Contents
What is TAR syndrome
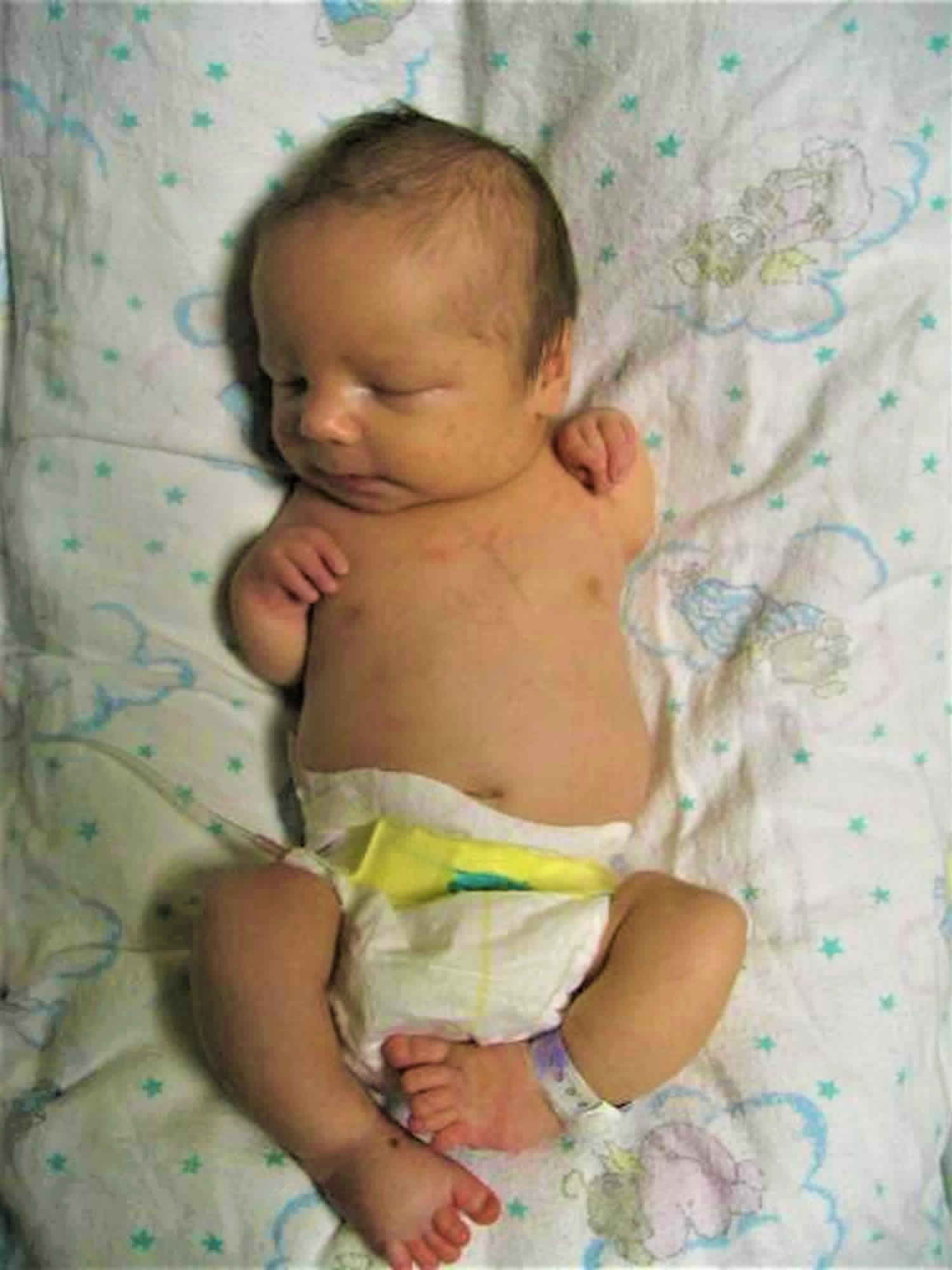
TAR syndrome is also known as thrombocytopenia-absent radius syndrome, which is a rare inherited condition where children with TAR syndrome have decreased production of platelets (the cells which help the blood to clot) and are missing a bone called the radius in each forearm. This platelet deficiency (thrombocytopenia) usually appears during infancy and becomes less severe over time; in some cases the platelet levels become normal.
