including hypercholesterolemia (high serum cholesterol), low serum albumin (hypoalbuminemia) and acute kidney injury with elevated creatinine. The lack of active sediments with the absence of blood in urine (hematuria) and red cell casts in urine microscopy differentiates it from nephritic syndromes. The nephrotic range proteinuria is attributed to podocyte injury and loss of membrane anionic charge barrier, causing albuminuria. This is in contrast to a nephritic syndrome, which involves an inflammatory process in the glomerular basement membrane 13.
Early in the course of disease, light microscopy may be nearly normal or may show thickening of the glomerular capillary loops. Immunofluorescence microscopy shows deposition of IgG (antibody) along capillary loops. Electron microscopy shows immune deposits (antibody) along the outside of the glomerular basement membrane, facing the urinary space and located underneath the podocyte).
The cause of membranous nephropathy can be primary or secondary. A kidney biopsy is used to confirm the diagnosis of membranous nephropathy. Immunosuppressive therapy plays a major role in the treatment of this disease.
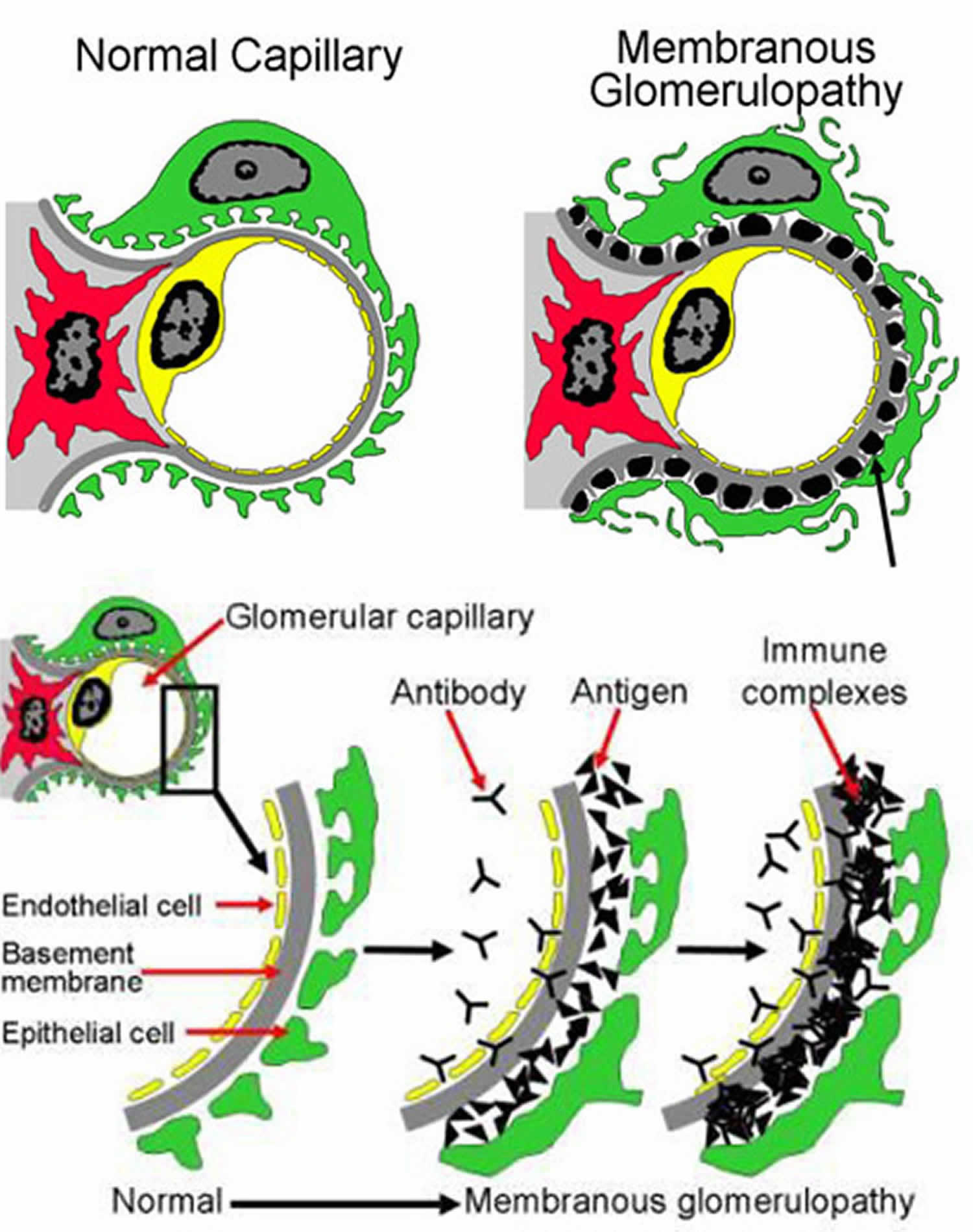
Figure 1. Membranous glomerulopathy
Footnotes: Top image shows part of a glomerulus, comparing a normal to one affected by membranous glomerulopathy (membranous glomerulonephritis). On the right, the black spots or lumps (there is an arrow pointing to one) are collections of immune complexes (antigen-antibody complexes). As more of these immune complexes build up between the layers of the filters, it becomes thickened. The kidney cells (green in this picture, called podocytes) that make up part of the filter become damaged from the immune complexes and the inflammation caused by the immune system, and stop working properly. You can see in the picture on the right that the grey layer (basement membrane) has become thicker and has started filling in the spaces between the black spots/lumps. You can also see that the green cell does not look the same as in the normal healthy filtering loop (capillary loop) on the left. Under a microscope, the filters (glomeruli) in the kidney become thickened, which is where the name membranous nephropathy comes from. The bottom image shows a cross section of part of the kidney filter (glomerulus). This includes different layers, including the cells making up the capillary blood vessel (endothelial cell, in yellow), the basement membrane (gray), and layer of kidney cells (podocyte, in green). Blood inside the capillary blood vessel is filtered across these layers and becomes urine. Antibodies (Y-shaped, black in the picture) in the blood stream attach to antigens (triangles, black in the picture) and form immune complexes that get stuck and build up between the layers of the filter (glomerulus). These immune complexes also activate the immune system causing inflammation. Buildup of these immune complexes as well as the inflammation cause the filter to stop working properly and can lead to kidney damage. Normally the filter (glomerulus) allows water, electrolytes and some waste materials through to become urine, and larger things like blood cells and proteins are too big to pass through the filter – so they stay in the blood. However, in this disease, protein and blood cells can leak into the urine because the filter is not working properly.
