Contents
- Multiple myeloma
- The Lymphatic System
- There are several types of plasma cell cancers
- Multiple myeloma symptoms and signs
- Multiple myeloma causes
- Complications of multiple myeloma
- Multiple Myeloma Diagnosis
- Multiple Myeloma Stages
- Multiple myeloma treatment
- Drug therapy
- Table 6. Major Treatment Regimens in Multiple Myeloma
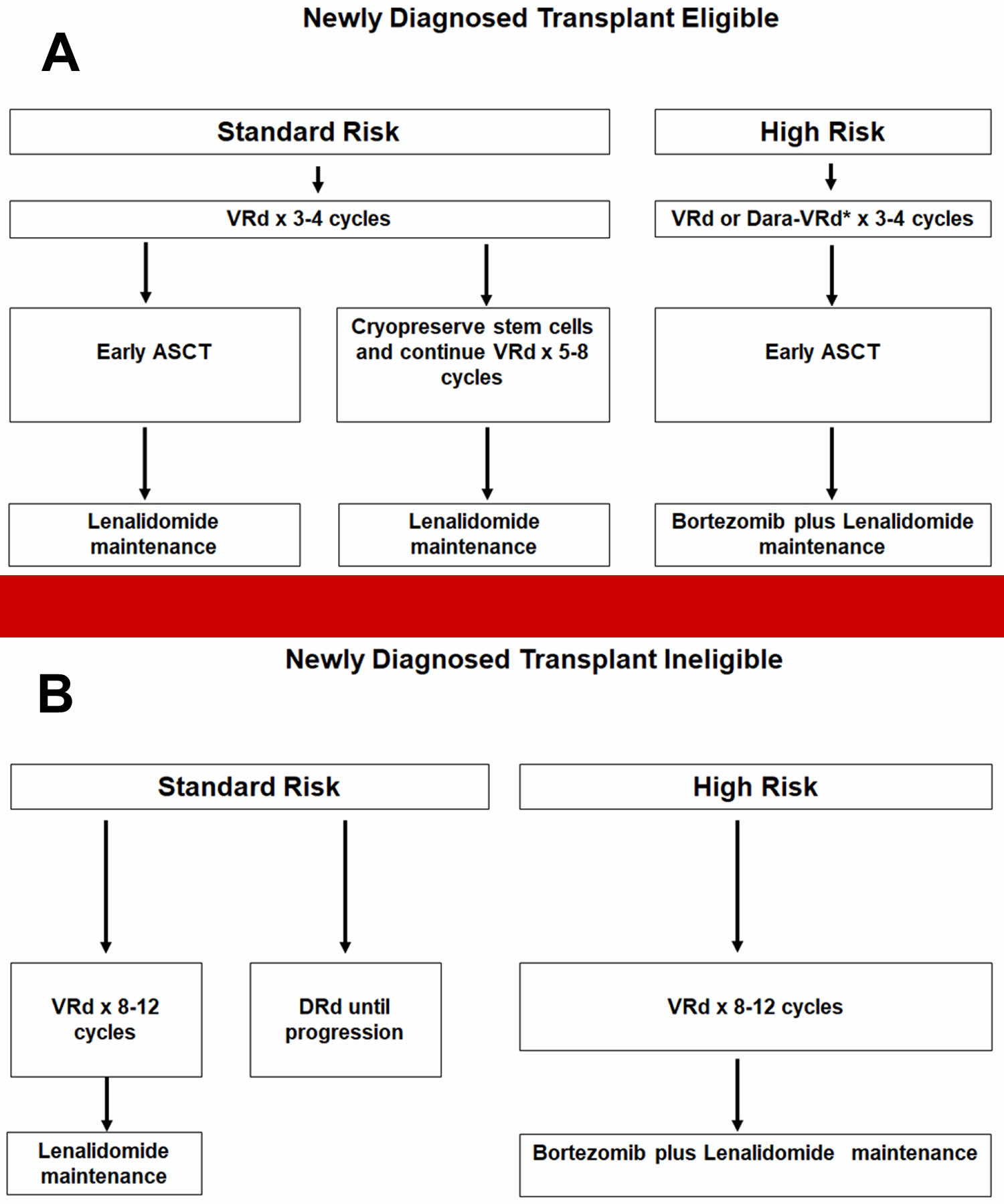
- Figure 13. Newly diagnosed multiple myeloma treatment algorithm
- Chemotherapy
- Corticosteroids (steroids)
- Immunomodulating agents
- Proteasome inhibitors
- Monoclonal antibodies
- Antibody-drug conjugates
- Nuclear export inhibitor
- Immunotherapy
- Bisphosphonates for bone disease
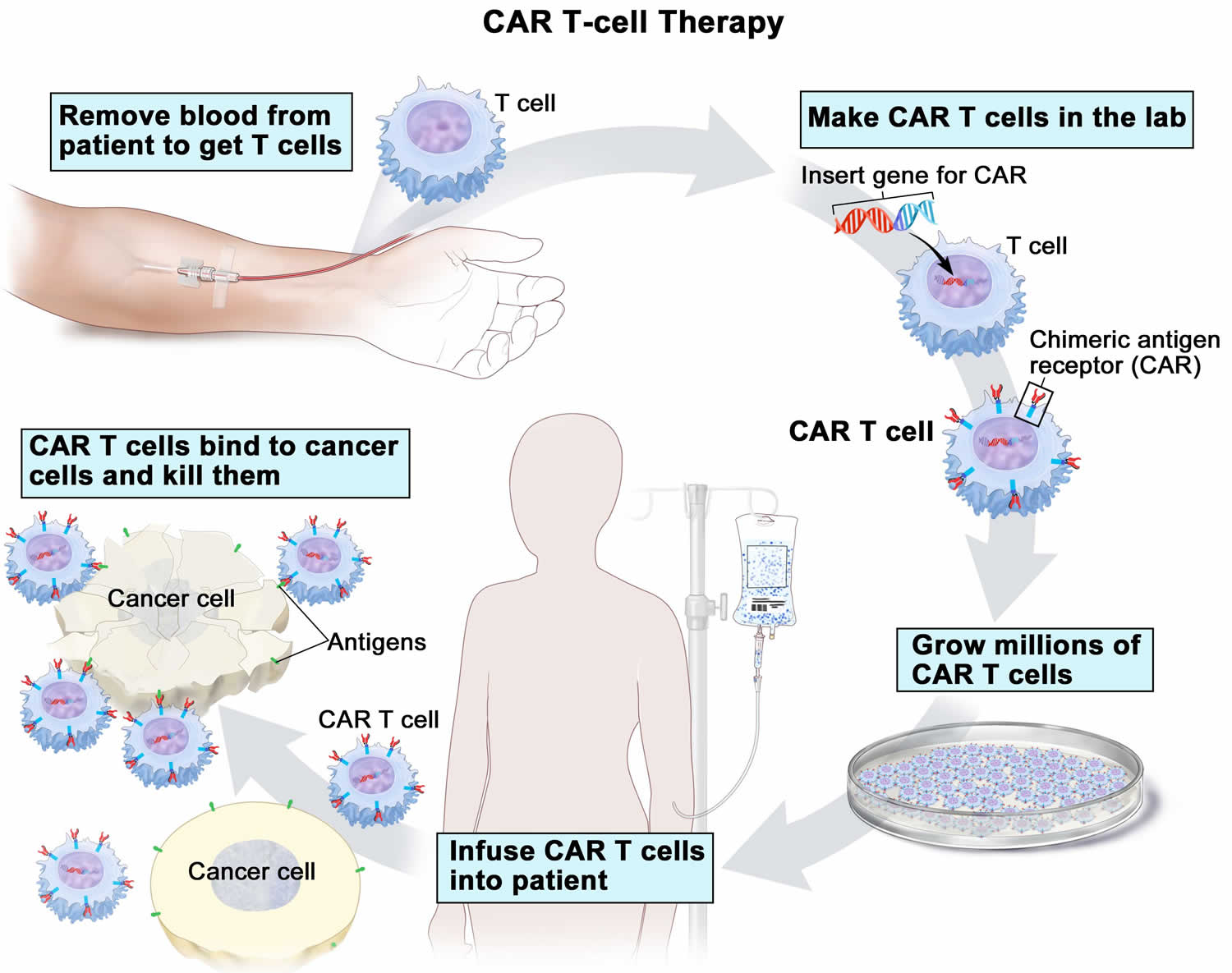
- CAR T-cell therapy
- Radiation therapy
- Stem Cell Transplant
- Supportive treatments
- Surgery
- Clinical trial
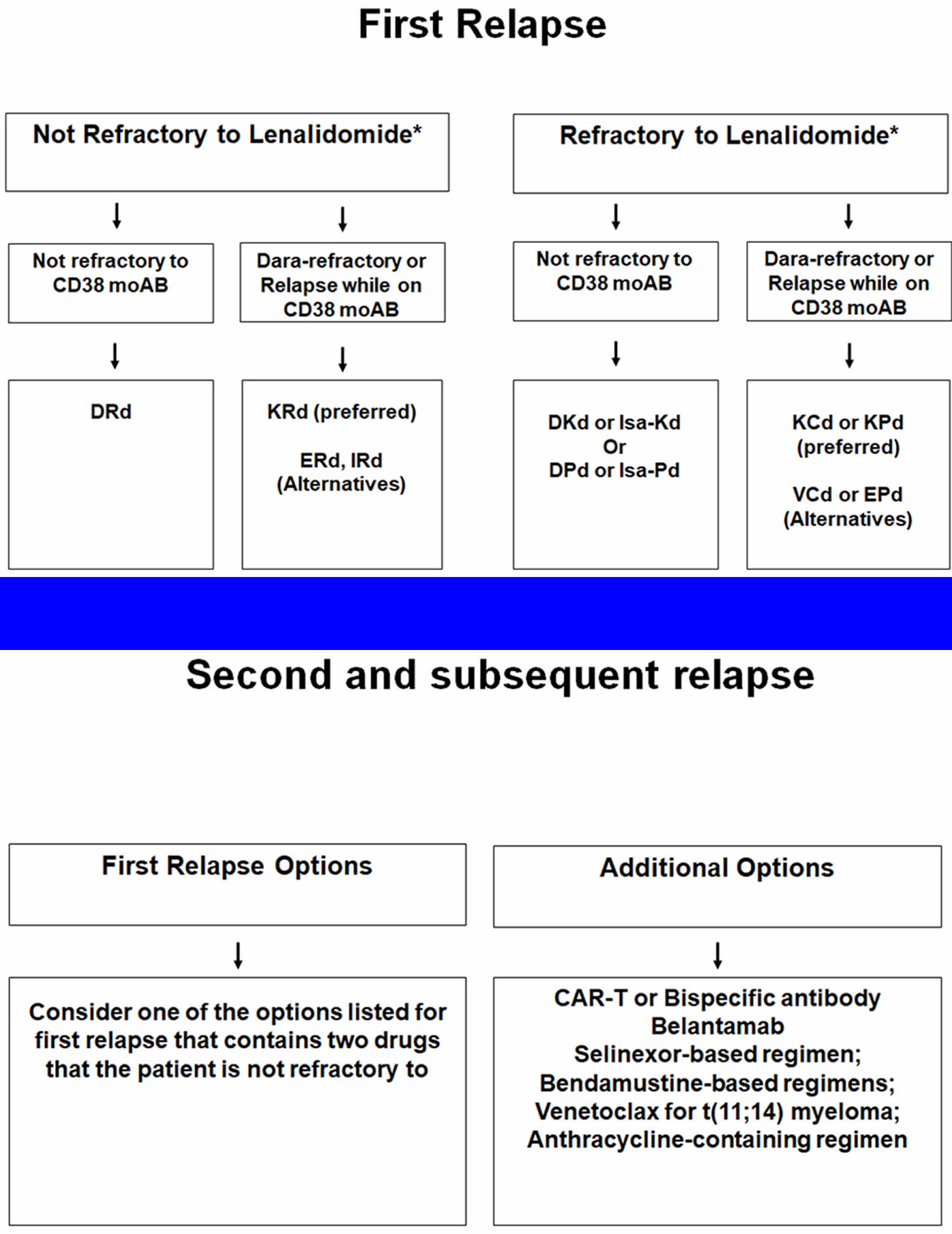
- Treatment of relapsed or refractory multiple myeloma
- Alternative medicine
- Treating complications
- Coping and support
- Treatment of Monoclonal Gammopathy of Undetermined Significance (MGUS)
- Treatment of Isolated Plasmacytoma of Bone
- Treatment of Extramedullary Plasmacytoma
- Drug therapy
- Multiple myeloma prognosis
- Multiple myeloma survival rates
Multiple myeloma
Multiple myeloma also known as plasma cell myeloma or myeloma, is a type of blood cancer that develops from cells in the bone marrow called plasma cells. Plasma cells are part of the immune system that develop from B lymphocytes (B cells), a type of white blood cell that is made in the bone marrow. Normally, when bacteria or viruses enter your body, some of the B cells (B lymphocytes) will change into plasma cells. The normal plasma cells make antibodies (also called immunoglobulins or Ig) to fight bacteria and viruses, to stop infection and disease. Each type of plasma cell make different antibodies (immunoglobulins) for different infections. Antibodies (immunoglobulins) attack and help to kill bacteria and viruses and so protect you from infections. They work with other parts of the immune system to help protect the body from germs and other harmful substances.
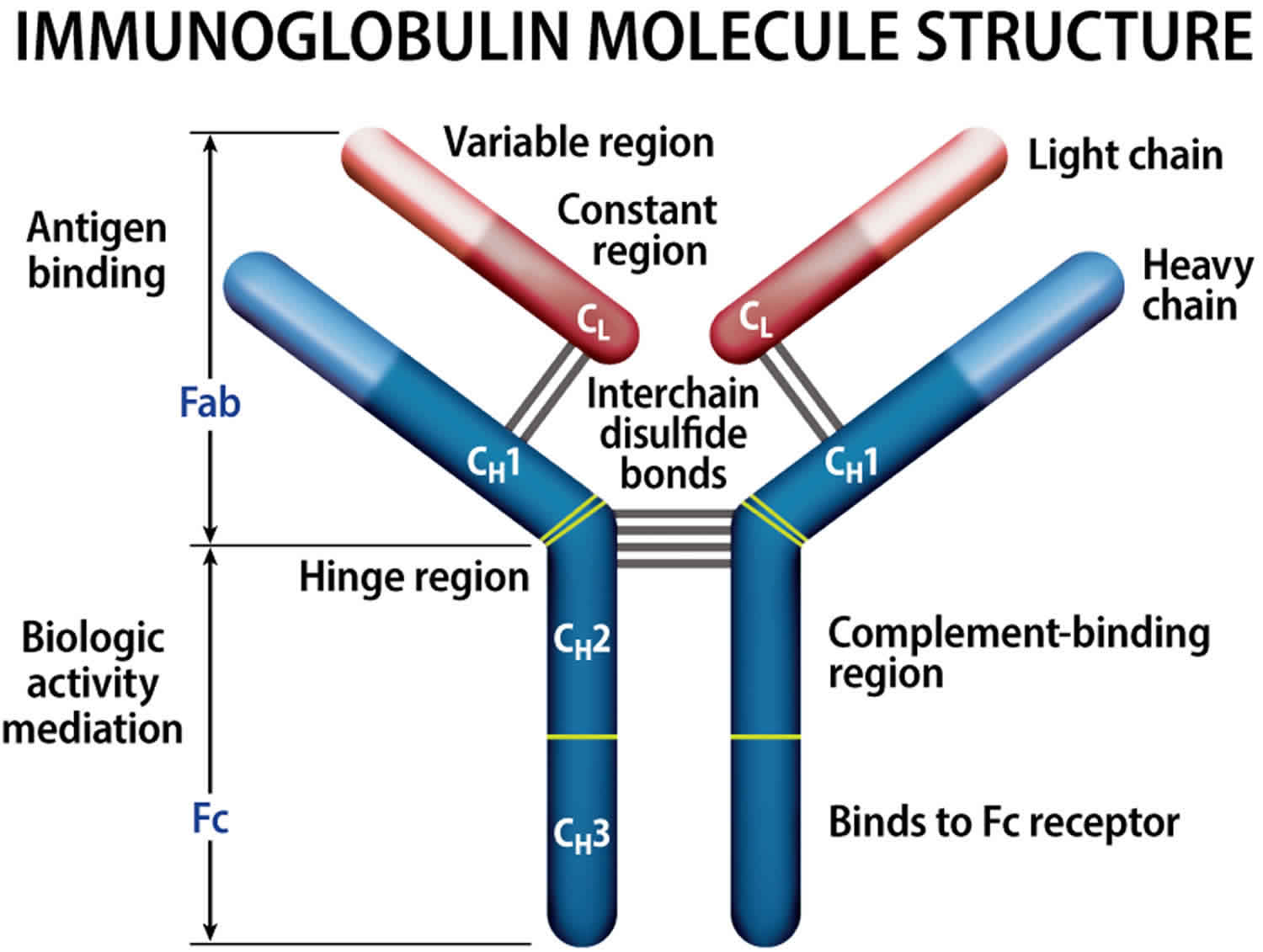
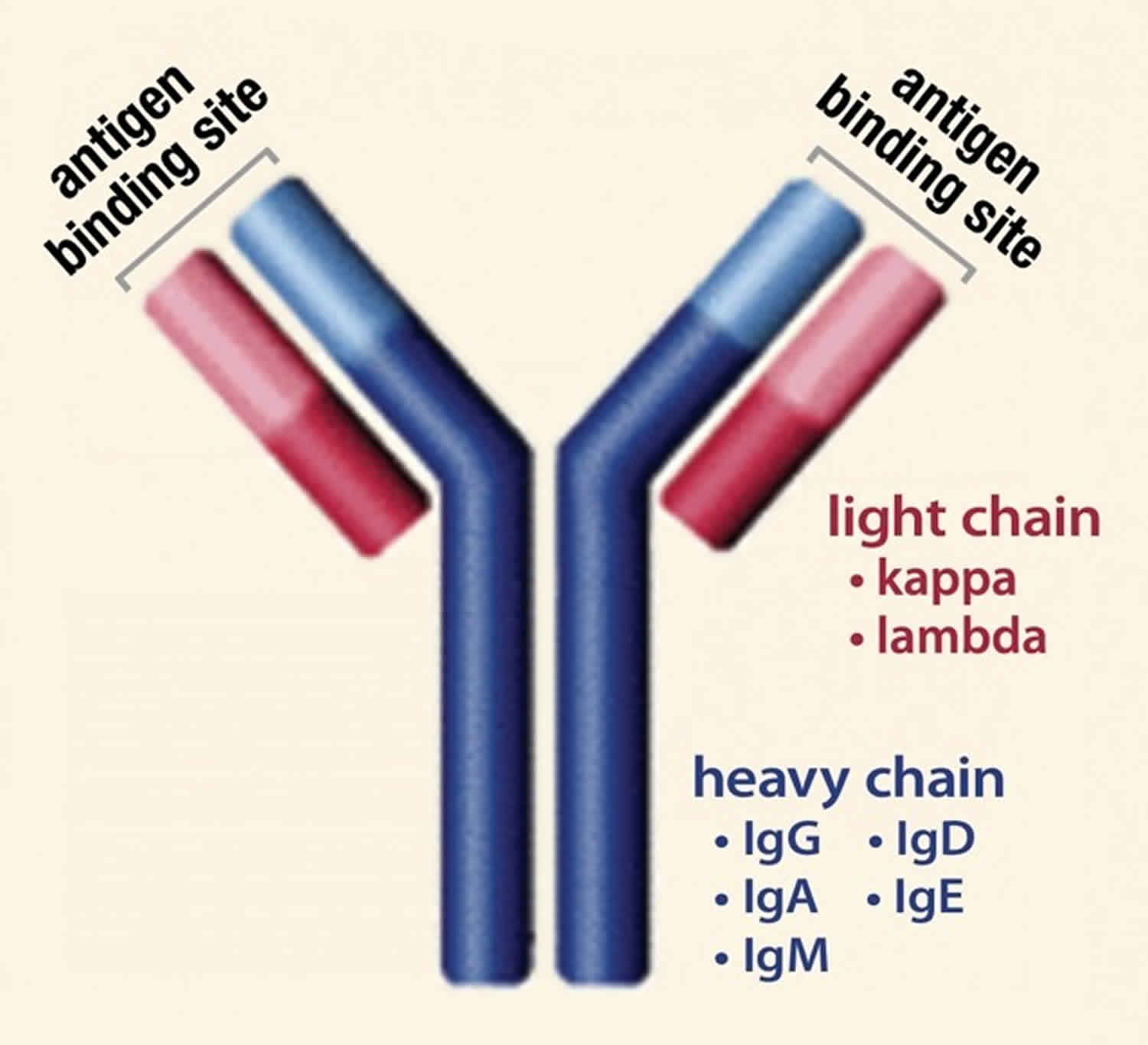
Normally, the body makes five different types of immunoglobulins – immunoglobulin G (Ig G), immunoglobulin M (Ig M), immunoglobulin A (Ig A), immunoglobulin E (Ig E) and immunoglobulin D (Ig D). Each one has slightly different immune system functions. Each type of immunoglobulin is composed of four protein chains – two identical heavy (long) protein chains and two identical light (shorter) protein chains (see Figure 8 below). The heavy chains may consist of one of five different types that correspond with the type of immunoglobulin produced: gamma (IgG), mu (IgM), alpha (IgA), epsilon (IgE) and delta (IgD). The light chains consist of one of two different types called kappa and lambda.
Within a plasma cell, two heavy chains of one type and two light chains of one type become attached to form one intact immunoglobulin molecule. Each particular plasma cell will produce only one type of immunoglobulin.
