Contents
- What is a nevus
- Atypical nevus or dysplastic nevus
- Blue nevus
- Congenital nevus
- What do congenital melanocytic nevus look like?
- Classification of congenital melanocytic nevus
- How common are congenital melanocytic nevi?
- Progression over time
- Descriptive names of some congenital nevus
- What causes a congenital melanocytic nevus?
- Do congenital melanocytic nevus cause any symptoms?
- Risk of developing melanoma within a congenital melanocytic nevus
- Congenital melanocytic nevus diagnosis
- Congenital melanocytic nevus treatment
- Epidermal nevus
- Spitz nevus
- Nevus sebaceous
- Melanocytic nevus
What is a nevus
The word nevus indicates a benign (non-cancerous) skin or mucosal lesion comprising an abnormal mixture of a tissue’s normal components, usually presenting at birth or at a young age. A nevus is a congenital (present at birth) or acquired growths or pigmented blemishes on the skin; birthmarks or moles. As an example, mole is a melanocytic nevus. A nevus may also form from other skin cells (e.g., vascular nevus, which are formed from blood vessels). Some of these are also congenital (present at birth).
Atypical nevus or dysplastic nevus
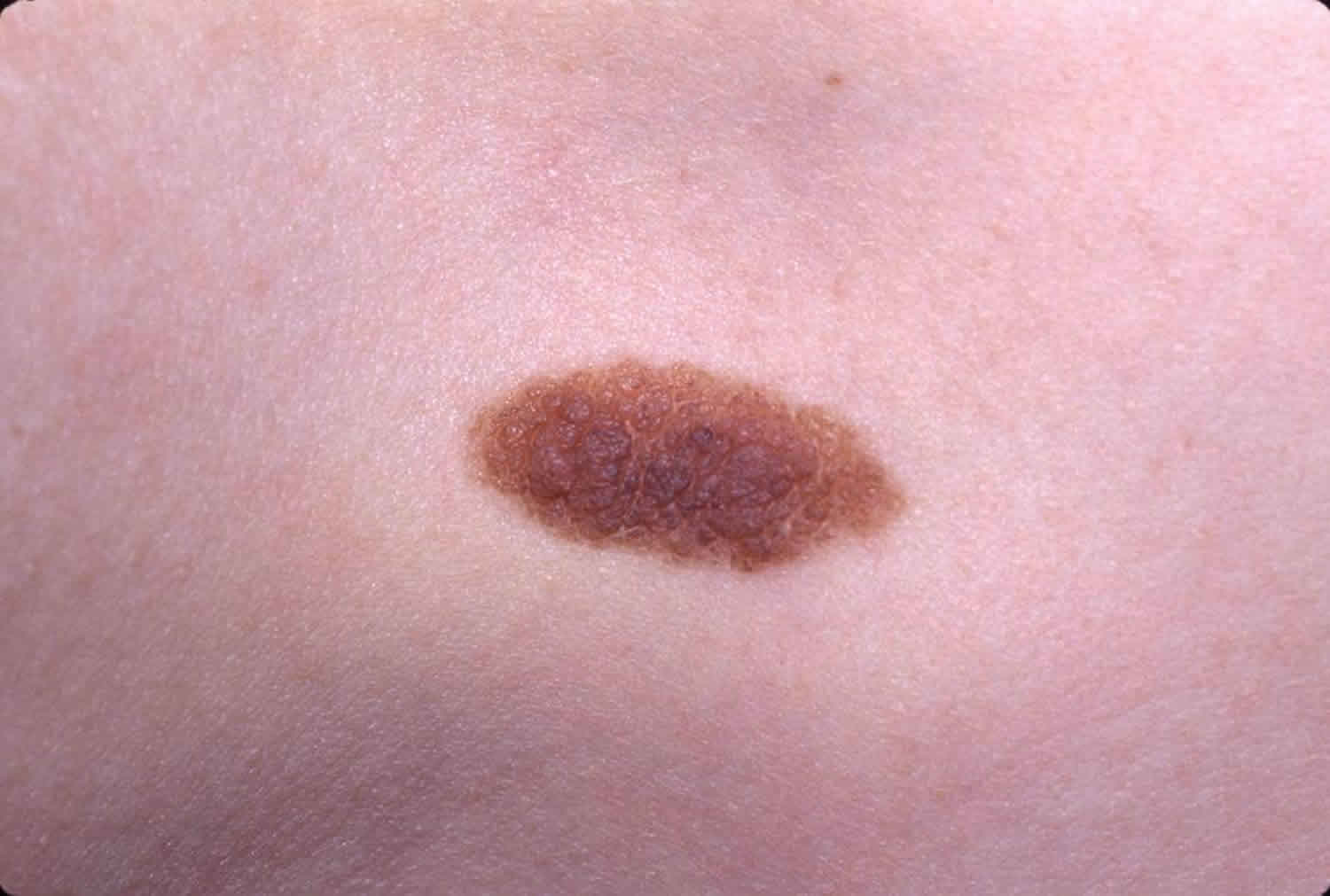
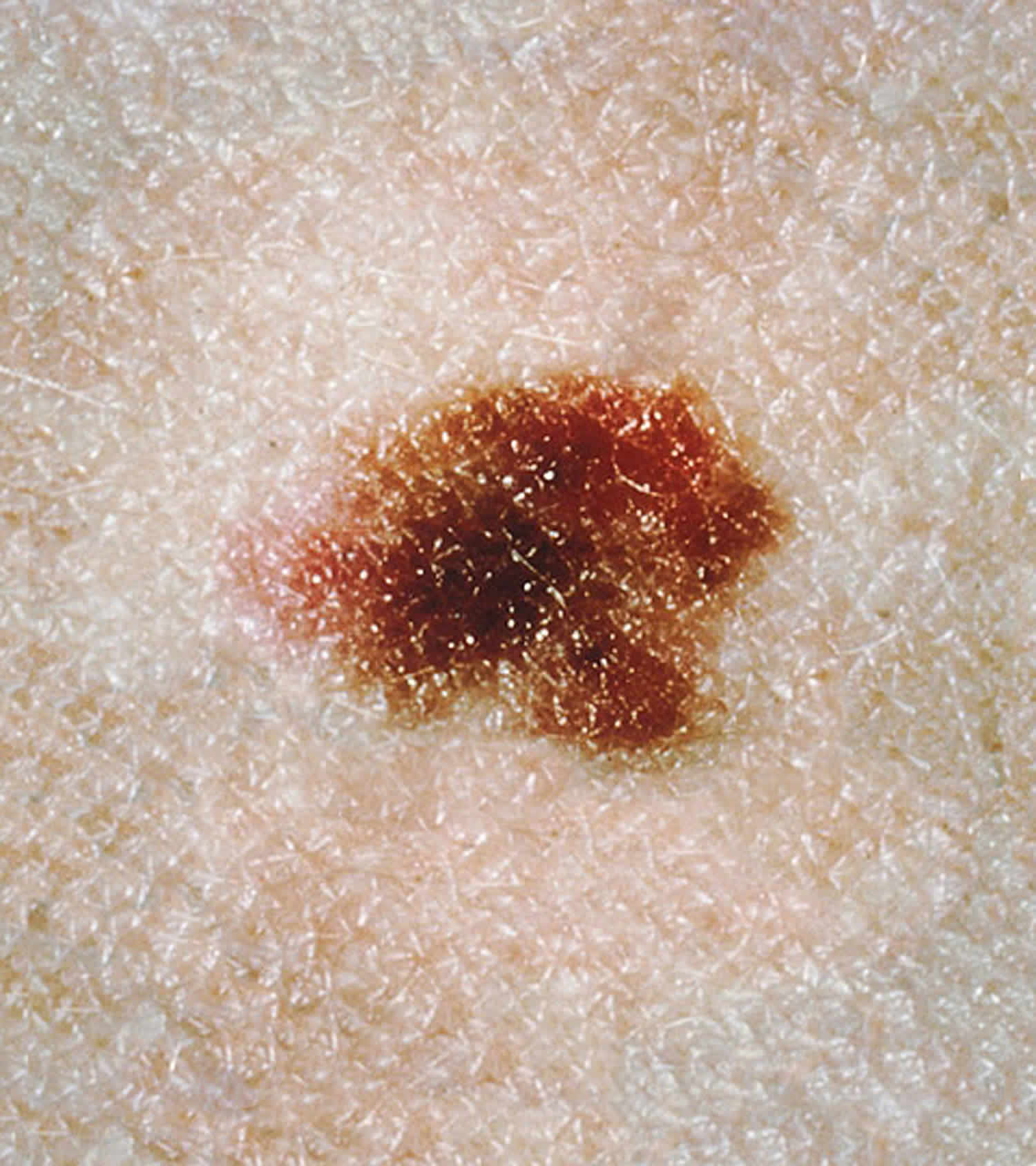
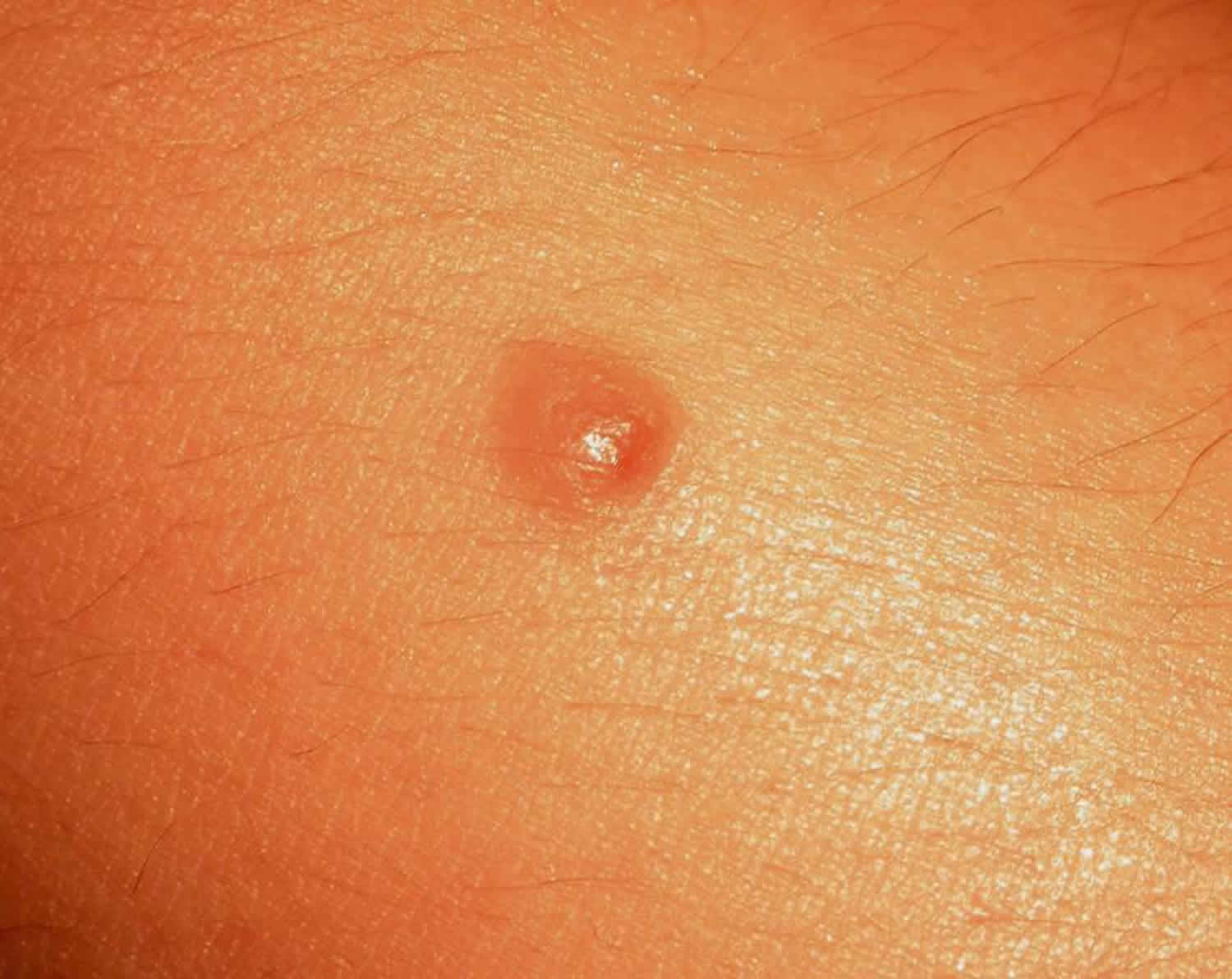
A dysplastic nevus or atypical melanocytic nevus, is a type of mole that looks different from a common mole. Some doctors use the term “atypical mole” or atypical melanocytic nevus to refer to a dysplastic nevus. “Atypical melanocytic nevus” is often shortened to “atypical nevus”. A dysplastic nevus may be bigger than a common mole, and its color, surface, and border may be different. A dysplastic nevus is usually more than 5 millimeters wide 1. A dysplastic nevus can have a mixture of several colors, from pink to dark brown. Usually, it is flat with a smooth, slightly scaly, or pebbly surface, and it has an irregular edge that may fade into the surrounding skin.