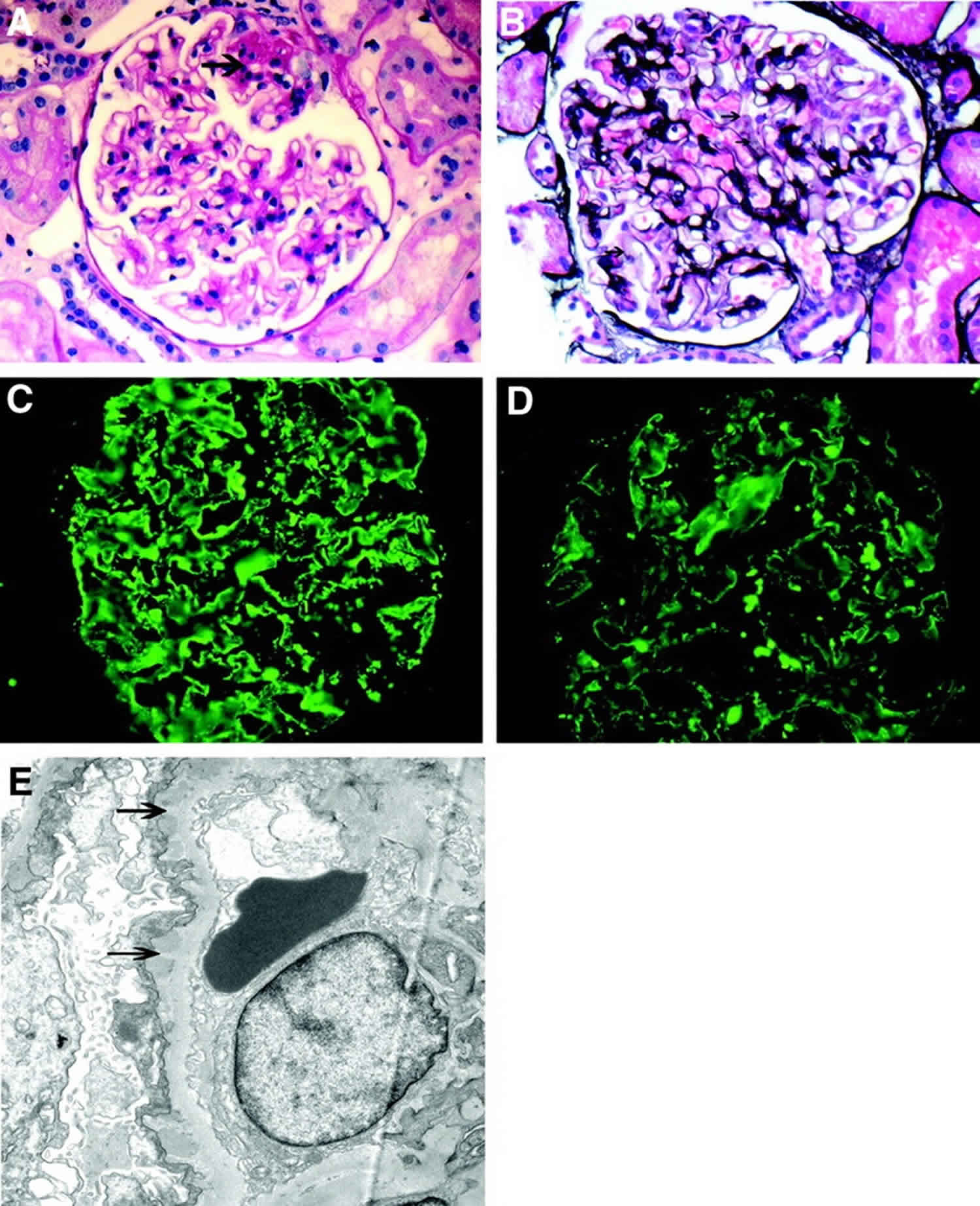
[Source 11 ]Figure 2. Membranous glomerulopathy pathology
Footnotes: (A and B) Light microscopy showing segmental sclerosis (arrow; A, periodic acid Schiff stain) and pinholes (arrow) along the glomerular basement membranes (B, silver stain). (C and D) Immunofluorescence microscopy showing granular IgG (C) and C3 (D) along the capillary walls. (E) Electron microscopy showing subepithelial electron-dense deposits and basement membrane spikes (arrows) separating the deposits. Magnification, ×40 in A, B, C, and D; ×7400 in E.
[Source 2 ]Will I have kidney failure because of membranous glomerulopathy?
You should talk with your doctor about your condition because the progression of the disease depends on many factors. Treatment can slow the process of kidney disease. Everyone is different in how they respond to treatment. Over time, some patients with membranous nephropathy gradually get worse until they reach kidney failure, If this occurs, they will need a kidney transplant or dialysis to stay alive. Some people respond well to treatment and may live with the disease for many years while being monitored for any signs of change.
Membranous glomerulopathy causes
Membranous glomerulopathy may be idiopathic (of unknown cause) also known as Primary membranous glomerulopathy (75% to 80% of cases) or associated with infections (hepatitis B) or stem cell transplant also known as Secondary membranous glomerulopathy (20% to 25% of cases) 1, 14.
Primary membranous glomerulopathy
Idiopathic (unknown cause) membranous glomerulopathy can attributed to the presence of one of the following antibodies and the absence of a secondary cause 7, 15:
- Antibodies against phospholipase A2 receptor (PLA2R) antigen, associated with HLA DQA1 (70% to 80% of primary cases)
- Antibodies against neural epidermal growth factor-like 1 (NELL-1) (15% to 20% of primary cases) 16
- Antibodies against thrombospondin (THSD7A) (1% to 5% of primary cases)
- Others: Antibodies against neutral endopeptidase (NEP) and exostosin (EXT1/EXT2). Membranous nephropathy podocyte antigens exostosin 1/eoxstosin 2 (EXT1/EXT2) -associated membranous lupus nephritis confers a better prognosis than EXT1/EXT2-negative membranous lupus nephritis 17. This may be due to EXT1/EXT2-related glycosylation of heparan sulfate, which protects the glomerular basement membrane (GBM) against immune injury. Contactin 1 (CNTN1) is associated with chronic inflammatory demyelinating polyneuropathy-related membranous nephropathy and protocadherin FAT1 (FAT1) is associated with allogeneic hematopoietic stem cell transplant-related membranous nephropathy 18, 19.
Membranous glomerulopathy is considered an autoimmune disease, which means that it caused by the body’s own immune system. Membranous glomerulopathy is caused by the build-up of immune complexes within the filters (glomeruli) of the kidney itself. The immune system normally creates antibodies to recognize and attach to something (called an antigen). When an antibody attaches to an antigen, this is called an immune complex. Antigens are normally foreign to the body, like a virus or bacteria. However, sometimes, the body can make antibodies that recognize and attach to something in the body itself (not foreign) – these types of antibodies are called autoantibodies. Immune complexes are normally cleared from the blood before causing any problems, but under certain conditions they can accumulate in different parts of the body. In membranous glomerulopathy these immune complexes (antibodies made by the immune system attached to antigens) get caught in the kidney filters (glomeruli). In most cases of membranous glomerulopathy, antibodies are made to an antigen that is part of the kidney filter (glomerulus) itself. Together these antibodies and antigens create immune complexes that get stuck in the kidney filter (glomerulus) and cause disease.
As with other kinds of autoimmune diseases (such as lupus, rheumatoid arthritis, or Crohn’s disease), scientists think that there are probably multiple things that contribute to membranous glomerulopathy developing – meaning multiple things that must occur for the immune system to target and attack/damage the body (rather than just targeting foreign things like infections). Some people may have a gene or genes that make them more likely to have an autoimmune disease (more susceptible to developing them). Though some people may be more likely to get autoimmune diseases if they have family members with autoimmune diseases, membranous glomerulopathy is not a genetic disease and it is not passed down from a parent to their child. In people who may be at higher risk of an autoimmune disease, certain events or triggers may cause the disease to ultimately develop – such as an infection or other inflammation in the body that might activate the immune system. However, these are hypotheses, and scientists do not understand at this point why one person gets membranous nephropathy when others do not.
Recently the antibody that causes most cases of membranous glomerulopathy was discovered and identified 9. In about 70-80% of patients with primary membranous glomerulopathy (meaning their membranous nephropathy is not associated with or due to other diseases or causes), an antibody called anti-PLA2R is found in the kidney and/or bloodstream 10. The anti-PLA2R antibody (short for anti-phospholipase A2 receptor antibody) attaches to the phospholipase A2 receptor (the antigen). The phospholipase A2 receptor is a protein found in the kidney filter (glomerulus), specifically within a cell called the podocyte which makes up part of this filter. Another antibody called anti‐thrombospondin type‐1 domain‐containing protein 7A‐antibodies (anti‐THSD7A) was also discovered but is found in a much smaller number of patients with primary membranous nephropathy, only about 2-3% 12. This is an antibody to a different antigen, THSD7A, that is also found in the kidney filter (another protein in the podocyte).
