Contents
- Pellagra
- What is Vitamin B3?
- What causes pellagra
- Pellagra prevention
- Pellagra symptoms
- Pellagra complication
- Pellagra diagnosis
- Pellagra treatment
- Pellagra prognosis
Pellagra
Pellagra is a disease caused by vitamin B3 or Niacin deficiency or tryptophan (an amino acid) deficiency 1. Since tryptophan (an amino acid) in the diet can be converted to Niacin in your body, both of these need to be deficient for pellagra to develop. Pellagra is characterized by 4 “D’s”: diarrhea, dermatitis, dementia, and death 2, 3, 4, 5, 6. Pellagra is characterized by a pigmented rash or brown discoloration on skin exposed to sunlight; the skin also develops a roughened, sunburned-like appearance (Figure 1) 3, 4, 5, 6. In advanced stages, increased pigmentation usually leads to thin varnish-like eruptive scales 5. The characteristic skin rash has a typical photosensitive distribution with welldefined borders, and is mostly observed on the face, the neck – forming the so-called Casal’s necklace -, the dorsa of the hands and the extensor surface of the forearms 7, 8, 9. In addition to skin changes, pellagra can cause a bright red tongue (glossitis) and changes in the digestive tract that lead to vomiting, constipation, or intractable diarrhea. The neurological symptoms of pellagrous encephalopathy can include depression; apathy; headache; fatigue; loss of memory that can progress to aggressive, paranoid, and suicidal behaviors; and auditory and visual hallucinations 4, 10, 3. As pellagra progresses, anorexia develops, and the affected individual eventually dies if pellagra is left untreated 10, 8, 11.
Pellagra is uncommon in industrialized populations and is mostly limited to people living in poverty, such as refugees and displaced people who eat very limited diets low in niacin and protein 12, 6. Pellagra was common in the early 20th century among individuals living in poverty in the southern United States and parts of Europe whose limited diets consisted mainly of corn or sorghum 10, 4. Pellagra was also common in the southern United States during the early 1900s where income was low and corn products were a major dietary staple 13. Interestingly, pellagra was not known in Mexico, where corn was also an important dietary staple and much of the population was also poor. In fact, if corn contains appreciable amounts of niacin, it is present in a bound form that is not nutritionally available to humans. The traditional preparation of corn tortillas in Mexico involves soaking the corn in a lime (calcium oxide) solution, prior to cooking. Heating the corn in an alkaline solution results in the release of bound niacin, increasing its bioavailability 14. Pellagra epidemics were also unknown to Native Americans who consumed immature corn that contains predominantly unbound (bioavailable) niacin 13.
Although frank niacin deficiencies leading to pellagra are very rare in the United States, some individuals have marginal or low niacin status 15, 5, 12.
Niacin deficiency or pellagra may result from inadequate dietary intake of NAD precursors, including tryptophan. Niacin deficiency — often associated with malnutrition — is observed in the homeless population, in individuals suffering from anorexia nervosa or obesity, and in consumers of diets high in maize and poor in animal protein 16, 17, 18, 19. Deficiencies of other B vitamins and some trace minerals may aggravate niacin deficiency 20, 21.
Niacin deficiency should especially be suspected in the following conditions: i) malnutrition (homelessness, anorexia nervosa or severe comorbid conditions like end-stage malignancy or HIV); ii) malabsorption (e.g. Crohn’s or Hartnup disease); iii) chronic alcoholism; iv) hemodialysis or peritoneal dialysis; v) drugs like isoniazid, ethionamide, 6-mercaptopurine and estrogens; vi) carcinoid syndrome (due to excess turnover of tryptophan, precursor of niacin, to serotonin) 7, 8, 11.
Malabsorptive disorders that can lead to pellagra include Crohn’s disease and megaduodenum 22, 23. Patients with Hartnup’s disease, a hereditary disorder resulting in defective tryptophan absorption, have developed pellagra. Carcinoid syndrome, a condition of increased secretion of serotonin and other catecholamines by carcinoid tumors, may also result in pellagra due to increased utilization of dietary tryptophan for serotonin rather than niacin synthesis. Furthermore, prolonged treatment with the anti-tuberculosis drug isoniazid has resulted in niacin deficiency 24. Other pharmaceutical agents, including the immunosuppressive drugs azathioprine (Imuran) and 6-mercaptopurine, the anti-cancer drug 5-fluorouracil (5-FU, Adrucil), and levodopa/carbidopa (Sinemet; two drugs given to people with Parkinson’s disease), are known to increase the reliance on dietary niacin by interfering with the tryptophan-kynurenine-niacin pathway 25. Finally, other populations at risk for niacin deficiency include dialysis patients, cancer patients 26, 27, individuals suffering from chronic alcoholism 8, and people with HIV/AIDS. Furthermore, chronic alcohol intake can lead to severe niacin deficiency through reducing dietary niacin intake and interfering with the tryptophan-to-NAD conversion 21.
The World Health Organization (WHO) recommends treating pellagra with 300 mg/day nicotinamide in divided doses for 3-4 weeks along with a B-complex or yeast product to treat likely deficiencies in other B vitamins 6.
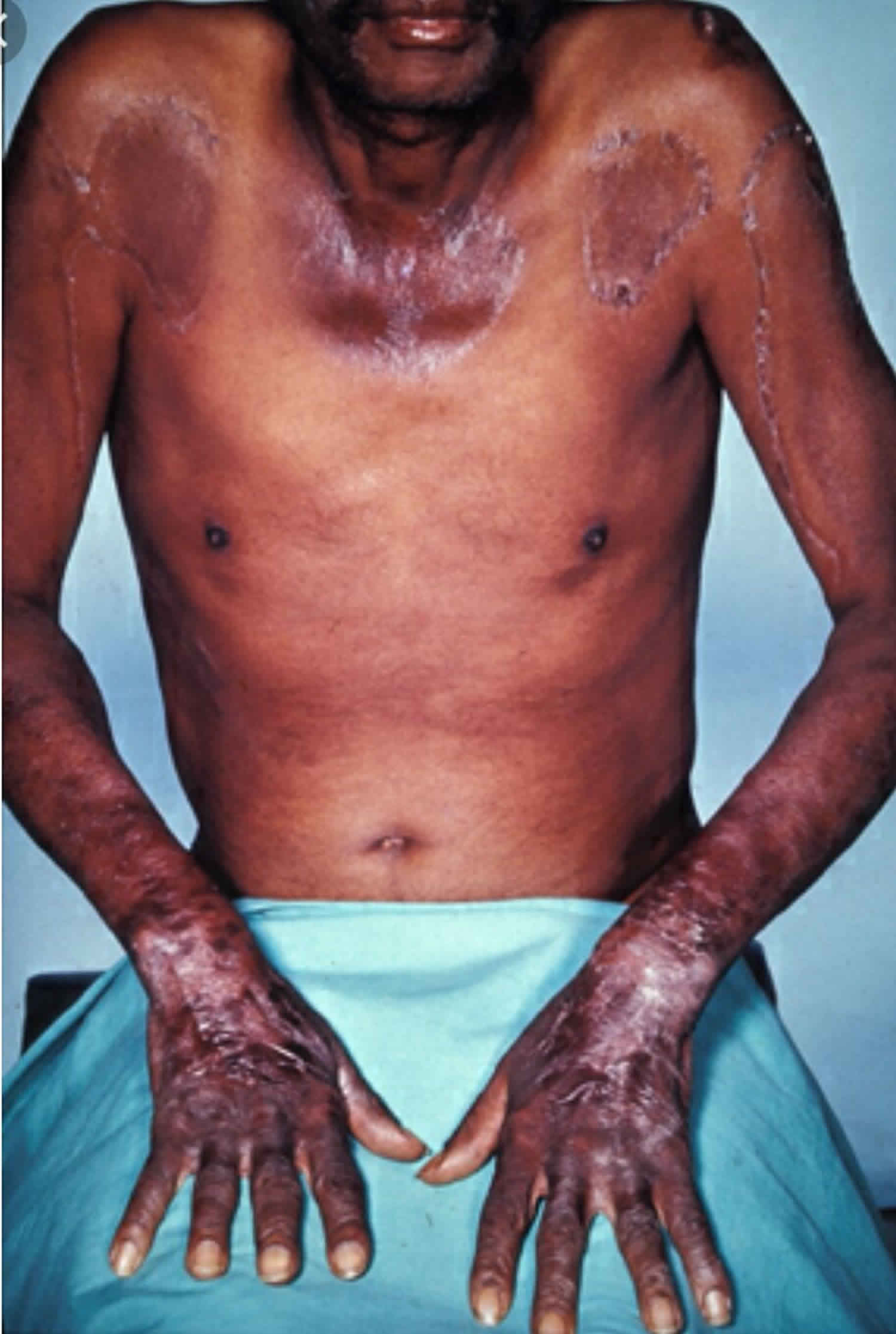
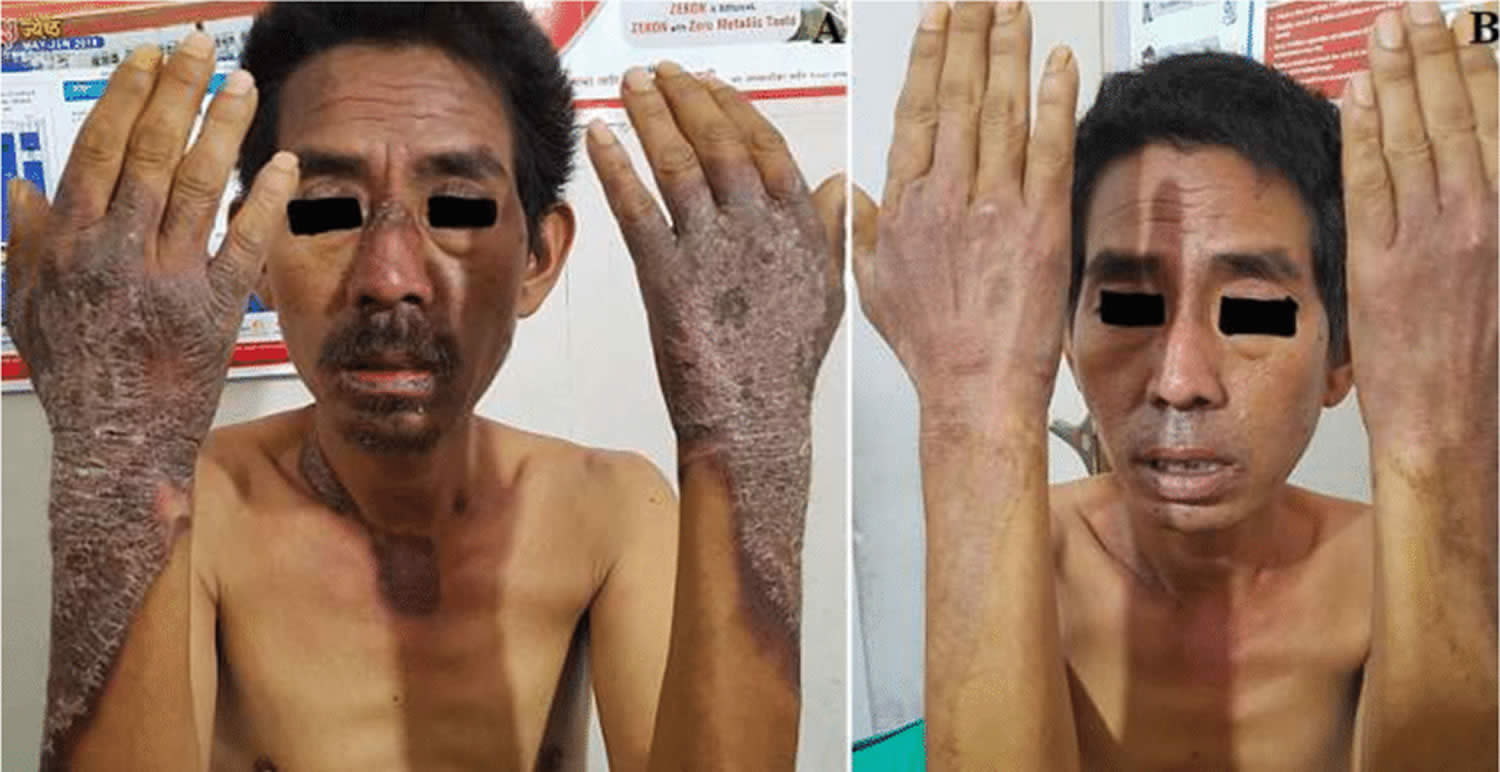
Figure 1. Skin lesions of niacin deficiency (pellagra) involving face, neck, hand and forearm (A), after treatment with niacin for 2 weeks (B)
[Source 28 ]Figure 2. Pellagra rash
Footnote: This is a classical case of pellagra. A 42 year old woman was admitted to hospital with a one month history of progressive forgetfulness, irritability and confusion. There was no history of tremor or confabulation. Reportedly, she also had fever two weeks prior to admission. There was no history of headache, neck-ache or neck stiffness. Further inquiry revealed she had developed rash around her neck and in the distal parts of all four limbs a month prior to the onset of the altered mental status. There was no history of rash involving the mucosae or of having taken any drugs in the period preceding the rash.