Myeloma begins when a plasma cell becomes abnormal. The abnormal cell divides to make copies of itself. These abnormal plasma cells are called myeloma cells. The cancerous plasma cells (myeloma cells) also make an abnormal monoclonal antibody protein known by several different names, including monoclonal immunoglobulin, monoclonal protein, myeloma protein, M-protein, M-spike, or paraprotein 1. The M protein (monoclonal protein) does not work properly, is not needed by the body and does not help fight infection as well as other antibodies. These monoclonal antibody protein (M protein or abnormal monoclonal immunoglobulins) build up in the bone marrow and can cause your blood to thicken or can damage your kidneys.
In time, myeloma cells collect in the bone marrow. They may damage the solid part of the bone. When myeloma cells collect in several of your bones (e.g., spine, skull, pelvis and ribs), the disease is called “multiple myeloma.”
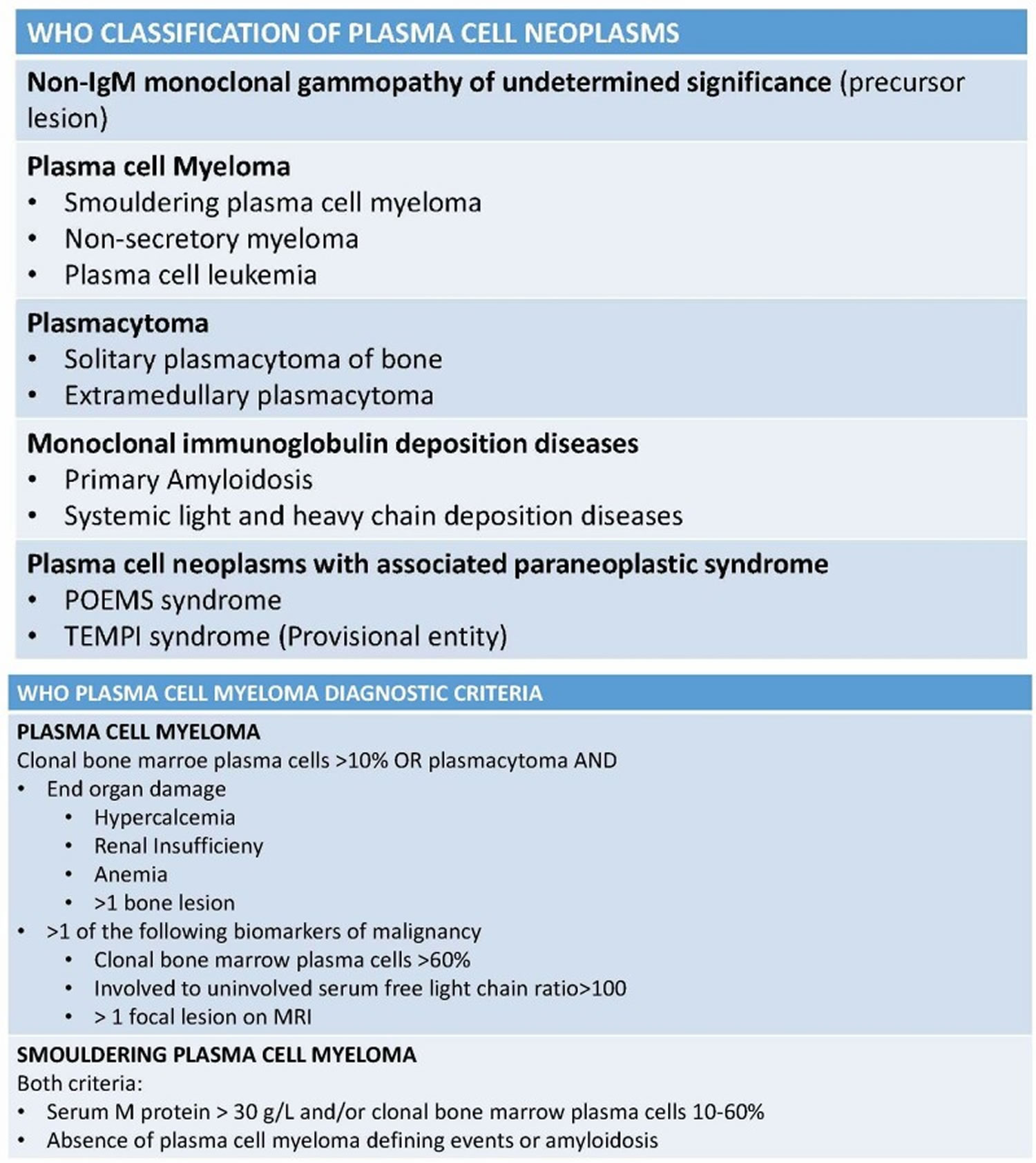
There are, however, other plasma cell neoplasms that also have abnormal plasma cells (myeloma cells) but do not meet the criteria to be called active multiple myeloma. Plasma cell neoplasms can be benign (not cancer) or malignant (cancer). According to the World Health Organization (WHO) classification in 2017 (see Figure 12 below), plasma cell neoplasms includes non-Ig-M monoclonal gammopathy of undetermined significance (MGUS), plasmacytoma, monoclonal immunoglobulin deposition diseases and multiple myeloma (plasma cell myeloma) and plasma cell neoplasms with the associated paraneoplastic syndromes 2, 3.
Multiple myeloma is the most common disease entity in this category because it is symptomatic and well-studied 3. Multiple myeloma is a relatively uncommon cancer comprising about 1% of malignant tumors, 10% to 15% of hematopoietic neoplasms, and causes up to 20% of the deaths in the hematologic malignancies category. In the United States, the lifetime risk of getting multiple myeloma is 1 in 132 (0.76%) 4. Multiple myeloma is a disease of older adults (greater than 50 years), with a median age at diagnosis of 66 years 3. There is increased risk in males and African Americans. Non-Ig-M monoclonal gammopathy of undetermined significance (MGUS) is a plasma cell myeloma precursor disease and presents in up to 4% of plasma cell neoplasms 5.
The American Cancer Society’s estimates for multiple myeloma in the United States for 2022 are 4, 6, 7:
- New cases: About 34,470 new cases will be diagnosed (19,100 in men and 15,370 in women).
- Deaths: About 12,640 deaths are expected to occur (7,090 in men and 5,550 in women).
- 5-year relative survival (based on cases diagnosed from 2012 to 2018 data): 57.9% (all stages combined); 78% for localized disease and 55% for distant disease. Relative survival is an estimate of the percentage of patients who would be expected to survive the effects of their cancer. It excludes the risk of dying from other causes. Because survival statistics are based on large groups of people, they cannot be used to predict exactly what will happen to an individual patient. No two patients are entirely alike, and treatment and responses to treatment can vary greatly.
- Percentage of All Cancer Deaths: 2.1%.
- Rate of New Cases and Deaths per 100,000: The rate of new cases of myeloma was 7.1 per 100,000 men and women per year. The death rate was 3.1 per 100,000 men and women per year. These rates are age-adjusted and based on 2015–2019 cases and 2016–2020 deaths.
- Lifetime Risk of Developing Cancer: Approximately 0.8 percent of men and women will be diagnosed with myeloma at some point during their lifetime, based on 2017–2019 data.
- In 2019, there were an estimated 159,787 people living with myeloma in the United States.
Multiple myeloma has the following three variants 8:
- Asymptomatic (smoldering) plasma cell myeloma show classic M protein and bone marrow findings with no symptoms.
- Non-secretory myeloma shows an absence of an M-protein; however, elevated serum free light chains and/or an abnormal free light chain ratio are detectable.
- Plasma cell leukemia (PCL) shows extensive and diffuse involvement by clonal plasma cells (greater than 60%) and association with extramedullary tissues infiltration as well as increased incidence of CRAB symptoms.
Doctors sometimes refer to the acronym, CRAB, to describe signs of multiple myeloma. The letters stand for:
- C – Calcium elevation (high levels of calcium in the blood; also known as “hypercalcemia”)
- R– Renal insufficiency (poor function of the kidneys that may be due to a reduction in blood-flow to the kidneys)
- A – Anemia (low red blood cell counts)
- B – Bone abnormalities (lesions).
Patients with one or more of these CRAB criteria, or with recurrent infections, are considered to have disease that requires treatment. Some patients have abnormalities which predict a very high probability of progression to active myeloma. Although they do not have symptoms, they may still require treatment. Other patients who do not exhibit any of these criteria are said to have “smoldering myeloma” or “asymptomatic myeloma,” and these patients may be followed with a watch-and-wait approach.
A diagnosis of multiple myeloma requires either 9:
- A plasma cell tumor (proven by biopsy) OR at least 10% plasma cells in the bone marrow AND
- At least one or more of the following CRAB features and myeloma-defining events:
- Calcium elevation (high blood calcium level or hypercalcemia) – serum calcium >0.25 mmol/L (> 1 mg/dL) higher than the upper limit of normal or > 2.75 mmol/L (> 11 mg/dL)
- Renal insufficiency – creatinine clearance < 40 mL per minute or serum creatinine > 177 umol/L (> 2 mg/dL)
- Anemia – hemoglobin concentration of > 2 g/dL below the lower limit of normal, or a hemoglobin concentration of < 10 g/dL
- Bony lesions – one or more osteolytic lesions found on skeletal radiography, CT or PET-CT scans. If bone marrow has <10% clonal plasma cells, more than one bone lesion is required to distinguish from solitary plasmacytoma with minimal marrow involvement.
- Any one or more of the following biomarkers of malignancy (myeloma-defining events):
- 60% or greater clonal plasma cells on bone marrow examination
- Serum involved / uninvolved free light chain ratio of 100 or greater, provided the absolute level of the involved light chain is at least 100mg/L (a patient’s involved free light chain either kappa or lambda is the one that is above the normal reference range; the uninvolved free light chain is the one that is typically in, or below, the normal range)
- More than one focal lesion on MRI that is at least 5mm or greater in size.
Treatment for multiple myeloma isn’t always necessary right away. If you have multiple myeloma but aren’t experiencing any symptoms (also known as smoldering multiple myeloma), you might not need treatment right away. Immediate treatment may not be necessary for multiple myeloma that is slow growing and at an early stage. However, your doctor will regularly monitor your condition for signs that the disease is progressing. This may involve periodic blood and urine tests.
If you’re experiencing symptoms, treatment can help relieve pain, control complications of the disease, stabilize your condition and slow the progress of multiple myeloma.
If you develop signs and symptoms or your multiple myeloma shows signs of progression, you and your doctor may decide to begin treatment.
For people with multiple myeloma who require treatment, a number of options are available to help control the disease include:
- Targeted therapy. Targeted drug treatments focus on specific weaknesses present within cancer cells. By blocking these abnormalities, targeted drug treatments can cause cancer cells to die.
- Immunotherapy. Immunotherapy uses your immune system to fight cancer. Your body’s disease-fighting immune system may not attack your cancer because the cancer cells produce proteins that help them hide from the immune system cells. Immunotherapy works by interfering with that process.
- Chemotherapy. Chemotherapy uses drugs to kill cancer cells. The drugs kill fast-growing cells, including myeloma cells. High doses of chemotherapy drugs are used before a bone marrow transplant.
- Corticosteroids. Corticosteroid medications regulate the immune system to control inflammation in the body. They are also active against myeloma cells.
- Bone marrow transplant. A bone marrow transplant, also known as a stem cell transplant, is a procedure to replace your diseased bone marrow with healthy bone marrow. Before a bone marrow transplant, blood-forming stem cells are collected from your blood. You then receive high doses of chemotherapy to destroy your diseased bone marrow. Then your stem cells are infused into your body, where they travel to your bones and begin rebuilding your bone marrow.
- Radiation therapy. Radiation therapy uses high-powered energy beams from sources such as X-rays and protons to kill cancer cells. It may be used to quickly shrink myeloma cells in a specific area — for instance, when a collection of abnormal plasma cells form a tumor (plasmacytoma) that’s causing pain or destroying a bone.
The treatment of multiple myeloma is usually done in phases:
- Induction therapy: This is the first phase of treatment. Its goal is to reduce the amount of disease, and may include one or more of the following:
- For younger, fit patients (eligible for a transplant):
- Chemotherapy.
- Targeted therapy with a proteasome inhibitor (bortezomib).
- Immunotherapy (lenalidomide).
- Corticosteroid therapy.
- For older, unfit patients (not eligible for a transplant):
- Chemotherapy.
- Targeted therapy with a proteasome inhibitor (bortezomib or carfilzomib) or a monoclonal antibody (daratumumab).
- Immunotherapy (lenalidomide).
- Corticosteroid therapy.
- For younger, fit patients (eligible for a transplant):
- Consolidation chemotherapy: This is the second phase of treatment. Treatment in the consolidation phase is to kill any remaining cancer cells. High-dose chemotherapy is followed by either:
- one autologous stem cell transplant, in which the patient’s stem cells from the blood or bone marrow are used; or
- two autologous stem cell transplants followed by an autologous or allogeneic stem cell transplant, in which the patient receives stem cells from the blood or bone marrow of a donor; or
- one allogeneic stem cell transplant.
- Maintenance therapy: After the initial treatment, maintenance therapy is often given to help keep the disease in remission for a longer time. Several types of treatment are being studied for this use, including the following:
- Chemotherapy.
- Immunotherapy (lenalidomide).
- Corticosteroid therapy.
- Targeted therapy with a proteasome inhibitor (bortezomib or ixazomib).
Figure 1. Blood composition
Note: Blood is a complex mixture of formed elements in a liquid extracellular matrix, called blood plasma. Note that water and proteins account for 99% of the blood plasma.
Figure 2. Bone marrow anatomy
Footnote: Anatomy of the bone. The bone is made up of compact bone, spongy bone, and bone marrow. Compact bone makes up the outer layer of the bone. Spongy bone is found mostly at the ends of bones and contains red marrow. Bone marrow is found in the center of most bones and has many blood vessels. There are two types of bone marrow: red and yellow. Red marrow contains blood stem cells that can become red blood cells, white blood cells, or platelets. Yellow marrow is made mostly of fat.
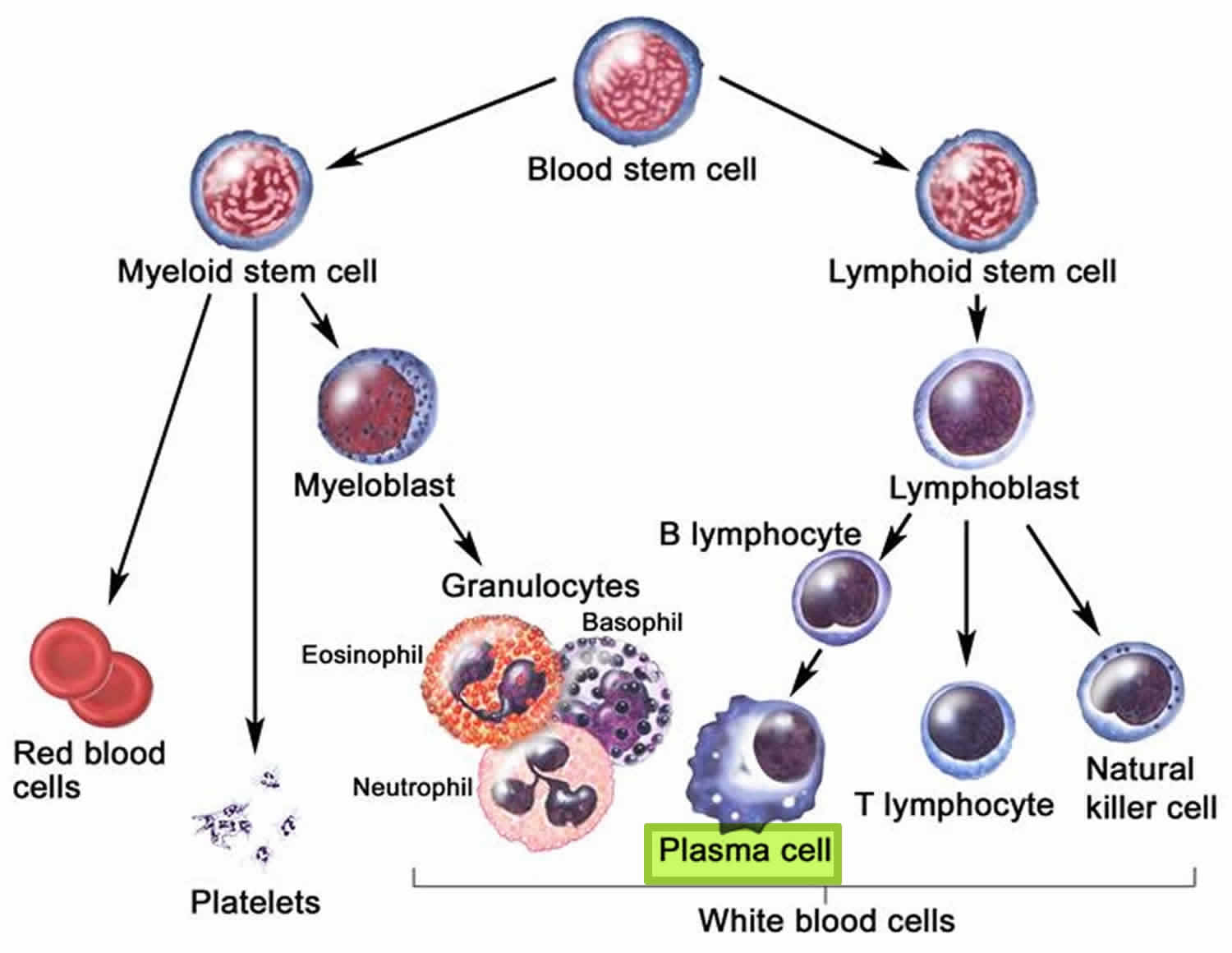
Figure 3. White blood cells development. A blood stem cell goes through several steps to become a red blood cell, platelet, or white blood cell
Figure 4. White blood cells development
Figure 5. Plasma cells
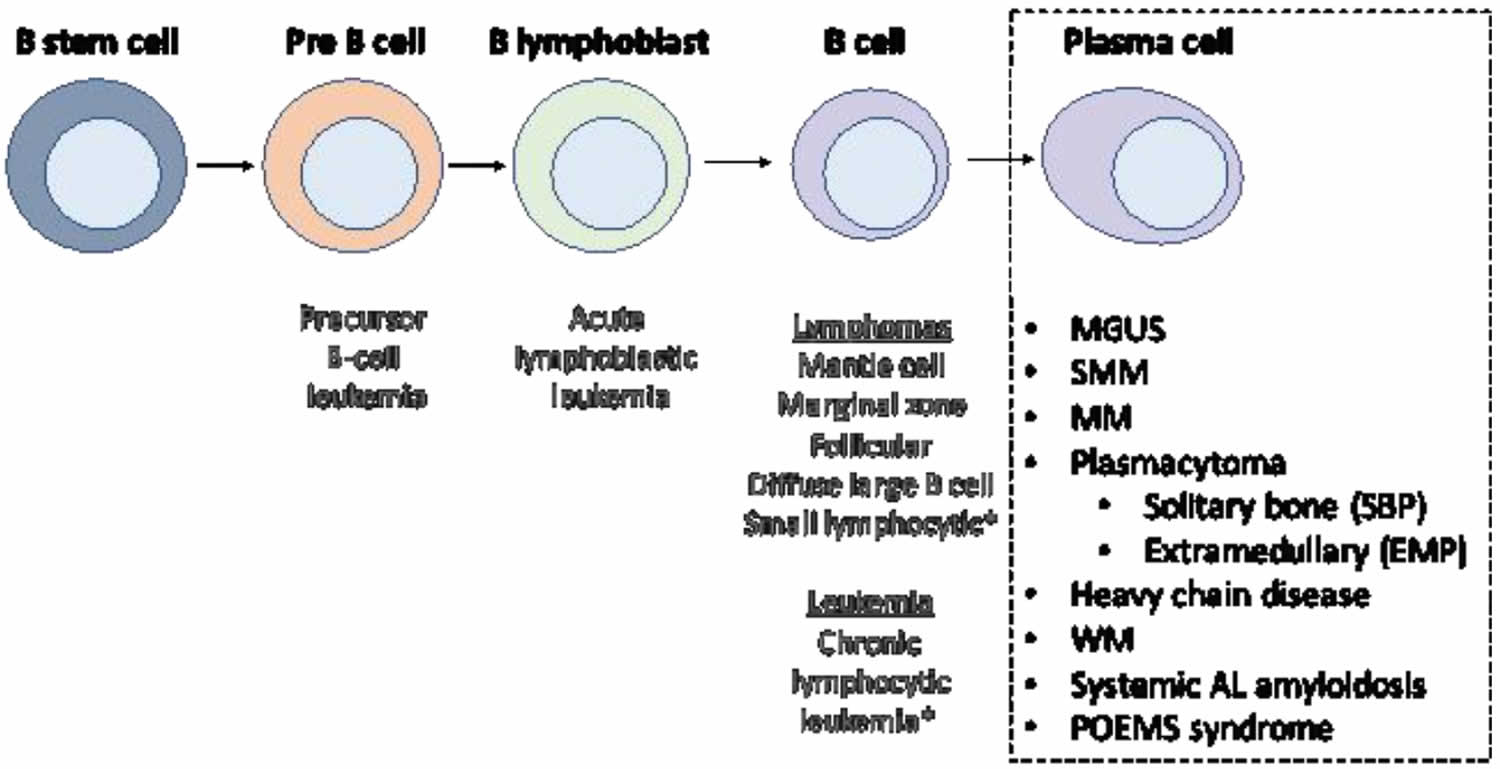
Figure 6. Stem cell origin of plasma cell neoplasms
Footnote: This figure demonstrates the origin of the monoclonal proliferation of plasma cells that characterizes plasma cell neoplasms.
