Contents
- What is pelvic inflammatory disease
- Can pelvic inflammatory disease be cured?
- What can happen if pelvic inflammatory disease is not treated?
- Who gets pelvic inflammatory disease?
- How do you get pelvic inflammatory disease?
- Pelvic inflammatory disease signs and symptoms
- Pelvic inflammatory disease causes
- Pelvic inflammatory disease prevention
- Pelvic inflammatory disease diagnosis
- Pelvic inflammatory disease treatment
What is pelvic inflammatory disease
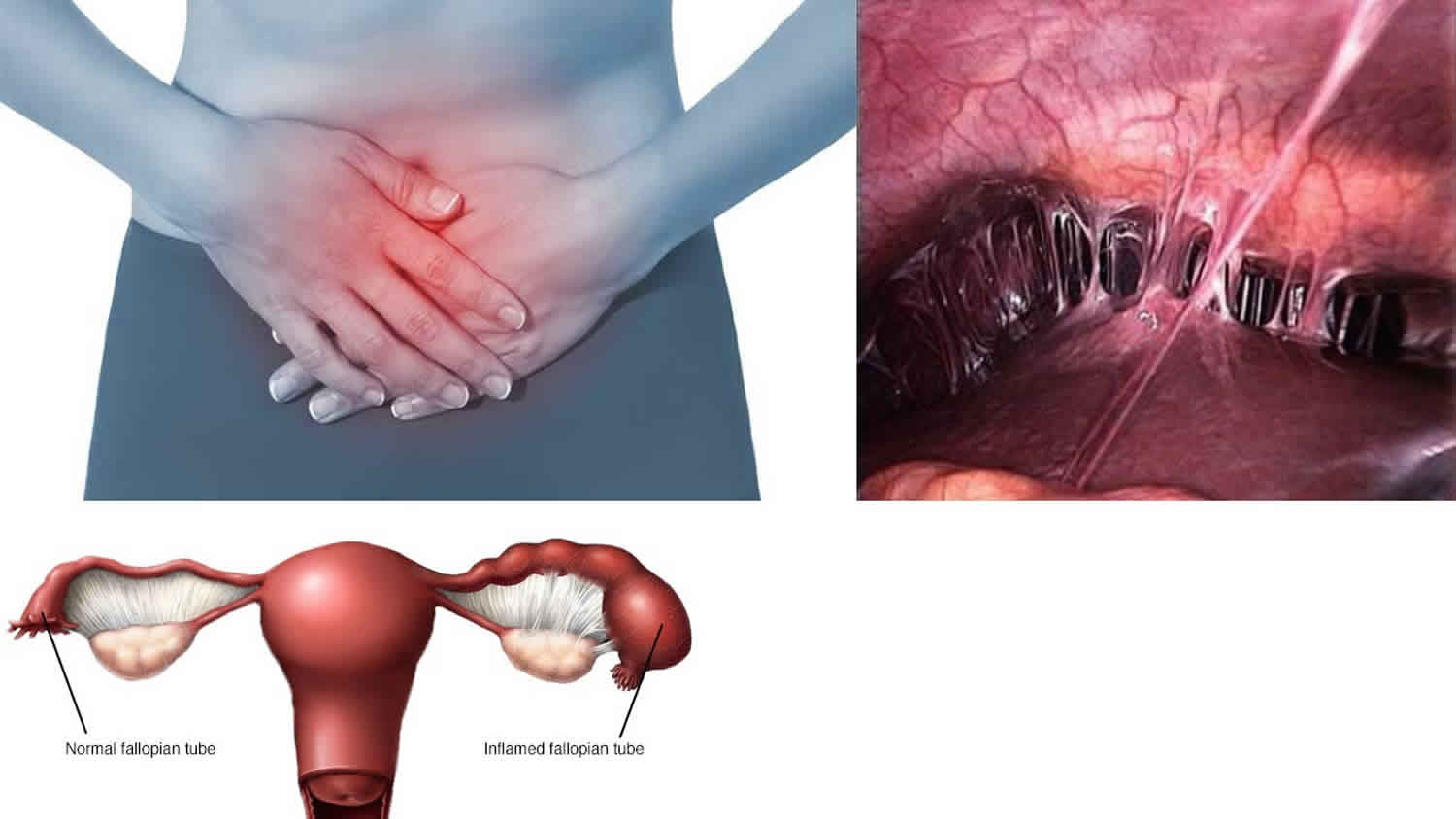
Pelvic inflammatory disease (PID) is an infection of a woman’s reproductive organs. The reproductive organs include the uterus (womb), fallopian tubes (uterine tubes), ovaries, and cervix. In 2013, about 88,000 women ages 15–44 in the United States were diagnosed with Pelvic inflammatory disease 1. Pelvic inflammatory disease is often caused by a sexually transmitted infection (STI). If left untreated, pelvic inflammatory disease can cause problems getting pregnant, problems during pregnancy, and long-term pelvic pain. 1 in 8 women with a history of pelvic inflammatory disease experience difficulties getting pregnant 2.
Pelvic inflammatory disease can be caused by many different types of bacteria. Usually pelvic inflammatory disease is caused by bacteria from sexually transmitted infections (STIs) also called sexually transmitted diseases (STDs). Sometimes pelvic inflammatory disease is caused by normal bacteria found in the vagina.
Any woman can get pelvic inflammatory disease, but you’re more likely to get it if you:
- have more than 1 sexual partner
- have a new sexual partner
- have a history of sexually transmitted infections (STIs)
- have had pelvic inflammatory disease in the past
- are under 25
- started having sex at a young age
Pelvic inflammatory disease (PID) comprises a spectrum of inflammatory disorders of the upper female genital tract, including any combination of endometritis, salpingitis, tubo-ovarian abscess, and pelvic peritonitis 3. Sexually transmitted organisms, especially Neisseria gonorrhoeae and Chlamydia trachomatis, are implicated in many cases. Recent studies suggest that the proportion of pelvic inflammatory disease cases attributable to Neisseria gonorrhoeae or Chlamydia trachomatis is declining; of women who received a diagnosis of acute pelvic inflammatory disease, <50% test positive for either of these organisms 4. Microorganisms that comprise the vaginal flora (e.g., anaerobes, Gardnerella vaginalis, Haemophilus influenzae, enteric Gram-negative rods, and Streptococcus agalactiae) have been associated with pelvic inflammatory disease 5. In addition, cytomegalovirus (CMV), Mycoplasma hominis, Ureaplasma urealyticum, and Mycoplasma genitalium might be associated with some pelvic inflammatory disease cases 6. Newer data suggest that Mycoplasma genitalium might play a role in the pathogenesis of pelvic inflammatory disease 4 and might be associated with milder symptoms 7, although one study failed to demonstrate a significant increase in pelvic inflammatory disease following detection of Mycoplasma genitalium in the lower genital tract 8. All women who receive a diagnosis of acute pelvic inflammatory disease should be tested for HIV, as well as gonorrhea and chlamydia, using nucleic acid amplification test (NAAT). The value of testing women with pelvic inflammatory disease for Mycoplasma genitalium is unknown, and there is no commercially available diagnostic test that has been cleared by FDA for use in the United States 9.
