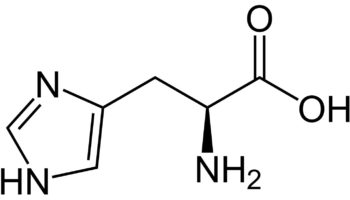
What is methionine
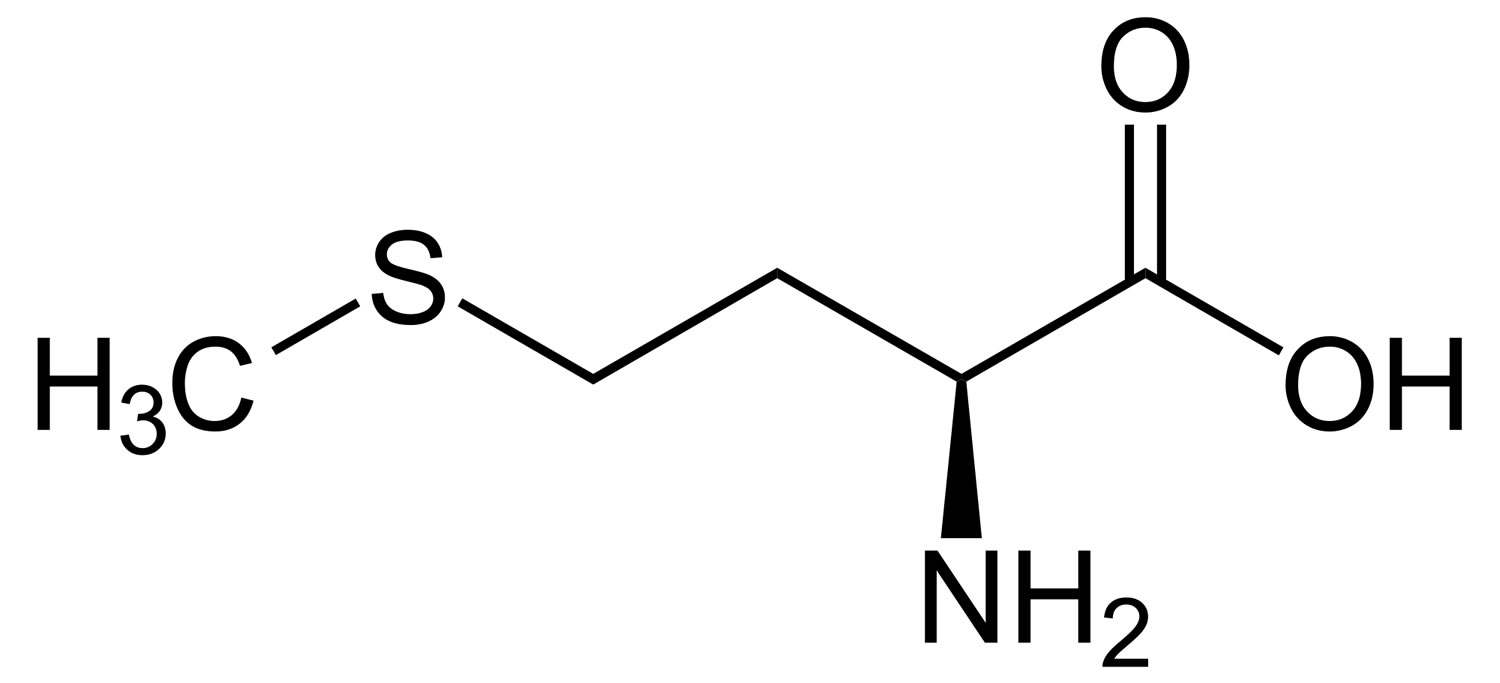
Methionine (C5-H11-N-O2-S) is one of nine essential amino acids in humans that needs to be provided by food. Your body cannot produce methionine from scratch, so it must come from food. Methionine is most abundant in animal products, such as lean meat and eggs, but also can be found in lower quantities in plant sources. Methionine is often the most limiting amino acid in the diets of the developing world’s population because of its low concentration in cereal grains 1, 2. Methionine is a sulphur-containing amino acid that can be obtained through the diet in protein or synthesized from homocysteine. Methionine is required for protein synthesis, metabolic processes, normal growth, tissue repair and development of humans 3. Methionine improves the tone and pliability of skin, hair, and strengthens nails. Burn patients may have an increased demand for methionine to maintain nitrogen balance 4. Methionine is involved in many detoxifying processes, sulphur provided by methionine protects cells from pollutants, slows cells aging, and is essential for absorption and bio-availability of selenium and zinc 5, 6. Methionine chelates heavy metals, such as lead and mercury, aiding their excretion. Methionine is also acts as a lipotropic agent and prevents excess fat buildup in the liver.
There is a general consensus concerning normal sulfur amino acid requirements. From the classical experiments of Rose 7 in healthy men, it was calculated that the minimal intake of methionine required to maintain nitrogen balance is 1.10 g/day, which comes down to ∼13 mg/kg body weight per day. The World Health Organization (WHO) recommendations amount to 13 mg/kg body weight per day in healthy adults. This amount is roughly doubled in artificial nutrition regimens. In a recent study it was shown that this intake also is sufficient to maintain glutathione synthesis in healthy volunteers 8. In 2023, Paoletti et 9 determined the total sulphur amino acids (SAAs) requirement provided as dietary methionine (no dietary cysteine) in healthy adults ≥ 60 years of age to be 26.2 and 17.1 mg/kg body weight per day for males and females, respectively. In infancy and childhood, the dietary requirement for sulfur amino acid is higher 10. Sulfur amino acid requirements in elderly subjects are unknown, but it has been suggested that the altered redox state reported in the elderly 11 requires increased ingestion of sulfur amino acid. In contrast, the safe upper limit of chronic sulfur amino acid supplementation may be reached earlier in the elderly because of accumulation of homocysteine, which is considered a risk factor for the development of atherosclerosis 12. Hyperhomocysteinemia may, however, be prevented by adequate vitamin B and folate supplementation.
