Contents
- What is a nerve conduction study
- Nerve conduction study indications
- Nerve conduction study side effects
- What happens during the nerve conduction study?
- What happens after an nerve conduction study?
- How painful is a EMG nerve conduction study?
- Specific nerve conduction study techniques
- Nerve conduction study results
- Nerve conduction study results interpretation
What is a nerve conduction study
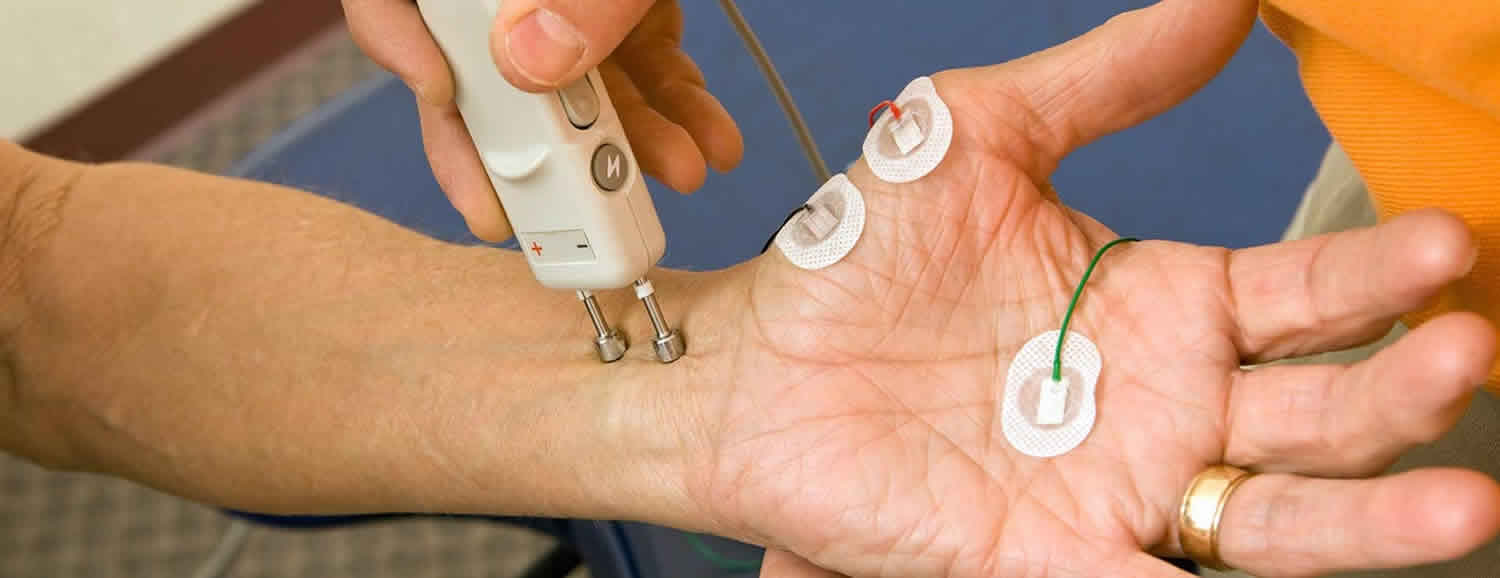
Nerve conduction study also called nerve conduction velocity, measures how fast an electrical impulse moves through your nerve. Nerve conduction study can identify nerve damage. Nerve conduction study is an electrical test which involves the stimulation of nerves along several points (usually as they run down the arms or legs). During the nerve conduction study, your nerve is stimulated, usually with electrode patches (small thin metallic discs) attached to your skin. Two electrodes are placed on the skin over your nerve. One electrode stimulates your nerve with a very mild electrical impulse. The other electrode records it. The resulting electrical activity is recorded by another electrode. This is repeated for each nerve being tested.
The speed is then calculated by measuring the distance between electrodes and the time it takes for electrical impulses to travel between electrodes.
A related test that may be done is an electromyography (EMG). Electromyography (EMG) is a diagnostic procedure to assess the health of muscles and the nerve cells that control them (motor neurons). EMG results can reveal nerve dysfunction, muscle dysfunction or problems with nerve-to-muscle signal transmission.
EMG is often done at the same time as an nerve conduction study. Both tests help find the presence, location, and extent of diseases that damage the nerves and muscles.
Principals of nerve conduction studies
Nerve conduction study involve the application of a depolarizing square wave electrical pulses to the skin over a peripheral nerve producing: (1) a propagated nerve action potential (NAP) recorded at a distant point over the same nerve: and (2) a compound muscle action potential (CMAP) arising from the activation of muscle fibres in a target muscle supplied by the nerve. In both cases these may be recorded with surface or needle electrodes.
Surface electrodes are designed to give information about the whole of a muscle stimulated, giving data for the time taken for the fastest axons to conduct an impulse to the muscle and the size of the response.
Needle electrodes for nerve conduction study give very accurate conduction time information, but because they record from only a small area of muscle or nerve, they give poor or, in the case of the latter, more complex information making numerical analysis difficult. However, needle recordings are most appropriate when severe muscle wasting has occurred, or when the depth of a muscle under study makes a surface recording impossible.
Nerves may be stimulated through the skin with surface stimulators, or via a needle placed close to a nerve or a nerve root. Spinal root and cerebral cortical stimulation may also be carried out using transcutaneous magnetic stimulation (TMS). Thus the full length of the motor pathway may be assessed from cortex to cord, root, neuromuscular junction, and the contractile apparatus. Choice of the stimulation points depends both on the desire to “bracket” above and below the point of a proposed focal lesion and the anatomical availability of the appropriate structure.
Peripheral nerves contain many nerve fibres of different diameters, degrees of myelination, and afferent or efferent connections. The nerve conduction study studies the fastest 20% of these fibres and the aim of the investigation is to document focal or continuous abnormalities in the length of the mixed, motor or sensory nerve. Particular attention is paid to the following questions as the test progresses:
- Is the fastest conduction velocity normal?
- Is the velocity gradient normal. Normally nerves closer to the neuraxis and more cephalad conduct faster than more distal and caudal nerves.
- Is the compound muscle action potential (CMAP) normal in size and shape?
- Does the compound muscle action potential (CMAP) alter in size, shape or duration between stimulation points?
- giving evidence for temporal dispersion.
- giving evidence for conduction block.
Is nerve conduction study painful?
Nerve conduction studies are safe and generally well tolerated. Patients typically experience only minor discomfort. Long term side effects occur rarely, if ever. Most patients describe the feeling as a ‘tingling’ or ‘tapping’ sensation.
Nerve conduction study indications
Nerve conduction study is often used along with an electromyography (EMG) to tell the difference between a nerve disorder and a muscle disorder. nerve conduction study detects a problem with the nerve, whereas an electromyography (EMG) detects whether the muscle is working properly in response to the nerve’s stimulus.
Diseases or conditions that may be checked with nerve conduction study include:
- Guillain-Barré syndrome. A condition in which the body’s immune system attacks part of the peripheral nervous system. The first symptoms may include weakness or a tingling sensation in the legs.
- Carpal tunnel syndrome. A condition in which the median nerve, which runs from the forearm into the hand, becomes pressed or squeezed at the wrist by enlarged tendons or ligaments. This causes pain and numbness in the fingers.
- Charcot-Marie-Tooth disease. An inherited neurological condition that affects both the motor and sensory nerves. It causes weakness of the foot and lower leg muscles.
- Herniated disk disease. This condition occurs when the fibrous cartilage that surrounds the disks of your vertebrae breaks down. The center of each disk, which contains a gelatinous substance, is forced outward. This places pressure on a spinal nerve and causes pain and damage to the nerve.
- Chronic inflammatory polyneuropathy and neuropathy. These are conditions resulting from diabetes or alcoholism. Symptoms may include numbness or tingling in a single nerve or many nerves at the same time.
- Sciatic nerve problems. There are many causes of sciatic nerve problems. The most common is a bulging or ruptured spinal disk that presses against the roots of the nerve leading to the sciatic nerve. Pain, tingling, or numbness often result.
Nerve conduction studies may also be done to find the cause of symptoms, such as numbness, tingling, and continuous pain.
Other conditions may prompt your healthcare provider to recommend nerve conduction study.
Nerve conduction study side effects
The voltage of the electrical pulses used during an nerve conduction study is considered very low.
Side effects depend on your specific medical condition. Be sure to discuss any concerns with your healthcare provider before the nerve conduction study and EMG procedure.
Certain factors or conditions may interfere with the results of nerve conduction study tests. This includes damage to the spinal cord, severe pain before the test, and body temperature.
There are very few contraindications to these investigations, but the most important is the presence of some cardiac pacemakers or cardiac defibrillators. With most there is no risk but discussion with the patient’s cardiologist is advised if (1) the nerve conduction study are likely to involve stimulation close to the chest wall, and (2) if a life threatening event would be risked should the pacemaker either be triggered or changed to a harmful default state if subject to an external voltage.
What happens during the nerve conduction study?
