Contents
What is metastasis
Metastasis is the movement or spreading of cancer cells from one organ or tissue to another that is responsible for >90% of cancer-related deaths. The main reason that cancer is so serious is its ability to spread (metastasize) in your body. Cancer cells can spread locally by moving into nearby normal tissue. Cancer can also spread regionally, to nearby lymph nodes, tissues, or organs. And cancer can spread to distant parts of the body. When this happens, it is called metastatic cancer. For many types of cancer, it is also called stage IV (four) cancer. The process by which cancer cells spread to other parts of the body is called metastasis.
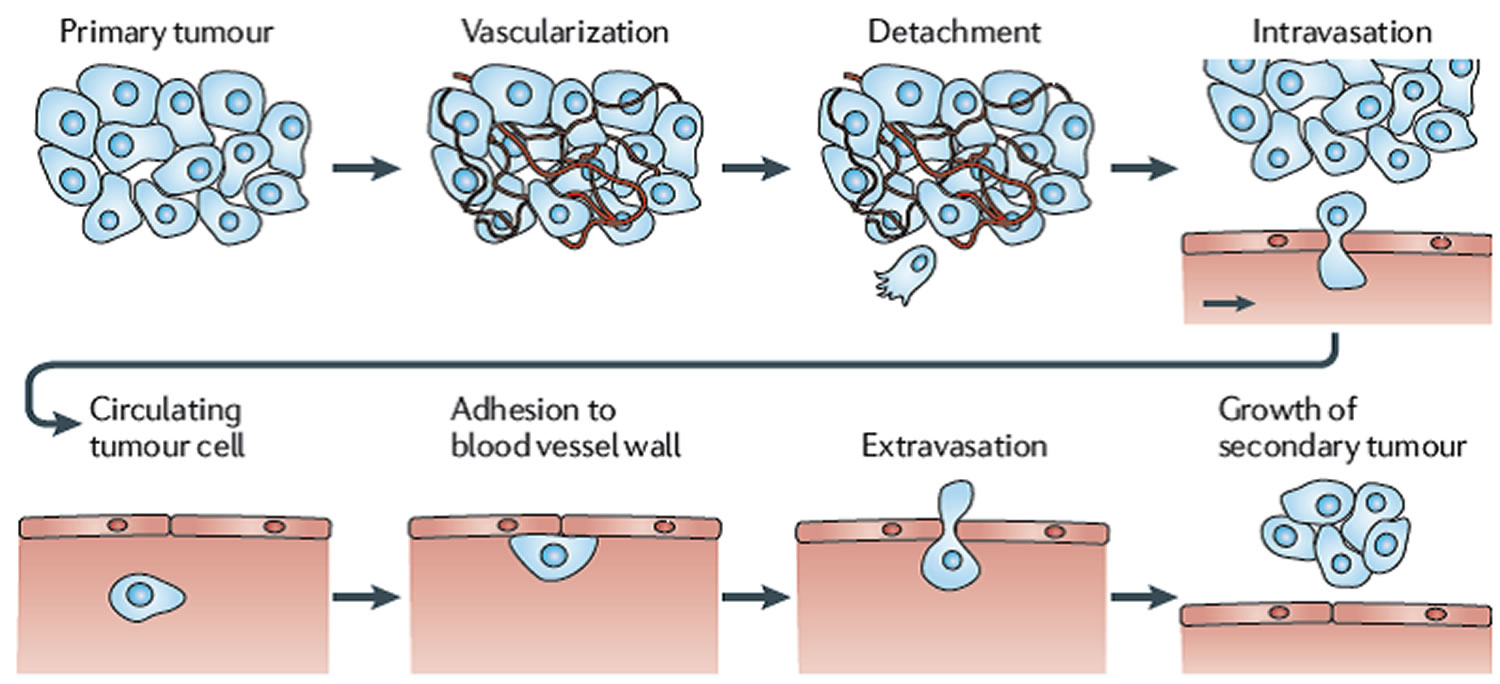
Cancer cells usually spread through the blood or the lymph system. If a cancer spreads, it is said to have “metastasized.” The course of cancer metastasis entails a series of complex, multistep process or stages that lead to the formation of secondary tumors in distant organs and is, largely, responsible for the mortality and morbidity of cancer (Figure 1) 1. Most cancer-related deaths are due to metastatic disease, primarily reflecting the lack of effective treatments once cancer has spread beyond the primary organ 2. In addition to genetic and external environmental factors, the physical interactions of cancer cells with their microenvironment, as well as their modulation by mechanical forces, are key determinants of the metastatic process. The emerging insight into these physical interactions may help to solve some long-standing questions in cancer progression and may lead to new approaches to developing cancer diagnostics and therapies. Investigations into the molecular and cellular processes underlying the metastatic cascade are vital for streamlining therapeutic approaches directed against disseminated disease.