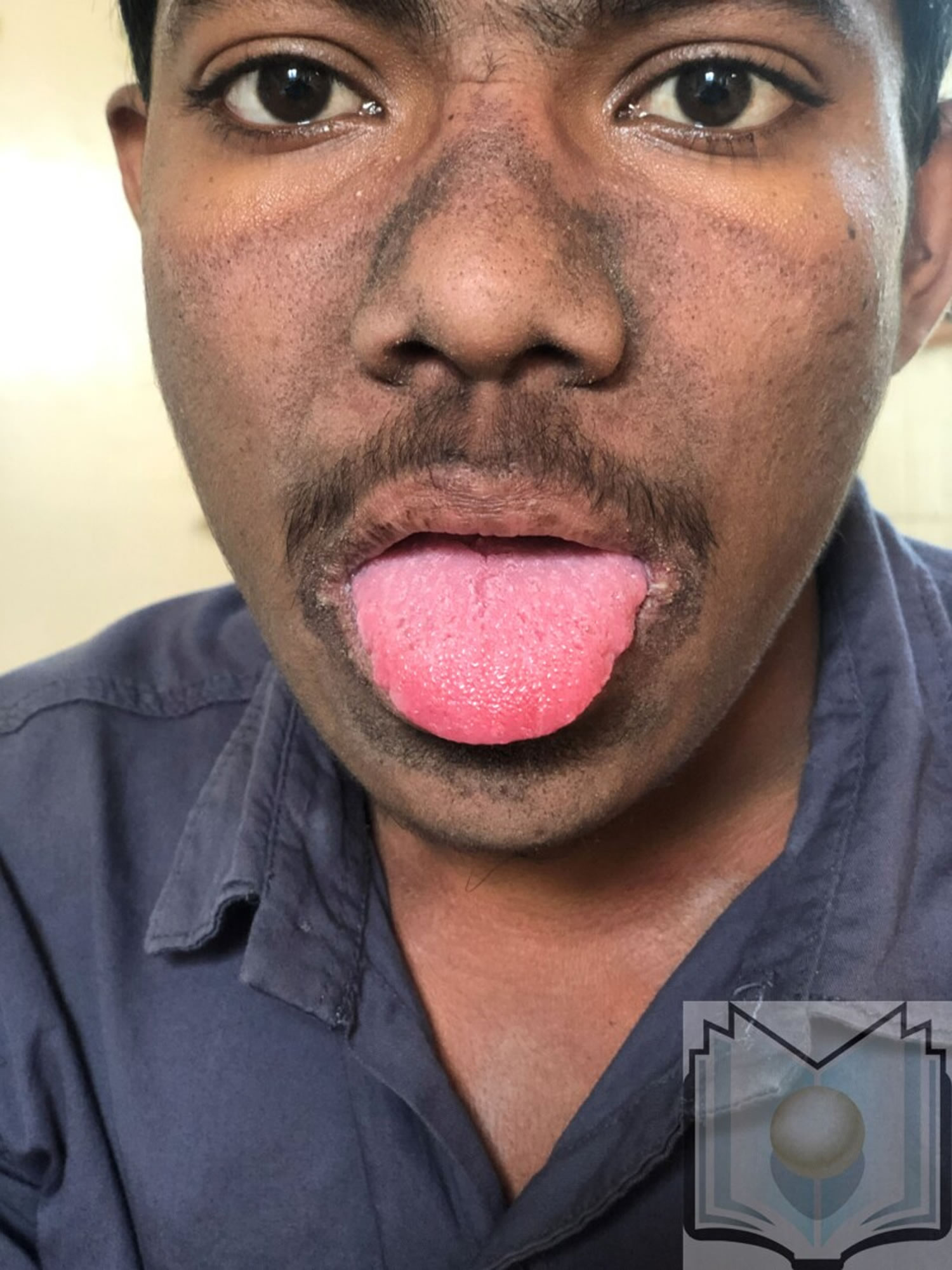
[Source 29 ]Figure 3. Pellagra tongue
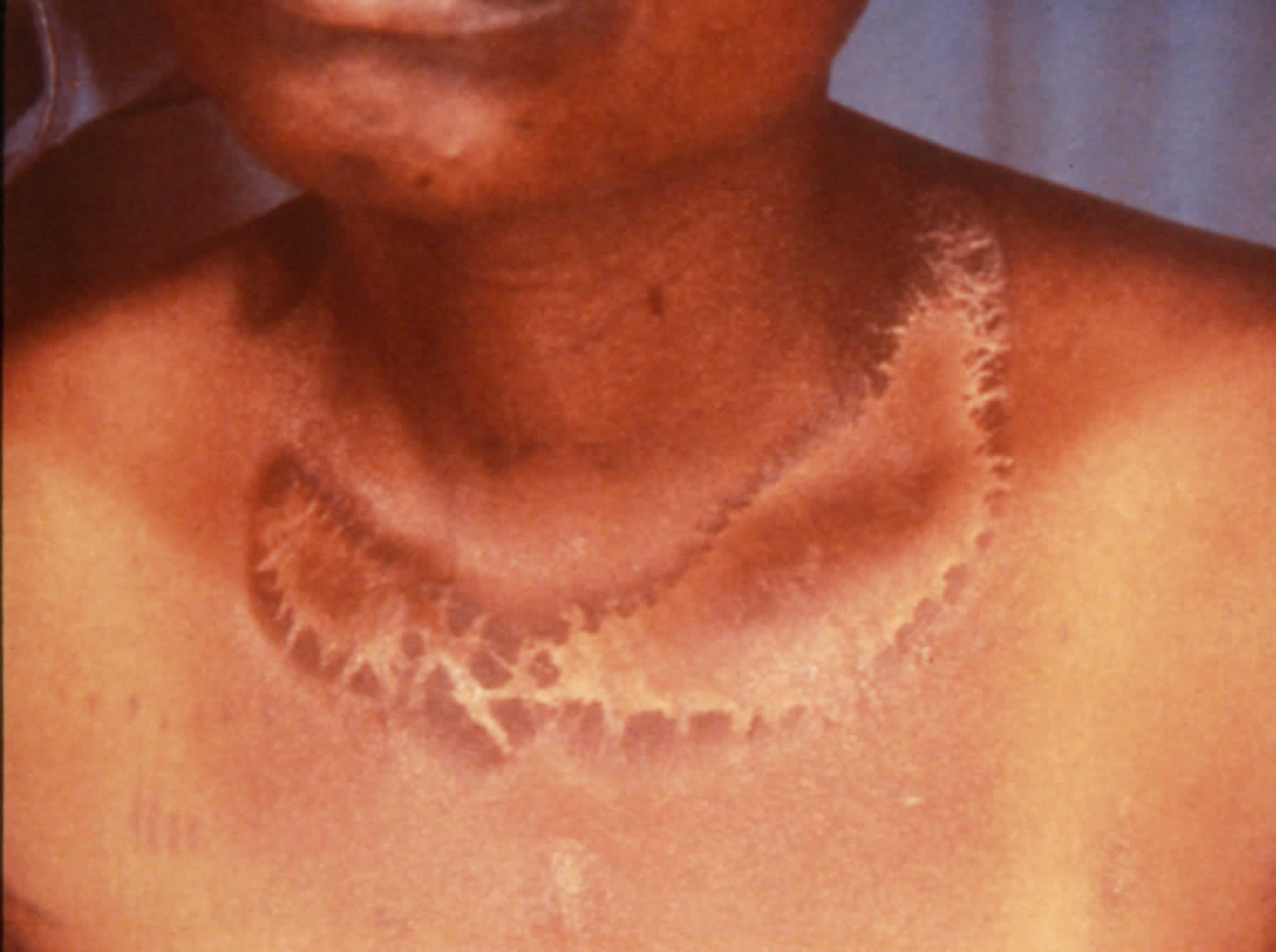
[Source 30 ]Figure 4. Casal necklace
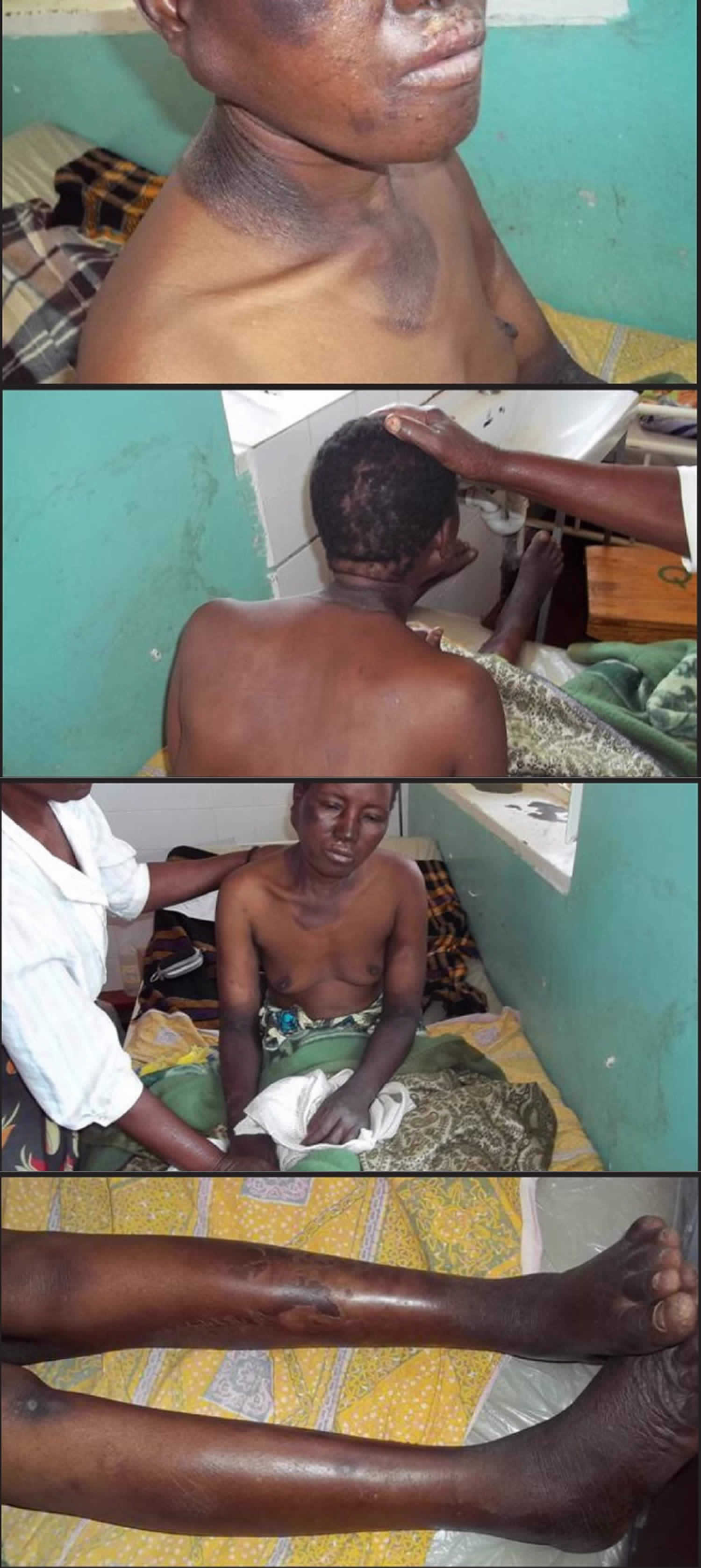
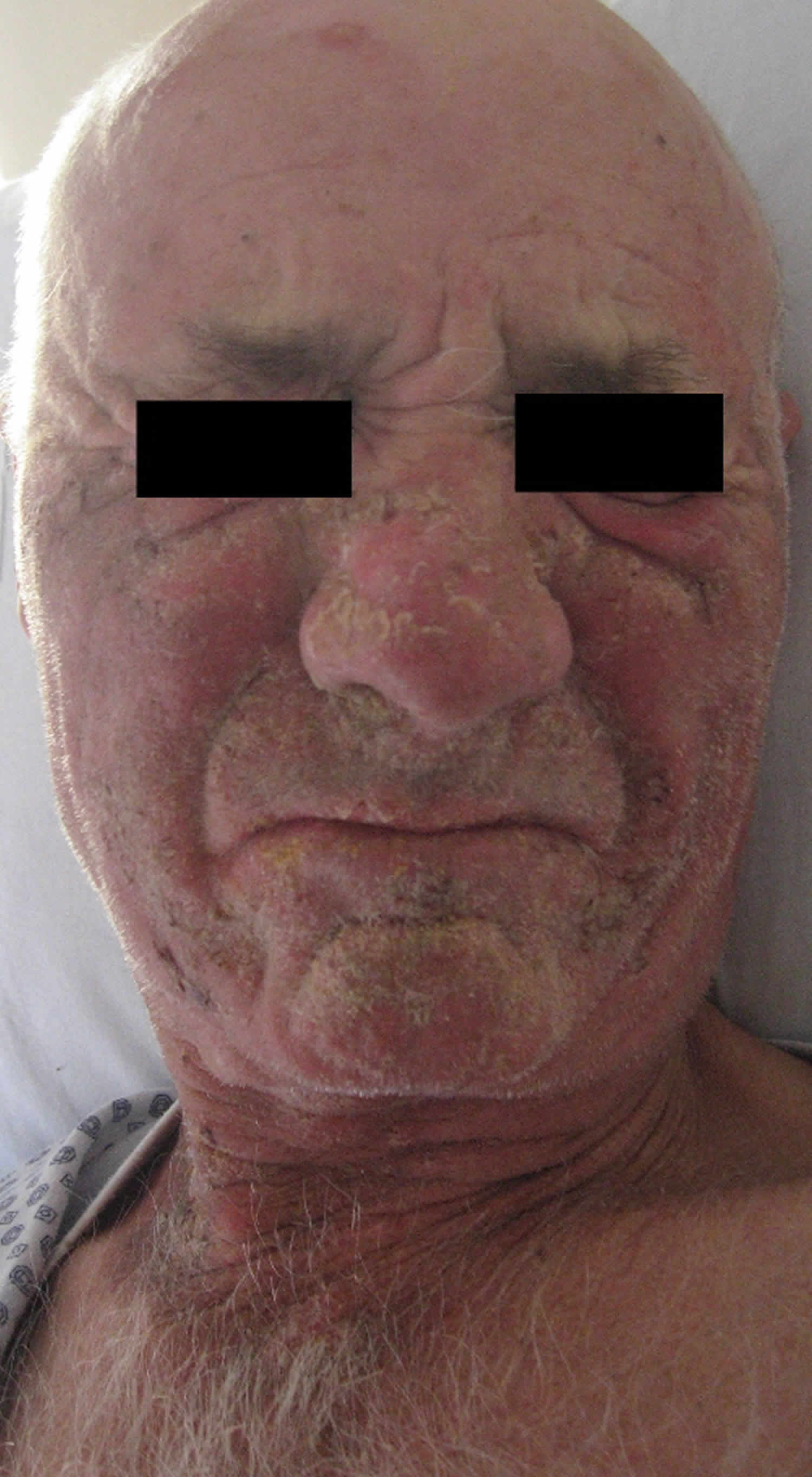
Figure 5. Pellagra dermatitis (scaly butterfly rash on the face)
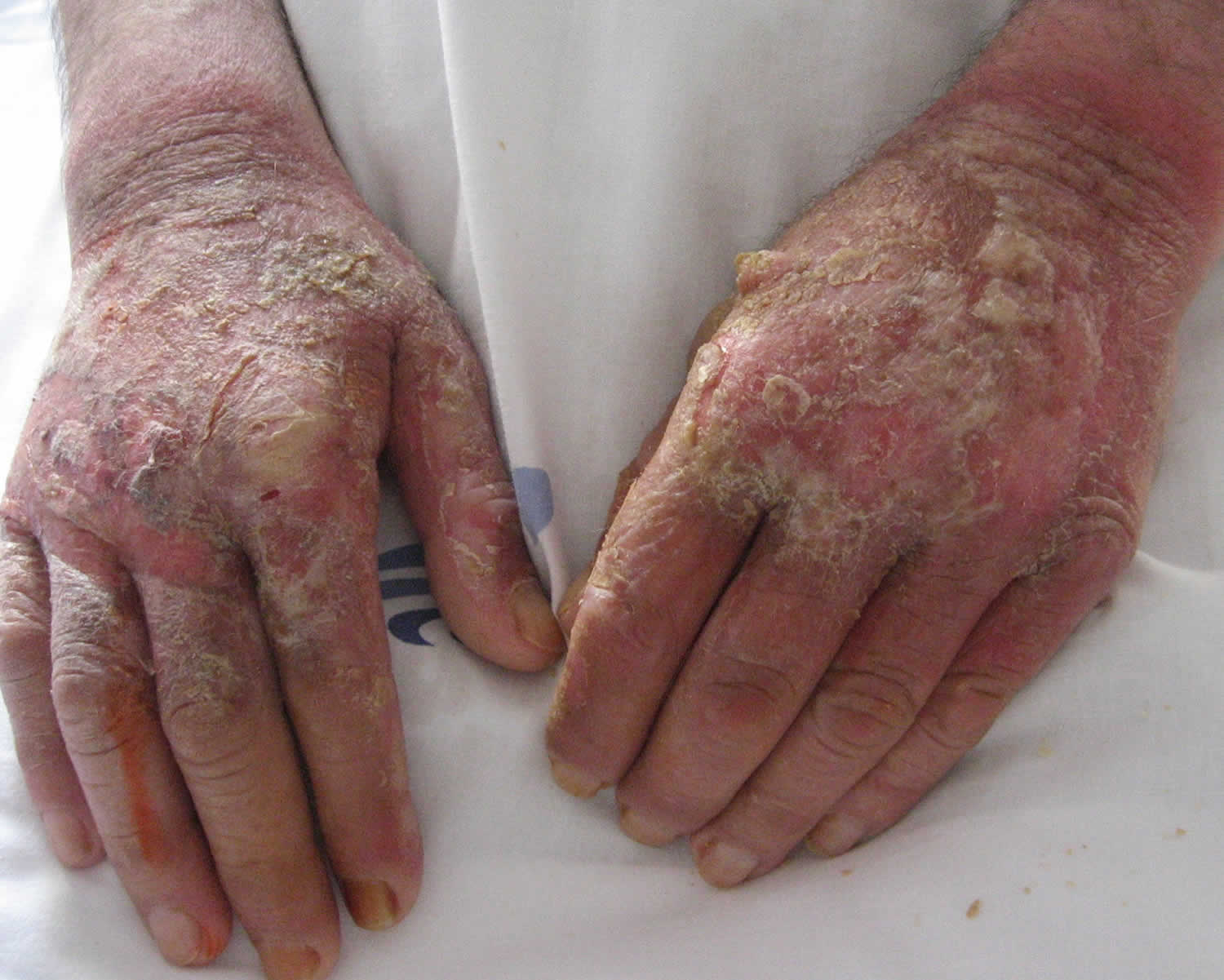
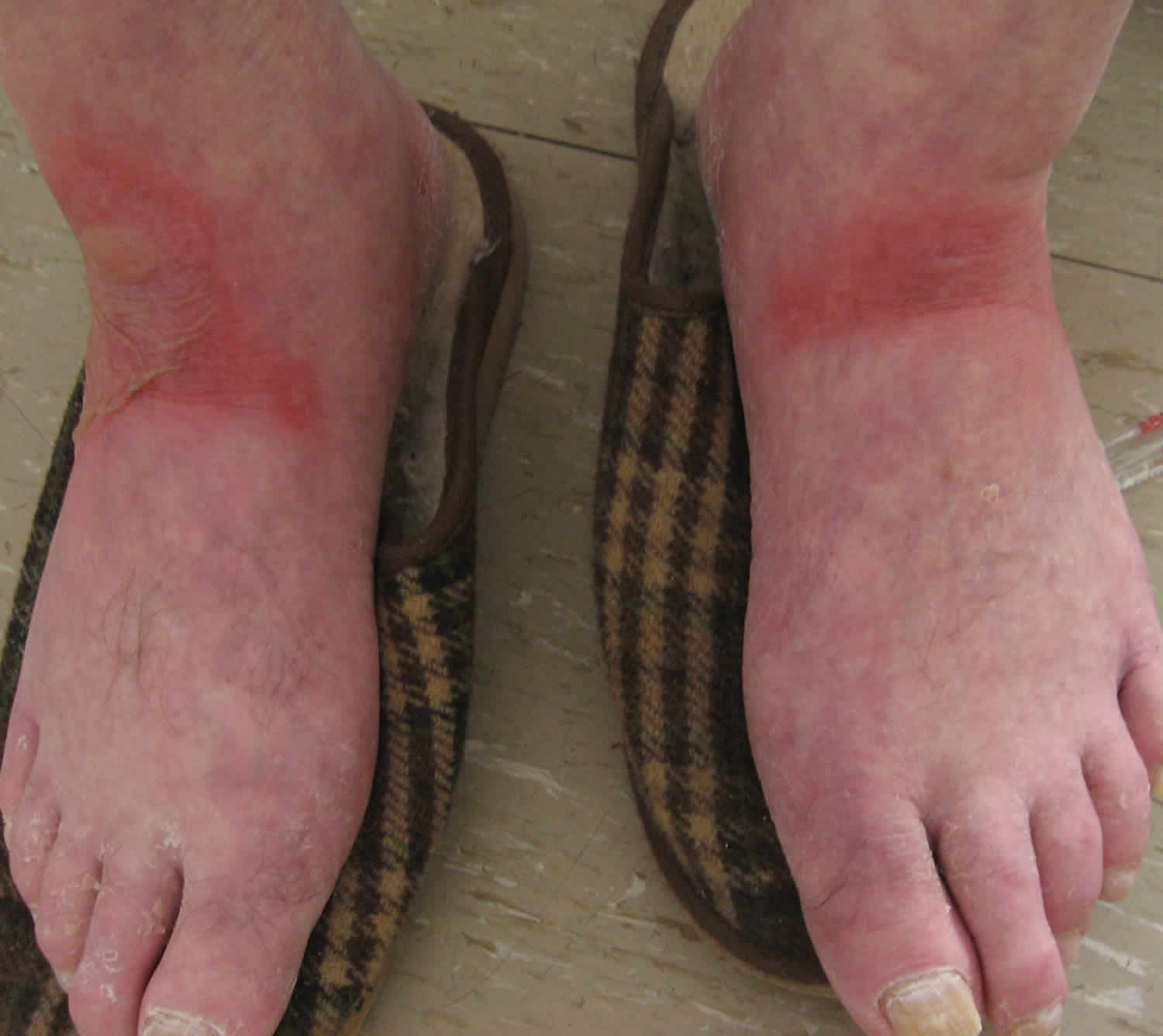
Footnote: A 63-year-old man was sent for dermatological evaluation because of a three-month history of an evolving erythematous, scaly and bullous skin rash involving the face, neck, hands, and feet. Although he was a cooperative patient, he had periods of disorientation and emotional lability. Skin examination revealed a symmetric scaly erythematous eruption on the face and neck as well as the presence of cheilitis (Figure 1). The dorsum of the hands exhibited bullae on an erythematous-brawny peeling base (Figure 2). The dorsa of the feet were also erythematous and had large bullae (Figure 3). In every affected location, a sharp demarcation between involved and healthy skin was evident. The patient lived alone and had scarce familial support. He admitted having a high alcohol intake, smoked 40 cigarettes a day, and wasn’t aware of any major health problems except for two surgeries for “gastric ulcers” in the past. He wasn’t taking any medication. When asked for associated symptoms, he reported repeated vomiting in the last three months, but denied diarrhea.
[Source 31 ]Figure 6. Pellagra dermatitis
[Source 31 ]Figure 7. Wet pellagra dermatitis
[Source 31 ]What is Vitamin B3?
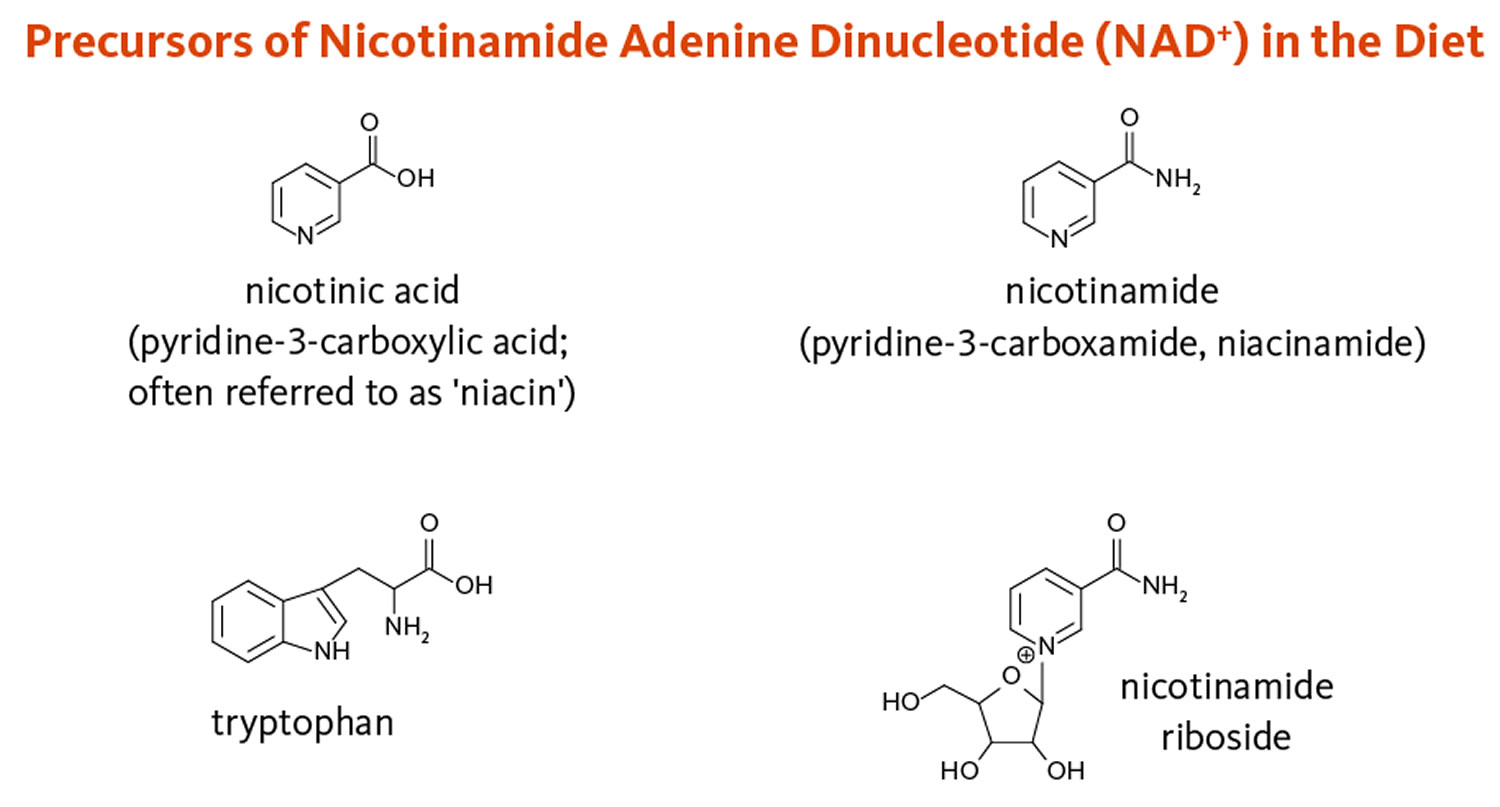