An nerve conduction study procedure may be done on an outpatient basis, or as part of your stay in a hospital. Procedures may vary depending on your condition and your doctor’s practices.
The nerve conduction study is done by a neurologist. This is a doctor who specializes in brain and nerve disorders. A technologist may also do some parts of the test.
Generally, an nerve conduction study procedure follows this process:
- You will be asked to remove any clothing, jewelry, hairpins, eyeglasses, hearing aids, or other metal objects that may interfere with the procedure.
- If you are asked to remove clothing, you will be given a gown to wear.
- You will be asked to sit or lie down for the test.
- A neurologist will locate the nerve(s) to be studied.
- A healthcare provider will attach a recording electrode to the skin over your nerve, using a special paste. He or she will then place a stimulating electrode away from the recording electrode, at a known distance.
- A mild and brief electrical shock, given through the stimulating electrode, will stimulate your nerve.
- You may experience minor discomfort for a few seconds.
- The stimulation of the nerve and the response will be displayed on a monitor.
What happens after an nerve conduction study?
The paste used to attach the electrodes will be removed from your skin.
After the test, you may return to your previous activities, unless your healthcare provider advises you differently. Your healthcare provider may instruct you to avoid strenuous activities for the rest of the day.
Your healthcare provider may give you other instructions after the procedure, depending on your situation.
How painful is a EMG nerve conduction study?
Electromyography (EMG) is a test that measures muscle response to nervous stimulation. A needle electrode is inserted through the skin into the muscle. Each muscle fiber that contracts will produce an action potential. The presence, size, and shape of the wave form of the action potential produced on the oscilloscope, provides information about the ability of the muscle to respond to nervous stimulation.
Motor neurons transmit electrical signals that cause muscles to contract. An EMG uses tiny devices called electrodes to translate these signals into graphs, sounds or numerical values that are then interpreted by a specialist.
During a needle EMG, a needle electrode inserted directly into a muscle records the electrical activity in that muscle.
You may feel some pain or discomfort when the needles are inserted. But most people are able to complete the test without problems.
Afterward, the muscle may feel tender or bruised for a few days.
Specific nerve conduction study techniques
Motor nerve conduction studies
Motor studies are performed by electrical stimulation of a nerve and recording the compound muscle action potential (CMAP) from surface electrodes overlying a muscle supplied by that nerve.
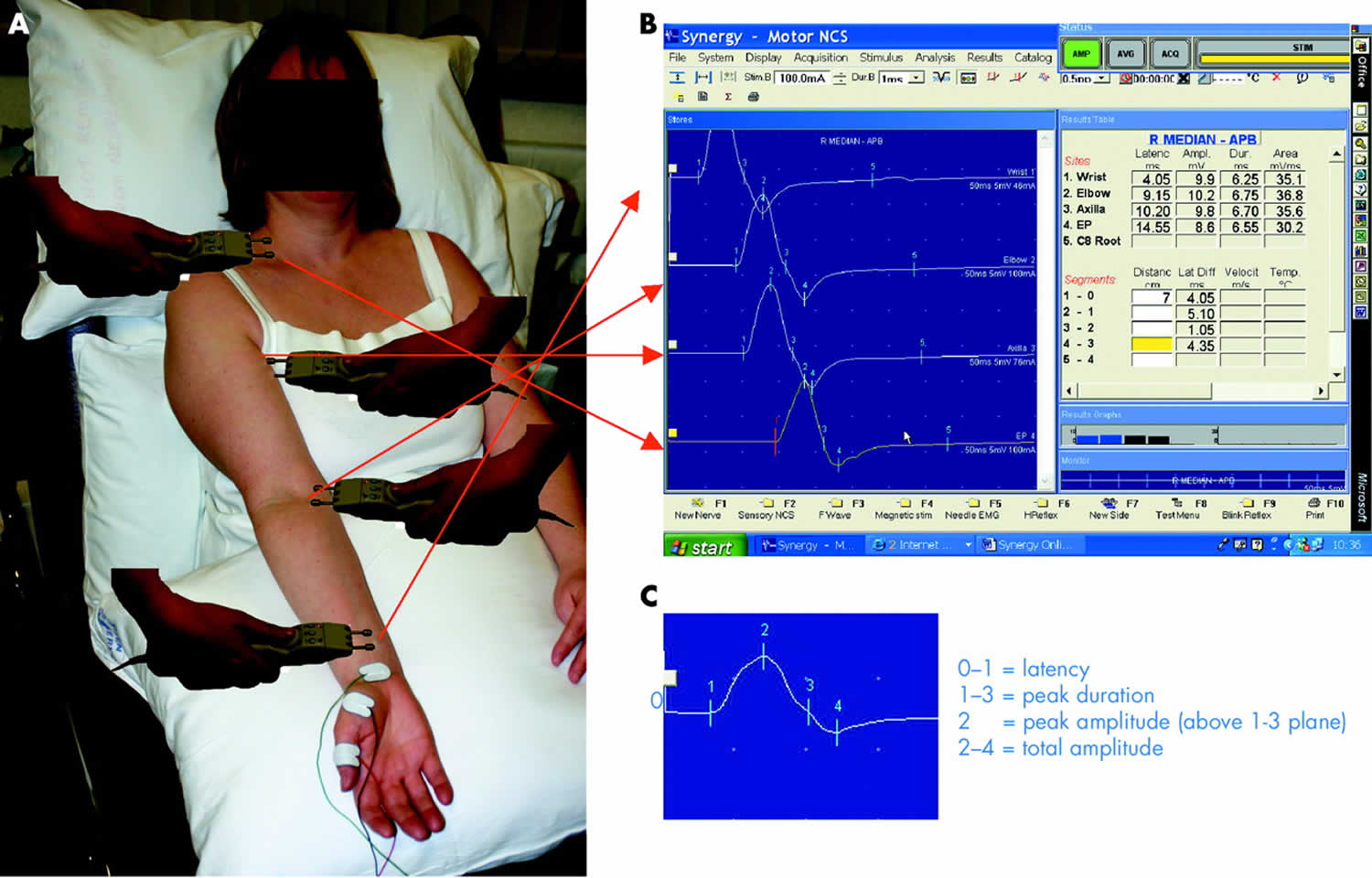
The recording electrodes are performed using adhesive conductive pads placed onto the skin overlying the target muscle. The active electrode is placed over the muscle belly and the reference over an electrically inactive site (usually the muscle tendon). A ground electrode is also placed somewhere between the stimulating and recording electrodes providing a zero voltage reference point. The median motor study might involve stimulation at the wrist, the elbow, and less frequently the axilla and the brachial plexus (Figure 1A,B).
The compound muscle action potential (CMAP) is a summated voltage response from the individual muscle fibre action potentials. The shortest latency of the CMAP is the time from stimulus artefact to onset of the response and is a biphasic response with an initial upward deflection followed by a smaller downward deflection. The CMAP amplitude is measured from baseline to negative peak (the neurophysiological convention is that negative voltage is demonstrated by an upward deflection) and measured in millivolts (mV) (Figure 1C).
To record the CMAP, the stimulating current or voltage is gradually increased until a point is reached where an increase in stimulus produces no increment in CMAP amplitude. It is only at this (supramaximal) point that reproducible values for CMAP amplitude and the latency between the stimulus and the onset of the CMAP can be recorded accurately.
The nerve is then stimulated at a more proximal site—in the median nerve this will be the antecubital fossa, close to the biceps tendon. In the normal state stimulating the median nerve at the wrist and the elbow results in two CMAPs of similar shape and amplitude because the same motor axons innervate the muscle fibres making up the response. However, the latency will be greater for elbow stimulation compared with wrist stimulation because of the longer distance between the stimulating and recording electrodes (Figure 1B). The difference in latency represents the time taken for the fastest nerve fibers to conduct between the two stimulation points as all other factors involving neuromuscular transmission and muscle activation are common to both stimulation sites. If one measures the distance between the two sites then the fastest motor nerve conduction velocity can be calculated as follows: FMNCV (m/s) = distance between stimulation site 1 and site 2 (mm)/[latency site 2 – latency site 1 (ms)].
Figure 1. Motor nerve conduction study
Footnote: (A, B). Median motor nerve conduction study. Active recording electrode is over the APB muscle, with stimulation at the wrist, elbow, axilla, and brachial plexus. Panel B shows the motor response from stimulation at all four sites. Responses are of the same shape but the latency is longer with more proximal stimulation. (C) The compound muscle action potential (CMAP) and its parameters.