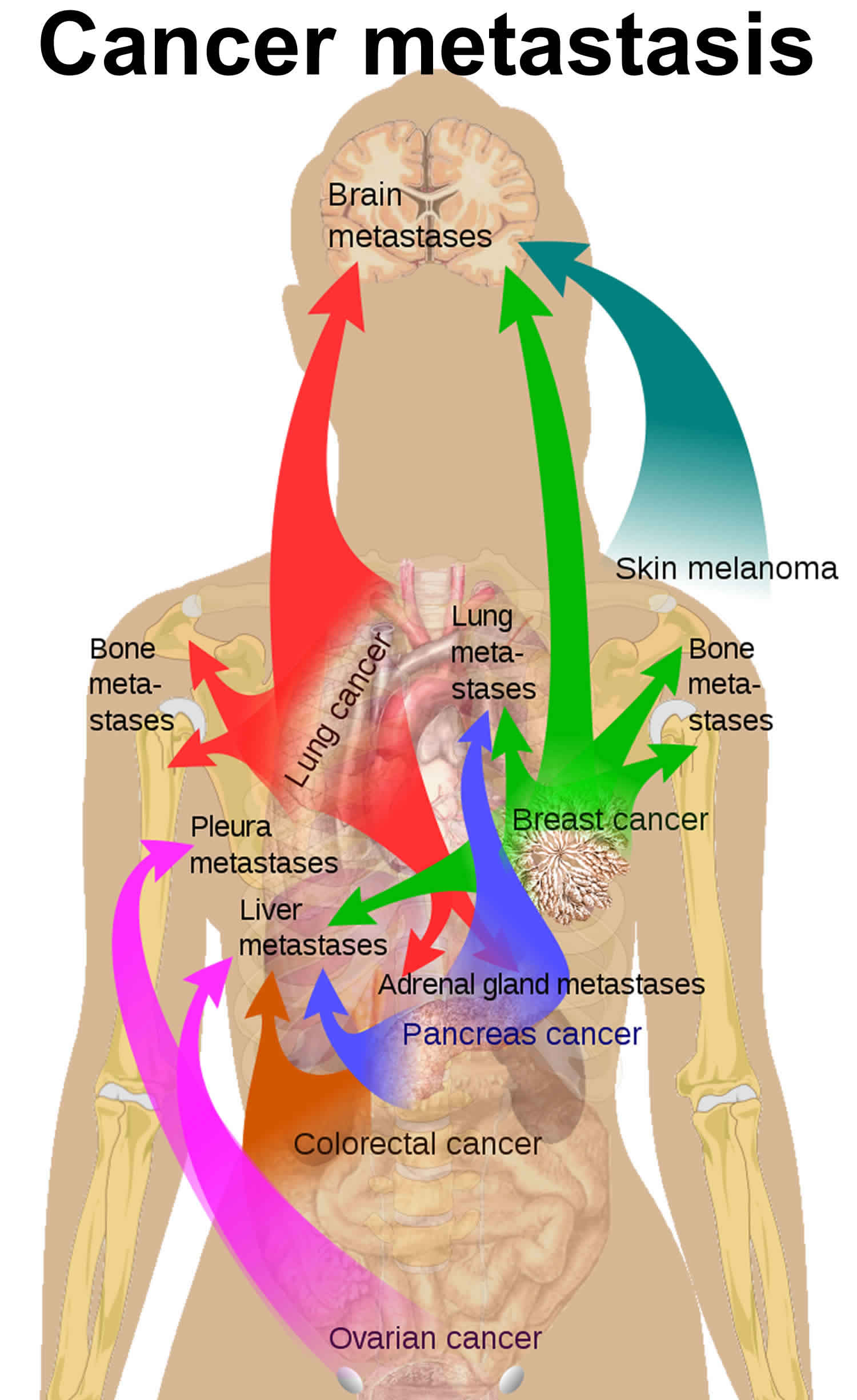
Figure 1. Cancer metastasis
When observed under a microscope and tested in other ways, metastatic cancer cells have features like that of the primary cancer and not like the cells in the place where the cancer is found. This is how doctors can tell that it is cancer that has spread from another part of the body.
Metastatic cancer has the same name as the primary cancer. For example, breast cancer that spreads to the lung is called metastatic breast cancer or lung metastasis, is not lung cancer. It is treated as stage 4 breast cancer, not as lung cancer.
Sometimes when people are diagnosed with metastatic cancer, doctors cannot tell where it started. This type of cancer is called cancer of unknown primary origin.
When a new primary cancer occurs in a person with a history of cancer, it is known as a second primary cancer. Second primary cancers are rare. Most of the time, when someone who has had cancer has cancer again, it means the first primary cancer has returned.