Secondary membranous glomerulopathy
- Infections (e.g., hepatitis B, hepatitis C, syphilis, malaria, human immunodeficiency virus [HIV], schistosomiasis, leishmaniasis)
- Neoplasms (e.g., adenocarcinoma and squamous cell carcinoma of lungs and gastrointestinal tract, hematological malignancies)
- Medications (e.g., non-steroidal anti-inflammatory drugs [NSAIDs], anti-tumor necrosis factor-alpha inhibitors, gold therapy, penicillamine, probenecid)
- Heavy metal poisoning (gold, mercury)
- Autoimmune diseases (e.g., systemic lupus erythematosus [SLE], Sjogren syndrome, rheumatoid arthritis, IgG4 related nephropathy)
- Thyroiditis
- Miscellaneous (hematopoietic stem cell transplant, graft versus host disease, diabetes mellitus)
Depending on the patient’s age, 4 to 20% have an underlying cancer, including solid cancers of the lung, colon, stomach, breast, or kidney; Hodgkin or non-Hodgkin lymphoma; chronic lymphocytic leukemia; and melanoma.
Because hepatitis and cancer can be associated with membranous glomerulopathy, anyone who is found to have membranous nephropathy should be tested for hepatitis and make sure they are up to date on age-appropriate cancer screening. A blood test can be done to check for hepatitis. Age appropriate cancer screening can include tests such as a pap smear, mammogram, colonoscopy, or CT scan of the lungs (in people who smoke or have smoked). Your doctor can clarify which of these tests are appropriate or necessary for you.
Membranous glomerulopathy is rare in children and, when it occurs, is usually due to hepatitis B virus infection, SLE, or autoimmune thyroid disease.
Membranous glomerulopathy pathology
Recent findings of anti‐phospholipase‐A2‐receptor‐antibodies (anti‐PLA2R) 10 and anti‐thrombospondin type‐1 domain‐containing protein 7A‐antibodies (anti‐THSD7A) 12 have improved understanding of the autoimmune pathophysiology of primary membranous glomerulopathy. Primary membranous glomerulopathy is caused by the subepithelial formation of immune complex deposits in the kidney’s glomerular basement membrane (GBM) 20. The exact mechanisms behind this remain unclear, however, there are a number of presumptive hypotheses. Firstly, systemically pre‐formed immune‐complexes may deposit in the glomerular basement membrane, suggesting a similar pathophysiological mechanism as in lupus‐associated nephritis 20. Secondly, circulating antigens (such as during infection) might be targeted by antibodies, thus forming immune complexes that deposit in this site. this has especially been observed in infection‐related (i.e. secondary) forms of membranous nephropathy, such as during infection with hepatitis B virus 20, 21, 22. Thirdly, a rat model of Heymann nephritis 23, podocyte‐antigens (such as megalin) may lead to binding of autoantibodies to the glomerular basement membrane’s podocytes which cause the subepithelial deposits that are present in primary membranous nephropathy 24. However, thus far, this connection has not been clearly established through the extraction of anti‐megalin‐antibodies in primary membranous nephropathy. Finally, the complement system and genetic factors might contribute to the autoimmune etiology of primary membranous nephropathy. So far, two associated genomic loci have been identified: chromosome 2q24 encodes for the anti‐PLA2R‐receptor auto‐antibody and chromosome 6p21 encodes for HLADQA1, which might play pivotal roles in the pathogenesis of primary membranous nephropathy 25, 26.
Membranous glomerulopathy is caused by the deposition of antigen-antibody complexes between the glomerular basement membrane (GBM) and podocytes. These complexes mainly consist of immunoglobulin IgG4, complement C3, and C5b-C9 membrane attack complexes (MAC). In the case of secondary membranous glomerulopathy (like systemic lupus erythematosus) complexes may also include IgG1/IgG3, IgA, IgM, or C1q, and rarely involve the mesangium (“full house pattern”) 6. The immune complexes activate the complement system and generate membrane attack complex (MAC), which releases proteases, cytokines, and oxidants, causing cellular and tissue damage. This leads to disruption of podocyte structure, hampering of slit diaphragm integrity, and loss of membrane anionic charge barrier, resulting in proteinuria. This nephrotic range proteinuria leads to hyperlipidemia, prothrombotic state, vitamin D deficiency, and hypertension. Studies have also suggested that an unknown cytokine in membranous nephropathy leads to decreased nephrin synthesis, a protein responsible for glomerular filtration barrier integrity 27.
Membranous glomerulopathy signs and symptoms
Membranous glomerulopathy happens slowly over time, so you may not notice anything is wrong. You may only see some signs on your own, while others may be found by your healthcare provider.
Signs and symptoms of membranous glomerulopathy include:
- Swelling in body parts like your legs, ankles and around your eyes (called edema)
- Weight gain
- Fatigue
- Foaming of the urine caused by high protein levels in the urine (called proteinuria)
- High fat levels in the blood (high cholesterol)
- Low levels of protein in the blood
The most common symptom of membranous glomerulopathy is swelling (edema). This can range from mild to severe. Most people with membranous glomerulopathy have some swelling and it is often the first symptom people notice. In membranous glomerulopathy (as opposed to some other diseases that cause protein in the urine and nephrotic syndrome), swelling usually comes on slowly (over weeks to months) but it can sometimes come on more quickly. It typically starts in the feet, ankles, or legs, but can occur anywhere in the body, including the abdomen, hands or arms, and face.
The swelling in membranous glomerulopathy occurs because of fluid building up in the body, and specifically in different tissues. When fluid builds up, it can sometimes go into the lungs (pulmonary edema) and cause difficulty breathing or shortness of breath. Pulmonary edema (excess fluid in the lungs) is less common than swelling (edema) but in people who have this it may be most noticeable when walking or exerting yourself or when lying down flat.
Some people with membranous glomerulopathy- in particular, people who have nephrotic syndrome (with large amounts of protein in the urine and low blood protein levels)- feel very tired or run-down. Doctors do not know exactly how or why this happens but some people with membranous nephropathy (and other diseases that cause nephrotic syndrome) notice this.
When protein gets through the filter in your kidney and into your urine, the urine can become foamy or bubbly. Some people may notice this change in their urine before they have other symptoms.
Renal vein thrombosis is more frequent in membranous glomerulopathy and is usually asymptomatic but may manifest with flank pain, hematuria, and hypertension. It may progress to pulmonary embolism.
There are other symptoms that can occur with membranous glomerulopathy, but the ones above are some of the most common and the ones that people with membranous nephropathy often notice first.
Nephrotic syndrome
Membranous glomerulopathy often causes nephrotic syndrome. Nephrotic syndrome is a group of symptoms or changes that often occur together in someone that is losing a lot of protein into the urine. Nephrotic syndrome can also happen in other diseases that cause a lot of protein to be lost into the urine. Although a lot of people with membranous glomerulopathy have nephrotic syndrome, though not everybody does. Nephrotic syndrome includes these findings:
- At least 3.5 grams of protein in the urine per day (proteinuria). This can be measured on a 24 hour urine collection but can also be estimated on a single urine sample. To estimate the amount of proteinuria from a single urine sample, the urine protein to creatinine ratiois used – this gives an estimate of how many grams of protein would be in a 24 hour urine sample.
