Contents
Sertoli cell only syndrome
Sertoli cell-only syndrome is a cause of male infertility. In sertoli cell only syndrome, only Sertoli cells (cells that nurture the immature sperm) line the seminiferous tubules (tubes inside the testicles where sperm develop) (see Figures 1 to 3). Therefore, there are not any sperm cells present in the seminiferous tubules. Sertoli cell only syndrome is classified into two histological patterns of the pure (primitive) form and the secondary (acquired) form 1. The pure form is a congenital disorder that is characterized by a normal tubule diameter. In the secondary or acquired form, the seminiferous tubule diameters range from almost normal to small 1. Men typically learn they are affected between ages 20-40 years when being evaluated for infertility and are found to have no sperm production (azoospermia). Other signs and symptoms are rare, but in some cases there could be an underlying cause of sertoli cell only syndrome that causes other symptoms, such as Klinefelter syndrome 2.
The prevalence of Sertoli cell-only syndrome in the overall population is estimated to be low. Approximately 10% of couples in the United States are affected by infertility; of these couples, approximately 30% have a pure male factor as the underlying cause, and another 20% have a combined male and female factor 2. Although precise figures are difficult to obtain, it is estimated that less than 5%-10% of these infertile men have sertoli cell only syndrome 2. Information is limited due to the nature of the condition, as only men who have been thoroughly evaluated for infertility (including a testicular biopsy, which is necessary for the diagnosis) have been reported. Sertoli cell only syndrome has no known racial predilection; however, sertoli cell only is more common in white men. In most series, most couples who present for evaluation of male infertility are white.
Most cases of sertoli cell only syndrome are idiopathic (unknown cause), but causes may include deletions of genetic information on regions of the Y-chromosome, especially on the azoospermia factor (AZF) region of Y-chromosome. Other causes include exposure to chemicals or toxins, history of radiation therapy, and history of severe trauma. Diagnosis of sertoli cell only syndrome is confirmed with testicular biopsy. Although there is currently no effective treatment, assisted reproductive technology may assist some men with sertoli cell only syndrome in being able to have children 3.
Couples faced with a diagnosis of sertoli cell only syndrome should be given the options of adoption, use of donor sperm with intrauterine insemination and testicular sperm extraction with in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI).
Although sperm may be successfully extracted from small pockets of spermatogenesis in up to 20%-40% of men with a diagnosis of sertoli cell only syndrome, the use of these sperm for IVF/ICSI is successful in only a small percentage of patients and thus should not be offered as a standard of care. Adoption is an effective option; however, couples must understand that adoption can be a lengthy and costly process. For intrauterine insemination, donor sperm may be obtained locally or nationally through sperm banks.
My son has been diagnosed some time ago with sertoli cell only syndrome, and there is now a question over cancer. How likely is this to occur?
Yes, several studies have found that the prevalence of testicular nodules and cancer in men with Sertoli-cell-only syndrome is increased compared to the general population 4. Some reports cite this number as a 26% risk of nodules and a 10.5% risk of testicular cancer. Due to this increased risk clinical testicular cancer screening by a doctor is recommended 5. However in 2016, a published study did not find an increased risk of testicular cancer for men with azoospermia, though the medical researchers suggested this may be due to the small number of men participating in the study who had azoospermia 6.