Whether or not cancer cells spread to other parts of the body depends on many things, including:
- The type of cancer
- The stage of the cancer
- Original location of the cancer
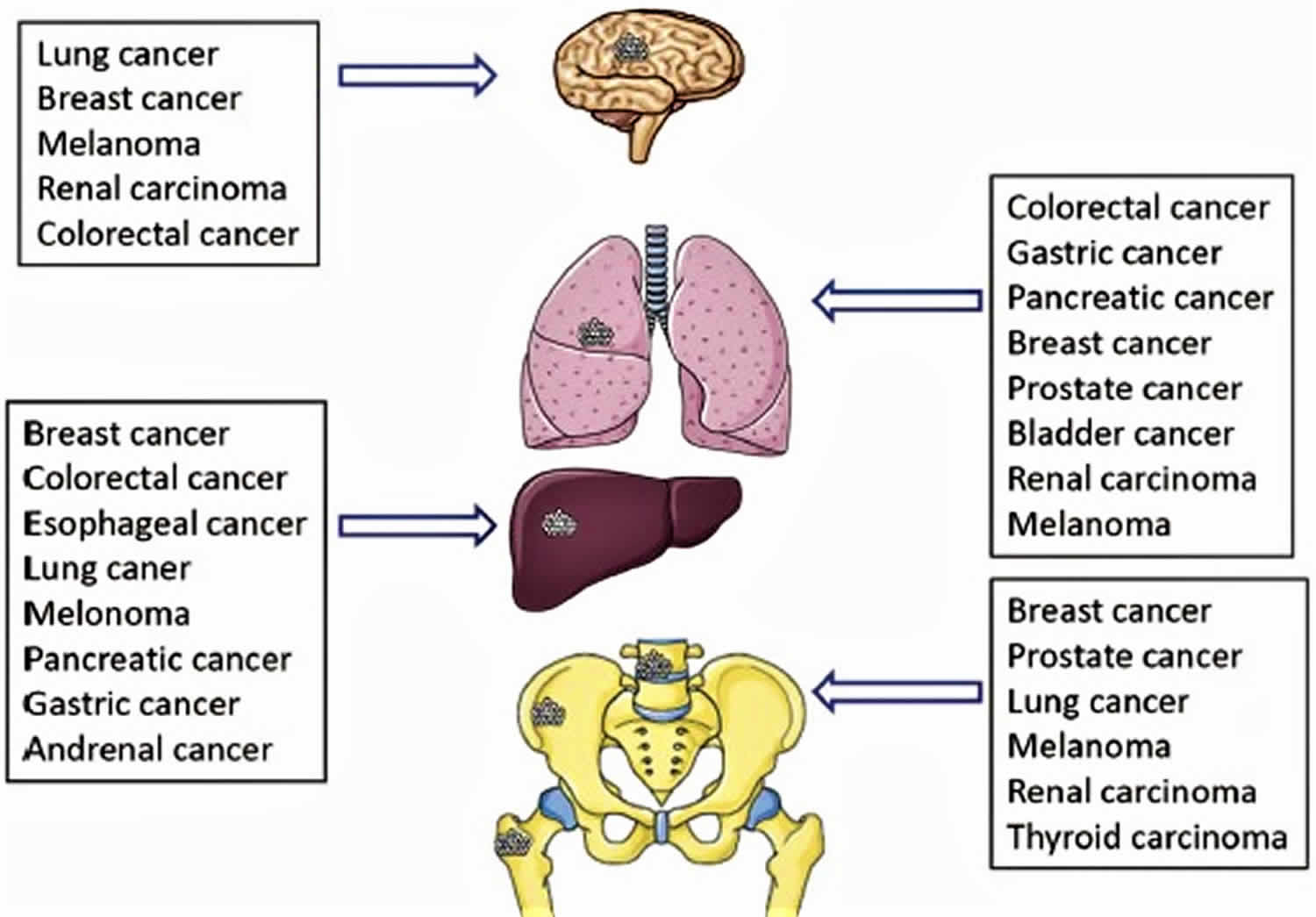
The organs mostly affected by metastases are lung, liver, brain and bone (see Figure 2) 3. Treatment depends on the type of cancer and where it has spread.
Figure 2. Organ specific metastases from primary tumors
[Source 4 ]Cancer metastasis
Cancer cells spread through the body in a series of steps. These steps include:
- Growing into, or invading, nearby normal tissue
- Moving through the walls of nearby lymph nodes or blood vessels
- Traveling through the lymphatic system and bloodstream to other parts of the body
- Stopping in small blood vessels at a distant location, invading the blood vessel walls, and moving into the surrounding tissue
- Growing in this tissue until a tiny tumor forms
- Causing new blood vessels to grow, which creates a blood supply that allows the tumor to continue growing
The metastatic process is an inefficient process whereby the vast majority of circulating tumor cells are not able to progressively grow at distant sites 1. Most of the time, spreading cancer cells die at some point in the metastatic process. But, as long as conditions are favorable for the cancer cells at every step, some of them are able to form new tumors in other parts of the body. Metastatic cancer cells can also remain inactive at a distant site for many years before they begin to grow again, if at all. A latent period may exist between infiltration of cancer cells at a distant site and colonization leading progressively to the growth of a secondary tumor. Such a period can be as long as a couple of years seen in some metastases of breast cancer after initial management, and it can also be as short as a few months in lung cancer which may develop a metastasis rapidly within a few months of diagnosis. The cellular origin, intrinsic properties of the tumor, tissue affinities and circulation patterns determine not only the sites of tumor spread, but also the temporal course and severity of metastasis to vital organs. In addition to the above aspects of metastases, certain metastatic cells exhibit tissue tropism, preferring to grow in certain organs (see Table 1). In breast cancer, for example, metastasis affects the bone and the lung, and less frequently the liver, brain, and adrenal medulla. Although the genetic and epigenetic basis of these metastatic properties is yet to be fully established, acquisition of the ability to complete each step involved in metastasis is thought to be driven by the accumulation of genetic mutations and epigenetic events that may result in a cells acquisition of metastatic traits during the process of developing a secondary tumor.