[Source 1 ]Sensory nerve conduction studies
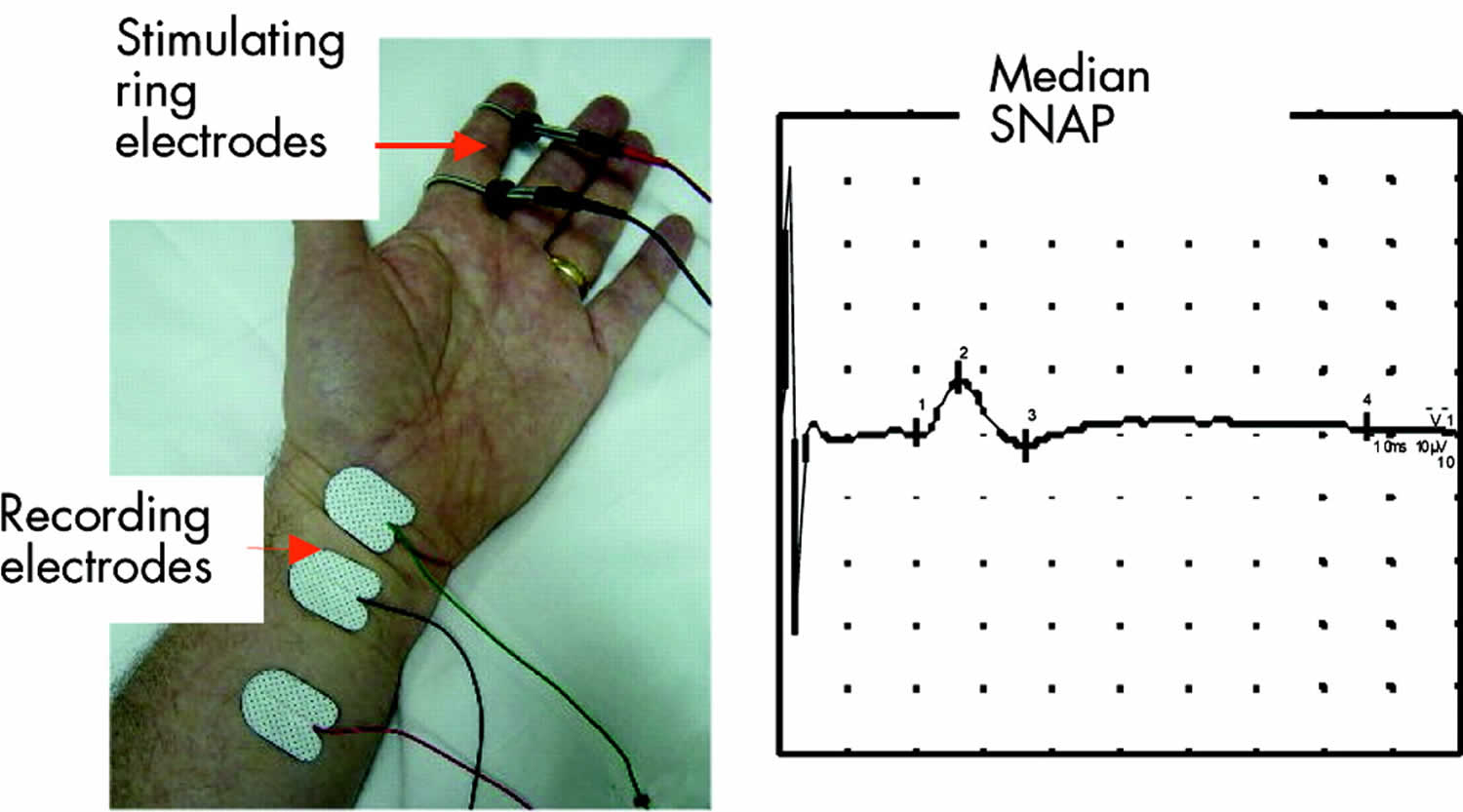
The sensory nerve action potential (SNAP) is obtained by electrically stimulating sensory fibers and recording the nerve action potential at a point further along that nerve. Once again the stimulus must be supramaximal.
Recording the sensory nerve action potential (SNAP) orthodromically refers to distal nerve stimulation and recording more proximally (the direction in which physiological sensory conduction occurs). Antidromic testing is the reverse. Different laboratories prefer antidromic or orthodromic methods for testing different nerves. An orthodromic median sensory study is shown in Figure 2. The sensory latency and the peak to peak amplitude of the SNAP are measured. The velocity correlates directly with the sensory latency and therefore either the result may be expressed as a latency over a standard distance or a velocity.
Only the 20% largest diameter and fastest conducting sensory fibres are tested using conventional sensory studies functionally supplying fine touch, vibration, and position sense. Predominantly small fibre neuropathies affecting the other 80% of fibres exist usually with prominent symptoms of pain and conventional sensory studies may be normal. In such cases quantitative sensory testing and autonomic testing will be required, which are beyond the scope of this article (see Interpretation pitfalls).
Figure 2. Sensory nerve conduction study
Footnote: Median orthodromic sensory study. The index finger digital nerves are stimulated via ring electrodes and the response recorded over the median nerve at the wrist.
[Source 1 ]F waves
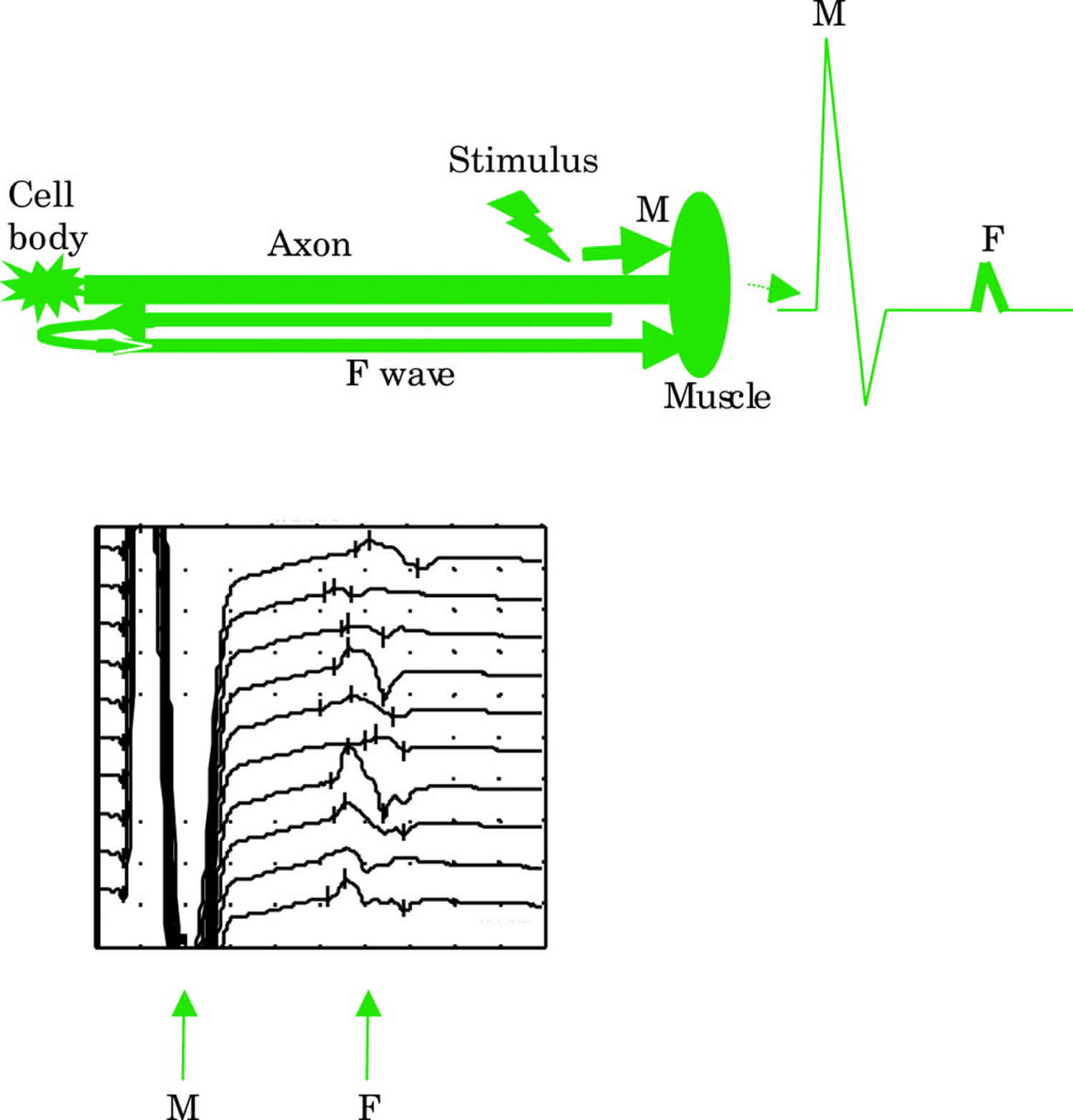
F waves (F for foot where they were first described) are a type of late motor response. When a motor nerve axon is electrically stimulated at any point an action potential is propagated in both directions away from the initial stimulation site. The distally propagated impulse gives rise to the CMAP. However, an impulse also conducts proximally to the anterior horn cell, depolarising the axon hillock and causing the axon to backfire. This leads to a small additional muscle depolarisation (F wave) at a longer latency. Only about 2% of axons backfire with each stimulus. Unlike the M response (Figure 3), F waves vary in latency and shape because different populations of neurones normally backfire with each stimulus. The most reliable measure of the F wave is the minimum latency of 10–20 firings.
