Contents
- Neisseria meningitidis
- Neisseria meningitidis transmission
- Risk factors for meningococcal disease
- Neisseria meningitidis prevention
- Neisseria meningitidis vaccine
- Who should Not get Neisseria meningitidis vaccines?
- Neisseria meningitidis symptoms
- Meningococcal disease complications
- Neisseria meningitidis diagnosis
- Neisseria meningitidis treatment
- Meningococcal disease prognosis
- What is neisseria gonorrhoeae
- Neisseria gonorrhoeae transmission
- Risk factors for getting gonorrhea
- Gonorrhea prevention
- Neisseria gonorrhoeae symptoms
- Signs of gonorrhea
- Gonorrhea complications
- Neisseria gonorrhoeae diagnosis
- Neisseria gonorrhoeae treatment
- Antibiotics for gonorrhea
- Uncomplicated Gonococcal Infections of the Cervix, Urethra, and Rectum
- Uncomplicated Gonococcal Infections of the Pharynx
- Gonococcal conjunctivitis
- Disseminated Gonococcal Infection
- Gonococcal Infections in Pregnancy
- Management of Antibiotic-Resistant Gonorrhea
- Allergy to Penicillins or Cephalosporin
- Management of Suspected Gonococcal Treatment Failure
- Follow-Up
- Management of Sex Partners
Neisseria meningitidis
Neisseria meningitidis also known as meningococcus, is a bacteria that cause meningococcal disease, which is a serious and life-threatening infection. Meningococcal disease includes infections of the lining of the brain and spinal cord (meningitis) and bloodstream infections (bacteremia or septicemia) and can result in permanent disabilities and even death. Neisseria meningitidis is spread through the exchange of respiratory and throat secretions like spit (e.g., by living in close quarters, kissing). About 1 in 10 people have Neisseria meningitidis bacteria in the back of their nose and throat with no signs or symptoms of disease; this is called being ‘a carrier’. But sometimes the Neisseria meningitidis bacteria invade the body and cause certain illnesses, which are known as meningococcal disease.
There are five serogroups (types) of Neisseria meningitidis — A, B, C, W, and Y — that cause most disease worldwide. Three of these serogroups (B, C, and Y) cause most of the illness seen in the United States 1. In 2014, the overall incidence of invasive meningococcal disease for the United States was 0.14/100,000 population 2. Of the cases reported, 25% of cases were bacteremia with a fatality of 20%. The serogroup distribution was 26% serogroup B, 36% serogroup C, 9% serogroup Y, and 28% other serogroups. In the United States, serogroup B is responsible for 65% of infant disease, and serogroups C and Y are responsible for adolescent disease. Among the unvaccinated, outbreaks are due to serogroup C.
Meningococcal disease in the United States peaks during the months of November through March. A progressive increase in the protective antibodies against meningococci is seen between 2 and 12 years. Passive in utero IgG antibodies transfer occurs in neonates if the mother has the anti-meningococcal antibody.
In developing countries, the incidence rate of invasive meningococcal disease is 10 to 25 per 100,000 inhabitants per year. The highest rate of 10 to 1000 per 100,000 per year is seen in a belt across sub-Saharan Africa, termed the meningitis belt, with recurrent epidemics of group A. Death occurs in 6% to 10% of cases and complications in 4.3 to 11.2% of cases 3. Group A was predominant during 2007 through 2009, while serogroup W135 predominated in 2010 through 2011 4. In 2013 and 2014, 2 outbreaks of meningococcal serogroup C, a strain relatively rare in Africa, occurred in Nigeria and Kebbi 5.
International outbreaks of Neisseria meningitidis infections occurred in 1987 and 2000 associated with the Hajj pilgrimage to Mecca. 1987 outbreak was due to serogroup A and 2000 outbreak due to serogroup W-135. Serogroup W has emerged in other regions, including South America and England. In European countries, invasive serogroup C disease has declined and serogroup B is causing 60% to 72% of cases of invasive disease. In Asia, large epidemics caused by serogroup A occurred historically in China, India, Nepal and Russia, more recently serogroup B and C emerged as the cause in this area 6.
An increase in the Neisseria meningitidis serogroup Y has been reported in the Nordic European countries 7.
Neisseria meningitidis transmission occurs by respiratory droplets and requires close direct contact. Children younger than 5 years do not have adequate immunity against the polysaccharide antigens of Neisseria meningitidis 2. The risk factors for infectious disease in child care facilities include immunologic susceptibility, lack of awareness and practice of good hygiene, a natural tendency to intimacy, frequent oral contact with objects in the environment.
The invasive meningococcal disease is seen in 2 age groups: infants who are vulnerable due to disappearance in the early life of the maternal antibodies and adolescents with a high rate of colonization of the nasopharynx 8.
Asymptomatic pharyngeal colonization is the initial step of infection with humans being the natural reservoirs 2. From the nasopharynx, the Neisseria meningitidis bacteria reaches the meninges translocating across the nasopharyngeal mucosa and along the perineural sheath of the olfactory nerve, through the cribriform plate of the ethmoid. Bloodstream spread to the meninges will cause meningitis. In some children, the predominant feature is cardiovascular collapse leading to septic shock.
Meningococcal disease can be devastating and often and unexpectedly strikes otherwise healthy people. Although meningococcal disease is uncommon, teens and young adults 16 through 23 years old are at increased risk 9.
Doctors treat meningococcal disease with antibiotics, but quick medical attention is extremely important. Keeping up to date with recommended vaccines is the best defense against meningococcal disease.
Neisseria meningitidis transmission
People spread Neisseria meningitidis bacteria to other people by sharing respiratory and throat secretions (saliva or spit). Generally, it takes close (for example, coughing or kissing) or lengthy contact to spread Neisseria meningitidis bacteria. Fortunately, Neisseria meningitidis bacteria are not as contagious as germs that cause the common cold or the flu. People do not catch Neisseria meningitidis bacteria through casual contact or by breathing air where someone with meningococcal disease has been 1.
Sometimes the Neisseria meningitidis bacteria spread to people who have had close or lengthy contact with a patient with meningococcal disease. Those at increased risk of getting sick include:
- People who live with the patient
- Anyone with direct contact with the patient’s oral secretions, such as a boyfriend or girlfriend
Close contacts of someone with meningococcal disease should receive antibiotics to help prevent them from getting the disease. This is known as prophylaxis. Health departments investigate each case of meningococcal disease to identify all close contacts and make sure they receive prophylaxis. This does not mean that the contacts have the disease; it is to prevent it. People who are not a close contact of a patient with meningococcal disease do not need prophylaxis.
Risk factors for meningococcal disease
Certain people are at increased risk for meningococcal disease. Some risk factors include:
- Age: Doctors more commonly diagnose meningococcal disease in infants, teens, and young adults.
- Infants, preteens, teens, and young adults have the highest rates of meningococcal disease in the United States.
- Community setting: Infectious diseases tend to spread wherever large groups of people gather together. Several college campuses have reported outbreaks of serogroup B meningococcal disease during the last several years.
- Certain medical conditions: Certain medical conditions and medications put people at increased risk of meningococcal disease. They include not having a spleen, having a complement component deficiency, and being infected with HIV.
- Travel: Travelers to the meningitis belt in sub-Saharan Africa may be at risk for meningococcal disease.
Neisseria meningitidis prevention
Keeping up to date with recommended immunizations is the best defense against meningococcal disease. Maintaining healthy habits, like getting plenty of rest and not having close contact with people who are sick, also helps.
Vaccination
Vaccines help protect against all three serogroups (B, C, and Y) of Neisseria meningitidis bacteria commonly seen in the United States. Like with any vaccine, meningococcal vaccines are not 100% effective. This means there is still a chance you can develop meningococcal disease after vaccination. People should know the symptoms of meningococcal disease since early recognition and quick medical attention are extremely important.
Antibiotics
Close contacts of a person with meningococcal disease should receive antibiotics to prevent them from getting sick. This is known as prophylaxis (pro-fuh-lak-sis). Examples of close contacts include:
- People in the same household or roommates
- Anyone with direct contact with a patient’s oral secretions (saliva or spit), such as a boyfriend or girlfriend
Doctors or local health departments recommend who should get prophylaxis.
Re-Infection
Although rare, people can get meningococcal disease more than once. A previous infection will not offer lifelong protection from future infections. Therefore, CDC recommends meningococcal vaccines for all preteens and teens. In certain situations, children and adults should also get meningococcal vaccines.
Neisseria meningitidis vaccine
The Centers for Disease Control and Prevention (CDC) recommends vaccination with a meningococcal conjugate vaccine for all preteens and teens at 11 to 12 years old, with a booster dose at 16 years old. Teens and young adults (16 through 23 year olds) also may be vaccinated with a serogroup B meningococcal vaccine 10.
There are two types of meningococcal vaccines available in the United States:
- Conjugate vaccines (Menactra® and Menveo®)
- Serogroup B (recombinant) vaccines (Bexsero® and Trumenba®)
Meningococcal Conjugate Vaccines
- Menactra®: Two doses are given to preteens and teens. It is also given to certain people at increased risk of meningococcal disease. It helps protect against four types of the bacteria that cause meningococcal disease (serogroups A, C, W, and Y).
- Menveo®: Two doses are given to preteens and teens. It is also given to certain people at increased risk of meningococcal disease. It helps protect against four types of the bacteria that cause meningococcal disease (serogroups A, C, W, and Y).
Serogroup B (Recombinant) Meningococcal Vaccines
- Bexsero®: It is given as a two-dose series to people 16 through 23 years old who are not at increased risk of meningococcal disease. It is also given as a two-dose series to people 10 years or older at increased risk of meningococcal disease. It helps protect against one type of the bacteria that causes meningococcal disease (serogroup B).