Screening and treating sexually active women for chlamydia reduces their risk for pelvic inflammatory disease 10. Although bacterial vaginosis is associated with pelvic inflammatory disease, whether the incidence of pelvic inflammatory disease can be reduced by identifying and treating women with bacterial vaginosis is unclear 5.
Many episodes of pelvic inflammatory disease (PID) go unrecognized. Although some cases are asymptomatic, others are not diagnosed because the patient or the health-care provider fails to recognize the implications of mild or nonspecific symptoms or signs (e.g., abnormal bleeding, painful sex, and vaginal discharge). Even women with mild or asymptomatic pelvic inflammatory disease might be at risk for infertility 11. Because of the difficulty of diagnosis and the potential for damage to the reproductive health of women, health-care providers usually maintain a low threshold for the diagnosis of pelvic inflammatory disease 12. The following recommendations for diagnosing pelvic inflammatory disease are intended to help health-care providers recognize when pelvic inflammatory disease should be suspected and when additional information should be obtained to increase diagnostic certainty. Diagnosis and management of other common causes of lower abdominal pain (e.g., ectopic pregnancy, acute appendicitis, ovarian cyst, and functional pain) are unlikely to be impaired by initiating antimicrobial therapy for pelvic inflammatory disease.
Presumptive treatment for pelvic inflammatory disease should be initiated in sexually active young women and other women at risk for sexually transmitted infections (STIs) if they are experiencing pelvic or lower abdominal pain, if no cause for the illness other than pelvic inflammatory disease can be identified, and if one or more of the following minimum clinical criteria are present on pelvic examination:
- cervical motion tenderness
OR - uterine tenderness
OR - adnexal tenderness.
The requirement that all three minimum criteria be present before the initiation of empiric treatment could result in insufficient sensitivity for the diagnosis of pelvic inflammatory disease. After deciding whether to initiate empiric treatment, clinicians should also consider the risk profile for sexually transmitted diseases (STDs).
Several types of antibiotics can cure pelvic inflammatory disease. Antibiotic treatment does not, however, reverse any scarring caused by the infection. For this reason, it is critical that a woman receive care immediately if she has pelvic pain or other symptoms of pelvic inflammatory disease. Prompt antibiotic treatment can prevent severe damage to the reproductive organs. The longer a woman delays treatment for pelvic inflammatory disease, the more likely she is to become infertile or to have a future ectopic pregnancy because of damage to the fallopian tubes.
Pelvic inflammatory disease is treated with a combination of antibiotics for at least 14 days. Most of the time, at least two antibiotics are used that work against many different types of bacteria. You must take all of your antibiotics, even if your symptoms go away. This helps to make sure the infection is fully cured. See your doctor or nurse again two to three days after starting the antibiotics to make sure they are working. During treatment you are advised not to have sex. If you or your partner are diagnosed with an sexually transmitted disease (STD) you may also need to contact previous sexual partners to inform them they should get checked. Your doctor or nurse can assist you with how to contact trace your sexual partners.
When pelvic inflammatory disease is treated early, it greatly reduces the risk of complications like lower fertility, chronic abdominal pain, ectopic pregnancy (foetus development in the fallopian tubes), miscarriage, premature birth and stillbirth.
Condoms are the best method of protection against both sexually transmitted infections and pelvic inflammatory disease. It is also important to have regular tests for sexually transmitted infections if you are sexually active and to have an sexually transmitted disease (STD) check before any gynecological procedure, such as intrauterine device (IUD) insertion or abortion.
Your doctor or nurse may suggest going into the hospital to treat your pelvic inflammatory disease if:
- You are very sick
- You are pregnant
- Your symptoms do not go away after taking the antibiotics or if you cannot swallow pills. If this is the case, you will need IV antibiotics.
- You have an abscess in a fallopian tube or ovary
If you still have symptoms or if the abscess does not go away after treatment, you may need surgery. Problems caused by pelvic inflammatory disease, such as chronic pelvic pain and scarring, are often hard to treat. But sometimes they get better after surgery.
How common is pelvic inflammatory disease in the United States?
Despite declining trends, pelvic inflammatory disease is a frequent and important infection that occurs among women of reproductive age. Based on data from the National Health and Nutrition Examination Survey (NHANES) 2013-2014 cycle, the estimated prevalence of self-reported lifetime pelvic inflammatory disease was 4.4% in sexually experienced women of reproductive age (18–44 years) 13. This equates to an estimated 2.5 million women in the United States with a reported lifetime history of pelvic inflammatory disease diagnosis . The prevalence was highest in women at increased risk, such as those with previous sexually transmitted infections (STIs) 13.
The significant burden of disease attributed to pelvic inflammatory disease comes predominantly from the long-term reproductive complications of tubal infection: tubal factor infertility, ectopic pregnancy, and pelvic adhesions, which can lead to chronic pelvic pain. The Centers for Disease Control and Prevention (CDC) knowledge of the longitudinal outcomes for affected women who experience pelvic inflammatory disease is primarily derived from data published using a Scandinavian cohort of inpatients diagnosed with pelvic inflammatory disease 14. Data from this study indicated that those women with pelvic inflammatory disease were more likely to have ectopic pregnancy (6 times increased rate), tubal factor infertility (ranging 8% after first episode to as high as 40% after three episodes) and chronic pelvic pain (18% following 1 episode).
Can pelvic inflammatory disease be cured?