F waves allow testing of proximal segments of nerves that would otherwise be inaccessible to routine nerve conduction studies. F waves test long lengths of nerves whereas motor studies test shorter segments. Therefore F wave abnormalities can be a sensitive indicator of peripheral nerve pathology, particularly if sited proximally. The F wave ratio which compares the conduction in the proximal half of the total pathway with the distal may be used to determine the site of conduction slowing—for example, to distinguish a root lesion from a patient with a distal generalised neuropathy.
Figure 3. F waves
Footnote: Schematic representation of the early M response from the distally propagated action potential and the later F wave from the proximally propagated action potential. The latter depolarizes the axon hillock causing it to backfire. Actual F wave responses are shown in the lower trace. F waves vary in latency and shape due to different populations of axons backfiring each time.
[Source 1 ]Errors
The main sources of non-biological error in nerve conduction study measurements are the identification and measurement of waveform onset and the measurement of the length of the nerve segment on the limb. Calculations have shown that in a nerve with a conduction velocity of 50 m/s, the 2×SD experimental error for velocity is 14 m/s over 10 cm and 4.7 m/s over 25 cm. Of the error, time measurement is 92.3% and distance 7.7%, so the use of the measuring tape is quite adequate in conventional nerve conduction study.
Nerve conduction study results
Nerve conduction study provides information to locate lesions in the length of a nerve, and pathophysiological information. Peripheral nerve pathology primarily affects axons or myelin. In reality, the two pathologies often co-exist but usually one predominates (Table 1). A patient with a radial nerve palsy at the spiral groove causing wrist drop is more likely to make a complete and speedier recovery (6–12 weeks) if this is mainly due to focal demyelination and/or conduction block (neuropraxia) compared with when significant axonal injury has also occurred (6–12 months).
In generalized processes it is also important to determine whether a peripheral neuropathy is demyelinating or axonal as this will affect further investigation and management. For example, acute inflammatory demyelinating polyneuropathy (AIDP or Guillain-Barré syndrome) results in a characteristic pattern of segmental nerve demyelination and may be treated with human immunoglobulin or plasma exchange. Conversely a length dependent axonal neuropathy developing in a patient on chemotherapy requires reassessment of the chemotherapy or addition of a protective agent.
Neuropathies may be classified pathologically in this fashion, anatomically or electrophysiologically.
Your doctor may order an EMG and nerve conduction study if you have signs or symptoms that may indicate a nerve or muscle disorder. EMG is most often used when a person has symptoms of weakness, pain, or abnormal sensation. It can help tell the difference between muscle weakness caused by the injury of a nerve attached to a muscle, and weakness due to nervous system disorders, such as muscle diseases.
Such symptoms may include:
- Tingling
- Numbness
- Muscle weakness
- Muscle pain or cramping
- Certain types of limb pain
EMG results are often necessary to help diagnose or rule out a number of conditions such as:
- Muscle disorders, such as muscular dystrophy or polymyositis
- Diseases affecting the connection between the nerve and the muscle, such as myasthenia gravis
- Disorders of nerves outside the spinal cord (peripheral nerves), such as carpal tunnel syndrome or peripheral neuropathies
- Disorders that affect the motor neurons in the brain or spinal cord, such as amyotrophic lateral sclerosis or polio
- Disorders that affect the nerve root, such as a herniated disk in the spine
Table 1. Typical nerve conduction study abnormalities seen with axon loss or demyelination
| Axon loss | Demyelination | |
|---|---|---|
| It is not necessary to have all the features of axon loss or demyelination to come to a conclusion. Some conditions only affect motor or sensory nerves, and some processes are length dependent and others universal. It can sometimes be quite difficult to decide whether a process is primarily demyelinating or demyelinating with secondary axonal changes as features of both may coexist. | ||
| Sensory responses | Small or absent | Small or absent |
| Distal motor latency | Normal or slightly prolonged | Prolonged |
| CMAP amplitude | Small | Normal (reduced if conduction block or temporal dispersion) |
| Conduction block/temporal dispersion | Not present (responses may disperse slightly) | Present |
| Motor conduction velocity | Normal or slightly reduced | Notably reduced |
| F waves minimum latency | Normal or slightly prolonged | Significantly prolonged |
Nerve conduction study results interpretation
Normal values for nerve conduction study
Age matched “Normal” values for nerve conduction study parameters are either derived from studies of groups of neurologically normal subjects or culled from the literature. In the view of Mallik et al. 1, the most frequent statistics used are limits of 95% or less frequently 99% confidence limits of a normal group to indicate abnormality of a single parameter.
This approach may mislead as a crude separation between “normal” and “abnormal” dilutes the information whereas a Z score, for example, indicating the separation between a single value and the group mean expressed in SD, may be more informative. Alternatively, (a) a number of electrophysiological parameters may be taken together either as an “index” or “score”, or (b) the neurophysiologist assesses a number of parameters together to make a judgement as to whether a clinically relevant numerical abnormality should be emphasised in the report interpretation or not.
There are a number of physical parameters that require correction or allowance for. The most important is temperature. The fastest motor nerve conduction velocity is reduced by approximately 1 m/s per °C temperature fall. Conventionally, studies are performed as close to a surface recorded temperature of 34 °C. If that is not achieved by adequate heating or the limb, rarely a temperature correction must be applied. Some measures of conduction require correction for limb length or height. Finally nerve conduction data alter with age. The motor conduction slows by 0.4–1.7 m/s per decade after 20 years and the sensory by 2–4 m/s.
Motor nerve conduction study
In axonal loss
The most striking abnormality is a reduction in CMAP amplitude as fewer functioning motor axons are connected to muscle fibres. Since myelin is unaffected, the remaining axons conduct normally and one would expect latencies and conduction velocities to remain normal. However, with increasing motor axon loss some of the largest fastest conducting fibres will be lost. Therefore distal motor latency may be slightly prolonged (< 120% of normal limit) and conduction velocity slightly slowed (> 80% of normal limit).
The dynamics and timing of an axonal insult can affect the abnormalities seen. Immediately after a traumatic complete transection of the nerve, the portion of the nerve distal to the lesion will be normal as there has not been time for axonal degeneration to occur. The CMAP amplitude will only start to fall a few days later. Conversely, if there is a very slow loss of axons in a generalised neuropathy, the remaining unaffected axons may have time to sprout new connections to muscle fibres that have lost their innervation (collateral reinnervation) and the CMAP may remain within the normal amplitude range even though the total number of nerve axons is smaller. However, the immature regenerating fibres have slower velocities due to the effect of the short internodal distances and this produces a more dispersed CMAP.
In demyelination
With loss of myelin thickness nerve conduction is slowed and, if severe enough, saltatory conduction fails (conduction block). nerve conduction study shows severely prolonged motor latencies and notably slowed conduction velocities. The precise changes seen depend on the site and extent of demyelination. If demyelination is very proximal then distal motor latency and conduction velocity may be normal in which case only F waves may show abnormalities.
