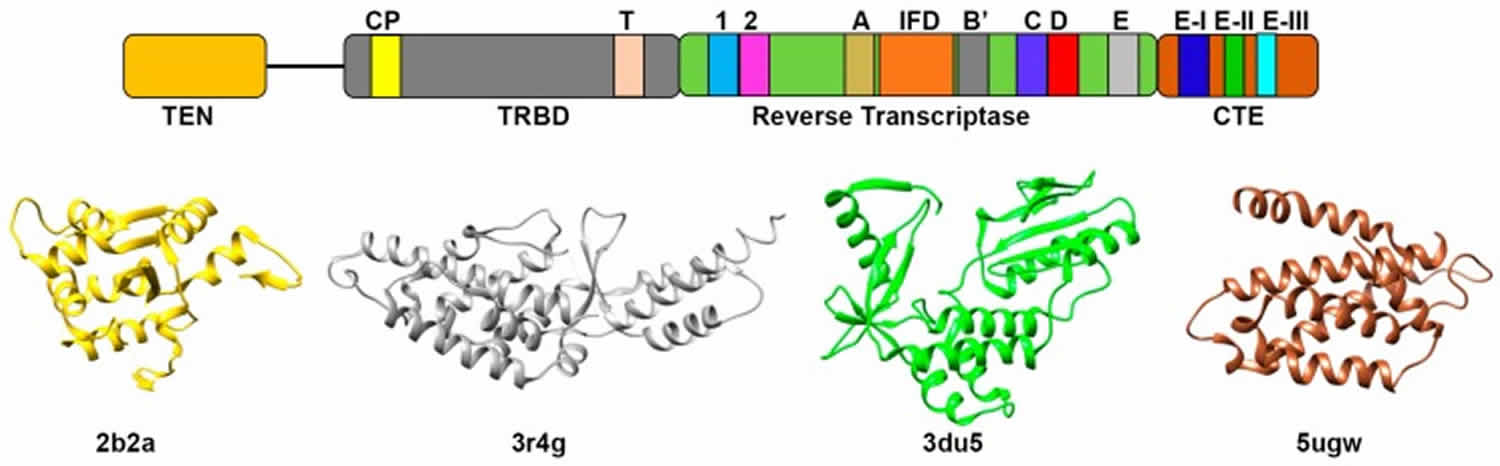
What is telomere
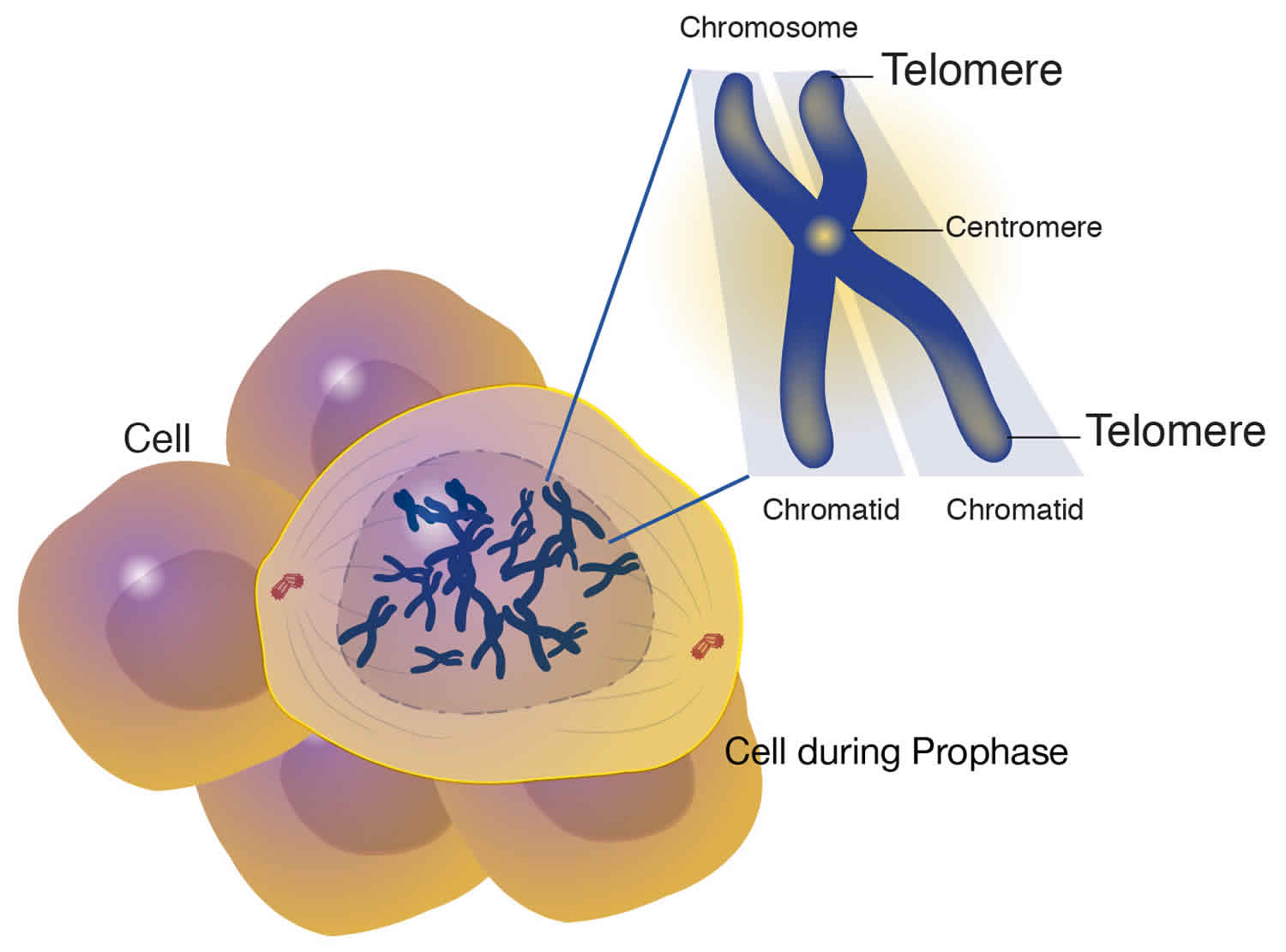
A telomere is the end of a chromosome, a specialized repetitive DNA sequences at the ends of the linear chromosomes, and associated proteins, that serve to maintain the integrity of the chromosomes. Telomeres are made of hundreds or thousands of repeats of the same short DNA sequence of non-coding DNA, which varies between organisms but is 5′-TTAGGG-3′ in humans, that protect the chromosome from damage. Each time a cell divides, the telomeres become shorter. Eventually, the telomeres become so short that the cell can no longer divide. Replicative capacity of a cell is strongly correlated with telomere length regulation. Aberrant lengthening or reduction in the length of telomeres can lead to health anomalies, such as cancer or premature aging. Telomerase is a ribonucleoprotein DNA polymerase complex that maintains telomere length. The complex comprises the protein telomerase reverse transcriptase (TERT, or hTERT in humans) and a catalytic telomerase RNA component (TERC) 1. Telomerase is a master regulator for maintaining replicative potential in most eukaryotic cells. It does so by controlling telomere length at chromosome ends. In the absence of telomerase activity telomeres progressively shorten. Telomerase activity is absent in most normal human somatic cells because of the lack of expression of the protein telomerase reverse transcriptase (TERT); catalytic telomerase RNA component (TERC) is usually present. Without telomerase, telomere shortening eventually limits the growth of cells, either by senescence, in cells with intact cell cycle checkpoints (a G1 cell cycle block), or by crisis in cells with inactivated checkpoints (telomeric end-to-end fusions cause chromosome breakage and mitotic catastrophe) 1. Expression of telomerase reverse transcriptase (TERT) in cells that otherwise lack telomerase activity causes cells to bypass senescence and crisis, and such cells are usually termed “immortalized.”