- Trumenba®: It is given as a two-dose series to people 16 through 23 years old who are not at increased risk of meningococcal disease. It is given as a three-dose series to people 10 years or older at increased risk of meningococcal disease. It helps protect against one type of the bacteria that causes meningococcal disease (serogroup B).
Vaccines that help protect against meningococcal disease work well, but cannot prevent all cases.
In studies demonstrating the efficacy of meningococcal conjugate vaccines:
- Menactra® in preteens and teens: Between 8 and 9 people out of every 10 vaccinated had a protective immune response one month after completing the series
- Menactra® in adults: Between 7 and 9 people out of every 10 vaccinated had a protective immune response one month after completing the series
- Menveo® in preteens and teens: Between 7 and 9 people out of every 10 vaccinated had a protective immune response one month after completing the series
- Menveo® in adults: Between 7 and 9 people out of every 10 vaccinated had a protective immune response one month after completing the series
In studies demonstrating the efficacy of serogroup B meningococcal vaccines:
- Besexero® in preteens, teens, and young adults: Between 6 and 9 people out of every 10 vaccinated had a protective immune response one month after completing the series
- Trumenba® in preteens, teens, and young adults: 8 people out of every 10 vaccinated had a protective immune response one month after completing the series
Preteens and Teens
There are two types of meningococcal vaccines for preteens and teens:
- Meningococcal conjugate vaccines (Menactra® or Menveo®)
- Serogroup B meningococcal vaccines (Bexsero® or Trumenba®)
Each type helps protect your child against different serogroups (strains) of meningococcal disease. Some meningococcal vaccines for preteens and teens are designed to protect against four serogroups (A, C, W, and Y), while others help protect against one serogroup (B). There currently is not a meningococcal vaccine that offers protection against all common serogroups in one shot.
The minimum time needed between doses of meningococcal conjugate vaccine is 8 weeks. However, the second dose is recommended at age 16 so that teens have boosted protection during the ages when they are at highest risk.
All 11 to 12 year olds should be vaccinated with a meningococcal conjugate vaccine, with a booster dose given at 16 years old. All teens may also be vaccinated with a serogroup B meningococcal vaccine, preferably at 16 through 18 years old, when they are at highest risk of meningococcal disease.
Protection from a single dose of meningococcal conjugate vaccination declines in most teens within 5 years. So, teens need a second dose at age 16 to boost their protection during the ages when they are at highest risk of meningococcal disease.
In addition to a meningococcal conjugate vaccine, certain preteens and teens should get a serogroup B meningococcal vaccine if they:
- Have a rare type of disorder (complement component deficiency)
- Are taking the medicine called Soliris®
- Have a damaged spleen or their spleen has been removed
- Are part of a population identified to be at increased risk because of a serogroup B meningococcal disease outbreak
Many colleges require proof of meningococcal conjugate vaccination within 5 years before starting school.
Even if it isn’t a requirement for your child’s school, CDC recommends all teens vaccinated before their 16th birthday get a booster dose for the best protection during the ages when they are at highest risk. Teens who receive their first dose of a meningococcal conjugate vaccine at or after age 16 do not need a booster dose.
Babies and Children
CDC recommends a meningococcal conjugate vaccine (Menactra® or Menveo®) for children who are between 2 months and 10 years old, if they:
- Have a rare type of disorder (complement component deficiency)
- Are taking the medicine called Soliris®
- Have a damaged spleen or their spleen has been removed
- Have HIV
- Are traveling to or residing in countries in which the disease is common
- Are part of a population identified to be at increased risk because of a serogroup A, C, W, or Y meningococcal disease outbreak
Talk to your child’s doctor to find out if, and when, they will need booster shots.
CDC recommends a serogroup B meningococcal vaccine (Bexsero® or Trumenba®) for children 10 years or older if they:
- Have a rare type of disorder (complement component deficiency)
- Are taking a medicine called Soliris®
- Have a damaged spleen or their spleen has been removed
- Are part of a population identified to be at increased risk because of a serogroup B meningococcal disease outbreak
Adults
Meningococcal vaccines are recommended for certain groups of adults at increased risk for meningococcal disease. Each meningococcal vaccine is listed below with which groups of adults are recommended to get it.
Meningococcal Conjugate Vaccine Recommendations
Adults should get a meningococcal conjugate vaccine (Menactra® or Menveo®) if they:
- Have a rare type of disorder (complement component deficiency)
- Are taking a medicine called Soliris®
- Have a damaged spleen or their spleen has been removed
- Have HIV
- Are a microbiologist who is routinely exposed to Neisseria meningitidis
- Are traveling to or residing in countries in which the disease is common
- Are part of a population identified to be at increased risk because of a serogroup A, C, W, or Y meningococcal disease outbreak
- Are not up to date with this vaccine and are a first-year college student living in a residence hall
- Are a military recruit
Talk to your doctor to find out if, and when, you will need booster shots.
Serogroup B Meningococcal Vaccine Recommendations
Adults should get a serogroup B meningococcal vaccine (Bexsero® or Trumenba®) if they:
- Have a rare type of disorder (complement component deficiency)
- Are taking a medicine called Soliris®
- Have a damaged spleen or their spleen has been removed
- Are a microbiologist who is routinely exposed to Neisseria meningitidis
- Are part of a population identified to be at increased risk because of a serogroup B meningococcal disease outbreak
Meningococcal vaccines possible side effects
Most people who get a meningococcal vaccine do not have any serious problems with it. With any medicine, including vaccines, there is a chance of side effects. These are usually mild and go away on their own within a few days, but serious reactions are also possible.
Mild Problems
Meningococcal Conjugate Vaccines
- Mild problems following meningococcal conjugate vaccination can include:
- Reactions where the shot was given
- Redness
- Pain
- Fever
If these problems occur, they usually last for 1 or 2 days.
Serogroup B Meningococcal Vaccines
- Mild problems following a serogroup B meningococcal vaccination can include:
- Reactions where the shot was given
- Soreness
- Redness
- Swelling
- Feeling tired
- Headache
- Muscle or joint pain
- Fever or chills
- Nausea or diarrhea
- Reactions where the shot was given
If these problems occur, they can last up to 3 to 7 days.
Problems that could happen after getting any injected vaccine
- People sometimes faint after a medical procedure, including vaccination. Sitting or lying down for about 15 minutes can help prevent fainting, and injuries caused by a fall. Tell the healthcare professional if you or your child feel dizzy, have vision changes, or have ringing in the ears.
- Some people get severe pain in the shoulder and have difficulty moving the arm where a shot was given. This happens very rarely.
- Any medicine can cause a severe allergic reaction. Such reactions from a vaccine are very rare, estimated at about 1 in a million doses, and would happen within a few minutes to a few hours after the vaccination.
- As with any medicine, there is a very remote chance of a vaccine causing a serious injury or death.
Who should Not get Neisseria meningitidis vaccines?
Because of age or health conditions, some people should not get certain vaccines or should wait before getting them. Read the guidelines below and ask your or your child’s healthcare professional for more information.
Tell the person who is giving you or your child a meningococcal vaccine if:
You or your child have had a life-threatening allergic reaction or have a severe allergy.
- Anyone who has ever had a life-threatening allergic reaction after a previous dose of a meningococcal vaccine should not get another dose of that vaccine.
- Anyone who has a severe allergy to any part of these vaccines should not get another dose of that vaccine. Your child’s healthcare professional can tell you about the vaccine’s ingredients.
You are pregnant or breastfeeding.
- Meningococcal conjugate vaccines may be given to pregnant women who are at increased risk for serogroup A, C, W, or Y meningococcal disease.
- Serogroup B meningococcal vaccines should only be given to pregnant or breastfeeding women who are at increased risk for serogroup B meningococcal disease who decide, after talking with a doctor, that the benefits of getting the vaccine outweigh the risk.
You or your child are not feeling well.
- People who have a mild illness, such as a cold, can probably get the vaccine. People who are moderately or severely ill should probably wait until they recover. Your or your child’s healthcare professional can advise you.
Neisseria meningitidis symptoms
Seek medical attention immediately if you or your child develops symptoms of meningococcal disease. Symptoms of meningococcal disease can first appear as a flu-like illness and rapidly worsen. The two most common types of meningococcal infections are meningitis and septicemia. Both of these types of infections are very serious and can be deadly in a matter of hours.
If you think you or your child has any of these symptoms, get medical attention right away.
Meningococcal meningitis
Doctors call meningitis caused by the bacteria Neisseria meningitidis meningococcal meningitis. When someone has meningococcal meningitis, the bacteria infect the protective membranes covering their brain and spinal cord and cause swelling.
The most common symptoms include:
- Fever
- Headache
- Stiff neck
There are often additional symptoms, such as:
- Nausea
- Vomiting
- Photophobia (eyes being more sensitive to light)
- Altered mental status (confusion)
Newborns and babies may not have or it may be difficult to notice the classic symptoms of fever, headache, and neck stiffness. Instead, babies may be slow or inactive, irritable, vomiting, or feeding poorly. In young children, doctors may also look at the child’s reflexes for signs of meningitis.
Meningococcal septicemia (meningococcemia)
Doctors call septicemia (a bloodstream infection) caused by Neisseria meningitidis meningococcal septicemia or meningococcemia. When someone has meningococcal septicemia, the bacteria enter the bloodstream and multiply, damaging the walls of the blood vessels. This causes bleeding into the skin and organs.