Yes, if pelvic inflammatory disease is diagnosed early, it can be treated. However, treatment won’t undo any damage that has already happened to your reproductive system. The longer you wait to get treated, the more likely it is that you will have complications from pelvic inflammatory disease. While taking antibiotics, your symptoms may go away before the infection is cured. Even if symptoms go away, you should finish taking all of your medicine. Be sure to tell your recent sex partner(s), so they can get tested and treated for sexually transmitted infections (STIs), too. It is also very important that you and your partner both finish your treatment before having any kind of sex so that you don’t re-infect each other.
You can get pelvic inflammatory disease again if you get infected with an sexually transmitted infection (STI) again. Also, if you have had pelvic inflammatory disease before, you have a higher chance of getting it again.
What can happen if pelvic inflammatory disease is not treated?
Without treatment, pelvic inflammatory disease can lead to serious problems like infertility, ectopic pregnancy, and chronic pelvic pain (pain that does not go away). If you think you may have pelvic inflammatory disease, see a doctor or nurse as soon as possible.
Antibiotics will treat pelvic inflammatory disease, but they will not fix any permanent damage done to your internal organs.
Pelvic inflammatory disease (PID) can sometimes lead to serious and long-term problems, particularly if the condition isn’t treated with antibiotics quickly.
But most women with pelvic inflammatory disease who complete their course of antibiotics have no long-term problems.
Repeated episodes of pelvic inflammatory disease
Some women experience repeated episodes of pelvic inflammatory disease. This is known as recurrent pelvic inflammatory disease. The condition can return if the initial infection isn’t entirely cleared. This is often because the course of antibiotics wasn’t completed or because a sexual partner wasn’t tested and treated.
If an episode of pelvic inflammatory disease damages the womb or fallopian tubes, it can become easier for bacteria to infect these areas in the future, making it more likely that you’ll develop the condition again.
Repeated episodes of pelvic inflammatory disease are associated with an increased risk of infertility.
Abscesses
Pelvic inflammatory disease can sometimes cause collections of infected fluid called abscesses to develop, most commonly in the fallopian tubes and ovaries (tubo-ovarian abscess). Abscesses may be treated with broad-spectrum antibiotics, but sometimes laparoscopic surgery (keyhole surgery) may be needed to drain the fluid away. The fluid can also sometimes be drained using a needle that’s guided into place using an ultrasound scan.
Women infected with HIV may be at higher risk for tubo-ovarian abscess. Mortality from pelvic inflammatory disease is less than 1% and is usually secondary to rupture of a tubo-ovarian abscess or to ectopic pregnancy.
Long-term pelvic pain
Some women with pelvic inflammatory disease develop long-term (chronic) pain around their pelvis and lower abdomen, which can be difficult to live with and lead to further problems, such as depression and difficulty sleeping (insomnia). If you develop chronic pelvic pain, you may be given painkillers to help control your symptoms.
Tests to determine the cause may be carried out. If painkillers don’t control your pain, you may be referred to a pain management team or a specialist pelvic pain clinic.
Ectopic pregnancy
An ectopic pregnancy is when a fertilized egg implants itself outside of the womb, usually in one of the fallopian tubes. If pelvic inflammatory disease infects the fallopian tubes, it can scar the lining of the tubes, making it more difficult for eggs to pass through.
If a fertilized egg gets stuck and begins to grow inside the tube, it can cause the tube to burst, which can sometimes lead to severe and life-threatening internal bleeding.
If you’re diagnosed with an ectopic pregnancy, you may be given medication to stop the egg growing or have surgery to remove it.
Infertility
As well as increasing your risk of having an ectopic pregnancy, scarring or abscesses in the fallopian tubes can make it difficult for you to get pregnant if eggs can’t pass easily into the womb.
It’s estimated about 1 in 10 women with pelvic inflammatory disease become infertile as a result of the condition, with the highest risk for women who delayed treatment or had repeated episodes of pelvic inflammatory disease.
But a long-term study in the US showed that women who’d been successfully treated for pelvic inflammatory disease had the same pregnancy rates as the rest of the population.
Blocked or damaged fallopian tubes can sometimes be treated with surgery. If this isn’t possible and you want to have children, you may want to consider an assisted conception technique, such as in-vitro fertilization (IVF).
In-vitro fertilization (IVF) involves surgically removing eggs from a woman’s ovaries and fertilizing them with sperm in a laboratory, before planting the fertilized eggs into the woman’s womb.
This technique can help you get pregnant if you can’t have children naturally. But the success rate is often low, depending on your age and other factors.
Can I get pregnant if I have had pelvic inflammatory disease?
Maybe. Your chances of getting pregnant are lower if you have had pelvic inflammatory disease more than once. When you have pelvic inflammatory disease, bacteria can get into the fallopian tubes or cause inflammation of the fallopian tubes. This can cause scarring in the tissue that makes up your fallopian tubes.
Scar tissue can block an egg from your ovary from entering or traveling down the fallopian tube to your uterus (womb). The egg needs to be fertilized by a man’s sperm and then attach to your uterus for pregnancy to happen. Even having just a little scar tissue can keep you from getting pregnant without fertility treatment.
Scar tissue from pelvic inflammatory disease can also cause a dangerous ectopic pregnancy (a pregnancy outside of the uterus) instead of a normal pregnancy. Ectopic pregnancies are more than six times more common in women who have had pelvic inflammatory disease compared with women who have not had pelvic inflammatory disease 15. Most of these pregnancies end in miscarriage.
Who gets pelvic inflammatory disease?
Pelvic inflammatory disease affects about 5% of women in the United States 16. Your risk for Pelvic inflammatory disease is higher if you 2:
- Have had an sexually transmitted disease (STD)
- Have had pelvic inflammatory disease before
- Are younger than 25 and have sex. Pelvic inflammatory disease is most common in women 15 to 24 years old.
- Have more than one sex partner or have a partner who has multiple sexual partners
- Douche. Douching can push bacteria into the reproductive organs and cause Pelvic inflammatory disease. Douching can also hide the signs of Pelvic inflammatory disease.
- Recently had an intrauterine device (IUD) inserted 17. The risk of Pelvic inflammatory disease is higher for the first few weeks only after insertion of an IUD (intrauterine device), but Pelvic inflammatory disease is rare after that. Getting tested for sexually transmitted infections (STIs) before the intrauterine device (IUD) is inserted lowers your risk for pelvic inflammatory disease.