Where cancer spreads
Cancer can spread to most any part of the body, although different types of cancer are more likely to spread to certain areas than others. The most common sites where cancer spreads are the bone, liver, and lung. The following list shows the most common sites of metastasis, not including the lymph nodes, for some common cancers:
Table 1. Common sites of cancer metastasis
| Cancer Type | Main Sites of Metastasis |
|---|---|
| Bladder | Bone, liver, lung |
| Breast | Bone, brain, liver, lung |
| Colon | Liver, lung, peritoneum |
| Kidney | Adrenal gland, bone, brain, liver, lung |
| Lung | Adrenal gland, bone, brain, liver, other lung |
| Melanoma | Bone, brain, liver, lung, skin, muscle |
| Ovary | Liver, lung, peritoneum |
| Pancreas | Liver, lung, peritoneum |
| Prostate | Adrenal gland, bone, liver, lung |
| Rectal | Liver, lung, peritoneum |
| Stomach | Liver, lung, peritoneum |
| Thyroid | Bone, liver, lung |
| Uterus | Bone, liver, lung, peritoneum, vagina |
Lung metastasis
The lungs are the commonest site of metastases for many primary tumors. However, there is a great difference in propensity between the cancers. It is just as high as 90% in melanomas at autopsy. The lungs serve as first filter for tumor cells spreading through blood circulation in malignancies whose venous drainage flows directly into the lungs. The tumors of testis, melanoma, osteosarcoma, and head and neck tumors have the highest incidence of pulmonary metastases 3.
Liver metastasis
The liver is one of the most common sites for metastatic disease, accounting for 25% of all metastases to solid organs 6. In the United States and Europe, secondary liver cancers are far more common than primary liver cancers. In the adult oncology patient, most are metastatic carcinomas, of which adenocarcinomas are the predominant subtype, followed by squamous cell carcinomas and neuroendocrine carcinomas. Other tumor types that metastasize to the liver include melanomas, lymphomas, and rarely sarcomas.
Brain metastasis
The most frequent metastasis to the brain occurs in patients with lung, breast, melanoma, renal, and colorectal tumors.148 In 2700 cases from the Memorial Sloan-Kettering Cancer Center in New York, the distribution of primary cancers was as follows: 48% lung, 15% breast, 9% melanoma, 1% lymphoma (mainly non-Hodgkin), 3% GI (3% colon and 2% pancreatic), 11% genitourinary (21% kidney, 46% testes, 5% cervix, 5% ovary), 10% osteosarcoma, 5% neuroblastoma, and 6% head and neck tumor . Once metastasis to the brain is diagnosed, the median survival of untreated patients is 12 mo. Bone metastases are most commonly seen in prostate, breast and lung cancer, which are leading malignancies in female and/or male having the highest incidence and mortality rates 7.
Bone metastasis
Bone metastasis usually leads to severe morbidities, which always persist until the death of patients, including bone pain, hypercalcemia, pathological fracture, spinal cord compression and consequent paralysis. In the following part, we generally reviewed the process and molecular mechanisms of organ specific metastases with a focus on bone metastasis.
Metastatic Course, Routes and Steps
At an early stage, cancerous cells are confined to the primary site within the boundary of certain surrounding tissues. As the disease progresses, some cancer cells, as the result of genetic/ epigenetic predisposition, environmental interaction/stimulation, and indeed the combination of these elements, become more aggressive and begin to breach the surrounding structure 1. These cells would either directly invade the surrounding tissue, or disseminate via lymphatic and hematogenous (blood) routes. Direct invasion may result in the spreading of cancer cells to surrounding tissues and neighboring organs. For example, the local invasion of prostate cancer, can affect the erectile nerves, seminal vesicles, bladder and rectum nearby the prostate. The lymphatic and vascular routes differ from cancer to cancer according to their primary sites, however, frequently result in the systemic spread of cancer cells to distant organs, including bones, lung, and liver. For example, the primary lymphatic drainage of the prostate is via the internal iliac, perivesical, external iliac, obturator, and presacral nodes. The secondary lymphatic drainage includes the inguinal, common iliac and parα-aortic nodes. These nodes are therefore prime locations when one searches for the involved positive lymph nodes. Since the end of last century, a new technique, sentinel lymph node dissection has been developed and introduced in the detection, staging and management of lymph node involvement in cancer. The detection of a positive sentinel node indicates the need for a wide dissection of lymph nodes during surgery.
Both lymphatic and hematogenous dissemination frequently occur, even during early stages of cancer, and are seen in a vast majority of the patients who have an advanced cancer. To determine if systemic spread ‘occurred’ or not is a highly controversial topic, a conclusion of which is dependent on a wide variety of factors, from the type of samples to test, location and timing of sampling, techniques to detect cancer cells, to the interpretation of the presence of cancer cells or a cancer cell in a sample. Nonetheless, brain, bone, lung and liver are the most leading hematogenous sites from certain solid tumors 8.