- Low blood protein (albumin) levels
- Swelling (sometimes called edema)
- High cholesterol
- Increased risk for blood clots
Membranous glomerulopathy complications
- Complications from a pulmonary embolism, deep vein thrombosis, renal vein thrombosis, and other systemic thromboembolic phenomena as well as increased risk of bleeding from systemic anticoagulation
- Membranous glomerulopathy leading to hyperlipidemia, hypertension, and chronic kidney disease, compromising cardiovascular health
- Progression of membranous glomerulopathy to chronic kidney disease (CKD) with reduced eGFR. CKD-related complications like anemia, bone-mineral disorders, and vitamin D deficiency
- Complications and side effects of immunotherapy:
- Increased risk of infections (fungal, viral, and bacterial)
- Increased risk of malignancies over time, like bladder cancer, specifically with cyclophosphamide
- Renal tubular acidosis, stones from chemotherapy (proximal renal tubular acidosis)
- Cytopenias due to bone marrow suppression
- Infertility risk with cyclophosphamide
- Mycophenolate mofetil: gastrointestinal side effects, cytopenias, teratogenicity, and hence the need for dual contraception during therapy
- Reactivation of infections like tuberculosis and hepatitis B with rituximab
- Infusion hypersensitivity reactions
- Calcineurin inhibitors associated nephrotoxicity and neurotoxicity, renal parenchymal fibrosis, hair loss, and pancreatic toxicity
- Steroids increase the risk of infections, bone diseases like avascular necrosis of large joints, metabolic syndrome, hypertension from salt and fluid retention, psychosis, and gastrointestinal irritation, to name a few
- End-stage kidney disease (ESKD) requiring renal replacement therapies and concomitant complications inherent to the procedure
- Risk of catheter-associated bacteremia, hypotension, neurological side effects, and accelerated cardiovascular morbidity and mortality
- Post-transplant recurrent membranous glomerulopathy 28:
- Prevalent in 30% to 50% of patients with positive anti-PLA2R antibodies on initial presentation
- Pathophysiology of recurrent (de novo) membranous glomerulopathy is different from primary membranous glomerulopathy
Membranous glomerulopathy diagnosis
Membranous glomerulopathy is an uncommon disease and diagnosis can sometime be delayed. Since swelling, the most common symptom, can be caused by a lot of different diseases or problems (including kidney, heart, or liver problems), the kidneys may not be identified right away as the cause. Most often it is diagnosed when evaluating someone for protein in the urine (normally there should not be protein in the urine). Some people go to their doctor because of symptoms (such as swelling) and urine tests reveal protein in the urine. Other times, a urine test may be done for another reason (on a routine physical for example) and protein in the urine is discovered. Protein levels can be measured (or quantified) on a 24 hour urine collection or estimated from a single urine sample. Other evaluation usually includes measuring kidney function (from a blood test called creatinine) and doing other bloodwork.
These tests are done to find out if you have membranous glomerulopathy:
- Urine test: A urine analysis will help find protein (proteinuria) and blood in your urine (hematuria). Urine microscopy for cellular casts, fatty casts, and oval fat bodies.
- Spot urine protein or urine albumin to creatinine ratio (UPCR). A 24-hour urine collection is rarely indicated and/or is based on clinician preference. It is done serially and aids in monitoring the clinical response.
- Blood test: A blood test will help find levels of protein, cholesterol, and wastes in your blood.
- Serum metabolic panel to assess blood urea nitrogen, creatinine, uric acid, electrolytes, vitamin D levels, and estimated glomerular filtration rate (eGFR) 29.
- Serum albumin and total protein to evaluate hypoalbuminemia and hypoproteinemia.
- Lipid profile to assess for dyslipidemia.
- Serum IgG with subtypes, for decreased serum levels 30.
- For primary membranous glomerulopathy, serum anti-PLA2R antibody and anti-thrombospondin by Western blot and indirect immunofluorescence.
- Detailed studies for respective secondary causes based on history and presentation (like heavy metal levels, infectious etiology like HIV, parasitic infections, autoimmune panel).
- Glomerular filtration rate (GFR): A blood test will be done to know how well your kidneys are filtering the wastes from your body.
- Kidney biopsy: In this test, a tiny piece of your kidney is removed with a special needle, and looked at under a microscope. The kidney biopsy may show if you have a certain type of a protein that helps your body fight infection, called an antibody. Your body usually makes this antibody when you have membranous glomerulopathy. The pathological specimen should be sent for light microscopy, silver stain, immunofluorescence, electron microscopy, and immunohistochemistry for anti- PLA2R antibody.
Imaging tests that may be done include:
- Abdominal CT scan
- Kidney ultrasound. Ultrasonography study of the kidneys to assess for radiological kidney disease, evidence of obstruction, and renal vein thromboembolism.
- Renal vein doppler and computed tomography (CT)/magnetic resonance angiography (MRA) to rule out renal vein thrombosis.
- CT angiogram of the chest to rule out pulmonary embolism.
- Chest x-ray
- Intravenous pyelogram (IVP)
- Lower extremity doppler to assess for deep vein thrombosis.
- The benefit from a contrast study outweighs the risk of contrast-induced nephropathy and should be based on the acuity of presentation.
Different kidney diseases – not just membranous glomerulopathy – can cause protein in the urine, and a kidney biopsy is ultimately needed to diagnose the specific disease causing the protein in the urine. A kidney biopsy is a procedure that involves using a needle to get a sample of kidney tissue to look at under the microscope. This allows the individual glomeruli (kidney filters) to be seen under high magnification. Additional tests can be done on the kidney tissue from the biopsy to help make a diagnosis. Blood tests and measuring protein in the urine can be helpful to understand the severity of the disease and to rule out or look for certain causes, so these tests are often done as part of the workup, but a biopsy is needed to diagnose membranous nephropathy.
Since most people with membranous glomerulopathy have anti-PLA2R antibodies, bloodwork can be done to check for this antibody. If it is positive, it is very likely that someone has membranous nephropathy. However, a negative test does not mean that someone does not have membranous glomerulopathy, and a kidney biopsy is important to confirm the diagnosis as well as to provide more information to guide management.
Membranous glomerulopathy histopathology
A kidney biopsy is the gold standard in confirming the diagnosis of membranous glomerulopathy or membranous nephropathy. It is used either exclusively or in combination with antibody assay, depending on the ease of availability. Antibody assay is replacing biopsy mostly due to the temporal relationship between proteinuria and circulating anti-PLA2R antibodies. It is a non-invasive method to monitor disease progression and outcomes. Since clinical disease lags behind immunological outcomes, antibody assay is an excellent biomarker for clinical management. Nevertheless, the following kidney biopsy findings specific for membranous glomerulopathy should be noted 6:
- Light microscopy: Diffuse capillary and glomerular basement thickening. These might be absent in earlier stages of membranous glomerulopathy.
- Immunofluorescence: Granular pseudo-linear IgG4 deposits are present all along the glomerular capillary wall.
- Silver stain: Spikes (represent staining of basement membrane between the deposits)
- Electron microscopy: Effaced podocytes. Ehrenreich and Churg classification based on the appearance of electron-dense deposits 2:
- Stage 1: Small, sparse, electron-dense deposits on the epithelial side of glomerular basement membrane (GBM)
- Stage 2: Larger deposits causing glomerular basement membrane (GBM) thickening, along with foot process effacement giving the characteristic “spike and dome” appearance
- Stage 3: Stage 2 plus intramembranous coarse granular deposits with “neomembrane” formation
- Stage 4: Irregular thickening, dissolution of deposits (holes), and sclerosis of glomerular basement membrane (GBM)
- Variants of membranous glomerulopathy with crescents have also been found 31.
- Chronic membranous glomerulopathy may show tubular atrophy, interstitial fibrosis, and nephrosclerosis on biopsy 32.
- Excessive protein reabsorption can lead to the deposition of hyaline droplets in the proximal convoluted tubules as seen in light microscopy 33.