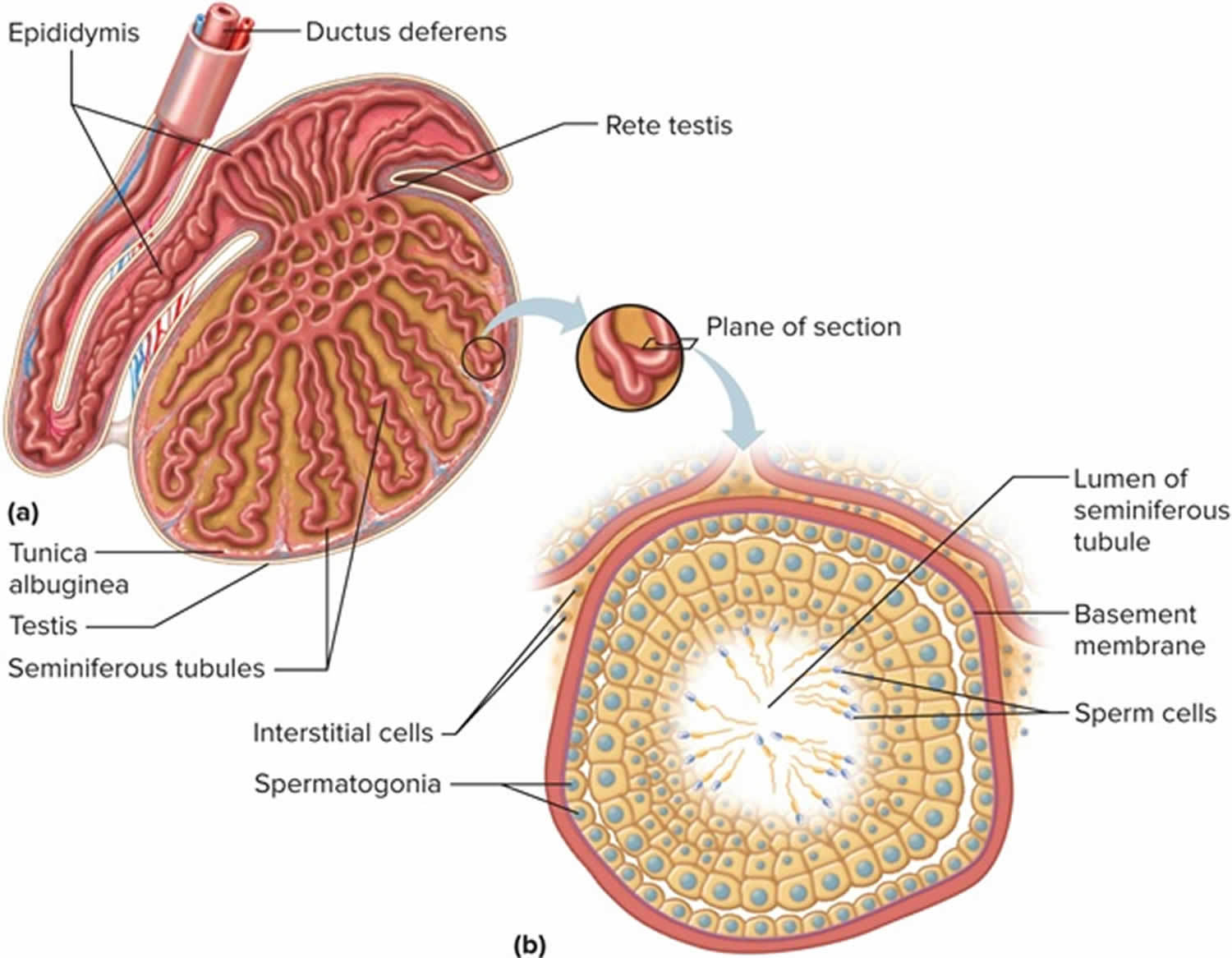
Male reproductive system
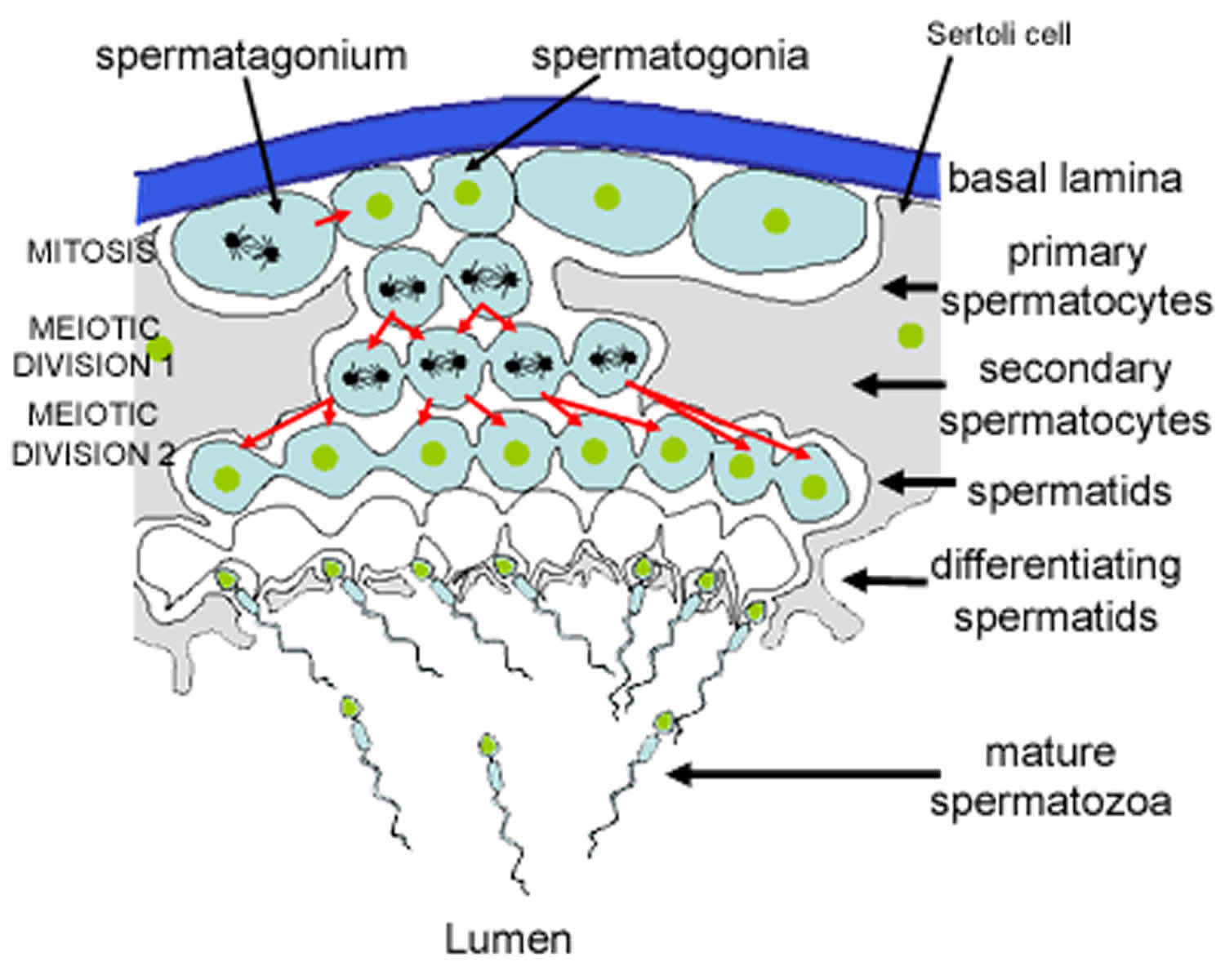
Spermatogenesis takes place in the seminiferous tubules. This includes mitosis and meosis to form haploid gametes, followed by maturation to form spermatocytes (called spermiogenesis). The stages that a developing spermatogonium passes through as it develops and matures to form a mature spermatozoon are covered in more detail here.
The seminiferous tubules are lined by a complex stratified epithelium containing two distinct populations of cells, spermatogenic cells, that develop into spermatozoa, and Sertoli cells which have a supportive and nutrient function.
Figure 1. Testis
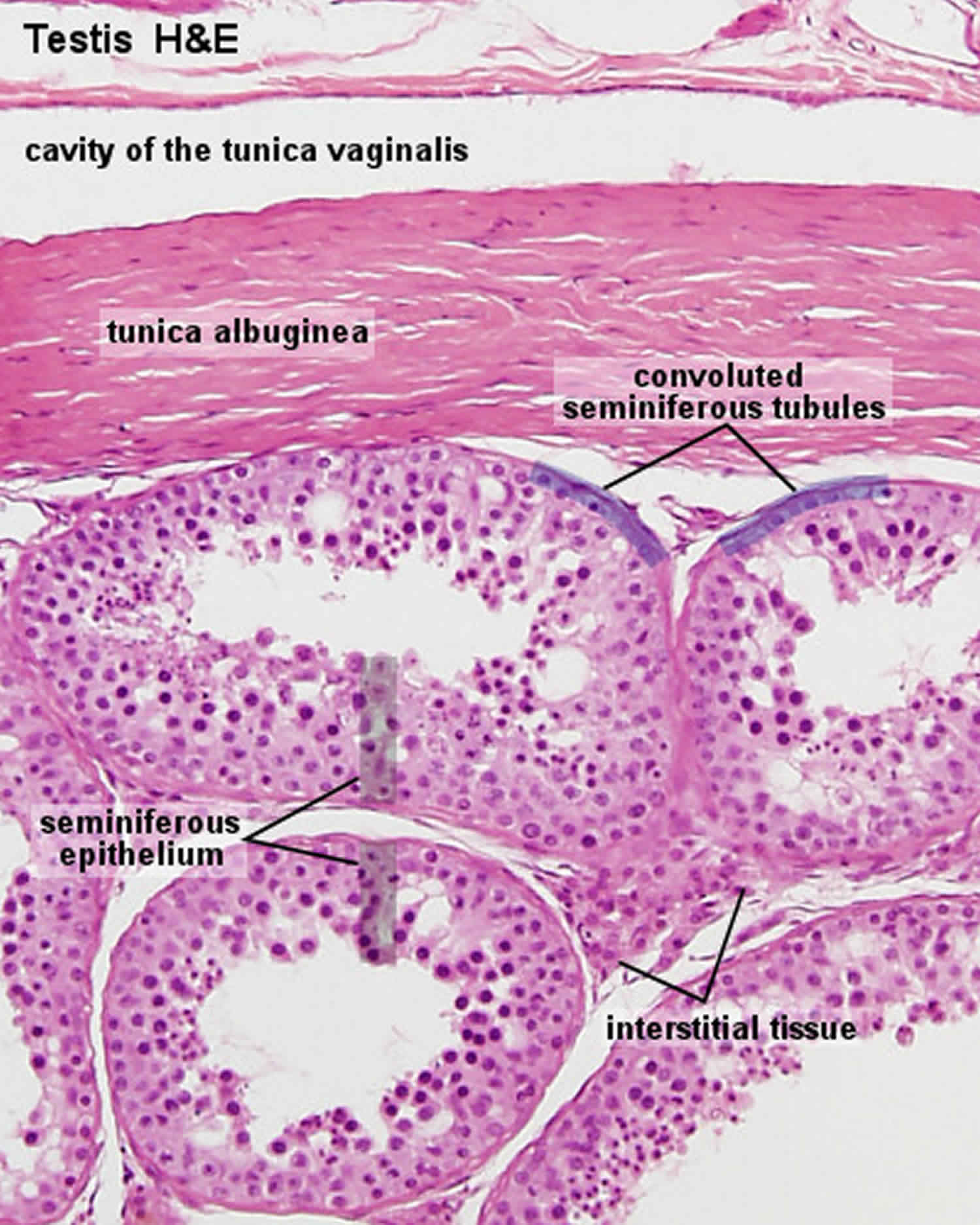
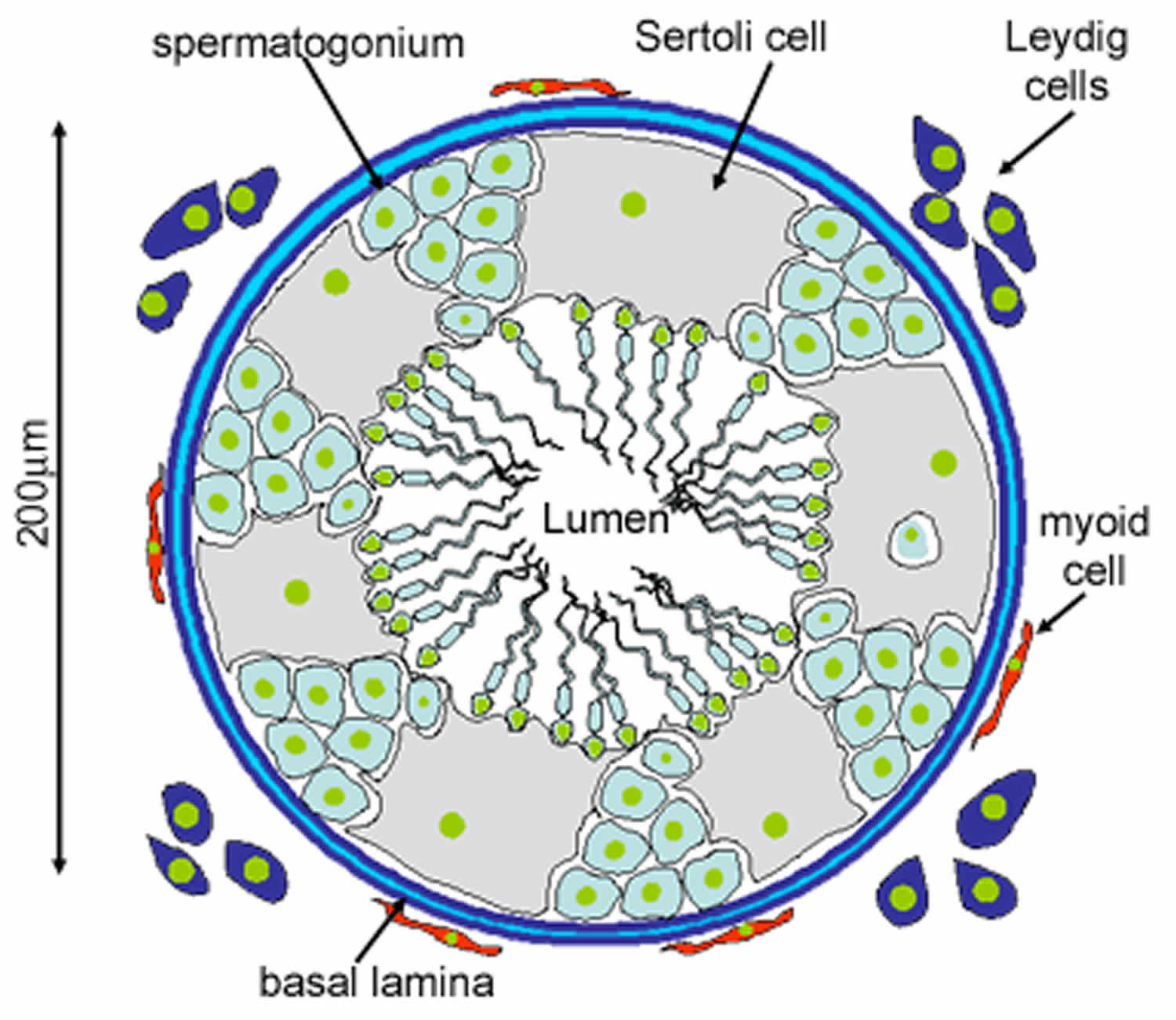
Figure 2. Seminiferous tubules