Modern interest in telomeres and telomerase has its roots in experiments carried out in the 1930s by two remarkable geneticists: Barbara McClintock, then at the University of Missouri at Columbia, and Hermann J. Muller, then at the University of Edinburgh. Working separately and with different organisms, both investigators realized that chromosomes bore a special component at their ends that provided stability. Muller coined the term “telomere,” from the Greek for “end” (telos) and “part” (meros). McClintock noted that without these end caps, chromosomes stick to one another, undergo structural changes and misbehave in other ways. These activities threaten the survival and faithful replication of chromosomes and, consequently, of the cells housing them.
The chromosome ends (telomeres) of mammalian cells contain tandemly arrayed hexanucleotide repeats with the sequence 5′-TTAGGG-3′ 2. This telomeric DNA is mostly double-stranded, but it terminates in a single-stranded 3′ overhang 3. In human somatic cells, each telomere is 4-12 kb long and the single-stranded overhang contains 100-200 nucleotides (Figure 1A). Telomeres need to be distinguished from double strand breaks (DSBs), to avoid being fused to each other by normal DNA repair mechanisms, because they have single-stranded overhangs, which “look like” damaged DNA. The overhang at the lagging strand end of the chromosome is due to incomplete end replication. The overhang at the leading strand end of the chromosome is actually generated by enzymes that cut away part of the DNA 4. In some species (including humans), the single-stranded overhangs bind to complementary repeats in the nearby double-stranded DNA, causing the telomere ends to form protective loops (Figure 1B) 5. Proteins associated with the telomere ends also help protect them and prevent them from triggering DNA repair pathways. Additionally, mammalian telomeres form a higher order structure by sequestering the 3′ overhang in cis within the duplex telomeric DNA, resulting in a telomere loop (t-loop) that likely contributes to the capping mechanism 6.