Symptoms may include:
- Fever
- Fatigue
- Vomiting
- Cold hands and feet
- Cold chills
- Severe aches or pain in the muscles, joints, chest or abdomen (belly)
- Rapid breathing
- Diarrhea
- In the later stages, a dark purple rash.
Meningococcal disease complications
- Complications of meningococcemia are skin necrosis (ischaemic infarction of skin and soft tissues), hearing loss, deafness, seizure, amputation, and skin scarring. Impaired organ perfusion due to hypovolemia, vasoconstriction and myocardial failure result in prerenal failure manifesting as oliguria or anuria or acute tubular necrosis.
- Immunologic or reactive complications like arthritis, cutaneous vasculitis, iritis, and pericarditis are due to deposition of immune complexes with polysaccharide antigen, IgG, and C3 resulting in acute inflammation.
Neisseria meningitidis diagnosis
Meningococcal disease is very serious and can be deadly in a matter of hours. Early diagnosis and treatment are very important.
Meningococcal disease can be difficult to diagnose because the signs and symptoms are often similar to those of other illnesses. If a doctor suspects meningococcal disease, they will collect samples of blood or cerebrospinal fluid. Doctors then test the samples to see if there is an infection and, if so, what germ is causing it. Cerebrospinal fluid (CSF) in meningitis shows Gram-negative intracellular and extracellular diplococci. If Neisseria meningitidis bacteria are in the samples, laboratorians can grow (culture) the bacteria. Growing the bacteria in the laboratory allows doctors to know the specific type of bacteria that is causing the infection. Knowing this helps doctors decide which antibiotic will work best. Other tests can sometimes detect and identify the bacteria if the cultures do not.
Neisseria meningitidis treatment
Doctors treat meningococcal disease with a number of effective antibiotics. It is important that treatment start as soon as possible. If a doctor suspects meningococcal disease, they will give the patient antibiotics right away. Antibiotics help reduce the risk of dying.
Depending on how serious the infection is, people with meningococcal disease may need other treatments, including:
- Breathing support
- Medications to treat low blood pressure
- Wound care for parts of the body with damaged skin
Antimicrobial Agents
Third-generation cephalosporin-ceftriaxone or cefotaxime are used for initial therapy.
Continued disease:
- Ceftriaxone (80 mg/kg per day in 1 to 2 divided doses intravenously [IV])
- Cefotaxime (200 mg/kg per day in 3 to4 divided doses IV)
- Penicillin G (50 mg/kg every 4 to 6 hours IV)
- Chloramphenicol (100 mg/kg/day in 4 divided doses, orally or IV)
- Meropenem for those with severe allergies
Recommended duration of therapy is 7 days for both meningitis and meningococcemia.
Adjunctive and Experimental Therapies
Corticosteroid therapy: Replacement doses (25 mg/m3 hydrocortisone 4 times) daily is useful in children with refractory shock associated with impaired adrenal gland response.
- Recombinant bactericidal permeability-increasing protein (rBPI), binds to endotoxin and blocks the inflammatory cascade. Children receiving rBPI had fewer amputation and blood product transfusions and improved functional outcomes.
- Of other adjunctive therapies in the management of septicemia are plasmapheresis, extracorporeal membrane oxygenation (ECMO), fibrinolysis and anti-mediator therapy.
Emergency Management
- After securing the airway, priorities in children with meningococcal disease are:
- Correction of cardiovascular shock and
- Control of raised intracranial pressure
- Aggressive fluid resuscitation with 0.9% NaCl solution in a volume of 20 ml/kg over 5 to 10 minutes is of importance and repeated until shock improves.
- Inotropic support is needed to maintain tissue perfusion.
- Human albumin solution can be used as an alternative.
- Anemia, coagulopathy is monitored and corrected.
- In cases of raised intracranial pressure, adequate cerebral perfusion should be ensured by correcting shock and providing neurointensive care.
Meningococcal disease prognosis
Even with antibiotic treatment, 10 to 15 in 100 people infected with meningococcal disease will die. About 11 to 19 in 100 survivors will have long-term disabilities, such as loss of limb(s), deafness, nervous system problems, or brain damage.
What is neisseria gonorrhoeae
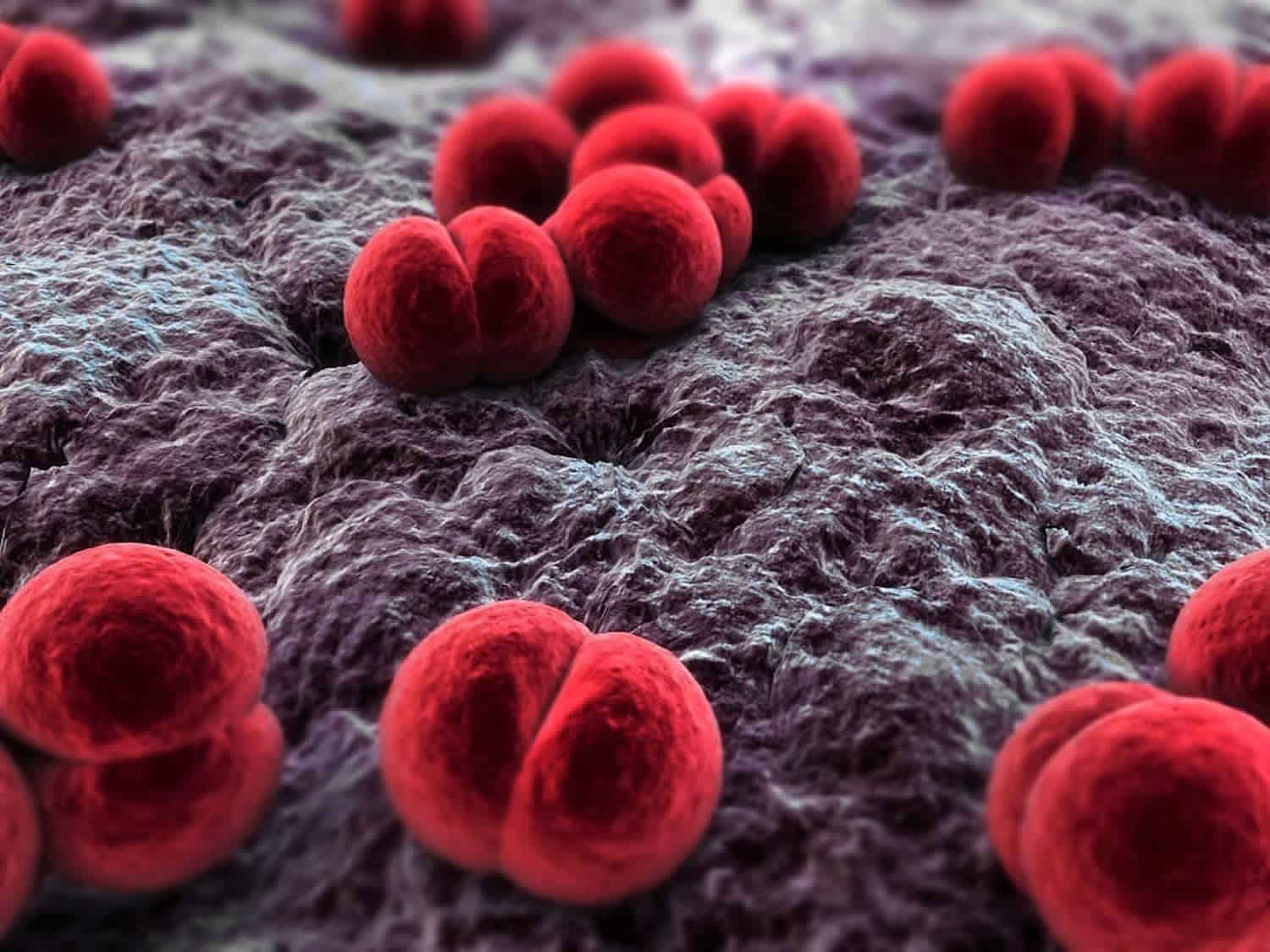
Neisseria gonorrhoeae bacterium causes the sexually transmitted disease (STD) gonorrhea that can infect both men and women, among other diseases. Neisseria gonorrhoeae infects the mucous membranes of the reproductive tract, including the cervix, uterus, and fallopian tubes in women, and the urethra in women and men. Neisseria gonorrhoeae can also infect the mucous membranes of the mouth, throat, eyes, and rectum. Neisseria gonorrhoeae is spread by sexual contact or through transmission during childbirth. The Centers for Disease Control and Prevention (CDC) recommends that all patients with gonorrheal infection also be treated for presumed co-infection with Chlamydia trachomatis 11.
Gonorrhea is a very common infectious disease. CDC estimates that approximately 820,000 new gonococcal infections occur in the United States each year, and more than half of these infections are detected and reported to CDC 12. CDC estimates that 570,000 of them were among young people 15-24 years of age. In 2017, 555,608 cases of gonorrhea were reported to CDC 13.
Who should be tested for gonorrhea?
Any sexually active person can be infected with gonorrhea. Anyone with genital symptoms such as discharge, burning during urination, unusual sores, or rash should stop having sex and see a health care provider immediately.
Also, anyone with an oral, anal, or vaginal sex partner who has been recently diagnosed with an sexually transmitted disease (STD) should see a health care provider for evaluation.
Some people should be tested (screened) for gonorrhea even if they do not have symptoms or know of a sex partner who has gonorrhea 14. Anyone who is sexually active should discuss his or her risk factors with a health care provider and ask whether he or she should be tested for gonorrhea or other sexually transmitted diseases (STDs).