Vitamin B3 also known as Niacin, nicotinamide (pyridine-3-carboxamide), nicotinamide adenine dinucleotide (NAD), nicotinic acid (pyridine-3-carboxylic acid) or nicotinamide riboside is one of the water-soluble B vitamins that occurs in many animal and plant tissues 32, 33, 25, 34. Vitamin B3 or Niacin is the generic name for nicotinic acid (pyridine-3-carboxylic acid), nicotinamide (niacinamide or pyridine-3-carboxamide), and related derivatives, such as nicotinamide riboside 35, 4, 10. Niacin is naturally present in many foods, added to some food products, and available as a dietary supplement. Food such as bran, yeast, eggs, peanuts, poultry, red meat, fish, whole-grain cereals, legumes, and seeds are rich sources of niacin or vitamin B3 36. The essential amino acid tryptophan can also be converted into nicotinamide adenine dinucleotide (NAD) via the kynurenine pathway, so tryptophan (an amino acid in protein) is considered a dietary source of niacin 25, 34. Note that none of the Niacin vitamers are related to the nicotine found in tobacco, although their names are similar. Likewise, nicotine — but not nicotinic acid — is an agonist of the nicotinic receptors that respond to the neurotransmitter, acetylcholine 25.
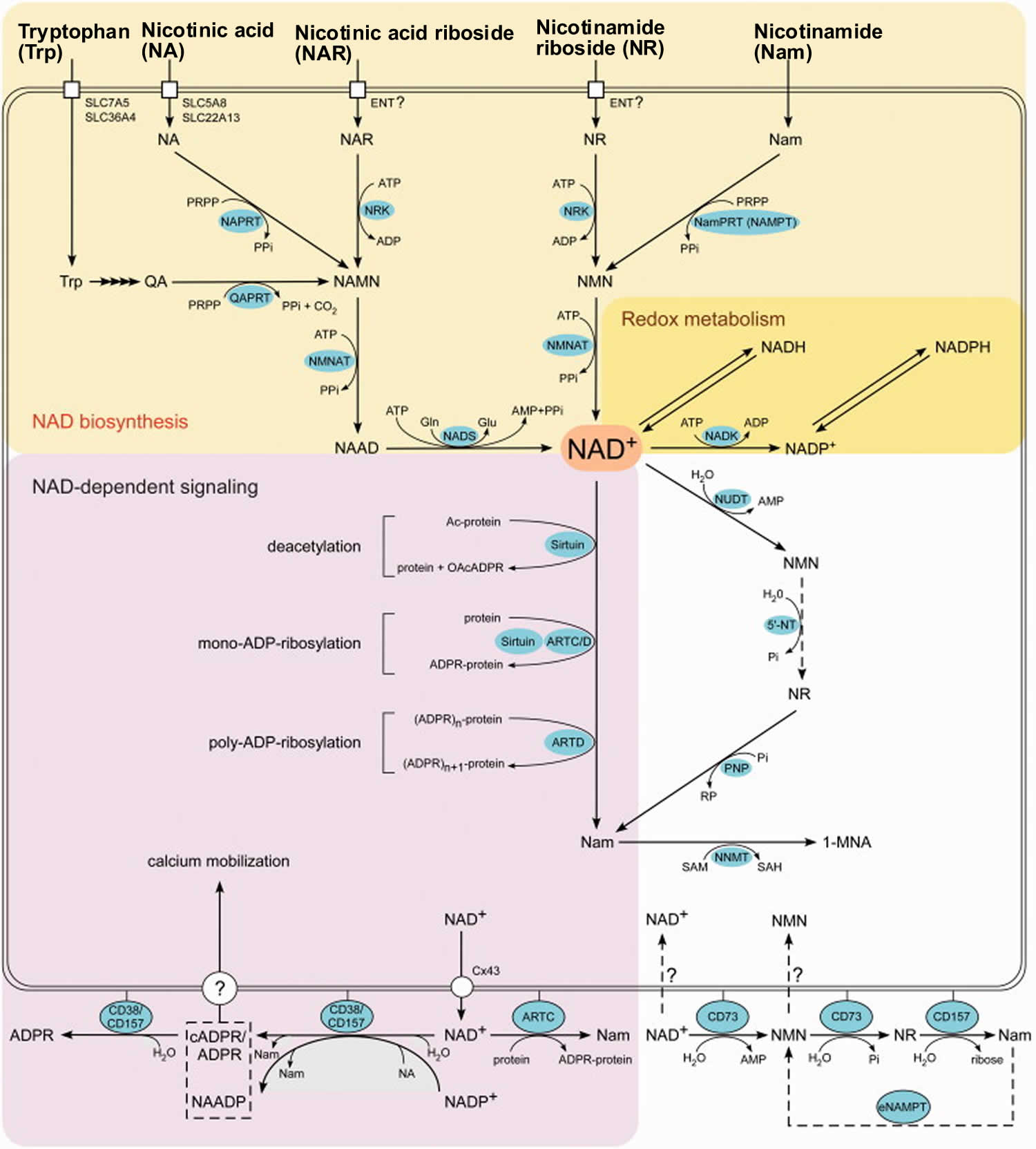
Essential to all forms of life, the coenzyme nicotinamide adenine dinucleotide (NAD) is synthesized in all tissues in your body from four precursors that are provided in the diet: nicotinic acid, nicotinamide, nicotinamide riboside, and tryptophan (Figure 8) 34. More than 400 enzymes require nicotinamide adenine dinucleotide (NAD) to catalyze reactions in your body, which is more than for any other vitamin-derived coenzyme 35. Nicotinamide adenine dinucleotide (NAD) is also converted into another active form, the coenzyme nicotinamide adenine dinucleotide phosphate (NADP), in all tissues except skeletal muscle 3.