In disease or after trauma, requirements may be altered for methionine, cysteine, and taurine. Although in specific cases of congenital enzyme deficiency, prematurity, or diminished liver function, hypermethioninemia or hyperhomocysteinemia may occur, sulfur amino acid supplementation can be considered safe in amounts exceeding 2-3 times the minimum recommended daily intake. Apart from some very specific indications (e. g. acetaminophen poisoning) the usefulness of sulfur amino acid supplementation is not yet established 10.
Methionine is needed by cells to repair damaged DNA and reduce oxidative stress, so depleting methionine from cancer cells targeted by DNA-damaging therapies, such as chemotherapy or radiation therapy, may enhance the ability of these treatments to kill the cancer cells. A 2020 study, researchers showed that feeding mice a diet very low in methionine improved the ability of chemotherapy and radiation therapy to shrink tumors 13. In that study, Dr. Locasale and his colleagues first measured whether cutting the amount of methionine in the diet of mice could quickly reduce the amount of methionine available to cells in the body 13. They found that switching mice to a diet about seven times lower in methionine than normal reduced the levels in cells after 2 days. They then fed the low methionine diet to mice carrying tumors derived from either of two types of human cancer cells. Methionine restriction alone greatly slowed the growth of tumors derived from one of the cell lines, and slowed it somewhat in tumors derived from the other cell line. The researchers next tested whether adding dietary methionine restriction to cancer treatments in mice could magnify the effects of those treatments. In mice bearing tumors derived from human colorectal cancer, low doses of the DNA-damaging chemotherapy drug 5-fluorouracil (5-FU) failed to shrink the tumors. But when the mice were fed the methionine-restricted diet during chemotherapy drug 5-fluorouracil (5-FU) treatment, their tumors shrank. Analyses of metabolism showed that the production of molecules needed to repair DNA, which requires methionine, had been altered as expected. The researchers also tested the combination of methionine restriction and radiation therapy in mice engineered to grow aggressive soft tissue sarcomas. The combination of the methionine-restricted diet plus radiation therapy slowed tumor growth by about 50% compared with the combination of a normal diet plus radiation. In a proof-of-concept follow-up experiment, six healthy middle-aged adults were recruited to eat a low methionine diet for 3 weeks. The “low methionine diet” contained about 80% less methionine than an average normal diet. Protein was mainly supplied through a methionine-free dietary supplement. The diet also included fruits, vegetables, and refined grains, which are naturally low in methionine. In the human study, the low methionine diet quickly reduced the amount of the amino acid available to participants’ cells and altered the cells’ metabolism, similar to what the researchers had observed in mice fed a methionine-restricted diet 13. However, the study was not designed to test the effect of methionine restriction on cancer treatment in humans and more research is needed because methionine is required for protein synthesis, metabolic processes, normal growth, muscle mass, nerve cells, tissue repair and development of humans and there could be side effects of restricting methionine in your diet in the long term 14, 15, 13.
Methionine is necessary for cell growth and differentiation as an essential amino acid and functions as an initiator of protein synthesis across species 16. Methionine also plays major roles in metabolism, including the methionine cycle, transsulfuration pathway, and salvage cycle, which are well conserved from Escherichia coli to humans 16. The first step in methionine metabolism is the methionine cycle, which is regulated by S-adenosylmethionine synthetase (SAMS-1) also known as methionine adenosyltransferase (MAT), which leads to the biosynthesis of S-adenosylmethionine (SAM), a major methyl donor, from methionine (Figure 1 and 2). The Methionine/S-adenosylmethionine (SAM) cycle is involved in the one-carbon metabolism and produces many important building blocks for the maintenance of your body 15. Methionine deficiency induces kidney apoptosis and cell cycle arrest 17. It has also been reported that methionine supplementation improves reproduction, egg quality, antioxidant status, and the immune response in breeder Japanese quail 18. Therefore, the balance of methionine composition in the body is important for maintaining healthy reproduction.
Methionine is the only essential sulfur amino acid and can provide sulfur for cysteine and taurine synthesis. Animal protein is generally considered to be a better source of sulfur amino acid than vegetable protein 10. This is primarily because the biological value of animal protein is higher than that of vegetable protein 19. Soy, which is the only vegetable protein used for artificial enteral nutrition, is also low in absolute sulfur amino acid content 19.