Thrombocytopenia prevents normal blood clotting, resulting in easy bruising and frequent nosebleeds. Potentially life-threatening episodes of severe bleeding (hemorrhages) may occur in the brain and other organs, especially during the first year of life. Hemorrhages can damage the brain and lead to intellectual disability. Affected children who survive this period and do not have damaging hemorrhages in the brain usually have a normal life expectancy and normal intellectual development.
The severity of skeletal problems in TAR syndrome varies among affected individuals. The radius, which is the bone on the thumb side of the forearm, is almost always missing in both arms. The other bone in the forearm, which is called the ulna, is sometimes underdeveloped or absent in one or both arms. TAR syndrome is unusual among similar malformations in that affected individuals have thumbs, while people with other conditions involving an absent radius typically do not. However, there may be other abnormalities of the hands, such as webbed or fused fingers (syndactyly) or curved pinky fingers (fifth finger clinodactyly). Some people with TAR syndrome also have skeletal abnormalities affecting the upper arms, legs, or hip sockets.
Other features that can occur in TAR syndrome include malformations of the heart or kidneys. Some people with this disorder have unusual facial features including a small lower jaw (micrognathia), a prominent forehead, and low-set ears. About half of affected individuals have allergic reactions to cow’s milk that may worsen the thrombocytopenia associated with this disorder.
TAR syndrome is a rare disorder, affecting fewer than 1 in 100,000 newborns.
TAR syndrome diagnosis is made by physical examination, in which the radius bones in the arms are found to be missing. Blood tests are done to assess the platelet count and for genetic analysis of chromosome 1. Affected individuals have a deletion (absence) of chromosome 1 at position 1q21.1.
TAR syndrome critical period is the first year of life. Platelet transfusions are required to prevent life threatening bleeding. For most children with TAR syndrome, platelet counts improve as they grow out of childhood. Surgery may also be required for skeletal abnormalities. Avoidance of cow’s milk to reduce the severity of gastroenteritis and to avoid exacerbations of thrombocytopenia 1. To reduce the risks of alloimmunization and infection, avoid platelet transfusion in older individuals whose platelet counts exceed a particular threshold (10/nL).
Has TAR syndrome been associated with abnormalities with white cells in the bone marrow?
In addition to problems with platelets, some individuals with TAR syndrome may, at times, make too many white cells. This is not leukemia in the sense of being a malignancy, but rather is called a leukamoid reaction – a reaction of the leukocytes or white cells during which large numbers (often exceeding 35,000 cells/mm3) of them are made 2. This most often occurs along with low platelets in infants and children who are very sick 2. Leukemoid reactions are generally short-lived 1.
The bone marrow may also make too much of a type of blood cell called the eosinophil. The eosinophil is a white blood cell that is easily identified under the microscope because of its reddish granules. It is usually associated with allergies and asthma. The reason that eosinophils are increased in some individuals with TAR syndrome is unknown 2.
Has TAR syndrome been associated with an increased risk for cancer?
While the National Cancer Institute lists leukemia (cancer of the blood and bone marrow) as a possible associated cancer, they clearly state that it is not clear whether patients with TAR syndrome are truly at increased risk for developing cancer 3.
TAR syndrome causes
Mutations in the RBM8A gene cause TAR syndrome. The RBM8A gene provides instructions for making a protein called RNA-binding motif protein 8A. This protein is believed to be involved in several important cellular functions involving the production of other proteins.