Most dietary niacin is in the form of nicotinic acid and nicotinamide, but some foods contain small amounts of NAD and NADP.
Humans are able to synthesize nicotinic acid from tryptophan – the liver can synthesize niacin from the essential amino acid tryptophan, but the synthesis is extremely slow and requires vitamin B6 (Pyridoxine); 60 mg of tryptophan are required to make one milligram of niacin 37. Bacteria in the gut may also perform the conversion but are inefficient. Another source for nicotinic acid is the gut flora. In humans there is no deamidation of nicotinamide to nicotinic acid in the gut. Nicotinamide is rapidly absorbed in stomach and small intestine. In plasma both the acid and the amide form are found. Red blood cells take up the nicotinic acid by a sodium dependent saturable transport system. Both the nicotinic acid and nicotinamide are able to pass the blood-brain barrier, however separate systems for uptake have been identified. Brain cells have a high affinity for nicotinamide, but not for nicotinic acid. Nicotinamide is the main substance that is transported between the different tissues as a precursor of NAD synthesis. The liver, kidneys, brain and red blood cells prefer nicotinic acid as a precursor for NAD synthesis, but testes and ovaries prefer nicotinamide. NAD nucleosidase cleaves NAD with nicotinamide as one of the products. This can be deamidated to form nicotinic acid (and re-converted to NAD) or methylated and released via urine. Excretion of the amide (and its metabolites) tends to be more extensive compared to the acid 38.
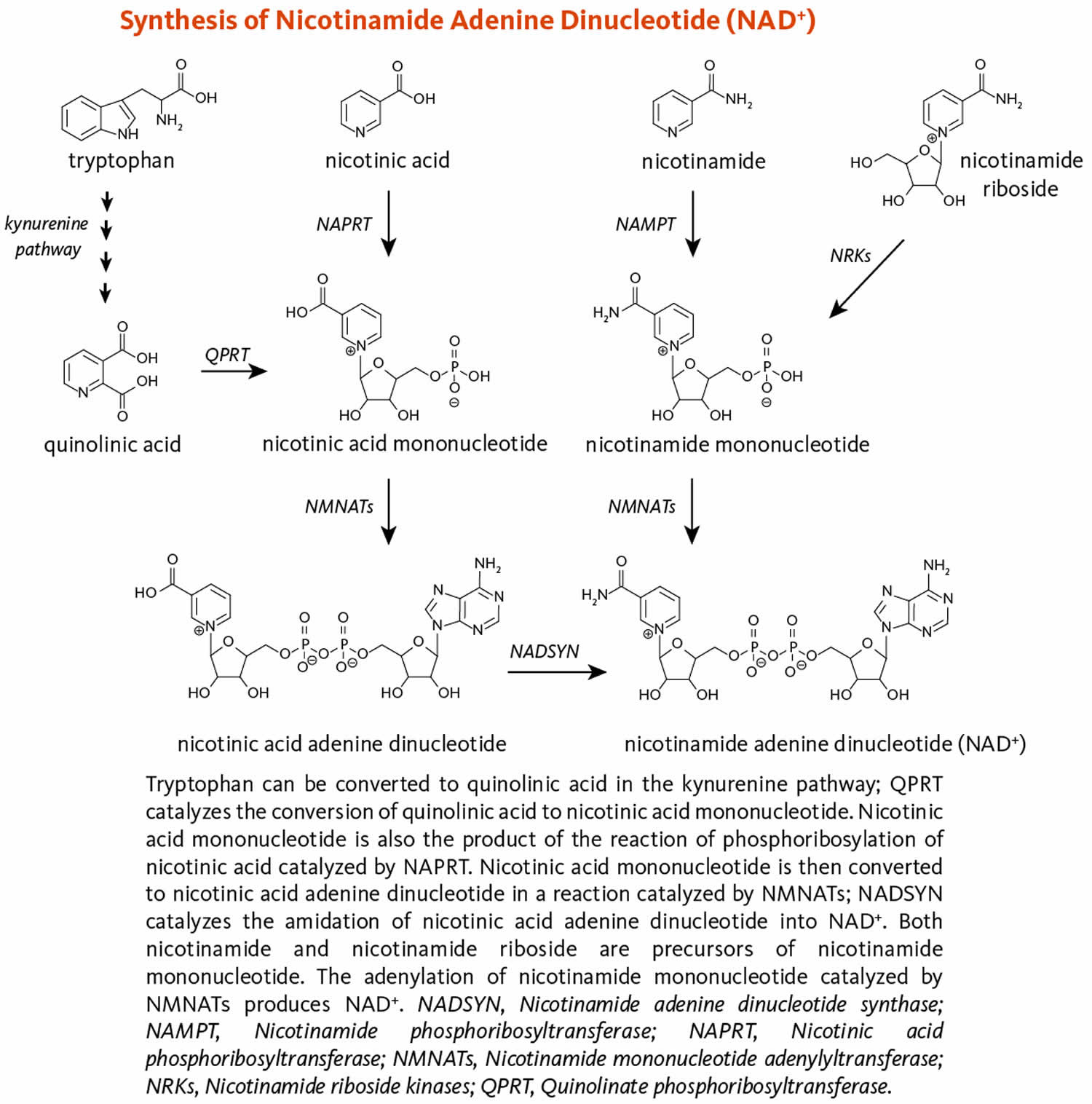
Figure 9 illustrates the separate biosynthetic pathways that lead to nicotinamide adenine dinucleotide (NAD) production from the various dietary precursors. Nicotinamide adenine dinucleotide (NAD) is synthesized from nicotinamide and nicotinamide riboside via two enzymatic reactions, while the pathway that yields nicotinamide adenine dinucleotide (NAD) from nicotinic acid – known as the Preiss-Handler pathway — includes three steps 25. The kynurenine pathway is the longest nicotinamide adenine dinucleotide (NAD) biosynthetic pathway: the catabolism of tryptophan through kynurenine produces quinolinic acid, which is then converted to nicotinic acid mononucleotide, an intermediate in nicotinamide adenine dinucleotide (NAD) metabolism. Nicotinamide adenine dinucleotide (NAD) is then synthesized from nicotinic acid mononucleotide in the Preiss-Handler pathway 39.
All pathways generate intermediary mononucleotides — either nicotinic acid mononucleotide or nicotinamide mononucleotide 25. Specific enzymes, known as phosphoribosyltransferases, catalyze the addition of a phosphoribose moiety onto nicotinic acid or quinolinic acid to produce nicotinic acid mononucleotide or onto nicotinamide to generate nicotinamide mononucleotide 25. Nicotinamide mononucleotide is also generated by the phosphorylation of nicotinamide riboside, catalyzed by nicotinamide riboside kinases (NRKs) 25. Furthermore, adenylyltransferases catalyze the adenylation of these mononucleotides to form either nicotinic acid adenine dinucleotide or nicotinamide adenine dinucleotide (NAD). Nicotinic acid adenine dinucleotide is then converted to nicotinamide adenine dinucleotide (NAD) by glutamine-dependent NAD synthetase (NADSYN), which uses glutamine as an amide group donor (Figure 9) 39. Of note, nicotinic acid adenine dinucleotide has been reported to form following the administration of high-dose nicotinamide riboside, suggesting that a potential deamidation could occur to convert nicotinamide adenine dinucleotide (NAD) to nicotinic acid adenine dinucleotide when the pool of nicotinamide adenine dinucleotide (NAD) is high 33.
When NAD and NADP are consumed in foods, they are converted to nicotinamide in the gut and then absorbed 3. Ingested niacin is absorbed primarily in the small intestine, but some is absorbed in the stomach 35, 4, 10.
Niacin or vitamin B3 helps turn the food you eat into the energy you need. Niacin is important for the development and function of the cells in your body 40.
As a drug, Niacin or vitamin B3, has two main indications 36:
- To treat hyperlipidemia (types 2A and 2B or primary hypercholesterolemia) (FDA approved use). Niaspan and generic niacin extended release (ER), available as a prescription medicine, provides 500-1,000 mg extended-release nicotinic acid. It is used to treat high blood cholesterol levels 34. The principal antilipemic effect of niacin appears to result mainly from decreased production of very low density lipoprotein cholesterol (VLDL-cholesterol) and is effective in lowering low density lipoprotein (LDL) cholesterol and raising high density lipoprotein (HDL) cholesterol, which makes this agent of unique value in the therapy of dyslipidemia 41, 42. Decreased production of VLDL-cholesterol by niacin may be related to the partial inhibition of free fatty acid release from adipose tissue, a decreased delivery of free fatty acids to the liver, and a decrease in triglyceride synthesis and VLDL-triglyceride transport. Enhanced clearance of VLDL-cholesterol and chylomicron triglycerides also may occur, possibly as a result of enhanced activity of lipoprotein lipase. Reductions in LDL-cholesterol concentrations may be related to decreased production and enhanced hepatic clearance of LDL-cholesterol precursors (i.e., VLDL-cholesterol). The mechanism by which niacin increases HDL-cholesterol concentrations has not been fully elucidated but may be related to a decreased hepatic clearance of apo A-I-containing particles and decreased synthesis of apo A-II. Niacin has no effect on cholesterol synthesis or fecal excretion of fats, sterols, or bile acids.
- To treat Niacin or vitamin B3 Deficiency, also known as pellagra (Italian for “rough skin”) 7, 8, 9. In the 1700s, pellagra first appeared in Italy, and the name translates into “pella” meaning skin, and “agra” meaning rough 5. Niacin or vitamin B3 deficiency causes pellagra, a disease characterized by the triad of dermatitis, diarrhea, and dementia that is endemic today in parts of India and China, and may result in death in severe cases 43, 44. Other symptoms include irritability, loss of appetite, weakness, and dizziness. Niacin deficiency is rare in the United States but may still be seen in alcoholics, dietary cultists, and patients with malabsorption syndrome 45. Some clinicians prefer niacinamide for the treatment of pellagra because it lacks vasodilating effects 46.
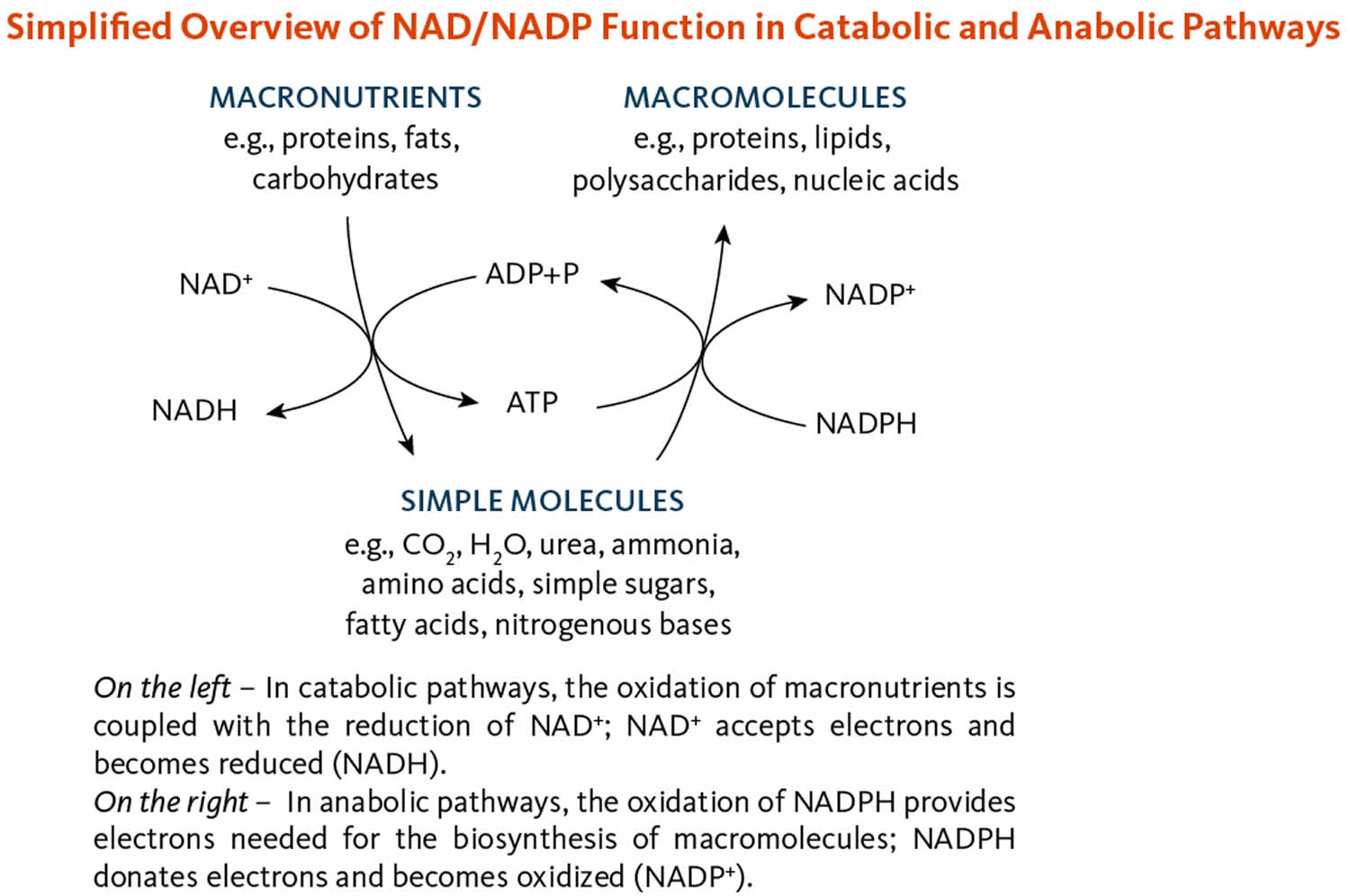
The coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) are required in most metabolic oxidation-reduction (redox) processes in cells where NAD and NADP are oxidized or reduced (Figure 10) 34. NAD is primarily involved in catabolic reactions that transfer the potential energy in carbohydrates, fats, and proteins to adenosine triphosphate (ATP), the cell’s primary energy currency 3. Nicotinamide adenine dinucleotide (NAD) is also required for enzymes involved in critical cellular functions, such as the maintenance of genome integrity, control of gene expression, and cellular communication 3, 10. Nicotinamide adenine dinucleotide phosphate (NADP), in contrast, enables anabolic reactions, such as the synthesis of cholesterol and fatty acids, and plays a critical role in maintaining cellular antioxidant function 34.