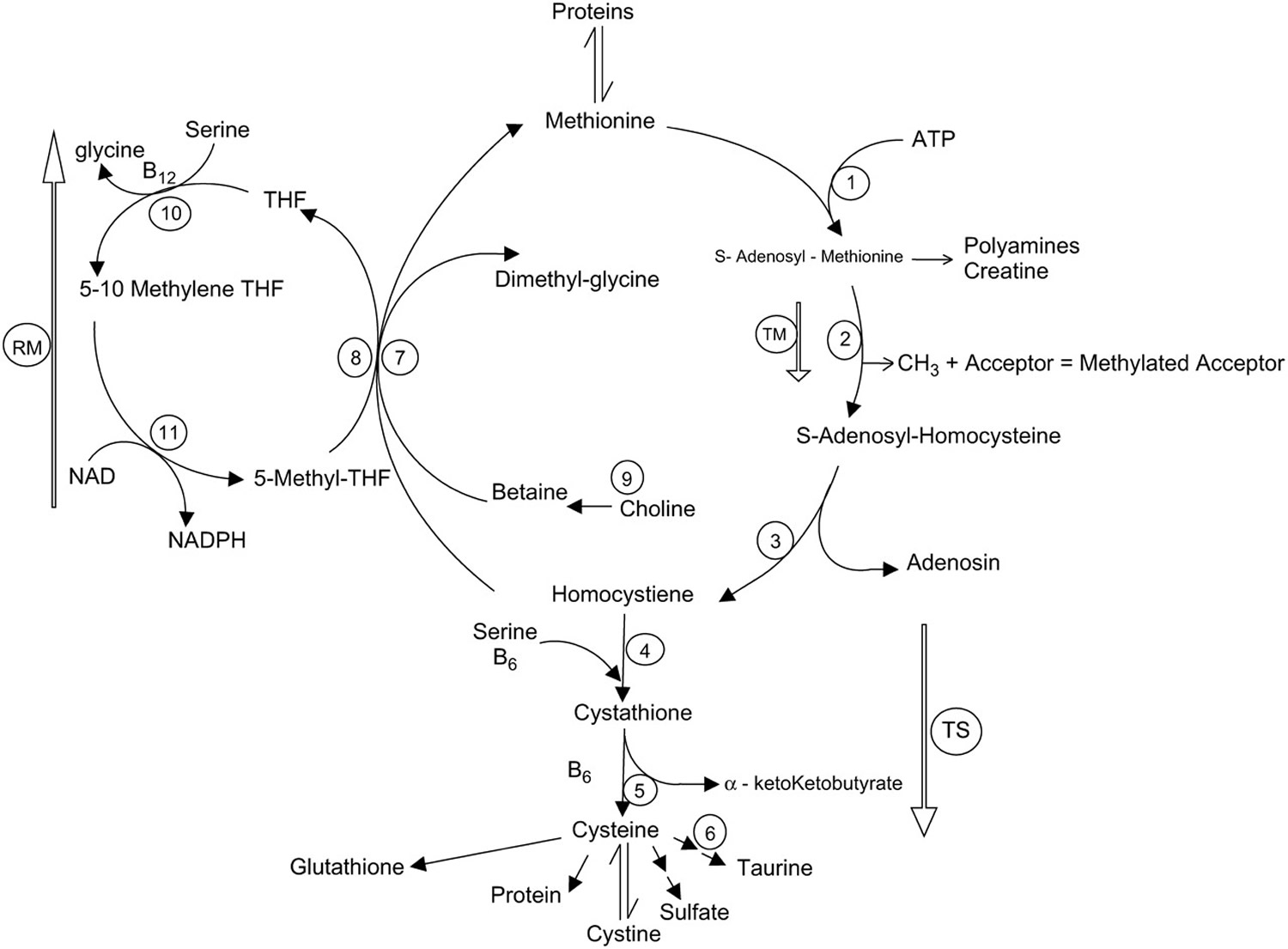
The sulfur amino acid, methionine, has gained renewed interest in recent years largely because of its relation to homocysteine and glutathione (Figure 1). Methionine breakdown occurs by transmethylation to homocysteine 20. The first step in this pathway is the conversion of methionine to S-adenosylmethionine, which can donate its methyl group for numerous methylation processes including DNA methylation. Separation of the methyl group yields S-adenosylhomocysteine, which can be converted to homocysteine. Homocysteine can be remethylated to methionine by the folate (vitamin B9) and vitamin B-12-dependent enzyme methionine synthetase or enter the irreversible transsulfuration pathway. Homocysteine is a central product in the metabolic pathway of methionine metabolism, and hyperhomocysteinemia has been identified as an independent risk factor for cardiovascular disease in adults 21, ischemic and hemorrhagic stroke in newborns and children 22 and Alzheimer’s disease in adults 23. A high plasma total homocysteine concentration is a risk factor for cardiovascular disease 24. However despite the function of methionine as a precursor of homocysteine, and the role of homocysteine in vascular damage and cardiovascular disease, there is no evidence that dietary intake of methionine within reasonable limits will cause cardiovascular damage 25. But intakes higher than 5 times normal (1.10 g/day or 13 mg/kg body weight per day) resulted in elevated homocysteine levels. These effects of methionine on homocysteine and vascular function are moderated by supplements of vitamins B-6, vitamin B-12, vitamin C, and folic acid 25.
The increase in total homocysteine induced by methionine, the sole dietary precursor of homocysteine, might be modulated by other amino acids present in dietary proteins. A randomized crossover trial was conducted in 24 healthy men. Each subject ingested 4 meals on separate days, which were separated by 1 week. Total homocysteine concentrations were measured in the fasting state and at 2, 4, 6, 8, 10, and 24 hours after meal ingestion. The meals were #1) a low-protein meal fortified with 30 mg methionine/kg body body weight (methionine only); #2) meal 1 additionally fortified with 60.6 mg serine/kg body body weight (Methionine + Serine); #3) meal 1 additionally fortified with 12.3 mg cystine/kg body body weight (Methionine + Cystine) and 4) a protein-rich meal containing 30 mg methionine, 60.6 mg serine, and 12.3 mg cystine per kg body body weight (Methionine + Serine + Cystine). RESULTS: The mean fasting total homocysteine concentration was 37% (Methionine + Serine), 32% (Methionine + Cystine) and 77% (Methionine + Serine + Cystine) lower. CONCLUSIONS: Dietary methionine increases total homocysteine much less than does free methionine. Serine and cystine reduce the total homocysteine-raising effect of free methionine (i.e. methionine supplement). Thus, dietary proteins with a high content of serine or cystine relative to methionine may lead to lower postprandial total homocysteine responses 24.
Methionine is also required for synthesis of cysteine. Undisputed functions of cysteine include protein synthesis and the biosynthesis of taurine, sulfate 26 and glutathione 27. However, the ability of cysteine to provide a portion of the total sulfur amino acid requirement in humans, thereby providing a sparing effect on the dietary methionine requirement, has been an area of considerable debate.
Methionine is accepted as the metabolic precursor for cysteine 28. Only the sulfur atom from methionine is transferred to cysteine; the carbon skeleton of cysteine is donated by serine 28. Cysteine is not a precursor for methionine because of the irreversibility of the cystathionase synthase reaction 29 (see Figure 1). Consequently, any substitution by cysteine for dietary methionine requirement can only be via inhibition of the sulfur amino acid pathway that leads to synthesis of the transsulfuration metabolites, including cysteine itself.
Glutathione, the most prevalent intracellular thiol 30 and an important endogenous antioxidant and scavenger 31, is synthesized de novo within all cells 32, and intracellular availability of cysteine is believed to be the most important rate-limiting factor for glutathione synthesis 27. More importantly, glutathione concentrations are reduced in several disease states including HIV 33, liver cirrhosis 34, diabetes 35 and Alzheimer’s disease 36. Glutathione concentration is also found to be reduced in surgical trauma patients 37, septic patients 38, premature infants 39, and in children with severe protein–energy malnutrition 40. Comprehensive understanding of sulfur amino acid requirements and their complex metabolism clearly has many implications for human health.