CDC recommends yearly gonorrhea screening for all sexually active women younger than 25 years, as well as older women with risk factors such as new or multiple sex partners, or a sex partner who has a sexually transmitted infection.
People who have gonorrhea should also be tested for other STDs.
Is gonorrhea curable?
Yes. Gonorrhea can be cured with the right treatment. The Centers for Disease Control and Prevention (CDC) recommends dual therapy or using two drugs, to treat gonorrhea – a single dose of 250mg of intramuscular ceftriaxone AND 1g of oral azithromycin. It is important to take all of the medication prescribed to cure gonorrhea. Medication for gonorrhea should not be shared with anyone. Although medication will stop the infection, it will not repair any permanent damage done by the disease. Antimicrobial resistance in gonorrhea is of increasing concern, and successful treatment of gonorrhea is becoming more difficult. If a person’s symptoms continue for more than a few days after receiving treatment, he or she should return to a health care provider to be reevaluated.
Neisseria gonorrhoeae transmission
Gonorrhea is transmitted through sexual contact with the penis, vagina, mouth, or anus of an infected partner. Ejaculation does not have to occur for gonorrhea to be transmitted or acquired. Gonorrhea can also be spread perinatally from mother to baby during childbirth, which can cause eye infection (neonatal conjunctivitis) and even blindness.
People who have had gonorrhea and received treatment may be reinfected if they have sexual contact with a person infected with gonorrhea.
Any sexually active person can be infected with gonorrhea. In the United States, the highest reported rates of infection are among sexually active teenagers, young adults, and African Americans 13.
The transmission of gonorrhea can occur in several ways:
- Male-to-female transmission of gonorrhea via semen occurs at a rate of approximately 50% to 70% per episode of vaginal intercourse with ejaculation; male-to-female transmission of gonorrhea can occur without ejaculation 15.
- An infected woman can transmit gonorrhea to the urethra of a male sex partner; the rate of transmission is approximately 20% per episode from vaginal intercourse, and it increases to approximately 60% to 80% after four or more intercourse exposures 16.
- Pharyngeal gonorrhea is readily acquired by fellatio; it is less efficiently acquired by cunnilingus. Gonorrhea can also be transmitted from the pharynx to the urethra during fellatio (and presumably to vagina with cunnilingus).
- Perinatal transmission (mother-to-infant) can occur during vaginal delivery, when the infected mother has not been treated during the perinatal period.
Rectal intercourse transmission rates have not been quantified, but rectal intercourse appears to be an efficient mode of transmission. - Gonorrhea is associated with increased susceptibility to HIV acquisition. It is also associated with an increase in HIV transmission, because gonococcal urethritis increases HIV shedding in men 17.
How does gonorrhea affect a pregnant woman and her baby?
If a pregnant woman has gonorrhea, she may give the infection to her baby as the baby passes through the birth canal during delivery. This can cause blindness, joint infection, or a life-threatening blood infection in the baby 18. Treatment of gonorrhea as soon as it is detected in pregnant women will reduce the risk of these complications. Pregnant women should consult a health care provider for appropriate examination, testing, and treatment, as necessary.
Risk factors for getting gonorrhea
Risk factors and risk markers for acquiring gonorrhea include:
- Younger age
- Being adolescent (especially female)
- A new sex partner
- Multiple sex partners
- A sex partner who has concurrent partners
- Inconsistent or incorrect condom use
- Living in an urban area where gonorrhea prevalence is high
- Having a lower socio-economic status
- Using drugs including alcohol (in association with higher risk sex)
- Exchanging sex for drugs or money
- African American race
- Previous gonorrhea diagnosis
- Having other sexually transmitted infections
Gonorrhea prevention
Practicing safe sex is the best way to prevent gonorrhea infection.
Latex condoms, when used consistently and correctly, can reduce the risk of transmission of gonorrhea 19. The surest way to avoid transmission of gonorrhea or other STDs is to abstain from vaginal, anal, and oral sex, or to be in a long-term mutually monogamous relationship with a partner who has been tested and is known to be uninfected.
Take steps to reduce your risk of gonorrhea:
- Always use condoms with a water-based lubricant if you choose to have sex. Abstaining from sex is the surest way to prevent gonorrhea. But if you choose to have sex, use a condom during any type of sexual contact, including anal sex, oral sex or vaginal sex.
- Always use dental dams for oral sex (a dental dam is a thin square of latex placed over the vulva or anus during oral sex).
- Limit your sex partners or have a long-term monogamous relationship where neither of you is already infected.
- Ask your partner to be tested for sexually transmitted infections. Find out whether your partner has been tested for sexually transmitted infections, including gonorrhea. If not, ask whether he or she would be willing to be tested.
- Don’t have sex with someone who has any unusual symptoms. If your partner has signs or symptoms of a sexually transmitted infection, such as burning during urination or a genital rash or sore, don’t have sex with that person.
- Avoid sex with someone infected with gonorrhea until after they have finished treatment and are cured.
- Have regular sexually transmitted infection (STI) check-ups. Annual screening is recommended for all sexually active women less than 25 years of age and for older women at increased risk of infection, such as those who have a new sex partner, more than one sex partner, a sex partner with concurrent partners, or a sex partner who has a sexually transmitted infection. Regular screening is also recommended for men who have sex with men, as well as their partners.
To avoid reinfection with gonorrhea, abstain from unprotected sex for seven days after you and your sex partner have completed treatment and after resolution of symptoms, if present.
Screening for gonococcal infection
Routine screening for gonorrhea infection in women is recommended in order to decrease morbidity as well as to reduce the burden of disease in the community 20. Urethral infections caused by gonorrhea among men usually produce symptoms that cause them to seek curative treatment soon enough to prevent sequelae, but transmission to others may occur in this interim. Among women, gonococcal infections are commonly asymptomatic until complications (such as pelvic inflammatory disease with resultant risk for infertility and ectopic pregnancy) have occurred. The following summarizes gonorrhea screening recommendations issued by the CDC and the U.S. Preventive Services Task Force (USPSTF) for different patient populations 20, 21:
- Sexually Active Women Who Have Sex with Men: The CDC 20 and the U.S. Preventive Services Task Force 21 recommend (1) annual screening for gonorrhea in all sexually active women younger than 25 years of age, and (2) annual screening for gonorrhea in sexually active women age 25 years and older if they are considered to have increased risk for gonococcal infection. The most important identified risk factors for gonococcal infection include a new sex partner, multiple sex partners, a sex partner with concurrent partners, or a sex partner with a sexually transmitted infection; additional factors that indicate risk of gonococcal infection include inconsistent condom use in persons not in a mutually monogamous relationship, exchange of sex for money or drugs, one or more previous sexually transmitted infections, or a coexistent sexually transmitted infection. Women diagnosed with gonorrhea infection should have repeat testing approximately 3 months after completing treatment.
- Women Who Have Sex with Women: The CDC recommends gonococcal screening for women who have sex with women should occur according to the current screening guidelines for sexually active women who have sex with men 22.
- Women Who are Pregnant: The CDC recommends screening for gonorrhea should be performed at the first prenatal visit for (1) women younger than age 25 and (2) women age 25 years and older who are at increased risk for gonorrhea (e.g. women with a new sex partner, a sex partner who has a sexually transmitted infection, more than one sex partner, or a sex partner with concurrent partners 22. Additional factors associated with increased risk of gonococcal infection include inconsistent condom use in persons not in a mutually monogamous relationship, exchange of sex for money or drugs, and previous or coexisting sexually transmitted infections. A repeat test for gonococcal infection should be performed during the third trimester for those at continued risk. Pregnant women diagnosed with gonorrhea infection should have repeat testing approximately 3 months after completing treatment 22.
- Men Who Have Sex Only with Women: Routine screening for gonococcal infection is not recommended by either the CDC or the USPSTF for men who have sex only with women.[10,27]
- Men Who Have Sex with Men: The CDC 22 recommends screening for gonococcal infection in men who have sex with men at least annually, regardless of a history of condom use during sexual contact; the sites tested should correspond with sites involved in sexual activity with other men during the prior year (e.g. urethral testing if insertive intercourse, rectal testing if receptive anal intercourse, and pharyngeal testing with receptive oral intercourse). The U.S. Preventive Services Task Force 21 does not recommend routine screening for gonorrhea in men, including men who have sex with men.
- Transgender Men and Women: The CDC 22 recommends screening for gonorrhea in transgender men (“trans-men”) and transgender women (“trans-women”) should be based on age, current anatomy, and sexual practices.
- Persons with HIV Infection: The CDC 23 recommends performing routine screening for gonorrhea for persons with HIV infection who are sexually active; testing for gonorrhea should be performed at the initial evaluation and at least annually thereafter (more frequent screening may be indicated based on risk). The testing should consist of obtaining samples from the anatomic sites of sexual exposure.
- Persons in Correctional Facilities: The CDC 22 recommends performing routine gonococcal screening at the initial intake in a correctional facility for women 35 years of age and younger and men younger than age 30.
Neisseria gonorrhoeae symptoms
Many men with gonorrhea are asymptomatic 24. When present, signs and symptoms of urethral infection in men include dysuria or a white, yellow, or green urethral discharge that usually appears one to fourteen days after infection 25. In cases where urethral infection is complicated by epididymitis, men with gonorrhea may also complain of testicular or scrotal pain.
Most women with gonorrhea are asymptomatic 26. Even when a woman has symptoms, they are often so mild and nonspecific that they are mistaken for a bladder or vaginal infection 27. The initial symptoms and signs in women include dysuria, increased vaginal discharge, or vaginal bleeding between periods. Women with gonorrhea are at risk of developing serious complications from the infection, regardless of the presence or severity of symptoms.