Even when taken in very high doses of 3–4 g, niacin is almost completely absorbed 34. Once absorbed, physiologic amounts of niacin are metabolized to nicotinamide adenine dinucleotide (NAD). Some excess niacin is taken up by red blood cells to form a circulating reserve pool. The liver methylates any remaining excess to N1-methyl-nicotinamide, N1-methyl-2-pyridone-5-carboxamide, and other pyridone oxidation products, which are then excreted in the urine 34. Unmetabolized nicotinic acid and nicotinamide might be present in the urine as well when niacin intakes are very high 34.
Levels of niacin in the blood are not reliable indicators of niacin status 34. The most sensitive and reliable measure of niacin status is the urinary excretion of its two major methylated metabolites, N1-methyl-nicotinamide and N1-methyl-2-pyridone-5-carboxamide 4. Excretion rates in adults of more than 17.5 micromol/day of these two metabolites reflect adequate niacin status, while excretion rates between 5.8 and 17.5 micromol/day reflect low niacin status 34. An adult has niacin deficiency when urinary-excretion rates are less than 5.8 micromol/day 34. Indicators of niacin deficiency such as this and other biochemical signs (e.g., a 2-pyridone oxidation product of N1-methyl-nicotinamide below detection limits in plasma or low red blood cell NAD concentrations) occur well before overt clinical signs of niacin deficiency 4. Another measure of niacin status takes into account the fact that NAD levels decline as niacin status deteriorates, whereas NADP levels remain relatively constant 35, 10, 47. A “niacin number” (the ratio of NAD to NADP concentrations in whole blood x 100) below 130 suggests niacin deficiency 48, 49. A “niacin index” (the ratio of red blood cell NAD to NADP concentrations) below 1 suggests that an individual is at risk of developing niacin deficiency 50. No functional biochemical tests that reflect total body stores of niacin are available 47.
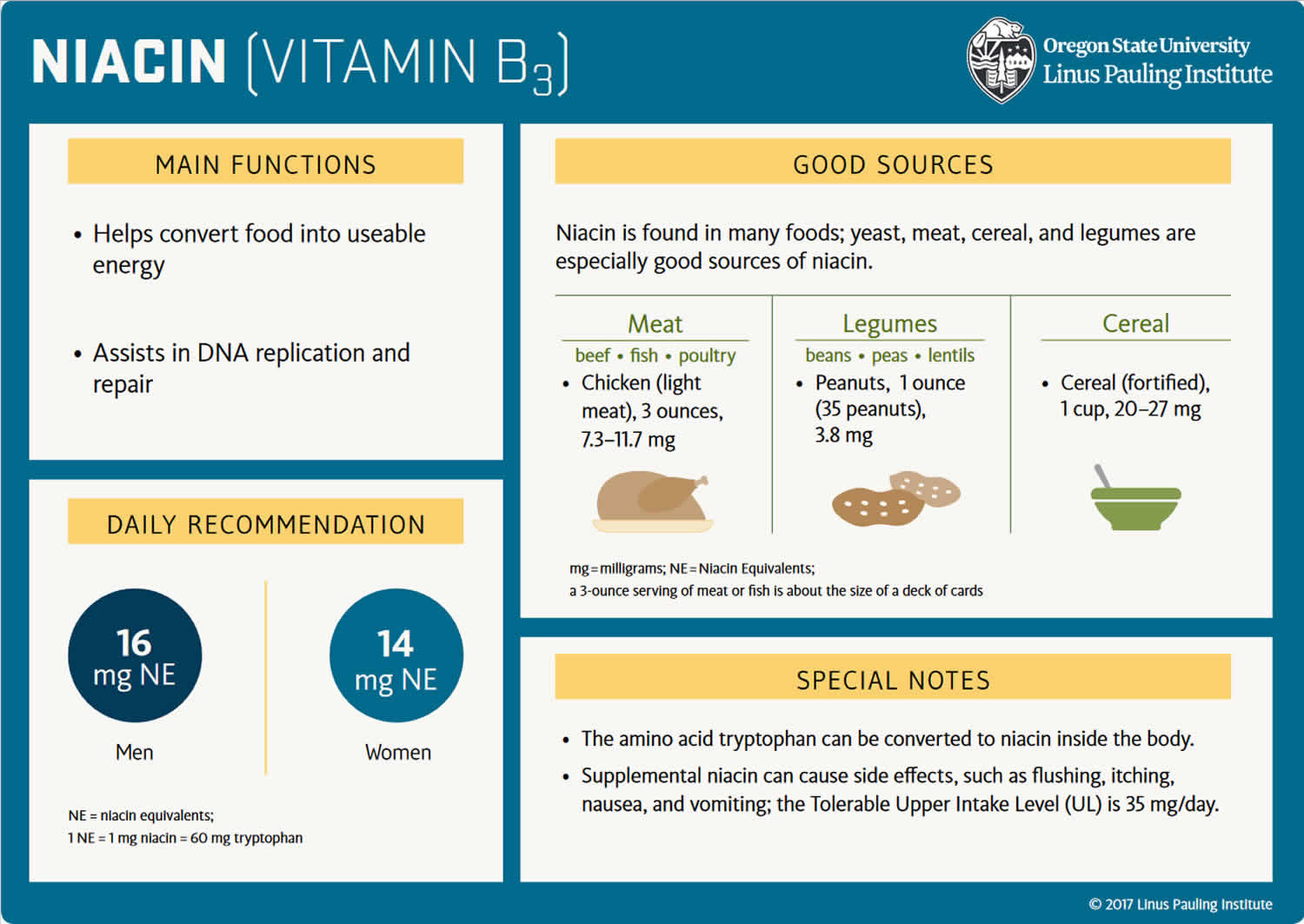
The recommended dietary allowance (RDA) of Niacin or vitamin B3 is 14 to 16 mg daily in adults, and slightly more for pregnant women (18 mg) and less for children (2 to 12 mg). No adverse effects have been reported from the consumption of naturally occurring niacin in foods 4, 42. However, high intakes of both nicotinic acid and nicotinamide taken as a dietary supplement or medication can cause adverse effects, although their toxicity profiles are not the same.
30 mg to 50 mg nicotinic acid or more typically causes flushing; the skin on the patient’s face, arms, and chest turns a reddish color because of vasodilation of small subcutaneous blood vessels 34. The flushing is accompanied by burning, tingling, and itching sensations 51, 52. These signs and symptoms are typically transient and can occur within 30 minutes of nicotinic acid intake or over days or weeks with repeated dosing; they are considered an unpleasant, rather than a toxic, side effect 34. However, the flushing can be accompanied by more serious signs and symptoms, such as headache, rash, dizziness, and/or a decrease in blood pressure. Supplement users can reduce the flushing effects by taking nicotinic acid supplements with food, slowly increasing the dose over time, or simply waiting for the body to develop a natural tolerance 34.
When taken in pharmacologic doses of 1,000 to 3,000 mg/day used in the therapy of hyperlipidemia, nicotinic acid can also cause more serious side effects 51, 52, 3, 4. Many of these effects have occurred in patients taking high-dose nicotinic acid supplements to treat hyperlipidemias. These adverse effects can include hypotension severe enough to increase the risk of falls; fatigue; impaired glucose tolerance and insulin resistance; gastrointestinal effects, such as nausea, heartburn, and abdominal pain; and ocular effects, such as blurred or impaired vision and macular edema (a buildup of fluid at the center of the retina). High doses of nicotinic acid taken over months or years can also cause liver injury; effects can include increased levels of liver enzymes; hepatic dysfunction resulting in fatigue, nausea, and anorexia; hepatitis; and acute liver failure 42, 4, 53, 51, 54. Liver injury is more likely to occur with the use of extended-release forms of nicotinic acid 55, 56, 51.
To minimize the risk of adverse effects from nicotinic acid supplementation or to identify them before they become serious, the American College of Cardiology and the American Heart Association recommend measuring liver transaminase (liver enzyme), fasting blood glucose or hemoglobin A1C, and uric acid levels in all supplement users before they start therapy, while the dose is being increased to a maintenance level, and every 6 months thereafter 53. The American College of Cardiology and the American Heart Association also recommend that patients not use nicotinic acid supplements or stop using them if their liver transaminase (liver enzyme) levels are more than two or three times the upper limits of normal; if they develop persistent high blood sugar level (hyperglycemia), acute gout, unexplained abdominal pain, gastrointestinal symptoms, new-onset atrial fibrillation, or weight loss; or if they have persistent and severe skin reactions, such as flushing or rashes 53.
Nicotinamide does not cause skin flushing and has fewer adverse effects than nicotinic acid, and these effects typically begin with much higher doses 51. Nausea, vomiting, and signs of liver toxicity can occur with nicotinamide intakes of 3,000 mg/day 4. In several small studies of participants undergoing hemodialysis, the most common adverse effects from 500-1,500 mg/day nicotinamide supplementation for several months were diarrhea and thrombocytopenia (low platelet count) 52, 57, 58, 59.
The Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine has established Tolerable Upper Intake Level (maximum daily intake unlikely to cause adverse health effects) for niacin that apply only to supplemental niacin for healthy infants, children, and adults 4. These Tolerable Upper Intake Levels (ULs) are based on the levels associated with skin flushing. The Food and Nutrition Board acknowledges that although excess nicotinamide does not cause flushing, a Tolerable Upper Intake Level for nicotinic acid based on flushing can prevent the potential adverse effects of nicotinamide 4. The Tolerable Upper Intake Level, therefore, applies to both forms of supplemental niacin. However, the Tolerable Upper Intake Level does not apply to individuals who are receiving supplemental niacin under medical supervision 4.
Niacin and its metabolites are rapidly excreted in urine. Following oral administration of single and multiple doses of an immediate-release (Niacor) or extended-release (Niaspan) niacin preparation, approximately 88 or 60-76% of the dose, respectively, was excreted in urine as unchanged drug and inactive metabolites 60.
Figure 8. Dietary precursors of nicotinamide adenine dinucleotide (NAD)
Footnote: Dietary precursors of nicotinamide adenine dinucleotide (NAD), including nicotinic acid, nicotinamide, and nicotinamide riboside, are collectively referred to as niacin or vitamin B3. The essential amino acid tryptophan can also be converted into NAD via the kynurenine pathway.
[Source 25 ]Figure 9. Nicotinamide adenine dinucleotide (NAD) synthesis
Footnote: Figure 9 illustrates the separate biosynthetic pathways that lead to nicotinamide adenine dinucleotide (NAD) production from the various dietary precursors. Nicotinamide adenine dinucleotide (NAD) is synthesized from nicotinamide and nicotinamide riboside via two enzymatic reactions, while the pathway that yields nicotinamide adenine dinucleotide (NAD) from nicotinic acid – known as the Preiss-Handler pathway — includes three steps 25. The kynurenine pathway is the longest nicotinamide adenine dinucleotide (NAD) biosynthetic pathway: the catabolism of tryptophan through kynurenine produces quinolinic acid, which is then converted to nicotinic acid mononucleotide, an intermediate in nicotinamide adenine dinucleotide (NAD) metabolism. Nicotinamide adenine dinucleotide (NAD) is then synthesized from nicotinic acid mononucleotide in the Preiss-Handler pathway 39.
[Source 25 ]Figure 10. Nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) functions
[Source 25 ]Figure 11. Overview of NAD biosynthesis and function
Footnotes: Overview of the NAD biosynthesis and function in humans. NAD can be synthesized from five precursors: tryptophan (Trp), the pyridine bases nicotinamide (Nam) and nicotinic acid (NA) or the nucleosides nicotinamide riboside (NR) and nicotinic acid riboside (NAR), which enter cells by different transport mechanisms. Quinolinic acid (QA), a Trp degradation product, is transformed to nicotinic acid mononucleotide (NAMN) by quinolinic acid phosphoribosyltransferase (QAPRT). Nicotinamide (Nam) and nicotinic acid (NA) are converted to the corresponding mononucleotides (NMN and NAMN) by nicotinamide phosphoribosyltransferase (NamPRT, also known as NAMPT) and nicotinic acid phosphoribosyltransferase (NAPRT), respectively. Nicotinamide mononucleotide (NMN) might also be synthesized by an extracellular NamPRT (eNAMPT). Nicotinamide mononucleotide (NMN) and nicotinic acid mononucleotide (NAMN) are also generated through phosphorylation of nicotinamide riboside (NR) and nicotinic acid riboside (NAR), respectively, by nicotinamide riboside kinases (NRK). Nicotinamide mononucleotide (NMN) and nicotinic acid mononucleotide (NAMN) are converted to the corresponding dinucleotide (NAAD or NAD+) by NMN adenylyltransferases (NMNAT). NAD synthetase (NADS) amidates NAAD to NAD+. Phosphorylation by NAD kinase (NADK) converts NAD+ to NADP+. The oxidized and reduced forms of the dinucleotides, NAD(P)+ and NAD(P)H, serve as reversible hydrogen carriers in redox reactions. Members of the Sirtuin family of protein deacetylases catalyze the transfer of the protein-bound acetyl group onto the ADP-ribose moiety, thereby forming O-acetyl-ADP ribose (OAcADPR). The transfer of a single (mono-ADP-ribosylation) or several (poly-ADP-ribosylation) ADP-ribose units from NAD+ to acceptor protein is catalyzed by diphtheria toxin-like ADP-ribosyltransferases (ARTD). Mono-ADP-ribosylation is also catalyzed by clostridial toxin-like ADP-ribosyltransferases (ARTC) and some Sirtuin proteins. NAD+ and NADP+ are also used for the synthesis of second messengers, nicotinic acid adenine dinucleotide phosphate (NAADP), cyclic ADP-ribose (cADPR) and ADPR, which mediate intracellular calcium mobilization. All the three molecules are synthesized by ecto-NAD glycohydrolases CD38 and CD157. The mechanism of how messengers reach their cytosolic targets is still debated. Signaling-independent interconversions of NAD and its intermediates include NAD hydrolysis to NMN and AMP by Nudix pyrophosphatases (NUDT); NMN dephosphorylation to nicotinamide riboside (NR) by cytosolic 5′-nucleotidases (5′-NT); phosphorolytic cleavage of nicotinamide riboside (NR) to nicotinamide (Nam) by purine nucleoside phosphorylase (PNP); and conversion of Nam to N-methylnicotinamide (1-MNA) by nicotinamide-N-methyltransferase (NNMT). NAD+ can possibly be released from cells through connexin 43 hemichannels (Cx43), and can be degraded to NR by ecto-nucleotidase CD73. Nicotinamide riboside (NR) is hydrolyzed to nicotinamide (Nam) by CD157. Whether cells can take up NAD or NMN is debated.