When present in sufficiently high levels, methionine can act as an atherogen and a metabotoxin. An atherogen is a compound that when present at chronically high levels causes atherosclerosis and cardiovascular disease 25. A metabotoxin is an endogenously produced metabolite that causes adverse health effects at chronically high levels. Chronically high levels of methionine are associated with at least ten inborn errors of metabolism, including cystathionine beta-synthase deficiency, glycine N-methyltransferase deficiency, homocystinuria, tyrosinemia, galactosemia, homocystinuria-megaloblastic anemia due to defects in cobalamin metabolism, methionine adenosyltransferase deficiency, methylenetetrahydrofolate reductase deficiency, and S-adenosylhomocysteine hydrolase deficiency. Chronically elevated levels of methionine in infants can lead to intellectual disability and other neurological problems, delays in motor skills, sluggishness, muscle weakness, and liver problems 25. Many individuals with these metabolic disorders tend to develop cardiovascular disease later in life. Studies on feeding rodents high levels of methionine have shown that methionine promotes atherosclerotic plaques independently of homocysteine levels 41. A similar study in Finnish men showed the same effect where it was found that “long-term, moderately high dietary methionine intake may increase the risk of acute coronary events in middle-aged Finnish men free of prior coronary heart disease”42.
In addition to being a substrate for protein synthesis, mehionine is an intermediate in transmethylation reactions, serving as the major methyl group donor in vivo, including the methyl groups for DNA and RNA intermediates. Methionine is a methyl acceptor for 5-methyltetrahydrofolate-homocysteine methyltransferase (methionine synthase), the only reaction that allows for the recycling of this form of folate, and is also a methyl acceptor for the catabolism of betaine. Methionine is the metabolic precursor for cysteine. Only the sulfur atom from methionine is transferred to cysteine; the carbon skeleton of cysteine is donated by serine 1.
Methionine is known to exacerbate psychopathological symptoms in schizophrenic patients, but there is no evidence of similar effects in healthy subjects. The role of methionine as a precursor of homocysteine is the most notable cause for concern. Acute doses of methionine can lead to acute increases in plasma homocysteine, which can be used as an index of the susceptibility to cardiovascular disease. Sufficiently high doses of methionine can actually result in death. Longer-term studies in adults have indicated no adverse consequences of moderate fluctuations in dietary methionine intake, but intakes higher than 5 times the normal amount resulted in elevated homocysteine levels. These effects of methionine on homocysteine and vascular function are moderated by supplements of vitamin B6 (pyridoxine), vitamin B12 (cyanocobalamin) and folate (vitamin B9) 25.
Figure 1. Methionine metabolism in mammalian tissue
Footnotes: Illustration of the pathways of methionine metabolism in mammalian tissue via transmethylation (TM), transsfulfuration (TS), and remethylation (RM). The numbers represent the following enzymes or reaction sequences: 1, L-methionine-S-adenosyl-transferase; 2, transmethylation reaction; 3, adenosylhomocysteinease; 4, cystathionine-β-synthase; 5, cystathionase; 6, multiple reactions leading from methionine to sulfate or taurine, cysteine used for protein or GSH or protein synthesis, or the reversible conversion between cysteine and cystine; 7, betaine-homocysteine methyl transferase; 8, methyltetrahydrofolate homocysteine methyltransferase; 9, choline dehydrogenase and betaine aldehyde dehydrogenase; 10, serine hydroxymethylase; 11, methylene tetrehydrofolate reductase.
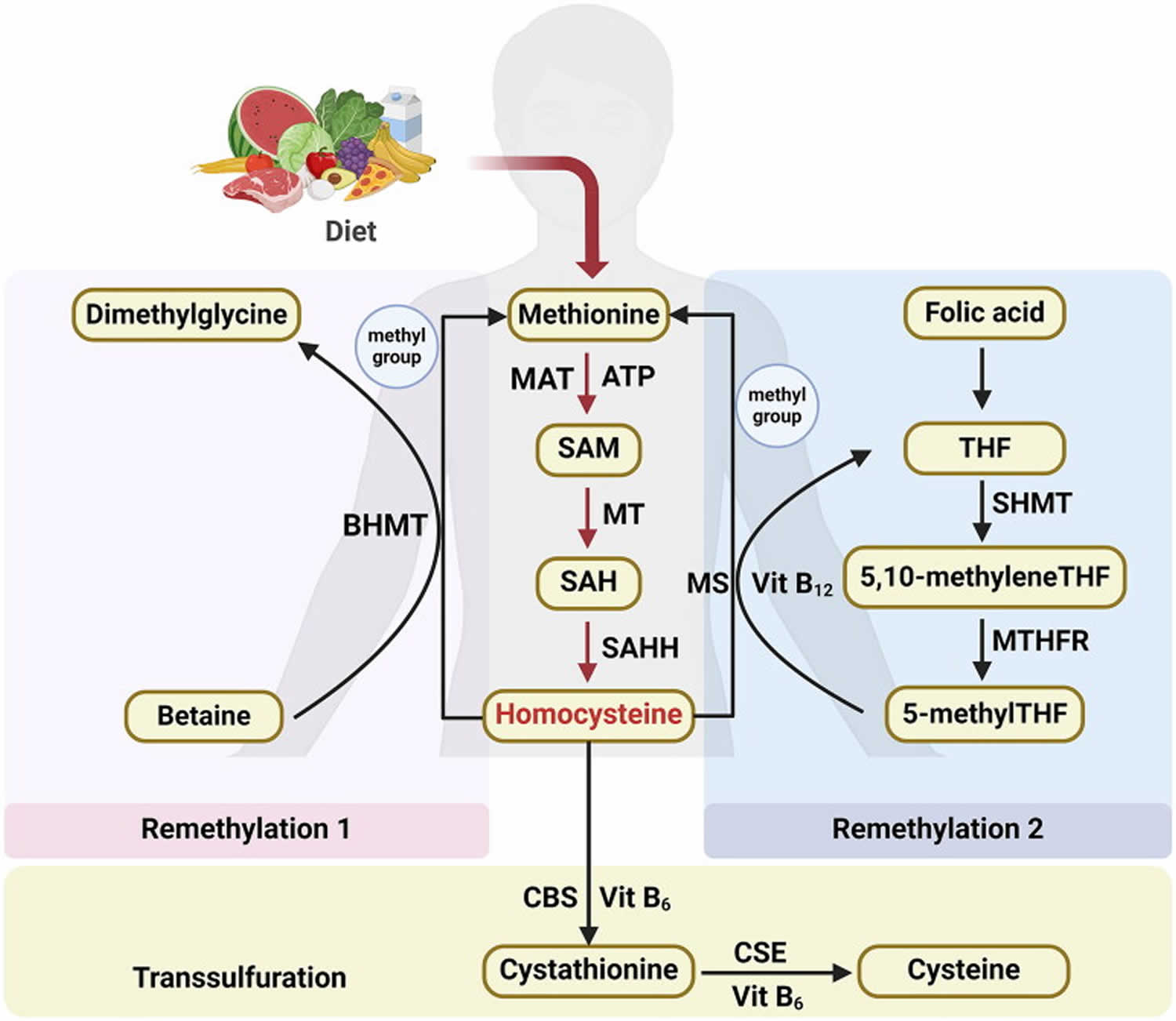
[Source 1 ]Figure 2. Homocysteine biosynthesis and metabolism
Footnote: Concise pathway for homocysteine biosynthesis and metabolism.