Atypical nevus or atypical melanocytic nevus is skin lesion whose clinical and histologic features sit somewhere on the proliferative continuum from a common mole to a melanoma, although they rarely progress to melanoma. As with other melanocytic nevi the majority disappear in time but some persist.
- Older names for atypical melanocytic nevus include Clark nevus and B-K mole.
- Some people prefer to use the term dysplastic nevus when referring to an atypical nevus, but strictly speaking dysplastic nevus is a histological description of a particular kind of atypical melanocytic nevus.
Atypical nevus may occur sporadically or they may be familial (inherited).
Sporadic atypical nevus
Sporadic atypical nevus mainly affect fair-skinned individuals with light colored hair and freckles (phototype 1 or 2). They are more numerous if they have been frequently exposed to the sun.
Atypical nevus may develop at any time but most of them develop within the first 15 years of life. Typically, people with sporadic atypical nevus have one to ten moles that are larger than 6 mm in diameter.
Familial atypical nevus
Atypical nevus that run in families may be part of the FAMM syndrome. FAMM is an abbreviation for Familial Atypical Mole and Melanoma. People with FAMM syndrome have the following:
- One or more first-degree or second-degree relative diagnosed with malignant melanoma at a young age (< 40 years);
- A large number of nevi (often more than 50), some of which are atypical;
- Nevus that are dysplastic on histopathology.
FAMM syndrome was previously known as the dysplastic nevus syndrome.
People with FAMM syndrome may have several hundred atypical nevi.
Figure 1. Dysplastic nevus
What is a dysplastic nevus?
Only a minority of clinically atypical nevi fulfil microscopic criteria for dysplastic nevus. Many histologically dysplastic nevus are clinically trivial (e.g., small in size, and uniform in color and in structure).
Histological dysplasia may be mild, moderate or severe. Distinct pathological criteria of a dysplastic nevus are listed here.
- The dysplastic nevus may be a junctional nevus (when the melanocytes are found at the epidermodermal junction) or a compound nevus (when the melanocytes are found at the epidermodermal junction and within the dermis).
- The nevus cells form a row along the dermoepidermal junction (this is reported as lentiginous proliferation), with or without nevus cells in nests (also called theques).
- These round or oval collection or nest of melanin-containing nevus are often irregular in size and shape and may ‘bridge’ or join together.
- The cells may be spindle-shaped (elongated) or epithelioid (broad, resembling epidermal keratinocytes).
- There may be cytologic atypia (cells that are smaller or larger than usual).
- There may be fibrosis or scarring in the dermis.
- Inflammatory cells may infiltrate the lesion.
- Associated blood vessels may be increased in number or enlarged.
Melanoma may arise within a dysplastic nevus, within a non-dysplastic, normal, melanocytic nevus, or more often, in normal-appearing skin.
What does an atypical nevus look like?
The term atypical nevus is sometimes used to mean any funny-looking mole, and sometimes to mean a melanocytic lesion that is suspected of being a malignant melanoma (a cancerous mole).
One definition of an atypical nevus is a mole with at least 3 of the following features.
- Size > 5 mm diameter
- Ill-defined or blurred borders
- Irregular margin resulting in an unusual shape
- Varying shades of colour (mostly pink, tan, brown, black)
- Flat and bumpy components.
What is the importance of atypical nevus?
People with 5 or more clinically atypical nevi have a higher risk than the general population of developing melanoma; the relative risk is reported to be six times that of people without atypical nevi. People with Familial Atypical Mole and Melanoma (FAMM) syndrome have an extremely high risk of developing melanoma.
Melanocytic nevi are harmless (benign) and do not need to be removed. However, it is not always easy even for an experienced dermatologist to tell whether a lesion is a nevus or a melanoma, especially if there are atypical features. Dermatoscopy in trained hands may help. If in doubt, a suspicious or changing atypical nevus should be removed by excision biopsy. Partial biopsy is best avoided, as the test may miss a small focus of melanoma. A pathologist will usually make the correct diagnosis, although sometimes deeper levels and/or a second opinion may be required.
People diagnosed with atypical nevi should be taught how to self-examine their skin for new skin lesions and for changes to existing moles that may indicate the development of melanoma. People with numerous moles should visit their family doctor or dermatologist regularly for a thorough skin check.
It is often helpful to keep photographic records of melanocytic nevi (preferably with dermatoscopic images); digital archiving at a photographic skin surveillance clinic is convenient for people with many moles or atypical nevi. The close-up photographs with dermatoscopic views should be repeated from time to time, so change can be detected early and its significance determined.
Careful sun protection is recommended for everyone but is especially important for people with many nevi or atypical nevi. Avoid excessive sun exposure and sunburn, dress up, and use a SPF50+ sunscreen when outdoors in the middle of the day or for prolonged periods.
Can a dysplastic nevus turn into melanoma?
Yes, but most dysplastic nevi do not turn into melanoma 2. Most remain stable over time. Researchers estimate that the chance of melanoma is about ten times greater for someone with more than five dysplastic nevi than for someone who has none, and the more dysplastic nevi a person has, the greater the chance of developing melanoma 1.
What should people do if they have a dysplastic nevus?
Everyone should protect their skin from the sun and stay away from sunlamps and tanning booths, but for people who have dysplastic nevi, it is even more important to protect the skin and avoid getting a suntan or sunburn.
In addition, many doctors recommend that people with dysplastic nevi check their skin once a month 3. People should tell their doctor if they see any of the following changes in a dysplastic nevus 4:
- The color changes
- It gets smaller or bigger
- It changes in shape, texture, or height
- The skin on the surface becomes dry or scaly
- It becomes hard or feels lumpy
- It starts to itch
- It bleeds or oozes
Another thing that people with dysplastic nevi should do is get their skin examined by a doctor 4. Sometimes people or their doctors take photographs of dysplastic nevi so changes over time are easier to see 4. For people with many (more than five) dysplastic nevi, doctors may conduct a skin exam once or twice a year because of the moderately increased chance of melanoma. For people who also have a family history of melanoma, doctors may suggest a more frequent skin exam, such as every 3 to 6 months 1.
Should people have a doctor remove a dysplastic nevus or a common mole to prevent it from changing into melanoma?
No. Normally, people do not need to have a dysplastic nevus or common mole removed. One reason is that very few dysplastic nevi or common moles turn into melanoma 1. Another reason is that even removing all of the moles on the skin would not prevent the development of melanoma because melanoma can develop as a new colored area on the skin 4. That is why doctors usually remove only a mole that changes or a new colored area on the skin.
Blue nevus
Blue nevus is a type of melanocytic nevus in which spindle-shaped nevus cells are located deep within the dermis.
Blue nevus presents mainly in older children and young adults. Unlike melanoma, once a blue naevus appears it tends to remain unchanged throughout life.
Blue nevi are twice as common in women as in men. Blue nevi are more prevalent among Asians, where the prevalence is estimated to be 3–5%, compared to approximately 1–2% in white skinned adults.
Different types of blue nevus include:
- Common blue nevus
- Cellular blue nevus
- Amelanotic blue nevus
- Combined blue nevus
- Sclerosing (desmoplastic) blue nevus
- Epithelioid nevus.
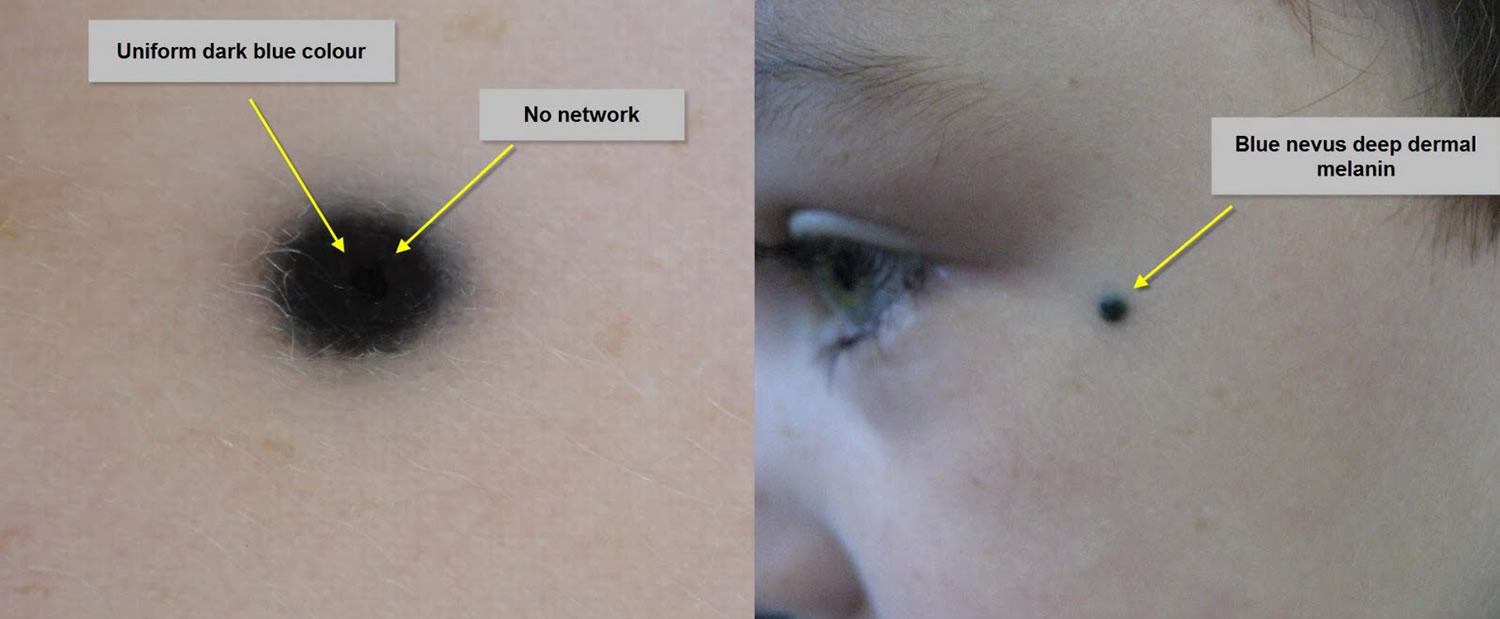
What are the clinical features of blue nevus?