Because conventional DNA replication machinery cannot copy extreme terminal sequences of the lagging-strand during replication of linear chromosomes, 50-200 base pairs of telomeric DNA will be lost during each successive cell division 7. Due to the end-replication problem 8, the ends of linear chromosomes (repeats that make up a telomere) shorten with each round of DNA replication (a telomere are eaten away slowly over many division cycles) 9. Telomere shortening has been connected to the aging of cells, and the progressive loss of telomeres may explain why cells can only divide a certain number of times 10. In human somatic cells, the progressive telomere shortening that occurs with continued proliferation eventually results in the triggering of a replicative checkpoint. Telomere shortening and the structural changes that it presumably causes, leads to a DNA-damage checkpoint response at the telomere and induction of a permanent p53- and Rb-dependent growth arrest (i.e., replicative senescence) 11. Because this limits the proliferative capacity of somatic cells, including those that have accumulated oncogenic mutations, telomere shortening and replicative senescence are a potent tumor suppressor mechanism.
If senescence pathways are absent, due for example to loss of p53 and Rb function, cells will continue to divide until the telomeres become almost completely eroded, leading to crisis, a period characterized by rampant chromosome end-to-end fusions and cell death 12. Formation of tumors is, in most cases, dependent on the evolution of cells that escape from the barriers that senescence and crisis present to unlimited proliferation. Cells that achieve this are referred to as “immortalized” and in all cases this requires the activation of a mechanism for preventing telomere shortening. In most cases this is accomplished by upregulating the activity of telomerase 13, a ribonucleoprotein enzyme that adds new telomeric repeats to chromosome termini. Telomerase has an important role in cells of the germ line and in normal somatic biology, especially in those tissue compartments that depend upon extensive cellular proliferation. Nevertheless, in normal somatic cells telomerase is not expressed at sufficient levels to prevent telomere shortening and telomere length maintenance in many cancers requires dysregulated levels of telomerase. A substantial minority of immortalized cell lines and tumors are telomerase-negative, however, and in these cells telomere length maintenance can be achieved instead by a telomerase-independent mechanism, termed Alternative Lengthening of Telomeres (ALT) 14.