The process of metastasis is complex and arduous, which incorporates multiple cells, factors and stages. During the development and progression of primary tumors, certain clones of tumor cells will have the required genotypic and phenotypic characteristics to enable themselves to interact with the local microenvironment. For example, tumor cells release vascular endothelial growth factor (VEGF) to initiate angiogenesis (formation of new blood vessels), thus enhancing the blood supply to the tumor. The stromal cells are rich sources of protein factors that directly act on cancer cells thus driving the growth of tumors and dissemination of cancer cells. On the other hand, some of the stromal cell derived factors will directly induce angiogenesis thus supporting the growth and spread of an aggressive tumor. A good example of these stromα-derived protein factors is hepatocyte growth factor (HGF), a cytokine secreted by the stroma cells, which has been implicated in the angiogenesis and the dissemination of tumor cells 9. The disruption of intercellular adhesion in the tumor causes some tumor cells to detach from the tumor mass (detachment), followed by these cells invading through the extracellular matrix, a process so-called invasion which incorporates the motility, migration of tumor cells and breakdown of extracellular matrix. Some tumor cells will penetrate the blood vessels, thus entering the circulation (intravasation). From this point, these tumor cells move away from the primary site and circulate in the blood circulation where, they would encounter resistance by the immune system and the mechanical stresses of blood flow. Some tumor cells will eventually survive and adopt a process to leave the blood circulation, known as extravasation, in which cells adhere and penetrate the blood vessel again (a virtual reversal of the intravasation process). Once the tumor cells escape from the circulation, they will have to survive and finally develop a secondary tumor at the other site, in this case in bone. This complex process also needs the integration of multiple factors and events, such as invasion of tumor, angiogenesis and the interaction between tumor cells and the local microenvironment at a distant site/organ.
Metastasis Regulators
The interrelated and sequential multi-steps of metastasis require certain transformations of cancer cells at each step, from primary site to metastatic site. Numerous genes and molecules have been implicated into this dynamic and adaptable evolution of metastatic cancer cells, including suppressors and promoters of metastasis which may be altered genetically or epigenetically in accordance with the requirements at each step. Initiating factors for tumor progression and metastasis are critical and essential, particularly for dissociation and invasion which allow cancer cells to leave primary sites. The genes that determine these activities have been defined as metastasis initiation genes 10. These genes could promote cell motility, epithelial mesenchymal transition (EMT), extracellular matrix degradation, angiogenesis or evasion of the immune system. For example, epithelial mesenchymal transition (EMT) is mediated by developmental programmes that are under the control of aberrantly regulated transcription factors, such as Twist1, Snai1 and Snai2 (also known as Slug). Other determinants of invasion are components and modulators of certain pathways which include hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF) and extracellular signal-regulated kinase (ERK) pathways. Metastatic growth is also initiated by the suppression of non-coding RNAs, such as miR-126 and miR-335 in breast and gastric carcinomas 11. Some of the initiating factors that allow transformed cells to invade the surrounding tissue and attract a supportive stroma facilitate the dissemination of cancer cells and probably continue to do so after cancer cells infiltrate distant tissues. This is why some prognosis signatures of a malignancy can also be utilized as a signature to predict metastases 12.
Metastasis suppressor genes are defined by their ability to inhibit metastasis at any step of the metastatic cascade. These metastasis suppressor genes inhibit metastasis of cancer cells, in vivo, without blocking tumorigenicity. To date, some metastasis suppressor genes have been identified, such as nonmetastatic gene 23 (NM23), Kangai 1 (KAI1), KISS1, mitogen-activated protein kinase 4 (MKK4), breast cancer metastasis suppressor 1 (BRMS1), Rho GDP dissociation inhibitor 2 (RhoGDI2), cofactor required for Sp1 transcriptional activation subunit 3 (CRSP3) and Vitamin D3 upregulated protein 1 (VDUP1). Deregulation of these metastasis suppressor genes has been indicated in certain solid tumors 13.
Metastasis symptoms
Metastatic cancer does not always cause symptoms. When symptoms do occur, their nature and frequency will depend on the size and location of the metastatic tumors. Some common signs of metastatic cancer include:
- Pain and fractures, when cancer has spread to the bone
- Headache, seizures, or dizziness, when cancer has spread to the brain
- Shortness of breath, when cancer has spread to the lung
- Jaundice or swelling in the belly, when cancer has spread to the liver
Metastatic cancer treatment
Once cancer spreads, it can be hard to control. Although some types of metastatic cancer can be cured with current treatments, most cannot. Even so, there are treatments for all patients with metastatic cancer. The goal of these treatments is to stop or slow the growth of the cancer or to relieve symptoms caused by it. In some cases, treatments for metastatic cancer may help prolong life.
The treatment that you may have depends on your type of primary cancer, where it has spread, treatments you’ve had in the past, and your general health.
When metastatic cancer can no longer be controlled
If you have been told you have metastatic cancer that can no longer be controlled, you and your loved ones may want to discuss end-of-life care. Even if you choose to continue receiving treatment to try to shrink the cancer or control its growth, you can always receive palliative care to control the symptoms of cancer and the side effects of treatment. Information on coping with and planning for end-of-life care is available in the Advanced Cancer section.
Ongoing research
Researchers are studying new ways to kill or stop the growth of primary and metastatic cancer cells. This research includes finding ways to help your immune system fight cancer. Researchers are also trying to find ways to disrupt the steps in the process that allow cancer cells to spread. Visit the Metastatic Cancer Research page to stay informed of ongoing research funded by National Cancer Institute here (https://www.cancer.gov/types/metastatic-cancer/research).
Metastasis survival rate
Survival rates tell you what portion of people with the same type and stage of cancer are still alive a certain amount of time (usually 5 years) after they were diagnosed. They can’t tell you how long you will live, but they may help give you a better understanding about how likely it is that your treatment will be successful.