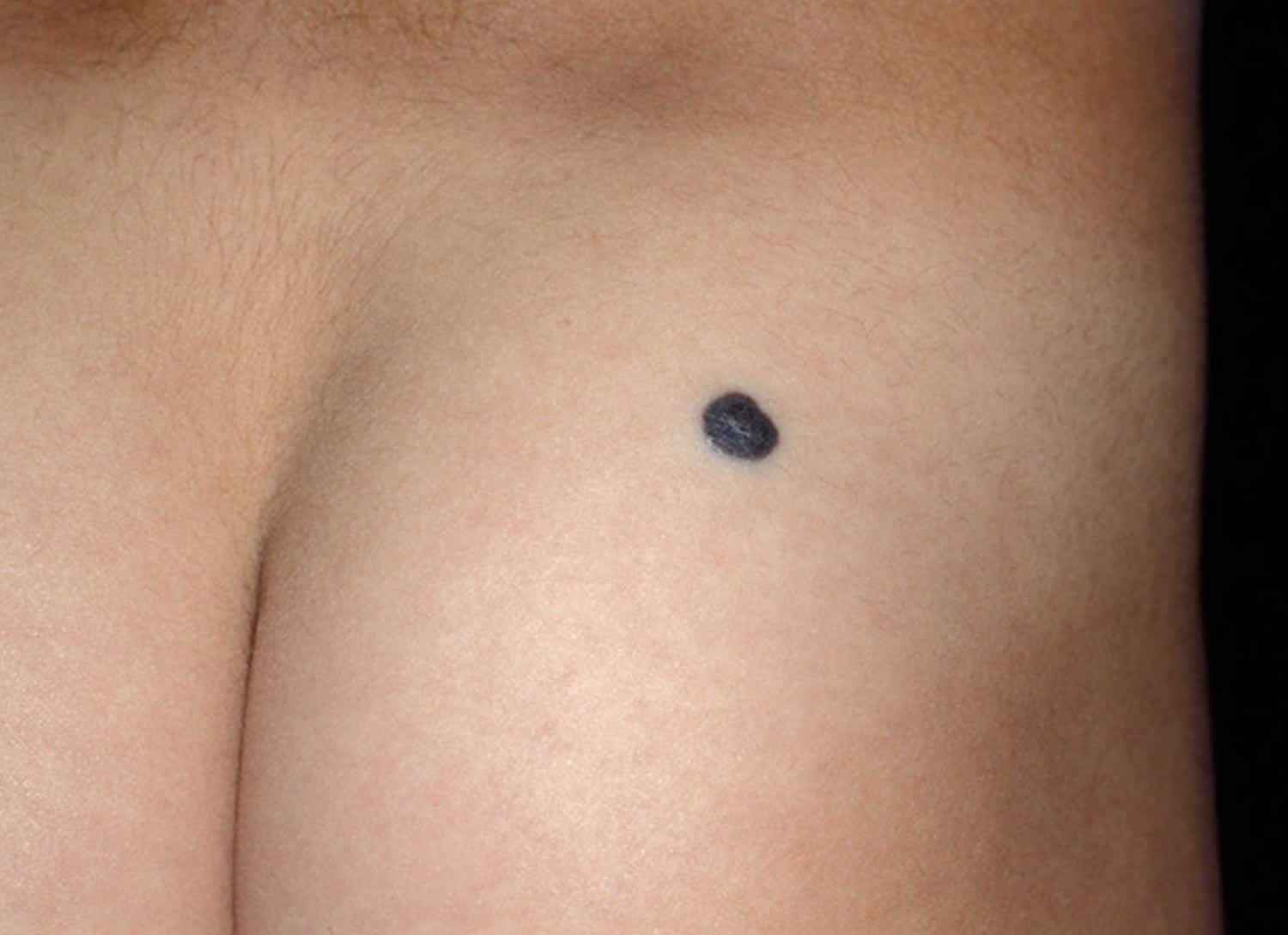
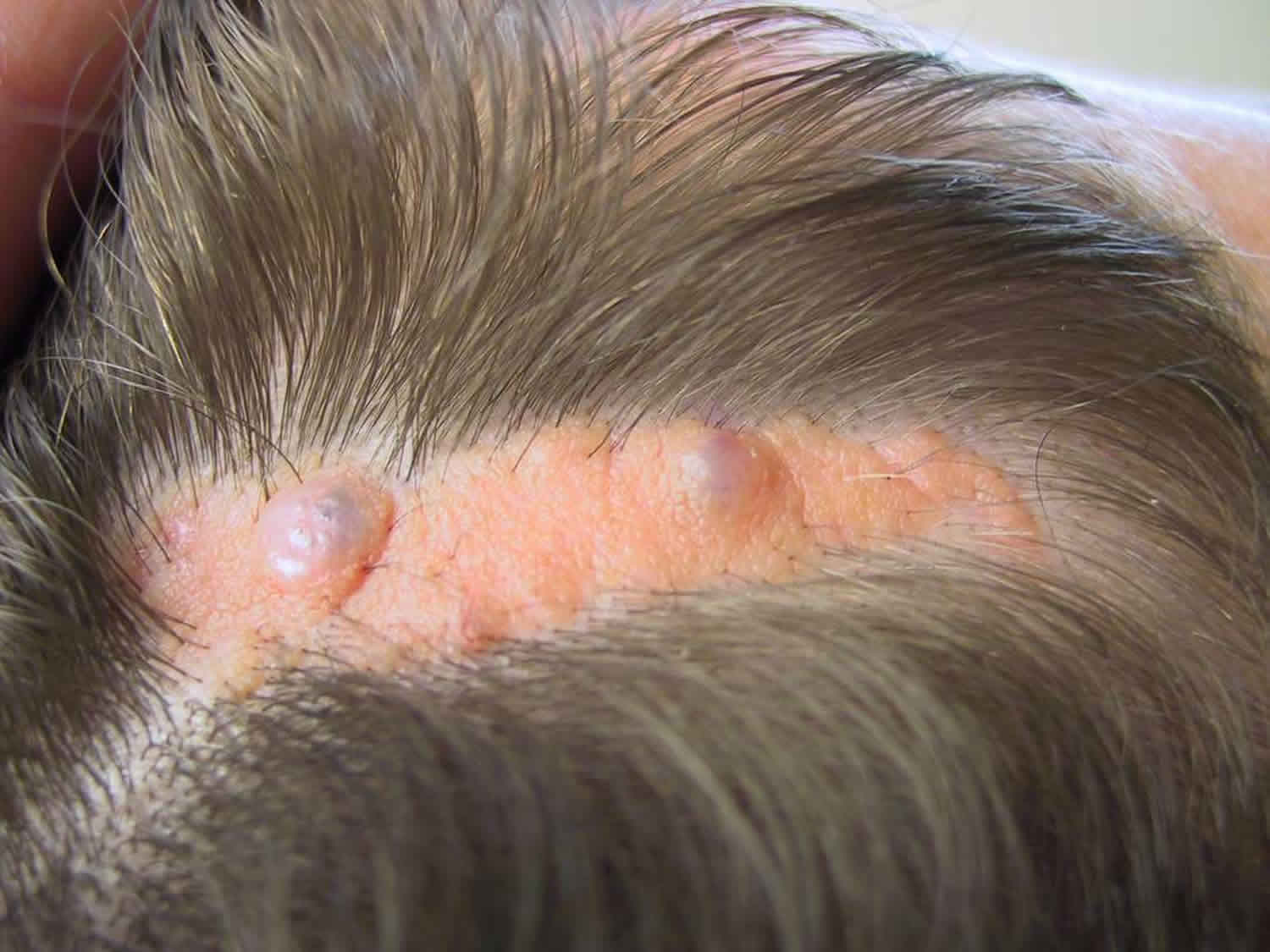
The common blue nevus is a solitary, bluish, smooth surfaced macule, papule or plaque. They are generally round or oval in shape.
- The common blue nevus is usually 0.5–1 cm in diameter. The cellular blue nevus is more nodular and is at least 1 cm in diameter.
- The color of blue nevi can also vary, usually being composed of blue to grey hues, but they are sometimes brown or yellowish.
- Blue nevi are usually found on the distal extremities (dorsum of hands and feet), buttocks, scalp and face although they can occur anywhere on the body.
- They have rarely have been reported in the vagina, the spermatic cord, the lymph nodes, the uterine cervix, the prostate and the oral mucosa.
Blue nevi may develop at any age but are rarely present at birth or within the first 2 years of life. The most common age of onset is late childhood or adolescence.
Figure 2. Blue nevus
Figure 3. Cellular blue nevus
What causes a blue nevus?
Blue nevus derive from an incomplete migration of melanocytes from the neural crest. The blue color is caused by the Tyndall effect, in which shorter wavelengths of incident light are scattered by the dermal melanocytes. The blue nevus is usually blue in color because the melanocytes are deeper than those of brown moles.
What are the complications of blue nevus?
Common blue nevi do not have any complications. They are benign and stay unchanged throughout life.
In contrast, cellular blue nevi can rarely transform into malignant cellular blue nevus (a type of melanoma).
How is blue nevus diagnosed?
Blue nevi are usually diagnosed clinically by their typical appearance. The diagnosis may be confirmed by finding a homogenous steel-blue ground-glass pattern on dermatoscopy. If there is any uncertainty about the diagnosis, an excision biopsy may be performed. The histopathology of blue nevus reveals spindle-shaped melanocytes in the dermis.
What is the treatment for blue nevus?
Usually, no treatment is required for a blue nevus.
Blue nevi that are bigger than 1 cm, changing or appearing in an older adult should be considered for histological evaluation to exclude melanoma. Blue nevi in the scalp are often removed as a precaution, because their history is mostly unknown and it is difficult in keep them under review.
A blue nevus can also be surgically removed for cosmetic reasons.
What is the outcome for blue nevus?
Unless surgically removed, blue nevi usually persist lifelong.