[Source 10 ]Figure 7. Multiple myeloma plasma cells
Figure 8. Immunoglobulin structure
Can multiple myeloma be found early?
It’s difficult to diagnose multiple myeloma early. Often, multiple myeloma causes no symptoms until it reaches an advanced stage. Sometimes, it might cause vague symptoms that at first seem to be caused by other diseases. Sometimes, multiple myeloma is found early when a routine blood test shows an abnormally high amount of protein in the blood.
People with MGUS (monoclonal gammopathy of unknown significance) or solitary plasmacytoma are at risk of developing multiple myeloma and have regular bloodwork to monitor for it. Multiple myeloma may be diagnosed sooner in those people than those who did not have MGUS or a solitary plasmacytoma.
The Lymphatic System
To understand multiple myeloma, it helps to know about the lymph system (also known as the lymphatic system) and the functions of lymphoid tissue in the body.
The lymph system is part of the immune system, which helps fight infections and some other diseases. The lymphatic system plays a role in:
- fighting bacteria and other infections
- destroying old or abnormal cells, such as cancer cells
The lymphatic system also helps the flow of fluids in the body.
The lymph system is made up mainly of cells called lymphocytes, a type of white blood cell. There are 2 main types of lymphocytes:
- B lymphocytes (B cells): B lymphocytes (B cells) respond to an infection by changing into a different type of cell called a plasma cell. Plasma cells make proteins called antibodies (also called immunoglobulins) that help the body attack and kill disease-causing germs like bacteria and viruses.
- T lymphocytes (T cells): There are several types of T cells. Some T cells destroy germs or abnormal cells in the body. Other T cells help boost or slow the activity of other immune system cells.
- Natural killer (NK) cells, which attack virus-infected cells or tumor cells.
The lymphatic system is a vast collection of cells and biochemicals that travel in lymphatic vessels, and the organs and glands that produce them. The lymphatic system includes a network of vessels (like the arteries and veins that carry blood) that assist in circulating body fluids (a colorless liquid called lymph), so it is closely associated with your cardiovascular system. Lymphatic vessels transport excess fluid away from interstitial spaces in most tissues and return it to the bloodstream (Figure 5). This fluid carries food to the cells and bathes the body tissues to form tissue fluid. The fluid then collects waste products, bacteria, and damaged cells. It also collects any cancer cells if these are present. This fluid then drains into the lymph vessels. Without the lymphatic system, this fluid would accumulate in tissue spaces. Special lymphatic capillaries, called lacteals, are located in the lining of the small intestine. They absorb digested fats and transport them to the venous circulation.
The lymphatic system is a system of thin tubes and lymph nodes that run throughout the body. Lymph nodes are bean shaped glands. The thin tubes are called lymph vessels or lymphatic vessels. Tissue fluid called lymph circulates around the body in these vessels and flows through the lymph nodes.
The lymph system is an important part of your immune system. It plays a role in fighting bacteria and other infections and destroying old or abnormal cells, such as cancer cells.
The major sites of lymphoid tissue are:
- Lymph nodes: Lymph nodes are bean-sized collections of lymphocytes and other immune system cells throughout the body, including inside the chest, abdomen, and pelvis. They are connected to each other by a system of lymphatic vessels.
- Spleen: The spleen is an organ under the lower ribs on your left side. The spleen makes lymphocytes and other immune system cells. It also stores healthy blood cells and filters out damaged blood cells, bacteria, and cell waste.
- Bone marrow: The bone marrow is the spongy tissue inside certain bones. New blood cells (including some lymphocytes) are made there.
- Thymus: The thymus is a small organ behind the upper part of the breastbone and in front of the heart. Thymus is an organ in which T lymphocytes mature and multiply.
- Adenoids and tonsils: These are collections of lymphoid tissue in the back of your throat. They help make antibodies against germs that are breathed in or swallowed.
- Digestive tract: The stomach, intestines, and many other organs also have lymph tissue.
The lymphatic system has a second major function— it enables you to live in a world with different types of organisms. Some of them live in or on the human body and in some circumstances may cause infectious diseases. Cells and biochemicals of the lymphatic system launch both generalized and targeted attacks against “foreign” particles, enabling the body to destroy infectious agents. This immunity against disease also protects against toxins and cancer cells. When the immune response is abnormal, persistent infection, cancer, allergies, and autoimmune disorders may result.
The larger lymphatic vessels lead to specialized organs called lymph nodes. After leaving the lymph nodes, the vessels merge to form still larger lymphatic trunks.
Figure 9. Locations of major lymph nodes
Figure 10. Functions of lymph nodes in the lymphatic system
Figure 11. Schematic representation of lymphatic vessels transporting fluid from interstitial spaces to the bloodstream. Depending on its origin, lymph enters the right or left subclavian vein.
There are several types of plasma cell cancers
Plasma cell neoplasms can be benign (not cancer) or malignant (cancer). According to the World Health Organization (WHO) classification in 2017, plasma cell neoplasms includes non-Ig-M monoclonal gammopathy of undetermined significance (MGUS), plasmacytoma, monoclonal immunoglobulin deposition diseases and multiple myeloma (plasma cell myeloma) and plasma cell neoplasms with the associated paraneoplastic syndromes 2, 3. Plasma cell neoplasms include the following:
Figure 12. Plasma cell neoplasm WHO classification
[Source 11 ]Table 1. International Myeloma Working Group Diagnostic Criteria for Multiple Myeloma and Related Plasma Cell Disorders
| Disorder | Disease Definition |
|---|---|
| Non-IgM monoclonal gammopathy of undetermined significance (MGUS) | All 3 criteria must be met: • Serum monoclonal protein (non-IgM type) <3gm/dL • Clonal bone marrow plasma cells <10%* • Absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, and bone lesions (CRAB) that can be attributed to the plasma cell proliferative disorder |
| Smoldering multiple myeloma (SMM) | Both criteria must be met: • Serum monoclonal protein (IgG or IgA) ≥3gm/dL, or urinary monoclonal protein ≥500 mg per 24h and/or clonal bone marrow plasma cells 10-60% • Absence of myeloma defining events or amyloidosis |
| Multiple Myeloma (plasma cell myeloma) | Both criteria must be met: • Clonal bone marrow plasma cells ≥10% or biopsy-proven bony or extramedullary plasmacytoma • Any one or more of the following myeloma defining events:
|
| Plasma cell leukemia | Both criteria must be met: • Meets diagnostic criteria for multiple myeloma • Presence of 5% or more plasma cells in conventional peripheral blood smear white blood cell differential count |
| IgM Monoclonal gammopathy of undetermined significance (IgM MGUS) | All 3 criteria must be met: • Serum IgM monoclonal protein <3gm/dL • Bone marrow lymphoplasmacytic infiltration <10% • No evidence of anemia, constitutional symptoms, hyperviscosity, lymphadenopathy, or hepatosplenomegaly that can be attributed to the underlying lymphoproliferative disorder. |
| Light Chain MGUS | All criteria must be met: • Abnormal serum free light chain (FLC) ratio (<0.26 or >1.65) • Increased level of the appropriate involved light chain (increased kappa FLC in patients with ratio > 1.65 and increased lambda FLC in patients with ratio < 0.26) • No immunoglobulin heavy chain expression on immunofixation • Absence of end-organ damage that can be attributed to the plasma cell proliferative disorder • Clonal bone marrow plasma cells <10% • Urinary monoclonal protein <500 mg/24h |
| Solitary Plasmacytoma | All 4 criteria must be met • Biopsy proven solitary lesion of bone or soft tissue with evidence of clonal plasma cells • Normal bone marrow with no evidence of clonal plasma cells • Normal skeletal survey and MRI (or CT) of spine and pelvis (except for the primary solitary lesion) • Absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, or bone lesions (CRAB) that can be attributed to a lympho-plasma cell proliferative disorder |
| Solitary Plasmacytoma with minimal marrow involvement** | All 4 criteria must be met • Biopsy proven solitary lesion of bone or soft tissue with evidence of clonal plasma cells • Clonal bone marrow plasma cells <10% • Normal skeletal survey and MRI (or CT) of spine and pelvis (except for the primary solitary lesion) • Absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, or bone lesions (CRAB) that can be attributed to a lympho-plasma cell proliferative disorder |
Footnotes:
* A bone marrow can be deferred in patients with low risk MGUS (IgG type, M protein <15 gm/L, normal free light chain ratio) in whom there are no clinical features concerning for myeloma
** Solitary plasmacytoma with 10% or more clonal plasma cells is considered as multiple myeloma
Monoclonal gammopathy
A monoclonal gammopathy is when plasma cells make too many copies of the same antibody. It is usually found on a routine blood test when looking for other conditions. Although people with multiple myeloma have a monoclonal gammopathy, not everyone with monoclonal gammopathy has multiple myeloma. It can also occur in other diseases, such as Waldenstrom macroglobulinemia and some lymphomas. It can also occur in a disorder known as monoclonal gammopathy of undetermined significance (MGUS), which does not cause problems like multiple myeloma does. However, some people with MGUS will eventually go on to develop multiple myeloma or other diseases.
Monoclonal gammopathy of undetermined significance (MGUS)
MGUS also called monoclonal gammopathy of undetermined significance, is a medical condition in which an abnormal protein known as ‘monoclonal protein’ or ‘M protein’ is in found your blood or urine 13. The monoclonal protein or M protein, is produced by a type of blood cell known as plasma cells which are found in the blood-producing soft tissues. These tissues are present at the center of the bones, also known as the bone marrow. Monoclonal gammopathy of undetermined significance or MGUS is characterized by serum M-protein less than 30 g/L (3 g/dL), bone marrow clonal plasma cells less than 10 percent, absence of multiple myeloma-related end-organ damage (CRAB symptoms such as hypercalcemia, renal insufficiency, anemia, or bone lesions) and absence of B-cell lymphoma or other diseases known to produce an M-protein 14. MGUS is benign condition (not cancer), but it is sometimes called pre-malignant plasma cell disorder because each year approximately 1% of people with MGUS will eventually develop cancers such as multiple myeloma (most commonly), light chain amyloidosis, lymphoplasmacytic lymphoma (Waldenstrom macroglobulinemia), or chronic lymphocytic leukemia, which may require therapy 14, 15, 16, 17. MGUS (monoclonal gammopathy of undetermined significance) usually does not affect a person’s health or asymptomatic 18. It doesn’t cause weak bones, high calcium levels, kidney problems, or low blood counts. MGUS (monoclonal gammopathy of undetermined significance) is most often found when a routine blood test finds a high level of protein in the blood and further testing shows the protein is a monoclonal antibody or ‘M protein’. However, in some patients, MGUS may later develop a more serious condition, such as amyloidosis, or cause problems with the kidneys, heart, or nerves.
Monoclonal gammopathy of undetermined significance (MGUS) is found in approximately 2% to 3% of adults over age 50 and in 5% of adults older than the age of 70. MGUS is more common in men than in women (1.5:1) and 2 to 3-fold more common in African Americans compared to Caucasians 14.
Doctors typically estimate a person’s risk of progressing soon after MGUS is diagnosed, using a test that measures the amounts of certain markers in the blood. The risk of MGUS progression is increased when M protein greater than or equal to 15 g/L and with an abnormal serum free light chain (FLC) ratio 19, 16.
Risk factors that predict MGUS progression include the following 19, 16:
- An abnormal serum free light chain (FLC) ratio (ratio of kappa to lambda free light chains).
- Non-IgG class MGUS.
- A high level of serum M protein (≥1.5 g/dL).
A Swedish cohort study confirmed that an abnormal serum free light chain (FLC) ratio and a high level of serum monoclonal protein (M protein) are high-risk factors 20. The study described the additional risk factor of immunoparesis, which is defined as the reciprocal depression of the other immunoglobulin classes (i.e., if a patient has an IgG kappa M protein, the IgM and IgA would be below normal levels with immunoparesis). Incorporation of gene-expression profiles to better assess risk is under clinical evaluation 21.
Plasma cells are part of the immune system that develop from B lymphocytes (B cells), a type of white blood cell that is made in the bone marrow. Normally, when bacteria or viruses enter your body, some of the B cells (B lymphocytes) will change into plasma cells. The normal plasma cells make antibodies (also called immunoglobulins or Ig) to fight bacteria and viruses, to stop infection and disease. Each type of plasma cell make different antibodies (immunoglobulins) for different infections. Antibodies (immunoglobulins) attack and help to kill bacteria and viruses and so protect you from infections. They work with other parts of the immune system to help protect the body from germs and other harmful substances.
Normally, the body makes five different types of immunoglobulins – immunoglobulin G (Ig G), immunoglobulin M (Ig M), immunoglobulin A (Ig A), immunoglobulin E (Ig E) and immunoglobulin D (Ig D). Each one has slightly different immune system functions. Each type of immunoglobulin is composed of four protein chains – two identical heavy (long) protein chains and two identical light (shorter) protein chains (see Figure 4 below). The heavy chains may consist of one of five different types that correspond with the type of immunoglobulin produced: gamma (IgG), mu (IgM), alpha (IgA), epsilon (IgE) and delta (IgD). The light chains consist of one of two different types called kappa and lambda.
Within a plasma cell, two heavy chains of one type and two light chains of one type become attached to form one intact immunoglobulin molecule. Each particular plasma cell will produce only one type of immunoglobulin.
Abnormal plasma cells make many copies of the same antibody called a monoclonal protein or M protein, which is sometimes found during a routine blood or urine test. In most patients, the amount of M protein stays the same and there are no signs, symptoms, or health problems. Moreover, the abnormal plasma cells in MGUS do not form an actual tumor or mass and do not cause any of the problems seen in multiple myeloma. In MGUS, the number of plasma cells may be increased, but they still make up less than 10% of the cells in the bone marrow and there is no cancer.
Patients with MGUS don’t need treatment, but they are watched closely to see if they get a disease that does need to be treated, such as multiple myeloma, amyloidosis, lymphoplasmacytic lymphoma (Waldenstrom macroglobulinemia), or chronic lymphocytic leukemia 22, 23. Patients with MGUS usually undergo clinical follow up every 6-12 months for signs of disease progression 24, 25.
Virtually all cases of multiple myeloma are preceded by a gradually rising level of MGUS 26, 27.
Monoclonal gammopathies that cause organ damage, particularly to the kidney, heart, or peripheral nerves, require immediate therapy with the same strategies applied for the conventional plasma-cell dyscrasias 28. A monoclonal gammopathy causing kidney dysfunction—by direct antibody deposition or amyloidosis—is referred to as monoclonal gammopathy of renal significance 29. Rising serum creatinine, dropping glomerular filtration rates, and increasing urinary–albumin excretion are all parameters that may signify renal damage and are assessed prospectively for high-risk MGUS patients. Although the N-terminal pro-brain natriuretic peptide is a very sensitive marker for amyloid involvement in the heart, the low specificity must be noted. These extra tests are included with the M-protein level, free light chain (FLC) levels, and free light chain (FLC) ratio when following patients with MGUS 30.
In a retrospective review of 6,399 patients with newly diagnosed multiple myeloma, 44 patients were found to have a biclonal IgG or IgA MGUS. The overall response rate of the myeloma clone to induction therapy was 93%, compared with 64% for the separate-clone MGUS 31. Many MGUS plasma cell clones were unresponsive to available myeloma therapy; this result highlights the need to lower expectations for response in situations in which an MGUS may require therapy because of end-organ damage.
Plasmacytoma
A plasmacytoma is a type of abnormal plasma cells (myeloma cells) growth that is cancerous. But rather than many tumors in different locations as in multiple myeloma, there is only one tumor in one place, hence the name solitary plasmacytoma.
There are two types of plasmacytoma 32:
- In isolated plasmacytoma of bone, one plasma cell tumor is found in the bone, less than 10% of the bone marrow is made up of plasma cells, and there are no other signs of cancer. Plasmacytoma of the bone often becomes multiple myeloma.
- In extramedullary plasmacytoma also called extraosseous plasmacytoma, one plasma cell tumor is found in soft tissue (such as the lungs or other organs) but not in the bone or the bone marrow. Extramedullary plasmacytomas commonly form in tissues of the throat, tonsil, and paranasal sinuses.
A solitary plasmacytoma often develops in a bone. Sometimes plasmacytoma can be cured. Solitary plasmacytomas are most often treated with radiation therapy. Sometimes surgery may be used. As long as no other plasmacytomas are found later on, the patient’s outlook is usually excellent. However, since many people with a solitary plasmacytoma will develop multiple myeloma, these people are watched closely for signs of this disease.
Signs and symptoms depend on where the tumor is.
- In bone, the plasmacytoma may cause pain or broken bones.
- In soft tissue, the tumor may press on nearby areas and cause pain or other problems. For example, a plasmacytoma in the throat can make it hard to swallow.
Smoldering multiple myeloma
Smoldering multiple myeloma is an early or asymptomatic (no symptoms) myeloma that is not causing any problems. People with smoldering myeloma have some signs of multiple myeloma, such as any of the following:
- A large amount of plasma cells in the bone marrow (between 10% and 60%)
- A high level of monoclonal immunoglobulin (M protein) in the blood
- A high level of light chains (small protein segments also called Bence Jones protein) in the urine.
But, they have normal blood counts, normal calcium levels, normal kidney function, no bone or organ damage, and no signs of amyloidosis.
People with smoldering multiple myeloma do not need treatment right away, because the disease can take anywhere from many months to years to become active (symptomatic) myeloma. Some people may have very slow disease that never becomes active myeloma. Smoldering multiple myeloma is an area of active research. There are smoldering multiple myeloma that have high risk features that put them at a greater chance of turning into active myeloma and studies are being done to see if they should be reclassified as “active” myeloma or if they should start treatment sooner. People with smoldering multiple myeloma are also watched closely for signs of myeloma.
Patients with smoldering multiple myeloma have a risk of progression of approximately 10% per year for the first 5 years, 3% per year for the next 5 years, and 1% per year thereafter 33. Patients with the highest risk of progression (ultra-high risk) have now been reclassified as having multiple myeloma by the new International Myeloma Working Group criteria 12. Within the current definition of smoldering multiple myeloma (Table 1), there are two groups of patients: high risk (25% per year risk of progression in the first 2 years) and low risk (~ 5% per year risk of progression) 34. Risk factors for high risk smoldering multiple myeloma are outlined below. Presence of 2 or 3 of these factors is associated with a median TTP to multiple myeloma of approximately 2 years, and is considered high risk smoldering multiple myeloma (Mayo 2018 criteria).
Criteria for High Risk Smoldering Multiple Myeloma 35, 36:
- Mayo 2018 criteria
- Any 2-3 of the following:
- Serum M protein > 2gm/dL
- Serum free light chain (FLC) ratio (involved/uninvolved) >20
- Serum M protein ≥30g/L
- Bone marrow plasma cells >20%
- Any 2-3 of the following:
- Other High Risk Factors
- Progressive increase in M protein level (Evolving type of smoldering multiple myeloma)†
- Bone marrow clonal plasma cells 50-60%
- t (4;14) or del 17p or 1q gain
- Increased circulating plasma cells
- MRI with diffuse abnormalities or with 1 focal lesion
- PET-CT with focal lesion with increased uptake but without underlying osteolytic bone destruction
Footnote: * The term smoldering multiple myeloma excludes patients without end-organ damage who meet revised definition of multiple myeloma, namely clonal bone marrow plasma cells ≥60% or serum free light chain (FLC) ratio ≥100 (plus measurable involved FLC level ≥100 mg/L), or more than one focal lesion on magnetic resonance imaging. The risk factors variables associated with a higher risk of progression of smoldering multiple myeloma, and identify patients who need close follow up and consideration for clinical trials. Patients who are high risk by Mayo 2018 criteria are candidates for prophylactic therapy with lenalidomide or lenalidomide plus dexamethasone in the absence of clinical trials.
†Increase in serum monoclonal protein by ≥25% on two successive evaluations within a 6 month period.
Light chain amyloidosis
Light chain amyloidosis also known as primary amyloidosis, is also a disorder of abnormal plasma cell growth, but with lower amounts of abnormal plasma cells in the bone marrow compared to multiple myeloma.
Monoclonal proteins (antibodies) are made up of joined protein chains – 2 short light chains and 2 longer heavy chains. In light chain amyloidosis, abnormal plasma cells make too many light chains which are shorter and weigh less than the heavy chains. The light chains build up in tissues as an abnormal protein known as amyloid.
The buildup of amyloid in certain organs can enlarge them and affect the way they work. For example, when amyloid builds up in the heart, it can cause an irregular heart beat and cause the heart to enlarge and get weaker. A weak heart can lead to a condition called congestive heart failure, with symptoms like shortness of breath and swelling in the legs. Amyloid in the kidneys can cause them to work poorly. This may not cause symptoms early on, but the poor kidney function may be found on blood tests. If it gets worse, it can lead to kidney failure.
Light chain amyloidosis is only one of the diseases where amyloid builds up and causes problems. Amyloidosis can also be caused by a genetic (hereditary) disease called familial amyloidosis. Long-standing (chronic) infection and/or inflammation can also cause amyloidosis. This is known as secondary or AA amyloidosis.
A diagnosis of light chain amyloidosis is made when the patient has ALL of the following:
- Signs and symptoms of amyloidosis
- A biopsy that shows amyloid in any tissue (fat, bone marrow, or organ such as the heart)
- A positive test showing the amyloid protein is a light chain and not a heavy chain
- Abnormal plasma cells in the bone marrow, high levels of M protein in the blood, or high levels of M protein in the urine.