Alternative Lengthening of Telomeres may resemble (or represent) the earliest telomere maintenance mechanism (TMM), which preceded the evolution of telomerase-dependent maintenance of chromosomal termini. While the possibility cannot be excluded that a low level of Alternative Lengthening of Telomeres-like activity occurs at normal mammalian telomeres, the telomere phenotype seen in Alternative Lengthening of Telomeres-positive immortalized cells and tumors is not found in normal cells. The current data strongly support Alternative Lengthening of Telomeres being a homologous recombination (HR)-mediated DNA replication mechanism, which occurs in the context of telomere instability resulting from loss of several controls over telomere function.
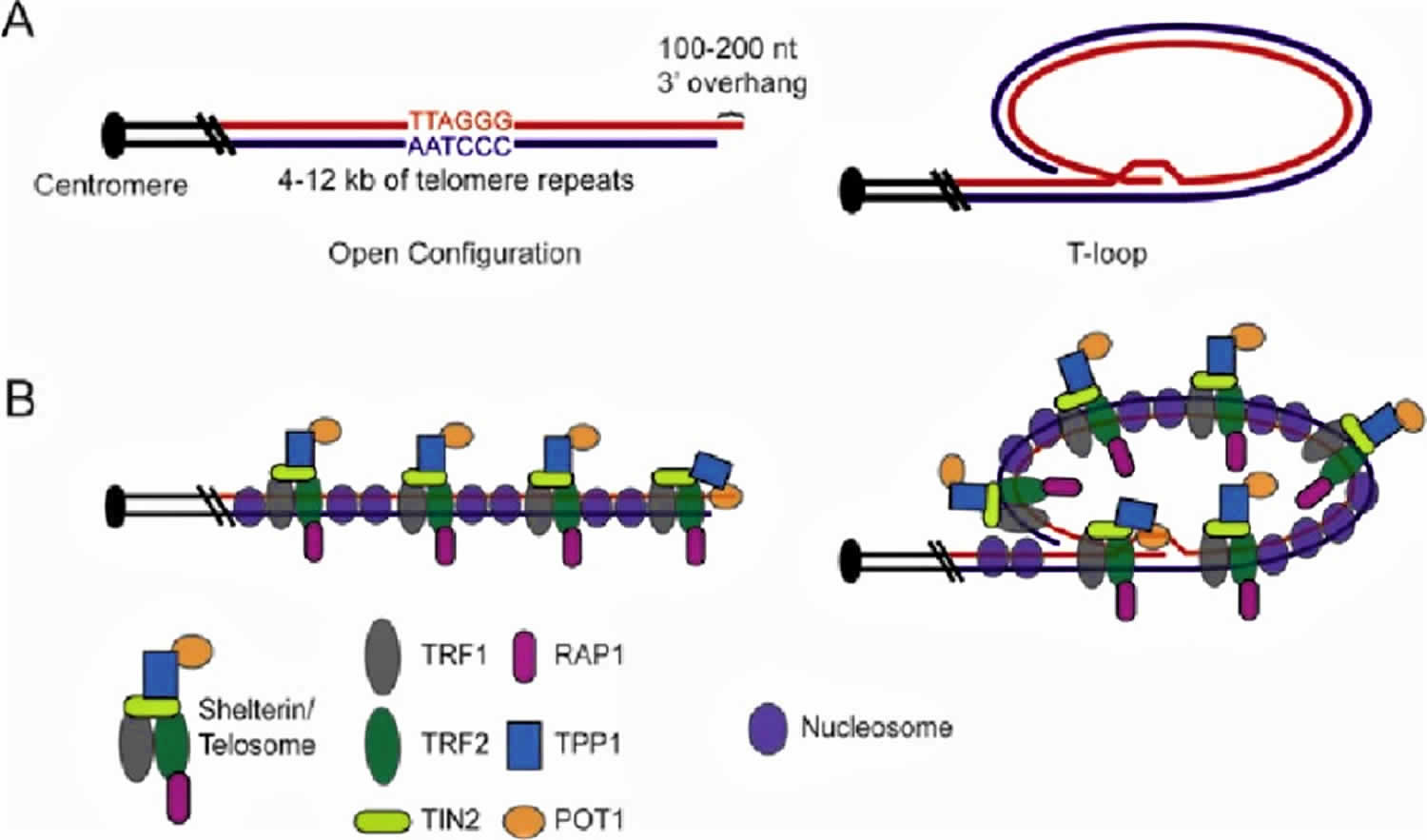
Figure 1. Human telomere components and structure
Footnote: Human telomere components and structure. A) Graphic representation of the telomeric DNA in human cells, which is normally composed of 4-12 kb of G-rich repeats (TTAGGG in red, AATCCC in blue), culminating in a 100-200 nt 3′ overhang. Open and t-loop configurations are shown. B) Graphic representation of telomeric DNA with associated proteins. The six subunit “shelterin” or “telosome” complex coats the length of the duplex telomeric DNA via the direct interaction of TRF1 and TRF2 with telomeric DNA. TIN2 interacts with both TRF1 and TRF2 but not with telomeric DNA. TPP1 (formerly PTOP, PIP1 or TINT1) bridges TIN2 with POT1. POT1 interacts specifically with single-stranded G-rich telomeric DNA, presumably at the chromosome end when the telomere is in open configuration, or at the single-stranded region within the t-loop junction. Telomeres are also assembled into chromatin.
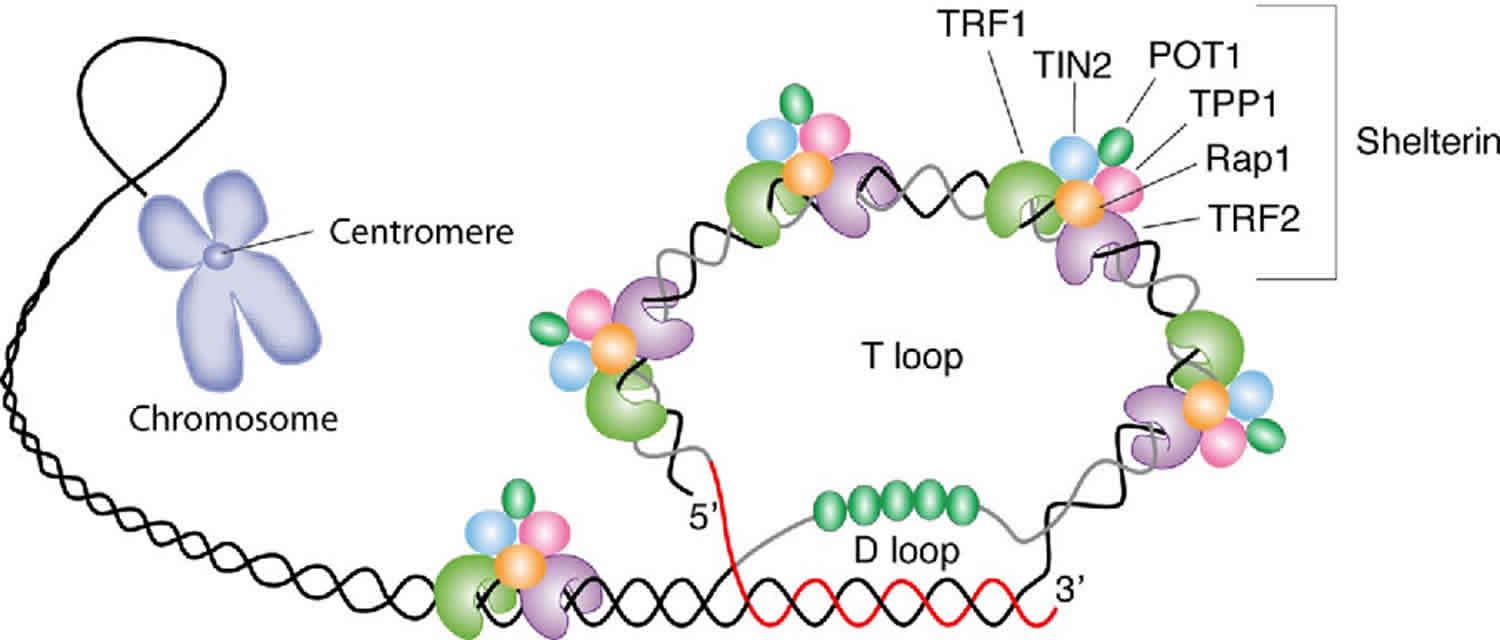
[Source 15 ]Figure 2. Telomere structure