5-year survival rate
Statistics on the outlook for a certain type and stage of cancer are often given as 5-year survival rates, but many people live longer – often much longer – than 5 years. The 5-year survival rate is the percentage of people who live at least 5 years after being diagnosed with cancer. For example, a 5-year survival rate of 90% means that an estimated 90 out of 100 people who have that cancer are still alive 5 years after being diagnosed.
Relative survival rates are a more accurate way to estimate the effect of cancer on survival. These rates compare women with breast cancer to women in the overall population. For example, if the 5-year relative survival rate for a specific type of cancer is 90%, it means that people who have that cancer are, on average, about 90% as likely as people who don’t have that cancer to live for at least 5 years after being diagnosed.
But remember, the 5-year relative survival rates are estimates – your outlook can vary based on a number of your specific factors.
Cancer survival rates don’t tell the whole story
Survival rates are often based on previous outcomes of large numbers of people who had the disease, but they can’t predict what will happen in any particular person’s case.
There are a number of limitations to remember:
- The numbers below are among the most current available. But to get 5-year survival rates, doctors have to look at people who were treated at least 5 years ago. As treatments are improving over time, people who are now being diagnosed with stage 4 metastatic cancer may have a better outlook than these statistics show.
- These statistics are based on the stage 4 cancer when it was first diagnosed. They do not apply to cancers that come back later, for example.
- Many other factors can affect a person’s outlook, such as age and health, the presence of hormone receptors on the cancer cells, the treatment received, and how well the cancer responds to treatment.
Your doctor can tell you how these numbers might apply to you, as he or she is familiar with your particular situation.
Table 2. Relative 5 Year Survival of Stage 4 Cancers (2008-2014)
| Oral Cavity and Pharynx | All races | Male and female | 39.1% |
|---|---|---|---|
| Male | 39.2% | ||
| Female | 38.6% | ||
| White | Male and female | 39.9% | |
| Male | 40.4% | ||
| Female | 38.0% | ||
| Black | Male and female | 29.1% | |
| Male | 28.9% | ||
| Female | 29.3% | ||
| Esophagus | All races | Male and female | 4.8% |
| Male | 4.3% | ||
| Female | 7.4% | ||
| White | Male and female | 5.0% | |
| Male | 4.6% | ||
| Female | 7.3% | ||
| Black | Male and female | 3.5% | |
| Male | 1.9% | ||
| Female | 7.4% | ||
| Stomach | All races | Male and female | 5.2% |
| Male | 5.4% | ||
| Female | 4.8% | ||
| White | Male and female | 5.0% | |
| Male | 5.4% | ||
| Female | 4.2% | ||
| Black | Male and female | 6.2% | |
| Male | 4.9% | ||
| Female | 8.4% | ||
| Colon and Rectum | All races | Male and female | 13.8% |
| Male | 12.8% | ||
| Female | 14.9% | ||
| White | Male and female | 14.3% | |
| Male | 13.4% | ||
| Female | 15.4% | ||
| Black | Male and female | 10.4% | |
| Male | 9.5% | ||
| Female | 11.3% | ||
| Liver and Intrahepatic Bile Duct | All races | Male and female | 2.4% |
| Male | 2.0% | ||
| Female | 3.5% | ||
| White | Male and female | 2.6% | |
| Male | 2.1% | ||
| Female | 3.9% | ||
| Black | Male and female | 0.9% | |
| Male | 1.1% | ||
| Female | 0.0% | ||
| Gallbladder | All races | Male and female | 1.7% |
| Male | 1.4% | ||
| Female | 1.9% | ||
| White | Male and female | 1.9% | |
| Male | |||
| Female | 2.3% | ||
| Black | Male and female | 1.7% | |
| Male | 2.6% | ||
| Female | 1.2% | ||
| Pancreas | All races | Male and female | 2.7% |
| Male | 2.6% | ||
| Female | 2.8% | ||
| White | Male and female | 2.7% | |
| Male | 2.7% | ||
| Female | 2.6% | ||
| Black | Male and female | 3.0% | |
| Male | 2.4% | ||
| Female | 3.4% | ||
| Larynx | All races | Male and female | 33.5% |
| Male | 33.3% | ||
| Female | 34.1% | ||
| White | Male and female | 33.8% | |
| Male | 33.8% | ||
| Female | 33.9% | ||
| Black | Male and female | 31.2% | |
| Male | 31.1% | ||
| Female | 31.6% | ||
| Lung and Bronchus | All races | Male and female | 4.7% |
| Male | 3.9% | ||
| Female | 5.7% | ||
| White | Male and female | 4.5% | |
| Male | 3.7% | ||
| Female | 5.5% | ||
| Black | Male and female | 4.6% | |
| Male | 3.9% | ||
| Female | 5.6% | ||
| Bones and Joints | All races | Male and female | 31.9% |