[Source 39 ]How much Vitamin B3 (Niacin) do I need?
The amount of Vitamin B3 or Niacin you need depends on your age and sex. Average daily recommended amounts are listed below in milligrams (mg) of niacin equivalents (NE) (except for infants in their first 6 months) 40. The mg niacin equivalents (NE) measure is used because your body can also make niacin from tryptophan, an amino acid in proteins. For example, when you eat turkey, which is high in tryptophan, some of this amino acid is converted to niacin in your liver. Using mg niacin equivalents (NE) accounts for both the niacin you consume and the niacin your body makes from tryptophan. Infants in their first six months do not make much niacin from tryptophan.
Table 1 lists the current Recommended Dietary Allowances (RDAs) for niacin as mg of niacin equivalents (NE) 4. The Food and Nutrition Board defines 1 NE as 1 mg niacin or 60 mg of the amino acid tryptophan (which the body can convert to niacin). Niacin RDAs for adults are based on niacin metabolite excretion data. For children and adolescents, niacin RDAs are extrapolated from adult values on the basis of body weight. The Adequate Intake (AI) for infants from birth to 6 months is for niacin alone, as young infants use almost all the protein they consume for growth and development; it is equivalent to the mean intake of niacin in healthy, breastfed infants. For infants aged 7-12 months, the Adequate Intake (AI) for niacin is in mg NE and is based on amounts consumed from breast milk and solid foods.
Most people in the United States get enough niacin from the foods they eat. Niacin deficiency is very rare in the United States 40. However, some people are more likely than others to have trouble getting enough niacin:
- Undernourished people with AIDS, alcohol use disorder, anorexia, inflammatory bowel disease, or liver cirrhosis
- People whose diet has too little iron, riboflavin, or vitamin B6; these nutrients are needed to convert tryptophan to niacin
- People with Hartnup disease, a rare genetic disorder
- People with carcinoid syndrome, a condition in which slow-growing tumors develop in the gastrointestinal tract
An analysis of data from the 2015–2016 National Health and Nutrition Examination Survey (NHANES) found that the average daily niacin intake from foods and beverages was 21.4 mg for ages 2–19 61. In adults, the average daily niacin intake from foods and beverages was 31.4 mg in men and 21.3 mg in women. An analysis of data from the 2009-2012 NHANES found that only 1% of adults had intakes of niacin from foods and beverages below the Estimated Average Requirements (the average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals) 62. Among all racial and ethnic groups, Hispanics had the greatest prevalence, 1.3%, of niacin intakes below the Estimated Average Requirement 63.
According to self-reported data from the 2013-2014 NHANES, 21% of all individuals aged 2 and older took a dietary supplement containing niacin 61. The proportion of users increased with age from 8% of those aged 12-19 years to 39% of men and 40% of women aged 60 and older. Supplement use doubled or tripled total niacin intakes compared with intakes from diet alone. According to data from the 2003-2006 NHANES, 10% of all individuals aged 2 and older who took dietary supplements had total niacin intakes that reached or exceeded the Tolerable Upper Intake Level (the maximum daily intake unlikely to cause adverse health effects) (see Table 2 below) 64.
Table 1. Recommended Dietary Allowances (RDAs) for Vitamin B3 (Niacin)
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months* | 2 mg |
| Infants 7–12 months* | 4 mg NE |
| Children 1–3 years | 6 mg NE |
| Children 4–8 years | 8 mg NE |
| Children 9–13 years | 12 mg NE |
| Teen boys 14–18 years | 16 mg NE |
| Teen girls 14–18 years | 14 mg NE |
| Adult men 19+ years | 16 mg NE |
| Adult women 19+ years | 14 mg NE |
| Pregnant teens and women | 18 mg NE |
| Breastfeeding teens and women | 17 mg NE |
Footnote: Recommended Dietary Allowance (RDA) is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
* Adequate Intake (AI) is intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an RDA.
[Source 40 ]Table 2. Tolerable Upper Intake Levels (ULs) for Vitamin B3 (Niacin)
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| Birth to 6 months | None established* | None established* | ||
| 7–12 months | None established* | None established* | ||
| 1–3 years | 10 mg | 10 mg | ||
| 4–8 years | 15 mg | 15 mg | ||
| 9–13 years | 20 mg | 20 mg | ||
| 14–18 years | 30 mg | 30 mg | 30 mg | 30 mg |
| 19+ years | 35 mg | 35 mg | 35 mg | 35 mg |
Footnote: * Breast milk, formula, and food should be the only sources of niacin for infants.
[Source 34 ]What foods provide Vitamin B3 (Niacin)?
Niacin is found naturally in many foods, and is added to some foods. You can get recommended amounts of niacin by eating a variety of foods, including the following:
- Animal based foods foods, such as poultry, beef, pork, and fish, provide about 5-10 mg niacin per serving, primarily in the highly bioavailable forms of NAD and NADP 10.
- Plant-based foods, such as nuts, legumes, and grains, provide about 2-5 mg niacin per serving, mainly as nicotinic acid.
- Some types of nuts, legumes, and grains. In some grain products, however, naturally present niacin is largely bound to polysaccharides and glycopeptides that make it only about 30% bioavailable 10, 3.
- Many breads, cereals, and infant formulas in the United States and many other countries contain added niacin. Niacin that is added to enriched and fortified foods is in its free form and therefore highly bioavailable 4.
Tryptophan is another food source of niacin because this amino acid—when present in amounts beyond that required for protein synthesis—can be converted to NAD, mainly in the liver 10, 47. The most commonly used estimate of efficiency for tryptophan conversion to NAD is 1:60 (i.e., 1 mg niacin [NAD] from 60 mg tryptophan). Turkey is an example of a food high in tryptophan; a 3-oz portion of turkey breast meat provides about 180 mg tryptophan, which could be equivalent to 3 mg niacin 34. However, the efficiency of the conversion of tryptophan to NAD varies considerably in different people 10.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing niacin arranged by nutrient content (https://www.nal.usda.gov/sites/www.nal.usda.gov/files/niacin.pdf).
Table 3. Vitamin B3 (Niacin) Content of Selected Foods
| Food | Milligrams (mg) per serving | Percent Daily Value (DV)** |
|---|---|---|
| Beef liver, pan fried, 3 ounces | 14.9 | 93 |
| Chicken breast, meat only, grilled, 3 ounces | 10.3 | 64 |
| Marinara (spaghetti) sauce, ready to serve, 1 cup | 10.3 | 64 |
| Turkey breast, meat only, roasted, 3 ounces | 10 | 63 |
| Salmon, sockeye, cooked, 3 ounces | 8.6 | 54 |
| Tuna, light, canned in water, drained, 3 ounces | 8.6 | 54 |
| Pork, tenderloin, roasted, 3 ounces | 6.3 | 39 |
| Beef, ground, 90% lean, pan-browned, 3 ounces | 5.8 | 36 |
| Rice, brown, cooked, 1 cup | 5.2 | 33 |
| Peanuts, dry roasted, 1 ounce | 4.2 | 26 |
| Breakfast cereals fortified with 25% DV niacin | 4 | 25 |
| Rice, white, enriched, cooked, 1 cup | 2.3 | 14 |
| Potato (russet), baked, 1 medium | 2.3 | 14 |
| Sunflower seeds, dry roasted, 1 ounce | 2 | 13 |
| Bread, whole wheat, 1 slice | 1.4 | 9 |
| Pumpkin seeds, dry roasted, 1 ounce | 1.3 | 8 |
| Soymilk, unfortified, 1 cup | 1.3 | 8 |
| Bread, white, enriched, 1 slice | 1.3 | 8 |
| Lentils, boiled and drained, ½ cup | 1 | 6 |
| Bulgur, cooked, 1 cup | 0.9 | 6 |
| Banana, 1 medium | 0.8 | 5 |
| Edamame, frozen, prepared, ½ cup | 0.7 | 4 |
| Raisins, ½ cup | 0.6 | 4 |
| Tomatoes, cherry, ½ cup | 0.5 | 3 |
| Broccoli, boiled, drained, chopped, ½ cup | 0.4 | 3 |
| Cashews, dry roasted, 1 ounce | 0.4 | 3 |
| Yogurt, plain, low fat, 1 cup | 0.3 | 2 |
| Apple, 1 medium | 0.2 | 1 |
| Chickpeas, canned, drained, 1 cup | 0.2 | 1 |
| Milk, 1% milkfat, 1 cup | 0.2 | 1 |
| Spinach, frozen, chopped, boiled, ½ cup | 0.2 | 1 |
| Tofu, raw, firm, ½ cup | 0.2 | 1 |
| Onions, chopped, ½ cup | 0.1 | 1 |
| Egg, large | 0 | 0 |
Footnotes: These values are for the niacin content of foods only. They do not include the contribution of tryptophan, some of which is converted to NAD in the body.
** DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed Daily Values (DVs) to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for niacin is 16 mg for adults and children aged 4 years and older 65. The FDA does not require food labels to list niacin content unless niacin has been added to the food. Foods providing 20% of more of the DV are considered to be high sources of a nutrient.
[Source 34 ]What kinds of Vitamin B3 (Niacin) supplements are available?
Vitamin B3 or Niacin is found in multivitamin or multivitamin-mineral supplements. Vitamin B3 or Niacin is also available in B-complex dietary supplements and supplements containing only niacin. The two main forms of niacin in dietary supplements are nicotinic acid and nicotinamide. Some niacin-only supplements contain 500 mg or more per serving, which is much higher than the Recommended Dietary Allowance (RDA) for this nutrient 34.Niacin (in the form of nicotinic acid) is also available as a prescription medicine used to treat high blood cholesterol levels.Nicotinic acid in supplemental amounts beyond nutritional needs can cause skin flushing, so some formulations are manufactured and labeled as prolonged, sustained, extended, or timed release to minimize this unpleasant side effect. Nicotinamide does not produce skin flushing because of its slightly different chemical structure 51. Niacin supplements are also available in the form of inositol hexanicotinate, and these supplements are frequently labeled as being “flush free” because they do not cause flushing. The absorption of niacin from inositol hexanicotinate varies widely but on average is 30% lower than from nicotinic acid or nicotinamide, which are almost completely absorbed 51, 66, 67. Two niacin-like compounds, nicotinamide riboside and nicotinamide mononucleotide (NMN; also referred to as β-NMN), are also available as dietary supplements, but are not marketed or labelled as sources of niacin 34. However, FDA ruled in November 2022 that nicotinamide mononucleotide (NMN) may not be legally marketed as a dietary supplement because it has been authorized for investigation by FDA as a new drug 68.
What happens if I don’t get enough Vitamin B3 Niacin?
You can develop niacin deficiency if you don’t get enough niacin or tryptophan from the foods you eat. Severe niacin deficiency leads to a disease called pellagra. Pellagra, which is uncommon in developed countries, can have these effects 40:
- Rough skin that turns red or brown in the sun
- A bright red tongue
- Vomiting, constipation, or diarrhea
- Depression
- Headaches
- Extreme tiredness
- Aggressive, paranoid, or suicidal behavior
- Hallucinations, apathy, loss of memory
In its final stages, pellagra leads to loss of appetite followed by death.
What causes pellagra
Niacin deficiency or pellagra is caused by having too little niacin or tryptophan in your diet. It can also occur if your body fails to absorb niacin or tryptophan.
Half of your body’s niacin requirement comes from dietary intake of niacin. The other half is synthesized in your body from the amino acid tryptophan. Niacin is found in many animal products (as nicotinamide) and plants (as nicotinic acid). A varied diet including milk, eggs, red meat, poultry, fish, peanuts, legumes, and seeds provides sufficient niacin and tryptophan.
Niacin deficiency or pellagra may also develop due to 69, 70, 71, 72:
- Primary pellagra results from inadequate niacin (vitamin B-3) and/or tryptophan in the diet (mainly in developing countries or poverty stricken areas)
- Secondary pellagra occurs when there is enough niacin in the diet but something prevents its absorption and processing. Causes of secondary pellagra include:
- Excessive alcohol use or chronic alcoholism
- Prolonged diarrhea
- Gastrointestinal diseases such as ulcerative colitis and Crohn disease
- Liver cirrhosis
- Anorexia nervosa
- Weight loss (bariatric) surgery
- Gastric cancer surgery
- Gastrectomy
- Carcinoid syndrome (group of symptoms associated with carcinoid tumors of the small intestine, colon, appendix, and bronchial tubes in the lungs)
- Hartnup disease (tryptophan metabolism disorder)
- HIV infection
- Tuberculosis of the gastrointestinal tract
- Hepatic cirrhosis
- Drugs e.g. isoniazid, azathioprine, pyrazinamide, 6-mercaptopurine, 5-fluorouracil, phenytoin, hydantoin, phenobarbital, chloramphenicol, and ethionamide
- Diet rich in leucine
Pellagra is most common among poor and food-limited populations. The disease is more common in parts of the world (such as certain parts of Africa) where people have a lot of untreated corn in their diet. Corn is a poor source of tryptophan, and niacin in corn is tightly bound to other components of the grain. Niacin is released from corn if soaked in limewater overnight. This method is used to cook tortillas in Central America where pellagra is rare. Pellagra is rare in the United States and may be associated with severe alcoholism or medical causes of malnutrition.
Groups at Risk of Niacin deficiency
Niacin inadequacy usually arises from insufficient intakes of foods containing niacin and tryptophan. It can also be caused by factors that reduce the conversion of tryptophan to niacin, such as low intakes of other nutrients 4, 12. The following groups are among those most likely to have inadequate niacin status.
People with undernutrition
People who are undernourished because they live in poverty or have anorexia, alcohol use disorder, AIDS, inflammatory bowel disease, or liver cirrhosis often have inadequate intakes of niacin and other nutrients 4, 12, 15, 5.
People with inadequate riboflavin (vitamin B2), pyridoxine (vitamin B6) and/or iron intakes
People who do not consume enough riboflavin (vitamin B2), pyridoxine (vitamin B6), or iron convert less tryptophan to niacin because enzymes in the metabolic pathway for this conversion depend on these nutrients to function 4, 12.