The diagnosis of membranous glomerulopathy is based on the following findings 2:
- Thickened glomerular basement membrane (GBM), often showing pinholes and spikes on silver and periodic acid-Schiff stains, and occasionally subepithelial fuchsinophilic deposits on trichome stains;
- Immunofluorescence microscopy showing granular Ig, usually IgG and C3, along the capillary walls; and
- Subepithelial deposits on electron microscopy.
Membranous glomerulopathy treatment
Treatment of membranous glomerulopathy is usually managed by a nephrologist (kidney specialist). After your nephrologist (kidney specialist) finds that you have membranous nephropathy, your doctor will follow up with you very closely without treatment. This waiting period allows time to see if you go into remission (the disease stops being active and causes no symptoms) without having to use strong drugs. During this waiting time you will be given supportive care (treatments that have little or no risk).
Supportive care includes:
- Angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin 2 receptor blockers (ARBs): These drugs help reduce high blood pressure and the amount of protein in the urine (proteinuria). These medications are generally the first step in treating membranous glomerulopathy. Though they were designed as blood pressure medicines, in membranous nephropathy they are used to lower the amount of protein leaking into the urine, and may have the added benefit of helping to control blood pressure if it is elevated. Sometimes these medications cannot be used if someone’s blood pressure is too low (since they can lower blood pressure) or if someone has high potassium levels in the blood (since they can raise potassium levels). However in most people, if there is no contraindication, one of these medications should be used.
- ACE-inhibitors (angiotensin converting enzyme inhibitors) include lisinopril (Zestril, Prinivil), enalapril (Vasotec), ramipril (Altace), benazepril (Lotensin), and quinapril (Accupril).
- Angiotensin 2 receptor blockers (ARBs) include losartan (Cozaar), valsartan (Diovan), irbesartan (Avapro), telmisartan (Micardis), olmesartan (Benicar), and candesartan (Atacand).
- Diuretics or water pills that cause you to urinate more. Diuretics can be used to treat swelling if it occurs. Swelling can be a very bothersome or problematic symptom of membranous glomerulopathy and so these medications can be an important part of managing the disease. However, they do not treat the membranous nephropathy itself. Diuretics include furosemide (Lasix), torsemide, bumetanide (Bumex), and sometimes others.
- Low-salt diet: Lowering salt may help to reduce edema (swelling in your body parts like your legs, and around your eyes)
If your symptoms do not go away after supportive care, you may be given treatments that affect your immune system (immunosuppressive medication). The goal of your treatment is to manage your symptoms. If you have nephrotic syndrome, your healthcare provider may give you pills to reduce the water in your body (diuretics).
Immunosuppressive medication– not all people with membranous glomerulopathy will need medication to suppress their immune system, but it is an important part of the treatment for many people who have membranous nephropathy. Reasons that someone might need immunosuppresive medication include: worsening kidney function, high levels of protein in the urine (especially if they do not get better after a period of observation), or complications from the nephrotic syndrome (such as a blood clot). Because membranous nephropathy is an autoimmune disease, in which the body’s immune system targets and injury the body’s own tissue (in this case, part of the glomerulus), medications to suppress or decrease the immune system are needed to treat the disease in many people. There are different immune suppressing medications that can be used to treat membranous nephropathy. Since these medications all suppress the immune system, all of them increase the risk for infection. However, they have different individual side effects besides this, and the different possible side effects can be a factor in deciding which one to use. It is very important to discuss the side effects of your treatments with your healthcare provider. If you are a woman, and are considering having children, be sure to speak with your doctor about how your treatments may affect this process.
Various immunosuppressive medications have been shown to induce remission in patients with membranous glomerulopathy, including steroids, cyclophosphamide, and cyclosporine. Edema is managed by dietary salt restriction and diuretics, either administered orally or intravenously.
- Cyclophosphamide (Cytoxan)– this is a medication that can be given as a monthly infusion or as a daily pill (by mouth). One of the most common regimens for treating membranous glomerulopathy includes alternating months of cyclophosphamide and corticosteroids for a total of 6 months (Ponticelli protocol).
- Rituximab (Rituxan), a chimeric anti-CD20 monoclonal antibody– this is a medication that is given as an infusion, either 4 weekly doses or 2 doses spaced 2 weeks apart. Its effects last about 6 months in the body, and sometimes a maintenance (repeat) dose can be given after 6 months 34, 35.
- Ofatumumab and obinutuzumab are humanized anti-CD20 monoclonal antibodies, which have successfully treated patients with primary membranous nephropathy who developed serum sickness to rituximab or anti-rituximab antibodies 36, 37, and demonstrated superior B-cell depletion compared to rituximab respectively 38. The efficacy of binutuzumab and belimumab [humanized monoclonal antibody against B-cell activating factor (BAFF)] in lupus nephritis, another disease mediated in part by autoreactive B cells, and CD19 CAR T cells in treatment-refractory SLE are also promising alternative B cell-depleting therapies, which require further evaluation in primary membranous nephropathy 39, 40, 41, 42.
- Calcineurin inhibitors (cyclosporine or tacrolimus)– these medications are taken as a pill by mouth, usually twice a day.
- Corticosteroids (prednisone)– this is a medication that is often used with one of other immunosuppressive medications above (most commonly with cyclophosphamide or with one of the calcineurin inhibitors). Corticosteroids are not effective by themselves for treating membranous glomerulopathy but can be used as part of other regimens.
Because cholesterol levels can be elevated in people with nephrotic syndrome, treatment may include a medication for this. The most common type of cholesterol medication is called a “statin” and these include atorvastatin (Lipitor), lovastatin, pravastatin (Pravachol), and rosuvastatin (Crestor).
Blood pressure management – blood pressure is often elevated in people with kidney disease, including membranous glomerulopathy. It is important to keep blood pressure well-controlled to prevent damage to the kidneys. In addition to an ACE-inhibitor (angiotensin converting enzyme inhibitor) or angiotensin 2 receptor blocker (ARB) that can help with protein in the urine, there are many other medications that can be used to treat high blood pressure. Limiting salt intake can help with blood pressure control as well as with decreasing swelling.
Blood thinners – because people with nephrotic syndrome (protein in the urine, low blood protein levels, and swelling) are at higher risk for blood clots, treatment may also include a blood thinner to prevent blood clots. The decision of whether to start a blood thinner is based on balancing the risk of having a clot with the risk of having bleeding from being on a blood thinner. There is a website that you can use with your doctor to help decide if a blood thinner would be beneficial for you. In most cases, the blood thinner should be stopped when the nephrotic syndrome gets better and blood protein levels go up.
Therapies of unproven long-term value include IV immuneglobulin and nonsteroidal anti-inflammatory drugs (NSAIDs).
Kidney transplantation is an option for patients with end-stage kidney disease. Membranous glomerulopathy recurs in about 10% of patients, with loss of graft in up to 50%.
Finally, if the membranous glomerulopathy is considered secondary to something else, then it is most important to treat the underlying disease (infection, cancer, etc.) or to stop the causative drug. This is often enough and treatment with immune suppressing medications can be avoided.
General management
Symptomatic management of membranous glomerulopathy is done with diuretics, statins, angiotensin-converting enzyme inhibitors (ACE inhibitors) OR angiotensin receptor blockers (ARBs), systemic anticoagulant therapy (newer direct oral anticoagulant agents or vitamin K antagonist therapy), antihypertensives, and dietary salt restriction. 1/3rd of the patients respond to these conservative measures and another 1/3rd will need one of the following immunosuppressive therapies 43, 44, 45.