Footnote: Schematic representation of telomere structure. Telomeres are at the extremities of chromosome DNA. The telomeric 3 end terminates as a single-stranded, G-rich overhang able to form the t-loop, in which the overhang invades the telomeric double helix, remodeling the DNA into a circle. Telomeres are capped by at least 6 proteins (TRF1, TRF2, TPP1, POT1, TIN2, and Rap1), collectively known as shelterin, that physically shield the DNA.19 TRF1, TRF2, and TPP1 specifically recognize and bind to doublestranded TTAGGG repeats; POT1 binds to the singlestranded telomeric overhang19,20; TIN2 and Rap1 complete the shelterin complex. Shelterin allows discrimination of telomeres from double-stranded DNA breaks; lack of shelterin allows telomeres to be identified as double-stranded DNA breaks and triggers DNAdamage pathways.
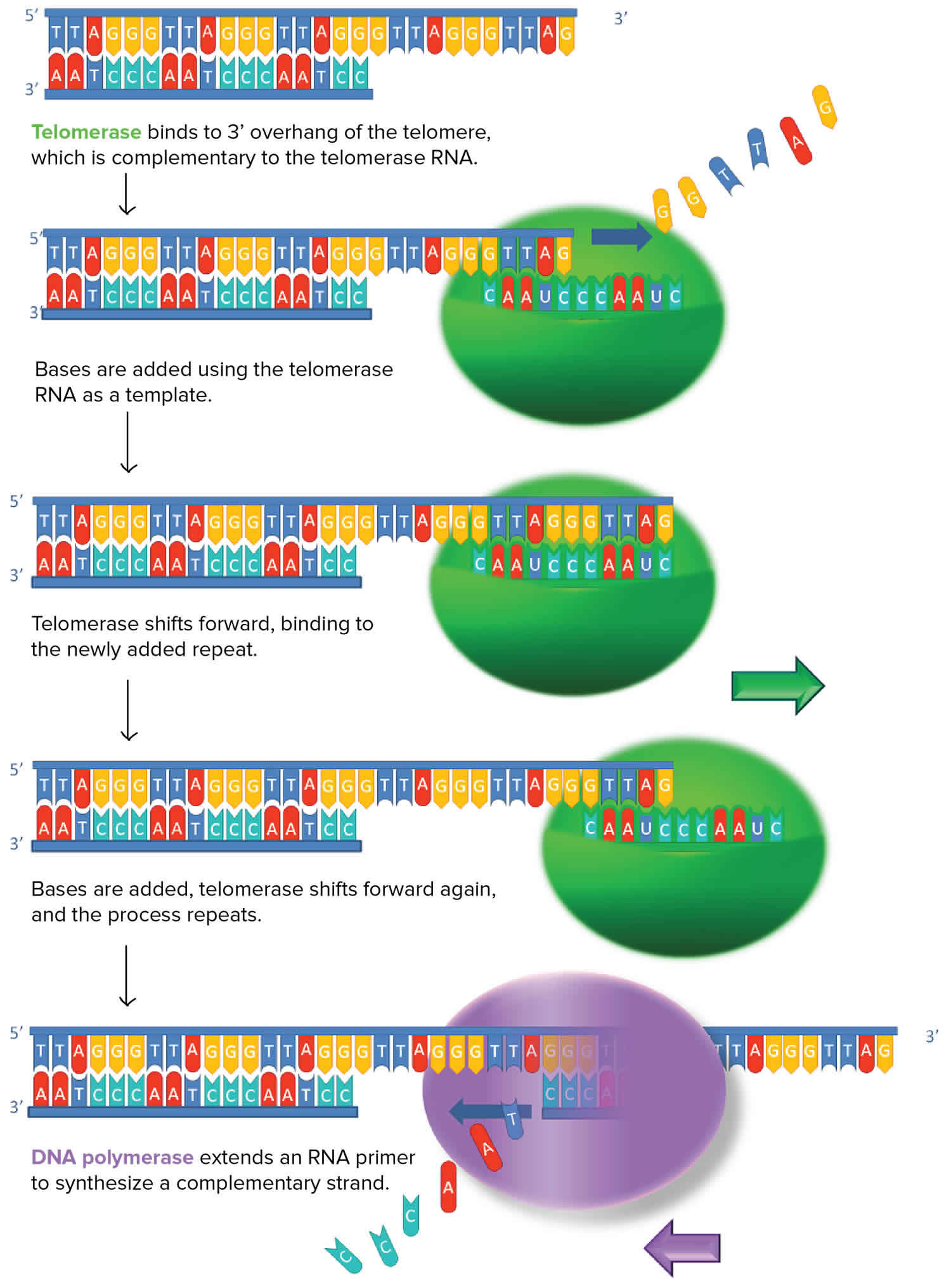
[Source 16 ]Figure 3. Telomerase
Figure 4. Telomerase structure
Replicative capacity of a cell is strongly correlated with telomere length regulation. Aberrant lengthening or reduction in the length of telomeres can lead to health anomalies, such as cancer or premature aging. Telomerase is a master regulator for maintaining replicative potential in most eukaryotic cells. It does so by controlling telomere length at chromosome ends. Akin to cancer cells, most single-cell eukaryotic pathogens are highly proliferative and require persistent telomerase activity to maintain constant length of telomere and propagation within their host.
Telomere dysfunction can result in enormously elevated rates of chromosomal alterations, particularly in subtelomeric regions. Interestingly, the chromosomal regions in the vicinity of telomeres are often among the most rapidly evolving in the genome. These facts could suggest that the protective capping function of telomeres has not evolved to be fail-safe but instead to permit a certain rate of failure that can foster evolution through subtelomeric rearrangements 17.
Just as the geography of the earth has influenced the nature and evolution of human communities, so too has the geography of chromosomes influenced the nature and evolution of genes. A prominent example of this is subtelomeric DNA. Subtelomeric regions are the frontier outposts of the genomes and existence for genes there can be precarious and not always governed by the laws that regulate genes in other parts of the chromosome. Like the inhabitants of frontier towns, the genes in subtelomeric regions are often not representative of those found elsewhere. Instead, subtelomeric regions are often enriched for genes that allow rapid adaptation to new environments.