Footnote: This diagram shows a single seminiferous tubule, and cells in the interstitial tissue outside the tubule. In thisconnective tissue between the seminiferous tubules there are large cells with eosinophilllic cytoplasm called Leydig cells. There are also cells called myoid cells surrounding the basement membrane, which are squamous contractile cells, and generate peristaltic waves in the tubules.
Figure 3. Formation of spermatozoa (sperm)
Footnote: This is a schematic diagram of part of a seminiferous tubule, showing the stages in the formation of spermatozoa.
Sertoli cells
Sertoli cells are the epithelial supporting cells of the seminiferous tubules. They are derived from the epithelial sex cords of the developing gonads. They are tall simple columnar cells, which span from the basement membrane to the lumen. They surround the proliferating and differentiating germ cells forming pockets around these cells, providing nutrients, and phagocytosing excess spermatid cytoplasm, not needed in forming the spermatozoa.
They are connected to each other by continuous tight junctions that seal the tubule into two compartments: the basal (close to the basal lamina) and adluminal (towards the lumen) compartment. Large molecules cannot pass between the basal and adluminal compartment – this is called the blood-testis barrier.
Like all epithelial cells, the Sertoli cells are avascular. Sertoli cells suport the germ cell progenitors and help to transfer nutrients from the nearby capillaries. The developing spermatogonia rely on the Sertoli cells for all of their nourishment. The blood-testis barrier formed by the Sertoli cells effectively isolates the developing spermatogonia, spermatocytes, spermatids and mature spermatozoa from blood. Differentiating spermatozoa nestle in pockets in the peripheral cytoplasm of these cells.
Sertoli cells also produce testicular fluid, including a protein that binds to and concentrates testosterone, which is essential for the development of the spermatozoa. They also help to translocate the differentiating cells to the lumen, and phagocytose degenerating germ cells and surplus cytoplasm remaining from spermiogenesis.
Blood-testis barrier
Importantly, the basal regions of the lateral borders of the Sertoli cells are connected to each other by continuous tight junctions. These divide the tubules into two separate compartments. The mitotic spermatogonia remain in the basal compartment. Differentiating progeny enter the adluminal compartment, and are sealed off from the basal compartment. If novel antigens are expressed on the haploid cells, then it is less likely that they will be detected by the immune system in this sealed off compartment. The developing cells are also in a very protective environment.