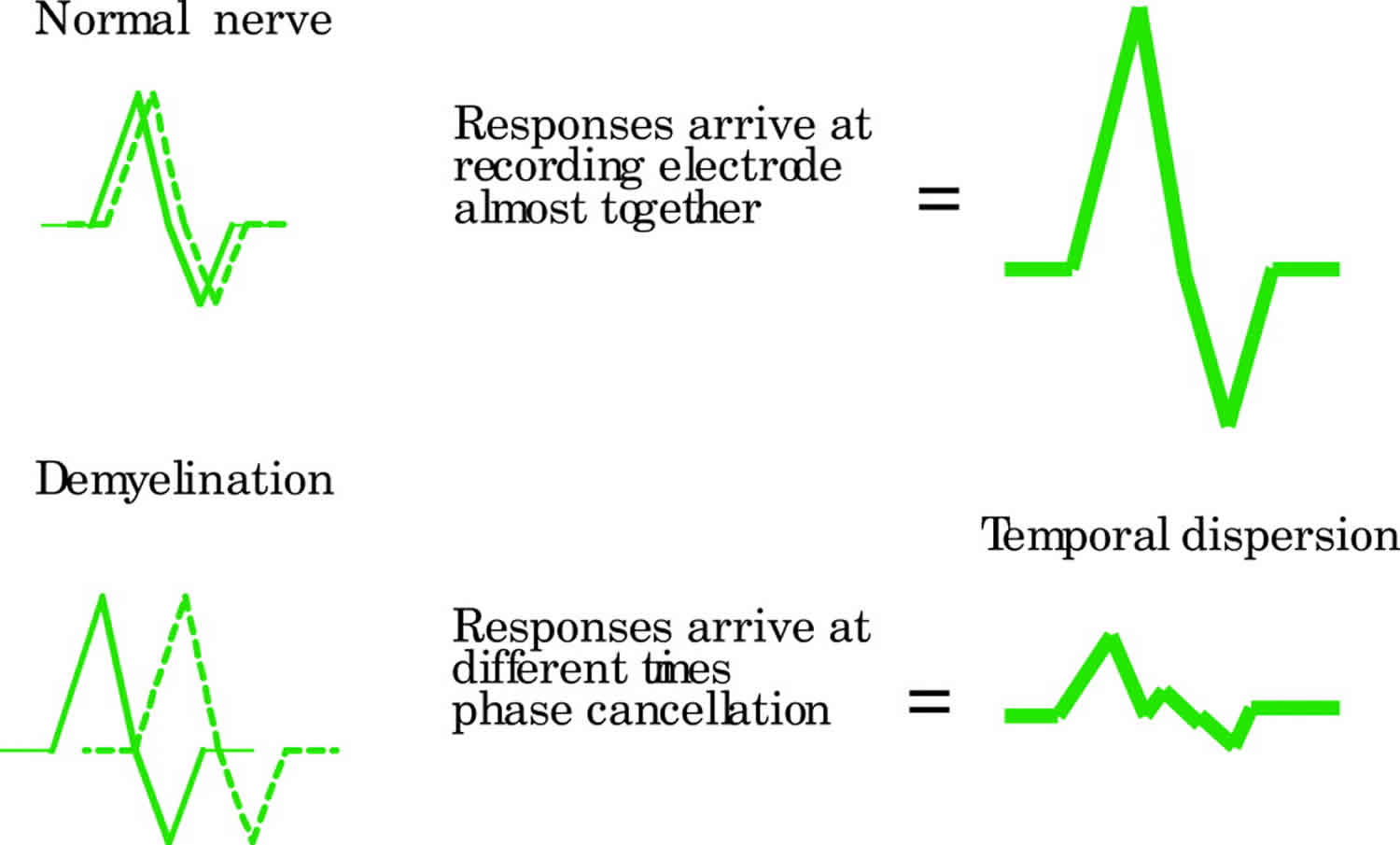
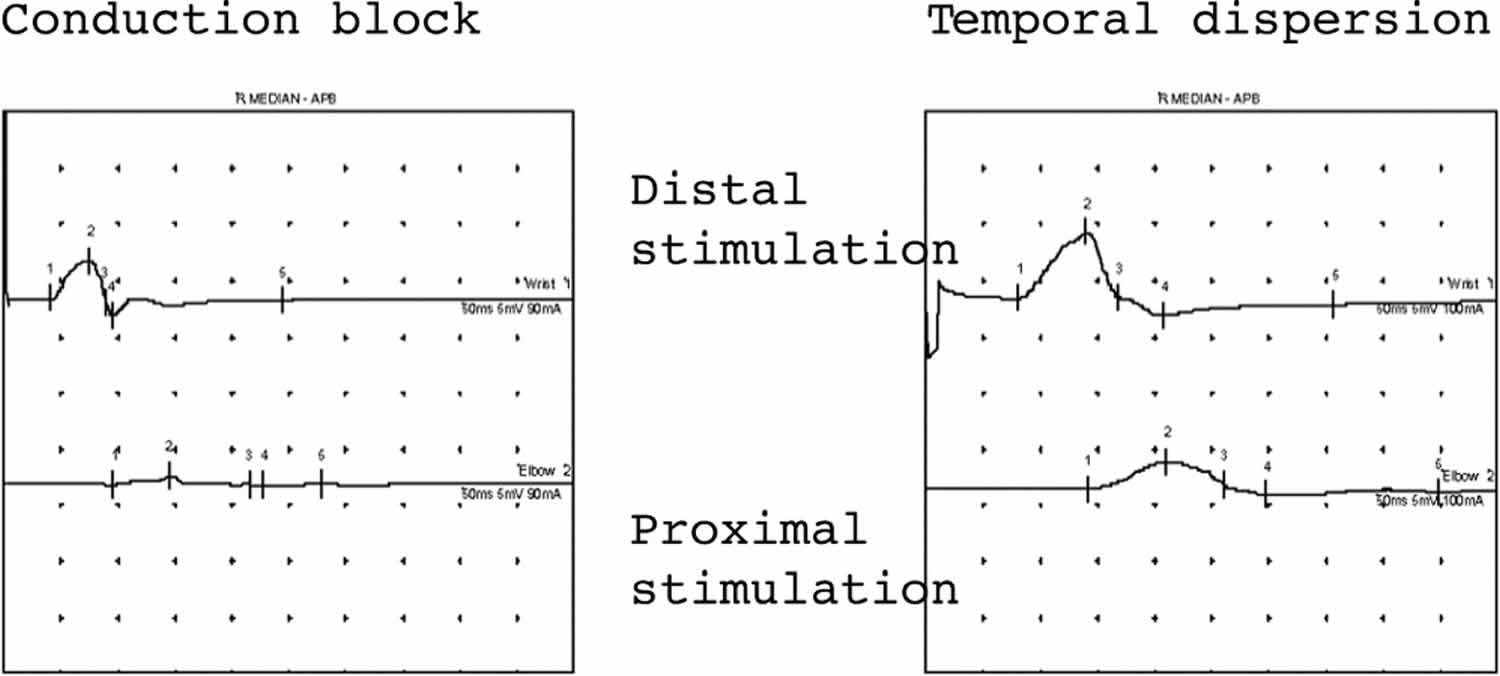
Conduction block or temporal dispersion both result in a reduction in CMAP amplitude. The CMAP area is used to assess the contribution of these two processes. In conduction block there is complete failure of conduction in some or all of the motor axons studied. Therefore the CMAP area with stimulation proximal to site of conduction block is smaller (> 20% reduction) compared with distal stimulation (Figure 5). For true conduction block to be detected, the proximal CMAP duration must not increase by > 20%. In temporal dispersion (Figure 5) there is a loss of synchrony in the nerve action potentials resulting in a loss of CMAP amplitude because the positive part of one muscle fibre action potential cancels out the negative part of another (phase cancellation) (Figure 4).
It is important to realize that slowing of conduction velocity alone without conduction block does not result in weakness as the impulses are still conducted from nerve to muscle. A good example of this is the presence of profound slowing of motor nerve conduction in totally asymptomatic primary relatives of patients with demyelinating hereditary motor and sensory neuropathy.
Figure 4. Demyelination nerve conduction study results
Footnote: Schematic representation of phase cancellation and temporal dispersion in demyelination. In the normal nerve, the responses are synchronised in time and therefore summate (amplitude is higher that that of the individual components). Temporal dispersion results in an increased duration and reduced amplitude of CMAP.
[Source 1 ]Figure 5. Demyelination nerve conduction study results
Footnote: Both these traces show demyelination in median motor studies. The trace on the left shows almost complete conduction block with an absent response with proximal stimulation. The trace on the right shows temporal dispersion where the CMAP duration increases by almost 40% with proximal stimulation. In both situations the CMAP amplitude with proximal stimulation is smaller.
[Source 1 ]Sensory nerve conduction study
In generalized disorders
In both axonal and demyelinating pathologies the sensory nerve action potential (SNAP) amplitude is reduced for different reasons. Sensory axonal loss will result in a smaller sensory nerve action potential (SNAP). Demyelination also produces small SNAPs but with prolonged durations. As they are of much shorter duration than compound muscle action potentials (CMAPs) they are more susceptible to phase cancellation (Figure 4).
The distribution of sensory nerve conduction study abnormalities may be helpful in determining aetiology. For example, the loss of smaller sensory nerve action potentials (SNAPs) in the lower limbs is common in an axonal dying back neuropathy related to drugs like vincristine, whereas equal involvement of upper and lower limb SNAPS raise the possibility of a sensory ganglionopathy such as that related to thalidomide treatment.
Focal lesions
Multiple sensory nerve conduction study allow the investigator to locate sensory neuropathies that involve single or multiple digital nerves distally (for example, vasculitis or hand arm vibration syndrome) right up to the major trunks, cords, and divisions of the brachial plexus proximally.