People with Hartnup disease
Hartnup disease is a rare genetic disorder caused by mutations in the SLC6A19 gene and is inherited in an autosomal recessive manner 73. The SLC6A19 gene produces a protein known as an amino acid transporter, which serves to assist the movement (or transport) of specific amino acids within the body. The amino acid transporter protein is especially active within the kidneys and the intestines, although these organs are otherwise unaffected and function normally. The amino acids affected include tryptophan, alanine, asparagine, glutamine, histidine, isoleucine, leucine, phenylalanine, serine, threonine, tyrosine, and valine 73. Hartnup disease interferes with the absorption of tryptophan in the small intestine and increases its loss in the urine via the kidneys 73, 4, 15. As a result, the body has less available tryptophan to convert to niacin.
The symptoms of Hartnup disease vary greatly from one person to another. The majority of affected individuals do not have any apparent symptoms (asymptomatic). When symptoms do develop, they most often occur between the ages of 3-9. In rare instances, symptoms first appear in adulthood.
The most common symptom are red, scaly light-sensitive (photosensitive) rashes on the face, arms, extremities, and other exposed areas of skin.
A wide variety of neurological abnormalities can occur including sudden episodes of impaired muscle coordination (ataxia), an unsteady walk (gait), impaired articulation of speech (dysarthria), occasional tremors of the hands and tongue, and spasticity, a condition marked by increased muscle tone and stiffness of the muscles, particularly those of the legs.
There have been reports of delayed cognitive development and, in rare instances, mild intellectual disability in some children. It is, however, unclear whether these symptoms are related to Hartnup disorder or incidentally occurred in the same individual and were therefore attributed to Hartnup disorder. Similarly, seizures, fainting, trembling, lack of muscle tone (hypotonia), headaches, dizziness and/or vertigo, and delays in motor development have been observed but may be unrelated. Some affected individuals may experience psychiatric abnormalities including emotional instability such as rapid mood changes, depression, confusion, anxiety, delusions, and/or hallucinations.
Some children experience growth delays and may be shorter than would be expected based upon age and gender (short stature). In some instances, the eyes may be affected and individuals may experience double vision (diplopia), involuntary rhythmic movements of the eyes (nystagmus), and droopy upper eyelids (ptosis).
Diarrhea may precede or follow an episode of this disorder. Some adults with Hartnup disease have been reported whose initial symptom was the onset of seizures during adulthood. Heartburn has been reported in adults with the disorder.
Hartnup disease has a good prognosis with treatment and symptoms tend to improve with age.
People with carcinoid syndrome
Carcinoid syndrome is caused by slow-growing tumors in the gastrointestinal tract that release serotonin and other substances. It is characterized by facial flushing, diarrhea, and other symptoms. In those with carcinoid syndrome, tryptophan is preferentially oxidized to serotonin and not metabolized to niacin 4. As a result, the body has less available tryptophan to convert to niacin.
Pellagra prevention
Pellagra can be prevented by following a well-balanced diet.
Get treated for health problems that may cause pellagra.
Pellagra symptoms
Symptoms of pellagra or niacin deficiency include 44:
- Delusions or mental confusion
- Diarrhea
- Weakness
- Loss of appetite
- Pain in abdomen
- Inflamed mucous membrane
- Scaly skin sores, especially in sun-exposed areas of the skin
The most common symptoms of niacin deficiency involve the skin, the digestive system, and the nervous system 25. The symptoms of pellagra are commonly referred to as the 4 “Ds”: sun-sensitive dermatitis, diarrhea, and dementia. A fourth “D,” death, occurs if pellagra is left untreated 74. In the skin, a thick, scaly, darkly pigmented rash develops symmetrically in areas exposed to sunlight. In fact, the word “pellagra” comes from “pelle agra,” the Italian phrase for rough skin. Symptoms related to the digestive system include inflammation of the mouth and tongue (“bright red tongue”), vomiting, constipation, abdominal pain, and ultimately, diarrhea. Gastrointestinal disorders and diarrhea contribute to the ongoing malnourishment of the patients. Neurologic symptoms include headache, apathy, fatigue, depression, disorientation, and memory loss and are more consistent with delirium than with the historically described dementia 8. Disease presentations vary in appearance since the classic triad rarely presents in its entirety. The absence of dermatitis, for example, is known as pellagra sine pellagra 25.
Skin
- The first sign is reddened skin with superficial scaling in areas exposed to sunlight, heat and friction. This may resemble severe sunburn then gradually subsides leaving a dusky brown-red coloration
- The rash is usually symmetrical with a clear edge between affected and unaffected skin
- There may be itching or a burning sensation
- In time the skin becomes thick, hard, scaly and cracked. Bleeding may result in blackened crusts
- Lesions may occur anywhere on the body especially the hands, arms, lower legs, feet, face, and neck (known as Casal’s necklace)
- Lips, tongue and gums may be sore and peeling
Dermatitis begins as erythema, resembles sunburn in the initial stages, but tanning occurs more slowly than typically seen in sunburn. The lesion is a bilaterally symmetrical eruption located at sites of sunlight exposure 75. Cutaneous patches on the neck are known as Casal necklace. It extends as a broad collar around the cervical dermatomes C3 and C4 in the neck.
Gastrointestinal
- Diarrhea occurs in 50% of cases
- Poor appetite, abdominal pain, nausea and vomiting are common
- It may be difficult to eat and drink, leading to further malnutrition
Gastrointestinal disturbances include diarrhea, nausea, vomiting, epigastric discomfort, poor appetite, abdominal pain, and increased salivation. Stools are typically watery but can be bloody or mucoid occasionally.
Neurological
- Initially symptoms of apathy and slight depression may go unnoticed
- Other symptoms include headache, confusion, irritability, restlessness, anxiety, tremor, delusions, disorientation and psychosis
- Patients eventually become stuporous, comatose and may die
Neurologic manifestation presents as nonspecific symptoms like confusion, hallucinations, irritability, psychomotor unrest, ataxia, and depression. As the disease advances, patients become confused, disoriented and delirious, then comatose and stuporous, and finally die.
Pellagra complication
Left untreated, niacin deficiency or pellagra can result in malnutrition and cachexia (wasting of the body), nerve damage, particularly in the brain. Progression of the neuropsychiatric symptoms can lead to delusions, hallucinations, psychosis, and eventually coma and death 5. Skin sores may become infected.
Pellagra diagnosis
Niacin deficiency or pellagra diagnosis is a clinical diagnosis and biochemical testing is rarely used 30. Investigations such as blood tests and skin biopsy are not diagnostic but may be used to exclude other diagnoses 76.
Pellagra diagnosis is challenging in the absence of the skin lesions and is more easily made if characteristic skin lesions are present and rapid response to oral niacin supplementation. The skin eruption is characteristically a photosensitive rash affecting the dorsal surfaces of the hands, face, neck, arms, and feet. In the acute phase it resembles sunburn with erythema and bullae (wet pellagra), but progresses to a chronic, symmetric, scaly rash that exacerbates following re-exposure to sunlight. The typical locations are neck (Casal necklace) and hands and forearms (pellagra gauntlet). As the disease advances, neuropsychiatric symptoms supervene and include photophobia, asthenia, depression, and memory loss that can evolve to frank psychosis and even death if the disease is not identified and treated 77.
Although the skin is inevitably the key to the diagnosis of pellagra, usually the first symptoms are gastrointestinal, with anorexia, abdominal pain, vomiting, and later watery diarrhea, accompanied by lassitude and irritability 78.
If needed, niacin deficiency can be assessed by two methods 79, 70, 5:
- Biochemical assessment. This approach is not widely used. Measurement of urinary N-methylnicotinamide or erythrocyte NAD: NADP (ratio) can be obtained to evaluate the metabolic rate of niacin in the body. The combined urinary excretion of N methylnicotinamide and pyridone of less than 1.5 mg in 24 hours indicates severe niacin deficiency. Low urinary levels of N-methylnicotinamide and pyridone suggest niacin deficiency and support the diagnosis of pellagra 80.
- Low serum niacin, tryptophan, NAD, and NADP levels can reflect niacin deficiency and confirm the diagnosis of pellagra.
- Blood counts (anemia), findings of hypoproteinemia, higher levels of serum calcium and lower levels of serum kalium and phosphorus, liver function test results, and serum porphyrin levels can help in diagnosing pellagra.
- Clinical assessment. It is more widely used by assessing for diarrhea, glossitis, and dermatitis. The skin is assessed for hyperpigmentation, dryness and scaling, facial butterfly sign, and/or Casal’s collar (necklace) in sun-exposed areas. It is important to note that diarrhea and dementia may not always be present.
The rapid response to niacin supplementation usually confirms the diagnosis.
Hartnup disease is diagnosed on the detection of neutral amino acids in the urine. Molecular genetic testing can confirm a diagnosis of Hartnup disease in some cases. Molecular genetic testing can detect genetic alterations in the SLC19A6 gene known to cause the disorder, but usually is not necessary to obtain a diagnosis 73.
Pellagra treatment
Pellagra can be effectively cured with intravenous or oral niacin or nicotinamide. The adult dose is nicotinamide 100 mg orally, every 6 hours for several days until relief of acute symptoms, followed by 50 mg 8 to 12 hourly until all skin lesions heal 1. In severe cases (marked neurological or gastrointestinal tract symptoms), 1 g 3 to 4 times a day can be given, initially by the parenteral route 1. For children, one can use 10 to 50 mg orally every 6 hours until symptoms of pellagra resolve 1. For mild endemic pellagra, smaller doses such as 10 mg per day can be used 1. Therapy should include other B vitamins, magnesium, and zinc as well as a calorie-rich diet. Sustained-release (SR) formulations have been developed which are available over-the-counter. Sustained-release niacin can be administered once daily and is less likely to cause flushing.
Maximum tolerated dose of niacin 1
- Immediate-Release formulation: 6 g/day in 2 to 3 divided doses
- Sustained-Release formulation: 2000 mg per day 81
An improvement in primary pellagra should be seen within two days of commencing treatment. A high protein diet supplemented with B-group vitamins is needed for complete recovery. Secondary pellagra may be harder to treat in view of its possible causes.
Skin lesions may be treated with topical emollients may reduce discomfort due to skin lesions 82. Topically zinc oxide and para-aminobenzoic acid ointment may be advised. Sun protection is important during the recovery phase. Cover up and apply a broad spectrum sunscreen to all exposed areas daily.
Individuals with Hartnup disease who do not develop symptoms will usually not require any treatment 73. Low protein diets (vegan or similar) may trigger symptomatic episodes, which can be reduced or avoided by maintaining good nutrition including a high protein diet, avoiding excess exposure the sun, and avoiding certain drugs such as sulphonamide drugs 73. Supplementing the diet with nicotinamide or niacin is also of benefit in preventing Hartnup disease episodes 73. In some instances, during a symptomatic episode, treatment with nicotinamide may be recommended. According to the medical literature, at least one individual showed an improvement of symptoms after treatment with the compound L-tryptophan ethyl ester, which restored tryptophan levels in both the serum and cerebrospinal fluid 73. Other treatment is symptomatic and supportive. Genetic counseling may be helpful for affected families.
Niacin side effects
Flushing
Niacin causes vasodilation of small subcutaneous blood vessels mediated by prostaglandin D2 that leads to a cutaneous flush, accompanied by an uneasy sensation of pruritus and warmth. Severe flushing may lead to hypotension and dizziness. Flushing appears earlier after dosing with immediate-release niacin (approximately 30 minutes) and delayed for sustained-release niacin (2 to 4 hours). Patients should avoid hot showers immediately after a dose, and if necessary, aspirin or ibuprofen is helpful.
Peptic Ulcer Disease
Niacin therapy may aggravate peptic ulcer disease. Niacin should be used cautiously with active or chronic gastrointestinal disorders.
Hepatotoxicity
The most serious adverse effect is niacin hepatotoxicity. Mild increase in hepatic transaminase levels up to twice the upper limit of the normal range is common.
Hyperglycemia
Decreased glucose tolerance and hyperglycemia can occur in individuals with diabetes and is thought to result from insulin resistance consequent to the free fatty acid rebound after moderately-sized doses.
Arrhythmia
Patients with supraventricular tachycardias (SVTs) may experience unusual chest sensations and palpitations even when the SVTs are controlled via concomitant antiarrhythmic therapy.
Eye Symptoms
Retinal edema or toxic amblyopia resulting in blurred vision is also reported 83.
Pellagra prognosis
Diarrhea and glossitis are the first to improve within days usually improve in 2 to 3 days, while recovery from dementia and dermatitis is seen within 7 days of treatment 84, 85, 70. The skin changes typically resolve within two weeks. However, a longer recovery may be seen in chronic cases 86.