Amyloid can build up in any tissue, and a biopsy may be able to diagnose this disease. Sometimes it can be seen on a bone marrow biopsy. The biopsy done most often to look for amyloid uses a needle to remove some fat from the wall of the abdomen (belly). This is after the skin over the biopsy site is numbed with medicine. A doctor uses a special stain on the removed fat to look for amyloid.
Because amyloid often affects the heart and kidneys, they may also be biopsied to look for amyloid. This is rarely needed to find out if a patient has light chain amyloidosis, but it is sometimes done in someone with amyloid if it isn’t clear that their heart or kidney problems are caused by the amyloid or some other problem.
Other tests are often done as well, to help confirm that the patient has light chain amyloidosis and not some other kind. These include a bone marrow biopsy, serum free light chains, and electrophoresis of the urine.
Waldenstrom macroglobulinemia
Waldenstrom macroglobulinemia also called Waldenstrom’s macroglobulinemia, Waldenström macroglobulinemia or lymphoplasmacytic lymphoma, is an uncommon type of blood cancer of the B lymphocytes (a type of white blood cell) 37. Waldenstrom macroglobulinemia is a type of non-Hodgkin’s lymphoma and more closely resembles indolent non-Hodgkin’s lymphoma in its progression as a disease 38. In addition, Waldenstrom macroglobulinemia can have symptoms that are similar to other non-Hodgkin’s lymphoma.
The cancer cells in people with Waldenstrom macroglobulinemia are similar to those of multiple myeloma and non-Hodgkin lymphoma (NHL) 39. Multiple myeloma is considered a cancer of plasma cells, and non-Hodgkin lymphoma is a cancer of lymphocytes. Waldenstrom macroglobulinemia cells have features of both plasma cells and lymphocytes and are called lymphoplasmacytoid 39. Even though Waldenstrom macroglobulinemia has a monoclonal gammopathy and is sometimes grouped into other plasma cell disorders, it is considered a type of non-Hodgkin lymphoma (NHL).
Waldenstrom macroglobulinemia cells (B lymphocytes that are turning into plasma cells) make large amounts of a protein called monoclonal immunoglobulin M (IgM) antibody, which is known as a macroglobulin 40. The “M” in immunoglobulin M (IgM) stands for macroglobulin and is where the naming macroglobinemia is derived from. Each antibody (protein) made by the Waldenstrom macroglobulinemia cells is the same, so it is called a monoclonal protein, or just an M protein. The buildup of this M protein (high levels of IgM antibody) in the bone marrow, spleen and blood, causing the blood plasma to become thicker and blood flow to various body organs may be impaired. This may cause signs or symptoms such as trouble seeing or hearing loss, heart problems, shortness of breath, headaches, dizziness, abnormal bleeding, confusion, and numbness or tingling of the hands and feet. Patients can also experience lethargy and fatigue, and are at increased risk of infection. This complication occurs in approximately 10-30% of people with Waldenstrom’s macroglobulinemia. Sometimes there are no signs or symptoms of Waldenstrom macroglobulinemia. It may be found when a blood test is done for another reason.
The Waldenstrom macroglobulinemia cells grow mainly in the bone marrow, where they can crowd out the normal cells that make the different types of blood cells. This can lead to low levels of red blood cells (called anemia), which can make people feel tired and weak. It can also cause low numbers of white blood cells (called leukopenia), which makes it hard for your body to fight infection. The numbers of platelets in the blood can also drop (called thrombocytopenia), leading to increased bleeding and bruising.
Lymphoma cells can also grow in organs like the liver and spleen, causing these organs to swell and leading to abdominal pain 41.
Waldenstrom macroglobulinemia is rare, with an incidence rate of about 3 cases per million people per year in the United States 42. About 1,000 to 1,500 people are diagnosed with Waldenstrom macroglobulinemia each year in the United States 42.
Waldenstrom macroglobulinemia is more common in men than it is in women and it is much more common among Whites than African Americans 42.
There are few cases of Waldenstrom macroglobulinemia in younger people, but the chance of developing this disease goes up as people get older. The average age of people when they are diagnosed with Waldenstrom macroglobulinemia is 70 42.
Waldenstrom macroglobulinemia typically progresses slowly and may be managed with a careful watch-and-wait approach for about 20% of patients 43. More often, Waldenstrom macroglobulinemia requires treatment. Treatment for Waldenstrom macroglobulinemia includes drugs used to treat multiple myeloma and non-Hodgkin lymphoma (NHL). The current therapies can control the disease for many years. The five-year-survival-rate is approximately 75% for patients with newly diagnosed Waldenstrom macroglobulinemia 43. New therapies have significantly increased our ability to control the disease and may extend the median overall survival of Waldenstrom macroglobulinemia patients for up to 15 years after diagnosis, although we are still collecting data to prove this projection. Nevertheless, recurrence is common in Waldenstrom macroglobulinemia patients, and the disease is still considered incurable 43.
Multiple myeloma symptoms and signs
Signs and symptoms of multiple myeloma can vary and, early in the disease, there may be no symptoms at all. This is called smoldering multiple myeloma or “smoldering myeloma” or “asymptomatic myeloma”. It may be found when a blood or urine test is done for another condition.
Doctors sometimes refer to the acronym, CRAB, to describe signs of multiple myeloma (plasma cell myeloma). The letters stand for:
- C – Calcium elevation (high levels of calcium in the blood; also known as “hypercalcemia”)
- R– Renal insufficiency (poor function of the kidneys that may be due to a reduction in blood-flow to the kidneys)
- A – Anemia (low red blood cell counts)
- B – Bone abnormalities (lesions).
Patients with one or more of these CRAB criteria, or with recurrent infections, are considered to have disease that requires treatment.
Others can have common symptoms of the disease include any of the following:
- Bone pain, especially in your spine or chest
- Bones that break easily
- Nausea
- Constipation
- Loss of appetite
- Excessive thirst
- Fever for no known reason
- Frequent infections.
- Easy bruising or bleeding
- Trouble breathing
- Weakness or numbness in your arms or legs
- Feeling very tired or fatigue
- Mental fogginess or confusion
- Weight loss
Make an appointment with your doctor if you have any persistent signs and symptoms that worry you.
Low blood counts
In multiple myeloma (plasma cell myeloma), abnormal plasma cells (myeloma cells) build up in the bone marrow and form tumors in many bones of the body. These tumors (overgrowth of plasma cells) may keep the bone marrow from making enough healthy blood cells by crowding out normal blood-forming cells, leading to low blood counts. Normally, the bone marrow makes stem cells (immature cells) that become three types of mature blood cells:
- Red blood cells that carry oxygen and other substances to all tissues of the body.
- White blood cells that fight infection and disease.
- Platelets that form blood clots to help prevent bleeding.
As the number of myeloma cells increases, fewer red blood cells, white blood cells, and platelets are made.
- This can cause anemia (a shortage of red blood cells). People with anemia become weak and fatigued.
- Multiple myeloma can also cause the level of platelets in the blood to become low (called thrombocytopenia). This can lead to increased bleeding and bruising.
- Another condition that can develop is leukopenia (a shortage of normal white blood cells). This can lead to problems fighting infections.
Bone problems
The myeloma cells can also damage and weaken the bone. Myeloma cells can interfere with cells that help keep bones strong. Bones are constantly being remade to keep them strong. Two kinds of bone cells work together to keep bones healthy and strong:
- Osteoclasts break down old bone
- Osteoblasts lay down new bone
Myeloma cells make a substance that tells the osteoclasts to speed up dissolving the bone. So old bone is broken down without new bone to replace it, making the bones weak and easy to break. Fractured bones are a major problem in people with myeloma. This increase in bone break-down can also raise calcium levels in the blood.
- Bone pain, which can be in any bone, but is most often in the back, the hips, and skull
- Bone weakness, either all over (osteoporosis), or where there is a plasmacytoma
- Broken bones (fractures), sometimes from only a minor stress or injury
High blood levels of calcium (hypercalcemia)
A tumor can damage the bone and cause hypercalcemia (too much calcium in the blood). This can affect many organs in the body, including the kidneys, nerves, heart, muscles, and digestive tract, and cause serious health problems.
Hypercalcemia may cause the following signs and symptoms:
- Feeling thirsty or extreme thirst, leading to drinking a lot
- Urinating (peeing) a lot
- Dehydration
- Kidney problems and even kidney failure
- Severe constipation,
- Abdominal (belly) pain
- Loss of appetite
- Nausea or vomiting
- Feeling very tired
- Muscle weakness
- Restlessness
- Feeling drowsy
- Confusion or trouble thinking
If the level of calcium gets high enough, you can even slip into a coma.
Kidney problems
Myeloma cells make abnormal monoclonal antibody protein (M protein) that can harm the kidneys, leading to kidney damage and even kidney failure.
Early on, this doesn’t cause any symptoms, but signs of kidney damage may be seen on a blood test or a urine test. As the kidneys start to fail, they lose the ability to get rid of excess salt, fluid, and body waste products. This can lead to symptoms such as:
- Weakness
- Shortness of breath
- Itching
- Leg swelling.
Nervous system symptoms
If myeloma weakens the bones in the spine, they can collapse and press on spinal nerves. This is called spinal cord compression and can cause:
- Sudden severe back pain
- Numbness, most often in the legs
- Muscle weakness, most often in the legs.
This is a medical emergency and you should contact your doctor right away or go to the emergency room. If spinal cord compression is not treated right away, there is a possibility of permanent paralysis.
Nerve damage
Sometimes, the abnormal proteins produced by myeloma cells are toxic to nerves. This damage can lead to weakness and numbness and sometimes a “pins and needles” sensation. This is also called peripheral neuropathy.
Hyperviscosity
In some patients, large amounts of abnormal monoclonal antibody protein (M protein) can cause the blood to “thicken.” This thickening is called hyperviscosity. It can slow blood flow to the brain and cause:
- Confusion
- Dizziness
- Symptoms of a stroke, like weakness on one side of the body and slurred speech
Patients with these symptoms should call their doctor. Removing the protein from the blood using a procedure called plasmapheresis can rapidly reverse this problem. (Note: This is not something that can be treated with drugs known as “blood thinners.”)
Infections
Abnormal plasma cells cannot protect the body from infections. As mentioned before, normal plasma cells produce antibodies that attack germs. In multiple myeloma, the myeloma cells crowd out the normal plasma cells, so that antibodies to fight the infection can’t be made. The antibody made by the myeloma cells does not help fight infections. That’s because the myeloma cells are just many copies of the same plasma cell – all making copies of the same exact (or monoclonal) antibody.
When someone with myeloma gets an infection, they may be slow to respond to treatment. That person may stay sick for a long time. Pneumonia is a common and serious infection seen in myeloma patients.
Multiple myeloma causes
Scientists still do not know exactly what causes most cases of multiple myeloma (plasma cell myeloma). However, chronic antigenic stimulation from infections, chronic disease, radiation, or specific toxic substances has been proposed 3. Studies have shown that there is increased risk due to exposure to specific substances in occupations such as cosmetologists and farmers as well as chronic laxative abusers. A hereditary factor is possible; in non Ig-M monoclonal gammopathy of undetermined significance (MGUS), an increased prevalence of the disease has been found in families with a lymphoproliferative disorder 5.
Recent studies have found that abnormalities of some oncogenes (genes that promote cell growth) such as MYC gene develop early in the course of plasma cell tumors 44. Changes in other oncogenes such as the RAS genes are more often found in myeloma cells in the bone marrow after treatment, and changes in tumor suppressor genes (genes that slow down cell growth or make cells die at the right time) such as the gene for p53 are associated with spread to other organs 44. In addition, other oncogenes such as NRAS, KRAS, and BRAF may participate in plasma cell proliferation 45.
Myeloma cells also show abnormalities in their chromosomes. In human cells, DNA is packaged into chromosomes. Although normal human cells contain 46 chromosomes, some cancer cells may have extra chromosomes called a duplication or have all or part of a chromosome missing called a deletion. One common finding in myeloma cells is that parts of chromosome number 17 are missing 44. These deletions appear to make the myeloma more aggressive and resistant to treatment.
In about half of all people with multiple myeloma, part of one chromosome has switched with part of another chromosome in the myeloma cells. This is called a translocation. When this occurs in a crucial area next to an oncogene, it can turn the oncogene on.
Researchers have found that patients with multiple myeloma have important abnormalities in other bone marrow cells and that these abnormalities may also cause excess plasma cell growth. Certain cells in the bone marrow called dendritic cells release a hormone called interleukin-6 (IL-6), which stimulates normal plasma cells to grow. Excessive production of IL-6 by these cells appears to be an important factor in development of plasma cell tumors.
A connection with MGUS
Multiple myeloma almost always starts out as a relatively benign condition called monoclonal gammopathy of undetermined significance (MGUS).
MGUS, like multiple myeloma, is marked by the presence of M proteins — produced by abnormal plasma cells — in your blood. However, in MGUS, the levels of M proteins are lower and no damage to the body occurs.
Risk factors for developing multiple myeloma
A risk factor is anything that changes a person’s chance of getting a disease such as cancer.
Factors that may increase your risk of multiple myeloma include:
- Increasing age. Your risk of multiple myeloma increases as you age, with most people diagnosed in their mid-60s (at least 65 years old). Less than 1% of cases are diagnosed in people younger than 35.
- Male sex. Men are more likely to develop multiple myeloma than are women.
- Black race. Black people are more likely to develop multiple myeloma than are people of other races. The reason is not known.
- Family history of multiple myeloma. Multiple myeloma seems to run in some families. If a brother, sister or parent has multiple myeloma, you have an increased risk of the disease. Still, most patients have no affected relatives, so this accounts for only a small number of cases.
- Personal history of a monoclonal gammopathy of undetermined significance (MGUS). Multiple myeloma almost always starts out as MGUS, so having this condition increases your risk.
- Overweight or obesity. Being overweight or obese increases a person’s risk of developing multiple myeloma.
- Environment. Some studies are investigating a link between the development of myeloma and one or more of the following factors: radiation or exposure to certain kinds of chemicals such as pesticides, fertilizers and Agent Orange.
- Presence of chronic immunodeficiency
- Presence of known inflammatory diseases or conditions (eg, cardiovascular disease or type II diabetes).
- Occupation. Some studies indicate that firefighters have a statistically significantly higher risk for multiple types of cancer than the general population.
Complications of multiple myeloma
Complications of multiple myeloma include:
- Frequent infections. Myeloma cells inhibit your body’s ability to fight infections. You may find you get frequent infections that last for a long time.
- Bone problems. Multiple myeloma can also affect your bones, leading to bone pain, thinning bones and broken bones.
- Reduced kidney function. Multiple myeloma may cause problems with kidney function, including kidney failure. Higher calcium levels in the blood related to eroding bones can interfere with your kidneys’ ability to filter your blood’s waste. The proteins produced by the myeloma cells can cause similar problems.
- Low red blood cell count (anemia). As myeloma cells crowd out normal blood cells, multiple myeloma can also cause anemia and other blood problems.
- Unusual bleeding. Some people with multiple myeloma have bruising and unusual bleeding (hemorrhage), such as frequent nosebleeds, bleeding gums and heavy periods. This is because the myeloma cells in your bone marrow can stop blood-clotting cells called platelets from being made.
- Hyperviscosity. In some patients, large amounts of abnormal monoclonal antibody protein (M protein) can cause the blood to “thicken.” This thickening is called hyperviscosity. It can slow blood flow to the brain and cause:
- Confusion
- Dizziness
- Symptoms of a stroke, like weakness on one side of the body and slurred speech
- Patients with these symptoms should call their doctor. Removing the protein from the blood using a procedure called plasmapheresis can rapidly reverse this problem. (Note: This is not something that can be treated with drugs known as “blood thinners.”)
Multiple Myeloma Diagnosis
Sometimes multiple myeloma is diagnosed when your doctor detects it accidentally during a blood test for some other condition. It can also be diagnosed if your doctor suspects you could have multiple myeloma based on your signs and symptoms.
Tests and procedures used to diagnose multiple myeloma include:
- Blood tests. Laboratory analysis of your blood may reveal the M proteins produced by myeloma cells. Another abnormal protein produced by myeloma cells — called beta-2-microglobulin — may be detected in your blood and give your doctor clues about the aggressiveness of your myeloma. Additionally, blood tests to examine your kidney function, blood cell counts, calcium levels and uric acid levels can give your doctor clues about your diagnosis.
- Urine tests. Analysis of your urine may show M proteins, which are referred to as Bence Jones proteins when they’re detected in urine.
- Examination of your bone marrow. Your doctor may remove a sample of bone marrow for laboratory testing. The sample is collected with a long needle inserted into a bone (bone marrow aspiration and biopsy). In the lab, the sample is examined for myeloma cells. Specialized tests, such as fluorescence in situ hybridization (FISH) can analyze myeloma cells to identify gene mutations.
- Imaging tests. Imaging tests may be recommended to detect bone problems associated with multiple myeloma. Tests may include an X-ray, MRI, CT or positron emission tomography (PET).
Multiple myeloma is often diagnosed based on tests, the patient’s symptoms and the doctor’s physical exam of the patient.
A diagnosis of multiple myeloma requires either 9:
- A plasma cell tumor (proven by biopsy) OR at least 10% plasma cells in the bone marrow AND
- At least one or more of the following CRAB features and myeloma-defining events:
- Calcium elevation (high blood calcium level or hypercalcemia) – serum calcium >0.25 mmol/L (> 1 mg/dL) higher than the upper limit of normal or > 2.75 mmol/L (> 11 mg/dL)
- Renal insufficiency – creatinine clearance < 40 mL per minute or serum creatinine > 177 umol/L (> 2 mg/dL)
- Anemia – hemoglobin concentration of > 2 g/dL below the lower limit of normal, or a hemoglobin concentration of < 10 g/dL
- Bony lesions – one or more osteolytic lesions found on skeletal radiography, CT or PET-CT scans. If bone marrow has <10% clonal plasma cells, more than one bone lesion is required to distinguish from solitary plasmacytoma with minimal marrow involvement.
- Any one or more of the following biomarkers of malignancy (myeloma-defining events):
- 60% or greater clonal plasma cells on bone marrow examination
- Serum involved / uninvolved free light chain ratio of 100 or greater, provided the absolute level of the involved light chain is at least 100mg/L (a patient’s involved free light chain either kappa or lambda is the one that is above the normal reference range; the uninvolved free light chain is the one that is typically in, or below, the normal range)
- More than one focal lesion on MRI that is at least 5mm or greater in size.
Blood counts
The complete blood count (CBC) is a test that measures the levels of red cells, white cells, and platelets in the blood. If there are too many myeloma cells in the bone marrow, some of these blood cell levels can be low. The most common finding is a low red blood cell count (anemia).
Blood chemistry tests
Levels of blood creatinine, albumin, calcium, and other electrolytes will be checked.
- Creatinine levels show how well your kidneys are working. High levels mean that the kidneys are not functioning well. This is common in people with myeloma.
- Albumin is a protein found in the blood. Low levels can be seen in myeloma.
- Calcium levels may be high in people with advanced myeloma. High calcium levels (hypercalcemia) can cause symptoms of fatigue, weakness, and confusion.
A blood test to measure lactate dehydrogenase (LDH) levels might also be done. It can be a useful indicator of a patient’s prognosis (outlook). High levels mean the disease is more advanced and may have a worse prognosis.
Urine tests
A routine urine sample is typically taken to look for myeloma protein that has filtered through the kidney. You most likely also will be asked to give a sample of urine that has been collected over a 24-hour period, so it can measure how much myeloma protein is present. These tests are called urine protein electrophoresis (UPEP) and urine immunofixation.
Quantitative immunoglobulins
This test measures the blood levels of the different antibodies (immunoglobulins). There are several different types of antibodies in the blood: IgA, IgD, IgE, IgG, and IgM. The levels of these immunoglobulins are measured to see if any are abnormally high or low. In multiple myeloma, the level of one type may be high while the others are low.
Electrophoresis
The antibody produced by myeloma cells is abnormal because it is monoclonal (all the exact same ). Serum protein electrophoresis (SPEP) is a test that measures the antibodies in the blood and can find a monoclonal antibody. Another test, called immunofixation or immunoelectrophoresis, is used to determine the exact type of abnormal antibody (IgG. IgA or some other type). Finding a monoclonal antibody in the blood may be the first step in diagnosing multiple myeloma. This abnormal protein is known by several different names, including monoclonal immunoglobulin, monoclonal protein (M protein), M spike, or paraprotein.
Antibodies are made up of chains of protein : 2 long (heavy) chains and 2 shorter (light) chains. Sometimes pieces of the abnormal myeloma protein are filtered through the kidney into the urine. This urine protein, known as Bence Jones protein, is the part of the antibody called the light chain. The tests used for finding a monoclonal antibody in urine are called urine protein electrophoresis (UPEP) and urine immunofixation. These are done most often on urine that has been collected over a 24-hour period, not just on a routine urine sample.
Serum free light chains
This blood test can measure the light chain levels in the blood and is done when looking for myeloma or light chain amyloidosis.
This is most helpful in the rare cases of myeloma in which no M protein is found by serum protein electrophoresis (SPEP). Since the serum protein electrophoresis (SPEP) measures the levels of intact (whole) antibodies, it cannot measure the amount of light chains only.
This test also calculates the light chain ratio which is used to see if there is one type of light chain more than the other. There are 2 kinds of light chains: kappa and lambda. Normally, they are present in equal amounts in the blood, giving a ratio of 1 to 1. If there is more of one type of light chain than the other, the ratio will be different, which can be a sign of myeloma.