Most people with TAR syndrome have a mutation in one copy of the RBM8A gene and a deletion of genetic material from chromosome 1 that includes the other copy of the RBM8A gene in each cell. A small number of affected individuals have mutations in both copies of the RBM8A gene in each cell and do not have a deletion on chromosome 1. RBM8A gene mutations that cause TAR syndrome reduce the amount of RNA-binding motif protein 8A in cells. The deletions involved in TAR syndrome eliminate at least 200,000 DNA building blocks (200 kilobases, or 200 kb) from the long (q) arm of chromosome 1 in a region called 1q21.1. The deletion eliminates one copy of the RBM8A gene in each cell and the RNA-binding motif protein 8A that would have been produced from it.
People with either an RBM8A gene mutation and a chromosome 1 deletion or with two gene mutations have a decreased amount of RNA-binding motif protein 8A. This reduction is thought to cause problems in the development of certain tissues, but it is unknown how it causes the specific signs and symptoms of TAR syndrome. No cases have been reported in which a deletion that includes the RBM8A gene occurs on both copies of chromosome 1; studies indicate that the complete loss of RNA-binding motif protein 8A is not compatible with life.
Researchers sometimes refer to the deletion in chromosome 1 associated with TAR syndrome as the 200-kb deletion to distinguish it from another chromosomal abnormality called a 1q21.1 microdeletion. People with a 1q21.1 microdeletion are missing a different, larger DNA segment in the chromosome 1q21.1 region near the area where the 200-kb deletion occurs. The chromosomal change related to 1q21.1 microdeletion is often called the recurrent distal 1.35-Mb deletion.
TAR syndrome inheritance pattern
TAR syndrome is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell are altered. In this disorder, either both copies of the RBM8A gene in each cell have mutations or, more commonly, one copy of the gene has a mutation and the other is lost as part of a deleted segment on chromosome 1. The affected individual usually inherits an RBM8A gene mutation from one parent. In about 75 percent of cases, the affected person inherits a copy of chromosome 1 with the 200-kb deletion from the other parent. In the remaining cases, the deletion occurs during the formation of reproductive cells (eggs and sperm) or in early fetal development. Although parents of an individual with TAR syndrome can carry an RBM8A gene mutation or a 200-kb deletion, they typically do not show signs and symptoms of the condition.
It is rare to see any history of autosomal recessive conditions within a family because if someone is a carrier for one of these conditions, they would have to have a child with someone who is also a carrier for the same condition. Autosomal recessive conditions are individually pretty rare, so the chance that you and your partner are carriers for the same recessive genetic condition are likely low. Even if both partners are a carrier for the same condition, there is only a 25% chance that they will both pass down the non-working copy of the gene to the baby, thus causing a genetic condition. This chance is the same with each pregnancy, no matter how many children they have with or without the condition.
- If both partners are carriers of the same abnormal gene, they may pass on either their normal gene or their abnormal gene to their child. This occurs randomly.
- Each child of parents who both carry the same abnormal gene therefore has a 25% (1 in 4) chance of inheriting a abnormal gene from both parents and being affected by the condition.
- This also means that there is a 75% ( 3 in 4) chance that a child will not be affected by the condition. This chance remains the same in every pregnancy and is the same for boys or girls.
- There is also a 50% (2 in 4) chance that the child will inherit just one copy of the abnormal gene from a parent. If this happens, then they will be healthy carriers like their parents.
- Lastly, there is a 25% (1 in 4) chance that the child will inherit both normal copies of the gene. In this case the child will not have the condition, and will not be a carrier.
These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
Figure 1 illustrates TAR syndrome autosomal recessive inheritance. The example below shows what happens when both dad and mum is a carrier of the abnormal gene, there is only a 25% chance that they will both pass down the abnormal gene to the baby, thus causing a genetic condition.