Congenital nevus
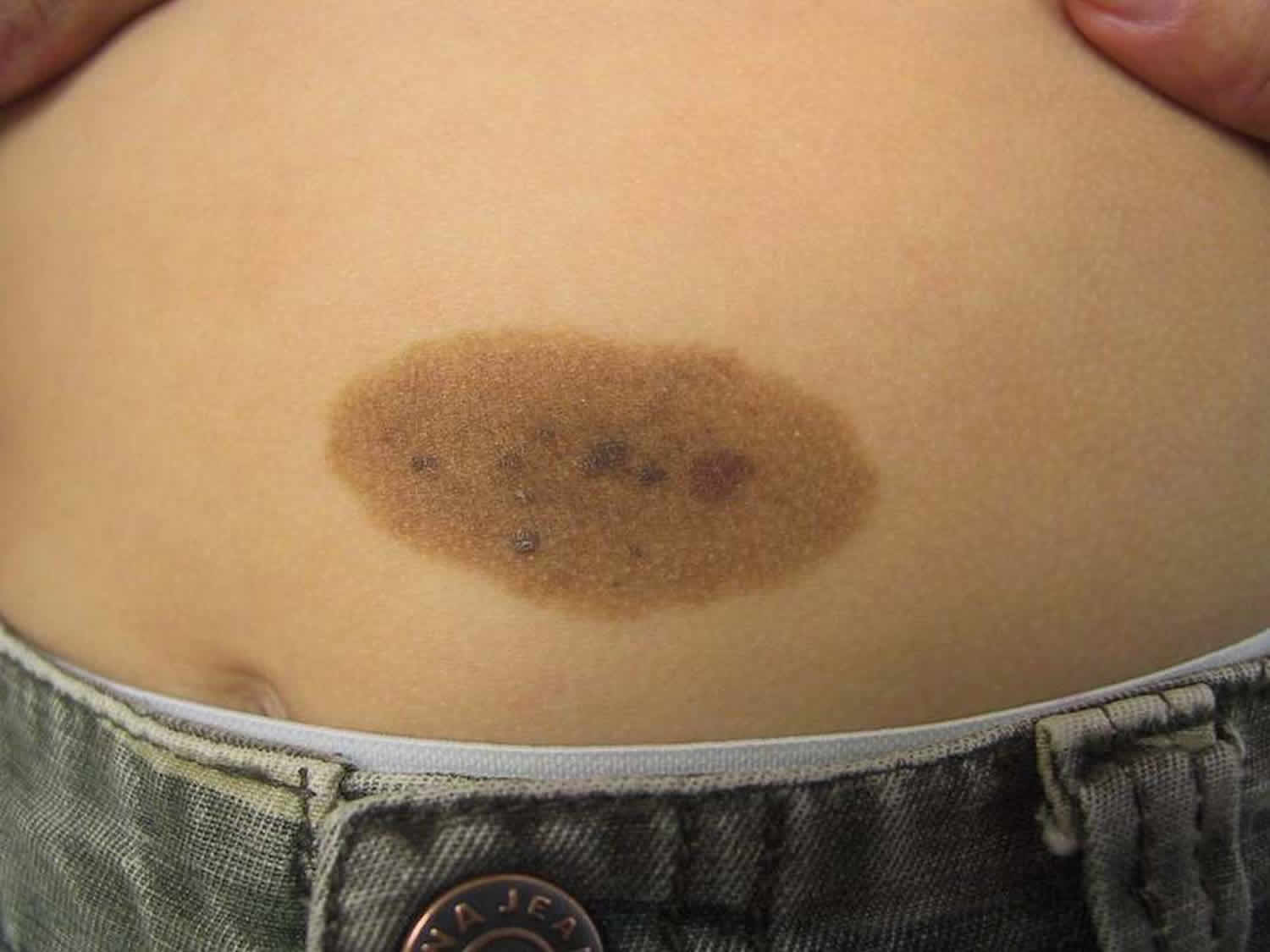
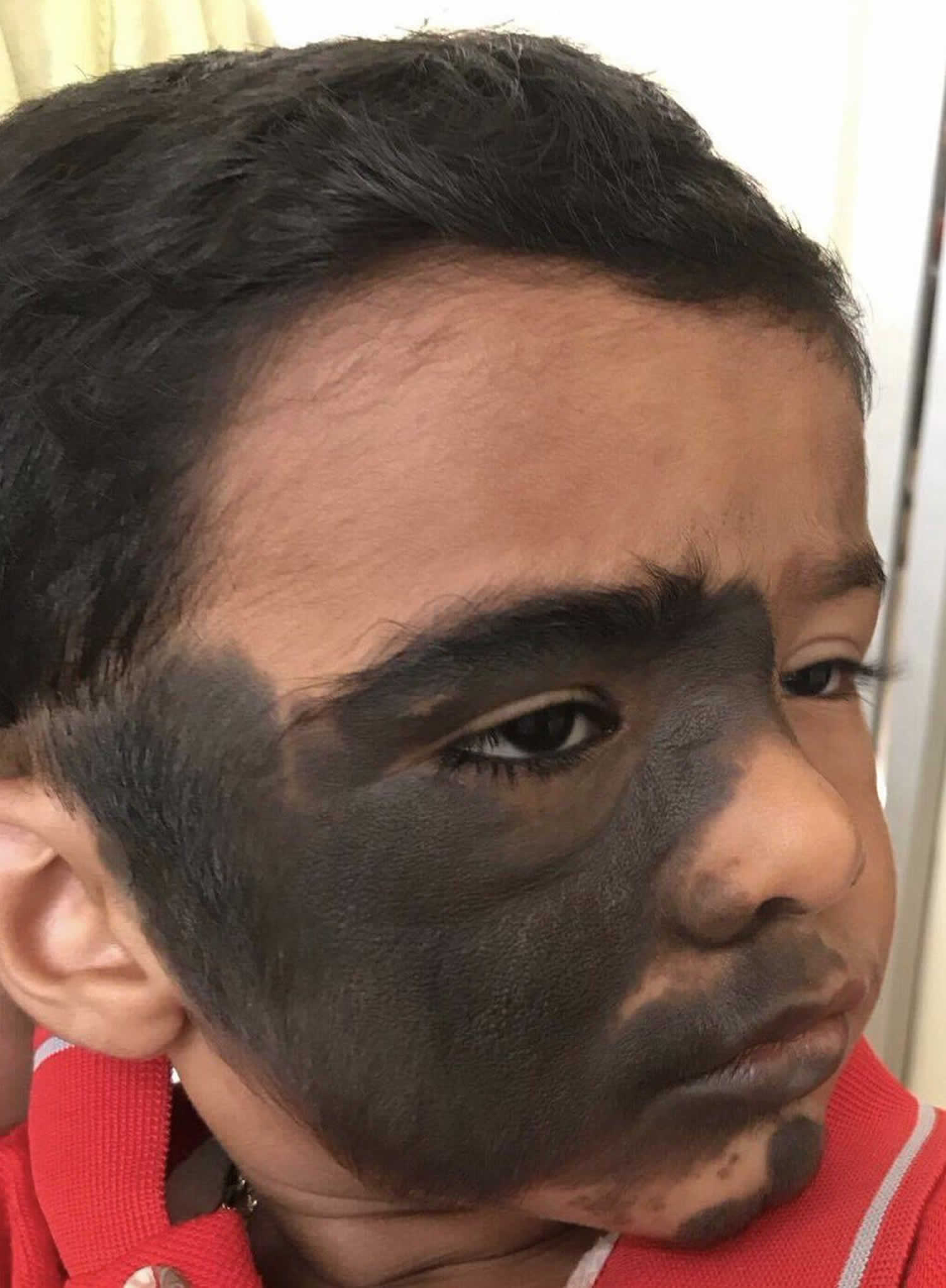
A congenital melanocytic nevus is a proliferation of benign melanocytes that are present at birth or develop shortly after birth 5. This form of a congenital nevus is also known as a brown birthmark. Similar melanocytic nevi, or moles that were not present at birth, are often called ‘congenital melanocytic nevus-like’ nevi, ‘congenital type’ nevi or ‘tardive’ nevi.
What do congenital melanocytic nevus look like?
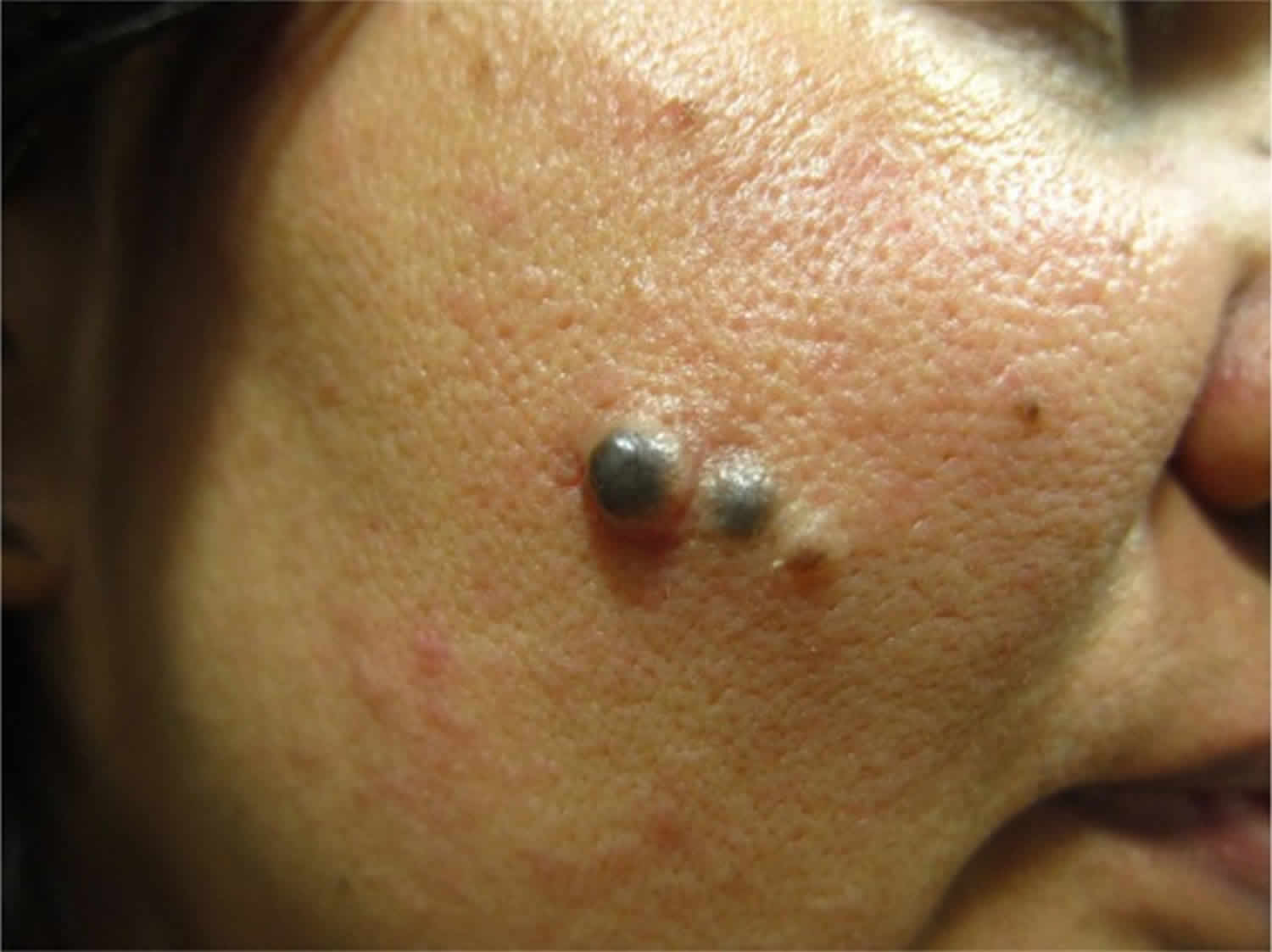
Congenital melanocytic nevus present as single or multi-shaded, round or oval-shaped pigmented patches 6. They may have increased hair growth (hypertrichosis). The surface may be slightly rough or bumpy.
Congenital nevus usually enlarge as the child grows but they may sometimes become smaller and less obvious with time. Rarely some may even disappear. However, they may also become darker, raised, more bumpy and hairy, particularly around the time of puberty.
Figure 4. Congenital melanocytic nevus
Figure 5. Congenital melanocytic nevus
Classification of congenital melanocytic nevus
Congenital melanocytic nevus are usually classified by their size in an adult. There are several different classifications.
- A small congenital melanocytic nevus is < 1.5 cm in diameter.
- A medium congenital melanocytic nevus is 1.5–19.9 cm.
- A large or giant congenital melanocytic nevus is ≥ 20 cm in diameter.
A modification of the above criteria is used in some centers 6 in an effort to increase the accuracy of classification.
- Small congenital melanocytic nevi are < 1.5 cm in diameter.
- Medium congenital melanocytic nevi are 1.5–10 cm.
- Large congenital melanocytic nevi are between 11–20 cm.
- Giant congenital nevi are >20cm in diameter, and are further subdivided into:
- G1 (21–30 cm)
- G2 (31–40 cm)
- G3 (> 40 cm).
2013 Classification of congenital melanocytic nevus
In 2013, a new categorization of congenital melanocytic nevus using predicted adult size was proposed 7:
- Small (< 1.5 cm)
- Medium (M1: 1.5–10 cm, M2: > 10–20 cm)
- Large (L1: > 20–30 cm, L2: > 30–40 cm)
- Giant (G1: > 40–60 cm, G2: > 60 cm)
- Satellite nevus: none, 1–20, > 20–50, and > 50 satellites.
Congenital melanocytic nevus should be described according to their body site, colors, surface features and whether or not there is hypertrichosis (hairs).
How common are congenital melanocytic nevi?
- Small congenital nevi occur in 1 in 100 births 5
- Medium congenital nevi occur in 1 in 1000 births 8
- Giant congenital melanocytic nevi are much rarer (1 in 20,000 live births) 6
They occur in all races and ethnic groups, and males and females are at equal risk.