How do you get pelvic inflammatory disease?
A woman can get pelvic inflammatory disease if bacteria move up from her vagina or cervix and into her reproductive organs. Many different types of bacteria can cause pelvic inflammatory disease. Most often, pelvic inflammatory disease is caused by infection from two common sexually transmitted infections (STIs): gonorrhea and chlamydia. The number of women with pelvic inflammatory disease has dropped in recent years. This may be because more women are getting tested regularly for chlamydia and gonorrhea 18.
You can also get pelvic inflammatory disease without having an sexually transmitted infection. Normal bacteria in the vagina can travel into a woman’s reproductive organs and can sometimes cause pelvic inflammatory disease. Sometimes the bacteria travel up to a woman’s reproductive organs because of douching. Do not douche. No doctor or nurse recommends douching.
You are more likely to get pelvic inflammatory disease if you:
- Have an sexually transmitted infection (STI) and do not get treated;
- Have more than one sex partner;
- Have a sex partner who has sex partners other than you;
- Have had pelvic inflammatory disease before;
- Are sexually active and are age 25 or younger;
- Douche;
- Use an intrauterine device (IUD) for birth control. However, the small increased risk is mostly limited to the first three weeks after the IUD is placed inside the uterus by a doctor.
Can women who have sex with women get pelvic inflammatory disease?
Yes. It is possible to get pelvic inflammatory disease, or an sexually transmitted infection (STI), if you are a woman who has sex only with women.
Talk to your partner about her sexual history before having sex, and ask your doctor about getting tested if you have signs or symptoms of pelvic inflammatory disease.
Pelvic inflammatory disease signs and symptoms
Many women do not know they have pelvic inflammatory disease because they do not have any signs or symptoms. When symptoms do happen, they can be mild or more serious.
Pelvic inflammatory disease signs and symptoms include:
- Pain in the lower abdomen (this is the most common symptom) or back pain
- Fever 100.4° F (38 °C) or higher, chills, nausea or vomiting.
- Vaginal discharge that may smell foul
- Painful sex, including bleeding after sex
- Pain when urinating
- Irregular menstrual periods or increased pain during periods
- Pain in the upper right abdomen (this is rare)
Most women have mild symptoms that may include 1 or more of the above symptoms. Pelvic inflammatory disease can come on fast, with extreme pain and fever, especially if it is caused by gonorrhea.
A few women become very ill with:
- severe lower abdominal pain
- a high temperature (fever)
- nausea and vomiting
If you have any of these symptoms, please see your doctor.
It’s important to visit your doctor or a sexual health clinic if you experience any of the above symptoms.
If you have severe pain, you should seek urgent medical attention from your doctor or local emergency department.
Delaying treatment for pelvic inflammatory disease or having repeated episodes of pelvic inflammatory disease can increase your risk of serious and long-term complications.
There’s no simple test to diagnose pelvic inflammatory disease. Diagnosis is based on your symptoms and the finding of tenderness on a vaginal (internal) examination.
Swabs will be taken from your vagina and cervix (neck of the womb), but negative swabs don’t rule out pelvic inflammatory disease.
Pelvic inflammatory disease causes
Most cases of pelvic inflammatory disease are caused by a bacterial infection that’s spread from the vagina or the cervix (entrance to the womb) into the reproductive organs higher up – the womb, fallopian tubes and ovaries.
If an infection spreads upwards from the vagina and cervix, it can cause inflammation of the:
- Womb lining (endometrium)
- Fallopian tubes
- Tissue around the womb
- Ovaries
- Lining of the inside of the abdomen (peritoneum)
- Pockets of infected fluid called abscesses can also develop in the ovaries and fallopian tubes (tubo-ovarian abscess).
Many different types of bacteria can cause pelvic inflammatory disease and it can sometimes be difficult for doctors to pinpoint which are responsible. The sexually transmitted pathogens Chlamydia trachomatis and Neisseria gonorrhoeae have been implicated in a third to half of pelvic inflammatory disease cases 19. However, in many other cases, pelvic inflammatory disease is caused by bacteria that normally live in the vagina, including gram positive and negative anaerobic organisms and aerobic/facultative gram positive and negative rods and cocci, found at high levels in women with bacterial vaginosis, also have been implicated in the pathogenesis of pelvic inflammatory disease 20. Newer data suggest that Mycoplasma genitalium may also play a role in pelvic inflammatory disease and may be associated with milder symptoms 21 although one study failed to demonstrate a significant increase in pelvic inflammatory disease following detection of M. genitalium in the lower genital tract 8. Because of the polymicrobial nature of pelvic inflammatory disease, a combination of antibiotics will be prescribed so a variety of bacteria can be treated.
Sexually transmitted infections (STIs)
In about a third to half of pelvic inflammatory disease cases, pelvic inflammatory disease is caused by a sexually transmitted infection (STI) such as chlamydia, gonorrhoea or mycoplasma genitalium.
These bacteria usually only infect the cervix, where they can be easily treated with a single dose of an antibiotic.
But if they’re not treated there’s a risk the bacteria could travel into the upper genital tract.
It’s estimated 1 in 10 women with untreated chlamydia may develop pelvic inflammatory disease within a year.
Other causes
In many cases, the cause of the infection that leads to pelvic inflammatory disease is unknown. Sometimes normally harmless bacteria found in the vagina can get past the cervix and into the reproductive organs. Although harmless in the vagina, these types of bacteria can cause infection in other parts of the body.
This is most likely to happen if:
- you have had pelvic inflammatory disease before
- there’s been damage to the cervix following childbirth or a miscarriage
- you have a procedure that involves opening the cervix (such as an abortion, inspection of the womb, or insertion of an intrauterine (IUD) contraceptive device)
Risk factors for developing pelvic inflammatory disease
Risk factors for pelvic inflammatory disease include factors associated with sexually transmitted infection (STI) acquisition, such as younger age, having a new or multiple sex partners, having a sex partner who has other concurrent sex partners, and inconsistent use of condoms during sex. Other factors that have been associated with pelvic inflammatory disease include a history of sexually transmitted infections (STIs) or prior pelvic inflammatory disease, and vaginal douching 13. A small increased risk of pelvic inflammatory disease associated with intrauterine device (IUD) use is primarily confined to the first three weeks after IUD insertion 22.