Immunosuppressive therapy
Immunosuppressive therapy should be considered only for patients with symptomatic idiopathic membranous glomerulopathy and for those most at risk of progressive disease. While there is no strong evidence that immunosuppressive therapy has a long-term benefit for patient or kidney survival, it appears to improve rates of remission and possibly progression to end-stage kidney disease (ESKD) 9, 46, 47. Older and chronically ill patients are at greater risk of infectious complications due to immunosuppressants.
No consensus protocol exists, but historically a common regimen included corticosteroids, followed by chlorambucil. However, chlorambucil is no longer preferred because of other therapeutic options with better safety profiles. Most experts favor use of a combination of rituximab and corticosteroids 46. Alternatives to rituximab include a calcineurin inhibitor and cyclophosphamide. The choice of agent is guided by disease severity and anti-PLA2R antibody levels.
Ponticelli regimen (6 months):
- Months 1, 3, and 5: Methylprednisolone (1 g) daily for 3 days followed by prednisolone (0.4 mg/kg/day) or prednisone (0.5mg/kg/day) for 27 days.
- Months 2, 4, and 6: Cyclophosphamide orally 2 mg/kg/day for 30 days.
Modified Ponticelli regimen (6 months):
- Months 1, 3, and 5: Methylprednisolone (1 g) daily for 3 days followed by oral prednisone (0.5mg/kg/day) for 27 days.
- Months 2, 4, and 6: Cyclophosphamide orally 2 to 2.5 mg/kg/day for 30 days.
To be given with appropriate pneumocystis pneumonia (PCP) and antiviral prophylaxis (trimethoprim-sulfamethoxazole and valganciclovir).
Rituximab a monoclonal antibody to CD20 antigen of B cell 48:
- Usually a second-line therapy after failure of steroids.
- As first-line therapy or in refractory cases, or after 6 months of conservative management.
- Dose: 375 mg/m² intravenously once weekly × 4. The maintenance dose is similar but is given every six months.
Alternate therapy with calcineurin inhibitors are less popular due to the high relapse rate 49. Either of the following can be used:
- Tacrolimus ( 0.025 to 0.040 mg/kg divided into twice a day ) for six months.
- Cyclosporine (1.5 to 2.5 mg/kg divided into twice a day) +/- 0.15 mg/kg prednisone for six months.
Other drug options are:
- Chlorambucil
- Mycophenolate mofetil
- Adrenocorticotropic hormone (ACTH) analogs
The various immunosuppressive treatments come with their inherent side effects like increased risk of infections, cancers, reduction in the number of red blood cells, white blood cells and platelets (cytopenias), side effects of long-term steroids like cataract, metabolic syndrome, avascular necrosis of joints, etc.
Management of progressive disease
In advanced oliguric (urinary output less than 400 ml per day or less than 20 ml per hour) or anuric (no urine or your kidneys aren’t producing urine) kidney injury, renal replacement therapy may be required.
Patients may need vitamin D and calcium supplementation, especially if treated with steroids.
Kidney transplant for patients with advanced chronic kidney disease (CKD Stage 5) or end-stage kidney disease (ESKD).
Membranous glomerulopathy treatment in children
It is very rare for a child to have membranous glomerulopathy, so it is important to check for anything that might be causing the disease, especially lupus. If your child needs treatment for membranous nephropathy, the treatment is usually the same as it is for adults. It is important to know that some people with membranous glomerulopathy go into spontaneous remission, which means it suddenly goes away without any treatments.
Will the membranous glomerulopathy come back in my kidney transplant?
There is a 40% chance that membranous glomerulopathy will return in a transplanted kidney with loss of graft in up to 50% of cases. Unfortunately, there are no factors that have been identified to give doctors an idea of patients at risk for this problem. Generally, recurrence of the disease will occur in the first 2 years after the kidney transplant.
Is there any treatment for membranous glomerulopathy that comes back in a kidney transplant?
There have not been any trials to evaluate different therapies for membranous glomerulopathy that comes back in a transplanted kidney. However, Rituximab is one option that has been used successfully for treatment.
Membranous glomerulopathy prognosis
Definition of membranous glomerulopathy prognosis or outcomes 50:
- Complete remission: Proteinuria <0.3 g/d or 300 mg/g on spot urine albumin to creatinine ratio (UPCR).
- Partial remission: Greater than 50% reduction of proteinuria from baseline or between 0.3 and 3.5 g/day, with relatively stable estimated glomerular filtration rate (eGFR).
- Relapse: Recurrence of greater than 3.5 g/day of proteinuria after remission.
- End-stage kidney disease (ESKD): GFR less than 15 ml/min or requirement for kidney dialysis or kidney transplant.
Rule of One-thirds 2
- Spontaneous remission in one-third of the patients with conservative management only.
- One-third have symptomatic proteinuria without progressing into renal failure and might benefit from immunosuppressive therapies.
- The remaining one-third are refractory to treatment and require dialysis due to end-stage kidney disease (ESKD). These should be evaluated for renal transplantation.
Risk Factors for Poor Prognosis 32:
- Male gender, White race
- Old age
- Hypertension on presentation 51
- Massive proteinuria (greater than 8 g/day) for 6 months
- Elevated creatinine or acute kidney injury at the time of presentation
- Extensive tubulointerstitial fibrosis on biopsy
Glomerular filtration rate (GFR) is typically normal at the outset of membranous glomerulopathy, but in some patients glomerular filtration rate will decline after months or years. Risk factors for progressive loss of kidney function include heavy proteinuria and male sex.
Up to a third of patients diagnosed with primary membranous glomerulopathy will go into remission spontaneously within 5 years, even without immunosuppressive therapy 52. With treatment, most patients’ disease will go into remission. Complete remission means stable (or improved) kidney function and protein levels in the urine decreased to normal. Partial remission means stable or improved kidney function plus protein levels in the urine decreased to less than half of original levels and not in the nephrotic syndrome range (less than 3.5 grams/day). Despite this, 15% to 50% of patients who do not receive immunosuppressive treatment progress to end‐stage kidney disease (ESKD) within 10 years 53, 54, 55.
However, relapse is common in membranous glomerulopathy, and patients that go into remission either spontaneously or in response to immunosuppressive can have the disease return later. Long term (over decades) about 1/3 of patients will progress to end-stage kidney disease (kidney failure needing dialysis or kidney transplantation), about 1/3 will have continued protein in the urine without kidney failure (persistent nephrotic syndrome), and about 1/3 will be in remission 8.
Women, children, and young adults with non–nephrotic-range proteinuria and patients with persistently normal renal function 3 years after diagnosis tend to have little disease progression. More than 50% of patients with nephrotic-range proteinuria who are asymptomatic or who have edema that can be controlled with diuretics will have a partial or complete remission within 3 to 4 years.
Risk of progression to renal failure is highest among patients with 56:
- Persistent proteinuria ≥ 8 g/day, particularly men age > 50 years
- An elevated serum creatinine level at presentation or diagnosis
- Biopsy evidence of substantial interstitial inflammation
Things that predict a better long-term outcome in membranous glomerulopathy include lower levels of protein in the urine, being female, being younger (less than 60), and achieving remission.