Progression over time
Congenital melanocytic nevus usually grow proportionally with the child. As a rough guide, the likely adult size of a congenital nevus can be calculated as follows:
- Lower limbs: adult size is x 3.3 size at birth
- Upper limbs/torso: adult size is x 2.8 size at birth
- Head: adult size is x 1.7 size at birth.
Descriptive names of some congenital nevus
Some congenital nevi are given specific descriptive names. Some of these are listed here.
Speckled lentiginous nevus
- Also called nevus spilus
- Dark spots on a flat tan background
- The number of spots may increase or decrease over time
Satellite lesions
- Found on the periphery of central congenital melanocytic nevus or elsewhere on the body
- Smaller melanocytic nevi similar in appearance to
- Present in > 70% of patients with a large congenital melanocytic nevus
Tardive nevus
- Melanocytic nevus that appears after birth
- Slower growth and less synthesis of melanin than congenital nevus 8
- Histopathology is similar to true congenital melanocytic nevi
Garment nevus
- The name relates to the anatomical location of nevus
- Bathing trunk nevus involves central areas usually covered by a bathing costume, for example, buttocks
- Coat sleeve nevus involves an entire arm and proximal shoulder
Halo nevus
- Affects some congenital and tardive melanocytic nevi
- Surrounding skin becomes lighter or white
- The central lesion may also become lighter and smaller and may disappear
- Due to immune destruction of melanocytes
What causes a congenital melanocytic nevus?
Congenital melanocytic naevi are caused by localized genetic abnormalities resulting in the proliferation of melanocytes; these are cells in the skin responsible for normal skin colour. This abnormal proliferation is thought to occur between the 5th and 24th weeks of gestation. If proliferation starts early in development, giant and medium-sized congenital melanocytic naevi are formed 5. Smaller congenital melanocytic naevi are formed later in development after the melanoblasts (immature melanocytes) have migrated from the neural crest to the skin 5.
Proto-oncogenes c-met and c-kit have important roles in the development of melanocytes. Hepatocyte growth factor, a cytokine (messenger protein) that regulates the proliferation and migration of melanocytes, may also be important in the development of congenital melanocytic naevi 5.
In some cases, there is also overgrowth of hair-forming cells and epidermis, forming an organoid nevus.
Very early onset of congenital nevus before the separation of the upper and lower eyelids results in kissing naevi, ie one part of the nevus is on the upper lid and the other part is on the lower eyelid.
Do congenital melanocytic nevus cause any symptoms?
Congenital melanocytic nevi are usually asymptomatic, however, some may be itchy, particularly larger lesions. It is thought there may be a reduced function of sebaceous (oil) and eccrine (sweat) glands, which may result in skin dryness and a heightened sensation of itch.
The overlying skin may become fragile and erode or ulcerate. Deep nests of melanocytes in the dermis may weaken the bonds between the epidermis and the dermis and account for skin fragility 8.
Congenital melanocytic nevi are often unsightly, especially when extensive, ie large or giant congenital melanocytic nevi. They may, therefore, result in anxiety and impaired self-image, especially when the lesions are in visible areas.
Giant melanocytic nevi, and to a lesser degree small lesions, are associated with increased risk of developing cutaneous melanoma, neurocutaneous melanoma and rarely other tumours (see below).
Neurocutaneous melanosis
Neurocutaneous melanocytosis is a rare syndrome defined by the proliferation of melanocytes in the central nervous system (brain and spinal cord) and the presence of a congenital melanocytic nevus 5. The majority of cases are associated with a giant congenital melanocytic nevus and satellite lesions.
It is estimated neurocutaneous melanosis affects 5–10% of people that have a giant congenital melanocytic nevus. However it is likely that the majority of cases remain asymptomatic, and the true incidence remains unknown 8. The melanocytes in the brain and spinal cord may often be detected by an MRI scan but the use of these scans is controversial because the condition is not easily treatable.
Neurocutaneous melanocytosis may present with symptoms of raised intracranial pressure 6, such as:
- A headache
- Vomiting
- Irritability
- Focal cranial nerve signs
- Seizures
- Hydrocephalus
- Delayed development.
Risk of developing melanoma within a congenital melanocytic nevus
The following characteristics of congenital melanocytic nevus are associated with the increased risk of development of melanoma (a skin cancer).
- Large or giant size
- Axial or paravertebral location (crossing the spine)
- Multiple congenital satellite naevi
- Neurocutaneous melanosis.
The risk of melanoma is mainly related to the size of the congenital melanocytic nevus. Small and medium-sized congenital melanocytic naevi have a very small risk, well under 1%. Melanoma is more likely to develop in giant congenital nevi (lifetime estimates are 5–10%), particularly in lesions that lie across the spine or where there are multiple satellite lesions. Melanoma can start deep inside the nevus or within any neuromelanosis found in the brain and spinal cord. Very rarely, other tissues that contain melanocytes may also be a source of melanoma such as the gastrointestinal tract mucosa. In 24% of cases, the origin of the melanoma cannot be identified 6.
Melanoma associated with a giant congenital melanocytic nevus or neuromelanosis can be very difficult to detect and treat.
The risk of development of melanoma is greater in early childhood; 70% of melanomas associated with giant congenital melanocytic naevi are diagnosed by the age of ten years 5.
Rarely, other types of tumour may develop within giant congenital melanocytic naevi including benign tumours (lipomas, schwannomas) and other malignant tumours (including sarcomas).
Melanoma can also develop within a small congenital melanocytic nevus. This is rare and likely to occur on the periphery of the nevus during adult life.
Is regular follow-up recommended?
- It can be useful to have a close-up photograph of the congenital nevus with a ruler beside it to assess for changes in size.
- Digital surveillance using dermoscopic images (mole mapping) may also be helpful to detect changes in structure. However, such changes are normal in childhood and should not usually give rise to concern.
- It is advisable to continue neurodevelopmental observation in those at risk of neurocutaneous melanosis 8.
Prognosis of melanoma associated with congenital melanocytic nevus
Unfortunately, when a rare melanoma arises within a giant congenital melanocytic nevus, the prognosis is unfavorable. This is due to the deeper origin of the tumour rendering it more difficult to detect on clinical examination, resulting in a later stage at presentation. The deeper location also facilitates earlier spread through blood and lymph vessels. In 24% of cases, the melanoma has already spread to other sites (metastases) at the time of the first diagnosis.
Congenital melanocytic nevus diagnosis
The diagnosis of a congenital melanocytic nevus is usually based on the clinical appearance. If there is any doubt, examining the lesion with dermoscopy or taking a sample of the lesion for histology (biopsy) may show characteristic microscopic features.
Dermoscopy
Evaluation of the congenital melanocytic nevus by dermoscopy will reveal the pattern of pigmentation and its symmetry or lack of symmetry. The most common global pattern of congenital or tardive melanocytic nevus is globular, but reticular, structureless and mixed patterns may occur. The nevus may have differing structures across the lesion, sometimes leading to overall asymmetry of the structure.
Pathology
Congenital melanocytic nevi are usually larger than acquired nevi (which are melanocytic nevi that appear after 2 years of age), and the nevus cells often extend deeper into the dermis, fat layer, and deeper structures. The nevus cells characteristically cluster around blood vessels, hair follicles, sebaceous and eccrine glands, and other skin structures. Congenital nevus cells tend to involve collagen bundles in the deeper layers of the skin more than is the case in an acquired nevus 5.
Congenital melanocytic nevus treatment
Management of a congenital melanocytic nevus must take into account the age of the subject, the lesion size, the location and depth, and the risk of developing malignant change within the lesion.
Giant congenital melanocytic nevus
- The only definite indication for surgery in a giant congenital melanocytic nevus is when melanoma develops within it 6.
Small congenital nevus
- If a small congenital nevus is growing at the same rate as the child and is not changing in any other way, the usual practice is not to remove it until the child is old enough to co-operate with a local anaesthetic injection, usually around the age of 10 to 12 years. Even then, removal is not essential.
Reasons to consider surgical removal may include:
- Unsightly appearance
- Difficulty in observing the mole (eg, scalp, back)
- A recent change in the lesion (darkening, lumpiness, increasing size)
- Melanoma-like appearance (irregular shape, variegated color).
Prophylactic surgical removal of a nevus
The following factors should be considered prior to prophylactic surgical removal of a nevus.
- Prophylactic excision of a small lesion may be delayed until an age when the patient is old enough to make an informed choice 8.
- Small or medium-sized congenital melanocytic naevi are at low risk for developing malignant change.
- Irregular, lumpy or thick lesions or lesions that are difficult to clinically assess may have a lower threshold for consideration of surgical excision, so as not to miss a melanoma.
- 50% of melanomas diagnosed in those with giant congenital melanocytic naevi occur at another body site such as within the central nervous system 6.
- Therefore surgical excision of the lesion may not eliminate the risk of melanoma.
- Large or giant melanocytic lesions may be too large to excise completely.
- Large lesions may require a skin flap or graft to close the surgical defect.
Complications of surgery
Complications that may occur after surgery include:
- Graft or flap failure
- Infection
- Wound breakdown
- Bleeding or hematoma
- Hypertrophic or keloid scar
- Irritable or itchy scar.
Other treatment options for a congenital melanocytic nevus
Dermabrasion
Dermabrasion can allow partial removal of a large congenital nevus; deeper nevus cells may persist. Dermabrasion may lighten the colour of the nevus but may not reduce hair growth within it. It can cause scarring.
Tangential (shave) excision
Tangential or shave excision uses a blade to remove the top layers of the skin (epidermis and upper dermis). This may reduce the pigmentation but the lesion may not be completely removed. Shave excision may result in significant scarring.
Chemical peels
Chemical peels using trichloroacetic acid or phenol may lighten the pigmentation of a superficial (surface) congenital nevus that is located in the upper layers of the skin.
Laser ablation
Laser treatment is considered if surgical intervention is not possible. They may result in lightening of the lesion. Suitable devices include:
- Ruby Q-switched laser
- Carbon dioxide resurfacing laser
Techniques that result in partial removal of a congenital nevus can make the lesion more difficult to assess during long-term surveillance 6.
Epidermal nevus
An epidermal nevus is due to an overgrowth of the epidermal keratinocytes. Epidermal nevi are present at birth (50%) or develop during childhood (mostly in the first year of life). The abnormality arises from a defect in the ectoderm, the outer layer of the embryo that gives rise to epidermis and neural tissue.
Types of epidermal nevi
The skin lesions most often referred to as epidermal nevi are due to an overgrowth of keratinocytes (horny skin cells).
- Linear epidermal nevus
- Epidermolytic verrucous epidermal nevus
- Acantholytic epidermal nevus (Hailey-Hailey or Darier disease-like)
- Linear porokeratosis
- Systematized epidermal nevi
When another component of the skin is predominant, the lesion is called an organoid nevus.
Whilst the majority of epidermal nevi only affect the skin and are of limited significance, it is important to differentiate between epidermolytic and non-epidermolytic verrucous epidermal nevi as patients with the former have an increased risk of having children born with epidermolytic hyperkeratosis (bullous ichthyosiform erythroderma), a rare but serious condition. With regards to non-epidermolytic verrucous epidermal nevi, rarely, such lesions are associated with a wide range of non-cutaneous abnormalities, especially the central nervous system (CNS), eyes and skeleton, which together are known as the epidermal nevus syndrome.
Figure 6. Verrucous epidermal nevus
Figure 7. Linear epidermal nevus
What causes an epidermal nevus?
There are two copies of every gene, one derived from the individual’s mother and the other from their father. It is thought that there are two populations of skin cells, containing either the mother’s genes or the father’s genes (mosaicism). If one of these populations of skin cells is abnormal, it may result in a localized area of thickened skin, an epidermal nevus. Epidermal nevi very rarely affect more than one member of the family. Mutations have been detected in FGFR3, PIK3CA and HRAS.