Intrauterine Contraceptive Devices
IUDs (intrauterine contraceptive devices) are one of the most effective contraceptive methods. Copper-containing and levonorgestrel-releasing IUDs are available in the United States. The risk for pelvic inflammatory disease associated with IUD use is primarily confined to the first 3 weeks after insertion 23. If an IUD user receives a diagnosis of pelvic inflammatory disease, the IUD does not need to be removed 24. However, the woman should receive treatment according to these recommendations and should have close clinical follow-up. If no clinical improvement occurs within 48–72 hours of initiating treatment, providers should consider removing the IUD. A systematic review of evidence found that treatment outcomes did not generally differ between women with pelvic inflammatory disease who retained the IUD and those who had the IUD removed 25. These studies primarily included women using copper or other nonhormonal IUDs. No studies are available regarding treatment outcomes in women using levonorgestrel-releasing IUDs.
Pelvic inflammatory disease prevention
You may not be able to prevent pelvic inflammatory disease. It is not always caused by an sexually transmitted infection (STI). Sometimes, normal bacteria in your vagina can travel up to your reproductive organs and cause pelvic inflammatory disease.
But, you can lower your risk of pelvic inflammatory disease by not douching. You can also prevent sexually transmitted infections (STIs) by not having vaginal, oral, or anal sex.
If you do have sex, lower your risk of getting an sexually transmitted infection (STI) with the following steps:
- Use condoms. Condoms are the best way to prevent sexually transmitted infections (STIs) when you have sex. Because a man does not need to ejaculate (come) to give or get sexually transmitted infections (STIs), make sure to put the condom on before the penis touches the vagina, mouth, or anus. Other methods of birth control, like birth control pills, shots, implants, or diaphragms, will not protect you from sexually transmitted infections (STIs).
- Get tested. Be sure you and your partner are tested for sexually transmitted infections (STIs). Talk to each other about the test results before you have sex.
- Be monogamous. Having sex with just one partner can lower your risk for sexually transmitted infections (STIs). After being tested for sexually transmitted infections (STIs), be faithful to each other. That means that you have sex only with each other and no one else.
- Limit your number of sex partners. Your risk of getting sexually transmitted infections (STIs) goes up with the number of partners you have.
- Do not douche. Douching removes some of the normal bacteria in the vagina that protect you from infection. Douching may also raise your risk for pelvic inflammatory disease by helping bacteria travel to other areas, like your uterus, ovaries, and fallopian tubes.
- Do not abuse alcohol or drugs. Drinking too much alcohol or using drugs increases risky behavior and may put you at risk of sexual assault and possible exposure to sexually transmitted infections (STIs).
The steps work best when used together. No single step can protect you from every single type of sexually transmitted infection (STI).
Pelvic inflammatory disease diagnosis
It can be difficult for a woman to tell if they have pelvic inflammatory disease. Many women with pelvic inflammatory disease have subtle or nonspecific symptoms or are asymptomatic. Some women may not have any symptoms, particularly when pelvic inflammatory disease is caused by a chlamydia infection.
However, there’s no single test for diagnosing pelvic inflammatory disease (PID). Pelvic inflammatory disease is diagnosed based on your symptoms and a gynecological examination. Your doctor will first ask about your medical and sexual history.
The next step is to carry out a pelvic examination to check for any tenderness and abnormal vaginal discharge.
Pelvic inflammatory disease can be diagnosed by taking vaginal and cervical swabs, but may also require a pelvic examination or other tests, particularly if there are no symptoms.
You may experience some discomfort during this examination, particularly if you do have pelvic inflammatory disease.
Swabs are usually taken from the inside of your vagina and cervix. These are sent to a laboratory to look for signs of a bacterial infection and identify the bacteria responsible.
A positive test for chlamydia, gonorrhoea or mycoplasma genitalium supports the diagnosis of pelvic inflammatory disease.
But most women have negative swabs and this doesn’t rule out the diagnosis.
As pelvic inflammatory disease can be difficult to diagnose, other tests may also be required to look for signs of infection or inflammation, or rule out other possible causes of your symptoms.
These tests may include:
- a urine or blood test
- a pregnancy test
- an ultrasound scan, which is usually carried out using a probe passed through the vagina (transvaginal ultrasound)
Most women with pelvic inflammatory disease have either mucopurulent cervical discharge or evidence of white blood cells on a microscopic evaluation of a saline preparation of vaginal fluid (i.e., wet prep). If the cervical discharge appears normal and no white blood cells are observed on the wet prep of vaginal fluid, the diagnosis of pelvic inflammatory disease is unlikely, and alternative causes of pain should be considered. A wet prep of vaginal fluid also can detect the presence of concomitant infections (e.g., bacterial vaginosis and trichomoniasis).
The most specific criteria for diagnosing pelvic inflammatory disease include:
- endometrial biopsy with histopathologic evidence of endometritis;
- transvaginal ultrasound or magnetic resonance imaging (MRI) techniques showing thickened, fluid-filled tubes with or without free pelvic fluid or tubo-ovarian complex, or Doppler studies suggesting pelvic infection (e.g., tubal hyperemia); or
- laparoscopic findings consistent with pelvic inflammatory disease.
A diagnostic evaluation that includes some of these more extensive procedures might be warranted in some cases. Endometrial biopsy is warranted in women undergoing laparoscopy who do not have visual evidence of salpingitis, because endometritis is the only sign of pelvic inflammatory disease for some women.
In some cases, laparoscopy (keyhole surgery) may be used to diagnose pelvic inflammatory disease.
A laparoscopy is a minor operation where 2 small cuts are made in the abdomen.
A thin camera is inserted so the doctor can look at your internal organs and, if necessary, take tissue samples.
This is usually only done in more severe cases where there may be other possible causes of the symptoms, such as appendicitis.
Admission to hospital
You may be urgently admitted to hospital if:
- you’re pregnant (especially if there’s a chance you may have an ectopic pregnancy)
- your symptoms are severe (such as nausea, vomiting and a high fever)
- you have signs of pelvic peritonitis
- an abscess is suspected
- you’re unable to take oral antibiotics and need to be given them through a drip (intravenously)
- you may need emergency surgery, such as for appendicitis
To diagnose pelvic inflammatory disease, doctors usually do a physical exam to check for signs of pelvic inflammatory disease and test for sexually transmitted infections (STIs). If you think that you may have pelvic inflammatory disease, see a doctor or nurse as soon as possible.