The long linear DNA molecules that constitute eukaryotic chromosomes can have two types of DNA ends, telomeric ends and broken ends 18. The fundamental difference between a telomere and a broken DNA end is that the telomere is the natural stable end of the chromosome and the broken DNA end is something that typically occurs from damage and that the cell will usually rapidly repair. Telomeres and broken DNA ends are therefore usually treated by the cell in opposite ways.
Telomeric ends are the normal targets for sequence addition by telomerase, which counteracts the gradual sequence loss from ends that occurs as a consequence of the ‘end replication problem’ 19. Telomeric ends also function to prevent chromosome ends from acting like broken DNA ends. This protective role is commonly known as capping. In the great majority of eukaryotes (organisms whose cells have a nucleus enclosed within membranes), the distinctive features that distinguish telomeres from broken ends result from the specific DNA sequences that make up telomeres as well as the specific proteins that bind them. In the large majority of eukaryotes, telomeric DNA is composed of tandem arrays of a short repeat (5-26 bp) the sequence of which is specified by the template region of the telomerase RNA 20. Telomere capping generally involves telomere-specific DNA binding proteins. These include proteins that bind double stranded telomeric repeats as well as those that bind the single stranded 3′ overhangs at telomeric termini 21. Paradoxically, some proteins that bind to broken DNA ends also make critical contributions to telomere capping. Additionally, some organisms appear to use t-loops, structures where the 3′ overhang is thought to be strand invaded into more internal telomeric repeats, as a means to help cap telomeres 22.
Telomeric repeat sequences are not always limited to being at chromosome ends. Many species have telomere-like repeat arrays present at interstitial locations in chromosomes. These likely arise from a number of mechanisms including telomere-telomere fusions and aberrant repair of DNA double strand breaks (DSBs) 23. In at least some cases, these interstitial telomeric repeats can influence chromosome structure and stability by being hotspots for chromosome breakage or seed sequences for formation of a new telomere.