Abbreviations: BHMT = Betaine-homocysteine S-methyltransferase; MAT = Methionine adenosyltransferase; SAM = S-adenosylmethionine; MT = Methyltransferase; SAH = S-adenosylhomocysteine; SAHH = S-adenosylhomocysteine hydrolase; THF = Hydrolase tetrahydrofolate; SHMT = Serine hydroxymethyltransferase; MS = Methionine synthase; 5-methylTHF = N-5-methyltetrahydrofolate; MTHFR = 5:10-methylenetetrahydrofolate reductase; CBS = Cystathionine beta-synthase; CSE = Cystathionine gamma-lyase; vit = Vitamin.
[Source 43 ]Methionine rich foods
Table 1. Foods high in methionine (ordered from highest to low)
| Food | Methionine (g) Value Per 100 gram |
|---|---|
| Egg, white, dried, powder, stabilized, glucose reduced | 3.2 |
| Egg, white, dried, flakes, stabilized, glucose reduced | 2.99 |
| Egg, white, dried, stabilized, glucose reduced | 2.99 |
| Egg, white, dried | 2.79 |
| Fish, cod, Atlantic, dried and salted | 1.86 |
| Seeds, sesame flour, low-fat | 1.66 |
| Egg, whole, dried, stabilized, glucose reduced | 1.56 |
| Egg, whole, dried | 1.5 |
| Whale, beluga, meat, dried (Alaska Native) | 1.35 |
| Seeds, sesame flour, partially defatted | 1.33 |
| Mollusks, whelk, unspecified, cooked, moist heat | 1.21 |
| Seaweed, spirulina, dried | 1.15 |
| Beef, New Zealand, imported, brisket point end, separable lean only, cooked, braised | 1.14 |
| Soy protein isolate | 1.13 |
| Soy protein isolate, potassium type | 1.13 |
| Nuts, brazilnuts, dried, unblanched | 1.12 |
| Cheese, parmesan, shredded | 1.11 |
| Beef, New Zealand, imported, bolar blade, separable lean only, cooked, fast roasted | 1.1 |
| Beef, New Zealand, imported, flat, separable lean only, cooked, braised | 1.09 |
| Lamb, New Zealand, imported, fore-shank, separable lean only, cooked, braised | 1.09 |
| Pork, cured, bacon, cooked, microwaved | 1.07 |
| Lamb, New Zealand, imported, hind-shank, separable lean only, cooked, braised | 1.06 |
| Beef, New Zealand, imported, chuck eye roll, separable lean only, raw | 1.05 |
| Beef, New Zealand, imported, chuck eye roll, separable lean only, cooked, braised | 1.05 |
| Beef, New Zealand, imported, brisket point end, separable lean and fat, cooked, braised | 1.05 |
| Beef, New Zealand, imported, flat, separable lean and fat, cooked, braised | 1.05 |
| Seeds, sunflower seed flour, partially defatted | 1.04 |
| Beef, New Zealand, imported, variety meats and by-products, heart, cooked, boiled | 1.03 |
| Beef, New Zealand, imported, bolar blade, separable lean and fat, cooked, fast roasted | 1.03 |
| Beef, New Zealand, imported, hind shin, separable lean only, cooked, braised | 1.03 |
| Lamb, New Zealand, imported, neck chops, separable lean only, cooked, braised | 1.02 |
| Seeds, sesame flour, high-fat | 1.02 |
| Lamb, New Zealand, imported, square-cut shoulder chops, separable lean only, cooked, braised | 1.01 |
| Beef, New Zealand, imported, flank, separable lean only, cooked, braised | 1.01 |
| Beef, New Zealand, imported, rump centre, separable lean only, cooked, fast fried | 0.99 |
| Beef, New Zealand, imported, flank, separable lean and fat, cooked, braised | 0.99 |
| Lamb, New Zealand, imported, fore-shank, separable lean and fat, cooked, braised | 0.99 |
| Beef, New Zealand, imported, cube roll, separable lean only, cooked, fast roasted | 0.99 |
| Beef, round, top round roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 0.99 |
| Beef, New Zealand, imported, rump centre, separable lean and fat, cooked, fast fried | 0.99 |
| Beef, New Zealand, imported, chuck eye roll, separable lean and fat, raw | 0.98 |
| Beef, New Zealand, imported, chuck eye roll, separable lean and fat, cooked, braised | 0.98 |
| Beef, New Zealand, imported, oyster blade, separable lean only, cooked, braised | 0.98 |
| Beef, round, eye of round roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 0.98 |
| Beef, New Zealand, imported, hind shin, separable lean and fat, cooked, braised | 0.98 |
| Beef, New Zealand, imported, oyster blade, separable lean and fat, cooked, braised | 0.98 |
| Beef, chuck, mock tender steak, boneless, separable lean only, trimmed to 0″ fat, choice, cooked, braised | 0.98 |
| Beef, New Zealand, imported, eye round, separable lean only, cooked, slow roasted | 0.98 |
| Beef, loin, top sirloin filet, boneless, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.97 |
| Beef, chuck, mock tender steak, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, braised | 0.97 |
Methionine supplement side effects
When present in sufficiently high levels, methionine can act as an atherogen and a metabotoxin. An atherogen is a compound that when present at chronically high levels causes atherosclerosis and cardiovascular disease 25. A metabotoxin is an endogenously produced metabolite that causes adverse health effects at chronically high levels. Chronically high levels of methionine are associated with at least ten inborn errors of metabolism, including cystathionine beta-synthase deficiency, glycine N-methyltransferase deficiency, homocystinuria, tyrosinemia, galactosemia, homocystinuria-megaloblastic anemia due to defects in cobalamin metabolism, methionine adenosyltransferase deficiency, methylenetetrahydrofolate reductase deficiency, and S-adenosylhomocysteine hydrolase deficiency. Chronically elevated levels of methionine in infants can lead to intellectual disability and other neurological problems, delays in motor skills, sluggishness, muscle weakness, and liver problems 25. Many individuals with these metabolic disorders tend to develop cardiovascular disease later in life. Studies on feeding rodents high levels of methionine have shown that methionine promotes atherosclerotic plaques independently of homocysteine levels 41. A similar study in Finnish men showed the same effect where it was found that “long-term, moderately high dietary methionine intake may increase the risk of acute coronary events in middle-aged Finnish men free of prior coronary heart disease”42.