If you have pain in your lower abdomen, your doctor or nurse will check for:
- Unusual discharge from your vagina or cervix
- An abscess (collection of pus) near your ovaries or fallopian tubes
- Tenderness or pain in your reproductive organs
Your doctor may do tests to find out whether you have pelvic inflammatory disease or a different problem that looks like pelvic inflammatory disease. These can include 17:
- Tests for sexually transmitted infections (STIs), especially gonorrhea and chlamydia. These infections can cause pelvic inflammatory disease.
- A test for a urinary tract infection or other conditions that can cause pelvic pain
- Ultrasound or another imaging test so your doctor can look at your internal organs for signs of pelvic inflammatory disease
A Pap test is not used to detect pelvic inflammatory disease.
Pelvic inflammatory disease treatment
If it’s diagnosed at an early stage, pelvic inflammatory disease (PID) can be treated easily and effectively with antibiotics.
These can be prescribed by your doctor or a doctor at a sexual health clinic. But left untreated, pelvic inflammatory disease (PID) can lead to more serious long-term complications.
Antibiotics
Treatment with antibiotics needs to be started quickly, before the results of the swabs are available.
Pelvic inflammatory disease is usually caused by a variety of different bacteria, even in cases where chlamydia, gonorrhoea or mycoplasma genitalium is identified.
This means you’ll be given a mixture of antibiotics to cover the most likely infections.
Antibiotics commonly prescribed to treat pelvic inflammatory disease include:
- Cefotetan
- Doxycycline
- Clindamycin
- Gentamicin
- Ceftriaxone
- Cefoxitin
- Azithromycin
- Metronidazole
Tell your doctor if you think you may be pregnant before starting antibiotic treatment, as some antibiotics should be avoided during pregnancy.
You’ll usually have to take the antibiotic tablets for 14 days, sometimes beginning with a single antibiotic injection.
It’s very important to complete the entire course of antibiotics, even if you’re feeling better, to help ensure the infection is properly cleared.
In particularly severe cases of pelvic inflammatory disease, you may have to be admitted to hospital to receive antibiotics through a drip in your arm (intravenously).
If you have pain around your pelvis or tummy, you can take painkillers such as paracetamol or ibuprofen while you’re being treated with antibiotics.
Parenteral Treatment
Several randomized trials have demonstrated the efficacy of parenteral regimens 26. Clinical experience should guide decisions regarding transition to oral therapy, which usually can be initiated within 24–48 hours of clinical improvement. In women with tubo-ovarian abscesses, at least 24 hours of inpatient observation is recommended.
Recommended Parenteral Regimens 17:
- Cefotetan 2 g IV every 12 hours PLUS Doxycycline 100 mg orally or IV every 12 hours
- OR
- Cefoxitin 2 g IV every 6 hours PLUS Doxycycline 100 mg orally or IV every 12 hours
- OR
- Clindamycin 900 mg IV every 8 hours PLUS Gentamicin loading dose IV or IM (2 mg/kg), followed by a maintenance dose (1.5 mg/kg) every 8 hours. Single daily dosing (3–5 mg/kg) can be substituted.
Because of the pain associated with intravenous infusion, doxycycline should be administered orally when possible. Oral and IV administration of doxycycline provide similar bioavailability. Although use of a single daily dose of gentamicin has not been evaluated for the treatment of pelvic inflammatory disease, it is efficacious in analogous situations.
When using the parenteral cefotetan or cefoxitin regimens, oral therapy with doxycycline 100 mg twice daily can be used 24–48 hours after clinical improvement to complete the 14 days of therapy. For the clindamycin/gentamicin regimen, oral therapy with clindamycin (450 mg orally four times daily) or doxycycline (100 mg twice daily) can be used to complete the 14 days of therapy. However, when tubo-ovarian abscess is present, clindamycin (450 mg orally four times daily) or metronidazole (500 mg twice daily) should be used to complete at least 14 days of therapy with doxycycline to provide more effective anaerobic coverage than doxycycline alone.
Limited data are available to support use of other parenteral second- or third-generation cephalosporins (e.g., ceftizoxime, cefotaxime, and ceftriaxone). In addition, these cephalosporins are less active than cefotetan or cefoxitin against anaerobic bacteria.
Alternative Parenteral Regimens
Ampicillin/sulbactam plus doxycycline has been investigated in at least one clinical trial and has broad-spectrum coverage 27. Ampicillin/sulbactam plus doxycycline is effective against Chlamydia trachomatis, Neisseria gonorrhoeae, and anaerobes in women with tubo-ovarian abscess. Another trial demonstrated high short-term clinical cure rates with azithromycin, either as monotherapy for 1 week (500 mg IV daily for 1 or 2 doses followed by 250 mg orally for 5–6 days) or combined with a 12-day course of metronidazole 28. Limited data are available to support the use of other parenteral regimens.
Alternative Parenteral Regimen
- Ampicillin/Sulbactam 3 g IV every 6 hours PLUS Doxycycline 100 mg orally or IV every 12 hours
Intramuscular/Oral Treatment
Intramuscular/oral therapy can be considered for women with mild-to-moderately severe acute pelvic inflammatory disease, because the clinical outcomes among women treated with these regimens are similar to those treated with intravenous therapy 12. Women who do not respond to IM/oral therapy within 72 hours should be reevaluated to confirm the diagnosis and should be administered intravenous therapy.
Recommended Intramuscular/Oral Regimens
- Ceftriaxone 250 mg IM in a single dose PLUS Doxycycline 100 mg orally twice a day for 14 days WITH* or WITHOUT Metronidazole 500 mg orally twice a day for 14 days
- OR
- Cefoxitin 2 g IM in a single dose and Probenecid, 1 g orally administered concurrently in a single dose PLUS Doxycycline 100 mg orally twice a day for 14 days WITH or WITHOUT Metronidazole 500 mg orally twice a day for 14 days
- OR
- Other parenteral third-generation cephalosporin (e.g., ceftizoxime or cefotaxime) PLUS Doxycycline 100 mg orally twice a day for 14 days WITH* or WITHOUT Metronidazole 500 mg orally twice a day for 14 days
*The recommended third-generation cephalsporins are limited in the coverage of anaerobes. Therefore, until it is known that extended anaerobic coverage is not important for treatment of acute pelvic inflammatory disease, the addition of metronidazole to treatment regimens with third-generation cephalosporins should be considered 29.