Symptoms of rectal infection in both men and women may include discharge, anal itching, soreness, bleeding, or painful bowel movements 28. Rectal infection also may be asymptomatic. Pharyngeal infection may cause a sore throat, but usually is asymptomatic 29.
Gonorrhea symptoms in men
In men, when symptoms do occur, they usually develop within one to three days. In men, gonorrhea symptoms may include:
- thick, yellow or white discharge from the penis
- pain, discomfort or burning sensation when passing urine
- pain or swelling in one testis (balls)
- redness around the opening of the penis
- anal discharge and discomfort
- sore, dry throat.
Urethritis
Urethritis is a common manifestation of gonorrhea in men. Most men develop overt, symptomatic urethritis, but a small percentage will develop asymptomatic (unrecognized) infection. Asymptomatic gonorrhea may act as a reservoir that perpetuates transmission in the community 30. The typical symptoms of gonococcal urethritis, when present, include a purulent or mucopurulent urethral discharge (Figure 12), often accompanied by dysuria. The discharge may also be clear or cloudy. The incubation period ranges from 1 to 14 days, with most men becoming symptomatic within 2 to 5 days after exposure 31.
Anorectal infections
Anorectal infection most often occurs in men who have sex with men, with acquisition of rectal gonorrhea occurring through receptive anal intercourse, but it also has been reported in women with gonococcal cervicitis who do not acknowledge rectal sexual contact. These infections may result from perineal contamination with infected cervical secretions. Most patients with anorectal infection are asymptomatic, although proctitis can occur. Symptoms of proctitis include anal irritation, painful defecation, constipation, scant rectal bleeding, painless mucopurulent discharge, anal pruritus, and tenesmus.[18] When proctitis is suspected, an anoscopic examination is recommended to assess for inflammation and mucosal injury. The anorectal mucosa may appear normal, but purulent discharge, erythema, or easily induced bleeding may be observable under anoscopy.
Complications of genital infection in men
Men with untreated gonococcal genital infection can develop epididymitis, with typical symptoms of unilateral testicular pain and swelling, and epididymal tenderness. Epididymitis is infrequent following gonococcal infection, but it is the most common local complication of gonorrhea infection in men. When it does occur, epididymitis is often associated with overt or subclinical urethritis. Urethral discharge may or may not be present. Notably, up to 70% of epididymitis caused by a sexually transmitted pathogen are due to Chlamydia trachomatis. Other less common complications associated with gonococcal infection in men include inguinal lymphadenitis, penile edema, periurethral abscess or fistula, accessory gland infection (Tyson’s glands), balanitis, urethral stricture, and prostatitis, and rarely perirectal abscess.
Gonorrhea symptoms in women
In women, when symptoms do occur, they usually develop within 10 days of infection. In women, gonorrhea symptoms may include:
- unusual vaginal discharge
- pain, discomfort or burning sensation when passing urine
- pelvic pain, especially during sex
- irregular vaginal bleeding, especially between periods or after sex
- abdominal or pelvic pain
- anal discharge and discomfort
- sore, dry throat.
Cervicitis
Symptomatic gonococcal infection in women most often manifests as cervicitis and/or urethritis, but at least 50% of women with genital gonococcal infection are asymptomatic. Symptoms of cervicitis vary and may include a nonspecific vaginal discharge, intermenstrual bleeding, dysuria, lower abdominal pain, and dyspareunia. Clinically, examination of the cervix may show mucopurulent or purulent cervical discharge and easily bleed with minimal contact. The incubation period in women is variable, but symptoms, when they do occur, usually develop within 10 days of the exposure.[19] Seventy to ninety percent of women with genital gonococcal infection have laboratory evidence of urethral infection (urethritis); dysuria may be present, but these women frequently do not have specific urethral symptoms.
Anorectal infections
Anorectal gonococcal infection is uncommon in women, but can occur via anal intercourse. Anorectal infection has been reported in women with gonococcal cervicitis who do not acknowledge rectal sexual contact, presumably these infections result from perineal contamination with infected cervical secretions.
Complications in genital infection in women
There are several complications associated with gonorrhea in women:
- Accessory gland infections: Infection of female sex accessory glands (Bartholin’s glands or Skene’s glands) is often a unilateral infection. Occlusion of the ducts of these glands due to inflammation may result in the formation of an abscess.
- Pelvic inflammatory disease (PID): If cervical gonococcal infection ascends to the endometrium and/or fallopian tubes, PID may develop, typically causing symptoms that include lower abdominal pain, vaginal discharge, dyspareunia, intermenstrual bleeding, and fever.[20] In some women, PID may also be asymptomatic. Presumptive treatment for PID should be considered if one or more of the following minimum criteria are present on pelvic examination—uterine or adnexal tenderness or cervical motion tenderness. The long-term sequelae of untreated PID can include chronic pelvic pain, tubal infertility, and increased risk for ectopic pregnancy.
- Perihepatitis (Fitz-Hugh-Curtis Syndrome): In situations where gonococcal infection ascends from the cervix, infection may produce inflammation of the liver capsule and the adjacent peritoneum. Most women with perihepatitis have associated PID, but perihepatitis can occur independently. Historically, perihepatitis was attributed only to gonococcal infection, but now it is often associated with chlamydial infection. Gonococcal perihepatitis is characterized by right upper quadrant pain, and may be accompanied by abnormal liver function tests.
Signs of gonorrhea
Gonorrhea can also affect these parts of the body:
- Rectum. Signs and symptoms include anal itching, pus-like discharge from the rectum, spots of bright red blood on toilet tissue and having to strain during bowel movements.
- Eyes. Gonorrhea that affects your eyes may cause eye pain, sensitivity to light, and pus-like discharge from one or both eyes.
- Throat. Signs and symptoms of a throat infection may include a sore throat and swollen lymph nodes in the neck.
- Joints. If one or more joints become infected by bacteria (septic arthritis), the affected joints may be warm, red, swollen and extremely painful, especially when you move an affected joint.
Pharyngeal Infection
Gonococcal pharyngeal infection is most often asymptomatic. The pharynx may be the sole site of infection if the only exposure was receptive orogenital intercourse. Exudative pharyngitis is rare. Symptoms of pharyngeal infection may include pharyngitis, tonsillitis, fever, and cervical adenitis.
Ocular Infection
Gonococcal infection of the eye, when it does occur, typically presents as conjunctivitis. Gonococcal conjunctivitis in adults most often results from autoinoculation in persons with genital gonococcal infection. Patients may initially develop a mild non-purulent conjunctivitis, that, if untreated, typically progress to marked conjunctival redness, copious purulent discharge, and conjunctival edema 32. Less often, the manifestations include an ulcerative keratitis. Untreated gonococcal conjunctivitis can cause complications that may include corneal perforation, endophthalmitis, and blindness.
Disseminated Gonococcal Infection
Disseminated gonococcal infection, a systemic gonococcal infection, occurs infrequently and is more common in women than in men. Disseminated gonococcal infection is associated with some gonococcal strains that have a propensity to produce bacteremia without associated urogenital symptoms. In addition, patients with complement deficiency have greater risk of developing disseminated gonococcal infection. Clinical manifestations of disseminated gonococcal infection include skin lesions, arthralgia, tenosynovitis, arthritis, hepatitis, myocarditis, endocarditis, and meningitis. Rates of disseminated gonococcal infection have decreased due to the declining proportion of gonococcal strains prone to disseminate 33.
Gonorrhea complications
Untreated gonorrhea can cause serious and permanent health problems in both women and men.
Untreated gonorrhea can lead to significant complications, such as:
- Infertility in women. Untreated gonorrhea can spread into the uterus and fallopian tubes, causing pelvic inflammatory disease (PID), which may result in scarring of the tubes, greater risk of pregnancy complications and infertility. PID is a serious infection that requires immediate treatment.
- Infertility in men. Men with untreated gonorrhea can experience epididymitis — inflammation of a small, coiled tube in the rear portion of the testicles where the sperm ducts are located (epididymis). Epididymitis is treatable, but if left untreated, it may lead to infertility.
- Infection that spreads to the joints and other areas of your body. The bacterium that causes gonorrhea can spread through the bloodstream and infect other parts of your body, including your joints. Fever, rash, skin sores, joint pain, swelling and stiffness are possible results.
- Increased risk of HIV/AIDS. Having gonorrhea makes you more susceptible to infection with human immunodeficiency virus (HIV), the virus that leads to AIDS. People who have both gonorrhea and HIV are able to pass both diseases more readily to their partners.
- Complications in babies. Babies who contract gonorrhea from their mothers during birth can develop blindness, sores on the scalp and infections.
In women, gonorrhea can spread into the uterus or fallopian tubes and cause pelvic inflammatory disease (PID). The symptoms may be quite mild or can be very severe and can include abdominal pain and fever 34. Pelvic inflammatory disease can lead to internal abscesses and chronic pelvic pain. Pelvic inflammatory disease (PID) can also damage the fallopian tubes enough to cause infertility or increase the risk of ectopic pregnancy.
In men, gonorrhea may be complicated by epididymitis. In rare cases, this may lead to infertility 35.
If left untreated, gonorrhea can also spread to the blood and cause disseminated gonococcal infection. Disseminated gonococcal infection is usually characterized by arthritis, tenosynovitis, and/or dermatitis 36. This condition can be life threatening.
Untreated gonorrhea can increase a person’s risk of acquiring or transmitting HIV, the virus that causes AIDS 37.
Neisseria gonorrhoeae diagnosis
Testing for gonorrhea involves taking a swab (sample) from the urethra in men and the cervix in women. It can also be tested by taking a urine sample.