Beta-2 microglobulin
This is another protein made by the myeloma cells. Although this protein itself doesn’t cause problems, it can be a useful indicator of a patient’s prognosis (outlook). High levels mean the disease is more advanced and may have a worse prognosis.
Bone marrow biopsy
People with multiple myeloma have too many plasma cells in their bone marrow. The procedure used to check the bone marrow is called a bone marrow biopsy and aspiration. It can be done either at the doctor’s office or at the hospital.
In bone marrow aspiration, the back of the pelvic bone is numbed with local anesthetic. Then, a needle is inserted into the bone, and a syringe is used to remove a small amount of liquid bone marrow. This causes a brief sharp pain. For the biopsy, a needle is used to remove a tiny splinter of bone and marrow. Patients may feel some pressure during the biopsy. There is some soreness in the biopsy area when the numbing medicine wears off. Most patients can go home immediately after the procedure.
The bone marrow tissue is examined in the lab to see the appearance, size, and shape of the cells, how the cells are arranged and to determine if there are myeloma cells in the bone marrow and, if so, how many. The aspirate (the liquid part of the bone marrow) may also be sent for other tests, including immunohistochemistry and flow cytometry, and chromosome analyses, including karyotype and fluorescent in situ hybridization (also known as FISH).
- Immunohistochemistry: a part of the biopsy sample is treated with special proteins which cause color changes and help identify myeloma cells.
- Flow cytometry: A sample of bone marrow is treated with special proteins that stick only to certain cells. This can help determine if those cells are abnormal and if they are myeloma cells, lymphoma cells, some other cancer, or a non-cancerous disease.
- Cytogenetics: A test that evaluates chromosomes (long strands of DNA) in normal bone marrow cells and myeloma cells. Some myeloma cells may have too many chromosomes, too few chromosomes, or other chromosome abnormalities (such as translocations and deletions). Finding these changes can sometimes help in to predicting a person’s prognosis (outlook). Cytogenetic testing usually takes about 2 to 3 weeks to get a result.
- Fluorescent in situ hybridization (FISH): It uses special fluorescent dyes that only attach to specific parts of chromosomes. It can find most chromosome changes (such as translocations and deletions) that can be seen in the lab in standard cytogenetic tests, as well as some changes too small to be seen with usual cytogenetic testing. It’s very accurate and results are often available within a couple of days.
Fine needle aspiration biopsy
Fine needle aspiration (FNA) uses a very thin needle and a syringe to withdraw a small amount of tissue from a tumor or lymph node. The doctor can aim the needle while feeling an enlarged lymph node near the surface of the body. If the abnormal area (tumor) is deep in the body, the needle can be guided while it’s watched on a computed tomography (CT) scan (see discussion of imaging tests later in this section). The main advantage of FNA is that it doesn’t require surgery. The disadvantage is that in some cases the thin needle cannot remove enough tissue for a definite diagnosis.
Core needle biopsy
This test is similar to fine needle aspiration (FNA), but a larger needle is used and a larger tissue sample is removed.
If an area looks abnormal on an x-ray, a biopsy may be needed to confirm that it’s a plasmacytoma. Most often, a needle biopsy (fine or core) is used.
Molecular Classification
Although multiple myeloma is still considered a single disease, it is in reality a collection of several different cytogenetically distinct plasma cell malignancies 46. On fluorescent in situ hybridization (FISH) studies of the bone marrow, approximately 40% of multiple myeloma is characterized by the presence of trisomies in the neoplastic plasma cells (hyperdiploid multiple myeloma), while most of the rest have a translocation involving the immunoglobulin heavy chain (IgH) locus on chromosome 14q32 (IgH translocated multiple myeloma) 47, 48. A small proportion of patients have both trisomies and immunoglobulin heavy chain (IgH) translocations. Trisomies and immunoglobulin heavy chain (IgH) translocations are considered primary cytogenetic abnormalities and occur at the time of establishment of MGUS 49. In addition, other cytogenetic changes termed secondary cytogenetic abnormalities arise along the disease course of multiple myeloma, including gain(1q), del(1p), del(17p), del(13), and secondary translocations involving MYC 49. Both primary and secondary cytogenetic abnormalities can influence disease course, response to therapy, and prognosis 49. Importantly, the interpretation and impact of cytogenetic abnormalities in multiple myeloma vary depending on the disease phase in which they are detected 50. Studies show that myeloma is associated with more than 400 canonical somatic mutations per patient, and the most commonly mutated genes include immunoglobulin heavy chain and light chain genes, NRAS, KRAS and BRAF 51.
Table 2. Primary Molecular Cytogenetic Classification of Multiple Myeloma
| Subtype | Gene(s)/chromosomes affected | Approximate Percentage of myeloma patients |
|---|---|---|
| Hyperdiploid multiple myeloma | Recurrent trisomies involving odd-numbered chromosomes with the exception of chromosomes 1, 13, and 21 | 45 |
| Immunoglobulin heavy chain (IgH) translocated multiple myeloma | 40 | |
| t(11;14) (q13;q32) | CCND1 (cyclin D1) | 20 |
| t(6;14)(p21;q32) | CCND3 (cyclin D3) | 5 |
| t(4;14) (p16;q32) | NSD2 | 10 |
| t(14;16) (q32;q23) | C-MAF | 4 |
| t(14;20) (q32;q11) | MAFB | <1 |
| Other immunoglobulin heavy chain (IgH) translocations, other cytogenetic abnormalities, or normal | 5 |
Footnote: * Requires absence of an immunoglobulin heavy chain translocation. If an immunoglobulin heavy chain translocation is present, classification will be based on that abnormality.
[Source 49 ]Imaging tests
Imaging tests use sound waves, x-rays, magnetic fields, or radioactive substances to create pictures of the inside of your body. Imaging tests may be done for a number of reasons, such as:
- To look at suspicious areas that might be cancer
- To learn how far cancer has spread
- To help determine if treatment is working
Bone x-rays
X-rays can detect bone destruction caused by the myeloma cells. Often doctors will do a series of x-rays that includes most of the bones. This is called a bone survey or skeletal survey.
CT scan (computed tomography scan)
A CT scan uses x-rays taken from different angles, which are combined by a computer to make detailed pictures of the organs. Sometimes, this test can help tell if your bones have been damaged by myeloma. It can also be used to guide a biopsy needle into an area of concern.
Magnetic resonance imaging (MRI) scans
Like CT scans, MRI scans show detailed images of soft tissues in the body. But MRI scans use radio waves and strong magnets instead of x-rays. A contrast material called gadolinium may be injected into a vein before the scan to see details better.
MRI scans are very helpful in looking at bones, the brain, and the spinal cord. Because MRI can find plasmacytomas that can’t be seen on regular x-rays, they can be helpful if the patient has pain in a bone but nothing abnormal is seen on the x-ray. MRI can also be used to look at the bone marrow in patients with multiple myeloma.
Positron emission tomography (PET) scans
For this test, a form of radioactive sugar is put into a vein and travels throughout the body. Cancer cells absorb high amounts of this sugar. A special camera then takes pictures that show the areas where the sugar collected throughout the body. A PET scan is often combined with a CT scan (known as a PET/CT scan).
When a patient appears to have a solitary plasmacytoma, a PET scan may be used to look for other plasmacytomas. Like MRI scans, PET scans can find plasmacytomas that can’t be seen on regular x-rays, so they are helpful if the patient has pain in a bone but the x-ray result is negative.
Echocardiogram (ECHO)
Amyloidosis often affects the heart, so if your doctor diagnoses or suspects you have this disorder, an echocardiogram (ECHO) may be ordered. This test is basically an ultrasound of the heart. It uses sound waves to look at the heart muscle and how well it’s working. The echocardiogram can see if the heart size is normal and if it is pumping normally. It also is especially helpful if amyloid is suspected because amyloid in the heart muscle looks different from normal heart muscle.
Multiple Myeloma Stages
If tests indicate you have multiple myeloma, your doctor will use the information gathered from the diagnostic tests to figure out if it has spread, and if so, how far. This process is called staging. The stage of a cancer describes how much cancer is in the body. It helps determine how serious the cancer is and how best to treat it. Doctors also use a cancer’s stage when talking about your prognosis and your treatment options. Cancer staging can be complex, so ask your doctor to explain it to you in a way you understand.
Your multiple myeloma may also be assigned a risk category, which indicates the aggressiveness of your disease.
Revised International Staging System
Multiple myeloma is staged using the Revised International Staging System (RISS) based on 4 factors 52:
- The amount of albumin in the blood
- The amount of beta-2-microglobulin in the blood
- The amount of lactate dehydrogenase (LDH) in the blood
- The specific gene abnormalities (cytogenetics) of the cancer.
Table 3. Revised International Staging System for multiple myeloma
| RISS Stage Group | Factors |
|---|---|
| 1 | Serum beta-2 microglobulin is less than 3.5 (mg/L) AND Albumin level is 3.5 (g/dL) or greater AND Cytogenetics are considered “not high risk” * AND Lactate dehydrogenase (LDH) levels are normal |
| 2 | Not stage 1 or 3 |
| 3 | Serum beta-2 microglobulin is 5.5 (mg/L) or greater AND “High risk” cytogenetics [t(4;14), t(14;16), or del(17p)]* AND/OR Lactate dehydrogenase (LDH) levels are high |
Footnote: * The bone marrow may be sent for tests to look at the chromosomes in the cancer cells. This test may also be called cytogenetics. Certain chromosome changes can mean a poorer outlook. For example, loss of a piece of chromosome 17 is linked to a poorer outcome. Another genetic abnormality that predicts a poor outcome is an exchange of material from chromosomes 4 and 14. This is called a translocation. A translocation involving chromosomes 14 and 16 is also linked to a poorer outcome. These 3 specific chromosome changes are considered high risk. Other chromosome abnormalities are considered standard risk or not high risk.
[Source 53, 52 ]Table 4. Cytogenetic Abnormalities on Clinical Course and Prognosis in Multiple Myeloma
| Cytogenetic Abnormality | Clinical Setting in which Abnormality is Detected | |
|---|---|---|
| Smoldering Multiple Myeloma | Multiple Myeloma | |
| Trisomies | Intermediate-risk of progression, median time to progression of 3 years | Good prognosis, standard-risk multiple myeloma, median overall survival 7-10 years Most have myeloma bone disease at diagnosis Excellent response to lenalidomide-based therapy |
| t(11;14) (q13;q32) | Standard-risk of progression, median time to progression of 5 years | Good prognosis, standard-risk multiple myeloma, median overall survival 7-10 years |
| t(6;14) (p21;q32) | Standard-risk of progression, median time to progression of 5 years | Good prognosis, standard-risk multiple myeloma, median overall survival 7-10 years |
| t(4;14) (p16;q32) | High-risk of progression, median time to progression of 2 years | Intermediate-risk multiple myeloma, median overall survival 5 years Needs bortezomib-based initial therapy, early autologous stem cell transplantation (if eligible), followed by bortezomib-based consolidation/maintenance |
| t(14;16) (q32;q23) | Standard-risk of progression, median time to progression of 5 years | High-risk multiple myeloma, median overall survival 3 years Associated with high levels of serum free light chain (FLC) and 25% present with acute renal failure as initial multiple myeloma defining events |
| t(14;20) (q32;q11) | Standard-risk of progression, median time to progression of 5 years | High-risk multiple myeloma, median overall survival 3 years |
| Gain(1q21) | High-risk of progression, median time to progression of 2 years | Intermediate-risk multiple myeloma, median overall survival 5 years |
| Del(17p) | High-risk of progression, median time to progression of 2 years | High-risk multiple myeloma, median overall survival 3 years |
| Trisomies plus any one of the immunoglobulin heavy chain (IgH) translocations | Standard-risk of progression, median time to progression of 5 years | May ameliorate adverse prognosis conferred by high risk immunoglobulin heavy chain (IgH) translocations, and del 17p |
| Isolated Monosomy 13, or Isolated Monosomy 14 | Standard-risk of progression, median time to progression of 5 years | Effect on prognosis is not clear |
| Normal | Low-risk of progression, median time to progression of 7-10 years | Good prognosis, probably reflecting low tumor burden, median overall survival >7-10 years |
Table 5. Mayo Clinic Risk Stratification for Multiple Myeloma (mSMART)
| Risk Group | Percentage of newly diagnosed patients with the abnormality |
|---|---|
| Standard Risk | 60% |
| Trisomies | |
| t(11;14) | |
| t(6;14) | |
| High Risk | 40% |
| t(4;14) | |
| t(14:16) | |
| t(14;20) | |
| del(17p) | |
| gain(1q) | |
| Double-Hit myeloma: Any 2 high risk factors | |
| Triple-Hit myeloma: Any 3 or more high risk factors |
Footnote: The Mayo Clinic mSMART risk stratification has additional detail that is valuable in formulating a therapeutic strategy.
[Source 54 ]Multiple myeloma treatment
If you have multiple myeloma but aren’t experiencing any symptoms (also known as smoldering multiple myeloma), you might not need treatment right away. Immediate treatment may not be necessary for multiple myeloma that is slow growing and at an early stage. However, your doctor will regularly monitor your condition for signs that the disease is progressing. This may involve periodic blood and urine tests. It’s important that your doctor is experienced in treating patients with myeloma or works in consultation with a myeloma specialist. This type of specialist is usually called a hematologist oncologist.
If you’re experiencing symptoms, treatment can help relieve pain, control complications of the disease, stabilize your condition and slow the progress of multiple myeloma.
If you develop signs and symptoms or your multiple myeloma shows signs of progression, you and your doctor may decide to begin treatment.
Standard treatment options for myeloma include:
- Targeted therapy. Targeted drug treatments focus on specific weaknesses present within cancer cells. By blocking these abnormalities, targeted drug treatments can cause cancer cells to die.
- Immunotherapy. Immunotherapy uses your immune system to fight cancer. Your body’s disease-fighting immune system may not attack your cancer because the cancer cells produce proteins that help them hide from the immune system cells. Immunotherapy works by interfering with that process.
- Chemotherapy. Chemotherapy uses drugs to kill cancer cells. The drugs kill fast-growing cells, including myeloma cells. High doses of chemotherapy drugs are used before a bone marrow transplant.
- Corticosteroids. Corticosteroid medications regulate the immune system to control inflammation in the body. They are also active against myeloma cells.
- Bone marrow transplant. A bone marrow transplant, also known as a stem cell transplant, is a procedure to replace your diseased bone marrow with healthy bone marrow. Before a bone marrow transplant, blood-forming stem cells are collected from your blood. You then receive high doses of chemotherapy to destroy your diseased bone marrow. Then your stem cells are infused into your body, where they travel to your bones and begin rebuilding your bone marrow.
- Radiation therapy. Radiation therapy uses high-powered energy beams from sources such as X-rays and protons to kill cancer cells. It may be used to quickly shrink myeloma cells in a specific area — for instance, when a collection of abnormal plasma cells form a tumor (plasmacytoma) that’s causing pain or destroying a bone.
Which combination of treatments you’re likely to receive will depend on whether you’re considered a good candidate for bone marrow transplant (stem cell transplant). This depends on the risk of your disease progressing, your age and your overall health. Options for bone marrow transplant (stem cell transplant) are discussed in Stem Cell Transplant.
- If you’re considered a candidate for bone marrow transplant, your initial therapy will likely include a combination of treatments, such as targeted therapy, immunotherapy, corticosteroids and, sometimes, chemotherapy. Your blood stem cells will likely be collected after you’ve undergone a few months of treatment. You may undergo the bone marrow transplant soon after your cells are collected or the transplant may be delayed until after a relapse, if it occurs. In some situations, doctors recommend two bone marrow transplants for people with multiple myeloma. After your bone marrow transplant, you’ll likely receive targeted therapy or immunotherapy as a maintenance treatment to prevent a recurrence of myeloma.
- If you’re not considered a candidate for bone marrow transplant, your initial therapy will likely include a combination of treatments, such as targeted therapy, immunotherapy, corticosteroids and, sometimes, chemotherapy.
- If your myeloma recurs or doesn’t respond to treatment, your doctor may recommend repeating another course of the treatment that initially helped you. Another option is trying one or more of the other treatments typically used as first line therapy, either alone or in combination.
Research on a number of new treatment options is ongoing, and you may be eligible for a clinical trial in order to gain access to those experimental treatments. Talk to your doctor about what clinical trials may be available to you.
Treatment for bone disease (bisphosphonates) is often started along with chemo. If the areas of damaged bone continue to cause symptoms, radiation therapy may be used.
Patients with multiple myeloma also receive supportive treatments, such as transfusions to treat low blood cell counts, and antibiotics and sometimes intravenous immunoglobulin (IVIG) for infections.
CAR T-cell therapy may be another treatment option for some people, especially if several other treatments have already been tried. This treatment helps the body’s own immune system attack the cancer cells. While it can be very effective for many people, it can also cause very serious side effects.
Drug therapy
Many different types of drugs can be used to treat plasma cell myeloma (multiple myeloma). The approach to treatment of symptomatic newly diagnosed multiple myeloma is outlined in Figure 13 and is dictated by eligibility for bone marrow transplant (stem cell transplant) and risk-stratification.
Although a single drug may be used to treat multiple myeloma, it is preferable to use at least 2 or 3 different kinds of drugs in combination because the cancer responds better. For example:
- Lenalidomide (or pomalidomide or thalidomide) and dexamethasone
- Carfilzomib (or ixazomib or bortezomib), lenalidomide, and dexamethasone
- Bortezomib (or carfilzomib), cyclophosphamide, and dexamethasone (called VCd or CyBord)
- Bortezomib, lenalidomide and dexamethasone (called VRd)
- Elotuzumab (or daratumumab), lenalidomide, and dexamethasone
- Bortezomib, liposomal doxorubicin, and dexamethasone
- Elotuzumab, bortezomib, and dexamethasone
- Melphalan and prednisone (MP), with or without thalidomide or bortezomib
- Vincristine, doxorubicin (Adriamycin), and dexamethasone (called VAD)
- Dexamethasone, cyclophosphamide, etoposide, and cisplatin (called DCEP)
- Dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide (called DT-PACE), with or without bortezomib
- Selinexor, bortezomib, dexamethasone
The choice and dose of drug therapy depend on many factors, including the stage of the cancer, the age, kidney function of the patient as well as how frail the patient may be and whether a stem cell transplant is planned. If a stem cell transplant is planned, most doctors avoid using certain drugs, like melphalan, that can damage the bone marrow.
Patients with active myeloma or light chain amyloidosis are often given a combination of 2 or 3 drugs. The drugs chosen depend on the patient’s health (including their kidney function) .
Often, a combination containing bortezomib, lenalidomide, and dexamethasone is used. Combinations containing bortezomib are especially helpful in patients with kidney problems and those whose myeloma cells contain certain high-risk chromosome abnormalities.
Many other combinations may be considered as well. If one drug combination stops working (or the myeloma comes back), other drugs can be tried.
Some patients are given additional cycles of treatment after a bone marrow transplant (stem cell transplant). This is called consolidation treatment and increases the chance of a complete response (where all signs and symptoms of the disease go away).
Some patients (even some who didn’t have a stem cell transplant) may be given long-term treatment with lenalidomide or bortezomib. This is known as maintenance treatment. It can help delay the return of the myeloma, but it can also cause serious side effects in some people.