Sertoli cell only syndrome causes
Sertoli cell-only syndrome can be caused by a variety of factors, but most cases occur for unknown reasons (idiopathic). Potential causes of sertoli cell only syndrome include 2:
- Genetic factors: the infertility is caused by specific genetic changes
- Hormonal factors: the decreased sperm production is caused by hormonal problems
- Toxin exposure: an exposure to a chemical caused decreased sperm cell production
- History of radiation: radiation to the testicular region caused decreased sperm cell production
- History of trauma: trauma to the testicular region caused decreased sperm cell production
- History of a viral infection affecting the testicles
Most cases of sertoli cell only syndrome are idiopathic 2. A congenital absence of germ cells due to failure of migration of gonocytes is theoretically possible.
A genetic basis for sertoli cell only syndrome is under intense investigation 7. Massive deletions in the azoospermia factor (AZF) region of the Y chromosome, specifically in AZFb/b+c, have been found in men with sertoli cell only syndrome. Five deletions arose from nonallelic homologous recombination between palindromes P5 and P1 and 2 between P4 and P1. In addition, two deletions were found at novel proximal breakpoints in the interval region between P4 and P3 8.
Y-chromosome microdeletions are also occasionally identified as a cause of sertoli cell only syndrome 9. As research continues, more genetic and chromosomal abnormalities associated with sertoli cell only may be found.
Expression of Fas, FasL, and active caspase-3 has been detected in Sertoli cells and hyperplastic interstitial cells. This may be associated with apoptotic elimination or altered maturation of Fas-expressing germ cells through the activation of caspase-3 10.
Exposure to chemicals and toxins may cause sertoli cell only; however, direct cause-and-effect relationships in humans have been difficult to document.
Klinefelter syndrome, 47 XXY, results in a characteristic biopsy appearance of sertoli cell only and Leydig cell hyperplasia 11.
Attempting to distinguish between primary (congenital) and secondary (acquired) sertoli cell only syndrome is of no prognostic significance.
Sertoli cell only syndrome inheritance
In some cases, Sertoli cell-only syndrome may be caused by a genetic change (mutation). One possible example of genetic causes of Sertoli cell-only syndrome include deletions of genetic information on regions of the Y-chromosome, especially on the azoospermia factor (AZF) region of the Y-chromosome 2.
If sertoli cell only syndrome is caused by a genetic change in a particular individual, it is unlikely that this change was inherited. This is because most genetic causes of sertoli cell only syndrome are caused by mutations of the Y chromosome. A male’s Y chromosome is inherited from his father, and men who have sertoli cell only syndrome are typically not able to have children unless they use specific reproductive technologies. Therefore, it would be unlikely for a person to have inherited the cause of sertoli cell only syndrome from his father. In some cases, though, a genetic change causing sertoli cell only syndrome may occur on another chromosome. In these cases, it is possible for sertoli cell only syndrome to run in families 12.
Sertoli cell only syndrome symptoms
The primary sign of Sertoli cell-only syndrome is a lack of production of sperm cells (azoospermia). Men are generally diagnosed with Sertoli cell-only syndrome when they are between 20 and 40 years of age and are being evaluated for infertility. Some patients with sertoli cell only syndrome may have small testicles 2.
In some cases, sertoli cell only syndrome can be caused by another underlying disease. In these cases, it is possible for affected men to have other symptoms. For example, some men with sertoli cell only syndrome are later diagnosed with Klinefelter syndrome, another cause of male infertility. The signs and symptoms associated with underlying causes of sertoli cell only syndrome can vary 3.
The most common presentation involving Sertoli-cell–only syndrome is a young man seeking evaluation for infertility. His semen analysis will have demonstrated azoospermia (absence of sperm on semen analysis). Less commonly, these men may have severely decreased sperm densities of less than 1 million sperm per mL. In this latter situation, the testes have foci of sertoli cell only syndrome and hypospermatogenesis.
Azoospermia may be due to spermatogenic failure or obstruction. Examples of causes of spermatogenic failure include genetic factors, hormonal factors, idiopathic factors, toxin exposure, history of radiation therapy, and history of severe trauma. These conditions may be associated with sertoli cell only syndrome. Obstruction would not be associated with sertoli cell only.