| Male | 32.5% | ||
| Female | 31.0% | ||
| White | Male and female | 32.0% | |
| Male | 32.5% | ||
| Female | 31.3% | ||
| Black | Male and female | 28.3% | |
| Male | 30.0% | ||
| Female | 26.3% | ||
| Soft Tissue including Heart | All races | Male and female | 16.4% |
| Male | 15.6% | ||
| Female | 17.4% | ||
| White | Male and female | 16.1% | |
| Male | 15.4% | ||
| Female | 17.0% | ||
| Black | Male and female | 17.3% | |
| Male | 17.8% | ||
| Female | 16.9% | ||
| Melanoma of the Skin | All races | Male and female | 22.5% |
| Male | 21.1% | ||
| Female | 25.5% | ||
| White | Male and female | 22.2% | |
| Male | 21.1% | ||
| Female | 24.7% | ||
| Black | Male and female | 27.2% | |
| Male | 13.5% | ||
| Female | 39.8% | ||
| Breast | All races | Male and female | 26.9% |
| Male | 23.3% | ||
| Female | 27.0% | ||
| White | Male and female | 28.1% | |
| Male | 23.6% | ||
| Female | 28.1% | ||
| Black | Male and female | 19.8% | |
| Male | 24.8% | ||
| Female | 19.7% | ||
| Cervix Uteri | All races | Male and female | |
| Male | |||
| Female | 17.2% | ||
| White | Male and female | ||
| Male | |||
| Female | 18.8% | ||
| Black | Male and female | ||
| Male | |||
| Female | 10.8% | ||
| Corpus and Uterus, not otherwise specified | All races | Male and female | |
| Male | |||
| Female | 16.3% | ||
| White | Male and female | ||
| Male | |||
| Female | 17.4% | ||
| Black | Male and female | ||
| Male | |||
| Female | 9.2% | ||
| Ovary | All races | Male and female | |
| Male | |||
| Female | 29.2% | ||
| White | Male and female | ||
| Male | |||
| Female | 29.6% | ||
| Black | Male and female | ||
| Male | |||
| Female | 22.3% | ||
| Prostate | All races | Male and female | |
| Male | 30.0% | ||
| Female | |||
| White | Male and female | ||
| Male | 29.1% | ||
| Female | |||
| Black | Male and female | ||
| Male | 30.0% | ||
| Female | |||
| Testis | All races | Male and female | |
| Male | 73.7% | ||
| Female | |||
| White | Male and female | ||
| Male | 74.3% | ||
| Female | |||
| Black | Male and female | ||
| Male | 69.7% | ||
| Female | |||
| Urinary Bladder | All races | Male and female | 4.8% |
| Male | 5.2% | ||
| Female | 4.0% | ||
| White | Male and female | 4.8% | |
| Male | 4.8% | ||
| Female | 4.4% | ||
| Black | Male and female | 3.4% | |
| Male | 4.6% | ||
| Female | 1.4% | ||
| Kidney and Renal Pelvis | All races | Male and female | 11.6% |
| Male | 11.3% | ||
| Female | 12.1% | ||
| White | Male and female | 11.7% | |
| Male | 11.8% | ||
| Female | 11.6% | ||
| Black | Male and female | 9.4% | |
| Male | 7.4% | ||
| Female | 13.2% | ||
| Eye and Orbit | All races | Male and female | 30.1% |
| Male | 22.5% | ||
| Female | 40.0% | ||
| White | Male and female | 27.7% | |
| Male | 20.5% | ||
| Female | 38.2% | ||
| Black | Male and female | – | |
| Male | – | ||
| Female | – | ||
| Brain and Other Nervous System | All races | Male and female | 32.7% |
| Male | 29.0% | ||
| Female | 37.7% | ||
| White | Male and female | 31.2% | |
| Male | 27.6% | ||
| Female | 36.3% | ||
| Black | Male and female | 38.6% | |
| Male | 38.0% | ||
| Female | 39.1% | ||
| Thyroid | All races | Male and female | 55.5% |
| Male | 50.9% | ||
| Female | 58.3% | ||
| White | Male and female | 55.8% | |
| Male | 50.7% | ||
| Female | 59.2% | ||
| Black | Male and female | 47.0% | |
| Male | 42.4% | ||
| Female | 48.7% | ||
| Hodgkin Lymphoma | All races | Male and female | 78.2% |
| Male | 78.3% | ||
| Female | 78.0% | ||
| White | Male and female | 78.4% | |
| Male | 79.2% | ||
| Female | 77.0% | ||
| Black | Male and female | 76.1% | |
| Male | 71.4% | ||
| Female | 83.1% | ||
| Non-Hodgkin Lymphoma | All races | Male and female | 64.1% |
| Male | 62.4% | ||
| Female | 66.3% | ||
| White | Male and female | 65.0% | |
| Male | 63.2% | ||
| Female | 67.3% | ||
| Black | Male and female | 57.6% | |
| Male | 55.3% | ||
| Female | 60.6% | ||
| Myeloma | All races | Male and female | 49.6% |
| Male | 49.2% | ||
| Female | 50.0% | ||
| White | Male and female | 48.7% | |
| Male | 48.6% | ||
| Female | 48.8% | ||
| Black | Male and female | 51.5% | |
| Male | 50.4% | ||
| Female | 52.6% | ||
| Leukemia | All races | Male and female | 61.4% |
| Male | 62.5% | ||
| Female | 60.0% | ||
| White | Male and female | 61.8% | |
| Male | 62.7% | ||
| Female | 60.7% | ||
| Black | Male and female | 55.4% | |
| Male | 56.9% | ||
| Female | 53.6% | ||
| Lymphocytic Leukemia | All races | Male and female | 79.7% |
| Male | 80.3% | ||