- Alok A, Yadav A. Membranous Nephropathy. [Updated 2023 Jun 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559169[↩][↩][↩][↩]
- Fervenza FC, Sethi S, Specks U. Idiopathic membranous nephropathy: diagnosis and treatment. Clin J Am Soc Nephrol. 2008 May;3(3):905-19. doi: 10.2215/CJN.04321007[↩][↩][↩][↩][↩]
- Hoxha, E., Reinhard, L. & Stahl, R.A.K. Membranous nephropathy: new pathogenic mechanisms and their clinical implications. Nat Rev Nephrol 18, 466–478 (2022). https://doi.org/10.1038/s41581-022-00564-1[↩]
- Keri KC, Blumenthal S, Kulkarni V, Beck L, Chongkrairatanakul T. Primary membranous nephropathy: comprehensive review and historical perspective. Postgrad Med J. 2019 Jan;95(1119):23-31. doi: 10.1136/postgradmedj-2018-135729[↩]
- Cattran DC. Idiopathic membranous glomerulonephritis. Kidney Int. 2001 May;59(5):1983-94. doi: 10.1046/j.1523-1755.2001.0590051983.x[↩]
- Menon S, Valentini RP. Membranous nephropathy in children: clinical presentation and therapeutic approach. Pediatr Nephrol. 2010 Aug;25(8):1419-28. doi: 10.1007/s00467-009-1324-5[↩][↩][↩]
- Ronco P, Debiec H. Molecular Pathogenesis of Membranous Nephropathy. Annu Rev Pathol. 2020 Jan 24;15:287-313. doi: 10.1146/annurev-pathol-020117-043811[↩][↩]
- Couser WG. Primary Membranous Nephropathy. Clin J Am Soc Nephrol. 2017 Jun 7;12(6):983-997. doi: 10.2215/CJN.11761116[↩][↩]
- von Groote TC, Williams G, Au EH, Chen Y, Mathew AT, Hodson EM, Tunnicliffe DJ. Immunosuppressive treatment for primary membranous nephropathy in adults with nephrotic syndrome. Cochrane Database Syst Rev. 2021 Nov 15;11(11):CD004293. doi: 10.1002/14651858.CD004293.pub4[↩][↩][↩]
- Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009 Jul 2;361(1):11-21. doi: 10.1056/NEJMoa0810457[↩][↩][↩][↩]
- Membranous Nephropathy. https://unckidneycenter.org/kidneyhealthlibrary/glomerular-disease/membranous-nephropathy[↩][↩][↩]
- Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RAK, Lambeau G. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014 Dec 11;371(24):2277-2287. doi: 10.1056/NEJMoa1409354[↩][↩][↩]
- Ramachandran R, Kaundal U, Girimaji N, Rakha A, Rathi M, Gupta KL, Kohli HS, Jha V. Regulatory B Cells Are Reduced and Correlate With Disease Activity in Primary Membranous Nephropathy. Kidney Int Rep. 2020 Mar 30;5(6):872-878. doi: 10.1016/j.ekir.2020.03.023[↩]
- Abe S, Amagasaki Y, Konishi K, Kato E, Iyori S, Sakaguchi H. Idiopathic membranous glomerulonephritis: aspects of geographical differences. J Clin Pathol. 1986 Nov;39(11):1193-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1140761/pdf/jclinpath00319-0029.pdf[↩]
- Couser WG. Primary Membranous Nephropathy. Clin J Am Soc Nephrol. 2017 Jun 7;12(6):983-997. doi: 10.2215/CJN.11761116. Epub 2017 May 26. Erratum in: Clin J Am Soc Nephrol. 2017 Sep 7;12 (9):1528.[↩]
- Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, Ravindran A, Buob D, Jadoul M, Fervenza FC, Ronco P. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020 Jan;97(1):163-174. doi: 10.1016/j.kint.2019.09.014[↩]
- Ravindran A, Casal Moura M, Fervenza FC, Nasr SH, Alexander MP, Fidler ME, et al.. In patients with membranous lupus nephritis, exostosin-positivity and exostosin-negativity represent two different phenotypes. J Am Soc Nephrol (2021) 32(3):695–706. doi: 10.1681/ASN.2020081181[↩]
- Le Quintrec M, Teisseyre M, Bec N, Delmont E, Szwarc I, Perrochia H, et al.. Contactin-1 is a novel target antigen in membranous nephropathy associated with chronic inflammatory demyelinating polyneuropathy. Kidney Int (2021) 100(6):1240–9. doi: 10.1016/j.kint.2021.08.014[↩]
- Sethi S, Madden B, Casal Moura M, Nasr SH, Klomjit N, Gross L, et al.. Hematopoietic stem cell transplant-membranous nephropathy is associated with protocadherin FAT1. J Am Soc Nephrol (2022) 33(5):1033–44. doi: 10.1681/ASN.2021111488[↩]
- Lai WL, Yeh TH, Chen PM, Chan CK, Chiang WC, Chen YM, Wu KD, Tsai TJ. Membranous nephropathy: a review on the pathogenesis, diagnosis, and treatment. J Formos Med Assoc. 2015 Feb;114(2):102-11. doi: 10.1016/j.jfma.2014.11.002[↩][↩][↩]
- Bhimma R, Coovadia HM. Hepatitis B virus-associated nephropathy. Am J Nephrol. 2004 Mar-Apr;24(2):198-211. doi: 10.1159/000077065[↩]
- Lai FM, To KF, Wang AY, Choi PC, Szeto CC, Li PK, Leung CB, Lai KN. Hepatitis B virus-related nephropathy and lupus nephritis: morphologic similarities of two clinical entities. Mod Pathol. 2000 Feb;13(2):166-72. doi: 10.1038/modpathol.3880031[↩]
- HEYMANN W, HACKEL DB, HARWOOD S, WILSON SG, HUNTER JL. Production of nephrotic syndrome in rats by Freund’s adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959 Apr;100(4):660-4. doi: 10.3181/00379727-100-24736[↩]
- Tramontano A, Knight T, Vizzuso D, Makker SP. Nested N-terminal megalin fragments induce high-titer autoantibody and attenuated Heymann nephritis. J Am Soc Nephrol. 2006 Jul;17(7):1979-85. doi: 10.1681/ASN.2005101144[↩]
- Bullich G, Ballarín J, Oliver A, Ayasreh N, Silva I, Santín S, Díaz-Encarnación MM, Torra R, Ars E. HLA-DQA1 and PLA2R1 polymorphisms and risk of idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2014 Feb;9(2):335-43. doi: 10.2215/CJN.05310513[↩]
- Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, Stephens HA, Laundy V, Padmanabhan S, Zawadzka A, Hofstra JM, Coenen MJ, den Heijer M, Kiemeney LA, Bacq-Daian D, Stengel B, Powis SH, Brenchley P, Feehally J, Rees AJ, Debiec H, Wetzels JF, Ronco P, Mathieson PW, Kleta R. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med. 2011 Feb 17;364(7):616-26. doi: 10.1056/NEJMoa1009742[↩]
- Akankwasa G, Jianhua L, Guixue C, Changjuan A, Xiaosong Q. Urine markers of podocyte dysfunction: a review of podocalyxin and nephrin in selected glomerular diseases. Biomark Med. 2018 Aug;12(8):927-935. doi: 10.2217/bmm-2018-0152[↩]
- Lim WH, Shingde M, Wong G. Recurrent and de novo Glomerulonephritis After Kidney Transplantation. Front Immunol. 2019 Aug 14;10:1944. doi: 10.3389/fimmu.2019.01944[↩]
- Zhang J, Pan M, Zhang J, You X, Li D, Lin F, Lu G. Serum uric acid is an independent predictor of renal outcomes in patients with idiopathic membranous nephropathy. Int Urol Nephrol. 2019 Oct;51(10):1797-1804. doi: 10.1007/s11255-019-02254-7[↩]
- Hou J, Cheng Y, Hou Y, Wu H. Lower Serum and Higher Urine Immunoglobulin G Are Associated with an Increased Severity of Idiopathic Membranous Nephropathy. Ann Clin Lab Sci. 2019 Nov;49(6):777-784[↩]