Sometimes swabs are also be taken from the throat and anus.
It is also important to get tested for other sexually transmitted infections such as syphilis, chlamydia and HIV.
To determine whether the gonorrhea bacterium is present in your body, your doctor will analyze a sample of cells. Samples can be collected by:
- Urine test. This may help identify bacteria in your urethra.
- Swab of affected area. A swab of your throat, urethra, vagina or rectum may collect bacteria that can be identified in a laboratory.
For women, home test kits are available for gonorrhea. Home test kits include vaginal swabs for self-testing that are sent to a specified lab for testing. If you prefer, you can choose to be notified by email or text message when your results are ready. You may then view your results online or receive them by calling a toll-free hotline.
Gonorrhea test
The approach to diagnostic testing for gonorrhea has evolved from traditional cultivation to widespread use of nucleic acid amplification tests (NAAT) 38. Gram’s stain, another non-culture test, is used for the diagnosis of urethral gonorrhea in symptomatic males. Culture is still recommended if antimicrobial resistance is a concern, especially in cases of treatment failure.
Nucleic Acid Detection Tests
There are two types of nucleic acid detection tests: non-amplified tests and amplified tests:
- Amplified Tests: The nucleic acid amplification tests (NAATs) include polymerase chain reaction (PCR) (Roche Amplicor; Cepheid GeneXpert CT/NG), transcription-mediated amplification (TMA) (Gen-Probe Aptima), and strand displacement amplification (SDA) (Becton-Dickinson BDProbeTec ET) 38. Amplified tests are FDA-cleared for endocervical specimens from women, urethral specimens from men, and urine specimens from men and women. Some NAATs are also cleared for vaginal swabs. For many of the commercially available tests, the same specimen can be used to test for Chlamydia trachomatis infection. NAATs are the most sensitive test to detect gonorrhea infections. NAATs are not FDA-cleared for rectal or oropharyngeal specimens, though many individual laboratories have validated NAAT for non-genital sites and this practice is becoming increasingly common 39. At present, antimicrobial susceptibility cannot be determined with NAATs, but research in this area is ongoing.
- Non-Amplified Tests: Non-amplified tests used for gonorrhea include the DNA probe (e.g. Gen-Probe PACE 2 and Digene Hybrid Capture II). A non-amplified test is less likely to be affected by transport conditions than culture, and has the potential for more timely results. These tests are FDA-cleared for endocervical specimens from women and urethral specimens from men. They are not FDA-cleared for pharyngeal, rectal, or urine specimens. The same specimen can be evaluated for Chlamydia trachomatis infection 38. Antimicrobial susceptibility cannot currently be determined with non-amplified tests.
Neisseria gonorrhoeae Gram stain
The use of Gram’s stain is a non-culture test that can make a presumptive diagnosis of gonorrhea. In the clinical setting, a Gram’s stain to detect gonorrhea is most often performed on a male with purulent urethral discharge. A Gram’s stain on a specimen positive for gonorrhea shows polymorphonuclear leukocytes (PMNs) with intracellular gram-negative diplococci. A Gram’s stain, with proper laboratory technique, has greater than 95% sensitivity and greater than 99% specificity for diagnosing symptomatic male gonococcal urethritis 38. Thus, the Gram’s stain is considered reliable both to diagnose and to exclude gonococcal urethritis in symptomatic men 20. The sensitivity of a Gram’s stain is lower for mane with asymptomatic urethral infection and thus not considered adequate to rule out infection in asymptomatic men 20. Performing a Gram’s stain is not recommended on endocervical, pharyngeal, or rectal specimens due to poor sensitivity 20.
Culture
Obtaining a bacterial culture is the historic standard for detection of gonorrhea. It has several advantages over non-culture tests, including low cost, use for a variety of specimen sites, and antimicrobial susceptibility testing can be performed if gonorrhea is isolated from the specimen. Despite having some advantages, culture is not as sensitive as NAAT and is more laboratory intensive, which has led to infrequent use in modern practice. At present, culture is primarily used for antimicrobial resistance surveillance by collecting specimens from either symptomatic urethral infections or from screen-positive sites of infection prior to treatment.
Testing for other sexually transmitted infections
Your doctor may recommend tests for other sexually transmitted infections. Gonorrhea increases your risk of these infections, particularly chlamydia, which often accompanies gonorrhea. Testing for HIV also is recommended for anyone diagnosed with a sexually transmitted infection. Depending on your risk factors, tests for additional sexually transmitted infections could be beneficial as well.
Neisseria gonorrhoeae treatment
Adults with gonorrhea are treated with antibiotics. Due to emerging strains of drug-resistant Neisseria gonorrhoeae, the Centers for Disease Control and Prevention (CDC) recommends that uncomplicated gonorrhea be treated only with the antibiotic ceftriaxone — given as an injection — in combination with either azithromycin (Zithromax, Zmax) or doxycycline (Monodox, Vibramycin, others) — two antibiotics that are taken orally.
Some research indicates that oral gemifloxacin (Factive) or injectable gentamicin, combined with oral azithromycin, is highly successful in treating gonorrhea. This treatment may be helpful in treating people who are allergic to cephalosporin antibiotics, such as ceftriaxone.
Gonorrhea treatment for partners
Your partner also should undergo testing and treatment for gonorrhea, even if he or she has no signs or symptoms. Your partner receives the same treatment you do. Even if you’ve been treated for gonorrhea, you can be reinfected if your partner isn’t treated.
Gonorrhea treatment for babies
Babies born to mothers with gonorrhea receive a medication in their eyes soon after birth to prevent infection. If an eye infection develops, babies can be treated with antibiotics.
Antibiotics for gonorrhea
The CDC treatment guidelines recommend using dual therapy for the treatment of gonococcal infections in adults and adolescents. Ceftriaxone is the most effective cephalosporin for treatment of gonorrhea and should be used in combination with azithromycin. The recommendation for dual therapy is based on the premise that using two antimicrobials with different mechanisms of action (e.g. a cephalosporin plus azithromycin) may improve treatment efficacy and potentially slow the emergence and spread of resistance. In addition, azithromycin and doxycycline will effectively treat concomitant C. trachomatis infection, if present. Azithromycin is preferred over doxycycline as a second agent due to convenience (single-dose therapy versus 7-day therapy) and the substantially lower prevalence of gonococcal resistance to azithromycin than with doxycycline, particularly for gonococcal strains that have an elevated cefixime MIC. In the case of azithromycin allergy or severe intolerance, doxycycline (100 mg orally twice a day for 7 days) can be used as a substitute for azithromycin, but doxycycline should only be used as an alternative, primarily because of the high prevalence of gonococcal tetracycline resistance. For details regarding these alternative regimens, refer to the section on gonococcal infections in the 2015 STD Treatment Guidelines 40. The following recommendations for treatment are based on the 2015 STD Treatment Guidelines.
Uncomplicated Gonococcal Infections of the Cervix, Urethra, and Rectum
Recommended Regimen
- Ceftriaxone 250 mg IM in a single dose
PLUS - Azithromycin 1g orally in a single dose
As dual therapy, ceftriaxone and azithromycin should be administered together on the same day, preferably simultaneously and under direct observation. Ceftriaxone in a single injection of 250 mg provides sustained, high bactericidal levels in the blood. Extensive clinical experience indicates that ceftriaxone is safe and effective for the treatment of uncomplicated gonorrhea at all anatomic sites, curing 99.2% of uncomplicated urogenital and anorectal and 98.9% of pharyngeal infections in clinical trials 41. No clinical data exist to support use of doses of ceftriaxone >250 mg.
Single-dose injectable cephalosporin regimens (other than ceftriaxone 250 mg IM) that are safe and generally effective against uncomplicated urogenital and anorectal gonococcal infections include ceftizoxime (500 mg IM), cefoxitin (2 g IM with probenecid 1 g orally), and cefotaxime (500 mg IM). None of these injectable cephalosporins offer any advantage over ceftriaxone for urogenital infection, and efficacy for pharyngeal infection is less certain 41.. Several other antimicrobials are active against gonorrhea, but none have substantial advantages over the recommended regimen, and efficacy data (especially for pharyngeal infection) are limited.
Alternative Regimens
If ceftriaxone is not available:
- Cefixime 400 mg orally in a single dose
PLUS - Azithromycin 1 g orally in a single dose
A 400-mg oral dose of cefixime should only be considered as an alternative cephalosporin regimen because it does not provide as high, nor as sustained, bactericidal blood levels as a 250-mg dose of ceftriaxone; further, it demonstrates limited efficacy for treatment of pharyngeal gonorrhea (92.3% cure); in older clinical studies, cefixime cured 97.5% of uncomplicated urogenital and anorectal gonococcal infections (95%) 41. The increase in the prevalence of isolates obtained through GISP with elevated cefixime MICs might indicate early stages of development of clinically significant gonococcal resistance to cephalosporins. CDC anticipates that rising cefixime MICs soon will result in declining effectiveness of cefixime for the treatment of urogenital gonorrhea. Furthermore, as cefixime becomes less effective, continued used of cefixime might hasten the development of resistance to ceftriaxone, a safe, well-tolerated, injectable cephalosporin and the last antimicrobial known to be highly effective in a single dose for treatment of gonorrhea at all anatomic sites of infection. Other oral cephalosporins (e.g., cefpodoxime and cefuroxime) are not recommended because of inferior efficacy and less favorable pharmacodynamics 42.