Figure 1. TAR syndrome autosomal recessive inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
TAR syndrome symptoms
Children with TAR syndrome are almost always diagnosed at birth. Signs and symptoms include the following:
- Bruising and bleeding as a result of the decreased platelets
- Missing radius bone from both lower arms (although the thumbs are present)
- Short stature and additional skeletal abnormalities, including underdevelopment of other bones in the arms and legs
- Malformations of the heart and kidneys
- Associated features may also include a small lower jaw (micrognathia), a prominent forehead, and low-set ears
- About half of affected individuals experience difficulty digesting cow’s milk
TAR syndrome can potentially affect multiple systems of the body, but it is especially associated with blood (hematological) and bone (skeletal) abnormalities. The two main findings are thrombocytopenia (low levels of the platelets) and radial aplasia. A variety of additional symptoms also occur. The specific symptoms vary from patient to patient. Affected individuals will not have all of the symptoms listed below. Some symptoms improve over time and may cause little or no problems in adulthood. Most affected individuals have normal intelligence, are able live independently, and many have married and have had their own children.
Thrombocytopenia may be congenital or may develop within the first few weeks to months of life. Approximately 90 percent of affected individuals develop symptoms related to low levels of the platelets (thrombocytopenia) in the blood during the first year of life. Platelets are specialized blood cells that clump together to form clots to stop bleeding. In TAR syndrome certain specialized cells in the bone marrow known as megakaryocytes are defective or improperly developed (hypoplastic). Megakaryocytes normally develop into platelets. The normal maturation of megakaryocytes into platelets does not occur in individuals with TAR syndrome, causing the low levels of platelets, which may be referred to as (hypomegakaryocytic thrombocytopenia). The exact reason why megakaryocytes fail to develop into platelets is unknown. In one review, it was noted that thrombocytopenia developed during the first week of life in only 59% 4. In general, thrombocytopenic episodes decrease with age, with most children with TAR syndrome having normal platelet counts by school age. However, cow’s milk allergy is common, and can be associated with exacerbation of thrombocytopenia.
In individuals with TAR syndrome, the level of platelets in the blood goes up and down. Episodes of thrombocytopenia are most frequent during the first two years of life. Episodes may be preceded or triggered by certain infections, such as viral illnesses (particularly digestive [gastrointestinal] illnesses), surgery, stress, or other factors, such as intolerance to cow’s milk (see below).
Low platelet levels can result in severe bleeding episodes (hemorrhaging). Specific symptoms of thrombocytopenia include frequent nosebleeds or gastrointestinal bleeding, which can result in the vomiting of blood (hematemesis) or bloody stools. In addition, affected individuals may develop bleeding (hemorrhages) within skin (dermal) layers or layers below the mucous membranes (submucosal), resulting in easy bruising (ecchymoses) and/or the appearance of pinpoint-sized, purplish or reddish spots on the skin (petechiae). In severe cases, bleeding episodes, particularly in the brain (intracranial hemorrhaging), may lead to potentially life-threatening complications during infancy. In addition, intellectual disability has been reported in some individuals who had a history of intracranial hemorrhaging. Otherwise, intelligence in individuals with TAR syndrome is usually unaffected.
As mentioned above, thrombocytopenia typically is most severe during the first year of life. By adulthood, platelet levels may improve to almost normal ranges. Therefore, adults may have few associated symptoms; however, affected women may have unusually heavy or prolonged menstrual periods (menorrhagia).
In addition to platelets, the two other main blood cell lines (red and white cells) may also be affected. Red blood cells deliver oxygen to the body and white blood cells help in fighting off infections. Low levels of circulating red cells (anemia) may occur. Anemia is associated with fatigue, pale skin, and weakness. In some cases, affected children may have an excessive amount of white blood cells called a “leukemoid reaction”. This occurs in infants with extremely low platelet levels. There may also be enlargement of the liver and spleen (hepatosplenomegaly). In some cases, increased levels of a specific type of white blood cell called an eosinophil (eosinophilia) may also occur. The cause of eosinophilia is not known. It is often associated with allergy or asthma and may occur in children with TAR syndrome who have cow’s milk intolerance.