Table 6. Major Treatment Regimens in Multiple Myeloma
| Regimen | Usual Dosing Schedule* |
|---|---|
| Bortezomib-Thalidomide-Dexamethasone (VTd)** 55 | Bortezomib 1.3 mg/m² subcutaneous days 1, 8, 15, 22 Thalidomide 100-200 mg oral days 1-21 Dexamethasone 20 mg oral on day of and day after bortezomib (or 40 mg days 1, 8, 15, 22) Repeated every 4 weeks x 4 cycles as pre-transplant induction therapy |
| Bortezomib- Cyclophosphamide-Dexamethasone** (VCd or CyBord) 56 | Cyclophosphamide 300 mg/m² orally on days 1, 8, 15 and 22 Bortezomib 1.3 mg/m² subcutaneous on days 1, 8, 15, 22 Dexamethasone 40 mg oral on days on days 1, 8, 15, 22 Repeated every 4 weeks† |
| Bortezomib-Lenalidomide-Dexamethasone (VRd)** 56, 57 | Bortezomib 1.3 mg/m² subcutaneous days 1, 8, 15 Lenalidomide 25 mg oral days 1-14 Dexamethasone 20 mg oral on day of and day after bortezomib (or 40 mg days 1, 8, 15, 22) Repeated every 3 weeks† |
| Carfilzomib- Cyclophosphamide-Dexamethasone (KCd) ‡ ‡ 58. | Carfilzomib 20 mg/m² (days 1 and 2 of Cycle 1) and 27 mg/m² (subsequent doses) intravenously on days 1, 2, 8, 9, 15, 16 Cyclophosphamide 300 mg/m² orally on days 1, 8, 15 Dexamethasone 40 mg oral on days on days 1, 8, 15, 22 Repeated every 4 weeks |
| Carfilzomib-Lenalidomide-Dexamethasone (KRd) ‡ ‡ 59 | Carfilzomib 20 mg/m² (days 1 and 2 of Cycle 1) and 27 mg/m² (subsequent doses) intravenously on days 1, 2, 8, 9, 15, 16 Lenalidomide 25 mg oral days 1-21 Dexamethasone 40 mg oral days 1, 8, 15, 22 Repeated every 4 weeks |
| Carfilzomib-Pomalidomide-Dexamethasone (KPd) ‡ ‡ 60 | Carfilzomib 20 mg/m² (days 1 and 2 of Cycle 1) and 27 mg/m² (subsequent cycles) intravenously on days 1, 2, 8, 9, 15, 16 Pomalidomide 4 mg oral on days 1-21 Dexamethasone 40 mg oral on days on days 1, 8, 15, 22 Repeated every 4 weeks |
| Daratumumab-Lenalidomide-Dexamethasone (DRd) 61 | Daratumumab 16 mg/kg intravenously weekly x 8 weeks, and then every 2 weeks for 4 months, and then once monthly Lenalidomide 25 mg oral days 1-21 Dexamethasone 40 mg intravenous days 1, 8, 15, 22 (given oral on days when no daratumumab is being administered) Lenalidomide-Dexamethasone repeated in usual schedule every 4weeks |
| Daratumumab-Bortezomib-Dexamethasone (DVd) ** 62 | Daratumumab 16 mg/kg intravenously weekly x 8 weeks, and then every 2 weeks for 4 months, and then once monthly Bortezomib 1.3 mg/m² subcutaneous on days 1, 8, 15, 22 Dexamethasone 40 mg intravenous days 1, 8, 15, 22 (given oral on days when no daratumumab is being administered) Bortezomib-Dexamethasone repeated in usual schedule every 4 weeks |
| Daratumumab-Pomalidomide-Dexamethasone (DPd) 63 | Daratumumab 16 mg/kg intravenously weekly x 8 weeks, and then every 2 weeks for 4 months, and then once monthly Pomalidomide 4 mg oral on days 1-21 Dexamethasone 40 mg intravenous days 1, 8, 15, 22 (given oral on days when no daratumumab is being administered) Repeated every 4 weeks |
| Daratumumab-Carfilzomib-Dexamethasone (DKd)** 64 | Daratumumab 1800 mg subcutaneously (or 16 mg/kg intravenously) weekly x 8 weeks, and then every 2 weeks for 4 months, and then once monthly Carfilzomib 56 to 70 mg/m2 days 1, 8, and 15 (Cycle 1, day 1 dose is 20 mg/m2) Dexamethasone 40 mg days 1, 8, 15, 22 Carfilzomib-Dexamethasone repeated in usual schedule every 4weeks |
| Ixazomib-Lenalidomide-Dexamethasone (IRd) 65 | Ixazomib 4 mg oral days 1, 8, 15 Lenalidomide 25 mg oral days 1-21 Dexamethasone 40 mg oral days 1, 8, 15, 22 Repeated every 4weeks |
| Elotuzumab-Pomalidomide-Dexamethasone (EPd) 66 | 10 mg/kg intravenously weekly x 8 weeks, and then 20 mg/kg every 4 weeks Pomalidomide 4 mg oral days 1-21 Dexamethasone per prescribing information Lenalidomide-Dexamethasone repeated in usual schedule every 4weeks |
| Isatuximab-Pomalidomide-Dexamethasone (Isa-Pd) 67 | 10 mg/kg intravenously weekly x 4 weeks, and then every 2 weeks Pomalidomide 4 mg oral days 1-21 Dexamethasone per prescribing information Pomalidomide-Dexamethasone repeated in usual schedule every 4weeks |
| Isatuximab-carfilzomib-Dexamethasone (Isa-Kd)** 68 | 10 mg/kg intravenously weekly x 4 weeks, and then every 2 weeks Carfilzomib 56 to 70 mg/m² days 1, 8, and 15 (Cycle 1, day 1 dose is 20 mg/m²) Dexamethasone 40 mg days 1, 8, 15, 22 Carfilzomib-Dexamethasone repeated in usual schedule every 4weeks |
Footnotes:
* All doses need to be adjusted for performance status, renal function, blood counts, and other toxicities
** Doses of dexamethasone and/or bortezomib/carfilzomib reduced based on other data showing lower toxicity and similar efficacy with reduced doses; dose of selinexor reduced based on better tolerability with once weekly dosing in subsequent randomized trial; subcutaneous route of administration of bortezomib preferred based on data showing lower toxicity and similar efficacy compared to intravenous administration
† The day 22 dose of all 3 drugs is omitted if counts are low, or after initial response to improve tolerability, or when the regimen is used as maintenance therapy; When used as maintenance therapy for high risk patients, further delays can be instituted between cycles.
‡Omit day 15 dose if counts are low or when the regimen is used as maintenance therapy; When used as maintenance therapy for high risk patients, lenalidomide dose may be decreased to 10-15 mg per day, and delays can be instituted between cycles as done in total therapy protocols.
‡ ‡ Carfilzomib can also considered in a once a week schedule of 56 to 70 mg/m² on days 1, 8 and 15 every 28 days (cycle 1, day 1 should be 20 mg/m²); Day 8, 9 doses of carfilzomib can be omitted in maintenance phase of therapy after a good response to improve tolerability; KCd dosing lowered from that used in the initial trial which was conducted in newly diagnosed patients
Figure 13. Newly diagnosed multiple myeloma treatment algorithm
Footnote: Approach to the treatment of newly diagnosed multiple myeloma in bone marrow transplant (stem cell transplant) eligible (A) and transplant ineligible (B) patients
Abbreviations: VRd = bortezomib, lenalidomide, dexamethasone; Dara-VRd = daratumumab, bortezomib, lenalidomide, dexamethasone; DRd = daratumumab, lenalidomide, dexamethasone; ASCT = autologous stem cell transplantation.
[Source 49 ]Chemotherapy
Chemotherapy (chemo) is the use of certain kinds of drugs that destroy or control the growth of cancer cells. These drugs can be taken by mouth or given in a vein or a muscle. They enter the bloodstream and reach almost all areas of the body. At one time, chemo was often part of the main treatment for multiple myeloma. As newer types of drugs have become available in recent years, chemo has become less important in treating myeloma, although it is still used in some situations.
Chemo drugs that can be used to treat multiple myeloma include:
- Cyclophosphamide (Cytoxan)
- Etoposide (VP-16)
- Doxorubicin (Adriamycin)
- Liposomal doxorubicin (Doxil)
- Melphalan
- Bendamustine (Treanda)
Often one of these drugs is combined with other types of drugs like corticosteroids and immuno-modulating agents (drugs that will change the patient’s immune response). If a stem cell transplant is planned, most doctors avoid using certain drugs, like melphalan, that can damage bone marrow.
Chemotherapy (chemo) side effects
Chemo drugs kill cancer cells but also can damage normal cells. They are given carefully to avoid or reduce the side effects of chemotherapy. These side effects depend on the type and dose of drugs given and the how long they are taken. Common side effects of chemo include:
- Hair loss
- Mouth sores
- Loss of appetite
- Nausea and vomiting
- Diarrhea or constipation
Chemotherapy often leads to low blood counts, which can cause:
- An increased risk of serious infection (from having too few white blood cells)
- Easy bruising or bleeding (from having too few blood platelets )
- Feeling tired or short of breath (from having too few red blood cells)
Most side effects go away after treatment is finished.
If you have side effects, your cancer care team can suggest steps to ease them. For example, drugs can be given along with the chemo to prevent or reduce nausea and vomiting.
Along with these short-term side effects, some chemo drugs can permanently damage certain organs such as the heart or kidneys. The possible risks of these drugs are carefully balanced against their benefits, and the function of these organs is carefully monitored during treatment. If serious organ damage occurs, the drug that caused it is stopped and sometimes replaced with another.
Corticosteroids (steroids)
Corticosteroids, such as dexamethasone and prednisone, are an important part of the treatment of multiple myeloma. They can be used alone or combined with other drugs as a part of treatment. Corticosteroids are also used to help decrease the nausea and vomiting that chemo might cause.
Common side effects of these drugs include:
- High blood sugar
- Increased appetite and weight gain
- Problems sleeping
- Changes in mood (some people become irritable or “hyper”)
When used for a long time, corticosteroids also suppress the immune system. This increases the risk of serious infections. Steroids can also weaken bones.
Most of these side effects go away over time after the drug is stopped.
Immunomodulating agents
The way immunomodulating agents affect the immune system isn’t entirely clear. Three immunomodulating agents are used to treat multiple myeloma. The first of these drugs to be developed, thalidomide, caused severe birth defects when taken during pregnancy. Because the other immunomodulating agents are related to thalidomide, there’s concern that they could also cause birth defects. That’s why all of these drugs can only be obtained through a special program run by the drug company that makes them.
Because these drugs can increase the risk of serious blood clots, they are often given along with aspirin or a blood thinner.
Thalidomide (Thalomid) was first used decades ago as a sedative and as a treatment for morning sickness in pregnant women. When it was found to cause birth defects, it was taken off the market. Later, it became available again as a treatment for multiple myeloma. Side effects of thalidomide can include drowsiness, fatigue, severe constipation, and painful nerve damage (neuropathy). The neuropathy can be severe, and might not go away after the drug is stopped. There is also an increased risk of serious blood clots that start in the leg and can travel to the lungs.
Lenalidomide (Revlimid) is similar to thalidomide. It works well in treating multiple myeloma. The most common side effects of lenalidomide are thrombocytopenia (low platelets) and low white blood cell counts. It can also cause painful nerve damage. The risk of blood clots is not as high as that seen with thalidomide, but it is still increased.
In patients, where the myeloma is in remission after either a stem cell transplant or initial treatment, lenalidomide may be given for maintenance therapy to prolong the remission.
Pomalidomide (Pomalyst) is also related to thalidomide and is used to treat multiple myeloma. Some common side effects include low red blood cell counts (anemia) and low white blood cell counts. The risk of nerve damage is not as severe as it is with the other immunomodulating drugs, but it’s also linked to an increased risk of blood clots.
Proteasome inhibitors
Proteasome inhibitors work by stopping enzyme complexes (proteasomes) in cells from breaking down proteins important for controlling cell division. They appear to affect tumor cells more than normal cells, but they are not without side effects.
Bortezomib (Velcade) was the first of this type of drug to be approved, and it’s often used to treat multiple myeloma. It may be especially helpful in treating myeloma patients with kidney problems. It’s injected into a vein (IV) or under the skin, once or twice a week.
Common side effects of this drug include nausea and vomiting, tiredness, diarrhea, constipation, fever, decreased appetite, and lowered blood counts. The platelet count (which can cause easier bruising and bleeding) and the white blood cell count (which can increase the risk of serious infection) are most often affected. Bortezomib can also cause nerve damage (peripheral neuropathy) that can lead to problems with numbness, tingling, or even pain in the hands and feet. The risk of nerve damage is less when the drug is given under the skin. Some patients develop shingles (herpes zoster) while taking this drug. To help prevent this, your doctor may have you take an anti-viral medicine (like acyclovir) while you take bortezomib.
In patients where the myeloma was put into remission after either a stem cell transplant or initial treatment, bortezomib may also be given for maintenance therapy to prolong the remission.
Carfilzomib (Kyprolis) is a newer proteasome inhibitor that can be used to treat multiple myeloma in patients who have already been treated with other drugs that didn’t work. It’s given as an injection into a vein (IV), often in a 4-week cycle. To prevent problems like allergic reactions during the infusion, the steroid drug dexamethasone is often given before each dose in the first cycle.
Common side effects include tiredness, nausea and vomiting, diarrhea, shortness of breath, fever, and low blood counts. The blood counts most often affected are the platelet counts (which can cause easier bruising and bleeding) and the red blood cell count (which can lead to tiredness, shortness of breath, and being pale). People on this drug can also have more serious problems, such as pneumonia, heart problems, and kidney or liver failure.
Ixazomib (Ninlaro) is a proteasome inhibitor that is a capsule taken by mouth, typically once a week for 3 weeks, followed by a week off. This drug is usually given after other drugs have been tried.
Common side effects of this drug include nausea and vomiting, diarrhea, constipation, swelling in the hands or feet, back pain, and a lowered blood platelet count (which can cause easier bruising and bleeding). This drug can also cause nerve damage (peripheral neuropathy) that can lead to problems with numbness, tingling, or even pain in the hands and feet.
Monoclonal antibodies
Antibodies are proteins made by the body’s immune system to help fight infections. Man-made versions (monoclonal antibodies) can be designed to attack a specific target, such as proteins on the surface of myeloma cells.
Antibodies against CD38
Daratumumab (Darzalex) is a monoclonal antibody that attaches to the CD38 protein, which is found on myeloma cells. This is thought to both kill the cancer cells directly and to help the immune system attack them. This drug is used mainly in combination with other types of drugs, although it can also be used by itself in patients who have already had several other treatments for their myeloma.
This drug is often given as an infusion into a vein (IV). A newer form of the drug, known as daratumumab and hyaluronidase (Darzalex Faspro), can be given as a subcutaneous (under the skin) injection, typically in the belly area over a few minutes.
Either form of this drug can cause a reaction in some people while it is being given or within several hours afterward, which can sometimes be severe. Symptoms can include coughing, wheezing, trouble breathing, tightness in the throat, a runny or stuffy nose, feeling dizzy or lightheaded, headache, rash, and nausea.
Other side effects can include fatigue, nausea, back pain, fever, and cough. This drug can also lower blood cell counts, which can increase the risk of infections and bleeding or bruising. Darzalex Faspro can also cause reactions at the injection site, such as swelling, itching, and redness.
Isatuximab (Sarclisa) is another monoclonal antibody that attaches to the CD38 protein on myeloma cells. This is thought to both kill the cancer cells directly and to help the immune system attack them. This drug is used along with other types of myeloma drugs, typically after at least 2 other treatments have been tried. It’s given as an infusion into a vein (IV).
This drug can cause a reaction in some people while it is being given or within a few hours afterward, which can sometimes be severe. Symptoms can include coughing, wheezing, trouble breathing, tightness in the throat, chills, feeling dizzy or lightheaded, headache, rash, and nausea.
The most common side effects of this drug include respiratory infections (such as colds or pneumonia) and diarrhea. This drug can also lower blood cell counts:
- Having too few white blood cells can increase your risk for infections.
- Having too few red blood cells (anemia) can make you feel tired and weak.
- Having too few blood platelets can increase your risk of bleeding and bruising easily.
This drug might also increase your risk of developing a second cancer.
Antibodies against SLAMF7
Elotuzumab (Empliciti) is a monoclonal antibody that attaches to the SLAMF7 protein, which is found on myeloma cells. This is thought to help the immune system attack the cancer cells. This drug is used mainly in patients who have already had other treatments for their myeloma. It’s given as an infusion into a vein (IV).
This drug can cause a reaction in some people while it is being given or within several hours afterward, which can sometimes be severe. Symptoms can include fever, chills, feeling dizzy or lightheaded, rash, wheezing, trouble breathing, tightness in the throat, or a runny or stuffy nose.
Other common side effects with this drug include fatigue, fever, loss of appetite, diarrhea, constipation, cough, nerve damage resulting in weakness or numbness in the hands and feet (peripheral neuropathy), upper respiratory tract infections, and pneumonia.
Antibody-drug conjugates
An antibody-drug conjugate is a monoclonal antibody linked to a chemotherapy drug. In this case, the antibody looks for and then attaches to the BCMA protein on myeloma cells, bringing the chemo directly to them.
Belantamab mafodotin-blmf (Blenrep) is an antibody-drug conjugate that can be used by itself to treat myeloma mainly in people who have already had at least 4 other treatments for their myeloma (including proteasome inhibitors, immunomodulatory drugs, and a monoclonal antibody to CD38). The drug is given in a vein (IV) typically every 3 weeks.
Common side effects include feeling very tired, fever, nausea, and reactions when the drug is given. Because this drug could cause severe problems in the eyes including blurry vision, dry eyes, vision loss, and damage to the cornea, it can only be obtained through a special program run by the drug company that makes it.
Nuclear export inhibitor
The nucleus of a cell holds most of the cell’s genetic material (DNA) needed to make the proteins the cell uses to function and stay alive. A protein called XPO1 helps carry other proteins from the nucleus to other parts of the cell.
Selinexor (Xpovio) is a drug known as a nuclear export inhibitor. It works by blocking the XPO1 protein. When the myeloma cell cannot transport proteins from its nucleus, the cell dies.
This drug is used with dexamethasone:
- for people whose myeloma has been treated with and no longer responds to at least 5 other myeloma drugs, including proteasome inhibitors, immunomodulatory drugs, and a monoclonal antibody to CD38 OR
- along with bortezomib for adults whose myeloma has grown on at least one other drug therapy.
It is a pill that can be taken on the first and third day of each week or weekly.
Common side effects include low platelet counts, low white blood cell counts, diarrhea, nausea, vomiting, not feeling hungry, weight loss, low blood sodium levels, and infections like bronchitis or pneumonia.
Immunotherapy
Immunotherapy drugs work to stimulate the body’s own immune system to help it recognize and attack cancer cells more effectively.
Bispecific T cell engager (BiTE)
Teclistamab (Tecvayli) is a type of immunotherapy know as a bispecific T cell engager (BiTE). Once it’s injected into the body, one part of the drug attaches to immune cells called T cells, while another part attaches to the BCMA protein on myeloma cells. This brings the two together, which helps the immune system attack the cancer cells.
Teclistamab can be used to treat multiple myeloma that has been treated and no longer responds to at least 4 other myeloma drugs, including proteasome inhibitors, immunomodulatory drugs, and a monoclonal antibody to CD38.
This drug is given as an IV infusion, typically once a week.
Common side effects of Teclistamab include:
- Fever
- Feeling very tired
- Headache
- Nausea
- Diarrhea
- Muscle and joint pain
- Pneumonia (infection of the lung)
- Low blood counts
Cytokine release syndrome is a serious side effect that can occur when T cells in the body release chemicals (cytokines) that ramp up the immune system. This happens most often within the first day after the infusion, and it can be life-threatening. Symptoms can include:
- High fever and chills
- Muscle weakness
- Trouble breathing
- Low blood pressure
- A very fast heartbeat
- Headache
Your health care team will watch you closely for possible signs of cytokine release syndrome, especially during and after the first few infusions. Be sure to contact your health care team right away if you have any symptoms that might be from cytokine release syndrome.
Bisphosphonates for bone disease
Myeloma cells can weaken and even break bones. Drugs called bisphosphonates can help bones stay strong by slowing down this process. They can also help reduce pain in the weakened bone(s). Sometimes, pain medicines such as non-steroidal anti-inflammatory drugs (NSAIDs) or narcotics will be given along with bisphosphonates to help control or lessen the pain. Bone pain can be a difficult symptom to treat during and after treatment for myeloma.
The drugs used most often for treating bone problems in people with myeloma are the bisphosphonates pamidronate (Aredia) and zoledronic acid (Zometa) and the drug denosumab (Xgeva, Prolia). These drugs are given intravenously (IV or into a vein) or subcutaneously (under the skin). Most patients are treated once a month at first, but they may be able to be treated less often later on if they are doing well. Treatment with one of these drugs helps prevent further bone damage and events related to weakened bones such as fractures, hypercalcemia (high calcium levels), and spinal cord compression in multiple myeloma patients.
These treatments can have a rare but serious side effect called osteonecrosis of the jaw. Patients complain of pain and doctors find that part of the jaw bone has died. This can lead to an open sore that doesn’t heal. It can also lead to tooth loss in that area. The jaw bone can also become infected. Doctors aren’t sure why this happens or how best to prevent it, but having jaw surgery or having a tooth removed can trigger this problem. Avoid these procedures while you are taking any of these medicines.One way to avoid these dental procedures is to maintain good oral hygiene by flossing, brushing, making sure that dentures fit properly, and having regular dental checkups. Any tooth or gum infections should be treated right away. (Dental fillings, root canal procedures, and tooth crowns do not seem to lead to osteonecrosis of the jaw.) If osteonecrosis of the jaw does occur, the doctor will stop the bone medicine.
Your doctor might recommend that you have a dental checkup before starting treatment. That way, any dental problems can be taken care of before starting the drug. They might also recommend taking calcium and Vitamin D supplements while on the medicine to help your body build bone.
CAR T-cell therapy
Chimeric antigen receptor (CAR) T-cell therapy is a type of cancer immunotherapy. It helps the body’s own immune system find and attack cancer cells. CAR T-cell therapy is also sometimes talked about as a type of cell-based gene therapy, because it involves altering the genes inside certain immune cells to help them attack the cancer. To make this treatment, immune cells called T cells are taken from the person’s blood during a process called leukapheresis. Blood is removed through an IV line and goes into a machine that takes out the T cells. The remaining blood then goes back into the body. This process typically takes a few hours, and it might need to be repeated. The T cells are then frozen and sent to a lab, where they are genetically altered so they have specific receptors (called chimeric antigen receptors, or CARs) on their surface. These receptors help the T cells attach to proteins on cancer cells. The T cells are then multiplied in the lab, which typically takes several weeks. Once the CAR T cells are ready, the patient gets chemotherapy for a few days to help prepare the body. Then the CAR T cells are infused back into the patient’s blood, where they can seek out the cancer cells and help the immune system attack them.
Idecabtagene vicleucel (ide-cel, Abecma) and ciltacabtagene autoleucel (cilta-cel, Carvykti) are CAR T-cell therapies that target the BCMA protein, which is found on myeloma cells. Either of these treatments can be used in patients who have already received several (typically at least 4) other types of treatment for their multiple myeloma.
These treatments can have serious or even life-threatening side effects, so they need to be given in a medical center that is specially trained in their use. Your health care team will watch you closely for several weeks after you get the CAR-T cells.