In contrast to telomeric ends, broken DNA ends (such as produced by DNA double strand breaks) are severe forms of DNA damage and are precursors of many if not most chromosomal rearrangements. DNA double strand breaks are typically repaired by either of two repair pathways, nonhomologous end joining or homologous recombination and are not normally substrates for addition by telomerase 24. Nonhomologous end joining is a ligation reaction requiring the specialized ligase IV enzyme plus certain additional proteins 25. Mitotic DNA double strand breaks repair by homologous recombination is thought to commonly proceed through a synthesis-dependent strand annealing mechanism that results in localized gene conversion but not in cross-overs. It requires an intact homologous sequence as a template and this is thought to be most commonly supplied by a sister chromatid. DNA double strand breaks repair through either nonhomologous end joining or homologous recombination will generally result in the two broken arms of a chromosome being rejoined. Homologous recombination will normally bring about precise repair (albeit sometimes with gene conversions) while nonhomologous end joining is imprecise and will often incorporate small insertions or deletions at the junction point.
Telomeres therefore serve as key guardians of chromosomal integrity. By blocking chromosome ends from being subjected to nonhomologous end joining or homologous recombination events, telomeres act to preserve the integrity of the genome, particularly in and around subtelomeric regions.
Immediate subtelomeric regions and their possible functions
Subtelomeric regions are the DNA sequences in the vicinity of chromosome ends. An exact definition is not possible but an approximate definition would be those sequences adjacent to the telomeres that have features that differentiate them from other regions of chromosomes. These regions are known from many organisms for unusual characteristics that include a complex repetitive structure, rapid evolution and a frequently heterochromatic nature 26.
Subtelomeric sequences can loosely be grouped into two categories; regions immediately adjacent to the telomeric repeats that often lack genes and are present at a large percentage of the chromosome ends and more internal gene-containing regions that are present at smaller subsets of chromosome ends. When subtelomeric sequences are present at more than one chromosome end, they strongly tend to have the same orientation with respect to the telomeres where they are present. The functions of the generally gene-free, immediate subtelomeric sequences are not fully clear and may vary between species. Although they do not appear to be vital to the basic protective capping function of telomeres, there is evidence that they can contribute to telomere length regulation 27. Subtelomeric sequences can also contribute to telomere position effect, the transcriptional repression that occurs next to telomeres 28.
Another function of immediate subtelomeric elements can sometimes be to serve as templates for telomere repair through homologous recombination. In S. cerevisiae, mutants lacking telomerase frequently amplify subtelomeric Y’ sequences and spread them to chromosome ends where they were not originally present 29. Y’ elements are DNA sequences of several kilobases in size that are present in one or two copies next to many but not all telomeres in wild type cells. In the related yeast, Kluyveromyces lactis, subtelomeric sequences can also be spread to other telomeres when telomeres become short and prone to recombination 30. These recombination events are thought to represent break-induced replication (BIR), a nonreciprocal homologous recombination event copying a sequence from one chromosome arm to another that acts to restore telomeric sequences to chromosome ends that have lost most or all of their telomeric repeats 31.
It is interesting to speculate that immediate subtelomeric repeats could sometimes have characteristics of selfish DNA elements. Replacement of part or all of such an element at one telomere by a break-induced replication event would clearly involve competition between other such subtelomeric elements in the cell. If subtelomeric break-induced replication events are not strictly limited to mutants with compromised telomere function, this would have the potential to select for elements particularly able to promote their own spread by break-induced replication events. The common presence of families of small direct repeats in immediate subtelomeric sequences conceivably might act to facilitate the homology search of a Rad51-coated DNA filament that would be expected to initiate most break-induced replication events.
Telomeres have been shown to be involved in the early stages of homologous chromosome pairing during meiosis 32. However, yeast subtelomeric sequences appear to be relatively resistant to being sites of meiotic cross over events. Work in S. cerevisiae has shown that subtelomeric sequences, even if moved away from a telomere, are relatively resistant to crossover formation 33. Those crossovers that do occur in subtelomeric DNA appear poorly able to bring about proper chromosome segregation at meiosis I 34. Consistent with these data, cleavage by Spo11, the nuclease that makes the DNA double strand breaks that initiate meiotic recombination, is infrequent in subtelomeric DNA 35. These data suggest that one function of subtelomeric DNA in yeast is to prevent Spo11-induced meiotic crossovers from occurring in regions of the chromosome where they would not be able to properly function. Meiotic recombination maps in humans, on the other hand, show an increase in recombination at the most distal markers 36.