Epidermal naevi are distributed along the lines of Blashko. These lines are the tracks taken by groups of genetically identical cells in the developing embryo. Skin cells that have the active abnormal gene spread out to form the epidermal nevus, whereas the remaining skin cells form the other areas of apparently normal skin.
New research has found point mutations in keratin genes that support this theory. The abnormal gene is found in the epidermal nevus cells but not in the normal skin. The same keratin 1 and keratin ten gene abnormalities have been found in parents who have epidermolytic epidermal nevus and in their offspring who have bullous ichthyosiform erythroderma (a rare form of ichthyosis). So the epidermolytic epidermal nevus is thought to be a mosaic form of this type of ichthyosis.
The ATP2A2 gene abnormality that arises in Darier disease has been detected in the affected cells of a patient with acantholytic epidermal nevus so that this type may be a mosaic form of Darier disease. Linear porokeratosis may be a mosaic form of disseminated superficial actinic porokeratosis.
What are the clinical features of epidermal nevus?
Linear epidermal nevus
An epidermal nevus usually arises on the trunk and limbs and is uncommon on the face or scalp. The majority are linear epidermal nevi — they form a line, usually just on one side of the body (unilateral, also known as nevus unis lateralis). When they first appear at birth or in infancy, they are flat tan or brown marks, but as the child ages, they become thickened and often warty (verrucous). The nevus may also become more extensive for a few years.
Systematized epidermal nevi
Systematized epidermal nevi are less common and are sometimes known as ichthyosis hystrix. There are multiple lesions that usually arise in a swirled pattern, arising on one or both sides of the body. In some patients, there are also other congenital abnormalities, particularly of the skeleton and central nervous system (CNS).
Histology of epidermal nevus
On skin biopsy, the epidermal nevus shows an increased thickness of the epidermis.
- The rare epidermolytic epidermal nevus subtype is characterized by a specific histological pattern called epidermolytic hyperkeratosis and resembles that seen in bullous ichthyosiform erythroderma.
- Acantholytic epidermal nevus has pathology that resembles Darier disease.
- Linear porokeratosis has pathology that resembles disseminated superficial actinic porokeratosis.
Epidermal nevus syndromes
The epidermal nevus syndromes refer to the association of a keratinocytic or organoid epidermal nevus with abnormalities in other organ systems derived from the embryonic ectoderm. These syndromes may involve the eyes, bones or nervous system. Many specific syndromes have been described.
The defect causing the skin lesions may also result in disorders of other internal organs such as the brain, eyes and skeleton. When multiple organs are involved the condition is referred to as an epidermal nevus syndrome.
Complications of epidermal nevus
Most epidermal nevus remain unchanged in adulthood and do not cause any problems.
What is the treatment for epidermal nevus?
There is no real effective medical treatment for epidermal nevus. Topical calcipotriol may reduce the thickness of the skin in some cases. If necessary, laser or surgical removal of epidermal nevus may be performed.
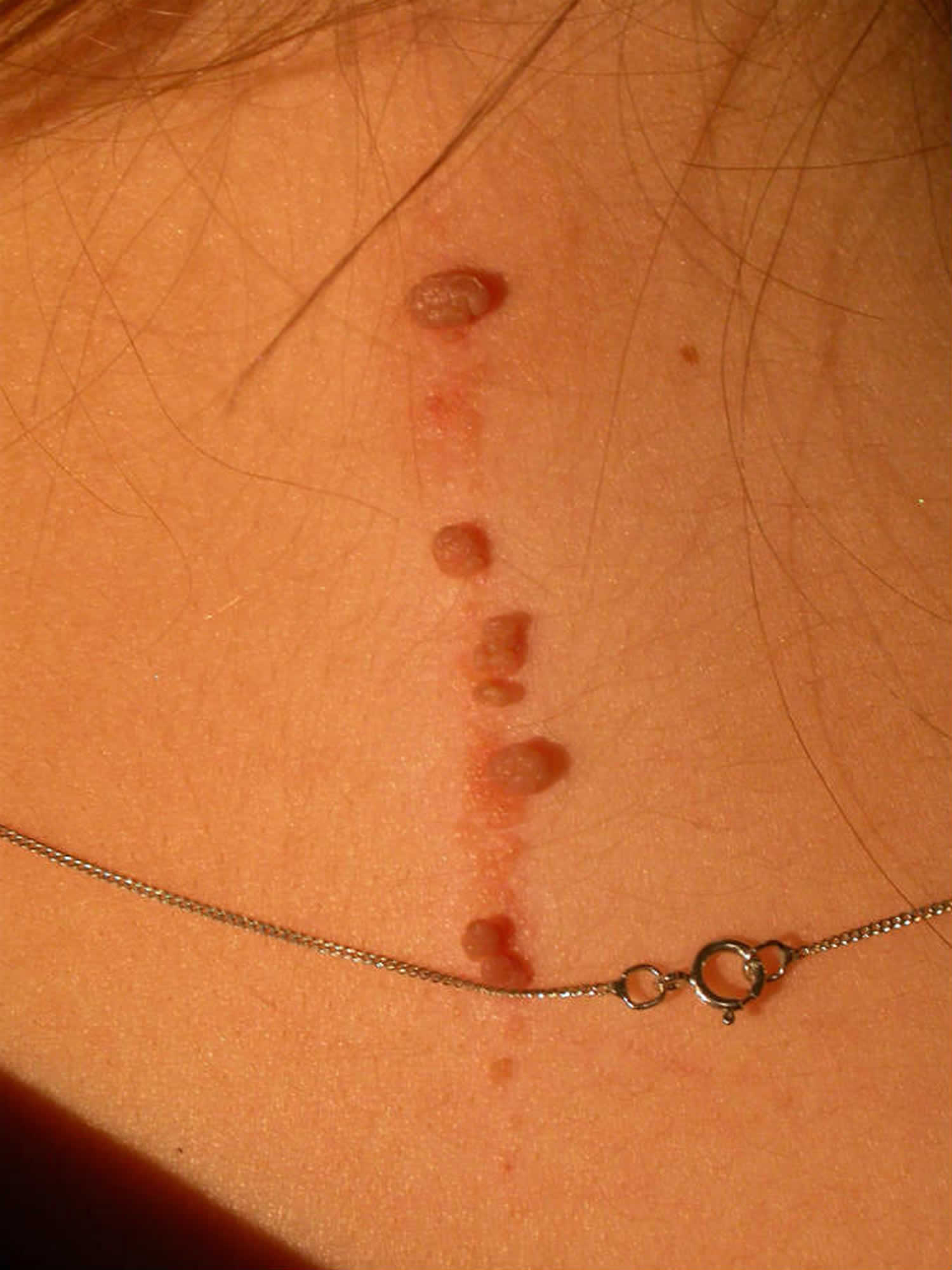
Spitz nevus
Spitz nevus is a benign, uncommon type of mole or melanocytic nevus. Spitz nevus is a variant of a compound nevus and is most commonly seen in children, 70% of cases diagnosed during the first 20 years of life. They may also arise in adults. They are most frequently found in fair-skinned individuals (skin phototypes 1 and 2) but can also affect those with dark skin.
The pigmented spindle cell nevus of Reed (Figure 10) is a variant of a compound nevus or occasionally a junctional nevus. There is debate as to whether the pigmented spindle cell nevus of Reed is an entity in its own right or whether it is a variation of a Spitz nevus. However, it may resemble a malignant melanoma clinically and microscopically, so they are often excised as a precaution.
Although both lesions are benign, they can have similar clinical and histopathological features to melanoma, making their assessment difficult and best left in the hands of a specialist.
What are the clinical features of a Spitz nevus?
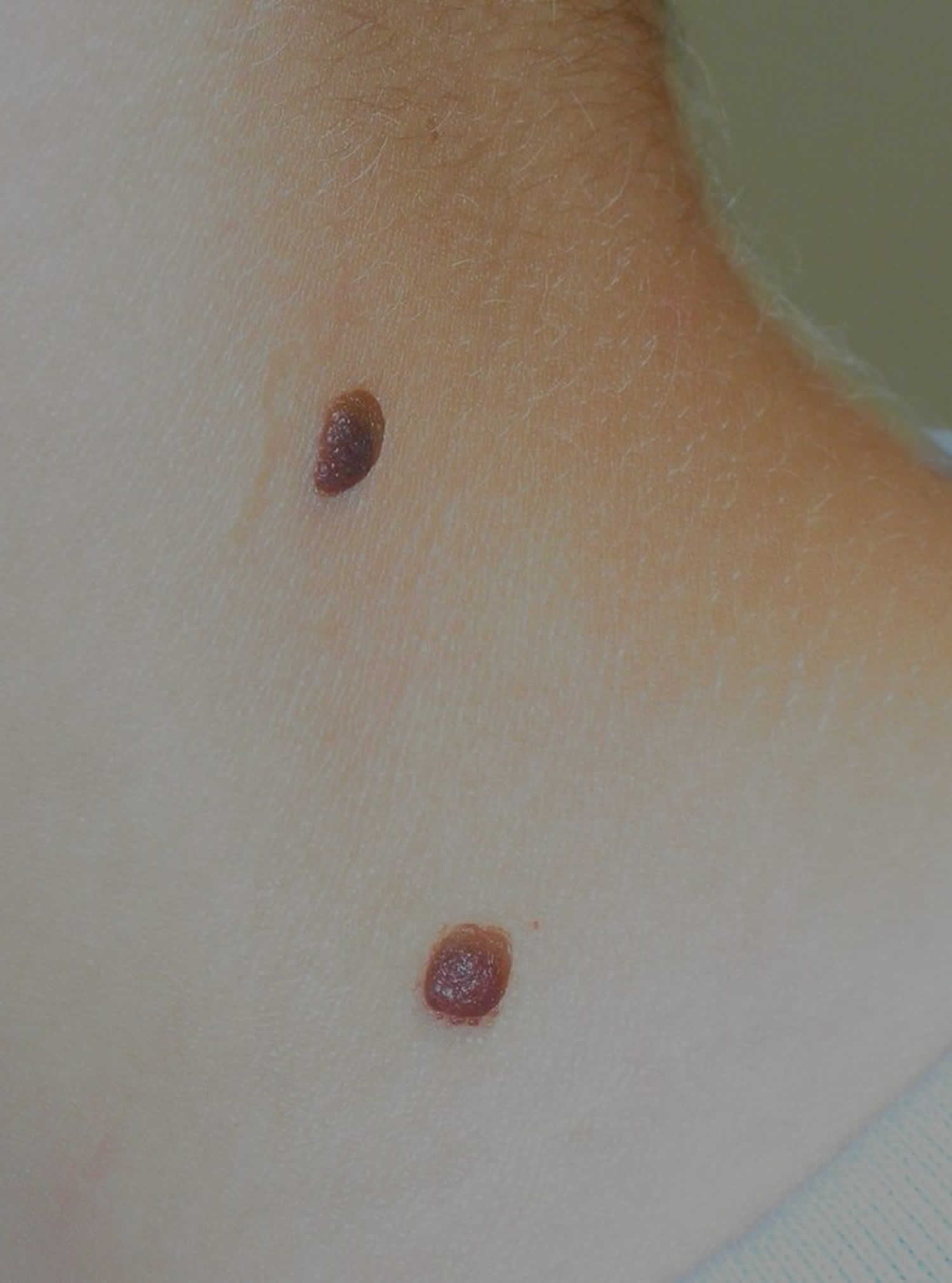
Spitz nevus is classified as classic, pigmented or spindle cell nevus of Reed.
- The classic Spitz nevus is typically a dome-shaped red, reddish-brown papule.
- A pigmented Spitz nevus is a tan or brown papule or nodule.
- A pigmented spindle cell tumor of Reed is a bluish or black papule.
There are clinical features in common for all three types of Spitz nevus:
- Their size ranges from a few millimeters to up to one or two centimeters in diameter.