A variety of limb anomalies (both upper and lower limbs) occur in individuals with TAR syndrome, although upper limb involvement tends to be more severe than lower limb involvement. The characteristic finding in individuals with TAR syndrome is bilateral absence of the radius. The radius is a long thin bone that extends from the elbow to the thumb side of the wrist. The thumbs are always present in individuals with TAR syndrome, a finding that distinguishes it from other disorders involving radii. The thumbs in individuals with TAR syndrome are of near-normal size, but are somewhat wider and flatter than usual. They are also held in flexion against the palm, and tend to have limited function, particularly in terms of grasp and pinch activities 5. The hands, fingers and thumbs are almost always unaffected, although the fingers may be abnormally short.
The upper limbs may also have underdevelopment or absence of the other bone of the forearm, the ulna. Sometimes the long bone of the upper arm (humerus), which extends from the shoulder to the elbow, may be underdeveloped. In some cases, the shoulder girdle may also be underdeveloped and affected individuals may have reduced upper body strength. In severe cases, the arms may be missing and the hands may be joined to the trunk by small, irregularly-shaped bone (phocomelia). Fingers may show syndactyly, and fifth finger clinodactyly is common.
In some cases, the lower limbs may be involved. Lower limbs are affected in almost half of those with TAR syndrome; hip dislocation, coxa valga, femoral and/or tibial torsion, genu varum, and absence of the patella are common findings. The most severe limb involvement is tetraphocomelia. The severity may range from barely noticeable changes to significant malformations.
Affected individuals may exhibit abnormalities of the knees including a loose kneecap that does not slide properly within its groove (patellar subluxation) and can potentially slide completely out of the socket (dislocate), absence of the knee cap (patella) or, in rare cases, the bones of the knees may be fused together. Dislocation of the hip, in which the head of the long bone of the upper leg (femur) does not fit properly into its socket in the hip, may also occur. Additional lower limb abnormalities often occur including improper inward rotation of the long bones of the legs (femoral and tibial torsion), bowing of the legs, and abnormalities affecting the feet and toes. Lower limb abnormalities can potentially affect the ability to walk (mobility). In most cases, individuals with severe involvement of the upper limbs are more likely to have abnormalities of the lower limbs.
Cardiac anomalies affect 15%-22% 6. Approximately one third of affected infants also have structural malformations of the heart (congenital heart defects). Such cardiac defects may include an abnormal opening in the fibrous partition (septum) that divides the upper chambers of the heart (atrial septal defect) or a malformation known as tetralogy of Fallot. The latter describes a combination of heart defects, including abnormal narrowing (stenosis) of the opening between the pulmonary artery (which carries blood to the lungs) and the lower right chamber (ventricle) of the heart, an abnormal opening in the partition between the lower chambers of the heart (ventricular septal defect); displacement of the major artery that transports oxygen-rich blood to most of the body (i.e., aorta); and enlargement of the right ventricle (hypertrophy).
Gastrointestinal involvement includes cow’s milk allergy and gastroenteritis. Both tend to improve with age.
Genitourinary anomalies include renal anomalies (both structural and functional) and rarely, Mayer-Rokitansky-Kuster-Hauser syndrome (agenesis of uterus, cervix, and upper part of the vagina) 7.
Leukemoid reactions have been reported in some individuals with TAR syndrome, with white blood cell counts exceeding 35,000 cells/mm³. These leukemoid reactions are generally transient 8.
Cognitive development is usually normal in individuals with TAR syndrome.
Growth. Most have height on or below the 50th centile.
Other skeletal manifestations, including rib and cervical vertebral anomalies (e.g., cervical rib, fused cervical vertebrae), tend to be relatively rare.
In addition, cow’s milk intolerance or allergy has frequently been reported in association with TAR syndrome. In such cases, introduction of cow’s milk to the diet may precipitate thrombocytopenic, eosinophilic, and/or “leukemoid” episodes (see above). Cow’s milk intolerance can also cause a variety of gastrointestinal symptoms including nausea, vomiting, diarrhea, and failure to gain weight and grow at the expected rate (failure to thrive).