Sertoli cell only syndrome diagnosis
Sertoli cell-only syndrome is typically suspected in men who present with infertility despite having normal testosterone levels. Tests to confirm the diagnosis include a determination of azoospermia (no sperm production) and increased FSH (follicle-stimulating hormone) levels. The diagnosis is typically confirmed by a biopsy of the testicles showing no sperm production 2.
Luteinizing hormone (LH) and prolactin testing are not routinely necessary. In addition, routine testing for male infertility includes at least two semen analyses. Results are as follows:
- Plasma testosterone levels are typically normal.
- Azoospermia and an elevated serum FSH (follicle-stimulating hormone) level (>2-3 times reference range) indicate spermatogenic failure.
- Azoospermia and a serum FSH level within the reference range suggest possible spermatogenic failure or an obstruction.
- The serum FSH level is typically (90%) elevated. Elevations of greater than 2.5-3 times the reference range are diagnostic for spermatogenic failure.
In its purest sense, Sertoli-cell-only (sertoli cell only) syndrome must present as azoospermia; however, a minority of men with the syndrome have foci of spermatogenesis in a testis that is predominantly sertoli cell only.
If a couple is considering intracytoplasmic sperm injection (ICSI), a micromanipulation technique in which a single sperm is injected into an oocyte, they should be offered genetic testing with a Y-chromosome microdeletion assay and karyotyping.
Other tests
Azoospermia should be documented with a semen pellet analysis. The semen pellet is performed by centrifuging a grossly azoospermic semen specimen for 10 minutes at 1500-2000 rpm. This pellet is considered standard for the diagnosis of azoospermia. The pellet at the bottom of the conical tube is examined under a microscope at a magnification of 400X. If sperm are identified, then patchy spermatogenesis within the testes is present.
Men with sertoli cell only syndrome who are azoospermic are at an increased risk of testicular nodules and cancer. As such, the roles of clinical evaluation, ultrasonography, and biopsy should be emphasized 13.
Sertoli cell only syndrome treatment
There is currently no available treatment for Sertoli cell-only syndrome. In some cases, men with sertoli cell only syndrome have very low levels of sperm production. In these situations, a procedure called testicular sperm extraction (TESE) can take place in order to remove sperm from the man’s testicles. The sperm can then be injected directly into a woman’s egg in a procedure called intracytoplasmic sperm injection (ICSI) 2.
Unfortunately, success rate for this procedure may be limited. Current reports suggest that about 13% of men with sertoli cell only syndrome had successful procedures that resulted in having a child 14. Success of the procedure depends on the presence of any sperm cells in the testes, and many men with sertoli cell only syndrome may have complete absence of sperm cells 15. The success of the procedure may be better at centers that specialize in treating men with infertility, including men with sertoli cell only syndrome 2. In cases where TESE-ICSI is used, genetic testing is recommended beforehand for more information about whether or not the syndrome could be passed on to his children 16.
Sertoli cell only syndrome prognosis
Men with Sertoli cell-only syndrome who wish to have children have a number of options. Other than the reproductive technology options mentioned above, men with sertoli cell only syndrome can choose to pursue adoption or their partners can choose to have their eggs inseminated with a donor sperm 2.
Sertoli-cell-only syndrome remains a stable condition with no appreciable improvement in prognosis or sperm production. Investigations have suggested that the prevalence of testicular nodules and cancer in patients with sertoli cell only syndrome is greater than that of the baseline. Initial reports show a 26% risk of nodules and a 10.5% risk of malignancy in testicles of men with pure sertoli cell only syndrome 13. Further studies would be helpful to support this initial report. It is important to discuss this risk with your doctor and discuss methods of looking for testicular cancer such as clinical evaluations, ultrasonography, or MRI 2.
A number of models have been developed to predict successful sperm retrieval with testicular sperm extraction, although efficacy of these models is moderate. In general, higher patient age, higher values for serum testosterone, and lower values for serum follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were predictive for successful sperm retrieval. Idiopathic nonobstructive azoospermia and the presence of an AZFc deletion were predictive for unsuccessful sperm retrieval 17.