Methionine has gained renewed interest in recent years largely because of their relation to homocysteine and glutathione (see Figure 1). Homocysteine is a central product in the metabolic pathway of methionine metabolism, and hyperhomocysteinemia has been identified as an independent risk factor for cardiovascular disease in adults 21, ischemic and hemorrhagic stroke in newborns and children 22 and Alzheimer’s disease in adults 23. Glutathione, the most prevalent intracellular thiol 30 and an important endogenous antioxidant and scavenger 31, is synthesized de novo within all cells 32, and intracellular availability of cysteine is believed to be the most important rate-limiting factor for glutathione synthesis 27. More importantly, glutathione concentrations are reduced in several disease states including HIV 33, liver cirrhosis 34, diabetes 35 and Alzheimer’s disease 36. Glutathione concentration is also found to be reduced in surgical trauma patients 37, septic patients 38, premature infants 39, and in children with severe protein–energy malnutrition 40. Comprehensive understanding of sulfur amino acid requirements and their complex metabolism clearly has many implications for human health.
Toxicity of methionine in liver disease was established halfway through the twentieth century by Dame Sheila Sherlock 45, who recognized that high methionine intake (∼10 g per day or 133 mg/kg per day for a 75 kg person) in patients with impaired liver function resulted in hyperammonemia and a deterioration of neurological functions, which could be alleviated with antibiotics. In addition, high levels of methionine may be directly hepatotoxic, and it has been suggested from results of animal studies that high amounts of methionine in feeding formulas are causally related to the onset of cholestasis during tube feeding or parenteral feeding 46. It has been shown that there is no relation between methionine intake and hyperhomocysteinemia, provided that vitamin status is optimal. In most cases hyperhomocysteinemia can be treated by vitamin B-12 (Cobalamin), vitamin B-6 (Pyridoxine), and/or folate (vitamin B-9) supplementation 47. In the old literature it is mentioned that methionine restriction and cysteine supplementation can prevent cognitive retardation in children with inborn errors of sulfur amino acid metabolism 48.
Oral methionine supplements (8.0 g daily for 4 days) were given to five normal volunteers who continued to eat their usual diet 49. This treatment resulted in a significant fall in serum folate (vitamin B-9) concentration. Three days after the end of treatment concentrations had not completely returned to control values. The fall in concentration was prevented by giving oral folic acid supplements. The study authors suggested that folic acid supplements should be given to patients who are receiving intravenous infusions of methionine amino acid mixtures.
As an essential amino acid, methionine is a standard component of artificial feeding formulas. According to the reported methionine amount and dose recommendation of standard tube feeds (Table 2), the daily amount of methionine administered to enterally or parenterally fed patients is ∼26 mg/kg per day, which apparently is twice the minimally required amount. Disease-specific feeds for malnutrition or metabolic stress are even higher in total protein and sulfur amino acid content and provide ∼4 times the recommended daily dose of sulfur amino acid 10.
Amino acid concentrations of pediatric protein and hydrolysate-based enteral formulas exceed that of breast milk, leading to a moderate increase of plasma levels of amino acids and urea nitrogen in enterally fed children 50. The consequences of this mild elevation of blood nitrogen concentration are difficult to assess in the light of the other differences between formula feeds and mother milk. However, no clinical data indicating specific amino acid toxicity in formula-fed children without errors in metabolism are known.
Methionine intake in infants at 14–28 days with various formulas may reach up to 120 mg/kg per day 51. A methionine-fortified hydrolysate providing up to 260 mg/kg per day to newborn healthy children was shown to induce only moderate elevations in methionine plasma level and no signs of methionine toxicity 52. In a retrospective case study of 10 children without enzyme deficiencies receiving the same methionine-fortified formula, however, severe hypermethionemia was found, which may have induced brain edema in 2 patients 51. A conclusive explanation for the increased sensitivity to high methionine intake was not given, but these findings led the authors to suggest that precaution is especially warranted in premature children, children with a very low birth weight, and children with liver disease.
Table 2. Sulfur amino acid content of commercially available feeding solutions
| Specific indication | Protein source, g/1000 mL | Sulfur amino acids, mg/100 mL | ||||
|---|---|---|---|---|---|---|
| Whey | Soy | Casein | Methionine | Cysteine | Total sulfur amino acid (SAA) | |
| General TPN (Total Parenteral Nutrition) and EN (Enteral Nutrition) | 4 | 133 | 12 | 145 | ||
| General TPN (Total Parenteral Nutrition) and EN (Enteral Nutrition) | 0.6 | 3.1 | 112 | 17 | 129 | |
| Ventilated ICU patients | 6.3 | 207 | 19 | 226 | ||
| Metabolic stress | 2.9 | 6.1 | 209 | 19 | 228 | |
| Malnourished cancer patients | 6.3 | 263 | 27 | 290 | ||
Footnotes: Typical sulfur-containing amino acid (SAA) content of some commercially available feeding solutions based on sulfur-containing amino acid (SAA) content of protein source.