These regimens provide coverage against frequent etiologic agents of pelvic inflammatory disease, but the optimal choice of a cephalosporin is unclear. Cefoxitin, a second-generation cephalosporin, has better anaerobic coverage than ceftriaxone, and in combination with probenecid and doxycycline has been effective in short-term clinical response in women with pelvic inflammatory disease. Ceftriaxone has better coverage against Neisseria gonorrhoeae. The addition of metronidazole will also effectively treat bacterial vaginosis, which is frequently associated with pelvic inflammatory disease.
Alternative IM/Oral Regimens
Although information regarding other IM and oral regimens is limited, a few have undergone at least one clinical trial and have demonstrated broad-spectrum coverage. Azithromycin has demonstrated short-term clinical effectiveness in one randomized trial when used as monotherapy (500 mg IV daily for 1–2 doses, followed by 250 mg orally daily for 12–14 days) or in combination with metronidazole 28, and in another study, it was effective when used 1 g orally once a week for 2 weeks in combination with ceftriaxone 250 mg IM single dose 30. When considering these alternative regimens, the addition of metronidazole should be considered to provide anaerobic coverage. No data have been published regarding the use of oral cephalosporins for the treatment of pelvic inflammatory disease. As a result of the emergence of quinolone-resistant Neisseria gonorrhoeae, regimens that include a quinolone agent are no longer routinely recommended for the treatment of pelvic inflammatory disease. If allergy precludes the use of cephalosporin therapy, if the community prevalence and individual risk for gonorrhea are low, and if follow-up is likely, use of fluoroquinolones for 14 days (levofloxacin 500 mg orally once daily, ofloxacin 400 mg twice daily, or moxifloxacin 400 mg orally once daily) with metronidazole for 14 days (500 mg orally twice daily) can be considered 31. Diagnostic tests for gonorrhea must be obtained before instituting therapy, and persons should be managed as follows.
- If the culture for gonorrhea is positive, treatment should be based on results of antimicrobial susceptibility testing.
- If the isolate is determined to be quinolone-resistant Neisseria gonorrhoeae or if antimicrobial susceptibility cannot be assessed (e.g., if only nucleic acid amplification test (NAAT) testing is available), consultation with an infectious-disease specialist is recommended.
Other Management Considerations
To minimize disease transmission, women should be instructed to abstain from sexual intercourse until therapy is completed, symptoms have resolved, and sex partners have been adequately treated. All women who received a diagnosis of acute pelvic inflammatory disease should be tested for HIV, as well as gonorrhea and chlamydia, using nucleic acid amplification test (NAAT).
Follow-up
In some cases, you may be advised to have a follow-up appointment 3 days after starting treatment so your doctor can check if the antibiotics are working. Women should demonstrate clinical improvement (e.g., defervescence; reduction in direct or rebound abdominal tenderness; and reduction in uterine, adnexal, and cervical motion tenderness) within 3 days after initiation of therapy.
If the antibiotics seem to be working, you may have another follow-up appointment at the end of the course to check if treatment has been successful.
If no clinical improvement has occurred within 72 hours after outpatient IM/oral therapy, hospitalization, assessment of the antimicrobial regimen, and additional diagnostics (including consideration of diagnostic laparoscopy for alternative diagnoses) are recommended. All women who have received a diagnosis of chlamydial or gonococcal pelvic inflammatory disease should be retested 3 months after treatment, regardless of whether their sex partners were treated (480). If retesting at 3 months is not possible, these women should be retested whenever they next present for medical care in the 12 months following treatment.
If you have an intrauterine device (IUD) fitted, you may be advised to have it removed if your symptoms haven’t improved within a few days, as it may be the cause of the infection.
Treating sexual partners
Any sexual partners you have been with in the 6 months before your symptoms started should be tested and treated to stop the infection recurring or being spread to others, even if no specific cause is identified. Men who have had sexual contact with a woman with pelvic inflammatory disease during the 60 days preceding her onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea, regardless of the etiology of pelvic inflammatory disease or pathogens isolated from the woman. If a woman’s last sexual intercourse was >60 days before onset of symptoms or diagnosis, the most recent sex partner should be treated.
Pelvic inflammatory disease can occur in long-term relationships where neither partner has had sex with anyone else.
It’s more likely to return if both partners aren’t treated at the same time.
Male partners of women who have pelvic inflammatory disease caused by Chlamydia trachomatis and/or Neisseria gonorrhoeae frequently are asymptomatic. Arrangements should be made to link male partners to care. If linkage is delayed or unlikely, Expedited Partner Therapy (EPT) and enhanced referral are alternative approaches to treating male partners of women who have chlamydia or gonococcal infections 32.
You and your partner should avoid having sex until you, your partner and their sex partners have completed the course of treatment (i.e., until therapy is completed and symptoms have resolved, if originally present).
If you haven’t had a sexual partner in the previous 6 months, your most recent partner should be tested and treated.
Your doctor or sexual health clinic can help you contact your previous partners.
Pregnancy
Pregnant women suspected to have pelvic inflammatory disease are at high risk for maternal morbidity and preterm delivery. These women should be hospitalized and treated with intravenous antibiotics.
HIV Infection
Differences in the clinical manifestations of pelvic inflammatory disease between women with HIV infection and women without HIV infection have not been well delineated. In early observational studies, women with HIV infection and pelvic inflammatory disease were more likely to require surgical intervention. More comprehensive observational and controlled studies have demonstrated that women with HIV infection and pelvic inflammatory disease have similar symptoms when compared with HIV-negative women with pelvic inflammatory disease 33, except they are more likely to have a tubo-ovarian abscess; women with HIV infection responded equally well to recommended parenteral and IM/oral antibiotic regimens as women without HIV infection. The microbiologic findings for women with HIV infection and women without HIV infection were similar, except women with HIV infection had higher rates of concomitant Mycoplasma hominis and streptococcal infections. These data are insufficient for determining whether women with HIV infection and pelvic inflammatory disease require more aggressive management (e.g., hospitalization or intravenous antimicrobial regimens).