Cytokine release syndrome: Cytokine release syndrome can happen when T cells release chemicals (cytokines) that ramp up the immune system. Cytokine release syndrome most often happens within a few days to weeks after treatment, and in some cases it can be life-threatening. Symptoms can include:
- High fever and chills
- Trouble breathing
- Severe nausea, vomiting, and/or diarrhea
- Feeling dizzy or lightheaded
- Headaches
- Fast heartbeat
- Feeling very tired
Nervous system problems: This treatment can sometimes have serious effects on the nervous system, which can result in symptoms such as:
- Headaches
- Changes in consciousness
- Confusion or agitation
- Seizures
- Shaking or twitching (tremors)
- Trouble speaking and understanding
- Loss of balance
Because of the risk of these side effects, you’ll be advised not to drive, operate heavy machinery, or do any other potentially dangerous activities for at least 8 weeks after you get your treatment.
Other serious side effects: Other possible serious side effects can include:
- Allergic reactions during the infusion
- A weakened immune system
- An increased risk of serious infections
- Low blood cell counts, which can increase the risk of infections, fatigue, and bruising or bleeding
It’s very important to report any side effects to your health care team right away, as there are often medicines that can help treat them.
Figure 14. CAR T-cell therapy
Radiation therapy
Radiation therapy uses high-energy rays or particles to kill cancer cells. Radiation may be used to treat areas of bone damaged by myeloma that have not responded to chemotherapy and/or other drugs and are causing pain or may be near breaking. It’s also the most common treatment for solitary plasmacytomas.
If myeloma severely weakens the vertebral (back) bones, these bones can collapse and put pressure on the spinal cord and spinal nerves. Symptoms include a sudden change in sensation (such as numbness or tingling), sudden weakness of leg muscles, or sudden problems with urination or moving the bowels. This is a medical emergency; patients with these symptoms should call their doctor right away. Prompt treatment with radiation therapy and/or surgery is often needed to prevent paralysis.
The type of radiation therapy most often used to treat multiple myeloma or solitary plasmacytoma is called external beam radiation therapy (EBRT). The radiation is aimed at the cancer from a machine outside the body. Having radiation therapy is much like having a diagnostic x-ray except that each treatment lasts longer, and the course of treatment can continue for several weeks.
Side effects of radiation can include:
- Skin changes in the area being treated, which can range from redness to blistering and peeling
- Fatigue (tiredness)
- Nausea
- Diarrhea (if the belly or pelvis is being treated)
- Low blood counts
These symptoms improve once treatment is over.
Stem Cell Transplant
In a stem cell transplant, the patient gets high-dose chemotherapy to kill the cells in the bone marrow. Then the patient receives new, healthy blood-forming stem cells. When stem cell transplants were first developed, the new stem cells came from bone marrow, and so this was known as a bone marrow transplant. Now, stem cells are more often collected from blood (a peripheral blood stem cell transplant).
Stem cell transplant is commonly used to treat multiple myeloma. Before the transplant, drug treatment is used to reduce the number of myeloma cells in the patient’s body.
Stem cell transplants (SCT) can be autologous or allogeneic.
Autologous transplants
For an autologous stem cell transplant, the patient’s own stem cells are removed from the bone marrow or peripheral blood before the transplant. The cells are stored until they are needed for the transplant. Then, the person with myeloma gets treatment such as high-dose chemotherapy, sometimes with radiation, to kill the cancer cells. When this is complete, their stored stem cells are given back into their blood through a vein.
This type of transplant is a standard treatment for patients with multiple myeloma. Although an autologous transplant can make the myeloma go away for a time (even years), it doesn’t cure the cancer, and often the myeloma returns.
Some doctors recommend that patients with multiple myeloma have 2 autologous transplants, 6 to 12 months apart. This approach is called tandem transplant. Studies show that this may help some patients more than a single transplant. The drawback is that it causes more side effects and as a result can be riskier.
Allogeneic transplants
In an allogeneic stem cell transplant, the patient gets blood-forming stem cells from another person – the donor. The best treatment results occur when the donor’s cells are closely matched to the patient’s cell type and the donor is closely related to the patient, such as a brother or sister. Allogeneic transplants are much riskier than autologous transplants, but they may be better at fighting the cancer. That’s because transplanted (donor) cells may actually help destroy myeloma cells. This is called a graft vs. tumor effect. In studies of multiple myeloma patients, those who got allogeneic transplants often did worse in the short term than those who got autologous transplants. At this time, allogeneic transplants are not considered a standard treatment for myeloma, but may be done as a part of a clinical trial.
Side effects
The early side effects from a stem cell transplant (SCT) are similar to those from chemotherapy and radiation, only more severe. One of the most serious side effects is low blood counts, which can lead to risks of serious infections and bleeding.
The most serious side effect from allogeneic transplants is graft-versus-host disease (or GVHD). This occurs when the new immune cells (from the donor) see the patient’s tissues as foreign and attack them. GVHD (graft-versus-host disease) can affect any part of the body and can be life threatening.
Supportive treatments
Supportive treatment also known as palliative care is aimed at preventing or relieving symptoms instead of trying to cure the cancer. The main purpose of this type of treatment is to improve the comfort and quality of life for someone diagnosed with cancer no matter what the cancer stage or the goal of treatment might be. You might also hear supportive care referred to as symptom management, or comfort care.
Zoledronic acid or pamidronate are recommended for all patients 49. A lower intensity schedule every 3-4 months may provide similar protection with less side effects compared to monthly administration 69. Denosumab, a high-affinity monoclonal antibody targeting RANKL is an alternative especially in patients with significant renal dysfunction 70. When denosumab is discontinued for any reason, a dose of bisphosphonate should be considered in order to avoid rebound osteoclast activity.
Prophylaxis with trimethoprim-sulfamethoxazole or alternative agent against pneumocystis jirovecii pneumonia should also be considered long-term in all patients receiving long-term steroid or anti CD38 monoclonal antibody therapy 49. Acyclovir or valacyclovir should be administered routinely for patients receiving proteasome inhibitors, anti CD38 monoclonal antibodies, or elotuzumab as prophylaxis against herpes zoster 49. Intravenous immune globulin (IVIG) should be considered in hypogammaglobulinemic patients on daratumumab who develop frequent respiratory tract infections 49.
Intravenous immunoglobulin (IVIG)
Patients with multiple myeloma often have low levels of the normal antibodies (immunoglobulins) needed to fight infection. This can lead to problems with lung and/or sinus infections that keep coming back. The level of antibodies in the patient’s blood can be tested, and if it’s low, antibodies from donors can be given into a vein (IV) to raise the levels and help prevent infections. The antibodies given are called IVIG or intravenous immunoglobulin. IVIG is often given once a month at first, but may be able to be given less often based on blood tests of antibody levels.
Treatment for low blood cell counts
Some patients develop low red blood cell counts (anemia) from multiple myeloma or its treatment. They might feel tired, lightheaded, or short of breath while walking. Anemia that’s causing symptoms can be treated with blood transfusions. These are often given on an outpatient basis.
Epoetin (Procrit) and darbepoetin (Aranesp) are drugs that can help improve low red blood cell counts and reduce the need for blood transfusions in some patients who are getting chemotherapy. But these drugs are used much less often because they have been linked to poorer survival in some patients with lymphoid cancers, such as multiple myeloma.
Plasmapheresis
Plasmapheresis can be used to remove myeloma protein from the blood. It’s helpful when certain myeloma proteins build up, thicken the blood, and interfere with circulation (called hyperviscosity).
Most often, this procedure is done through a large catheter (tube) placed in a vein in the neck, under the collarbone, or in the groin. This catheter is hooked up to a machine, and blood flows into the machine. The machine separates the blood cells from the blood plasma (liquid part of the blood), and then returns the blood cells to the patient, along with either salt solution or donor plasma. The plasma that’s removed, which contains the abnormal antibody protein made by the myeloma cells, is discarded.
Although plasmapheresis lowers the abnormal protein level and can relieve symptoms for a time, it does not kill the myeloma cells. That means that without further treatment, the protein will just build up again. For this reason, plasmapheresis is often followed by chemotherapy or some other type of drug treatment to kill the cells that make the protein.
Surgery
Surgery is sometimes used to remove single plasmacytomas, but it’s rarely used to treat multiple myeloma. When spinal cord compression causes paralysis, severe muscle weakness, or numbness, emergency surgery may be needed. Surgery to attach metal rods or plates can help support weakened bones and may be needed to prevent or treat fractures.
Clinical trial
For some patients, taking part in a clinical trial may be the best treatment choice. Clinical trials are part of the cancer research process. Clinical trials are done to find out if new cancer treatments are safe and effective or better than the standard treatment.
Some clinical trials only include patients who have not yet received treatment. Other trials test treatments for patients whose cancer has not gotten better. There are also clinical trials that test new ways to stop cancer from recurring (coming back) or reduce the side effects of cancer treatment.
Clinical trials are taking place in many parts of the country. For more information on trials at the Clinical Center, see National Cancer Institute Center for Cancer Research 71 and Developmental Therapeutics Clinic 72.
Many of today’s standard treatments for cancer are based on earlier clinical trials. Patients who take part in a clinical trial may receive the standard treatment or be among the first to receive a new treatment.
Patients who take part in clinical trials also help improve the way cancer will be treated in the future. Even when clinical trials do not lead to effective new treatments, they often answer important questions and help move research forward.
Treatment of relapsed or refractory multiple myeloma
Almost all myeloma patients will experience relapse (the cancer returns after a successful course of treatment) and/or the disease will become refractory (the cancer does not respond to treatment). The choice of a treatment regimen at relapse is affected by many factors including previous therapy, rate of relapse, patient health, and genetic abnormalities.
In some instances, if the patient had a good response to a drug or combination of drugs initially, that treatment option may be repeated.
Treatment of relapsed or refractory multiple myeloma may include the following:
- Watchful waiting for patients whose disease is stable.
- A different treatment than treatment already given, for patients whose tumor kept growing during treatment.
- The same drugs used before the relapse may be used if the relapse occurs one or more years after initial treatment.
- Stem cell transplantation. The use of high-dose chemotherapy followed by autologous stem cell transplantation may also be an option for some relapsed/refractory myeloma patients, who have either not been treated with a transplant before or who had a good durable response to a prior transplant.
Drugs used may include the following:
- Targeted therapy with monoclonal antibodies (daratumumab or elotuzumab).
- Targeted therapy with proteasome inhibitors (bortezomib, carfilzomib, or ixazomib).
- Immunotherapy (pomalidomide, lenalidomide, or thalidomide).
- Chemotherapy.
- Antibody-drug conjugate therapy with belantamab mafodotin.
- Corticosteroid therapy.
- A clinical trial of CAR T-cell therapy. CAR-T drugs approved for treatment of adult patients with relapsed or refractory multiple myeloma include:
- Ciltacabtagene autoleucel (Carvykti)
- Idecabtagene vicleucel (Abecma)
- A clinical trial of targeted therapy with a small molecule inhibitor (selinexor) and corticosteroid therapy.
- A clinical trial of targeted therapy with a BCL2 inhibitor (venetoclax).
Figure 15. Treatment of relapsed multiple myeloma
Footnotes: Suggested options for the treatment of relapsed multiple myeloma in first relapse (A) and second or higher relapse (B)
Abbreviations: DRd = daratumumab, lenalidomide, dexamethasone; KRd = carfilzomib, lenalidomide, dexamethasone; ERd = Elotuzumab, lenalidomide, dexamethasone; IRd = ixazomib, lenalidomide, dexamethasone; DKd = daratumumab, carfilzomib, dexamethasone; Isa-Pd = isatuximab, carfilzomib, dexamethasone; DPd = daratumumab, pomalidomide, dexamethasone; Isa-Pd = isatuximab, pomalidomide, dexamethasone; KCd = carfilzomib, cyclophosphamide, dexamethasone; KPd = carfilzomib, pomalidomide; VCD = bortezomib, cyclophosphamide, dexamethasone; EPd = Elotuzumab, pomalidomide, dexamethasone; ASCT = autologous stem cell transplantation
*Consider salvage autologous stem cell transplantation in patients eligible for autologous stem cell transplantation who have not had transplant before; Consider 2nd auto stem cell transplantation if eligible and had >36 months response duration with maintenance to first autologous stem cell transplantation
[Source 49 ]Alternative medicine
No alternative medicines have been found to treat multiple myeloma. But alternative medicine may help you cope with the stress and side effects of myeloma and myeloma treatment.
Talk to your doctor about your options, such as:
- Art therapy
- Exercise
- Meditation
- Music therapy
- Relaxation exercises
- Spirituality
Talk with your doctor before trying any of these techniques to make sure they don’t pose any risks for you.
Treating complications
Because multiple myeloma can cause a number of complications, you may also need treatment for those specific conditions. For example:
- Bone pain. Pain medications, radiation therapy and surgery may help control bone pain.
- Kidney complications. People with severe kidney damage may need dialysis.
- Infections. Your doctor may recommend certain vaccines to prevent infections, such as the flu and pneumonia.
- Bone loss. Your doctor may recommend bone-building drugs to help prevent bone loss.
- Anemia. If you have persistent anemia, your doctor may recommend medications to increase your red blood cell count.
Coping and support
A cancer diagnosis can be shocking and devastating. With time, you’ll find ways to cope with the stress and uncertainty of living with cancer. Until you find what works best for you, consider trying to:
- Learn enough to make decisions about your care. Learn enough about multiple myeloma so that you’re able to participate in decisions about your treatment and care. Ask your doctor about your treatment options and their side effects. You may find additional help gathering information through your local library and online. Start with the National Cancer Institute (https://www.cancer.gov) and the International Myeloma Foundation (https://www.myeloma.org).
- Maintain a strong support system. Having a strong support system can help you cope with issues and anxieties that might occur. Your friends and family may be willing to offer support. You might also find that support from a formal support group or others coping with cancer may be helpful. Friends you meet in support groups may be willing to share practical advice for coping with cancer and cancer treatment. Support groups are also available online.
- Set reasonable goals. Having goals helps you feel in control and can give you a sense of purpose. But don’t choose goals you can’t possibly reach. You may not be able to work a 40-hour week, for example, but you may be able to work at least part time. In fact, many people find that continuing to work during cancer treatment can be helpful in maintaining some normalcy.
- Take time for yourself. Eating well, relaxing and getting enough rest can help combat the stress and fatigue of cancer. Also, plan ahead for the downtimes when you may need to rest more or limit what you do.
Treatment of Monoclonal Gammopathy of Undetermined Significance (MGUS)
Treatment of monoclonal gammopathy of undetermined significance (MGUS) is usually watchful waiting. Regular blood tests to check the level of M protein in the blood and physical exams to check for signs or symptoms of cancer will be done.
Treatment of Isolated Plasmacytoma of Bone
Treatment of isolated plasmacytoma of bone is usually radiation therapy to the bone lesion.
Treatment of Extramedullary Plasmacytoma
Treatment of extramedullary plasmacytoma may include the following:
- Radiation therapy to the tumor and nearby lymph nodes.
- Surgery, usually followed by radiation therapy.
- Watchful waiting after initial treatment, followed by radiation therapy, surgery, or chemotherapy if the tumor grows or causes signs or symptoms.
Multiple myeloma prognosis
Multiple myeloma is highly treatable and potentially curable when it presents as a solitary plasmacytoma of bone or as an extramedullary plasmacytoma 73. The median survival in the prechemotherapy era was about 7 months 73. After the introduction of chemotherapy, prognosis improved significantly with a median survival of 24 to 30 months and a 10-year survival rate of 3% 73. Even further improvements in prognosis have occurred because of the introduction of newer biologic therapies and better salvage options, with median survivals now exceeding 60 to 90 months 74. Data from randomized controlled trials using modern therapy show that the median survival in multiple myeloma is approximately 6 years 75. In the subset of patients eligible for autologous stem cell transplantation, 4-year survival rates are more than 80% 76; the median overall survival among these patients is approximately 8 years 77. Among elderly patients (age >75 years), median overall survival is lower, and is approximately 5 years 75. These numbers likely underestimate current survival probabilities since they predate the arrival of monoclonal antibodies and several other new agents that have been introduced in the last 3 to 5 years. On the other hand, they may be overestimates of the true population-based survival since they are derived from randomized controlled trials where patients with poor performance status and comorbidities are typically excluded. Nevertheless, these estimates are valuable benchmarks, and appear generalizable to newly diagnosed myeloma patients in good performance status 78.
Patients with plasma cell leukemia or with soft tissue plasmacytomas (often with plasmablastic morphology) in association with multiple myeloma have poor outcomes 79, 80. Racial disparities because of biology, socioeconomic factors, and structural racism are under evaluation 81.
Factors other than stage that affect survival
- Kidney function. The blood creatinine level shows how healthy the kidneys are. Kidneys eliminate this chemical from the body. When they are damaged by the monoclonal immunoglobulin, blood creatinine levels rise, predicting a worse outlook.
- Age. Age is also important. In the studies of the international staging system, older people with myeloma do not live as long.
- Your overall health. Your overall health can affect the outlook of your myeloma. Poorly controlled health conditions, such as diabetes or heart disease, for example, can predict a worse prognosis.
Multiple myeloma survival rates
Survival rates tell you what percentage of people with the same type and stage of cancer are still alive a certain amount of time (usually 5 years) after they were diagnosed. They can’t tell you how long you will live, but they may help give you a better understanding about how likely it is that your treatment will be successful.
Keep in mind that survival rates are estimates and are often based on previous outcomes of large numbers of people who had a specific cancer, but they can’t predict what will happen in any particular person’s case. These statistics can be confusing and may lead you to have more questions. Your doctor is familiar with your situation; ask how these numbers may apply to you.
A relative survival rate compares people with the same type and stage of cancer to people in the overall population. For example, if the 5-year relative survival rate for a specific stage of multiple myeloma is 60%, it means that people who have that cancer are, on average, about 60% as likely as people who don’t have that cancer to live for at least 5 years after being diagnosed.
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database tracks 5-year relative survival rates for multiple myeloma in the United States, based on how far the cancer has spread. The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database, however, does not group cancers by the Revised International Staging System (stage 1, stage 2, stage 3). Instead, it groups cancers into localized, regional, and distant stages:
- Localized: Only one tumor (a solitary plasmacytoma) is growing in the bone or outside the bone.
- Regional: This stage does not apply to myeloma, because this type of cancer does not spread to the lymph nodes.
- Distant: Many tumors are found inside or outside the bones, or multiple myeloma has been diagnosed.
Table 7. 5-year relative survival rates for multiple myeloma
| SEER stage | 5-year relative survival rate |
|---|---|
| Localized (solitary plasmacytoma) | 78% |
| Regional | Not applicable |
| Distant (multiple myeloma) | 55% |
| All SEER stages combined | 56% |
Footnotes: These numbers are based on people diagnosed with plasmacytomas or multiple myeloma between 2011 and 2017. And these numbers don’t take everything into account. Survival rates for myeloma are generally based on if a single plasmacytoma is found or if multiple myeloma is diagnosed. But other factors, such as the tumor’s cytogenetics (chromosome changes), the levels of certain proteins and other substances in the blood, your kidney function, and your age and overall health, can also affect your outlook. People now being diagnosed with multiple myeloma may have a better outlook than these numbers show. Treatments have improved over time, and these numbers are based on people who were diagnosed and treated at least five years earlier.