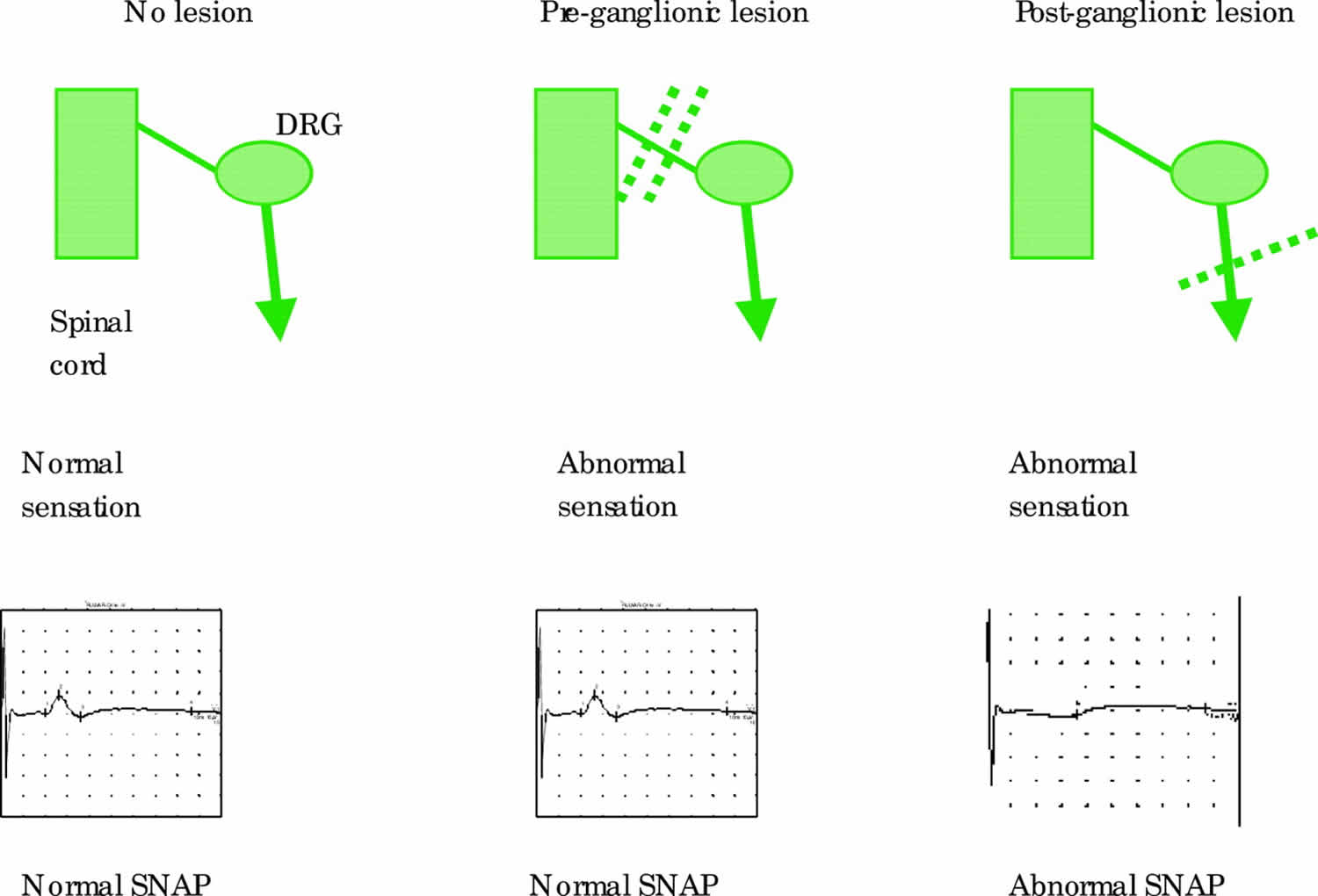
In proximal nerve trauma, maintenance of the sensory potential depends on the intact cell bodies in the dorsal root ganglia. Thus sensory nerve conduction study are extremely useful in localizing a peripheral nervous system lesion as either pre- and/or post-ganglionic. In a patient with a clinically suspected C8, T1 root lesion and with appropriate anaesthesia in that dermatome, the absence of the ulnar and medial antebrachial cutaneous sensory potential places the lesion distal to the dorsal root ganglion (DRG) in the lower trunk of the brachial plexus and not at root level (Figure 6). Needle EMG can then be used to define this further.
Figure 6. Sensory nerve conduction study results
Footnote: Sensory responses are normal in pre-ganglionic lesions even though sensation may be abnormal clinically. Post-ganglionic lesions result in abnormal sensory responses. DRG = dorsal root ganglion.
[Source 1 ]F waves
Generalized disorders
F waves are sensitive to all forms of generalised peripheral neuropathy with their absence or a prolonged minimum latency occurring early. For example, in AIDP where demyelination may be segmental, proximal and patchy, F wave abnormalities may be the earliest and (in mild cases) the only electrophysiological abnormality seen.
In axonal pathology F wave latencies may also be mildly delayed in keeping with the motor conduction velocity slowing secondary to the loss of the fastest conducting motor axons.
In motor neuronopathies such as the motor neurone diseases, prolongation of any F wave latency is strong evidence either that this is the incorrect diagnosis (such as in multifocal motor neuropathy) or that a second pathological process is present.
Focal lesions
F waves may be absent in focal peripheral nerve or anterior spinal disorders. They were initially also thought to be very useful in identifying individual root distribution abnormalities. However, particularly in the upper limbs, the substantial overlap of segmental innervation in the distally available peripheral nerves makes this test on its own of low sensitivity and anatomical specificity. In addition, the effect of demyelination is diluted by the length of the path over which the F wave passes. In distinguishing the presence of a distal or proximal lesion, the use of the F wave ratio which compares the F wave latency in the upper and lower halves of the limb (conventionally using knee and elbow as the dividing line) may be useful.
Repetitive nerve stimulation
Repetitive nerve stimulation is used in the evaluation of patients with suspected neuromuscular transmission disorders such as myasthenia gravis or Lambert-Eaton myasthenic syndrome. Repetitive nerve stimulation is a modified motor nerve conduction study where instead of recording CMAPs with single supramaximal electrical stimuli, a train of 8–10 stimuli is applied and the sequential response amplitudes and/or areas measured. This may be carried out at low (3–4 Hz) or high frequency stimulation (20–50 Hz). In the latter case the train is prolonged to allow 2–10 seconds of continuous data to be measured. Both distal and proximal muscles/nerves should be studied in every patient suspected of an neuromuscular transmission disorder as the sensitivity of the test is greatly increased by this means.
With low frequency stimulation in normal subjects, the CMAP amplitude and/or area falls over the first 4–5 stimuli by a maximum of 10–12%. The maximum fall should be between potentials 1 and 2. A number of department specific protocols have been published to study the repetitive nerve stimulation over time both before and after a period of maximum voluntary contraction of the muscle to pick up early or late neuromuscular transmission failure (Figure 7).
There are many pitfalls in the repetitive nerve stimulation test and artefact almost always gives rise to an abnormal test. Thus adherence to a strict protocol and heightened suspicion on the part of the clinical neurophysiologist to an abnormal result is essential as are repeated studies for reproducibility of abnormalities.
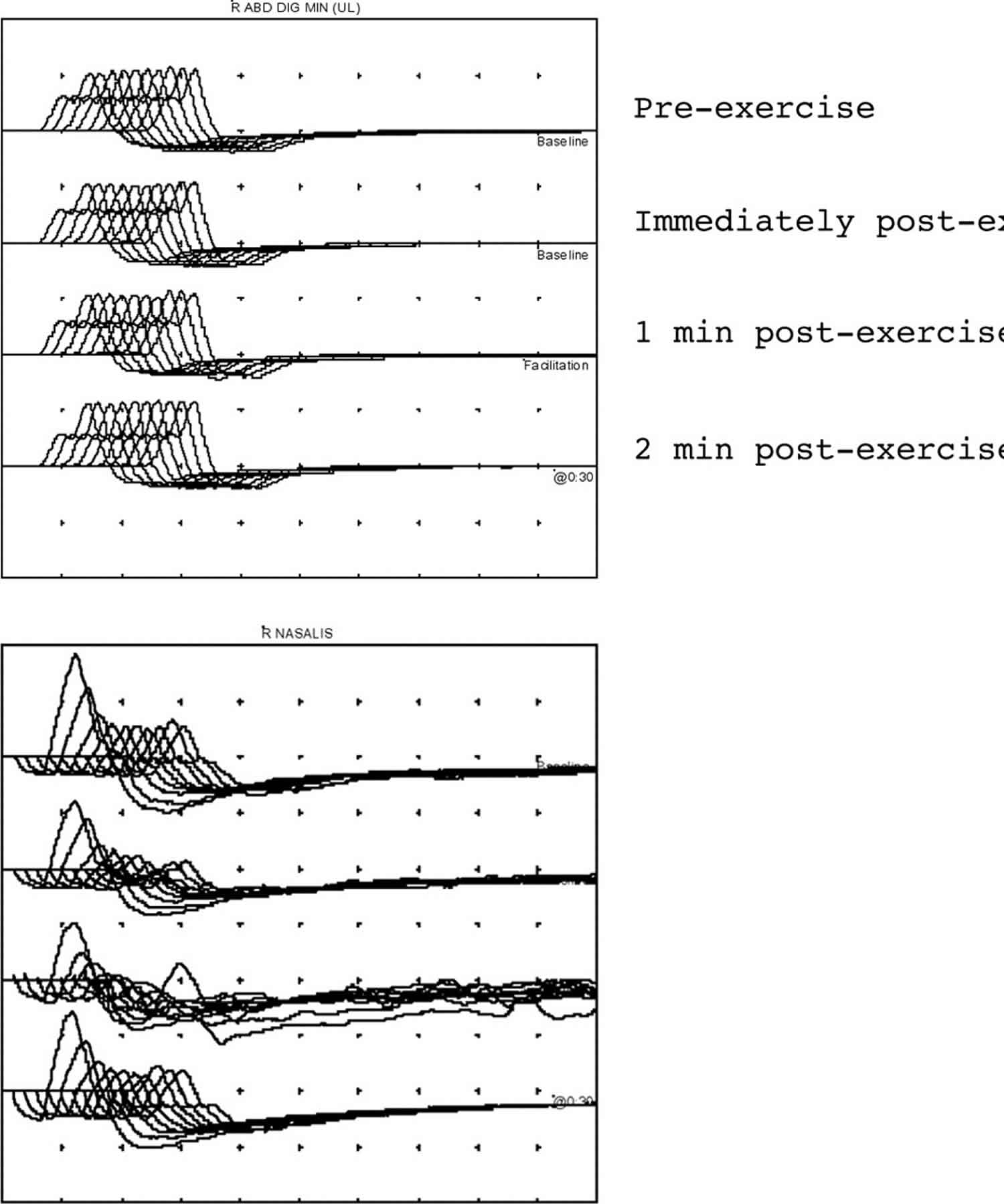
Figure 7. Repetitive nerve stimulation results
Footnote: (A) Normal repetitive nerve stimulation from abductor digiti minimi muscle in the hand. The amplitude of the CMAPs within each train does not decrement nor is there any significant increment in CMAP amplitude after exercise. (B) Repetitive nerve stimulation in myasthenia gravis from the nasalis muscle. Four stimulus trains are given—all at baseline with no exercise. A reproducible decrement of 55% is seen (train 3 is technically unsatisfactory because of movement artefact).