- Anniballo R, Ubaldi F, Cobellis L, et al. Criteria predicting the absence of spermatozoa in the Sertoli cell-only syndrome can be used to improve success rates of sperm retrieval. Hum Reprod 2000; 15: 2269–2277.[↩][↩]
- Sertoli-Cell-Only Syndrome. https://emedicine.medscape.com/article/437884-overview[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Behre HM, Bergmann M, Simoni M, et al. Primary Testicular Failure. [Updated 2015 Aug 30]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279076[↩][↩]
- Eisenberg ML, Betts P, Herder D, Lamb DJ, Lipshultz LI. Increased risk of cancer among azoospermic men. Fertil Steril. 2013;100(3):681–685. doi:10.1016/j.fertnstert.2013.05.022 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3759541[↩]
- ManciniM, Carmignani L, Gazzano G, Sagone P, Gadda F, Bosari S, Rocco F, and Colpi GM. High prevalence of testicular cancer in azoospermic men without spermatogenesis. Human Reproduction. January 12, 2007; 22:1042-1046. http://humrep.oxfordjournals.org/content/22/4/1042.full.pdf[↩]
- Hanson HA, Anderson RE, Aston KI, Carrell DT, Smith KR, Hotaling JM. Subfertility increases risk of testicular cancer: evidence from population-based semen samples. Fertil Steril. 2016;105(2):322–8.e1. doi:10.1016/j.fertnstert.2015.10.027 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4744156[↩]
- Miyamoto T, Koh E, Tsujimura A, Miyagawa Y, Saijo Y, Namiki M, et al. Single-nucleotide polymorphisms in the LRWD1 gene may be a genetic risk factor for Japanese patients with Sertoli cell-only syndrome. Andrologia. 2013 Feb 28.[↩]
- Yang Y, Ma MY, Xiao CY, Li L, Li SW, Zhang SZ. Massive deletion in AZFb/b+c and azoospermia with Sertoli cell only and/or maturation arrest. Int J Androl. 2008 Dec. 31(6):573-8.[↩]
- Hadjkacem-Loukil L, Hadj-Kacem H, Hadj Salem I, Bahloul A, Fakhfakh F, Ayadi H. Genotyping of Tunisian azoospermic men with Sertoli cell-only and maturation arrest. Andrologia. 2011 Jul 6.[↩]
- Kim SK, Yoon YD, Park YS, Seo JT, Kim JH. Involvement of the Fas-Fas ligand system and active caspase-3 in abnormal apoptosis in human testes with maturation arrest and Sertoli cell-only syndrome. Fertil Steril. 2007 Mar. 87(3):547-53.[↩]
- Stouffs K, Gheldof A, Tournaye H, Vandermaelen D, Bonduelle M, Lissens W, et al. Sertoli Cell-Only Syndrome: Behind the Genetic Scenes. Biomed Res Int. 2016. 2016:6191307[↩]
- Stouffs K, Gheldof A, Tournaye H, Vandermaelen D, Bonduelle M, Lissens W, and Seneca S. Sertoli Cell-Only Syndrome: Behind the Genetic Scenes. BioMed Research International. January 26 2016; 6191307. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4746273[↩]
- Mancini M, Carmignani L, Gazzano G, Sagone P, Gadda F, Bosari S, et al. High prevalence of testicular cancer in azoospermic men without spermatogenesis. Hum Reprod. 2007 Apr. 22(4):1042-6.[↩][↩]
- Vloeberghs V, Verheyen G, Haentjens P, Goossens A, Polyzos NP, and Tournaye H. How successful is TESE-ICSI in couples with non-obstructive azoospermia?. Hum Reprod. August 2015; 30(8):1790-6. http://humrep.oxfordjournals.org/content/30/8/1790.long[↩]
- Althakafi SA, Mustafa OM, Seyam RM, Al-Hathal N, and Kattan S. Serum testosterone levels and other determinants of sperm retrieval in microdissection testicular sperm extraction. Translational Andrology and Urology. April 2017; 6(2):282-287. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5422690[↩]
- Stouffs K, Gheldof A, Tournaye H, et al. Sertoli Cell-Only Syndrome: Behind the Genetic Scenes. Biomed Res Int. 2016;2016:6191307. doi:10.1155/2016/6191307 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4746273[↩]
- Cissen M, Meijerink AM, D’Hauwers KW, Meissner A, van der Weide N, Mochtar MH, et al. Prediction model for obtaining spermatozoa with testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod. 2016 Sep. 31 (9):1934-41.[↩]