[Source 10 ]- Ronald O. Ball, Glenda Courtney-Martin, Paul B. Pencharz; The In Vivo Sparing of Methionine by Cysteine in Sulfur Amino Acid Requirements in Animal Models and Adult Humans, The Journal of Nutrition, Volume 136, Issue 6, 1 June 2006, Pages 1682S–1693S, https://doi.org/10.1093/jn/136.6.1682S[↩][↩][↩]
- Navik U, Sheth VG, Khurana A, Jawalekar SS, Allawadhi P, Gaddam RR, Bhatti JS, Tikoo K. Methionine as a double-edged sword in health and disease: Current perspective and future challenges. Ageing Res Rev. 2021 Dec;72:101500. doi: 10.1016/j.arr.2021.101500[↩]
- Fomon SJ, Ziegler EE, Nelson SE, Edwards BB. Requirement for sulfur-containing amino acids in infancy. J Nutr. 1986;116:1405–22.[↩]
- Martensson J, Larsson J, Schildt B. Metabolic effects of amino acid solutions in severely burned patients: with emphasis on sulfur amino acid metabolism and protein breakdown. J Trauma. 1985;25:427–32.[↩]
- Sanderson SM, Gao X, Dai Z, Locasale JW. Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nat. Rev. Cancer. 2019;19:625–637. doi: 10.1038/s41568-019-0187-8[↩]
- Kanarek N, Petrova B, Sabatini DM. Dietary modifications for enhanced cancer therapy. Nature. 2020;579:507–517. doi: 10.1038/s41586-020-2124-0[↩]
- Rose WC. Amino acid requirements of man. Nutr Rev. 1976;34:307–9.[↩]
- Jackson AA, Gibson NR, Lu Y, Jahoor F. Synthesis of erythrocyte glutathione in healthy adults consuming the safe amount of dietary protein. Am J Clin Nutr. 2004;80:101–7.[↩]
- Paoletti A., Pencharz P.B., Ball R.O., Kong D., Xu L., Elango R., Courtney-Martin G. The Dietary Requirement for Total Sulphur Amino Acids in Adults ≥60 Years Appears Higher in Males than in Females. Am. J. Clin. Nutr. 2023;118:538–548. doi: 10.1016/j.ajcnut.2023.06.015[↩]
- Marcel C. G. van de Poll, Cornelis H. C. Dejong, Peter B. Soeters; Adequate Range for Sulfur-Containing Amino Acids and Biomarkers for Their Excess: Lessons from Enteral and Parenteral Nutrition, The Journal of Nutrition, Volume 136, Issue 6, 1 June 2006, Pages 1694S–1700S, https://doi.org/10.1093/jn/136.6.1694S[↩][↩][↩][↩][↩]
- Erden-Inal M, Sunal E, Kanbak G. Age-related changes in the glutathione redox system. Cell Biochem Funct. 2002;20:61–6.[↩]
- Fukagawa NK, Galbraith RA. Advancing age and other factors influencing the balance between amino acid requirements and toxicity. J Nutr. 2004;134:1569S–74S.[↩]
- Gao X, Sanderson SM, Dai Z, Reid MA, Cooper DE, Lu M, Richie JP Jr, Ciccarella A, Calcagnotto A, Mikhael PG, Mentch SJ, Liu J, Ables G, Kirsch DG, Hsu DS, Nichenametla SN, Locasale JW. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature. 2019 Aug;572(7769):397-401. doi: 10.1038/s41586-019-1437-3[↩][↩][↩][↩]
- Parkhitko A.A., Jouandin P., Mohr S.E. Methionine metabolism and methyltransferases in the regulation of aging and lifespan extension across species. Aging Cell. 2019;18:e13034. doi: 10.1111/acel.13034[↩]
- Ducker GS, Rabinowitz JD. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017 Jan 10;25(1):27-42. doi: 10.1016/j.cmet.2016.08.009[↩][↩]
- Min H, Kim J, Lee M, Kang S, Shim YH. Methionine Supplementation Alleviates the Germ Cell Apoptosis Increased by Maternal Caffeine Intake in a C. elegans Model. Nutrients. 2024 Mar 20;16(6):894. doi: 10.3390/nu16060894[↩][↩]
- Song B., Zeng Q., Liu Y., Wu B. Effect of methionine deficiency on the apoptosis and cell cycle of kidney in broilers. Res. Vet. Sci. 2021;135:228–236. doi: 10.1016/j.rvsc.2019.09.013[↩]
- Kalvandi O., Sadeghi A., Karimi A. Methionine supplementation improves reproductive performance, antioxidant status, immunity and maternal antibody transmission in breeder Japanese quail under heat stress conditions. Arch. Anim. Breed. 2019;62:275–286. doi: 10.5194/aab-62-275-2019[↩]
- Luiking YC, Deutz NE, Jakel M, Soeters PB. Casein and soy protein meals differentially affect whole-body and splanchnic protein metabolism in healthy humans. J Nutr. 2005;135:1080–7[↩][↩]
- Finkelstein JD. Pathways and regulation of homocysteine metabolism in mammals. Semin Thromb Hemost. 2000;26:219–25.[↩]
- Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62.[↩][↩]
- Hogeveen M, Blom HJ, van Amerongen M, Boograms B, van Beynum IM, van de Bor M. Hyperhomocysteinemias a risk factor for ischemic and hemorrhagic stroke in newborn infants. J Pediatr. 2002;141:429–31.[↩][↩]
- Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–77.[↩][↩]
- Verhoef P et al; Am J Clin Nutrition 80 (3): 674-9, 2004[↩][↩]
- Peter J. Garlick; Toxicity of Methionine in Humans, The Journal of Nutrition, Volume 136, Issue 6, 1 June 2006, Pages 1722S–1725S, https://doi.org/10.1093/jn/136.6.1722S[↩][↩][↩][↩][↩][↩][↩]
- Griffith O. Mammalian sulfur amino acid metabolism: an overview. Methods Enzymol. 1987;143:366–76.[↩]