Uncomplicated Gonococcal Infections of the Pharynx
Most gonococcal infections of the pharynx are asymptomatic and can be relatively common in some populations 43. Gonococcal infections of the pharynx are more difficult to eradicate than are infections at urogenital and anorectal sites 44. Few antimicrobial regimens, including those involving oral cephalosporins, can reliably cure >90% of gonococcal pharyngeal infections 45. Providers should ask their patients with urogenital or rectal gonorrhea about oral sexual exposure; if reported, patients should be treated with a regimen with acceptable efficacy against pharyngeal gonorrhea infection.
Recommended Regimen
- Ceftriaxone 250 mg IM in a single dose
PLUS - Azithromycin 1 g orally in a single dose
Gonococcal conjunctivitis
In the only published study of the treatment of gonococcal conjunctivitis among adults, all 12 study participants responded to a single 1 g intramuscular injection of ceftriaxone 46. Nevertheless, due to concerns for emergence of antimicrobial resistance with gonorrhea, the CDC’s recommendation is to treat with ceftriaxone 1 g intramuscular injection once and azithromycin 1 g orally as a single dose. In addition, a one-time lavage of the infected eye with saline should be considered.
Recommended Regimen
- Ceftriaxone 1 g IM in a single dose
PLUS - Azithromycin 1 g orally in a single dose
Disseminated Gonococcal Infection
Disseminated gonococcal infection frequently results in petechial or pustular acral skin lesions, asymmetric polyarthralgia, tenosynovitis, or oligoarticular septic arthritis. The infection is complicated occasionally by perihepatitis and rarely by endocarditis or meningitis. Because of the possibility of potentially severe sequelae associated with these complications, the 2015 STD Treatment Guidelines recommend hospitalization and consultation with an infectious diseases specialist for patients suspected of having disseminated gonococcal infection. The recommended initial therapy is ceftriaxone 1 g intramuscularly or intravenously every 24 hours plus azithromycin 1 g orally in a single dose. The first dose is given ideally after promptly obtaining cultures and NAATs from multiple sites, as indicated, including skin, synovial fluid, blood, and cerebrospinal fluid. The duration of therapy for disseminated gonococcal infection with arthritis-dermatitis syndrome is at least 7 days and the ceftriaxone can transition to oral therapy if antimicrobial sensitivity testing shows an effective oral choice 20. For patients with meningitis, parenteral therapy should continue for 10 to 14 days and with endocarditis parenteral therapy should be given for at least 4 weeks 20.
Treatment of Arthritis and Arthritis-Dermatitis Syndrome
Recommended Regimen
- Ceftriaxone 1 g IM or IV every 24 hours
PLUS - Azithromycin 1 g orally in a single dose
Alternative Regimens
- Cefotaxime 1 g IV every 8 hours
OR - Ceftizoxime 1 g IV every 8 hours
PLUS - Azithromycin 1 g orally in a single dose
When treating for the arthritis-dermatitis syndrome, the provider can switch to an oral agent guided by antimicrobial susceptibility testing 24–48 hours after substantial clinical improvement, for a total treatment course of at least 7 days.
Treatment of Gonococcal Meningitis and Endocarditis
Recommended Regimen
- Ceftriaxone 1–2 g IV every 12–24 hours
PLUS - Azithromycin 1 g orally in a single dose
No recent studies have been published on the treatment of disseminated gonococcal infection. The duration of treatment of disseminated gonococcal infection has not been systematically studied and should be determined in consultation with an infectious-disease specialist. Treatment for disseminated gonococcal infection should be guided by the results of antimicrobial susceptibility testing. Pending antimicrobial susceptibility results, treatment decisions should be made on the basis of clinical presentation. Therapy for meningitis should be continued with recommended parenteral therapy for 10–14 days. Parenteral antimicrobial therapy for endocarditis should be administered for at least 4 weeks.
Gonococcal Infections in Pregnancy
As with other patients, pregnant women infected with gonorrhea should be treated with recommended cephalosporin-based therapy in combination with azithromycin. Pregnant women infected with gonorrhea should be treated with dual therapy consisting of ceftriaxone 250 mg in a single IM dose and azithromycin 1 g orally as a single dose. Pregnant women should not be treated with any fluoroquinolone or any tetracycline drug. Because spectinomycin is not available in the United States, pregnant women who cannot tolerate a cephalosporin should be evaluated by an infectious diseases specialist.
Management of Antibiotic-Resistant Gonorrhea
Although there are no confirmed cases of treatment failure due to cephalosporin-resistant gonorrhea in the United States, the gradual upwards trend of MICs documented by the United States Gonococcal Isolate Surveillance Project remains worrisome 47. Criteria for resistance to cefixime and ceftriaxone have not been defined by the Clinical and Laboratory Standards Institute, but isolates with cefixime or ceftriaxone MICs equal to or greater than 0.5 μg/mL are considered to have decreased susceptibility. Only five isolates with ceftriaxone MIC equal to or greater than 0.5 μg/mL have been reported during the history of the United States Gonococcal Isolate Surveillance Project. Notably, isolates with high-level cefixime and ceftriaxone MICs (cefixime MICs 1.5–8 μg/mL and ceftriaxone MICs 1.5–4 μg/mL) have been identified in Japan, France, and Spain 48.
Allergy to Penicillins or Cephalosporin
Allergic reactions to first-generation cephalosporins occur in less than 2.5% of persons with a history of penicillin allergy and are less common with third-generation cephalosporins such as ceftriaxone and cefixime 49. Ceftriaxone is contraindicated in patients with a history of IgE-mediated anaphylaxis to penicillin. Given these considerations, expert consultation with an infectious diseases specialist (and possibly also an allergy specialist), is recommended for treating gonorrhea among persons who have documented severe cephalosporin allergy. Cephalosporin desensitization is preferred but impractical in many settings. Potential therapeutic options in this situation for adults and adolescents include (1) dual treatment with single doses of oral gemifloxacin 320 mg plus a single dose of oral azithromycin 2 g, or (2) dual treatment with single doses of intramuscular gentamicin 240 mg plus a single dose of oral azithromycin 2 g 20. Note that since May 2015, gemifloxacin has not available for use in the United States because of a legal dispute regarding the license to manufacture and distribute this drug. For patients with documented severe cephalosporin allergy, recent evidence supports superior effectiveness of dual therapy when compared with azithromycin monotherapy. In this setting, spectinomycin monotherapy has been effective in clinical trials, curing 98.2% of uncomplicated urogenital and anorectal gonococcal infections, but it has poor efficacy against pharyngeal infection and is not currently available in the United States. Although true allergic reactions to third-generation cephalosporins are uncommon among persons who report a history of penicillin allergy, use of ceftriaxone is contraindicated in persons with a history of IgE-mediated penicillin allergy.
Management of Suspected Gonococcal Treatment Failure
Clinicians who diagnose gonorrhea infection in a person with suspected cephalosporin treatment failure should (1) perform culture and susceptibility testing of all relevant clinical specimens; (2) obtain expert opinion for guidance in clinical management (through the STD Clinical Consultation Network [https://stdccn.org], a local STD/HIV Prevention Training Center clinical expert, the CDC, or an infectious diseases specialist); and (3) report the case to the CDC through state and local public health authorities 20. Isolates that grow gonorrhea should be saved and sent to the CDC through state public health laboratory mechanisms. Health departments should prioritize notification and culture evaluation for sex partner(s) of persons with gonorrhea infection suspected for cephalosporin treatment failure or persons whose isolates demonstrate decreased susceptibility to cephalosporins. In this setting, a test-of-cure at relevant clinical sites should be obtained 7 to 14 days after retreatment; culture is the recommended test, preferably with simultaneous NAAT and susceptibility testing of gonorrhea if isolated. For patients considered to have high likelihood of true treatment failure, especially those with a documented elevated cephalosporin MIC for gonorrhea, the 2015 STD Treatment Guidelines suggested options consist of (1) single dose oral therapy with gemifloxacin 320 mg plus azithromycin 2 g, or (2) single dose oral therapy with azithromycin 2 g plus a single intramuscular injection of a 240 mg dose of gentamicin. Note that since May 2015, gemifloxacin has not available for use in the United States because of a legal dispute regarding the license to manufacture and distribute this drug.
Follow-Up
In general, a test-of-cure is not recommended for patients who have uncomplicated gonorrhea and are treated with any of the recommended regimens. Patients who have persistent symptoms should be evaluated by culture for gonorrhea, and any gonococci isolated should be tested for antimicrobial susceptibility. A routine test-of-cure at day 14 after treatment with NAAT or culture is recommended for patients with pharyngeal gonorrhea treated with an alternative regimen. All patients diagnosed with gonorrhea should have repeat testing in 3 months at the anatomic site of exposure, regardless of whether they have symptoms. Infections identified after treatment with one of the recommended regimens usually result from reinfection rather than treatment failure, indicating a need for improved patient education and referral of sex partners. Patients who have persistent infection despite treatment with a recommended regimen and who deny sexual exposure after treatment should be evaluated with culture of clinical specimens and susceptibility testing. Clinicians should promptly notify the local STD program of such cases, and local or state STD programs should notify the CDC.
Management of Sex Partners
Recent sex partners (within the 60 days preceding onset of symptoms or gonorrhea diagnosis) should be referred for evaluation, testing, and presumptive dual treatment. The most recent sex partner should be treated regardless of interval from diagnosis. To avoid reinfection, sex partners should be instructed to abstain from unprotected sexual intercourse for 7 days after they and their sex partner(s) have completed antimicrobial treatment and symptoms have resolved.