- Shay J, Wright W. Hallmarks of telomeres in ageing research. J. Pathol. 2007;211:114–23[↩][↩]
- Moyzis RK, Buckingham JM, Cram LS. et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622–6626[↩]
- Makarov VL, Hirose Y, Langmore JP. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell. 1997;88:657–666[↩]
- Chow, T. T., Zhao, Y., Mak, S. S., Shay, J. W., and Wright, W. E. (2012). Early and late steps in telomere overhang processing in normal human cells: The position of the final RNA primer drives telomere shortening. Genes Dev., 26(11), 1168. http://dx.doi.org/10.1101/gad.187211.112.[↩]
- Vega, L. R., Mateyak, M. K., and Zakian, V. A. (2003). Getting to the end: Telomerase access in yeast and humans. Nature Reviews Molecular Cell Biology, 4, 951. http://dx.doi.org/10.1038/nrm1256[↩]
- Griffith JD, Comeau L, Rosenfield S. et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514.[↩]
- Greider, C.W. and E.H. Blackburn (1985) Cell 43:405.[↩]
- Watson JD. Origin of concatemeric T7 DNA. Nat New Biol. 1972;239:197–201[↩]
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460[↩]
- Bartlett, Z. (2014, November 14). The Hayflick limit. In The embryo project encyclopedia. Retrieved from https://embryo.asu.edu/pages/hayflick-limit[↩]
- d’Adda di Fagagna F, Reaper PM, Clay-Farrace L. et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426(6963):194–198.[↩]
- Counter CM, Avilion AA, LeFeuvre CE. et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929 [↩]
- Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791[↩]
- Bryan TM, Englezou A, Dalla-Pozza L. et al. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med. 1997;3:1271–1274[↩]
- Cesare AJ, Reddel RR. Alternative Lengthening of Telomeres in Mammalian Cells. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6486[↩]
- Calado, Rodrigo T. and Neal S. Young. “Telomere maintenance and human bone marrow failure.” Blood 111 9 (2008): 4446-55. https://pdfs.semanticscholar.org/cc3e/b79d4c3054d3f740af751a29692a55f35c66.pdf[↩]
- McEachern MJ. Telomeres: Guardians of Genomic Integrity or Double Agents of Evolution? In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6483[↩]
- de Lange T, Lundblad V, Blackburn EH.eds.Telomeres, second edition Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press 2006[↩]
- Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337(6205):331–337[↩]
- Autexier C, Lue NF. The Structure and Function of Telomerase Reverse Transcriptase. Annu Rev Biochem. 2006;75:493–517[↩]
- Bertuch AA, Lundblad V. The maintenance and masking of chromosome termini. Curr Opin Cell Biol. 2006;18(3):247–253[↩]
- Griffith JD, Comeau L, Rosenfield S. Mammalian telomeres end in a large duplex loop Cell. 1999. pp. 503–514[↩]
- Azzalin CM, Nergadze SG, Giulotto E. Human intrachromosomal telomeric-like repeats: sequence organization and mechanisms of origin. Chromosoma. 2001;110(2):75–82.[↩]
- Aylon Y, Kupiec M. DSB repair: the yeast paradigm. DNA Repair (Amst). 2004;3(8-9):797–815[↩]
- Daley JM, Palmbos PL, Wu D. et al. Nonhomologous end joining in yeast. Annu Rev Genet. 2005;39:431–451[↩]
- Mefford HC, Trask BJ. The complex structure and dynamic evolution of human subtelomeres. Nat Rev Genet. 2002;3(2):91–102[↩]
- Berthiau AS, Yankulov K, Bah A. et al. Subtelomeric proteins negatively regulate telomere elongation in budding yeast. EMBO J. 2006;25(4):846–856[↩]
- Cryderman DE, Morris EJ, Biessmann H. et al. Silencing at Drosophila telomeres: nuclear organization and chromatin structure play critical roles. EMBO J. 1999;18(13):3724–3735.[↩]
- Lundblad V, Blackburn EH. An alternative pathway for yeast telomere maintenance rescues est1- senescence. Cell. 1993;73(2):347–360[↩]
- McEachern MJ, Iyer S. Short telomeres in yeast are highly recombinogenic. Mol Cell. 2001;7(4):695–704[↩]
- McEachern MJ, Haber JE. Break-induced replication and recombinational telomere elongation in yeast. Annu Rev Biochem. 2006;75:111–135[↩]
- Bass HW. Telomere dynamics unique to meiotic prophase: formation and significance of the bouquet. Cell Mol Life Sci. 2003;60(11):2319–2324.[↩]
- Barton AB, Su Y, Lamb J. et al. A function for subtelomeric DNA in Saccharomyces cerevisiae. Genetics. 2003;165(2):929–934[↩]
- Ross LO, Maxfield R, Dawson D. Exchanges are not equally able to enhance meiotic chromosome segregation in yeast. Proc Natl Acad Sci USA. 1996;93(10):4979–4983[↩]
- Gerton JL, DeRisi J, Shroff R. et al. Global mapping of meiotic recombination hotspots and coldspots in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2000;97(21):11383–11390[↩]
- Matise TC, Sachidanandam R, Clark AG. et al. A 3.9-centimorgan-resolution human single-nucleotide polymorphism linkage map and screening set. Am J Hum Genet. 2003;73(2):271–284[↩]