- They usually appear on the face or limbs.
- Usually, a Spitz nevus is solitary, but sometimes they erupt as multiple lesions.
- A Spitz nevus grows rapidly for a few months. After the initial growth period, if untreated, it may remain static for years.
- A Spitz nevus may disappear spontaneously after some time.
The atypical Spitz nevus or spitzoid nevus is so called because its features differ from those seen in the majority of Spitz naevi.
Spitz nevus
- Distribution: The face is the most common site, but other areas can be affected
- Morphology: A raised, red and firm papular / nodular lesion. They can be pigmented
Pigmented spindle cell nevus of Reed
- Distribution: The thigh is the most common site
- Morphology: Regular, densely pigmented blue / black, palpable lesions measuring 5-10 mm in size
Figure 8. Classic Spitz nevus
Figure 9. Pigmented spindle cell nevus of Reed (Spitz nevus)
What causes a Spitz nevus?
A Spitz nevus is a type of melanocytic nevus, that is, it is a mole composed of melanocytes — these are cells that normally produce pigment, melanin, and are responsible for skin color. The melanocytes in Spitz nevus may be inactive. Thus the lesion may be pink rather than brown. The genetic pattern of the DNA in Spitz nevus is characteristic with kinase fusions being prominent.
It is not known why Spitz nevi occur.
How is a Spitz nevus diagnosed?
A Spitz nevus is often suspected clinically by its characteristic dome-shaped appearance and rapid growth over a few weeks to months.
- Dermatoscopy of a classic Spitz nevus often reveals uniform rounded structures with prominent punctate or rounded blood vessels.
- A pigmented Spitz nevus may show starburst or globular pattern of pigmentation but later evolves to a homogeneous or structureless dermatoscopic pattern.
- A spindle cell tumor of Reed typical has a deeply pigmented starburst pattern with pseudopods.
- An atypical or spitzoid nevus may have more than one pattern, asymmetry of structure, and unusual dermatoscopic features.
In older children and adults, the diagnosis of Spitz nevus is usually confirmed by an excisional skin biopsy. The pathology of Spitz nevus usually shows a symmetrical compound nevus composed of nests of characteristic epithelioid cells in classic and pigmented Spitz, and fusiform cells in spindle cell tumor of Reed. Both forms can co-exist.
Spitz nevus treatment
In children under the age of 12 years, a Spitz nevus may be kept under review using digital dermatoscopic surveillance (monitoring photographs of the lesion’s dermatoscopic appearance). It is expected to enlarge uniformly over a year or two and then to stop growing.
Because of the difficulty in definitively predicting the outcome of Spitz naevi in older children or adults, or if the structure of the lesion is not uniform, they are generally excised.
An atypical Spitzoid tumor or a spitzoid tumor of uncertain malignant potential (STUMP) may be difficult to distinguish histologically from spitzoid melanoma and is best widely excised. Sentinel node biopsy should not be undertaken as the results can be misleading.
What is the outcome for a Spitz nevus?
If it hasn’t been excised, a Spitz nevus may disappear over a few years.
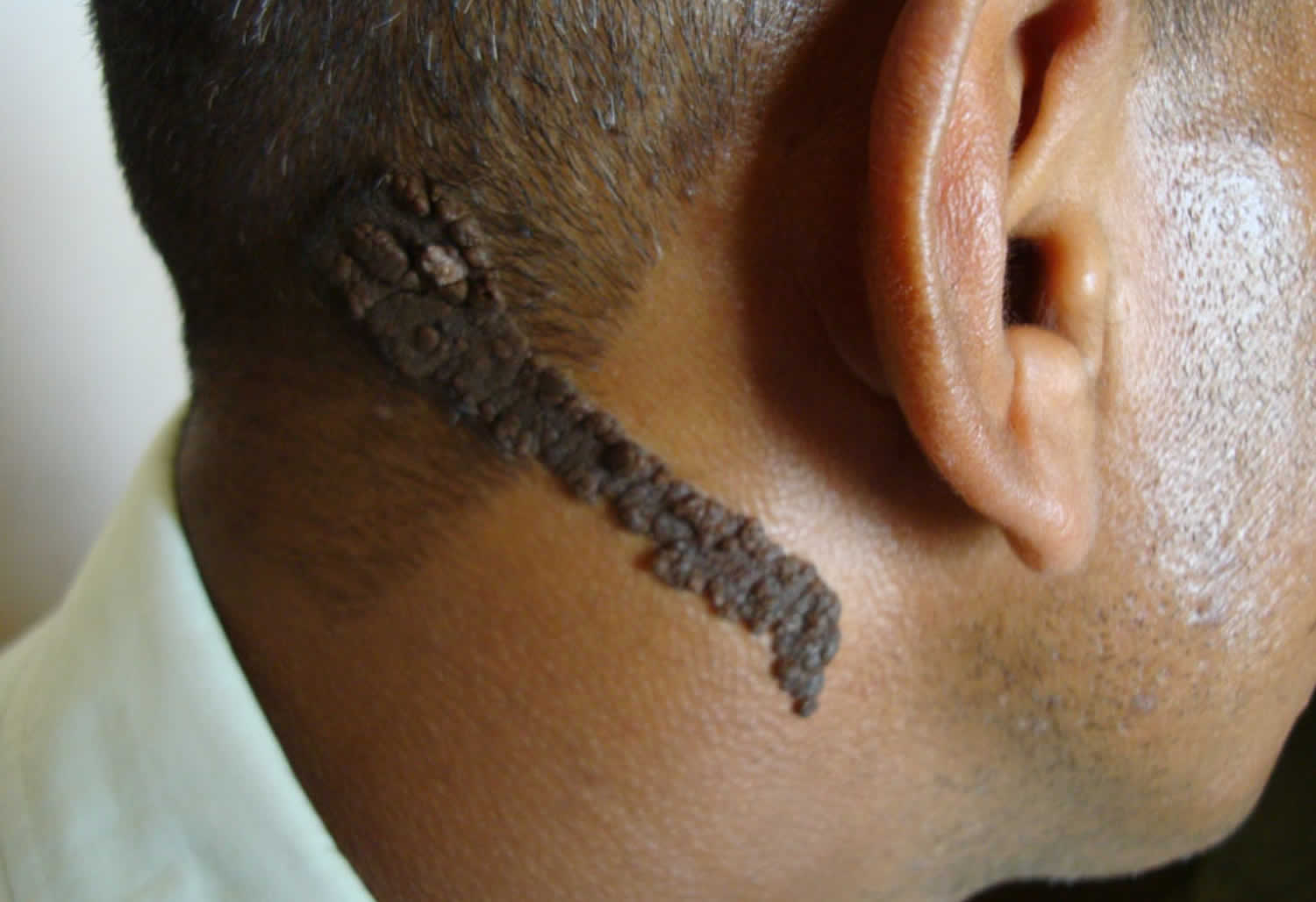
Nevus sebaceous
A sebaceous nevus is an uncommon type of birthmark. Present at birth, sebaceous nevus is most often found on the scalp, but sebaceous nevus may also arise on the face, neck or forehead. Sebaceous nevus consists of overgrown epidermis (upper layers of the skin), sebaceous glands, hair follicles, apocrine glands and connective tissue. Sebaceous nevus is a type of epidermal nevus and is classified as a benign hair follicle tumor. A sebaceous nevus is also called an organoid nevus because it may include components of the entire skin.
Sebaceous nevus syndrome
Sebaceous nevus syndrome refers to the rare association of a large sebaceous nevus with disorders of the eye, brain and skeleton.
Sebaceous nevus syndrome may result in eye tumors and the skull may be asymmetrical. Characteristic associated neurological features may include:
- Developmental delay
- Epileptic seizures, especially infantile spasms
- Hemiparesis (paralysis of half the body) or cranial nerve palsies (paralysis of individual nerves)
- X-ray images and ultrasound evaluation may be quite normal
- Various structural abnormalities may be found within the brain
Phakomatosis pigmentokeratotica
Phakomatosis pigmentokeratotica is the association of a sebaceous nevus and a speckled lentiginous nevus. Speckled lentiginous nevus is a flat, light-brown birthmark with darker spots within it, and is classified as a type of congenital melanocytic nevus.
Neurological defects in phakomatosis pigmentokeratotica may include hemiatrophy (in which one side of the body under-developed), muscle weakness, sensory nerve abnormalities and hyperhidrosis (exessive sweating).
Tumors arising within sebaceous nevus
Most sebaceous nevi remain unchanged throughout life and do not cause any problems. However, another tumor may grow within the lesion. This is most often benign (in 0-50% of cases), most often trichoblastoma. Other hair follicle unit lesions (trichilemmoma, infundibular cyst and sebaceoma) and sweat gland tumors (eccrine poroma, syringocystadenoma papilliferum) may also arise.
Less commonly (in 0-22% of cases), a skin cancer may grow within a sebaceous nevus: basal cell carcinoma, squamous cell carcinoma, sebaceous carcinoma (oil gland tumor), or an adnexal carcinoma (apocrine or eccrine carcinoma).
What does sebaceous nevus look like?
In an infant or young child, sebaceous nevus presents as a solitary, smooth, yellow-orange hairless patch, often oval or linear in shape.
Sebaceous nevus becomes more pronounced around adolescence, often appearing bumpy, warty or scaly.
Figure 10. Sebaceous nevus
What is the cause of sebaceous nevus?
The abnormality resulting in sebaceous nevus arises from a defect in the ectoderm. This is the outer layer of the embryo that gives rise to epidermis and neural tissue.
Sebaceous nevi are thought to be due to a mosaic genetic abnormality, i.e. a line of cells with a genetic error. In some patients, an abnormality of the PTCH gene has been detected.
How is sebaceous nevus diagnosed?
Sebaceous nevus has a characteristic clinical appearance and is therefore often diagnosed in childhood or adolescence. Sometimes the diagnosis is made by a pathologist after the lesion has been surgically removed.
A biopsy undertaken during childhood will show a hairless lesion with immature hair follicles and sebaceous glands. In adults, there is thickening of the epidermis and mature hair follicles and prominent sebaceous glands. Sometimes a second tumor is found in the specimen.
How should sebaceous nevus be treated?
A sebaceous nevus should be monitored. If a lump or sore appears within a sebaceous nevus, arrange for it to be reviewed by a dermatologist. It may require a biopsy or the whole sebaceous nevus may be excised (cut out).
Full skin thickness excision may also be arranged for cosmetic reasons but a scar is inevitable.
Lasers have been used to treat sebaceous nevus with varying success.
Melanocytic nevus
Melanocytic nevus is also called a mole or a nevocytic nevus, which is a common benign skin lesion due to a local proliferation of pigment cells (melanocytes). A brown or black melanocytic nevus contains the pigment melanin, so may also be called a pigmented nevus.
A melanocytic nevus can be present at birth (congenital melanocytic nevus) or appear later (acquired melanocytic nevus). There are various kinds of congenital and acquired nevus.
Almost everyone has at least one melanocytic nevus:
- About 1% of individuals are born with one or more congenital melanocytic nevus. This is usually sporadic, with rare instances of familial congenital naevi.
- Fair-skinned people tend to have more melanocytic nevus than darker skinned people.
- Melanocytic nevi that appear during childhood (aged 2 to 10 years) tend to be the most prominent and persistent melanocytic nevus throughout life.
- Melanocytic nevi that are acquired later in childhood or adult life often follow sun exposure.
Most white-skinned people have 20–50 melanocytic nevi.
What are the clinical features of melanocytic nevus?
Melanocytic nevus vary widely in clinical, dermatoscopic and histological appearance.
- They may arise on any part of the body.
- Melanocytic nevus differ in appearance depending on the body site of origin.
- They may be flat or protruding.
- They vary in colour from pink or flesh tones to dark brown, steel blue, or black.
- Light skinned individuals tend to have light-colored melanocytic nevus and dark-skinned individuals tend to have dark brown or black melanocytic nevus.