Some individuals with TAR syndrome may exhibit short stature. A variety of additional physical abnormalities have been reported to be associated with TAR syndrome including an abnormally small jaw (micrognathia), incomplete closure of the roof of the mouth (cleft palate), one or more pink or dark red irregularly shaped patches of skin (hemangiomas) on the face caused by dense collections of small blood vessels (capillaries), or minor abnormalities affecting the spine and ribs. Kidney (renal) defects may also be present, such as a malformation in which the two kidneys are abnormally joined at the base (horseshoe kidney) as well as underdevelopment (hypoplasia) and improper function of the kidneys. Some of these findings have only occurred in a few reported cases and researchers do not know whether these are coincidental occurrences or whether individuals with TAR syndrome have a greater risk of developing these manifestations.
TAR syndrome diagnosis
In most cases, the diagnosis of TAR syndrome is made at birth based upon a thorough clinical examination, identification of characteristic physical findings, and a variety of specialized tests. Such testing may include blood studies to confirm the presence of thrombocytopenia, anemia, and/or other hematologic abnormalities as well as a radiograph (X-ray) of the forearm and renal ultrasonography of the kidneys. Thrombocytopenia; usually <50 platelets/nL (normal range: 150-400 platelets/nL).
The first step in molecular genetic testing is deletion/duplication analysis for the region of chromosome band1q21 that contains the RBM8A gene. Diagnosis of TAR syndrome is confirmed if a deletion is present in an individual with bilateral absence of the radius and presence of thumbs. However, lack of identification of this deletion is not sufficient to rule out the diagnosis. Sequence analysis of the RBM8A gene should be done if no deletion is identified, or to identify the second RBM8A gene mutation for confirmation of the diagnosis.
Clinical testing and work-up
Cardiac evaluation may also be recommended to detect any heart abnormalities that may be associated with the disorder. Such evaluation may include a thorough clinical examination, during which heart and lung sounds are assessed through use of a stethoscope, and specialized tests that enable physicians to evaluate the structure and function of the heart (e.g., x-ray studies, electrocardiography [EKG], echocardiography, cardiac catheterization).
TAR syndrome treatment
The treatment of TAR syndrome is directed toward the specific symptoms that are apparent in each individual. Such treatment may require the coordinated efforts of a team of medical professionals, such as pediatricians, surgeons, physicians who diagnose and treat disorders of the skeleton, joints, muscles, and related tissues (orthopedists), specialists in the study of the blood and blood-forming tissues (hematologists), physicians who specialize in heart disease (cardiologists), and/or other health care professionals.
Physicians may recommend preventive measures to help affected infants and children avoid infection, stress, or other factors that may precipitate thrombocytopenia. In addition, experts indicate that cow’s milk should be avoided, since its introduction may precipitate thrombocytopenic, eosinophilic, or “leukemoid” episodes.
Management of the disorder may include ongoing monitoring and supportive hematologic measures as required, such as platelet transfusions or transfusions with whole blood products. In some cases, the use of certain medications or other measures may be recommended to help prevent or treat hematologic complications. As noted above, thrombocytopenia typically improves with age. Bone marrow transplantation is generally not indicated, given the transient nature of the thrombocytopenia.
In individuals with TAR syndrome, various orthopedic techniques may also be recommended, such as splints, corrective braces, and/or certain surgical measures. In some cases, the use of adaptive and/or artificial devices (prosthetics) and mobility aids, such as wheelchairs or motorized carts, may also be beneficial.
For affected individuals with congenital heart defects, treatment with certain medications, surgical intervention, and/or other measures may be necessary. The surgical procedures performed will depend upon the severity and location of the anatomical abnormalities, their associated symptoms, and other factors.
Early intervention may be important to ensure that children with TAR syndrome reach their potential. Special services that may be beneficial include special education, physical therapy, and/or other medical, social, or vocational services.
Genetic counseling is recommended for affected individuals and their families. Other treatment for this disorder is symptomatic and supportive.