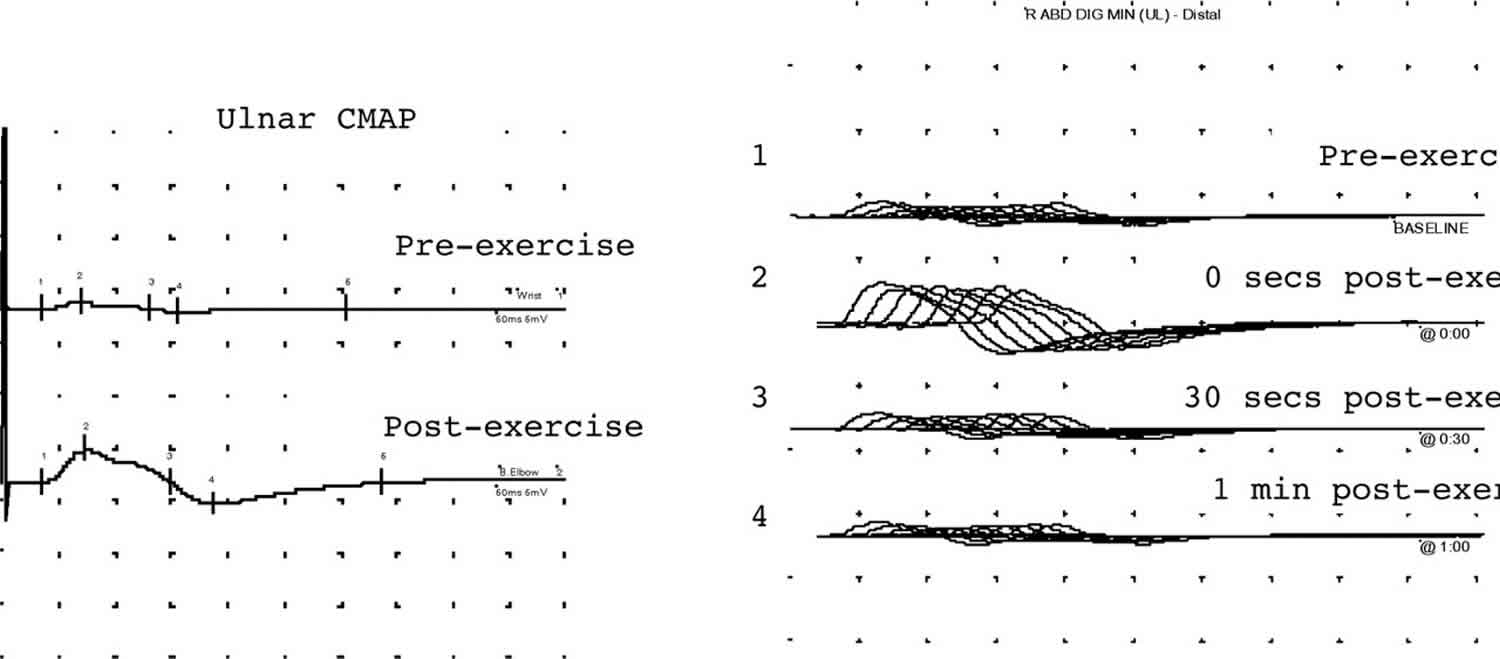
[Source 1 ]High frequency stimulation may be used to discover evidence of a post-synaptic transmitter release disorder like Lambert-Eaton myasthenic syndrome. It is painful and requires considerable patient tolerance. There is evidence that recording low frequency repetitive nerve stimulation immediately before and after a 20–30 second period of maximum voluntary contraction by the patient is equally sensitive and is more humane (Figure 8).
Figure 8. Repetitive nerve stimulation results
Footnote: These traces show typical electrophysiological features of a pre-synaptic neuromuscular transmission disorder in a patient with Lambert-Eaton myasthenic syndrome. The traces on the left show a small amplitude ulnar CMAP that after exercise increases fourfold in amplitude. The traces on the right show repetitive nerve stimulation studies. The amplitude increases post-exercise. Trains 1, 3, and 4 show a decrement of 30% which repairs with exercise (train 2).
[Source 1 ]Physiological basis for the repetitive nerve stimulation
The neuromuscular junction consists of the motor axon terminal, the synaptic cleft, and the post-synaptic muscle membrane. As the motor axon potential depolarises the nerve terminal, voltage gated calcium channels open increasing the concentration of calcium in the pre-synaptic nerve terminal. This in turn facilitates the release of quanta of acetylcholine (ACh) from the nerve terminal into the synaptic cleft. Acetylcholine binds to receptors on the post-synaptic membrane causing depolarisation (end plate potential). The size of the end plate potential is dependent on the amount of acetylcholine released and its binding to receptors. In the healthy state, the end plate potential reaches a threshold level and causes an action potential to be propagated along a muscle fibre resulting in muscle contraction. Normally there is a large safety factor for neuromuscular transmission with the amount of acetylcholine released per impulse several times that required to generate a threshold level end plate potential.
In low frequency repetitive nerve stimulation, the rate of stimulation is such that the end plate physiology is stressed, but not to the level that produces the natural facilitation of neuromuscular transmission at greater stimulation frequencies. Thus an abnormal fall (decrement) in CMAP amplitude and/or area at low stimulation rates indicates a drop in the safety factor for transmission whether from a pre- or post-synaptic cause.
In high frequency stimulation natural facilitation is enhanced by pre-synaptic Ca++ influx and this may counteract a process such as Lambert-Eaton myasthenic syndrome where quantal release is depressed.
Repetitive nerve stimulation in disease
Neuromuscular transmission disorders may be congenital or acquired and in broad terms can be thought of as pre-synaptic or post-synaptic depending on where the defect lies.
Post-synaptic disorders of neuromuscular junction transmission
The archetypal post-synaptic disorder is myasthenia gravis (MG) where antibodies to acetylcholine receptors (AChR) cause degradation and increased turnover of receptor as well as macrophage initiated post-synaptic membrane simplification. In Myasthenia gravis the safety factor is lost because as AChRs are depleted, less post-synaptic depolarisation occurs and some end plate potentials do not reach threshold for genesis of a propagated muscle membrane potential producing neuromuscular block. If this process affects a significant proportion of the tested muscle end plates, the repetitive nerve stimulation baseline train will show a significant decrement (greater than 10%). The decrement is usually measured by comparing the amplitude of the third or fourth CMAP in the train to the first (Figure 7B). Very often some slight facilitation reduces the decrement over potentials 7–10 of the train.
An abnormal decrementing repetitive nerve stimulation test is non-specific and can be seen in a number of circumstances where muscle contraction processes may fail with repetitive stimulation (see repetitive nerve stimulation pitfalls).
Pre-synaptic disorders
In Lambert-Eaton myasthenic syndrome there are antibodies to voltage gated calcium channels (pre-synaptic disorder) causing impaired release of ACh quanta. Low frequency repetitive nerve stimulation stimulation may produce exactly the same decrement as seen in Myasthenia gravis with additionally a small initial CMAP amplitude. Here calcium influx into the nerve terminal is reduced due to the action of voltage gated calcium channel antibodies and in turn ACh release into the synaptic cleft is reduced and some end plate potentials will be sub-threshold.
However, the diagnostic abnormality is of a significant > 100% increment in the CMAP amplitude after exercise or fast repetitive stimulation. Exercise increases calcium influx and the CMAP amplitude may increase by up to 10 times. In this case we are just comparing the amplitude of the first CMAP in the train before and after exercise (Figure 8). Despite this increment, within each low frequency train a further decrement may occur due to ACh depletion.