- Although mostly round or oval in shape, melanocytic nevus are sometimes unusual shapes.
- They range in size from a couple of millimeters to several centimeters in diameter.
Figure 11. Melanocytic nevus
Figure 12. Intradermal nevus
What causes melanocytic nevus?
Although the exact reason for the local proliferation of nevus cells is unknown, it is clear that the number of melanocytic nevus a person has depends on genetic factors, on sun exposure, and on immune status.
- People with many melanocytic nevus tend to have family members that also have many melanocytic nevus, and their melanocytic nevus may have a similar appearance.
- Somatic mutations in RAS genes are associated with congenital melanocytic naevi.
- New melanocytic naevi may erupt following the use of BRAF inhibitor drugs (vemurafenib, dabrafenib).
- People living in Australia and New Zealand have many more nevi than their relatives residing in Northern Europe.
- Immunosuppressive treatment leads to an increase in numbers of nevi.
Classification of melanocytic nevus
Congenital melanocytic nevus
Congenital melanocytic naevi are classified according to their actual or predicted adult size in maximum dimension and on specific characteristics.
- A small congenital melanocytic nevus is < 1.5 cm in diameter.
- A medium congenital melanocytic nevus is 1.5–19.9 cm.
- A large or giant congenital melanocytic nevus is ≥ 20 cm in diameter.
- Hairy congenital nevus grows thick long hairs.
- Cafe au lait macule is a flat brown patch.
- Speckled lentiginous nevus is a flat brown patch with darker spots.
- Nevus of Ota is a bluish brown mark around forehead, eye and cheek.
- Mongolian spot is a large bluish mark most often seen on buttocks of a newborn.
The pathological classification of melanocytic nevus relates to where nevus cells are found in the skin.
- Junctional nevus: A junctional nevus has groups or nests of nevus cells at the junction of the epidermis and the dermis. A flat mole.
- Dermal nevus (intradermal nevus): A dermal or intradermal nevus has nevus cell nests in the dermis. A papule, plaque or nodule with a pedunculated, papillomatous (Unna nevus) or smooth surface (Miescher nevus).
- Compound nevus: A compound nevus has nests of nevus cells at the epidermal-dermal junction as well as within the dermis. A central raised area surrounded by a flat patch.
- Combined nevus: A combined nevus has two distinct types of mole within the same lesion – usually blue nevus and compound nevus.
Dermatoscopic patterns of melanocytic nevus
Dermatoscopy has given rise to a new classification based on the pigment patterns of melanocytic nevus. Examples include:
- Reticular nevus: Reticular nevus reveals a lattice of intersecting brown lines.
- Globular nevus: Globular nevus characteristically shows aggregated brown oval structures.
- Blue nevus: The blue nevus is a uniform structureless lesion, steel blue in color.
- Starburst nevus: Starburst nevus reveals radial lines around the periphery of the lesion.
Site-related nevus:
- Facial nevus reveal pseudo-network around hair follicles
- Acral nevus (these are on palms and soles) tend to be made up of parallel lines.
- Nevi with special features include eczematised nevus, irritated nevi and halo nevi
- Unclassifiable nevus doesn’t have any of the other patterns.
Acquired melanocytic nevus
Ordinary moles that appear after birth may be referred to as acquired nevi. Acquired melanocytic nevi are given a variety of names and there is considerable overlap of descriptions.
- Common nevus: A common nevus is a flat mole with a single uniform color.
- Nevus in dark skin: In dark skin, nevi are often black in color.
- Atypical nevus: People with multiple atypical nevi are at increased risk of melanoma (cancerous mole).
- Dysplastic nevus: Dysplastic nevus describes an atypical mole that has specific microscopic criteria.
- Blue nevus: Blue nevus is a deeply pigmented type of dermal nevus.
- Cellular nevus: Cellular nevus is a non-pigmented dermal nevus.
- Miescher nevus: Miescher nevus is a dome-shaped smooth dermal nevus often found on the face.
- Unna nevus: Unna nevus is a papillomatous dermal nevus that is in the shape of a raspberry.
- Meyerson nevus: Meyerson nevus is a nevus affected by a halo of eczema/dermatitis.
- Halo nevus: Halo nevus or Sutton nevus has a white halo around the mole. The mole gradually fades away over several years.
- Spitz nevus: Spitz nevus or epithelioid cell nevus is a pink (classic Spitz) or brown (pigmented Spitz) dome-shaped mole that arises in children and young adults.
- Reed nevus: Reed or spindle cell nevus is a very dark-colored mole with spindle-shaped dermal melanocytes, usually found on the limbs.
- Recurrent nevus: Recurrent nevus refers to the reappearance of pigment in a scar following surgical removal of a mole – this may have an odd shape.
- Agminated nevus: An agminated nevus is a cluster of similar moles or freckles.
- Acral nevus: Acral nevus refers to one on the palm or sole.
- Nail unit nevus: Nail unit nevus causes a uniform longitudinal band of pigment on a nail.
Signature nevi are the predominant group of nevi in an individual with multiple moles.
- Solid brown nevus: Solid brown nevi have uniform brown pigmentation.
- Solid pink nevus: Solid pink nevi are seen in fair-skinned individuals and lack melanin pigmentation.
- Eclipse nevus: Eclipse nevus has a ring, or segment of a ring, of darker pigment around a tan or pink center. Often found in the scalp.
- Cockade nevus: Cockade, or nevus en cocarde/cockade, has a central dark nevus surrounded by concentric circles of light and dark pigmentation like a rosette.
- Nevus with perifollicular hypopigmentation: Nevi with perifollicular hypopigmentation have white spots around each hair. Easier to see by dermoscopy.
- Fried-egg nevus: Fried-egg nevus is a compound nevus with a flat rim of pigment around a bumpy central portion – the bump can be lighter or darker than the pigmented rim.
- Lentiginous nevus nevus with eccentric pigmentation: Lentiginous nevi are small, dark brown or black, flat lesions, often with a slightly paler rim – people with multiple lentiginous naevi have been said to have cheetah phenotype. The Bolognia sign refers to a harmless, small area of darker color on one side of the nevus.
Uncommon types of melanocytic nevus include:
- Spitz nevus or epithelioid cell nevus: a pink (classic Spitz) or brown (pigmented Spitz) dome-shaped mole that arises in children and young adults.
- Reed nevus: darkly pigmented type of Spitz nevus with starburst dermatoscopic pattern
- Agminated naevi: a cluster of similar moles
- Kissing nevus: adjacent melanocytic naevi on upper and lower eyelids, due to nevus formation prior to separation of eyelids in utero.
The term atypical nevus may be used in several ways.
- A benign mole that has some clinical or histopathological characteristics of melanoma
- A mole with specific characteristics: large (> 5 mm); ill-defined or irregular borders; varying shades of colour; with flat and bumpy components.
- Or, any funny-looking mole; large, or different from the patient’s other moles.
Atypical nevi usually occur in fair-skinned individuals and are due to sun exposure. They may be solitary or numerous. Pathology is reported as dysplastic junctional or compound nevus and has specific histological features (the Clark nevus).
What are the complications of melanocytic nevus?
People worry about melanocytic nevus because they have heard about melanoma, a malignant proliferation of melanocytes that is the most common reason for death from skin cancer.
- At first, melanoma may look similar to a harmless mole, but in time it becomes more disordered in structure and tends to enlarge.
- People with a greater number of melanocytic nevus have a higher risk of developing melanoma than those with few melanocytic nevus, especially if they have over 100 of them.
Melanocytic nevus sometimes change for other reasons than melanoma, for example following sun exposure or during pregnancy. They can enlarge, regress or involute (disappear).
- A Meyerson nevus is itchy and dry because it is surrounded by eczema.
- A Sutton or halo nevus is surrounded by a white patch and fades away over several years
- A recurrent nevus is one that appears in a scar following surgical removal of a mole — this may have an odd shape.
Melanocytic nevus diagnosis
Melanocytic nevus are usually diagnosed clinically by their typical appearance. If there is any doubt about the diagnosis, an expert may be consulted in person or with the help of clinical and dermatoscopic images. This is especially important if:
- A mole changes size, shape, structure or color
- A new mole develops in adult life (> 40 years)
- It appears different from the person’s other melanocytic nevus (a so-called ugly duckling)
- It has ABCD characteristics (Asymmetry, Border irregularity, Color variation, Diameter > 6 mm)
- It is bleeding, crusted or itchy.
Most skin lesions with these characteristics are actually harmless when evaluated by an expert using dermatoscopy. Short-term digital dermatoscopic imaging may be used in equivocal flat lesions to check for change over time.
A nevus that remain suspicious for melanoma are excised for histopathology (diagnostic biopsy). A partial biopsy is not recommended, as it may miss an area of cancerous change.
Can melanocytic nevus be prevented?
The number of melanocytic nevus can be minimized by strict protection from the sun, starting from birth. Sunscreen alone is not sufficient to prevent new melanocytic nevus from appearing.
- At any age, sun protection is important to reduce skin ageing and the risk of skin cancer.
- Cover up. Wear a hat, long sleeves and long skirt or trousers. Choose fabrics designed for the sun (UPF 40+) when outdoors.
- Apply sunscreen to areas you can’t cover. Choose broad-spectrum high protection (SPF 50+) sunscreens, applied frequently to exposed areas.
Melanocytic nevus treatment
Most melanocytic nevus are harmless and can be safely left alone. Melanocytic nevus may be removed in the following circumstances:
- To exclude cancer
- The melanocytic nevus is a nuisance: perhaps irritated by clothing, comb or razor
- Cosmetic reasons: the mole is unsightly.
Surgical techniques include:
- Excision biopsy of a flat or suspicious mole
- Shave biopsy of a protruding mole
- Electrosurgical destruction
- Laser to lessen pigment or remove coarse hair.
Melanocytic nevus prognosis
Most melanocytic nevus that appear in childhood remain forever. Teenagers and young adults tend to have the greatest number of melanocytic nevus. There are fewer in later life because some of them slowly fade away.
To increase the chance of spotting melanoma early, doctors recommend:
- Self-skin examination monthly
- Significant changes in melanocytic nevus or new lesions are reported to doctor or dermatologist
- Regular skin examinations in patients with many melanocytic nevus, atypical nevus or previous skin cancer
- Total body photography and digital dermatoscopic imaging (mole mapping) for patients at high risk of melanoma, especially if they have many melanocytic nevus.
- Friedman RJ, Farber MJ, Warycha MA, et al. The “dysplastic” nevus. Clinics in Dermatology 2009; 27(1):103–115.[↩][↩][↩][↩]
- Tucker MA. Melanoma epidemiology. Hematology/Oncology Clinics of North America 2009; 23(3):383–395.[↩]
- Cyr PR. Atypical moles. American Family Physician 2008; 78(6):735–740.[↩]
- Goodson AG, Grossman D. Strategies for early melanoma detection: approaches to the patient with nevi. Journal of the American Academy of Dermatology 2009; 60(5):719–738.[↩][↩][↩][↩]
- Viana ACL, Gontijo B, Bittencourt FV. Giant congenital melanocytic nevus. An Bras Dermatol. 2013; 88(6): 863-78.[↩][↩][↩][↩][↩][↩][↩][↩]
- Eds. Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3rd ed. Elsevier Saunders; 2012. P 1871-6[↩][↩][↩][↩][↩][↩][↩][↩]
- Krengel S, Scope A, Dusza SW, Vonthein R, Marghoob AA. New recommendations for the categorization of cutaneous features of congenital melanocytic nevi. J Am Acad Dermatol. 2013 Mar;68(3):441-51. doi: 10.1016/j.jaad.2012.05.043[↩]
- Kovalyshyn I, Braun R, Marghoob A. Congenital melanocytic naevi. Aust J Derm. 2009; 50: 231-40.[↩][↩][↩][↩][↩][↩]