| Female | 78.8% | ||
| White | Male and female | 79.9% | |
| Male | 80.4% | ||
| Female | 79.3% | ||
| Black | Male and female | 70.2% | |
| Male | 72.1% | ||
| Female | 67.4% | ||
| Acute Lymphocytic Leukemia | All races | Male and female | 68.1% |
| Male | 67.8% | ||
| Female | 68.5% | ||
| White | Male and female | 68.2% | |
| Male | 67.5% | ||
| Female | 69.1% | ||
| Black | Male and female | 63.6% | |
| Male | 66.0% | ||
| Female | 60.2% | ||
| Chronic Lymphocytic Leukemia | All races | Male and female | 84.2% |
| Male | 84.4% | ||
| Female | 83.8% | ||
| White | Male and female | 84.2% | |
| Male | 84.4% | ||
| Female | 83.9% | ||
| Black | Male and female | 73.1% | |
| Male | 74.3% | ||
| Female | 71.2% | ||
| Myeloid and Monocytic Leukemia | All races | Male and female | 40.4% |
| Male | 40.1% | ||
| Female | 40.7% | ||
| White | Male and female | 39.4% | |
| Male | 39.1% | ||
| Female | 39.9% | ||
| Black | Male and female | 43.7% | |
| Male | 42.6% | ||
| Female | 44.7% | ||
| Acute Myeloid Leukemia | All races | Male and female | 27.4% |
| Male | 26.3% | ||
| Female | 28.6% | ||
| White | Male and female | 26.9% | |
| Male | 25.9% | ||
| Female | 28.0% | ||
| Black | Male and female | 28.8% | |
| Male | 26.5% | ||
| Female | 30.9% | ||
| Acute Monocytic Leukemia | All races | Male and female | 23.2% |
| Male | 21.3% | ||
| Female | 25.4% | ||
| White | Male and female | 23.8% | |
| Male | 21.7% | ||
| Female | 26.4% | ||
| Black | Male and female | 25.2% | |
| Male | 21.1% | ||
| Female | 28.4% | ||
| Chronic Myeloid Leukemia | All races | Male and female | 67.6% |
| Male | 66.4% | ||
| Female | 69.3% | ||
| White | Male and female | 66.4% | |
| Male | 65.4% | ||
| Female | 68.0% | ||
| Black | Male and female | 70.0% | |
| Male | 67.8% | ||
| Female | 72.3% | ||
| Mesothelioma | All races | Male and female | 7.2% |
| Male | 5.2% | ||
| Female | 13.1% | ||
| White | Male and female | 7.2% | |
| Male | 5.3% | ||
| Female | 13.1% | ||
| Black | Male and female | 5.6% | |
| Male | 0.0% | ||
| Female | 14.8% | ||
| Kaposi Sarcoma | All races | Male and female | 45.0% |
| Male | 47.3% | ||
| Female | – | ||
| White | Male and female | 50.3% | |
| Male | 50.3% | ||
| Female | |||
| Black | Male and female | – | |
| Male | – | ||
| Female | – |
- Martin TA, Ye L, Sanders AJ, et al. Cancer Invasion and Metastasis: Molecular and Cellular Perspective. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK164700[↩][↩][↩]
- Pearson HB, Pouliot N. Modeling Metastasis In Vivo. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK100378[↩]
- Debois JM. 2002. TxNxM1: The Anatomy and Clinics of Metastatic Cancer, Kluwer Academic Publisher.[↩][↩]
- Martin TA, Ye L, Sanders AJ, et al. Cancer Invasion and Metastasis: Molecular and Cellular Perspective. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK164700[↩]
- Metastatic Cancer. https://www.cancer.gov/types/metastatic-cancer[↩]
- Abbruzzese JL, Abbruzzese MC, Lenzi R, Hess KR, Raber MN. Analysis of a diagnostic strategy for patients with suspected tumors of unknown origin. J Clin Oncol. 1995;13:2094–103.[↩]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. http://dx.doi.org/10.3322/caac.20006[↩]
- Nguyen DX, Bos PD, Massagu J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. http://dx.doi.org/10.1038/nrc2622[↩]
- Jiang WG, Martin TA, Parr C, Davies G, Matsumoto K, Nakamura T. Hepatocyte growth factor, its receptor, and their potential value in cancer therapies. Crit Rev Oncol Hematol. 2005;53:35–69. http://dx.doi.org/10.1016/j.critrevonc.2004.09.004[↩]
- Chiang AC, Massagu J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–23. http://dx.doi.org/10.1056/NEJMra0805239[↩]
- Yang R, Dick M, Marme F, Schneeweiss A, Langheinz A, Hemminki K, et al. Genetic variants within miR-126 and miR-335 are not associated with breast cancer risk. Breast Cancer Res Treat. 2011;127:54954. http://dx.doi.org/10.1007/s10549-010-1244-x[↩]
- Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest. 2005;115:44–55[↩]
- Malik FA, Sanders AJ, Kayani MA, Jiang WG. Effect of expressional alteration of KAI1 on breast cancer cell growth, adhesion, migration and invasion. Cancer Genomics Proteomics. 2009;6:205–13[↩]
- SEER Survival Statistics. https://seer.cancer.gov/canques/survival.html[↩]
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2017 Sub (2000-2015) , National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2018, based on the November 2017 submission.[↩]