- Alawieh R, Brodsky SV, Satoskar AA, Nadasdy T, Parikh SV, Rovin B, Cassol CA. Membranous Nephropathy With Crescents. Kidney Int Rep. 2020 Jan 30;5(4):537-541. doi: 10.1016/j.ekir.2020.01.010[↩]
- Zhang XD, Cui Z, Zhang MF, Wang J, Zhang YM, Qu Z, Wang X, Huang J, Wang F, Meng LQ, Cheng XY, Wang SX, Liu G, Zhao MH. Clinical implications of pathological features of primary membranous nephropathy. BMC Nephrol. 2018 Aug 28;19(1):215. doi: 10.1186/s12882-018-1011-5[↩][↩]
- Sato S, Kitamura H, Ghazizadeh M, Adachi A, Sasaki Y, Ishizaki M, Inoue K, Wakamatsu K, Sugisaki Y. Occurrence of hyaline droplets in renal biopsy specimens: an ultrastructural study. Med Mol Morphol. 2005 Mar;38(1):63-71. doi: 10.1007/s00795-004-0272-1[↩]
- Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, et al.. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med (2019) 381(1):36–46. doi: 10.1056/NEJMoa1814427[↩]
- Scolari F, Delbarba E, Santoro D, Gesualdo L, Pani A, Dallera N, et al.. Rituximab or cyclophosphamide in the treatment of membranous nephropathy: The RI-CYCLO randomized trial. J Am Soc Nephrol (2021) 32(4):972–82. doi: 10.1681/ASN.2020071091[↩]
- Podestà MA, Ruggiero B, Remuzzi G, Ruggenenti P. Ofatumumab for multirelapsing membranous nephropathy complicated by rituximab-induced serum-sickness. BMJ Case Rep (2020) 13(1):e232896. doi: 10.1136/bcr-2019-232896[↩]
- Boyer-Suavet S, Andreani M, Lateb M, Savenkoff B, Brglez V, Benzaken S, et al.. Neutralizing anti-rituximab antibodies and relapse in membranous nephropathy treated with rituximab. Front Immunol (2019) 10:3069. doi: 10.3389/fimmu.2019.03069[↩]
- Tobinai K, Klein C, Oya N, Fingerle-Rowson G. A review of obinutuzumab (GA101), a novel type II anti-CD20 monoclonal antibody, for the treatment of patients with b-cell malignancies. Adv Ther (2017) 34(2):324–56. doi: 10.1007/s12325-016-0451-1[↩]
- Amoura Z, Remy P, Quintana Porras L, Chiche L, Chauveau D, Roccatello D, et al.. Alternative renal response definitions in a randomized, controlled trial of obinutuzumab for proliferative lupus nephritis [abstract]. Arthritis Rheumatol (2020) 72(suppl 10):104. doi: 10.1136/annrheumdis-2020-eular.2983[↩]
- Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, et al.. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med (2020) 383(12):1117–28. doi: 10.1056/NEJMoa2001180[↩]
- Mougiakakos D, Krönke G, Völkl S, Kretschmann S, Aigner M, Kharboutli S, et al.. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med (2021) 385(6):567–9. doi: 10.1056/NEJMc2107725[↩]
- Mackensen A, Müller F, Mougiakakos D, Böltz S, Wilhelm A, Aigner M, et al.. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med (2022) 28:2124–32. doi: 10.3410/f.742323959.793595617[↩]
- Mirkovic K, Frenay AS, van den Born J, van Goor H, Navis G, de Borst MH; NIGRAM consortium. Sodium restriction potentiates the renoprotective effects of combined vitamin D receptor activation and angiotensin-converting enzyme inhibition in established proteinuric nephropathy. Nephrol Dial Transplant. 2017 Aug 1;32(8):1293-1301. doi: 10.1093/ndt/gfv304[↩]
- Bomback AS, Fervenza FC. Membranous Nephropathy: Approaches to Treatment. Am J Nephrol. 2018;47 Suppl 1:30-42. doi: 10.1159/000481635[↩]
- Cattran D, Brenchley P. Membranous nephropathy: thinking through the therapeutic options. Nephrol Dial Transplant. 2017 Jan 1;32(suppl_1):i22-i29. doi: 10.1093/ndt/gfw404[↩]
- Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, Jefferson JA, Gipson PE, Rizk DV, Sedor JR, Simon JF, McCarthy ET, Brenchley P, Sethi S, Avila-Casado C, Beanlands H, Lieske JC, Philibert D, Li T, Thomas LF, Green DF, Juncos LA, Beara-Lasic L, Blumenthal SS, Sussman AN, Erickson SB, Hladunewich M, Canetta PA, Hebert LA, Leung N, Radhakrishnan J, Reich HN, Parikh SV, Gipson DS, Lee DK, da Costa BR, Jüni P, Cattran DC; MENTOR Investigators. Rituximab or Cyclosporine in the Treatment of Membranous Nephropathy. N Engl J Med. 2019 Jul 4;381(1):36-46. doi: 10.1056/NEJMoa1814427[↩][↩]
- Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, Melis P, Valzorio B, Sasdelli M, Pasquali S, Pozzi C, Piccoli G, Lupo A, Segagni S, Antonucci F, Dugo M, Minari M, Scalia A, Pedrini L, Pisano G, Grassi C, Farina M, Bellazzi R. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol. 1998 Mar;9(3):444-50. doi: 10.1681/ASN.V93444[↩]
- Bomback AS, Derebail VK, McGregor JG, Kshirsagar AV, Falk RJ, Nachman PH. Rituximab therapy for membranous nephropathy: a systematic review. Clin J Am Soc Nephrol. 2009 Apr;4(4):734-44. doi: 10.2215/CJN.05231008[↩]
- Goździk M, Płuciennik A, Zawiasa-Bryszewska A, Nowicka M, Nowicka Z, Wągrowska-Danilewicz M, Kurnatowska I. Acute Kidney Injury Following Exposure to Calcineurin Inhibitors in a Patient with Idiopathic Membranous Nephropathy. Drug Saf Case Rep. 2019 Oct 5;6(1):9. doi: 10.1007/s40800-019-0103-x[↩]
- Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry Group. Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int. 2004 Sep;66(3):1199-205. doi: 10.1111/j.1523-1755.2004.00873.x[↩]
- Lu W, Gong S, Li J, Wang Y. Clinicopathological features and prognosis in patients with idiopathic membranous nephropathy with hypertension. Exp Ther Med. 2020 Apr;19(4):2615-2621. doi: 10.3892/etm.2020.8506[↩]
- Polanco N, Gutiérrez E, Covarsí A, Ariza F, Carreño A, Vigil A, Baltar J, Fernández-Fresnedo G, Martín C, Pons S, Lorenzo D, Bernis C, Arrizabalaga P, Fernández-Juárez G, Barrio V, Sierra M, Castellanos I, Espinosa M, Rivera F, Oliet A, Fernández-Vega F, Praga M; Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Española de Nefrología. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010 Apr;21(4):697-704. doi: 10.1681/ASN.2009080861[↩]
- Deegens JK, Wetzels JF. Diagnosis and treatment of primary glomerular diseases. Membranous nephropathy, focal segmental glomerulosclerosis and IgA nephropathy. Minerva Urol Nefrol. 2005 Sep;57(3):211-36.[↩]
- Ponticelli C, Passerini P. Management of idiopathic membranous nephropathy. Expert Opin Pharmacother. 2010 Sep;11(13):2163-75. doi: 10.1517/14656566.2010.494599[↩]
- Waldman M, Austin HA 3rd. Controversies in the treatment of idiopathic membranous nephropathy. Nat Rev Nephrol. 2009 Aug;5(8):469-79. doi: 10.1038/nrneph.2009.101[↩]
- Membranous Nephropathy. https://www.msdmanuals.com/professional/genitourinary-disorders/glomerular-disorders/membranous-nephropathy[↩]