Nerve conduction study pitfalls
Table 2. Nerve conduction study interpretation pitfalls
| Pitfall | Explanation |
|---|---|
| Abbreviations: CN = clinical neurophysiologist; LEMS = Lambert Eaton myasthenic syndrome; MG = myasthenia gravis; PNE = peripheral neurophysiological examination; SNAPS = sensory nerve action potentials. | |
| Overinterpretation of results | • A limited numerical abnormality must be reported but placed in the context of the clinical problem—for example, the finding of a subclinical carpal tunnel abnormality on NCS does not make the diagnosis of carpal tunnel syndrome • An NCS abnormality may relate to a previous and currently irrelevant injury or operation |
| Over simplification of results | • All studies should be interpreted together in case multiple diagnoses are present. Occum’s razor may be too sharp. For example, in a patient with obvious polymyositis, NCS should ideally be performed to delineate any additional coexisting neuropathy |
| Misinterpretation of NCS results | • If the FMNCV is normal, the patient may still have slowing of conduction of other nerve fibres related to a neuropathy • If the SNAPS are normal in all respects the patient may still have a small fibre sensory neuropathy producing symptoms and signs • If a nerve is reported unstimulatable, think of anomalous innervation or anatomical variation of nerve position before concluding pathology |
| Misinterpretation of RNS results | • If a decrement continues throughout the RNS train, this is probably artefact • If a decrement is found in RNS, it is not pathognomic of myasthenia gravis. It has been found to be abnormal in a number of conditions with abnormal nerve terminal function (motor neurone disease, active axonal neuropathy) or muscle membrane instability (polymyositis) • While MG and LEMS are the most common NMT disorders, the unwary will miss correctly diagnosing congenital myasthenic syndromes, botulism, drug and toxins (for example, organophosphates), all of which have characteristic PNE patterns when all tests are interpreted together with the clinical picture |
| For the referrer | • If the referring doctor has not asked a specific question of the CN investigator, they should not be surprised if occasionally their request for a PNE is misunderstood • A PNE request should never be couched in “please exclude” terms. While probabilities may be discussed, total exclusion of a diagnosis depends on non-biological errors such as sampling • In the UK, CN are a scarce resource, often struggling with large waiting lists. Thus all referrals should be considered in terms of: (a) what knowledge will I gain about my patient from this investigation? (b) Will the result change my management? (c) How urgent should the request be |
Table 3. Nerve conduction study performance of tests pitfalls
| Technique | Pitfall | Explanation |
|---|---|---|
| Abbreviations: CMAP = compound muscle action potential; DL = distal latency; FMNCV = fastest motor nerve conduction velocity; NAP = nerve action potential; NCS = nerve conduction studies; NMT = neuromuscular transmission; RNS = repetitive nerve stimulation. | ||
| Motor NCS | Temperature | A low FMNCV or long DL may relate to low skin temperature. It should always be recorded and cool limbs heated |
| F waves | No pathway length allowance | Because of the long pathway, normal values have to be related to limb length, pathway measurement or body height |
| Motor NCS | Waveform measurements | If the onset of the CMAP is positive—that is, a downward deflection—the active recording electrode is misplaced away from the motor point and spurious latency and amplitude values will occur |
| If an initially positive going waveform is only seen in proximal median nerve stimulation there is a likelihood of an anatomic anomaly in the forearm | ||
| Motor and sensory NCS | Inadequate numbers of nerves studied | In order to determine whether abnormalities are focal, generalised, length dependent, a sample of nerves in upper and lower limbs usually need to be studied |
| Even in simple entrapment neuropathies, studies should be bilateral and include at least one other nerve not under suspicion to exclude an underlying generalised process | ||
| Motor and sensory NCS | Inadequate range of stimulus sites | Dependent on the clinical picture stimulation may be required proximally to detect focal proximal abnormality. However, particularly with root and plexus stimulation, care in ensuring supramaximal stimulation is vital |
| Motor NCS | Electrode positioning | If optimal positioning not used, the indifferent electrode may contribute significantly to amplitude measurements |
| Motor NCS | Nerve length measurement | A flexible ruler/tape is necessary to follow the nerve path accurately |
| Some measurements around joints are affected by flexion/extension of the joint. For example, the least errors are found in ulnar NCS if the elbow is flexed to >100° | ||
| Motor NCS | Not supramaximal | If the CMAP is not recorded supramaximally at all sites, the measured FMNCV will be in error. Spurious conduction block may be seen if proximal nerve stimulation is not supramaximal |
| Motor NCS | Over stimulation | If a nerve is stimulated with too high a current/voltage, adjacent nerves may be recruited and produce spurious results |
| Sensory NCS | Stimulus artefacts | Particularly in the lower limb nerves the stimulus may cause an artefact which alters the NAP shape. This must be allowed for in calculation if it cannot be abolished |
| Sensory NCS | Absent potentials | In some nerves, using the orthodromic technique, the 95% confidence limits may include zero microvolts. Thus an absent potential may not be “abnormal” |
| Motor and sensory NCS | Normal values | Failure to use age specific normal values in the very young and very old may lead to error |
| RNS | Inadequate positioning and movement restriction | If the recording electrodes do not lie right over the motor point, a spurious decrement may be created as the muscle contracts repeatedly. Care over positioning and ideally splintage of the muscle so that it remains isometric helps |
| Inadequate facilitation | Patients may need continuous encouragement to obtain 30–30 second maximal voluntary contraction | |
| Too short an analysis period | RNS trains should be followed for at least 3 minutes (preferably 5) to pick up the occasional post-facilitation exhaustion | |
| Limited sites | The sensitivity of RNS in NMT disorders is greatly increased by studying both proximal (nasalis, trapezius) muscles as well as distal (abductor digiti minimi). | |
| Wrong test | As a rule of thumb, a patient who has an unequivocally normal edrophonium test will frequently also have a normal distal and proximal RNS test. In these circumstances the authors go straight to single fibre electromyography of an intermediate and proximal muscle as a more sensitive test. | |
EMG Normal Results
There is normally very little electrical activity in a muscle while at rest. Inserting the needles can cause some electrical activity, but once the muscles quiet down, there should be little electrical activity detected.
When you flex a muscle, activity begins to appear. As you contract your muscle more, the electrical activity increases and a pattern can be seen. This pattern helps your doctor determine if the muscle is responding as it should.
Abnormal EMG and nerve conduction study
An EMG can detect problems with your muscles during rest or activity. Disorders or conditions that cause abnormal results include the following:
- Alcoholic neuropathy (damage to nerves from drinking too much alcohol)
- Amyotrophic lateral sclerosis (ALS; disease of the nerve cells in the brain and spinal cord that control muscle movement)
- Axillary nerve dysfunction (damage of the nerve that controls shoulder movement and sensation)
- Becker muscular dystrophy (muscle weakness of the legs and pelvis)
- Brachial plexopathy (problem affecting the set of nerves that leave the neck and enter the arm)
- Carpal tunnel syndrome (problem affecting the median nerve in the wrist and hand)
- Cubital tunnel syndrome (problem affecting the ulnar nerve in the elbow)
- Cervical spondylosis (neck pain from wear on the disks and bones of the neck)
- Common peroneal nerve dysfunction (damage of the peroneal nerve leading to loss of movement or sensation in the foot and leg)
- Denervation (reduced nerve stimulation of a muscle)
- Dermatomyositis (muscle disease that involves inflammation and a skin rash)
- Distal median nerve dysfunction (problem affecting the median nerve in the arm)
- Duchenne muscular dystrophy (inherited disease that involves muscle weakness)
- Facioscapulohumeral muscular dystrophy (Landouzy-Dejerine; disease of muscle weakness and loss of muscle tissue)
- Familial periodic paralysis (disorder that causes muscle weakness and sometimes a lower than normal level of potassium in the blood)
- Femoral nerve dysfunction (loss of movement or sensation in parts of the legs due to damage to the femoral nerve)
- Friedreich ataxia (inherited disease that affects areas in the brain and spinal cord that control coordination, muscle movement, and other functions)
- Guillain-Barré syndrome (autoimmune disorder of the nerves that leads to muscle weakness or paralysis)
- Lambert-Eaton syndrome (autoimmune disorder of the nerves that causes muscle weakness)
- Multiple mononeuropathy (a nervous system disorder that involves damage to at least 2 separate nerve areas)
- Mononeuropathy (damage to a single nerve that results in loss of movement, sensation, or other function of that nerve)
- Myopathy (muscle degeneration caused by a number of disorders, including muscular dystrophy)
- Myasthenia gravis (autoimmune disorder of the nerves that causes weakness of the voluntary muscles)
- Peripheral neuropathy (damage of nerves away from the brain and spinal cord)
- Polymyositis (muscle weakness, swelling, tenderness, and tissue damage of the skeletal muscles)
- Radial nerve dysfunction (damage of the radial nerve causing loss of movement or sensation in the back of the arm or hand)
- Sciatic nerve dysfunction (injury to or pressure on the sciatic nerve that causes weakness, numbness, or tingling in the leg)
- Sensorimotor polyneuropathy (condition that causes a decreased ability to move or feel because of nerve damage)
- Shy-Drager syndrome (nervous system disease that causes bodywide symptoms)
- Thyrotoxic periodic paralysis (muscle weakness from high levels of thyroid hormone)
- Tibial nerve dysfunction (damage of the tibial nerve causing loss of movement or sensation in the foot)