Expedited partner therapy
In settings where prompt referral and treatment are unavailable or impractical, providers should consider expedited partner therapy 50. This entails provision of appropriate antibiotics as well as educational and pharmacy information for the partner. The documentation should include notification that partner(s) have been exposed, information about the importance of treatment, signs and symptoms of potential complications, as well as possible therapy-related potential allergic reactions and adverse effects 20. The expedited partner therapy regimen for sex partners of patients with gonorrhea infection is cefixime 400 mg and azithromycin 1 g, with delivery of the prescription to the partner by either the patient, a disease investigation specialist, or a collaborating pharmacy as permitted by law 20. It is essential to check with one’s state health department to clarify the policies, as the use of expedited partner therapy is not legal in all states. The CDC maintains an updated information page Legal Status of Expedited Partner Therapy that identifies the legal status of expedited partner therapy in each state in the United States, as well as providing links to each state for more detailed state policies. Notably, provision of expedited partner therapy alone is not sufficient and each partner should ideally be seen in follow-up for repeat testing to confirm resolution of infection and check for reinfection. Although offering expedited partner therapy to female partners is acceptable, this approach may result in undertreatment of pelvic inflammatory disease. The use of expedited partner therapy for gonorrhea is contraindicated in a female partner who have current signs or symptoms that are suggestive of PID. Female partners who have current signs and symptoms suggestive of PID should undergo prompt evaluation by a health care provider. In addition, the use of expedited partner therapy should not be considered a routine partner management strategy in men who have sex with men with gonorrhea for several reasons, including the high risk for coexisting infections (especially HIV and syphilis infection), inadequate data regarding the efficacy of expedited partner therapy in this patient population, and concerns regarding the increased proportion of gonococcal isolates among men who have sex with men with reduced susceptibility to cefixime.
- Meningococcal Disease – Causes and Spread to Others. https://www.cdc.gov/meningococcal/about/causes-transmission.html[↩][↩]
- Siddiqui JA, Gulick PG. Meningococcemia. [Updated 2019 Jan 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534849[↩][↩][↩]
- Bosis S, Mayer A, Esposito S. Meningococcal disease in childhood: epidemiology, clinical features and prevention. J Prev Med Hyg. 2015 Aug 31;56(3):E121-4[↩]
- Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, McIntyre P, Ramsay ME, Sáfadi MA. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012 May 30;30 Suppl 2:B26-36[↩]
- Funk A, Uadiale K, Kamau C, Caugant DA, Ango U, Greig J. Sequential outbreaks due to a new strain of Neisseria meningitidis serogroup C in northern Nigeria, 2013-14. PLoS Curr. 2014 Dec 29;6[↩]
- Batista RS, Gomes AP, Dutra Gazineo JL, Balbino Miguel PS, Santana LA, Oliveira L, Geller M. Meningococcal disease, a clinical and epidemiological review. Asian Pac J Trop Med. 2017 Nov;10(11):1019-1029[↩]
- Törös B, Thulin Hedberg S, Jacobsson S, Fredlund H, Olcén P, Mölling P. Surveillance of invasive Neisseria meningitidis with a serogroup Y update, Sweden 2010 to 2012. Euro Surveill. 2014 Oct 23;19, 42[↩]
- Harrison LH. Epidemiological profile of meningococcal disease in the United States. Clin. Infect. Dis. 2010 Mar 01;50 Suppl 2:S37-44[↩]
- Meningococcal Vaccination for Preteens and Teens: Information for Parents. https://www.cdc.gov/vaccines/vpd/mening/public/adolescent-vaccine.html[↩]
- Meningococcal Vaccination: What Everyone Should Know. https://www.cdc.gov/vaccines/vpd/mening/public/index.html[↩]
- [Guideline] Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015 Jun 5. 64 (RR-03):1-137.[↩]
- Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis, 40(3), 187–193, 2013.[↩]
- CDC. Sexually Transmitted Disease Surveillance, 2017. Atlanta, GA: Department of Health and Human Services; September 2018.[↩][↩]
- U.S. Preventive Services Task Force. Screening for gonorrhea: recommendation statement. Ann Fam Med 2005, 3, 263–267[↩]
- Hooper RR, Reynolds GH, Jones OG, et al. Cohort study of venereal disease. I: the risk of gonorrhea transmission from infected women to men. Am J Epidemiol. 1978;108:136-44.[↩]
- Lin JS, Donegan SP, Heeren TC, et al. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J Infect Dis. 1998;178:1707-12.[↩]
- Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868-73.[↩]
- Thadepalli H, Rambhatla K, Maidman J, Arce JJ, Davidson EC Jr. Gonococcal sepsis secondary to fetal monitoring. Am J Obstet Gynecol 1976, 126(4), 510–512[↩]
- Holmes KK, Levine R, Weaver M. Effectiveness of condoms in preventing sexually transmitted infections. Bull World Health Organ 2004, 82(6), 454–461[↩]
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. Gonococcal infections. MMWR Recomm Rep. 2015;64(No. RR-3):1-137.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- LeFevre ML; U.S. Preventive Services Task Force. Screening for Chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:902-10.[↩][↩][↩]
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. Special populations. MMWR Recomm Rep. 2015;64(No. RR-3):1-137.[↩][↩][↩][↩][↩][↩]
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. HIV infection: detection, counseling, and referral. MMWR Recomm Rep. 2015;64(No. RR-3):1-137.[↩]
- Peterman T, Tian L, Metcalf C et al. High incidence of new sexually transmitted infections in the year following a sexually transmitted infection: a case for rescreening. Ann Intern Med 2006, 145(8), 564–572.[↩]
- Harrison WO, Hooper MR, Wiesner PJ et al. A trial of minocycline given after exposure to prevent gonorrhea. N Engl J Med 1979, 300(19), 1074–1078[↩]
- Platt R, Rice PA, McCormack WM. Risk of acquiring gonorrhea and prevalence of abnormal adnexal findings among women recently exposed to gonorrhea. JAMA 1983, 250(23), 3205–3209.[↩]
- McCormack WM, Johnson K, Stumacher RJ, Donner A, Rychwalski R. Clinical spectrum of gonococcal infection in women. Lancet 1977, 1(8023), 1182–1185[↩]
- Klein EJ, Fisher LS, Chow AW, Guze LB. Anorectal gonococcal infection. Ann Intern Med 1977, 86, 340–346[↩]
- Wiesner PJ, Tronca E, Bonin P, Pedersen AHB, Holmes KK. Clinical spectrum of pharyngeal gonococcal infection. N Engl J Med 1973, 288(4), 181–185[↩]
- Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. Asymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significance. N Engl J Med. 1974;290:117-23.[↩]
- Harrison WO, Hooper RR, Wiesner PJ, et al. A trial of minocycline given after exposure to prevent gonorrhea. N Engl J Med. 1979;300:1074-8.[↩]
- Wan WL, Farkas GC, May WN, Robin JB. The clinical characteristics and course of adult gonococcal conjunctivitis. Am J Ophthalmol. 1986;102:575-83.[↩]
- Bleich AT, Sheffield JS, Wendel GD Jr, Sigman A, Cunningham FG. Disseminated gonococcal infection in women. Obstet Gynecol. 2012;119:597-602.[↩]
- Svensson L, Westrom L, Ripa K, Mardh P. Differences in some clinical and laboratory parameters in acute salpingitis related to culture and serologic findings. Am J Obstet Gynecol 1980, 138(7), 1017–1021[↩]
- Berger R, Alexander E, Harnisch J et al. Etiology, manifestations and therapy of acute epididymitis: prospective study of 50 cases. J Urol 1979, 121(6), 750–754[↩]
- Holmes KK, Counts GW, Beaty HN. Disseminated gonococcal infection. Ann Intern Med 1971, 74, 979–993[↩]
- Fleming D, Wasserheit J. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm DIs 1999, 75(1), 3–17[↩]
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae–2014. MMWR Recomm Rep. 2014;63:1-19.[↩][↩][↩][↩]
- Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis. 2008;35:637-42.[↩]
- 2015 Sexually Transmitted Diseases Treatment Guidelines. Gonococcal Infections. https://www.cdc.gov/std/tg2015/gonorrhea.htm[↩]
- Newman LM, Moran JS, Workowski KA. Update on the management of gonorrhea in adults in the United States. Clin Infect Dis 2007;44(Suppl 3):S84–101[↩][↩][↩]
- Yu RX, Yin Y, Wang GQ, et al. Worldwide susceptibility rates of Neisseria gonorrhoeae isolates to cefixime and cefpodoxime: a systematic review and meta-analysis. PLoS One 2014;9:e87849.[↩]
- Schachter J, Moncada J, Liska S, et al. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008;35:637–42.[↩]
- Ota KV, Fisman DN, Tamari IE, et al. Incidence and treatment outcomes of pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections in men who have sex with men: a 13-year retrospective cohort study. Clin Infect Dis 2009;48:1237–43.[↩]
- Newman LM, Moran JS, Workowski KA. Update on the management of gonorrhea in adults in the United States. Clin Infect Dis 2007;44(Suppl 3):S84–101.[↩]
- Haimovici R, Roussel TJ. Treatment of gonococcal conjunctivitis with single-dose intramuscular ceftriaxone. Am J Ophthalmol. 1989;107:511-4.[↩]
- Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-6.[↩]
- Deguchi T, Yasuda M, Hatazaki K, et al. New Clinical Strain of Neisseria gonorrhoeae with Decreased Susceptibility to Ceftriaxone, Japan. Emerg Infect Dis. 2016;22:142-4.[↩]
- Novalbos A, Sastre J, Cuesta J, et al. Lack of allergic cross-reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy. 2001;31:438-43.[↩]
- Golden MR, Whittington WL, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676-85.[↩]