[Source 82 ]- What Is Multiple Myeloma? https://www.cancer.org/cancer/multiple-myeloma/about/what-is-multiple-myeloma.html[↩]
- Rajkumar SV. Multiple myeloma: 2012 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2012 Jan;87(1):78-88.[↩][↩]
- Kaseb H, Durer C, Fazal S, et al. Plasma Cell Cancer. [Updated 2022 Sep 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507913[↩][↩][↩][↩][↩]
- Key Statistics About Multiple Myeloma. https://www.cancer.org/cancer/multiple-myeloma/about/key-statistics.html[↩][↩]
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004 Oct 28;351(18):1860-73. doi: 10.1056/NEJMra041875. Erratum in: N Engl J Med. 2005 Mar 17;352(11):1163.[↩][↩]
- Myeloma. https://cancerstatisticscenter.cancer.org/#!/cancer-site/Myeloma[↩]
- Myeloma — Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/mulmy.html[↩]
- Caers J, Paiva B, Zamagni E, Leleu X, Bladé J, Kristinsson SY, Touzeau C, Abildgaard N, Terpos E, Heusschen R, Ocio E, Delforge M, Sezer O, Beksac M, Ludwig H, Merlini G, Moreau P, Zweegman S, Engelhardt M, Rosiñol L. Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European Expert Panel. J Hematol Oncol. 2018 Jan 16;11(1):10. doi: 10.1186/s13045-017-0549-1[↩]
- Rajkumar, SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020; 95: 548– 567. https://doi.org/10.1002/ajh.25791[↩][↩][↩]
- Rowell, Sean & Ho, Matthew & Anderson, Kenneth. (2019). Plasmacytoma (Solitary bone plasmacytoma, extramedullary plasmacytoma). Atlas of Genetics and Cytogenetics in Oncology and Haematology. 23. 10.4267/2042/69762[↩]
- Kaseb H, Durer C, Fazal S, et al. Plasma Cell Cancer. [Updated 2022 Sep 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [Figure, Plasma Cancer Cell. Hatem Kaseb, MD, Ph.D., M.P.H.] Available from: https://www.ncbi.nlm.nih.gov/books/NBK507913/figure/article-41398.image.f1[↩]
- Rajkumar, V., Dimopoulos, M., Palumbo, A., Blade, J., Merlini, G., Mateos, M.-V., Kumar, S., Hillengass, J., Kastritis, E., Richardson, P., Landgren, O., Paiva, B., Dispenzieri, A., Weiss, B., Leleu, X., Zweegman, S., Lonial, S., Rosinol, L., Zamagni, E., & San Miguel, J. (2014). International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncology, 538-548. doi:10.1016/S1470-2045(14)70442-5[↩][↩]
- Landgren O. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: biological insights and early treatment strategies. Hematology Am Soc Hematol Educ Program. 2013;2013:478-87. doi: 10.1182/asheducation-2013.1.478[↩]
- Kaseb H, Annamaraju P, Babiker HM. Monoclonal Gammopathy Of Undetermined Significance. [Updated 2022 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507880[↩][↩][↩]
- Zingone A, Kuehl WM. Pathogenesis of monoclonal gammopathy of undetermined significance and progression to multiple myeloma. Semin Hematol. 2011 Jan;48(1):4-12. doi: 10.1053/j.seminhematol.2010.11.003[↩]
- Kyle RA, Larson DR, Therneau TM, Dispenzieri A, Kumar S, Cerhan JR, Rajkumar SV. Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N Engl J Med. 2018 Jan 18;378(3):241-249. doi: 10.1056/NEJMoa1709974[↩][↩][↩]
- Bird, J., Behrens, J., Westin, J., Turesson, I., Drayson, M., Beetham, R., D’Sa, S., Soutar, R., Waage, A., Gulbrandsen, N., Gregersen, H., Low, E. and (2009), UK Myeloma Forum (UKMF) and Nordic Myeloma Study Group (NMSG): guidelines for the investigation of newly detected M-proteins and the management of monoclonal gammopathy of undetermined significance (MGUS). British Journal of Haematology, 147: 22-42. https://doi.org/10.1111/j.1365-2141.2009.07807.x[↩]
- Kristinsson SY, Tang M, Pfeiffer RM, Björkholm M, Goldin LR, Blimark C, Mellqvist UH, Wahlin A, Turesson I, Landgren O. Monoclonal gammopathy of undetermined significance and risk of infections: a population-based study. Haematologica. 2012 Jun;97(6):854-8. doi: 10.3324/haematol.2011.054015[↩]
- Rajkumar SV, Kyle RA, Therneau TM, Melton LJ 3rd, Bradwell AR, Clark RJ, Larson DR, Plevak MF, Dispenzieri A, Katzmann JA. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005 Aug 1;106(3):812-7. doi: 10.1182/blood-2005-03-1038[↩][↩]
- Turesson I, Kovalchik SA, Pfeiffer RM, Kristinsson SY, Goldin LR, Drayson MT, Landgren O. Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid malignancies: 728 cases followed up to 30 years in Sweden. Blood. 2014 Jan 16;123(3):338-45. doi: 10.1182/blood-2013-05-505487[↩]
- Dhodapkar MV, Sexton R, Waheed S, Usmani S, Papanikolaou X, Nair B, Petty N, Shaughnessy JD Jr, Hoering A, Crowley J, Orlowski RZ, Barlogie B. Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120). Blood. 2014 Jan 2;123(1):78-85. doi: 10.1182/blood-2013-07-515239[↩]
- Go RS, Gundrum JD, Neuner JM. Determining the clinical significance of monoclonal gammopathy of undetermined significance: a SEER-Medicare population analysis. Clin Lymphoma Myeloma Leuk. 2015 Mar;15(3):177-186.e4. doi: 10.1016/j.clml.2014.09.004[↩]
- Sigurdardottir EE, Turesson I, Lund SH, et al. The Role of Diagnosis and Clinical Follow-up of Monoclonal Gammopathy of Undetermined Significance on Survival in Multiple Myeloma. JAMA Oncol. 2015;1(2):168–174. doi:10.1001/jamaoncol.2015.23[↩]
- McLellan L, Pohlman B, Rybicki L, et al. Distress screening scores of malignant and benign hematology patients: Results of a pilot project. Blood. 2012;120.[↩]
- Khimani F, Curley B, Almubarak M. Survey of Patients Referred to a University Cancer Center for Benign Hematology: Quality Measures and Patient Understanding. J Oncol Pract. 2015 Jan;11(1):26-9. doi: 10.1200/JOP.2014.001543[↩]
- Bladé J, Rosiñol L, Cibeira MT. Are all myelomas preceded by MGUS? Blood. 2009 May 28;113(22):5370. doi: 10.1182/blood-2009-03-207241[↩]
- Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009 May 28;113(22):5418-22. doi: 10.1182/blood-2008-12-195008[↩]
- Fermand JP, Bridoux F, Dispenzieri A, Jaccard A, Kyle RA, Leung N, Merlini G. Monoclonal gammopathy of clinical significance: a novel concept with therapeutic implications. Blood. 2018 Oct 4;132(14):1478-1485. doi: 10.1182/blood-2018-04-839480[↩]
- Leung N, Bridoux F, Nasr SH. Monoclonal Gammopathy of Renal Significance. N Engl J Med. 2021 May 20;384(20):1931-1941. doi: 10.1056/NEJMra1810907[↩]
- Merlini G. Determining the significance of MGUS. Blood. 2014 Jan 16;123(3):305-7. doi: 10.1182/blood-2013-12-539940[↩]
- Campbell JP, Heaney JLJ, Pandya S, Afzal Z, Kaiser M, Owen R, Child JA, Cairns DA, Gregory W, Morgan GJ, Jackson GH, Bunce CM, Drayson MT. Response comparison of multiple myeloma and monoclonal gammopathy of undetermined significance to the same anti-myeloma therapy: a retrospective cohort study. Lancet Haematol. 2017 Dec;4(12):e584-e594. doi: 10.1016/S2352-3026(17)30209-0[↩]
- Plasma Cell Neoplasms (Including Multiple Myeloma) Treatment (PDQ®)–Patient Version. https://www.cancer.gov/types/myeloma/patient/myeloma-treatment-pdq#section/all[↩]
- Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM, Larson DR, Plevak MF, Jelinek DF, Fonseca R, Melton LJ 3rd, Rajkumar SV. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007 Jun 21;356(25):2582-90. doi: 10.1056/NEJMoa070389[↩]
- Rajkumar SV, Landgren O, Mateos MV. Smoldering multiple myeloma. Blood. 2015 May 14;125(20):3069-75. doi: 10.1182/blood-2014-09-568899[↩]
- Lakshman A, Rajkumar SV, Buadi FK, Binder M, Gertz MA, Lacy MQ, Dispenzieri A, Dingli D, Fonder AL, Hayman SR, Hobbs MA, Gonsalves WI, Hwa YL, Kapoor P, Leung N, Go RS, Lin Y, Kourelis TV, Warsame R, Lust JA, Russell SJ, Zeldenrust SR, Kyle RA, Kumar SK. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 2018 Jun 12;8(6):59. doi: 10.1038/s41408-018-0077-4[↩]
- Mateos MV, Kumar S, Dimopoulos MA, González-Calle V, Kastritis E, Hajek R, De Larrea CF, Morgan GJ, Merlini G, Goldschmidt H, Geraldes C, Gozzetti A, Kyriakou C, Garderet L, Hansson M, Zamagni E, Fantl D, Leleu X, Kim BS, Esteves G, Ludwig H, Usmani S, Min CK, Qi M, Ukropec J, Weiss BM, Rajkumar SV, Durie BGM, San-Miguel J. International Myeloma Working Group risk stratification model for smoldering multiple myeloma (SMM). Blood Cancer J. 2020 Oct 16;10(10):102. doi: 10.1038/s41408-020-00366-3[↩]
- Leblond V, Kastritis E, Advani R, Ansell SM, Buske C, Castillo JJ, García-Sanz R, Gertz M, Kimby E, Kyriakou C, Merlini G, Minnema MC, Morel P, Morra E, Rummel M, Wechalekar A, Patterson CJ, Treon SP, Dimopoulos MA. Treatment recommendations from the Eighth International Workshop on Waldenström’s Macroglobulinemia. Blood. 2016 Sep 8;128(10):1321-8. https://doi.org/10.1182/blood-2016-04-711234[↩]
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 May 19;127(20):2375-90. doi: 10.1182/blood-2016-01-643569[↩]
- What Is Waldenstrom Macroglobulinemia? https://www.cancer.org/cancer/waldenstrom-macroglobulinemia/about/what-is-wm.html[↩][↩]
- Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML, Morra E, Pangalis GA, San Miguel JF, Branagan AR, Dimopoulos MA. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003 Apr;30(2):110-5. doi: 10.1053/sonc.2003.50082[↩]
- Mazzucchelli M, Frustaci AM, Deodato M, Cairoli R, Tedeschi A. Waldenstrom’s Macroglobulinemia: An Update. Mediterr J Hematol Infect Dis. 2018 Jan 1;10(1):e2018004. doi: 10.4084/MJHID.2018.004[↩]
- Key Statistics About Waldenstrom Macroglobulinemia. https://www.cancer.org/cancer/waldenstrom-macroglobulinemia/about/key-statistics.html[↩][↩][↩][↩]
- Waldenström macroglobulinemia (WM). https://www.lls.org/research/waldenstrom-macroglobulinemia-wm[↩][↩][↩]
- What Causes Multiple Myeloma? https://www.cancer.org/cancer/multiple-myeloma/causes-risks-prevention/what-causes.html[↩][↩][↩]
- Pasca S, Tomuleasa C, Teodorescu P, Ghiaur G, Dima D, Moisoiu V, Berce C, Stefan C, Ciechanover A, Einsele H. KRAS/NRAS/BRAF Mutations as Potential Targets in Multiple Myeloma. Front Oncol. 2019 Oct 24;9:1137. doi: 10.3389/fonc.2019.01137[↩]
- Kumar SK, Rajkumar SV. The multiple myelomas – current concepts in cytogenetic classification and therapy. Nat Rev Clin Oncol. 2018 Jul;15(7):409-421. doi: 10.1038/s41571-018-0018-y[↩]
- Fonseca R, Bailey RJ, Ahmann GJ, Rajkumar SV, Hoyer JD, Lust JA, Kyle RA, Gertz MA, Greipp PR, Dewald GW. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood. 2002 Aug 15;100(4):1417-24. https://doi.org/10.1182/blood.V100.4.1417.h81602001417_1417_1424[↩]
- Seidl S, Kaufmann H, Drach J. New insights into the pathophysiology of multiple myeloma. Lancet Oncol. 2003 Sep;4(9):557-64. doi: 10.1016/s1470-2045(03)01195-1[↩]
- Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022 Aug;97(8):1086-1107. doi: 10.1002/ajh.26590[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Rajan AM, Rajkumar SV. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer J. 2015 Oct 30;5(10):e365. doi: 10.1038/bcj.2015.92[↩][↩]
- Miller A, Asmann Y, Cattaneo L, Braggio E, Keats J, Auclair D, Lonial S; MMRF CoMMpass Network, Russell SJ, Stewart AK. High somatic mutation and neoantigen burden are correlated with decreased progression-free survival in multiple myeloma. Blood Cancer J. 2017 Sep 22;7(9):e612. doi: 10.1038/bcj.2017.94[↩]
- Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S, Lahuerta JJ, Facon T, Bringhen S, Gay F, Attal M, Passera R, Spencer A, Offidani M, Kumar S, Musto P, Lonial S, Petrucci MT, Orlowski RZ, Zamagni E, Morgan G, Dimopoulos MA, Durie BG, Anderson KC, Sonneveld P, San Miguel J, Cavo M, Rajkumar SV, Moreau P. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015 Sep 10;33(26):2863-9. doi: 10.1200/JCO.2015.61.2267[↩][↩]
- Multiple Myeloma Stages. https://www.cancer.org/cancer/multiple-myeloma/detection-diagnosis-staging/staging.html[↩]
- https://static1.squarespace.com/static/5b44f08ac258b493a25098a3/t/6244859445f2cf719cca6b93/1648657812271/RiskStrat+3.0_Mar2022_FINAL_REV.pdf[↩]
- Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M, Di Raimondo F, Crippa C, Zamagni E, Palumbo A, Offidani M, Corradini P, Narni F, Spadano A, Pescosta N, Deliliers GL, Ledda A, Cellini C, Caravita T, Tosi P, Baccarani M; GIMEMA Italian Myeloma Network. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010 Dec 18;376(9758):2075-85. doi: 10.1016/S0140-6736(10)61424-9. Epub 2010 Dec 9. Erratum in: Lancet. 2011 Nov 26;378(9806):1846.[↩]
- Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, Stewart AK, Turturro F, Rifkin R, Wolf J, Estevam J, Mulligan G, Shi H, Webb IJ, Rajkumar SV. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012 May 10;119(19):4375-82. doi: 10.1182/blood-2011-11-395749[↩][↩]
- Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, Avigan DE, Xie W, Ghobrial IM, Schlossman RL, Mazumder A, Munshi NC, Vesole DH, Joyce R, Kaufman JL, Doss D, Warren DL, Lunde LE, Kaster S, Delaney C, Hideshima T, Mitsiades CS, Knight R, Esseltine DL, Anderson KC. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010 Aug 5;116(5):679-86. doi: 10.1182/blood-2010-02-268862[↩]
- Bringhen S, Petrucci MT, Larocca A, Conticello C, Rossi D, Magarotto V, Musto P, Boccadifuoco L, Offidani M, Omedé P, Gentilini F, Ciccone G, Benevolo G, Genuardi M, Montefusco V, Oliva S, Caravita T, Tacchetti P, Boccadoro M, Sonneveld P, Palumbo A. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood. 2014 Jul 3;124(1):63-9. doi: 10.1182/blood-2014-03-563759[↩]
- Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, Hájek R, Rosiñol L, Siegel DS, Mihaylov GG, Goranova-Marinova V, Rajnics P, Suvorov A, Niesvizky R, Jakubowiak AJ, San-Miguel JF, Ludwig H, Wang M, Maisnar V, Minarik J, Bensinger WI, Mateos MV, Ben-Yehuda D, Kukreti V, Zojwalla N, Tonda ME, Yang X, Xing B, Moreau P, Palumbo A; ASPIRE Investigators. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015 Jan 8;372(2):142-52. doi: 10.1056/NEJMoa1411321[↩]
- Shah JJ, Stadtmauer EA, Abonour R, Cohen AD, Bensinger WI, Gasparetto C, Kaufman JL, Lentzsch S, Vogl DT, Gomes CL, Pascucci N, Smith DD, Orlowski RZ, Durie BG. Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood. 2015 Nov 12;126(20):2284-90. doi: 10.1182/blood-2015-05-643320[↩]
- Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Komarnicki M, Suzuki K, Plesner T, Yoon SS, Ben Yehuda D, Richardson PG, Goldschmidt H, Reece D, Lisby S, Khokhar NZ, O’Rourke L, Chiu C, Qin X, Guckert M, Ahmadi T, Moreau P; POLLUX Investigators. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016 Oct 6;375(14):1319-1331. doi: 10.1056/NEJMoa1607751[↩]
- Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV, Mark TM, Qi M, Schecter J, Amin H, Qin X, Deraedt W, Ahmadi T, Spencer A, Sonneveld P; CASTOR Investigators. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016 Aug 25;375(8):754-66. doi: 10.1056/NEJMoa1606038[↩]
- Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ, Weiss BM, Krishnan A, Lentzsch S, Comenzo R, Wang J, Nottage K, Chiu C, Khokhar NZ, Ahmadi T, Lonial S. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017 Aug 24;130(8):974-981. doi: 10.1182/blood-2017-05-785246[↩]
- Dimopoulos M, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, Weisel K, Yang H, Klippel Z, Zahlten-Kumeli A, Usmani SZ. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020 Jul 18;396(10245):186-197. doi: 10.1016/S0140-6736(20)30734-0. Erratum in: Lancet. 2020 Aug 15;396(10249):466.[↩]
- Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, Sandhu I, Ganly P, Baker BW, Jackson SR, Stoppa AM, Simpson DR, Gimsing P, Palumbo A, Garderet L, Cavo M, Kumar S, Touzeau C, Buadi FK, Laubach JP, Berg DT, Lin J, Di Bacco A, Hui AM, van de Velde H, Richardson PG; TOURMALINE-MM1 Study Group. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016 Apr 28;374(17):1621-34. doi: 10.1056/NEJMoa1516282[↩]
- Dimopoulos MA, Dytfeld D, Grosicki S, Moreau P, Takezako N, Hori M, Leleu X, LeBlanc R, Suzuki K, Raab MS, Richardson PG, Popa McKiver M, Jou YM, Shelat SG, Robbins M, Rafferty B, San-Miguel J. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N Engl J Med. 2018 Nov 8;379(19):1811-1822. doi: 10.1056/NEJMoa1805762[↩]
- Attal M, Richardson PG, Rajkumar SV, San-Miguel J, Beksac M, Spicka I, Leleu X, Schjesvold F, Moreau P, Dimopoulos MA, Huang JS, Minarik J, Cavo M, Prince HM, Macé S, Corzo KP, Campana F, Le-Guennec S, Dubin F, Anderson KC; ICARIA-MM study group. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019 Dec 7;394(10214):2096-2107. doi: 10.1016/S0140-6736(19)32556-5. Epub 2019 Nov 14. Erratum in: Lancet. 2019 Dec 7;394(10214):2072.[↩]
- Moreau P, Dimopoulos MA, Mikhael J, Yong K, Capra M, Facon T, Hajek R, Špička I, Baker R, Kim K, Martinez G, Min CK, Pour L, Leleu X, Oriol A, Koh Y, Suzuki K, Risse ML, Asset G, Macé S, Martin T; IKEMA study group. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet. 2021 Jun 19;397(10292):2361-2371. doi: 10.1016/S0140-6736(21)00592-4[↩]
- Himelstein AL, Foster JC, Khatcheressian JL, Roberts JD, Seisler DK, Novotny PJ, Qin R, Go RS, Grubbs SS, O’Connor T, Velasco MR Jr, Weckstein D, O’Mara A, Loprinzi CL, Shapiro CL. Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients With Bone Metastases: A Randomized Clinical Trial. JAMA. 2017 Jan 3;317(1):48-58. doi: 10.1001/jama.2016.19425[↩]
- Nea Raje. An International, Randomized, Double Blind Trial Comparing Denosumab With Zoledronic Acid (ZA) for the Treatment of Bone Disease in Patients (Pts) With Newly Diagnosed Multiple Myeloma. 16th International Myeloma Workshop (IMW); Mar 1-4, 2017; New Delhi, India, Abstracts Abstract 546 2017.[↩]
- National Cancer Institute. https://ccr.cancer.gov/clinical-trials/patients[↩]
- Developmental Therapeutics Clinic. https://dtc.cancer.gov/trials/search.htm[↩]
- Plasma Cell Neoplasms (Including Multiple Myeloma) Treatment (PDQ®)–Health Professional Version. https://www.cancer.gov/types/myeloma/hp/myeloma-treatment-pdq[↩][↩][↩]
- van de Donk NWCJ, Pawlyn C, Yong KL. Multiple myeloma. Lancet. 2021 Jan 30;397(10272):410-427. https://doi.org/10.1016/S0140-6736(21)00135-5[↩]
- Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, Thakuri M, Reu F, Reynolds CM, Sexton R, Orlowski RZ, Barlogie B, Dispenzieri A. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017 Feb 4;389(10068):519-527. doi: 10.1016/S0140-6736(16)31594-X[↩][↩]
- Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, Arnulf B, Macro M, Belhadj K, Garderet L, Roussel M, Payen C, Mathiot C, Fermand JP, Meuleman N, Rollet S, Maglio ME, Zeytoonjian AA, Weller EA, Munshi N, Anderson KC, Richardson PG, Facon T, Avet-Loiseau H, Harousseau JL, Moreau P; IFM 2009 Study. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med. 2017 Apr 6;376(14):1311-1320. doi: 10.1056/NEJMoa1611750[↩]
- Goldschmidt H, Lokhorst HM, Mai EK, van der Holt B, Blau IW, Zweegman S, Weisel KC, Vellenga E, Pfreundschuh M, Kersten MJ, Scheid C, Croockewit S, Raymakers R, Hose D, Potamianou A, Jauch A, Hillengass J, Stevens-Kroef M, Raab MS, Broijl A, Lindemann HW, Bos GMJ, Brossart P, van Marwijk Kooy M, Ypma P, Duehrsen U, Schaafsma RM, Bertsch U, Hielscher T, Jarari L, Salwender HJ, Sonneveld P. Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia. 2018 Feb;32(2):383-390. doi: 10.1038/leu.2017.211[↩]
- Paquin A, Kumar SK, Buadi FK, et al. Overall survival of transplant eligible patients with newly diagnosed multiple myeloma: comparative effectiveness analysis of modern induction regimens on outcome. Blood. 2017;130:3138.[↩]
- Pagano L, Valentini CG, De Stefano V, Venditti A, Visani G, Petrucci MT, Candoni A, Specchia G, Visco C, Pogliani EM, Ferrara F, Galieni P, Gozzetti A, Fianchi L, De Muro M, Leone G, Musto P, Pulsoni A; for GIMEMA-ALWP (Gruppo Italiano Malattie EMatologiche dell’Adulto, Acute Leukemia Working Party: coordinator Sergio Amadori). Primary plasma cell leukemia: a retrospective multicenter study of 73 patients. Ann Oncol. 2011 Jul;22(7):1628-1635. doi: 10.1093/annonc/mdq646[↩]
- Bladé J, Fernández de Larrea C, Rosiñol L, Cibeira MT, Jiménez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011 Oct 1;29(28):3805-12. doi: 10.1200/JCO.2011.34.9290[↩]
- Marinac CR, Ghobrial IM, Birmann BM, Soiffer J, Rebbeck TR. Dissecting racial disparities in multiple myeloma. Blood Cancer J. 2020 Feb 17;10(2):19. doi: 10.1038/s41408-020-0284-7[↩]
- Survival Rates for Multiple Myeloma. https://www.cancer.org/cancer/multiple-myeloma/detection-diagnosis-staging/survival-rates.html[↩]