- Lyons J, Rauh-Pfeiffer A, Yu UM, Lu XM, Zurakowski D, Tompkins RG, Ajami AM, Young VR. Blood glutathione synthesis rates in healthy adults receiving a sulfur amino acid-free diet. Proc Natl Acad Sci USA. 2000;97:5071–6.[↩][↩][↩]
- Du Vigneaud V, Kilmer GW, Rachele JR, Cohn M. On the mechanism of the conversion in vivo of methionine to cystine. J Biol Chem. 1944;155:645–51.[↩][↩]
- Rose WC. The nutritive significance of the amino acids. Physiol Rev. 1938;18:109–36.[↩]
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–60.[↩][↩]
- Wernerman J, Floke H. Modulation of endogenous glutathione availability. Curr Opin Clin Nutr Metab Care. 1999;2:487–93.[↩][↩]
- Reid M, Jahoor F. Methods of measuring glutathione concentration and rate of synthesis. Curr Opin Clin Nutr Metab Care. 2000;3:385–90.[↩][↩]
- Jahoor F, Jackson A, Gazzard B, Philips G, Sharpstone D, Frazer ME, Heird W. Erythrocyte glutathione deficiency in symptom-free HIV infection is associated with decreased synthesis rate. Am J Physiol. 1999;276:E205–11.[↩][↩]
- Bianchi G, Brizi M, Rossi B, Ronchi M, Grossi G, Marchesini G. Synthesis of glutathione in response to methionine load in control subjects and in patients with cirrhosis. Metabolism. 2000;49:1434–9.[↩][↩]
- Ghosh S, Ting S, Lau H, Pulinilkunnil T, An D, Qi D, Abrahani MA, Rodrigues B. Increased efflux of glutathione conjugates in acutely diabetic cardiomyocytes. Can J Physiol Pharmacol. 2004;82:879–87.[↩][↩]
- Liu H, Harrell LE, Shenvi S, Hagen T, Liu RM. Gender differences in glutathione metabolism in Alzheimer’s disease. J Neurosci Res. 2005;79:861–7.[↩][↩]
- Luo JL, Hammarqvist F, Andersson K, Wernerman J. Surgical trauma decreases glutathione synthetic capacity in human skeletal muscle tissue. Am J Physiol Endocrinol Metab. 1998;275:E359–65.[↩][↩]
- Lyons J, Rauh-Pfeiffer A, Ming-Lu Y, Lu XM, Zurakowski D, Curley M, Collier S, Duggan C, Nurko S, et al. Cysteine metabolism and whole blood glutathione synthesis in septic pediatric patients. Crit Care Med. 2001;29:870–7.[↩][↩]
- Vina J, Vento M, Garcia-Sala F, Puertes IR, Gasco E, Sastre J, Asensi M, Pallardo FV. L-Cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency. Am J Clin Nutr. 1995;61:1067–69.[↩][↩]
- Reid M, Badaloo A, Forrester T, Morlese JF, Frazer M, Heird WC, Jahoor F. In vivo rates of erythrocyte glutathione synthesis in children with severe protein-energy malnutrition. Am J Physiol Endocrinol Metab. 2000;278:E405–12.[↩][↩]
- Sulfur amino acids and atherosclerosis: a role for excess dietary methionine. Ann N Y Acad Sci. 2016 Jan;1363:18-25. https://doi.org/10.1111/nyas.12962[↩][↩]
- High dietary methionine intake increases the risk of acute coronary events in middle-aged men. Nutr Metab Cardiovasc Dis. 2006 Mar;16(2):113-20. Epub 2005 Nov 2. https://www.nmcd-journal.com/article/S0939-4753(05)00109-2/fulltext[↩][↩]
- Tian W, Ju J, Guan B, Wang T, Zhang J, Song L, Xu H. Role of hyperhomocysteinemia in atherosclerosis: from bench to bedside. Ann Med. 2025 Dec;57(1):2457527. doi: 10.1080/07853890.2025.2457527[↩]
- United States Department of Agriculture Agricultural Research Service. USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/search/list[↩]
- Phear EA, Ruebner B, Sherlock S, Summerskill WH. Methionine toxicity in liver disease and its prevention by chlortetracycline. Clin Sci (Lond). 1956;15:93–117.[↩]
- Moss RL, Haynes AL, Pastuszyn A, Glew RH. Methionine infusion reproduces liver injury of parenteral nutrition cholestasis. Pediatr Res. 1999;45:664–8.[↩]
- Righetti M, Tommasi A, Lagona C, La Rosa L, Uccellini M, Sessa A. Effective homocysteine-lowering vitamin B treatment in peritoneal dialysis patients. Perit Dial Int. 2004;24:373–7.[↩]
- Perry TL, Hansen S, Love DL, Crawford LE, Tischler B. Treatment of homocystinuria with a low-methionine diet, supplemental cystine, and a methyl donor. Lancet. 1968;2:474–8.[↩]
- Connor H, Newton DJ, Preston FE, Woods HF. Oral methionine loading as a cause of acute serum folate deficiency: its relevance to parenteral nutrition. Postgraduate Medical Journal. 1978;54(631):318-320. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2425156/pdf/postmedj00257-0026.pdf[↩]
- Hernell O, Lonnerdal B. Nutritional evaluation of protein hydrolysate formulas in healthy term infants: plasma amino acids, hematology, and trace elements. Am J Clin Nutr. 2003;78:296–301.[↩]
- Harvey Mudd S, Braverman N, Pomper M, Tezcan K, Kronick J, Jayakar P, Garganta C, Ampola MG, Levy HL, et al. Infantile hypermethioninemia and hyperhomocysteinemia due to high methionine intake: a diagnostic trap. Mol Genet Metab. 2003;79:6–16.[↩][↩]
- Borschel MW, Baggs GE. Cysteine (Cys) and methionine (Met) intakes associated with normal growth of healthy, term infants fed casein hydrolysate formula (CHF) [abstract]. FASEB J. 1998;12:A848.[↩]