- Peechakara BV, Gupta M. Vitamin B3. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526107[↩][↩][↩][↩][↩][↩]
- Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004 Jan;43(1):1-5. https://www.ncbi.nlm.nih.gov/pubmed/14693013[↩]
- Bourgeois C, Moss J. Niacin. In: Coates PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD, eds. Encyclopedia of Dietary Supplements, 2nd ed. New York, NY: Informa Healthcare; 2010:562-9.[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press, 1998.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Savvidou S. Pellagra: a non-eradicated old disease. Clin Pract. 2014 Apr 28;4(1):637. doi: 10.4081/cp.2014.637[↩][↩][↩][↩][↩][↩][↩][↩]
- Pellagra and its prevention and control in major emergencies. https://apps.who.int/iris/rest/bitstreams/63163/retrieve[↩][↩][↩][↩]
- Russel RM. Vitamin and trace mineral deficiency and excess. : Kasper DL, Braunwald E, Fauci AS, et al., Harrison’s principles of internal medicine. 16th ed New York: McGraw-Hill; 2005. pp 404-405[↩][↩][↩]
- Oldham MA, Ivkovic A. Pellagrous encephalopathy presenting as alcohol withdrawal delirium: a case series and literature review. Addict Sci Clin Pract. 2012 Jul 6;7(1):12. doi: 10.1186/1940-0640-7-12[↩][↩][↩][↩][↩][↩]
- A. MacDonald , A. Forsyth, Nutritional deficiencies and the skin, Clinical and Experimental Dermatology, Volume 30, Issue 4, 1 July 2005, Pages 388–390, https://doi.org/10.1111/j.1365-2230.2005.01787.x[↩][↩]
- Kirkland JB. Niacin. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease, 11th ed. Baltimore, MD: Williams & Wilkins; 2014:331-40.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Pipili C, Cholongitas E, Ioannidou D. The diagnostic importance of photosensitivity dermatoses in chronic alcoholism: report of two cases. Dermatol Online J. 2008 Nov 15;14(11):15.[↩][↩]
- Li, R., Yu, K., Wang, Q., Wang, L., Mao, J. and Qian, J. (2016), Pellagra Secondary to Medication and Alcoholism. Nutrition in Clinical Practice, 31: 785-789. https://doi.org/10.1177/0884533616660991[↩][↩][↩][↩][↩]
- Kirkland JB. Niacin. In: Zempleni J, Suttie JW, Gregory III JF, Stover PJ, eds. Handbook of Vitamins. 5th ed. Boca Raton: CRC Press; 2013:149-190.[↩][↩]
- Gregory JF 3rd. Nutritional Properties and significance of vitamin glycosides. Annu Rev Nutr. 1998;18:277-96. doi: 10.1146/annurev.nutr.18.1.277[↩]
- Crook MA. The importance of recognizing pellagra (niacin deficiency) as it still occurs. Nutrition. 2014 Jun;30(6):729-30. doi: 10.1016/j.nut.2014.03.004[↩][↩][↩]
- Jagielska G, Tomaszewicz-Libudzic EC, Brzozowska A. Pellagra: a rare complication of anorexia nervosa. Eur Child Adolesc Psychiatry. 2007 Oct;16(7):417-20. doi: 10.1007/s00787-007-0613-4[↩]
- Dawson, B., Favaloro, E.J., Taylor, J. and Aggarwal, A. (2006), Unrecognized pellagra masquerading as odynophagia. Internal Medicine Journal, 36: 472-474. https://doi.org/10.1111/j.1445-5994.2006.01108.x[↩]
- Kertesz SG. Pellagra in 2 homeless men. Mayo Clin Proc. 2001 Mar;76(3):315-8. doi: 10.4065/76.3.315[↩]
- Prakash R, Gandotra S, Singh LK, Das B, Lakra A. Rapid resolution of delusional parasitosis in pellagra with niacin augmentation therapy. Gen Hosp Psychiatry. 2008 Nov-Dec;30(6):581-4. doi: 10.1016/j.genhosppsych.2008.04.011[↩]
- Majewski M, Kozlowska A, Thoene M, Lepiarczyk E, Grzegorzewski WJ. Overview of the role of vitamins and minerals on the kynurenine pathway in health and disease. J Physiol Pharmacol. 2016 Feb;67(1):3-19.[↩]
- Abdulla A.-B. Badawy, Pellagra and Alcoholism: A Biochemical Perspective, Alcohol and Alcoholism, Volume 49, Issue 3, May/June 2014, Pages 238–250, https://doi.org/10.1093/alcalc/agu010[↩][↩]
- Rosmaninho A, Sanches M, Fernandes IC, Pinto-Almeida T, Vilaça S, Oliveira A, Selores M. Letter: Pellagra as the initial presentation of Crohn disease. Dermatol Online J. 2012 Apr 15;18(4):12.[↩]
- Zaraa, I., Belghith, I., EI Euch, D., Karoui, S., Mokni, M., Fillali, A. and Ben Osman, A. (2013), A Case of Pellagra Associated With Megaduodenum in a Young Woman. Nutrition in Clinical Practice, 28: 218-222. https://doi.org/10.1177/0884533612464783[↩]
- Bilgili SG, Karadag AS, Calka O, Altun F. Isoniazid-induced pellagra. Cutan Ocul Toxicol. 2011 Dec;30(4):317-9. doi: 10.3109/15569527.2011.574303[↩]
- Niacin. https://lpi.oregonstate.edu/mic/vitamins/niacin[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Dreizen S, McCredie KB, Keating MJ, Andersson BS. Nutritional deficiencies in patients receiving cancer chemotherapy. Postgrad Med. 1990 Jan;87(1):163-7, 170. doi: 10.1080/00325481.1990.11704531[↩]
- Nogueira A, Duarte AF, Magina S, Azevedo F. Pellagra associated with esophageal carcinoma and alcoholism. Dermatol Online J. 2009 May 15;15(5):8.[↩]
- Paudel V, Chudal D. Classical pellagra, the disease of 4 Ds, the forgotten entity. Pan Afr Med J. 2020 Jul 27;36:219. doi: 10.11604/pamj.2020.36.219.24806[↩]
- Segula D, Banda P, Mulambia C, Kumwenda JJ. Case report–A forgotten dermatological disease. Malawi Med J. 2012 Mar;24(1):19-20. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3588195[↩]
- Mousa TY, Mousa OY. Nicotinic Acid Deficiency. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557749[↩][↩]
- Nogueira, A., Duarte, A. F, Magina, S., & Azevedo, F. (2009). Pellagra associated with esophageal carcinoma and alcoholism. Dermatology Online Journal, 15(5). Retrieved from https://escholarship.org/uc/item/5z50c578[↩][↩][↩]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press (US); 1998. Available from: https://www.ncbi.nlm.nih.gov/books/NBK114310/ doi: 10.17226/6015[↩]
- Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016 Oct 10;7:12948. doi: 10.1038/ncomms12948[↩][↩]
- Niacin. https://ods.od.nih.gov/factsheets/Niacin-HealthProfessional[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Penberthy WT, Kirkland JB. Niacin. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition, 10th ed. Washington, DC: Wiley-Blackwell; 2012:293-306.[↩][↩][↩][↩]
- Peechakara BV, Gupta M. Vitamin B3. [Updated 2022 Jun 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526107[↩][↩]
- Nicotinic acid. http://www.hmdb.ca/metabolites/HMDB01488[↩]
- Organization for Economic Cooperation and Development; Screening Information Data Set for 3-Pyridinecarboxamide (Nicotinamide) CAS #: 98-92-0 p.40 (2002). Available from, as of February 20, 2007: http://www.inchem.org/pages/sids.html[↩]
- Nikiforov A, Kulikova V, Ziegler M. The human NAD metabolome: Functions, metabolism and compartmentalization. Crit Rev Biochem Mol Biol. 2015;50(4):284-97. doi: 10.3109/10409238.2015.1028612[↩][↩][↩][↩]
- Niacin. https://ods.od.nih.gov/factsheets/Niacin-Consumer[↩][↩][↩][↩][↩]
- McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1715[↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Niacin. [Updated 2020 Jul 9]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548176[↩][↩][↩]
- Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 483 (2005[↩]
- Pellagra. https://medlineplus.gov/ency/article/000342.htm[↩][↩]
- Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1022[↩]
- McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3622[↩]
- Gibson, RS. Principles of Nutritional Assessment, Second Edition. New York: Oxford University Press. Copyright 2005.[↩][↩][↩]
- Jacobson EL, Jacobson MK. Tissue NAD as a biochemical measure of niacin status in humans. Methods Enzymol. 1997;280:221-30. doi: 10.1016/s0076-6879(97)80113-9[↩]
- Shah GM, Shah RG, Veillette H, Kirkland JB, Pasieka JL, Warner RR. Biochemical assessment of niacin deficiency among carcinoid cancer patients. Am J Gastroenterol. 2005 Oct;100(10):2307-14. doi: 10.1111/j.1572-0241.2005.00268.x[↩]
- Fu CS, Swendseid ME, Jacob RA, McKee RW. Biochemical markers for assessment of niacin status in young men: levels of erythrocyte niacin coenzymes and plasma tryptophan. J Nutr. 1989 Dec;119(12):1949-55. doi: 10.1093/jn/119.12.1949[↩]
- Douglas MacKay and others, Niacin: chemical forms, bioavailability, and health effects, Nutrition Reviews, Volume 70, Issue 6, 1 June 2012, Pages 357–366, https://doi.org/10.1111/j.1753-4887.2012.00479.x[↩][↩][↩][↩][↩][↩][↩]
- Clara Minto and others, Definition of a tolerable upper intake level of niacin: a systematic review and meta-analysis of the dose-dependent effects of nicotinamide and nicotinic acid supplementation, Nutrition Reviews, Volume 75, Issue 6, June 2017, Pages 471–490, https://doi.org/10.1093/nutrit/nux011[↩][↩][↩]
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014 Jul 1;63(25 Pt B):2889-934. doi: 10.1016/j.jacc.2013.11.002. Epub 2013 Nov 12. Erratum in: J Am Coll Cardiol. 2014 Jul 1;63(25 Pt B):3024-3025. Erratum in: J Am Coll Cardiol. 2015 Dec 22;66(24):2812.[↩][↩][↩]
- McKenney JM, Proctor JD, Harris S, Chinchili VM. A Comparison of the Efficacy and Toxic Effects of Sustained- vs Immediate-Release Niacin in Hypercholesterolemic Patients. JAMA. 1994;271(9):672–677. doi:10.1001/jama.1994.03510330050033[↩]
- McKenney J. New perspectives on the use of niacin in the treatment of lipid disorders. Arch Intern Med. 2004 Apr 12;164(7):697-705. doi: 10.1001/archinte.164.7.697[↩]
- Leung K, Quezada M, Chen Z, Kanel G, Kaplowitz N. Niacin-Induced Anicteric Microvesicular Steatotic Acute Liver Failure. Hepatol Commun. 2018 Sep 25;2(11):1293-1298. doi: 10.1002/hep4.1253[↩]
- Shahbazian H, Zafar Mohtashami A, Ghorbani A, Abbaspour MR, Belladi Musavi SS, Hayati F, Lashkarara GR. Oral nicotinamide reduces serum phosphorus, increases HDL, and induces thrombocytopenia in hemodialysis patients: a double-blind randomized clinical trial. Nefrologia. 2011;31(1):58-65. doi: 10.3265/Nefrologia.pre2010.Nov.10734[↩]
- Takahashi Y, Tanaka A, Nakamura T, Fukuwatari T, Shibata K, Shimada N, Ebihara I, Koide H. Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int. 2004 Mar;65(3):1099-104. doi: 10.1111/j.1523-1755.2004.00482.x[↩]
- Cheng SC, Young DO, Huang Y, Delmez JA, Coyne DW. A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin J Am Soc Nephrol. 2008 Jul;3(4):1131-8. doi: 10.2215/CJN.04211007[↩]
- McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1716[↩]
- Food Surveys Research Group: Beltsville, MD. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/[↩][↩]
- Blumberg JB, Frei BB, Fulgoni VL, Weaver CM, Zeisel SH. Impact of Frequency of Multi-Vitamin/Multi-Mineral Supplement Intake on Nutritional Adequacy and Nutrient Deficiencies in U.S. Adults. Nutrients. 2017 Aug 9;9(8):849. doi: 10.3390/nu9080849[↩]
- Blumberg JB, Frei B, Fulgoni VL III, Weaver CM, Zeisel SH. Contribution of Dietary Supplements to Nutritional Adequacy in Race/Ethnic Population Subgroups in the United States. Nutrients. 2017 Nov 28;9(12):1295. doi: 10.3390/nu9121295[↩]
- Fulgoni VL 3rd, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J Nutr. 2011 Oct;141(10):1847-54. doi: 10.3945/jn.111.142257[↩]
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels. https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels[↩]
- Keenan JM. Wax-matrix extended-release niacin vs inositol hexanicotinate: a comparison of wax-matrix, extended-release niacin to inositol hexanicotinate “no-flush” niacin in persons with mild to moderate dyslipidemia. J Clin Lipidol. 2013 Jan-Feb;7(1):14-23. doi: 10.1016/j.jacl.2012.10.004[↩]
- Norris, R.B. (2006), “Flush-Free Niacin”: Dietary Supplement May Be “Benefit-Free”. Preventive Cardiology, 9: 64-65. https://doi.org/10.1111/j.1520-037X.2006.04736.x[↩]
- B-Nicotinamide Mononucleotide (NMN) from Inner Mongolia Kingdomway Pharmaceutical Limited. https://www.regulations.gov/document/FDA-2022-S-0023-0051[↩]
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: Dermatitis, dementia, and diarrhoea. Int J Dermatol 2004;43:1–5. 10.1111/j.1365-4632.2004.01959.x[↩]
- Terada N, Kinoshita K, Taguchi S, Tokuda Y. Wernicke encephalopathy and pellagra in an alcoholic and malnourished patient. BMJ Case Rep. 2015 Oct 21;2015:bcr2015209412. doi: 10.1136/bcr-2015-209412[↩][↩][↩]
- Karthikeyan, K. and Thappa, D.M. (2002), Pellagra and skin. International Journal of Dermatology, 41: 476-481. https://doi.org/10.1046/j.1365-4362.2002.01551.x[↩]
- Okan, G., Yaylaci, S. and Alzafer, S. (2009), Pellagra: will we see it more frequently?. Journal of the European Academy of Dermatology and Venereology, 23: 365-366. https://doi.org/10.1111/j.1468-3083.2008.02883.x[↩]
- Hartnup Disease. https://rarediseases.org/rare-diseases/hartnup-disease[↩][↩][↩][↩][↩][↩][↩][↩]
- Penberthy WT, Kirkland JB. Niacin. In: Erdman JW, MacDonald I, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Ames: International Life Sciences Institute; 2012:293-306.[↩]
- Isaac S. The “gauntlet” of pellagra. Int. J. Dermatol. 1998 Aug;37(8):599.[↩]
- Pellagra. https://dermnetnz.org/topics/pellagra[↩]
- Pipili C, Cholongitas E, Ioannidou D. The diagnostic importance of photosensivity dermatoses in chronic alcoholism: Report of two cases. Dermatology Online Journal 14 (11): 15.[↩]
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia and diarrhea. Int J Dermatol 2004, 43, 1-5.[↩]
- Pitche PT. Pellagre et érythèmes pellagroïdes [Pellagra]. Sante. 2005 Jul-Sep;15(3):205-8.[↩]
- Jen M, Shah KN, Yan AC. Cutaneous changes in nutritional disease in Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffel DJ. Fitzpatrick’s Dermatology in General Medicine, 7th ed, McGraw Hill 2008 USA. Pp 1209-1211 [↩]
- Kashyap ML, McGovern ME, Berra K, Guyton JR, Kwiterovich PO, Harper WL, Toth PD, Favrot LK, Kerzner B, Nash SD, Bays HE, Simmons PD. Long-term safety and efficacy of a once-daily niacin/lovastatin formulation for patients with dyslipidemia. Am. J. Cardiol. 2002 Mar 15;89(6):672-8.[↩]
- Pownall HJ, Jackson RL, Roth RI, Gotto AM, Patsch JR, Kummerow FA. Influence of an atherogenic diet on the structure of swine low density lipoproteins. J. Lipid Res. 1980 Nov;21(8):1108-15.[↩]
- McKenney JM, Proctor JD, Harris S, Chinchili VM. A comparison of the efficacy and toxic effects of sustained- vs immediate-release niacin in hypercholesterolemic patients. JAMA. 1994 Mar 02;271(9):672-7.[↩]
- Segula D, Banda P, Mulambia C, Kumwenda JJ. Case report–A forgotten dermatological disease. Malawi Med J. 2012 Mar;24(1):19-20. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3588195/[↩]
- Prousky JE. Pellagra may be a rare secondary complication of anorexia nervosa: a systematic review of the literature. Altern Med Rev. 2003 May;8(2):180-5.[↩]
- Matapandeu G, Dunn SH, Pagels P. An Outbreak of Pellagra in the Kasese Catchment Area, Dowa, Malawi. Am J Trop Med Hyg. 2017 May;96(5):1244-1247. doi: 10.4269/ajtmh.16-0423[↩]