Pregnancy management
Fewer than ten pregnancies have been reported in women with TAR syndrome. Almost all develop thrombocytopenia during pregnancy. In one, corticosteroids appeared to be fairly successful in treating the thrombocytopenia 9. In one pregnant woman with TAR syndrome, exacerbation of her thrombocytopenia preceded the development of preeclampsia.
Other considerations during pregnancy include potential difficulties with administration of regional anesthetics (given potential difficulties with vascular access) and difficulties accessing the airway for general anesthesia 10.
TAR syndrome prognosis
The first two years of life are the most critical in TAR syndrome 11. During this time, children frequently develop life-threatening bleeding episodes due to extremely low platelet levels (thrombocytopenia). These episodes decrease with age, and platelet counts are usually normal by the time a child goes to school 11. Many individuals with TAR syndrome are allergic to cow’s milk, which can also exacerbate the symptoms of thrombocytopenia 1. Intellectual development is usually not affected by TAR syndrome, though some individuals have intellectual disability due to complications from bleeding within the brain 11. People with TAR syndrome may be at increased risk of developing acute leukemia during childhood or adulthood 11.
TAR syndrome life expectancy
TAR syndrome affected children who survive life-threatening episodes of severe bleeding (hemorrhages) and do not have damaging hemorrhages in the brain usually have a normal life expectancy and normal intellectual development.
- Toriello HV. Thrombocytopenia Absent Radius Syndrome. 2009 Dec 8 [Updated 2016 Dec 8]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2019. Available from: https://www.ncbi.nlm.nih.gov/books/NBK23758[↩][↩][↩]
- TAR Syndrome. A Review. http://www.ivh.se/TAR/?page_id=40[↩][↩][↩]
- Inherited Bone Marrow Failure Syndrome Study (IBMFS). https://www.marrowfailure.cancer.gov/disorders/othersyndromes.html[↩]
- Hedberg VA, Lipton JM. Thrombocytopenia with absent radii. A review of 100 cases. Am J Pediatr Hematol Oncol. 1988;10:51–64[↩]
- Goldfarb CA, Wustrack R, Pratt JA, Mender A, Manske PR. Thumb function and appearance in thrombocytopenia: absent radius syndrome. J Hand Surg Am. 2007;32:157–61[↩]
- Greenhalgh KL, Howell RT, Bottani A, Ancliff PJ, Brunner HG, Verschuuren-Bemelmans CC, Vernon E, Brown KW, Newbury-Ecob RA. Thrombocytopenia-absent radius syndrome: a clinical genetic study. J Med Genet. 2002;39:876–81[↩]
- Ahmad R, Pope S. Association of Mayer-Rokitansky-Küster-Hauser syndrome with Thrombocytopenia Absent Radii syndrome: a rare presentation. Eur J Obstet Gynecol Reprod Biol. 2008;139:257–8[↩]
- Klopocki E, Schulze H, Strauss G, Ott CE, Hall J, Trotier F, Fleischhauer S, Greenhalgh L, Newbury-Ecob RA, Neumann LM, Habenicht R, König R, Seemanova E, Megarbane A, Ropers HH, Ullmann R, Horn D, Mundlos S. Complex inheritance pattern resembling autosomal recessive inheritance involving a microdeletion in thrombocytopenia-absent radius syndrome. Am J Hum Genet. 2007;80:232–40[↩]
- Bot-Robin V, Vaast P, Deruelle P. Exacerbation of thrombocytopenia in a pregnant woman with thrombocytopenia-absent radius syndrome. Int J Gynaecol Obstet. 2011;114:77–8[↩]
- Wax JR, Crabtree C, Blackstone J, Pinette MG, Cartin A. Maternal thrombocytopenia-absent radius syndrome complicated by severe pre-eclampsia. J Matern Fetal Neonatal Med. 2009;22:175–7[↩]
- Thrombocytopenia-Absent Radius Syndrome. https://reference.medscape.com/article/959262-overview[↩][↩][↩][↩]