- 2013 Sexually Transmitted Diseases Surveillance. https://www.cdc.gov/std/stats13/tables/45.htm[↩]
- Pelvic Inflammatory Disease (PID) – CDC Fact Sheet. https://www.cdc.gov/std/pid/stdfact-pid.htm[↩][↩]
- Wiesenfeld HC, Sweet RL, Ness RB, et al. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis 2005;32:400–5.[↩]
- Wiesenfeld HC, Hillier SL, Meyn L, et al. Mycoplasma genitalium – is it a pathogen in acute pelvic inflammatory disease (PID)? STI & AIDS World Congress 2013 (Joint Meeting of the 20th ISSTDR and 14th IUSTI Meeting); July 14-27, 2013; Vienna, Austria.[↩][↩]
- Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol 2005;162:585–90.[↩][↩]
- Haggerty CL, Totten PA, Astete SG, et al. Mycoplasma genitalium among women with nongonococcal, nonchlamydial pelvic inflammatory disease. Infect Dis Obstet Gynecol 2006:30184.[↩]
- Short VL, Totten PA, Ness RB, et al. Clinical presentation of Mycoplasma genitalium infection versus Neisseria gonorrhoeae infection among women with pelvic inflammatory disease. Clin Infect Dis 2009;48:41–7.[↩]
- Oakeshott P, Aghaizu A, Hay P, et al. Is Mycoplasma genitalium in women the “New Chlamydia?” A community-based prospective cohort study. Clin Infect Dis 2010;51:1160–6.[↩][↩]
- 2015 Sexually Transmitted Diseases Treatment Guidelines. https://www.cdc.gov/std/tg2015/pid.htm[↩]
- Jares EJ, Sanchez-Borges M, Cardona-Villa R, et al. Multinational experience with hypersensitivity drug reactions in Latin America. Ann Allergy Asthma Immunol 2014;113:282–9.[↩]
- Wiesenfeld HC, Hillier SL, Meyn LA, et al. Subclinical pelvic inflammatory disease and infertility. Obstet Gynecol 2012;120:37–43.[↩]
- Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol 2002;186:929–37.[↩][↩]
- Kreisel K, Torrone E, Bernstein K, Hong J, Gorwitz R. Prevalence of Pelvic Inflammatory Disease in Sexually Experienced Women of Reproductive Age — United States, 2013–2014. MMWR Morb Mortal Wkly Rep 2017;66:80–83.[↩][↩][↩]
- Rein, D B. Kassler, W J. Irwin, et al. Direct medical cost of pelvic inflammatory disease and its sequelae: decreasing, but still substantial. Obstet & Gynecol. 2000;95:397-402.[↩]
- Weström, L., Joesoef, R., Reynolds, G., Hagdu, A., Thompson, S.E. (1992). Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sexually Transmitted Diseases; 19(4): 185–192.[↩]
- Leichliter, J., Chandra, A., Aral, S.O. (2013). Correlates of Self-Reported Pelvic Inflammatory Disease Treatment in Sexually Experienced Reproductive-Aged Women in the United States, 1995 and 2006–2010. Sex Transm Dis; 40(5):413–418.[↩]
- Pelvic Inflammatory Disease (PID). 2015 Sexually Transmitted Diseases Treatment Guidelines. https://www.cdc.gov/std/tg2015/pid.htm[↩][↩][↩]
- Centers for Disease Control and Prevention. (2014). STDs in Women and Infants. https://www.cdc.gov/std/stats13/womenandinf.htm#pid[↩]
- Haggerty CL, Ness RB. Epidemiology, pathogenesis and treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2006;4:235-47.[↩]
- Hillier SL, Kiviat NB, Hawes SE, et al. Role of bacterial vaginosis-associated microorganisms in endometritis. Am J Obstet Gynecolo 1996;175;435-41.[↩]
- Cohen CR, Mugo NR, Astete SG, et al. Detection of Mycoplasma genitalium in women with laparoscopically diagnosed acute salpingitis. Sex Transm Infect. 2005;81:463-466.[↩]
- Grimes DA. Intrauterine device and upper-genital-tract infection. Lancet 2000;356:1013–9; Viberga I, Odlind V, Lazdane G, et al. Microbiology profile in women with pelvic inflammatory disease in relation to IUD use. Infect Dis Obstet Gynecol 2005;13:183–90.[↩]
- Viberga I, Odlind V, Lazdane G, et al. Microbiology profile in women with pelvic inflammatory disease in relation to IUD use. Infect Dis Obstet Gynecol 2005;13:183–90.[↩]
- CDC. U.S. Selected practice recommendations for contraceptive use, 2013: Adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd ed. MMWR Recomm Rep 2013;62(No. RR-05).[↩]
- Tepper NK, Steenland MW, Gaffield ME, et al. Retention of intrauterine devices in women who acquire pelvic inflammatory disease: a systematic review. Contraception 2013;87:655–60.[↩]
- Ness RB, Hillier SL, Kip KE, et al. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet Gynecol 2004;104:761–9.[↩]
- McGregor JA, Crombleholme WR, Newton E, et al. Randomized comparison of ampicillin-sulbactam to cefoxitin and doxycycline or clindamycin and gentamicin in the treatment of pelvic inflammatory disease or endometritis. Obstet Gynecol 1994;83:998–1004.[↩]
- Bevan CD, Ridgway GL, Rothermel CD. Efficacy and safety of azithromycin as monotherapy or combined with metronidazole compared with two standard multidrug regimens for the treatment of acute pelvic inflammatory disease. J Int Med Res 2003;31:45–54.[↩][↩]
- Walker CK, Wiesenfeld HC. Antibiotic therapy for acute pelvic inflammatory disease: the 2006 CDC Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis 2007;28[Supp 1]:S29–36[↩]
- Savaris RF, Teixeira LM, Torres TG, et al. Comparing ceftriaxone plus azithromycin or doxycycline for pelvic inflammatory disease: a randomized controlled trial. Obstet Gynecol 2007;110:53–60.[↩]
- Judlin P, Liao Q, Liu Z, et al. Efficacy and safety of moxifloxacin in uncomplicated pelvic inflammatory disease: the MONALISA study. Br J Obstet Gynaecol 2010;117:1475–84.[↩]
- Golden MR, Whittington WL, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med 2005;352:676–85.[↩]
- Irwin KL, Moorman AC, O’Sullivan MJ, et al. Influence of human immunodeficiency virus infection on pelvic inflammatory disease. Obstet Gynecol 2000;95:525–34.[↩]